




















As always, we are delighted to welcome all of our readers to a new issue of The CHR VOICE. Our May issue is again made up of a compendium of articles that will hopefully satisfy the broad spectrum of our readership, made up of current and past CHR patients, many thousands of steady visitors to our website every month, clinical and research colleagues, research collaborators, and friends in various medical fields with interest in reproductive medicine and infertility
This month’s lead article explores the hypothesis that, based on evolutionary theory, our fertility may reflect our overall health and, therefore, possibly our potential longevity. Should this hypothesis be confirmed, the potential research opportunities that would present themselves through polygenic risk scoring and AI would be nothing but astonishing.
Especially, if having failed several IVF cycles before, a question almost every new patient asks us at first consultations is, “what will the CHR do differently from our prior IVF cycles elsewhere?”, and our usual answer is, “practically everything!”. Because we encounter this question so frequently, we decided it was time to summarize for all infertility patients who are considering treatment at the CHR, and for all colleagues who are considering referring patients to the CHR, why and how the CHR individualizes treatments and how many of those differ from most other IVF centers in the U.S. and elsewhere.
Have you heard about the CHR’s three ongoing PRP trials? PRP stands for platelet-rich plasma (a fraction of our blood) which, when injected into the ovaries, has been alleged to recruit more and better follicles into maturation and by many fertility centers is widely advertised under the name “ovarian rejuvenation.” We in one of our announcements especially invite patients to join our PRP III trial which is open to women up to age 45 and is a prospectively randomized study that, blinded, prospectively compares outcomes following injection into ovaries of either PRP or a control plasma fraction of the patient’s own blood. The purpose of this protocol is to determine whether potential positive effects of PRP treatments are caused by the PRP itself or are the consequence of the mechanical process of needling the ovaries, which also is known to, possibly, activate dormant follicles.
In his monthly “A Piece of My Mind” series of articles, the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, this time addresses the long time (on average 17 years) it takes in all fields of medicine for new discoveries to reach routine clinical practice. Though some efforts are underway in attempts to shorten this time, he is skeptical of their likely success. He, moreover, suggests that there may be an advantage to the length of the process in allowing the marketplace (i.e., practicing physicians and their patients) to eliminate the many useless “garbage” treatments that increasingly enter medical care with infertility practice, of course, in many ways having become a posterchild for many of these useless treatments.
We also again answer four questions we received from patients and/or the public and want to take the opportunity to encourage our readers to send us questions they want to be addressed in future issues of The VOICE and/or any other communication they consider relevant for this newsletter. Since the month of May is Mental Health Awareness Month, we are also offering some thoughts about mental health as it relates to infertility and infertility treatments. As always, concluding this issue of The VOICE, we offer once again a quite lengthy literature review of recently published articles that we considered of relevance to our field of medical practice. Due to the subject’s relevance to all medicine, we in this month especially recommend our commentary on “evidence-based medicine” in the section on “The business of infertility.”
We very much hope that you will enjoy this May issue of The VOICE and want to remind you that you, now, can also subscribe to a printed version of this newsletter by indicating your interest to social@thechr.com.


That based on changes in inherited traits throughout successive generations, mankind is a current-day product of millennia of evolution, is now a widely accepted concept.1 The geneticist Theodosius Dobzhansky’s 1964 statement that “nothing in biology makes sense except in the light of evolution,”2 has, therefore, become widely quoted as a reflection of this understanding. As Charles Robert Darwin (1809-1882) already in the mid-19th century concluded, natural selection acts on populations of organisms with varying traits and changes them over time in response to environmental changes, leading to natural selection (i.e., survival and reproduction) of individuals in those populations.3 This principle is also often described as “survival of the fittest,” made famous in the 5th edition of his “On the Origin of Species” book in 1869.4 It applies as much to the rapidly evolving SARS-CoV-2 strains in response to developing immunity in human populations, as to how, for example, contemporary cell phone use, undoubtedly, will affect human evolution, - to note only one among many currently likely very influential environmental influences affecting the further evolution of mankind.
As already noted above, survival of the fittest is, however, very obviously not only dependent on selection of certain inherited traits, but also on the transmission of those traits into future
Continued on page 6
generations, and that can only be achieved through a successful reproductive process, - i.e., normal female and male fertility. That for mankind this is not necessarily any longer a quantitative, but more of a qualitative issue, is well demonstrated by very obvious, yet in an evolutionary sense very logical, reproductive limitations in humans such as single-follicular natural cycles and ovulations and others, recently well described by pediatric geneticist Mark Lubinsky. 5 However, if our fertility is so closely related to our evolutionary survival, should it not also be reflected in our general health and, therefore, our longevity?
As several papers recently addressed this question, we are here offering a brief review of what currently is available in the medical literature to support such a hypothesis. If one assumes this hypothesis to be correct, a diagnosis of female and/or male infertility would, of course, assume significant additional public health relevance.
Based on many studies, it is widely accepted that poor health in both sexes adversely affects fertility; but whether poor fertility is a potential reflection of future poor health and, therefore, potentially also of longevity, is less well understood. In males, for example, infertility has been associated with testicular cancer,6 cancer in general,6,7 as well as general mortality.8 In females even more examples come to mind: certain phenotypes of polycystic ovary syndrome (PCOS) have for many years been strongly associated with metabolic syndrome, characterized by diabetes hypertension and cardiac disease9 and, therefore, unquestionably with premature death. Diminished ovarian reserve has been associated with shortened telomere length,10 a typical finding in age-related diseases.
In 2016, the subject was already considered important enough for the National Institute of Child Health and Human Development (NICHHD) and the Center for Disease Control (CDC) to organize a workshop bringing together experts in somatic diseases and reproductive medicine. They in 2017, summarized in an article in Human Reproduction Open 11 As a possible explanation for such an association the paper suggested four categories for biological explanations: genetic factors, hormonal factors, in-utero factors, and lifestyle/health factors. Though pointing out that such an association by that point had not been established and may, indeed, be rejected by future research, the workshop, nevertheless, concluded that investigations were urgently needed. The expert opinion furthermore noted the importance of interdisciplinary studies since current departmental compartmentalization into medical specialties did not allow for appropriate longitudinal studies to be conducted in only either reproductive medicine or adult general medicine.
Within this context, it is also important to note that a link between infertility and later disease and/or lifespan may also go the other way since it has been known for some time that childbirth may protect mothers from certain diseases. What here comes to mind are possible protective effects of pregnancy on breast and ovarian
cancer and/or general rejuvenating effects of pregnancy plasma, as observed in animal experiments through transfusion of younger animal plasma into older animals.12 The same group of investigators then, indeed, demonstrated a transient effect of pregnancy on muscle regeneration for up to two months, suggesting it may be caused by fetuses in pregnancy partially sharing a circulation with their mother that allowed for regenerative effects by activation of dormant muscle progenitor cells via the Notch signaling pathway.13 A similar effect on maternal tissue regeneration has also been suggested from fetal stem cells.14
More recent studies offer further support for an association between infertility and future health. Especially the association between infertility and cardiovascular diseases has attracted considerable evidence in recent years: A study in infertile men from Taiwan, for example, demonstrated a significantly increased risk of cardiovascular disease in comparison to fertile controls.15 Two recent studies also identified later cardiovascular diseases as a substantial risk factor for infertile women.16, 17
Associations in males were not too long ago in detail reviewed by Choy and Eisenberg:18 Like other authors on the subject, they divided associations suggesting male infertility to be a harbinger of future health into genetic, developmental, and lifestyle associations, as well as various disease groupings, including cancer, cardiovascular, and other chronic diseases. An even more recent study reported that almost 10% of men presenting with primary infertility (sic., an important distinction from men with secondary infertility or studies no differentiating between the two) demonstrated decreases in overall health status within 10 years from initial infertility diagnosis (therefore, likely, at already quite young ages.)19 Men with non-obstructive azoospermia, indeed, demonstrated the worst health status, leading to a recommendation for careful follow-up. Related, another recent study investigated through polygenic risk scores male childlessness as a proxy for cardiovascular risks and mortality, concluding that childless men and men with severe infertility problems may be an important target group for the prevention of cardiovascular risks.20
Associations in females appear to be similar: Female infertility was in a recent study associated with a hazard ratio of 1.26 for premature death largely driven by death from cancer. It was moreover stronger in women diagnosed at earlier ages. A greater risk of all-mortality was especially also associated with ovulatory infertility.21 U.S. investigators recently also reported that atherosclerotic cardiovascular disease in postmenopausal women was more frequent in women with a history of infertility than in women without.22 This risk was 13% higher in nulliparous infertile women and 36% higher in nulliparous women with additional history of pregnancy loss. In addition,
Continued on page 7
Continued from page 6
because, as a next step, one could institute preventative interventions for infertile women as well as men to protect them from threatening diseases and, by doing so, extend life spans.

1. Forbes A, Krimmel BA. Nat Ed Knowledge 2010;3(10):6
2. Dobzhansky T. Am Zoologist 1964;4:443-452
3. Tibell LAE, Harms U. Science & Education 2017;26:953-973
4. https://www.britannica.com/science/survival-of-the-fittest
5. Lubinsky M. J Asist Reprod Genet 2018;35:2133-2139
6. Hotaling JM, Walsh TJ. Nat Rev Urol 2009;6:550-556
7. Eisenberg et al., J Urol 2015;193(5):1596-1601
8. Eisenberg et al., Hum Reprod 2014;29(7):1567-1574
9. Hopkinson et al., BMJ 1998317(7154)329-332
10. Butts et al., J Clin Endocrinol Metab 2009;94(12)4835-4843
11. Cedars et al., Hum Reprod Open 2017;2:hox008
12. Falick Michaeli et al., Fertil Steril 2015;103(5):1125-1128
13. Falick Michaeli et al., Aging Cell 2015;14(4):698-700
14. Zhong JF, Weiner LP. Gene Reg Syst Biol 2007;1:111-115
15. Chen et al., World J Mens Health 2022;40(3):490-500
16. O’Kelly et al., Circ Res.2022; 130(4):652-672
17. Farland et al., J Am Heart Assoc 2023;12(5):e027755
18. Choy JT, Eisenberg ML. Fertil Steril 2018;110(5)”810-814
19. Boeri et al., Andrology 2022;10:128-136
20. Elenkov et al., Sci Reports 2021;11:18526
21. Wang et al., Lancet Reg Health -Americas 2022;7:100122
22. Murugappan et al., Fertil Steril 2022;117(5):1038-1046
23. Zahid et al., Am J Cardiol 2023;186:126-134
a recent study in infertile women undergoing treatments with assisted reproductive technologies between 2008 and 2019 had a higher risk of preeclampsia, heart failure, arrhythmias, and stroke during delivery hospitalizations.23
Here-described associations between female as well as male infertility and subsequent risk for several medical conditions, also linked to variations in lifespan, appear intriguing and support the hypothesis that infertility has the expected relevance for “survival of the fittest” and, therefore, for evolution. This area of research appears especially well suited for so-called polygenic risk scoring, a relatively new technology involving whole genome amplification to predict the risk of developing a disease at more advanced ages based on polygenic mutation profiles. The VOICE discussed polygenic risk scoring in recent months repeatedly, though within the very different context of risk scoring preimplantation-stage human embryos for medical risks, blue eyes, etc. Unsurprisingly, though already offered by several IVF centers and genetic testing laboratories in the U.S. as a purchasable test akin to PGT-A, the CHR considers such testing in human embryos currently unethical. Such risk scoring of infertile in comparison to fertile individuals, however, could be of potentially great importance
“Such
of infertile in comparison to fertile individuals, however, could be of potentially great importance...”
We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO (jtapper@thechr.com). All submissions are considered confidential correspondence.
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

