



THE OCTOBER 2022 in this month’s issue When a Woman with Underlying Medical Problems is Trying to Conceive DHEA, Brain Development, and Evolution Injecting Platelet-rich Plasma (PRP) Into the Ovaries: The Preliminary CHR Experience A Piece of My Mind: Not Only in Politics do Repetitions Make Falsehoods Believable and Lead to “Group-Think” The CHR in the Media The CHR’s Interpretation of Recent Literature, Relevant to Reproductive Medicine 03 07 11 15 19 21
the last issue
The VOICE?
previous issues on



The CHR is known as a
center of last resort,” primarily serving patients who have previously failed treatments elsewhere.
CHR’s areas of special expertise are treatments of “older” ovaries, whether due to advanced female age or premature ovarian aging (POA), immunological problems affecting reproduction, repeated pregnancy loss, endometriosis, polycystic ovary syndrome (PCOS), tubal disease, male factor infertility, etc.


the VoiCe

HOW ABSURD CAN INSURANCE COMPANIES ACT?

More so than even we expected!
Here is a shortened summary of an anonymized letter one the CHR’s physicians recently sent to a major insurance company. The absurdity of the situation should be obvious. The CHR not only fights for every egg and embryo for our patients, but also for every dollar!
To Whom It May Concern:
My patient X informed me that insurance coverage of her recent fertility related preconcep tion screening panel was denied ($3,023.31) because included was a test for Vitamin D defi ciency. In support of this denial your company relied on a meta-analysis (Pacis et al., JARG 2015;32(3):323-335).
In Support of such screening, I, however, note a more up to date meta-analysis (Zhao et al., Reprod Biol Endocrinol 2018;16:13), which concludes that: “Deficient vitamin D was associated with decreased probability of live birth after IVF/ICSI. So, vitamin D should be supplied to women with deficient level of vitamin D.” Vitamin D deficiency, moreover, is listed among your covered diagnoses; how could one make the diagnosis without testing?

To deny coverage of an entire diagnostic panel because of one test appears egregious. While in your letter you claimed to have informed our practice of a new vitamin D policy in January of 2022, I never heard of this policy till today. I am extremely disappointed by the way you have handled this matter.
Sincerely, XXXX, MD
WITH THE CHR
OCTOBER 2022 2 | o C to B e R 2022 | The Voice www.thechr.com @CHRNewYork @CHRNewYork@CHRNewYork CONNECT
Missed
of
Access
thechr.com
“fertility
Among
Some Background
This year we celebrate the 100year anniversary since Canadian physician scientists at the University of Toronto, Frederic Banting, MD and Charles Best, MD, administrated for the first time on January 11, 1922, insulin to a type I diabetic by the name Leonard Thomson. 1 Up to that point a diagnosis of Type I diabetes was a death sentence at very young ages. Leonard Thomson at that time was 14 years old, was 5’11”, weighed only 64 pounds, and drifted in and out of diabetic coma. Though not as radical, type II diabetics also had significantly shortened life spans. Typically, neither type I nor type II diabetics, indeed, had ever children because type I diabetics were too sick to conceive even at young ages, while type II diabetics, even if they on rare occasions conceived, usually miscarried.
All of this changed with the introduction of insulin, and over recent decades women with diabetes, if well controlled, therefore have basically almost normal pregnancy and delivery chances, though especially type I diabetics, still, face increased risk for congenital anomalies in offspring and increased perinatal morbidity/mortality. Unfortunately, large numbers of diabetic women in reproductive years are not well controlled when conceiving,2 clearly a failure of many health care systems even in developed countries, in the U.S. at least partially driven by exorbitantly high insulin costs, only recently at least partially addressed for Medicare recipients by Congress in the socalled Inflation Reduction Act.3 Diabetes in women is, therefore, an excellent example for how progress in medicine has increasingly allowed women with a significant underlying medical problem to
ONLY A FEW DECADES AGO, HAVING A SIGNIFICANT MEDICAL PROBLEM FOR A WOMAN USUALLY MEANT NOT TO HAVE CHILDREN: EITHER THE UNDERLYING DISEASE CAUSED INFERTILITY OR PHYSICIANS WERE RELUCTANT TO CLEAR PATIENTS FOR PREGNANCY. ALL OF THIS HAS, HOWEVER, RADICALLY CHANGED IN RECENT DECADES. NOWADAYS, FOR A WOMAN NOT TO BE ABLE TO GO THROUGH PREGNANCY BECAUSE OF AN UNDERLYING SIGNIFCANT MEDICAL PROBLEM, AS LONG AS SHE RECEIVES APPROPRIATE AND COORDINATED MEDICAL CARE FROM A MULTISPECIALTY TEAM OF PHYSICIANS, IS A RARITY.
safely conceive, and to deliver healthy offspring. Other examples abound, from maternal cardiac diseases to autoimmune diseases, from surgeries in pregnancies almost always leading to a loss of pregnancy, to not only most surgeries avoiding the occurrence of pregnancy losses, to even intrauterine fetal surgeries allowing for continued pregnancies. 4
This very positive development is, however, counterbalanced by another demographic development in most countries around the globe, the aging of the obstetrical population, as wom en increasingly delay childbirth. This latter development is, of course, especially apparent in infertility centers, and the aging of the infertility population has, therefore, been a repeated subject of discussion in the pages of the VOICE since the CHR, now for almost two decades, has been at the cutting edge of this development by serving the by-far oldest patient population of
any IVF center in the U.S. (and likely in the world), with a median age of over 43 years. This means that at the CHR, over half of newly presenting patents are over 43 years old at time of their presentation.
As women are getting older, medical problems accumulate and get worse. Infertility patients, therefore, now present with medical problems almost never seen in infertility practice 20-30 years ago, like coronary heart disease, after renal, hepatic, or even cardiac transplants, with severe autoimmune diseases, etc. Fortunately, the CHR has been for several reasons wellprepared for these developments: The first reason is the special expertise of the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, regarding medical problems in pregnancy. Not very widely known and appreciated, before founding one of the first U.S. IVF centers in the U.S. in 1981 in Chicago, his primary academic
The V oice | o C to B e R 2022 | 3Continued on page 4
WHEN A WOMAN WITH UNDERLYING MEDICAL PROBLEMS IS TRYING TO CONCEIVE
clinical and research interests were, indeed, medical problems in pregnancy and he then edited several editions of what to this day is considered “the bible” textbook on this subject, “Principles and Practice of Medical Problems in Pregnancy.”5
Together with his friend and cardiology colleague, Prof. Uri Elkayam, from the University of Southern California, Los Angeles (USC-LA), he also edited several editions of “Cardiac Problems in Pregnancy,”6 to these days the principal reference textbook for management of cardiac disease in pregnancy.
His special expertise in the management of medical disease in pregnancy has, therefore imprinted patient management at the CHR from the beginning and has resulted in disproportionally many women with medical problems seeking advice at the CHR before planning on pregnancies. Consequently, the CHR, likely, has more experience in managing such patients than most other fertility centers, which, of course, builds upon itself because more experience, of course, means more practice and more familiarity.
Some specifics
It is, of course, impossible to discuss the management of all medical problem in pregnancy in a short article like this. What we, however, can comfortably provide here are some important principles, and we already alluded to one above:
Patients must be prepared for pregnancy
Patients with a significant medical problem should never be allowed to conceive “accidentally.” The reason is that at time of conception they should be in bets possible
shape and not, for example, in the midst of an autoimmune flair, in even mild congestive heart failure, in poor glucose control with abnormally high HgA1c (below 5.8% if possible), or hypertensive.
Practically this means that women with significant medical problems should be on proper birth control and come off it only once maximized in their medical status and ready to conceive.
pregnant, care can be automatically transferred from the fertility center to the obstetrical team that is already awaiting the patient. At the CHR we, therefore, also recommend that the patient choses all of her subspecialists at the hospital where she intends to deliver. It does not make much sense for her to deliver at hospital X, while her cardiologist has only privileges at hospital Y.
This preparation period rarely lasts less than 3-6 months and should involve a collaborating team of specialists, covering all of the patients’ medical problems, of course including the patient’s tentative obstetrician and, in most cases, also a collaborating perinatologist, so that, once
The interplay between pregnancy and disease Medical treatments in pregnancy must always be aware of 4 core issues: (i) Pregnancy affects every disease; (ii) every disease affects pregnancy; (iii) because medical management in pregnancy affects 2 patients (mother and fetus), treatments often must differ from the non-pregnant state; and (iv) pregnancy complication for the mother can carry over into the postpartum period – by some now called the 4th semester of pregnancy. Here are a few examples for each of the 4: Pregnancy affects every disease: Pregnancy, for example, automatically dilates blood vessels and increases the blood volume in a pregnant woman by approximately 40%. This can have good and bad effects. In some women with hypertension, the dilatation of blood vessels may result in temporary improvements in hypertension. In women on the border to congestive heart failure (CHF), the additional fluid load can lead to CHF. Similarly, pregnancy is a diabetogenic state. Consequently, insulin requirements will go up in pregnancy or even nondiabetic women may become diabetic (so-called gestational diabetes). Or pregnancy can lead to autoimmune flairs at two important periods: in early pregnancy by preventing development
Continued from page 5
“Diabetes in women is, therefore, an excellent example for how progress in medicine has increasingly allowed women with a significant underlying medical problem to safely conceive, and to deliver healthy offspring”
4 | o C to B e R 2022 | The Voice
hiRinG
AT THE CHR
ONE or TWO-YEAR CLINICAL FELLOWSHIP in RE-I

If you failed in securing a formal fellowship position in RE & Infertility or simply want a change out of gener al OB/GYN practice, the CHR offers a fellowship position in RE-I which after 1 year will greatly improve competitiveness for a formal fellowship position and in 2 years establishes independent competence for establishing an infertility practice. The center’s last 1-year fellow was accepted into the NIH-fellowship program after failing to secure a position in the preceding year.
Qualified candidates must be OB/GY board-eligible or certified, be eligible for hospital privileges and for a New York state license to practice medicine. As CHR maintains a very active research program in affiliation with Rockefeller University, CHR fellows gain substantial research experience, with great likelihood resulting author- or co-authorships in peer-reviewed publications. Board eligible candidates who still must accumulate surgical cases will be given the opportunity to do so.
Chosen candidates will receive a very competitive salary and a generous benefit package, including health and malpractice insurance as well as paid vacation time. If you feel that you qualify and are interested in a career in RE & I, please submit your CV and a brief application letter to our COO, Ms. J. Tapper, at jtapper@ thechr.com. The position is available as of January 1, 2023. All submissions are considered confidential.
of normal tolerance of the maternal immune system for the fetal semi-allograft, and at the end of pregnancy, - by terminating tolerance prematurely.
Diseases affect every pregnancy: Again, there are many examples, with premature labor, for a variety of reason, likely being the most frequently observed effect of medical diseases on pregnancy. Autoimmune diseases for example have a very close relationship with pregnancy since both are immunological phenomena. An early pregnancy flair causes miscarriages; and a late flair is frequently the cause of premature labor; so-called gestoses of pregnancy, and preeclampsia/ eclampsi are also often the result of early termination of adequate tolerance. Maternal heart disease can slow fetal growth and lead to small for gestational date pregnancies, hypertensive diseases of pregnancy can have the same effect and often lead to small placentas; diabetes can lead
to very large babies in mild cases and to small babies in more severe cases; and we can go on and on with examples.
Treating 2 patients: As many medications cross the placenta, every treatment prescribed in pregnancy must consider potential effects on the fetus. This is a reason why, for example, many antihypertensive treatments women receive prior to pregnancy must be

changed once they are pregnant. As most pharma companies unfortunately do not want to spend the money and/or take the risk of testing their new products in pregnant women, the safety of many new drugs in pregnancy has not been established and those drugs, therefore, often cannot be used in pregnancy until many years after they have been approved for non-pregnant patients.
The 4th semester of pregnancy: Here the best example is again autoimmunity since autoimmune diseases frequently flair in the postpartum period for up to five months post-delivery. This is a very important and a too rarely communicated point to women with autoimmune diseases or a genetic predisposition toward autoimmunity, which then often leads to a first clinical manifestation of disease postpartum. The most typical example for postpartum flairs and/or first presentation is autoimmune thyroid disease.
Conclusions
The news for women with medical problems who want to conceive, therefore, are overall good: Most women should have no problem going through pregnancy, as long as their medical condition(s) can be stabilized before they conceive. To run behind
ADVERTISEMENT
Continued on page 6 The V oice | o C to B e R 2022 | 5
and start treatments only once a pregnancy or a patient is already in trouble, is much more difficult, and clearly much less successful.
But there are, of course, some women who are so badly affected by their medical problems that carrying a pregnancy can absolutely not be recommended. Examples are women with so-called Eisenmenger syndrome (an opening between both sides of the heart in association with pulmonary hypertension) or with primary pulmonary hypertension. In both conditions pregnancy is contraindicated because of very high maternal mortality.
And then there are the “questionable” cases. What we mean by that is that, somewhat understandably, many physicians often feel uncomfortable in clearing patients with medical problems for pregnancy and delivery. They then, frequently, without really good reasons recommend the use of gestational carriers in what we often consider a premature decision. Like with egg donation, the philosophy of the CHR has always been to consider these two options as last resort options. When in doubt, we, therefore, always recommend patients to get a second opinion before agreeing to give up on such important life-determining issues, like use of donor eggs and/or gestational carriers (surrogates).
REFERENCES
1. https://jdrf.org.au/case-studies/leonardthompson-a-100-year-old-recipe-for-amiracle/
2. Newman et al., Diabetes Res Clin Pract 2021;173:108685
3. Konish L. CNBC. https://www.cnbc. com/2022/08/09/inflation-reduction-actaims-to-trim-insulin-costs-for-medicareusers.html
4. https://www.mayoclinic.org/testsprocedures/fetal-surgery/about/ pac-20384571
5. https://link.springer.com/ book/10.1007/978-1-4613-2415-7
6. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC6655557/
congratulations to our DirEctor oF
tHE clinical iVF PrograM
DaViD H. baraD, MD., Msc.
for receiving the honor and recongnition as an exemplary reviewer for his outstanding service to the Fertility and Sterility journal from the American Society for Reproductive Medicine (ASRM).

