
10 minute read
Small pipes, large benefits
Tyler Schertz, Mettler Toledo, Switzerland, details the process of measuring small pipelines with tunable diode laser (TDL) using a multi-reflection folded optical path.
The chemical and petrochemical industries require real-time information on the dynamics of the production of an end product, in order to control the process and improve both safety and efficiency. To conform to these aspects, significant layers of automation are implemented to report the dynamic readings in the process, by means of various devices that detail the process variables such as pressure, temperature and concentrations.
Measurement techniques
Concentration readings are provided by an analyser or chemical sensor. One notable example of this automated measurement is the assurance that oxygen does not ingress into a flare pipeline between the knock-out vessel and the liquid seal to prevent explosive mixtures from forming. To obtain these measurements, a large number of classical analytical methods have been adapted and packaged so that the measurement of a gas such as oxygen can be taken extractively or in situ in a process stream without the need for laboratory analysis by taking an aliquot. Analytical technologies such as paramagnetic (oxygen measurement), nondispersive infrared sensor (NDIR), ultraviolet-visible (UV-vis) and electrochemical are used extensively in the aforementioned industries. Of these analyses, many relate to classical absorption spectroscopy and, more recently, tunable diode laser absorption spectroscopy (TDLAS or TDL).
TDL is a mature analytical method and although it was primarily an extractive methodology and continues to be used as such, in recent years, multiple instrumentation vendors have established in situ measurements as cross-stack and probe designs. Mettler Toledo has also introduced a wafer process adaption for measurement in extremely small pipeline diameters of 5 – 10 cm (2 – 4 in).

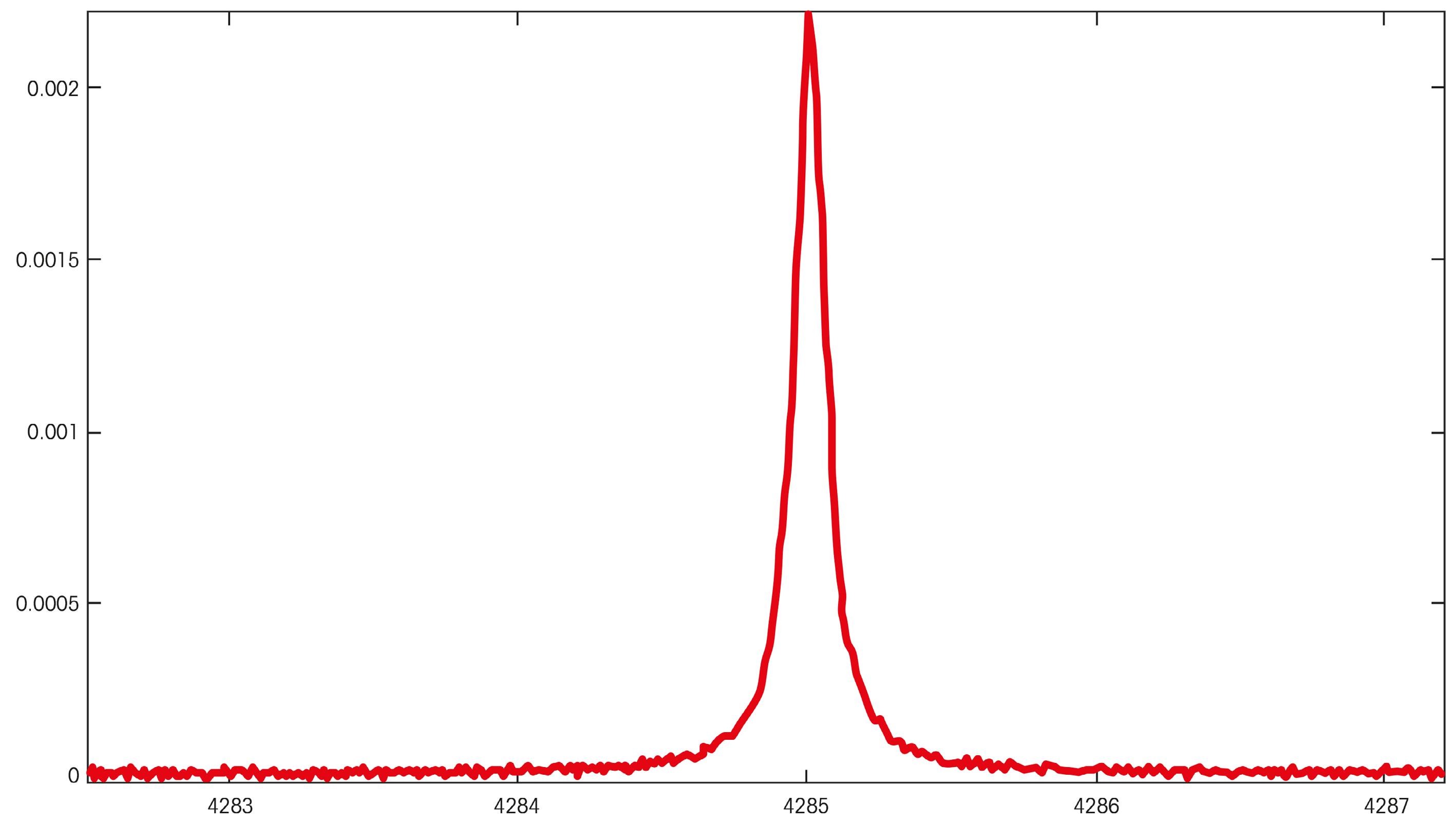
Figure 1. Spectroscopic differences between UV-vis absorption (top) and TDL absorption (bottom). Note the contrast in wavelength specificity.