With over 90% of new patients arriving at the CHR after a usually lengthy infertility journey through often multiple prior IVF cycles, frequently at several different IVF clinics, the one question that sooner or later always comes up in a first consultation is, “and what does the CHR do differently from what we experienced in prior IVF rounds?” And the answer to that question usually is also always the same, -“almost everything,” and that is by no means an exaggeration. A summary of all of these differences in an article in the VOICE, therefore, seemed timely. In the June issue, we will follow up with an article that will describe what the CHR does differently from other fertility centers outside of IVF.
Before addressing this subject, it is important to point out that the CHR has several potential economic conflicts in addressing some of the subjects in this article. These conflicts relate to the ownership of several U.S. user-patents by the CHR and some of its employees, as well as the receipt of royalty payments from some of these patents by the CHR and/or some of its employees from Fertility Nutraceuticals, LLC. Most of these patents claim treatment benefits from supplementation of selected infertile women with androgen hormones, especially dehydroepiandrosterone (DHEA) but also other androgens. Other patents refer to anti-Müllerian hormone treatments in infertile women. Employees of CHR and the CHR also own shares in Fertility Nutraceuticals, LLC. We, therefore, advised our readers to consider these potential conflicts especially when reading this article about the clinical utilization of androgens and, especially, of DHEA.
The general principles of IVF at the CHR, of course, remain the same: Ovaries are stimulated with fertility medications (in principle gonadotropins) and follicle growth is carefully monitored with ultrasound and blood tests for estradiol, but beyond that, almost everything else at the CHR greatly differs for most patients when compared to other IVF clinics. Changes do not only start during the IVF cycle; they already start 6-8 weeks before IVF cycle start because one of the big differences in how the CHR views the IVF process is based on the recognition that in many women, the performance of their ovaries can and must be maximized before cycle start.
What is meant by this statement is the assumption that ovaries, often, do not
Continued on page 12
perform at peak-performance levels and that pretreatments prior to IVF cycle start, therefore, can improve their performance. This concept specifically applies to two possible pre-cycle supplementation schedules, supplementation with androgens (male hormones) and supplementation with human growth hormone (HGH). Both of these treatments have remained controversial as general concepts; when using these treatments, the CHR, however, moreover follows a quite different protocol than most other IVF centers.
We here are not planning to address the rationales for discussed treatments. The rationale for androgen supplementation has, indeed, been repeatedly presented on these pages and in the literature.1
Our standard supplementation dosage is DHEA 25mg TID, orally, though we recently noticed a suspected increase in the potency of DHEA and, therefore, often see a need to reduce the dosage after an initial four weeks of supplementation.2
Like every medication, DHEA will only be effective if patients have a reason for taking it, which in this case, is hypo androgenism. Therefore, supplementation does not make sense in women with normal testosterone and SHBG levels. If supplementation is indicated, it, however, must be started at least 6-8 weeks before the IVF cycle start. The reason is that androgen synergy with FSH on granulosa cells is especially pronounced at small-growing follicle stages (primary through small antral follicle stages). These follicles, however, require still at least 6-8 weeks to reach gonadotropin sensitivity, which makes them responsive to gonadotropin stimulation. If androgens are supplemented for shorter time periods, future generations of follicles beyond any next IVF cycle will benefit, - but not the intended cycle. Once we initiate androgen supplementation, we continue uninterrupted until the patient is pregnant or terminates fertility treatments with autologous oocytes. We strongly prefer supplementation with DHEA rather than transdermal testosterone gel because over-dosaging is almost impossible with DHEA, but very easy with testosterone.
Like androgens, HGH works through insulin growth factor-1 (IGF-1)
synergistically with FSH on granulosa cells, thereby enhancing the growth and development of follicles, especially during small-growing follicle stages. Since HGH does not directly act upon the ovary, whether HGH supplementation is indicated, therefore, does not depend on whether HGH levels are low but whether IGF levels are low.
For inexplicable reasons, this fact is not widely appreciated and HGH is often used in women with perfectly normal and, at times, even abnormally high IGF1 levels. That makes therapeutically little sense because it is like giving a patient aspirin for a headache who really does not have a headache. Unsurprisingly, the literature is, therefore, mixed in reported results from HGH supplementation.
The CHR considers HGH supplementation very effective if IGF-1 levels are really low (at the CHR, <125ng/mL) and supplements at a dosage of 3IU/ day, once again, and for the same reason, for at least 6-8 weeks before cycle start, uninterrupted till pregnancy. In contrast to androgen supplementation, which is almost universally indicated in all women above age 40 and most younger women with premature ovarian aging (POA), HGH supplementation is, only rarely indicated, and not even necessarily age-related.3

The CHR applies a variety of stimulation protocols, usually highly individualized to every patient. Some principal differences, however, do apply in comparison to most other IVF centers. For example, most IVF centers use GnRH-antagonists routinely; having become convinced that antagonists adversely affect oocyte quality and that this is especially the case in older women and younger women with POA, the CHR hardly ever uses antagonists, - not even in egg-donor cycles.
Convinced by Pharma-interests, most IVF clinics almost exclusively stimulate only with FSH products. The CHR, in contrast, stimulates almost all patients with a combination of FSH and hMG (FSH/LH mixture) and strongly believes
that at least older ovaries must receive some LH. Moreover, the CHR interprets the literature as demonstrating that practically every decently planned head-to-head comparison of an hMG product with a pure FSH product, uniformly demonstrated outcome benefits in pregnancy and live birth rates for the hMG product. Pergonal, Serono’s, and the world’s original gonadotropin is by many old-timers in IVF, to this day, considered to have been the best gonadotropin ever introduced to the market. The importance of LH in ovarian stimulation is also confirmed by improved pregnancy rates with pure FSH stimulation if LH is added.4
makes absolutely no sense.
The CHR also does not subscribe to related concepts, like the allure that milder stimulation produces better quality eggs and that gonadotropin dosages above 225 IU/day do not further increase egg numbers. CHR investigations have demonstrated a pretty straight correlation between gonadotropin dosage and egg numbers up to a dosage of 450 and, possibly, as much as 600 IU.5 The younger the patient, the longer in higher dosages the correlation is maintained. In older patients, when higher dosages, indeed, may no-longer add egg numbers, milder stimulation may also have the additional advantage of prolonging, otherwise, very short cycles.
Almost since the inception of IVF, a to these days mostly maintained dogma has been that IVF cycles should be triggered at lead follicle sizes of 18-23 mm, with the goal being to obtain a high as possible percentage of mature (so-called M2) oocytes and as few a possible immature (M1) and very immature (GV) oocytes. The CHR’s researchers questioned this dogma for the first time in in 2015, when they for the first time reported that follicles prematurely luteinize increasingly early with advancing female age, suggesting that in order to prevent this from happening, retrievals must be performed earlier and earlier.6
As steady readers of the VOICE are likely already aware, the CHR also does not support the concept of mild ovarian stimulation and/or natural cycle IVF. The only exception considered is the young patient with a normal ovarian reserve who wants only one more pregnancy. In all other cases, mild stimulation is considered inferior to standard stimulation protocols and the principal rationale is obvious: after female age, the number of available embryos for transfer is the second-most important predictor of pregnancy and live birth chances.
To, a priori, voluntarily reduce egg and embryo numbers in an IVF cycle because of milder stimulation, therefore, logically
They confirmed these findings in 2018 in a follow-up study, furthermore demonstrating that such earlier retrievals did not increase percentages of immature MI and GV oocytes to significant decrees but extended pregnancies and deliveries following the use of autologous oocytes into more advanced ages.7 The CHR, therefore, currently holds the world record for the oldest woman to have conceived in IVF with the use of her own eggs less than 2 weeks short of age 48 and having delivered a healthy baby close to age 49.8
CHR’s investigators have given this revolutionary treatment the acronym HIER (highly individualized egg retrieval), and it has been routine treatment at the CHR since 2015, with triggers at lead follicle
sizes as small as 11-12 mm having become a daily routine. Remarkably, even though the CHR’s researchers have published extensively on this subject, so far, at most, only a handful of IVF centers worldwide have followed in the CHR’s footsteps in this regard, among them a prominent center in The Emirates, a small center in Europe, and an academic center in Florida. But reading this month’s “A Piece of My Mind” by the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, who can be surprised? After all, it takes on average 17 years to bring newly developed scientific evidence into clinical practice,9, 10 and we are here only in year 8!
While almost all IVF clinics routinely try to culture all embryos to blastocyst-stage, the CHR considers such an approach poorly advised. Once again quite regularly discussed in the pages of The VOICE, we do not wish to be repetitive and, therefore, only so much: The CHR simply does not believe that it is in the best interest of patients to have all of their embryos routinely cultured to blastocyst if achieving maximal pregnancy and live birth chances is their primary objective. The reasoning is also, once again, simple, and straightforward:
(i) Cumulative pregnancy and live birth chances from all embryos in an IVF cycle cohort are always a little better with cleavage-stage than with blastocyst-stage transfer. (ii) Outcome advantages in first-cycle transfers for blastocyst culture to this day were only demonstrated in good-prognosis patients and even there they were minimal. (iii) The older a patient and/or the poorer her functional ovarian reserve (FOR) is, the less likely will her embryos survive extended culture to blastocyst. Such culture, therefore, in such patients eliminates even relatively small pregnancy chances with cleavage-stage transfers.
The CHR, therefore, practically never cultures embryos to blastocyst-stage in
women above age 40 and/or in younger women with low FOR. In contrast to many other IVF centers, the CHR in poor prognosis patients also avoids embryo cryopreservation, unless a patient produces more embryos than can be transferred in a cycle. The reason is that the poorer eggs and embryos are, the poorer they freeze and thaw, and the bigger the embryo loss will, therefore, be from cryopreservation.
From this statement, it will also already be obvious that the CHR also does not support the concept of elective single embryo transfer (eSET) except in young, good prognosis patients and, of course, in patients who do not wish to conceive twins and/or have medical contraindications to carrying twins. In all other cases, the CHR considers twins a desirable IVF outcome, - but, of course, only for women/couples who are desirous of twins.11
Differences in how the CHR pursues the IVF process are, however, not only limited to the clinical side but also involve the embryology laboratory and, in many ways, are not less profound. Like on the clinical side, our laboratory is also fully committed to the CHR’s motto, “we fight for every egg and embryo.” In practice, this means what the motto states: every egg and every embryo count in a universal attempt to bring as many embryos
to transfer into the uterus as possible. This, of course, does not mean transferring “garbage” embryos with no realistic chance of pregnancy, but it does mean that every known effort will be made to achieve in an IVF cycle the largest number of potentially transferrable embryos.
This also means that the CHR manages its embryology laboratory conservatively. What we mean by that is that we do not automatically jump at every new “fashion of the moment.” Indeed, whenever the literature suggests a possible improvement for embryology laboratories, the CHR, first of all, tests it out in-house. Substantial changes in the laboratory’s routine are then only implemented if those in-house tests confirm the claimed outcome advantages.
There are two principal reasons for being so cautious: First, much of what is published in even good medical journals is often simply not true. “Fake news” is as prevalent in medical publishing as, likely, in all publishing venues. But there is also a second important reason for the CHR’s caution that specifically and almost exclusively only applies to the CHR, and that is the center’s unique patient population. What we learned already many years ago is that what works in general infertility populations does not always work in patients strongly adversely selected out because of poor prognosis. And since such patients represent the vast majority of the CHR’s patient population,
“Like on the clinical side, our laboratory is also fully committed to the CHR’s motto, “We fight for every egg and embryo.” In practice, this means what the motto states, every egg and every embryo count in a universal attempt to bring as many embryos to transfer into the uterus as possible.”
what works at other IVF clinical does not always also work at the CHR. The wisdom of CHR’s caution was just very recently once again confirmed when Dutch colleagues published a paper in the prestigious medical journal, The Lancet, convincingly demonstrating no outcome benefits from uninterrupted embryo culture in contained incubators with concomitant time-lapse-based embryo selection,12 – originally named “embryoscope” after the first such instrument brought to market around 2015. Convinced by highly biased marketing efforts by several companies, many – if not most – IVF centers in the U.S. immediately converted their embryology laboratories. Cautious as always, the CHR, however, secured a loner instrument from one of the companies and conducted two prospective, controlled studies in donor egg cycles (representing “best” ovaries) and in regular patients (representing the center’s usually adversely selected patient population), neither of which demonstrated any of the promised advantages for the instrumentation. On the contrary, in good-prognosis egg donation cycles, egg quality, indeed, deteriorated. On the economic front, promised time savings in the embryo laboratory also were not apparent. The center’s embryologists, indeed, spent significantly more
time on every cycle than they did with standard manual embryology. The CHR published these data13 and, obviously in contrast to other IVF centers, did not convert the lab to closed incubation.
On a side note, in doing so, the CHR not only saved the CHR significant expense, but also saved our patients from significant additional IVF cycle costs because many IVF clinics added special charges to their IVF cycle fees for use of these systems in order to appreciate the purchasing costs for these units (in excess of US$ 100,000 per unit). One prominent IVF clinic in NYC was alleged to have bought not less than 10 such units. One wonders how they feel after the publication of the recent paper from The Netherlands.12

At the clinical practice level, the biggest difference in the CHR’s embryology laboratory is probably the handling of immature oocytes. Many IVF center, still, discard many, if not most, immature eggs. The CHR for years has not been following such a policy and attempts to mature in vitro every immature egg, whether an M1 (mildly immature) or a GV oocyte (very immature).14 In the process our researchers recently made some very surprising and consequential observations that have not been
known so far. We, currently, cannot reveal those yet because a manuscript describing these findings is currently under review. Once the manuscript is published, we, however, will, of course, immediately report on these findings on these pages.
In summary, the CHR, indeed, “fights for every egg and embryo!”
1. Shohat-Tal et al., Nat Rev Endocrinol 2015;11(7):429-441
2. Gleicher N, Barad DH. F S Reports 2023l In press;
3. Gleicher et al., J Assist Reprod Genet 2022;39(2):409-416
4. Mochtar et al., Cochrane database Syst rev 2017;5(5):CD 005070
5. Gleicher et al., Reprod Biol Endocrinol 2012;10:48
6. Wu et al., J Endocrinol 2015;226(3):167-180
7. Wu et al., J Ovarian Res 2018;11(1):23
8. Gleicher et al., Reprod Biomed Online 2018;37(2):172-177
9. Morris et al., J R Soc Med 2011;104(12):510-520
10. Green et al., Annu Rev Public Health 2009;30:151-174
11. Adashi EY, Gleicher N. Ranbam Maimonides Med J 2017;8(2):e0022
12. Kieslinger et al., Lancet 2023; S01406736(23)00168-X. doi: 10.1016/S01406736(23)00168-X.Online ahead of print.
13. Wu et al., Reprod Biol Endocrinol 2016;14:19
14. Lee et al., Endocrine 2016;52(1):165-171
The whole staff is attentive, supportive, and vested in a positive outcome for their patients. So glad I switched from my local clinic... it has still been a tough journey, but worth it!
- DBDr. Norbert Gleicher took his time to explain all options available to us. He is also extremely patient when my husband and I had some additional questions for him..