Continued from page 5
6 | o C to B e R 2022 | The Voice
DHEA, Brain Development, and Evolution
 By Yu. Kizawa, Founding Editor of the VOICE
By Yu. Kizawa, Founding Editor of the VOICE
Now Director of Marketing at Ovaterra by Fertility Nutraceuticals and Ovaterra Institute
CONFLICT STATEMENT: Please be advised that the CHR has an ownership interest in Ovaterra by Fertility Nutraceuticals and receives from the company royalty payments for DHEA patents.
You may think of DHEA primarily as a supporter of your ovarian health, but it also potentially plays important roles in the baby’s brain development during pregnancy and cognitive health after birth. It may have even helped us become human on our evolutionary journey, according to an intriguing analysis by a biologist-anthropologist.1
It’s a bit more speculative than our usual explainers, but it’s a fun one. Let’s break it down.
DHEA May Support Fetal Brain Development
We know that the fetus produces a large amount of DHEA in the adrenal glands – enough to increase the amount of DHEA in the mom’s body during pregnancy, in fact. This extra DHEA is primarily taken up by the placenta and converted into an estrogen, which helps the uterus and placenta grow with the baby to maintain a healthy pregnancy. Doctors think, however, that a part of this additional DHEA is also used to support fetal brain development during pregnancy.2 The mechanism is not very well understood, but scientists are working for a better understanding. A review of animal studies, for example, suggested that DHEA may regulate the effects of another steroid hormone, glucocorticoids,
A CONTRIBUTION FROM THE OVATERRA INSTITUTE
The V oice | o C to B e R 2022 | 7
necessary for normal brain development.3
DHEA may also play an important role in brain development long after birth. DHEA, as a neuroactive neurosteroid, may modulate the baby’s early brain development.⁴ The increase in DHEA levels as our adrenal glands start producing it at around ages 5-11 has also been suggested as a mechanism to support cognitive and behavioral maturation before and during puberty.²
What Does This Have to do With Human Evolution?
So, what does DHEA’s role in the baby’s brain development have to do with human evolution? Biologist James Michael Howard posits that an increase in DHEA (and testosterone) levels in humans – especially in females – may have played a significant role in the survival and success of our species in a challenging climate.
In an article published in the journal Rivista di Biologia/ Biology Forum and reposted here, Howard explains:⁵
• Thermogenesis: DHEA increases how much heat our body can produce from the food we eat. At the end of the Cretaceous period, when an impact winter from an asteroid wiped out three-quarter of the species on the planet, early humans may have been able to survive partly because of the thermogenic benefit of increased DHEA levels, as they were able to stay warm from the limited amount of food available.
• Larger brains and stronger bones: DHEA (and testosterone) levels, especially in females, may

have led our species to have advantage over other hominid species. Higher DHEA levels gave rise to some of homo sapiens’ defining characteristics: Larger brains, stronger and larger bones and females closer to males in body size.
• Further frontal brain growth: As the climate warmed back up, more of the DHEA may have been diverted from gener ating heat to driving brain growth, especially in the frontal region – which further differentiated homo sapiens (that’s us) from other hominids and primates.
The Takeaway
While certainly speculative, Howard’s argument is intrigu ing, given what we know about DHEA’s role in the development of the physical brain, as well as its functions. It’s also a reminder that – despite the “male hormone” moniker –androgens like DHEA and testosterone play important roles in women’s health.
Too much androgen in women is of course a problem, but we shouldn’t automatically consider androgens as detri mental to women’s reproductive or prenatal health. They are necessary for many biological processes that support our lives, including reproduction.
For a more detailed history of how we came to be as a spe cies and what role testosterone and DHEA may have played in that evolution, read Howard’s full post.⁵
8 | o C to B e R 2022 | The Voice
“When I started this journey over 3 years ago, I knew I would have good days and bad days, but nothing can ease the difficult days more than a compassionate team, that’s CHR. They are group of phenomenal human beings, knowledgeable, professional, caring, honest, approachable, kind, and com passionate. Dr. Gleicher, Dr. Barad and Dr. Benor are skilled, experienced, and honest physicians that keep communica tion as one of the key elements in their relationship with the patient. Even when things did not go as expected, they made sure to comfort me and maintained a positive attitude without being unrealistic. The day I had my first Skype consulta tion with Dr. Gleicher, I knew they were the ones, and that they will give me my so desired baby.”

PATIENT TESTIMONIALS PATIENT TESTIMONIALS PATIENT TESTIMONIALS
PATIENT TESTIMONIALS
“Dear Dr. Norbert, We sincerely want to thank you for all the guidance and kind sup port you’ve given us. We’re so grateful to have you as our doctor and are so happy to have our treatment here at CHR.”
- Lia & Steven
“
“
- A Patient The V oice | o C to B e R 2022 | 7 9
EMBRYOLOGY LABORATORY SUPERVISOR FOR



The CHR is searching for a candidate for the newly created position of Embryology Laboratory Supervisor for Research. The CHR’s em bryology laboratory, under a single laboratory director, is in the process of being reorganized into three distinct areas with separate supervisory responsibilities: (i) clinical, (ii) administrative, and (III) research.
Supervisors in all three areas must hold PhD degrees (or equivalent) and be fully trained human embryologists with sufficient historical professional experience to hold a supervisory position.
While such human embryology experience is preferred for this new position as well, priority qualifications are a record of excellence in reproductive biology research, documented by publications in prestigious peer-reviewed journals and, in absence of human IVF experience, at least substantial animal IVF experience allowing for relatively quick in-house training in human IVF.

Besides a competitive salary and benefit package, the CHR also offers in this position a unique financial incentive-struc ture linked to the success of the center’s research activities, as demonstrated by publications in prestigious peer-reviewed journal. Moreover, this position will also be eligible for the opportunity to earn shared ownership in research-driven new start-up companies and the center itself.
If you feel that you qualify for this position, please submit your CV and a brief application letter to the CHR’s COO, Ms. J. Tapper, at jtapper@thechr.com. The position is available immediately. All submissions are considered confidential.
RESEARCH
hiRinG AT THE CHR Learn more by accessing our library of educational videos: https://www.centerforhumanreprod. com/contents/video-gallery Center for Human Reproduction 10 | o C to B e R 2022 | The Voice ADVERTISEMENT ADVERTISEMENT
INJECTING PLATELET-RICH PLASMA (PRP) Into the Ovaries: The Preliminary CHR Experience
Platelet-rich plasma (PRP) is a fraction of human blood which is enriched for the number of platelets it contains. Platelets are very important for the initiation of wound healing and secrete in the process several growth factors, among those 3 isomers of platelet-de rived growth factor, 2 among many known as transforming growth fac tors-beta , vascular endothelial growth factor and epithelial growth factor. In addition, PRP also contains cell adhe sion molecules for osteo-conduction and matrix for bone, connective tissue, and epithelial migration.1 When PRP purified from a patient’s own blood is injected back into a patient, her own healing system is, therefore, used to achieve whatever goal is clinically pursued.
PRP was first used in soft tissue healing but gained popularity and increasing scrutiny when used on some prominent athletes suffering from sport-injuries, mostly affecting joints. Treatments with PRP have in recent decades gained increasing popularity in various medical fields, from rheumatology to plastic surgery and has become a primary treatment for male-pattern hair loss. Despite increasing utilization in sports medi cine, orthopedics, and rheumatology, PRP has, however, remained a contro versial procedure. Only recently, for example, several frequently performed orthopedic utilizations were found to be clinically ineffective.
Greek investigators were the first to report on intraovarian PRP injections
in 2016, claiming restauration of ovarian function and menstruation in ³ allegedly perimenopausal and meno pausal women with a history of one year of amenorrhea.⁶ Published experi ence with intraovarian PRP treatments has unfortunately largely remained restricted to anecdotal experiences and, at best, to small case series. Yet, as with so many other “add-ons” to IVF in the last two decades, the use of PRP has proliferated among IVF centers with none reporting truly interpretable results. Nevertheless, the procedure is widely advertised by many IVF centers, often under the obviously misleading, though equally obviously “sexy” name “ovarian rejuvenation.”
That there exists no convincing evidence so-far that ovarian PRP

2
4,5
The V oice | o C to B e R 2022 | 11
a single bolus injection into the hilus of the ovary, as was practice at many IVF centers. Moreover, we secured new equipment that allowed the lab to quantitate platelet counts in each patient’s PRP sample before injection under ultrasound control in a reversed egg retrieval set-up, with fluid in jected into, rather than withdrawn from, the ovary. In other words, CHR, supported by the center’s Institutional Review Board (IRB), not only decided to offer PRP treatment to our pa tients, but to do so within the frame work of a registered prospective clin ical trial that within 2-3 years (based on power analysis) should permit us to learn whether PRP in these patients really made any clinical sense.

As this article is written, this so-called PRP-I trial at the CHR requires only one more patient with a diagnosis of POI/POF before closure of enroll ment and analysis of data. PRP-1 was, however, not meant to remain the sole PRP trial the CHR was to con duct because women with very poor ovarian reserve and/or early meno pause above age 40, who did not qualify for enrollment under PRP-1, because of all the advertisement of the procedure they saw mostly on the Internet, were complaining that the CHR was discriminating against them by not allowing them to participate in the trial. The CHR, therefore, a few months later, initiated and registered in parallel to PRP-I a second trial (PRP-II), serving women above age
treatment “rejuvenates” ovaries, is in very much detaile and objectively explained in a recent review in Human Reproduction by Atkinson et al.7 We recommend this articles strongly for readers who wish to fully understand the status of ovarian PRP treatments as of this moment and, in addition, also want to understand the potential physiological background for why the injection of PRP into ovaries may have a physiological logic.
Why and how the CHR got interested in PRP?
Observing the proliferation of PRP treatments in IVF centers around the world during 2018-2019 and, increas ingly frequently asked why the CHR did not offer “ovarian rejuvenation,” we decided to join the bandwag on, - though with one distinctive difference: we would offer PRP only to a clearly defined patient popula tion, made up of young women with primary ovarian insufficiency (POI), also called premature ovarian failure (POF) or premature menopause This meant that only women in early menopause (FSH above 40.0mIU/ mL) and under age 40 were eligi ble for PRP; and the treatments in informed consents were described as “experimental.”
We, moreover, chose an FDAapproved PRP kit and defined in advance an injection technique into the ovaries that required multiple subcapsular injections (where primor dial follicles are located), rather than
40 with very low ovarian reserve (2 or less oocytes in a prior IVF cycle at age above age 40 and even into early 50s) or with early menopause (above age 40 but below age 50).
Considering the CHR’s unusual patient population with very advanced female ages, unsurprisingly PRP-II attracted much larger patient numbers than PRP-I. Because the patient population in this trial, however, is much less ho mogenous, such a population requires larger patient numbers to achieve proper statistical power. This study, therefore, will, likely, take around another year before end of enrollment is reached, though CHR’s investigators recently published an interim anlysis.8 Though obviously limited in its ability to inform about PRP in general, the study, likely, already allows for the rather unsurprising conclusion that PRP is not a “wonder drug” for poor ovarian reserve. Equally unsurprising, these preliminary data also suggested that whatever benefits PRP may offer, will be primarily only visible in women up to age 42. Interestingly, we, how ever, since publication of this paper have witnessed in PRP-II an ongoing pregnancy in a woman in her mid-40s.
And then there is the most recent PRP trial (PRP-III) registered and initiated in order to answer a very important secondary question regarding PRP, the other two trials cannot answer. As we still do not know how PRP exactly works (assuming it does), there exist
"We, moreover, chose an FDA-approved PRP kit and defined in advance an injection technique into the ovaries that required multiple subcapsular injections (where primordial follicles are located), rather than a single bolus injection into the hilus of the ovary, as was practice at many IVF centers."
Continued from page 12
12 | o C to B e R 2022 | The Voice
two basic possibilities: The first is the assumption that PRP results in the re lease of biologically active factors from platelets and in some ways, by doing so, activates follicles into maturation which prior to PRP were no longer responsive. There, however, is also a second possible explanation for PRP treatment effects, namely that it is not the PRP that does the job by activating primordial follicles through the Hippo pathway but the mechanical fragmen tation of ovarian tissue by the needle that delivers the PRP to the ovary.9

