VOICE
IN THIS MONTH’S ISSUE
THE CHR LETTER DOES FEMALE AND MALE FERTILITY REFLECT UPON A HEALTHY LIFE, AS WELL AS LONGEVITY?
A PIECE OF MY MIND: PRIVATE MEDICINE - AT ITS END OR AT A NEW BEGINNING?
WHAT HAPPENS BEFORE IVF CYCLE START IS NOT ANY LESS IMPORTANT THAN THE CYCLE ITSELF
IN RECOGNITION OF INFANT- LOSS AWARENESS MONTH, A FEW WORDS ABOUT MISCARRIAGE RISK, INCLUDING A NEW “CASE REPORT OF THE MONTH”

NEW CHR PUBLICATIONS
QUESTIONS PATIENTS AND THE PUBLIC ASK
THE CHR’S INTERPRETATION OF RECENT LITERATURE RELEVANT
Investigating the association of vitamin D levels in newly presenting infertile women at the CHR, AMH levels were, as the figure demonstrates, inversely related to vitamin D levels: Best AMH levels were associated with lowest vitamin D levels. This unexpected finding, however, almost completely vanished once data were adjusted for female age, suggesting that this initial observation was mostly driven by patient ages and, therefore, did not suggest any causality.

See article on pages 25-28

OCTOBER 2023 THE CENTER FOR HUMAN REPRODUCTION
TO REPRODUCTION 3 11 13 19 21 24 25 29
Figure: Correlation between vitamin D levels and functional ovarian reserve, as assessed by AMH levels
Ribbon for Pregnancy and Infant Loss Awareness Month
The CHR is known as a “fertility center of last resort,” primarily serving patients who have previously failed treatments elsewhere. Among CHR’s areas of special expertise are treatments of “older” ovaries, whether due to advanced female age or premature ovarian aging (POA), immunological problems affecting reproduction, repeated pregnancy loss, endometriosis, polycystic ovary syndrome (PCOS), tubal disease, male factor infertility, etc.







CONNECT WITH THE CHR Missed the last issue of The VOICE? Access previous issues on thechr.com
www.thechr.com @CHRNewYork @CHRNewYork @CHRNewYork 2 | OCTOBER 2023 | The Voice ADVERTISEMENT
ThE VOICE
We want to thank the unusually large number of readers who commented on our September issue in writing to us. Of course, we welcome any kind of response, however short it may be. We would also like to take this opportunity to remind our readers that The VOICE welcomes the submission of longer pieces for publication, which can be in response to prior articles in the newsletter or may address a new subject in reproductive medicine that is of potential interest to the community of our readers.
We especially value opinions from colleagues that diverge from the opinions held by the CHR and that are routinely reflected in The VOICE because the culture of the CHR fully recognizes the relativity of much of what we do in medicine, even if considered to be routine. Sooner or later, because of newly evolving knowledge, even most routines are replaced.
Per usual, this issue of The VOICE contains a series of articles, answers to questions from the CHR’s patients and/or readers of this newsletter, news about recently in-print CHR publications, and this month again the discussion of a very long list of interesting papers in the medical and scientific literature.
As we announced last month, our lead article this month is about the relationship of female as well as male fertility with good health and longevity. Though mentioned in The VOICE on prior occasions, investigators at the CHR are getting increasingly fascinated by this subject and therefore convinced the editorial team of The VOICE to give this subject more recognition in the pages of this month’s issue.
Another important subject addressed in this issue is whether better IVF outcomes are really in the best interest of IVF clinics. After all, a majority of failed cycles are followed by another cycle and, therefore, additional revenue. In contrast, a successful IVF cycle leading to pregnancy, assuming it is not miscarried, shortly thereafter terminates income for the clinic until another pregnancy is desired (if ever).
Finally, in a short article, we also reemphasize the importance of a comprehensive infertility evaluation for every newly presenting couple. We are often surprised at how little testing was done on a female/couple before the start of the IVF cycle. The CHR always strongly believed that a detailed history and laboratory testing in almost all cases would allow for a presumed diagnosis. Like with any other disease, first comes the diagnosis, which then can be followed by a treatment recommendation. How treatments can be decided considering the frequent poly-causality of infertility has always been somewhat astonishing to us. Understanding causality would appear to us like a rather universal prerequisite for newly presenting couples.
This will do for the month of October. Just a quick reminder that on December 1- 3, 2023, the FRMC (Foundation for Reproductive Medicine Conference) will again be organized in collaboration with the CHR in NYC. We would love to see as many of you as possible at the conference. This is the first time after a 3-year break due to COVID. Again, we were able to recruit an amazing faculty of world leaders in their respective fields. As always, the conference offers day passes at greatly reduced rates to students, residents, fellows, and infertility patients (whether the CHR’s or from other clinics). For further details, please see the program on pages 6-10.
OCTOBER 2023 The V oice | OCTOBER 2023 | 3
The Editorial Staff of The CHR VOICE

4 | OCTOBER 2023 | The Voice ADVERTISEMENT
TRYING TO REACH THE INFERTILITY COMMUNITY?

Have you thought about advertising in the VOICE?
This newsletter every month goes electronically to ca. 80,000 infertility patients, medical professionals in the field, and members of the media, with over 25% (an unusually high number) also opening the VOICE.

For further information, please contact: Ms. Alexandra Rata (212) 994-4400 or e-mail to arata@thechr.com

ADVERTISEMENT 12 | s E p TE m BER 2023 | The Voice The V oice | OCTOBER 2023 | 5 ADVERTISEMENT
Scan the QR code to watch the FMRC 2023 introduction video!




ADVERTISEMENT 6 | OCTOBER 2023 | The Voice

Continued from page 6 ADVERTISEMENT The V oice | OCTOBER 2023 | 7

Continued from page 7 ADVERTISEMENT 8 | OCTOBER 2023 | The Voice

Continued from page 8 ADVERTISEMENT The V oice | OCTOBER 2023 | 9

Continued from page 9 ADVERTISEMENT 10 | OCTOBER 2023 | The Voice
Does female and male fertility reflect upon a healthy life, as well as longevity?


Older evolutionary theories of aging predicted that increased life spans would come at the cost of reduced fertility.1 However, exactly the opposite conclusion appears also logical: Since it is in an evolutionary paradigm, reproductive success leads all biological activities in importance, reproductive success and therefore, fertility/fecundity, must be closely intertwined with good health and longevity. Reversing this paradigm, infertility could also be expected to offer a prospective assessment of disease risks and potentially shortened lifespans.
However, some later studies were unable to confirm these older evolutionary theories. For example, in 2011 when Dutch investigators studied suspected associations of female fertility with age at menarche, menopause, and mortality in 3,575 married women, they failed to demonstrate associations between fertility and age at menarche and menopause but was inversely associated with mortality: Those with 2-3 children had significantly lower mortality than women with no children, though this survival advantage disappeared with larger numbers of children.2
Other studies were more supportive of the older evolutionary theories: A more recent Chinese study of 1,623 older adults on the other hand reported a (marginal – p<0.05) correlation between mortality and larger numbers of children.3 Moreover mortality trended upwards with more male offspring and showed a declining trend with more female births. A study of European populations also found a strong adverse effect of large offspring numbers on longevity.4
It has been suggested that aging may be the consequence of an accumulation of mutations, leading to tissue dysfunction, a notion supported by the findings of a recent study performed in Utah that
demonstrated age-adjusted mutation rates, indeed, associated with higher all-cause mortality in both sexes combined. Those in the top quartile of mutations demonstrated more than double the mortality of those in the lowest quartile (p=0.008), with the medial survival difference being 4.7 years. When it came to fertility, women with higher mutation rates demonstrated significantly lower birth rates and a younger age at first birth. The authors concluded that germ lime mutation rates offer a measure of reproductive as well as systemic aging.5
Two articles in a special issue of Fertility & Sterility recently offered somewhat superficial reviews on the subject in females6 and males.7 That associations between factors relating to reproduction and general health have become a very “chic” new subject of scientific exploration, was recently also demonstrated by a paper in The Journal of the American Heart Association which reported
Continued on page 12
The V oice | OCTOBER 2023 | 11
“Reversing this paradigm, infertility could also be expected to offer a prospective assessment of disease risks and potentially shortened lifespans.”
Continued from page 11
an apparently causal role of several reproductive health factors on cardiovascular disease.8 Specifically, earlier age at first birth increased the odds of coronary heart disease (p=3.72x10-7), heart failure (p=0.009), and marginally for stroke (p=0.048). A higher number of births increased the risk of atrial fibrillation, heart failure, ischemic stroke, and stroke. Earlier menarche increased the risk of coronary heart disease (p=1.68x10-6) and heart failure (p=5.06x10-7), - all pretty remarkable findings.

Assuming that these associations are reversible, one could propose a thought experiment along the following lines: If high fertility shortens life spans, do circumstances that prolong life spans adversely affect fertility/fecundity? An interesting example to investigate within such a context would, for example, be the new weight-loss drugs, like Vegovy, Ozempic, or Munjaro. This family of drugs is now being investigated for additional benefits, including positive effects on cardiovascular diseases in women and men and, possibly, longevity. Under the assumption of reversibility of associations, these drugs then should adversely affect fertility/fecundity. Then again, who would be surprised if the new “wonder drugs” also would improve fertility/fecundity? After all, obesity is in both sexes clearly associated with infertility.
REFERENCES
1. Kirkwood TBL. Nature 1977; 270:301-304
2. Kuningas et al. Age (Dord) 2011; 33(4):615-622
3. Zhou et al. BMC Public Health 2022; 22:682
4. Hsu et al. PLoS One 2021; 16(8):e0255528
5. Cawthon et al. Sci Rep 2020; 10(1):1001

6. Huttler et al. Fertil Steril 2023; 120(3):421-428
7. Belladelli et al. Fertil Steril 2023; 120(3):429-437
8. Ardissino et al. Am J Heart Assoc 2023; 12(5):e027933
12 | OCTOBER 2023 | The Voice ADVERTISEMENT
MY A OF
PIECE MIND

Private medicine - at its end or at a new beginning?
By Norbert Gleicher, MD Founder, Medical Director, and Chief Scientist The CHR, New York, NY

Some history to start with
That private medicine in the U.S. has radically changed over the last decade by now must be obvious. Major changes started in the late 1990s, when Wall Street for the first time concluded that medical care, then mostly provided through small mom-and-pop practices, was inefficient and, therefore, a huge potential financial opportunity for investors. The frequently heard analogy at the time was the evolution from mom-and-pop grocery stores to local, or even multi-state, supermarket chains.
Investors started buying up mom-and-pop practices (and small group-practices) in various medical specialties in attempts to establish dominant nationwide chains of provider practices, literally within months producing several chains with billion-dollar Wall Street valuations. The underlying assumptions were that such national clinic chains would benefit from the usual advantages of size in the availability of financial resources for growth, purchasing power, and
Continued on page 14
The V oice | OCTOBER 2023 | 13
overall cost control. Such clinic chains were also assumed to quicken the pace toward unified best practices and, therefore, improve the quality of medical care by producing improved outcomes.
Even though most U.S. states at that time prohibited the practice of medicine by medical corporations not owned by physicians (laws to these days still in place in most states), corporate lawyers quickly developed legally valid ways to circumvent these laws, which allowed investor-owned medical practice chains to exert virtually full administrative as well as medical control over those companies.
Unsurprisingly, hospitals started feeling threatened in their referral patterns and, consequently, started competing for medical practices, thereby raising already excessive acquisition costs. These competing efforts clearly accelerated the decline in physician-owned private practices all over the country, based on data from the American Medical Association (AMA), by 2016 resulting in a majority of private practices no longer being owned by physicians. Only 47.1% of physicians by that point were still practice owners, the same percentage was employed by a medical practice without ownership, and 5.9% worked as independent contractors.1 As of 2023, almost three-quarters of physicians in the U.S. are salaried employees, and at least half of all physician practices are owned by hospital or investor-owned corporate entities.2
During those early stages of private practice consolidation in the 1990s, hospitals, in the end, were actually more successful in integrating acquired practices because the first wave of large billion-dollar national clinical practice networks in all specialties imploded in the late 1990s even quicker than they were initially put together in what Wall Street later came to call the “physician practice management bubble.” Well summarized by Becker’s Healthcare Website, 3 in 1998, two major physician practice management companies (PPMCs), as these practice networks were then described, in different specialty areas reported disappointing earnings and shortly thereafter declared bankruptcy. By 2002, eight of the 10 largest PPMs had declared bankruptcy
and the figure below best summarizes the complete obliteration of the combined market cap of publicly traded PPMCs during literally only a few short months, obliterating billions of dollars in investments.3


With some personal history added
Then still living and working full-time in Chicago, I was heading the CHR, at the time the largest provider of fertility services in the city by far and, likely, the second- or third-largest IVF provider in the nation. Considering what was happening in other fields of medicine, it was not surprising that the CHR attracted the attention of Wall Street and was aggressively pursued by several prominent Wall Street firms, which saw the CHR, as a key anchor practice for a dominant nationwide PPMC in the infertility field. Though initially hesitant, Wall Street, ultimately convinced us that we should not pass on this opportunity for the CHR, which at that time was owned by over 10 physician-partners and provided fertility services in eight locations throughout the larger Chicagoland area, performing over 2,000 IVF cycles annually, - in those days considered a huge number.
Among several Wall Street firms that had pursued the CHR, we chose Smith Barney as the primary Wall Street sponsor to help in establishing a PPMC and take it public, which was likely the leading Wall Street firm in this market niche at the time. Simply based on the announcement that Smith Barney was sponsoring the CHR, a large local insurance company and a PPMC in another medical specialty area invested several million dollars into the newly formed infertility PPMC which, as of that point, only was represented by the CHR. Funds from those investments were then used over the following year to purchase five additional fertility clinics around the country and to establish a brand new practice in NYC. With a public offering planned within months, Smith Barney strongly felt that the new company had to have a presence in NYC. Because the city at that time did not offer suitable IVF clinics for purchase, a decision was reached to start a brand new CHR-New York (the forerunner of today’s CHR).
Continued from page 13 14 | OCTOBER 2023 | The Voice
Figure 1
As the word got out that Chicago’s CHR was planning on coming to NYC, I was contacted by colleagues from the local Mount Sinai as well as Columbia University fertility programs both experiencing a strong need to move out of their own hospital facilities for their own very special reasons at that time, but not having the necessary financial resources to do so. A collaborative effort with the CHR offered an attractive opportunity. Similarly, for the CHR, the opportunity to work with well-established colleagues in the city from two prestigious academic institutions, of course, represented a unique opportunity to enter the NYC marketplace with considerable immediate visibility and credibility.
There was only one problem: New York medical institutions were not (and to this day are not) known for their collaborative spirits and neither our Columbia nor our Mount Sinai colleagues were very excited about the possibility of not having exclusivity in the relationship with the CHR. Considering my personal experience with academic institutions (which included a fellowship, residency, and two years of full-time faculty membership at Mount Sinai), I strongly felt that the new PPMC in a market as large as NYC should not only become identified with, and dependent on, only one academic institution and, ultimately, indeed, succeeded in convincing both academic institutions that such a solution was also in their best interests.
After contracts were signed, the PPMC rented a large space in mid-town on Madison Avenue and initiated a multimillion-dollar buildout, large enough to accommodate the fertility programs of both institutions. Roughly halfway through construction, rumors started swirling around NYC that Mount Sinai and NYU might merge. Though the CEO of Mount Sinai at the time assured us that such a merger was not as close as suggested in the media and, even if it were to happen, would not affect the agreement, only roughly two weeks later, Mount Sinai informed us that, because of the imminent merger with NYU (which really never happened), Mount Sinai was withdrawing from the agreement and merging instead with the NYU IVF program (which also never happened). Pointing out the previously signed contract, we were advised, that the contract mandated approval by Mount Sinai’s Board of Directors which had not yet met. Mount Sinai considered the contract null and void.
Because of the by then already scheduled public offering of the new PPMC’s shares through Smith Barney and two other Wall Street sponsors, we were advised against pursuing the matter further, with the consequence being that the PPMC found itself exactly in the position we had attempted to avoid, - being identified with, and dependent on, only one academic program. Almost as expected, the arrangement between the PPMC and the Columbia group only survived for barely one year, resulting in yours truly over a period of almost three years having to assume responsibility for the management of centers in Chicago and NYC and, therefore,
weekly splitting time between these two cities. This craziness only ended in 2002, when I finally relocated full-time to NYC after selling the Chicago operations to a local colleague.
Most current observers are unaware that many of the changes we have been observing over the last decade, practically are reruns of the 1990s. Witnessing over the recent decade the resurgence of PPMCs in all medical specialties, including infertility, it became quickly apparent that investors in this second wave of investments in the private practice of medicine learned important lessons from mistakes made in the first round, allowing the quick evolution of seemingly highly successful and very valuable PPMCs of a much larger scale than even the initially most successful PPMCs in the 1990s achieved. At the same time, I would argue that, despite having been more successful than in the first round, current generations of PPMCs and of private practice clinic networks owned by hospital organizations have started to show similar stress symptoms as observed in the late stages of the first PPMC bubble in the 1990s.
Why the bubble burst?
In 1998 we started a roadshow with Smith Barney with a pre-offering valuation of the newly formed infertility PPMC of US$ 110 million (in those days a very high valuation for a start-up). On the second day of the roadshow, the offering was completely sold out and Smith Barney was planning on raising the offering price per share. On that same evening, both the above-noted large PPMCs, however, reported disappointing earnings and we had to interrupt the roadshow. Though Smith Barney had anticipated a quick recovery of the sector and expected a return to a public offering within months, this, of course, never happened and, the whole sector, as the figure above so well demonstrates, basically disappeared (as, apropos, did Smith Barney as well, - a few years later).
Our PPMC still struggled for almost a year but, finally, shut down after selling the individual clinics back to their prior owners. Since the NYC clinic had no prior owner, I purchased it, determined to apply the lessons I learned over the preceding years. Today’s CHR is, therefore, the product of this process, at least to a degree explaining why the CHR, as a fertility center, is so distinctively different from most other providers of fertility services.
But before we get to that, a few reasons why PPMCs failed the first time around: Bill Frack and Nurry Hong in a succinct 2014 postmortem suggested that those failures occurred because the concept at that time “was premature and poorly executed – but not unsound (in its underlying hypotheses) …. and has now a clear strategic rationale and value proposition,” 3 implying that both of those were not existing in the 1990s. Though I agree with their overall conclusion that, for several reasons, the concept may have been premature and was executed poorly, I can agree with this
The V oice | OCTOBER 2023 | 15 Continued on page 16
conclusion only with some further caveats: In my opinion the most important is that investors, still to these days, overestimate the ability of PPMCs to “fill fundamental gaps in the capabilities of traditional physician offices.”3 They, indeed, do have much better abilities to make required capital investments, create data-sharing infrastructures, and achieve administrative efficiency. They also unquestionably have better abilities to acquire new patients, negotiate sophisticated risk-sharing agreements, and drive health care costs lower. But, whether all of these abilities in the end, as promised, improve the quality of health care, in my opinion, is highly questionable.
As we will further discuss below, rapidly evolving evidence suggests the opposite: these developments appear to adversely affect the quality of medical care and, in addition, actually appear to increase costs. Also, based on my experience in managing what likely represented the first (small) clinic network acquired in the fertility field, this is for the following four reasons not surprising: (i) Purchasing physicians’ medical practices, even in the presence of strong financial incentives, instantly significantly reduces their productivity. (ii) Even more importantly, the process adversely affects their creativity (on which past successes of the practice were often based). (iii) The quality of cost-effectiveness evaluations for new capital expenditures declines, and the (iv) introduction of best practice innovations can be equated to “herding cats” and, considering the prevalent natural individualism of medical practice, in general, is either an almost impossible task or if imposed, leads in the long run to an increasingly toxic practice environment between clinic and administration.
As a consequence, profitability expectations are routinely missed, setting into motion “more drastic administrative managerial interventions,” often highly unpopular with the clinical staff and, therefore unsurprisingly, also quickly perceived by patients. What once was a thriving private practice setup, therefore, quickly turned into a “clinic-like” setup, with very different patient expectations. As patient expectations change, the patient population the practice serves changes in parallel, with the important fraction of self-pay patients for every fertility clinic declining and, with it, profit margins narrowing.
Here is a personal example of what happens: Being myopic (nearsighted), I require regular vision checks, for over a decade until recently received from a very personable ophthalmologist in a small and crowded office, - though, despite substantial use of technical support staff, always characterized by an opportunity to interphase with the physician. One day, he unfortunately moved out of the city and sold his practice to an ophthalmologic PPMC. “Wanting to introduce himself to a colleague,” I met the new physician on my first subsequent visit only for a brief handshake. On my second visit a few months later, I saw only staff and decided to switch to a new ophthalmology office.
Because it is only within walking distance from the CHR, I again chose an office owned by a (different) PPMC. During my
first visit, I met a very personable but obviously rushed ophthalmologist who, as a colleague, apologized for “how long she had left me waiting in the exam room.” Returning a little over six months later in response to a routine reminder notice from the office, I was left abandoned in the waiting room for over 45 minutes, even though I, on purpose, had made an early morning appointment before my own office hours started. I finally walked out (without examination), with the reception clerk for the first time noticing me and profusely apologizing for “the office being short on staff.” I am now, of course, again searching for a new ophthalmology practice; this time it will, however, not be a PPMC-owned practice if such a possibility still exists these days in Manhattan!
What all of this means for medicine in general Estimates suggest that, following practice acquisitions in a $ 2 billion acquisition spree in 1997, in 1998, 39 public and 125 still private PPMCs (like ours) existed in the U.S.,3 not counting practice networks created by hospital systems. How could such a seemingly attractive and successful industry then, literally, vanish within months?
The answer is, likely, not as simple as suggested by the principal explanation proposed in the literature: Significant overpayment for physician practices, especially large enough physician groups that were most interesting for investors is indisputable as a contributing factor. These groups were often purchased at 50100% above cash flow basis. But, there were also other important causes why expected profit margins were routinely missed by surprisingly large margins: PPMCs, for example, were hardly ever able to bend anticipated cost curves. Prediction models, built on growth in especially IVF cycle utilization, often also did not pan out. Not only was growth not as rapid as anticipated but, even more importantly, increasing insurance coverage for IVF services, widely anticipated to significantly contribute to cycle number increases, did so much slower than anticipated (insurance companies restricted IVF access, often mandating other treatment modalities before authorizing the use of IVF). Also not anticipated in many forecasts were the effects of increasing insurance coverage for IVF on cycle reimbursement rates. The reimbursement insurance companies were willing to pay, of course, did not even come close to what private market rates had been, further exacerbating the observed deficits from projections.
However, the likely and ultimate reason for the failure of the first wave of PPMCs was a rapidly growing level of dissatisfaction of the medical staff involved. They were not only frustrated by poor management but also by the loss of alleged value of the newly established PPMCs. Because many of the medical practice purchases included equity participation in the PPMC that acquired their practices, physicians saw the value of the sale prices for their practices declining and, ultimately collapsing.3
Though PPMCs in the current second wave claim to have a much clearer strategic rationale and value proposition, I am
16 | OCTOBER 2023 | The Voice
Continued from page 15
not convinced that this is really the case. To me, it still appears that practice acquisition values are often highly exaggerated and that does not only apply to billion-dollar acquisitions of whole networks in the fertility field as we have witnessed (more about that below), but also to million-dollar acquisitions of group-practices, once again often partially paid with equity participation and, therefore, depending on continuous improvement in practice values if sellers are to remain satisfied.
However, the largest danger in my opinion comes from societal dissatisfaction with the trend toward a reorganization of private practice away from physician-owned toward investor-owned practices, which is based on the perception that this is just another profit-motive-driven idea of Wall Street, leading to poorer quality medicine and higher costs.2,4,-6 The field of infertility also must confront the inherent economic conflict represented by the fact that failure to achieve pregnancy in an IVF cycle, more often than not, leads to another IVF cycle (and, therefore, additional revenue for the clinic), while success in establishing pregnancy, assuming no miscarriage occurs, ends the current relationship with the patient (and, therefore, ends all revenue-generation). To a degree, economic incentives in IVF are unnaturally stacked against success.
What all of this means for infertility practice
All of this has major additional significance for the fertility field, where PPMCs are already in possession of a highly significant and ever-growing share of the national market.7 This newsletter in the literature review section routinely offers updates on new acquisitions and sales of individual clinics and/or clinic networks. The most remarkable has been the recent sale of the international IVIRMA network at an amazing enterprise value that exceeded US$ 4 billion.
Our Spanish colleagues who built this empire must be congratulated on an unprecedented business achievement. When first rumors started circulating that IVIRMA was seeking a buyer at a valuation exceeding US$ 1 billion, nobody (except, of course, the company’s ownership) considered this a serious proposition. Barely a year later, the purchase price had quadrupled. Considering standard business models followed by equity investors which expect a highly profitable resale of purchased companies within 3-7 years, one must wonder not only about the overall feasibility of such a venture in the infertility arena, but also about the impact that such a time-restricted, aggressively profit-driven business plan may have on how, and at what cost, such a business model will be pursued by IVIRMA and other similarly-large IVF clinic chains.
One must assume that a hard-driving management structure that, considering existing time pressure, must maximize profitability, will have to drive down the cost structure of the company that offers fertility services to patients and, at the same time, must increase the revenue generated by the company. That billion-dollar acquisitions in health care, consequently, can threaten equitable access to such health care, raise cost, and
reduce the clinical autonomy of physicians has, therefore, raised serious concerns.8
In fertility practice, several years ago we already pointed out the close association with declining live birth rates all over the world because of the increases in numbers and utilization of mostly unvalidated and often even harmful (to outcomes) “add-ons” to IVF practice.9 One, of course, must wonder about potential motivations!
Indicating the dependence of current IVF practice on “addons,” a Wall Street analyst who interviewed me over a year ago, claimed that an analysis he did suggested that roughly a third of U.S. IVF clinics would have to shut down or otherwise reorganize if they lost current PGT-A revenues, as they reflect most of those clinics’ profit margins. Considering low IVF insurance reimbursements and the fact that PGT-A fees (which insurance companies uniformly do not cover) are direct cash payments from patients to IVF clinics, those payments have become crucially important contributions to average cycle revenue for many clinics. Who then can be surprised about increasing PGT-A utilization in the U.S. despite increasing evidence that PGT-A does not improve IVF outcomes in general populations and, in many women, actually reduces pregnancy chance? We, moreover, in an abstract that was accepted at the annual 2023 ASRM meeting in New Orleans and submitted for publication as a full-length paper,10 demonstrated based on a national U.S. data set of reporting IVF clinics that the highest PGT-A utilizing clinics (80-100% of cycles utilized PGT-A) had significantly higher equity and venture capital ownership than lowest utilizing clinics (0-20% utilization). These data suggest the possibility that financier-owned clinics in efforts to raise revenue, consciously or unconsciously favor the utilization of useless “add-ons.”
Conclusions for the CHR
This brings us back to the basic question of this column of whether we are witnessing the end of private medicine or a new beginning. Observing the evolution of several different healthcare systems around the world, my strong suspicion as of this moment is that we in the U.S., indeed, are witnessing the evolution of a new private practice model, which in the infertility field will be bifurcated: a majority of patients will, indeed, receive their treatments in multiple-provider clinic setups through highly regulated and protocol-driven PPMCs and contracted hospital provider networks, while only a small percentage of private practice care (my estimate is ca. 15-20%) will continue to be provided at higher costs in a more traditional model of private practice, characterized by a more personalized physician-patient relationship.
In some specialties, it will be in the format of what nowadays in the U.S. is called “concierge medicine;” in some European countries insurance companies already offer similar services under “supplemental insurance plans” which can be purchased as additions to standard private essential insurance coverage.
The V oice | s E p T m EBER 2023 | 9
on page 10
Continued
The V oice | OCTOBER 2023 | 17 Continued on page 18
In the infertility field, I expect the bifurcation to be not only service-oriented but, based on the recognition that high-volume and low-cost fertility clinics are simply incapable of offering at current reimbursement levels satisfactory services to poor prognosis patients, also qualitative. In other words, I predict that the current discrimination of poor prognosis patients by the medical insurance industry (by, for example, age restrictions for coverage, limitations of cycle attempts, etc.) will expand in parallel to improvements in the general infertility coverage for good- and average-prognosis patients, - not dissimilar to the coverage of IVF services in Scandinavian countries, where IVF services are widely available under government-managed health insurance plans, but cut off at age 40 to 42.
In the early 2000s, the decision that the CHR, going forward, would strive to develop special expertise in managing the “aging ovaries,” we also soon recognized that the medical efforts required to serve a primarily poorer-prognosis patient population would significantly exceed what was required for more standard patients. The CHR at that point saw no other option but to terminate a majority of insurance contracts the center had signed on to before, and also had to reject joining the New York State Program that covered IVF services, as none of these insurance contracts were even close to reimbursement rates the CHR required to reach break-even.
One does not have to hold an MBA degree to understand that caring for much older than average infertile women who have already failed multiple IVF cycles, often at multiple clinics, mandates more involved, personalized, and individualized treatment efforts than are required for on-average, much younger infertility patients, often undergoing their first IVF cycles. The CHR, consequently, has consciously avoided the “clinic atmosphere” that has evolved in many other IVF clinics, especially with the rebirth of PPMCs and hospital-owned provider networks.