We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratorysupervisor for research


To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

Have you thought about advertising in the VOICE?
This newsletter every month goes electronically to ca. 80,000 infertility patients, medical professionals in the field, and members of the media, with over 25% (an unusually high number) also opening the VOICE.
For further information, please contact: Ms. Alexandra Rata (212) 994 4400 or e-mail to arata@thechr.com


Fundamental to all biological systems is the ability of cells to divide. For sperm and eggs, these divisions occur through a specialized process known as meiosis, something that we only see in the testis or ovaries. For the remaining cells of the body, the process that yields two daughter cell from one mother cell is known as mitosis. In this image of a filed of actively dividing HeLa cells, the structures upon which chromosomes (green) first converge and then separate are known as microtubules (magenta), a vital part of the living cells’ infrastructure that performs more tasks than those assigned to it during mitosis.
Here we see an illustration of microtubules in a human oocyte demonstrating the pattern of organization expected prior to the oocyte undergoing its meiotic division as mentioned above. Note the microtubules (green) fill the oocyte cytoplasm and surround the nucleus (also known as the GV or germinal vesicle, blue) and closely abut the outermost region of this large cell which is stabilized by a network of actin filaments (red).

Once the egg is fertilized, it begins its journey to become a blastocyst by undergoing a series of mitotic divisions. In this image of a compacting morula (taken from earlier published studies from Dr Albertini’s lab) one cell (upper right) has initiated the process of mitosis while its neighbors are uniformly maintained in a non-dividing state, awaiting their signal to proceed into mitosis. By studying the mechanics of cells in gametes and embryos using these kinds of imaging tools at the CHR, we hope to gain a more complete understanding of why and how human gametes and embryos are prone to making errors during such a vital part of our reproductive legacy.

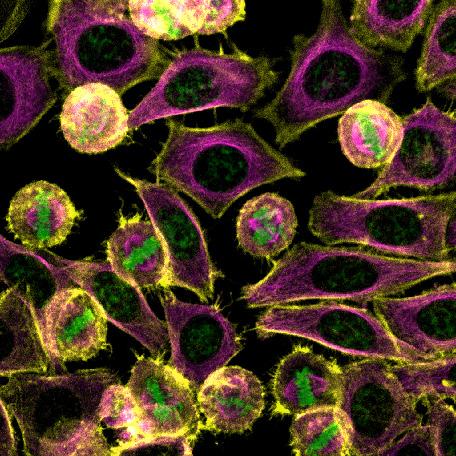 Image 2
Image 3
Image 2
Image 3

 By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist The CHR, New York, N.Y.
By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist The CHR, New York, N.Y.

That the translation of obtained evidence into clinical practice takes too long has been decried for decades.1 It indeed has been repeatedly suggested that the average time between the development of evidence and clinical translation is 17 years.1, 2 In many cases it can take even longer even when seemingly shorter (for example, in the record-breaking development of mRNA-based anti-COVID-19 vaccines), basic concepts (in that case using mRNA to induce immune responses) have often already been around for decades. Had the pandemic not mandated a speedier response than usual to develop vaccines, the world would, likely, still be mostly unaware of the possibility of mRNA-based vaccines: mRNA, indeed, by 2014 was already satisfactorily defanged off its instability and strong immunogenicity to permit clinical applications in humans.3
Continued on page
The shortcomings of evidence-based medicine make the long time to translate evidence into clinical practice worthwhile
An average translational time period from bench to clinical practice of 17 years is, therefore, of course, just a rough estimate, as some investigators suggested, “hiding complexities relevant to policy and practice which would benefit from greater understanding.”1 A recent article in JAMA by science writer Rita Rubin, brought the subject again to the forefront. In the process, she introduced many readers to a new field of study in medicine, - so-called “implementation science.”4 Aware of the 17-year translational timespan for evidence to reach clinical practice, I was, nevertheless, surprised to learn from her article that only 1 in 5 so-called evidence-based interventions then makes it into routine clinical practice.4 As her article also points out, this evidence-to-practice gap, unsurprisingly, is even more pronounced in historically marginalized populations.
“Implementation science” can be viewed as an outgrowth over the last few decades of the worldwide fascination with so-called evidence-based medicine. As a new field of expertise in the medical universe, it is supposed to facilitate the timelier spread of evidence-based practice.5 Like in most new medical specialty areas, these developments spawned a new medical journal, Implementation Science, and NIH already in 2007 organized a conference around this new specialty. New specialty areas, of course, quickly also breed new “specialists,” representing this new knowledge base and becoming advocates for the field’s interests. Those specialists, of course, also become the “experts” in this new field of medical practice and/or science.
The significant limitations of expert opinions, relegating them to the very bottom of the evidentiary pyramid in medicine, have been a subject of special interest in this newsletter on repeated prior occasions. The principal reason for this repeated attention is that, despite supposed almost universal support for practicing evidence-based medicine, the truth is that expert opinions still prevail in all specialty areas throughout medicine.
In our specialty area of reproductive medicine and infertility one, for example, just has to look at the leading medical journal in the field, Fertility and Sterility, the primary official journal of the ASRM. Though obviously propagating evidence-based medicine, over the recent decade expert opinion articles (commentaries, editorials, invited review articles, etc.) have steadily increased and original research articles, of course, decreased in parallel. This did not only happen on the margins, but to very substantial degrees, with some issues of the journal expert-opinion pages exceeding pages of original research.
Often addressed over centuries by philosophers and more recently mostly in behavioral science articles, the subversive opinions of experts have, however, rarely been better described in the literature than by the British political theorist Harold J. Laski (1893-1950), who in 1931 in his pamphlet in the Fabian Society noted that “experts” bring with them “an occupational disability,” which he defined as the inability to see beyond their
particular subject (i.e., their small area of expertise and, at times, personal interests as well as economic conflicts). As a consequence, they become intolerant of novel views, especially if they come from “experts” in other fields. They lack humility and, consequently, “fail to see the obvious before their very noses.” Moreover, their proposals for what to do about facts are often rooted in unexamined premises, not shared by those likely most affected. In short, “the expert tends to make his (her) subject the measure of life, instead making life the measure of his subject.” 6
This, of course, not only sounds familiar but, among many other areas of human existence, describes well the environment of current medicine in all of its corners, including our own medical specialty: How many “unexamined premises” have we, for example, been presented with by “experts” regarding preimplantation genetic testing for aneuploidy (PGT-A) over more than 20 years? Indeed, how many so-called “add-ons” to IVF have “experts” been selling us, especially since roughly 2010?
It, therefore, seems somewhat curious when Rubin in her article suggests that the COVID-19 pandemic offered good examples for the purposes of “implementation science.” One, indeed, would hope the pandemic taught the public health community better than that: If COVID-19 has taught us one important lesson, then it is the danger of relying on only a few experts. As Laski almost 100 years ago already perfectly well understood, the world, of course, needs experts; their expertise, however, must be consumed with appropriate caution. Nothing demonstrates this argument better than a retrospective comparison of expert opinions that ended up determining policy during most of the COVID-19 pandemic, contrasted with opinions of equally-well qualified experts who not only were not listened to, but who, often, were muzzled by state and federal government, media, and – yes – professional medical organizations and medical journals. Such an objective retrospective comparison would, of course, clearly demonstrate that the latter group was more often correct, reaffirming Laski’s point that “experts” serve an important function in governance, but only if decision-makers listen to “experts” with widely diverging opinions before reaching final conclusions.

If one considers how evidence is established in medicine, the process is at every step basically driven by “experts:” They select study themes, devise study protocols, analyze data, write manuscripts, review manuscripts, and accept or reject manuscripts for publication. These are only the preliminary steps in evidence development. What follows are study selections for literature reviews and meta-analyses, and, of course, their interpretations and analyses before publication. The end-product of this lengthy process then again undergoes peer review which ends with either acceptance
Continued on page 19
or rejection. What then is published especially in more prestigious journals heavily contributes to the establishment of “evidence” and “standard of care.” The final determining factor is, however, usually one additional “expert”- driven process, - the issuance of guidance documents by professional organizations.
Common wisdom considers this process to be objective; but on closer examination, it is important to reemphasize that, in every phase, of this lengthy process, it is exclusively driven by small groups of “experts” and, therefore, deeply endangered in objectivity by the inherent biases of those “experts”. These biases have been, likely, best investigated and described in the literature regarding the peer review process that submitted manuscripts undergo. A few simple examples probably suffice: With few exceptions, editors are mostly still in part-time positions; in most of their professional life, they are, therefore, practicing physicians and/or bench scientists with opinions about what is going on in their field. In other words, like any other “expert,” they have inherent biases. Those biases will influence their choice of reviewers, favoring reviewers with similar opinions to their own and, thereby, actually amplifying their own opinions (experienced editors, indeed, in most cases can predict their reviewers’ responses). These “expert” biases then continue amplifying themselves throughout the complete, above-described evidence development process because it is human nature to preferably choose “more similar” than “more distant” people to participate in decisions within our social and professional environments.
To assume that the concept of evidence-based practice is pure, precise, and absolute is, therefore, not credible. Of course, it also can never be timely because the publication of even only a single contradictory study currently considered “best practice” and/or “best evidence” in a prestigious medical or science journal can radically change what just the day before was fully accepted as such.

Returning to the original question, whether 17 years between the development of evidence and (only 1/5) treatments reaching general practice, is too long a timespan and should be shortened, I have to acknowledge that I have somewhat changed my mind: Though 17 years still does appear to reflect an unnecessarily long process, I no longer view the length of time as the quintessential problem. Biases in defining what at any given moment represents “best evidence” appear to me like a much bigger and more costly problem (cost-savings have been a basic argument for “implementation science” and speeding up the process1).
One, indeed, can argue that counting on “the wisdom of the crowd” (involving physicians as well as patients), a lengthier process, at least to a degree, will filter out garbage science

and maintain the more valid treatments. Isn’t it, indeed, rather astonishing that the above-outlined current process of evidence development at no point involves patients and/ or the general public? Such a conclusion is also supported by current estimates that only less than 20% of general medicine is based on established best medical evidence.7 It is also supported by the fact that currently, well over 90% of all IVF centers will refer women over ages 42-43 automatically into third-party egg donation and/or follow in large majority treatment schemes which simply make no logical sense. An interesting study would, indeed, be a prospective comparison of which practice patterns become obsolete quicker, those based on alleged high-quality evidence or those which have, simply, risen to the top because physicians and their patients have concluded that they work well. I wouldn’t bet against the latter.
REFERENCES
1. Morris et al., J R Soc Med 2011;104(12):510-520
2. Green et al., Annu Rev Public Health 2009;30:151-174
3. Brachet J. In the Nucleic Acids (ed. Chrgaff E, Davidson JN), Vol 2, pp. 475-519, Academic Press, New York
4. Rubin R. JAMA 2023;doi:10.1001/jama.2023.4387
5. Bauer et al., BMC Psychol 2015;3(1):32
6. Laski HJ. Society 2020;57:371-377
7. Ebell et al., Evid Based Med 2017;22(3):88-92





Ozone therapy is not only a very controversial alternative medicine treatment, but the Food and Drug Administration (FDA) actually prohibits all medical uses of ozone in any medical condition in which the use of ozone has not been verified as an effective treatment.1 The reason is obvious: ozone is a toxic gas with no known useful medical application (and this included infertility). In order to have germicidal effects, the gas must be used in concentrations known to be toxic for animals and humans.
There is no serious evidence in the medical literature that would suggest effectiveness in any gynecological condition, including infertility. Claims thrown around in some dubious review articles in low-level medical journals lack serious support.2,3 We, therefore, would consider offering ozone therapy to women who are trying to conceive to be not only potentially harmful but unethical, and strongly recommend against consuming such “treatments.” Unfortunately, they are offered by a very small number of infertility centers; but even one would be too much!
REFERENCES
1. Code of Federal Regulations Title 21 Sec. 801.415 Maximum acceptable level of ozone (FDA Website, as of 2022-07-18)

2. Merhi et al., Med Gas Res 2019;9(2):101-105
3. Merhi et al., Medicine (Baltimore). 2019;98(34):316865
According to the Centers for Disease Control and Prevention (CDC)1 and the World Health Organization (WHO), 2 infertility is, indeed, rapidly increasing all around the world, with the WHO increasing the prevalence from 1:8 to 1:6. Annual reported increases have been running at approximately 5% which, if correct, would be huge and, obviously, unsustainable.
Continued on page 21
Some causes are known and very obvious, others are suspected and more controversial, and much – we are convinced – is actually still unknown. A clearly contributing factor to reported increases in prevalence are women having children ever later in life. As recently as in the 1950s, if women developed infertility after age 35, they celebrated because they already had all the children they wanted. Nowadays, most women only start thinking about having children at age 35 (or even later). With these women now being counted as infertile, the number of infertile women requiring treatment, indeed, increases significantly. These women, however, do not reflect “more” infertility in the population because; because of later childbirths, a presumed similar prevalence in the population now, however, requires more therapeutic interventions. Had these women, like their forebearers, conceived earlier in life they nowadays would not be patients requiring infertility treatments.
The announcements by CDC and WHO, that the prevalence of infertility (in the population) is dramatically increasing, therefore, may technically be correct; but etiologically it, likely, is not or is, at least not as pronounced. There, indeed, is objective evidence that human fertility is declining. For example, after almost a decade of small declines, the prevalence of pelvic inflammatory disease (PID) is once again on the rise,3 and more PID leads to more tubal infertility. Even more concerning is the significant decline in worldwide sperm counts,4 obviously contributing to a significant rise in male infertility.5
Why sperm counts are dropping so quickly is also not clear, though endocrine-disrupting chemicals are widely held responsible6 and are also believed to affect female fertility.7
REFERENCES
1. https://www.cdc.gov/reproductivehealth/infertility/index.htm
2. https://www.who.int/health-topics/infertility#tab=tab_1



