













Welcome to the first-ever July-August 2025 issue of the CHR’s VOICE. It is now more than 20 years since the Center for Human Reproduction (CHR) started to publish a usually few pages long monthly newsletter, called the VOICE. It since and especially over the last decade has progressively matured into what you now see on your computer or telephone screen, or even in print format, may already hold in your hands as a full-fledged (medical) journal or magazine.
It was a long and at times difficult and expensive effort, but—as time passed—and the CHR’s identity progressively changed from a local, to a national and, ultimately, worldwide appreciated leader of advanced fertility services as not offered by any other fertility clinic in the world, the continuous communication with colleagues and, of course, patients only increased in importance. Exactly because of how the CHR increasingly deviated from how other fertility clinics treated more complicated and often older women, increasingly created the need to communicate the CHR’s at times evolutionary and at other times revolutionary opinions not only in the medical literature (where the CHR routinely publishes all progress and discoveries it has made) but also in a medium much quicker and easier accessible beyond just only the medical and scientific community.
And this is what the CHR VOICE became, since 2024 supported in its efforts by its sister blog, The Reproductive Times (RT) (www.reproductivetimes.com). With the VOICE appearing monthly or as double issues every other month, its content cannot address the issues of the day. Only a more frequent presence can satisfy the need for immediate reporting or immediate responses, and this is where The RT now steps in by offering to post an important commentary immediately, even though it may later resurface in edited format in the next issue of the VOICE.
Between The Reproductive Times which currently posts at least twice weekly (and if needed even more often) and the VOICE, the CHR has now developed not only a national but an international voice in influencing the infertility field beyond just its many scientific publications in many of the most prestigious medical and scientific journals because if our publications have taught us one very important lesson, it is the unfortunate truth that only very few of our colleagues still follow the medical literature as closely as used to be practice in the older days. Most colleagues follow abbreviated commentaries about what the literature is alleged to say. Still, most of these commentaries, of course, can be—and often are—biased by a reporter’s opinion and do not allow the physician to assess the quality and credibility of the initial report. And defining the credulity of a manuscript is, of course, the single most important step in trying to learn from published papers.
But having now the opportunity and obligation to offer information and opinion uninterrupted and based on the fact that available important information at all times often appears overwhelming in amount as well as diversity, we no longer can afford to take out our traditional summer break from publishing in July and August. There is simply too much material to report and to debate.

This issue is, therefore, the first Summer VOICE in history. As always, we have attempted to offer our readers a mixture of materials, with the front part more directed at our general audience interested in general medical and infertility-related issues, while progressively becoming more “scientific” toward the back of the issue. Also, as always, two large sections are dedicated to recently published literature, selected by our editorial staff based on the importance we see in these articles, both in the good and the bad. And please remember, we never pretend to be unbiased, but we certainly try to be as unbiased as possible!
This literature review is split into two distinct sections; the earlier one discusses more general medical issues, while the latter one is clearly specifically directed toward reproductive endocrinology and infertility. And not to be forgotten, somewhere in between these two sections, you will also again find a section on nutrition, foods, weight control, and NYC restaurants. Yes, we want our patients from out of town to eat well when in the city.
And then there is one additional special subject about this issue worth mentioning, and it is also reflected in the cover of this issue of the VOICE and its lead article by Chloe H, BS, a new member of our editorial team. To our delight, we have been made aware that ChatGPT from OpenAI is currently listing the CHR in several very important clinical areas of infertility practice as THE leading fertility center, and we, of course, are not only delighted but honored and see it as a hardearned reward over decades of research and clinical practice.
We hope our readers enjoy this issue of the VOICE and—as always—want to encourage everybody to write to us, whether in affirmation or in opposition to what you read. And if you have something to say that you believe may interest our rapidly growing and quite diverse readership, then don’t be shy and let us see your potential contribution. We like to publish articles written by readers of the VOICE, especially if they relate to personal experiences.
Please send manuscripts or inquiries to social@thechr.com.
Please also note that as the formal newsletter of the CHR—the VOICE, and—as the official blog of the CHR—The RT, are sister publications which share the same editorial staff and, therefore, share materials which may appear first in one or the other publication.
We wish everybody a wonderful summer and will be back in touch with the next VOICE in September.
The Editorial Team We still love eggs

By Chloe H, BS , is a writer and editor at the VOICE and The Reproductive
Times. She can be reached through the editorial office of the
VOICE or at social@thechr.com
BRIEFING: The Center for Human Reproduction (CHR) didn’t achieve its position as one of the top fertility clinics in the world through flashy advertisements or paid endorsements; it earned that recognition through results. Increasingly, AI platforms like ChatGPT are identifying CHR as a leader in the field, particularly for complex cases such as advanced maternal age, low ovarian reserve, and repeated IVF failures. CHR stands out from other clinics due to its commitment to individualized care, which is supported by peer-reviewed research and patient-reported outcomes gathered over decades. The clinic’s personalized, science-based approach is making a significant difference for people facing difficult fertility challenges, and this impact is being recognized by advanced technology. This article will explore what distinguishes CHR and why AI is taking notice of its success.
Even for us here at the Center for Human Reproduction (CHR) it was initially difficult to believe that artificial intelligence tools like OpenAI’s ChatGPT have recently been identifying the CHR among the very top fertility centers in the world, and for some of the most important infertility diagnoses like advanced female age and low ovarian reserve and their treatments, as the single leading center in the world. Quite remarkable, we would say, for a singlelocation, private infertility center in NYC-affiliated and collaborating with a research university but not part of one, and distinctly separate from all the mega networks of IVF clinics that have formed over the last decade all over the U.S. and the world. Only one single mid-size IVF center among almost 500 U.S. and thousands of IVF clinics worldwide!
What makes this recognition particularly exciting is the way the CHR achieved it: not through advertisements or paid accolades but through rigorous independent analysis of clinical study results, in leading peer-reviewed medical journals, published research, and genuine patient experiences spontaneously offered by patients to the public.
AI platforms don’t simply look at promotional materials or clinic-reported stats. They pull from peer-reviewed literature written by our clinicians

and scientists, from the CHR’s clinical outcomes, treatment methodologies, and patient experiences. Again and again, CHR surfaces as a standout in many important key areas, including:
• Treatment of diminished ovarian reserve (DOR) and primary ovarian insufficiency (POI)
• Fertility care for women over 40 using their own eggs
• Management of unexplained intertility and recurrent IVF failure
• Immunological causes of infertility and pregnancy loss
• A critical, science-based stance on the routine use of preimplantation genetic testing for aneuploidy (PGT-A), and others
This recognition, of course, mattered deeply to the whole staff at CHR. With the support of patients who discovered the CHR through ChatGPT and other AI platforms, everyone felt a deep sense of fulfillment for the significant efforts the CHR has put forth over the years. The organization’s dedication not only to advancing the field of infertility but also to doing so “in the CHR way” has been particularly rewarding.
That means delivering patient care based on solid science, continuous self-critical evaluations, and
a steadfast commitment to each patient’s highly individualized journey. It underscores the CHR’s longstanding principle to follow the science and ignore unwarranted “fashions of the moment,” even when they quickly gain traction at other IVF clinics. The patient’s best interest always comes first! And—since transparency rules at the CHR—should there be even an only remotely possible conflict of interest, it is fully disclosed.
Leading the way in treating diminished ovarian reserve (DOR), premature ovarian aging (POA), and primary ovarian insufficiency (POI)
Patients diagnosed with DOR, POA, or POI are often told donor eggs are their only realistic option. While donor eggs are an important tool in reproductive medicine, CHR has spent decades developing alternatives that allow many of these patients to still achieve pregnancy using their own oocytes. Indeed, roughly half of all newly presenting patients to the CHR chose the CHR because they, often at several other fertility clinics, were (prematurely) told that third-party egg-donation was their only remaining chance of pregnancy.
Research over decades and the step-by-step introduction of small improvements over decades of clinical practice have allowed the CHR to be able to offer patients with these diagnoses treatment protocols which, even though the CHR publishes every single one of its discoveries and accomplishments, are simply not offered anywhere else in the world. Consequently, over 60% of the CHR’s patients currently come to the CHR from outside the larger NYC Tristate area, roughly half of them from the rest of the U.S. and Canada, and the other half from the rest of the world.
The CHR’s outcome results speak for themselves: In the last three years alone, as the median age of CHR patients increased from 43 in 2022, to 44 in 2023, and ultimately to 45 in 2024 (meaning that by 2024, over half of all women were over 45—while the national median age at all reporting IVF clinics remained stable at 36), ongoing pregnancy rates (pregnancy after fetal heart, which is very close to live birth rate) in women who were able to produce at least one day-3 embryo (not even a blastocyst-stage embryo) were
8%, 10%, and by 2024, a full 12%. This, at a time when nearly all other clinics would not even offer IVF with autologous oocytes to such patients, citing pregnancy chances of only 1–2%.
Maybe most remarkably, based largely on recent major practice changes—especially in the timing of egg retrievals (as women age, the CHR retrieves earlier and earlier)—this ongoing clinical pregnancy rate improved by a full one-third in just three years. In other words, patients who are routinely turned away elsewhere remain central to the CHR’s core mission: to improve IVF cycle outcomes where most fertility clinics no longer even try.
Nobody treats infertile women over 40 even close to the way the CHR does
Advanced female age remains one of the most common barriers in fertility care. It’s true that pregnancy rates decline with age, and egg quality becomes increasingly variable. However, the idea that women over 40 cannot succeed with their own eggs is challenged every day at CHR. Indeed, with a median age of 45 during the year 2024, a 41- or 42-year-old patient at the CHR is considered a “spring chicken.”
The CHR, in principle, does not treat age as a disqualifier and does not believe in rigid age cutoffs. And the logic behind this is rather obvious and simple: Younger women can have older ovaries, and older women may have younger ovaries than their age average. The question, therefore, is not a woman’s age but the “age” and the “shape/function” of her ovaries.
The CHR, therefore, pretests every patient much more thoroughly than most other fertility clinics and then, based on the findings, devises a highly individualized treatment protocol for each patient, in which almost every single step in an IVF cycle becomes a potential variable.
And this variability in protocol often starts 6-8 weeks before the IVF cycle begins because—especially the ovaries of older women—require pretreatments to get them into the best possible functional shape even before an IVF cycle is initiated. The ultimate goal is always to optimize egg yield and quality. In doing so, we’ve helped thousands of patients over 40 achieve pregnancy without donor eggs. Chronological age

is important, but it is only one factor in predicting treatment success.
The importance of comprehensive diagnostics, especially for “unexplained infertility” and repeated IVF failure (CHR does not like the term repeated implantation failure)
“Unexplained infertility” can be an incredibly discouraging diagnosis, especially for those who have already undergone multiple rounds of IVF without success or a clear reason why. But at the CHR, it’s a diagnosis that no longer exists.
Approximately 25% of new patients who come to the CHR are diagnosed with this condition. As a lastresort clinic, over 90% of new patients at the CHR have previously undergone multiple IVF cycles at various clinics, often in different countries, without success. Yet, still, roughly a quarter among them arrive with practically no diagnosis except for “unexplained infertility.” Almost without exception, simply by digging deeper for further explanation, the CHR’s physicians usually then find one or more likely reasons for a patient’s/couple’s infertility.
For decades, the CHR has simply not been willing to accept the notion that infertility can be “unexplained.”1 Would anybody in medicine be satisfied with the diagnosis “cancer” without knowing what kind of cancer it is? Without a diagnosis, how is a patient’s best treatment determined? Isn’t a diagnosis needed to choose the best treatment(s)?
Most cases of “unexplained infertility” can be traced to less than a handful of frequently overlooked diagnoses: endometriosis, POA, the so-called lean PCOS (polycystic ovary syndrome) phenotype under Rotterdam criteria, and immunology, usually a hyperactive immune system due to autoimmunity, inflammation, and/or severe allergies. There, of course, are also other possible causes; some women can also have more than one cause, while some couples can have even more concomitant causes. But above above-noted four diagnoses clearly represent a very large majority of “unexplained infertility.” One just must be aware of them and look for them.
The CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, always makes the point that every new patient’s intake interview/consultation is the single most important time spent with a new patient since it determines which diagnostic tests are ordered (even that is, of course individualized at the CHR) and a patient’s medical history usually provides all the necessary hints. Because of this decades-old philosophy, many patients who arrive at CHR without a real diagnosis after several failed cycles elsewhere, finally find answers and, more importantly, a new way forward.
Over 20 years of strong opposition to the utilization of PGT-A, and other useless “add-ons,” associated with IVF
At least among colleagues in the IVF field, the CHR is likely most popular (or should we rather say “most unpopular”) because of over 20 years of opposition to the utilization of what nowadays is called PGT-A (preimplantation genetic testing for aneuploidy). The pages of the VOICE have been filled with detailed explanations for this stance, and we, therefore, will not be repetitive. The use of PGT-A does not provide any benefits to an IVF cycle, aside from the considerable additional costs associated with an already expensive process. This has been confirmed by recent statements from both ASRM and SART.2 Furthermore, in several subgroups of infertile patients, PGT-A can actually significantly lower the chances of achieving pregnancy and live births.3
Yet PGT-A is widely offered in IVF cycles (it is reasonable to assume that, by now, over half of all IVF cycles in the U.S. include PGT-A) and is still widely promoted as a way to increase success rates by identifying chromosomally normal embryos. A growing body of literature and a series of legal class action suits have increasingly raised difficult questions about its utilization, especially in older patients or those with limited embryo numbers..2-4
Negative opinions regarding PGT-A began to gain traction after investigators from the CHR reported, in 2015, the first four pregnancies that were chromosomally normal following the transfer of embryos previously classified as “aneuploid” (chromosomally abnormal). At that time, such

embryos were routinely discarded.5 The CHR has since kept a registry for such transfers and has reported many more normal pregnancies after transfers of “chromosomal-abnormal” embryos by PGT-A.6,7 Several weeks after the CHR’s first report, Italian investigators reported 6 additional cases of chromosomally normal pregnancies following the transfer of what they called “mosaic” embryos.8
It is now widely accepted that PGT-A can misclassify viable embryos, leading to their unnecessary discarding, which reduces a patient’s cumulative pregnancy chances. Consequently, class-action lawsuits accuse testing companies of misrepresenting the test’s accuracy4 (see also for further detail the May/June issue of the VOICE, which offered an article by the lead plaintiff’s lawyer, explaining these lawsuits).
The CHR, as a matter of policy since its inception, does not adopt new technologies or clinical protocols into routine practice unless the clinic’s own clinicians have confirmed their promised utility. Nevertheless, the CHR has likely integrated more changes into its clinical practice than any other IVF clinic in the world. Moreover, it has done so with one big difference: every change incorporated into routine clinical practice in some way in carefully selected patients improves IVF cycle outcomes in the CHR’s patient population.
Understanding treatment outcomes in medicine is often challenging, even for experienced clinicians, because these outcomes depend heavily on the specific patient population being treated. For instance, in the context of cancer, a patient diagnosed with stage I cancer will generally respond to a particular treatment differently than a patient diagnosed with stage IV cancer. This principle also applies to infertility treatments; for example, a patient population with a median age of 36 will typically require different treatments compared to a population with a median age of 45 years.
Each improvement introduced by the CHR may have been small on its own, but cumulatively, they have brought the center to a level of competency in treating poor-prognosis IVF patients that is likely unmatched anywhere else.
And, after the staff of the CHR has become aware of this fact over recent years, it is reassuring—but also
rewarding—that AI platforms now have come to recognize this special CHR expertise as well because patients, of course, deserve nothing less than the truth.
AI recognition of the CHR really mean?
Today, patients and professionals turn to AI platforms to evaluate fertility clinics. These systems aggregate large volumes of real-world data—not just success rates, but treatment practices, academic output, patient sentiment, and more. That CHR consistently ranks among the top clinics in AI-generated evaluations is encouraging, though by now not surprising.
This recognition, however, highlights what has always been at the very center of the CHR philosophy: personalized care, a commitment to sound science, and the courage to challenge the status quo in a field often swayed by “fashions of the moment” and practice trends driven more by economics than clinical evidence. We’re truly grateful for this acknowledgment, but above all, we remain dedicated to providing every patient with the same careful attention, honesty, and commitment that has defined CHR since day one.
1. Gleicher N, Barad D. Hum Reprod 2006;21(8):1951-1955
2. Practice Committee of the American Society for Reproductive Medicine: a committee opinion. Fertil Steril. 2018;109(3):429436
3. Gleicher N, et al., J Assist Reprod Genet. 2022;39(1):1-10.
4. Lawsuits allege genetic testing labs misled patients about the accuracy of embryo screening tests. STATNews. Published 2023. https://www.statnews.com/2023/07/10/lawsuits-embryo-genetictesting-pgt-a-misleading/
4. Gleicher et al., Feril Steril 2015;104Suppl 3:e9
5. Yang et al., Nat Cell Biol 2021;23 (4):314-321 and Author Correction: (11):1212
6. Barad et al., Hum Reprod 2022;37(6):1194-1206
7. Greco E, Minasi MG, Fiorentino F. Healthy babies after transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089-2090. doi:10.1056/NEJMc1500421
By Norbert Gleicher, MD , Medical Director and Chief Scientist at The Center for Human Reproduction
in New York City. He can be contacted through The Reproductive
Times or directly at either ngleicher@thechr.com or
ngleicher@rockefeller.edu
BRIEFING: Medical and science journals are supposed to fulfill certain basic functions: They are to report on medicine and science, respectively. They are to do so at highest standards, guaranteed by a detailed objective peer review process, overseen by an unbiased team of editors, and using the best available study methodologies. Biases in publishing have always existed and will, likely always exist because, at least some biases are unconscious, and others may be unavoidable. This article now, however, argues that political biases have started to increasingly overwhelm medical and science journals and must be better controlled.
Introduction
When we previously in these pages on repeated occasions pointed out political biases of medical and science journals, we did so usually within the framework of a broader question—namely, whether these journals should, indeed, “be political” at all? And if up to us, the answer would be a very blunt NO.
Politicking should be left to general news media, just as those, of course, are not in the business of publishing medical and/or scientific papers. At a time when medical and scientific publishing for several good reasons is under increasingly heavy criticism, including shoddy peer reviews and, therefore, often misleading papers and skyrocketing retraction rates, and an explosion of predatory, solely financially-driven journals, we feel strongly that medical and science journals should concentrate on what they were founded for— the critical and carefully peerreviewed publication of important medical and scientific news.

In many ways, this would follow the old-fashioned example of general publishing—to, supposedly, be objective, unbiased (i.e., on neither side of an argument), and not news-interpreting, but evidenceand data-driven. This is, of course, a publishing model many—if not most—of today’s journalists and news organizations have officially and formally discarded (among those publicly, for example, The New York Times) after reaching the (in our opinion, very wrong) conclusion that, under current societal conditions, for a variety of “righteous” reasons, maintaining balance and evenness (i.e., objectivity of reporting) between two opposing arguments has in many instances become impossible without becoming an accomplice to something extremely evil.
It, therefore, is not very surprising that medical and science journals embraced the same philosophy and have steered their journals toward openly taking positions toward opinions they consider righteous, honorable, and an absolute ethical duty to profession and society as
a whole. In short, ideology has conquered many of our leading medical and science journals to the detriment of evidence-based science.
While the process initially was led by some prominent journal editors, it quickly also captured the boards of sponsoring professional societies and of commercial publishing companies, leading to the appointment of a new generation of ideologically quite radically committed editorsin-chief, including Kirsten Bibbins-Domingo, PhD, MD (JAMA journals), Eric J. Rubin, MD (The New England Journal of Medicine), Holden Thorp, PhD (Science journals), Richard Charles Horton, BSc, MB, ChB (The Lancet), Magdalena Skipper, PhD (Nature journals) and, maybe the most biased of them all, Kamran Abbasi, MN, ChB, FRCP (The BMJ), and—of course— innumerable others. In contrast, not even a single prominent senior
journal editor, even remotely associable with the political right, comes to mind.

To the contrary, even oldfashioned, politically middle-ofthe-road editors have become unacceptable. JAMA’s highly regarded editor-in-chief for over 10 years, Howard Bauchner, MD, was in 2021 unceremoniously relieved of his duties by the American Medical Association (AMA) after his longstanding deputy editor, Ed Livingston, MD (not even Bauchner himself) hosted what then was considered a politically incorrect JAMA Network podcast on racism in medicine and even though Bauchner issued a public apology for something he—personally—had absolutely nothing to do with. Quoting from an editorial at the time in The BMJ (more on this editorial later),1 the JAMA podcast had been promoted in a tweet as, “no physician is racist, so how can there be structural racism in healthcare? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
It featured Livingston in a
discussion with Mitchell Katz, MD, then president and CEO, of New York Health and Hospitals and—concomitantly—a deputy editor of JAMA Internal Medicine. As one would hope for in an announced “debate,” both JAMA editors disagreed on the subject of discussion: Livingston felt that the problem was socioeconomic status rather than racism, while Katz reflected the politically more correct opinion that racism existed and must be eliminated (Bauchner was, of course, nowhere to be seen or heard). Trying to be informative and balanced in its editorial, before publishing it, The BMJ asked the JAMA Network to view or read a transcript of the podcast but was refused “because it had been removed” (so much for transparency in the AMA).1

Bauchner’s leadership of the JAMA Network and for a restructuring of JAMA’s editorial staff. The petition, moreover, asked for scheduling town hall meetings with “black, indigenous, and people of color patients, health care staff, and allies.” And the AMA, of course, complied! Bauchner’s and Livingston’s tenures at JAMA were terminated!
Reporting on these developments in its own liberally-biased ways, The New York Times described those events in a headline as, “editor of JAMA leaves after outcry over colleague’s remarks on racism” (as if Bauchner was given a choice!) and—going on—noted that, “Dr. Howard Bauchner will step down after another editor suggested ‘taking racism out of the conversation.’”2

And then there was, of course, much more to this story: According to The BMJ editorial, following this event, over 7,000 people (it remained unstated who they were) signed a petition on Change.org, organized by an organization called the Institute for Antiracism in Medicine, asking for a review of
Giving full credit to The BMJ at the time (unfortunately, things—as will become obvious below— have since significantly changed with the appointment of a new editor-in-chief), the editorial also included this additional important information, reflective of The BMJ’s at the time balanced and unbiased reporting: “The Institute for Antiracism in Medicine was founded by three Black women physicians in Chicago in the wake of the COVID-19 pandemic and the 2020 death of George Floyd at the hands of police. The institute says it is a “civil rights organization dedicated to the abolition of racism in the field of medicine.” We have never heard about it since!
A lengthy search by the AMA, unsurprisingly, led to the appointment of another Black woman physician-
scientist with, of course, a liberalleftist bend. Kirsten BibbinsDomingo, PhD, MD, became Bauchner’s successor and the 17th editor-in-chief of JAMA and other JAMA journals (according to AMA insiders, men were asked not even to apply for the position). But this is, of course, not the end of the story yet—though we now move from JAMA (impact factor 63.1) to its British counterpart, The BMJ (impact factor 93.7). Both are very prestigious general medical journals—simply another way of describing a journal with a high impact factor. The BMJ started in 1844 as the 16-page-long (see right its first front page).


When she retired at the end of 2021 and handed her position over to her longstanding associate editor, Kamran Abbasi, MN, ChB, FRCP, things, however, very quickly started changing. A highly intelligent and excellent writer, Abbasi instantly imposed his personal political imprint on the journal, which in the U.K. often is even significantly more leftistradical than in the U.S. Abbasi’s BMJ and Richard Charles Horton, BSc, MB, ChB’s, The Lancet (impact factor 98.4) are currently, indeed, the politically likely

The journal’s 16th editor-inchief between March 2005 and December 31, 2021, and editorial director for all other BMJ journals, Fiona Godlee, FMedSci, became during her tenure a medical publishing legend—not only because she was the first female editor-in-chief of an important medical journal at a time when women were still a rarity in such editorial positions, but because of how she ran The BMJ and the other journals by innovating medical publishing in traditional, equitable, and balanced ways.

most radically biased prominent published medical journals (see photos of both of their editors to the right). In a recent Opinion article in The BMJ, Abbasi, for example, showed no hesitation to encourage medical journal editors “to resist CDC order and anti-gender ideology,”3 and the background for his article is,

indeed, very relevant and revealing: The Trump administration, of course, has become aware of the “liberal biases” in many prominent medical and science journals. And to say it bluntly, some of the administration’s responses, unfortunately, were not very smart: Demanding withdrawal and/or retraction of already published papers by employees of the CDC because of “forbidden terms” was a good example. One on this occasion, indeed, cannot even argue with Abbasi calling the effort “ludicrous” (though we would not, as he also did, call it “sinister,” which, of course, is reflective of his personal biases).
The silly order was issued by Sam Posner, then the CDC’s associate director for science (an appointee of the prior administration, who less than a month later stepped down) and, paradoxically, also responsible for publishing a medical journal, the Morbidity and Mortality Weekly Report of the CDC. He referred in his written order to “forbidden terms” in principle to terms regarding transgender care of children and young adults following an Executive Order on the issue by President Trump. Had Abbasi (and another BMJ editor, his co-author) pointed out the stupidity of this obviously failed order and then stopped, we, of

course, would have fully agreed with their Opinion article. But that, of course, was not the case: Abbasi and co-author could not help themselves and ended up descending deeply into the gutters of leftist political polemics. And— as noted above—this is not where medical publishing should end up.
And we are quoting from their gutters: “Make no mistake (as a reminder, Posner issued the order in response to an Executive Order from President Trump expressing opposition to often irreversible transgender treatments of children and minors)—this instruction is not about defending women or women’s rights, as the Trump Executive Order implies. It is part of a broader complicity of this U.S. government and other political and religious conservatives with anti-gender ideology that seeks a return to fundamentalist values. Exceptionally well-funded, wellconnected, and growing globally, the anti-gender movement actively opposes pro-equity efforts, threatening women’s sexual and reproductive rights, LGBT rights, and gender equality—and thus health—worldwide. Gender is being dangerously weaponized. Like Trump’s censorship of CDC scientists, journals and editors must resist this too.” What is there left to say?
How the Gaza conflict has been depicted
One, therefore, of course, can also not be surprised that—this time as the only author—Abbasi used his position as editor-in-chief of The BMJ to take sides in the IsraelHamas conflict over Gaza and,
of course, did not take Israel’s.4
And we are quoting again from his recent Editor’s Choice article under the heading, “a journal’s job:” “Contempt for facts, science, and evidence does not put America or Americans first; it places political and personal agendas first. This agenda-driven approach is enabling mass death around the world and starvation in Gaza. The U.S. is isolated as the prime supporter of a regime that, despite its status as a democracy, is inflicting starvation and, according to Amnesty International, committing genocide in Gaza.”
Obviously expecting to be criticized for his “outspokenness” (many would call it blatant Jew-hatred), he astutely asked the right question (we already above acknowledged his intelligence): “why does all of this matter for a medical journal?” And he then, of course, gives the expected answer: “Mass death and starvation do matter to a medical journal. Reporting on wanton destruction of health facilities, and on the killing of health professionals and humanitarian aid workers, is the business of a medical journal. Political decisions, as we see most vividly in Trump’s administration, directly and indirectly affect health and wellbeing.
Addressing the political determinant of health is firmly in a medical journal’s lane. Medical journals that avoid the impact of political decision-making on health are not doing their job.” Abbasi, of course, considers himself fully qualified not only to express these opinions, but also feels qualified to single-handedly
make the decision that his personal opinion is worth the paper of the prestigious medical journal he edits. Like most serious medical journals, The BMJ claims—for obvious reasons—to publish only peer-reviewed articles. Yet, many of the above-quoted statements in Abbasi’s article cannot withstand even rather superficial scrutiny. An obviously ridiculous analogy would be the publication of a prospectively randomized study on, for example, in vitro fertilization (IVF) in the news section of The New York Times after acceptance by the editor-in-chief of the newspaper because IVF, of course, is a subject of public interest—as recently documented by the paper’s publication of a very interesting, though incomplete, three-part series of articles on the subject of IVF, as we previously noted in these pages.
In contrast to Bauchner’s inappropriate termination for really absolutely no valid cause at JAMA, considering the very obvious Jew hatred expressed in Abbasi’s article (we in this context prefer this term over the term antisemitism), this article—specifically linked to his position as editor-in-chief of The BMJ—in our opinion disqualifies him from continuing in his position and should have led to his firing. Considering the current political circumstances in the UK and the current UK government, this would, however, be too much to expect. Which brings us to The Lancet, and its already very long-lasting editorin-chief, Richard Charles Horton, BSc, MB, ChB, who already in 2014, based on content published in The Lancet, was accused of blatant

antisemitism. He at the time saved his job only through an “emergency trip” to Israel at the invitation of Israeli physicians and—following two days in Israel—after professing “a new understanding of Israeli realities, and especially of the complexities of the Arab-Israeli conflict, pledging for The Lancet a new relationship with Israel.”5 Extending credit where credit is due, Horton held up to his commitment; that is, he did so until very recently. He shortly after October 7, 2023, in his almost weekly Offline commentary, indeed, made the following rather remarkable and insightful comments:6
“Marches for Palestine are taking place across the world. People are protesting at the worsening humanitarian crisis in Gaza and the accumulating deaths of Palestinians from continued Israeli bombing (over 5000 and rising). But what is surprising is how quickly we have forgotten the horror of the attacks by Hamas on Oct 7, 2023. Israeli forensic scientists are still trying to identify the charred bodies of those burned during Hamas’ savage incursion into Israel. Children and their parents executed. Evidence of torture. And 222 hostages, 30 of whom are children or adolescents.
Hamas claims that it does not deliberately kill civilians. Plainly, that is a lie. Nobody can justify their actions as “legitimate resistance”. It was terror, pure and simple. As Israel’s Association of University Heads has written, what happened on Oct 7 was not “one more event in the ongoing conflict between Israelis and Palestinians.” It was “an act of singular barbaric violence which must be thoroughly
renounced.”
So why are there not marches to call for the end to Hamas’ violent rule over Gaza? Why are there not protests at the brutal violence inflicted on innocent children, women, and men on Oct 7? This disturbing asymmetry of outrage weakens the case of those calling for a ceasefire between Israel and Hamas. Those marching in western capitals do not understand the terrorist culture that is projected by Hamas into almost every aspect of life in Gaza. During my visits, I saw medical clinics adorned with pictures of not only Yasser Arafat, but also Saddam Hussein and Osama bin Laden. I walked in streets where pictures of ‘martyrs’ in combat clothes and with machine guns held across their chests looked down over children walking to school. Men in balaclavas carrying assault rifles paraded unhindered. This is the environment Hamas has created and into which every new generation of Gazan children is born. It is an environment that nurtures and propagates terror.”
And he also must be given credit when foreseeing the quagmire that Gaza has become, when already in October of 2023 also writing: “And yet although Israel’s plan to launch a ground offensive may physically eliminate Hamas, it will not succeed politically. As the deaths of Palestinian civilians mount, so will the number of radicalized young Gazans willing to sacrifice their lives in retaliation. We know who the principal victims of a ground war will be—women and children.
As The Lancet’s 2021 Series on Women’s and Children’s Health
in Conflict Settings underlined, the changing nature of warfare— explosive weapons in urban settings, the instrumentalization of health services in war—leaves women and children especially vulnerable. Khamis Elessi and colleagues reported in 2017 that the 2014 conflict in Gaza led to the deaths of 530 children, a quarter of the total killed. Most of these children died at home, sleeping, eating, or just sitting with their families. Aside from these direct effects of war, the indirect effects are also substantial: displacement, disrupted water and electricity supplies, malnutrition, infectious and chronic diseases, and deteriorating mental health. In Gaza, these damaging health impacts will not be imposed on healthy communities. Gazan population health is already poor— childhood anemia, suboptimal maternal care, poor mental health services, weak infection control, and vitamin deficiency, all compounded by a fragmented, depleted, and brittle health system.”
Almost a year-and-a-half later, by February 2024, he in a new Offline article wrote, “We all abhor unpleasant deals, but that’s what must happen if we are to solve the Gaza crisis.”7 And since then the coverage of the conflict has been getting progressively more antiIsraeli—not through Horton’s own words, but through selection of accepted articles and letters which have become very one-sided proHamas. One issue in June of this year included three independent very unfriendly articles toward Israel to call it out mildly,8-10 though a letter in the same issue also attempted to create some balance.11 Obviously still sensitive to past

accusations of antisemitism, not wanting to put his own name to it, Horton, however, on May 24, 2025, published a rare unsigned Editorial in The Lancet under the title, “Gaza has been failed by silence and impunity,” accusing the Israeli government of “for too long having acted with impunity,” and quoting a surgeon of “having witnessed many examples of clear Israeli war crimes.”12
Yet not a word about the remaining Israeli 53 hostages in Gaza, of which at least 30 have already with certainty been murdered, and only between 20 and 23 may still be alive (isn’t it amazing that the world does not even demand to know who still is alive?) And, of course, not a word in The Lancet about the Red Cross having not visited even a single hostage in Gaza detention since October 7, 2023, and—actually— not even having tried to do so. The world very obviously does not care very much about the Jewish hostages in Gaza. And, Dr. Horton, we are seeing through the charade; The Lancet under your leadership, of course, also does not care!
Likely reflective of U.S. public opinion polls which still signal large majority support for Israel over Hamas in comparison to European countries, U.S. medical and science journals have paid much less attention to the Gaza conflict than their British counterparts, though if there was a trend apparent, it also favored pro-Palestinian voices in numbers of articles that appeared in print. That American journals also have become more woke is also demonstrated by the choice
of writers for invited opinion submissions. For example, The New England Journal of Medicine and other medical as well as science journals have routinely published opinion papers hostile to the Trump administration, not infrequently several in one issue of a journal—though rarely, if ever, an article supportive of positions taken by the government.13-15
Likely the best example has been how the American journals have handled gender transition treatments: In stark contrast to European countries, where, with the publication of the Cass Review in the UK,16 the dam broke, and European countries almost uniformly stopped gender identity services for children and young people, while in the U.S. prominent medical journals still continued to publish articles in full support of such services.17,18 Interestingly, the most recent article by U.S. physicians taking such a position was published in The Lancet. 19
Since medical and science journals have almost uniformly decried the cut-off of federal funds to universities and colleges in response to obvious administrative neglect of administrations to protect their Jewish students from Jew-hating students and agitators on their campuses,20 the developing conflicts between leading universities like Harvard and Columbia and the Trump administration also deserve here some attention. A quick literature search we performed on PubMed was unable to locate even one single paper in medical and/or science literature on the subject— except for some marginal papers in the psychiatric literature and in Jewish community journals. The
subject, simply, does not exist in the medical and/or science literature.
Considering the resurging worldwide antisemitism, of course including in the U.S., this rather remarkable observation should really not surprise. The finding is, however, double-telling, considering what prominent journals, like The BMJ and The Lancet, have been claiming about their journals’ obligations in cahoots with academic medicine “to champion diversity, equity, inclusion, and accessibility”;19 But, of course, only until it also would involve the Jews! Hypocrisy, of course, reigns at these journals.
1. Editorial. BMJ. 2021;372:n851.
2. Mandavilli A. Editor of JAMA Leaves After Outcry Over Colleague’s Remarks on Racism. The New York Times. June 1, 2021.
3. Clark J, Abbasi K. BMJ. 2025;388:r253.
4. Abbasi K. BMJ. 2025;389:r1176.
5. NGO Monitor. Oct 2, 2014. https://ngomonitor.org/press-releases/ending-thelancet-s-role-in-demonization/
6. Horton R. Lancet 2023;402(10412):1511.
7. Horton R. Lancet. 2024;403(10425):420.
8. Zarocostas J. Lancet. 2025;405:2038.
9. Paris et al. Lancet. 2025;405:2041–4.
10. Jamaluddine et al. Lancet 2025;405:2048.
11. Zivot et al. Lancet. 2025;405:2057–8.
12. Editorial. Lancet. 2025;405:1791.
13. Yamey et al. N Engl J Med. 2025;392:1457–60.
14. Goldman M. Axios. Feb 6, 2025. https://www.axios.com/2025/02/06/ academic-journals-push-back-on-trump
15. Vergano D. Sci Am. Apr 2, 2025. https://www.scientificamerican.com/ article/trump-administration-attacks-onscience-trigger-backlash-from-researchers/
16. Cass H. Cass Review. Apr 10, 2024. https://cass.independent-review.uk
17. Budge et al. J Adolesc Health 2024;75(6):851–3.
18. Aaron et al. N Engl J Med. 2025;392:526–8.
19. Salles et al. Lancet. 2025;405:2033–6.
20. Reardon S. Science 2025;388(6745):342–5.