By no means meant as a criticism of these clinics, these clinics serve an important function as providers of fertility services for a majority of usually younger and uncomplicated infertile women. Like other fields in medicine, infertility has been evolving, - in the process converting much of what was difficult in the early days of IVF into clinical routine, fully and conveniently accessible to patients in their local neighborhoods at basically the same quality and with similar outcomes as in “famous” IVF centers to which infertility patients in early days of IVF often had to travel over significant distances. By reaching the decision 20 years ago to concentrate on poorer-prognosis patients, the CHR also recognized that these women often would not receive the best care in their neighborhood’s routine IVF clinics, and, therefore, would have to continue to travel to more “specialized” IVF centers (like the CHR).
We feel that the earlier discussed changes in medical practice, and especially in how infertility care is provided, confirm the evolution of a bifurcated fertility field, as we envisioned 20 years ago. We, however, are surprised that this bifurcation apparently
has not yet been recognized by Wall Street and other investors in the fertility field. Consequently, business models to this day appear flawed because they ignore the “most expensive” 15% of infertile women, - poorer prognosis patients. Older women, moreover, represent the age group in which the utilization of IVF is increasing more rapidly than at any other age. One would think that this kind of information would be of interest to investors and insurance companies.
As things stand, poorer prognosis patients are not identified early in their infertility journeys. Therefore, they often unnecessarily fail multiple IVF cycles in such patients’ unqualified IVF clinics. Not only is this emotionally painful and often costly for these patients but those efforts also, often, represent wasted valuable time for patients who can least afford time wasted. Particularly insurance companies should be interested in identifying these patients early because, once pregnant, they, because of unusually high obstetrical complication rates, also represent excessive obstetrical and perinatal costs.
REFERENCES
1. Murphy B. AMA, https://www.ama-assn.org/about/research/ first-time-physician-practice-owners-are-not-majority#:~:text=Ac cording%20to%20data%20drawn%20from,been%20evident%20in%20 recent%20years
2. Zhu et al. N Engl J Med 2023; 389(11):965-968
3. Frack B, Hong N. LEK Consulting. https://www.beckershospitalre view.com/hospital-physician-relationships/physician-practice-manage ment-a-new-chapter.html?oly_enc_id=1505B4491778G9P
4. Borsa et al. BMJ 2023; 382:e075244
5. Goozner M. BMJ 2023; 382:p1396
6. Rotenstein et al. JAMA Health Forum 2023; 4(3):e230299
7. Patrizio et al. J Assist Reprod Genet 2022; 39(2):305-313
8. Shah et al. N Engl J Med 2023; 388(2):99-101
9. Gleicher et al. Hum Reprod Open 2019; 2019(3):hoz017
10. Patrizio et al. Abstract accepted to ASRM 2023 and submitted for publication.
18 | OCTOBER 2023 | The Voice
Continued from page 17
ADVERTISEMENT
CYCLE START IS NOT ANY LESS IMPORTANT THAN THE CYCLE ITSELF
Understandably, everybody is often in a rush to get IVF cycles started: patients are anxious and IVF clinics’ cycles are the principal revenue generator. But many – if not most – IVF cycles require preparation. What we mean by this is that as last-resort fertility treatments, IVF cycles are emotionally more draining than other fertility treatments, very costly (especially without insurance coverage), time-consuming for patients, and highly complex for fertility service providers. The latter point is especially relevant for those fertility clinics that seriously attempt to individualize cycle protocols, as has been a routine practice at the CHR for almost 20 years.
Many, if not most IVF clinics unfortunately still routinely utilize only one or two standard IVF protocols and even this small number of protocols defines an IVF cycle as a complex process, even though the complexity greatly increases with increasing numbers of potential cycle protocols. Moreover, complexities increase even further with the likelihood that an initially selected protocol may change in the middle of a cycle because of unanticipated observations. As utilizers of highly individualized IVF protocols, we are emphasizing the difference between using only a few standard protocols and true individualization of protocols because the latter is only possible if infertile couples are extremely well “understood” in their often-multifactorial pathology that underlies their infertility. Such an understanding can only be obtained through a very detailed history and laboratory investigation at the intake of new patients into a fertility program.
Such a detailed historical evaluation of new patients at presentation is only a first step in ultimately being able to define the “best” IVF cycle protocol. The patients’ histories then dictate their diagnostic testing process and these two steps in preparing them for their “best” treatment protocol are mutually dependent on each other: A better history will lead to a more thorough diagnostic work-up and vice-versa.
Recognizing that infertility, much more often than not, will be multifactorial (with one or more than one cause in females and males), it is not only of crucial importance to diagnose all possible contributions to a couple’s infertility, but it is maybe even more important to treat all of these contributing causes concomitantly. Just missing one, may invalidate all other treatments, - even if administered perfectly. This is where, at times,
patience is required from patients as well as the treating physician because not all treatments follow the same time schedule.
One of the more crucial steps in preparing many infertile women for an IVF cycle is attempting to improve ovarian performance. Please note that we are attempting to improve ovarian performance and not ovarian reserve! The difference between these two concepts is important because ovarian reserve, defined as the number of remaining follicles/oocytes in a woman’s ovaries, very likely cannot be improved with current therapeutic abilities. Ovarian performance, or what we years ago started calling the “functional ovarian reserve (FOR),” defined as the small growing follicle pool immediately after follicle recruitment between primary follicle and small antral follicle stages, on the other hand, can be improved in many infertile women, but such improvements take time – at least six to eight weeks. The reason is that small growing follicles between primary and small antral stages still require at least six to eight weeks of further growth and maturation before they reach the so-called gonadotropin-dependent stage (i.e., where they become dependent for further development on the gonadotropin hormones routinely used in IVF cycle stimulations).

CONFLICT STATEMENT
The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product. Since the following paragraphs address androgen supplementation with DHEA, readers of this paragraph are advised that opinions expressed in this paragraph may be biased by financial interests.
We often explain these treatments of ovaries with a car analogy: If a car engine does not function well anymore and we do not want to replace it, we usually “clean it out,” trying to make it perform at its best, while fully recognizing that a new engine would do better. The same principle applies here: As we cannot yet “make new eggs,” we are doing the next best thing, - we are trying to make our patients’ ovaries work better, fully appreciating that young donor eggs, of course, would do better.
WHAT HAPPENS BEFORE IVF
The V oice | OCTOBER 2023 | 19 Continued on page 20
In principle, this is done in two ways: If infertile women have low androgen levels (male hormones, with the most important one being testosterone), we supplement with androgens, in most cases with a hormone called dehydroepiandrosterone (DHEA), from which our bodies make testosterone.1 If insulin growth factor -1 (IGF-) is low, we supplement with human growth hormone (HGH) because HGH stimulates the production of IGF-1.2
Both of these treatments make only sense if either androgens or IGF-1 levels are low. Androgens as well as IGF-1 decline with age. Androgens especially may also be low in some younger women. Ovaries, however, require both of these substances at normal levels if the “engine” is to work at its best because both support the hormone FSH during small growing follicle stages in making follicles grow and mature. Once they have done their job, the so-affected follicles still need six to eight weeks to reach gonadotropin dependency. Consequently, if patients with low androgens and/or IGF-1 are properly prepared for IVF, they must be on supplementation for at least six to eight weeks before IVF cycle start.
However, this is not the only preparation that may take time before an IVF cycle is initiated. Another very important point is the integrity of the uterine cavity. Replacing embryos into an abnormal endometrial cavity makes little sense. Therefore, every endometrial cavity should be explored before IVF, at least through a hystero-sonogram. If this test does not offer reassurance that the cavity is normal, the test must be followed up by an MRI of the uterus and/or a diagnostic hysteroscopy. If a pathology is found, it in most cases should be removed. Such pathologies may be scar tissue in the cavity, endometrial polyps, or
into the cavity protruding fibroid tumors (so-called submucous myomas).
Whether surgery should be performed or not, is not always an easy decision. If it involves minor interventions, as with mild adhesions or with small polyps, surgery is usually indicated before initiations of IVF cycles because such minor surgeries usually do not delay an IVF cycle by much. The situation can be very different if a myomectomy (surgical removal of a fibroid) appears indicated. Such a procedure can delay an IVF cycle often by at least three to four months, which especially in older patients may have a significant impact on pregnancy chances.

This brings us to the last point this communication wants to make: Ultimately, an IVF cycle should never be started without, first, having an in-depth discussion with the involved patients. In the CHR’s opinion decisions that are either black or white are relatively rare in medicine. Most decisions reflect varying gray tones. Involvement of well-informed patients in the decision-making process, therefore, at the CHR is considered essential. As we always explain to our patients: “We are not qualified at all to tell our patients how to live their lives. We, however, are very well qualified to advise them what their options are (with risks and benefits). In the end, the choice must always be theirs!”
REFERENCES
1. Gleicher N., BJOG 2016;123(7):1106
2. Gleicher et al., J Assist Reprod Genet 2022;39(2):409-416
3. Gleicher et al., Hum Reprod 2013;28(4):1084-1091 ADVERTISEMENT
20 | OCTOBER 2023 | The Voice Continued from page 19
& INFANT LOSS
AWARENESS MONTH
a few words about miscarriage risk, including a new “Case Report Of The Month”

Pregnancy and Infant-loss Awareness Month reminds us how prone mankind is to pregnancy loss: roughly onethird of implanting embryos are miscarried so early that these miscarriages are called “chemical pregnancies.” That such early pregnancy losses existed was not even recognized until IVF came on the scene a little over 40 years ago because no one obtained early enough blood tests to test for pregnancy in those days. If menstruation was delayed by a few days, this was usually attributed to physiological irregularities in menstrual patterns. These pregnancies are called “chemical” because they are only diagnosed by chemical means via a pregnancy test and are not yet visible by ultrasound.
Another third of pregnancies experience spontaneous miscarriages after clinical diagnosis by ultrasound at approximately 5.5 gestational weeks (3.5 weeks post-implantation) when a gestational sac with a fetal pole and fetal heart can be visualized with good ultrasound equipment.
What these statistics demonstrate is the extreme inefficiency of human reproduction, resulting in the loss of approximately two-thirds of all embryos that are already implanted. In addition, only a relatively small minority of embryos available for implantation do so, further aggravating this inefficiency.
Considering this sparsity of successful pregnancy from an evolutionary standpoint, it must offer significant benefit for the survival of our species to have been retained by evolution over so many thousands of years. What this likely benefits seems to be very obvious in this case: a high loss rate of embryos and already established pregnancies very obviously protects our species from much larger numbers of abnormal births than we currently experience.
In practical terms, this means that we may not want “to fight” a large majority of pregnancy losses and, indeed, may not view them as “adverse” events, but, rather, consider them as an integral part of one of nature’s evolutionary ground rules, - “survival of the fittest.”
This is an important message that is not always well understood by the public and should be appropriately reemphasized this month. However, at the same time, this is also a message that does not apply to all pregnancy losses. A
minority of pregnancy losses happen to what are considered normal pregnancies, with the term “normal” used to describe pregnancies that are chromosomal-normal and do not demonstrate obvious congenital morphologic abnormalities. It does not describe a truly fully “normal” pregnancy because truly “normal” pregnancies will not get lost.
A better way to make this point is probably to differentiate pregnancy losses based on whether they are caused by, the fetus or the mother. For the fetus being the responsible party, either the egg, embryo, and/or fetus must demonstrate abnormalities. If that is not the case, one can assume that the reason for the pregnancy loss must be the mother’s. It is of utmost importance that this differentiation is made correctly.
The reason why this differentiation is of such significant importance brings us back to the fact that fetal abnormalities, with very rare exceptions, are random events (i.e., bad luck) and, therefore, do not indicate recurrence risks. They rarely demand treatments. Maternal causes of pregnancy losses often lend themselves to treatments and must be pursued to try to avoid repeat losses. That usually means that affected women must undergo appropriate diagnostic testing, followed by treatment.
Maternal causes of losses encompass a wide variety of possibilities and one, therefore, faces the question of when such diagnostic workups should be initiated. Medical textbooks to this day suggest that they should not be pursued until a woman has experienced three consecutive pregnancy losses. The CHR does not subscribe to this opinion and believes that it causes unnecessarily preventable pregnancy losses. We here at the CHR do not believe that there exists one miracle number of prior miscarriages that, automatically, defines who should be tested. Instead, the CHR pursues (as in almost all of its clinical decisions) an individualized approach, in this case, based on a patient’s detailed past medical and family histories.
Here is our “CASE REPORT OF THE MONTH” as a good example: A 32-year-old woman came to the CHR because she had not conceived in over a year after experiencing a prior spontaneous pregnancy that unfortunately had been miscarried at 10 weeks gestational age, a fetal heart was seen on ultrasound in an apparently normal singleton
IN RECOGNITION OF
PREGNANCY
Continued on page 18 The V oice | OCTOBER 2023 | 21 Continued on page 22
Continued from page 17 pregnancy several weeks after. The only unusual finding noted in the ultrasound report at the time was a small (and asymptomatic) subchorionic hematoma.
Discussion: This patient presented to the CHR at a relatively young age because of so-called “secondary” infertility of over one-year duration (this diagnosis is reached if infertility sets in after at least one prior pregnancy). It is important that she did not present because of concerns about her earlier miscarriage, even though that miscarriage occurred relatively late (at 10 weeks and after a positive fetal heart) and was accompanied by a subchorionic hematoma by ultrasound. We will return to why these two findings are of importance. Her past medical history was otherwise negative, - except that her medical chart reflected a maternal history of systemic lupus erythematosus (SLE), - an autoimmune disease.
The female and her partner underwent a routine diagnostic infertility workup at the CHR. Based on mildly high FSH (10.8 miU/mL) and mildly low AMH (0.8ng/mL) for her age, the female was diagnosed with premature ovarian aging (POA). She, in addition, demonstrated a mildly positive ANA of homogenous and speckled type at a titer of 1:160 (a relatively low level).
In summary, the patient was a young woman with secondary infertility, POA, and with evidence of a hyper-active immune system based on a positive ANA and a maternal family history of SLE (autoimmunity is highly familial). She, in addition, in her prior miscarriage demonstrated a subchorionic hematoma, which is a finding associated with the presence of autoimmunity (especially ANA and antiphospholipid antibodies) as well as increased miscarriage risk. 1,2
Suddenly, this patient who initially presented as an infertility patient, based on her own past medical history and family history was revealed as a woman at significant risk for repeat miscarriages. After only a single prior miscarriage, she had to be viewed as a potential repeat immune aborter. She, in the CHR’s assessment, instantly, was no longer an infertility patient, but also a potential miscarriage patient.

