






Welcome to the first issue of the VOICE for the 2024/2025 academic year. After taking the customary summer break during July and August, we are pleased to be back with what we think is an exciting September issue. In addition, building on the amazing and all-expectations-exceeding growth in content and readership the VOICE has experienced during the preceding academic year, we reached important conclusions about where we wanted to take the VOICE going forward; here is a brief summary of what we believe will be a very exciting future.
The VOICE was born as an irregularly appearing newsletter of the Center for Human Reproduction (CHR), only a few pages long and primarily addressed to past, present and, possibly, future CHR patients, CHR staff, and a small number of colleagues at other institutions with which the CHR had special relationships, usually involving research. Over the years, the newsletter appeared more regularly, expanded its content, and also grew its electronic mailing list. During the 2020/2021 academic year it became obvious that the VOICE had outgrown its “immaturity” and, if further progress was to be achieved, the newsletter had to become a more professionally produced product. Therefore, the CHR for the first time hired dedicated fulltime staff and radically changed the design and production of the VOICE, a process of maturation that has not stopped since, as demonstrated by the steady expansion and improvement of its content, design, and readership.
The June 2024 issue of the VOICE contained no fewer than 74 pages, electronically distributed to a mailing list of almost 80,000 individuals (and growing), all associated with reproductive biology and medicine, with, of course, special attention to reproductive endocrinology and infertility (REI). The response the VOICE received, moreover, exceeded all expectations, best characterized by amazing opening rates for our electronic mailings and unexpectedly long reading times, both parameters progressively increasing from issue to issue. In addition, the VOICE has received a steadily growing stream of comments to articles that appeared in the newsletter, often from totally unexpected sources.
This unexpected degree of success with the VOICE, however, raised the question of where do we want to go with the newsletter from here and what is/are the goal(s) we want to achieve? It seemed obvious that the VOICE in many ways had outgrown its purpose as the CHR’s newsletter, but what should be its future direction?
We are now very pleased to report that after many months of discussion, we over the summer decided on what we believe is not only an excellent next step for the VOICE, but at the same time the birth of a brand-new project that reaches far beyond the initial intent of publishing a newsletter for the CHR: On Monday, September 2, 2024, a new media product will go live with a new URL under the name THE REPRODUCTIVE TIMES. Its goal, independent of the CHR, will be to offer a steady stream of information from all over the world, relevant to reproductive biology, medicine and, of course, the REI field, posted immediately when available.
Content will, like in the VOICE, include articles meant to inform, personal opinions—whether the editorial board shares them or not—an ongoing review of the relevant medical literature, debates, interviews, and some lighter fare. In other words, THE REPRODUCTIVE TIMES will be a new format of news in the reproductive field as it currently does not exist, offering anybody interested in and/or professionally connected to reproduction new and reliable information on an ongoing basis, clearly differentiating between information and opinion.
This, however, does not mean that the VOICE will disappear. To the contrary, the VOICE will continue its monthly schedule (except for July and August) and will pull each issue together from selected materials in the prior month published by THE REPRODUCTIVE TIMES, at times accentuated by content that specifically relates to the CHR.
As the CHR will have the opportunity to use articles from THE REPRODUCTIVE TIMES for the VOICE, so will—for a nominal fee—fertility clinics all over the world be given the opportunity to utilize materials for their own instruments of communication. THE REPRODUCTIVE TIMES, indeed, will offer fertility clinics customized newsletters and/or other products for their own exclusive use. If you want to contact THE REPRODUCTIVE TIMES to discuss such opportunities, you can do so at hello@reproductivetimes.com. We hope that you access our new website at reproductivetimes.com as often as possible to enjoy our regular posting. We also encourage you to also let your friends and colleagues know about THE REPRODUCTIVE TIMES.
Which brings us back to this issue of the VOICE which, because of the summer break in publishing, is again quite substantial and contains the usual sections. As always, we encourage our readers to communicate with us regarding articles in the VOICE or if you are interested in contributing an article. Please send your communications to social@thechr.com
Welcome to the new academic year!

We still love eggs!

H. Duncan, MFA, is a writer and copy editor of the VOICE
BRIEFING: This article digs into recent headlines about IVF access in the United States, where it is increasingly seen as the next battle in the culture war regarding reproductive rights. States’ so-called “fetal personhood laws,” such as the one that enabled the Alabama Supreme Court in February to rule that frozen embryos are considered children, raise worrying questions about the ability to access fertility treatment. In this election year, both major political parties are scrambling to respond to the recent wave of public attention to IVF, a procedure which is, despite these controversies, largely supported by Americans.
In February, the Alabama Supreme Court ruled that frozen embryos are considered persons with a constitutional right to life. The case in question was brought by parents whose embryos created through IVF at a reproductive medicine center were accidentally destroyed; in its decision, the Court cited the state’s 2018 constitutional amendment affirming the rights of “unborn children.”
The decision sparked a nationwide outcry and prompted IVF clinics in the state to close, leaving couples undergoing fertility treatment in limbo; the following month, the Alabama legislature passed a law instituting protections to ensure clinics could resume operations without fear of liability. The ruling and the backlash against it thrust the practice of IVF, increasingly used by people experiencing fertility challenges, into the national spotlight in an election year in which reproductive freedoms remain a hot-button issue after the 2022 fall of Roe v. Wade and the legal ramifications of “fetal personhood” are under more and more scrutiny.
Since the first birth of an IVF-conceived baby in 1978, the procedure has brought more than ten million children into the world, and today it accounts for about two percent of U.S. births. As more and more women delay childbirth, IVF is a vital tool, and one generally viewed favorably: according to Pew Research Center, more than 4 in 10 adults say they’ve used fertility services or personally know someone who has, and a majority believe fertility treatment should be covered by insurance (Goddard, Aragao, Pew Research Center). Another poll found that more than sixty percent of Americans support protecting IVF access (Associated Press, WGCU).
At the heart of the conservative push against IVF lies the destruction of embryos that’s inherent to the procedure: it usually takes multiple embryos
before achieving a successful pregnancy, and the unused embryos are most often discarded. This is where the notions of fetal and embryonic personhood come into play: According to a 2023 report by advocacy group Pregnancy Justice, which sounds the alarm that “fetal personhood” is no longer a fringe idea, “at least 11 states have extremely broad personhood language that could be read to affect all state law” in conveying rights to embryos or fetuses (Pregnancy Justice).
Though most Americans support IVF and voters often reject new fetal personhood ballot measures, there is still ambivalence. In June, the 13-million-member Southern Baptist Convention, considered “a bellwether of American evangelicalism,” voted to condemn the practice of IVF (Graham, New York Times). During debates on the resolution, many members acknowledged the pain and struggle of those facing infertility, but maintained that they could not condone what they saw as the destruction of human life. This points to an important contradiction at the heart of the matter: As The Economist journalist Sacha Nauta has noted, “Over 8 in 10 Americans think IVF is morally acceptable, but only about half of Americans think it is morally acceptable to destroy these excess embryos. So that’s an inconsistent view” (WABE).
A few salient points help put today’s debates over IVF into context and illuminate whether Americans seeking the treatment should fear restrictions.
First, political views about the use of embryos have been affecting IVF for decades, starting with the sphere of research. Since 1996, a federal appropriations amendment signed into law by President Bill Clinton has banned federal funding from being used for human embryo experimentation (Wadman, Nature).
As noted by a Medical News Today special report, “a convoluted state-by-state system means that embryo research is only legal in five states and ‘vaguely’ legal in another 13”; as well, less than three percent of unused frozen embryos sitting in storage throughout the country are available for research, hampering the progress that could be made on the procedure with more widespread access to embryos (Yockey, Medical News Today).
Second, Republicans are distancing themselves from backlash to anti-IVF measures. Republican Presidential nominee Donald Trump, after the Alabama ruling, said he supports IVF (Pollard, et al., Associated Press). His running mate, Senator J.D. Vance, a conservative on issues of reproductive rights, voted against the federal Right to IVF Act in June, but in the wake of increased attention to his pronatalist viewpoints and verbal attacks on childless women, he has attempted to clarify that he supports fertility treatments despite his vote (Weixel, The Hill; Dobkin, Newsweek).
The Republican Party platform is murky on the issue. It calls for “supporting mothers and policies that advance Prenatal Care, access to Birth Control, and IVF (fertility treatments)”, yet it endorses that states can use the 14th Amendment to ensure fetal personhood—another contradictory position given that the notion of fetal personhood is incompatible with the practice of IVF. Likewise, Project 2025, a policy handbook for the Republican Party that was written by the conservative Heritage Foundation and is seen as a right-wing wish list for a second Trump term, does not call for restrictions to IVF but contains language supportive of human rights beginning at conception. (The Trump campaign has disavowed Project 2025.)
Democrats are seizing the moment to capitalize on the growing alarm over anti-IVF rhetoric and restrictions to women’s rights more broadly. President Biden, when still campaigning for re-election, released a campaign ad featuring an infertility patient stating that “Thanks to Donald Trump, IVF is now at risk.” Now the Democratic Presidential nominee, Vice President Kamala Harris–long a vocal advocate for reproductive rights–has become outspoken about protecting IVF access, slamming the Alabama ruling, issuing a campaign statement on World IVF Day in July and inviting an Alabama IVF patient to share her experiences at the White House. And Democratic elected officials themselves are sharing their personal stories of relying on IVF; Harris’s running mate, Minnesota Governor Tim Walz, has spoken about using IVF to build his family, and Arizona Senator Mark Kelly penned a People Magazine op-ed on the topic with his wife, former U.S. Representative Gabby Giffords
(Kelly, Giffords, People.)
Finally, even without the vicissitudes of the national political theater, access to IVF is already uneven. The high cost of IVF and widespread lack of health insurance coverage for the procedure is an existing barrier for many Americans, with more and more patients resorting to going abroad for cheaper treatment (see page 8). It is clear that in some regards, Americans’ access to IVF could begin to mirror abortion access, in that it may come to depend on the laws of the state one lives in and how much money one has to pursue the procedure.
The extent to which the political climate could further erode Americans’ access to IVF remains to be seen, and will depend upon the decisions of courts, the results of the November elections, and the efforts of advocates to position IVF as an essential family-building tool.
Isabel Goddard and Carolina Aragao. Pew Research Center. September 14, 2023. https://www.pewresearch.org/shortreads/2023/09/14/a-growing-share-of-americans-say-theyve-hadfertility-treatments-or-know-someone-who-has/
Associated Press. WGCU. July 15, 2024. https://news.wgcu.org/ health/2024-07-15/more-than-6-in-10-u-s-adults-support-protectingaccess-to-ivf-poll-finds
Pregnancy Justice US. 2023. https://www.pregnancyjusticeus.org/ wp-content/uploads/2022/12/fetal-personhood-with-appendix-UPDATED-1.pdf
Ruth Graham. New York Times. June 12, 2024. https://www.nytimes. com/2024/06/12/us/ivf-vote-southern-baptists.html WABE. July 10, 2024. https://www.wabe.org/tv-episodes/afteroverturning-roe-v-wade-will-ivf-be-americas-next-culture-warozhtac/
Meredith Wadman. Nature. February 1, 1996. https://www.nature. com/articles/379386a0.pdf
Katie Yockey. Medical News Today. July 4, 2024. https://www. medicalnewstoday.com/articles/ivf-research-what-are-the-latest-advances-and-what-obstacles-stand-in-the-way#Decades-old-legislationlimiting-new-research
James Pollard, Michelle L. Price, Meg Kinnard and Bill Barrow. Associated Press. February 24, 2024. https://apnews.com/article/trump-ivf-abortion-alabama-republicans-8215336740a5963b57bbd970b47bb3a7
Nathaniel Weixel. The Hill. June 13, 2024. https://thehill.com/ homenews/senate/4720506-senate-republicans-block-ivf-legislation/
Rachel Dobkin. Newsweek. July 27, 2024. https://www.newsweek. com/jd-vance-ivf-views-explained-childless-cat-ladies-fallout-1931082
Mark Kelly and Gabby Giffords. People. June 20, 2024. https://people.com/mark-kelly-gabby-giffords-ivf-reproductive-freedom-exclusive-essay-8664641
Yash Kulkarni, BS (Bioengineering) is a writer, designer, and editor of the VOICE
BRIEFING: In the June VOICE, this author presented the birth rate crisis in the developed world and the reasons behind it. This article focuses on the United States and the affordability of fertility healthcare coverage, or rather the lack thereof. The astounding price of fertility treatment, the lack of coverage by both government and private healthcare, and the growing financial burden that children represent effectively reserves the right to procreate for privileged social classes. Medicaid exemplifies some of the biggest and most flagrant inequalities in fertility healthcare, while private insurers use a variety of religious and bureaucratic excuses to withhold coverage. LGBTQ people are also often short-changed by these companies’ exploitation of federal and state policy. Because this issue is both bipartisan and critical to much of the public and their representatives, progress is being made in the form of state mandates, but still too slowly to turn the tide. This has led to a surge of Americans going abroad for treatment, further illuminating the inequality crisis.
My last article in the June VOICE presented an analysis of the dropping birth rates across the first world. Among a myriad of factors, the unmanageable financial burden of children on young people seems to be a prominent reason. The recent inflationary trends in the US economy and across the world do not help. Especially in America, as the cost of living surges and companies shrink health coverage, more women are choosing their careers over family building. While this decision is more financially secure, it of course poses fertility challenges, leading to an ever-growing reliance on fertility treatments like prophylactic egg-freezing and IVF. Though these technologies make rapid progress, their affordability becomes an escalating problem exacerbated by lagging government health plans and private insurance coverage.
America has a lengthy history of unjust reproductive legislation, manifested most blatantly in the eugenics movement. The movement sought to improve the human race by preventing unfit individuals from having children through policies like forced sterilization, and of course this fitness was defined by race, ethnicity, and demography. While the injustices of eugenics reached their peak in Nazi Germany, plenty of revered Americans were proponents of the movement here at home.
Politicians like Woodrow Wilson, scientists like Alexander Graham Bell, and even many physicians believed that eugenics was a moral and ethical obligation of medicine. One of the more prominent figures was Margaret Sanger, a birth control activist and sex educator who founded Planned Parenthood, but whose association with the movement
has allowed some to reposition the organization as a sinister force of racism. These arguments are still present in medicine today, especially with regards to genetic abnormalities, cognitive impairments, and physical disabilities, of which Sanger said, “the most urgent problem today is how to limit and discourage the over-fertility of the mentally and physically defective” (Jennifer Latson, TIME).
Though our healthcare system has made much progress since the days of eugenics, it is no surprise that our current state and federal healthcare programs still leave plenty of room for systemic failures, especially affecting the most vulnerable Americans.
While the World Health Organization (WHO) defines infertility as a disease, government healthcare does not cover infertility. Many private health plans decline to cover fertility treatments, and state laws requiring coverage are influenced by powerful insurance companies.
The greatest problem is with Medicaid, which only provides fertility coverage in two states and only covers small portions of treatment. For people with incomes low enough to qualify for Medicaid, the up to $20,000 price tag of a single cycle makes IVF unattainable, and of course conception in the first cycle attempt is highly unlikely. The usual retort against expanding coverage is that those who can’t pay for treatment couldn’t afford to raise a child anyway. Ironically, this highlights the designed failure of the system for these vulnerable people and exposes a sentiment of superiority of those with the financial freedom to have children over those
without. Quoting friend of the CHR Dr. Eli Adashi, these policies “[stand] out as a sore thumb in a nation that would like to claim that it cares for the less fortunate” (Michelle Andrews, CBS News).
According to a 2022 Duke University study, the cost of IVF remains the greatest barrier to infertility care. Private insurers have used the same excuses as the government to deny coverage, but states have the ability to mandate some coverage under laws that define infertility as a disease. So far, 21 states have passed mandates, with 14 including limited IVF treatment. While still in Chicago in 1991, the CHR’s Norbert Gleicher, MD was actively involved in an uphill battle to pass the nation’s second such mandate in Illinois, called the Family Building Act. More recently, insurers have been met with additional motivations, as many employees will favor jobs with fertility coverage as a part of comprehensive insurance packages.

Of course, insurers have found a variety of convenient objections, from the religious doctrine cited by Providence Health Plan in Oregon to Sanford Health Plan in the upper Midwest using obscure North Dakota laws to inflate premiums and create red tape. Bills in Oregon, North Dakota, Illinois, Wisconsin, Connecticut, Washington, New York, and other states failed to pass in 2023. North Dakota State Representative Mike Brandenburg (R) has thrice unsuccessfully attempted to pass a bill mandating coverage and says he will keep trying. Wisconsin Governor Tony Evers (D) added a mandate to the budget in 2023 which was shot down by the legislature. These issues are clearly not partisan, but the insurers’ bottom line continues to prevail.
Despite the difficulty in passing legislation around it, infertility coverage could and should be rather inexpensive. Financially, it makes very little sense for a company to decline coverage, as the vast majority will experience little to no cost increase, as not all the employees will require expensive procedures.
Patients with fertility coverage are obviously more likely to undergo important screenings use multiple embryo transfers during IVF, and experience fewer complications in pregnancy, ultimately reducing expenses for both employees and employers. In cases in which more expensive procedures like IVF or surgeries are required, those expenses can also be tax deductible Taxpayer Publication 502 includes possible deductions for medical and dental expenses, including fertility enhancement procedures (Lauren Cuddeback, Benefits Pro). These deductions only apply if the expenses are more than the taxpayer’s adjusted gross income and do not include the cost of surrogates.
This area of the law is still quite murky and gets increasingly uncertain for couples that aren’t heterosexual. Infertility is defined by the US Center for Disease Control and Prevention (CDC) as “not being able to conceive after one year of unprotected sex”, which obviously excludes same-sex couples. While the lead of the CDC’s Assisted Reproductive Technology Surveillance and Research Team clarifies that the language is “not intended to guide any recommendations about the provision of fertility care services”, many insurance providers still use similar language in their policies. One of these, Blue Cross Blue Shield of Illinois, was sued last year for this definition on the basis that it openly discriminates against LGBTQ couples (Bailey Shulz 2, USA Today).
Their policy requires infertile couples that cannot conceive because of their sexual orientations to pay for a full year of assisted conception before Blue Cross’s coverage starts. According to Patience Crozier, the Director of Family Advocacy at GLAD (GLBTQ Legal Advocates and Defenders), there is legislation in the works to address this issue, though the hundreds of anti-LGBTQ bills introduced just this year have stalled progress. House Bill 6617, introduced in Connecticut last year, would have required private insurance companies to offer fertility coverage to LGBTQ and single people but was tabled for the legislative session.
While change through legislation is gradual by design, political pushback in recent years has not helped. Crozier worries about the impact on reproductive rights of the 2022 Supreme Court overturn of Roe v. Wade, especially for the LGBTQ community. In response, more American women are going abroad for fertility treatments. In contrast to the aforementioned cost in the US, patients in Colombia pay around $7,000 for a round of IVF. In Barbados, that cycle costs about $6,500, $6,000 in Spain, and $5,800 in Mexico (Ivana Sarik, Axios).
Of course, even international options aren’t always available for lower- and middle-class Americans and aren’t necessarily affordable even for those with some means. Between interests of the legislatures, insurers, clinics, and citizens must lie a solution somewhere, but not likely one that fulfills everyone’s needs. Any real solution must make access for fertility treatment available for everybody who needs it, and also urgently considering the birthrate crisis. The US, supposedly the most prosperous country in the world, does fewer IVF cycles per capita than almost any other developed nation, simply
because its citizens cannot afford it, yet it hosts some of the largest health insurance companies.
Maybe something to learn for the U.S government and other countries in the developed world which experience low birth rates! Since a country’s birth rates are an economic argument determining a nation’s future, maybe, governments would understand the underlying issue in favor of covering fertility services better if explained in this way.
Reading List:
Jaimme Ducharme. TIME. April 10, 2024. https://time. com/6965267/women-having-kids-later/
Michelle Andrews. CBS News. February 22, 2024. https://www. cbsnews.com/news/ivf-fertility-treatment-costs-insurance-coverage-medicaid/
Linda Villarosa. The New York Times Magazine. June 8, 2022. https://www.nytimes.com/2022/06/08/magazine/eugenics-movement-america.html
Resolve: The National Infertility Association. June 17, 2024. https:// resolve.org/learn/financial-resources-for-family-building/insurance-coverage/insurance-coverage-by-state/
Tim Henderson. Stateline. July 28, 2023. https://stateline. org/2023/07/28/fertility-health-coverage-is-still-hard-to-come-byin-many-states/
Ivana Saric. Axios. July 25, 2023. https://www.axios. com/2023/07/25/americans-fertility-treatments-abroad
Bailey Shulz. USA TODAY. Jan 29, 2024 https://www.usatoday. com/story/money/taxes/2024/01/29/is-ivf-tax-deductible/72340462007/
Lauren Cuddeback. Benefits Pro. May 21, 2024. https://www. benefitspro.com/2024/05/21/fertility-benefits-more-affordable-than-employers-think/?slreturn=20240709151633
Bailey Shulz. USA TODAY. June 13, 2023. https://www.usatoday. com/story/money/2023/06/13/ivf-cost-higher-for-lgbtq-couples/11135417002/
Jennifer Latson. TIME. October 14, 2016. https://time. com/4081760/margaret-sanger-history-eugenics/

by Sònia Gayete Lafuente, MD, PhD, FRM research fellow at the CHR
BRIEFING: Female infertility increases with advancing age. As women all around the world for several social as well as economic reasons are increasingly delaying pregnancies into older age, egg-freezing, in parallel, has for psychological as well as clinical reasons become an increasingly popular tool to support this decision. Unsurprisingly, the increasing demand for egg-freezing cycles has attracted economic interests, resulting in a booming “egg-freezing industry” with “egg-freezing clinics” and long-term cryopreservation facilities at the center of the industry, often owned by investors and not physicians. In contrast to regular IVF clinics, they first primarily—and at times even exclusively—performed egg-freezing cycles, a practice model that proved to be economically disappointing, leading to quick shutdowns of some of these first-generation egg-freezing clinics. Others changed their model to more general IVF practice, though still with emphasis on egg-freezing which, therefore, is currently offered in two distinct scenarios: either by exclusive egg-freezing clinics or in regular IVF clinics, like the CHR, serving a general infertility population. As this article will explain, to be able to distinguish between these two formats of egg-freezing services is important and, often, not well understood by the public.

Since women are born with all of their eggs, two negative things occur to a woman’s original egg-pool in both of her ovaries:
(i) It constantly shrinks in size, as eggs are constantly recruited out of this pool, biologically speaking, going to waste. (ii) Those eggs that are not recruited out of the pool remain in the ovaries but age as the female ages. And, as eggs age, they lose pregnancy potential. The egg of a healthy 25-year-old, for example, offers at least a 35% pregnancy chance, while the egg of
a 45-year-old woman offers only approximately a 5% pregnancy chance. In parallel, with advancing age poorer egg quality also means more chromosomal abnormalities in eggs and embryos and, therefore, more miscarriages as women age. The principal idea of egg-freezing, therefore, is to interrupt this biological “double-whammy” of fewer and poorer quality eggs by freezing at relatively young ages a sufficiently large number of eggs to later in life—with certain probability—be able to conceive one or more children, should the woman’s remaining egg pool at that age no longer permit pregnancy.
Once frozen, eggs preserve their pregnancy potential at a woman’s age at which they were cryopreserved.
So, who then may benefit from egg-freezing at young ages?
First, we must differentiate between women at risk to lose their fertility because their ovaries need to be surgically removed and/ or who, because of cancer or other diseases, must be treated with gonadotoxic medications which will wipe out most or all of their eggs (medically indicated egg cryopreservation) and those who wish to freeze their eggs to preserve their ability to conceive into older ages where they—naturally—will likely no longer have the ability (non-medical or social egg-freezing). This latter group is made up of only two potential clinical scenarios:
§ Biological females planning on undergoing gender-affirmative treatments who want to maintain the option of later producing biological children themselves or using their eggs in creating embryos carried by a female partner.
§ Women who consciously choose to delay motherhood for personal reasons, with pursuit of a professional career, for the longest time were widely believed to represent the major motivation. Recent research, however, has demonstrated that, with the ticking time clock of aging ovaries in mind, the most frequent motivation appears to be a woman’s inability to find her “right” partner.
It is this second scenario we are addressing here, with the first to be addressed in a separate article in the VOICE in the near future, as the CHR, of course, also offers egg-freezing services to females undergoing gender-affirming medical treatments. What, then, are the most important issues for women who choose to freeze their eggs because they consciously wish to secure their ability to achieve pregnancy with use of their own eggs at some future time?
Should I freeze my eggs? As already noted, all women are born with a finite supply of ovarian follicles (which contain the eggs), which constitutes the so-called ovarian reserve (OR). OR gradually diminishes over time, with a notable acceleration between ages of 37 to 40 years, until the OR reaches a minimal threshold in numbers of remaining follicles and women enter menopause. As also already noted, concurrently, egg competence also diminishes, accelerating from approximately age 35, and more significantly from ages 37-40, although individual variability can be substantial. The declines in egg quantity and quality are the primary factors contributing to decreasing fertility in women during their late thirties and beyond.
Once frozen, eggs will after thawing retain their reproductive potential at the level commensurate with the age of the woman when her eggs were frozen. This, however, does not necessarily mean over all age-ranges that the younger you freeze eggs, the better. Recent data, indeed, indicates that elective freezing should likely not be done before age 23. Optimal timing should, moreover, should not only take into consideration the female’s OR (a factor often not even assessed in primary egg-freezing clinics) but also social factors characteristic of the patient (and, yes, egg-freezing should not be viewed as just a social interaction, but—very definitely—as a medical procedure).

Some social factors deserving attention are the costs not only of one retrieval cycle (as patients are often quoted) but for all cycles a patient will, likely, need to accomplish a large enough frozen oocyte pool that meets her expectation for one or more deliveries at a later point. Of similar importance is an assessment (as uncertain as it may be) of whether the patient likely will or will not return to use her frozen eggs. In some cases, even psychological evaluations may be indicated before committing young patients to these costly interventions (once again, rarely a concern expressed in clinics primarily serving as egg-freezing providers).
Freezing eggs in the 20s may be biologically highly efficient but, at the same time, can be expected to lag in cost-effectiveness, since utilization of frozen eggs will be low due to the likely later occurrence of spontaneous pregnancies. In contrast, cost-effectiveness may increase with advancing female age, likely reaching a peak around ages 37-38 years old. The mean number of eggs obtained per retrieval under age 30 is comparable but starts significantly declining after 35 years. Moreover, in non-infertile individuals, fertilization and pregnancy rates obtained from the eggs of women in their late twenties or early thirties are comparable to those obtained in early twenties, and better than from eggs of younger girls below ages 22-23.
The best timing for egg freezing in cases of normal OR is, therefore, between midto late-20s until mid-30s. Considerations, however, change dramatically if women at younger ages already demonstrate evidence of low OR, a diagnosis called premature ovarian aging (POA). Once a diagnosis of POA is reached in a young woman, the recommendation is straightforward: get pregnant soon or start immediately to freeze your eggs.
Several reasons support such a recommendation: (i) Medicine has so far not yet learned to arrest POA and POA is, of course, a progressive condition. (ii) Even
though POA in a large majority of cases only progresses slowly, in a small subgroup of women deterioration can occur very quickly and to date we have not learned to predict who falls into this small subgroup. Consequently, all POA patients need to be prudently approached assuming a worstcase scenario.
Like in an in vitro fertilization (IVF) cycle, egg freezing requires that a woman goes through ovarian stimulation with injectable fertility drugs called gonadotropins. But there should not be a uniform protocol for every patient (as is unfortunately often the case in clinics where egg-freezing is the most often performed indication for an IVF cycle). Instead, understanding egg-freezing as the complex medical procedure it really is, every patient should beforehand have a highly individualized assessment and, accordingly, a personalized and tailored ovarian stimulation plan. And as with all IVF cycles to achieve pregnancy, this requires close monitoring of the patient over an average of 10-14 days, during which patients should be seen frequently in the treating clinic, not only by support staff, but by a responsible physician who is experienced in assessing hormone levels and pelvic ultrasounds, both essential components of monitoring a cycle’s progress.