3. Kreisel et al., J Infect Dis 2021;224(Suppl2):S103-S112
4. Levine et al., Hum Reprod Update 2023;29(2):646-659
5. Ravitsky V, Kimmins S. Biol Reprod 2019;101():872-874
6. Rehman et al., Transl Androl urol 2018;7(3):490-503
7. Rattan et al., J Endocrinol 2017;233(3):R109-R129
Any discussion of Hashimoto’s thyroiditis must start with the fact that this condition is an autoimmune disease. Why is this important? Because almost no other group of diseases (except for, maybe, cardiovascular diseases) has as intimate a physiological relationship with pregnancy as autoimmune diseases.
2 Fathers - 1 Family

The principal reason for that is obvious: In autoimmune diseases, the immune system is hyperactive, with most of this hyperactivity directed against a patient’s own body/tissue components. When our immune system is activated, it usually responds to a stimulus from one or more epitopes. This is also what happens when an embryo implants, which under most basic rules of immune function should be rejected by the maternal immune system because of the 50% “foreign” paternal genetic contribution to the embryo (100% “foreign” if the embryo is the product of a third-party egg donation).
The purpose of activation of the maternal immune system is to induce so-called tolerance pathways which make the growing embryo “invisible” to the maternal immune system and, therefore, prevent its immunological rejection. The current hypothesis is that at least some of these induced tolerance pathways share functional features with autoimmunity, which would explain why some autoimmune diseases have been suggested to improve during the mid-trimester of pregnancy.1
As an autoimmune disease, Hashimoto’s thyroiditis, therefore, shares many pregnancy complications with other autoimmune diseases. Those include among others increased risk for the following: Pregnancy loss (likely because of deficiencies in initial tolerance pathway induction), premature labor and delivery (likely the result of premature termination of immunological tolerance), small for gestational age infants, preeclampsia, third-trimester gestoses of pregnancy, and as one of the most characteristic risks, late in pregnancy and especially postpartum flares of disease for up to 5 months post-delivery and/or first clinical occurrence of disease. No other autoimmune disease, indeed, demonstrates this risk as profoundly as autoimmune
thyroid disease, which can take the form of hyper- or hypothyroidism or even present as hyperthyroidism that over weeks turns into hypothyroidism (the so-called thyroid postpartum syndrome), highly associated with the presence of anti-thyroid peroxidase (TPO) antibodies. These antibodies, apropos, are also highly associated with the risk to develop postpartum depression.
Hashimoto’s thyroiditis is an autoimmune disease where immune activities are directed specifically against the thyroid gland (the disease is characterized by so-called “thyroid-antibodies, mostly anti-thyroglobulin (TG) and anti-TPO antibodies. The presence of these autoantibodies is usually (though not always) associated with hypothyroidism (abnormally low function of the thyroid gland). Autoimmunity against the thyroid and abnormal thyroid function, therefore, represent distinct disease states of the thyroid, even though they often correlate. Pregnant women can be hypo- or hyperthyroid (the latter mostly characterized by anti-thyroid receptor antibodies) with or without concomitant autoimmunity, and thyroid autoimmunity may be present despite normal thyroid function.2
Antibodies to the thyroid, and especially TPO antibodies, however, even in absence of abnormal thyroid function, almost uniformly are indicative of future abnormal thyroid function, which frequently for the first time presents clinically late in pregnancy and especially postpartum. Patients with Hashimoto’s thyroiditis, whether their thyroid function is normal or not, therefore, must be carefully followed regarding their thyroid function with TSH tests in every trimester of pregnancy and up to 5-6 months postpartum. If hypothyroid and on thyroid hormone supplementation, medication dosages will likely have to be adjusted up in pregnancy (because of significantly expanding plasma volume in pregnancy) and down again after delivery.3
Hashimoto’s thyroiditis also shares one more characteristic with other autoimmune disease: Patients who have one autoimmune disease are at greatly increased risk of also developing other autoimmune diseases. Once again, those secondary autoimmune diseases often present peripartum and especially postpartum. The combination of two autoimmune diseases is called “poly-autoimmunity” (Hashimoto’s is often associated with rheumatoid arthritis, for example); an association with three or more autoimmune diseases is called the “multiple autoimmune syndrome,” separated into Types 1 through 3. Hashimoto’s thyroiditis is included in Type 2, which includes in addition to Sjögren’s syndrome, rheumatoid arthritis, primary biliary cirrhosis, and scleroderma.4 A very recent study, also briefly discussed in our literature review section of this newsletter, demonstrated, using several genetic approaches, a strong genetic relationship between hypothyroidism and systemic lupus erythematosus (SLE).5
Finally, one more distantly related issue: The CHR’s investigators already many years ago pointed out the many similarities, endometriosis, one of the most consequential diseases of women in reproductive years, shares with autoimmune diseases.6 A recent paper in Scientific Reports pointed out that women who suffer from endometriosis in presence of concomitant autoimmunity demonstrate more severe stages of endometriosis.7 This point is made here also within the context of women demonstrating a much higher prevalence of autoimmune diseases than men.8
1. Tan et al., J Autoimmun 2022; 132:102864
2. De Leo S, Pearce EN. Lancet Diabetes Endocrinol 2018;6(7):575-586
3. Mammen JSR, Cappola AR. JAMA 2021;325(23):2392-2393
4. https://www.autoimmuneinstitute.org/comorbidities-multiple-autoim mune-syndrome/Liu et al., J Clin Endocrinol Metab 2023;108:941-949
5. Gleicher et al., Obstet Gynecol 1987;70(1):115-122
6. Stell Vanni et al., Sci Reports 2021;11:15372
7. Xing et al., J Invest Dermatol 2022;142(3Pt B):857-866

CoQ10, a so-called antioxidant, is very widely prescribed in female infertility. Its use in male infertility is, however, less well appreciated. This is despite the fact that there is considerable evidence that oxidative stress is closely associated with poor seminal fluid quality. Antioxidant therapy is, therefore, considered a cornerstone of empirical treatments of male infertility.1
The CHR uses CoQ10 as its preferred antioxidant in cases of male infertility and uses it at the same dosage as prescribed for women (600mg/day).

REFERENCE
1. Salvio et al., Antioxidants (Basel) 2021;10(6):874

That the U.S. is in the midst of a gigantic mental health crisis is easily apparent in the streets of many of our cities. Unprecedented numbers of homeless, many obviously afflicted by psychiatric diseases, are visible everywhere. But that is only the visible tip of a much larger iceberg. According to an in 2020, amid the COVID-19 pandemic, released CDC study, 40% of adults in the U.S. reported seriously struggling with mental health challenges and/or substance abuse.1 It appears that things since then have only gotten worse: Over half of all teen girls (57%) persistently feel sad and hopeless – double the rate of teen boys, in itself already a much too high number.2 The United Nations Foundation attributes much of the crisis to the COVID-19 pandemic.3 Though the pandemic unquestionably contributed, it appears irrational to overestimate this contribution since especially our younger generation of teenagers and young college students already expressed abnormally high mental health problems even before the pandemic.
One more general comment: The treatment of psychiatric disorders appears to stand on the verge of revolutionary changes because of an emerging circuit-based understanding of psychiatric diseases, recently very well-reviewed in a Nature Medicine article which summarized the progress made with truly revolutionary brain-circuit-based interventions leading the way toward precision psychiatry.4
That mental health, especially persistent depression (dysthymia) and anxiety, and infertility are linked, has been acknowledged for a long time and, though clear differences exist between women and men, and clinical expression varies with the permanency of infertility.5 Psychotherapists, therefore, unsurprisingly have suggested a role for psychotherapy in infertility situations.6 Studies on the subject are, however, limited in quality as well as numbers.7 A long-term follow-up study 20-23 years following IVF treatments demonstrated most patients to be in good mental health. Childless women or those who are left without a partner even later in life, were, however, still found to be vulnerable.8 Another long-term study reported similar results: While most women demonstrated resilient trajectories during and after IVF
treatments, 37% showed temporary or chronic maladjustments during IVF and 10% remained maladjusted 11-17 years post-IVF.9 It appears that follow-up mental health care should especially concentrate on those women (and men) who failed fertility treatments.
Antipsychotic drugs can induce hyperprolactinemia which, in turn, can cause infertility by reducing estradiol levels and causing ovarian dysfunction and lack of ovulation, leading to menstrual irregularities or even complete amenorrhea (cessation of menses). The following medications are known to do this: chlorpromazine, prochlorperazine, haloperidol, risperidone, metoclopramide, methyldopa, cimetidine, some older antidepressants like amitriptyline, SSRI antidepressants like sertraline or fluoxetine, and many others In males, anti-psychotic drugs in a recent study resulted in lower sperm counts and poorer progressive motility.10
The CHR is cognizant of recipients being very concerned about donors with mental health issues of any kind and/or with family histories of mental diseases. The CHR, therefore, disqualifies such donors from joining the center’s egg donor pool. We recognize that many donor candidates with selected mental health issues would, still, make excellent donors but their health issues would have to be disclosed to recipients, with an overwhelming majority of recipients then rejecting them. Other causes for rejection of donors at the CHR are heritable psychiatric disorders, substance abuse, close relatives with substance abuse, use of psychoactive medications, and a history of sexual or physical abuse.
1. Czeisler et al., MMWR 2020;69(32):1049-1057
2. https://www.cnn.com/2023/02/13/health/teen-health-risks-cdcsurvey/index.html
3. https://givingcompass.org/article/how-the-pandemic-is-spur ring-a-youth-mental-health-crisis?gclid=EAIaIQobChMIlLWwu vqp_gIVMgtlCh05XwAZEAAYAyAAEgLSsvD_BwE
4. Scangos et al., Nat Med 2023; 29: 317-333
5. Klemetti et al., Acta Obstet Gynecol 2010;89:677-682
6. Hart VA. Issues Mental Health 2002;23(1):31-41
7. Ying et al., J Assist Reprod Genet 2016;33:689-701
8. Vikström et al., BMJ Open 2015;5:e009426
9. Ganeiro et al., Hum Reprod 2016;31(8):1788-1798
10. Mazzilli et al., Front Endocrinol 2021;12:620936





ADVERTISEMENT

ADVERTISEMENT
The CHR is searching for a candidate for the newly created position of Embryology Laboratory Supervisor for Research. The CHR’s embryology laboratory, under a single laboratory director, is in the process of being reorganized into three distinct areas with separate supervisory responsibilities: (i) clinical, (ii) administrative, and (III) research.
Supervisors in all three areas must hold PhD degrees (or equivalent) and be fully trained human embryologists with sufficient historical professional experience to hold a supervisory position.
https://www.centerforhumanreprod.com/contents/video-gallery


While such human embryology experience is preferred for this new position as well, priority qualifications are a record of excellence in reproductive biology research, documented by publications in prestigious peer-reviewed journals and, in absence of human IVF experience, at least substantial animal IVF experience allowing for relatively quick in-house training in human IVF.

