




Assuming no need for immediate communication to our readers, this issue of the VOICE closes the volume for the current academic year. After the usual annual summer break during July and August, we then will return in early September with the first issue of the new volume for academic year 2024/2025.
Occurrences in the past year on many different levels mandated deviations from “usual” practices at the VOICE. We, consequently, found ourselves wading into territories we never before had covered in these pages and that must be explained.
Nothing exemplifies this point more than the world’s reaction to what happened on October 7, 2023, in Israel. As horrific as the unprecedented inhumanities of that day were (the biggest slaughter of Jews since the gas chambers of Nazi Germany), they could be viewed as deranged exceptional behavior of Hamas, a fringe terrorist group (so defined for a long time by most Western countries). But the shock brought on by this day was then even exceeded by what the world’s reaction to October 7 revealed about the antisemitic underbelly of even most Western societies, including our own.
We found it impossible to ignore that professional medicine – including, shamefully, many of the principal societies in our medical specialties of Obstetrics & Gynecology and Reproductive Endocrinology & Infertility - allegedly committed to the defense of women’s rights - remained silent when women (and, hardly ever mentioned, males as well) were not only systemically raped and sexually mutilated as part of a strategic act of war, but then also literally slaughtered in most gruesome ways in the hundreds.
And, though the perpetrators, themselves, proudly filmed how they “played ball” with cut-off breasts and other mutilated organs of their victims and how they brutally murdered children in front of their parents and parents in front of their children, cut babies out of pregnant women’s bellies and beheaded them, and burned whole families alive, hugging each other in their gruesome death, not a word from women’s organizations worldwide.
The U.N. leadership took weeks to publicly address the atrocities, even though the perpetrators’ videos were all over the Internet. Even the Nazis were trying to keep their atrocities secret. Here, there was no such shame. To the contrary, these actions were considered “heroic.” For example, a widely circulated tape allowed us to witness firsthand how a proud Gazan mass murderer called his parents on his cell phone to report “how many Jews he already had murdered with his own hands;” and his parents – just regular civilians in Gaza – congratulated him on this achievement!
And then there was, of course, the welcome as “returning heroes” these murderers received from their civilian brethren upon returning with live and dead hostages to Gaza, as they were dragging dead bodies (some from the waist down naked women) behind their trucks though dusty streets and showing off on their trucks still alive (often wounded) hostages of all ages. To the time of this writing, after eight months have passed, still over 120 hostages – most as of this moment no longer believed to be alive – are held by Hamas. The International Red Cross has not even visited one!
How the world (this country included) reacted to these atrocities was, however, even more motivating for us in deciding to speak out in these pages. It was not only the shameful silence of women’s groups, academic institutions (among those many of the most prominent universities in New York City, Chicago, and Los Angeles), professional organizations, and many politicians (among the latter surprisingly also many prominent Jewish politicians), but - almost impossible to believe considering the video-evidence provided by the perpetrators of these atrocities themselves - many voices actually denied that these atrocities really ever had happened and/or defended them as acceptable resistance to a decades-long occupation by an oppressed Palestinian people (more on this argument below).
Many were well-known intellectual leaders, of course including politicians, journalists, and other media personalities, prominent academicians (including from leading New York Universities, such as Columbia University and NYU). And then there, of course, were also the students of those professors at prominent universities, setting up protest camps, while advocating in favor of Hamas, first at Columbia and, once they discovered how much visibility they were receiving from the media for their often-abhorrent statements and pronouncements, the encampments spread to many of the most famous universities in the U.S. and overseas.
Such seemingly highly-educated Hamas supporters – one would assume - should, of course, also have enough intellectual curiosity to familiarize themselves with subjects before addressing them publicly in their exercise of free speech. Their ignorance of essential historical facts, however, proved over-and-over again astonishing: When questioned, almost none, indeed, appeared to have even most basic knowledge about geography (which river and which sea, when pronouncing “a Palestine from the river to the sea”) and history of the region.
Almost none were, indeed, cognizant of the fact that a Palestinian people and/ or Palestine state never really existed in the Middle East. The concept of such a separate Palestine people (from just being part of the Middle East’s Arab-Muslim world) was for the first time only suggested by Yasir Arafat in the 1960s with establishment of the Palestine Liberation Organization (PLO) (at the time basically almost exclusively a terror organization specializing in airline hijackings). Only in1988, the PLO publicly “reupdated terrorism in all of its forms, including state terrorism” and became the widely accepted “representative” of the newly discovered Palestinian people under the new name of the Palestinian Authority (PA). Though supposedly having rebuked “all forms of terrorism” (in contrast to Hamas), the PA to this day pays life-long stipendia to families of Palestinian terrorists and, not only has failed to condemn the October 7 attack by Hamas but announced that families of all fallen and/or imprisoned Hamas fighters would also be eligible for these payments.
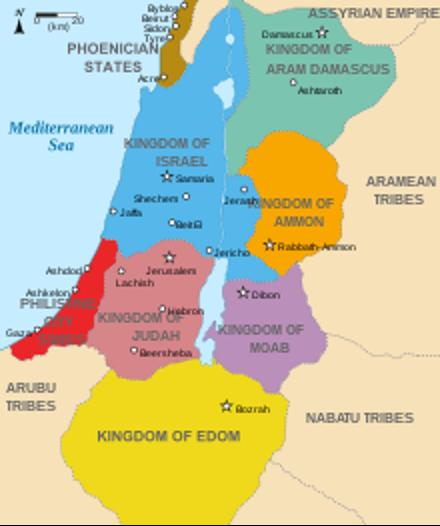
Members of the “intellectual elites” in Western societies supporting Hamas (including here in the U.S.), thus, either are unaware of or (more likely) on purpose completely ignored the historical fact that until the 1960s the Muslim population in the region was, indeed, not considered Palestinian (as a people) but “Arabic.” in Arabic called “al-filiastiniyyin” (after the Biblical Philistines), often also referred to in Arabic as “al-arabi il-filastini.” And, as an interesting historical sidenote, the Philistines mostly lived in the territories now called Gaza (see below the map of the region in the 9th century BC).
If their professors did not know these historical facts (or, more likely, found them to be a rather inconvenient truth), who then can be surprised that almost all the students creating havoc at U.S. (and other) universities in recent months usually proved to be historically and geographically uninformed about the Middle East. We here, however, do not wish to further regurgitate the amazing ignorance of the very visible part of the politically surprisingly effective worldwide proHamas movement. Nor do we intend to address here the invisible part of this movement, including the movement’s financial resources and organizational structure. What we like to concentrate on is that we, like so many others in this country and elsewhere, were surprised by how deeply this country was already in the midst of a very serious educational crisis in higher education (already starting in middle-school or even before), having produced a generation of mis- or under-educated youth, yet radically indoctrinated in Marxist and related theories. Unfortunately, this also applies to medical schools.
All of these schools of higher learning, therefore, share in the blame of having produced a generation of graduates highly motivated in often rather aggressive advocacy, yet characterized by an astounding lack of factual knowledge, and not only in subjects they advocate for politically, but also in subjects they are studying for lifelong careers. How, because of increasing numbers of primarily equity-driven admission to medical school, medical students at UCLA’s medical school have in recent years increasingly been failing basic tests of medical competence (now up to half of all students) is only one – but rather well documented - example, sourced to whistleblowers, recently reported on May 23, 2024, by Aaron Sibarium in the Washington Free Beacon. As it involves medical education, it, of course, resonates at the CHR.
Differentiation between indoctrination and education in medicine is essential, as medical practice must be based on best available evidence and not personal beliefs and/or ideologies. Nobody, of course can disagree with the notion that maximal efforts to achieve equity in admissions to medical schools (and medical practice in general) must be made. This, however, does not mean that considerations about equity should outweigh other qualifying characteristics for admission to medical schools (or other positions in society). Meritocracy must remain the leading consideration if schools are to maintain educational excellence. Abandoning meritocracy in medicine, moreover, would not only be harmful for schools of higher education, but society as a whole, a position we acknowledge –some of our readers and colleagues may disagree with (see also the letter-to-the editor section below).
By now, engulfed in a circle of political and social discovery, the VOICE could no longer remain silent. It had started on October 7, 2023, with the slaughter of almost 1,500 Jews, followed by very disappointing worldwide responses to this tragedy and, in intensity and in its aggressiveness almost shockingly surprising support Hamas received on college and university campuses, often crossing over into blatant antisemitism.
Contrary to most parents’ expectations, the not insignificant tuition fees they had paid for their children’s education, instead of education, had bought firstclass Marxist indoctrination. It suddenly was obvious how deep the U.S. advanced education system had sunk into crisis.
As a consequence, many youths (as during the demonstrations became apparent, in a large majority surprisingly young women rather than men) have come to divide the world into the oppressed (i.e. Palestinians) and their oppressors (i.e., Jews and Israel). And – on a side note – the protestors also followed the widely shared practice among radical Muslims of describing Israel and the U.S. as, respectively, the “small and big Satan”).
We consequently expect to remain involved in these subjects into the coming academic year (and maybe even beyond). As noted before, we understand that not all of our readers and colleagues will agree with our interpretation of facts and resulting conclusions, but we are used to that from the CHR’s clinical work: The CHR is, after all, well known all around the world for “thinking differently” when it comes to treating infertile patients when others have failed. Though we in clinical practice have much more often proven to be right than wrong, we, of course, are by no means perfect and, therefore, welcome opposing opinions (see letter below as an example).
Our intent, moreover, is not to become “political” or even “partisan,” but we have come to accept that we live in politically revolutionary times, where truth must be spoken to rebut harmful and illogical clinical practices, whether during a pandemic like COVID-19 or in infertility practice, but also when it comes to illogical and dangerous opinions and ideologies that threaten the quality of medicine and society as a whole.
As we do not ever presume exclusivity in knowledge or even wisdom, we strongly encourage and welcome our readers’ written participation in discussion of everything published in the VOICE, especially if subjects are controversial and our readers do not share our opinions. Please address your opinions, therefore, in submissions to FEEDBACK at social@thechr.com.
Aside from societal and social issues, we in this month’s VOICE, also, of course, again address more directly- “medical” issues which, as always, encompass a broad range of subjects and a detailed medical literature review of articles with special relevance to our specialty area of reproductive endocrinology and infertility. We hope you, as always, will enjoy the read.
Besides apologizing for the unusual length of this note to our readers, we also want to take the opportunity to wish you a wonderful and hopefully peaceful summer, wherever you may spend the summer months. To our Israeli and Palestinian friends and colleagues, we, of course, wish the same, and in addition hopefully an end to the war and, most importantly, “LET THEM COME HOME NOW!” The same also applies to our Ukrainian and Russian friends and colleagues and, of course, to friends and colleagues anywhere else in this crazy world where people shoot at each other and kill each other instead of speaking to each other.
The Editorial Team of the VOICE
We love eggs!

BRIEFING: The VOICE welcomes communications for the FEEDBACK section and publishes communications received whenever space allows. Any received written communication regarding the VOICE is considered a submission for consideration. Accepted publications will be published anonymously, unless we received specific written confirmation from the authors that she/he wishes the FEEDBACK to appear under her/his name.
My feedback is that I used to look forward to reading your newsletters to learn about your fertility research. However, I’m now deeply troubled by the aggressive evidence-free political stances expressed in this recent issue [Ed: May issue]. For example, the current safety problems at Boeing are a result of diversity practices?
Where is the evidence for this? I see that it’s a Twitter thread by Elon Musk and musings by Bari Weiss. Your nuance free evaluation of Covid policy and long Covid was also deeply problematic.
How disappointing and troubling. Although I am forever grateful to Dr. Barad, this newsletter makes me question your overall practice's ability to evaluate evidence and makes me reconsider recommending CHR to anyone else in the future.
We are always sorry to disappoint but things are not always as they appear; We quoted Elon Musk in the “News” section of the May issue of the VOICE (p. 20), within a discussion of DEI (diversity, equity, inclusion) in medical schools. As this June issue of the VOICE quite well demonstrates, this subject, indeed, appears to gather interest and readers will, therefore, find it addressed repeatedly here in the June issue.
We never said that diversity practices at Boeing were the cause of the company’s recent problems. We just reported and referenced several publications – among those the New York Post - quoting Elon Musk that Boeing in recent years had shifted financial incentives for employees away from quality to such issues as DEI. Interestingly, Boeing, since, announced “shifting employee bonuses back to safety and quality” (CNN, March 8, 2024).
The other article referred to as “musings of Bari Weiss,” principal founder of The Free Press and former senior editor at The New Yok Times, was really written by Franceska Block and Olivia Reingold (though in The Free Press) and did not address Boeing but Google’s catastrophic A.I. chatbot launch which widely was attributed to biased data entry and, therefore, was by some called “Woke” AI Disaster (Business Insider, February 28, 2024). Similarly, what the letter called out a “nuance-free and problematic evaluation of COVID policy and Long Covid,” was also a –somewhat longer – blip in the “News” section, quoting articles in The Wall Street Journal, The Free Press, and in prestigious medical journals, like PNAS as well as a World Health Organization (WHO) publication.
The only comment that, however, really troubled us here at the VOICE was the suggestion that, based on all the above, the writer had to questions the “overall ability of the CHR to evaluate evidence.: This is like saying, because I don’t like the opinion of the food critic of The New York Times, I no longer believe anything the newspaper writes. This, of course, makes little sense but, unfortunately, reflects the intolerance for differences in opinion our society has been experiencing and we daily feel more and more around us. In this case the alleged transgressions were not even opinions but reports in the media on a subject that quickly appears to attract increasing attention. We, hopefully, will hear more from this writer, as we continue to cover the subject.
---THE EDITORS
BRIEFING: The title of this article includes a question we hear from a surprisingly large number of infertility patients in a variety of ways. Often, indeed, the version differs a bit when patients, after being diagnosed with PCOS at the CHR, note that their own reading made them suspect this diagnosis, but their physicians denied it, either because they were not obese, were ovulatory with regular menses, and/or were not hyperandrogenic. This Feuilleton article tries to explain why so many cases of PCOS are missed even in, otherwise, competent fertility centers and why nutrition and supplements play an ever-bigger role in the treatment of PCOS.
Despite being one of the most common diagnoses in reproductive medicine, Polycystic Ovary Syndrome (PCOS) is still often misdiagnosed. Interestingly, misdiagnoses can go both ways, sometimes PCOS is under- and at other occasions it can be over-diagnosed. There are several reasons for this confusion but the understanding of any medical condition, of course, starts with its definition.
If diagnoses cannot be made consistently, accurately, and reproducibly, how can a condition then be properly investigated? It can’t, and that has been the crux of the matter since PCOS was first “identified” as the Stein-Leventhal Syndrome, an independent diagnosis associated with female infertility. Only years later was it proposed to be made up of several supposedly distinct phenotypes, which over ensuing years and several international gatherings of “experts” (NIH, Rotterdam, etc.) were defined not based on evidence obtained through studies but based on “expert” opinions. Yes, “experts” literally voted based on personal opinions how those phenotypes should be defined and whenever medicine almost exclusively relies on “expert opinions,” we, by now, of course already know that no good can come of it. Consequently, PCOS to this day has likely remained the least understood among most important infertility diagnoses.
But that, of course, does not mean that there has been no progress in our understanding of PCOS. Quite to the contrary: we now understand that PCOS in at least some of its proposed phenotypes is later in life closely associated with metabolic syndrome (a risk for diabetes, hypertension, and heart disease) and, yes, we more recently have also learned that PCOS likely has a male counterpart. Most importantly, however, recent clinical (at the CHR) as well as genomic studies (at the medical endocrinology department of Mount Sinai in NYC) have suggested that what to this day is called PCOS, likely, represents two distinct genomic conditions, one mostly “metabolic” and the other associated with a hyperactive immune system.
Traditionally, PCOS has been defined as presence of two among three of the following factors: irregular menstrual cycles and/or anovulation, clinical or biochemical signs of hyperandrogenism (excessive “male hormone” levels), and/or ultrasound evidence of polycystic ovaries typically showing 12 or more small follicles in each ovary or increased ovarian volume. But one of the most important factors overlooked over decades by the “experts” charged with defining PCOS, has been the absence of age considerations in the diagnosis of PCOS since PCOS, of course, is not a static condition but changes with advancing age.
The absence of age considerations in defining/diagnosing PCOS greatly contributed to misdiagnoses in PCOS. For example, the presence of multi-follicular ovaries in young post-menarcheal women with normal ovarian function and response is not necessarily PCOS, even if they skip some periods. Lack of age considerations also led to the incorrect definition of the D-phenotype of PCOS (under Rotterdam criteria) as “normo-androgenic.” As the CHR’s investigators, however, demonstrated, this phenotype between menarche and approximately age 25 is just as hyper-androgenic as all other (A, B, and C phenotypes). Its androgen levels, however, after age 25 start to decline because adrenal androgen production becomes increasingly insufficient. Rotterdam criteria considered the D-phenotype normo-androgenic because between ca. ages 25 and 35 (when over 90% of PCOS diagnoses are made) these women’s androgens are in normal range, only to fall into abnormally low levels after age 35. The CHR, therefore, renamed this phenotype after age 35 as the hyper/hypo (HH) PCOS because it requires androgen supplementation at this point if women are to conceive in their IVF cycles (please see conflict statement).
Because phenotype-D women in addition are not obese (they are also called the “lean” phenotype), have usually regular menses and are ovulatory, they very
frequently are not diagnosed as PCOS patients and often are tagged with the diagnosis of “unexplained” infertility.
Implications of PCOS diagnosis on fertility, based on the phenotype
Based on their published research, Dr Gleicher and Dr Barad recently proposed to classify the different PCOS phenotypes in two distinct entities based on the individual androgen levels profile: a hyperandrogenic (H-PCOS) and a hyper-/hypoandrogenic phenotype (HH-PCOS). Understanding ovarian behavior and response to IVF treatments based on these classification, allows optimizing clinical and reproductive management. On the one hand, H-PCOS is characterized by being associated with metabolic syndrome like overweight and tendency to develop diabetes. Women with H-PCOS very often suffer from irregular periods because of delayed ovulation or lack of it in some cycles, which can lead to difficulty conceiving. If this is your case, the good news is that losing about 10% of body weight and addressing ovulation might overcome infertility. Moreover, if needed, response to IVF is usually good due to the high ovarian reserve, which actually needs to be watched to avoid overresponse (the so-called ovarian hyperstimulation syndrome). On the other hand, the HH-PCOS appears hyperandrogenic with advancing age becoming a hypo-androgenic phenotype, in about 85% of the cases presenting with a hyperactive immune system, allergies, inflammation and autoimmune conditions like hypothyroidism or recurrent miscarriages. We have observed that women with HH-PCOS frequently become relatively resistant to ovarian stimulation for IVF from age 35 years and later in life, which however understandably improves with androgen supplementation using DHEA for at least 4 weeks. Therefore, due to its significance in clinical care, it's crucial to pursue a thorough fertility expert evaluation for accurate characterization of PCOS if you've ever been informed of its possibility!
The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces a DHEA product and other fertilityrelated supplements. Since this article, among other subjects, addresses androgen supplementation with DHEA, readers are advised that expressed opinions in this paragraph, therefore, may be accordingly biased.
A comprehensive clinical approach toward PCOS in many cases must also involve dietary interventions. In obese phenotypes that means caloric restrictions and, as we discussed in the May issue of the VOICE, potentially pharmacologic interventions with the new family of GLP-1 weight loss drugs.
Women with HH-PCOS, however, of course do not need to lose weight; they, however, in over 85% demonstrate evidence of a hyper-active immune system and, therefore, may significantly benefit from an anti-inflammatory diet.
As in most instances in medicine “one-size-fit-all” approaches are nonsensical. This also applies to the use of supplements which in the infertility field has reached at times almost bizarre excesses. It now is not uncommon to see women taking between 10 to 20 supplements, often not even knowing what they are supposed to do. Not only does such exaggerated supplement use in the end prove costly, often simply makes no sense, but, at times, can be even harmful. A good example for the latter is the uncontrolled use of myoinositol in PCOS patients. There, indeed, is good evidence that myoinositol is effective in lowering androgen levels in hyper-androgenic PCOS women; but if above described HH-PCOS patient takes this supplement, she will only further aggravate her already existing hypoandrogenism.
Inositols are naturally occurring compounds belonging to the vitamin B family of molecules. They are present in fruits, beans, grains, and nuts, and can also be synthesized by the human body, while playing roles in various cellular functions such as signal transduction, lipid metabolism, and insulin signaling. Inositol, particularly myo-inositol (MI) and D-chiro-inositol (DCI), have shown promise in managing hyperandrogenic PCOS because of potentially beneficial effects on insulin sensitivity and hormonal regulation and because of decreasing androgen effects. In addition to improving acne and hirsutism (excess of facial hair growth), it has also been suggested to support follicle maturation, ovulation, regular menstrual cycles and, hence, fertility.
DHEA is a hormone naturally produced by ovaries and adrenal glands which serves as substrate in our bodies for the production of testosterone. DHEA, there-
fore, increase the levels of testosterone and to a lesser degree after aromatization estrogen. This can lead to a range of positive effects in hypo-androgenic women, including improved sexual function, muscle mass and strength, bone density, mood and cognition, reduced body fat, and enhanced ovarian function and egg quality. Since these hormones can interact with other hormones and medications, DHEA should always be prescribed by a professional.
By helping to increase and maintain appropriate androgen levels, DHEA supplementation can play a key role in the hormonal ovarian environment of women over 35 years of age with HH-PCOS, enhancing follicular growth and egg maturation. Evidence suggests that DHEA supplementation may improve egg quality and, consequently, success in natural conception. Moreover, in IVF, it also improves ovarian response to stimulation, possibly leading to better egg yield following retrieval.
Reading list
Dapas M, Dunaif A. Diagnising of polycystic ovary syndrome: Genomic insights into PCOS causal mechanisms and classifications. Endocr rev 2022;43(6):927-965
Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol 201;9:67
Gleicher N, Darmon S, Patrizio P, Barad DH. Reconsidering the Polycystic Ovary Syndrome (PCOS). Biomedicines. 2022 Jun 25;10(7):1505. doi: 10.3390/biomedicines10071505. PMID: 35884809; PMCID: PMC9313207
Kurzrock R, Cohen PR. Polycystic ovary syndrome in men: Stein-Leventhal syndrome revisited. Med Hypotheses 2007;68(3):480-483
Shohat-Tal, Sen A, Barad DH, Kushnir V, Gleicher N. Genetics of androgen metabolism in women with infertility and hypoandrogenism. Nat Rev Endocrinol 2015;11(7):429-441
ADVERTISEMENT
The only prenatal on the market following the latest science!


