
• larger volume of acquisition
• multiple signal averaging First (H) proton MRS published in 1985…
MR spectroscopy in pediatric neuroradiology
https://pmc.ncbi.nlm.nih.gov/articles/PMC8107850/



• larger volume of acquisition
• multiple signal averaging First (H) proton MRS published in 1985…
MR spectroscopy in pediatric neuroradiology
https://pmc.ncbi.nlm.nih.gov/articles/PMC8107850/


Larmor’s equation: Resonance frequency in a perfect world…



Real world…each nucleus is influenced by local magnetic field resulting in a shielding effect (diamagnetic or paramagnetic effects) = isotope/molecule resonates at a lower resonance frequency.

• Interaction between two neighboring
• Direct: “through space” interaction
• Indirect: “bonding” electrons
• Coupling constant “J” in units of Hz.
• Lactate:
• J constant = 6.9 Hz at 1.33 ppm

• Indirect interaction of CH3 protons “phase” of the doublet:
Positive phase at TE 33, 288
Negative phase at TE 144
“composite peak”…

•


About 20 metabolites present…Post Processing software…phase correction, base line correction, elimination of macromolecule signals…


Must be in concentrations of greater than 1 mmol/kg tissue to be detected as “peak”
MRS Techniques
• Single voxel MRS
• PROBE: 1995, PROton Brain Examination
• Techniques:
• PRESS
• “point resolved spectroscopy”
• Doubles SNR, more artifact
• STEAM
• “stimulated echo acquisition mode”
• Short TE only, lower SNR than PRESS
• Multivoxel
• MRSI
• “MR Spectrographic Imaging”
• CSI
• “Chemical Shift Imaging”
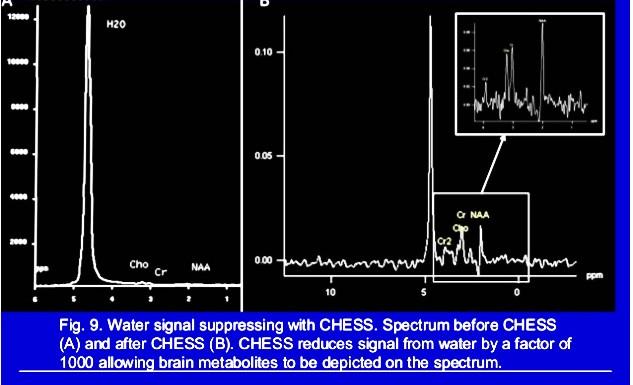
Water Suppression Techniques:
CHESS: Chemical Shift Selective
VAPOR: Variable Pulse Power & Optimized Relaxation Delays
WET: Water Suppression Enhanced Through t1 effects
IR: Inversion Recovery



Appearance depends on:
Age of Patient
Location of ROI
Timing of Injury
Technical Factors:
Type of MRS Sequence
Strength of Magnet
TE: See lipids, macromolecules, certain neurotransmitters, myo-inositol (mI), and trace lactate at short TE due to short T2 relaxation values.
Shim
Line Width


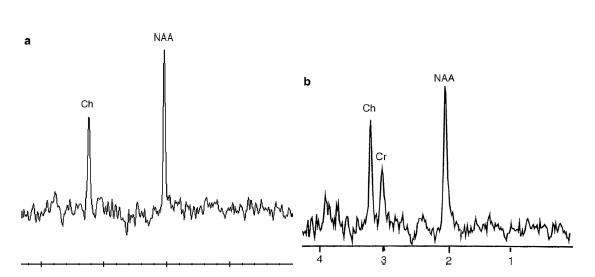
• NAA: N-acetyl aspartate 2.0 ppm
• Second most abundant free amino acid in the adult brain after GLU
• Synthesis in neuronal mitochondria
• Transported across membrane
• Cleaved in cytosol to aspartate and acetate
• Function:
• Marker for Neuronal & Axonal viability
• Transporter of acetyl groups for lipogenesis
• Neuronal osmolite (removes excess water from neurons)
• Neuronal energy metabolism of mitochondria and brain fatty acids
• Neuronal reservoir for GLU
• Substrate for NAAG
• Neurons, oligodendrocytes, microglia
• Signals astrocytes about state of neurostimulation, & requirements for energy and waste removal