Figure 2. The conceptual approach to path length multiplication by the White cell.
The Beer-Lambert law
Absorption spectroscopies in the domain of process industries include UV-vis, infrared (IR), NDIR, TDL and Raman, and are a consequence of the fact that molecules absorb light in a specific fashion. For example, light of specific frequencies is absorbed by a specific molecule in an exact and repeatable manner. The implication here is that a particular molecule at a specific concentration will absorb light of the same wavelengths and the resulting absorption of light will be proportional to the concentration. The physical law that is at the heart of this concept is the Beer-Lambert law that follows as equation 1:
I = I0 e-acL (1)
The absorption of light by molecules can be widely different. UV-vis absorptions are quite broad and use light in the UV and visible wavelengths. In contrast, near-infrared (NIR) spectroscopy, where TDL resides, has extremely narrow wavelengths and this is a major benefit to selectively choosing an absorption line that is unique to one analyte in a multicomponent process stream.
Equation 1 relates the absorption (I) by the analyte to the total light emitted (I0). This absorption is proportional to the absorption coefficient (a) multiplied by the path length and the concentration. The absorption coefficient itself is dependent upon the pressure and temperature of the gas. Fundamentally, the Beer-Lambert law is summarised as: the absorbance of light energy is proportional to the path length of light passing through the sample, multiplied by the concentration of the species.
It is easy to observe from equation 1 that low concentrations will present a very small absorption of light and that to increase the absorption of light, one must increase the optical path length (OPL) presented for the laser beam to transit or increase the concentration of the absorbing species. This article will further discuss the means by which a measurement for very low concentrations of analyte with TDL can be improved by increasing the OPL through the use of unique optical component arrangements, and the pros and cons of each.
Absorption spectroscopy
Returning to the presentation of absorption spectroscopy and the major difference between TDL and others such as UV-vis, note the spectral wavelengths presented for a typical absorption in the graphs in Figure 1. Clearly, the advantage with TDL is the narrow absorption line(s) which is often unique for a particular analyte of interest and fits perfectly with the narrow spectral range presented by laser light. Other technologies such as paramagnetic analysers are susceptible to interferences, measure indirectly and cannot measure in the process itself.
The Beer-Lambert law clearly implies that one of the major limitations to obtaining low level (ppm and ppb) measurements by TDL is potential limitations to the overall OPL. A general constraint of obtaining a TDL measurement is related to the commercial availability of lasers of the required wavelengths. Oxygen is a notoriously weak absorbing species with respect to the NIR range lasers on the market today. This critical gas measurement relies greatly on the ability to increase the OPL.

The process industries clearly demonstrate that the limitation here is most commonly the physical dimensions of the process piping in which the gas sample is contained for in situ measurements. For flue and other ducts of 1 – 3 m dimensions, path length does not become a major obstacle to the measurement in situ, even for weakly-absorbing species such as oxygen. However, when the diameter of the pipe is less than 38 cm (15 in.), the direct OPL for an in situ measurement by cross-pipe or probe design becomes significantly more difficult when measuring for oxygen below 5000 ppm (0.5%).
TDL measurements in the latter example present only a few notable designs on the market for increasing the OPL of extractive measurements, and for in situ TDL there is only one prominent and unique process adaption by which to measure in small pipe diameters for oxygen. Additionally, when the cross stack is mounted longitudinally, the purge gas protecting the optics from contamination leads to the dilution of the sample stream. This is a significant consideration for the accurate measurement of the lower explosive limit (LEL) of oxygen in a process stream.
Common methods to increase the OPL typically fall under the following three categories: n White cell. n Herriott cell. n Optical prism arrangement (multi-reflection device).
White cell
The White cell (Figure 2) was first detailed by John White in 1942 in the Journal of the American Optical Society as a means to increase the OPL using a compact arrangement of three spherical mirrors with the same radius of curvature.1 In this arrangement, the primary mirror is diametrically opposed to the secondary mirrors to provide an increase in path length that is always four-fold the distance between the primary and secondary mirrors.
Although this accomplishes the goal of increasing the path length to achieve the desired detection sensitivity, it suffers from placing the optics in contact with the process gases, which can be caustic or contain contaminants such as dust and condensable materials.
The other detriment to using this arrangement is that aligning three mirrors and spacing them accordingly can be rather difficult, and the precision wave front (smoothness) of the mirrors is an added expense and is easily destroyed by contamination from dust and condensates.
Herriott cell
The Herriott cell is another popular option with some TDL manufacturers to increase the OPL.2 This method relies on two spherical, highly-reflective and polished mirrors to propagate an incident laser beam between two spherical mirrors, as shown in Figure 3. Depending on the angle of the incident beam through a small hole on the primary mirror, path lengths of many metres are possible, providing very low detection limits.
The Herriott cell has an advantage over the White cell in that the mirror arrangement is typically more stable and easier to align, with the primary factor being that there is one
Your Specialist for PRESSURE RELIEF SOLUTIONS
Consulting. Engineering. Products. Service. T +49 2961 7405-0 T +1 704 716 7022

Figure 3. A general perspective of the Herriott cell and the propagation of the beam line.
Figure 4. The optical arrangement for the multi-reflection prism arrangement.
less mirror in the arrangement to carefully position. Here again, however, there are a few disadvantages in that the optics are in contact with the process gases and the size and materials of construction generally relegate this to an extractive measurement.
The disadvantages of both methods are significant considerations when the majority of industries utilising TDL consider in situ measurements as a significant factor for form and function of an analyser.
Optical prism arrangement
In the last two years, a new process adaption has been introduced into the TDL marketplace. The in situ self-aligning probe design offered by Mettler Toledo addresses customary pain points. Namely, it addresses the need to make a measurement within the process at critical measurement points for process safety and control, and provides a modular, universal design that is easy to maintain.
One of the limitations to enclosing the optical components in a rigid body as designed by the company for in situ measurements is that often the pipeline diameter for the in situ measurement of oxygen, such as a flare header pipe, is limited to very small diameters. This often limits the insertion depth of the probe to 20 cm and thus restricts the lower detection limit of oxygen to thousands of ppm.