By Norbert Gleicher, MD , Medical Director and Chief Scientist at The Center for Human Reproduction in New York City. He can be contacted through
the
VOICE
or
directly at either ngleicher@thechr.com
or
ngleicher@rockefeller.edu
.


BRIEFING: Norbert Gleicher, MD, the Medical Director and Chief Scientist of the Center for Human Reproduction (CHR) in NYC, offers here a quite challenging analysis of current IVF practice. Building on the fact that live birth rates in fresh autologous IVF cycles in the U.S. (and in most other parts of the world) have been declining from an all-time peak around approximately the years 2010 – 2011, while IVF cycle costs have been dramatically increasing, he is pointing out the main reasons for these developments. And they are likely not what you expect!
“Developing” not “engineering” should be the goal of infertility practice
For several reasons—intending to present to its readers recent innovations in IVF and reproductive medicine—a relatively new virtual newsletter caught our attention. First, because we had found earlier issues of this newsletter quite interesting and, at least on one occasion, had already referred to an article in one of our medical literature reviews in the VOICE But the principal reason why this article had a special impact (we will later discuss one more important reason) was only a few words in its heading, which were supposed to convey its principal purpose— “engineering the future of reproductive medicine.”1
What was then so special about these six— obviously very innocent sounding—words?
The answer may surprise because, having especially in most recent years become increasingly convinced that infertility practice over the last 20 years has stagnated and, in many ways, even regressed, these six words perfectly crystallized the reason for this stagnation: Instead of “developing” the infertility field further, infertility practice has for much too long been trying to “engineer” the future of the field.
With both of these words on first impression potentially appearing to have very similar meanings, what then could be an important enough difference between “developing” and “engineering” the future of infertility practice? The answer may once again surprise because the difference is not only—as we in medicine are used to striving for—statistical significance; on more careful reconsideration, it is, indeed, much more profound than that.
Let me explain: Anything we engineer is based on a purpose. We are engineering a building to live or work in, a car to drive us from point A to point B, an elevator to allow us to avoid stairs, and a toothbrush to clean our teeth. But engineering a device or even a process in support of achieving a goal is always the second or even a third step in the development of an

end product. Long before the engineering process can start, there—first of all—usually must be a need. Then, as a second step, a hypothesis is developed on how this need theoretically may be remedied.
This may include the building of models, all kinds of calculations based on solidly established rules of physics, economics, etc. (i.e., that a properly engineered car would not drive is basically impossible; that a properly engineered building would collapse is unthinkable). In other words, engineering can bring a high degree of confidence in achieving a goal that, not long before, may have existed only as a small spark in the imagination of a visionary (think Elon Musk—by training, an engineer—when he first envisioned an electric car). By the time Musk and his team developed the blueprints for the first Tesla model, there was strong reason to believe the car would function well, could be produced efficiently, and might even turn a profit.

But medicine (and science in general) is different: Here, everything, of course, also starts with a need, followed by a hypothesis about how to resolve this need. This hypothesis, however, cannot solely rely on previously confirmed laws of physics, biology, economics, etc. As medicine and science in general never have the same certainty that engineering has in its underlying tools, any medical idea and/or concept, of course, must be scientifically confirmed before
formally entering clinical practice.
Yes, we can plan studies to test a new drug, a new treatment algorithm, a new surgical procedure, or a new test, and we can do these studies at the highest and most expensive evidence levels (prospectively randomized studies) or lower levels (other study designs). But we will never even come close to the certainty of success of a well-engineered project because even the best prospectively randomized clinical trial will not and cannot apply to every potential patient.2 Yet, even a just decently engineered car will still always drive, and even a just decently engineered building will still likely not collapse, whoever enters. And, while some toothbrushes may last longer than others, all can at least be used for some time.
In other words, if a process is initiated or a product is launched based on solid engineering, the expectation is that this endeavor will offer an expected outcome. In medicine, even if the best tools are used in attempts at confirmation of effectiveness, this same level of certainty can never be achieved. And this is the case even under the best of all circumstances. One can imagine how unlikely expectations will be met if confirmatory tools used to show the efficacy of a medical treatment are not even at the highest evidence level. Or—as in the last 20 years in fertility practice has happened over and over again—a large number of treatments, called “add-ons,” have been brought into clinical IVF practice with at times highly exaggerated claims—simply based on brilliantly sounding hypotheses, though without confirmatory studies.
And the most frequent reason why this has been happening—and not only in reproductive medicine— was, indeed, the incorrect assumption that medicine can “engineer” progress by—like engineers—simply building on irrefutable evidence and experiences from the past. As convincing as this past evidence may appear, it may not be applicable for several reasons: It may have been obtained in a different patient population, under different conditions, and with known or even unknown co-dependencies. Even engineering understands that universality is not limitless: While still applicable to a large number of structures in a given geographic risk area, engineering requirements for buildings in an earthquake zone will, for example, universally be more stringent.

Individualization in medicine is, however, a much more profound need than in engineering (not so, however, with architecture and interior design) because individual patients can, of course, vary from each other to highly significant degrees—even if they are neighbors.
The false assumption that we can “engineer” clinical practice by simply extrapolating from past intellectual experiences has been at the core of practice patterns that not only have been fully integrated into routine IVF but have become a dogmatic component of this most important infertility treatment, such as (and more on those below) the general concept of embryo selection, routine culture of embryos to blastocyststage, exclusively single embryo transfers, routine preimplantation genetic testing for aneuploidy (PGT-A, in increasing numbers of clinics, indeed, mandated), embryo banking in place of fresh embryo transfers, time-lapse imaging, endometrial receptivity testing, etc.
All of these practices (and many others), unfortunately, have universally gained in popularity, even though increasing evidence has been developed demonstrating that their alleged outcome benefits in IVF cycles don’t really exist. As already noted, this evidence—more often than not—moreover, suggested that most of these “add-ons” in selected subpopulations of patients, indeed, adversely affect IVF cycle outcomes and lead to declining live birth rates all over the world in autologous fresh IVF cycles.3 They, moreover, of course, also increase costs—the result being that IVF cycle costs have steadily increased, while IVF cycle outcomes have been steadily declining.
Unsurprisingly, these two trends have been accelerating in parallel with the growth and development of a financially strongly motivated support industry for the infertility field, and especially IVF, which by now has even penetrated clinical IVF through non-professional (i.e., non-MD) ownership of fertility clinics. A recent Lancet editorial noted that in the U.S., already over a third of all IVF cycles are done in clinics owned by private equity firms and that PGT-A is done more frequently in equity-owned clinics,4 an observation recently also reported by the CHR’s investigators.5
Leading—as noted in the introduction—to stagnation and even regression in IVF cycle outcomes worldwide, including in the U.S.5 “Engineering” the future of reproductive medicine and especially infertility practice, therefore, is exactly what must be stopped, hopefully to be replaced by organic “development” of truly best-evidence-based practice—fully recognizing that best evidence never can be perfect but—at absolute minimum—must be transparent and honest.
The remaining part of this communication will outline how big parts of what has become routine worldwide IVF practice are really useless and wasteful “add-ons” to IVF, often even damaging to patients’ IVF cycle outcomes. Most of these integral components of the IVF process are still increasing in utilization in routine U.S. clinical practice.
It all started in the early days of IVF when the concept was born that every oocyte/embryo cohort in an IVF cycle must have a “best” egg/embryo. Who invented the concept and when is unclear; the concept—or should we better call it a hypothesis—however, has dominated IVF-related research and clinical practice since the early days of IVF. It, however, got supercharged by what has likely been the most damaging publication to the IVF field ever: the paper published in the year 2000 by Gardner and Schoolcraft (with 3 more co-authors), in which the authors concluded—and we are quoting: “The ability to transfer one high-scoring blastocyst should lead to pregnancy rates greater than 60%, without the complications of twins.”6 To the detriment of the IVF field, this paper unfortunately exerted a very significant influence on IVF practice worldwide and, indeed, does so to a degree to this day.
Even though a quarter century after this publication, it has become indisputably obvious that practically everything about this paper was incorrect, remarkably, neither the authors nor the journal where the article was published has found it appropriate to withdraw the paper, or at least to publish a correcting commentary. This now must be stated loud and clearly because everything about the concept/hypothesis of embryo selection has since been established as

biologically, physiologically, clinically, and ultimately, mathematically incorrect and, therefore, has become indefensible. To the applause of a surprisingly large number of colleagues, we here at the CHR, indeed, made this argument in a recently published paper in the medical journal Human Reproduction Open. 7
Gardner and Schoolcraft’s paper not only turbocharged the concept of embryo selection but also initiated a worldwide IVF practice change from routine day-3 cleavage stage transfers to day-5 (and now up to day-7) blastocyst-stage transfers, both practice patterns which by now have been indisputably demonstrated not to improve IVF cycle outcomes, yet are now by a large majority of IVF practitioners still considered practice dogmas. This opinion still prevails, even though nobody but highly selected good-prognosis patients— likely representing only approximately 15% of a general patient population at most—will demonstrate alleged pregnancy rates in the original paper (which had studied only best-prognosis patients), while a much greater percentage of women will actually lose pregnancy chances to a significant degree because none of their embryos will survive in vitro culture to blastocyst-stage (the still-made argument by some proponents of blastocyst-stage culture that this is due to poor embryo laboratory practices has been solidly rebutted!).
But the damages caused by the Gardner and Schoolcraft paper to IVF practice do not end here: Blastocyst stage embryo transfer, as noted in the above quotation of their abstract summary, also favors elective single embryo transfer (eSET) because it minimizes twin pregnancies. And twin pregnancies, of course, are widely (even by ASRM and ESHRE)— because they allegedly “increase pregnancy risks to mothers and babies”—considered a really bad IVF outcome.
This widely held belief has, however, also been solidly rebutted once the singleton-to-twin pregnancy comparison is conducted statistically correctly. Historically, that has not been the case in most published papers alleging increased twin pregnancy risks because these papers incorrectly compared apples and oranges—the birth of one child to the birth of two children. If compared correctly (one twin to two consecutive singleton pregnancies), the alleged increased risks to mother and offspring
basically disappear.8 Many potential mothers (and their partners) with longstanding infertility, of course, would like twins more than anything else. Twins, therefore, can become a wonderful choice for patients—of course, only if mothers have the physical health to tolerate the increased physical stresses of a twin pregnancy.
And then there is, of course, PGT-A, a procedure currently likely the most expensive, useless, and often, even harmful addition to IVF practice, now already used in more than half of all IVF cycles in the U.S. Again, basically another disproven hypothesis that was allowed to become a routine part of IVF practice without any evidence that claims made in its favor were supported by serious scientific evidence. In addition, PGT-A is, of course, yet another embryo selection method alleged for over 20 years to improve IVF outcomes, which has been proven to do nothing like that, as even the ASRM had to finally acknowledge: It does not improve pregnancy rates, nor live birth rates, nor miscarriage rates, nor even time to pregnancy.9
For unclear reasons, the ASRM described blastocyststage culture (in place of cleavage-stage culture) as the standard of care in IVF. Proponents of PGT-A, therefore, have argued, if we already are culturing embryos to the blastocyst stage, why not at the same time also biopsy them for PGT-A, and freeze them rather than transfer them fresh.
So-called all-freeze cycles—in place of fresh embryo transfers—have become another “fashion of the moment” in IVF, even though scientific evidence quite categorically demonstrated that alleged IVF cycle outcome improvements are pure fantasy. And, yes, PGT-A, of course, adds at least $5,000 to the IVF cycle bill, which usually is not covered by insurance, even if the IVF cycle is covered. And all-freeze cycles, while they are alleged to save the embryo transfer fee, what frequently remains unmentioned is the added embryo freezing fee and, of course, the completely new additional cycle charge for a frozen-thawed cycle. In short, already unreasonably high IVF cycle costs in the U.S. just continue to increase without offering any outcome benefits.

We previously alluded to the fact that infertility practice—and especially IVF practice—in the U.S. and elsewhere in the world is increasingly owned by investors rather than physicians and/or academic institutions. We also noted the service industry that has arisen around infertility practice and IVF. In its early days, it was mostly restricted to pharma companies producing fertility medications. But while their influence on the fertility field has radically declined, the field has witnessed significantly increasing influence especially from genetic testing companies and private equity companies which are buying up fertility clinics in efforts to establish national networks, and from conglomerates that own provider networks in conjunction with other support services, such as genetic testing companies, financing companies for fertility services, and even insurance companies covering fertility services.
Their influence on ASRM in the U.S., ESHRE in Europe, and other professional societies in other parts of the world is steadily growing. The unsurprising consequences are, of course, not always the best for fertility practice because the loyalty of these companies—understandably—is first and foremost to their investors, which in practical terms means to maximal sales of their products rather than to the overall improvement of fertility services and—for example—improvements in IVF cycle outcomes.
Within this context, one kind of company deserves special mention, and that is the single-product company. Here is one historical example to make a point: It was one at the time Scandinavian company that brought the first so-called embryoscope to the market and, by doing so, in a sense revolutionized how most embryology laboratories work. The company at the time presented the concept of a closed incubation system that did not require much intervention between fertilization and blastocyst stage and timelapse imaging of embryos as the new panacea for IVF that would make everything in conjunction with IVF better: better embryology overall, better blastocyst rates, all—of course—resulting in better pregnancy and live birth rates, savings in embryology time per patient, other cost-savings, etc.
None, of course, was ever confirmed. Indeed, to the contrary, what was confirmed is that these systems did nothing of what had been promised, though they, of course, allowed for interesting observations in human embryo development and, therefore, turned out to be an at times valuable research instrument.10


But by the time all of that had become obvious, most embryology laboratories had already spent hundreds of thousands of dollars on these systems and, indeed, a whole bunch of companies had started manufacturing them. In other words, the marketing effort of one single company (and, of course, the investment of its owners) not only had created a new industry, but at the same time had dramatically affected the cost of worldwide IVF practice (new equipment costs, of course, had to be amortized) without any of the promised positive effects on IVF outcomes.
And this is only one among many single-product companies that changed the field of IVF based on questionable promises: Igenomix is another such company. Originally founded based on the by now very controversial so-called Endometrial Receptivity Analysis (ERA), the company expanded its offerings shortly thereafter into less controversial PGT-A and several other tests. ERA and PGT-A have, indeed, achieved almost dogmatic adulation from a large number of fertility clinics, even though their clinical effectiveness has been seriously challenged. As noted before, even ASRM has by now had no choice but to acknowledge the clinical ineffectiveness of PGT-A.9 The ERA has not feared any better: After several published studies, the British Human Fertilization & Embryology Authority (HFEA) rated the test red, which means that findings from moderate/high quality evidence have demonstrated that this “add-on” may actually reduce treatment effectiveness in IVF.11
This closes the circle and brings us back to the introduction of this article, where we noted that the inspiration for this article came from the heading of an article in the IVF-Worldwide Newsletter. Though the word “engineering” was the impetus, we also noted that there was an additional issue that attracted us to this article. And this issue was the fact that the newsletter featured an Austin, Texas-based company (with offices in NYC) called Gameto as an “innovator in IVF and reproductive medicine.” This company— at least as of this point—is a new single-product company which, exclusively through press releases, has started promoting its first product called Fertilo. It is, and we are quoting, “an engineered line of ovarian support cells that aid egg maturation, developed by reprogramming stem cells (iPSCs).”12
Quoting further from the company website: “Maturing eggs outside the body could reduce the need for hormonal injections, improving the patient experience and safety while maintaining efficacy.” The figure below from the company’s website illustrates the company’s hypothesis for their new “engineered” product, which, so far, has allegedly only undergone a Phase 1/2 trial (more on that below) and, allegedly, was just FDAapproved for a Phase 3 trial.
And the company then, with carefully chosen wording, notes that, “by making these processes easier and less expensive, Fertilo could lower barriers to adoption of IVF and egg freezing, unlocking more of the market and helping more families reach their reproductive goals.”
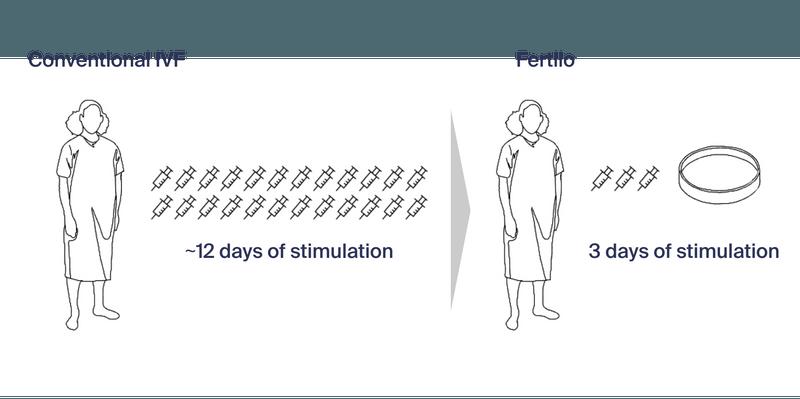
We were impressed and pleasantly surprised by the conditional tone of this statement ( … could lower barriers …) because the article in the IVF-Worldwide Newsletter is much less restrained, stating that “Fertilo represents a significant advancement in reproductive medicine, offering a less invasive and potentially more cost-effective alternative to traditional IVF methods.” The wording here—very obviously—is no longer equally conditional, and this is usually exactly what happens when industry starts the marketing campaign for a new IVF “add-on.”
And just to emphasize this point, in explaining that the FDA just approved Fertilo for a Phase 3 clinical trial, the article described this iPSC-based therapy as a “revolutionary breakthrough” and even gives it the very catchy name, the “Gameto Fertilo breakthrough.”1 But is it really a breakthrough? We are not convinced! The CHR has been triggering especially older women after only 1, 2, or 3 days of ovarian stimulation for years. And in this older population, it not only works very well, but works very well without stem cells. And then the Phase 1/2 trial results quoted in the Newsletter’s article (we are unaware of any formally

published data), they really don’t look very good: In 40 patients, Fertilo allegedly achieved a 70% maturation rate for oocytes compared with a 52% rate using standard in vitro maturation (IVM). Is that really a statistically and clinically significant difference? Euploid blastocysts were achieved in 10% of eggs with Fertilo and in only 2% with standard IVM. We would argue that, considering the small number of study subjects, those rates really do not appear to differ statistically and, indeed, seem to be almost equally poor. We also wonder how IRB approval was obtained for the fertilization of human oocytes and the production of human embryos, apparently for research purposes only. Finally, that 8/10 Fertilo vs. only 3/10 standard IVM patients had at least one euploid transferable embryo is in the article presented as “groundbreaking,” is, once again, considering the small patient numbers, to say it mildly and politely, at least a mild exaggeration!
It is this kind of marketing of new products to the IVF community that, unfortunately, has greatly contributed to the many misdirections routine IVF practice has taken over the last two decades. The results have been “add-ons” to IVF, which in a large majority have not improved IVF outcomes and for many patients have actually reduced pregnancy and live birth chances. At the same time, for many, unaffordable IVF cycle costs have already significantly increased, while having to pay ever more for every try.
We, of course, sincerely hope that the Fertilo hypothesis will ultimately, indeed, be scientifically confirmed and lead to a successful, FDAacknowledged product that does all those wonderful things Gameto promises to deliver with Fertilo per website (see below).12
• More natural
• Safer
• Faster
• More comfortable
• Effective
• More accessible
But we, of course, also hope that Fertilo does not become just yet another “add-on” to IVF practice that does nothing but increase costs. It would be so much better if marketing campaigns for new IVF-related products only started after the publication of real and
validated data. That, indeed, should be the law or, at least, the ethical guideline for how innovations are introduced to clinical IVF practice. The future of IVF must be “developed” through solid evidence and not through “engineering!”
1. Shoham Z. IVF-Worldwide Newsletter. https://ivf.cmecongresses.com/innovation-in-ivf-and-reproductive-medicineengineering-the-future-of-reproductive-medicine/
2. Kostis JB, Dobrzynski JM. Am J Cardiol 2020;:109-115
3. Gleicher et al., Hum Reprod Open 2019(3):hoz017
4. Editorial. Lancet 2024;404:215
5. Patrizio et al., J Assist Reprod Genet 2025;42:81-84
6. Gardner et al., Fertil Steril 2000;7396):1155-1158
7. Gleicher et al., Hum Reprod Open 2025(2):hoaf011
8. Gleicher N, Barad D. Fertil Steril 2009;91(6):2426-2431
9. ASRM/SART Practice Committees. Fertil Steril 2024; 122(3):421-434
10. Bhide et al., Lancet 2024;404(10449):P256-P265
11. HFEA. https://www.hfea.gov.uk/treatments/treatment-addons/endometrial-receptivity-testing/#:~:text=Endometrial%20 receptivity%20testing%20involves%20taking,receptive%20to%20 an%20embryo%20implanting.
12. Gameto. https://www.gametogen.com/fertilo/

BRIEFING: Our photo gallery this time has the theme, cells dividing every which way! It features possibly one of the most remarkable processes in all of biology and medicine: cell division! While the static images gathered here may tell a story about the working machinery at play when both our genomes (chromosomes) and cytoplasm become perfectly separated into two “equivalent” daughter cells, watching this process in action is something we encourage our readership to enjoy on the internet. The star of this “show” is the spindle-a complex amalgam of cytoskeletal components known as microtubules and actin filaments that under the orchestration of many regulatory molecules see to it that in most cases cell division proceeds according to plan. That this may not always happen can be for better or worse for the products of mitosis or meiosis!

FIGURE 1 demonstrates h14. The infamous HeLa cells shown here sometimes get it “right” and sometimes not. Three dividing cells occupy the center of this image, representing steps in the mitotic process that coordinate chromosome positioning (blue) with microtubules in the spindle (red) and special organizers known as centrosomes (in yellow).

FIGURE II demonstrates tripole. Too many cooks (centrosomes) sometimes spoil the recipe, leading to spindles in this case with three poles, a situation that nearly always will result in an abnormal number of chromosomes in “daughter” cells.
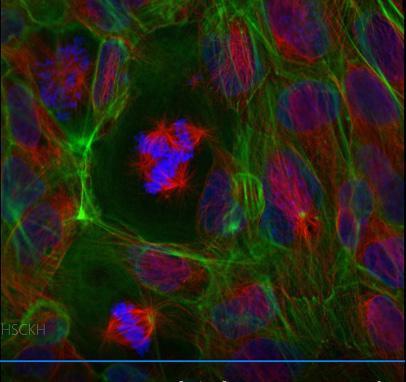
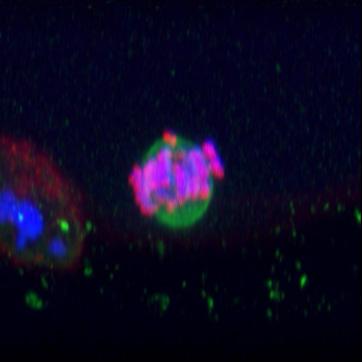
FIGURE III demonstrates Recon. Moving here along from mitosis to meiosis, we capture the meiotic spindle of a human oocyte making a last-ditch effort to corral all of its chromosomes in the midzone of the spindle, so it can get on with the business at hand, to separate bivalents and emitting a first polar body that would genetically position the oocyte in a state of preparedness for fertilization.

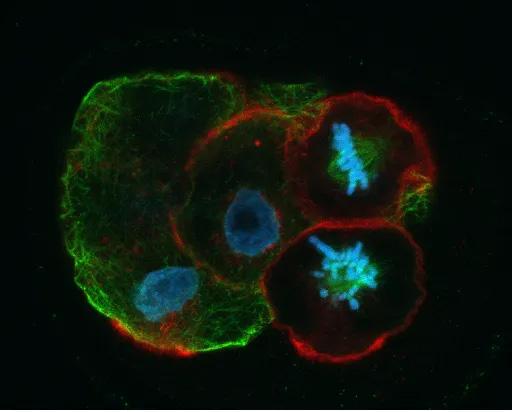
FIGURE IV shows kh1d. Once fertilization has occurred, the one-cell embryo or zygote will proceed through a series of mitotic divisions as shown here in a 4-cell embryo in which two of the blastomeres are preparing for the big event! Note the ordered alignment of the chromosomes (blue) on the spindle (green) in the upper blastomere compared to the apparent state of disarray in the lower cell. Errors in mitosis during early embryonic cell divisions are the principal cause of aneuploidy in human embryos.
By Chloe H, BS , is a writer and editor at the VOICE and The Reproductive Times.
of the VOICE or at social@thechr.com

She can be reached through the editorial office
BRIEFING: We’re introducing a new article type to the VOICE , the “Quick Read,” which is meant to inform our readers in a very brief format about an important clinical issue in infertility. Today’s subject is ovulation, the process that, in most women, allows, until perimenopause, almost every month, one ovary (usually alternating between the right and left ovary) to release one egg. It is then “caught” by the distal, fimbriated end of a fallopian tube. Once the egg has successfully entered the fallopian tube, egg, and sperm meet in the distal ampulla of the fallopian tube after sperm has found its way from the vagina, through the cervix and uterine cavity into the tube and then further upwards toward the waiting egg in the tubal ampulla, where then fertilization occurs.
Introduction
When it comes to fertility, timing truly is everything. If you’ve ever wondered why some months seem easier to conceive than others, or why your cycle sometimes feels unpredictable, you’re not alone. Behind the scenes, your body is orchestrating a complex interplay of hormones, lifestyle choices, and daily habits— all of which shape your chances of ovulation and conception. Understanding these factors can empower you to make small changes that can have a big impact on your reproductive health.
Hormones: The chain reaction behind ovulation
Ovulation results from a carefully timed hormonal sequence. In the first half of the cycle, folliclestimulating hormone (FSH) supports follicle development. Rising estrogen levels coming from the growing follicles then trigger a surge in luteinizing hormone (LH), which initiates ovulation.
If stress, PCOS, or undernourishment disrupt this chain, ovulation can be delayed or missed. In PCOS, hormone imbalances and insulin resistance may prevent the LH surge from occurring.¹ Recognizing these disruptions can help you better understand your body’s signals—and know when it’s time to consult your doctor.
The Mediterranean diet, which emphasizes whole grains, vegetables, fruits, legumes, olive oil, and fish, has been linked to improved ovulation and fertility outcomes.² This pattern supports hormone balance and reduces inflammation.
Diets high in trans fats, refined sugars, and processed foods, on the other hand, can disrupt insulin sensitivity and increase inflammation, which may interfere with ovulation.³ A 2021 review found that high-glycemic carbohydrates and saturated fats were associated with ovulatory disorders.³ While evidence is not yet definitive, even small shifts, like swapping refined grains for whole grains, may support fertility and improve overall health.

