A subcutaneous pen sryinge injection of FSH (Follicle Stimulating Hormone) into the abdominal wall for ovarian stimulation










A subcutaneous pen sryinge injection of FSH (Follicle Stimulating Hormone) into the abdominal wall for ovarian stimulation









We very enthusiastically welcome you to the April issue of The CHR VOICE because over the last month, we had the opportunity to put together our most expansive issue so far (44 pages). We also, luckily, came across a new record number of interesting articles in the literature for the Medical Literature Review section of this newsletter.
Subjects addressed in this issue include the rapid expansion of the donor egg bank industry and how these developments impact IVF practice. Mild ovarian stimulation, at times in the CHR’s opinion too aggressively promoted by colleagues, has remained a highly controversial issue in infertility practice and - we felt - deserved a CHR update. Interestingly, the subject also came up among questions we received and, therefore, is addressed twice in this newsletter.
In another timely piece, we explore why where patients start their infertility journey matters. The IVF field has made remarkable progress over its existence. While if one looks at the medical progress as measured by pregnancy and live birth rates, the field has stagnated and to some degree even regressed, access to treatment, with IVF centers practically at every corner, the progress has been nothing but astonishing. This has mostly been the consequence of Wall Street discovering infertility as a presumed growth industry and basically buying up the field, - both on the clinical side and in support functions, like laboratories, cryopreservation, donor gamete banks, and even financing. With ever larger conglomerates providing more and more clinical care (in Australia three companies control approximately 80% of the market), how fertility services are provided, therefore, has drastically changed in recent years.
In here published articles, we, therefore, present the argument that large provider organizations, which are rapidly increasing their share of the total infertility market, must strive for unified “best-practice” protocols if they want to provide the best services to the largest number of their patients. This requires rigid protocols which will produce the best results for the largest number of patients because they usually represent mostly younger and uncomplicated cases. Such protocols, however, are often not suited for older and/or more complex younger infertility patients who require more individualized care.
Where patients, therefore, start their fertility treatments does matter: While it is logical to seek out providers locally in one of the evolving IVF clinic chains if you are one of those younger, straightforward cases, - if you are older or face a more complex medical situation with other so-called “co-morbidities” even at relatively young ages, a center that individualizes care, like the CHR, may be a better choice from the beginning. You then from the beginning will not become part of a protocol-driven machine and will not automatically be advised to use third-party donor eggs if you are over age 42 and/or have too high FSH or too low AMH to fit into one of the center’s protocols. Moreover, in infertility time is rarely on the patient’s side, and time, therefore, matters.
Truth in medicine is this month’s subject of Norbert Gleicher’s column, “A Piece of My Mind.” In surveying this subject, the CHR’s Medical Director and Chief Scientist expresses concern about increasing difficulties in determining what represents “truth” in general which, of course, also greatly affects the medical field.
Then there also are the questions we receive from patients and readers and their answers, how the CHR last month participated in the general media landscape, and newly published peer-reviewed papers by the CHR’s researchers and clinicians and, of course, our by now famous (or, as some would say, infamous) review of the medical literature. What is not to be forgotten and - we hope - is getting your attention are our announcements of CHR-sponsored events, such as GrandRounds and the annual Foundation for Reproductive Medicine Conference (FRMC) on December 1-3, 2023 in NYC, co-sponsored by The CHR, which has opened registration (https://whova.com/portal/ registration/frmc_202301/) and abstract submissions (https://www.foundationforreprodmed.com/frmcabstract).
Not the least because of messaging by the VOICE’s, last month’s GrandRounds were a big success in attracting onsite and virtually a very interesting crowd of attendees, mostly colleagues from local medical schools and, among them, to our delight, some of the leading opponents of opinions the CHR is representing. The discussion, consequently, at times was heated but always remained collegial and in bounce.
We very much hope to continue such discussions of controversial topics among representatives of different opinions not only in public events the CHR organizes, but also in the VOICE, and want to reemphasize to colleagues with different opinions that this newsletter always welcomes contributions with differing opinions, whether in response to previously published articles or because colleagues are interested in distributing a message to a large readership interested in the infertility field. NYC is blessed by a very large community of infertility providers who, understandably and to the benefit of the public, fiercely compete with each other. However, this does not mean that all of this talent can, concomitantly, not also collaborate in improving the practice of infertility for everybody.
Since for many years, egg (oocyte) cryopreservation technically lagged behind semen cryopreservation, frozen sperm banks preceded frozen egg banks by decades. The immediate impetus for the creation of frozen sperm banks came in the mid-1980s from reported HIV transmissions through intrauterine inseminations with untested donor semen samples, - a very common practice at the time.1 Decades later, the primary impetus for frozen donor egg banks came from improvements in oocyte cryopreservation techniques, especially the universal switch from slow freezing to vitrification, perfected by Japanese colleagues around the year 2000.2 Practical and financial considerations in the in vitro fertilization (IVF) world further added to the unusually quick acceptance and subsequent expansion of this new approach toward egg donation.

Today, frozen donor egg banks are everywhere and likely exceed the number of frozen sperm banks. Considering how much lower the costs for a frozen semen sample are than for a frozen egg cohort, this should not surprise: selling eggs, simply, offers a much better business opportunity with larger profit margins, which is an observation leading us directly into this article’s first subsection.
The selling of semen samples was common practice in gynecologic infertility practice (in those days a subspecialty of reproductive endocrinology and infertility did not yet exist) long before the establishment of the first frozen sperm banks in the mid-80s. Medical students and residents at many institutions in those days routinely supplemented their income by donating semen samples to affiliated gynecology practices that used them without further screenings of the donors, simply based on the knowledge that they came from medical students and residents. Historically, a first third-party
Continued on page 6
donor semen use goes back for much longer and is, for example, strongly suspected to have involved Henry IV (1425-1474), also nicknamed “the Impotent,” who in 1455 married Princess Juana (the sister of the king of Portugal) who only six years later gave birth to a daughter, later claimed not to be Henry’s genetic child.3
In conjunction with IVF, the issue of third-party egg donation arose relatively quickly after the world’s first IVF offspring was born when a first donor-egg pregnancy was reported in 1983 in Australia, shortly thereafter followed by the first donor-egg pregnancy in a California IVF center.4 On a societal level, interestingly, third-party egg donation became, ethically, a much more “relevant” issue than the long-standing practice of third-party semen donation. This is best demonstrated by the observation that nobody has ever even addressed the issue of semen donation payments; yet, once women started donating eggs, suddenly, the payment for their gametes became a major issue of controversy, with everybody (including major professional organizations) agreeing that the payment for gametes (in this case oocytes) assigned a commercial value to eggs and, therefore, was unethical.
Due to the unanimity on the subject and the still, nevertheless, very obvious need for egg donors, the IVF community, under the auspice of the American Society for Reproductive Medicine (ASRM) looked for an ethically acceptable solution that would allow the continuation of third-party egg donation. Such an ethically acceptable solution was found quickly by offering an obvious ruse: (i) It reaffirmed that payments for eggs were, indeed, unethical. Egg donors, therefore, had to donate their eggs as an “altruistic” act and without receiving payments in return (semen donors, of course, had never been asked for such a “favor”). (ii)
Ethical considerations, however, did allow for payments to egg donors “for their efforts, time, and expenses in being an egg donor.” Shortly thereafter, ASRM, took the additional step of defining the appropriate payment range for IVF centers to their egg donors, to reimburse them for all of their donation-related efforts, - a decision the ASRM would come to regret.
The recommended donor compensation per donation was US $5,000, with special considerations permitting a range of up to US $10,000 per donation cycle. As described in detail in an article by Kimberly D. Krawiec, JD, in the American Medical Association Journal of Ethics in January of 2014.5
Lindsay Kamakahi, a potential U.S. egg donor, “caused an international stir” by filing a class action lawsuit against the ASRM, its sister society the Society for Assisted Reproductive Technology (SART), member fertility clinics, and egg agencies that agreed to abide by ASRM/SART egg donor compensation guidelines on behalf of herself and other egg donors,6 challenging those guidelines as restrictive to free trade and, therefore, as illegal. She quickly received considerable support in the media, including in an editorial in The New York Times, which claimed that the absence of a free market for egg donors unjustly shortchanged them from earning more from their egg donation than they received under those guidelines. In an opinion piece in Fertility & Sterility, the official organ of the ASRM, three prominent officers at different branches of the National Institutes of Health (NIH), moreover, suggested that a first step toward a fairer model of egg donor compensation would be the abandoning of the concept of “altruistic” egg donation.7
By February 2016, the ASRM agreed to a settlement under which the society agreed to remove language from their guidelines that stipulated that total payments to donors in excess of US$ 5,000 required justification and sums above US $10,000 were inappropriate. In addition, the society agreed to pay US $1.5 million to compensate the plaintiff’s counsel for fees and costs and US $150,000 for notice to the class.8
The ethical importance of this settlement by the ASRM cannot be overemphasized because it, practically, removed ethical considerations and concerns from the agenda when it comes to the commercialization of the human IVF process. One, indeed, can argue that, on similar legal grounds, not only the trading of gametes (semen and oocytes), but also of human embryos should be permitted since human embryos, in many states are considered private property.9 Yet, current ASRM guidelines clearly state that, “though the practice may charge potential recipients a professional fee for embryo thawing, embryo transfer procedure, cycle coordination and documentation, and infectious disease screening and testing of both, recipients and donors, the selling of embryos per se is ethically unacceptable.”
Indeed, one could further argue that IVF clinics could produce embryos for donation “on-spec” from donor eggs and donor sperm. Though in New York State, every embryo produced under
local state law must have “parents,” this rule does not exist in all states, and clinics in some states, indeed, do produce embryos “on-spec,” keeping them cryopreserved for clients (i.e., patients) on a first-come, first-served basis, exactly like sperm and egg banks now keep gametes in cryo-storage. The commercialization of human reproduction, therefore, appears increasingly unstoppable.
The subject of human egg banks cannot be properly addressed without pointing out the differences between using fresh and frozen eggs in the IVF process. Whether fresh and cryopreserved oocytes offer the same pregnancy chances has been the subject of controversy from the very beginning of frozen egg banking. The initial discussion, indeed, prominently involved CHR investigators who, from very early on claimed that frozen egg-bank oocytes produced lower pregnancy rates.10 Colleagues, however, disagreed with the CHR’s investigators arguing that differences in favor of fresh oocytes would disappear with increasing use of and with more experience with frozen donor eggs.11 A national follow-up study by the CHR’s investigators three years later, however, demonstrated an even expanding margin,12 and a 2018 comparison of national data by a frozen donor egg bank reported an even larger difference:13 According to the Center for Disease Control (CDC) registry, fresh outperformed frozen eggs by 57.1% to 44.2% (+12.9%) and by SART registry by 49.4% to 39.3% (+9.15),a, thus, even further expanding margin in favor of fresh egg donation.
Therefore, it appears that increasing the utilization of frozen eggs has not led to better results, as suggested by colleagues,10 but, as argued by the CHR,10,12 had the opposite effect. This, indeed, should not surprise because moving a treatment from an academic setting, where original studies are performed, into wider practice utilization, usually reduces the effectiveness of treatments. This may, however, not be the only reason for deteriorating success rates with frozen donor egg-bank oocytes. The CHR has also been noticing a decline in quality since COVID-19 started, especially over the last year in oocytes purchased by the center’s patients from several of the leading national egg banks that are licensed in the State of New York.
It is difficult to ascertain why that may be, but a few possibilities come to mind: (i) COVID-19 for some reason appears to have reduced the number of good available egg donors. Egg banks, therefore, may have to fall back on poorer-quality donors. CHR has seen some evidence for this hypothesis in that some donors rejected by the CHR (yes, we still maintain our own donor bank and frozen donor pool) later appeared in pools of donor egg banks. (ii) With the establishment of many new frozen donor egg banks, the competition for good egg donors has significantly increased. (iii) Donor egg banks are usually dependent on multiple IVF centers for their supplies of
donor eggs. Different IVF centers differ in how they manage their cycles and, therefore, also in the quality of oocytes they produce. (iv) With the increasing demand for frozen donor oocytes (according to most recent data, over half of all U.S. donor-egg cycles now utilize frozen donor eggs), egg banks may be short of supply and more willing to use marginal oocytes.
Whatever the reasons, the increasing national par between fresh and frozen donor eggs in pregnancy and live birth outcomes is, however, of obvious concern and is closely watched by the CHR. Frozen donor eggs, however, also offer distinct advantages over fresh eggs, and these advantages extend to patients as well as treating physicians: First and foremost, the availability of frozen eggs greatly simplifies the egg donation process since it eliminates the need for a preceding full fresh egg retrieval cycle of an egg donor since the required donor eggs are already cryopreserved and instantly available. With it, this also eliminates concerns and uncertainties over the number of available oocytes and their quality which, both, are never certain in a fresh cycle. Finally, for many IVF centers, the availability of large numbers of donor eggs without waiting periods also eliminated their own need to maintain a (fresh) oocyte donor program and, therefore, saves them considerable expense, as well as effort.
In the early days of frozen egg donor banks, the industry also had good reason to argue that the use of frozen donor eggs was less costly than fresh donor egg cycles. After significant price increases at practically all egg banks since COVID-19, and especially over the last
year, these advantages, however, likely no longer exist. Moreover, a large variety of package deals offered by egg banks, involving varying numbers of eggs and all kinds of guarantee programs (see for further detail below), often lead to increasingly less transparent pricing and progressively greater difficulties in comparing costs between egg banks, - but also between frozen and fresh cycles.
It is also important to reemphasize that frozen donor egg banks are businesses, with the primary goal of being profitable and creating economic value for their owners. Like any other business, they, therefore, will blow their own horn wherever and whenever possible, and their statements and representations must, therefore, be viewed with caution. A good example is the repeated observation in viewing their published materials that frozen and fresh eggs produce similar pregnancy and live birth rates. As already demonstrated above, this is, of course, blatantly incorrect as all U.S. data show; yet, this statement can be found in many, if not most, websites and or other representations from the egg-freezing industry.
As already noted, frozen egg banks now offer a confusing array of packages that, unfortunately, progressively appear to get less and less transparent. Those packages used to be simple and easy to understand, offering oocytes either on a per-egg basis or as cohorts of usually 5-6 mature eggs. The per oocyte cost, therefore, was easy to understand. However, packages offered now include costs with or without “treatment” (in itself something of an oxymoron since egg banks are not licensed to offer
Continued on page 8
“DIFFERENT IVF CENTERS DIFFER IN HOW THEY MANAGE THEIR CYCLES AND, THEREFORE, ALSO IN THE QUALITY OF OOCYTES THEY PRODUCE”
treatments), different mature egg numbers, and all kinds of “guarantees” that usually sound good but, if missed, really mean that the provided eggs must, literally, have been dead. Increasingly, egg banks also offer at exorbitant costs refunds and/or replacement guarantees for poor eggs.
Earlier this year, we discussed refund programs in IVF in general terms in The VOICE and made the point that, like any insurance contract, those offers are based on careful actuarial calculations under which patients who conceive quickly subsidize those who conceive late or not at all and, therefore, become eligible for, usually, only partial refunds. We at that point also noted that the profit margins for IVF centers in those refund plans are actually higher than in single cycles. In other words, and overall, these refund package plans are usually more expensive than single-cycle payments.
When it comes to donor egg cycles, those multiple-cycle offerings, whether with or without refund options, unfortunately, are even more complex. The principal reasons are the various guarantees attached to each plan which can include different numbers of initial egg numbers, minimum numbers of either cleavage- and/or blastocyst-stage embryos for transfer, the occurrence of pregnancy (or not), and ultimately, whether there is a live birth (or not). Each of these offerings has, of course, different pricing, with some, for example, one extreme package, promising a 100% refund after up to six (sic!) unsuccessful donor egg cycles (a donor-egg cycle number that should virtually guarantee close to 100% pregnancy chances). Though other offerings of this egg bank are listed with estimated pricing, interested parties in this 100% refund offer are requested “to contact the center for further information.” We can only imagine the costs for this program!
Often just noted in fine print, eligibility for different refund programs is, of course, restricted to good-prognosis patients. In short, many offerings from, otherwise, seemingly reputable egg banks, even if not on purpose misleading, clearly do not fulfill even minimal requirements of transparency, an impression not only obtained from reviewing websites but also confirmed from contacts with patients who ordered oocytes from some of these egg banks with, at times, disturbing results.
Not only do many patients apparently not understand the packages they committed to but, as we noted in prior issues of
The VOICE this year, we have also been witnessing increasing resistance from donor egg banks to fulfill commitments under minimum guarantees they had issued, even if the egg quality of sold oocytes on by egg banks provided photographs - even before attempted thaws - was obviously poor.
Reviewing advertised pricing on various websites, we almost uniformly calculated under the varying offerings average oocyte costs between approximately US$ 3,5004,500. In some instances, costs were even higher but, because they were outliers and, often almost impossible to interpret in their cost structure, we are leaving them out of here presented discussion. Oocyte costs, however, of course, depend on egg donor costs, and as noted above, donor fees nowadays are whatever the market dictates. Increases in oocyte costs as we have witnessed in recent years, unquestionably are at least partially the consequence of higher fees paid to egg donors.
At least equally likely, by supplying egg banks with their oocytes, IVF centers also contribute to the price increases and, currently, have little incentive to reduce fees. However, that may change as increasing numbers of U.S. citizens are pursuing third-party egg donation overseas, where egg donors to this day often are still not allowed to be paid for their donations (except for efforts and expenses) and medical costs also are much lower than in the U.S.
Concomitantly, frozen egg banks from overseas are attempting to enter the U.S. market which currently is still restricted for them because their donors are usually not pretested in FDA-approved laboratories (an FDA-mandated condition for using an egg donor in the U.S.). However, efforts are underway to find ways to get those overseas donors appropriately pretested. Some overseas egg banks, indeed, have already started to offer frozen oocytes in the U.S., claimed to be “FDA-tested” because the donors’ blood was sent to U.S. FDA-approved laboratories for analysis. While costs for these overseas eggs are significantly lower than customary costs at U.S. egg banks, our review of advertised egg costs suggests that IVF clinics usually do not pass through these potential cost savings to patients, as costs for U.S.- and overseas- oocytes are usually the same. Despite lower costs from egg banks, overseas eggs, therefore, only unlikely will lower patient costs.
Continued on page 9
The use of such overseas – oocytes in the U.S. furthermore appears problematic because the ability to trace involved donors appears highly questionable, as one wonders who guarantees the authenticity of the blood sent for testing to U.S. labs. The state of New York, for example, rightly mandates that IVF centers in other states that contribute oocytes to U.S. egg banks be registered for regular inspection like N.Y.-based IVF centers if their eggs are to be used in the state of N.Y. That guarantees of such traceability may be lacking was just a few years ago demonstrated when more than 60 European individuals, among them physicians, medical staff, and lawyers were arrested in Greece, accused of human egg trafficking, and running an illegal adoption ring involving economically vulnerable women from poor neighboring countries.14 The utilization of economically deprived young women from poor countries, of course, in addition also raises the issue of coercion. It appears simply impossible to ascertain whether egg donors in overseas countries have the same level of legal protection they are privy to here in the U.S. The CHR, therefore, as of this moment rejects the use of frozen donor eggs obtained from outside of the U.S.
Like in all medical matters, the CHR’s policy is characterized by full transparency and free patient choice. The CHR’s staff, therefore, offers no preference for either fresh or cryopreserved third-party donor-egg cycles, nor does the CHR express preference for individual egg banks (or egg donor agencies, here not discussed). The CHR, indeed, does not even express a preference for its own donor egg program, even though the center still maintains its own pool of donors for fresh egg donation cycles as well as a small frozen donor pool and strongly believes that the thoroughness and transparency of the center’s donor selection are second to none.
Several years ago, when donor reimbursements changed because of the above-described lawsuit,5 The CHR, out of ethical concerns, concluded that the sale of oocytes should not become a profit motive for the center. The center, therefore, decided to only serve as a conduit for anonymous payments from egg recipients to donors and, accordingly, priced oocyte charges only to cover the center’s egg-donor expenses. Consequently, the CHR’s egg-donor program can offer eggs at significantly lower costs than almost all other sources.
At the same time, the staff of the center, however, constantly reemphasizes that The CHR considers the patients’ choice of donor as a quintessential decision a patient, alone, can make. That patients really like their donors and are certain in their ultimate choice is, therefore, of great importance for the CHR because we do not want our patients ever, later in life, to have to second-guess their choice.
By this point, our readers will, hopefully, fully have understood what a revolution the IVF field has been undergoing with the increasing availability of frozen donor egg banks over recent years. Much easier access to donor eggs has, however, one additional highly significant consequence which The CHR is not very happy to recognize: By having made egg donation procedurally “easier,” unfortunately, patients are, even more often than before, prematurely directed into third-party egg donation. The CHR for many years, both in these pages and in the medical literature,15,16 has strived to educate patients as well as colleagues that many patients, advised that their only realistic chance of pregnancy is through third-party donor eggs, still have decent chances with autologous eggs, if only given the chance.
We know this for a fact because these patients represent a significant portion of the CHR’s patient population. While we, of course, very much welcome them, we often wonder how much more successful we could have been in helping them conceive if they had presented to the CHR just a little bit earlier, - maybe before they failed their last three IVF cycles elsewhere. We love to be the “last resort” center for so many of our patients; but we also often wonder why, at least some of these patients, did not find their way to our center just half a year or a year earlier The difference in outcomes can be highly significant!
(see also “Where you start your infertility journey matters”)
1. Araneta et al., JAMA 1995;273(11):854-858
2. Kuwayama et al., Reprod Biomed Online 2005;11(3):300-308
3. Ombelet W, Van Robays J. Facts Views Vis bgyn 2015;7(2):137-143
4. https://www.eggdonationfriends.com/egg-donation-guide/ egg-donation-history/
5. Krawiec KD. Am Med Assoc J Ethics 2014; 16:1:57-62
6. Lindsay Kamakahi v American Society for Reproductive Medicine, class action complaint, case no. 3:11-CV-1781 (N D Cal, filed April 12, 2011).
7. Bayefsky et al., Fertil Steril 2016;105(5):1153-1154
8. http://marketdesigner.blogspot.com/2016/02/caps-on-payment-toegg-donors-abolished.html
9. Koboska, Caroline, “Embryo Litigation: The Legal Categorization of Embryos as Protected Humans or Property” (2019). Theses, Dissertations and Culminating Projects. 282. https://digitalcommons. montclair.edu/etd/282
10. Kushnir et al., JAMA 2015;314(6):623-624
11. Grifo et al., JAMA 2015314(23):2569-2570
12. Kushnir et al., J Ov Res 2018;11(1):2
13. https://donornexus.com/blog/fresh-vs-frozen
14. Harley N. The National News.com. September 26, 2019; https://www. thenationalnews.com/world/more-than-60-arrested-in-500-000-eu ropean-human-egg-trafficking-and-illegal-adopt ion-ring-1.915464#:~:text=More%20than%2060%20people%2C%20 including,by%20an%20organised%20crime%20gang.
15. Gleicher et al., J Endocrinol 2016;230(1):F1-6
16. Gleicher et al., J Assist Reprod Genet 2020;37(7):1583-1588
We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO (jtapper@thechr.com). All submissions are considered confidential correspondence.
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.