BRIEFING: In this ongoing series of articles on “testing the tests,” the author this time directs his attention toward carrier screening for recessively inherited diseases. Such screening was initiated decades ago for a handful of high-prevalence conditions but has in recent year progressively expanded, now encompassing hundreds of tests in so-called “expanded” carrier testing. As new technology now allows to do this at similar costs to older tests for only a handful of recessive diseases, the primary argument of the genetic testing industry has been that such expanded testing, therefore, is cost-effective. As in so many other areas of medicine, the genetic testing industry here, however, may once again not be telling the whole story, as Dr. Barad here well outlines.
Carrier screening is used to identify couples who might share recessive mendelian genetic traits that could negatively affect the health of their prospective children if both parents were carriers for the same recessive disease (creating a 2.5% risk of having a child affected by the disease). Screening for carrier states was first introduced in the 1960s with early efforts exclusively focused on screening of African American couples for sickle cell disease.
In the 1970s, carrier screening was expanded to the Ashkenazi Jewish population after advances in testing methods led to the development of carrier screening programs for Tay-Sachs disease. Further technological progress with the advent of DNA technology in the 1980s allowed for the screening for the CFTR gene for cystic fibrosis (CF), adding by the 1990s, for the first time, a routinely recommended screening test for the general population in women planning on pregnancy or already pregnant. Both earlier tests had exclusively been recommended only based on racial/ ethnic backgrounds of patients. Moreover, in general only the woman was tested and male partners were tested only if the female proved to be positive as a carrier.
By the early 2000s general (expanded) screening, however, started to include progressively more high-prevalence disorders in the general population, including spinal muscular atrophy (SMA). Recommended maternal testing also for the first time included a condition not inherited as a recessive trait but as a so-called “expansive” gene passed on through the mother (sex-linked), the fragile X mental retardation-1 (FMR1) gene (which is located on the X chromosome), and causes the Fragile X syndrome (FXS).
An offspring is at risk for FXS (when his/her gene has >200 CGG repeats), with males more severely affected than females (who have the benefit of 2 X-genes), if the mother has between 55-200 CGG repeats in one of her FMR1 (i.e., a 50% risk), because her gene in one generation can expand from that CGG-range to >200 repeats. In addition, the genetic testing industry also started to offer increasing testing opportunity for single gene diseases inherited in dominant fashion (I.e., 50% risk for offspring).
Polymerase chain reaction (PCR) and other new molecular techniques continued to enhance the accuracy and efficiency of all of such screening, while concomitantly reducing the costs. Unsurprisingly, professional organizations, including the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMGG) started to support the use of general carrier screening for prenatal/preconception population for common single-gene autosomal recessive disorders.
The development of next-generation sequencing (NGS) significantly improved the ability to screen for multiple genetic disorders simultaneously. Once again, unsurprisingly, expanded carrier screening panels continued to expand, initially involving a few dozens of diseases and by now including hundreds of genetic conditions, though basically at a fraction of the expense of a single-gene test in the early days of carrier testing.
As so often appears to be the case, commercial laboratories continued adding diseases to routine testing panels with little evidence-based guidance as to which genes did and did not make sense to test for.
Yet, the list has continued to grow despite lack of evidence that this “super”- expanded carrier screening, including diseases with progressively lower prevalence, still served useful clinical purposes. Nevertheless, such testing has become routine practice in many general Ob/Gyn and infertility clinics, at least to a degree driven by marketing efforts of the genetic testing industry which, of course, has an obvious profit motive (more on that below).
But before we get to that, here is a little introduction to the science of genetics to better understand this issue: Even though it is now possible to test for thousands of genetic mutations, it is important to understand that the mere presence of a genetic mutation does not necessarily predict the exact risk this presence represents for expressing a disease and the reason is that, like a thermostat, mutations can have different degrees of expressions. Moreover, mutations in our genes are very common, so much so that with routine testing for hundreds of mutations (as is done regularly nowadays), most individuals will test positive as carriers of at least one or two mutations.
And this is where the problem starts: In a large majority of cases, it is, still, the woman who gets tested first. Since most women will be found to be carriers for something, most male partners will now also choose to be tested (of, course a very attractive proposition for the genetic testing industry). Such partner testing will now be performed even if the prevalence of the female’s mutation in the general population may be so low that it statistically equates the probability of winning in the lottery.
The likelihood of the male also testing heterozygous for the same genetic trait as the female and, therefore, the risk that this couple may have a one-in-four chance of having an affected offspring, depends firstand-foremost on the prevalence of this mutation in the population: the lower the prevalence, the less likely will the male demonstrate the same mutation (though he, of course, may have others that don’t matter since the female does not have those). Assuming, however, both partners do share a common mutation, the question arises what to do next, given that the couple does not wish to take the risk of having a child with the disease?
In principle, such a circumstance offers two options: The first is to ignore the risk, proceed with conceiving, and test the pregnancy through CVS or amniocentesis to find out whether the pregnancy is affected or not.
As noted, the odds are that 3:1 that the pregnancy will not be affected. This option, however, requires being comfortable with the notion of terminating the pregnancy in case it proves to be affected.
The second option is a procedure called preimplantation genetic diagnosis for monogenetic diseases (PGT-M), in which the couple undergoes IVF, produces embryos which are tested by PGT-M to see whether an embryo is affected by the disease or not. Under this procedure, only unaffected embryos are transferred.
This is, however, where the discussion must return back to the already previously addressed point that the clinical expression of a monogenic diseases can have variable expression, from very mild to very severe disease. When an embryo is diagnosed as “affected by PGT-M,” it is, in most cases, impossible to know whether the embryos would give rise to a minimally or maximally affected offspring. If a minimally affected embryo is not transferred or even discarded, that, of course, reduces the cumulative pregnancy chance of the IVF cycle.
An ACOG Committee Opinion published in 2017 and reaffirmed in 2023 is probably the last authoritative word on the subject, making the following points:
• All patients should be offered carrier screening for cystic fibrosis and spinal muscular atrophy as well as screening for thalassemia and hemoglobinopathies. Fragile X premutation screening is recommended for women with a family history of fragile X-related disorders.
• Couples with consanguinity should be offered counseling to discuss their progeny’s increased genetic risks.
• Carrier screening panels should be limited to those conditions with a carrier frequency of 1% or greater, have a well-defined phenotype, have detrimental effect on quality of life, cause cognitive or physical impairment and require intervention early in life.
• Carrier screening should not include conditions primarily associated with disease in an adult.
This is, however, exactly why current testing practice appears excessive because most of the conditions screened in large carrier screening panels now offered by the genetic testing industry have a carrier frequency of far less than 1 in 100.
Some have a carrier frequency of less than 1 in a million, which is the reason for the analogy of winning the lottery since this means that even if a woman is a carrier for such a rare condition, the chance that her reproductive partner would also be a carrier for the same condition would be less than 1 in a million.
One more point: Some of the conditions screened for in the larger carrier screening panels may be more common; but are obviously not lethal, and only affect quality of life. One such condition is so-called non-syndromic hearing loss, accounting for ca. 70% of all inherited hearing loss cases. The carrier frequency of this condition among couples of European ancestries may be as high as 4%, though at 2%, it is lower in Asian populations. It is, however, the most prevalent sensory disorder, affecting 1 in 1,000 newborns with hearing impairment. The prevalence of sensorineural hearing loss (SNHL) increases as children grow, reaching 2.7 in 1,000 children by the age of 5.
While hearing loss, of course, affects life to significant degrees, it does not preclude an otherwise healthy and normal life. Couples who are both carriers for SNHL and have only one embryo left after an IVF cycle, which was found by PGT-M to be affected by the disease, may choose to transfer this embryo after all. As noted before, there is only a 25% risk of having an affected child. The chance of having an unaffected child is three-times better. Parents, therefore, may choose to forgo all genetic testing in such a case and just trust nature in 75% of cases to produce an unaffected child.
Finally, the medical literature demonstrates that many conditions nowadays routinely tested in expanded carrier screening have been reported worldwide in
CONDITION
3-BETA-HYDROXYSTEROID DEHYDROGENASE TYPE II DEFICIENCY (HSD3B2)
3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase Deficiency (HMGCL)
3-Methylcrotonyl-CoA Carboxylase 1 Deficiency
3-Phosphoglycerate Dehydrogenase Deficiency (PHGDH)
6-Pyruvoyl-Tetrahydropterin Synthase (PTPS) Deficiency (PTS)
Abetalipoproteinemia (MTTP)
Achondrogenesis, Type 1B (SLC26A2)
Achromatopsia, CNGB3-Related (CNGB3)
Acrodermatitis Enteropathica (SLC39A4)
Acute Infantile Liver Failure, TRMU-Related (TRMU)
Acyl-CoA Oxidase I Deficiency (ACOX1)
Aicardi-Goutières Syndrome (SAMHD1)
Alpha-Mannosidosis (MAN2B1)
Alpha-Thalassemia (HBA1/HBA2)
Alport Syndrome, COL4A3-Related (COL4A3)
Alstrom Syndrome (ALMS1)
Andermann Syndrome (SLC12A6)
only very few cases. The chance of a partner to also test positive would, therefore, be infinitesimally small (i.e., even smaller than winning in the lottery). The table below demonstrates how small!
Why, then, do the testing? Because genetic testing companies make a lot of money with extended carrier testing. And they more often than not make it twice because if the lady of the house turns out to be a carrier (and, as noted before, the more diseases we test for, the more women will be carriers of something), the gentleman of the house must now also be tested.
What does it cost? The webpage of one company recently made the claim that average “out of pocket” expenses for patients who have met their insurance deductible end up being less than $249. But what if the list price is $5,000 (even if insurance company reimbursements are, of course, greatly discounted) and you have not met any of your deductibles yet? We all know the answer: The patient will get a bill for $5,000 for the testing of one person and for $10,000 if both partners have been tested. The time appears right for less testing, lower costs, and more transparency!
Reches A, Ofen Glassner V, Goldstein N, Yeshaya J, Delmar G, Portugali E, Hallas T, Weinstein A, Kurolap A, Berkenstadt M, Mantsour T, Abu-Gutstein L, Ries-Levavi L, Reznik-Wolf H, Behar DM, Yaron Y, Pras E, Baris Feldman H. J. Med Genet 2024 May 6:jmg-2023-109629.doi: 10.1136/jmg-2023-109629. Online ahead of print.
Van Tongerloo AJAG, Verdin H, Steyaert W, Coucke PJ, Janssens S. Accepting or declining preconception expanded carrier screening: An exploratory study with 407 couples.
J Genet Couns. 2024 Apr 12. doi: 10.1002/jgc4.1899. Online ahead of print.
Tan L, Qi Y, Zhao P, Cheng L, Yu G, Zhao D, Song YX, Xiang YG. Clinical application value of pre-pregnancy carrier screening in Chinese Han childbearing population. Mol Genet Genomic Med. 2024 Apr;12(4):e2425. doi: 10.1002/mgg3.2425.PMID: 38562051 Free PMC article.
DISEASE PREVALENCE 1:1,000,000 1:125,000 1:36,000 1:1,000,000 1:66,200 1:1,000,000 1:40,000 1:30,000 1:500,000 1:266,666,667 1:266,666,668 1:20,000,000 1:500,000 1:10,000 1:5,000 1:1,000,000 1:1,000,000
BRIEFING: Here the author discusses the ongoing crisis in fertility across the world. Many first-world countries like Italy, South Korea, and Spain have decreasing fertility rates that could have catastrophic consequences in the future, while the lowest-income countries have the highest rates in the world. The article discusses reasons for this widening gap and its effect on the demographics and economies of these countries. However, these statistics are often used as a means for political scapegoating in the media, clouding the real problem. While some countries have begun to address the issue, major institutional change will be required to reverse this trend (see also page 29).
The falling birth and “fertility” rates across the first world are a well-known trend, at this point covered multiple times in this issue of the VOICE alone.
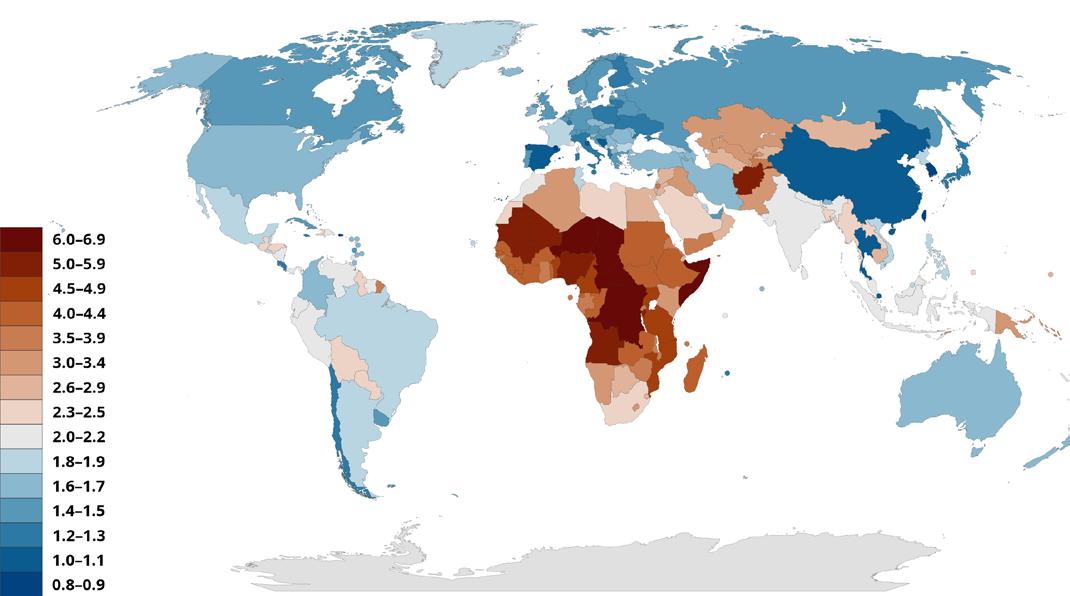
Between 1950 and 2021, the global Total Fertility Rate (TFR) more than halved from 4.84 to 2.23, with more than half of all countries falling below the replacement level of 2.1.1 The countries projected to remain the “most fertile” in the future tend to be the lowest-income countries, with sub-Saharan Africa forecasted to produce over half the world’s livebirths by 2100. On the other hand, countries like Spain, Italy, and South Korea have legitimate fertility crises, with the lowest rates in 2022 belonging to the EU and East Asia.2 Reporting on and solutions to this problem are as complex as the factors that drive this global shift. The Global Burden of Diseases (GBD) 2021 Fertility and Forecasting Collaborators, for example, noted the importance of girls’ education, contraceptive access, and fertility policies as some of the most important predictors of a country’s TFR.3 The relationships between these factors and the crisis at hand becomes apparent after understanding the distinction between “fertility” and the more appropriate word, “fecundity.”
Fertility is defined by the number of offspring a population (or an individual) actually produces. In contrast, fecundity is the possible number of offspring a population or person has the potential to produce over a lifetime.
Although the poorest countries in the world have the highest birthrates, they are by no means the most fertile. These countries usually sport very young populations, demonstrating immense potential for pregnancies (i.e., fecundity), but because of poor healthcare and lack of appropriate financial resources, they also have some of the highest infertility rates in the world,4 yet they produce the most children due to failing education systems and lack of access to contraceptives. Their fecundity (i.e., chance of spontaneous pregnancy) is extremely high but, for various reasons (a high prevalence of sexually transmitted diseases, high pregnancy loss rates, poor obstetrical services, etc), their ultimate fertility can be lower.
As countries become richer, natural fecundity declines. In the United States (U.S.), hundreds of articles have been written in the last decade about how and why millennials won’t have more kids. Their content remains consistent: women prioritize careers and wait too long, the cost of living is unmanageable, institutional barriers trap the working class, etc.5 The result is smaller, older families, and although older maternal ages also affect fertility, access to good healthcare and fertility services at least partially compensate. Ultimately, this leads to wealth consolidation in a continuously decreasing population and an increasing wealth gap between the wealthiest and poorest nations.
Especially when it comes to religion, both sides of the political spectrum have at times turned this issue of low birth rate into a political football. In May, the Vatican got involved in a press cycle about Italy’s historically low 2023 birth count when Pope Francis, historically a liberal icon, came under scrutiny. He conflated this “hemorrhage of life” with what he called “the most profitable investments [right now] ... the manufacture of arms and contraceptives –one destroys life, the other prevents life.”6 While this was likely only meant as a catchy soundbite, it sparked some equally irrelevant back-and-forth in the press, highlighting an important part of the fertility discussion.
That religion matters when it comes to birth rates appears obvious. Here is, however, another example why religion and politics are not a good match. Because most of the high-fertility countries are Arab and/or have Islamic-majorities, discussions about birthrates often devolve quickly into fearmongering. Even in the most populous country in the world, Indian Prime Minister Narendra Modi uses fear of “infiltrators” and “those who have more children” to rally political zealots.8 Discourse about these complex and contextual statistics can often become a political trojan horse for xenophobia.
Regardless of the noise surrounding the worldwide crisis of declining birth rates, the issue remains serious. Grossly oversimplified, an aging population leads to a shortage of labor and puts strain on the economy while widening the wealth gap. In short, repercussions can be bad. Beyond the usual political grandstanding, most countries have not taken serious steps to address the problem. In the U.S., pro-fertility legislation like prolonged paid parental leave would require substantial institutional changes and would be in opposition to half-century-long trends in corporate practices.
The least fertile city in the least fertile country in the world, Seoul, South Korea, has recently begun to seek some solutions.7 For example, an allocation of $73,000 per person was given to men to incentivize 100 to reverse their vasectomies and women to “untie” their fallopian tubes. In addition, the government announced a rather pitiful $5.9 million in medical care for pregnant patients over 35 and $1.6 million for public wedding venues. South Korea now also subsidies egg freezing, offers financial allow-
ances for parents with newborns, and incentives corporations to financially aid their employees in family building. Seoul’s fertility rate last year was a frightfully low 0.55 and was largely blamed on rising costs of living.
While incentives like these are not a new idea for South Korea, it’s unclear whether similar incentives in the Western world would work equally well. To battle quickly growing fundamental societal problems reaching from China to Europe and the U.S., structural and institutional change will be necessary and will only occur if incentives in economic structures are initiated – and soon – that encourage women to have more children and, if possible, at younger ages. The culturally Western country with the highest current birth rate is Israel at rank #57, with a birth rate of 2.9 (the Palestinian population with a rate of 3.3 is at rank #46). At the very bottom is Hong Kong (#204, rate 0.8), South Korea (#203, rate 0.9), Singapore, San Marino, Malta, Macau, China, and Aruba (#202 to 197, rate all at 1.2). The breakeven for replacement, a rate of 2.1, is occupied by 5 countries at #99 to 91, meaning that out of 204 listed countries/territories, only 94 were above replacement levels.9
1. GBD 2021 Fertility and Forecasting Collaborators (2024). Global fertility in 204 countries and territories, 1950-2021, with forecasts to 2100: a comprehensive demographic analysis for the Global Burden of Disease Study Lancet 2021. 403: 2057-2099
2. Yanatma S. EuroNews., May 14, 2024. www.euronews.com/ health/2024/05/14/europes-fertility-crisis-which-european-country-is-having-the-fewest-babies
3. Mburu, Gitau, et al. Towards a nuanced view and response to global fertility trends. Lancet 2024;403:1953-1956
4. Issanov, A. et al. (2022). Impact of governmental support to the IVF clinical pregnancy rates: differences between public and private clinical settings in Kazakhstan-a prospective cohort study. BMJ Open. 2022;12(2)
5. Ducharme J. Time Magazine, April 10, 2024. https://time. com/6965267/women-having-kids-later/
6. Allen Jr. JL. Angelus May 16, 2024; https://angelusnews.com/voices/pope-pro-fertility-campaign/
7. Zitser J. Business Insider. May 30, 2024. www.businessinsider. com/south-korea-pay-reverse-vasectomy-tubal-ligation-birth-rateslow-2024-5
8. Chakraborty P. India Today; April 22, 2024; https://www. indiatoday.in/elections/story/asaduddin-owaisi-pm-modi-congress-wealth-infiltrators-rally-rajasthan-lok-sabha-elections-2530005-2024-04-22
9. Wikipedia. List of Countries by Total Fertility Rate; May 24, 2024; https://en.wikipedia.org/wiki/List_of_countries_by_total_fertility_rate
THINK DIFFERENTLY – A CHR OPINION
Norbert Gleicher, MD is the Medical Director and Chief Scientist at the CHR
BRIEFING: In our ongoing efforts to educate the field of REI about the importance of the female immune system for successful reproduction, we here point out how the maternal immune system in pregnancy not only affects the pregnant woman’s brain but also that of her offspring.
During pregnancy the maternal brain undergoes long-lasting structural changes involving reductions in gray matter volume in regions sub serving social cognition.1 On a long-term basis, neural and biological states of the adult female are significantly altered by parity and motherhood.2 What induces this remarkable degree of plasticity of the brain is, however, still largely unknown. In rats, microglia, the brain’s innate immune cells, were remarkably reduced in density during late pregnancy and postpartum period in basolateral amygdala, medial prefrontal cortex, nucleus accumbent shell and dorsal hippocampus as well as in all four brain regions but unaffected in the motor cortex, while IL-6 and IL-10 levels were postpartum increased in the hippocampus.3 This is exactly the time period in which postpartum depression and other psychiatric disorders frequently become clinically apparent in humans.4
These observations suggest a quite significant shift in the maternal neuroimmune environment during the peripartum period, which appears to contribute to the significant neural as well as behavioral plasticity occurring during the transition to motherhood. For quite

a number of reasons, pregnancy-induced changes to maternal brains deserve, however, more attention than they currently receive.
Our understanding of how the immune system affects maternal brains was significantly enhanced in 2015 with the reported discovery of a structural as well as functional lymphatic system in the brain.5 Up to that point, conventional wisdom assumed that, because the so-called blood-brain barrier (a semipermeable membrane) prevented cellular components of blood, and therefore immunologically-important cells, from accessing the central nervous system (CNS), the immune system could not interact with the brain. Over just a few short years it has now become obvious that, not only does the immune system have access to the brain, but that interactions between the brain and immune system are extremely intimate and may be responsible for a variety of neurological and psychiatric diseases in women who go through pregnancy. The degree of interaction, indeed, appears so intense, that a prominent neuro-immunologist designated the immune system as the “seventh sense.”6

Since it is now confirmed that blood products and cells have access to the CNS during pregnancy, the behavior of a number of neurological and psychiatric diseases in association with pregnancy deserve renewed scrutiny. Pregnancy has, for the longest time, been considered a stress test for all female organs. Evolutionary theory predicts accelerated aging in parallel with increasing pregnancy numbers because of diversion of more energy during pregnancy from routine somatic body maintenance, and was recently confirmed in a study of Filipino women.7 Because pregnancy so typically serves as a universal stress test, phenotypical disease presentations in association with pregnancy8 and cardio-vascular disease are common.9
Likely the most typical so-associated pregnancy phenotype is maternal autoimmunity in all of its presentations, even if only sub-clinically present. Because of inadequate induction of tolerance pathways for the implanting embryo (a paternal semi-allograft) in women with hyper-active immune systems, autoimmunity in early and late pregnancy is typically associated with maternal immune system hyperresponsiveness toward the fetus. In early pregnancy, this results in implantation failure and enhanced miscarriage risks in pregnancy,10 while in late gestation, premature termination of tolerance leads to premature initiation of labor, a typical presentation in practically all autoimmune diseases.11 The most characteristic phenotypical presentation of autoimmunity is, however, first expression and/ or flaring of autoimmune diseases with and/or after termination of pregnancies, establishing a maternal risk period of up to five months into the postpartum period.12,13
Several neuropsychiatric disorders, especially depression (in the form of so-called postpartum depression or postpartum “blues”) and bipolar disorders typically present with above-described autoimmune phenotype, characterized by initial presentation or flaring peripartum and in the postpartum period (hence the name postpartum depression). Women with depression typically also present with premature labor,14,15 practically a uniform finding in women with all autoimmune diseases.11An autoimmune etiology is also supported by the strong association between thyroid autoimmunity and postpartum dysphoria.16-18 The CHR, for quite some time, have been proposing a potential autoimmune etiology for the psychiatric disease of depres-
sion, even if not related to pregnancy15,19 and even before publication of the groundbreaking discovery that, contrary to prior assumptions, the CNS is privy to a complex immune system (reviewed in reference20).
Maternal immune system activation, however, does not affect the mother alone. There is even more evidence that it indeed represents a highly significant risk factor for neuropsychiatric disorders (including autism) in offspring,21-27 summarized by Estes and McAllister in a special section on neuro-immunology in Science 28
It is also in this context of interest that the late-pregnancy complication of preeclampsia, a likely consequence of preterm declines in maternal tolerance levels,10 has been statistical associated with maternal depression and anxiety.29 Moreover, postpartum females appear to exhibit significant changes in expression of cytokines within the brain that are associated with depressive-like behavior.30
J.S. Scott already suggested in 1966 that pregnancy offered investigators a natural “disease model” for the investigation of autoimmunity.31 With this contribution, we would like to make the same suggestion to neurobiologists who are trying to decipher the effects of the immune system on neuro-psychiatric diseases in mothers and their offspring.
1. Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco E, Garcia-Garcia D, Soliva JC, Tobeña A, Desco M, Crone EA, Ballesteros A, Carmona S, Vilarrova O. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci 2016;doi: 10.1038/nn.4458
2. Bridges RS. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm Behav 2016;77:193-203
3. Haim A, Julian D, Albin-Brooks C, Brothers HM, Lenz KM, Leuner B. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun 2017;59:67-78
4. Meltzer-Brody S, Howard LM, Bergink V, Vigod S, Jones I, Munk-Olsen T, Honikman S, Milgrom J,. Postpartum psychiatric disorders. Nat rv Dis Primers 2018;4:18011
5. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske HD, Derecji NC, Castle D, Mandell JW, Kevin L, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatics. Nature 2015;523(7560):337-341
6. Kipnis J. The Seventh Sense. Sci Am 2018;319(2):28-35
7. Ryan CP, Hayes MG, Lee NR, McDade TW. Jones MJ, Kobor MS, Kuzawa CW, Eisenberg DTA. Reroduction predicts shorter telomers and epigenetic age acceleration among young adult women. Sci rep 2018;8(1):11100
8. Podymow T, August P. Hypertension in pregnancy. Adv Chronic Kidney Dis 2007; 14:178-119
9. Rottenkolber M et. al. The diabetes risk phenotype of young women with recent gestational diabetes. J Clin Endocrinol Metab 2015;




SUPPORTS ENERGY METABOLISM PROTECTS AGAINST OXIDATIVE STRESS

100MG OF ACTIVE UBIQUINOL FORM OF COQ10 IN EACH SOFTGEL
PATENTED VESISORB TECHNOLOGY FOR BETTER ABSORBTION
16. Goer MW, Vaughan JH. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J Obstet Gynecol Neonatal Nurs 2014;
17. Pedersen C, Leserman J, Garcia N, Stansbury M, Meltzer-Brody S, Johnson J. Late pregnancy thyroid-binding globulin predicts perinatal depression. Psychoneuroendoecrinology 2016;65:
18. Brochetta A, Traccis F, Mosca E, Serra A, Tamburini G, Loviselli A. Bipolar disorder and antithyroid antibodies: review and case series. Int J
19. Gleicher N. Postpartum depression, an autoimmune disease? Autoimmune
20. Mueller KL, Hines PJ, Travis J. Neuroimmunology. Science 2016;
21. Mandal M, Donelly R, Elkabes S, Zhang P, Davini D, David BT, Ponzio NM. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun 2013;33:33-45
22. Wolff AR, Bilkey DK. Prenatal immune activation alters hippocampal place cell firing characteristics in adult animals. Brain Behav Immun
23. Aavani T, Rana SA, Hawkens R, Pittman QJ. Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum 2015;14:491-505
24. Choid GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal intrleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016;351:933-939
WWW.MYOVATERRA.COM
10. Gleicher N, Kushnir VA, Barad DH. Redirecting reproductive immunology research toward pregnancy as a period of temporary immune tolerance. J Assist Reprod Genet 2017;34(4):425-430
11. Gleicher N. Does the immune system induce labor? Lessons from preterm deliveries in women with autoimmune diseases. Clin Rev Allergy Immunol 2010; 39: 194-206
12. Østensen M, Andreoli L, Brucato A, Cetin I, Chambers C, Clowse ME, Costedoat-Chalumeau N, Cutolo M, Dolhain R, Fenstad MH, Förger F, Wahren-Herlenius M, Ruiz-Irastorza G, Koksvik H, Nelson-Piercy C, Shoenfeld Y, Tincani A, Villiger PM, Wallenius M, von Wolff M. State of the art: Reproduction and pregnancy in rheumatic disease. Autoimmun Rev. 2015;14:376-86. doi:
13. Tincani A, Dall'Ara F, Lazzaroni MG, Reggia R, Andreoli L. Pregnancy in patients with autoimmune disease: A reality in 2016. Autoimmun Rev. 2016;15:975-977.
14. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod 2009; 24:146-153
15. Gleicher N, Weghofer A. Why depression is associated with increased risk toward premature labor. Hum Reprod 2009;24:760-761
JOLANTA@MYOVATERRA.COM
25. Chow KH, Yan Z, Wu WL. Induction of maternal immune activation in mice as mid-gestation stage with Viral Mimic Poly(I:C). J Vis Exp 2016;25,109):e53643
26. Nardone S, Elliott E. The interaction between the immune system and epigenetics in the etiology of autism spectrum disorders. Front Neurosci 2016;10:329
27. Rose DR, Caraega M, Van de Water J, McAllister K, Bauman MD, Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain behave Immun 2016;pii: S0889-1591(16)30522-0
28. Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016;353:772-777
29.Logue OC, George EM, Bidwell GL 3rd. Preeclampsia and the brain: neural control of cardiovascular changes during pregnancy and neurological outcomes of peeclampsia. Clin Sci (Lond) 2016;130:1414-1434
30. Possilico CK, Schwartz JM. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behavior. Behav Brain Res 2016;298(Pt B):218-228
31. J.S. Scott. Immunological diseases and pregnancy. Brit Med J 1966;1:1559-1567
IMAGE 1: In every IVF cycle after eggs are retrieved the cells supporting the oocyte through its lengthy journey to ovulation are removed and - having done their job – are usually discarded. At the CHR, however, they are sometimes preserved and used in research trying to resolve several yet unknown issues regarding their functions. This figure shows these granulosa cells as they appear in the confocal microscope shortly after retrieval. The mostly blue-greenish spherical structures are nuclei of cells that have died through a process known as apoptosis. In contrast, many of the other cells show reddish borders indicating that they are active and perhaps continuing to provide factors needed for the oocyte to develop into an embryo after fertilization.
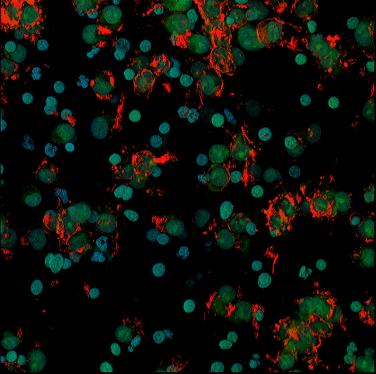


IMAGE 2: Here the same kind of granulosa cells are shown after they have been placed in 24hour culture after retrieval. Cells that remained alive anchored themselves to a glass or plastic substrate and provided researchers at the CHR with opportunity to learn why these cells from some patients are prone to die, whereas the same cells from other patients remain not only viable but capable of producing hormones which are considered indicators of ovarian health.
BRIEFING: We here offer brief notes regarding news which in preceding months caught our attention that are relevant to the practice of reproductive medicine and infertility.
New FDA rule finally tightens scrutiny of socalled “laboratory-developed” tests (LDTs): but will it also – as it should - apply to LDTs that have come to dominate reproductive medicine?
After literally talking about it for decades – but not really doing anything about it – the U.S. Department of Health and Human Services Food and Drug Administration (FDA) on April 29, 2024, finally issued a ruling regarding how it will start actively overseeing so-called “laboratory-developed tests” (LDTs).
LDTs represent a group of diagnostic lab tests, designed and executed in a single laboratory, the FDA always claimed to have a right to oversee, but for decades chose not to bother with because, when initially defined as a separated testing category, it was meant to apply, in principle, to only very simple and very basic tests mostly performed in small private laboratories of country doctors.
The laboratory testing industry, however, quickly recognized the usefulness of this designation for newly developed tests to circumvent the otherwise cumbersome approval process diagnostic laboratory tests have to undergo in order to receive FDA approval. As The Wall Street Journal pointed out on April 30, 2024, this led to a large number of very diverse tests, from illicit drug-testing to highly sophisticated genetic testing for disease risks, or determination of treatment choices for cancer patients based on highly sophisticated genetic testing being marketed to the public without any prior FDA scrutiny.1 The article notes that, according to an organization called Grand View Research, LDTs over the last two decades have come to represent ca. half of all diagnostic tests in medicine. Reuters reported that LDTs represented ca. 5% of Labcorp’s and ca. 10% of Quest Diagnostics total diagnostic testing volumes (source Jeffries Brokerage).2 The final rule is available in the Federal Register 3
The Wall Street Journal article, moreover, also quotes as a basic motivation of the FDA to, finally, initiate supervision over LDTs, as the agency’s recognition that “inaccurate tests marketed in recent years could have led to patients getting the wrong treatments for heart disease, cancer, or being incorrectly diagnosed with autism or
Alzheimer’s disease” (to quote only some examples).
Assuming supervision of such a huge number of tests will, of course, take time. As the Wall Street Journal article also reported, the FDA will initially concentrate on “complicated and high-stakes” tests and, though such selective initial scrutiny is understandable, it, of course, raises the question what (and who) defines what the most complicated a high-stakes LDTs are in the market?
And here is where we feel reproductive medicine must speak up because, at least the lay-media have – at least so-far – not come to recognized how extremely reproductive medicine, and especially the infertility field, has been affected by LDTs. Though articles, of course correctly, point out the dangers that can come from false diagnoses in cancer, heart disease and other important human diseases, we did not even find a single mention in the media that preimplantation genetic testing of human embryos during IVF cycles for aneuploidy (PGT-A) and polygenic risk (PGT-P) are LDTs, and daily lead to inaccurate diagnoses of human embryos and, often, to their non-use and/ or disposal, leading to wasted pregnancy chances for thousands of women and unneeded and premature referrals into third-party egg donations. Moreover, we have also not seen any FDA statements that would indicate that the agency understands especially the degree of misdiagnoses in PGT-A, which now affects over half of all IVF cycles performed in the country.
As we have discussed these issues in the VOICE repeatedly before, our readers will, likely already know that the only hint that the FDA has recognized a potential problem from LDTs in reproductive medicine came from a warning mailing on April 19, 2022, in which the FDA warned about the possibility of false-positive diagnoses of chromosomal abnormalities in early first-trimester non-invasive prenatal testing (NIPT) via cell-free DNA.4 The FDA has, however, so far failed to acknowledge the fact that this risk is minuscule in comparison to the risks that come from false positive diagnoses with PGT-A testing.`
1. Essley White L. The Wall Street Journal. April 30, 2024; pA3
2. Reuters April 29, 2024; https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-publishes-final-rule-laboratory-developed-tests-2024-04-29/
3. https://www.federalregister.gov/d/2024-08935
4. https://www.fda.gov/medical-devices/safety-communications/genetic-non-invasive-prenatal-screening-tests-may-have-false-results-fda-safety-communication
Certain subjects are, of course, pretty automatically meant to make headlines and nobody can, therefore, be very surprised that a relatively recent paper in the Annals of Internal Medicine (a pretty prestigious medical journal) did exactly that.1 It was, after all, widely interpreted in the media to have demonstrated that women make better physicians than men. But, as we all by know only too well understand, one cannot always believe what the media are reporting, and another good example for considerable misinformation by the media, therefore, does not surprise.
That women may in certain aspects be better physicians than men has been suggested before: female physicians were reported to have better screening rates for diseases, have better patient outcomes, and lower mortality rates.2 But before anybody falls into the media trap of simplistic explanations, it seems worthwhile to ponder the underlying question to all further considerations: what defines a physicians as better or poorer, and that is definitely not an easy question to answer because – like in any other occupation – a professional in medicine may have weaker and better performance levels within the individual’s medical specialty area. A physician may, for example, be a terrific surgeon but have horrible bedside manners. What does that make her/him, a great or a horrible doctor? And the answer may not only depend on the physician; it equally may be dependent on the patient and what she/he perceived as the more important physician characteristic.
But, luckily, we this time have been offered excellent help in addressing this issue (and this paper) from Perry Wilson, MD, MSCE, an Associate Professor of Medicine (nephrology) and Public Health at the Yale School of Medicine in New Haven, CT, who is also the Director of the Clinical and Translational Research Accelerator at the university and is widely recognized as an expert in interpreting (often complex) medical studies. He uses this special ability in his commentaries in the Impact Factor, a weekly publication by Medscape in which he comments on selected articles in the medical literature. As recently above noted articles in the Annals became the subject of such a commentary, he once again demonstrated that only very few have the ability to explain complicated concept as well as he does.3
We, of course, do not have the space enough to go into much detail (we, indeed, for that purpose recommend Wilson’s complete article). Only so much: This was a surprisingly solid study which with considerable sophistication attempted to determine whether there as a detectable difference in clinical outcomes (defined as hospital mortality and readmission rates) by physician sex as well as patient sex. Four dyads were basically investigated: Male patient - male doctor; Male patient – female doctor; Female patient – male doctor; Female

patient – female doctor, using almost one million patients treated by approximately 50,000 physicians. Interestingly, patients appeared like a homogenous real-world patient group (above age 65) but the treating hospitalists varied by additional factors than just sex (yes, doctors giving care in hospitals now are usually so-called hospitalists. And that, of course, adds yet another dimension to the discussion because, as physicians know better than most others, physicians’ medical specialty areas attract quite different personality traits.
So, what were then the ultimate findings? Females showed significantly lower 30-day mortality rates than males; but they did even better if they received treatments from female physicians. The sex of physicians, however, only barely affected male outcomes. Sex-based differences between physicians were, thus, really barely different and could be relatively easily explained away by confounders among physicians. Though Wilson does not outright say so, what the study reports in observed differences between female and male physicians is only statistical noise, he notes that the effect size of the study ended up being “incredibly small and only at the border of statistical significance.” As we are making a point regarding this subject very frequently especially when discussing papers in the literature. Just because a study produces a P< 0.05, therefore, does not necessarily suggest a clinically relevant finding. And then, there is, of course, the especially sensitive subject of sex and gender in today’s society, which Wilson also handled exquisitely well by, first of all, apologizing if his words had been “indelicate” and by acknowledging that, after all, “he was only a male doctor.”
1. Miyasaki et al., Ann Int Med 2025; https://doi.org/10.7326/M23-3163; ahead of print.
2. https://www.mdlinx.com/article/do-women-make-better-physicians/7tns77uBbTG0wnrjyBPbLZ
3. https://fperrywilson.medium.com/are-women-really-better-doctors-than-mene4e1e91b6247
More on diversity, equity, and inclusion (DEI)
how
It is difficult to say whether medical schools are drowning more in DEI activities than other graduate schools; but one thing is very obvious, two professionals most people do not want assigned based on DEI but prefer to be chosen based on merit are pilots and physicians (or maybe even more so, surgeons), though we could think about some other professional activities where selection based on merit would appear logical (how about other medical specialties, soldiers, and, yes, how about university or college presidents).
Congress now discovered the “problem” and has started discussions on how to prevent DEI excesses from affecting medical schools adversely. On March 19 a proposal was introduced in Congress with the aim to ban “race-based mandates in medical schools.”1 At time of this publication the fate of this bill is still in question; but, as the article notes, this legislation highlights the large national backlash against considering race and ethnicity in higher education after the Supreme Court overturned affirmative action in the summer of 2023. If it becomes law, medical schools will be forbidden from “establishing, maintaining or contracting with DEI offices or any other functional equivalent.” Schools must also agree that they will not force students or faculty to acknowledge that “America is an oppressed nation” or that “individuals should be adversely treated on the basis of their sex, race, ethnicity, religion, color, or national origin.” Non-compliant medical schools would no longer receive federal funding or be eligible to participate in guaranteed student loan programs.
The next generation of physicians
So – if you haven’t already known - there exists an organization called the American Medical Student Association (AMSA) which, within the current “Zeitgeist,” of course are committed to a mission of “educating and empowering diverse future physicians to become unapologetic advocates for justice in healthcare.”2 May 30 through June 1, 2024, AMSA’s annual meeting will take place in Washington, D.C. under the heading “Future Physicians for Change.” The (political) agenda says it all and includes abortion restrictions, DEI funding cuts, attacks on LGBTQIA+ healthcare, and, of course, the ongoing humanitarian crisis in Gaza (though, of course, not a word about over 130 hostages still kept by Hamas – many likely not even alive anymore). One, of course, also wonders who finances this organization?
In their recent mailing the organization noted that, “in solidarity, AMSA established dedicated brave spaces (a community space where different points on a journey of learning and growing are acknowledged) to empower students in their advocacy efforts, fostering dialogue and support to address injustices in Gaza, ensuring that all members are well-informed and actively involved in advocacy endeavors.” The mailing went on to state that, “as students, the AMSA claims a role in shaping the future of medicine and society, advocating for justice and equity in healthcare worldwide.”
If this organization should really represent the future of organized medicine in the U.S., medicine in this country may be in more trouble than we think!
Has the pendulum regarding DEI already started swinging back?
Yes, especially after the 2023 U.S. Supreme Court decision to strike down any consideration of race in college admission, there are those who believe that DEI has peaked at universities, colleges, and in corporate America; but we would consider this as a very premature conclusion. Nothing makes this point better than a recent “Editorial” in Science magazine by Shirley M. Malcolm, PhD, a senior advisor to the CEO and Director of the STEM Equity Achievement (SEA) at the American Association for the Advancement of Science (AAAS), the publisher of Science, which actually called for “strengthening the case for DEI.”3

