

My Blood My Health
"Bridging Health with Knowledge and Advocacy"


What is CAR T-cell Therapy, and Why is it Important?
New hope for people with sickle cell disease and beta-thalassemia
Finally, a treatment for anemic patients with myelofibrosis!
New treatment options for AML and iron deficiency
Are you ready for ASH 2024? December 2024
2024 Health Canada Approval in Blood Diseases
Newhopeforpeoplewithsicklecelldiseaseorbetathalassemia
Finally,arealtreatmentoptionforanemicpatientswith myelofibrosis!
What’snewforAMLpatients?
Twonewoptionsforpatientswithirondeficiency

Welcome to My Blood, My Health Digital Magazine
Welcome to the fourth issue of "My Blood, My Health" magazine. This December edition is dedicated to innovative treatment approaches. CAR-T cell, Gene therapy, and new targeted treatments have been made accessible recently to patients afflicted with blood disorders. These innovations are crucial to improving patientlifeexpectancyandqualityof life(QoL).
With this magazine, we aim to pursue Heal Canada's mission to empower patients to access better andequitableservices.We'redeeply grateful to our readers for their continued interest and support. Yourengagementdrivesustobring insightful and valuable content to encourage patient-centricity in healthcare.
Thank you for participating in our journey, and welcome to this enlighteningissue!
Fromyourdedicatedteam



Cheryl Petruk, MBA B.Mgt. CEO & Founder of Heal Canada

Brigitte Leonard, Ph.D Chief Scientific Officer
My Blood, My Health Director
Heal Canada is a registered Not For Profit Organization in Canada healcanadaorg
Disclaimer: The Patient Advocacy Digital Magazine provides general information and resources to promote patient empowerment and awareness. The content is not a substitute for professional medical advice or treatment. Always consult with qualified healthcare professionals for personalized guidance regarding your specific medical condition or situation

What is CAR T-cell Therapy, and Why is it Important?
Cheryl Petruk, MBA
Chimeric Antigen Receptor (CAR) T-cell therapy represents a breakthrough in the field of immunotherapy, offering hope to patients with certain types of cancer that have resisted traditional treatments like chemotherapy and radiation. This innovative approach involves genetically engineering a patient's own immune cells to fight cancer more effectively. By harnessing the body's natural defence mechanisms, CAR Tcell therapy has opened new avenues for treating blood cancers and is expanding into the treatment of solid tumours.
In this article, we explore the fundamentals of CAR T-cell therapy, its importance in the fight against cancer, how it works, the types of diseases it treats, and the future of this groundbreaking treatment. We also discuss the challenges and potential risks associated with CAR T-cell therapy and highlight why patient advocacy is crucial for expanding access and improving outcomes.
How Does CAR T-cell Therapy Work?
At its core, CAR T-cell therapy is a type of adoptive cell transfer. It is a form of immunotherapy in which a patient’s immune cells are collected, modified, and then infused back into the body to target cancer cells. CAR T-cell therapy specifically focuses on T-cells, a type of white blood cell that plays a crucial role in the immune response.