Opinions in the medical literature regarding so-called “mild” ovarian stimulation of ovaries in infertile women have differed for decades and these differences, unfortunately, have not narrowed over the years. The CHR has actively participated in this discourse in the pages of The VOICE and the medical literature,1,2 not only based on the center’s study results, - but also based on the careful interpretation of published literature by others (both, proponents as well as opponents of mild stimulation), consistently reaching the same conclusion that except in rare young best-prognosis patients [and, of course, in women at risk for ovarian hyperstimulation (OHSS), especially with polycystic ovary syndrome ( PCOS)] so-called mild stimulation not only produces poorer IVF cycle outcomes than standard ovarian stimulation but, logically, simply does not make any sense. We, therefore, decided to address this issue once more in more detail, including comments regarding more recently published literature.
Whenever disputes exist in the medical literature, the first question that comes to mind is whether we really understand the world behind this dispute. This involves many different questions but one of the most basic ones is whether proponents of opposing opinions are actually addressing the same issues. A second crucially important question is are the opponents in their respective opinions addressing the same patient populations? Also as here demonstrated, the answer to both of these questions, unfortunately, is a very clear no, - proponents and opponents, now for decades, have been talking by each other; that this dispute has remained unresolved, therefore, should not surprise.
Everything starts with the indisputable fact that there exists no universally accepted, consistent definition of what mild stimulation is.3 Several years ago, a group of proponents of mild stimulation proposed a definition that was based on desired oocyte numbers retrieved in an IVF cycle: 2-7 with “mild” stimulation, and 8 or more with conventional “standard” stimulation.4 Others have proposed to define “minimal” stimulation by a target of 5 oocytes and “mild” stimulation by 10 oocytes.5
Remarkably, neither these two nor many other attempts at defining mild stimulation ever took into consideration the overwhelming importance of patients’ ages and functional ovarian reserves (FOR) in not only defining what mild stimulation means for an individual patient but also how these quintessential patient characteristics interphase with each other and IVF cycle outcomes within the context of mild versus standard ovarian stimulation. Returning to the almost bizarre concept of defining the intensity
Continued on page 12
of ovarian stimulation by an arbitrarily chosen goal of retrieved oocytes, 5 eggs in a 25-year-old, of course, have a very different meaning than in a 45-year-old patient. Moreover, why are 5 oocytes the set target, - why not 7, 9, or 12? Also, how much do egg numbers retrieved in an IVF cycle really mean? Though egg numbers relate to embryo numbers, and embryo numbers to pregnancy and live birth rates, they, of course, do so only in age- and FOR-dependent associations.
In absence of a universally agreed to definition of what represents mild stimulation at various ages and with different levels of ovarian reserve, even an uninformed reader on the subject will, therefore, immediately comprehend that the existing literature on the subject must be largely worthless because comparing apples with oranges is never acceptable for recovery of valid scientific information.
If one follows the history of IVF, the importance of egg and embryo numbers becomes immediately obvious because IVF only succeeded in becoming a clinically valid treatment after the Norfolk IVF program (the U.S.’ first and most important IVF program in the early stages of IVF) introduced ovarian stimulation with gonadotropins to IVF, which before that relied only on natural cycles and/or clomiphene citrate stimulation.6,7 It also very quickly became obvious that, among other distinctive
“Whenever disputes exist in the medical literature, the first question that comes to mind is whether we really understand the world behind this dispute”
Continued from page 11
contributing factors, the dosing of the gonadotropin stimulation had to vary between patients depending on age and FOR. Paradoxically, colleagues, however, at some later point for clearly inexplicable reasons (as we will explain further below), decided to revert course and go back to concepts like natural cycle IVF and mild stimulation cycles, like the so-called Kato protocol,8 that not only became the dominant protocol in Japan but also found followers in the U.S and other countries.
The motives for this regressive development to this day are unclear because proponents of mild stimulation IVF, astutely, refrained from exaggerated IVF outcome claims that have characterized many so-called “add-ons” to IVF in recent years. In contrast, mild stimulation proponents described their cycle outcomes more realistically as “similar” to standard IVF stimulation cycles or as “reasonable” or “acceptable,”9 while claiming secondary benefits, such as fewer twin pregnancies, less patient discomfort, better patient-friendliness, fewer (or no) injections (see the CHR’s comments on needle-free IVF in the March issue of The VOICE), lower OHSS risk, better quality eggs, better endometrial receptivity, better egg and embryo quality and, finally, lower costs.
Unfortunately, none of these claims is, however, validated either: First of all, though a recent systematic review and meta-analysis of poor responders claimed equal efficacy for mild versus conventional ovarian stimulation,10 results of this study and its conclusions cannot be taken seriously. A detailed critique of this study would exceed the space allotment to this article, but we strongly urge interested readers to look up this reference and reach your own conclusions. The senior author of this paper, Greeta Nargund, MBBS, FRCOG, a British colleague and Medical Director at Create Fertility in the UK, is to this day a leading proponent in the world of mild ovarian stimulation. She is also, together with Bart CJM Fauser, MD, a principal in The International Society for Mild Approaches in Assisted Reproduction, the latter at the time being the Editorin-Chief of the journal where most of her papers on the subject appeared in print,11 with the last even trying to make the point that efforts at maximizing live birth rates in IVF are often exaggerated and other equally important consequences should be considered.12
Multiple studies, of course, have repeatedly demonstrated that even highly educated and risk-informed infertility patients value live births over practically any other considerations when seeking out IVF services. This does not mean that other considerations do not count; they, of course, do; but, only too often, physicians are willing to give up significant percentages of their patients’ pregnancy and live birth chances for secondary benefits, as they perceive them, while their patients, unrecognized by them, have often very different priorities. This, unfortunately, is a widespread phenomenon in current infertility practice, which the CHR does not endorse, as we see achieving pregnancies and safe as well as healthy deliveries as our primary responsibility.
Mild stimulation, thus, indeed reduces twin pregnancies, - but most infertile women will gladly accept the associated increased risks, increase patient discomfort, and more injections. Significant OHSS risk almost no longer exists with modern fertility treatments. That mild stimulation produces better eggs and embryos is a myth and has repeatedly been disproven, and even equal outcomes are a myth. Where outcomes have been reported as equal, the
reason was always biased patient selection against regular ovarian stimulation. Not a single study in the literature, of course, could ever demonstrate outcome benefits for mild stimulations. And how about costs? They, of course, on a cycle basis are lower; but if you need more cycles to achieve the same pregnancy and live birth rates, that more than compensates. The best example is Japan, where the dominance of the mild Kato protocol8 led to a decline in national live birth rates by two-thirds.13 In comparison, Japan over the same time period tripled IVF cycle starts. It is hard to imagine that three mild Kato stimulation cycles are less costly than one standard-stimulation cycle. We, indeed, would suggest the opposite is the case: mild stimulations, likely, increase cumulative IVF costs because of increased cycle numbers.
As already noted earlier, all of this, of course, does not mean that there are not circumstances where mild stimulations are preferable. We already noted the risk of OHSS in association with abnormally high FOR, as is seen in PCOS patients. At the other extreme, women with very low FOR, often, also have very short cycles. Attempts at lengthening cycles may, at times, require lower stimulation dosing. As a recent study well documented, the number of oocytes associated with maximal cumulative live birth rates, of course, change with advancing female age,14 and why ovarian stimulation should always be aimed at safely maximizing oocyte yields was recently well summarized.15
Because female age affects egg numbers and egg quality, and egg quality reflects ca. 95% of embryo quality, female age is by far the most important predictor of IVF outcomes. The second-most-important predictor of cumulative pregnancy chance is, however, the available number of transferrable embryos. If ovarian stimulation is not maximized to retrieve maximal egg numbers for that age, pregnancy chances are given away, and that makes little sense since compensatory benefits cannot make up for lost pregnancy chances (even if some physicians think otherwise12). Therefore, we consider the concept of mild stimulation (whatever it may mean at different IVF centers in absence of a uniformly accepted definition) antithetical to our obligation to offer the best pregnancy and live birth chances to our patients. There simply is no logic behind such a clinical approach except, maybe, in young best-prognosis patients who want only one more child.
1. Gleicher et al., Reprod Biomed Online 2012;24(4):396-402
2. Orvieto et al., Rprod Biol Endocrinol 2017;15(1):48
3. Baker VL. J Assist Reprod Genet 2013;30(2):197-202
4. Nargund et al., Hum Reprod 200;22:2801-2804
5. Zarek Sm, Muasher SJ. Fertil Steril 2011;95:2449-2455
6. Jones et al., Fertil Steril 1982;38(1):14-21
7. Garcia et al., J In Vitro Fert Embryo Transfer (now JARG) 1984;1(1):24-28
8. Kato et al., Reprod Biomed Online 2012;10:35
9. Zhang et al., Reprod Biomed Online 2010;21(4):485-495
10. Kumar Datta et al., Reprod Biomed Online 2020;41(2):225-238
11. Nargund et al., Reprod Biomed Online 2022;45(6):1133-1144
12. Nargund G, Kumar Datta A. Reprod Biomed Online 2022;44(4):587-589
13. Gleicher et al., Hum Reprod Open 2019;2019(3):hoz017
14. Law et al., Hum Reprod 2019;34(90:1778-1787
15. Ata B. Reprod Biomed Online 2023;S1472-6483(23)00050-0. Doi: 10.1016/j.rbmo.2023.01.016. Online ahead of print.
Always makes such difficult times that much easier. In completely and capable hands and then some….

Very friendly environment and very clean everyone is so pleasant and polite I loved my experience at the clinic. Thank you all.
We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratorysupervisor for research


To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.
Have you thought about advertising in the VOICE?
This newsletter every month goes electronically to ca. 80,000 infertility patients, medical professionals in the field, and members of the media, with over 25% (an unusually high number) also opening the VOICE.

For further information, please contact:
Ms. Alexandra Rata (212) 994 4400 or e-mail to arata@thechr.com

We elsewhere in this issue of The VOICE were wondering how much more successful the CHR could be with many of our infertility patients if they only had come to us earlier. Unfortunately, we can only speculate because, as of this moment, over 90% of the CHR’s patients seek out services at the center only after having repeatedly failed IVF cycles elsewhere, - often at multiple IVF centers. Being a “center of last resort” for patients from all over the world, is, of course, a great honor and daily motivates all of the CHR staff. Being able to help an infertility patient to conceive is always highly rewarding; but achieving a pregnancy for a couple that has been through multiple failed prior IVF cycles, often at multiple IVF centers, understandably, adds significant further levels of satisfaction.
Considering how impressive the center’s results are with what, undisputably, is the oldest and most adversely selected patient population of any U.S. IVF center (and likely in the world), it is only reasonable to assume that being able to apply some of the CHR developed treatments earlier in a patient’s fertility journey, will result in even better results. To prove this point, patients will have to find their way to the CHR earlier, - and that is difficult to achieve because many, if not most patients at the beginning of their infertility journey, indeed, do not really need a fertility center with the kind of specialization the CHR offers. Others, however, might even at earlier stages of their journey benefit from a more specialized fertility
Continued on page 16
center and, since the differences between fertility centers are not always well understood, we thought it was time to summarize them here in an organized fashion.
Starting from the beginning, it will probably be obvious to most that not all IVF centers are the same. IVF centers, indeed, since the early days of IVF never were the same, and in those days fiercely competed with each other based on the pregnancy rates they were achieving. For several reasons, this competition over time receded: First, with increasing insurance coverage for IVF, patients selected less based on pregnancy rates and more based on which centers were part of their insurance networks. Also, while IVF clinics in the early days were sparse, patients were willing to travel longer distances for services. With an IVF center on every second corner nowadays, why should patients travel for daily cycle monitoring, if they can get it done in their own neighborhood? As a frequently overlooked contribution, it also must be acknowledged that infertility practice increasingly deemphasized pregnancy rates by no longer considering pregnancy and live birth rates as the primary outcome goal of IVF cycles (see also the article on “mild ovarian stimulation” in this issue of The VOICE).1 However, the likely and biggest contribution to the
current situation in infertility practice came from the industrialization of IVF, - especially over the last decade, which also removed direct incentives to offer quickly best pregnancy and live birth rates, - not the least because failure in most cases just offered another full IVF cycle for the business. 2,3
The establishment of IVF clinic networks mandated a completely different approach to IVF than centers had pursued earlier. Whether change involved the local spread into one or more central units with several satellite clinics attached, the establishment of national clinical networks, or even international multi-continental spread, the larger clinic networks grew, the more industrialized they had to become in establishing uniform best practices and best protocols, everybody was expected to follow. If the development of the best practice is handled well, this can be an excellent approach to offer good infertility services to a large majority of patients who are mostly young and uncomplicated. But the more complex patients become, the higher the probability of her/him being lost in the cracks of an otherwise very well-functioning system that simply cannot afford required deviations from routine protocols and practice.
The natural history of this evolution in the provision of infertility care, therefore, is the rise of a double-tear system, as we have been increasingly witnessing in recent years: Patients enter infertility care at the local level in one of these industrialized clinic systems, with a majority quite quickly achieving their goals of pregnancy and live births. But roughly 15-20% of patients fail, - uninformed that, considering their specific conditions, such an industrialized set-up was not in their best interest because it could not offer the individualization of care they required. This latter group of patients, therefore, suffers a double insult: they over several attempts do not conceive and, especially if older, in addition, lose valuable time. And, in infertility treatments, time is rarely on a patient’s side!

Large volume programs, especially if multi-local, must rely on consistent and spelled-out treatment protocols which usually allow for only very limited individualization of patient care. Such an uncompromising protocol-driven approach in this kind of setting clearly offers the best possible IVF cycle outcomes for the largest number of patients undergoing IVF cycles. As already noted above, these programs, consequently, simply cannot afford to deviate too much from these preset protocols. Here are some observations that prove the point: The most obvious one is, of course, that most IVF centers after ages 42 to 43 automatically – and without really even trying their patients’ own eggs - refer almost all women to third-party egg donation. Another good example discovered in a still unpublished study, the CHRs investigators found an almost perfect correlation between organization size and utilization of preimplantation genetic testing for aneuploidy (PGT-A). Congressionally, mandated reports from U.S. IVF centers demonstrated convincingly that the larger provider organizations were, the higher was the percentage of their IVF cycles in which they utilized PGT-A.
Similarly, practically every clinic network that reports to either CDC and/ or ASRM/SART, almost universally cultures patients indiscriminately to blastocyst-stage, whether younger, older, or with normal or abnormal functional ovarian reserve (FOR). Many, especially older women, but also younger women with premature ovarian aging (POA), may, however, benefit from cleavage-stage transfers because, cumulatively, cleavage-stage transfers to a minor degree appear to beat out blastocyst-stage transfers, an observation only explainable by some embryos, not even in good laboratories reaching blastocyst-stage, if transferred at cleavage-stage, still offering healthy pregnancies and normal births. Though a controversial issue, recent publications increasingly support such
an explanation: For example, if only one embryo was available on day-3, cleavage-stage transfer of this embryo significantly beat out blastocyst-stage culture of this embryo in combination with PGT-A.4 Moreover recognizing the limitations of a retrospective study and trying to overcome it by matching patients by propensity score, a recent study reported a strong trend in favor of day-3 transfers in live birth rates with reference cycle start (15.2% vs. 12.4 %, P=0.160) and a somewhat less obvious trend also in favor of cumulative live births (17.7% vs. 16.8%).5
Whether to pursue cleavage or blastocyst-stage transfer is, of course, easy to resolve in younger, good-prognosis patients, where blastocyst-stage transfers may speed up time to pregnancy and where this outcome gain may be an appropriate compensation for a few “lost” embryos on the way to blastocyst-stage because these women will, still, have embryos for likely cryopreservation. However, in poorer-prognosis patients with small embryo numbers, this can become a crucially important issue that must be individualized if such patients are to be offered best overall chances. Even more importantly, since blastocyst-stage transfer in such poor-prognosis patients may, indeed, reduce pregnancy chances if none of their embryos makes it to blastocyst-stage, the controversial issue is no longer only how to maximize chances but how not to adversely affect pregnancy and live birth chances?
Though most women under age 40, likely derive neither benefits nor harm from blastocyst-stage culture,6 routine extended culture for everybody in protocol-driven programs, therefore, makes logical sense. However, for that small group of younger women under age 40 who either suffer from POA or have other reasons for producing only small egg and embryo numbers, every embryo counts. It is this group of younger women who not only derive no benefit from such protocol-driven programs but may, actually, be harmed in their IVF cycle outcomes by receiving the same treatments
as good- and average-prognosis patients. Therefore, it is this relatively small group of younger women, generally assessed as representing between 15-20% of patients who, likely, would not only fail to benefit from entering such protocol-driven IVF programs, thus actually producing poorer cycle outcomes. They also would lengthen time to pregnancy in comparison to receiving more individualized treatments from the very beginning of their fertility journey. To help women to recognize early that they may fall into this group of patients is the central purpose of this article.
As noted above, ca. 15-20% of infertile women under age 40 are relatively poor-prognosis patients, who, likely, would benefit from individualized treatment schedules. A big part of this population are women with POA which affects approximately 10% of all women, independent of race and/or ethnic background. Practically, this means that 1 in 10 women will develop low FOR (LFOR) before age 40 and most nowadays will not even know that this is happening because the condition called POA is quiescent and mostly asymptomatic. Moreover, at young ages, hormonal contraceptive use further covers up some of the very few potential signs and symptoms that may cause suspicion like, for example, changes in menstrual patterns.
Paradoxically, POA is a diagnosis that 50 years ago did not exist because women used to have children at much younger ages. If by age 35, they no longer fell pregnant, they often celebrated because they already had sizable families and no longer wanted more children. As women now are increasingly delaying pregnancy and since POA reaches an infertility threshold at approximately age 35 (though some women can experience clinically overt POA already at much younger ages), the diagnosis is becoming more frequent and POA patients, obviously, can be found concentrated in fertility clinics.
When coming off their hormonal contraceptives after often many years of uninterrupted use, these patients are usually not only surprised about having a diagnosis of infertility but find themselves also out of most treatment options. Under the best of circumstances, they by that point will need IVF and, in worst cases, only third-party egg donation will do.