We fully trust in the creativity of U.S academia to circumvent the court decision. Once again, Malcom said it well: “To push back against critics (of DEI) it is important to remember why DEI efforts are so important and agree on the best ways to gauge their success.” And then, of course, comes the subtle attack on meritocracy in academia: “Success is (after all) measured not only by publications and head counts of underrepresented groups but also by creating a culture of inclusion and respect.” And she continues: “It’s crucial not to lose sight of the kind of scientific community the federal government aims to support. In this vein, DEI advocates, the scientific community, universities, and federal agencies must together consider where processes can be modified within institutions to remove barriers to participation by all.” What a gentle and elegant way to describe how the Supreme Court ruling can (and will) be subverted by academia.4
Within this context, The Massachusetts Institute of Technology - by no mean known as a bastion of conservatism – officially became the first elite school to allegedly “eliminate” DEI hiring requirements under the claim that “they don’t work.”5
MIT President, Sally Kornbluth, PhD (of Congressional hearing fame for being the only among the three presidents to have kept her job - so far) apparently decided to eliminate the previously mandatory diversity statement every job candidate had to sign during the school’s hiring process at MIT. Her – actually precinct -rational was that “compelled statements impinge on freedom of expression and don’t work.” But it, of course, remains to be seen whether this step by MIT, in fact represents a formal change in institutional DEI-driven hiring practices or only a tactical change to appear in compliance with the recent Supreme Court decision.
At least a somewhat older generation of physicians feels increasingly forced into “woke indoctrination” as part of their CME requirements. So-far seven states have, indeed, gone as far as passing a mandatory “implicit bias training” session as a requirement for license renewal and, according to a recent Opinion article in The Wall Street Journal by Stanley Goldfarb, MD, Chairman of an organization called “Do No Harm,” 25 additional states are considering similar mandates.6
Tired of being indoctrinated, his organization decided to launch on May 1 (what an appropriate date!) a CME course that fulfills Michigan’s implicit-bias-training mandate, created in 2021 and updated in 2023. It is considered one of most expansive mandates in the


nation. What made this such an interesting announcement is, as Goldfarb claims, the fact that this CME course goes into more ethical and less political directions, and thereby, while fulfilling all by the state mandated requirements for such a course, basically turns the table on the concept of “implicit bias” by making the central point that medical practice should not reemphasize racial differences but, to the contrary, be color-blind.
Whether this line of thinking on the subject is, indeed, less political remains open to discussion. One, however, cannot deny that “implicit bias training” sounds more like a mandated activity during Mao’s Cultural Revolution in China than in current times under the U.S. constitution. We can’t wait for Governor Gretchen Whitmer’s response. But, independent of how the state will respond to this challenge, maybe it is time that government officials be mandated to take an ethics course under the tile “first of all, do no harm.”
Related, recent experiences with Artificial Intelligence (AI) image generators clearly demonstrated that the resulting images are often racists and sexist. A recent article in Nature magazine, therefore, questioned whether such results can be easily fixed?4 What brought this issue to the broad attention of the public was, of course, Google’s experience which, among other bizarre results, in response to a request for an image of a 1943 German soldier, the system produced images of Black and Asian Nazis.
That systems can and will be improved can be assumed with more and better focus on existing biases in data sets used to train the A.I. systems; but even then, the question remains (with no easy answer) what the “right” answer should be. Should it, for example, reflect the reality in current societal output, even if that reality is unfair? Who will – or should – decide?
And if we already talk about A.I biases, as FOX News recently reported, A.I.-driven facial recognition technology and human raters were able to predict the political orientation of individuals from images of expressionless faces even when controlling for demographics and self-presentation.7 The model was derived from standard images controlled for age, gender, and ethnicity and the findings, of course, have significant repercussions for privacy.
1. Weber s. Medscaped Medical News. March 21, 2024; https://www.medscape. com/viewarticle/proposed-bill-could-end-stu..._mscpmrk_pcp_hospitalist_ etid6464419&uac=223637CN&impID=6464419
2. https://www.amsa.org/amsa-stands-in-solidarity-with-student-protests-across-thenation-our-commitment-to-creating-brave-spaces-for-student-organizing-and-activism/#:~:text=In%20solidarity%2C%20we%20have%20established,actively%20 involved%20in%20advocacy%20endeavors.
3. Malcolm S. Science 2024;383(6690):1395
4. Ananya. Nature 2024;627:723
5. Sahakian T. FOX News. https://www.foxnews.com/media/elite-university-eliminates-dei-hiring-requirement-work
6. Glodfarb S. The Wall Street Journal; April 27-26, 2024. pA11
7. Kosinski et al., American Psychologist 2024; https://doi.org/10.1037/ amp0001295; ahead of print.
For probably too long, the VOICE has, on purpose, been staying away from addressing the swirling controversy of gender identity-related medical services because – frankly – we had a difficult time sorting out the greatly varying arguments around this subject. Quite significant new developments, mostly in Europe, however, no longer permit continuous silence. A recent editorial opinion in The Lancet – of course, by no means a conservative medical journal – made this very clear.
In this article Jacqui Thornton, 1 an award-winning health journalist writing for the Lancet, the BMJ, and Nature magazine, addresses a review into British gender identity services for children and young people, conducted by Dr. Hilary Cass, a pediatrician and former President of The Royal College of Pediatrics, making the point that this 388-long report2 will fundamentally change the way such services will be delivered in the U.K in the immediate future.

We are quoting: Dr Hilary Cass submitted her final report and recommendations to NHS England in her role as Chair of the Independent Review of gender identity services for children and young people. The Review was commissioned by NHS England to make recommendations on how to improve NHS gender identity services and ensure that children and young people who are questioning their gender identity or experiencing gender dysphoria receive a high standard of care, that meets their needs, is safe, holistic, and effective. The report described what currently is known about the young people who are seeking NHS support around their gender identity and set out the recommended clinical approach to care and support they should expect, the interventions that should be available, and how services should be organized. It also makes recommendations on the quality improvement and research infrastructure required to ensure that the evidence base underpinning care is strengthened. In making her recommendations, Dr Cass had to rely on the currently
available evidence and had to think about how the NHS can respond safely, effectively, and compassionately, leaving some issues for wider societal debate.
CONTEXT: Exploration of identity is a completely natural process during childhood and adolescence and rarely requires clinical input. However, over the past five – ten years the number of children and young people being referred for NHS support around their gender identity has increased rapidly. As a result, young people are waiting several years to receive clinical support and during this time they and their families are left to make sense of their individual situations, often dealing with considerable challenges and upheaval. There has been a similar pattern in other Western countries, with clinicians noting not only the rising number but also a change in the case mix of the young people seeking support (this makes this report also so relevant for the U.S).
There have been many more birth-registered females being referred in adolescence, marking a shift from the cohort that these services have traditionally seen; that is, birth-registered males presenting in childhood, on whom the previous clinical approach to care was based. Clinicians also noted that these young people often had other issues that they were having to manage alongside their gender-related distress.
The Independent Review set out to understand the reasons for the growth in referrals and the change in case-mix, and to identify the clinical approach and service model that would best serve this population. To provide an evidence base upon which to make its recommendations, the Review commissioned the University of York to conduct a series of independent systematic reviews of existing evidence and new qualitative and quantitative research to build on the evidence base. Dr Cass also conducted an extensive program of engagement with young people, parents, clinicians, and other associated professionals.
• There is no simple explanation for the increase in the numbers of predominantly young people and young adults who have a trans or gender diverse identity, but there is broad agreement that it is a result of a complex interplay between biological, psychological, and social factors. This balance of factors will be different in each individual.
• There are conflicting views about the clinical approach, with expec tations of care at times being far from usual clinical practice. This has made some clinicians fearful of working with gender-question ing young people, despite their presentation being similar to many children and young people presenting to other NHS services.
• An appraisal of international guidelines for care and treatment of children and young people with gender incongruence found that that no single guideline could be applied in its entirety to the NHS in England.
• While a considerable amount of research has been published in this field, systematic evidence reviews demonstrated the poor quality of the published studies, meaning there is not a reliable evidence base upon which to make clinical decisions, or for children and their families to make informed choices.
• The strengths and weaknesses of the evidence base on the care of children and young people are often misrepresented and overstated, both in scientific publications and social debate.
• The controversy surrounding the use of medical treatments has taken focus away from what the individualized care and treatment is intended to achieve for individuals seeking support from NHS gender services.
• The rationale for early puberty suppression remains unclear, with weak evidence regarding the impact on gender dysphoria, mental or psychosocial health. The effect on cognitive and psychosexual development remains unknown.
• The use of masculinizing / feminizing hormones in those under the age of 18 also presents many unknowns, despite their longstand ing use in the adult transgender population. The lack of long-term follow-up data on those commencing treatment at an earlier age means we have inadequate information about the range of out comes for this group.
• Clinicians are unable to determine with any certainty which chil dren and young people will go on to have an enduring trans identity.
• For most young people, a medical pathway will not be the best way to manage their gender-related distress. For those young people for whom a medical pathway is clinically indicated, it is not enough to provide this without also addressing wider mental health and/or psychosocially challenging problems.
• Innovation is important if medicine is to move forward, but there must be a proportionate level of monitoring, oversight and regula tion that does not stifle progress, while preventing creep of un proven approaches into clinical practice. Innovation must draw from and contribute to the evidence base.
The recommendations set out a different approach to healthcare, more closely aligned with usual NHS clinical practice that considers the young person holistically and not solely in terms of their gender-related distress. The central aim of assessment should be to help young people to thrive and achieve their life goals.
• Services must operate to the same standards as other services seeing children and young people with complex presentations and/or additional risk factors.
• Expand capacity through a distributed service model, based in paediatric services and with stronger links between secondary and specialist services.
• Children/ young people referred to NHS gender services must re ceive a holistic assessment of their needs to inform an individual ized care plan. This should include screening for neurodevel opmental conditions, including autism spectrum disorder, and a mental health assessment.
• Standard evidence based psychological and
psychopharmacological treatment approaches should be used to support the management of the associated distress from gender incongruence and cooccurring conditions, including support for parents/carers and siblings as appropriate.
• Services should establish a separate pathway for pre-pubertal children and their families. ensuring that they are prioritized for early discussion about how parents can best support their child in a balanced and non-judgmental way. When families/careers are making decisions about social transition of pre-pubertal children, services should ensure that they can be seen as early as possible by a clinical professional with relevant experience.
• NHS England should ensure that each Regional Center has a follow-through service for 17–25-year-olds; either by extending the range of the regional children and young people’s service or through linked services, to ensure continuity of care and support at a potentially vulnerable stage in their journey. This will also allow clinical, and research follow up data to be collected.
• There needs to be provision for people considering detransition, recognizing that they may not wish to re-engage with the services whose care they were previously under.
• A full program of research should be established to look at the characteristics, interventions and outcomes of every young person presenting to the NHS gender services.
• The puberty blocker trial previously announced by NHS England should be part of a program of research which also evaluates out comes of psychosocial interventions and masculinizing/ feminizing hormones.
• The option to provide masculinizing/feminizing hormones from age 16 is available, but the Review recommends extreme caution. There should be a clear clinical rationale for providing hormones at this stage rather than waiting until an individual reaches 18. Every case considered for medical treatment should be discussed at a national Multi- Disciplinary Team (MDT).
• Implications of private healthcare on any future requests to the NHS for treatment, monitoring and/or involvement in research, and the dispensing responsibilities of pharmacists of private prescriptions needs to be clearly communicated.
Though the report makes 32 recommendations, Thornton points out several key points, none likely as profound and important as the recommendation that clinicians should be “extremely cautious” in providing hormones to people younger than 18 years (and considering such caution, how much more cautious should clinicians, therefore, be in having people younger than 18 years undergo life-changing irreversible surgeries). She also points out that the UK has also already banned the use of puberty blockers under age 18, unless in clinical trials.
Forbes magazine pointed out that in Europe political divisions on this topic are not “nearly as conspicuous” as in the U.S., with the debate, therefore, being much more fact based.3 Consequently, minors in several European countries besides the UK (Norway, Sweden, Denmark, France, etc.) can access puberty blockers and cross-sex hormones only if they meet strict eligibility requirements, usually in tightly controlled research settings.
On April 18, 2024, Scotland’s only gender clinic also announced that it had paused prescribing puberty blockers to persons younger than 18 years, while new minors in treatment would no longer receive any hormone treatments.4
The Dutch government ordered an investigation into physical and mental health outcomes of children prescribed puberty blockers. A report generated by an investigational board in Norway recommended that puberty blockers and hormonal as well as surgical gender confirmation treatments for children and young people be defined as “experimental.” The National Academy of Medicine in France recommended that puberty blockers and transitioning hormones be used in children and adolescents only with “greatest reserve,” and only with parental consent. The latter point is, of course, worthwhile mentioning, considering prominent voices in the U.S. arguing that such interventions should be offered to children without parental consent (and even behind their backs). Sweden considers the risks of puberty blockers and gender-affirming hormones under age 18 to outweigh potential benefits. Greece, Luxembourg, and Ireland apparently do not have any gender services for children and adolescents.
Unsurprisingly, the European Academy of Pediatrics very recently also published a very cautious (and politically much more considered) statement on the clinical management of children and adolescents with gender dysphoria than above cited Cass Report.5 But, considering how much less aggressive even this document is in comparison to U.S. practice at some of the most prominent medical institutions in the U.S., one really must wonder what really motivates U.S health care to an so-far undeterred much more aggressive interventional treatment approach in even children and young adolescents?
We are again quoting: Gender issues have become a polarized and political subject in modern pediatrics and indeed, in broader society. These include the management of infants with disorders of sex development and transgender sports participation, but especially recently regarding the management of gender dysphoria. The European Academy of Pediatrics (EAP) acknowledges that there are deeply held beliefs about this issue based on conscience and social norms. Several European countries, led by the UK, have recently reviewed the management of gender dysphoria in children and young people. Recognizing the need for far more research into treatments such as pubertal suppression and cross-sex hormones in children and young people, we review the current ethical and legal dilemmas facing children with gender dysphoria, their families and the clinical teams caring for them. We suggest an approach that maintains the child’s right to an open future whilst acknowledging that the individual child is the crucial person affected by decisions made and must receive appropriate support in decision-making and care for any associated mental health or psychological issues. Noting that national approaches to this vary and are in flux, the EAP advocates a child-centered individual rights-based analytical approach.

The almost complete absence from this debate of prominent medical voices from leading medical institutions in the U.S. is, indeed, surprising. Media attention to the changes that have recently taken place in Europe came mostly from JK Rowling’s (of Harry Potter fame) very public engagement with this subject and her strong support for Dr. Hilary Cass, when noting: “Follow the money may be a conspiratory theorist trope and I hate the X cliché – let that sink in – but when a respected pediatrician is advised not to travel by bus (referring to Dr. Cass out of security concerns) and when lobby groups fall over themselves to discredit a meticulous medical review, both might seem to apply.”6
1. Thornton J. The Lancet 2024;403:1529
2. Cass H. Independent review of gender identity services for children and young people. https://cass.independent-review.uk/
3. Cohen J. Forbes, December 2, 2023; https://www.forbes.com/sites/joshuacohen/2023/12/02/europe-and-us-diverge-on-treatment-of-gender-incongruence-in-minors/?sh=3e21eda920d0
4. Harris S. Medscape Medical News. April 25, 2024. https://www.medscape.com/ viewarticle/europe-and-puberty-blocker-debate-2024a1000831?form=fpf
5. Brierley et al., Front. Peadiatr 2024;12:1298884
6. Penley T. FOX News . April 21, 2024. https://www.foxnews.com/media/jk-rowling-defends-pediatrician-avoid-public-transit-gender-study-controversy-follow-money
The truth is that, except maybe for a handful exceptions, most medical journals even to these days simply do not like to get involved “in wars.” How do we know? Just look how many times in the last 30 years we have seen articles about health effects of wars in major leading medical journals (except, maybe in The Lancet); and has there ever been a time when there wasn’t a war ongoing somewhere in the world? Things recently started to change a little bit (but really not too much) because for the first time since Clinton’s Balkan war (which really was just a relatively small skirmish in contrast to what the world is currently facing) we are living through two major land wars in Europe (after Russia invaded the Ukraine) and in the Middle East (after Hamas’s murderous and beyond heinous October 7 attack on Israel). Though Russia’s incursion into Ukraine has caused significant more deaths and destruction and, on top of it millions of Ukrainian refuges all over the world, the Israel-Hamas conflict has in the U.S. and Europe, paradoxically, generated much more political upheaval. Unsurprisingly, therefore, even medical publishing has, therefore, been unable to escape involvement.
We have previously noted in these pages the surprisingly balanced coverage of this conflict by The Lancet, in the past not always known as a fair observer of Israel, which has given both sides of the conflict approximately equal opportunity to comment. Somewhat surprisingly, JAMA – previously never known as particularly interested in commenting on wars – also opened its correspondence section up to both sides of the conflict. It was an exchange of opinions between colleagues and the editors of this journal that, indeed, caught our attention.
After participating in back-and-forth articles to JAMA after they considered some articles on the conflict to be one-sided in support of Hamas, three colleagues from Northwestern University Feinberg School of Medicine in Chicago and Charleston, South Carolina, penned a “Viewpoint” article to the journal arguing that, “to preserve the principle of ‘do no harm,’ medical and public health journals assiduously should discourage authors from taking (political) sides in their medical articles.”
Though they supported their argument with some well-thought-out comments,1 we found ourselves in more agreement with the response they received from the two senior editors of the journal who pretty outright rejected their arguments by rightly describing them as content moderation.2
We, indeed, would on two separate levels go even further: First, in general terms, wars do horrible things to people and medicine, therefore, must be a presence in every discussion of war. In regard to the Israel-Hamas war, we, however, would make exactly the opposite argument to the position of Greenland et al., and would actually argue that we – to this day – have been waiting for a formal
statement of JAMA journals (and yes, the AMA) that unequivocally condemned Hamas’s atrocities of October 7, without buts and/or any other equivocation.
As we have previously in these pages decried the silence of ACOG, ASRM and other societies from within the reproductive medicine community, we find the silence of the AMA and its medical journals not less shameful. Not to speak out is not as bad as suppressing speech; but it comes pretty close!
1. Greenland et al., JAMA 2024;331(16):1361-1362
2. Curfman G, Bibbins-Domingo K. JAMA 2024;331(16):1368
Who is really responsible for the not so “little”
One can argue that the recent wars between Russia and Ukraine and Israel and Hamas/Hezbollah have refreshed the conscience of an emotionally passive and desensitized world toward the horrible cruelties of war. But even in the midst of this awakening, when civilian death rates and injuries of children, women, and men increase daily, there is often no emotional space left for the many relatively “smaller” tragedies in that same war which, however, for affected parties can still be highly impactful.
As providers of fertility services, charged with the secure maintenance of thousands of human gametes, embryos, and reproductive tissues, IVF clinics face no more demanding responsibility than to secure these biological specimens. The loss of thousands of cryopreserved human embryos in an IVF clinic in Gaza reported by REUTERS® , 1 therefore, greatly resonated with us, even though, objectively of course, it cannot be compared to the daily loss of innocent life brought on by the Gaza and Russian-Ukrainian wars.
The loss of these embryos, therefore, is one of those relatively little tragedies on the periphery of much larger daily tragedies of war. By the media attributed to an Israeli air strike, as recent history repeatedly demonstrated, to which side of the conflict the media attributed the resulting carnage following an explosion in Gaza, later often turned out to be incorrect. And since the press core in Gaza is practically 100% Hamas-friendly (reporters from Western countries are in contrast almost exclusively located in much safer Tel-Aviv and, therefore, rely in their reporting on what they hear from their local Arab colleagues in Gaza), the obviously skewed reporting with proHamas bias does not surprise.
But who the culprit is in an isolated incidence like here addressed loss of IVF embryos, is really not even the issue, as painful as this one incidence may be for involved couples who lost their embryos. Responsible for their loss (and all other damages caused by the war) is, of course, Hamas, the party that started the war.
And we do not even address here the unimaginable cruelties and war crimes imposed on Israel’s civilian population on October 7, 2023, by this by the U.S. and many other Western (and even some Arab) countries as a terrorist defined organization.
Remarkably, this is, however, not how most of the international press (and many professors and students at U.S. elite universities) have come to view the Gaza conflict: October 7, 2023, if that day really ever existed in their consciousness – by now appears completely erased from memory. The brutal intended murder of over 1300 civilians in their homes, at a music festival propagating peace, the depraved rape and sexual mutilation of hundreds of women and men, the beheading and burning of babies, etc., all by the perpetrators themselves documented on video - apparently never really happened or, at least, never entered cognitive consciousness among so many of our youths who proudly occupy universities, bridges, and roadways in celebration of a murderous terrorist organization with absolutely zero regard even for its own civilian population which they are more than willing to serve up as fodder for as long as it serves their own propaganda and war machine .
For our indoctrinated youth at U.S. elite universities (and many of their Marxist teachers), if October 7, indeed, did happen after all, so what, no big deal because it only represents the “normal” reaction of the oppressed to their oppressors! Yes, murdering, raping, burning, and abducting babies, children, their parents, and grandparents is ok, for as long as the perpetrators are alleged victims of oppression. One wonders how the U.S. government would have reacted if, at the order of the Mexican government, hordes on Mexican soldiers had crossed into the U.S., murdered, raped, and abducted U.S. citizens under the same assumptions, whatever their depraved excuses otherwise might have been? We, of course, all know how the U.S. government reacted when two-handful of terrorists flew airplanes into the World Trade Center towers in 2001 under similar claims of oppression.
But since it is Israel - dam the 130 hostages Hamas is allegedly still holding in abominable conditions in contradiction to all rules of war (there is increasing concern, of course, that most may be already dead), and not even once visited by the Red Cross (imagine the uproar if Israel did not allow the Red Cross visits to Hamas prisoners). Which leaves us with the question, independent of who’s bomb or rocket it was that destroyed above-noted embryology laboratory, who is the ultimate guilty party in this destruction and responsible for all the other suffering brought on by the Gaza war? The answer seems obvious; and it is not Israel!
REFERENCE
1. Salem et al., REUTER. April 17, 2024. https://www.reuters.com/podcasts/podcastgazans-shattered-ivf-dreams-nazi-slogan-coral-reef-stress-2024-04-18/