Center for Human Reproduction

Besides a competitive salary and benefit package, the CHR also offers in this position a unique financial incentive-structure linked to the success of the center’s research activities, as demonstrated by publications in prestigious peer-reviewed journal. Moreover, this position will also be eligible for the opportunity to earn shared ownership in research-driven new start-up companies and the center itself.
If you feel that you qualify for this position, please submit your CV and a brief application letter to the CHR’s COO, Ms. J. Tapper, at jtapper @thechr.com. The position is available immediately. All submissions are considered confidential.
Mostly placed into a clinical context, we in this section of the newsletter offer a survey of articles in the English literature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. These articles, however, nevertheless often point out where clinical practice will likely go and, therefore, serve an important translational purpose. Translational research has been the CHR’s principal research goal since its founding in 1981, has produced a significant number of U.S. patents over the years, and has propelled the CHR into its current position as a worldwide center of last resort for infertile patients who have failed treatments elsewhere.
Based on a report in The Wall Street Journal on April 6, 2023, New York’s KKR, which recently closed on the blockbuster purchase of IVIRMA at an enterprise valuation of over US$ 4.2 billion, has run into some troubles with another mega acquisition in the health care field, Envision Health Care, which was acquired in 2018 for US$ 6 .0 billion.1 The company is apparently in negotiations with creditors after missing a deadline in March to report fourth-quarter financials by March 31, triggering a technical default under the company’s loan agreements, giving it 10 business days to cure. Some of the company’s bonds and loans, according to the article, currently trade at 15% to the dollar, considered a sign that investors do not expect to get paid back in full.
This comes following the unusual occurrence in association with the IVIRMA purchase we reported previously in these pages, when all leading banks that had committed to the financing of
Continued on page 29
the IVIRMA purchase, sold off their commitments before the closing of the deal at a loss and KKR & Co announced that they would obtain financing from other sources. IVIRMA’s sale appears to have been timed perfectly for the sellers. Only the future will tell whether timing (and valuation) was at least equally well timed by KKR & Co.
1. The Wall Street Journal. April 6, 20123. p.B11
According to a report by Ron Shinkman in an e-mail to fertility centers by Griffin Jones (Inside Reproductive Health) on April 4, 2023, U.S. Fertility, one of several fertility center networks billing itself as the “largest” physician practice management platform in the infertility field, is to acquire Ovation Fertility, which differs from all other fertility networks in that the company, in principle, only manages IVF -related laboratories. U.S. Fertility is a three-year-old company created by Amulet Capital and Shady Grove Fertility, one of the largest IVF clinic chains in the U.S. Ovation Fertility, in contrast, is
in principle owned by Morgan Stanley Capital Partners with an additional investment from WindRose Health Investors, according to the article.
Interestingly, U.S. Fertility described the transaction allegedly as a merger, while Morgan Stanley described it as a sale, basically removing the company as a major investor from the infertility niche. Considering Morgan Stanley’s earlier enthusiasm about the infertility field, this is an unexpected development and may be another sign that major finance institutions have changed their optimism about the field. Such an interpretation is also supported by the fact that the terms of the deal were not disclosed and, therefore, the valuation of Ovation Fertility was not revealed. We would not be surprised if it was much lower than one would have expected after recent deals, especially the acquisition of IVIRMA by KKR & Co
We have over the last year repeatedly pointed out examples where we, here at the CHR, felt that insurance carriers went to almost absurd lengths in denying authorizations for certain infertility treatments and/or practically prescribed the order of treatments if treatments were to be covered under a patient’s insurance plan. We, therefore, were pleasantly surprised to learn in several media reports that the insurance unit of UnitedHealth, one of the strictest and, at times most restrictive insurance companies in the industry, announced that they were going to cut down on the need for prior authorization.1
Yet, as of this point, our expectations are rather limited because United will reduce the use of prior authorizations as of this point by only 20% and this reduction will be restricted to some non-urgent surgeries and procedures only. Moreover, as of this point, we were unable to receive information on whether any, and if so which, infertility treatments may be affected by this announcement. We also do not know as of the time of this writing whether these changes also apply to the Oxford Health Plans, owned by UnitedHealth. So let us know whether you are noticing any changes for the better if you do and, of course, also especially if you don’t!
REFERENCE
1. https://www.medscape.co/viewarticle/990239?ecd=WNL_trdairt_ pos1_230401&uac=223637CN&impID=5299672
A Homeland Security Committee report by its Democrat members demonstrated that important drug shortages between 2021 and 2022 increased by almost 30%. Especially affected were children’s medications and antibiotics. For 2022 this meant that a whopping 295 different drugs were insufficiently available by the end of 2022.1 The chairman of the committee summarized the
significance of these findings as indicative of not only a healthcare crisis but also a national security problem of major significance. Reliance on foreign manufacturing sources (our comment, -mainly China and India) and inadequate “visibility” of supply chains were described as the major causes, with, paradoxically, even neither the Pharma industry nor the federal government having the ability to understand the complete supply chain. Over 15 important clinical drugs, therefore, have been in short supply for over 10 years, most of them injectables, over twice as often in short supply than oral and topical medications. Over a third of drugs in short supply are antibiotics, with obvious consequences for the treatment of infectious diseases. Propofol, a standard intravenous sedative routinely used in IVF, has been in and out of shortness for many years.
If anybody has any doubts about the incompetence (or should we say the not caring) of Big Pharma and the government, these facts say it all! It is becoming increasingly obvious that Big Pharma really does not care about the general health of people. All they care about are big-selling drugs. If a drug is not a least a billion-dollar seller, nobody bothers anymore? But this is where government should come in and government, as in so many other areas of governance, is unfortunately becoming increasingly incompetent. That it is nowhere more obvious than here because it does not take a genius to recognize that the nation needs these medications and it is not that difficult to get them reliably manufactured, whether in or outside of the U.S. One just has to recognize the problem and get it done!
1. Shabad R, Tsirkin J. NBC News. https:www.nbsnews/politics/congress/ drug-shortages-are-rising-pose-national-security-rik-new-repot-warnsrcna75959.
In reading the literature, one starts feeling sorry for academia because everybody, suddenly, appears to have recognized that academia has “lost luster” as an article by Clare Watson in Nature Medicine (among several others) recently correctly pointed out.1 Yet, in reading her and other writers’ articles on the subject, one is somewhat surprised about the surprise in their voices and in the banality of their explanations.
First of all, there has always been a brain drain from academia to industry and it was mostly (though, of course, not always) a healthy selection process: the good scientists who did not make it to becoming independent lab directors and/or department chairs, left academia and, thereby, offered important skills to industrial research labs that they had absorbed in academia. The best, however, usually remained behind because they were the ones who were given the opportunity to run their independent research laboratories and/or departments. They, therefore, continued to train great physicians and scientists, thereby propagating a healthy academic as well as industrial circle of permanent enhancement in creativity and knowledge.
Watson (and many others) now claims that today’s exodus from academia is based on a desire for better work conditions and “real-world impact.” She, however, in our opinion completely misreads what is happening these days in academic medicine and research. We concur that the current younger generation of researchers (and physicians) is more centered on quality-of-life issues than earlier generations – a point we have made in these pages repeatedly – but this is not why we see the highly significant and rather unprecedented flight from academia.
How do we know? We know because the people who nowadays flee academia are often no-longer those in the second category who failed in reaching the peaks of an academic career, - but, significantly more often than in the past, highly accomplished individuals who either already rose to the very top at their institutions but now realize that academia no longer offers the almost limitless freedom of thought and activities it allowed in the past, - or those who deserved to reach those peaks based on their accomplishments but were passed over because nowadays academic appointments, first (and second, and third) of all, are primarily based on political correctness rather than objective qualifications of candidates.
Paradoxically, for mostly pure economic reasons, meritocracy still counts for something in industry and, in addition, of course, pays more (much more!). We therefore know of world-famous scientists at leading research institutions who for years were pursued by industry and rejected financially extremely generous offers but over the last 2-3 years changed their minds. Also, we know many more among medium-aged scientific leaders in their respective areas of expertise who are actively looking for opportunities to leave their academic positions, an idea they would have not even given a thought to only three years ago.
This brings us back to the young generation of academicians, whether physicians or basic scientists, who, indeed, may have different worldviews from older generations. As we have frequently noted in these pages, they undoubtedly, are more protective of their social life than older generations were at those ages. They also, as Watson suggested, may have bigger dreams about having a “real-world impact,” though what many among them may consider a significant world impact may, obviously differ from what Salk considered to be a worthwhile impact when he invented his polio vaccine. In short, like in any generation, younger people have
different priorities from those their parents and grandparents had. Then, there is another big difference between individuals in the prior young generation that pursued medicine or other science areas as their careers: First, they were almost 100% male; and second, they were striving personalities and, therefore unsurprisingly, at the top of their classes. Today in contrast, over half of all medical school classes and PhD programs are female-dominated and we lost the aggressive males at the top of their high school classes a long time ago, first to Wall Street and finance, and more recently to the start-up world. No wonder, therefore, our young physicians and scientists look at the world differently from prior generations. They simply see no logical reasons to stay in academia for either academic, personal, or economic reasons.
We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO (jtapper@thechr.com). All submissions are considered confidential correspondence.

