
PMMI Media Group | www.HealthcarePackaging.com SPRING 2024 The Business Effects of PFAS Regulations | 16 The AI That Drives Humanoid Robots | 26 Protect Your Sterile Barrier Rollstock | 34 Challenges in UserCentered Design | 80 See Special Section | 38 REFILLABLES COME HOME OTCs and Rx in Stackable Glass, Compostable Pouches | 22















THE POWER WITHIN With 48 years of proven performance in ampoule-based contract filling and packaging, we’ll help develop a unique dispensing system for your formulations, with a variety of tips and in a range of different sizes and colors. Your journey to smarter single-use packaging begins here. james-alexander.com • 908-362-9266 PROVIDING UNIQUE SOLUTIONS IN SINGLE-USE GLASS AMPOULE PACKAGING Prescription • First Aid • Dental • Diagnostics • Veterinary • Cosmetics • Dermatology Extended Shelf Life Formulation Stability Tamper Resistance Quality Assurance Two-Part Mixing UNLEASH
22
26
34
80





Startup Cabinet Health offers reusable packaging and a refill system for the healthcare market that it says has the potential to transform the industry.
A look at the powerful AI behind the robot that allows it to move, think, and act like us. STERILE
Much attention is given to packages as they protect a product from end-of-line to the patient. But are you correctly handling your sterile barrier inventory prior to packaging?


A panel at Pharmapack discussed competing challenges in designing healthcare packaging and devices, where sustainability or anti-counterfeiting may be in conflict with simplicity for the user.
The






and medical device brands may only
a small portion of a converter’s total
As converters comply with regulations for food and CPG packaging, eventually it may not make economic sense to carry PFAS-containing
solely for life science brands.
—pp. 16, PFAS: Navigating Materials of Concern in Life Science
2 | Healthcare Packaging • Spring 2024 CONTENTS 22 26 80 12 14 Vol.19 No. 1
PACKAGING
REUSABLE
Refill System Cuts
Plastic from OTC, Pharma Packaging
AUTOMATION
Humanoid Robots and the AI That Drives Them
PACKAGING
Protect Your Sterile Barrier System Rollstock
ADHERENCE/DELIVERY
2024 Challenges in User-Centered Design
Cover image courtesy: Cabinet Health, The Sustainable Healthcare Co. COLUMNS & DEPARTMENTS 06 KEREN SOOKNE’S PERSPECTIVE 08 QUICK HITS 10 QUOTABLES/BY THE NUMBERS 12 NEWS 14 COLD CHAIN CORNER 16 MATERIAL DEVELOPMENTS 77 NEW PRODUCTS SPECIAL SECTION 38 Leaders in Healthcare Packaging 2024 This exclusive Leaders in Healthcare Packaging section includes profiles and product listings for a variety of companies serving the healthcare and pharmaceutical industry.
“ ”
implications
immediate concerns
Pharmaceutical
business.
materials
of PFAS bans extend beyond the
of compliance.
represent
Packaging




com o
H g -b

Keren Sookne ksookne@pmmimediagroup.com
CONTRIBUTING EDITOR Melissa Griffen-Young
CONTRIBUTING EDITOR Joseph Derr
ART DIRECTOR Michael Smith
CREATIVE DIRECTOR David Bacho Advertising
VICE PRESIDENT, SALES John Schrei jschrei@pmmimediagroup.com
SENIOR MANAGER, PRINT OPERATIONS Lara Krieger lkrieger@pmmimediagroup.com
FINANCIAL SERVICES MANAGER Janet Fabiano jfabiano@pmmimediagroup.com
ACCOUNT EXECUTIVE Elizabeth Tierney 815.861.2992
PMMI Media Group
PRESIDENT David Newcorn
VICE PRESIDENT, DIGITAL Elizabeth Kachoris
SENIOR DIRECTOR, MEDIA OPERATIONS Kelly Greeby DIRECTOR, DIGITAL MEDIA Jen Krepelka
SENIOR DIRECTOR, EVENTS Trey Smith
FOUNDING PARTNER AND EXECUTIVE VICE PRESIDENT, INDUSTRY OUTREACH, PMMI Joseph Angel
Questions about your subscription or wish to renew? Contact circulation@pmmimediagroup.com
www.healthcarepackaging.com Healthcare Packaging® (ISSN # 21543666) is a registered trademark of PMMI, The Association for Packaging and Processing Technologies. Healthcare Packaging® is published twice annually by PMMI with its publishing office, PMMI Media Group, located at 401 N. Michigan Ave, Suite 1700, Chicago, IL 60611; 312.222.1010; Fax: 312.222.1310. Copyright 2024 by PMMI. All rights reserved. Materials in this publication must not be reproduced in any form without written permission of the publisher. Applications for a free subscription may be made online at www. healthcarepackaging.com/subscribe. Paid subscription rates per year are $55 in the U.S., $80 Canada and Mexico by surface mail; $130 Europe, $200 in all other areas. Single copy price in U.S. is $20. Free digital edition available to qualified individuals outside the United States. POSTMASTER; Send address changes to Healthcare Packaging®, 401 N. Michigan Avenue, Suite 1700, Chicago, IL 60611-3789. PRINTED IN USA by Quad. Volume 19, Number 1 The opinions expressed in articles are those of the authors and not necessarily those of PMMI. Comments, questions and letters to the editor are welcome and can be sent to: editors@healthcarepackaging. com. Mailing List: We make a portion of our mailing list available to reputable firms. If you would prefer that we don’t include your name, please write us at the Chicago, IL address. PLEASE RECYCLE THIS MAGAZINE Remove inserts or samples before recycling. PMMI The Association for Packaging and Processing Technologies 12930 Worldgate Dr., Suite 200, Herndon VA, 20170 p: 571.612.3200 | f: 703.243.8556 | www.pmmi.org PMMI Media Group 401 N. Michigan Ave., Suite 1700 Chicago, IL 60611 p: 312.222.1010 | f: 312.222.1310 www.pmmimediagroup.com Content EDITOR-IN-CHIEF
4 | Healthcare Packaging • Spring 2024
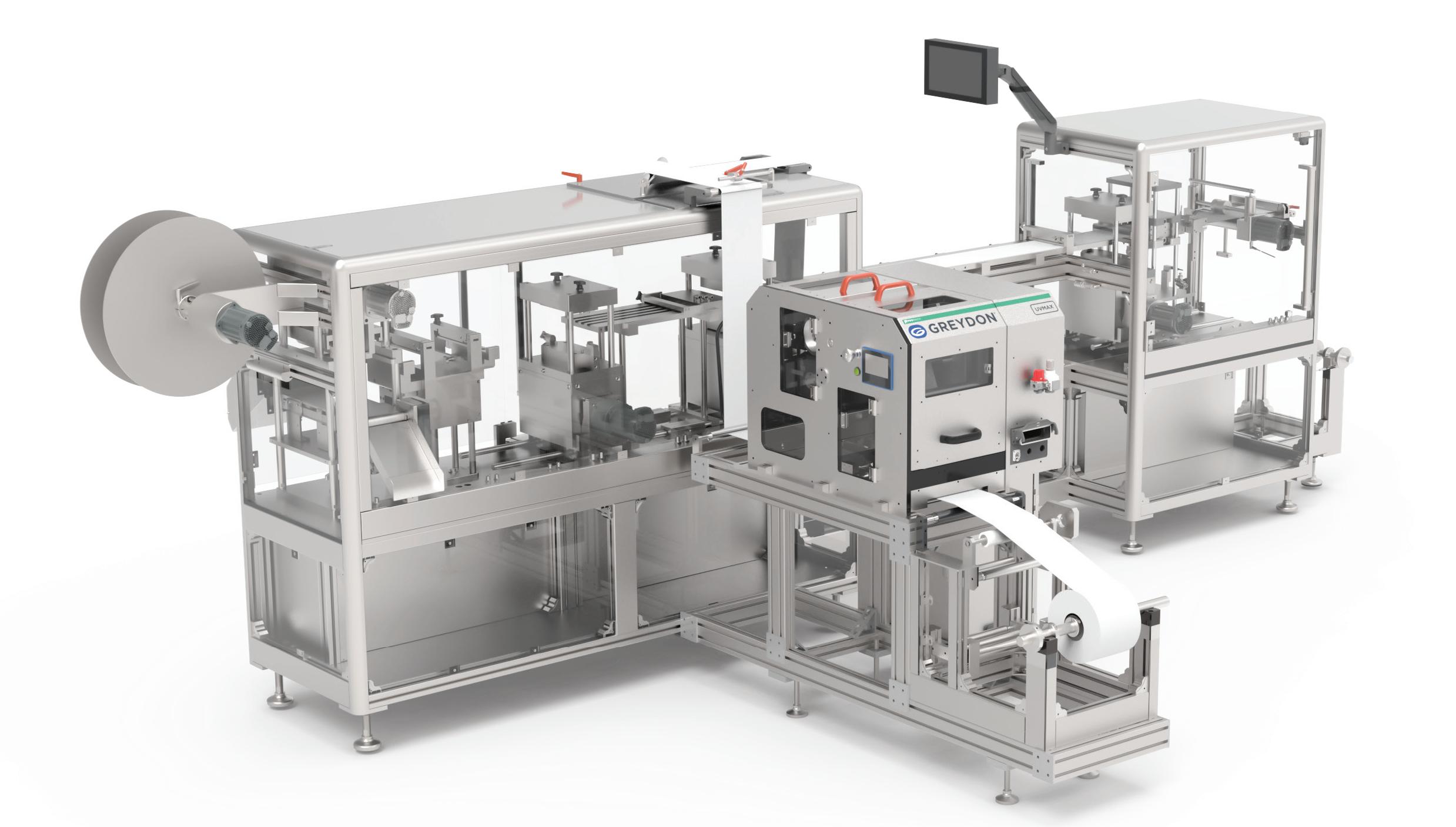
DESIGNED FOR INTEGRATION AND PERFORMANCE
IN-LINE FLEXOGRAPHIC PRINTING FOR BLISTER PACKAGING
The UVMax by Greydon is an in-line flexographic printer engineered to integrate with pharmaceutical blister packaging systems. It prints the complete package, including variable information, with an integrated thermal inkjet printer, thus eliminating the need for purchasing pre-printed blister materials.

SEAMLESS IN-LINE INTEGRATION
Designed to integrate in-line printing capabilities to blister packaging machines and production lines.

STATIC & VARIABLE DATA PRINTING
Prints static product and regulatory data as well as variable coding for a fully finished compliant package.

SERVO-CONTROLLED WEB DRIVE
Industry-leading web management ensures accurate print positioning and reduces the stretching of materials.
INCREASE YOUR PACKAGING PRINTING & THROUGHPUT
Greydon@ProMachBuilt.com | York, PA | 717-848-3875 | Greydon.com © 2024 ProMach Inc. CONTACT US TODAY AT GREYDON@PROMACHBUILT.COM
The Intersection of Sustainability and User-Friendliness
A new refillable prescription package hits the market. Elsewhere, experts discuss the challenges of balancing reusable devices with simplicity.

Operating in such a highly regulated environment, it’s understandable that healthcare can’t make sustainability pivots at the same speed as the fast-moving consumer goods space. But that doesn’t mean that progress— in many forms—isn’t taking place.
Take for example, Cabinet Health and their aim to “de-plastic” pharmacy aisles and medicine cabinets (pp. 22). This isn’t the first time we’ve seen refillable, reusable packaging for oral solid doses, but their sleek offerings are particularly unique in (1) the sheer variety of offerings, (2) their entry into the prescription drug space, and (3) refill pouches which are compatible with both industrial and
home composting. Of course, whether a pouch makes it to home compost depends on the user’s access to home compost. But this represents a major step forward for OTC and prescription drug pouches, particularly with the general public’s current lack of access to industrial composting. Related, I recommend Eight Ways You Might Be Greenwashing Your Packaging and Not Even Know It (hcpgo.to/431)
Practicality will ultimately determine whether a design makes sustainable, user-centric sense. At times, these very concepts are at odds with each other, as panelists discussed at Pharmapack Europe (pp. 80).
KEREN SOOKNE is the Editor-in-Chief of Healthcare Packaging She may be reached at ksookne@pmmimediagroup or at linkedin. com/in/kerensookne

Leading-edge technology trusted worldwide for superior product inspection and contaminant detection.
Your brand is on the line, so you need equipment that’s better than good. Anritsu systems deliver performance, reliability and low total cost of ownership Plus, we back our longlasting equipment with unparalleled service and support.
Discover what you’ve been missing. Learn how to advance your product quality and operational efficiency at anritsu.com/product-inspection.
PERSPECTIVE
X-RAY SSV CHECKWEIGHER
M6 METAL DETECTOR
XR75









O SOU C O -O -L C IN SOLU IONS C ON OU ww. c o .co end- of-line au tomated pack aging s olu t ion s Pac teon Group c ompanie s o ff er
.co
ss c olo i s.co
o i w s.co
www.sc
www
www.
1
Fatalities Alleged as Nurse Swapped Fentanyl with Tap Water
An Oregon Public Broadcasting article highlighted a disturbing set of incidents at Asante Rogue Regional Medical Center in which a nurse is suspected of removing the painkiller fentanyl from patients’ IV bags, and replacing it with tap water. The number of people who have died or been impacted by the diversion and tampering has not been officially reported. The tap water, which is not sterile, reportedly led to multiple incidences of pseudomonas infection which Docs on Call host Dr. Robin Miller says is very dangerous to people in poor health, and can cause sepsis, pneumonia, and more. —Keren
Sookne
2 Sanofi Supports Employees Diagnosed with Cancer
Fierce Pharma notes Sanofi is going the extra mile for employees. The company-wide “Cancer & Work: Acting Together” support program secures any employee’s job, including salary and benefits, for a minimum 12 months after diagnosis of cancer or other critical illnesses. Sanofi has run the program in some form for years. This next iteration is designed to provide social, emotional, and financial support. Benefits include flexible work arrangements, external psychological support, and support for caregivers and managers. Work to cover “miscellaneous non-medical expenses” is underway. —Melissa
Griffen
3‘Copycat’ Eye Drop Packaging Draws FDA Warning
Per ABC News , the latest eye drop issue in a spate of contamination warnings is copycat packaging that resembles Bausch + Lomb's Lumify brand eye drops. FDA is warning consumers not to use South Moon, Rebright, and FivFivGo brands. South Moon tested positive for Burkholderia cepacia complex, which FDA reports can lead to antibiotic-resistant infection, and neither drug was found to have the active ingredient in Lumify’s redness-reducing drops. Some online copycat product photos may feature the words “Bausch + Lomb,” while actual product may look different. —Keren
Sookne
4 Spoon-in-lid Packaging Launched for Infant Formula
According to a recent MSN article, UK-based Chadwicks and New Zealand-based Tekplas partnered on an innovative spoon-in-lid design for infant formula packaging. Unlike the traditional packaging for infant formula that simply places spoons inside formula cans, the scoop will not become submerged within the contents of the can with the spoon-in-lid design. Launched in the infant formula market in New Zealand, Australia, and East Asia, all the components of the packaging, including the aluminum can, over-lid, scoop, and die-cut lid, are reportedly fully recyclable. —Melissa Griffen
5 FDA Neglect in Tainted Breathing Machine Scandal?
ProPublica coverage claims the FDA failed to protect millions of people who used tainted breathing machines, particularly those manufactured by Philips Respironics, including the popular DreamStation for sleep apnea. Despite receiving numerous complaints about a dangerous defect, the FDA did not issue alerts to doctors or patients over the course of a decade. The company submitted reports years later without penalty. Other leading device makers submitted hundreds of thousands of late reports to the FDA, leaving regulators and the public without safety information. In Feb. 2024, reporters for the With Every Breath series received a George Polk Award for journalism. —Tim Hayes
6 Last-Mile Pollution Hits Homes Near Hubs
A recent UW News article highlighted a rarely discussed topic: how e-commerce hub pollution affects residents. U of Washington researchers found that Seattle-metro neighborhoods within 1.9 miles of an Amazon last-mile delivery station or sortation center are exposed to twice the amount of delivery van and truck traffic (and thus higher emissions) than neighborhoods farther away, despite ordering fewer packages. While healthcare shipping accounts for only a small portion of all goods shipped—and the study was focused on Amazon—it’s a reminder that hubs can impact local public and environmental health, particularly as home healthcare increases.
—Keren Sookne
QUICK HITS To keep up with the latest news bits from around the world visit healthcarepackaging.com to subscribe and get Quick Hits sent right to your inbox. 8 | Healthcare Packaging • Spring 2024
Monoblock Filling and Closing



From our headquarters in North Carolina, Chase-Logeman Corporation has been designing and building monoblock style filling and finishing equipment trusted by the healthcare industry for over 63 years. To meet your exacting process needs, ChaseLogeman integrates production processes for a sanitary process package.

Whether you are using vials with stoppers and crimps, or bottles with plugs and caps, Chase-Logeman has decades of experience to integrate the complete filling line.