The process of CAR T-cell therapy involves several key steps: Collection of T-cells: First, blood is drawn from the patient, and T-cells are isolated through a process called leukapheresis These cells are then sent to a laboratory for genetic modification.
1. Genetic Modification: In the lab, the T-cells are genetically engineered to express a chimeric antigen receptor (CAR) on their surface. This receptor enables the T-cells to recognize and bind to a specific antigen found on the surface of cancer cells.
2. Expansion of Modified T-cells: Once modified, the CAR T-cells are expanded in large numbers This process ensures there are enough engineered cells to target and destroy the cancer effectively.
3. Infusion: The modified CAR T-cells are infused back into the patient, where they begin hunting down and destroying cancer cells. These engineered cells continue proliferating in the patient’s body, providing ongoing surveillance against cancer.
5.
4. Targeting Cancer Cells: The CAR T-cells are designed to recognize specific proteins on the surface of cancer cells, such as CD19, which is commonly found in certain blood cancers When the CAR T-cells bind to these proteins, they trigger a potent immune response, destroying the cancer cells.
The Importance of CAR T-cell Therapy
CAR T-cell therapy represents a significant advancement in cancer treatment for several reasons:
1. Long-lasting Effects: Unlike traditional treatments like chemotherapy, which often need to be repeated, CAR T-cells can persist in the patient's body for months or even years. This long-term presence allows the immune system to continue fighting cancer cells, reducing the risk of recurrence.
3.
Effective for Refractory Cancers: One of the most remarkable aspects of CAR T-cell therapy is its effectiveness in treating cancers that have not responded to other forms of treatment. Patients with advanced, relapsed, or refractory cancers have seen complete remissions with CAR T-cell therapy when other treatments have failed
2. Personalized Medicine: CAR T-cell therapy is a highly personalized treatment. Since it uses a patient's own T-cells, it is tailored to the individual's specific cancer, improving the chances of success and reducing the risk of rejection or adverse immune reactions
4.
Innovative Approach to Solid Tumors: Although CAR T-cell therapy has seen the most success in treating blood cancers, there are ongoing efforts to expand its use to solid tumours, such as breast cancer, lung cancer, and glioblastoma. While solid tumours present unique challenges, advances in CAR T-cell technology hold promise for treating these more complex cancers.
Diseases Treated with CAR T-cell Therapy
CAR T-cell therapy has shown remarkable success in treating certain types of blood cancers. Below are a few examples of diseases where this therapy is being used:
1. Acute Lymphoblastic Leukemia (ALL)
CAR T-cell therapy has been particularly effective in treating patients with B-cell acute lymphoblastic leukemia (ALL), a cancer of the blood and bone marrow that primarily affects children. Patients with relapsed or refractory ALL who have undergone CAR T-cell therapy have seen significant remission rates. For example, the FDA-approved CAR Tcell therapy Kymriah (tisagenlecleucel) was the first gene therapy available in the U.S. for ALL, demonstrating high rates of durable remission in patients (Maude et al., 2018). Health Canada approved the medication manufactured by Novartis in May 2019.

2. Diffuse Large B-cell Lymphoma (DLBCL)
Another area where CAR T-cell therapy has made a profound impact is in treating diffuse large B-cell lymphoma (DLBCL), the most common form of non-Hodgkin lymphoma. For patients who have not responded to other treatments, CAR T-cell therapies like Yescarta (axicabtagene ciloleucel) have provided new hope, leading to high response rates (ORR) (a), prolonged response (DOR) (b) and prolonged survival (OS)(c)(Neelapu et al., 2017).

aORR:OverallResponseRate
CR:CompleteResponse
SD:Stabledisease
PR:PRogressivedisease
n:numberofpatients
bDOR:Durationofresponse
NR:Notreached

NE:Notevaluable c
OS:Overallsurvivald
NE:Notevaluable

3. Multiple Myeloma
While CAR T-cell therapy has shown the most success in B-cell malignancies, its application in multiple myeloma is gaining traction Multiple myeloma is a cancer of plasma cells, and CAR T-cell therapies targeting the B-cell maturation antigen (BCMA) have shown promising results. Idecel (idecabtagene vicleucel), the first CAR T-cell therapy approved for multiple myeloma, has led to significant tumour responses in patients with relapsed or refractory disease (Munshi et al., 2021).
4. Chronic Lymphocytic Leukemia (CLL)

CAR T-cell therapy is being explored in patients with chronic lymphocytic leukemia (CLL), a slower-growing blood cancer. Although CLL has been more resistant to CAR T-cell therapy than ALL and DLBCL, ongoing clinical trials are working to optimize CAR T-cell treatments for this patient population.
Challenges and Risks of CAR T-cell Therapy
Despite its transformative potential, CAR T-cell therapy is not without challenges and risks:
1. Cytokine Release Syndrome (CRS)
One of the most serious side effects of CAR T-cell therapy is cytokine release syndrome (CRS). This occurs when the engineered T-cells trigger an intense immune response, leading to high levels of inflammatory molecules called cytokines. Symptoms can range from mild (fever, fatigue) to severe (low blood pressure, difficulty breathing), and in some cases, CRS can be lifethreatening. Early detection and prompt management are crucial for minimizing the risks associated with CRS.