The time, therefore, may have come for academia to look at itself and reconsider what reorganizations are needed to make academic careers again worthwhile for younger and more senior physicians and/or basic scientists. Should that not happen soon, academia will enter an irreversible cycle of negative selection, with a steadily deteriorating quality of faculty, and will, ultimately, also lose the privilege of training future generations of physicians and scientists.
1. Watson C. Nat Med 2023; 29:6-9
Readers of this newsletter will be aware that what represents “evidence” in medicine has over recent months repeatedly been the subject of discussion in these pages. We have now for some time been sensing increasing dissatisfaction with the concept of EBM, which has been at the very core of medical practice since 1990, when Gordon Guyatt, MD, from McMasters University in Canada came up with the term and proposed the concept in a paper co-authored by his mentor at the university, David Sackett, MD. 1
EBM was nothing less than a long-coming revolution in medicine which historically up to that point primarily had been based on expert opinions. EBM now argued that through correctly conducted clinical studies (i.e., wherever possible through blinded prospectively randomized studies, BPRSs) and so-called meta-analyses of such studies, conducted at different places around the world studying different patient populations, “true evidence” could be developed leading clinical practice much better than expert opinions, classified as the least effective level of evidence after all other study formats.
The concept was brilliant and logical and, therefore, unsurprisingly, almost without criticism or even skepticism, widely accepted, quickly becoming the most widely heard and propagated slogan in medical research. What the medical community without proper prior validation studies overlooked, however, is a critical error in medical research in all specialty areas: Great-sounding ideas, proposed by smart “experts” in a field are, indeed, much more often incorrect than correct. How do we know that? From many examples but, likely, the most convincing is the failure of new drug studies which fail in over 90%, even though drug companies have tons of very knowledgeable “expert” advisers very carefully selecting drug candidates for clinical studies because of the enormous costs of clinical drug trials (now assessed as on average $ 1 billion).2
Consequently, why should we be surprised that brilliantly sounding ideas in medical practice, even if proposed by seemingly brilliant “experts” in the field, will be wrong significantly more often [in our specialty by now engrained ideas such as routine blastocyst culture, elective single embryo transfer (eSET) for everybody, mild ovarian stimulation and, of course, PGT-A]? Moreover, this fact alone one can argue, establishes incontrovertible evidence that important clinical practices should not be integrated into routine clinical care before hypotheses are tested in properly conducted clinical trials,just as drug companies are mandated to do. This fact also explains why despite innumerable new treatment additions to IVF, pregnancy and live birth rates, as of ca. 2010, have worldwide been declining.3 Considering all the promises of those “add-ons” to IVF, shouldn’t pregnancy rates not have instead improved?
Yet, paradoxically, brilliant investigators who conceived and supported EBM then ended up making the same mistakes the medical field has been making in toto: for decades (i) It implemented rules without, first, testing their effectiveness. (ii) It ignored variables in study populations that, simply, cannot be ignored in interpreting study outcomes. (iii) It, therefore, ended up with meta-analyses that
simply made no sense because differences in study populations often obfuscate outcomes in specific patient subpopulations. In other words, the old IBM dictum, “garbage in garbage out,” applies here as well! Also (iv), probably most paradoxically, EBM which was supposed to replace expert opinions, instead ended up reemphasizing them (consider, for example, why there are such strong differences of opinion in our specialty regarding blastocyst culture for all, eSET, and PGT-A).
For most of you – the reader of this commentary – it is not because you have been studying the literature on the subject but because, like you are doing right now, you are consuming a very subjective and, therefore, at least to a degree, biased “expert opinion.”
Though we at the CHR have been expressing our discomfort with how EBN is practiced for a good number of years, we were pleasantly surprised to see that we no longer appear to be in only a small minority, as over the last few months several very important journals, suddenly discovered the need to “rethink evidence in medicine.” Nature Medicine announced a new series of articles on, “evidence in medicine to discuss new approaches to assessing the safety and efficacy of cutting-edge health technologies and treatments.”4 Another article in the same journal pointed out how the poor management of the COVID-19 pandemic may have been an important contributing factor in recognizing the need for revaluation of EBM.5 Its sister journal, Nature, also started to address the subject by acknowledging that even pure politics has entered evidence by penning an editorial in defense of political endorsements (of Joe Biden in 2020),6 but, fairly, also publishing a contradicting opinion which, in our opinion correctly, noted that such political endorsements can affect the credibility of a medical journal (one would like to see more acknowledgment in other medical journals of this controversial subject).7
1. Smith et al., JAMA.2014;311(4):365-367
2. Sun et al. Acta Pharmaceutica Sinica. B.2022;12(7):3049-3062
3.Gleicher et al. Hum Reprod Open. 2019;2019(3): hoz017
4. Editorial. Nat Med. 2023; 1:1
5. Subbiah V. Nat Med 2023; 29:49-58
6. Editorial. Nature. 2023; 615:561
7. Lupia A. Nature. 2023; 615:590
An increasing number of Chinese women go through gestational carrier IVF cycles in the U.S. because surrogacy is illegal in China. They use American gestational carriers, who deliver in the U.S. and, thereby, produce future U.S. citizens. Now comes a researcher from the conservative Heritage Foundation who makes the argument that, as future U.S. citizens, these children may represent a future security risk for the U.S., creating long-term pathways to infiltrate industry and government in the future even though they will be raised in China. The researcher noted that California IVF clinics are the epicenter of this “rent a womb” trend, with hundreds of Chinese babies being born this way every year, and nobody knowing who they are and whether their parents have
any connections with the Chinese government. She also urged Congress to look closer into this practice.1
On a side note, Reuters also recently discussed commercial surrogacy in the world after a Spanish celebrity received considerable attention for using an American surrogate to produce an offspring with her prematurely deceased son’s semen. She did this in the U.S. because in Spain, interestingly for decades the place where many Europeans travel to for egg donation, surrogacy of any kind is prohibited.2
Also, according to the article, for-profit (commercial) surrogacy is prohibited in Canada, Denmark, New Zealand, Brazil, Britain, and Australia, though these countries allow cases of “altruistic” surrogacy. Bulgaria, France, Germany, Italy, Portugal, Taiwan, and Spain prohibit all forms of surrogacy whether commercial or “altruistic.” Until the war started a year ago, Ukraine was a worldwide center for commercial surrogacy, and hundreds of surrogates and the international couples they served were caught in the early days and weeks of the war. Then, there are countries such as India and Thailand, which served as prominent hubs of commercial surrogacy, until their governments clamped down and prohibited surrogacy, in the case of Thailand only for foreign families.
1. Sahakian T. Fox News Digital. April 25, 2023
2. Reuters. April 6, 2023; https://www.medscape.com/view article/9990457?
According to an article by medical writer, Heidi Ledford, in Nature magazine,1 a recent international summit on Human Genomic Editing in London, UK, concluded that “heritable genomic editing remains at this time unacceptable” because safety and efficacy have not been established. Very much to the contrary: Kathy Niakan, PhD, from Cambridge, UK, as well as Shoukhrat Mitalipov, PhD, from Oregon, US, reported large unintended deletions on edited chromosomes.
We reported in last month’s VOICE that He Jainku, PhD, of past infamy about allegedly transferring CRISPR-edited embryos into two women and producing three babies, is back in business after allegedly having served a 2-year term in prison. We also noted that he refused at that meeting to disclose details about these three allegedly alive children. Therefore, it is still unknown whether he originally succeeded in his edits and what the possible side effects on the children have been.
The same meeting, however, also touted rapid clinical advances in somatic cell gene editing by presenting a patient with sickle cell disease who was successfully treated. Indeed, she was the first patient to have her blood stem cells CRISPRed to compensate for the sickle cell mutation and had those re-engineered red cells returned to her body. As noted in a commentary by
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/ Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.
medical writer, Kay Kupferschmidt, in Science magazine, the intent of presenting the patient at the conference was to emphasize the distinction between inherited germ cell editing (as He Jainku did when editing human embryos) and non-heritable somatic genetic editing.2 The writer also interpreted the concluding statement of the conference that heritable genome editing remains at this time unacceptable, as a quite significant change from the prior conference in Hong Kong, five years earlier, because, “at this time” strongly suggests that debates and discussions should go on even on this subject.
1. Ledford H. Nature 2023; 615:568-569
2. Kupferschmidt K. Science 2023;379(6637):1074-1075
Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BPM15), both secreted by oocytes, are known to have several important effects on ovaries by modulating the cell fate of somatic granulosa cells and quality as well as developmental competence of the maturing egg.1 Chinese investigators now in a paper in JARG investigated the levels of both in follicular fluids of younger poor prognosis IVF patients (POSEIDON classification 3) in comparison to a young control group. Unsurprisingly, considering what has been known about these two substances for quite some time, both were lower in follicular fluid of poor prognosis patients who, - also expected, had poorer IVF outcomes than control patients.1 Interestingly, the same group of Chinese authors already in 2021 published in The Journal of Ovarian Research a very similar paper, in which in a similar (and larger) patient population they investigated the gene expression of GDF9 and BPM15 genes in follicular fluid and granulosa cells with
the same kind of underwhelming results of basically showing not much more than declining activity with advancing age.2
Unfortunately, both papers are good examples of what we, here at the CHR have come to call “self-fulfilling prophecy papers,” which wastes time, resources, and efforts of researchers. Moreover, by publishing a clinical paper following a genetic expression study on the same subject, one here must even wonder about double publication. The only factual addition in the more recent publication was the number of authors which went from 6 to 11.
1. Belli M, Shimanski S. Vitam Horm 2018;107:317-348
2. Gong et al J. Ovarian Res 2021;14(1):1
A longstanding hypothesis in IVF suggests that oocyte aging in older women to a significant degree is the consequence of mitochondrial aging in the cytoplasm of oocytes. Consequently, mitochondrial replacement in older women has been suggested as a potential treatment to improve the function of their oocytes but early experiments were interrupted by a somewhat controversial prohibition from the FDA which currently also prohibits mitochondrial transplants to prevent inherited mitochondrial disease.1
Now unrelated to infertility or reproduction in general, a cancer study offers interesting insights into transferred mitochondria in eLife 2 Unexpectedly, transferred macrophage mitochondria were found to be dysfunctional and accumulated reactive oxygen species in the recipient (cancer) cells, which activated ERK signaling and, with it, cancer cell proliferation. These downstream effects of transferred mitochondria, of course, raise significant concerns about the safety of mitochondrial transfers and offer support for the FDA decision.
1. Pompei M, Pompei F. J Assist Reprod Genet 2019;36(3):383-393
2. Kidwell et al., Elife 2023;12:e85494
Likely the “hottest” topic in reproductive biology is in these days the production of so-called “synthetic” or “artificial” embryos from
stem cells. Two of the most prominent laboratories in the field, indeed, got into a very public rift (we reported before in these pages), the laboratories of Magdalena Zernicka-Goetz, PhD (currently supervising laboratories at Cambridge University in the UK, and the California Institute of Technology, in the US) and the laboratory of Jacob H. Hanna, MD, PhD., at the Weitzman Institute in Israel. The latter was rightly upset that MZG, after visiting his lab, used a technology he developed, but downplayed the significance of his contribution, even though she could never have published her paper1 without using his technology. It also did not help that she got Nature magazine to speed up her publication, so it would appear ahead of Hanna’s publication in Cell. 2 Both investigators at that point worked in mouse models and, therefore, produced synthetic mouse embryos.
The next step for both laboratories, however, will, likely, be to take the concept of making synthetic embryos into the human experience by making human synthetic embryos from human stem cell lineages. The importance of this next step can, of course, not be overemphasized for our basic understanding of reproductive physiology. We at present are still living with an invisible “black box” when it comes to embryo development after implantation, downstream from the blastocyst-stage, and, therefore, have little detailed understanding of implantation. Moreover, such models would allow the ethical manipulation of developmental steps, without having to resort to real human embryos.
A recent paper in Cell Stem Cell by Chinese investigators also represents a remarkable next step in the same direction: They produced from naïve monkey ESCs what they called cynomolgus monkey blastoids (i.e., synthetic embryos) that had similar characteristics to natural monkey blastocysts. Remarkably, they also had the capacity of in vitro gastrulation with three germ-layer differentiation and in vivo early pregnancy with gestational sac followed up to 10 days in selected gestational carrier female monkeys.3 A commentary by medical writer, Mitch Leslie, in Science rightly described the findings as a milestone in the development of stem-cell derive embryo models.4
A recent review paper in Reproduction, therefore, is timely when offering a comparison of mouse and human preimplantation development.5 An accompanying commentary especially addresses some ethical issues that are arising from all of these developments and raises issues deserving of further bioethical research.4
1. Amadei et al., Nature 2022;610 (7930):143-153
2. Tarazi et al,, Cell 2022;185(18):3290-3306.e25
3. Li et al., Cell Stem Cell 2023;30:362-377
4. Leslie M. Science 2023;380(6640):21-122
5. Biondic et al., Reproduction 2023; 165:R103-116
6. Villalba et al., Reproduction 2023;165:V1-V3
Japanese researchers recently reported in Nature magazine an amazing experimental achievement in a mouse model: Using the known observation that pluripotent stem cells gain and/or lose chromosomes in prolonged in vitro cell culture, thereby producing aneuploidy, they were able to convert XY to XX cells. They did this by converting mouse embryonic stem cells (mESCs) from male to female. They then guided those sex-converted cells to form eggs that were fertilizable and yielded healthy offspring.1
An accompanying commentary appropriately described this paper as a “milestone” in reproductive biology and noted that the authors’ protocol opens up new avenues in reproductive biology and fertility research.2 They couldn’t be more correct!
1. Murakami et al., Nature 2023;615:900-906
2. Bayerl j, Laird DJ. Nature 2023;615:805-806
Pathogenic DNA variants in primary ovarian insufficiency (POI)
POI, defined by FSH hormone levels over 40.0mIU before age 40 and amenorrhea, is in the literature generally described to affect 1% of the female population but, recently, was estimated to have a 3.7% worldwide prevalence.1 In a research briefing,2 the authors summarized their full-length study,3 to this day, likely, the largest whole-exome sequencing study of POI patients.2 The study identified 20 new disease-associated genes which, combined with already known genes, represent only approximately a quarter (23.5%) of the complete POI risk.3 Over half of all POI risk is currently still considered “idiopathic.”
The study involved 1030 POI patients and discovered several interesting new findings: women with POI with primary amenorrhea had a higher genetic contribution than POI with secondary amenorrhea (25.85% vs. 17.8%). They also had more biallelic variants (2.5% vs. 1.9%). Though the authors identified in their study 20 new POI-associated genes, even in top-ranked genes, pathogenic variants were rare, - found in only 1.25% of POI patients. Variants in genes associated with meiosis, DNA repair, oxidoreductase activity, fatty acid metabolism, and mitochondrial function were enriched in POI.
As geneticists usually do, the authors recommended genetic testing for currently recognized markers, arguing that such screening would allow for a definite diagnosis and would also permit genetic family testing. We are not certain that this makes sense, considering that over three-quarters of patients would be negative, and POI already is known to be a “familial” condition. Counseling about early functional ovarian reserve testing is, therefore, already recommended to first-degree relatives of POI patients.
We, however, do fully agree with another conclusion the authors reached: They correctly pointed out the likely, common etiology between POI and premature ovarian aging (POA), thereby suggesting that POA may demonstrate similar pathogenic DNA variant associations as POI. CHR’s investigators already years ago pointed such similarities out in association with autoimmune findings.4
1. Golezar et al., Climacteric 2019;22:403-411
2. Che Z-J., Nat Med 2023;29:315-316
3. Ke et al., Nat Med. 2023; https://doi.org/10.1038/s41591-022-02194-3
4. Gleicher et al., Hum Reprod 2009;21(8):1951-1955
In an e-mail to membership on March 31, 2023, SART/ASRM reported selected, preliminary national IVF outcome data for 2021. Interestingly getting the headline in the mailing and considering the marketing efforts by the egg-freezing industry rather unsurprisingly, egg-freezing cycles jumped in numbers much more than clinical IVF cycles by, indeed, a whopping 31% - from 16,786 to 24,558.
What, however, should have instead created a “screaming” headline is the fact that national IVF birth rates in 2021 improved over 2020. How do we know that? Following the lead story of the above-noted dramatic increase in egg-freezing cycles, the mailing informs us that the data show an uptick in babies born from 73,602 in 2020 to 89,208 in 2021 and attributes this increase primarily to 19% more IVF cycles started in 2021. The increase in live births, however, was 21.2%; in other words, an absolute gain over cycle starts of 2.1%, - clearly a very positive development if confirmed by final reported results for 2021. Our analysis is further supported by twin pregnancies having declined from 6% to 5% and, therefore, not contributing to larger birth numbers.
These preliminary released data, however, also inform on several other interesting issues relating to IVF practice in the U.S., which we will be addressing in more detail in the June issue of the VOICE, usually the last issue for this academic year and the two-month summer break.
This time Chinese investigators are the ones overselling AI to the IVF community, claiming in a paper in eLife that using AI will boost IVF success rates by identifying embryos most likely to result in a healthy birth.1 Here is why this is just yet another so-called “self-fulfilling prophecy paper:” The five most predictive parental factors for good embryos AI discovered were: (i) maternal age; (ii) day of blastocyst stage embryo transfer; (iii) the number of pre-retrieval follicles; (iv) number of eggs retrieved; and (v) endometrial thickness. Wow, what a surprise!
The only interesting finding in this study was that a heat map suggested that the AI program, when it came to embryo morphology, mainly focused on image regions of inner cell mass and
trophectoderm. But do we really need AI to figure out above noted five patient characteristics that define good-prognosis patients? We don’t think so, but AI is now the fashion of the moment, and millions of US dollars have flown into start-ups with the purpose of improving IVF outcomes. If that sounds familiar, we hope that in this case, at least some succeed because we all would, of course, benefit from such a success. Forgive us, however, for being skeptical: even the best AI can be helpful only if it can distinguish between at least two binary characteristics. If there, simply, is not enough distinction, even the best AI cannot make it up (indeed, hopefully, does not make it up).
We suggest being careful with promises from “interested parties” before either investing in start-ups or, even more importantly, investing in your patients’ best IVF interests. Remember how quickly the start-ups selling closed incubation systems with around-theclock closed-circuit monitoring of embryos through time-lapse disappeared, followed by the start-ups that (so far) were unable to establish validated embryo selection algorithms from all those hours of close embryo observation between cleavage and blastocyst-stages (see also the next section).
Maybe, “what is obvious is obvious,” in embryology and has already been codified for decades by competent clinical embryologists in what we call oocyte and embryo grading? Maybe, there just are no more (or better) visual characteristics that will predict better implantation and pregnancy chances? Maybe, AI will only have a chance to further contribute if, either instead or in combination with looking at egg and embryo pictures, AI takes a closer look at the mother (and maybe even the father), her/his medical history, family history, etc.?
1. Liu et al., Elife 2023;12:e83662
It was again, as in recent times on many important occasions in infertility practice, for our Dutch colleagues to provide an ultimate answer on a controversial issue, based on their unique country-wide cooperative of fertility centers that cooperate on largescale prospectively randomized studies. This time, our Dutch colleagues wrote the final word regarding “Time Lapse,”1 yet another “fashion-of-the-moment,” a big part of the fertility field succumbed to over 10 years ago.
In a three-armed, multicenter, double-blinded, prospectively randomized controlled trial, with couples and physicians being masked to treatment, patients were compared for use of early embryo viability assessment by time-lapse EEVA (TLE) and uninterrupted culture. The timelapse routine (TLR) group received routine embryo selection and uninterrupted culture, while the control group received routine embryo selection and interrupted culture. Primary study endpoints were cumulative pregnancy rate within 12 months in all women and ongoing clinical pregnancy rate after fresh single embryo transfer (eSET) in a good-prognosis patient population, with analysis performed by intention to treat (cycle start).
Results were clear and convincing: Neither embryo selection with time-lapse using the EEVA test nor uninterrupted culture in a closed time-lapse incubator improved IVF outcomes compared to non-time-lapse routine embryology.
Think how much money IVF centers have spent on time-lapse and, as elsewhere in this issue already mentioned, how much more patients have been charged for their IVF cycles, either because IVF centers quietly raised their cycle fees after purchasing the expensive time-lapse equipment or, as some centers did, they “marketed” their state-of-the-arts” laboratory that now employed time-lapse and, therefore, now transparently, could charge an extra fee for every embryo placed into such a closed incubation system.
That is, however, not even all the damage done by the time-lapse craze: Like other non-sensical procedures in IVF (for example, PGT-A), time-lapse practically automatically commits the cycle to extended embryo culture to blastocyst-stage. The embryos of older women or of younger women with premature ovarian aging often do not survive to blastocyst-stage. How many women, therefore, have been told that they can no longer conceive with their own eggs, simply because all their embryos were automatically cultured to blastocyst-stage in a closed incubation system?
As also already noted elsewhere in this issue before, the CHR did not convert its embryology to time-lapse incubation because we, by principle, do not run after “fashions-of-the-moment.” Before making substantial changes in the center’s routine practice, whether on the clinical side or in the laboratory, we always compare “old” with
We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratory supervisor for research
To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