Strange things are happening these days all over the world and the IVF field is no exception. As reported in an April 18, 2024, e-mail from Griffin Jones’s Inside Reproductive Health newsletter, one of only two IVF clinics in Nashville, TN, the Nashville Center for Reproductive Health, suddenly – and with no prior announcement – closed doors in the first week of April. Two days after receiving Jones’ notice, the CHR was contacted by one of the center’s patients who wanted to transfer her cryopreserved embryos to the CHR and was still unaware of what was going on at the center. A “To Patients”1 note since posted on the Internet suggests that the clinic, together with several related entities, is in bankruptcy court and indicates that the receiver is currently focused on initiating a quick and safe transfer of patient records and genetic materials to new providers.1
As such, requests for updates related to pending claims and operational inquires will be addressed after the patient transfer is complete. Interested non-patients may submit any questions or requests via email to CRH@Resolutecommericial.com or can leave a message at (480) 744-4103. To obtain a copy of medical records or to have records sent to another healthcare provider, requests should be mailed to CRH-records@crhnashville.com with full name, date of birth, contact information for the recipient of records, and details of what is requested. To move frozen genetic material to another clinic/ facility, email the request to CRH-records@crhnashville.com with full name, date of birth, and contact information for the clinic/facility.
In another e-mail of April 4 Griffin Jones offered the news that the UK government closed down an IVF clinic, the Homerton Fertility Centre in London, a regional healthcare provider affiliated with Britain’s National Health Service and has been licensed since 1995. The newsletter described the reason for the temporary suspension of license to be three incidences in seven months resulting in loss of embryos after not following proper embryo freezing procedures. As, however, since reported, the HFEA License Committee was since informed of four cases of missing embryos and a risk that 153 embryos might not be found on thaw, with 45 patients or couples potentially affected.2 In a mailing on May 3, 2024, Homerton Healthcare confirmed that the HFEA investigation was still ongoing and that it involved 32 patients.3
Ovation Fertility faces four separate lawsuits alleging negligent embryo loss
This is a developing story, originally based on an e-mail newsletter from Griffin Jones on May 2, 2023, and an article by Ron Shinkman in this newsletter. The claim here appears to be that embryos mistakenly were in the embryology lab exposed to hydrogen peroxide. The lawsuits were filed in Orange County, CA. Superior Court by 17 different plaintiffs. As the article noted Ovation’s official statement appears to confirm at least parts of alleged damages. The company, however, denied another allegation, namely that embryos were transferred into patients’ uteri even after they were recognized as damaged.4
Ovation Fertility was in 2023 sold by Morgan Stanley Capital Partners to US Fertility which is financed by Amulet Capital Partners, a middle-market private equity investment firm that manages over $1.7 billion. US Fertility advertises itself as the largest physician-led IVF clinic network in the U.S. It arose out of Shady Grove Fertility and now in addition to Ovation Fertility and Ovation Genetic Laboratories, involves Fertility Centers of Illinois (FCI), Reproductive Medicine Associates of New York (RMA-NY), also known as the Mount Sinai IVF Program, IVF Florida, and others.
Have plaintiff lawyers discovered preimplantation genetic testing for aneuploidy (PGT-A) as a target of lawsuits? In researching above-described lawsuits against Ovation Fertility, we discovered that those four lawsuits may not be the company’s only legal problems: What we discovered was that the plaintiff personal injury law firm Lieff Cabraser Heimann & Bernstein is investigating reports that a significant number of recent preimplantation genetic tests (PGT) performed by Ovation Genetics/ Ovation Fertility on IVF patients’ embryo samples are unreliable and inconclusive.5
In its announcement the firm notes that these tests are performed on viable embryos before implantation, seeking to confirm the presence or absence of serious birth defects and other illnesses. Individuals have reported receiving PGT results from Ovation Genetics/ Ovation Fertility and making significant family-planning decisions based on these results, including the implantation or destruction of precious embryos, only to later discover the reports they received were faulty.
Another Personal Injury law firm, Rocky McElhaney, is also advertising interest in Ovation Fertility, explaining it the following way: Due to a testing disaster and subsequent cover-up at Ovation’s laboratory, many reports on IVF samples are unreliable. We are currently investigating reports that a number of preimplantation genetic tests (PGT) performed by Ovation Fertility were faulty, leading people to make important family planning decisions on inconclusive data, including implanting or destroying embryos.6
And more on the CooperSurgical lawsuits
We previously reported on the many lawsuits filed by couples against CooperSurgical amid claims that embryo culture media supplied by the company to IVF clinics destroyed embryos.7 The
case, however, only appears to be growing, with increasing numbers of plaintiff firms advertising for clients in this case, including such prominent firms as Weitz & Luxenberg8, again Lieff Cabraser Heiman & Bernstein (they appear to like the IVF field)5 and Myers & Flowers 9
Plaintiffs’ lawyers have suggested that in the affected batch of medium magnesium was lacking, while the company claims that the investigation is still going on. What the media, however, have not yet picked up on is the fact that CooperSurgical is also one of the biggest – if not the biggest – provider of PGT-A laboratory services in the world.
to get better at
We wrote extensively about the shake up in the administration of Kindbody in last month’s issue of the VOICE. And here are, based on an e-mail from Griffin Jones on April 11, 2024, and an article in the mailing by Rosemary Scott, further updates, involving six new hires for the executive suit. Concomitantly, the company announced further expansion plans with four new clinics in Charlotte, NC, Miamai, FL, Newport Beach, CA, and San Diego, CA. One wonders where they expect to get the staff from?
New staff in old roles is a new Chief Financial Officer, a Chief Revenue Officer, a Chief Scientific Officer (CSO), – quite a strange story because we reported last month the hiring of a person for this position; but she apparently decided to stay in her prior job in First Fertility. They now hired Jason Barritt, PhD, who was the Lab Director at RMA-NY for 10 years before moving on as Lab Director at Southern California Reproductive Center in Beverly Hills, CA.
Angie Beltsos, MD, who had the title of CEO-Clinical, has now a new title as Chief Executive Physician. The company, indeed, appears packed with titles, including three Chief Medical officers, and a Chief Growth Officer. And then there are all the newly created titles: Chief Operating Officer, Chief Compliance Officer, Chief Accounting Officer, etc. Too many generals for too few soldiers?
REFERENCES
1. https://www.crhnashville.com/our-location-and-facilities/
2. Wise J. BMJ 2024;384:q621
3. https://www.homerton.nhs.uk/fertility/
4. Limehous J. USA Today; April 26, 2024; https://www.usatoday.com/story/news/ health/2024/04/26/ovation-fertility-newport-beach-lawsuit-ivf/73457205007/
5. https://www.lieffcabraser.com/injury/ovation-genetics/?gad_source=1&gclid=EAIaIQobChMIyIaM-q33hQMVjjcIBR3_gwrJEAAYASAAEgIlUPD_BwE
6. https://www.rockylawfirm.com/ovation-fertility-preimplantation-genetic-test-failures/
7. Lawrence L. STAT News. March 8, 2024; https://www.statnews.com/2024/03/08/ ivf-doctors-embryo-coopersurgical-lawsuit/
8. https://info.weitzlux.com/coopersurgical-ivf/?utm_source=adwords&utm_medium=paidsearch&utm_campaign=coopersurgical&utm_content=search-ad&utm_ term=cooper%20surgical%20lawsuit&c_id=C-9952&gad_source=1&gclid=EAIaIQobChMImvbNh733hQMVw8s8Ah26aQYJEAAYASAAEgK5W_D_BwE
9. https://www.meyers-flowers.com/lp/cooper-surgical-ivf-liquid/?campaign=21095981218&content=693244884390&keyword=coopersurgical%20 lawsuit&gad_source=1&gclid=EAIaIQobChMImvbNh733hQMVw8s8Ah26aQYJEAAYBCAAEgLTjPD_BwE
Wherever one looks in health care, one sees horizontal as well as vertical consolidations, from relatively small scale (for example, medical practice purchases by private equity, frequently discussed before in these pages) to gigantic stages involving hospital consolidations in big city markets or even vertical consolidations between health insurers and, for example, pharmacy chains or physician payment companies (on behalf of insurers).
Physicians find themselves, of course, at the very bottom of these consolidation processes, basically increasingly powerless and at the mercy of ever more powerful hospital administrations, insurance companies, and other financial interests from outside the medical field. The consequences – as also repeatedly before addressed in these pages – have been new practice mandates imposed on physicians from all sides: governments do it based on the all-consuming new wokeness in society and especially young society, hospital systems, mostly not less woke than governments and their agencies, however, in addition have become economically increasingly aggressive in insisting “on owning the practice of medical care,” no longer satisfied with just providing the infrastructure for care; and then, of course, medical insurance companies which – since they consider themselves to be paying for everything – feeling entitled to tell physicians how to practice medicine.
With increasing consolidation in the various spheres of the medical industry, an interesting new phenomenon, however, appears to attract increasing attention: clashes among so-called medical corporate giants. Insurance companies increasingly not only scrutinize physician costs but also charging patterns of hospital chains, pharmacy mangers, pharma companies, and other powerful interest groups. One, therefore, nowadays hears constantly about such clashes of in their respective interests highly conflicted giants. The ultimate loser is, however, because of higher prices and/or insurance premiums always the patient-consumer.
This became once again very obvious in a recent article in The Wall Street Journal which reported (how totally unsurprising) that hospital-care prices rose significantly after hospital mergers.1 Based on studying 322 hospital mergers between 2010 and 2015 using data from CVS Health ‘s Aetna, United Healthcare, and Humana, prices rose by 1.6% over the two years after mergers but by 5.2% where the mergers resulted in significantly increased market power.
Who can be surprised about these findings? We could have predicted this study outcome even without having to perform such a study because this is how markets work. But what is surprising is that governments continue allowing formation of these almost- monopolies. But what could then be an alternative, – giving government a monopoly? Don’t think so!
REFERENCE
1. Evans M. Wall Street Journal, April 25, 2024; pA6
We last months in these pages reported on a paper by Canadian colleagues which quite unequivocally demonstrated that how national maternal mortality rates had been reported in the U.S. was bogus; but, of course, nobody had been interested in finding out because rising maternal mortality, especially amongst minorities, not only mashed perfectly with the current political climate, but was also a God-given argument to receive more financial support for the field of Obstetrics.
But, now, low and behold, after years of media reports that told us what a horrible country we have been for, even in these days, producing worse and worse maternal mortality rates year after year, what a surprise – the U.S. maternal mortality rate, suddenly, as CNN reported, significantly declined, based on the CDC’s National Center for Health Statistics from a whopping 32.9/100,000 live births in 2021 by almost a third to 22.3/100,000 in 2022 (see figure above). Unsurprisingly, the rate for Black women was still higher than for Whites, Hispanic, and Asian women. What may be the reason(s) has remained unresolved.
And then there is the U.S. fertility rate. According to a news release from the CDC on April 25, 2024,2 the 2023 rate once again dropped to another historic low of 3% below the 2022 rate. More specifically, they declined in women 20-39 years and remained unchanged at ages 10-14 (really, how bizarre!) and 40-49.
Teenagers aged 15-19 demonstrated a 3% drop; women between ages 20 and 24 reached a record low birth rate of 55.4/1000,000. Preterm births remained unchanged at 10.4% and almost one-third of pregnancies (32.4%) were delivered by Cesarean section.
The average American family has only 1.6 children; a country’s replacement rate is 2.1 children per family. Barring emigration, the U.S. would not even be able to replace its dying population. Other developed countries are even worse off. The Free Press recently ran an article under the heading that “more than 150 schools in South Korea had 0 first graders this year.”3 Many European countries are not far behind but are actively replacing their population with migrants, mostly from the Middle East (see also page 13).
The increasing importance of IVF for birth rates
Considering the radically declining birth rates in so many countries, the utilization of IVF as a toll of improving birth rates will come increasingly into focus. On a recent release by SART on April 18, 2024, the society announced that in 2022 a full 2/5% of all U.S. births were IVF births. At the same time, the announcement also included some disappointing numbers, not the least the number of IVF clinics which in 2022 stood only at 368 (in comparison to almost 500 reporting clinics to the CDC).4
CDC warns about bird flu
So why is there, suddenly, so much talk about the Avian or Bird flu? The answer is not really clear because as in recent history so-often, it takes reading tea leaves to at times understand what the CDC is trying to communicate. So here is a little bit of history to, maybe, understand things a little better: Bird (Avian) flu, as the name already indicates is in principle a bird virus and, often a very lethal bird virus. Avian influenza A, also called H5N1, therefore, is not a virus inherent to humans. The virus has, however, a proclivity of jumping to other animals, including primates, and this is where the current story circulating the globe got its start: Several U.S. states discovered cows infected with the virus. Moreover, pasteurized milk allegedly tested positive for viral component, even though no risk of infection from drinking pasteurized milk.5
As we are writing this commentary, a letter-to-the-editor in The New England Journal of Medicine reported an eye infection with the highly pathogenic H5N1 virus in a dairy farm worker.6 He, however, had no respiratory symptoms.
So, what is the current advice of the CDC? “The risk to the public remains low; but the agency encourages states to make response plans in case this turns into another pandemic.” And on a side note, vaccines against this virus already exist. What we hear from other experts is that things could go either way.
1. Howard J. CNN Health, May 2, 2024; https://www.cnn.com/2024/05/01/health/ maternal-mortality-cdc-report/index.html
2. CDC, National Center for Health Statistics; April 25, 2024. https://www.cdc.gov/ nchs/
3. Sussman AL. The Free Press ; April 16, 2024. https://www.thefp.com/p/south-korea-america-having-kids
4. https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/general-information/success-rates/
5. Evans M. Wall Street Journal, April 25, 2024; 6. Uyeki, et al., N Engl J Med May 3, 2024. DOI: 10.1056/NEJMc2405371; ahead of print.
After formally identifying women’s health as one out of four potential high-growth areas for its business, Labcorp took a decisive step in expanding its offering in reproductive testing by purchasing assets from Bioreference Health, as subsidiary of Opko Health (a diversi-
fied healthcare company with presence in more than 30 countries) for $237.5 million. As reported by MedtechDive, this involved a loss-making Opko unit that had recently laid off 252 people in an effort to return to profitability.1
1. Talor NP. MedtechDive. March 29, 2024; https://www.medtechdive.com/news/ labcorp-buys-bioreference-reproductive-women-health-assets/711713/
What is it about the state of Utah that leads it to repeatedly facilitate by the FDA – unapproved medical treatments? Not very widely known is, for example, the fact that since deceased Utah’s long-serving senator Orrin Hatch was instrumental in defining the hormone dehydroepiandrosterone (DHEA) in the U.S. uniquely as a food product and not – like any other hormone – as an FDA- regulated drug. A powerful politician in his days, he was probably the most responsible individual for the 1994 passage of the Dietary Supplement Health and Education Act, which legitimized the sale of dietary supplements.1
Now Utah, as a recent article in Science well described, once again flouts the authority of the FDA by passing a law that allows patients to receive medical therapies with placental stem cells, a treatment currently not approved by the FDA, yet by the FDA considered to be under the agency’s regulatory mandate.2 Under this new Utah law, all forms of placental stem cell therapies are permitted, as long as patients sign an informed consent that clearly states that this treatment has not been approved by the FDA. Eligible as “providers” are physicians, naturopaths, chiropractors, podiatrists, pharmacists, nurses, and midwifes who’s scope of practice includes stem cell therapy (whatever that may mean).
Considering the complete absence of guidelines for use of placental stem cells, vetting of manufacturing standards, safety, sterility, etc. this law is of considerable concern, especially since other states may follow. And then there is another issue we have previously addressed in these pages, the proliferation of specialty societies with ever smaller specialty areas but ever bigger pretentions of being qualified to establish guidelines for the field and, of course, determine clinical practice, usually, of course, in collaboration with industry. That then economic conflicts of interest abound cannot surprise.
And her is promptly another excellent example for this practice: Did you, for example, within the here discussed subject know that there exists a Perinatal Stem Cell Society (PSCS) which, recently per e-mail (April 24, 2024, www.perinatalstemcells.com) “called upon cell manufacturers of non-FDA approved stem cell, exosome, and tissue products’ with the purpose of “convening an industry coalition of perinatal product manufacturers.”
To quote the society’s announced plan, “the PSCS plans to create industry guidelines for self-regulation to ensure the safety of perinatal stem cell and tissue products and invites members of industry to join the society in shaping the future of stem cell therapy in the U.S.”
This at a time when the FDA on April 9, 2024, issued a consumer alert regarding regenerative medicine products including stem cells and exosomes encouraging patients to report adverse events related to stem cells, exosomes or other products marketed as regenerative medicine.3
1. Pray WS. J Child Neurol 2012;27(5):561-563
2. Wadman M. Science 2024;384(6691):14-15
3. https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/consumer-alert-regenerative-medicine-products-including-stem-cells-and-exosomes
A recent article by Hannah Rahim, a Student fellow (JD/MPH candidate) at the Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics at Harvard Law School and T.H. Chan School of Public Health, in the Perie-Flom Blog, addressed the increasingly important issue of international commercial surrogacy. As our heading indicated, the term “surrogacy” is incorrect because a surrogate would be expected not only to carry a pregnancy (as a gestational carrier does), but also to contribute her own eggs to the pregnancy. The article, therefore, really addressed the internationally quickly growing business of using gestational carriers over long distances.1
The article defines the process as, “people sending their gametes or embryos to be gestated by a woman residing in another country.” After birth of the child, the birth mother then gives up the rights to the child to the commissioning parent(s) in return for financial compensation. International arrangements like this are often driven by legal prohibitions of such arrangements in several countries but can also be the consequence of exorbitant agency and gestational carrier fees in countries like the U.S.
Without having the space to go into detail, the article is an excellent summary of the subject, addressing ethical issues as well as arguments in favor of regulatory interventions in better defining the process. We strongly recommend this paper for anybody interested in the subject because of personal or professional reasons.
1. Rahim H. Petrie-Flom Blog. March 18, 2024; https://blog.petrieflom.law.harvard. edu/2024/03/18/regulating-international-commercial-surrogacy/#more-32393
Are paper retractions now also becoming subject to political yo-yo?
We are, of course, on record as strong proponents of paper retractions if published papers conveyed incorrect information. At the
same time, we, however, are very concerned about the possible weaponization of the process, which, as a recent article in Retraction Watch suggested, may already be under way. As we are writing this note, Retraction Watch, a publication that has made its goal “to track retractions as a window into the scientific process,” published an article on the retraction of three papers by a medical journal (Health Services Research and Managerial Epidemiology) and its publisher (Sage), all relating to what in current-day America, still is, likely, the most controversial public health issue – abortions. One of these three papers, indeed, has been used as “evidence” in the current mifepristone case before the Supreme Court.
With accusations flying on both sides of the issue, we are not in a position to judge which side is right; but only so much: All but one author of these three papers have been active pro-life advocates with affiliations with several pro-life advocacy groups, yet declared “no conflict” when submitting their manuscripts for publication. In short, they lied!
Though that does not automatically mean that they also “lied” in their submitted manuscripts, this fact, at minimum, created doubts about the authors’ ability to operate with objective insights and warranted an investigation. Incorrect representations of conflicts are, however, unfortunately a wide-spread problem in medical publishing, in reproductive medicine often blatantly obvious to informed observers, yet, unfortunately, equally blatantly overlooked by obviously knowing editors. Authors (and, yes, even editors of prominent medical journals) almost routinely publish papers listing no personal potential conflicts, when such conflicts have been only too obvious and are well recognized in editorial offices. Yet, these papers are, nevertheless, often allowed to slip through the peer review process.
And what applied to above-noted three papers, should also apply to all of these papers with obviously incorrect conflict statements in the reproductive medicine literature; Authors who lie in their conflict statement, must also be scrutinized in their data presentation and opinions.
Which brings us back to the question we asked at the beginning and were unable to answer: We don’t know whether the data presented by the authors in these three retracted papers were biased by the authors’ strong personal believes regarding the abortion issue and, therefore, were properly retracted. We have no objections to defining any substantial failure of pointing out potential conflicts in the by most journals mandated disclosure statements as a good reason for a paper’s retraction; but if those are the rules, then they should apply equally to somebody who does not disclose personal political or personal financial conflicts. There cannot be a bifurcation between disclosure of permissible financial and impermissible philosophical or political conflicts.
REFERENCE
1.https://retractionwatch.com/2024/02/06/papers-used-by-judge-to-justify-abortion-pill-suspension-retracted/
Whether ovaries should be removed at time of hysterectomy has been a decade-old debate, with arguments in favor and against battling it out in literature and clinical practice. Con voices, however, appear to have gained an at least temporary upper hand it appears after early removal of ovaries was associated in several studies with increased dementia risk. This relatively newly recognized association appears to have broken the camel’s back, with the pendulum increasingly clearly swinging toward retention of ovaries, as a recent article in The Wall Street Journal appeared to suggest.1
But things are, of course, never as simple as they appear on first impression: The here debated issue is no exception and like in practically all medical decisions, there are risks that must be weighed against benefits. The results of such considerations are, however, not necessarily always very clear, one reason why medicine to these days by many is described not only as a scientific but also an artsy profession requiring a degree of intuition good artists carry art the core of their artistic individuality.
We, therefore, would summarize the current status of the debate as follows: (i) In principle, no surgery should be performed without good reason. In other words, just “because we are already there (because of another surgery), does not mean we have to do additional surgery. Removing ovaries is not the only case where this argument has become fashionable; the number of appendectomies performed worldwide under this rational by far exceeds the number of unwarranted oophorectomies by many orders. (ii) If an additional surgery is indicated, there must be a good reason: i.e., a risk-benefit calculation must clearly come out on the side of the additional intervention; and, (iii) we also must consider alternatives.
As noted in above cited Wall Street Journal article, it appears that hormone replacement therapy (HRT), for example. can make up for some of the oophorectomy-associated increased risks (which besides more dementia, also entails more heart disease and more strokes). But HRT, of course, has its own risks which then also must be considered in an appropriate risk-benefit evaluation.
In other words, if a woman is a BRACA carrier with highly significant risks of developing breast and/or ovarian-tubal cancers, her risk exposure may very well warrant prophylactic oophorectomies as well as mastectomies; but the risk-benefit, of course, looks very different if one is not a BRACA carrier.
In short, one of the most basic rules at the CHR applies here more than in many other clinical circumstances, always be skeptical if a medical treatment is proposed to everybody!
REFERENCES
1. Reddy S. The Wall Street Journal. April 24, 2-24; pA12
The CHR has for decades pointed out immunologic similarities between peripartum cardiomyopathy and preeclampsia, making the point that both conditions very likely are reflections of abnormal tolerance of the fetal-placental semi-allograft in late pregnancy. The latest brief comment on the subject was a very recent letter-to-the-editor from the CHR’s Norbert Gleicher, MD, in the New England Journal of Medicine in which he, in regard to a literature review in The Journal, once more bemoaned how the maternal immune system was being ignored in discussions of peripartum cardioopmyopathy (see also page 34).1
Interestingly, only weeks after that communication a large group of investigators from all around the world published in a very interesting paper in Science Translational Medicine which demonstrated that placentas from patients with peripartum cardiomyopathy and/ or preeclampsia had increased gene expression and tissue signatures typical of senescence, a process, of course, linked to aging and cellular stress. This was also confirmed in a mouse model of peripartum cardiomyopathy. Senolytics compound or monoclonal antibodies targeting a senescence protein in the model during midto late-pregnancy reduced placental senescence and, interestingly, improved heart function, suggesting according to the authors that peripartum cardiomyopathy could be treated through reduction of cellular senescence in the placenta.
Once again, however, the investigators overlook the immunological connection because they do not ask why the placenta in these two conditions demonstrates increased senescence? An at least theoretical answer appears obvious: The placenta will demonstrate increased senescence whenever maternal tolerance pathways in protection of the fetal semi-allograft become insufficient. In women with hyper-active immune systems (autoimmunity, inflammation, allergies), this happens prematurely, leading not only to peripartum cardiomyopathy and preeclampsia but, universally, to early labor, and peri- and post-partum autoimmune disease flares. Please continue reading on the subject in the next section where Dr. Gleicher’s recent letter-to-the-editor is further discusses.
1. Gleicher N. N Engl J Med 2024;390(15):1443
2. Roh et al., Sci Transl Med 2024;16(743):eadi0077

On May 3, 2024, the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, was notified by ScholarGPS™ that his “prolific publication record, the high impact of his work, and the outstanding quality of his scholarly contributions placed him in the top 0.05% of all scholars worldwide.” As if this was not enough of an honor, his rankings in several specific specialty-related areas were even more remarkable. Here they are:
HIGHLY RANKED SCHOLAR – LIFETIME WORLDWIDE RANKING
# 9,046 Overall – all fields
# 2,307 Medicine
# 37
Obstetrics and Gynecology
# 12 Assisted reproductive technology (ART)
# 17
Reproductive biology
# 94 Pregnancy
HIGHLY RANKED SCHOLAR – LAST 5 YEARS WORLDWIDE RANKING*
# 3
# 6
Reproductive biology
Genetic testing
ScholarGPS™ is California-based company that applies A.I., data mining, machine learning, and other data science techniques to its massive database of over 200 million publications and 3 billion citations to rank over 30 million scholars and 55,000 institutions worldwide. For more information, you are invited to visit ScholarGPS.com.
*reflecting his research activities in most recent years.
cardiomyopathy. N Engl J Med 2024;390(15):1443
We already above briefly noted the recent publication of a letter-to-the-editor by the CHR’s Norbert Gleicher, MD. 1 It related to his long-standing interest in reproductive immunology and especially in the delicate interplay between pregnancy tolerance by the maternal immune system and autoimmunity. One of the very characteristic features of this interplay is the widely known fact that autoimmune diseases tend to flare once a pregnancy ends (this applies not only to term or near-term pregnancies, but flares often also occur after miscarriages or terminations of pregnancy).2
Though the reasons for these disease flares are not yet well understood, they obviously must have something to do with the end of immunological tolerance of the maternal immune system toward the fetal-placental semi-allograft (full allograft if donor eggs are involved). As the CHR understands the process, so-called tolerance pathways a maternal immune system induces during implantation to make the implanting embryo immunologically “invisible,” are terminated.3 The obvious consequence is a re-awakening of the maternal immune system in its ability to recognize the fetal placental unit as “foreign,” leading to an inflammatory immune response, now widely believed to be the signal for onset of labor. Consequently, autoimmune diseases are practically universally also characterized by early onset of labor and premature deliveries.4
Like pregnancy, tolerance of “self” is also dependent on tolerance pathways. That tolerance pathways for pregnancy can be cross-reactive with other tolerance pathways has been, for example demonstrated in certain parasitic diseases, where survival of parasites within a host is obviously also dependent on tolerance pathways in the host that prevent rejection of the parasites. In certain infections with helminths (worms), protective tolerance pathways against helminths have also been demonstrated protective of pregnancy.5 Cross-protection between tolerance for “self” and pregnancy is well demonstrated by the observation that certain autoimmune diseases clinically improve in pregnancy, once full pregnancy tolerance has been established (mid-trimester).6
Gleicher has been exploring this issue for decades in his writing, repeatedly pointing out the importance of the peripartum timing of certain disease flares as a strong suggestion that the
underlying medical conditions must in their respective etiologies be autoimmune in nature. This specifically applies to two conditions, what is now called peripartum depression (previously called postpartum depression or “postpartum blues”)7,8 and, as previously noted, peripartum cardiomyopathy.9 In both of these diseases, investigators are currently still seeking an underlying etiology/pathophysiology. They, therefore, are not yet considered to be autoimmune diseases and Gleicher’s letter to The New England Journal excellent review article about peripartum cardiomyopathy in the journal, which mentioned the possibility of an (auto)immune etiology in only one short sentence. Gleicher’s arguments regarding an autoimmune etiology for peripartum cardiomyopa thy is now, of course, also further strengthened by the recently published article in reviewed.


1. Gleicher N. N Engl J Med 2024;390(15):1443
2. Eudy et al., Ann Rheum Dis 2018;77(6):855-860
3. Gleicher et al., J Assist reprod genet 2017;34(4):425-430
4. Gleicher N. Clin Rev Allergy Immunol 2010;39(3):194-206
5. Blackwell et al., Science 2015;350(6263):970-972
6. Piccinni et al., Clin Mol Allergy 2016;14:11
7. Gleicher N. Autoimmune rev 2007;6(8):572-576
8. Gleicher N, Weghofer A. Hum Reprod 2009;24(3):760-761
9. Gleicher N, Elkayam U. Autoimmune Rev 2009;8(5):384-387
10. Roh et al., Sci Transl Med 2024;16(743):eadi0077


“My retrieval process went very smoothly and the doctors did not give up on me! They fought for every egg to give me the best possible outcome. The entire staff is so supportive and always always always available for anything you might need. Dr Gleicher and Dr Barad are the best in the industry”
-Anonymous
“Besides his great expertise and high scientific knowledge, Dr. Barad is compassionate, kind, patient. He supported me in the worst moment of taking the decision to transfer my poor quality embryos on Day 3 holding my hand and genuinely caring for my wellbeing. Because of the pandemic, I had to wait a couple of years before being treated at CHR - I definitely recommend Dr. Barad and hope to get his advice also in the near future.”
-Anonymous


Plan ahead to the always unique 2024 FOUNDATION FOR REPRODUCTIVE MEDICINE CONFERENCE (FRMC) on Translational Reproductive Biology and Clinical Reproductive Endocrinology in New York City, December 6-8, 2024
VENUE: CONVENE Conference Center: 237 Park Avenue, between 45th and 46th Street, accessible from Park and Lexington Avenues





Building on the success of the 2023 conference, the FRMC over 3 conference days will once again present a unique program in reproductive medicine, connecting in a single lecture hall between basic science and clinical practice, facilitating translational connections between bench and clinic in the process. As in preceding years, the principal purpose is demonstrating to clinicians what is possible and to scientists what is needed. The intent is not to dream about the future but to demonstrate what can already be achieved.
“To think differently” has been the principal motto of the FRMC since the beginning and will remain that, as questioning mainstream thinking has been at the core of the conference’s success since its beginning. The FRMC, in addition, also frequently premieres new treatment paradigms.
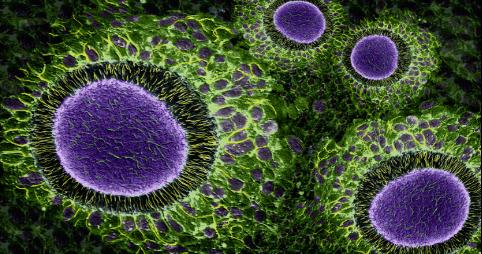
As new findings are reported in basic science and clinical journals, the conference content evolves over the preceding year. Since the embryo genetically contains universal information about almost everything in human biology - life, death, regeneration, immune tolerance, etc. - the earliest stages of embryo development never fall out of fashion and have always occupied an important place in the program of the FRMC. While several big themes, such as reproductive genetics, the aging of infertile patient populations, “add-ons” to IVF practice, difficulties in IVF cycle outcome reporting, the relevance of the female immune system to fertility, and others that have been addressed before will return with hopefully new data, brand-new subjects can also be expected. For example, obesity is a rapidly growing worldwide health problem also adversely affecting female and male fertility as well as fertility treatment success. Very successful, newly-to-market anti-obesity drugs, therefore, must be integrated into routine infertility care of obese patients. Another big theme in the 2024 FRMC conference will be the ethics of infertility practice because the rapidly expanding takeover of infertility practice by outside financial interests is a subject gaining urgency by the day.
The FMRC can proudly point out to have affected the engrained ineffective and, at times, harmful daily practice patterns in IVF long before other conferences even noticed. This included preimplantation genetic testing for aneuploidy (PGT-A), and more recently, the long-standing concept of embryo selection in general, single embryo transfer for everybody, and blastocyst culture for everybody. Worldwide declines in IVF live birth rates since 2010-2013 have still not attracted the attention they deserve. Never bashful in addressing controversies, FRMC 2024, therefore, will stay the course in attempting to define the underlying causes for this observation. The publication crisis in medicine will, of course, also have to be addressed, considering the constantly increasing number of paper retractions, even in very prominent journals involving very prominent individuals. Also related is the recognition that what is considered evidence-based medicine must be accompanied in parallel by “real world data” studies, as carefully planned prospectively randomized studies can never fully reflect what happened in “real-world” applications of treatments.
In short, following the annual lighting of New York City’s famous Christmas tree at Rockefeller Center that formally opens the city’s Christmas season by only several days, the 2024 FRMC will not only offer what, likely, is the most interesting professional conference in reproductive medicine anywhere in the world but also a unique time to visit New York City in one of the city’s most beautiful periods.
There is simply no better time to visit NYC than in the weeks before Christmas!



• Attempting to offer a teachable moment, we on a monthly basis present in this section of the VOICE an anonymized case report from the files of the CHR.
• This case involves a 26-year-old G0 woman who presented with a principal diagnosis of very low functional ovarian reserve for age, 2 failed IVF cycle attempts, and a recommendation from 3 infertility clinics to advance into third-party egg donation.
• After receiving appropriate treatment, she experienced an unexpected outcome which well demonstrates an important underlying pathophysiology observed in practi cally all women with premature ovarian aging (POA) but is often ignored by fertility clinics.
The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces an androgen (male hormone) supplementation product called dehydroepiandrosterone (DHEA) and other fertility-related supplements. Since this article, among other subjects, addresses androgen supplementation with DHEA, readers are advised that expressed opinions in this paragraph may be biased.