Operative Setting for embryo transfer at the CHR
Ovarian stimulation occurs through the above-noted daily self-injection of subcutaneous gonadotropins (FSH, LH, or both in combination). During a natural menstrual cycle, these hormones are secreted by the pituitary gland at only low amounts and produce only 1 or 2 growing follicles (the small cystic spaces in which eggs live, Figure 1); but under external and much higher-dosed amounts of hormone, ovaries, especially in younger women, will produce much larger numbers of follicles and eggs. Though not every follicle yields an egg, some younger women – and even older women with PCOS – can easily produce over 20 eggs in such a cycle. Since the goal of egg-freezing is to get a certain minimum number of eggs, there always exists the risk that ovaries are hyper-stimulated. In most extreme cases that can lead to the so-called ovarian hyperstimulation syndrome (OHSS), a potentially dangerous complication of ovarian stimulation which, at times, can even be life-threatening. Fortunately, OHSS in experienced hands has by now become an extremely rare event.
At the end of ovarian stimulation, when leading follicles reach the appropriate diameter, a “trigger” injection for final follicular maturation is administered, and eggs are collected 34-36 hours later.
With the patient asleep, eggs are aspirated with a long needle under ultrasound control. Using this technology, the operating physician (and at the CHR every egg retrieval is performed by a physician) can carefully lead the needle tip, which is clearly visible on ultrasound, into every single follicle.
Women almost universally tolerate (careful) ovarian stimulation and egg retrievals well, with the latter done under conscious sedation of the patient and, at the CHR, given by an anesthesiologist. The process may cause mild bloating and abdominal cramping, but most women do not report additional symptoms. The length of the procedure depends, of course, on the number of follicles, but rarely lasts more than 15 minutes, and patients are discharged in about one hour.
SIDE NOTE: The first vaginal egg retrieval in the world for IVF was performed by CHR physicians at CHR-Chicago in 1983 and was reported in the same medical journal in the same letter-to-the-editor format [Gleicher et al., Egg retrieval for in vitro fertilization by sonographically controlled vaginal culdocentesis. Lancet 1983;2(8348):508-509] as the first birth of an IVF baby had been reported five years earlier [Steptoe and Edwards. Lancet 1978;2(8085):366]
Since not all retrieved oocytes are mature and will successfully fertilize, develop into viable embryos, implant in the uterus, and result in a pregnancy and, ultimately, a live birth, how many oocytes a woman who decides to pursue egg-freezing should cryopreserve is likely the most important— but also the most difficult—question to answer. And yet, it is the one question that should be answered at the very beginning of the decision-making process because it ultimately determines how many retrieval cycles will be needed and at what cost. 4
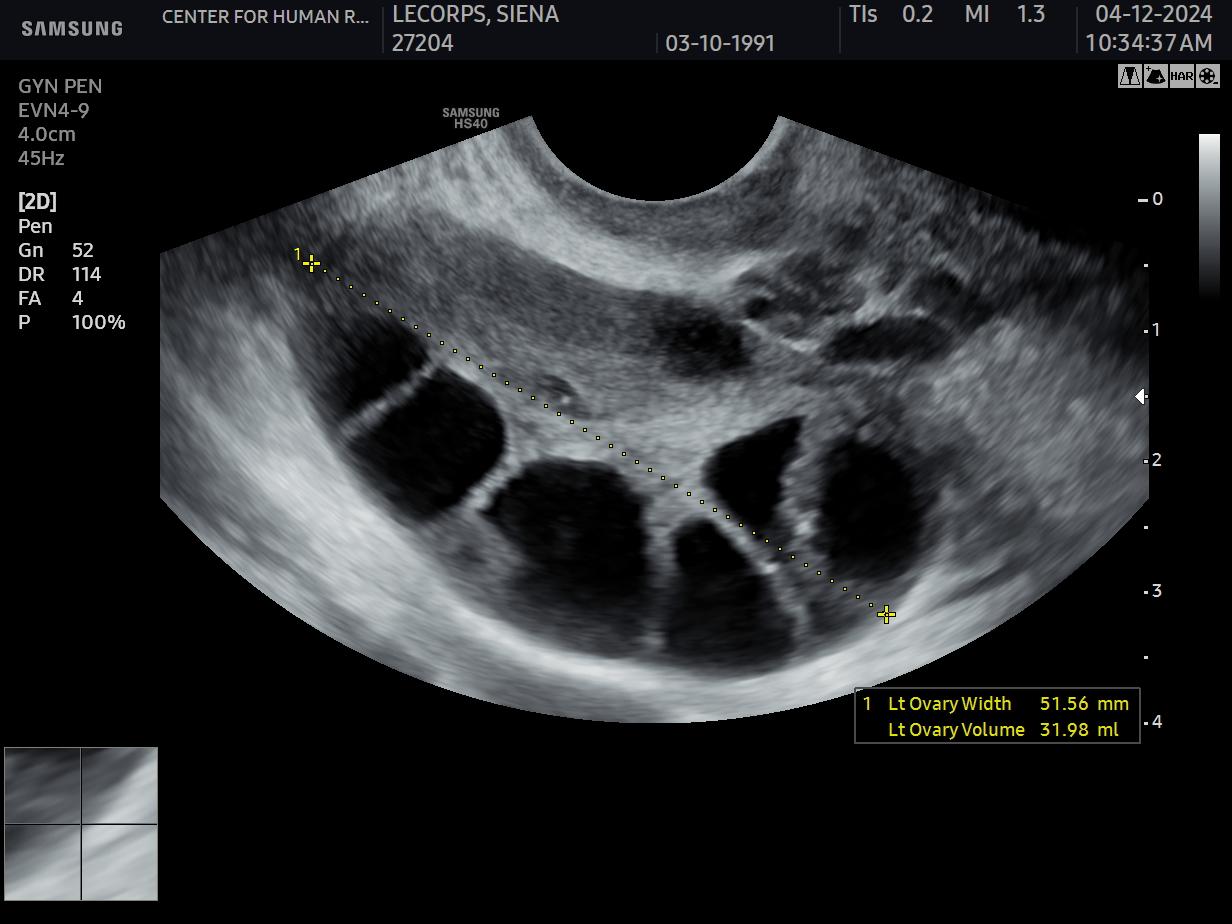
The answer is so difficult because it depends on so many varying factors: The female’s age and her OR are obviously the two most important ones; but one should not forget to find out how many children the patient may, ultimately, want to conceive with help of her frozen eggs. To make an educated recommendation to a given patient, therefore, is always only an educated guess because what cannot be predicted with certainty is how well (or poorly) the frozen eggs will thaw out since this depends to a large degree on the inherent quality of those oocytes which at the time of freezing is not always obvious.
Moreover, in a circular pattern, egg quality declines with advancing female age but also with declining OR, even if such declines happen prematurely in younger women. Consequently, in the CHR’s opinion, superficial patient evaluations often made when giving patients recommendations – because younger women are (falsely) automatically assumed to have good quality eggs – are insufficient. Hence, the CHR investigates young women in the same detail as infertility patients before reaching judgments and making recommendations.
Assuming no unusual findings in a patient, the literature suggests that, up to age 35, at least 8-12 mature oocytes should be cryopreserved (note the wide range!) per desired child, with likelihood of success, of course, increasing with more stored eggs. In other words, to determine how many eggs a woman should freeze per desired child also greatly depends on the coveted level of certainty for the prediction given: 8-12 mature oocytes, therefore, likely offer an approximately 75-80% probability, which still means that 20-25% of women with that (wide) range of egg numbers will not have enough eggs frozen to achieve even only one live birth.
And, as already noted above, achieving pregnancy cannot be equated with achiev-
ing healthy live birth, as pregnancies can be lost for a variety of reasons which, again, are highly patient-dependent. Miscarriages and practically all other pregnancy complications increase with age, and so do all potential medical problems a patient may have that may affect pregnancy, again pointing out the importance of a proper and detailed medical evaluation of every woman planning on freezing eggs.
Once an estimate has been made of how many eggs a woman should freeze to meet her expectations, the next big question arises: how many stimulation cycles will the patient require to achieve this goal? And the answer, once again especially in first cycles, can only be crude estimate. Therefore, unfortunately, insufficiently informed women often start their egg-freezing experience believing that all they will need is one cycle of oocyte preservation. When after that cycle they learn that not enough eggs were retrieved and they need one or more additional cycles, they often are surprised and unable to afford them. The consequence is finding themselves in limbo, with only an insufficient number of frozen eggs leaving them far from meeting their desired probability of success from the egg-freezing process they so enthusiastically had pursued in the unrealistic expectation of needing only one retrieval cycle.
Does egg-freezing adversely affect fertility? A question frequently asked is whether egg-freezing reduces a woman’s remaining egg pool (her OR). And the answer is a categorical no! The reason is simple: no additional eggs are lost that the patient would not have lost in this month anyhow. What so-called fertility drugs do is not recruit more eggs; the egg recruitment process is naturally going on uninterrupted all the time. The drugs prevent some of those steadily recruited eggs from degenerating. In other words, the eggs obtained in an egg-freezing cycle (or in a regular IVF cycle) are saved from otherwise being lost.
Consequently, there is no impact on the remaining OR, nor is the timing of menopause affected.
What happens after the retrieval cycle is over? You will very likely resume your usual menstrual cycle pattern within 1-2 months. Your frozen eggs will remain stored until you wish to use them. If you freeze your eggs at the CHR, they remain in the CHR’s highly secured egg-freezing laboratory. At many clinics, the eggs are shipped out into long-term banking facilities. The CHR does not do that and, therefore, you have access to your eggs on short notice at any time. Every quarter, the CHR automatically bills a nominal storage fee for your frozen eggs.
What happens if the frozen eggs are used or never needed? Whenever you decide to use your frozen eggs, storage costs end, and you can start an IVF cycle very quickly. Storage costs also end if you decide to move your eggs to another clinic, since they can be shipped out instantly. Storage also automatically ends if you decide to donate your eggs anonymously or openly (per your decision) to an infertile woman under treatment at the CHR or if you decide to donate your unused eggs to the CHR’s research program (often conducted in collaboration with colleagues at other institutions). While donations for academic purposes seems to have become the most popular option in recent years, most data suggest that disposition decisions often evolve throughout a woman’s fertility journey, highlighting the importance of ongoing contact between clinic and patient, as intentions and plans can change over time.
Please address your feedback, questions, and comments to this article to the author at social@thechr.com.
Reading list:
Caughey LE, Lensen S, White KM, Peate M. Disposition intentions of elective egg freezers toward their surplus frozen oocytes: a systematic review and meta-analysis. Fertil Steril. 2021;116(6):1601-1619.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of Maternal Age on Oocyte and Embryo Competence. Front Endocrinol (Lausanne). 2018;9:327.
Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105(3):755-764.e8.
Guzman L, Inoue N, Núñez D, Meza J, Bendezu P, Pino P, Portella J, Noriega-Portella L, Noriega-Hoces L. What advice should we give our patients to preserve their fertility and avoid needing oocyte donation in the future? - A Social Fertility Preservation program. JBRA Assist Reprod. 2019;23(2):106-111.
Mesen TB, Mersereau JE, Kane JB, Steiner AZ. Optimal timing for elective egg freezing. Fertil Steril. 2015;103(6):1551-6.e1-4.
Pennings G. Elective egg freezing and women’s emancipation. Reprod Biomed Online. 2021;42(6):1053-1055.
Solé M, Santaló J, Boada M, Clua E, Rodríguez I, Martínez F, Coroleu B, Barri PN, Veiga A. How does vitrification affect oocyte viability in oocyte donation cycles? A prospective study to compare outcomes achieved with fresh versus vitrified sibling oocytes. Hum Reprod. 2013;28(8):2087-92.
Wu YG, Barad DH, Kushnir VA, Wang Q, Zhang L, Darmon SK, Albertini DF, Gleicher N. With low ovarian reserve, Highly Individualized Egg Retrieval (HIER) improves IVF results by avoiding premature luteinization. J Ovarian Res. 2018;11(1):23.
ADVERTISEMENT

David Barad, MD, MS is a Senior Scientist and Head of Clinical IVF and Research at the CHR

The figure demonstrates between +...+ a nuchal translucency measurement
BRIEFING: This month’s reviewed test is nuchal translucency testing by ultrasound in early pregnancy. It is used as a screening test for certain chromosomal and morphological abnormalities a pregnancy can suffer from and demonstrates well the difference between a screening and diagnostic test.
Introduction
Nuchal translucency (NT) testing is an early ultrasound test that assesses the risk of chromosomal abnormalities in a pregnancy, mostly Down’s syndrome (also called trisomy 21). NT refers to a physiological accumulation of fluid at the back of a fetus’s neck, detectable in all fetuses during the first trimester of pregnancy (see the figure above). An increased NT measurement can indicate various fetal pathological conditions, including congenital cardiac anomalies, chromosomal abnormalities, and genetic disorders.
NT testing was first introduced in the early 1990s. The development of this screening method is credited to Professor Kypros Nicolaides and his team at King’s College Hospital in London, who published pioneering research on the correlation between increased nuchal translucency and chromosomal abnormalities, particularly Down’s syndrome. This research laid the foundation for the widespread use of NT testing in prenatal screening.
The test is conducted when the fetus is between 11 and 14 weeks old, when the translucency measurements are most accurate. If an abdominal ultrasound is used, the patient may need to have a full bladder, which is not required with a vaginal ultrasound.
To correctly perform the test, the ultrasound image must be a mid-sagittal view. This means the ultrasound image should be a clear, longitudinal view of the fetus, showing a profile that includes the neck, nasal bone and the palate. The fetus’s head should be in a neutral position, not tilted too far back (hyperextended) or too far forward (flexed). Moreover, the head should be in line with the spine.
The sonographer then measures the thickness of the nuchal translucency. This measurement is done by placing calipers on the ultrasound image to measure the maximum thickness of the translucent area. At 11 weeks, a normal NT measurement should not exceed 2 mm. At 13 weeks and 6 days, a normal measurement is up to 2.8 mm, though some practices consider normal to be up to 3.5 mm.
NT testing is a screening test, not a diagnostic test. This is a crucially important distinction, often not properly explained to patients. It helps in estimating the likelihood that the fetus may have a chromosomal abnormality but does not provide a definitive diagnosis. As a screening test, unlike some diagnostic procedures, NT testing is non-invasive and poses no risk to the fetus. If the NT test indicates an increased risk of a chromosomal abnormality, further diagnostic testing, such as amniocentesis or chorionic villus sampling (CVS), analyzing fetal chromosomes directly, may be recommended for confirmation or refutation of the NT-diagnosis.
Though originally described as a screen for chromosomal aneuploidies (i.e., Down’s syndrome), it was later discovered that NT testing can also detect congenital cardiac anomalies due to genetic disorders not associated with chromosomal aneuploidies.
This means that NT testing can be positive even in the presence of a normal chromosome analysis (karyotype). The incidence of both fetal structural abnormalities and chromosomal disorders, however, rises significantly with increasing nuchal translucency (NT) measurements, ranging from 30% for NT measurements between 3.5 and 4.5 mm to approximately 70% for NT measurements greater than 6.5 mm.
Karyotype analysis of amniotic fluid (amniocentesis) or of placental tissue (CVS) is then typically the initial step in the further evaluation of fetuses with increased NT, diagnosing chromosomal abnormalities in 30%–40% of cases. When the karyotype is normal, array Comparative Genomic Hybridization (array-CGH) can identify submicroscopic imbalances, such as microdeletions and microduplications, offering an additional diagnostic level with an additional yield of approximately 5%. Non-chromosomal genetic mutations such as Noonan’s syndrome can also demonstrate increased nuchal translucency.
One recent study of 114 pregnancies with increased nuchal translucency and normal karyotype as well as normal array-CGH testing found that there was still a 33% risk of serious fetal morbidity even though the karyotype was normal. Further testing for specific genetic syndromes (like Noonan’s syndrome) and a normal mid-trimester anomaly scan reduced the risk among the remaining cases to 9.4%.1
Since NT is a screening test, it should not be used as sole indication to terminate a pregnancy. Elevated NT measurements should be followed up with genetic testing to rule out aneuploidy and other genetic causes. The risk of major cardiac anomalies increases with the size of the NT, with over 50% of fetuses with an NT measurement greater than 6 mm having cardiac anomalies. Many pregnancies with positive NT tests will, however, prove to be normal, though to be safe extensive further testing is required.
A current CHR patient, for example, in a prior pregnancy was transferred with a by PGT-A testing chromosomal-normal embryo[H1] , conceived and on ultrasound was found to be NT positive. The patient underwent further testing with micro-array CGH and second trimester anatomy screening, and, in the end, it was a perfectly normal female pregnancy. She recently returned to the CHR for a second pregnancy attempt and, after conceiving, once again turned out to be NT testing positive. Her follow-up testing at time of this writing is still pending.
Another patient we recently saw at the CHR was found to have an NT of over 9 mm. Her obstetrician unfortunately had advised her to have an immediate D&C without further testing of the pregnancy. Chromosomal testing of the fetal tissue after pregnancy termination, however, revealed a normal karyotype. Chromosomal testing of products of conception cannot rule out that this pregnancy had a cardiac (or other) anomaly. It, however, is also possible that in this case a normal
pregnancy was mistakenly terminated.
Any abnormality suggested by NT screening, therefore, should be confirmed by more definitive diagnostic testing before final decisions are made about pregnancy termination.
Reference 1. Spataro et al., Int J Gynaecol Obstet 2023, 161 (3), 1040-1045).
ADVERTISEMENT

Norbert Gleicher, MD is Medical Director and Chief Scientist at the CHR
BRIEFING: There are over 80 diseases which are already fully recognized as socalled autoimmune diseases (AIDs) and/ or inflammatory diseases (IDs) and, undoubtedly, there are many more waiting to be discovered as such. One characteristic they all have in common is their high degree of familial occurrence. Most diseases are, indeed, familial, but the degree of risk one inherits (the “penetrance”) if another member of the family is afflicted can greatly vary, and no group of diseases except so-called single gene diseases or so-called sex-linked disease, where the risk is either 25% (recessive) or 50% (dominant and/or sex-linked), has greater penetrance than autoimmunity. And autoimmunity as well as inflammation have considerable effects on especially female fertility as well as conception, and significantly increase risks for pregnancy. For young women in general and especially for young women planning on delaying pregnancy and considering fertility preservation through egg-freezing, knowing whether they are at risk for autoimmunity and/ or inflammation, therefore, is of crucial importance, and this article will explain why that is, how you can find out, and what can be done in protection of reproductive health.
Our immune system has one principal function, which is protection from invaders. In other words, it is our border police and military, all at once. If it malfunctions, bacteria, viruses, parasites, and other foreign agents are free to enter our bodies without meeting resistance. This is, for example, the reason why older people–who have weaker immune systems than younger individuals–are at greater risk for viral infections like COVID or why individuals born with complete immune deficiency used to have to live in complete isolation in “bubbles” (who remembers the movie about David Vetter, nicknamed ‘Bubble
Boy,’ born in 1971 with combined immunodeficiency – basically complete absence of an immune system – who until his death at age 12 lived in a sterile plastic bubble?).
To function properly our immune systems also have to be able to “recognize” when something is “foreign” because, short of this ability, the immune system could and would start attacking normal components of our own bodies. Our immune system, therefore, has an innate ability to distinguish “self” from “foreign.” When this ability malfunctions and the immune system by mistake starts attacking components of our own body, the affected individual demonstrates autoimmunity. And when this malfunction of the immune system leads to clinical symptoms, the individual has an autoimmune disease (AID). The distinction between demonstrating autoimmunity and having an AID is important because individuals with only autoimmunity will mostly be asymptomatic and, therefore, will likely be unaware of their autoimmunity. Adverse effects of autoimmunity on reproduction can, however, already be present at those early stages. In regard to infertility this means that many cases of socalled “unexplained” infertility in reality are cases of autoimmunity.1 This is an important reason why the CHR does not believe in the diagnosis of “unexplained infertility,” which textbooks and many even relatively recent publications still claim to represent at least 15% of all infertility cases.2
Over 80 AIDs have so far been described in the literature and experts agree that many more likely exist, still unrecognized in their autoimmune etiology. Table 1 presents the most frequent ones. Roughly one in fifteen people in the U.S. is affected by an AID, women much more frequently than men, though men usually have more severe disease.
Table 1. The most frequent autoimmune/ inflammatory* diseases
· Rheumatoid arthritis (RA)
· Systemic lupus erythematosus (SLE)
· Autoimmune thyroid disease
Hashimoto’s thyroiditis – Hypothyroidism
Grave’s disease -Hyperthyroidism
· Inflammatory bowel disease (IBD)*
Ulcerative colitis
Crohn’s disease
· Multiple sclerosis (MS)
· Type 1 diabetes
· Guillain-Barre syndrome (GBS)
Chronic inflammatory demyelinating polyneuropathy (CIDP)
· Psoriasis and psoriatic arthritis
· Sjögren’s syndrome
· Celiac disease
· Autoimmune adrenal disease
Addison’s disease – adrenal insufficiency
Cushing’s syndrome – hyperadrenalism
· Dermatomyositis
· Myasthenia gravis
· Pernicious anemia
· Vasculitis etc.
Like most diseases, AIDs are so-called polygenic diseases. In contrast to monogenic diseases, polygenic diseases are not inherited through only one single chromosomal mutation in any one of 46 chromosomes, but by an inheritance pattern that is dependent on the presence of multiple mutations in multiple chromosomes. This kind of polygenic inheritance pattern does not result in exactly predictable inheritance risks (of 25% or 50%), but basically means only the following: (i) The risk of getting the disease is bigger than it would be if no close relative had the disease (how much bigger varies between diseases but is much lower than the 25% in a recessive single-gene inheritance) and is, therefore, also called a “familial”-inheritance pattern; (ii) In cases of autoimmunity where the risk involves many different autoimmune diseases, it also means that the individual at risk, after expressing one AID, will also be at increased risk to develop other AIDs. More-
over, the number of affected close relatives matters since the risk increases with more affected close relatives. A currently widely accepted hypothesis regarding autoimmune diseases suggests that the predisposition toward autoimmunity is inherited in above-described polygenic fashion; but by which individual autoimmune disease(s) an individual may express during her/his lifetime, is then determined by environmental factors she/he was exposed to in utero or after birth.
Autoimmunity affects the reproductive success of women in several quite distinct ways,3,4 from being closely associated with premature ovarian aging (POA) in young women during their reproductive years and, therefore, with female infertility, to–likely–the implantation process, almost universally an increased miscarriage risk, and increased risk for several pregnancy complications, with premature labor and premature delivery almost representing hallmarks of all AIDs,5 and an increased preeclampsia risk also being highly significant.3,4
But AIDs demonstrate yet another very typical general characteristic of medical problems in pregnancy, namely that medical diseases not only affect pregnancy, but that
pregnancy–in reverse–also often affects medical diseases. And, once again, AIDs demonstrate this fact probably better than most other diseases because–as yet another almost universal characteristic–they tend to flare peripartum and especially post-partum.6 Peri- and post-partum clinical presentations, from preeclampsia, over premature labor, and peri-as well as post-partum disease flares are, likely, all the results of caseation of immunological tolerance of the fetal-placentals semi-allograft by the maternal immune system and, probably, the best evidence how closely tolerance pathways of “self” and of pregnancy are interwoven.7
But among all of these associations, what relates to the topic of this communication is only the long-known association of autoimmunity/inflammation with POA, including its end stage, primary ovarian insufficiency (POI).8 Abnormally low age-specific ovarian reserve has been reported with many–if not most–autoimmune diseases (Table 2).
And here is why all of this is important information for young women: Approximately 10% of all women–independent of race and ethnic background–end up prematurely aging their ovaries (i.e., will be suffering from POA).
Approximately 10% of these women (1% of all women) will experience early menopause (i.e., POI), which is the end stage of POA.
Unfortunately, POA is usually an asymptomatic disease. In other words, so-affected young women in most cases have no idea that their ovaries are aging prematurely, until they discover that they no longer can conceive. By that point their functional ovarian reserve (i.e., the number of remaining eggs in their ovaries) has already fallen below a certain minimal threshold, thereby preventing them from conceiving. Consequently, they will require fertility treatments, in most cases in vitro fertilization (IVF). And if they waited too long, IVF may also no longer be an option and what is then left is only third-party egg donation.
And these are exactly the situations which, through this article, we are trying to help young women avoid: it all starts with being conscious as a young woman about one’s risk status for developing POA. In other words, just like young women have become increasingly conscious about the possibility of freezing their eggs, so they should–at even younger ages–become conscious about the importance of finding out if they are among those 10% of all women who are at increased risk for developing POA. And since autoimmunity is highly associated with
Table 2. Abnormally low age-specific functional ovarian reserve reported with autoimmune diseases*
• Autoimmune thyroid disease
Hashimoto’s thyroiditis
Subclinical hypothyroidism
• Chronic inflammatory rheumatic diseases
Rheumatoid arthritis (RA)
Behçet’s diseases
Spondyloarthritis
Systemic lupus erythematosus (SLE)
Juvenile SLE
Dermatomyositis
Primary antiphospholipid syndrome
Sjögren’s syndrome
• Neurological AIDs
Multiple sclerosis
Neuromyelitis optica spectrum disorder
• Other endocrine AIDs
Addison’s disease
Type I diabetes
Mouse model
Most common AID associated with POI
No difference in AMH but difference in AFCs
Can be associated with autoimmune oophoritis
REFERENCES 9 10, 11, 12, 13 14, 15 16, 17, 18 16 16 19, 20, 21 22 23 24 25, 26 27, 28, 29 30 31 32, 34 34
AFC, antral follicle count; AIDs, autoimmune diseases. *Diseases in the literature described as primarily “inflammatory,” such as inflammatory bowel disease (ulcerative colitis and Crohn’s disease) and endometriosis share many features with AIDs. For example, practically every AID is also an “inflammatory” condition, and patients with inflammatory bowel disease and endometriosis demonstrate typical features of AIDs, such as production of pathological autoantibodies. Moreover, like AIDs, inflammatory bowel disease35 and endometriosis36 are associated with increased risk for premature deliveries.
POA, knowing about one’s family history of autoimmunity is of great importance.
In a moment we will discuss how a risk assessment for POA can be done at young ages; but before we do so, it is also important to note that finding out whether one is at risk for POA is also extremely relevant for egg-freezing because those at risk are well-advised to freeze their eggs as early as possible. The later in life eggs are frozen, the fewer eggs will be produced in every retrieval cycle and the more cycles of egg-freezing will be necessary (i.e., costs will go up significantly). Moreover, the poorer the quality of obtained eggs will be and egg quality, of course, translates into embryo quality and pregnancy chances once eggs are thawed out.
One more very important point: Beyond egg-freezing women considered at risk for POA also usually still have an alternative option to egg-freezing. They can simply decide to have children earlier than otherwise was planned, and that may even still be possible without medical help. What makes this such an important discussion, however, is the fact that it offers young women maximal control over their reproductive future. When to start their families remains their decision and is no longer dependent on their genetically inherited baggage for POA.
“What’s my fertility?” is a very simple program for which the CHR was awarded a U.S. patent roughly 10 years ago, which allows for the prediction of whether a young woman is likely (a) not at risk for POA; (b) at risk for POA; or (c) already has evidence of POA.37 It is based on a handful of short questions and blood tests. One of the very important questions is whether the young woman has relatives with autoimmune diseases. If the answer is affirmative, it automatically puts her into the “at-risk” group. That’s it; it’s that simple!

ADVERTISEMENT
Individuals who are judged not to be at risk don’t have to return for 3-5 years for a
repeat evaluation. Individuals judged at risk enter, depending on their risk assessment, into a 6- or 12-month retesting program with an appropriately timed recommendation for egg-freezing. Women already found to show evidence of POA are advised to attempt conception right away or start egg-freezing instantly. If their functional ovarian reserve is already significantly diminished, they are immediately started in preparations for IVF.
The CHR’s approach to IVF in POA patients significantly differs from what is offered by most other IVF clinics. This is not the moment to summarize all the important differences; therefore, only so much: As noted earlier, autoimmunity is statistically highly associated with POA, and autoimmunity, like POA, can be asymptomatic. Therefore, risk-screening for POA also involves some immune testing. Such testing is not only important for POA screening; if a woman is found to have evidence of subclinical autoimmunity (i.e., immune abnormalities in her blood but without any clinical symptoms of an AID), this denotes that–without proper treatment–she likely would be at increased risk to miscarry once she conceives. And of course, the last thing we would want to happen after conception is a miscarriage that is preventable with proper treatment.
It should now be crystal-clear why the CHR during every initial consultation with new patients places so much emphasis on obtaining very detailed family histories. And it, moreover, it should also be very obvious why knowing one’s own immune system is so important for young women in general, but especially if considering delaying pregnancy and planning on freezing one’s eggs.
REFERENCES
1. Gleicher N, Barad D. Hum Reprod 2006;21(8):19511955
2. Carson SA, Kallen AN. JAMA 2021;326(1)65-76
3. Gleicher N. Acta Haematol 1986;76(2):68-77
4. Gleicher et al., J Autoimmun 2012;38(2-3):J74-J80
5. Gleicher et al., Clin Rev Allergy Immunol 2010;39(30):194-206
6. Gleicher N. J Autoimmun 2014;50:83-86
7. Gleicher et al., J Assist Reprod Genet 2017;34(4):425430
8. Kunicki et al., J Reprod Immunol 2024;164:104253
9. Ayesha et al., J Clin Diagn Res 2016:10(10):QC10QC12
10. Li et al., Int J Immunopharm 2022;108:108670
11. Safarian et al., Int J Med Sci 2023;24(50:4705
12. Hsieh Y-T, Ho JYP. Hum Reprod 2021;36(6):16211629
13. Chen et al., Thyroid 2017;27(9):1194-1200
14. Rao et al., Thyroid 2020;30(1):95-105
15. Li et al., Thyroid 2022;32(7):841-848
16. Henes et al., Rheumatology (Ox ford):2015;54(9):1709-1712
17. Valdeyron, et al., Rheumatology (Oxford) 2021;60(4):1863-1870
18. Zhang et al., Int Arch Allergy Immunol 2022;183(4):462-469
19. Luo et al., Ann Paliat Med 2020;9(2):207-215
20. Angley et al., Lupus Sci med 2020;7(1):e000439
21. Lourenç0 et al., Clin Rheumatol 2021;40(9):36513658
22. Medeiros et al., Lupus 2009; 18(1):38-43
23. De Souza et al., Clin Exp Rheumatol 2015;33(1):4449
24. Yamakami, et al., Lupus 2014;23(9):862-867
25. Karakus, et al., J Obstet Gynaecol Res 2017;43(2):303-307
26. Mao et al., Reprod Biol Endocrinol 2024;22(1):57
27. Thöne et al., Mult Scler 2015;21(1):41-47
28. Carbone et al., Int J Gynaecol Obstet 2013;163(1):1122
29. Pelayo et al., Mult Scler Relat Disord 2023;79:105012
30. Thöne et al., Front Neurol 2018;9:446
31. De Bellis et al., Eur J Endocrinol 2017;177(4):329-337
32. Filardi et al., J Biol Regs Homeostat agents 2020;34(5):1959-1962
33. Yang et al., Endocrine 2022;77(2):205-212
34. Li et al., Mol Cell Endocrinol 2020;500:110627
35. Tondreau et al., Arch Gynecol Obstet 2024; doi: 10.1007/s00404-024-07521-2. Online ahead of print.
36. Breintroft et al., Acta Obstet Gynecol Scand 2022;101:417-423
37. https://www.facebook.com/whatsmyfertility/
ADVERTISEMENT

This month’s images portray a circumstance rather rarely addressed by either researchers or clinicians: while pretty-much everybody understands that in ovaries eggs develop within follicles, far less appreciated is the fact that muscular forces participate in follicular growth and egg maturation. This subject, nevertheless, has recently been attracting increasing attention, as we learn to confront the many factors underwriting why– especially as a function of a woman’s age–human eggs are variable in their quality. What investigators at the CHR have been able to visualize, indeed, offers interesting new insights:


Image 1 shows the appearance of a young ovary labeled to show in white the distribution of muscle cells. Note the follicle in the center of the field and the pronounced bands of muscle tissue creating a swirling like configuration that encloses smaller follicles (circles) near the bottom of the image. If we manipulate this image to emphasize only those cells resembling muscle, you see what is displayed in Image 2. The red fibers coursing around the follicles and through the ovarian tissue emphasize what we now believe reflects active force generation that likely is highly regulated as follicles are selected and prepared for ovulation during natural or stimulated cycles.
Image 3 is a highly magnified version of an ovarian follicle that in this case has been labeled to demonstrate muscle cells shown in green. Notice the muscular envelope surrounding not only the follicle itself but also the egg and demonstrating many finger-like extensions known as transzonal projections, a topic we will visit in greater detail in the next issue of the VOICE


BRIEFING: The VOICE in this section offers opinions about news which in preceding months attracted our attention and which we believe to be relevant to the practice of medicine. As these are opinions–in medicine widely recognized as the lowest level of evidence–we fully recognize their limitations due to inherent and unpreventable biases of our writers and their respective privileges (or lack thereof), whatever those may or may not be. Unable to offer consistent “truth” (assuming that something like that really exists), the VOICE, however, strongly believes that coming at least as close as possible to the most likely “truth of the moment” mandates openness to a wide offering of opinions. We therefore welcome submissions from our readers that address opinions voiced in this newsletter even, or especially, if they contradict our own. They should be submitted with the subject line “Opinion” to social@thechr.com.
From antisemitism to failing school systems, a severely biased media landscape, and unprecedented levels of government corruption, now also increasingly contaminating medicine
One cannot help but wonder how historians will look back on the current period in time after big parts of this country’s political and media elites in close cooperation with the federal government for years outright lied to the nation’s citizens about the increasingly obvious cognitive and physical impairment of the nation’s president. Once his mental and physical deterioration, however, could no longer be hidden from the public, his own party eliminated him from renomination for a second term which he by that point already had secured based on a never-before-seen cover-up, also involving physicians. Considering the unprecedented reach of this unprecedented conspiracy, historians will undoubtedly have to conclude that the nation in those years (and this means in our present existence) faced unprecedented perils.
But unprecedented perils for this country do not only exist domestically. Undoubtedly, historians will also consider the period as the most dangerous for the world since the end of World War II; talk about the threat of WW3, indeed, has become an almost daily event in the media, with two large active wars in Europe (Ukraine and Russia) and the Middle East (Israel and
Iran through its proxies) not being the only potential sources for a worldwide conflict.
What may be even more complex to decipher for historians about this moment in history than even the above-noted government conspiracy, may be the world’s response to the surprise Hamas attack on October 7, 2023, on Israel’s south leading to the slaughter of over 1,300 children, women, and men and the abduction of over 230 hostages, including babies, toddlers, and older children, women, and men of all ages. And, as if that was not enough, many of those murdered–females and males–were sexually assaulted and raped either before or after being killed in the most gruesome ways,1 often recorded by perpetrators themselves and sent out into the world to demonstrate their “heroism” to their communities in Gaza.
Much of the world acknowledged at least some of these atrocities for somewhere between five and ten minutes, before starting to blame the victims–in this case the state of Israel–for acting inhumanely by striking back at where all of this evil originated. And as if all of this evil wasn’t enough, Israel was attacked daily with literally thousands of missiles, almost exclusively directed at the country’s civilian population. And these missiles came not only from Gaza but, at the other, northern end of the country, from a second Iranian proxy, Hezbollah.
One can only imagine how the U.S. would have reacted to such a
multi-faceted assault, or Brazil, or Turkey, or Russia, or China, or for that matter, any other country in the world? One just must look at what Turkey has been doing for decades to its Kurdish minority. The U.S. invaded and occupied Afghanistan after 9/11 to go after al-Qaeda which had killed approximately 3000 U.S. citizens (ca. 0.00009091% of the population), and without hostages being taken, missiles being directed at civilians in U.S. cities, or children, women, and men being raped, children murdered in front of their parents and parents murdered in front of their children. For Israel, just the loss of 1,300 murdered citizens represented a loss of 0.01625% of her population and, based on the comparison in both nations’ populations would in the U.S. represented 232,373 victims of 9/11. The difference should be only too obvious!
And not only have countries and worldwide organizations like the United Nations, the World Health Organization, The International Red Cross, etc., not spoken out about the atrocities committed by Hamas in timely fashion–or at all–but neither have professional organizations (in our medical specialty, for example, ACOG and ASRM) and/or local or international women’s organizations. But who has spoken out in timely fashion loudly and clearly has been in the U.S and elsewhere the radical street, though not in defense of Israel but in support of Hamas and Hezbollah, both by most countries formally designated to be terrorist organizations.

How that could be, will leave historians with several challenges as to how this response of the street can be explained. That much of this response can be traced to the schooling of our youth in high schools, colleges, and universities appears obvious after witnessing administrators of some leading universities–as congressional hearings and the behavior of protestors in demonstrations on campuses and in the streets so well demonstrated–can now be unequivocally attributed to resurgence of antisemitism around the world unprecedented since World War II. Innumerable occurrences all over the world very clearly demonstrated that much of these (well-financed and organized) activities were not, as claimed by some anti-Israeli or anti-Zionist, but outright anti-Jewish and, therefore, antisemitic.
Though characterized by participation and leadership of disproportionate numbers of recent Muslim immigrants and/or their children, the movement also included large numbers of other participants, usually on the extreme political left and, disturbingly – many even of Jewish background. As antisemitism apparently unites, one also at times could notice in the crowd members of the extreme right. A socially very interesting observation, though almost unnoticed by the media, has also been a very significant preponderance of female over male participants, with females frequently also in leadership positions during demonstrations. One wonders how historians will explain this phenomenon.
What is happening at our universities is truly astonishing. Aside from widely publicized examples of antisemitism at such elite universities as Columbia in New York City (three senior academic administrators had to be dismissed because they were caught exchanging antisemitic comments via e-mail2) Harvard in Boston,3 and the University of Pennsylvania in Philadelphia4 (asked
their Presidents to resign) because they could not bring themselves in a Congressional hearing to consider antisemitism to be incompatible with university policies, and both of these schools (as well as many others) are under investigation by Congress and the Justice Department under federal discrimination statutes and/or have been sued by some of their Jewish students.
Similar biases (to use a polite term) permeate our media. When terrorists from Hezbollah killed 12 Israeli Druse children between ages 10 and 16 playing soccer on a Saturday evening and wounded many more, CNN and the BBC reported this gruesome event as “children in Israel died.” When they reported on children killed in Gaza because Hamas uses them as outlooks and/or shields, “they were killed.” And The New York Times didn’t find the news of this tragedy “fit to print” on the frontpage, publishing a brief article in the Sunday edition only on page A7.6
How bad have things become in medical schools? Apparently quite bad, as Jewish patients as well as staff, for example, publicly have been expressing concerns about antisemitism at UCSF Medical Center, one of the nation’s most prominent and highly ranked medical schools,7 and when 3,000 (mostly Jewish) academics found it necessary to publish a letter condemning academic boycotts of Israel and calling on university officials to protect academia from caprices of politics.8 And just on a side note, after withholding degrees from some of the most aggressive pro-Hamas protestors on campus, Harvard University, just a few weeks later, announced that most of them were “restored their good standing” and were granted their degrees after all.9 And, as the next short article demonstrates, things are not any better in medical publishing.
1. The Times of Israel. July 26, 2024. https://
www.timesofisrael.com/four-survivors-told-authorities-they-were-sexually-assaulted-on-october-7-report/
2. Egan M. CNN. July 8, 2024. https://www. cnn.com/2024/07/08/business/columbia-deans-antisemitic-tropes/index.html
3. Haidar EH, Kettles CE. The Harvard Crimson, January 2, 2024. https://www.thecrimson.com/article/2024/1/3/claudine-gay-resign-harvard/
4. Arkin D. NBC News. December 9, 2023. https://www.nbcnews.com/news/us-news/ university-pennsylvania-president-steps-criticism-antisemitism-testimo-rcna128712
5. World Israel news. July 28, 2024. https:// worldisraelnews.com/cnn-bbc-headlinesclaim-druze-in-isra...tm_term=CNN_2C+bbC+Won_27t+Say+Israeli+Children+_27Were+Killed_27
6. Rasgon A, Boxerman A. The New York Times. July 28. pA7. https://www.nytimes. com/2024/07/27/world/middleeast/israel-golan-heights-lebanon-rocket.html
7. Or-El A. The Jewish Journal. July 16, 2024. https://jewishjournal.com/community/373172/jewish-patients-and-staff-expressconcerns-over-antisemitism-at-ucsf-medical-center/
8. Pierre DJ. The Algemeiner. July 24, 2024. https://www.algemeiner. com/2024/07/24/3000-academics-denounce-boycotts-israel-new-letter/
9. Idem. The Algemeiner. July 25, 2024. https:// www.algemeiner.com/2024/07/25/harvarduniversity-grants-degrees-pro-hamas-protesters-says-students-restored-good-standing/
Richard C. Horton and The Lancet apparently just don’t like Jews!
So here we go again, and it’s the same old story: The Lancet, and especially its longstanding editor-in-chief, Richard Charles Horton OBE,FRCPCH, FMedSci, don’t really appear to like Jews. That became rather obvious apparently [H1] already years ago–shortly after Horton assumed his position at The Lancet–when he basically offered free access to the pages of the medical journal to a cohort of prominent British and Arab voices under the leadership of Sir Iain Geoffrey Chalmers, FRCOG, FRCPE, FMedSci, a pioneering health services researcher (amongst other areas in Perinatology), and a founder of the Cochrane Collaborations, who never made any secret of his animus toward Israel and his personal close association with Pales-

tinian positions, in those days mostly represented by Arafat’s PLO. Before becoming a renowned health services researcher and promoter of evidence-based medicine, he practiced as a clinician in the UK and, of all places, in the Gaza Strip (!).1

Like the worldwide post-October 7 discussion, this group’s opinions often veered beyond just criticism of Israel into what was widely perceived as straight, good-old-fashioned antisemitism (which Chalmers and colleagues, of course strongly denied). Their opinion pieces, however, attracted enough criticism of Horton’s leadership of The Lancet (especially after some opinion pieces he wrote himself that were perceived as antisemitic), to allow rumors to circulate that his days as editor-in chief of The Lancet were coming to an end.
Paradoxically, his job was, however, saved by a group of Israeli physicians at Haifa’s Rambam Hospital who invited him to visit Israel after The Lancet in 2014 published a letter which falsely accused Israel of a “massacre” in Gaza (sounding familiar?). “Re-educated” in his three-day visit by the opportunity to interface with Arab-Israeli physicians at Rambam Hospital and elsewhere, and blaming his prior misinformation on shortcomings of what was reported in the British press, Horton condemned the contributors of the letter to The Lancet for “promoting explicitly antisemitic materials, expressed a new understanding of Israeli realities including the complexities of the Arab-Israeli conflict, and pledged a new relationship with Israel,” while inviting Israelis “to tell the Israeli story in The Lancet in parallel to the Palestinians.”
Subsequently the letter in question
was formally retracted by The Lancet2 and, while the journal by no means developed an Israel-favoring outlook on the Middle East, it did maintain a reasonable balance between diverging opinions mostly expressed in form of letters-to-the-editors. After the unimaginable brutality of October 7 perpetrated by Hamas operatives upon several southern Israeli settlements, the VOICE, indeed, lauded Horton for a personal commentary in which he unequivocally condemned the events of the day and attacked the mostly young pro-Hamas protestors around the world who denied those events as “uninformed.”
But in the end, Horton apparently could not help himself after all: it did not take very long before the tone of his own commentaries started to change,3 as did The Lancet’s overall direction, with the journal not only making headlines for publishing incorrect facts about the current Gaza conflict,4 but publishing truly bizarre claims, like that the death toll in Gaza could reach 186,000.5 While the former communication was allowed to be refuted,6 the latter was widely picked up by similarly biased news outlets.7
The antisemitic bias of The Lancet under Horton is difficult to overlook, considering how selectively these biases exist only when it comes to letters, articles, and Norton’s own commentaries in The Lancet about Israel. Israel’s border closure is denying health care to Gazans (in Israel).8 Another article calls for closure of Israel’s detention center and hospital for captured Hamas terrorists.9 We, of course, have seen no articles about the fact that the (still alive) hostages in Gaza were not even once allowed visits by a Red Cross representative, as international war conventions require. Hamas even refuses to account for which hostages are still alive.
There have been no weekly editorials and letters-to-the-editor in The Lancet
about the truly hundreds of thousands Muslims killed in the third Sudanese civil war in the last few decades, this time also with the help of Iran (yes, again Iran!). A first article was published only recently.10 And how about the millions of Muslims in Chinese reeducation and concentration camps, and the hundreds of thousands of Syrian Muslims killed by the Syrian president Azad in that country’s civil war, of course, also with the help of Iran.
What all of this once again demonstrates is that–in a medical sense–antisemitism is to be viewed like a drug addiction: For those who have the predisposition, once an opportunity to relapse presents itself, they will relapse. Horton, The Lancet, and–unfortunately–too many even well-educated professionals in education, media, and politics have demonstrated to be only too good examples for this terrible disease! The world urgently needs a booster shot to get this resurging pandemic under control.
Recognizing the fact that emotions are high on both sides of the argument when it comes to the current situation in the Middle East it is easy for editors to get caught between the extremes. In contrast to The Lancet, here is something JAMA under its new editor-in-chief has been doing much better in understanding that under such circumstances it becomes the editor’s responsibility to offer equal opportunity for opposing arguments. When Israeli authors more or less made the argument that medical journals should stay away from reporting alleged health consequences of wars,11 the JAMA’s senior editors –in our opinion, correctly, disagreed,12 an opinion recently supported by two Arab authors.13 We would only add one caveat to their opinions: publish pro- and con- opinions together in the same issue of a journal.
1. Sanai L. BMJ 2005;331(7525):s214

2. Times of Israel, October 2, 2014. https;// www.timesofisrael.com/lancet-editor-deeply-regrets-publishing-gaza-letter/amp/
3. Horton R. Lancet 2023;402(10418):2180
4. Abi-Rached JM, Reinhart E. Lancet 2024; 403(1:2285
5. Khatib et al., Lancet 2024;;404 (10449):237238
6. Marmon et al., Lancet .2024;404(10448):124
7. Chalabi M. The Guardian, July 12, 2024; https://amp.theguardian.com/world/article/2024/jul/12/gaza-death-toll-indirect-casualties
8. Ahsan S. Lancet 2024;403(10448):2581
9. Devi S. Lancet 2024;404(10449):229-230
10. Idem. Lancet 2024;404(10449):231-232
11. Greenland et al., JAMA 2024;331(16):13611362
12. Curfman G, Bibbins-Domingo K. JAMA 2024;331(16):1368.
13. Ijaz N, Habib AR. JAMA2024;332(1):13-14
...Woke universities are demoralizing for their best people
We recently came across a short article by the Russian-British satirist, author, and libertarian pundit Konstantin Kisin who (with Francis Foster) also cohosts the TRIGGERnometry podcast.1 In the article he made the point that in all the criticism woke universities have recently been exposed to, an important point has been missed: woke academia, ultimately, starts driving away some of

the best and brightest faculty members as well as students.
As also reported in the media, new enrollment even in some elite universities has been crashing, especially by young males. His own department at a prosperous university had lost roughly a third of its faculty since 2020 because people became demoralized to learn and work at the university.
He suggested three main reasons for this increasing level of dissatisfaction: A first was “unseriousness.” As an ex-
ample, he offered the contrast between a wealthy woke university and a much poorer regional school, both attempting to recruit more students of color. The wealthy school in their strategy sessions ended up concluding that “there wasn’t even a point in trying to recruit more students of color until the campus works on its own racism” (without definition how that should be done). At the poorer regional school, the conclusion of their brainstorming sessions was, “keeping enrollment costs down, adding degree programs in skilled trades, emphasizing shared unifying values including love, humility, and courage. And sending their president to disadvantaged feeder schools to tell students about these initiatives and values.” Their enrollment skyrocketed!
As a second reason he identified “purposeful blindness.” He described it as “not seeing obvious things,” then explaining further that over the last 10 years or so, universities uniformly decided that ending bigotry and promoting emotional well-being were their two most important objectives. They then, however, “put some of the most bigoted and emotionally unwell people on campus in charge of achieving these goals.” As an example, he described an anti-racism training module recommended on a campus to everyone, in which one of the trainers stated that he used to physically assault white people who offended him because “white supremacy culture made him do it.” And regarding the enhancement of wellness and belonging of students, he noted that recent research clearly demonstrated that ideologies pushed by universities in these efforts actually make young people feel unwell and isolated.
As the third reason he identified “rule lessness,” with made-up rules often enforced and actual, logical rules often broken with impunity. He concluded by noting that it usually is only a small but very vocal minority of faculty, staff,
and students who are responsible for these problems; “but almost everyone else either enables them, keep their heads down and go along, or quietly leave out of frustration, for this collective cowardice sharing in the blame for the current state of higher education.” I could not agree more!
1. Kisin K. https://www.konstantinkissin. com/p/how-woke-universities-demoralize
…Diversity, equity, inclusion programs in medicine are evolving, but…
And here is a little more on the subject: It at times is almost overwhelming how much attention diversity, equity, and inclusion (DEI) these days receive in academia–including medical schools. Three Boston- and New York City-based colleagues recently penned a relevant “Viewpoint” article,1 making the valid point that DEI, in the three basic concepts expressed in those three letters, emerged at universities out of awareness that many universities historically had systematically excluded many individuals from their ranks based on sex, religion, race, and ethnicity. Correcting such practices, of course, fully aligns with what goals of any academic institution should be.
The article then acknowledged that a small minority of DEI programs strayed from this mission, though it suggested that because of such DEI programs academic health institutions are now overall more diverse, equitable, and inclusive. While we of course agree that significant progress has been made over recent decades in all three areas, we would argue that many universities not only “strayed” from the original mission but purposefully replaced one set of discriminations with a new set of discriminations against different groups of individuals. The question, moreover, appears not to be whether things over the years have changed to the better, but whether they have changed more to the better than they would have had formal DEI

programs never existed?
The article–in my opinion incorrectly–for example suggested that the enormous gain in numbers of female medical students has largely been a consequence of DEI programs (since 2019, women have been a majority2). While such programs may, indeed, have contributed, substantial other changes in women’s standing in society, of course, contributed far more to women by 2020 already representing 53.7% of matriculating medical students,3 in medical fields like obstetrics and gynecology reducing males in many institutions to less than 10% of residents.
From this very obvious change, the article deduced that the focus of DEI programs cannot remain static and must evolve. Claiming moreover that DEI was never intended to be only about race and ethnicity, the authors then, however, failed to explain why most programs nevertheless ended up looking exactly like that was, indeed, their primary purpose.
Though desperately trying to appear measured and “post-woke” by, for example, pointing out the recently undeniable “unprecedented schism - including an alarming increase in antisemitism” on campuses, unable to overcome their wokeness, they in the same breath had to add to the sentence “… and islamophobia.” As though, since the October 7 events in Israel, there had been even a single anti-Muslim demonstration or encampment at any U.S. college or university (in contrast, of course, to innumerable antisemitic and pro-Hamas events).
The irony in using the phrase “increase in antisemitism and islamophobia” speaks for itself. It is an excellent example for an amazing lack of self-awareness about the authors’ own wokeness, these days shared by so many faculty members in academic environments, including medical
schools. This despite the fact that the three authors very obviously wrote their paper with good intentions when arguing that DEI efforts should go “back to basics and original principles.” That will, however, not be possible, unless–as the preceding article alluded to–academia lives up to its primary responsibility, which is to educate students on how to think independently rather than indoctrinate them in controversial futuristic social, economic, and political hypotheses.
Whether in social or medical sciences, hypotheses are there to be explored and to be either confirmed or refuted. Their purpose never was or will be to be taught as if they were “absolute truth.” This has been a longstanding theme in how the VOICE has been addressing (and often criticizing) medical practice. The same principles also apply at all levels of education and–in a straight line–then automatically lead to a meritocratic social system. In contrast to widely held beliefs, DEI is compatible with meritocracy as long as its three principal goals are pursued without finding new targets for discrimination. As we have repeatedly pointed out in these pages before, if academia gives up on meritocracy in pursuit of supposedly more important and allegedly more humane goals, academia as we have known it will have ceased to exist, and mankind will suffer for it. A few more fitting points follow in the next article.
1. Cozier et al., JAMA 2024. doi:10.1001/ jama.2024.9951; ahead of print.
2. Association of American Medical Colleges/ December 9, 2019. https://www.aamc.org/ news/press-releases/majority-us-medical-students-are-women-new-data-show
3. Murphy B. AMA. September 29, 2021. https://www.ama-assn.org/education/ medical-school-diversity/women-medical-schools-dig-latest-record-breaking-numbers
… Concern about medical education in today’s political environment Which brings us more directly to medical education: In the weekend edition of July 27 and 28, 2024, Travis J. Morell, MD, a Colorado physician and senior fellow at Do No Harm, a national association of medical professionals “combating the attack on the healthcare system by woke activists,” published an op-ed in The Wall Street Journal1 under the heading, “Ideology in medical schools threatens everyone’s health.” He in the piece described his experiences when, “in an attempt to protect children from radical transgender ideology,” he filed at the Colorado Medical Society (CMS) a potential policy resolution for acceptance, built around an already existing policy against female genital mutilation in the society, which is also considered a federal crime.
Unsurprisingly, considering recent important developments in gender-determining medicine following Dr. Hilary Cass’s comprehensive report to NHS England about gender identity services (which we discussed in detail in the VOICE’s June issue on pages 23-25) and which drastically changed medical practice in the UK and in many other European countries, his fellow physicians in CMS overwhelmingly supported the resolution when, shortly before closure of voting, the tide suddenly turned.
A physician by the name of Frank Merritt, MD, an assistant professor at the University of Colorado School of Medicine, had e-mailed the student body requesting that the students vote against the resolution, and boy, did they! Over 150 medical students who, otherwise, in a majority likely would not even have known that, according to CMS rules, they were eligible to vote, within less than 24 hours ended up voting against the resolution, resulting in its rejection.

There are several reasons why this is an important story that offers important evidence about what brought our education system–and in this instance our medical education system–to the extremes we have been witnessing: first, it demonstrates the power of faculty–even junior faculty–over the student body. Second, it demonstrates the willingness of the student body to exert their power through political activism without really understanding the issues.
Less than 24 hours was, of course, not enough time for the medical students to educate themselves about as complex an issue as gender reassignment treatments in children and young adults. One can bet that none of the over 150 medical students who voted against the well-thought-out and evidence-based resolution proposed by Morell even looked at the 388-pagelong report issued by Dr. Hilary Cass just a few months earlier which radically changed how most of the developed world views the subject.
Yet lack of time is no excuse for the ignorance about subjects students these days are “willing to fight for.” This was well demonstrated by the many protesters in recent pro-Hamas rallies who, chanting “from the river to the sea, Palestine will be free,” had absolutely no idea which river and which sea they were referring to.
Third, faculty can be shameless in pursuing political activism when they, instead, should teach their students critical thinking. And, as this case also so well demonstrates, medical faculties these days often do not hesitate to follow ideology rather than medical evidence in how they treat patients. Morrell, therefore, was correct in pointing out in his article “the coming dangers to patients nationwide” from such faculty. This danger, indeed, applies not only to patients but, concomitantly, represents an even more substantial danger to young physicians
in training when being taught ideology by faculty instead of absolute obligation to gather evidence and follow best available evidence. By following the assistant professor’s request to vote against the submitted evidence-based resolution, they instead shamefully sided with unproven, unethical, and anti-scientific medical interventions.
A few more words on gender-affirming care follow in the next article.
1. Morell TJ. Wall Street Journal. July 27 & 28, 2024. pA13. https://www.wsj.com/articles/ ideology-in-medical-schools-threatens-everyones-health-f6887ae3
Gender-affirming care seems everywhere. Why do we know so little about it?
... Gender-affirming care: Elon Musk speaks from personal experience As noted above, the June issue of the VOICE not only offered a discussion of the complexities surrounding gender-affirming medical care in children and young adults, but also explained why it took us so long at the VOICE to address the issue. And, as we explained, it was not the controversy which now, for several years, has been engulfing the subject that we wanted to avoid; considering the often diametrically opposing opinions found on the subject in the published literature, we simply felt “uninformed” and, therefore, unqualified to chime in. All of this changed after Dr. Hilary Cass in the U.K. published her extremely researched and documented report on the subject. Either concomitantly or as a consequence of this report–as we noted in our June article–several European countries and/or their medical organizations in addition uniformly repealed what in many of those countries up to that point–like in the U.S.–had been a very proactive approach toward treating children and young adults with gender dysphoria who expressed a desire to transition. We noted then–and nothing has
changed since–that, somewhat surprisingly, U.S. medical organizations have remained mostly mum. And, while the marketing efforts in support of gender-affirmative care for children and young adults in the country have quite visibly diminished, children in the U.S., even at some prominent medical institutions, still undergo irreversible treatments which, based on the Cass report’s findings, as of this moment have to be considered unethical and maybe even criminal.
It was no surprise when Elon Musk, not known to shy away from addressing controversial issues, chimed in on the subject; the reason for entering the discussion was, however, a big surprise since it involved one of his many children, who when born was named Xavier and now goes by Vivian.
But let’s take a step back: It all started with famous (or, as some would argue, infamous) Canadian psychologist and Professor Emeritus at the University of Toronto, Dr. Jordan Peterson, holding a livestreamed conversation with Elon Musk meant to address subjects such as AI, declining birthrates in most of the world, religion, and Musk’s life philosophy.1 And though each segment of this hours-long conversation was worthwhile listening, none received the amount of media attention as the segment of the conversation that turned to gender-affirming medical care. Musk got surprisingly personal, relating the story of his son Xavier, now 20 years old, who at age 16 was placed by treating physicians on socalled puberty blockers. Estranged from her father, she now lives under the name Vivian Wilson, 2 her mother’s maiden name. She is one of six children Musk had with his first wife, Justine Wilson, a writer.
In his conversation with Peterson, Musk suggested that he was “tricked” (presumably by his son’s health care providers) into consenting to his son’s treatments,

which resulted in him “losing [his] son”. He apparently was told by medical staff that his son might commit suicide if he wasn’t allowed to take puberty blockers which he did not know were irreversible in their effects. In more crass terms he described his son, as a consequence of these treatments, now as “dead,” and characterized the treatments as “child mutilation and sterilization” (for life).
Later on X (the former Twitter, which Musk now owns), he further explained that his son was born “gay and slightly autistic”2 (Musk, himself in 2022 publicly confirmed that he in his youth in South Africa was diagnosed with Asperger Syndrome,3 now considered part of the autism spectrum), and that these traits led his son to develop gender dysphoria3 and made his father an “ardent opponent of gender-affirming care” ever since, and on a quest to “destroy this woke mind virus.” He also reaffirmed that he was only referring to the abusive use of puberty blockers and surgery in children and young adults and fully supported those choices for adults capable of deciding for themselves.
Musk’s position is, therefore, fully in line with the recent report by Cass and associates and with recent restrictions on gender transition tools in children and young adults implemented in the UK and in several other European countries. Regarding his claim of having been misled about his son’s risk for suicide, a recent commentary in the BMJ also appears to be supportive: A recent audit in the UK by a professor of psychiatry at the University of Manchester and commissioned by the British Health and Social Care Secretary demonstrated no evidence whatsoever that bans of puberty blockers had led to a surge in suicides, as had been claimed by some health care providers, especially in the U.S.4
A recent editorial in the same journal described the Cass review as a “signif-
icant event” for medicine and urged medical institutions (listen America!) to consider what they have contributed, both negative and positive, to the report’s damning conclusion.5 In The New York Times, columnist Pamela Paul in a very detailed and scathing opinion noted that there is no basis to rush putting kids on an irreversible path of medicalization, especially in absence of evidence to support it.6
1. https://x.com/jordanbpeterson/status/1815427698703090085
2. Telford, et al., The Washington Post. July 26, 2024. https://www.washingtonpost.com/ business/2024/07/26/musk-transgender-vivian-grimes/
3. King H. Axios. April 15, 2022. https://www. axios.com/2022/04/15/elon-musk-aspergers-syndrome
4. Feinmann J. BMJ 2024;386. Doi: https//doi. org/10.1136/n,j.q1638
5. McCartney M. BMJ 2024;385:q1189
6. Paul P. The New York Times. July 12, 2024. https://www.nytimes.com/2024/07/12/opinion/gender-affirming-care-cass-review.html
... A positive byproduct: more interest in exploring sex and gender
Maybe we are too optimistic in interpreting the scientific Zeitgeist, but it seems worthwhile mentioning that several prominent journals suddenly appear to have discovered that research of sex and gender has been a stepchild for much too long. In an unsigned editorial noting that many scientists are reluctant to investigate questions regarding sex and gender, fearing their studies will be “misused,” Nature magazine announced a series of article on these subjects.1
In a first such article, two Canadian colleagues made this point, concomitantly calling for a more nuanced approach.1 In a second article in the same issue, other investigators point out that female-male comparisons offer powerful biomedical research (and are done much too infrequently). They noted that binary sex studies have been denounced as too simplistic, but see them as a long-neglected area of research in biomedicine, and we agree.
1. Editorial. Nature 2024;629:7-8
2. Ritz SA, Greaves L. Nature 2024;629:34-36
3. Sabra ApA, et al., Nature 2024;629:3740
... increasing warranted worries about academic medicine
Considering all the uproar in academic medicine, it is not surprising that several prominent medical journals have picked up on the subject. But what is surprising is the issues they have chosen to pursue. One very prominently-addressed issue in several articles in several eminent journals was the concept of “neutrality” of academic institutions. H. Holden Thorp, PhD, Editor-in-Chief of the Science family of journals, was one of the prominent authors addressing the issue in an editorial.1
The subject surfaced after Harvard University, following severe criticism of the institution’s handling of the pro-Hamas student demonstrations on campus, announced “political neutrality” as a new, formal institutional policy.2
Holden Thorp defined the concept as the idea that universities (and especially their leading administrators, such as presidents, deans, etc.) should be politically neutral. He also pointed out that this was not a new idea since the University of Chicago already in 1967 (following the Six-Day War of Israel with her Arab neighbor countries, an interestingly analogy, considering that much of the issue arose out of demonstrations over the current strife in the Middle East) issued the so-called Kalven Report, which stated that “the university is the home and sponsor of critics; it is (however) not itself the critic.”1
He then basically endorsed institutional neutrality “when it comes to political issues,” but raised the question of whether such neutrality was appropriate when it comes to science.

While by now, at least among academic opinion leaders, there appears to be a clear plurality for neutrality regarding geopolitical issues (for example boycotting Israel academically or not), the academic world is more split about where universities should draw the line when it comes to reporting of research outcomes and their conclusions. We in that regard also sense a slight advantage for those who argue that institutions should not have opinions in general, but the support for this notion is clearly smaller than support for geopolitical neutrality.
Thorp argued that for scientists absolute neutrality by universities (and, of course, medical schools) is a “two-edged sword,” quoting a prominent university president who supported wholesale neutrality based on the belief that universities should be places where “popular and dissenting viewpoints are thoughtfully subject to reason and evidentiary debate.”
Prominent physician-scientist Eric Topol, MD, frequently quoted in the pages of the VOICE, disagreed and is quoted by Thorp to have told him that he considered such an approach (among other adjectives) “spineless.”1 Somewhat less outspoken, he nevertheless, as co-author in a recent opinion article in The New England Journal of Medicine, argued that institutional voices were a prerequisite for academic freedom.3
Both of his co-authors in this paper are from Stanford University, a campus where inter-faculty disputes during the COVID-19 pandemic made headlines, with the university ending up not looking very good in the eyes of the public after not necessarily siding with those faculty members who in the end proved to have been more correct than the other side of the debate. Therefore, rather unsurprisingly, Stanford just announced the shut down of its Disinformation Research Program. 1 One wonders how many other universities
currently still maintain such–or similar–programs here in the U.S. (though, of course, not in Russia or China).
And now to the editor-in-chief of the BMJ, Kamran Abbasi, MB, ChB, FRCP, who in a recent editorial, announcing a BMJ Commission on the Future of Academic Medicine, wrote the following two introductory paragraphs:4
“Academic medicine is broken. Worldwide, it has been for decades. Perverse incentives, entrenched power imbalances, deteriorating career pathways, restricted funding, and health service pressures are breaking it further. The complex challenges are global, with regional and national subtleties. Scan the landscape of commercialized life sciences, wasteful research and development, and exploitative scientific publishing—taking in a colossal waste of public money — and you quickly realize that this one system failure sits at the center of the Venn diagram (a Venn diagram uses overlapping circles - or other shapes – to illustrate logical relationships between 2 or more sets of data/items. Academic medicine is, however, not an irrelevant silo.
Science should form the basis of clinical practice and patient care. It should be central to medical education and training. It should advance diversity and inclusion. It should be the guiding light for government policy. The fact that it isn’t, and is drifting further from the center in each sphere, is a damning indictment of what society now values. The case for urgent solutions to this global crisis has never been stronger.”
I couldn’t agree more! More on the business of medicine in the next section.
1. Holden Thorp H. Science 2024;385(6707):347
2. Haidar E, Kettles CE. The Harvard Crimson. May 28, 2024. https://www.thecrimson.com/ article/2024/5/28/harvard-institutional-neutrality-report/
3. Mullen et al., N Engl J Med 2024;391(1):1-3
4. Abbaso K. BMJ 2024;385:q1294
business of medicine
... is the rapidly growing role of private equity in medicine in trouble?
Because the infertility field in recent years has been a primary target, the takeover of medicine by private equity has been a repeated subject of our comments in the VOICE. In recent months, we, moreover, especially in several fertility clinic networks, repeatedly pointed out warning signs about impending troubles, such as unexpected changes in senior executive positions (often repeatedly), earnings shortcomings, unexpected sales by one private equity to another without expected profits, and others. Now those signs are flashing even stronger.
Medscape Medical News recently reported on the unhappiness of physicians working in private equity-owned provider settings.1 The article reported that one in four emergency rooms is now understaffed if owned by private equity, leading to longer wait times, deterioration in patient care, and higher bills.
Though not strictly a private equity investor, United Health Group Inc (UHG), one of the nation’s biggest health insurance companies, now also owning provider clinics, is also cited in the article as gaining “unhealthy” market influence. This company alone now employs roughly 10% of active U.S. physicians, mostly employed through its subsidiary, Optum Health, which primarily owns provider organizations for primary, urgent, and surgical care but also in other medical areas is increasingly active in purchasing physician-owned practices, often in competition with private equity investors.
Optum has already over two million patients–of course, all insured through

UHG–for which UHG pays Optum under a payment model, now fashionably called “value-based care.”2 Under this model the provider clinic is paid by UHG a set amount for all care in a given practice area, very similarly to what Health Maintenance Organizations (HMOs) decades ago used to do through so-called “capitated contracts.” Under the newly fashionable term “value-based care,” this means nothing else but the reintroduction of such capitated services, which in their earlier iteration became so unpopular with the public that HMOs almost completely disappeared.
Like in the old HMO days, the current claim is that this kind of payment arrangement incentivizes preventive care and leads to less waste. In practice, the incentives, however, go into only one direction: the withholding of medical services from patients. The conflicts of interest that exist in such an arrangement when both contractual partners have the same ownership are, of course, glaring. Under HMOs there was at least an adversarial relationship between insurer and provider that united providers and patients in a common interest for adequate services. Under “value-based care,” the patient is left out of the loop completely and the motto is: as few services as possible.
In a recent Perspective article in The New England Journal of Medicine, 3 the authors pointed out that UHG today is the largest health insurance company, the single largest employer of physicians, and third-largest pharmacy benefit manager in the U.S. The company also operates pharmacies and banks.
The big-three pharmacy benefit managers–UHG’s subsidiary obviously one of them–were supposed to keep drug pricing low for patients and insurance companies. According to several recent media reports, however, their interventions actually increased drug costs.
And, as the article further reports,
UHG is not the only health insurance company undergoing this kind of vertical consolidation: Humana is another one, and has already become the largest provider of primary medical services to seniors and post-acute in-home care; and other major insurance companies have started following similar vertical strategies.4 Concern has, therefore, been rising that these conglomerates’ growth tactics potentially lead to market abuses, raise costs, undermine the competition, and, ultimately, erode patient-care quality. The article suggested that UHG (and other similar conglomerates) may be an outright health threat to everybody.
Three federal agencies have announced investigations of how private equity purchases of health care providers and the resulting consolidation (by some economists considered a good thing) affect quality of medical care and costs. And, as the article points out, physicians in private equity-owned set-ups are increasingly unhappy and talking to the agencies. The involved federal agencies are the U.S. Department of Justice’s Antitrust Division, the Federal Trade Commission (FTC), and the Department of Health and Human Services.
Nobody, of course, likes such excessive federal attention, and while it is unclear how guilty the feds are in this development, private equity investments have apparently sharply slowed down, as reported in the Wall Street Journal on June 5, 2024.5 As the article pointed out, this especially appears to affect roll-ups of doctor clinics–maybe the most fundamental strategy in the private-equity playbook. A recognition that such a strategy may no longer warrant the huge investments of the past can, of course, also be expected to affect current valuations of already existing roll-ups.
In infertility such roll-ups have, indeed, been at the core of the field’s industrialization and commoditization
and one, therefore, can expect further upheaval beyond what recently already made headlines. Here are a few examples we recently read or heard about:
Women’s Care Enterprises, founded by Lindsay Goldberg LLC, a New York-based private equity firm that raised $20 billions of equity capital and has invested in over 60 platforms, a seemingly successful multispecialty women’s health company “dedicated to transforming the delivery of women’s health care,” after in principle working mostly in general obstetrics and gynecology, ventured in 2020 into the fertility arena by purchasing Beverly Hills’ well known Southern California Reproductive Center (SCRC). And now both sides are keeping lots of lawyers busy, trying to figure out how to exit from an obviously failed marriage. SCRC, under the leadership of Mark Surrey, MD, has been a mainstay of the California IVF scene through its Beverly Hills office and satellite offices in surrounding areas. We sincerely hope the center will recover from this experience intact.
In our June issue before the summer break, we reported on yet another major executive shuffle at Kindbody; and from a July 21, 2024, mailing from our friend, Griffin Jones’s Fertility Bridge with attached interview, we then learned that our June report was already outdated because Kindbody’s founder, first CEO, and serial entrepreneur, Gina Bartasi, was back at the helm at the company as also its latest CEO. These things obviously don’t happen when things are running well. While her interview with Griffin Jones did not offer any real insights, her return is clear evidence that things within the company are not running the way they are supposed to. We also hear about a lot of unhappiness from some of the most senior physicians–all also shareholders. We wish Gina and her team well. If anybody can clean up the mess at Kindbody, it is Gina Bartasi!