“new” before making changes. The CHR many years ago learned that simply because of differences in patient population, not everything that worked at other IVF clinics would also work at the CHR. When we tested time-lapse, we very quickly noticed that in our patient population it not only offered no outcome advantages but harmed outcomes in some patients and increased rather than decreased (as promised by industry) embryologist-time per patient.2
1. Kieslinger et al., Lancet 2023; S0140-6736(23)00168-X. doi: 10.1016/S01406736(23)00168-X.Online ahead of print.
2. Wu et al., Reprod Biol Endocrinol 2016;14:19
This is a question this newsletter has repeatedly addressed in the past and, as things are looking, will have to continue to address, because there appear to be no easy answers. This is again confirmed by a recent paper in the American Journal of Obstetrics and Gynecology in which U.S. investigators from Amherst and Yale investigated whether state insurance mandates to cover fertility treatments under general health insurance packages, assumed to open access to infertility treatments irrespective of ethnicity and/or race, would, therefore, improve utilization as well as outcome disparities.1
Yet, as so often before when these issues were investigated, the results surprised: Though, as has been reported before, state mandates improve utilization in general, the increase was the greatest for non-Hispanic Asians and non-Hispanic Whites. As a result, the difference in fertility utilization between NonHispanic Whites and Black/African American women, indeed, increased from being higher at 23.5 per 10,000 women IVF cycles in non-mandated states to 56.2 per 10,000 cycles higher in mandated states. The authors, of course, concluded that state mandate did not enhance equity. One, indeed, could argue that state mandates increase inequity for Black/African American women. As so often before, one is left wondering what else can be done: Black women clearly deserve better!
REFERENCE
1. Correira et al. Am J Obstet Gyncol 2023;228:313.e1-8
One of the most interesting studies we are presenting this month was a research letter by Swedish and British colleagues in JAMA. 1 As IVF pregnancies uniformly produce increased adverse outcomes in comparison to spontaneously conceived pregnancies, this has mostly been attributed to the infertile patient populations and not the IVF procedures. In other words, the adverse outcomes have been attributed to co-factors included in the diagnosis of infertility. Here discussed paper challenged this conclusion by introducing a new control population, -female same-sex couples who, obviously, usually do not enter IVF because of an infertility diagnosis.
As this study quite clearly demonstrated, same-sex couples, indeed, had very similar pregnancy outcomes to spontaneous conception, significantly strengthening the hypothesis that infertility, per se, causes observed adverse IVF outcomes. Now we just need to figure out why that is.
A somewhat related paper from China, this time in JAMA Network Open, compared genetic profiles of neonates, either conceived with or without IVF and low and behold, did not find significant differences.2 Even though one can assume that after over 8 million IVF births worldwide, we would have noticed if IVF caused systemic genetic effects, such studies are always reassuring.
REFERENCE
1. Goisis et al., JAMA 2023329(13):1117-1119
2. Huang et al., JAMA Network Open 2023; 6(4):e236537
Systemic lupus erythematosus (SLE) is one of the most common autoimmune diseases in women during reproductive years and, therefore, is not infrequently seen in infertility practice. As frequently pointed out in these pages, autoimmunity, and pregnancy exhibit strong mutual dependency: SLE can affect pregnancy in different ways and vice versa, - pregnancy can affect SLE in a multitude of ways. Especially because of the CHR’s Norbert Gleicher, MD’s longstanding interest in reproductive immunology (he was a founding member of the American Society of Reproductive Immunology and its first Vice President and was the founding
editor-in-chief of its official organ, The American Journal of Reproductive Immunology (AJRI), the CHR, likely, sees proportionally more autoimmune cases than most other fertility centers.
When the Society for Maternal-Fetal Medicine publishes a guidance paper on SLE in pregnancy,1 we, of course, want our patients to be aware of it. Robert Silver, MD, a very knowledgeable individual in the field, is the first author of this paper by the Publication Committee of the society. While we do not necessarily agree with all the many treatment recommendations made in the document, most recommendations make sense and often limited knowledge levels are properly acknowledged. For everybody with an interest in SLE in pregnancy, it is a worthwhile read.