2. Neurotoxicity
Another potential side effect is neurotoxicity, which can cause confusion, seizures, and other neurological symptoms. While the exact mechanism behind CAR T-cell-induced neurotoxicity is not fully understood, it appears to be linked to the intense immune activation triggered by the therapy. Most cases of neurotoxicity are reversible with appropriate medical intervention.

3. Access and Affordability
CAR T-cell therapy is complex and expensive, with treatment costs reaching hundreds of thousands of dollars. This raises concerns about access and affordability, particularly for patients in low-income settings or countries with limited healthcare coverage Expanding access to CAR T-cell therapy requires ongoing efforts in patient advocacy, healthcare policy, and innovative financing models.
The Future of CAR T-cell Therapy
As research advances, the future of CAR T-cell therapy looks promising. Scientists are refining CAR T-cell technology to improve its safety, effectiveness, and applicability to a broader range of cancers. Some areas of ongoing research include:
Tackling Solid Tumours: Researchers are developing new CAR T-cell therapies that can overcome the unique challenges of solid tumours, such as the dense tumour microenvironment and immune evasion mechanisms.
Reducing Side Effects: Efforts are underway to develop CAR T-cell therapies that minimize the risk of CRS and neurotoxicity One promising strategy involves using "onoff" switches that allow clinicians to control CAR T-cell activity more precisely.
Combining Therapies: Combining CAR T-cell therapy with other treatments, such as checkpoint inhibitors or traditional cancer therapies, may enhance its effectiveness and broaden its use.
The Role of Patient Advocacy
Patient advocacy is crucial in expanding access to CAR T-cell therapy and ensuring that patients understand their treatment options. Advocacy organizations can help bridge the gap between patients, healthcare providers, and researchers by promoting awareness, supporting research funding, and lobbying for policy changes that increase access to these life-saving therapies.
Additionally, advocates can work to ensure that clinical trials for CAR T-cell therapies are designed with patient needs in mind, helping to optimize treatment protocols and expand eligibility for a broader range of patients.
Conclusion
CAR T-cell therapy represents one of the most exciting advancements in cancer treatment, offering hope to patients with few remaining options Its ability to provide durable remissions in previously refractory cancers has revolutionized the treatment of blood cancers, and ongoing research is expanding its reach to other types of malignancies. While challenges remain, particularly in managing side effects and expanding access, the future of CAR T-cell therapy is bright. As patient advocates continue to push for broader access and better understanding, this therapy has the potential to save countless lives.




Emerging Treatments in Blood Diseases: Health Canada approval in 2024
Brigitte Leonard, Ph.D
According to the Health Canada database, the agency has evaluated and decided on the faith of 53 products in 2024. Of these 53, only 44 were authorized for commercialization in Canada (83%). Of these 44, only 26 were new active substances and seven touch blood diseases.
In this article, you will find a summary of 5 products approved in 2024 and how they can make a difference in the patient's life. Among them, there is a real breakthrough innovation and 1st in-class approval.
Thank you to Vertex Pharmaceutical, GSK, Servier, CSL Behring and KYE Pharmaceutical for developing these new treatments. Your support for Canadians afflicted with blood diseases to improve their life is appreciated.
New hope for people with sickle cell disease or beta-thalassemia
Health Canada approved Casgevry for commercialization in September 2024 to treat two genetic blood disorders: sickle cell disease and beta-thalassemia.
In these two inherited diseases, the production of hemoglobin is affected. Hemoglobin binds and carries oxygen from the lungs to other parts of the body (Figure 1) Each red blood cell circulating in blood vessels contains several hundred million hemoglobin molecules.
Hemoglobin always contains four units called chains, which all contain a heme linked with iron (Figure 2). Hemoglobin chain types are different before and after birth. The predominant form of hemoglobin (Hemoglobin F) in the fetus will contain four chains: two alpha and two gamma. The body will stop producing this variant around 12 weeks after birth At that time, the Hemoglobin A variant, which contains four chains (two alpha and two beta), will become the most predominant and stay during the rest of our lives (Figure 2). Both types of chains and their structure are essential for the function of hemoglobin.


