










The purpose of this procedure is to establish and maintain chemical hygiene and safety standards for Purity Laboratories, Inc. (Purity). This procedure outlines the variety of hazards that personnel will encounter in the laboratory setting. These hazards include but are not limited to: chemical, biological and physical. This procedure details the proper way these hazards are handled and prevented. This policy helps to ensure that all laboratory operations are conducted using safe working practices and that all applicable local, state and federal regulations and policies are being correctly implemented
Due to the hazardous chemicals used within the laboratory, occupational exposure to such chemicals needs to be monitored This procedure addresses the specific chemical hazards, the location of the chemicals, how to properly handle, store and dispose of them This procedure will include the proper documentation steps necessary to ensure these chemicals are properly controlled
Biological hazards will also be used within the laboratory, and certain personnel will be potentially exposed to these hazards This procedure will outline the types of control needed in order to reduce personnel exposure to hazardous biological samples as well as the various levels of risk associated with each hazard
Physical hazards are also prevalent in the laboratory; these include but are not limited to: fire, electrical, ventilation, equipment and glassware. This procedure outlines the potential danger associated with each of these hazards and how to prevent them as well as how to handle a potentially hazardous situation.
This procedure applies to all personnel employed at Purity Laboratories, Inc. and to anyone otherwise working on the premises.

Normal chemistry laboratory precautions apply, including eye protection, lab coat and other protective precautions as required Depending on the severity of the hazardous chemical used, specific personal protective equipment will be required in the handling of said hazards
Extra personal protective equipment is available in the laboratory depending on the severity of the hazard Thicker gloves, full coverage safety glasses, particle masks and aprons may be used if chemicals with higher GHS ratings are to be handled
4 1 PPE - Personal Protective Equipment: protective clothing or garments designed to protect the wearer’s body from injury by objects, heat, chemicals, electrical components, infections and other potential hazards that he or she may be exposed to at his or her occupation.
4.2 MSDS: Safety Data Sheet: a standardized chemical information sheet that provides workers with procedures for handling and working with materials and substances in a safe manner. It has sixteen standardized categories that include but is not limited to: product identification, hazard identification, composition information, first aid measures, accidental release measures, handling and storage, exposure controls, physical and chemical properties, stability and reactivity, and disposal considerations.
4.3 CHP: Chemical Hygiene Plan: a document that covers the specific areas of the laboratory and outlines the potential hazards.
4 4 OSHA: Occupational Safety and Health Administration: OSHA standards are rules that describe the methods that employers must use to protect their employees from hazards
4 5 GHS: Globally Harmonized System of Classification and Labeling of Chemicals is a standardized system for classifying and displaying chemical hazards The system contains nine hazard categories, and uses symbols, signal words, and hazard statements to communicate
4 6 NFPA 704: National Fire Protection Association 704 is a standard system for the identification of the hazards of materials for emergency response It was commonplace prior to the adoption of GHS, and is included here for completeness A placard placed on all chemicals denoting their stability and the hazard that they possess There are four color coded squares on the placard, blue is health, red is flammability, yellow is reactivity and white is special (including but not limited to: reacts with water, oxidizer, asphyxiant gas). Each has a scale of 0-4 depending on severity of hazard with 4 being the most hazardous.
4.7 BSL: Biosafety level. The level of the biocontainment precautions required to isolate dangerous biological agents in an enclosed facility. The levels of containment range from 1-4 depending on the danger of the hazard with 4 being the most hazardous.
4.8 Bloodborne Pathogen: Any disease that can be spread through contamination via blood or any of its constituents including blood and blood plasma.
4 9 OPIM: Other potentially infectious material This category of biohazard includes semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva and, any other bodily fluid that is contaminated with blood
4 10 Clinical Laboratory: A workplace where diagnostic or other screening procedures are performed on blood or other potentially infectious materials

4.11 Exposure Incident: A specific occurrence where personnel are exposed to blood or other potentially infectious materials via the eyes, mouth, mucous membranes or skin through duties performed by their job.
5.0 Responsibility
5.1 Chemical Hygiene Officer (CHO): This person is responsible for implementing the Chemical Hygiene Plan He or she is responsible for knowing the CHP and training employees on its contents The CHO is also responsible for implementing new chemical and safety procedures when necessary; including but not limited to when new chemicals are being used in the laboratory
5.2 Safety Officer: This person is responsible for all OSHA related tasks and reports as well as giving a monthly safety topic to all personnel on relevant safety related issues This person is responsible for following the proper protocol including corrective action for safety violations and incidents This person is also responsible for updating safety forms and investigating safety concerns
5.3 Chief Operations Officer: The COO is responsible for working with the CHO to control and coordinate chemical hygiene and safety. He or she is responsible for the control of chemicals as well as the proper disposition of them.
5.4 Quality Department: The Quality Department is responsible for ensuring personnel are following all standard operating procedures and safety protocols. The Quality Department is responsible for any and all disciplinary action that results in violations by personnel of safety protocols.
5.5 Personnel:
5 5 1 It is the responsibility of personnel to understand and follow all safety procedures as well as know the GHS and NFPA rating system and Biosafety Levels

5 5 2 If a new chemical comes into the lab, personnel are responsible for notifying the CHO so it can be properly labeled and documented
5 5 3 Personnel must be familiar with all safety and hygiene procedures as well as how to respond during an emergency situation
5 5 4 Personnel are required to wear proper personal protection equipment (PPE) at all times within the laboratory setting This includes but is not limited to: lab coats, eye protection, long pants, closed-toe shoes and gloves
5 5 4 1 Other PPE will be available to all personnel who might need extra protection against various hazards This includes aprons, full coverage eye protection, heavy-duty gloves, and respirators
1.0 Outline
This section outlines all the basic safety precautions as well as the general rules for working in the laboratory. These rules include but are not limited to: general laboratory conduct, hygiene policies, personnel training and workplace rules.
2.1 Eating and drinking in the laboratory is strictly forbidden due to the potential crosscontamination of food to samples as well as the hazardous nature of various chemicals and microbiological standards used. All eating and drinking is to take place in the kitchen, lunch room or front office/ administrative area.
2 2 The kitchen and lunch room are to be kept clean and free of clutter All extra food will be stored in cabinets or labeled in the refrigerator The microwave and convection oven in the kitchen shall not be left unattended while actively in use The refrigerator in the kitchen is for personnel use only and no samples will be stored within Conversely, all sample refrigerators within the laboratory are for sample storage only
2 3 Employees are not to keep personal belongings in the laboratory area; this is to keep samples from getting contaminated from the outdoors and to keep personal belongings from getting damaged from the various chemicals and standards in the laboratory
2 3 1 Laboratory area includes but is not limited to: any and all areas where test methods are being conducted, sample storage, chemical storage and laboratory material storage
2.3.2 Personnel will be provided with a place to store their personal belongings that is not within the laboratory testing area.
2.4 The laboratory is to be kept neat and orderly. Chemicals and standards not being used will be stored in their proper location depending on nature of the substances. All instruments and equipment will be maintained according to their specifications and stored properly when not in use.
2.5 Laboratory personnel are responsible for cleaning up a work area subsequent its use. This includes but is not limited to: scales, smaller mobile equipment, glassware, countertops, sinks, hoods and other laboratory equipment
2 6 Finished samples will be disposed of and stored according to LP2005 Test Sample Storage, Dispersion, Retention, and Disposition When chemicals used within the laboratory are either exhausted or no longer in use, they will be disposed of according to LP3003 Chemical Hygiene Plan and Safety Manual Section III: Chemical Hazards

3.1 In order to prevent any cross contamination from samples and chemicals that are used in the laboratory to personal food and drinks, lab coats are required to be removed before entering the lunch room and kitchen.
3.2 Lab coats are required to be removed prior to entering the restroom. No lab coats will be permitted in the restroom to prevent any cross-contamination to samples in the lab.
3.3 Lab coats are not permitted outside. Personnel are required to remove lab coats prior to exiting the building. If an employee needs to dispose of something that is hazardous or messy, he or she may wear a lab coat while disposing the item, but he or she needs to use a new lab coat once re-entering the laboratory and place the soiled lab coat in the proper location
3 4 Smoking is prohibited in the building If employees wish to smoke, they must go to a designated smoking area outside Designated smoking areas are required to be at least 10 feet away from any building entry point
3 5 Hand washing is required after using the restroom, after eating or drinking and before returning to the workstation When an employee is working with a sample or chemical that is repugnant, he or she is required to wash hands intermittently while working with that substance

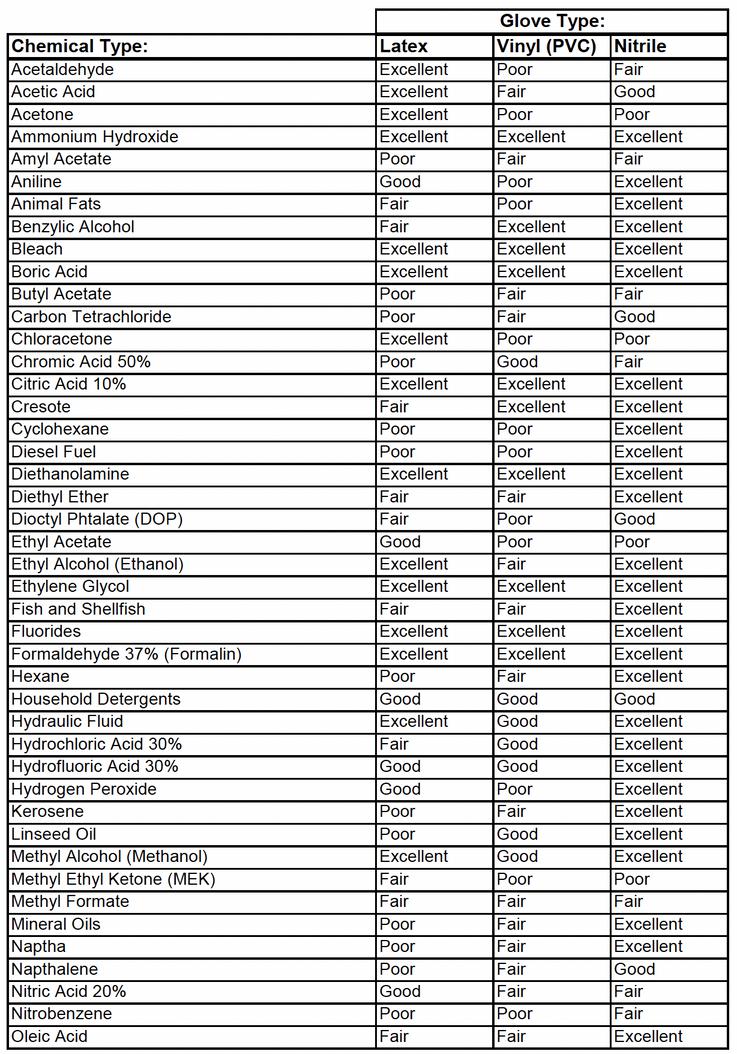
3 6 Gloves are required to be worn while working with any and all chemicals and samples in the laboratory. In order to prevent cross contamination, gloves should be changed frequently. Frequent glove changing also keeps them from wear and tear when working with harsh chemicals. Some gloves are not compatible with certain chemical uses. To determine what gloves to use, see LP3009 Appendix A: Glove Comparison Chart.
3.6.1 Hands should be clean before donning gloves and washed every time gloves are changed.
3.6.2 Gloves should be changed when entering a different testing area.
3.7 Personal hygiene and grooming:
3.7.1 Lab coats are required to be worn whenever working in the laboratory.
3.7.2 All personnel are expected to dress and present themselves with professionalism. Certain clothing choices are prohibited from being worn in the laboratory due to the fact that they may increase an employee’s exposure to various hazards All laboratory personnel are required to wear long pants and closed-toe shoes that completely cover the foot Laboratory
personnel should have long hair secured and should not wear dangling or obstructive jewelry. Administrative employees are allowed to wear clothing that isn’t as protective, but will not be allowed in the laboratory area unless properly covered. Clothing that is prohibited for laboratory personnel include: open-toed shoes, pants that do not cover the legs or ankles, bare-midriff tops, skirts and dresses that are worn bare-legged. All personnel will be required to wear eye protection when in the laboratory area.
4.1 Visitors: Any non-laboratory personnel are required to sign in before entering the laboratory. Each visitor will be assigned a name badge. Most visitors will be asked to stay in the blue tiled areas of the laboratory to avoid any work benches or hazardous chemicals, and under supervision of Purity staff Visitors are required to wear protective eyewear When the visitor has completed his or her business in the laboratory, the visitor will need to sign out and return his or her name badge and any protective gear loaned
4 1 1 Non-laboratory personnel includes but is not limited to: visitors, vendors, volunteers, family, children, visiting scientists and instructors

4 2 Security: The main entrance to the laboratory is to remain unlocked during normal business hours which are Monday through Friday 7:30 am to 4:30 pm Access to the laboratory is only permitted to personnel or visitors that have signed in
4 2 1 The doors will all automatically lock at 4:30 pm on weekdays and remain locked until 8:00 am the following work day The doors will be locked all day on the weekends and holidays
4.3 Testing hours may be outside the span of normal business hours. If personnel are at the laboratory before or after normal business hours, the main entrance is to remain locked. All personnel working outside normal business hours are to be given a key card and security code to the main entrance.
4.4 The alarm is to be turned on by the last employee leaving the laboratory and disabled the next day by the first employee entering the laboratory. All approved employees will be trained how to arm and disarm the alarm system.
4 5 Alarm System:
4 5 1 Each employee will be given a key fob that has a unique identification number Human Resources controls all the key fobs and number assignments
4 5 2 To unlock the front door, wave the key fob in front of the security box adjacent to the door handle
4 5 3 When the red light flashes green, the door will be unlocked Once an employee enters through the door, the door will automatically close and re-lock
4 5 4 If there are problems with the alarm system, the employee is to contact the Chief Operating Officer
4.6 At the end of the day, the last employee is to complete the following checks:
4.6.1 Ensure the warehouse lights are off.
4.6.2 Ensure all employees have exited the building.
4.6.3 Ensure all exit doors are closed to prevent the alarm from activating.
4.6.4 Set the alarm by entering in the 4 digit code on the keypad. The system is now armed and will display a 60 second countdown. Exit the building before the 60 seconds to prevent the alarm from being activated.
4.6.4.1 If the security code was entered into the system incorrectly, press the “#” button to clear and then re-enter the code.
4 7 Employees are not to work alone in the laboratory area It is required that a minimum of two employees are in the laboratory area during scheduled working hours This is to minimize any hazards or accidents that may occur in the laboratory and to keep personnel safe If an employee needs to work outside his or her scheduled hours, the employee needs to inform management so the proper arrangements for another employee to work at the same time can be made
4 7 1 In extreme circumstances such as illness or other unplanned absences, an employee may work alone in the lab He or she is required to contact a supervisor via phone or email at the minimum of three times per shift to ensure his or her safety
4 7 2 Employees may however, work alone in administrative and front office areas alone This does not include working in the laboratory area, even if the work is administrative in nature The employee working alone must notify supervisor before working alone