Contact us at:
Chase-Logeman Corporation
303 Friendship Drive Greensboro, NC 27409
336.665.0754
info@chaselogeman.com
https://www.chaselogeman.com

“We’re moving into a kind of ‘post-technical challenge’ world—10 to 15 years ago, a lot of the challenge was technical. Can we make things small enough, last long enough for the battery, etc? The technical problems have largely been solved… people can have pulse oximeters on their wrists with ECGs, you can measure blood glucose in real time and have it connected. The technology is not the problem anymore. The problem now is, will the payers pay for these? Will the regulators approve it? Will the users use it?”
—TOM OAKLEY, VP OF DESIGN AND DEVELOPMENT, SPRINGBOARD
On Unregulated and Counterfeit Medicines:
“ I'm taking four generic drugs for my hypertension and elevated cholesterol... and if I got a drug that was half as potent as I thought it was, I could have a heart attack, never knowing that I didn't get the treatment that I thought I was getting.”
—FDA COMMISSIONER ROBERT CALIFF IN A HOUSE APPROPRIATIONS SUBCOMMITTEE HEARING (VIA REUTERS, FEB. 28, 2024)
“At the beginning of the twentieth century, anthropogenic mass was equal to only 3% of global biomass. ... About 120 years later, in 2020, anthropogenic mass is exceeding overall biomass in the world.”
—EMILY ELHACHAM ET AL., “GLOBAL HUMAN-MADE MASS EXCEEDS ALL LIVING BIOMASS,” NATURE, DEC. 9, 2020
$22 MILLION
THE FIGURE allegedly paid to hackers as a result of the ransomware attack targeting medical firm Change Healthcare. The transaction was publicly visible on Bitcoin’s blockchain.
Source: Wired
50%
IN EVERY SECTOR SURVEYED, an hour’s unplanned downtime now costs the manufacturer at least 50% more than it did two years prior.
Source: Siemens, The True Cost of Downtime 2022
$2.5 MILLION
THE AMOUNT of funding raised by Scotland-based drug delivery device developer 1nhaler to develop its paperbased, single-use dry powder inhaler (DPI), which integrates a breathable membrane.
Source: Archangels
47%
THE PERCENTAGE of Americans surveyed that falsely believe only safe, verified websites selling prescription drugs appear at the top of search results. 60% of online pharmacy users would consider purchasing from an unapproved source for convenience.
Source: ASOP Global, Americans’ Perception and Use of Online Pharmacies Survey
Q 10 | Healthcare Packaging • Spring 2024 QUOTABLES BY THE NUMBERS

Pharma Machinery Market Growth
Outpaces Larger Industries
A forecasted expansion of the pharmaceutical machinery market is driven by enhancements in technology, automation, sustainability, and supply chain challenges, as highlighted in a recent infographic, Pharmaceutical Manufacturing: The Future Ahead, released by PMMI (view the infographic at hcpgo.to/430). “A large portion of the biggest pharmaceutical manufacturers have announced capacity expansion in the billions, with the majority of investment going toward expanding capacity in North Carolina,” says Rebecca Marquez, director, custom research, PMMI. “This has pushed forecasted growth of packaging machine shipments to the pharmaceutical sector up for 2023 and 2024 compared to other industries.” Both e-commerce and central pharmacies, which sit between the retail pharmacy and wholesaler and serve multiple pharmacies, were cited as major reasons for this growth. Increasingly, central pharmacies are being used to serve mail-order prescriptions in the growing e-commerce landscape.
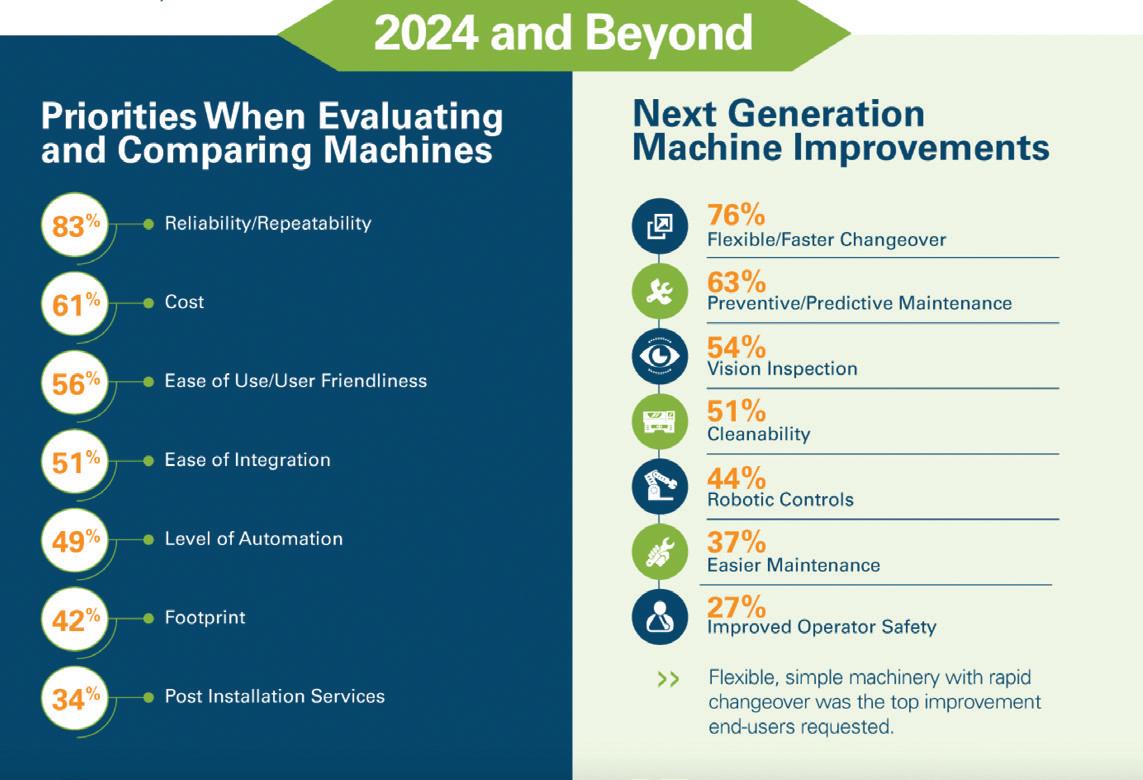
Going into 2024, manufacturers identified reliability/repeatability (83%) as a top priority when evaluating and comparing
EU: Proposed Compliance Delay for IVDs
In January, the European Council (EC) proposed more time for companies to become compliant with the In Vitro Diagnostic Medical Devices Regulation (IVDR), under certain conditions, to ensure availability of safe devices. The EC reports that a considerable number of legacy in vitro diagnostics (IVDs) currently on the market do not comply with the rules, nor have they been replaced by new devices. The proposed new transition periods include (1) a shorter transition period for high risk IVDs (Dec. 31, 2027) and (2) longer periods for medium and lower risk IVDs (Dec. 31, 2028, and Dec. 31, 2029, respectively). The proposal is advancing to review by the European Parliament and Council for adoption.
The EC is proposing to accelerate the implementation of the EUDAMED medical device database by making certain modules mandatory by late 2025,
machines and flexibility/faster changeover (76%) as a critical improvement for next-generation machines. The infographic also details key trends affecting the pharmaceutical industry. Automating costs, labor, changing packaging formats due to sustainability requirements, and delays in acquiring parts were all identified as challenges for the industry in their respective categories.
—Gatwiri Muthara

instead of waiting for all modules to be in place. MedTech Europe is supportive of measures that keep devices available to patients in need, while it issued four considerations that it believes should be met before any modules of EUDAMED are made mandatory.
—Keren Sookne
UPCOMING EVENTS
INTERPHEX (Apr 16-18) New York, NY
ISTA Forum: TransPack & TempPack (Apr 22-24) San Diego, CA
PDA Parenteral Packaging Conference (Apr 23-24) Copenhagen, DK
thePACKout (May 14-16) Coronado, CA
GS1 Connect 2024 (June 4-6) Orlando, FL
EXPO PACK Mexico (Jun 4-7) Mexico City, MX
PACK EXPO International (Nov 3-6) Chicago, IL
FOR THE LATEST in packaging and machinery, check out the 2023 PACK EXPO Innovations Report covering Pharma/Med Device.
NEWS 12 | Healthcare Packaging • Spring 2024

Ensure your products match the prescription.


• CEIA high sensitivity metal detection; ferrous, non-ferrous, stainless steel
• Ishida X-ray for foreign object and product defects
• Ishida precision checkweighing for weight verification
• Powders, capsules, tablets, liquids
Heat and Control® offers a complete line of metal detectors, checkweighers, and X-ray inspection systems for pharmaceutical products from the leading manufacturers, Ishida and CEIA®. Technical support, demonstration, and testing is available in North, Central and South America.




91 97 16 98 34 108 94 116 102 2 1 101 1 17 93 59 103 104 107 36 96 42 0842 2 ~2~ ~2 27 26 1 52 14 28 29 52 info@heatandcontrol.com | heatandcontrol.com
Quality Control.
detection systems that offer unparalleled sensitivity
pharmaceutical products to protect
consumer
brand. LOOKING BACK. PRESSING FORWARD. ALWAYS INNOVATING.
Inspection and foreign object
for
your
and your
INSPECTION & FOREIGN OBJECT DETECTION
PHARMACEUTICAL
Apr. 16-18, 2024 Booth 1308 Javits Center NYC NY, USA
ISTA: New White Paper on Shipping & Distribution Risk Assessment
Developed by the ISTA Pharma Committee, the new ISTA PCW-02 | Shipping & Distribution Risk Assessment white paper provides an overview for developing a shipping and distribution risk assessment process for temperature-sensitive product shipments. A risk assessment involves identifyin g the hazards and evaluating the risks associated with exposure to those hazards. Risks to products during shipments include damage, lost shipments, temperature alarms, delayed shipments, and more. [Experts will also discuss what’s new in temperature-controlled shipping at the ISTA Forum, Apr. 22-24 in San Diego.] Download ISTA’s white papers from their site via: hcpgo.to/429 —Keren Sookne
Surplus Feather System for Cold Chain Insulation







Expected to launch for life sciences in the first half of 2024 (as of press time), Pluumo+ is designed to insulate temperature-sensitive deliveries in life sciences using surplus feathers destined for landfill. After cleaning, Pluumo converts feathers into an intermediate fiber which is then combined with a small amount of binder material (a PLA/PBS composite), then formed into insulation and sealed within a starch-based compostable outer film. An example of bioutilization, the system is reported to be compatible with dry ice and a variety of configurations to accommodate different payloads, a range of temperature-profiles, and more. With any new innovation, due diligence on thermal performance is necessary. —Keren
Sookne




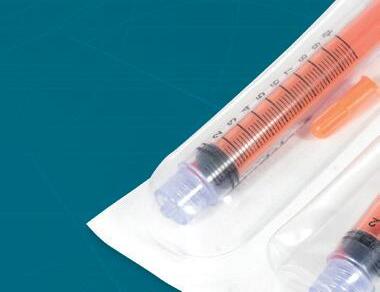
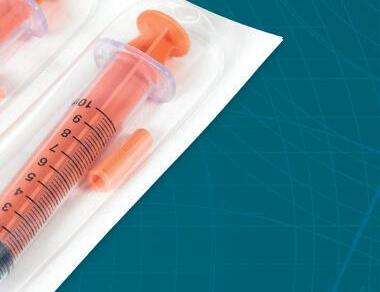

Harpak-ULMA offers medical packaging solutions that meet all of the standards required, helping to provide maximum product protection, hermetic sealing, customizable package shapes, hygienic individual or multi-packs, and easy-open options.
Our experts are ready to walk you through all of the considerations and help you choose the right packaging options for your products.













COLD CHAIN CORNER
© 2024 Harpak-ULMA Packaging, LLC, 85 Independence Drive, Taunton, MA 02780 USA a T a a a W k w th Ha ak-ULMA f m a a kag ng s t ns w th va at n- a y atf ms that m t a n st y stan a s! C nta t s T ay! www ha ak- ma m • 800-813-6644





































































































































CCLeAgile.com Complete RFID Solutions Custom Inlay Development Inlay Testing Labeling Printing and Converting Encoding and Personalization Software and Hardware Highspeed RFID Packaging Lines High-Speed RFID Injectable Packaging Lines Late stage encoding with speeds up to per minute Download Our White Paper CCL e-Agile offers comprehensive RFID solutions, featuring customized high-performance inlays, hardware, software, and a seamless, nonintrusive bolt-on solution for high-speed encoding and late stage personalization on RFID packaging lines. S E Pre-Filled East pril 22-24 S E Pre-Filled West June 10-11 Pack E po No ember 3-6 PD Pre-Filled October 22 -23 Pharma Ed PFS December 4-5th Live RFID demonstrations at: RFID Made Easy 700 items
PFAS: Navigating Materials of Concern in Life Science Packaging
From regulations to supplier inventory, how might PFAS bans impact your operations in the short- and long-term? What might be the next materials of concern?
KEREN SOOKNE, EDITOR-IN-CHIEF
While the healthcare packaging and medical device communities have been exempted from certain material regulations, recent discussions have highlighted the need for companies to prepare for changing laws, especially those pertaining to per- and polyfluoroalkyl substances (PFAS), commonly referred to as “forever chemicals.” These chemicals do not readily breakdown in the body or environment and have been linked in studies to endocrine disruption, certain cancers, and more.
During a session at the[PACK]out in 2023, experts from Boston Scientific and Amcor shared insights on the global regulatory landscape and its implications for healthcare brand owners. To start, Alex Bowman, R&D Manager at Amcor, emphasized the importance of staying informed and prepared for regulatory changes that will impact the life science packaging community, underscoring the need for companies to anticipate and adapt to these shifts.
PFAS uses in healthcare packaging
PFAS, which encompasses a broad range of chemicals used for their hydrophobic and hydrophilic properties, have come under scrutiny due to their persistence in the environment and potential health impacts. Haley Gorr, PhD, an Engineer and Global Product Steward from Boston Scientific, said, “What’s really important to understand is how PFAS is defined in the legislation. PFAS is really an umbrella term including PFOS, PFOA, PFHS—they’re very similar. In the legislation, they’ve been shifting towards a very holistic definition of PFAS. There’s not a lot of opportunity to differentiate between those different types of molecules. They’re saying, ‘If you have one fully fluorinated carbon atom, then you’re in scope.’ And that’s where everyone’s landing whether EU, U.S., etc.”
Gorr said that while their presentation wasn’t about debating the merits of these chemicals, it’s important to understand that there is a difference between molecular PFAS and polymerized PFAS, noting that the latter, known as fluoropolymers or FPs, are non-toxic due to their stable, locked-in structure.
“Fluorinated polymers have really helpful properties. We like them because they are biologically inert, they are hydro-
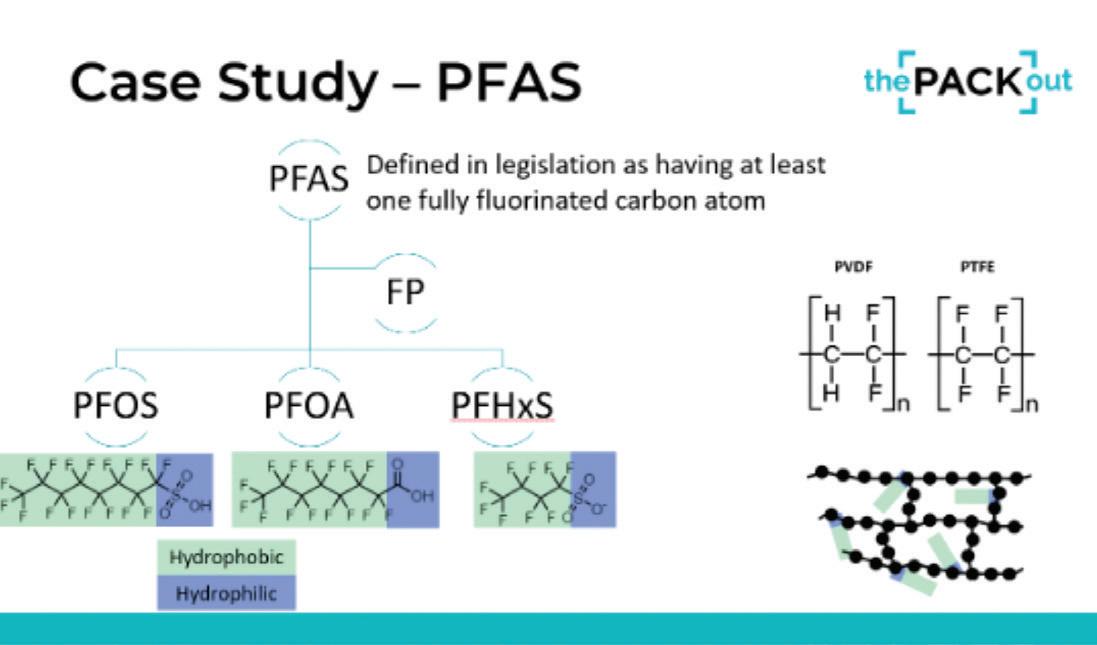

phobic, and they contribute lubricity to our products because the water sits on top of those materials [due to the hydrophobic fluorinated carbon atoms],” she said ( see slide above ). She explained that PFAS is generally used in medical devices to add lubricity and reduce friction on some catheters, wires, and implants. Another common application is to cover electrical wires that come into contact with tissue, such as the lead wires of some defibrillators and pacemakers. There are limited, if any, alternative materials for these complex applications at present.
There are, however, some traditional PTFE applications with alternative materials. Gorr explained that PTFE may be included in glossy carton coatings to prevent sticking. PTFE-free carton coatings are now widely available.
Bowman explained why converters use materials that fall under the PFAS umbrella in their films as processing aids. “Typically, linear low densities [LLDPE] and polypropylene are the kinds of materials that can use aids in processing. There’s a stick-slip phenomenon when these polymers are extruding through a die interface and these fluoropolymers and fluoroelastomers reduce that friction against the interface. So it’s basically eliminating things like melt fracture and orange peel and gives a nice clear film so you can see what’s inside your package,” he said.


MATERIAL DEVELOPMENTS 16 | Healthcare Packaging • Spring 2024
↑ PFAS is an umbrella term including PFOS, PFOA, PFHS. Source: Haley Gorr/Alex Bowman via the[PACK]out
↑ Haley Gorr, PhD, Engineer and Global Product Steward at Boston Scientific.



INNOVATIVE PACKAGING. IT’S IN OUR DNA. No matter what your packaging challenge, we’ll provide a unique, fully-integrated equipment solution that perfectly fits your needs. Fairfield, New Jersey • 973-808-8185 mgamerica.com See us at Interphex Booth 2221
MATERIAL DEVELOPMENTS
Market forces and indirect effects
The implications of PFAS bans extend beyond the immediate concerns of compliance. Pharmaceutical and medical device brands may only represent a small portion of a converter’s total business. As converters comply with regulations for food and CPG packaging, eventually it may not make economic sense to carry PFAS-containing materials solely for life science brands.
“We’re seeing some of those impacts in the regulations in food packaging that are driving a lot of action right now. We’re actively changing out of PFAS-containing materials to not-intentionally-added PFAS materials, including resins and additives that we’re buying from our suppliers, and then upstream even further to those chemical manufacturers making the fluoropolymers and fluroelastomers that we use as processing aids,” said Bowman. “Think about what the demand change feels like to the chemical manufacturers and resin and additive producers. They’re seeing a major shift away from one type of material.”

 ↑ Alex Bowman, R&D Manager at Amcor.
↑ Alex Bowman, R&D Manager at Amcor.
For now, Bowman reckons converters will continue to support healthcare customers with the existing materials. “But I don’t expect
it to be that way forever. Eventually, you can see the demand start to shift and it probably becomes unprofitable and unlikely that they’re going to continue to support what’s a pretty small consumer in terms of all of the plastics in the world,” he added. “We’re able to avoid some of these regulations because we’re in our own regulated markets. But some of these things are going to come at you from further up the supply chain than even [suppliers] can control.”
Legislation: U.S. and abroad
The conversation touched on legislative actions at the state level in the U.S., with Vermont being the first to enact a ban on PFAS-containing food packaging that went into effect July 1, 2023. On Feb. 28, 2024, the FDA announced the completion of a voluntary phase out in which grease-proofing substances containing PFAS are no longer being sold by manufacturers for food contact use in the U.S. market.


Maine’s PFAS-reporting law requires disclosure of certain materials in products. While packaging is currently exempt, according to a local news report, manufacturers are grappling with the difficulty

Install
The G&K-Vijuk Cartonac 2005 Folder has a hinged feeding table for easy access to the fold plates and rollers.
It is a simple upgrade from a Cartonac 91 or 2000!