In sickle-cell disease and beta-thalassemia, a mutation occurs in the gene that produces the beta chain on chromosome 11. This mutation causes the production of abnormal beta chains and disrupts red blood cell production and oxygen transport.

Casgevy (exa-cel: exagamglogene autotemcel) is a gene therapy developed by Vertex Pharmaceuticals and CRISPR Therapeutics The FDA approved it in the United States for treating sickle cell disease in December 2023 and betathalassemia in January 2024 EMA approved it for the European market in February 2024
Casgevy is the 1st gene therapy approved that does not use a viral vector. Casgevy uses CRISPR/Cas9 technology to edit the patient's DNA cells that produce red blood cells. Casgevy does not repair the mutated gene on chromosome 11. It stimulates the production of the fetal form of hemoglobin silenced after birth The fetal form (gamma chains) replaces the defective beta chains in the composition of hemoglobin to eliminate issues with the abnormal beta chain.
Casgevy in sickle cell disease
In the clinical study CLIMB SCD-121, patients who experienced more than three crises yearly became free from crises after Casgevy treatment For a year, 97% of patients with sickle cell disease were free from crises. Also, participants didn't need hospitalization due to crises during the year. The clinical benefits are due to participants' capability to have early and sustained increases in total and fetal hemoglobin levels, reaching near-normal to normal levels at 6 months. Patients were able to maintain better blood count over time.
How does it work?
1) In the hospital, patients' stem progenitor cells from bone marrow that produce mature blood cells are harvested.
2) Patient cells are sent to a central facility to be processed:
Enrichment of progenitor cells
Modification of progenitor cells with Casgevy (called editing)
Proliferation of modified progenitor cells
Cells will be tested, frozen, and shipped back to the hospital.
3) Upon confirmation of viable modified cells, patients will receive intensive chemotherapy (busulfan) to destroy all stem progenitor cells in their bone marrow.
4) After the chemotherapy, modified stem progenitor cells will be infused back into the patients. Patients will need a month to recuperate and get a normal - near normal blood cell production.

