


















We welcome you to the June issue of The VOICE, as always, the last issue of the academic year before our publishing break during July and August. There have been years where important news mandated special issues during those two months, but for that to happen, the news must really be of immediate importance. For that reason, we already want to take the opportunity to wish all of our readers a wonderful and hopefully relaxing and peaceful summer. We all deserve it! We are looking forward to welcoming you back in early September for the new 2023/2024 academic year.
For the CHR, the summer months are never very quiet since many among our long-distance patients like to use their vacation time during these two months for treatment cycles and, considering what appears to be waiting in the wings, and the absence of any negative surprises, we expect the CHR to be especially busy this year. Since the CHR serves so many long-distance patients (over 60% of our patients), the center, in contrast to many other fertility centers, never closes down during the summer months. Indeed, we gladly offer coverage for patients of other fertility centers during this time period if their patients require services.
Coming to this issue of The VOICE, we, as always, offer a broad potpourri of article subjects for our very diverse mailing list of over 80,000 former, as well as current patients, hopefully, many future patients from all over the world, clinical and research colleagues and, not to be forgotten, members of print, social and all other forms of local, national, and international media. Here are a few pointers: How can a young woman from early on know that she may be at risk for infertility? We in this issue offer the answer.
Especially these days, when most women already at young ages, often for decades of uninterrupted use initiate hormonal contraceptives, they have almost no possibility of knowing if, quietly, a fertility problem is developing. This is not only a hypothetical scenario; it happens to large numbers of women all the time when they, only in their mid- to late-30s, coming off birth control for the first time, are surprised to discover that, for some reason, they cannot conceive. The CHR’s investigators, already several years ago patented a diagnostic algorithm that, based on only a handful of historical facts and few very basic blood tests, allows for early diagnosis of women at risk for premature ovarian aging (POA), the by-far most frequent underlying cause for these surprises. Indeed, no less than 10% of women of all races and ethnic backgrounds end up suffering from POA. They called the program, “What’s my fertility?”
As one of the world’s leading IVF centers “of last resort,” the CHR has no choice but to develop new treatment approaches because doing the same things that failed patients repeatedly before coming to the CHR, of course, makes little sense. In the May issue of The VOICE, we described how going through IVF at the CHR differs from most other fertility centers. In this issue, in the second half of this series of articles, we describe how treatments other than IVF differ at the CHR.
We in this issue also draw attention again to the clinical trials of intra-ovarian PRP injections at the CHR. Three studies are currently ongoing addressing this treatment, by some also called “ovarian rejuvenation,” under the heading PRP-I, II, and III. A first preliminary report on PRP-II was already published¹ (though the study is continuing). PRP-I and PRP-III , however, still need additional patients. PRP-I is restricted to women with early menopause before age 40, while PRP-III is open to all women up to age 45.
Another interesting subject addressed in this issue is where IVF centers and commercial frozen egg banks in the U.S. increasingly are getting their donor eggs from. This is a story that may surprise many and should worry even more potential recipients of these donor eggs. It is also an issue that should start attracting more media and government scrutiny. In his monthly essay, the CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, again addresses the increasing utilization of “add-ons” to IVF, - but does so from a new statistical vantage point that addresses what happens to IVF cycle outcomes when “add-ons” are added on top of each other.
Of course, we as always answer questions we receive from patients and/or readers of The VOICE and, as promised last month, are also offering a preliminary take on the preliminary 2020/2021 SART registry outcome data of national IVF cycles. Finally, as is by now tradition, our monthly review of interesting papers that relate to reproductive medicine in one way or the other, either because they are interesting or, as a warning, because they are just plainly bad.
REFERENCE
1. Barad et al. Hum Reprod Open 2022;(3):hoac027
Edmond Confino, MD, Professor Emeritus of Obstetrics and Gynecology at the Feinberg School of Medicine, Northwestern University in Chicago, Illinois, passed away on May 25, 2023, at the age of 71. His family emigrated from Bulgaria to Israel in 1952. He received his MD degree from Tel Aviv University in 1977 and completed a residency in OB/GYN in 1983.

He joined the OB/GYN Department at Mount Sinai Hospital in Chicago, under the chairmanship of Norbert Gleicher, MD, in 1985, and with it, the CHR, which in those days was located in Chicago. He already in 1986 co-authored with Dr. Gleicher his first paper.1 By the time he left to pursue a fellowship in Reproductive Endocrinology and Infertility at Rush Medical College in 1990, 37 more co-authored papers had been published.
In 1993, he joined the Division of Reproductive Endocrinology and Infertility at Northwestern, where he practiced until his retirement in 2018,- though he continued teaching activities as an Emeritus Professor. Shortly before his retirement, the university established an endowed chair in his name.
He is survived by his wife of 32 years, Maryann Hayes , his two adult sons, Rafael and Cary, and his brother Dr. Alex Confino, who resides in Venice, Italy.
So, you just turned 36, - happily married for six years and are clearly at the ascent of a promising professional career. Until recently, the idea of having children barely came up, of course, unless your parents came by for a visit. Your husband, like you, was also pursuing a busy career, and life was good the way it was. Suddenly, something changed: both of you started noticing more pregnant women in the neighborhood, and how about all those cute little babies in their carriages or in the arms of their parents? Suddenly, having a baby like this of your own, appeared almost more important than anything else in life. A diagnosis was easy to reach: You finally succumbed to the most basic of all instincts, - the drive to reproduce. It suddenly seemed so obvious that it was time to throw away your birth control pills and, while you missed the daily routine over 20 years of taking the pill when you brushed your teeth in the morning, it felt like a relief.

You and your husband tried for three months, - six months, - and nothing happened. Finally, you decided to see your gynecologist, and she ordered some blood tests. The telephone call you just hung up on was your gynecologist’s, informing you of the results. You were shocked, - indeed more than shocked: How was it possible that, for all of your life blessed with impeccable health and committed to a healthy lifestyle of no smoking or drinking, no drugs, and always watching what you ate, you out of all people, could have a problem conceiving? Yet, this was exactly what your test results were showing: You, indeed, have a fertility problem!
According to the World Health Organization’s latest bulletin, worldwide, 1 in 6 people are affected by infertility.1 Though statistics in the literature somewhat vary and in recent years appear in more flux than before, in couples after 12 months of unsuccessful attempts to conceive defined as “infertile,” roughly 55% demonstrate one or more female problems, ca. 45% are found to have one or more male problems, and in 25% of couples there is at least one problem on each side. On both sides, multiple different causes can lead to infertility. Therefore, to conceive, both partners must be fertile. Another way of saying the same thing is that one or more problems in one or both of the partners can render a couple infertile. You having a fertility problem, does not mean your husband does not have a problem as well.
In both partners, infertility can have different causes, and their correct discovery is always an essential first step in determining the best treatment for a couple. How deeply one searches, of course, will determine how accurate a final diagnosis will be. Therefore, The CHR does not subscribe to the still widely used diagnosis of “unexplained infertility” in textbooks and infertility literature and considers this diagnosis an oxymoron.2 That something “unexplained” can represent a diagnosis, logically simply does not make any sense because what, ultimately, determines the accuracy and completeness of an infertility diagnosis is only dependent on how deep one digs in trying to reach a real diagnosis. Whenever a patient presents to The CHR with a diagnosis of “unexplained infertility,” we know that something has been overlooked in the diagnostic work-up of the couple.
Since reaching a correct diagnosis is much more complex in the female than in the male, most of the time the overlooked problem will be found in the female. Male fertility is relatively easy to assess quickly: If the male does not have a history of a functional sexual disorder, it basically comes down to his semen analysis. If the semen analysis is normal, he in almost 100% of cases (there are some very rare exceptions) can be ruled out as a contributing partner to a couple’s infertility. On the woman’s side, the diagnostic work-up is much more involved. Even more than in the male, obtaining a detailed past medical history is crucial and, indeed, will in most cases immediately point in the right direction.
For example, a history of past tubal infection or endometriosis will immediately suggest that there may be a problem with the fallopian tubes. A history of fibroids in the uterus may suggest an implantation or miscarriage problem.
However, the purpose of this article is not to rehash the many well-known underlying causes for female infertility which can be easily deduced either because of medical history or symptoms. What we in this article are trying to point out is the fact that one of the most important diagnoses in female infertility, to this day is frequently overlooked for years by women as well as their gynecologists, even though this is a condition that affects a full 10% of all women, independent of race and/or ethnicity. Indeed, even once women affected by this condition reach fertility treatments in fertility clinics, this condition is, still, often overlooked, and this condition is premature ovarian aging (POA), by some also called occult primary ovarian insufficiency (oPOI). The word “occult” in the latter term comes from the fact that this condition sneaks up on women quietly and mostly without symptoms and, if there are minimal symptoms, they usually are covered up by hormonal means of birth control.3

Consequently, young women usually have absolutely no idea that they are among those 10% destined to develop POA/oPOI as they go unconcerned through their young lives, - until, as noted in above cited case, usually sometimes in their 30s, they come off their hormonal contraception and try to conceive.
POA/oPOI is a very easily defined condition: Affected women have fewer eggs left in their ovaries than 90% of their age-peers.
It is also a progressive condition that we, so far, have not learned to arrest yet. This means that affected women at young ages are perfectly fertile, but once they fall below a certain threshold in remaining egg numbers, they become infertile. Though the age when this will happen can vary, age 35 is often the approximate threshold. The diagnosis of POA/oPOI did not exist 50 years ago because women had children so much earlier in life. If by approximately age 35, they no longer fell pregnant, they often welcomed the change since they already had several children. With many women these days not even thinking about children until the mid-30s, POA/oPOI has exploded in frequency as a cause of infertility; yet, as noted above, even in competent fertility clinics, the diagnosis is still often overlooked and patients, not infrequently, are considered “unexplained.”
As in the above-described case, large numbers of women, later in life deciding to try to conceive are surprised to find out that they have a fertility problem. Also, a big majority among them are women with POA/oPOI. They are disproportionally represented because of the quiet nature in which this condition progresses in contrast to many other infertility-causing conditions. If diagnosed with POA/oPOI only at advanced ages, treatment options are limited, with most women ending up in IVF. Many, indeed, are lucky if this is the case; if their condition has progressed too far, it may require the use of third-party egg donation.
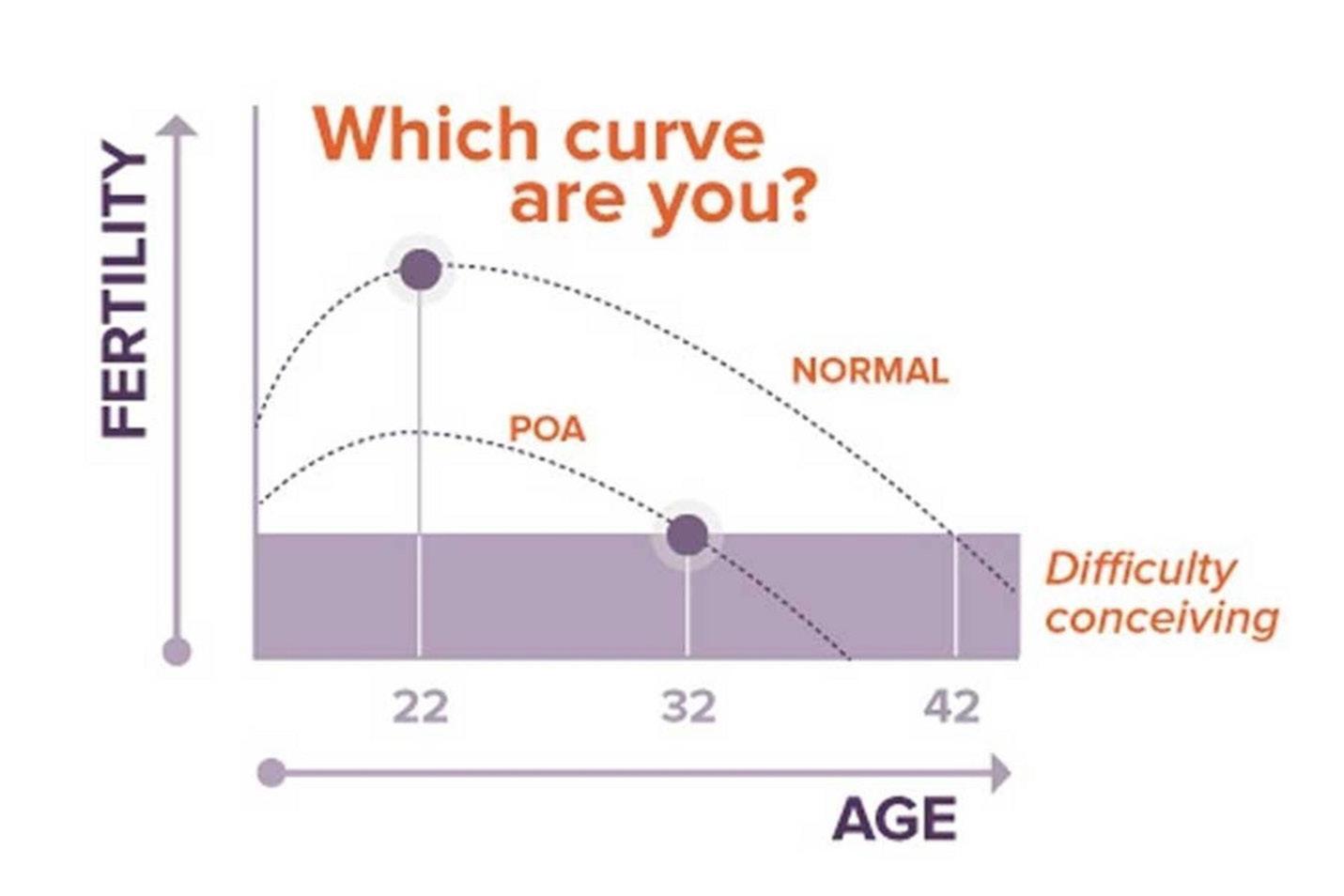
This fact alone raises the question of whether there is something that can be done for women at much younger ages that will allow them to recognize that they may be at increased risk for POA/ oPOI. Since the CHR has been interested in the investigation of POA/oPOI-related issues for decades (and in the process has become the likely leading fertility center in the world in managing POA/oPOI patients), the center’s investigators have been pursuing the idea of early risk assessment in young women for many years.4 They, indeed, developed a very simple algorithm based on a small number of historical facts and an even smaller number of blood tests, earning a U.S. patent in the effort. Based on this algorithm, young women very quickly can be separated into, (i) likely not at risk; (ii) at risk; and (iii) already affected.
Young women found not to be at risk can safely go on hormonal contraceptives for 3-5 years before being retested, while if women defined at risk do initiate hormonal contraception, they are advised to come off them every 1-2 years and be retested in their functional ovarian reserve (FOR).5-7 This program is available at The CHR under the name “What’s my fertility?,” and we strongly recommend it to young women, especially if they are planning to start using hormonal contraceptives for a prolonged time period. As already noted, we have not yet learned to arrest POA/oPOI; but, if we can advise young women that they are at increased risk to develop the condition, or already have evidence of early-stage POA/oPOI, those women, at least, have additional options: They can decide to advance their plans for childbearing into younger ages or they can make a timely effort to freeze their eggs.
Egg freezing is quickly gaining popularity among young women as a general concept. However, what most young women do not understand at all is that the urgency to freeze eggs even at relatively young ages vary. If you are on the verge of developing POA/oPOI the urgency is, of course, much bigger than when your screening suggests no increased risk. If you, your girlfriend, your sister, your daughter, or your granddaughter is considering freezing her eggs, consider “What’s my fertility?” – testing at the CHR first!
1. WHO. https://www.who.int/news/ item/04-04-2023-1-in-6-people-globally-affected-by-infertility
2. Gleicher N, Barad D. Hum Reprod 2006;21(8):1951-1955
3. Nelson LM. N Engl J Med 2009;;360(6):606-614
4. Gleicher et al., Reprod Biol Endocrinol 2015;13:34
5. Kushnir et al., Reprod Biomed Online 2014;29(5):527-529
6. https://www.youtube.com/watch?v=S7NNBLywukw
7. https://www.youtube.com/watch?v=S7NNBLywukw
We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@ thechr.com. All submissions are considered confidential correspondence.
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