We were significantly less enthused to read a supposed systematic review and meta-analysis of maternal and infant outcomes of pregnancy in association with anti-SSA/Ro antibodies.2 This paper appeared to be one of those by now fairly typical Chinese paper-mill products that have been flooding the literature. Many of our Chinese colleagues apparently have nothing better to do with their time than to perform systematic reviews and meta-analyses on everything. In this case, it is the presence of anti-SSA/Ro antibodies, independent of who they are present, whether in association with scleroderma, SLE, or in association with other conditions, at what titers, etc.
How ridiculous this paper is, can be best seen in the pooled estimates they list in their study populations for such common events as termination of pregnancy (4%) and spontaneous abortion (5%), basically suggesting that SSA/Ro-antibodies are protective against abortions of all kinds. This is clearly a candidate for “worst paper” in this issue of the VOICE.
1. Silver et al., Am J Obstet Gynecol;2023(3):B41-B60
2. Sheng et al., Ped Rheumatol 2023;21:22
A somewhat rarer but seemingly increasing occurrence in pregnancy is a diagnosis of cardiomyopathy, most often in the peripartum period and, therefore, also often called peripartum cardiomyopathy (PPCM). The world’s leading expert on this condition, Uri Elkayam MD, a professor of cardiology at USC-Los Angeles, will, indeed, be a speaker at the December FRM Conference we are organizing again this year. We are quite certain that the subject will come up in his talk.
However, we are raising it here because another paper in the American Journal of Obstetrics and Gynecology pretends to present a systematic review and meta-analysis on perinatal outcomes from pregnancies affected by cardiomyopathy. This paper by British colleagues is again one of these disappointing papers that not only do not offer any new data but do not even try to be original. Unsurprisingly, the paper’s conclusions are equally bland when stating that women with this condition should receive “detailed counseling regarding the risks of pregnancy and have their pregnancies managed by experienced multidisciplinary teams.” Really?!
REFERENCE
1. Eggletonet al., Am J Obstet Gynecol 2023;228(3):283-291
Though fortunately most of what we learned during the COVID-19 pandemic regarding the infection’s effects on mothers and offspring
has been holding up, several somewhat worrisome observations have been reported recently, including severe brain infections with the SARS-CoV-2 virus in two offspring of mothers with the disease, one of which, indeed, died.1 Those cases, like chorioamnionitis from the virus, now also reported in several cases (and previously noted in these pages) are, however, exceedingly rare and, therefore, almost anecdotal.
That is, however, not the case with a study recently reported in JAMA Network Open, which reported that in a cohort study of 18,355 infants delivered to mothers who experienced COVID-19 during pregnancy, male infants (but not females) were more likely to receive a neurodevelopmental diagnosis in the first 12 months of life than offspring from uninfected mothers, even after accounting for preterm deliveries.2 Those are, of course, very worrisome findings that undoubtedly will be followed up.
1. Benny et al. Pediatrics 2023;6:e2022058271
2. Edlow et al., JAMA Network Open 2023;6(3):e:234415
Let us start with the definition of a “genomic autopsy.” This term has been used by geneticists when trying to determine causes of miscarriages or deaths in utero at more advanced gestational ages. The first question is always, - is there a chromosomal cause? Traditionally, the answer then came from chromosomal testing of products of conception. Our current knowledge about chromosomal pregnancy loss, whether earlier or later in gestation, is based on data from such analyses of products of conception following pregnancy losses.
Why is this important? Because specimens were always taken from placental tissue rather than from the embryo or fetuses, with the reason being that fetal biopsies only rarely produce successful cell cultures. Also, successful cell cultures are required for successful chromosome spreads, - the exclusive basis of chromosomal diagnoses in those days.
As now is finally well understood, whether the extraembryonic cell lineage (placenta) of a pregnancy or the embryonic cell lineages (fetus) is biopsied, matters because both do not always offer the same chromosomal diagnoses. Indeed, especially when aneuploid cells in a pregnancy are the consequence of mitotic (rather than meiotic) aneuploidies, there can be significant differences between what biopsies of embryonic and extraembryonic cell lineages may reveal. And the same, of course, applies to cell cultures from these two tissue sources: biopsies from the extraembryonic cell lineage, which are the routine, will always demonstrate more aneuploidies than are found in fetal tissues. In other words, what we think we know about aneuploidies in miscarriages and late pregnancy losses is obviously biased toward exaggerations of aneuploidies and, therefore, must be reevaluated.
Such reevaluations, however, do not necessarily have to depend on old-fashioned chromosome spreads but can utilize modern genomic testing methods. Indeed, those methods now also allow quite reliable non-invasive testing of early pregnancies (at this point at least for the most frequent trisomies). A preliminary determination of whether a
miscarriage was likely of chromosomal nature, therefore, can be done gnomically with the same simple blood test now used for early prenatal genetic diagnoses.1 If the test is negative, a chromosomal cause for a pregnancy loss becomes very unlikely and products of conception can, still, be gnomically tested for confirmation, if one wishes to be certain.
1. Kimmelman D, Pavone ME. Best Pract Res Clin Obstet Gynecol 2021;70:51-62
As if a cancer diagnosis in pregnancy wasn’t bad enough, now come Canadian investigators who investigated long-term mortality in women diagnosed with cancer during pregnancy or postpartum.1 They investigated 1,014, 3,074, and 20,219 women diagnosed respectively with various cancers during pregnancy, postpartum, and remote from pregnancy. While 1-year survival was similar, 5-year survival was lower for women diagnosed in pregnancy and postpartum. Rates also varied with cancer type: Increased mortality was seen with breast, ovarian, and gastric cancers diagnosed in pregnancy, and breast as well as melanoma diagnosed postpartum.
The maternal immune system is sending us once again a very clear message: the tolerance pathways it is forced to induce in pregnancy to allow a pregnancy to thrive within the maternal host, even though being a 50% allograft, also appear to reduce the effectiveness of the immune system against the development of cancer. This is the same phenomenon observed in associations with autoimmune diseases, like SLE, where disease, paradoxically, improves especially in mid-trimesters because the same tolerance pathways that allow for a tolerance of the pregnancy and, as this study suggests of cancer, also improve tolerance to self. In breast cancer, similar observations have been reported before.2
1. Cairncross et al., JAMA Oncol 2023;6:e230339
2. JAMA Netw Open 2019;2(1):e186997
The most recent CDC report on the prevalence and characteristics of autism spectrum disorder (ASD) among children at age 8 in the U.S. reports further increases: For 2022, 1/36 children are ages 8 years, representing 4% of boys and 1% of girls, was suspected to have ASD, higher estimates than for the prior period of 2000-2018. Moreover, for the first time, White children demonstrated a lower prevalence than non-White, and Black children with ASD were still more likely to have a concurring intellectual disability. As so often, one is left with the question, why is all of this happening?
1. Manner MJ, et al., MMWR 2023;72(2):1-10
It was just a formality after the manufacturer withdrew Macena (hydroxyprogesterone caproate) from the market a few weeks earlier (as reported in these pages), the FDA now withdrew its 2011 approval of the drug which had been approved based on rather flimsy data that claimed benefits in preventing preterm births. Post-market studies since then have clearly demonstrated the ineffectiveness of the drug. It was time!
1. https://www.fda.gov/news-events/press-announcements/fda-commission er-and-chief-scientist-announce-decision-withdraw-approval-makena
Combined oral contraceptives including estrogen as well as progestogen have been associated with a small increase in breast cancer risk. Australian investigators now attempted to determine in a nested case-control study whether progestogen-only contraceptives also increased the risk. The study that appeared in PLoS Medicine, 1 The findings were interesting: The relative risk to get breast cancer rose about 20-30% with recent use and this increase in risk was independent of the mode of delivery of the hormone. Moreover, though percentage-wise a significant increase, in absolute risk the increase was minuscule: For use between ages 16-20 from 0.084% to 0.0935, and for use between 35 and 39 from 2.0% to 2.2%. Considering the obvious advantages of contraception during reproductive years, it would appear that the use of progestogen contraceptives is a reasonably good choice.
REFERENCE
1. Fitzpatrick et al., PLoS Med 2023;PMID:36943814
News about PGT-A is never lacking, and the last month was no exception. Here are a few articles we found of interest: Antonio Capalbo’s group (The CHR’s Norbert Gleicher, MD just publicly debated him on PGT-A at the 10th Annual IVIRMA Conference in Malaga, Spain, on April 28) published a paper in Human Reproduction asking the questions which copy numbers (percentage of aneuploid vs. euploid DNA) in a single trophectoderm biopsy (TEB) most accurately predict real mosaicism and concluded that cut-offs of 20-80% as the “mosaic” range was inferior to 30-70%. In addition, they suggested that the pre-2016 binary reporting of embryos as only either euploid or aneuploid was the most accurate one.1
The whole premise of the paper, however, makes absolutely no sense since an on average 5-6-cell TRB can, as so many times
explained in these pages (and in the medical literature), for biological, technical, ad mathematical reasons never reflect the accurate diagnosis of an embryo.2 If a single TEB demonstrates intermediate copy numbers between either 20-80% or 30-70%, the only thing we can be sure about is that this embryo is really “mosaic,” as at least 80% of blastocyst-stage embryos are. The problem, however, starts below and above that range at allegedly “euploid” and allegedly “aneuploid ranges because in both of these ranges a majority of embryos are still “mosaic.”
The authors conclude that fewer copy numbers (lower aneuploid DNA levels) are better than higher in predicting adverse outcomes. To reach such a conclusion one does not need a study; - simple logic suffices. But the question remains whether the risk of having an aneuploid embryo increases linearly with increasing copy numbers or not, as previously claimed by some authors and here once again suggested by this paper. At least 2 independent studies, one of them by CHR authors using the data set of a study that had claimed such a correlation, however, were unable to confirm such a linear correlation at a significant-enough correlation to allow clinical judgments regarding the transferability of embryos below certain cut-off mosaicism levels.2 Those laboratories which distinguish between low and high mosaics and/or have returned to binary euploid/aneuploid reporting (most do so at 50% aneuploidy in a single TEB), therefore, are still withholding significant pregnancy and live birth chances from their patients by not transferring and/or discarding embryos with aneuploidies above this cut-off. It, of course, is entirely possible, even likely, that the percentages of so-wasted embryos decline with increasing aneuploidy percentages in a TEB, - but, likely, not linearly. The initial mouse data on self-correction of embryos downstream from blastocyst-stage, for example, demonstrated that even at 50% aneuploidy within the inner cell mass, still, approximately one-third of pubs were chromosomally completely normal.3
A recent paper by Chinese investigators in JARG also attracted our attention, though, here too, we have significant questions regarding reported results.4 As seen so many times in the literature, Chinese colleagues love to perform meta-analyses and this paper is in principle a meta-analysis, even though they also report on
448 of their patients. The principal question the study attempted to answer was an important one: Among embryos by PGT-A reported as “mosaic,” how IVF cycle outcomes differed with different chromosomal abnormalities in those allegedly “mosaic” embryos. On purpose, we used the word “allegedly,” because, as many times discussed before in these pages, current reporting terminology in virtually all PGT-A laboratories uses an incorrect definition of mosaicism and, therefore, incorrectly designates many embryos as either “euploid” or ‘aneuploid” which in reality are truly mosaic.2
The study then comes to several contradictory conclusions from what other studies have reported. Like most prior studies, this study, too, concluded that pregnancy rates after the transfer of “mosaic” embryos are lower than after the transfer of “normal-euploid” embryos. Interestingly, however, the authors found no difference in IVF cycle outcomes between single transfers of monosomies and trisomies. This is a surprising finding because, based on miscarriage studies, the assumption has been that, except for rare exceptions (X0 for example), monosomies generally do not implant. Moreover, all sub-types of mosaicism were found to reduce pregnancy and implantation rates but only for women above age 35. This is a surprising finding because recent studies suggested that, if there is a subgroup of women who may demonstrate marginal benefits from PGT-A, it is older women. The authors also concluded that higher “mosaicism” levels reduced implantation and clinical pregnancy rates, a subject we already addressed in the discussion of the first paper in this section. With lower “mosaicism” only segmental gain reduced implantation and pregnancy rates. This again is an unexpected finding because several reported experiences (including the CHR’s experience) suggest that segmental abnormalities produce the highest pregnancy rates among all aneuploidy subgroups. The authors also claimed to be surprised by the observation that type of chromosomal abnormality had more of an impact on miscarriage rates than the degree of mosaicism. From the discussion above, it will already be obvious that these findings, indeed, should not be considered surprising. What, however, again is very surprising is the reported close association with low as well as high “mosaicism” of monosomies. Miscarriage risk has traditionally always been much more associated with trisomies than monosomies.
Why all of these differences? We frankly don’t know for sure, but meta-analyses follow the old IBM dictum “garbage in, - garbage out.” The most likely explanation are obvious selection biases in patient populations in studies used to compile the reported meta-analyses. That PGT-A studies are almost characteristically affected by this “disease” has, of course, also been discussed in these pages over and over again. We, therefore, would warn against considering this paper-reported data as valid.
Yet, another study addressing the “mosaicism” diagnosis with PGT-A was also published in JARG and is basically a propaganda piece from the genetic testing industry (2 of the authors are employees of CooperGenomics, the, likely largest provider of PGT-A services in the world). This paper is almost too absurd in its conclusion to give it attention; but as the clear winner of “the worst paper” this month, we could not pass on the opportunity.
Based on what is claimed by CooperGenomics, a study of 115 clinics from one central diagnostic laboratory the study aimed to identify what proportion of (by current incorrect PGT-A criteria) as “mosaic” reported embryos should be considered for transfer. Based on the percentage of aneuploid DNA in a single TEB and based on involved chromosomes, they did this in patients who had no euploid embryos by categorizing embryos into 3 groups: high, medium, and low priority. To demonstrate once again the current anarchy in PGT-A laboratories, here is how this company categorized embryos: Highest priority: <40% aneuploid DNA (i.e., neither 29 or 30% or 50% as the previously discussed studies did); and only 1 affected chromosome, among chromosomes #1, 3, 4, 5, 6, 8, 9, 10, 11, 12, 17, 19, 20. Medium priority: >40% aneuploid DNA and only 1 affected chromosome among #1, 3, 4, 5, 6, 8, 9, 10, 11, 12, 17, 19, 20. Lowest priority: “mosaic” biopsy involving 2 or chromosomes or only 1 chromosome from among # 2, 7, 13, 14, 15, 16, 18, 21, 22, X, Y.
That these criteria have no, or at best only minimal validity, should by now, following the above discussions, already be obvious. Unsurprisingly, therefore, the results this paper reports are absolutely nonsensical: Only 1.7% of cases were classified as having at least 1 high-priority embryo available; 2.8% had no high priority but at least 1 medium-priority mosaic embryo available; and 6.0% of cases had only low priority mosaic embryos available. How nonsensical these numbers are is best demonstrated by the fact that assuming all of these so-selected “mosaic” embryos were added up and all would lead to pregnancy and delivery (i.e., a 100% live birth rate), the combined live birth rate would still be only 10.5% of all patients., when the live birth rate in the last 50 consecutive transfers of chromosomal-abnormal embryos reported by the CHR (and not only restricted to “mosaic” but also including by PGT-A diagnosed as “aneuploid” embryos) was 14% (and because of two iatrogenic-caused miscarriages even a few percentage points higher).6
In short, this paper is pure phantasy with the obvious goal of falsely promoting the efficacy of PGT-A. This paper, therefore, deserves the award of “the worst paper” in this issue of the VOICE.
Considering all the confusion that reigns even within the reproductive endocrinology and infertility community regarding PGT-A, it can be no surprise that general OB/GYNS demonstrate significant knowledge gaps regarding the procedure, as another paper in JARG recently reported.7 And since general OB/GYNs are often the first advisers patients seek out when confronting infertility problems, this fact is also not helpful in presenting an objective opinion about PGT-A to the public. It appears high time to educate our colleagues in general OB/GYN better.
1. Girardi et al., Hum Reprod 2023; dead049. doi: 10.1093/humrep/ dead049. Online ahead of print PMID: 36928183
2. Gleicher et al., Trends Mol Med 2021;27(8):731-742
3. Bolton et al., Nat Commun 2016; 7:11165
4. Wang et al. J Assist Reprod Genet 2023;40:639-652
5. Sanders et al., J Assist Reprod Genet 2023;40:653-664.
6. Barad et al., Hum Reprod 2022;37(6):1194-1206
7. McNamee et al., J Assist Reprod Genet 2023;40:665-669
The CHR has had a longstanding interest in the FMR1 gene, well documented by a large number of publications. This is why yet another rather unusual paper, this time by Israeli authors attracted our attention; unfortunately, however, again for all the wrong reasons: In trying to explain the purpose of this paper, the authors correctly note that so-called premutations of the FMR1 gene are closely associated with ovarian dysfunction, including an increased risk of developing primary ovarian insufficiency (POI), also often called premature menopause (before age 40). Yet, things get strange because they suggest that because of the gene’s association with POI, the FMR1 premutation-range genotype may also be associated with numeric sex chromosome variations. But why one would suspect such an association is unclear.1
We, therefore, made this manuscript the third finalist for “the worst paper” in this issue of the VOICE but, obviously, nothing can beat the beforehand-noted winner.
1. Malcov et al., J Assist Reprod Genet 2023;40:683-688
The CHR recently in response to a query to the New York Department of Health regarding the use of semen from HIVpositive males in making embryos for transfer into a gestational carrier, received the following information from Matthew Kohn, PhD, Director of the Tissue Resource Program. We felt that the distribution of this policy may be reassuring for all potentially affected parties.
10 NYCRR part 52 requires that all semen donors be tested and found negative for HIV-1 and HIV-2 and precludes the use of semen from a donor who has tested positive for either virus.
Since 2017, it has been the Department’s policy that U = U, or “Undetectable = Untrasmittable.” As per the policy, “people living with HIV (PLWH) who have achieved and continue to maintain an undetectable viral load, do not sexually transmit HIV.
Under Part 52-3.8 the Department may exempt a tissue bank (like, for example, the CHR) from specific requirements in Part 52 under limited circumstances. Requests for exceptions to allow assisted reproductive procedures, using semen from directed (known) donors who are living with HIV may be provided under the following conditions:
• The semen donor is taking antiretroviral therapy as prescribed and has an undetectable viral load by blood testing concurrent with the collection of the semen specimen(s) to be used.
• The recipient, including a gestational carrier, is fully informed and counselled about the risks by the tissue bank medical direc tor or attending physician, with documentation of such.
• The recipient is offered pre- and post-exposure prophylaxis for HIV, including 20 days preceding embryo transfer and 28 days post-embryo transfer procedure.
• The tissue bank follows CDC’s Universal precautions in handling the reproductive tissues.
• To prevent accidental misuse of the reproductive tissue, the tissue bank sequesters the semen specimens, and any resulting embryos, from other donor samples.
• If a gestational carrier is used, the tissue bank is registered as an Assisted Reproductive Technology Service Provider, the surro gacy agreement adheres to the requirements of the ChildParent Security Act, and the gestational carrier is provided with the Gestational Surrogate’s Bill of Rights.
The strange infectious disease outbreaks around the world appear to continue. The CDC issued this Health Alert Network (HAN) Health Advisory to inform clinicians and public health departments in the United States about two confirmed outbreaks of Marburg virus disease (MVD)—one in Equatorial Guinea and one in Tanzania. Currently, there is no evidence to suggest that these two outbreaks are related; most experts agree that these represent two independent animal-to-human spillover events. To date, no confirmed cases of MVD related to these outbreaks have been reported in the United States or other countries outside Equatorial Guinea and Tanzania. This Health Advisory provides information about these outbreaks to increase awareness of the risk of imported cases in the United States. It also summarizes CDC’s recommendations for case identification, testing, and clinical laboratory biosafety considerations in the United States.
1. https://emergency.cdc.gov/han/2023/han00489.asp
With autoimmune thyroid disease being the most common female autoimmune condition in women of reproductive years and systemic lupus erythematosus (SLE) being one of the most common autoimmune diseases with significant relevance for pregnancy, the interplay between these two conditions, of course, is of interest to reproductive medicine. This is why a recent paper by Chinese investigators in the JCEM attracted our attention which, to our knowledge for the first time, pointed out a complementary genetic bidirectional relationship between these two important diseases.1 Odds ratios (ORs) of SLE on primary hypothyroidism were 1.037 (95% CI. 1.013-1.061; P=2.00x10-3) and 1.007 (95% CI. 1.0011.013; P=0.032). Interestingly, TSH levels were not associated with
SLE. Colocalization analysis, moreover, demonstrated shared causal variants between the two diseases.
These findings, of course, do not surprise, as these two conditions (at least in Chinese patient populations) have been known for some time to demonstrate associated prevalence.2 Moreover individuals with one autoimmune disease for decades have been known to be at increased risk for other autoimmune diseases. The likely principal clinical conclusion from this study, therefore, is that, like with other autoimmune diseases, women with these two conditions must be watched for evidence of other autoimmune diseases and, in this case, a close association between hypothyroidism and SLE (and vice versa).
Based on anecdotal clinical experiences, we actually would have expected a stronger association between these two conditions, raising the question of whether the rather weak association reported in this manuscript does not more reflect the general association between autoimmune diseases rather than the in this paper claimed “special” association between SLE and hypothyroidism.

1. Liu et al., J Clin Endocrinol Metab 2023;108:941-949
2. Li et al., Am J Med Sci 2020;08.026
Fighting for every egg and embryo
The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400.
The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiology, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved.
For letters to the editor, comments, and suggestions, please contact Micah Elias at melias@thechr.com. For all advertisements or sponsorships in The VOICE , please contact Alexandra Rata at arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR. .R