And, yes, roll-ups are still continuing: Dresner Partners on June 5, 2024, in an email from its partner, Mitchell Stern, announced it had advised the Reproductive Science Center of New Jersey in its sale to Inception Fertility which in NYC, of course, includes the Prelude Network, represented by the NYU program.
1. Weber S. Medscape Medical News May 2, 2024. https://www.medscape.com/viewarticle/docs-vent-feds-investigate-private-equity-consolidation-2024a10008hd
2. Wainer D. The Wall Street Journal in Heard on the Street. June 14, 2024. pB10.
3. Rooke-Ley et al., N Engl J Med 2024391(2):97-99
4. Essley Whyte L. Wall Street Journal July 9, 2024. https://www.wsj.com/health/healthcare/big-pharmacy-benefit-managers-increase-drug-costs-ftc-says-c6a40ee6
5. Cumming C. The Wall Street Journal. June 5, 2024, pB1. https://www.wsj.com/articles/private-equity-puts-brakes-on-healthcare-rollups-after-government-scrutiny-6fc64f5a
...the growing business of infertility –the good, the bad, and the indifferent THE GOOD: Coming back to Griffin Jones: In an email dated July 25, 2024, he announced that his outfit, Inside Reproductive Health, has commissioned a comprehensive digital catalog with a directory of 400-500 companies who provide products and services for fertility centers and IVF labs. Though not announced, we suspect that this registry, given the interesting name–IVF Heroes Universe–may at some point turn into a marketplace. For the time being companies (for a registration fee) may be formally registered in 18 categories in alphabetical order from (i) At Home testing to (xviii) Supplements.
Current subscribers to Inside Reproductive Health will receive the digital catalog in their inbox on Thursday October 17, 2024, just in time for the annual ASRM conference, held this year in Denver, CO.
Congratulations to Griffin on a great idea!
THE BAD: Is the fertility industry profiting from the vulnerability of our patients?
This is, indeed, exactly what an unsigned editorial in The Lancet claims.1 That a medical journal as prominent as The Lancet would publish a formal, unsigned editorial–and not just an opinion piece by some invited experts–appears telling and indicative of a significant level of frustration that has built up at the journal regarding this issue. It also means that the time may have come for some introspection by the infertility field.
Furthermore, remarkable beyond its challenging title without even a question mark (“The fertility industry: profiting from vulnerability”), is the relative “banality” of the editorial’s content, basically offering nothing we all have not already known for some time. Unmentioned, the editorial, therefore, raises the question: where have you–providers of fertility services–been for so long without doing anything about it?
The editorial fairly points out the amazing gains the fertility field has achieved over the last almost 50 years, primarily a consequence of the evolution of in vitro fertilization (IVF), now in some high-income countries contributing almost 10% of births to a birth-starved world. But it also points out that the fertility field spawned an “industry” around the field that, often, does not only not improve quality of care and affordability, but in its actions often has had opposite effects.
This argument has, of course, been at the very center of much of the CHR’s research and educational efforts for decades.2 We, therefore, cannot help but agree with the sentiment of this editorial, which appears motivated by several recently published papers in the literature (among those two articles with their own respective commentaries in the same issue of The Lancet, 3-6 which we in more detail will
be addressing in the Literature Review section of this issue of the VOICE), demonstrating the clinical futility of widely utilized practices in IVF, often described as “add-ons” (to previously existing routine IVF practice).
Consensus is increasing that most “add-ons” over the last 20 years have proven to be useless and some have been demonstrated to even be harmful to selected sub-populations.7,8 This, of course, also means that those “addons” were allowed to enter routine IVF practice before proper prior validation studies confirmed alleged benefits and/or allowed for the determination of which patient populations should or should not be exposed to them. The list is long, indeed too long: from routine extended embryo culture to blastocyst-stage, to routine elective single embryo transfer, to preimplantation genetic testing for aneuploidy (PGT-A), and, more recently, routine embryo cryopreservation with delayed frozen-thawed embryo transfer, likely, being the most consequential. But of course, there are many more!
As these “add-ons” uniformly involve clinical practice, one may ask where “industry” comes into play? The answer is, indeed, simple: with rapidly growing ownership worldwide of IVF practice by private equity (see also earlier discussion above), which in the U.S. by now is alleged to control over half of all annual IVF cycles (and rapidly growing), infertility practice, unfortunately, has, indeed, become a central part of the rapidly growing fertility industry the Lancet editorial referred to.
Through private equity ownership motivated by similar time and profit considerations to the above-discussed medical insurance industry, clinical IVF practice is also becoming increasingly vertically integrated, in the process creating exactly the same conflicts of interest we described above for the medical insurance industry.

Likely, the most advanced example is Inception Fertility which in the Prelude Network® not only owns what the company describes as the fastest-growing network of fertility centers in North America,9 but in MyEggBank® owns one of the largest egg bank networks in North America, in BUNDL™, owns a fertility treatments financing service, and in Inspire RX™ owns a pharmacy. And then, of course, there is also what is widely understood to represent the real “industry,” meant to produce services and products for the infertility field. Into this category, for example, fall the suppliers of laboratory equipment for the IVF field, among those producers of closed embryo incubation systems with time-lapse monitoring of embryos, a new concept that burst onto the scene around 2014 to 2015 with a product called the “embryoscope.”
Based on the promise of better IVF outcomes through better embryo selection and better standardization of embryology, as well as lower staffing costs in laboratories, industry very quickly convinced fertility clinics worldwide to spend hundreds of millions of dollars on, as one of the above referenced studies in The Lancet now finally quite unequivocally demonstrates, clinically ineffective time-lapse equipment.3 Costs of this quite expensive new equipment were, of course, recouped through higher IVF cycle fees, expenses that were out of reach for too many infertile women. Some clinics in their marketing efforts, indeed, suggested that higher cycle costs were more than compensated for “by now being able to offer patients photo albums of their growing embryos.”
Even more devastating for the fertility field has, however, been the explosive growth of the testing industry around infertility and this, of course, all started with genetic testing (in detail discussed in the past by the CHR in the VOICE’s “Testing the Tests” series of articles by the CHR’s Director of Clinical IVF
and Research, David H. Barad, MD, MS and in innumerable articles in the literature by the CHR’s investigators). And this not only involved the quickly spreading practice of PGT-A but also involves such bizarre issues as expanded carrier testing for by now hundreds and hundreds of diseases with carrier prevalence often similar to the chance of winning a lottery jackpot (addressed by Dr. Barad in the June issue of the VOICE). And it, of course, also involves several non-genetic tests.
Because PGT-A has been a core subject of the CHR’s criticism for many years, only too often discussed in these pages (and in articles in the medical literature), we will not be repetitive here. Moreover, even ASRM and SART by now recognized that PGT-A has no useful clinical purpose for the general infertility population.10
Regarding non-genetic tests the industry has offered, they almost uniformly have been demonstrated not to fulfill promised diagnostic consequences on fertility treatment outcomes. Besides PGT-A which, of course, created a worldwide PGT-A testing industry around a single test, other aggressively marketed tests have also contributed to the growth of several new companies worldwide (see also below). Remarkably, despite convincing published evidence for their uselessness, these companies have continued marketing them, often still maintaining claims refuted by independent studies.
Which really brings us to the punchline of this discussion, which reflects back to the article by Konstantin Kisin, we earlier discussed in a preceding subsection of this Monthly News section: One can, of course, criticize with great ease the conditions in which we live and work, but isn’t it time to face up to our own responsibilities in allowing these conditions to arise and then to fester? And this question does not only apply to us health care providers as individuals. One also wonders
where for example, our professional organizations have been all of this time?
Here is one highly relevant and very blatant example why professional organizations matter: Even if overdue by many years, the above-noted combined ASRM and SART opinion that PGT-A has no clinical utility in unselected patient populations,10 is, of course, very welcome; but how come both societies then failed in also taking the logical next step which, of course, should be that if something does not work, it should no longer be used! Unfortunately, however, this logical conclusion was–and still is–nowhere to be found.
And why is that? That is a difficulty question to answer; but let us offer a hypothesis: Because of often absurd federal restrictions, professional organizations, which since their initial creation have always been dependent on industry for most of their support (membership covers only a small portion of their budget), have become increasingly desperate to maintain financial support from industry. In the fertility field, this support for many years came primarily from “big pharma,” which in those days meant Serono, Organon, and starting a little later, Ferring. As real big pharma swallowed Serono and Organon, their motherships lost interest in the fertility field which, in contrast to Wall Street and private equity, they no longer viewed as a potential growth area since there were no blockbuster drugs (expected sales over $1 billion) in sight (indeed the infertility field has not even seen a single new drug with clinically significant consequences come to market in decades). FDA regulations for even bringing generic drugs to the U.S., moreover, are so cumbersome and costly that companies which produce gonadotropins (still the mainstay of infertility treatments) for European and Asian markets do not find it economically worthwhile to enter the U.S. market.

Less competition in the U.S. than in those other markets, moreover, not only deprived the U.S. of “investments” (which always includes the financing of professional societies) by these other pharma companies (which are very active in Europe and Asia) but, actually, also diverted marketing funds of the three remaining companies in the U.S. elsewhere since competition is, therefore, in the U.S. much weaker than in Europe and Asia. As a consequence, reproductive medicine in the U.S. has been starving in financial support from pharma and has made U.S. - based fertility – related conferences increasingly dependent on support from non-pharma industries. And who would that be? One only has to visit the exhibition halls to see the answer: It’s, of course, the testing industry!
Our professional societies, therefore, have become economically increasingly dependent on a part of industry that is selling the field often useless and, sometimes even harmful, tests and services. As The Lancet in its editorial, therefore, so succinctly argued, at least certain parts of the fertility industry, indeed, are, therefore, profiting from not only the vulnerability of infertility patients, but also from our vulnerability as individual health care providers who, often, are deprived of objective advice.
But, as the CHR demonstrates, it does not always have to be so: The CHR does not–and never did–culture all embryos to blastocyst-stage; it does not–and never did–transfer only single embryos but individualizes the number; it does not–and never did–possess even a single time-lapse set-up because, before changing important practice patterns that have proven their effectiveness over time, the CHR initiates its own analyses of the literature and–if required–its own outcome studies before changing practice simply based on representations of others.
When the time-lapse craze started, the
CHR, for example, insisted on testing such equipment out before purchasing it. Already in 2016, in two studies of different patient populations at the CHR, we determined that such systems would NOT improve our patients’ outcomes and, in some patients, indeed, would reduce their chances.11 It took almost 10 years from when the CHR for the first time understood the futility of investing for clinical purposes in time-lapse systems (such systems, of course, can be good research tools), until the field now, finally, may be catching up. 3,4 It took the CHR’s investigators over two years to get their paper published because–as it so totally contradicted what almost everybody at the time believed–most journals rejected it.
It took an even much longer 18 years from the time the CHR developed doubts about the utility of PGT-A, before ASRM and SART, recently agreed at last.10 The CHR reached this conclusion for the first time in 2006 but could not get a paper into print making this point until 2008.12 Again, because the CHR’s conclusion were so contradictive of the common wisdom at that time, the paper was rejected by the reviewers of innumerable medical journals. Only after Dutch colleagues published a paper in 2007 reaching the same conclusions,13 Fertility & Sterility – which previously had been among the journals that had rejected the CHR’s paper–“recalled” and published it.
In contrast to most colleagues, the CHR, therefore, never recommended PGT-A to patients over all of these years, though, respecting our patients’ right to self-determination, we of course have been offering the test to patients who still wanted it. Eighteen years later, the CHR, however, can be proud to have utilized PGT-A only very rarely, thereby saving many of CHR’s patients not only the remorse many patients these days feel for, after PGT-A, having discarded so many
of their embryos, but also for having saved them considerable costs and probably at least a small number of pregnancies.
What the CHR is most proud of, however, is that in all of those years, the CHR in its practice always put the patients’ interests first, even if, as in the case of PGT-A, this over so many years meant the significant loss of revenue, relating to non-utilization of PGT-A alone, in the millions of dollars. To protect infertility patients from the vulnerability they almost automatically face has, indeed, been a principal ethical obligation for the CHR since its establishment.
THE INDIFFERENT: What we under this subheading want to communicate is that–likely not different from other medical specialty fields–the industrialization and commoditization of reproductive medicine is continuing and, indeed, expanding. The principal motivation and, indeed, purpose of any for-profit venture is–as the term already indicated–maximal profit. The assumption should always be that profit should only be obtained by ethical means. But what is considered ethical in many circumstances, of course, leaves room for consideration.
What the legal limits are for companies in promoting questionable outcome benefits from tests, such as PGT-A, polygenic risk scoring (PGRS) of embryos, or from endometrial receptivity (ER) testing, and/or vaginal or uterine microbiome studies, and many others still remains to be determined. As mostly so-called laboratory-developed tests (LDTs), they in the past have not been subject to FDA scrutiny and have been brought to market by industry basically exclusively based on the industry’s representation of effectiveness. The FDA recently announced that this has changed, and at least some LDTs would become subject of the agency’s approval.

As health care providers, when many of the claims made for such tests by manufacturers or laboratories are not supported by the literature, one should, however, be even more concerned about the ethical considerations in discussing these controversial and usually very costly tests with patients.
How quickly some companies have grown by exclusively selling LDTs is, indeed, quite surprising. IGENOMIX, founded by Carlos Simón, PhD, Antonio Pellicer, MD, and José Remohi, PhD (the latter two also were the founders of IVI) is such a company. It was sold by EQT Private Equity in 2021 (after owning it for only two years) to Vitrolife for $1.48 billion and is now one of the best known and most successful diagnostic laboratories in reproductive medicine worldwide. It started by offering an ER- assay and PGT-A testing, and has been pioneering microbiome testing, all three LDTs and to this day highly controversial tests. If the FDA were to conclude that, contrary to claims by the company, PGT-A, ER-, and microbiome-testing in infertility practice do not offer any clinical benefits, IGENOMIX overnight, therefore, would have very little work left to do (the company–apropos–just announced improvements in its EMMA and ALICE microbiome tests).14
Where borders in marketing questionable medical laboratory services should be drawn was recently raised by the British Medical Journal (BMJ)15,16 and The New York Times17 in regard to cord blood banking. The argument made was that marketing efforts by privately owned biobanks for cord blood storage may be misleading expectant parents about the value of cord blood storage for their offspring. In the UK such storage costs between £550 and £3,000, with the claim being that these cells, if later in life needed by the offspring, can become any body tissue and can even be used to grow new organs. That, however, at least at current
knowledge levels, cannot be achieved and, again at current knowledge levels, is, indeed, believed not to be possible. Moreover, as The Times noted, the sostored stem cells are only rarely useful and, sometimes even contaminated and therefore useless.
And then, as a final chapter in addressing inappropriate marketing in medicine, especially after the world’s COVID-19 experience, medicine must learn to recognize the impact public pronouncements by professionals can have on the lay public and such statements, therefore, must be made responsibly. Here is one recent example that affected the CHR and, we are certain, many other IVF clinics as well: The CHR recently received innumerable calls after the British Guardian newspaper published an article (then picked up by several U.S. outlets) under the highly provocative title: “Dream come true: study suggests drug could extend women’s fertility by five years. ”
The article referred to an ongoing study at Columbia University here in NYC under direction of the geneticists Yousin Suh, PhD, and REI colleague Zev Williams, MD, PhD, whom the VOICE previously referred to in an earlier issue. We then pointed out that this study pursued an interesting hypothesis by trying to determine whether treatment of women with a drug called rapamycin could delay ovarian aging.
The Guardian article now claimed that the preliminary results of the Columbia trial suggested that rapamycin could, indeed, extend a woman’s fertility by five years and help them live longer and better lives. But this study–planning to involve 1000 women–had enrolled only 34 participants so far!
It is difficult to understand how a so-far only very small study with only very short follow-up can suggest anything even close to the magnitude
suggested by the Guardian article. It most certainly, as of this point, is not even close to being able to support the alleged benefits claimed in the newspaper article. Anybody with even only minimal knowledge about clinical trials, therefore, has to view the Guardian article as “fake news.”
The only issue that remains to be determined is whether Dr. Suh overhyped still-very-preliminary data or whether the journalist, Amelia Hill, either overinterpreted or misunderstood what she had been told. Either way, following this article, the CHR staff for several days had to explain to a lot of callers that they had fallen victim to a “fake news” article.
1. Editorial. Lancet 2024; 404(10449):215.
2. Von Schondorf-Gleicher, et al., J Assist Re prod Genet 2022;39(3):591-604
3. Bhide et al. Lancet 2024;404(10449):256-265
4. Bergh C, Lundin K. Lancet 2024;404(10449):217-119
5. Ho et al., Lancet 2024;404(10449):266-275
6. Pinborg A, Løssi K. Lancet 2024;404(10449): 219-220
7. Moffett et al., Hum Reprod 2023;38(11):2062-2104
8. Feng et al., Hum Reprod 2024;39(3):448-453
9. https://inceptionfertility.com/about-us/ Practice Committee of the ASRM and SART. Fertil Steril 2024; May 18: S00150282(24)00241-3. doi: 10.1016/j.fertn stert.2024.04.013. Online ahead of print.
11. Wu et al., Reprod Biol Endocrinol 2016;14(1):49
12. Gleicher et al., Fertil Steril 2008; 89(4):780788
13. Masstenbroek et al., N Engl J Med 2007;357(1):9-17
14. Igenomix USA, May 14, 2024; via e-mail
15. Kwan J. BMJ 2024;386:q1581
16. Abbasi K. BMK 2024;386:q1648
17. Kliff S, Ghorayshi A. The New York Times, July 15, 2024. https://www.nytimes. com/2024/07/15/health/cord-blood-stor age-contamination.html

We, of course, have known (and written about) so-called predatory medical journals, so called because they published “junk science,” often produced in paper mills in China. But we did not know that there also exist now predatory medical and scientific conferences. This is exactly what Christine Ro from Nature magazine taught us in a recent article.1 As it turns out there appear to exist lots of what the journal calls dud and non-existent conferences.
As the article notes, like predatory journals, their conference counterparts are at times difficult to define. In the end it means that there appears to be no intent to seriously deliver science; the only real purpose is for the organizer to make money. Though the article introduced some characters, apparently fooled into registering for such conferences, we must say that we are skeptical. It does not seem that difficult–it would seem to us–to figure out whether a conference is for real or not. More likely, whether consciously or subconsciously, people who fall for these kinds of pseudo-conferences probably just like to travel, or need a tax write off or an excuse to charge the trip’s costs to a grant or an institution.
But predatory medical journals are for real and can do real harm on many different levels. Even The Wall Street Journal on May 15, 2024, addressed the issue, publishing a frontpage (A1) article with the alarming title: “Counterfeit studies are infecting scientific journals.”2 When even Wiley, a 217-year-old publisher and leading publisher of medical journals–now based in Hoboken, NJ–announced the closing of 19 journals, some of which the publisher finally discovered “to be infected by large scale research fraud,” things must have gone seriously off the rails.
One wonders how come the editors of these journals didn’t notice this right away, and the publisher didn’t notice that the editors did not notice. Based on the Journal article referenced, Wiley retracted about 11,300 papers in the last two years alone. And it took them two years to close down these 19 journals? What a scam, and Wiley is not the only publisher involved in this dirty business!
But even the serious medical and scientific publishing business seems to be slipping deeper and deeper into troubles. A widely cited 2016 Alzheimer disease study from a prominent laboratory in Nature magazine (it does not get any more prestigious than publishing in Nature) was just retracted by the authors because they could no longer hide that the paper contained manipulated images.3 We have made the point repeatedly now in these pages: something stinks in scientific publishing in general and, while everybody is talking about it, nobody appears to be doing anything serious about it, even while the numbers of retracted manuscripts are skyrocketing. Biomed retractions have quadrupled over the last 20 years, mostly attributed to data falsification and other forms of clear misconduct.4
And since we are already talking about Alzheimer’s, a U.S. grand jury just indicted Hoau-Yan Wang, PhD, a tenured professor at CUNY Medical School in NYC, on felony charges of defrauding the National Institutes of Health (NIH) of not less than $16 million in grants by faking data in grant applications.5 Considering that, obviously, only a small minority of such misdeeds are ultimately discovered, how much must there exist!
Integrity concerns about NIH research have, indeed, been surging since 2018, when NIH started tracking. Science magazine in the June 14 issue published a graph of the number of allegations ranging from harassment to research misconduct, peer review,
grant fraud, and others.6 We also recommend a quick look at the last published Science Integrity Digest of June 2024, which well reflects the mess we are in.7
1. Ro C. Nature 2024;631:921-923
2. Subbaraman N. Wall Street Journal 2024; June 15, 2024. P1A ; https://www.realclearscience.com/2024/05/15 /flood_ of_ fake_science_forces_multiple_journal_closures_1031624.html
3. Piller C. Science 2024; 384 (6700):1055
4. Else H. Nature 2024;630:280-281
5. Brainard J. Science 2024;385(6704):8
6. Science 2024; 384(6701):1157
7. Eliesbic. Science Integrity Digest, August 1, 2024. https://scienceintegritydigest. com/2024/08/01/science-integrity-digest-june-2024/
There is no business without legal business
... equal rights for LGBTQ+ when it comes to infertility insurance
As Ron Shinkman reported in Griffin Jones’ e-mails of May 23, 2024, Aetna, owned by CVS Health, got caught discriminating against LGBTQ+ enrollees in the way the insurance company offers coverage for same-sex and heterozygous couples. As we already noted elsewhere in this issue, Aetna required that–mimicking coverage rules for heterozygous couples who under a certain age are mandated to try for at least one year to conceive on their own spontaneously, same sex couples were required by Aetna–at their own expense–to have 12 consecutive insemination (IUI) cycles before becoming eligible for coverage with IVF. As reported by Associated Press, plaintiffs were represented in this class action lawsuit by the National Women’s Law Center. 1
In a settlement, the insurance company, Aetna, will make coverage for IUI standard for all insured and will also make sure IVF access is equal for everybody including members of the LGBTQ+ community. The principal plaintiff in the case was a same-sex female couple that claimed $50,000 in IUI out of pocket costs who expect to

get reimbursed.
... it’s not worth interfering with patient reviews
And a plastic surgeon in Washington State was found guilty in federal court and fined $5 million for breaking federal law and the Consumer Review Fairness Act (CRFA) because he required all of his patients to sign nondisclosure agreements before giving them any treatment. In some cases, the clinic threatened litigation after poor reviews, while in others the clinic offered free services or payments in exchange for removing reviews. They then, however, were required to sign agreements that implicitly forbade the posting of future negative reviews and threatening a $250,000 penalty in case of breach of the agreement. He, moreover, also faked positive reviews to compensate for the bad ones, and claimed that the whole lawsuit was instigated by a competitor.
... medicine also requires protecting whistleblowers
We have addressed elsewhere how differently Europe and the U.S. have come to regulate transgender medicine in children and young adults. As the New York Post reported on June 10, 2024, what has been happening at Texas Children’s Hospital reemphasizes this discrepancy:3 this hospital has been continuing treatment of minors with puberty blockers and other medical interventions, including surgeries, who self-identified as “trans.” As public pressure increased–especially after the elsewhere described British report was published–the CEO of the hospital announced that he was shutting down its “Child Gender Clinic”, though that never seemed to happen, as the doctors of the clinic continued treatments, including surgeries of children as young as one year old, as if nothing had happened.
A surgical resident, Eithan Haim, MD, felt morally obliged to make this charade known and spoke to a local
reporter about it. But instead of receiving applause, the Justice Department is threatening him with prosecution for breaking HIPAA rules. Amazing what the Justice Department does find time for, when so much more important things should be investigated and prosecuted.
1. The Associated Press , May 3, 2024. https:// www.nbcnews.com/nbc-out/out-news/ aetna-agrees-settle-lawsuit-fertility-coverage-lgbtq-customers-rcna150658
2. Washington State, office of the Attorney General. April 15, 2024. https://www.atg.wa.gov/ news/news-releases/federal-judge-rules-plastic-surgeon-acted-illegally-restricting-online-reviews
3. New York Post, June 10, 2024. p25
... quo vadis ACOG?
It seems that everybody these days not only has an opinion about everything, but also wants his/her opinions to rule the world: ACOG, once a proud representative of all of the OB/GYN brotherhood, has turned into a zealous proponent of radically liberal ideas, for example the idea that residency programs should now teach obviously liberal social science concepts, like DEI. How else is it explainable that ACOG sent out on April 1, 2024 (not a joke!) a statement from Verda J. Hicks, MD, President of the American College of Obstetricians and Gynecologists, stating that ACOG opposes legislative efforts to erode DEI inclusion initiatives in medical training. How absurd; who says so, for what purpose, and who decided that this is the opinion of the College?
And then on June 3, 2024, members of ACOG received an e-mail form ACOG’s Collective Action against Racism (whatever address within ACOG this may be) inviting registration for DEI Community Events on June 17 and June 24. ACOG very obviously has a new center of gravity!
And who would have guessed that ACOG has a Collective Action Strategy for transforming the culture of medicine. Doesn’t ACOG even have a difficult time serving its membership with such basics as decent journals with respectable impact factors, or annual meetings that anybody would be really interested to attend.
... 8 hours of CME for federal narcotic prescribing licenses?
Yes, this is what the federal government apparently requires if a physician wants to renew her/his prescribing licenses and it is not even free; there is $888 charge for each license, according to a Commentary on Medscape by Melissa Walton-Shirley, MD. 1 We can thank the Medication Access and Training Expansion Act (MATE) for this wonderful addition to our medical life. We, however, can also thank our federal bureaucracy which for many years has allowed “big pharma” to lie to the medical community about the narcotics the companies were pushing into the marketplace. Where was the FDA in these cases?
As we already noted above, it has at times become difficult in medicine to distinguish between real and fake news. But it is easy to imply that it was all the doctor’s fault that the country experienced an iatrogenic drug crisis and let them, therefore, waste eight hours of their valuable time on learning how to administer Narcan, as Walton-Shirley correctly stated. How crazy and how great that fertility patients don’t usually need narcotics.
1. Walton-Shirley. Medscape. June 13, 2024. https://www.medscape.com/ viewarticle/dea-training-mandate-8hou...oexpansion-algo_20240622_eetid6615686&uac=223637CN&impID=6615686



Norbert Gleicher, MD, reappointed to an Adjunct Professorship at the Medical University of Vienna, Austria
Norbert Gleicher, MD, the CHR’s Medical Director and Chief Scientist, was on May 13, 2024, notified that, by recommendation of the university’s Department of Obstetrics and Gynecology, he was reappointed at the Medical University of Vienna in Vienna, Austria, as Adjunct Professor.
Dr. Gleicher started his medical studies at the Medical University of Vienna in 1966 but switched after the preclinical years to the medical school of Tel Aviv University in Israel, where he graduated in 1973 and earned his medical degree after a year of internship and completion of a thesis in 1974.
He has held this appointment, which mandates renewal every three years, for several decades. The reappointment letter noted that “this award is dedicated to you personally and reflects our sincere appreciation of your professional achievements and of the mutual and successful cooperation in research between our institutions. We would like to take this opportunity to congratulate you for your outstanding work and for enhancing the close collaboration between your institution and our Department of Obstetrics and Gynecology at Medical University of Vienna. ”
The President of the Republic of Austria in 1991 also awarded Dr. Gleicher the Ehrenkreutz für Wissenschaft und Kunst (the Honorary Cross for Science and the Arts), the highest civilian decoration of the Republic of Austria.


Medical students – free attendance
Residents, fellows, postdocs – discounted attendance
Obstetricians & Gynecologists
Endocrinologists & Infertility Specialists
Reproductive Biologists - discounted attendance
Embryologists - discounted attendance
Geneticists
Ethicists
Fertility nurses and nurse practitioners - discounted attendance
Sonographers - discounted attendance
Science journalists – free attendance
Investors & administrators in the field
Industry interested in the field
Lawyers interested in the field
A Not-For-Profit Research Foundation www.foundationforreprodmed.com
The Center for Human Reproduction (CHR)
An International Fertility and Research Center www.centerforhumanreprod.com
(i) To demonstrate to clinicians in the field what ongoing bench research can contribute to better understanding of physiological processes relevant to clinical practice.
(ii) To demonstrate to basic scientists in the field what the basic knowledge needs of clinicians are.
(iii)To learn to “think differently” by critically assessing current clinical practice patterns in attempts to improve clinical outcomes
PROGRAM OBJECTIVES
• To present most up-to-date basic science as well as clinical knowledge to lead to a better understanding between basic scientists and clinicians in reproductive medicine.
• To demonstrate how improved cooperation between basic science and clinical medicine can improve patient care.
• To outline an alternative thinking pattern that allows for the critical evaluation of clinical practice patterns in their respective effectiveness.
The conference will award up to 20 CME credits.
A Not-For-Profit Foundation
CONFERENCE ADMINISTRATION
Trebron Management, Inc. New York, N.Y. ComtecMED Medical Congresses, Israel
21 East 69th Street, New York, N.Y 10021; T.646.882 0840, F.212.988 0250
CONFERENCE HOTELS – all within 2 blocks of the conference venue
HOTEL NAME STARS
Club Quarters Boutique Hotel, ***
ADDRESS
COMMENTS
128 East 45th Street Closest to venue Grand Central
The Lexington Hotel, Autograph Collection ****
511 Lexington Avenue/48th Headquarter hotel by Marriott
Fitzpatrick Hotel Grand Central ****
InterContinental New York Barclay
Building on the success of the 2023 conference, the FRMC will present over three conference days a unique program in reproductive medicine, connecting in a single lecture hall between basic science and clinical practice, in the process facilitating translational connections between bench and clinic. As in preceding years, the principal purpose is demonstrating to clinicians what is possible and to scientists what is needed. The intent is not to dream about the future, but to demonstrate what can be achieved now.
“To think differently” has been the principal motto of the FRMC since its establishment in 2015, as questioning mainstream thinking and premiering new treatment paradigms has been at the core of the conference’s success. The conference content always evolves over the preceding year, as new findings in basic science and clinical practice are reported. Since the embryo possesses universal information about human biology, the earliest stages of embryo development, together with other big themes, always occupy an important place on the program. This includes reproductive genetics, reproductive aging, widely overlooked reproductive immunology and, this year for the first time, the utilization of GLP-1 agonists in the treatment of obesity, which now allows serious consideration of obesity as a treatable infertility diagnosis. With societal controversies more than in any other medical specialty area accompanying infertility practice since the inception of IVF, ethics will again feature prominently in discussion, further enhanced by the dramatic changes in clinical infertility practice caused by changes from physician ownership of fertility clinics to mostly investor-owned clinics and clinic networks and the resulting industrialization and commoditization of the field.
141 East 44th St./Lex. Ave.
111 East 48th St./Lex Ave.
The FMRC can proudly note to have pointed out new treatment paradigms as well as ineffective and, at times, harmful practice patterns long before other conferences and authoritative bodies, the most important likely being preimplantation genetic testing for aneuploidy (PGT-A), only this year by the ASRM, finally, formally described as “not able to improve IVF outcomes”–a conclusion the FRMC already offered to its audience in 2015 (and ever since). The conference, however, does not only address clinical controversies relevant to reproductive medicine. A subject repeatedly addressed in prior years, and again finding attention this year, is the publication crisis in medicine, reflected in exploding numbers of paper retractions, even in eminent journals, involving very prominent individuals. Related is also the recognition that what is considered evidence-based medicine must be accompanied in parallel by “real world data” studies, as carefully planned prospectively randomized studies can never fully reflect what happened in “real-world” applications of treatments.
Following only a few short days after the annual lighting ceremony of New York City’s famous Christmas tree at Rockefeller Center (page 38), the 2024 FRMC not only offers the most interesting annual conference in reproductive medicine, but holds the conference at the most exciting period of the year to visit New York City. We very much are looking forward to welcoming you to what at that time of the year is a city of lights that never sleeps.
-The Conference Chairs on behalf of the sponsoring organizations






The importance of recognizing that infertility often is multifactorial and must be treated as such
· Attempting to offer a teachable moment, we present in this section of the VOICE an anonymized case report from the files of the CHR.
· This month’s case is what, under Rotterdam criteria, is described as the D-phenotype of PCOS, also called the “lean” PCOS phenotype because patients do not demonstrate the usual truncal obesity of PCOS patients with other phenotypes. This phenotype also–at least at more advanced ages–is ovulatory and, therefore, in contrast to other phenotypes, presents with a history of regular menses.
· The literature also describes it as the only non-hyperandrogenic (or only normo-androgenic) phenotype, with all other phenotypes being hyper-androgenic. Here the Rotterdam criteria, however, erred since between menarche and approximately age 25 the D-phenotype is also hyper-androgenic. In contrast to phenotypes A, B, and C, it, however, does not remain hyper-androgenic.
· Instead - because of declining adrenal androgen production – it demonstrates steadily declining androgen levels. At approximate age 35, after roughly a 10-years in normal (and declining) androgen range, these women, however, enter hypo-androgenic ranges. Between ages 25 and 35, however, ca. 90% of PCOS diagnoses are made. Rotterdam criteria, therefore, erred in describing PCOS-D as normo-androgenic. It in reality is the only phenotype going with advancing age from hyper- to hypo-androgenic.
· And because ovaries need normal androgen levels to make good eggs, so-affected women in parallel go from being fertile to being infertile, while often being undiagnosed as PCOS patients and frequently considered “unexplained.”
· And in parallel, these women’s immune systems demonstrate increasing evidence of hyper-activity, increasing the risk of miscarriage, even if they do conceive.
· Successful treatment of PCOS-D patients after age 35, therefore, in most cases requires androgen supplementation and appropriate immune suppression.
· But recognizing all of all of these very important facts regarding the patient’s PCOS diagnosis very likely would not have resulted in successful treatment, had important comorbidities not been also diagnosed and treated in parallel. Infertility is frequently a multifactorial condition which can be treated successfully only if all co-morbidities are therapeutically addressed at the same time, - as this case so well demonstrates.
CONFLICT STATEMENT: The CHR and some of its employees were awarded certain U.S. patents claiming treatment benefits from supplementing hypo-androgenic infertile women with the male hormone DHEA. The CHR and these employees, therefore, receive license fees from the use of DHEA in hypo-androgenic infertile women. Readers of this article should be aware of this fact since it may bias opinion expressed by the authors of this article regarding androgen supplementation in hypo-androgenic infertile women.


A 37-year-old G3P0 presented to the CHR for the first time through a Second Opinion Internet Consultation request. She had been in infertility treatment at four different clinics since age 30, when she first consulted a fertility clinic after having experienced two consecutive spontaneous miscarriages, one a blighted ovum, and the second a loss of fetal heart at eight weeks gestational age. Karyotyping of this second pregnancy revealed a chromosomal normal 46, XY pregnancy and, therefore, suggested the possibility of a non-chromosomal cause for repeated pregnancy loss.
Upon initial presentation to the first fertility clinic, her FSH was 8.9 mIU/mL and her AMH was 5.8ng/mL. Her BMI was 22.4 and her menses was regular every 26-28 days. Her past medical history was otherwise basically negative except for intermittent outbreaks of eczema, mostly in the face and hands.
Though her general gynecologist at age 21 had once noted that she may be a Polycystic Ovary Syndrome (PCOS) patient (she did not remember what this diagnosis was based on), all four fertility clinics she later attended rejected the diagnosis, despite recorded AMH values between 4.8 and 6.5 ng/mL. When repeatedly raising the possibility with her physicians during her treatments, she was always told that she could not be a PCOS patient since she had such a low BMI and, on top of it, a regular ovulatory menses pattern. Moreover, she demonstrated no evidence of hyper-androgenism in either blood tests or phenotypical appearance.
Prior to presenting to the CHR, she for seven years received infertility treatment at the above-noted four different fertility clinics in the Middle East and Africa, starting with an unclear number of natural and medicated intrauterine insemination cycles, followed by at least eight IVF cycles under different stimulation protocols (there could have been more cycles), from so-called “mini-IVF” cycles to regular stimulated cycles. Three “mini-IVF” cycles yielded between seven and 15 oocytes, while cycles under regular stimulation protocols yielded between 23 and 34 oocytes. Because of changes back and forth between these four clinics and constant protocol changes, trends were difficult to discern, but oocyte yield appeared to decline with advancing age. Moreover, despite obviously large egg numbers, embryo yield at blastocyst-stage in all cycles were comparatively small and embryo quality was poor, with most embryos reaching blastocyst-stage only on days six and seven after fertilization. Two out of the four clinics at some stages recommended donor eggs, an option the patient was not open to for religious reasons.
In all above-noted treatment cycles only one positive pregnancy test was obtained at age 33. Because the pregnancy was a blighted ovum, products of conception did not undergo karyotyping after evacuation. The patient presented to the CHR with a request for an electronic Second Opinion Consultation after her last unsuccessful IVF cycle at age 37.
Based on the above-noted history, the patient received a written Second Opinion from the CHR, suggesting that her principal diagnosis of PCOS-phenotype D had, most likely, been overlooked in her prior fertility treatments. Because of several obvious missing data in the patient’s submitted past medical history, the CHR’s opinion, moreover, suggested a virtual follow-up consultation (the patient resided outside of the U.S.) before further and more detailed testing of the patient would be pursued.
The patient, however, preferred a face-to-face meeting and within two weeks traveled to NYC for an in-person consultation. This also allowed for a relatively quick and comprehensive follow-up investigation of the patient which fully confirmed the suspicion of phenotype-D PCOS, now, however, already in a severely hypo-androgenic phase and, therefore, by the CHR described in the literature as Hyper-Hypo-androgenic PCOS (HH-PCOS).1,2 Free and total testosterone levels were very low and her sex-hormone binding globulin (SHBG)–which usually goes in the opposite direction to testosterone–was abnormally elevated.



Unsurprisingly, considering her very low testosterone, the patient upon query also reported low energy levels and extremely low libido. A more detailed family history furthermore revealed several first- and second-degree relatives with autoimmune diseases, another typical finding in HH-PCOS patients who, themselves, at that point in ca. 85% of cases demonstrate evidence of a hyperactive immune system.
That was, indeed, also the case, as the female demonstrated evidence of inflammation with an elevated C-reactive protein (CRP) and elevated sedimentation rate (ESR), a positive ANA at a titer of 1:160 – speckled, but no outright obvious clinical symptomatology for an autoimmune condition. She also demonstrated gammopathies in total IgA and IgE levels, in both cases excessively high levels, though, except for her lifelong eczema outbreaks noted above, she reported no evidence for specific allergies. Laboratory findings supporting a hyperactive immune system, moreover, also added evidence to the suspicion that her three miscarriages were immunologically induced and presented a recurrence risk for any future pregnancy that also had to be addressed. In other words, this patient needed the CHR’s help not only to conceive but, likely, also to maintain her pregnancy.
As a somewhat unexpected finding, the patient was in her work-up also found to have unusually low insulin growth factor-1 (IGF-1) levels. Like androgens, IGF-1 works synergistically with follicle stimulating hormone (FSH) in supporting follicle growth and development. Like androgens, normal IGF-1 levels, therefore, are important for good egg quality. Since IGF-1 is induced by human growth hormone (HGH), this meant that in addition to androgen supplementation (with 25mg DHEA, TID), this patient also had to be pre-supplemented for 6-8 weeks with HGH.
Moreover, because the patient demonstrated clear evidence for excessive inflammation in her body–and especially in absence of obesity that often can be the cause of such inflammation–the patient underwent an endometrial biopsy to rule out chronic endometritis. The biopsy was, indeed, positive and did not correct after two weeks of intensive antibiotic treatment, leading to the conclusion that her endometritis was, likely, inflammatory in nature.
Treatment and results
Because of her inflammatory findings, the patient was placed on Plaquenil 200mg BID (for at least 4 weeks) before IVF cycle start.
Conclusions
• Especially young POA patients are too often–in this case by three IVF clinics–prematurely referred to third-party egg donation.
• As this case of even spontaneous pregnancy after androgen supplementation well demonstrates, their ability to still achieve pregnancy with autologous oocytes is at most fertility clinics greatly underestimated.
• To maximize pregnancy and live birth chances, such patients must, however, receive individualized precision treatment, involving specific preparation of ovaries for at least 6-8 weeks with androgens and, on rarer occasions, GH. proper individualized ovarian stimulation, and potential prevention of miscarriages if such a risk is diagnosed based on evidence of a hyper-active immune system.
• Because of the patient’s spontaneous conception, this cased did not offer the opportunity also to discuss HIER (highly individualized egg retrieval) which refers to individualized earlier ovulation trigger at smaller lead follicle sizes7 and embryology management8 based on ovarian age.
• Pregnancy and live birth chances, of course, decline with advancing age of the female.
REFERENCES
1. Gleicher et al., Hum Reprod 2013;28(4):1084-1091
“Dr Gleicher is a king, he is on the highest level of experience and knowledge. I met some IVF doctors before and no one came even close to his professionalism and wisdom. This generation of doctors are the real doctors, that know IVF inside out. And fight for their patients and not treat them like numbers like most others in that industry. They want the best outcome and actually investigate your issue based on their vast knowledge, almost like a detective. I am so thankful I met him.”
“The whole staff is attentive, supportive, and vested in a positive outcome for their patients. So glad I switched from my local clinic... it has still been a tough journey, but worth it!”


"Hi Dr Barad and all CHR staff,
Our beautiful baby girl was born on July 29, 2024 after 3 years of IVF and 7 transfers.
Thank you for making our family complete."



P r e s e n t :
G R A N D R O U N D S

SPEAKER: SHUO XIAO, PH.D. D
e a r n e d h i s M B B S i n P r e v e n t i v e M e d i c i n e a n d M S i n T o x i c o l o g y f r o m
P e k i n g U n i v e r s i t y , f o l l o w e d b y a P h D i n F e m a l e R e p r o d u c t i v e B i o l o g y a n d
T o x i c o l o g y f r o m U n i v e r s i t y o f G e o r g i a . H e c o m p l e t e d h i s P o s t d o c t o r a l
T r a i n i n g
Norbert Gleicher, MD Medical Director and Chief Scientist at The CHR
This opinion piece addresses the excessive enthusiasm in medicine on so-called evidence-based medicine (EBM) in both affirming an/or refuting medical treatments. This does not mean that EBM cannot be included in assessments of medical practice; its should just not be a dominant component. As EBM, itself, acknowledges that physician knowledge and expertise, ultimately, must be part of the EBM process, so does this article argue that, ultimately nothing (except, sometimes in the future, possibly, A.I., and even that is questionable), can replace the final judgment of the well-trained/educated physician. At the same time, knowledge must not be equated with “truth” because knowledge advances at ever quickening pace, while “truth” has historically, of course, always been viewed as static and unchangeable. For physicians it, therefore, appears essential to understand that the existing evidence for “something” always remains a moving target. Colleagues who regarding any subject in medicine believe to know what represents “absolute truth,” therefore, must always be challenged. Unless a physician/scientist in the back of her/his brain maintains a degree of self-doubt about any of her/his believes, she/he, indeed, should not even trust their own judgments.
“Evidence suggests serving as proof of the actuality or existence of something” -Merriam-Webster Dictionary
Prologue


Though it had been for centuries a widely used word in the English language, in medicine the word “evidence” achieved prominence only after Gordon Guyatt, MD, MSc, a Canadian physician and Professor at McMaster University in Hamilton, Canada, first in a lecture in April of 1991, and shortly thereafter (with 31 co-authors, - the Evidence-Based Medicine Working Group) proposed the term Evidence Based Medicine (EBM) in a paper in JAMA under the title, “EBM, a new approach to teaching the practice of medicine.”1 He and co-authors with this paper initiated a cultural as well as scientific revolution in medicine. The principal idea behind the new concept of EBM was well explained in the first paragraph of their paper:
“A new paradigm for medical practice is emerging. Evidence-based medicine de-emphasizes intuition, unsystematic clinical experience, and pathophysiologic rationale as sufficient grounds for clinical decision making and stresses the examination of evidence from clinical research. Evidence-based medicine requires new skills of the physician, including efficient literature searching and the application of formal rules of evidence evaluating the clinical literature.”

A much more recently electronically published book chapter defined EBM like this:2
“It uses the scientific method to organize and apply current data to improve healthcare decisions, combining the best available science with the healthcare professional’s clinical experience and patients’ values to arrive at the best medical decisions for patients.”
How well is this practically accomplished?
So how is all of this accomplished? In principle, in five steps: (i) One establishes a clinically useful question to either prove or disprove a currently existing practice pattern. (ii) Now starts a (literature) search for the best evidence available in the medical literature. (iii) This is followed by a critical appraisal of this best evidence. (iv) Once conclusions are reached from best evidence, they are clinically applied; and, finally, (v) the performance of this application of EBM is validated.
From these five points it will be apparent that conducting an EBM-based study requires a complex effort. The question that EBM is expected to answer, therefore, should be worth the effort. In practical terms this means that the study–if an unequivocal answer is reached–should have real outcome consequences. Unfortunately, nowadays this is the case in only a small minority of EBM publications. Many–and possibly by now most–published systematic reviews (of the literature) and meta-analyses [stages (ii) and (iii)], unfortunately, are basically worthless. They are either self-fulfilling prophecies (questions with only too obvious answers) or questions which, as the review of existing literature [stage (ii)] already very obviously reveals, simply cannot be answered with reasonable certainty. Consequently, more often than not, conclusions of such EBM-based publications include the phrase (or similar), “there is currently insufficient evidence to reach conclusions (regarding the raised question); consequently, further studies are required.”
Such EBM-based studies, nevertheless, have been growing exponentially in numbers in almost all medical journals but can be found in especially high concentrations in many potentially predatory open-access journals,3 often produced by Chinese (or other) paper mills.
Evidence in medicine is widely stratified into 4 levels, with two of those being stratified into A and B sub-levels (see Table 1). Though the table is mostly self-explanatory, a few comments, nevertheless, appear of importance at both ends of the table: That prospectively randomized clinical trials (PRCTs) represent the gold standard of clinical evidence is widely accepted. This acceptance, however, over the years achieved followers with almost religious zeal, whenever the phrase PRCT appeared in the materials and methods section of a paper. What, however, very often is widely overlooked is the fact that many PRCTs are poorly designed and, therefore, can produce biased outcomes. Their negative effects on medicine then, however, will be even more pronounced than those from poor-quality lower-evidence level studies, because of the almost cult-like and uncritical admiration practically almost all PRCTs these days receive in medicine.
Table 1. The levels of evidence
• Level IA: Evidence from a meta-analysis of multiple, well-conducted, and well-designed randomized trials. Randomized trials in general offer the strongest clinical evidence of any study format, and if repeated and combined in a meta-analysis, results are assumed to be even stronger.
• Level IB: Evidence obtained from only a single well-conducted and well-designed randomized controlled trial. When well-designed and well-conducted, a randomized study is a gold standard for clinical medicine.
• Level IIA: Evidence from at least one well-designed and executed non-randomized controlled study. Without randomization, there may be more bias in the study.
• Level IIB: Evidence from at least one well-designed case-control or cohort study.
• Level III: Evidence from at least one non-experimental study, typically case series or not well-designed case-control or cohort studies.
• Level IV: Expert opinions from respected authorities on the subject based on their clinical experience.

Misleading PRCTs, therefore, exist in abundance. A good example in reproductive medicine were, for example, PRCTs regarding preimplantation genetic testing for aneuploidy (PGT-A), almost unopposed for several years successfully claiming outcome advantages in IVF after randomizing patients only if at least one embryo reached blastocyst-stage. This patient selection criterion, of course, to highly significant degrees biased patient selection of women who were allowed to participate in the study and, therefore, falsely skewed results in favor of PGT-A utilization.4 Nevertheless, such studies to this day are frequently cited in support of PGT-A utilization.
At the other end of evidence levels–denoting the lowest level of evidence–rests “expert opinions from respected authorities.” And that, of course, is one of the principal paradoxes of EBM because the concept then mandates that clinicians use their professional and clinical experience to extrapolate the scientific evidence from an EBM study, as it applies to a specific patient [(iv) and (v)].2 And this is, of course, where the lowest evidence level is reintroduced into the concept of EBM, with expert opinion, indeed, coming back into the picture as the dominant force in reaching a diagnosis, whether in the form of a teacher, a department chair, department policy, an editorial in a prominent medical journal by an “expert,” etc.
In many ways EBM, therefore, is nothing but a vicious circle returning upon itself, with expert opinion–the lowest level of evidence–still dominating the clinical decision-making process. This conclusion is, moreover, even strengthened by the fact that it is also expert opinion that selects and classifies the level of evidence of studies for EBM in stages (ii) and (iii).
How severely selection biases can affect outcomes was recently well demonstrated in another, maybe even more important sphere of data selection, namely the artificial intelligence (A.I.) field. Google’s disastrous Gemini A.I. launch, widely attributed to its “woke” image generator (i.e., image selections on which their A.I. program was based) is, of course, a good example why several American presidents were misidentified as African American and why many other rather bizarre results were produced by the program.5
In that sense, EBM shares with A.I. the vulnerability of being dependent on the data input upon which each system is built. If the literature selection or interpretation and/or classification of published papers is biased, results of the EBM process will, of course, be biased as well. Much too often systematic reviews (with associated meta-analysis) in published papers were very obviously based on biased literature selection and/or analysis/classification. Motivations, of course, can vary from simply being ignorant about how literature should be selected to “wokeism” in attempts to prove and/or reconfirm an at the moment popular clinical treatment and/or opinion. And, yes, as the COVID pandemic better than any other recent medical event demonstrated that opinions can be “woke,” not only in politics but also in medicine.
Consequently, in EBM the old IBM-dictum “garbage in, garbage out” fully applies and one often wonders how much better time, energy, and resources could be spent on other than clinically completely useless EBM-driven study efforts that have been increasingly flooding many of our medical journals.
Concerns about the concept of EBM are, of course, nothing new, and the literature has, indeed, selectively addressed most of them, including publication- and PRCT-biases.2 But, interestingly, basically nothing until very recently, was able to break the cultlike believe that EBM, alone, if only properly and aggressively enough applied, will offer the ultimate truth on almost any subject in clinical medicine.
More recently, however, for in principle two reasons, this dam starts showing some cracks: A first reason is that, mostly for ethical reasons but also at times for cost reasons, some questions simply cannot be addressed by PRCTs. And a second somewhat paradoxical reason is that the more accurately a study population is selected–excluding patients if they even in the slightest deviate from the study protocol–the less representative will such a study’s results be for the tested treatment in an unselected “real-world” patient population.

The paradox, in other words here is, that the more disciplined patient selection was in an initial PRCT leading to approval of a drug by the Food and Drug Administration (FDA), the more will the study’s results vary when this drug, after FDA approval, is used in relatively less-stringently selected patient populations.
The FDA understood this problem and, therefore, introduced post-marketing study requirements for many drugs as part of their approval process; but the same principle also applies to non-drug related PRCTs: The “real world” application of a treatment will practically never completely reflect outcomes obtained by even well-designed PRCTs. It, therefore, is high time to view even well-designed PRCTs as imperfect in predicting a treatment’s efficacy accurately in routine clinical practice. With this conclusion as background, one, therefore, cannot be surprised about the recent ascent in all of medicine in the importance of so-called “real-world” studies.6
Which brings us to the ultimate goal of creating evidence which by many in medicine is understood as determining the ultimate “truth” about “something.” But, as we by now all understand, medical evidence is anything but static. This is one reason why textbooks have lost most of their luster because by the time they appear in print, half of their content is outdated. So, how come so many among us still equate best evidence with “truth”?
The answer is simple: nobody to this day knows how to define “truth,” as various theories and views continue to be debated among scholars, philosophers and theologians. Based on our theological as well as philosophical upbringing, we, however, believe to know what it is not: namely, something that changes. And since best evidence always changes, how can any evidence then be considered “truth.” In other words, maybe one can define best evidence as “truth-of-the-moment,” but it very obviously cannot be viewed as “truth – forever.”
I, therefore, always admire the self-confidence of colleagues who in a debate are able to project absolute certainty about an argument, while in the back of my own brain–even if I feel certain in my conclusions, there always remains that nagging little doubt that, what I consider to be the “truth-of-the-moment” or best-evidence, may already be outdated and replaced by something new. Though I do secretly admire colleagues for their ability to believe in “absolute truth” (they must enjoy more restful nights), this kind of absolute certainty is at the same time quite disturbing and intellectually difficult to trust. I, indeed, would argue that these colleagues would be well-advised to consider starting to distrust themselves at least a little bit in their own judgments, if they really want to offer their patients good medical care.
REFERENCES
1. Guyatt et al., JAMA 1992;268(17):2420-2425
2. Tenny S, Varacallo M. October 24, 2022; https://www.ncbi.nlm.nih.gov/books/NBKA70182/
3. https://beallslist.net/
4. Tiegs et al., Fertil Steril 2021;115(3):627-636
5. Gilbert D. WIRED. February 22, 2024. https://www.wired.com/story/google-gemini-woke-ai-image-generation/
6. Blonde et al., Adv. Ther 2018;35(1):1763-1774
BRIEFING
In this still relatively new section of the VOICE, we are trying to inform about the importance of food for general health and reproduction, with a little bit of lighter fare dispersed between pages. Food can have positive effects, at times even acting like a medicine, or do the opposite and harm the body. And food is, of course, closely linked to the subject of obesity, a condition in women and men closely associated with infertility and lowered success rates for fertility treatments. With the availability of ever more-effective GLP-1 drugs for the treatment of obesity, weight loss with this family of drugs has, therefore, assumed the role of a new fertility treatment. GLP-1 drugs in this sense can also be understood as the first substantial new family of drugs the pharma industry has provided to the infertility field in decades. GLP-1 drugs in this sense can also be understood as the first substantial new family of drugs the pharma industry has provided to the infertility field in decades.
Since postmenopausal women are constantly trying to convince us that postmenopausal hormone replacement may keep them slim and/or prevent them from gaining weight, it appears time to address this issue. Surprisingly, there is relatively little literature on this subject and we, therefore, will refer to an article published on the subject not too long ago in the JCEM by longtime CHR friend, Nanette F. Santoro MD.1

ogy at the University of Colorado Anschutz Medical Campus will be a faculty member at the 2024 FRM Conference (FRMC) in NYC between December 6 and 8, talking about the effects of weight loss on female and male infertility, a big subject at this year’s conference.

Basically to model the concept of elective bilateral oophorectomy, she addressed in the article a study of women who were planning cancer risk-reducing oophorectomy because of genetic predisposition and compared them to matched controls who retained their ovaries. After two-years of follow-up, there was no weight difference detectable between the two groups. But an interesting and unexpected observation threw a potential wrench into the conclusion because women who underwent elective bilateral oophorectomy demonstrated greater regional fat accrual in the abdominal visceral compartment and these findings were unaffected by whether women received hormone replacement therapy or not.
On first impression, the long standing habit in gynecology of, once a woman is in menopause, taking out her “no-longer useful “ovaries,” if she for unrelated reasons already is undergoing abdominal surgery (“since we are already there,” – as the saying goes), at least within two years of follow up does not appear to induce weight gain. But what about longer follow-up. Because one, of course, cannot be certain that, with longer follow-up, weight may not be gained because accumulating abdominal fat may, ultimately, be a factor in weight gain. Moreover, women like beer-bellies even less than men.
And on a side note, Nanette Santoro, MD, Professor and E. Stewart Taylor Chair, Division of Reproductive Endocrinology & Reproductive Sciences, Department of Obstetrics and Gynecol-
And if we already are talking about myths, Eric Topol, MD, who we are routinely citing because of his EXCELLENT substack, GROUND TRUTHS, on June 26 interviewed Christopher Labos, MD, a cardiologist and epidemiologist at McGill University in Montreal, Canada, who recently published a very successful (and funny) book with the title, “Does Coffee Cause Cancer? And 8 More Myths about the Food We Eat.”2 We are here quoting from the interview.
The eight questions the title refers to are listed below and not only reflect the author’s refreshing humor but to a significant degree reveal our ignorance about what we routinely ingest day after day. If you want to get the information, order the book ($24.95 CAD); but if you are interested in two brilliant minds discussing our myths regarding the food we are eating listen to Eric Topol’s podcast or read the transcript (both are free).
• Does vitamin C prevent the common cold? And if it works, why does it only work in Canadian soldiers, ultramarathon runners, and skiers?
• Was red meat really declared a carcinogen by the WHO? Does that mean I should become a vegetarian? And who decides what gets labeled as red meat and white meat?
• Is salt really not that bad for you and did a group of researchers really want to experiment on prisoners to prove the point?

• Does coffee cause cancer or heart attacks? Why did a California court say coffee needed a warning label?
• Is red wine really good for your heart, and what makes the French Paradox such a paradox?
• Why did the New England Journal of Medicine link eating chocolate with winning a Nobel Prize?
• Why were eggs once bad for you but now good for you again? Does that mean I don’t need to worry about cholesterol?
• Should I be taking vitamin D?
1. Santoro N. J Clin Endocriol Metab 2024;109:e858-e859
2. https://ecwpress.com/products/does-coffee-cause-cancer
... how did mankind’s foods evolve over time? This is a question a very interesting article by Kate Wong in Scientific America recently explored.1 Considering all the different diets nowadays being propagated by influencers, dieticians, and even physicians, claiming to reflect our evolutionary ancestry, this appears like a subject worth a little attention. And in contrast to what is widely claimed by some, it wasn’t meat where everything started. Hominis – what our oldest ancestors are called around 6 million years ago – indeed, like monkeys and apes, for the first half of our history exclusively lived on plants. First archaeological evidence for meat in the diet was found only ca. 3.4 million years ago but, as the article points out, that did not mean that our ancestors switched from plants to meat; they instead expanded their menu from plants to meat. As one scientist interviewed for the article noted: humans have always been omnivores.
Concluding her article the author has no specific diet recommendations to make but suggested that we should feel liberated to try different diets and find the one that “fits us best.” Maybe even more importantly, she recommends that when somebody states that there is only one correct way to eat, stop listening because that person is wrong.
... the puzzling association between obesity and disease
Everybody, of course, knows that obesity and disease are linked or, as we say in medicine, associated. Yet some skinny people can be very sick, and some very obese people can be very healthy. And this contradiction was the subject of another very interesting article in the same issue of Scientific America. 2 The first important point the article makes is that there apparently exists metabolically healthy obesity, defined as a BMI over 30, yet metabolically healthy and–one can argue–the healthy counterpart to the metabolic syndrome, as frequently before dis-
cussed in these pages a typical association for all PCOS patients except for phenotype D (under Rotterdam criteria).
And then there is the good (brown) and the bad (yellow) body fat, with healthy fat cells being smaller and unhealthy fat cells bigger, the latter also hosting more inflammatory immune cells. Consequently, yellow fat cells are much more inflammatory than brown fat cells. Research now involves the conversion of unhealthy into healthy fat cells. Moreover, location of fat cells in the body also appears to matter.
... and then there are, of course, GLP-1s
Under the heading, “Turning down the food noise,” Lauren J. Young offers one of the shortest and clearest descriptions of how GLP-1s works. While she does it with beautiful graphics, we here borrow just the pathways:3

Gut releases GLP-1 into bloodstream
GLP-1 binds to vagus
Person feels full
GLP-1 binds to nerve relaying signal receptors throughout to brain stem and may the brain - spreading also enter the brain appetite suppression directly in small amount signals
Neurons in brain stem (some also synthesize GLP-1) activate other areas of the brain controlling energy balance
Much more on GLP-1s below.
REFERENCES
1. Wong K. Scientific America 2024; July/August:23-27
2. Aschwangen C. Scientific America 2024; July/August: 29-35
3. Young LJ. Scientific America 2024; July/August: 37-41
... they are much more than just anti-obesity drugs
Common sense would suggest that any significant weight loss will be associated with secondary health benefits. Such an assumption follows pretty automatically from the long-known association of obesity with poor health. Removal of obesity from consideration, therefore, would be expected to lead to improvements in general health. But, as noted in the preceding section, not every obese person has metabolically bad obesity; some obese individuals, indeed, are metabolically very healthy, while

some very skinny people may be metabolically in quite poor shape. Some older data, indeed, have demonstrated that mortality in very low-weight individuals is greater than in heavier people.
In a study of 50,000 Canadian women, the skinniest women, with a BMI of less than 22.5, which included underweight as well as normal weight women, demonstrated a 44% higher risk of dying during approximately seven-years of follow-up, while at the other end of the spectrum, women with more than 38.7% total body fat had only a 19% higher death rates. had only a 19% higher death rates. For men with BMI below 23.8 the death rate in the follow-up period was 45% higher, whereas men in the highest body fat group (more than 36% body fat) had a 59% higher death rate.1
All of this, of course, does not negate beneficial effects of weight loss on general health, but demonstrates that weight, of course, is not the only determining factor of health and that–like almost every medical treatment–weight loss may also have negative effects. And this is exactly what makes new discoveries on how drugs like semaglutide and other GLP-1 receptor-agonists, affect not only weight, but also individual organ systems is of so much interest and, as a recent paper in Nature Medicine noted, why cardiologists and other clinicians must start familiarizing themselves with prescribing them. 2
So, what else beyond weight loss can we expect from these drugs? An excellent summary was recently offered in Science magazine:3 In it the author summarized GLP-1 effects the following way: GLP-1s directly activate T cells through their GLP-1 receptor (GLP-1R), thereby reducing inflammation. They, as noted above, at least partially indirectly, of course induce weight loss through PGP-1Rs in the brain, and–through loss of fat cells–weight loss also reduces inflammation. Consequently, there already exists evidence that GLP-1s reduce the risk of myocardial infarction, Atherosclerosis, diabetic kidney disease, and metabolic liver disease.
A reduction in inflammation, however, also benefits the brain. In other words, GLP-1s are also neuroprotective with already established declines in stroke risk and several studies underway to confirm preliminary suggestions that these drugs may also have beneficial effects on Parkinson’s disease and Alzheimer’s disease. And the same also applies to several neuropsychiatric diseases, including substance use disorders and compulsive behavior, including several additions beyond substance abuse. Yale University’s F. Perry Wilson, MSCE, MD, in his excellent weekly commentary, the Impact Factor, recently noted that data also demonstrated that the drug Ozempic (and other GLPs) not only reduced calories but also alcohol consumption, compulsive
shopping (any family members as treatment candidates?) and could be viewed as “fundamentally anti-consumption drugs in a society that may be considered plagued by overconsumption. A small study also suggested a potential beneficial effect on smoking caesation.4
A significant concern in using GLP-1s for weight loss has been that significant weight loss can induce muscle wasting, physical frailty and/or sarcopenia. A recent Viewpoint article in JAMA, however, did not support this notion based on so-far published data.5 That is good news, - but should not prevent - especially older people - from exercising their muscles.
Another concern with these drugs has been that, once stopped, much of the lost weight appears to return. A recent paper investigating long-term weight loss from semaglutide treatment, therefore, was timely.6 Weight loss continued over 65 weeks and was sustained for up to 4 years. By 208 weeks mean reduction in weight had fallen to a level of -10.2%, a waist circumference to -7.7cm a still highly significant level vs, placebo (P<0.0001). Whether and how GLP-1s should be discontinued to maintain maximum weight loss, however, still remains to be resolved.
Despite the resistance of the insurance industry, the use of GLP1s for weight loss has, of course, exploded. This was confirmed by a recent Research Letter in JAMA Health Forum investigating national U.S. data over all payment methods (commercial insurance, Medicaid, Medicare Part D, and cash), representing 92% of prescriptions for semaglutide (in form of 3 brands) in retail pharmacies between January 2021 and December 2023. 7 Interestingly, persistently, a majority of all such prescriptions were filled through commercial insurance.
This, of course, should not surprise, considering how expensive these drugs are. Unfortunately, those high prices have also given rise to a rapidly growing industry which offers the public a whole variety of generic GLP-1s, with often false claims of 100% mimicking the expensive brand names. Indeed, almost half of all online pharmacies have started selling weight loss drugs.8 Many, indeed, do so without requiring prescriptions or with virtual prescribers offering prescriptions through the seller. The Food and Drug Administration (FDA) and other international regulatory authorities, moreover, have warned the public about fake versions of these drugs.
Another Research Letter, this time in JAMA Network Open, therefore, was extremely timely, well describing the dangers of what is going on in this relatively new marketplace.9 The study

confirmed that semaglutide products are actively sold without prescriptions by, often, illegal online pharmacies. Products sold are often unregistered and/or fake. U.S. poison control centers, moreover, registered a 1500% increase in semaglutide-related calls.
This, of course, does not mean that there are not legitimate pharmacies, licensed and capable of compounding GLP-1s properly; but pharmacies willing to dispense these medications without prescriptions should be suspect.
In other words, obesity is a disease and requires medications, and these medications should be prescribed by licensed physicians and produced by licensed manufacturers or compounding pharmacies.
The original studies of GLP-1s, of course, go back decades and started with investigators trying to determine how peptides could help regulate glucose levels after food intake in diabetic patients. That these medications could also be helpful in fighting obesity came into focus only relatively recently. An article in Science magazine offers a detailed history on occasion of the awarding the Mani L. Bhaumik Breakthrough of the Year Award to Lotte Bjerre Knudsen (chief scientific adviser at Novo Nordisk) and Richard DiMarchi (Distinguished Professor of Biochemistry and Gill Chair in Biomolecular Sciences at Indiana University) for their role in ensuring that GLP-1R agonists were developed beyond diabetes.10 As the article noted, both awardees “stayed strong in the face of critics who doubted that obesity was disease at all.”
And, finally, a little bit of new science: At least with initial use, GLP-1s can cause side effects, including, on rare occasions, severe nausea. Investigators now discovered in a mouse model that the neurons that produce this sick feeling are distinct from those that–after food intake–provide the feeling of fullness.11 In the process, they also discovered that GLP-1Rs in the so-called hindbrain were responsible for the weight loss, though not for the nausea.
REFERENCES
1. https://www.cnn.com/2016/03/14/health/low-bmi-higher-death-rate/index.html#::text=The%20researchers%20found%20that%20the,%2Dyear%20follow%2Dup%20 period.
2. Sattar et al., Nat Med 2024;30:1830-1831
3. Drucker DJ. Science 2024;385(6706)”258-260
4. Wilson FP. Impact Factor, July 30, 2024. https://www.medscape.com/viewarticle/ Ozempic-curbs-hunger-and-not-just-food-2024a1000dw7?form=fpf
5. Conte et al., JAMA 2024;332(1):9-10
6. Ryan et al., Nat Med 2024;30:2049-2057
7. Scannell et al., JAMA Health Forum 2024;5(8):e242026
8. Szabo L. NBC News. August 2, 2024. https://www.nbcnews.com/health/healthnews/nearly-half-online-pharmacies-selling-weight-loss-drugs-are-operating-rcna164935
9. Reza Ashraf , et al., JAMA Network Open 2024;7(8):e2428280
10. Phelan M. Science 2024;384(6699):968970
11. Lenharo M. Nature 2024;631:493-494
... what fathers eat affects their sons’ metabolic health It is hard to believe, but, as a recent study in Nature magazine demonstrated in mice and humans, the food fathers ingest affects their male offsprings’ metabolic health.1 Certain types of RNA on sperm can be influenced by environmental factors and will influence the traits in male offspring, which can determine whether they will or will not develop metabolic disorders. So far, only the principles have been elucidated; how and what exactly the sperm does to affect an offspring’s health remains to be explained. But in the meantime, men had better watch what they eat (and probably drink) while they are trying to become fathers.
And if we already are talking again about diets, as part of its article series, “Nutrition in Medicine,” The New England Journal of Medicine just published a splendid review article on diets. It communicates everything important regarding dieting. An article we warmly recommend!
Another recent article we really liked was a comparison of a “personalized” dietary program versus general advice. Over 18 weeks, the personalized program used food characteristics, individual postprandial glucose levels and triglyceride responses to food microbiomes. Individuals with personalized protocol demonstrated significant reductions in triglycerides and health history to produce food scores. Controls received standard care and patients were randomized. 3
The results were as follows: triglycerides were reduced, but cholesterol was unchanged. Secondary outcomes showed only marginal improvements (P<0.05) in weight, waist circumference, HbA1c, diet quality, and microbiome, - but mostly only in highly adherent patients to protocol. No changes were noted in blood pressure, insulin, glucose, C-peptide, apolipoprotein A1 and B, and postprandial triglycerides. Though the authors claimed “some improvements in cardiometabolic health,” we have a hard time seeing them. Even highly rated journals like Nature Medicine unfortunately do, at times, publish garbage articles.
Those among us who love uni (the Japanese word for sea urchin) may soon experience withdrawal symptoms, since a mysterious sea urchin plague is rapidly spreading from ocean to ocean.4 Alarm bells started ringing in late 2022 when first reports became public that sea urchins had begun to die in the northern
terminus of the Gulf of Aqaba, in Jordan. By April all were dead.
As it turned out, this was not the first incident of this kind: In early 2022, reports from the Caribbean reported mass mortality among a species called Diadema antillarum, caused by a single-celled organism which now was also identified as the culprit in the Gulf of Aqaba and appears to be spreading with considerable speed around the world. By 2022 the coasts of Greece were affected. From the Gulf of Aqaba, it now has spread to the Red Sea, and may soon reach Australia’s Great Barrier Reef.


Besides obvious effects on the international restaurant scene, where uni has been integrated into many menus beyond just in Japanese restaurants, these losses could also have devastating environmental effects because corals can be cut off from sunlight by algae overgrowth.
1. Tomar et al., Nature 2024;630:720-722
2. Yannakoulia, Scarmeas N. N. Engl J med 2024;390(22):2098-2106
3. Bermingham et al., Nat Med 2024;40: 1898-1897
4. Cummings S. Science 2024;384(6699(:944-945
... a former favorite NYC restaurant finally closes for good So, there was, once upon a time, an absolutely gorgeous fairytale-like restaurant, called Verõnika, on the second floor of an equally gorgeous old building–a converted church–on the east side of Park Avenue and 22nd Street that now houses a very edgy photography center/museum called Fotografiska. The restaurant was possibly the most beautiful room in NYC, similar in architecture to the relatively recently opened Café Carmellini on lower Fifth Avenue in the Fifth Avenue Hotel (and one of our current favorites). But it had a sad history.
It first opened in 2020, just before COVID hit, and very quickly closed. It then reopened after indoor seating became possible again in 2021 with Wolfgang Ban, a well-known Austrian chef whose own restaurant in NYC had been Micheline-starred, leading the kitchen and the same “Gestalt” as a grandiose European traditional restaurant with distinctively Habsburgian reach from Russia, over Poland, to Austria as before, and, of course, with French influences as in those long-gone days was the tradition. The food was amazing, the atmosphere was electric, and we loved it, until one day–while the restaurant was still in its running-in period–we came by for drinks at the most beautiful bar in NYC and found the restaurant closed and locked up.
It reopened again in 2022 with a new chef whose name we don’t want to mention. The food was simply atrocious. The restaurant was no longer run by the same restaurant management company, but the owner of Fofografiska took over with a very different and in many ways bizarre menu, giving dishes familiar names which often had nothing in common with what one had ordered. In the hope things would improve, we gave the restaurant several chances, but it did not get better. We finally gave up on it and never went back.
Media reported that the photography center/museum is moving but did not say where. Hopefully, a real restaurateur will take over this gorgeous restaurant space. In combination with good food and the amazing bar one passes on the way into the main seating area, this space is destined to be a big success.
... a new restaurant review: Café Boulud
100 East 63rd Street; Phone: (212) 772-2600
When it comes to Daniel Boulud it is difficult to be critical because New York owes him so much. Daniel, his first restaurant off Madison Avenue on 76th Street, was a revolution for the city’s dining scene when it opened, serving authentic French gourmet food with reasonable pricing, and it made his name as one of the city’s most important French chefs, if not its most important. He quickly outgrew his namesake restaurant and moved it a few blocks more downtown into its current position on the corner of Park Avenue and 65th Street, while turning his prior Daniel into


the new Café Boulud, with both thriving, Daniel in the process earning three Michelin stars (he has since lost one).
Under a series of different chefs, including fabulous Andrew Carmellini of current Café Carmellini fame, the Café bloomed and boomed, and we continued loving it until the same fate befell Daniel Boulud’s rapidly growing restaurant empire that only too often befalls prominent chefs: their restaurants become luxury cafeterias, managed not by a creative chef with skin in the game every day in the kitchen but by a young chef who, if she/ he has talent, will be restricted in her/his creativity and self-fulfillment by the corporate structure he reports to and will leave.
Those that stay must largely give up on their talents and creativity to fit into the corporate structure. The food becomes increasingly standardized and, therefore, uninteresting; flavors disappear; everything tastes the same.
This is, unfortunately, where we found the new Café Boulud to be. After having had two very disappointing dinners shortly after it opened, we wanted to give it time to recover. In two further recent visits, however, we unfortunately didn’t find its food


improved. Very much to the contrary, while perfectly prepared, practically every dish was bland and tasteless. The restaurant also lost some of its comfort by having rather forcefully added tables to the dining room, in the process stealing from its prior elegance. Somewhat surprising, service is sporadic and can be very good or quite bad.
For us, this is it. No further visits are planned. It is, unfortunately, not a restaurant we recommend to our patients.

All listed restaurants are in Manhattan unless otherwise noted. Like all opinions about restaurants, ours are sub- jective and are to be understood as such. If you visit one of them, let us know whether you agree with our ratings. We value your feedback.
PRICES FOOD QUALITY
SPECIAL COMMENTS
$ Inexpensive - Not worth the trip + Overall favorite of the CHR
$$ Moderately expensive • Good v Special vibe
$$$ Expensive •• Very good M Michelin starred
$$$$. Special event expensive ••• Excellent V Vegetarian/vegan dishes on •••• Uniquely delicious the menu
ADDRESS
AUSTRIAN
Koloman +/•••/$$/v 16 W 29th Street
Wallsé +/•••/$$/v/* 344 W 11th
CHINESE
Hwa Yuan Szechuan +/•••/$$/v
42 East Broadway
TELEPHONE
COMMENTS
(212) 790-8970 V; Excellent desserts
(212) 352-2300 V; Excellent desserts
(212) 966-6002 V; Authentic Szechuan
Mr. Chow Downtown +/••/$$$/v 121 Hudson St., Tribeca (212) 965 9500 V Uptown 324E, 57th Street (212) 751 9030 V
CONTINENTAL
425 •••/$$$/v
425 Park Avenue
(212) 751-6921 V; Gorgeous restaurant FRENCH
Le Gratin ••/$$
5 Beekman St.
(212) 597-9020 V; Try the gratin potatoes
Le Charlot +/•• /SS 19 E 69th St. (212) 794-6419 V; Best steak au poivre & Thai mussels; great desserts
Le Bernadine +/••••/$$$$/ MMM 155 W 51st
ITALIAN
Cipriani Downtown +/••/$$/v 376 West Broadway
(212) 554-1119 V; Mostly seafood. Likely the best NYC restaurant
(212) 343 0999 Great food, beautiful people & Uptown 781 5th Avenue
Elio’s +/•••/$$/v 1621 2nd Ave.
Principe ••/$$/v
Sistina •••/$$$
50 West Broadway
24 E 81st St.
(212) 753-5566 Great food and high society
(212) 772-2242 Best Italian home cooking and where everybody meets
(212) 335-0509 V; mostly seafood but one of the best chicken dishes
(212) 861-7660 V; Amazing wine list FRENCH-ITALIAN
Café Carmellini •••/$$$$/v 250 Fifth Avenue
(212) 231 9200 V; Gorgeous place, top service; sophisticated and adventurous food JAPANESE
SUSHI
Sushi Ann +/••• /$$$$
8 E 51st St.
(212) 755-1780 V; Best quality fish; reservation at the bar GENERAL
Sakagura +/•••/$$
211 E 43rd St.
Yakitori Torishin +/+++/$$$/M 362 West 53rd Street
(212) 953-7253 V; Amazing food; Best sake selection
(212) 757-0108 Unique and amazing skewer restaurant KOREAN
Jungsik •••/$$$$/ MM 2 Harrison St.
Oiji Mi +/•••/$$/v / M 17 W. 19th St.
(212) 219-0900 Where NYC’s Korean restaurant revolution was born …
(212) 256-1259 … and has continued GREEK
Elias Corner (Queens) ••/$ 24-02 31st St.
(718) 932-1510 Mostly seafood HAMBURGERS
Jackson Hole Burgers (the “original”) +/••/$ 232 E 64th St.
(212) 371-7187 Not a place for vegetarians MIDDLE EASTERN-ISRAELI
Dagon ••/$$ 2454 Broadway
(212) 873 2466 Best hummus in NYC NEW YORK JEWISH DELI
P. J. Bernstein’s Deli •/$ 1215 3rd Avenue
(212) 879-0914 All the great classics; pastrami, chicken soup PERUVIAN
Mission Ceviche •••/$$ 1400 2nd Avenue
(212) 650-0014 If you like Peruvian – the best PIZZA
San Matteo Pizzeria e Cuccina ••/$ 1559 2nd Avenue
(212) 861-2434 True Napoli POLISH
Karczma (Brooklyn) +/•/$ 136 Greenpoint Avenue
(718) 349-1744 Great authentic Polish food - and dirt-cheap ROMANIAN-JEWISH
Sammy’s Roumanian Steak House** $$ 112 Stanton St.
UKRAINIAN/RUSSIAN
Caviar Russe +/••••/$$$$/ M 538 Madison Avenue
Russian Samovar •/$/v
256 West 56th St.
(212) 673 0330 Authentic Jewish-Romanian Steak House, with entertainment
(212) 980-5908 V; Most underrated restaurant in NYC
(212) 757 0168 Great Russian/Ukranian food and music
Any suggestions and/or comments, please write to social@thechr.com
BRIEFING: The VOICE in this section of the newsletter offer commentaries on a broad survey of recent articles in the English literature, which the CHR found of interest, - even if, at times, not immediately applicable to daily clinical practice. Articles are mostly chosen for their translational value to clinical medicine, often helping in determining where clinical practice will likely go.
Translational research is, since its founding in 1981, one of the CHR’s principal goals and has over the years produced some milestone discoveries and a good number of U.S. patents. Such research has also propelled the CHR into its current status as a worldwide center of last resort for infertile patients who have failed treatments elsewhere. This section of the VOICE, demonstrates and makes public the process through which the CHR for decades has been following and interpreting the published literature, a process always at the very core of how research and clinical practice have evolved at the CHR.
Primarily directed at physicians and basic scientists, this section of THE VOICE to our surprise has also attracted many of the CHR’s patients, from which we assume that it also has found a readership among the public. The VOICE, therefore, strives through its writing style, to make this section also accessible and understandable for a general audience.
How important really is Vitamin D [25(OH)D]?
It is unclear why recent months have offered a clear uptick of published papers on supplementation with Vitamin D. This publication frenzy reached a peak when the Endocrine Society on July 12, 2024, in the same issue of the JCEM, published on the subject a Clinical Practice Guideline,1 as so-called Clinical Practice Communication,2 and a Clinical Practice Guidelines Systematic review.3
The first document addressed Vitamin D [25(OH)D] supplementation for prevention of disease and suggested empiric supplementation between the ages of one and 18 and in adults over age 75, as well as during pregnancy and with high-risk pre-diabetes. The document, however, recommended against routine blood testing for Vitamin D in absence of established indications and stressed the need for further investigations to reach disease-specific recommendations and determine optimal hormone levels.
The second document addressed Vitamin D insufficiency and epistemic “humility.” Practically this means that the Endocrine Society no longer endorses its prior definition of hormone “sufficiency” of at least 30ng/ mL (75nmol/L) or its prior definition
of “insufficiency” [>20ng/mL (50nmol/L) but < 30ng/mL (75nmol/L)]. The reason for this decision was absence of well-designed prospectively randomized studies and shortcomings of reported observational studies.
Finally, the third document reported on the systematic review supporting the new clinical practice guidelines. Out of 37,000 citations, the review included 151 studies. At very young ages low-certainty evidence suggested a reduction in respiratory tract infections from Vitamin D supplementation. Up to age 75 no obvious benefits were observed and above age 75 a small reduction in mortality was observed with high certainty. Only low-certainty evidence for any pregnancy benefits on maternal and/or fetal outcomes, and in prediabetics, moderate certainty suggests a reduction in progression to diabetes.
Summarizing all of this fancy language in only one short sentence: If vitamin D supplementation has any benefits, they are minimal.
1. Demay et al., J Clin Endocrinol Metab 2024; 109(8):1907-1947
2. Mccartnet et al., J. Clin Endocrinol Metab 2024;109(8):1948-1954
3. Shah et al., J. Clin Endocrinol Metab, 2024;109(8):1961-1974)
Why we do not like inflammation – and not only in reproduction
The CHR’s patients and everybody reading the VOICE probably knows by now how much the CHR dislikes inflammation, and we were just given some additional reasons for our strong feelings on this subject. One is that chronic inflammation contributes to chronic diseases associated with aging. As we grow older and our bodies accumulate increasing cell damage, the immune system can mistake this as evidence for a possible infection and may respond accordingly, causing further damage leading to autoimmune diseases and even cancer.
As investigators have now discovered, a well-known promotor of inflammation, Interleukin-11 (IL-11), a member of the IL-6 family, may play a crucially important role in these processes. Older rats and mice also demonstrated much higher IL-11 levels than younger animals. Blocking IL-11 function in older mice then boosted their metabolism, reduced frailty significantly, and increased their lifespan by a whopping 25%.1
Why is this of interest for reproductive medicine? Because anti-IL-11 therapy with anti-IL-11 antibodies is currently in early-stage clinical trials for fibrotic lung disease and cancer.2 Fibrosis appears also to be one of the major factors in ovarian aging; IL-11 blockage, therefore, may be a potential candidate to treat ovarian aging.
It appears that these anti-IL-11 antibodies have a similar effect on aging as rapamycin. Earlier in this issue of the VOICE we noted a Columbia University ongoing clinical trial of rapamycin under the hypothesis that the drug could slow ovarian aging (page 33). But a recent news article in Nature magazine, in comparing those two approaches to battle fibrosis, quoted an expert as noting that rapamycin had much more clinical side effects than anti-IL-11 antibodies and, therefore, “was good for lifespan extension but not for health span.”2
What this exciting new study reemphasizes among other things is the close association of aging with inflammation and, therefore, the immune system. However, as another commentary in Nature magazine points out, it also suggests that “researchers now can move beyond broad generalizations to engage with the nuances that modulate the pace of aging.”3 For reproductive biology and clinical infertility all of this additionally means that the female immune system is even more important for reproductive success than we have been aware of so far. It is not only responsible for tolerance of the fetal-placental allograft but also–at least to some degree–for the aging process of ovaries and other reproductive organs. In other words, for those colleagues who for the longest time have been arguing that attention to the female immune system in infertility practice is unnecessary, it appears time to reconsider and catch up.
And since we are already talking about inflammation, here is another very interesting piece of news: Researchers at Vanderbilt University in a large genomic association study apparently found that coronary artery disease and major depression appear genetically interconnected, with the common link being low-level inflammation.4 This finding is of considerable interest for us here at the CHR because the CHR’s investigators have for many years been
arguing that depression–in its postpartum clinical expression pattern–exactly follows the pattern of typical autoimmune diseases.5,6 The data also showed a similar connection between coronary artery disease and cardiomyopathy, which in the form of peripartum cardiomyopathy, because of disease timing, has also been tagged by CHR investigators as most likely being an autoimmune disease.7
1. Widjaja et al., Nature 2024;632(8023):157165
2. Ledford H. Nature 2024;631:716-717
3. Miller RA. Nature 2024;632:35-36
4. Snyder B. Vanderbilt University Medical Center News, April 9, 2024. https://news. vumc.org/2024/04/09/heart-disease-depression-linked-by-inflammation-study/
5. Gleicher N, Weghofer A. Hum Reprod 2009;24(3):760-761
6. Gleicher N. Autoimmune Rev 2007;6(8):572576
7. Gleicher N, Elkayam U. Autoimmune rev 2009;8(5):384-387
A Swedish study in eClinicalMedicine (a Lancet journal) recently set off alarm bells in the tattooing community by reporting in a population-based case-control study an increased risk for malignant myeloma after tattoo exposure.1 Since one underpowered study in the literature had suggested a risk for malignant lymphoma from tattoos, Swedish investigators set out to conduct an epidemiological study including all cases of malignant lymphoma between 2007 and 2017 in Sweden with three sex-matched controls per case and they found a 21% higher risk with prior tattoos, with the risk being the strongest with diffuse large B cell lymphoma and follicular lymphoma. The authors’ conclusion was–unsurprisingly–that the chemical composition of tattoo ink must be regulated.
1. Nielsen et al., Lancet 2024; 72:102649
The Vatican’s secret role in IVF
A recent, fascinating article in Vanity Fair1 attracted our attention, and not only because the article described in detail the enormous role longstanding CHR friend and colleague Bruno Lunenfeld, PhD, played in IVF and in the history of modern infertility care in general, but because it revealed how important the Vatican was in making IVF possible. And that is, of course, a contradiction to basic Catholic dogma which to this day does not support IVF.
As the article recounts, it all started on Ash Wednesday in 1957 in Rome, when Lunenfeld–then barely 30 years old–gave “one hell of a presentation” to a group of men of science and men of faith, gathered to learn important new facts about female fertility. But the true purpose of his talk was to secure urine from “the brides of Christ”–in other words, from nuns.
By that time, Lunenfeld had been working in Israel for four years on a treatment for anovulatory women that would make them ovulate. He now was seeking the help of a pharma company, the Istituto Farmacologico Serono (which we all for over 50 years know as fertility pioneer Serono, which later became the first company to bring a gonadotropin product, Pergonal, to market. Many IVF old-timers, including the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, still hold that this was clinically the best gonadotropin product ever produced.)
According to the article, the company’s own researcher, Piero Donini, PhD, a chemist who in 1949 had purified gonadotropin from the urine of postmenopausal nuns, had been working on the same idea and had facilitated Lunenfeld’s trip from Israel to Rome. The crowd listened to Lunenfeld’s talk with interest, but didn’t bite; he was told that no help was forthcoming since it appeared unlikely that
anybody would be able to procure the vast quantities of urine that would be required to produce pharmacological amounts of gonadotropins.
Severely disappointed by this rejection, Lunenfeld, however, was approached after the talk by one of Serono’s board members, the Italian prince Don Giulio Pacelli, whose family’s name –unknown at the time to Lunenfeld–not only carried significant weight in Rome but also represented strong personal connections to the Pope at the time, Pius the XIIth, whose pre-papal name until his 63rd birthday had been Eugenio Maria Giuseppe Giovanni Pacelli. Don Giulio Pacelli had an idea, and the idea was to recruit, with the help of the Vatican, postmenopausal nuns as donors of postmenopausal urine which was rich in gonadotropins.
In the first year, the program recruited 100 nuns, which produced 30,000 liters of urine, which produced 100mg of hormone (with impurities) which produced 9,000 vials of 75IUs of what later became Pergonal, the world’s first clinically used gonadotropin. And, while Pergonal was not involved in the birth of the Brown baby in the UK–the first IVF baby in the world–it was used in the IVF cycle that led to the first U.S. IVF birth, the birth of Elizabeth Carr. And, considering how low pregnancy rates were with IVF until the Norfolk program in the U.S. started using gonadotropin stimulation in place of natural cycles and/or clomiphene citrate stimulation of ovaries, IVF, most definitely, would not have become the success it became without availability of gonadotropins for ovarian stimulation.
Lunenfeld tested the first batches at the Weizmann Institute on himself, we learned from the article, and it took five rounds of steadily improving purification before he did not develop a fever following self-injection and clinical studies could be started. He
also never patented his findings and, therefore, did not benefit financially from the clinical utilization of gonadotropins. 1962 was the year when the first anovulatory woman treated with gonadotropin gave birth. Lunenfeld also was never awarded a Nobel Prize, even though millions of people would not be on this earth were it not for his work.
According to the article, current Pope Francis in 2023 reaffirmed the Vatican’s anti-IVF stance with the following statement: “For those committed to a serious and evangelical renewal of thought, it is essential to call into question even settled opinions and assumptions that have not been critically examined.”
IVF, however, would not be what it has become without the help of the Catholic church and the church’s nuns. Close to 8 million IVF babies–now citizens of this world–of course must be convinced that God works at times in unexpected ways!
1. Weir K. Vanity Fair. April 29, 2024. https:// www.vanityfair.com/style/story/pope-secret-history-ivf
How useless is the appendix?
Apparently not as much as was thought
Even though the literature suggests otherwise, appendectomy remains the standard treatment for uncomplicated appendicitis. Until very recently this practice pattern was controversial only because studies have quite convincingly demonstrated that most such cases can be very successfully treated with antibiotics, thereby avoiding a need for surgery.
The anti-surgery argument has, however, over recent years found additional supporters due to a much more significant concern: the possibility that the removal of an appendix may increase the risk of GI problems, including irritable bowel syndrome and
even colorectal cancer. While the data are still preliminary, they already cause concern because they are supported by certain previously unknown biological facts. For example, the appendix may act as a kind of safe house for “favorable” gut bacteria and as a kind of “training ground for the immune system.” Moreover, species with appendices live longer than species without. There is also evidence that the appendix has a role in preventing diarrhea.1
Appendectomies have also been associated with increased risk for type 2 diabetes, systemic lupus erythematosus, and other autoimmune diseases, with women–as one would expect considering their much higher prevalence of autoimmune diseases–being especially affected.
Suggested positive associations with Parkinson’s disease and ulcerative colitis have remained very controversial, strengthening the suspicion that the appendix may have an immune-system-related function. Appendectomies apparently also reduce CD3+ and CD8+ T cells which, if correct, would mean that appendectomies weaken immune surveillance which, in turn, could increase cancer risks.
1. Zaraska M. Medscape Medical News. June 10, 2023. https://www.medscape.com/ viewarticle/useless-appendix-more-fascinating-than-we-thought-2024a1000au9?form=fpf
Tumor-infiltrating lymphocytes (TILs), a new tool in precision cancer immunotherapy, and other cancer news
And since we are talking about cancer, here is some good and interesting news. The FDA just approved a new concept in cancer immunotherapy, the use of so-called tumor infiltrating lymphocytes (TILs), when approving the first such treatment for malignant melanoma. Additional trials for lung and cervical cancer are underway.1
TILs are white blood cells which invade solid tumors and attack them after recognizing a specific surfa antigen of the tumor. After excision of a solid tumor, samples are then sent to a lab where TILs are isolated from the specimen and grown in cell culture for ca. three weeks. IL2 helps in this process. Once the culture has grown to several billion cells, they are returned to the patient by I.V. infusion. Certain pre- and post-treatments are required, making this an in-hospital therapy requiring hospitalization for ca. two weeks. Another seemingly successful step in precision cancer therapy, which has been a guiding light for precision medicine in general!
Precision health care is, indeed, gaining ground in many medical specialty areas as health care becomes more data driven. A series of articles in Nature Medicine recently asked what it will take to realize the benefits of precision medicine in local as well as global contexts, especially considering that “precision health”–at least in high income countries–is already becoming big business.2
Mammography: When women should start having regular mammograms has been a controversial and basically unresolved issue for decades. No wonder recommendations and starting ages for regular screening are all over the map in different countries. In the U.S., age 40 has been widely considered an appropriate start, but official recommendations have pointed at age 50. In Europe, to our best knowledge, not a single country starts routine screening before age 45, and most at age 50.
Citing rising breast cancer rates in young women (cancer risk–as we noted in a prior VOICE–has recently in general been significantly increasing in young individuals), an expert panel of the U.S. Preventive Services Task Force, in a reversal of the panel’s earlier opinion, now recommends universal breast cancer screening formally to
start at age 40 (in 2009, the same task force had raised the age to 50).3 The panel still recommends mammograms every two years, but many physicians recommend annual exams.
It is important to note that these recommendations/guidelines are meant for general populations and not for high-risk patients, patients with suspicious findings upon physical exam or through self-examination, or for patients with special circumstances, like, for example, planning to conceive. Pregnancy of course disqualifies one from doing mammograms (though MRIs are possible) but once lactation is initiated, testing becomes almost impossible. And dense breasts are a completely separate story because they make mammography less reliable and really mandate an MRI, which many insurance companies do not pay for when the indication is routine breast exams and neither does Medicare What this task force recommends is that insurers then are obliged to reimburse.
The article also noted that Black and Jewish (mostly Jewish Ashkenazi) women, because of their high prevalence of BRCA 1/2 carrier status, along with other minorities, more frequently have breast cancer and also more frequently die before age 50 from the disease.
1. Healey N. Nat Med 2024;30:1795-1796
2. Editorial. Nat Med 2024;30:1793-1794
3. Rabin RC. The New York Times. April 30, 2024; https://www.nytimes.com/2024/04/30/ health/breast-cancer-mammography-ultrasound.html
And as hard it is to understand why younger people in increasing numbers develop cancers and die, we were shocked to see a recent paper in JAMA Network that reported on suicide rates in preteens at ages 8-12 during the years 2001-2022.1 Who would
even think about the possibility that children that young would commit suicide, or indeed, would know how to do it.
But as it turns out, such youth suicide has become a very significant public health concern, as the article noted, leading in the National Institute of Mental Health in 2021 to convene a research roundtable to address the issue. The study revealed a significant increase in suicides in this age group over the study period, with female suicides growing faster in numbers than male suicides, thereby significantly narrowing the historical gap between the sexes that used to significant favor males. Suicide was, indeed, the 11th leading cause of death in female preteens between 2001 and 2007 and became the fifth leading cause between 2008 and 2022. Suicide in male preteens maintained its position as the fifth leading cause throughout the study period. Black preteens had the highest rates for both periods and Hispanic preteens had the highest increase. Hanging and suffocation were the predominant methods of suicide for the study period, but the largest increase was seen with use of firearms. One wonder where they are getting those from?
1. Ruch et al., JAMA Network Open 2024;7(7):e2424664
What pooping patterns tell us
Yes, everybody poops; but when and how often varies, according to a recent paper in Cell Reports Medicine by investigators from the University of Washington, Seattle, WA.1 It turns out that bowel movement frequency (BMF) matters because it impacts the gut microbiota and has been linked to chronic kidney disease and dementia. Constipation leads to a switch from fiber fermentation and short-chain fatty acid production to more detrimental protein fermentation and production of toxins.
The study revealed differences in abundances of gut microbial genera, blood metabolites, and variations in lifestyle across BMF categories relating to inflammation (here we go again!!), heart health, liver function, and kidney function. Accumulation of microbiota-derived toxins associated with abnormal BMF appears to precede organ damage and my contribute to chronic, aging-related diseases.
1. Johnson-Martinez, et al., Cell Rep Med 2024;5(7):101646
treatments using assisted reproductive technologies (ART)
Starting with the embryology laboratory … IVF culture media: Two leading voices in the international infertility arena, Richard J. Paulson, MD, and Eli Adashi, MD, recently published an important commentary in Reproductive Medicine Online, 1 which was, somewhat confusingly, placed by the editors into a manuscript category called “countercurrent” and, therefore, is supposed to represent opposing opinions to currently held beliefs in the field. The subject addressed by the two authors in their paper, however, is anything but controversial; 99.9% of embryologists and IVF providers will probably agree that producers of human embryo culture media do not inform adequately on the constituents of their culture media and that this is unacceptable and frankly, a scandal that must stop.
If manufacturers of media feel that their media contain ingredients with special unreported effects on the IVF process, they should apply for patents which then will protect their intellectual property. User patents offer such protection even for compounds that are already known and manufactured, if inventors can demonstrate previously unknown effects from those ingre-
dients.
Nobody, of course, prescribes medications to patients without knowing what they contain. And like patients, embryos in our embryology laboratories, in this instance through the media they are cultured in, are exposed, at least theoretically, to positive as well as adverse long-term effects. The authors in their commentary pointed out the importance of the culture period in embryology laboratories for epigenetic influences on the embryo which will last for a lifetime. And they also noted the huge differences between culture media, suggested by a handful of analytical studies, without being able to trace purposes and problems of ingredients and being able to compare outcomes of IVF cycles accordingly. The field, indeed, currently even lacks adequately powered comparable studies between media sold. Imagine the uproar, if a new cancer drug claiming benefits over older drugs, would make claims of superiority without scientific backup from a properly executed comparative study! Why do we accept this in our embryology practice?
Time-lapse: Elsewhere already briefly addressed in this issue of the VOICE, an excellent study by mostly British investigators offered a final verdict on the clinical effectiveness and safety of time-lapse imaging closed incubation systems and the news was not what most anticipated: this very expensive addition to many–if not most–embryology laboratories in the world did not significantly increase the odds of live births compared to standard embryology without time-lapse.2
This, of course, does not mean that closed incubation systems with timelapse imaging do not have a purpose in IVF–they of course do especially for research–but this publication once more lays bare the irresponsible introduction of “newness” into the IVF field based on unproven hypotheses. In this case the false claims were that time-
lapse would standardize and improve IVF outcomes and reduce manpower demands in embryology; none of these promises were kept. One must wonder how much better some of the funds spent on most of this new time-lapse equipment could have been used elsewhere and/or how much lower IVF cycle costs would now be.
As also already noted before in this issue of the VOICE, because the CHR never makes significant changes in the IVF laboratory without first testing them out on the center’s very unique patient population, we never fell into this trap, as our tests failed to confirm all of the claimed benefits by manufacturers already by 2016. The CHR’s investigators then published these result at the time, basically reaching similar conclusions as the here discussed (much larger and better quality) study did3–but very few of our colleagues listened.
A.I. for oocyte assessment: An obviously related subject to time-lapse imaging is the currently ongoing A.I. frenzy surrounding the embryology laboratory. We have previously noted in these pages that, for biological and statistical reasons, we have for years rejected the hypothesis that selecting–beyond basic embryology as practiced in every decent embryology laboratory–one (or more) “best” embryos from among a single IVF cycle embryo cohort would to significant degrees improve IVF outcomes. From rejecting the unproven hypothesis of embryo selection then follows automatically the conclusion that all the commotion about embryo selection with A.I. will lead to the same kind of disappointment as just reported in here noted recent paper about time-lapse.
That A.I. may, however, potentially find different applications was recently suggested at the annual ESHRE conference in Amsterdam, instead of using A.I. to assess embryos, assessing freshly retrieved oocytes with A.I. As
one of the first oral presentations of the conference ([Abstract O-004], a Canadian start-up [Future Fertility, Toronto, Canada] presented a novel A.I. model to predict ploidy status of a developing blastocyst based on a given mature MII a oocyte. The AUC of the model (0.61) to be predictive of aneuploidy was, however, clinically still very obviously insufficient but can be expected to improve with more learning. Moreover, the authors of the abstract acknowledged serious limitations for obvious selection biases in embryos used to build their model and should be able to correct for those.
An A.I. model from Mexico [Abstract O-003] attempted to determine whether it could enhance the prediction of mature MII oocytes prior to stripping from cumulus-oocyte complexes (COCs) and claimed that the program improved the judgment before denudation in Grade III and Grade IV COCs (representing more difficult to predict COCs before denudation) (P<0.001). The abstract unfortunately does not allow for a clear enough understanding of the study protocol to judge its ultimate validity; but assuming the results hold up, this may be a potentially clinically important application of A.I. in IVF.
We are happy to see that that the interest in oocyte morphology has been perking up. The CHR, after all, has for many years been arguing that IVF over-scrutinizes embryos and under-scrutinizes oocytes.
Extracellular vesicles: And as the last ESHRE abstract in this section, Swiss colleagues finally demonstrated that extracellular vesicles, which currently are the target of research in every last corner of the human body, apparently also play a role in oocyte maturation in follicles. More specifically, it appears the vesicles from mature oocytes - after being taken up by immature oocytes – help in their maturation. [Abstract O-005]. If confirmed, we find this to be
a potentially very significant observation because, retrieving IVF cycles in selected patients much earlier than most other IVF clinics, investigators at the CHR have–based on clinical observations–for some time suspected that the largest lead follicles affect the fates of their smaller counterpart. Specifically, these observations suggested for example that once lead follicles overmatured, they adversely selected the quality of smaller, second-generation follicles. That this effect may be mediated by extracellular vesicles would not surprise.
1. Paulson RJ, Adashi EY. RBMOnline 2024;48(3):103645
2. Bhide et al., Lancet 2024;404:256-265
3. Wu et al., Reprod Biol Endocrinol 2016;14(1):49
Continuing with alleged new treatments …
A secret drug to improve implantation: And to remain at the ESHRE event, Spanish and Polish colleagues reported on OXO-001 which allegedly improves implantation through a direct effect on the endometrium. What the drug does as well as its name or chemical description remains a secret. But based on a randomized, double blinded, placebo-controlled multicenter phase II clinical trial with three parallel arms conducted in 28 European centers between 9/2021 and 1/2023, this pilot study improved implantation rates by more than 10% 46.3% vs. 35.7%). Considering that this study was performed in donor egg-recipient cycle of women under age 40, the pregnancy rate in controls of 35.7% appears low, while the reported pregnancy rate after use of the new “wonder-drug” of 46.3% may just qualify as the expected rate. We assume that we soon will be hearing more about this mysterious new drug which, supposedly, is not a hormone, but from those preliminary data, we are somewhat skeptical.
Platelet rich plasma (PRP): The VOICE has reported on the use of
PRP repeatedly. Moreover, we have, of course, also reported on the three registered clinical trials of PRP we have been conducting at the CHR. One has closed and a paper has been submitted for publication after an earlier interim report was published on this trial.1 It has been replaced by a fourth trial that was just initiated, so that the CHR is currently again conduction three different PRP trials on three different patient populations.
Since its initial introduction into clinical medicine in the sports medicine field years ago, we in more recent years, of course, noticed that PRP has also become very popular at many IVF clinics after initially being introduced to the field by Greek colleagues. We, however, have questioned its popularity under such names as “ovarian rejuvenation” (without any evidence that it really rejuvenates anything) and similar enticing names because only so little has been published about its use in female infertility. Moreover, after initially being injected into the ovary in attempts to improve egg numbers and egg quality, it quickly also found application in uterine perfusions, either for so-called implantation failure (whatever this diagnosis may mean) or for chronically thin endometrium.
Though published data are still very sparse, slowly more papers are published, with two of them here briefly noted: A first involved intraovarian administration of PRP and was a prospectively randomized study of young women (<age 38) with prior poor ovarian response, 2 or more prior cycles with <3 retrieved oocytes. The study involved only 83 patient cycles (41 PRP+ and 42 PRP-), not an adequate study size to ever reach conclusions regarding pregnancy or even live birth rates. The authors, therefore, used as primary outcome the number of MII oocytes.
As we would argue for several reasons that make us question study outcome
outcome and interpretation, the authors found no difference in MII oocyte numbers and, therefore, concluded that intraovarian PRP does not improve IVF outcomes in women under age 38. Based on these results, they furthermore recommended against the use of PRP in this kind of infertility population.
Though the CHR to this day remains undecided about the effectiveness of PRP, this study does not warrant their conclusions and here is why: Though the study was allegedly powered to define significance at a difference of one MII oocyte, that does not appear to represent a valid cut-off. To make the point more bluntly, whether a 32-yearold produces three or four MII oocytes likely does not make much practical difference. More importantly, however, the CHR’s researchers recently reported that as ovaries age their ability to produce good-quality embryos declines, while the ability of immature oocytes improves.2 MII oocytes, therefore, reflect only a part of the cumulative pregnancy chance of an IVF cycle. The researchers, therefore, should have used the total oocyte number retrieved (including MI and GV oocytes and not only MIIs) as their primary endpoint.
This does not necessarily mean that the conclusions the paper presented may, in the end, not turn out to be correct; but in this paper presented data do not allow for the conclusions the authors reached. We basically still don’t know whether intraovarian PRP improves IVF chances and, if so, in which kind of infertility patients.
The second article is a review of PRP use for endometrial perfusions.3 As a review article, one cannot expect major new revelations and that applies also to this paper. It, however, offers a single source for what little is known about endometrial applications of PRP.
As for intraovarian applications, good quality studies for endometrial pur-
poses are, therefore, urgently needed but as with intraovarian applications, we expect many fertility clinics to integrate this treatment quickly into their routine protocols before its effectiveness has been validated. This is, after all, how most “add-ons” have found their way into routine infertility practice. Even though the CHR likely has more experience with PRO than most other IVF providers, PRP at the CHR, whether intraovarian or endometrial, is still considered an experimental procedure and treated as such in the informed consent process with patients and in clinical practice, with every treatment outcome recorded in detail for later analysis, which then will be shared with colleagues through publication in a peer-reviewed journal.
1. Barad et al., Hum Reprod Open 2022(3):hoac027
2. Nicholas et al., iScience 2023;26:107308
3. Karadbhanje et al., Cureus 2024;;16(5): e59728
Follicular volume: Spanish colleagues published in Fertility and Sterility a superficially very daring paper, suggesting that follicular volume (i.e. taking current standard follicular diameter measurements from 2 into 3 dimensions) and concluding that a follicular volume of > 0.56 cm3 denoted oocyte maturity. It is quite rare that we can judge a paper as completely misleading by simply reading the title, but this is one example, because suggesting that–independent of age and other parameters affecting oocyte maturity like functional ovarian reserve–one cut-off value for follicle volume defines maturity is complete nonsense. It furthermore demonstrates a surprising lack of understanding of the physiology underlying ovarian aging, characterized by oocytes with advancing female age reaching maturity at progressively smaller follicle sizes (i.e., therefore also–as a given–by progressively smaller follicle volumes).
To argue that one follicle volume at all ages defines likelihood of maturity and impending ovulation, therefore, very clearly warrants a WORST PAPER AWARD for this issue of the VOICE.
Semen exposure after egg retrieval? Because acceptance of the fetal semi-allograft by the maternal immune system is crucially important for implantation and early post-implantation development of pregnancies, and because historically reproductive immunologists have argued that exposure of the female immune system to paternal semen (i.e., paternal HLA antigens) prepared the maternal immune system for the paternal allogeneity of the implanting embryo, the hypothesis that post-retrieval semen exposure may benefit IVF outcomes has been around since practically the beginning of IVF. Now Swedish investigators, however, seem to have put an end to it.2
In a double-blind placebo (saline)-controlled prospective trial of 792 couple cycles (393+ and 399-), served as the study population, with primary outcome being live birth. In the seminal plasma group positive pregnancy test was recorded in 35.4% of cycles and clinical pregnancy in 28.8% (-6.6%), and live birth rates 26.6% (-8.8%) while in controls the corresponding numbers were 37.3%, 33.6% (-3.7%), and 29.8% (-7.5%). While the paper correctly noted no effect of paternal semen exposure on live birth rates, we on purpose here calculated miscarriage rates because they also demonstrate no difference. This paper, therefore, very clearly makes the point that post-retrieval inseminations are useless.
Another still controversial issue in IVP practice has been the question of which is the best method to prepare a woman’s endometrium for frozen embryo transfer (FET). A Vietnamese study of 1428 patient cycles (476 in each of 3 group) we briefly mentioned earlier in this issue of the VOICE3
motivations for the recent Lancet editorial (page 440) accusing the fertility industry of abusing patients’ vulnerabilities,4 now addressed this question in a randomized open label study and found no difference between natural, modified natural, or artificial endometrial preparation. Considering for how long this question has remained unresolved, this is unsurprising, and could be expected since any obvious difference should have been already detected long ago.
So where does this leave the field? We would argue for natural cycles, whenever women have regular ovulatory cycles, augmented natural cycles, when their natural cycles proof insufficient in generating a good endometrium, and artificial cycles for the rest of patients. And this is exactly what the CHR has been doing for several years.
Effects of air pollution on IVF: Another ESHRE 2024 abstract deserves mention in regard to this issue (Abstract O-075) because Australian investigators within this context reported that increases in particulate matter (in the study defined as PM2.5 and PM10) prior to oocyte collection were found to be associated with significantly decreased live birth rates in subsequent frozen embryo transfers. More specifically, exposure to PM10 (10 micron in diameter and can include dust, pollen, and mold5) particles decreased odds of live birth by 38%. Finer particles of PM2.5 size, usually produced by vehicle exhaust and industrial pollution, were also associated with lower odds of live birth, but to lesser extent.
The investigators performed a retrospective study of 3,657 FET cycles between 1/2013 and 12/2021 which besides PM10 and PM2.5 data from the government also included SO2, CO, O3, and NO2 levels at 24 hours, 2 and 4 weeks, and 3 months prior to egg retrievals but only increases in particulate matter affected IVF outcomes.
These are for several reasons interesting results: First, because one would expect current IVF laboratories to filter out these large-size particles. And if this were to be correct, then exposure effects would not be directly in the laboratory on gametes and embryos, but on patients themselves. And, if this were, indeed, to be the case, how would these effects so quickly translate into effects on IVF results? Does that mean our Australian colleagues’ IVF laboratories do not have clean enough air?
Of course, we don’t know the answer, but some clarity may come from a study in which the CHR participated. This study assessed IVF cycle results during the days in 2023 when huge wildfires in Canada brought major air pollution to the East Coast. While we at the CHR saw no effects on our IVF cycles during those days, our center’s cycle numbers on those days were too small to reach conclusions. We, therefore, are awaiting the combined results of all participating IVF clinics.
Uterine infusion strategies: We above briefly discussed uterine perfusions with PRP. But endometrial perfusions are now performed for several reasons, using varying agents. This, besides PRP, includes granulocyte colony stimulating factor (G-CSF), while blood cells, human chorionic gonadotropins, etc. As expected, based on above described PRP experience, all of these treatments have remained controversial because reported outcome data are insufficient to judge their effectiveness.
A recent review article by Chinese colleagues, however, summarized this limited experience and, therefore, is a worthwhile read.6 We, however, cannot agree with the authors’ conclusions that all of these treatments represent promising treatment strategies, and that PRP is likely the best. We would have preferred a more cautious tone, suggesting that some of these strategies may turn out to be effective in specific
patients for specific clinical conditions. Moreover, since none of these strategies has been established as effective, their use should be limited to study protocols.
Mosaicism in PGT-A: It is impossible to discuss IVF cycle outcomes these days without at least one reference to preimplantation genetic testing for aneuploidy (PGT-A). The opportunity presented itself with publication of a review article in Genes which attempted to explain reported live births after transfer of by PGT-A as “mosaic” reported embryos. The three authors specifically wanted to contribute an explanation to the “disappearance” of aneuploid cells during early stages of embryo development.7
Frankly, for several reasons they failed to do so: First, they still failed to acknowledge the basic difference between how PGT-A laboratories define mosaicism and how the rest of the biological world does. We have made this point repeatedly in the medical literature and in these pages, but as this paper so well demonstrates, a repeat explanation may not hurt: PGT-A uniformly defines mosaicism through an on average 5-6-cell trophectoderm biopsy. Such a definition, however, contradicts the worldwide accepted biological definition of mosaicism which refers not only to 5-6 cells of an embryo but refers to all cells in a in a complete organism (i.e., in this case a complete blastocyst-stage embryo).
Considering the importance of this distinction will clarify why we really did not like this review article: A second–in this case aneuploid–cell lineage in 5-6 random cells of a blastocyst-stage embryo, of course, is significantly different in its interpretation from presence of such a second cell lineage in a complete 250 to 350 cell blastocyst-stage embryo. Here in the below table are the differences in order of likelihood.
PGT-A RESULT
Euploid
Mosaic*** Aneuploid
DIAGNOSTIC OPTIONS**
COMPLETE BLASTOCYS
Mosaic
Euploid Mosaic Mosaic Aneuploid
*Trophectoderm biopsy; **In order of likelihood; ***Often reported as low or high
As will be apparent from the table, whatever the official PGT-A diagnosis is, it will in a majority of cases be incorrect because most embryos signed out as euploid will be actually mosaic. This is likely also correct for most embryos signed out as aneuploid, though it is reasonable to assume that the diagnosis of aneuploidy will be more often correct than a diagnosis of euploidy. That a diagnosis of euploidy involves a majority of really mosaic embryos is indirectly acknowledged by the PGT-A laboratory community in how euploidy is defined by most laboratories: Almost all laboratories define an embryo as euploid if it has less than 20% intermediate DNA counts (i.e., <20% of a second aneuploid cell lineage) which, of course, even at 5 or 10% defines an embryo as mosaic. Some PGT laboratories, moreover, now have extended the definition of an euploid diagnosis to as much as 50% aneuploid DNA.
That the authors of above cited paper completely omit these facts in their review seems strange because not only should the correct definition of mosaicism be the basis for every diagnosis of an embryo by PGT-A, but should also be obvious considering that single cell studies of human embryos now have irrefutably demonstrated that, at blastocyst-stage, at least ca. 80% of embryos have aneuploid cells (i.e., are mosaic).
bility that PGT-A may overestimate mosaicism is absurd and earns these authors another WORST PAPER AWARD for this issue.
Chemical pregnancies: What causes chemical pregnancies, which represent ca. half of all pregnancy losses, is still unknown. Spanish colleagues now published an “interesting” paper in Human Reproduction8 after trying to determine whether biochemical pregnancy loss was due to the embryo or the endometrium. They did this by testing autologous IVF cycles embryo ploidy (by PGT-A and, therefore, with all of above outlined inaccuracies) and embryo transfer after performance of an endometrial receptivity assay (ERA, of course, another highly controversial test as repeatedly discussed in these pages) or with the use of donor oocytes, with embryos transferred in a frozen embryo transfer (FET).
They concluded that the risk of chemical pregnancy did not differ between autologous and donor eggs and with use of PGT-A and /or ERA. Maybe, the more correct interpretation, however, is that PGT-A and ERA simply don’t work.
They further hypothesized that chemical pregnancies “may be related to a mechanism not associated with the embryos’ chromosomes or transcriptome of the endometrium.” Considering their study design alone, this manuscript also deserves a WORST PAPER AWARD for this issue, even though their conclusion that neither embryo nor endometrium are at fault may be correct, after all. The literature, indeed, suggests that chemical pregnancies may in many cases have an immune etiology.9
1. Rodriguez-Fuentes et al., Fertil Steril 2024;121(6):991-998: WORST PAPER AWARD for this issue.
5. Thmson D. HealthyDay. July 8, 2024. https:// www.healthyday.com/health-news/pregnancy/air-pollution-exposure-tied-to-40-dropin-live-births-among-ivf-pateints
6. Xiet et al., Reprod Biol Endocrinol 2024;22:44
7. Campos et al., Genes 2024;15:18: WORST PAPER AWARD for this issue
8. Munoz et al., Hum Reprod 2024; deae106. doi: 10.1093/humrep/deae106. Online ahead of print.
9. Coulam CB, Roussev R. Am J Reprod Immunol 2002;48(5):323-328
Social/planned oocyte preservation/freezing
Two recent papers from Israeli colleagues deserve motioning under this title. In a first, the authors conducted a systematic literature review on the subject1 and involved 3,847 records but included only 10 studies performed between 1999 and 2020 involving 8,750 cycles, a remarkably small number reflective of how little is really know about the efficacy of this clinical concept. Their mean age at time of cryopreservation was 37.2 years, a reflection of the previously reported fact that most women fail to pursue egg-freezing at best ages. The return rate was only 11.1%, an especially low number considering the relatively advanced ages of these women at time of cryopreservation. Among those who returned, the mean age at cryopreservation was, indeed, even higher than for the complete group (38.1 years). The average number of cryopreserved oocytes, moreover, was only 12.6, likely barely enough for a reasonable live birth chance for one child.
Oocyte survival was recorded in 78.5% of eggs, and the live birth rate per patients was only 28%. Among women who cryopreserved above age 40, the live birth rate was only 19.0%, while women under age 35, demonstrated a live birth rate of 52.0%.
That these authors, therefore, even in the title of the article raise the possi-
2. Liffner et al., Fertil Steril 2024;122(1):131138
We do not find these data very reassuring, especially as one can assume that reported outcomes in the literature are positively selected toward academic centers with likely better overall 5-6
3. Ho et al., Lancet 2024;404:266-275
4. Editorial. Lancet 2024; 404(10449):215.
outcomes than average community clinics can expected to produce. Moreover, the message appears clear. The concept of social egg-freezing to extend reproductive capacity into more advanced years works reasonably well if pursued at relatively young ages. The field has, however, so far not succeeded in educating the public appropriately. The responsibility for this education of especially younger women for practical reasons falls, however, primarily on our colleagues in general obstetricis and gynecology. It is our responsibility in the infertility field to educate them!
We found the second paper of special interest because in this study–in an attempt to maximize retrieval cycles in women undergoing fertility preservation cycles–the authors concentrated on the timing of oocyte retrieval.2 As readers of the VOICE, of course already know, one of the most crucial differences between how the CHR manages IVF cycles in comparison to most other IVF clinics, is that patient age strongly determines the time of retrieval, with cycles getting progressively shorter with advancing female age.
In this study the investigators matched 140 women undergoing fertility preservation cycles with 140 women undergoing IVF for male factor infertility. As one would expect, fertility preservation patients were stimulated more aggressively with higher gonadotropin dosages and, consequently, produced more oocytes. In this group of patients, the investigator noted a negative correlation between number of large follicles and number of retrieved oocytes. Though there was no association between number of large follicles and oocyte maturity for the whole study group, age stratification revealed a negative correlation.
The authors concluded correctly that these findings challenge traditional beliefs regarding oocyte retrievals; they are, however, completely in line with CHR’s practice over almost a decade
to adjust retrieval timing to patient age and other specific patient characteristics.
1. Hirsch et al., Hum Reprod Update 2014; dmae009. doi: 10.1093/humupd/dmae009. Online ahead of print.
2. Haikin Herzberger et al., J Assist Reprod Genet 2024;41:1863-1870
The CHR and some of its staff members own shares in a company (Fertilty Nutraceuticals, LLC, doing business under the name Ovaterra), which produces an androgen (male hormone) supplementation product called dehydroepiandrosterone (DHEA) and other fertility-related supplements. Since we here, among other subjects, address androgen supplementation with DHEA, readers are advised that expressed opinions in this paragraph, therefore, may be biased.
Androgen supplementation in female infertility: The CHR has been instrumental in bringing androgen supplementation into the fertility field and the Center’s investigators have, per PubMed, published over 44 peer-reviewed papers related to this subject over almost 20 years since the first paper appeared in print in 2005, reporting increased oocyte yield in certain infertile women following androgen supplementation with DHEA. We also have discussed this subject repeatedly in the VOICE and, therefore, do not want to be repetitive. Only so much: Primarily for lack of properly powered, well designed prospectively randomized studies, androgen supplementation in female infertility has remained controversial.
The CHR has twice attempted to conduct a prospectively randomized study, but had to give up both times because we were unable to recruit patients in adequate numbers who were willing to be randomized to a placebo (since patients who need androgen supple-
mentation usually have low functional ovarian reserve, once cannot blame them for not wanting to lose potentially valuable time on a placebo). Several colleagues around the world have also tried, only to end up with the same experience.
Infertility is not unique in this kind of inability to perform prospectively randomized studies, widely considered the highest level of evidence of any study format. What then usually happens in medicine is that studies are performed in other study formats, considered of lower evidence levels. The CHR and others have done many of such lower evidence studies, though with one big difference between the CHR’s studies and practically all other studies published in the literature. While the discovery of androgen supplementation at the CHR in the form of DHEA (dehydroepiandrosterone) supplementation was accidental (a patient started using it without the CHR’s knowledge), the CHR’s investigators in collaboration with colleagues at a university who worked on a mouse model very quickly learned why androgens improved fertility treatments in selected infertile women.
The answer was, indeed, very simple and logical: women who responded to DHEA had abnormally low androgen levels including testosterone, and follicles need good testosterone levels to grow well during so-called small growing follicle stages between primary and small antral follicle stages. This understanding led the CHR to include androgen level testing into the routine initial checklist for patients and, of course, led the CHR toward androgen supplementation only in women with low androgen levels. And the CHR chose to do this with DHEA rather than androgens because our bodied make testosterone from DHEA. In contrast to supplementation with testosterone directly, DHEA supplementation, moreover, poses almost no risk for overdosing.
Most infertility clinics, even if they use DHEA or testosterone for androgen supplementation, to this day do not test androgen levels in infertile women. To the best of our knowledge, no DHEA or testosterone supplementation study–of course, aside of those published by the CHR–ever restricted the patients they treated with androgens to proven hypo-androgenic patients. But if one treats headache with aspirin in women who do not have a headache, aspirin will obviously not work. And this is an important reason why the DHEA (and testosterone) literature is so confusing with many reports claiming to strongly demonstrate effects and others showing no effects of such treatments.
Other reasons are that many studies didn’t take into consideration that androgens affect only small growing follicle stages. This means that these follicles still need 6-8 weeks to reach gonadotropin sensitivity (i.e., become responsive to fertility drugs). This means that they need to be started at least 6-8 weeks before IVF cycle start and continued until the patient is pregnant. Many published studies treated only during the IVF cycle or for only 1-2 weeks before, thereby not affecting the cycle they studied at all.
There are many other good reasons why the androgen supplementation literature has remained controversial but going into further detail would be excessive. We offer this lengthy introduction because, as happened in pretty regular intervals, somebody (in this case colleagues from New Zealand and Australia) performed a systematic review of androgens (either DHEA or testosterone) for women undergoing IVF and concluded that pre-treatment with testosterone likely improves, and pre-treatment with DHEA likely results in little to no difference, in live birth and clinical pregnancy rates in women undergoing IVF who have been identified as poor responders. DHEA and T probably do not decrease
miscarriage rates in women under IVF treatment.
Poor response to ovarian stimulation, of course, can have multiple causes. As noted, in a quick survey of the 20 studies included in their analysis, not even one–as far as we could see–paid any attention to androgen levels before starting patients on androgen supplementation. Dosages and length of treatment before IVF cycles were also all over the page. In short, one more–to follow the old IBM dictum–“garbage in, garbage out.”
One more point, however, must be made, and that is an argument of proportionality. The CHR has been very critical of many “add-ons” to IVF, with significant additional costs being one strong argument (consider the costs of PGT-A, universal cryopreservation, single embryo transfer, time lapse, etc.) and compare those costs to the costs of androgen supplementation. Yet, many of the very outspoken critics of androgen supplementation routinely utilize PGT-A; some even mandate it and other useless “add-ons” without hesitation. For qualified women, androgen supplementation is, likely, the most economic infertility treatment there is, cheaper than clomid cycles. We, therefore, have the honor of awarding this paper another WORST PAPER AWARD for this issue.
1. Naik et al., Cochrane Database Syst rev 2024; 6(6):CD009749. WORST PAPER AWARD for this issue.
Some news on FSH effects on ex-vivo (mouse) folliculogenesis Though based on mouse work, a recent paper on FSH effects of ex-vivo folliculogenesis by investigators from Rutgers University in New Jersey recently attracted our attention1 because it reported several interesting findings: At least in the mouse a minimum FSH threshold appears required for follicle maturation into the high estradiol-secreting preovulatory stage, inducing
distinct expression patterns of genes related to follicle maturation, follicular transcriptomics, and follicular cAMP.
The most interesting finding was, however, that unusual high FSH levels (20-30 mIU/mL) resulted in premature luteinization, high androgen production and production of proinflammatory factors, and reduced expression of genes related to energy metabolism in oocytes. Are we seeing here the FSH toxicity the CHR has recently been suspecting with use of higher gonadotropin dosage during ovarian stimulation especially in older ovaries, which have led us to reduce ovarian stimulation in older women?
1. Zhan et al., Endocrinology 2024;165(7):bqae054
Avoiding Clomiphene Citrate periconceptionally?
Again in mice–and, yes, mice are not men–Australian investigators fed mice within 36 hours after mating Clomiphene Citrate at body-weight-adjusted dosage similar to human use and recorded a dose-dependent adverse effect on pubs at a dose of 0.75 mg/ kg: Viable pregnancies were reduced by 30%, fetal weight was reduced by 16%, and approximately 30% of fetuses demonstrated delayed development and congenital abnormalities in multiple organs not seen in controls. They also saw a 30-hour delay in birth, together with increased early postnatal death of pups.
These are surprisingly severe consequences and, of course, raise concern about periconception exposure to Clomiphene Citrate in humans. Since this medication is in principle used in anovulatory females with oligoamenorrhea, early pregnancies may be missed. These findings suggest that a pregnancy before treatment start with Clomiphene Citrate may not be a bad idea.
REFERENCE
1. Chin et al., Endocrinology 2024;165(7):bqae047
Hypopituitarism
It is rare to see women with complete deficiency of anterior or posterior pituitary hormone production in infertility practice; but for anybody interested to read an excellent comprehensive (16 pages), and up-to-date summary on the subject we recommend a recent “Seminar” by several experts on the subject in The Lancet. 1 Quoting some of their conclusions, treatment aims at replacement of missing hormones. Increased mortality may, however, persist despite treatment, particularly in younger patients, especially in females, and in those with arginine vasopressin deficiency. Patients with complex diagnoses, pregnant patients, and adolescent patients transitioning to adulthood should ideally be managed at a pituitary tumor center of excellence.
REFERENCE
1. Fleseriu et al., Lancet 2024;403:2632-2648
Polycystic ovary syndrome (PCOS)
So here is an interesting story on PCOS regarding a recent publication (in short form as a Research Article Summary) by Chinese investigators in Science magazine, which reported that compounds of artemisinins, derived from Artemesia plants and often used to treat malaria, apparently successfully targeted all clinical aspects of PCOS by suppressing ovarian androgen production.1
The paper reported that these compounds decreased testosterone levels in blood and eliminated the ovarian phenotype in a PCOS mouse model and improved implantation rate and litter size in a rat model (one, of course, must wonder why two different small animal models?). Most importantly, however, dihydroartemisinin
treatment ameliorated hyperandrogenemia, reduced AMH (anti-Müllerian hormone) levels, improved ovarian polycystic morphology, and normalized menstrual patterns in 19 PCOS patients.
If confirmed by more data (19 patients are really not enough and neither mice nor rats are men), we may have found a very promising new treatment approach to hyperandrogenic PCOS and that is, of course, potentially great news. After all, PCOS is widely believed to affect at least 10-13% of all women in reproductive years and this may, indeed, be an underestimate. Unsurprisingly, the potential importance of this paper was further reflected in an accompanying commentary in Science2 (which was longer than the original article summary) and a brief summary in the News Section of JAMA. 3
But here is what really fascinated us here at the CHR about all three of these publications (original paper and both commentaries): all three introduced their respective manuscripts by describing PCOS as characterized by hyperandrogenemia,1 by elevated androgen levels,2 and by elevated levels of male sex hormones known as androgens,3–all obviously incorrect statements because they ignored what, under so-called Rotterdam criteria, is the D-phenotype (also called “lean” or “ovulatory” phenotype).
Though this phenotype after menarche–and until approximately age 25–is also hyper-androgenic, it after that age develops progressive insufficiency of adrenal androgen production (in females adrenals and ovaries produce, each, approximately half of all androgens), resulting in steadily declining androgen levels. For approximately 10 years, between ages 25 and 35, these women remain in what generally are considered age-appropriate androgen levels, but after age 35 they enter the hypo-androgenic range and become
increasingly hypo-androgenic with advancing age.4-6 The roughly 10-year period of being normo-androgenic is also the age range when most PCOS cases are diagnosed. It is therefore unsurprising that the original Rotterdam criteria erroneously described phenotype D PCOS as “normo-androgenic.”
We are reviewing all of this in conjunction here discussed recently published paper1 because by describing PCOS as a hyper-androgenic condition–and this is, indeed, how PCOS is almost universally described in the medical literature–three important negative consequences follow:
(i) PCOS phenotype D diagnoses will often be missed; PCOS is therefore even more prevalent in the general population than is widely appreciated, with prevalence of the D phenotype being widely underestimated.
(ii) While lowering androgens in the other three phenotypes makes sense (whether with myoinositol, as these days widely practiced or in the future, possibly, with artemisinins), in D phenotype lowering androgens will only further aggravate already existing hypo-androgenism. Though these women, while still normo-androgenic, often still spontaneously conceive, once hypo-androgenic, they
(iii) not only become infertile (ovaries need normal androgen levels to produce good quality eggs in good numbers), but often, indeed, become IVF treatment-resistant until their hypo-androgenism is corrected.6
One last point regarding the phenotype D: While the other three PCOS phenotypes later in life are associated with metabolic syndrome (diabetes,
CONFLICT STATEMENT: The CHR and some of its employees were awarded certain U.S. patents claiming treatment benefits from supplementing hypo-androgenic infertile women with the male hormone DHEA. The CHR and these employees, therefore, receive license fees from use of DHEA in hypo-androgenic infertile women. Readers of this article should be aware of this fact since it may bias opinion expressed by the authors of this commentary regarding androgen supplementation in hypo-androgenic infertile women. hypertension, and cariad disease), this does not apply to the lean PCOS phenotype. This phenotype has, however, another associated risk, and that is a hyperactive immune system (autoimmunity, inflammation, allergies). And a hyperactive immune system, if not appropriately treated, predisposes to miscarriages. Often, therefore, these women not only need help in getting pregnant but also may need help in maintaining their pregnancy.
And as a final word regarding PCOS in general, we find it encouraging that other voices are starting to speak out as well about the fact that PCOS represents a broad spectrum of disease that has been widely ignored in the study of PCOS (likely being the principal reason why progress in PCOS research has been so inadequately slow). In a commentary in Fertility and Sterility two U.S. colleagues made this point in discussing two other articles on PCOS in the same issue of the journal.7 Unusually blunt, they made the point that–though both articles they addressed represented seminal contributions from well respected authors–neither adequately addressed this fact.7 Unless we follow their advice of investigating individual sub-groups of PCOS within the broad PCOS family of conditions separately and individually and stop throwing everything that carries the name PCOS into the same basket, we will never figure out what PCOS really is.
1. Liu et al., Science 2024;384(6701):1187
2. Stener-Victorin E. Science 2024;384(6701(:1174-1175
3. Harris E, JAMA 2024;332(4):274
4. Gleicher et al., J Steroid Biochem Mol Biol 2017;167:144-152
5. Gleicher et al., Endocrine 2018; 59(3):661676
6. Gleicher et al., Biomedicines 2022;10(7):1505
7. Rebar RW, Keator CS. Fertil Steril 2024;121(6):934-936
Endometriosis and PCOS: Endometriosis has much in common with PCOS. Like PCOS, endometriosis is one of the most common diagnoses encountered in fertility practice; and like in PCOS, progress in our understanding of this condition has been painfully slow; and like in PCOS, one of the main reasons has been our inability to reach accurate diagnoses of those conditions. Though our textbooks still tell us that a diagnosis through laparoscopy is accurate, that is a misrepresentation because likely a majority of endometriosis cases are only microscopic and, therefore, not visible to the naked eye during laparoscopy. Indeed, multiple random peritoneal biopsies–suggested by some as a way to improve diagnostic accuracy–also can never with certainty rule out the presence of endometriosis elsewhere. And whenever we cannot rule out a possible diagnosis, one has to automatically assume that control populations in studies will be contaminated with the condition and, therefore, biased.
Endometriosis and “unexplained infertility:” With this introduction, we here offer a recent study by Belgian colleagues which in the format of a systematic review, an apparently increasingly fashionable study format in our medical journals which, after all, does not require recruitment of even a single patient and, with only the help of a laptop can be performed from anywhere, attempted to determine “the prevalence of endometriosis in unexplained infertility.”1
That this title of the paper already rep-
resents an oxymoron, did apparently not become obvious to the authors: if a patient is diagnosed with “unexplained infertility,” how can she in parallel have a diagnosis of endometriosis?
We here also do not wish to rehash the absurdity of the concept of “unexplained infertility” since we have discussed this already too often in these pages and in the literature;2 but how long something remains “unexplained,” of course, depends on how deep one digs. To quote the authors, this is how deep their dig went: They performed a systematic search “to identify all studies reported on pelvic pathologies found by laparoscopy in couples diagnosed with unexplained infertility.”
Do we have to say more? With this as the basis for the authors’ literature search, can the 44% of patients by laparoscopy diagnosed pathology being endometriosis really be taken seriously? We don’t believe so! That a significant portion of cases with the pseudo-diagnosis of “unexplained infertility” are endometriosis cases has been known for a long time; but so are other diagnoses as well.2
We brought this paper up, just to demonstrate how bad a paper can be these days that still will be published. That it succeeded to receive a WORST PAPER AWARD for this issue, therefore, should be obvious.
Endometriosis and the immune system: One other overlooked diagnosis often misdiagnosed as “unexplained infertility” is a hyperactive immune system (endometriosis in itself has for decades, of course, been associated with a hyperactive immune system, especially due to autoimmunity and inflammation.2-4 )
Now Bruce A Lessey, MD and colleagues published an interesting paper relevant to this issue,5 which supported a role for endometriosis in unex-
-plained euploid embryo transfer failure.5 More specifically, the authors concluded that suppression of endometriosis with a GnRH antagonist prior to embryo transfer may improve IVF success by addressing underlying inflammatory and epigenetic changes found in so-affected patients.
This is, of course, not a new idea; CHR investigators and colleagues, indeed, already in 1988 suggested that suppression of endometriosis may have beneficial immunological effects on women with endometriosis but, already then, also demonstrated that the effects of a GnRH -agonist were significantly inferior to the effects of the androgen, danazol, which in those days was still a preferred drug in suppressing endometriosis.6 Danazol was then, several years later, demonstrated to have an immunomodulatory effect on the autoimmune response in endometrium associated with endometriosis.7
Endometriosis and cancer risk: That endometriosis has an association with ovarian cancer risk has been known for some time. A recent study in JAMA now not only reemphasizes this risk but defined it in further detail:8 Women with endometriosis had a 4.2-fold higher cancer risk than women without (known) endometriosis. This risk increased to 9.7-fold in women with ovarian endometriomas and/or deep infiltrating endometriosis. Moreover, associations were higher with type I (endometrioid, clear cell mucinous, and low grade serous) than type II (high grade serous) ovarian cancers. An accompanying editorial–like the manuscript–reemphasized that these data support the importance of counseling especially women with deep infiltrating and/or ovarian endometriosis about their increased ovarian cancer risk.9 The editorial raised two additional important points, especially in more high risk situations with deep endometriosis and/or ovarian endometriosis: the possibility of prophylactic surgery, and–as a potentially
important hypothesis–the question whether endometriosis in certain cases can be considered a precursor condition of ovarian cancers.
Endometriosis and adenomyosis are distinct clinical entities with almost identical pathophysiological features. In a commentary, several European colleagues recently argued that both are hormonally active diseases (frankly, we don’t understand what that means) which need to be treated by controlling local hyper-estrogenism and progesterone resistance. The suggested purpose for this commentary according to the authors was to stimulate discussion and spark interest in research of these two condition.10
We, of course, agree with this sentiment, though, unfortunately, their paper did not offer much help. We, question whether the basic underlying premise that adenomyosis is nothing more than endometriosis of the uterus is correct. It appears to us that proliferation of endometrium-like cells within muscular tissue must have very different physiological characteristics proliferation of endometrium-like cells within pelvic and abdominal cavities. Related, JAMA Surgery published an editorial about the intersection of endometriosis and ovarian cancer prevention.11 Its worth the read!
1. Van Gestel, et al., Reprod Biomed Online 2024; 49(3):103848. WORST PAPER AWARD for this issue
2. Gleicher N, Barad DH. Hum Reprod 2006;21(8):1951-1955
3. Gleicher N, Pratt D. Int J Obstet Gynecol 1993; 40 Suppl S21-S27
4. Gleicher et al., Obstet Gynecol 1987;70(1):115-122
5. Lessey et al., Int J Mol Sci 2024;25(13):6852
6. el-Roeiy, et al., Fertil Steril 1988;50(6):864871
7. Ota et al., Fertil Steril 1996;65(3):545-551
8. Barnard et al., JAMA 2024; 332(6):482-489
9. McHale MT. JAMA 2024;332(6):460-461
10. Cozzolino et al., Reprod Biomed Online 2024;48(4):103737
Everything about pregnancy
Pregnancy brain: The term “pregnancy brain” has been around forever, suggesting that pregnant women experience problems with memory, poor concentration, and absentmindedness. Now, as The Wall Street Journal (WSJ) reported in May, one neuroscientist’s experience with her pregnancy seems to have opened up unexpected research avenues after she volunteered to have her brain scanned at various stages of pregnancy.1 Liz Chrastil, PhD, an associate professor in Neurobiology and Behavior at the School of Biological Sciences at the University of California, Irvine, as the WSJ noted, turned her whole pregnancy into a science project, getting 26 brain MRI examinations between 3 weeks before conception, roughly every two weeks during pregnancy, and periodically after birth for two years.
Though it was known from animal experiments that the mother’s brain significantly changes during pregnancy, everybody linked to the study was still surprised about the degree of change they saw in the scientist’s brain. The total brain volume significantly decreased, with gray matter and cerebral cortex shrinking. But, previously unknown, the function of the white matter significantly improved. This aspect of the brain’s elasticity had been unknown because–as it turned out–the white matter after pregnancy quickly returned to its pre-pregnancy state. Why all of this happens is still unclear, but the suspicion is that it is essential for developing the strong mother-infant bond. The more brains change, the stronger mothers score in regard to maternal attachment. So, “pregnancy brain” really exists!
Gestational diabetes (GD): GD is the most common medical complication in pregnancy and, therefore, a condition of extraordinary importance.
Moreover, like diabetes in general, it appears on the rise and not only in economically more advanced nations. An exquisite three-article series in The Lancet on the subject, therefore, caught our attention and the series of articles really offers everything (and more) one may want to know on the subject. Article I addressed pathophysiology from preconception through pregnancy and beyond.2 Article II reviewed the epidemiology of GD and how it should be managed;3 Article III called to action for a life course approach.4 Everybody even minimally interested in this subject should look up these three papers.
Autoimmunity in pregnancy: One does not often find papers reporting on 164 pregnant women with Sjögren’s syndrome; but trust the Chinese, they always have the numbers and in here presented paper compare 164 patients with the disease to 328 age-matched controls in a 1:2 match. Women with the syndrome had a higher incidence of miscarriages, both spontaneous (12.80% vs. 1.52%; P<0.001) and therapeutic (6.10% vs. 0.91%; P<0.05). They also demonstrated significantly more congenital heart disease in offspring (27,34% vs 12.03%; P<0.5)
Interestingly, 22.56% of women with the disease required ART to conceive, a much larger percentage than among controls. They also demonstrated an increased risk for preterm births and small for gestational age infants. In short, Sjögren’s behaves exactly how one would expect an autoimmune disease to behave.
Measles: The measles virus, by many long-ignored and almost forgotten, is unfortunately experiencing a worldwide resurgence. The U.K. and many other countries are, indeed, already in the midst of large outbreaks, and it appears just a matter of time until the U.S. finds itself in the same situation.
Most U.S. citizens were vaccinated in
childhood and, likely, maintain immunity. But in some individuals immunity wears off, and especially among younger citizens the MMR vaccine (measles, mumps, and rubella) was not as uniformly administered as in older generations. The CHR, therefore, routinely tests patients for immunity to MMR and, if found lacking, strongly recommends vaccination at least one month before fertility treatment start (the MMR vaccine is a live virus vaccine and, therefore, should not be administered within 30 days of potential pregnancy).
A measles infection in pregnancy can be dangerous to mother and offspring: mothers are increased risk for measles pneumonia, and pregnancies are at risk for fetal loss, premature delivery, and neonatal death, as an article in eClinicalMedicine (a Lancet journal) recently reemphasized.6 The article also noted that measles infections–though fortunately a rare complication–can also result in subacute sclerosing panencephalitis, a very severe and always fatal neurodegenerative condition, which usually manifests only years after infection, but in pregnant women can have shorter latency with fulminant course in pregnant women.
Preeclampsia and hypertension: A recent paper in the American Journal of Obstetrics & Gynecology addressed the two likely principal physiological processes implicated in preeclampsia at term, what has been described as an anti-angiogenic state and intravascular inflammation. An international team of investigators, led by (be ready for a long name) the Perinatology Research Branch, Program for Perinatal Research and Obstetrics in the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Bethesda, MD and Detroit, MI. reported in 2023 that patients with early preeclampsia demonstrate an abnormal predisposition toward anti-angiogenic influence in virtually 100% of cases.
Term preeclampsia in contrast, however, demonstrates favoritism toward anti-angiogenic effects in the angiogenic/ anti-angiogenic balance in only about 50% of cases, which the investigators then supposedly were able to divide into two specific clusters,7 even though our review of the paper left us somewhat unconvinced about the claimed difference in clinical presentation that was alleged because significance was low (P<0.05).The same group now followed up on their prior paper in the same medical journal (where one of the senior authors, apropos, is the senior editor for perinatal medicine) with a study that investigated the plasma profile of selected cytokines and chemokines in term preeclampsia in women with normal and abnormal angiogenic profiles.8 Their conclusion was that term preeclampsia with anti-angiogenic state was also coupled with an excessive intravascular inflammatory process, whereas the other alleged cluster does have neither of these characteristics.
Unfortunately, the data in support of these conclusions were less than impressive because, without offering much detail, they were based on the investigation of 6 cytokines (IL-6, IL8, IL-12/IL-23p40, IL-15, and IL-16) and 7 chemokines (eotaxin, eotaxin-3, interferon-gamma inducible protein 10, monocyte chemotactic protein-4, macrophage inflammatory protein 1beta, macrophage derived chemokine, and thymus and activation-regulated chemokine). Why these cytokines and chemokines were selected for the study went unexplained. The only thing noted was that these cytokines and chemokines (in unison) were more abnormal among term preeclampsia patients than among normal pregnancy controls. Frankly, the whole data set appear questionable and only barely escaped a WORST PAPER AWARD.
In contrast a paper in Science Translational Medicine seems of special interest, even though on first
impression it may appear odd in claiming that the pathophysiologic signature of placental senescence is shared between preeclampsia and peripartum cardiomyopathy.9 But on closer scrutiny, this should actually not surprise, as both of these conditions very clearly share in having an immunological–and likely at least to a degree–an autoimmune etiology.10,11
And as the final article in this section, a paper in Nature Medicine, based on a large population study, reported that excessive heat exposure in early pregnancy induces a risk for preterm birth in a process mediated by the occurrence of maternal hypertension.12 Does this mean that global warming will result in more cases of hypertensive diseases in pregnancy and higher prematurity rates?
Genetics: Since past Expert Reviews at the American Journal of Obstetrics & Gynecology have not always deserved this categorization, we are very pleased to be able to report this time on an excellent review article on placental, maternal, fetal, and technical origins of false-positive cell-free DNA screening results.13 Especially after just having experienced once again in one of the CHR’s patients after discharge - without prior confirmation by chorionic villous sampling (CVS) or amniocentesis – a termination of pregnancy after false-positive cell-free early pregnancy screening (also called non-invasive prenatal testing, NIPT), we strongly recommend this article to every general Ob/Gyn in obstetrical practice.
Smoking in pregnancy: In a reviewed preprint in eLIfe, Canadian investigators, after leveraging well established CpGs for maternal smoking, constructed a cord blood epigenetic score of maternal smoking validated in one of the European-origin cohorts (n=347).14 It then was tested for associations with smoking status, secondary smoke exposure during pregnancy, and general health outcome in off-
spring in white European (n=397) and South Asian (n=504) pregnant patient cohorts. Findings revealed a strong association between smoking status (P=5.5x10-33) and more hours of self-reported smoking exposure per week (P=7.8x10-9) in White Europeans, but not with self-reported exposure in South Asians. The same score was, moreover, consistently associated with smaller birth size (P=0.0023) in South Asians and lower birth weight in both cohorts combined (P=0.0011).
The authors concluded that this cord blood epigenetic score can help identify offspring exposed to maternal smoking and assess its long-term impact on growth. It, moreover, confirmed a consistent association between the DNAm signature of maternal smoking and low birthweight.
1. McGinty JC. The Wall Street Journal. May 10, 2024. https://www.wsj.com/science/pregnancy-brain-0aa642c5
2. Hivert et al., Lancet 2024;404:158-174
3. Sweeting et al., Lancet 2024;175-192
4. Simmons et al., Lancet 2024;404:193-214
5. Tan et al. RMD Open 2024 ;10:e003616
6. Khalil et al., eClinicalMedicine 2024;72:102594
7. Chaiworapongas et al., Am J Obstet Gynecol 2023;228(5):569e1-569e24
8. Chaiworapongas et al., Am J Obstet Gynecol 2024
9. Roh et al., Sci Transl Med 2024;16(743):eadi0077
10. Gleicher N. Am J Obstet Gynecol 2007;196(1):5e-7e
11. Gleicher N, Elkayam U. Autoimmune Rev 2009;8(5):384-387
12. Wang et al., Nat Med 2024;30:1974-1981
13. Raymond et al., Am J Obstet Gynecol 2024;230(4):381-389
14. Deng WQ., et al., eLife 2024. https://elife science.org/reviewed-preprint/93260?utm source=conte...tm_content=full text&utm campaign=22-July-elife-alert#assessment
Here is another oral presentation from the 2024 ESHRE meeting in Amsterdam, The Netherlands (O-315) which we initially considered to be of interest and in which Danish colleagues concluded that familial endocrine diseases increased the risk of pregnancy loss,
including repeated pregnancy loss. Using a nationwide Danish data set, they defined endocrine diseases as diabetes, thyroid disease, PCOS, and etc. (etc. being an unfamiliar endocrine condition to us but at the core of why we ended liking this abstract less than we initially thought).
Though at least in the abstract not disclosing which other endocrine disease were included among etc., the authors reported that in the study population a family history of endocrine diseases was associated with increased miscarriage risk overall and, in addition, with increased risk for repeat pregnancy loss. They furthermore concluded that women with repeated pregnancy loss (defined as 2 or 3 losses) should be investigated for endocrine diseases and family history for endocrine diseases.
But, once again, one must wonder about the judgment of peer reviewers: We do not recall any published study describing the study population under “etc.” This appears especially ridiculous considering that we are unaware of any mechanism that would or could connect all endocrine diseases with elevated miscarriage risk. We, however, know several endocrine diseases with autoimmune etiology which–in autoimmunity–do have a straight link with miscarriage as well as repeat miscarriage risk. Among those are, of course, type I diabetes, autoimmune thyroid disease and–at least in phenotype D–PCOS, suggesting that the association may not be with endocrine diseases but with being an autoimmune and/or inflammatory endocrine disease. And that, of course, makes quite a differ

Preimplantation genetic testing for aneuploidy (PGT-A) and polygenic testing of embryos (PGT-M)
To a degree it is rewarding for us here at the CHR to acknowledge how much the discussion over PGT-A has changed over the last year. Commentaries regarding the procedure are getting more cautious–can we say more skeptical?1,2 Even Chinese colleagues, in the past unanimous in their admiration of PGT-A, now report that PGT-A does not improve cumulative live birth rates of a cycle cohort of embryos3 (how could anybody ever believe that it did?).
Somewhat amazingly, people still publish papers on PGT-A from cell free spent culture media not understanding that, if PGT-A does not work for embryo biopsies, how can it work for cellfree spent media? (We, of course feel for all the investors in start-ups built on the idea of non-invasive PGT-A. But even those still trying to prove the unprovable at least no longer claim to have discovered the nirvana of IVF.)4
Apparently, some have still not given up: At least the question for those is now “to transfer or not to transfer mosaic embryos,” as a recent review article pretended to do.5 They, however, finally at least admit that some embryos they for long years described as untransferable and discarded, can safely be transferred. Interestingly, it is usually the members of “special interest groups” in reproductive genetics–i.e., the supposed “experts”–who take the longest to understand.
But what we are still missing in the still ongoing PGT-A discussion is the answer to a very simple question: if something does not work, why are we continuing to use it?
Paradoxically, we may, finally, have discovered a real “medical indication” for
PGT-A, the new abortion laws in some states which prohibit abortions after 6 weeks gestational age (as information for the politicians who passed these laws, this means 4 weeks after conception), when most women don’t even know yet that they have conceived. One wonders what will remain for our still unconvinced colleagues to convince patients to undergo PGT-A once the courts end the 6-week restrictions? We can see only one remaining argument: the fear of the unknown!
And then there is PGT-M, increasingly offered by IVF clinics and genetic testing laboratories. And why shouldn’t they. In contrast to PGT-A, PGT-M at least offers a logical proposition in claiming to be able to predict whether a given embryo carries in its few cells a special predilection toward a polygenic disease, like diabetes or heart disease. That PGT-A does not work in accurately differentiating between chromosomally normal and abnormal embryos should have been understood 20 years ago. To find out whether PGT-M is even able to predict the risks for a given disease accurately enough to reach a logical decision to dispose of an embryo will take probably at least 20 years to find out.
And all of this does not even start to address all the ethical concerns that come with PGT-M6 and, after several European societies (including ESHRE) now also have the American College of Medical Genetics and Genomics concerned on the rapid integration of PGT-M into clinical IVF.7
1. Barret F, Molinaro T. Fertil Steril 2024;122(1):68-69
2. Cooper AR, Viotti M. Fertil Steril 2024;122(1):74-75
3. Hu et al., Fertil Steril 2024;122(1):121129
4. Nakhuda et al., Dertil Steril 2024;122(1):4250
5. Muñoz et al., Reprod Biomed Online 2024;48(3):103664
6. Sierman et al., J Assist reprod genet 2024;41:1719-1726
7. Grebe et al., Genet Med 2024;26(4):101052
How RNA has been underestimated
A very interesting recent article in Scientific American made the point that RNAs–and not as widely believed, DNAs–really rule our genome.1 The article presented with this headline based on in recent years discovered thousands of active RNA molecules which in a variety of ways have controlling functions in our bodies.1
It all started once it became clear that the initial belief that only 1-2% of our DNA was “working” in producing proteins by using RNA as messenger that travel to cells where those proteins are produced. Under this false assumption, 98-99% of DNA was considered socalled useless “junk DNA.” This, however, raised the question, if only 1-2% of DNA was through RNA encoding proteins, what was the rest doing?
The answers started coming in as recently as in 2012, when first papers appeared in the literature suggesting that much more of the genome was “functional” in that a much larger percentage of DNA was producing so-called noncoding RNA (i.e. it does not encode proteins as mRNA does) but, still, engages with other molecules, in the process fulfilling a huge array of biochemical tasks. As the article notes, by 2020, the so-called ENCODE project had identified at least 37,600 noncoding DNA in DNA stretches with instructions for RNA molecules that do not code for proteins (ncRNA). If correct–and final numbers are still awaiting confirmation–this would mean that non-coding DNA genes are almost twice as common as genes that code for proteins.
Once again nature has in this way created a thermostat effect for coding genes (we previously have used this analogy in explaining the effects of environmental factors of coding gene functions in a process called epigenetics) because what ncRNA does is fine-tunes the functions of coding
genes, in the process becoming the final determinator of whether protein is made and at what quantities. Unsurprisingly ncRNAs have also been found to play important roles in diseases. The argument that RNA–and not DNA–rules our genome, therefore, has considerable merit.
REFERENCE
1. Ball P. Scientific America, May 14, 2024. https://www.scientificamerican.com/article/ evolutionary-genetics-research-shows-rnamay-rule-our-genome/
Epigenetics
And since we are already talking about epigenetics, Mitinori Saitou’s lab at Kyoto University in Japan published another important paper in Nature magazine, reporting on the in vitro reconstitution of epigenetic reprogramming in the human germ line,1 which has remained a fundamental challenge. It resets parental epigenetic memories and differentiates primordial germ cells (PGCs) into mitotic pro-spermatogonia or pro-oogonia, thereby ensuring sexually dimorphic germ cell development for totipotency. In the paper the authors report on epigenetic reprogramming and differentiation of pluripotent stem-cell-derived human PGC-like cells into mitotic pro-spermatogonia and oogonia, followed by extensive amplification (~>1010-fold). So-produced calls still failed to fully activate genes vital for spermatogenesis and oogenesis. The study, however, provided a remarkable framework for epigenetic reprogramming in humans and represented an important step forward in the potential ability to produce human gametes from stem cells sometime in the not-too-distant future.
1. Murase et al., Nature 2024;631:170178
Female mosaic chromosome loss
As recently has become known as a side product of studies of age-related changes in clonal hematopoiesis, healthy adults experience with advancing age increased sex chromosome
aneuploidies. In females this especially includes loss of one X chromosome (mostly the inactivated one), producing a mosaic XO (Turner syndrome) karyotype, usually in low single digit percentages in peripheral leukocytes. Because it primarily (though not exclusively) affects the inactivated X chromosomes and, likely, because the mosaicism affects only a very small percentage of cells (the percentages can vary between organs), patients are clinically generally not likely to be affected, though this mosaicism apparently increases the risk of developing leukemia. That observation alone, of course, opens the possibility that this kind of low-level XO mosaicism may also have other medical effects (especially if the mosaicism affects the active X chromosomes).
Since the X chromosome represents ca. 5% of the genome and contains several genes relevant to immune function and cancer susceptibility, one can also not preclude Turner syndrome-like effects on fertility at older ages in association with this age-dependent mosaicism, and we have anecdotally seen at the CHR over the years a handful of women with low functional ovarian reserve for age who during karyotyping surprisingly were found to demonstrate this low-level mosaicism. Valid associations with infertility have so-far, however, not been demonstrated. Interestingly, these X chromosome losses are in women much more frequent than losses in autosomes and losses of the X chromosomes in men are much rarer.
All of this information is meant as introduction of a paper by a large number of authors from many universities around the world that reported on genetic drivers and cellular selection of female mosaic X chromosome loss.1 Using data from 883,574 females from 8 biobanks, 12% demonstrates detectable X0 mosaicism in on average 2% of leukocytes. They, indeed, demonstrated an increased prevalence
of myeloid and lymphoid leukemias. They also demonstrated 56 common genomic variants associated with XO, implicating genes with roles in in chromosomal missegregation, cancer predisposition, and autoimmune diseases. A polygenic risk score including 44 allelic shift loci correctly inferred the lost X chromosome in 80.7% of mosaic XO cases in the top decile.
1. Liu et al., Nature 2024;631:134-141
Finally, a treatment for Huntington’s disease?
There are not many worse diseases than Huntington’s disease, an inherited neurodegenerative disease that causes nerve cells in the brain to break down and die. It usually becomes clinically symptomatic in middle age and causes involuntary muscle movements (chorea) and changes in cognition and behavior. After diagnosis, life expectancy is ca, between 15-25 years. It is dominantly inherited from either mother or father (which means one parent usually has the disease) and women as well as men can get it.
A company based in the Netherlands called uniQure recently received FDA Regenerative Medicine Advanced Therapy Designation of an investigational gene therapy (AMT-130 for Huntington’s disease1) and preliminary reports on this treatment have been very positive, leading to a rise in the company’s stock price by over 60%.2
The gene therapy involved in the trial is designed to thwart production of a protein called huntingtin. It normally is helpful in brain function but, when it is mutated, becomes toxic to brain cells. The most recently published interim data suggest that AMT-130 is slowing progression of disease in a dose-dependent way. And so-far has data on 20 patients followed over 24 months; but this is just the beginning of still a tall mountain to climb before a final judgment about this treatment
1. UniQure. June 3, 2024. https://uniqure.gcsweb.com/news-releases/news-release-details/ uniqure-receives-fda-regenerative-medicine-advanced-therapy-rmat
2. Bell J. Biopharma Dive. July 9, 2024. https://www.biopharmadive.com/ news/uniqure-huntingtons-gene-therapy-stock-neurofilament/720856/
Is bird flu the next pandemic?
So-far reported cases in the U.S. are sparse, but at least one report suggests that a good number of cases are going undetected because it seems almost impossible to assume that only 13 cases have occurred in humans. In a small study of farmworkers’ blood samples, 2/14 supposedly never infected individuals demonstrated antibodies to the virus; that is 15.4%. Both had a history of respiratory complaints in the past. It increasingly seems that what we know is only the tip of the iceberg.1
And now, for a change, some good news: Studies suggest that some people, because of prior flu exposure, may be better protected from bird flu.2 The H5N1 strain that is now running the show turned out to be highly lethal in birds, but for now it does not seem to spread easily between people, though it clearly made the jump to humans and experts worry that it could easily become more contagious and spark a pandemic, especially since it is genetically very different from seasonal flu viruses circulating now and in recent years.
But here is some further good news, at least for some people: A 2016 paper in Science magazine found, after analyzing almost 20 years of severe infections caused by H5N1 and H7N9 flu strains, that people remain largely unaffected by flu strains that best matched their first exposure in life to a flu virus, while remaining vulnerable if a strain is mismatched with their first infection. This is important for people born
before 1968 because H5N1 infections were then predominant. In contrast, people born after 1968 can expect to be the lucky ones should a H7N9-like strain start circulating because this is when the H7N9 strain was prevalent.
Should the current bird flu expand, older people, therefore, can expect to be spared, and younger people can expect to be affected.
1. Maxmen A. KFF Health News. July 31, 2024
2. Kozlov M. Nature 2024;631:491-492
For two reasons, the literature about COVID-19 has been overabundant over the last few months: A first one was a rather unexpected summer wave of infections, when expert expected a flu-like late fall-early winter wave1,2 (so much again about COVID experts!) and the increasing attention to long COVID, which now is assumed to have affected at least 7% of U.S. adults.3
KP.3 and KP.3.1.1 now account together for over half of all new cases in the U.S. and long COVID is still only poorly understood.4 However, it increasingly looks like the immune system plays an important role,5-6 possibly involving rogue antibodies.7 Likely at least partially motivated by long COVID, The Lancet has been arguing that medicine should take persistent physical symptoms more seriously (rather than assuming they were supratentorial).8-10
And if all of this were not already enough, here is a little more bad news: As it turns out, those who have recovered from long COVID can suffer relapses or flare-ups of their past COVID not only from fresh COVID infections but also from other viral infections, like cold or flu viruses. Called “viral interreference” this phenomenon has also been seen in patients with HIV and other infections and is characterized by myalgias and the so-called encephalomyelitis/chronic fatigue syndrome.11
Finally, another excellent and very important study in the American Journal of Obstetrics & Gynecology reporting on INTERCOVID-2022, a large prospective observational study between November 2021 and June 2022, when the omicron variant of the COVID virus was dominant. During that study period, newborns of unvaccinated mothers had an increased risk of neonatal death. Neonates of vaccinated mothers, in contrast, had a decreased risk of preterm deliveries and adverse neonatal outcomes. Because the protective effect of anti-COVID vaccines declines over time, the authors of this study recommend that mothers should receive vaccine boosters no more than 14 weeks before any expected delivery.12
A lesson to be remembered in the current COVID wave, even though disease expression is milder than during omicron, and there may be another fall-winter wave on the way, and who knows how malignant the dominant strain(s) will be.
1. CDC, National Wastewater Surveillance System. July 7-11, 2024. https:/www.cdc.gov/ nwss/rv/COVID19-currentlevels.html
2. Edwards E, Syal A. NBC News,July 25, 2024, https://www.nbcnews.com/health/healthnews/covid-isolation-guidelines-cdc-updated-positive-cases-rcna163292
3. Fang et al., JAMA 2024;332(1):5-6
4. Perry Wilson F. Impact factor, Medscape, June 18, 2024. https://www.medscape.com/ viewarticle/we-wont-solve-long-covid-untilwe-decide-what-it-2024a10000b9g?form=fpf
5. Israelow B, Iwasaki A. Nature 2024;631:33-35
6. Lindeboom et al., Nature 2014;631:189
7. Wong C. Nature 2024;630:798-799
8. Editorial. Lancet 2024;403:2565
9. Endresen Reme S. Lancet 2024;403:2569
10. Lõwe et al., Lancet 2024; 403:2649-2662
11. Ready T. Medscape Medical News. July 3, 2024. https://www.medscape.com/view article/cold-flu-virus-can-trigger-longcovid-relapses-2024a1000cau
12. Barros et al., on behalf of the INTER COVID -2022 International Consor tium. Am J Obstet Gynecol 2024; https:// doi.org/10.1016/j.ajog.2024.02.008; on line.
Are infections going bonkers on us?
Doesn’t it suddenly seem like viruses in our environment are out of control? Besides all the above-described viral problems, Dengue fever is reaching historical levels in the Americas, including in the U.S., where close to 3000 cases have been reported to the CDC 1 (real numbers can, of course, expected to be even higher), inducing the CDC to issue an official Health Advisory.2
And a little-known virus, called Oropouche virus, linked to brain malformations and stillbirths, is also surging in Brazil,3 sounding very similar to when the Zika virus started in Brazil several years ago and caused fear in the whole hemisphere. And–though a bacterium and not a virus–even Chlamydia trachomatis, very frequently encountered in gynecology, is surging in the U.S., in Las Vegas demonstrated to peak after holidays and major sporting events, as discovered through wastewater surveillance.4
1. CDC, Cuevas E. USA Today. July 18, 2024. https://www.usatoday.com/story/news/ health/2024/07/18/dengue-fever-us-symptoms-transmission/74373970007/
2. CDC Health Alert Network, June 25, 2024. https://www.cdc.gov/locs/2024/06-272024-Lab-Advisory_CDC_Issues_Alert_Increased_Risk_Dengue_Virus_Infections_ United_States.html
3. Moutinho S. Science 2024.385(6707):355
4. Curtin C. Genomweb. Hune 17, 2024. https://www.genomeweb.com/infectious-disease/wastewater-surveillance-chlamydia-las-vegas-finds-peaks-after-holidays-major
More evidence on the dangers of cannabis in pregnancy
This is, of course, a subject the VOICE has been addressing for ages and, as evidence of the dangers has been increasing–yet seemingly ever-expanding use of cannabis products in pregnancy–we have progressively become exasperated about how little of this message is going out to the public, either through the government or
As we do not want to be too repetitive, only two recent publications as examples: U.S. investigators looked at self-reported prenatal cannabis use in 316,722 pregnancies and maternal pregnancy outcomes.1 Though overall 6.3% screened positive, only 2.9% self-reported; 0.6% used daily, 0.7% weekly, 1.5% monthly or less, and the rest were unknown. Prenatal use was associated with increased risk of gestational hypertension, preeclampsia, smaller or larger than recommended weight gain, and placental abruption.
As an observational study, cannabis use cannot be considered directly responsible for these findings since outcomes obviously could not be adjusted for confounders, including social confounders. The data, nevertheless, are concerning because they just reaffirm what other studies have shown.
An essay by Megan Brooks after interviewing several experts on the subject for Medscape Medical News concentrated on the effects of current-day high potency cannabis (not the fathers’ and grandfathers’ strength) on the adolescent brain because this age group’s brains have been reported especially vulnerable to cannabis. Yet cannabis use (or should we call it “abuse”) has since 2000 in the U.S. increased by about 254% (!!). The consequences in adolescents and young adults are especially concerning because those are also the ages when psychoses often
for the first time become apparent and cannabis, especially in young males, has been shown to trigger psychotic episode.2
1. Young-Wolff et al., JAMA Internal Medicine 2024;e243270
2. Brooks M. Medscape Medical News. May 13, 2024. https://www.medscape.com/ viewarticle/high-potency-cannabis-tied-impaired-brain-development-2024a1000935?form=fpf
We have repeatedly reported in these pages about concerns expressed by investigators, ethicists, and laypeople about the lack of ethical guidelines for an abundance of rapidly evolving laboratory-grown embryo models. Now the U.K developed and published in July a first code of behavior in the use of human embryo models.1
The creation of the Code – its full title being, “Code of Practice for the Generation and Use of Human Stem Cell-Based Embryo Models,” was led by Cambridge Reproduction, an interdisciplinary research center at the University of Cambridge – in partnership with the Progress Educational Trust, a charity, and was funded by the BBSRC, the University of Cambridge Impact and Knowledge Exchange Fund, and UKRI Sciencewise. The complete guideline can be downloaded at: www. repro.cam.ac.uk/scbemcode.

As a brief commentary in Nature magazine noted, countries are grappling with how to regulate research that uses stem-cell-based embryo models. They will be watching the U.K.’s voluntary approach2 (described in this document).
Somewhat related, Chinese investigators, who have made enormous progress in the field, in a reviewed preprint in eLife reported on a human receptive endometrial organoid with the purpose of deciphering the black box of implantation.3 The journal’s editorial assessment of this preprint was: “This study presents a valuable development of endometrial organoid culture methodology that mimics the window of implantation. Functional validation to demonstrate its robustness is lacking; therefore, the study is considered incomplete. The data may be interesting to embryologists and investigators working on reproductive biology and medicine.”
One question this model and the editorial comment immediately raises is, whether the above noted British Code would allow for implantation experiments of artificial human embryos using this implantation model?
1. https://www.repro.cam.ac.uk/scbemcode
2. Mallapaty A. Nature 2024;631:259-260
3. Zhang et al., 2024. https://doi.org/10.7554/eLife.90729.2. July 19, 2024
The amazing plasticity of the primitive endoderm
Another amazing paper, this time from Danish investigators, reported that the primitive endoderm of a blastocyst-stage embryo (derived from the split of the inner cell mass into embryonic epiblast producing the fetus – embryonic cell lineage) and primitive endoderm producing trophectoderm and placenta – extraembryonic cell lineage) has so much plasticity and potency that it can regenerate a complete blastocyst which continues post-implantation development.1
This is not only an astonishing finding but should convince even the most extreme skeptics that a blastocyst-stage embryo can self-correct from having aneuploid cells.
1. Linnenberg-Agerholm et al Cell 2024;S0092-8674(24)00595-6


What prospective patients should know about the CHR:
We guarantee first appointments within 2 weeks.
We reserve daily spots for emergency same-day appointments.
We offer per-need appointments after regular hours (5:00 PM) and on weekends.
The CHR is open 7 days a week and 365 days a year, including holidays.
Contact us:
Appointments: (212) 994-4400
https://www.centerforhumanreprod.com
The Center for Human Reproduction has a long and impressive history of IVF innovations. The team works closely with people from all walks of life, such as single parents, couples, LGBTQ+ community members, or those from overseas. The team has helped more than 18,000 clients become pregnant, many through IVF. We are one of the only fertility clinics that adjusts your treatment plan daily based on your body’s response.