Being one of the world’s “fertility centers of last resort” with over 90% of the CHR’s new patients having failed prior IVF cycles at other IVF clinics – often many times over and at multiple clinics, the CHR must approach their treatments differently, because why would the same treatments that failed elsewhere, suddenly, work at the CHR? Therefore, we felt that it was time to explain to our readers how fertility treatments differ at the CHR in comparison to most other fertility centers and started the discussion in last month’s May issue of The VOICE by addressing how the center’s IVF practice varied in comparison to most other fertility clinics. In this issue, we continue this discussion by addressing other principal differences, which we feel are responsible for the CHR’s still excellent results in what likely represents the most adversely selected patient population of any fertility center in the U.S., - if not the
Before addressing this subject, it is important to point out that the CHR has several potential economic conflicts in addressing some of the subjects in this article. These conflicts relate to the ownership of several U.S. user-patents by the CHR and some of its employees, as well as the receipt of royalty payments from some of these patents by the CHR and/or some of its employees from Fertility Nutraceuticals, LLC. Most of these patents claim treatment benefits from supplementation of selected infertile women with androgen hormones, especially dehydroepiandrosterone (DHEA) but also other androgens. Other patents refer to anti-Müllerian hormone treatments in infertile women. Employees of the CHR and the CHR also own shares in Fertility Nutraceuticals, LLC. Therefore, we advised our readers to consider these potential conflicts especially when reading this article about the clinical utilization of androgens and, especially, of DHEA.
That all fertility treatments start with getting a history from the patient, of course, does not, surprise, since this is the first step in meeting physicians in all medical specialties. How this history may be obtained will, of course, differ and, like everything else in medicine, will be greatly influenced by our routine behavioral patterns as physicians, - but also by our inherent biases. Especially the second point is of major importance because, to offer just one example, if a physician does not believe that the female immune system plays an essential role in allowing pregnancy to occur (unfortunately, still a widely held believe among many colleagues), obtaining a patient’s past medical history will, likely, avoid or at least deemphasize any inquiry regarding the patient’s immune system. At the CHR, we are trying very hard to avoid such subconscious omissions and our intake consultations, therefore, are usually quite lengthy.
Such perception biases, of course, also greatly depend on how physicians follow the medical literature and how they interpret what they read. It is no secret that physicians have been following the literature less and less through medical journals, leading a prominent cardiologist to wonder “whether anyone still reads medical journals at all?”1 Instead, physicians in all medical specialties, unfortunately, increasingly rely on usually radically shortened summaries and/or commentaries, thereby removing physicians from the responsibility to judge the scientific validity of published information, - of course an essential step in how new literature should be consumed. Reliance on data interpretations by others then, as repeatedly discussed before in this newsletter, fosters the power of opinion leaders (i.e., so called “experts”) to greatly influence the debate on a subject, enhancing “groupthink.”
However, this is not how the CHR practices medicine. Indeed, it is steadily expanding its reading list of journals, probably best documented by the monthly Review of the Literature section in this newsletter. Therefore the CHR’s staff is not only always up to date on newest developments and insights gained on relevant subjects, even if outside of the infertility field, but critically assesses every publication. As a result, as repeatedly noted in these pages, the CHR does not automatically follow all the “fashions of the moment,” as many other clinics do but, at the same time, is in the position to integrate new knowledge that may be beneficial quickly.
The website of almost every fertility clinic advertises research and alleged publications of its physicians as evidence of its academic pursuits, but a closer look at best demonstrates not more than a handful of publications, often during residency or fellowship (and not since) and mostly in low-impact journals. The truth is that the number of notable research programs in fertility centers is small,
Continued on page 10
Continued on page 12
Continued from page 11
and usually restricted to clinics in academic hospital settings and/or associated with academic institutions. The CHR is one of the very few exceptions to this rule, as demonstrated not by a handful publications in mediocre journals, but by over 500 publications in medical journals of the specialty but, often, also in some of the leading general medical and science journals with very high impact factors. Even among academic programs, there are only relatively few who can compete with the CHR’s quantitative and qualitative research output to the literature. Over the 42 years of its existence, the CHR, therefore, contributed many important “firsts” to the field of reproductive endocrinology and infertility (see the Table below).
Year Subject Reference
1981 Common denominators of pregnancy and malignancy
1983 1st transvaginal egg retrieval in the world
1984 NK cells in pregnancy
1984 T-cells in preeclampsia
1985 Lupus anticoagulant syndrome in habitual aborters
1986 1st transvaginal tubal catheterization
1986 First uterine transplant model in rabbit
1987 Endometriosis as an autoimmune disease
1988 1st description of the Reproductive Autoimmune Failure
Gleicher N, Siegel I. Progr Clin Biol 1981;70:93-114
Gleicher et al., Lancet 1983;2(8348):508-509
Toder et al., J Clin Lab Immunol 1984;14(3):123-127
Idem, J Clin Lab Immunol 1984:14(3):149-154
Siegel I, Gleicher N. Am J Obstet Gynecol 1984:149:583
Gleicher N, Frieberg J. JAMA 1985;253(22):3278-3281
Confino et al., Fertil Steril 1986;46(5):963-966
Confino et al., Int J Obstet Gynecol 1986;24(4):321-325
Gleicher et al., Obstet Gynecol 1987;25(2):155-157
Gleicher N, el-Roei A. Am J Obstet Gynecol 1988;159:223 Syndrome (RAFS)
1990 1st multicenter study of transvaginal tubal re-canulation
1991 Autoimmunity in reproductive failure
2005 1st report on increased oocyte yields after DHEA
2006 1st report of age-based ovarian stimulation
2008 CHR’s 1st critical article on PGS/PGT-A
Confino et al., JAMA 1990;264(16):2079-2082
Gleicher N. Ann NY Acad Sci 1991;626:537-544
Barad DH, Gleicher N. Fertil Steil 2005.83(6):1888-1889
Gleicher N, Barad DH. Fertil Steril 2006;86(6):1621-1625
Gleicher er al., Fertil Steril 2008;89(4):780-788
2009 CHR’s first paper on the importance of the FMR1 gene Gleicher et al., Reprod Sci 2009;16(5):462-467
2009 Declining miscarriage rates after DHEA
2010 Race/ethnicity differences in the FMR1 gene
Gleicher et al., Reprod Biol Endocrinol 2009;7:108
Gleicher et al., Reprod Biomed Online 2010;20(4):485-491
2010 All autoimmune diseases are characterized by premature Gleicher N. Clin Rev Allergy Immunol 39(3):194-206 deliveries
2011 1st successful treatment of thin endometrium with G-CSF Gleicher et al., Fertil Steril 2011;95(6):2123.e13-7
2011 1st association of FMR1 genotypes with different Gleicher et al., PLoS One 2011;6(4):e18781 races/ethnicity on IVF outcomes
2012 1st AMH and FSH ratios per retrieved oocyte Gleicher et al JCEM 2012;97(3):995-1004
2013 1st report of association of hypoandrogenism with low Gleicher et al., Hum Reprod 2013;28(4):1084-1091
functional ovarian reserve
2013 1st report on clinical relevance of FSH/AMH relationship in Gleicher et al., JCEM 2013;98(5):2136-2145
Infertile women
2013 1st proposition of therapeutic intervention in early follicle Gleicher et al., Endocrinology 2013;154(10):3498=3501
maturation as a new treatment principle
2014 Biological explanation of how androgens regulate follicle Sen et al., Proc Nat Acad Sci USA 2014;111(8):3008-3013
development
2014 How endocrine autoimmune diseases interact with female Sen et al., Nat Rev Endocrinol 2014;10(1):37-50
infertility
2014 1st suggestion to screen ovarian reserve before long-term Kushnir et al., Reprod Biomed Online 2014;29(5):527-529
hormonal contraception initiation
2014 Further clarification of androgen action in ovaries. Prizante et al., J Endocrinol 2014;222(3):R141-151
2015 1st report of normal live births following transfer of Gleicher et al., Fertil Steril 2015;104:e59; “chromosomal abnormal” embryos
2015 Genetics of androgen metabolism in hypo-androgenic Shohat-Tal, et al. Nat Rev Endocrinol 2015;11(7):429-441
infertile women
2015 1st proposal to prospectively assess premature ovarian Gleicher et al., Reprod Biol Endocrinol 2015;13:29
aging risk in young women
2015 1st report that poor prognosis patients at least up to age Gleicher et al., Fertil Steril 2015;104(6):1435-1441
45 can have better live birth rates than widely assumed
2015 1st report that women with CGG repeats < 25 demonstrate Gleicher N, Transl Res 2015;166(5):502-7.e1-2
early declines in ovarian reserve
2015 1st report of premature follicle maturation with advancing Wu et al., J Endocrinol 2015;226(3):167-180 age mandating earlier retrievals
2015 1st report that frozen donor eggs produce lower pregnancy Kushnir et al., JAMA 2015;314(6):623-624 rate than fresh donor eggs
2016 1st report that oocyte scoring is predictive and additive Lazzaron-Tealdi et al., PLoS One 2015;10(120:e0143632 to embryo scoring
2016 1st report on adverse effects of embryo banking on Kushnir et al., PLoS One 2016;11(5):e0154620 national IVF outcome reporting
2016 1st report that hypo-androgenism in infertility is usual of Gleicher et al., Reprod Biol Endocrinol 2016;14:23 adrenal origin
2016 1st report that hypo-androgenism in infertile women is Gleicher et al., J Steroid Biochem Mol Biol 2016;158:82-89 also associated with low cortisol
2016 1st report of recue IVM in women with poor ovarian reserve Lee et al., Endocrine 2016;52(1):165-171
2016 New information on how AMH intracellularly regulates Hayes et al., Mol Cell Endocrinol 2016;433:56-65 follicular development
2016 1st report that PGS is compromised by degree of mosaicism Gleicher et al., Reprod Biol Endocrinol 2016;14(1):54 in embryos
2016 1st prospectively randomized study disputing alleged IVF Vega et al., Reprod Biomed Online 2016;33(3):370-375 outcome advantages from time lapse systems
2016 1st report that In national U.S. data PGS actually reduces Kushir et al., Fertil Steril 2016;106(1):75-79 pregnancy chances
2016 1st report of an inverted U effect of increasing AMH levels Gleicher et al., J Transl Med 2016;14(1):172 on IVF pregnancy and live birth rates
2017 1st report redefining PCOS phenotype-D as an often- Gleicher et al., J Steoid Biochem Mol Biol 2017;167:144-152 overlooked major infertility diagnosis
2017 1st report that a single biopsy mathematically can in Gleicher et al., Reprod Bio Endocrinol 2017;15(1):33 PGT-A never represent a complete embryo
2017 1st suggestion to include insufficiency of the adrenal Gleicher et al., JCEM 2017102(9):3560-3570 zona reticularis in definition of adrenal function
2018 1st definition of HIER (Highly Individualized Egg retrieval) Wu et al., J Ovarian Res 2018;11(1):23
2018 The CHR reports the so-far oldest woman in the world to Gleicher et al., Reprod Biomed Online 2018;37(2):172-177 conceive with her own eggs
2018 Further insights into AMH expression in granulosa cells. Roy et al., Endocrinology 2018;19(9):3433-3445
2018 1st report of reduced RNA expression in women with Wang et al., PLoS One 2018; 13(12):e0209309 low CGG repeats (<26) in the FMR1 gene
2019 1st report that DHEA improves sexual function in Kushnir et al., Endocrine 2019;6363(3):632-638 premenopausal infertile women with low androgens
2019 The association of “add ons” to IVF with declining Gleicher et al., Hum Reprod Open 2019;2019(3):hoz017
Worldwide live birth rates in IVF
2020 1st ever conflict resolution analysis in reproductive
Mochizuki L, Gleicher N. JARG 2020;37(3):669-672 medicine to resolve PGT-A disagreements
2021 The “rebound effect: may rescue IVF cycles in no-responders Gleicher et al.,. J Ovarian Res 2021;14(1):11
2021 1st report of secondary ovarian insufficiency due to Gleicher et al., Endocrine 2021;19(1):23 adrenal hypo-androgenism
2021 1st reported evidence in human embryos for the ability Yang et al. Nat Cell Biol 2021 72(1):260-267 to self-correct aneuploidy downstream from blastocyst
2021 Cytoplasmic granulation patterns in poor prognosis patients Hu et al., Fertil Steril 2021;116(2):431-443 and young egg donors (2 papers)
Idem: Fertil Steril 2021;116(5):1330-1340
2022 The uncertain science of preimplantation and prenatal Nat Med 2022;28(3):442-444 genetic testing.
2022 Further molecular insights into normal ovarian function Roy et al., Endocrinology 2022;163(5):bqac047 and fertility
2022 50 consecutive patients who had chromosomal “abnormal” Barad et al., Hum Reprod 202237(6):1194-1206 embryos transferred by CHR, previously refused transfer
2022 Reconsidering PCOS based on the new understanding of Gleicher et al., Biomedicines 2022;10(7):1505 phenotype D
*out of over 500 peer-reviewed publications overall
** in bold type, papers that have or will contribute to changes in clinical practice worldwide
Probably quite an exaggeration, a guideline from the Canadian Fertility and Andrology Society claimed that “as many as 50% of couples seeking infertility care present with a diagnosis of “unexplained infertility” (UEI),2 a diagnosis the CHR does not accept as a real diagnosis,3,4 even though it, to this day, is considered a valid infertility diagnosis.
A relatively recent issue of Seminars in Reproductive Medicines, edited by Ben W. Mol and Roger J. Hart, both well-respected investigators in reproductive medicine, was fully dedicated to UEI. In their introduction to the issue, the two editors define this condition as “the non-occurrence of conception after 12 months of unprotected intercourse without identification of cause.” Actually highlighting the absurdity of this diagnosis, they then continue their introduction by pointing out motivations for the various articles in the special issue. For example, the role of sperm and oocytes in unexplained infertility is presented in the article “to explore the more subtle perturbations in gamete function.”5 Even these two, otherwise among the most highly aware and logical investigators in reproductive medicine, thus quite astonishingly, do not perceive the very obvious contradiction between their definition of UEI and a discussion of a “role of sperm and oocyte in UEI.”
The reason why the CHR rejects the concept of UEI is, therefore, obvious: it is an oxymoron! Whether one or more real tentative diagnoses will be made in a couple, always depends on how hard one looks and how deep one digs. If one looks hard enough, one will always find possible reasons why a couple does not conceive. The CHR, of course, always searches hard and deep! One, however, must give Mol and Hart credit at least in one regard: In the concluding paragraph of their introduction, they do reach the correct conclusion that, “the better we understand infertility, the less the contribution of UEI will be.”5 They, of course, are correct with that statement, though it also invited a reminder regarding the relativity of every infertility diagnosis. Since infertility often is multifactorial, it is essential to understand that, in contrast to a diagnosis of sterility, infertility diagnoses are always “presumed” diagnoses.
Like all medical fields, infertility practice is characterized by many ingenious new ideas by many brilliant practitioners in the field. Again, like in any other specialty, many of these seemingly brilliant ideas in clinical practice disappoint. Frequently a subject before in this newsletter, reproductive medicine appears to have lower thresholds for the integration of such “fashions of the moment” into routine clinical practice than other specialties. The reason may be, that in many other areas of medicine, new ideas often require new pharmacological compounds and/or new equipment, both subject to regulatory approval processes.
An idea like “mild” ovarian stimulation in infertility practice, extended embryo culture to blastocyst for everybody, elective single embryo transfer, closed incubation of embryos with time-lapse, and - yes – preimplantation genetic diagnosis for aneuploidy (PGT-A), in contrast, required no such approval. All it took was a good-sounding idea propagated in unvalidated publications, and practitioners started integrating these new treatments (and many others) into their clinical practices. Most remarkably, many of these new treatments/diagnostic tests retained their popularity, even once serious new evidence argued against their use.
So-called “mild” stimulation holds a special place among those “fashions of the moment” as one of the first such treatments reaching worldwide acceptance after initially being proposed by a group of Japanese clinicians at the Kato Ladie’s Clinic in Tokyo, Japan,6 even though, on purely logical grounds, mild stimulation for an overwhelming majority of patients, for one simple reason, never made sense: Except for female age, the obtained number of eggs and embryos in one IVF cycle, is the most important predictor pregnancy and live birth rates, as years ago reported by CHR investigators7 and just recently again confirmed8
“The reason why the CHR rejects the concept of UEI is, therefore, obvious: it is an oxymoron! Whether one or more real tentative diagnoses will be made in a couple, always depends on how hard one looks and how deep one digs. ”
and commented on.9 CHR investigators also already in 2016 reported in a prospectively randomized study that, even in good prognosis patients, mild stimulation actually reduced pregnancy chances.10

Though not even a single appropriately performed study in the literature could ever demonstrate non-inferiority (none, of course, demonstrated superiority), mild stimulation is, still, widely advocated, mostly based on alleged (and universally unproven) secondary gains, like better “patient-friendliness,” lower costs, less ovarian hyperstimulation, etc. The above-cited, just published study in Fertility & Sterility8 established beyond reasonable doubt that fertility clinics, considering the achievement of safe pregnancy as their principal goal, have no good reason to use mild ovarian stimulation except, maybe, in young women with excellent functional ovarian reserve who want only one child. Especially in women who are desirous of more than one child, there is never a reason to choose a mild stimulation cycle which, a-priori, will produce fewer eggs and embryos and, therefore, will reduce future chances from cryopreserved excessive embryos from a first cycle.
The CHR does not follow “fashion”, but the best available evidence at any given moment. As frequently noted in this newsletter, the best evidence, of course, changes all the time, mandating the continuous review of newly published data, as has been practice at the CHR for decades, and as our readers can testify, are presented every month in this newsletter.
1. Packer M. MedPage Today 2018; https://www.medpagetoday.com/opinion/revolutionandrevelation/72029
2. Buckett W, Sierra S. Reprod Biomed Online 2019;39(4):633-640
3. Gleicher N, Barad D. Hum Reprod 2006;1951-1955
4. Gleicher et al., Lancet 2018;392(10157:1516-1517
5. Mol BW, Hart RJ. Semin Reprod Med 2020;38(01):001-002
6. Kato et al., Reprod Biol Endocrinol 2012;10:35
7. Gleicher et al., J Transl Med 2016;14(1):172
8. Fanton et al., Fertil Steril 2023;119(5):762-768
9. Kim HH. Fertil Steril 2023;119(5)770-771
10. Gleicher et al., Am J Obstet Gynecol 2016;214(3):412-413
I am so glad I decided to pursue treatment with Dr. Barad. He’s one of the most caring and compassionate physicians I have ever encountered, and this is saying a lot because I am a physician myself. Too soon to test if I’m pregnant yet, but I know he has been helping me do everything possible to achieve my dream of having a baby. I cannot recommend him more highly!
- EB
“I have been with CHR since 2018. I came in for a second opinion when my last fertility Dr. advised I would need to consider egg donation. I was devastated. When I consulted with Dr. Gleicher on 12/5/2018 he told me you will get pregnant with your own egg. I left positive that I was going to get pregnant. He got to the root of my infertility which the previous Dr. did not and managed my treatment to my need. 2/3/2019 I went in for retrieval 2/7/2019, transfer on 2/19/2019. I was pronounced pregnant & on 10/19/2019 my baby girl made me a mommy. Because of Dr. Barad & Dr. Gleicher I have been blessed.
In the U.S. and in many other countries, third-party egg donation has become a growth industry. There are three principal reasons for this development. The first important reason is how the infertility field views egg donation: Instead of seeing the need for egg donation as a last-resort option and failure of standard infertility treatments with autologous oocytes, as the CHR does,1 most IVF clinics, consider the much better pregnancy and live birth rates with younger donor-eggs “preferable” because of better effectiveness, lower costs for patients, and more convenience for patients as well as providers. The second major reason, related to the last point, is the rapid growth in the frozen egg-bank industry, which has not only significantly improved the convenience of egg donation, but has also significantly increased donor choice and reduced matching times, and is further enhanced by (questionable) claims of lower cycle costs for patients. Related to this last point, one also must acknowledge that third-party donor-egg cycles represent the most profitable IVF cycle for most IVF clinics. As a consequence of all of these developments, over half of all donor-egg cycles in the U.S. now use frozen rather than fresh eggs, with this trend actually accelerating.

However, the purpose of this article is not yet another argument in favor of not giving up on patients’ use of autologous oocytes too early. Patients who seek out fertility treatments obviously almost universally do so with the idea of conceiving with the use of their own eggs. Nor is the purpose of this article to discuss the use of fresh or frozen donor eggs (fresh donor eggs clearly offer somewhat better pregnancy and live birth rates2-4). The impetus for this article came from a very new development, which raises serious medical as well as ethical concerns, - the increasing practice of importing frozen donor eggs from overseas.
To explain the complex issues arising from this observation, a little bit of background information is required: Third-party gamete donation, whether oocytes or semen, in the U.S., is under close FDA supervision. IVF clinics and donor-semen as well as donor-egg banks are under close federal government supervision by the FDA and undergo regular inspections to assure that all players follow all guidance rules issued by the FDA. In addition, several states, New York included, have a separate inspection process with, often, additional rules that build upon the FDA guidance.
One very essential FDA rule is that all donors must be tested for infectious diseases before donation in FDA-licensed commercial laboratories. Since very few FDA-licensed laboratories exist outside of the U.S., this rule for the longest time prevented the import of donor eggs into the U.S. from other countries. However, this is very quickly changing, and increasing numbers of IVF clinics in the U.S. started purchasing donor eggs from overseas countries, circumventing the FDA’s laboratory testing rule by sending the blood of donors to U.S.-based FDA-approved laboratories for testing.
Though this practice on paper, at least partially, fulfills FDA requirements, in reality, it does not because it does not guarantee what is called the “chain-of-custody.” What is meant by this term, is that every tested specimen in a laboratory is supposed to go from hand-to-hand in a controlled and traceable fashion: The blood drawer signs off on a slip that he/she drew the blood from a certain person; the laboratory that receives the specimen has a tracing mechanism that shows who received the specimen, who tested it and recorded the result and, ultimately, reports the result to the ordering physician. Moreover, laboratories licensed by the FDA not only are inspected for performing the required tests correctly, but also for maintaining this “chain-of-custody” properly. How that would be possible for blood drawn from Ukrainian donors who, for example, as we were told, cross into Poland for the day for testing and egg retrievals, is unclear.
That such controls of “chain-of-custody” is of crucial importance is well understood by The New York State Department of Health (NYSDH), which therefore, in our opinion very appropriately, mandates that donor eggs from frozen egg banks brought into the state for use by New York IVF clinics, not only must come from state-licensed (and inspected) frozen egg-banks, but that the IVF clinics that obtained those eggs from a donor (on behalf of the egg-bank) must also be licensed (and subject of inspection) by the NYSDH That those IVF clinics in Europe or elsewhere would subject themselves to FDA inspections, of course, appears rather unlikely. Unfortunately, not all states have similar rules to those in New York, and “chain-of-custody,” therefore, always remains questionable.
Moreover, one must wonder how one, under such circumstances, can trust medical and family histories provided for these donors, as neither donor, retrieving clinic, nor business intermediaries in these transactions are easily accessible under U.S. laws, should misrepresentations occur.
Serious further ethical concerns arise from who these donors usually are. Only a few years ago, over 60 people, including lawyers and physicians, were arrested in Europe in
a large human egg trafficking and illegal adoption ring that targeted vulnerable Bulgarian women who were brought over the border into Greece to serve as egg donors and gestational carriers.5
Based on information we’ve received, many of the egg donors who used to supply the egg exports to the U.S. come from impoverished countries, like Ukraine, Bulgaria, Romania, Albania, etc. They are often paid significantly less than 10% of what represents customary payments to U.S. egg donors. Consequently, egg merchants can offer these eggs to IVF clinics and egg banks at significantly reduced costs and, still, make a significant profit. Interestingly, we have found that these reduced costs are not even passed on to the clinics’ and egg banks’ U.S. patients. Pricing for patients of imported eggs, indeed, appears to be the same as for eggs produced by U.S. donors. That this suggests human abuse, is to state it mildly and, simply, for ethical reasons must be questioned.
Years ago, the CHR already decided against considering the sale of donor eggs as a profit center (we just anonymously pass donor costs through to the recipient). The CHR has no economic interest in patients using either fresh or frozen eggs from its own egg-donor pool or frozen eggs from frozen egg-banks. Therefore, we encourage patients to work with donor eggs from wherever they find their “best” donor. However, having become aware of the new circumstances described in this article in the egg-freezing industry, we feel obliged to inform our patients and readers.
The CHR is fortunate to be located in New York state, where not only egg banks but also their supplying IVF clinics must be licensed to allow for the use of their frozen eggs by the CHR (and other IVF centers). Before accepting eggs from a frozen egg-donor bank, our embryology staff makes sure that the eggs were retrieved in a clinic that is licensed in New York State. However, this may not happen everywhere (as noted, it is not required in all states). Wherever you are or wherever you try to find your “ideal” egg donor, we suggest you stay away from imported oocytes.
1. Gleicher et al., J Assist Reprod Genet 2020; 37(7): 1583-1588
2. Kushnir et al., J Ovarian Res 2018;11(1):2
3. Setti et al., Zygote 2021;29(30:234-238
4. Stan Williams et al., Fertil Steril 2022;117(2):339-348
5. Harley N. N World. https://www.thenationalnews.com/world/morethan-60-arrested-in-500-000-european-human-egg-traffickingand-illegal-adoption-ring 1.915464#:~:text=More%20than%2060%20 people%2C%20including,by%20an%20organised%20crime%20gang.

Patients arrive for egg retrieval at the CHR hoping that the physicians and embryologists will bring good news to them soon. This image, taken at the CHR, illustrates the first signs that good news is on the horizon. It depicts a freshly retrieved cumulus-oocyte complex with the oocyte seen as a large central sphere partially obscured by the thousands of cumulus cells radiating outwards. As noted before in The Voice, the next step is for the embryologist to remove these cells so they can further evaluate the health and maturity status of oocytes, which if mature, will then undergo ICSI to launch embryonic development.
What happens when the cumulus cells are removed? This image shows that sometimes it is difficult to remove all of the cumulus cells as seen at 12-3 o’clock. Note that at 3 o’clock a single round nucleus is evident which tells us that this oocyte has not yet completed the maturation process that should have been triggered by the last injections. In fact, this is what we call an immature GV (germinal vesicle) stage oocyte that would have undergone overnight rescue maturation before attempting ICSI the following day.
Much of the research at the CHR has focused on why some oocytes are fully mature after trigger and why others fail to either initiate or complete the maturation process. Dr. Albertini has spent much of his career trying to understand the fascinating dialogue that exists between the oocyte and cumulus cells and here in this image is an example of one of his CHR research projects. Looking down on the surface of a human oocyte, many cumulus cells can be seen and using special staining techniques. Cumulus cells “reach out and touch” the oocyte using fine extensions referred to as Transzonal Processes or TZPs for short. TZPs serve as conduits for supplying the oocyte with both nutritional and informational molecules that tell the oocyte to mature and prepare for fertilization, key elements in the underlying biology that will dictate which oocytes go on to develop as an embryo-or not.
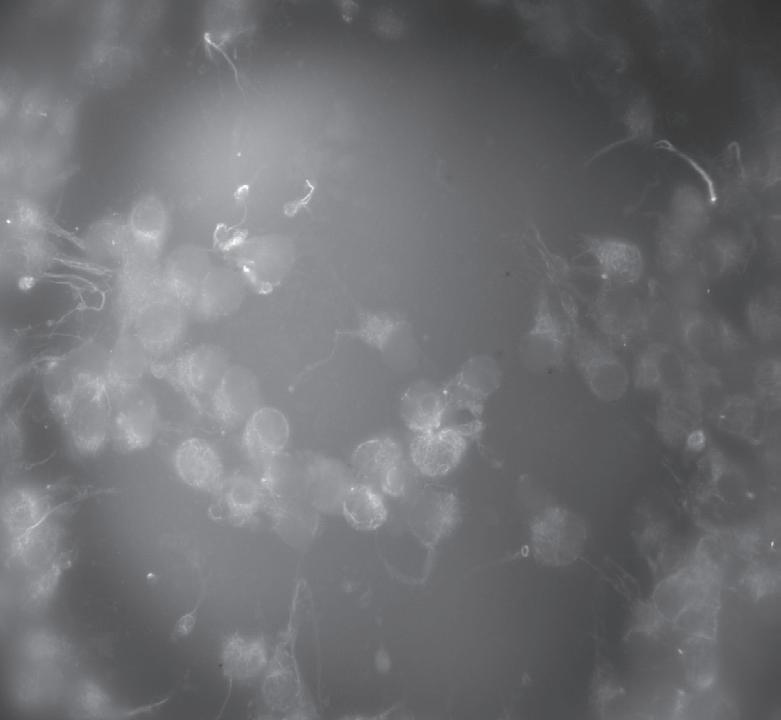
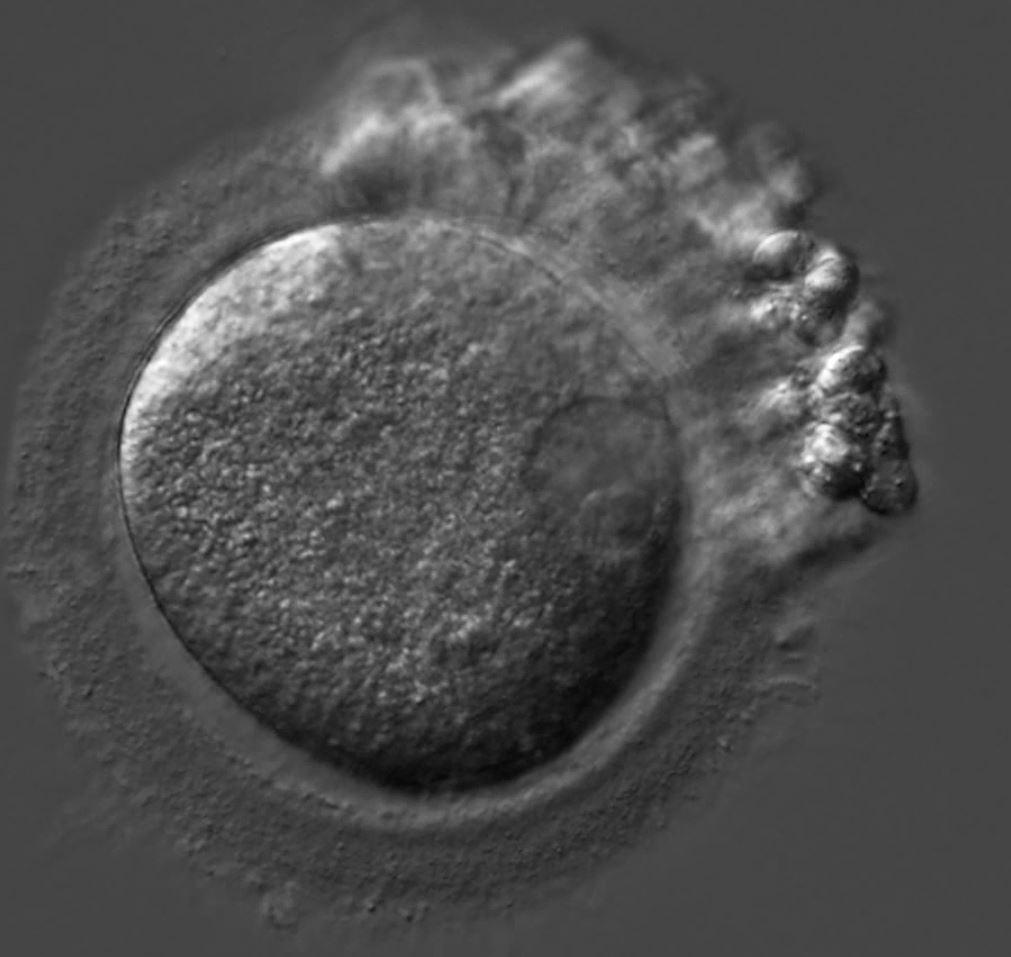
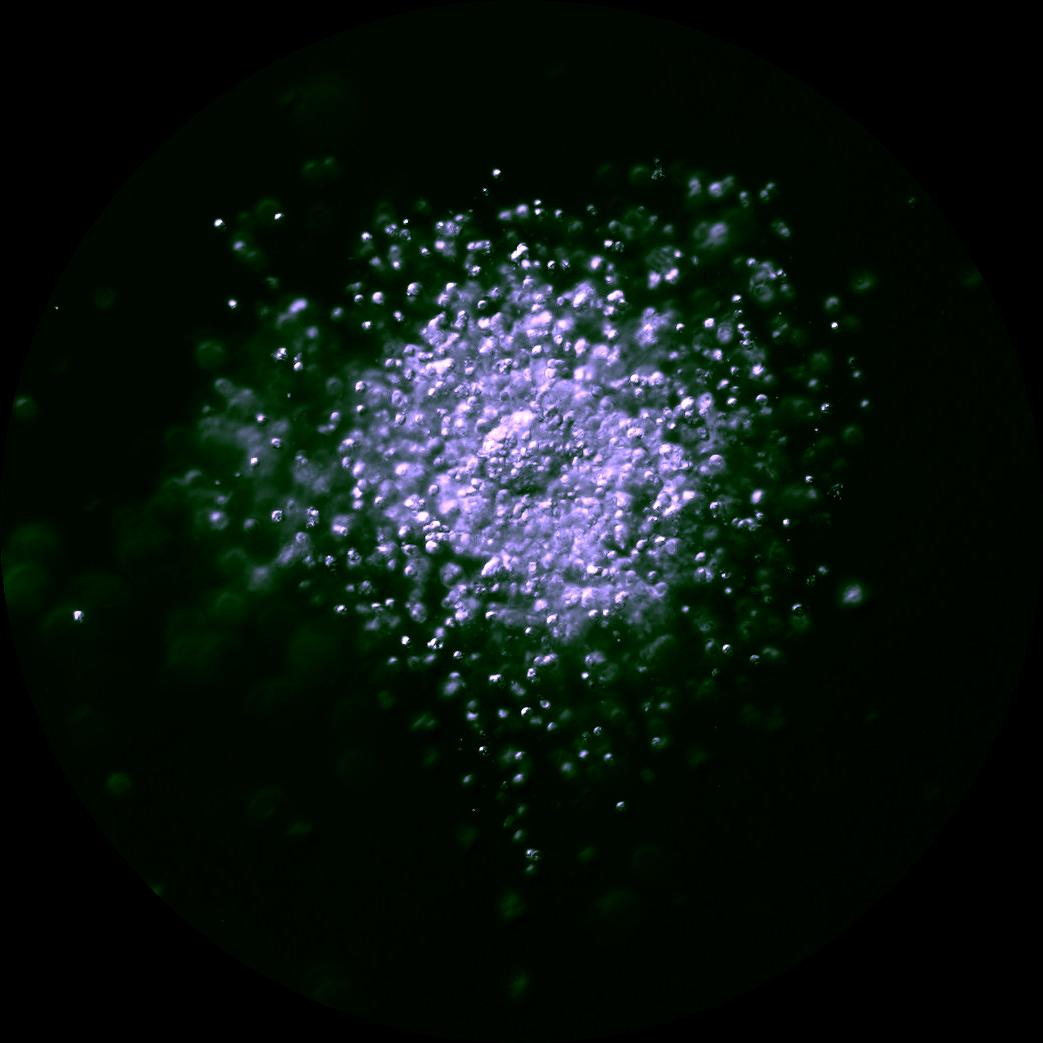 Image 2
Image 3
Image 2
Image 3


Since so-called “add-ons” to IVF in the last 10-15 years have come to dominate IVF practice, some of them, of course, are constantly addressed in this newsletter. However, an important related subject has so far been left out of these discussions, - not only in The VOICE - but in the general medical literature as well, while growing in importance, as the number of “add-ons” continues to expand.
One must assume that so-called “add-ons” to IVF are introduced to routine clinical practice with the laudable goal of improving outcomes in IVF. However, as by now widely accepted in the literature, when it comes to these “add-ons,” good intent, unfortunately, does not always translate into good medical practice.1 It, indeed, is not only becoming increasingly obvious that

many of these well-intended new treatments not only do not improve IVF cycle outcomes, but in many instances, actually adversely affect pregnancy chances in IVF. In other words, not only are patients unnecessarily exposed to treatments, and often face significant additional costs to an already exorbitantly expensive IVF cycle, but they also do not benefit from those treatments and, actually, diminish their already low pregnancy chances (why else would they be in infertility treatment?) even further.2
This is, of course, a deeply troubling proposition, but what renders it even more concerning is the subject of this essay,the potential cumulative adverse effects of multiple “add-ons” in the same IVF cycles, which, as multipliers, can greatly aggravate adverse outcome effects into logarithmic scales. An excellent example is the chain link of: (i) extended embryo culture to blastocyst-stage; (ii) elective single embryo transfer (eSET), and (iii) preimplantation genetic testing for aneuploidy (PGT-A).
It starts with the “add-on” hypothesis that extended embryo culture represents a functional method of embryo selection, - that culturing embryos to blastocyst-stage filters out “best” embryos with the best pregnancy and live birth chances. As frequently before discussed in this newsletter, but a crucially important point that must be made over and over if the currently excessive utilization of extended embryo culture is to be remedied, relatively minor outcome benefits from extended embryo culture were only shown in good-prognosis patients. Every attempt to demonstrate such outcome benefits in studies in general infertility populations, unsurprisingly, in past meta-analyses either demonstrated none or only marginal benefits from extended culture.3 Since embryos of poor prognosis patients often do not survive to blastocyst-stage, recent data, moreover, suggests that cleavage-stage transfers in such cases offer significantly improved outcomes.4 Another way of saying the same thing is that extended embryo culture to blastocyst in some patients will reduce pregnancy and live birth chances. Yet, all of this does not even take into consideration recent reports which suggest significant adverse epigenetic effects on embryos from extended culture.5
In good-prognosis patients (approximately 20% of an average population), blastocyst-stage culture marginally improves IVF outcomes. In a large majority of average-prognosis patients, it will not demonstrate any effects on IVF cycles, and in poor-prognosis patients (again ca. 20% in a general population), extended culture will actually cause negative effects on cycle outcomes. Now imagine, that all three patient populations in addition have exposure to another “add-on,” let’s say, eSET. For many years, a widely heard argument in support of routine blastocyst culture has, indeed, been that, by selecting the “best” embryos, extended embryo culture facilitates the efficacy of eSET in achieving pregnancy and, concomitantly reduces the
risk of twin pregnancies, which much of the IVF establishment has (incorrectly) come to consider an adverse IVF outcome.6
Aside from the fact that the CHR strongly believes that twin pregnancies for some infertility patients are not an adverse, but the “best” outcome,6 one here must point out what the chain-link between blastocyst culture and eSET statistically really does: eSET in comparison to 2-embryo transfer (2ET), indeed, reduces twin pregnancies. However, it also in parallel significantly reduces pregnancy and live birth rates, requiring at least one additional frozen-thawed cycle to catch up.7 Who will be most exposed to this loss, if not once again, the poor-prognosis patient? So it goes: a large majority of average prognosis patients remain mostly unaffected (and why then use all of these shenanigans?), for poor-prognosis patients the fall from the cliff becomes, however, more and more pronounced, while best-prognosis patients, who even without “add-ons” already demonstrate excellent outcomes, may gain yet another minimal outcome advantage that may not be worth the additional efforts and additional costs in this patient population (though that is still worthwhile a good study).
However, singleton versus twin pregnancy outcomes is only one endpoint of this chain-link: There is also still PGT-A, like eSET dependent on universal extended embryo culture but, in addition, also dependent on eSET because, like blastocyst-stage culture, it is at least partially considered motivated by eSET, so is eSET motivated by PGT-A: The transfer of a “euploid” embryos is by many still (falsely) considered to offer much better pregnancy and live birth chances than the transfer of single chromosomal-“abnormal” embryos. To suggest their inaccuracies, 8-10 we in accordance with CHR practice, here, place chromosomal PGT-A diagnoses between exclamation marks.
What has been going on with “add-ons” to IVF over the last 10-15 years can, therefore, be best described as a series of Ponzi schemes: First came extended embryo culture with the exaggerated claim of improving IVF outcomes at best, while at least significantly reducing pregnancy chances for at least a subgroup of poor prognosis patients. When that became increasingly obvious, universal eSET was added as a new reason for extended embryo culture with the absolutely nonsensical argument that every twin pregnancy must be avoided.11 In parallel, we experienced what initially was called preimplantation genetic screening (PGS) with the claim of improving pregnancy and live birth rates and reducing miscarriage rates. As those claims became no longer sustainable, the procedure was renamed PGT-A and, like extended embryo culture, eSET became the motivation for utilization of PGT-A, arguing that “in accordance
Continued on page 21

Continued from page 20
with ASRM guideline,12 the use of PGT-A also may allow for eSET.”,13 In other words, one ineffective new “add-on” is used to support another one, all pushed on the IVF community and infertility patients by the same commercial interests. The end product, not surprisingly, has been declining live birth rates after IVF all over the world from the U.S to Japan,14 increasing numbers of third-party donor egg cycles, often for women who only were made to believe they needed them because their own still perfectly fine embryos were falsely declared to be aneuploid and 20either not transferred or even discarded.15,16
We have here described only one such linked chain of “addons” because it very likely is the most harmful one regarding adverse overall effects on worldwide IVF outcomes. Yet, there are many others, starting with closed incubation systems plus timelapse, by definition, of course, also linked to extended embryo cultures and eSET, as well as several other useless tests which since their commercial launch have been reported to not offer promised outcome benefits.17
Once a test or another intervention has been introduced into routine clinical practice without proper prior validation studies, its ineffectiveness is often difficult to prove. Paradoxically, proponents then often shift to other parties their own initial obligation of proving efficacy for a test or intervention before introduction into routine clinical practice. PGS/PGT-A has been an excellent example: Rather than the proponents being forced to demonstrate effectiveness, opponents of PGS/PGT-A utilization were forced to demonstrate the test’s ineffectiveness in improving IVF outcomes, while proponents of the test, at every step were allowed to discredit the opponents’ evidence without, themselves, ever being able to shore up their own claims for the test.
In the process, it became increasingly clear that in general, neither in infertility populations like in U.S. registries,18 nor in good-prognosis infertility populations,19 has PGT-A improved IVF outcomes. Therefore, the first step in rebutting the continued utilization of ineffective intervention is a demonstration of non-superiority. Once that has been accomplished, logic, however, suggests that only two possibilities remain: either the intervention has no effect, or it is actually harmful. The recent evolution of knowledge regarding PGT-A, indeed, reflected this well: A large multicenter, prospectively randomized study of cumulative live birth chances in IVF with and without PGT-A by Chinese investigators was reported by the authors to demonstrate non-inferiority for the non-use of PGT-A over the use of PGT-A.19 A corrected reanalysis of the study by Israeli colleagues and CHR investigators, however, revealed actual inferiority in outcomes in association with PGT-A usage.20 At approximately the same time, an analysis of U.S. IVF registry data
of all reporting IVF centers also demonstrated that PGT-A utilization not only failed to confer an outcome benefit over non-use, but actually reduced live birth chances in this national data set.18
That both studies suggested overall adverse outcome effects in general infertility populations from the utilization of PGT-A strongly suggests that there had to be a subgroup of patients in whom the test/intervention caused significant harm to IVF outcomes. This conclusion, in principle, applies to every “add-on” and, indeed, to every therapeutic intervention in medicine that does not significantly improve desired outcomes. An unchanged outcome for a complete study group is always the likely result of three subgroups in a study population: a group gaining, a larger group remaining unchanged, and a third group losing regarding expected outcomes, with gaining and losing groups, basically, balancing each other out. An unselected general population will, therefore, demonstrate positive or negative effects from a treatment only if those are extremely pronounced. When the proposition becomes that everybody should be treated, as has increasingly been the case when it comes to extended embryo culture, but also to eSET, PGT-A, and other “add-ons,” even existing effectiveness in sub-population will, therefore, often disappear. Whenever treatments are proposed “for everybody,” it is, therefore, always time to sound the alarm because that usually means that there is a subgroup of patients who are really seriously harmed.
1. Glatthorn HN, Decherney A. J Asist Reprod Genet 2022;39(3):581-589
2. Wilkinson et al., Fertil Steril 2019;112(6):973-977
3. Glujovsky et al., Cochrane Database Syst Rev 2022;19(5):CD002118
4. Xiao et al., Reprod Biomed Online 2019;39:916-923
5. Sciorio et al., Clin Genet 2023;103(2):133-145
6. Gleicher N, Orvieto R. Reprod Biomed Online 2022;44(1):1-4
7. McLennon et al., BMJ 2010;341:C6945
8. Gleicher et al., Clin Chem 2022;68(4):501-503
9. Gleicher et al., Trends Molec Med 2021;27(8):731-742
10. Gleicher et al., Nat Med 2022;28(3):442-444
11. Wang et al., JAMA Netw Open 2021;4(9):e2123634
12. Practice Committee of the ASRM. Fertil Steril 2017;107:901-903
13. Tiegs et al, Fertil Steril 2020;115(3):627-637
14. Gleicher et al., Hum reprod Open 2019;(3):hoz017
15. Barad et al., Hum Reprod 2022;37(6):2730-2734
16. Barad et al., Hum Reprod 37(9):2216-2218

17. Kieslinger et al., Lancet 2023;401(10386):1438-1446
18. Kucherov et al., J Assist Reprod Genet 2023;40(1):137-149
19. Yan et al., N Engl J Med 2022;385:2047-2058
20. Gleicher et al., Hum Reprod 2022;37(12):2730-2734
Have you thought about advertising in the VOICE?
This newsletter every month goes electronically to ca. 80,000 infertility patients, medical professionals in the field, and members of the media, with over 25% (an unusually high number) also opening the VOICE.

For further information, please contact: Ms. Alexandra Rata (212) 994 4400 or e-mail to arata@thechr.com
We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratorysupervisor for research

To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.



“Shared egg-freezing” (SEF) has, suddenly become a much-discussed item on the Internet and we are getting frequent questions. What it practically means is that a young woman “donates” part of the eggs she produces in an egg-freezing cycle for fertility preservation purposes for herself, as “payment” for her egg-freezing cycle to the infertility center. The infertility center, in turn, can sell those eggs to a patient who needs third-party donor eggs to conceive. Several fertility centers in the U.S. offer this service and some among those, indeed, quite aggressively promote it on their websites and social media. In some countries where the commercialization of egg donation (i.e., the paying of egg donors) is forbidden, SEF arrangements are sometimes the only option to get donor eggs legally.

Except if the donor and recipient are in some fashion related, the CHR does not recommend and, therefore, does not offer SEF, and we believe that that there are several good reasons why, medically as well as ethically, this is the right choice. Starting with the most obvious one, though some proponents claim that SEF “is a unique program that serves both sides of the fertility gap – young women looking to freeze their eggs and individuals and couples seeking donor eggs,”1 we see it more as a program that serves both sides of the fertility gap rather poorly: The young woman who wants to freeze her eggs, is in principle poorly served for two reasons: First, and mostly a practical point, she gets only a fraction of the eggs she produces in a full stimulation cycle (the rest going to a usually anonymous recipient), when even all the eggs of a cycle only rarely are enough to offer her a realistic security blanket for later in life. Most young women need at least 2-3 cycles to accumulate enough frozen eggs to reach such a point. Our second concern regarding the egg donor is of ethical nature: We consider this kind of “deal” to be, at least marginally, coercive.
Regarding the recipient couple, they too end up compromising because they also, ultimately, do not get what they would receive if they simply chose a fresh donor or frozen eggs from a frozen donor bank. First, it is reasonable to assume that the donors in SEF are not equally well-vetted as regular donors. For example, many younger women decide to freeze their eggs because they already know that they have risk factors for infertility. Though such studies have not been reported, we would strongly suggest that freezing outcomes from 100 carefully selected egg donors would be significantly better than freezing outcomes from 100 donors in SEF. A second disadvantage for recipients is that they don’t have the opportunity of purchasing all the eggs of a donor cycle (if they so wish).
It is one thing to make second-best choices when the law prohibits best choices, as it still does in some European countries in this case. Yet, even there, patients usually prefer to cross borders and pursue unlimited donor-recipient cycles where they are legal, rather than chasing SEF cycles in their own country.
For young women who have approached the CHR with the idea of being an egg donor in SED cycles we, therefore, always advise to apply as regular egg donors to the CHR’s website (at https://www.centerforhumanreprod.com/contents/egg-donation) – or, for that matter, elsewhere. If they are accepted and matched, they will receive an egg donor pay that far exceeds the costs of one egg-freezing cycle and, often may pay for two or even three cycles. However, if they are not chosen as donors, they will likely be told why. Especially if there is a medical reason, they practically will have received a free infertility consultation that can then help them in setting priorities for their future fertility.
For infertile individual patients and couples who need donor eggs, we here at the CHR always stress that in our opinion, nothing is as important for them as their donor choice: A gamete donor choice should never be a compromise because we do not want our patients, later in life as they see their child growing up, to ever have second thoughts and/or regrets regarding their choice of donor.
REFERENCE
1. https://freezeandshare.com




Before addressing this subject, it is important to point out that the CHR may have several potential economic conflicts in addressing this subject relating to the ownership of U.S. user-patents by the CHR and some of its employees and receipt of royalty payments from these patents by Fertility Nutraceuticals LLC, a company, among other supplements, producing an androgen product (DHEA, dehydroepiandrosterone). The CHR in multiple studies claimed treatment benefits from the supplementation of selected infertile women with DHEA. Employees of CHR and the CHR also own shares in Fertility Nutraceuticals, LLC.
This is, as we will explain below in some detail, a very timely question. But before we get to specific risks, some background information is required: Dehydroepiandrosterone (DHEA) is the precursor hormone of testosterone which, in turn, is the principal male hormone in our bodies in both
men and women, - though, of course, at very different concentrations. The CHR prefers supplementation with DHEA over supplementation with testosterone products directly, because the best testosterone levels vary in our organs. Supplementation with testosterone floods the whole body with the same level of the hormone, relatively easily leading to overdosing. Also, testosterone levels that are too high may be even more harmful to ovarian function than underdosing. By administering DHEA, the precursor of testosterone, every organ, ovaries included, will take out of circulation only as much DHEA as it needs to produce desired testosterone levels in that organ. At a daily dosage of up to 75mg of DHEA, overdosing with DHEA is, therefore, almost impossible.
The importance of normal testosterone levels for normal ovarian function was not fully understood and appreciated until approximately 2010, when Sen and Hammes in a mouse androgen receptor knockout model demonstrated how essential its effects were on granulosa cell function, especially during small growing follicle stages immediately after recruitment of follicles out of primordial stages.1 This explanation came along roughly five years after CHR’s investigators with help from a patient stumbled over the clinical observation that DHEA apparently improved ovarian reserve in some women with low functional ovarian reserve (LFOR) and, therefore, improved their IVF cycle outcomes.2,3 This discovery initiated a serious research effort over many years to further elucidate a better understanding of this observation, to this date resulting in over 40 publications in peer-reviewed journals by the CHR’s investigators.
DHEA supplementation of women with LFOR has, since, become a widely utilized treatment all over the world, likely
involving up to half of all IVF cycles. For several reasons, it, however, has remained controversial despite irrefutable animal data in various small and large animal models confirming the initial study of Sen and Hammes that demonstrated the importance of good androgen levels for normal ovarian follicle development.4
The principal reason for this skepticism about DHEA has been the absence of properly powered prospectively randomized studies of DHEA in infertile women. Though a good number of severely underpowered prospectively randomized studies have been published, that such inadequate studies resulted in contradictory results cannot surprise. Indeed, almost identical studies in the same medical journal of almost identically selected infertility patients, reported within a two year span, improved5 and unchanged6 IVF cycle outcomes.
A second major reason for contradictory outcomes of DHEA supplementation studies lies in the fact that practically none of the published studies preselected patient participants based on confirmed low testosterone levels. Indeed, we are unaware of even a single study that tested androgen levels before deciding whether to include a patient in a DHEA supplementation study. Just as studying whether aspirin is an effective headache treatment will not succeed in patients who do not suffer from headaches, a study of DHEA supplementation in infertile women makes only sense if those women have low androgen levels.
Finally, a third major reason for contradictory findings is the fact that DHEA supplementation is prescribed in very different ways: in timing, in dosing, and in product quality. As noted, androgen effects on ovaries occur primarily (though not exclusively) at small growing follicle stages. At those stages, follicles still require at least 6-8 weeks to reach gonadotropin-sensitivity, when follicles become sensitive to the gonadotropin medications administered in IVF cycles to further stimulate the growth and maturation of follicles. To pretreat with androgens for less than at least 6-8 weeks before IVF cycle start, therefore, physiologically makes little sense. Yet many published DHEA studies did exactly that.
Daily treatment dosing also greatly varies between studies, as does the quality of DHEA products on the market. A recent report from Consumer Lab.com for example reported that one tested product on the market contained only 14.7% of the listed DHEA amount.7 Moreover, particle sizes in DHEA products vary, and absorption rates of DHEA vary with particle size. The same daily dosing may, therefore, have varying effects on androgen levels. As reviewed under “CHR-publications” elsewhere in this issue of The VOICE, CHR’s investigators recently also reported that the potency of a least some DHEA products on the market appears to have increased, requiring more frequent down-dosing than in the past.8
For all of these reasons, again unsurprisingly, literature reviews and meta-analyses that tried to make sense of reported underpowered studies, also produced contradictory results, based on which randomized studies they included in their respective analyses.4,9,10 Attempts to conduct proper trials have, however, been made repeatedly in the past (including twice by the CHR after obtaining financing for those attempts), but failed because patients with LFOR (understandably), usually because of the involved potential time-loss they often feel they cannot afford, refuse randomization to a placebo.
In addressing the “risks” of DHEA supplementation, contradictory opinions on the utilization of DHEA, therefore, must be recognized as a first issue. Like with any medication, a second risk is, as already noted, over-dosing, but this risk is relatively minimal if androgen supplementation occurs with DHEA rather than testosterone and does not exceed 75mg daily.
The most frequent side effect especially in women with oily skin is more oily skin and acne. Rarely, patients complain about hair loss and all three of these complications can be easily reversed by reducing the dosage or stopping DHEA completely. DHEA has also been associated with gastric symptoms, fatigue, and changes in menstrual patterns, though we see those effects only very rarely. Side effects noted in the literature are also facial hair growth (hirsutism), hypertension, and/or deepening voice, though, likely since infertility patients never exceed dosages of 75mg per day, we have not experienced these alleged side effects.
Liver disease and diabetes are relative contraindications to supplementation with DHEA because the hormone has been alleged to make liver problems worse and because it increases insulin resistance and, therefore, potentially blood glucose levels. Considering the low dosage usually used in fertility treatments and the relatively short time period of use, those contraindications only rarely apply to infertile women. Since a small amount of DHEA also turns into estrogen, contraindications to estrogen treatments (like, for example, estrogen receptor-positive breast cancers) must also be considered.
Though DHEA in the U.S. for rather strange reasons is considered a food supplement and, therefore, is available over the counter without prescription, in most of the world, like every other hormone, it is considered a medication, requiring a prescription from a physician. Since DHEA (in much higher dosages) is often abused (especially by bodybuilders), in several countries it is even considered a controlled substance.
1. Sen A, Hammes SR. Mol Endocrinol 2010;24(7):1393=1403
2. Barad DH, Gleicher N. Fertil Steril 2005;84(3):756
3. Gleicher N, Barad DH. Hum Reprod 2008;23(12):2868-2870
4. Neves AR, et al., Front Endocrinol (Lausanne) 2021;12:653857
5. Kara et al., Eur J Obstet Gynecol Reprod Biol 2014;173:63-65
6. Kottb et al., Eur J Obstet Gynecol Reprod Biol 2016; 200:11-15
7. https://www.nutraceuticalsworld.com
8.

9. Qin et al., J Gynecol Obstet Hum Reprod 2017;46(1):1-7
10. Fouany MR, Sharara FI. J Assist Reprod Genet 2013;30(9):1239-1244

This is another very timely question because, if the chat on social media is representative, the interest in prenatal vitamins appears to grow day by day. We, indeed, used to believe that prenatal vitamins are, more or less, all the same; but that has changed: Nowadays ,the public has become much more selective and there is good reason for that because prenatal vitamins are now more variable than they have ever been. Yet recent research suggests that only very few prenatal vitamins meet all the nutritional needs pregnancy demands. A recent pamphlet based on information from the NIH Environmental Influences on Child Health Outcomes (ECHO) Program, indeed, suggested so-much:1 Since almost all pregnant women do not meet nutrition requirements during pregnancy from their nutrition alone, prenatal vitamin supplementation has been one pregnancy practice almost universally accepted for decades. In the U.S., this policy recommendation differs from what is customary in many other countries, where the utilization of a multivitamin is often replaced by selective Folate and, maybe, vitamin D supplementation. What NIH recommends is much more complex and involved, and involves a multitude of so-called Dietary Reference Intakes (DRIs).2
DRIs are a set of reference values used to plan and assess the nutrient intake of healthy people. They vary by age and sex, and include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient require ments of nearly all (97–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally ade quate diets for them; can also be used to assess the nutri ent intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
The Health.gov DRI Activities Update always offers the latest update on the subject but, like with any medication, what is best, is never the same for everybody. At the core of every prenatal vitamin must be supplementation for calcium and Vitamin D since they contribute to the development of teeth and bones in the baby. Also of importance is iron supplementation. Once a woman has conceived, her total blood volume starts increasing. Very quickly, her, what is called plasma volume, can increase by approximately 40%, which, of course, dilutes out substances in the blood to significant degrees. This is, for example, why women in pregnancy become anemic: as their plasma volume increases, their iron is significantly diluted. All of this also applies to vitamins C, A, E, and B, as well as other elements of blood, like zinc and iodine.
Everybody agrees that all of these items must be part of a good prenatal vitamin and must reflect their respective DRIs. However, here is where the new brave world of prenatal vitamins starts because patients now demand more than just the basics from their prenatal vitamins. A good example, and at the center of considerable attention recently, has been choline, recently reviewed,3 and by the American Academy of Pediatrics recognized as “brain building2” for the fetus.
Especially for women above age 35 who start demonstrating declining androgen levels, the addition of 25mg of DHEA to a standard prenatal vitamin has been suggested in attempts to improve a woman’s fecundity [Please note the above Conflict Statement regarding DHEA and other androgen supplementation methods]. We here note these two examples for extra-additions to standard prenatal vitamins to reemphasize the point that there is no one “best” prenatal vitamin for everybody. Like any medication and/ or supplementation, prenatal vitamins must be individualized to a patient’s needs.
1. https://www.prnewswire.com/news-releases/nih-research-sug gests-few-prenatal-supplements-meet-all-nutrition-recommenda tions-for-pregnancy-301789887.html. April 4, 2023
2. https://ods.od.nih.gov/HealthInformation/nutrientrecommenda tions.aspx
3. Korsmo et al., Nutrients 2019;11(8):1823
4. Schwarzenberg SJ, Georgieff MK. Pediatrics 2018;141:e20173716
This is another very good question, - not the least because of a recent editorial in Scientific America, which strongly recommended that the FDA assume a regulatory function over nutraceuticals. The editorial describes nutraceuticals as “ something between vitamins and processed foods,” with approximate global sales of US $400 billion.1
Before addressing this subject, it is, however, important to point out that the CHR may have potential economic conflicts in addressing this subject since the CHR and some of its employees own shares in Fertility Nutraceuticals, LLC., which produces and sells nutraceuticals under the name Ovaterra.
That this, moreover, is not just a straightforward question with an equally straightforward answer becomes immediately obvious when one looks up definitions of what a “nutraceutical” is: Wikipedia defines a nutraceutical as, “a pharmaceutical alternative which claims physiological benefits. In the U.S., under the authority of the Federal Food, Drug, and Cosmetic Act, nutraceuticals are largely unregulated, as they exist in the same category as dietary supplements and food additives.”2 Moreover, the term “nutraceutical” is not defined by U.S. law: Depending on its ingredients and the claims it is marketed with, it can be regulated as a drug, dietary supplement, food ingredient, or food.


The medical literature, in turn, defines nutraceuticals as “pharma-foods” or as “a food or part of a food that provides medical or health benefits, including prevention or treatment of a disease, partially overlapping with the definition of a food supplement. Both, indeed, claim beneficial effects for health but, while nutraceuticals are made from food or parts of a food, food supplements are single substances used alone or in mixtures with the scope of adding micronutrients when the body needs them. In
contrast to food supplements, nutraceuticals, therefore, necessitate clinical evidence substantiating the health efficacy of nutraceuticals based on safety, efficacy, and known mechanism of action.3
The editorial correctly noted that nutraceutical companies under current rules can do their own testing for safety and purity without any requirements of submission of results and or supportive evidence for clinical effects being verified by independent reviewers, as the FDA mandates for drugs.1 The editorial, however, in this regard is not completely correct because the FDA is actually quite serious in policing statements about alleged clinical benefits of supplements and not only disallows such statements, but actively persecutes supplement companies which, in the agency’s opinion, breach this prohibition.
The CHR, nevertheless, agrees with the editorial in Scientific America that called for tighter regulation of nutraceuticals by the FDA, especially when it comes to safety and purity testing of nutraceuticals (and vitamins) because, as the editorial also noted, active compounds in these nutraceuticals can be in different chemical forms and/or quantities than what is stated on the labels, rendering dosing inconsistent.” At the same time, the FDA should also establish clearer guidelines regarding what represents allowable and not-allowable efficacy statements because current interventions by the FDA at times can appear rather arbitrary, issuing warning letters to some companies but not to others with very similar statements.
The CHR also agrees with the editorial when it called for a bill that would require manufacturers to do third-party testing for safety, the concentration of ingredients, and efficacy, and report results to the FDA, with the agency being empowered to verify chemically all ingredients, and allowing the FDA to enforce recalls or even product bans if the wellbeing of the public is threatened. Consumers should never be exposed to hyped claims and/or shoddily produced nutraceuticals. Many supplement companies voluntarily already farm out quality testing of their products to third parties. A few bad apples will, however, continue ruining the image of the whole industry, unless the FDA steps in.
REFERENCES
1. The Editors. Scientific America, June 2023; p8
2. https://en.wikipedia.org/wiki/Nutraceutical
3. Santini A, Novellino E. Foods 2017;6(9):74

Like other bodily cavities, the vagina has its own, typical microbiome. That the vagina has its own, typical bacterial population and that this population changes with different

vaginal pH, has been known for decades. However, more detailed studies have been performed only in more recent years as especially microbiomes in the GI tract were found to be highly significant for a large variety of physiological processes as well as disease states.1 The vaginal microbiome appears to change significantly at menarche, switching from a mixture of E.coli and anaerobes to domination by Lactobacilli. 2 Further changes have been reported with sexual activity, menstrual cyclicity, contraceptives, pregnancy, and menopause. VALENCIA, the most extensive database, classifies test samples according to their diversity by allocating any microbial community to one of 13 community state types (CSTs). A healthy vaginal microbiome harboring mostly different Lactobacilli are primarily CST I, II, III, and V, while whole CST IV usually demonstrates dominance by other bacteria than Lactobacilli. CST III is associated with bacterial vaginosis, and vaginal microbiomes also vary with race and geographic location.3
Though there have been claims of a concomitant endometrial microbiome, more recent research suggests that those data were based on contaminations and that the normal endometrium may, indeed, be, as has been argued for decades, mostly sterile. A sterile endometrium would also argue against claims made by some research groups that endometrial microbiomes were associated with recurrent implantation failure (RIF). The likely absence of an endometrial microbiome would also argue against an association with IVF cycle outcomes, a claim recently made by Investigators from The Netherlands.4,5
On the other hand, considering how important the gut microbiome has now been established to be for an individual’s overall health, including brain activity,1 it would not at all be surprising should vaginal and/or (if really existing) endometrial microbiota could statistically relate to IVF outcomes, especially negative outcome. Yet, a final answer regarding this question if, likely, is still years away.
1. WebMD, https://www.webmd.com/digestive-disorders/ss/slide show-how-gut-health-affects-whole-body ; 1/28/23.

2. Farage M, Maibacj H. Arch Gynecol Obstet 2006;273:195-202
3. Xu et al., Curr Opin Obstet Gynecol 2022;34(3):122-132
4. Kedooder et al., Hum Reprod 2019;34:1042-1054
5. Schoenmakers S, Laven NJ. Curr Opin Obstet Gynecol 2020; 32:169-178
If you have never heard the term “cultural competence” in association with discussions regarding fertility care given to gay, bisexual, transgender, and queer people, we recommend a relatively recent review article in Fertility and Sterility, 1 even though this rather comprehensive literature review could offer only quite limited insights, as the literature on the subject is still surprisingly sparse. The term describes the ability to recognize and respect cultural differences among individuals.
The authors identified as key barriers to participation in treatments gender dysphoria, heteronormativity, still, stigmatization (amazingly even these days), and psychological distress. They furthermore found significant concern about the lack of LGBTQ+-tailored specific information, while, in contrast, positive findings and solutions included the existence of tailored information, psychosocial interventions, gender-neutral language, and inclusive intake processes. Unsurprisingly, they concluded that the LGBTQ+ community, still, faces unique barriers in their access to fertility care, as suggested by patients as well as medical providers.
Targeted solutions for culturally competent care included (see table below):
Lack of LGBTQ+-tailored services Targeted resources and information handouts, partnerships with the community, further research, more established guidelines, needs-based assessments, long-term research.
Heteronormativity/cisnormativity
Psychological distress/triggering situations
Stigmatization and discrimination
Inclusive intake forms, gender-neutral washrooms, use of preferred pronouns and gender-neutral language.
Cultural awareness and sensitivity of providers, psychological assessments and follow-up, comfort for patients during triggering examinations.
Welcoming signage and written statements including LGBTQ+ patients, training for health care providers, policies to prevent discrimination, knowledge translation tools for providers.
Another recent study commented on pregnancy outcomes in the LGBTQ+ community since data suggest that they are worse than among heterosexual patients.2,3 The commentary noted that the ACOG recently recognized that the marginalization of the community leads to poor health outcomes and acknowledged the need for additional training of health care providers for this community.4 What specifically causes worse obstetrical outcomes is still largely unknown. Most has been reported about lesbian and bisexual women, who often, even before conception, already underuse routine health care. For transgender men and non-binary individuals (as noted before) the risks of gender dysphoria, and potential pregnancy complications including miscarriages, may be most traumatic and, often, lead to postpartum depression.
The authors of the commentary then summarized their conclusions as follows:
1. Research
- Require justification for the exclusion of LGBTQ+ individuals from studies.
- Fund studies focused on perinatal care of LGBTQ+ populations.

- Support, develop, and nurture researchers focused on the study of a variety of sexual identities, with an emphasis on gender‐expansive populations.
- Ensure diverse representation of researchers, inclusive of LGBTQ+ individuals.
- Build and increase participation in national and interna tional LGBTQ+ research networks focusing on pregnancy research.
2. Clinical care
- Promote outreach from clinical practices to communities and organizations serving LGBTQ+ individuals.
- Create universal staff training and office guidelines to promote a safe and friendly environment for LGBTQ+ individuals.
- Create or participate in a multidisciplinary network with clinicians providing gender‐affirming and patient‐centric services.
3. Education
- Add curricula focusing on gender-inclusive healthcare to medical schools and training programs for all healthcare professionals.
- Provide continuing medical education focusing on gen der‐affirming care and health disparities within pregnant LGBTQ+ populations.
- Provide educational and community resources for pregnant patients and families.


1. Kirubarajan et al., Fertil Steril 2021;115(5):1294-1301


2. Everett et al., Mat Child Health 2019;23(1):72-81
3. Hodson et al., BJOG 2017;124(3):393-402
4. Croll et al., BJOG 2022;129(10):1625-1629
The Society for Assisted Reproductive Technology (SART) together with mothership, ASRM, on March 30, 2023, released preliminary 2020/2021 national IVF outcome data in a press release.1 Since this release happened just before the publication of The VOICE’s May issue, we were able to only include a brief commentary, pointing out that the announcement, in our opinion, overlooked what appeared to be the most important, development in comparison to 2019/2020 results. Therefore, we promised for the June issue a more detailed discussion, which is now helped by final SART and CDC reports having been published for this year.2,3
As already noted last month, live birth rates from IVF mildly improved from the preceding year. Since approximately 2010, when national IVF live birth rates started plateauing before, a few years later, plunging to levels not seen since the mid1990s,4 an improvement in live birth rates over the preceding year is really news, suggesting that the observed declines since 2010 hopefully, may have finally been arrested, offering hope for a return to better cycle outcomes.
Somewhat surprisingly, this was not the headline. That spot was taken by the observation that the number of egg-freezing cycles in 2021 jumped by 31%.1 In some ways, it should not surprise that SART chose this headline over the one we would have chosen because this choice reflects, especially over approximately the last decade, a very obvious trend in IVF practice, increasingly turning into a social service from once having been a real medical treatment.
After fighting for decades to get infertility recognized as a medical disease entitled to medical treatment like any other disease, we, by increasingly emphasizing social egg-freezing (yes, we know that some colleagues do not like this terms because they fully understand the potential consequences that arise from the use of this term), but oocyte cryopreservation to “extend female fertility,” is, of course, much more of a social than a medical service.
These cycles increased from 16,786 to 24,558 (31%), while IVF cycles in general, likely due to penned-up demand from the COVID-19 period, increased by only 19%. In other words, social egg-freezing cycles grew approximately 40% quicker than all clinical IVF cycles. If this trend continues (and why shouldn’t it?), we soon can expect to perform more fertility preservation cycles than active infertility treatment cycles, a trend, we are certain is very much welcomed by Wall Street investors in the fertility field, but is it a healthy trend for the fertility field and ultimately for our patients?
We do not think so, and the reasons are only too obvious: Fertility preservation through oocyte cryopreservation mostly
exists in a vacuum. There is hardly any quality control,not because we don’t want quality control, but because there can be no real quality control when only a small fraction of women return to use their cryopreserved oocytes. Not only is the effectiveness of social egg-freezing, therefore, still unknown,5 but IVF centers have, practically, no reasonable ways to assess the quality of their oocyte freezing programs. No wonder the popularity of egg and embryo freezing cycles (aside from obvious financial benefits); who would not prefer to practice IVF without being judged by the outcomes of their IVF cycles?

Although they are not identical national registries, the CDC’s ART Success Rates for 2020 have also been published.3 According to this data set, out of 326,468 total IVF cycles 123, 304 (37.8%) were either egg or embryo banking cycles. In other words, almost 40% of all cycle starts did not result in an immediate cycle outcome and, moreover, required an additional treatment cycle to have such a potential cycle outcome. What is the basis for such a dramatic practice change from fresh embryo transfer to an embryo banking concept? We do not know the answer to this question because we are unaware of any convincing evidence in the literature that would suggest better cycle outcomes from such delays in an overwhelming number of cases (there, of course, are exceptions when all-freeze cycles are indicated); all we see are delays in treatment, lower live birth rates, and higher costs for the patient. Indeed, all recently published evidence argues against the concept of embryo banking on clinical grounds,6 and a meta-analysis investigating this issue has been announced.7
The CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, elsewhere in this issue of The VOICE describes the logarithmically increasing adverse cumulative effects on IVF cycle outcomes from congruent utilization of multiple add-ons in one IVF cycle. Here is a good example for this argument.
Continued on page 31
Here are also a few additional observations: According to SART, twin pregnancies declined by almost 20% from 6% to 5%, an observation very much welcomed by SART and, likely, most colleagues who consider twin pregnancies an adverse IVF outcome. That The CHR does not consider every twin pregnancy an adverse outcome requires no repeating here; we have repeatedly explained in these pages and the literature why we (and many patients) see the risk-benefit considerations for twins often differently from an obvious majority of our colleagues. Yet, increased use of elective single embryo transfer is, of course, in principle responsible for this change.
Another important observation is that, according to SART, 50.8% of all IVF cycle starts took place in women under age 35, and only 3.9% in women above age 42. To contrast this with The CHR experience, in the 1st quarter of 2023, 73% of IVF cycles at the CHR occurred in women above age 43. We are presenting this contrast to demonstrate that these small national 1st cycle numbers in women above age 42 do not correspond with the reported aging of infertility patients in the U.S. In practical terms, this, simply, means that U.S. IVF centers, still direct women above age 42 pretty automatically into egg donation, - and that is a pity!
Furthermore, to address the old question of whether fresh and frozen donor eggs result in similar outcomes: Live birth rates were 44.7% and 41,4%, respectively a difference of 3.3 absolute percentage points and 7.4% relative difference in favor of fresh donor eggs. Unsurprisingly, donated embryos had the lowest live birth rate at 40.5%.
What are then the ultimate conclusions from another year of national outcome data? Many national practice patterns, still, simply make no sense and, unfortunately, our IVF laboratories and practice patterns have become increasingly inefficient. How do we know that? Based on the least-biased observation point in IVF, the use of fresh donor eggs, where there are the least differences in practice patterns between IVF clinics and patient populations (i.e., young egg donors) are most similar. In 2005, the clinical pregnancy rate in fresh egg donor cycles was 52%. Even assuming an unrealistically high miscarriage rate that still demonstrates that even in third-party donor egg cycles, we, still are worse off today than we were in 2005. What is the excuse for that?
1. https://www.sart.org/news-and-publications/news-and-research/press-releases-and-bulletinsegg-freez ing-cycles-jumped-31-in-2021/#:~:text=a%20marked%20increase%20in%20fertility,in%202021%20compared%2to%22020.
2. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx

3. https://www.cdc.gov/art/reports/2020/summary.htm
4. Gleicher et al., Hum Reprod Open 2019(3):hoz017
5. Wang et al., BMJ Open 2019;9:e030700
6. Ben Rafael Z. Hum Reprod 2020;35(10):2179-2184
7. Wang et al., BMJ Open 2022;1297):e62578
The CHR mourns the premature deaths of Adina Azarian and her daughter, Aria, who perished on June 4th, 2023 in a plane crash in rural Virginia. We extend our condolences to the Rumpel family who lost their daughter and granddaughter.

Gleicher N, Mochizuki L, Barad DH, Patrizio P, Orvieto R, on behalf of the International Do No Harm Group in IVF (IDNHG-IVF). A review of the 2021/2022 PGDIS Position Statement on the transfer of mosaic embryos. J Asst Reprod Genet 2023; 40:817-826
In collaboration with colleagues from Israel and Miami with endorsement by the IDNHG-IVF, the CHR’s investigators published what we consider to be a very important opinion paper because literally, line by line, it critically reviews the most recent guideline document issued by the PGDIS (Preimplantation Genetic Diagnosis International Society), this time claiming to address the transfer of mosaic embryos. To understand the importance of this obviously quite critical commentary, some historical information is essential: The PGDIS until 2016 was known as a very small and rather obscure special interest group, mostly made up of geneticists involved in what then was called PGD (preimplantation genetic diagnosis) and mostly meant genetic testing of embryos for single-gene diseases. The idea of biopsying embryos to derive genetic information from them came about in the 1990s when investigators recognized that every cell in an embryo, of course, contains the embryo’s complete genetic code. Consequently, if a genetic defect causing a single gene disease was present in an embryo, every biopsied cell from this embryo should demonstrate this defect. Therefore, PGD for single gene defects from the very beginning was a very accurate and clinically useful test.
However, the same cannot be said for a test first introduced in the late 1990s, then called preimplantation genetic screening (PGS). This test also relied on the idea that biopsying cells off an embryo can provide genetic information on the embryo, - but this time, the target was not a genetic mutation present in every cell of the embryo. This time, the hypothesis (since demonstrated to be incorrect) was that, like single disease-causing mutations can be found in every cell of an affected embryo, chromosomal abnormalities would also be detectable in all cells. Of course, that turned out not to be the case. Whether out of scientific ignorance or blinded by recognizing the opportunity to vastly enlarge the use of genetic embryo biopsies during IVF cycles, the genetic testing community incorrectly started claiming to have the ultimate panacea for improving IVF cycle outcomes, - the deselection of aneuploid embryos before embryo transfer, thereby improving implantation, pregnancy, and live birth rates for remaining embryos.
Though only based on a hypothesis and never really validated, by 2010, the idea of PGS had caught fire within the IVF community and increasing numbers of IVF centers started utilizing the procedure almost routinely in all IVF cycles. It helped that roughly half of the income from the procedure went to IVF centers (for performance of the embryo biopsy). To this day, not reimbursed by any health insurance plan because of the above-noted lack of validation studies (even if IVF represents a covered benefit), the additional cash income for IVF centers from this procedure was increasingly welcome, as expanding insurance coverage reduced general IVF reimbursement rates for many clinics. The utilization of chromosomal testing of embryos in IVF cycles, therefore, has continued to expand unabated to this day.
By not recommending such testing, the CHR became an exception and increasingly argued in several publications against the utilization of PGS because data generated at the center and reanalysis of data published by colleagues from around the world, impressed upon the CHR’s investigators that PGS, as practiced in those days, simply did not make biological and/or medical sense. In 2014, the CHR on an experimental basis started transferring selectively so-called chromosomal- “abnormal” embryos, by PGS reported as “aneuploid.” In 2015, the CHR reported the first chromosomal-normal births after the transfer of allegedly chromosomal-“abnormal” embryos.1 A presentation on the last day of the annual ASRM Conference in Baltimore in 2015 became a sensation, further supported by a report from Italian colleagues who a few weeks later also reported chromosomal-normal births after the transfer of what they called “mosaic” embryos.2 Since there, simply, could be no better evidence for the futility of PGS than deliveries of chromosomal-normal newborns from supposedly chromosomal-abnormal embryos, the genetic laboratory testing community and IVF practitioners recommending PGS, suddenly, found themselves in the midst of a considerable credibility crisis. This is when in 2016, the PGDIS stepped out of the shadows by publishing a first formal guideline document regarding the practice of PGS, from a marketing standpoint also conveniently
Continued on page 34
renamed at the time to preimplantation genetic testing for aneuploidy (PGT-A), the acronym to this day used for the procedure.
The PGDIS by that point was still a small and barely known special interest group of mostly laboratory geneticists, run out of a genetic testing laboratory in Chicago, where, originally, the idea of PGS was born and where the PGDIS was founded. The guidance document the PGDIS published in 2016 had no authors, had not even a single reference attached, did not fulfill even the most basic criteria for publications of medical practice guidance documents suggested by the literature, did not provide any information on who collected and interpreted data, did not disclose the obvious economic conflicts of practically all members of the PGDIS, starting with the leadership of the society, and, likely most remarkably, was just published as an e-mail to a mailing list and deposited on the website of the PGDIS. Suffice it to say, there was also no evidence of any form of peer review of the document before publication.
Yet, beyond astonishing, in the absence of formal guidance documents on the subject from ASRM and ESHRE at the time, this PGDIS guidance from its publication in July of 2016 on, has dictated to this day, despite its unprecedented shortcomings, the practice of what now is called PGT-A. Moreover, the PGDIS since 2016 published two additional guidance documents, with the latter being the document in detail reviewed by our here-cited recent publication. According to this PGDIS guidance, it supposedly replaced the two earlier guidance documents of the PGDIS
It would exceed the purpose of this column and available space to go into further detail about why our paper mostly rejected this most recent guidance document of the PGDIS. For this purpose, we refer interested readers to the publication (you can order a reprint by contacting Alexandra Rata at arata@thechr.com). Only so much though, the second and third guidance documents from the PGDIS have improved and were published in a legitimate journal (with direct ownership conflict with the PGDIS, as both organizations were founded by the same individual), though quite obviously without proper peer review, the most recent document still lacks any of the widely accepted criteria regarding how medical guidance documents are supposed to be published. Most importantly, the PGDIS must finally be seen for what it is: A highly biased and conflicted small special interest society whose members almost exclusively make a living from performing PGS/PGT-A. This is not the kind of scientific body that should issue guidance documents; yet, both ASRM and ESHRE, in their own related guidance documents, amazingly, quite often refer to PGDIS statements in the society’s guidance documents. Considering how obviously conflicted the PGDIS is regarding PGT-A, one must wonder how that is possible.
1. Gleicher et al., Fertil Steril 2015;104: e59
2. Greco et al., N Engl J Med 215;373:20892030
Gleicher N, Barad DH. Some caution about DHEA supplementation. F S Rep. https://www.fertstert.org/ news-do/some-caution-dhea-supplementation
This manuscript so far appeared only as an electronic preprint, but is worthwhile noting because the CHR’s clinicians here report a rather unusual observation. Having for many years been treating hypo-androgenic infertile women with DHEA at a daily starting dosage of 25mg TID, they only rarely found the patients’ androgen levels to overshoot desired ranges. However, that appeared to change during the COVID-19 pandemic, when the CHR’s physicians increasingly found themselves having to reduce the daily dosage patients were receiving because their androgen levels did overshoot. After documenting that this, indeed, happened more often than before, the CHR decided to communicate this finding to colleagues through the above-noted publication because most DHEAprescribing IVF clinics, still, do not follow their patients’ androgen levels. Consequently, they may not even be aware that this has recently been happening.
Though as of this point, the cause(s) for this change are unclear, the suspicion is that the raw material for all DHEA products in the U.S. come basically exclusively from China, possibly from mostly only one factory, and this raw product appears to have increased in biological potency. The CHR’s investigators concluded that this was the most likely explanation because it saw excessive androgen levels in patients who received DHEA products from different U.S. manufacturers. The CHR in this manuscript strongly recommended that IVF clinics prescribing supplementation with DHEA to their patients, check androgen levels at regular intervals and adjust the dosage of daily DHEA accordingly.





ADVERTISEMENT
The CHR is searching for a candidate for the newly created position of Embryology Laboratory Supervisor for Research. The CHR’s embryology laboratory, under a single laboratory director, is in the process of being reorganized into three distinct areas with separate supervisory responsibilities: (i) clinical, (ii) administrative, and (III) research.
Supervisors in all three areas must hold PhD degrees (or equivalent) and be fully trained human embryologists with sufficient historical professional experience to hold a supervisory position.
https://www.centerforhumanreprod.com/contents/video-gallery Center for Human Reproduction



While such human embryology experience is preferred for this new position as well, priority qualifications are a record of excellence in reproductive biology research, documented by publications in prestigious peer-reviewed journals and, in absence of human IVF experience, at least substantial animal IVF experience allowing for relatively quick in-house training in human IVF.

Besides a competitive salary and benefit package, the CHR also offers in this position a unique financial incentive-structure linked to the success of the center’s research activities, as demonstrated by publications in prestigious peer-reviewed journal. Moreover, this position will also be eligible for the opportunity to earn shared ownership in research-driven new start-up companies and the center itself.
If you feel that you qualify for this position, please submit your CV and a brief application letter to the CHR’s COO, Ms. J. Tapper, at jtapper @thechr.com. The position is available immediately. All submissions are considered confidential.
Mostly placed into a clinical context, we in this section of the newsletter offer commentaries to a broad survey of articles in the English literature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. Nevertheless, these articles, however, often point out where clinical practice will likely go and, therefore, serve an important translational purpose. Translational research has been the CHR’s principal research goal since its founding in 1981, has produced a significant number of U.S. patents over the years, and has propelled the CHR into its current position as a worldwide center of last resort for infertile patients who have failed treatments elsewhere. The here-presented section for the first time makes public how the CHR for decades has been interpreting the published literature, a process that always has been at the very core of how research and clinical practice evolved at the CHR.
In order to shorten the summer break, this June issue of The VOICE appears ca. two weeks later in the month than usual and the number of here-reviewed papers is significantly larger than usual. We this month, especially recommend our discussion of three papers: (i) RCTs vs. “real world evidence” in the section on “Important general medical news;” (ii) In the section on “Reproductive genetics,” the discussion on why aneuploidy in cancers has major importance for reproductive medicine and, (iii) In the section on “Reproductive endocrinology and infertility,” the discussion of a Chinese paper on 10 rather unique PCOS patients.
Healio.com. in an excellent article published on May 26, 2023, reviewed the impact of private equity investments in medicine.1 Though the article does not specifically refer to the infertility field, the observations reported throughout several practice areas in medicine in general, of course, also apply to infertility. The article noted that since 2010, nearly $11 trillion has been invested in U.S. health care across multiple specialty areas through thousands of acquisitions of hospitals and individual practices and/or practice groups. While 325 such deals were announced in 2010, by 2021, there were in excess of 1,000. More than half of a total of $2.6 trillion in worldwide investments into health care took place in the U.S., in 2021 alone exceeding 150 billion. Initial attractive specialty areas, like anesthesiology, dermatology, and ophthalmology were joined
by newly popular areas of practice, like rheumatology (and, of course, infertility).
In addressing the consequences of what the article calls “corporatization” (and we in the past have called “industrialization”), a prominent rheumatologist in Texas is quoted as stating the obvious: “Corporatization is changing medicine and, in many cases, not for the better.” Of course, this is a sentiment also expressed on several occasions in these pages. Another prominent rheumatologist is quoted asking, “Whether the business of making money (obviously the principal goal of private equity investments) can coexist with providing optimal care?”
Consequences also extend to physicians, who increasingly end up being employed by hospitals, health systems, and/or private equity-owned commercial entities rather than “hanging a shingle themselves,” as used to be common practice. The article notes that, in the U.S., currently only 15% of rheumatologists are left in private practice. We, of course, have repeatedly pointed out that over recent months, the number of private practitioners has fallen below 50% nationwide and continues to decline at an accelerating pace, as an older generation of physicians is retiring.
Trends caused by these developments discovered by studies, according to the article, included increases in the volume of medical services, increased profits, improved payor mix, increased payments per patient visit, increased use of mid-level practitioners (whatever that may mean), decreased physician autonomy, and decreased physician salaries, - overall, therefore, not great news!
Among physicians who sold their practices, complaints also abound and appear not only to be a consequence of going from being an employer to being employed: One also frequently hears concern about having to see many more patients than before, and not being able to give those patients adequate time and attention. Moreover, as one prominent physician quotes: “Moving forward, doctors will no longer have the option to remain independent and, as employees, will not receive the monetary benefits of their work efforts. As with any venture, private equity will be seeking returns on their investment and that will have to come from reduced provider income. It is as simple as that, and it is already happening.”
Although this kind of view about the future of medicine in the U.S. is a possibility, one can also imagine a very different future. Under such an alternative scenario, private equity (and other organizations employing physicians) because of quickly growing physician shortages in practically almost all countries of the Western world, including the U.S, Canada, the U.K., Australia, New Zealand, Israel, Austria, and Germany, to name only a few, employers may, actually, have to pay higher salaries (the infertility field is only one example where, in the U.S., this is already happening). These staffing shortages do not only apply to physicians; they may be even more acute in the nursing environment, where droves of nurses have left the profession after the COVID-19 pandemic. Foreseeing increasing staffing shortages in all areas of medical services, and the then rather automatic following increases in provider costs, one must wonder whether earnings projections of private equity investors (and other medical provider organizations) in hands-on health care may not be overly optimistic.
As the article also notes, health care in the U.S., as a whole, is moving (or stated even better, “merging”) toward increasingly larger group entities (so-called “super-groups”), whether this means larger and larger chains of clinics in a specialty (as witnessed in the fertility field, especially over the last decade), hospital chains gobbling up medical practices of private providers, insurance companies buying up “super-groups” and, effectively, creating private single payor systems, longitudinally and horizontally integrated under one ownership umbrella. United Health Group, as the article notes, is for all practical purposes already a single-payer health care system, having their own insurance company, pharmacy benefit management company, specialty pharmacies, multi-specialty groups, imaging centers, as well as surgical centers. What differentiates such an organization from a government-run single-payer system?
Probably, as of this point, still better economic management, and efficiency, but the bigger these organizations will become, the less efficient and the more conflicting will internal economic interests be between different divisions of such a single provider
organization. With profits mostly going to shareholders rather than innovations of the system, one must increasingly wonder about the overall logic behind what is driving our evolving healthcare systems in the U.S., - and where everything is moving to. Limited to a maximum of 5-7 year frameworks, investment strategies in health care are often short-term. Often accumulating debt with every sale, as investors cash out their short-term profit gains, companies get holed out from the inside, under increasing stress to produce ever more revenues, - yet, often, lacking the financial resources to support growth.
Unsurprisingly, bankruptcies in health care, therefore, appear already on the rise. Among relatively small players in the infertility field on April 30, 2023, for example, Zhang Medical P.C. DBA New Hope Fertility Clinic filed for bankruptcy in the U.S. Bankruptcy Court for the Southern District of New York (Manhattan) under petition #:23-10678.3 Among much bigger players, as we reported in these pages already last month, partially backed by private equity giant KKR (holding a ca. 30% stake in the company), GenesisCare, a large provider of cancer-services recently was reported to have filed for bankruptcy with over $1.7 billion in debt.4 Envision Healthcare, another healthcare provider in physician staffing also backed by KKR, filed for chapter 11 a few weeks earlier, with KKR writing off their complete investment of $3.5 billion. 5 Though these two developments are not directly related to the fertility field, indirectly they are much more connected than one might think, since KKR just recently closed under somewhat unusual circumstances at a record enterprise valuation of over $4 billion on the purchase of what claims to be the largest network of IVF clinics in the world, IVIRMA Global SL.6 While the deal was initially financed by some of the world’s biggest banks, they allegedly in unison sold their financing commitments at significant losses before the deal even closed.7
Venture capitalists, nevertheless, are still raising huge amounts of cash to invest in health care, - though much of this cash has remained on the sideline, as investments in startups have been significantly slowing.8 In an e-mail on May 4, 2023, from Griffin Jones (Inside Reproductive Health), Fertility Bridge, LLC, this was also indirectly confirmed when Ron Shinkman in an article noted that the M&A of fertility clinics in the first four months of 2023 halved in comparison to 2022. The reasons appear obvious: borrowing costs have quintupled, inflation is never good for sales, and raised concerns about the demand for future fertility services in an inflationary environment cannot surprise, even though patient demand in 2023 appears to have remained strong so far. At the same time, fertility center acquisitions in the U.S. continue, as the article also noted, with several such purchases of individual clinics described, though with their purchase prices significantly down in multiples of EBITDA.
Concerns about the growing influence of private equity in health care delivery were recently also expressed in a “Viewpoint” article in JAMA, in which the two authors offered a potential policy framework as to how arising problems may be addressed.9 Their conclusions made sense: Considering increasing and concerning emerging evidence of changes in economic and clinical outcomes
in association with rapidly growing private equity acquisitions, enforcing existing laws would be a first point. Currently, only mergers above a transaction value of $111.4 million are subject to federal regulatory review. This cut-off, however, excludes over 90% of private equity transactions from such review, and federal regulatory agencies are understaffed for even the small volume of reviews they currently are conducting. The authors, therefore, suggested that state governments may be better suited to respond by decreasing reporting threshold, expanding executive actions, and increasing transparency for such transactions.
1. https://www.healio.com/news/rheumatology/20230524/private-equity-ishere-investment-deluge-in-medicine-may-be-saving-grace-or-its-doom
2. Smith Y. News Medical Life Sciences. https://www.news-medical.net/ health/Physician-Shortage.aspx
3. https://bkdata.com/business-bankruptcies/ manhattan=newyork/04-30-2023/zhang-clinic-10678
4. Yerak B. The Wall Street Journal. https://www.wsj.com/articles/ kkr-backed-cancer-treatment-provider-genesiscare-files-for-bankrupt cy-953132cf
5. Gladstone A, Biswas S. The Wall Street Journal, May 26, 2023. https:// www.wsj.com/articles/kkr-backed-healthcare-provider-genesiscare-prepsfor-bankruptcy-filing-within-days-e838a82e
6. Market Screener. January 12, 2023. https:// www.bloomberg.com/news/articles/2022-09-08/ banks-give-up-on-kkr-s-ivirma-deal-pay-private-funds-to-take-it#x j4y7vzkg
7. Ruckin C. Bloomberg. September 8, 2022. https:// www.bloomberg.com/news/articles/2022-09-08/ banks-give-up-on-kkr-s-ivirma-deal-pay-private-funds-to-take-it#x j4y7vzkg
8. Gormley B. The Wall Street Journal. May 25, 2023. https://www.wsj.com/ articles/venture-fundraising-in-healthcare-rises-as-investment-in-start ups-slows-67f3bd7c
9. Cai C, Song Z. JAMA 2023;329(18): 1545-1546
The PitchBook Platform reported on May 12, 2023, that dealmaking for women’s and reproductive health-tech companies has slowed.1 That includes companies offering family planning services, including contraception and abortions, but also fertility services. The slowdown has originally been, at least in part, attributed to the June 2022 Dobbs decision by the Supreme Court, but after that decision’s impact has waned, the report suggests that its impact was quickly replaced by the banking crisis and shrinking capital availability. Consequently, funding rounds appear to now take four to six months to close, while in better times during 2021, they used to close in as little as four weeks.
Venture capital deals in healthcare IT, in contrast, appear to have picked up from a low point in late 2022, according to a May 31, 2023, report in the PitchBook Platform 2 $1.3 billion raised in Q1 2023 in ca. 70 rounds suggest a bullish market for health care IT, especially relating to generative artificial intelligence (AI) that supports interoperability. As Marina Temkin in a May 22, 2023, PitchBook Platform analysis noted, generative AI early-stage investors are now living in two distinct worlds: One is reflected in the existing potential for generative AI, while the other is defined by helping existing portfolio companies to survive the current funding slump.

We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO of the CHR jtapper@thechr.com. All submissions are considered confidential correspondence.
Some generative AI startups without any revenue are now raising Series A rounds at valuations around $250 million. For the rest of the early-stage companies, the expectation is that the graduation rate from seed to Series A rounds will fall significantly in the coming quarters.
1. https://pitchbook.com/news/articles/ femtech-womens-health-abortion-startups-venture-capital
2. https://pitchbook.com/news/ reports/q1-2023-healthcareit-report?utm_ter&utm_ source=daily_pitch&utm_ content=q1_2023_health care_it_report
Continued on page 42
In an article in Forbes magazine, Matt Novak, founder of Paleofuture.com, recently suggested that Google was about to turn the online publishing industry upside down,1 making us wonder whether the same concerns may not also apply to medical publishing. The reason for these concerns was Google’s announcement that the company was planning changes for how it presented search engine results by utilizing generative AI. Novak argued that, consequently, Google Search users will no longer have to visit pages that contain the content they were searching for because generative AI in a narrative-style answer will offer answers to hard questions without any longer requiring visits to pages of origin for those answers. Yet, it is this visit to the original page that allows the online publishing industry to monetize their publications, whether it is large publishers like newspapers or individual authors who are writing for individual websites.
Here at The VOICE, we are not too concerned about this possibility. We believe that our patients, colleagues, and other readers will continue coming to our website, even if Google’s generative AI may include our materials in its responses. In contrast to general knowledge, medical knowledge is and will remain, much more reference-dependent. If you do not want to be misled by medical publications, as so often noted in these pages, medicine almost always requires a careful direct evaluation of published materials before their acceptance. After all, how can we ever know whether the results of a paper are real, unless we determine that the study design and analysis were conducted appropriately, and conclusions reached by the authors made sense?
1. https://www.forbes.com/sites/mattnovak/2023/05/11/google-is-about-todrop-a-nuclear-bomb-on-the-online-publishing-industry/?sh=29777cea3faa
Who does not remember the two cryo-tank failures on the same day on March 4, 2018, on both coasts in two well-respected IVF clinics? In another e-mail from Griffin Jones on May 25, 2023, Ron Shinkman this time reports that Chart Inc. reserved over $300 million for settlements of the Westcoast cryogenic tank failure at Pacific Fertility Center in San Francisco, ruining over 4,000 frozen eggs and embryos of approximately 600 patients. The IVF clinic, and its owner, Prelude Fertility, claiming over 85 clinics in their Prelude Network in the U.S., in NYC including two NYU Langone Fertility Centers, were, according to the article, accused of negligence for failing to monitor the liquid nitrogen tank more closely. Chart Industries, the manufacturer of the malfunctioning tank was later added to over 550 cases currently in courts or in private arbitrations, among which some 217 suits involve Chart Inc.
Since most contracts with patients contained an arbitration clause, the clinic in 2019 succeeded in obtaining a court ruling forcing most
lawsuits into arbitration, with more than 340 already arbitrated. Since Chart Inc. did not have such contractual protection, it could not pursue a similar legal strategy. In a 2021 federal trial, the jury found that Chart Inc. was aware of potential defects in the tank’s electronics, but failed to take steps to repair or recall the tanks. Overall, in a lawsuit involving only five patients, the jury awarded them $ 14.975 million, with Chart Inc. held responsible for 90% and Pacific Fertility for 10%.
For the second time in a year, an IVF pregnancy produced at Assuta Medical Center in Tel-Aviv, Israel, one of the country’s largest and most prominent IVF centers, was found not to have a genetic link to one of the parents, in this case, the father, according to a report published in the Jerusalem Post on May 18, 2023.1 The parents apparently initially requested that no inquiry be made and that their privacy be protected. However, only a few days later, they claimed that the hospital had offered them “hush money,” so that the case (which occurred over two years before the discovery) would not be reported to the Health Ministry, as required by law.2
By May 22, 2023, Israel’s Health Ministry launched a formal investigation of the national IVF health system while announcing the transfer of all IVF from private hospitals to public hospitals.3 Since most IVF cycles in the country are paid for by the government, this may reflect an epochal change in the Israeli IVF system.
Whether such a change will be for the better is, however, questionable since the principal problem in our opinion does not appear to lie in the difference between public and private embryology laboratories. but in excessive government control over how IVF is practiced in Israel overall: Israel allows for only a relatively small number of licensed mega-IVF laboratories, which are geographically distributed throughout the country. These laboratories, in turn, serve individual physicians in large numbers of private office practices who manage their own patients independently, but share with colleagues in those mega-embryology laboratories and attached egg-retrieval facilities. These embryology laboratories, therefore, serve patients from large numbers of different clinics, with different management philosophies and, therefore, also with different cycle outcomes. Proper quality control is practically impossible since it becomes impossible to determine whether a cycle outcome is a consequence of good versus bad patient preparation or good versus bad laboratory performance. Moreover, serving so many different physician masters also makes the management of such mega-laboratories much more difficult and unnecessarily complex. Moving the problem from private hospitals into public hospitals will not solve existing problems in embryo laboratories; it will only move the problems from private to public hospitals.
As we see it, the problem lies in the interruption of the “chain-of-responsibility” process, as it is practiced in most U.S. IVF centers, where clinics (or chains of clinics) under single ownership and under single administration, provide preparatory
patient services as well as retrieval and laboratory services. To maintain “chain-of-responsibility,” which involves proper preparation of patients for egg retrieval and transfer as well as management of gametes in the embryology laboratory under strict “chain-of-custody” rules for every egg and every semen sample, therefore, is less complex and much easier to oversee.
In the U.S. (including in NYC), also exists a small number of embryology laboratories that are “open” to multiple physicians who practice IVF without having their own laboratories. Such laboratories, however, are the exception and, as one would expect, considering their small number, they also have been disproportionally in the news. Based on their practice model, “open” laboratories, simply by definition, must be at increased risk for “adverse events.”
As noted above, this second case reported in a year by the same private hospital chain is, of course, alarming and must be investigated. However, we caution against jumping to conclusions prematurely and reaching incorrect conclusions. First, though an unlikely cause, when it is paternity that is found not to match (in contrast to when maternity does not match) non-laboratory related explanations can be the cause: women in IVF cycles have been reported to have conceived spontaneously unrelated to their IVF cycle4 and paternities in spontaneous pregnancies, of course, can vary. Discrepancies from expected paternity are, indeed, quite common.5 The other possibility is a laboratory mix-up of two semen samples, which under “onespecimen-at-the-time” and “chain-of-custody” rules in most embryology laboratories should not occur. Yet, the busier a laboratory is at any given time, the likelier becomes the possibility of such a mix-up if these two basic laboratory rules are not followed.
1. https://www.jpost.com/breaking-news/article-743548
2. https://www.jpost.com/health-and-wellness/pregnancy-and-birth/ article-743834
3. https://www.jpost.com/health-and-wellness/pregnancy-and-birth/ article-743846
4. Bavan et al., Case Rep Obstet Gynecol 2019;1804948
5. Grethel et a., Fam Relations 2022;1-17
In a Medscape article, Roxanne Nelson noted that according to data from the American Society of Health-System Pharmacists (ASHP), drug shortages in the country have not been as bad in over a decade. The clinically most significant shortages affect cancer drugs. Moreover, what turns these shortages into a double whammy is the fact that many of these cancer drugs have no alternative drugs, and therefore, shortages have become life-threatening for some cancer patients. As of early May, 15 such cancer drugs were on the FDA’s shortage list. Except for oncology drugs, other affected drug classes were drugs used in
Continued on page 44
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.
CNS disorders, antimicrobials, fluids and electrolytes, and hormones. Over the last year, the infertility field has witnessed shortages in several popular drugs.
REFERENCE
1. Nelson R. Medscape. https://www.medscape.com/viewarticle/992144
As we previously reported, significant concern has been expressed over the last year by the FDA that breast implants may be associated with increased breast cancer risk, mostly of squamous cell carcinoma (SCC) and especially anaplastic large-cell lymphoma (ALCL) 1,2 Now a study published as a research letter in JAMA Surgery offered reassuring data regarding SCCs.3 However, these tumors are distinct from ALCLs, which have been the subject of a black box warning for all saline and silicone gel-filled breast implants since 2020.
In this new study across 421,227 person-years of follow-up, only one case of SCC (2.37/million versus 1.02/million in the general population; 95% CI, 0.06-13.0) was recorded, which did not reflect an increased incidence.
However, it is important to point out that the study also identified five cases of ALCL, which corresponds to a prevalence of 11.9/million compared to 0.29/million in the general population. This reflects a robust, statistically significant association. The following is an explanation of ALCL from the FDA. 4
Breast Implant-Associated Lymphoma (BIA-ALCL) is not breast cancer - it is a type of non-Hodgkin’s lymphoma (cancer of the immune system).5
In most cases, BIA-ALCL is found in the scar tissue and fluid near the implant (see figure), but in some cases, it can spread throughout the body. An individual’s risk of developing BIA-ALCL is considered to be low; however, this cancer is serious and can lead to death, especially if not treated promptly. In most patients, it is treated successfully with surgery to remove the implant and surrounding scar tissue, and in some patients, also treated with chemotherapy and radiation therapy. The risk of BIA-ALCL is higher for textured surface implants versus smooth surface implants.
The main symptoms of BIA-ALCL are persistent swelling, the presence of a mass or pain in the area of the breast implant. These symptoms may occur well after the surgical incision has healed, often years after implant placement. Upon evaluation by a health care provider, evidence of fluid collection around the breast implant (seroma) is often observed. Some patient reports indicated that a lump under the skin or capsular contracture (thick and noticeable scar capsule around the implant) was present.