In IVF cycles, these POA patients, moreover, will require different approaches to women at the same ages, - but without POA and, still, will have lower pregnancy chances. Diagnosing such patients, therefore, preferably before age 35 (or actually as early as possible), therefore, should be a priority at fertility clinics and, especially young women on long-term hormonal contraceptives, should at least every few years interrupt this contraception, so that FOR can be properly assessed. The CHR’s investigators have proposed such early diagnosis of POA risks for several years and were, indeed, awarded a patent for an algorithm for such early diagnoses under the title “What’s my fertility?” 7 which is able to predict risk for POA or not. If such an increased risk exists, we have not yet learned to prevent overt clinical POA from developing. However, such women will be at least given the opportunity to make choices regarding their reproductive future and will not be surprised by first finding out that they suffer from infertility when already at advanced stages of clinically overt POA.
Once young women between ages 20 and 30 are diagnosed as “at risk,” testing will reveal how close they may be to infertility, which will offer the options of either advancing the planned timing of pregnancies (hopefully, still without the need for fertility treatments) or, in an attempt at fertility preservation, they will still have the opportunity of freezing good numbers of good quality eggs for future use. The CHR, therefore, strongly recommends that young women on hormonal contraceptives of any kind, which can “hide” menstrual irregularities, often the only sign of
impending POA, come off these contraceptives for at least one month, every two to three years so that their FOR can be properly assessed.
Under the name ”What’s my fertility?”, the CHR has been offering for several years such early diagnoses to young women. The testing process involves a few very short questions and a one-time blood draw that establishes whether a young woman is at no detectable risk, is at risk, or already is affected by POA. Women at no risk, are, depending on age, recommended to return for repeat testing in three to five years; those at risk, depending on the presumed degree of risk, enter a much shorter follow-up schedule, while already afflicted patients are immediately referred into either fertility preservation or outright fertility treatments.
Through its ”What’s my fertility?” program, the CHR is striving to increase among its patients the number of younger patients, either at risk or already affected by POA, because, as a fertility center with highly individualized protocols, the CHR is the ideal place for such patients to receive their treatments early in their treatment journey. As we have noted on repeated occasions before in these pages, if we can see these young women before they have failed large numbers of IVF cycles elsewhere, we not only can, likely, do better than protocol-driven IVF clinics in achieving pregnancies, but can save patients in time to pregnancy. Almost nothing is more important for infertile women than time!
1. Nargund G, Kumar Datta A. Reprod Biomed Online 2022;44(4):587-589
2. Von Schondorf-Gleicher et al., J Assist Reprod Genet 2022;39(3):591-604
3. Patrizio et al., J Assist Reprod Genet 2022;39(2):305-313; CORRECTION: Idem 39(2):315
4. Xiao et al., Reprod Biomed Online 2019;39(6):916-923
5. De Croo et al., Hum Reprod Open 2022;(3):hoac031
6. Glujovsky et al., Cochrane Database Syst rev 2022;5(5):CD002118
7. Gleicher et al., Reprod Biol Endocrinol 2015;13:34
Communication between patients, physicians, nurse coordinators and embryologists is the key to success at the CHR. Timing from cycle start to embryo transfer not only depends on an interactive platform between all participants but also on the biological underpinnings that enable sperm and egg to collaborate in the process of fertilization and embryo development. This image from professor Albertini’s archives documents a case where upon retrieval this human oocyte was found to be immature and therefore not ready for fertilization. It happens that many patients get to retrieval only to yield a small percentage of oocytes that have not been able to fully mature. Not to worry, the embryologists who recognize this situation will give such eggs a “second chance” by culturing them overnight upon which about 50% of oocytes will have reached the mature metaphase-2 state. This picture reveals a telltale sign of the problem and if you look closely, you may be able to see many fine threads sending information from the surrounding cumulus cells through the black zona pellucida that may, or may not signal the oocyte to mature.
If we take a closer look inside the shell covering of the oocyte (red sphere lower right corner) these threads serve as telephone cables transmitting information from the cumulus cells into the oocyte. Just what kind of information is conveyed to the oocyte is under investigation but Dr. Albertini and colleagues over the years have discovered that important fuels like ATP are among the critical molecules the egg receives during ovulation that will propel the activities of the embryo following fertilization.
This elaborate communication system between the oocyte and its companion cells is not unique to humans. This figure illustrates using confocal microscopy one of the first images taken from a rhesus monkey oocyte that was collected just prior to the animal undergoing ovulation. Only a small portion of the oocyte is visible within the zona pellucida but it is clear that each of the surrounding cumulus cells are interconnected with each other and with the oocyte by these specialized information bearing structures. Here at CHR we take pride in continuing our research on the role communication plays in determining the quality of the oocytes our patients provide on the long and winding road to parenthood.
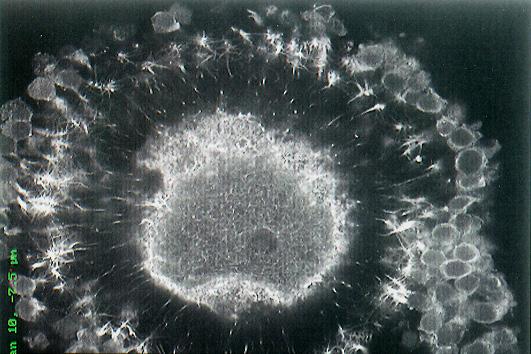
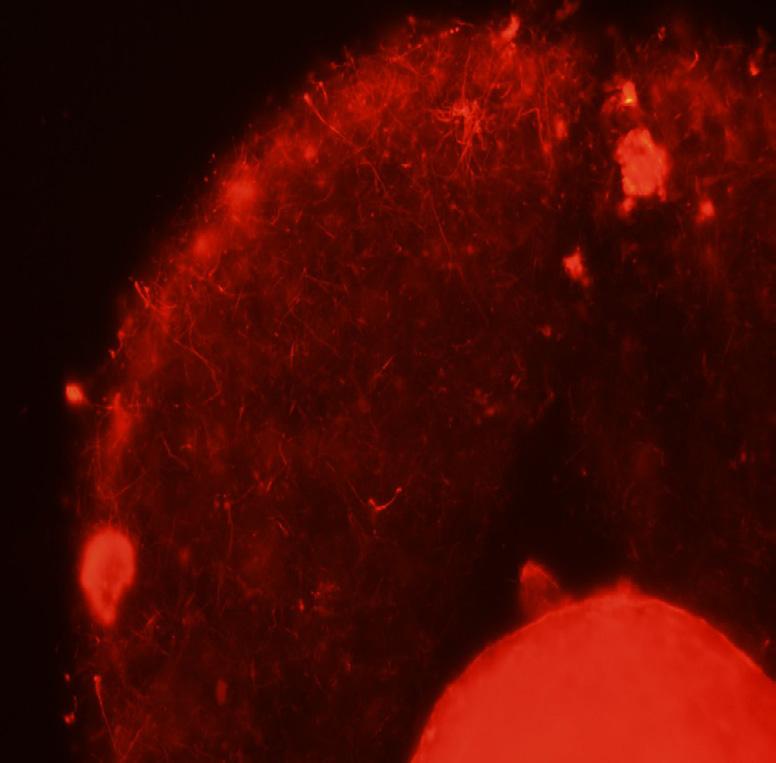
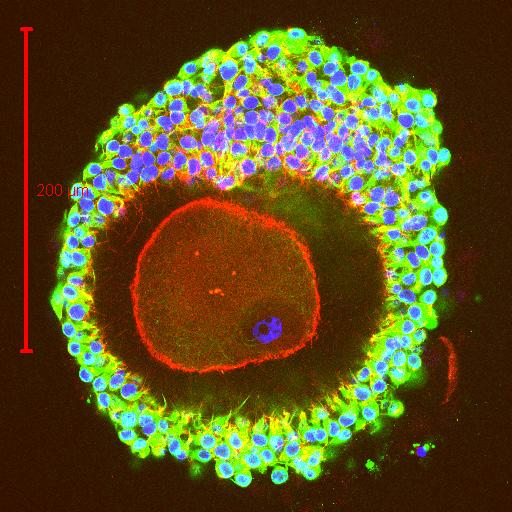 Image 2
Image 3
Image 2
Image 3

 By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist The CHR, New York, N.Y.
By Norbert Gleicher, MD Founder, Medical Director and Chief Scientist The CHR, New York, N.Y.

Until only relatively recently by most people believed to represent a rather straightforward effort, searching for truth in contemporary America has, suddenly, become highly complex and controversial. This, of course, not only relates to truth in medicine but to our complete existence as an intelligent species, by thousands of years of evolution and history imprinted with a restless desire for finding “truth” in religion, governance, art, science, medicine, relationships, and anywhere else.
What does represent truth has over millennia and centuries constantly been challenged, with traditional battlelines usually drawn between supporters of “old” truth ( “conservatives”) and supporters of “new” truth (“progressives”). This historical fact, alone, defines “truth” not as a constancy but as a fluid and constantly changing concept, thereby contradicting widely
Definitions Source
The body of real things, events, and facts. Merriam-Webster1
The state of being the case.
A judgment, proposition, or idea that is true or accepted as true. The body of true statements and propositions. The property of being in accord with fact or reality. Fidelity to an original or to a standard.
Sincerity in action, character, and utterance.

GOD
That which is true or in accordance with fact or reality. Oxford Languages2 A fact or belief which is accepted as true.
All the facts about “it,” rather than things that are imagined or invented. Collins Dictionary3
The true facts about something, rather than the things that have Oxford’s Learner’s Dictionaries4 been invented or guessed.
A fact or principle that is thought to be true by most people. Cambridge University Press5
distributed definitions of the word which, mostly, ignore this fluidity and, therefore, imply permanence (selected definitions and their sources are offered in Table 1). Remarkably, as the table also demonstrates, definitions not only differ, but often utilize words, which, in themselves, require definitions not less complex than the word “truth.” Some, indeed, use the word “true” to define the word “truth,” in itself somewhat of an oxymoron.
This subject has occupied philosophers from the times of Plato, Aristotle, and Socrates, with the latter’s quote, “I am the wisest man alive, for I know one thing, and that is that I know nothing,” 6 indeed representing to this day, likely, the wisest definition of “truth” because, once we acknowledge how little we know, one must conclude that, contrary to what textbooks teach us, and religions as well as ideologies impose, there is no universal truth beyond shorter or longer moments in time.
Having for some time though about the subject of truth in medicine for a future “Piece of My Mind” article, how “truth” has historically been defined in medical practice, has, therefore, seriously occupied my mind now for months. Unsurprisingly, I, however, struggled in preparing the usual outline for an article in my mind, stuck at first base in,
basically, trying to come to grips with what I would be really writing about. If “truth” is only a transient, time-dependent concept, not even requiring that almost all (only “most”) people at any given moment consider something to be true,5 how would it ever make sense to attempt writing about “truth” in medical practice?
One day, while watching a news program on the renewed national interest in the origins of the COVID-19 pandemic, one of the medical experts, however, helped me over this hump when reminding me that, like “the truth,” best evidence in medicine is always transient (a theme in the past, indeed, repetitively addressed in The VOICE). Best medical evidence and what in science (and other spheres of life) is considered “truth,” therefore, share in the characteristic of always being time-limited and, consequently, always being open to challenges. In contrast to religions and political ideologies, where the source of alleged evidence is really mythical, in modern medicine, “truth,” is, however, supposed to depend on -in the moment - best available evidence. What that is, as the COVID-19 pandemic so well demonstrated, can often become highly controversial. “Truth” in medicine, therefore, is not only transient but, at times, characterized by more than only one presentation.
Efforts to silence diverging medical opinions, therefore, not only are unconstitutional, but impractical and self-defeating, with the COVID-19 pandemic, once again serving as an excellent example in demonstrating that the interruption of necessary conflicts of opinion in developing best medical evidence and the medical “truth” of the moment, will only lead to inferior medical care and poorer treatment outcomes.
That open discourse between opposing opinions is essential for progress in science has been recognized for ages; yet, increasingly, attempts at silencing diverging opinions appear to come into vogue again: For the first time since McCarthyism during the country’s “red scare’ in the 1950, we are again witnessing organized efforts by government and/or other major interest groups to silence unpopular opinions. Paradoxically, the emotional “pain” especially our current very sensitive youth is apparently experiencing from unpopular opinions, supported by some often rather surprising protagonists considering their historically rather fervent supporters of free speech, including colleges, universities, and general as well as social media, is considered reason enough to support restrictions on unpopular speech.
That the suppression of free expression is dangerous in all spheres of democratic civilization seems obvious; but not in many areas of daily life will the consequences be as quickly apparent as in medicine. Take for example the recent law that the state of California passed (fortunately at least temporarily put on ice by the courts) threatening physicians with loss of license if they are found to have deviated in their medical opinions from “standard of care,” not as defined by their peers but as concluded by a government-appointed bureaucratic body.7 Or consider that the American College of Obstetricians and Gynecologists (ACOG), supposedly the national organization of all OB/GYNs in the country, recently banned a club of “pro-life” colleagues from hosting an exhibition booth at the annual ACOG Conference because “they did not represent the ACOG’s value system.”8 As a life-long “pro-choice” advocate, to me this very obvious example of intolerance not only appears shameful on collegial grounds, but contradicts everything medicine should stand for in supporting and, indeed, preserving differences in opinion on how we all view science and the world.
Considering these increasing pressures toward suppressing uncomfortable speech and, therefore, opinions, serious concerns are timely because, as of now, one can still reasonably believe what we read, hear, see, and what general as well as social media offer. Computer-generated imagery has, however, in recent years increasingly eaten away on this assumption, and things will get even much worse in the very near future, as we are entering a time when “deepfakes” of
images, videos, and audios, can be produced with increasing ease through “deep learning,” a subset of artificial intelligence (A.I.), - even allowing you to put words into someone’s mouth.9 The New York Times addressed this subject very recently in a frontpage article in the business section.10
And then, there are all the concerns surrounding the introduction of A.I. chatbots, also recently subject of an article in The New York Times 11 In other words, instead of improving the world’s ability to determine “reality” and the from reality resulting evidence and “truth,” it appears that we purposefully go into the opposite direction. This is obviously a very worrisome development not only for medicine and science in general, but for all of our understanding of the building blocks for all of our perceptions and, therefore, for all of our knowledge, making the world even more vulnerable to manipulation of minds than it already has become over recent years through the mind-bending effects of social media.
Considering furthermore how much difficulty we currently already experience in determining what represents “truth,” one cannot but wonder whether in the future it will be even worthwhile trying to find out what the truth of the moment is in medicine or in any other sphere of our daily existence. I, therefore, worry for our children and grandchildren; they may be spending their lives in a largely “unreal” world, manipulated by large companies and/or governments without the ability of regular citizens to distinguish between what is real and/or fake information, - a truly frightening prospect!
REFERENCES
1. https://www.merriam-webster.com/dictionary/truth
2. https://languages.oup.com/google-dictionary-en/

3. https://www.collinsdictionary.com/us/dictionary/english/sim ple-truth#:~:text=uncountable%20noun,that%20are%20imagined%20 or%20invented
4. https://www.oxfordlearnersdictionaries.com/us/definition/english/ truth#:~:text=the%20truth,have%20been%20invented%20or%20 guessed
5. https://dictionary.cambridge.org/us/dictionary/english/truth
6. https://www.brainyquote.com/authors/socrates-quotes#:~:tex t=The%20only%20true%20wisdom%20is%20in%20knowing%20 you%20know%20nothing.&text=I%20am%20the%20wisest%20 man,is%20that%20I%20know%20nothing.&text=I%20know%20 that%20I%20am,know%20that%20I%20 know%20nothing.
7. https://www.nytimes.com/2022/08/29/technology/california-doc tors-covid-misinformation.html
8. https://www.catholicnewsagency.com/news/253768/ pro-life-obgyns-are-banned-from-taking-part-in-medical-conference
9. https://mashable.com/article/deepfake-video-altered-text
10. https://www.nytimes.com/2023/03/12/technology/deepfakes-cheap fakes-videos-ai.html
11. https://www.nytimes.com/2023/02/08/technology/ai-chatbots-disin formation.html
[https://www.fertilitybridge.com/inside-reproductive-health/172gleicher]
A successful CHR intervention with New York State on behalf of gay HIV-positive males with undetectable viral load
The CHR is very pleased to report, and grateful to Matthew Kohn, PhD, the Director of the Tissue Resources Program at the New York Department of Health (James V. McDonald, MD, MPH, Acting Commissioner; Megan E. Baldwin, Acting Deputy Commissioner), that a query from the CHR has resulted in a very significant change in policy by the Department regarding the use of donor semen from HIV-positive donors: New York state regulations, 10 NYCRR Part 52 requires that all semen donors be tested and found negative for HIV-1 and HIV-2, and precludes the use of semen from a donor who has tested positive for either virus. Since 2017, it, moreover, has been the Department’s policy that U = U, or “Undetectable = Untransmittable.” As per the policy, “people living with HIV (PLWH) who have achieved and continue to maintain an undetectable viral load, therefore, are not considered to sexually transmit HIV.” Yet, their semen could not be used for the creation of transferrable human embryos into a third party (like a gestational carrier) not, otherwise, through intercourse, exposed to the individual’s semen.
In response to a query from the CHR regarding a same-sex male couple that is desirous of having a child with help of a gestational carrier, - but where the potential semen contribution would have come from an HIV-positive individual, the Department decided to review its policy regarding donor semen use from HIV-positives males. Under so-far existing FDA and New York State rules, all HIV-positive males, even if “Untransmittable,” were prohibited from contributing semen, which included the possibility of creating embryos with such semen that then would be transferred into a gestational carrier. The recent review of the subject by the Department then led to the following letter received by the CHR (and we presume other IVF centers in the state):