• CHO: Choline 3.2 ppm
• Free Cho, phosphoryl-Cho (Pcho) , glycerylphosphoryl-Cho (GPC), taurine and others
• Linked to membrane biochemistry
• disruption, cell proliferation (tumors) or inflammation by macrophages
• Cho product of myelin breakdown
• Increased in tumor
• Increased cellular density, tumor growth with proliferation of membrane phospholipids
• Increased in WM diseases
• Reflects precursors of myelin synthesis
• Degradation products of myelin degradation


• CR: Creatine 3.0 ppm, (3.9 ppm)
• Creatine Cr, phosphocreatine pCr
• Most stable
• ‘internal reference”
• 50% from diet/50% endogenous
• Metabolized to creatinine, excreted by kidneys
• Marker of metabolism and energy production


• Lac: Lactate 1.3 ppm
• Anaerobic metabolism, cell death
• Also…mitochondrial impairment in an inflammatory response with macrophage infiltration
• HIE, seizures, metabolic disorders
• Acute inflammation with macrophage accumulation
• Poor washout in cysts, abscess, NPH/CSF and within cystic or necrotic tumors
• J-coupling: interaction of lactate methyl and methane proton peaks at 7-8 Hz, results in double peak
• Doublet can be “smoothed” with shimming issues


mI: Myo-inositol mI (3.5 ppm): simple sugar, marker of intracellular sodium content and glial activation, correlated with osmolarity conditions, synthesized in glial cells (almost exclusively astrocytes) and absent in neurons therefore considered a glial/astrocyte marker, therefore elevated in gliosis and reactive astrocytosis; can not cross the blood brain barrier. Elevated in the setting of gliosis, astrocytosis, Alzheimer’s dementia, inflammation, breakdown of myelin.
Amino Acid Peaks: composite peaks at 2.2 and 2.6 ppm, (3.6ppm)
Ala: Alanine: 1.48 ppm/doublet: Role in citric acid cycle, found in some meningiomas.
Gln: Glutamine: primary derivative for Glu
Glu: Glutamate: 2.2-2.4 ppm: neurotransmitter, most abundant AA in the brain
GLX: combined Gln & Glu, increases with destructive processes
GABA (gamma-aminobutyric acid): important inhibitory neurotransmitter
Asp: Aspartate
Glc or Gc: Glucose: 3.43 and 3.8 ppm: dominates fuel supply, correlates with flood flow, therefore increased in DM and HIE
Lipid/Macromolecules: .9 & 1.3 ppm: broad composite peaks at .9 and 1.3 ppm, elevated in tumors, also stroke, MS, demyelination, necrosis in tumors.


• Acquire T2 Flair straight
• Try to position nose, head straight

• Line width below 15 but would like below 10

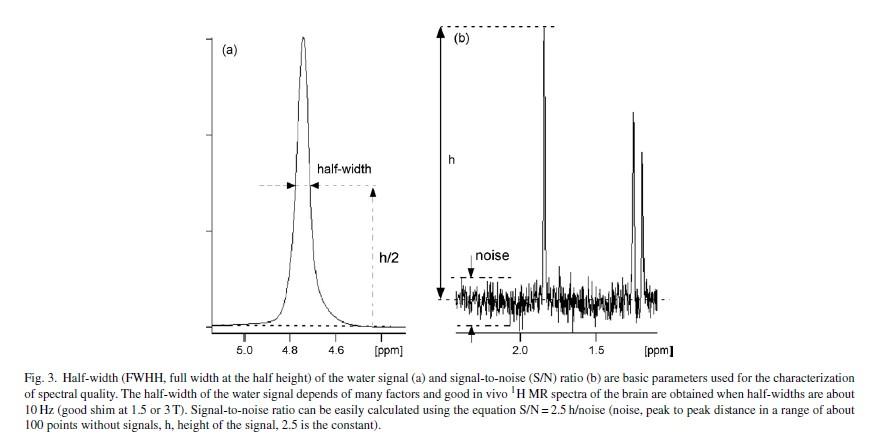
• Line width
• FWHH=full width at half height
• Around 10 Hz or below at 1.5 or 3.0 T
• Limited by voxel size
• large voxel means increase in overall inhomogeneity of B0 field


Increase SNR by increasing number of acquisitions, but also increases time… Increase SNR by increase voxel size
• Broadened with accumulation of Gad based contrast agents
• …so do prior to contrast…maybe?…
Decide whether Single vs Multivoxel is needed:
Single Voxel (TE 34, 144, 288)…2-3 cm.
Better detection of key metabolites such because of relatively short T2 relaxation times.
Short scan time.