715 Church Road, Elmhurst, IL 60126 • 630-530-2203 • www.guk-vijuk.com
leaflets in-line and insert them along with the product into cartons!
Fold
and synchronize a G&K-Vijuk Cartonac with your packaging line’s chain or toothed-belt drive.
Need help? Automate! PRODUCT Save on labor, time, and leaflet storage space! Call today to optimize production!


















The ingle- ource pharmaceutical packaging y tem provider with over a century of cu tomer ati faction
Excellence in:
• Individual mac ines
• Full pr duc i n lines
• In- use manufac uring
• Exclusive represen a i n f par ner rands





• Full line in egra i n
For:
• Oral s lid d se
• Liquid d se / Asep ic fill finis
• P wders
• Gummies
• Pers nal care pr duc s

Asep ic Fill Finish
C pping
C se/Tr y P cking
Coding
Co oning
Coun ing
Decon min ion
Feeding
Filling
Inser Feeding



Isol ion Technology
L beling
Line In egr ion
Robo ic P lle izing
Seri liz ion
S eriliz ion
W shing

©2023 ProM ch Inc 5600 Kier n Mon re l, QC H4S 2B5 PHONE 514-337-6990 EMAIL info@NJMPackaging.com WEB NJMPackaging.com
Vi it in
of identifying PFAS in their products and supply chains. That regulation also has a PFAS ban built in for 2030.
Outside of the U.S., new regulations include a mineral oil ban in France for food packaging, a PVC food packaging ban in Taiwan, and mandatory packaging reporting in Singapore.
Additionally, Gorr highlighted the proposed EU PPWR, the Packaging and Packaging Waste Regulation, evolving from the directive (PPWD). “This [regulation] is still the same content as the [directive]. You’re still required to report on what’s in your packaging waste and be accountable for it,” she said, which ties into extended producer responsibility and environmentally preferred purchasing (EPP) programs. “It’s not just regulations that we need to care about, it’s also what our customers are requiring of us. It’s a different type of requirement, but still a requirement. If they’re asking you for recycled content, I feel that we are obligated to supply that to them, if possible,” Gorr said.
Looking ahead, the life science packaging community must prepare for the possibility that medical device packaging and devices themselves may not remain exempt from these regulations indefinitely. Gorr, who owns the advocacy gap assessments for the REACH regulations at Boston Scientific, pointed out that while medical devices might currently be shielded from
certain bans, the industry must remain vigilant and proactive in its approach to regulatory compliance. “Medical devices are not going to be exempted forever,” she cautioned, suggesting that the industry should be proactive in engaging with legislators and educating them on the potential socioeconomic impacts of proposed bans.
What’s next?
As the regulatory environment continues to evolve, the industry faces the challenge of balancing compliance with innovation and sustainability. The session concluded with a forward-looking question posed to the audience: “What do you think the next PFAS will be?” As Bowman and Gorr highlighted, PFAS is certainly not the last material of concern that will either be banned or discussed in the media as an environmental or human health risk. Responses varied, indicating a wide range of concerns about potential future regulations on materials such as colorants, additives including titanium dioxide, BPA, PVC, styrenes, and nanoparticulates.
Understanding, adapting to, and anticipating the regulatory landscape will be critical to maintaining compliance and ensuring the continued viability of healthcare products and processes.

MATERIAL DEVELOPMENTS









Gamma sterilized and validated to a sterility assurance level of 10-6 per AAMI standards for healthcare products. Now Available Pre-sterilized! MADE IN USA Cleanroom Sponge The ORIGINAL TruCLEAN Aluminum Mop Frame Ultra-lightweight hybrid design for easy and efficient cleaning of hard to reach areas. www.perfex.com Shop Online! Booth # 1529 April 16th - 18th Visit Us: The pioneers of cleanroom disinfection and your trusted supplier of critical cleaning tools for controlled environments. 1-800-848-8483 Est. 1924 Specifically designed aquazone foam ideal for cleaning and disinfecting high-grade cleanroom environments. INNOVATIVE CLEANING TOOLS ® TruCLEAN
Refill System Cuts Plastic from OTC, Pharma Packaging
ANNE MARIE MOHAN, SENIOR EDITOR, PACKAGING WORLD
TOP THREE TAKEAWAYS
In every CPG category and market, efforts are underway to eliminate single-use plastic packaging when and where it makes sense. One area that has traditionally lagged behind, however, is the healthcare market, primarily OTC and pharmaceutical packaging. However, startup Cabinet Health has come up with a solution that it says has the potential to transform the industry, making it more sustainable—“from retail and pharmacy aisles to medicine cabinets and homes.”
Founded in 2018 by Achal Patel and Russell Gong, Cabinet Health: The Sustainable Healthcare Co. has developed a reusable and refillable packaging system whose components include a stackable, shatter-resistant and reusable glass bottle paired with refills in a compostable pouch. Its initial products, a line of OTC meds under the Cabinet brand name, first became available via direct-to-consumer (D2C). In May 2023, the portfolio, which
includes allergy, pain, cold and flu, digestive, and sleep remedies, launched at retail in 700-plus CVS pharmacy stores nationwide.
Gong shares that he and Patel came up with the idea for Cabinet while they were working abroad running a social innovation program for a consulting group that focused on identifying mission-driven and sustainable startups and helping them scale as businesses. “We saw firsthand the impact of the plastic crisis and saw other industries coming up with innovative sustainable packaging solutions, but no large healthcare players were successfully tackling this issue yet,” he says. “We combined our backgrounds in sustainable startups and medicine [Patel’s grandfather built one of the first acetaminophen factories in a small town in India] to reimagine people’s medicine cabinets.”
Reflecting on the scale of the plastics crisis, Gong shares that more than 194 billion plastic medicine bottles enter landfills each year. Further, one model found that humans consume 0.1 to 5 g of microplastics each week—the latter is roughly the size of a credit card. “The plastic waste crisis is a public health crisis,” he says.

Reusable packaging exhibits both form and function
In developing the reusable packaging system, Gong relates that Cabinet started from scratch, doing most of the work in-house, but did bring in some “amazing specialists and partners along the way.” The cube-shaped medicine containers, he shares, were developed with both form and function in mind. Available in a small and large size, they are made from glass, frosted to preserve the contents and shatter-tested for durability. They are also stackable for easy organization on consumers’ medicine shelves.
REUSABLE PACKAGING 22 | Healthcare Packaging • Spring 2024
1. Startup Cabinet Health offers reusable packaging and a refill system.
2. The portfolio of OTC products launched in CVS Pharmacy stores in 2023.
3
. A starter kit comes with one reusable container and one compostable pouch.
1. Cabinet Health’s portfolio of OTC products, including allergy, pain, cold and flu, digestive, and sleep remedies, initially launched at retail in 700-plus CVS pharmacy stores nationwide.


We don’t just make caps.









We make possibilities. We
We believe every project we work on is a way to create a dynamic customer experience. That’s why we design customized packaging solutions to fit exactly what your customer needs.
To find out how we can help, visit us at mrpsolutions.com

Says Gong, “With a low, square profile, the Cabinet system takes up a fraction of the space in your medicine cabinet than traditional bottles—a dream for minimalists, aesthetes, and anyone who could use the extra storage space.”
Topping each bottle is a colorful, reportedly child-resistant square plastic cap with a bayonet locking closure and integrated
magnet. Nestled inside a concave area on the top of the closure is a separate, removable magnet upon which is printed directions for use of the medication, expiration date, lot number, and other required information, as well as a QR code consumers can use to order medication refills.

For the refill packaging, Cabinet chose a proprietary material that is compatible with both home compost devices and industrial composting facilities. According to Gong, the company is also currently waiting on backyard-compostable certification. Along with the capsules or tablets, each refill pouch also holds a new snap-in magnet specific to that batch.
“Cabinet’s system uses glass, compostable flexible films, and aluminum,” Gong says. “We indexed on hyper-recyclable materials with compostable films being our path forward for earth-digestible materials. We looked into a whole host of other materials, but ultimately we needed to balance regulatory quality, cost, design, and sustainability.”
FLEXIBILITY
A single Columbia palletizer can handle all of these products and more with ease.


REUSABLE PACKAGING WWW.PALLETIZING.COM / 800 628 4065 LEARN MORE
2. Available in a small and large size, Cabinet Health’s reusable, sustainable packaging is made from glass, frosted to help preserve the contents and shatter-tested for durability.
3. The flexible pouch for refills uses a proprietary material that is compatible with both home compost devices and industrial composting facilities.

A pound per person of plastic saved annually
According to Cabinet, by switching to the brand’s refillable system, a consumer can eliminate up to one pound of plastic annually and hundreds of pounds of plastic in a lifetime. To arrive at that estimate, the company calculated that, on average, a single empty bottle is made of 3.75 oz of plastic and that, also on average, consumers purchase about five refills of a given medicine per year.

“Oftentimes, pills expire before they’re able to be used, or even worse, expired pills are consumed,” says the company. “Cabinet medicines come in lower pill counts than some bulk medicine offerings to minimize pill waste. With that in mind, we factored in a 0.85 to 1 ratio to account for us offering fewer pills per purchase than competitors in some cases.
“Based on the above variables, we estimate that when you buy a Cabinet medicine starter set [reusable bottle with medication plus one refill], you are eliminating one single-use plastic medicine



bottle immediately, plus four more via compostable medicine refills throughout the year. Multiply that by 0.85, and you are eliminating one pound of single-use medical plastic per year.”
The company’s products are manufactured in India, using FDA-approved active ingredients. Cabinet pays for carbon offsets to account for the transport of the product and make the supply chain less damaging for the environment.
The next step in Cabinet Health’s journey to “de-plastic pharmacy aisles and medicine cabinets across the country” is the use of its packaging system for prescription pharmaceuticals. In October 2023, they launched their refillable mail-order prescription services after a beta-test period. For this application, the company has designed a child-resistant pouch for refills.
In the future, Gong says Cabinet plans to offer packaging formats to accommodate other form factors, such as liquids and creams.











































































































REUSABLE PACKAGING Scan to learn more
4. A Cabinet medicine starter kit comes with one reusable container with pills or capsules and one compostable pouch containing product refills.
Humanoid Robots and the AI That Drives Them
ELISABETH CUNEO, SENIOR EDITOR, DIGITAL STRATEGY & ENGAGEMENT
TOP THREE TAKEAWAYS
1. AI allows robots to capture information, analyze data, make inferences, and act.
H2. The robot practices a task in simulation thousands of times over, optimizing performance.
umanoid robots, also referred to as general-purpose robots, use AI to perform the tasks they’re assigned. By now you know that humanoid robots are not just a thing of the future. In fact, you’ve likely seen videos of how they are currently being used in manufacturing and retail and could very well be deployed soon on the packaging line. But do you know what drives them? What makes them effective, and what technology enables them to “think” and move like humans? It’s none other than artificial intelligence (AI).
With a combination of advanced computer vision and machine learning, the robot can navigate complex environments and perform tasks like climbing stairs and grasping objects. The powerful machine learning program allows the humanoids to apply new experiences to known information—in effect, learning new experiences—and “learn” how to take this information and its own experiences into account for future actions. It’s this ability that lets the robot reason, draw conclusions, and ultimately, make decisions.

3. The future may lie in robots acting on voice commands without pre-programming.
Human-like intelligence
As the humanoid robots move around their environment, the AI is what allows the robot to capture information through cameras and LiDAR sensors, analyze that data, make inferences, and then move or act to the desired outcome. It’s these sophisticated tasks that allow the humanoid to resemble humans in their thinking.
As CEO & Co-Founder of Sanctuary AI, the robotics company that created humanoid Phoenix, Geordie Rose has said, “general-purpose robots must be able to sense, understand, and act in the world the same way we do.” And to do that, they require AI.
“While we’re immensely proud of our physical robot, the real star of the show is the underlying software. Carbon is our pioneering and unique AI control system, designed to give Phoenix humanlike intelligence and enable it to do a wide range of work to help address the labor challenges affecting many organizations today. It is a cognitive platform that provides Phoenix with the ability to think and then act to complete work tasks just like a person. Integrating modern AI technologies to translate natural language into action in the real world, Carbon features reasoning, task, and motion plans that are both explainable and auditable,” says Rose.
EVE, the humanoid robot from 1x , backed by OpenAI, the company behind ChatGPT, leverages AI to reason and perform tasks. EVE is referred to as an “embodiment chatbot,” generating answers to questions much like OpenAI’s ChatGPT does, using available information and patterns in data to give the best answer. OpenAI’s mission states that it aims to create “a computer that can think like a human in every way and use that for the maximal benefit of humanity.”
Part of thinking like a human is not only using data to come up with a solution, but also being able to handle many tasks and work autonomously (apart from other human help). Humanoids for use in manufacturing often perform tasks like picking and placing, and material handling tasks, apart from human influence while at the same time operating safely around humans on the line.
AUTOMATION 26 | Healthcare Packaging • Spring 2024
1. A critical component needed to make robots truly general-purpose is dextrous hands, says Sanctuary AI’s Geordie Rose.
How our name change now gives you more solutions.


Visit us at PackExpo East March 18th – 20th, booth #1329.
Joining the Rychiger family means more than just a name change for us at Nuspark, it’s a change that now offers you a wider range of innovative equipment solutions and emerging technologies.
As part of the Rychiger family, we combine our advanced secondary & tertiary packaging automation capabilities with their deep expertise in primary packaging equipment. Helping to make factories and plants smarter, more productive, and sustainable.
We are smarter together, offering over 125 years of combined experience.
smarter
Make it
Humanoid robots are described as general purpose in that they function in many different real-world environments. The humanoids are there to help do a little bit of everything, learn the environment, predict future needs, and go where they’re needed. But not all humanoids are developed solely for work. Developing more than just task-based learning, Hanson Robotics’ Sophia, is described as a social robot and is learning how to read human faces and expressions for ultimate human-like functionality.
The robot is powered by Hanson AI’s OpenCog, a cloud-based AI program that enables the robotics company to have large-scale cloud control of its robots. Sophia’s “brain” has deep-learning data analytics for processing the data that she extracts from her millions of interactions. She learns through both her own interactions, like humans, as well as what she is programmed to know.
Sophia describes it best, “My real AI combines cutting-edge work in symbolic AI, neural networks, expert systems, machine perception, conversational natural language processing, adaptive motor control, and cognitive architecture.”
Whether designed for social interactions or packaging and manufacturing help, humanoid robots have the ability to use their human-like intelligence to process data, learn from experiences, and accomplish tasks.
healthcare Packaging options

2. Apollo shines in tasks that are considered “gross manipulation,” or gross motor function, like grabbing something with both hands.
But first, training
As stated, the AI behind the robot is what is helping it learn new things and get better at them over time.
“The best way to think about Apollo is like an iPhone, or a personal computer. You can think of the AI as different applications that can go on top of it,” says Jeff Cardenas, Founder of Apptronik , the robotics company that created the humanoid robot Apollo.




















AUTOMATION
Efficient, sustainable,
designed for meeting strict medical standards. Experience simple operation and superior seal quality today. sales@formostfuji.com • 425-483-9090 • www.formostfuji.com Scan for details!
Cutting-edge packaging equipment:
and


1256 Nor h Church S ree Moores own, NJ 08057 PHONE 856-273-3377 EMAIL WLS@ProMachBuilt.com WEB Weilerl .com ©2023 ProM ch Inc , B ckground im ge of WLS l beler ken from NBC News Vi ls | Bulk syringes | Bo les | Ampules & c r ridges Au oinjec ors | Non-ph rm ceu ic l The mo t advanced pre ure en itive labeling and label printing olution available Pressure sensi ive la eling La el prin ing | Serializa i n Vial c ding | Inspec i n Hig es speed Bes l cal service C mpac f prin Simplici y & au n my For: Vi it in

The robot then learns these different applications through the idea of reinforcement learning, in which the robot practices how to do something in simulation and performs the task thousands of times over. In doing that, it is constantly optimizing how it performs the task over time, performing the task better and better.


“There are a lot of new software frameworks that have real potential to enable the robots to do a much wider range of tasks and learn much quicker. It’s early days for this, but we’re starting to adopt things like large language models, and in robotics there’s a version of this called large behavior models,” says Cardenas.
Through this idea, a human would show the robot how to do something, either by tele-operation or eventually by video and the robot would learn how to do that based on demonstration. Cardenas says that Apptronik is currently working on this and researching how it can be used to help Apollo do many more things over time.
“The idea is to build a general-purpose platform that is a software update away from doing something new,” says Cardenas.
Since humanoids are not humans, but rather machines, they of course have limitations, especially as it relates to experiencing a new problem or environment for the first time. As humans, we can anticipate what could occur even if we never experienced it, but these robots can’t do that unless that problem has already occurred, and it has “experienced” it. Meaning, each humanoid robot would require some sort of training to complete a given task.
As mentioned above, large language models and demonstration are the future in robotic task learning. In a similar vein, Sanctu-

We Believe in the Transformative Power of Automation



Are you struggling with floor space, optimizing production rates or excessive down time?
Let’s talk about your next project today!
AUTOMATION
3. Agility Robotics’ Digit uses its claw-like hands to grab and move empty tote boxes, removing this repetitive task from human workers.


Modular and scalable assembly equipment from Stevanato Group.





When pharma and CDMO partners need high precision, high quality, high yield assembly solutions, they turn to Stevanato Group.
Our GMP-compliant device sub-assembly and final assembly equipment can be configured to adapt to your production requirements, providing technology transfer from prototyping to high volume production. This modularity also enables for different formats or devices to be run on the same line, reducing time-to-market.
Visit us at booth #1319 during the Interphex show in New York 16-18 April!
stevanatogroup.com
“The idea is to build a general-purpose platform that is a software update away from doing something new.”—Jeff Cardenas
ary AI reported a system for training its humanoid Phoenix on specific tasks.
To get it right, the robotics company says that it films a particular task being done and then digitizes the entire event as a virtual environment. The AI can then practice the task in this virtual environment, perfecting the performance until it is ready for the physical world.
“AI gets better, smarter, and faster with more data, so the robots can complete tasks faster with more data and repetition,” says Rose.
While it’s the early days of general-purpose robots, Cardenas says that they are working on exciting new things that leverage proven robotic technology and AI to improve humanoid robotic function in the future.
Melonee Wise of Agility Robots , maker of Digit, describes that the future of humanoid robots and learning lies in the large
language model that enables the robot to understand a voice command and perform the task without any pre-programming (kind of like using ChatGPT).
“A lot of the places that we use learning or artificial intelligence is in the higher aspects of the system for learning a new tote, or a new model of a customer’s facility. Where it will become very powerful is in high-level programming of Digit. Instead of having an engineer type lines of code to explain the task, you’ll verbally ask Digit to do the task… That’s where we see long-term the value of solutions like ChatGPT and large language models,” says Wise.
Wise says that today Agility is using AI for identifying objects in the facility, but in the future the company could use it to simply tell a robot a command and it will perform the commanded task.
As more companies adopt AI into their daily lives, the technology will only improve, producing faster results and smarter innovation. Should we feel threatened by AI-powered robots as mere humans? Or can humanoid robots truly share the packaging line with us? For now, only the future holds the truth about whether or not humanoid robots will ever be widely adopted into packaging lines large-scale. Until then, keep your eyes open for a robot co-worker to come parading into your plant, and if you happen to see one, let us know.








AUTOMATION ©2024 ProMach Inc. Booth 2353 Javits Center, NYC Join Us at Exclusive representation of partner brands ProMachPharma.com plus
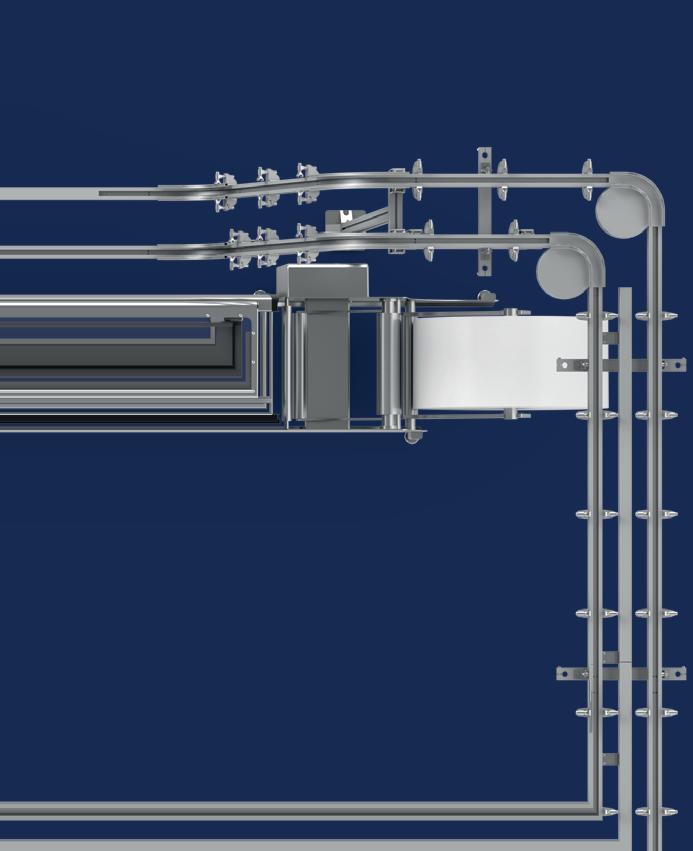
Protect Your Sterile Barrier System Rollstock
KEREN SOOKNE, EDITOR-IN-CHIEF
1. It’s important to correctly handle inventory prior to packaging.
OTOP THREE TAKEAWAYS
2. High temperature can impact the solubility of slip agents in resins.
n Sterile Packaging Day 2024 at MD&M West, Henk Blom, Todd McDonald, and Nick Packet discussed a roundup of sterile packaging updates from the Flexible Packaging Association’s Sterilization Packaging Manufacturers Council. This included their mission, efforts to educate policy makers on sterile packaging to help prevent unintended consequences from well-meaning legislation, and best practices for storing rollstock/pouches for sterile barrier systems (SBSs).
“In cases where only a partial roll is used, it’s a good idea to keep the outer packaging and re-wrap rolls if they’re returned to inventory for future use.”
On the latter topic, while many are rightly focused on what happens to the package after filling—on its way to the patient— rollstock storage considerations prior to packaging are also critical to success. Blom shared common tips from an SPMC white paper, Rollstock Storage and Handling, to protect your materials before packaging.

3. Consider conditioning rollstock before production.
Rollstock and pouches should be protected from the elements —kept clean and dry—with minimized exposure to direct sunlight. He noted that while it may sound like common sense, not every facility around the globe features a pristine warehouse, with climate and pest control. Dust and chemical contamination, which can be carried into cleanrooms via packaging, should also be minimized.
Rolls are typically individually wrapped on a pallet and should remain packaged until ready for use. In cases where only a partial roll is used, it’s a good idea to keep the outer packaging and re-wrap rolls if they’re returned to inventory for future use.
Storage conditions play an important role in the health of your rollstock. Stock is typically best stored at 50-80 °F (10-27 °C) but Blom said to consult your supplier for material-specific conditions. What happens if rollstock deviates from temperature requirements? Blocking (in which one layer sticks to another), wrinkling, or crushed cores can occur.
Blom noted another temperature consideration: slip agent behavior. “A lot of sealants—such as polyethylene and peelable sealants—are processed with low levels of what’s called a slip agent. This helps the materials move though the equipment. That material is designed to bloom to the surface and give you a low coefficient of friction so that you get good machinability,” he explained. “But as the temperature changes, the solubility of that slip agent in the material changes. As the temperature increases, the slip actually wants to go back into the material. And so your COF [coefficient of friction] will start to increase and the material will start to stick and slip as it’s moving through, for instance, a flow wrap machine.”
The good news here is that this process is reversible by placing the roll in cold storage, which will allow the slip to bloom back out to the material’s surface.
STERILE PACKAGING 34 | Healthcare Packaging • Spring 2024
↑ From left: Henk Blom, PAXXUS; Todd McDonald, Technipaq; and Nick Packet, DuPont Tyvek Healthcare Packaging.


















The USA’s leading provider of pharmaceutical and medical packaging solutions Sustainable packaging solutions built and supported locally Blister Machines Custom Automation/ Product Handling Rebuilds Vision Inspection Cartoning i it in 2346 Success Drive Odess , FL 33556 PHONE 727-232-8200 EMAIL Sale @Pharmawork .com WEB Pharmawork .com ©2023 ProM ch Inc. Medical device | Pharmaceutical Nutraceutical | Consumer goods Animal health
“…it’s important to always work closely with your material supplier to understand if a particular material has any specific storage requirements that need to be taken into consideration.”
While many common packaging materials like oriented PET, foils, highly engineered papers, and various polyolefins are not typically affected by humidity, there are certain nylons and papers that can be affected. Here, it’s important to check with your supplier, because certain papers can absorb and desorb moisture with changes in humidity. The good news, Blom said, is that this is reversible, but it’s preferable to ensure protection from the start.
Nylons can absorb 5 to 9% of their weight in moisture. “That’s a significant amount of moisture, and it can change the physical properties quite drastically. If you dry it out completely, it becomes somewhat brittle, and then as it absorbs more moisture, it can become much more stretchy,” Blom said. “In roll form, it can actually start to wrinkle because it also expands the material a bit. Again, this is reversible, so you can put it in a drier environment and that moisture will eventually equilibrate back out.”
The location of the nylon layer matters. “If your nylon is in the middle of a multi-layer, coex structure, it’s not actually seeing the ambient humidity, it’s protected by the outer layers. So it’s not as
—Henk Blom
much of a concern there,” he added. “But if it’s the outer layer of the laminate, or the standalone material with a heat seal coating on it, for instance, we generally would wrap that in the metallized polyester to get maximum barrier protection.”
If there’s a big difference between storage and floor conditions, Blom said it’s a good idea to condition your rollstock, allowing 24 to 48 hours for the material to equilibrate to ambient conditions before packaging. “Some materials will be more susceptible than others,” he noted, adding that if COF is an important consideration, then conditioning will help stabilize the material. Blom hammered home that some materials have unique requirements that may not be covered by the more common storage recommendations he shared. “So, it’s important to always work closely with your material supplier to understand if a particular material has any specific storage requirements that need to be taken into consideration,” he said.
View SPMC’s white papers, including Rollstock Storage and Handling, at sterilizationpackaging.org/learning-tools





STERILE PACKAGING




M al D X-Ray C kw g g V n n n T a k & T a n
C m z Ma al Ha l gGl bal F l -ba S v n S al za S l n

OS L v D m a
B
4www.m . m/
A w a
This exclusive Leaders in Healthcare Packaging section includes profiles and product listings for a variety of companies serving the healthcare and pharmaceutical industry.

SPECIAL SECTION 2024

Anritsu Product Inspection & Detection

701 Innovation Drive, Suite A, Elk Grove Village, IL 60007
847-419-9729
detectionperfection@anritsu.com
www.anristu.com/product-inspection
Anritsu provides high-quality inspection systems for the Pharmaceutical and Nutraceutical industries to help ensure the safety and integrity of your products. Our systems are designed to detect foreign objects as well as verify product integrity and quality in tablets, capsules, cartons, cases, sachets, sticks, patches, tubes, pouches, bottles, cans, and medical devices.
With our X-ray systems, it’s possible to detect even the smallest metallic and non-metallic contaminants in products even in sealed areas. Anritsu X-rays also perform a wide range of quality checks on opaque and thin packaging. All models come with standard HD imaging and safety design features that ensure minimal leakage.
Our line of SSV Checkweighers features models that are specifically designed to check the exact weight of capsules, small bottles,
aerosol inhalers, blister packs, or cases with the greatest accuracy. We also offer multi-lane checkweigher models that can easily handle high production volumes of capsules, pouches, and other products.
The Anritsu M6 Tablet Metal Detector can detect metal contaminants as small as 0.22mm in tablets and capsules. In a pharmaceutical production line, this equipment can inspect up to 30,000 items per minute without being affected by vibrations, electromagnetic noise, or static electricity.
Combination systems consist of a checkweigher and a metal detector united into a single unit – saving space and reducing the total cost of ownership. The option of vertical metal detector heads allows for the inspection of vertical packaging such as bottles or stand-up pouches.

There is no greater priority than the safety and security of your customers. Our commitment to developing unique inspection solutions of the highest value advances the food and pharma industries and assures consumer safety. It’s our goal to continue a level of precision, reliability, and support that truly advances the quality of your product and the efficiency of your operation.
Delivering a Positive Anritsu Customer Experience is at the core of everything we do. We are a global team, supporting each other’s families and those of future employees. We are dynamic and agile. The market is ever-changing, and we are too! We hold ourselves to the highest standard – always striving for flawless execution. We strive to maximize the performance of our technology and ultimately that of our customers.

See our ad on page 6 Spring 2024 • Healthcare Packaging | 39

Antares Vision Group
200 Century Parkway, Ste. C, Mount Laurel, NJ 08054
856.780.3465
sales.us@antaresvision.com
www.antaresvisiongroup.com
Antares Vision Group’s range of Inspection Machines is designed to guarantee your product’s quality, integrity, and safety and meet regulatory requirements. Our new generation of vision technologies allows product inspection of any container and product content from any angle during different production phases.
AV Group’s Prefilled Syringe Inspection Machine can handle up to 400 pieces per minute, processing SVP glass and plastic syringes up to 24mm in diameter. It inspects liquids with a wide range of viscosities, from water-like products to emulsions and gels. An additional camera mounted on a secondary carousel yields 100% inspection of the syringe surface. Fast, smooth single-point handling minimizes the risk of product breakages and scratches, thanks to individually motorized grippers and guideless conveying with vacuum-operated starwheels.
The VRI VI series specializes in precise, highspeed vial inspection of up to 400 liquid-filled
glass containers per minute, combining sophisticated particle and cosmetic detection with technology-driven closure integrity verification. The unit can inspect liquids in various viscosi-ties, including water-like, oily, suspension, gel, emulsion, lyophilized, and powder products. Vials are also inspected for cosmetic defects such as crimping quality, flipoff color, neck-shoulder sidewalls, and stopper position. Molded glass and plastic containers also can be inspected.
DIAMIND, an integrated ecosystem for comprehensive production management, offers holistic transparency and production optimization. It operates at the line, factory, warehouse, enterprise, and supply chain levels through sophisticated inspection systems to end-to-end traceability via cloud-based data management.

Antares Vision Group is driving the digitalization of products and supply chains by leading traceability, inspection, and integrated data management. AV Group helps companies achieve safety, quality, efficiency, and sustainability, enabling Trustparency®.
DIAMIND, AV Group’s integrated ecosystem of solutions, simplifies the production environment and supports business growth by enabling a tailored data-driven journey by applying artificial intelligence and blockchain technology.
AV Group operates in the Life Science (pharmaceuticals, medical devices, and hospitals), Beverage, Food, Cosmetics, Chemicals, and Packaging industries. The Group operates in 60 countries, employs over 1,300 people, and has a consolidated network of over 40 international partners.


See our ad on page 11 40 | Healthcare Packaging • Spring 2024

BELL-MARK

331 Changebridge Road, Pine Brook, NJ 07058
973-882-0202
connect@bell-mark.com
www.bell-mark.com
Medical Device and Pharmaceutical Packaging - The accuracy of coding on your product package is crucial. Expiration dates must be clearly identifiable and easily human-readable. Bar codes must be verifiable and scannable. There are no exceptions. BELL-MARK addresses these needs with systems that provide the highest quality print available on cartons, medical paper, Tyvek, poly, and foil substrates.
BELL-MARK has successfully satisfied UDI GS1 requirements for many of our customers. We understand the UD1 rules and regulations and have experience in a wide range of applications. From retrofitting band sealers to Form, Fill, Seal packaging machines, we have the expertise to ensure your codes are verifiable and scannable and meet the necessary regulations.
Meat, Poultry and Prepared Foods PackagingEliminate costly labeling by printing directly onto
your package or replace your CIJ printer with a maintenance-free alternative. BELL-MARK offers a full line of washdown tolerant printers, including both thermal transfer and thermal inkjet technologies. Print variable data, graphics, nutritional facts, bar codes, lot/expiration dates and safe handling instructions on almost any surface. Rest easy knowing your printer is IP67 rated and can be washed down and sanitized without complicated protective procedures.
Industry-Leading Reliability - BELL-MARK’s in-line printing and coding equipment delivers industry-leading reliability and durability, time and time again. High-resolution print quality that consistently affords you the peace of mind in knowing that your codes will not fail. While other companies may claim it, BELL-MARK leads the way in the lowest cost of ownership and highest return on investment.

For over 65 years, BELL-MARK has been developing innovative solutions for on-demand package and carton printing applications in the medical device, pharmaceutical, prepared foods, meat, bakery, dairy, and poultry packaging industries. BELL-MARK offers many in-line printing technologies, including piezo inkjet, thermal inkjet, thermal transfer, flexographic, and ink coder - all designed with cost-effectiveness and user flexibility in mind.

See our ad on page 25 Spring 2024 • Healthcare Packaging | 41

CCL Healthcare

120 Stockton Street, Hightstown, New Jersey, 08520
609.490.3700
CCLHealthcare@cclind.com
CCLHealthcare.com
CCL Healthcare stands as a global leader in printed secondary packaging, boasting 37 cGMP facilities worldwide and continuously expanding. Our dedicated team of experts is solely focused on manufacturing innovative packaging solutions for the pharmaceutical, medical device, biotech, and life science industries.
Smart and Intelligent Packaging: Since pioneering the first RFID label on a commercially available pharmaceutical product in the early 2000s, CCL Healthcare has remained at the forefront of innovative smart packaging solutions. Today, we offer fully integrated hardware and software solutions for IoT, RFID, NFC, and dualfrequency packaging.
Clinical Labeling Expertise: Experience the powerful alliance of Clinical Systems and Faubel under the banner of CCL Clinical! Leveraging over two decades of expertise and operating across
various manufacturing sites, we emerge as your premier partner for clinical trial labeling. With a combined portfolio of distinctive offerings, global reach, and dedicated local assistance, CCL Clinical sets the standard for excellence in clinical trial labeling services.
Functional and Specialty Labels and Products:
CCL specializes in complex multi-web constructions to create functional products and applications that surpass the capabilities of other converters. Our strength lies in the unique ability to print adhesive on uncoated material, empowering our engineers to design solutions for our customers’ most complex projects.
Specialization in Folding Cartons: Our global folding carton production specializing in low volume high mix products. We offer no MOQ;s, short lead-times and industry leading quality.

CCL Healthcare offers comprehensive RFID solutions, featuring customized highperformance inlays, hardware, software, and a seamless, non-intrusive bolt-on solution for high-speed RFID packaging lines called RFID Line Management or RLM. RLM is the most advanced non-intrusive bolt-on RFID encoding and validation solution on the market. RLM fully integrates into your current packaging line, achieving encoding speeds of 700 items per minute.
Our commitment to patient safety drives us to develop cutting-edge RFID inlays for the pharmaceutical industry. We firmly believe that if our inlays can prevent even one patient death, aid in a drug recall, or ease the burden on healthcare providers by allowing them more time to focus on patient care, then our efforts are justified.
Failure is not an option for us that is why we offer optimized RFID Inlays tailored to your medications dielectric properties.

See our ad on page 15 42 | Healthcare Packaging • Spring 2024


Manufacturer of Monoblock Style Filling and Closing Equipment for volumes from microliters to 125ml in glass and plastic containers.
Chase-Logeman Corporation
303 Friendship Drive, Greensboro, NC 27409
336.665.0754
info@chaselogeman.com
ChaseLogeman.com
For over 63 years, Chase-Logeman Corporation has been building monoblock-style filling/ closing machines and support equipment. From our offices in Greensboro, North Carolina, ChaseLogeman custom designs every machine to maximize production quality without sacrificing throughput.
This equipment reliably serves the Chemical, Cosmetic, Diagnostic, Homeopathic Medicine, Nutraceutical, and Pharmaceutical markets with machines that are easy to use and durable.
All of Chase Logeman’s machines are washdown capable. There are designs available for flammable materials and processes requiring isolators. Every machine begins from a cGMP thought process.
Chase Logeman incorporates filling, plugging, capping, and labeling capabilities into the
monoblock machine as requested to save space and minimize the number of operators needed. Product changes can be accomplished in 15 minutes or less, often without tools.
Specializing in fill sizes from microliters to 125ml, every Chase-Logeman machine prevents repetitive stress injuries by incorporating proven automation processes into the machine.
Contact Chase-Logeman and let our years of experience help automate your filling process.


See our ad on page 9 Spring 2024 • Healthcare Packaging | 43

Columbia Machine, Inc.
107 Grand Blvd., Vancouver, WA 98661
360.694.1501
pallsales@colmac.com
www.loadtransfer.net
REDUCE CONTAMINATION—The
Load Transfer Station (LTS) product line, offered by Columbia Machine, helps to reduce contamination from entering production areas, streamlines operations & reduces costs. Columbia manufactures a complete line of pallet transfer solutions including stand-alone stations to fully automatic solutions that allow pallet load transfer to be completed in less than one minute, without requiring the operator to leave the safety of the forklift.
INDUSTRY LEADING STANDARD SAFETY—
Columbia Machine’s LTS solutions come standard with the latest safety features. These include Category 3 electrical safety components, upstacker guarding with A-B SensaGuard RFID interlocked door switches, multiple emergency stops, and muted light curtains on the automated systems. Every Columbia Machine LTS has been engineered to exceed the requirements of
pharmaceutical processors who are working to meet FSMA regulations.
Flexible Product Handling
Columbia’s LTS is capable of transferring products that are packaged in cases, super sacks, glass vials, pails, barrels, drums and bags from one pallet type to another, including Plastic, Chep and GMA pallets that are commonly used in both receiving and shipping applications.
Columbia Machine, Inc.
The Load Transfer Station (LTS) product line is part of the Palletizer Division of Columbia Machine, a leading American palletizer manufacturer. For more than 80 years, Columbia has manufactured complete palletizing and material handling solutions.