The most common side effects in the CLIMB SCD-121 study can primarily be attributable to Busulfan treatment. They include low platelets and white blood cell levels, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, febrile neutropenia (fever and low white blood cell count), headache, and itching
Casgevy in beta-thalassemia
In the CLIMB THAL-111 clinical study, patients with a heavy need for transfusion have been included. They received, on average, 34 blood units per year, representing one transfusion every week to every two weeks Despite the level of transfusion received, they had a hemoglobin level below normal. Also, these patients had a high excess of iron in their blood, liver and heart, putting them at risk for complications due to organ damage. Treatment with Casgevy resulted in transfusion independence in 91% of patients enrolled in the trial. The safety profile was generally consistent with intensive chemotherapy busulfan. No deaths or cancers occurred
In conclusion
The data from these two clinical trials show that a one-time infusion of Casgevy provides early and sustained increases in total and fetal hemoglobin levels, resulting in durable improvement of patients' conditions and quality of life.
Finally, a treatment option for anemic patients with myelofibrosis!
Myelofibrosis is a rare cancer characterized by an overproduction of abnormal blood cells and inflammation markers called cytokines. Treatment of myelofibrosis has improved significantly since the 1st JAK inhibitor, Jakavi, was approved in 2011 JAK inhibitors are targeted therapies for cancer, like myelofibrosis, because the disease is caused by mutations that overactivate the JAK/STAT pathway.
Currently, the FDA has approved a total of four JAK inhibitors:
Jakafi/Jakavi (Ruxolitinib) 2011
Inrebic (Fedratinib) in 2019
Vonjo (Pacritinib) in 2022
Ojjaara (Momelotinib) in 2023
Until last month, Health Canada only approved two JAK inhibitors:
Jakafi/Jakavi (Ruxolitinib) 2012
Inrebic (Fedratinib) in 2023
In November 2024, Health Canada approved Ojjaara produced by GSK.
Why is Ojjaara's addition to the Canadian treatment options good news for patients?
Anemia is often present at diagnosis and eventually develops in nearly all patients due to cancer progression. Anemia and blood transfusion are risk factors linked with leukemic transformation and mortality. Most available treatments for myelofibrosis exacerbated disease-related anemia. To avoid complications related to anemia, physicians will prescribe a small dose of JAK inhibitor, which limits its efficacy.
Ojjaara is as efficient as other JAK inhibitors. However, unlike other therapies, it can improve anemia by facilitating the production of blood cells. SIMPLIFY trials have demonstrated that patients can reach healthier blood levels and become transfusionindependent. Some patients could even delay complex procedures such as transplantation due to this treatment
This unique benefit of Ojjaara (MMB) is due to its capacity to target a molecule called ACVR1. By inhibiting ACVR1, Ojjaara reduces hepcidin production. Hepcidin levels are often higher than usual in myelofibrosis patients. The reduction of hepcidin allows better iron management and stimulates red blood cell production in patients with myelofibrosis
Conclusion


Now, patients with myelofibrosis who are anemic will be able to have access to a JAK inhibitor that can improve their symptoms, reduce their enlarged spleen, and prolong their life while increasing their quality of life (QoL).
What’s new for AML patients?
In July 2024, Health Canada approved TIBSOVO to treat two types of cancers positive for a specific mutation called IDH1: acute myeloid leukemia (AML) and cholangiocarcinoma. Tibsovo (Ivosidenib) was developed and is supported by Servier.
Patients with IDH1-positive AML have an inferior outcome when treated with the current therapy than patients without the mutation. Only half of the IDH1+ AML patients tend to be alive after two years.
During the clinical trial in AML, adding TIBSOVO to the standard treatment provided significant short-term and long-term clinical benefits with an acceptable safety profile.
Adding TIBSOVO to the standard treatment increased the complete response rate by three times. The ratio of participants who became transfusion-independent also increased by three times. Being transfusionindependent is vital to improve QoL in patients
Adding TIBSOVO to the standard treatment increased the survival rates, and the improvement is maintained over time. With TIBSOVO, the odds of staying alive are increased by 67%, and the median survival is 24 months versus 7.9 months.
In conclusion




Based on medical experts in AML, Adding TIBSOVO to standard treatment should become the new standard care for IDH1+ AML patients because it improves response rate, prolongs survival, reduces transfusion needs and improves QoL.
Two new options for patients with iron deficiency
Iron (Fe) is an essential mineral that plays several crucial roles in the human body. Iron links to several proteins necessary for proper functioning; Iron is vital for oxygen transport and energy production. Without iron, the brain, muscles and immune system cannot function properly. Also, adequate iron levels are essential for growth and development, especially in infants, children, and pregnant women.
Iron level is tightly regulated in a healthy person because excessive iron leads to several serious health issues due to organ damage. The regulation involves several mechanisms: absorption from nutrients and recycling. Unlike other minerals, the body has no active mechanism for excreting excess iron, making the regulation of absorption crucial.
Iron deficiency can occur for several reasons, and understanding these causes can help manage and prevent it.