5.1 Emergencies: All personnel will have access to phones, fire extinguishers, first aid kits, safety showers and eyewash stations in the event of an emergency. For more information on emergencies, see LP3006 Section VI: Emergency and Accident Plan.
5.2 Training: Only trained personnel are authorized to operate laboratory equipment and handle potentially hazardous materials and chemicals, including bio-hazardous materials. All training must be documented on LF2018 Training Record.
5.3 OSHA Requirements: All OSHA standards for safe laboratory practices will be followed Things that are prohibited in the laboratory are:
5 3 1 Horseplay
5 3 2 Running
5 3 3 Handling multiple chemicals or carrying multiple containers
5.3.4 Mouth pipetting
5.3.5 Using compromised glassware
5.3.6 Using unlabeled chemicals
5.3.7 Making unauthorized modifications to equipment or procedures

5.3.8 Improper chemical storage
5.3.9 Laboratory personnel are required to: Wear and use proper PPE
5.3.10 Dispose of chemicals and samples properly
5.3.11 Maintain records of chemical receipt, storage and disposition.
5.3.12 Follow all laboratory procedures and protocol
5.3.13 Be trained in all areas in which they work. This training is to be documented.
5 3 14 Notify management of all medications that can interfere with work; drowsiness or other side effects
5.4 Safety Committee: Each month, the Safety Committee will create an applicable safety topic and present it at the first laboratory meeting of the month The topic can range from chemical safety to physical hazards an employee might encounter at the lab
5 4 1 The topics are to be saved and stored in the proper secure location in the Safety Committee Binder
5 4 2 A training document for the monthly safety topic will be filled out and signed by all personnel present
5 4 3 If an employee is absent for the first meeting of the month, the Safety Officer is responsible for training the employee and filling out the proper training document
5 4 4 The Safety Committee will be comprised of a representative from each department in the laboratory This includes: Microbiology, Chemistry, Quality, Administrative and Clinical
5.4.5 The Safety Committee will appoint one representative as the Safety Officer, the officer position will be held for one year, and then a new appointment will be made.
5.4.6 The Safety Committee will meet at minimum of once a quarter, but meetings can be called more often when needed.
5.4.7 It is the responsibility of the Safety Officer to find and present concers to the quarterly lab technicians' meetings. He or she will inform the Safety Committee of the topic to determine if the committee wants to add anything to the topic.
5.5.1 Any employee that gets injured or falls ill at work or due to the working environment is to fill out LF1002 Accident Report This will document the nature of the injury and if medical attention is needed
5 5 2 Even if an injury or illness does not seem serious at the time, they are still required to be document in the event they become worse
5 5 3 If an employee is injured to the point he or she needs urgent medical attention, the Workers’ Compensation Call-In Script Form is required to be filled out This form is not required to be taken to the medical office at the time of the emergency Emergency handling takes precedence, but once the employee has been treated, the form should be filled out and taken to the medical office
5 5 4 If an employee is on a medication prescribed by a doctor, the doctor must confirm the employee’s ability to work and complete tasks at hand Certain medications may interfere with an employee’s ability to safely and competently complete a task, or can contaminate certain tests conducted.

6.1 LP2005 Test Sample Storage, Dispersion, Retention, and Disposition
6.2 LP3003 Chemical Hygiene Plan and Safety Manual Section III: Chemical Hazards
6.3 LP3006 Section VI: Emergency and Accident Plan
6.4 LP3009 Appendix A: Glove Comparison Chart
6 5 LF1002 Accident Report
6 6 LF2018 Training Record
This section outlines all the basic safety precautions for working with chemicals in the laboratory It also outlines the protocols for chemical handling, storage and disposition The laboratory is responsible for controlling all types of chemicals and there is a rating system in place for chemical monitoring and handling
There are many types of chemical hazards that Purity personnel will encounter while working in the laboratory environment They include but are not limited to:
Acids
Bases
Flammables
Toxic
Corrosive Oxidizing Irritants

Non-Hazardous
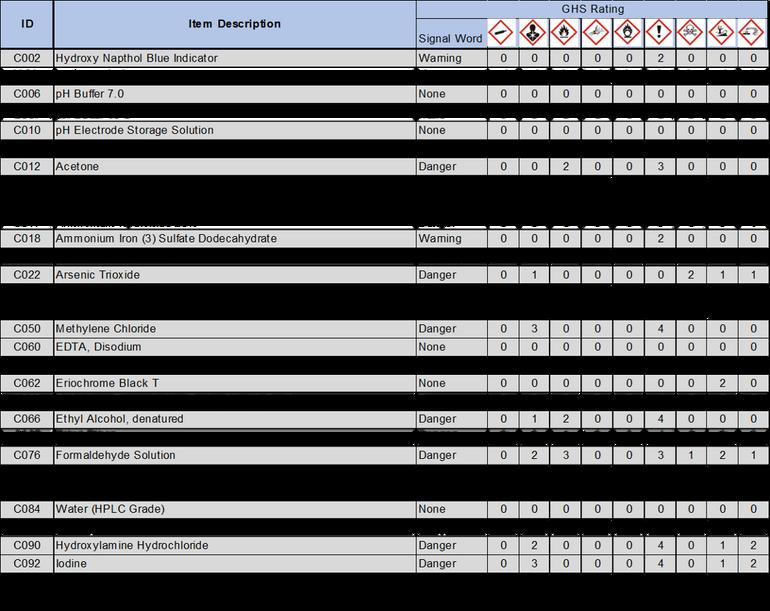
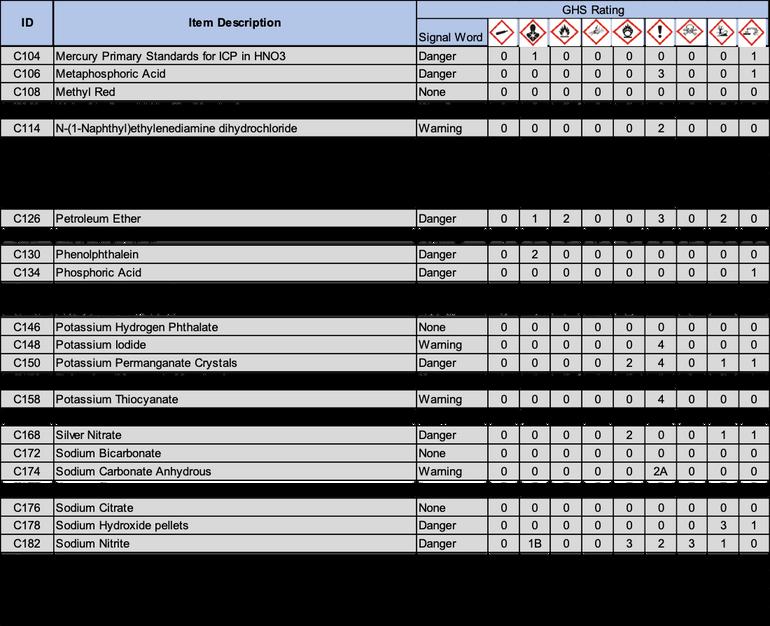
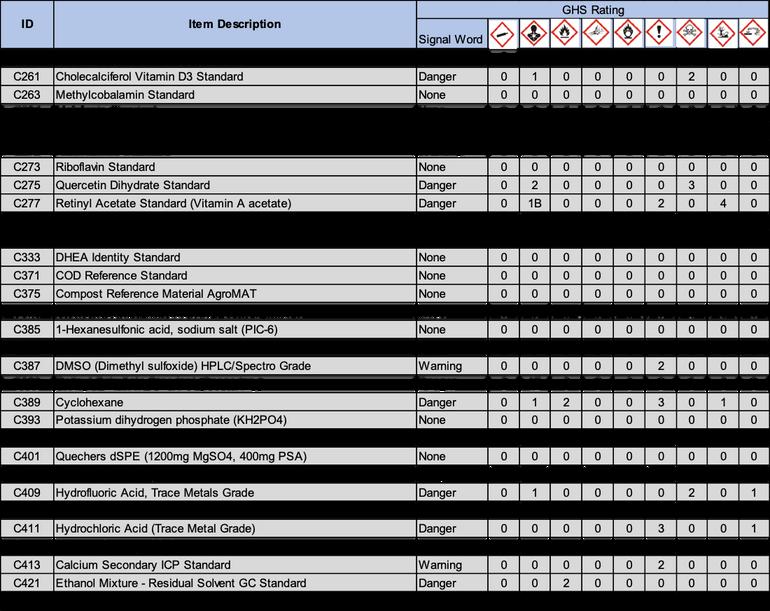
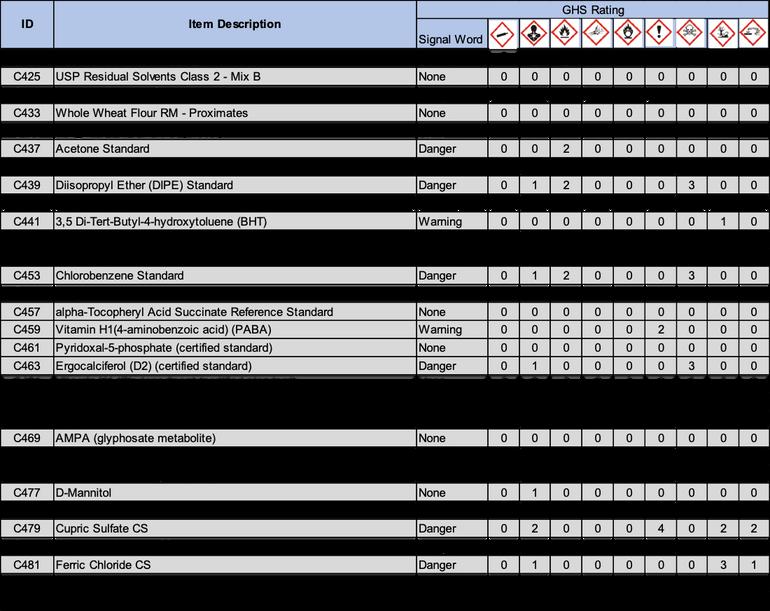
2 1 Following OSHA requirements, all chemicals must now be labeled under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) This system includes nine classes, or types, of hazards Numerical categories designate the severity of the hazard, with 1 being the most severe Categories are usually not present on chemical labels, but are listed in section two of the standardized SDS Labels in accordance with this system include three standardized elements: symbols, signal words, and hazard statements
2 1 1 Symbols: Symbols, or hazard pictograms, represent the nine hazard classes Each contains a black and white illustration within a red diamond The table below illustrates the nine hazard symbols and lists their meanings
2 1 2 Signal Words: Two signal words are used to denote the severity of the hazard “Danger” is used for more severe hazards, and “Warning” for less severe ones If a substance is hazardous, one of these words will appear on the label below the hazard symbols
2.1.3 Hazard statements: Hazard statements are standardized phrases that describe the associated hazards in more detail. For example “Harmful if swallowed”.


2 2 The NFPA 704 system for classifying chemical hazards was used prior to the adoption of GHS In the event NFPA labels are present, the system is described here An AFPA label includes four color coded squares with numbers corresponding to the severity of the hazard:
2 2 1 Flammability (red): Depending on how easily something will burn or ignite, the NFPA has a 0-4 rating system, with 4 being the most severe
0: Will not burn
1: Must be heated for ignition to occur; flash point over 93°C
2: Must be moderately heated or exposed to relatively high ambient temperature before ignition can occur. Flash point between 38°C and 93°C.
3: Liquids and solids that can be ignited under almost all ambient conditions. Liquids have a flash point below 23°C and have a boiling point at or above 38°C, or have a flash point between 23°C and 38°C.
4: Will rapidly or completely vaporize at normal atmospheric pressure and temperature, or is readily dispersed in air and will burn readily. Flash point below 23°C.
2.2.2 Health (blue): Depending on severity of adverse health effects, the NFPA has a 0-4 rating system, with 4 being the most severe.
0: Poses no health hazard, no precautions necessary.
1: Exposure would cause irritation with only minor residual injury.
2: Intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury
3: Short exposure could cause serious temporary or moderate residual injury
4: Very short exposure could cause death or major residual injury
2 2 3 Reactivity (yellow): Depending on how easily a substance will react, the NFPA has a 0-4 rating system, with 4 being the most severe
0: Normally stable, even under fire exposure conditions, and is not reactive with water
1: Normally stable, but can become unstable at elevated temperatures and pressures
2 2 4 Special (white): This category includes substances that require special care when using or handling
OX: Oxidizer
W: Reacts with water in an unusual or dangerous manner
SA: Simple asphyxiant gas.
Nitrogen Helium Neon Argon Krypton Xenon

2 2 5 Other: These symbols are not a part of the NFPA 704 standard but will also be present on the white section of the hazard diamond For pictures of the symbols see Appendix D
TOX: Toxic or poisonous
CORR: Corrosive

IRR: Irritant
BIO: Biological hazard
2.3 Chemical Control:
2.3.1 New Chemicals:
2.3.1.1 All new chemicals brought into the laboratory will be documented and it is responsibility of the CHO and Quality Department to update the LF2021 Stock Chemical Logbook whenever a new chemical is brought into the laboratory.
2.3.1.2 All new chemicals will be evaluated to ensure hazard labels are in compliance with GHS standards. These will then be updated in the logbook.
2.3.1.3 All chemicals must be stored in the proper location according to the Chemical Hygiene plan.
2.3.1.4 Each container of new chemical arriving to the lab will be marked with received date, opened date, expiration date and initials of analyst. If multiple containers of the same chemical were received, this will also be noted on the container
2 3 1 5 Every reagent, chemical, standard, media or other substance that is received in the laboratory will need to be documented via the LF2021 Stock Chemical Logbook This includes new chemicals as well as ones that have been received in the past
2 3 1 6 If not provided by the manufacturer, expiration dates will be determined from the chemical composition and reactivity information provided in the SDS
2 3 1 7 If a reagent, chemical, standard, media, or other substance is used up, the consumed by date must be documented in LF2021 Stock Chemical Logbook
2 3 2 Chemical Handling:
2 3 2 1 Only personnel that have been trained to properly handle hazardous chemicals should use them Both hands are required when picking up and transporting large jugs of chemicals If a chemical is too large or difficult to handle, use a cart, hand-truck or ask a fellow employee for help.
2.3.2.2 If a chemical bottle looks damaged or otherwise deemed unfit for use, transport to a proper container or dispose of accordingly. Notify the CHO and Quality Department of unfit chemicals found in the laboratory.
2.3.2.3 If a chemical is transferred to a secondary container, that container must be labeled with a GHS hazard label unless the worker who made the transfer uses all of its contents during their work shift.
2 3 3 Chemical Storage:
2 3 3 1 See LP3012 Appendix D: Proper Chemical Storage for chemical storage locations
2 3 3 2 All chemicals in GHS flammable categories 1-4 are required to be stored in a flammable cabinet
2 3 3 3 All acids will be stored in the acid cabinet
2 3 3 4 All bases will be stored in a separate safety cabinet from acids and solvents
2 3 3 5 All reactive, toxic and corrosive chemicals will be stored in a safety cabinet
2 3 3 6 Dry chemicals can be stored in general storage cabinets as long as they don’t have a low category value in any GHS hazard class The storage of these chemicals is at the discretion of the CHO and Quality Department
2.3.4 Chemical Disposal:
2.3.4.1 LF2021 Stock Chemical Logbook, under the Chemical Inventory ID tab, will have information on the hazard of each chemical, as well as the SDS
2.3.4.2 Innocuous chemicals can be disposed of down the drain.
2.3.4.3 Hazardous chemical waste must be disposed of in proper waste receptacles, and either neutralized/decontaminated to be disposed of down the drain or removed by a chemical waste removal company.
2.3.4.3.1 The disposition of waste by a chemical waste removal company must be documented via LF3001 Chemical Disposition Logbook.