Under Part 52-3.8, the Department may exempt a tissue bank from specific requirements in Part 52 under limited circumstances. Requests for exceptions to allow assisted reproductive procedures¹, using semen from directed (known) donors² who are living with HIV, may be provided under the following conditions:
- The semen donor is taking antiretroviral therapy as prescribed and has an undetectable viral load by blood testing concurrent with the collection of the semen specimen(s) to be used.
- The recipient, including a gestational carrier, is fully informed and counseled about the risks by the tis sue bank medical director or attending physician, with documentation of such.
- The recipient is offered pre- and post-exposure prophylaxis for HIV, including 20 days preceding embryo transfer and 28 days post-embryo transfer procedure.
- The tissue bank follows CDC’s Universal Precautions in handling the reproductive tissues.
- To prevent accidental misuse of the reproductive tissues, the tissue bank sequesters the semen speci mens, and any resulting embryos, from other donor samples.
- If a gestational carrier is used, the tissue bank is registered as an Assisted Reproductive Technology Service Provider, the surrogacy agreement adheres to the requirements of the Child-Parent Security Act, and the gestational carrier is provided with the Gestational Surrogates’ Bill of Rights.
As a reminder, FDA requirements, as found in Title 21 of the Code of Federal Regulations (CFR) Part 1271, apply to all tissue banks in New York State.
The reminder regarding the FDA was very appropriate because IVF clinics operate under the federal supervision of the FDA. The newly announced very logical policy of the New York State Department of Health created a rational for the CHR to contact the FDA with a request to review the agency’s current policy, even-though current FDA policy allows use of otherwise unqualified donor semen if the recipient is appropriately informed and stored specimens are appropriately marked.
As the CHR is serving a rapidly growing population of individual HLGPT patients and couples, the CHR considers it an essential responsibility to act as an advocate for this patient population in trying to overcome impediments to successful reproduction.
The CHR’s investigators participated in two publications that appeared electronically and/or in print over the last month, both addressing the subject of PGT-A. We here offer brief insights into both:
Gleicher N, Mochizuki L, Barad DH, Patrizio P, Orvieto R on behalf of the International Do No Harm Group in IVF (IDNHG-IVF). A review of the 2021/2022 PGDIS Position Statement on the transfer of mosaic embryos. J Assist Reprod Genet. 2023 Mar 9. doi: 10.1007/s10815-02302763-6. Online ahead of print.
This manuscript was a detailed, almost line-by-line, critique of a recently published guidance document by the Preimplantation Genetic Diagnosis International Society (PGDIS) in the medical journal Reproductive Biomedicine Online 1 Some background: The PGDIS is a relatively small society, made up of almost exclusively individuals making a living from performing PGT-A. In 2016, this society published out of the blue a first guidance document for PGT-A on its website which, paradoxically, did not have authors, references, or any other explanation for how this
guidance had come about. Even more paradoxically, this guidance document became worldwide the guiding light for PGT-A and, therefore, IVF practice. Due to the PGDIS having recently removed this guidance after six years (replacing it with only a very short summary), it unfortunately, is no longer possible to demonstrate how absurd this guidance was in almost all of its components.
In 2019, the PGDIS then published a second guidance document on PGT-A, this time, however, in a medical journal.2 Though this document, for a change, had authors and included references, it still lacked literally all of the most basic requirements of a medical guidance document, including information on how data was collected and analyzed, how conclusions were reached, who fulfilled what responsibilities in the process and in writing the paper, and, finally, whether anybody involved in the process had conflicts of interest (and, if so, what they were). The CHR’s researchers and an international group of colleagues, therefore, critically reviewed this guidance in a manuscript that was submitted to the same journal where the guidance had been published. However, the journal’s editors rejected the submission without even bothering with an outside peer review. Therefore, the paper had to be published in a different medical journal in the field.3
Here-noted CHR publication addressed a third guidance document of the PGDIS regarding PGT-A, published again in the same medical journal as the second one. CHR’s investigators and colleagues again composed a critique of the document and, again, submitted it to the same medical journal, where it, again, was rejected without external peer review. Remarkably, the rejection letter this time was written by a senior editor of the journal, - herself a prominent member of the PGDIS
However, the background story of this manuscript got even more interesting: The rejected manuscript was, of course, resubmitted to another medical journal (different from the journal that had published the prior critique), where it was accepted after revisions and published last month.4 The peer review process of the manuscript, however, took a highly unusual turn: One of the reviewers noted that some of the allegedly verbatim quotes in the critique of the PGDIS paper, were inconsistent with how the final, printed version of the PGDIS guidance read in Reproductive Biomedicine Online. Astutely, the anonymous reviewer compared the submitted critique to the several months earlier electronically published version of the PGDIS guidance in the journal, and, low and behold, suddenly, the verbatim quotes in the submitted critique manuscript of the PGDIS guidance document were a perfect fit. The reviewer, therefore, correctly concluded that the authors of the critique paper apparently had worked off the journal’s electronic version of the PGDIS paper, which preceded the print version by several months. As the evidence now suggested, this electronic version of the PGDIS manuscript, however, between electronic and print publications, had apparently still undergone significant edits.
While on occasions manuscripts are further edited between electronic and print versions, such late changes are, however, extremely rare. Moreover, they are usually transparently disclosed and explained to readers. This was not the case here! Miraculously, the changes made to the manuscript, in addition, and without exception, only involved criticisms of the PGDIS guidance pointed out in the originally submitted critique to the medical journal (Reproductive Biomedicine Online) which this journal had rejected without external peer review.
What made the authors of the PGDIS guidance change their original manuscript in this way after their manuscript had already been published electronically, therefore, becomes an interesting question to ponder. There, of course, are only two possible explanations: Either the authors, suddenly, acquired divine insights into many of the mistakes in their electronically published manuscripts that exactly coincide with criticisms by the journal rejected critique of their manuscript, or the editors of the medical journal, after rejecting the critique-manuscript, turned its content over to the authors of the PGDIS paper, - so they could make appropriate corrections between electronic and in print publications.
Becoming aware of what had transpired during the peer review process, the authors of the recently published and here-noted critique paper, of course, had to make significant revisions to their manuscript. In explaining these changes to readers, they reported this sequence of events but politely refrained from being accusatory.4 However, assuming hypothetically that editors of the journal provided the authors of the PGDIS document with a copy of
the critic paper they had rejected and, again hypothetically, also allowed (or even encouraged) the authors of the PGDIS document to correct sections in their manuscript between electronic and print publications based on criticisms in the rejected critique-paper, this would not only represent an inexcusable breach of confidentiality in medical publishing, but would also represent a simply inexcusable breach of scientific publication ethics, disqualifying any editor responsible for such conduct from ever again being allowed to assume responsibilities of a medical editor.
Within this context, it is also important to note the close historical connection between the PGDIS and Reproductive Biomedicine Online, as both organizations were founded by the same individual and the journal counts the PGDIS among its affiliated organizations.5
To understand the seriousness of potential ethical transgressions in this case, we strongly recommend you read the recently published paper by the CHR’s investigators and colleagues in its full length. The manuscript points out well the liberties the PGT-A industry has now been taking for over 20 years in misrepresenting established facts.
Gleicher N, Patrizio P, Mochizuki L, Barad DH. Previously reported and here added cases demonstrate euploid pregnancies followed by PGT-A as “mosaic” as well as “aneuploid” designated embryos. Reprod Biol Endocrinol 2023; 21:25
The CHR has been transferring embryos by PGT-A reported to be chromosomal “abnormal” since 2014, when the test was still called preimplantation genetic screening (PGS) and embryos were only differentiated as either “euploid” (normal) or “aneuploid” (abnormal). By 2016, Next Generation Sequencing (NGS) allowed for the recognition of a second cell lineage in a trophectoderm biopsy and, with the renaming of the procedure to PGT-A, laboratories also started classifying embryos as either “euploid,” “mosaic,” or “aneuploid.”
To this day, a large majority of IVF centers only transfer embryos by PGT-A reported as “euploid.” Especially over the last year, increasing numbers of colleagues have, however, also started transferring selected “mosaic” embryos. The CHR has never differentiated between “low-” or “high-mosaic” or between “mosaic” and “aneuploid” because we are convinced that PGT-A, as currently performed, cannot accurately discriminate between those options. In transferring “abnormal” embryos the CHR, therefore, does not discriminate between “mosaic” and “aneuploid,” fully recognizing that there may be differences in pregnancy and live birth chances between “abnormal” embryos, - but convinced that any differentiation would only increase non-use and/or disposal of embryos with significant pregnancy potential.
With the same (false) certainty with which many proponents of PGT-A for the longest time opposed transfers of all chromosomal-“abnormal” embryos (some of which they now suddenly themselves transfer), they have been (falsely) claiming that no embryo, by PGT-A reported to be “aneuploid,” has ever led to a normal delivery. The CHR, in contrast, has argued for years that this is just one more incorrect conclusion of the PGT-A industry and, indeed, reported a small number of such cases in the literature.6,7 Though every healthy birth following the transfer of an allegedly “aneuploid” embryo represents irrefutable evidence that at least some of these embryos can establish healthy and normal births, those cases did not convince the naysayers, thereby, however, convincing the CHR team to conduct a review of the literature of all cases so-far reported.
Since the CHR to this day is almost alone in transferring selected by PGT-A as “aneuploid” diagnosed embryos, it was not surprising that the literature so far revealed only one such case outside of the CHR experience;8 but the here addressed recently published CHR paper, nevertheless, beyond reasonable doubt once again reaffirmed that proponents of PGT-A often simply do not know what they are talking about or, maybe, simply don’t want to know. At some point ignorance can no longer be acceptable as a valid excuse: First PGT-A (then called PGS) was supposed to improve pregnancy and live birth rates in IVF. Then the goal was to reduce miscarriages by eliminating chromosomal “abnormal” embryos. Then, PGT-A laboratories claimed that in blastocyst-stage embryos mosaicism was rare (in single digits), even though single-cell studies have demonstrated that almost all blastocysts contain aneuploid cells and, therefore are mosaic.
Then the “experts” claimed most aneuploidies at blastocyst stage were meiotic; except, in reality, almost 80% are mitotic, which, of course, has major relevance for the accuracy of a single trophectoderm biopsy in determining the status of a complete embryo. In short, following the history of PGS/PGT-A for over 20 years, the procedure’s proponents have been wrong over and over again. And as here presented paper demonstrated, they are also once again wrong when rejecting the idea of transferring by PGT-A as “high-mosaics” and or “aneuploid” classified embryos. For as long as colleagues consciously or subconsciously ignore basic human embryology and physiology, they will continue to waste pregnancy and live birth chances for their patients by not using, or even discarding embryos with pregnancy and normal live birth potential. It would also behoove them to consider all of those women who are wrongly advised that their only chance of motherhood is through third-party egg donation because all of their embryos were repeatedly reported as “aneuploid.” The CHR’s experience has demonstrated indisputably that such women do, still, have considerable pregnancy and live birth chances with the use of their own allegedly ‘abnormal” embryos.7,9

1. Leigh et al., Reprod Biomed Online 2022;45(1):19-25
2. Cramm et al., Reprod Biomed Online 2019;39(1):e1-4
3. Gleicher et al., Reprod Biol Endocrinol 2020;18(1):57
4. Gleicher et al., J Assist Reprod Genet 2023; doi: 10.1007/s10815-023-02763-6. Online ahead of print.
5. https://www.rbmojournal.com/content/societyinfo
6. Yang et al., Nat Cell Biol 2021;23(4):314-321, CORRECTION idem 23(11):1212
7. Barad et al.., Hum Reprod 2022;37(6):1194-1206
8. Lin et al., F S Reports 2022;3(4):294-295
9. Barad et al., Hum Reprod 2022;37(9):2216-2218








We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratory supervisor for research

Learn more by accessing our library of educational videos:

https://www.centerforhumanreprod.com/contents/video-gallery
Center for Human Reproduction

To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.





So, you were told that intrauterine inseminations (IUIs) were the right treatment for you. You did not conceive in the first cycle and now you are concerned, wondering what to do next.

The likely answer is that there is no reason yet to worry because one failed cycle does not mean very much, - just as, even at young and fertile ages, one cannot every time expect to get pregnant spontaneously in the first month of trying. The general recommendation is that patients should undergo only three IUIs before going on with more intensive treatment which usually means in vitro fertilization (IVF). However, some colleagues have recommended up to eight IUI cycles.1 Three-cycle recommendations stem from the observation that pregnancy rates drop significantly after the first three cycles have been unsuccessful, but this does not mean that there are no pregnancies occurring at all after three tries.

A more complex related question is whether IUIs are indicated at all in a given patient and, unsurprisingly, the answer is, therefore, also more complex: There are many colleagues who (especially at more advanced female ages) because of relatively low pregnancy chances, recommend against all utilization of IUIs. At the opposite extreme are those colleagues who insist that all women with patent fallopian tubes should undergo at least three IUIs before advancing into IVF. The latter position has also been assumed by many insurance carriers in demanding that patients be offered IVF only after three failed IUI cycles.
The CHR’s position on this issue falls somewhere in the middle. We find the insurance dictum of three IUI cycles for everybody to be ridiculous because older women and/or women with low functional ovarian reserve (FOR) will with IUIs have even lower pregnancy rates than most other women. These patients also often cannot risk the unnecessary loss of time and therefore, simply for that reason, in the CHR’s opinion should quickly be advanced into IVF. However, for younger women with normal FOR, we see no reason why they should not start their fertility treatments with up to three IUI cycles, as long as the patient understands that, in comparison to IVF, IUI cycles result in a much bigger risk for multiple births, - even beyond twins, into high-order multiples.2
The reason is that by deciding how many embryos are transferred, we are quite reliably able to control the multiple, and especially the high-order multiple risk.2 However, with in vivo conception, it is nature (or mostly luck) that determines how many eggs a woman ovulates after treatment with fertility drugs and how many among those will be fertilized by partner semen after intercourse.
One more important additional point: IUIs require at least one patent fallopian tube (and preferably two). A so-called hysterosalpingogram (HSG) before starting IUIs is, therefore, a must. It is always embarrassing to discover only after a patient has failed several IUI cycles to discover that she has occluded tubes and, therefore, never really had a chance of conceiving. All of these efforts, will, of course, be for naut if the egg and sperm cannot meet because of obstructed fallopian tubes.
1. Custers et al., Hum Reprod 2008;23(4):885-888
2. Gleicher et al., N Engl J Med 2000;343(13):2-7
“Mini-IVF” always involves mild ovarian stimulation in an IVF cycle and mild stimulation has been already extensively addressed in a full-length article on page 11. Having read through this preceding article, our readers should, therefore, already have an answer to this question. However, should you have missed this article, only so much: No, “mini-IVF” is really never better than standard IVF if, what in principle defines “being better,” are pregnancy and live birth rates. As also extensively referenced in the preceding article and noted in the literature by CHR’s investigators already in 2011, “mini-IVF” uniformly actually produces in IVF cycles poorer pregnancy and live birth rates than standard stimulations.1
Since, in contrast to many colleagues, the CHR still considers safe and best pregnancy and live birth rates the central desire of every infertility patient, and because not a single properly conducted study has ever been able to demonstrate outcome benefits from “mini-IVF” over standard protocols, we cannot find rational for the utilization of “mini-IVF,” except, maybe, in rare cases of very young women with normal FOR and excellent egg production. Concomitantly, we also reject many of the false claims made by proponents of “mini-IVF,” among those that milder stimulation produces better egg quality.2 If this principal claim were to be true, why does “mini-IVF” produce only “acceptable,”2,3 and not better or at least equal outcomes to standard IVF protocols?
1. Gleicher et al., Reprod Biomed Online 2011;23(3):274-278
2. Zhang et al., Reprod Biomed Online 2010;21(4):485-495
3. Abe et al., J Assist Reprod Genet 2020;37(2):297-304

As the term “chemical” indicates, a chemical pregnancy differs from a “clinical” pregnancy by when and how it is diagnosed: The former is diagnosed early by only chemical means (i.e., usually a pregnancy test in the blood) before turning again negative, while the latter is seen by clinical means (usually an ultrasound examination) and remaining visible by ultrasound.
A chemical pregnancy is basically a very early, before visibility, miscarriage. How do we know that? This is because (except for some tumors) only an embryo produces the hormone, chorionic gonadotropin (hCG), which is the basis of every pregnancy test. hCG, therefore, will be detected in a woman’s blood only if an embryo has dug deeply enough into the uterine lining (endometrium) to establish contact with the mother’s blood supply. Once that contact has been established, hCG will become detectable in the mother’s blood (usually 10-12 days after an embryo transfer in an IVF cycle), which means 8-10 days after implantation (as embryos spend ca. 48 hours in the cavity unattached before implantation).
Symbolically and practically, a chemical pregnancy, therefore, means that a woman’s eggs were fertilized by her partner’s sperm (i.e., that the male partner’s semen very likely is normally functioning) and that the couple’s embryos can (and do) implant. In other words, a chemical pregnancy makes an implantation problem less likely. In most cases, a single chemical pregnancy, therefore, has no adverse meaning and most women with chemical pregnancies end up having normal pregnancies uneventfully.

Losing pregnancies is a very common phenomenon in humans and is part of mankind’s very limited reproductive potential: Ca. 15% of all clinical pregnancies are lost to miscarriage. The

existence of chemical pregnancies was only learned after IVF became a clinical treatment and ca. 22% of IVF pregnancies were demonstrated to end in chemical pregnancies. Up to that point, their existence was unknown because besides, maybe, a short delay in their respective menstruation starts, affected women most of the time had no symptoms. Combined between chemical and clinical miscarriages, roughly half of all pregnancies, are thus lost after implantation.
The innocence of a single pregnancy loss, however, changes if it repeats itself: Whether it is chemical or clinical, after two to three losses (it can be one or the other), pregnancy loss is no longer a random event and, with increasing repeats, increasingly can be expected to have an underlying cause which, of course must be diagnosed if appropriate treatments are to be applied to stop those losses.
REFERENCE
1. https://www.everydayhealth.com/chemical-pregnancy/guide/

This is definitely the most frequently asked question received from infertility patients in every fertility center. It often is also linked to the difficult to combat and often mistaken guilt-feelings of many infertility patients who believe that their infertility problem must have been caused by something they did. Though female as well as male infertility may, of course, have behavior-related causes, in most cases, once patients are in fertility treatments, there is little they can do beyond following medication instructions. Indeed, even if there were obvious behavioral interventions from them
Continued from page
that could help in achieving improvements, those usually require significant delays in treatment, which especially in older women or in women with LFOR at already young ages would be more harmful to pregnancy chances than no intervention.
A good example is obesity. In women as well as men, extreme obesity very clearly affects the success of infertility treatments, and even relatively minor weight loss would already have positive outcome effects.1 Yet attempts at structured weight loss have in principle been unsuccessful in such patient populations. For example, bariatric surgery apparently did not improve fertility treatment outcomes.2
If patients receive reasonably individualized infertility treatments, it appears likely that, should patients derive benefits from out-of-usual-protocol treatments, their REs will already have instituted these treatments. As discussed on page 15 of this newsletter, so-affected patients may benefit from choosing an IVF center for their treatments that is not too protocol-driven; but patients at the CHR receive usually only the following answer to this question: There is not much you can do in addition to the treatments you are already scheduled for. Yet, there are some important additions to these treatments for which only you can be responsible: (i) Watch your diet,though this is not the time to start a concentrated effort to lose weight. However, eat healthy and stay away from food that gives you any form of indigestion because such foods are often inflammatory, and we do not like inflammation in women who are trying to conceive. (ii) Don’t use any medications without, first, asking the CHR staff, - even if prescribed by another physician. (iii) Of course, stay away from drugs, including cannabis. (iv) Make sure you get enough good sleep. Female infertility was recently again associated with sleep disorders.3

1. Meldrum DR. Fertil Steril 2017; 107(4)P831-832
2. Grezegorczyk-Martin et al., Hum Reprod 2020;35(12):2755-2762
3. Zhao et al., Sleep Breath 2023; doi: 10.1007/s11325-023-02802-7. Online ahead of print.Mostly placed into a clinical context, we in this section of the newsletter offer a survey of articles in the English literature, usually published in the preceding month, which the CHR found of interest to the current practice of clinical reproductive endocrinology and infertility, - even if at times not immediately applicable to daily clinical practice. These articles, however, nevertheless often point out where clinical practice will likely go and, therefore, serve an important translational purpose. Translational research has been the CHR’s principal research goal since its founding in 1981, has produced a significant number of U.S. patents over the years, and has propelled the CHR into its current position as a worldwide center of last resort for infertile patients who have failed treatments elsewhere.
An increasingly conflicted world mandates progress in conflict resolution
Not dissimilar to the 1930s, the world is experiencing complex times, with internal political conflicts in countries all around the world becoming more confrontational and disagreements between nations becoming more dangerous. With internal political confrontations on the rise, the medical field has not been spared. Naked politics now also affecting the medical field was probably best demonstrated by the political vitriol that involved the management of the COVID-19 pandemic, with the political left and right widely diverging in how they perceive their own respective realities. The consequence is in many countries a level of internal “political hatred” unprecedented since World War II that can only be described as pre-revolutionary.
Considering the seriousness of the situation, it is not surprising that even basic science journals have started addressing the problem. Nature magazine, for example, recently published an article by Saima May Sidik in which she summarized strategies that researchers are
pursuing “to bring people (again) closer together.”1 We strongly recommend this article not only because of the information it offers on what kind of research is going on “to calm political hatred,” but, even more importantly, because it reemphasizes the need for more and better research into societal conflict resolution in general.
That improvements in conflict resolution in medicine are absolutely essential was indisputably demonstrated by the COVID-19 pandemic which demonstrated that, simply excluding opinions from the debate, never works. That other remedies to resolve strong differences of opinion in medicine must be found was a point made by investigators from the CHR already several years ago, when publishing a conflict resolution analysis on the highly confrontational conflict surrounding the use of preimplantation genetic testing for aneuploid (PGT-A).2 In having a Columbia University-trained expert on conflict resolution, Ms. Lyka Mochizuki, MSc, on staff, the CHR research team has become schooled in understanding the importance of conflict resolution capacity in all spheres of life, including local as well as international politics.
A related topic was the subject of a recent “perspective” article in The New England Journal of Medicine,3 in which two authors (a physician and a lawyer) addressed the fact that the management
of the COVID-19 pandemic has resulted in a clear loss in trust in organized medicine. However, their article unfortunately concentrates on “protecting the legitimacy of medical expertise” by preventing physicians from voicing publicly obviously “false” information.
Examples they present in their article are physicians who insisted “that viruses don’t cause disease” or “that COVID-19 vaccines magnetize people or connect them to cell towers,” failing to recognize that the loss of trust in expert medical opinion was not caused by a few very obvious “cuckoos” in the profession with crazy ideas, but by main-stream representatives of the profession (i.e., the Anthony Faucis of the world), major authoritative government bodies, as well as professional organizations. Nobody argues with “cuckoos” losing their license (after objective prosecution, of course); but, how come so many highly respected physicians and scientists were silenced during the pandemic , - just because they differed in their opinions from the politically correct Fauci line of thought?
Therefore, we do not face “a broad cultural attack on expertise and authority,” as the article claims, but a trust in selective and imposed expert opinion. Philosophers and behavioral scientists have for centuries been skeptical about expert opinions because experts are known for imprinted biases by their expertise and for ignorance about how the rest of the world affects their tiny area of special expertise. How a majority of the medical field cowed to obviously flawed politically correct opinions during the COVID-19 pandemic was, nevertheless, somewhat of a surprise because medicine is very much aware of the limited reliability of expert options and, therefore, relegated them to the bottom of the medical evidence pyramid.
The authors instead see the loss of credibility in medical authority caused by the pandemic as part of a “broader cultural attack,” which “increasingly sees expertise and authority as means for elites to establish and support existing hierarchies.” Though the authors acknowledge “that there may be some substance to this argument,” they basically end up rejecting it, thereby devaluing the public in their ability to differentiate between “cuckoo”- and valid opinions and ignoring their right to self-determination. It is just a little more of good old-fashioned patriarchal medicine that believes that physicians know better what is good for their patients than patients, themselves.
1. May Sidik A. Nature 2023;615:26
2. Mochizuki L, Gleicher N. J Assist Reprod Genet 2020;37(3):677-687
3. Baron RJ, Coleman CH. N Engl J Med 2023;388(8):676-678
As recently warmly welcomed by the ASRM, the U.S. Office of Personnel Management (OPM), which manages the Federal Employee Health Benefits (FEHB) program in its 2024 carrier letter announced plans to include artificial inseminations (and medications), and medications for up to three IVF cycles annually.1 The CHR, of course, also welcomes this development as a right step toward inclusion of infertility, as a disease state, into general medical insurance coverage.
1. https://www.asrm.org/news-and-publications/news-and-re search/press-releases-and-bulletins/asrm-applauds-opms-updat ed-health-benefits-for-federal-employees-with-expanded-art-cov erage/
Unfortunately, breast implants are once again in the news. What we mean by that is best demonstrated by a short history lesson: in 1962, the silicone breast implant is invented. In 1976, the FDA regulates silicone breast implants. In 1982, because of adverse effect reported in the literature, the FDA places silicone breast implants into the more rigorous class III category. In January of 1992, primarily because of an alleged association with autoimmune diseases, the FDA calls for a voluntary moratorium on the use of silicone gel breast implants until safety has been reviewed. Several manufacturers then withdraw from the market. In April of 1992, the FDA advises that silicone implants should only be used for reconstructive surgery or to correct congenital deformities. In 2006, the FDA lifts restrictions on silicone breast implants. In 2011, the FDA notes a potential link between silicone breast implants and Breast Implant Associated Lymphoma (BIAALCL), a cancer of the immune system.2 In 2019, the FDA called upon Allergan, a manufacturer of textured silicone breast implants, to recall their BIOCELL model from the market and announced that these implants could cause “adverse health consequences and potential death.” Asymptomatic women are, however, not advised to remove the implants since such removals may increase the chances of developing BIA-ALCL. Symptomatic implants are, however, to be removed immediately.
However, now comes a publication on healio.com,3 in which Shaher W. Khan, MD, an expert in breast implant illness in Michigan, argues that, eventually, all silicone breast implants require removal, and the longer a person has these implants, the greater the chance of complications.
1. https://www.reuters.com/article/us-france-implants-pip/ timeline-a-short-history-of-breast-implants-idUSTRE80P12V20120126
2. https://nwhn.org/why-is-the-fda-asking-for-the-removal-of-certain-breastimplant-models-from-the-market/
3. https://www.healio.com/news/womens-health-ob-gyn/20230306/ qa-all-silicone-breast-implants-eventually-require-removal#:~:tex t=All%20silicone%20breast%20implants%20eventually%20require%20 removal.,upon%20removal%20of%20the%20implants
An article in a recent series on breast feeding in The Lancet claimed, “that an estimated US $341.3 billion is lost globally each year from unrealized benefits of breastfeeding to health and human development due to inadequate investments in protecting, promoting, and supporting breastfeeding.”1 In accompanying unsigned editorial the journal noted that, “for decades the commercial milk formula industry has used underhand marketing strategies, designed to prey on parents’ fears and concerns at a vulnerable time, to turn the feeding of young children into a multibillion dollar industry.” Unsurprisingly, therefore, the journal titled its editorial, “unveiling the predatory tactics of the formula milk industry.”
The editorial team of The Lancet often expressing rather radical positions is not new. They usually express opinions of the political left, which should also not be a surprise, but it is worth noting that all articles in the series, including the editorial, of course, were supportive of one side of the story, -without giving the other side a chance to respond.
The health benefits of breast feeding are, of course, well established and beyond reproach. They, therefore, do not require much further explanation. One, however, once again very clearly notices an authoritarian drift in this series of articles (the editorial included) which not only is intended to inform but clearly attempts to “destroy” the other side, - in this case the “predatory” formula milk industry.”
Readers of the VOICE, of course, know how often this newsletter in representation of the CHR’s opinion has been critical of industry and how often it has pointed out abusive marketing tactics, but at the same time, we always attempt to also present the other side’s position. When medical writing deviates from medical facts and supposes that formula milk is imposed on newborns to shorten maternity leaves, medical reporting becomes political advocacy and, therefore, self-defeating because it loses objectivity and, therefore, credibility.
One comes to wonder whether an article addressing the now almost year-long shortness of sufficient amounts of baby-formula in the U.S. would not have been a much better opportunity for a positive message. For example, how about addressing young mothers with a message that explains all the good reasons why breast feeding may, indeed, be a better and healthier options than formula for them and their offspring.
1. Pérez-Escamilla et al., Lancet 2023; 401:472485
2. Editorial. Lancet 2023;401:409
Based on the opinion of at least one physician recently quoted in an article by Amanda Loudin on medscape.com this is, indeed, the case.1 The same physician continued the quote by stating that, “anyone who cares about patients is doomed to burnout.”
Medical staff burn outs, including physician burn outs, seem to be on everybody’s mind. It’s discussed everywhere, in hospital hallways, medical conferences, medical and lay journals, and especially in social media; and one has to wonder why that is?
Is everybody working harder than in the past? During the COVID pandemic maybe, but before and after, is there really evidence for that?
Medicine traditionally always claimed that prior generations worked harder: Resident hours and working conditions, however, have truly improved; but what is often overlooked is that who practices medicine has dramatically changed. Women now
represent over half of all graduating physicians and their needs, often, of course differ significantly from those of their male colleagues. But even males who choose medical school nowadays are different from those who chose a medical career 30-40 years ago.