1. https://www.fda.gov/medical-devices/safety-com munications/update-reports-squamous-cell-carcinoma-scc-capsule-around-breast-implants-fda-safety-communication
2. Worcester S. Medscape https://www.medscape. com/viewarticle/991177
3. Kinslow et al., JAMA Surg2023;doi:10.1001/ jamasurg.2023.0262
4. https://www.fda.gov/medical-devices/breast-im plants/questions-and-answers-about-breast-im plant-associated-anaplastic-large-cell-lympho ma-bia-alcl
5. https://www.fda.gov/medical-devices/breast-im plants/questions-and-answers-about-breast-im plant-associated-anaplastic-large-cell-lymphomabia-alcl
Like the preceding subject, the rapidly increasing utilization of legalized pot has also repeatedly been a subject of discussion in these pages, - mostly because of concerns about offspring following great increasing maternal use in pregnancy and increasing use by teenagers. In both cases, the concerns mostly relate to the effects of cannabis on the young brain, with the active chemical ingredient being delta-9-tetrahydrocannabinol (THC)
David Hitzenrath in an excellent article in Clinical Advisor, recently contributed to the discussion by pointing out that legal pot is more potent than ever but, still, largely unregulated.1 He in his article points out that only a few decades ago, the THC content in weed was less than 1.5%, while current products in the market exceed 90%. The consequences are obvious, as he also points out: “The buzz of yesterday has given way to something more alarming: Marijuana-related medical emergencies have landed hundreds of thousands of people in the hospital and millions are dealing with psychological disorders linked to cannabis use, according to federal research.”
Higher THC concentrations increase the risk of physical dependency and addiction. Yes, the pot of today can be addictive! Moreover, increasing dosages also in parallel increase the risk of psychiatric conditions, including anxiety, paranoia, and psychosis, including schizophrenia. Especially young males appear at increased risk for schizophrenia.2 He further notes that according to the federal Department of Health and Human Services (DHHS) in 2021, 16.3 million people in the U.S. –5.8% of all citizens over age 12, experienced so-called marijuana use disorder (MUD) within the preceding year, a much larger number than the combined total of substance abuse with cocaine, heroin, methamphetamine, prescription stimulants (i.e., such as Adderall), or prescription analgesics, such as fentanyl and OxiContin. Indeed, 1 in 7 cases had severe MUD, which can be equated with “addiction.”
Current cannabis excesses, indeed, have resulted in new medical diagnoses, such as “cannabis dependence with psychotic disorder with delusions” and “cannabinoid hyperemesis syndrome,” a condition of unstoppable vomiting. Recreational or adult use is now legal in 22 states and DC.
The article also points out the absurdity of current policymaking in this country, with all of these relatively recent developments being caused by the widespread legalization of cannabis, while the FDA is almost completely sidelined, yet at the same time claiming maximal efforts to combat smoking and vaping. To demonstrate the absurdity of the situation even further, the federal Drug Enforcement Administration (DEA), part of the Justice Department if one can believe that, considering the above-outlined effects, still correctly, classifies marijuana as a controlled substance.
Yet, out of political opportunism, the Justice Department did not even respond to the author of the article when he asked for some questions to be answered. However, the blame goes much further: Congress has been doing nothing but limiting enforcement of the DEA’s classification of cannabis as a controlled substance. President Biden in October of 2022 directed the secretary of HHS and the attorney general to “review the federal government’s stance toward marihuana.” Yes, there are already “Marijuana Anonymous” meetings for recovering marijuana addicts all over the country! This is only the beginning: If current-day marijuana can do all of the above-described things to adult brains, imagine what it can do to developing brains in-utero when the mother uses, or in middle school and high school students, where use has also exploded.
1. Hilzenrath D. Clinical Advisor. May 12, 2023. https://www.clinica ladvisor.com/home/topics/practice-management-information-center/ legal-pot-is-more-potent-than-ever-and-still-largely-unregulated/
2. Hjothøj et al., Psychol Med 2023;1-7. Doi: 10.1017/S0033291723000880
A recently published study in JAMA1 and an exquisite editorial to this study, 2 should be read by anybody interested in how studies in medicine should be conducted and interpreted. Both manuscripts address a core issue in medical research, namely that prospectively randomized controlled trials (RCTs) represent the peak of the evidentiary pyramid,3 yielding the strongest inference about the effects of medical treatments and, therefore, allow conclusions about the effects of treatments and/or of causal relationships. Theoretically, all medical practice should, therefore, be based on such studies; yet only somewhere in the 10-20% range of all current medical practice has been considered to be evidence-based,4,5 even though we, supposedly, live in an area of evidence-based medicine.
Unsurprisingly, one will find no phrase more frequently in concluding paragraphs of published papers than, “more RCTs are still required …”, even though everybody knows that they will never be conducted. There are, of course, good reasons why they never will be conducted, such as patient resistance to participate because they do not want to take the chance of being a placebo designee (a very frequently encountered problem especially in older women with infertility, who correctly argue that they may have not the time to lose six months to a placebo). Yet, the most frequent cause is impracticability: RCTs are complicated to set up, time-consuming, and, often, unaffordable for investigators, left to pursue study
designs at lower levels of the evidence hierarchy and, therefore, in the literature often dismissed as insufficient.
So-called observational studies are one such example and are generally believed not to be able to lead to causal inferences and, therefore, can only describe potential associations and correlations and must not lead to cause-and-effect conclusions. Yet, as the editorial correctly noted, such observational studies, increasingly derived from very large data sets (consider, for example, the national annual CDC and SART/ASRM IVF outcome reports), often represent “real-world evidence” and play an increasingly prominent role in health care. Moreover, the idea that such data can greatly contribute to our understanding of treatment effects – possibly to even similar degrees as RCTs, has been around for some time.3 However, Wang et al now expanded on the size of a correlation study in which they attempted to emulate the design of 30 completed and two ongoing RCTs with database studies using observational analogues (population, intervention, comparator, outcome, etc.) and to quantify agreement in RCT database study pairs.
What they found was very similar to earlier attempts at testing the same point: “Real-world evidence can reach similar conclusions as RCTs when design and measurements can be closely matched, though may at times be difficult to achieve. Here is why we liked R. Christopher Sheldrick’s editorial so much:2 he did not, as many other commentators may have done, end up reciting the usual cliches of RCTs being the only ultimately acceptable study format. Instead, he logically argued that there are cases in which RTCs cannot be closely emulated. He then made the important point that, paradoxically, “real-world-evidence” in those cases, does not fully emulate an RCT may actually represent a particularly important feature of “real-world-evidence,” as it may be more reflective of actual clinical care. How “true” is the perfection of a treatment application in a RCT, if such perfection cannot be achieved in “real-world-clinical practice” (a point also made by others6)?
Though he did emphasize that database studies are not a substitute for RCTs, comparisons between both offer insights into the validity of each study design and can contribute to the knowledge of sufficient causation. To offer in conclusion of the discussion of these two remarkable papers an example in the documentation of how important a point this is, consider the PGT-A debate in which the CHR (and several colleagues outside of the CHR) for years have been arguing based on several studies that PGT-A not only fails to improve IVF outcomes but, for many patients, actually adversely affects outcome chances. Only once colleagues recently analyzed a national data set, did this message finally attract attention.7
1. Wang et al., JAMA 2023;329(16):1376-1385
2. Sheldrick RC. JAMA 2023;329(16):1352-1353
3. Concato et al., N Engl J Med 2000;342(25):1887-1892
4. Ebell et al. BMJ Evidence-Based Med. 2017; https://ebm.bmj.com/ content/22/3/88
5. Howick et al., J Clin Epidem2020;126:154-159
6. Franklin et al., Clin Pharmacol Ther 2020;107(4):327-35
7. Kucherov et al., J Assist Reprod Genet 2023;40(1):137-149
Of course, it is widely accepted that research involving human embryos must be carefully regulated. However, how and by whom this regulation should be organized is undetermined. Moreover, one hears increasing concern that research, especially in genome editing and other biotechnologies (for example A.I. applications), is moving too fast for regulators to even keep up. The latter was, indeed, the subheading of a recent feature article in Nature magazine1 and yet another article that started with comments on the 3rd International Summit on Human Genome Editing that took place in March in London, U.K. (on which we reported repeatedly in these pages since then).
The article noted that currently, neither the World Health Organization (WHO), nor many other international organizations have the power to create rules for research. Of course, there is HFEA (Human Fertilisation and Embryology Authority) in the U.K. that controls IVF practice and, therefore, by extension also related areas such as, for example, the experimental use of nuclear and/or spindle transfer procedures to treat mitochondrial diseases by using in this way third-party donor plasma with unaffected mitochondria. Then, there is the FDA in the U.S. which already decades ago prohibited experiments of partial cytoplasm exchange in infertile women because this creates three-parental embryos, with the donors of cytoplasm becoming a third genetic parent by contributing mitochondrial DNA.
The author also noted that most scientists feel bound by rules published by professional bodies like the International Society for Stem Cell Research (ISSCR), but opinions of professional societies have neither legal force, nor do they always reflect general societal concerns. The consensus appears to evolve that somatic-cell editing (which is not heritable) is increasingly warranted in treating medical conditions, such as sickle cell disease (the conference hosted a talk by a woman with sickle cell disease who had been successfully treated by somatic-cell editing of her body’s red blood cells). Yet, the current consensus also holds that germline editing is a very different story and requires much more caution.
The article concludes that regulatory approval simply cannot happen at the same speed as scientific development. Regulation will always run behind, not the least because regulation in most democratic countries involves query and inclusion of public opinions, -uniformly a very time-consuming process.
REFERENCE
1. Ball P. Nature 2023;617:242-243
In last month’s issue of The VOICE, we already reported about the rather mind-blowing work of Japanese scientists who in mice, produced new eggs from male cells. Those eggs then were fertilized and produced healthy pups.1 First publicly reported at the above-noted ISSCR on March 8, and shortly thereafter published in Nature magazine, we (and others) described this work as a milestone in reproductive medicine. Now, two science writers reported also in Nature how all of this happened.2 Researchers have been working on accomplishing this for many years. In 2018, researchers already reported using embryonic stem cells, produced from either sperm or eggs, to generate pubs with either two fathers or two mothers. Interestingly, those made from two mothers survived and were able to produce pups, themselves. However, those, produced by two fathers, lived only for a few days.3 By 2022, Katsuhiko Hayashi, PhD, who recently moved his laboratory to Osaka University in Japan, published his by now famous mouse paper, in which he completely reconstructed the reproductive cycle in the ovary, producing eggs, capable of fertilization, and producing multiple generations of pups.4
By having all of this knowledge, Hayashi et al reprogrammed in preparation for the here-discussed paper, cells from an adult male mouse and created stem cell-like induced pluripotent stem cells from them. They grew those cells over many generations in culture, knowing that under such circumstances, a small number of these cells spontaneously lose their Y-chromosome (a normal male has
We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratory supervisor for research
To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

an XY karyotype). Using a trick also used by the CHR’s investigators and their collaborators in a not-too-long-ago published paper in Nature Cell Biology to induce aneuploidy (chromosomal abnormalities),5 they then treated those cells with a substance called reversine, and hoped that would create some cells which would by accident be female, having two X chromosomes (XX karyotype). From there on, things were relatively easy because all they had to do is to repeat the induction cycles, they previously had reported in their mouse model that had led to healthy births of multiple generations of pubs. 4
The survival rate of transferred embryos this time was very low: only 7/630 transferred embryos developed to pubs, - a much lower number than in their earlier experiments. However, those that did survive were normal and fertile.
As exciting as this new accomplishment of the Hayashi team is for our understanding of basic reproductive biology, clinical applications in humans, as the recent Nature article suggested, will still take time. So far, nobody has been able to repeat Hayashi’s earlier accomplishment in the mouse, of producing new eggs in ovaries that have the ability to be fertilized in humans and result in normal human births. That this and similar experiments will not happen soon is not necessarily based on the fact that, “man is not mouse,” but that the necessary experiments are simply not ethically possible in humans. However, seeing them soon done in large primates, would not surprise!
1. Murakami et al., Nature 2023;615(7954):900-906
2. Kenharo M, Wolf L Nature 2023;615:379-380
3. Li et al., Cell Stem cell 2018;23:665-676
4. Yoshio et al., Science 2021;373.abe0237
5.Yang et al. Nat Cell Biol 2021;23:314-321
Speaking of large primates, two recent papers in Cell by two different Chinese groups of investigators reported on exiting new ex utero 3D monkey models which were able to offer new insights into early post-implantation periods of organogenesis in a monkey model. The first paper examined advanced gastrulation and early neurulation of the cynomolgus monkey embryo between days 7 to 25 post-fertilization,1 while the second paper concentrated in the same monkey species at weeks 3 to 4 on developmental milestones, including gastrulation and formation of organ primordia.
Both manuscripts basically demonstrated similar results, confirming that 3D culture systems of monkey embryos apparently allow for longer ex-vivo embryo cultures than 2D systems. Moreover, both in principle also demonstrated that this monkey species on single cell levels functions very similarly to humans as well as smaller animal models. Since limitations of available human model systems and practical as well as ethical limitations of human embryo research post-implantation create major problems for the investigation of early embryo developmental stages in human pregnancy, both of these studies, in principle, confirm on a molecular level, that the cynomolgus monkey can serve as a very good substitute.
1. Zhai et al., Cell 2023;18:2078-2091
2. Gong e al., Cell 2023;186:2092-211
In the earliest stages of mammalian development (in the mouse only at zygote and 2-cell stage), do these cells have true totipotent qualities, reflective of unrestricted potential to form a completely new organism. To be able to grow such cells in the laboratory has been a longstanding goal of biological research and based on a recent publication in Nature magazine (again by Chinese investigators),1 it appears that, at least in the mouse, we are getting closer to reaching this goal. In this study, the investigators demonstrated (in a mouse model) that a “defined chemical cocktail” can induce long-term maintenance of such totipotent stem cells from mouse pluripotent stem cells using a combination of three small molecules: the retinoic acid analogue TTNBP, 1-azakenpaullon, and the kinase blocker WS6.
Based on an earlier pioneering recognition that cultures of mouse embryonic stem cells made up of pluripotent cells also contain a small group of cells that resemble cells at the 2-cell stage of mouse embryos (which, as noted, are still totipotent),2 suggested to the investigators that there must be a way to maintain totipotent cells in culture. The researchers in the here-addressed paper, therefore, started a search for small chemicals that could enhance the expression of a fluorescent reporter-gene active in the 2-cell minority cell population, thereby identifying the above-noted three molecules and naming the resulting cells, chemically induced totipotent stem cells.
The so-created totipotent cells demonstrated bidirectional developmental potential and produced embryonic as well as extraembryonic cells in vitro and in teratomas. After injecting into 8-cell embryos, those cells, moreover, were highly efficient in contributing to embryonic (fetus) as well as extraembryonic (placenta) cell lineages.
A commentary on this article in Nature3 notes that two additional recent papers 4,5 also have pointed out the progress that is being made by the research community in obtaining renewable cultures of totipotent stem cells in the laboratory. Though the long-term culture of totipotent stem cells in humans has so far not been accomplished, the recent commentary in Nature was optimistic that limitations still existing will soon be overcome.3 The author, furthermore, suggested that assuming from totipotent stem cell-derived embryos becoming endowed with the ability to implant in uteri and undergo normal pregnancy, broad new horizons – along with significant ethical challenges – would, of course, open up. The relevance of infertility treatments is instantly obvious, but the relevance of all of this for regenerative medicine may be even greater.
1. Hu et al., Nature 2023;617:792-797
2. Baker CL, Pera MF. Cell Stem Cell 2018;22:315-324
3. Pera MF. Nature 2023;617:683-684
4. Yang et al., Cell Stem cell 2022;29:400-418
5. Mazid et al., Nature 2022; Nature 2022;605:315-324
Since we are already talking about totipotency, a recent review article in Reproduction, once again mostly in the mouse, discusses very well oocyte biology, which, of course, is a prerequisite for totipotency post-fertilization.1 An interesting aspect of this review article is the authors’ discussion of the stoichiometry of different reprogramming and remodeling factors present in the oocyte, their balance, and how these factors in combination lead to the formation of new organisms.
1. Fulka et al., Reproduction 2023;165: R75-R89
Under the overall title, “When a delicate balancing act goes wrong,” a special section in a May issue of Science magazine was recently dedicated to autoimmunity. Even though we in these pages pretty constantly point out how closely pregnancy and autoimmunity are interwoven, that by itself would, nevertheless, not be reason enough to feature this section here. The principal reason why we, however, are pointing attention toward this special section is that tolerance is a recurrent theme. And tolerance is, of course, the essential physiological phenomenon without which there would be no pregnancies.
Autoimmunity is the phenotypical presentation of a breakdown in self-tolerance by an immune system. Since pregnancy is the ultimate example of temporary tolerance of a rapidly growing solid semi-allograft by the maternal immune system (and if third-party donor eggs are involved, the allograft is 100% allogenic). Just like to this day, what causes autoimmune diseases is in principle, unknown,1 we also to this day do not know how the maternal immune system established tolerance for a logarithmically in size increasing graft and does so for only on average 40 weeks. Yet, just like tolerance to self can break down, so can
tolerance of the fetal-placental graft, because how else is the onset of labor and, especially, premature onset of labor to explain, - but as an abrupt ending of tolerance?
The introductory perspective article has, per se, nothing to do with pregnancy, since it addresses the current unexplained observation that autologous hematopoietic stem cell transplants (AHSCTs), originally developed to combat hematopoietic malignancies, have been found to be an effective treatment for autoimmune diseases, such as multiple sclerosis, systemic sclerosis, Crohn’s disease, and systemic lupus erythematosus.1 Interestingly, nobody understands why that is, though the article attempts to offer some suggestions. The basic point is that the conditioning treatment with chemotherapy and biological therapeutics ablating all lymphocytes also eradicates the very obviously “out-of-balance” disease-causing factor in the immune system that, in the first place, induced the autoimmune disease. Reinfusion of the patient’s own previously collected hematopoietic progenitor stem cells then reestablishes the patient’s own “rejuvenated” hematopoiesis, apparently short of the disease-inducing factor, - whatever that may be and, with it, reestablishes self-tolerance. Who does not believe that there may not be something to learn from this, for example, about improving the weakening tolerance of the fetal-placental graft of pregnancy?
The second article in the special section, which is a review of spatiotemporal regulation of T cell tolerance,2 has even more relevance and directs attention to the fact that the large volume of gut microbiota we all carry around with us must be tolerated. Considering how important our gut microbiome has been demonstrated over the last decade to be for health as well as various diseases and their treatments, this article addresses the, at times very complex and recently discovered facts, that underlie the establishment of intestinal tolerance in general. The manuscript, therefore, emphasizes the intestine as a model tissue for peripheral T cell tolerance in general, in the process comparing immunity to pathogens, tolerance to self-antigens, and tolerance to microbiota and demonstrating that during the development of the immune system in early life, distinct waves of tolerogenic immune cells emerge from the thymus and in the periphery. Somewhat disappointingly, the article does not differentiate between female and male immune systems, - obviously, a subject of general neglect in immunology, - yet, equally obviously, an important question to be answered, considering that female immune systems must acquire the unique ability to tolerate a rapidly growing solid (semi)-allograft for on average 40 weeks, and male immune systems, of course, do not ever have to face such a challenge.
A third article, again a review, addresses how inborn errors of innate immunity can instigate B cell
autoimmunity, causing all kinds of autoimmune syndromes, from chilblain to systemic lupus erythematosus (SLE), and severe interferonopathies.3 The article suggests that the tipping point between antiviral immunity and autoimmunity hinges on the strength of nucleic acid sensing and the amount of interferon I (IFN I) production. Unsurprisingly, genes involved in IFN I production linked to viral susceptibility genes, have also been recognized as SLEassociated genes. Pathway protecting hosts against viral infections, therefore, can also cause autoimmunity if signaling is excessive or if there is an overabundance of ligands. Most interestingly, in species in which viruses can threaten reproductive success, autoimmunity may, therefore, from an evolutionary vantage point, be considered an essential trade-off that protects reproduction, in an evolutionary sense, of course, always the first priority.
Finally, a fourth review article discusses common genetic factors among autoimmune diseases and points out connections to pathogen-driven infectious selection pressures for an increased prevalence of autoimmune diseases.4 That the occurrence of autoimmunity is highly familial has been known for decades and the general assumption has been for some time that a general genetic predisposition toward autoimmunity is polygenetic inherited, with epigenetic environmental factors then deciding which autoimmune condition is phenotypically expressed. As the article notes, autoimmune diseases display a high degree of comorbidity within individuals and families, suggesting shared risk factors for the occurrence of varying autoimmune diseases in families, but also for the occurrence of more than one autoimmune disease in individuals. Interestingly, over 90% of putative causal genetic variants associated with autoimmune diseases have been found in noncoding regions of the genome. Such predominantly common variants are present in over 5% of the population.
The high prevalence of autoimmune alleles in the general population must be the consequence of evolutionary preservation of risk alleles for a higher purpose and, as already noted above, that purpose must have significant importance, in this case being the presumed ability to protect from infections by improving an individual’s overall resistance to infection.
Unrelated to the special section in Science magazine, The Washington Post on June 1, 2023, addresses the interesting increasingly obvious connection between psychiatric conditions and at least some autoimmune diseases.5 The newspaper did this as part of a report about a catatonic woman with schizophrenia who after 20 years awakened after receiving aggressive treatment for SLE, after one physician raised the possibility, she could suffer from SLEinduced autoimmune disease of her brain.5
The understanding of immune system effects on the brain for the longest time was hampered by the false belief that the brain was an immunologically privileged site in the body without a functioning immune system. With the discovery of a fully functioning immune system in the brain only relatively recently, the field of autoimmune neurology has made rapid progress.6 Within this context, it
is important to note that the CHR’s medical director and Chief Scientist, Norbert Gleicher, MD, already in 2007 penned an article in Autoimmune Reviews, in which he suggested that, simply based on the timing of presentation, postpartum depression, and possible cases of depression in general, may be autoimmune diseases.7 The above-cited article now, indeed, suggests once again that autoimmunity may play a more important role in psychiatry than widely appreciated. For reproductive medicine that, indeed, sounds logical, considering the peripartum flair pattern of several psychiatric diseases, which is so typical for autoimmune diseases.
1. Muraro PA. Science 2023;380(6644):470-471
2. Brown CC, Rudensky AY. Science; 380(6644):472-477
3. Vinuesa et al., Science 380(6644):478-484
4. Harroud A, Hafler DA. Science 2023;380(6644):485-490
5. Sima R. Washington Post, June 1, 2023, https://www.washingtonpost. com/wellness/2023/06/01/schizophrenia-autoimmune-lupus-psychiatry/
6. Ramathatan et al., Nat rev Neurol 2023;19(3):172-190
7. Gleicher N. Autoimmu Rev 2007;6(8):572-576
Human mosaicism is, finally, recognized as an important subject for investigation. On May 11, the NIH launched a $140 M effort to investigate genetic variations in normal human cells and tissues.1 It appears high time for such an effort, called the Common Fund’s Somatic Mosaicism Across Human Tissues (SMaHT) Network. The aim is to determine how much genetic variation exists in cells and tissues throughout our bodies and how they change with advancing age. Such changes can have a wide-reaching impact on health. Because somatic mosaicism can occur in every cell type in the body, the subject goes beyond the reach of any one institute within the NIH, which is why this funding initiative comes out of the NIH Common Fund program.
Though the announcement, for example, refers to the importance of somatic mosaicism in cancer, it, interestingly, does not specifically mention the potential importance of mosaicism in preimplantation stages of embryos and, throughout all of pregnancy, in the placenta, both subjects repeatedly addressed in these pages in the past. Nevertheless, one would hope that some of these funds would go toward such research which, likely, would greatly contribute to our understanding of how the maternal immune system tolerates the rapidly growing fetal-placental semi-allograft of pregnancy.
1. NIH. May 11, 2023. https://www.nih.gov/news-events/news-releases/ nih-launches-140-million-effort-investigate-genetic-variation-nor mal-human-cells-tissues#:~:text=The%20National%20Institutes%20\ of%20Health,and%2 0tissues%20throughout%20our%20bodies.
Like mosaicism, single mutations in DNA also accumulate with age. A recent paper in eLife addresses the issue regarding mutations in mitochondrial (mi)DNA,1 which is inherited exclusively from the mother and represents the only extra-nuclear DNA in cells, since it is located in mitochondria in the cytoplasm. Using a mouse model, the researchers investigated what happens to miDNA with advancing age and reported somewhat surprising results: After detecting >89,000 independent somatic miDNA mutations and demonstrating, as expected, significant tissue-specific increases with age, those increases, however, did not correlate with mitochondrial content and tissue function. Though findings were tissue-specific, there was universally, a lack of accumulation of oxidative damage-linked mutations with age, suggesting a life-long dynamic clearance of either the oxidative lesions or of miDNA genomes harboring oxidative damage.
Should this mouse work be reflective of what happens in humans, this paper may have major significance on current thinking regarding the effects of advancing female age on oocyte quality because current beliefs still hold that declining mitochondrial function in oocytes with advancing female age is a central cause for declining oocyte quality. Building on this belief, cytoplasmic exchange has, therefore, been advocated as a potential treatment approach for older infertile women.2,3 Assuming that in the here-discussed paper observed findings are also relevant to aging human oocytes, replacement of cytoplasmic mitochondria would, however, be futile as a treatment of ovarian aging.
1. Sanchez-Contreras et a., eLife 2023;12:e83395
2. Liu et al., Hum Reprod 2003;18(9):1903-1907
3. Christodulaki et al., Front Endocrinol (Lausanne) 2021;12:635370
Here at the CHR, we, of course, can never get enough new information on PGT-A and that is especially true when an excellent statistician, like Jack Wilkinson, PhD, from the Centre for Biostatistics, Division of Population Health, Health Service Research and Primary Care at the University of Manchester in the U.K., publishes a critical article on how PGT-A has been widely reported in the medical literature. He in this “Views and reviews” article, makes the point that randomized controlled trials (RCTs) must be properly designed and analyzed if they are to provide valuable results. He also notes that IVF offers many opportunities for errors in statistical analyses because of the multi-stage nature of this treatment. As a result, cycle results may be analyzed using different denominators, while excluding potential participants who did not reach certain milestones, and such analyses, therefore, do not produce sound results. This is, of course, a point more than frequently made in these pages, but cannot be often enough repeated, as long as proponents of PGT-A use incorrect statistics in attempts to appear correct. Wilkinson uses a recent study, previously reported and discussed here, as an example.2 On the basis of PGT-A, he then demonstrates how a common statistical error in the analysis of
an RCT, in this case, an analysis of cycle outcome with reference to embryo transfer, can be severely misleading. He furthermore correctly points out that this kind of error is not only restricted to PGT-A studies, but appears unusually frequently in infertility-related RCTs in general. We could not agree more!
1. Muraro PA. Science 2023;380(6644):470-471
2. Yan et al., N Engl J Med 2021;385:2047-2058
When PGT-A is criticized, the complaint usually is the high false-positive rate of diagnoses, leading to the non-use or disposal of potentially normal embryos and wasted pregnancy chances. However, what is often forgotten is that, like any laboratory test, PGT-A can also have false-negative results, i.e., can miss abnormal diagnoses in embryos. The CHR recently experienced such a false-negative test, when an allegedly euploid embryo was transferred and an overall healthy offspring was born but, genetically, was demonstrated to have a segmental chromosomal abnormality, hopefully of no clinical significance. The laboratory that reported this PGT-A result is still exploring how this oversight might have happened.
Related, IVF patients undergo extensive single-gene testing for recessively inherited conditions, which can give rise to affected children if both parents carry the same recessive gene. Errors in this kind of genetic testing should really not occur, but such an error was just reported in a case report in JARG, when a case of X-linked Menkes syndrome was diagnosed in a newborn in Denmark, a disease caused by mutations in the ATP7A gene that regulates the metabolism of copper.
That any form of medical testing will have false-positives and false-negatives, therefore, must be accepted. However, every such case must be closely investigated as part of a quality assurance process, - to keep errors as rare as possible.
1. Gerdes et al., J Assist Reprod Genet 2023; 40(4):811-816
It has been estimated that approximately 5.2% of the human genome is made up of segmental duplications (duplicons), which means regions of more than 1 kb in length. They are either segmental on the same chromosome (3.9%) or inter-chromosomal between chromosomes (2.3%). Most of the duplicons are in the pericentromeric regions.1 In other words, they are frequent and, therefore, like their counterpart – chromosomal deletions- quite commonly detected in human embryos during PGT-A. Though duplications can cause rare congenital birth syndromes in affected offspring like the Pallister Killian syndrome (extra chromosome #12 material), they are much more
rarely associated with congenital syndromes than segmental deletions, which are much more frequently causing such syndrome, like Cri-du-chat, Prader-Willi, and Wolf-Hirschhorn syndromes.2
Now U.S investigators in a paper in Nature magazine reported that human segmental duplications are characterized by increased numbers of mutations and gene conversions.3 The authors suggest that the observed very distinct mutation properties help to maintain an overall higher GC content (high cytosine/ guanine DNA sequences are more stable) of DNA in segmental duplications than in unique DNA, which is likely driven by GCbiased conversions between paralogous sequences.
Interestingly, the CHR’s investigators reported that segmental duplications and deletions produced the highest pregnancy rates among embryos with PGT-A diagnoses of all kinds of chromosomal abnormalities.4

1. https://www.sciencedirect.com/topics/ biochemistry-genetics-and-molecular-biology/human-genome
2. Clancy S, Shaw KM. Nat Education. 1(1):23. https://www.nature.com/ scitable/topicpage/dna-deletion-and-duplication-and-the-associated-331/
3. Vollger et al. Nature 2023;617:325334
4. Barad et al., Hum Reprod 202;37(6):1194-1206
Continued on page 52
A group of U.S. investigators developed a CRISPR-based technology to study chromosome-specific aneuploidy by targeting human centromeres. The authors designed highly specific sgRNAs for 19 of 24 chromosomes, thereby describing an innovative (though quite complex) technology to create and study missegregation and aneuploidy within the context of cancer but also beyond that.1
1. Bosco et al., Cell 2023;186:1985-1991
Science magazine did something recently that is rarely done, - it invited CHR-friend and collaborator, Samuel F. Bakhoum, MD, PhD, to pen a Prize (for innovation) Essay,1 essentially summarizing his spectacular research career as a young physician-scientist (he is an attending, practicing radiation oncologist, and group-leader in the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center). Starting with his PhD thesis, his research interest has been directed by chromosomal instability and, therefore, chromosomal abnormalities (aneuploidy) in cancer. He, indeed, discovered that cancers can develop resistance to treatment through chromosomal instability and aneuploidy, which allows them to circumvent tumor checkpoints our immune systems have set up to prevent and/or fight early cancer.