After chromosomal abnormalities, a hyperactive maternal immune system (mostly due to autoimmunity and inflammation) is widely considered the second-most common (and most controversial) cause of repeat miscarriages.2 Here is an additional very important point: If one looks at the so-called natural history of women with repeated immune miscarriages, it starts with one or more miscarriages (likely more often male than female pregnancies). This is not where things end. At a certain point, these women, suddenly, no longer get pregnant. In other words, they develop secondary infertility, usually associated with POA. Almost uniformly, when we see patients with a history of one or more miscarriages followed by POA and/or secondary infertility, the common denominator is usually a hyperactive maternal immune system, most frequently evidence for autoimmunity and/or inflammation.
Then, such-affected women not only require help in achieving pregnancy, but also need treatments to be able to maintain their pregnancy without miscarrying. The earlier such patients are identified, the more miscarriages can be prevented. Consequently, the CHR strives to diagnose these women not only after they already have lost three pregnancies but, as this case report so well demonstrates, hopefully, much earlier.
Two more important points: Textbooks to this day are not only behind when suggesting that it takes at least three consecutive clinical pregnancy losses to diagnose a woman as a repeat (immune) aborter. They are also mistaken when demanding that these losses be “clinical.” “Chemical” pregnancies, have, indeed, been demonstrated to have the same diagnostic significance as “clinical” miscarriages in reaching a diagnosis of repeat (immune) pregnancy loss. The other important point to remember is that, while a majority of miscarriages are the consequence of chromosomal abnormalities in the fetus, considering the “wisdom of nature,” those are mostly early miscarriages before the fetal heart. Therefore, the later a miscarriage occurs, the less likely is it due to a chromosomal abnormality in the fetus and the more likely is the cause a maternal immune problem.
Finally, there also exists several other additional maternal problems that can lead to pregnancy losses, including uterine abnormalities, medical maternal problems during pregnancy, and pregnancy complications. However, the diagnoses of all of these problems is usually much more obvious.
REFERENCES
1. Li et al., Ann Med 2021;53(1):841-847
2. Baxi LV, Pearlstone MM., Am J Obstet Gynecol 1991;165(5):P1423-1424
3. FERTILE BATTLE. Odendaal et al., Fertil Steril 2019;112(6):1002-1006
22 | OCTOBER 2023 | The Voice Continued from page 21
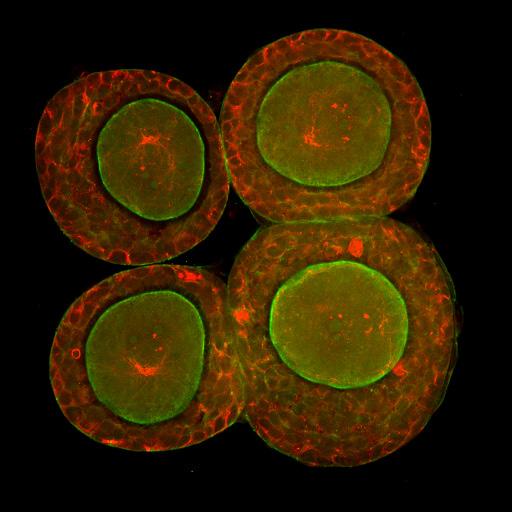
A longstanding collaboration with the Brivanlou laboratory at The Rockefeller University produced this image of a human embryo that was donated for research. Investigators used a variety of antibodies to stain this embryo with different fluorescent dyes allowing them to identify the various cell types that emerge to form both precursors of the placenta and yolk sac as well as the embryo proper.

Photo Gallery
Capturing oocytes at the earliest stages of their development is one of the challenges being confronted by investigators at the CHR. Here we see four early stage so-called pre-antral follicles that have been labelled to understand how the oocyte cytoskeleton contributes to establishing the shape and physical relationships with outer granulosa cells. The green staining reflects the protein actin at the oocyte boundary whereas the red stain illustrates the network of microtubules both within the oocyte (center of each of the four follicles) and in associated granulosa cells.
DR. ALBERTINI’S
Image 2
The V oice | OCTOBER 2023 | 23
Image 1
NEW CHR PUBLICations

Gleicher N, Darmon S, Patrizio P, Barad DH. The utility of all-freeze IVF cycles depends on the composition of study populations. J Ovarian Research 2923;16:190
The CHR’s investigators in this manuscript tackle a subject dear to our heart, - the importance of patient populations in whom studies are performed for the interpretation of study outcomes and the applicability of conclusions to different patient populations. This manuscript addresses this issue regarding the claim made by some colleagues that all-freeze cycles with subsequent thaw cycles offer better IVF outcomes than immediate fresh transfers.1,2 Even though this claim has been convincingly rebutted,3,4 the practice, like many other “add-ons” to IVF, unfortunately, continues rather unabetted, partially encouraged by the ASRM by recognizing it in its annual clinic reporting scheme as a formal cycle outcome option.
To explain the discrepancies in reported outcomes with all-freeze cycles, the CHR’s investigators in this study that was modelled from retrospective anonymized electronic CHR data selected patient populations and compared in those varying patient populations the clinical utility of all-freeze IVF cycles. As expected, depending on patient populations in which these comparisons were made, the effects of all-freeze cycles varied greatly, reemphasizing the point that medical journals in the infertility field must pay more and better attention to the, unfortunately, widely-spread practice in the field of performing studies in highly selected (often best-prognosis) patient populations and then extrapolating the conclusions of these studies to completely different patient populations.
REFERENCES
1. Shapiro et al., Fertil Steril 2013;99(2):389-392
2. Shapiro et al., Fertil Steril 2014;102(1):3-9
3. Wong et al., Cochrane Database Syst Rev 2019;61(1):52-57
4. Maheshwari et al., Hum Reprod 2022;37(3):476-487
CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR
ADVERTISEMENT 24 | OCTOBER 2023 | The Voice
UESTIONSPATIENTS AND THEPUBLIC ASK
QCan I use DHEA and myo-inositol together?

We see with increasing frequency, patients who were prescribed DHEA as well as myo-inositol, and that, in most cases makes little sense because both supplements oppose each other in a very important function: As the precursor of testosterone, DHEA increases androgen (male) hormones, while myo-inositol reduces androgens. Consequently, DHEA supplementation is often indicated in women with infertility who demonstrate low androgen levels, such as women above age 38-40, women with premature ovarian aging, and several other rarer causes of female infertility. In contrast, myo-inositol is indicated in women with abnormally high androgen levels (most but not all PCOS patients), but also has several other potential indications, mostly relating to insulin and gonadotropin signaling.1

The first question to ask when considering either DHEA or myo-inositol supplementation is what are a patient’s androgen levels? DHEA should be only prescribed if androgens are low, and myo-inositol should only be prescribed if androgens are too high. If this first ground rule is followed, there will be very few instances where the possibility of prescribing DHEA and myo-inositol together will even come up. These possibilities will arise only if other than high androgens demand the prescribing of myoinositol.
What could those be? There have been suggestions of marginally improved pregnancy chances in IVF cycles,2 but the quality of this and other studies claiming outcome advantages in fertility treatments was uniformly quite poor. In short, there is no serious evidence for improvements in outcomes of fertility treatments in women with normal (or low) androgen levels if supplemented with myo-inositol.
We usually agree with many, if not most, recommendations Rebecca Fett, the author of ”It Starts with the Egg,” makes in her book (a 3rd edition was just published), and recommend it to our patients. In a recent mailing to her followers on August 20, 2023, she, however, seeded confusion when correctly noting that “the value of myo-inositol outside of PCOS is still uncertain. She then continued into somewhat controversial areas when stating that with “insulin resistance or high fasting insulin levels, which can cause lower percentages of mature eggs in IVF and low fertilization rates as well as recurrent miscarriages … women should be talking to their doctor about supplementing with myo-inositol.” She further claimed that “myo-inositol appears to help ovarian follicles respond to FSH,” and that “new studies indicate that in women with very high FSH levels and poor follicle growth in IVF, supplementing with myo-inositol may help ovarian cells respond better to FSH.” The latter is exactly what androgens (and IGF-1, - i.e., human growth hormone) are doing. Her bottom line was that, “if you have low testosterone and low DHEA but also have insulin resistance and low fertilization rate in IVF, it is possible that taking both, myo-inositol and DHEA, could be helpful.”


Continued on page 26 The V oice | OCTOBER 2023 | 25
Everything “is possible,” but to practice medicine is to make risk-benefit decisions whenever an intervention is considered. If this is done, considering the potential risks of adversely affecting female fertility by reducing androgens - against no realistic potential benefits - we would argue that combining DHEA and myo-inositol supplementation, simply, doesn’t ever make sense. If a patient has insulin resistance, why not place her on metformin, even that should be done only cautiously.
REFERENCES
1. Gambioli et al., Pharmaceuticals (Base); 202114(6):504
2. Lisi et al., Reprod Biol Endocrinol 2012;10:52
I am HIV-positive, and we want a baby: what now?

The first question is, who is HIV-positive? One partner or both? If it is only one partner, are you making sure you are protecting the other partners from getting infected? Is the infected partner taking prescribed medications? If both of you are infected, are both of you regularly taking your medications?
Being infected with the virus is no longer the death sentence it used to be in the early days of the AIDS epidemic. If infected women and men take their prescribed anti-viral medications, their viral load usually becomes “undetectable,” AIDS becomes a chronic condition, and patients can experience a normal lifespan.
When it comes to having a baby, there are differences between infected males and females. Let’s start with males in this case: If a male has an undetectable viral load, the semen is with overwhelming likelihood not infectious. Most fertility centers, therefore, have no problem handling such semen. The story is a little different if the male’s viral load is not at undetectable levels. In such a case, the semen is still infectious and a male with a detectable viral load can (and likely will) infect his female partner if a condom is not used during intercourse. For fear of contamination, most fertility centers in such cases will refuse to work with the semen of such individuals until their viral titers become “undetectable” after appropriate treatments.
If the female is infected but has undetectable viral levels and wants to become pregnant, she is free to proceed for as long as she continues to take her medications uninterrupted throughout her pregnancy. The reason is that, if her newborn baby then receives anti-HIV medications after birth for 2-6 weeks, the chance of the baby being infected by HIV becomes negligible (less than 1%).1
A final word on HIV and getting pregnant: There exists increasing evidence that infertility may be increased in women with HIV. One reason is that their ovarian reserve (the number of remaining eggs in ovaries) appears to be diminished.2
A low ovarian reserve may also be a price to pay for getting great treatment with contemporary anti-retroviral drugs because, at least in some small animal models, these drugs have been demonstrated to disrupt follicle development.3 If you are a young HIV-positive woman who is in treatment and is planning to have a baby sometime in the future, we recommend that you ask your gynecologist to regularly, every year or two, check your ovarian reserve. You also may want to consider freezing your eggs while you are still young.
Anti-viral HIV treatments have also been reported to adversely affect semen analyses (mostly motility).4 However, the reason why declines in male fertility have been reported in association with positive HIV status, is not clear yet.5
REFERENCES
1. HIV.gov. https://www.hiv.gov/hiv-basics/ hiv-prevention/reducing-mother-to-child-risk/ preventing-mother-to-child-transmission-of-hiv/
2. Van omen et al., AIDS 2023;37(5):769-778
3. Aijbaye O, Taylor-Robinson SD., J Exp Pharmacol 2023;15:267-278

4. Jerónimo et al., Hum Reprod 2017; 32(2):265-271
5. Akang EN, et al., Andrologia 2022;54(11):e14621
Do Vitamin D levels affect IVF outcomes?


The effects of Vitamin D levels on female and male infertility have remained controversial. A recent review of the literature regarding the effects of the vitamin on males concluded that vitamin D plays a role in male reproduction, exerting positive effects on semen quality, especially on sperm motility in, both, fertile and infertile men.1 Available data for clinically significant effects on IVF cycle outcomes, however, are lacking.
The situation on the female side is similar: Vitamin D supplementation appears to impact gene expression in granulosa cells,2
Continued from page 25 26 | OCTOBER 2023 | The Voice
and does not appear to have any effects on cytokine/chemokine levels in uterine fluids.3 Effects have been suggested in PCOS patients and a prospectively randomized study of vitamin D supplementation before IVF in women with PCOS was announced in 2020 but has not been published yet.4
The CHR supplements patients with low vitamin D, we recently looked retrospectively at a large number of women who underwent IVF cycles at the CHR at their initial presentation to the center to see how their vitamin D levels related to their AMH levels (representing ovarian reserve). Interestingly, at first glance, the results suggested an opposite effect from what had been expected (see the Figure below): AMH levels were the best with the lowest vitamin D levels. Once this correlation was adjusted for female age, most of the gradient disappeared. In other words, at least on first look (and not published yet), the CHR’s new patients did not demonstrate a statistical association between vitamin D levels and ovarian reserve.
Stem cell treatments in infertility?

It suddenly seems like stem cells are everywhere in fertility treatments. It all started over 12 years ago when Indian investigators reported the first case of an allegedly successful autologous bone marrow stem cell transplant in a woman with severe Asherman’s syndrome.1 This was followed in 2014 by a report by Yale University investigators on a murine model that supported the notion of bone marrow-derived stem cells improving Asherman’s syndrome.2 In the same year, another Indian group reported successful injection of bone-marrow-derived mononuclear cells sub-endometrial into the endometrial cavity.3 The concept entered more mainstream in the field around 2016 when Spanish investigators first reported in a mouse model that human CD133+ mesenchymal bone marrow-derived stem cells promote endometrial repair after injury.4 This was followed up by the allegedly successful treatment by the same group of investigators of 11 women with Asherman’s syndrome with autologous CD133 + bone marrow-derives stem cells injected into the uterine vasculature.5 So far, this group of investigators unfortunately have not followed up since with a larger series of patients, as one by now would have expected. Nor have others reported on experience with such treatments with sufficiently large case numbers to reach serious conclusions about the efficacy of such treatments, though additional small case series have been reported,6 as have been further animal model studies in support of the concept.7, 8
Investigating the association of vitamin D levels in newly presenting infertilewomen at the CHR, AMH levels were, as the figure demonstrates, inversely related to vitamin D levels: Best AMH levels were associated with the lowest vitamin D levels. This unexpected finding, however, almost completely vanishedonce data were adjusted for female age, suggesting that this initial observation was mostly driven by patient ages and, therefore, did not suggest any causality.

This does not mean that vitamin D levels may not have positive or negative effects in IVF cycles on some subpopulations, but, overall, there appears no strong effect one way or the other.
REFERENCES
1. Calagna et al., Nutrients 2022;14(16):3278
2. Makieva et al., Hum Reprod 2021;3691):130-144
3. Cermisoni et al., Hum Repod Open 2022;(2):hoac017
4. Hu et al., BMKJ Open 2020; 10(12):e041409
This concept was seriously questioned when Australian, Chinese, and U.S. investigators questioned the ability of bone marrow-derived stem cells to transdifferentiate into the endometrial stroma, epithelium, and/or endothelium.9 Since then, an additional study reporting on 25 patients receiving bone-marrow-derived stem cells, injected sub-endometrial, has been reported by the previously noted group of Indian investigators.10 Moreover, Chinese investigators suggested that cord-derived mesenchymal stem cells may (at least in animal models) also be effective in repairing Asherman’s syndrome.11 Another group of Chinese investigators found umbilical cord stem cells installed over two consecutive cycles into the endometrial cavity effective in treating thin endometrium following Asherman’s syndrome.12 A single case from the Indian group reported on the successful treatment of a woman with bone marrow-derived stem cells after genital tuberculosis.13 Turkish investigators reported improvements in endometrium after two injections of bone marrow-derived mononuclear cells into the endometrial-myometrial junction within one week.14
The variability of methodologies and the anecdotal nature of published reports based on small numbers of patients and variability in underlying uterine conditions at present, therefore, raise serious questions about the utility of the concept of treating Aschermann’s syndrome (and thin endometria) with mesenchymal stem cells.
Injection of mesenchymal stem cells into ovaries in women with POI/POF or for what widely has been called “ovarian rejuvenation,” has also been proposed.15 Available valid data in support of this proposition is just as limited, even though the number of publications significantly exceeds stem cell use for uterine pathology. Here are selected findings of interest: Human amniotic epithelial cells are
Figure: Correlation between vitamin D levels and functional ovarian reserve, as assessed by AMH levels
Continued on page 28 The V oice | OCTOBER 2023 | 27
of low immunogenicity and have similar features to stem cells. They have been used in animal models to test the hypothesis that stem cell injections into ovaries can reverse cyclophosphamide-induced POI/POF.16 Iranian investigators demonstrated that intra-ovarian placement of autologous adipose-derived mesenchymal stromal cells appears safe and leads to (inconsistent) declines in FSH levels in POI/POF patients.17
Aside from innumerable animal studies suggesting a “rejuvenating” effect of stem cell injections into POI models, two cases of allegedly successful intraovarian autologous stem cell treatment for low functional ovarian reserve in women were again reported by Indian Investigators.18 Iranian investigators claimed improved outcomes following intraovarian administration of autologous menstrual blood-derived mesenchymal stromal cells in women with the ESHRE definition of POI/POF (which is FSH below 20.0mIU/mL rather than the U.S. definition which requires an FSH of >40.0mIU/mL), 19 which under CHR criteria represents POA patients. A recent Chinese systematic review and meta-analysis reported 26 animal studies and six clinical studies on the subject. The clinical studies suggested that stem cell therapy may marginally decrease FSH levels (P = 0.04) but very significantly may increase antral follicle count (p < 0.00001), pregnancy rate (p < 0.01), and live birth rate (p < 0.001). Yet, interestingly, the study demonstrated no improvement in menstrual function, oocyte numbers retrieved, and embryo numbers obtained.20 Moreover, the Spanish group of investigators previously referred to regarding the use of stem cells for Asherman’s syndrome, in a review of the subject concluded that follicle reactivation techniques in models appear promising but current evidence is still too scarce to warrant clinical use 21 (beyond experimental protocols with appropriate consents).
REFERENCES

1. Nagori et al., J Hum Reprod Sci 2011;4(1):43-48.
2. Alawadhi et al., PLoS One 2014;9(5):e96662
3. Singh et al., J Hum Reprod Sci 2014;7(2):93-98
4. Cervelló et al., Fertil Steril 2015;104(6):1552-1560
5. Santamaria et al., Hum Reprod 2016;31(5):1087-1096


6. Zhao et al., Sci China Life Sci 2017;60(4):404-416
7. Sahin Ersoy et al., ol Ther Methods Clin Dev 2017;4:169-177
8. Liu et al., J Cell Mol Med 2018;22(1):67-76
9. Ong et al., Stem Cells 2018;36(1):91-102
10. Singh et al., J Hum Reprod Sci 2020;13(1):31-37
11. Lv et al., Mol Reprod Dev 2021;88(6):379-394

12. Zhang et al., Stem Cell Res Ther 2021;12(1):420
13. Patel et al., Clin Exp Reprod med 2021;48(3):268-272
14. Arikan et al., J Assist Reprod Genet 2023;40(5):1163-1171
15. Gao et al., Tissue cell 2022;74:101676
16. Zhang et al., Am J Transl Res 2020;12(7):3234-3254
17. Mashayekhi et al., J Ovarian Res 2021;14(1):5
18. Tandulwadkar et al., J Obstet Gynecol India 2022;72(suppl 2):458-460

19. Zafardoust et al., Arch Med Res 2023;54(2):135-144

20. Hu et al., Arch Gynecol Obstet 2023;doi: 10.1007/s00404-023-07062-0, Online ahead of print
21. Pellicer N, Cozzolino M, Diaz-García C, et al. Reprod Biomed Online. 2023;46(3):543-565
Continued from page 27 ADVERTISEMENT 28 | OCTOBER 2023 | The Voice
RECENT LITERATURE, relevant to REPRODUCTIVE MEDICINE
Mostly placed into a clinical context, we in this section of the newsletter offer commentaries to a broad survey of articles in the English literature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. Since this is the first issue after the newsletter’s summer break in July and August, we this month offer almost three months of literature review.
Articles are mostly chosen for their translational value for clinical medicine, even if, superficially, a direct link to clinical practice may not always be instantly obvious. Chosen articles, even if, for example, basic science or business articles, often help in determining where clinical practice will likely go and, by doing so, serve an important translational purpose.
Translational research has been the CHR’s principal goal since its founding in 1981 and has over the years produced a significant number of U.S. patents. It has also propelled the CHR into its current status as a worldwide center of last resort for infertile patients who have failed treatments elsewhere. This section of The VOICE, demonstrates and makes public the process through which the CHR for decades has been following and interpreting on an ongoing basis the published literature, a process always at the very core of how research and clinical practice has evolved at the CHR.
Ethics in reproductive medicine
More on embryo models
Several prominent laboratories from around the world over recent months have published human embryo models produced from pluripotent human stem cells. Unsurprisingly, as these models increase in similarity to natural human embryos, their production and utilization in research have attracted increasing attention and controversy. The head of one leading research lab in this arena, Nicolas C. Rivron, PhD, (apropos, also a speaker at the upcoming FRM Conference, December 1-3, 2023 in NYC), from the Institute of Molecular Biotechnology of the Austrian Academy of Sciences at the Vienna Biocenter, in Austria, published an interesting “Perspective: article in Cell in which he (and co-authors from other European laboratories) attempted to propose an ethical framework for human embryology with embryo models, starting with a more refined legal definition of what represents a “human embryo” and suggest a tipping point at which further advancements in similarity of models toward natural embryos, such models would have to be awarded similar protection to that of natural embryos.1
This paper is probably so-far the most thoroughly thoughtout document addressing this increasingly controversial issue and should be read by everybody interested in the subject. Unsurprisingly, Nature magazine picked up on the subject in a new article by Philip Ball, reemphasizing the point made by Rivron et al that sometime in the not-too-distant future, a tipping point will be reached where scientific advances will narrow biological gaps between model and natural embryos to such a degree that any ethical distinction between the two will have to disappear.
REFERENCES
1. Rivron et al., Cell2023;186:3548-3557
2. Ball P., Nature 2023;620:928-929
Should pregnant and lactating women be included in clinical trials?
At first glance for very valid reasons, pregnant and lactating women have traditionally been excluded from clinical trials. Likely the most obvious reason was concern about potential adverse effects on offspring. But, there was also a second important reason for the recognition that pregnancy affects and modifies practically all physiological processes in the body of a woman.
the
chr’s interpretation of
Continued on page 30 The V oice | OCTOBER 2023 | 29
Not being the first to address this issue in recent years, but probably gaining more than the usual attention by being published in Nature Medicine, two female Canadian physicians now effectively argued for the inclusion of pregnant and lactating women in clinical trials for cardiovascular diseases.
They argue that “excluding women who are pregnant or lactating or have childbearing potential rather than obtaining their consent for trial participation breaches the ethical principle of autonomy, beneficence, and justice.” They further argue that excluding this patient population hurts mothers and offspring by depriving them of new high-quality evidence for treatments they may need during pregnancy, as a consequence depriving mother and child often from effective treatments.
The authors also acknowledge that this is not an “all or nothing” proposition, recognizing “justifiable reasons” why some women should still be excluded from trials: (i) If the studied condition does not affect pregnancy and/or lactation, (ii) There is a likelihood of harm from a trial intervention, (iii) Pre-clinical or clinical data already suggest such harm, (iv) The intervention already does have known benefit for a pregnant woman or is clinically indicated (i.e., participation in the trial would be potentially harmful by preventing the woman to receive the best treatment), and (v), of course, in absence of proper informed consent.
REFERENCE
1. Assadpou E, Van Spall HGC., Nat Med 2023;29:1897-1899
Gender-affirming surgery
The subject of gender-affirming surgery (GAS), especially in young children and teenagers has not only split the medical community, but has also become a highly volatile political issue, almost daily making headlines in newspapers and other media and, likely, trending toward becoming a major issue in the 2024 national elections we are quickly approaching. A recent publication in JAMA Network Open, therefore, had major relevance when the investigators from NYC and California reported on such surgeries between 2016 and 2020 based on national statistics for the U.S.1
Among 48,019 patients identified to have undergone GAS, 20,999 (52.35%) were between ages 19 and 30. Remarkably, 3,678 (7.7%) GAS were performed in children and teenagers between ages 12 and 18. Procedures rose from 4,552 in 2016 to 13,011 in 2019, and mildly declined in 2020 to 12,818. Most procedures performed were breast and chest surgeries, but genital procedures increased with advancing age.
REFERENCE
1. Wright et al., JAMA Network Open 2023;6(8):e2330348
Posthumous use of semen
Cappy Rothman, MD, an old friend of the CHR from California, as a urologist, was not only an infertility pioneer on the male side of infertility treatments by establishing the country’s best-known donor sperm bank, California Cryobank (now under different ownership), but as a recent article by Jenny Kleeman in the New York Post (September 10, 2023) reminded us, Rothman was the first to collect
semen from freshly expired individuals for potential posthumous use. He did this, in a very public way after Robin Cranston, the son of late Senator Alan Cranston (D-Calif), in 1980 at age 32 had died at the senator’s request. And as Rothman notes in the article, “the ASRM was not supportive at all.” Though this semen sample was never clinically used, the precedent made history, and postmortem sperm retrievals from deceased males can now be viewed as almost routine even though they occur only relatively infrequently.
Kleeman in her article points out that New York State requires a court order before sperm can be extracted from a dead person and whoever makes the request, whether parents or a partner, must demonstrate that the deceased wanted to have a child before his death. In contrast, in Israel, the posthumous use of sperm according to the article happens so often, that it no longer is considered news. For posthumous use, sperm cryopreservation prior to deployment has also become popular in militaries, from Ukraine to Russia, the U.S., and Israel. Therefore, posthumous use of semen is here to stay.
REFERENCE
1. Kleeman J., New York Post. 9/10/23. https://nypost.com/2023/09/09/ posthumous-conception-is-changing-motherhood/
Are we ready for the artificial uterus?
In 2017, we reported in these pages for the first time on the use of an artificial uterus in premature lambs. By now, this technology has advanced to a possible point where the FDA is considering how scientists may test such an artificial uterus (not a real uterus, but a concoction made up of a plastic bag with connected tubes that deliver amniotic fluid and/or oxygen as well as medications).
As Liz Essley White reported in The Wall Street Journal (September 14, 2023),1 the project is driven by a Philadelphia company, called Vitara Biomedical, which also posted photos of lamb fetuses in such an environment in 2017. Limits of potential viability currently hover between 21 and 22 weeks, as Essley White noted in her article. The purpose of an artificial womb, at least in the early stages of development, would be to lower this threshold, maybe, by a few more weeks.
The subject also attracted Nature magazine, where Max Kozlov offered an even more insightful article, which not only reported on the scientific history of efforts to develop an artificial womb in the lamb model in preparation for upcoming human trials in more detail, but also head on addressed ethical concerns. That the development of an artificial womb could one day replace pregnancy as we know it, unsurprisingly, appears to be a dire concern, but based on interviews with several researchers in the field, Kozlov dismisses this concern as “too far in the distant future to make any current discussion worthwhile.”
He also does not skirt around the “current politically charged environment for reproductive rights,” following the recent US Supreme Court abortion decision to strike down Roe v. Wade and return abortion decisions to the states. Artificial wombs do have the potential to further reduce the age of viability of human embryos. Considering that the original Roe v. Wade decision protected fetuses from being aborted after viability was reached, this makes the concept of artificial wombs a highly relevant issue for the abortion debate.
Continued from page 29 30 | OCTOBER 2023 | The Voice
Will we ever be able to offer extrauterine pregnancies to maturity and delivery? Somewhat in disagreement with Kozlov, we would suspect that, with evolving AI, this goal is probably closer than we currently can even imagine.
REFERENCES
1. Essley White L., The Wallstreet Journal, September 14, 2023, https://www. wsj.com/health/fda-artificial-womb-rules-human-clinical-trials-fcb923a4
2. Kozlov M., Nature 2023;621:458-460
Counseling and compassionate medical care in the face of poor chance and/ or clinical futility
It is rare that we, here at the CHR, agree with colleagues when it comes to the management of poor prognosis patients. Most of the time, for several reasons, we disagree: (i) We believe that patients are often too early directed into third-party egg donation. (ii) We consider egg donation and excellent treatment options, though only as a last resort. (iii) We are not qualified to tell patients how to live their lives, however, we feel exceptionally well qualified to advise them regarding their options and the potential outcomes of those options. The decision is always the patient’s.
A recent article by Chicago-based reproductive endocrinologist
Sigal Klipstein, MD, is an exception in that there is absolutely nothing in an article on the role of compassionate reproductive care and counseling in the face of futility, published in Fertility and Sterility, 1 to which we would disagree. It is an exceptionally well-thought-out, researched, and written piece, which we recommend to everybody dealing with poor prognosis patients in their fertility practice.
REFERENCE
1. Klipstein S., Fertil Steril 2023;120(3);409414
Reproductive endocrinology &
infertility
Is there a stress effect on ovaries?
If you believe a group of investigators from Harvard’s TH Chan School of Public Health, in Boston, MA, then the answer is yes. However, if you read the paper published in Reproductive Biomedicine Online, you get an almost perfect lesson on data manipulation. In other words, how one should not analyze, report, and interpret research data.
Using the Perceived Stress Scale 4 (PSS-4) only out of 520 women seeking fertility care and enrolled in the Environment and Reproductive Health Study between 2005 and 2019 had ovarian
reserve markers including antral follicle count (AFC), FSH, and AMH values. In other words, the study did not involve 520 patients as the introduction seems to suggest, but only 185 patients. The first question to ask is why these 185 women had their ovarian markers drawn and the remaining 335 did not? Could the answer be that those 185 patients had more evidence of fertility problems than the other 335? In other words, was initial patient selection already biased to a degree that does not allow for universally applicable conclusions?
The authors then claimed that higher total PSS-4 scores were negatively associated with AFCs and AMH levels. The authors had split their already shrunk study group of 185 patients into tertials (of ca. 61 patients) of stress levels from mild to severe (please remember, the PPS-4 is based on self-reporting). Levels II and III together barely demonstrated lower AFCs than women in the 1st tertial (both p<0.05). Though also claiming lower AMH, no p-values are offered. Moreover, without presenting any hard statistical data, the authors claimed that these reported associations varied based on “several socioeconomic factors” and were observed among younger women, minorities, college graduates, and those with household incomes of less than $100,000.
The VOICE has not selected the “worst paper” of the month in a long time, however, this paper definitely deserves this rare honor.
REFERENCE
1. Minguez-Alarcón et al., Reprod Biomed Online 2023;46(6):956-964 WINNER of “WORST PAPER” OF THE MONTH!
Another example of how androgen supplementation studies should not be performed
CONFLICT STATEMENT: This commentary addresses the clinical use of androgen supplementation in women with infertility. The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product. Since the following paragraph addresses androgen supplementation, readers are advised that opinions expressed in this paragraph may, therefore, be biased.
The authors of this paper are very lucky that the preceding paper was published in the same month otherwise this paper would have won the award for “worst paper” of the month. Let’s start from the beginning: This Danish group of investigators wanted to investigate whether
on page 32 The V oice | OCTOBER 2023 | 31
Continued
androgen supplementation in women with low ovarian reserve improves IVF outcomes. This is still a reasonable question to ask, considering existing differences of opinion. One then must ask why would they go about designing their study in such ridiculous ways?
(i) If one wishes to find out whether androgen supplementation may be helpful, should one, first, not determine whether patients need androgen supplementation (i.e., have low androgens? After all, aspirin cures headaches only in people who do have a headache! Here androgen levels were not assessed before treatment.
(ii) If androgen is to be supplemented, should it not be done most reliably and straightforwardly by either testosterone or DHEA administration? Why use a “concept” that involves “androgen priming with a 3-day administration of hCG before IVF (of course, developed by the senior author who “investigated it in normal responders with regular menstrual cycles”) which is not the study population here.
(iii) The authors note that this method of androgen induction only affects ovarian androgen production by theca cells. Infertilityassociated hypo-androgenism is, however, virtually in 100% of cases, the consequence of adrenal androgen insufficiency.
(iv) Androgen supplementation in hypo-androgenic infertile women must start at least 6-8 weeks before IVF cycle start because androgen effects on follicle growth and maturation occur primarily at small-growing follicle stages. For that reason, short-term androgen supplementation does not work. Small growing follicles still need at least 6-8 weeks to reach gonadotropin dependency, when they become responsive to gonadotropin stimulation in IVF cycles.
Finally, a few words about statistics:
(v) Using patients as their own controls favors “regression to the mean.”
(vi) This study was based on 20 patients chosen from 30 eligible patients. Why they were chosen is not provided, but it really does not matter because there is no IVF study in the world where only 20 patients can ever offer a statistically significant result.
It is astonishing that this study could make it through peer review, therefore, we award it the honorable price of “runner up” to “worst paper” of the month. It was truly a hard choice!
REFERENCE
1. Friis Wang et al., Hum Reprod 2023;38(4):716-725 RUNNER UP TO “WORST PAPER” OF THE MONTH!
Further evidence for the adverse effects of autoimmunity on fertility and pregnancy
Though it is likely because of its association with psoriatic arthritis in recent decades more visible as an autoimmune disease, psoriasis has generally not been particularly associated with infertility and/or
pregnancy complications. The CHR has for many decades advanced the viewpoint that autoimmunity in general is the culprit, and not individual diseases.1 This model of autoimmune effects on pregnancy (and vice versa pregnancy effects on autoimmunity) follows the similar observation that autoimmunity is a highly familial occurrence, even though phenotypical expression of disease type may vary in families. It is believed that the familial occurrence of autoimmunity follows a polygenic inheritance pattern, but the expression of the disease phenotype will depend on epigenetic environmental influences.
Now come British investigators who report on national trends in the UK from psoriasis on fertility and adverse pregnancy outcomes, offering interesting further insights. The data included over 6 million patients between 1998 and 2019 who were of childbearing ages between 15 and 44 years.
Patients with moderate to severe psoriasis had lower fertility rates (p < 0.001) and a higher pregnancy loss ratio once pregnant (p < 0.001). An increased risk of venous thromboembolism dissipated after adjustments. Moreover, the study, somewhat surprisingly, did not find an increased risk for antenatal hemorrhages, preeclampsia, and/or gestational diabetes. We would have especially expected preeclampsia to be increased.
REFERENCES
1. Gleicher N, Friberg J., JAMA 1985;253(22):3278-3281
2. Chen et al., JAMA Derm 2023;159(7):736
Increased stroke risk after fertility treatments?
We would not be surprised if the paper discussed here relates to the previous one at least indirectly. In a cohort study of 31,339,991 pregnant women between 2010 and 2018, women treated for infertility demonstrated a distinctively increased risk of stroke within one year in comparison to normal-fertile women.1 The risk for hemorrhagic stroke was greater than the risk for ischemic stroke.
Every time medical problems manifest themselves postpartum, the specter of autoimmunity must be considered because disease flares postpartum (up to 5 months) is a typical characteristic of autoimmune diseases.
REFERENCES
1. Sachdev et al., JAMA Network open 2023;6(8):e2331470
“Add-ons” in reproductive medicine
We don’t know what drives our field toward an almost endless stream of guidelines, good practice recommendations, and/or other forms of “expert opinions.” This trend appears to accelerate even though everybody accepts that expert opinions are at the very bottom of the evidence pyramid and are therefore basically worthless. Add to this that by the time a serious assessment of the literature has been completed and written up, concluding opinions are often already outdated by newer data.
A good example of the increasing absurdity of such expert opinions is the recent “good practice recommendation on add-ons in reproductive medicine,” published by the ESHRE Add-ons working group.2 Employing large numbers of “working groups,” ESHRE is the largest
Continued from page 31 32 | OCTOBER 2023 | The Voice
abuser among societies issuing practice guidelines in reproductive medicine. As in this case, they practically end up being just another set of useless expert opinions.
As often expressed in The VOICE, the CHR is most definitely not friendly toward the utilization of many, if not most, “add-ons” in infertility practice. At the same time, the CHR also opposes the expression of opinions in opposition to newly proposed treatments that are based on unreasonable demands for evidence. Once again, the above-referenced ESHRE publication is a good example: Here, 14 prominent experts who constituted the working group, all very credible in their own rights, were willing to issue practice recommendations for not less than 42 “add-ons” by the group identified as involved in the practice of reproductive medicine. At 43 pages, the manuscript is already unusually long but, considering other sections of the manuscript, on average offers less than one page of discussion per reviewed “add-on.”
Unsurprisingly, many reviews of subjects are superficial. Unfortunately, they often are also characterized by selective reference citations, raising suspicion about biases by whoever aggregated data and, ultimately, reached a conclusion, with details of this process not even disclosed. Also, one cannot be surprised about an unusually short materials and method section, considering the size of this project. This paper in many aspects is more a caricature than an example of how a rare “expert opinion” should be reached and communicated. The number of subjects this working group chose to tackle, in itself, reflects the absurdity of this project and, in principle, of similar publications.
REFERENCES
1. Sachdev et al., JAMA Network open 2023;6(8):e2331470
2. ESHRE Add-ons working group, et al., Hum Reprod 2023; dead184. doi: 10.1093/humrep/dead184. Online ahead of print.
Human embryo implantation
In many ways, embryo implantation in humans is still a black box waiting to be explored. But progress in recent years has been quite remarkable and anybody interested in the subject must read the recent review article by Muter et al. in Development.1 It is a masterpiece and deserves the time it takes to get through it. The authors point out that implantation in humans is “interstitial,” which means that the entire conceptus embeds in the endometrium before the trophoblast invades beyond the uterine mucosa into the myometrium. The article offers within an evolutionary framework, a detailed up-to-date review of what is known about human implantation. The only important issue we found missing is the remarkable observation that normal uterine development, up to at least day 10 after fertilization, is completely independent of any maternal contribution.2 The absence of any direct interphase between host and invader during these days, very obviously, allows the maternal immune system “to get used to the invader” by developing tolerance pathways. Short of that “get-to-know” period, the maternal immune system, very likely, would instantly attack the invading semi-allograft (or in the case of egg donation, a complete allograft).
REFERENCES
1. Muter et al., Development 2023;150(10):dev201507
2. Deglincerti et al., Nature 2016;533(7602):251-254
In Vitro Fertilization (IVF)
Perinatal outcomes after cleavage and blastocyst stage embryo transfers
After several reports in the literature, including some from Scottish colleagues in Aberdeen, reporting on distinct differences in perinatal outcomes between cleavage and blastocyst stage embryo transfers, now comes a paper from the Aberdeen group comparing 60,962 IVF cycles, 18,249 cleavage stage to 42,677 blastocyst stage cycles, without being able to demonstrate significant differences in perinatal outcomes.1 Most surprisingly, they reported no weight differences, a previously significant finding by several groups, who noted larger weight post extended culture to blastocyst.2
Thus, this cohort study contradicts the earlier meta-analyses by the same investigators without being able to offer a good explanation for this discrepancy in results. The authors, however, did point out that blastocyst-stage transfers, often, are restricted to good prognosis patients, while cleavage-stage transfers usually are applied to poorer prognosis patients. They furthermore suggest that cleavage stage transfers in good prognosis patients at least theoretically, may demonstrate better perinatal outcomes than blastocyst stage transfers. However, this hypothesis remains to be confirmed.
REFERENCES
1. Marconi et al., Hum Reprod Update 2021;28:1-27
2. Marconi et al., Fertil Sterile 2023;120(2):312320
More on how oocytes change with advancing age
We, in last month’s VOICE reported on a recently published paper by the CHR’s investigators which reported on how the efficiency to produce good quality embryos of eggs at different maturity grades changes as women get older.1 Now, U.S. investigators report in Molecular Human Reproduction in a mouse model, controlling separately for age and FSH administration, that advanced maternal age increases the susceptibility of eggs to high FSH levels by producing fewer total number of oocytes as well as viable oocytes, while inducing increases in ootoxicity and aneuploidy. 2
Commenting on these findings, the authors offer two explanatory hypotheses: (i) High FSH is ootoxic to ovulatory oocytes and, therefore, the cause of low pregnancy rates in women with high FSH levels; and (ii) Advanced maternal age increases the susceptibility of oocytes to FSH-induced ootoxicity and aneuploidy.
From their study, results the authors concluded that maternal age and gonadotropins, independently, contributed to oocyte quality. They furthermore hypothesized that ovulatory oocytes from older women are more susceptible to the induction of spindle defects (and, therefore aneuploidy) than younger eggs and that FSH in those older eggs, therefore, functions as an “involuntary killer” of oocytes.
This is an interesting observation because it suggests once again that the age of oocytes greatly matters and that younger and older oocytes must be treated differently. Assuming that what the authors of this
Continued on page 34
The V oice | OCTOBER 2023 | 33
study observed in mice is also occurring in human eggs (and mouse and human eggs, of course, are not equal and, indeed, often greatly differ in functionality), this would mean that older women should likely be treated with the lowest possible gonadotropin dosages that still elicit an appropriate ovarian response because smaller numbers of better eggs are, obviously, preferable over larger numbers of poor eggs.
That female age adversely affects IVF cycle outcomes also independent of embryo ploidy, was recently demonstrated by Italian colleagues.3 An accompanying editorial (reflection) speculated on possible causes.4 Neither authors nor commentators mentioned, however, the fact that poorer prognosis patients, including older women, usually receive higher gonadotropin dosage. Based on the above-noted mouse paper, those could then be the culprits.
REFERENCES
1. Nicholas et al., iScience 2023; 26:107308
2. Bernstein et al., Mol Hum Reprod 2023;29:gaad030
3. Vitagliano et al., Feril Steril 2023;129(2):251-264
4. Cromack SC, Pavone M.,Fertil Steril 2023;120(2):266-267
More on the hypothesis of embryo selection
The hypothesis that an IVF cycle’s embryo cohort must have a “best” embryo has been at the core of IVF practice almost since its inception. However, it is important to point out that this idea has remained an unproven “hypothesis,” which investigators at the CHR have increasingly come to doubt in its clinical relevance.
A series of articles on the subject of Fertility and Sterility, therefore, is timely. A very astutely and carefully worded introduction to three articles on various aspects of embryo selection is deserving of mention.1 Three papers following the introduction are less impressive.2-4 Giménez et al presented a review of time-lapse imaging and, in our opinion, clearly overshooting in their conclusion after allegedly having reviewed 47 articles. To state that, “time-lapse imaging has emerged as a powerful tool for creating predictive models of IVF outcomes,” is simply incorrect!
Documentation of continued embryo development through timelapse imaging has proven to be an interesting research tool and has contributed to our understanding of embryo development; but we
are unaware that it, to this point, has demonstrated any positive impact on pregnancy, live birth rates, and/ or IVF cycle outcomes in general. This may not be the fault of time-lapse imaging because, assuming that most embryo cohorts, after adjusting for oocyte maturity at the time of egg retrieval, may not be variable enough in pregnancy and live birth potential to allow for the selection of “best” embryos, time-lapse cannot be held responsible for the failure to do so.
Time-lapse in our opinion is left with only one remaining chance of proving itself as a potentially valuable tool in selecting the “best” embryo(s) and that is the possibility that developmental observations made by time-lapse in combination with selected patient characteristics, through artificial intelligence (AI) learning will ultimately find in a multifactorial model enough differences between embryos to be able to define the “best” embryos and, indeed, improve pregnancy and live birth rates (a possibility further explored below). However, that will only happen if there is enough of a difference among embryos in a cohort that exceeds the sensitivity threshold of traditional embryology and that is in no way assured.
In another paper in the series, Jiang and Borman suggest that AIbased ploidy predictions of embryos have the potential to improve IVF pregnancy rates and reduce costs for IVF.3 Once again, we question the optimism of the authors because it appears that based on two separate unproven assumptions: (i) that PGT-A (whether invasive or non-invasive ) at blastocyst-stage can reliably predict an embryo’s ploidy, and (ii) there is enough difference in embryo quality between embryos of a single cycle cohort to select the “best” embryos and, in doing so, achieving significant improvements in IVF cycle outcome. Increasing evidence, however, suggests that PGT-A (in whatever format) does not improve IVF outcomes and in some subgroups of infertile women, as repeatedly pointed out before in these pages, actually adversely affects pregnancy and live birth chances.
In a third paper, Cinnioglu et al offer a systematic review of non-invasive (ni) PGT-A.4 Though they correctly conclude that niPGT-A is not yet ready for primetime, the authors failed to mention the principal problem with niPGT-A, which is that it, still, is PGT-A! In other words, even if potential adverse effects on the embryo from the trophectoderm biopsy are avoided, the question remains whether a blastocyst-stage embryo, in any diagnostic approach, can reflect chromosomal-accurate an embryo downstream from blastocyst stage. As already noted, because many embryos to a significant degree can self-correct downstream from blastocyst stage, the answer to the question is, likely a rather self-evident no by now.
Then, there is an article by Gardner and Sakkas in the following (September) issue of Fertility and Sterility on “making and selecting the best embryo in the laboratory.” Being among the most prominent proponents of the hypothesis of embryo selection (Gardner et al., after all, brought blastocyst-stage culture into IVF as a tool for best” embryo selection), they appear convinced that AI will allow for the development of complex selection algorithms which, in
Continued from page 33 34 | OCTOBER 2023 | The Voice
turn, will allow to determine the “best” embryos as already described above as the “last chance” of time-lapse imaging.
They predict that this will be successfully done by recording the metabolic functions of embryos, cell-free DNA, and morpho kinetics observed by time-lapse imaging. In this way, mountains of data are generated, only interpretable by AI. But what if even AI cannot find a clinically relevant difference between embryos in a single IVF cohort?
In summary, no subject in the history of IVF has attracted as much research effort and research funding as the hypothesis of embryo selection - in our opinion with no evidence of improving IVF outcomes. The CHR’s current thinking about embryo selection is that it shortens the time to pregnancy to a relatively minor degree in good-prognosis patients only, but it cannot improve the collective cumulative pregnancy chance of an IVF cycle’s embryo cohort and, therefore, does not warrant current research efforts and funding.
Some alleged methods of embryo selection, including routine blastocyst culture for every IVF cycle and PGT-A have been demonstrated to be harmful for selected sub-populations of infertile women. The CHR over the years has become very suspicious of the concept of embryo selection, increasingly advocating that research resources in the infertility field be directed elsewhere.
Highly relevant to the preceding discussion of embryo selection, is a recent paper in Human Reproduction by U.S. and German investigators who reviewed the current status of noninvasive metabolic imaging of cumulus cells, oocytes, and embryos in attempts to select the “best” oocytes and embryos.6 They suggest that so-called fluorescence lifetime imaging (FLIM) of the auto-fluorescent coenzymes NAD(P)H and flavine adenine dinucleotide (FAD+), which are reflective of many metabolic pathways will offer robust information on NAD(p)H and FAD+ levels in individual cells, which, in turn, allows for robust conclusions on the metabolic state of cells, in this context representing a promising approach in selecting “best” oocytes and embryos.
Though that remains to be established, even assuming the technology works appropriately, whether it will reveal the “best” oocytes and/or embryos will depend on whether there will be meaningful difference found between eggs and embryos of equal maturity grades in single cycle cohorts, reaffirming that this remains the quintessential question that, ultimately, must be answered if the hypothesis of embryo selection is ever to be confirmed or rejected.
Also relevant to this subject is a recent paper by Israeli investigators in Advanced Science. 7 Considering that this group of investigators is promoting an AI product in the marketplace, their statement that “high-content time-lapse embryo imaging assessed by machine learning is revolutionizing the field of IVF,” reflective of quite obvious excessive exuberance, is not surprising. Their paper is of interest because it, to our knowledge, is the first to consider “sibling” embryos from the same cohort in machine-learning models for embryo selection. They, in other words, use information encoded in “sibling” embryos from the same cohort to add to their prediction model for implantation, pregnancy, and live birth of individual embryos in the same cohort.
We would argue, not unexpectedly, that they concluded that: (i) Implantation outcomes of individual embryos correlate with attributes derived from cohort siblings. (ii) These additional data boost the prediction of implantation. Moreover, they were able to identify sibling cohort properties especially linked with false predictions. Whether consciously or subconsciously, they, thereby, acknowledge our principal criticism of the embryo selection hypothesis, that embryos in a cycle embryo cohort, in principle, are similar. The question that remains on the table is, whether the relatively minute differences between individual embryos in a single embryo cohort not detectable by morphological observation in routine embryology are significant enough to discriminate between clinically relevant better and poorer chances for implantation, pregnancy, and live birth.
This is an important paper that we strongly recommend and are pointing out because it was not published in a journal frequently read in the infertility field. Somewhat hyperventilating in interpreting the results on the company website as a “breakthrough research, enhancing IVF implantation prediction,”8 this paper represents an important step in maximizing the potential of machine learning in embryo selection. But just because the study demonstrated a statistically significant improvement in implantation rate, does not mean that this improvement renders the algorithm clinically more effective. The question remaining is, whether all of these different efforts at finding the “best” embryos from amidst a single embryo cohort really make sense?
REFERENCES
1. Widra EA., Fertile Steril 2023;120(2):217
2. Giménez et al., Fertil Steril 2023;120(2):218-227
3. Jiang VS, Bormann CL., Fertil Steril 2023;120(2):228 -234
4. Cinnioglu et al., Fertil Steril 2023;12(2):235-239
5. Gardner DK, Sakkas D., Fertil Steril 2023;12(3)457-466
6. Venturas et al., Hum Reprod 2022; 38(5):799-810
7. Tzukerman et al., Advanced Sci 2023;2207711:1
8. https://aivf.co/ema-platform/
The Polycystic Ovary Syndrome (PCOS)
As one of the most common causes associated with female infertility (somewhere between 10-20% of young women have been reported afflicted) and the most common cause for anovulation (some scientists believe that PCOS also has a male counterpart), PCOS is a steady subject of interest in every fertility center. The CHR has had a strong research interest in certain aspects of PCOS, indeed, quite often also addressed in the past in The VOICE. The condition has, however, in recent years become more controversial, as the subdivision of PCOS into four so-called phenotypes (A, B, C, and D) has been increasingly questioned.
As in the September issue of The VOICE recently discussed in the literature review section under the subheading of “IVF in PCOS patients,” CHR investigators1 and colleagues from Mount Sinai here in NYC2 independently concluded that PCOS, likely, is a condition made up of only two distinct genomic entities, a “metabolic” condition, later in life associated with the metabolic syndrome old phenotypes A, B, and C) and an “immunologic,” by Mount Sinai’s colleagues called a “reproductive” subtype.
Continued on page 36
The V oice | OCTOBER 2023 | 35
Now come Kotlyar and Seifer presenting a review article about IVF in PCOS patients that based on references and content, appears outdated and not only because the paper does not at all refer to the above-noted unhappiness with the by now decades-old Rotterdam criteria that divided PCOS into 4 phenotypes. Among 84 references, 83 were published before 2022. The manuscript apparently was in submission circulation for quite some time before, finally, finding a home for publication. More importantly, the article appears not only outdated, but also outclassed by the 2023 “International Evidencebased Guideline for the assessment and management of PCOS,” just recently published by ESHRE, a 258-page-long document.4 What then is the purpose of this rather superficial paper?3
Another PCOS-related paper in the same medical journal, this time by Chinese investigators, concluded that so-called progestin-primed ovarian stimulation (PPOS, according to the authors “an increasingly popular stimulation method”) demonstrates in older women significant adverse effects on IVF cycle outcome in comparison to antagonist cycles: Blastocyst formation was lower (p=0.036) and euploidy rate in PPOS cycles was only 20% of the rate in antagonist cycles.5
This paper unquestionably also deserves the title of “runner up” to the “worst paper of the month” because almost nothing makes sense here; Though PPOS cycles may be popular in China, they to the best of our knowledge are still a rarity in the rest of the world. In a retrospective data review, there must have been reasons why patients had one or the other stimulation. To automatically attribute lower blastocyst formation and higher aneuploidy to PPOS, therefore, makes no sense. Equally probable is the explanation that poorer prognosis patients more likely received PPOS than antagonist cycles (by no means an unreasonable decision).
Adjustments for ovarian reserve were not made. In addition, the CHR’s experience suggests that, unless patients are predominantly good-prognosis patients, IVF studies require study participation from at least 150 patients/cycles to offer statistically valid outcome data (and in many cases even larger study groups). Although in a Chinese study where patient numbers are rarely the problem, here, cycle numbers were quite ridiculously low.
Finally, how could PPOS increase aneuploidy? Should we before making such a suggestion not at least have a potential physiological pathway that could explain such a finding?
REFERENCES
1. Gleicher et al., Biomedicines 2023;10:1505
2. Daps M, Dunaif A., Endocrine Rev 2023;23:440
3. Kotlyar AM, Seifer DB., Reprod Biol Endocrinol 2023;21:70
4. ESHRE 2023;doi.org/10.26180/23625288.v1
5. Pai et al., Reprod Biol Endocrinol 2023;21:72
RUNNER UP TO ‘WORST PAPER” OF THE MONTH!
A little more about social egg-freezing
It is only a coincidence that the subheading of “social egg freezing” in this issue of The VOICE falls right between the headings of “IVF” and “The business of infertility.” But, it is also easy to see this as a symbolic positioning because social egg-freezing involves all the techniques and knowledge of IVF and has become a huge business sector of its own within reproductive medicine.
We know that some colleagues do not like the use of “social” as an addendum to the term egg-freezing to contrast egg-freezing to extend the time of potential pregnancy into older ages from “medically mandated” egg-freezing, done to save pregnancy potential before toxic treatments for ovaries has become necessary in women during reproductive years. The CHR believes that a clear distinction between these two indications for egg-freezing is essential for proper informed consent and full transparency and is not provided by calling social egg-freezing by any other terms. There simply is no better term to describe the decision to freeze one’s eggs with the intent of delaying pregnancy for social reasons.
How relevant this issue has become for a quickly increasing number of mostly younger women was recently once more documented in a report by FOX News that described egg-freezing as “exploding among some age groups.”1
Last month The VOICE published a full-length article on egg-freezing, with this FOX News piece covering most of the same points and adding that U.S. egg-freezing cycles increased between 2020 and 2021 from 16,786 to 24,558, an almost 50% increase. In the U.K. egg storage between 2019 and 2021 increased by 64%. The subject of egg freezing has so far attracted ca. 75 million views on TikTok, which means that even younger women are “educated’ (or should we say “indoctrinated”?) about the subject. The article further notes that almost 3/5 GenZers and millennials admit to being worried about their fertility, yet egg-freezing is becoming more popular as women learn more about the procedure, stay single longer, and are putting off having kids because “careers come first.”
Like last month’s article in The VOICE, the FOX article also reemphasized that egg-freezing does not always guarantee a baby, a point that cannot be overemphasized, considering how many disappointed women we see at the CHR after having run out of frozen eggs without conception. And, as the VOICE article last month prominently noted, egg-freezing makes only sense if an adequately large age-specific number of eggs is cryopreserved to have a reasonable chance of conception (a number that must increase with advancing female age at egg-freezing) and also must consider how many children may be desired. Almost always, this means that more than one egg-freezing cycle will be necessary, with some IVF clinics charging up to ca. $20,000 per cycle.
What this article left out is that some centers – and no fertility center more aggressively than the CHR – fully cognizant of these facts, offer multiple-cycle egg-freezing packages, where per cycle costs are reduced to a fraction of the above-quoted cycle cost. Therefore, U.S. $20,000 should pay for much more than just a single cycle.
Yale University professor of Anthropology, Marcia Inhorn, PhD, just published a book, titled “Motherhood On Ice, 2 which points out a mating gap between the sexes and why women freeze their eggs. The book is the result of several years of studies in which Inhorn interviewed women undergoing egg-freezing, with the CHR, indeed, being one of her collaborating fertility centers. We strongly recommend this book, and not only because Prof. Inhorn has been a longstanding collaborator and friend of the CHR. This book is simply essential for everybody interested in the practice of egg freezing.
Continued from page 35 36 | OCTOBER 2023 | The Voice
Under the heading, “A good man is hard to find: Egg freezing and the ‘mating gap,’” the Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics at Harvard Law, very favorably commented in its blog on the book.3
The blog recently also addressed another egg-freezing-related issue, - concerns with employer-funded elective (“social”) egg-freezing,4 a growing trend among large companies like Meta, Google, and Netflix 4 Acknowledging the obvious benefits, the article by Katie Hammond also discusses the limitations and effective downsides of such offerings by large companies.
We also recommend a recent opinion article in Fertility and Sterility by Anne Z. Steiner, MD, MPH, 5 who in her “inklings” article acknowledges that egg-freezing at times creates an additional conundrum, namely whether a patient who has frozen eggs stored and is ready to attempt conceiving, at that point uses those frozen eggs or tries natural conception. She in this short article very well spells out the advantages and disadvantages of both options, - exactly how proper informed consent is to be given to patients under such circumstances.
REFERENCES
1. Stabile A., FOX News. August 18, 2023. Hhtp://www.foxnes.com/ health/egg-freezing-exploding-age-groups-what-women-must-know
2. https://anthropology.yale.edu/news/ professor-marcia-inhorn-publishes-new-book-egg-freezing
3. Harwood K., August 30, 2023. https://blog.petrieflom.law.harvard. edu/2023/08/30/a-good-man-is-hard-to-find-egg-freezing-and-themating-gap/
4. Hammond K., September 6, 2023. https://blog.petrieflom.law.harvard. edu/tag/employer-funded-egg-freezing/
5. Steiner AZ., Fertil Steril 2023;120(3):573-574
The business of medicine
That infertility has become “big business” can no longer be denied after companies providing fertility services through networks of clinics have reached billion-dollar valuations and increasingly are expanding into related industrial areas, like diagnostic laboratory services, cryopreservation companies, supplement manufacturing, insurance coverage for fertility services, and even the financing of IVF and egg-freezing cycles. Here is the latest news from the business world of fertility services.
More international IVF clinic network news
That infertility has become a worldwide business is again confirmed by an e-mail to his mailing list from Griffin Jones from Inside Reproductive Health on August 17, 2023, in which he reports that Hong Kong-based investment firm BPEA EQT (formerly Baring Private Equity Asia) was set to acquire 60% for a $656.6 million majority stake in Indira IVF (a very large Indian fertility services provider), establishing a new valuation of over $1.1 billion on the whole company, which operates 116 IVF clinics across India and currently performs roughly 33,000 IVF cycles annually.
The 60% purchase involves shares owned by TA Associates and shares owned by the founders of Indira IVF, who are retaining a minority stake in the company and continue to lead it.
Interestingly, TA Associates also used to own a stake in CCRM, another mostly U.S.-based network of fertility clinics, which the company already sold in 2021.
This follows a post-transaction value of approximately $4.2 billion for IVIASRM Global SL after being purchased by KKR&Co, and Kindbody’s market valuation of S1.15 billion after the acquisition of the Vios Fertility Institute and network of clinics.
According to another e-mail from Griffin Jones from Inside Reproductive Health, IVIRMA Global SL hired as of July 17, 2023, as its new CEO the former Chairman and CEO of Iberia Airline, the Spanish national carrier. He replaced Antonio Pellicer, MD who founded the company when opening the first fertility clinic in 1990 in Valencia, Spain, that later became IVI. Pellicer will be a speaker at the 2023 annual FRMC in NYC, co-sponsored by the CHR, with the topic of his talk being the evolution of IVIRMA Global SL under his leadership.
Related, IVIRMA Global SL, as of August 23, 2023, entered, according to an announcement by Dresner Partners, 1 a “partnership” (financial terms were not disclosed) with Conceptions Reproductive Associates of Colorado, thereby expanding its footprint in the U.S., which to this point mostly consisted of former RMA locations. Conceptions operated a fertility center with four locations throughout Colorado, representing the principal competition to the CCRM chain of fertility clinics at its home base in Colorado.
Finally, again based on an e-mail from Griffin Jones from Inside Reproductive Health on September 21, 2023, Twig Fertility, a new start-up in Toronto, Canada, secured a second round of funding for Canadian $ 8 million from the venture capital firm Rhino Ventures, with the intent of opening another clinic in Toronto and expanding to other Canadian provinces. Their first round was secured through family and friends and had to be quite small (and remains undisclosed). Such early funding through venture capital is quite unusual; but their early status and cash flow would, likely, not have qualified for a private equity investment.
REFERENCES
1. Dresner Partners., August 23, 2023. https://www.globenewswire.com/ en/news-release/2023/08/23/2730708/0/en/Dresner-Partners-AdvisedConceptions-Reproductive-Associates-of-Colorado-on-Its-Partnership-WithIVIRMA-Global.html
Concerns about the corporate practice of medicine are increasing
The VOICE has repeatedly expressed concern about the seemingly increasing corporate practice of medicine by health insurance companies under the guise of giving medical “experts” (physicians employed by those corporations and obliged to follow corporate guidelines developed by other “experts” hired by the corporations) the right to refuse coverage for services recommended by physicians for their patients. Now an academic physician and two law professors expressed similar concerns in a “perspective” article in the New England Journal of Medicine, of course, a much more prominent pulpit than The VOICE
Continued on page 38 The V oice | OCTOBER 2023 | 37
The article points out that these concerns arose for the first time in the late 1800s when corporations for the first-time started hiring physicians in the U.S. and profited from their services without being bound by the same professional ethical considerations, physicians are supposed to follow. It was then that the American Medical Association (AMA) revised its Principles of Medical Ethics by defining any contractual agreement that interferes with a physician’s independent and free practice as “unprofessional.” Individual states saw the wisdom in this new definition, with many adopting prohibitions to “corporate practice of medicine,” barring unlicensed entities (to practice medicine) from owning and controlling medical practices. As also discussed elsewhere in this issue of The VOICE, the industrialization of medical practice since those years, and especially over the last few decades, what the article calls “the corporate land grab in health care”, has been overwhelming and unopposed by the government and medical organizations, including the AMA. The article strongly argues in favor of strengthening prohibitions against the corporate practice of medicine.
REFERENCES
1. Zhu et al., N Engl J Med 2023;389(11):965-968
Costs through hospital-owned outpatient care greatly outpace costs at physician-owned practices
Primarily motivated by two considerations, hospital organizations, whether for-profit or not-for-profit, have been buying up private physician practices at a very rapid pace: (i) To secure and/or purchase referral patterns for in-patient care, and (ii) because current laws and federal as well as insurance company billing rules offer them better profit margins than private physicians in owning these practices, not the least because they in contrast to practicing physicians are permitted to add facility charges to usual professional fees. This also includes fertility practices.
A recent study by Blue Health Intelligence, an independent licensee of the Blue Cross Blue Shield Association, not unexpectedly, reported a recently completed study which demonstrated that between 2017 and 2022, prices for six commonly provided procedures in outpatient settings were not only overall significantly higher when delivered by hospitals, but the difference grew rapidly over the six years of the study period.1
Investigating a data set of 133 million lives insured by a PPO product (unfortunately, the studied services did not include fertility-related services), but there is no reason to believe that those would have behaved differently), costs were compared between ambulatory surgery centers (ASC), hospital outpatient departments (HOPD) and physician offices. Almost uniformly, physician offices demonstrated the by far lowest costs and with some procedures even mildly declined over the study period. ASCs followed, and HOPDs were, the by far highest. They, in addition, increased significantly over the study period.
From an obvious viewpoint of self-interest, the document concluded that site-neutral payments would result in substantial savings for employers, employees, and patients. What the report left out is the obvious reality that physician practices face unfair economic competition from ASCs, and especially from HOPDs providers. Moreover, because they are usually invisible to insured patients, the public is unaware of the fact that the fees they pay for services outside of physician-owned offices are much
higher. Though never specifically investigated, it seems reasonable to conclude that this also applies to outpatient infertility treatments, like IVF. Something to think about!
REFERENCES
1. Blue Health Intelligence, Issue Brief, September 2023. https://www.bcbs. com/sites/default/files/file-attachments/site-neutral/BHI-Site-Neutral-IssueBrief.pdf
Selling lab tests directly to consumers
Selling laboratory tests directly to consumers is becoming increasingly popular, also affecting the fertility field. A recent paper investigated the selling of AMH tests directly to consumers, reporting several disturbing findings:1 Investigating 27 websites across seven countries, the authors of the study found that most websites included false and misleading claims falsely leading consumers to believe that AMH can reliably predict fertility potential and age at menopause. The consequences of such misunderstandings of what test results really mean can be significant.
REFERENCE
1. Johnson et al., JAMA Network Open 2023;6(8):e2330192
Demand for donated sperm soars, and the cost with it
A recent article in The Wall Street Journal offered interesting information, the medical literature to the best of our knowledge so far has not yet addressed: The availability of donated sperm is shrinking due to increasing demand and, as one would expect in a free market economy, costs are increasing.1
The days of the $150 semen sample are long gone. Requirements for increasingly extensive genetic donor screening, increasing demand by ever larger numbers of single women pursuing fertility treatments, and the reluctance of potential semen donors to donate in view of new, already existing disclosure rules in some states (with other states expected to follow) once offspring conceived through third-party semen donations reach age 18, have led to an explosion in semen sample pricing, more than doubling over prices before 2019 to upward from US $ 2,000 per specimen.
The article notes that California Cryobank, one of the largest sperm-donor banks in the country is down to ca. 300 available donors from over 550 in 2019, and now spends millions of dollars a year on digital advertisement, when in the past all the company had to do was to post fliers on telephone poles at colleges. The cost of specimen vials increased at the bank from approximately $1,000 in 2021 to now being $1,895.
Even insurance plans that cover infertility services usually do not cover donor semen costs those are not the only costs patients face: Sperm banks in addition also charge shipping costs, frozen storage fees, and subscription costs for viewing virtual donor catalogs.
REFERENCE
1. Wolfe R., The Wall Street Journal. September 25, 2023; pA14. https://www. wsj.com/personal-finance/sperm-donor-cost-pregnant-babies-fde53e2f
Continued from page 37 38 | OCTOBER 2023 | The Voice
Smaller companies are starting to offer fertility benefits in their health plans
As the Wall Street Journal recently reported, more hourly jobs now offer fertility benefits.1 Employers are weighing the costs of offering IVF, egg-freezing, and other fertility-related treatments against having to attract employees. Apparently, 25% of employers in the U.S. now offer IVF coverage. In a recent article in the blog of the Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics at Harvard Law, Dafni Lima recently even went a step further by suggesting that, “now that parental leave has proven it works, it is time to talk about assisted reproduction leave”.2
REFERENCES
1. Torry H., The Wall Street Journal, August 22, 2023; pA2. https://www.wsj. com/economy/jobs/hourly-jobs-with-fertility-benefits-offer-some-workersa-path-to-parenthood-e56b4177
2. Lima D., The Petri—Flom Blog. August 29, 2023. https://blog.petrieflom. law.harvard.edu/2023/08/29/parental-leave-has-proven-it-works-it-is-timeto-talk-about-assisted-reproduction-leave/#:~:text=Parental%20leave%20 has%20proven%20it%20works%20when%20it%20comes%20 to,Professor%20at%20Durham%20Law%20School.
The Mount Sinai Hospital system to close Beth Israel Hospital – a symptom of increasing hospital troubles?
After years of rumors, Mount Sinai Health finally made it official: Beth Israel Hospital (now called Mount Sinai Beth Israel), founded in 1889 to serve a mostly Jewish population in the Lower East Side, will gradually close down. Its ultimate complete closure is expected to take a few years.
According to Becker’s Hospital Review, 1 the principal reasons are the underutilization of its beds at only 20% of capacity and mounting losses expected to reach $150 million this year. It is difficult to understand why its utilization has been so poor, considering that most NYC hospitals experience significantly higher utilization. A Mount Sinai news release claimed that “as a leading non-profit institution, we must take immediate steps to preserve our ability to continue providing healthcare services to the greater NYC community.” It continued by stating that “continuing to keep the hospital open, would jeopardize the mission of the Mount Sinai Health System.”
However, other sources suggested that the real reasons may lie elsewhere. Supposedly, the closure of Beth Israel has been seriously considered since the hospital became part of the Mount Sinai Health System. The decision to proceed now may have been accelerated by other developments, including poorer-than-expected collections (also see below) and massive financial losses at Sema4, a Mount Sinai Health System venture into commercial genetic laboratory testing company founded in 2017.
As Becker’s Hospital Review recently reported, hospitals “are in a world of denial.”2 What was meant by this phrase was that hospitals are increasingly facing the same problems physicians have bee facing for a long time: their charges get rejected by insurers, often denying large claims in toto because of disagreement over
some minor issues. In other words, insurance companies have started to penny-pinch hospitals in the same obnoxious ways as physicians. Like physicians, hospitals also face longer and longer collection times and have higher and higher billing costs.
It will be interesting to see what the future will bring in this regard. What we foresee are gigantic clashes between ever larger monopolistic hospital systems with ever larger and often monopolistic insurance companies. Not a very attractive prospect to look forward to!
REFERENCES
1. Thomas N., September 14, 2023; Becker’s Health Care. https://www.becker shospitalreview.com/finance/mount-sinai-to-close-beth-israel-campus-innew-york.html
2. Gamble M., August 14, 2023; Becker’s Health care. https://www.beckershos pitalreview.com/finance/hospitals-are-in-a-world-of-denial.html
General medicine
Artificial intelligence (AI) in medicine
Though, as prior economic and technological revolutions, AI will, likely, affect every sphere of our existence, and medicine is projected to likely be among the earliest beneficiaries. In many ways, AI is already everywhere, as a large number of recently published opinion pieces amply demonstrate. Some applications have already started: As Mike May in a recent news feature article in Nature Medicine pointed out, how clinical trials are conducted is already changing, with randomizations and placebo control patients giving way to biomarkers, real-world data from wearables, and multicenter and adaptive trials.1
At the same time, two knowledgeable physicians warn against overhyping at least in the near term, the ability of AI to significantly contribute to reaching clinical diagnoses.2 They acknowledge that AI, if effectively integrated, can potentially improve the quality of care, but also point out that, “in its current form, the advantages of AI do not effectively account for certain real-world aspects of making clinical diagnoses.” Another group of authors in The New England Journal of Medicine suggests considering biased data in AI-assisted health care as informative artifacts.3 They point out that biased algorithms (i.e., so-called algorithmic bias) can also lead to algorithmic discrimination.
Three other authors in the same issue of JAMA as the first article addresses the challenges of regulating the medical utilization of ChatGPT (and similar language models), concluding that we are only at a possible starting point as of this stage because new risks and benefits will emerge as language models evolve and are coming into greater use in the medical field.4 Finally, two other authors (lawyers) in the same journal issue address liability risks for physicians and safety issues for patients from increasing use of generative AI in health care.5 They concluded that physicians must continue warning patients of risks associated with relying on AI-generated medical information and also expressed concern that, should something go wrong, there may be nobody to hold responsible.5
A few weeks later, and again in JAMA, three authors summarized the obvious in a “special communication” article: Large language models in medicine must be actively shaped in medicine by using relevant training data, specifying desired benefits, and then evaluating those alleged benefits in real-world experiences.6 They reemphasized in their article that training AI tools with skewed data will bias those tools.
Continued on page 40 The V oice | OCTOBER 2023 | 39
REFERENCES
1. May M., Nature Med 2023;29:1884-1886
2. Kulkarni PA., Singh H. JAMA 2023;330(4):317318
3. Ferryman et al., N Engl J Med 2023;389(9):833838
4. Minssen et al., JAMA 2023;330(4):315-316
5. Duffourc M., Gerke S. JAMA 2023;330(4):313-314
6. Shah et al., JAMA 2023;330(9):866-869
Cancer statistics
According to the American Association for Cancer Research (AACR): (i) the overall cancer rate in the U.S. has been steadily declining since the 1990s, avoiding between 1991 and 2020 over 3.8 million cancer deaths. (ii) This decline is primarily driven by declines in mortality from breast, colon and rectum, lung, and prostate cancers. (iii) As of January 2022, over 18 million cancer survivors were living in the U.S. (iv) Progress, however, varied between cancers. (v) The country suffers from stark inequities in the cancer burden, occurring across the cancer continuum, and are driven largely by social factors. (vi) The economic burden from cancers, however, is expected to rise in coming years, highlighting the need for more research to accelerate progress.1
At the same time, another study suggested that cancer in younger individuals was on the rise. Surveying cancer incidences between January 1, 2010, and December 31, 2019, in a U.S. cohort of 562,145 patients of which 324,138 (57.7%) were 40-49 years old, 62.5% were female with early onset cancer. Between 2010 and 2019, early onset cancers increased overall (p=0.01) and in females (p<0.001), but not in males, which decreased (p<0.001). By 2019, the highest incidence of early cancer involved the breast, gastrointestinal cancers had the fastest-growing incidence between 2010 and 2019.2
REFERENCES
1. AACR. Cancer Progress Report 2023. https://cancerprogressreport.aacr. org/progress/cpr23-content/cpr23-cancer-in-2023/#driving
2. Koh B, et al., JAMA Network Open 2023;6(8):e2328171
Relevance of recent aging research for fertility
Though there exists no consensus on the definition of aging, an excellent recent “perspective” article in Cell still offers a stellar review of the subject of biomarkers of aging for the identification and evaluation of longevity interventions.1 With aging becoming an increasingly studied subject in association with several other specialty areas, including reproductive medicine (aging of ovaries is, of course, a central issue in fertility
research), we strongly recommend this manuscript for everybody interested in the subject.
Approximately 10 years ago, it was first reported that blood from younger mice could restore youthful properties in older mice.2,3 Platelet factors have recently been demonstrated to positively affect various physiological processes, including wound healing, attenuate inflammation, and rescue cognition in aging mice. 4 Another team reported that platelet factors, including PF4 induced by longevity factor Klotho, enhances synaptic plasticity.5 Yet, another team reported that PF4 is involved in the formation of new neurons.6
A news article in Nature magazine now reports that two biotech companies are testing such molecules that have been demonstrated to be associated with aging in attempts to develop treatments that might promote regeneration and healthy aging in general. PF4 is one such molecule.7
Why does all of this have relevance to reproductive medicine?
PRP (platelet-rich plasma), as repeatedly discussed in these pages before, has found significant worldwide utilization in infertility in attempts to improve ovarian reserve 8 and the endometrium.9 The CHR is still in the midst of three separate trials using PRP injections into ovaries in different patient populations in attempts to improve functional ovarian reserve and IVF cycle outcomes, with a preliminary report of one of the trials recently published.10 The hypothesis behind PRP use is that this fraction of human plasma contains platelet-derived factors such as PF4. It would be interesting to see whether the PRP used in infertility practice contains PF4.
REFERENCES
1. Moqri et al., Cell 2023;186(18):3758-3775
2. Katsimpardi et al., Science 2014;344:630-634
3. Villeda et al., Nature Med 2014;20:659-663
4. Schroer et al., Nature 2023;620:1071-1079
5. Park et al., Nat Aging 2023; 3:1967-1078
6. Leiter et al., Nat Vommun 2023;14:4375
7. May Sidik S., Nature 20123;620:709
8. Tremellen K., Pacella-Ince L., Aust N Z J Obstet Gynaecol 2022;62(5):767-772
9. Dogra et al., JBRA Assist Reprod 2022;26(1):13-21
10. Barad et al., Hum Reprod Open 2022;2022(3):hoac027
Anti-obesity drugs and their potential relevance to infertility
As a recent feature article in Nature magazine pointed out, it is rare to see a Big Pharma product be so successful that its makers stop advertising. But, that is exactly what has been happening to the launched new family of weight-loss drugs in recent years, starting with semaglutide and tirzepatide because manufacturers could not keep up with demand.1
Obesity is increasingly recognized as a chronic, though treatable neuro-metabolic disease with rapidly increasing prevalence
Continued from page 39 40 | OCTOBER 2023 | The
all over the world. By 2035 it is expected to affect up to one-quarter of the world’s population.2 It has tripled over the last 50 years in incidence.1 The adverse effects of obesity on fertility3 as well as infertility treatments 4 are well established. How the medical field and society as a whole will manage the enormous potential consequences of increasing utilization of this new family of drugs in treating the world’s obesity problem will have significant effects on fertility in all populations around the world.
It is somewhat surprising how little the fertility literature so far has paid attention to this very rapidly evolving new specialty area in medicine and a clear impetus for The VOICE to offer here a brief summary of recent developments. The above-noted article in Nature offers an excellent summary by addressing four principal questions everybody is interested in regarding this highly effective new group of weight-loss drugs (for more information on available drugs, please see the Information Box below).
INFORMATION BOX
Semaglutide (trademark Ozempic), initially developed as a diabetes drug, was the firstFDA-approved drug as an obesity treatment in 2021. It is a so-called glucagon-like peptide (GLP) 1 Analogue (the hormone glucagon has been known for some time to curb appetite). In a 2022 study, Semaglutide was found to induce much higher weight loss than previously known glucagon analogues.5 Since then, even more, potent weight-loss drugs have been developed: Tirzepatide (Mounjaro), already approved for diabetes and imminently expected to receive FDA approval for weight loss, mimics in addition to glucagon a second hunger-suppressing hormone glucose-dependent insulinotropic polypeptide (GIP), and was demonstrated within 18 months to cause even more substantial weight loss than Ozempic (average weight loss 21% on the highest dose of 15mg of weekly s.c. injection). Moreover, a third drug from the manufacturer of Mounjaro called Retatrutide, mimicking three appetite/hunger-suppression-related hormones has been reported to be even more effective than Mounjaro (loss of 24% of body weight in less than 1 year on maximal dose). This drug, however, also activates the sympathetic nervous system and, therefore, increases heart rate and, potentially, blood pressure (that seems to peak at 6 months and then recedes). This drug will likely be reserved only for people in excellent health.
The questions the article addressed were:
(i) How do these medications work? The honest answer is that we do not yet fully understand how they work. As noted in the Information Box, they in several different ways, suppress a person’s appetite, both in the gastrointestinal tract (feeling of fullness, slowing of gastric emptying) and brain (diminished reward-feeling associated with eating). The latter led to these drugs now also being investigated as anti-addiction medications to lower cravings for alcohol, smoking, and even gambling.
(ii) Does everybody lose weight equally? The answer is, NO! For reasons still unclear, a small percentage of patients do not respond well to GLP-1 drugs. In addition, type 2 diabetics lose less weight than individuals without diabetes in response to GLP-1 mimics. In a recently published Retatrutide trial, women at all doses lost more
weight than men.6 Animal data of this drug demonstrated that the more overweight mice were at the beginning of treatment, the more weight they lost with treatment,7 suggesting that triple (or more) agonist treatments should be reserved for the most extremely, morbidly obese individuals.1
(iii) What are potential long-term risks? Short-term side effects of this family of drugs appear already well-defined: Most patients have little or no side effects if medications are started gradually. Nausea, vomiting, diarrhea, and other GI symptoms are not infrequent but, in most individuals, quickly disappear. Rarely are initial side effects so severe that patients stop the treatment. One study reported an increased risk of intestinal blockage after GLP1 use,8 and there has been some talk about increased suicide thoughts on the medication.
One potentially more significant issue is that weight loss does not only include fat, but muscle and bone may also account for up to 40%.9 Another important factor to consider is that these treatments will likely have to be maintained lifelong because withholding these medications in almost all participants was shown to lead to the regaining of lost weight. Detailed enough studies on how to take these medications in the long run have not been performed yet. For example, one can imagine lowering the dosage once a desired weight has been reached to, simply, maintain this weight or extending the time between injections for weekly to longer intervals may have similar effects.
Since GLP-1 receptor agonists have been FDA-approved since 2005 and long-term adverse effects have been non-existent, longterm use is, likely, very safe. But, as with all medications, one can never exclude the possibility of surprises. Long-term follow-up studies will have to be performed, but should not delay the continuous rapid expansion of utilization of these medications in the fight against obesity.
(iv) Do these medications change how we view obesity?
The Nature paper reemphasizes correctly that this new family of medications demonstrates that obesity is not due to a lack of willpower because the human brain dominates the decision of how much to eat. Others, however, are concerned that they may exacerbate the occurrence of eating disorders and weight stigma and emphasize that there is no “right weight” for people.
What the article, however, does not address is all the secondary health benefits that can be expected from successful weight reduction, and that also includes fertility and fertility treatments. The relationship between fertility and obesity appears to be complex and multifactorial.10 Though studies have unsurprisingly reported mixed results, it appears that, at least in subgroups of patients, even rather minor weight loss can positively affect menstrual cycle cyclicity, fertility, and fertility treatments.11 In rodents, even moderate caloric restriction sustains female reproductive function into advanced chronological ages.12 Overfed dairy heifers benefitted in embryo production from reductions in weight.13 Studies on the use of this new group of weight-loss medications in infertile women and men are urgently needed. The only review of GLP-1 in reproduction in 2019 is outdated.14
Continued on page 42 The V oice | OCTOBER 2023 | 41
Within this context, another recently published study in Nature magazine described the neural basis for fasting activating the hypothalamic-adrenal axis.15 Considering the importance of this hormonal axis for fertility, there is not much doubt left for the urgent need to explore the importance of weight loss further.
REFERENCES
1. Prillaman M., Nature 2023;620-28-30
2. World Obesity Federation. 2023. https://data.worldobesity.org/ publications/?cat=19
3. Amiri M, Ramezani Tehrani F., Int J Endocrinol Metab 2020;18(3):e101776
4. Wan et al., Obes Res 2023;24(10):e13603
5. Rubino et al., JAMA 2022;327(2):138-150
6. Jastreboff et al., N Engl J Med 2023;389(6):514526
7. Jall et al., Mol Metab 2017;6:440-446
8. Faillie et al., Clin Pharm Therap 2022;111:272-282
9. Wilding et al., N Engl J Med 2021;384:989-1002
10. Armstrong et al., Curr Opin Obstet Gynecol 2022;34(4):184-189