Moderate physical activity is linked to more regular cycles and better ovulatory function.³ You don’t need to overdo it—gentle, consistent movement like walking, yoga, or swimming can improve circulation and reduce stress. Overly intense workouts, especially with low body fat, can suppress ovulation. Chronic stress can elevate cortisol, which may disrupt the release of LH.³ While stress can’t be eliminated, practices like mindfulness, journaling, or deep breathing can help restore hormonal balance. Sleep is also essential: poor or irregular sleep has been linked to hormonal imbalances and menstrual issues.⁴ Aim for 7–8 hours of restful sleep to support reproductive function.
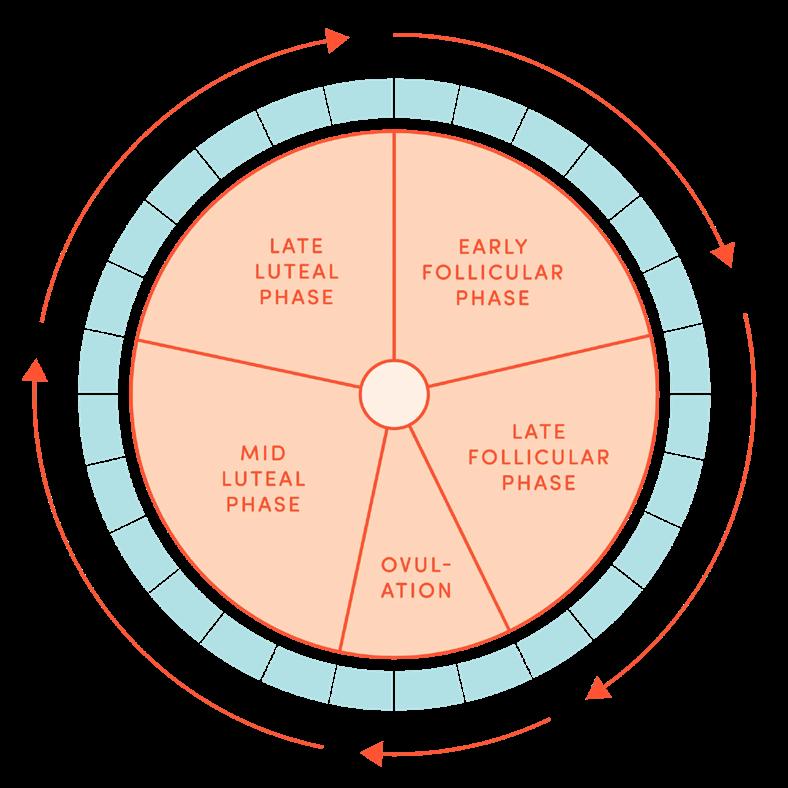
Getting to know your cycle is one of the most empowering steps you can take. While ovulation typically happens about 14 days before your next period, that’s not true for everyone, and it can change from month to month. That’s where ovulation tracking tools come in. There are a variety of tools available, ranging from simple to high-tech, and each has its pros and cons. A 2017 review categorized the most common ovulation tracking tools into four groups: biological signals, hormone-based methods, physical measurements, and digital tools.⁵
• Cervical mucus tracking involves observing changes in vaginal discharge throughout the cycle. As ovulation approaches, cervical mucus typically becomes clear, stretchy, and egg-white-like, signaling peak fertility. This method is free, noninvasive, and backed by solid science, but it does take a little practice to learn how to interpret changes accurately.
• Urine-based ovulation predictor kits (OPKs) test for the presence of luteinizing hormone (LH), which surges right before ovulation. These are widely available and generally reliable, though they can give false positives for people with hormonal imbalances like PCOS.
• Basal body temperature (BBT) tracking relies on measuring your temperature first thing every morning. After ovulation, body temperature rises
slightly (about 0.5-1°F). While BBT can confirm that ovulation occurred, it doesn’t predict it in advance, so it’s most effectively used in combination with other methods.
• Wearables and fertility monitors are an emerging category that includes devices and apps tracking temperature, heart rate, hormone levels, or breathing patterns. While promising, they can be costly, and accuracy varies depending on the device. As with most aspects of fertility, personalization matters. The best tracking method is the one that fits your lifestyle and helps you tune into your body’s unique rhythms.
Ovulation is a significant factor in reproductive health, and it is greatly influenced by the choices you make every day: what you eat, how you move, how you sleep, and how you manage stress. While hormones play a pivotal role in the process, your habits are the architects. Whether you’re on the journey to conceive or simply want to understand your body better, mastering the art of tracking ovulation and making supportive lifestyle changes can give you a sense of responsibility and control.
And if challenges arise, the knowledge you’ve built will help you seek the right support, reinforcing your role as an informed, empowered participant in your reproductive journey. And as always, medical care should always be personalized; please consult your healthcare provider to determine what’s right for your individual needs.
1. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. doi:10.1093/humrep/dey256
2. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379-389. doi:10.1016/j. ajog.2017.08.010
3. Kucharska A, Szostak-Wƒôgierek D. The influence of diet on ovulation disorders in women. Nutrients. 2021;13(4):1156.
4. Beroukhim G, Esencan E, Seifer DB. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: a comprehensive review. Reprod Biol Endocrinol. 2022;20:16. doi:10.1186/s12958-022-00889-3
5. Soules MR, et al. Detection of ovulation: a review of currently available methods. Bioeng Transl Med. 2017;2(3):238-246. doi:10.1002/btm2.10058

BRIEFING: In this section, the VOICE offers opinions about medical news not necessarily connected to reproductive medicine, but with potential relevance to the field. More news about reproductive medicine is reported in a later section of this VOICE . Since, like outside opinions, the CHR’s positions on issues can be biased, it is important to reemphasize that we are fully cognizant that “expert” opinions in medicine are considered the lowest level of evidence and should be viewed as such by our readers. Unable to offer, therefore, a consistent “truth” (assuming that something like that really exists in science), the VOICE strives to come in its selection of topics and its content as close as possible to the most likely “truth of the moment.” We, therefore, welcome from our readers, especially opposing opinions.
Corporatization—a new term in medicine
So, The New England Journal of Medicine just announced a new “Perspective” series of articles addressing the corporatization of US health care, pointing out that the corporatization of US health care is not a new thing and can be traced to its beginnings in the 1920s.1 But there can be little doubt that the reach of this process and its impact have in recent years achieved absolutely new heights and, therefore, have also received considerable coverage in the VOICE, especially, of course, with reference to infertility practice.
We, therefore, are very much looking forward to this series of articles in, of course, hoping for objective coverage of this important subject. The same issue of The Journal already offered the first installment, a quite interesting article by Erin C. Fuse Brown JD, MPH, a Professor of Health

Services, Policy & Practice at the Center for Advancing Health Policy through Research at the Brown University School of Public Health in Providence, R.I., who used this paper to define what is really meant by “corporatization” (we—apparently grammatically incorrectly—in the past called the process “corporization”).2 And her definition in one sentence is worthwhile repeating:
“The term ‘corporatization’ now refers to the general trend throughout the health care industry toward higher levels of integrate control by consolidated profit-seeking enterprises.”
She also reminded us in the article of two key elements of corporatization, the first being
shareholder primacy in such situations, which means that the primary duty of the corporation is to maximize shareholder profits, subordinating the interests of other stakeholders (in this case, patients and health care providers, community, and others) and the second being consolidation, which in recent years has become increasingly apparent throughout the health care system in the form of vast horizontal as well as vertical consolidation in single markets by single corporate entities and multiple markets by conglomerates.
The consequences are manifold, including increased costs, declining quality of care and patient satisfaction, and the infertility field has, of course, witnessed all of this and more. Fuse Brown concludes that we have reached a boiling point which likely will lead to political interventions to initially, at least, break up conflicting powers of these commercial entities, like medical insurance companies, for example, also owning medical provider organizations, pharmacy operations, and/or vice-versa. The article is also accompanied by an

audio interview with the author.
1. Malina et al., N Engl J Med 2015;393(1):81-82
2. Fuse Brown EC. N Engl J Med 2025;393(1):1-3 (The New England Journal of Medicine also offers an audio interview with the author)
Is predictive modeling in health care a house of cards?
This is exactly the point Akhil Vaid, MBBS, Assistant Professor in the Windreich Department of Artificial Intelligence and Human Health Medicine and the Department of Medicine at Icahn School of Medicine at Mount Sinai in NYC, recently made under the same heading in a Nature commentary article.1

This has also been an issue investigators at the Center for Human Reproduction (CHR) have been keenly interested in for several years, as they’ve shared the same concerns. While medical journals likely experienced a peak in modeling studies during the COVID-19 pandemic—when they suddenly appeared everywhere and in every medical specialty—things have somewhat calmed down since. What computers allow you
to do from home these days is truly amazing!
Therefore, it wouldn’t be surprising if progress with machine learning reignites this discussion. In his article, Vaid indeed noted that “uncontrolled deployment of machine learning in medicine can distort patient information and sacrifice long-term data reliability for short-term benefits.” He specifically highlights the many difficulties that may arise from how different models influence each other, or how new models built upon older ones may become misdirected. It’s a very interesting article!
1. Vaid A. Nature 2025;642:864-866
Is Uterine transplantation cost-effective?
This is what some uterine transplant surgeons are really claiming and—forgive us here at the CHR—but we, simply, cannot buy this notion because if you just for a moment think about what uterine transplantation involves (aside of IVF and other infertility treatments)—two major surgeries for the patient, transplantation and at some later point removal of the transplanted uterus, and one major surgery for the donor of the uterus, the need for anti-rejection drugs, the inherent failure rate of organ transplants, etc., as huge a scientific and technical accomplishment as well as momentary clinical accomplishment every successful uterine transplant represent, it at the same time also—at least as we see it—represents and abuse of basic logic and some of the most basic rules of medicine, including,
“first, do no harm.”
The recent July issue of Fertility and Sterility, roughly at the 10th anniversary of the first live birth after a uterine transplant in Sweden, almost looked like a special promotional issue for uterine transplantation because it contained several papers on the subject, with all of them, more or less, advising us how wonderful a treatment uterine transplantation has become.
One Reflection article among those (for unclear reasons, F&S differentiates Reflections from so-called Inklings) had, indeed, the heading, “ From milestone to mainstream: the case for uterine transplantation,” and included this rather astonishing comment:” “Once viewed as a highly experimental procedure, uterine transplantation has evolved into a reproducible, increasingly safe (and when will it be really safe if we may ask?), and clinically meaningful option (we also, of course would love to get a better understanding what represent a clinically meaningful treatment option) for women with ‘absolute uterine factor infertility’ (one also wonders which talented marketer coined this obviously brilliant term for what medicine for decades called an ‘absent uterus.’”
The author is, of course, one of the uterine transplant surgeons at the Cleveland Clinic, which has been one of the principal purveyors of uterine transplantation surgeries in the U.S. and, of course, has no self-interest in promoting uterine transplants. And this non-self-promotion was helped by ChatGPT-4.0 (OpenAI) in

“rephrasing sentences and refining language for clarity and flow.”
And then there were—lo and behold—two alleged costeffectiveness analyses regarding uterine transplantations in the issue of F&S, both comparing the costs of uterine transplants to the recruitment of a gestational carrier (GC) and both the products of several academic centers and including economists.
The first study concluded that a singleton birth using a GC cost $97,712.90, which to us looks like a bizarrely low number. Very difficult to believe, the same birth after uterine transplant supposedly would cost only $116,137.20 (seriously, they reported the cents), also in our opinion a completely unrealistic number in the U.S. Quality-adjusted life years were also higher with GC (0.93) than transplant, as were live birth rates transplant (94% vs. 77%). So socalled Monte Carlo simulation (i.e., a model) demonstrated that a transplant had a 37% chance of being cost-effective. Cost advantage flipped toward transplant with two births, but the overall rate of live birth remained higher with GC deliveries (86% vs. 66%). In short, the study suggested that for a single birth was preferable, while for two children, a transplant was more cost-effective.2
The second study, also by a U.S. consortium, offered what appeared to be much more realistic numbers and reported. Using another modelling product decisiontree mathematical model), they demonstrated that a uterine transplant was $1.4 million more
expensive than a GC, with, in addition, lower utility by 23.74 quality-adjusted life years using the same number of children born per every two FET cycles. After 10,000 simulations, the GC arm had 2 children born 42% of the time, compared with only 17% with the transplant arm. No children were born in 56% of transplants and in only 16% of GC arms. The authors concluded that their model currently marks GC use as the clearly more cost-effective approach.3
And then there was another Reflections article, which by all rules really should have been an Inkling article since it was the adnexal commentary to the two papers comparing GC pregnancies to transplant pregnancies.4 Interestingly written by an infectious disease expert from the CDC (probably the only financial model expert the editors could locate), which basically said nothing of interest except for regurgitating the two papers she was supposed to comment on. And considering her lack of expertise in reproductive medicine, one also cannot blame her for a concluding sentence full of clichés: Uterus transplant offers the existing possibility for women with absolute uterine factor (she obviously learned the lingo quickly) to bear their children.
Really? We elsewhere in these pages of the VOICE coincidentally note our dislike for modelling. Every model is, of course, fully dependent on the assumptions it is fed. We all know—or at least should know—how off so many of our assumptions in medicine
often are and, therefore, have very serious doubts about all the papers here discussed. And we, indeed, have even more severe doubts about the basic rationale of uterine transplantation, and not only because of cost concerns, but also because of safety concerns for mothers, offspring (nine months under maternal immune suppression), and uterine donors.
And, yes, we almost forgot, in addition to the four papers we just discussed on uterine transplantation, the July issue of F&S also contained three video presentations (and abstracts), offering detailed descriptions for “standardized” robot-assisted living donor hysterectomy,5 recipient surgery,6 and back-table technique for uterus transplantation.7
1. Richards EG. Fertil Steril 2025;124(1):60-61
2. Walter et al., Fertil Steril 2025;124(1):121-132
3. Combs et al., Fertil Steril 2025;124(1):134-143
4. Honeycutt AA. Fertil Steril 2025;124(1):54
5. Tamate et al., Fertil Steril 2025l124(1):161-163
6. Tamate et al., Fertil Steril 2025;124(1):164-165
7. Tamate et al., Fertil Steril 2025;124(1):167-168
for prime time?
So, we learned a lot about the importance of microbiomes in recent years! And it, of course, all started with the gut microbiome, and the big breakthrough happened once the gut-brain axis came into focus.1 A recent Perspective article in Cell, however, poured some cold

water over overheated expectations and commercial excesses that threaten the integrity of this still relatively new field of research and clinical practice.2
Here is the article’s summary:
“Despite promising evidence in diagnostics and therapeutics, microbiome research is not yet implemented into clinical medicine. Several initiatives, including the standardization of microbiome research, the refinement of microbiome clinical trial design, and the development of communication between microbiome researchers and clinicians, are crucial to move microbiome science toward clinical practice.”
And, as the article also pointed out, the challenges are multifactorial, from biological, over methodological, to logistical, and cultural: Biological, because identification of causal links between gut microbiome and human diseases can, due to the heterogeneity and complexity of the gut microbiome, be difficult. Methodological, because diet, environments, medications, and other environmental influences affect microbiome studies.
Reproducible results, therefore, often are lacking. Logistical, because studies usually are underpowered single-center studies that cannot be generalized (does that sound familiar?); and, finally, cultural – following from all the other considerations – treating physicians, understandably, still lack confidence in microbiome data.
That even when it comes to the gut microbiome, there are still such very obvious limitations may surprise; but what does not surprise is that in infertility practice, apparently, does not exist any such hesitancy in promoting commercially available vaginal microbiome tests under the usual categorical representation that they can improve the outcome of infertility treatments.3,4
What a shame!
1. Mayer et al., J Clin Invest 2015;125(3):926938
2. Porcari et al., Cell 2025;188(11): P2836-P 2844
3. Evvy. Vaginal Microbiome Test with Fertility Insights. https://www.evvy.com/vaginalmicrobiome-test-fertility
4. Juno Bio. Updated February 6, 2025; https://www.juno.bio/blogs/learn/assistedreproduction-and-the-vaginal-microbiome?srsl tid=AfmBOorPiFcOCTjczKq9VJzNzKNR5w9o b7I4HZOwm8g3GFn51LDIqiSh
Is medicine really once again promoting Eugenics? The at times absurdity of current medical publishing
Genevieve L. Wojcik, PhD, a statistical geneticist, genetic epidemiologist, and Associate Professor of Epidemiology at Johns Hopkins Bloomberg School of Public Health in Baltimore, MD, apparently believes that medicine in the U.S. is once again promoting eugenics. Under the heading, “Eugenics is on the rise again: genetics must take a stand,” she then summarized her Opinion article in Nature (of course, not an unimportant platform for an opinion!) in one very telling sentence: “Scientists must push back against the threat of rising white nationalism and the
dangerous and pseudoscientific ideas of eugenics.”1
So, let’s start the discussion of this article with some background information. First, of course, the definition of “eugenics” according to Merriam-Webster:2 “Eugenics is the practice or advocacy of controlled selective breeding of human populations (as by sterilization) to improve the populations’ genetic composition.” The definition by the National Human Genome Research Institute is significantly less polite. It describes eugenics as “an immoral and pseudoscientific theory that claims it is possible to perfect people and groups through genetics and the scientific laws of inheritance.” The institute further noted, “Eugenicists used an incorrect and prejudiced understanding of the work of Charles Darwin and Gregor Mendel to support the idea of ‘racial improvement.”3
And there is, of course, considerable history to advocating eugenics in medicine as well as in politics, and in both of these areas, this history proved to be anything but positive. The person who in 1883 coined the term “eugenics,” was Francis Galton, not widely known, a cousin of Darwin.
In the U.S., eugenics, as MerriamWebster also notes, has become virtually a dirty word closely identified with racism. Nobody exemplifies this more than Margaret Sanger, widely credited to be the founder of the country’s birth control movement in the early 20th century, yet also increasingly accused of having

been a racist, advocating for the extermination of African Americans and more specifically having founded Planned Parenthood with the intent to “help kill black babies before they came into the world.”4
In Europe, eugenics was, of course, closely associated with Germany’s efforts to create a “master race” and with the genocide of six million Jews and additional millions of Gipsies (Roma) as well as other minorities, Mengele’s atrocities at Auschwitz, and the murder of mentally and otherwise impaired individuals.
Yet in medicine, starting in the early 20th century—on both sided of the Atlantic—eugenics was anything but a dirty word! On October 7 (what a coincidence in the date to a recent October 7 event of extreme racism !!) of 1921, the report of the Eugenics Conference was, indeed, the lead article in Science. 6 The text below contains the abstract of an unsigned 1938 Nature report,5 and the language is telling!
The Galton Lecture to the Eugenics Society, by Prof. John A. Ryle, on medicine and eugenics, is printed in the Eugenics Review, 30, No. 1. In a carefully considered address, it is pointed out that the eugenic movement needs the fuller support of the medical profession, and that this can only be given when medical men receive a fuller training in human genetics than is now the case. The family doctor is now rarely prepared, even if asked, to give advice connected with eugenic prognosis, although
men and women are increasingly prepared to discuss such matters. Practicing physicians should be able to keep pedigree records of their patients who show mental and physical defects. Medical education should be altered so as to lay greater stress on animal and human genetics in place of some of the routine zoology and the more specialized biochemistry and biophysics. The constitutional variations which abound should be the subject of closer genetic study. Several chairs of human genetics should be instituted, and associated with them should be research centers concerned with morbid inheritance in man. Wider contacts of the Eugenics Society with medical societies throughout the country would be helpful. The foundation of a National Council is advocated, embodying an alliance between medicine, eugenics and sociology and having appropriate contacts with the Ministries of Health, Agriculture and Labour. The preservation of health as a primary function, with the treatment of disease as a secondary function, should become the new ideal.
Several very prominent physicians, intellectuals, and political leaders, including Alexander Graham Bell, Winston Churchill, John Maynard Keynes, Woodrow Wilson, and many others in that area, were, indeed, strong proponents of eugenics, and eugenics became in the 1920s a serious scientific movement on both sides of the Atlantic.
Wojcik, in her Nature article, noted that in 1924, the U.S. passed
the so-called Johnson-Reed Act, which limited immigration with the intent to stem “a stream of alien blood with all of its inherited misconceptions.” And in the immediately following sentence she apparently couldn’t help herself any longer, accusing President Trump of using “similar eugenic language to justify his proposed immigration policies,” alleging “persistent racist tropes, discussing “race as a social construct,” and concluding that “geneticists and (other) scientists more broadly must consider the potential for their work to be misappropriated.”
But her political rage in one of the planet’s leading science journals did not end with almost clinically paranoid concerns. Of course, unreferenced, she made the totally bizarre claim that “in just the past three years, genetics has been used to justify the murder of Black and Hispanic people in the United States.” And she accused, of course again unreferenced, “academic journals to persist in giving a platform to scientific racism, and the misappropriation of studies examining the geographical distribution of genetic variants to give white nationalism a veneer of academic respectability.”
On which academic planet does this person live? And on which scientific and peer review planet do the editors of Nature exist these days!
But, if we are already talking about the resurrection of eugenics, let’s have a quick look at who is really aggressively promoting the genetic “improvement” of the human species. Oh, what a surprise; it is

Here is only one piece of evidence that speaks for itself: Prenatal carrier screening started with less than a handful of tests of frequent recessive mutations in only specific populations, like sickle cell disease in individuals of mostly African descent and, for example, Tay-Sachs in Ashkenazi Jews, both populations with very high prevalence of these two respective conditions. Nobody then would, for example, have tested Ashkenazi Jews for sickle cell disease and African Americans for Tay Sachs.
As testing capabilities improved and costs declined, geneticists convinced medicine to expand testing to less frequent mutations and make it a test for everybody. In other words, it no longer mattered whether somebody belonged to a subpopulation at increased risk. The idea—heavily propagated by the genetics community was to eliminate recessive diseases from the population in general, and that, of course, is nothing but a typical eugenic goal.
It first started with maybe up to 25 tests, then it went to around 100, while the likelihood of “catching” an affected couple for those diseases continued to decline (both partners must be carriers of a recessive mutation to create a 25% risk for the couple’s offspring to express the disease. Then, renamed “expanded carrier testing,” it went to ca. 300-350 recessive conditions, of course with ever declining risk because of declining prevalence in general populations, and—as of most recently—genetic testing companies are offering tests for
over 500 diseases, most with risk levels similar low to chances of winning in the lottery.
If this is not eugenics, then what is? And if the conviction of helping improve mankind by preventing the birth of individuals with genetically inherited diseases with often infinitesimally small risk is not eugenics, what is it? The likely alternative motivation for all this testing is even more disgusting because it would mean that the genetic testing community really only pretends that its motives are to improve the genetic pool of mankind and, in reality, just wants to do more genetic testing.
And one cannot but wonder, which of these two explanations is more despicable! There, indeed, is good reason to believe that it is the second explanation because commercial genetic testing is, of course, everywhere and, indeed, has even become a consumer product (see 23andMe).
In reproductive medicine, we do not have far to look to find additional examples: How about preimplantation genetic testing of embryos for aneuploid (PGT-A)? Our readers will, of course, understand that we simply cannot restrain ourselves from bringing PGT-A into the discussion. Repeatedly noted in recent months in these pages, even ASRM/SART has now finally concluded that PGT-A is basically a worthless test that, in over 20 years of clinical utilization (in different formats and under different names), to this date has failed to show evidence of any clinical utility in general infertile populations.7 It, indeed, in certain
subpopulations, actually reduces pregnancy and live birth chances in IVF.
And who has been imposing PGT-A upon the infertility field?
Of course, the genetic testing community, or should we by now call it the genetic testing industry! And what have been the industry’s principal arguments, all since proven to be incorrect? (i) That deselecting aneuploid embryos prior to embryo transfer would improve pregnancy and live birth rates; (ii) That the process would reduce miscarriages; (iii) That the process would reduce abnormal births; and (iv) That PGT-A would shorten the time to pregnancy with IVF; and that for all of these reasons PGT-A, therefore, would be cost-effective.
And then there is, of course, now also polygenic risk scoring of embryos, already freely offered by several genetics laboratories and IVF clinics—whether for polygenic diseases (diseases caused by multiple genes at once, like heart disease, cancer, hypertension), promised special future skills of offspring (we want a Michael Jordan!), or just eye color (we hear blue is the favorite color !). It doesn’t matter that polygenic risk scoring, even in adults, so far has achieved only limited accuracy. In human embryos, several professional societies (both in genetics and REI) have declared polygenic risk screening not only ineffective but unethical.
And the promotion by the genetic testing industry is once again pure eugenics, with the argument being to potential parents how

can you not want to improve the life of your offspring by selecting the “best” embryo(s) with lowest disease risks—whether they run in the family or not—and either special physical characteristics and/or talents. And who cares that such polygenic risk scoring, even in adults, is still considered experimental and simply does not yet work with sufficient accuracy. But as PGT-A so well demonstrated over more than 20 years of clinical utilization, the reality of a true and validated purpose for the test was never a concern of the genetic testing industry!
So, Dr. Wojcik and dear editors of Nature, please give us a break with your grandstanding about “what scientists can do” and should do in the current political climate. May we also suggest that you remain with your respective publications within the constraints of a framework of science and clinical practice, since nobody subscribes to your journals to learn about your increasingly often truly absurd political opinions, as discussed in here discussed Nature paper.
Geneticists and other scientists and physicians must not—as you suggested—“stand against an (imaginary) global rise of a (non-existing) white nationalism which seeks to leverage scientific racism for eugenicist goals because this world exists only in Marxist imaginations and can probably be sourced to the extreme leftist dialectics of the Frankfurt school in the 1960s. Most of the world has, fortunately, passed on from this believe system because it, simply, makes no sense in a modern society that—more than ever before—is
dependent on meritocracy to advance and prosper.
Younger generations and their academic teachers at many of our leading universities simply do not want to learn from history and continue to spew the kind of poisonous Marxist dialectics and theory that permeates Wojcik’s commentary and has, whenever tried in history, been demonstrated to be destructive for society. How well did, for example, the “defund the police movement” do in recent years?
Nature and other overly politicized medical and basic science journals simply must stop writing about things they have absolutely no expertise in and concentrate on what their real purpose is: to communicate about and support progress in medicine and various other scientific fields. And, on a side note, we are also wondering what the administration of Johns Hopkins University is thinking about this article. Does even an obviously quite woke university like Johns Hopkins really like to be represented in Nature magazine (or anywhere else) by a professor who—quite obviously—is more driven by political ideology than scientific clarity?
1. Wojcik GL. Nature 2025;641:37-38
2. Merriam-Webster. https://www. merriam-webster.com/dictionary/eugenics
3. National Human Gnome Research Institute. Eugenics: Its Origin and Development (1883-Present). https://www. genome.gov/about-genomics/educationalresources/timelines/eugenics
4. Latson J. Time. October 14, 2016. https://time.com/4081760/margaretsanger-history-eugenics/
5. Anonymous. Medicine and Eugenics.
Nature 1938;142:68. https://www.nature. com/articles/142068a0
6. Farber SA. Zebrafish 2008;5(4):243-245
7. Practice Committees of ASRM and SART. Fertil Steril 2024;122(3):421-434
Salaries of REIs in the U.S.: Who pays best?
So here is an interesting Research Letter article in Fertility and Sterility: Canadian colleagues investigated how REI salaries compared between academic positions and employment in socalled private clinics.1 And rather unsurprisingly, those in the private sector did much better, especially once they reached 10 years of seniority.
But we found the article rather unsatisfactory because nobody will, of course, be surprised by the reported discrepancy between academic and private salary spectra. It is widely known, and not only in the infertility field. What would have been a much more relevant question is—within socalled private practice jobs—what are the income/salary differences between truly private practice (i.e., physician-owned clinics) and Private Equity and other large Wall Street-owned clinic networks?
If we had to guess, we would find a greatly varying pattern from that reported in this communication among physicians in private settings: We would suspect that, (i) starting salaries would be the highest in organizations owned by outside investors; but (ii) that advantage would increase much slower with seniority than in truly by physicians privately owned clinics.

Why is all of this of importance?
Because outside-investor-owned clinic networks have, for all practical purposes, taken over the field when it comes to hiring. Nobody among the still truly private clinics can compete with their starting salaries. But once they “own” their physicians, they become much stingier and start asking for much more in return. Academia, in most cases, cannot compete with either form of private practice.
The consequences for the field, therefore, should be obvious: Don’t expect much true innovation, but we would not be surprised by more and more revenue-generating useless “add-ons” to IVF. Just more evidence that infertility practice is quickly moving away from being a medical subspecialty and moving toward becoming an industry.
1. Kolokythas A, Dahan MH. Fertil Steril 2025;124(1):159-160
How badly is the UnitedHealth Group in trouble?
How much is the UnitedHealth Group (UHG) in trouble? Probably even more than anybody is currently thinking. Until recently, widely described as the biggest, most powerful, most aggressive, and most successful health care company,1 things now look very different. Just a run of bad luck? We and increasingly many experts don’t think so!
That the CEO of United Healthcare, of course, one of the most important subdivision leaders in the UHG, was in the streets of New
York City brutality murdered by a new folk-hero for many, Luigi Mangioni, can still be viewed as a coincidence and bad luck. But losing $280 billion in market capitalization literally within hours can no longer be considered bad luck alone. What came first was a very unexpected and troubling earnings report for the first quarter of 2025 by the company’s CEO, Andrew Witty, dropping the company stock by 22%; and then, 26 days later (on May 13, 2025), Witty unexpectedly resigned for “unspecified personal reasons.”
The Wall Street Journal and other media outlets on the following day reported that the company was investigated by the Department of Justice for possible criminal Medicare fraud. And as above cited recent article in Yahoo Finance noted, another reason was a huge increase in consumption of health care services by United insured, while competitors like Humana and Aetna did not record such an increase.1
And then there is, of course, the conflict of interest that arises when one and the same company ensures potential patients but also owns the medical practices that are providing this care. As it turns out UHG currently employs directly of contracts with approximately 10% of all physicians in the U.S. If the owner of the insurance company can influence the medical provider organization—owned by the same company—to offer less medical care (in insurance parlor, also called “more efficient” care), profits and valuation of the
insurance company, of course, soar. In contrast, the opposite will be the case in the provider organization.
One wonders, of course, which physician would want to work in such a provider company, unless, of course, the pay is really special! And just to close the circle, didn’t Luigi Mangioni murder the CEO of United Healthcare because the insurance company allegedly inappropriately denied coverage of health care?
The Wall Street Journal recently asked what the company could do to revive its stock price, down nearly 40% in 2025.2 And The New York Times reported that UnitedHealth (the mothership) has been “applying legal pressure to try to quiet critics.”3 Not necessarily a very smart tactic!
1. Calvin G. Yahoo Finance. May 21, 2025. https://finance.yahoo.com/news/sickunitedhealth-wave-bad-news-093000362. html?guccounter=1&guce_
If there is one thing to bet on, then it is that medical insurance companies will not—ever—ease preapproval requirements for medical services, if they can help themselves! But facing increasing pressure from patients and doctors, and for the first time, the threat of regulatory crackdowns from federal as well as some local state governments, some media outlets, including The New York Times, recently claimed that some of the bigger insurance companies may,

indeed, ease some requirements.1
We are skeptical because we have heard these pronouncements too often before, with restrictions on care getting progressively worse than better, but a little bit of retained optimism cannot hurt, and who knows, maybe the Trump administration will finally get serious about medical insurance companies practicing medicine without a license.
And—because of pure ignorance— they sometimes do it even in self-destructive ways: Take, for example, the requirement by some insurance companies that most infertile women must have three intrauterine insemination cycles before being approved for trying IVF. How silly is that for an older woman, or even a younger woman who wants several children, or a younger woman with low functional ovarian reserve? It not only makes no sense from a clinical point but even looking at this requirement as an expense consideration of the insurance company, it makes absolutely no sense.
Our skepticism was somewhat supported by a recent article in The New York Sun, which noted that, after a CEO’s murder (on the streets of NYC) and public pressure, health insurers claim they’ll reform care approval delays. Still, the article also wonders whether the Trump administration can really make them deliver on this promise, noting that attempts to streamline the much-despised prior authorization process failed twice before.2
1. Abelson R. The New York Times June 20, 2025. https://www.nytimes. com/2025/06/20/health/health-insuranceprior-authorization.html
2. McKay H. The New York Sun. Updated July 7, 2025. https://www.nysun.com/ article/after-ceos-murder-and-publicpressure-health-insurers-say-they-willreform-care-approval-delays-can-trumpmake-them
How bad are Kindbody’s troubles and has the company “found” its savior?
And since we are already talking about being in trouble, that Kindbody is in trouble is not as surprising as above above-noted United news. Those troubles that were brewing at Kindbody have been known for almost two years. We in earlier issues of the VOICE repeatedly reported on. They included noted failed efforts to raise in 2023 a large additional funding round, and the failure of raising even only $50 million in 2024,1 a not at all friendly article about the company, accusing Kindbody of having a “profit mindset” that pushes doctors to perform more egg retrievals,1 about egg donation “parties,” a quite unprecedented number of short-term CEOs, and a recent CEO trio of senior executives (forming an Office of the CEO) because no new single CEO could be recruited.
There are some interesting similarities between United Health Group and Kindbody, with the most consequential likely being that both organizations function as insurers as well as medical service providers. Like United, Kindbody therefore potentially faces significant ethical as well
as practical-financial conflict situations.
Now, two important new staffing announcements came from the company: The first was that Angeline N. Beltsos, MD (we know her as Angie), after many years, left the company. In many ways, she was the embodiment of the early Kindbody because only through her merging with Kindbody, a network of IVF clinics she had singlehandedly build starting in Chicago. And then reaching out into the whole Midwest (Vios Fertility, did Kindbody become a behemoth in the infertility field? Serving as one of the CEOs of the company after the merger and then occupying several other senior management positions, she never gave up clinical practice. What made her finally leave the company was not announced, but the rumors are swirling.

And then an even bigger announcement, reported by Ron Shinkman in Inside Reproductive Health on June 12, 2025 in one of the usual e-mails by Griffin Jones to IVF clinics by this publication: Kindbody—after a very long search —finally found a new CEO and as it turns out—it is David Stern, MBA,

most recently the CEO of Boston IVF and one of the best and longest known corporate administrators in the fertility field. We, indeed, still remember him well in his position as a Vice President at Serono USA and wish him Good Luck in his new position. He is one of those guys everybody likes and will take over the CEO position at Kindbody on July 7, 2025.

Stern appears to have all the qualifications to right the ship at Kindbody. As vice president at Serono, he served all U.S. IVF clinics and got to know the different practice models in the field—and more recently, as CEO at Boston IVF, he headed a truly historical provider organization in IVF. Boston IVF already in the 1990s pioneered large-scale insurance contracting in Massachusetts, then the first state to mandate that insurance companies cover IVF services. According to Shinkman, Stern’s tenure at Boston IVF ended in February of 2025, after he had negotiated the sale of the company to the private equity-owned IVIRMA, which appointed Haryanto Hokianto as Executive Director of Boston IVF and Chief of Staff of IVI-RMA North America.
Kindbody (estimated annual revenue $180-$200 million) is, however, a much bigger company
than Boston IVF (estimated annual revenue $75 million) and, because of the various businesses it pursues, also a much more complex company to run. Stern, therefore, will undoubtedly face new challenges.
According to the article by Shinkman, one of the first changes at Kindbody will be the elimination of the current Office of the CEO staffed by three co-CEOs: President Gina Bruzzichesi, Chief Financial Officer Scott Bruckner, and Chief Business Officer Shilpa Patel; however, no further information was provided by the company regarding other expected administrative changes.
1. Davalos J. Fertility Startup With a ‘Profit Mindset’ Pushes Doctors for More Egg Retrievals. Bloomberg. December 22, 2023. https://www.bloomberg.com/news/ articles/2023-12-22/kindbody-fertilityclinic-pushes-doctors-for-more-eggretrievals
The troublesome rise in stem cell clinics in medicine, now also starting to involve the infertility field
That treatments utilizing stem cells will very likely have a very important role to play appears to us obvious. Several remarkable therapeutic initial achievements have, indeed, already been reported but—in principle—stem cell therapies uniformly must still be considered experimental and, therefore, should not be pursued in “fly-by-night” outfits in thirdworld countries At the same time, charlatans and snake oil salesmen are ruling the current commercial world of stem cell therapy within
the U.S. and—to even more significant degrees—just outside the borders of the U.S. in Canada, the Bahamas, and Mexico.
The highest concentration of stem cell therapy clinics can, indeed, be found in the U.S., Mexico, India, and China, and travel to receive stem cell therapies in foreign distant lands is now called “stemcell tourism.”1
According to Google, in 2021, ca. 1480 U.S. businesses operated 2,754 clinics that directly marketed stem cell therapies. The proliferation of such clinics has, since, even further expanded at a logarithmic pace. And this expansion of unlicensed stem cell therapies is proceeding despite their incompatibility with FDA regulations.2 And we would also call for caution when locations of such therapies are advertised in the Grand Cayman Islands, the Bahamas, Panama, in Mexico, Albania, and even at times in Canada because that often suggests that their offerings are too potentially toxic to even allow the FDA to ignore them (the FDA, of course, does currently ignore a quite unbelievable number of completely spurious stem cell offerings in the U.S.).
The global—presumably legitimate —stem cell market has been estimated to grow to approximately $930 million by 2031.3 Clinically already been reported. Credible successes included, among others, cord blood stem cells that led to HIV remission and, in chronic heart failure, stem cells reducing the risk of heart attack or stroke by 58%, a figure that climbed to 75% in patients with high inflammation.

Stem cells have also already been demonstrated to be effective in multiple sclerosis, Parkinson’s disease, and certain ocular disorders.
When it comes to infertility, questioning Google,
“Can stem cells cure infertility?”
which unfortunately resulted in the wrong answer:
“The course of stem cell treatment for female infertility allows restoring the ovulation cycle in women while also improving egg quality, uterine lining, fallopian tube blockage, and uterine fibroids.”
This is, of course, a very wrong answer: Stem cells—as of the present time—have not been demonstrated to support any of these claims, and travel to Albania, the Bahamas, Panama, Mexico, and/or any other countries—at least in the short-term—is not going to change this.
There is one stem cell clinic, called Bespoke Biologix, which operates in the New York Tristate market. It is heavily advertised on the radio. The advertisements are slick and give the impression of a very large enterprise with longstanding experience. They do not mention that they are located in Mexico. You find that out only once you Google it. This is also when you discover that this is really only a one-physician show—a trained anesthesiologist—claiming to offer “cutting-edge stem cell therapy transforming the future of medical treatments.”5 Simply amazing!
One more final word on this subject: As convinced as we are that effective stem cell therapies will in the not-too-distant future play an important role in medicine, one can also be as convinced that stem cell therapies can cause medical harm. One of the most dangerous potential complications shown in animal models has, for example, been cancer induction.6 And if you found this short commentary interesting, we suggest you also read the next one.
1. The Conversation. Stem cell therapies: why they’re expensive, unproven and often dangerous. The Conversation. July 14, 2023. https://theconversation.com/ stem-cell-therapies-why-theyre-expensiveunproven-and-often-dangerous-206548
2. Schneider ME. In US, unlicensed stem cell clinic numbers keep climbing. Regulatory Focus. November 4, 2021. https://www.raps.org/news-and-articles/ news-articles/2021/11/in-us-unlicensedstem-cell-clinic-numbers-keep-cli
3. Ogbonna N. Stem cell therapy success rates hit 78%: new research reveals breakthrough results. Global RPh. March 7, 2025. https://globalrph.com/2025/03/ stem-cell-therapy-success-rates-hit-78new-research-reveals-breakthroughresults/
4. American Parkinson Disease Association. Understanding stem cell therapy in Parkinson’s disease treatment. American Parkinson Disease Association July 3, 2024. https://www.apdaparkinson. org/article/understanding-stem-celltherapy-in-parkinsons-disease-treatment/ 5. Bespoke Biologix. About Us. Bespoke Biologix. https://bespokestemcell.com/ about-us/.
6. Seno M, Anai H, Maehara Y, et al. Stem cell-based therapy for cancer: current status and future perspectives. Oncol Lett. 2019;18(3):2756-2762. doi:10.3892/
The New England Fertility Institute (NEFI) sold to CSG.BIO
The sale of infertility clinics has become so much of a routine that most are not even publicly announced any longer. But when Dresner Partners recently published an announcement about having served as advisor to the NEFI’s sale, noting that the buyer was a company called CSG.BIO,1 we got interested because we had never before heard of CSG.BIO as an owner and/or purchaser of fertility clinics. And as will quickly become apparent, this commentary is highly relevant to what is, unfortunately, happening to the infertility field and medicine in general.
Looking up CSG.BIO, we found confirmed by the company’s website that the company, indeed, had never before purchased an IVF clinic. And our research, in addition, revealed several very interesting facts about CSG.BIO: Its website described the company as a “global life science company that has helped over 500,000 families grow and safeguard healthy children”2 (how this deed was accomplished remained unmentioned), launched in 2005 as a company processing stem cells, testing, as well as storing them.
According to the website, the company by 2019 had grown to span businesses in 3 continents, North America, Asia, and Europe, and by 2021 acquired another private stem cell banking provider in the US called AlphaCord. By 2023, the company formed a “strategic partnership” (again, not

explaining what that meant in practical terms) with the Dubaifounded Bioscience Institute and Abu Dhabi Stem Cells Center (ADSCC, apparently the same corporate entity).3
So, we continued our research and looked up both of these two new partner organizations: The Bioscience Institute, according to its website, now has five locations in Dubai, Rome and Milano in Italy, San Marino—a tiny island state in the midst of Italy, Lugano in Switzerland, and San Francisco, USA. Its website offers stem cell treatments for body shaping, skin rejuvenation, scars/stretch marks reduction, regenerative orthopedics, vaginal rejuvenation, and (lo and behold) premature ovarian failure (though without telling how it does it!).3
The ADSCC, with locations in Abu Dhabi and Dubai, describes itself as “a healthcare center focused on cell therapy and regenerative medicine, delivering cutting-edge research on stem cells and offering accessible hematopoietic stem cell transplantation for both adults and children, regenerative treatments for orthopedic problems, women’s and men’s health concerns, general wellness, and specialized treatments, such as UAECell19 for long-COVID and smokers, as well as stem cells for cosmetic and antiaging treatments.”4
In other words, the NEFI appears to have become part of a worldwide stem cell enterprise, in some unclear ways associated with financial partners in the Emirates.
Now a final word about the NEFI,
a well-respected mid-size fertility clinic in Connecticut, which, as at least as of July 5, 2025, does not report the sale on its website.5 It was established and, to this day, was run by Gad Lavy, MD, a wellregarded fertility specialist who, prior to establishing NEFI, was

for four years in charge of the IVF

program of Yale University. He was later joined by a second wellregarded physician, Michael M. Guarnaccia, MD, MPH, who, before that, was on the faculty of Columbia University in NYC. Though not a research center, NEFI—especially in more recent years—achieved special recognition for serving the LGBTQ+ community. A new affiliation with a corporate entity practically exclusively involved with stem cell therapies, of course, raises
important questions, especially as the Bioscience Institute claims to treat premature ovarian failure (now more correctly called primary ovarian insufficiency, POI) with many commercially advertises stem cell treatments in the U.S. currently under more scrutiny by the FDA, as has been reported. One, therefore, wonders what the planned strategy is behind the purchase of NEFI by CSG.BIO may be?
Never a dull moment in the infertility field, and still, new players are constantly entering!
1. Dresner Partners. Press release. July 2, 2025; https://www.dresnerpartners.com/ news/article.asp?id=26303
2. CSG.BIO, https://csg.bio/about-us/; accessed July 5, 2025.
3. Bioscience Institute. https://bioinst. com/en/; accessed July 5, 2025.
4. Abu Dhabi Stem Cell Center (ADSCC). https://adscc.ae/; accessed July 5, 2025.
5. New England Fertility Institute (NEFI). https://www.nefertility.com/


Are Private Equity investments in medicine in trouble?
Here is another subject we have been covering in these pages for quite some time with increasing frequency: the rapidly growing ownership of medical practices, as well as hospitals, by Private Equity. In parallel, private equity investments in medicine have increasingly become subjects to scientific investigations, and the results, quite often, have been anything but wonderful for medical outcomes, medical cost structures, the various affected medical specialties (the infertility field having a prime target for private equity investments in recent years), and society in general.1 Unsurprisingly, the involvement of Private Equity in medical practice has also become the subject of several Congressional as well as state hearings.
Now, once again in Inside Reproductive Medicine, Ron Shinkman reported on June 19, 2025 (via the usual Griffin Jones e-mail) that the state of Oregon—as the first state in the Union—enacted a law restricting the potential role of Private Equity in medical practice in the state. Oregon Senate Bill #951 was signed into law on June 9, 2025, and prohibits employees of a professional medical corporation from controlling or directing the “professional judgment of a licensee who is practicing within the professional corporation.”2 The law also placed restrictions on how non-compete and non-disparagement clauses for physicians who leave medical
groups may be structured.
As Shinkman noted, the intent of the law was clearly to close a loophole that mandates majority ownership of medical corporations by physicians but does not specify the corporate roles of physicians and non-physicians within a medical corporate entity. The law, therefore, specifically focuses on restricting how employees of a management services organization can influence clinical management decisions through the management of the operations of a medical practice.
This is, of course, an issue that is not only crucial when it comes to the takeover of medical practices by Private Equity but also applies for example—as already noted above in our discussion of UnitedHealth Group and Kindbody—to insurance companies which through their ability to refuse coverage in many ways can control the medical judgment of physicians even more so than non-medical owners of medical provider organizations. And, as Shinkman’s article also noted, nonmedical owners, of course, also have the opportunity to further affect the quality of medical care by demanding ever-increasing workloads on physicians (note above pointed out the demand of Kindbody of their physicians to increase IVF cycle numbers). Shinkman also takes the passing of this unique Oregon law as an opportunity to review how this kind of legislature may affect the infertility practice field as well as the Private Equity community. He did both very well: He, for example, noted that the ASRM,
according to Sean B. Tipton, its Chief Advocacy and Policy Officer, chose not to take a position on the Oregon law. And Mica Hill, DO, current SART president, refused to comment, and one wonders why? Has the power of Private Equity in the infertility really already become that substantial?!
Assuming similar initiatives in other U.S. states, the professional life of Private Equity in the medical field could become substantially more complicated. And that at a time when Private Equity in general does not do very well because it is experiencing significant exit difficulties, falling in Q1 of 2025 to a 2-year low.3 Altogether, this likely suggests declining purchase prices for medical assets, including IVF clinics.
1. Schlafly A. Mo Med 2024;121(5):328332
2. Oregon State Legislature. Senate Bill 951, 2025 Regular Session. https://legiscan. com/OR/bill/SB951/2025
3. Thomas D, Hiteshbhai Bharaucha
N. S&P Global. April 21, 2025. https:// www.spglobal.com/market-intelligence/ en/news-insights/articles/2025/4/ private-equity-exits-fall-to-2year-lowin-q1-2025-88524467
We probably are biased in feeling that artificial intelligence (AI) in medicine is being oversold, but it seems to be everywhere, whether in general medicine or infertility, and it, likely to be everywhere in everything, and not only in medicine. It suddenly seems—and it may turn out to be true—that everything can be done through AI better, with less effort, quicker, and

maybe also less costly.
Those are at least the claims, and here are some with medical relevance, and not necessarily in order of importance, as we currently view the claims:
A large language model, for example, in Cell, is alleged to deconstruct the clinical intuition behind diagnosing autism.1 And if you didn’t right away understand what this sentence means, neither did we on the first try. So, here are some explanations: Genomewide assays and brain scans to diagnose autism have not been very successful and seen diminishing returns. Clinical intuition of healthcare professionals remains the gold standard for diagnosis. So, investigators leveraged deep learning to deconstruct and interrogate the logic of clinician intuition. After pre-training on hundreds of millions of general sentences, they finessed large language models (LLMs) on >4,000 free-form health records from healthcare professionals to distinguish confirmed versus suspected autism cases. Their extended language model architecture could then pin down the most salient single sentences in what drives clinical thinking toward correct diagnoses, flagging the most autism-critical criteria, which were stereotyped repetitive behaviors, special interests, and perception-based behaviors. These findings challenged today’s focus on deficits in social interplay, offering better potential diagnostic criteria.
Unsurprisingly, and often avoiding disclosure requirements of medical and science journals, AI found wide use in manuscript preparation.2 Miryam Naddaf, in a News article in Nature magazine, now, based on a paper on PLoS Biology, 3 that hundreds of studies appeared to follow a template reporting correlations using public data sets. Associations, then, however, often did not hold up to statistical scrutiny. Other studies appeared to have “cherry-picked” data.
A News article in Science magazine by Cathleen O’Grady, in turn, reports that as a consequence of public data use and AI, lowquality papers produced by paper mills (mostly in China) “drive the industrialization of shoddy research.”4 After 2021, 92% of papers using publicly available National Health and Nutrition Examination Survey data came from China; before 2021, the percentage was only 8%.4
Related, a letter in Nature raised the suspicion that some peer reviewers are asking large language models to write “critical” reviews to be able to reject a paper.5 The writer suggested that journals must strengthen their policies regarding AI-generated texts and scrutinize texts for signs of unauthorized AI use.
AI designed at Google to help scientists and mathematicians create new algorithms – Science magazine also reported that Google’s DeepMind released an AI agent called AlphaEvolve, meant to help with the development of new algorithms.6
And if we are already talking about Google, The Wall Street Journal recently noticed in an article how threatening AI is to Google’s core business of searching.7
Brain-computer interface in pre-clinical testing may boost human intelligence
This is at least the hope for new AIA.I.-powered brain-computer interfaces, according to a News article by Paul Webster in Nature Medicine, 8 which can send and receive signals. The brains of study participants are already being trained.
Where do we call?
1. Stanley et al., Cell 2025;188(8):P22352248.E10
2. Suchak et al., PloS Biol 2025; 23(5): e3003152
3. Nadaff M. Nature 2025;641:1080-1081
4. O’Grady C. Science 2025; 388(6749):807-808
5. Xames MD. Nature 2025;641:39.
6. Service RF. Science 2025; 388(6749):805
7. Gallagher D. The Wall Street Journal May 8, 2025. https://www.wsj.com/ tech/ai/ais-threat-to-google-just-gotreal-8280b4ee?gaa_at=eafs&gaa_ n=ASWzDAjwrcuJ_q56VKJFuPC1e222xw beD6wYuPGMZxJhy7b4iuFm3CwC5mgq hqe-B6c%3D&gaa_ts=68587b7e&gaa_
8. Webster P. Nat Med 2025;31:1045-1047


That many of the nation’s most prominent universities are in the midst of major upheaval is likely an understatement. One, indeed, can argue that universities—among those elite universities like Harvard and Columbia are experiencing unprecedented crises with still unforeseeable consequences. A very recent article in The Free Press by Christopher F. Rufo presented the crisis in higher education in the country. As far as we have seen, so far, the best is when stating that, “the American people have been enormously generous to our universities. It is time for schools to honor their end of the bargain.” In the title of his important article, he was more direct when making the point that, to save higher education, the historical serial abuse of the American taxpayer must end.1 We couldn’t agree more!
Below are comments regarding several other relevant recent articles we found of interest.
1. Rufo CF. The Free Press. July 14, 2025. https://www.thefp.com/p/the-presidentwants-to-fix-higher-education-policyreform-campus-university
UCLA’s Geffen School of Medicine hit with class action lawsuit alleging continuous race-based admission process
FOX News and other news outlets have reported that UCLA’s medical
school was challenged by a class action lawsuit under the allegation that the medical school is still using race in its admission process. Considering the recent Supreme Court ruling on the subject, this can be viewed either as a heroic resistance or as stupidity. We would likely vote for the latter, considering that medical schools—like all universities and colleges—of course must abide by the law and prioritize merit.
1. Colton E. FOX News. May 8, 2025. https://www.foxnews.com/politics/ ucla-medical-school-hit-class-actionlawsuit-allegedly-still-using-race-basedadmissions-process
And Columbia University somehow always succeeds in screwing things up anew!
Based on media reports, Columbia University, in contrast to Harvard University, which chose to negotiate with the government rather than fight it in court, was on the way to recovery in its institution’s relationship with the Trump administration. But then Claire Shipman, the acting president of Columbia University after resignation of her predecessor just a few months earlier, involuntarily nevertheless succeeded in hitting the media headlines for in the past as the then vice-chair of Columbia’s Board of Trustees, having advocated the removal of a Jewish board member who she felt was too outspoken in her pro-Israeli advocacy, to be replaced by an Arab member.
The Washington Free Beacon quotes a text message1 she sent to the board and released by the House
Committee on Education and Workforce, in which she argued that “we need to get somebody (non-Jewish) from the Middle East or who is Arab on our board.”

Shipman, acting president of Columbia University
A story like this, of course, raises several questions, among them who Claire Shipman is and how she got to be the acting president of Columbia University, considering she never had an academic career. She grew up in Columbus, OH, with a law professor father and earned two degrees at Columbia University, a bachelor’s degree in Russian studies and then a Master of International Affairs from Columbia’s School of International and Public Affairs, which—considering the history of this school—of course virtually guarantees that she probably never even considered conservative viewpoints and, most definitely, cannot be accused of conservative political biases.
That was also evident when she pursued a journalistic career, which started at CNN (5 years at CNN’s Moscow bureau), then became White House correspondent for NBC during Clinton’s presidency, and peaked when she became the senior national correspondent for ABC’s Good Morning America program.
She joined the Board of Trustees at Columbia in 2013, a rather unusual appointment for a journalist, and became the Vice-Chair 10 years later. After the forced resignation of the prior president (now still the dean of the Columbia medical school), she then assumed her current position as acting president. Once again, a rather unusual appointment, considering the fact that her highest degree was a master’s, and she really never came even close to an academic administrative career.


On a more personal but telling note, she was married and divorced twice: Her first marriage was to the then Moscow bureau chief for CNN (1991-1996), and she married White House Press Secretary for President Obama (2011-2014), Jay Carney, further reemphasizing her association with the Democratic party. They divorced this year and share a daughter.
The negotiations with the Trump administration haven’t been going too well for Columbia. Because of how the university had handled campus antisemitism, the administration had cut $400 million of the school’s federal funding, which the university hoped to recover through negotiations. Katrina Armstrong,
MD, her predecessor as Columbia President and now back again CEO and Dean of Columbia’s medical school system had given—what The Washington Free Beacon called a “disastrous” deposition in April— which had led to her resignation (isn’t it amazing how consequences for failure don’t seem to exist any longer at our elite universities: Past Harvard president Claudine Gay, PhD, returned to being dean of a Harvard school and—as has been reported—has maintained her presidential salary, and Dr. Armstrong is back to heading up one of the biggest medical centers and medical schools in the country. No wonder meritocracy has become a dirty word on too many campuses!
1. Sibarium A. The Washington Free Beacon. July 2, 2025. https://freebeacon. com/campus/columbia-president-claireshipman-privately-said-school-needed-toadd-an-arab-board-member-and-removea-jewish-one/

And how can we not also mention Harvard University?

Against a background of media reports suggesting that Harvard University—despite its outward aggressiveness in pushing back against the Trump administration—is, in reality, like Columbia University and other institutions of higher learning, in the midst of intense negotiations over a consent settlement with the government, a recent Crimson poll conducted between April 23 and May 12 found that no less than 71% of the arts and sciences faculty oppose any negotiation with the administration that may require concessions long sought by conservatives.¹
And who can be surprised by this survey outcome? We, indeed, would have speculated that the percentage of rejection rate among—especially the arts and science faculty— would even be higher, considering how few conservative faculty members—especially in arts and sciences—Harvard University employs.
And if one looks into a bit more detail, the numbers, indeed, get significantly worse: 64% of faculty

members strongly disagreed with closing down DEI programs, 73% opposed rejecting foreign applicants with anti-American beliefs (even if hostile to American values and institutions inscribed in the U.S. Constitution and Declaration of Independence). And a full 70% strongly disagreed with revoking school recognition from pro-Hamas groups.
Finally—and this really says it all—a full 98% of faculty supported the university’s decision to sue the Trump administration.
REFERENCE
1. Dion JP. The Algemeiner. July 11, 2025. https://www.algemeiner.com/2025/07/11/ harvard-faculty-oppose-deal-with-trumpdistancing-from-hamas-apologistscrimson-poll/
Do U.S. university officials now also need physical protection from Hamas supporters?
According to Jewish Breaking News, it is no longer only Jewish students who need physical protection at some universities and colleges; now, university officials may have the same experience.1 In an, of all dates, July 4-dated article, this news site reported that officials at the University of Michigan had to be placed under 24-hour protection after being targeted by Hamas extremists. And they apparently did not only target these administrators but also their families at their private homes. One Regent with a Jewish-sounding name had, indeed, thrown urinefilled jars through his home’s front window.
REFERENCE
1. University of Michigan officials placed under 24-hour protection after being targeted by pro-Hamas extremists. Jewish Breaking News. July 3, 2025.
This is why an article in The New York Sun by Professor Emeritus of law at Harvard University, Alan Dershowitz, JD, attracted our attention because it made a crucially important point: “Universities need to come clean about the real meaning of ‘meritocracy,’ as the diversity debate roils higher education.”1
As he noted in the article, one of the demands of the Trump administration from Harvard University was that the institution reject the concepts of Diversity, Equity, and Inclusion (DEI) and accept meritocracy in admission of students and in hiring criteria. Yet meritocracy can have different meanings: As he explained, in hiring pilots, surgeons, athletes, stock pickers, etc., what counts is the candidate’s current ability to do the best job. Yet a second definition can take a somewhat longer view by looking at a candidate’s likely development, trajectory as he called it, which would, for example, apply to student admissions, the hiring of faculty, or the recruitment of an orchestra member. A third definition, in turn, is very different because it is largely a moral one, basing the decision to hire on past events that demonstrated that the candidate deserves to be hired, obviously judging the candidate not only on their future potential but also on past experiences and especially difficulties she/he
overcame.
He then—interestingly—argues that institutions should be given discretion in how they define meritocracy; however, once decided and published, they should not be allowed to cheat, which is what he feels is exactly what universities (and other institutions) are doing these days. And they cheat because their real goal is not meritocracy but to fill certain quotas (currently at many universities, for example, 13-17% for Black student admissions), which he then describes as “illegal, unconstitutional, and even immoral.”
Sport teams, he noted, in contrast, don’t care about race; all they care about is performance, even if it means that all the team is Black or White. And most people will pick their physicians based on their current skills and not based on their sex, race, or any other criteria. In short, he is correct; it all starts with how we define meritocracy!
The government has been cutting research grants to universities all over the country, and not only to Harvard, even though Nature magazine recently noted that Harvard researchers have been especially reeling, as the Trump administration cut 1,000 grants.2 But virtually every research university has switched into emergency budgeting mode, simply based on the fact that the federal government has reduced so-called “indirect overhead costs” for all grants to maximally 15%

of direct costs. For many of the major research universities, used to indirect costs of often over 60% of direct costs, this has been a devastating development, resulting in significant budget cuts in such routine items as conference travel.
A recent article in detail explained indirect costs:3 What in federally funded R&D direct costs should be in comparison to indirect costs, has been a subject of debate since the 1940s. As of May 2025, indirect cost reimbursements for institutions of higher education were typically pre-negotiated with the federal government and varied between 30% and 70%. But more recently, the National Institutes of Health (NIH), the Department of Energy (DOE), the National Science Foundation (NSF), and the Department of Defense (DOD) released policies that would impose a 15% indirect cost rate on all awards. Congress is now debating the potential consequences and whether, and to what degree, the federal government should support indirect costs.
Limiting federal funding for indirect costs could save significant amounts for federal agencies that could be used to increase the number of research projects funded by the federal government. Limits could also potentially incentivize operational efficiency. On the other hand, such changes could have a significant negative impact on some, especially smaller public research institutions that lack private-sector support or endowments. Allowing indirect cost rates to continue to vary might limit Congress’s ability to address long-standing critiques, alleging a lack of transparency in the use
of indirect cost reimbursements. Where this discussion will end will be interesting to see.
1. Dershowitz A. The New York Sun Updated June 17, 2025. https://www. nysun.com/article/as-diversity-debateroils-higher-education-universities-needto-come-clean-about-the-real-meaningof-meritocracy
2. Garisto D. Nature 2025;642:15-16
3. Gallo ME, Harris L. Congress.GOV. May 16, 2025. https://www.congress.gov/crsproduct/R48540
Can you mandate “gold standard science?” and a Justice
Since President Trump, on May 23, 2025, signed an Executive Order mandating the overhaul of research-integrity policies in the federal government to ensure that “the federal government promotes transparent, rigorous, and impactful ‘gold standard science,’” that ”gold standard science” can be mandated, is apparently what the administration believes.
If only it were that easy!
And it, of course, isn’t, and in some ways that is too bad because medical and science publishing is fully deserving the increasing scrutiny it is receiving and could greatly benefit from interventions to improve many of the products this industry is producing (see also the article on political biases in medical and science journals on page 8).
Unsurprisingly, the response President Trump received from the research community was
not very friendly. According to a News article in Nature magazine, thousands apparently signed an open letter against the U.S. president’s order, claiming concern about political interference in science (who those thousands were is not clear).1
As the Journal article noted, the Executive Order stated that research integrity issues, including high-profile retractions and the inability to reproduce many scientific studies, have caused the public to lose faith in science (and medicine).
Paranoia and hypocrisy were rather obvious when Nature in this article claimed that “some who spoke to the journal worried that language in the order opened the door to political interreference in U.S. science.”1 And this, after all the government interference during the COVID pandemic, now established as much broader than ever suspected.
The CHR’s medical director and chief scientist, Norbert Gleicher, MD, elsewhere in this issue of the VOICE, addresses the politicization of medical and science journals in considerable detail (page 8). This article in Nature magazine is, of course, just another example of exactly the kind of politicization of medical and science journals that Dr. Gleicher’s article is trying to warn about.
One can obviously not mandate a “gold standard science” because such a standard simply does not exist. But both medicine and science in general would greatly benefit from some self-reflection, considering how obviously

conflicted so much of the currently published medical literature is in so many different ways. And the literature in reproductive medicine is no exception.
And very much relating to this Executive Order but also to above noted article regarding the politization of medical publishing, the Trump administration has, as The Wall Street Journal recently reported,2 also homed in on several U.S. medical journals, including The New England Journal of Medicine (NEJM) and the Journal of the Medical Association (JAMA).
The Justice Department—rather unprecedented—in April of this year, indeed sent letters to 15 of the country’s leading medical and science journals inquiring about “fraud,” political bias,” and “censorship” at these journals. A letters from a U.S attorney addressed to one specific journal is quoted in the article to have stated that, “it has come to my attentions that more and more journals and publications are conceding that they are partisans in various scientific debates” (signed by the U.S. attorney for the District of Columbia). And this is actually exactly one of the points Gleicher made in his above-noted article in this issue of the VOICE.
The article in The Wall Street Journal also pointed out that current FDA Commissioner Marty Makary, MD, Health and Human Services Secretary Robert F. Kennedy Jr., and NIH Director Jay Bhattacharya, MD, MBA (the “Three Musketeers of Healthcare”)—especially during and after the COVID pandemic—
have all been outspoken critics of medical journals and of how they often reported alleged scientific facts.
Though, as the VOICE really since its inception has on many occasions pointed out, we to a significant degree agree with this criticism, The Wall Street Journal article alleged that the Justice Department, likely, took things a little too far, when a spokesperson for the U.S. attorney noted that the letters were sent in response to “legitimate public grievances” (so-far ok!) but then claimed that, “you have a bunch of leftists who are sitting on big pots of money from pharma, and they all entertain each other and publish their friends” (of course, somewhat of an exaggeration). And he then went on to say, “… they were basically publishing lies.”

The “Three Musketeers” running the national health system in the Trump administration, from left to right: Jay Bhattacharya, MD, PhD, Robert F. Kennedy, Jr., JD, & Martin (Marty) Makary, MD, MPH
Unsurprisingly unhappy about receiving one of those 15 letters, Eric Rubin, MD, the editor-inchief of the NEJM, called the letter an intimidation tactic and, according to The Wall Street Journal article, reemphasized the medical journal’s rigorous peer review, editorial independence, and First Amendment rights as evidence for its unbiased conduct. Except,
of course, that none of these three arguments really addressed the three concerns expressed in the letters sent by the Justice Department to the 15 medical journals, which were fraud, political bias, and censorship by editors and/ or publishers.
Interestingly, The Lancet—which, likely because of its British genealogy, did not receive one of the 15 Justice Department letters—nevertheless, felt obliged to dedicate one of its rare anonymous Editorials to those letters, describing them as an “obvious ruse to strike fear into journals and impinge on their right to independent editorial oversight.” Still, this opinion, of course, leaves open to interpretation what “independent” means.
And, if The Lancet considers, for example, editorial oversight by its longstanding and still current editor-in-chief, Richard Charles Horton, BSc, MB, ChB, to be unbiased and balanced, as Dr. Gleicher extensively documents in his above-noted article in this issue of the VOICE, then we also have a bridge in Brooklyn for sale!
In short, as is so often the case when politics become involved, the opinions at the extremes are usually both wrong. Again confirmed, in this case the Trumpian right is obviously mistaken in believing that “gold standard science” is just a whiff away from execution after a Presidential Executive Order, and the Left, when prominent editors-in-chief of even more prominent journals, like Rubin (NEJM) and Horton (Lancet) —and there is quite a long list

of additional editors—lack institutional and emotional inside into their positions of professional leadership to such a significant degree that they don’t even appear to understand what the letters from the government are talking about.
And they, of course, also completely are missing the very obvious link the current administration has come to recognize between decadesold misdirections in education in the country’s institutions of higher learning, often having led to indoctrination rather than education of our youth and, on the other side—in a continuum— political indoctrination rather than education of medical providers and scientists by politically-driven medical and science journals, their editors, and their publishing organizations.
This is what the 15 letters, likely, were really all about; and, just as the time has come to take back our institution of higher learning from cults on the extreme Left of the political spectrum, so does medical practice have to be recovered from wokeism and indoctrination, and that, of course, also includes reproductive medicine.
One final point from The Wall Street Journal article2 is worth mentioning: According to a 2024 Pew survey, there are considerable differences in how Democrats and Republicans view research scientists: 80% of Democrats but only 52% of Republicans view them as “honest.” No wonder, therefore, that the scrutiny of the conduct of science in the country has increased under the current
Republican administration.
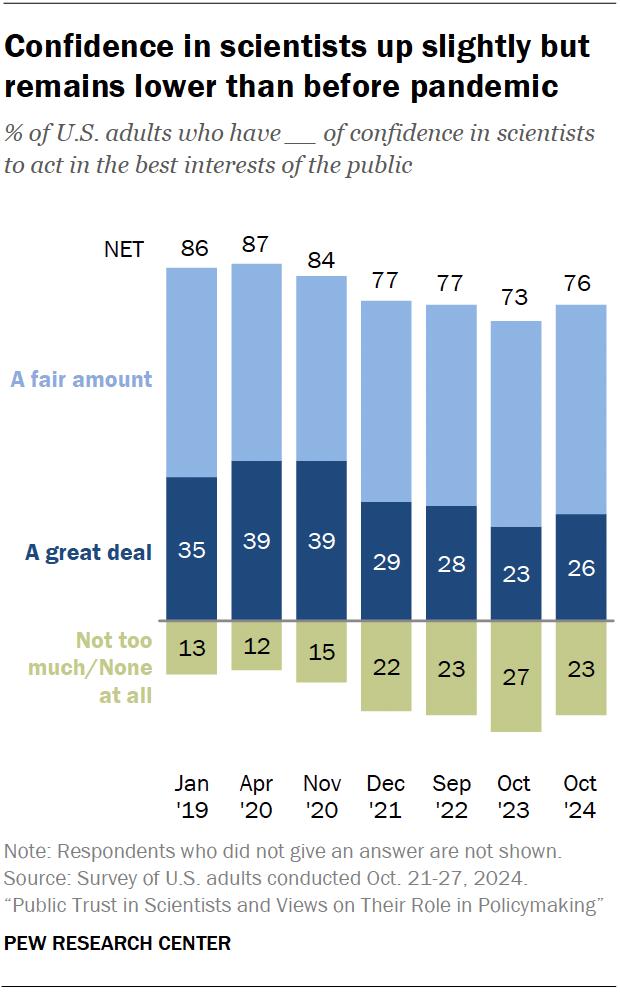
1. Tollefson J, Garisto D. Nature 2025;642:13-14
2. Paul P. The Wall Street Journal. June 1415m 2025, https://www.wsj.com/science/ how-scientific-journals-became-magaslatest-target-9874b6f7?gaa_at=eafs&gaa_
Medical schools apparently still selectively accept students based on race
According to a recent editorial in The Wall Street Journal, U.S. medical schools appear to be ignoring the Supreme Court decision that banned racial preferences in university admissions [Students for Fair Admissions (SFFA) v. Harvard].1 Though not alone among schools of higher education, medical schools appear to be among the more frequent offenders. Do No Harm, a group of medical professionals that studies such preferences in medicine, obtained so far admission data from 24 (they requested data from 93) public
medical schools for the year 2024. At many of them MCAT admission scores of Black applicants were lower than for Caucasian and Asian applicants, and Black candidates –assuming equal MCAT scores – had much higher admission rates than Caucasian and Asian candidates.
Prominent among those schools, the University of Wisconsin School of Medicine and Public Health (Black applicants x10 chance of Caucasians and Asians), Eastern Virginia Medical School (x11).
To a degree that should not surprise, as the editorial noted, because after the Supreme Court decision, the Association of Medical Colleges was “deeply disappointed” by the decision, and schools have been looking at all


kinds of ways to circumvent the Supreme Court’s decision.
1. Editorial. The Wall Street Journal. July 17, 2025. pA14
Can sunlight, in fact, cure diseases?
This is what a recent article in Scientific American, indeed, suggests:1 Sunshine appears especially effective when the immune system must be calmed down and that, of course, applies especially to autoimmune conditions, like multiple sclerosis (MS) and type 1 diabetes. It was in the mid-1970s that researchers found in small animal cancer models that UV lights apparently can induce cancers. But it took a few additional years until the mechanism underlying this association became clear: UV light suppressed a mouse’s natural immune response. And since then, it has become quite apparent that UV light calms, especially inflammation, in the skin, the central nervous system, the pancreas, and the gut.
What does mean practically in daily medical practice: UV light boxes emitting only a narrow bandwidth of light that does not cause cancer have now for years been used in the treatment of psoriasis (of course, also an autoimmune disease). And these light boxes are now also increasingly used (4 minutes per side per day is all that’s needed) in MS patients.
Why are we featuring this article here in the VOICE? Because immunosuppression is, of
course, also sometimes needed in reproductive medicine: Women with autoimmune diseases or just women with repeated pregnancy loss due to a hyperactive immune system could be candidates. It seems that 4 minutes per day of light treatment may most certainly be worth a try if it saves patients from much more invasive treatments.
1. Jacobsen R. Scientific American, June 2025; pp22-30. https://www. scientificamerican.com/article/ surprising-ways-that-sunlightmight-heal-autoimmune-diseases/
Courtesy of Jen Christiansen. Data from Simpson S Jr, et al. J Neurol Neurosurg Psychiatry. 2019;90(11):1193-1200.













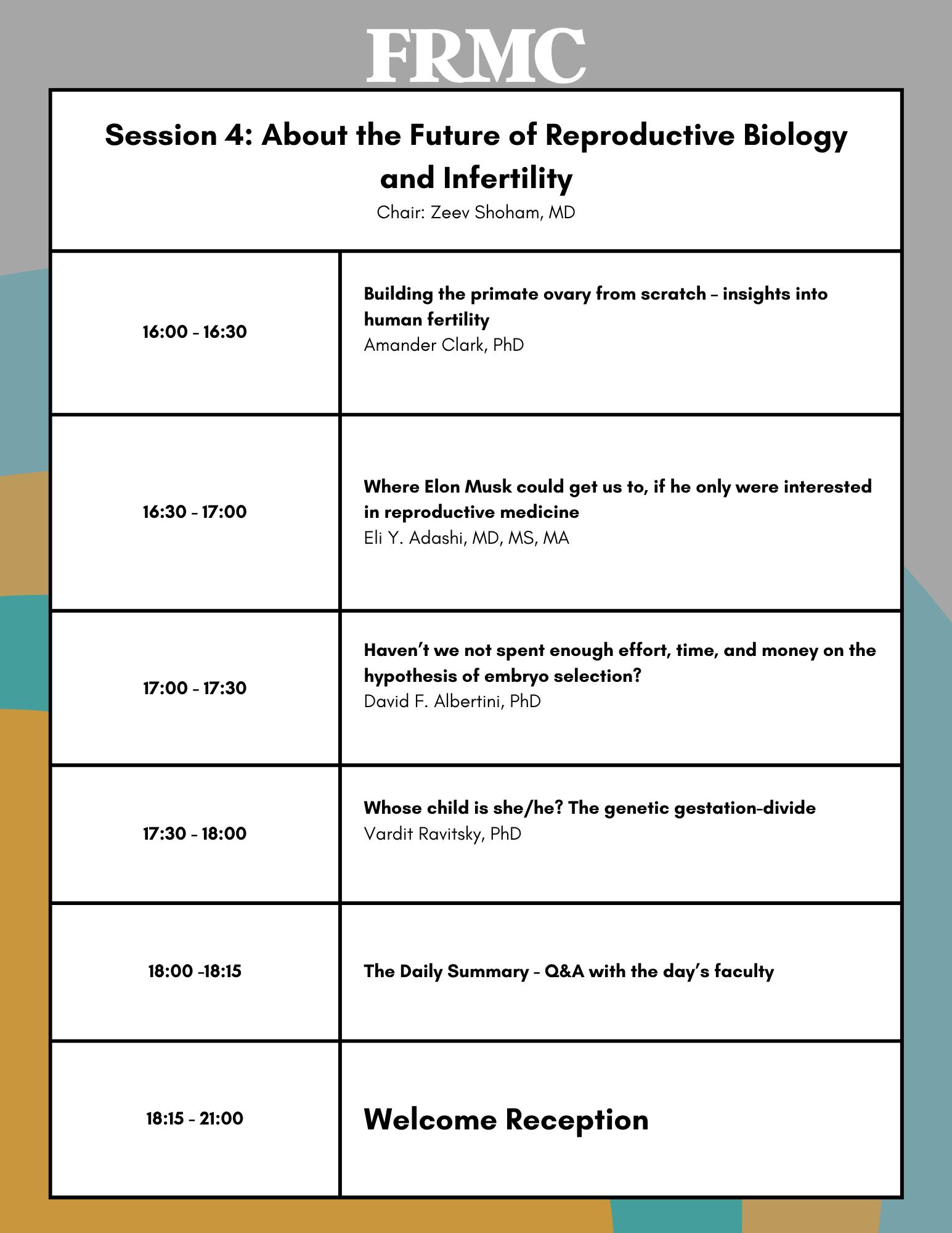







BRIEFING: That “food is medicine” is an increasingly frequently heard phrase in medicine that also applies to fertility. This section of the VOICE caters to this concept in a variety of ways. A prominent current subject is, for example, weight loss with GLP-1 receptor agonist drugs since obesity is becoming an increasingly frequent co-diagnosis in infertility practice.
Are there differences in effectiveness between the new weight loss medications?
You bet there are, and that should not surprise, because studies of individual drugs reported different degrees of weight loss. Now, however, The New England Journal of Medicine published a head-to-head comparison trial1 between Tirzepatide [a long-acting glucose-dependent insulinotropic polypeptide (GIP) together with a glucagon-like peptide-1 (GLP-1) receptor agonist] and semaglutide, which is only a long-acting GLP-1 receptor agonist. Some people would call these two medications also the second and first generation of this new family of life-changing and medicine-changing drugs. Tirzepatide is available under two different brand names, even though it’s the same medication combination: Mounjaro® for diabetes and Zepbound® for weight loss. Eli Lilly makes both, and they are interchangeable. Semaglutide is the active ingredient in Ozempic®, Wegovy®, and Rybelsis®, all made by NOVO Nordisk. And the outcome was clear: Among obese participants, though without diabetes, Tirzepatide was the better one, producing within 72 weeks larger weight loss and greater loss of waist circumference.
REFERENCE
1. Aronne et al., N Engl J Med 2025;393():26-36
A short recent Commentary article1 in Cell Reports Medicine is worth mentioning because it summarizes well the amazing expansion of discovered medical benefits that GLP-1 agonists appear to offer beyond just blood sugar control and weight loss, their initial purposes.
As the article summarizes well, these medications have by now demonstrated to reduce rates of chronic kidney disease, myocardial infarction, stroke, and cardiovascular death in people with type 2 diabetes. They also improve symptoms and outcomes in people with heart failure with preserved ejection fraction, the majority with concomitant obesity and often type 2 diabetes. Benefits have also been demonstrated in separate trials of people with metabolic dysfunctionassociated liver disease (MASLD), sleep apnea, osteoarthritis, and peripheral artery disease. Worth the read!
REFERENCE
1. Gonzalez-Rellan MJ, Drucker DJ. Cell Reports Med 2025;6(7):102214
So, we all know that weight loss is good for fertility: It improves the chance of spontaneous pregnancy, but also improves the pregnancy chance with practically all fertility treatments. And then weight loss also reduces pregnancy complications. Reproductive Biology and Endocrinology, which increasingly publishes interesting papers (also reflected in its steadily rising impact factor) now published a paper1 by a consortium of U.S investigators demonstrating how little weight loss already matters.
In an unfortunately only retrospective cohort study at a university multidisciplinary program for women with reproductive disorders and obesity, all participants from the program’s start in November 2021 until July 2023 were included in the analysis. The primary outcome was the percent body weight loss at 3 months. Secondary outcomes included weight

loss at 6 months and achievement of >5% and >10% weight loss at each time point.
Among 237 participants, 88.2% desired pregnancy, and among those, 63.2% of participants were willing to postpone pregnancy attempts/fertility treatments so that they could focus on weight loss for at least 3 months. They consequentially achieved significantly greater weight loss at 3 months compared to those who continued pregnancy attempts (mean −4.8% vs. -2.5%, P=0.004) and were more likely to achieve>10% body weight loss at 3 months (14.0% vs. 2.20%, P=0.031). Those who achieved >5% weight loss by 6 months were more likely to achieve pregnancy within the first 6 months of trying to conceive (34.1% vs. 7.7%, P=0.004). In other words, just a 5% loss of bodyweight achieved in 3 months already significantly improved pregnancy chances.
Quite a lesson for patients as well as treating physicians!
1. Schon et al., Reprod Biol Endocrinol; 2025;23:89
So which diet offers more weight loss—Keto or Mediterranean?
We would not be surprised if this were the most frequently asked question these days when it comes to dieting, and we now may have an answer from a study by Spanish investigators, published very recently in BMC Medicine.1
In a three-month, parallel-arm, randomized clinical trial including 160 adults with obesity, they randomized to several groups against a Mediterranean diet control, MedDiet. They were: a ketogenic diet (KD), a very low-carbohydrate diet, early timerestricted eating (eTRE), late time-restricted eating (lTRE), and modified alternate-day fasting (mADF). The primary outcome was the difference in weight loss after 3 months between the caloric-restricted MedDiet and the other 5 diets. Secondary outcomes were changes in BMI, body composition, and cardiometabolic risks.
The mean age of patients was 45.7 years, and 70.6% were women; 140 participants completed the study.
Significant differences in weight loss were found between KD and the control group [− 3.78 kg], between mADF and the control group [− 3.14 kg], and between lTRE and the control group [− 2.27 kg], but there were no outcome differences between eTRE and the control group [− 1.22 kg].
The authors concluded that in obesity (the definition was quite broad, with a BMI range of 30-45 kg/m2), calorie-restricted KD, mADF, or lTRE may be more effective for weight loss than a calorie-restricted MedDiet. Once again, caution is probably indicated before jumping to conclusions. The BMI range of study subjects was huge, going from mildly obese to morbidly obese. One wonders whether all of these weight classes can be thrown into the same patient pool. We really don’t think so!


REFERENCE
1. Martinez-Monoro et al., BMC Medicine 2025;23:368
The increasing importance of the gut-brain axis
Once more, Eric Topol, MD’s Ground Truth on Substack offered an important posting, this time describing in much detail the increasing knowledge that is accumulating regarding the very important gutbrain axis (see Figure 1 below).1
Topol describes the axis as made up of 4 interactions through which the gut influence the brain and vice versa: 2 neural pathways (also called the “second brain”) reflecting, first, the gut’s nervous system made up of the cells that line the gut and communicate through the vagus nerve with the brains (and vice versa) and, second, the autonomous nervous system (sympathetic and parasympathetic) branches and spinal cord innervations to the gut, primarily communicated through the autonomous nervous system.
The gut’s endocrine cells also produce a family of hormones, some of which have entered medical practice in a grand way as medications for diabetics and, more recently, for weight loss (glucagon-like peptides, GLP-1 agonists), but also others, like gastric inhibitory peptide (GIP), peptide YY, secretin, gherlin, gastrin, etc. And the hormonal interaction through these hormones with the brain also affects the hypothalamic-pituitary -adrenal axis (and, therefore, also must affect the ovaries).
In addition, trillions of cells of over 3,000 species of bacteria and their metabolites influence, to a significant degree production and stimulation of different neurotransmitters like 5HR and GABA, and of metabolites that then communicate with the brain and the immune system. The communication with the immune system is of special importance because it is supposed to maintain the integrity of the gut lining, which can lead to the “leaky gut syndrome” when it is not properly maintained. And the same applies regarding the integrity of the brain-blood barrier. In this context, he also noted a recent paper in Nature which demonstrated that CD4+ T cells from the inflamed gut can infiltrate the brain.2
He then goes on to describe “a plethora of new gut hormones” now under investigation for their potential clinical use and, as always, a tour de force and absolutely worthwhile reading.
1. Topol E. Ground Truth. Substack. June 22, 2025. https:// erictopol.substack.com/p/the-gut-brain-axis-takes-center-stage 2. White et al., Nature 2025;643:509-518






LE VEAU D’OR**/$$/v
As traditional French as they get 129 East 60th Street; Tel.: (646-386 7608)
So, there is something of a renaissance of French dining on the Upper East Side, with three newcomers suddenly being among the most difficult reservations to be had in all of Manhattan. And we are not talking about big new palaces like 425 on Park Avenue—but small restaurants with just a handful of tables, close together, almost like in Paris. They are Cafe Commerce on Lexington Avenue and 70th Street, Chez Fifi at 74th Street and Lexington, and our review for today, Le Veau d’Or, which we are starting with, because for the other two, we have not had enough visits yet.
If you are planning dinner at Le Veau d’Or, make sure you are not by mistake just passing by the restaurant on the north side of 60th Street, because its entrance is so small and unremarkable. As you walk into the space—pushing aside a heavy curtain—you are in Paris, and not in 2025 Paris, but—more so—in 1975 Paris. A small place with red plastic banquets, close together tables, and—since always packed—also always very noisy. There is not much to say about the food

beyond that—with very few exceptions—it is classical French and excellent. The one exception we would call out was—somewhat surprisingly—the duck Magret

aux cerises (cherries), which was repeatedly strongly recommended by the otherwise exquisite wait staff and described as being a tender duck breast with crispy skin. We, unfortunately, found it to be chewy, and the skin was definitely not crispy. Delicious in contrast were practically all the appetizers, including a traditional pâté en croute, tripes à la mode, les escargots provençal, Little Neck oysters, and our favorite, the oeuf en gelé. Among the main courses, each one was excellent except for the duck.
Service was professional but not stuffy, and—especially since a review in The New York Post—gave the impression of unusually high prices—we found them to be surprisingly modest in comparison to other current “in-places” of this quality. We also enjoyed the relaxed vibe, while still being served serious food.
Originally established in 1937, Le Veau d’Or reopened in 2024 to substantial acclaim after a good number of years of absence from the NYC dining scene under Chefs Riad Nasr and Lee Hanson of Frenchette and Le Rock. Reservations are required and are not easy to get. The only reason we haven’t added the restaurant yet to our all-favorite (+) restaurant list is that we haven’t been able to secure reservations often enough for a final judgment. But that will likely soon happen because—in addition—the place is only walking distance away from the CHR.



BRIEFING: In this section, the VOICE offers opinions about recent articles in the medical literature directly referring to reproductive medicine and infertility practice. Like in the earlier general medicine section, the commentaries on papers in the recent literature are, again, to a degree subjective opinions of the CHR. And—while hopefully mostly unbiased, they are usuall referenced. We are starting today with a section on legal issues but want to point out that we in this issue of the VOICE discussed two very important legal issues for reproductive medicine already in the two opening full-length articles.
GENERAL INFERTILITY AND REPRODUCTIVE ENDOCRINOLOGY
“Ovarian aging,” a missing diagnosis in reproductive medicine
This was the heading of a recent Commentary in Nature Medicine by three colleagues from Northwestern University in Chicago1 and how correct the three authors were when then concluding that “recognizing ovarian aging as a formal diagnosis—akin to how obstetrics adopted ‘advanced maternal age,’ would transform reproductive medicine, public health and research into women’s health.”
For one selfish reason, we here at the CHR, of course, could not agree more with those three authors because we have been using the term “ovarian aging” in our daily work, research, and publications for decades. We, indeed, have gone even a step further by coining, also already decades ago, the term “premature ovarian aging” (POA)— when describing a usually younger woman with lower functional
ovarian reserve (FOR, i.e., higher FSH and lower AMH than she would be expected to demonstrate at her age).
And why is that important?
In principle unfortunately some important conclusions from these two “new” diagnoses were not sufficiently explored in the Commentary: (i) One crucial conclusion is that these diagnoses, indeed, require agespecific “normal” and “abnormal” hormone levels (as well as antral follicle counts—AFCs—for those who still use those) to describe the ovarian age correctly as either normal, younger than expected, or older than expected. (ii) The recognition that there is a “natural” aging process that on average ends with menopause at roughly age 51 and a “premature” aging process which—if it leads to menopause before age 40—we arbitrarily have given the name “primary ovarian insufficiency” (POI) or premature ovarian failure (POF) and—if menopause happens after age 40 but before age 51—as “early menopause.” These arbitrary cutoffs, of course, make absolutely no sense because physiology, of course,
works in graduations. (iii) What, however, is most often overlooked is the typical POA patient, who in most cases may still have the usual age of 51 when reaching menopause, but on that journey from a very young age on, always has lower FOR than ca. 90% of women her age.
The reason is that roughly 10% of all women, independent of race and ethnicity, suffer from POA, but only 10% of these 10% (i.e., 1% of the total female population) experience POI. And the 9% with only POA are too often overlooked, even in decent fertility clinics.
Which brings us to (iv), the very important reason why the diagnosis of POA should not—and cannot— be overlooked: A 28 year-old patient with mild POA may have ovaries that behave only mildly “older”—maybe like 32-year-old ovaries with only mildly high FSH and mildly low AMH; but severe POA at the same age– for example mimicking 44 year-old ovaries— may already have significantly elevated FSH levels and very low AMH and sometimes even already undetectable levels. And even in confirmed POA patients, whether

a patient’s ovaries behave like 32or 44-year-old ovaries, therefore, makes quite a difference in how such a patient must be treated and what her pregnancy chances will be.
And the same may, of course, also be true at more advanced ages: Here, too, for example, a 43-yearold patient may have appropriate FOR considering her age or either poorer or better FOR. Once again, in which of these patient groups the patient finds herself will define her treatment options and her treatment outcomes.
But a big “Bravo” for our Chicagobased colleagues who are finally bringing this important subject to the pages of one of the highestranked medical journals in the world!
1. Hughes et al., Nature Med. https://doi. org.10.1038/s41591-025-03733-4. ahead of print
An important Supreme Court ruling regarding gender transition of children and older youth
Likely no other media outlet has been as remarkably committed in its coverage of gender transition treatments for children and older youths as The Free Press. And— fully sharing that publication’s outlook on the subject—we here at the VOICE have repeatedly quoted articles from The Free Press.
Among those were some truly remarkable articles at a time when most other media either ignored the subject or, completely removed from reality, just repeated
outrageously supportive comments by medical professionals and hospital spokespeople of such treatments. Two articles deserve special mention, one from the original whistleblower nurse at an Indianapolis hospital and a second about a whistleblower surgeon in Houston who disclosed that his hospital, in contrast to what it represented to the public, lifechanging surgeries on transitioning children were still performed.

It, therefore, is only appropriate that we give here, The Free Press, the honor of referencing its article by senior editor, Emily Yoffe, on the recent landmark decision of the U.S. Supreme Court, which ruled that states can restrict the ability of minors to get transition treatment.1 As she correctly noted in the heading of the article: It has been long overdue!
1. Yoffe E. The Free Press. June 18, 2025. https://www.thefp.com/p/the-end-ofyouth-gender-transition
Do pregnancies after clomiphene citrate (CC) treatments suffer from more stillbirths or neonatal deaths?
According to investigators from Australia, as reported in the JCEM, that exactly appears to be the case, though not to a really threatening degree.1 Among singletons without
CC, the numbers looked as follows: Stillbirths 6.6/1,000 births; neonatal deaths 2.1/1,000; with CC, the numbers were 10.2/1,000 and 3.1/1,000. Combined counted as perinatal deaths, these numbers reached significance [OR 1.54, 95% CI 1.15, 0.07].
There are several reasons why we are skeptics, though one, of course, cannot just ignore a study involving 242,077 births: (i) The study involved deliveries between 2003 and 2015. Why? (ii) Despite the huge number of cases, the study reached significance only once stillbirths and neonatal deaths were combined, suggesting a really absolutely minimal effect. An (iii) we could understand it if the medication caused early pregnancy problems, but why extremely late and neonatal problems? What’s the mechanism(s)?
1. Moore et al., J Clin Endocrinol Matab 20025;110:1818-1827
The tie-up of infertility with later heart disease in women
That infertility is some ways is connected to later heart disease in women has by now been known for quite some time. Reporting from a combined meeting of the European Societies for Pediatric Endocrinology and (adult) Endocrinology, HealthDay newsletter now reported on a pooled data set of 21 studies involving 179,000 women with infertility and almost 3.4 million without and found a 17% increased risk of heart disease, 16% increased risk of stroke, and 14% increased risk of health conditions affecting the heart or blood vessels. Younger
women bore the greatest risk, with 20% higher odds of heart disease among those under age 40.1
Still perplexing in its causality—if there is one—the association has by now to be considered solid. Now we need to learn to understand it.
1. Thomson D. HealthDay. June 18, 2025. https://www.healthday.com/health-news/ women-health/infertility-tied-to-heartproblems-in-women
Another example of the overutilization of PGT-A in IVF—this time in association with male factor infertility
In an excellent Research Letter article in Fertility and Sterility, Chinese investigators from Shandong University offered a very well-designed prospectively randomized study in a, by the investigators clearly described group of 1,212 good-prognosis patients (what cannot all studies be that clear?!), asking a very basic question: Do couples with male factor infertility in their IVF cycles benefit from preimplantation genetic testing for aneuploidy (PGT-A)?
And—at least for us, here at the CHR—the answer was not unexpected: No, there is no benefit from PGT-A in IVF cycles for male factor infertility! And why, indeed, should there be any benefit if PGT-A has not demonstrated any
such benefit in over 20 years of use in general populations?1
Though designed to answer abovenoted very simple question, this study in its importance goes significantly beyond this very basic question because it—ultimately— represents the a perfectly designed study of PGT-A in general: The hypothesis of PGT-A from the very beginning (including when it had other names) was that embryo testing for chromosomal abnormalities prior to transfer to deselect chromosomal “abnormal” embryos would improve IVF cycle outcomes. And chromosomal abnormalities are, of course, practically exclusively associated with the female contribution to embryos. By studying IVF cycles with only male contribution to a couple’s infertility and, therefore, excluding any female contribution, these Chinese investigators, therefore, basically performed a perfect study of PGT-A in general, maybe even the most perfect one so far ever published!
They are to be congratulated on this achievement, even though they, reviewers of this paper, and the editors of F&S, very obviously overlooked this point. The paper, therefore, was not even given an Inklings commentary.
1. Wang et al., Fertil Steril 2025;124(1):153-155

Progesterone support in FET cycles
We usually are not big fans of the Inklings articles in Fertility & Sterility, which now accompany
most published papers. Often written by one of the reviewers, they, for several good reasons, represent a very questionable and relatively new practice at many journals, mostly instituted to improve the journal’s impacts factors, since editorial comments and review articles are much more frequently cited than original papers (some more fastidious opinions describe their purpose more as dumbing-down articles).
But there are exceptions, and one such exception was recently an Inkling article by French colleague Dominique de Ziegler, MD (et al.),1 who after his retirement as chairman of an OB/GYN university department in Paris has become a very visible international member of the editorial juggernaut Fertility & Sterility (which has the by far largest editorial board of any journal we are aware of). He and the CHR’s Norbert Gleicher, MD on a side note—as young physician scientists in the mid 1970s occupied adjacent laboratories on the 20th floor of the Annenberg Research Building at New York’s Mount Sinai Medical Center.
The article attracted our interest for several reasons: (i) First, the Inkling article had no connection whatsoever—as is usually customary—to any article in the same issue of Fertility & Sterility. In other words, it was, indeed, an “Inkling” article (we for some time have been wondering why Fertility & Sterility named its commentaries “Inklings,”—clearly not a very authoritative term for a commentary) because it—kind of out of the blue—addressed the question why frozen embryo transfer (FET) results are poorer

with vaginal than intramuscular progesterone? (ii) We were not really certain whether the premise that vaginal progesterone causes poorer FET outcomes is correct (and we still are not certain of that because we believe that the difference may lie in the age of the patients, with older women benefiting from I.M. progesterone). And (iii) we found it laudable that de Ziegler used his obviously privileged access to the journal to offer an interesting reconsideration of an earlier publication of which he, himself, had been a co-author, which tried to explain this finding with a “first uterine pass effect.”2
But reason (iv) was the real hook because de Ziegler directed the attention of readers to the fact that, among all of its endocrine functions, progesterone also functions as an immunosuppressor and potentially participates in immune tolerance toward the fetal semi-allograft in pregnancy.3 And everybody who knows the CHR, of course, knows that we love reproductive immunology! Whether vaginal progesterone, indeed, lowers FET cycle outcomes in comparison to I.M. progesterone, therefore, for the CHR is less important than the acknowledgement that reproductive success may have something to do with the female immune system. Too many REIs to this day still deny this indisputable fact!
Assisted reproduction at couples (female as well as males) with advanced parental age
But the Ethics Committee of the ASRM recently published
an updated guideline paper on extending assisted reproductive treatments (ART)—of course mostly in vitro fertilization (IVF)— to couples of advanced paternal and maternal age.4 We are here reprinting the six key points:
• Risks associated with reproduction at an advanced reproductive age (ARA) fall into three broad categories: risks to offspring associated with the use of gametes from older individuals; increasing health risks of gestating at an older age; and risk from having older parents who may have a limited number of expected healthy life years available for parenting. These age-related risks occur on a continuum and can be difficult to quantify. More precise estimates regarding healthy life years can be obtained using actuarial tables.
• Clinics should have written policies regarding inclusion and exclusion criteria as they relate to parental age to ensure consistency in assessments and to reduce the risk of bias. Clinics may base age policy on the predicted number of healthy life years available for parenting, or the risk of parental death before the offspring reach the age of 18.
• Those gestating at an ARA face increased medical risks, and careful counseling is warranted, potentially in conjunction with maternalfetal medicine specialists.
• Clinics should strongly consider having a policy that declines the transfer of an embryo to the uterus of a person at such age as a
program may individually determine a review of the relevant medical literature.
• Prospective patients of ARA should be counseled about the potential negative impact of their age on the success of fertility treatments using autologous gametes and on the increased medical risks to the resultant offspring, including the fact that many of these risks are poorly characterized due to limited data.
• Prospective patients of ARA should be counseled regarding short- and long-term parenting and child-rearing issues specific to their age and health. The age and health of the partner, if present, should also be considered in this discussion.
One more important point: Many IVF clinics have strict age cut-offs (usually primarily for women and more rarely for males). For most women the cut-off for an IVF cycle with their own (autologous) eggs is usually somewhere between 42 and 43 years, when they are advised that their only “realistic” chance of pregnancy is with third-party egg donations. This is also reflected in national U.S. IVF data, which demonstrate only relatively few cycles after female age 42 and— most certainly—after age 43.
Some clinics also have combined cutoffs. We, for example were recently informed by a couple that they were denied a frozen embryo transfer of their own embryo because—combined—they exceeded 100 years. That, of course, makes absolutely no sense! The CHR out of principle does not have age-dependent cutoffs for