In the process of conducting research, Bakhoum has won practically every young investigator award offered in biology research, starting with the Outstanding Young Investigator Award from the American Society for Clinical Investigations and the American Academy of Physicians. In 2018, he was honored with the NIH Director’s Early Independence Award and the Burroughs Welcome Fund Career Award for Medical Sciences, as well as the Blavatnik Family Foundation Regional Award for Young Scientists and the Radiological Society of America’s Roentgen Research Award. In the same year, he was also named a NextGen Start by the American Association for Cancer Research
In his essay in Science, he explained that his research started with the basic biology underlying chromosome segregation during cell division, then turned to computational approaches that model the behavior of tumor cell populations, and most recently, used experimental tools to selectively interrogate chromosomal instability in tumor evolution and metastasis.1 For further details, we refer our readers to his essay which tells the history of his lab’s research brilliantly and in more detail.
Shortly after his essay was published in Science, a true tour-de-force paper appeared in Nature (of course the, likely, two most prestigious science journals in the world), in which his lab beautifully demonstrated how chromosomal instability alters genomic copy numbers but also promotes epigenetic reprogramming and heterogeneity in cancer.2
What does all of this have, likely, to do with reproduction? The answer is very simple: what is bad (from our point of view as patients) for cancer is good for pregnancy. Based on Bakhoum’s work, which explains how cancer via chromosomal instability and subsequent aneuploidy circumvents rejection, so does the fetal-placental semi-allograft likely circumvent rejection by the maternal immune system through persistent aneuploidy in the placenta. Without the ability of the maternal immune system to tolerate the big “tumor” of pregnancy, we all, likely, would not be here!
1. Bakhoum SF, Science 2023;380(6640)
2. Agustinus et al., Nature 2023; doi: 10.1038/s41586-023-06084-7. Online ahead of print. PMID: 37286593
It has become increasingly clear in recent years that there is a male equivalent to female polycystic ovary syndrome (PCOS) when it comes to hormonal, metabolic, and clinical phenotype features.1
Now a group of mostly Swedish investigators reports transgenerational transmission of reproductive and metabolic dysfunctions in the male progeny of women with PCOS. Specifically, they report that sons of women with PCOS are frequently obese and dyslipidemic. Moreover, if offspring of obese and/or androgen-exposed mothers, they also demonstrate reproductive and metabolic problems across generations, mediated by small RNAs dysregulation in sperm. Moreover, in PCOS-sons and PCOS mothers, altered miRNAs target PCOS risk genes, and small RNAs in sperm imply transgenerational phenotype transmission. The authors correctly conclude that their work strengthens prior mouse work and further amplifies still underappreciated risks of reproductive and metabolic alterations across generations via the male germline. Viva “male-PCOS!”
1. Di Guardo et al., Int J Fertil Steril 2020;14(2):79-83
New York State Department of Health (NYSDH) updated donor (gamete) (infectious disease and other)
The following changes in rules guiding tissue donations in NY State were announced by the NYSDH in qualifying gamete (and other) donors:
All required clinical laboratory testing shall be performed by a laboratory operating under a permit issued by the (N.Y. State) department. For out-of-state tissue acquisitions by N.Y. State-licensed banks, all required clinical laboratory testing shall be performed by a laboratory that is approved by the state’s regulatory authority, the (U.S. Health Care Financing Administration) Centers for Medicare and Medicaid Services, or by the Department.
Blood samples from all allogeneic donors of tissue for clinical use, except oocyte donors tested in accordance with section 52-8.6([h]i) of this part shall be tested for evidence of infection with HIV-1, HIV-2, hepatitis B virus (HBV), including hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) and, except for donors of eye tissue or tissue to be virally inactivated, human T-lymphotropic virus type I (HTLV-1) for purposes of donor selection.
Client depositors are defined as males who deposit reproductive tissue prior to intended or potential use in IUIs or IVF solely on himself or themselves (if involving a sexual partner) or women who deposit reproductive tissue for processing into embryos and subsequent implantation into the same woman.
For reproductive tissue banks located within N.Y. State, all required clinical laboratory testing shall be performed by a laboratory operating under a permit issued by the department.
For out-of-state reproductive tissue banks, all required clinical laboratory testing shall be performed by a laboratory that is approved by the state’s regulatory authority, the (U.S. Health Care Financing Administration) Centers for Medicare and Medicaid Services, or by the Department.
Embryos shall not be created for donation (from donor oocytes and donor semen) except at the request of a specific patient who intends to use such embryos for her own treatment.
A recent study of over 72,000 blood donors revealed that 96% had antibodies against the SARS-CoV-2 virus.1 In addition, 48% had so-called hybrid immunity, a combination of immunity made up of immunity from infection and immunity from vaccines, now widely accepted as the most effective immunity; 23% had antibodies only from infection and 26% had antibodies only from vaccines. In practical terms, this means that only roughly a quarter of the population is only protected by vaccine-induced immunity, obviously not a very reassuring number. Hybrid immunity, moreover, not only appears to be the best but also appears to last the longest.
Related, The Lancet Infectious Diseases recently published a paper, that demonstrated that COVID-19 booster vaccinations produced significant protection against severe disease for up to one year, though declines in protection against the Omicron variant were already observed after seven months.2
1. Jones et al., MMWR Morb Mortal Wkly Rep 2023; 72(22):601-605
2. Wu et al., Lancet Infect Dis 2023;S1473-3099(23)00199-8
One sometimes wonders why certain studies are published because expected outcomes appear so obvious. We call them “self-fulfilling prophecies.” One such study was recently published by U.S. investigators in Fertility and Sterility. It then reported in the usual big and red headlines that “a higher number of oocytes retrieved is associated with an increase in fertilized oocytes, blastocysts, and cumulative live birth rates.”
By “really,” we do not mean to denigrate the authors’ study; we, indeed, want to congratulate them on having executed and published this study because the rather absurd truth about our
medical specialty is that this so very obvious message, very obviously, is not as obvious to everybody as it obviously should be!
Who really can doubt that more eggs are better than fewer eggs, will produce more embryos, and, ultimately, therefore, more pregnancies and live births? If that is the case, how come so many colleagues still believe in natural cycle IVF, mild stimulations, elective single embryo transfers, etc.? For all of those who still believe in these essentially completely illogical treatments, read the paper by Fanton et al; and if, after that, you continue offering your patients those routine mild stimulation cycles, nobody can really help you,and your patients.
1. Fanton et al., Fertil Steril 2023119;762-769
And the bad news for the ERA is piling up. We, only a few months ago, reported in these pages on the excellent clinical trial of ERA conducted by colleagues in Maryland which demonstrated no outcome benefits from the test. Now two colleagues, completely unrelated to the original study group, took their data and reanalyzed them in order to determine whether the ERA is even able to determine a correct implantation window.2
For us unsurprisingly, they demonstrated that the ERA was, indeed, unable to identify the window of implantation, as proponents of the test have now claimed for practically a decade. Yet, even more significantly, they in the process also demonstrated that utilization of the ERA reduced live birth rates rather than, as claimed, increased those. Therefore, they conclude that so-called “personalized” transfers in IVF, based on ERA testing, should immediately be stopped.
A few additional comments are at this point in order: First, the CHR never introduced the test into practice because several of its claims physiologically never made sense to us. Second, as also discussed elsewhere in this issue of The VOICE, what usually happens to unvalidated “add-ons” to IVF once they have been
Continued on page 54
“sold” to the field is, that studies, in the first step, demonstrate that an “add-on” does not result in promised IVF outcome improvements (fails to meet superiority of outcomes). Shortly thereafter, studies demonstrate that this “add-on” not only does not meet the criteria of superiority, but actually meets the criteria of inferiority, meaning that the “add-on,” actually reduces pregnancy and live birth chances in IVF for at least some patient sub-populations.
We have seen this sequence of events play out in conjunction with routine extended culture to blastocyst-stage, PGT-A, and now also for utilization of the ERA. Who then can be surprised that live birth rates in IVF have been declining all over the world after 2010,3 when “add-ons” reached a peak in popularity?
1. Doyle et al., JAMA 2022;328(21):2117-2125
2. Richter KS, Richter ML. Hum Reprod 2023;dead083
3. Gleicher eta l., Hum Reprod Open 2019(3):hoz017
A recent paper by Chinese investigators, published in JCEM, caught our attention, and did so for several reasons: The first, and in many ways for us the most interesting reason was the fact that, in trying to study their PCOS patients, the authors noted that “in their center, most women with PCOS were of non-hyperandrogenic phenotype,”1 which is exactly the observation we made under similar circumstances several years ago, when initiating a PCOS study on the CHR’s patients.2 Since the Chinese investigators subsequently excluded
the few hyperandrogenic PCOS patients they had among their PCOS patients, their study is the first “clean” study of, likely, only phenotype-D PCOS patients (also called the “lean” PCOS phenotype) we have seen in the literature since we published the CHR’s data set. As such, their results cannot be understood as representative of all PCOS patients but only of phenotype-D PCOS patients. However, the authors deserve credit for, even in the title of their paper, pointing out that they studied only normo-androgenic PCOS patients.
They investigated only 10 such women and 10 non-PCOS control patients, with the PCOS group being very young (mean age 28.5 ± 1.78 years). This means that between the approximate ages of 25 and 35, the study’s non-hyperandrogenic PCOS patients, indeed, must be expected to be in the normal androgen range, - after which phenotype D PCOS women drop into hypo-androgenic ranges.2-4 Since this is also the age when most PCOS cases are diagnosed, these women are considered ”not-hyperandrogenic” under Rotterdam Criteria, even though we now know that before approximately age 25, they too were hyperandrogenic, but demonstrate after age 25 a steady decline in androgen levels that, by approximately age 35, makes them reach hypo-androgenic levels.2-4 This is only one clear distinction between phenotype D and other phenotypes. The D-phenotype also distinguishes itself from especially A- and B- phenotypes by being “lean” rather than obese and being mostly ovulatory and, therefore, demonstrating regular menses. This PCOS phenotype, therefore, is often overlooked, and affected women go through life undiagnosed. This is not all that distinguishes the phenotype-D from other
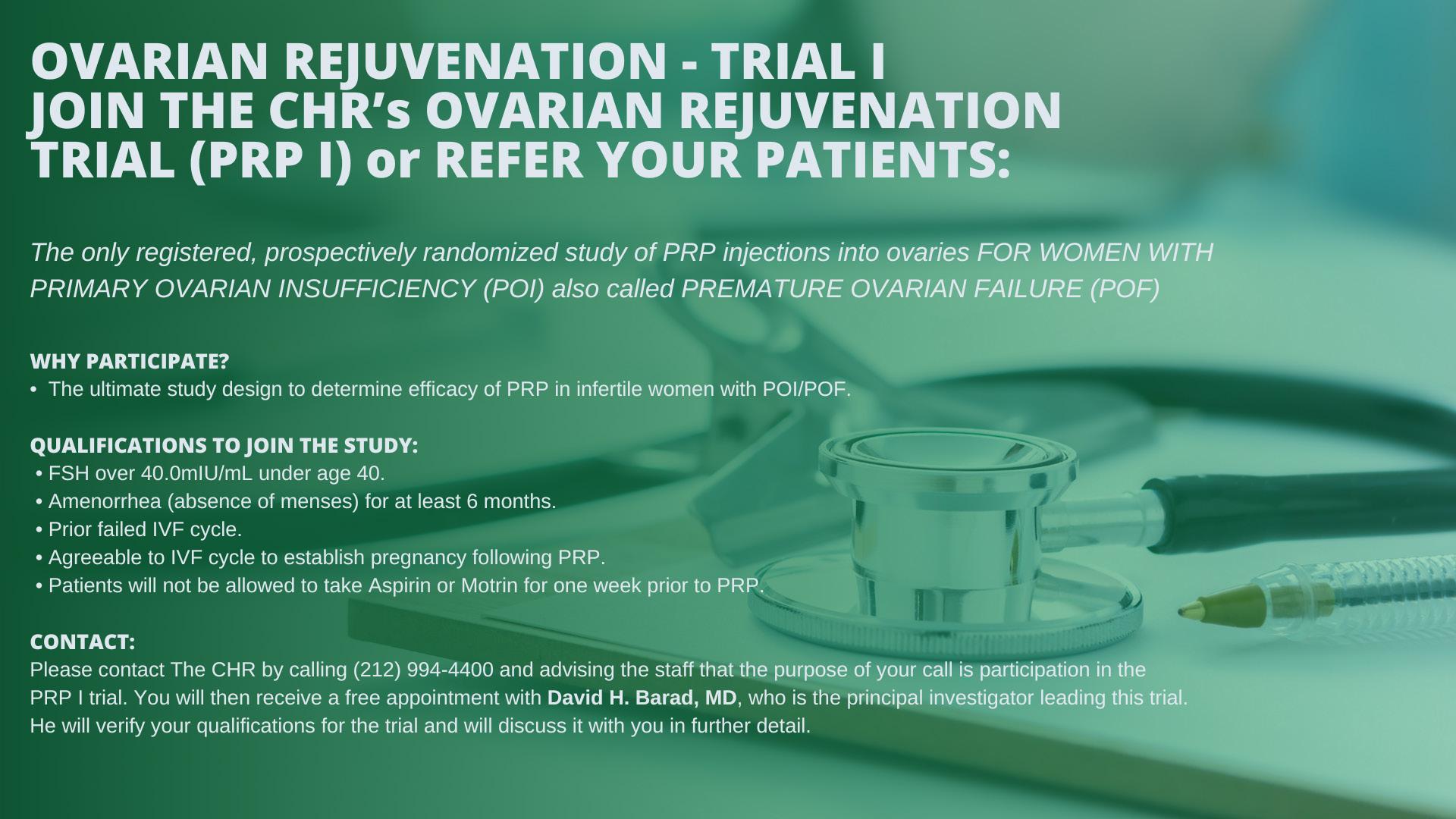
PCOS phenotypes: The D-phenotype also does not demonstrate the association with metabolic syndrome at later ages as other phenotypes are known for but, instead, demonstrates a very high association with a hyperactive immune system, manifested as autoimmunity, inflammation, and/or of being hyper-allergic.3,4 Moreover, because the D-phenotype after age 25 is usually ovulatory, it usually does not cause infertility until affected women become hypo-androgenic after age 35. Consequently, a large majority of infertile D-phenotypes present to infertility clinics only after age 35. Since more and more women, these days, start attempts at conception only at older ages, this frequently overlooked diagnosis is increasing in infertility centers.
That our Chinese colleagues were able to find only 10 such patients for their study suggests a very young patient population and also suggests that the authors were unaware of the CHR’s revised description of the ontogeny of phenotype-D PCOS with advancing age. They apparently also were unaware of our suggestion that PCOS probably should no longer be described as a syndrome made up of four phenotypes, but as two genetically, likely, distinct conditions, one encompassing what under Rotterdam Criteria are phenotypes -A, -B, and, likely, -C, and the other what is currently described as phenotype-D.5 Her discussed paper, indeed, supports such a contention.
Now to the also very interesting results of the paper that supports a very typical physiology in cases of phenotype-D PCOS:1 The authors in this study investigated whether follicular extracellular vesicles (EVs) in PCOS patients interfere with oocyte quality. EVs are small membrane vesicles that in recent years have attracted considerable interest in various areas of research because of the diversity of proteins and nucleic acids they carry with them as cargo and deliver to cells. In genetically unstable oncogene-driven cancer cells, EVs were recently demonstrated to trigger micronuclei formation in endothelial cells.6 If the term micronuclei sounds familiar by now, as we earlier in these pages noted when discussing why aneuploidy in cancer is important for reproduction, Sam Bakhoum’s laboratory recently demonstrated how chromosomal instability alters genomic copy numbers but also promotes epigenetic reprogramming and heterogeneity in cancer and does so, at least partially, through the content of micronuclei which are then reintegrated into nuclei of cells.7
Liu et al then reported that EVs of PCOS and control patients were internalized by oocytes within one hour, but PCOS oocytes following this internalization demonstrated significantly reduced oocyte maturation in comparison to control oocytes and, in addition, demonstrated an increased rate of abnormal mitochondrial distributions, and abnormal spindles, as well as increased reactive oxygen species (ROS) and excessive expression of catalase (CAT), glutathione synthetase (GSS), and superoxide dismutase (SOD) in oocytes. Their data suggested that EVs in normo-androgenic young phenotype-D PCOS patients adversely affected mitochondria and spindles in oocytes, and inhibited oocyte maturation, with oxidative stress being a potential cause.
1. Liu et al., J Clin Endocrinol Metab 2023;108:1394-1404
2. Kushnir et al., J Ovarian Res 2015;8:45
3. Gleicher et al., J Steroid Biochem Mol Biol 2017;167:144-152
4. Gleicher et al., Endocrine 2018;59(3):661-676
5. Gleicher et al., Biomedicine 2022;10:1505
6. Chennakrushnaiah et al., Sci Rep 2020;10:8532
7. Agustinus et al., Nature 2023; doi: 10.1038/s41586-023-06084-7. Online ahead of print. PMID: 37286593
Colleagues from Stanford University recently published an interesting paper in Fertility and Sterility, when demonstrating that MI oocytes, after undergoing “rescue”-maturation overnight demonstrated useful, though lower, developmental competence than oocytes retrieved as MIIs. Interestingly, single embryo transfers at blastocyst stage revealed no outcome differences in pregnancy and live births between embryos derived from natural MII and matured MI oocytes. These data, of course, strongly suggest that “rescue” maturation of MI oocytes should be routine, raising the follow-up question, what to do with GV oocytes?
The CHR’s investigators will, hopefully, soon be able to report on a related study performed at the CHR, which is currently still in the revision stage at a very prominent science journal and does report on MI as well as GV oocytes.
1. He-Moon J, et al., Fertil Steril 2023;119;690-696
In one of these mega-studies with huge numbers of authors and participating IVF centers that only China can produce, a recent paper reported on a prospectively randomized clinical trial comparing treatment with oral prednisone and placebo in IVF cycles of women with recurrent implantation failure. The authors concluded that prednisone treatment did not improve live birth rates.1
Even though accepted in a prestigious medical journal (JAMA), this manuscript demonstrates significant shortcomings. Therefore, we suggest that the conclusions from this study be considered with caution. Though the study included 715 women, the study also involved 8 academic facilities, an average of only 89 patients per clinic. The study offers no treatment comparisons between those eight clinics but both cleavage-stage and blastocyst-stage transfers were included, embryo numbers were allowed to vary, and other differences in management are not described. If patient numbers were not evenly distributed between the eight clinics, one or two larger clinics could greatly affect study results if their practice patterns differed from other clinics.
Likely the biggest criticism comes from patient selection, where two failed embryo transfers with good-quality embryos were considered adequate for inclusion into the study with a diagnosis of implantation failure. Therefore, one must conclude that a significant number
of women in this study did not, in fact, suffer from implantation failure. Yet, aspirin is effective against headaches only in patients with headaches. If given to patients who do not have a headache, it, of course, will be ineffective. If the number of over-diagnosed patients in a clinical trial is too large, they will dilute results to a degree, where a type II error in outcome assessment becomes possible or even likely. The study also involved a very young, good prognosis patient population (median age 32 years), a patient age, and a patient population where implantation failure is rare.
Finally, why would anybody prescribe prednisone to implantation failure in the first place, unless one suspects an immunological cause of the alleged implantation failure? Not only does this fact reemphasize the above-made point that aspirin works only in patients with headaches, but why expose patients to the side effects of prednisone treatment without an immunological reason for such treatment? In short, despite the prominence of the medical journal that published this paper and the publicity this study therefore elicited, it is basically a very poorly designed and executed study that should never have been accepted in its current format. Not the “worst” paper of the month, but close to being a candidate.
1. Sun et al. JAMA 2023;329(17):1460-1468
In an article in Fertility and Sterility, colleagues from Canada and Israel reported that diminished ovarian reserve (DOR), defined as an AFC of <6, was associated with a higher incidence of preeclampsia and placental vascular lesions.1 Though this is a finding one would anticipate, considering that DOR is highly associated with hyperactive immune systems,2 which, in turn, of course, are associated with increased preeclampsia risk,3 a good study confirming these clinical observations would, of course, be welcome. This is, however, not what this study offers.
Deliveries of 110 DOR patients were retroactively compared with 772 controls. However, as one would expect from a poorly designed and controlled, retrospective study, DOR patients were significantly older than controls (P=0.02). Noting this fact, the investigators at least adjusted their outcome for age and noted a significant difference in preeclampsia incidence of 8.1% vs. 2.7% (P=0.003). To define DOR at all age groups by the same number of <6 antral follicles, however, makes absolutely no physiological sense, and every study, of course, starts with the patient population that is chosen for investigation. Less than 6 antral follicles may denote DOR in young women, but in women above age 40-42, this may be a perfectly normal ovarian reserve. Though at mean ages of 36.3 and 35.3, the number of older women, likely, was small, planning a study of DOR patients without properly defining the basic diagnosis that selects the patient population, immediately devaluates the study. Too bad!
Here we go back to the hot topic of small extracellular vesicles (EVs) – this time of placental nature – and the question can they be helpful in the early detection of preeclampsia. More specifically, Canadian investigators from Toronto here investigated whether fibronectin, elevated in placentae of preeclamptic pregnancies, and an oxygen sensor that regulates placental fibronectin called JMJD6, may be useful. They found that fibronectin was significantly elevated in circulating placental EVs as early as at 10-14 weeks gestation, accompanied by depletion of JMJD6 content.3 A new test for early preeclampsia risk detection? We will see! If confirmed, the next question, of course, would be how to prevent it?
Likely, the clinically most valuable future approach to early diagnosis of preeclampsia (and gestational hypertension) is, however prediction through polygenic risk scoring 5 In a very interesting paper in Nature Medicine, investigators searched for maternal DNA sequence variants in genomes in 20,064 women with preeclampsia and in 703,117 controls. In parallel, they also did the same for 11,027 women with gestational hypertension and 412,788 controls.
They identified 18 independent loci associated with preeclampsia/eclampsia and/or gestational hypertension, of which 12 were not known before and which highlighted the role of natriuretic peptide signaling, angiogenesis, renal glomerular function, trophoblast development, and (surprise, surprise!!) immune dysregulation. Polygenic risk scores derived from these observations predicted preeclampsia/eclampsia as well as gestational hypertension independent of clinical risk factors. This allowed for the reclassification of eligibility for treatment with low-dose aspirin in the prevention of preeclampsia.
We, here at the CHR, were, of course, especially pleased to see the association with immune dysregulation variants because we have viewed the occurrence of preeclampsia/eclampsia for years as a consequence of the termination of normal immune tolerance of the fetal-placental semi-allograft.6
Since we are already talking about low-dose aspirin for the prevention of preeclampsia, the American Journal of Obstetrics & Gynecology recently published a systemic review article with meta-analysis by British investigators, examining the effect of low-dose aspirin in preventing superimposed preeclampsia on women with chronic hypertension.7 Somewhat surprisingly, the meta-analysis did not demonstrate outcome advantages from low-dose aspirin on the occurrence of so-called superimposed preeclampsia, small- for gestational age, and/or perinatal mortality. However, the study, interestingly, did detect a beneficial protective effect from the treatment on the occurrence of preterm births which, in the authors’ opinion, warrants continued aspirin prophylaxis in women with chronic hypertension. We agree!
Finally, a so-called “care plan for individuals at risk for preeclampsia” was developed by a group of 19 U.S. experts with the goal of formulating “a shared approach to care that will facilitate
prevention of preeclampsia and its attendant short- and long-term morbidity in patients identified as at risk for development of this disorder.”8 We, of course, wish we could believe that; but inherently skeptical of “expert opinions,” we consider them in most cases superfluous.
1. Ganer Herman et al., Fertil Steril 2023;119(5):794-800
2. Vega et al., Am J Reprod Immunol 2016;76(4):333-337
3. Gleicher N. Am J Obstet Gynecol 2007;196(1): P5.E1-5.E7
4. Alahari et al., Endocrinology 2023;164:1-16
5. Honigberg et al., Nat Med 2023; doi: 10.1038/s41591-023-02374-9. Online ahead of print
6. Gleicher et al., J Assist Reprod Genet 2017;34(4):425-430
7 Richards et al., Am J Obstet Gynecol 2023;228(4):395-408
8. Roberts et al., Am J Obstet Gynecol 2023; S0002-9378(23)00260-0.doi: 10.1016/j.ajog.2023.04.023. Online ahead of print.
The pharma company Janssen recently announced the successful prevention of severe hemolytic disease (HDFN) of fetus/newborn in pregnant women with the investigational drug nipocalimab, a drug developed to bind to the (neonatal) Fc receptor (FcRn) and, thereby, reduce or block passage of anti-red cell antibodies from mother to fetus. According to a news article in Nature Medicine,
Continued on page 58
a majority of 14 participants in an open-label phase 2 UNITY trial achieved live births after 32 weeks of gestational age without requiring intrauterine transfusions.1
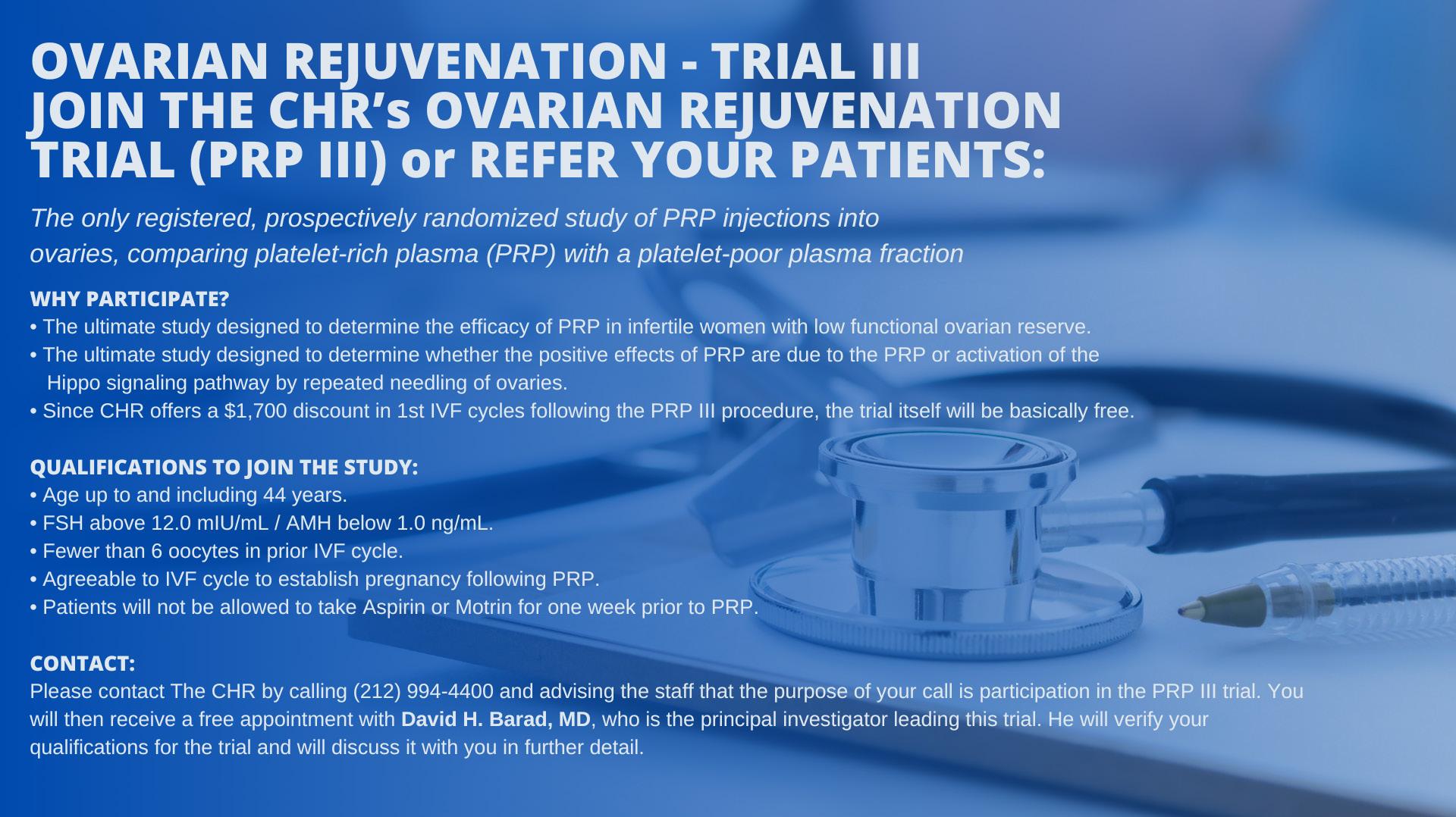
1.
Carvalho T. Nat Med 2023;29:757758We previously on repeated occasions have pointed out in these pages and in other sections of The VOICE that increased medical risks pregnant women face, often, can carry over into the postpartum period. Examples abound, with likely the two leading ones being cardio-vascular and autoimmune conditions, both of which can flair postpartum. Especially autoimmune diseases have been reported to flair for up to 5 months postpartum. However, increased medical risks for new mothers continue for up to a full year, as a recent article in The New York Times noted, reporting on CDC data between 2017 and 2019 of 1,018 pregnancy-related deaths: A third occurred during pregnancy or on the day of delivery, a second third ensued in the first 6 weeks postpartum, but a full 30% of deaths occurred between 6 weeks postpartum and one full year from birth.2 No wonder, more and more experts
are referring to the time after delivery as the “fourth trimester of pregnancy,”3,4 thereby indicating that maternal health and well-being after delivery deserved more medical attention.
One condition almost typically occurring postpartum is peripartum cardiomyopathy, therefore sometimes also called postpartum cardiomyopathy. Cardiologists define the condition as an ejection fraction of less than 45% in the absence of other causes of heart failure.5 While most textbooks describe the etiology of the condition as still unknown, an autoimmune history has been suspected for decades,6 including by investigators at the CHR,7 not the least based on the timing of the condition’s clinical presentation, - perior post-partum. It has been recently suggested that the incidence of this condition may be increasing.8
1. Piccinni et al., Clin Mol Allergy 2016;
2. Caryn Rabin R, The New York Times, May 28, 2023; https://www.nytimes. com/2023/05/28/health/pregnancy-childbirth-deaths.htm
3. Assaf-Balut et l., J Clin Med 2019; PMID: 31546914
4. Wu et al., Eur Cardiol 2021;PMIDL 34603511
5. Bauersachs et al., Eur J Heart Fail 2019;21(7):827-843
6. Sundstrom et al., Autoimmune Rev 202;1(1-2):73-77
7. Gleicher N, Elkayam U. Autoimmune Rev 2009;8(5):384-387
8. Winnicki M. Clinical Advisor, 5/15/23. https://www.clinicaladvi sor.com/home/topics/cardiovascular-disease-information-center/ postpartum-cardiomyopathy-diagnosis-treatment/
In a study of 8,012,433 participants of up to age 49 in JAMA Pediatrics, Swedish investigators in 174.4 million person-years,
11,464 had atrial fibrillation by age 49, preterm birth, and large for gestational age weight were significantly associated with a later history of atrial fibrillation later in life. Preterm birth was more associated with the risk in childhood than in adulthood, while large children’s increased risk occurred in the first 18 years of life, - but no longer after that age.
1. Yang et al., JAMA Ped 2023. Doi: 10.1001/jamapediatric.2023.0083. Online ahead of print.
U.S. investigators recently reported surprisingly clear associations between endometriosis as well as fibroid tumors of the uterus and ovarian cancer. Black and White participants with endometriosis had a higher risk of ovarian cancer. Performance of a hysterectomy, however, modified this association among White participants, though not among Black patients. Leiomyomas, on the other hand, were associated with an increased risk of ovarian cancer in both racial groups. Interestingly, this risk was modified by hysterectomy in both groups. The authors correctly concluded that these data may affect risk reduction strategies in different racial groups. Another important question that remains to be answered is, of course, why do endometriosis and fibroids predispose to ovarian cancer?
REFERENCE
1. Harris et al., Obstet Gynecol 2023; doi: 10.1097 AOG.0000000000005191. Online ahead of print.
That endometriosis is associated with increased miscarriage risk has been known for many decades, with this association being mostly attributed to the hyperactive immune system found in most endometriosis patients, often demonstrating inflammatory and/or autoimmune markers.1 It, nevertheless, is important to get reminded of this fact from time to time, and this is what a recent paper in Fertility and Sterility accomplished which in a nationwide cohort study found endometriosis associated with pregnancy loss in general, but also with repeat pregnancy loss. Indeed, the association strengthened with increasing numbers of miscarriages. Something to remember when dealing with endometriosis patients! In such women being, at times, proactive may prevent first and second miscarriages.
1. Gleicher et al., Am J Obstet Gynecol 1989;160(6):1376-1380
2. Dyhrberg Boje et al., Fertil Seril 2023;119(5):826-833
Being born prematurely, too small, or too heavy, increase the risk of atrial fibrillation
On June 9, The Wallstreet Journal reported that laboratorydeveloped tests will be facing new FDA regulations.1 This has been a topic of repeated discussion in the pages of The VOICE and has been addressed by the CHR’s investigators in the literature because most genetic tests, including preimplantation genetic testing for aneuploidy (PGT-A), are laboratory-developed tests and, under current rules, are not reviewed and/or licensed by the FDA. The CHR has criticized this omission for a long time and, therefore, would be very pleased if the FDA, finally, established oversight over these tests.
The article refers mostly to cancer-related genetic tests where differences in reporting have been substantial, but one would have to assume that, once laboratory-developed tests attract the attention of the FDA, new rules will apply to all such tests, including those affecting infertility and early pregnancy, meaning PGT-A as well as non-invasive prenatal testing (niPT). The results of these tests have varied not less than cancer tests and, indeed, likely even more so.
As the article, however, also notes, some legal authorities argue that the FDA does not have the legal authority to develop laboratory-developed tests without legislation by Congress. Though so far never implemented, the FDA has, however, always claimed the right to do so,
1. Whyte LE. The Wall Street Journal, June 9, 2023, pA3
2. Gleicher et al, Nat Med 2022; 28(3):442-444
The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400. The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiology, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved.
For letters to the editor, comments, and suggestions, please contact Micah Elias at melias@thechr.com. For all advertisements or sponsorships in The VOICE , please contact Alexandra Rata at arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR. .R