A 26-year-old GO presented to the CHR after 2 failed IVF cycles at 2 IVF clinics and after being advised by 3 fertility centers that her only chance of conception was through third-party egg donation. After developing menstrual cycle irregularities, she, based on abnormally FSH (23.4 mIU/mL), one year earlier had been referred to a fertility specialist who confirmed her elevated FSH and also recorded an abnormally low AMH for age (0.7 ng/mL).
She was advised that she had diminished ovarian reserve and, likely, would soon be in premature menopause (primary ovarian insufficiency, POI, also called premature ovarian failure, POF). Since she and her partner at that time had no intention of conceiving, they decided to cryopreserve eggs instead of pursuing pregnancy right away. Within one month from initial consultation at the fertility clinic, the female was in an egg-freezing cycle, which only produced 2 eggs, of which only 1 was judged cryo-preservable.
Disappointed by this cycle outcome, the couple switched clinics and, after further consultation at that clinic, decided to change strategy and attempt pregnancy right away. Once again, they entered cycle stimulation within a very short time and without any preparation of ovaries but, in contrast to their first cycle, the patient this time received only mild stimulation with clomiphene citrate and 75IU of FSH every second day. The female went to retrieval with only 1 follicle of 19mm at time of trigger, but no egg was retrieved.
At this point the couple was advised by the clinic that, going forward, their only realistic chance of pregnancy was with use of donor eggs. Unwilling to consider such an option, the couple consulted with two other fertility clinics but, at both, received exactly the same advice. This is when she consulted for the first time with the CHR.
At the CHR, they were advised that, based on age of the female and the level of her FSH and AMH abnormalities, they should still have a very good chance of conception with autologous eggs, though chances with young donor eggs, of course, would be higher. We, however, were very confident that, allowing for several tries, they would end up being successful.
The CHR’s routine diagnostic work-up confirmed this assessment, when her FSH was recorded at 22.7 mIU/mL and her AMH at 0.5ng/mL, thereby also confirming that she to significant degrees was prematurely aging her ovaries (i.e., had much fewer eggs left in her ovaries based on her age than she should). Overall, she was assessed to suffer from a moderate degree of premature ovarian aging (POA, with options being mild, moderate, and severe).


Once this diagnosis was reached, two automatic questions arose: (i) Why did this woman suffer from POA which affects ca. 10% of women, independent of race and ethnicity? And (ii), how should she be pre-treated before any further IVF attempts and in her subsequent IVF cycles?
Both of these questions exemplify how differently the CHR approaches the treatment of POA from most other fertility centers. The importance of the first question lies in the fact that POA patients in almost all cases demonstrate significant laboratory abnormalities which most fertility clinics not even look for: A first is hypo-androgenism (low male hormone levels), a finding affecting virtually 100% (a rare thing in medicine) of all women with POA (please note above Conflict Statement).1 Ovaries, however, require good androgen levels in order to make eggs in good quantity and of good quality.2 POA patients with low androgen levels, therefore, must be pre-supplemented with androgens for at least 6-8 weeks before IVF cycle start (the CHR prefers pre-supplementation with DHEA to pre-supplementation with testosterone gel), which in over half of all cases will positively affect AMH levels.3
Similarly, ovaries also require normal levels of insulin growth factor-1 (IGF-1) which, in turn, is stimulated by human growth hormone (GH). In other words, women with low IGF-1 must, therefore, be pre-supplemented with GH for 6-8 weeks before IVF cycle start, though, fortunately, a much rarer occurrence than low androgen levels. That IGF-1 deficiency is rarer is fortunate because GH supplementation is much more expensive than the relatively inexpensive androgen supplementation.
As expected, this patient turned out to have very low androgen levels (total and free testosterone, DHEA, DHEAS) and abnormally high sex hormone binding globulin (SHBG) which goes in opposite direction to androgen levels and, therefore, can be used to judge androgen levels. Her IGF-1 levels were, however, in normal range. She, therefore required only pre-treatment of her ovaries with DHEA (from which our organs make testosterone).
A second very important issue often overlooked by fertility clinics is the fact that POA is often associated with a hyperactive immune system.4 This is important for several reasons: first because it suggests a close link between the risk of developing POA and the female immune system but, on a more practical level, because hyper-active immune systems are also closely associated with increased miscarriage risk.5 The last thing one, of course, would want to happen is to help a patient to conceive, just to experience a potentially preventable miscarriage. And this patient, indeed, demonstrated a positive antinuclear antibody (ANA) as well as positive antiphospholipid antibodies in IgM and IgG isotypes, suggesting that she needed prophylactic treatment to prevent such a possible miscarriage.


At a return consultation all of these findings were discussed with the couple. We also raised the additional question, whether they would want more than one child? The rational for this question was that should this female conceive she, because of pregnancy and breast feeding, would be at least ca. 18 months out of the bay-making business. Considering her moderate POA at such young age, that could mean that her ovarian reserve may have so-far deteriorated by then that, even autologous IVF, might no longer be possible.
This concern, therefore, raised the question whether before a first immediate pregnancy attempt, we should try to freeze some eggs or embryos for such a circumstance. Because of the relatively small number of expected eggs/embryos, the couple, however, decided against such an option because they felt they did not have the financial resources for the potentially required number of cycles to make such a strategy worthwhile.
The patient was restarted on prenatal vitamins which she had stopped. We also initiated supplementation with CoQ10, 200mg TID, and, of course, with DHEA, 25mg TID. The plan was to retest her androgen levels after four weeks, when they would be expected to be back in normal range. Once that was confirmed, the patient would then start another IVF cycle with the following menses.
Her androgen levels after four weeks, indeed, had normalized, as had her SHBG. She, therefore, was entered into the CHR’s routine preparation protocol for a precision- IVF cycle to be initiated on day-2 of her next menses, expected approximately two weeks later. As a routine recommendation, the couple was encouraged to maintain intercourse during this time period since we have witnessed a small number of spontaneous pregnancies in women with POA after start of androgen supplementation alone, while waiting for IVF cycle start.6 Couples are, moreover, advised to immediately notify the CHR if they are late for their menses, so they can be immediately tested for pregnancy since studies have demonstrated that the effectiveness of miscarriage-preventing treatments quickly declines in early pregnancy.
One day after missed menses we received a call and found out that the patient was spontaneously pregnant. She was started on the lowest level of immunosuppressive treatment the CHR uses in women with hyperactive immune systems, consisting of 1 baby-aspirin (up to 36 weeks of pregnancy) and low dose prednisone (only until confirmation of fetal heart in absence of an immune system flare) and ended up delivering a healthy male infant at 39 weeks. We expect the couple back soon to try for a sibling.
• Especially young POA patients are too often – in this case by 3 IVF clinics -prematurely referred into third-party egg donation.
• As this case of even spontaneous pregnancy after androgen supplementation well demonstrates, their ability to still achieve pregnancy with autologous oocytes is at most fertility clinics greatly underestimated.
• To maximize pregnancy and live birth chances, such patients must, however, receive individualized precision treatment, involving specific preparation of ovaries for at least 6-8 weeks with androgens and, on rarer occasions, GH. proper individualized ovarian stimulation, and potential prevention of miscarriages if such a risk is diagnosed based on evidence of a hyper-active immune system.


• Because of the patient’s spontaneous conception, this cased did not offer the opportunity also to discuss HIER (highly individualized egg retrieval) which refers to individualized earlier ovulation trigger at smaller lead follicle sizes7 and embryology management8 based on ovarian age.
• Pregnancy and live birth chances, of course, decline with advancing age of the female.
1. Gleicher et al., Hum Reprod 2013;28(4):1084-1091
2. Barad D, Gleicher N. Hum Reprod 2006;21(11):2845-2849
3. Gleicher et al., Reprod Biomed Online 2010;21(3):360-365
4. Gleicher et al., Menopause 2009;16(4):760-764
5. Gleicher et al., Autoimmunity 1993;16(2):115-140
6. Shohat-Tal et al., Nat Rev Endocrinol 2015;11(7):429-441






As ethical and financial conflicts in medicine seem to be everywhere, only physician-driven interventions can preserve at least some self-regulation of medical practice
Norbert Gleicher, MD Medical Director and Chief ScientistThe CHR


BRIEFING
Professional societies have been valuable participants and contributors to the remarkable progress medicine has made especially over the last half century. To this day, they play important roles in self-regulation of medical practice and medical research by the medical profession. With special emphasis on reproductive medicine, this opinion-article, however, argues that the standing of medical societies as pillars or self-regulation is quickly diminishing due to increasing conflicts of interest, a development that ultimately must lead to increasing government control and less physician influence in shaping the future of medicine, unless medical societies find the courage of addressing these conflicts.
When I started my training in general Ob/Gyn in 1975 at what then was called The Mount Sinai Medical Center here in NYC, even though subspecialty societies already existed, the OB/GYN field was still clearly ruled by the American College of Obstetricians and Gynecologists (ACOG). Because the special expertise in subspecialty areas was so minimal, on an organizational level, they were still almost irrelevant.
Regarding reproductive endocrinology and infertility (REI), though this subspecialty field’s society was initiated already in 1944 as the American Society for the Study of Sterility and in 1965 was renamed the American Fertility Society, it only with the invention of IVF reached functional maturity, assuming its current name as the American Society for Reproductive Medicine (ASRM) in 1994.

Like in practically all areas of medicine, subspecialty societies not only proliferated in numbers, but also rapidly gained membership. That did not necessarily mean that the mothership, ACOG, lost members (we all, simply, became members of multiple societies in our practice fields) but the importance of ACOG (and, therefore, the financial support the society received from other than membership fees) took a beating. And, while AFS/ASRM initially completely controlled the infertility field, this splintering, of course, continued, as additional sub-sub-specialty societies formed for example for surgical treatments of infertility, reproductive genetics, reproductive immunology, and, of course, assisted reproductive technologies (Society for Assisted Reproductive Technologies, SART), smartly organized by the ASRM as a daughter society of ASRM before anybody else could make a claim to this increasingly important part of REI.
That is, indeed, a good question and definitely worth updated considerations since it seems increasingly obvious that these purposes have evolved. Medical societies have existed since the dawn of modern medicine (and who knows, maybe even before that). From the very beginning, they had a duality of purpose: first, they served as a professional forum for the exchange of knowledge, and second, there was also always a social component to them that facilitated not only friendships, but at times also professional cooperation.
One can argue that both of these purposes have survived centuries and – though in obviously advanced formats – still represent the backbone of professional societies (and not only in medicine). Until modern electronic communications came into the picture, societies had to be based on personal gatherings. As modes of travel improved, these gatherings increased in size and the concept of congresses evolved, and with it, not only was a new purpose for professional societies created, but because congresses also started to be exhibition opportunities for industry, they became increasingly important income sources for professional societies. And since medical societies suffer from the same addiction to administrative growth as other bureaucracies, societies became increasingly dependent on industry in supporting their growing program diversity and, of course, bureaucracies.
But even bureaucracies require reasons to grow, and nothing provides more reason for their existence than additions to the purposes of a society. Trying to avoid too much government control, self-regulation of medical practice was, of course, an irresistible add-on to the most basic purposes of medical societies, and this is how the “guideline industry” came into existence.
Because guidelines (like A.I.) are dependent on the material that is used for analysis, and because humans, with all of their conscious and unconscious biases (as we in recent A.I. failures painfully learned), select and interpret the studies that serve as the basis for the establishment of guidelines, I was never impressed by their value but have increasingly come to the conclusion that, overwhelmingly, they not only have absolutely no value but can often be outright misleading.

And consider the effort, manpower, and expense that goes into the publication of many guidelines, especially if they reach the level of absurdity of the recent “Draft for Review” sent out in collaboration by the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) of suggested “Guideline on the management of premature ovarian insufficiency,” claiming to answer not fewer than 142 key questions in a not less than a 227 pages-long document.1
Considering how long it must have taken to produce this document, like most textbooks, even assuming its conclusions were originally valid, a significant portion of those must, however, already be outdated by formal publication date. Moreover, one can only wonder how many fertility service providers and/or members of ESHRE and ASRM will really read these 227 pages-long guidelines when published in final format and will, after that, change anything in their practice patterns as a consequence. The answer very likely would not withstand a cost-effectiveness analysis.
As physician membership fees of course do not suffice, societies often expand their membership beyond physicians, also including related professions, and even lay peoples (i.e., patients) and, yes, of course, at special premium costs – also members of industry. And to prevent competition, some societies (sic., ASRM with the Society for Assisted Reproductive Technology, SART) even form sister societies they own.
What then happens is, likely, the biggest problem in medicine today that nobody is talking about: We created steadily increasing financial dependency of professional medical societies on financial support from commercially interested parties who are trying to sell to the practice field. This is very obvious in REI. One just must look at the exhibition halls at the annual meetings of ASRM and ESHRE, which until ca. 20 years ago were dominated by the big pharma companies, producing fertility drugs. After Big Pharma lost interest in the field, it now is the genetic testing industry that rules the REI world. Who then can be surprised that ASRM, which in the old days was very protective of its relationship with pharma, now has become equally protective of the genetic testing industry. No surprise, therefore, that the ASRM only just now published a guideline that politely stated that PGT-A does not work (see page 71). That use of polygenic risk scoring of human embryos (PGT-M) is unethical still remains to be said. Even the European Society for Human Genetics has made a public statement that PGT-M is unproven and unethical practice, while U.S. medical societies still have maintained complete silence.
Who then can be surprised that forming sub-sub-specialty societies has become kind of a business for some individuals. To stay within our specialty, one does not have to look far. The VOICE has repeatedly pointed out the very questionable circumstances of the Preimplantation Genetic Disease International Society (PGDIS), made up mostly of individuals making a living from performing PGT-A and literally run by a PGT laboratory company, making the absurd claims to be qualified to issue guidelines for PGT-A.2,3 What, however, of course is even more bizarre is the fact that the ASRM in its own strange PGT-A guidelines regarding “mosaic” PGT-A results, extensively quotes these non-sensical PGDIS guidelines.4
Or take the much younger International Perinatal Stem Cell Society (IPSCS) founded already in 2013 - yes established even before any established clinical use of perinatal stem cells, as its website states - ”focused on working with industry and regulators to bring the field of perinatal stem cells and (resulting) tissue regenerative medicine products to patients.”5 And, yes, like the PGDIS, the IPSCS also already issues guidelines.6 Just as there are serial entrepreneurs, so characterized because they serially create start-up companies, so we now apparently also have to contend with serial medical society founders, as the founder of the IPGDIS well demonstrates.

Unsurprisingly, these serial society founders then usually circulate in the industry between societal and corporate jobs.
And those societies, of course, also organize conferences paid for by industry, even if hardly anybody shows up, as the recent PGDIS Conference in Kuala Lumpur (out of all places) so well demonstrated.
No wonder, then, that medicine is ever deeper caught in a web of ethical and financial conflicts that become increasingly difficult to untangle. Wherever one looks, the influence of physicians has been declining, while the influence of money from outside the primary medical field has been increasing. Corporate interests not only own ever bigger parts of provision of health care, but they also increasingly control our conferences (who does or does not speak), what is or is not published, and how medicine is practiced. And all of this is happening with the law in most states still prohibiting the corporate practice and ownership of medical practice.
In REI practice, we have, moreover, especially over the last decade, witnessed the unprecedented proliferation of, simply, useless products which, often, not only fail in what they are advertised to do but, often, even harm patients. This includes several diagnostic tests which in the U.S. have been brought to market as so-called “laboratory-developed tests” which, as such, did not have to undergo FDA scrutiny. As we repeatedly covered in these pages, the FDA has been for years planning to clamp down on abuses with so-designated test and, indeed, recently, announced a new policy for approval of such tests. Whether – considering how much catching up the FDA will have to do on these tests at least in the beginning - this will involve tests affecting our specialty appears as of this moment highly questionable. But, sooner or later, they will get to it. Shouldn’t we, however, not clean our own house first if we want to maintain even a relatively small level of self-determina tion in our practice field?
ADVERTISEMENT
15% OFF WITH THE CODE:
1. ESHRE and ASRM, April 2024; Draft for review. Guideline on the management of premature ovarian insufficiency. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency.aspx
2. Gleicher et al., Reprod Biol Endocrinol 2020;18(1):57
3. Gleicher et al., J Assist Reprod Genet 2023;40(4):817-826
4. Practice Committee and Genetic Counseling Professional Group (GCPG) of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org. Feril Steril 2020;114(2):246-254
5. Perinatal Stem cell Society. https://www.perinatalstemcells.com/
6. Perinatal Stem Cell Society. https://www.isscr.org/guidelines
7. U.S. FDA. https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests




BRIEFING
Obesity in women and men adversely affects spontaneous fertility, but also adversely impacts every fertility treatment. For the longest time, attempts at weight loss in infertile patient populations were, however, either unsuccessful and/or lost too much time which, especially older infertility patients, could not afford. With the ascent of GLP-1 agonists (and soon also antagonists), these considerations have changed because these drugs allow for relatively quick weight loss over three months, which is sufficient to make a difference on treatment outcomes. Studies have demonstrated that a weight loss of as little as 15 pounds and/or 5% of total body weight is sufficient to see improved treatment outcomes. Considering obesity, therefore, now as a new infertility diagnosis, we have dedicated this section of the VOICE to this subject.
Possibly, and maybe even probably, as a recent article in prestigious Nature Medicine pointed out.1 Overall, the writer concluded that evidence is accumulating to suggest that dietary interventions can be effective in treating and/or delaying selected disease. A substantial section of the article was dedicated to women’s health, noting that dietary interventions in women may be especially effective in endocrine (hormonal) conditions such as polycystic ovary syndrome (PCOS) and in endometriosis.
We agree with these conclusions, even though how these two conditions may benefit from dietary interventions in our opinion may differ somewhat. Both of these diagnoses are associated with an increased prevalence of inflammation and both, therefore, in such cases my benefit from an anti-inflammatory diet. But selected PCOS patients (we here are referring to hyperandrogenic PCOS patients) may also reduce their androgen (male hormone) levels if they lose weight. A Mediterranean diet is both anti-inflammatory and weight loss-producing. In PCOS, diet and exercise are considered, therefore, good treatment especially in obese PCOS patients.
How truly revolutionary this concept of food as medicine has become is also well demonstrated by how much visibility nutrition received in the medical literature lately. The New England Journal of Medicine, for example, just announced a new review article series in nutrition in medicine.2
And, finally for this section, that obesity in women can lead to hypogonadism is well established; but Eng et al in Endocrine Rev just published probably the best review on the subject in a long time, which we strongly recommend.3
1. Venkatesan P. Nat Med 2024;30:916-919
2. Lee et al., N.Engl J Med 2024;390(14):1324-1325
3. Eng et al., Endocrine Rev 2024;45:171-189
By far the most interesting news regarding the new GLP-1 agonists now widely used worldwide is publication of a first large study using these medications in early pregnancy and the news were excellent.1 In a short report in JAMA, children of 50,000 pregnant women (in 6 countries) who were exposed to GLP-1 agonists for diabetes treatments during early pregnancy were followed up, and they demonstrated no increase in congenital abnormalities in newborns.
This is, indeed, excellent news but not yet enough to continue treatment with these drugs into pregnancy because mouse studies did suggest possible effects of these drugs on offspring. And there are many highly significant differences between mice and men and, based on what we know so-far about this family of drugs, we would be very surprised if there were increased birth defects from these medications; but there may be other adverse effects on babies and the CHR, therefore, remains cautious about use of these drugs in pregnancy.
For the foreseeable period, the CHR, therefore, will continue mandating a one-month washout period for these drugs before attempts at preg nancy can be made.
For those interested in reading more detail about these weight loss drugs, the BMJ just published an excellent up-to-date review with a little peek into the near future, when even more effective weight loss drugs are expected to come to market.2 The authors conclude that the future for pharmacotherapy for obesity is bright.
One still largely unresolved issue regarding these drugs is whether, and if so how, these medications can be stopped once a certain desired weight has been achieved. So-far data suggested that abrupt discontinuation will, within six months, result in significant gain of much of the lost weight. If confirmed, this would mean that obesity must be viewed as a lifelong disease, requiring lifelong treatment.






Now, however, two poster presentations at the 2024 European Congress on Obesity in Venice, Italy, suggested that slow tapering of semaglutide in combination with lifestyle changes (food, exercise) allowed patients to maintain the weight loss.3 If confirmed, that would be, of course, great news!
How truly revolutionary these new weight loss drugs are for medicine in general is also well demonstrated by how much visibility nutrition received in the medical literature lately. The New England Journal of Medicine, for example, just announced a new review article series in nutrition in medicine.
1. Harris E. JAMA 2024;331(4): 280
2. Henderson Lewis, et al., BMJ 2024;384:e072686
3. Medscape. https://www.medscape.com/viewarticle/weight-loss-maintained-slow-taper-semaglutide-2024a100095i?form=fpf




Support our mission to showcase the latest research connecting obesity as an infertility diagnosis.
ADVERTISEMENT



All listed restaurants are in Manhattan unless otherwise noted. Like all opinions about restaurants, ours are subjective and are to be understood as such. If you visit one of them, let us know whether you agree with our ratings. We value your feedback.
SYMBOLS WE USE IN OUR RATINGS
$ Inexpensive
$$ Moderately expensive
$$$ Expensive
$$$$. Special event expensive
- Not worth the trip + Overall favorite of the CHR
• Good v Special vibe
•• Very good M Michelin starred
••• Excellent V Vegetarian/vegan dishes on
•••• Uniquely delicious the menu
AUSTRIAN
Koloman +/•••/$$/v 16 W 29th Street (212) 790-8970 V; Excellent desserts
Wallsé +/•••/$$/v/* 344 W 11th (212) 352-2300 V; Excellent desserts
CHINESE
Hwa Yuan Szechuan +/•••/$$/v 42 East Broadway (212) 966-6002 V; Authentic Szechuan
Mr. Chow Downtown +/••/$$$/v 121 Hudson St., Tribeca (212) 965 9500 V Uptown 324E, 57th Street (212) 751 9030 V
CONTINENTAL
425 •••/$$$/v 425 Park Avenue (212) 751-6921 V; Gorgeous restaurant FRENCH
Le Gratin ••/$$ 5 Beekman St. (212) 597-9020 V; Try the gratin potatoes
Le Charlot +/•• /SS 19 E 69th St. (212) 794-6419 V; Best steak au poivre & Thai mussels; great desserts
Le Bernadine +/••••/$$$$/ MMM 155 W 51st (212) 554-1119 V; Mostly seafood. Likely the best NYC restaurant
ITALIAN
Cipriani Downtown +/••/$$/v 376 West Broadway (212) 343 0999 Great food, beautiful people & Uptown 781 5th Avenue (212) 753-5566 Great food and high society Elio’s +/•••/$$/v 1621 2nd Ave. (212) 772-2242 Best Italian home cooking and where everybody meets
Principe ••/$$/v 50 West Broadway (212) 335-0509 V; mostly seafood but one of the best chicken dishes
Sistina •••/$$$ 24 E 81st St. (212) 861-7660 V; Amazing wine list FRENCH-ITALIAN
Café Carmellini •••/$$$$/v 250 Fifth Avenue (212) 231 9200 V; Gorgeous place, top service; sophisticated and adventurous food
JAPANESE SUSHI
Sushi Ann +/••• /$$$$ 8 E 51st St. (212) 755-1780 V; Best quality fish; make reservation at the bar
GENERAL
Sakagura +/•••/$$
211 E 43rd St. (212) 953-7253 V; Amazing food; Best sake selection
Yakitori Torishin +/+++/$$$/M 362 West 53rd Street (212) 757-0108 Unique and amazing skewer restaurant
KOREAN
Jungsik •••/$$$$/ MM
Oiji Mi +/•••/$$/v / M
2 Harrison St. (212) 219-0900 Where NYC’s Korean restaurant revolution was born …
17 W. 19th St. (212) 256-1259 … and has continued GREEK
Elias Corner (Queens) ••/$ 24-02 31st St. (718) 932-1510 Mostly seafood HAMBURGERS
Jackson Hole Burgers (the “original”) +/••/$ 232 E 64th St. (212) 371-7187 Not a place for vegetarians
MIDDLE EASTERN-ISRAELI
Dagon ••/$$ 2454 Broadway (212) 873 2466 Best hummus in NYC
NEW YORK JEWISH DELI
P. J. Bernstein’s Deli •/$ 1215 3rd Avenue (212) 879-0914 All the great classics; pastrami, chicken soup
PERUVIAN
Mission Ceviche •••/$$ 1400 2nd Avenue (212) 650-0014 If you like Peruvian –the best
PIZZA
San Matteo Pizzeria e Cuccina ••/$ 1559 2nd Avenue (212) 861-2434 True Napoli POLISH
Karczma (Brooklyn) +/•/$ 136 Greenpoint Avenue (718) 349-1744 Great authentic Polish food – and dirt-cheap
ROMANIAN-JEWISH
Sammy’s Roumanian Steak House** $$ 112 Stanton St. (212) 673 0330 Authentic JewishRomanian Steak House, with entertainment
UKRAINIAN/RUSSIAN
Caviar Russe +/••••/$$$$/ M 538 Madison Avenue (212) 980-5908 V; Most underrated restaurant in NYC
Russian Samovar •/$/v 256 West 56th St. (212) 757 0168 Great Russian/ Ukrainian food and music
Any suggestions and/or comments, please write to social@thechr.com.



112 Stanton Street, New York, NY 10002
Phone: (646) 410-2427


It took a while, but Sammy’s is finally open again in a new space on Stanton Street. Those who do not recognize the name must be newcomers to New York City because everybody used to know this place until it closed during COVID in 2021. Before that, Sammy’s was for over four decades a New York institution on the Lower East Side om Chrystie Street founded by Stan Zimmerman and later taken over by his son, David Zimmerman. It was located steps down in a basement space with low ceilings and walls plastered with photographs of family, celebrities, regular clients, and events (see photos above).
When the CHR several years ago was, still, running monthly lecture events for the general Ob/Gyn community in rotating restaurants, Sammy’s was not only a steady location, but registrants almost every year voted it their favorite place (and against serious competition).


Thank God, the food has not changed, and is still an unavoidable cholesterol poisoning, especially if you don’t wash it down with the vodka, still served in full bottles parked in a big brick of ice. Still also the best chopped liver with lots of browned onions, especially if slightly “diluted” with the schmaltz, still served liquid in dispensers at the table (in the background of above photo next to the antique Selzer bottle which also is still being offered); very good gefilte fish and, of course, broiled chicken livers with “unborn” eggs, and Romanian karnatzlacks, (delicious ground meat patties for those with lots of tolerance for garlic) among several other appetizers.
It is difficult not to be full after appetizers; but be warned, it is worthwhile leaving some space for the entrees and desserts. The place is, of course a steak house and that means guests are given a choice of several different steaks; but look at the sizes (picture above). In addition, they serve excellent lamb chops and breaded veal chops, of course similarly oversized. Food is served family style and even the portions of salmon (and other fishes) are, huge. Consider this when ordering but almost nobody has ever left Sammy’s without a doggy bag.
And one cannot review Sammy’s without a big shout out to Dani Luv (his real name is Daniel Lubnitzki, see photo right) who like at the previous location, every night sits behind his piano at the very end of the space, just that in the old space it was the right, and in the new space it is the left corner (see picture) and entertains the room. In a full-page article in The New York Times before the reopening of Sammy’s, he was described as the “resident lounge singer and pianist, where entertainment is as schmaltzy as the chopped liver, delivering smutty insults to diners.”
Dani, of course, has also a longstanding relationship with the CHR. Moreover, over 20 years ago, the CHR’s Dr. Gleicher flew Dani to Chicago to perform at his older daughter’s Bat Mitzvah. Unsurprisingly, the CHR has added Sammy’s to its most favorite restaurant list (see above). It’s a party every night!


We are writing this letter of gratitude to Dr. Norbert Gleicher and the entire staff at the Center for Human Reproduction. My wife and I are so very thankful that we listened to Dr Gleicher's advice back on October 1st, 2021 during a consultation. He advised us to not allow our fertilized eggs to be discarded and killed. Our two eggs were tested and declared to be irregular by another IVF lab. We kept the eggs frozen and, after 4 more failed IVF cycles, came to Dr. Gleicher for another try. We asked Dr. Gleicher if it was worth a try to have eggs transferred into my wife and he studied the data and approved of the procedure.
On February 7th, 2024 we had 2 frozen eggs transferred and waited for the results. Finally, in February after the 13 day wait, we were informed of the blood test that confirmed that we were pregnant with an XX embryo from the transfer. XX is a girl and a huge feeling of warmth and happiness overwhelmed both of us. We had several ultrasounds during the 4-5 weeks following and all were very positive with strong heartbeat and great growth. Today my wife is approaching her 18th week pregnant and all is going great with the development and follow up visits. Our baby is growing by the day and we look forward to our 20 week ultrasound for further development rate.
If we had not discussed and listened to Dr Gleicher back in 2021 we would never have been so far with a pregnancy. The entire staff is wonderful and nurse Hui Na was very helpful and communicative through the entire process with my wife. Please accept our sincere thank you and heartfelt praise for the team. Our baby is due in Oct 2024 and we intend to make the trip from our home to New York to say thanks in person. Blessings to all!