To differentiate between these two possibilities, the PRP III trial injects, blinded to patient and physician, either PRP or another fraction of the patient’s own plasma. Assuming that PRP is not what causes positive effects on follicle growth in ovaries, and all of these effects are the consequence of tissue fragmentation by the nee dle, the expectation would be that both plasma fractions produce iden tical results. This study was started much later than PRP-I and PRP-II and recruitment is, unfortunately slow because many patients do not want to be randomized, even though the code is broken after cycle completion and women who received the placebo and did not conceive in the immediate next cycle automatically receive PRP. Our expectation, therefore, is that PRPIII will take ca. another two years to complete.
What is different about PRP at the CHR?
As in many other treatments, how the CHR administers PRP differs from most other centers. We already above noted that where, and in how many individual injections, we administer PRP to ovaries differs. In contrast to most other IVF centers, the CHR also does not just send the patient home with instructions to call if “something happens.” Instead, patients are closely followed after their PRP treatments, which means that for two weeks they
are scanned by ultrasound every 3-4 days, to immediately recognize any follicle growth. Since patients in all here described trials are women with exceedingly low ovarian reserve, every visualized follicle counts and is then immediately supported in its growth with low-dosage gonadotropin support. A small percentage of women do show such an immediate response (ca. 15% of cases). Women who do not demonstrate an immediate response within 2 weeks, ca. one month after
become convinced that some patients with very low ovarian reserve will, indeed, benefit from PRP. We, however, still do not know how to select them in advance, though it appears that, like with almost all infertility treatments, PRP works better in younger than older patients. We also are by now quite certain that peak effects last for ca. 3-4 months, at which time some patients may benefit from a repeat procedure.
Since ovarian aging is progressive and unstoppable, patients with low function al ovarian reserve at all ages, of course, have no time to waste. If you, therefore, want to be considered for one of CHR’s three PRP trials, the sooner you con tact us, the better. Please call our main telephone number at 1212 994 4400 and advise our staff that you are calling as a potential PRP-study-participant. You then will be eligible for an expedited and free first consultation with our center’s Director of the Clinical IVF Program, David H. Barad, MD, MS, who is heading up all three PRP studies at the CHR. (For related information, please also look in this newsletter’s Literature Review at the article discussed under heading “A, for several reasons, interesting ”hypoth esis” article relating to intra-ovarian PRP injections.”)
REFERENCES
the PRP procedure automatically are entered into a gonadotropin stim ulation cycle and, if responding, go through a routine IVF cycle. Once a patient goes at the CHR through PRP, she, therefore, is under constant supervision for at least 3-4 months, whether she lives in the larger New York area and is followed at the center or whether arrangements must be made with a local center under CHR’s directions.
Conclusions and recommendations
Still recruiting for all three PRP trials, we from preliminary observations have
1. Marx RE. J Oral Maxillofac Surg 2004;62:489-496
2. https://www.hss.edu/condition-list_prp-injec tions.asp
3. https://www.hopkinsmedicine.org/ health/treatment-tests-and-therapies/ plateletrich-plasma-prp-treatment
4. Hurley et al., Am J Sports Med.2019;47(3):753-761
5. Kearney et al., JAMA 2021;326(2):137-144
6. Pantos et al., Cell Transplant 2016;v28(9-10); PMC6767896
7. Atkinson et al., Hum Reprod 2021;36(7): 1737-1750
8. Barad et al., Hum Reprd Open 2022(3):hoac027
9. De Roo C, et al., Hum Reprod Open 202 (4);hoaa048
The V oice | o C to B e R 2022 | 13
Image 1
For years now, scientists have been busy at work attempting to identify the working parts of cells. Trained as a cell biolo gist back in the 1970s, Dr. Albertini has been enamored with the power of microscopes to begin to tell us what cells are made of and how their various components are orchestrated to accomplish the remarkable things that cells, tissues and organs do. The cell pictured (image 1) is one of those found inside the ovarian follicle-a granulosa cell and one the CHR has been focused on for years given its importance in the ovaries abil ity to make and secrete steroid hormones like estrogen. The im age itself has been artistically rendered using digital image processing to accentuate different parts of the cell. The orange oval in the center is the cell nucleus.And the blue and red stripes represent a special organelle called microtubules which serve as railroad tracks for moving around chromosomes when a cell divides; notably this cell was caught at a time when it was not dividing but this process of cell division is one that we at the CHR continue to take aim at given the many divisions an embryo must undergo on its way to becoming a blastocyst.
ALBERTINI’S
Image
Speaking of blastocyst, here is an im age (image 2) of a human blastocyst as it would appear some 5 days after fer tilization by IVF or ICSI. In blue, all of the nuclei are evident for each of the cells which amount to some 100-120 at the blastocyst. The image was tak en at the CHR as part of our ongoing investigation into genetic integrity of embryos made by human IVF, a high ly controversial matter given all of the press coverage over the past sev eral years concerning whether or not tests like PGT-A can truly be relied upon as a measure of genetic integrity. To gain more insights into this prob lem, we at the CHR have teamed up with the Brivanlou laboratory at Rockefeller University to take an even closer look at the genetics of human embryos, as shown in the next image.

Using a specialized imaging technique known as confocal microscopy, the CHRRockefeller collaboration began to examine human blastocysts such as this in which the embryo is labeled with specific anti bodies to a portion of each chromosome in the human genome. In green with yel low blobs on it is the zona pellucida that is known to protect the early embryo from its environment(image 3). Within the zona, as in the earlier image, the blue denotes each of the nuclei in the balstocyst and note that each nucleus has a series of fine spots or foci that appear pink. These foci represent centromeres, specific regions on each of the 46 chromosomes and by analyses like this, our work is continuing to de cipher exactly where and when the chromo somes get positioned during development.
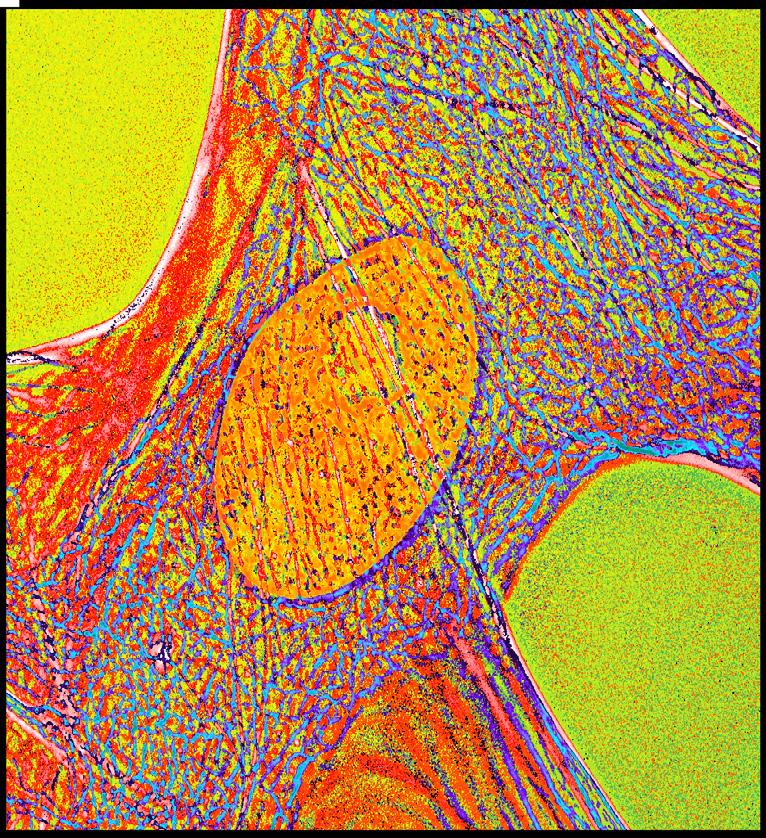
Image 3

DR.
Photo Gallery
2
14 | o C to B e R 2022 | The Voice
A OF

PIECE
MY MIND
By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist
 The CHR, New York, N.Y.
The CHR, New York, N.Y.
Those not aware of what in cognitive psychology is called the “illusory truth effect”, have definitely gone through life somewhat light-hearted. Joseph Goebbels, Nazi Germany’s infamous propaganda minister, used this phe nomenon as the “Big Lie” long before cognitive psychologists in 1977 first time proposed the term,1 when openly acknowledging that, “ if you tell a lie big enough and keep repeating it, people will eventually come to believe it.”2 Politicians have already made use of this concept even long before Goebbels and, certainly, increasingly consciously, after him (see the current Russian Federation in the Ukraine war), and so has the advertising industry, social and general media and, yes, medicine, as probably best demonstrated by the excesses of the Covid-19 pan demic. And even if “lie” in medical applications may often be too strong a word, the same applies to the repetition of “falsehoods;” and those, of course, exist in medicine in abundance.

Continued on page 16
Not only in politics do repetitions make falsehoods believable and lead to “Group-Think”
The V oice | o C to B e R 2022 | 15
A quite recent study resurfaced the subject by demonstrating that audience-belief in all statements is increased by repetition, occurs across all levels of plausibility, and strengthens with increasing ambiguity of an item,3 - a point I will return to. Consequently, even highly implausible statements will become more plausible through repetition. This, of course, should not surprise since we all know that brainwashing and indoctrination work. and medical education as well as clinical practice, to this day, to significant degrees is characterized by both. Briefly addressed last month in the VOICE, the repetitiveness of the process then, ultimately, contributes to the highly prevalent “group-think” in medicine.

Whether in medical school or postgraduate education, our current medical education system still uniformly indoctrinates us to believe that the “truth of the moment” represents “absolute truth,” when true education (in place of indoctrination) would instead emphasize the constantly required changes in medical knowledge that are necessary for advancements. When this column in last month’s issue of the VOICE addressed medical guidelines, we therefore made the point, unless constantly updated, how irrational static guidelines are.

Once an adequate knowledge plateau has been reached, to have static opinions may be quite comfortable, while being open to change means steady work in developing new evidence from the literature. Yes, we all under most state licensing laws accumulate continuous medical education (CME) credits; but who really does this with a primary purpose of developing new knowledge? In most instances we pursue credits to satisfy licensing or privilege requirements, and we do CME by just regurgitates knowledge (i.e., repeating the old), further hardening our already fixed opinions and thereby, indeed, feeding into “group-think.”
While the number of medical journals, and with it the number of published articles, has been multiplying exponentially over recent decades, one sometimes wonders who is reading all of these papers? As attention spans are getting shorter and shorter, even those who still read medical journals are usually too busy to read beyond abstracts. Medical journals have recognized this trend, and even some of the most prestigious journals, therefore, now offer further “shortcuts” from abstracts.
But it is not all the physicians’ fault: Now in the U.S in a rapidly expanding majority no longer independently self-employed,4 physicians- are increasingly judged not by clinical and intellectual performance but by how well they perform as employees of corporate structures in filling beds and generating practice revenue. With financial incentives appropriately structured by Wall Street and/or hospital chains with seemingly insatiable appetite to control certain medical specialties, including infertility medicine,5 economics consideration play an ever-increasing role in how medicine is practiced. Within an evolving new health care system that eliminates the economic independence of physicians in private practice, “group-think” will inevitably grow because uniform thinking in practice is very much in the interests of the rapidly expanding corporate entities that now employ most physicians.
Though in the U.S. physicians supposedly work in a “capitalistic” (i.e., a privately owned) health care system, the ultimate product is now mostly dictated by financial corporate interests of private insurance companies, hospital networks and/or practice-aggregators financed by Wall Street. Like in government-driven socialist medical systems
Continued from page 15 16 | o C to B e R 2022 | The Voice
this, ultimately switches the independent decision-making processes in clinical medicine from the patient-physician relationship to bureaucrats (usually not licensed to practice medicine). They then impose clinical practice mandates either based on “confidential and/or proprietary” criteria and/or based on alleged “expert-opinions,” which, of course, are mendable and freely available in the marketplace, often knowingly representing falsehoods (as noted on the June issue of the VOICE) or simply being the consequence of within this context warmly welcomed (and incorrect) “group-think.”

The capitalistic medical system in this way mirrors socialistic medicine, ultimately leading to the same excesses and disregard for best possible patient care, except at even higher cost; because what is rewarded in fee-for-service medicine (as most of infertility services are) is “doing more,” and not, as one would hope, “to do better.” This, of course, results in more revenue and better profitability for corporate ownership but, if conflicts are to be avoided with corporate management, incentives for physicians are aligned against independent thinking, creativity, and progressive change, all essential components in achieving improvements in treatment outcomes.
All of this, of course, also applies to infertility medicine. There are several reasons why IVF outcomes all over the world have dramatically declined since 2010 6,7 (a subject hardly ever discussed in the literature). Ultimately the most important one, likely, is the changes in the economic model the infertility field has been undergoing in recent years.5,8,9 Unsurprisingly, not the least through constant repetition of falsehoods, it has driven the specialty to an unprecedented level of misdirection and “group-think” in the over 40 years of modern infertility practice.
Encountering colleagues with absolute certainty in their professional opinions, therefore, appears on the rise. I must at times acknowledge a whiff of jealousy because I always experience a little bit of gnawing doubt in the back of my mind after analyzing and interpreting the results of an experiment, reviewing the methodology of a newly published study, or simply listening to a great talk. Life must be so much easier if one’s own absolute certainties always suffice, and there is only one absolute overruling truth, almost a religiosity of thought.
Medicine, indeed, has its own religiosities, ideologies and, of course, conflicts of interest. And then there are the myopias of “experts,” exclusively directed at the narrowest field of alleged expertise, and therefore, often totally blinded to co-variables from outside these narrow realities, almost a century ago well described in the classical text of Harold J. Lasky,10 with no better contemporary example than America’s own Anthony Fauci, MD. “Experts” like him
who cannot accept that there can be a truth beyond their own believe systems, unfortunately, to these days populate medicine to astonishing numbers.
Obviously devoid of any self-doubt, he, indeed, equated disagreements with his opinions with attacks on all of science,11 publicly apponting himself as the (only) true voice of “science” (in grandiosity truly a Napoleonic designation). “Napoleons,” of course, are not lacking in medicine. Repeating their rigid believes over and over again, they then become a driving force in the development of “group-think” and intolerance toward dissenting opinions.
Examples in reproductive medicine, of course, abound, from the promotion of elective single embryo transfers (also addressed elsewhere in this issue regarding the recent publication by Katler et al. on the subject in the AJOG 12), to almost universal routine utilization of extended embryo culture to blastocyst stage, even though in unselected patient populations never shown to improve outcomes and, a repeated subject in the VOICE, the rapid expansion of preimplantation genetic testing for aneuploidy (PGT-A) by now affecting approximately 50% of U.S. IVF cycles; and, not to forget, of course, also the increasing utilization of many other so-called “add-ons” to IVF.
Which closes the circle and returns us to the beginning of this column, where we noted that repetition of falsehoods becomes especially believable when it addresses issues of ambiguity. Suffice to say that, of above noted issues, all, of course, fully qualify as controversial and ambiguous. As recently pointed out in reference to PGT-A, a fortunately inevitable occurrence in science is that, sooner or later, “in the end truth always wins.”13 For many patients, considering current circumstances in reproductive medicine, the wait for that
happen may, however,
REFERENCES
to
take too long!
1. Hasher et al., J Verb Learn Verb Behave 1977;16:107-112 2. https://www.jewishvirtuallibrary.org/ joseph-goebbels-on-the-quot-big-lie-quot 3. Fazio et al., Psycho Bull Rev 2019;26:1705-1710 4. https://www.ama-assn.org/press-center/press-releases/ ama-analysis-shows-most-physicians-work-outside-private-practice 5. Patrizio et al., J Assist Reprod Genet 2022;39(2):409-416; Correction: idem:315 6. Gleicher et al., Hum Reprod Open 2019(3):hoz017 7. Gleicher et al., Reprod Biol Endocrinol 2021;19(1):110 8. Mochizuki K, Gleicher N., J Assist Reprod Genet 2020;37(3):677-687 9. Von Schondorf-Gleicher et al., J Assist Reprod Genet 2022;39(3):591-604 10. https://digital.library.lse.ac.uk/objects/lse:wal303heb 11. https://thehill.com/policy/healthcare/557602-fauci-attacks-on-meare-really-also-attacks-on-science/ 12. Katler et al., Am J Obstet Gynecol 2022;227(2):129-135) 13. Barad et al., Hum Reprod 2022;deac 151. Doi: 10.1093/hum rep/ deac151. Online ahead of print Continued from page 16 The V oice | o C to B e R 2022 | 17
PATIENT TESTIMONIALS
PATIENT TESTIMONIALS PATIENT TESTIMONIALS PATIENT TESTIMONIALS
“