Remember to screen save your ROI image with the appropriate MRS, automatic with single voxel.
Spectrum automatically generated, saved to PACS.

Single Voxel TE 35: Single Voxel Long TE 144 or 288
Loose your lipid peaks at 0.9 & 1.4 ppm due to short T2 relaxation time…
288)
Multiple voxels (minimum volume of each voxel 1.0 cm) acquired at the same time. Less influenced by gradient-induced distortions therefore better baseline. Greater “voxel bleeding” therefore quantification not precise.


• Suppress water from brain
• Stay out of fat, scalp, & blood!
• Use saturation bands…
• GE: total of 9, 6 automatically prescribed orthogonal to voxel but hidden on set up
• can add 3 more but if add >3, will remove one of preexisting sat bands





Save as a screen shot…
Adjust scale of tallest peak But stay consistent across all boxes!







How do I choose the TE? It’s all about flipping that lactate peak!
• LOW TE 34
• Used often in Single voxel
• More metabolites
• Lac peak UP (pos phase, above the baseline)
• HIGH TE 144
• Single or multivoxel
• Interrogate complex tumors or larger areas
• Lac peak DOWN (neg phase)
• HIGH TE 288
• single or multivoxel
• Lac peak UP (pos phase)
• Increased SNR
• Improved Spatial resolution
• Increased Spectral (peak) resolution
• Improved Temporal Resolution (shorter acquisition times)

J-modulation artifacts
• Loss of “Lac doublet” or signal at 3T on 144, should reappear on 288
• Chemical shift misregistration
• Magnetic field stability and “drift”
• Radiofrequency coil efficiency



GE Phillips Siemens



“White Brain”


ROI #1: Basal Ganglia

Single Voxel MRS Spectrum automatically generated, saved to PACS.
MRS ROI #2: Watershed White Matter

Multivoxel : Remember to manually screen save your ROI image.








to drop, resolution of Lac,
If see increase Lac early in ischemia, Recommend 10 day follow up to follow NAA and Lac peaks

2 “near drowning” patients in coma…prognosis… when to intervene…


3 “near drowning” patients in coma…prognosis… when to intervene…


Normal MRS
Good Prognosis
Depression of NAA
Elevation of LAC
Poor prognosis
5 day old neonate with hypotonia/seizure…HIE?

5 day old neonate with hypotonia/seizure…HIE?






Abnormal wide doublet at 0.9 ppm = BCAAs (Branched chain amino acids) & BCKAs (Branched chain ketoacids)



• Diffusion restriction corticospinal tracts, basal ganglia, cerebellum and dorsal brainstem, Ageneses CC
• Elevated glycine peak at 3.55 ppm, higher than choline.
• What else lives at 3.5 and 3.6, and creates a doublet?



• Diffusion restriction corticospinal tracts, basal ganglia, cerebellum and dorsal brainstem, Ageneses CC
• Elevated glycine peak at 3.55 ppm, higher than choline.
• What else lives at 3.5 and 3.6, and creates a doublet?
• MyoInositol, shorter than Choline






Term infant, hypotonia, HIE?… what is the TE?...Missing?


• Creatine Synthesis
• Last step involves methylation by guanidinoacetate methyltransferase (GAMT) to form creatine

• 3.5 months treatment with oral creatine supplementation







Falling Naa/Cr, ^^ Cho/Cr, ^^ Lactate peak
=Acute demyelination



3 zones:
Inner: astrogliosis
Intermediate: active inflammation/lymphocytic infiltration
Outer: active demyelination, edema
disorder of B-oxidation of very long chain fatty acids (VLCFA), defect in ALD protein gene product in the peroxisomal membrane, accumulation of VLCFA

• Accumulates VLCFAs
• Posterior predominance
• T2 hyperintense signal within WM
• Peripheral enhancement
• Involves corpus callosum/splenium
• ADC values increase from peripheral to central, restricting along the enhancement margin
• MRS: Active Demyelination
• Decreased NAA
• Increased Cho
• Increased Lactate

1 year followup
^Cho, ^mIns,
Dec NAA, ^ ?Lac
2 year followup
Progression
^mIns, ^Cho
Dec NAA
^ Lac
^Mobile lipids
Dec GLU, ^ GLN