The alleged high current burnout rate among physicians then very clearly suggests that medical practice is not adjusting quickly enough to changes in who our physicians will be for the foreseeable future. If we want them to give us good medical care, we have to make sure that we give them the medical environment in which they not only can (barely) survive but will prosper (whatever that may mean for the current generation).
1. Loudin A, https://www.medscape.com/viewarticle/988422
The CHR offers a very competitive salary with incentive bonus structure, an excellent benefit package, and a generous partnership schedule over either a 3-year or 5-year track. Most importantly, however, the CHR offers a unique practice model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If you are the physician-scientist we are looking for, please send your CV to Ms. Jolanta Tapper, COO (jtapper@thechr.com). All submissions are considered confidential correspondence.
Another subject on everybody’s mind in medicine is, of course, the clinical use of Big Data (BD) with the help of Machine Learning (ML). Everybody agrees that BD + MC = THE FUTURE OF MEDICINE; but when and to what degree remains to be determined.
We are looking for an RE, equally experienced in clinical practice and clinical research, interested in a leadership position in one of the country’s best known private fertility centers with a substantial research program
A recent opinion piece in JAMA Health Forum by Joan M. Teno, MD, MS, offers some important insights and is, therefore, strongly recommended to our readers interested in this subject. The principle point she is trying to drive home in her article is that BD + MC are not necessarily enough to achieve adequate predictability of results. Everything depends on the integrity of the data set used (i.e., the quality of the BD).
This is important to understand because without having a clear understanding of the data set used, neither FDA nor medical journal editors can really judge how good a conclusion is reached from BD+MC.
1. Teno JM, JAMA Health Forum 2023;4(2):e230397
The incoming president of the Royal College of Psychiatrists in the U.K., recently called for a public information campaign about the consequences of regular Cannabis consumption.1 He specifically, warned that high consumption was associated with developing psychosis later in life and described this fact as “a ticking time bomb,” with only once a week use creating within years a five-times higher risk.
One can only wonder how come nobody in the U.S. medical establishment is sounding the alarm in this country. Of course, this is not the only adverse consequence of legalizing Cannabis. We have on repeated occasions in these pages pointed out the many adverse consequences to mothers and their offspring in pregnancy. Currently available cannabis is significantly more potent than what was available only several years ago and increasing use, in combination with increasing potency sounds, indeed, like a “ticking atom bomb.” The hesitancy to point this out in this country because of political correctness is regrettable.
What can be learned from female animals about women’s health or human health in general, was recently reported in a very interesting article in Scientific America. The author, for example, points out that certain animals have to maintain certain functions (like, for example, the ability to maintain speed of movement) in pregnancy, which humans lose. In investigating what allows animals to maintain these abilities, can greatly contribute to our understanding of these abilities and, therefore, allow us to “ find solutions in nature to human health problems.”
We have on prior occasions pointed out in the VOICE that adrenals and ovaries share an embryonic primordium and that, after ovaries, adrenals have the largest number of AMH receptors of any organ in the body. Both of these observations strongly point to a considerable interplay between adrenals and ovaries that is currently still not understood. This is why a recent review of adrenal research from ancient anatomy to contemporary molecular biology caught our attention.3 This monster of an article will remain a reference source for adrenal physiology for years to come.
Immediately following this article in the same journal, another excellent article reviewed estrogen receptor signaling in the immune system. The immune system responding to hormonal simulations, including estrogens, has, of course, been known for a long time. This article, however, does it in a very systematic and easily understandable approach and, therefore, also will with certainty become a widely referenced paper for many years to come.4
1. https://www.bmj.com/content/380/bmj.p407.full
2. Natterson-Horowitz B. Scientific America, February 14, 2023. https://www.scientificamerican.com/article/ what-scientists-are-learning-about-womens-health-from-other-female-ani mals/
3. Miller WL, White PC. Endocrine Rev 2023;44:70-116
4. Chakraborty B et al., Endocrine Rev 2023;44:117-141
Laboratory reorganizations and even closures in the genetic testing industry have been covered in the pages of this newsletter in the last three issues. Last month (February 17, 2023) The Wall Street Journal chimed in with an article about the industry leader Illumina Noting that new startups are taking on the industry leader by cutting sequencing costs and finding new applications for the technology.1 Illumina is, however, not sitting on its hand either and is supposedly coming out with a new instrument series, NovSeq X, that reduces sequencing costs to $200 per sample.
Rachel Leland reported in Inside Reproductive Health, and e-mailed out on February 23, 2023 to IVF centers by Griffin Jones that among 12 publicly traded companies in the reproductive health space, 11 demonstrated declining profits over the last year. Among those the best known are Monash IVF, one of Australia’s three dominant IVF clinic chains, Natera, a prominent genetic testing company, EDM Serono and Organon, two of the world’s largest pharma companies producing fertility drugs, and Progyny, the largest fertility benefit and insurance company in the field. It will be interesting to see what this means for the future of the infertility field.
In a March 9, 2023 e-mail to IVF centers from Griffin Jones, Natasha Spencer reported that Natera, with revenue over US$ 800 million reported a net loss of almost US$ 600 million for 2022.
Another major company in the field, Kindbody, announced a US$ 100 million new funding round “to further accelerate growth.”2 In an e-mail from Griffin Jones to IVF centers on March 16, 2023, Erika Riley commented on this funding round as well, noting that the Series C funding round valued the company at US$ 1.8 billion and that the company’s founder supposedly announced a public offering still in this year. It will be interesting to see whether current economic circumstance will really allow for that.
The article also notes that only ca. 20% of the company’s revenues come from direct access services (i.e., to their IVF clinics); 30% come from providing fertility benefits to employers, and another 30% from managed cares services. Through recent acquisitions, Kindbody also owns the genetic testing company Phosphorus Labs and a surrogacy agency, called Alternative Reproductive Resources. Like other companies in the field, Kindbody, thus is pursuing a multiprong approach that involves having representation in multiple aspects of the fertility market.
The company currently apparently serves 112 corporate clients, including Walmart and employs 34 REs as well as 52 embryologists and was founded in 2018 by Gina Bartasi, the same entrepreneur
who also founded Progyny, which currently has a market value in excess of US$ 3 billion.
Another serial entrepreneur in the infertility field is Martin Varsavsky, who founded Prelude and other companies, and recently made the news, when his latest company, Gameto, licensed technology from Harvard University and developed at the Wyss Institute for Biologically Inspired Engineering that allows for in vitro-growth of ovary-like structures, so-called human ovarian organoids.3 Gameto is a biotechnology company using cell engineering to develop therapeutics for diseases of the female reproductive system. A paper reporting on the licensed technology just appeared in print,4 and will be discussed later in the sub-section, “Basic science.”
Infertility practice is, fortunately, not a very heavily litigated medical specialty. Lawsuits are, therefore, paid close attention to. Recently, Brenda Pierson from Reuters Health Information reported that a Pasadena, California, fertility clinic was sued under the allegation that the clinic allegedly implanted an embryo carrying a rare gene that causes a deadly gastric cancer and then falsified records to cover up the mistake.
The couple apparently went through IVF and PGT-M (preimplantation genetic diagnosis for monogenetic diseases) in an attempt , “to break the family curse of cancer and early death” since other family members had succumbed to this rare cancer, called hereditary diffuse gastric cancer. The couple had an embryo transfer with a single unaffected embryo by the gene and conceived but, unfortunately, miscarried. The patient consequently was told by a staff member at the clinic that she has another unaffected embryo, - but this information turned out to be incorrect. In January of 2021, she transferred this embryo, conceived, and delivered a male infant. The parents did not find out the facts of the case until after his birth when they saw everything mentioned in a handwritten note on a report of the January embryo transfer.
The couple then requested a copy of the medical record and received such a copy, though without the alleged handwritten note.
We, of course, have no way of knowing whether this note really existed or not; but the first rule in the defense against malpractice suits is never to change medical records post-factum. Whether guilty or not guilty in a malpractice case, evidence that medical records were changed makes you look guilty, whether you committed malpractice or not.
1. Gormley B. The Wallstreet Journal. February 17, 2023; https://www.wsj.com/articles/ startups-take-on-illumina-in-race-for-cheaper-dna-sequencing-9aa ceecb
2. https://kindbody.com/kindbody-announces-100-million-in-new-fund ing-to-further-accelerate-growth/
3. Brownell L. Wyss Institute. February 21, 2023, https://kindbody.com/ kindbody-announces-100-million-in-new-funding-to-further-acceler ate-growth/
4. Brieno-Enriquez et al., Nat Commun 2023;14(1):670
The CHR now offers paid 1-year clinical-, or 2-year clinical and research - fellowships to general OB/GYNs, which lead to independent clinical competence in practicing reproductive endocrinology and infertility medicine

To qualify, candidates must be graduates of a licensed Ob/ Gyn residency program and must be eligible for a New York state license to practice medicine. The CHR offers a very competitive salary and an excellent benefit package. Most importantly, the CHR offers a unique educational model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting important research. If all of this excites you and you feel that such a fellowship would suit your career plans, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.
Can there be too many studies on a subject?
Ethical behavior is a basic building block of medical research and clinical practice. In a recent editorial in JAMA, two authors (one an editor of the journal) addressed a very interesting issue in medical research which we previously discussed in the VOICE, though within a different framework: What we previously discussed on several occasions in these pages was the question why investigators wasted their valuable time, efforts, and finances on, at times, ridiculous projects. We in that instance especially referred to studies that represented so-called “self-fulfilling prophecies;” in other words, studies that had an obvious answer, even before a study was initiated.
The recent paper in JAMA basically addressed the same issue from an ethical vintage point which they described as “managing persistent uncertainty.”1 What they meant by using this heading is, as extensively discussed elsewhere in this issue (page 19), because “truth” in medicine is always only temporary, one can argue that the concept of best treatment or “standard of care,” also must be viewed as temporary concepts. Consequently, the question arises, when are data secure enough to, for ethical reasons (which we will discuss below in more detail), consider the initiation of further studies unethical and, therefore, inappropriate.
As an example, they cited a study also published in the JAMA, in which 1,206 COVID-19 patients were randomized to the controversial drug Ivermectin and placebo and no significant benefits were obtained from the drug, thereby confirming prior reports on this medication, including three other prospectively randomized studies. The two authors, moreover, discovered that currently 10 more trials of the drug are registered
on ClinTrials.
gov, and concluded that, “decisions about what investigations to undertake must be responsive to the relative social value of continuing to reduce uncertainty around one intervention, and stakeholders must consider whether scarce time, resources, and participant effort could be better invested examining other questions.”
They based their conclusion of the definition of clinical “equipoise,” which is defined as the ethical permission to randomize patients if no consensus exists about a best treatment. They further argue that this definition also means that it is impermissible to randomize to a treatment known to be worse that existing alternatives. Citing the COVID-19 experience they also noted that, even after well-executed studies, uncertainty can often persist, raising the question how to manage such persistent uncertainty ethically.
One of the answers they propose is that funding bodies and editors of medical journals consider, “whether authors performed a comprehensive evidence synthesis through living and systematic reviews of a subject before initiating studies,“ and idea that seems eminently logical. They also correctly argue that “equipoise” mandates that involving patients ethically in the effort of a clinical trial, mandates that the patients’ efforts are not wasted, when they could be redirected toward better and more useful uses.
This is exactly where this editorial runs into problems because, while such redirection of patient efforts on first impression seems logical, it is basically impossible to execute. That this issue represents an ethical problem , moreover, appears somewhat far-fetched. To us, doing unnecessary studies is illogical and, therefore, simply does not make sense and being more of a practical and economic than ethical issue that does not warrant the involvement of already overburdened ethics boards. One more point: Does anybody really expect researchers who do not recognize that they, for example, are pursuing “self-fulfilling prophecies” in their research, expect them to produce research results of even minimal value? We don’t think so! And if they cannot figure it out on their own, they will hopefully quickly find out. The answer to unnecessary studies is not more rules and a growing bureaucratic effort for everybody, - but better peer review.
And now let’s talk about real ethical concerns because, released from jail, He Jainkui is again in the news. If the name is not immediately familiar, this is the Chinese biophysicist who in 2018 used CRISPR-Cas9 to edit the CCRS gene (it encodes an HIV virus receptor) in several human embryos, with the goal of making them resistant to HIV. Ignoring international conventions, he then allegedly implanted two so manipulated embryos in one, and a third into another woman, allegedly producing three live births, - one set of twins and one singleton. We use “allegedly” to describe his activities because, to this day, he has not reported any of this in the medical literature. Consequently, nobody really knows whether he succeeded in editing the genomes of these three embryos (now over 4 years old offspring).
After last year being released from prison in China for “violating medical regulations in China,” paradoxically invited to speak at on February 12 at a bioethics conference at the University of Kent in Canterbury, UK, he, however refused to answer questions from the audience. Other participants noted that his refusal to address his past activities did not further help his already marginal credibility.2 Over 80 researchers from 13 countries attended the virtual conference, while he attended (with 20 academics and students), out of all places, from the now rather infamous city of Wuhan (as in the Wuhan Institute for Virology).
In failing to address his CRISPR-Cas9 experiments, he gave a 25 minutes-long talk, describing his future research plans that involve the development of a gene-editing drug for muscular dystrophy. How he is viewed in China is quite interesting because, rather than like a scientific outcast, he seems to have become something of a national science hero in his home country, also raising questions about the seriousness of the punishment he allegedly received under Chinese law.
A 2020 review found that only approximately 70 countries currently outright prohibit genome editing in human clinical practice.3 Raising further concerns about how seriously China is taking this prohibition and He’s past transgressions, he is now freely fundraising for his future not-for-profit research institute which, being outside of academia, is apparently not even bound by the Chinese government’s newly issued CRISPR-Cas9 rules and regulations, a “scary situation,” as a British stem-cell biologist was quoted in a recent article in Science. 4
1. John London A, Seymour CW. JAMA 2023; doi: 10.1001/ jama.2023.1675. Online ahead of print.
2. Mallapaty S. Nature 2023;614:599-60
3. Baylis et al., CRISPR J2020;3:365-377
4. Normile D. Science 2023;379(636):970
For the longest time has there been the suspicion that men and women respond differently to human growth hormone (HGH) supplementation. Now a study in JCEM confirmed that women benefit less from HGH supplementation than men.1 IGF-1 scores remained subnormal more often in women, while scores above normal were more frequent in men. Women also recorded more adverse events, mostly symptoms of fluid retention and required more often dosage reductions or temporary stops in treatments. Discontinuations of treatment were similar between sexes but, interestingly males demonstrated in the long-term more malignancies.
1. Slagboom et al., J Clin Endocrinol Metab 2023;dgad013, https://doi. org/10.1210/clinem/dgad013. Online ahead of print
Vitamin D
There is almost no area in medicine where Vitamin D has not been alleged to effect clinical outcomes. In most areas - and that includes reproductive medicine – the evidence has remained sparse. Now come Chinese investigators and offer a systematic review and meta-analysis on reproductive outcomes in infertile patients, claiming to have demonstrated beneficial outcome effects.1 A closer look at the paper, however, raises serious questions: For example, vitamin D supplementation resulted in significantly increased clinical pregnancy rates; yet, however implantation rates, chemical pregnancy rates, miscarriages, and multiple pregnancy rates did not differ. Moreover, improvements in clinical pregnancy rates were dose dependent. How that can be, remains unexplained. The CHR evaluates all patients’ vitamin D levels at admission and supplements women only if they demonstrate abnormally low levels.
Herbal supplements
Like vitamin D, herbal supplements have been in vogue for quite some time, prescribed not only in infertility clinics but also by acupuncturists, herbalists, and many general OB/GYNs, as well a general practitioner. A systematic review and meta-analysis, however, could not demonstrate outcome benefits in IVF,2 even though innumerable reports have made all kind of, mostly poorly supported, claims. A recent review of herbal supplement use among women of reproductive age, furthermore, suggested high rates of moderate-risk supplement-drug interactions and, possibly, harmful effects in early pregnancy.3 Because of concerns about estrogenic compounds in many Chinese herbal products, the CHR in most cases recommends that women discontinue their use before IVF cycle starts.
1. Meng et al., Reprod Biol Endocrinol 2023;21:17
2. Cao et al., PLoS One 2013;8(12):e81650
3. Friedman et al., F S Rep 2023;4(1):2666-3341
That survival of the species and, in individual cases, survival of individuals is the primary goal of evolution, is by now well understood. Survival of species is, however, practically impossible, unless reproduction functions efficiently. Based on pure logic, one, therefore, must conclude that fertility must also be of essential importance for evolution. And, once this premise is accepted, on must furthermore conclude that an individual’s fertility must also relate to her/his general health and, likely, longevity.
This is, indeed, a subject on the VOICE’s list to be covered in a future issue because more and more literature suggests in women as well as men that fertility status offers a strong hint about an individual’s future health. This was also again demonstrated in a brief research letter in The New England Journal of Medicine in which U.S. investigators reported that unexplained infertility in women was clearly associated with several specific genetic disease variants mostly reflecting risk heart problems and cancer. Medically actionable genes included well-known ones, like BRCA1 and BRACA2 ( breast, ovarian, pancreatic cancers), and lesser-known ones, like MYH11 (aortic dissection), GLA (Fabry disease), PKP2 (arrhythmogenic right ventricular dysplasia or cardiomyopathy), KCNQ1 (familial atrial fibrillation and long Q syndrome). SCN5A (6 arrythmias and the Brugada syndrome), RYR1 (central core disease of muscle, malignant hyperthermia), APOB (familial hypercholesterolemia), and CACNA1S (hypokalemic periodic paralysis). Approximately 17% of women with unexplained infertility, indeed, demonstrated pathogenic or likely pathogenic disease variants. What this short study, however, did not tell us was what the prevalence of these variants is in a fertile female population.
Another relevant paper to addressing the question posed above in this sub-section is a recent paper in the Journal of the American Heart Association2 which in more detail will be addressed in the “Pregnancy” section. Only so much here: Through a Mendelian randomization study the authors demonstrate that sex-specific reproductive factors augment cardiovascular disease risk in women.
1. Daughtery et al., N Engl J Med 2023;388:11:1055-1056
2. Ardissino et al., J Am Heart Assoc 2023;12:e027933
We in these pages previously noted how amazing the scientific effort of European colleagues was in bringing the concept of uterine transplantation from small to big animal models and, ultimately, making it a clinical service in women, now successfully offered in several places around the world. We, however, were also critical of this procedure because we felt it caused significant risks through quasi semi-elective surgeries (because of at least three surgeries, implant and removal in recipient and removal in donor of the uterus) because a safer and more cost-effective alternatives is available through the use of gestational carriers, though we, of course, do recognize that there is no absolute equivalency between these two options.
Now the concept is apparently even further expanded into transgender women: As an “inklings” article in Fertility & Sterility by colleagues from Cleveland Clinic (likely the leading U.S. uterine transplantation site) now indicated, “a first uterus transplant in transgender women is very likely in the near future.”1
This, of course, raises several interesting additional concerns: As the authors pointed out, transgender uterus transplants technically, of course, will significantly differ from female transplantations. This, of course, must be reflected in preparations, training of surgeons, and informed consents. Whether risks will be similar to routine female transplants also remains to be determined and above outlined concerns in female uterus transplants of significant risks and quite abhorrent costs, of course, also apply. Moreover, one also must wonder how transgender women will tolerate the hormonal as well as physical changes a pregnancy imposes on the body and, ultimately, how a pregnancy will develop in a transgender hormonal environment.
We, therefore, are somewhat skeptical whether a uterine transplant into a transgender woman is really just around the corner and waiting to happen. It, indeed, appears ethically questionable to go straight into human reproduction without conducting, first, preliminary investigations in animal models which, to our knowledge, have so-far not been reported. Paradoxically, an argument can, however, be made that experiencing a pregnancy may be for a transgender woman psychologically more important than for an infertile woman with no uterus. We truly live in interesting times!
1. Richards et al., Fertil Steril 2023;119(3):390-391
Are IVF outcomes affected by race and ethnic background? There are innumerable studies in the IVF literature that have demonstrated that IVF cycle outcomes in Caucasian women are significantly better than in women of African descent, with Asian women (usually of Chinese Han descent) falling in-between. What, however, is less obvious are the causes behind these observations. Now come British colleagues and report on an analysis of HEFA (Human Fertilization & Embryology Authority) data, the British counterpart to SART and CDC registries, which, unsurprisingly, demonstrated exactly the same outcome differences.1 The authors conclude that some of the differences may be mediated by age, with Black women entering fertility treatments two years later. An accompanying editorial, however, points out how many explanations have been offered in the literature, with none really offering a final word.2
Interestingly, explanations have gone from social and economic discrimination to the reported increased incidence of uterine myomas in Black women, but none of these explanations really explains the hierarchical decline from Caucasian to Asian, and African American (or Afro-British) women, - that is, except for one study published years ago by the CHR’s researchers which demonstrated distinct distribution patterns in CGG repeats in the FMR1 (fragile X) gene that followed exactly the same distinct pattern from Caucasian, to Asian, and African descent. That the FMR1 gene is closely associated with ovarian function has in recent years become increasingly obvious4 but is, still, best demonstrated by the strong association between primary ovarian insufficiency risk and premutations (55-200 CGG repeats) in the gene.5
Colleagues from the Weill Cornell program in NYC reported on two cases of complete fertilization failure which they attributed to a combined oocyte- and sperm-related oocyte activation deficiency.6 They, furthermore, claimed in the paper in Fertility & Sterility Reports to have overcome this combined insufficiency successfully by optimizing oocyte response to chemical activation with calcium ionophore. The authors fully acknowledged that none of the treatments they utilized in these two cases was new but claimed that the combined application of treatments to sperm and egg represented new information.
To overcome oocyte-related oocyte activation deficiency, they extended in vivo (time between hCG trigger and retrieval) and in vitro oocyte maturation in order to enhance ooplasmic maturation. And to address sperm-related oocyte activation deficiency, so-called assisted gamete treatment was used to trigger oocyte activation which previously had failed. Following these combined treatments, these two couples achieved respective fertilization rates of 63.0% and 43.8%, and both patients conceived.
It is, of course, difficult to jump to final conclusions based on two reported cases (especially since it is unclear whether other attempts were made that failed); but the concept the investigators pursued sounds logical, and they are to be congratulated on a very interesting report, which further strengthens an important point which, now for years, has been at the core of the CHR’s treatment philosophy: the time for precision medicine in infertility has come, which means we must abandon rigid protocols applied to everybody and individualize care.
We are recruiting an experienced RESEARCH BIOLOGIST with animal IVF experience to join or clinical embryology team in the function of laboratory supervisor for research
To qualify, candidates must have a PhD degree and have a publication list in evidence of independent research experience. Though human embryology experience is preferred, it is not a precondition since we are willing to train an, otherwise, well-qualified candidate. Substantial prior animal IVF experience is, however, a minimum requirement. The CHR offers a very competitive salary and excellent benefit package. Most importantly, however, the CHR offers a unique model for the infertility field by being a privately-owned fertility center with strong academic links and with academic discipline in practicing medicine and conducting and publishing important research. By becoming a member of our embryology team, you will be splitting your time between providing clinical IVF services and conducting research. If your current research position is no longer what you are looking for and a combination of bench and clinic potentially excites you more, please send your CV to Ms. Jolanta Tapper, COO of the CHR at jtapper@thechr.com. All submissions are considered confidential.