“Dear Dr. Norbert, We continue to feel cared by everyone and that really made our journey here feel more at ease. Happy to see you again. Take good care and we hope to see you again next time.”
- Lia and Steven
“
“Dr Gleicher was wonderful. He was very generous and took the time to answer all my questions and explain different processes.” - A Patient
18 | o C to B e R 2022 | The Voice
the chr in the media
TIME MAGAZINE IN PRINT AND ON VIDEO
Under the title “How Researchers Are Working to make IV More Effective”, TIME magazine featured in an article and accompanying video CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, addressing the subject of preimplantation genetic testing for aneuploidy (PGT-A)¹ [https://time.com/6211312/ivf-more-ef fective/]. Article and video also featured a California patient who had been refused transfer of her allegedly “abnormal” embryos but, after consulting with the CHR, was able to convince her local reproductive endocri nologist to transfer against his medical advice one of the abnormal embryos; and, low and behold, the patient conceived. In the video, the patient in moving terms described her reaction and, even more interestingly, the astonishment of her physician, demonstrating well the depth of disinformation about PGT-A the IVF physician community is unfortunately exposed to. The CHR has, after all, been transferring selected so-called “abnormal” embryos already since 2014 and has extensively reported excellent outcomes, as have others.
The level of disinformation is also further demonstrated by the representations of Zeev Williams, MD, head of Reproductive Endocrinology and Infertility at Columbia University, who in article and video was featured as representative of the pro-PGT-A community, and who in favor of PGT-A could only present the (incorrect) arguments that PGT-A reduces miscarriages and saves patients from disappointments of not conceiving and/ or miscarrying. Both of these representations are blatantly incorrect since PGT-A has now conclusively been demonstrated not to improve pregnancy and live birth rates in general populations,² and was in a just pub lished Canadian study again demonstrated to adversely affect IVF outcomes in poor prognosis patients with small egg and embryo numbers.³ That PGT-A reduces miscarriages has by now also been disclosed as myth.²
The time appears right for colleagues to, finally, stop using the failed argument that PGT-A is meant to protect patients from the disappointment of failing to conceive and/or experiencing increased mental trauma from miscarriages because, if one carried this argument to its logical conclusion, humanity would have in the future to stop natural conception and convert to IVF + PGT-A as the standard way to conceive, -obviously a highly unrealistic concept that, in addition, would, however, make absolutely no difference in outcomes, except to reduce birth opportunities in older women and other poorer prognosis patients.
Therefore, more than ever before, PGT-A has remained an expensive “add-on” to IVF in search of a clinical uti lization. That a brilliant physician-scientist, like Dr. Williams, has remained to such an extreme degree support ive of PGT-A is, therefore, somewhat surprising, though a side-comment in the article may offer some insights into motivations. Dr. Williams and colleagues recently announced by press release that his laboratory had developed a speeded-up PGT-A assay that offers results within hours rather than the currently customary up to two weeks.4 If properly validated (so-far no peer-reviewed publication has appeared), such a test on a technical level, would, indeed, mean significant progress because it would allow avoidance of embryo cryopreservation and fresh embryo transfers. A quicker test, however, does not change the clearly established fact by now that a single trophectoderm biopsy can never correctly represent a complete embryo5,6 and, therefore, even a quicker test, will not improve the utility of PGT-A. A patent for such a test, however, of course, offers significant finan cial incentives to its inventors, - though that is only the case if PGT-A testing in association with IVF continues at its current absurd rate.

REFERENCES
1. https://time.com/6211312/ivf-more-effective/
2. Yan et al. N Engl J Med 2021;385:2047-2058
3. Mahesan et al., J Assist Reprod Genet 2022;doi: 101007/s10815-022-02588-9. Online ahead of print.
4. https://www.nih.gov/news-events/news-releases/nih-funded-researchers-develop-same-day-test-detect-abnormal-fetal-chro mosomes
5. Mastenbroek et al. N Engl J Med 2021385:731-742
6. Gleicher et al. Nat Med 2022;28(3):442-444
Continued on page 20 CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR
The V oice | o C to B e R 2022 | 19
CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR CHR
EPSILOON MAGAZINE

(FRANCE)
This time in a French science magazine1 . CHR’S Medical Director and Chief Scientist, Norbert Gleicher, MD, was again quoted on genetic embryo selection through polygenic risk scoring. As on multiple occasions in the pages of the VOICE and in the literature,2 Dr. Gleicher explained his strong opposition to the current clinical utilization of risk scoring of human blastocyst-stage embryos, which is already offered by some IVF centers and genetics laboratories in the U.S.

Among many arguments against current utilization of polygenic risk scoring, the article quotes his con cern, in the literature also expressed by many other experts, about the possibility that our current genome is the end-product of thousands of years of evolution which as primary goal always maintained the survival of the species. One, therefore, can ague that current polygenetic pre dispositions toward certain diseases would not have survived over all of this time if they, at the same time, did not also offer a contribution to survival of the species. Eliminating these polygenic risk combinations from the population through poly genic risk scoring, therefore, may ac tivate detrimental risk in other areas relevant to survival of humanity.
CHR
This, of course, is only one among may arguments against clinical utilization of polygenic risk scoring in human IVF. This newsletter will in a future issue address this issue in more detail (see also below in the literature review comments on the effects of modern infertility practice on evolution of mankind).
REFERENCES
1. Mérat M-C. epsilon.com; Septembre 2022;p34-37
Gleicher et al. Nat Med 2022;28(3):442-444
hiRinG
AT THE CHR
BOARD-CERTIFIED RE-I AT ASSOC. PROF/PROFESSOR LEVEL
The CHR is looking for another senior board-certified RE-I (or international equivalent) to join our growing national and international practice. Though private, our center is organized along academic principles in that physi cians are expected to excel not only as clinicians but also as researchers. The center’s uniquely structured compensation package, therefore, incentiv izes both activities. We are looking for an individual with documented ex cellence in both areas, commensurate with associate professor/professor level. Besides a competitive salary, incentive bonus structure, and excellent benefit package, the CHR also offers partnership along either a 3-year or 5-year equity track and, ultimately, part or complete ownership, as the current leadership is expected to retire within that time-period.
If you feel qualified for the position and share our philosophy of being a physician-scientist, please let us know by submitting your C.V. and a brief letter of interest to our COO, Ms. J. Tapper, at jtapper@thechr.com The position can be filled immediately. All sub missions are considered confidential.

20 | s eptem B e R 2022 | The Voice Continued from page 19
2.
CHR CHR
CHR CHR CHR CHR CHR Continued from page 19 20 | o C to B e R 2022 | The Voice
ADVERTISEMENT
The CHR’s interpretation of RECENT LITERATURE, relevant to REPRODUCTIVE MEDICINE
Mostly placed into a clinical context, we in this section of the newsletter offer a survey of articles in the English liter ature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. These articles, however, nevertheless often point out where clinical practice will likely go and, therefore, serve an im portant translational purpose. Translational research has been the CHR’s principal research goal since its founding in 1981, has produced a significant number of U.S. patents over the years, and has propelled the CHR into its current position as a worldwide center of last resort for infertile patients who have failed treatments elsewhere.
Pregnancy
Does the status of the maternal immune system predispose offspring to autism?
A just published nationwide cohort study from Sweden together with a polygenic risk score analysis in a general population-based UK cohort, and a Mendelian randomization analyses in one of the most prestigious medical journals in the world,1 not only suggested that maternal inflammatory bowel disease (IBD) predisposes offspring to autism, but invigorates the for many years brewing controversy whether a hyperactive maternal immune system, and especially autoimmunity and/or inflammation, in general predis pose to an increased mental health risks in offspring.2
The Swedish population demonstrated evidence suggestive of associations between IBD, Crohn’s disease (CD), as well as ulcerative colitis (UC) and autism in offspring. A sub-co hort of UK mothers suggested associations between maternal genetic liability for CD as well as UC with autistic traits in children, further confirmed by mendelian randomization analyses. This paper, thus, added evidence to earlier reports which demonstrated that a diagnosis of IBD may be associ ated with autism in offspring but, in addition, demonstrated that a maternal genetic liability to IBD was associated with the autism risk. Maternal immunological (and potentially other) characteristics, therefore, very obviously can affect the uterine environment in which the fetus develops, as an accompanying
The V oice | o C to B e R 2022 | 21
“research briefing of two of the paper’s authors pointed out.3
That maternal autoimmunity may predispose to autism in off spring has been swirling around the literature for a good number of years. Such associations have been reported with systemic lupus erythematosus (SLE)4 and with antibodies in general,5 but likely never before has a study involved so many study subjects and, therefore, has had the statistical power of here presented manuscript in Nature Medicine.
The study, therefore, raises serious concerns as well as questions but may also offer some explanations: Starting with the latter, the world has been wondering about the significant and unexplained increase in the prevalence of autism in recent decades, which, as now must be noted, runs in parallel with increases in autoimmune disease prevalence, from rheumatoid arthritis6 to autoimmune liver diseases,7 and likely many other autoimmune diseases. Could those two parallel developments be causally related?
Additional questions that arise, of course, center around prevention, - which means that the potential pathophysiology of how a hyperactive maternal immune system potentially affects the fetal brain must be better understood. For the longest time, and until only several years ago, the brain was believed to lack an active immune system. Today we know that the brain, indeed, houses a very active immune system and produces highly sig nificant immune responses. Multiple sclerosis, for example, is a typical autoimmune disease in which lymphocytes are activated against myelin autoantigens in the central nervous system,8 while in Alzheimer’s disease astrocytic and microglial cells modulate neuroinflmammation.9
Similar to maternal IgG SSA/SSB antibodies entering the fetal compartment via the placental barrier in scleroderma and/or SLE, thereby causing congenital fetal heart blocks, it appears that SLE antibodies can also cross the blood-brain barrier (BBB) and cause cognitive impairment.10 And if antibodies can do that in an adult blood-brain barrier, why should they not be able to do it in fe tuses during nine months of pregnancy? On a sidenote, increasingly unknown side effects of autoimmune diseases are being discovered beyond just effects on the brain: A recent Lancet study, for example, demonstrated, especially in younger
patients, surprisingly clear effects of various autoimmune diseases on cardiovascular risks.11
REFERENCES
1. Sadik et al., Nat Med 2022;28:1406-1411
2. He et al., JAMA Netw Open 2022;5(4):e227503
3. Dardani C, Rai D. Nat Med 2022;28:1353-1354
4. Vinet et al., Arthritis Rheumatol 2015;67(12)3201-3208
5. Libbet JE, Fujinami RS. Autism res 2010;3(4):147-152
6. Finckh et al 2022; Nat Rev Rheumatol 2022;doi: 10. 1038/s41584/s41584022-00827-y. Online ahead of print.
7. Trivedi PJ, Hirschfield GM. Gut 2021;70(10):1989-2003
8. Karami et al., IET Syst Biol 2022;doi: 10. 1049/syb2.12048. Online ahead of print.
9. Singh D. J Neuroinflammation 2022;19(1):206
10. Hanly et al. Lupus Sci Med 2022; 9(1):e000668
11. Conrad et al. Lancet 2022;400:733-743
More questions about the mode of delivery and subsequent immunological diseases in offspring
There has been a growing pool of literature that suggests that, because of different bacterial exposures, Cesarean section (CS) deliveries increase the risk of newborns to develop later in life immune system-related disorders. Now Swedish investigators in a way confirmed this hypothesis by demonstrating in a population of 1,102,468 individuals (88.4% vaginal- and 11.6% CS deliver ies) that CSs after appropriate adjustments, indeed, were asso ciated with increased risk of developing Crohn’s disease (other GI conditions, including ulcerative colitis, were not enriched).1 Considering steadily rising CS rates in developed counties, this is, of course, of some concern and raises several question: (i) Whether the difference between the maternal vaginal and the abdominal microbiome is really the primary culprit? Or (ii) whether the maternal microbiome is only the inducing conduit in a genetically predisposed offspring; and/or (iii) whether such increased risk for offspring applies to all, selected, or only very few autoimmune/inflammatory diseases in mothers? So many questions, and so few answers!
REFERENCES
1. Hellsing et al., Acta Obstet Gynecol 2022;00:1-7; DOI: 10.1111/aogs.14427
Animal models to understand preeclampsia better
Preeclampsia is still a major reason for maternal as well a fetal morbidity and even mor tality. Though certain aspects of pathophysiology are better understood, the condition has largely remained a mystery for our obstetrical colleagues.
Continued on page 23 22 | o C to B e R 2022 | The Voice
From an immunological viewpoint, the condition increasingly looks like the consequence of premature abatement of immunological tolerance of the maternal immune system toward the fetal-placental semi-allograft of pregnancy. Somewhat surprisingly though, this has not been a research pathway that is very actively pursued by research laboratories.
A recent “mini-review” in Endocrinology demonstrates this fact once more, when, in principle, only concentrating on animal models of preeclampsia in offering an update on mechanistic insights and promising therapeutics. In that sense the paper will offer new infor mation to most readers. But if the purpose was to offer a better un derstanding of what really causes preeclampsia, we are left waiting.
REFERENCES
1. Taylor EB, George EM. Endocrinology 2022;163:1-12
More on vaginal microbiotas in pregnancy and their effects on mothers and their offspring Investigating a large cohort of paired maternal-infant vaginal and stool specimens, the investigators here identified shared bacteria and microbial gene functions that related to atopy in newborns. Concomitantly, they were able to demonstrate that vertically transmitted Lactobacilli were able to prevent the allergy.1 They thus demonstrate that: (i) Prenatal vaginal microbiotas related to mater nal health and exposures; (ii) Variability of heritable bacteria and of functions related to markers of allergy in newborn; (iii) Fetal Lactobacillus jensenii inhibited primary human-antigen-presenting cell activation; (iv) Vertically transmitted bacteria suppressed airway allergic responses in vivo. In short, the make-up of vaginal microbi otas matters!
REFERENCES
1. McCauley et al., Cell Rep Med 2022;3:100713
Clinical infertility
What modern infertility treatments do to human evolution: Is it time to start worrying? Progress in modern medicine over the last 50 years has been nothing but miraculous and is only further accelerating. But, as Norbert Gleicher, MD, the CHR’s Medical Director and Chief Scientist else where in this newsletter already noted (see “A Piece of My Mind”), practically every treatment in medicine not only offers benefits, but also may have side-effects, - many unforeseen and often undetected, unless studied prospectively over often prolonged time periods.
Nobody has, however, ever – to the best of our knowledge - seriously looked at the question what will be the consequences for the future of mankind from the rapid progress mankind has been making in curing and/or even eliminating certain diseases? Mo medical prac tice framework, however, requires such an assessment more urgently than reproductive medicine and infertility because infertility which, in an evolutionary sense, of course, are crucial in either blocking or allowing inheritance.
Consider for example diabetes mellitus (DM), a so-called poly genic disease, which means that the predisposition to the disease is not based on only a single gene (as in so-called single-gene disease) but depends on the coordinated activities of several different genes. As already noted in this month’s lead article in the VOICE, before insulin was discovered, women with DM almost never conceived and, if they did, they almost always miscarried. In an evolutionary sense, nature, thus, prevented birth of chil dren from diabetic parents, thereby preventing the transmission polygenic predispositions toward diabetes into the next genera tion. Improving abilities to treat diabetes, however, did exactly the opposite!
Though current fertility and live birth rates of diabetic women are basically equal to those of non-diabetics - obviously a colossal accomplishment for mankind – it appears time to start quanti tating the price mankind is paying for this accomplishment and, even more importantly, to start pondering how to minimize those long-term consequences, as all of those diabetic women are now transmitting their polygenic diabetic predispositions into the next generation, and so will their offspring, etc. Unsurprisingly, the end-result is all over the world an explosion of DM and of many other polygenic diseases which initially were associated with either female or male infertility that blocked polygenic inheritance into future generations. A similar example to DM are many mater nal autoimmune diseases universally associated with high miscar riage risk if untreated or male factor infertility which, if overcome by intracytoplasmic sperm injection (ICSI), leads to an increase in urogenital congenital abnormalities in male offspring.
Now two Norwegian scientists offered for the first time a fascinat ing review of the potential impact of IVF on human evolution.1 Since space is limited, here are only a few key points: The authors point out that evolution, of course, is relevant to all aspects of life. Within that context, IVF changes selection pressures on gametes, on embryos as well as on fetuses. In the process selection process es on adults also change. The authors’ principal conclusions are that, since IVF bypasses some resource-demanding processes that reproduction by coitus mandates, IVF redirects resources away from natural reproduction. Consequently, IVF renders humans increasingly dependent and adapted to technological means of reproduction.
To demonstrate the potential wisdom of these conclusions we refer interested readers to the recently published TIME magazine article, discussed in this newsletter under the heading “CHR in the media.” The increasing utilization of PGT-A and the recent introduction of polygenic risk scoring of embryos quite obviously are moving IVF unquestionably toward increasing dependence on technology-driven conception. Paradoxically, Martin Varsavsky, one of the pioneering investors leading to the explosive industrial ization of IVF over the last decades, may therefore have been right after all, when already in 2016 predicting that companies would store gametes of young individuals at peak fertility, produce em bryos when the time has come, test them genetically and transfer the allegedly “best” in an IVF cycle.2
Continued from page 22 The V oice | o C to B e R 2022 | 23
Whether this is really the reproductive future that mankind is looking toward is, hopefully, still open for discussion. It seems, however, high time to initiate the discussion.
REFERENCES
1. Hanevik HI, Hessen DO. Hum Reprod Update 2022;28(4):457-479
2. Helft M. Forbes 2016. https://www.forbes.com/sites/miguelhelft/2016/10/17/ prelude-fertility-200-million-startup-stop-biological-clock/?sh=11cfc2837260
“Fertility fraud”
Did you even know that a legal term like “fertility fraud” exists? Do you know what it means? Did you know that 17 states already have passed legislation or at least have pending laws, defining it as “delib erately misrepresenting the source of sperm, eggs, or embryos used to treat infertility,” as a recent timely commentary in The New England Journal of Medicine pointed out, while also making the point that this development represents “a turning point for informed consent” when it comes to assisted reproduction.1
Importantly, the doctrine behind this legislation demands that plain tiffs not only are able to proof that a meaningful medical risk was not disclosed, but also that this failure of disclosure led to significant damage for the plaintiff. Originally spawned by the large number of gynecologists who through modern-day genetic ancestry testing were discovered to have used their own semen to inseminate pa tients, while, deceptively, advising them that donor semen had been used, these legislative initiatives will, of course, as always have much wider implications. Our recommendation to fertility centers in the U.S. is, therefore, to review their informed consents and adjust them accordingly.
rates, without considerations given to health outcomes of women and babies and cost of treatments. They, therefore, propose that a successful IVF cycle should be redefined as “birth of a healthy singleton baby at term, without compromising the health and safety of the woman and baby, and achieved at lowest possible cost.”1
One, of course, cannot argue with the basic premise that there must be a way to define a “successful” IVF cycle, and that such a definition should not only rely on pregnancy and live birth rates. But it is remarkable that the two authors completely overlooked the likely most important determinant of what represents “successful” IVF cycle outcomes, - and that, of course, is represented by a patient’s wishes and desires, - and certainly not by those of her physician. A strong proponent of mild stimulations and elective single embryo transfers (eSET) for decades, we have never seen Nargund expressing a regard for patients’ wishes and desires in any publications; yet strong physician opinions are always prominent. Patients, after all, have a right to self-de termination even if their wishes do not correspond to those of their medical providers. A good example is, indeed, their right to be desirous of twin pregnancies which, from the two above cited authors’ writing, is obviously not something they favor.
Treatments without ever being able to demonstrate objective and rational reasoning for them beyond, as again in this paper, incorrectly claiming a mantle of “holistic” medicine and of cost-effectiveness is neither clinically appropriate nor ethical. Cost-effectiveness is, moreover, not only determined by IVF cycle costs alone, but by IVF cycle costs in combination with pregnancy and live birth rates: A cycle cost of $5,000 may be a bargain with conception and healthy delivery but the same cycle cost for a failed cycle is, of course, costly. The argument that mild-stimulation cycles are necessarily less expensive is, therefore, misleading because these cycles also produce signifi cantly lower pregnancy and live birth rates.
What are desirable performance indicators for IVF?
In a “countercurrent” article in Reproductive Biomedicine Online Geeta Nargund and Adrija Kumar Datta offer the argument that IVF success currently is measured only by pregnancy and live birth
Neither Nargund nor any of the other proponents of mild stimulation and or eSET have ever demonstrated objective out come and or cost advantages from the alternative treatments to routine IVF cycles they have proposed. To the contrary, every study ever performed using mild stimulation and/or eSET demonstrated lower pregnancy and live birth rates than regular stimulations or 2-embryo transfers. Preaching against “add-ons,” moreover appears cynical, considering that mild stimulations and eSETS are among the most frequently utilized “add-ons” to IVF practice after blastocyst-stage culture and PGT-A.
We, therefore, fully agree with the title of here discussed manuscript, “Maximizing live birth rates cannot be the only key performance indicator of IVF.” But unless the authors,
REFERENCES 1. Fox D. N Engl J Med 2022;387(9):770-772
Continued on page 25 24 | o C to B e R 2022 | The Voice
first and foremost, accept that well-educated and informed patients have a right to self-determination, all of their arguments fall flat.
REFERENCES
1. Nargund G, Datta AK. Reprod Biomed Online 202244(4):587-589
Uterus transplant a reality, - but logical? In a news article in JAMA, the medical writer Anita Slomski charac terizes uterine transplants as a “reality” in medical practice and as “a viable way to help some patients with absolute uterine-factor infer tility (AUFI), a condition that allegedly affects 1 in 500 women.”1 She reached these conclusions based on a cohort of 33 women reported in JAMA Surgery2 that underwent this surgery at three major U.S. in stitutions over a 5-year period. This comes to 11 cases per institution in 5 years, which means 2.2 cases on average per year.
Does this represent a surgical reality? We don’t think so. Does such a low surgical volume allow for skill maintenance and appropriate training for additional surgeons? We again don’t think so, and this raises in our opinion serious ethical concerns.
But the numbers are even worse than that because at 1-year post-sur gery only 23/33 (69.7%) had a viable graft and only 19/33 (57.6%) had at least one live birth. But even these numbers don’t tell the complete story because we are here not only talking about 33 uterine transplant surgeries: 14 transplanted uteri came from living donors who, of course, had to undergo a hysterectomy with more than usual extensive sidewall dissection to obtain long enough vessels for anas tomosis. In addition, by time of the report 19/23 of the uterine grafts were already removed by hysterectomy, with the remaining expected to be removed. In short, the number major surgeries performed in this patient cohort was 33+14+33 = 80.
We don’t even want to get into the cumulative costs per delivered newborn and so-far incompletely available neonatal outcomes. The fact that median gestational age at birth was 36 weeks and median weight was 2860 gram (the 58th percentile at that gestational age) appears reassuring; but can these results really be considered “a clinical reality?” We would more tend to describe them as a “clinical phanta sy” of ethically very questionable pro portions. Though an
accompanying commentary noted that “concerns remain,”3 it in our opinion also overemphasized the accomplishment.
We have on prior occasions in these pages noted what a remarkable research effort our Swedish colleagues undertook over many years when pioneering uterine transplants, first in animal models and later in the human experience.4 But that does not mean that, just because we can do it, whatever the reasons we also should, even if uterine loss occurred due to cancer.5
REFERENCES
1. Slomski A. JAMA 2022;328(9):817
2. Johannesson et al., JAMA Surg 2022;e222612. Doi: 10.1001/jama surg.2022.2612.Online ahead of print.
3. Forbes RC, Karp S. JAMA Surg 2022; doi: 101001/jamasurg.2022.2652. Online ahead of print.
4. Brännström et al., Lancet 2015;385(9968):607-616
5. Dam-Kähler et al., BMJ. http://dx.doi.org/10.1136/ijgc-2020-001804
Do frozen embryos produce an increased cancer risk for offspring?
Because our Scandinavian colleagues have better national data sets than most other countries, they often lead in epidemiological stud ies. In a just published study in PLOS Medicine, Swedish research ers now reported that, in contrast to fresh-transferred embryos, frozen-thawed embryo transfers may lead to an increased cancer risk in offspring.1 As is often the case in such studies, numbers went from extremely small to very small, but the difference was sta tistically significant. Astutely, the authors point out the small num ber of cancer cases observed and caution from overinterpretation of results but they, correctly, also emphasize that, while individual risks appear small, the huge increase in frozen-thawed cycles, brought on by recent change in IVF practice in many centers that favor universal cryopreservation of embryos with delayed embryo transfer, may demonstrate an impact at a population level.
The data on which these conclusions were reached were based on 7.9 million children in four Scandinavian countries, among which 172,000 (2.2%) were born through IVF and 22,630 (13.2%) who were born after frozen-thawed cycles. Though short-term adverse effects from frozen-thawed embryos have been reported,2 this is the first re port of potentially serious long-term effects. Surprisingly little can be, indeed, found in the literature on the epigenetic consequences of embryo cryopreservation. Available data is mostly restricted to bovine embryos,3 and it would appear naïve to assume that cryopreservation of human embryos does not affect epigenetic expressions to significant degrees. Just for that reason alone (though there are several others), we reemphasize the CHR’s longstanding policy to
Continued from page 24 The V oice | o C to B e R 2022 | 25
only cryopreserve excessive embryos. In other words, CHR only cryopreserves embryos when there is no other choice in achiev ing best possible outcomes for infertile women and, as we have noted repeatedly in these pages before, we, therefore. strongly oppose one of the relatively recent new “fashions of the moment” in IVF, - routine all-freeze cycles with delayed frozen-thawed transfer cycles.
REFERENCES
1. Sargisian et al. PLoS Med 2022;19(9):e1004078
2. Hwang et al., Fertil Steril 2019;112(5):900-907
3. Maldonado et al. Cryobiology 2015;71(3):481-485
Yes, we have “vanquished” multiple pregnan cies in IVF, - but at what cost?
In a rather astonishing “expert review” article in the AJOG, sev eral prominent colleagues from different academic departments celebrated the “vanquishing” of multiple pregnancies in IVF in the U.S over the last 25 years.1 They, of course, are correct that multiple pregnancies have dramatically declined but in every other aspect this manuscript leaves a lot to be desired.
First of all, the authors fail to differentiate between twin and higher order multiple pregnancies, incorrectly suggesting that they are one and the same, when in reality nobody will argue with avoidance of high-order multiples, but what should be done about twin pregnancies has remained a highly controver sial issue (extensively discussed in the past in these pages and in the literature on repeated occasions). Without any evidence in support, they, furthermore, then attribute this “success” in vanquishing multiple pregnancies to the introduction of elective single embryo transfer and (eSET) and preimplantation genetic testing for aneuploidy (PGT-A), both subjects in these pages so often addressed in the past that we here do not want to waste further space beyond making the point that, when it comes to twins, most of the authors’ representations regarding eSET and PGT-A, were simply incorrect. Several CHR staff, indeed, felt obliged to correct their representation and wrote a letter-to-the editors of AJOG, though we don’t know as of this point whether the journal will choose to publish the letter.
Only so much here: A Canadian experiment, in which a Canadian province struck a deal with the local IVF provider community several years back called for the IVF community to adhere to eSETs, and the provincial government, in return, agreed to expanded insurance coverage for IVF.2 The agreement, indeed, “vanquished” twin pregnancies to a large degree but, nevertheless, did not last for very long once the government rec ognized that expected cost savings were not realized. As has also been noted before, while in force, the Canadian province lost ca. 40% of its IVF births from lower IVF pregnancy rates from eSETs and loss of second-twin deliveries.2 One really wonders how many infertile women would really choose eSET, if they were correctly advised that by choosing eSET they reduce their live birth chances of a healthy newborn by ca. 40%? Our guess is, not many!
REFERENCES
1. Katler et al., Am J Obstet Gynecol 2022;227(2):129-135
2. Gleicher N. Reprod Biomed Online 2011;23(4):274-278
A potentially new test for PCOS?
One of the most interesting researchers in reproductive medicine in Europe is Claus Yding Andersen, PhD, a great Danish biologist and embryologist, with important contributions especially to fertility preservation. In managing the national ovarian tissue-freezing bank in Denmark, he and his team have the unique opportunity at time of cryopreserving ovarian cortical tissues, to extract large numbers of oocytes from follicles of different sizes which, of course, represent great research material.
This group of investigators now published in JCEM an interesting study in which they determined in ovaries with polycystic ovary phe notype intrafollicular concentrations of GDF9 and BMP15 in follicles of different sizes. Both are secreted by oocytes into follicles. As it turned out, women with PCO ovarian phenotype had a significantly higher GDF9/BMP9 ratio., leading to the authors’ suggestion that a high ratio could be utilized as diagnostic test for polycystic ovary syndrome (PCOS).
Though an interesting concept, the study, unfortunately, has some of the same shortcomings plaguing most PCOS studies, starting with the definition of who is a PCOS patient, and once that has been established, which of the four phenotypes of PCOS a patient rep resents. To the first point, the authors were careful in only talking about PCO patients (i.e., patients with polycystic ovaries) and from their selection criteria they basically only considered the (“classical”) phenotype-A (and, maybe, some phenotype B and C women) and, only very unlikely, phenotype-D (“lean”) patients. To which PCOS patients this ratio, therefore, would apply is of this moment unclear.
REFERENCES
1. Kistensen et al., J Clin Endocrinol Metab 2022;107:e3374-33383
A, for several reasons, “interesting” article relating to intra-ovarian PRP injections
We earlier in this newsletter offered a summary of CHR’s preliminary experience with injecting platelet-enriched plasma into ovaries (page 11). One of the leading pioneers of this procedure in the U.S. was a reproductive endocrinologist in San Diego, CA, by the name Eric Scott Sill, MD., who recently published an interesting “hypothesis” article, speculating on one of the principle unresolved questions sur rounding PRP: if it, indeed, does improve ovarian function in some women, how does it do it?1
An unmentioned sidenote in the article is, however, the fact that Sills in 2019 was charged with murdering his wife, is awaiting trial, and recently also lost his medical license.2 Our listing of his paper as “in teresting,” therefore, refers to this rather unusual circumstance but, independently, also to the fact that this article offers a very compre hensive background review for everybody interested in PRP.
Continued on page 27 26 | o C to B e R 2022 | The Voice
REFERENCES
1. Sills ES, Wood SH. J Clin Transl Res 2022;8(1):49-53
2. https://www.cbs8.com/article/news/local/carlsbad-fertility-doctor-chargedwith-murdering-his-wife-in-2019-has-medical-license-pulled/509-904d5e556d16-4fef-b0ef-553d02ce54f2
Reassuring data regarding fertility preservation efforts in freshly diagnosed women with breast cancer
Breast cancer is the most frequent indication for “medical” fertility preservation. It is, therefore, very reassuring that a recent study in JAMA Oncology demonstrated no adverse long-term consequences from brief fertility preservation efforts before initiation of treatments for freshly diagnosed breast cancer cases.1 Neither relapse of cancer nor disease related mortality were affected. This, of course, raises the interesting question how much time may be spent on fertility preservation efforts under such circumstances since, currently, those usually involve only one month (i.e., one stimulation or two stimula tions in a double stimulation cycle), which in most patients does not yield large oocyte numbers. Could we spend more time on collecting oocytes without harming a patient’s’ cancer survival chances?
REFERENCES
1. Marklund et al., JAMA Oncol 2022; doi: 10.1001/jamaoncol.2022.3677. Online ahead of print.
Artificial intelligence (AI) in IVF
These days everybody seems to be working on AI projects in associa tion with IVF, suggesting potential clinical applications. The question that then arises obviously is, why have we not seen yet any really useful AI application feeding into IVF practice?
One of the answers, of course, is that in order to be a useful tool, AI needs to work on a detectable pattern. Assuming such a detectable pattern does not exist, even the most sophisticated AI program will, obviously, not discover one. This should be of concern when thinking about the most frequently targeted goal in association with IVF, - the decades-old dream of embryo-selection: i.e., finding from among an IVF cycle cohort of embryos, the one best for transfer.
But what if this concept of embryo selection, pursued by the IVF field from almost the very beginning of IVF,1 was to be incorrect? What if an embryo cohort in an IVF cycle, short of the obvious (i.e., easily detectable differences in morphology), does not possess “a best” em bryo? Would Ai in such a case still be useful?
That, indeed, appears to be doubtful and, considering the numerous unsuccessful studies published utilizing time lapse imaging, a call for caution appears indicated before, once again, expecting (and/or promising) too much. To better understand the realistic potential of AI, we recommend a recent article by Carol Lynn Curchoe, PhD, in JARG2 which, though in our opinion a little too optimistically, in very understandable format lays out the potential place of AI “on the ART roadmap.”
Plachot et al., Ann Genet 1987; 30(1):22-32
Endometriosis
First single-cell analysis of endometriosis Colleagues from neighboring Connecticut recently published the first single-cell analysis of endometriosis lesions.1 They characterized peritoneal and ovarian endometriosis lesions and compared them to the patients’ matched eutopic endometrium, to endometrium from by endometriosis unaffected women, and to organoids derived from those tissues. In total the study involved 122,000 single cells from 14 individuals and spatially localized cell types with imaging mass cytometry.
Several of their findings were of interest: (i) Only peritoneal le sions revealed a perivascular mural cell with apparent dual role in promoting angiogenesis as well as immune cell trafficking. (ii) They also identified an immune-tolerant peritoneal niche, potentially explaining how endometriotic implants are not rejected by the maternal immune system. (iii) The investigators also described important differences between eutopic endo metrium and microenvironments in endometriotic lesions, potentially raising questions about commercial assays currently offered which are alleged to diagnose endometriosis through endometrial biopsies.2 (iv) Finally, the investigators also described a previously unknown progenitor-like epithelial sub population of cells which clearly requires further exploration.
In toto, this study does not offer surprises but suggests further directions to be explored. For us at the CHR, the most inter esting finding – though not surprising – was the discovery of a seemingly immune-tolerant peritoneal niche. It definitely should be at the forefront of additional research efforts.
REFERENCES
1. Tan et al. Nat Cell Biol 2022;24:1306-1318
2. Ahn et al., Fertil Steril 2017;107(3):523-532
And a fiFIrst single cell analysis of menstrual endometrial tissue with poten tially profound clinical implications Here colleagues from Long island’s Feinberg Institute investi gated through single-cell RNA-sequencing of menstrual flow from 33 subjects, including known endometriosis patients and controls. As validation controls the researchers utilized patients not diagnosed with endometriosis but experiencing typical symptomatology. Their results were very interesting because they suggested that menstrual flow may allow for the accurate diagnosis of endometriosis and, possibly, also of chronic endo metritis without the need for an endometrial biopsy.
If confirmed by further independent studies, this opens up the long-awaited ability to diagnose endometriosis (and chronic endometritis) non-invasively. One, indeed, could speculate that the concept of obtaining biological data from menstru al effluent could be expanded to other pathologies affecting the reproductive tract. Because of the potential clinical and
REFERENCES 1.
2. Curchoe CL. J Assist Reprod Genet 2022;39:1493-1496
Continued from page 26 The V oice | o C to B e R 2022 | 27
importance of this paper, the CHR invited the two senior authors of the manuscript, Christina N. Metz, PhD and Peter K. Gregersen, MD, to present their study at the center’s GrandRounds, co-sponsored by the Foundation for Reproductive Medicine (FRM), on October 11, 2022. Seating is limited and res ervation for this dinner event is open to REs and embryologists as well as researchers in reproductive medicine by e-mail [to Micah Elias, melias@thechr.com) till October 7, for as long as seating is available.
(CAH) based on circulating steroid hormones. Though one can always differ on what represents clinically useful sensitivity and specificity of a test, the authors appeared satisfied and concluded from their results that steroidogenic score they used was bio chemically interpretable and showed high accuracy in diagnosing CAH, especially when due to non-classical 21-alpha hydroxylase deficiency (21 OHD).2
For infertility treatments the differentiation of CAH from PCOS may be academically of interest; but clinically is of minor impor tance since treatments are similar. But, overall, the in this paper presented option appears attractive.
Reproductive Endocrinology
News about the adrenal glands
For several reasons, the CHR considers the adrenal glands the most overlooked glandular organs in infertility practice: First, adrenals and ovaries share an embryological primordium, which means that arise from similar stem cell populations; and second, rarely pointed out in the literature, after ovaries (and testes), the adrenals have the highest concentration of AMH receptors of any organ in the body. One wonders what the purpose of those receptors might be because no AMH function has been ascribed to adrenals so-far. And then, there is, of course, the important fact that adrenals produce roughly half of a woman’s androgen hormones and that it is almost universally the adrenal portion of androgen production that becomes insufficient, when hypoadrogenism is diagnosed in infertile women.
We, therefore, found two recently published papers in the JCEM of interest: A first manuscript described the changing pattern of drug-induced adrenal insufficiency in the FDA’s Adverse Event Reporting System. 1 This paper confirms recently noted likely increases in drug-induced adrenal insufficiency from various causes.
This paper had relevance for the CHR since we, in two patients who presented to the CHR with longstanding infertility and an incorrect diagnosis of “unexplained” infertility (in itself an oxymoron since how well we diagnose infertility always depends on how deeply one explores), recently observed adrenal insuffi ciency as the underlying cause of their infertility. As it turned out, long-term exogenous glucocorticoid treatments had resulted in adrenal insufficiency by shutting down pituitary ACTH, thereby suppressing androgen production in the zona reticularis of the adrenals which, in turn, interrupted follicle maturation in ovaries and created a clinical presentation of secondary ovarian insuf ficiency (SOI). Based on increasing numbers of drug-induced adrenal insufficiency (and not only from corticosteroids), the authors of the manuscript urged an urgent search for markers that may predict causes of drug-induced adrenal insufficiencyIn a second paper in the same journal, Chinese authors offered a system to identify and subtype Congenital Adrenal Hyperplasia
REFERENCES
1.
Immunology
Immunologic testing for subfertility
Very prematurely diseased, Alan Beer, MD, based on his earlier collaboration with Ruppert E. Billingham, FRS, was, especial ly during his early days in Ann Arbor, MI, clearly one of the founding fathers of clinical reproductive immunology. He then moved his laboratory to the Chicago Medical School in North Chicago, now called the Rosalind Franklin University of Medicine and Science, before joining for a few years the CHR in Chicago. At Chicago Medical School he pioneered the concept of the commercial reproductive immunology laboratory. After leaving Chicago Medical School, one of his disciples, Joanne Kwak-Kim, MD, MPH, took over his lab and, together with Alice GilmanSachs, PhD, built it into, likely, the most widely utilized clinical reproductive immunology laboratory in the U.S. (and probably the world).
translational
REFERENCES 1. Shih et al., BMC Med 2022;20(1):315
Raschi et al., J Clin Endocrinol Metab 2022;107:e3107-e3114 2. Ye et al., J Clin Endocrinol Metab 2022;107:e3304-e3312
Continued on page 29 28 | o C to B e R 2022 | The Voice
When Fertility& Sterility recently dedicated its front-part to a series of articles on reproductive immunology, Kwak-Kim and her group, unsurprisingly, were chosen to cover immunological testing in infertility practice in a review article.1 Though we recommend the article to our readers as a comprehensive summary of what immu nological infertility testing today mostly entails, the publication of this article, however, also lays bare how little benefit immunological testing has contributed to successful infertility practice since Alan Beer, MD initiated the concept. And, yet, many patients are unnec essarily spending thousands of dollars, often uncovered by insur ance, on such testing at various so-called reproductive immunology laboratories.
A principal reason for all medical testing is the assumption that test ing results can, and will, change subsequent treatments. Otherwise, why test at all? For several understandable reasons, investigating new treatments in pregnant women face often unsurmountable bar riers. Basic treatments of what are considered immunological condi tions contributing to infertility have, therefore, barely changed since the 1970s. And if changes were made (several proposed by KwakKim and associates), supporting data have been sparce. Indeed, even the definition of what is being treated has remained largely unclear.
Take for example here discussed paper, where the abstract starts with the statement that, “unexplained subfertility and implantation failures not only are emotionally and physically distressing but also become a significant obstacle to reproductive-age couples who wish to build their family.” But though the intent is to telegraph that im mune testing and subsequent “immune modulation” will help, one wonders how Kwak-Kim et al even define “unexplained subfer tility” and “implantation failure” before testing and treating their patients?” A diagnosis of “unexplained subfertility” is, of course, an oxymoron because what remains “unexplained” mostly depends on how deep one digs in a diagnostic work-up. And if one looks at how “implantation failure” is defined in the literature, almost every study
has its own – and differing – definition. Our skepticism about currently offered huge and very costly commercial testing packages and about many currently applied, often also very costly, treatment options, however, by no means should suggest that the CHR, suddenly, has become supportive of immune-skeptics in the infertility field who usually completely reject the concept of abnormal immune function at times leading to reproductive failure and, simply, don’t wish to hear about immu nology. Very much to the contrary, as frequently before addressed in these pages, the CHR’s considers pregnancy primarily an im mune system-driven condition and only secondarily as hormonally driven, as most colleagues view pregnancy. To recognize whether an infertility patient is affected by immunological infertility, in CHR’s opinion, however, does not require many thousands of dollars in testing cost and neither do most useful treatments (though some may, indeed, be relatively costly).
To treat immunological reproductive failure appropriately, it takes besides general knowledge about the immune system, a degree of self-awareness that recognizes how little we really still know about diagnosis and treatment of immunological infertility. At the CHR we believe we have this self-awareness; and this is what has kept us modest!
REFERENCES
1.
Infectious diseases
Covid-19 news
How did it all start?
Here we are, over two-and-a-half years into the Covid-19 pandem ic, and we still do not know how and where everything started. If we were reading a crime novel or watching a movie, we as of this point would have concluded that the secretive and obstructive
Kwak-Kim et al. Fertil Steril 2022;117(6):1132-1143
Continued from page 28 The V oice | o C to B e R 2022 | 29
behavior of the Chinese government leaves little room for doubts that China is hiding “something.” And, if the SARS-CoV-2 virus really at the Wuhan animal market for the first time from a yet undetermined intermediate carrier between bats and humans was transmitted to humans, all of this secrecy would, of course, be completely unnecessary. That there is so much secrecy and obstruc tion to international investigations, therefore, strongly suggests that the by China alleged Huanan Seafood Market origin in downtown Wuhan is “fake-news,” trying to prevent the world from learning the true source of the pandemic. Reason for such a major effort at hiding the truth can only be that the truth would to significant degrees compromise the Chinese government; and the only obvi ous source for the start of the pandemic that would, indeed, deeply embarrass the Chinese government would be the involvement of the government-owned Wuhan Institute of Virology, which is under the control of the Chinese military.
Jon Cohen in a news-article in Science magazine now reports that China frantically insists that the Covid-19 pandemic, indeed, did not even start within China’s borders but that the reason why China reported the first cases was that the SARS-CoV-2 virus was brought to China from outside its borders. Paradoxically, but not surprising for a political dictatorship, some of these absurd arguments are vehemently supported by many Chinese scientists, with, as Cohen notes, “a flurry of papers pointing the fingers elsewhere.”1 We strongly recommend this article to our readers for its openess.
Cellular T cell immunity to the SARS CoV-2 virus
In the same issue of Science, two U.S. investigators in a “viewpoint” article address T cell immunity (i.e., cellular immunity),2 an im portant aspect of anti-SARS-CoV-2 immunity that for the longest time did not receive the appropriate attention by investigators who primarily were concentrating on humeral (antibody) immunity. As a commentary, the article does not present new data; but it does offer a detailed description of why T-cell immunity against the Covid-19 virus appears critical for long-term protection by Covid-10 vaccines. Reading this very informative article, one won ders why, with so many diagnostic antibody tests on the market, there still does not exist a commercially available diagnostic Covid-19 test that tells us how much antibody- as well as T-cell immunity an individual has.
Long Covid
It also appears important to point out in these pages that so-called “long covid” (i.e., the persistence of significant clinical symptoms beyond the acute phase of Covid-19 infections) is becoming a highly significant societal problem. Long covid appears to be a very heterogenous condition but, as pointed out in another recent news article in Science by Jennifer Couzin-Frankel, some clues have been coming up: 3 Affected patients often complain about intense fatigue, brain fog and exercise intolerance. Blood tests usually reveal low cortisol levels, though ACTH levels are normal. The cause for the low cortisol as of this point is unclear. These patients, further more, demonstrate exhaustion in a certain category of T cells that
usually surge with exposure to pathogens, suggesting that their immune system fights (or recently fought) “something.” What that “something” is, remains to be determined but some studies sug gested that it may be a reactivated Epstein-Barr virus, while other researchers suggested that it may be reactivated SARS-CoV-2 virus, released from immunologically protected niches.
An interesting additional paper from British investigators also addresses long covid.4 Here the authors in a huge population study of Covid-19 involving 486,149 Covid-19 patients and 1,944,580 non-Covid controls, defined the most common persisting symp toms, reporting surprisingly significant reproductive problems among the top-10. In declining frequency, the following symptoms were the most common: anosmia (loss of smell), hair loss, sneezing, ejaculation difficulties, reduced libido, shortness of breath at rest, fatigues, pleuritic chest pain, hoarse voice, and fever.4 Until reading this paper, we were unaware that Covid-19 was associated with ejac ulation problems and loss of libido.
The impact of past Covid-19 on frozen-thawed IVF cycles
After hearing for the longest time how fortunately limited the impact of Covid-19 infection was on IVF, there is increasing “noise” in the literature that things may be a bit more complex than initially presented. One paper making this point, was recently published by Israeli investigators in JARG, 5 suggesting that frozen-thawed embryo cycles, in which oocytes were retrieved prior to a SARSCoV-2 infection, resulted in significantly poorer pregnancy rates than controls for ca. 60 days post-infection. If confirmed, one must conclude that a SARS-CoV-2 infection exerts influence as to how the affected female’s immune system manages the induction of toler ance that is required in order to prevent immunological rejection of an implanting embryo. For up to 60 days post-acute Covid-19, the maternal immune system apparently is prevented from inducing appropriate tolerance pathways, resulting in fewer implantations and pregnancies.
This is a potentially worrisome observation that fits well into an evolving picture in the medical literature that suggests that Covid-19 vaccines (and maybe the SARS-CoV02 virus itself) may have sup pressive effects on certain aspects of the immunes systems that go beyond just resistance to the SARS-CoV-2 virus, actually increasing infection risks with other pathogens. In a still ongoing study at the CHR under the leadership of David H. Barad, MD, the investiga tors, indeed, discovered in infertility patients a significant decline in certain natural autoantibody levels after immunizations to the virus, suggesting that the anti-Covid-19 vaccinations suppressed an unrelated immune response that usually produces those antibodies (Barad et al, unpublished data). One also may start wondering why some long-absent infections, like monkeypox and polio, suddenly, are raising their ugly heads. We will carefully follow this subject and advise you accordingly.
Continued on page 31 30 | o C to B e R 2022 | The Voice
REFERENCES
1. Cohen J. Science 2022;377(6608):805-809
2. Wherry EJ, Barouch DH. Science 2022;377(6608):821-822
3. Couzin-Franke J. Science 2022;377(6608):803
4. Haroon S, Subramanian A. Nat Med 2022;28:1554-1555
5. Youngster et al., J Assist Reprod Genet 2022 39:1565-1570
Monkeypox
As previously noted in these pages, monkeypox is a virus currently actively spreading especially among networks of men who have sex with men but there is a risk of it breaking out into other popula tions.1 Though New York also declared an “emergency” regarding monkeypox, likely due to improved education of high-risk groups,2 the case load in NYC, which led all other major U.S. cities in case numbers, appears to have plateaued and may have even started to decline. A first death in the U.S. from the disease was reported in Texas,3 a second death apparently occurred recently in New York; but the risk of death from this disease is infinitesimally small, and an effective vaccine is available for high-risk groups. HIV/AIDS patients are at increased risk for severe disease if affected with monkeypox, especially if their CD4 count is below 200.4 For those interested in more detail, we recommend a recent JAMA article.5
REFERENCES
1. https://www.greaterthan.org/videos/what-you-need-to-know-about-mon keypox/?gclid=Cj0KCQjwjvaYBhDlARIsAO8PkE1QgTDmicTh8pzdSxpdil b9ARw5XvcJWpLOvc1Ch2tqxwzwPEA0ODYaAk0wEALw_wcB
2. https://www.greaterthan.org/campaigns/monkeypox/?gclid=Cj0KCQjwj vaYBhDlARIsAO8PkE2CvlqBuLgySa4ArjFg61Ejgeqdliu-HQPZdMe2CFL WVcjm-xmm_7IaAj3UEALw_wcB
3. https://dshs.texas.gov/news-alerts/ texas-confirms-first-death-of-a-person-with-monkeypox/
4. https://www.greaterthan.org/videos/what-you-need-to-know-about-mon keypox/?gclid=Cj0KCQjwjvaYBhDlARIsAO8PkE1QgTDmicTh8pzdSxpdil b9ARw5XvcJWpLOvc1Ch2tqxwzwPEA0ODYaAk0wEALw_wcB#stories-2
5. Del Rio C, Malani PN. JAMA 2022;328)10):921-922
Polio
And, yes, New York has also declared a medical emergency regarding polio, even though so-far only one case of paralytic polio has been reported in the state (in Westchester). The emergency was declared because tests of wastewater in different parts of the state have increasingly shown positive results, suggesting subclinical spread of the virus into the general population at much larger degrees than a single case would predict. But polio can be spread by asymptomatic carriers and most polio infections are not leading to paralysis and may, indeed, be overlooked and/or considered a mild flu-like infection.
Authorities have pointed out that those who are fully vaccinat ed with three sequential vaccines are probably well protected for a lifetime. A single booster injection is, however, never theless, recommended for individuals who (i) are not certain that they were fully vaccinated in their youth; (ii) are above age 60; (iiI) have suppressed immune systems; and (iv) are at risk because of increased potential contacts with polio carriers and/ or contaminated fluids, like wastewater.
REFERENCES
1. https://www.cnbc.com/2022/09/09/new-york-declares-state-of-emer gency-over-polio-to-boost-vaccination-rate.html
Genetics
The consolidation in the genetic test ing industry in reproductive medicine is continuing
We in the September issue of the VOICE reported on two genetic testing companies either scaling back or completely closing down certain product lines, like PGT-A testing. It now appears that consolidation in the genetic testing industry is continuing, as Becker Hospital Review reported significant upheaval at yet another major genetic testing provider, Sema4, a pioneer in expanded carrier testing for single gene diseas es, and one of several spinoff companies from Mount Sinai Health System here in NYC. The company cut 250 jobs, among those also the company’s founder, Eric Schadt (who appar ently resigned) to be replaced as chief technology and product officer by Matthew David of the genetic testing company which, as we last month here reported, just shut down its national PGT-A testing laboratory program. Sema4 also announced that it was shutting down its somatic tumor-testing business and closing a clinical laboratory in Connecticut. With ca 1,600 remaining employees, the company, however, still remains a giant in the industry.1
REFERENCES
1. https://www.beckershospitalreview.com/digital-health/mount-sinaispinoff-lays-off-250-employees-founder-exits.html
Continued from page 30 The V oice | o C to B e R 2022 | 31
Genetic testing in reproductive medicine after the Supreme Court’s decision on Roe vs. Wade
The recent Supreme Court decision to return the abortion issue to the jurisdiction of states, of course, has potential repercussions beyond just whether abortions are permitted or not. For practical purposes this decision also returned related and/or associated issues to the states. Contrary to some recently published opinions expressing concerns about the ability to offer continuous IVF services in currently available formats in some states,1,2 we have previously expressed the opinion in this newsletter that a signif icant effect appears unlikely even in states that may prohibit or significantly restrict abortions because IVF - even among pro-lif ers- has become too engrained in U.S. society. But extreme political decisions in the current political climate cannot be completely ruled out and the subject, therefore, requires continuous attention by the IVF field.
A related issue has, however, only more recently attracted atten tion, and that is preimplantation and prenatal genetic testing be cause of the very obviously associated issue of discarding embryos and/or aborting a pregnancy. Regarding IVF, the concept of PGT-A is, likely, the most vulnerable subject to attack because there is now significant evidence in the literature that the process leads to nonuse and/or disposal of quite large numbers of embryos with signif icant pregnancy potential. In a discussion of both of these issues, a recent “commentary” in Cell Reports Medicine, pointed out that in the opinion of the two authors, the high commercialization of the fertility industry with its significant lobbying power on federal as well as state levels, should help IVF overcome whatever pressures may come from the right to life movement.3 One, of course, can only hope that these authors are correct. We recommend their article for those interested in these issues.
REFERENCES
1. Greely HT. J Law Bisci 2022;9(2):lsac019. Doi: 10.1093/jib/lsac019. eCollec tion 2022
2. Cohen et al., JAMA 2022;328(1):15-16
3. Allysee MA, Michie M. Cell Rep Med 2022;3:100690
More on PGT-A and chromosomal mosaicism in embryos
The confusion continues
We have previously in these pages repeatedly pointed out the current confusion caused by PGT-testing laboratories, which follow greatly varying definitions of “abnormal” when reporting out PGT-A results. As a consequence, as we have noted in the past, what in one lab may be a “mosaic: embryo may in another lab be signed out as “normal-euploid;” or what in one lab may be an “aneuploid” embryo, may elsewhere be reported as a “mosaic” embryo.
These highly confusing circumstances IVF centers face, was recently reemphasized in a chainmail CHR’s physicians received from Igenomix®, a leading international PGT-A laboratory (August 12, 2022; gc@igenomix.com) in which the company “opened an important discussion on the topic of chaotic results on PGT-A.” This
is another issue of controversy because Igenomix® considers an embryo “chaotic” only if it demonstrates more than 6 chromo somal abnormalities. Other PGT-A laboratories, however, will assign such a definition to an embryo already with more than 2 abnormalities.
The company in its mailing furthermore stated that, “traditionally it was assumed that these embryos were the least viable of all; but emerging data suggest that there is more to the story.” And, indeed, there was surprisingly more to the story because the company observed in re-biopsied embryos previously classified as “chaotic,” “a surprising 38% chance of euploid results,” suggesting that “some embryos reported as chaotic could have more reproductive potential (i.e., pregnancy and live birth chances) than previously thought.”
Though the company must be given credit for disseminating this message apparently as soon as they became aware of the data (and even before in print), one must ask, what took so long and why would this be “surprising” to one of the leading PGT-A labora tories in the world? These data are, obviously, not surprising to the CHR because the observation that re-biopsies of embryos demonstrate in a very high percentage radically different results was a main message of CHR’s first already in 2015 published study reporting healthy births from transfers of supposedly chromosom al-“abnormal” embryos.1,2 This study was, indeed, a strong factor why the CHR since then has been actively advocating against the routine clinical use of PGT-A in IVF.
The company furthermore concludes that, assuming a “euploid” re-biopsy. “the clinical management could be based on the eu ploid result, and embryo transfer could be considered with careful counseling.”
The circular thinking behind this mailing is nothing but aston ishing: Since the same observation obviously also applies to other than “chaotic” PGT-A outcomes, what here issued professional advice from a leading PGT-A laboratory basically says is, that a 1st PGT result, cannot be believed and, therefore, should be followed by a repeat biopsy, which is more believable and then, finally, can be believed and acted upon. Hallelujah, the PGT-A industry just significantly further increased its testing volume, even though it just, once again, demonstrated how useless PGT-A really is.
Speeding up chromosomal testing of embryos (PGT-A) and early pregnancy (NIPT)
One of the big technical drawbacks of PGT-A testing is that, after a trophectoderm biopsy has been obtained for a blastocyst-stage em bryo, the embryo must be frozen and cannot be transferred fresh because receiving testing results from central laboratories, where those biopsies have to be sent to, takes days. Now Zev Williams, MD, head of Columbia University’s Reproductive Medicine and Infertility division, and colleagues reported in research letter format in the prestigious New England Journal of Medicine a new testing method that allows IVF centers (or OB/GYN offices during early pregnancy testing), using a handheld rapid nanopore sequencing-base screen for aneuploidy testing to obtain testing
32 | o C to B e R 2022 | The Voice Continued on page 33
results within three hours, which, therefore, would avoid the need for cryopreservation of transferrable embryos and allow still fresh transfers at blastocyst stage.3
As an earlier 2018 publication demonstrates,4 Williams and col leagues have been working on this idea already for several years but now, appear to have matured it for potential clinical appli cations in early pregnancy testing as well as in PGT-A. Such an instrument, of course, potentially creates, significant commercial business competition for the genetic testing industry which, as CHR’s researchers recently calculated, based on current PGT-A utilization levels in the U.S. alone, annually collects revenues in the $300-500 million range. Potential revenue from non-invasive pre natal testing (NIPT) can expected to be even higher. Revenues in excess of a billion dollars may, therefore, be at risk annually for the genetic testing industry if ever fertility center and every obstetrical office of decent size can do this testing in house and have results within 3 hours.
An article in The New York Times by senior medical writer Gina Kolata on August 17, 2022, noted that the reported results by the Williams group still require confirmation from outside studies.5 But assuming that such confirmation is obtained, technically this would represents highly significant progress with consid erable translational consequences for reproductive medicine. Williams and colleagues are, therefore, to be congratulated on their accomplishment!
As, however, also noted elsewhere in this newsletter (see “CHR in the Media”), this new instrument does not change the CHR’s neg ative opinion regarding the utilization of PGT-A. Even assuming that this new instrument works perfectly, a 5-6-cell trophectoderm
biopsy, still, cannot accurately determine the total chromosomal make-up of a blastocyst-stage embryo and PGT-A, therefore, still, in principle remains a useless test for most IVF patients and even a harmful test for women with few eggs and embryos, as recently again demonstrated in a study, further discussed in the next paragraph.6
Further confirmation that PGT-A in poor-prognosis patients harms IVF outcomes.
The CHR has made the point that PGT-A (then called PGS) was hurting IVF cycle outcomes in older women and other poor prog nosis patients since 2008, - with substantial additional evidence published since then and frequently discussed in this newsletter. We, therefore, do not wish to be repetitive; but when a new group of investigators previously not actively involved in this subject en ters the field confirming the point, it is still worth noting.6 In a ret rospectively matched cohort study of 130 poor-prognosis PGT-A and 130 non-PGT-A poor-prognosis patients based on small egg and embryo numbers, matched for age, BMI blastocysts and em bryo quality, the authors reported similar live birth numbers and miscarriage rates with reference point embryo transfer. However, when outcomes were assessed with reference point retrieval, live birth rates in non-PGT cycles were 43% and only 23% in PGT-A cycles. The authors with their study not only confirmed that in poorer-prognosis patients PGT-A is harmful to IVF outcomes but, also, that outcome studies with reference embryo transfer skew and bias studies by eliminating poorer prognosis patients. Such studies, nevertheless, are still widely quoted by proponents of PGT-A;rather shameful, we would argue!
The V oice | o C to B e R 2022 | 33 Continued from page 32
Mosaicism in preimplantation-stage human embryos
It does not often happen that one falls in love with a paper. Here we recommend a “review” paper by two Scottish colleagues from the University of Edinburg, 7 we, indeed, have fallen in love with, since this manuscript presents the highly complex subject of mo saicism in preimplantation-stage human embryos in a way that is relatively easily understood by even currently completely confused clinicians.
Rarely addressed in the infertility literature, the manuscript among many other important issues, points out the discrepancy between embryonic cell lineage in which aneuploidy usually does not survive, and extraembryonic cell lineage where aneuploidy per sists until birth in the form of confined placental mosaicism. We strongly recommend this manuscript for everybody who, when it comes to PGT-A, still, does not understand what PGT-A laborato ries are really reporting when it comes to “mosaicism,” and/or who still does not understand why PGT-A for physiological reasons simply cannot work.
REFERENCES
1. Gleicher et al., Fertil Steril 2015; 104:e59
2. Gleicher et al., Reprod Biol Endocrinol 2016;14(1):54
3. Wei et al., N Engl J Med 2022;18:387(7):658-660
4. Wei et al., Fertil Steril 2018;110(5):910-916.e2. doi: 10. 1016/j. fertnstert.2018.06.014
5. Kolata G. The New York Times; 8/17/22. https://www.nytimes. com/2022/08/17/health/birth-defects-testing.html
6. Mahesan et al., J Assist Reprod Genet 2022; doi: 10.1007/s10815-02202588-9. Online ahead of print.
7. West JD, Everett CA. Reprod Fertil 2022;3:R66-R90
Aneuploidies are the result of replication stress that impairs chromosome segregation Aneuploidy in preimplantation-stage embryos is so common that it is now by many considered normal. What causes this chro mosomal instability is, however, not very clear yet. Now come Palmerola et al (from Dieter Egli, PHD’s laboratory at Columbia University) with a paper that offers some insights.1 Quoting from their abstract: The S phase at the 1-cell stage shows replication fork stalling, low fork speed, and DNA synthesis extending into G2 phase. DNA damage foci consistent with collapsed replica tion forks, DSBs, and incomplete replication form in G2 in an ATR- and MRE11-dependent manner, followed by spontaneous chromosome breakage and segmental aneuploidies. Entry into mitosis with incomplete replication results in chromosome break age, whole and segmental chromosome errors, micronucleation, chromosome fragmentation, and poor embryo quality. Sites of spontaneous chromosome breakage are concordant with sites of DNA synthesis in G2 phase, locating to gene-poor regions with long neural genes, which are transcriptionally silent at this stage of development. Gene poor regions in the human embryos due to DNA replication stress are, thus, exposed to fragility, which leads to aneuploidy.
Similar chromosomal instability is also observed in metastatic malignant tumors,2 and, one, therefore, wonders whether these processes are also the cause of aneuploidy in cancer. As reported in human embryos by the Egli laboratory, one end product are micro nucleations,1 in chromosomal-unstable cancer cells also a common byproduct of cell division, they play an important role in cancer metastasis and in introducing in subsequent cell cycles chromo somal abnormalities into the primary cell nucleus.3
REFERENCES
1. Palmerola at al., Cell 2022;185(16):2988-3007
2. Al-Rawi DH, Bakhoum SF. Curr Opin Genet Dev 2022;74:101913
3. Augustinus AS, Bakhoum S. Methods Cell Biol 2022;172:51-66
Do maternal premutation range FMR1 mutations affect embryo quality?
Maternal premutations in the FMR1 (fragile X) gene have several very important clinical implications for reproductive medicine. What primarily drove this gene into the field and resulted in FMR1 testing becoming routine in fertility as well as obstetrical practice, was the recognition that a mother’s premutation (56-200 CGG repeats) in one generation may expand into a full mutation (>200 CGG repeats), thereby causing the fragile X syndrome, especially in male offspring a rather horrible birth defect, characterized by mental retardation and autism. Prenatal and later preimplantation genetic diagnosis, therefore, became important if a mother was recognized to be a carrier of such a premutation.
It furthermore became obvious that women who carried the premutation experienced a significantly increased risk for pri mary ovarian insufficiency (POI), also called premature ovarian failure (POF). What, however, to this day is often forgotten is that POF/POI has a precursor stage, during which women have low functional ovarian reserve (LFOR) for age (and, therefore, often infertility) but are not yet in menopause, by the CHR years ago giv en the name premature ovarian aging (POA) and by others also called occult primary ovarian insufficiency (oPOI). Indeed, while POI/POF affects only approximately 1% of women, POA/oPOI affects ca. 9%. Every woman with POA/oPOI, therefore, should have her FMR1 status evaluated since premutations (but not full mutations) are the most frequent genetic cause for POA/oPOI and POI/POF.
Males with premutations in their single FMR1 gene (males have only 1 gene since the FMR1 gene is located on the X chromosome) are also potentially greatly affected since they are at significant risk to develop (at middle ages) a neurodegenerative disease, called the fragile X-associated tremor/ataxia syndrome (FXTAS). Premutation of the FMR1 gene, thus have significant potential ad verse impacts on women and men who are carriers and, of course, can cause full fragile X syndrome in offspring of women-carriers. What, interestingly, however, so-far has never before been inves tigated is whether a woman with an FMR1 premutation produces poorer quality embryos? That this would make sense follows from
34 | o C to B e R 2022 | The Voice Continued on page 35
the well-known fact that LFOR, of course, is associated with poorer IVF outcomes. Since POA/oPOI and POI/POF are defined by LFOR, such an association, therefore, should not surprise.
Israeli researchers now offer an at least preliminary answer after retrospectively investigating 529 embryos from 126 IVF cycles of 39 FMR1 premutation carriers who underwent genetic testing for monogenic diseases (PGT-M).1 Using time lapse, they then compared the development of premutation carriers’ embryos to embryos of control patients: Morpho-kinetic parameters did not differ between the two groups of embryos between fertilization and day-3. Blastulation rates were also similar; the start of, blastula tion was, however, delayed in premutation carriers (P=0.001) and percentage of top-quality embryos was lower, though that differ ence must be viewed with caution because of marginal statistical significance (25.6% vs. 38.8%; P=0.04).1
Overall, this is, however, not an unexpected finding considering earlier work (much done by CHR investigators) which clearly demonstrated that the number of CGG repeats on the FMR1 gene in general can affect female fertility to significant degrees.2-4 This paper is now, however, a potentially important additive contribution which further suggests that the FMR1 gene, likely, plays a much more important role in ovarian physiology than is currently appreciated and, therefore, requires further investigation.
REFERENCES
1. Shulman et al. Reprod Biomed Online 2022; S1472-6483(22)00430-8.
2. Wang et al., PLoS One 2018;13(12):e0209309
3. Barad et al., Reprod Biol Endocrinol 2017;15(1):34
4. Gleicher et al., Transl Res 2015;166(5):7.e1-2; doi: 10.1016/j. trsl.2015.06.014. Epub 2015 Jul 6.PMID: 26209748
Basic sciences
The fif irst chromosomal fusion in mammals Engineering/fusing chromosomes has so-far only been achieved in yeast. Now Chinese investigators reported in Science construction of a sustainable mouse karyotype through programmed chromo some fusion.1 By using haploid embryonic stem cells and gene editing, they fused chromosomes of similar sizes, discovering that chromatin conformation and stem cell differentiation were only minimally affected. Karyotypes with large, fused chromosomes, like #1 and #2, resulted in arrested mitosis, polyploidization, and embryonic lethality, while karyotypes of smaller fused chromo some, like #4 and #5, were able to be passed on to homozygous offspring. The authors presented their study as the first evidence for the feasibility of chromosomal engineering in mammals. Since we are mammals as well, this is one more step into potentially very controversial areas of research.
REFERENCES
1. Wang et al., Science 2022;377(6609):967-975
What causes the arrest of in vitro fertilized human embryos?
In a for us much more interesting paper (also) out of China, investigators identified metabolic and epigenetic dysfunctions in human embryos that lead to arrest of development in human embryos during IVF. Since a majority of embryos arrest between 3- and 8-cell stages, what causes these arrests, and whether they are potentially preventable and/or even treatable, are, of course, important questions.
They now were addressed by Yang et al in a very interesting paper in PLoS Biology,1 in which the authors were able to demonstrate that embryos that arrest enter a senescent-like state as a conse quence of cell cycle arrest. They further identified three separate types of arrests; Type I fails to complete the maternal-zygotic tran sition; Types II and III have low glycolysis and either high (Type II) or low (Type III) oxidative phosphorylation. Most interestingly, resveratrol or nicotinamide treatments reversed the arrest, obvi ously now warranting treatment trials with arrested embryos in human IVF.
REFERENCES
1. Yang et al.,
PLoS Biol 2022;20(6):e3001682 The V oice | o C to B e R 2022 | 35 Continued from page 34
More on synthetic embryos generated ex utero from mouse naive ESCs
That stem cells can contribute to embryonic or extraem bryonic tissues (the two initial embryonic cell lineages) after injection into preimplantation stage embryos has been demonstrated before. Whether stem cells can, how ever, independently, give rise to a normally developing embryo, has remained open for discussion. Whether this question really still had remained open for discussion, indeed, became subject to a nasty public exchanges (since withdrawn) between the senior author of a manuscript that just appeared in Cell,1 Jacob H. Hanna, PhD (in Israel), and one of the leading investigators in this field, Magdalena Zernicka-Goetz, PhD (U.K. and now also in the U.S.), which suggested that, fairly or not, the lady may have actually beaten the gentleman to the punch with a slightly earlier publication.2
Whoever was first, both laboratories deserve enormous credit for, at least in the mouse, demonstrating the feasibil ity of making normal embryos from stem cell lineages. If it can be done in the mouse, we will also be able to do it in the human experience. The question is only how soon?
REFERENCES
1. Tarazi et al., Cell 2022;185:3290-3306
2. Bao et al., Nat Cell Biol 2022; doi: 10.1038/s41556-022-00984-y. Online ahead of print.
Can GnRH improve cognition
According to a recent study by Manfredi-Lozano et al in Science this is exactly what apparently happens.1 In mouse models of Down’s syndrome and Alzheimer’s disease and in men with Down’s syndrome they demonstrated that pulsatile administration of gonadotropin-releasing hormone (GnRH), indeed, improved cognitive function and did so, interestingly, without changing reproductive hormones. Because of concern the GnRH could interrupt female menstrual patterns, in humans this phenomenon was only investigated in males. As an accompanying “perspective,” however, correctly noted, to confirm the full clinical value of GnRH in fostering cognitive function, a randomized study in both sexes will be required.2
As the commentary, however, also points out, this surpris ing finding opens up a large variety of potential clinical applications for pulsatile GnRH. We all may become con sumers as we grow older.

REFERENCES
1. Manfred-Lozano et al., Science 2022;377(6610):eabq4515
2. Hoffmanm HM., Science 2022;377(6610):1042-1043
AT THE CHR
LABORATORY
The CHR is searching for a qualified candidate to help the laboratory division in performing blood tests, semen analyses, and help with completion of administrative paperwork. Qualified candidates should have at least one year experience in working in a diagnostic laboratory setting.
This position offers a competitive salary and benefit package and is available immediately. For further information or to submit a resume, please contact CHR’s COO, Ms. J. Tapper

hiRinG
TECHNICIAN
at jtapper@thechr.com 36 | o C to B e R 2022 | The Voice ADVERTISEMENT ADVERTISEMENT Continued from page 35



The Center for Human Reproduction Fighting for every egg and embryo Conne C t with us! www.the C h R . C om so C ial@the C h R . C om 212.994.4400 21 e 69th s t n ew Yo R k, n ew Y o R k 10021 ADVERTISEMENT
















 By Yu. Kizawa, Founding Editor of the VOICE
By Yu. Kizawa, Founding Editor of the VOICE














 The CHR, New York, N.Y.
The CHR, New York, N.Y.