• Frontal ROI:
• Persistent lesional profile
• Depressed NAA
• Elevated Cho, Lac
• Occ ROI:
• Improved neurochemical profile
• Reduced Cho, Lipid/Lac
• NAA still reduced relative to Cr




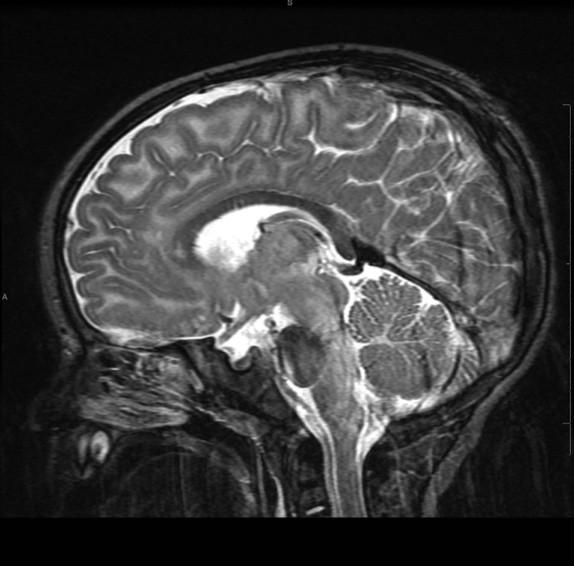
• Macrocephaly – swollen astrocytes
• Homogeneous high T2 throughout WM
• Not “Hypomyelination”
• Spares internal capsule and CC
• Early involvement of subcortical U-fibers

Mutation in ASPA gene, deficiency in enzyme aspartoacylase, required to hydrolize NAA




• Congenital, infantile, and juvenile forms
• Macrocephaly – swollen astrocytes
• Homogeneous high T2 throughout WM
• Early involvement of subcortical U-fibers
• Involves globus pallidi & thalami
• Spares caudate/putamen, internal capsule and CC

• MRS: elevated NAA (also in urine)


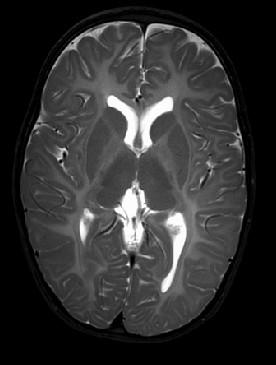





MRS: Mainly decreased Cho in the WM due to hypomyelination
• Classic (late infancy) vs Connatal (birth to early infancy) forms
• Mutation in proteolipid protein 1, essential to integrity of myelin
• Diffuse CNS Hypomyelination
• Involves subcortical U-fibers, internal capsule
• May have “tigroid” appearance along perivascular areas Metachromatic Leukodystrophy