Blood loss: menstruation, gastrointestinal bleeding, surgery or injury
Inadequate iron intake: diets
Increased iron needs: infant, children, and pregnancy
Poor iron absorption due to medical conditions, medications, and diets.
Iron supplements can be needed when patients have symptoms of iron deficiency, such as low energy, fatigue, lack of concentration, and breathlessness during exercise.
In August 2024, Health Canada approved ACCRUFER (ferric maltol), an oral treatment for iron deficiency anemia. The FDA approved this product in 2019. KYE Pharmaceutical licensed Accrufer for the Canadian market from Shield Therapeutics. The AEGIS-H2H study has shown that Accrufer was non-inferior to intravenous iron therapy. With Accrufer, it is possible to improve iron-deficiency anemia without requiring hospital administration. It is also a non-salt formulation of ferric iron, providing an alternative to salt-based oral iron therapies. It also carries fewer gastrointestinal side effects typically observed with salt-based therapies.
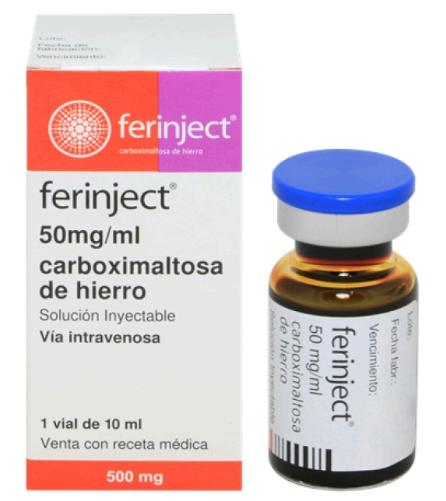
Health Canada approved FERINJECT (ferric carboxymaltose) in March 2024, which was distributed by CSL Behring. It is an intravenous iron supplement used to treat iron deficiency anemia (IDA) in one-year and older patients when oral iron preparations are neither tolerated nor effective. FERINJECT can also treat iron deficiency (ID) in adult patients with heart failure to improve exercise capacity.




Are you ready for ASH 2024?
Just over 30,000 hematologists and hematology-oriented investigators are expected to attend the 66th American Society of Hematology (ASH) Annual Meeting & Exposition in San Diego. Last year, 113 countries were presented by the ~32,000 individuals who attended the conference. Mark your calendars now to attend the world’s most comprehensive hematology event of the year
The ASH annual meeting is the world's largest and most comprehensive hematology event of the year. With hundreds of sessions on the hottest trends in hematology, ASH offers multiple ways for attendees to find exactly what they are looking for.
In April 1958, the first official meeting of the American Society of Hematology (ASH) was held in Atlantic City, New Jersey, where more than 300 hematologists gathered to discuss clinical and research matters related to blood and blood diseases. Since that initial meeting, ASH has played an active and important role in the development of hematology as a discipline. For more than six decades, ASH has sponsored its annual meeting, the premier education and scientific event in the field of hematology, and has published the journal "Blood", the most cited peer-reviewed publication in the field.
ASH is committed to building and nurturing a global hematology community and workforce inclusive of diverse perspectives, talents, and experiences as it works toward one collective goal: helping hematologists conquer blood diseases worldwide. With more than 18,000 members from nearly 100 countries, ASH is the world’s largest professional society, serving both clinicians and scientists who are working to conquer blood diseases.

C l i n i c a l T r i a l s a n d S u r v e y s




A l l i a n c e s a n d
C o l l a b b o r a t i o n s


Join Us
KYB invites individuals, healthcare professionals, community leaders, and organizations to join this mission. Together, we can work towards reducing health disparities and improving outcomes for individuals with blood disorders in the African/Caribbean community. Whether through donating blood, volunteering, or simply spreading the word, every contribution helps create a healthier future.
For more information on how you can get involved, send an email to: knowyourblood2024@gmail.com follow us on social media: Instagram: @Knowyourblood; TikTok: know.your.blood
Together, we can make a difference