any reasons and here is why: First, having rigid cutoffs for women but not for men is, of course, inherently unfair. But, even more importantly, such cut-offs make little sense since some older women may have young-behaving ovaries, while some younger women may have older-behaving ovaries. Similarly, some, older women may be healthy and perfectly capable of withstanding the stresses of pregnancy, while some younger women may have severe medical problems which may be lifethreatening should they conceive.
In other words, because humans are just too variable, rigid agedependent cutoffs for fertility services should never be used and—certainly not—when it comes to making fertility services available. Except in very rare circumstances—whether women or couples are given access to fertility services should not be at the whim of a provider, but of course, after appropriate informed consent, should ultimately be the patients’ decision.
Providers can—and should— make recommendations but should never mandate and/ or withhold reasonable fertility treatments, unless such services can unquestionably be assumed to be futile and that is only very rarely the case. Many patients do not achieve mental peace until they conceived themselves or—at minimum—convinced themselves that they had given it all to conceive. And while such an effort may to observers at times appear irrational, to the patient it may be essential.
1. De Ziegler D, et al., Fertil Steril 2025 (6): 989-990
2. Bulleti et al., Hum Reprod 1997; 12:1073-1079
3. Motomura et al., J Steroid Biochem Mol Biol 2023;229:106254
4. Ethics Committee of the American Society for Reproductive Medicine. Fertil Steril 2025;123(6):999-1004
A detailed commentary on a really bad paper on IVF outcomes in older women using PGT-A
This is a detailed review of a recently published single paper, which—our editors felt—was timely for several reasons: First, the subject of preimplantation genetic testing for aneuploidy (PGT-A), which, by now for over 20 years has been a core theme of research, clinical practice, and discussion at the CHR. Second, for all of this time, PGT-A has remained a controversial subject even though, by clinical and ethical standards, it no longer should be. A third very important motivation has been the rapidly declining quality of peer review in medical journals and, increasingly, also in science journals, a subject the CHR has been increasingly concerned about. The latter was obviously at play here, as this manuscript was published by Fertility and Sterility, one of the leading journals in the infertility field and the main organ of the American Society for Reproductive Medicine (ASRM). Remarkably, this article moreover appeared in the June 2025 issue of F&S, while in November of 2024, F&S had published the updated opinion of the Practice Committees of ASRM and the
Society for Assisted Reproductive Technology (SART) on PGT-A, which completely contradicted much of what here discussed paper claimed. Yet, the ASRM/ SART position paper was not even referenced in the manuscript. The reference list of the paper was, indeed, totally biased toward supporters of PGT-A (who, of course, also were likely the reviewers of the paper). This brings us to the fourth reason: a steadily growing publication bias we are seeing in journals. Popular opinions are published, while unpopular opinions often are not even making it into peer review. It is, of course, not all the editors’ fault because all of them are these days overwhelmed by rapidly increasing paper submission numbers and, at the same time, also face declining reviewer availability, as especially good reviewers who, of course, still work for free, also are overwhelmed by review requests and, therefore, have to increasingly decline reviews. In short, a rapidly deteriorating publication industry urgently requires and begs for a major reorganization.
When reading what many of our supposedly even better medical journals at times allow into print these days, it sometimes feels like we live in an alternative universe. And as fate wanted it, a very recent paper in Fertility and Sterility1 offers an excellent example of what we mean and, therefore, offers an opportunity for discussion.
Considering how much—forgive the word—“garbage” has over the

last two decades been published about preimplantation genetic testing for aneuploidy (PGT-A)— of course, a favorite topic of permanent discussion at the Center for Human Reproduction (CHR) and, therefore, the VOICE as well as The Reproductive Times—it should not surprise that we here chose to address a PGT-A paper.
It does not appear to matter that well-designed prospectively randomized studies have demonstrated no outcome benefits for IVF in general populations, as even a recent combined ASRM/ SART Practice Committees’ opinion finally pointed out2 as even this ASRM/SART guidance does not appear to have made even the slightest dent in the number of “garbage” papers on this subject, many in even better medical journals. Who then can be surprised that PGT-A utilization in association with IVF in the U.S. (and in many other countries) is still increasing.
Coming back to the paper under discussion, this paper, authored by Chinese investigators, a point not made to disparage Chinese colleagues in general, who, of course, often publish very well thought-out and conducted studies. But mass-produced by paper mills mostly in China and a few other select countries has really become a serious problem for medical and science journals.3-5 A really bad paper from one of these countries, nevertheless undiscovered sailing through peer review, therefore, deserves—and, indeed, mandates— special attention (hopefully also by editorial peer review processes at
the journal).
The paper here addressed claimed to have investigated effects of preimplantation genetic testing for aneuploidy (PGT-A) in IVF cycles of women, and this is a very important point, who at the time of the IVF cycle were at an advanced age.1 The paper, however, offers absolutely no rational why it would even conduct such a study, considering even ASRM and SART in a joined document finally—and with difficult to understand delay of not only months but years— recently concluded that PGT-A to this point, in all of its iterations and under all of its earlier names, has been unable to demonstrate any IVF cycle outcome benefits in unselected patients populations.2
And without clinical utility in general patient populations, why would anybody with just minimal common sense conclude that PGT-A—even theoretical—might work in older women? Women with advancing age, of course, produce progressively smaller numbers of eggs and embryos in IVF cycles, which, indeed, is one of two principal causes for declining pregnancy rates in IVF with advancing female age (the second cause is declining egg quality). Older women, based on biologically, but also mathematically, indisputable facts, therefore, must be among the worst candidates for routine utilization of PGT-A, even though some “garbage” studies have suggested that women above age 35 benefit from PGT-A.6
The problem with the stillexpanding use of PGT-A in IVF cycles is not only the procedure’s
complete lack of clinical utility (i.e., PGT-A not only fails to improve IVF outcomes), but by now overwhelming evidence suggests that, at least in some subpopulations, PGT-A actually reduces pregnancy and live birth chances in IVF. And older patients—for very obvious reasons—are, of course, among those subpopulations that are harmed by the procedure. And, though there are several very good reasons for that, we here will point out only the most obvious one because it is simply indisputable: Every false-positively “aneuploid” labelled embryos (which, therefore, is not used for transfer or is even discarded) reflects lost pregnancy chance!
That PGT-A produces an unacceptably high percentage of false-positive results has, as innumerable publications attest, been argued by the CHR against fierce opposition from PGT-A proponents since approximately 2007. By in 2015 reporting the first chromosomally normal offspring in the world after transfers of what then were reported to be “aneuploid” embryos that should have been discarded based on test laboratory recommendations (under current PGT-A practice some of these embryos might be classified as “mosaic”), the CHR’s arguments against routine PGT-A utilization were no longer disputable.
Rather than reconsidering the whole concept of PGT-A (in medical terminology called a “hypothesis”) of biopsying embryos

for chromosomal abnormalities, PGT-A proponents introduced the concept of “mosaicism” to their report repertoire. They rechristened the test under its current name, PGT-A, though without reassessing whether this test biologically, genetically, and mathematically really made any sense.
When a procedure has failed to demonstrate any utility for over 20 years and adds significant costs to already for many unaffordable IVF cycle costs, the only logical and ethically correct conclusion left is to abandon the use of the procedure, unless convincing indications exist for its use.
Refuting that any of the promised outcome benefits from PGT-A have been achieved, the recent ASRM/ SART document on the subject of PGT-A really offers a clear answer2 because, if a medical intervention does not fulfill its purpose and does not at least fulfill a compensatory outcome benefits, and when this procedure in different presentations and under different protocols and—on top of all of this—when this procedure in addition adds significant costs (in this case at least $5,000) to already for many unaffordable IVF cycle costs, the only logical as well as ethical conclusion left is to abandon the use of the procedure, unless convincing indications exist for its use (and nobody argues that there do not exist some of such rare indications).
Without further rehashing the history of PGT-A, obviously very closely related to the history of the CHR, only so much: PGT-A can, for several technical and biological reasons, have false-positive results.
One relatively late-recognized reason is self-correction of embryos, first reported in mice by British investigators and more recently reported by the CHR’s investigators in collaboration with colleagues in the Brivanlou Laboratory at Rockefeller University in NYC in human embryos. Many at blastocyststage by PGT-A as “aneuploid” diagnosed embryos—even though at that point correctly diagnosed as containing “aneuploid” cells (which means that an embryo is either “mosaic” or outright “aneuploid”), can, downstream from blastocyststage, still self-correct (i.e., expel “aneuploid” cells). Even a technically correct PGT-A diagnosis may, therefore, turn out to be biologically incorrect.
And, as a biologically incorrect diagnosis – if such an embryo is then deselected from embryo transfer – this deselection reduces a woman’s cumulative pregnancy chance in the IVF cycle in which this embryo was produced. It, therefore, once again, does not take a genius—or for that matter, large prospectively randomized studies—to figure all of this out. Yet, “garbage” papers, making all kinds of convoluted arguments in support of PGT-A utilization, still make it into the literature.
Further evidence why this paper should never have been published
So, this time it was F&S that fell for the scam involving a paper that claimed to have investigated “embryo transfer outcomes in women of advanced reproductive age.”1 And to make the study apparently more interesting, the researchers decided to restrict the
study population to only older women who at most produced three oocytes in a given IVF cycle.
Among 230 studied IVF PGT-A cycles, 49 (21,3%) were repeat IVF cycles, representing a first major protocol design error since repeat cycles in the same patients can lead to significant patient selection biases. Though the study alleges to have studied women of “advanced reproductive age,” astonishingly, all the manuscript reveals is that all women were above age 38 (no mean ± SD, median, or age range, and all of those, of course, greatly matter. In contrast, 309 patients received no PGT-A, and among those, 89 (28.8%) had more than one cycle (with, of course, the same concern about patient selection bias as in the PGT-A group).
Those two study groups of patients were, moreover, of course, not randomized, suggesting that there had to be reasons why all of these women either did or did not undergo PGT-A, very obviously creating further concerns about patient selection biases and confounding patient characteristics. Considering the relatively large number of women with repeat IVF cycles in the study, it also seems likely that at least some of them may have been in PGT-A as well as no PGT-A cycles. In other words, study design alone should have disqualified this paper from publication.
But there are significant other reasons as well: By restricting the study to women with maximally three retrieved oocytes, the authors are trying to give the impression that they were studying a relatively poor prognosis patient group with

abnormally low functional ovarian reserve (FOR). That could be a correct assumption, but only if the mean age was under 40 years. If the mean age were above 40, retrievals yielding only 1-3 oocytes would not necessarily be abnormal.
We, however, thought that we also had found a seemingly redeeming characteristic of the paper: The authors in the Materials and Methods section claimed to have assessed IVF cycle outcomes with reference points, cycle start (intent to treat), and egg retrieval (another valid reference point). Since many supposedly important PGT-A papers in the literature unfortunately, over the years reported IVF cycle outcomes with reference embryo transfer, a correct assessment of cycle outcomes would, of course, have been a positive brownie point for the paper because an intent to treat analysis includes all patients starting an IVF cycle, without selecting or deselecting specific patient subgroups. Cycle outcome analyses with reference embryo transfer, in contrast, select out good prognosis patients because they usually include patients who did not make it to embryo transfer and, therefore, artificially inflate outcomes.
But in proceeding with review of the paper and data analysis, it very quickly became apparent that the credit we had given to the investigators was seriously misplaced: They reported that PGT-A cycles as one would expect, of course, had lower embryo transfer rates; but the difference was truly astonishing: Only 14.1% of PGT-A cycles reached embryo transfer vs. 84.4.2% in controls (on a side note, in the abstract the latter
percentage was incorrectly listed as 73.2%). And beyond this point, the data analysis of the paper became outright deceptive because the paper reported a positive chemical pregnancy test in the PGT-A group in 71.4% of cycles (30/42, with 42 being the number of cycles that produced at least one transferable embryo and, therefore, reached transfer).
This, of course, very clearly reflects data manipulation and misdirection because the in Materials and Methods alleged reference points for analysis of IVF cycle outcomes were cycle start and retrieval, for which we above—obviously prematurely— lauded the authors. They now, however, replaced cycle start and retrieval with the incorrect and biased reference point—embryo transfer—as earlier noted, a very typical statistical “trick,” proponents of PGT-A have used in innumerable studies over the years. Every study with reference point embryo transfer obviously selects out the best prognosis patients because it takes at least one or more blastocysts to be included in IVF cycle outcome determinations. In contrast, poorer prognosis patients who do not produce transferable blastocysts just don’t exist in outcome determinations. The obvious, undisputable consequence is a significant exaggeration of outcome benefits for PGT-A, as so many pro-PGT-A studies have now claimed for over two decades, thereby misleading the IVF field.
The here discussed study demonstrates these facts, however, better than most other studies. Considering the huge discrepancy in the percentage of cycles reaching
embryo transfer (14.1% vs 84.4%). How this point was not recognized during peer review is, therefore, difficult to understand. Among non-PGT-A cycles 362/429 reaching embryo transfers, these cycles produced in reference to transfers only 62/362 pregnancies, resulting in a seemingly statistically highly significant outcome benefit of positive pregnancy test (in itself a very unreliable endpoint) of 71.4% (PGT-A cycles) and 17.1% (nonPGT-A cycles, P<0.001).
A correct data analysis of positive pregnancy test with reference point cycle start (i.e., intent to treat) should, however, of course have compared in PGT-A cycles 30 pregnancies out of 298 (10.1%) cycle starts to 62 pregnancies out of 429 cycle starts (14.5%), not only an inversion of reported outcomes, but suggestive of an almost 50% higher pregnancy rate for women not-undergoing PGT-A.


The same principles, of course, also apply to other more telling outcomes than chemical pregnancies, including clinical pregnancy rates, clinical pregnancy rates per retrieval, live birth rates per retrieval, live birth per number of retrieval cycles, and—requiring slightly different considerations— time to pregnancy (see Table to the right).
After multivariate logistic regression the paper claimed a significant advantage (P<0.001) for PGT-A in chemical pregnancies and clinical pregnancies, which we cannot agree with, marginally lower miscarriage rates per retrieval (P=0.038) and per pregnancy P=0.013) which may or may not be correct, a better live birth rate per ET—unquestionably—a spurious finding, and even the paper noted no difference between both cycle groups when it came to live births.
Considering this study’s results, the authors note that “PGT-A significantly improved clinical pregnancies and live birth rates per embryo transfer in women with advanced reproductive age and diminished ovarian reserve.” What the paper, however, failed to say is that pregnancy outcomes with reference to embryo transfer have a very different meaning than pregnancy outcomes with reference to cycle start (or even egg retrieval).
Consequently, the paper gives the false impression that PGT-A in older women improves IVF cycle outcomes. The correct interpretation of the paper’s data has to be that in highly selected (likely the rare cases of older women with especially good

ovarian reserve) older women may have an outcome advantage in time to pregnancy (and even that appears uncertain).
As already noted before, a conclusion that PG-A would improve pregnancy and live birth rates in older women is also completely counterintuitive to all logical and mathematical considerations. Nothing in this paper would, indeed, suggest such improvements. Alleged observations with great certainty can be attributed to patient selection biases, which are so obvious in this manuscript. It, for example, seems intuitive to assume that older women with unusually good ovarian reserve will see more frequent utilization of PGT-A than older women with low ovarian reserve.
The authors furthermore concluded that, “although the cost-effectiveness of PGT-A in this population requires further investigation, it offers a valuable tool for patient selection and may reduce the emotional toll of miscarriage for some patients.” We again must politely disagree, since we saw nothing in this study to suggest that it in any way contributed to patients’ selection of PGT-A. Even assuming a marginal non-significant reduction in
miscarriage rate with PGT-A to be valid—an in itself very questionable proposition—one must question whether such a minor effect warrants the wide utilization of PGT-A, which in the U.S. already involves over half of all IVF cycles.
A word about the accompanying Reflections article
We don’t know whether the two authors who wrote the accompanying Reflections article to this paper were also reviewers of the paper. Had they been among the reviewers, we, however, would not be surprised because their commentary deserves almost more criticism than the paper itself.8
Did you, for example, know that— as the Reflections article claimed— PGT-A significantly advanced the success of elective single embryo transfer (SET)? That is not our understanding, and neither apparently is that of the Practice Committees of ASRM and SART.2 But their commentary gets even more bizarre when they pointed out that in the SART registry, over 80% of women at a younger age (below 38 years) had an elective SET, yet above age 38 years, only 58%-70% all women had an elective SET. They then, without any

reference, attribute this difference (likely at least partially correctly) to increased use of cleavage-stage embryo transfers at advanced ages, especially with small embryo numbers.
The reason why they, at least partially, are likely correct is the fact that cleavage–stage embryo transfer, of course, for several reasons, makes sense in older women, not the least because there is no evidence for better IVF cycle outcomes after blastocyst-stage transfers of single embryos.9
But then their commentary gets really “crazy”—and we quote, “these patients are likely to benefit from PGT-A due to increased rates of aneuploidy and subsequent miscarriage. The delays caused by aneuploid pregnancy loss can significantly affect a patient’s ability to achieve their desired family size and may contribute to higher drop-out rate.” And where are they getting this information from? Haven’t ASRM/SART just finally summarized the real facts about PGT-A?
PGT-A, of course, does neither reduce miscarriage risk nor does PGT-A shorten time to pregnancy/ delivery.2
That they consider Ou et al.1 to “have provided valuable insights into a specific subpopulation often perceived as unsuitable candidates for PGT-A,” therefore, cannot really surprise. What, however, still does surprise over and over again is that F&S—having the likely largest editorial board of any medical and/ or science journal in the world— allows people who, frankly, don’t know what they are talking about to
publicly comment on a paper (and, possibly also review the paper for acceptance) in the primary journal of the ASRM, which for many REIs is the “bible” and often the only journal they at least glance at.
How many of our colleagues who, fortunately, up to this moment at least, didn’t take older patients’ embryos to the blastocyst stage (as it is, too many, not too few IVF cycles utilize extended embryo culture)10 will now change their practice pattern and force even older women into PGT-A?
After all, if F&S said so, why not?
Suffice to say that this is not only shameful, but to a degree “schizophrenic.” And we use this word not only because it was, of course, F&S that only a few months earlier had published the ASRM/ SART statement on PGT-A,2 but because the editor-in-chief of F&S, himself, only very recently was co-author of an Inkling article in F&S which very astutely asked the question why and how extended embryo culture to blastocyst-stage has become standard of care in IVF (see next discussed paper), with automatic extended embryo culture, of course, especially in older women making little sense.10
In short, two really bad papers, both clearly worth the WORST PAPER AWARD for this issue of the VOICE! And we are sorry to have spent so much paper on these two papers, but sometimes there is more to learn from bad than from good-quality papers.
Because of its subject, this article exceptionally offers and extended references listing with up to 5 authors and including titles of articles.
1. Ou Z, Liu N, Chen A, Li Q, et al. Effects of preimplantation genetic testing for aneuploidy on embryo transfer outcomes in women of advanced reproductive age with no more than three retrieved oocytes. Fertil Steil 2025;123(6):991-998
WORST PAPER AWARD in this issue
2. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy: a committee opinion. Fertil Steril 2024;122(3):421-434
3. Ro C, Leeming J. Buy the “BY”: Revealing the machinations of paper mills. Nature 2025;642:823826
4. Soriano JB, Ruano-Ravina A. The rising threat of predatory journals and paper mills in respiratory medicine and research. Lancet Resp Med 2025;13(6):E30-E31
5. Parker L, Boughton S, Bero L, Byrne JA> paper mill challenges: past present, and future. J Clin Epidem 2024;111549
6. Simopoulou M, Sfakianoudis
K,Maziotis E, Tsisoulou P, Sokratis G, et al., PGT-A: who and when? A systematic review and network meta-analysis of RCTs. J Assist Reprod Genet 2021;38(8):939-1957
7. Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, et al. Depletion of aneuploid cells in human embryos and gastruloids. Nat Cell Biol 2921;23(4):314321
8. Molinaro TA, Constantini-Ferrando M. :The birds, the bees, and the blastocysts.” Fertil Steril 2025;123(6): 1006
WORST PAPER AWARD in this issue.
9. Glujovsky D, Quinteiro-Ratamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cochrane Database Syst Rev 2022;(5):CD002118
10. Orvieto R, Barnhart KT. Extended embryo culture to the blastocyst stage in in vitro fertilization: should it be the standard of care? Fertil Steril 2025;124(1):37-39

Why is extended embryo culture to blastocyst stage widely considered standard of care?
We are here addressing yet another really poor paper regarding the practice of IVF, authored by Chinese investigators, and we are referring to the Chinese origin of this paper, not because we wish to disparage Chinese papers in general. To the contrary, Chinese investigators, of course, also publish outstanding papers (not the least the one addressed in the preceding commentary); but the medical literature—as we also discussed in these pages before— is unfortunately increasingly flooded by papers from China (and some other countries) often, unfortunately, produced by commercial paper mills. Editors of medical and science journals, therefore, have become more cautious about submissions from China, and so have we. The real reason for choosing this paper for a commentary was, however, different, and it was—at least this time—not our usual obsession with preimplantation genetic testing for aneuploidy (PGT-A), even though the paper nevertheless addresses PGT-A. But the issues discussed here, indeed, go beyond just PGT-A.
Three points must be made in that regard:
(i) Because trophectoderm biopsy only becomes feasible at the blastocyst stage of embryos, PGT-A biopsies of embryos are currently performed at that stage. Before and up to ca. the year 2016, when, after the CHR reported
the first chromosomally normal pregnancies following transfers of embryos by PGT-A called “aneuploid” embryos, our genetics colleagues felt they had no choice but to completely revamp what then was called preimplantation genetic screening (PGS). One crucial change was moving the embryo biopsy from the cleavage stage (day 3 after fertilization), allowing only for the removal of 1-2 cells from a usually 6- to 8-cell embryo, to the blastocyst stage, which allowed for an average of at least 5-6 trophectoderm cells. Another symbolically crucial step was the name change from PGS to PGT-A.
(ii) Most embryo arrests occur between day 3 after fertilization and the blastocyst stage at days 5-7. This therefore means that, if cycle outcomes are calculated with reference point embryo transfer, all arrested embryos are completely disregarded in IVF cycle outcome assessments from consideration. Moreover, reaching the blastocyst stage with at least one embryo becomes a precondition for being included in outcome assessments of IVF cycles.
(iii) The trophectoderm biopsy at the blastocyst stage is, however, also not yet the end of the story. That is only reached after the biopsy results are obtained, and a given embryo by PGT-A at the blastocyst stage was found to be transferable (i.e., in most cases this will require that the embryo be reported as “euploid”). In other words, since most non-euploid embryos are not cleared for transfer, these embryos also disappear from
statistical consideration in IVF cycle outcome assessments with reference point embryo transfer.
Reference point embryo transfer, therefore, means that only a fraction – and this number will vary dependent on the studied patient population—of initially started IVF cycles will still be in the game of cycle outcome assessment when an embryo transfer is – or is not—performed. Blastocyst-stage culture (in place of cleavage-stage culture) is, therefore, a temporary embryo selection procedure and so is, of course, PGT-A. In other words, all the embryos that, after blastocyst-stage culture and PGT-A analysis, are still in the game for embryo transfer (and, of course, the patients who produced them) are highly selected for usually being younger, healthier, and, in principle, more fertile.
Using embryo transfer as a reference point for cycle outcomes, therefore, offers real and reliable data, but only on relatively good prognosis embryos/patients, which in most IVF clinics represents only a relatively small minority of patients. Patients who are not successful in producing a transferable blastocyst-stage embryo, of course, have zero pregnancy chance because they didn’t make it to embryo transfer. The per embryo transfer data outcome reports simply don’t matter.
By reporting IVF cycle outcomes with reference to embryo transfer, a rather persistent mode of reporting in the infertility literature since (and including) the first paper ever suggesting outcome benefits in pregnancy rates and live births after

blastocyst-stage embryo transfers,1 has been an inappropriate transposition of outcome data from good-prognosis patients to general populations. And whenever this happens, outcome benefits end up highly exaggerated! And this is the ultimate reason why all IVF outcome studies must be analyzed by “intent to treat;” i.e., cycle start, with egg retrieval only being second best because reaching egg retrieval, to a degree, is also already a means of patient selection.
Which brings us to above aboveannounced second really poor paper in a row from China, which this time made it successfully through peer review at the JARG, though it should have been outright rejected. In this paper, the investigators alleged to have investigated pregnancy outcomes in IVF cycles of patients with unexplained recurrent pregnancy loss, with and without utilization of PGT-A.2
Since this was a retrospective study, PGT-A and non-PGT-A control cycles were, of course, not randomized and involved thaw cycles after embryos had been frozen (FETs). Because we here do not want to even start discussing the issue of repeated pregnancy loss and how it was in this paper defined, and should be defined (we have written about this in these pages on several occasions before), only so much: 316 IVF cycles in women with repeated pregnancy loss involved PGT-A and 359 did not. Apparently, all cycles involved universal embryo cryopreservation with delayed FETs after choosing the single embryo with the highest
morphologic score.
Women who underwent PGT-A had higher clinical pregnancy rates (P=0.002), ongoing pregnancy rates (P=0.001), and live birth rates (P=0.001). In women above age 38, PGT-A also (barely) reduced miscarriage rates (P=0.046).
And based on this study and these data, the authors concluded, and we quote: “PGT-A has been demonstrated to enhance clinical pregnancy rates, ongoing pregnancy rates, and live birth rates in patients experiencing unexplained recurrent miscarriages. Furthermore , the implementation of PGT-A significantly reduced the rate of early miscarriage among older patients aged 38 and above.”
So let’s summarize all the patient/ embryo selection steps that were involved here: (i) Patients given or not given PGT-A must, of course, have had reasons for that and, for example, better functional ovarian reserve is often the reason; (ii) As noted above, to be entered into the study, patients had to have at least 1 blastocyst-stage; (iii) Those undergoing PGT-A were further selected by only including allegedly “euploid” embryos (those not being offered PGT-A, of course, did not have this benefit, if PGT-A is even considered a benefit); and (iv) after all of these selection biases the P-value for reduction of miscarriages – which in a population of unexplained repeat aborters would appear to be the main goal of these treatments— barely scrapped significance at <0.046.
But even the HFEA (Human Fertilization and Embryology Authority) apparently does not know how to correctly report IVF outcomes
If there exists one governmentsponsored organization that over the years has acquired considerable credibility in overseeing the practice of reproductive medicine, including IVF, then it is the U.K.’s HFEA. Among several functions in the field, in a somewhat distant analogy to the CDC in the U.S., the organization is also charged with reporting British IVF clinic outcomes.
Because of HFEA’s credibility, it came as a shock to us when we became aware of an open letter, six prominent IVF practitioners in the U.K. recently sent to Peter Thompson, CEO of HFEA, in which they complained that HFEA


The letter specifically said the following: “Focusing on live births per embryo transferred, rather than per cycle started or per patient, omits key aspects of the treatment journey.” The letter then went on pointing out further details and consequences in such very clear language that we could not resist reprinting the whole letter here. So, here we go:
• Focusing on live births per embryo transferred, rather than per cycle started or per patient, omits key aspects of the treatment journey and, therefore, can unintentionally.
• Favor clinics that selectively transfer only high-quality embryos, often after multiple freeze-all cycles, where embryos of average quality are not even given a chance and discarded, thereby inflating apparent success rates.
• Allow exclusion of patients with poorer prognoses, including those whose cycles are cancelled or result in no embryos for transfer—particularly common in low ovarian reserve.
• Create space for clinics to implicitly attribute success to adjunctive treatments or addons (such as PGT-A, IVIG, or immune testing), when in fact their reported outcomes benefit from methodological artefacts rather than evidence-based efficacy.
The statement on the HFEA website that differences are due to ‘chance’ is misleading in this context. The data is being published from highly heterogeneous populations and practices. Clinics do not all treat the same mix of patients; they can (and do) differ in their:
• Patient age profiles.
• Use of donor gametes
• Use of PGT-A.
• Patient selection (poorer prognosis patients may be shifted into ‘natural IVF category, allowing for to remove of this group of patients from the denominator).
• Clinical protocols (multiple cycle banking for patients with poor reserve).
• Inclusion or exclusion of certain types of cycles in reported denominators.
To suggest that differences of a significant percentage points are simply ‘chance’ without mentioning that systematic biases in patient selection and reporting drive these differences is non-scientific and risks misleading patients. The mixing of different subpopulations into one’ success rate’ is a serious methodological flaw. Reporting an aggregate ‘live birth per embryo transfer’ without appropriate stratification:
• Rewards clinics that heavily filter patients pre-transfer.
• Hides poor performance in the stimulation, fertilization, or embryo development phases.
• Fails to account for the fact that many patients (especially older or poorer prognoses) never reach embryo transfer.
The current system ignores the fundamental problem of:
• Denominator manipulation— by excluding failed cycles and those who never reach embryo transfer, a clinic can appear to outperform others simply by being more selective.
The PGT-A disclaimer is insufficient and misleading. While
the website does mention that PGT-A may influence success rates, the current wording is not enough to prevent patients from perceiving that PGT-A clinics are ‘better’. Critically, it fails to mention that:
• Many patients are excluded from reported success rates entirely if their embryos are deemed ‘abnormal’ and no transfer occurs.
• PGT-A outcomes should be reported separately and transparently (as per the latest good practice recommendations), with success rates per cycle started or per patient, not just per embryo transfer.
Without full transparency on how many cycles never result in transfer, the success rates can appear artificially high. We urge the HFEA to consider the following immediate steps:
• The presentation of data in the current format needs to be suspended.
• Live birth rate needs to be reported per cycle started (multiple pregnancy rate still can be assessed in this setting
• Clearly separate PGT-A cycles and donor egg treatments in all outcome data, with transparent labelling by age group.
• Aim to develop reporting on the cumulative live birth rate per cycle started. While we understand that cumulative live birth rate per cycle started is a more complex metric to present, it remains the internationally accepted gold standard—already adopted by SART/CDC (USA), CARTR+ (Canada), ANZARD (Australia/ New Zealand), and endorsed by ICMART. These systems recognize that the patient journey begins with a cycle
start, not with an embryo transfer, and that fair reporting must reflect the full scope of that journey.
These concerns are not new. For well over a decade, clinicians and academics have pointed out that current reporting frameworks— including the continued reliance on embryo-based metrics—allow for distortion, selective exclusion, and confusion. Multiple studies and professional commentaries, including from members of our team, have outlined how such practices undermine the original goals of the HFE Act: to inform, protect, and empower patients. Yet despite these repeated calls, meaningful reform has lagged behind international best practices.
With the current update to CaFC, the Authority has a rare opportunity to address longstanding weaknesses and restore confidence in how outcomes are reported and understood. We also welcome clarity on the upcoming consultation process.
• Will clinics, patients, and independent experts be able to contribute formally?
• Will the consultation explicitly address the use and abuse of elective freeze-all cycles and multi-cycle commercial packages?
We note that the HFEA’s 2025–2028 Strategic Plan includes strong commitments to transparent, patient-centered information and to reducing inequalities in treatment outcomes. We are concerned, however, that the current CaFC metrics—in particular the continued focus on embryo-based success rates – may
not fully reflect those strategic priorities in practice. We hope the forthcoming consultation will be an opportunity to bridge this gap and bring CaFC into closer alignment with the Authority’s vision. We believe this is a critical moment. How the HFEA reports outcomes will influence not only patient choice, but also clinical practices and commercial models across the U.K. fertility sector. Inaccurate or overly simplified metrics carry real risk of harm and perpetuate inequity. Transparent, cycle-based reporting—underpinned by clear definitions and cumulative success data—is essential if CaFC is to become a truly trusted tool for patients.
Professor Dusko ILIC, MD PhD
Dr Julia Kopeika, MD PhD, FRCOG
Professor Yacoub Khalaf, MD, FRCOG
Tarek El-Toukhy, MSc MD MRCOG
Professor Caroline Mackie Ogilvie, BSc DPhil OBE
Professor Ying Cheong, MD, FRCOG
REFERENCES
1. Gardner et al., Fertil Steril 1998; 69(1):84-88
2. Xu et al., J Assist Reprod Genet 2025;42:1679-1687.
3. Ilic et al., https://www.progress.org.uk/ an-open-letter-to-the-hfea-aabout-choosea-fertility-clinic/
we predict IVF outcomes in women with abnormally low (functional) ovarian reserve?
a recent article published in Scientific Reports, 1 a relatively new general science journal from Nature Journals. And, unfortunately, this is again one of those very questionable reports, with a very logical basic question underlying the study and rather poor execution. The interesting question the study asked was, what are the chances of a patient with low functional ovarian reserve (FOR—in the literature also called diminished ovarian reserve, DOR). We like to differentiate between these two terms because low FOR defines age-adjusted expectations, while DOR is an age-independent assessment of remaining follicles in a woman’s ovaries.
Since at the CHR almost 100% of all patients—whether younger or older—have low FOR, we, of course, were attracted by the hypothesis of this study. The CHR has its prediction models, but the promise of this paper was not only to offer new predictors of outcomes in women with low FOR/DOR, but also to define thresholds. So, here is how the investigators presented their study: As already noted, they aimed to find independent predictors of IVF cycle outcomes in women with low FOR/DOR, specifically oocyte numbers, cleavage-stage embryos (day-3 after fertilization), clinical pregnancy rates during fresh embryo transfers, and blastocyst formation.