3.0 References / Cross-References
3 1 LP3012 Appendix D: Proper Chemical Storage
3 2 LF2021 Stock Chemical Logbook
3 3 LF3001 Chemical Disposition Logbook
1.0 Outline
This section outlines all the basic safety precautions for working with biological standards and samples in the laboratory. It also outlines the protocols for handling, storage and disposition of these biological hazards. The laboratory is responsible for controlling all types of standards and samples and there is a rating system in place for monitoring and handling.
There are many types of biological hazards that Purity personnel will encounter while working in the laboratory environment. They include but are not limited to:
2 1 There are a variety of potential biological hazards present at the laboratory that personnel could be exposed to These hazards include, but are not limited to: microbiological positive control standards, human blood, human serum, human urine and OPIM
2.2 Biosafety Levels: This category is for microbiological hazards such as positive control standards. The range of BSL is 1-4 with 4 being the most hazardous.
2.2.1Strains of microorganisms not known to cause disease in healthy human adults. Normal safety procedures apply.
2.2.2 Moderate risk microorganisms that are associated with less serious human diseases. Extra precautionare necessary such as PPE and limited lab access.
2.2.3 High risk microorganisms with a true potential for infection via inhalation. PPE required such as respirators, double gloves and gowns. Work only to be conducted in an isolated laboratory.
2 2 4Easily transmitted very high risk microorganisms which cause life threatening diseases for which there is no treatment Hazmat suits required as well as chemical showers
2 3 Microbiological Hazards:
2 3 1 There are a variety of positive control standards used by the microbiology department
Each of these standards comes with a BSL (Biosafety Level) rating Depending on the degree of the rating, various precautions must be taken when using these standards


2.3.2 The list below is all the microbiological positive control standards used in the laboratory along with their corresponding BSLs. Regular PPE is required to be worn including labcoats, proper footwear, protective eyewear and gloves. Aprons, facemasks and respirators will be made available for personnel wanting extra protection while working with BSL 2 organisms.

2 4 Bloodborne Pathogens and OPIM:
2 4 1 Bloodborne pathogens and OPIM will be controlled under LP3050 Bloodborne Pathogen Exposure Control Plan All normal safety standards are required when working with these hazards and they will be controlled and documented accordingly
2.4.1.1 All procedures for handling an exposure incident are detailed in LP3050 Bloodborne Pathogen Exposure Control Plan.
2 4 2 Any employee who will be in direct contact with a bloodborne pathogen or OPIM is required to complete and pass bloodborne pathogen training
2 4 2 1 The training may be administered by any private or public organization as long as proper documentation is completed and the training is administered by a knowledgeable healthcare professional

2 4 2 2 All personnel that go through the bloodborne pathogen training will need to obtain documentation that they have completed the course
2 4 2 3 Personnel that have not completed bloodborne pathogen training are not permitted to work with any OPIM, blood or its constituents.
2.4.2.4 All personnel that complete the bloodborne pathogen training will have the option to be vaccinated against certain blooodborne pathogens. Vaccination is not required and will be paid for by the company.
2.4.2.4.1 Documentation of an employee’s choice of vaccination will be kept in his or hers employee file.
There are many types of hazards that Purity personnel will encounter while working in the laboratory environment There are a variety of precautions and regulations in place to handle each of the hazards present in the laboratory The physical and environmental hazards present in the laboratory include but are not limited to:
Flammables
Toxic
Irritants
Non-Hazardous
Electrical Equipment
Gas
Labware
Noise
Ventilation

2.1 There are a variety of physical hazards personnel can encounter in the laboratory. Caution must be taken while working with and around these hazards Extra PPE will be available to all personnel regardless of the various hazards presented day to day to each employee
2 2 1 There are multiple electrical hazards within the laboratory, this includes but is not limited to: circuit boards, various equipment, appliances, outlets and IT accessories There are multiple tactics for working safely in the laboratory in order to reduce the risk of electrocution presented by these electrical hazards The following is an outline of OSHA safety practices that help to reduce this risk:
2 2 1 1 Always follow manufacturer’s recommendations for using electrical equipment
2 2 1 2 Do not use electrical equipment to perform a task for which it was not designed
2 2 1 3 Safely designed equipment includes either a three-pronged plug or double insulation.
2.2.1.3.1 If a product does not have one of these features, it will not protect the use from electric shock.
2.2.1.4 Use a fused power strip that will automatically shut off if too much power is used when needing multiple pieces of equipment for one outlet.
2.2.1.5 Any outlets near a sink or water source should be Ground-Fault Circuit Interrupter (GFCI) protected.
2.2.1.5.1 Periodically check the GFCI outlet by plugging a piece of equipment into it and pushing the “test” button, once the equipment shuts off, press “reset” to turn it back on.
2.2.1.6 Do not disable any electrical safety feature
2.2.1.7 Before turning equipment on, check that all power cords are in good condition
2.2.1.8 Do not use extension cords as a substitute for permanent wiring
2.2.1.9 In the event that an employee gets electrocuted, do not touch them. Instead turn off the power by pulling the plug or tripping the circuit breaker. Or use a non-conductive material to pull the employee away from the object. Then call 911 immediately.
2.3.1 All the equipment in the laboratory will be handled and equipped with proper safety precautions. These safety features must not be tampered with or disabled. However, certain pieces of equipment still are considered hazardous, so certain precautions are required to be taken against them
2 3 2 Autoclaves:

2 3 2 1 Autoclaves can cause burns from handling hot items being taken out or from the autoclave itself while it’s running
2 3 2 2 Personnel are to wear the proper PPE when using an autoclave such as oven mitts for hot items being removed or steel mesh gloves for sharps
2 3 2 3 Personnel are to exercise caution when opening autoclaves due to pressure buildup and steam ventilation
2 3 3 Centrifuge:
2 3 3 1 Due to the high speed of centrifuges, they have the potential for causing injury
2 3 3 2 The most common source of injury from a centrifuge is user error In order to prevent this, ensure the centrifuge is balanced.
2.3.3.2.1 If there are an uneven number of samples, blanks are to be made using a comparable material.
2.3.3.2.2 Ensure all samples and blanks are placed in the same test tube type.
2.3.3.3 When working with hazardous material, employees are to wait for ten minutes after it has completed its cycle before removing samples.
2.4 Gas:
2.4.1 Compressed gases can be toxic, flammable, oxidizing, corrosive or inert. Leakage of any of these types of gases can be hazardous.
2 4 2 Inert gases can displace the air quickly which results in an oxygen deficient air
2 4 3 Toxic gases can create a poisonous atmosphere
2 4 4 Flammable or reductive gases can result in fires or exploding cylinders
2 4 5 The pressure of the gas in the cylinder is also considered a hazard If the valve is broken off, the gas cylinder can become a projectile and cause damage
2.4.6 The gas cylinder is large and heavy. It can fall over and cause damage to people and surrounding objects. Cylinders are to be kept in a specific location on a protective non-slip surface. They are also required to be chained or secured to a wall or stable surface.
2.4.7 All cylinders are to be stored upright.
2.4.8 Cylinders are never to be dropped or allowed to strike each other with force.
2.4.9 Transportation of compressed gas cylinders requires protective caps to be in place.
2.4.10 The cylinders are not to be rolled or dragged; but must be moved with an appropriate handcart.
2.4.11 All cylinders are to be equipped with pressure relief devices. These are to be maintained by the gas supplier.
2 4 12 Some cylinders will vent when they build up pressure This is a normal occurrence; however precaution should be taken to prevent alarm Purchasing of whisper valves can help to alleviate the startling noise the tanks make when they vent
2 5 1 All glassware in the lab is to be used solely for its intended purpose
2 5 2 If glassware is broken or damaged in any way, it is to be disposed of in the broken glass receptacle
2 5 3 Broken glassware with blood or OPIM on them will be disposed of in a sharps container
2 5 4 If an employee gets cut from broken glassware, dispose of the piece of glassware in the sharps container and follow the first aid protocol in LP3006 Section VI: Emergency and Accident Plan
2.6.1 Personnel are exposed to a variety of noises throughout the laboratory. This includes but is not limited to: instruments, fans, exhaust systems, fume hoods, centrifuges, sonicators, stirrer motors and autoclaves.

2.6.2 Many of these objects are constantly running, which can make personnel habituate to the loudness of them. Constant noise at high decibels can cause irreversible hearing damage. All personnel should wear proper PPE in order to prevent this.
2.6.3 Hearing damage occurs at levels of 85 decibels or greater. If laboratory noise reaches this level, precautions should be taken to protect employees. This can include PPE as well as enclosing certain pieces of equipment that generate a lot of noise
2 6 4 Ear plugs will be provided to all personnel for hearing protecprotection If ear plugs are not sufficient protection, other ear protection will be available This includes noise cancelling headphones and earmuffs
2 6 5 The laboratory will be tested for decibel level any time a new piece of equipment is added that generates noise as well as on an annual basis If the decibel level increases, the necessary precautions will need to be taken to prevent employee hearing damage
2 6 5 1 Decibel testing may be performed in house or by an outside vendor Records will be kept of the test and results of inquiry
2.6.5.2 Decibel testing will be recorded in the LF3002 Decibel Testing Logbook.

2.7.1 The laboratory will be equipped with proper ventilation systems, separated out between each department. This is done to reduce the potential of airborne cross-contamination.
2.7.2 The air ventilating throughout the lab will be filtered as to not bring in any external environmental contamination.
2.7.3 The internal laboratory temperature will be constantly maintained at a specified range. This is to ensure all testing being performed is done in the proper environment.
2.8 Chemical Fume Hoods:
2 8 1 Chemical fume hoods are to be used by personnel when they need to work with certain hazardous chemicals
2 8 2 If chemicals are irritating to the skin, eyes or respiratory tract, personnel must work with them exclusively under the fume hood
2 8 3 Only trained personnel are to operate fume hoods
2 8 4 Before using a fume hood, check to ensure the air is circulating properly
2 8 5 The sash is to be kept as low as possible Employee’s heads should never be below the level of the sash
2 8 6 PPE is required to be worn when using a hood
2 8 7 It is prohibited for chemicals to be stored in the fume hood
2 8 8 Personnel are to work with as little clutter as possible in a fume hood Having too many items in the hood can increase the risk for spilling or can block the air flow and circulation.
2.8.9 All materials in the hood are to be kept at least six inches away from the sash.
2.8.10 The sash is to be kept closed when running but not actively in use.
2.8.11 The air flow and circulation is to be checked by a qualified outside vendor once a year. LF4001 Equipment Maintenance Form is to be completed after the check.
2.8.12 Equipment that is required to operate in a fume hood must not be utilized in a fume hood containing stove top burners.
2.8.13 Flammable solvents are not to be utilized in a fume hood containing stove top burners nor can they be utilized simultaneously with any equipment that generates heat, including but not limited to muffle furnaces or hot blocks.
3 1 LP3006 Section VI: Emergency and Accident Plan
3 2 LF3002 Decibel Testing Logbook
3 3 LF4001 Equipment Maintenance Form
In the event of an emergency, the laboratory will have specific handling procedures in place
2.0 Chemical Spills
2.1 General Spills:
2.1.1 If a spill is significant i.e. greater than 100 mL, caution must be exercised and other personnel should be alerted prior to containment.
2.1.2 If a spill includes broken lab ware, proper PPE must be worn to prevent cuts and scrapes. Broken glassware should be disposed of in the glass disposal box.
2.1.3 If a chemical spill occurs that cannot be handled by laboratory personnel, notify all personnel and begin evacuation procedures outlined in section 4.0 below.
2.2 Concentrated Acids and Bases:
2 2 1 In the event of a spill, remain calm and assess the area If the spill is less than 100 mL, don the proper PPE (including acid/base resistant gloves) and clean spill using spillcontainment kit located nearest to spill area
2 2 2 To contain a minor spill:
Make a circle around the spill using the PIG Sock If necessary, more than one PIG Sock can be used
Next, pour the appropriate neutralizing solution on the spill and wait for the color change
Once the solution is neutral, use a PIG Haz-Mat pad to soak up the liquid
Dispose of pad in chemical waste if toxic or appropriate garbage receptacle
Document No : LP3006 6 Page 2 of 9
2 2 3 For minor base spills:
Use the acidic pink solution. It has a pH of 2.
Once poured on a basic spill, it will turn blue while it’s neutralizing.
Once the solution is completely neutral, the pH will be between 7-9. The solution will be yellow.
Once the solution turns yellow, it’s safe to clean up.
2.2.4 If a spilled acid or base contacts clothes or skin, immediately remove the affected garments and rinse area with sufficient quantities of water.

2.3.1 For small spills, less than 100 mL, clean up with paper towels and leave in a fume hood overnight to evaporate.
2.3.2 For larger spills between 100 and 500 mL:
2.3.2.1 Make a circle around the spill using the PIG Sock. If necessary, more than one PIG Sock can be used.
2.3.2.2 Use a PIG Haz-Mat pad to soak up the liquid.
2.3.2.3 Dispose of pad in chemical waste if toxic or appropriate garbage receptacle.
2 3 2 4 For spills greater than 500 mL, notify all personnel then follow emergency evacuation procedures outlined in section 4 0 below
3 1 1 If glassware is broken in the laboratory, personnel are to use the proper PPE prior to cleaning up
3 1 2 Broken glass is to be disposed of in the broken glass disposal box
3 1 3 If the glassware broken had a solution in it, follow the spill procedure in section 2 0 above
3 1 4 If the glassware broken had a solid substance in it, attempt to separate out the solid matter from the broken glassware using proper PPE and caution Dispose of the solid material in the proper waste receptacle.
3.1.5 It is highly unlikely that all the solid material can be separated from the glassware, attempt to put the large pieces of broken glassware in the broken glass disposal box.
3.1.6 Smaller pieces of glass may be disposed of in the garbage can or other suitable container, as long as the substance it is being disposed with is non-hazardous.
3.2.1 If an employee gets hurt in the laboratory, he or she is to notify other personnel immediately for assistance.

3.2.2 If the injury includes a solvent, acid, or base spill, the employee is to remove any article of clothing that is affected, and use the safety shower
3 2 3 If a chemical got in an employee’s eyes, he or she is to rinse vigorously under the eye wash for at least 15 minutes
3 2 4 If an employee gets hurt to the point he or she needs medical attention, other personnel are to call 911, and stay with him or her to help provide assistance to the injured person as needed
3 2 5 The first aid kit is located on the wall in the general laboratory area Personnel can use it when needed and are to assist others to its contents when they are unable to help themselves
3.2.6 Certain employees are trained on CPR. In the event of cardiac arrest, one of these employees is to administer CPR while waiting for professional medical attention.
3.2.7 Certain employees are trained in bloodborne pathogen handling. If an employee is exposed to a potential bloodborne pathogen, one of the trained employees is to assist him or her in various decontamination procedures while waiting for professional medical attention.
3.2.8 A list of all trained personnel on CPR and bloodborne pathogen handling will be posted throughout the laboratory and controlled by HR.
3.2.9 All injuries, no matter how minor, are required to be reported to upper management and HR. The injured employee is required to fill out LF1002 Accident and Injury Report Form.
3.3 Gas Leaks:
3 3 1 Argon, Helium and Nitrogen Tanks:

3 3 1 1 Gas tanks are used in the laboratory for certain mass spectroscopy testing These tanks are to be monitored daily to ensure they are functioning properly
3 3 1 2 Argon, helium and nitrogen are asphyxiants, this means they are minimally toxic gases that displace the normal oxygen in breathing air
3 3 1 3 Because many of the gases used in the laboratory are colorless, tasteless and odorless, it is imperative that employees are diligent on monitoring the tanks
3 3 1 4 Tanks should be kept away from other objects as well as not kept too close together to minimize them from impact
3 3 1 5 Gas tanks should be monitored to ensure they are free from punctures and dents The gauges and valves should be monitored to ensure they are functioning properly
3.3.1.6 If a valve is leaking gas and cannot be closed, notify fellow personnel and exit the building. Call the proper authorities (gas vendor or qualified maintenance company) to fix the defective tank.
3.3.2 Testing fumes:
3.3.2.1 If a test method performed in the laboratory creates fumes, it is to be conducted in the hood to prevent personnel from breathing these potentially toxic substances.
4.1 General:
4.1.1 In the event of an emergency, the alarm system at the front of the building is equipped with three buttons to contact authorities There is one for the fire department, police department and medical assistance
4 1 1 1 By pressing one of these buttons in the event of an emergency, the corresponding authority will be contacted and they will arrive at the laboratory shortly
4.2.1 Remember “RACE” during an emergency:
R = Rescue/Remove all occupants
A = Activate the alarm system
C = Confine fire by closing doors (if safe to do so)
E = Evacuate/Extinguish

4.2.2 Shut as many doors as possible after exiting during an evacuation.
4.2.3 All personnel are required to be trained in proper emergency and evacuation protocol. This includes knowing where fire extinguishers are located, emergency exits, alarm locations, eyewash station locations, first aid locations and the facility layout This training is covered in LF3008 Laboratory Safety Tour Checklist
4 2 4 Fire routes will be shown on facility maps, LF3005 Emergency Evacuation Plan, that will be posted throughout the laboratory in conspicuous locations
4 2 5 In the event of a fire or other emergency requiring evacuation, all personnel evacuating the building are required to meet in the landscaping area across the street from the lab The Lab Manager or department leads are responsible for ensuring all personnel and visitors are accounted for
4 2 6 If an employee’s clothes are on fire, attempt to get him or her under the safety shower and pull the lever If the shower is not close enough, have the employee implement the “Stop, Drop and Roll” method until flames are extinguished A fire blanket or extinguisher may be used.
4.3.1 In the event of an earthquake, personnel are to go to the nearest safe shelter (e.g. doorways, tables or desks without chemicals that could fall, etc.) within the laboratory.
4.3.2 If personnel are near an exit when the earthquake starts, they should exit the laboratory. However, personnel should not attempt to exit the building if they are not near a door. Moving during an earthquake can be more dangerous.
4.3.3 If personnel are unable to evacuate the laboratory, they should find proper safe shelter during the impact and hold on. Proper shelter during an earthquake includes:
4 3 3 1 Door frames that are strongly protected and load bearing
4 3 3 2 Under stable desks
4 3 3 3 Under sturdy tables
4 3 3 4 Under other pieces of furniture that is secure and sturdy
4 3 4 Once the earthquake stops, personnel should evacuate the building once turning off all potentially dangerous equipment This includes:
4 3 4 1 Gas valves
4 3 4 2 Any relevant heat sources (including but not limited to):
4 3 5 Once the earthquake stops, personnel should evacuate the building once turning off all potentially dangerous equipment This includes:
4.3.5.1 Gas valves
4.3.5.2 Any relevant heat sources (including but not limited to):
Hotblocks
Protein digesters
Stove tops
Microwaves
Water baths
4.4 Fire:
4.4.1 Fire is an extremely common hazard in a laboratory setting. A fire can start from electrical sources, chemicals, gas or other flammable objects Laboratories have a high risk for flash fires, explosions, rapid spread fires and high toxicity of products of combustion due to the nature of chemicals and solvents used in the laboratory
4 4 2 The following is a guideline of safety tips to prevent fires and minimize the risk of them in the laboratory:
4 4 2 1 Minimize materials being used at one time
4 4 2 2 Observe proper housekeeping and minimize clutter in all areas
4 4 2 3 Segregate solvents to designated areas of the laboratory and keep them in flammable storage cabinet when they are not being used
4 4 2 4 All chemicals used in the laboratory will be labeled with the proper NFPA sticker and their flammability rating will designate their storage
4 4 2 5 Use all safety precautions on equipment including fume hoods and fans

4.4.2.6 Wear proper PPE.
4.4.2.7 Never work in the laboratory alone.
4.4.2.8 Only use open flames under a fume hood while constantly attended.
4.4.2.9 Do not heat solvents using a hot plate.
4.4.3 Fire extinguisher training:
4.4.3.1 All fire extinguishers in the laboratory are ABC dry chemical extinguishers.
4.4.3.2 It is required that at least one employee on every shift is trained in how to use a fire extinguisher. If more employees want to be trained, it will be available to them.
4.4.3.3 Training will be conducted by a qualified company and will follow OSHA 29 CFR 1910.157.
4 4 3 4 A list of all personnel who are trained in fire extinguisher operation will be posted throughout the laboratory and controlled by HR
4 4 3 5 Extinguisher locations will be placed on facility maps which will be posted throughout the laboratory in conspicuous locations
4.4.3.6 Remember “PASS” when using an extinguisher:
P = Pull the pin
A = Aim extinguisher nozzle at the base of the fire
S = Squeeze the trigger while holding the extinguisher upright
S = Sweep the extinguisher from side to side; covering the fire with the spray
4.5 Floods:
4.5.1 During a flood warning, personnel should prepare to evacuate the building.
4.5.2 Personnel should ensure all chemicals are closed and placed in the proper chemical storage cabinets. If possible, they should be moved to a more secure location to prevent spreading contamination during the flood Pathogenic or OPIM should be stored and secured as well
4 5 3 Personnel should turn off all equipment to prevent fire or electrocution This includes but is not limited to:
4 5 3 1 All heat sources
4 5 3 2 Gas valves
4 5 3 3 Computers
4 5 3 4 Testing equipment
4 5 3 5 Once personnel have evacuated the building and are all accounted for, they are to follow emergency protocol for heading to a safer environment
4.6.1 All laboratory personnel are encouraged to call the police or any other proper authorities if in the position of feeling threatened by outside people or coworkers.

4.6.2 Weapons:
4.6.2.1 Weapons are strictly prohibited on the laboratory premises and property, regardless if the person is licensed to carry them.
4.6.3 Violence:
4.6.3.1 Fighting or other types of workplace violence is prohibited on the laboratory premises. All employees are strongly encouraged to report any dangerous behavior to management.
4 6 4 Dangerous person:
4 6 4 1 In the event a dangerous person is around the laboratory premises, the laboratory will lock down A lock down entails locking all doors and means of entering/exiting the laboratory and closing the blinds and or turning off the lights within the laboratory to discourage the dangerous person from attempting to enter the building Personnel will be asked to stay away from doors and windows.
4.6.4.1.1 To lock down the front door, an employee has to access the alarm system through a computer. At least one employee on weekday shifts will be trained to do so.
4.6.4.2 If able, laboratory personnel are to alert authorities by calling “911” and remain locked down until the situation is resolved. Or personnel can press the police button on the alarm system keypad.
4.7.1 An active shooter/ hostile intruder is an individual actively engaged in killing or attempting to kill people in a confined and populated area by any means including but not limited to firearms (most frequently used), bladed weapons, vehicles, or any tool that in the circumstance in which it is used constitutes deadly physical force In most cases, there is no pattern or method to their selection of victims Most active shooter situations are unpredictable, evolve quickly, and are over within minutes

4 7 2 EVACUATE – Run: If there is an accessible escape path, attempt to evacuate the premises Be sure to:
4 7 2 1 Have an escape route and plan in mind
4 7 2 2 Evacuate regardless of whether others agree to follow
4 7 2 3 Leave your belongings behind
4 7 2 4 Help others evacuate, if possible
4.7.2.5 Call 911 when you are safe.
4.7.2.6 Prevent individuals from entering an area where the active shooter may be.
4.7.2.7 Keep your hands visible.
4.7.2.8 Follow the instructions of any police officers.
4.7.2.9 Do not attempt to move wounded people.
4.7.3 SHELTER-IN-PLACE – Hide: If evacuation is not possible, find a place to hide where the active shooter is less likely to find you. Your hiding place should:
4.7.3.1 Be out of the active shooter’s view.
4 7 3 2 Provide protection if shots are fired in your direction (i e an office with a closed and locked door)
4 7 3 3 Not trap you or restrict your options for movement
4 7 3 4 To prevent an active shooter from entering your hiding place:
4 7 3 4 1 Lock the door
4.7.3.4.2 Blockade the door with heavy furniture.
4.7.3.5 If the active shooter is nearby:
4.7.3.5.1 Lock the door.
4.7.3.5.2 Silence your cell phone and/or pager.
4.7.3.5.3 Turn off any source of noise (i.e. radio, television).

4.7.3.5.4 Hide behind large items (i.e. cabinets, desks).
4.7.3.5.5 Remain quiet.
4.7.4 PROTECT YOURSELF – Fight: As a last resort, and only when your life is in imminent danger, attempt to disrupt and/or incapacitate the active shooter by:
4.7.4.1 Acting as aggressively as possible against him/her.
4 7 4 2 Throwing items and improvising weapons
4 7 4 3 Yelling
4 7 4 4 Committing to your actions
4 7 5 WHEN POLICE ARRIVE
4 7 5 1 Put down any items in your hands
4 7 5 2 Keep hands visible
4 7 5 3 Follow all instructions
4 7 5 4 Avoid making quick movements towards officers
4 7 5 5 Do not stop to ask officers for help or direction when evacuating, just proceed in the direction from which officers are entering the premises
5.1 LF1002 Accident and Injury Report Form
5.2 LF3005 Emergency Evacuation Plan
5.3 LF3008 Laboratory Safety Tour Checklist
All laboratory personnel are required to be trained in all safety procedures of the laboratory before beginning work. This training is to be documented according to LF2018 Training Record Form.
All laboratory personnel are required to be trained in a certain test method before performing the test The training is to be documented according to the Training Record Form

Certain personnel may qualify to be trained in fire extinguisher use, bloodborne pathogens, first aid/CPR, or other relevant safety and hygiene areas of the laboratory Documentation from the outside vendor conducting the training will need to be provided and the individuals participating in the training will be documented according to the Training Record Form
All laboratory personnel are required to follow all procedures in the laboratory Safety and hygiene procedures are created to promote a safe and healthy work environment and are written with personnel’s best interests in mind All personnel are responsible for following set procedures after they have been properly trained on them. Personnel are to be held accountable according to LP1011 Code of Conduct.
Any employee who fails to follow safety or hygiene procedure may be subject to disciplinary action. The disciplinary action taken against this employee is up to the discretion of management, but depending on the severity of the offense can include anything from verbal warning up to termination. If disciplinary action is to be taken against an employee, it is to be documented using LF2005 NonConformance Record.
Any incidents, including a dangerous person causes a threat, an accident occurs, theft or police activity, will need to be documented via LF1003 Incident Report This report may be completed by any employee and given to HR to file
Any injury that occurs on laboratory premises, no matter how minor, will need to be documented according to LF1002 Accident and Injury Report Injuries that do not require medical attention will still need to be documented via the Accident and Injury Report Injuries that require medical attention will also need to be documented according to Purity's insurance company's Worker Compensation form and brought to the medical office or hospital the employee attends Accidents and injuries need to be reported to management as soon as possible in case they worsen and medical attention is required



















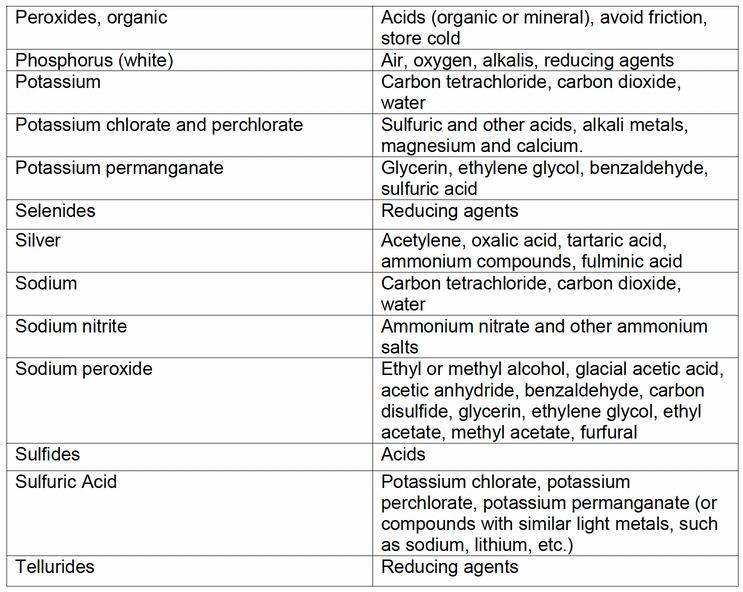
Certain chemicals can become reactive when stored with other chemicals