Whether female autoimmunity affects IVF treatments has remained a controversial subject,7 with several prominent colleagues to this day completely rejecting the concept. Two recent papers, however, offer limited new insights on how autoimmunity interphases with IVF. In a first report investigators from Russia and Israel reported on IVF-related outcomes in TPO-antibody-positive women with thyroid disease who were compared to matched controls. Presence of TPO antibodies was associated with lower AMH values and lower antral follicle counts. Soaffected patients also, therefore, unsurprisingly, showed poorer response to ovarian stimulation, lower fertilization rates, and lower numbers of high-quality embryos.8
None of this is, of course, surprising because, though as noted above rejected by some, laboratory findings suggesting a hyperactive immune system have been associated with poorer IVF outcomes for decades. The remaining open question is, however, what to do about it.
Likely the most investigated autoimmune disease in pregnancy after thyroid disease, is systemic lupus erythematosus (SLE). In a second paper Chinese investigators in a retrospective multicenter study reviewed pregnancy outcomes in women with SLE. Once again, the results of the study did not offer any major new insights, but they confirmed that pregnancy outcomes in women with stable SLE are “satisfactory, though not perfect.”9
As suggested by the literature for practically all autoimmune diseases in pregnancy,10 the leading adverse outcome was prematurity in 42.9% of pregnancies. Also not unexpectedly, preeclampsia prevalence was significantly associated with prematurity (P=0.012), and prematurity was associated with low birth weight and very low birthweight (P<0.0001). Disease flares were considered mild and moderate (none were considered severe) and uniformly had “favorable” outcomes.
Since these disease flares occurred in second and third trimesters, they were the only surprising findings because autoimmune diseases, including SLE, are widely believed to have two primary period of increased risks for flares, the early pregnancy stages (causing miscarriages) and late pregnancy reaching into what by many is now called the “4th trimester of pregnancy, - the postpartum period (typical disease flare and/or initial clinical presentation).11
Autoimmune diseases are, indeed, widely believed to improve in second and early third trimesters because of improving induction of tolerance
pathways for the fetus by the maternal immune system. In this paper reported outcomes contradict this and suggests that close patient surveillance for disease flares in SLE must be maintained throughout pregnancy. Reassuringly, IVF on its own, also had no adverse effects on SLE.
Using human growth hormone (HGH) in association with IVF The pendulum regarding the use of HGH in association with IVF has been swinging back substantially in favor of utilization over the last decade, even though, as we have noted in these pages repeatedly, the literature basically offers no valid support for such treatments since reported studies have been highly contradictory. We, however, also noted that we believe to know why study results have differed so significantly and have proposed that the reason lies in unselected use of HGH supplementation: in other words, most colleagues do not have a specific and valid reason to initiate HGH, but “throw it in” when nothing else has helped.
The utilization of HGH in an IVF cycle should, however, have one very clear indication, and that is, obviously, low IGF-1 (insulin-like growth factor 1) levels. The reason is equally obvious: HGH works through IGF-1; HGH, indeed, induces the production of IGF-1 by granulosa cells, which then, similarly to ovarian androgens, synergistically with FSH (follicle stimulating hormone) drives growth and maturation of follicles. Consequently, if IGF-1 levels are normal, supplementing a patient with HGH makes little sense. Only women with low IGF-1 levels, therefore, should be supplemented for at least 6-8 weeks prior to IVF cycle start. This time period is required because, again like androgens, IGF-1 effects are the strongest at small growing follicle stages, which then need this time to reach the gonadotropin-sensitive stage, where they become responsive to exogenous gonadotropin stimulation in IVF cycles.
So, why does all of this explain why worldwide HGH studies in IVF have been so contradictive? Here, too, the answer appears obvious: only a small minority of infertile women have low IGF-1 levels. Giving them HGH supplementation is like giving somebody aspirin for headache who does not have a headache. In other words, the differences in reported HGH supplementation reported in the literature are, very likely, caused by which patients a study selected. If many had low IGF-1, the study, likely, showed positive results, if everybody had normal IGF-1 levels, why should the use of HGH show any effects?
All of this is meant as an introduction for a paper Chinese colleagues recently published in Reproductive Biology and Endocrinology, a relatively young journal with rapidly improving impact factor, in which the authors investigated the effects of HGH on the metabolome of follicular fluid in patients with low functional ovarian reserve (LFOR).12 The study identified 134 metabolites, among which 24 significantly varied between women treated with HGH and controls. HGHtreated cycles also produced marginally
more oocytes (3 vs.2, P=0.04). The authors concluded that supplementation with HGH may improve oocyte development in ovaries of women with LFOR by altering metabolites found in follicular fluids. Our analysis of the paper, however, revealed many of the usual shortcoming seen in prior studies on the subject, including no preselection of patients based on IGF-1 levels, retrospective and uncontrolled utilization of HGH supplementation which obviously biases outcomes visa-vis controls, and very marginal statistically significant findings.
Male factor infertility: Paternal alcohol use in IVF
A recent paper from Texas A&M in Molecular Human Reproduction, chosen as an “editor’s choice,” demonstrated in a mouse model decreased embryo survival and pregnancy success rates after preconception alcohol exposure of the paternal animal. Strengthening the results, the findings were, moreover, dose-depending.13 The authors also presented evidence that alcohol exposure caused these effects by disrupting embryonic gene expression, including the genes Fgf4 and Egfr, both critical regulators of trophectoderm stem cell growth and placental patterning, with lasting impact on the histological organization of late-term placentas. Moreover, these changes were also accompanied by changes in regulation of pathways that control mitochondrial function, oxidative phosphorylation, and selected imprinted genes.
The authors, reasonably, concluded that, male alcohol use (in this mouse model) significantly impedes overall IVF success. But what must be remembered is that “men are not mice“ and, therefore, one cannot automatically assume that, in humans, paternal alcohol use during IVF treatments of the female partner will have similar effects. The in this paper reported findings are, however, serious enough to mandate quick follow-up studies in human IVF. In the meantime, it may not be a bad idea to recommend that male partners refrain from alcohol intake while their partners are in infertility treatments or, at least, reduce their alcohol intake.
1. Henderson et al., Fertil Steril 2023;119(2):241-248
2. Ghidei L, Wiltshire A. Fertil Steril 2023;119(2):250-251
3. Gleicher et al., Reprod Biomed Online 2010;20(4):485-491
4. Ferder et al., Reprod Fertil Dev 2022;34(16):1034-1042
5. Poteet et al., J Assist Reprod Genet 2023;40(1):179-190
6. Xie et al., F S Rep 2023;4(1)72-76=
7. Simopoulou et al., Int J Mol Sci 2019;20(4):892