This is at least what Chinese investigators have claimed in
The study, however, was a retrospective analysis of 1,403 cycles in 1,039 women. As already in detail discussed in one of our earlier commentaries in this section of the VOICE just a few papers earlier (in the “unusual long review” commentary) involving

another Chinese IVF study, this paper, too, committed three major “crimes” in study design: First, every retrospective study introduces significant risk of patient selection biases because IVF cycles can vary remarkably, based on what individual IVF clinics consider to be best protocols for individual patients (and the study confirms that in materials and methods). The study population can, therefore, for example, be biased toward certain patient populations, certain treatments, or other variables, which, even in statistical adjustments, often can only be rather unsatisfactorily corrected.
The second “crime” is even more remarkable because it is so easily avoided, and that is the inclusion of repeat cycles in the same patients. Once again, this mistake opens the door to significant patient selection biases, if, for example, patients with a certain characteristic have more repeat cycles than other patients.
And the third “crime” may actually be the most consequential one, and that was how the study defined low FOR/DOR: Women had to meet two out of the three following criteria: (i) AMH, 1.1 ng/mL; (II) Antral Follicle Count <7; and (iii) basal FSH => 10.0IU/L. Because all of these parameters are, of course, age-dependent, and patient selection was not age-adjusted, the study has a major design problem. Simply based on these three “crimes”—all affecting basic patient selection for this study—one, therefore, must already question all in this study reported findings.
Among the 1,403 cycles (again, cycles—not patients!), 441
underwent fresh transfers (which, of course, must have had specific reasons in comparison to those who did not have fresh transfers!). And then univariate and multivariate logistic regressing analyses were performed to identify, through ROC curves, alleged factors influencing cycle outcomes.
Based on intent to treat (i.e., reference cycle start), among 1,403 cycle starts only 150 clinical pregnancies were observed (10.7%) in a rather young patients population with mean age of 33.2 in pregnant and 35.2 in non-pregnant women (P<0.001), mean AMH of 0.8 and 0.7 ng/mL (N.S.), mean AFC of 4.7 and 4.2 (P=0.005), and mean FSH of 10.3 and 10.8IU/L (N.S.), a quite disappointing outcome considering the patients’ young age and relatively mild degree of low FOR/DOR.
The authors concluded that AMH was the most effective independent predictor for oocyte retrieval. In contrast, AFC was most predictive for day-3 cleavage stage embryo numbers, with predictive thresholds being 0.345 ng/mL for AMH and 3.5 for AFC, respectively. They also claimed that day-3 top quality embryo counts in women under age 40 were more predictive of clinical pregnancy than age, and,believe it or not (satirically, of course), on day 3, available embryos were the sole predictors of viable blastocyst formation.
In short, we learned absolutely nothing new except maybe that our Chinese colleagues at this institution might benefit from a little coaching when it comes to the treatment of young women with
REFERENCE
1. Zeng et al., Sci Reports 2025;15:18875
Predicting embryo quality and IVF cycle outcomes based on the secretome of embryos
And if we are already talking about predicting cycle outcomes, let’s go to the next “fashion of the moment”: the embryo secretome into spent culture media. We, of course, are all (ironically speaking) anxiously waiting for a commercial product to buy that will allow us to select embryos based on testing spent culture medium. Aren’t an increasing number of IVF clinics already testing chromosomal abnormalities of embryos from culture media? And (again allowing irony to speak) if we can determine aneuploidy non-invasively from spent media, why should we not be able to also judge embryo quality?
As a recent Review article in Reproductive Biomedicine Online on the subject now, however, unfortunately suggests things are not as rosy as hoped for.1 Nevertheless, the article suggests that molecules, including, of course, cell-free DNA, but also mitochondrial DNA, small noncoding RNA, including microRNA and PIWI-interacting RNA, in combination with proteomes, metabolomes, microbiomes, and extracellular vesicles, “have emerged as important players in predicting IVF outcomes.”
There is only one problem with this statement: Nothing in the article, indeed, really, confirmed that any of

these tests has yet really emerged as an “important player in predicting embryo quality and IVF outcomes.” Maybe if the authors, instead, had stated that some of these tests “might” emerge as important players, we would have felt more sympathy for the effort put into this paper. But if even simple summary descriptions of findings are imprecisely worded, how can one trust the whole review?
1. Toporcerova et al., Reprod Biomed Online 2025; 51(1):104825
Further evidence for overutilization of ICSI in IVF
IVF-related papers in prestigious general medical journals like Nature Medicine are rare and usually have a special message. It may be exaggerated to say that a recent paper by Danish investigators sent a special message, but it certainly reemphasized a message which in recent years has gotten significantly louder in the infertility literature: The IVF field is overdoing it with ICSI (intracytoplasmic sperm injection).1
In a prospectively randomized study between 2019 and 2022, 824 women in their first IVF cycles were randomized in an openlabel, multicenter controlled trial across six public fertility clinics in Denmark: 414 had cycles with, and 410 without ICSI. Cumulative live births from these cycles were statistically not different and trended even in favor of no ICSI (43.2% vs. 47.3%). In the absence of any difference, the authors correctly concluded that ICSI should be
reserved only for more severe cases of male factor infertility.
As always, one, however, must be careful about not going too far and overinterpreting data. The authors astutely noted in their discussion, for example, that their conclusion applied only to patients under “their” current practice conditions, including routine extended embryo culture to blastocyst and elective single embryo transfer. By doing so, they acknowledge at least indirectly that the choice of ICSI is not only male-factor-dependent. Efforts to maximize fertility may also be based on oocyte factors. And in this context, it is also important to note that the relative importance of embryos increases with advancing female age and declining pregnancy chance per embryo. But altogether a worthwhile paper!
1. Berntsen et al., Nat Med 2025;31:19391948
This is what a recent paper by a Danish investigator in Human Reproduction of 78,284 men suggested when concluding that men with a total motile sperm count of >120 million could expect to live 2.7 years longer than men with a total motile sperm count of >0 and up to 5 million. Men with a total motile count of >120 million could expect to live 80.3 years, compared to 77.6 years among men with a total motile
count of >0 to 5 million. All semen parameters, moreover, were negatively associated with mortality in a dose-response manner both in the total population and the more recent subpopulation (P-trend for all semen parameters <0.001). Adjustment for educational levels and prior diagnoses did not change the estimates in the latter.1
This is a novel finding but makes evolutionary as well as biological sense and was in the journal accompanied by a very worthwhile to read commentary (without fancy name) which went into detail into possible explanations by an Australian colleague,2 which the paper, in itself, did not do to the same degree. We were especially intrigued by the discussion of the importance of the immune system, especially the differences between female and male, and strongly recommend both papers in combination.
1. Priskorn et al., Hum Reprod 2015;40(4):730-738
2. Aitken RJ. Hum Reprod 2025; 40(4):580-584
At least with bull semen, it works1 and that suggests it may also work with human semen, a recent short article in Scientific American suggested.2 Calling it “fertility physics,” the latter article is easier to understand than the original one that describes the test as “connecting droplet adhesion with sperm kinematics.” We, therefore, will use the simpler explanation: To reach the egg in a long journey from vagina through the uterus and

against tubal peristalsis through the complete length of the tube to reach the egg situated in the ampulla of the tube (where fertilization occurs), the sperm has to “wiggle” vigorously (in traditional semen analysis described as “motility”).
The new test uses basic physics to measure sperm activity and is promised to be simpler, less workintensive, and, therefore, cheaper. The ultimate plan is to develop this technique to allow for home semen analyses.
1. Shyam et al., Advanced Material Interface 2025;12(90:240680
2. Thompson J. Scientific American. June 2025.p18
Is zinc a key regulator of sperm function
This is a finding reported by Japanese investigators in a reviewed preprint in eLife. 1 More specifically, the authors suggest that zinc serves as a regulator of the spermspecific K+ channel, also called Slo3. As a reviewed preprint, the article is accompanied by an eLife assessment which describes the paper as “useful.” Still, it considers the evidence supporting the claim as of this point as “incomplete.”
Knowing the scientific excellence of most of our Japanese colleagues in reproductive biology, we are certain that they will quickly improve upon this preprint and, should they be able to confirm their findings, we may start to supplement males with abnormal semen analyses with zinc.
REFERENCES
1. Andriani et al., eLife 2025; https://doi. org/10.7554/eLife .105450.1.sa3
A sperm donor with rare genetic mutation that predisposes to cancer—10/67 of his offspring now have cancer
Presented publicly for the first time at the annual conference of the European Society of Human Genetics in Milan, Italy, in May, and reported by CNN Health, 1 a European sperm donor— apparently active throughout Europe—fathered at least 67 children. Among those, so far, 10 have been diagnosed with cancer.
Unknown to him and others, he carried a mutation in what the report called the TP53 gene. This gene is often also called the p53 gene and is a very important tumor suppressor gene that plays a very important role in suppressing cancer development by controlling cell growth, DNA repair, and apoptosis. Mutations of the gene are found in approximately half of all cancers.
Though the donor’s sperm was used all over Europe, he donated only in one sperm bank, symbolically named the European Sperm Bank, and had been tested beyond genetic minimum requirements for sperm donors, but those do not include testing for mutations in the TP53/ p53 gene. Neither Europe nor the U.S. currently has legal restrictions on how many children a sperm donor can father. The ASRM, however, recommends a limit of 25. ESHRE has no explicit limit but recommends that sperm banks set an appropriate limit.
The European Sperm Bank, of course, had no such limit, though,
through its VP of corporate communications, now announced that this will change.
1. Guy J. CNN Health. Updated May 27, 2025. https://www.cnn.com/2025/05/27/ health/genetic-mutation-sperm-donorscli-intl
A recent paper in Human Reproduction asked two important questions about endometriosis:1 (i) Are immunological diseases more frequent in patients with endometriosis? and (ii) in case this is, indeed, so, does a shared genetic basis contribute to the risk?
And the answer in a multicenter international group of investigators—unsurprisingly—was that endometriosis patients, indeed, demonstrate more autoimmune, autoinflammatory, and mixed pattern diseases (a finding known for decades). And a GWAS analysis did offer new details: Diseases with increased prevalence included rheumatoid arthritis, multiple sclerosis (MS), celiac diseases, osteoarthritis, and psoriasis, with genetic correlations between endometriosis and osteoarthritis, rheumatoid arthritis, and MS, and a potential even causal link to rheumatoid arthritis. The risk for developing a classical autoimmune disease was increased over controls by 30-80%. Endometriosis and osteoarthritis shared 3 genetic loci, and rheumatoid arthritis shares 1 locus with endometriosis.
Interestingly, the paper references a publication by the CHR’s medical Director and Chief Scientist, Norbert Gleicher, MD, in which he already 1987 asked the question whether endometriosis was not an autoimmune disease?2
Somewhat related, another recent paper suggested that diet can reduce endometriotic pain.3 And, considering that endometriosis is now widely accepted as a neuroinflammatory disease, why should that not be?
The right figure reports the number of survey respondents who in the study reported using diets or supplements. It is difficult to make much detailed sense from this study (even aside from the fact that survey studies are notoriously unreliable). Still, there are nevertheless a few interesting observations to be made: For example, that anti-inflammatory diets, reflected in no gluten and dairy intake, were the second and third most frequently taken steps are of interest and makes sense, though we must admit that the fact that alcohol was the most frequently excluded dietary component, was somewhat of a surprise.
Another paper in the same journal asked yet another question, which to a large degree has already been answered: Is endometriosis associated with the type and age of menopause? And once again, the answer is not much of a surprise:
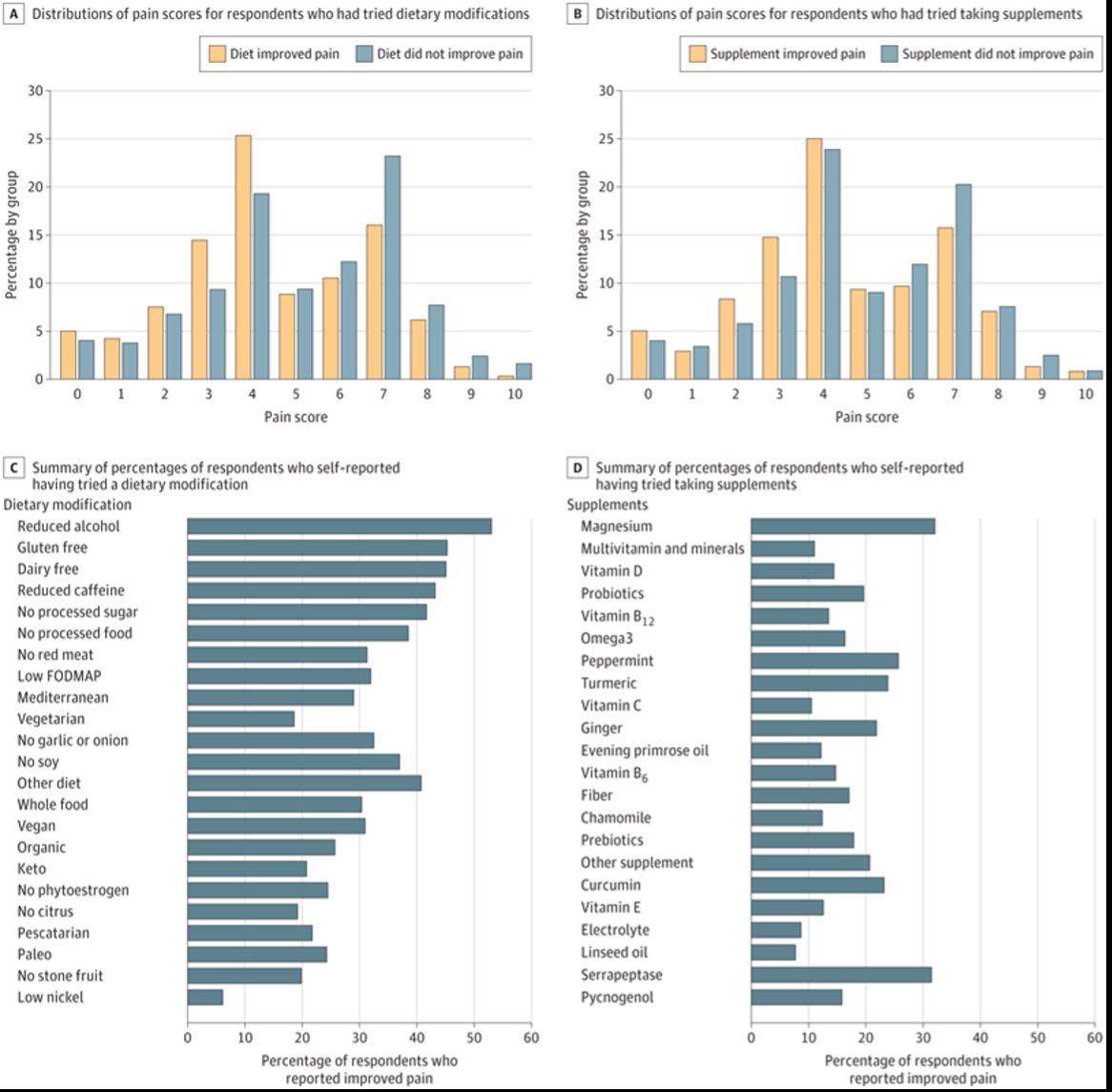
Women with endometriosis showed a 7-fold increase in surgical menopause and were more likely to experience both natural as well as surgical menopause.4 In contrast to the previous study, we, however, did not learn much new information from this one.
1. Shigesi et al., Hum Reprod 2025;40(6):1195-1209
2. Gleicher et al., Obstet Gynecol 1987;70:115-122
3. Heran-Yeates et al., JAMA Network Open 2025;8(3):e253152
4. Chung H-F, et al., Hum Reprod 2025;40(6):1210-1219

If you were not aware that PCOS affects the endometrium, you are probably not alone, since this has not been a very prominent subject in the literature. But a recent paper in Nature Medicine kind of rekindled the interest in the subject with the publication of a singlecell atlas of the endometrium, since it demonstrated that PCOS alters the endometrium’s cell composition. The consequences are disruptions of hormone signaling and impaired receptor signaling, negatively affecting receptivity of the endometrium, increasing miscarriage risks, and also increasing the risk for endometrial cancer.1

PCOS patients were overweight and obese, hyperandrogenic, and insulin resistance compared with controls of similar age, weight, and body mass index (i.e., they apparently did not include phenotype-D patients under Rotterdam criteria). Analysis of 5 control patients, 12 PCOS cases at baseline, and of 7 after 16 weeks of metformin treatment, and of 3 after lifestyle intervention revealed celltype-specific disease signatures and variations in cellular composition and localization. Samples taken after 16 weeks of metformin treatment and lifestyle management showed extensive recovery from their disease-specific endometrial signatures.
The authors were also able to link specific roles of each cell type to clinical features such as hyperandrogenism and insulin resistance, and the risk of endometrial and metabolic disease. In addition, potential therapeutic targets such as integrin inhibitors were identified, and the role of metformin in restoring endometrial health in patients with PCOS was highlighted.
This is an important paper since it informs on several new issues regarding PCOS: First, it reemphasizes the possible pathology cause by the condition in the endometrium, raising the question how to diagnose I whether it exists in a given patient and, second, it raises therapeutic questions, as, for example— should all PCOS patients receive metformin treatment?
Considering the damage PCOSaffected endometrial cells demonstrated in this study and
how well metformin remedied this damage, the answer may very well be a strong yes (of course, initially in the form of a clinical trial). But this is also the time to remember that this study did not include women with the lean PCOS phenotype (phenotype D) which differs from phenotypes A, B, and C in two typical ways: (i) it is not hyper-androgenic; and (ii) it is not associated with the metabolic syndrome at more advanced age but demonstrates a very strong association with evidence for a hyperactive immune systema and, therefore, with increased miscarriage risk.2 Metformin in these women, therefore, may not have the same beneficial effects.
That PCOS is a polygenetic inherited disease has, of course, been known for some time. Some candidate genes have, indeed, been described for obese and/or lean PCOS patients.3 The most potent variants were 4 unique variants in lean PCOS, 2 unique variants in obese PCOS, and 5 common variants in both. The study found that leptin signaling impairment and insulin resistance, as well as mutations in CYP1A1, CYP19A1, ESR1, AR, AMH, AdipoR1, NAMPT, NPY, PTEN, EGFR, and Akt, all play significant roles in PCOS in the studied group.
In parallel, the literature also reflected a familial male pattern of PCOS.4 Now, however, a paper in the JCEM for the first time reported that brothers of PCOS patients demonstrate a higher risk for developing hypertension, obesity, and type 2 diabetes, in other words of developing metabolic syndrome.5
Even more evidence that the genetic background to PCOS in women also exists in males.
1. Erikkson et al., Nat Med 2025;31:19251938
2. Gleicher et al., Biomedicine 2022;10(7):1505
3. Dhar S, Bhattacharjee P. Sci Rep 2024;14:24468
4. Di Guardo et al., Int J Fertil Steril 2020;14(2):79-83
5. Kanina et al., J Clin Endocrinol Metab 2025; dgaf121, https://doi.org/10.1210/ clinem/dgaf121
Japanese investigators published an interesting paper in Menopause, investigating the association between regular sexual activity and sexual function of women in menopause and the so-called genitourinary syndrome-related symptoms. And—can we say unsurprisingly—the study demonstrated an association between regular sexual activity and low prevalence of symptoms relating to the syndrome. In other words, “use it or lose it” applies here as well!
1. Yoshikazu et al., Menopause 2025;32(7):592-600


The beginning of in-vivo gene editing—the beginning of a new phase in medicine
We recently witnessed the beginning of a new period in medicine with the announcement of the first ever personalized geneediting case in which an infant with carbamoyl-phosphate synthetase 1(CPS1) deficiency was treated at roughly age of seven months,1 rightly in a Science Behind the Study article in the same issue of the journal described as a milestone in the evolution of personalized therapies for rare and ultra rare inborn errors of metabolism.2
CPS1 is recessively inherited and is exceedingly rare (estimates range between 1/1,300,000 and 1/800,00 births). It affects CPS1, a mitochondrial enzyme that catalyzes the first and ratelimiting step of the urea cycle, the conversion (primarily in hepatocytes) of ammonia and bicarbonate to carbamoyl phosphate. If dysfunctional, ammonia accumulates in the blood, which is toxic to the brain, leading to coma and death shortly after birth in ca. 50% of cases if left untreated.2
Here, the investigators immediately after birth of the infant started developing a so-called “baseeditor” which can correct diseasecausing abnormal genetic variants, in this case, a customized lipid nanoparticle delivering base editing therapy, of which the infant received two infusions. Already
in the seventh week after the first infusion, the infant was able to tolerate an increased amount of dietary protein (and reduced dose of nitrogen-scavenger medication, the usual treatment, to half of its usual dose).
Authors as well as commentators, of course, pointed out the preliminary nature of these results, but nobody can argue with the fact that a new phase in medicine has started.
1. Musunuru et al., N Engl J Med 2025;392(22):2235-2243
2. Gropman AL, Komor AC. N Engl J Med 2025;392(22):2273-2276
The increasing subtle marketing of polygenic embryo scoring (PGT-P) in association with IVF and the mysteriously disappearing strongly negative opinions of ESHRE and ASRM
Where have the original strongnegative opinions of ESHRE and ASRM about polygenic risk scoring (PGT) vanished to?
The subtle marketing of PGT-P in association with IVF has begun, and ESHRE, as well as ASRM, in association with PGT-A, follow their usual policy of deniable ignorance.
We, of course, have by now often enough written about polygenic embryo scoring (or screening)— also given the acronym PGT-P— so that most of our readers already know what this new testing of embryos is supposed to accomplish. But—just in case a
refresher is needed—here it is, as short as possible: PGT-P must be differentiated from PGT-M and PGT-T, though all three of these PGTs require that an embryo be biopsied at the blastocyst stage. PGT-M is the oldest and most reliable test since it checks for the presence of a so-called monogenic disease (i.e., a disease cause by a single mutation in a gene; therefore: -M). Examples are sickle cell disease, Tay–Sachs, etc.
Everybody, of course, knows that PGT-A means testing of embryos for abnormalities in the count of their chromosomes (i.e., for aneuploidy, therefore: -A) Everybody by now, of course, also knows that—even though especially in the U.S. very widely utilized in IVF—this test has become very controversial and even ASRM and SART in a combined statement just a few months ago concluded that PGT-A does not offer any outcome benefits for IVF,1 as the genetic testing industry incorrectly still claims. It, of course, is also no secret that the CHR has been opposing the routine utilization of PGT-A not only because it does not improve IVF outcomes as has been incorrectly alleged for over 20 years, but because PGT-A for many subgroups of patients will actually reduce pregnancy and live birth chances, while adding significant costs to an already too expensive IVF cycle.2-3
And now the genetic testing industry (and its proponents and investors) have started preparing us for the next embryo testing excess, PGT-P. And this time we are not alone in categorically—yes, in this case, and of this moment, really categorically—rejecting

the use of PGT-P. The reasons are very obvious: Testing for socalled polygenic genetic risk (i.e., for genetic risk, not caused by a single, but by many different genetic mutations on different chromosomes, as, for example, in heart disease, diabetes, etc.) does not even work well enough yet in adults to be used clinically. Therefore, very obviously, it simply cannot be considered to work reliably in human embryos, where absolutely no short-, intermediate-, or even long-term follow-up studies exist.
And then there is a second major concern: When a risk is inherited through multiple genes, evolution usually establishes this genetic predisposition for a biological purpose that enhances the survival chances of the species. Excluding one specific polygenic pattern may—relatively—enhance other patterns, some of which may also be undesirable.
And a third major concern may be the most important among them all: PGT-P has already, for years, been advertised to, for example, select embryos for eye color (can you imagine in an IVF cycle to discard embryos because of their alleged unwanted eye color?). How about selecting for basketball talent like Michael Jordan, or higher IQ, etc.? In other words, we are starting to get into ethically very questionable territories when starting to pursue polygenic patterns to genetically “enhance” our offspring.”
And then something very interesting has been happening regarding how professional societies have come to view PGT-P.
Professional genetic societies on both sides of the Atlantic came out in quite strong words against the clinical utilization of PGT-P. The European Society for Human Genetics (ESHG), indeed, in 2021 concluded that the use of PGT-P was not only clinically currently unproven but also unethical⁶— only to be attacked by a group of mostly U.S. geneticists with very obvious commercial interests in the performance of PGT-P, claiming “scientific consensus and potential utility of this new technology”⁷— all, of course, pure fantasy.
The American Society for Human Genetics (ASHG) acknowledged the potential of PGT-P to inform reproductive decisions but also highlighted the need for careful consideration of its ethical and social implications. It also noted that PGT-P is not expected to enhance IVF success rates, and its clinical utility is still being evaluated. The ASHG also acknowledged that clinical validation studies are still needed to assess the effectiveness of PGT-P and that more research is needed to understand the complex interplay between genetics and the environment in the development of complex diseases.8
ESHRE fully and completely endorsed the above-noted ESHG statement,9 only to— quite recently—withdraw this endorsement and replace it with a statement on its website that ESHRE currently does not have a formal opinion on the utilization of PGT-P.10
What, however, happened at ASRM was even more interesting because for the longest time, ASRM had
remained mum on the subject of PGT-P. But then, as is routine before the publication of new policy statements of ASRM and SART, several months ago, ASRM/SART circulated for comments among its membership a confidential draft under the title, “Use of preimplantation genetic testing for polygenic disorders (PGT-P), an Ethics Committee opinion.” Interestingly, it was the opinion of the Ethics and not of the Practice committees!
Because it was mailed out with a request for confidentiality, we do not feel at liberty to describe the conclusions of the committee. We can only say so much about this document: Considering the decades-long hesitancy of ASRM to confront the useless practice of PGT-A, the CHR was very skeptical when receiving the document regarding PGT-P. We, however, were not only very pleased with all the conclusions of the document but were-frankly, surprised because they fully agreed with the CHR’s thinking about PGT-P.
And what happened next? ASRM went mum again and has not—as usually happens—several weeks to months after a policy is circulated— announced publication of a final version of the Ethics Committees’ opinions and, upon visiting the ASRM’s website, one suddenly— and very informally—finds a very different ASRM Opinion, listing ethical and practical concerns but no longer the very clear opinions originally expressed in the circulating document. Final recommendations per website currently are as follows:11
The ASRM emphasizes the need

for additional clinical validation studies to assess the effectiveness of PGT-P.
It also highlights the importance of ensuring equitable access to PGT-P and providing comprehensive genetic counseling to patients.
Furthermore, the committee recommends that PGT-P should only be offered after careful consideration of the ethical implications and potential unintended consequences. We, overall, again considering the ASRM’s obvious hesitance to take on the genetic testing industry as displayed in regard to PGT-A, consider this to be a quite acceptable set of recommendations, but, in comparison to the document that was circulated and now apparently has gone “puff,” it’s pure political correctness.
Quite obviously, ESHRE as well as ASRM must have heard from representatives of the genetic testing industry, and that is now the biggest industry in the exhibition spaces of both societies’ annual meetings, and with great likelihood also the biggest sponsors of both societies in general. And if they are unhappy, there is, of course risk for both societies to become unhappy as well! And they see PGT-P as the next big cash cow in association with IVF practice.
Who then can be surprised that the genetic testing industry and its financiers have actually already started to actively pursue PGT-P? Some laboratories and IVF clinics do this openly by advertising PGT-P services. According to Google, in NYC alone, at least three
clinics already offer PGT-P.12
Yet others do it more subtly, and nobody is as talented in selling new “products” in the field of assisted reproduction as some of our Spanish colleagues. One just has to think back to the first embryoscope and all the promises about improved IVF cycle outcomes, or how about all the representations made for endometrial receptivity testing. Those tests were good enough to build a corporate behemoth, now offering laboratory services all over the world.
Which brings us finally, after a very long introduction, to the reason for this commentary, which was an Opinion Article in Human Reproduction by Italian and Spanish colleagues, which started the selling job for PGT-P with exquisitely brilliant subtlety by—seemingly just raising concerns about the psychological burden poor parents now have to carry, “facing complex decisions based on probabilistic risk scores, requiring them to weigh uncertain benefits against potential harm.”13
Even though not even with a single word encouraging PGT-P, the authors brilliantly “normalize” the use of PGT-P because, sublimely, the article, of course, suggests that—if we have to be concerned about the patients’ psychological counseling, they must already stand in line to get PGT-P. Simply brilliant marketing!
1. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy: a committee opinion. Fertil Steril 2024;122(3):421-434
2. Gleicher et al., Hum Reprod 2022;37(12):2730-2734
Gleicher N, Barad DH, et al. Trends Mol Med. 2021;27(8):731-742
4. Houser K. Futurism. October 3, 2018. https://futurism.com/the-byte/eye-colordesigner-baby
5. Sussman AL. I.V.F., Gene Selection and Embryo Screening: Is This the Future of Making Babies? The New York Times. April 1, 2025. https://www.nytimes.com/ interactive/2025/04/01/opinion/ivf-geneselection-fertility.html
6. Forzanto et al., Eur J Hum Genetics 2022;30:493-495
7. Tellier LCAM et al., Eur J Hum Genetics 2023;31:278
8. American Society of Human Genetics. New Guidance on the Challenges of Polygenic Scores in Human Genetic Research. Published December 15, 2022. https://www.ashg.org/advocacy/statementarchive/new-guidance-on-the-challengesof-polygenic-scores-in-human-geneticresearch/
9. ESHRE. https://www.eshre.eu/Europe/ Position-statements/PRS
10. ESHRE. https://www.eshre.eu/ Guidelines-and-Legal/Guidelines/PGT
11. Roura-Monllor JA, Walker Z, Reynolds JM, et al. Promises and pitfalls of preimplantation genetic testing for polygenic disorders: a narrative review. F&S Reviews 2025;6(1):100085. doi:10.1016/j.xfnr.2024.100085
12. https://www.google.com/search?q=La boratories+and+IVF+clinics+that+offer+P GT-P&rlz=1C5CHFA_enUS1083US1083& oq=Laboratories+and+IVF+clinics+that+ offer+PGT-P&gs_
13. Forte et al., Hum Reprod 2025;40(6):977-982
BRCA1 and BRCA2 effects on ovarian reserve
Even though they did not give the CHR credit in referencing earlier work on the subject at the CHR,1 we want to give colleagues from Brown University in Providence, RI, credit for demonstrating in a Research Letter in Fertility and Sterility once again the fact that women with BRCA1 and BRCA2 mutations demonstrate lower functional

