1 minute read
What’s new for AML patients?
from MBMH Issue 4 English
by healcanada
In July 2024, Health Canada approved TIBSOVO to treat two types of cancers positive for a specific mutation called IDH1: acute myeloid leukemia (AML) and cholangiocarcinoma. Tibsovo (Ivosidenib) was developed and is supported by Servier.
Patients with IDH1-positive AML have an inferior outcome when treated with the current therapy than patients without the mutation. Only half of the IDH1+ AML patients tend to be alive after two years.
During the clinical trial in AML, adding TIBSOVO to the standard treatment provided significant short-term and long-term clinical benefits with an acceptable safety profile.
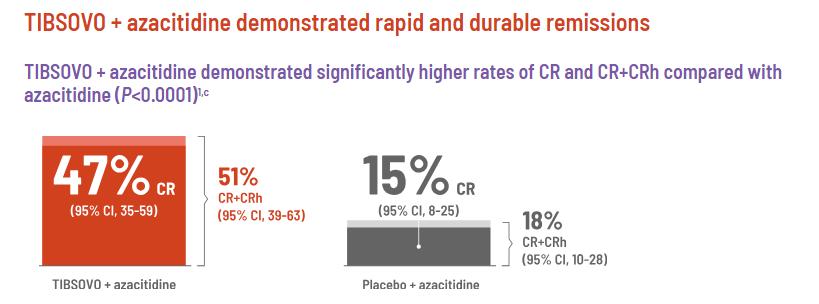
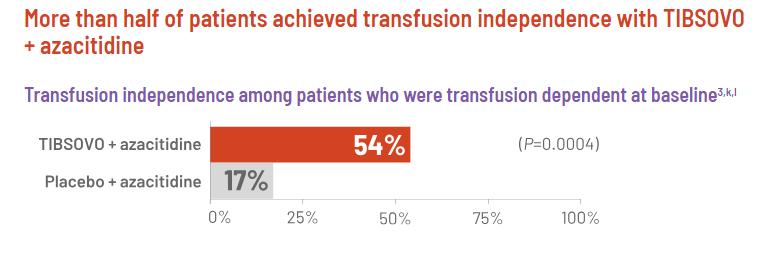
Adding TIBSOVO to the standard treatment increased the complete response rate by three times. The ratio of participants who became transfusion-independent also increased by three times. Being transfusionindependent is vital to improve QoL in patients
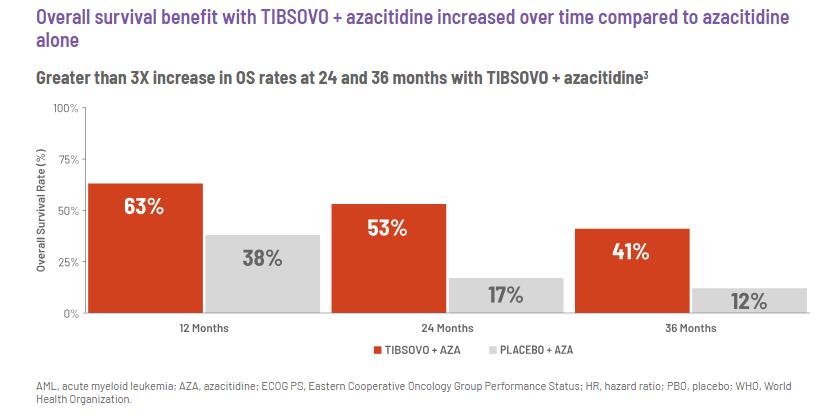
Adding TIBSOVO to the standard treatment increased the survival rates, and the improvement is maintained over time. With TIBSOVO, the odds of staying alive are increased by 67%, and the median survival is 24 months versus 7.9 months.
In conclusion
Based on medical experts in AML, Adding TIBSOVO to standard treatment should become the new standard care for IDH1+ AML patients because it improves response rate, prolongs survival, reduces transfusion needs and improves QoL.