Below is a list of chemicals and what they might react with




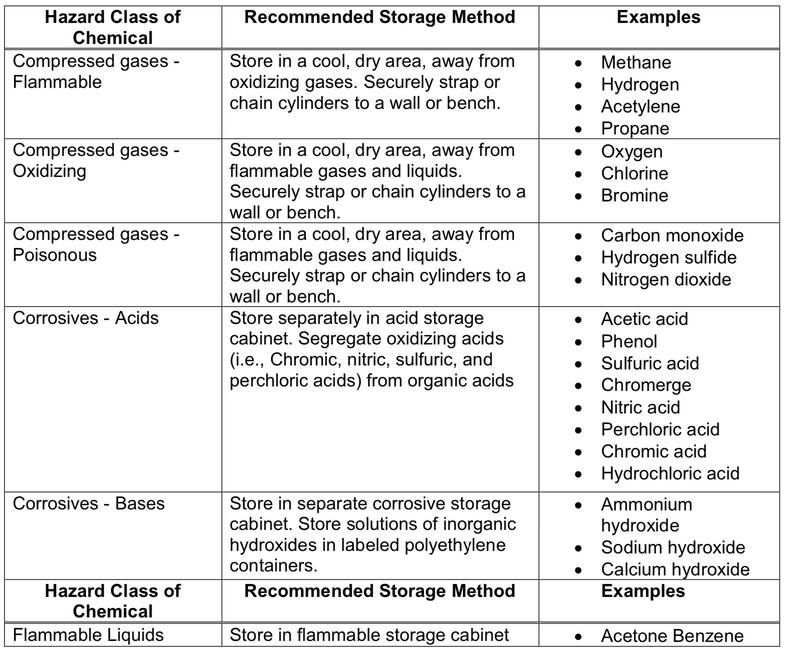
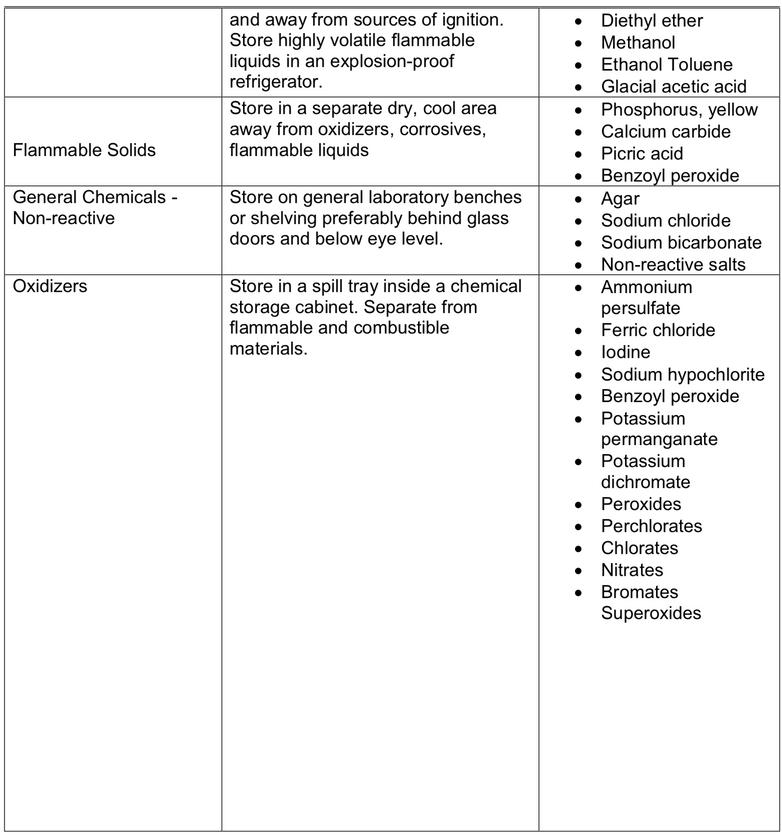
All chemicals have specific storage requirements. They will be listed on their corresponding SDSs. Below is a chart of the types of chemical, storage conditions and examples of these chemicals


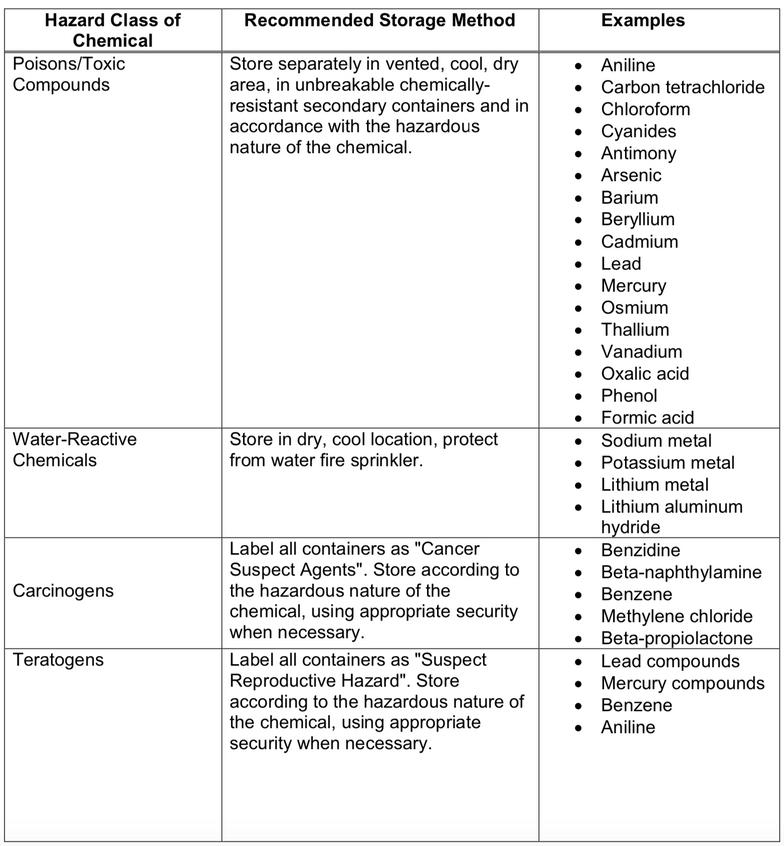



1.0 Purpose
The purpose of this procedure is to establish and maintain standards for aseptic technique for Purity Laboratories, Inc. (Purity) in the Microbiology Department. The purpose of aseptic technique is to minimize the possibility of microorganism transfer between the environment and test samples, standards or preparation materials.
2.0 Scope
This procedure applies to all personnel employed at Purity Laboratories, Inc. and to anyone otherwise working on the premises
3.0 Safety
Normal chemistry laboratory precautions apply, including eye protection, lab coat and other protective precautions as required
4.0 Definitions
4 1 Aseptic Technique: (Sterile Technique) performing a test method under sterile conditions All activities aiming to control the existence, growth and proliferation of contamination in areas of the testing laboratory All sterile surfaces must only be touched and exposed to other sterile surfaces Exposure to the air is to be kept at a minimum
4.2 Contamination: Accidental introduction of foreign material that can affect the results of the test performed. This can be accidental sterilization of the sample or introduction of microorganisms by laboratory personnel.

4.3 Good Microbiological Practices: Includes aseptic technique and other practices that are necessary to prevent contamination of the laboratory with the agents being handled and contamination of the work with agents from the environment. They are in place to ensure contamination does not affect test results.
4.4 Personal Protective Equipment (PPE): protective clothing or garments designed to protect the wearer’s body from injury by objects, heat, chemicals, electrical components, infections and other potential hazards that he or she may be exposed to at his or her occupation
4 5 Universal Precautions: An approach to infection control where all samples including those containing human constituents are treated as if known to be infectious
4 6 Biosafety Level (BSL): The level of the biocontainment precautions required to isolate dangerous biological agents in an enclosed facility The levels of containment range from 1-4 depending on the danger of the hazard with 4 being the most hazardous
4.7 Bloodborne Pathogen: Any disease that can be spread through contamination via blood or any of its constituents including blood and blood plasma.
4.8 Antiseptic: An agent that inhibits or destroys microorganisms on living tissue. This includes but is not limited to: skin, oral cavities and open wounds.
4.9 Sporicidal Agent: An agent that destroys bacterial and fungal spores when used in sufficient concentration for a specified contact time. It is meant to kill all vegetative microorganisms.
4 10 Sterilant: An agent that destroys all forms of microbial life including fungi, viruses and all forms of bacteria and their spores
4 11 Disinfectant: A chemical or physical agent used to destroy or remove infectious fungi, viruses and bacterial This does not ensure the spores from these infecting agents will be destroyed Disinfectants only target pathogenic microorganisms
4 11 1 Chemical Disinfectant: A chemical agent used on inanimate surfaces and objects to destroy infectious materials
4 11 2 Physical Disinfectant: The use of primarily heat as an agent to destroy infectious materials on objects
5 0 Responsibility
5.1 Microbiology Personnel: the microbiology personnel are responsible for understanding the principles and following the protocols of aseptic technique. Microbiology personnel are not altering these procedures in any way unless instructed to do so by the Micro Lead.
5.2 Microbiology Lead: The Lead is responsible for understanding aseptic technique and ensuring the protocols within this procedure are being implemented correctly. It is the responsibility of the Lead to ensure the Microbiology Personnel are effectively trained and understand the importance of aseptic technique. Any modifications to testing protocols that may affect the aseptic technique procedure are to be documented and are to be done only with approval by the Lead.
5 3 Other Laboratory Personnel: Non-Microbiology personnel are responsible for knowing the importance of aseptic technique They are to respect the rules of the laboratory and are not to enter the Microbiology Department without purpose They are required to follow all hygiene and safety protocols as outlined in LP3001 – LP3008 Chemical Hygiene Plan and Safety Manual
6 1 There are two types of sterilization implemented in the Microbiology Laboratory They are chemical and mechanical and will be outlined below

6.2 Chemical:
6.2.1 All work surfaces, counters and preparation areas will be cleaned daily prior to use. This is to ensure there is no cross contamination from surfaces to sample.
6.2.2 The chemical used to clean surfaces will be one of the following: 70% Isopropyl Alcohol Cleaning Solution (M147), an all-purpose cleaner, or Sporicidin Solution (M167)
6.2.3 Any changes to the cleaning chemical used is to be documented via LF2017 Deviation Record Form.
6.2.4 Once per week, the work surfaces, counters, and sample storage areas will be cleaned with a glutaraldehyde based sporical cleaning solution (M167). This is to prevent any spores from forming on work surfaces and contaminating test samples or materials.

6 3 Mechanical:
6 3 1 Items that cannot be disinfected or sterilized via chemical agents, will be cleaned via autoclaving
6 3 2 Prepared media, broths and solutions will be autoclaved according to their corresponding LSs This will be documented via LF3004 Autoclave Logbook
6 3 3 Operation of the autoclave is to be done by authorized and trained personnel only, the autoclave is to be operated via LP4010 Autoclave Operation, Cleaning and Maintenance
6 3 4 Autoclaves will be monitored for effectiveness via a few controls Autoclave cycles will be documented on LF3004 Autoclave Logbook This will monitor sample type, time and temperature of the autoclave The temperature of the cycle will be monitored internally to ensure the proper temperature for the cycle is reached
6.3.5 The samples being autoclaved will be marked with heat activated indicator tape also to ensure the samples reached the proper temperature.
6.3.6 Each cycle the autoclave is run, a biological indicator will be added to the cycle. This ensures the autoclave reaches the proper temperature as well.
6.3.7 All of these systems will be documented via LF3004. The autoclave is to be cleaned daily and maintained via LP4010 Autoclave Operation, Cleaning and Maintenance.
7.1 Even with proper aseptic technique, samples can still become contaminated and therefore compromised via various sources of contamination. The following is a list of most likely sources of contamination
7 1 1 Supplies:
7 1 1 1 The supplies used in the microbiology lab are sterile, one time use supplies These include sterile plastic loops, sterile plastic needles, sterile plastic spoons, sterile pre-filled pipette racks and sterile plastic Whirl-Bags
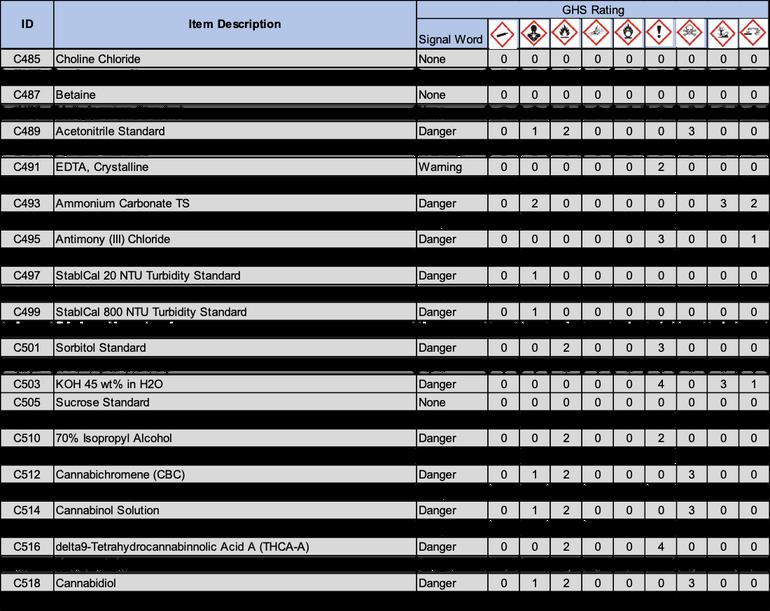
7.1.1.2 Sterile supplies are only to be used one time, then discarded. Plastic loops and needles, plastic spoons, pipette tips and Whirl-Bags are to only be used for one material and not transferred from sample to sample.
7.1.1.3 Supplies may be compromised during shipping. Upon receipt, supplies are to be inspected to ensure the integrity of the sterilization was maintained.
7.1.1.4 If supplies look damaged or compromised in any way, they are not to be used. The Microbiology Lead will be notified and the compromised supplies will be removed and replaced.
7 1 2 Media and Reagents:
7 1 2 1 Stock chemicals and reagents will be purchased pre-sterilized Upon receipt, Microbiologists are to inspect the stock chemicals and determine if they were compromised or contaminated in any way Suspicious looking chemicals or reagents will not be used and the Microbiology Lead will dispose of the chemical or reagent
7 1 2 2 Prepared media, broth and solutions will be autoclaved according their corresponding Laboratory Solutions (LSs) The autoclave will be cleaned, checked and operated via LP4010 Autoclave Operation, Cleaning and Maintenance
7.1 3 Environmental and Airborne Particles:
7 1 3 1 Airborne particulates can be a significant source of contamination The microbiology lab will be equipped with MERV (Minimum Efficiency Reporting Value) 8 Standard Pleated Air Filters on all vents to prevent the entry of any potentially contaminated material Air filters will be maintained and serviced by an outside agency quarterly to ensure they are in working order. They will be replaced every quarter regardless of their condition. This will be documented via LF4001 Equipment Maintenance Log.
7.1.3.2 Insects can be a source of contamination. There will be a pest control system in place throughout the lab. The system will be maintained once per month by an outside agency. Findings will be issued by the outside agency’s report.
7.1.3.3 One source of environmental contamination is other people visiting or working in the laboratory. The microbiology laboratory is to be segregated from other testing areas and access to the microbiology portion will be limited. Non-microbiology personnel are not to enter the microbiology laboratory without authorization from a microbiologists. Visitors and non-microbiology personnel are to adhere to all sanitation procedures while being in the microbiology laboratory This includes but is not limited to: eye protection, closed-toe shoes, long pants, long hair is secured and hand sanitation
7.1 4 Equipment
7 1 4 1 Incubators:
7 1 4 1 1 Incubators will be monitored for cleanliness and temperature via LP4011 Incubator Use, Cleaning and Maintenance

7.1.4.1.2 The temperatures of the incubators will be checked and monitored twice daily via LP2001 Temperature Monitoring.
7.1.4.1.3 Once per Quarter, all incubators will be cleaned with the current microbiological cleaning solution as well as Sporicidin (M167).