BRIEFING: The VOICE in this section of the newsletter offer commentaries on a broad survey of recent articles in the English literature, which the CHR found of interest, even if, at times, not immediately applicable to daily clinical practice. Articles are mostly chosen for their translational value to clinical medicine, often helping in determining where clinical practice will likely go.
Translational research is, since its founding in 1981, one of the CHR’s principal goals and has over the years produced some milestone discoveries and a good number of U.S. patents. Such research has also propelled the CHR into its current status as a worldwide center of last resort for infertile patients who have failed treatments elsewhere. This section of the VOICE, demonstrates and makes public the process through which the CHR for decades has been following and interpreting the published literature, a process always at the very core of how research and clinical practice have evolved at the CHR.
Primarily directed at physicians and basic scientists, this section of the VOICE to our surprise has also attracted many of the CHR’s patients, from which we assume that it also has found a readership among the public. The VOICE, therefore, strives through its writing style, to make this section also accessible and understandable for a general audience.
The hot new topic in infertility: stem cells Have you noticed, stem cells, suddenly, appear to be everywhere in infertility treatments. A PubMed search regarding stem cells in infertility currently already yields over 3000 papers. The craze started a little over a decade ago with the concept of regenerating endometrium from stem/progenitor cells in Asherman’s syndrome.1 By 2018 Spanish colleagues proposed use of autologous ovarian stem cell transplants2 and then a year later expanded the theme to treating Asherman’s syndrome as well as what they called premature ovarian failure allegedly with bone marrow-derived stem cells.3 As mesenchymal stem cells are much more accessible, they, of course, also entered the fray,4 shortly followed by induced pluripotent stem cells (iPSCs).5 Male infertility, of course, also got into the game, using spermatogonial stem cells.6
The reason we are raising this subject here is publication of a review article which offers a rather comprehensive assessment of work that has been so-far done on clinical neo-oogenesis with the use of ovarian stem cells.7 Even though it by now appears well established that all organs in the body have their own stem cells, the definition of ovarian stem cells is, at best, incomplete. Skeptics, indeed, still question their existence. Mostly based on interesting work done in mouse models in Japan8 what, however, can no longer be disputed is that ovarian stem cell-like cell lineages can be used to generate gametes.
There is of course a substantial difference in the complexities involved in producing gametes in mouse models and humans. But it seems we are quickly getting closer, and since we will be welcoming Katsuhiko Hayashi, PhD, one of the leading Japanese magicians in
this field of exploration, between December 6 and 8 at the annual FRM Conference here in NYC (which the CHR co-sponsors), we expect a detailed update.
1. Deane et al., Curr Opin Obstet Gynecol 2013;25(3):193-200
2. Harraiz et al., Fertil Steril 2018;110(3):496-5-5e1
3. Herraiz et al., Curr Opin Obstet Gynecol 2019;31(3):156-162
4. Estandyari et al., Cells 2020;9(10):2253
5. Saha et al., Cells 2021;10(7):1613
6. Di Persio S, Neuhaus N. Hum Reprod 2023;38(1):1-13
7. Grettka et al., Biomedicines 2024;12(6);1139
8. Hayashi et al., Nature 2016;539)7628):299-303
Evidence-based guideline for premature ovarian insufficiency 2024: a draft by ESHRE and ASRM circulating before formal publication among stakeholders9
The CHR’s Norbert Gleicher, MD, commented in his monthly “A Piece of My Mind” commentary in this issue of the VOICE already on the new guideline document on the management of premature ovarian insufficiency (POI) by ESHRE and ASRM at time of this writing circulating among stakeholders for comments and we have to agree with him - it is a monster.9 One really must wonder what those responsible for this 227-pages-long document, claiming to answer 142 POI-specific questions really set out to achieve? How many stakeholders do they believe will really sit down and read though a 227 pages-long document to adjust their practice pattern, - especially since a majority of the answers to the 142 questions the document pretends to answer note how flimsy the available data to answer the questions are, therefore requiring further studies.
But especially these days when, because of significant hiccups in the launch of Google’s (and others’) Large Language Model (LLM)
A.I. programs everybody has been sensitized to the importance of unbiased, fairly, and evenly selected source materials, one also must wonder how the medical establishment still can maintain this almost religious believe in the validity and value of allegedly evidence-based guidelines? Don’t they represent exactly the same data selection process (just in smaller volume) as LLMs?
They, of course, do and they, therefore, of course, also are always representative of the “common momentary wisdom” of the crowd, with the crowd (in this case represented by publications) selected by a usually biased “expert” whose selection process, of course, is driven by her/his own biases. The question, therefore, must be asked whether the huge efforts and the substantial expenses required to generate this monster of a guideline could not have been applied for better purposes?
REFERENCE
1. ESHRE and ASRM. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency.aspx
Some original thinking about (repeated) implantation failure
Ben Willem Mol, MD, has for several decades been one of the most original thinkers in reproductive medicine, at times, indeed, maybe even too original for the medical establishment. This was - as rumored at the time – a reason why he left the Netherlands, where he grew up and had experienced a stellar academic career at quite young age before moving down under to Australia. Many years later, Australia still appears to be where he keeps his central office at Monash University, but he also holds an academic position in Scotland at the Aberdeen Centre for Women’s Health Research at the University of Aberdeen, and in a way in the U.S. as well, because he is now a very active Associate Editor of Fertility and Sterility

This introduction is offered because he is the senior author of a paper that asked the provocative question whether (repeated) implantation failure (IF) has a treatable cause (i.e., really exists as a clinical diagnosis) or occurs simply by chance?1 This is, of course, a question the CHR has pondered for a long time, as also reflected repeatedly in these pages. Our skepticism regarding this diagnosis has had several reasons but has, not the least, been based of rather ridiculous criteria under which most papers in the literature have defined IF (2-3 embryo transfers), and, usually, without any consideration of patient age.
As readers of this newsletter likely by now are aware of, the CHR is usually not a big fan of modelling studies. There are, however, circumstances where modelling studies are pretty much the only way to get at least the hint of an answer and IF is such a circumstance. And ignoring the usual unavoidable weaknesses of modelling studies, this was a well-thought out and executed study of 1000 women undergoing 4 repeated IVF cycle.
And the answer was, as we at the CHR have argued for decades, that IF likely is a totally iatrogenic-produced clinical entity as a consequence of inherently low IVF success rates (more likely failure than success in an overwhelming majority of cycles) and, of course, heavily dependent on patient-age. One more important contribution, Ben (and associates). Congratulations!
And if we are already talking about IF, how about a paper that claims to have investigated the effectiveness of treating the non-existing diagnosis of IF with granulocyte colony stimulating factor (G-CSF) in a systematic review?2 As a systematic review, one, of course, cannot be surprised that the study originated in China, clearly the world’s headquarter for such reviews and, as noted above in our comments regarding the newly circulated ESHRE/ASRM guideline for POI, very often a classic example for the old IBM dictum, “garbage in garbage out” and, therefore, clearly an perfect candidate for this issue’s “worst paper designation.” No surprise then that the authors concluded after their literature review that “G-CSF has (‘to some extent’) a beneficial effect on pregnancy outcomes in women who experience at least one implantation failure” Ridiculous!
REFERENCES
1. Cutting et al., Reprod Biomed Online2024;48(6):103845
2. Su et al., Front Endocrinol 2024;15:1370114 (“WORST PAPER IN ISSUE AWARD” RECIPIENT)
ASRM does not stop giving: This time it is the Practice Committee of the ASRM that gives us its wisdom after appropriate (of course, meant facetiously) literature reviews about smoking in pregnancy, whether tobacco or weed.1 Fortunately this opinion update is only 11 pages long (references included). It does not offer much news beyond smoking tobacco is on several levels bad for you but – politically correct as always – “marijuana use has not been consistently associated with male or female fecundity, time to pregnancy, reproductive hormone levels, semen parameters, or ART outcomes.” And how about all the data published in recent months on the effects of marijuana of the fetus and especially on the fetal brain, among other consequences, leading to increases in autism and ADHD in offspring?2
We live in a truly upside-down world: On the one hand the world is spending fortunes in attempts to reduce tobacco-use; yet on the other hand, the world has not only been liberalizing Marijuana use, but actually promoting it by suppressing “bad news” and by considering arguments against this liberalization as politically incorrect. Its use since 2008 has skyrocketed with the per capita rate of reporting last-year increasing by 120%. Daily or nearly daily use increased by 218%, with daily cannabis use for the first time exceeding daily alcohol use.3 This, of course, also means increasing use in pregnancy, often daily and it also means a dramatic increase in psychiatric disorders, permanent brain damage especially in teenagers, and other serious harm.4 How come ASRM and ACOG are so quiet on the subject?
1. Practice Committee of the ASRM. Fertil Steril 2024;121(4):589-602
2. European Psychiatric Association. News release, April 8, 2024; https://www.europsy.net/app/uploads/2024/04/EPA-2024.-Cannabis-use-during-pregnancy_Press-Release_13MAR24_FINAL.pdf
3. Caukkins JP. Addiction 2024. https://doi.org/10.1111/ad.16519
4. Finley A. The Wall Street Journal 2024;May 11-12, 2024, pA11
Application of machine learning to embryo selection in IVF
We above mentioned the use of stem cells in infertility treatments as a new “fashion of the moment.” It, however, is even substantially exceeded by the number of reports claiming IVF outcome benefits from deep-learning-based A.I. programs. Already in the preceding issue of the VOICE discussed in some detail, we here want to add two more recent publications, further strengthening our skepticism about the ability of A.I to significantly improve current IVF cycle outcomes.
Both papers were generated in China, with the first suggesting that machine learning – guided non-invasive chromosome screening of embryos was able to select embryos for ploidy, thereby improving IVF cycle outcomes.1 Unfortunately, the paper for several reasons lacks all credibility and here is why, pointing out only the most blatant criticisms of this study (there are many more):
Ignorance of basic biology: It is by now well established that over 80% of human embryos at blastocyst stage contain aneuploid cells. Somewhere around 15-20% of these embryos with aneuploid cells have meiotic aneuploidy generated during the first cell division and, therefore, involving all cells of an embryo. The remaining 80-85% of embryos have mitotic aneuploidies which, therefore, are clonal and are present in embryos as small islands of aneuploid cells. It is only this second group of aneuploid embryos which has the ability to self-correct in the embryonic cell lineage forming the embryo. The extra-embryonic cell lineage, forming the trophectoderm and later the placenta does not have the same ability to self-correct and, therefore, maintains significant aneuploidy until birth in placentas even in normal, euploid pregnancies. Considering these facts alone, an A.I. program recognizing “aneuploidy” alone – without the ability
to differentiate between meiotic and mitotic aneuploidy – cannot be enough, because a large majority of so selected embryos will be mitotic and, therefore, subject to self-correction downstream from timing of the embryo biopsy.
Materials and methods: The authors used an embryo screening method first reported by Chinese investigators in 2022 in Reproductive Biomedicine Online. 2 This study, however, trained their machine learning algorithm based on non-invasive (ni) PGT-A (chromosome testing) results of spent media rather than trophectoderm biopsy results which - practically universally – have been demonstrated to be inferior in accuracy to trophectoderm biopsy. And since even trophectoderm biopsy by now are proven not to improve IVF outcomes, the hypothesis that an even inferior diagnostic method would result in improvements in IVF cycle outcomes is, simply, non-sensical.
Results: Without again going into all the criticisms about study design and result reporting, just one example why the reported results simply make no statistical sense: The authors report differences between study and control group pregnancy rates as highly significant (p<0.001) at 70.0% vs. 54.0% and 58.9% vs. 44.7%) but as not statistically significant at 61% vs 46,9%, 57.9% vs. 34.8%, and 33.0% vs 0%.
The second paper by a different group of Chinese investigators in the same medical journal in principle attempted to follow the same hypothesis that deep learning-based embryo scoring for non-invasive aneuploidy prediction would ultimately offer outcome advantages in IVF cycles.3 They, to their credit, were, however, significantly more cautious in their conclusions.
These authors trained their neural network model on embryos that had undergone invasive PGT-A through trophectoderm biopsy, establishing an embryo score trained on time lapse videos to predict fetal heartbeat and demonstrated that higher scores statistically correlated with embryo aneuploidy. The area under the curve (0.612) was, however, not very impressive and, even with addition of clinical patient and embryo characteristics improved only to 0.688.
Though they, too, concluded that their embryo score may in the future offer an inexpensive and non-invasive alternative for selected patients undergoing IVF, they correctly noted that the accuracy of ploidy determination in human embryos during IVF would, of course, still depend on the accuracy of the PGT-A hypothesis (i.e., the biological criticism we outlined above in discussing the prior paper).
In summary, we reemphasize that in the CHR’s opinion non-invasive aneuploidy testing of human embryos will not be successful, unless the technology learns to differentiate between meiotic and mitotic aneuploidies detected in embryos.
Once that is possible, one, indeed, may expect outcome improvements from excluding exclusively only embryos with meiotic aneuploidies from transfers. But, as we have repeatedly noted in discussions of the utility of PGT-A in general in these pages, even then an expected improvement in cumulative pregnancy and live birth chances for a cycle’s embryo cohort will not be achieved because it is predetermined upwards by number of transferrable embryos. The only outcome advantage that can be achieved using embryos selection (by whatever means) is time to pregnancy and, even that, is only achievable in good-prognosis patients with large numbers of transferrable embryos.
Whether all the investment dedicated to deep learning-based embryo scoring models is, therefore, worthwhile the effort, is at least very questionable.
1. Li et al., Reprod Biol Endocrinol 2024;22:61
2. Chen et al., Reprod Bimed Online 2022;45(1):26-34
3. Ma et al., Reprod Biol Endocrinol 2024;22:58
A recent paper in JAMA Network Open attracted our attention because it investigated whether a diagnosis of endometriosis adversely affects IVF cycle outcomes in otherwise similar infertility patients. This has remained a controversial issue in the IVF field. Donor oocyte cycles, of course, mostly remove the ovarian factor from consideration and, therefore, allow concentration on whether endometriosis affects implantation and, possibly, normal pregnancy progression. The study relied on two sources, - an amalgam of 7,212 published oocyte donation cycles and 162,082 cycles from two registries (SART in the USA and HFEA in the U.K.). Both analyses detected a very modest reduction in live birth rates in women with endometriosis, leading the authors to conclude that endometriosis may have a minor negative effect on implantation.
According to our interpretation the authors, however, missed some
important points in their own study: Implantation and live birth rates, overall, to a minor degree, indeed favored control patients. This difference, however, varied depending on the source of donor eggs and whether cycles occurred in the U.S. (SART data) or the U.K. (HEFA data): For example, in every single category analyzed, U.S. implantation and live birth rates were significantly higher than U.K. rates (see table on the next page). Moreover, if donor oocytes were fresh, implantation and live birth rates in the U.S. registry showed practically no difference whatsoever between controls and endometriosis and live birth rates improved over implantation rates. In contrast, the much lower U.K. implantation rates were very similar between controls and endometriosis patients, live birth rates, however, improved over implantation rates only in controls.
Endometriosis patients, interestingly, did not experience this improvement which is not only explainable by an implantation problem in the U.K., but also could represent a loss of pregnancies between implantation and live birth, - i.e., an increased number of miscarriages. As endometriosis is statistically highly associated with autoimmune-induced pregnancy loss,2 this observation raises the possibility that IVF practice in the U.K. may be less inclined to prophylactically treat immune-miscarriage risk.
As this paper well demonstrates, merging study populations from different parts of the world can be dangerous and misleading and can obfuscate outcomes in subgroups of combined populations by including opposing data sets.
This study, indeed, offers further interesting insights: It, for example, confirms that frozen oocytes produce significantly lower implantation and live birth rates than fresh oocytes. Though by now repeatedly reported, this has still remained a somewhat controversial issues,3 especially as frozen donor eggs are now used in over 75% of U.S. donor egg cycles.4 In frozen donor egg cycles U.S. and U.K patients moreover varied in outcomes between endometriosis and control patients in that endometriosis patients uniformly did worse than controls in implantation as well as live birth rates.
Registry and type of oocytes
SART
Fresh oocytes
Frozen oocytes
Thawed embryos
Total HFEA
Fresh oocytes
Frozen oocytes
Thawed embryos
Total
This observation clinically creates for endometriosis patients disproportionate incentives to use fresh rather than frozen donor eggs. On a more basic level, the observation raises the question why this is happening with frozen donor eggs?
A likely answer contradicts the original conclusion of the paper that endometriosis affects IVF only marginally and, likely, primarily through the implantation process. It, indeed, suggests that donor oocytes after fertilization impose on embryos in the environment of endometriosis patients diminished tolerance for cryopreservation and thawing. If confirmed, then donor oocytes in endometriosis patients, whenever possible, should only be used fresh.
Finally, a word about the use of frozen-thawed embryos, which in recent years, due to increasing utilization of all-freeze cycles and embryo banking, also has become a controversial issue in IVF practice.5 The study here again demonstrates clearly lower implantation and live birth rates in the U.K. than the U.S., but both rates remain similar between control and endometriosis patients and neither U.S. nor U.K. patients demonstrate a bump in live birth rates in comparison to implantation rates. Finally, Table 2 of the paper in implantation rates of control patients contains a very obvious typo (the rate cannot be 3.1% but likely is mean to be 30.1%).
Which leaves us with the final question this paper raises, - why are there still such highly significant outcome differences in IVF outcomes in practical all cycle categories between the U.S. and the U.K.? IVF cycle outcomes between the U.S. and Europe have differed for decades,6 and have generally been attributed to differences in health care systems and IVF practice patterns. As insurance coverage for IVF in the U.S. has improved and as practice patterns have appeared to become increasingly similar, one would have expected a narrowing of the par between the two continents. Comparing U.S. to U.K data from this paper, this, however, does not appear the case in the European country, perceived, most closely related in its practice to the U.S. The study discussed here, therefore, demonstrates that this old question is still waiting for an answer.
And since we already are talking about the effects of endometriosis on IVF outcomes, another paper from Chinese investigators recently suggested that IVF outcomes in endometriosis patients improved after short-term treatment of patients with rapamycin.7 If that were, indeed, to be the case, this, of course, would be a significant addition to our treatment armamentarium in infertile women with endometriosis.
As the authors note in the introduction, rapamycin is an FDA –approved prescription drug easily available in the marketplace and defined as a potent mechanistic target of rapamycin (mTOR) inhibitor which has immunosuppressive, anti-cancer, and anti-angiogenic properties. Characteristically the mTOR pathway is activated when tissue and/or cell senescence takes place. The drug in recent times has also found a strong following in anti-aging medicine, where it is considered to be age-defying, to increase the lifespan in mice,
and to lack significant side effects even in older people. It has also been demonstrated to inhibit growth of endometrial lesions in mice, though how that happens is still unresolved. We in last month’s issue of the VOICE noted that several studies of the drug are under way in treating ovarian aging, one, indeed, here in NYC at Columbia University
1. Paffoni, et al., JAMA Netw Open 2024;7(1):e2354249
2. Gleicher et al., Am J Obstet Gynecol 1989;160(6):1376-1380
3. Kushnir et al., JAMA 2015;314(6):623-624
4. SART. https://sartcorsonline.com/Csr/Public?ClinicPKID=0&reportingYear=2022&newReport=True
5. Ben RZ. Should we still offer elective freezing of all embryos in all IVF cycles? Hum Reprod. 2020;35(10):2179–81.
6. Gleicher et al., Fertil Steril 20007;87(6) 1301-1305
7. Fan et al., Reprod Biomed Online 2024;48(1):103319
How primary Sjögren syndrome (PSS) affects female fertility and pregnancy outcomes is not well understood. A multi-center retrospective cohort study by Chinese investigators, therefore, appears timely. PSS is a systemic autoimmune disease which mostly occurs in isolation from other autoimmune conditions. It affects primarily salivary and lacrimal glands and often results in sicca symptoms and tissue damage in various organs, including pulmonary interstitial fibrosis and cirrhosis. It also is associated with an increased lymphoma risk. In the laboratory, it is associated with the presence of anti-SS antibodies (SS-A and SS-B), with presence of SS-A antibodies being associated with a ca. 1% risk of congenital heart block in offspring. This antibody can also be found in women with systemic lupus erythematosus (SLE).
The rarity of this disease in pregnancy is well demonstrated by the fact that, despite this being a multicenter study at three university-affiliated hospitals in China, which is well-respected for large-scale multicenter medical studies, here only 47 cases are reported. Results, therefore, have to be viewed with caution but are, nevertheless, of interest. They were based on propensity score matching with a control population.
PSS patients demonstrated significantly lower AMH (p<0.001), as were large follicles, total oocytes and MII oocyte numbers, good quality day-3 embryos, and blastocyst numbers. Unsurprisingly considering all of these findings, implantation rates were also reduced (p<0.022), cumulative live births were decreased, and the premature delivery rate was increased as in practically all autoimmune diseases.2 In summary, PSS behaves like most typical systemic autoimmune diseases behave in many regards when it comes to fertility and pregnancy outcomes.
Also timely in view of the recently enhanced interest in the study of obesity as a disease is a paper by U.S. investigators of morbidly obese women undergoing IVF and their subsequent pregnancies.3
Investigating 48,365 ART pregnancies established between 2012 and 2015, 45,125 (93.3%) were non-obese, 2445 (5.1%) were class I and class II obese, and 795 (1,6%) were class III obese.
OBESITY CLASSIFICATION
Class I
BMI
30.0 - 34.9
Class II 36.0 - 39.9 Class III
Class III patients were more likely to have a hypertensive disease, diabetes mellitus, large for gestational age neonates, and intrauterine fetal demise. Hypertension and diabetes even significantly differed in comparison to class I and II obese patients. After adjustments, class III patients were 70% more likely to experience severe maternal morbidity at delivery, and observation not made with class I and II patients.
REFERENCES
1. Mao et al., Reprod Biol Endocrinol 2024;22:57
2. Gleicher N., Clin Rev Allergy Immunol 2010;39(3):194-206
3. Song et al., J Assist Reprod Genet 2024;41:903-914
Does birth after ART increase cancer risk?
This French study of 260,236 born after intrauterine inseminations or embryo transfer in IVF (fresh and frozen).1 Reassuringly, the overall risk of cancer was not increased. The authors, however, determined – based on 20 cases only – that the risk of acute lymphoblastic leukemia was marginally higher than in controls after frozen embryo transfer (HR 1.61; 95 % CI, 1.04 to 2.50 per million person-years). Somewhat bizarrely, neonates born only between 2010 and 2015, moreover, demonstrate an increased leukemia risk after fresh embryo transfer (HR 1.42; 95% CI, 1.06 to 1.92), adjusted RD, 19.7; 95% CI, 2.8 to 43.2 per million person years.
These data make little logical sense and, likely, artefacts commonly seen in such large and poorly controlled population studies but the authors, in our opinion correctly, concluded that this matter should be prospectively watched.
REFERENCE
1. Rios et al., JAMA Network Open 2024;7(5):e249429
Rescue in vitro maturation has recently, as discussed in these pages, repeatedly been in the literature, suggesting increasing utilization. A paper by Spanish investigators attempting to determine whether such rescue maturations may affect the first meiotic division, and thereby meiotic aneuploidy, seems timely.1 The study reassuringly demonstrated no adverse effects.
Chinese investigators tackled another still quite controversial intervention into IVF, patient supplementation with growth hormone
(GH).2 Once again a subject repeatedly addressed in these pages, GH can be an important contribution to an IVF cycle but, of course, only if there is a purpose to such supplementation. And whether such a purpose exists can be easily determined by measuring a woman’s insulin growth factor-1 (IGF-1) levels) because GH works on the ovary through IGF-1; If IGF-1 is low, GH supplementation will improve IVF cycles. IF IGF-1 levels are in range, why would one even assume that GH supplementation may help?
Much of the confusion surrounding the use of GH in association with IVF stems from the fact that IVF clinics either use GH in everybody or nobody and do not rely on IGF-1 levels; indeed, those in most IVF clinics are not even tested. Like a headache pill will successfully treat a headache only if the patient really has a headache, so will GH not work unless IGF-1 is low. That, however, means that IGF-1 must be assessed before a decision is made whether to supplement with GH or not.
And this, unfortunately was not done in this Chinese study. Instead of determining IGF-1 levels to decide who should receive GH, they administered the hormone to everybody in a group of 3,043 women who in this retrospective study in the previous cycle had demonstrated “poor embryonic development.” These patients were then propensity score - matched to women without GH supplementation, - quite obviously not an adequate study design. The author reported superior pregnancy rates with GH supplementation, though obstetrical outcomes of offspring did not differ. In short, anything but a credible study!
And, finally, one more systematic review from Chinese colleagues, this time of platelet rich plasma (PRP) injections into ovaries. Once again, this is, of course, not only a familiar topic at CHR since four separate clinical trials are underway involving this treatment, but also because several recent reviews on the subject have been discussed here. So, here is one more:3 The authors conclusions, nothing new: PRP allegedly has the potential to improve IVF outcomes in women with poor prior ovarian response, increases success rates of IVF in such patients, and may do so by improving embryo quality without creating significant risks.
Since this study was performed based on only 7 prospective and 3 retrospective cohort studies, this appears to be yet another study not wort even the paper it was printed on. What a waste and what a shame for BMC Women’s Health, the journal where this article appeared.
REFERENCES
1. Esbert et al., Reprod Biomed Online 2024;48(1):103379
2. Liu et al., Reprod Biol Endocrinol 2024;22(22):53
3. Li et al., BMC Women 2024;24:263
It is impossible to review the reproductive medicine literature without coming across a topic with more published articles than PCOS. Consequently, it always comes down to being selective and in choosing what the CHR considers the most impactful subjects and papers. A recently published systematic review by mostly Australian investigators in the JCEM this month, therefore, attracted our attention because it addressed a topic receiving increasing attention in the field and did it well. The topic of the paper was the use of the supplement inositol – either in the form of myo-inositol or as D-chiro-inositol – in women with PCOS.1
Inositol products for PCOS patients are now the among the most advertised and most widely offered product offerings of fertility-related supplement companies and it is not that the VOICE has not covered them before in these pages. Our prior coverage was, however, mostly restricted to one issue of considerable importance, - the question which PCOS patients might benefit from supplementation with inositol. In other words, the CHR warned about the widely made claim by supplement companies that inositol was beneficial in all PCOS patients. More specifically, the CHR warned about use of inositols in PCOS women with the D-phenotype (under Rotterdam criteria), - also widely called the lean phenotype.
The reason was simple: This phenotype of PCOS, in contrast to all other phenotypes (A, B, and C) does not remain hyper-androgenic into advanced age but – roughly around age 25 -starts demonstrating declining androgen (male hormone) levels. While for approximately a decade – between age 25 and 35 – in what is considered normal range, androgens after age 35 fall into a range below normal.
The CHR for this stage, therefore, coined the term hyper/hypo-androgenic PCOS (HH-PCOS). Among other functions, once of the main reasons why inositol has been claimed to be effective in treating PCOS has been its ability to lower androgens in hyper-androgenic PCOS patients. But since phenotype D patients after age 25 only very rarely still are hyper-androgenic, they no longer require this benefit. To the contrary, as they are on the way to becoming hypo-androgenic by roughly age 35, treating them with inositol before that will, indeed, cause even earlier hypo-androgenism and, once they are hypo-androgenic, increase the severity of their hypo-androgenism. And, as readers of the VOICE, of course, by now well understand, ovaries need normal androgen levels in order to make good quality eggs in good numbers (please note the conflict statement below).
The CHR and some of its staff members own shares in a company (Fertility Nutraceuticals, LLC, doing business under the name Ovaterra), which produces an androgen (male hormone) supplementation product called dehydroepiandrosterone (DHEA) and other fertility-related supplements. Since this article, among other subjects, addresses androgen supplementation with DHEA, readers are advised that expressed opinions in this paragraph may be biased.
The VOICE, however, never really addressed the value of inositol supplementation in PCOS beyond this one issue, and this is exactly what the paper of Fitz et al did;1 and they did it well, relying on 30 trials involving 2230 patients, 1093 interventions, 1137 controls, in 19 pooled meta-analyses and the results were quite surprising: Comparisons were made for 13 outcomes, 3 of them by meta-analysis. Both inositols resulted in selected minor metabolic benefits for patients and D-chiro inositol (but not the more frequently used myo-inositol) demonstrated improved ovulation. But that was it; no other benefits on fertility were found! The authors concluded that the evidence for use of inositol in the management of PCOS is limited and so-far at best inconclusive.
It will be interesting to see whether these conclusions change anything in the current promotion patterns for inositol products by the supplement industry. Since we are not convinced that this will be the case, addressing this issue here appeared timely. Moreover, since PCOS is, of course, closely associated with obesity and metabolic syndrome (here again the phenotype-D does not demonstrate these associations), the following recent publications have also relevance: One interesting study – also in the JCEM - attempted to determine whether metabolomics could figure out why that was the case, and failed in doing so.2
To the CHR this failure does not come as a surprise because a principal underlying cause for so many failures in PCOS research appears always to be the same, repeatedly discussed in the VOICE: all phenotypes are included in studies of PCOS without sub-analyses, including above noted phenotype-D which so greatly differs from phenotypes-A, B, and C and, therefore, counter-powers many finding in these three phenotypes.
This study, indirectly, supports this conclusion by some of the findings the authors considered of secondary importance: The identified novel signaling pathways, with one of them involving bile acid function on the adrenal gland.2 This is a fascinating – and likely by the authors underappreciated finding because the adrenals, of course, appear to be a main-player in the D-phenotype that becomes hypo-androgenic because of insufficiency in adrenal-androgen production. The authors, indeed, note in their discussion of the paper that bile acids, therefore, may be candidate signaling molecules involved in explaining the connection between obesity and hyper-androgenism and associations between bile acids and hyperandrogenism have, indeed, been reported before and the bile acid receptor, farnesoid X receptor (FXR) was found expressed in human adrenal glands.
Clearly a very interesting study!
And since we are already talking about hyper-androgenism, here is a third paper from the JCEM which, also in form of a review (a “mini-review” this time) attempted to clarify the cardiometabolic risk women face from hyper-androgenism pre- and post-menopausally.3
The conclusion the author reached was in general not surprising but in one aspect did surprise: In premenopausal women hyper-androgenism is- independent of BMI - clearly associated with increased cardio-vascular disease risks as well as mortality. In postmenopausal women, these risks, including stroke risk, are dependent on the female’s BMI and register only with obesity (high BMI). The overall cardiac disease in postmenopausal women, indeed, is similar to controls. What causes tis difference cardiovascular risk between preand post-menopausal women is still unclear.
And now some recently published data from the always somewhat controversial Women Health Initiative (WHI), addressing how the metabolic syndrome and obesity affect breast cancer in postmenopausal women.4 The study involved 63,330 such women with no breast cancer, normal mammograms, and normal (negative) metabolic.