8. Safarian et al., Int J Molec Sci 2023;24:4705
9. Lao et al., Arthritis Res Ther 2023;25(1):13. CORRECTION: Idem 25(1):18
10. Gleicher N. Clin Rev Allergy Immunol 2010;39(3):194-206
11.https://www.cdc.gov/lupus/basics/pregnancy.htm
12. He et al., Reprod Biol Endocrinol 2923;2:21
13. Roach et al., Molec Hum Reprod 2023;29(2):gaad002
Evaluating baseline self-reported sleep duration, sleep mid-point, social jetlag, and shift work, U.S. investigators from several institutions found no clinically relevant associations between sleep characteristics and live birth rates. Some observed weak associations, however, did not rule out minor effects and additional studies, therefore, would appear indicated.
1. Freeman et al., Fertil Steril 2023119(2):252-262
This has, of course been a longstanding debate in the IVF field and was recently subject of some opinion articles in Fertility & Sterility. We here, however, want to especially point out the article by Quinn and Paulson which, though brief, hit the right tone by pointing out that different patients require varying dosing regimens.1 The article by Kuokkanen and Pal, in contrast, followed the usual argument of mild stimulation
Continued on page 46
proponents, that studies that claim better outcomes with higher dosing are of poor quality and, therefore, must be dismissed, but ignore that the studies they usually quote in support of mild stimulation are of at least equally bad quality but, moreover, uniformly also demonstrate that regular stimulations produce better pregnancy and live birth rates than mild stimulations.
One very important point both papers failed to address is that, especially in older women, higher gonadotropin dosages also speed up cycles and therefore, shorten already usually short cycles. Reductions in gonadotropin dosages may, therefore, be indicated to lengthen cycles a little bit, - even if that may mean smaller oocyte yields.
1. Quin MM, Paulson RJ. Fertil Steril 2023;119(2):170-172
2. Kuokkanene A, Pal L. Fertil Steril 2023;119(2):166-169
Pregnancy complications and their epigenetic consequences on newborns
In a monster cohort study, U.S. investigators studied mother-infant pairings in several large and diverse population resources with special reference to gestational diabetes (GD), gestational hypertension (GH), and preeclampsia (PE), attempting to determine the effects of these three conditions in association with biological gestational age on the epigenetic clocks in newborns.1 Not only was the design of this study involving 1801 children remarkable, but so were the findings: PE and GD were significantly associated with decelerated gestational age; and these associations were more pronounced in female than male offspring. Interestingly, PH did not demonstrate such associations.
This paper, thus, adds further evidence to a theme recently discussed in these pages on several occasions, when other studies indicated maternal epigenetic disease effects on offspring. What makes this study, however, so interesting besides the large and diverse sample size of study subjects, were the findings which demand answers to several newly arising questions: A first that comes to mind is, what drives the observed differences between female and male offspring? Considering that the maternal immune system responds differently to female and male pregnancies,2 it would not be surprising if it became at least part of the answer. Another obvious question is why were these effects seen with GD and PE, but not with GH. What is especially interesting is the dichotomy between PE and GH, which in the perinatology literature suggest a certain overlap yet, here, appear as distinctively separate, thereby suggesting distinctive disease pathways in the mother, - one (PE) pregnancy-induced and the other (GH), likely, a chronic disease pathway, by pregnancy only enhanced.
Is the health of mothers before pregnancy more important than their age?
A study last month presented at the American College of Cardiology
Annual Scientific Session together with the World Congress of Cardiology suggested that in recent years observed dramatic increases in pregnancy complications in the U.S. were primarily not, as widely believed, due to delayed maternity into older age but due to more health problems pregnant women experience even before conceiving. Since the study has not been published yet, this conclusion is, however, difficult to verify because deteriorating health is of course a quite typical characteristic of
advancing age.
Zachary Hughes, MD, the lead author of the study, an internist at Northwestern Memorial Hospital of the Northwestern University Feinberg School of Medicine in Chicago, nevertheless, got considerable media attention by claiming that rising rates of adverse pregnancy outcomes, including hypertensive disorders of pregnancy, low birthweight, and preterm births, are, all, largely attributable over the years 2011-2019 (involving 3.7 million births) to the health of mothers before they conceived, rather than their age at the time of pregnancy.3 If more scrutiny of these data is confirmed, this would be a very important finding because it would suggest that the rather poor birth outcomes in the U.S. in comparison to other major countries in the developed world could be improved by concentrating on improving the health of women who are planning on conceiving.
Another paper from the Northwestern University, Feinberg School of Medicine, though this time from the OB/GYN department, also attracted our attention because of where it was published and because of the potential significant impact on preconception counseling and treatment of very obese women increasing utilization of this surgery may have. In this “opinion” article in JAMA, 5 colleagues reviewed the impact of bariatric surgery on risks for adverse pregnancy outcomes and concluded that, “given the increasing incidence of bariatric surgery in reproductive-age women, clinicians must be prepared for nutritional, postsurgical, and other potential complications of bariatric surgery in subsequent pregnancies.5 Likely unintentionally, this “insights” article did not mention an article that approximately at the same time had appeared in Obstetrics&Gynecology, authored by perinatologists from several academic centers in the U.S.6 which demonstrated in an ethnically diverse patient cohort that bariatric surgery prior to pregnancy reduced the risk of preeclampsia, gestational diabetes, impaired fasting glucose, and large gestational age neonates (with all of their well-known complications). The only negative impact of the surgery was an increase in small-for-gestationalge neonates. Interestingly, no outcome differences were noted, once again, in association with gestational hypertension and, somewhat surprisingly, in preterm births and cesarean section rates.
The issue is, however, in our opinion far bigger than that, involving two in recent years accelerating trends: first rapidly growing obesity defined by BMIs above 30.0, by now affecting 39.7% of U.S. women in reproductive years,5 and the very recent discovery that very significant weight loss can be achieved in a relatively short time through a new family of injectable weight loss drugs,7 as non-surgical options for many patients are greatly preferable.
We in the prior section of this literature review addressed the effects of autoimmunity on IVF. A recent paper in Arthritis Care & Research reported on birth outcomes and rehospitalization of pregnant women with rheumatoid arthritis (RA) and SLE as well as their offspring.8 Reported results especially reemphasized the dangers in the postpartum period, now by some perinatologists therefore, called the “4th trimester of pregnancy.” Remarkably, those risks extend not only to mothers but also to their offspring and appear to be more common and severe in SLE than RA. SLE women were especially affected by the need for rehospitalization
for up to six months postpartum. Infants of SLE patients also demonstrated an increase in birth malformations (9% vs. 6%; RR 1.46, 95%CI 1.21-1.75) and increased rehospitalization as well as mortality for up to two years (RR 2.11, 95%CI 1.21-3.67). This paper, therefore, reemphasizes not only the need for close maternal and fetal surveillance during pregnancy (as also noted in the previous section), but also that women with autoimmune diseases, and especially SLE and RA, and their offspring must be also closely watch for up to six months in the postpartum period.
Continuing the discussion of whether infertility was predictive of future health which we started in the “fertility” section of this literature review, British investigators published last month a very important paper in the Journal of the American Heart Association, in which they quite convincingly were able to associate female fertility with cardiovascular disease risk.9 The findings were, indeed, very surprising: Based on uncorrelated (r2 <0.001) genome-wide significant (P<5x10-8) single-nucleotide polymorphisms and inverse-variance weighted Mendelian randomization, earlier genetically predicted age at first birth increased odds for coronary heart disease [OR 1.49 (95% CI, 1.27-1.74), P=3.72x10-7], for heart failure [OR1,27 (CI 1.06-1.53), P=0.009], for stroke [OR 1,25 (95% CI, 1.00-1.56), P=0.048] with partial mediation by BMI, type 2 diabetes, blood pressure, and cholesterol traits. Higher genetically predicted numbers of live births increased the risk for atrial fibrillation(P=0.023), heart failure (P=0.001), ischemic stroke (P=0.039), and stroke in general (P =0.007). Earlier genetically predicted ge at menarche increased the risk of coronary heart disease (P=1.68x10-6) and heart failure (P=5.06x10-7), both at least partially mediated by BMI.
The authors appropriately concluded that certain reproductive factors in women play a causal role in women later in life developing cardiovascular diseases.
1. Ladd-Acosta e al., JAMA Network Open 2023;6(2):e230672)
2. Amanda et al., Brain Behav Immun 2017;60:32
3. https://www.news-journal.com/rise-in-pregnancy-complications-down-tohealth-not-age/article_5de43e7a-c954-5b29-b977-9b9489e317c9.html
4. https://www.news-journal.com/rise-in-pregnancy-complications-down-tohealth-not-age/article_5de43e7a-c954-5b29-b977-9b9489e317c9.html
5. Fisher et al., JAMA 2023;329(9):758-759
6. Boller et al., Obstet Gynecol 2023;141(3):583-591
7. https://www.economist.com/briefing/2023/03/02/a-new-class-ofdrugs-for-weight-loss-could-end-obesity?utm_medium=cpc.adword. pd&utm_source=google&ppccampaignID=17210591673&ppca dID=&utm_campaign=a.22brand_pmax&utm_content=conversion.di rect-response.anonymous&gclid=Cj0KCQjw8e-gBhD0ARIsA JiDsaXb6Phs_DYaYKy5uBeN_zTC2UYeoX_ZTsjLuzz0h7RDUdbaytKrycaAgEnEALw_wcB&gclsrc=aw.ds
8. Singh et al., Arthritis Care Res (Hoboken) 2023; doi: 10.1002/ acr.25087. Online ahead of print.
9. Ardissino et al., J Am Hert Assoc 2023;13:e027933
Prevention of preeclampsia with aspirin
That aspirin in high-risk patients significantly reduces the prevalence of preeclampsia can now been considered an established fact.1
Several details regarding this treatment, have, however, remained open to discussion, among those the best daily dosage of aspirin and, as recently addressed by Spanish investigators,2 how far into pregnancy daily aspirin treatment should be maintained. The latter point is of importance because aspirin treatment beyond 36-37 weeks may be associated with an increased risk of peripartum bleeding events. The Spanish investigators, therefore, performed a multicenter, open-label, randomized phase 3, noninferiority trial of aspirin 150mg daily to determine whether discontinuation of aspirin between 24-28 weeks, and better selection of high-risk patients in the first trimester of pregnancy, may mitigate the bleeding risk in comparison to patients continuing aspirin till 36 weeks (which is also the current CHR recommendation for high-risk patients). The study defined noninferiority by the higher 95% CI for the difference in preeclampsia incidence was less than 1.9%, - and this criterion was met. Moreover, they also found a higher incidence of minor antepartum bleeding episodes in the longer-use aspirin group [12.31% vs. 7.61%; OR -4.70 (95% CI, -8.53 to -0.871].
An accompanying editorial pointed out that several authoritative organizations in the UK and the U.S. have issued guidelines in recent years supporting the use of 75-100mg aspirin, initiated between 12- and 16-weeks gestational age in attempts to reduce preeclampsia prevalence in high-risk patients. 3 This study, however, utilized 150mg of aspirin daily, a quite significantly higher dosage than recommended. For this and several other reasons, the editorial wondered whether these Spanish data applied to U.S. populations. We support this cautionary note for one additional reason: As worse perinatal outcome data in the U.S. in comparison to most of Europe demonstrate, patient populations and general obstetrical practice patterns differ on both sides of the Atlantic, within the study, for example, only 3.4% being Black and 93% being White. We have in our commentaries on these pages repeatedly criticized inappropriate generalizations of study outcomes in highly selected patient populations to other patient groupings. This study is just one more example!
More preeclampsia after IVF? Not really, even if a recent study so claims
A presentation at the recent American College of Cardiology Scientific Session by a Northwell Health physician from Staten Island in NYC reported that pregnancies from IVF were twice as likely to develop
preeclampsia than spontaneous conceptions.4 Using data from the National Inpatient Sample Database, the researchers assessed the rates of cardiovascular complications for 5,874 technology-assisted pregnancies and more than 2.2 million traditional pregnancies from 2016-2018. Considering that women with infertility, of course, represent a different patient profile than women with spontaneous conceptions, it cannot surprise that the study revealed significant differences in pregnancy outcomes: IVF patients demonstrated more gestational diabetes (12.1% vs. 7.5%), cardiac problems, including supraventricular tachycardia (0.3% vs 0.1%), conduction abnormalities )0.4% vs 0.2%), acute pulmonary edema (0,2% vs 0%), and, yes, preeclampsia (10.2% vs. 5.0%), all also leading to longer hospital stays (4.1 vs 2.7 days).
However, confirming that observed increases in preeclampsia risk, likely, have little to do with IVF 5 (as the report tried to imply4), 190 IVF pregnancies in surrogates (in obviously younger and generally healthier women) demonstrated a lower preeclampsia risk, which is, likely, the most interesting finding since those pregnancies are a 100% allograft for the carrier’s immune system, while as spontaneously conceived pregnancy is always only a semi (50%) – allograft. Since preeclampsia is increasingly viewed as a reflection of prematurely waning tolerance of the maternal immune system of the fetus,6 gestational carrier pregnancies would, indeed, be expected to have higher preeclampsia risk if patient populations had similar characteristics.
1. Rolnik et al., N Engl J Med 2017;377(7):613-622
2. Mendoza et al, JAMA 2023;329(7): 542-550
3. Emeruwa et al., JAMA 2023;329(7):539541
4. American College of Cardiology, February 24, 2023; https://www.acc.org/About-ACC/Press-Releases/2023/02/23/18/46/ Technology-
Assisted-Pregnancies-Have-Twice-the-Risk-ofPreeclampsia#:~:text=People%20
who%20became%20pregnant%20using,the%20World%20Congress%20of%20 Cardiology.
5. Watanabe et al., BMC Pregnancy Childbirth 2014;14:69
6. Saito et al., J Reprod Immunol 2007;76(1):30-39
A Danish study recently reported on a cohort of 333 spontaneous pregnancy losses, in their etiology investigated by cell-free fetal DNA, Gestational ages ranged between 35-149 days (mean 70.5 or 10 weeks plus 1 day). The tests demonstrated sensitivity for aneuploidy in 85% (95% CI 79-90) and specificity of 93% (95% CI 88-96) in comparison to direct sequencing of products of conception (POCs); 4% of tests suggested multiple aneuploidies and 11% were inconclusive.
Though the authors concluded that this validation study of cell-free fetal DNA testing confirms the feasibility of this method to distinguish euploid from aneuploid pregnancy loss, we have serious reservations about this conclusion. Whether a miscarriage is euploid or aneuploid especially matters in women with either unknown causes for repeated pregnancy loss or in already diagnosed immune aborters already in treatment, where whether another miscarriage is euploid or aneuploid will determine whether an already applied treatment is insufficient or may be sufficient.
Accuracy of diagnosis, therefore, is of importance if potentially avoidable further miscarriages are to be prevented. We, therefore, would be hesitant to employ a test with only 85% sensitivity and 11% inconclusive findings in addition. The genetic testing industry, once more, is apparently trying to launch yet another clinically insufficiently vetted test. An accompanying editorial also calls for caution before jumping to a premature conclusion. The editorial, however, did point out a potential benefit from this kind of testing if POCs are not available for testing. For testing, though, even this potential indication remains questionable until it has been established at what gestational age cell-free fetal DNA can be detected with reliability in the blood of an early-pregnant woman.2
1. Schlaikjær Hartwig et al., Lancet 2023;401:762-771
2. Duoff L. Lancet 2023;401:710-711
are more pregnant women dying in the U.S. than elsewhere?
Per new data from the CDC, the rate of women dying in childbirth surged by almost 40% during 2021, the second year of the COVID-19 pandemic.1 Even worse, approximately 80% of maternal U.S. deaths have recently been determined to be preventable2 and, primarily, affected Black women. In total 1,205 women died in childbirth in 2021 compared to 754 in 2019, with maternal mortality increasing from 20.1/100,000 live births in 2019 to 23.8/100,000 in 2020, and 32.9/100,00 in 2021. For (non-Hispanic) Black women the 2021 rate was, however, 69.9/100,000, 2.6 times higher than the rate in (non-Hispanic) White women. Rates also increased with age during the COVID-19 years of 2020 and 2021, with women over age 40 demonstrating a 6.8-times higher rate than women under age 25. The consensus appears to be that, though COVID-19 likely contributed to these numbers, the country’s healthcare system is flawed in how minority populations receive pregnancy care. Maternal mortality rates in France, the UK, and Canada were in comparison during the same period only 8, 10, and 11/100,000.3 Interestingly, the rate of premature births (the leading cause of infant mortality), however, fell during COVID-19 worldwide4 Causal pathways for all of these developments are, therefore, likely more complex than widely assumed, and urgently warrant further research. This was also confirmed by the recently reported observation that higher adverse maternal outcomes in Black women are not only restricted to poorer socioeconomic individuals; childbirth for Black women is equally more deadly even when they are rich.5
1. Hoyert DL. NCHS Health E-Stats. 2023. DOI: https://dx.doi.org/10.15620/ cdc:124678.
2. CDC. September 19, 2022; https://www.cdc.gov/media/releases/2022/ p0919-pregnancy-related-deaths.html
3. Toy S. The Wall Street Journal, March 16m 2023, https://www.wsj.com/ articles/u-s-maternal-mortality-hits-highest-level-since-1965-f9829776
4. Calvert et al., Nat Hum Behav 2023; https://doi.org/10.1038/ s41562-023-01522-y
5. Cain Miller et al. The New York Times, February 19, 2023; https://www. nytimes.com/interactive/2023/02/12/upshot/child-maternal-mortali ty-rich-poor.html
A very interesting study from The Netherlands investigated microbial transmission routes from mothers to their infants across six maternal and four infant body sites. Interestingly, transmission patterns varied depending on niche: The most important role was played, however, by breast milk, reemphasizing the importance of breast milk for newborns. Another important finding was that, while losing in quantity fecal microbial transmissions from their mothers, infants delivered by cesarean section compensated for this loss by other routes.
1. Bogaert et al., Cell ost Microbe 2023;31:447-460Hoyert
That many women report memory loss and brain fog in pregnancy and/ or postpartum has given rise to the presumptive diagnosis of “mommy brain,” also called baby brain, mom brain, momnesia, and pregnancy brain is not reason enough to conclude that a mother’s brain no longer functions well is scientifically not correct, as a recent “viewpoint” article in JAMA Neurology suggested.1 The authors do not deny minor potential deceases in aspects of cognitive function that may affect memory and cause mental fogginess. They, however, also note that the brains of mothers gain faculties during this period of life. They, for example, show a boost in learning about baby-related items and demonstrate better long-term memory than never pregnant women. Birthing parents also demonstrate structural plasticity in their brains, with reductions in gray matter in several regions (important for memory) lasting for up to six years.
We are struck, however, by the fact that the literature apparently does not at all address some of the similarities between how pregnant women describe their lapses in cognitive function and, for example, many longCOVID-19 patients describe theirs.’ Do these similarities not suggest that what widely is described as “mommy brain” may be more of what should be described as an “immune-response brain?”
That the immune system in pregnancy, and especially upon termination of pregnancy, can cause havoc in maternal brains is now with an evolving understanding of postpartum psychiatric disorders increasingly obvious.2 Maybe, the term “mommy brain,” therefore, should not be abandoned as suggested of the authors of the paper we here are reviewing but, instead, be viewed as a physiologically normal adaption of the brain to a radically changed maternal immune system.
1. McCormack et al., JAMA Neurology 2023; doi: 10.1001/jamaneu rol.2022.5180. Online ahead of print.
2. Deligianniis KM, Clayton AH. J Clin Psychiatry 2023;84(Suppl1):SG22945SU1C.
“ACOG is aware of the decision by the manufacturer of Makena to voluntarily withdraw the product—currently the only FDA-approved medication to help prevent recurrent spontaneous preterm birth—from the market in the coming months. ACOG is still uncertain as to whether that timeline
will be implemented as we await a potential final decision by the FDA. Until further information is available, ACOG’s current guidance, ‘Prediction and Prevention of Spontaneous Preterm Birth,’ will remain in effect.
ACOG understands that its members who provide care to patients with prior preterm birth will soon lose access to a treatment option that was previously available to them. It is critical that other effective interventions be identified to prevent recurrent preterm birth for the health and well-being of our patients and their families.1
1. ACOG. March 8, 22023; https://www.acog.org/news/news-releases/2023/03/ acog-statement-on-announcement-regarding-the-voluntary-remov al-of-makena-17-ohpc-from-the-market#:~:text=%E2%80%9CACOG%20 is%20aware%20of%20the,market%20in%20the%20coming%20months
It is somewhat telling that the best recent article addressing menopausal hormone replacement therapy (HRT) did not appear in an Ob/ Gyn journal but in Circulation, a leading cardiology journal and was executed by the American College of Cardiology Cardiovascular Disease in Women Committee, along with leading gynecologists, women’s health internists, and endocrinologists.1 Though somewhat surprising, there is a certain logic to the initiative coming from the cardiology community, because the radical change in how medicine viewed postmenopausal HRT that took effect a little over 20 years ago,2 was mostly the consequence of alleged excess cardiovascular risks from combined estrogen/progestin HRT. As the article in Circulation now points out, much has been learned about this risk over the last 20 years. This mostly refers to how age and time from menopause and route of administration of HRT relate to cardiovascular risks.
The pendulum which swung aggressively against all post-menopausal hormone replacement around the year 2000, in recent years started to swing back, with four major professional societies now agreeing that in significantly symptomatic postmenopausal women, HRT is indicated. For those interested in this subject, the paper offers a detailed discussion of how patients for HRT should be selected and which format of hormone replacements should be utilized.
Importantly, as Leslie Cho, MD, director of the Cleveland Clinic’s Women’s Cardiovascular Center, recently pointed out in a healio.com interview,3 HRT is not recommended to decrease future cardiovascular risks in post-menopausal women as was widely believed until approximately the year 2000 (HRT does not do that at all), but for the purpose of alleviating post-menopausal symptoms solely. The ideal candidates for HRT are women under age 60 within 10 years from the onset of menopause who, upon structured cardiovascular risk assessment, have a 10-year estimated risk of less than 5%, and do not have an increased risk for breast cancer and/or thromboembolic events.
This does not necessarily mean that symptomatic women who do not fully qualify under these strict criteria should not be offered HRT but women with “intermediate” cardiovascular risks, like patients with diabetes, smokers, women with hypertension, obesity, metabolic
syndrome, and/or autoimmune diseases, should be offered more individualized approaches. Transvaginal estrogen administration is generally preferred, transdermal HRT, however, appears to reduce the east associated with increases in cholesterol, and blood pressure and offers lower risks of abnormal clotting events.
1. Cho et al., Circulation 2023;147:597-610
2. Nelson et al., JAMA 2002; 288(7):872-881
3. healio.com., February 14, 2023. https:// www.healio.com/news/cardiology/20230214/ menopausal-hormone-therapy-safe-for-most-women-at-low-cvd-risk
The Ovarian Cancer Research Alliance just published a Symptom Awareness Consensus Statement on ovarian cancer that we found of interest, - not the least because the statement acknowledges the current limitations in the diagnosis of this terrible condition. The statement, therefore, was chosen to reflect messages that have been proven to improve outcomes in ovarian cancer. They are:
- Encouraging women, or anyone born with ovaries, to know their risk.
- Promoting genetic testing to at-risk populations, engaging in the consideration of population-based genetic testing, and taking action when necessary is more beneficial — a sure way to decrease incidence and mortality.
- Encouraging those who are undergoing pelvic surgeries for benign conditions (hysterectomy, tubal ligations, cysts, endometriosis) to consider having their fallopian tubes removed. As the fallopian tube is the origin of most high-grade serous cancers, fallopian tube removal has been shown to dramatically reduce the risk for a later ovarian cancer diagnosis. This has been referred to as “opportunistic salpingectomy”).
- Educating about the symptoms of ovarian cancer, so that patients may receive a prompt diagnosis, which may ease psychological dis tress and facilitate treatment.
- Educating to ensure every person diagnosed with ovarian cancer is seen by a gynecologic oncologist and has access to the best standard of care.
- Educating patients to utilize genomic testing for more personalized treatment.
- Encouraging cascade testing for patients’ family members when a mutation that increases ovarian risk is discovered.
- Promote consideration of participation in clinical trials.
Now some good news: A recent study in JAMA Network Open demonstrated in a case-control study that near-daily use of aspirin reduces the risk of non-mucinous ovarian cancer across most strata of genetic risk by polygenic risk score.2 This is an important finding because it suggests that genetic susceptibility for ovarian cancer (ovarian cancer is a highly “familial” cancer with strong genetic predispositions even beyond BRACA1/2) does not negate the protective effects of aspirin.
1. Ovarian Cancer Research Alliance. https://ocrahope.org/news/ ovarian-cancer-screening-and-symptom-awareness-consensus-statement/
2. Hurwitz et al., JAMA Network open 2023;6(2):e230666
We have discussed the effects of nutrition on endometriosis repeatedly before in the VOICE, making the point that, though much too little research has been done on this subject, our subjective and, therefore, potentially biased opinion is that an anti-inflammatory diet can represent highly effective treatment in obtaining symptom relief in patients affected by endometriosis. Whether diet can also reduce the endometriosis imprint is, however, unclear.
Now a so-called “mini-review” offers a brief summary regarding the prevention and treatment of endometriosis by nutritional means.1 This is a rather superficial paper but for those interested in a quick read on the subject, it may be worth it.
1. Barnard et al. Front Nutrition 2023;10:1089891
As a concept in adult medicine and preimplantation genetic testing, this newsletter has addressed PRS in the past on repeated occasions. Moreover, the CHR’s staff has also commented on the subject in the medical literature.1 As a reminder, the concept of PRS is based on looking at thousands of genetic variants across an individual’s (or an embryo’s) genome and then, to assess the risk of this person (embryo) based on detected genetic variants to develop specific diseases. Because each genetic variant in polygenetic-inherited diseases represents only a relatively small risk, it takes multiple (the more than better) of such genetic variants cumulatively to achieve a clinically significant enough disease model to predict future disease risk. This “polygenic” risk stands in contrast to so-called single gene disorders, where risks are depending on only one gene, and where one gene, therefore, can establish highly significant and predicable risk, based on Mendelian inheritance.
In adult medicine, PRS is still mostly considered an experimental procedure to predict the risk of developing a disease in the future, with the hypothesis mostly being that such knowledge may permit early interventions that may either delay or, hopefully, completely avoid a clinical expression of disease later in life. In reproductive medicine, PRS has attracted attention mostly because it is already being offered at some IVF centers and genetic testing laboratories under the dubious claim of being able to predict future disease risk in embryos, thereby giving parents allegedly another screening tool for their embryos in IVF cycles beyond just preimplantation genetic testing for aneuploidy (PGT-A) which, of course, in itself is a very controversial clinical test, repeatedly previously addressed in these pages.
Though PRS is increasingly entering medical practice in adult medicine, a recent opinion article in the BMJ made the important point that realistic expectations are key to realizing the potential benefits of polygenic scores (also the title of the paper). The article furthermore noted that the enthusiasm around polygenic scores must not lead to forgetting bigger, more modifiable, and, therefore, often more relevant factors.2 Within this context, the authors then offer three key message:
(i) PRS will always be limited in predicting disease, as much as disease risk is determined by factors PRS cannot measure. (ii) In effective communication of this limitation, there is risk of overemphasizing the
potential, and role, of PRS, which can undermine currently effective screening programs. This is, of course, a point that especially applies to the PRS of human embryos in IVF. (iii) The enthusiasm around PRS must not distract from efforts to tackle modifiable risk factors for disease. In expanding on this point, one also can argue that PRS must not distract from helping patients to achieve pregnancy cost-effectively.
In another remarkable paper on PRS this time involving PRS in human embryos, in Science magazine, U.S. investigators reported on a nationally representative U.S. survey-based experiment on the attitude of 6,823 people toward PRS, gene-editing and, as a non-genetic control for the attitude toward interventions that in general are interventions targeting the college admission test SAT.3 The authors noted in their conclusions that results were somewhat surprising since many media reports on PRS presented PRS as a “fringe issue,” while the survey revealed considerable interest by the public in these subjects.
They further noted the dramatic change in public attitude toward IVF as an example of how “progressive” the public has become toward reproductive interventions which only a few decades ago were widely opposed. In confirmation, they noted that 78% of surveyed individuals now view IVF as morally acceptable and only 6% described it as morally wrong. In parallel, reproductive gene editing reached a rather surprising 41% acceptance rate in the general public (far exceeding what likely currently represents the prevailing opinion in the scientific community).
The authors, therefore, concluded that in a democracy public opinion matters. Experts who currently still strictly oppose ethically controversial procedures like PRS and genetic germline editing in embryos, should not assume that the public shares those opinions. At the same time, they, however, also acknowledge in the manuscript that the public does not necessarily match the scientists’ knowledge. They also acknowledge that “much more should be learned about the public’s reflexive as well as considered judgment.” For example, it is unclear for and against which possible traits the public may or may not wish to select in PRS. Unquestionably, a very interesting and unusual paper!
1. Gleicher et al., Nat Med 2022;28(3):442-444
2. Sud et al., BMJ 2023;380:e073149
3. Meyer et al., Science 2023;379(6632):541-543
AI is, of course, fully dependent on the definitions it is asked to work with. For example, if a trophectoderm biopsy of on average 5-6 cells is used to define not only that biopsy but, based on that biopsy, is asked to define a complete blastocyst-stage embryo, which biologically and mathematically in a large majority of embryos is impossible, the AI response will be “stupid.” As every expert will acknowledge, though the old IBM dictum, “garbage in, garbage out,” preceded AI, it fully also applies to AI. Consequently, if we accept the notion that, for biological reasons we have extensively discussed in the literature,1,2 a single trophectoderm biopsy can represent a complete embryo only if the aneuploidy is meiotic (it then exists in every cell of the embryos), we automatically must conclude that in a large majority or embryos (indeed ca. 80%) this is not the case, - because this is the approximate percentage of mitotic aneuploidies which are clonal and, therefore, exist in small arbitrarily positioned cell clusters.
If one, furthermore, defines the three diagnostic options of a PGT-A diagnosis as “euploid,” “mosaic,” and “aneuploid” incorrectly, then even the best AI in the world will in its analysis be “stupid.” And this is exactly what happened in a recent paper by colleagues from the Prelude Network at NYU Langone Fertility Center in collaboration with what likely is the largest PGT-A laboratory in the country, the genetic laboratory division of CooperSurgical Inc., Trumbull, CT,3 when they defined embryos based on a single trophectoderm biopsy as “euploid” if the biopsy contained between 0% and 20% abnormal DNA that deviated from either 46, XX or 46, XY, as “mosaic” if the percentage of “abnormal” DNA was between 21% and 80%, and as “aneuploid,” if that percentage was between 81% and 100%. Moreover, embryos containing three or more “mosaic” chromosomes were considered “aneuploid.”
However, these definitions on simply biological grounds are incorrect since the correct biological definition of an embryo as “mosaic” is not based on just a single 5-6-cell trophectoderm biopsy, but refers to a complete organism that arose from a single cell. In other words, even a theoretical single aneuploid cell in a blastocyst would define the embryo as “mosaic.” Moreover, the study’s definition of “euploid” with aneuploid DNA of up to 20% already defines most of these alleged “euploid” embryos as really “mosaic” embryos. The same applies at the
other extreme, where even embryos with 81% and above in aneuploid DNA in a single biopsy are not necessarily “aneuploid.” Many, indeed, will still be “mosaic” as well. In short, if we feed AI with garbage, what we are getting out is only more garbage.
1. Gleicher et al., Nat Med 2022;28(3):442-444
2. Gleicher N et al., Trends in Mol Med 2021;27(8):731-742
3. Buldo-Licciardi et al., J Assist Reprod Genet 2023;40:289-299
A paper so-far only been published on the preprint server for biology, bioRxiv, and, therefore, not yet peer-reviewed, for several reasons, nevertheless, deserves mention: A very good reason is that Rajiv C. McCoy, PhD from Johns-Hopkins University, and Alan H. Handyside, PhD from the University of Kent in the U.K., two leading researchers in the PGT-A arena, are the two lead authors. A second good reason is some of the data the manuscript presents regarding a pesty problem in embryology, - the fact that many embryos that make it relatively well to day-3 of culture, subsequently, however, arrest and do not make it to blastocyst-stage.
The investigators in this study now followed a large number of embryos in IVF cycles with time-lapse imaging and determined through PGT-A how meiotic and (mostly) mitotic aneuploidies of mostly the first two cleavage divisions related to embryo arrests. This paper now reports (still unreviewed) that aneuploidies among arrested embryos were exceedingly high (94%), mostly representing mitotic aneuploidies affecting multiple chromosomes. Among abnormally dividing embryos on time-lapse, 51% of embryos had mitotic aneuploidies, - a much higher rate than normally dividing embryos demonstrated (23%). For somewhat unclear reasons, the authors conclude that the observed increase in miotic aneuploidies may have been exacerbated by sub-optimal embryo culture conditions. To the best of our knowledge, there is so far no human evidence for such a conclusion, though some animal work and theoretical considerations do, indeed, support such a possibility.
The study, thus, confirms that most aneuploidies in preimplantation-stage embryos are mitotic and not meiotic. Paradoxically, this is still a controversial issue for some proponents of PGT-A and considerable importance because meiotic abnormalities exist in all cells of an embryo, while mitotic aneuploidy is clonal and, therefore, restricted to the smaller or bigger island of cells, turning a single trophectoderm biopsy of on average 5-6 cells (the usual process in PGT-A) into a crapshoot event that, simply, cannot be representative of a complete embryo.
1. McCoy et al., bioRxiv 2022; doi: https://doi.org/10.1101/2022.07.03.498614
Israeli investigators for the first time generated two identical human cell lines from the same person, except that they differed in chromosomal sex, - one was XX and the other XY. As the involved investigators from the Hadassah Medical Center in Jerusalem explained, they intend to use this model to differentiate between hormonal and chromosomal effects which often are difficult to distinguish. Moreover, these cell lines should also allow better distinction between the effects of X and Y chromosomes, while ruling out additional contributions from the genome of the research subject.
A second article in NewScientist offers interesting evolutionary information regarding the X chromosome. 2 It appears that, after the exodus of mankind from Africa, the X chromosome evolved into a “selfish” X chromosome containing regions that promote their DNA’s spread by killing Y carrying sperm. It furthermore appears that, over time, the Y chromosome evolved counter mechanisms, which is likely the reason why all of this had not been discovered before. It, thus, appears that we have been in a “war of the sexes” for millennia!
1. Kwon D. Scientific Amrica March 2023: 14-15
2. Le Page M. New Scientist Spring 2023. https://www.newscientist.com/arti cle/2348335-modern-humans-evolved-a-selfish-x-chromosome-after-afri ca-exodus/
Two review articles deserve mention: A first describes “the changing face of Turner syndrome,“(XO), offering many new insights into the genomic architecture of the syndrome.1 This is an excellent recent review article on this subject.
A second excellent review addresses a very different theme; In this paper, Swiss researchers address PIWI-interacting RNAs (piRNAs) in mouse sperm function and as they refer to embryo viability. In spermatogenesis, they have been known for some time to play an indispensable role. The review now, however, summarizes very recent discoveries, which demonstrated the importance of these small piRNAs. Paternal piRNAs may, in addition, play a significant intergenerational role.2
While hypoactive female sexual desire disorder has been of interest to investigators (including investigators at the CHR3) for years, hypo-active sexual desire disorder in males has been a stepchild in reproductive research. Now, however, investigators from the U.K. reported that treatment with the reproductive neuropeptide, kisspeptin, offers the first apparently effective remedy.4 It appears to substantially modify sexual brain processing in men with the condition, increasing penile tumescence and behavioral measures of sexual desire and arousal.
1. Gravholt et al., Endocrine Rev 2023;44:33-69
2. Perillo et al., Reproduction 165(3): R91-R102
3. Kushnir et al., Endocrine 2019;63(3):632-638
4. Millis EG., et al. JAMA Network Open 2023;6(2):e2254313
Supercharging again the COVID-19 pandemic?
A recent news article in Science magazine raised the possibility that the popular antiviral drug, Molnupiravir (Lageviro™) may again help “supercharge” the COVID-19 pandemic.1 An analysis of SARSCoV-2 genomes submitted to a global database demonstrated a significant amount of guanine-to-adenine mutations induced by this drug. Further suspicion arose, from the fact that counties where the drug was in wide use (U.K., U.S., Australia) had more of such mutations than countries with lesser utilization (France, Canada).
Studies, moreover, also questioned the effectiveness of the drug against COVID-19, therefore, as the author of the article noted, raising the question of whether it is worthwhile to take any risks with continued use of the drug.
A systematic review and meta-analysis of the literature in The Lancet, 2 moreover, established with a high degree of certainty that people with prior vaccination as well as prior infection with the SARS-CoV-2 virus (called under updated nomenclature “hybrid immunity”) demonstrate better protection against the Omicron variant than individuals with only a past infection (97% at 12 months vs. 75%). The same advantage remained regarding reinfections (42% vs, 25%). The paper suggested that in deciding on future booster timing, the protection from past infection should be weighed along with protection from vaccinations, a point demanded by several experts for some time, - yet so far mostly rejected by U.S. government agencies.
The Florida Department of Health through the State Surgeon General on February 17, 2023, issued a “health alert” concerning the COVID-10 vaccine rollout, reporting a 1,700% increase in the Vaccine Adverse Event Reporting System (VAERS) in comparison to only a 400% increase in overall vaccine administration for the same time period. Reports of life-threatening conditions increased by over 4,400%, an increase not seen during the 2009 H1N1 vaccination campaign (see figure).3 Quoting several public health experts, many media outlets, however, reacted with a rather skeptical commentary regarding the validity of those data from the Florida State Surgeon General, 4 who has repeatedly faced a rather hostile press core when the state in COVID-19 policies deviated from what was widely perceived recommended practice. The figure below, however, appears to speak for itself!
Though the authors considered it necessary to make special notice of the need to ensure equitable global access to publicly funded new healthcare technologies, they, curiously, failed to notice the fact that the rest of the world, as unfortunately has become customary, did not equitably participate in the development of the mRNA technology, - yet obviously benefitted from the U.S. investments. Moreover, especially Russian and Chinese efforts to develop competing vaccines against the SARS-CoV-2 virus very obviously fell flat in producing only vaccines with very low levels of immunogenicity.
An accompanying editorial added further interesting information” Moderna and Pfizer, the two principal manufacturers of the COVID-19 mRNA vaccines, accumulated over US$ 100 billion in global sales. Estimated to cost between US$ 1-3 per dose, both companies announced that, going forward, they would charge the public (and insurers) over US$ 120 per dose once automatic government payments for all COVID-19 vaccines would be paused.6 No wonder Pfizer has been able to afford the acquisition of another large pharma company valued at US$ 43 billion7 and Moderna was able to make its first-ever acquisition in purchasing Japanese DNA manufacturer OriCiro for a meager US$ 85 million.8
By Congressional hearings finally almost convinced that COVID-19 started as a lab-leak at the Wuhan Virology Institute, miraculously a new theory appeared on the scene claiming evidence after 3 years of futile attempts that animals at the Wuhan Seafood Market, after all, may have been the culprits. 9
However, the story sounds fishy: A team of international investigators suddenly noticed fresh/old evidence that intermediate-animals at the market, after all, spread the SARS-CoV-2 virus to people. This evidence is based on initial washings of the surfaces at the market which in early pandemic days were routinely genetically sequenced by Chinese scientists and publicly posted but then, after a few days, at instruction from the Chinese government, were removed. Miraculously, they again became public this January and were “spotted “ by the international team in early March. After contacting Chinese scientists who had produced those data, 665 gigabytes of data the international group was especially interested in again disappeared and Chinese investigators so-far refused to turn this sample over.
A paper in the BMJ reported on the unprecedented public investment the U.S. government made in the development of mRNA COVID-19 vaccines:5 The study identified 34 related NIH grants which combined with other identified U.S. government grants came to a total of US$ 31.9 billion, of which US$ 337million were already invested prior to the pandemic. The authors concluded that this sizable investment translated into millions of saved lives and was crucial for the development of MRNA vaccine technology which will have substantial future applications not only in the development of vaccines against viruses but will, likely, also play a substantial role in other areas of medicine.5