7.1.4.2 Pipettes:
7.1.4.2.1 Pipettes will be used according to LP4004 Pipette Use and Maintenance Plan. Only trained personnel are authorized to use pipettes.
7.1.4.2.2 Pipettes that are visibly damaged are not to be used and are to be given to the Quality Department for service.
7.1.4.2.3 If pipettes are dirty or suspected to be contaminated via liquid entering the shaft of the pipette, do not continue to use Alert the Quality Department for service and/or autoclaving
7 1 4 2 4 Some pipettes are able to be autoclaved, the manual should be consulted prior to autoclaving and only trained and authorized personnel are to operate the autoclave
7 1 4 2 5 A new pipette tip is required to be used when switching solutions, this is to ensure cross contamination does not occur All pipette tips are to be bought presterilized or autoclaved prior to use in the Microbiology Laboratory
7 1 5 Work Surfaces:
7 1 5 1 Work surfaces are to be cleaned and disinfected every day prior to beginning testing They will be cleaned with the current cleaning chemical
7 1 5 2 Work surfaces will be sterilized with a sporicidin solution once per week to prevent spore growth
7.1.5.3 Work surfaces are to be cleaned whenever there is a sample spill, positive control spill, or if they appear especially dirty.
7.1.5.4 Storage of materials, media, equipment and samples will be done in a clean fashion.
7.1.5.5 Storage shelves and areas will be cleaned quarterly with the current chemical as well as with the sporicidin solution. They will then be rinsed with deionized water and wiped dry.
8.1.1 When new samples are received, they are not to be opened or tampered with in any fashion After being logged in according to LP1004 Test Sample Receiving and Processing, samples are to be delivered directly to their proper destination without delay
8.2 Preparation:
8.2.1 When a sample is delivered to the Microbiology Department, the sample is to be initially inspected by the Microbiologist setting the sample.
8.2.2 If the sample is deemed acceptable for testing, the Microbiologist is to ensure the work space is cleaned and sanitized.
8.2.3 All solutions, media and broths to be used are to be sterilized in the autoclave via LP4010 Autoclave Operation, Cleaning and Maintenance. This is to be documented via LF3004 Autoclave logbook.
8.2.4 All test tubes, petri dishes, plastic loops, plastic spoons, pipettes, pipette tips and materials used in setting the sample are to be either pre-sterilized or sterilized in the autoclave

8 2 5 The Microbiologist performing the test is to ensure his or her hands are sanitized with soap and water or hand sanitizer prior to donning gloves
8 2 6 The Microbiologist is required to use gloves prior to handling the sample He or she is to change gloves whenever changing test methods or if the gloves become dirty or damaged
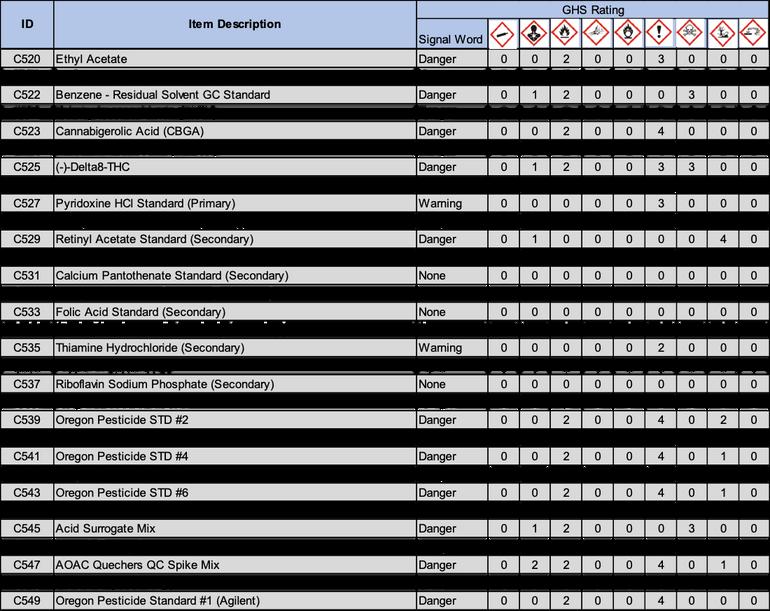
8 2 7 Using alcohol based hand sanitizer on the gloves can also be implemented, but should not be used in place of replacing damaged or dirty gloves
8 3 Sample Setting:
8 3 1 Attempt to work as efficiently as possible to reduce the sample’s exposure to the air
8 3 2 Solid Form or Powdered Samples:
8 3 2 1 Prepare a whirl-bag for each sample by labeling with sample identification and test type.
8.3.2.2 After preparing the work space as outlined in section 8.2, sanitize hands and don gloves.
8.3.2.3 Open the pre-labeled sample whirl-bag.
8.3.2.4 Place a plastic basket on the scale, then place the pre-labeled whirl-bag in the plastic bag and tare the scale.
8.3.2.5 Next, open the sample bag or sample container.
8.3.2.6 Open a sterile plastic spoon and scoop the appropriate amount of sample into the whirl-bag on the scale as indicated by the test method.
8.3.2.6.1 Never place the spoon on the counter.
8 3 2 7 After scaling, record the weights on the appropriate Laboratory Forms (LFs), close whirl bag and sample container
8 3 2 8 Throw away plastic spoon in trash and repeat with remaining samples
8.3.3 For Liquid Samples:
8.3.3.1 Prepare a whirl-bag for each sample by labeling with sample identification and test type.
8.3.3.2 After preparing the work space as outlined in section 8.2, sanitize hands and don gloves.
8.3.3.3 Open the pre-labeled sample whirl-bag and place in a plastic basket.
8.3.3.4 Next, open the sample bag or sample container.
8.3.3.5 Open a sterile box of pipette tips and slowly pipette the appropriate volume into the whirl-bag.
8.3.3.5.1 Never place the pipette with the tip attached on the counter.
8 3 3 6 After transfer, record the volumes on the appropriate LFs, close whirl bag and sample container
8 3 3 7 Eject pipette tip in autoclave bag and repeat with remaining samples
8 3 4 To transfer samples to other bags:
8 3 4 1 Prepare a new whirl-bag for each sample by labeling with sample identification and test type
8 3 4 2 After preparing the work space as outlined in section 8 2, sanitize hands and don gloves
8 3 4 3 Open the second pre-labeled sample whirl-bag
8 3 4 4 Place a plastic basket on the scale, then place the pre-labeled whirl-bag in the plastic bag and tare the scale
8 3 4 5 Next, open the original whirl-bag containing the sample
8.3.4.6 Open a sterile plastic spoon and scoop the appropriate amount of sample into the whirl-bag on the scale as indicated by the test method. Or, open a sterile box of pipette tips and slowly pipette the appropriate volume into the whirl-bag.
8.3.4.6.1 Never place the spoon or the pipette with the tip attached on the counter.
8.3.4.7 After scaling, record the weights on the appropriate LFs, close whirl-bags and store samples appropriately.
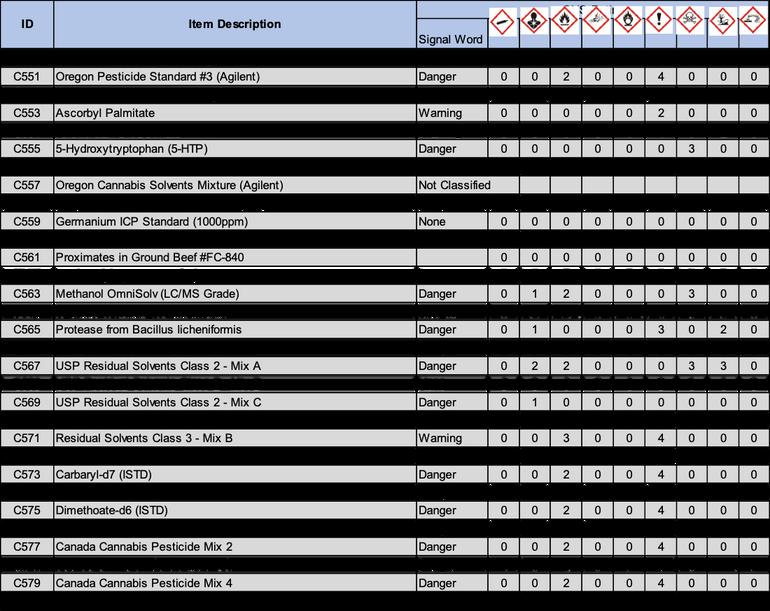
8.3.4.8 Throw away plastic spoon in trash and eject pipette tips in an autoclave bag and repeat with remaining samples.
8.3.5 To transfer samples to broth test tubes:
8.3.5.1 Label a sterilized test tube with sample identification and test type.

8 3 5 2 After preparing the work space as outlined in section 8 2, sanitize hands and don gloves
8 3 5 3 Open the sample bag
8.3.5.4 Open a sterile box of pipette tips and slowly pipette the appropriate volume into the labeled test tube, replacing the cap immediately after transfer.
8.3.5.4.1 Never place the pipette with the tip attached on the counter.
8.3.5.5 Repeat for remaining dilutions using a new pipette tip with every solution.

8.3.6 To streak samples onto a slant tube:
8.3.6.1 Label a sterilized test tube with sample identification and test type.
8.3.6.2 After preparing the work space as outlined in section 8.2, sanitize hands and don gloves.
8.3.6.3 Open the sample bag.
8.3.6.4 Open a sterile plastic loop, and insert the loop into the sample bag.
8 3 6 5 Open the cap of the test tube, and streak the loop on the surface of the slanted agar Ensuring to do so quickly and evenly in a zig-zag pattern
8 3 6 5 1 Do not allow the loop to puncture the agar surface
8 3 6 5 2 Never place the loop on the counter
8 3 6 6 After streaking, replace the cap quickly and dispose of gloves in trash and dispose of loop in autoclave bags
8 3 6 7 Repeat with remaining samples ensuring to use a new loop for each sample and tube
8 3 7 To inoculate the butt or stab of test tube:
8 3 7 1 Label a sterilized test tube with sample identification and test type
8 3 7 2 After preparing the work space as outlined in section 8 2, sanitize hands and don gloves
8.3.7.3 Open the petri-dish containing colonies to be used for inoculation.
8.3.7.4 Open a sterile plastic loop, and collect 1 colonies (1 loopful.)
8.3.7.5 Open the cap of the test tube, and stab the loop into the middle of the surface of the agar, refer to the corresponding test method to determine the depth the loop needs to go into the agar. Stabs will go down part-way and butt inoculations will go further towards the bottom of the tube.
8.3.7.5.1 Do not allow the loop to touch the sides of the test tubes.
8.3.7.5.2 Never place the Loop on the counter.
8.3.7.6 After inoculation, replace the cap quickly and dispose of gloves in trash and dispose of loop in autoclave bags.
8 3 7 7 Repeat with remaining samples ensuring to use a new needle for each sample and tube
8 3 8 To transfer samples to petri dishes:
8 3 8 1 Follow the diagram in figure one to follow the proper T-streak pattern
8 3 8 2 Label a sterilized petri dish with sample identification and test type
8.3.8.3 After preparing the work space as outlined in section 8.2, sanitize hands and don gloves.
8.3.8.4 Observe the petri dish and ensure there are no water droplets on the surface of the agar. If so, use a sterile cotton swab and lightly wipe the condensate away, ensuring not to damage the agar in the process.

8.3.8.5 Open the sample bag.
8.3.8.6 Open a sterile plastic loop, and insert the loop into the sample bag.
8.3.8.7 Open the lid of the petri dish, and streak one third of the dish. Ensure to do so quickly and evenly in a zig-zag pattern.
8.3.8.7.1 Do not allow the loop to puncture the agar surface.
8 3 8 7 2 Never place the loop on the counter
8 3 8 8 Using a new sterile loop, streak the second sector of the dish by streaking once across the first sector, and dragging to the second sector using the same zig-zag pattern
8 3 8 9 Using a new sterile loop, streak the third sector of the dish by streaking once across the second sector, and dragging to the second sector using the same zig-zag pattern

8 3 8 10 After streaking, replace the lid of the petri dish quickly and dispose of gloves in trash and dispose of loop in autoclave bags
8 3 8 11 Repeat with remaining samples ensuring to use a new loop for each sample and tube
8 3 8 12 Flip petri dishes upside down and incubate in this manner to ensure condensation doesn’t form on the surface of the agar
9.1.1 In order to ensure air and filter quality, air plates will set out every day twice per day. An air plate will be placed in the general Microbiological Lab.
9.1.2 A biological indicator will be run along with every cycle the autoclave is run. The indicator that will be used is Bacillus stearothermophilus. This will be documented via LF3004
Autoclave Logbook.
9.1.3 A positive control will be ran along with every batch of samples on pathogenic test methods. This is to ensure the test method was executed properly.
9.1.4 Two controls will be ran along with every batch of samples on all test methods. This is to ensure the media, broths and supplies used are properly sterilized. One will be to monitor sterility, the other will be to monitor growth.

1.0 Purpose
The purpose of this procedure is to establish and maintain standards for Purity Laboratories, Inc. (Purity) with regards to the accommodations and environmental conditions of the laboratory.

2.0 Scope
This procedure applies to all personnel employed at Purity.
3.0 Safety
Normal laboratory precautions apply, including eye protection, lab coat and other protective precautions as required.
4.0 Procedure
4 1 1 Purity will follow all applicable local, state, and national legislation regarding the health and safety of staff Additional procedures regarding the health and safety of staff can be found in LP3001 to LP3012 Chemical Hygiene Plan and Safety Manual
4 1 1 1 Purity will provide personal protective equipment including eye protection, lab coats, gloves, and other protective equipment as needed
4 1 1 2 Purity will also ensure that there is adequate and proper storage for all chemicals, reagents, media, controls, and standards to ensure the safety of personnel and to reduce risk of spills, fire, or other catastrophic events
4 1 2 Management must ensure that the laboratory facility has adequate space and resources to conduct analysis as needed.
4.1.2.1 When equipment is purchased or moved to a new location within the laboratory, management must address the specific needs of the equipment. This includes, but is not limited to: contamination; electrical needs; venting; water; accessibility for performing maintenance, cleaning, and calibration; and any other specifications given by the manufacturer.
4.1.3 Purity will give consideration regarding the comfort of personnel in areas including, but not limited to: work station ergonomics; provision of adequate “people amenities” such as lunchroom, eating areas, and restrooms; and provision of adequate heating and cooling, ventilation, and lighting.
4 1 4 To prevent cross-contamination or compromise of test results, effective separation will be established where incompatible activities occur in an area
4.1.4.1 The microbiological testing department will be physically separated from all other testing to prevent cross-contamination and access to department will be limited.
4.1.5 To ensure the safety of all visitors, access to the laboratory is controlled and limited. LP3002 Chemical Hygiene Plan and Safety Manual Section II: General Laboratory Safety details laboratory access.
4.1.6 Purity will have separate storage areas for test samples, references (including positive controls and standards), and chemicals, media, and reagents. Samples should also not be handled or stored in the same area that controls or standards are being prepared. Media should also not be prepared or stored in the same area as positive controls.
4.1.6.1 Procedures regarding the storage, handling, and disposal of chemicals, media, and reagents can be found in LP3001 to LP3012 Chemical Hygiene Plan and Safety Manual.
4.1.6.2 See LP2005 Test Sample Storage, Retention, and Disposition for procedures regarding test samples.
4 2 1 Purity will ensure that the environmental conditions do not invalidate the testing results or adversely affect the required quality of any measurement

4 2 2 This will be done by controlling, monitoring, and recording environmental conditions as required by relevant specifications, methods, and procedures, or where they influence the quality of the results Environmental conditions include, but are not limited to: biological sterility, dust, electromagnetic disturbances, radiation, humidity, electrical supply, temperature, and sound and vibration levels
4.2.3 When a test method or procedure spells out specific environmental conditions for the conduct of an analysis, the measures taken to resulting data defining conditions recorded. Environmental conditions will be monitored either directly or indirectly as needed by the test method or procedure.
4.2.3.1 Direct measurements are documented within the original observations or in a logbook dedicated to such measurements.
4.2.3.2 Indirect measurements include quality control samples, such as certified reference materials or spikes.
4.2.4 Environmental conditions will be monitored at frequencies according to test method or procedures. Activities will be stopped if conditions could be detrimental to an analysis. If equipment or analysis is performing within acceptable ranges, the conditions are considered acceptable.
4.2.5 The internal temperature of the laboratory will be constantly maintained by a functioning heating/cooling ventilation system. The system will be fitted with filters to ensure external contamination is not introduced to the laboratory.
4.2.5.1 Filters will be replaced at least quarterly.
4.2.5.2 The ventilation system will be arranged in such a way as to prevent crosscontamination from incompatible activities and/or departments.
4.2.5.3 Certain environments within the laboratory will have the room temperature monitored and recorded to ensure the temperature does not affect testing.
4.2.5.3.1 Room temperatures to be recorded will be listed on LF2001 Temperature Log Sheet. See LP2001 Temperature Monitoring for procedures regarding the monitoring and recording of temperatures.
4.3.1 All personnel are responsible for general housekeeping of their work areas. Personnel are also responsible for cleaning after themselves in common areas such as lunchroom Housekeeping policies can be found in LP3002 Chemical Hygiene Plan and Safety Manual Section II: General Laboratory Safety
4 3 2 Purity will contract a janitorial service to provide basic cleaning services with instructions on what they are to clean

4 3 3 In areas of the laboratory needing biological sterility or specific cleaning processes, procedures addressing this will be written In some cases, the cleaning must be documented
4 3 4 Purity will utilize a pest control company to aid with the control of unwanted pests
4.3.4.1 The pest control company will perform an inspection at least monthly and provide a report of inspection.
4.3.4.2 Reports from the pest control company will be kept in a book maintained by the pest control company.
4.3.4.3 Laboratory personnel are to notify management if any pests are noticed. Laboratory personnel are to follow all housekeeping policies to avoid attracting any pests.
5.0
5.1 The Quality Department will ensure that all environmental conditions are clearly defined in procedures and test methods and that all tests were performed within specified conditions.
6.1 LP2001 Temperature Monitoring
6 2 LP3001 to LP3012 Chemical Hygiene Plan and Safety Manual
6 3 LF2001 Temperature Log Sheet
The purpose of this Exposure Control Plan (ECP) is to establish and maintain bloodborne pathogen safety standards for Purity Laboratories, Inc (Purity) These standards will help to eliminate or minimize occupational exposure to bloodborne pathogens in accordance with OSHA standard 29 CFR 1910 1030 This procedure outlines the determination of employee exposure, methods of compliance, personal protective equipment, engineering and work practice controls This procedure details the proper way biological hazards are handled and exposure to pathogens is minimized and prevented This policy helps to ensure that all laboratory operations are conducted using safe working practices and that all applicable local, state and federal regulations and policies are being correctly implemented. Employees that will have occupational exposure to blood and or other potentially infectious materials (OPIM) are required to be trained in bloodborne pathogen handling and exposure as outlined in this procedure. This training will include all handling, testing and verification of blood, OPIM or blood materials and will include the universal precautions for potential exposure.
This procedure applies to all clinical personnel employed at Purity Laboratories, Inc. and to anyone otherwise working on clinical processes on the premises.

3.0
Normal chemistry laboratory precautions apply, including eye protection, lab coat and other protective precautions as required.
4.0 Definitions
4 1 BSL: Biosafety level The level of the biocontainment precautions required to isolate dangerous biological agents in an enclosed facility The levels of containment range from 1-4 depending on the danger of the hazard with 4 being the most hazardous
4 2 Bloodborne Pathogens: pathogenic microorganisms that are present in human blood and can cause disease in humans These pathogens include, but are not limited to, hepatitis B virus (HBV) and human immunodeficiency virus (HIV)
4.3 CLIA: Clinical Laboratory Improvement Amendments. Regulatory quality standards for all laboratory testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed.
4.4 Clinical Laboratory: a workplace where diagnostic or other screening procedures are performed on blood, specimens derived from humans or other potentially infectious materials.
4.5 Engineering Controls: controls (e.g., sharps disposal containers, self- sheathing needles, safer medical devices, such as sharps with engineered sharps injury protections and needleless systems) that isolate or remove the bloodborne pathogens hazard from the workplace
4 6 Exposure Incident: A specific occurrence where personnel are exposed to blood or other potentially infectious materials via the eyes, mouth, mucous membranes or skin through duties performed by their job
4.7 Hazmat suit: Hazardous material suit that covers the entire body and is impermeable to hazardous materials.
4.8 MSDS: Material Safety Data Sheet: a sheet that provides workers with procedures for handling and working with materials and substances in a safe manner. It includes but is not limited to physical properties of the substance, toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment required and spill handling procedures.
4.9 Occupational Exposure: reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious materials that may result from the performance of an employee's duties.
4.10 OPIM: Other potentially infectious material. This category of biohazard includes semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva and, any other bodily fluid that is contaminated with blood.
4 11 OSHA: Occupational Safety and Health Administration: OSHA standards are rules that describe the methods that employers must use to protect their employees from hazards
4 12 PPE: Personal Protective Equipment: protective clothing or garments designed to protect the wearer’s body from injury by objects, heat, chemicals, electrical components, infections and other potential hazards that he or she may be exposed to at his or her occupation
4.13 Universal Precautions: An approach to infection control where all human blood and certain human body fluids are treated as if known to be infectious for HIV, HBV, and other bloodborne pathogens.

5.1 CLIA Supervisor: the supervisor is responsible for overseeing all clinical laboratory personnel and practices The supervisor is responsible for compliance of clinical testing to standard operating procedures created by Purity as well as regulatory standards

5 2 CLIA Laboratory Director: the director is responsible for overall operation and administration of the laboratory as well as employing competent personnel The director is responsible for ensuring that all CLIA regulations are met and are being implemented correctly
5.3 Personnel: clinical laboratory personnel are required to follow all procedures set in place by the CLIA supervisor and follow all regulatory standards for a clinical laboratory. Personnel are required to wear proper personal protection equipment (PPE) at all times within the laboratory setting. This includes but is not limited to: lab coats, eye protection, long pants, closed-toe shoes and gloves.
5.3.1 Other PPE will be available to all personnel who might need extra protection against various hazards. This includes aprons, full coverage eye protection, heavy-duty gloves, and respirators.
6.1 Biosafety Levels: This category is for microbiological hazards such as positive control standards. The range of BSL is 1-4 with 4 being the most hazardous.
6.1.1 Strains of microorganisms not known to cause disease in healthy human adults. Normal safety procedures apply.
6.1.2 Moderate risk microorganisms that are associated with less serious human diseases. Extra precautions are necessary such as PPE and limited lab access.
6.1.3 High risk microorganisms with a true potential for infection via inhalation. PPE required such as respirators, double gloves and gowns Work only to be conducted in an isolated laboratory
6 1 4 Easily transmitted very high risk microorganisms which cause life threatening diseases for which there is no treatment Hazmat suits required as well as chemical showers
6 2 With the exception of extraordinary circumstances in which the samples are suspected to contain extremely contagious pathogenic material, clinical laboratory samples are to be handled under BSL-2 Level 2 is the level for which HIV, HBV and HCV are handled and are the most likely pathogens a clinical laboratory employee will encounter.
6.3 The focus of BSL-2 is the prevention of exposure to pathogenic material via mucosal membranes and percutaneous injuries.
6.4 BSL-2 requires the laboratory to use primary barriers such as PPE, universal precautions and a biosafety cabinet to prevent splashing and spraying of droplets of potentially pathogenic material.
6.5 The clinical laboratory area is to be segregated from any other testing being performed in the laboratory The clinical area is only to be accessed by trained and authorized personnel
7.0 Exposure Determination:
7 1 Due to the nature of the tests performed at Purity using dried blood and serum spots, everyday sample testing will provide minimum risk for pathogen exposure However, verification of testing methods will require the use of liquid whole blood and serum samples

7.2 Exposure to a bloodborne pathogen is due to everyday tasks performed in the laboratory; known as occupational routes of exposure. Occupational routes of exposure are the most likely sources where an employee will be most susceptible to pathogenic material.
7.3 The bodily route in which a pathogen will take once a person is exposed to pathogenic material is known as a human route of exposure.
7.4 In the event of an exposure, follow Section 11.0 Exposure Incident.
7.5 Occupational Routes of Exposure:
7.5.1 Analysis of whole blood or serum samples
7.5.2 Creating dried spots from whole blood or serum
7.5.3 Disposal of contaminated items
7.5.4 Decontamination of work areas upon completion of testing
7 6 Human Routes of Exposure:
7 6 1 Percutaneous Injuries: injuries that result from needle sticks, cuts or abrasions from a contaminated item These injuries are especially critical due to direct path to the bloodstream
7 6 2 Inhalation of Aerosols: some testing and sample preparation requires the use of sonicators and vortexes This could cause the aerosolization of the materials within Face shields and eye protection will be provided to minimize the risk
7 6 3 Mucous Membrane: mucocutaneous exposure can occur due to splashes of potentially contaminated material to the eyes, nose or mouth. Face shields and eye protection will be provided to minimize the risk.
7.6.4 Ingestion: accidental ingestion of potentially contaminated material can occur when proper hygiene policies are not being followed. All personnel are to be trained in LP3001-3012 Chemical Hygiene and Safety Manual to minimize this risk.
8.0 Communication of Hazards
8 1 Bloodborne pathogens aren’t the only potential hazard in the laboratory Hazardous chemicals may also be used in the various clinical test methods performed
8.2 All personnel will be trained on the handling, storage, use, labeling and disposition of hazardous chemicals. This will be covered in the LP3001-3012 Chemical Hygiene Plan and Safety Manual.
8.3 The CLIA supervisor is responsible for handling and allocating the control and monitoring the chemicals used for the test methods and controlling corresponding MSDSs.
8.4 All MSDSs will be kept in a MSDS binder in a central location for ease of access. All clinical laboratory personnel will know the location and will be able to access the binder at any time. They will be trained in how to properly read MSDSs.
8.5 Clinical personnel are sometimes required to perform non-routine tasks that are potentially hazardous. Proper training is required by authorized supervisor prior to completing these tasks. This training will be documented on the LF2018 Training Record Form.
8 6 Personnel are to understand the risks associated with performing hazardous tasks and have access to corresponding MSDSs
8 7 Purity is responsible for providing contractors and other employers information about the types of hazards and chemicals their employees may be exposed to while working in the clinical laboratory This will include a list of the chemicals as well as their corresponding MSDSs

9.0 Prevention:
9.1 Engineering Controls:
9.1.1 Controls will be put into place to prevent or minimize an employee’s exposure to bloodborne pathogens.
9.1.2 The following is a list of controls that will be in place in the laboratory to minimize the risk of exposure:
9.1.2.1 A designated separate area will be made for working with blood and OPIM within the laboratory.
9.1.2.2 Only authorized and trained personnel will be permitted to work in the blood and OPIM area. Even if clinical personnel are not working with a potential hazard, non-authorized personnel are not permitted in the area.
9 1 2 3 Sharps disposal containers and other biohazard waste containers will be monitored to ensure they are being disposed of properly and in a timely manner
9 1 2 4 Disposition of biohazard material will be done via the autoclave by authorized and trained personnel only. This will be documented in the LF3004 Autoclave Logbook.
9.1.2.5 Employees may not eat, drink, smoke, apply any cosmetics, or handle contact lenses where occupational exposure can occur. Food and beverages may not be stored in refrigerators, freezers and other sites used to store blood and OPIM.
9.2.1 PPE will be made available to employees at no cost to them. Employees will be shown how to obtain PPE and will be trained on any instructions for using the PPE.
9.2.2 All laboratory personnel are required to wear long pants and closed-toe shoes that completely cover the foot. Laboratory personnel should have long hair secured and should not wear dangling or obstructive jewelry.

9.2.3 In order to prevent any cross contamination from samples and chemicals that are used in the laboratory to personal food and drinks, lab coats are required to be removed before entering the lunch room and kitchen.
9.2.4 Lab coats are required to be removed prior to entering the restroom. No lab coats will be permitted in the restroom to prevent any cross-contamination to samples in the lab
9 2 5 Lab coats are not permitted outside Personnel are required to remove lab coats prior to exiting the building If an employee needs to dispose of something that is hazardous or messy, he or she may wear a lab coat while disposing the item, but he or she needs to use a new lab coat once re-entering the laboratory and place the soiled lab coat in the proper location
9 2 6 Employees are required to change lab coats when entering another area in the lab Due to the potential cross-contamination of pathogenic material, PPE worn in the clinical laboratory area may not be worn elsewhere in the laboratory.
9.2.7 Gloves are required to be worn while working with any and all chemicals and samples in the laboratory. In order to prevent cross contamination, gloves should be changed frequently. Frequent glove changing also keeps them from wear and tear when working with harsh chemicals. Some gloves are not compatible with certain chemical uses. To determine what gloves to use, see LP3009 Appendix A: Glove Comparison Chart.
9.2.7.1 Hands should be clean before donning gloves and washed every time gloves are changed.
9.2.7.2 Gloves should be changed when entering a different testing area.
9 2 8 Immediately remove any garment contaminated with blood or OPIM Place the garment in a biohazard container or in a plastic bag with a biohazard label
9 2 9 Garments contaminated with blood or OPIM are required to be autoclaved by authorized and trained personnel. This is to be documented via the LF3004 Autoclave Logbook.
9.2.10 All personnel are required to wear protective eyewear while working in the laboratory. This will be provided by Purity. Depending on the nature of test method or chemical being worked with, various levels of protection may be needed. All types of eyewear will be provided to personnel.
9.2.11 Other forms of PPE such as respirators, thicker gloves, face shields and smocks may be needed depending on nature of work being performed. This will be provided by Purity.

9.3.1 The clinical laboratory area will be kept neat and orderly to minimize mistakes and to ensure precision is upheld. This is also to minimize the risk of cross contamination of samples.
9.3.2 Only trained and authorized personnel are permitted to clean up spills in the clinical laboratory area. This is to ensure they are following proper safety protocols when working with blood and OPIM.
9.3.3 Work areas will be cleaned as soon as possible after working with blood or OPIM. Work areas will be cleaned with a 1:10 solution of liquid household bleach or a chemical germicide approved for use as a hospital disinfectant and effective against HIV
9 3 4 Biohazard waste will be placed in containers which are closable and constructed to all contents and prevent leakage Biohazard waste containers will be appropriately labeled or colorcoded
9 3 5 Any contaminated sharps must be discarded as soon as possible in containers that are closable, puncture-resistant and leak proof on the sides and bottoms Sharps disposal containers will be appropriately labeled or color-coded.
10.1 All the equipment in the laboratory will be handled and equipped with proper safety precautions. These safety features must not be tampered with or disabled. However, certain pieces of equipment still are considered hazardous, so certain precautions are required to be taken against them.
10.2.1 Autoclaves can cause burns from handling hot items being taken out or from the autoclave itself while it’s running.
10.2.2 Personnel are to wear the proper PPE when using an autoclave such as oven mitts for hot items being removed or steel mesh gloves for sharps.
10 2 3 Personnel are to exercise caution when opening autoclaves due to pressure buildup and steam ventilation
10.3 Centrifuge:
10.3.1 Due to the high speed of centrifuges, they have the potential for causing injury.

10.3.2 The most common source of injury from a centrifuge is user error. In order to prevent this, ensure the centrifuge is balanced.
10.3.2.1 If there are an uneven number of samples, blanks are to be made using a comparable material.
10.3.2.2 Ensure all samples and blanks are placed in the same test tube type.
10.3.3 When working with hazardous material, employees are to wait for ten minutes after it has completed its cycle before removing samples.
10.4 Glassware:
10.4.1 All glassware in the lab is to be used solely for its intended purpose.
10.4.2 If glassware is broken or damaged in any way, it is to be disposed of in the broken glass receptacle.
10.4.3 Broken glassware with blood or OPIM on them will be disposed of in a sharps container.
10 4 4 If an employee gets cut from broken glassware that has potentially infectious materials on it, dispose of the piece of glassware in the sharps container and follow the first aid protocol in Section 10 0: Exposure Incident
10 4 5 If an employee gets cut from broken glassware, dispose of the piece of glassware in the sharps container and follow the first aid protocol in the LP3001-3012 Chemical Hygiene Plan and Safety Manual
10 5 Biological Safety Cabinets (Biosafety Cabinets):
10.5.1 Airflow gauges should be checked prior to use to ensure they are working properly.
10.5.2 The air flow and circulation is to be checked by a qualified outside vendor once a year. The LF4001 Equipment Maintenance Form is to be completed after the check.
10.5.3 Extra supplies must not be stored in the biosafety cabinets. Only materials needed for immediate use should be kept in the biosafety cabinet, and then returned to a proper storage place after use.
10.5.4 Containers of chemicals or biohazardous materials should not remain open in the cabinet.
10.5.5 Move in and out of the cabinet slowly to limit the disruption of the air curtain.
10.5.6 PPE is required to be worn while working in the biosafety cabinet. This includes more protective eyewear, double gloves and aprons if necessary.
10 5 7 The grille should be kept clear of all objects including work materials, papers and arms
10 5 8 The cabinet should be turned on and allowed to purge for 3-5 minutes prior to use This allows for the particulates to be flushed through the system for a clean environment
10.5.9 Aseptic technique must be practiced when working within the biosafety cabinet.
10.5.10 Decontamination procedures must be followed after working with or spilling any biohazardous material. See Section 11.0.

10.5.11 The biosafety cabinet must be cleaned after every use. This is to ensure no biohazardous material contaminates other experiments or other areas of the laboratory. The cabinet must be cleaned with approved antimicrobial cleaning solution and any contaminated items must be autoclaved and then disposed of.
10.5.11.1 Cleaning of the biosafety cabinet will be documented in the LF3003 Biosafety Cabinet Cleaning Logbook.
10.5.11.2 Autoclaving of materials will be documented on the LF3004 Autoclave Logbook.
11.0 Exposure Incident:
11.1 An exposure incident to bloodborne pathogens is defined as an eye, non-intact skin, parenteral, mouth or other mucous membrane contact with blood or OPIM that results from tasks performed by an employee.
11 2 For other laboratory accidents and injuries, see the LP3001-3012 Chemical Hygiene Plan and Safety Manual
11 3 In the event of an exposure incident, implement the following protocols:
11 3 1 Biological Spill Over a Large Part of the Body Incident: 11 3 1 1 Alert surrounding employees to call 911
11.3.1.2 Personnel who are trained in Bloodborne Pathogens are to assist injured employee. 11.3.1.3 Remove all contaminated clothing. 11.3.1.4 Remove any jewelry or obstructive material. 11.3.1.5 Wash the exposed area with copious amounts of water and soap for 15 minutes. 11.3.1.6 Do not use any neutralizing chemicals on the spill.
11.3.1.7 Once medical attention arrives, inform them on the amount and type of potentially infectious material the employee was exposed to. 11.3.2 Percutaneous (skin-prick) Incident: 11.3.2.1 Alert surrounding employees to call 911. 11.3.2.2 Personnel who are trained in Bloodborne Pathogens are to assist injured employee.
11 3 2 3 Remove all contaminated clothing
11 3 2 4 Remove any jewelry or obstructive material
11 3 2 5 Wash the wound with copious amounts of water and soap for 15 minutes
11.3.2.6 Do not use any neutralizing chemicals on the spill.
11.3.2.7 Once medical attention arrives, inform them on the amount and type of potentially infectious material the employee was exposed to.
11.3.3 Eye Exposure Incident:
11.3.3.1 Alert surrounding employees to call 911.
11.3.3.2 Personnel who are trained in Bloodborne Pathogens are to assist injured employee.
11.3.3.3 Remove all contaminated clothing.
11.3.3.4 Remove any jewelry or obstructive material.

11.3.3.5 Remove contact lenses if applicable.
11.3.3.6 Wash eyes with copious amounts of water under the eyewash station for 15 minutes.
11.3.3.7 Do not use any eye drops.
11.3.3.8 Ensure to hold both eyelids open while washing.
11.3.3.9 Once medical attention arrives, inform them on the amount and type of potentially infectious material the employee was exposed to.
11.3.4 Inhalation Incident:
11 3 4 1 Personnel who are trained in Bloodborne Pathogens are to assist injured employee 11 3 4 2 Remove any clothing that obstructs breathing
11 3 4 3 Take the employee to a clean area with fresh air
11 3 4 4 Promptly get medical attention
11 3 4 5 Once medical attention arrives, inform them on the amount and type of potentially infectious material the employee was exposed to
11 3 5 Ingestion Incident:
11.3.5.1 Alert surrounding employees to call 911.
11.3.5.2 Personnel who are trained in Bloodborne Pathogens are to assist injured employee.
11.3.5.3 Bring employee to a more comfortable area and position, try to comfort employee to prevent shock.
11.3.5.4 Cover employee with blanket or other covering to prevent shock.
11.3.5.5 Once medical attention arrives, inform them on the amount and type of potentially infectious material the employee was exposed to.
11 4
11 4 1 Once an employee has received medical attention, the LF3053 Post Bloodborne Pathogen Exposure Incident Evaluation and Follow-Up form needs to be filled out
11.4.2 Any medical documents and test results are to be placed in employee’s medical file.
11.4.3 The exposed employee must give consent if he or she wants to be tested for HIV, HBV and HCV. This will be documented on the LF3053 Post Bloodborne Pathogen Exposure Incident Evaluation and Follow-Up form.
11.4.4 If the employee does not give consent for HIV serological testing during collection of blood for baseline testing, preserve the baseline blood sample for at least 90 days; if the exposed employee elects to have the baseline sample testing during this waiting period, perform testing as soon as feasible.
11.4.5 All post-exposure testing will be done at the expense of Purity.
11.5.1 The circumstances involving an exposure incident will be reviewed by the CLIA Supervisor and/or the Quality Department to determine the following:
11.5.1.1 engineering controls in use at the time
11.5.1.2 work practices followed
11 5 1 3 any device or equipment being used 11 5 1 4 protective equipment or clothing being used
11 5 1 5 location of the incident
11 5 1 6 procedure being performed when the incident occurred
11 5 1 7 employee’s training
11 5 2 This investigation will review this Exposure Control Plan to determine if changes need to be made to prevent an exposure incident from happening in the future
11.5.3 The exposure incident will be documented on the LF3051 Exposure Incident Investigation Form.
12.1.1 Any employee who will be in direct contact with a bloodborne pathogen or OPIM is required to complete and pass bloodborne pathogen training.
12.1.2 Training is required prior to beginning any work with blood or OPIM.
12.1.3 The training may be administered by any private or public organization as long as proper documentation is completed and the training is administered by a knowledgeable healthcare professional.

12 1 4 All personnel that go through the bloodborne pathogen training will need to obtain documentation that they have completed the course This will be kept in their personnel files
12 1 5 Personnel that have not completed bloodborne pathogen training are not permitted to work with any OPIM, blood or its constituents.
12.1.6 All employees who undergo bloodborne pathogen training are required to be competent in the following areas:
12.1.6.1 Epidemiology, symptoms and mode of transmission of pathogenic diseases.
12.1.6.2 An explanation of activities and tasks that may involve exposure to blood or OPIM
12.1.6.3 How appropriate engineering controls, work practices, and PPE will prevent or reduce exposure
12.1.6.4 The basis for the selection of PPE; the types, use, location, removal, handling, decontamination, and disposal procedures
12.1.6.5 Hepatitis B vaccine information including that the vaccine is provided at no cost, the benefits of being vaccinated and methods of administration
12.1.6.6 Employer responsibilities for post-exposure evaluation and medical follow-up; how and who to contact should an exposure incident occur
12.1.6.7 An explanation of the signs and hazard labels
12.1.6.8 An explanation of the OSHA bloodborne pathogen standard and this ECP along with how to obtain a copy of each document
12 2 Documentation:
12 2 1 All training will be documented on the LF2018 Training Record Form
12 2 2 Training records will be maintained for three years and will include the following information:
12 2 2 1 the date of the training session
12.2.2.2 the contents or a summary of the training session
12.2.2.3 the name(s) and qualifications of person(s) conducting the training session
12.2.2.4 the names of all people attending the training sessions
12.2.3 Medical records for employees are confidential and are kept for the duration of employment plus 30 years.
12.2.4 Employee medical records will be provided upon request of the employee or to anyone having written consent of the employee.
12.2.5 Medical records include the employee’s name, Social Security number, and hepatitis B vaccination status (including dates of hepatitis B vaccination and any medical records relative to the employee’s ability to receive the vaccination).

12 2 6 In the event of an exposure incident, the following records will be kept in the employee’s medical file:
12 2 6 1 The results of any examination, medical testing, and follow-up procedures
12.2.6.2 A copy of the health care professional’s written opinion to Purity Laboratories
12.2.6.3 A copy of all information provided Purity Laboratories to the health care professional regarding the exposure incident.
12.2.7 All exposure incidents determined to meet OSHA’s Recordkeeping Requirements will be recorded in the OSHA 300 log.
12.2.8 All injuries from contaminated sharps will be recorded in a LF3054 Sharps Injury Log. Information recorded on the Sharps Injury Log will be retained for 5 years.
12.2.9 The Sharps Injury Log will include the following information: date of the injury type and brand of the device involved work area where the incident occurred explanation of how the incident occurred
12.3 Vaccinations:
12.3.1 All personnel that complete the bloodborne pathogen training will have the option to receive the hepatitis B series vaccines Vaccination is not required and will be paid for by the company
12 3 2 An employee may decline to take the vaccination series but must sign a declination statement Any employee who initially declines the vaccination may request and obtain the vaccination at a later date at no cost
12 3 3 Any previously vaccinated employees must provide a vaccination record that includes the vaccination dates
12.3.4 Purity Laboratories will retain copies of employee vaccination records and documentation of refusal of the vaccination. This will be kept in the employee’s medical file.

12.4 Labeling of biohazards:
12.4.1 Sharps disposal containers and other biohazard waste containers must be completely red in color or labeled with a biohazard warning label.
12.4.2 Refrigerators and freezers used for containing and storing blood and OPIM must be labeled with a biohazard warning label.
12 4 3 Biohazard warning labels must be fluorescent orange or orange-red and must contain the biohazard symbol and the word BIOHAZARD in a contrasting color The labels must be attached to the necessary objects by string, wire, adhesive, or another method which will prevent the loss or removal of the label.
12.4.4 All hazardous chemicals will be labeled according to the LP3001-3012 Chemical Hygiene Plan and Safety Manual.
12.5 Exposure Control Plan Updating:
12.5.1 The Exposure Control Plan will be reviewed and updated annually.
12.5.2 Any changes made to the Exposure Control Plan will require employees to have training on the changes made.
12.5.3 If test methods are changed or if new research proves a method or equipment used to be more or less hazardous, the Exposure Control Plan will be updated to reflect this new research.