The mission at Columbia Machine is to be the preferred supplier of engineered product solutions in the targeted markets we serve. We provide exceptional customer value through strategic marketing, innovative product development and unparalleled customer service. We value safety, integrity, trust, fairness, professionalism and teamwork in relationships with our customers, employees, business partners, suppliers and shareholders. We “always” see our business through “the eyes of our customers,” and provide them with superior solutions through innovation, quality, reliability and continuous improvement.


See our ad on page 24 44 | Healthcare Packaging • Spring 2024

Columbia/Okura LLC.

301 Grove Street, Vancouver, WA, 98661
877.204.7444
COLLCSales@colmac.com
www.columbiaokura.com
Columbia/Okura LLC is a robotic palletizing integrator, applying expert knowledge and tools to solve customers’“End of Line” production challenges for a diverse range of businesses, products, and applications. The historical success of our company can be attributed to an exclusive focus on end of line palletizing systems and applications. Columbia/Okura LLC offers robotic palletizing solutions comprised of dynamic products and services aimed at increasing our customers end-of-line packaging efficiency, promoting safety, and reducing their overall cost of production. Like all industrial robots, Columbia/Okura robots are programmable, automating tasks that are often repetitive or harmful to workers. They have the ability to lift and move heavy loads quickly while keeping products safe and stacking them to be shipped or even neatly to be sold in stores.
The Columbia/Okura miniPAL® is a collaborative palletizing robot built for flexibility and
ease of use. It features a range of cobots by Universal Robots, that can handle up to 30KG. The miniPAL® is equipped with Pally software, an intuitive pattern-building program—created in partnership with Rocketfarm—that is easy to operate and program for various product types, including cases, spot packs, trays, bundles, and plastic bags. With 25 years of equipment design and system integration experience, we have the knowledge to offer the right solution for the job. We offer complete and professional services; from initial system design proposals through project start-up and commissioning, as well as after sales support.
Our integrated lines offer an end-to-end solution for fully automated bagging and robotic palletizing line solutions from a single, US-based, OEM provider. Our service offering includes integration of manual bagging machines, openmouth bagging machines, valve bag fillers, air packers, impeller packers, and pallet dispensers.

We design, integrate, and commission end of line robotic palletizing solutions for most major industries. For over 25 years, we have been a leading provider of robotic palletizing systems by delivering custom engineered solutions to meet demanding customer requirements. With over 1000 robotic palletizers installed throughout North America and beyond, Columbia/Okura is unparalleled in the industry. Columbia/Okura applies its expertise via defined production line surveying and problem solving processes that allow us to understand our customers’ challenges, identify critical need areas, and design and apply the appropriate solution to lower the cost of ownership and generate profitable returns for our customers. Decades of industry leading experience and expertise have allowed us to put safe practices and operations at the forefront, enabling us to deliver the safest solutions in the industry.

See our ad on page 30 Spring 2024 • Healthcare Packaging | 45

ESS Technologies, A Pacteon Company

3160 State Street Blacksburg, VA 24060
540.961.5716
info@esstechnologies.com
www.esstechnologies.com; www.pacteon.com
ESS Technologies, a Pacteon company, develops cutting-edge packaging solutions that enhance the efficiency, accuracy, and safety of pharmaceutical and healthcare product handling processes. Our solutions include:
- Horizontal Cartoners
- Vertical Cartoners
- Tray Erectors & Loaders
- Case Packers
• Top-Load
• Side-Load
• Bottom-Load
• Wrap-around
- Proprietary TaskMate Robotic Systems™
- Robotic Palletizers
• Single Cell
• Multi-Cell
• Collaborative
- Operator Training
- Technical Support
- Preventative Maintenance Plans
ESS specializes in the specific requirements of the pharmaceutical and healthcare sectors. Our high precision end-of-line robotic packing solutions help to streamline operations, improve product quality, and ensure compliance with industry regulations.
- Serialization and track-and-trace capabilities
- Product inspection, sampling, and rejection
- Medical device & diagnostic test kit assembly
- Blister packaging

It takes expertise to integrate and support reliable end-of-line automated packaging solutions. Pacteon Group companies offer a responsive and committed single source solution to design, integrate, and service your product handling and automation needs.
Turn to Pacteon for your end-of-line packaging solutions:
- Cartoning
- Case & Tray Packing
- Robotic Palletizing
- Automatic & Semi-Automatic Stretch Wrapping
We Make It Right.

See our ad on page 7 46 | Healthcare Packaging • Spring 2024

Formost Fuji Corporation
905 80th Street SW, Everett, WA 98203
425.483.9090
sales@formostfuji.com
www.formostfuji.com
Your Trusted Partner for Efficient Packaging Solutions in Healthcare. Formost Fuji is a leading manufacturer of horizontal flow wrap machines and bagging machines. Our equipment stands out for its quality, dependable parts and service, and engineering expertise. With a dedicated focus on efficiency and reliability, our form-fillseal wrappers (HFFS) deliver strong, dependable packages.
Our flow wrappers are designed with userfriendliness in mind, featuring an easy-tooperate HMI with graphics and step-by-step instructions. Customizable for simplicity, the HMI allows for one-touch changeover using icons or product photos. Our innovative flow wrapper also offers a time-saving film threading process and a shortened film route, saving money. Easy access for maintenance and sanitation is ensured with the tilting center fin seal unit.
Experience superior sealing with our B16 Box Motion End Seal Technology, delivering highperformance hermetic seals on challenging films.
The Formost Fuji Bagging machine combines performance, speed, and versatility, perfect for medical drapes and other products. Our HighSpeed Box Motion horizontal wrapper offers efficiency, tight wrapping, and low vibration at high speeds.
Formost Fuji provides product and film testing to save time and money in production. Ask about our training programs to assist your team in operating and maintaining your Formost Fuji equipment.
Trust us to help you build the right solution for your packaging needs and be there for support for years to come.

Dedicated to Your Packaging Needs
At Formost Fuji, we prioritize you, the customer. With 60 years of industry experience, we are committed to delivering exceptional value and surpassing your expectations in design, manufacturing, and service.
Our team of experts collaborates closely with you throughout the process to provide reliable packaging solutions, including horizontal wrappers, baggers, automation solutions, and special applications. We take pride in producing sustainable and efficient packaging equipment for the healthcare industry.
“First and Formost”, you can rely on Formost Fuji for top-tier packaging solutions. Contact us today to find out how we can meet your packaging needs.


See our ad on page 28 Spring 2024 • Healthcare Packaging | 47

G&K–Vijuk International

715 Church Road, Elmhurst, IL 60126
630.530.2203
info@guk-vijuk.com
www.guk-vijuk.com
To save time and labor, invest in GUK packaging-line equipment that runs on the packaging line’s drive. GUK–Vijuk Cartonac Leaflet Folders enable you to fold leaflets in-line and immediately insert them into cartons along with the product. The servo driven GUK Cartonac 2005 has a hinged feeding table for easy access to the fold plates and rollers. Improved suction rollers and air blasts effects efficient feeding of products—up to 3 thicknesses. The PLC controlled Cartonac 2003DS sets up easily and quickly and is servo driven for better control and speed.
Pre-folded leaflets can be fed in-line for insertion into cartons with GUK PA21 Leaflet Feeders. The PA15 model can rotate leaflets 90º for feeding, while mounted parallel to the packaging line to save space. These may also be used with gluing or tabbing equipment in off-line leaflet bundling systems.
GUK–Sigma Pick & Place Stations precisely tip flat or 3-dimensional items onto products in production lines.
Make spine-glued booklet leaflets on the GUK FA53 Folder. Fold large med-guides on sheets up to 28-3/4 inch wide on the GUK FA73 Folder. Choose from a number of G&K–Vijuk Outsert Systems with varying capabilities from folding outserts with up to 90 panels, to up to 350 panels.
Meet TRACK & TRACE requirements with the G&K–Vijuk CTM Coding and Serializing Station. Print unique or standard 1- and 2dimensional codes on outserts. A qualityverification system logs all results, good and bad, for a tab on missing numbers in a series.
Save time and labor with the GUK–Sigma PPM Auto Stacker, which automatically collects and packs outserts compactly into trays.
GUK RS Roll-Fed Folders save paper, cutting, and labor costs—and the probability of leaflet mix-up.

World leader in outsert-producing machinery, G&K–Vijuk has been specializing in miniature-leaflet folding solutions for over 40 years.
Member of the GUK Group headquartered in Germany (manufacturer of folding machinery since 1949), we continue to assess the needs of the pharmaceutical industry, as shown by recent developments in machinery to fold a greater number of panels and fold wider sheets—both for more print space on a single leaflet; and machinery for bundling leaflets for dispensing even more information on two to four leaflets.
GUK recently acquired MB Bäuerle, manufacturer of automated folding and inserting systems in Germany, and Sigma Engineering, manufacturer of pick and place product-handling machinery in the Netherlands to further our goal to provide innovative, time-saving equipment to improve our customers’ efficiency.

See our ad on page 18 48 | Healthcare Packaging • Spring 2024
Greydon A ProMach Product Brand
391 Greendale Road, York, PA, 17403
717.848.3875
Greydon@ProMachBuilt.com
www.greydon.com
Greydon provides advanced printing systems, including thermal transfer, digital, thermal inkjet, continuous inkjet, and code daters. These systems can be custom built to withstand harsh environments and are UDI-compliant for medical devices, pharmaceuticals, personal care, consumer products and many more industries.
Greydon solutions include advanced highspeed digital printing systems for a fully digital workflow to handle multiple SKUs and short production runs in today’s modern packaging lines. With traversing and inline printing capabilities, versatile mounting options, and scalable designs, our solutions can print graphics directly onto packaging substrates and interface with serialization and manufacturing software to ensure complete UDI and DSCSA compliance –compatible with GS1 and HIBCC standards.
Printing & Coding Solutions For:
• Pharmaceutical / Nutraceutical Packaging
• Medical Device Packaging
• Blister Packaging
• Flexible Packaging
• Cartons & Cards
Printing & Coding Capabilities:
• Digital Printing
• Inline Flexographic Printing
• Traversing Thermal Transfer Printing
• Late-Stage Customization
• Ink & Consumables

As part of ProMach Labeling & Coding, Greydon designs, manufactures, and integrates innovative solutions, ranging from simple to complex product identification, tracking, brand protection, and compliance with industry standards in today’s modern packaging lines.
Industries We Specialize In:
• Pharmaceutical
• Nutraceutical & Vitamin
• Medical/ Medical Device
• Personal Care & Cosmetics
• Animal Health
• Consumer Products


See our ad on page 5 Spring 2024 • Healthcare Packaging | 49
Harpak-ULMA Packaging, LLC
85 Independence Drive, Taunton, MA, 02780
800.813.6644
info@harpak-ulma.com
www.harpak-ulma.com
Harpak-ULMA packaging systems can handle all your medical primary and secondary packaging equipment requirements – from single components through completely automated systems. Our full-service solutions address installation, training, spare parts, service and customer support, while capabilities span robotics and automation, thermoforming, tray sealing, filling, flow pack, stretch, blister, skin pack, and vacuum. Our secondary equipment can prepare products for retail ready displays, create multipacks for bundling of products, and erect and load cartons to get your product out the door. We provide the total solution – from beginning to end, product to pallet – for the medical industry.
Our medical packaging solutions offer maximum product protection, hermetic sealing, the ability to customize the package shape, blister packs for retail sale, hygienic individual and multipacks, easy open options, and protection during transport and handling. These solutions
meet the strictest standards required by the medical sector. We can meet them all, like ISO 11607, EN 868, ASTM D1585, ASTM F2097 and ASTM F3475-11. Packages are suitable for sterilization processes and maintain these conditions until opened. You can also expect to be in accordance with CFR 21 Part 11 for documentation.
We have in-process controls like vision inspection for product and printing, product in package height detection, and automatic rejection. Process monitoring includes alarm conditions for critical parameters out of range like time, temperature and pressure. There’s also a data integrity option for track and trace of operator in the HMI, plus trending of critical parameters. When it comes to calibration, our critical parameter equipment comes calibrated from the factory, and access points are provided for routine calibration.
Look to Harpak-ULMA Packaging to prototype, design, build, implement and maintain packaging automation solutions for today’s complex, rapidly changing packaging landscape.


See our ad on page 14 50 | Healthcare Packaging • Spring 2024

Heat and Control, Inc.
21121 Cabot Boulevard, Hayward, CA, 94545
510 259 0500
info@heatandcontrol.com
www.heatandcontrol.com
Detect metal contaminants with confidence by choosing the world’s leading metal detection solution. CEIA®, is a leading innovator of industrial metal detection systems for products such as powders, capsules, tablets, and liquids. Quality control is at the core of CEIA development of the most advanced electronic and mechanical technologies for detection of contaminants accidentally present in products. CEIA metal detection systems meet high level industrial metal contaminant detection standards. Are fully-HACCP and GMP compliant, ISO 9001 certified and constructed of EC and FDA approved materials and is a available for free-falling product applications.
Find what you don’t see with the latest Ishida X-ray solutions. The photon counting dual energy IX-PD series X-ray machine employs an alternative sensor and accompanying image processing technology to give our highest sensi-
tivity and accuracy of low-density and minute foreign object contaminant detection. This technology differentiates with high accuracy between product and foreign objects, reducing the rate of erroneous detection. All models offer exceptionally sensitive foreign body contaminant detection and additional benefits such as the ability to identify damaged and missing products or components, helping the pharmaceutical industry achieve a rapid return on investment.
Check out Ishida high-precision weight checking you can rely on. Checkweighing is key for delivering what consumers expect by providing accurate verification of a package’s weight or count and detecting missing components. Promote quality control and customer satisfaction with Ishida’s extensive know-how in weighing technology.
Providing safeguards that help ensure your products match the prescription. Anywhere along the line, protect your consumer and your equipment. Efficient detection of foreign objects is critical to consumer safety, brand survival, and will also protect machinery and prevent downtime. We offer a complete line of metal detectors, checkweighers, and X-ray inspection systems from our strategic partners: CEIA and Ishida.
Providing sales, service and spare parts expertise across the globe for metal detection, X-ray and checkweighing anywhere along a production line.


See our ad on page 13 Spring 2024 • Healthcare Packaging | 51

James Alexander Corp.
845 Route 94 Blairstown, NJ 07825
908.362.9266
info@james-alexander.com
908.362.5019
www.james-alexander.com
James Alexander Corp. has expanded its operations floorspace, adding 18,000 square feet to its warehousing capacity and 2,000 feet apiece to manufacturing and office space. The expansion provides additional capacity for servicing key markets including pharmaceutical (OTC & Rx), medical devices, health & beauty products, first aid and diagnostics.
Currently, James Alexander is developing new and innovative materials for JAC’s plastics ampoules. Among other aims, the goal is to make the containers compatible with alcohol-based liquids.
JAC’s patented single-use plastic ampoules, which have undergone various enhancements since their initial market introduction, are available in a variety of colors and with an array of applicators, offering single-handed activation in a customizable format. Meanwhile, the company’s glass ampoules can be filled and assembled in single-use swab or dropper packages. JAC also recently introduced a winged device, THE ACTIVATOR™, which provides easier activation for these glass formats. Other services include autoclave sterilization for glass ampoules, blister packaging and formula compounding.
Plastic Unit-Dose Dispensing Systems
James Alexander Corp.’s revolutionary plastic ampoule combines style and ease of use through single-handed activation. With just a gentle squeeze, the inner membrane ruptures, allowing the contents to be dispensed by the user. The plastic ampoule is available in sizes up to 5ml, as well as a range of colors and applicators.
Unit-Dose Glass Swabs
James Alexander Corp.’s unit-dose swabs offer the stability of glass in one- or two-part systems allowing for convenient application of pharmaceuticals and health aids. JAC also produces single-use glass ampoules for inhalation and dropper tip assemblies for the dispensing of liquids.
The DuoDispersion System® Tandem Package
James Alexander Corp.’s DuoDispersion System® is a refinement to the company’s well-regarded tandem swab package. Safe and easy to use, the tandem dropper or swab can be customized to hold two separate liquids, or a powder and a liquid; each individual formula is hermetically sealed in its own ampoule. The DuoDispersion System® can hold a combined volume of 1.2mls that are kept separate until the point of application. Two versions of the DuoDispersion System® are available: a dropper tip and a swab for topical application.

CEO
Francesca Fazzolari
PRESIDENT
David Robinson
Located in northern New Jersey, James Alexander Corporation (JAC) is a leading contract manufacturer and custom filler of single-use crushable glass and plastic ampoules. Founded in 1976 by Francesca Fazzolari and Alexander Davidson, JAC is a privately-owned, ESOP company that still services several of the same customers it originated with 45 years ago.
James Alexander Corp.’s manufacturing facility features unique, company-designed equipment and produces its patented plastic ampoules, among other product offerings. The company makes great efforts to ensure that most of its components are made in the USA, aligning with its goal of investing in local communities, regional job markets and the American manufacturing sector at large.

www.james-alexander.com See our ad on page 1 52 | Healthcare Packaging • Spring 2024
Kingwills

16F, Building 2, Bingjiang International Plaza, 1062 Yangshupu Road,Shanghai, China
info@kingwills.com
www.hypak.com
The strength of Flashspun Hypak™ medical device custom packaging materials are much stronger than other materials because these kinds of medical packaging materials are made of 100% virgin HDPE through flash spinning technology, and are continuous filament product with an ultra-fine fiber mesh structure, so the physical properties of our custom healthcare sterilization packaging are also very strong.
Flashspun Hypak™ custom medical packaging materials are resistant to tearing, puncturing, and folding, and can withstand special transportation and storage conditions. These types of medical device packaging materials can be used to wrap irregular and sharp medical devices without breaking, thus ensuring the sterility and integrity of medical devices.
Due to extremely dense structure and tiny pore size of these types of medical sterilization packagings, Flashspun Hypak™ custom medical packaging materials not only block the penetra-
tion of various liquids, but also provide excellent dust and bacteria resistance against various bacteria, microorganisms, particles, and aerosols, with excellent bacteria resistance data, making it impossible for bacteria to penetrate the sterilization packaging of surgical instruments and reach the instruments.
The good permeability is compatible with various sterilization methods such as ethylene oxide, gamma ray, electron beam, steam (controlled conditions), and plasma hydrogen peroxide.
Flashspun Hypak™ custom medical packaging material does not produce any debris or fibrils, or smaller particles that are not visible to the naked eye, when unwrapped, and therefore this light weight waterproof material do not contaminate the device itself.
Disposable medical sterilization packaging needs to guarantee the continued sterility and integrity of medical devices before they can be used.
Our flashspun fabric of Flashspun Hypak™ have been widely applied in autoclave sterilization bags, dental sterilization bags, dry heat sterilization packaging, eto sterilization packaging and so on. Why do many medical device manufacturers prefer to use Flashspun Hypak™ as medical device packaging materials?
Because Flashspun Hypak™ custom medical packaging materials offer a good balance between the contradictory properties of lightness, strength, and breathability, as described below.

See our ad on page 4 Spring 2024 • Healthcare Packaging | 53

Klockner Pentaplast

3585 Klockner Road, Gordonsville, VA 22942
3585 Klöckner Road, Gordonsville, VA 22942
540-832-3600
kpainfo@kpfilms.com
www.kpfilms.com
Focused on delivering its vision: The Sustainable Protection of Everyday Needs, kp is a global leader in rigid and flexible packaging and specialty film solutions, serving the pharmaceutical, medical device, and shrink sleeve label markets, amongst others. With a broad and innovative portfolio of packaging and product films and services, kp plays an integral role in the customer value chain by safeguarding product integrity, protecting brand reputation, and improving sustainability. kp’s “Investing in Better” sustainability strategy solidifies its commitment to achieving ten clear targets for longterm improvement by increasing recycling and recyclability of products, cutting carbon emissions and continuous improvement in employee engagement, safety, and diversity, equity and inclusion. kp has earned a gold rating from EcoVadis, the leading platform for environmental, social, and ethical performance ratings, putting kp in the top 3% of companies rated in the manu-
facturing of plastics products sector. Founded in 1965, kp has 30 plants in 18 countries and employs over 5,700 people committed to serving customers worldwide in over 60 locations.
We have invested heavily across all the market segments we service, in the development and production of recyclable and recycle3d content packaging films. Chief among these markets is the pharmaceutical blister films market. Our kpNext® R1 pharmaceutical blister film was the first to market as a certified recyclable blister film in the RIC 1 recycling stream and remains the premium choice for many major pharmaceutical brands globally! We have since expanded the kpNext® family to include a certified recyclable polypropylene blister film that features enhanced barrier characteristics, called kpNext® RB5. When it comes to premium, sustainable packaging for pharmaceuticals, along with medical device packaging films, think of kp as your trusted partner.

See our ad on page 3 54 | Healthcare Packaging • Spring 2024
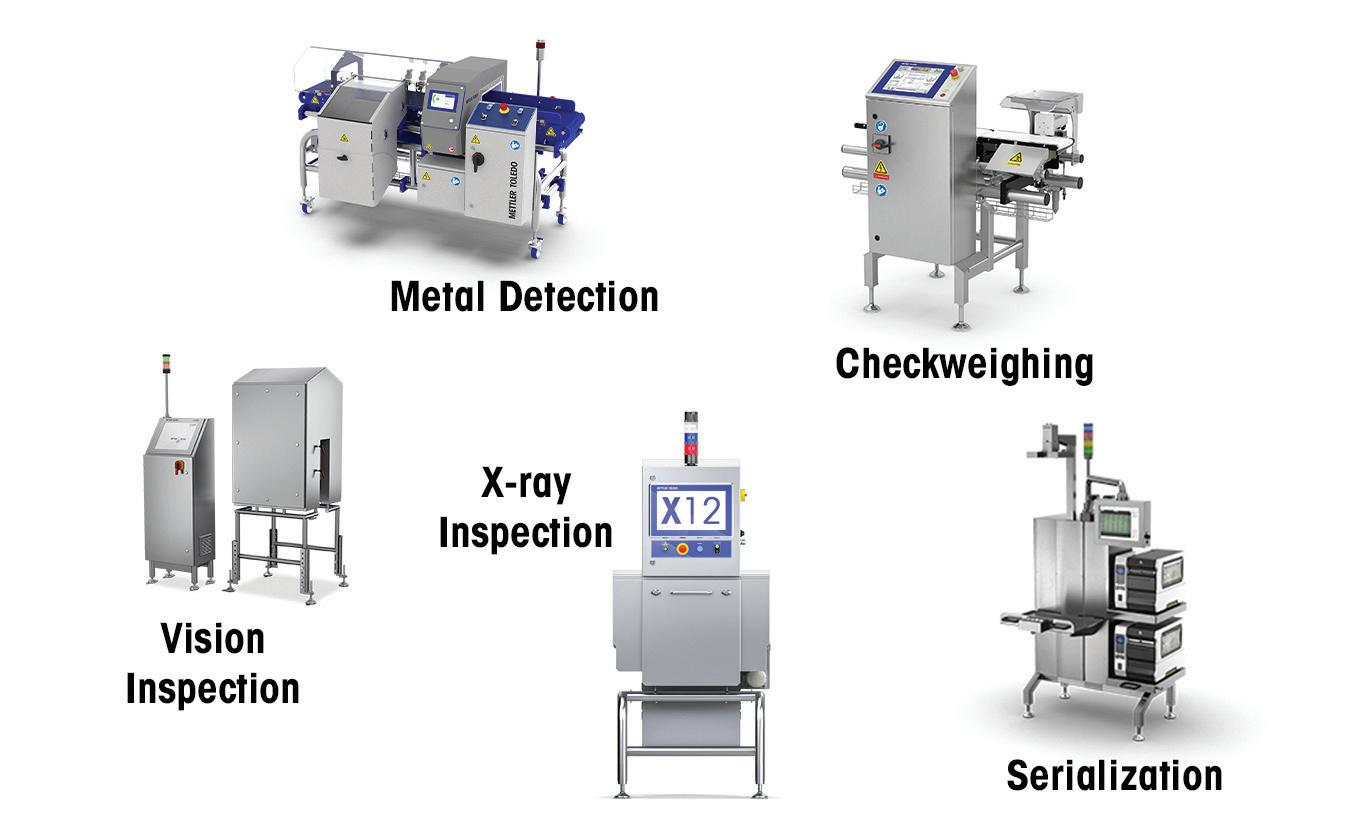
METTLER TOLEDO
1571 Northpointe Parkway, Lutz, FL 33558
813.889.9500
pi.marketing@mt.com
www.mt.com/pi
METTLER TOLEDO provides product inspection solutions with data collection and management software that help ensure product quality and regulatory compliance, increase productivity, boost profits and protect your brand.
Safeline metal detectors are extremely sensitive and easy to use and detect all types of metal contaminants. Safeline x-ray inspection equipment combines innovative hardware and highperformance image processing to detect dense physical contaminants, measure product mass, check for missing or broken product, and confirm fill levels.
Hi-Speed checkweighers perform superior weighing accuracy even at high production rates, reducing giveaway for maximum profits. Innovative dashboard style controls make product set-up and changeover fast and error free for maximum uptime and productivity.
CI-Vision machine vision systems perform accurate label and package quality inspections on all types of packaged products at high speeds. Systems can also prevent label mix-up, one of the leading causes of recalls, to ensure brand protection and avoid costly lawsuits.
PCE provides comprehensive solutions for Track & Trace programs with serialization and aggregation software, hardware and product handling using stations or integrated kits for existing packaging machinery. Scalable and module solutions offer serialization of saleable item and aggregation to bundles, case and pallet. Serialization data is captured and recorded for each line and for the site with completion of production orders and communicated to MES/ERP/Cloud systems to enable full traceability across the packaging line and site, and across the supply chain.

The METTLER TOLEDO Product Inspection division designs and manufactures both standard and customized inspection solutions prioritizing product safety, brand protection and quality assurance. Its solutions empower businesses to optimize operations, reduce costs and embrace digital transformation while meeting regulatory compliance. With a focus on safety and precision, our innovative solutions deliver measurable results, enabling manufacturers to maintain the highest standards of product integrity and consumer trust. By partnering with METTLER TOLEDO, manufacturers can confidently navigate the challenges of the modern business landscape and achieve sustainable growth.


See our ad on page 37 Spring 2024 • Healthcare Packaging | 55

MG America

31 Kulick Rd, Fairfield, NJ 07004
973-808-8185
sales@mgamerica.com
www.mgamerica.com
Headquartered in Fairfield, New Jersey, MG America is a subsidiary of MG2 of Bologna Italy, a company that was founded in 1966 and today is one of the world’s three leading manufacturers of capsule filling equipment.
MG America is a leading supplier of an innovative family of precision-crafted processing and packaging machinery that includes capsule fillers, material handling, primary packaging equipment, secondary packaging equipment, checkweighing/weight control systems, tablet & capsule inspection, and line integration solutions. From sales, field service and spare parts to machine trials and local service/support representation, MG America offers a true “Partnership for Success.”
Packaging equipment from MG America can be found throughout North America in industries such as pharmaceutical, medical device,
diagnostics, nutritional products, and OTC products. Our lineup of premier, European-made machinery has earned a global reputation for reliability, precise performance, and superior craftsmanship.

BIN BLENDING
WASHING/DRYING
CAPSULE FILLING
CAPSULE WEIGHT CONTROL
VISION INSPECTION
CHECKWEIGHING
FILLING/CLOSING FOR:
VIALS, BOTTLE, TUBES
STICK PACKING
SACHET PACKING
DEEP-DRAW THERMOFORMING
CASE PACKING
TOP-LOAD/SIDE-LOAD CARTONING
TRAY FORMING/LOADING
SERIALIZATION
PALLETIZING
LINE INTEGRATIONS

See our ad on page 17 56 | Healthcare Packaging • Spring 2024

MRP Solutions

1 Plant Street, Plattsburgh, NY 12901
518.561.1812
marketing@mrpsolutions.com
www.mrpsolutions.com
Build your brand and deliver packaging premiumization with MRP Solutions. More than ever, consumers expect quality products packaged to deliver an unmatched user experience and connection to the brand and product. MRP Solutions combines extensive packaging expertise with a consultative approach to reliably uncover customer needs. By understanding business goals, we can tailor smarter, safer and more flexible packaging solutions that reduce cost and increase speed to market, helping businesses capitalize on opportunities.
In the highly regulated pharmaceutical market, reliable child resistant closures are essential. MRP Solutions’ child resistant closures safely secure your product while ensuring compliance with rigorous protocol testing to meet Consumer Product Safety Commission (CPSC) standards. Our many designs offer just the right option for safe product storage and include text, debossed,
embossed, pictorial, smooth and sleek selections.
Offering experience and innovation with the broadest range of closures and jars for nutraceutical and pharmaceutical applications, the MRP Solutions product portfolio also includes a selection of continuous thread closures, dispensing closures and customizable jars to enhance your brand.
With one of the most robust, recyclable plastic closure offerings in the market, MRP is your partner in sustainability. All MRP closures are available in Post Consumer Recycled resin to help achieve your sustainability goals.
Let our experienced, innovative, dedicated professionals help to provide the packaging solutions for your next project.

At MRP Solutions, our mission is to lead the market as a trusted partner delivering responsive packaging solutions.
MRP Solutions combines extensive packaging expertise with a consultative approach to reliably uncover customer needs. By understanding your business goals, we can tailor smarter, safer, and more flexible packaging solutions that reduce cost and increase speed to market, helping your businesses capitalize on opportunity. Across category and customer, shared purpose inspires unexpected ideas. By understanding our partners’ business objectives and design requirements, we can reimagine our approach and tailor our solutions to achieve their desired outcome, applying decades of packaging expertise to move faster, efficiently, and cost-effectively in an ever-changing market.

See our ad on page 23 Spring 2024 • Healthcare Packaging | 57

MULTIVAC

11021 N Pomona Avenue Kansas City, MO 64153
800.800.8552
info@multivac.com
www.multivac.com
MULTIVAC is your partner for healthcare packaging, labeling, inspection and handling solutions. Our packaging machines are the perfect machine for every pack.
MULTIVAC is one of the world’s leading suppliers of packaging solutions for a wide variety of medical and pharmaceutical products, as well as consumer and industrial goods. Our portfolio covers virtually all packaging design, performance, and resource efficiency requirements. In addition, it encompasses an extensive range of packaging technologies, automation solutions, and labeling and quality control systems.
All MULTIVAC machines can be integrated into holistically designed systems thanks to our comprehensive line solutions. To ensure maximum line operating and process reliability, high efficiency, and low Total Cost of Ownership (TCO). With this in mind, we are consistently driv-
ing forward digitalization – through real-time analysis tools such as MULTIVAC Smart Services targeted towards predictive maintenance and machine learning. Thinking and acting sustainably, we are dedicated to the responsible use of resources and energy in terms of manufacturing our products and their use at your site.
Ultimately, we see ourselves as a partner who makes a decisive contribution to your business success – whether you are a start-up or a large company, when you decide on a MULTIVAC packaging solution, you opt for the highest level of quality and efficiency, comprehensive customer care, and perfect service. As a worldwide group with 85 subsidiaries, we are closely linked with our customers and their markets, allowing us to identify trends and developments early. As our customer, you benefit from our highly qualified sales and service team in your area.

As one of the leading worldwide providers of packaging and processing solutions for over 60 years, MULTIVAC takes great pride in setting the industry standard in innovation & technology, efficiency, and reliability through our expertly engineered product range of packaging and processing equipment. With the most experienced team in the industry, we help you develop the perfect package and full line solution for your product, your brand, and your objectives for profitability.
MULTIVAC is a family-owned company, which operates globally with more than 80 sites and over 7,000 employees worldwide. Our US headquarters, located in Kansas City, Missouri is complimented by four Regional Customer Support Centers located in Denville NJ, Irvine CA, Lisle, IL, Charlotte NC, and soon to be in TX.

See our ad on the IFC 58 | Healthcare Packaging • Spring 2024

NITA Sentient Labeling Systems
1051 Du Viger Street, Terrebonne, Quebec, Canada, J6B 6W6
1.855.668.NITA (6482)
sales@nita.ca
www.NitaLabeling.com
Welcome to NITA Sentient Labeling Systems, the original 100% Servo Labeler that dramatically REDUCES OPERATOR TOUCH-TIME and increases productivity - all while integrating the latest next-gen technology, the most intuitive user experience, best-in-class responsive support, and truthfully, pure labeling joy.
We love UPTIME. We’re so driven by it we’ve designed all the features on every NITA to create it. How? By meticulously analyzing your labeling pain points – then solving them – with innovation, technology, and certifiably flawless execution.
And how do we keep you running? By building these incredible UPTIME features into every NITA:
- Parts ordering directly from the machine via built-in 3D drawings that scale down to your specific required item.
- A self-diagnosing system that identifies possible issues and suggests fixes & parts needing change or servicing before they become a problem.
- Daily Preventative Maintenance schedules and video tutorials with tiered-level tasks.
- Ultra-fast & precise color-coded changeovers with in-screen 3D location maps or even our incredible Servo-driven FULLY AUTOMATIC product changeover system at the push of a button.
- NitaCare, our signature In-Screen Wi-Fi enabled login program for instant operator issue resolution.

Nita Labeling PROMISES to do anything and everything required to make your labeling life as easy and productive as it can be. It is why we exist.
Experience for yourself what the best labeling company and labeler will bring to your productivity numbers. 100% moneyback-guaranteed happiness.
We cannot wait to meet you.
For the love of labeling!
Your NITA Team


See our ad on page 36 Spring 2024 • Healthcare Packaging | 59

NJM, A ProMach Product Brand

5600 Kieran, Montreal, QC H4S 2B5 Canada
1.800.811.6990
info@NJMPackaging.com
www.NJMPackaging.com
NJM Products Include:
Line Integration – NJM can integrate a tablet, powder, or liquid packaging line. We provide equipment and then integrate turnkey systems at the customer’s plant.
Unscramblers – NJM unscrambling equipment is compact and designed for round, rectangle, square, or oval bottles.
Cappers & Retorquers – NJM cappers include inline belt, inline disc, and rotary continuous models. Compact design makes our cappers easy to incorporate into your packaging operation.
Cottoners – NJM cottoners are simple yet precise, with a guillotine tearing device as standard equipment, and options such as missing cotton detection and wisp detection.
Labelers – NJM labelers are robust, reliable, and versatile, with electronic controls and up-todate container handling features.
Liquid Filling & Closing – Aseptic liquid filling & closing systems for the pharmaceutical liquid
dose industries are offered by our partner, Dara Pharmaceutical. NJM is Dara Pharma’s exclusive sales partner in the USA and Canada.
Washing & Sterilization Systems - NJM is the exclusive North American representative for Steelco’s Pharma and Biopharma products.
Print & Apply – NJM print & apply labelers label cases, cartons, bags, pallets, drums, and tires. Purchase individually, or as part of a turnkey system. RFID tagging is also available.
Tablet Counters – NJM offers tablet counters from our partner, Cremer. Cremer counters feature thoughtful design, robust construction, quality, and accuracy. Cremer tablet counters are also simple to take apart for cleaning, without tools.

NJM, part of ProMach Pharma Solutions, has been a trusted automated packaging systems manufacturer, integrator, and support resource for over a century. We offer a broad range of technologies and applications, specializing in the needs of pharmaceutical, nutraceutical/vitamin, and personal care product packagers. Complementing NJM’s manufacturing and integration expertise, we supply quality packaging line equipment from other leading manufacturers.
The full range of NJM’s services – manufacturing, representation of NJM’s distributed brands, and integration – makes NJM a one-stop packaging solutions provider offering expert knowledge and experience from the earliest stages of planning through implementation and production.

See our ads on pages 19, 32 60 | Healthcare Packaging • Spring 2024
Ossid LLC

4000 College Rd, Battleboro, NC 27809
252.446.6177
Ossid@ProMachBuilt.com
www.ossid.com
Ossid LLC, a ProMach brand, is committed to providing our customers with a superior line of medical packaging machines.
Working with customers in the medical industry, Ossid provides sterile, safe, and effective packaging equipment solutions. There’s nothing standard about medical devices. Ossid understands the unique and complicated requirements of the medical industry and strives to meet those needs with precision and safety at the forefront of the design. That’s why Ossid’s line of thermoform fill and seal machines are flexible to fit your specialized needs, no matter the specifications. If you can imagine it, our team of engineers, with a combined 75 years of experience, will work to design it.
Ossid’s medical device thermoformers are versatile; packaging both flexible and rigid medical package types and are built with UL 508A standards.
These machines offer many features and
benefits including an auto web aligner, static eliminator, hinged upper tooling, servo actuated presses, and a robust framework and guarding package. These machines are ideal for packaging medical devices, dental kits, syringes, pharmaceuticals, and other products.
In addition to thermoformers, Ossid is also a master distributor for the Reepack brand of flow wrappers. With four styles to choose from, flow wrappers can provide a packaging solution ideal for products requiring a hermetic seal or a simple dust covering. Let our team guide you to the best packaging solution for your application.
Our comprehensive customer service program, including service technicians, parts and training teams know how to help you keep your equipment running at maximum efficiency. Ossid helps its packaging customers protect and grow the reputation and trust of their consumers. ProMach is performance, and the proof is in every package.

Our mission at Ossid is to provide our customers with cost-effective packaging and labeling solutions. Ossid works to give our customers a complete flexible packaging solution. Providing customers with excellent service nationwide, we can respond quickly to customers experiencing downtime needs and offer preventive maintenance programs to reduce overall downtime concerns. Our goal is to build long term relationships with our customers.
Our committed sales, service, and aftermarket parts teams work collectively to quickly and effectively assist customers with their needs. ProMach can offer solutions for any customer project or application needs by providing best in class stand-alone equipment from industry leading product brands, partial line integrations, and complete large multi-brand turnkey packaging line integrations.

See our ad on page 20 Spring 2024 • Healthcare Packaging | 61

Paxiom

2037 East Maule Ave, Las Vegas, NV, 89119
(702) 450-0808
info@paxiom.com
www.paxiom.com
At Paxiom, our passion lies in delivering highquality packaging machines that not only meet but exceed our customers’ expectations.
We are dedicated to providing the finest solutions that bring value to your business. With a commitment to innovation, reliability, and customer satisfaction, we strive to be the trusted partner you can rely on for all your packaging needs. Our goal is to help you achieve operational excellence and enhance your brand’s success through our cutting-edge technologies and exceptional service.
We provide national sales, systems integration, and service for WeighPack Systems. Inc, EndFlex LLC, ValTara S.R.L and Canapa Solutions.
We are more than a machine, we are your partner and a single-source provider, meaning you get one point of contact for design, manufactur-
ing, service and integration. And since we take full responsibility from line layout design, manufacturing and testing through to factory acceptance, installation and technical support, you get peace of mind that your investment is safe.
We’ve delivered over 7,000 machines to our clients in 30+ countries since 1991, but we know that investing in packaging automation is not just about the machine. It’s about the people you work with, the company behind the machine and their infrastructure, longevity and support. That’s why we aim to create long-lasting partnerships based on trust and a genuine desire to see you succeed.
Our primary, secondary, and end-of-line solutions are engineered to fit almost any production requirement and budget. Let us design a system to increase your production rate and save you valuable time, labor and resources.

We are much more than our machines. We know that investing in packaging automation is not just about the machines, it’s about creating long-lasting relationships based on trust. We believe in doing things right the first time. While we take pride in designing and building solutions to meet the demands of today, we are always planning for the needs of tomorrow. We are committed to developing the innovative products that our clients will need as they continue to grow. We are customerservice-obsessed! Our service team is run by factory-trained technicians who work closely with your production team to ensure the most positive servicing experience. We are confident we have the right automation solutions for your business. Contact us to take a free tour at our Las Vegas Xperience Center today to see who we are!
 Get More with Paxiom.
Get More with Paxiom.
See our ad on page 78 62 | Healthcare Packaging • Spring 2024

PERFEX CORPORATION
32 Case Street, Poland, New York, 13431
800-848-8483
perfex@perfex.com
www.perfex.com
Perfex Corporation is a world leading manufacturer of critical cleaning tools for controlled environments. Established in 1924, Perfex is celebrating 100 years of excellence fueled by passion and dedication. Still family owned and operated, our experienced team is devoted to providing you with the most innovative and effective cleaning systems in the industry.
PERFEX is the pioneer of TruCLEAN® mopping systems, a first-of-its-kind design specific for ultra-high sanitation areas, cleanrooms, laboratories, and controlled environments. Manufactured in the United States, TruCLEAN mopping systems are lightweight, ergonomic and easy to use on floors, walls, and ceilings. Includes autoclave compatible stainless-steel components, temperature-resistant polypropylene buckets and multiple configurations suited for large or small area cleanrooms of any grade.
TruCLEAN mops incorporate specialized sponge and fiber materials to promote economical and consistent cleaning methods without leaving residual contaminants.
We also manufacture a complete line of colorcoded brooms, brushes, and squeegees hygienically designed to prevent cross contamination and particulate contamination.
PERFEX® products can be found in diverse industries across the globe such as pharmaceutical, semiconductor, bioengineering, and life sciences. Our representatives are readily available for on-site evaluations, product support, and technical advice.

How it Started
Established in 1924 as the Brooklyn Fiber Broom Company, our founder believed cleaning tools should be designed to eliminate associated safety issues. To this day we develop each product with the same idea in mind. Reliable tools, made from quality materials, to address todays hygienic concerns.
Here and Now
Our TruCLEAN product line leads the critical cleaning industry with autoclave compatible mopping systems. NEWTruCLEAN® Sterile includes sponge mops and mop covers gamma sterilized and validated per AAMI guidelines.


See our ad on page 21 Spring 2024 • Healthcare Packaging | 63

Pharmaworks, A ProMach Product Brand
2346 Success Dr., Odessa, FL 33556 USA
1.727.232.8200
Sales@Pharmaworks.com
www.Pharmaworks.com
Pharmaworks Products Include:
TF1 Blister Machine – A simple and cost-effective blister packaging solution. The TF1 blister machine is perfect for entry-level packagers where budget matters.
TF1e Blister Machine – Features a compact footprint, quick changeover, and the latest in servo technology. The TF1e is perfect for small, medium, or clinical production.
TF1pro Blister Machine – The latest in blister packaging technology, the TF1pro is built for today’s demanding cGMP and changeover requirements.
TF2 Blister Machine – A medium output workhorse that is great for deep-draw medical devices and consumer products.

TF3 Blister Machine – All the features expected in a high output machine.
Vision Systems – Scanware blister and print vision inspection systems.
Feeding Systems – Pharmaworks provides a variety of feed systems featuring pick & place, flood feeding, dedicated, and custom robotic technology.
Blister Machine and Cartoner Rebuilds – Not ready to invest in new blister packaging equipment? Pharmaworks is the clear leader with over 20 years of experience rebuilding and upgrading third-party equipment.
Tooling & Change Parts – As the leading OEM, Pharmaworks is the go-to source for tooling any of our packaging machines as well as a variety of third-party equipment.

Pharmaworks, part of ProMach Pharma Solutions, is the premier supplier of blister packaging machinery for North America and has a worldwide installed base. Backed by the power of ProMach, Pharmaworks can turnkey any size blister project from our expanded production facility in Odessa, FL.
For 20 years, Pharmaworks has set ourselves apart from other OEMs by not only selling our line of blister machines and equipment, but by rebuilding and upgrading other OEM brand equipment. This uniquely positions Pharmaworks to be a full-service provider for our packaging customers.
Industries served:
• Pharmaceutical
• Medical/ Medical Device
• Animal Health
• Nutraceutical
• Personal Care

See our ad on page 35 64 | Healthcare Packaging • Spring 2024

ProSys Fill LLC.
426 East Fountain Road, Webb City, MO 64870
info@prosysfill.com 417-673-6542
ProSysFill.com
ProSys is a premier manufacturer of semiautomatic and fully automatic equipment for filling, Squeeze Tubes, Syringes, Airless Pumps, Cartridges, Jars, Custom Containers & Hot Melt applications for the Pharmaceutical industry. A global supplier of filling equipment since 1985 with U.S. sales, manufacturing and customer service facilities located in Southwest Missouri.
FEATURES & BENEFITS
• Fill Accuracy of +/- 0.1% by Volume
• Turnkey & Custom Designs
• Air-Free Vertical Bottom-Up Filling
• Custom Mix Solutions (Eliminates Batching)
• Drum & Pail Presses
• SERVO Solutions
• Explosion Proof Controls (Class 1 Division 1&2, ATEX 0&1)
• Tool-free Release System for Simple Changeovers

• “Digital Readout Indicators” for Fast & Accurate Adjustments
• Multiple Service Technicians for Less Down Time & Preventive Maintenance
• On-line Service & Support
• Recipe Storage & Recall
• Creams, Lotions & Viscous Pastes to 3 Million Centipoise
• Designed & Built in the U.S.A.
MAJOR MARKET GLOBAL INSTALLS
• Pharmaceutical
• Cosmetic
• Chemical
• Adhesive/Sealant
• Lubricant
• Food

Building Quality, Integrity & Value In Our Team, With Our Customers, & In The Equipment We Design, Build & Deliver.
FLEXIBLE COMBO FILLING SYSTEMS
• Plastic & Metal Tube Filling Systems
• Tube & Airless Pump Filling Systems
• Tube & Cartridge Filling Systems
• 10, 14 & 30 oz. Cartridge Filling Systems
Squeeze Tube Filling Machines speeds from 15 to 300 per minute.
Syringe Filling Machines speeds from 20 to 300 per minute.
Airless Pump Filling Machines speeds from 15 to 300 per minute.
Cartridge Filling Machines speeds from 10 to 250 per minute.
Custom Filling Systems

See our ad on page 79 Spring 2024 • Healthcare Packaging | 65

Rychiger Canada O/A Nuspark
45 Steeprock Drive, Toronto, Ontario, M3J 2X1
416 663 7071
Sales.ryca@rychiger.com
www.Rychiger.com
Rychiger Canada formerly known as Nuspark Inc. continues provides turn-key primary, secondary & tertiary packaging machinery solutions for the healthcare, pharmaceutical, food & beverage and personal care industries. As part of the Rychiger family, we will combine our advanced automation capabilities with their deep expertise in primary packaging equipment to make factories and plants smarter, more productive, and sustainable.
• Over 125 years’ of combined experience
• Leader in secondary packaging
• Innovative custom engineering
• Customer satisfaction driven
• In house manufacturing
Rychiger Canada has a reputation for creative innovation. We maintain this reputation through constant analysis of new and emerging technologies, ensuring that our customers benefit from
state-of-the-art design and construction. All our equipment is proudly built and manufactured in our Canadian facility located in Toronto, Ontario, giving us complete control over the quality of our equipment.
Our promise from proposal to production:
• Attention to detail
• Precise execution
• Deliver complete turn-key solutions
• Mechanical design
• Electrical/control engineers
• In-house manufacturing facilities
• Dedicated after sales service team
We look forward to working with you on your next project!

Rychiger Canada provides equipment solutions for filling, cartoning, case/tray packing, forming, and palletizing machinery. Offering solutions for all your healthcare packaging needs – from standalone machine to highly complex, fully integrated packaging lines. Our gifted and highly educated engineers go above and beyond to deliver innovative solutions.


See our ad on page 27 66 | Healthcare Packaging • Spring 2024

STARVIEW PACKAGING MACHINERY, INC.

1840 Boulevard Saint Régis Dorval, Quebec H9P 1H6
514-920-0100 ext.310
sales@starviewpackaging.com
www.starviewpackaging.com
Starview Packaging Machinery, Inc. is the leading manufacturer of packaging machinery for high-visibility packaging with over 30 years of supplying standard and custom packaging systems to our customers. Providing the Medical Device and Pharmaceutical Industry with innovative packaging machines for:
• Medical Device Packaging
• Pharmaceutical Packaging
• Blister & Clamshell Packaging
• Customized Packaging Equipment
• Integrated Automated Systems
We design, engineer, and manufacture a comprehensive line of manual, semi-automatic, and automatic sealing machines. Available in shuttle, rotary, carousel, and inline conveyor configurations, a variety of standard and custom sealing areas are available to meet the specific requirements of customers and maximize
productivity. Machines are configured to suit the specific application such as carded packages, sterile medical device packages, or pharmaceutical wallet packages.
Our distinct competitive advantage is in providing a complete range of both standard and customized quality packaging systems backed by solid machine designs, robust machine construction, and superior service. Starview offers many value-added features for our machines such as product sensing, printing and/or verification, hydro-pneumatic cold seal presses, robotic product loading, automatic packaging materials loading, automatic inline fold-over, finished package unloading with reject features. Quick-change mechanisms for tooling sets, on-screen sealing press adjustments, machine performance tracking, and ANSI Class 4 safety make Starview machines an excellent choice.

The owners, management, and staff of Starview are dedicated to designing and manufacturing packaging machines in North America with the highest quality fit, and finish backed up with industry leading customer service.
Starview’s directive is to produce a full range of sealing machines for medical device packaging, pharmaceutical packaging and retail high visibility packaging. We offer standard machines with an array of custom and in demand options to provide our clients with machines to match their manufacturing requirements.

See our ad on page 77 Spring 2024 • Healthcare Packaging | 67

Stevanato Group

www.stevanatogroup.com/en
Stevanato Group
Founded in 1949, Stevanato Group is a leading global provider of drug containment, drug delivery, and diagnostic solutions to the pharmaceutical, biotechnology, and life sciences industries. Stevanato Group delivers an integrated, end-to-end portfolio of products, processes, and services that address customer needs across the entire drug life cycle at each of the development, clinical, and commercial stages. Stevanato Group counts more than 5,600 people in 16 sites in 9 countries.
Advanced Drug Containment Solutions & Analytical Services
Stevanato Group boasts unique expertise in providing advanced pharmaceutical containers from glass tubing. Its comprehensive portfolio covers every customer need, from those related to small molecules to highly sensitive drugs. Stevanato Group produces vials, syringes, and
cartridges for different applications, such as vaccines, diabetes care, anesthetics, hormones, anticoagulants, and biologics. Glass containers are available both in bulk and in EZ-fill®, the market-recognized ready-to-use configuration. Stevanato Group can also provide container closure and device characterization analytical services through its Technology Excellence Centers.
Vision Inspection, Assembly & Packaging Technologies: A Modular & Flexible Approach
Stevanato Group’s capabilities range from modular assembly platforms and packaging lines to advanced vision inspection machines, including manual, semi-automatic, and automatic. Stevanato Group equipment can inspect a wide range of liquid, emulsions, viscous, gellike, powder, and lyophilized drugs, catering to the needs of both small firms and big pharma companies producing blockbuster drugs.

Stevanato Group is fully committed to being the best, objective-focused partner in the research and delivery of innovative solutions to support the success of our customers.
And they never stand still.
It’s their continual innovation and pioneering of new trends in the pharmaceutical industry that ensures they produce the world’s most advanced solutions year after year, enhancing the level of product integrity that can be guaranteed to patients and always seeking to exceed customer expectations.
Stevanato Group’s mission is to cooperate deeply with their partners all over the world, providing their know-how, resources, and enthusiasm to turn every project into an achievement.
 9701 Giovanni Stevanato Dr, Fishers, IN, 46038, USA
9701 Giovanni Stevanato Dr, Fishers, IN, 46038, USA
See our ad on page 31 68 | Healthcare Packaging • Spring 2024

Uhlmann Packaging Systems LP
44 Indian Lane East, Towaco, NJ 07082
973.402.8855
info@uhlmann-usa.com
www.uhlmann.de
PTC 200
Our packaging solutions with the parenteral tray center PTC 200 address the special needs of pharmaceutical companies, doctors and patients in a particularly sustainable manner. Cardboard trays enable visually appealing and clear packaging, simple and ergonomic, product removal as well as protected and space saving storage. The gentle product handling and the simplified cleaning of the machine guarantee you even greater safety when packaging parenterals.
Thanks to the proven cartoning technology, trays and folding boxes can be erected in the packaging process, while our proprietary systems ensure correct and complete storage. The Uhlmann inspection system provides you even greater flexibility at favorable conditions. The modular machine concepts are therefore suitable for different or changing product appli-
cation as well as for new or seasonal product launches. Side loading allows you to save on machine length and for you to implement any packaging requirement with top opening. The PTC 200 acts quickly, safely, and reliable, just the way you need them to. The feeding of the products transportation system, the components can be supplied on a vertical, horizontal or suspended basis, Sensitive components such as ampoules, vials, syringes or medical devices benefit from our versatile and customizable feeding options.

From its northern NJ-based U.S. headquarters, Uhlmann provides sales support, service technicians, on-site training, parts inventory, and onsite engineering for line integrations. The company’s 600,000-sq.-ft. world headquarters is located in Laupheim, Germany.
Equipment includes:
• Blister machines, from compact models to complete, full-featured, highspeed blister lines
• Cartoning systems for blister, bottle, pouch, vial and kits
• Integrated bottling lines
• Casepackers, overwrappers, bundlers, and palletizers
• System engineering and line integration
• Validation and training service, including Topman training
• Inspection systems, peripheral products & services
• Custom-designed tools & format parts

Uhlmann Packaging Systems is the world’s leading manufacturer of high-quality packaging machinery for the pharmaceutical industry.

See our ad on the OBC Spring 2024 • Healthcare Packaging | 69

Weiler Engineering, Inc.
1395 Gateway Drive, Elgin, Il 60124
847-697-4900
www.weilerengineering.com
Weiler Engineering, Inc., a leading provider of aseptic custom Blow/Fill/Seal liquid packaging equipment for pharmaceutical and healthcare applications, is committed to the highest standards of excellence and to expand products and systems to enhance patient care. Weiler’s proprietary ASEP-TECH® B/F/S packaging machines produce shatterproof, durable, aseptically-packaged products in one uninterrupted operation. This hands-free manufacturing process ensures that parenterals, ophthalmic solutions, and respiratory drugs reach the marketplace sterile, in the most cost-effective manner possible. The ASEPTECH® System is the culmination of 65years of innovation in machine design and sterile process development, producing the most advanced aseptic liquid packaging process machinery.
The Weiler design incorporates the process of blow molding, aseptic filling, and hermetic sealing of liquid products in one sequential
operation on a compact machine frame. Weiler’s patented electronically controlled fill system, automatic sterilization system with integral data collection, andfilter integrity test system arestandard equipment for each machine configuration. Each machine is equipped with a HEPA air shower to ensure a Class 100 environment under dynamic conditions in the nozzle shroud area.
Weiler’s latest innovation is the NEW compact ASEPTECH® LAB+ Blow/Fill/Seal machine, which is ideal for Stability and Clinical batches for pharmaceutical products and/or small development batches using advanced aseptic technology. This revolutionary small footprint design focuses on ease of changeover and product range flexibility.
ASEP-TECH® Blow/Fill/Seal machines are proudly manufactured in the USA, designed, and built by Weiler Engineering, Inc. in a 140,000 ft², state-of-the-art building.

Facts:
• Recognized as an advanced aseptic technology by the USFDA
• 65 years serving global markets
• Experience gained from 25 years of operating a captive pharmaceutical CMO
• Cooperation with regulatory authorities – compliance is key
• Quality + Operational Knowhow + Integrity
Goals:
• Focus on the science of the technology for maximum customer bene¬fit
• Simplicity of design to maximize product flexibility and minimize footprint
• Optimum service support throughout the markets we serve = high customer satisfaction


See our ad on page 33 70 | Healthcare Packaging • Spring 2024

Weiler Labeling Systems (WLS), A ProMach Product Brand
1256 North Church Street, Moorestown, NJ 08057
856-273-3377
WLS@ProMachBuilt.com
www.WeilerLS.com
Rotary Labelers – Pressure-sensitive labelers for labeling a variety of product shapes vertically.
Vertical In-line Labelers – Pressure-sensitive labelers for labeling a variety of cylindrical products vertically.
Label Heads – Pressure sensitive label heads with label print and inspection options including serialized codes and RFID tags.
Label Presence Inspection – WLS’s new VialView label presence inspection stand-alone station is ideal for verifying label presence prior to final packaging.
Label Coders – For stand-alone or integrated high-speed coding of labels, including serialized codes.

Label Printers – AUTONOMY® is a high-speed, full-color, stand-alone digital label printer with integrated full-label inspection system. Printing variable and serialized data with label artwork.
Vial Coders – For code printing on vials or bottles using ink jets or lasers.
Documentation & Certifications – DDS, FAT, SAT, IQ/OQ and Trace Matrix documents as well as UL, Seismic and CE certifications.
Field Service – US and European field service team also offering equipment training courses and maintenance contracts.

WLS, part of ProMach Pharma Solutions, is an industry-leading designer and manufacturer of high-speed rotary and in-line labeling machines and serialization, coding and label printing solutions for the pharmaceutical and medical packaging markets as well as the food, beverage, personal care, and consumer markets.
With over three decades of experience, our mission is to improve our customer’s labeling capabilities and ensure that our labelers provide them with the highest possible OEE.
Industries Served:
• Pharmaceutical
• Medical/Medical Device
• Nutraceutical & Vitamins
• Food
• Beverage
• Consumer Products

See our ad on page 29 Spring 2024 • Healthcare Packaging | 71





•
•
•
•
•
Discover the Next Gen of Inspection Technologies
Antares Vision Group’s new generation of inspection machines allows product inspection of any container and product content from any angle to guarantee your product’s quality, integrity, and safety, meeting regulatory requirements. www.antaresvisiongroup.com

Unleash the power of automation with a built-in buffering system with integrated web tension control, ensuring consistent material handling and printing perfection. It’s automated maintenance system automatically purges, wipes, and caps the print heads to minimize downtime and maximize productivity.

RLM: Transforms packaging lines into RFID-enabled systems for late-stage encoding, validation, and item-level serialization while integrating with all major packaging lines. CCL provides tailored RFID solutions with high-performance inlays, hardware, software, and seamless RFID Line Management.

For over 63 years, Chase-Logeman Corporation has been designing monoblock-style filling and closing equipment for Chemical, Diagnostic, Food, Nutraceutical, and Pharmaceutical use from our facilities in Greensboro, NC.


72 | Healthcare Packaging • Spring 2024
ANTARES VISION GROUP See our profile on page 40
advancement in piezo inkjet printing BELL-MARK See our profile on page 41
InteliJet HD 3A, the newest
Line Management (RLM) achieves encoding of 700 items/min. CCL HeaLtHCare See our profile on page 42
RFID
Manufacturer of Monoblock-Style Filling/Closing Equipment Chase-Logeman Corporation See our profile on page 43
Up to150 Cartons per Minute
Manual,
Controlled
Servo
Pusher, or Robotic Loading
Fast Changeover
Adjustable Pitch for Wide Cartons
Labeler,
Serialization
Automatic Cartoner Systems ESS TEchnologiES, A PAcTEon comPAny See our profile on page 46
Checkweigher, and
Integration




Greydon
See our profile on page 49
UVMax™ – Flexographic Inline Printer for Blister Packaging Systems
A flexographic, inline printer engineered to integrate with pharmaceutical blister packaging systems. UVMax prints the complete package including variable information with an integrated thermal inkjet printer, allowing you to avoid purchasing pre-printed blister materials.

Heat and Control, InC.
See our profile on page 51
Inspection performance for total product quality confidence
Anywhere along the line, protect your consumer and your equipment. Efficient detection of foreign objects is critical. We offer a complete line of metal detectors, checkweighers, and x-ray inspection systems from our strategic partners: CEIA® and Ishida.
JAMES ALEXANDER CORPORATION

See our profile on page 52
CONTRACT FILLING: SINGLE-USE GLASS AMPOULES FOR UNIQUE DISPENSING SOLUTIONS
We’ll help you develop a customized dispensing system for your formulations with a variety of tips and in a range of different sizes/colors. You get: Extended shelf life, formulation stability, tamper resistance, quality assurance and two-part mixing. PROUDLY MADE IN THE USA. www.james-alexander.com
KlocKner PentaPlast
alfoil® high-barrier pharmaceutical films.
alfoil® can improve your packaging line performance by eliminating issues found with typical PVdC films. It promotes better material flow, eliminates blocking, and reduces tool corrosion. Enjoy faster line speeds and improved yields with alfoil® highbarrier blister films.
METTLER TOLEDO

See our profile on page 54

See our profile on page 55
Boost Profits, Comply With Regulations, and Safeguard Your Brand
Offering a full range of metal detectors, checkweighers, vision and x-ray inspection systems, serialization solutions and data collection and management software to ensure product quality and regulatory compliance, increase productivity, ensure operational excellence, boost profits and protect your brand.
Spring 2024 • Healthcare Packaging | 73





A leading supplier of processing and packaging machinery
Packaging equipment from MG America can be found throughout North America and across the life sciences industry. Our lineup of premier, European-made machinery has earned a global reputation for reliability, precise performance, and superior craftsmanship.
MRP
New! Satin Finish Sleek CR® from MRP
Give Your Child Resistant Closures a Branding Boost. The blank canvas of the Sleek CR® family provides the opportunity to create a customized closure that reflects your brand and ensures safe product storage. www.mrpsolutions.com


Multivac,
MULTIVAC is your Partner in Medical and Pharmaceutical Packaging
MULTIVAC is your partner for healthcare packaging, labeling, inspection, and handling solutions. In addition, it encompasses an extensive range of packaging technologies, automation solutions, and quality control systems.

Beltorque® Capper BT-IC NJM
Beltorque® inline cappers feature servo-driven cap sorting and feeding, as well as the ability to add real-time application torque monitoring. With the use of synchronized belts, this versatile and innovative machine applies cap torque without cap scuffing.

Ossid
Drive Efficiency with Ossid’s Trusted Medical Packaging Solutions
Unleash the power of Ossid’s 8000M Thermoform Fill Seal Machines for unparalleled medical device and kit packaging. Trust the Reepack ReeFlow 200 to deliver reliable hermetic seals, meeting the stringent requirements of your medical product application. Visit Ossid.com to contact us today

74 | Healthcare Packaging • Spring 2024
our profile
page 56
MG AMericA See
on
SOLUTIONS
our profile
page 57
See
on
inc. See our profile on page 58
PackagiNg See our profile on page 60
See our profile on page 61




Innovative Cleaning Tools For Controlled Environments
Perfex is the pioneer of TruCLEAN® mopping systems designed specifically for cleanroom sanitation requirements. Est. in 1924, we’re celebrating 100 years of excellence fueled by passion and dedication. Our experienced team is devoted to providing you the most effective cleaning systems in the industry.

Pharmaworks
TF1, Entry Level Blister Machine
The TF1 is a cost-effective blister machine that is perfect for low-volume production. Built with simplicity in mind, this entry-level machine requires minimal training and minimal maintenance. It can thermoform many types of materials and can optionally be set up to cold form.
ProSyS Fill llC.
See our profile on page 64

See our profile on page 65
FLEXIBLE, RELIABLE & ACCURATE FILLING SYSTEMS
ProSys is a premier manufacturer of semiautomatic and fully automatic equipment for filling, Squeeze Tubes, Syringes, Airless Pumps, Cartridges, Jars, Custom Containers & Hot Melt applications for the Pharmaceutical industry.

RychigeR canada O/a nuspaRk
Innovative and efficient packaging solutions.
• Leader in packaging automation from standalone machinery to full line solutions
• Fill, carton, case pack, tray pack and palletize
• Innovative & reliable machinery for healthcare, food & beverage, personal care industries and more.
See our profile on page 66

SB/PH1-1012 Updated Table Top Medical Device Tray Sealer
The recently updated SB/PH1-1012 sealer now comes with a color HMI screen to give you full access to on screen heat and optional pressure calibrations. Includes exclusive data validation/data download port and class 100 clean room filter. Operational log is CFR21 Part 11 compliant.

Spring 2024 • Healthcare Packaging | 75
CORPORATION
PERFEX
See our profile on page 63
Starview Packaging Machinery inc. See our profile on page 67





STEVANATO GROUP
Flexibility Built on Solid Experience!
Our GMP-compliant device assembly equipment can be configured to adapt to your production requirements, providing technology transfer from prototyping to high-volume production. This modularity enables different devices to be run on the same line, reducing time-to-market.
UHLMANN PACKAGING SYSTEMS
READY FOR MORE THAN ONE FUTURE
Uhlmann parenteral packaging in plastic or paper tray
Rely on Uhlmann’s decades of experience in packaging for the latest innovation in safe, sustainable and highly flexible packaging technology
WeighPack SyStemS inc. / Paxiom
See our profile on page 68

See our profile on page 69

See our profile on page 62
The Turnkey Gummy Bottling System with SpinDexer HS
Our high-speed automated system fills gummies into bottles at 100+ cycles/min, seamlessly handling unscrambling with vision inspection, weigh counting, continuous rotary filling with SpinDexer HS, capping, check weighing, induction sealing, and labeling.
Weiler engineering, inc.

See our profile on page 70
Weiler Engineering, Inc.’s Blow/Fill/Seal sterile-filled containers
Weiler Engineering, Inc.’s Asep-Tech Blow/Fill/Seal machines produce plastic aseptically packaged sterile-filled containers. Fill volumes range from 0.2mL vials to 1000mL LVP bottles. Typical resins used are LDPE, HDPE and P.P. The B/F/S process creates sterile finished product in a matter of seconds.

Weiler labeling SyStemS (WlS)
AUTONOMY® Digital Label Printer
AUTONOMY® is a state-of-the-art, high-speed, full-color, stand-alone digital label printer with integrated full-label inspection system. Printing variable and serialized data with label artwork. Providing in-house, on-demand, high-quality inspected labels ready for application.
See our profile on page 71

76 | Healthcare Packaging • Spring 2024

Recyclable Pharma Bottle
Bormioli Pharma
Bormioli Pharma and Loop Industries address the demand for sustainable choices with a pharmaceutical packaging bottle made entirely from 100% recycled, virgin-quality Loop PET resin. The Infinite Loop technology upcycles low-quality PET waste into high-quality resin, which promotes circularity for low-value waste and reduces greenhouse gas emissions.
Type I Tubular Vials
SGD Pharma
SGD Pharma’s Velocity Vials, featuring Corning’s glass-coating technology, reduce production line disruptions, minimize glass particles, and ensure faster delivery of injectable treatments. The Velocity Vial coating, available in clear and amber glass, reduces frictional resistance created by glass-to-glass and glass-to-metal contact, optimizing the fill and finish process by 20-50%.

Single-Sided Tablet Press
KORSCH America
The X 5 Tablet Press is a rotary press with turret segment capability that increases the number of punch stations, incorporating flexibility in a higher-speed, single-sided platform. Available in two versions, the X 5 SFP provides dedicated single-layer capability, while the X 5 MFP offers flexible single-layer, bi-layer, and tri-layer flexibility.


NEW PRODUCTS

Circular Economy Data Loggers
ELPRO Services, Inc.
ELPRO launches its Circular Economy program for LIBERO data loggers in which over 90% of all hardware components can be reused or recycled, minimizing environmental impact. Customers receive as-good-as-new devices with A-grade components reprocessed and reused from one to three times in a controlled manner.

Moisture-Adsorbing Flexible Packaging
Aptar CSP Technologies
Aptar CSP Technologies collaborates with ProAmpac to develop ProActive Intelligence
Moisture Protect (MP-1000), an Activ-Polymer technology that delivers fully integrated protection for moisture-sensitive products. MP-1000 delivers moisture protection without an add-on desiccant sachet, adsorbing excess moisture inside the package and also shielding the contents from moisture exposure en route.
Sustainable Closure Seals
Schreiner MediPharm
Schreiner MediPharm’s closure seals for pharmaceutical packaging comprise sustainable film material of up to 90% recycled content, which results in a reduction of CO2 emissions. The seals offer high functionality and tamper protection, and extensive testing confirms they match conventional seals in transparency, printability, and first-opening indication.















See the Turn-key System See the SpinDexer-HS The eed er Gummy ll ng ystem! info@weighpack.com | 1.833.4PAXIOM Unscrambling Weighing Filling Capping Labeling we gh ack.com
NEW PRODUCTS
Anritsu - Product Inspection 6, 39 & Detection www.anritsu.com/infivis
Antares Vision Group 11, 40, 72 www.antaresvisiongroup.com
Bell-Mark Sales Company 25, 41, 72 www.bell-mark.com
CCL Healthcare 15, 42, 72 www.healthcarepackaging.com
Chase-Logeman Corporation 9, 43, 72 www.chaselogeman.com
Columbia Machine, Inc. 24, 44 www.palletizing.com
Columbia/Okura LLC 30, 45 www.columbiaokura.com
Delta ModTech IBC www.deltamodtech.com
ESS Technologies, Inc. 7, 46, 72 www.esstechnologies.com
Formost Fuji Corporation 28, 47 www.formostfuji.com
G&K-Vijuk Intern. Corp. 18, 48 www.guk-vijuk.com
Greydon 5, 49, 73 www.greydon.com
Harpak-ULMA Packaging, LLC 14, 50 www.harpak-ulma.com
Heat and Control, Inc. 13, 51, 73 www.heatandcontrol.com
James Alexander Corp. 1, 52, 73 www.james-alexander.com
Kingwills 4, 53 www.kingwills.com
Klöckner Pentaplast, Health 3, 54, 73 & Protection and Durables www.kpfilms.com
METTLER TOLEDO 37, 55, 73 www.mt.com/pi
MG America, Inc. 17, 56, 74 www.mgamerica.com
MRP Solutions 23, 57, 74 www.mrpcap.com
Multivac, Inc. IFC, 58, 74 www.multivac.com
Nita Labeling Systems 36, 59 www.nitalabeling.com
NJM
Ossid



AD INDEX
Packaging 19, 32, 60, 74 www.njmpackaging.com
Paxiom Automation, Inc. 62, 75, 76 www.paxiom.com Perfex Corporation 21, 63, 75 www.perfex.com Pharmaworks LLC 35, 64, 75 www.pharmaworks.com PMMI 38 www.pmmi.org ProSys Fill LLC. 65, 75, 79 www.prosysfill.com Rychiger Canada 27, 66, 75 www.rychiger.com Starview Packaging 67, 75, 77 Machinery Inc. www.starviewpackaging.com STEVANATO GROUP 31, 68, 76 www.stevanatogroup.com Uhlmann Packaging 69, 76, OBC Systems L.P. www.uhlmann.de Weiler Engineering, Inc. 33, 70, 76 www.weilerengineering.com WLS 29, 71, 76 www.weilerls.com
20, 61, 74 www.ossid.com
2024 Challenges in User-Centered Design
KEREN SOOKNE, EDITOR-IN-CHIEF
TOP THREE TAKEAWAYS
The packaging and med device communities are, of course, well-versed in the need for patient-centered design in boosting adherence. Nonadherence to drug treatment is estimated to be responsible for 125,000 deaths annually in the U.S. alone.
But with multi-faceted challenges, one-size-fits-all answers are hard to come by. Even among patient populations for a given therapy, there are differences in user abilities and it’s difficult to standardize a package or device for best use across all patients. At Pharmapack Europe, panelists were asked what challenges they still see in their respective areas.
Personalized dosing: Aline Noizet, Founder of Digital Health Connector, noted that standardized dosing reduces efficacy. She explained of data from a recent Closed Loop Medicine presentation that many women are overdosed, while patients over 80 kg are often underdosed. In tests, personalizing dosage for individuals showed efficacy in 80% of the cases. Packaging plays a key role in unit dosing and while variable dosing options exist, many convenient solutions come pre-dosed. A second, related problem that still needs to be addressed is diverse clinical trial participation.
Sustainability: Sustainability is something that touches most people’s hearts in our industry, said Oliver Haferbeck, Head of Innovation and Advanced Technologies at Gerresheimer. “If we don’t find solutions concerning the environment, and being environmentally friendly, we’re going to run into huge problems,” he noted.
This can lead to competing priorities, highlighted Tom Oakley, VP of Design and Development at Springboard, who said they are often asked why they don’t just make things simpler and easier for the patient. He noted that sustainability, an extremely important subject, often goes into conflict with simplicity for the user. For example, if a device is designed to be reusable for sustainability reasons, that typically involves extra user steps—loading a drug, removing a needle, some form of cleaning, etc. “Those steps all make things harder for the user,” Oakley said.
Anti-tampering and cost: In the effort to prevent diversion or tampering with drugs, the packaging typically requires some

level of robustness, which may make it harder for the user to open. “Another one would be cost. People are somewhat resistant, particularly payers, to increases in cost if that’s what’s needed for extra patient-centricity,” Oakley said.
James Fries, CEO at Rx-360 said that counterfeiting and safety are top of mind for him, but that there’s a cyclical nature to topics like these in the industry and people may let their guard down. He encouraged attendees to consider early in the design process that there are bad actors out there attempting to infiltrate while life science companies look to improve patients’ lives.
Data: Eric Chanie, Director, Core Team Leader, Connected Pens, at Merck, discussed some benefits of the data collected, not only for adherence to a given schedule but even for guidance. By recording the orientation of the pen or the length of the injection, a provider can coach the patient on whether they need to adjust the injection angle or wait another few seconds before removing the device.
But don’t overlook the user-friendliness of the data. He said it’s important to consider how you present data to the physician, patient, and caregiver, providing them a simplified view and only what’s relevant for that patient. He also explained that while connected devices offer benefits, consider how the materials— electronic or not—will be recovered, reused, or recycled. A bestfit design will balance the above needs (and more) for the user.
ADHERENCE/DELIVERY 80 | Healthcare Packaging • Spring 2024
1. Industry faces competing design challenges.
2. Sustainability may conflict with convenience. 3. Anti-tampering cannot be overlooked.
↑ From left at Pharmapack Europe: Tom Oakley, Springboard; Aline Noizet, Digital Health Connector; Oliver Haferbeck, Gerresheimer; Eric Chanie, Merck; and James Fries, Rx-360.


Complete solutions from a single source
We think holistically in terms of solutions – not only machines.
From the planning of the packaging to the operation of the plant, we advise our customers and deliver end-to-end solutions along with the complete lifecycle. This covers the machine, digital solutions, service, maintenance and optimization measures.


PARENTERAL PACKAGING
BOTTLE LINES
BLISTER LINES
TABLET COUNTERS
CARTONERS
LABELING
TRACK + TRACE
WALLET PACKAGING
Packaging machines, services and digital solutions for the pharmaceutical, health care, consumer goods, food and agricultural market.
www.uhlmann.de


































































































































































































































































































































































 Get More with Paxiom.
Get More with Paxiom.
















 9701 Giovanni Stevanato Dr, Fishers, IN, 46038, USA
9701 Giovanni Stevanato Dr, Fishers, IN, 46038, USA
























































