The study assessed breast cancer incidence, mortality by evaluating the relative contributions of metabolic syndrome scores and obesity over more than 20 years. During the follow-up 4562 breast cancers were diagnosed, of which 659 died from breast cancer and 2073 died after breast cancer.
The findings were interesting: Having metabolic syndrome (scores 3 & 4 vs. 0), adjusted for BMI, was significantly associated with poor prognosis breast cancer (ER+/PR-) but was not associated with higher breast cancer risk overall. Likely related to poorer prognosis, higher metabolic syndrome scores were also associated with higher risk of dying from breast cancer but also with higher death risk after breast cancer. Once obesity was, however, adjusted for metabolic syndrome score, it was associated with higher incidence of overall breast cancer; these cancers were, however, mostly good prognosis breast cancers (ER+/PR+). Obesity was overall not associated with mortality risk from breast cancer but was so associated with severe obesity, defined as BMI >35. Interestingly obesity in general was, however, associated with more death after cancer.
The conclusion of this study was that metabolic syndrome and obesity affect breast cancer independently and that targeting obesity – now so much easier with the new family of GPL-1 agonists (and soon antagonists) – will, likely, reduce breast cancer incidence but not mortality from the cancer. To achieve that, metabolic syndrome scores will have to be targeted to identify potential poor prognosis ER+/PR- breast cancer patients early. 4
And, finally, just a few words about yet another systematic review
and network meta-analysis by Spanish investigators regarding the comparative efficacy of exercise, diet and/or pharma interventions on BMI, ovulation, and hormonal profile in overweight and obese women.5 And what a surprise (facetiously), the authors’ summary of their literature review revealed that the optimal treatment strategy for women with overweight or obesity wishing to conceive must consider exercise, diet, and pharmacological interventions during shared decision making process.
Not only is this one of those absolutely useless studies on a subject that no longer requires any more studying but, considering all the new GPL-1 medications, it, of course, becomes more outdated every day.
1. Fitz et al., J Clin Endocrinol Metab 2024;109:1630-1655
2. Calvert et al., J Clin Endocrinol Metab 2024; 109:1328-1333
3. Lindén-Hirschberg A. J Clin Endocrinol Metab 2024;109:1202-1213
4. Chlebowski et al., Cancer 2024; https://doi.org/10.1002/cncr.35318
5. Ruiz-Gonzálet et al., Hum Reprod Update 2024g
Finally, a more “robust” tool for the diagnosis of ovarian cancer?
Diagnostically – and probably otherwise as well – ovarian cancer has remained the most problematic among all gynecologic cancers. This, of course, has several reasons, among those not the least that, as is now widely recognized, most ovarian cancer cases are not really ovarian – but distal tubal – cancers. Whatever the underlying cancer, diagnosing ovarian cancer at early stage, therefore, has remained elusive and most cases are diagnosed at very advanced stages.
At the recent annual meeting of the American Association for Cancer Research in San Diego, CA, April 5-10, 2024, by investigators from John Hopkins University (Medina JE., et al., Abstract 6986), according to a report by Healio®,1 a liquid biopsy in combination with an AI-driven algorithm offer a first realistic promise for promise, with detection rates of 69% for stage I, 76% for stage II, 85% for stage III, and 100% for stage IV cancers.2 These are, of course, very promising results, even-though a miss-rate of ca. 30% in stage I disease is, still, quite bothersome.
1. https://www.healio.com/news/hematology-oncology/20240410/ai-partners-with-liquid-biopsy-for-robust-identification-of-women-with-ovarian-cancer
2. https://www.aacr.org/about-the-aacr/newsroom/news-releases/artificial-intelligence-analysis-of-dna-fragmentomes-and-protein-biomarkers-noninvasively-detects-ovarian-cancer/ April 9, 2024
Do hypertension, cardiovascular, female reproductive factors, and sex hormones increase the risk of developing fibroids?
It may sound like a crazy question to ask whether hypertensive and cardiovascular diseases may be risk factors for the development of uterine fibroids, but there, indeed, have been suggestions that this may be the case.1 Consequently, it is not as surprising as it may sound on first impression that investigators from several U.S. institutions got together to further investigate this issue. In a paper in JAMA Network Open, they now reported that women with untreated and new-onset hypertension demonstrated increased risk for newly diagnosed fibroids. Those, however, taking anti-hypertensive medications had clearly lower risk, leading the authors to suggest that medical blood pressure control may be a “new strategy” to prevent fibroids.2
This was overall a well-executed longitudinal study with repeated measurements in study as well as control patients and demonstrated in women with new-onset hypertension a 45% increased risk of new fibroids, while women with untreated hypertension demonstrated a 19% increased risk. Patients in treatment for their hypertension had, indeed, a 20% lower risk of fibroids than women without hypertension and an overall 37% lower risk compared to untreated hypertensive patients (with ACE inhibitors, indeed the lower risk grew to 48%).
Interestingly, however, the authors struggle in explaining how fibroid growth may be related to development of hypertension, though they suggested that the renin-angiotensin-aldosterone pathway may play a role. To their credit, they, however, also acknowledged the possibility that their results could be the result of bias and we, of course, agree. It appears to us that the differentiation between “new” or no “new” fibroids is, at best, very complex and, more likely, really impossible.
It seems less far fetched to ask whether reproductive female factors and sex hormones may be associated with risk for myomas, and this is what Chinese investigators recently did through a Mendelian randomization study.3 And what they found was actually quite interesting: They found risk for uterine leiomyomata was, interestingly, statistically highly associated with age at menarche (OR 0.84; p<0.0001), age at menopause (OR 1.08; p<0.0001), number of live births (OR 0.25; p<0.001), and total testosterone (TT) (OR 0.9; p<0.001). While, as noted above, late menopause increased the risk fibroids, a higher number of live births and higher TT were protective of myomas.
Considering how many infertile women are hypo-androgenic and the CHR’s longstanding interest in hypo-androgenism as a cause of female infertility, this paper attracted our interest since in the discovered association between production of myomas and female
hypo-androgenism, the study basically demonstrated a second female fertility-reducing effect of hypo-androgenism (besides adverse effects on folliculogenesis in ovaries). This finding, moreover, also creates a second incentive for regular androgen level monitoring in women as they get older.
1. Kirschen et al., Reproduction 2021;162(2):R1-R18
2. Mitro et al., JAMA Network Open 2024;7(4):e246832
3. Wang et al., Reprod Biomed Online 2024;48(2):103584
Some genomic data in the literature suggested certain similarities between endometriosis and several gynecological cancers. Saudi Arabian investigators decided to explore this association further using a U.S. data set (National Inpatient Sample) for the years 2016-2019. Comparing in this data set women with to women without endometriosis, the former demonstrated significantly higher odds of developing ovarian (46.56% vs. 20.28%) and endometrial cancers (34.27% vs. 13.46%) but both differences were only barely significant at p<0.05. Those associations remained significant and even strengthened (P<001). Other gynecologic cancers demonstrate no increased risk: Endometriosis actually appeared to reduce breast cancer risk and showed no association with cervical cancer. 1 Quite confusing results, we must admit, and we, kind of, do not know what to do with them.
1. Al-Badawi et al., Current Onc 2024;31:472-481
In a recent paper in Fertility and Sterility, Eli Y. Adashi, MD, returned (with co-authors) to a subject he has previously written and lectured about (also at prior FRM Conferences, co-sponsored by the CHR): what are the contributing factors – and in what importance – to the excessive prevalence of multiple births in association with fertility treatments.1 This, of course, has also been a repeated subject of interest at the CHR. Building on CDC data, which can claim much larger clinic participation than the ASRM ART registry, the study describes national trends in twin and larger multiple births between 1967 and 2021, concluding that ovulation induction treatments and ovarian stimulation (for all purposes) are the leading iatrogenic contributors to multiple births. The authors furthermore conclude that these two treatments, therefore, should be the target of interventions to reduce adverse maternal and neonatal outcomes associated with multiple – or as they call it – “plural” births.
These data are not surprising; they, indeed, have been known for decades. In 2000, the CHR’s investigators – then still in Chicago –pointed out in a by now classical paper in The New England Journal of Medicine that the nation’s exploding multiple birth rate was disproportionally the consequence of intrauterine inseminations (IUIs) following gonadotropin-based ovulation induction rather than IVF and that IVF actually represented the ideal remedy to reduce this excessive multiple pregnancy rate by controling the number of embryos transferred in an IVF cycle.2
The authors, however, added interesting new detail: They, for example demonstrated that women above age 35 significantly contributed to national multiple birth rates, - an obviously important finding, considering that older women now percentagewise represent the most rapidly growing age group of women having children. In other words, older women are increasing, while younger women demonstrate steadily declining birth rates. Also interesting, the relative contribution of ovulation induction and ovarian stimulation has remained stable, the contribution of unassisted conception (i.e., conception without treatments) has increased at all ages, while the contribution of IVF – likely because of increasingly stringent elective single embryo (eSET) policies has actually decreased.
The only criticism we have regarding this paper is not content-related and does not relate to interpretation of data - both of which are excellent – but with the fact that this paper, like basically all of the literature over the last 20 - or so – years has done regarding the issue of multiple births: It did not clearly enough point out the important differences between twin and higher-order pregnancies, throwing all multiple births into the same single pot of condemnation, basically designating all multiple pregnancies in infertility practice as adverse outcomes.
This is, however, a point where we, here at the CHR, divert from the current consensus because in our analysis of the literature the statistically correct risk profile for twin-pregnancies (mothers and offspring) is by no means prohibitive. As we in innumerable publications, and on many occasions in these pages, have in detail explained, for many infertile women/couples, twins, indeed, represent a great and desirable outcome. We, therefore, will here not be repetitive but want to reemphasize that for twins, for as long as there exist no obvious contraindications like maternal medical problems or advanced maternal age, at the CHR a patient’s right to self-determination is also upheld when it comes to her desire for a twin pregnancy. This is one reason why we differentiate so clearly between twin and higher-order pregnancies because we, of course, agree with the consensus that with higher-order multiple pregnancies the risk profile for mother and offspring becomes too negative.3
REFERENCES
1. Adashi et al., Fertil Steril 2024;121(5):656-764
2. Gleicher et al., N Engl J Med 2000;343:2-7
3. Gleicher N, Barad D. J Assist Reprod Genet 2013;30(4):575-579
Chromosomal testing of products of conception after pregnancy loss (RPL) and other miscarriage-related issues
That antiphospholipid antibodies (APAs) are associated with increased miscarriage risk has been known for decades. What universally, however, is not equally well accepted it that this does not mean that APAs necessarily cause miscarriages. ACAs can be found in many autoimmune-related conditions, but also in association with HIV and, yes, after COVID, and/or in association with Long COVID, supporting the notion that their presence can be viewed as a marker for a hyper-active immune system. And it is the hyper-active immune system (even in absence of APAs) that appears to be the disease entity that is associated with increased miscarriage risk. In other words, everything that denoted a hyper-active immune system, concomitantly also denotes miscarriage risk.
For that reason, a recent paper by Chinese investigators attracted our attention which compared in 118 women with either low or medium-high APA titers and a history of repeated miscarriages in 124 pregnancy episodes whether levels of APA titers mattered.1 And there were, indeed, some differences: Low titers were associated with higher prevalence of IgM anti-cardiolipin antibodies (ACAs), while medium-high titers demonstrated statistically more IgG and IgM anti-beta2 glycoprotein-1 antibodies and had more elevate ESRs; but no significant differences were seen in terms of maternal and neonatal pregnancy outcomes and miscarriage rates, reaffirming the CHR’s longstanding understanding of the importance of APAs (in all titers) in predicting miscarriage risk.
These findings strongly support the CHR’s longstanding believe that APAs, themselves, are not directly causing miscarriages because, if that were to be the case, one would expect more miscarriages with higher APA-titers. They also support the CHR’s longstanding believe that our rheumatology colleagues have been mistaken for decades by ignoring low-level APA-titers.
Related, as a longstanding member of the reproductive immunology community, William H. Kutteh, MD, has historically over decades been an important contributor to the APA literature regarding miscarriages. He recently also was the lead author of a review article that addressed the role of chromosomal analyses with 24 chromosomal microarray (CMA) analysis of products of conception (POCs) in managing early pregnancy loss.2 And as long as this paper stuck to the question of karyotyping POCs, the manuscript did well; but once it also expanded into recommendations regarding the utilization of PGT-A, things became somewhat blurry.
And here is why: The review article was based on 65,333 miscarriages, a prospective evaluation of 378 couples with repeated pregnancy losses (more on the definition later) who underwent advanced karyotyping, and 1020 couples who underwent basic diagnostic testing of mothers (as currently recommended by ASRM) but without advanced karyotyping of POCs.
Aneuploidy was found to be the (alleged) cause of miscarriage in 218/378 cases (57.7%) of cases, - compatible with data from the literature that suggests that aneuploidy can usually be found in a little over half of all POCs (more on why we describe chromosomal test results as “alleged” below). In contrast, the authors noted that the routine evaluation currently recommended by ASRM guidelines for pregnancy loss, which does not include advanced karyotyping of POCs, identified a potential cause for the miscarriage in only 600 out of 1398 cases (42.9%). Combining both approaches would identify a likely cause for RPL in 347/378 90.0%) of cases. Consequently, without advanced karyotyping couples with unexplained loss under ASRM guidelines represented 41.0% of cases.
Up to this point, we agree with the authors: They, however, based on these findings concluded that to categorize a pregnancy loss as “explained” vs. “unexplained” combining standard ASRM-recommended testing with advanced karyotyping, may help to identify patients who would benefit from expectant management versus PGT-A in a next pregnancy attempt. This statement, in our opinion, however, requires clarifications: The CHR fully agrees with the authors’ conclusion that current ASRM guidelines for the management of couples with RPL are outdated. We, however, differ in why we reached this conclusion and, even more importantly, regarding diagnostic consequences.
First, once cannot automatically assume that diagnosing POCs after a miscarriage as aneuploid, automatically defines the pregnancy loss as caused by this aneuploidy. Some aneuploidies (Trisomies 15, 18, 21, and sex chromosome abnormalities, as well as segmental aneuploidies, often, are not spontaneously aborted). Their presence, therefore, does not necessarily denote a chromosomal cause for the pregnancy loss. Moreover, standard karyotyping does not preclude maternal contamination of the POCs in cases of female karyotype (though advanced karyotyping does). A “normal” report as a consequence of maternal contamination, therefore, could be a false-negative result.
Second, biopsies of POCs for karyotyping are usually taken from the trophoblast and not the fetus and, therefore, represent the extraembryonic cell lineage which produces the placenta which maintains islands of aneuploidy until birth. The embryonic cell lineage which produces the fetus, in contrast, often self-corrects resulting in fullyeuploid fetuses. Trophoblast biopsies of POCs, therefore, over-report aneuploidies, resulting in false-positive results.
Third, even if biopsy results are “real,” not every aneuploidy denotes an abnormal fetus. For example, as already noted above, segmental aneuploidies, often, have no repercussions for a fetus. Indeed, many segmental abnormalities do not have any adverse outcome implications for the fetus (though some do). In other words, standard karyotyping of POCs should no longer be used - period!
As repeatedly discussed before in the VOICE, the CHR, moreover, especially in infertile couples considers the concept of RPL to be an oxymoron. In this study women were considered to have a diagnosis of repeated pregnancy loss after two pregnancy losses and studied
patients were between ages 25 and 40.
This kind of patient definition as repeat aborter is, however, completely non-sensical, - as everybody should understand that two losses in a 25-year-old have a different meaning than in a 45- yearold woman. Moreover, fertility clinics deal with infertile couples who by definition are at increased risk for miscarriages. To wait in such patients – whatever their age – for an arbitrary-chosen number of pregnancy losses, seems absurd: every miscarriage in an infertile woman should nowadays undergo advanced karyotyping because, how else, can an appropriate treatment plan be developed for such a patient’s next pregnancy attempt?
If advanced karyotyping the does not reveal a specific aneuploidy associated with miscarriage risk, the automatic conclusion must be that the pregnancy loss was not chromosomal and, therefore, must be accordingly investigated and treated. Whether patients after miscarriages that demonstrated a likely chromosomal cause (in absence of an abnormal parental karyotype) then should be advised to undergo PGT-A in the next pregnancy attempt is, in the CHR’s opinion also questionable considering the now much better understood shortcomings of PGT-A, but may be considered – though, primarily, only for psychological reasons since PGT-A, otherwise, does not really offer any significant outcome advantages at that point.
A very different issue, yet also related to increased miscarriage risk, is the use of the psychotropic drug, benzodiazepine, in pregnancy. This issue has raised considerable concern because of reported harmful effects of this class of drugs on neonates. The drug has also been reported to increase miscarriage risk,3 but data have so-far been limited. Investigators from Taiwan now reported an island-wide, population-based case-time-control study involving patients who experience miscarriages between 2004 and 2018 and used the drug in pregnancy during a defined risk period between days 1-28 before miscarriage. They were then matched with controls. The study involved 3,067,122 pregnancies in 1,957,601 women who experienced 136,134 miscarriages (4.4%). After accounting for a solid number of confounders benzodiazepine was found to be significantly associated with increased miscarriage risk [OR, 1.69; 95% CI, 1.52-1.87].4 The authors correctly concluded that risk-benefit considerations for using benzodiazepine in pregnancy must, therefore, be weighed very carefully.
And now some good news on the psychiatry front: A recent study reported in Nature Medicine by U.S. and U.K. investigators, reported that anxiety-focused cognitive behavioral therapy to prevent postnatal depression, indeed, works.5 The study involved 755 women, - among them 375 (49.7%) in the intervention arm. Odds of having a major depression episode or moderate-to-severe anxiety demonstrated 81% - reduced odds randomized to intervention [OR 0.19; 955CI 0.13-0.28]. Overall, 12% of women in the study group developed a major depressive episode by 6 weeks postpartum, vs. 41% among controls. Odds of an anxiety episode was reduced by 74%. Remarkable, the effective cognitive therapy consisted of only 6 one-
on-one intervention sessions in pregnancy and was delivered by non-specialist providers.
Related, British investigators reported that in women with prenatal depression (which is a very strong predictor of postpartum depression) a single low dose of esketamine after childbirth significantly decreased in a randomized trial major depressive episodes up to 42 days postpartum by about three-quarters.6
A study from China asked the question what the proteomic and phosphor-proteomic profiling differences in endometrium are between women with RPL and controls.7 The study revealed dysregulation in the endometrium of women with RPL in insulin/cAMP and AMPK/ mTOR signaling, accompanies by altered expression of 4 proteins: PRKAR1B, ADCY3, PRKAA2, and LPN2. Those findings may lead to potential treatments, once the causes for the dysregulation in those two signaling pathways has been determined.
Finally, one cannot discuss miscarriages these days without some reference to endometrial microbiota (and possibly vaginal of even GItract microbiota) and the opportunity to do that presents itself based on publication of a recent paper in Human Reproduction which quite well summarizes suggested associations between endometrial microbiota and early miscarriages.8 Before addressing this revie article, it must be pointed out, however, that whether the endometrium really has a distinct microbiome or whether what studies have reported in reality represents contamination from the vaginal microbiome, is really to this day not fully resolved, - even though the evidence in support of a relatively independent endometrial microbiome is growing. The article claims that recent research suggests that endometrial microbiota not only really exist, but that they may play an important part in early miscarriages, while at the same time acknowledging that the mechanisms behind such effects remain to be deciphered.
Data about the vaginal microbiome is more solid, suggesting that any interruption of the dominance of Lactobacillus species is detrimental to reproductive success and a woman’s health in general.
Depletion of vaginal Lactobacillus, indeed, has been associated with euploid miscarriages and inflammatory cytokines, including IL-1beta and IL-6. How this dysbiosis of the vaginal microbiome may cause, for example, miscarriages has, however, not been clarified to this point, and – as the authors noted – the research focus, therefore has somewhat shifted to the endometrial microbiome. Studies comparing vaginal and endometrial microbiomes have disappointingly varied, with some suggesting – more less – a continuum, while others described clear differences. Everything else in the paper from this point on are, however, still hypotheses. For those interested in this subject, we nevertheless recommend this paper as an excellent introduction to the subject.
1. Chen et al., Clin Rheumatol 2024;43:1327-1334
2. Kutteh et al., Reprod Biomed Online 2024;49(1):103738
3. Sheehy et al., JAMA Psychiatry. 2019;76(90:948-957
4. Meng et al., JAMA Psychiatry 2024;81(4):366-3735
5. Surkan et al., Nat Med 2024;30:675-682
6. Wang et al., BMJ 2024;385:e0728218
7. Chen et al., Reprod Bomed Online 2024;45(1):103585
8. Odendaal et al., Hum reprod 2024;39(4):638-646
We recently discussed in these pages in a prior issue of the VOICE a paper by Dutch investigators that reported an increased risk for Alzheimer’s disease and other dementias in women who experience hypertensive diseases of pregnancy.1 Now French investigators in a nationwide study of deliveries between 2010 and 2018 reported the occurrence of dementia at young ages in women with preeclampsia.2 The study involved 1,966,323 individuals with mean age 34.6 years of which 128 (<1%) developed dementia. Those who developed young dementia were older at time of pregnancy (36,4 vs, 34,6 years), were smokers, (14,8% vs. 9,0%), and had diabetes (2.3% vs. 0.7%). Risks were even higher if preeclampsia occurred before 34 weeks or was superimposed on chronic hypertension. Interestingly, however, severe preeclampsia was not associated with young-onset dementia.
This brief report reemphasizes the association of hypertensive diseases in pregnancy with occurrence of dementia and, therefore, adds to the growing evidence that preeclampsia is a systemic disease with lifelong repercussion. This strengthening association mandates quick and maximal efforts in improving early diagnosis and achieving prevention of preeclampsia.
1. Schliep et al., Alzheimers dement (AMST( 2023;15(2):e12443
2. Olié et al., JAMA Network open 2024;7(5):e2412870
In a nationwide birth cohort study of 3,251,594 infants (representing 2,369,322 paired mothers with mean age of 32.1 years, South Korean investigators reported somewhat surprising findings when claiming that opioid use during pregnancy was not associated with a substantial increase in risk for neuropsychiatric disorders if offspring, though what the authors described a “slightly increased risk” was obesrved.1 These findings were especially obvious in sibling-controlled analyses. Observed effects were observed and limited only to use of high opioid doses, more than one opioid, longer duration of exposure, exposure during early pregnancy, and were restricted to only specific neuropsychiatric diseases. An accompanying commentary in the BMJ had nothing substantial to add.2
Assuming these data hold up, this would mean that the effects of opioid on the fetal brain is less severe than cannabis effects, which were repeatedly discussed in these pages during the current academic publication year.
REFERENCES
1. Kang et al., BMJ 2024;385:e077664
2. Wen et al., BMJ. 2024;385:q803
We noted elsewhere that the CDC, currently, still does not perceive a large threat for a bird flu pandemic in humans; but with every infected human the risk, of course, increases. So-far according to the CDC, only 2 workers involved with different animal species have contracted the bird flu in the U.S. A first was a person in Colorado in 2022 who apparently got the virus from infected poultry. The now reported second case in the U.S. was transmitted by life stock. Cows are apparently an animal species that has become a primary reservoir for the virus (H5N1), with viral fragments (which are not infectious) detected in cow milk.1 In addition Mexico reported through the WHO the death of a 59 year old man who was hospitalized on April and later diagnosed with bird flu.2 Through which animal contact this individual was contaminated but media reports suggested that he (and family) may have eaten infected poultry.
More cases are expected, and federal agencies allegedly have already prepared ca. 4.8 million doses of well matched vaccine to the currently circulating H5N1 virus.
REFERENCES
1. Mandavilli A, Anthes E. The New York Times. May 22, 2024. https://www.nytimes. com/2024/05/22/health/h5n1-bird-flu-dairy.html
2. Steenhuysen J, Barrerra A, Reuters Health Information, June 5, 2024. https://www. medscape.com/s/viewarticle/person-bird-flu-died-mexi...oexpansion-algo_20240608_ etid6580338&uac=223637CN&impID=6580338
A WORD ABOUT VACCINES: With COVID now an endemic virus in the U.S., we better get used to its presence and seasonal returns, - just as we have gotten used to the annual flu-season (and related vaccines). Despite all the continuing political disputes surrounding the management of the COVID-19 pandemic by the U.S. and many other governments (including vaccination mandates), the CHR still recommends that women planning a pregnancy (this includes women in fertility treatments) or women already pregnant receive booster shots against the currently circulating SARS-CoV-2 strain, against influenza (the flu), and against syncytial virus infections. Such boosters are also recommended for everybody who for whatever reason is immuno-compromised, or is above age 65.
This may also be the right moment to remind all fertility patients to make sure they are still immune to Measles, Mumps, and Rubella, all three bad childhood disease to get during pregnancy which, often, will – cause problems to their pregnancies.
WE NOW MUST DEAL WITH 2 DISTINCT CLINICAL EXPRESSIONS OF COVID: There is the sporadic infections COVID infection that clinically can present similarly to the flu; and then there is what now officially is called Long COVID, which is a still very controversial and poorly understood disease that affects individuals after recovery from an acute COVID infection and can last for months or even years.
The currently circulation virus is highly infectious, - but causes only relatively mild and short disease. While some patients of the first 1-2 days can be still “knocked off their feet,” recovery is quick, and the CDC recommends returns to normal activities (including work) once the fever breaks and symptoms disappear or at least greatly improve. For most patients this will happen within 5 days. If patients do not improve within 1-2 days, they should see a physician and receive anti-viral medications.
Long COVID is, however, a very different story. For those not familiar with the condition, we recommend two Ground Truths podcasts on Apple and Spotify by Eric Jeffrey Topol, MD, one on May 201 and the other on May 30, 2024.2 Topol is, of course, a legend in academic medicine in the U.S. as a cardiologist, geneticist, and physician-scientist and much more than that since he, after all, published only over 1,300 peer-reviewed papers with over 340,000 citations, was elected to the National Academy of Medicine, has written several bestsellers on the future of medicine, and is now - after a long career - at major East coast universities - founder and director of the Scripps Research Translational Institute in La Jolla, CA and a professor of Molecular Medicine and Executive VP at the Scripps Research Institute.
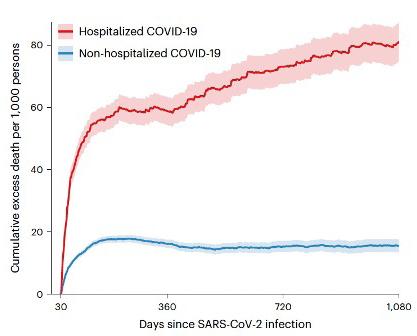
Figure 1. Total mortality of mild (non-hospitalized) and severe (hospitalized) COVID patients over 3 years after disease onset. While mild cases peaked in mortality at roughly 165 days, severe cases plateaued till approximately day 300, only to continue with increasing mortality up to 3 years. Source Substack, May 30. 2024
His podcasts are definitely worth listening to and it is interesting that he dedicated two recent ones to Long COVID. Figure 1 (previous page) from the May 30 podcast demonstrates one of the most basic characteristics of Long COVID, the difference in long-term cumulative mortality rate between non-hospitalized (i.e., mild) and hospitalized (i.e., severe) COVID patients.
As the figure very clearly shows, non-hospitalized patients peak in mortality at roughly 165 days from the beginning of the pandemic, with overall mortality after that again declining to baseline levels, while hospitalized patients (obviously demonstrating much higher immediate overall mortality) at that same first time point experience a short-lived plateau, after which - reflective of Long COVID - mortality again continues to rise for the following two years.
It is this secondary rise of continuous symptomatology and, as Figure 1 demonstrates, continuing increases in mortality, that have puzzled the research community now for a long time. Quite remarkably –except for the number - the curves of out- and in-patient total deaths until roughly six months into the pandemic run basically in parallel; but at approximately one year, patients with severe COVI, suddenly, appear to develop a new secondary condition leading continuously through the three-year point to ever higher death rates, while rates in mild COVID patients basically remain at pre-COVID baseline levels.
In Figure 2, Topol and Al-Aly in their May 30 podcast, moreover, demonstrated that in patients with severe COVID major adverse outcomes were persistent, including multi-system consequences, as well as disabilities and even death persisted until (and likely beyond) a 3-year follow up. Interestingly, however, no new adverse outcomes
were seen that had not been seen before in this patient cohort, actually rejecting the idea of a “a new condition.” And, if it is not a “new” condition, these finding can only mean that already prior-existing conditions became worse., As Figure 2 demonstrates, these conditions encompassed wide swaths of the human body. Who then can be surprised about the multitude of symptoms patient with Long COVID complain about.
Not represented in Figure 2 are the effects of COVID on the immune system That COVID challenges the patient’s immune system into activating autoimmune processes is now widely accepted. As previously noted, this tendency toward viral activation of autoimmunity is by no means only restricted to the SARS-CoV-2 virus. Taiwanese investigators now reported that COVID-19 infections were during a 12-months follow up after COVID associated with increased risk of subsequent thyroid dysfunction, including hypo- and hyperthyroidism, regardless of age and/or sex.3 Considering that autoimmune thyroid disease is the most prevalent autoimmune condition in women during reproductive years, this observation, of course, has relevance for the infertility field. It also suggests that the increase in autoimmune thyroid disease may reflect prevalence increases in other autoimmune conditions as well.
COVID (and other infectious) DIAGNOSES FROM A PERSON’s COUGH? This is exactly what a recent news article in Nature magazine reported.4 According to this article, Google scientists developed a machine learning tool in which AI detects and monitors medical conditions based on the analysis of noises created by breathing and coughing. The system apparently was able to diagnose COVID, tuberculosis, and was able to determine lung function overall.
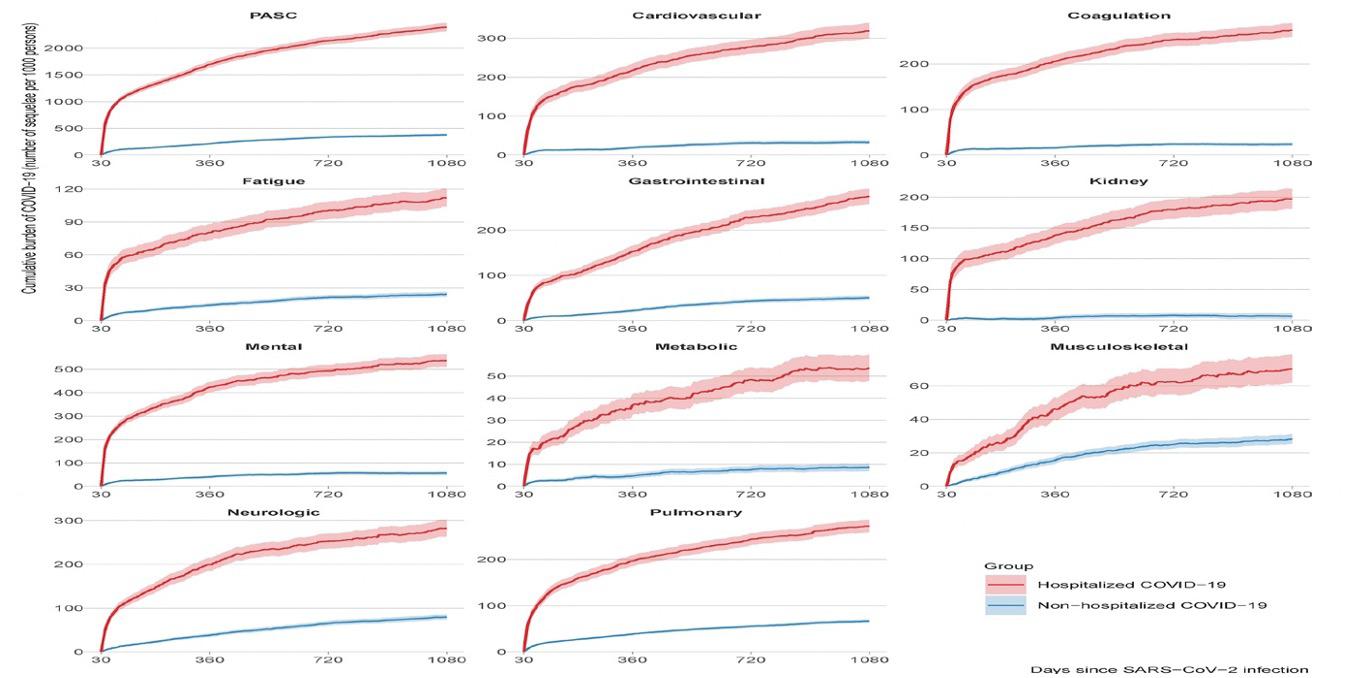
PREGNANCY INCREASE PREECLAMPSIA RISK: So-far only presented at the 2024 Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists (ACOG) in San Francisco, CA, between May 17 and 19, 2024 (Abstract # 2683535), Massachusetts’ investigators reported through the NTK Institute increased preeclampsia risk in mothers who experience COVID during the 1st or 2nd trimester of their pregnancy.5 The rate of infection with SARS was 19.4% for patients in the 1st trimester, 40.8% in the 2nd, and 39.8% in the 3rd trimester. The preeclampsia rate was 19.2% in patients who caught COVID in the 1st trimester, 18.2% for those who got sick in the 2nd trimester, and 11.2% for those who contracted COVID in the 3rd trimester, and 11.7% with COVIF in the 3rd trimester (p< 0.001). After appropriate adjustments significance was, however, only maintained for 1st trimester SARS infections.
As interesting as this finding may be overall (though contradicted by other earlier publications), assuming this association can, ultimately, be confirmed, this should not surprise, - and her is why: We above reviewed a paper that reported the association of COVID with later occurring hypoandrogenism or Grave’s disease,3 making the point that viruses can, in association with several autoimmune conditions, activate immune systems toward autoantibody production. Which brings us full circle to the hypothesis of preeclampsia as an immunologically mediated disease, induced by diminishing and/or problems with maternal tolerance of the fetal-placental semi-allograft. In other words, both of these physiological conditions are characterized by abnormalities in tolerance (either pregnancy or self).6
1. Substack. Topol E. Ground Truth. https://erictopol.substack.com/p/svetlana-blitshteyn-on-the-front
2. Substack. Topol E., Al-Aly Z. Ground Truth. https://erictopol.substack.com/p/longcovid-at-3-years
3. Huang L-A., et al., Thyroid 2024;34(4):442-449.
4. Lenharo M. Nature 2024;628:19-20.
5. Ramos E. NTK Institute, May 23, 2024; https://ntk-institute.org/article/sars-co-2infection-during-first-trimester-linked-to-increased-risk-of-pre-eclampsia.
6. Gleicher N. Am J Obstet Gynecol 2007;196(1):5.e1-7
Inflammation
Readers of the VOICE by now know that the CHR does not like inflammation in women who are trying to conceive or are already pregnant, with the main reason being that excessive inflammation is a manifestation of a hyperactive immune system. Women who have a hyperactive immune system then have difficulties in inducing the normal and required tolerance pathways in their immune systems, with the result being inadequate tolerance of the fetal-placental semi-allograft (in cases of egg donation a complete allograft).
Reports in the literature on the effects of systemic inflammation in medicine in general, therefore, always attract our attention. Which brings us back to above noted Eric Topol, MD and his Substack
blog called Ground Truths which, in its June 9 issue, featured in detail the importance of systemic inflammation in cardiovascular disease.1 He notes in his article that there are now relatively simple methods available to measure body-wide inflammation and many ways to reduce systemic inflammation, whether by diet and other lifestyle modifications or relatively inexpensive medications. But, like in infertility practice, none of these interventions have been so-far integrated into cardiology practice. And, like the CHR has for many years stressed that inflammation must be taken more seriously in infertility practice, so does Topol in this article make the argument for cardiology.
The occasion arose publication of an article in The Lancet which reported on a randomized trial of 0.5 mg daily of colchicine (yes, the good, old-fashioned drug used for decades in the treatment of gout) in 3100 patients with non-severe, non-embolic stroke or TIA (transient ischemic attack).2 Primary endpoints were – combined - heart attack, stroke, cardiac arrest, or hospitalization for unstable angina. A 16% reduction after colchicine treatment was not statistically significant but further adjustments increased this difference to 20%, which was statistically significant. Moreover, colchicine also was shown to significantly reduce high-sensitivity CRP (C-reactive protein), - a widely used inexpensive marker of systemic inflammation.
Inflammation is widely accepted as a major driver of atherosclerosis and atherothrombosis. As Topol further noted, colchicine in June of 2023 was, therefore, approved by the FDA as the first anti-inflammatory agent for reducing cardiovascular events. His article, furthermore, described the absence of appropriate anti-inflammatory treatments in cardiology as a “great miss.” The same term, in the CHR’s opinion, is currently also applicable to infertility treatments and the CHR, therefore, initiated pre-treatment with colchicine 0.5mg daily for infertile women with elevated CRP.
1. Topol E. Substack. Ground Truths; June 9, 2024. https://erictopol.substack.com/p/ the-big-miss-inflammation-and-cardiovascular
2. Kelly et al., Lancet 2024; https://doi.org/10.1016/S0140-6736(24)00968-1; Online ahead of print.
Investigators from Finland and the UK recently published an interesting nationwide registry study of 5,339,804 Finnish citizens born between 1964 and 1984 and diagnosed with any one of 19 immune-mediated diseases before age 30 (men before age 35) and matched them with 20 controls by birth year, sex, and education in order to study the effects of these diseases on reproductive performance. Several of these rheumatic diseases, especially Juvenile Idiopathic Arthritis (JIA), seropositive rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) were associated with higher rates of childlessness and fewer children in general.
Many of these diseases also demonstrated the expected pregnancy complications historically associated with them, including preeclampsia, small-for-gestational-age infants, preterm-deliveries, non-elective Cesarean section, and need for neonatal intensive care. These risks were especially pronounced for SLE, systemic sclerosis (SS), type 1 diabetes, and Addison’s disease, where they exceeded controls by more than two-fold. Most other diseases demonstrated a 1.1 to 1.5fold risk, including in inflammatory bowel disease (IBD), celiac disease, Immunological thrombocytopenia (ITP), and psoriasis. Interestingly, asthma – obviously a primarily allergic condition – also demonstrated the latter risk profile, reemphasizing an opinion long held at the CHR that (severe) allergies can also trigger the usual tolerance problems of a hyper-active immune system.
And since we are already talking about rheumatic diseases, The Lancet recently published a comprehensive update on SLE.2 The only shortcoming of the article was that pregnancy considerations were addressed in only one short paragraph (12 lines in one column of a two-column page). Consequently, this is not an article to at all inform on SLE and pregnancy; but it is a great article to inform on SLE in general.
And who would ever have believed that RA and cardiovascular disease share polygenic overlap! But this is exactly what a recent paper in BMC Medicine recently reported.3 The authors concluded that this shared genetic architecture may facilitate improved clinical management. One wonders?!
REFERENCES
1. Kerola et al., Rheumatology 2024;20:keae122. Online ahead of print.
2. Hoi et al., Lancet 2024;403”23262338
3. Sun et al., BMC Medicine 2024;22:152
That slowing removal of apoptotic cells from tissues is a typical feature of aging is now well established. Using a mouse model, investigators now demonstrated that depleting an expanding pool of aberrant stem cells in age mice using specific antibodies rebalanced blood cell production, diminished age-associated inflammation, and strengthened adaptive immune responses.1 In this investigation cell surface proteins of hematopoietic stem cells (HSCs) were the target of antibodies, depleting myeloid-biased HSCs; but the same principle should be appliable with other stem cells as well.
Commenting on the study in the same issue of Nature magazine, Arfat et al., noted that eliminating drivers of ageing lies at the core of prevention of age-related diseases and is the basic reason for the development of a class of drugs at the center of these efforts and, therefore, called “senolytics.”2 These drugs in principle target senescent cells which, if not timely removed, are a principal cause of inflammation. Several senolytics are currently under investigation in attempts to counteract ovarian aging; we, however, are unaware of anybody having attempted above noted antibody methodology for such a purpose. Something to think about!
Switching from ageing to cancer (of course, also a major cause of shorter lifespans) Tumor-infiltrating B cells are now well recognized as major players in cancer immunity which can serve as predictors of response to immunotherapies. They now are also known to execute various functions, most importantly through their ability to become plasma cells which produce antibodies. Chinese investigators now compiled a blueprint of B cell heterogeneity and two dynamic differentiation pathways of human cancers and highlighting unfavorable clinical outcomes to one of those.3 These observations should open the door to new B-cell targeted immunotherapies against several cancer types.
Finally, we want to point out an editorial in Cell on the occasion of the journal’s 50th anniversary in 2024 under the heading, “Immunology is for everyone” and with the intent of celebrating “immunology’s energy of ongoing discovery.”4 This feeling of immunology playing and important part everywhere in medicine permeated medicine also in the 1970s and 1980s, when immunology promised to decipher many – if not most – unresolved medical issues and then bitterly failed. But the success of immunology over the last decade has, indeed, been nothing but miraculous and, very much, deserves the celebration and enthusiasm expressed by this editorial. In addition, the journal then offers three reviews on big themes in immunology (innate, immunology, adaptive immunity, and – most interestingly – new perspectives), which we very much recommend to our readers with interest in the field of immunology.5-7
This is, after all, the CHR’s founder’s (Norbert Gleicher, MD) original field of research interest. He in the process not only contributed to the establishment of reproductive immunology as a distinct sub-specialty area in reproductive medicine in the world (and also served for 20 years as founding Editor-in-Chief of the American Journal for Reproductive Immunology and was in 1979 the founding Vice President of the American Society for Reproductive Immunology) but literally “impregnated” the CHR with a keen understanding of the importance of immunology for the establishment of normal and abnormal pregnancies that has been maintained, best documented by the large number of patients with immune problems the CHR to this day serves and the CHR’s research which never ceased to involve immunological subjects.
REFERENCES
1. Ross et al., Nature 2024;628:162-170
2. Arfat et al., Nature 2024;628:43-45
3. Ma et al., Science 2024; 383:eadj4857
4. Editorial. Cell 2024;187:2029
5. Carpenter et al. Cell 2024; 187:2030-2051
6. Chi et al., Cell 2024; 187:2053-2078
7. Medzhitov R, Iwasaki A. Cell 2024; 187:2079-2094
Preimplantation genetic testing, of course, involves the testing of preimplantation-stage human embryos at blastocyst stage (post-fertilization days 5-7). That testing can, however, have various purposes. If tests are done to see whether an embryo is afflicted by a mono-genetic disease (PGT-M, with M standing for monogenetic), embryos are biopsied and tested for the one specific gene that the embryo potentially has inherited for one or both parents. And this is a very accurate test.
If the question, however, is whether the embryo is afflicted by a chromosomal abnormality, the embryo is biopsied to test its chromosomal complement; in other words, whether the embryo is euploid (normal) or aneuploid (abnormal) and is, therefore called PGT-A (with A for aneuploidy). As readers of the VOICE by now unquestionably are aware of, the CHR considers the latter to be a rather “lousy” test, because it offers only inaccurate and uninterpretable results. The CHR’s patients, therefore, only extremely rarely undergo PGT-A; but the CHR, nevertheless, sees many patients who underwent PGT-A at other clinics, with all of their embryos reported as “aneuploid.” As most clinics still refuse transfers of “aneuploid” embryos, such patients often move their “abnormal” because we, since 2014, has been transferring carefully selected “abnormal” embryos with very good success.1
And then there is PGT-P, testing for polygenic disorders, which is the latest snake oil portion the genetic testing industry has started to sell to IVF clinics and their patients. It involves the testing of embryos for practically all other diseases which either are not monogenetic (i.e., inherited but through many different genes working together). Such polygenic risk scoring has made great progress in medical research for adults, - mostly as a tool to predict disease occurrence risk. But even in adults, polygenic risk screening (PGRS) is still considered an experimental test. Testing embryos is, moreover, even more complex and, therefore rather unsurprisingly, has by ESHRE and other professional organizations (including in the genetic field) induced to issue formal opinions that PGT-P is not ready for routine clinical practice and its use must at this moment be considered unethical.2,-5
All of this was an introduction to the various forms of PGT, which is necessary to understand here discussed recent publications regarding PGT: A paper – interestingly involving economists as well as ethicists, recently offered a review article attempting to address ethical, legal, and social issues regarding PGT which the authors felt academic debate often fails to reflect.6 For unclear reasons the authors reviewed only 506 publications between 1999 and 2019,while, especially in PGT-A and PGT-P, the most dramatic changes have taken place between after 2019. They, nevertheless, reached the correct conclusion that research on PGT does not align well with clinical practice. One can hardly argues with such a conclusion, though a more in-depth attack on the subject than this paper offered would have been more useful.
It is now for some time that we haven’t had the opportunity to discuss in these pages a real “self-fulfilling prophecy” paper. But, to our delight, a good example for such a paper – out of all places from Harvard –matched our criteria perfectly; it provided us a-priori with a study outcome that was virtually 100% predictable: That multiple embryo biopsies utilizing PGT-A may result in inferior clinical outcomes was really no revelation!7 Was there, even before publication of this paper, really anybody in the IVF field, who ever considered the possibility that an embryo undergoing yet another thaw-freeze-thaw cycle, would not be adversely affected in IVF outcome? What a waste of time and effort!
And then there were colleagues from the equally excellent Northwestern University program who apparently had nothing better to do than to use 22 studies published between 2008 and 2023 to assess the cost-effectiveness of PGT, with 64% of cases relating to PGT-A and 36% PGT-M. Again, to probably nobody’s surprise, the authors identified the fact that benefits of PGT-A have remained controversial (to say it mildly), therefore rendering a cost-effectiveness analysis “more difficult.”8 And this at a time when even the ASRM and SART have finally concluded that PGT-A does not improve IVF outcomes (see statement below).9
The Box below offers a word-by-word copy of an abstract, currently only electronically published, for a new combined 2024 ASRM & SART Practice Committee opinion which for the first time acknowledges what the CHR has been communicating in innumerable papers since 2007: PGT-A (its earlier name being preimplantation genetic screening, PGS) is ineffective in improving IVF outcomes. Finally, acknowledging this fact, one is left wondering why PGT-A then should be performed at all.
One is also left wondering why this opinion does not – loudly and clearly - also state that there now are equally solid data to demonstrate that in certain patient populations PGT-A not only does not offer outcome improvements, but, actually, reduces a woman’s chance of pregnancy and delivery.
Unfortunately, this new ASRM and SART opinion is, therefore, still missing its logical conclusions and treatment recommendations. A statement that, as a procedure – which at substantial costs (to return to above cited paper on the cost-effectiveness of PGT-A)does not offer patients any outcome benefits, PGT-A cannot be cost-effective and, therefore, should not be offered to patients, except in experimental settings and with full informed consent.
What also is missing is a reckoning by the infertility field why it had to take so many years to reach these conclusions, when the CHR already in 2015, after having offered several other important arguments why PGT-A should not be utilized, reported the first healthy euploid offspring after transfer by PGT-A as “abnormal” declared embryos.6