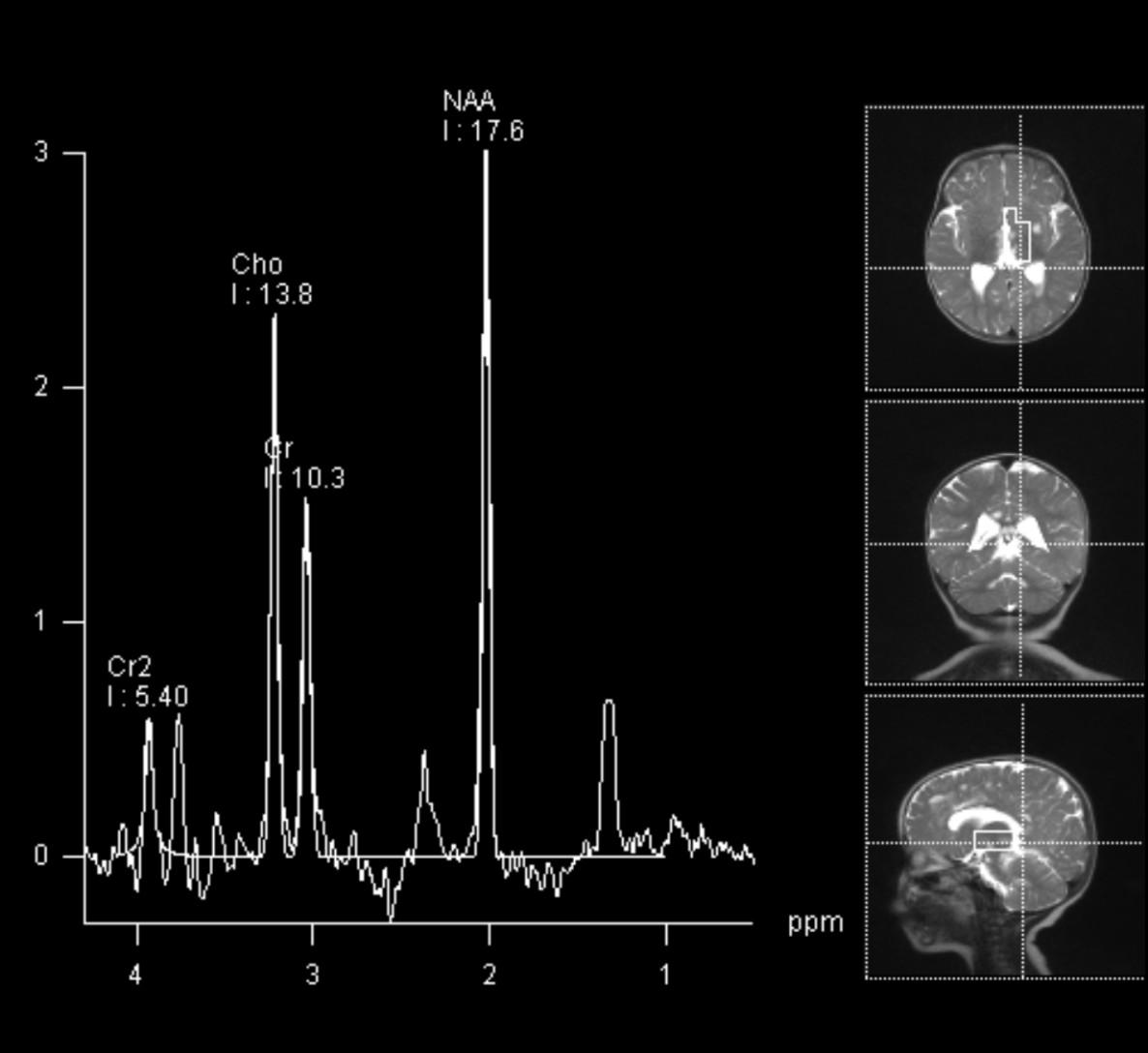



• Disorder or respiratory metabolism seen in infants
• Spongionecrotic lesions in the putamen, caudate nuclei, globi pallidi, periaqueductal GM, dorsal pons, cerebellar nuclei, WM late in disease
• Restricted diffusion
• MRS: Nonspecific Elevated Lac
MRS in Mitochondrial Diseases (Leigh’s, MERRF, KearnsSayre, MELAS):
Lac: ^^^ in normal and abnormal brain…GM (occ)/cerebellum > WM
Cho: Elevation (demyelination) or Drop (decreased maintenance)
NAA: Variable drop in NAA neuronal dysfunction, hard to appreciate early…
Leigh’s, ^ Lac in “normal” WM

look




Solvent for antiseizure meds which accumulates in neonates due to metabolic immaturity

Both

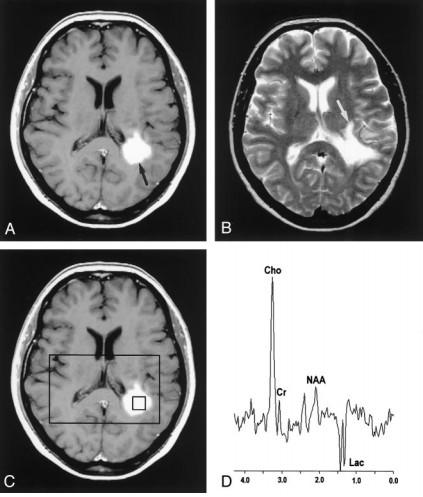
• Marked elevation Cho…both
• Membrane breakdown
• Cellular proliferation
• MS: Gliosis, inflammation
• Infarctions…gliosis
• Inflammation…glial proliferation
• Lac…both
• Marked decrease in NAA
• Greater in TumeMS than in high grade glioma
• Astrocytomas Grade I & II
• MyoInositol is elevated in low grade gliomas



c/o H. Rowley, UW-Madison

c/o T. Kennedy, UW-Madison

Elevated choline indicative of membrane turnover
c/o H. Rowley, UW-Madison

Loss of metabolites (“flat line” pattern) or lipid / lactate only c/o H. Rowley, UW-Madison