Improving the Lives of MPN Patients
The Global MPN Scientific Foundation (GMPNSF) is a charitable non-profit organization dedicated to advancing the research and management of MPNs. Our ultimate goal is to raise awareness, facilitate collaboration, and improve the lives of people affected by MPNs.
Our Mission
AtGlobalMPNScientificFoundation,weunderstandthechallengesandgapsinthe currentmanagementofMPN,suchas:
Lack of awareness and education among patients, physicians, and scientists
Limited access to specialized testing and care
Variability in diagnostic criteria and treatment guidelines
Insufficient data on the natural history and outcomes of MPN
Limited availability of effective therapies and clinical trials
That's why we are committed to providing a source of professionally backed information, building and facilitating an MPN community, and advocating for patients affected by this rare group of blood cancers Global MPN Scientific Foundation is run by volunteers comprising MPN patients and healthcare professionalswhosharethisvision.
On our platform, you will find a wide range of unbiased and medically backed information, including leaflets, newsletters, and forums where you can meet and hear about the latest MPN research. You will also learn about our investment in research and support for clinical trials. Moreover, you will discover how we are developing links with MPN groups worldwide to become more visible in advocating forMPNpatients.
We invite you to join us in our mission to improve the lives of people living with MPN. Whether you are a patient, a physician, or a pharma representative, you will find valuable resources and opportunities to connect with others who share your interestinMPN.
Together, we can make a difference! www.gmpnsf.org.




Created in 2017, the Acute Leukemia Advocates Network (ALAN) is an independent global network of patient organizations dedicated to changing the outcomes of patients with acute leukemia by strengthening patient advocacy in that area Our role at ALAN is to support our members in supporting patients and their families, raise awareness of leukemia, and conduct work that is best done internationally, like generating data and evidence. All our activities are patient-led, collaborative, and evidence-based.
Who are we?
In March 2024, the network counts 56 member organizations from 52 countries and is governed by a steering committee. All steering committee members are leaders of national patient organizations focusing on leukemia and blood cancers from different regions to ensure global representation.
How do we work?
At ALAN, we are convinced that:
A strong collaboration between contributors of a multi-stakeholder project will likely generate benefits for all parties involved.
Patients' and patient advocates' perspectives are unique. Therefore, they should be considered specialist knowledge complementary to other experts, academia, and industry.
So, we collaborate on various projects with the pharmaceutical industry, other patient organizations, medical societies, researchers, clinicians, and other experts.
What do we do?
1- Capacity Building:
2- Raising awareness
3- Evidence-based advocacy



Partners







My Blood, My Health Team
Brigitte Leonard, Ph.D

Brigitte Léonard is the Chief Scientific Officer at My Blood, My Health, a registered not-for-profit organization advocating equitable access to quality healthcare across Canada. She had the privilege of working in Pharma for over 20 years, contributing to bringing lifechanging treatments to patients with the highest ethical standards. Now, she wants to share her knowledge and utilize her scientific, strategic, and communication skills to help the patients' community.
She obtained her Ph.D. in Biomedical Sciences from Université de Montréal in 2003. Her doctoral research was conducted under the supervision of Dr. Denis-Claude Roy at Guy-Bernier, MaisonneuveRosemont Hospital Research Center. She developed a quantitative diagnostic assay in non-Hodgkin's lymphoma and evaluated the relevance of this marker in the patient's outcome.
Cheryl Petruk, MBA B.Mgt.

Cheryl A. Petruk is a multifaceted professional whose career spans across patient advocacy, business, and post-secondary education, showcasing her dedication to making a significant impact in each of these areas.
Cheryl's transition into patient advocacy was driven by a passion from her family circumstances and a deep commitment to ensuring patients' rights and access to care Cheryl has worked tirelessly to bridge the gap between the healthcare system, patients, and pharma stakeholders, ensuring that patients' voices are heard and their needs are met. Her work involves collaborating with Stakeholders and Patient Advocacy Organizations, lobbying for patient centricity, and providing patient support and guidance. Cheryl's empathetic approach and dedication to advocacy have made her a respected figure in this field, admired by patients, healthcare professionals, and fellow advocates.
Cheryl also leads and is the lead faculty member at CACHEducation, Patient Advocacy Training.