The use of preimplantation genetic testing for aneuploidy: A committee opinion
The use of preimplantation genetic testing for aneuploidy (PGT-A) in the United States has been increasing steadily. Moreover, the underlying technology used for 24-chromosome analysis continues to evolve rapidly. The value of PGT-A as a routine screening test for all patients undergoing in vitro fertilization has not been demonstrated. Although some earlier single-center studies reported higher live-birth rates after PGT-A in favorable prognosis patients, recent multicenter, randomized control trials in women with available blastocysts concluded that the overall pregnancy outcomes via frozen embryo transfer were similar between PGT-A and conventional in vitro fertilization. The value of PGT-A to lower the risk of clinical miscarriage is also unclear, although these studies have important limitations. This document replaces the document of the same name, last published in 2018.
How many embryos with substantial pregnancy potential have over all these years been disposed? How many infertile women have, therefore, been prevented from conceiving or - at least from conceiving with use of their own eggs - because they were advised that only donor eggs could help them conceive? The answer is mind-blowing.
One of the great things about medicine and science in general is the fact that, ultimately, the truth always prevails. Unfortunately, that does not help those women who, because of PGT-A, lost the chance of motherhood with use of their own eggs.
AND NEWS ON THE INCREASING POPULARITY OF PGT-P: Considering the disaster brought upon the infertility field by PGT-A, it is amazing to watch how history appears to repeat itself: Mimicking the beginning of PGT-A (then called PGS), the utilization of PGT-P in association with IVF has been starting slowly. Only a handful of genetic laboratories and their affiliated fertility clinics are currently offering this method of embryo testing and they do so despite – as noted above – widespread agreement among professional organizations that have issued relevant opinion papers (for references, see above) that PGT-P is scientifically not ready for clinical use in association with IVF and, therefore, defining its use as “unethical.”
That, in itself, is, of course, astonishing and one, once again, must wonder where are ASRM, SAR, ACOG and the FDA in addressing this issue? But reading the recent literature on the subject, the growth in PGT-P utilization should not surprise. PGT-P, indeed, increasingly appears to be promoted as the “next big thing” in IVF. For genetics laboratories and IVF clinics, PGT-P may, indeed, be destined to replace the expected utilization loss that may occur as a consequence of above noted recent ASRM opinion on PGT-A1 and, based on several recently published papers, could become the next big revenue source for the IVF field and the genetic testing industry.
Unsurprisingly, none of these papers offer any scientific outcome
data on PGT-P, - because such data really do not exist. Instead, several papers proclaimed to query the attitude toward the clinical utilization of PGT-P. So, for example, a U.S. group of investigators performed so-called semi-structured interviews with REIs and IVF patients and concluded that both groups held favorable views of screening human embryos for physical or psychiatric conditions, though REIs expressed certain “specific caveats.” In general, the paper described REIs as “more skeptical” than patients and patients as “more interested” in utilization of PGT-P than REIs.7 The authors, though, must be given credit for describing PGT-P at least as “uncertain terrain.”
Basically the same group of authors (though in different order) – published almost in parallel, though obviously performed later since this paper quotes the former - a more profound paper on the same subject in a different journal, basically investigating the same questions in the form of a survey in two distinct general study populations representing the general public, one stratified sample and one nonprobability sample.8 Whether the study populations for the two papers overlapped in some ways is unclear but the results were practically identical to above described first paper: 72.0% of 1,427 individuals in sample 1 approved of the use of PGT-P and even more among respondents to the survey undergoing IVF expressed interest in the procedure (81.9%). As noted in the earlier study, interest was especially high for embryo selection for physical and psychiatric issues (77.7% and 72.0%, respectively). One, however, also must note that there was significant concern about the potential of promoting eugenic practices in both surveyed groups (53.3% and 54.8%).
The authors concluded that, despite quite high societal concern, there was high approval and even higher interest regarding PGT-P reflected in public sentiment. Commenting on the clinical availability of PGT-P, the authors also pointed out that this practice is obviously unregulated and, therefore, should generate “informed dialogue and guidance.”
We, of course, could not agree more, - but are wondering how well-informed study participants really were about PGT-P?
That they were only poorly informed is almost certain, as most of their information, likely, came from parties interested in PGT-P. Considering how uninformed over decades even many REIs remained about PGT-A because they received most of their information from obviously “interested parties” in the performance of PGT-A (i.e., genetic testing laboratories and IVF clinics which usually shared in the revue generated from PGT-A), one must conclude that insufficient objective information about PGT-P must be even more prevalent (both in general populations and among REIs) because understanding the shortcomings of PGT-P is even more complex than understanding the shortcomings of PGT-A.
Interestingly even though all PGT-P related publications express serious concerns about its current clinical utilization, none of them calls for the discontinuation of these services. This, for example, also applies to a relatively recent Canadian paper.9 Concerns about PGT-P and the creation of “designer babies” have also been expressed on ethical and legal grounds, accompanied by demand for proper regulatory controls.10 But special credit is due to a group of international colleagues with lead author Antonio Capalbo from Italy, several among them originally among very committed proponents of universal PGT-A use, who recently published an excellent review article on the subject of PGT-P (or as named by them, polygenic embryo screening, PES) in Human Reproduction Update which we strongly recommend to everybody interested in PGT-P.11 These authors concluded and we are quoting: “Given the large number of practical limitations and possible harms, particularly unnecessary IVF treatments, and discarded viable embryos, PGT-P should be offered only within a research context,” while concomitantly stressing the need for unbiased genetic counseling. We couldn’t agree more!
And for those readers in general interested in the concept of PGRS, here is a very interesting paper in cell genomics, proposing a new method for developing enhanced ancestry-specific polygenic risk scores via Bayesian hierarchical modeling and ensemble learning leveraging summary statistics from genome-wide-association studies across multiple ancestry groups, which has the potential to reduce the current performance gap in polygenic risk prediction across different ancestry populations.12 If this sounds complicated, - it is! This, however, demonstrates how, even in adults PGRS still faces many unresolved issues before it can enter into routine medical care.
1. Practice Committee of the ASRM and SART. Fertil Steril 2024;May 18-S00150282(24)00241-3. Doi:10.1016/j.fertnstert.2024.04.013. Online ahead of print.
2. Gleicher et al., Hum Reprod 2022;37(12):2730-2734
3. Gleicher et al., Nat Med 2022;28(3):442-444
4. Gleicher et al., Trends Mol Med 2021;27(8):731-742
5. Nadgauda et al., Fertil Steril 2024;121(4):693-702
6. Gleicher et al., Fertil Steril 2015;104:e59
7. Barlevy et al., J Assist Reprod Genet 2024;41(5):1221-1231
8. Furrer et al., JAMA Network Open 2024;7(5):e2410832
9. Ginod P, Dahan MH. Reprod Biomed Online 2023
10. Rahim H. Petri-Flom Center at Harvard Law School. https://blog.petrieflom.
law.harvard.edu/2024/03/11/designer-babies-the-ethical-and-regulatory-implications-of-polygenic-embryo-screening/
11. Capalbo et al., Him Reprod Update 2024; dmae012.doi: 10.1093/humupd/ dmae012. Online ahead of print.
12. Jin et al., Cell Genomics 2024;4:100539
As the CHR and increasingly more other fertility clinics have started to transfer by PGT-A as “abnormal” diagnosed embryos, segmental chromosomal abnormalities have come into focus as the chromosomal abnormalities with – if transferred - practically normal pregnancy and live birth rates in comparison to “euploid” embryos. This should not surprise because segmental abnormalities quite frequently can be found in karyotypes of many completely normal individuals. Some, however, have been associated with congenital abnormalities and disease states.
One of those is the common 22q11.2 deletion which in the past has been associated with rare cono-truncal congenital heart defects.1 Now an international group of investigators reported in Science magazine that this deletion also mediates the risk of congenital menigomyocele.2 This, of course, does not mean that every embryo with this deletion will have a congenital heart lesion and/or meningomyelocele; but in counseling infertility patients who desire to transfer allegedly “abnormal” embryos with deletions, this newly described risk must now be included.
Coincidentally, Myriad Genetics just announced in an e-mail on April 16 results of a new study in Prental Diagnosis using Myrad’s cell-free DNA (pcfDNA) screen called Prequel® to diagnose 22q11.2 deletions, which incorporated fetal fraction amplification and confirmed the diagnosis in 100% of 22 patients with the deletion.3
1. Zhao et al., Am J Hum Genet 2020;106(1):26-40.
2. Vong et al., Science 2024;384(6695):584-590
3. Hammer et al., Prenat Diagn 2024; doi. 10.1002/pd.6562. Online ahead of print.
More interesting work from Magdalena Zernicka-Goetz, PhD
MZG has been one of the most important experimental reproductive biologists for many years. Initially located at Cambridge University in the U.K., she now oversees two laboratories, one in Cambridge and the other here in the U.S. at Caltech, CA, and her research creativity appears to have no borders. Her lab’s latest accomplishment is a paper in Cell in which the investigators demonstrate that the first two blastomeres of a human embryo contribute unequally to the developing embryo.
Through labeling and life imaging the study revealed that a majority of the future embryo originates form only one of first two blastomeres. At the 8-cell stage, moreover, descendants of this blastomere add more asymmetric divisions which then generate the small number of founding cells for the epiblast. These early asymmetric division are a bottleneck controlling the embryo’s clonal composition, - of course including aneuploidy, a subject we will return to.
With the majority of the epiblast (inner cell mass, ICM) origination from only one of the original two first blastomeres, only 1-3 cells (much fewer than in the mouse) become internalized at 8-16-cell stage and are more frequently derived from the first cell to divide at 2-cell stage.
As the authors point out, genomic instability and the resulting aneuploidy have previously been suggested to be drivers of asymmetric 2-cell clonal contribution in human embryos but, interestingly, most of the embryos investigated appeared to be euploid. Some aneuploid embryos did not demonstrate any specific clonal bias toward ICM or trophectoderm. These observations, thus, contradict the prevalent believe that genomic instability causes the asymmetrical lineage association.
1. Junyent et al., Cell 2024;187:1-17
Investigators from the University of Michigan, Ann Arbor, recently published a complete cellular atlas of the human ovary by profiling over 18,000 genes in 257 regions of 2 premenopausal donors. In addition, the investigators sequenced 21,198 individual cells from 3 more donors of ovaries, while identifying 4 major cell types in the ovary and 4 immune cell subtypes and distinct gene activities for oocytes, theca cells, and granulosa cells.1 These discoveries, in turn, allowed for the establishment of panels of specific genes for all three of these ovarian components, allowing for a better understanding of what drives follicle development. Moreover, the study also discovered previously unknown variations of hormone and extracellular matrix remodeling activities. These findings can be expected to lead to further interesting information about normal and abnormal ovarian function.
REFERENCE
1. Jones ASK, et al., Sci Adv 2024; 10(14)::eadm7506
The endometrium
In basically a rat-model study Chinese investigators demonstrated the ability to transplant endometrium between animals.1 While endometrium survived well, it formed a fibrotic zone (scar) between transplant and recipient which the authors do not further define in its consequences. Endometrial thickness and endometrial gland numbers to were similar to sham-operated controls but vascular density at 8 weeks was reduced. Transplanted endometrium also retained expression of leukemia inhibitory factor (LIF) and vascular endothelial growth factor. A mean of 5.0 fetuses developed in the horn that
received a transplant and full-term live fetuses were delivered.
To our best knowledge, these were the first attempts at full endometrial transplants and, while outcomes obviously were not perfect, they clearly looked promising. The authors, moreover, studied he possibility of obtaining transplantable human endometrium and reported apparent success in doing so. An interesting beginning!
And since we are already talking about human endometrium and LIF, as another recently published study demonstrated endometrial receptivity appears to be enhanced by extracellular seminal vesicles from semen (those mini-vesicles nowadays appear to be everywhere) through LIF.2 More specifically the authors of this paper suggest seminal extracellular vesicles promote expression and secretion of LIF in human endometrial cells which, in turn, enhances endometrial receptivity. Could this be the reason why some old-timers in IVF to this day recommend inseminations at time of retrieval?
1. Tian wt al, Reprod Biomed Online 2024;48(2):103370
2. Wang et al., Endocrinol 2024;165(5):bqae035
At least in the rat, such trans plants already work, as Japanese investigators recently reported (only the Japanese can pull something like this off!) and they did it by transplanting kidney tissue from one rat fetus to another one, as reported in a news article in Nature magazine.1 The goal is one day to transplant fetal pig kidneys in utero into human fetuses who don’t have functioning kidneys. The investigators had previously already transplanted mouse kidney tissue into rats to test whether xenotransplants would survive, and they did. They also had transplanted pig-to-pig 38 pig fetuses into 11 sows, and 18 piglets were born. The next step will involve monkeys.
And Mitinori Saitou’s laboratory at Kyoto University reported in Nature the in vitro reconstitution of epigenetic reprogramming in the human germ line.2 This is a little complicated to explain: As the authors note, epigenetic reprogramming resets prenatal memories and differentiates primordial germ cell (PVGs) into mitotic pro-spermatogonia Male precursor cells) and/or oogonia (female precursor cells), has, however, remained challenging in vitro (outside the body). And this is exactly what Saitou’s group now reported to have established in very robust ways. This paper, therefore, represents yet another (among many) milestone for this laboratory and this laboratory head, and through generation of pre-spermatogonia and pre-oogonia-like cells represents a very important step in what currently has become a central goal of reproductive biology, - in vitro gametogenesis.
REFERENCES
1. Mallapaty S. Nature 2024;629:267-268
2. Murase et al., Nature 2024; doi: 10.1038/s41586-024-07526-7. Online ahead of print

The Center for Human Reproduction has a long and impressive history of IVF innovations. The team works closely with people from all walks of life, such as single parents, couples, LGBTQ+ community members, or those from overseas. The team has helped more than 18,000 clients become pregnant, many through IVF. We are one of the only fertility clinics that adjusts your treatment plan daily based on your body’s response.
What prospective patients should know about the CHR:
We guarantee first appointments within 2 weeks. We reserve daily spots for emergency same-day appointments.
We offer per-need appointments after regular hours (5:00 PM) and on weekends.
The CHR is open 7 days a week and 365 days a year, including holidays. Contact us:
Appointments: (212) 994-4400
https://www.centerforhumanreprod.com







The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400. The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiolo- gy, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved. For letters to the editor, comments, and suggestions, please contact jbeebe@thechr.com. For all advertisements or sponsorships in The VOICE , please contact arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR.