IN THIS MONTH’S ISSUE
A QUICK REALITY CHECK ON EGG-FREEZING
TOO MUCH TALK ABOUT VALUE-BASED HEALTHCARE?
NEW DISCOVERIES REGARDING THE EVOLUTIONARY PURPOSE(S) OF MASTURBATION
A PIECE OF MY MIND: PERSONAL COMMENTS ABOUT A RECENT ARTICLE IN THE WASHINGTON POST
CONFERENCE CHAIRS OF THE FRMC 2023

SEPTEMBER
THE CENTER FOR HUMAN REPRODUCTION
VOICE
2023
THE CHR LETTER THE RESURGENCE OF “REAL WORLD EVIDENCE” IN MEDICINE
NEW CHR PUBLICATIONS QUESTIONS PATIENTS AND THE
ASK THE CHR’S INTERPRETATION OF
LITERATURE, RELEVANT TO REPRODUCTIVE MEDICINE 3 5 9 13 17 19 23 25 37
PUBLIC
RECENT
DECEMBER 1-3, 2023 INTERCONTINENTAL TIMES SQUARE HOTEL, NEW YORK, NY
The CHR is known as a “fertility center of last resort,” primarily serving patients who have previously failed treatments elsewhere. Among CHR’s areas of special expertise are treatments of “older” ovaries, whether due to advanced female age or premature ovarian aging (POA), immunological problems affecting reproduction, repeated pregnancy loss, endometriosis, polycystic ovary syndrome (PCOS), tubal disease, male factor infertility, etc.







CONNECT WITH THE CHR Missed the last issue of The VOICE? Access previous issues on thechr.com
www.thechr.com @CHRNewYork @CHRNewYork @CHRNewYork 2 | SEPTEMBER 2023 | The Voice
ThE VOICE
After our annual summer break during July and August, we welcome you reenergized and ready to rock’n’roll with our September issue into the new academic year 2023/2024. It is for several reasons a very special issue: As the first newsletter following a two-month break, quite a significant amount of interesting new literature has accumulated over the summer that we feel must be communicated to our readers. But this is not all: We also had time to create a list of what we felt were interesting subjects that should be covered in the new academic year in The VOICE and are very pleased to get started in this issue.
One subject that suddenly seems to be everywhere is so-called “real-world evidence” when it comes to assessing outcome data for a given treatment. Study design and the correct interpretation of data have, of course, been repeated subjects of discussion in this newsletter, but they usually involved prospectively randomized studies, widely accepted as the best level of evidence in medicine. But, as also repeatedly expressed in these pages, medicine, for several good reasons, cannot only be based on prospectively randomized trials and, indeed, it isn’t. Only a very small part of all medicine is based on such trials. The vast majority of information currently used for treatments comes from other methods of study design, including “real-world evidence” studies.
With such studies now, suddenly, popping up in all medical specialties, it appears time to address this subject within the context of infertility practice. Our lead story in this issue of the journal is, therefore, the recent resurgence of “real world evidence” as a, finally, appropriately valued contribution to clinical research.
Another subject increasingly addressed in several areas of medicine is “value-based medicine.” Again, by no means a new subject to consider in medicine because, like in education, government services, and any form of business, it appears reasonable to assume that a principal goal of all activities is to create the best possible value in return for the investment made in these activities. Yet, like in education and many government services, while expending on a per-unit-of-service- basis more money than all other developed nations, outcomes in medical practice are often inferior. How infertility treatments might become more value-based, therefore, seemed to us like an interesting subject for further exploration and pursuit, especially considering substantial new reported evidence over the summer that the field may, indeed, be moving in exactly the opposite direction.
Then there are several other subjects addressed in this issue of The VOICE, such as answering questions from our patients and/or readers, and the monthly column of our Medical Director and Chief Scientist, Norbert Gleicher, MD, who this month addresses a rather unusual article that appeared during the summer in The Washington Post. We hope you enjoy this month’s newsletter and want to remind all of our readers that we very much welcome comments and/or proposals for original contributions.
SEPTEMBER 2023 The V oice | SEPTEMBER 2023 | 3
The Editorial Staff of The CHR VOICE

4 | SEPTEMBER 2023 | The Voice
The Resurgence of Real World Evidence” in Medicine
First introduced into the medical literature in 1992,1 the three words, evidence-based medicine (EBM), have dominated the medical discourse in recent decades more profoundly than any other phrase. Though as a philosophical concept already discussed in mid-19th century Paris,2 the idea that the practice of medicine should not only be driven by personal experiences but by the judicious use of best evidence, wherever available, has especially in recent decades been an omnipresent idea in medicine. However, as such, EBM is not a new idea, but an obvious logical conclusion that follows from the ancient desire of medicine to offer the most accurate diagnoses and best possible treatments at all times. The foundation of any such effort must, of course, be based on attempting to secure the best available evidence from wherever it may be obtainable.
Forging ahead with EBM, as one would expect, the medical bureaucracy went to work in attempting to define how EBM should be formalized. First of all, any such formalization demands a quality scale that allows the determination of the quality of available evidence. Through consensus, the “evidence pyramid” evolved (Figure 1), initially ranking the production of unfiltered information based on the kind of studies that generated the information and then, on top, filtering the information through stages of critical review.
Among the methods of generating unfiltered information, the randomized controlled clinical trial (RCT) is widely considered the gold standard, while the endpoint of filtering available information and, therefore, the peak of the pyramid is the systematic review based on a final critical appraisal of prior critical appraisals of unfiltered research data from various studies, preferentially RCTs.
However, this sorting and resorting of data misses a crucially important point: the purity of study populations demanded from RCTs (and often other study formats) can never be reflected in real-world data. In other words, highly selected patient populations in clinical studies never reflect real-world patient populations who then receive the same treatments in daily clinical practice. Therefore, to assume that any treatment in a carefully controlled RCT will always yield the same results as under real-world circumstances in private practice is simply naïve, and illogical, - yet to this day has been a widely held basic assumption of drug and device approvals and of much of the medical literature we daily consume.

Continued on page 6
Figure 1. Source: “EBM Pyramid.” Digital Image. Eli M. Oboler Library, 27 May 2016
The V oice | SEPTEMBER 2023 | 5
Here is just a small and relatively insignificant example from recent reproductive medicine history, considering how huge a problem this really is in all of medicine: As we in the past reported in these pages, when approximately a decade ago, commercial frozen egg banks appeared on the scene for the first time, proponents reported that frozen eggs produced identical pregnancy and live birth rates to fresh donor eggs. To the CHR this claim, for several reasons, appeared far-fetched and we, therefore, started to investigate this issue based on U.S. national data. Unsurprisingly, we found that IVF cycle outcomes using previously cryopreserved oocytes lagged behind freshly retrieved oocytes by a few percentage points.
Colleagues from another prominent academic IVF center in NYC were very upset about the CHR’s conclusions, claiming in an exchange of articles in JAMA in 2015, that they did not find such a difference in their center’s experience. What made their statement so pertinent to this here-discussed subject is that they also predicted that once their academically acquired knowledge in how to work with frozen (rather than fresh) eggs would reach IVF clinics in the community, any outcome difference would disappear with improving the experience in the community of IVF clinics in dealing with frozen rather than fresh donor eggs. CHR’s researchers, in contrast, argued the opposite, predicting that as the use of cryopreserved oocytes would increase in the IVF community, the difference would further grow because variability in patient characteristics, but also in physician competence, would increase. Indeed, this is what happened.3
It has been known for decades that real-world experiences with many, if not most, treatments vary for reasons that are hard to understand and have not received the recognition they deserve. The worldwide push toward EBM was, likely, an important reason because, philosophically, real-world evidence was everything EBM was attempting to outcompete. Yet, the Food and Drug Administration (FDA) has had since 2018 a comprehensive framework for a Real-World Evidence (RWE) Program, 4 primarily geared at supporting the approval of new indications for drugs already approved. Such post-market surveillance is often also ordered for newly approved drug treatments if concerns arise about potentially not yet fully recognized longer-term side effects.
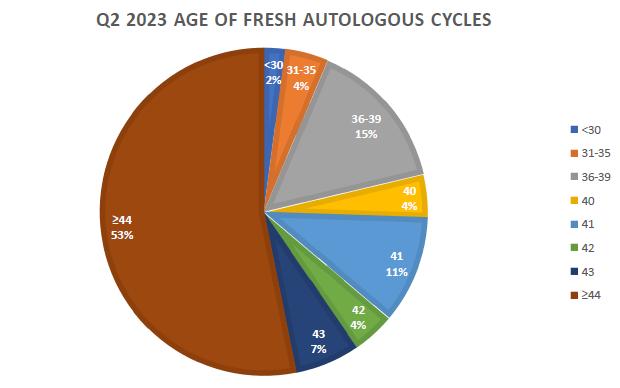
Unfortunately, to this day it is not well understood that many, if not most, RCTs we are basing routine treatments on, are not generalizable. Because of the CHR’s unique patient population (see, for example, the CHR’s remarkable and unique patient-age distribution in Q2 of this year below in Figure 2), the CHR, many years ago, learned this the hard way, when several standard practices that were very successful at other IVF clinics, in the CHR’s patient population failed. However, we also learned that the opposite may be true, - in that certain treatments may turn out to be especially effective in our very
adversely selected patient population and less effective in regular average infertility populations. Here is again an example: The CHR just published a paper in a prestigious science journal in which we demonstrated that so-called “rescue in vitro maturation” of very immature GV oocytes in older poor-prognosis women can be very successful, while in younger, better-prognosis patients the process remains highly inefficient.5
Figure 2. A parameter for the uniqueness of the CHR’s patient population is the much- more advanced age of the CHR’s infertility patients in comparison to all other reporting U.S. infertility centers. As this pie-chart for 2nd quarter IVF cycles in 2023 at the CHR demonstrates, only 21 % of patients were under age 40 and 53% were over 43 years old, for the first time inthe CHR’s history exceeding a median age of 44 years. The patients’ median age at the CHR in recent years has been uniformly over age 43. This compares to a median age of ca. 36 for all other U.S. IVF centers. Advanced age is, of course, only one patient parameter indicative of poor cycle prognosis in IVF. Another important parameter is functional ovarian reserve (FOR),indicated by FSH and AMH levels. Therefore, it is also important to point out that, even the 21% of women under age 40, almost uniformly, were characterized by abnormally high agespecific FSH and abnormally low age-specific AMH.
Recognizing the crucial importance of the interpretation of study results and who a study’s subjects had been, the CHR for years has in all of its publications emphasized the uniqueness of its patient population and very clearly stated that the outcomes obtained by the CHR in the center’s patient population, cannot automatically be extrapolated to other, usually very different infertility populations. Unfortunately, a large majority of studies published in the infertility literature do not emphasize this indisputable fact, thereby clearly sending an incorrect message to the community that has led to the integration of many treatments into routine IVF which only have been demonstrated to be effective in good-prognosis patients, - but now are routinely applied to everybody. Examples abound, from routine extended embryo culture to blastocyst-stage, to elective single embryo transfer, to preimplantation genetic testing for aneuploidy (PGT-A), as well as many others.
Continued from page 5 6 | SEPTEMBER 2023 | The Voice Continued on page 7
To this day, RCTs too often are still considered an absolute gold standard for all infertility patients, completely overlooking that results achieved in any study population, even if achieved through impeccable randomization, should only be applied to patient populations with at least similar genetic backgrounds. This conclusion has major relevance for the fertility field: Take for example the fact that several studies in the IVF field which in recent years attracted wide attention were conducted in China, practically involving exclusively ethnic Han Chinese individuals. Therefore, it is questionable that the results from these studies are in their totality applicable to U.S. populations. As another example, colleagues in The Netherlands in recent years have been very successful in offering several excellent multi-center studies on important subjects in the fertility field. But any thought that Dutch populations, in which these studies were performed, offer enough similarities with U.S. populations to render those study results automatically applicable to the U.S. experience is naïve, considering the diversity of U.S. patient populations in comparison to the relative homogeneity of Dutch patients.
The interpretation of the results of even very well-conducted clinical studies and, even more importantly, the application of conclusions from such studies to a fertility center’s patient population can be much more complex than it may appear. Variability in study populations can, however, also lead to other problems, - with the variability of study outcomes being another issue of major importance. Before continuing with this thought by offering an example, a brief CONFLICT STATEMENT: The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product. Since the following paragraph addresses androgen supplementation with DHEA, readers of this paragraph are advised that opinions expressed in this paragraph may, therefore, be biased by financial interests.
The CHR’s patients and readers of The VOICE, of course, likely already know about the longstanding research interest of the CHR in DHEA supplementation, which has over many years produced over 40 peer-reviewed publications on the subject, the latest just recently published in Fertility and Sterility Reports 6 Even though it was basically introduced to the fertility field by the CHR in 2005 7 and is now widely practiced all around the world, DHEA supplementation has remained controversial (see also the CHR’s monthly “A Piece of My Mind” article by the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, in this month’s issue of The VOICE), with the principal reason being the absence of properly powered RCTs and, the consequential, insufficiently-powered RCTs and studies of lower evidentiary power producing greatly varying results.
One factor never pointed out by critics of DHEA utilization is that, to the best of our knowledge, not a single DHEA study in the literature except for studies reported by the CHR, ever
selected female infertility patients for DHEA supplementation based on verified low androgen levels before DHEA supplementation start. Patients with normal androgen levels, however, cannot be expected to demonstrate benefits from such supplementation, - just as aspirin would not cure a headache in patients who do not have a headache. Again, except for the CHR’s studies, practically all other DHEA studies were performed utilizing different DHEA products at different dosages and with different absorption characteristics. No wonder they differed in outcomes.
When good-quality studies are lacking, studies representing RWE assume even greater importance and, therefore, should not automatically be dismissed as “inadequate” because they were not perfectly controlled RCTs. Animal studies in many different small and big species, which have delineated the importance of normal androgen levels for normal follicle development and maturation, especially during small growing follicle stages,8 and RWE studies by the CHR and other investigators since 2005 have established a clear framework for the clinical utilization of androgen supplementation in properly selected female infertility patients, while concomitantly allowing for a thorough understanding of the underlying physiology that explains why androgen supplementation works. Considering the worldwide experience that infertile women, for good reasons, are usually not willing to be randomized in RCTs, such evidence would never have been acquired without the help of RWE studies.
Therefore, it appears time for the IVF field to recognize that DHEA supplementation on a cost-basis, in properly selected patients likely represents one of the most cost-effective infertility treatments available. One, indeed, can only wonder why some of the loudest voices in opposing androgen supplementation in hypo-androgenic infertile women are among the strongest proponents of so much more costly routine blastocyst-stage culture and PGT-A which do absolutely nothing for a large majority of infertile women, nowadays routinely exposed to these treatments, and in significant subgroups of patients actually harm pregnancy and live birth chances. No clinically significant harm has ever been reported from androgen supplementation at recommended dosages and time periods.
REFERENCES
1. Evidence-Based Medicine Working Group. JAMA 1992;268(17):2420-2425
2. Saket DL. Sem Perinat 1997;21(1):305
3. Kushnir et al., J Ovarian Res 2018;11(1):2
4. U.S. Food & Drug Administration. Framework for FDA’s Real-World Evidence Program; December 2018. https://www.fda.gov/media/120060/ download#:~:text=Real%2DWorld%20Data%20(RWD),derived%20 from%20analysis%20of%20RWD
5. Nicholas et al., iScience 2023;26:107308
6. Gleicher N, Barad DH. Fertil Steril Rep 2023; www.fertstert.org/news-do/ some-caution-dhea-supplementation
7. Barad DH, Gleicher N. Fertil Steril 2005;84(3):756
8. Prizant et al., J Endocrinol 2014;222(3):R141-151
The V oice | SEPTEMBER 2023 | 7 Continued from page 6


ADVERTISEMENT 8 | SEPTEMBER 2023 | The Voice ADVERTISEMENT
A quick reality check on egg-freezing
Egg freezing is in fashion! All young women (and their parents, grandparents, and friends) appear to be thinking about it, but only relatively few among them are taking the plunge. Why is that?
Here are a few key points to consider that you may not hear about elsewhere:
What is the purpose of freezing eggs? There are two principal reasons why some women may benefit from freezing their eggs: First, younger eggs produce better pregnancy chances than older eggs and, second, because women are born with all of their eggs and constantly lose some of this “ovarian reserve,” they at some age may run out of eggs. This may happen as a natural development due to aging or due to medical problems such as quicker than normal loss of eggs, called premature ovarian aging (POA), surgical loss of ovaries, or chemo- or radiation therapy for cancer or other bad diseases.

The idea here is that, even though women are increasingly delaying pregnancies, most women will, and still, spontaneously conceive and have children the traditional way and, therefore, may never need frozen eggs. Consequently, we know that among women who currently freeze some of their eggs, most will never use those eggs. The principal purpose of egg-freezing is to create a “reserve” of eggs if a mostly unforeseen emergency should arise that requires additional (and preferably “younger” eggs).
Not every woman needs to freeze her eggs: If you are a healthy young woman with regular periods in your 20s, if you have a serious partner, and don’t plan on delaying having children because you want to have an MD and PhD degree before age 30 and be the CEO of a start-up with a billion dollar valuation by age 35, you very likely will be just fine with your own eggs. Even then, unexpected things may happen; - you, for example, may get a divorce at a more advanced age, remarry, and may want to start another family.
But, if you are in your 20s and have regular periods (without being on birth control pills), it’s much more likely that you never will need donor eggs than that you will need them. Though not recommended by many colleagues, we recommend that, before initiating any long-term (at least over 6 months) hormonal contraceptives, you have your ovarian reserve (OR) tested (that means getting an estimate of whether you have a roughly normal number of eggs left for your age in your ovaries). This is done by testing three hormones on the second or third day of the period: FSH, estradiol, and AMH. Assuming your OR is age-appropriate, you are likely safe for at least three years. We in such cases recommend repeated OR testing every three years, which means that 2-3 months before testing all hormonal contraceptives must be discontinued.
The V oice | SEPTMEBER 2023 | 9 Continued on page 10
Continued from page 9
When to freeze eggs? Eggs can be frozen at almost any age after menarche. We, however, do not recommend that everybody freezes their eggs by age 21. If you know that you will not have children before the age of 35, that is another story, and you may seriously consider freezing eggs once you turn 21 because the younger you are at the time of freezing the better. If by age 35, you still have not met the love of your life and started making babies, it, indeed, should be time to seriously consider freezing your eggs.
We just don’t want you to become a victim of the egg-freezing industry which, of course, wants to freeze eggs for everybody. There is no right or wrong time for egg freezing. It all depends on a young woman’s plans for life. If you plan on having all of your children before the age of 35, you likely will not need to resort to egg-freezing. But, if you see yourself having at least some children at later ages, egg-freezing may be an appropriate strategy.
In general, there is also no right or wrong age for freezing your eggs, with the principal reasons being that (i) the older the women at the time of freezing, the lower will later be the pregnancy chances from those eggs, (ii) the younger eggs are frozen, the better will be their chances to lead to pregnancy, but the lower the chance that they ever will be used. And, not to be forgotten, (iii) egg-freezing isn’t cheap and, often, not covered by health insurance and/or employer benefit packages.
The decision of when to freeze eggs is often a complex one, and should not be negotiated in “egg-freezing parties”, but in medical consultations with a fertility expert. Because of declining egg numbers obtained with advancing age in a single egg retrieval and declining pregnancy chances from older eggs, many fertility centers have age cut-offs for egg freezing. Since the CHR, in general, does not believe in arbitrary age cut-offs and all fertility-related treatments individualizes the decision-making process based on the patient’s characteristics, the CHR also does not have an age-determined cut-off for egg-freezing. But, if social circumstances allow, the CHR usually recommends against egg-freezing and pro-immediate pregnancy attempts, after age 38 years. We, of course, do recognize the power of social circumstances in making at times difficult decisions.
What are the pregnancy chances with frozen eggs?
In advertising egg-freezing services, the concept of egg-freezing is often misrepresented as an “insurance policy.” It is, indeed, anything but that! When purchasing health insurance, a car, or property insurance, we know exactly what we are purchasing and what we can expect to receive should the covered insurance situation come about. When freezing eggs, that is clearly not the case because nobody knows with reasonable certainty what your frozen eggs at that point will offer you in pregnancy and live birth chances. In other words, egg-freezing, therefore, is not an insurance; all it offers is the hope of somewhat improving your chances of conceiving and having a child with the use of your own eggs when age or other circumstances, otherwise, would no longer allow it.
The fact that you have eggs frozen does not even guarantee that, by the time you want to use them, they will still be functional. The CHR, unfortunately, quite frequently sees women who thought they had a good enough number of frozen eggs in storage but, once they decided to thaw them in an attempt to conceive, discovered that only very few, or even none, survived thawing. Unsurprisingly, The New York Times’s medical writer, Gina Kolata, in an article last fall described outcome information from egg-freezing as “sobering.”1
What we can say about subsequent pregnancy chances is, therefore, only very limited. A recent study reaffirmed what appears logical: the younger a woman is at the time of egg freezing, the higher her chances that her eggs will thaw well and the higher the pregnancy chances will be from those eggs.2 But that is practically all that can be said as of this point because there, simply, are not enough studies in the literature that would allow more specific comments.
A few additional conclusions, however, appear logical, with the most important likely being that egg freezing is not, as often advertised, a social service, but a serious medical procedure which, like every medical procedure, can be done better or poorer. In other words, where you freeze your eggs matters!

10 | SEPTEMBER 2023 | The Voice
Continued on
11
page
ADVERTISEMENT
This is important because egg freezing has given rise to its own “industry.” A good number of relatively new IVF clinics were specifically founded for egg-freezing purposes. Among those who survived this temporary fad and still exist, most have since expanded into general IVF services, though often still do a majority of IVF cycles for egg-freezing purposes.
Why is this important? Because such clinics may freeze a lot of eggs. However, because it often takes many years before patients ask to have their eggs thawed out, they frequently have no way of judging how well (or poorly) they are freezing eggs. Full-service IVF centers, in contrast, usually freeze and thaw eggs and embryos constantly and, therefore, have constant quality control over their freezing quality. Therefore, we strongly recommend that egg freezing be considered a serious medical procedure and, like any other fertility service, be pursued only in competent fertility centers with transparent quality assurance programs. And on a side note, how well such a center freezes eggs is much more important than whether it charges a few dollars more or less in monthly storage fees!
How many eggs should, therefore, be frozen? This is again an almost impossible question to answer, except, maybe, with a general statement, - “the more the better.” Moreover, since chances for pregnancy per frozen egg decline, with advancing female age, the number should increase with advancing female age. At the CHR this means that we at age 35 recommend at least 15 eggs per desired child. If the patient is younger, it may be a little less; if she is older, it will have to be a little more. As always in medicine, the key is individualization, since there are 35-year-olds with younger- and older-behaving ovaries.
A final word about freezing cycle numbers: Another deeply upsetting observation we make at the CHR all the time is how many women underwent only one single egg-freezing cycle. The reason why this is such an upsetting observation is that a single egg-freezing cycle very rarely produces enough eggs. Therefore, it is disturbing to see that many women who have undergone egg freezing claim to never have been informed about this fact. They then find themselves having spent whatever benefit coverage or cash they had available, for an insufficient number of eggs.
The CHR strongly recommends that everybody considering egg-freezing, first and foremost, establish with their physician how many eggs should be cryopreserved. Once that number has been agreed upon, the next question to be answered must be how many retrievals will it take to get to this number? This number will vary with age: Egg numbers produced in a cycle, unfortunately, decline with advancing age, when bigger numbers become necessary because pregnancy chances from individual eggs decline. It is also important to consider that not all eggs obtained in a retrieval are freezable. Indeed, only good

quality mature eggs should be cryopreserved and, therefore, considered in the final count.
This is why multiple-cycle packages are of such importance in egg freezing. Almost all patients require more than one retrieval cycle to reach minimal frozen egg numbers. That number, however, will differ between patients. Every patient, before signing up for a first freezing cycle, should be advised how many cycles she likely, will need. Because cycle packages (at least at the CHR) offer increasing cycle discounts with increasing package sizes, it seems unethical to withhold this information from patients in an attempt to induce them to have at least one cycle, if that one cycle at best will produce only insufficient egg numbers to fulfill a patient’s expectations.
REFERENCES
1. Kolata G. The New York Times. September 23, 2022. https://www. nytimes.com/2022/09/23/health/egg-freezing-age-pregnancy.html
2. Cascante et al., Fertil Steril 2022;118(1):158-166
The V oice | SEPTEMBER 2023 | 11
ADVERTISEMENT
TRYING TO REACH THE INFERTILITY COMMUNITY?

Have you thought about advertising in the VOICE?
This newsletter every month goes electronically to ca. 80,000 infertility patients, medical professionals in the field, and members of the media, with over 25% (an unusually high number) also opening the VOICE.



For further information, please contact: Ms. Alexandra Rata
(212) 994 4400 or e-mail to arata@thechr.com
ADVERTISEMENT 12 | SEPTEMBER 2023 | The Voice ADVERTISEMENT
TOO MUCH TALK ABOUT VALUE-BASED HEALTHCARE?


subject, suddenly, appears once again of special concern everywhere. Considering how U.S. society in recent years has approached certain other issues, the resurgence of interest in VNHC in our mind raises concerns.
Rising health care costs, likely accelerated by the recent inflation in all of the U.S. economy, are probably a dominant reason for all the attention given to VBHC. Even before the current spike in inflation, health care costs in the U.S. were already by far the highest among Western democracies, without being able to show much extra benefit. Those extra expenses, moreover, are perceived to come at the expense of other social spending that might offer better benefits to society.1 These kinds of ideas, however, of course, raise the question of where do health care costs end and does other social spending start? They also raise the specter of repeating the mistakes made in many communities in defunding the police under the claim that those funds could and would do better if applied to certain other social services. The verbiage, indeed, sounds eerily similar, - then claiming that budget allocations demonstrated prioritization of law enforcement over crime prevention, and now claiming that current budget allocations prioritize acute illness care over care that may maintain health.1
C(VBHC), it is not surprising that a recent article asked whether VBHC is just another buzzword.1 Like noted elsewhere in this issue of The VOICE, the phrase real-world evidence (RWE) has seemingly become omnipresent in the discussion of clinical studies and the concept of VBHC appears omnipresent in all discussions relating to health care reform.
Just as RWE by no means reflects a new approach to how evidence is generated, VBHC seems boringly obvious. The desire to improve the quality of care and, in parallel, lower healthcare costs has forever, of course, been at the core of healthcare policy planning. But, whether a new Office of Population Health at Johns Hopkins Medicine includes value-based care planning as one of its four policy priorities,2 wheth er surgery attempts to redefine value in endocrine surgery,3 or whether the BMJ Open explores the question of whether VBHC supports patient-centered care or not,
It is widely accepted that improvements in healthcare systems are considered successful if they achieve three specific goals: (i) Improvement of population health; (ii) reducing per capita costs; and (iii) improving the patient experience.5 In observing in many of the CHR’s Canadian patients the national Canadian health care
believe, we recently saw a woman in her 40s for consultation, who already 10 years earlier had been diagnosed with breast cancer and successfully treated with surgery and radiation only. Apparently cured, she had failed to conceive in Canada and, therefore, consulted with the CHR. Based on her breast cancer history, the CHR insisted that she have an MRI of her breast before further fertility treatments since a supposedly normal mammogram had reported “very dense breast tissue,” which at times can obscure cancerous lesions and lead to false-negative mammogram readings. Since MRIs can penetrate even dense breast tissue, this becomes the screening test of choice in women with dense breasts, especially if, like this patient, they are at increased risk for breast cancer.
Based on the claim that she did not need an MRI, in the Canadian health care system she was repeatedly denied an MRI examination for over five months. Only because of her relentless insistence, she finally succeeded in getting it done, and was, promptly, diagnosed with fresh breast cancer.
This is why we here at the CHR are concerned about all of the resurgent talks about VBHC: Like almost everybody among healthcare recipients and healthcare providers (who, of course also are healthcare consumers), the CHR strongly supports everything that will lead to improvements in population health and will reduce costs, fully recognizing that superficially, these two goals may appear contradictory; but, acknowledging that current medical practice is often very wasteful and, therefore, with better management potentially allowing for substantial savings. However, we are deeply concerned that the third requirement for improvements in health care – the improvement in patient experience – as the Canadian patients described above so well demonstrates, is often very quickly forgotten.
While in countries like Canada governments control resource utilization mostly in nationalized health care systems, similar draconian and often nonsensical utilization controls are increasingly also applied in the U.S. system. The only difference lies in the fact that, here, except under federally run programs like Medicaid and Medicare (and partially now even in those federal programs), an ever-shrinking number of giant and in many markets monopolistic health insurance companies (now increasingly also under cross-ownership with local hospital and pharmacy networks), however, behave exactly like government agencies in nationalized health care systems, often restricting care for all the wrong reasons. In employing physicians as alibi gatekeepers, those large insurance companies thereby for all practical purposes practice medicine because their physician-employees are not independent in their decision-making when denying patients medical care ordered by their physician. When denying treatments, we in physician-to-physician talks, indeed, hear from these
gatekeeper-physicians almost routinely that they “personally” agree with the request before them, “but that their hands are bound by established corporate policies.”
Quality of care and patient satisfaction are at least as important as cost controls, - if not more so. That the BMJ Open recently had to conclude that, at least so far, VBHC has not supported patient-centered care4 is, therefore, not reassuring.
REFERENCES
1. Clement F. Healthcare Policy 2023;18(4):18-25

2. Berkowitz et al., Am J Manag Care 2023;29(7):e189-191
3. Ayoub et al., Otolaryngol Head Neck Surg 2023;doi: 10. 1002/ohn.427. Online ahead of print.
4. Kidanemariam et al., BMJ Open 2023; 12:e070193
5. Berwick et al., Health Affairs 2008;27(3):759-762
14 | SEPTEMBER 2023 | The Voice


ADVERTISEMENT The V oice | SEPTEMBER 2023 | 15 ADVERTISEMENT
Developing mouse ovary showing the ingrowth of blood vessels (white) the cells of which will nourish the follicles that develop with each menstrual cycle in the adult.
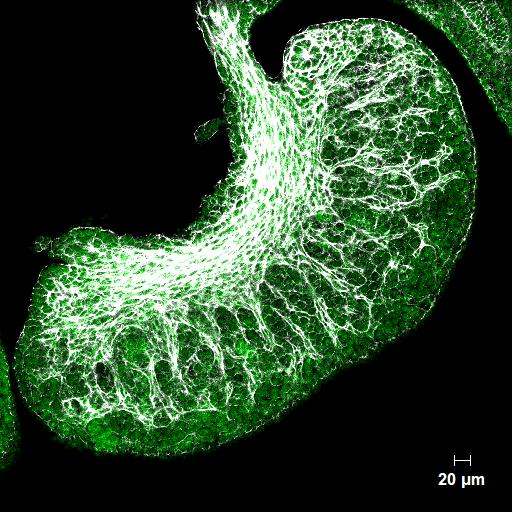
Striations show segments of the ovary where the very first follicles are forming, most of which will have degenerated by the time a woman reaches adulthood.
DR. ALBERTINI’S
Photo Gallery
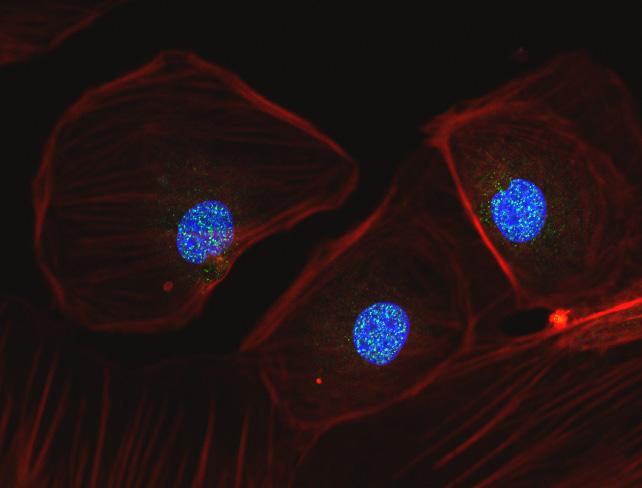
As discussed previously, during oocyte retrieval many cells lining the follicle are removed along with the oocyte that are known as granulosa cells. Research at the CHR continues to exploit the importance of these cells and the role they play in making an oocyte that has the potential to become an embryo. This picture shows three such granulosa cells that have been maintained in culture and labeled to demonstrate the actin cytoskeleton (red), the nucleus (blue) and within the nucleus, many bright yellow spots that reflect ongoing repiar of nuclear DNA.
Image 2
16 | SEPTEMBER 2023 | The Voice
Image 1
NEW DISCOVERIES REGARDING THE EVOLUTIONARY PURPOSE(S) OF MASTURBATION
This is definitely the first-ever article1 on masturbation in The VOICE and, very likely, the last one for some time to come, but it is the result of a publication in the Proceedings in Biological Sciences of the Royal Society which addresses an evolutionary paradox that has made scientists wonder for quite some time:1 Masturbation is widely observed in many animal species and, indeed, is especially common among non-human primates. Evolutionary scientists, however, for decades have wondered what the potential fitness benefits from this activity may be, having allowed this behavior to survive evolutionary pressures for millennia since, on first impression, it would appear to waste time, energy, and reproductive resources. In other words, if an activity survives evolutionary selection for so long, it must have benefits that, so far, have not been recognized. Now, British investigators claim to have, likely, found at least some of the underlying purpose(s).1
Some background is required: Especially because of its high prevalence in non-human primates, so-called autosexual behavior, the frequently used scientific term for masturbation, in contrast to the allosexual behavior that has puzzled evolutionary biologists for some time since it challenges evolutionary theory: It, after all, does not appear to directly increase survival (more on that later), excludes reproductive partners (and, therefore, pregnancy opportunity), but incurs significant costs in time lost, attention paid, and energy use.
Evolutionary biologists developed several hypotheses for this behavior, with none, however, really being able to fully explain the high prevalence of masturbation especially among non-human primates: Several similar so-called “non-functional hypotheses tried to explain the behavior as pathological or as a byproduct of high sexual arousal. In contrast, “functional hypotheses,” in masturbation saw adaptive benefits. A so-called “Postcopulatory Selection Hypothesis,” proposed that masturbation improves fertilization chances, while a “Pathogen Avoidance Hypothesis,” as the name indicates, suggests that masturbation helps in cleansing bacteria from the genital tract.1
In a recent article in Science News, Darren Incorvaia, PhD, following an interview with the lead author of the paper, summarized the British investigators’ findings as follows: An evolutionary history of masturbation in primates almost unbelievably can be traced back for at least 40 million years. In at least male primates, masturbation, moreover, appears to help their readiness when the opportunity for mating arises and also favors them staying free of infectious diseases.
Though the study could not define an exact date for when masturbation practices started, the authors of the study felt comfortable in stating that “ from ca. 40 million years ago, the ancestors of all monkeys and apes appear to have masturbated,” which is when simians (apes and monkeys) split from tarsiers (tiny primates at the time living in Southeast Asia). 2
The study further found in male primates (though not in females) that multiple partners and the prevalence of infectious pathogens were associated with masturbation. The lead author, however, indicated in the interview that a similar association might also be discovered in females with more available data. She further suggested that the stigma affecting the study of masturbation (and other sexual behaviors) has been easing and to, therefore, expect more data on the subject seemed realistic. She also noted in the interview that, considering that autosexual behavior is also common in other mammals, birds, and reptiles, “to obtain a more comprehensive evolutionary picture of this behavior requires a look beyond primates.”2
One cannot address the subject of masturbation without noting that practically all major religions to this day condemn the practice. Though the public’s perception over recent decades has obviously significantly changed from the days when school children were taught that masturbation leads to blindness. As a recent survey, however, again reaffirmed, the public’s feelings about masturbation have, nevertheless, remained somewhat ambivalent:3 The most frequent given reasons for not masturbating were lack of interest, being in a committed relationship, conflict with moral values, and religious opposition. Women who desired partnered sex “much more often” or “a little more often,” were 3.89 times and 2.07 times, respectively, more likely to report higher frequencies of masturbation than those with no desire for more sex. Men who desired sex “much more often” and “a little more often” were 4.40 times and 2.37 times, respectively, more likely to report higher frequencies of masturbation activity.
A recent European survey of older individuals (mean age 67, range 60-75 years) revealed that between 41% and 65% of men and 27% and 40% of women reported masturbation in the preceding month. Satisfactory sexual activity and moral attitudes toward disapproval of “sex without love” were significant predictors of masturbation in females and males. Interestingly, age, education, self-perceived health, and depression were predictive of men’s masturbation habits, but not of women’s behavior. In both sexes, those believing that sex is beneficial to older people were more likely to masturbate. Less permissive attitudes toward masturbation, as one would
NEW BASIC SCIENCE NEWS
Continued on page 18 The V oice | SEPTEMBER 2023 | 17
Continued from page 17
expect, decreased the likelihood of masturbation.4
Returning to the evolutionary purpose(s) of masturbation, it seems to us that, in finding potentially evolutionary “benefits” from masturbation primarily in males, and only regarding their readiness for reproductively efficient events and limitations of infectious risks from multiple partners, the recent British study, likely, at best just scratched the surface in discovering evolutionary benefits from masturbation. That it not only survived evolutionary selection pressures for over 40 million years but, with the advancing development of species, apparently increased over time in prevalence, strongly suggests that masturbation must fulfill much more important functions that benefit the survival of the species than suggested by the recent British study. We would argue that at least the 40-million-year history of masturbation strongly points to a very significant potential contribution for mankind from autosexual behavior.

Practically all published studies on masturbation in their respective introductions point out how “understudied” the subject is. In considering featuring the British study in this month’s VOICE, we were struck by the fact of how little
published data we could find on a possible association between masturbation, frequency, and longevity. To be specific, we found only one 1997 British study, which reported a 50% lower mortality in men with high orgasmic frequency.5 Though this study was criticized for failure to consider energy use as a potential cofounder,6 one would expect a paper demonstrating such substantial effects on longevity to initiate millions of dollars in funding for follow-up studies. That, instead, absolutely nothing like this happened, even though the study was published in a very prestigious medical journal, can only be viewed as evidence for masturbation (even within the science community) still being considered as mostly a taboo subject. Now you know why The VOICE this month features the subject!
REFERENCES
1. Brindle M et al., Proc Biol Sci 2023;290(2000): 20230061.
2. Incorvaia D. Science News, June 6, 2023; https://www.sciencenews. org/article/masturbation-evolve-primates
3. Herbenick et al., Arch Sex Behav 2023;52(3):1317-1331
4. Fischer et al., Arch Sex Behav 2022;51(3):1385-1396
5. Davey Smith et al., BMJ 1997;315(7123):1641-1644
6. Batty D. BMJ 1998; 316(7145):1671
18 | SEPTEMBER 2023 | The Voice
ADVERTISEMENT
MY A OF
PIECE MIND
 By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist
By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist

The CHR, New York, N.Y.
CONFLICT STATEMENT
The CHR and some of its staff members (including the author of this column) own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product. Since the following paragraph addresses androgen supplementation with DHEA, readers of this paragraph are advised that opinions expressed in this paragraph may, therefore, be biased by financial interests.
The number of articles about the CHR and/or the writer of this column over more than 40 years of the CHR’s history has been substantial. Some were short blips, others were full-length articles mostly relating to scientific publications, but sometimes they were more personal, like
Continued on page 20
Personal comments about a recent article in TheWashingtonPost The V oice | SEPTEMBER 2023 | 19
a weekend feuilleton a few years ago in The New York Times about Sunday habits.1 Some were more accurate, and some were more friendly than others. But none ever came close to a recent article in The Washington Post by Yeganeh Torbati2 (more about her later), usually writing about political and economic issues. She, recently, seemingly driven by a dislike of how fertility services were provided in this country, appeared to have discovered a new leading subject to write about.
In her first article on the subject, she quite fiercely attacked several prominent fertility clinics for raising storage fees for frozen eggs and embryos.3 However, her second effort targeted the CHR - or more accurately – the author of this column. Under what later turned out to be a mostly false pretext of being interested in DHEA supplementation, she had requested an interview.
As a consequence of having introduced DHEA supplementation to the treatment of female infertility, the CHR receives such requests quite routinely. The CHR, after all, has published over 40 peer-reviewed publications on the subject since 2005, when the first paper appeared in print. 4 Based on the CHR’s research, it and some of its researchers had also been awarded several U.S. user patents, claiming in selected infertile women clinical benefits from androgen supplementation, including supplementation with DHEA. These patents protected the sale of androgens (including DHEA) in such women for fertility purposes, - not differently from how patents protect new prescription medications for a predetermined number of years from being copied. Always willing to inform colleagues and the public on when and how to use DHEA, a virtual video interview was, therefore, set up with the reporter.
In retrospect, this interview from the beginning should have raised suspicion about the reporter’s real intent. Her prior article was rather “unfriendly” (to say it mildly) toward several well-known IVF clinics after they had raised egg and embryo storage fees, considering overall IVF cycle costs, by rather inconsequential amounts. Why would a writer who previously exclusively covered politics and economics, now suddenly, pivot to infertility?
She, moreover, appeared unprepared for the subject she had claimed she was covering. Her computer set-up “malfunctioned” as her camera did not work. Open to at least some psychological conjecture, she, thus, remained “invisible” during the interview. And she was surprisingly uninformed about and uninterested in, the clinical utilization of DHEA. This was, however, contrasted by a surprisingly detailed interest in very peripheral issues regarding the CHR’s past DHEA research: She, for example, could not get enough information about the CHR’s index patient who in 2004/2005 inspired the CHR to investigate DHEA utilization in female infertility and became the subject
of the CHR’s first DHEA publication.4 Even regarding this patient, her interest, however, did not extend to the likely most interesting question, - why and how she, on her own, decided to supplement with DHEA without the CHR’s knowledge? Instead, the reporter was very curious why the CHR had assigned her a part-ownership in later awarded DHEA patents.
The patient, indeed, had been awarded a small share of co-ownership in the CHR’s DHEA patents because, had she not (without CHR’s knowledge) self-administered DHEA, the CHR would never have had reason to investigate DHEA and would never have earned any DHEA patents.
It later turned out that the reporter had attempted to interview the patient, indeed, even before contacting the CHR, - but, to our surprise, had been rebuffed. This was surprising because this patient had been very forthcoming in granting interviews on several earlier occasions after the CHR published in 2005 a paper about her case.4 She apparently must have sensed something in this Washington Post reporter she did not like.
Over ensuing weeks, the primary target of the reporter became increasingly obvious and, somewhat surprisingly, it was neither DHEA, nor the above-noted CHR patient, but - yours truly - the writer of this column. CHR started receiving calls from prior employees and physician colleagues in other cities after being contacted by the reporter with, mostly personal questions “about Dr. Gleicher.” They practically in unison reported that the reporter showed only relatively minimal interest in DHEA but expressed “considerable interest in Dr. Gleicher, - personally,” even going back over 20 years, when he still resided in Chicago, IL. This interest was not at all directed at his clinical and academic achievements, but mostly concentrated on his private life, including seemingly banal and inappropriate questions such as, what car he was driving, what real estate he owned at different times, and even whether it was true that he had a girlfriend. Reports also came back from several patients who had agreed to be interviewed after being treated with DHEA at the CHR, without exception, reporting how surprised they were about the reporter’s obvious hostility toward Dr. Gleicher and the CHR.
Intrigued by the reporter’s possible motivations but forewarned by all of these reports the CHR had received, we at that point granted the reporter, nevertheless, a follow-up interview (this time via telephone). Unsurprisingly, her questions this time quickly became very personal: What about my “mansion” in Chicago? I never owned a mansion in Chicago, - just a nice modern house on a quiet side street, across from a church. Was it true that The Fugitive had been
Continued on page 21
Continued from page 19 20 | SEPTEMBER 2023 | The Voice
filmed at the house? Yes, true, a few scenes were filmed at the house. I, indeed, am listed in the credits of the film as a professional consultant. Do I also own the brownstone in New York City where the CHR is located and how much rent does the CHR pay monthly? What was my salary? Yes, there was a reason after all for the interest in our above-noted index patient: In the reporter’s opinion, awarding her a fractional ownership in our patents, reflected “being in business with the patient,” and that in her opinion was “unethical.” Though supposedly primarily a business reporter, she very obviously was unaware that assigning fractional co-ownership to contributors of patents is routine in academic institutions and corporations. Being informed of this fact, however, did not change her mind.
After her next question, I ended the conversation. Quoting her quasi-verbatim, it was more a statement than a question, when she asked, “Aren’t you ashamed to live such a life of luxury at the expense of poor infertility patients?” Her motivation for the planned article was now obvious! Like in her earlier piece in the Washington Post in which she accused several prominent IVF clinics of abusing infertility patients by raising freezing costs by negligible amounts, this time it was the turn of yours truly to be the bad guy, “who lived a life of luxury at the expense of poor infertility patients.”
When weeks later her article appeared in print, it, as expected, was quite personal and, at times, because of selective reporting, simply unprofessional. Obvious misrepresentations and/or omissions of information she had received during interviews with individuals who reported back to us, demonstrated clearly that she was not interested in discovering truth and reality, but was only looking for evidence of an already preconceived narrative, she had been following from the very beginning.
A nice modern house, with such a mindset, became a mansion, and through many years of hard work earned a comfortable lifestyle, a life of luxury. Whether DHEA was a valuable treatment or not, indeed, no longer mattered. That DHEA patents were earned based on considerable financial investments in research and through hard work also no longer mattered. What mattered was that these patents had the “evil” potential of earning income for their owners through licensing fees. That they had been granted based on solid research data did not matter. Supporting those claims in conversations with patients had to be “evil” because it did not represent scientifically established facts, but greed for self-enrichment on the back of poor infertility patients. In other words, the underlying ideology remains unchallenged, even if the truth contradicts the narrative.
Who then can be surprised that Ms. Torbati was beyond uninterested in finding out why the CHR started producing its own DHEA? And once told, she simply ignored the information. The motivation was actually to reduce costs for the CHR’s patients. When the CHR started the investigation of DHEA, available over-the-counter-DHEA was of very poor and uneven quality and, therefore, unsuitable for clinical treatments and serious scientific studies. Consequently, patients had to purchase a pharmacy-compounded DHEA, which was very expensive.
She was similarly disinterested in finding out why there still existed differences in opinion about the utilization of DHEA in female infertility. That, we too (and other colleagues she spoke to) tried to explain to her, - though with no avail. We explained to her that differences in opinion in medicine are omnipresent and, indeed, are a very important component of maintaining progress. It was also explained to her that any medication will only work if given to patients who suffer from the underlying problem for which the medication is prescribed. For example, aspirin works against headaches only if the patient suffers from headaches. Similarly, treating infertile women with DHEA makes only sense if their androgen levels are low. Yet, not even one of the studies reported not to support DHEA supplementation investigated androgen levels before the initiation of DHEA supplementation. Moreover, many of those studies also used insufficient dosing and incorrect timing of DHEA.
Neither was the reporter really interested in understanding the relationship between the CHR and Fertility Nutraceuticals, LLC, the company originally established to produce a cheaper DHEA for the CHR’s patients. Called Fertinatal®, without any marketing efforts, the word about the high quality of this DHEA product in comparison to other over-the-counter products quickly spread, and the company started selling this DHEA at the same price to patients outside of the CHR. For several years DHEA was the only product the company produced and sold. As sales grew, the required overhead grew with it. It also became increasingly clear that the company had to become independent from the CHR and had to expand its product offerings if it wanted to survive economically. This separation took place after some outside investors joined in the ownership of the company, just before the COVID pandemic started.
Under the brand name Ovaterra, the company now produces several additional over-the-counter products for infertile female and male patients, all with the same quality parameters that have maintained Fertinatal® as the undisputed market leader among DHEA products when it comes to female infertility. A principal reason is that it exactly reflects in quality and micronization the compounded DHEA that the CHR’s
Continued from page 20 The V oice | SEPTEMBER 2023 | 21
investigators used in their early studies of DHEA. Representing to patients the likely effects of DHEA based on those CHR studies, patients, therefore, are assured to receive the same DHEA used in those studies.
The CHR’s affiliation with this company was always transparent, starting with exhibits of all the fertility-supporting supplements the company produces in displays at the front desk, visible to all patients. Advertisements for the company’s products, clearly marked as such, are dotting The VOICE every month, and every publication in either The VOICE or in medical journals by employees of the CHR referring to androgen hormones, always contains a “Conflict Notice” (as this column does) which clearly discloses the CHR’s association with the company. And it is not that Ms. Torbati wasn’t made aware of all of these efforts by the CHR to be transparent. She, indeed, received copies of The VOICE and CHR publications to see examples. But, as noted earlier, once a narrative is set, why care about the facts?
Though, as in much of medicine, properly-powered prospectively randomized studies of DHEA supplementation in female infertility for several good reasons do not exist, “real world” data and many excellent animal studies demonstrating the effectiveness of androgen supplementation when androgen levels are low, have well documented how androgen supplementation improves ovarian function and, therefore, IVF outcomes.5 Moreover, Chinese investigators (with obviously no connection whatsoever to the CHR, just very recently published an updated meta-analysis of DHEA use in infertile women with poor ovarian responses and reported improved clinical outcomes.6 They did so, even though none of the included studies in the meta-analysis pretested androgen levels before initiating DHEA supplementation. Though supportive of DHEA supplementation, the analysis was actually objectively biased against demonstrating such benefits. Though referring to older meta-analyses, Ms. Torbati did not refer to this most recent one in her article.
We sincerely hope that Ms. Torbati’s obvious bitterness and misperceptions about how women currently receive fertility services in this country are only based on ideological bias and misinformation. Nobody deserves to be left with so much bitterness, especially when it’s regarding the creation of life. At the same time, it must be stated that no fertility center in this country deserves this kind of narrative less than the CHR, which never has offered medical services based on considerations that favor the center’s revenue generation. Very much to the contrary, the CHR’s longstanding position on the utilization of preimplantation genetic testing for aneuploidy (PGT-A) probably better demonstrates this fact than any other example. By not utilizing PGT-A in over 99% of IVF cycles, the CHR foregoes 15-20% of cycle revenue in almost every IVF cycle, at a time when increasing numbers of IVF clinics mandate PGT-A for almost all of their cycles.
The center’s physicians (this writer included), its scientists, nurses, as well as other staff members, day-in and day-out, serve the most unfavorable patient population of any IVF center in the U.S. (and likely the world). A majority of this center’s patients were, indeed, refused further treatment with the use of their own eggs at other fertility clinics before presenting to the CHR, and were advised that their only option was third-party egg donation.
That in such patient populations’ pregnancy chances will be low and live birth chances even lower is, of course, obvious, and always presented by the CHR’s physicians with what we uniformly describe as “brutal honesty.” But if patients, after receiving this information, still, wish to receive treatments utilizing their own eggs, who are we to deny them these services? Many women, understandably, require certainty that they “tried it all” in order to reach closure that then, often, allows them to proceed into third-party egg donation, - after all. That our philosophy makes sense is, of course, well supported by many healthy deliveries in women who, elsewhere, were told that their only chances of becoming a mom was with egg donation, among those also the two oldest women in the medical literature ever to conceive through IVF with use of their own eggs.7
What enticed Ms.Torbati to write her article and who fed her much of the very obviously biased information she appears to have relied on, we can only surmise: Based on who we believe she talked to (but did not mention in her article), we believe that her initial source was a colleague who was caught breaching the CHR’s DHEA patents, was offered a very fair licensing agreement (like others are holding), but rejected it. As a business reporter, Ms. Torbati should have had better judgment in choosing her source, by understanding the legal need of protecting intellectual property from breaches (what other purposes do patents otherwise, indeed, have?). But when one is just looking for confirmation of an already preconceived narrative, one, of course, is easily swayed.
REFERENCES
1. Boncompagni T. The New Yor Times, January 18, 2019; https://www. nytimes.com/2019/01/18/nyregion/how-norbert-gleicher-fertility-ex pert-spends-his-sundays.html
2. Torbati Y. The Washington Post July 30, 2023; https://www.washington post.com/business/2023/07/30/fertility-supplement-dhea-gleicher/
3. Torbati Y. The Washington Post, April 14, 2023; https://www.washing tonpost.com/business/2023/04/12/egg-freezing-storage-prices/
4. Barad DH, Gleicher N. Fertil Steril 2005;84(3):756
5. Prizant et al., J Endocrinol 2014;222(3):R141-R151
6. Zhu et al., Reprod Biol Endocrinol 2023;21:64
7. Gleicher et al., Reprod Biomed Online 2018;37(2):172-177
22 | SEPTEMBER 2023 | The Voice ADVERTISEMENT
Continued from page 21
NEW CHR PUBLICations
The summer months of July and August produced three new publications this year by CHR investigators, briefly discussed below. As always, reprints are available by contacting arata@thechr.com
Nicholas C, Darmon S, Patrizio P, Albertini DF, Barad DH, Gleicher N. Changing clinical significance of oocyte maturity grades with advancing female age advances precision medicine. iScience 2023; 26:107308
We, for several reasons, consider this publication one of the more important among recent publications from the CHR: First, it upends a long-held dogma in IVF that maturity grades of oocytes do not change in their ability to produce embryos, with MII (mature) oocytes having the by far best capacity, GV (very immature) oocytes the by far worst, and MI (mildly immature) oocytes falling in-between those two extremes. In this paper, the CHR’s investigators for the first time demonstrate that these three maturity grades of oocytes to significant degrees change in their respective abilities as women advance in age: MIIs greatly deteriorate with advancing female age, MIs remain mostly static, while GVs greatly improve.
These observations not only overturn a physiological dogma that existed since the inception of clinical IVF, but they also call for significant changes in current IVF practice, further refining HIER (Highly Individualized Egg Retrieval), practiced at the CHR since 2015.1,2 Indeed, all oocytes in this study were obtained after HIER cycles, meaning that all were retrieved early, many with ovulation triggers given as early as 11-12mm lead-follicle size. As already reported in the center’s earlier HIER studies, early retrievals did not increase oocyte immaturity. Cycles with HIER, indeed, still routinely demonstrate similar spreads in maturity grades, as observed with usual trigger sizes at 18-22mm. This study not only confirmed this fact once more, but also suggested that with advancing age MII oocytes become less and less desirable, and retrieval times, on purpose may have to be even further advanced to rather get immature MI and GV oocytes than overmatured MIIs. The next question to be answered now is how early can oocytes be retrieved?
The third important conclusion from this study was that overnight rescue in vitro maturation (rIVM) of MI and GV oocytes is worthwhile. Again, the CHR has been practicing such rIVM since 2015 and reported on outcomes for the first time in 2016.3 A very recent study from Human Fatemi’s group in Abu Dhabi, UAE, also confirmed the clinical value of rIVM.4 However, most IVF centers still quite automatically discard GV oocytes. At least in older women, this recent CHR study suggests that this may be a waste.
Finally, this manuscript further strengthens the concept the CHR has been advocating since 2015 that in trying to develop precision medicine in association with IVF, retrieval timing, based on patient age and some other patient characteristics, must be individualized. Here presented recently published paper reemphasizes this message and further enhances precision.
REFERENCES
1. Wu et al., J Endocrinol 2015;226(3):167-180
2. Wu et al., J Ovarian Res 2018;11(1):23
3. Lee et al., Endocrine 2016;52(1):166-171
4. Elkhatib et al., Hum Reprod 2023;38(8):1473-1483
Patrizio P, Gleicher N. A new test for preimplantation genetic testing for aneuploidy (PGT-A) and structural chromosomal imbalances (PGT-SR) is non-inferior to current platforms but still not clinically useful. Clin Chem 2023;69(8):791-792
In this “Editorial” for the journal Clinical Chemistry, Drs. Patrizio and Gleicher were asked to comment on a newly developed test for PGT-A and PGT-SR, which by the authors was demonstrated to be non-inferior to currently used platforms. We in answering a question from a patient in the next section of this newsletter, make the point that in current infertility practice, a considerable amount of unnecessary and, at times, to final treatment outcomes
Continued on page 24
CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR
The V oice | SEPTEMBER 2023 | 23
harmful testing is performed (in that case regarding reproductive immunology). But the same argument can be also made regarding PGT-A and has been made in these pages much too often to be repeated here again.

The main point this editorial made was that before planning on developing a new diagnostic test in medicine and running it through appropriate validation studies (which in reproductive medicine also only too often is not the case), one, of course, should consider whether this test will serve any valid clinical purpose? In association with PGT-A, which increasingly, for biological and mathematical reasons, is recognized as simply no longer a viable hypothesis, what is the purpose of yet another test if the underlying hypothesis has been mostly refuted?
Gleicher N, Barad DH. Some caution about DHEA supplementation. Fertil Steril Rep 2023. www.fertstert.org/news-do/some-caution-dhea-supplementation
In this brief report, the CHR’s investigators report that DHEA products in recent years appear to have increased in potency, requiring more frequent step-downs from the routine supplementation dosage of 25mg TID. They also point out the need to test androgen levels in patients before and during supplementation with DHEA.
CONFLICT STATEMENT: Please be advised that the CHR and the authors of this paper may have commercial conflicts regarding the addressed issue: The CHR and both authors co-own several patents that claim treatment benefits from supplementation of hypo-androgenic women with androgens, including DHEA. All parties also receive royalties from these patents. N.G., moreover, owns shares in a company (Fertility Nutraceuticals, LLC, d.b.a. Ovaterra) that produces a DHEA product (Fertinatal) and is a member of the Board of the company.
CHR
CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR
CHR CHR CHR CHR CHR CHR CHR
24 | SEPTEMBER 2023 | The Voice Continued from page 23
UESTIONSPATIENTS AND THEPUBLIC ASK
QWhat is the purpose of HLA DQ-Alpha testing?
We greatly appreciate this question because it gives us an opportunity to transparently address the current limitations that the field of reproductive immunology does not always correctly reflect. This simple-sounding question in reality is anything but simple because Human Leukocyte Antigen (HLA) really sits at the very core of the human immune response.
As readers of The VOICE know, whenever we start discussing the interplay of pregnancy with the maternal immune system, issues get complicated and often controversial. Many colleagues, several among whom we greatly appreciate, therefore, “don’t believe that the maternal immune system matters much in infertility,”



As we recently reviewed in an earlier issue of The VOICE, the CHR in contrast believes exactly the opposite: Pregnancy in our opinion, first and foremost, is not, as widely believed, primarily an endocrine (hormone-dependent), but an immune system-dependent condition. Since the implanting fetus is a semi-allograft (in 50% a mini-organ transplant from the father), before anything else happens, it must be immunologically tolerated by the maternal immune system.
For over 40 years, the CHR has been able to claim special expertise in reproductive immunology because our Medical Director and Chief Scientist, Norbert Gleicher, MD, has been an active researcher in reproductive immunology since 1975. Already in 1976, while a 2nd-year OB/GYN resident at Mount Sinai Medical Center in NYC, he secured grant funding and established one of the first reproductive immunology research laboratories in the world. By 1979, he was appointed head of the first reproductive immunology division in an OB/ GYN department in the country and was a co-founder of the American Society for Reproductive Immunology (and the Society’s first VicePresident). In the same year, he also became the founding Editor-in-Chief of the society’s journal, The American Journal of Reproductive Immunology, which he edited for almost 20 years. Reproductive immunology has, therefore, been “at home” at the CHR for over 40 years.
The CHR’s long interest in, and association with, reproductive immunology is not pointed out to in any way claim superior knowledge and/or expertise. Very much to the contrary, the CHR’s historical association over many decades with the evolution of reproductive
Continued on page 26 The V oice | SEPTEMBER 2023 | 25
immunology in the U.S. provides the CHR with a bird’s-eyeview which gives us the timidity to appreciate how limited progress has been over the decades and how little to this day is known about key-issues in reproductive immunology. To make progress and, even more importantly, to do so with scientific credibility, mandates transparency in determining what is and what is not known. For example, we must acknowledge that to this day, we only have a very limited understanding of one of the most magical miracles underlying our existence as a species: how our mothers’ immune systems do not reject us when we invaded them as microscopic-size embryos like, otherwise, only “foreign” parasites do. We then, again like parasites, (in most cases) take residency up in our mothers’ uteri, though, in contrast to parasites, for only a limited time period of on average nine months before, in an also still only poorly understood process, being expelled into the world during labor.
We have noted before in these pages and in the medical literature that to become “invisible” to a mother’s immune system, the implanting embryo – if everything works wellestablishes a crosstalk with her immune system that induces so-called tolerance pathways in the maternal immune system.1 If things do not work well, implantation may not take place, the immune system of the mother may not be “blinded” and may start attacking an already implanted pregnancy, leading to pregnancy loss. In later pregnancy, tolerance may be lost early, likely leading to early labor and/ or delivery, preeclampsia, and other complications of pregnancy. Remarkably, we still do not understand the underlying pathophysiology well enough for practically all these complications of pregnancy. Considering how much progress has been made in the understanding of the pathophysiology of, for example, cardiovascular diseases or cancer, it becomes difficult to ignore how much reproductive immunology has been lagging.



Much of what is currently “sold” on the Web and by some laboratories, claiming a better understanding of what represents normal vs. abnormal tolerance of the fetal semi-allograft unfortunately, is not based on biological realities, can often not be reliably tested out with laboratory tests and, most importantly, offers no established treatments. Assuming you don’t believe us, we suggest a short trip across some of the websites claiming “special” knowledge, which will often reveal completely contradictory claims, often lacking biological realities. Take, for example, the obsession of some colleagues with testing peripheral natural killer (NK) cell counts, even though it is widely acknowledged that peripheral NK cells and endometrial NK cells (which interact with the implanting embryo) neither correlate in numbers nor in functionality.2 Much of the testing often ordered on patients with suspected immunological reproductive failure, whether due to failure to implant or repeated pregnancy loss,
is, indeed, completely invalidated. From this follows that the diagnoses reached from such testing and treatment recommendations based on these unvalidated diagnoses, often make little sense.
This brings us, with much apology for the delay, finally to the question of what the purpose of HLA DQ-Alpha testing is. The table below explains how HLA is divided into Class I, Class II, and nonclassical HLA-G on the extravillous cytotrophoblast. With paternal semen and the embryo carrying paternal HLA haplotypes, they are for the maternal immune system part of the message, leading to the induction of tolerance pathways. If a father and mother share haplotypes, which means that they are genetically more similar, the current theory holds that signals to the mother’s immune system weaken with increasing similarity, leading to an increased miscarriage risk because the maternal immune system fails to adequately reprogram itself.3
Human Leukocyte Antigen (HLA)
Class I: HLA-A
HLA-B
HLA-C
Class II: HLA-DQA1*
HLA-DQB1
HLA-DRB1
HLA-DRB3/4/5

HLA-G
*here discussed
Continued from page 25 26 | SEPTEMBER 2023 | The Voice
The DQA gene is located on chromosome 6. Embryos inherit 2 haplotypes from mother and father, respectively. Matches in DQA can be heterozygous (x1) or complete (x2). Under heterozygous circumstances, 50% of embryos will carry the matched allele. If both alleles match, 100% of embryos will carry the allele. The clinical meaning of these findings has remained highly controversial, with alleged consequences in the literature greatly varying.
If a couple matches 100%, they are frequently advised that their road to pregnancy is limited, and treatment recommendations include such extremes as the use of third-party donor sperm, a gestational carrier, donor embryos, or adoption.4 Others have associated the excess sharing of HLA with primary and secondary recurrent abortions (including chemical pregnancies),5 but to the best of our knowledge, nobody has credibly been able to demonstrate that HLA sharing between partners adversely affects implantation and, therefore, fertility. Two HLA DQ-Alpha compatible alleles were more frequent in repeat aborters than fertile controls and births with two such matches demonstrated a deficit which, however, is more likely the consequence of pregnancy loss (including chemical pregnancies) than implantation failure. Chemical pregnancies appear especially associated with two matches. At least one small study has demonstrated no effects of HLA sharing on fecundity.6
Testing for HLA-DQ-Alpha is a good example of what we described above: an immune test widely ordered, often misinterpreted in its effects, and quite commonly leading to clinical treatments that are unnecessary. What the literature also frequently does not reflect is the fact that the few studies available in the literature to guide the decision-making process regarding HLA matches are often based on very small patient numbers with very questionable statistical significance in findings.
One final point: Increased sharing in the HLA system has been associated with increased miscarriage risk, but also with increased immune system abnormalities in mothers during early pregnancy, including absent paternal cytotoxic antibodies, ant-idiotypic antibodies, and mixed lymphocyte reaction blocking antibodies, overactive T-helper cell cytokines, and NK cells.5 However, increased miscarriage risks are also independently associated with maternal immune abnormalities, especially with the evidence of a hyperactive immune system (autoimmunity, inflammation, allergies). Several years ago, the CHR’s investigators reported a fairly typical implantation rash over the lower face, neck, and chest, occurring several days post-embryo transfer in couples with excessive Class II HLA matches and repeated prior pregnancy losses.7 This confluence of miscarriage risk, parental HLA matches, and immune system abnormalities appears to be factual and makes biological sense, considering the importance of the HLA system in guiding immune responses against invading organisms.
Adding up what is known and unknown regarding HLA testing in general, the CHR does not recommend routine HLA testing in infertility and sees no evidence in the literature that increased HLA matches are significantly associated with infertility. Class II matches, however, appear associated with repeat chemical and later occurring pregnancy loss and we do recommend HLA testing if no other causes for repeat pregnancy losses are suspected. This recommendation is based on the fact that the CHR’s clinical (anecdotal) experience couples with repeated pregnancy loss who demonstrate excessive HLA matching demonstrate best outcomes with a combination of immunosuppression with glucocorticosteroids and intravenous gamma globulin (IV-Ig), while most patients with only isolated autoimmune and/or inflammatory repeat pregnancy loss, at least as a first-line treatment, do not require IV-Ig. In the CHR’s experience, couples with excessive HLA sharing rarely require more drastic treatments, like gamete or embryo donation and/or the use of gestational carriers.
REFERENCES
1. Gleicher N. J Transl Med 2018;16(1):149
2. Marron K, Harrity C. J Assist Reprod Genet 2023;40(2):381-387
3. Tang et al., Compu Intell Neurosci 2022;8005538
4. http://www.pathtobirth.com.au/path-to-birth-blog-post/dq-alpha-and-ttc
5. Kwak-Kim et al., https://www.ncbi.nim.nih.gov/books/NBK6615/
6. Check et al., Clin Exp Obstet Gynecol 2001;28(3):142-143
7. Gleicher N, Barad DH. Dermatology 2011;222(3):206-211

EMBRACE and other so-called non-invasive chromosomal tests of embryos in IVF
If we already are talking about unnecessary and potentially harmful tests, let us talk about EMBRACE (which astutely stands for Embryo Analysis of Culture Environment),1 the latest commercial product from Igenomix (part of Vitrolife Group), one of the world’s largest provider of PGT testing services and the company that gave the IVF world the invaluable gift of endometrial receptivity testing (of course stated facetiously), as receptivity testing only recently in an excellent study by U.S. colleagues in JAMA was finally revealed as yet another useless test pushed upon IVF clinics.2 Like PGT-A, receptivity testing in general populations does not, as is claimed, improve IVF outcomes and actually, reduces pregnancy and live birth chances in some subgroups. Igenomix, is, thus, a company built on the highly successful marketing efforts of two allegedly validated diagnostic tests, inappropriately claiming to improve IVF outcomes.
With EMBRACE, the company now offers yet another supposedly new concept in IVF, - so-called non-invasive PGT-A (niPGT-A), in Australia already shut down by Monash IVF, one of the country’s largest IVF clinic chains, because of a major class action suit,3 and in the U.S., at least in one IVF center, allegedly under FDA-scrutiny.
Igenomix describes EMBRACE as, “the non-invasive test that allows us to prioritize transfer of embryos that are the most likely to be
The V oice | SEPTEMBER 2023 | 27 Continued on page 28
euploid.”1 If one untangles those carefully chosen words, here is what they are meant to say: (i) This is not your regular PGT-A that requires an invasive embryo biopsy. We are instead “non-invasively” testing the culture media in which the embryo was cultured. (ii) We are getting the same results as traditional “invasive” PGT-A, though with much less “pain” for the embryo. And (iii) we can’t be sure which embryo is euploid, but we can tell you which embryo is most likely euploid, which should improve pregnancy and live birth chances.
Unfortunately, only the first point is correct: whether you call it EMBRACE or give it another marketing-friendly name, it is absolutely correct that in contrast to traditional PGT-A, this test is less “invasive.” But this is where the truth ends, and, false claims, even if carefully worded, take over: To the best of our knowledge – and we are watching the literature very carefully - not a single study in the literature has been able to demonstrate similar accuracy for niPGT-A in comparison to traditional PGT-A. Though quite a number of comparative studies have been published, practically all demonstrated comparatively lower accuracy than traditional biopsy-driven PGT-A. And that should not surprise because if aneuploid (chromosomal-abnormal) DNA is discovered in the spent media, one does not know where it is coming from (trophectoderm or inner cell mass) and whether its concentration in media reflects that only a few or many cells of an embryo are aneuploid, - and that, of course, matters.
But most importantly, as also discussed in a recent editorial, Drs. Patrizio and Gleicher were asked to write for a medical journal that published a paper about a different new diagnostic PGT-A platform still involving embryo biopsy (briefly reviewed under “New CHR publications”),4 who cares how PGT-A is done if the whole basic hypothesis of PGT-A, namely that PGT-A allows accurate differentiation between euploid and aneuploid embryos, is incorrect. In such a case, how PGT-A is done almost no longer matters. But if more accurate biopsy-driven PGT-A does not improve IVF outcomes, why would the less accurate niPGT-A, EMBRACE, work? But apparently, that does not matter for Igenomix (and some other providers of niPGT-A). After all, it is just one more among many useless tests in IVF practice. Who cares if some patients’ pregnancy chances are actually adversely affected?!
REFERENCES
1. Igenomix. https://www.igenomix.eu/our-services/embrace/; accessed August 8, 2023

2. Doyle et al., JAMA 2022;328(21):2117-2125
3. Tran D. https://www.abc.net.au/news/2020-12-23/class-actionagainst-monash-ivf-fertility-clinic/13010682, Accessed August 8, 2023
4. Patrizio P, Gleicher N. Clin Chem 2023;69(8):791-792
The supplement PQQ in infertility
PQQ, among several names also called pyrroloquinoline quinone, has been used by some IVF clinics for over 10 years. It is not only found in fruits and vegetables but also breast milk. What it exactly does in the human body is not well understood yet. What is known is that PQQ acts as a very potent antioxidant, which is apparently more powerful in fighting free radicals than Vitamin C. Though human studies are sparse, and studies in women practically do not exist, there are suggestions that PQQ improves mitochondrial numbers and function. If confirmed, this could be of special importance for older women and women with abnormally low functional ovarian reserve because both of these conditions are believed associated with poorer mitochondrial function.
PQQ also is alleged to have anti-inflammatory functions, lowering CRP (C-Reactive Protein) and IL-6 (Interleukin-6), which also may be helpful in some infertility patients since we, here at the CHR, do not like abnormally high inflammatory markers such as high CRP and IL-6, suggestive of a hyperactive immune system.1,2 Finally, PQQ has also been reported to be nootropic, which means it allegedly helps memory, attention, and learning, especially helping older people with poor memory.
Though widely used in IVF clinics, the literature lacks studies on the use of PQQ in IVF and female infertility in general. The utilization of PQQ in IVF, therefore, has little objective basis. The only study we were able to find in relation to infertility treatments was a report of PQQ added to a culture medium, which may have improved mitochondrial activity in oocytes.3
Little is known about the potential side effects of PQQ supplementation, but we at the CHR have concerns about the combined use of PQQ with CoQ10. As both of these substances are antioxidants, and excessive antioxidant activity can be toxic to cells,4 we recommend against co-use of the two, which, unfortunately, is quite common in many IVF clinics.
REFERENCES
1. Kushnir et al., Am J Reprod Immunol 2016;75(6):672-677
2. Weghofer et al., Acta Gynecol Obstet 2020;301(3):831-836
3. Santiquet et al., Mol Hum reprod 2017;23(9):594-606
4. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/ antioxidant-supplements-what-you-need-to-know
28 | SEPTEMBER 2023 | The Voice Continued from page 27
Has the utilization of HIER at the CHR changed since it was introduced to IVF?

HIER stands for Highly Individualized Egg Retrieval, a concept the CHR introduced into its practice in approximately 2014 after discovering through basic molecular research that follicles with advancing age luteinize at progressively earlier sizes.1 Once follicles are luteinized, their eggs are over-matured and will not lead to pregnancy. The CHR, therefore, started to adjust lead follicle size at ovulation trigger for retrieval according to the age of the patient.


A first study set a lead follicle size of 16mm at trigger for women at age 43.1 This stood in contrast to lead follicle trigger sizes between 18mm and 23mm which have been the routine for all IVF cycles since the inception of IVF and to this day are the customary trigger sizes at practically almost all IVF clinics.
Following this initial study, the CHR’s investigators, however, discovered that for many women, a 16mm trigger size was already too late.2 The CHR, therefore, has since routinely retrieved women with as small as 11mm to 12mm lead follicle sizes.2 The CHR’s (and also the world’s) two oldest women to conceive with the use of their own eggs, both only days from their 48th birthdays at the time of embryo transfers, indeed, were triggered at lead follicle size 12mm.3

Though there was originally an assumption that so much earlier egg retrievals would increase the number of immature eggs, this did not happen. The spread of egg maturity, surprisingly, remained rather constant. This meant that some eggs would still be mildly immature (MI oocytes) or severely immature (GV oocytes). Because most of the CHR’s patients produce only small egg numbers, the CHR, in contrast to many other IVF clinics, since ca. 2014 has not been discarding MI and GV oocytes but, with quite a success, has been attempting so-called rescue in vitro maturation (rIVM) on such oocytes overnight.4 Only very recently colleagues in Abu Dhabi, UA, also confirmed the clinical value of this practice,5 which has been routine at the CHR since 2014.
Practicing rIVM over many years, the CHR’s embryologists and physicians relatively recently noticed that the success of rIVM appeared to vary depending on patient age. To explore this clinical impression further, they retroactively investigated their experience with rIVM in women of different ages and made another quite groundbreaking discovery that changed how we now view MII, MI, and GV oocytes at different ages: A recently published in the science journal iScience, and in more detail discussed in this issue of The VOICE under “New CHR publications,” the longstanding dogma that oocyte maturity grades maintain their functional quality of producing good quality eggs was proven wrong.6 As the study very surprisingly demonstrated, MII oocytes lost ability and GV oocytes gained ability with advancing age, while MI oocytes more-less maintained their ability to produce good quality embryos.
These results were surprising and in several ways are practice-changing: (i) They reaffirm the validity of rIVM because all immature MI and GV oocytes in this study had undergone rIVM. (ii) The study also reaffirmed the importance of HIER because all patients in this study had undergone HIER cycles. (iii) This study also suggested that egg retrievals may have to be triggered at even smaller lead follicle sizes than 11mm to 12mm as women get into ages beyond 45 because most desired maturity targets at such ages may no longer be MII oocytes but MI and GV oocytes. (iv) Finally, this study also suggests that immature oocytes no longer should be disposed of but should undergo rIVM overnight.
The ultimate message from this recent CHR publication is that the days of routine ovulation triggers in IVF between 18-22mm are over and are to be replaced by increasingly finetuned and individualized ovulation trigger times, based on smaller and smaller lead follicle sizes, ultimately leading to an advancement toward precision medicine in IVF for every infertility patient.
The V oice | SEPTEMBER 2023 | 29 Continued on page 30
REFERENCES
1. Wu et al., J Endocrinol 2015;226(3):167-180
2. Wu et al., J Ovarian Res 2018;11(1):23
3. Gleicher et al., Reprod Biomed Online 2018;37(2):172-177
4. Lee et al., Endocrine 2016;52(1):166-171
5. Elkhatib et al., Hum Reprod 2023;38(8):1473-1483
6. Nicholas et al.,iScience 2023;26:107308


How is adrenal insufficiency linked to female infertility?

This is another interesting and very opportune question because, while the two adrenal glands, in general, are not considered among reproductive organs, the CHR’s investigators over the last decade have increasingly come to view the adrenals in women as a very integral part of the reproductive organ system. There are three major reasons for this, but all are not very widely appreciated: (i) Adrenal glands and ovaries share an embryonic primordium, In other words, in very early organ development adrenals and ovaries were one organ. (ii) After ovaries, the adrenal glands have the greatest density of AMH receptors; yet no AMH function has been so far attributed to the adrenals. And (iii) through theca cells and zona reticularis, respectively in ovaries and adrenals, women share androgen production between those two organs in approximately equal parts.
CONFLICT STATEMENT: Please be advised that the CHR may have commercial conflicts regarding the here-addressed issue: The CHR and some of its employees co-own several patents that claim treatment benefits from supplementation of hypo-androgenic women with androgens, including DHEA. These parties also receive royalties from these patents, own shares in a company (Fertility Nutraceuticals, LLC, d.b.a. Ovaterra) that produces a DHEA product (Fertinatal), and are members of the Board of the company.
It was the recognition that androgens are important for ovarian function,1 that attracted investigators at the CHR to the adrenals. First recognizing hypo-androgenism as a typical finding in premature ovarian aging (as well as primary ovarian insufficiency),2 and later also in other female infertility diagnoses, including so-called secondary ovarian insufficiency (SOI)3 and so-called hyper/hypo-androgenic polycystic ovary syndrome (HH-PCOS), practically the D phenotype of PCOS, though only after age 35,4 they discovered that all of these cases of infertility-related hypo-androgenism in women were caused by adrenal insufficiency in androgen production (and not ovarian).
Since abnormally low androgens often adversely affect ovarian function by inhibiting normal follicle growth and maturation,1 the adrenal glands through their androgen production can affect ovarian function. This, therefore, establishes an adrenal-ovarian functional hormonal axis of potentially significant relevance for female fertility. Adrenals are, consequently, defined as reproductive organs not only based on embryology but also function. The CHR’s investigators already suggested several years ago that the insufficiency of adrenal androgen production in the zona reticularis – currently excluded – should be included in the diagnosis of adrenal insufficiency. This diagnosis currently only includes hormone insufficiencies created by the other two zonae.5
REFERENCES
1. Prizant et al., J Endocrinol 2014;222(3):R141-R151
2. Gleicher et al., Hum Reprod 2013;28(4):1084-1091

3. Gleicher et al., Endocrine 2021;72(1):260-267
4. Gleicher et al., Biomedicines 2022;10(7):1505
5. Gleicher et al., J Clin Endocrinol Metab 2017;102(9):3569-3570
30 | SEPTEMBER 2023 | The Voice Continued from page 29
Scan the QR code to watch the FMRC 2023 introduction video!




ADVERTISEMENT The V oice | SEPTEMBER 2023 | 31

Continued from page 31 32 | SEPTEMBER 2023 | The Voice ADVERTISEMENT

Continued from page 32 The V oice | SEPTEMBER 2023 | 33 ADVERTISEMENT

34 | SEPTEMBER 2023 | The Voice Continued from page 33 ADVERTISEMENT

The V oice | SEPTEMBER 2023 | 35 Continued from page 34 ADVERTISEMENT


36 | SEPTEMBER 2023 | The Voice ADVERTISEMENT ADVERTISEMENT
RECENT LITERATURE, relevant to REPRODUCTIVE MEDICINE
Mostly placed into a clinical context, we in this section of the newsletter offer commentaries to a broad survey of articles in the English literature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. Since this is the first issue after the newsletter’s summer break in July and August, we this month offer almost three months of literature review.
Articles are mostly chosen for their translational value for clinical medicine, even if, superficially, a direct link to clinical practice may not always be instantly obvious. Chosen articles, even if, for example, basic science or business articles, often help in determining where clinical practice will likely go and, by doing so, serve an important translational purpose.
Translational research has been the CHR’s principal goal since its founding in 1981 and has over the years produced a significant number of U.S. patents. It has also propelled the CHR into its current status as a worldwide center of last resort for infertile patients who have failed treatments elsewhere. This section of The VOICE, demonstrates and makes public the process through which the CHR for decades has been following and interpreting on an ongoing basis the published literature, a process always at the very core of how research and clinical practice have evolved at the CHR.
The business of
infertility
Does greed stand in the way of IVF changing health care forever?
Lorin Gu, a founding partner at Recharge Capital, just before the summer published an interesting opinion article in Femtech World, 1 under the challenging title, “IVF has the potential of changing healthcare forever, but greed is in the way.” This is for several reasons an interesting opinion piece and offers a very good introduction to this section of the literature review, even if we do not agree with several statements.
To start with, the article offers interesting technical information on the IVF business: First, the U.S. IVF market is unquestionably rapidly growing, with cycle numbers between 2015 and 2020 increasing by roughly 41%, at an annual growth rate of over 7%. At alleged average cycle costs of US$ 20,000-25,000, he feels that much of the potential patient population is, still priced out of the market.
We frankly don’t know where these numbers are coming from and, especially based on increasing insurance coverage – with attached lower cycle reimbursement rates – we consider them highly exaggerated. This, however, does not mean that for many, IVF is still unaffordable and, therefore, unavailable.
We also disagree with his representation that IVF cycle outcomes over this time period significantly improved. However, this is not the place to discuss the complexities of cycle outcome assessments. Where things get more interesting is when he begins to make projections into the future by claiming that many AI start-ups have been established with the purpose of further improving outcomes and, by doing so, reducing required cycles to achieve a pregnancy/delivery. Indeed, nearly US$100 million has apparently been raised “from top investors” for that purpose. Most of these projects apparently are geared at embryo selection, which, as readers of The VOICE by now are probably aware of, is a hypothesis the CHR is very skeptical about. We simply do not believe that embryo selection is worth the huge investments made into this hypothesis almost since embryo selection cannot improve cumulative pregnancy/live birth chances of a cycle’s embryo cohort; all it
the
chr’s interpretation of
Continued on page 38 The V oice | SEPTEMBER 2023 | 37
at best can do, is minimally improve time to pregnancy, and that is not worth those huge investments.
However, he then comes to the centerpiece of his article, which is the very obvious conflict between fertility clinics, as business enterprises, obviously being motivated to produce profits, and the equally obvious fact that higher success rates would reduce profit.
This, too, has been a repeated subject of The VOICE, noting that what represented competition between IVF clinics has changed drastically in recent years: while in the earlier days of IVF clinics competing based on cycle outcomes (better pregnancy rates meant more business), the increasing industrialization of IVF through creation of mega-clinics and national clinic networks has dramatically changed the fabric of competition, away from clinical competition based on outcomes to a more traditional marketing competition emphasizing in principle secondary issues, mostly unrelated to cycle success rates. He in this context notes that over the last decades, over half of all U.S. IVF clinics have become parts of clinic chains, mostly controlled by private equity and – who can blame them - the purpose of private equity is, of course, maximal profit for their investors.
Over the summer months, an onslaught of articles in some of the leading medical journals pointed out how equity takeovers of aspects of health care is harming patient care more than had even been understood before. Private equity in the U.S. currently owns 11% of U.S. skilled nursing facilities and 4% of hospitals. As medical care in all of its aspects is always a long-term enterprise, harm to this system is baked into every takeover by private equity because the business model of every private equity fund is to hold purchases for only 3-7 years, at which point they must be sold at maximal profit.2 A recent systematic review mostly only applicable to the U.S. of health outcomes, costs, and quality after private equity takeovers can only be described as “devastating.” To quote the authors: “Trends in private equity ownership rapidly increased across almost all healthcare settings studies. Such ownership is often associated with harmful impacts on costs to patients or payors and mixed with harmful impacts on quality (of medical care).3
This article attracted media coverage, but this coverage was surprisingly subdued, considering the obvious importance of national health care, - but also for the U.S. economy. A very detailed report on the subject was also recently published under the title,” Monetizing Medicine: private equity and competition in physician practice markets,” in collaboration with the American Antitrust Institute, The Petris Center of the School of Public Health at the University of California, Berkley, and the Washington Center for Equitable Growth. 4 We are here quoting verbatim the report’s conclusions, which also attracted media attention.5
• PE acquisitions of physician practices are increasing. We find that private equity (PE) firms have been increasingly acquiring physician practices across a number of physician specialties since 2012, increasing from 75 deals in 2012 to 484 deals in 2021, or more than a six-fold increase in only 10 years.
• PE firms are amassing high market shares in local physician
practice markets. At the local level, we find that individual PE firms are acquiring competitively significant shares of physician practice markets. In particular, in 28% of metropolitan statistical areas (MSAs), a single PE firm has more than 30% market share by full-time-equivalent physicians, and in 13% of MSAs, the single PE firm market share exceeds 50%.
• PE acquisitions are associated with price and expenditure increases. In 8 of the 10 physician practice specialties we study, we find statistically significant price increases associated with PE’s acquisition of a practice. These price increases range from 16% in oncology to 4% in primary care and dermatology. PE acquisitions are also associated with per-patient expenditure increases for 6 of 10 specialties, ranging from 4% to 16% depending on the specialty.
• Price increases associated with PE acquisitions are exceptionally high where a PE firm controls a competitively significant share of the local market. When we focus our analysis on markets where a single PE firm controls more than 30% of the market, we find further elevated prices associated with PE acquisitions in each of the 3 specialties with statistically significant results, for gastroenterology (18%), obstetrics and gynecology (16%), and dermatology (13%).
• Increased attention to the competition impacts of PE in physician markets is urgently needed. The vast majority of the PE acquisitions studied in this report took place without federal antitrust scrutiny and with limited state antitrust scrutiny. The market share and price results reported here indicate that more scrutiny is warranted on PE’s impact on competition. The pace at which PE is entering these markets and monetizing medicine makes a quick response imperative.
• Changes to Hart-Scott-Rodino (HSR) requirements, reimbursement policies, and tax policies are needed. At a minimum, federal antitrust reporting requirements must be adapted to modern business models, including PE, to ensure regulators have the information they need to evaluate the competitive impacts of these deals. The FTC has recently begun that process, which we applaud. HSR changes alone, however, are not enough, and we also recommend changes to Medicare reimbursement policy and tax policies that are driving conclusions and PE opportunities.
• More study is needed to understand the impact of PE acquisitions on healthcare. More study, starting with the development of better data sets, is needed to understand the complex impact of PE ownership in physician practice and healthcare markets generally. Expansion of healthcare ownership transparency beyond nursing homes is an important first step. Public reporting of PE deals would also facilitate understanding. Finally, building consensus around healthcare quality measures and the adoption of mandatory reporting would both enable better study of competition impacts and, potentially, incentivize PE funds and others who are monetizing medicine to seek high-quality healthcare, not just high profits.
38 | SEPTEMBER 2023 | The Voice Continued from page 37
In discussing excessive costs brought into health care through acquisitions, private equity is, however, not the only guilty party. As a recent editorial by former Louisiana governor (2008-2016) Bobby Jindal (and a co-author) in The Wall Street Journal noted, similar price gauging also occurs when hospital systems acquire private office practices. Here it is the addition of “facility fees” by the hospitals, even if services are provided in the same offices by the same physician providers, that will significantly inflate costs. Examples given in the editorial were a US$ 1,262 facility charge added to an arthritis treatment of a retiree which increased his co-pay ten-fold, and a US$ 847 facility fee for a telehealth consultation.6
And who acquired over the last five years the most physician practices? Bruce Giles recently offered the answer in a brief article in Becker’s Hospital Review:7 Private equity was the clear winner with 65%, followed by physician medical groups (14%), payers (11%), hospitals and health systems (8%), and others (4%).
REFERENCES
1. Gu L. https://www.femtechworld.co.uk/opinion/ivf-has-the-potential-tochange-healthcare-forever-but-greed-stands-in-the-way/
2. Goozner M. BMJ 2023;382:p1396
3. Borsa et al., BMJ 2023;382;e075244
4. Schefflet et al., https://www.antitrustinstitute.org/wp-content/up loads/2023/07/AAI-UCB-EG_Private-Equity-I-Physician-PracticeReport_FINAL.pdf
5. Whoiriskey P. The Washinton Pos. https:// www.washingtonpost.com/business/2023/07/10/ private-equity-raising-prices-doctors-practices-private-equity-doctors/
6. Jindal B, Katebi C. The Wallstreet Journal, July 27, 2023; pA17. https:// www.wsj.com/articles/doctors-office-care-at-hospital-prices-dishon est-billing-identifier-number-out-patient-fe670b3e
7. Giles B. Becker’s Hospital Review, June 29, 2023. https://www.beckershos pitalreview.com/disruptors/payers-big-tech-who-acquired-the-most-phy sicians-in-past-5-years-per-aha.html
Related IVF business news from Griffin Jones and others
On August 1, 2023, Griffin Jones reported in an e-mail that US Fertility acquired RMA of New York (RMANY), supposedly the largest fertility center in NYC. RMA-NY, in its original location housed in a space originally built out by the CHR, was upon its founding affiliated with RMA-NJ, an affiliation that did not last for very long. Both organizations, however, held on to the RMA name, just differentiated by the respective affixes, NY and NJ. RMA-NJ was several years ago acquired by the Spanish conglomerate IVI, which in turn, as we recently reported in The VOICE under
the name IVIRMA Global SL was sold for an enterprise value of over US$ 4 billion to KKR&Co
The price paid for the acquisition of RMA-NY has not been disclosed and this purchase represents the second big purchase made by US Fertility in 2023 after purchasing Ovation Fertility in April from Morgan Stanley. Whether Shady Grove-NYC and RMA-NY will be merged or will remain independent of each other has not been announced.
Through its Shady Grove brand, US Fertility had a presence in NYC already before the purchase, with locations in Uptown Manhattan, Soho, and Brooklyn. Its current Medical Director, Tomer Singer, MD, is, however, allegedly after the summer announced to return to a leadership position at his old training grounds at Lenox Hill Hospital of Northwell Health.
That the IVF market in NYC is amid a shake-up, is also demonstrated by the announcement that Oma Robotics, the parent company of Oma Fertility was reported to have gone out of business, according to a second e-mail by Griffin Jones on August 2, 2023. Oma’s principal physician was Michael Guarnaccia, MD; its laboratory director was Barry R. Behr, PhD, HCLD, a well-respected embryologist. Oma was owned by Indian interests, which also own an IVF clinic network in India.
In the same e-mail, Griffin Jones announced that First Fertility’s CEO, Derek Larkin, suddenly, stepped down “to pursue another opportunity.” Three IVF groups in the larger NY area are part of the First Fertility network: RMA-Long Island, The Fertility Institute of New York, and the Center for Advanced Reproductive Services at the University of Connecticut.
Finally, Mitchell Stern from Dresner Partners in an e-mail on August 1, 2023, announced the sale of the Advanced IVF Institute in Illinois, owned by Charles, E. Miller, MD, a former partner in CHR-Illinois, to CCRM Fertility, a large chain of fertility clinics originally founded by William Schoolcraft, MD, in Colorado.
The issue of AI and data analysis in general in reproductive medicine
It suddenly seems that AI is everywhere. We, in the introduction to this section, noted the major investments that are being made in medicine, including reproductive medicine with expectations of leading to improvements and medical care. There are major advances to be expected, especially when it comes to the many
The V oice | SEPTEMBER 2023 | 39
40
Continued on page
revelations that can be expected to come from big data. But, at the same time, it also feels like we are in the midst of a bubble, - a bubble of unrealistic expectations.
We note above our belief that embryo selection, as a hypothesis, makes little mathematical as well as economic sense. It is also important to note that differences between findings, outcomes, or other characteristics medical science seeks to discover with AI between different patient populations require one indisputable reality if AI is to offer results: there must be a difference in the first place. Even AI cannot find a difference where it does not exist.
Contrary to the general perception of AI being everywhere, objective data suggests that the adoption of AI in healthcare delivery is lagging behind other areas. An excellent recent review article on AI in U.S. healthcare delivery explains why.1 One important point lies in the interpretation of what it really means when one is addressing this subject. Administrative uses of AI in health care, for example in claims processing, must be clearly differentiated from clinical utilization in, for example, clinical diagnoses. The quality of care and safety assessments and their improvements likely lie somewhere in between those first two potential applications.
David M. Cuttler, MD from the Department of Economics at Harvard University recently offered five very astute “observations” about the possible effects of AI on medicine: (i) AI is likely to substitute for routine current human activities, such as common office work, including billing, appointment scheduling, and facility management, leading to substantial savings in a range of US$ 200-360 billion. (ii) In contrast, he astutely suggests that in clinical care, AI will not replace but complement clinicians. As already noted above, we could not agree more! (iii) Through newly developed applications, AI can be expected to greatly enhance efficiency through less expensive monitoring methods, diagnoses, and personnel needs, which, in turn, will lead to lower costs for diagnoses. (iv) While not replacing human thinking processes, AI algorithms should be able to extend and improve them, - not the least by eliminating biases we all carry around with us. Lastly (v) he notes that machine learning can be excellent in large data sets in finding patterns that human intuition cannot decipher. This, of course, especially applies to multifactorial patterns that benefit from cluster analyses.
Earlier in this issue of The VOICE, we, in detail, addressed the newly rediscovered popularity of so-called “real-world health data,” recently also discussed in an excellent article by Amy Abernethy MD, PhD, a former Principal Deputy Commissioner of the FDA, and current President of Product development and Chief Medical Officer of a company, in Nature Medicine. 3 Noting that real-world data reflects the diversity and lived experiences of individuals and, therefore, must be a central pillar of clinical research as well as clinical practice, she makes the absolute correct point that real-world data must be developed to its full potential so that it can serve as a true patient-centric research modality.3 AI, of course, can be expected to be very helpful in achieving this goal.
Returning to the specifics of reproductive medicine, we want to point out two recent publications with very similar messages: The
first was a commentary by Jack Wilkinson, PhD, a very accomplished British biostatistician, who in Fertility and Sterility in very easily understandable terms explained why in trials of PGT-A, the use of outcome analyses with reference embryo transfer, simply, does not make any sense.4 It is the best and most transparent explanation we have ever read, and well explains why so much of the published PGT-A literature is not worth the paper these studies are printed on. It’s too bad that most of the IVF field, to this day, has not come to understand this!
Similarly, Mark A. Clapp, MD, MPH in an editorial in Obstetrics & Gynecology, in very clear language demonstrates why even best-designed randomized controlled trials often are not generalizable in broad clinical practice in unselected patient populations when the study populations were well-selected.5
Both of these articles address crucially important issues in how study results should be presented and, maybe even more importantly, how published studies should be analyzed and interpreted when read. Both also point out that in reproductive medicine this, unfortunately often is not the case. This is a problem that can only be tackled in the editorial offices of medical and scientific journals. Maybe it is time to shrink the editorial board and reviewer lists to individuals who understand that.
REFERENCES
1. Sahni NR, Carrus B. N Engl J Med 2023;389(4):348-358
2. Cutler DM. JAMA Helth Forum 2023;47):e232652
3. Abernethy A. Nat Med 2023;29:1317
4. Wilkinson J. Fertil Steril 2021;119(6):910-912
5. Clapp A. Obstet Gynecol 2023;142(2):236-238
Basic science news
Why do synthetic embryos spark so much controversy?
The British reproductive biologist of Polish origin, Magdalena Zernicka-Goetz, PhD, who now oversees laboratories at the University of Cambridge in the U.K. and the California Institute of Technology in the U.S., has now for some time been recognized as one of the leaders in the field. That, however, does not appear sufficient because she strives for even more attention, and she gets it!
At the annual meeting of the International Society of Stem Cell Research (ISSCR) in June, she, as on so many occasions before, was once again the center of attention after reporting that “synthetic” human embryos could be cultures beyond what in “natural” embryos would be the equivalent of 14 days post-fertilization of eggs when gastrulation occurs, and symmetry is broken. First published in the Warnock Report in 1984, international ethical consensus, in one of the most internationally agreed to rules in reproductive science and medicine in general, the so-called “14-day rule” appeared to have settled the issue and became the law of the land.1 The controversy resurfaced in 2016 when the Cambridge laboratory of Zernicka-Goetz2 and the New York laboratory of Ali Brivanlou, PhD at Rockefeller University (with which the CHR maintains a close collaboration) demonstrated that human
Continued from page 39 40 | SEPTEMBER 2023 | The Voice
embryos could be cultured in the lab up to day 13 (and probably even longer, though neither lab tried). By 2021, the ISSCR, indeed, loosened the rules.4,5
When Zernicka-Goetz told a newspaper before her presentation at the Boston conference that her lab had developed the first three-lineage human embryo model from stem cells that allowed for the identification of amnion and germ cell precursors, the media went ballistic. It did not help that some scientists spoke out of both ends of their mouths. A prominent British stem cell expert, for example, reaffirmed that stem cell-derived synthetic embryos would offer “an awful lot of information about how we begin development..., without having to use (real) early embryos for research.” Yet in the next moment, he added, “If the intention is to develop these model embryos to mimic normal embryos, then they should be treated the same.”
To us, this sounds like an absurd and circular logic: synthetic embryos derived from stem cells are not human embryos. Why then should they be treated like human embryos? They are models; and as so-called “integrated embryo models” under the above-noted new ethics guidelines, they can be cultures “for a minimum amount of time” beyond the 14-day threshold.6 Another commentator suggested that Zernicka-Goetz “potentially broke the to this point existing distinction between a ‘natural’ embryo usually produced in an IVF cycle and ‘integrated embryo models,’ derived from stem cells.” We do not see things this way and the main reason is that these so-called “integrated embryo models,” while offering some insights into blastocyst-stage embryos, really are distinctively different as well. Nobody argues with the basic principle that research on synthetic embryos should be done responsibly, but that appears adequately controllable through routine IRB processes.
But, this is also not where this story ended: Well-known NYU medical ethicist, Arthur L. Caplan, PhD, astutely warned about getting too excited about oral reports claiming major new achievements and correctly suggested that waiting for these data to appear in print, may allow for a deep breath before getting too excited.7 But what nobody knew is that, barely a few days later, Zernicka-Goetz8 and stem cell biologist Jacob Hanna, PhD, MD, from the Weitzman Institute in Rehovot, Israel,9 published two preprints posted to the BioRxiv server on June 15 in which they described self-assembled embryo-like-structures to self-assemble from human embryonic stem cells, some of which converted to cells resembling placental stem cells and yolk sac stem cells.10
Those two investigators share a not-too-friendly competitive history, we reported about in The VOICE in the spring regarding earlier publications both laboratories published almost concomitantly on the same subject using mouse embryos.
Mitch Leslie in Science magazine contrasted their two human embryo models: Hanna started with embryonic stem cell lines and with stem cells converted from adult cells. The latter was successfully converted into extraembryonic cell lineages, which
in natural embryos produce the trophectoderm and the placenta and support the embryos, the product of the embryonic cell lineage, throughout pregnancy. Those lineages were then mingled with stem cells, resulting in cell clusters containing trophoblast and other hallmarks of post-blastocyst cells.11 The Zernicka-Goetz lab, in contrast, generated their extraembryonic cell lineages from two kinds of human embryonic stem cells after they were exposed to doxycycline. Those were then mixed with unmodified human embryonic stem cells, thereby producing the above-referred to embryonic cell clumps.
He furthermore reports on two more laboratories that have created human embryo models but have not yet published them: one at the University of Pittsburgh, and the other is a team of investigators in China.
As Philip Ball recently pointed out in Nature, 12 the two labs are not alone: Ali Brivanlou’s laboratory at Rockefeller University also has already a preprint in place that reports another synthetic stem cell-derived human embryo model that demonstrates a signature compatible with gastrulation in natural embryos at approximately day 12 after fertilization.13
The reasons for so much intellectual and financial investment being directed at human embryo models at so many leading research labs are obvious: They are expected to offer significant new insights into early human developmental stages in peri- and post-implantation-stage embryos, to this day a black-box in reproductive research.11 A better understanding of this time period in human development would, likely, produce significant translational applications, including advances in clinical IVF practice, a better understanding of miscarriages and their potential prevention where applicable, and of causes of developmental birth defects and their potential prevention. Therefore, we can expect the speed of new discoveries only to accelerate, with the participation of many additional research laboratories, especially in Asia.
REFERENCES
1. Appleby JB, Bredeoord AL. EMBO Mol Med 2018;10(9):e9437
2. Shahbazi et al., Nat Cell Biol 2016;18:700-708
3. Deglincerti et al., Nature 2016;533:251-254
4. Associated Press. May 26, 2021; https:// www.nbcnews.com/health/health-news/ new-guidelines-suggest-lifting-14-day-rule-growing-human-embry os-n1268628
5. Subbaraman N. Nature 2021;594:18-19
6. Meredith S. Medscape; June 16, 2023; https://www.medscape.co.uk/ viewarticle/synthetic-human-embryos-grown-lab-first-time-2023a1000db3
7. Genetic Engineering & Biotechnology News. https://www.genengnews.com/ news/art-caplan-on-the-ethics-of-creating-human-synthetic-embryonic-mod els/
8. Watherbee BAT et al., Preprint at bioRx 2023;https://doi. org/10.1101/2023.05.15.545082
9. Oldak B, et al., Preprint at bioRx 2023;https://doi. org/10.1101/2023.06.14.544922
10. Reardon S. Nature 2023;618:654-655
11. Leslie M. Science 2023;380(6651):12061207
12. Ball P. Nature 2023;618:653-654
13. De Santis et al., Preprint at BioRxiv 2023; https://doi. org/10.1101/2023.05.16.541017
Continued on page 42
The V oice | SEPTEMBER 2023 | 41
Nuclear DNA shedding during blastocyst expansion and biopsy
A recent paper from a multinational group of investigators, some prominent names in the IVF field in the U.S., U.K., and Spain recently received considerable attention, - not the least because it was published in Cell 1 which, of course, is one of the most prestigious science journals in the world. With Nicholas Plachta’s lab at the University of Pennsylvania apparently having led the effort, all participating scientists deserve credit for this achievement.
Appropriately arguing that ethical restrictions of genetic manipulations in human embryos and lack of dynamic imaging methods in early-stage human embryos have so far restricted studies of major events in early development to mouse models, they in this paper claim to have overcome especially the latter by combining fluorescent dyes with live imaging for the first time. This, in turn, allowed them to visualize the dynamics of such essential processes as chromosome segregation, compaction, polarization, blastocyst formation, and hatching in human embryos.
Interestingly, as the title of their paper suggests, they considered the observation that blastocyst expansion mechanically constrains trophectoderm cells, causing nuclear budding and DNA shedding into the cytoplasm, as the study’s central finding. They also reported that cells with lower levels of perinuclear keratin were more prone to DNA loss. A clinically relevant finding for IVF was the fact that the trophectoderm biopsy is necessary for PGT-A, increased DNA shedding. For reasons that are not clear to us, the authors concluded from this observation that aneuploidies observed in a large majority of blastocyst-stage embryos may not only originate from chromosome segregation errors, but also from shear-stress-related nuclear DNA shedding.
However, we see no direct evidence for this suggestion in their published work and would furthermore argue that by observing shedding in only ca. 5% of cells, this observation only unlikely contributes to in blastocysts observed aneuploidy which likely involves at least 80% of all blastocyst-stage embryos. It is also puzzling why when blastocyst expansion is a hallmark of mammalian embryos in
general, this mechanical stressor would not also be related to DNA shedding in other species.
Interestingly, if the authors’ suggestion of shedding in human embryos contributing to aneuploidy were to be correct, they would have presented yet another argument against trophectoderm biopsies and, therefore, utilization of PGT-A in association with routine IVF, which, as readers of The VOICE by now of course know, has been a strongly voiced opinion of the CHR for many years. Interestingly, the paper, however, did not mention this very obvious clinical conclusion from their suggested finding, and one wonders why?
The paper was, nevertheless, considered important enough to warrant an article by Miryam Naddaf in Nature magazine,2 in which she quotes Columbia University Infertility Program’s head, Zev Williams, MD, PhD, suggesting that “knowing when aneuploidies occur allows us to get opportunities to intervene and try to correct the issue.” Though we don’t know whether this was what he meant, if applied to the still increasing utilization of PGT-A, he, of course, would be absolutely correct. The required intervention would be that more leaders in the field (and professional organizations like ASRM and ESHRE) stop talking around the issue of PGT-A and finally come out with a clear statement that a costly test that does not improve IVF outcomes and, in addition, in some subgroups even harms outcomes, should only be used in exceptional and rare circumstances. This raises the question of why, aside from the CHR, so few IVF clinics are willing to do so.
REFERENCES
1. Domingo-Muelas, et al., Cell 2023;186:3166-3181
2. Naddaf M. Nature 2023;619:448-449
Generating primordial germ cells efficiently and scalable
The preceding topic offered a good example of the importance of human stem cells, not only as a basic physiological reality in all tissues, but also for research and potential therapeutic purposes. When it comes to human infertility treatments, the ultimate goal would be to produce de-novo functional gametes, whether oocytes or spermatozoa. If that were to be achieved (in the mouse Japanese investigators achieved this goal already several years ago), the source would likely have to be newly generated human induced pluripotent stem cells (hiPSCs). Currently, available methods to differentiate human primordial germ cell-like cells (hPGCLCs) are very labor-intensive and inefficient. Dutch investigators, however, now reported a much more robust method of hPGCLC differentiation,1 which should help in advancing the journey toward in vitro gametogenesis.
REFERENCES
1. Overeem et al. Cell Methods 2023;3:10488
Continued from page 41 42 | SEPTEMBER 2023 | The Voice
How early does sex-specific splicing occur in embryos?
Demonstrated in as Drosophila model, but likely applicable universally in all species, investigators just determined convincingly that sex-specific splicing genome-wide occurs much earlier in embryogenesis than has been assumed so far.1 To be specific differences already arise concurrent with zygotic genome activation. The study demonstrates that a maternally deposited pioneer transcription factor TF CLAMP (chromatin-linked adapter for MSL proteins) likely contributes to the generation of these differences. Loss of CLAMP leads to altered sex-specific splicing of genes which are involved in a diverse pool of biological processes in development. As a consequence, sex-specific differences in transcript diversity exist already at the earliest stages of development. Now one wonders how this diversity is expressed phenotypically.
REFERENCES
1. Ray et al., eLife 2023;12:e87865
How important are fallopian tubes for the development of good-quality embryos?
Oviduct fluid and extracellular vesicles (EVs) secreted by epithelial cells of the oviduct have been reported in several animal models to enhance fertilization, reduce the risk of polyspermy, and improve embryo quality.1 As we discussed in more detail in the June issue of The VOICE, EVs are increasingly recognized in all areas of medicine for their many physiological functions. They are nano-sized membranous vesicles that easily fuse with cell membranes and, thereby, distribute their content of miRNAs, mRNAs, proteins, lipids, and even metabolites.2 A recent study in pregnant cows even suggested that the immunological crosstalk between embryo and mother already starts in the oviducts (and not the endometrial cavity where embryos ‘reside” for ca. 48 hours before implantation), mediated by the modulation of miRNAs in oviduct epithelial cells and EVs.
Now comes a new study from Korea that reemphasizes the importance of EVs derived from oviduct epithelial cells for the ultimate developmental competence of embryos in a porcine model.4 Assuming all of these reports to be correct, one could argue that our culture media currently in use must be rather “primitive,” and that further studies of what characterizes the tubal milieu during the embryo’s passage would allow for much more “competent” IVF culture media.
REFERENCES
1. Harris et al., Int J Mol Sci 2020; 21:8280
2. Jeppesen et al., Cell2019; 177:428-455.e18
3. Mazzarella et al., Front vet Sci 20218:639752
4. Fang et al., Mol Med Reports 2023; 27:13009
Cryopreserving living tissues
A feature article in Science magazine recently reviewed the greatly diverse research efforts that are underway to preserve whole organs or even complete organisms.1 Whether it be rat kidneys or whole
ovaries, somebody is likely working on it. The article mentions Sebastian Giwa, a former hedge fund manager, who has launched several startups, all linked to cryopreservation. One, called GaiaLife, is learning how to vitrify whole ovaries. In a sheep model, 4 out of 5 ovaries survived thawing and reimplantation, as evidenced by progesterone production.
Coincidentally, news media all over the world reported on the successful “resuscitation” thaw of an ancient nematode (roundworm) after being frozen for 4,600 years in Russia’s permafrost tundra.2
Some of the methodologies explored are quite mind-blowing, while others are simpler. As we, in IVF centers, well understand after decades of cryopreserving gametes, embryos, as well as small ovarian tissue samples on large scales, almost everything in cryopreservation depends on preventing ice-crystal formation. Consequently, significant efforts are extended, and significant funds are spent on learning to improve the cryopreservation of larger living tissues and/or complete organisms. Many experts in this practice arena are openly starting to speak about human organ banks, which would do for organ transplantation, what frozen egg banks have done over just a few short years for third-party donation after the vitrification of oocytes improved enough to allow for the creation of frozen egg banks.
REFERENCES
1. Cornwall W. Science 2023; 380(6652):1313-1317
2. https://www.nytimes.com/2023/07/29/science/roundworm-nematodes-si beria-permafrost.html
General clinical medicine
Differences in stress response between female and male brains
In a beautifully executed study, investigators from Germany’s Max Planck Institute in Munich and Israel’s Weizmann Institute in Rehovot for the first time offered in mice, descriptions for molecular processes that, as has been known for some time, differ in how female and male brains respond to stress.1 They identified cell-type-specific signatures of acute restraint stress in the paraventricular nucleus of the hypothalamus, which is known as a central location of stress responses in both sexes. They further demonstrated that prolonged mild chronic stress changed this signature in distinctively different ways between female and male mice, with oligodendrocytes the targets of these sex-specific effects. The authors offered their work as an online interactive app, including the transcriptomes of thousands of individual cells a molecular resource for other investigators. The paper also serves another important purpose in pointing out yet another example of how females and males in core physiological characteristics differ, a subject in the current political climate of increasing importance not only for scientists to understand.
REFERENCES
1. Brivio et al., Cell Rep 2023; 42(8):112874
Continued on page 44
The V oice | SEPTEMBER 2023 | 43
Can memory loss be successfully treated?
Klotho was first measured in 20101, and is an apparent longevity factor that declines with advancing age.2 Now U.S. investigators from several medical schools reported that steady low doses (but not high doses) of klotho enhanced memory in older nonhuman primates.3 The findings suggest that this treatment may also be effective in aging humans.
However, how Klotho achieves these effects is still unclear, even though the authors validated the rhesus form of the Klotho protein in its effectiveness in mice by demonstrating that it increased synaptic plasticity and cognition. Since Klotho does not cross the blood/brain barrier, it must act through intermediaries. Experts have already called for clinical trials in humans.4
We find the apparent crossover responses of being a longevity as well as cognitive promoters of potentially great interest for reproductive medicine since there is reason to suspect that such effects may also be relevant to ovarian function, whether based on local or central nervous system effects. Such consideration may also include the now widely discussed new generation of weight loss drugs, from Ozempic, Wegovy, to Mounjaro, with more to come in the near future which, beyond weight loss, now also have been demonstrated to lower cardiovascular mortality in cardiac patients.
REFERENCES
1. Yamazaki et al., Biochem Biophys Res Commun 201; 98:513-518
2. Semba et al., J Gerontol A Biol Sci Med Sci 2011
3. Castner et al., Nat Aging 2023; 3:931-937
4. Tozer L. Nature 2023; 619:234
Highest suicide and homicide rates in decades for youth and young adults
A recent CDC report indicated that suicide and homicide rates for children and young adults between ages 10 and 24 during 2021 were the highest in decades, being the second- and third-leading causes of death overall.1 Since 2010, suicide rates have been higher than homicide rates in this age group, reaching levels that have not been seen since 1968. In 2021 (the first year of the COVID pandemic) the big spike in homicides, however, approximated those two rates once again. Between ages 10 and 14, suicides remained twice as frequent as homicides.2 Increasing suicide rates in the very young have also been reported in other studies, with social media, and especially TikTok being often accused of responsibility.3 It is quite remarkable how little attention these developments have attracted from the media, especially state and federal governments.
REFERENCES
1. Tsai B. June 15, 2023. https://blogs.cdc.gov/nchs/2023/06/15/7396/
2. McPhillips D, CNN, June 15, 2023. https://www.cnn.com/2023/06/15/health/ youth-suicide-homicide/index.html
3. Carville O. Bloomberg.com. April 20, 2023. https:// www.bloomberg.com/news/features/2023-04-20 tiktok-effects-on-mental-health-in-focus-after-teen-suicide
Who are better doctors, the younger or older ones?
This is a question, we are sure, many patients are asking. It is also a question the medical community finds itself confronted with especially when renewing hospital privileges. Can physicians be too old is a very relevant question that must be asked.1 Two journalists recently did exactly that in The Wall Street Journal, 2 reaching interesting conclusions after reviewing the available literature.2
It turns out that when it comes to physicians’ effectiveness, it is not age that matters: what does matter is the continuous involvement of physicians with medical practice (i.e., how many patients they see) and, maybe underappreciated even within the medical profession, how well they keep up with new medical developments in their fields (i.e., how well they follow the medical literature, attend conferences, etc.)
REFERENCES
1. DeMaria A. Struct Heart 2023; 7(2):100165
2. Jena AB, Worsham C, The Wall Street Journal, July 8-9, 2023,pC6, https://www.wsj.com/articles/ do-younger-or-older-doctors-get-better-results-25800b7c
Reproductive endocrinology & infertility
The absurdity of the diagnoses of Unexplained Infertility and Recurrent Implantation Failure
There are many diagnoses in reproductive medicine where we still only know much too little, but we are always amazed how much is made of some diagnoses where we practically have absolutely no knowledge or, indeed, where diagnoses are, simply, made up out of thin air.
Probably the most obvious example of the latter is “unexplained infertility,” which The VOICE routinely places into quotation marks because, as readers of this newsletter by now are aware of, we do not believe that such a diagnosis really exists. Whether a likely diagnosis (one in infertility, of course, can never be absolutely certain what causes infertility) for an infertile couple can be found, always depends on how hard one looks.1,2 In other words, the poorer a diagnostician a physician is, the more diagnoses of “unexplained infertility” they will make. That such a concept could be rewarded by the formal recognition by an academic profession through the creation of a formal “diagnosis” called “unexplained infertility,” appears not only absurd, but demeaning to the profession.
Not far behind in the gravity of misjudgment by the profession comes the diagnosis of “implantation failure”. The reason why this diagnosis is almost as absurd as the diagnosis of “unexplained infertility”, is that nobody really knows what defines “implantation
Continued from page 43 44 | SEPTEMBER 2023 | The Voice
failure” which also deserves its exclamation marks. If one searches the literature, one can find an abundance of varying definitions, from two, three, or more failed IVF cycles to three, five, or more “good quality embryos” transferred in IVF (with what represents a good quality embryo, of course greatly varying). The logical inconsistency of all of these definitions with our understanding of basic reproductive physiology is, simply, mind-blowing.
Take, for example, the fact that in young couples the basic definition of infertility is based on unsuccessful regular intercourse over 12 months in the absence of obvious causes of infertility. In practical terms, this means that a young woman with a formal diagnosis of infertility will have produced one embryo monthly with regular intercourse for a total of 12 months. This means that in young women, the internationally accepted definition of infertility practically says that it takes at least 12 good-quality embryos not leading to pregnancy, to reach a diagnosis of infertility. As women age, this number of embryos would be expected to even grow. How, then, does it make sense to allege a diagnosis of “implantation failure” before the transfer of at least 12 good-quality embryos (or more in older women)?
Yet, ESHRE found it appropriate to just publish an over 120-pagelong “guideline” for the management of “unexplained infertility,”3 shortly followed by an almost 120-page-long “good-practice recommendation” on “recurrent implantation failure.”4
Both documents reinforced the absurdity of these two respective diagnoses already in their introduction: The “unexplained infertility” document stated in its first sentence that “approximately 30% of infertile couples are considered to experience ‘unexplained infertility’.” Does anybody really believe that this statement, referenced by two 2019 and 2020 citations, really makes any sense? The absurdity of the “recurrent implantation failure” document is even more obvious when the abstract notes that ”recurrent implantation failure is a challenge in the ART clinic, with a multitude of investigations and interventions offered and applied in clinical practice, often without biological rationale or with unequivocal evidence of benefit.” Exactly! But why then two exhaustively long documents about nothing?
Related, ESHRE is not alone in this “game of opinions about nothing”. As if the lengthy statement by ESHRE on recurrent implantation failure was not enough, another group of European “experts,” this time joined by several U.S. colleagues, published a “consensus statement” on implantation failure following a workshop in Lugano, Switzerland, in July of 2022.5 This document considers this diagnosis to be “extremely uncommon,” defined as in less than 5% of infertile couples. We most appreciated the definition of repeated implantation failure in this document, characterized by the following statement: “It would be reasonable not to assign this diagnosis to a patient until she has failed at least 3 euploid embryo transfers (or the equivalent number of unscreened embryos, adjusted for age).” What a crystal-clear consensus statement that is which says absolutely nothing! And, yes, Fertility & Sterility considered this paper the “Seminal Contribution” article of the July issue.
REFERENCE
1. Gleicher N, Barad D. Hum Reprod 2006; 21(8):1951-1955
2. Gleicher et al., Lancet 2018; 392(10157):1516-1517
3. ESHRE 2023; https://www.eshre.eu/Guidelines-and-Legal/Guidelines/ Unexplained-infertility
4. ESHRE Working Group on Recurrent Implantation Failure. Hum Reprod Open 2023 (3):hoad023
5. Pirtea et al., Fertil Steril 2023; 120(1):45-58
How frequent are spontaneous pregnancies after prior IVF births?
British investigators recently asked the interesting question of what if the chance of spontaneous conception following a successful IVF delivery? Following a systematic review and meta-analysis of published studies, the authors concluded that the chance for that to happen was 20% (95% CI, 0.17-0.22). From this outcome, one could conclude, (as the authors also did) that spontaneous conceptions following IVF deliveries are not uncommon, but the subliminal point behind these findings was that maybe these 20% of women did not need IVF after all, and would also have conceived on their own. And this is, as our British colleagues would say, hogwash.
While most IVF cycles are performed to overcome infertility, a good number may be indicated for other reasons. Examples of such other reasons are immune problems that require specific timing of immune treatments, multiple pregnancy risks in PCOS patients overstimulated during IUI cycles, etc. In this context, it is also important to remember the CHR’s first-ever IVF pregnancy in 1981 (in those days in Chicago) which occurred in a young woman who without ever conceiving had undergone three major prior surgeries for stage IV endometriosis at the hands of the world’s best surgeons at the time. After her IVF pregnancy and delivery, she went on to have three more pregnancies - all spontaneously.
The only possible explanation we ever considered was, that the growing uterus, by ascending upwards into the abdominal cavity, persistently stretched her dense scar tissue from endometriosis, ultimately freeing her fallopian tubes better than the surgeries succeeded in doing. In the end, we will never know for certain how her IVF pregnancy later allowed her to conceive spontaneously. Just another example of how little we still know about infertility!
This British paper should not be understood as suggesting that on average, 20% of women who undergo IVF do not need the procedure. While, undoubtedly, such cases exist, the number is, fortunately, likely, very much smaller.
REFERENCE
1. Twaites et al., Hum Reprod 2023; 38(8):1590-1600
No more anticoagulation for recurrent miscarriages and inherited thrombophilia
Among medications that patients with immunological infertility and pregnancy loss most dislike have been anticoagulants. Here at
Continued on page 46 The V oice | SEPTEMBER 2023 | 45
the CHR, we have had reservations about the utility of these blood thinners for some time, but we did not want to cease the use of these drugs in these circumstances because this treatment was considered “standard therapy.” Now an excellent study by British and Dutch colleagues in The Lancet1 confirmed our suspicions and we are pleased to report that the CHR will no longer use anticoagulation for these conditions unless they are linked in individual patients to direct evidence for the presence of a hypercoagulable state.
This study confirmed that anticoagulation does not improve live birth rates in women with two or more miscarriages and confirmed inherited thrombophilia. The CHR never considered thrombophilia a reason for treatment in women with miscarriages because we were never convinced that thrombophilia contributed to repeat pregnancy loss. Consequently, we never saw an indication to treat with anticoagulants for that specific indication. However, miscarriages due to a suspected hyperactive immune system were treated with these medications in combination with other treatments, which continue to be used.
An accompanying editorial2 noted that this study is likely generalizable since patients from several countries and study locations were utilized in this study as well as different anticoagulants. We agree!
REFERENCE
1. Quenby et al., Lacet 2023; 402:54-64
Are infertility, miscarriages, and stillbirth associated with premature or early menopause?
Another study by a group of European investigators from various countries now published a study that confirmed what we, here at the CHR, have suspected for quite some time: A history of prior infertility, miscarriages, and stillbirth is, indeed, statistically associated with early menopause. Interestingly, this association is variable in strength based on race/ethnicity, with Asian women being the most affected.
The common link is in our opinion likely a hyperactive immune system, which is a finding more commonly seen in women with all these of these conditions.
REFERENCE
1. Liang et al., Am J Obstet Gynecol 2023; 229;47: e1-e9
Is the FDA finally really ready to regulate genetic tests for aneuploidy in all of reproductive medicine?
A brief article on July 2, 2023, in The New York Times1 suggested that the FDA is finally planning on regulating chromosomal blood tests in early pregnancy because of their high rate of false-positive results that mislead patients and their physicians. These so-called NIPTs (non-invasive prenatal tests), have become common practice and are based on the same concept (and technical methodologies) as PGT-A (preimplantation genetic diagnosis for aneuploidy). As some of the CHR’s investigators not too long ago in an opinion article in Nature Medicine noted, NIPTs were once before the subject of a “warning letter” from the FDA.2 The article pointed out the similarities between NIPTs and PGT-A testing and especially noted that false-positive diagnoses are even more common with PGT-A. Yet, the FDA, neither in their warning letter nor in the more recent announcement the Times article was based on, also included the PGT-A procedure.
One really must wonder why that is, considering that significantly larger numbers of embryos are misdiagnosed and not used or even discarded with PGT-A than pregnancies are misdiagnosed with early NIPDs. The one big difference between these two tests is that a false-positive NIPT can be corrected in a timely fashion by a follow-up CVS or amniocentesis, while a false-positive PGT-A test usually is automatically acted upon by not using an embryo or disposing of it. How the FDA can put so much emphasis on NIPTs, while ignoring PGT-A, is startling!
REFERENCES
1. Kliff S. The New York Times, June 27, 2021 p21; https://www.nytimes. com/2023/06/27/upshot/prenatal-testing-misleading-fda.html
2. Gleicher et al, Nat Med 2022;28(3):442-444
Is menopausal hormone therapy increasing dementia risks?
A nationwide Danish nested case-control study in the British Medical Journal reported disturbing findings regarding the association of menopausal hormone replacement therapy and the development of all causes of dementia and Alzheimer’s disease.1 Based on a study of 5,589 dementia cases and 55,890 age-matched controls, this study reported an increased rate of all causes of dementia with hormone therapy (hazard ratio 1.24). Increasing the duration of hormone therapy resulted in higher hazard ratios, from 1.21 (use of 1 year or less) to 1.74 (>12 years of use). Use of estrogen/progesterone was positively associated with the development of dementia, whether continuous (hazard ratio, 1.31) or
Continued from page 45 46 | SEPTEMBER 2023 | The
cyclic (hazard ratio, 1.24). The findings were consistent, even if supplementation started before age 55.
These are extremely worrisome findings that require serious consideration and follow-up studies in patients of more different genetic backgrounds than a national Danish study can offer. If confirmed, these findings may represent the so-far strongest argument against postmenopausal hormone replacement.
Related, Nature magazine recently published a feature article, summarizing “how the run-up to menopause changes the brain.” In it, the author, a senior reporter for Nature in London, UK, notes that considering current lifespans, women now spend approximately a third of their lives in the postmenopausal period. How the transition into menopause (the so-called perimenopause) affects the brain, is only now slowly becoming apparent and may “set the stage for brain health in menopause.”2 The article also notes that the whole subject of perimenopausal hormone replacement has remained controversial and requires additional research. In that context, the article immediately following the same issue of Nature addressed the gender gap in funding conditions that affect women and men. Finally, The Wall Street Journal recently suggested in an article’s heading (of course somewhat exaggerated) that menopause “could be gotten rid of,” while in the body of the article, describing that what really was meant, was to boost women’s health and extend female fertility into older age by developing methods to slow-down ovarian aging.4
It recently has become increasingly apparent that the age of menopause and longevity are statistically linked: every year of later menopause appears associated with a two-year longer lifespan, while the opposite also appears to be true; earlier menopause also means earlier death from, for example, cardiac diseases. Because this is a rapidly evolving subject in the medical literature, The VOICE in the October issue will address it in more detail.
Finally, just a short note about non-hormonal treatments for menopause symptoms. The North American Menopause Society (NAMS) released its 2023 Position Statement for allegedly evidence-based non-hormonal treatments (how this evidence was obtained is somewhat unclear). They included: (i) Cognitive therapy, (ii) Clinical hypnosis, (iii) Weight loss, (iv)Stellate ganglion blockers, (v) Selective serotonin reuptake inhibitors/serotonin-norepinephrine uptake inhibitors, and (vi) Gabapentin, oxybutynin, and fezolinetant.5 Specifically not recommended are: paced respiration, supplements/herbs, cooling techniques, avoiding triggers, exercise/ yoga, mindfulness-based interventions, relaxation, suvorexant, soy products, cannabinoids, acupuncture, calibration of neural oscillation, chiropractic interventions, clonidine, dietary modifications, pregabalin.
REFERENCES
1. Pourhadi et al., BMJ 2023; 381:e72770
2. Ledfod H. Nature 2023; 617:25-27
3. Smith K. Nature 2023; 617:28-29
4. Reddy S. The Wall Street Journal, July 15-16, 2023; C1-C2; https://www.wsj.
com/articles/what-if-we-could-get-rid-of-menopause-7adbc4e0
4. NAMS Advisory panel. Menopause 2023; 30(6):573-590 https:// pubmed.ncbi.nlm.nih.gov/37252752/#:~:text=Recommended%3A%20 Cognitive%2Dbehavioral%20therapy%2C,(Levels%20II%2DIII).
What happens to hormones as we age?
We all know that hormone levels change with advancing ageusually declining. The Endocrine Society recently published an excellent succinct summary of what happens to all hormonal axes in the body with aging.1 This includes the hypothalamic–pituitary–ovarian axis in women and males but, also of interest to reproduction, other axes, like the pituitary-adrenal axis (though, unfortunately still omitting its continuation to the ovary (which the CHR’s investigators have previously pointed out2). This article is the perfect quick refresher course on medical reproductive endocrinology.
REFERENCES
1. Cappola et al., J Clin Endocriol 2023; 108:1835-1874
2. Gleicher et al., J Clin Endocriol Metab 2017; 102(9):3569-3570
Childbearing among women physicians
Investigators at Northwestern University in Chicago in a cross-sectional study of 1,056 cisgender women physicians with a mean age of 38.3 years reported on their childbearing practice: 82.2% were married or had a partner, 65.3% already had at least one child, 78.0% correctly identified age 35 as the onset of a significant decline in female fertility; and 75.6% reported to have delayed childbearing. A total of 38.8% experienced infertility and, among those, 51.4% ended up utilizing IVF.
When asked if they would do things differently if they were given the opportunity, 45.7% would have conceived earlier, 44.8% stated that they wanted to reduce work hours, 38.8% wanted to take extended leave, and 28.4% wanted to utilize oocyte cryopreservation. However, what really happened was that 28.8% took an extended leave; 24.8% changed specialty; 47.1% reduced their work hours; 24.8% changed their practice setting; and 47.2% passed up an opportunity for career advancement after they had a child. Most remarkably, 4.3% left medicine entirely.
Unsurprisingly, the authors of the study concluded that these findings suggested, “that fertility and family building concerns among women in medicine may contribute to ongoing gender disparities and attrition and represent a potentially critical area for policy reform and future change.” Considering that women now at most medical schools represent a majority of students, these data have profound implications for the future staffing of U.S. health care with physicians, which is already looking at rather bleak projections.2 The fertility field carries special responsibilities in helping to address this subject.
REFERENCES
1. Bakkensen et al., JAMA Netw Open 2023; 6(7):e2326192
Continued on page 48 The V oice | SEPTEMBER 2023 | 47
2. Prober CG, Desai SV. Acad Med 2023; doi: 10.1097/ ACM.0000000000005262. Online ahead of print.
Clinical IVF
IVF in PCOS patients
CONFLICT STATEMENT: This commentary in sections addresses the clinical use of androgen supplementation in women with infertility. The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product. Since the following paragraph addresses androgen supplementation, readers are advised that opinions expressed in this paragraph, therefore, may be biased by financial interests.
Boston colleagues recently published an interesting paper investigating IVF cycle outcomes in PCOS patients in that they separated in a retrospective study of PCOS patients into only two groups:1 Obese (mean BMI 33.8) and lean (BMI 22.7). In practical terms, they divided their PCOS patients into Rotterdam criteria phenotypes, A, B, and C (“obese”) and phenotype D (“lean”). Putting several major weaknesses of this study design aside, this study caught our eye because of the CHR’s considerable research interest in PCOS, recently summarized.2 Based on clinical observation, the CHR’s researchers concluded that PCOS is not a condition of four subtypes (called phenotypes A through D) but of two genomic likely distinct entities, one with a “metabolic” phenotype (A, B, C) and a second with an “immune” phenotype (D). At approximately the same time, based on genomic studies, local colleagues at Mount Sinai Medical Center in NYC also concluded that PCOS reflected two distinct entities, giving them the name “metabolic” and “reproductive” sub-types,3 and describing them with almost identical clinical phenotypes as the CHR’s researchers had observed.
The here-addressed paper, unfortunately, appears to have been in multi-journal peer review without proper updates of the literature for too long (we are making this comment because its alphabetic arranged references, somewhat surprisingly, do not fit the reference style of the journal where the manuscript finally appeared). This is further demonstrated by the authors being unaware of the above-noted publications of the CHR and the Mount Sinai group, as both were unreferenced.
Which brings us to this paper’s findings and why the authors should have been aware of the CHR’s and Mount Sinai’s work: First, it is important to point out that the mean ages of both of their study groups were low, 33.2 years in their “obese” and 32.8 years in their “lean” group. Why is this important? Because, as the CHR reported,2 lean PCOS patients until approximately age 35 are more-or-less normally fertile, becoming infertile only after age 35, when their androgen levels drop below normal. Therefore, it would be expected that “lean” patients in this study exhibited higher pregnancy rates. It also does not surprise that miscarriage rates were higher in “obese” patients because “lean” patients develop evidence of a hyperactive immune system mostly only after age 35 (though
some may have already much earlier evidence), at which time they become prone to increasing miscarriages.
In other words, unknowingly and, likely, unwillingly, these authors in their study offered further evidence for the proposition made by the CHR’s investigators and the Mount Sinai group that PCOS is not made up of four phenotypes but, more likely, represents an amalgam of two distinctively different genomic entities. Had the authors continued to follow the literature while their manuscript travelled the frequently quite lengthy journey of repeat rejections before acceptance, and updated their manuscript during this time period, it, likely, would have been more impactful upon publication. Just another lesson to learn!
Related, Chinese investigators recently published a PCOS study 4 with interesting intent, but with the unfortunate and almost universal error of PCOS studies in the medical literature that patient selection (i.e., diagnosis) is determined by Rotterdam criteria, but the four phenotypes under those criteria then are co-mingled. This creates irreproducible results because those will vary, depending on the distribution of the four phenotypes within the study population.
Here, the question of the study was whether PCOS patients, because of better functional ovarian reserve (FOR) at advanced ages than other infertility diagnoses (in this case tubal infertility), had also better IVF outcomes. According to the study’s results, the answer was a clear no.
Simply on logical grounds, this appears to be the wrong answer because, after female age, the numbers of eggs and embryos generated in an IVF cycle represent the second most important predictor of pregnancy chances in IVF, and a better FOR always leads to better oocyte and embryo yields which, in turn, lead to better cumulative pregnancy and live birth rates. The principal error in this study was, however, likely the design of this retrospective cohort study which ended up co-mingling all four PCOS phenotypes into a single study population. Phenotype D (lean) PCOS patients after age 35 (this study investigated women above age 35 only) become hypo-androgenic and, therefore, treatment-resistant in IVF unless prior to their IVF cycle for at least six weeks supplemented with androgens.2 Since this manuscript does not indicate that D phenotypes received such supplementation, they, unquestionably, depressed the PCOS group’s combined IVF outcomes.
As many times noted in The VOICE before, proper patient selection makes or breaks a study. In this case, as in unfortunately so many other PCOS studies as well, it clearly invalidated the study from the very beginning by co-mingling PCOS phenotypes.
48 | SEPTEMBER 2023 | The Voice Continued from page 47
REFERENCES 1. Fouks et al., J Assist Reprod Genet 2023; 40:1437-1445 2. Gleicher et al., Biomedicines 202310:1505 3. Dapas M, Dunaif A. Endocrine Rev 2022; 43:927-965 4. Zhang et al., BMC Pregnancy and Childbirth 2023; 23:440
The endometrial scratching saga continues
An international collaborative individual participant data meta-analysis published in Human Reproduction Update once again brought to the forefront endometrial scratching, now a controversial issue since in 2000, it was first suggested by Israeli colleagues as a possible strategy to improve implantation and, therefore, pregnancy and live birth chances in IVF.1 Because reports on the success or failure of endometrial scratching often varied in how the procedure was performed, the authors of this study reviewed individual cases in the various studies that were included in their meta-analysis. This paper to date must be considered the most reliable meta-analysis published on this subject, and the results were surprising to many of the authors (personal communication).
The study involved data contributions from 15 randomized controlled trials (RCTs, 14 published, 1 unpublished) out of a total of 37 published and 15 unpublished RCTs, the authors were aware of, and involved 4,112 vs. 7690 patients. The risk of bias was low” for 10/13 evaluated studies. One-stage intention to treat analysis of scratch vs. no scratch/sham procedure demonstrated improved live birth rates [OR 1.29 (95%CI 1.02-1.64)]. A secondary analysis including women undergoing an embryo transfer and a treatment covariate interaction analysis demonstrated very similar results [OR 1.22 (95% CI 0.96-1.54) and OR 1.25 (95% CI 0.99-1.57)] and [OR 1.26 (95% CI 1.03-1.550)]. Covariate interaction analysis demonstrated no evidence for interaction with age, the number of earlier failed embryo transfer treatment types, and infertility diagnosis. In other words, it appears that endometrial scratching works, after all, and as the authors suggest, may make sense in selected patients with appropriate informed consent.
However, several major issues remain to be resolved: (i) The authors’ apparent reluctance to recommend routine scratching is interesting because if, as their study suggests, scratching universally improves IVF outcomes, why not use it in everybody? (ii) Why and how does scratching work? Medicine is always reluctant to apply treatments without knowing how they achieve their effectiveness. Indeed, without this kind of understanding, it becomes impossible to fine-tune treatments. (iii) How and exactly when should scratching be performed? In other words, there is still much work left when it comes to scratching but, based on this study, the CHR has again started offering it to selected patients.
REFERENCES
1. Van Hoogenhuze et al., Hum Reprod Update 2023; dmad014
2. Granot et al., Fertil Steril 2000; 73:381-386
Why properly conducted PRP studies are needed
That injections of autologous platelet-rich plasma (PRP) into ovaries improve oocyte yields was first proposed by Greek
colleagues in 2016 and 20191,2 and quickly became a widely used “add-on” to IVF. As we have unfortunately witnessed with several other “add-ons” since 2010, they then quickly became “routine treatments” without ever undergoing adequately powered validation studies. The clinical utilization of PRP has been following this pattern to the point: From the first above-referenced study which claimed to have treated a handful of women with primary ovarian insufficiency (POI) but, in reality, mostly described women with premature ovarian aging (POA), to this date, not a single adequately powered study on PRP use in female infertility has been published. This is the reason why three prospective trials of PRP in distinctly different patient populations are currently underway at the CHR. An interim report on the CHR’s PRP II Trial was recently published,3 but the study continues. The other two studies are also still collecting cases.
It is interesting to note that a PRP study by colleagues from IVIRMA-NJ in Basking Ridge, NJ, presented at the annual ESHRE conference in Copenhagen, Denmark, attracted media interest when it reported that PRP failed to improve oocyte yields in young women with diminished ovarian response.4 Though this study was an RCT, it involved only 83 patients (PRP group n=41; controls n=42). That fact alone should have resulted in the rejection of this abstract because there is simply no way how such small a number of studyand control patients can be sufficient to determine whether IVF outcomes differ in a relatively poor prognosis patient population with rather limited pregnancy chances in the first place.
A principal reason why the CHR decided to initiate three PRP studies was a degree of skepticism about the quick increase in unvalidated utilization of PRP in IVF centers. However, this does not mean that inadequate negative studies, like here discussed ESHRE presentation, should be favored over poorly designed studies that report positive results. That this study did not demonstrate beneficial outcome effects can simply be attributed to the inadequate size of the investigated patient population. One in this case can almost expect a type 2 error in the data analysis: just because an underpowered study demonstrates no statistical difference between two study groups does not mean that a properly powered study would not demonstrate such a difference. Our recommendation is to simply ignore this ESHRE presentation despite the media exposure it got. Hopefully, by the time our colleagues publish a full-length paper on the subject, they will have added more patients to the study. Interestingly, they noted several other potential “limitations” of their study, but the very small number of cases was not among them and, as we in The VOICE on so many occasions have pointed out, unless patient selection and description are appropriate, it is not worthwhile to continuing reading.
REFERENCES
1. Pantos et al., Hum Reprod 2016; 31: Issue suppl_1:i301 (ESHRE Abstract)
2. Pantos et al., Cell Transplant 2019; 28:1333-1340
The V oice | SEPTEMBER 2023 | 49 Continued on page 50
3. Barad et al., Hum Reprod Open 2022 (3):hoac027
4. Baez D.NTK Institute. https://ntk-institute.org/article/intraovarian-prpinjection-fails-to-improve-oocyte-yield-in-young-patients-with-poor-ovarian-response
Rekindling the idea of mitochondrial replacement in IVF
A recent paper in Fertility and Sterility1 brought back to attention the hypothesis that repeated IVF failure may be caused by poor and/or abnormal mitochondrial function of oocytes. Based on animal data,2,3 this hypothesis has been around for decades and was expanded into the human experience by performing ooplasmic transfers from young donor oocytes to older patient oocytes,4-6 thereby producing heteroplasmy.7 This was then also the reason why the FDA stepped in and prohibited further experiments involving ooplasmic transfers without an FDA-approved IND (Investigational New Drug Development).8
The idea behind the ooplasmic transfer was to at least partially replace aged autologous mitochondria in the ooplasm of oocytes with younger mitochondria from healthy egg donors, with the hypothesis being that this would improve the effectiveness of oocytes from primarily older women to still produce healthy pregnancies. However, the original reports,4-6 were too small in numbers to offer a judgment on the hypothesis that mitochondrial replacement may be an effective treatment for aging oocytes.
Mitochondrial replacement is potentially even more important in cases where mothers transmit so-called mitochondrial genetic diseases to their offspring. These fortunately rare diseases are inherited exclusively through mitochondrial DNA (mitochondria are the only extranuclear structures that have their DNA, so-called miDNA) only from the mother. Consequently, the potential prevention of inheritance would require the complete replacement of cytoplasm in eggs of an affected woman, and this can be achieved to almost 100% with either so-called nuclear- or spindle cell-transfer into an egg donor’s denucleated cytoplasm. Under the above-noted FDA guidance, these procedures are currently prohibited in the U.S. in the absence of an approved IND, but on a case-by-case basis, they have been permitted to prevent the inheritance of mitochondrial diseases in the U.K. But even in that clinical context, the effectiveness of such an almost complete exchange of cytoplasm has not sufficiently been established as of this point.
Yet now a substantial group of in part very prominent investigators in reproductive medicine from both sides of the Atlantic published a pilot study of spindle cell transfer in women from couples with so-called idiopathic (“unexplained”) infertility who experienced repeated IVF failure (from 3 to 11). The purpose was just to test out the concept of performing spindle cell transfers to potentially improve IVF outcomes mostly in women with idiopathic (“unexplained”) infertility. The study involved 25
infertile couples and 28 cycles but excluded couples with severe male factors. Metaphase II spindles from patient oocytes were transferred into enucleated donor oocytes. This was followed by ICSI using partner semen, extended embryo culture to blastocyst-stage, trophectoderm biopsy for PGT-A, and embryo vitrification. Only embryos reported as euploid were transferred in frozen-thawed cycles.
The 28 cycles produced 19 embryo transfers (67.87%), 7/28 pregnancies (25%), 6 live births (21.43%), and 1/7 pregnancy loss (14.29%). Newborn follow-ups for 12-24 months were uneventful; DNA fingerprinting revealed that for 5/6 children miDNA was over 99% from the egg donor. The 6th child, however, even though at blastocyst stage also demonstrating over 99% donor miDNA, demonstrated significant “drifting,” with 30-60% of miDNA still being maternal at birth.
We must acknowledge that because of several prominent U.S. and U.K. scientists among authors, we were surprised when we saw this paper because we knew that neither country would permit such a study under current conditions. We have been aware that a Greek center in collaboration with Spanish investigators was offering spindle cell transfer as a “treatment” for female infertility. It appears that this study was performed at that Greek center under the approval of the Greek Authority of Assisted Reproduction (license 437/23.9.2016) and the IASO Maternity Hospital Institutional Review Board with periodic renewal.
What does this small case series tell us? Unfortunately, not much: (i) The patient population was young between 32-39 years. For such an age group, the reported pregnancy rate was reasonable, but likely really below expectations. (ii) The same applies to a live birth rate of 21.43%, which, considering the young age of the patients, must also be considered subpar. Based on an offered outcome comparison to a 2014 study by Franasiak et al (not even referenced beyond that), they claimed similar aneuploidy rates. Why would one expect those to be affected in the first place? (iii) That 1/6 (16.67%) newborns demonstrated clear “drifting” (i.e., return of the mother’s mitochondrial DNA) is also not surprising because that was shown by Egli’s laboratory at Columbia University already in 2016.9
In short, we are not very impressed by the presented data in this paper because we do not see an improvement in expected outcomes in this patient population. We, moreover, are concerned that the 28 cycles presented in this manuscript were “selected” since it appears unlikely that a study protocol originally approved for infertility patients on a much broader scale in 2016, would have produced only 28 cycles in 6 years to a very restricted infertility population. Spindle cell transfers were also performed for infertility purposes in an IVF center in the Ukraine before the war broke out and, since that center did not publish their experience, one can only rely on the grapevine, - which also was not very favorable.
Continued from page 49 50 | SEPTEMBER 2023 | The Voice
In practical terms, this means that mitochondrial replacement only unlikely will “cure” the aging process in oocytes. Somewhat surprised by the fact that Fertility and Sterility published this paper, it was pleasing to see that an accompanying editorial by two prominent U.S. fertility experts emphasized caution in overinterpreting the reported results. At the CHR, we have the freedom to be somewhat less diplomatic! Nevertheless, one has to give the authors credit for having the courage to publish these data. Knowing since ca. 2016 that Ukraine and Greece were doing this, we were wondering why nothing was being published.
REFERENCES
1. Costa-Borges, et al., Fertil Steril 2023; 119(6):964-972
2. Malter HE, Cohen J. Reprod Biomed Online 2002; 5(1):26-35
3. Ramires Ferreira et al. Biol Reprod; 82(3):563-571
4. Cohen et al., Mol Hum Reprod 1998; 4(3):269-280
5. Brenner et al., Fertil Steril 2000; 74(3):573-578
6. Barritt et al., Hum Reprod 2001; 16(3):513-516
7. Barritt et al., Hum Reprod 2000; (Suppl2):2:207-217
8. U.S. Food & Drug Administration 3/16/18; https://www.fda.gov/vac cines-blood-biologics/cellular-gene-therapy-products/advisory-legal-re strictions-use-mitochondrial-replacement-techniques-introduce-do nor-mitochondria
9. Yamada et al, Cell Stem Cell 2016; 18(6):749-754
The rationale for maximizing oocyte and embryo yields
Maybe the most interesting section in Reproductive Biomedicine Online has been its periodic “countercurrent” article, which is meant to offer opinions that usually are somewhat out of the mainstream of “common wisdom” in the profession. That also applies to a recent article by a colleague from the Turkish Republic and Dubai, UAE, regarding oocyte yields making the point that “the more eggs one retrieves, the better.”1 This is of course, an opinion, the CHR has held for a very long time because, after a woman’s age, the size of an embryo cohort available for transfer is the second most important predictor of cumulative pregnancy and live birth chances in an IVF cycle. This is also the main reason, why the CHR was never a big fan of mild ovarian stimulations.
Another way of maximizing embryo yields is “rescue” in vitro culture overnight of immature oocytes, which in many cases allows for the fertilization of matured oocytes the following morning and, therefore, enlarges a cycle’s cumulative embryo cohort of transferrable embryos. Though most IVF centers, because of only very limited success especially with GV oocytes, usually discard most immature oocytes, the CHR has been practicing “rescue” maturation since approximately 2015. Now colleagues from Abu Dhabi, UAE, under the leadership of Human Fatemi, MD, PhD, reported a well-performed study in Human Reproduction, demonstrating a significant contribution of in vitro–matured oocytes to the cohort of embryos that could be biopsied at blastocyst-stage for PGT-A especially in women of advanced ages and/or with low egg and embryos numbers in general.
REFERENCES
1. Ata B. Reprod Biomed Online 2023; 46(4):655-658
2. Elkhabit et al., Hum Reprod 2023; ahead of print
Acupoint transdermal stimulations, DHEA, CoQ10, and growth hormone supplementation in poor responders undergoing IVF
CONFLICT STATEMENT: This commentary in sections addresses the clinical use of androgen And CoQ10 supplementation in women with infertility. The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA and CoQ10 product product. Since the following paragraph addresses androgen and CoQ10 supplementation, readers are advised that opinions expressed in this paragraph, therefore, may be biased by financial interests.
Chinese physicians and scientists have become masters of systematic reviews and meta-analyses. Every medical journal is filled with Chinese meta-analyses these days and reproductive medicine is no exception. However, it is still somewhat unusual when one paper offers systemic reviews and meta-analyses (in plural!) on four different clinical interventions. This is exactly what a recent paper in Reproductive Biology and Endocrinology did when claiming to report a systematic review and network meta-analysis of transcutaneous electric acupoint stimulation (TEAS, in other words, acupuncture), DHEA (please note conflict statement), CoQ10, and growth hormone supplementation in women with poor ovarian response to stimulation.1
Using Bologna criteria for the definition of poor ovarian reserve, the authors identified 2,323 women in 16 RCTs for the network analysis. Compared to a control group that did not supplement CoQ10 [OR 2.22, 95% CI (1.0 to 4,710)] and DHEA OR 1.92, 95% CI (1.16 to 3.16)] offered what the authors considered obvious advantages in improving clinical pregnancy chances. CoQ10 also significantly increased the live birth rate, while DHEA improved the number of oocytes retrieved, the implantation rate, and the number of high-quality embryos. Growth hormone supplementation also increased oocyte numbers. The least effective adjuvant treatment was TEAS.
These kinds of studies easily create associations with the old IBM dictum, “garbage in, garbage out,” because meta-analyses always depends on the studies chosen for inclusion and their respective quality assessments, both rather subjective decisions. The conclusions of this study should be considered with appropriate caution.
REFERENCE
1. Zhu et al., Reprod Biol Endocrinol 2023; 21:64
FSH alone or in combination with LH?
Whether FSH alone is as effective in stimulating ovaries in fertility treatments has remained controversial since Serono removed its initial urinary gonadotropin Pergonal, a 50/50 FSH/LH product,
Continued on page 52 The V oice | SEPTEMBER 2023 | 51
from the market to replace it with a pure recombinant FSH product. Initial results, heavily promoted by the manufacturer at the time, claimed equivalence in IVF cycle outcomes between the old and new product, but sporadic articles in the literature almost uniformly reported slightly better outcomes with FSH/LH mixture than pure FSH products.
Now comes likely one of the better studies on the subject, based on a German database,1 reaffirming the impression that, overall, an FSH/ LH combination produces slightly better pregnancy and live birth rates than a pure FSH product alone (29.8% vs. 27.8% and 20.3% vs. 18.0%, respectively).
With ovarian stimulation being such an essential part of infertility practice, the time for more serious investigations on what represents the “best” stimulations for selected patient populations appears to have come. The fact that FSH/LH combinations practically uniformly outperform FSH-only stimulation in general populations, suggests that, like in all studies of general populations, there likely must exist distinct sub-populations of infertile women who benefit, show no effects, or have adverse effects from FSH/LH combination therapy. It is quite astonishing that we still have not figured out who those patients are.
REFERENCE
1. Bielfeld et al., Best Pract Res Clin Obstet Gynecol 2023; 89:102350
Reproductive genetics
News about the FMR1 (fragile X) gene
Fragile X syndrome (FXS) is an X-linked (transmitted by mothers only) neurodevelopmental condition, caused by >200 CGG repeats in the expansive FMR1 gene. When this number of CGG repeats is reached, the production of FMR1-protein (FMRP) is interrupted, leading to severe intellectual disability. The condition is the most frequently known cause of genetic autism and also causes mental retardation, with both more severe in affected males than females. Individuals affected by CGG expansions in the 55-200 range have so-called FMR1 permutations, which are not characterized by the interruption of FMRP production but by sex-specific other disorders: Affected women are at an increased risk of developing early menopause [(primary ovarian insufficiency (POI)], while males are at increased risk of developing at middle-ages a neurodegenerative condition called the fragile X tremor ataxia syndrome (FXTAS).
The CHR has had a longstanding research interest in the FMR1 gene and has extensively published on the subject. This is why a recent paper in Cell immediately attracted our attention.1 In this paper U.S. investigators from Harvard Medical School in Boston for the first time report a potential treatment approach toward FXS that would allow for the correction of the genetic defect that in FXS patients shuts off the FMRP production. The investigators accomplished this task by recruiting endogenous DNA repair mechanisms, which excise the excessively long CGGn and restore the production of FMRP. As the authors note, this discovery for the first time opens the possibility of treating individuals with FXS.
REFERENCE
1. Lee et al., Cell 2023; 186:2593-2609
Genetics of primary ovarian insufficiency (POI)
Though the infertility field is not very disciplined in defining POI, this diagnosis is widely defined by age under 40 years, FSH over 40.0mIU/mL, and amenorrhea. It affects ca. 1% of the female population and is considered a heterogenous disease, sometimes monogenic, at other times polygenic, with several underlying causes, likely, not even yet known. Recently noncoding RNAs (ncRNAs) have attracted considerable interest regarding POI. Chinese investigators offered a review article on such ncRNAs in association with POI, concluding that dysregulation of ncRNAs plays a significant role in the development of POI. They further concluded that the potential utilization of ncRNAs in the treatment of POI warrants further investigation.
At roughly the same time, British and Australian investigators searched for single pathogenic determining variants for POI. In the process, they investigated in over 100,000 women, 105 genes previously associated with POI, and rather surprisingly found that these genes showed no pathogenicity for POI in the general population. Heterozygous predicted damaging variants were equally common in women with normally timed menopause. This study offers good evidence that contrary to the above-noted widely held belief that POI can have mono- and polygenic causes, it more likely is a polygenic disorder.2,3
REFERENCES
1. Zhang et al., J Clin Endocrinol Metab 2023; 108:1898-1908
2. Murray A, Perry J. Nat Med 2023; 29:1617-1618
3. Shekari et al., Nat Med 2023; 29:1692-1699
Polygenic risk scoring
Polygenic risk scoring (PGRS) has been repeatedly the subject of discussion in The VOICE. These discussions mostly centered on what we consider to be premature, attempts of introducing PGRS to IVF, as yet another rather irresponsible new commercial product (at least as of this point) of the genetic testing industry that some commercial laboratories have started to offer and some IVF centers have started to use to estimate an embryo’s genetic predisposition to develop later in life a genetically “complex “(i.e., polygenic) condition or disease.
PGRS in adults to predict disease risk has, however, become a center of attention in different medical specialty areas. A recent paper in Nature magazine pointed out that the accuracy of PGRS even in adults varies with the person’s genetic ancestry.1 The authors emphasize the need to abandon customary genetic ancestry clusters towards a continuum of genetic ancestry. This argument would also apply to the utilization of PGRS in embryo diagnoses.
REFERENCE
1. Ding et al., Nature 2023; 618:774-781
Continued from page 51 52 | SEPTEMBER 2023 | The Voice
We cannot help ourselves, but here is more on PGT-A
This time, it is a Chinese paper on PGT-A, claiming to report on outcomes in a retrospective cohort study involving 10,701 elective single embryo transfers, of which 3,125 underwent PGT-A and 7,576 did not. The authors then simply compared IVF cycle outcomes between these two groups, allegedly with adjustments for confounders, but what those supposedly were, was not stated. Unsurprisingly, they found that, except for ages 20-24, PGT-A cycles had significantly better live birth rates than non-PGT-A cycles at all other ages. The reason why this represents an unsurprising finding is not that it reflects any kind of reality but because this study repeats errors in statistical evaluations of PGT-A which are as old as the PGT-A hypothesis itself. Uncontrolled comparisons in general populations that compare PGT-A with nonPGT-A cycles are always biased in favor of PGT-A cycles because:
(i) To be considered a PGT-A cycle, at least one embryo must have in good quality reached blastocyst-stage to become eligible for inclusion. This, however, concomitantly means that all cycles that did not produce at least one good-quality blastocyst were excluded. The bias in patient selection for inclusion is obvious.
(ii) In this study there, is also, a second important bias entered the picture: Only roughly one-third of patients underwent PGT-A, while two-thirds did not. Why a decision was reached for or against PGT-A is not explained, but it is reasonable to expect that better-prognosis patients had PGT-A, and poorer prognosis did not.
In short, the conclusions of this paper make little sense. They contradict several recent studies that have demonstrated that in the general population, overall, and if properly analyzed, PGT-A reduces live birth rates. Moreover, age analysis, demonstrated that most harm from PGT-A comes to younger women under age 40. Indeed, as a somewhat surprising finding, over age 42-43 there no longer appears to be harm associated with PGT-A and there may even be a small benefit from PGT-A.2
This is why at least one finding in this paper is of interest: While the study reported that live birth rates were, as expected, significantly reversely related to female age, there was no such correlation found in PGT-A cycles. This can be well explained by the adverse impact of PGT-a especially under age 40 and, possibly, a slightly positive impact above age 43 (at least if one looks at outcomes with reference point embryo transfer).
Then there is the increasing utilization of non-invasive PGT-A (niPGT-A) by IVF clinics, which omits embryo biopsy and diagnoses embryos based on cell-free DNA in spent media of embryos. There is only one problem with this, theoretically, great sounding new approach to PGT-A: it so far does not work!
Not a single published study to our best knowledge was able to even match traditional PGT-A with biopsy, and consider how
poor a test this already is. No wonder a major fertility clinic chain in Australia is amid a class action suit because of prematurely offering niPGT-A and a U.S. clinic got a warning from the FDA. How IVF clinics are willing to reach a judgment on whether to use or dispose of embryos based on niPGTA is, therefore, puzzling.
A recent review of the subject in the same medical journal as the previously referenced article discusses the issue of cell-free DNA in spent embryo culture media.3 While well explaining where tested DNA may be derived from with niPGT-A, considering currently available technology, the article was unable to find a real argument in support of niPGTA. All it had to offer were speculations about potential future utilization. A recent systematic review with meta-analysis, of course by Chinese investigators, suggested a “high” accuracy rate of niPGT-A, with sensitivity, specificity, and area under the SRO curve, respectively, being 0.84, 0.85, and 0.91. The addition of blastocyst fluid to the analysis did not improve the accuracy of diagnosis.4
Considering what has been published on niPGT-A, one must wonder how the authors got to such surprisingly good values; but they must have wondered as well because their abstract suggests the need for future studies to determine the (real) detection value of niPGT-A.
REFERENCES
1. Zheng et al., J Assist Reprod Genet2023; 40(6):1417-1427
2. Kucherov et al., J Assist Reprod Genet 2023; 40(1):136-149
3. Handyani et al. J Assist Reprod Genet 2023; 40(1):231-1242
4. Huang et al., J. Assist Reprod Genet 2023; 40:1243-1253
A final word on genetic testing from the British Medical Journal
In a very brief piece in the BMJ, 1 the author pointed out that a study of 30,000 people from a UK biobank reported that people who participate in genetic studies are genetically predisposed to participate in genetic studies.2 This “footprint” of genetic bias was distinct from other traits. The tendency to participate in genetic
Continued on page 54
The V oice | SEPTEMBER 2023 | 53
studies was, moreover, passed down through families. Studies may, therefore, be affected by the participation of predisposed individuals in many different studies over a lifetime. This can lead to so-called “ascertainment bias,” which means that the genetic data collected is not representing the intended study population. As if we didn’t have already enough biases in our published studies; now we also have to fear participation biases!
REFERENCES
1. Looi M-K. BMJ 2023; PMD: 37460068
2. Benonisdottir S, Kong A. Nat Genet 2023; 55(8):1413-1420
Reproduction-related immunology
Pregnancy in women with multiple sclerosis
Like all autoimmune diseases, multiple sclerosis (MS) has very distinct interactions with pregnancy, characterized by the typical pregnancy characteristics of autoimmune diseases: increased miscarriage risk, increased risk for intrauterine fetal demise (loss of fetal heart later in pregnancy), slow fetal growth, premature labor and/or delivery, preeclampsia, dermatoses of pregnancy, and, likely the most characteristic feature, - postpartum flairs of disease for up to five months post-termination of pregnancy.
Like many other autoimmune diseases, MS, during pregnancy, however, actually improves, as demonstrated by a significant decline in disease fairs especially in the third trimester, as also noted in a recently published manuscript that reports on prominent epigenetic and transcriptomic changes in CD4+ and CD8+ cells during pregnancy.1 Uniquely among autoimmune diseases, MS patients have also been alleged to flair during ovarian stimulation with gonadotropins.2
Through DNA methylation analysis and DNA sequencing, the above-noted study revealed peaks in regulation in the third trimester which reversed postpartum, thereby associating observed peaks in regulation with improvements in MS flair activity, while their withdrawal postpartum was again associated with previously noted increased postpartum flair risk. The study also identified several enriched genes and pathways previously associated with MS important for this pregnancy-related regulation.
The authors concluded that pregnancy induces profound changes in peripheral T cells, likely associated with modulation of inflammation and MS activity. Considering that the flair pattern of MS is so similar to other autoimmune diseases, these findings are, likely also typical for other major autoimmune diseases with similar flair behavior.
REFERENCES
1. Zenere et al., J Neuroinflammation 2023; 20:98
2. Hellwig K., Correale J., Clin Immunol 2013; 149(2):219-224
Antiphospholipid antibodies (APAs)
The CHR has been at the forefront of much of the initial research in reproductive medicine that first defined how APAs (and other autoantibodies) affect pregnancy.1 The literature on the subject has, however, in recent years been rather sparse. A recently published review,2 appears timely. Though there is nothing new to report regarding APAs, pregnancy, and miscarriage risk, the paper is worth a quick read to refresh our knowledge of what is known regarding APAs.
REFERENCES
1. Gleicher et al., Autoimmunity 1993; 16(2):115-140
2. Grygiel-Górniak B, Mazurkiewicz L. J Thromb Thrombolysis 2023;56(2):301-314
From COVID-19 to current COVID
From COVID-19 to protection from COVID in 2023
It appears that we are once again in the midst of a COVID wave, which may further intensify as we go into fall and winter. It also appears that the newly circulating strains, while highly infectious, do not cause severe disease. The CHR, nevertheless, recommends booster vaccination for individuals above age 65 and everybody of all ages with any form of immune deficiency. We also recommend booster vaccinations for women planning on conception (spontaneous or through infertility treatments) or are already pregnant. The principal reason for the latter recommendation is the indisputable fact that, like the flu, COVID-19 infections in pregnancy are more severe than in non-pregnant women.
The boosters are scheduled to become available in early September. Getting annual boosters against the SARS-CoV-2 virus is something we will have to get used to, - just as we have gotten used to annual flu shots in the fall before the flu season starts. Flu shots are available at the CHR, but COVID-19 refresher boosters are unfortunately not yet offered to medical offices. Once they become available, the CHR will, of course, offer them at the center. They should be available in large chain pharmacies in early September.
What are COVID-19’s effects on fertility after all?
Though early reports were contradictory, there is now consensus that even mild COVID cases in males can affect semen quality. Men not only demonstrated adverse effects on semen parameters but, in some cases, have even been reported to become azoospermic. Because the length of spermatogenesis and epididymal sperm maturation is 78 days, azoospermia would suggest a viral attack on the germline even before the patient became infected with the virus. Reductions in sperm count and motility are observed even before the preceding occurrence of azoospermia.
Continued from page 53 54 | SEPTEMBER 2023 | The Voice
Spermatogenesis is reinitiated even before the virus is completely cleared, though spermatozoa demonstrate significant evidence of oxidative stress.1
A recent study by Spanish investigators reported at ESHRE 2023 in Copenhagen, Denmark in males with mild cases of COVID described roughly 20% declines in semen volume, a 26.5% decline in sperm concentration, and a 3.5% decline in sperm count. By the 100-day mark, semen analyses had not fully recovered.2 A Chinese study in JAMA Network Open compared the effects of SARSCoV-2 infections during ovarian stimulation based on oocyte and embryo-related outcomes. The infected group had impaired oocyte and embryo quality, 2PN cleavage rate, blastocyst formation, and quality of embryos. The male-positive group had fewer blastocysts. Infection ultimately was shown to adversely affect men as well as women.3
In summary, like so much else that is alleged about the SARSCoV-2 virus before, earlier reports of no significant effects on female and male fertility were incorrect, an important lesson to learn for the future!
REFERENCES
1. Gharagozioo et al., Transl Androl Urol 2022;11(1):110-115
2. Núñez-Calonge et al., Oral presentation: O-020, ESHRE 2023, HALL D, June 26, 2023. https://www.eurekalert.org/news-releases/993399
3. Tian et al., JAMA Network Open 2023; (6):e2323219
Exposed to COVID, why do some not get sick?
We all know people who repeatedly were in close contact with COVID-19-infected individuals and never got clinically sick. This appears to apply to ca. 20% of the population.1 When tested, they often demonstrate antibodies to the SARS-CoV-2 virus, but they never had even the slightest symptoms to suggest they had been infected. Why is that?
A new study in Nature magazine now offered an explanation:2 It appears that genes coding for human leukocyte antigen (HLA) are responsible. These genes encode molecules essential to immune responses to all infectious agents. Enrolling 29,947 individuals in the study for whom high-resolution HLA genotyping data were available, they selected 1,428 unvaccinated individuals who had demonstrated positive antibody levels for the SARS-CoV-2 virus. In those, they tested for associations with disease course/symptoms for five HLA-A loci and found in two independent cohorts a strong association between asymptomatic infection and the HLA locus HLA-B*15:01.
This association suggests preexisting T cell immunity and the investigators confirmed in pre-pandemic bloods that individuals with HLA-B*15:01 were reactive to an important immunodominant peptide of the saRS-CoV-2 virus but also cross-reactive to a peptide of a seasonal coronavirus. In other words, individuals with HLA-B*15:01 had preexisting anti-SARS-CoV-2 immunity because T cell immunity they had acquired earlier against a
seasonal cold virus also offered cross-reactive immunity against the SaRS-CoV-2 virus.
This HLA allele is found in ca. 10% of individuals with European ancestry and is less common among other ethnicities/races.1 In an interview with a JAMA reporter,1 the senior author of the study emphasized the fact that susceptibility toward the SARS-CoV-2 virus, of course, was multifactorial, with several genetic and non-genetic causes contributing in variable ways in different individuals. The reported association with HLA-B*15:01 in this paper is, however, fascinating!
REFERENCES
1. Abbazi J., JAMA 2023; 330(8):683-684
2. Augusto et al., Nature 2023; 620(7972):128-136
3. Ledford H., Nature 2023; 618:656
Gynecology
Interesting news on endometriosis
Japanese investigators in a recently published study suggested a possible causal association between endometrial infections with Fusobacterium and endometriosis.1
Among 155 study subjects, 64% of women with endometriosis, but only less than 10% of negative controls, demonstrated positive cultures for the bacterium, which is often found in the mouth, in the gut, and the vagina. It has been associated with gum disease.2 The authors also reported in a mouse model of endometriosis, that inoculation with the bacterium resulted in a significant increase in endometriosis lesions which could be prevented by antibiotic treatment. They concluded that the eradication of endometriosis may be possible with antibiotic treatments and clinical studies are already underway.
Though this study is impressive in its execution, it is important to remember that one reason why endometriosis studies are so difficult to perform is that control populations are never clean of contaminants with endometriosis because this condition often is only microscopic.3 A recent Finish study in Human Reproduction again reminded us of this fact, when reporting on first live birth rates in women before surgical verification of endometriosis,4 and discovering that their birth rates were lower than in controls. This confirms the obvious, namely that endometriosis preexists in its diagnosis and initial treatment attempts.
And, yes, there is also more evidence for endometriosis having characteristics of an autoimmune disease, a suggestion made by the CHR’s Norbert Gleicher, MD already in 1987,5 and the publication quotes another of his studies, demonstrating the effectiveness of reducing autoantibody load in endometriosis patients.6
In this study, the authors confirm significant intraperitoneal autoimmunity in a significant subset of and to much higher degrees in endometriosis patients than in controls. The tumor suppressor protein p53 was identified as the most frequent peritoneal fluid autoantibody
Continued on page 56 The V oice | SEPTEMBER 2023 | 55
target and proposed as a target for potential immune therapy of endometriosis. Once again, the 1988 paper by Gleicher and associates proposed exactly this kind of approach by treating endometriosis with danazol rather than GnRH antagonists.6
REFERENCES
1. Muraoka et al., Sci Transl Med 2023;15(700)
2. Ledford H. Nature 2023;618:656
3. Redwine DB. Gynecol Obstet Invest 2003;55(2):63-67
4. Tuominen et al., Hum Reprod 2023;38(8):1520-1528
5. Gleicher et al., Obstet Gynecol 1987;70(1):115-122
6. El-Roeiy et al., Fertil Steril 1988;50:864-871
Mifepristone for adenomyosis?
Often also described as endometriosis of the uterus, adenomyosis to some degree has remained an even more poorly understood condition than endometriosis. Whether and, if so to what degrees, it affects female fertility and/or IVF success, has remained controversial. Even the widely accepted association between adenomyosis and dysmenorrhea does not appear to be linear, and no good treatment has been established so far.
Now come Chinese investigators who offered the widely used progesterone receptor modulator, mifepristone, widely used as an abortifacient and more recently also reported to shrink uterine myomas, as an effective treatment of adenomyosis or, at least, as effective in treating the conditions dysmenoorhea:1 91.8% vs. 23.1% of patients had effective remission of symptoms after 12 weeks of treatment of 10mg of mifepristone or placebo, 88.5% vs. 6.2% had complete remission. Secondary outcomes, including menstrual blood loss, hemoglobin, CA125, platelet count, and uterine volume, all also significantly improved with treatment in comparison to placebo, while there were no differences in side effects. These data suggest mifepristone is an effective treatment for symptomatic adenomyosis.
REFERENCE
1. Che et al., JAMA Network Open 2023; 6(6):e2317860
Ovarian cancer
Chinese investigators recently also published a study of the uterine fluid metabolome in an attempt to diagnose ovarian cancer earlier.1 This very interesting study in Cell Reports Medicine identified a seven-marker panel that accurately detects early stages of ovarian cancer based on seven metabolite markers: (i) vanillylmandelic acid; (ii) norepinephrine; (iii) phenylalanine; (iv) beta-alanine; (v) tyrosine; (vi) 12S-HHT, and (vi) crithmumdiol. The study was initially based on 96 ovarian cancer patients and later validated on a set of 123 patients. The discrimination between early ovarian cancer patients and controls produced an area under the curve of 0.957 [95% CI, 0.894-1.0]. These are impressive numbers. If confirmed, this study offers what, likely, is to this day the best diagnostic option for earlier non-invasive diagnosis of ovarian cancer.
Another nationwide population study of Danish women reported in the International Journal of Cancer, concluded that women with a history of PCOS have double the ovarian cancer risk of control patients.2
Considering the increased follicle activity observed in PCOS patients, even though often anovulatory, this finding may not surprise, but unfortunately, the study does not discriminate between PCOS phenotypes. As elsewhere discussed in more detail in this issue of The VOICE, it appears increasingly clear that what, to this day is considered a syndrome of four phenotypes, likely, represents only two distinct genomic entities, what the CHR calls, a “metabolic” and an “immunological” subtype. It, of course, would be very important to know whether both or only one of these subtypes carries this risk.
An opinion article in JAMA, recently also discussed the concept of prophylactic salpingectomy to prevent ovarian cancer.1 With research now having established that the highly malignant serious type of presumed ovarian cancer often really originates in fimbriated ends of fallopian tubes, the concept of prophylactic salpingectomy, at least in high-risk patients, is becoming increasingly more popular. The authors, however, consider current usage still insufficient and make the likely correct point that a more expanded use probably will depend on the collaboration between surgical specialties that routinely access the abdominal cavity.
Finally, which professions are at the highest risk for ovarian cancer? If you guessed hairdressers, you were correct.4 Hairdressers have a three-fold risk after 10 years on the job and are followed by accountants with a two-fold risk. But who would have guessed the latter?
REFERENCES
1. Wang et al., Cell Rep Med 2023; 4(6):101061
2. Frandsen CLB et al., Int J Cancer 2023;15395):958-968
3. Stone et al., JAMA 2023; 329(23):2015-2016
4. Wilson FP. Medscape, July 10, 2023. https://www.medscape.com/ viewarticle/994113
Obstetrics & Perinatology
Does maternal rheumatoid arthritis increase autism risk in offspring?
This is exactly what a recent cohort study conducted by investigators from around the world in a Swedish patient population suggested:1 Among 3,629 children born to mothers with rheumatoid arthritis (RA), 70 (1.94%) were diagnosed with autism compared with only 1.92% of 28,892 children born to 1,503,908 mothers without RA. This represented a significantly increased risk (HR=1.43, 95% CI 1.11-1.84), with findings being especially significant with seronegative RA. No associations with autism were found with paternal RA, maternal sister RA, and RA diagnosed postpartum but maternal arthralgia demonstrated a similar risk to RA.
This is a difficult study to make sense of and is likely the reason why this study appeared in a psychology journal. That autism risk appears increased in the offspring of mothers with RA is not
Continued from page 55 56 | SEPTEMBER 2023 | The Voice
news, and has been reported before.2,3 Why this association should be more pronounced with seronegative RA and, similarly, with arthralgia in isolation is unclear. Both of these observations would suggest that the autism association is not with what is called RA but with only a mild form of RA, maybe at the initiation stages of the disease.
REFERENCES
1. Yin et al., Psychol Med 2023; 1-9. doi: 10.1017/S0033291723000855. Online ahead of print.
2. Rom et al., J Am Acad Child Adolesc Psychiatry 2018;57(1):28-31.e1
3. Sun et al, Front Med (Lausanne) 2022;9:1052806
Revisiting the impact of autoimmunity on reproductive success
As repeatedly discussed before in these pages, the impact of autoimmunity on reproductive success is, of course, considerable. A recent publication by British colleagues now once more confirms this fact in association with moderate and severe maternal psoriasis in a matched case control study. As expected with autoimmunity in general, this study again demonstrated lower fertility (RR, 0.75; 95% CI, 0.69-0.83) and higher miscarriage rates (OR, 1.06; 95% CI, 1.03-1.10) in women with this autoimmune disease.1 Preeclampsia and gestational diabetes risks did not vary between patients and controls.
Paradoxically, the authors conclude that “future research should identify the mechanism of increased risk of pregnancy loss among patients with psoriasis,” completely ignoring the fact that the observed association has little to do with the diagnosis of psoriasis and everything to do with the fact that psoriasis is an autoimmune disease.
REFERENCE
1. Chen et al, JAMA Dermatol 2023;159(7):736-744
Are ARTs associated with later cardiovascular risks?
Studies must make sense! In other words, before a study is planned, there should exist a logical hypothesis that the study then should confirm or reject.
Such a hypothesis appears to be lacking in a recent study by Scandinavian investigators which concluded that there was no increased risk for maternal cardiovascular diseases following the use of assisted reproductive technologies (ARTs), mostly IVF. That makes sense because why would an IVF cycle later in life increase the risk of cardiovascular disease unless the patient already had cardiovascular disease and/or a condition predisposing her to cardiovascular disease?
The authors interestingly point out the fact that infertility-associated conditions, like for example metabolic (hyperandrogenic) PCOS, which is associated with metabolic syndrome, are known to be associated with cardiovascular diseases later in life. The question that arises in such cases is not whether there is an association between ART and later occurring cardiovascular disease, but
whether the ART cycle increases preexisting risks such a question cannot be answered, as this study attempted by simply comparing risks between women with and without ART activities since both study groups contain at-risk patients, even though infertile populations, likely, have more. The way to investigate this question correctly then would be to see whether any existing risk increases with the number of ART cycles a patient underwent. In short, what a wasted effort, when there is so much need for studies that make sense!
REFERENCE
1. Chen et al, JAMA Dermatol 2023; 159(7):736-744
Hypertensive diseases of pregnancy
As repeatedly discussed in The VOICE, a good example of pregnancy conditions that predispose to later cardiovascular disease in women is hypertensive diseases in pregnancy. We here recommend an excellent recent review article on the subject by British and Taiwanese colleagues in the British Medical Journal (BMJ). 1
On the research side, one paper on this subject stood clearly out over the summer, - when researchers performed multi-ancestry genome-wide meta-analyses of women with preeclampsia/eclampsia and gestational hypertension. In the process, they identified 12 susceptibility loci associated with one or both of these diagnoses. Following those analyses, they were able to establish polygenic risk scores that in separate cohorts of patients allowed the prediction of hypertensive conditions of pregnancy. This allowed the determination of who should receive low-dose-aspirin prevention for preeclampsia. Astutely, and reemphasizing our comments regarding the earlier psoriasis paper in the preceding section, the authors also pointed out in their manuscript that the discovered genetic risks, including associated cardiovascular risks, are not specific to pregnancy, “but instead are unmasked by pregnancy.”2
Another interesting paper, this time in Obstetrics & Gynecology, explored pregnancy-associated maternal stroke outcomes in association with hypertensive disorders in national US data.3 This analysis demonstrated an increasing trend in postpartum strokes (in contrast to antepartum strokes). Moreover, as one would expect, almost half of all hospitalized patients for pregnancy-associated strokes were also hypertensive. Interestingly, while risks of adverse pregnancy outcomes were increased with postpartum strokes, neither postpartum nor hypertension-associated strokes demonstrated increased mortality.
REFERENCES
1. Wu et al., BMJ 2023;381:e071653
2. Honigberg MC, et al., Nat Med 2023;29:1540-1549
3. Bitar et al., Obstet Gynecol 2023;142(2):393-401
U.S. obstetric demographics
The good news is that a recent cross-sectional study found that delivery-related mortality in U.S. hospitals decreased for all racial and ethnic groups between 2008 and 2021. The bad news, however, was that severe maternal morbidity increased for all patients but especially for racial and ethnic minorities and age.
ADVERTISEMENT Continued on page 58 The V oice | SEPTEMBER 2023 | 57
Independently, advanced maternal age, racial or ethnic minority status, cesarean delivery, and comorbidities were associated with higher odds of mortality as well as severe morbidity.1 These are important findings for an general aging and multi-racial and multi-ethnic patient population in fertility clinics. The role of maternal age in increasingly severe maternal morbidity rates in the U.S. was further explored by another paper in Obstetrics & Gynecology, 2 further discussed below.
Further evidence for differences based on ethnic/racial backgrounds comes from another study, this time in JAMA, 3 looking at maternal mortality ratios (MMRs) on a state-by-state basis. What the researchers found was the following: In 1919, MMRs were higher among Native Americans and Black populations than in other racial/ethnic groups. Between 1999 and 2019, MMRs increased among Native American and Alaska Natives (14.0 to 49.2), in Black populations (26.7 to 55.4), among Asian, Native Hawaiian, and other Pacific islanders (9.6 to 20.9), among Hispanics (9.6 to 19.1), and among Caucasians (9.4 to 16.3). Between 1999 and 2019, each year, Black populations had the highest median state MMR. The largest increase in those years was seen among Native Americans and Native Alaskans. Those numbers speak for themselves and demonstrate how much work remains to be done to improve obstetrical outcomes, especially in minority populations.
Interestingly, risk due to advancing maternal ages appears to vary between races/ethnicities:2 Overall, changing age trends had little effect on severe maternal morbidity. What appears to be happening is that severe preexisting morbidities have been increasing overall in the general population and this increase is especially apparent in younger people. In other words, advancing age is not the culprit; the declining overall health of the female population that conceives appears to be the real problem. Only among the non-Hispanic Black population did age come into play and contributed 17-34% of the rise in severe maternal morbidity.
For fertility care these findings are very important because they point out the importance of detailed patient screening at the beginning of their infertility treatment journey. To find out that they have a medical problem must be viewed as a responsibility of the provider of fertility services before the patient conceives.
REFERENCES
1. Fink et al., JAMA Network Open 2023;6(6):e2317641
2. Berger et al., Obestet Gynecol 2023 142(2):371-380
3. Fleszar et al., JAMA 2023;330(1):52-61
More on cannabis use in pregnancy
Sarah C.M. Roberts, DrPH, has been one of the most prolific researchers of cannabis use in pregnancy. In a recent paper in JAMA Network Open, she added an interesting observation to her quickly growing oeuvre, demonstrating that warning signs at point of sale have no effect whatsoever on cannabis use in pregnancy. 1 The only thing they may do is to create more stigma among non-users.
On a side note, and reconfirming the very substantial increase in cannabis use since legalization in most U.S. states, 17.2% of study participants acknowledged the use of cannabis during pregnancy. This allows for the conclusion that real numbers must be even bigger and that probably at least a quarter of U.S. pregnant women during pregnancy now use cannabis in one way or the other. Considering by now well-recognized adverse effects on mothers and especially offspring from such use during pregnancy, these are, of course, very concerning numbers.
REFERENCE
1. Roberets et al., JAMA Netw Open 2023;6(6):e2317138
Vaginal seeding of newborns after Cesarean sections?
Cell Host Microbe is usually not a journal where Nature magazine picks up paper for commentary. This is, however, exactly what happened after Chinese investigators published a paper in the journal in which they reported on a prospectively randomized study of vaginal microbiota transfer (VMT) from mother to offspring after Cesarean section delivery where offspring are not exposed to the usual vaginal microbiota they encounter during vaginal passage in a vaginal delivery.1
The study involved 68 Cesarean deliveries in which newborn infants were randomized to VMT or placebo (saline). While adverse effects did not differ, the infants’ neurodevelopment after VMT treatment was at six months, based on a widely used developmental assessment method, was significantly advanced. VMT in those infants also significantly advanced gut microbiotic maturation and regulated certain fecal metabolites and metabolic functions, including carbohydrate, energy, and amino-acid metabolism within 42 days from birth.
The commentary in Nature was written after the journalist interviewed several experts in the field and can be summarized as follows: (i) These are exciting data that may change practice, but they require confirmation before universal introduction into practice. (ii) The practice appears to be safe, but only if the mother’s microbiome is, first, checked. (iii) Do observed effects last and does it make any difference in the long-term whether the practice is utilized?
REFERENCES
1. Zhou et al., Cell Host Microbe 2023;31(7):1232-1247.e5
2. Callaway E. nature 2023;618:659-660
Is amnioinfusion to treat early-onset anhydramnios ethical?
In a “viewpoint” article in JAMA, three neonatologists/pediatricians raised an important ethical question for all of medicine: when is a treatment we are pursuing too much and, therefore, unethical? They do this in this article for one procedure (amnioinfusion), in one condition (anhydramnios), and in one medical specialty (neonatology/pediatrics), but the answer applies to many such circumstances in, likely, most medical specialties.
Continued from page 57 58 | SEPTEMBER 2023 | The Voice
The authors point out that this treatment currently is still considered “experimental” and is not standard of care for fetuses with prenatal kidney failure and anhydramnios, which can be caused by numerous congenital and genetic abnormalities. Survival of so-affected infants is almost always associated with life-long medical problems and often requires major changes within the affected family unit. Given these facts, they made the plea to fellow neonatologists to withhold amnioinfusion in cases of early anhydramnios until a currently ongoing clinical trial is published, which is hoped to offer enough outcome data to allow proper informed consent for affected parents.
We fully agree with their recommendation since the two possible alternatives to following their recommendation in our opinion are both unethical: To ignore the fact that this currently is still an experimental procedure and treat it as routine is unacceptable and unethical. Similarly, it seems unethical to give parents informed consent if proper outcome information is unavailable. This, of course, leaves parents with the option of insisting on such treatment; and that is their right if they are willing to acknowledge in writing that such treatment is given “against medical advice.”
REFERENCE
1. Soffer et al., JAMA 2023;22:1913-1914
Prenatal vitamins before and during pregnancy
CONFLICT STATEMENT: This commentary addresses the clinical use of dietary supplements in the form of prenatal vitamins before, in, and after pregnancy. The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a prenatal vitamin. Readers of this commentary, therefore, are advised that opinions expressed in this paragraph may be biased by financial interests.
In the U.S., supplementation with a multivitamin before and during pregnancy, and carrying over into lactation, is considered routine. In other countries, this is not the case, and is often the only supplementation recommended is folic acid and supplementation of diagnosed specific deficiencies. What
should be in a multivitamin that is called a “prenatal vitamin,” has, however, remained somewhat controversial.
We were pleased to recently see a paper by several nutritional experts who, on behalf of program collaborators for Environmental Influences on Child Health Outcomes, surveyed the literature for six key nutrients with appropriate dosing that should be in any prenatal vitamin. The CHR here offers this paper not as an endorsement of these recommendations (the CHR only endorses products of Fertility Nutraceuticals, LLC doing business under the name Ovaterra because the CHR’s staff is closely involved in the creation of these products and is familiar with the quality control processes involved in the manufacturing of these products). We were impressed by the details and opinions expressed by the authors in this paper.
REFERENCE
1. Sauder et al., Am J Clin Nutrition 2023;117(4):823-829
The CHR
Fighting for every egg and embryo
NEWSLETTER INFORMATION
The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400. The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiology, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved.
For letters to the editor, comments, and suggestions, please contact Micah Elias at melias@thechr.com. For all advertisements or sponsorships in The VOICE , please contact Alexandra Rata at arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR. .R
Continued from page 58
The V oice | SEPTEMBER 2023 | 59




The Center for Human Reproduction Fighting for every egg and embryo www. T h EC h R . COM SOCI al@ T h EC h R . COM www.thechr.com @CHRNewYork @CHRNewYork @CHRNewYork 21 E 69 T h S T N E w Y OR k, N E w YOR k 10021 212.994.4400 C ONNECT w IT h u S !




























 By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist
By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist
