The interest came from the allegation of the international investigator group of having in the brief time those data were visible discovered evidence that some of the surface swabs from the market contained strings of genetic material from the virus as well as from raccoon dogs, Malayan porcupines, hoary bamboo rats and other mammals sold at the market, - often for food. What was considered the most relevant sample was one that contained the virus and thousands of sequences of raccoon dogs but with almost no human genetic material. Even if true, this would not mean that raccoon dogs were, in fact, the long-sought intermediate species that infected mankind with the virus because there are many possibilities for explaining the presence of raccoon DNA with the virus in absence of human DNA, - not the least, specimen manipulation.
The media, however, immediately and enthusiastically described the alleged findings as “backing up” earlier reports that the market (and not the virology lab) in Wuhan was the source of the pandemic,9 even though all the coincidences in this scenario appear somewhat
excessive: Where did those alleged test results suddenly come from and why were they not published in late 2019 when samples were really obtained at the market, and before research access to the market was prohibited by the Chinese government? Why did the specific specimen of interest again disappear? After all, confirmation of a market rather than lab origin for the virus would support the Chinese government’s (and Dr. Fauci’s) contentions?
Then there is the immediate demand by a WHO spokesperson that the Chinese government give the international group of investigators access to the allegedly twice withdrawn specimen.10 The WHO was never before so proactive in pressuring the Chinese government to facilitate research into the real origin of the pandemic. Our strong suspicion, therefore, is that we are witnessing is the first stage of another attempt at gaslighting world opinion: in a second, by public opinion supposedly “forced” stage, Chinese investigators will then “give in” and, reluctantly, turn over to the WHO a manipulated DNA sample in an attempt to, once and forever, prove that the Wuhan Virology Institute was not the origin of the worldwide COVID-19 pandemic. And media as well as Western governments will gladly accept the explanation!
For why scientists got the COVID lab leak wrong, we recommend an opinion article by Tim Trevan in the March 6, 2023, Wall Street Journal under the same title.11 Why the WHO and governments have shown no real interest in transparency probably does not need much explanation. And since we already are recommending recent newspaper articles of interest on the subject, we strongly recommend an article by Apoorva Mandavilli in the ScienceTimes section of The New York Times on March 21, 2023, under the title (that says it all), “Politial meddling and the pandemic,” in which CDC scientists recall their despair as COVID-related alerts and research were squelched by the U.S. government.12
The newest infectious threats
It appears like no week goes by without the CDC warning of yet another new infectious threat: This time it is shigellosis, a bacterium causing diarrhea which often can be prolonged and bloody with as well, abdominal cramps and fever. It used to infect mostly only young children under the age of 4; but now the FDA warns of drug resistant cases in adults, mostly among gay men, international travelers, people with HIV and the homeless, The disease can be especially severe in immune-compromised individuals.13
Have you heard about Candida auris, a fungus for the first time discovered 15 years ago in Japan, and now present all over the world? As a recent article by Dominique Mosbergen in The Wall Street Journal of March 21, 2023,14reported, this at times deadly fungus has now been detected in most U.S. states and is especially prevalent in long-term medical care facilities. While not everybody gets sick when carrying the fungus on the skin, once it gets into wounds, the blood steam, or organs, effects can quickly become life-threatening.
Nature magazine recently reported the third person in the world, cured of HIV after receiving virus-resistant cells.15 Unfortunately, however, the treatment is not yet ready for mass implementation because risks and costs of the procedure, requiring a bone marrow stem cell transplant, are too big.
1. Service RF. Science 2023;379(6632):526
2. COCID-19 Forecasting Team. Lancet 2023;401(10379):833-842
3. https://www.floridahealth.gov/newsroom/2023/02/20230215-updat ed-health-alert.pr.html
4. https://www.tampabay.com/news/health/2023/02/17/ ladapo-surgeon-general-coronavirus-vaccines-mrna-vaers-safety/
5. Lalani et al., BMJ 2023;380:e073747
6. Roy V. BMJ 2023;380”p413
7. https://pharmanewsintel.com/features/a-closer-look-at-the-most-re cent-pfizer-acquisition-deal-seagen-inc
8. https://www.fiercepharma.com/pharma/moderna-makes-its-first-ev er-acquisition-buying-japanese-dna-manufacturer-oriciro
9. Maxmen a. The Washington Post, March 25, 2023. https://www.washingtonpost.com/opinions/2023/03/25/ release-genetic-sequences-wuhan-market-covid-origins/
10. https://www.cnbc.com/2023/03/17/covid-origins-who-urges-china-torelease-data-on-wuhan-market.html
11. https://www.wsj.com/articles/how-scientists-got-the-covid-lab-leakwrong-wuhan-institute-of-virology-wet-market-theory-virus-chi na-hubei-psychology-hypothesis-99a9481b
12. Mandavilli A., The New York Times , March 21, 2023; https://www. nytimes.com/2023/03/21/health/covid-cdc.html
13. https://emergency.cdc.gov/han/2023/han00486.asp
14. https://www.wsj.com/articles/ deadly-fungus-detected-in-most-u-s-states-1eb3453a
15. Reardon S. Nature 2023;615:13-14
The naked mole-rat may not be very pretty, but the species makes up for that on many grounds, including as a model of longevity and, relevant to reproductive medicine, as a wonderful model for the study of oogenesis because in this animal oogenesis in its entirety occurs postnatally. A leading investigator of this model has for many years been Ned J Place, PhD, MD from Cornell University in Ithaca, NY, who is also the senior author of a very interesting paper in Nature Communications, which demonstrates how exceptionally large ovarian reserve is in this model animal, maintaining over a 30-year reproductive lifespan normal fertility into very advanced age.
The authors discovered that highly desynchronized germ cell development and maintenance of a small number of primordial germ cells (PCGs), which can expand upon reproductive activation, represent unique strategies in accomplishing this unique fete naked mole rats experience.
We liked this paper so much that we invited Prof. Place to give us more insights into this unique model in our CHR GrandRounds on April 25, 2023, co-sponsored by The Foundation for Reproductive Medicine. If you are interested in attending (on a first-come, firstserve basis please contact Micah Elias at melias@thechr.com.
REFERENCE
1. Brieño-Enriquez, et al., Nat Commun 2023;14(1):670
Another very interesting paper appeared in eLife by investigators from Harvard and Duke, which attracted a lot of attention1 not only because of its scientific values but also because the technology used in this paper was licensed to a start-up company, chaired, as noted in the March issue of The VOICE, by famous serial infertility entrepreneur, Martin Varsavsky. 2 With the senior author of the paper being Harvard’s legend, Prof. George M. Church, PhD, geneticist, molecular engineer, chemist, serial entrepreneur, and pioneer in personal genomics and synthetic biology,3 - no wonder everybody spoke about this publication.
What was so special about this paper? If for the first time reported success in producing granulosa-like cells from human induced pluripotent stem cells (hiPSCs), thereby becoming able to recapitulate key ovarian phenotypes, including follicle formation and steroidogenesis. Moreover, aggregation with human primordial germ cell-like cells (hPGCLCs), the investigators were able to obtain ovary-like organoids (named ovaroids). Moreover, the hiPSCs supported hPGCLCs development from pre-migratory to gonadal stages. The researchers rightly conclude that this model system may provide excellent opportunities for studying human ovarian biology and may enable the development of new fertility treatments. And Varsavsky apparently believes so, too!
1. Pierson Smela, et al., eLife 2023;11:e83291
2. Helft M. Forbes, October 17, 2016. https://www.forbes.com/sites/mi guelhelft/2016/10/17/prelude-fertility-200-million-startup-stop-biologi cal-clock/?sh=28f86f697260
3. https://wyss.harvard.edu/team/core-faculty/george-church/
In a series of two articles in Cell, 1,2 and previewed in a third article in the same journal,3 once more Harvard investigators, discovered in their first paper that controlling organoid symmetry breaking uncovered an excitable system underlying human axial elongation.1 In their second paper, the group reported that controlling human organoid symmetry breaking revealed that signaling gradients drove segmentation clock waves.2 The preview article, summarizing both papers, did so by concluding that they demonstrated that spatial coupling of human stem cell organoids induced coherent progression through developmental transitions, thereby allowing the dissection of molecular circuits underlying human development. Clearly not a small feat!
1. Anand et al., Cell 2023;186:497-512
2. Ilker Yaman, et al., Cell 2023;513-527
3. Thomson M. Cell 2023;186:461-463
The CHR VOICE is the newsletter of The Center for Human Reproduction (CHR), an independent, academically affiliated infertility and research center located at 21 East 69th Street in Manhattan, New York, N.Y 10021. www.centerforhumanreprod.com. Telephone +212 994 4400.
The CHR VOICE attempts to inform and engage a global community of infertility patients, infertility service providers, and researchers in reproductive medicine, physiology, and biology. The mission of The CHR is clinical care, research, and education, all at highest standards, with empathy, honesty, integrity, and equity.The newsletter is published 10 times a year (except July and August). Copyright © 2023 by The CHR. All rights reserved. Print ISSN 2836-3086. Online ISSN 2836-3094. Copyright © 2023 by The CHR. All rights reserved.
For letters to the editor, comments, and suggestions, please contact Micah Elias at melias@thechr.com. For all advertisements or sponsorships in The VOICE , please contact Alexandra Rata at arata@thechr.com. Advertisements appearing in The CHR VOICE do not necessarily reflect the opinions of The CHR. .R