11. Brewer CJ, Balen AH., Reproduction 2010;140(3):347-364
12. Selesniemi et al., Aging Cell 2008;7:622-9
13. Freret et al., Reprodution 2006;131:783-794
14. Jensterle et al., Hum reprod Update 2019;25(4):504-517
15. Douglas et al., Nature 2023;620:154-162
Are women better surgeons than men?
A recent Swedish study in JAMA Surgery attracted wide attention because it suggested that female surgeons had better outcomes in cholecystectomy surgeries than their male colleagues.1 In a study involving 150,509 patients with 64.9% undergoing elective and 35.1% acute care cholecystectomies, involving 849 (33.3%) female and 1,704 (67.7%) male surgeons the comparison was not even close: males had more surgical (OR, 1.29; 95%CI, 1.19-1.40) and total complications (OR, 1.12; 95%CI, 1.06-1.19). Female surgeons demonstrated significantly longer operation times, while male surgeons converted to open surgery more often in acute care surgeries and had longer hospital stays. Thirty-day mortality, however, did not differ. The authors concluded that it appears that women prioritize thoroughness and safety over speed in comparison to their male colleagues.
There may be a lesson to learn from these data for all surgical fields.
REFERENCES
More on increasing cannabis use in society
The VOICE has recently repeatedly pointed out concerns in the medical community about the increasing use and abuse of cannabis in society. Now investigators from several European countries offered an excellent systematic review of associations between cannabis, cannabinoids, and cannabis-based medicines on human health.1
As the authors noted, observational evidence has suggested that cannabinoids are associated with numerous health outcomes and have been tested for several medical conditions in prospectively randomized trials (PRTs). A comprehensive analysis of these data has, however, been missing and was the goal of their study.
They reached the following conclusions, and we quote: (i) Most outcomes associated with cannabinoids are supported by only weak evidence (mostly observational studies), demonstrate very low certainty (if involving PRTs), or do not demonstrate statistically significant findings (both study formats). (ii) Convincing or converging evidence recommends avoidance of cannabis during adolescence, and early adulthood in people prone to have or already having mental health disorders, who are pregnant, and while driving. (iii) Cannabidiol is an effective treatment for epilepsy, notably in children, while other cannabinoids can be effective in multiple sclerosis, chronic pain, inflammatory bowel disease, and in palliative care.
Notably, the authors concluded that the scientific reasoning behind extreme or ideological legislation and commercialization of cannabis even in young adults versus complete prohibition, and the different legislative requirements between cannabis and alcohol in disclosing to consumers the associated risks (unfortunately) remain unclear.
Related, Ingrid Wickelgren recently offered a feature article in Science magazine warning about the dangers of cannabis for the developing brain.2 The article was mostly based on an interview with Yasmin Hurd, PhD, who is the Ward-Coleman Chair of Translational Neuroscience and the Director of the Addiction Institute at Mount Sinai’s Icahn School of Medicine in NYC. Hurd sees growing evidence that cannabis use puts children and adolescents at risk for a variety of psychiatric problems, from dependence on cannabis and other drugs to schizophrenia. Exposure to cannabis, she is now convinced, can cause mental health problems in childhood and beyond, and her conviction is based on extensive experiments in mice as well as clinical observations.
Considering that so far 23 states and the District of Columbia have legalized recreational cannabis use for adults (New York state’s first dispensary opened in January of this year) her work is becoming increasingly pertinent. The article also includes a figure demonstrating how average THC concentrations (the psychoactive ingredient in cannabis) increased from 3.96% to 15.34% between 1995 and 2020. Studies demonstrated that the use of cannabis (as previously reported in The VOICE between 2002 and 2017 women between ages 12 and 44 more than doubled during pregnancy. Estimates now exceed a quarter of all pregnancies, causing significant alterations to the fetal brain’s dopamine system, including decreased expression of dopamine receptors in the amygdala and nucleus accumbens, which is a reward center, strongly hinting at cannabis interfering with emotional regulation and boosting vulnerability to all kinds of addictions.
Hurd also found additional changes in the fetal brain exposed to cannabis which, when recreated in a rodent model system, were associated with clear behavioral changes. Male rats more readily self-administered heroin as adults and in utero exposed adult rats, in general, demonstrated low motivation, depression-like traits, and increased sensitivity to stress. In following newborns over a year in collaboration with colleagues, she demonstrated that after in utero exposure to cannabis, the children at ages 3-6 demonstrated increased hyperactivity and heightened anxiety as well as aggression.
Also relevant to this issue is the recent announcement that the U.S. Department of Health and Human Services (DHHS) just recommended the rescheduling of marijuana from Schedule I to Schedule III.3
1. Blohm et al., JAMA Surgery 2023
Continued from page 41 42 | OCTOBER 2023 | The Voice
REFERENCES
1. Solmi et al., BMJ 2023382:e072348
2. Wickelgren I., Science 2023;381(6661):936-940
3. Knight R., August 31, 2023, https://cannabusiness.law/ top-us-health-agency-recommends-marijuana-reclassified-to-sched ule-iii/
Genetics in reproductive medicine
More on why aneuploidy in cancer likely can help in understanding aneuploidy in human
embryos and vice-versa
It appears increasingly likely that human cancers and human embryos (and likely human microbiomes as well as human parasites) share similar (and maybe even to some degree cross-reactive) tolerance pathways that allow the survival of the invading embryo, cancer, microbiome, or parasites in the host despite a normally functioning immune system that, however, has been “blinded” against these invaders. How immune systems are “blinded” may involve several overlapping pathways in timing (for example between the original stages of invasion and maintenance of invasion). One process by which tolerance appears to be induced early during invasions is aneuploidy in the invader. A large majority of cancers demonstrate mosaic aneuploid, with increasing aneuploid being associated with increasing aggressiveness of cancers and metastatic spread.
An approximately similar percentage of human embryos as cancers (both ca. 80%) demonstrate aneuploidy at the time of invasion of the host. In embryos, this means aneuploid at preimplantation stages, with this aneuploidy in many embryos self-correcting downstream between preimplantation cleavage stages and early fetal stages after implantation at blastocyst-stage. Such self-correction does not occur to the same degree in the trophectoderm which later becomes the placenta. The placenta, in even chromosomal completely normal births, still often maintains mosaic aneuploidy.
This ingenious dichotomy between embryo (a product of the embryonic cell lineage) and placenta (a product of the extraembryonic cell lineage) allows the fetal-placental unit to benefit from the persistent aneuploidy in the placenta by maintaining maternal tolerance toward this growing fetal-placental “parasite,” while the fetus can completely cleanse itself from all aneuploidies shortly before and after implantation.
Among aneuploidies in cancers, approximately 25% demonstrate gains of the q arm of chromosome 1. Despite the high prevalence of this aneuploidy, its role in cancer has remained unknown.1 Some cancers have been shown to demonstrate so-called “oncogene addition,” which means that the loss or blockage of a single cancer gene can be sufficient to induce cancer regression. For example, blockage of KRAS in pancreatic cancer is at times sufficient to induce cancer regression through apoptosis.
Now investigators from several U.S. laboratories and institutions have taken the concept of “cancer addiction” one step further by
speculating that the non-random distribution of chromosomal abnormalities in cancers may suggest that some may be “addicted” to certain chromosomal abnormalities (i.e., that certain chromosomal abnormalities are more permissive for occurrence of cancer than others).2
This is exactly what they found:1 Using what they described as ReDACT (for Restoring Disomy in Aneuploid cells using CRISPR Targeting), they produced a panel of isogenic cells that either have or lack common aneuploidies in cancers. They then demonstrated that the chromosome 1q trisomy is required for malignant growth in cancers by demonstrating that eliminating the trisomy of chromosome 1q almost completely ended the cancer’s independent growth and xenograft formation and, as a control experiment, elimination of trisomy 1q from a non-malignant cell line blocked RAS -mediated malignant transformation. Prolonged culture in vitro or in vivo after elimination of aneuploidy in cancer cell lines led to changes in karyotype, with 1q disomic cells being outcompeted by recovered 1q trisomic cells, while the removal of other trisomic chromosomes (not involving chromosome 1q) from cancer cells, had variable effects on malignant growth. The authors concluded that different aneuploidies phenotypically affect cancer cells differently.
Clinical sequencing data demonstrated that 1q gains arose early during tumorigenesis and were mutually exclusive with mutations in the tumor suppressor gene TP53, which could suggest that 1q trisomies may be mutation-independent in blocking p53 signaling. This was further supported by ReDACT-driven elimination of 1q trisomies increased expression of p53 target genes in TP53 wildtype cell lines, traced to a triplication of MDM4, a p53 inhibitor encoded on chromosome 1q. In addition, the deletion of a single copy of MDM4 impaired the growth of 1q-trisomic cells, while the over-expression of MDM4 rescued the growth of 1q disomic cells.
The investigators further demonstrated that chromosome 1q gains caused overexpression of UCK2, a nucleotide kinase encoded on chromosome 1q, also required for cytotoxicity of several anticancer nucleotide analogs and some 1q trisomic cell lines displayed enhanced sensitivity to these compounds because of upregulation of UCK2. This also disclosed a potentially useful cancer vulnerability of 1q aneuploidy.
Assuming that similar “aneuploidy addiction” also exists in tolerance of the fetal-placental unit during pregnancy, it would be worthwhile to start investigating chromosomal abnormalities in chromosomal-normal deliveries in determining which aneuploidies are preferentially associated with euploid-normal and successful pregnancy. In reverse, it may also be interesting to have a closer look at specific aneuploidies associated with spontaneous miscarriages. Flexible chromosome engineering methodologies like ReDACT could be very helpful in determining whether certain aneuploidies maintained in placentas favored or punished human pregnancies.
Differentiating polyploidy from aneuploidy
Somewhat related Elizabeth Pennisi in a feature article in Science magazine very well summarized the recent discovery that polyploid cells may help tissue respond to injuries.3 Describing mostly the
Continued on page 44 The V oice | OCTOBER 2023 | 43
Vicky Losik, PhD (now at Boston College) who together with Don Fox, PhD (then both postdocs at the Carnegie Institution for Science), studying wound repair in fruit flies, to their surprise noted cells behaving in unexpected ways around wounds created by needle sticks. Those strange cells grew and prepared to divide by duplicating their DNA. They then, however, stopped their new development, remaining enlarged cells, containing multiple copies of their genomes. Having expected to see stem cells helping in the process of wound healing, the two postdocs were a few days later surprised that the job was apparently done by these enlarged polyploid cells.
The author of the article points out that polyploid cells generally have been considered to be potentially harmful anomalies, driving cancer growth. This, however, no longer appears reasonable as a singular function, - and not only in the fruit fly. In adult human hearts, indeed, 80% of cells appear to be polyploid, with no polyploid cells seen after birth. Unsurprisingly polyploidy of cells has now attracted researchers in such divergent fields as cancer, developmental biology, embryology, evolution, cell biology, immunology, and even agricultural cell scientists. All got together in May of this year in Florida for a first meeting. The consensus reached appears to suggest that polyploidy represents an essential response of many tissues and organs to stress and injuries, diseases, and damages in human lungs, liver, and kidneys, basically serving as alternatives to stem cells.
Among plants, approximately 30% are fully polyploid; so are several salamanders. Evolutionary biologists have come to believe that polyploidy is meant to protect species from catastrophic environmental changes. It, moreover, appears that polyploidy can do good and bad. It, indeed, is associated with cancer but also appears essential in wound healing. Eliminating genes required for polyploidy prevented wounds from healing. Making the connection to reproductive medicine, over 10 years ago, Wu-Min Deng, PhD, a fruit fly biologist at Tulane University, reported this observation in wounded fruit fly ovaries. Again with considerable relevance to ovaries, Losik’s lab recently reported that scarring after injury, including fibrosis, may be necessary to constrict polyploidy because too much polyploidy may be dangerous and, indeed, potentially induces cancer and supports resistance to chemotherapy. Looking at the multitude of seemingly at times opposing functions, one must wonder where physiologic effects of micronuclei may come into play here, especially as those have recently been closely associated with aneuploidy, cancer, treatment resistance in cancer, and epigenetic dysregulation.4
REFERENCES
1. Girish et al., Research Article Summary, Science 2023;381(6660):848
2. Girish et al., Science 2023;38(6660):eadg4521
3. Pennisi E., Science 2023;381(6660):825-829
4. Agustinus et al., Nature 2023;619(7968):176-183
More mostly bad science regarding PGT-A
Papers on PGT-A keep coming into print in large numbers. Unfortunately, most repeat the same mistakes in analyzing data repeatedly pointed out in the literature (and here in The VOICE).
Yet, editors still accept these papers. Who then can be surprised that the field and public remain confused about the utility of PGT-A. We will be short in noting most of the recent PGT-A papers. Some, however, deserve a closer look, starting with a publication by the NYU group, among the country’s leading proponents of PGT-A.
Their new publication in Fertility and Sterility Reports, which just appeared electronically,1 aimed to determine how often non-euploid results from a single trophectoderm biopsy tested with NGS (next generation sequencing) would be concordant with a SNP (single nucleotide polymorphism) array-based PGT-A platform. On first impression, this seems like a reasonable question to ask, and the investigators apparently did an excellent job in attempting to answer the question, using not fewer than 100 for research donated blastocysts for the study (an issue we will come back to).
Among those 100 embryos, 40 had at least 1 whole chromosome full copy number aneuploidy (in short, were “aneuploid” on original PGT-A report), 20 had a single whole chromosome intermediate copy number (reported as “mosaic”), 20 had a single full segmental aneuploidy, and 20 had a single segmental intermedia copy number (i.e., were “segmental-mosaic on the initial report).
The results of the study can be summarized as follows: NGS and SNP were highly concordant if the initial SNP diagnosis had been “aneuploidy.” However, all other abnormal initial diagnoses (i.e., 60% of tested blastocysts) were in many cases discordant between initial NGS and SNP. The obvious question that arises is, what is the meaning of these findings for the utilization of PGT-A in IVF?
Here the authors once again fail, as they have failed in over 20 years of promoting PGT-A. The conclusion from their study – and we quote – was: ”Whole mosaicism, segmental aneuploidies, and segmental mosaicism were less concordant, reinforcing that embryos with these results may have reproductive potential and be suitable for transfer.” This conclusion is not only misleading but outright false. Also, it is at several levels (and this must at some point be stated) unethical.
Let us start with why it is false:
(i) The authors’ conclusion suggests that in the 40% of cases where NGS and SNP largely concurred, the original PGT-A result of “aneuploidy” was correct and such embryos should not be transferred under any circumstances. We strongly dis agree because, even if both methods concurred, this does not yet mean that an “aneuploid” biopsy of on average, 5 trophectoderm cells will accurately represent the complete embryo. That this is an incorrect assumption was demonstrated by investigators at the CHR when they reported chromosomal normal pregnancies and newborns after the transfer of fully “aneuploid” embryos. 2,3
Granted, the likelihood of a normal delivery with full “aneuploid” transfers is much smaller than all the other chromosomal abnormalities noted above, but it is not zero. Most patients who have no other embryos left for transfer will gladly take a chance on their remaining “aneuploid” embryos even if that chance is small (we do not recommend transfers of survivable aneuploidies such as trisomies 15, 18, and 21 and sex chromosome abnormalities).
work of
Continued from page 43 44 | OCTOBER 2023 | The Voice
(ii) Are all other embryo abnormalities really suitable for transfer, as suggested by the authors? We do not believe so: As noted above, we do not recommend the transfer of embryos with survivable trisomies (affecting chromosomes 15, 18, and 21) and with sex chromosome abnormalities. This would be especially pertinent to Turner syndrome embryos (XO), which frequently are mosaic.
(iii) Here is the likely most important argument as to why the authors’ conclusion is outright false: They commented on a test which in 40% of cases demonstrated congruence between two testing methods, but at the same time demonstrated significant divergence in 60% of cases. How can such a test be trusted to determine which embryos should be transferred and which embryos should be discarded? How do they know which testing platform to believe (if any)?
Now to the ethical arguments:
(i) For many years, we at the CHR have been wondering how our colleagues, who for almost 20 years have been arguing that, with any chromosomal abnormalities detected through PGT-A, embryos should be disposed of, account for the huge numbers of potentially transferrable embryos in those years unnecessarily discarded? We are still waiting from all longstanding proponents of PGT-A for a mea culpa for all the embryos with pregnancy chances they disposed of and, even more importantly, for the even larger numbers of unnecessarily disposed of embryos by other colleagues because of their “scientific” advice.
(ii) Under international understanding, human embryos should be used for research purposes only if no other methods exist to determine important new scientific information and, even under those circumstances, in only the smallest possible numbers.4 Did this recent study from our NYU colleagues warrant the destruction of 100 human blastocyst-stage embryos? What new information did we learn from this study?
Another study deserving of more detailed comments investigated the nature of embryonic mosaicism across female age by analyzing PGT-A results of 21, 345 PGT-A cycles.5 Before diving into results and their interpretation, it is important to point out that the definition of “mosaicism” in this paper is the incorrect definition under which PGT-A laboratories to this day report PGT-A results. In these reports, “mosaicism” is defined as intermediate copy numbers (of aneuploid DNA) by NGS recorded in the DNA of on average 5 trophectoderm-derived cells. However, the correct biological definition of mosaicism is the presence of a second (or more) aneuploid cell line anywhere in an organism (in this case, the complete embryo). Such a result confirms an embryo as being mosaic, but neither a completely “euploid” nor completely “aneuploid” result of a 5-cell trophectoderm biopsy does not mean that the embryo is, still, truly mosaic.6 The here-discussed paper, therefore, does not offer a complete picture of mosaicism across female age.
With this observation as a caveat, the paper reported that among all tested embryos 44% were reported as “euploid,” 40.2% as
“aneuploid,” and only 15.8% were underreported as “mosaic.” Interestingly, mosaicism was more prevalent at younger ages (both low- as well as high-level), in itself an interesting, here for the first time reported to our knowledge, finding with potential significance for the physiological purpose of the almost universal presence of aneuploid cells in preimplantation-stage embryos. As “mosaicism” diagnoses declined with age, diagnoses of complete “aneuploidies” and of complex aneuploidies increased with age, supporting a tentative summary statement that would suggest that “mosaicism” is associated with youth (therefore can be viewed as actually a positive finding), while full “aneuploidy” and complex “aneuploidy” is associated with advanced age (and likely a negative finding).
Interestingly, the authors in the interpretation of their results missed the very apparent principal finding of their study: To have a mosaic embryo is actually a good prognostic sign considering the likely importance of aneuploidy in overcoming immunological rejection (of products of conception and cancer, as discussed above under “Genetics in reproductive medicine”). Who can be surprised that the accompanying commentaries by three likely reviewers of the paper also completely overlooked this finding, while extensively dwelling on many usual, and mostly false, cliches that have overtaken PGT-A commentaries from early on.7,8 At least one of these reviewers in the past, and points out in his comments here again, how PGT-A, ultimately, adversely affects IVF outcomes in many patients.8
The Bernabeu Group in Alicante, Spain, interestingly, recently published a systematic review and meta-analysis of factors associated with embryo mosaicism.9 They reported that “mosaicism” was not related to either morphologic embryo quality or semen quality assessments, though slow embryos (day 6 & 7 blasts) had the highest mosaicism rates. Like the previously discussed papers, the study revealed mosaicism was higher in younger than older patients, while aneuploidy went the opposite way.
Another paper, this time in Human Reproduction, is also somewhat surprising, in that it took 19 authors to report two cases of “mosaicism” diagnosed by PGT-A, which later was also diagnosed in products of conception after termination.10 Of course, not every “mosaicism” present at blastocyst-stages and diagnosed by PGT-A will self-correct downstream, and prenatal diagnosis should be recommended. This was done in both of these cases, confirming in one case a 1p36 deletion syndrome, with 2.5% mosaicism in the infant’s brain. In the other case, a mosaic +21-trisomy (Down’s syndrome) embryo produced a pregnancy with 16% +21 cells in amniotic fluid. As already noted above, our center does not recommend transfers of embryos by PGT-A diagnosed with +21 embryos, whether “aneuploid,” “mosaic,” or segmental-abnormal. Why this manuscript was written and accepted, is not clear to us. It just reported the obvious!
Mosaicism in products of conception from abortuses are understudied. A major reason is that they often are clonal and only detectable through multiple biopsies, which is rarely done. Consequently, mosaicism is, likely widely underreported when cited in ca. 5%.11, 12
For completion’s sake, we also have to mention another allegedly systematic review and meta-analysis, this time by British colleagues, and published in JARG 13 How this paper slipped through the review process is difficult to understand, especially considering several
Continued on page 46 The V oice | OCTOBER 2023 | 45
publications over the last 12-18 months and the fact that the paper reports IVF cycle outcomes still with reference to embryo transfer. That such a paper at this point can still be allowed to be published is difficult to understand. That this paper claims that PGT-A increases composite outcomes for ongoing pregnancy and live birth rates and reduces miscarriage rates compared to morphological assessment alone, is moreover not at all surprising.
Fortunately, JARG also published a short, to-the-point opinion paper by Robert F. Casper, MD made clear that the conclusion of the preceding paper was simply ridiculous.14 Good for him!
REFERENCES
1. Cascante, et al., Fertil Steril 2023;S0015-0282(23)00767-7. Doi: 10.1016/ fertnstert.2023.08.010. Online ahead of print

2. Yang et al., Nat Cell Biol 2021;23(4):314-321; Author Correction: 2021;23(11):1212
3. Barad et al., Hum Reprod 2022;37(9):2216-2218
4. Ethics in Embryo Research Task Force, Ethics Committee of the American Society for Reproductive Medicine. Fertil Steril 2020;113(2):270-294
5. Armstrong et al., F S Rep 2023;4(3):256-261
6. Gleicher et al., Hum Reprod 2022;37(12):2730-2734
7. Willson S, Molinaro T., F S Rep 2023;4(3):252-253
8. Schattman G., F S Rep 2023;4(3):251
9. Cascales et al., A. Assist Reprod Genet 2023;40(10:2317-2324
10. Greco et al., Hum Reprod 2023;38(2):315-323
11. Sahoo et al., Genet Med 2017;19:83-89
12. Soler et al., Cytogenet Genome Res 2017;152:81-89
13. Kasaven et al., J Assist Reprod Genet 2023; 40:2297-2316
14. Casper RF., J Assist Reprod Genet 2023;40:2325-2332
Mechanisms of self-correction in human embryos
More interesting than most of the PGT-A literature was a reviewed preprint we came across in eLife by Belgian researchers, trying to investigate further how embryos downstream from blastocyst-stage self-correct.1 The introduction section of their manuscript is an extremely well-written up-to-date summary on how embryos self-correct. This part of the manuscript alone is worth the read. The manuscript goes beyond that by investigating the cellular response to complex aneuploidy during human preimplantation development, with a focus on stress response and lineage segregation events.
Using 85 human blastocysts, RNA-sequencing of trophectoderm cells demonstrated a transcriptional signature compatible with a deregulated p53 pathway and apoptosis proportional to chromosomal imbalance. Aneuploidy triggered proteotoxic stress, autophagy, and apoptosis, with cell numbers declining in trophectoderm as well as epiblast/primitive endoderm. Apoptosis appeared responsible for lower cell numbers in trophectoderm, but aneuploidy impaired especially segregation of the second lineage and formation of primitive endoderm, likely explaining why a large majority of at this developmental stage fully “aneuploid” embryos do not further develop. As already previously reported by CHR and Rockefeller investigators, human embryos 2 self-repair differently from mouse embryos3 by triggering autophagy and p53-mediated apoptosis and impairing second lineage segregation.
REFERENCES
1. Regin et al., Reviewed Preprint. eLife 2023;https://doi.org/10.7554/ eLife.88916.1
2. Yang et al., Nat Cell Biol 2023;23:314-321
3. Bolton et al., Nat Commun 2016;7:11165
Immunology in reproduction
A new side effect of anti-TNF-alpha therapy
With increasing biologicals used in infertility patients as well as during pregnancy, their side effects should be known. Tumor necrosis factor (TNF) inhibitors are one class of drugs increasingly used, and recently even demonstrated to reduce the risk of preeclampsia in women with inflammatory bowel disease treated during pregnancy with TNF inhibitors. One such side-effect has now been reported in patients with rheumatoid arthritis, who, following anti-TNF treatments, in post-marketing safety reports to the FDA were at surprising rates shown to develop psoriasis.
This finding is counterintuitive because anti-TNF therapies are widely used in treating psoriasis. A recent study, however, confirmed this associated risk in comparison to patients with rheumatoid arthritis treated with methotrexate.2
REFERENCES
1. Patel et al., Dig Dis Sci 2023;68(9):3557-3561
2. Joulfayan et al., Sci Reports 2023;13:10448
Ginger as a new immunosuppressant 6-gingerol, the most abundant phytochemical in ginger root inhibits phosphodiesterase inhibition which, in turn, counteracts neutrophil hyperactivity, which we quite frequently see in infertile women. A recent paper now reported that in a mouse model and human studies, daily ginger intake for as short as one week boosted in healthy volunteers cAMP inhibited neutrophil extracellular trap formation in response to (autoimmune) disease stimuli, and reduced its levels.
Considering how frequently we see neutrophilia (usually in combination with lymphopenia) in infertile women, and how much we
Continued from page 45 ADVERTISEMENT 46 | OCTOBER 2023 | The Voice
dislike evidence of hyperactive immune systems in our patients (whether through inflammatory or immune markers, ginger supplementation may make sense. The authors of this paper used a ginger supplement that offered a daily dose of gingerols of 2040mg/ day.
REFERENCE
1. Ramadan et al., JCI Insights 2023;8(18): e172011
A truly fascinating case of fetal-induced graft-versus-host disease (GVHD)1
A 32-year-old female with a negative past medical history gave birth to twins (both males). The delivery was associated with a vaginal hemorrhage, requiring transfusion with 2 units of nonirradiated packed red cells. Three weeks post-delivery, she developed fever, severe pancytopenia (assumed to be transfusion-dependent) with undetectable neutrophiles, diarrhea, and a desquamating rash all over her body. A detailed infectious and autoimmune workup was negative. Suspecting a transfusion-associated GVHD, skin, and bone marrow biopsies were performed, demonstrating cell infiltrates with predominantly T-cells with an increased proportion of CD8 cells (cytotoxic T-lymphocytes). On further examination, 20% of inflammatory cells in the skin and 30% of CD3+ selected cells in the bone marrow were haploidentical to the mother (i.e., reflecting fetal DNA). In addition, cytogenetic analysis of the bone marrow biopsy revealed XY in 3/15 cells.
The patient recovered within two weeks after initiation of treatment with glucocorticoids, - not requiring an urgent stem cell transplant. The treating physicians speculated that such a stem cell transplant did not become necessary (as they had expected) since the tolerance pathways induced by pregnancy, likely, rendered the fetal T cells more susceptible to glucocorticoids than usual in association with transfusion-associated GVHD.
In closing their fascinating report, the authors raise the important point that lower-grade versions of this condition very likely occur more frequently and remain undiagnosed. Milder cases have been reported before.2 Also important to note in this context is the similarity of many immunological findings between preeclampsia and GVHD, pointed out by the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, already in 2007.3
REFERENCES
1. Schreiber et al., N Engl J Med 2023389(7):668-670
2. Lo et al., Am J Hum Genet 1999;64:218-224
3. Gleicher N., Am J Obstet Gynecol 2007;196(1):5.e1-7
Infectious diseases and reproduction
We are again/still talking about the SARS-CoV-2 virus
It appears like we just went through a kind of mini-wave of new infections with the current mutants of the SARS-CoV-2 virus
during late August and September. Fortunately, the disease was mostly mild, and numbers now also appear to be again on the decline. At the same time, experts predict a, likely, bigger wave in the coming months, as temperatures get colder.
The CHR recommends, per CDC recommendations, that individuals at increased risk of developing severe disease “may” receive booster vaccination.1 Interestingly, this is a much weaker recommendation from the CDC than in the past, where the word “should” rather than “may” was usually used. Moreover, the Advisory Committee on Immunization Practices voted 13-0 (1 abstention) that people ages 60-64 receive the booster, but only 9-5 for above age 65.
Pregnant women as well as women attempting pregnancy, have been demonstrated to be at increased risk if infected with the virus during pregnancy. Vaccination also has been demonstrated to protect newborns from infection.
We are so far unaware of a CDC recommendation directly addressing pregnancy when it comes to the now by the FDA-approved vaccine boosters by Pfizer and GSK.
Here are several interesting papers regarding the virus that appeared recently in the literature:
How come SARS-CoV-2 infections in some people are completely asymptomatic?
Even in the early days of the COVID-19 pandemic, when the disease was at its most dangerous, some people converted to being positive for anti-SARS-CoV-2 antibodies (i.e., went through being infected) without exhibiting any symptoms whatsoever. Studies suggested that the number of asymptomatic infections with the virus may be at least 20%.2 A recent paper in Nature magazine, finally, offered an answer.3
Assuming that the answer may be linked to variations in the human leukocyte antigen (HLA) loci, an international group of researchers investigated 29,947 individuals for whom high-resolution HLA genotyping results were available. The primary study cohort ended up being 1,428 unvaccinated positive individuals in anti-SARS-COV-2 antibody testing. After testing for associations with five HLA loci with disease course, they discovered a strong association between HLA-B*15:01 and completely asymptomatic infections with the virus.
Continued on page 48 The V oice | OCTOBER 2023 | 47
On further investigations, the researchers also demonstrated that this finding in this specific group of individuals likely, was the consequence of preexisting T-cell immunity to NQKLIANQF, an immunodominant S-derived peptide of the SARS-CoV-2 virus. In other words, these individuals’ T lymphocytes demonstrated a cross-reactive memory phenotype with seasonal past coronaviruses. Immunity from past infections with these other coronaviruses protected these individuals from becoming symptomatic, even when exposed to the SARS-CoV-2 virus. It’s all in our genes!
Related, another genetic characteristic of mankind, our blood groups, also appear to have a relationship to how we face the SARS-CoV-2 virus. Considerable evidence suggests people with type O blood experienced lower risks during the COVID pandemic.4 Exact reasons are not clear yet, but it may not be that much that blood type O protects, but that, for example, blood type A makes individuals more prone to infection. In one study, the virus bound to red blood cells most in individuals with type A and least with type O. A Danish study of healthcare workers demonstrated lower infection rates with group O than A, B, or AB.
And
how come others are developing Long COVID?
Still unclear has also been why so many individuals develop so-called Long COVID (LC), a post-acute infection syndrome (PAIS) that can occur after several acute viral diseases. Now U.S. investigators determined distinguishing features in so-affected patients through immune profiling.5
Enrolling 273 individuals with and without LC in a study involving multidimensional immune phenotyping and unbiased machine learning, marked differences were found between study and control patients in circulating myeloid and lymphocyte populations. Affected patients also demonstrated exaggerated humoral responses against the virus, but also against non-SARS viruses, especially the Epstein-Barr virus. The investigators also found differences in soluble immune mediators and hormones, with cortisol levels in LC patients being lower than in controls. Unbiased machine learning models, moreover, identified certain key features strongly associated with LC.
This study, therefore, opens new research options for a better understanding and, hopefully, treatment, of LC. Related, investigators recently also reported in Cell that severe SARS-CoV-2 infections through IL-6 induce (in a mouse model as well as in humans) persistent epigenetic signatures in hemopoietic stem cells and their myeloid progenitors associated with increased inflammatory potential.6 A better understanding of LC is coming into sight and is discussed by Prof. Dr. Birgit Sawitzki from the Berlin Institute of Health at Charité (BIH) in a preview article in Cell 7
REFERENCES
1. CDC. June 21-23, 2023. https://www.cdc.gov/vaccines/acip/meetings/ slides-2023-06-21-23.html.
2. OranDP, Topol EJ., Ann Int Med 2020;173:M20-3012
3. Augisto et al., Nature 2023;620:128-136
4. Rubin R., JAMA 2023;330(9):795-796
5. Klein et al., Nature 2023; doi: 10.1038/s41586-023-06651-y. Online ahead of print.
6. Cheong et al., Cell 2023;186(18):3882-3902
7. Sawitzki B., Cell 2023;186:3753-3754
A new important antibiotic?
That we are running out of antibiotics because of increasing bacterial resistance is no secret. Neither is the fact that the search for new antibiotics over the last decade has been very disappointing. This why the news that researchers from The Netherlands, Germany, and the U.S. have been studying a new antibiotic that seems to be capable of combating multidrug resistance is very good news. Clovobactin, as the antibiotic has been named, was isolated from bacteria which, until recently, nobody could grow in the laboratory, so-called “bacterial dark matter,” unculturable bacteria, now open to studies on a so-called iCHip device, developed in 2015.1 Teixobactin, is another truly new antibiotic recently developed through this device.
REFERENCE
1. Shulka et al., Cell 2023;186(19):4059-4073.e27
FDA approves firsv maternal vaccine to prevent respiratory syncytial virus (RSV) infection in infants
It has been known for some time that some vaccines given to the mother can also establish immunity for offspring. For example, anti-COVID vaccines given to pregnant women were, likely, due to transplacental passage of antibodies, created in the mother in response to the vaccines, also protecting newborn infants, On August 21, 2023, the FDA approved the first vaccine against RSV given to mothers to “immunize” their offspring.1
Infants are among the highest risk patients for severe disease, leading to hospitalization. In a news release, the FDA advised that “this approval provided an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease.
REFERENCES
1. FDA. https://www.fda.gov/news-events/press-announcements/ fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
Obstetrical care
Here are a few briefly summarized interesting items regarding obstetrical practice:
•Delivery at 39 weeks provides the lowest perinatal risks in pregnancies conceived through fertility treatments.1
•According to a mouse model, small amounts of fetal cells (microchimerism) in the mother confer partner-specific protection after parturition against future pregnancy complications. They become replaced by new cells in subsequent pregnancies, a process essential for the expansion of Treg cells, with maternal microchimeric cells in female offspring being replaced by their offspring. maternal cells in female fetuses get replaced when the daughters, themselves, get pregnant. Mothers remember their offspring more durably, while daughters forget their mothers with new pregnancy-imprinted immunological memories.2
Continued from page 47 48 | OCTOBER 2023 | The Voice
•An excellent review article recently described the management of immune thrombocytopenia in pregnancy. 3
•Can a blood test measuring cell-free DNA methylation predict pre-eclampsia? Based on the hypothesis that changes in DNA methylation in the placenta might be detectable in blood (because placental DNA is released into the circulation), Belgian investigators recently in a paper in Nature Medicine offered preliminary evidence for exactly such a test.4
•The Society for Maternal-Fetal Medicine offers a “Special Statement” strongly endorsing the prophylactic use of low-dose aspirin in the prevention of preeclampsia in patients at risk.5
•In maternal depression, selective serotonin reuptake inhibitors (SSRIs) are the most frequently prescribed antidepressant medications. Gestational SSRI exposure may be adversely associated with offspring brain and behavioral development. These concerns are balanced by concerns that untreated maternal depression in pregnancy may be equally concerning. A recent article in JAMA Psychiatry addresses this complex issue in an editorial.6
•10 international pediatric endocrine societies worldwide published an international consensus guideline on the management of small for gestational age management from infancy to early adulthood.7 These pages in the past repeatedly expressed our discomfort with the proliferation of societal “mega” opinions, a concern here also applicable.
•The currently unacceptably high maternal mortality rate in the U.S. in a “Viewpoint” article by CHR friend and colleague (and September GrandRounds speaker) Eli Y. Adashi, MD, in JAMA is appropriately attributed in part to postpartum events. The article logically urges the extension of Medicaid coverage into the postpartum period.8
•We elsewhere in this issue already discussed cannabis use in general. Here, we want to point out another: Viewpoint” article in JAMA which addressed perinatal research on cannabis use, concluding that, “despite research advances and changes in public health practices, there remains a significant gap in knowledge regarding the effects of cannabis use and perinatal health outcomes.”9
On a side note, we are impressed by a visibly increased presence of articles relating to pregnancy and female health in JAMA since a new (female) editor-in-chief was appointed!
REFERENCES
1. Hamilton et al., JAMA Network Open 2023;6(8):e.2328335
2. Shao et al., Science 381(6664):1324-1330
3. Bussel et al., N Engl J Med 2023;389(6):540-548
4. De Borre et al., Nat Med 2023;29(9)::2206-2215
5. Society for Maternal-Fetal Medicine, Am J Obstet Gynecol 2023;229(2):
B2-B9
6. Talati A., JAMA Psych 2023; doi:10.1001/jamapsychiatry.2023.2664. Online ahead of print.
7. Hokken-Koelega et al., ENDOCRINE REV 2023;44:539-565
8. Adashi et al., JAMA 2023;330(10:911-912
9. Lo et al., JAMA 2023;330(10):913-914
Gynecological care
Here are a few briefly summarized interesting items regarding gynecological practice:
•Can excessive heat lead to miscarriages? Animal research supports such a link, according to a recent news article in Science magazine.1 A reproductive and environmental epidemiologist at Boston University, as the article noted, is currently trying to explore this question in humans, and has enrolled already over 100 participants who are tracked with temperature sensors while trying to conceive. This is, of course, an interesting question to get answered; but we are concerned that the number of research subjects required for such a study to offer statistically solid answers is, likely, practically unachievable.
•An interesting recent article in the Wall Street Journal by Sumathi Reddy raised the interesting question, “what if we could get rid of menopause?” In a brief but well-researched summary, she correctly concluded that boosting female fertility by extending it into older ages new tools must be developed to slow ovarian aging.2
•An editorial in the BMJ recently pointed out that the interaction between NSAIDs (non-steroidal anti-inflammatory drugs) and hormonal contraceptives deserve more attention.3 We fully agree!
•An article in Menopause recently attempted to address the decades-long controversial issue of what the consequences are of bilateral oophorectomy at young ages.5 After a median follow-up of 22 years. After surgery under age 46, women demonstrated increased risks of arthritis, asthma, obstructive sleep apnea, and bone fractures. They also walked a shorter distance on a 6-minute walk test. If the surgery was performed between age 46 and 49, they still demonstrated increased arthritis and obstructive sleep apnea risks. Importantly, neither age group demonstrated cognitive impacts. These observations must be considered before the performance of bilateral oophorectomies in younger women. Especially the impact on the immune system (arthritis, asthma) appears of concern.
•The use of menopausal hormone replacement therapy (HRT) has been associated with risk of non-alcoholic fatty liver disease (NAFLD). Now a paper published in Scientific Reports claimed that the route of estrogen supplementation matters: Investigating 368 postmenopausal women for 12 months on HRT, divided into transdermal (n=75) and oral (n=293) administration of estrogen. Somewhat surprisingly, NAFLD decreased from 24.0 to 17.4% with transdermal administration and increased from 25.3% to 29.4% with oral administration.5 WARNING: There is good reason to question the conclusion of the authors that transdermal estrogen supplementation is beneficial in reducing NAFLD in comparison to oral administration. The reason is the retrospective analysis these conclusions are based on: If our Korean colleagues treated only approximately 1 in 5 postmenopausal patients with transdermal and 4 in 5 with oral medications, one must, based on such an uneven distribution, suspect a conscious or unconscious patient selection bias, even if baseline characteristics did not differ between both groups (Table 1 in the paper).
Continued on page 50 The V oice | OCTOBER 2023 | 49
recent paper in the American Journal of Pathology points toward potentially new treatment options for endometriosis patients.6 As pain in women with endometriosis is attributed to neuroinflammation nociceptor nerves (“nociception” refers to the processing of peripheral noxious stimuli by nerves and the CNS), by the authors hypothesized to be activated by IL-1ß they validated the presence of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in endometrial tissue and functionally tested dose responses, with IL-1ß eliciting higher TrkA/B (receptor) responses in endometrial stromal tissue from endometriosis patients than cells of controls, effects mediated by the c-Jun N-terminal kinase (JNK) pathway. Peritoneal fluid, however, demonstrated only a trend toward higher BDNF concentrations. Based on these findings, the authors postulated that inhibitors of JNK, like SP600125, may reduce neuroinflammation in endometriosis.
Related, JAMA Network Open recently reported that endometriosis patients demonstrate an increased prevalence of mental disorders,7 raising the question of whether endometriosis is not as much a disease of the nervous system (including the brain) as it is a gynecological disease.
•The BMJ recently reported that the High Court in London allowed a group litigation order (the British counterpart to a Class Action in the U.S.) for 200 women in England and Wales against Bayer over alleged harm from the permanent contraceptive device Essure. 8 The U.S. cannot be far behind.
REFERENCES
1. Wadman M., Science 2023;381(6657):469
2. Reddy S., The Wall Street Journal, July 15-16, 2023. pp C1-C2. https://www. wsj.com/articles/what-if-we-could-get-rid-of-menopause-7adbc4e0
3. Schmidt M., BMJ 2023;382:p1990
4. Mielke et al., Menopause 2023; doi: 10.1097/ GME.0000000000002254. Online ahead of print.
5. Kim et al., Sci Rep 2023;13:15461
6. Yu et al., Am J Pathol 2023;193(8):1046-1058
7. Koller et al., JAMA Netw Open 2023;6(1):e2251214
8. Dyer C., BMJ 2023;382. Doi.org/10.1136/bmj.p2044. Online ahead of print
Basic Science Research
Trying to look into the black box of embryo development and uterine implantation in the peri-implantation period
How to model implantation in vitro in the laboratory has been a longstanding issue in many research laboratories, which recently has become even more acute with the development of several human embryo models produced from stem cells, which potentially allow research on implantation without numeric restrictions on natural human embryos mandate for any research involving human embryos.
Now scientists report the development of a synthetic extracellular matrix that allows cells to interact in an environment that mimics human physiological conditions.1 They describe their achievement as ”the development of a fully synthetic matrix to sustain the dynamic
changes of the endometrial microenvironment and support its applications to understand menstrual health and endometriotic diseases.”
A paper in Human Reproduction by Spanish and Italian investigators, somewhat unsurprisingly, reports that endometrial cells secret extracellular vesicles that contain miRNAs relevant to implantation which human blastocysts ingest.2 Why is this unsurprising? First and foremost because extracellular vesicles are now among the “hottest” items in bench research wherever one looks, from immune cells3 to cancer cells,4 and, yes, from human blastocyst-stage embryos, probably already in fallopian tubes, sending through them messages to the to the maternal immune system.5
We in preceding issues of The VOICE reported on several embryo models derived from stem cells and commented on their importance in offering not only models for investigating human embryos beyond blastocyst-stage, but also the quantity of research material which in all studies of human embryos is always restricted for practical as well as ethical reasons. Now, yet another in vitro stem cell model of human embryos capable of replicating human blastulation has been reported in Cell 6 This model’s synthetic embryos termed “peri-gastruloids,” encompass embryonic (epiblast) as well as extra-embryonic (hypoblast) cell lineages. Lacking trophoblast, and not “viable” (a term used by the authors which we find somewhat inappropriate), they do recreate important stages of early human peri-gastrulation development, including amniotic and yolk sac cavities, bilaminar and trilaminar embryonic disc, primordial germ cells, gastrulation initiation, and even early neurulation and organogenesis.
A potentially new application of these embryo models, which we have not read before, is the potential development of human fetal tissue for use in regenerative medicine. The article was also commented on in an accompanying opinion piece that offered a smart summary on the subject under the creative title, “Seven days in the life cycle of Homo sapiens,” making the point that the second week of embryonic development, an important time in embryonic life, has been largely inaccessible to investigators.7
Animal models can also contribute, as a recent paper in Cell Reports Methods demonstrates which showed how rat epiblast-derived stem cells can recapitulate attributes of pre-gastrulation epiblasts.8
Regarding early human development, an additional important new step was the recently announced cell atlas of the yolk sac.9 Acting as a transient extraembryonic organ, the yolk sac at those stages delivers vital functions to the developing embryo, with the reported cellular information, as the authors suggest, offering potential leveraged for tissue engineering and cellular therapy.
REFERENCES
1. Gneccoe et al., Med 2023;4(8):554-579.e9
2. Segura-Benítez et al., Hm Reprod 2023;38(8):1547-1559
3. Abbot A., Nature 2023;621:462464
4. Agustinus et al., Nature 2023;619(7968):176-183
5. Godakumara et al., Reprod Domest Anim 2022;57(suppl5):14-21
6. Liu et al., Cell 2023;186:3776-3792
7. Pera MF., Cell 2023;186:3755-3757
8. Pragathi Masamsetti et al., Cell Rep Methods 2023;3(8):100575
9. Goh et al., Science 2023;381, eadd7564. DOI: 10.1126/science.add7564
•A
Continued from page 49 50 | OCTOBER 2023 | The Voice
How is ovarian reserve built?
In a paper we almost overlooked had Science magazine not printed a short blog about it, Canadian investigators identified the orphan nuclear receptor, Steroidogenic factor (SF-1) as a contributing regulator for the formation of what is called the (resting) ovarian reserve (i.e., the number of remaining primordial follicles in ovaries of a woman. This does not include already recruited follicles out of the resting stage, which are called growing follicles and represent only a small fraction of resting follicles, - also called primordial follicles). Depletion of SF-1 resulted in a dramatic reduction in resting ovarian reserve due to impaired follicle formation and increased oocyte death. As a next step, SF-1 must be investigated in several infertility-related conditions, like premature ovarian aging (POA) and of course, primary ovarian insufficiency (POI). This recent paper also suggests the likelihood that SF-1 may determine how large an ovarian reserve a woman going through menarche starts her reproductive lifespan with.
REFERENCE
1. Hughes et al., PNAS 2023;120(32):.e2220849120
A promising new way to improve oocyte quality in older women
Chinese investigators just published a very interesting Reviewed Preprint in eLife in which they claim that exogenous supplemented C-type natriuretic peptide (CNP) in a mouse model of older animals improved oocyte quality significantly by inhibiting PINK1/Parkin mediated mitophagy.1
CNP has been for some time known as a meiotic inhibitor and has been, as such, used in in vitro maturation to mitigate the damage of higher reactive oxygen species (ROS, seen in older women). The authors of this paper now explain these observations by demonstrating that CNP concentrations in serum are highly correlated with oocyte quality. Exogenous supplementation of CNP improved follicle growth and ovulation in aged mice and enhanced meiotic competence, fertilization of oocytes, and cytoplasmic maturation of aged oocytes. Cytoplasmic maturation was improved based on improvements in spindle/chromosome morphology and redistribution of organelles. CNP treatment also ameliorated DNA damage and ROS-induced apoptosis. The improvements were
mediated by the restoration of mitochondrial oxidative phosphorylation and the elimination of excessive PINK 1/Parkin-mediated mitophagy. Mice were injected intraperitoneally with 120ug/kg.
REFERENCE
1. Zhang et al., Reviewed Preprint, eLife 2023; https://doi.org/10.7554/ eLife.88523.2
We finally have the complete sequence of the human Y chromosome
It took many years and, finally, not less than 87 authors, to publish the complete sequence of the Y chromosome.1 As small as it is (it is by far the smallest chromosome), it has been notoriously difficult to sequence, as the authors pointed out, because of its complex, repeat structure, including long palindromes, tandem repeats, and segmental duplications. This accomplishment closes an important process in the research of the human genome and, therefore, also represents a fitting paragraph to close this October issue of the CHR VOICE.
REFERENCE
1. Rhie et al., Nature 2023;621:344-364
The CHR
Fighting for every egg and embryo
NEWSLETTER INFORMATION
The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400.
The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiology, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved.
For letters to the editor, comments, and suggestions, please contact Micah Elias at melias@thechr.com. For all advertisements or sponsorships in The VOICE , please contact Alexandra Rata at arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR. .R
ADVERTISEMENT
The V oice | OCTOBER 2023 | 51




The Center for Human Reproduction Fighting for every egg and embryo www. T h EC h R . CO m s OCI al@ T h EC h R . CO m www.thechr.com @CHRNewYork @CHRNewYork @CHRNewYork 21 E 69 T h sT , N E w Y OR k, N E w YOR k, 10021 212.994.4400 C ONNECT w IT h us!





















