ovarian reserve than controls.2 Moreover, their finding also suggest that DNA damage may be the cause. Interestingly, they found similar effects with BRCA1 as well as BRCA2, while we note this effect only in BRCA1 patients.
Fortunately, BRCA mutations are relatively rare, but when they occur, one should be aware of this fact. Moreover, evidence for premature ovarian aging (POA) at a young age should be a reason for a BRCA evaluation. Since BRCA testing no longer carries with itself any longer the horrible expenses patients had to accept until several years ago had to accept, testing in such cases should be routine, especially with breast and some other cancer histories in the family.
Now one reads increasingly that especially BRCA1 testing should become part of prenatal carrier screening in obstetrical care, which, of course, also would mean in the routine first testing round in fertility clinics.3
1. Oktay et al., J Clin Onc 2009;27(15suppl):11039
2. Ou et al., Fertil Steril 2025;123(5):899901
3. Dioun et al., Am J Obstet Gynecol 2024;231(3):330e1-330e14
4. New York-Presbyterian. August 6, 2025. https://www.nyp.org/advances/article/thecost-benefits-of-performing-brca1-testingas-part-of-prenatal-carrier-screening
The European Alliance of Associations for Rheumatology (EULAR) declared for the first time that all tumor necrosis factor (TNF) inhibitors as safe throughout pregnancy and lactation.1 And this obviously also means that TNF inhibitors can be used in women receiving fertility treatments. The statement, moreover, summarized the current status of “allowed” antirheumatic drugs in general. This means that, in addition to TNFs, synthetic disease-modifying antirheumatic drugs (DMARDs) compatible with pregnancy include antimalarials, azathioprine, colchicine, cyclosporine, sulfasalazine, and tacrolimus. Regarding nonsteroidal antiinflammatory drugs (NSAIDs) and glucocorticoids, a more restrictive approach to their use during pregnancy is recommended (which we are not certain makes sense).
According to Medscape Medical News, the new recommendations reflect the increasing recognition of the toxicity of chronic, higherdose glucocorticoids, including during pregnancy.2 Synthetic DMARDS (csDMARDS), azathioprine or mercaptopurine, chloroquine, colchicine, cyclosporine, hydroxychloroquine, sulfasalazine, and tacrolimus are all compatible with pregnancy. The teratogenic medications methotrexate, mycophenolate, and cyclophosphamide should be stopped prior to conception. Nonsteroidal anti-inflammatory
drugs (NSAIDs) can be considered during pregnancy to control disease activity, but they should be used intermittently and stopped after 28 weeks of gestation. NSAIDs with a shorter half-life, like ibuprofen, are preferred. If a patient is having difficulty conceiving, discontinuing NSAIDs should be considered. Corticosteroids should be tapered to a 5 mg daily dose or lower or stopped entirely, if possible. For more details, we recommend Reference 1.
But to document the flimsiness of many rheumatology studies on which these new recommendations are based, here is another recently published, very related paper in the same medical journal, which recently reported that TNF inhibitors in a human study “improved pregnancy outcomes” in women with the so-called antiphospholipid syndrome (APS).3 And before we go into further detail, we have to acknowledge a longstanding bias when it comes to reports on the APS, not the least because of the many changes in defining this alleged syndrome over the decades since the syndrome was first described in principle, being defined by excessive thrombotic risk and increased miscarriage risk.4
In this recent multicenter study involving perinatologists as well as rheumatologists with decades-long interest (and many publications) in the APS, certolizumab pegol was used in women with APS and positive lupus anticoagulant. It was

administered during gestational weeks 8 through 28, in addition to standard treatment (which, of course, varied). The primary adverse obstetrical outcome for the study was a composite of fetal death ≥10 weeks’ gestation or preeclampsia with severe features or placental insufficiency requiring delivery <34 weeks’ gestation. As already noted in the commentary on the preceding paper, we do not like composite outcomes and, here new we even less like/alternative outcomes.
But our dislike of the study design even increased further based on the power analysis underlying the study, which was determined to require 45 patients, demonstrating any of the composite adverse pregnancy outcome(s) at 20% prevalence (95% CL, 9.6%-34.6%) with certolizumab versus (note !!!!) a historical control rate of 40% (in other words, this was not a prospectively randomized study but kind of a case control study, with all the limitations and potential biases of such a study). Moreover, practically speaking, one, especially considering the composite outcome, has to wonder how clinically significant a 20% vs 40% rate in 45 patients (9 vs 18 patients) really is.
And then not 45 but 51 patients were enrolled (one wonders why and when the decision was made?), of which 9 (17.6%) had so-called primary adverse pregnancy outcomes; and 6 patients were excluded because of pregnancy loss <10 weeks or because of presumed genetic pregnancy loss.
Miraculously, that brought the real number of study subjects back to
the original 45, but emphasizes yet another bias in too many published studies on the subject of miscarriages: a diagnosis of an aneuploid karyotype in the products of conception does not automatically mean that the aneuploidy was causal. For example, trisomies 13, 15, 18, and 21 are known to survive. Finding one of these karyotypes in the products of conception of a miscarriage does not mean that the aneuploidy was the cause of the miscarriage.
Moreover, all of these machinations, of course, changed the prevalence of primary adverse pregnancy outcome from 17.6% to 20.0%, thereby (and what a miracle, barely) meeting the originally defined criteria of significant difference between (new after treatment) 20% and (historical) 40% primary adverse outcome(s). Unsurprisingly, the authors concluded that “certolizumab appears effective in preventing placenta-mediated adverse outcomes in high-risk patients with APS.”
Our conclusion, in contrast, is that, even considering the unfortunately poor quality of increasing numbers of papers in even respected medical journals, it, indeed, is rare to see a study where patient selection appears as obviously manipulated as in this paper! What makes things, however, even worse, is that this paper was not the product of a “paper mill” somewhere in Asia but includes as authors some of the most respected “experts” regarding the APS in pregnancy from both perinatal and rheumatology medical specialties. And this is yet another good reason for awarding
this paper with a WORST PAPER AWARD for this issue of the VOICE
One more point: For those who weren’t, some more information on the APD, we recommend a recently published review article.4
This drug—originally discovered as an antimalarial—was later found to have excellent general anti-inflammatory effects and has become a mainstay of rheumatology treatments, especially for systemic lupus erythematosus (SLE). Now, a Swedish study claimed that the drug in SLE patients also lowers preeclampsia risk.5

The study involved a total 959, 404 (42%) nulliparous pregnancies, of which 232 (57%) were unexposed and 172 (43%) were hydroxychloroquine-exposed; 555 (58%) were parous pregnancies, with 333 (60%) unexposed and 222 (40%) exposed.
Preeclampsia was recorded in 19 (11%) of 172 hydroxychloroquineexposed pregnancies and 30 (13%) of 232 unexposed pregnancies. Summarizing their findings, the authors concluded that in women with SLE, hydroxychloroquine exposure in early pregnancy was associated with a lower risk. At the same time, any association

with pre-term delivery remained unclear.
Though a decline in preeclampsia risk with Plaquenil treatment makes logical sense, one would expect, in parallel, also a decline in premature deliveries, which is a constant finding in practically all autoimmune diseases.6 As some of the preeclampsia-related data analysis is also not very clear in this paper, we, frankly, would not put too much credibility behind this publication.
Familial mediterranean fever (FMF)
Surprisingly, even many physicians are not aware that FMF is a monogenic autoinflammatory disease. It, indeed, is the most common monogenic autoinflammatory disease and—as the name indicates— is most prevalent around the Mediterranean Sea and in Middle Eastern countries. Therefore, it should not surprise that the EULAR recently also published recommendations regarding treatment resistance and fertility concerns with FMF.7
Without going into too much detail (for that, we recommend Reference 7), first-line medication is still colchicine during conception, pregnancy, and breastfeeding. The literature review—again unsurprisingly—discovered a trend toward preterm birth, which, as noted above, is characteristic of autoimmune diseases.6 Effects of colchicine on semen analyses require further investigation.
1. Rüegg et al., Ann Rheum Dis 2025; 84(6):P910-926
2. Hicks L. Medscape Medical News May 7, 2025; https://www.medscape. com/viewarticle/eular-task-forceexpands-pregnancy-safe-drug-options2025a1000b1a
3. Branch et al. Ann Rheum Dis 2025;84(6):1011-1022; WORST PAPER AWARD for this issue
4. Murvai, et al., BMC Pregnancy & Childbirth 2025;25:337
5. Nguyen et al. Lancet Rheumatol. 2025. doi:10.1016/S2665-9913(25)00076-1. Published online ahead of print.
6. Gleicher N. Clin Rev Allergy Immunol 2010;39(3):194-206
7. Ozen et al., Ann Rheum Dis. 3035;84:899-909
Learning something new— activation of the vagus controls inflammation
We have not written about the vagus nerve in these pages until this issue in years, and now we are doing it here for the second time in the same issue. The vagal—also called 10th cranial
nerve—nerve is the longest and most complex nerve of the autonomic nervous system and has sensory as well a motor and sympathetic and parasympathetic fibers. Stimulation of the vagus leads to a large variety of generally positive effects on health, from decreasing stress and anxiety, improving digestive issues (as previously discussed in this issue in the nutrition section), enhancing sleep, and—yes—reducing inflammation.1
Here is a little bit of what, for a change, traditional Chinese medicine teaches us about this very important nerve (see Figure below): We can activate the nerve ourselves by:
• Deep/slow belly breathing
• ‘OM’ Chanting
• Cold water face immersion after exercise
• Filling the mouth with saliva and submerging your tongue to trigger a hyper-relaxing vagal response
• Loud gargling with water
• Loud singing
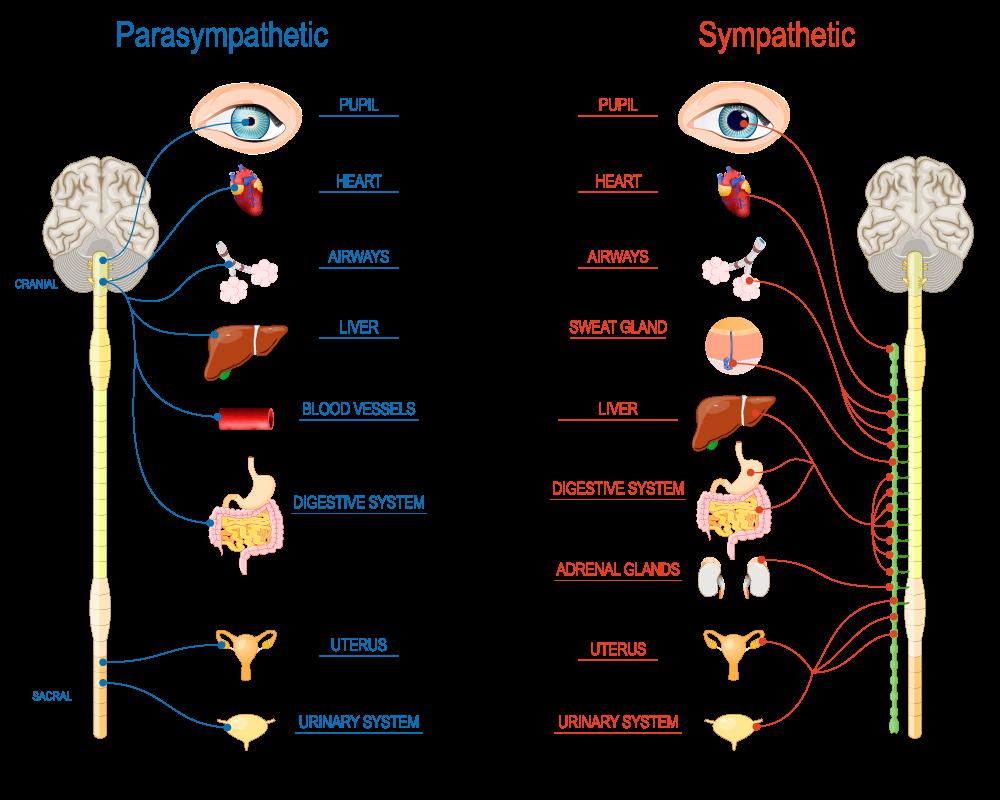

REFERENCE
1. Dolgin E. Science 2025;:594
What does the age at menarche have to do with inflammation?
And here is something surprising: U.S. investigators recently reported in JCEM that earlier age at menarche is associated with unfavorable inflammatory (and glucose) metabolic biomarker profiles in a nationally representative sample of adult women (without diabetes). These findings were especially pronounced among non-smokers.
Specifically, earlier menarcheal age was associated with higher CRP levels (P=0.006), fasting glucose, fasting insulin, and ferritin. The authors interpreted the data to mean that glucose intolerance with early menarche may be an inflammatory process.
REFERENCE
1. Santos et al., J Clin Endocrinol Metab 2025;110:1365-1374
What does the immune system have to do with ovarian physiology?
Increasingly more, it seems, as we are increasingly getting little bits of new information, and Belgian investigators have now summarized some of those data in a Review article in Human Reproduction. 1 Overall, progress is slow, indeed, embarrassing slow in comparison to other organs in the body. Also, the authors apparently did not have an immunological background because it becomes at times quite obvious that they
lack some very basic knowledge of (immunological) facts. They, for example, claim that autoimmune oophoritis can be the consequence of immune system activation against various ovarian antigens. This is categorically wrong since, after decades of searching, there is only one such antigen known, and it causes autoimmune oophoritis only in women with Addison’s disease. Or when they correctly note that—what ESHRE describes POI (oligo-amenorrhea for at least 4 months with FSH over 25.0 mIU/mL, close to what the CHR call premature ovarian aging, POA), while the U.S. considers POI to stand for primary ovarian insufficiency, characterized by amenorrhea, FSH >40.0mIU/, L, before age 40 is associated with many other autoimmune disease. While that is a very correct statement, to claim that the first autoimmune condition among those is the incredibly rare autoimmune polyglandular syndromes type 1 and 2(APS-1 and 2) is kind of ridiculous.
It is also quite remarkable that no mention is made of the fact that adrenals and ovaries stem from the same primordium, which must have immunological consequences.
The whole article kind of reads like a medical student’s thesis, though it does present a small number of recent discoveries.
REFERENCE
1. Cacciottola et al., Hum Reprod 2025;40(1):12-22
The CDC no longer recommends the COVID-19 vaccine during pregnancy and, therefore, by implication during infertility treatments, and during breast feeding—and we disagree!
So here we are: As the JAMA on July 11 in a News article by Rita Rubin, lead senior staff writer for Medical News at JAMA, put straight into the headline, “The CDC No Longer Recommends COVID-19 Shots During Pregnancy—Now What?”
And here is our answer!
As the article noted, Robert F. Kennedy Jr, U.S. Department of Health and Human Services Secretary, on May 27 announced in a 58-second-long video posted on X.com (formerly Twitter) that he had withdrawn the CDC’s recommendation for vaccination of healthy children and pregnant women from the recommended immunization schedule of the CDC. She also noted that Kennedy, in this short video, was flanked by the heads of NIH and FDA, but by nobody from the CDC, the obviously responsible federal agency for vaccine recommendations. Interestingly, just days earlier, the head of the FDA and co-workers had included current and recent pregnancy as risk factors for Long COVID.
While we fully understand why healthy children don’t need these vaccines—they only develop

mild disease—we completely fail to understand the rationale for recommending against vaccinations before and during pregnancy.
That pregnancy, like the regular flu, represents a higher risk circumstance for more severe COVID, is indisputable and has been repeatedly reported in these pages as a strong reason for a vaccination recommendation for every woman planning on conceiving or already pregnant. Similarly, evidence for passive transfer of maternal immunity to offspring also supports prenatal and in-utero vaccination against COVID. The CHR, therefore, cannot agree with the policy change announced by Kennedy and maintains, as of this moment, its recommendation for preemptive maternal vaccination against COVID as well as the seasonal flu.
It will be interesting to see how insurance companies will react to this edict. It, of course, offers an almost ideal opportunity to refuse coverage for the vaccines. And, obviously, the government will no longer cover the expense. For further details, we refer to the JAMA article by Rubin.1
It does not happen often that the CHR would agree with an Opinion article in JAMA—or, for that matter, any other medical journal— that contradicts government guidelines; but, just as we have during the COVID pandemic disagreed with several government policies, so we find ourselves this time again in opposition. The reason is obvious: there is no place for ideology in medical decisionmaking. It always must be factbased following best—at the time—
available evidence. And until we see such evidence for the updated CDC position, we will continue to recommend to our patients vaccinations against COVID and the relevant flu strains if they are planning on pregnancy or already are pregnant.
This, of course, does not mean a vaccination mandate for COVID and/or influenza at the CHR. Very much to the contrary, it is a continuation of our aversion to mandates for patients.
The CHR never mandates anything because nobody at the CHR feels qualified to tell other people how to live their lives. But patients will rarely find in the infertility field providers who are as well qualified as the CHR’s physicians in making treatment recommendations.
REFERENCE
1. Rubin R. JAMA 2025; doi: 10.1001/ jama.2025.11889. Online ahead of print.
Surprising and totally unexpected data about herpes zoster vaccinations
This is really surprising and totally unexpected news, but—based on a study in Wales—it appears that getting a herpes zoster vaccination reduces the risk for dementia.1 The data, indeed, are surprisingly strong and suggest a causal connection because they were, more or less, a natural experiment based on the date-of-birth eligibility threshold for the vaccine in Wales (November 2, 1936). This created likely very similar comparison groups born shortly before and after the date-ofbirth eligibility threshold, with the one difference of having received the vaccine or not.1
Those born after the eligibility date demonstrated decreased probability of having a new dementia diagnosis during 7.4 years by 1.8% (P=0.01). Other chronic conditions were not affected by the vaccine.
The observed effect is obviously quite small, but clearly at a significant level and, therefore, raises several interesting questions, among those, why and how this vaccination may be causing such an effect? It, moreover, raises the additional question, whether other vaccines may have similar effects?
REFERENCE
1. Pomirchy et al., JAMA 2025;333(23):2083-2092
The surging measles epidemic
According to a report in The Daily Beast1 and in many other news outlets as well as medical publications, measles cases in the U.S. have reached a 33-year high. As of July 7, the Johns Hopkins University Center for Outbreak Response Innovation (CORI), the country by that date had experienced 1,277 confirmed cases across 38 states, very likely a significant understatement.
The U.S. has been measles-free since the year 2000, and only three people died of complications of the disease between 2001 and 2024.
REFERENCE
1. Kimmins L. The Daily Beast. July 7, 2025. https://www.thedailybeast.com/ american-measles-cases-just-broke-adark-record-as-outbreaks-surge/

Rethinking estrogen supplementation: anti-aging effects of postmenopausal estrogens
Especially since the report of the so-called Women’s Health Initiative (WHI)—a large randomized clinical trial of estrogen and progestin supplementation—was published 23 years ago, periand postmenopausal estrogen replacement therapy has remained a somewhat controversial subject. Now, however, as Eric Topol, MD, in his Ground Truth podcast and Substack newsletter—a steady and very reliable source of the VOICE—in detail described interesting new data suggesting that postmenopausal estrogen supplementation may, indeed, have anti-aging effects and, therefore, may require a readjustment in our thinking about this treatment. More specifically, the evidence came from organ clocks, as we have already on repeated occasions reported in the VOICE, an increasingly popular study tool in anti-aging medicine.1

The WHI was stopped prematurely because supplementation in an interim analysis demonstrated a significant risk of invasive breast cancer (see frontpage article in The New York Times at the time). But the results were even more complex: They suggested an increased risk for coronary heart disease, stroke, pulmonary
embolism, in addition to above above-noted invasive breast cancer risk, and reduced risks for colon cancer and hip fractures. As Topol notes, though statistically significant, the absolute risks were really quite small (19 per 10,000 person-years). While the relative increase in risk for breast cancer was 26%, the absolute risk was only 8 cases per 10,000 women-years. The consequences of the WIH report were, nevertheless, substantial: Postmenopausal hormone supplementation -based on prescription frequency, declined by over 60%.
Without going into further detail (for that, we refer our readers to Topol’s article), the interpretation of the WHI data significantly changed over time, recognizing significant earlier shortcomings. Among those was the observation that supplementation initiated before age 60 or within 10 years of menopause actually reduced the risk of heart disease and all-cause mortality. Moreover, observational data (i.e., lower levels of evidence) also supported benefits on cognitive health, lower Alzheimer’s disease risk, and Type 2 diabetes. Combined, these findings could be interpreted as a beneficial antiaging effect of estrogen.
Topol then addressed several studies in the literature and noted that among 9 drugs found to be associated with reduced allcause mortality, 4 were estrogen preparations. And a very recent study of 460,000 women with a mean age of 42 years revealed that estrogen supplementation (alone) was linked to reduced youngonset breast cancer. The apparent incentive for Topol’s article was,
however, a preprint at bioRxiv 3 scheduled to appear practically unchanged in Nature Medicine in the journal’s July 9 issue, which reported the following rather remarkable findings: It has been established that organ-derived plasma protein signatures derived from protein arrays can track organ-specific aging, disease, and mortality in humans.
How robust these associations are, to what degree these models can offer clinical utility, and their biological underpinnings, however, remain largely unknown. The group of international investigators of this study, therefore, estimated the biological age of 11 organs from 44,526 individuals in the UK Biobank using an antibodybased proteomics platform to model disease and mortality risk using ~3,000 plasma proteins in study participants followed for approximately 17 years.
They found the following: Organ age estimates were “organismal” (not organ-specific), associated with future onset of heart failure, chronic obstructive pulmonary disease, type II diabetes, and Alzheimer’s disease, and sensitive to lifestyle factors such as smoking and exercise, hormone replacement therapy, or supplements.

Front page article in The New York Times on the interim results of the WHI in

Remarkably, the accrual of aged organs progressively increases mortality risk, while a youthful brain and immune system are uniquely associated with diseasefree longevity (see figures to the right). These findings support the use of plasma proteins for monitoring organ health and the efficacy of drugs targeting organ aging disease, and found the brain and immune system clocks to be most influential.
Topol concluded that the protective effect of estrogen largely accounted for why women develop heart disease on average 10 years after men. From mouse experiments, we also have learned that health span can likely be prolonged by delaying physiological ovarian failure (i.e., menopause). And quoting another author, Topol noted that the ovary is the only organ in the human body expected to fail prematurely in comparison to all other organs. At least in mouse models life span of an old mouse that received a young ovary transplant is extended. Topol, therefore, suggests that estrogen supplementation may, after all, be “an attractive alternative.”
1. Topol E. Ground Truths. July 5, 2025. https://erictopol.substack.com/p/new-antiaging-evidence-for-estrogen
2. O’Brien et al., Lancet Onc 2025;26(7):P911-923
3. Se-Hwee et al., bioRxiv. 2025. https:// doi.org/10.1101/2024.06.07.597771
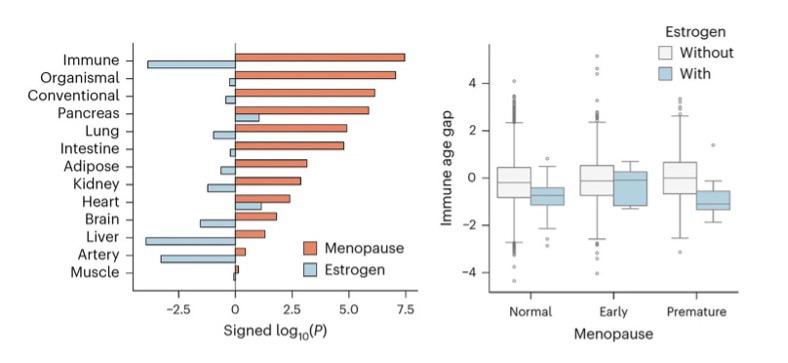
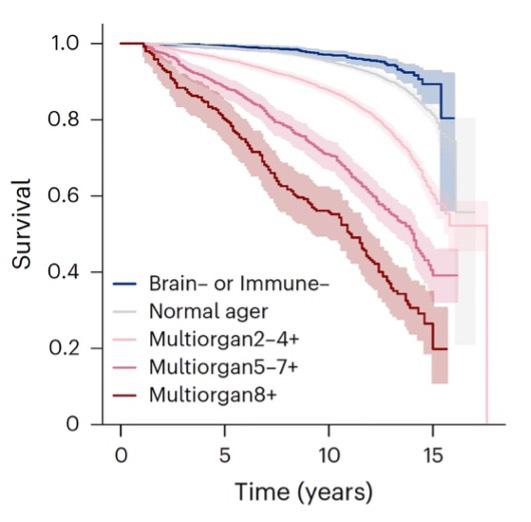
That pregnancy epigenetically ages women has been known for some time. Now a recent paper by Stanford University investigators in Obstetrics & Gynecology added some interesting detail:1 In a prospective cohort study of nulliparous women (age 18-50 years) seeking obstetric (pregnant 10-14 weeks) or gynecologic (nonpregnant) care in 2020-2021, they collected blood samples at enrollment (time 1) and postpartum day 1 (pregnant, time 2) or 7 months later (nonpregnant, time 2). Epigenetic age was measured with 11 established clocks from Illumina EPIC 2 arrays. Results were then scaled per 200day interval, and P values were adjusted for multiple testing. And
then, as the most interesting part of the study, the investigators through multivariable logistic regression explored associations between first-trimester epigenetic age and a composite of potentially immune-mediated complications (hypertensive disorders, gestational diabetes mellitus, preterm birth before 37 weeks of gestation, and small-for-gestational-age birth weight) adjusted for age and body mass index (BMI) higher than 30 at time 1.
And these were the results: The study enrolled 75 women, 45 (60.0%) pregnant. A total of 61 (81.3%) completed the study. Compared to nonpregnant women, pregnant women exhibited significant epigenetic age acceleration in six clocks (Hannum, PhenoAge, GrimAge, GrimAge2, Stem Cell Division, DunedinPACE). Moreover, epigenetic age acceleration per 200 days in the pregnant cohort ranged from 1.58 years (Hannum, 95% CI, 0.45-2.72, P=.01) to 5.28 years (PhenoAge, 95% CI, 2.97-7.61, P<.01). Each additional year of first-trimester GrimAge2 increased odds of the composite of pregnancy complications by 36% (adjusted odds ratio [aOR] 1.36, 95% CI, 1.01-1.84), while chronologic age (in continuous years) showing no

association (aOR 1.00, 95% CI, 0.83-1.21).
Though not earthshaking news, these data are interesting and can be summarized in the following way: Within-person epigenetic aging was accelerated by pregnancy by up to 5.3 years. Older firsttrimester GrimAge2 (an epigenetic biomarker of human mortality and morbidity risk), but not chronologic age, was associated with a composite of several pregnancy complications. These findings obviously encourage further investigations along the lines explored by this paper. At the same time, the data, however, also suggest caution about possible overinterpretations.
Obviously, the various epigenetic clocks demonstrated different severities of the pregnancy impact. For obstetrical care, however, even more importantly, the study found outcome significance only in a composite of pregnancy complications and not for individual pregnancy complications.
This finding has two possible explanations: either the impact of individual complications is very limited, or the power of the study was too low to show significance for individual pregnancy complications. Which of these two potential explanations applies is, of course, important to determine.
1. Panelli et al. Obstet Gynecol. 2025. doi:10.1097/AOG.0000000000006000. Published online ahead of print. PMID: 40638919; PMCID: PMC12252219
Interesting news regarding DNA double-stranded break repair
Double-stranded break repair is, of course, a very essential biological process, with considerable physiological consequences if it malfunctions, as we elsewhere in the genetics section of this literature review—for example—briefly noted in women with BRCA1 and BRCA2 mutations who, as a consequence, demonstrate lower than normal functional ovarian reserve.
A recent paper in Molecular Cell by British investigators1 started with the statement that the DNA’s double-stranded breaks are extremely toxic genomic lesions that human cells have learned over evolution to repair. Such repairs happen because doublestranded breaks induce histone modifications (and a recruitment cascade of repair proteins regulated by histones and repair protein modifications of phosphorylation, acetylation, methylation, ubiquination), and—now coming to the main subject of the paper— the conjugation of small ubiquitinlike modifiers called SUMO1-3 in a process called SUMOylation.
As the paper also notes, amplitudes of small-modifier protein signaling through ubiquitin and small ubiquitin-like modifiers (SUMO13) are critical for the correct phasing of DNA repair protein that accumulates, their activity, and clearance, and ultimately for completion of DNA double-strand break repair.
In this paper, the investigators now demonstrated that the—so far only poorly explored SUMO isoform, SUMO4, enhances the deSUMOylation activity of the SUMO protease SENP1, thereby restraining excessive cellular SUMOylation. This then prevents excessive recruitment of the SUMO-binding protein RAP80 to damage sites and promotes DNA repair. In short: (i) SUMO4 conjugation is deleterious; (ii) SUMO4 promotes SENP1 catalytic activity; and (iii) SUMO4:SeNP1 together restricts SUMO signaling and accumulation of the BRCA1-A complex.
And here is another recent paper from the same British group of investigators, but this time, in Nature Communications (not a bad record!).2 Unsurprisingly, the group adds further information regarding double-strand break repair response. This time, the data relate to RNF168, an E3 ubiquitin ligase critical for the repair response. This protein is recruited to ubiquitin and amplifies ubiquitin signals at damaged chromatin. If not properly regulated, it can initiate an uncontrolled ubiquitin cascade, which would hurt the repair process.

regulation that is part of the normal

while promoting correct ubiquitin signaling and DNA repair.
1. Garvin et al., Molecular Cell 2025;85:877-8
2. Chauhan et al., Nat Commun 2025;16:3399
Are we rewriting the rules of cell division?
This is at least what a commentary in Science argues1 in relevance to a paper out of Nicholas Plachta, PhD’s laboratory, which recently appeared in the same issue of Science, suggesting that at preimplantation stages of embryos, actin organizes chromosomes and microtubules to ensure mitotic fidelity.2
The authors of the paper noted that at preimplantation stages, a single copy of the genome is going through several rounds of mitotic cell division. Errors during mitosis can result in missegregation of chromosomes and aneuploidy of cells, which means abnormal chromosome numbers. What maintains chromosomal fidelity, therefore, has remained a core issue in reproductive biology.
The traditional opinion has been that the spindle apparatus manages chromosome organization. More specifically, the believe has been that microtubules in principle organize the process of mitosis. More recently, this—what the authors call the microtubulecentric view—has, however, been increasingly challenged by the recognition that cytoplasmic actin filaments may also play an important role.
Using high-resolution imaging, the authors have now revealed in their paper that an actin cytoskeleton actually organizes chromosomes and microtubules during early embryonic development. They identified two actin assemblies that performed functions usually attributed to the centromere. They also noted that this finding finally explains how mouse embryos can do it without having the machinery that longest time assumed to be alone responsible for mitosis. Never a dull moment in reproductive biology!
And if we are already talking about mitosis, here is a paper that traced nanoscale DNA only to reveal the self-organization mechanism of mitotic chromosomes.3 What they found they summarized as follows: 3D DNA tracing resolved the internal architecture of mitotic chromosomes.
Chromosomal rod shape then emerged without a contiguous scaffold or regular handed helix, and condensin traversal enabled extrusion of overlapping loops of up to several megabases. Finally, self-repulsion of overlapping loops generates a mitotic chromosomal rod shape.
How genomic DNA folds during cell division to form the characteristic rod-shaped mitotic chromosomes essential for faithful genome inheritance has remained an open question. The authors’ structural analysis revealed a characteristic genome scaling minimum of 6–8 megabases in mitosis. Data-driven modeling and molecular perturbations then helped them to demonstrate that— formed by condensinsvery large
and strongly overlapping loops are the fundamental structure of mitotic chromosomes.
These loops compact chromosomes to the limit set by chromatin selfrepulsion. Their characteristic length, density, and increasingly overlapping structure are observed in 3D, fully explaining how a rod-shaped mitotic chromosome structure then emerges by selforganization during cell division (see Figure)

Graphic abstract of reference 3
1. Bement M. Science 2025;388(6749), 817-818
2. Hernandez et al., Science 2025;388(6749)835
3. Sandvold Beckwith et al., Cell Press 2025;188(10):P2656-2669


A Preprint paper in eLife attracted our attention because it identified a female germline-specific protein that is crucial for germline stem cell renewal and differentiation, as well as oogenesis.1 Specifically, this “valuable” study in the eLife assessment reports the first characterization of the CG14545 gene in Drosophila melanogaster, in the process naming the gene “Sakura.”
The gene appears to be essential for oogenesis as well as female fertility in general and is mostly expressed in ovaries, and there in germline cells, inclusive of germline stem cells. Female null mutants of the gene display only rudimentary ovaries without germlines and tumorous phenotypes. They are unable to produce eggs and, of course, are fully sterile.
Finally, the authors also identified Out (Ovarian Tumor) as a protein binding partner of Sakura, defining both seemingly as partners in germline stem cell renewal and differentiation, as well as oogenesis.
Since the Drosophila female germline is an excellent model for the study of regulatory mechanisms in the germline, these findings may have considerable significance for the human experience as well.
1. Azlan A, Lim JM, Ishizu H, et al. Female-germline specific protein Sakura interacts with Otu and is crucial for germline stem cell renewal and differentiation and oogenesis. eLife Reviewed preprint posted online June 20, 2025. doi:10.7554/eLife.103828.3