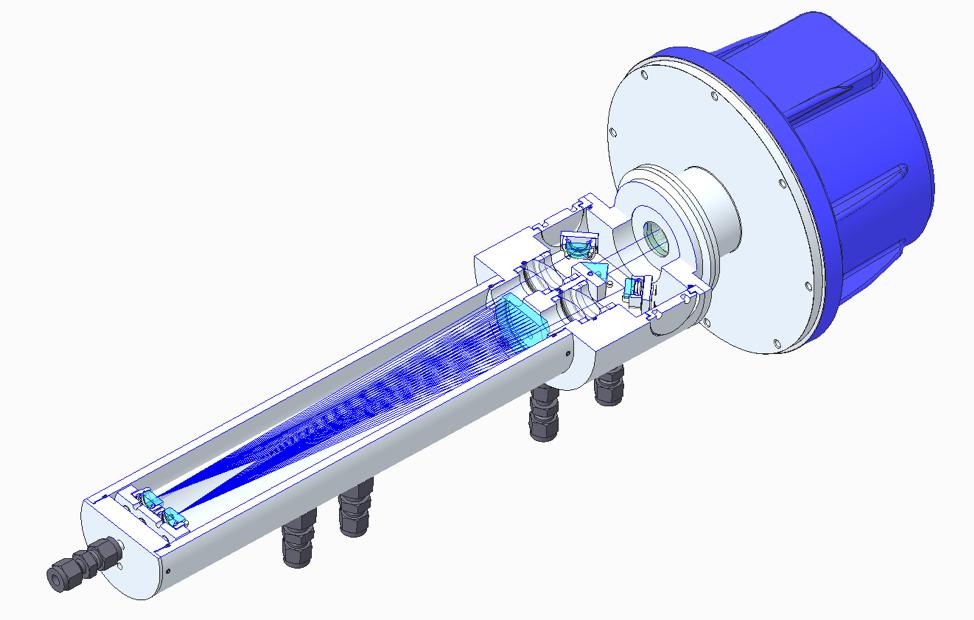
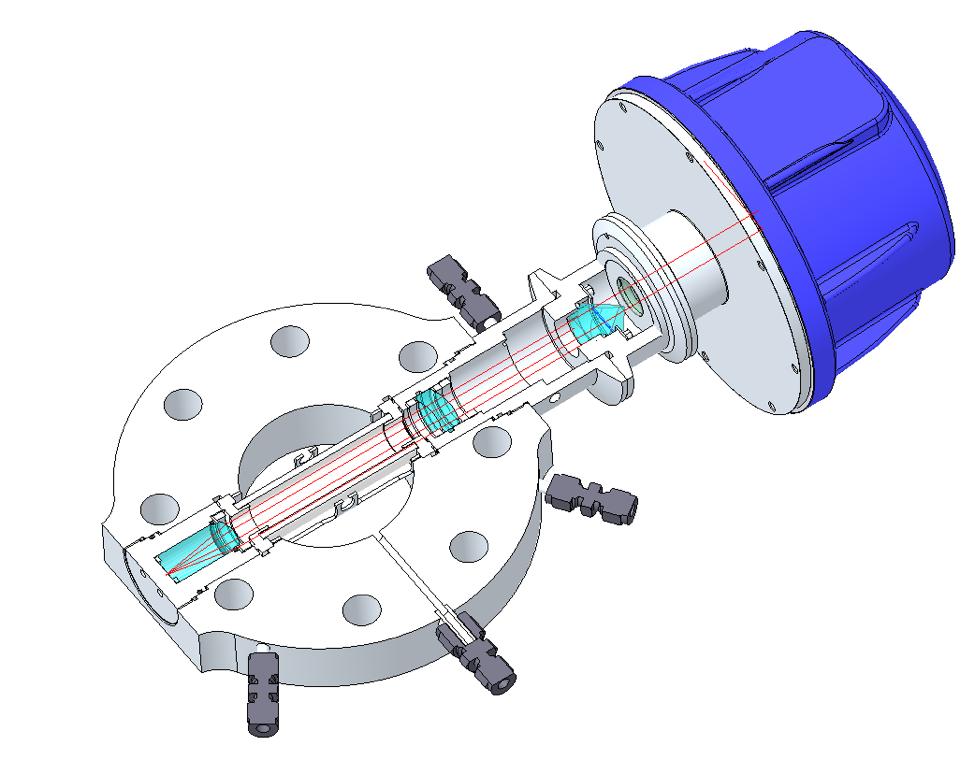
Another significant form factor in the product portfolio is the unique process adaption aptly named the wafer, which functions as a spool piece within the pipe without obstructing the process flow. This can be fitted to pipe diameters as small as 10 cm (2 in. OPL). To address the severely shortened OPLs in these measurements, a new optical means was developed to increase the OPL in situ and fit with the form and function of the product line – the multi-reflection device (MRx).
This new, commercially-available means to increase OPL is unique compared with the Herriott and White cell arrangements in that it does not rely on mirrors in a properly conditioned sample stream. The laser beam path in this optical arrangement is simply doubled or tripled by utilising optical prisms, as shown in Figure 4. The secondary optic (prism) in this in situ measurement is not in contact with the process gas, which is a significant benefit for use in harsh processes that could introduce optical fouling. It is also a significantly more compact technique than the other methods to increase the OPL, and this can benefit installations where the external physical dimensions are limited.
The same secondary optical prism is used with both the two-fold (MR2) and three-fold (MR3) OPL adaptions. To achieve the three-fold reflection, an uncoated sapphire ‘cat eye’ lens is used, which is not only extremely durable and chemically-compatible, but also has the distinct advantage of eliminating flat reflective surfaces that could induce etalon effects and therefore improve the baseline noise that is inherent to direct absorption spectroscopy.
Conclusion
TDL spectroscopy is often considered by the process industries based on its long life, analyte specificity and capability to make fast in situ measurements for reasons of process safety and control with oxygen as the primary species of measurement. Oxygen unfortunately suffers from being a weakly-absorbing species in the perspective of the choice of adequate laser diodes. As such, means to increase the OPL are critical in TDL measurements of oxygen.
A number of methods with regards to increasing the OPL have been introduced in this article. Two of the aforementioned methods suffer from notable drawbacks for use in harsh industrial processes. However, the MRx from Mettler Toledo helps to meet the needs and long operational life of TDL in chemical, petrochemical and other industries, while addressing the critical need to fit within compact pipeline diameters to measure low concentrations of oxygen for safety and process control. In the near future, it is anticipated that other path length increasing optical methods that correspond with the form and function of today’s needs and desires within the scope of TDL measurements will become available.
References
1. WHITE, J, U., ‘Long Optical Paths of Large Aperture,’ Journal of the
Optical Society of America, Vol. 32, No. 5 (1942), pp. 285 – 288. 2. HERRIOTT, D., and SCHULTE, H., ‘Folded Optical Delay Lines’,
Applied Optics, Vol. 4, No. 8 (1965), p. 883.










