Leczenie Ran

Polish Journal of Wound Management
Kwartalnik | Zeszyt 2 | Tom 22 | Rok 2025
ISSN: 1733-4101
eISSN: 1733-7607
MNiSW: 20 IC: 86,65

breastfeeding
Ekspozycja na materiał potencjalnie zakaźny i profilaktyka poekspozycyjna




Kwartalnik | Zeszyt 2 | Tom 22 | Rok 2025
ISSN: 1733-4101
eISSN: 1733-7607
MNiSW: 20 IC: 86,65

breastfeeding
Ekspozycja na materiał potencjalnie zakaźny i profilaktyka poekspozycyjna



ISSN: 1733-4101 | e-ISSN: 1733-7607
Redakcja
Redaktor naczelny
Arkadiusz Jawień 0000-0001-8380-9371
Katedra i Klinika Chirurgii Naczyniowej i Angiologii, Szpital Uniwersytecki Nr 1 im. dr. A. Jurasza w Bydgoszczy, e-mail: ajawien@ceti.com.pl
Zastępcy redaktora naczelnego
Maria T. Szewczyk 0000-0002-0511-0685
Katedra Pielęgniarstwa Zabiegowego, Collegium Medicum UMK, Szpital Uniwersytecki nr 1 im. dr. A. Jurasza w Bydgoszczy
Beata Mrozikiewicz-Rakowska 0000-0002-1160-9204
Klinika Diabetologii i Chorób Wewnętrznych, Warszawski Uniwersytet Medyczny
Maciej Sopata
Katedra i Klinika Medycyny Paliatywnej, Hospicjum Stacjonarne Uniwersytetu Medycznego im. Karola Marcinkowskiego w Poznaniu Mariusz Kózka
Oddział Kliniczny Chirurgii Naczyniowej, Szpital Uniwersytecki w Krakowie
Sekretarz Redakcji
Katarzyna Cierzniakowska 0000-0002-4657-8321
Katedra Pielęgniarstwa Zabiegowego, Collegium Medicum UMK, Szpital Uniwersytecki nr 2 im. dr. J. Biziela w Bydgoszczy, e-mail: katarzyna.cierzniakowska@biziel.pl
Konsultant ds. statystyki
Andrzej Tukiendorf
Pracownia Biostatystyki Klinicznej, Zakład Radioterapii, Narodowy Instytut im. Marii Skłodowskiej-Curie w Gliwicach
Redaktorzy językowi
Timothy Alexander Monika Ślusarska
Rada Naukowa
Magdalena Annersten Gershater 0000-0003-4395-2522
Department of Care Science, Faculty of Health and Society, Malmö University, Malmö, Sweden
Dariusz Bazaliński 0000-0003-1717-1319
Instytut Nauk o Zdrowiu, Kolegium Nauk Medycznych, Uniwersytet Rzeszowski, Rzeszów, Polska
Piotr Ciostek 0000-0001-7461-0221
Katedra i Klinika Chirurgii Ogólnej i Naczyniowej, Warszawski Uniwersytet Medyczny, Polska
Gaye Filinte 0000-0003-2583-2922
Plastic, Reconstructive and Aesthetic Surgery Department, Faculty of International Medicine, University of Health Sciences, Istanbul, Turkey Kartal Dr Lütfi Kırdar City Hospital, Kartal Burn and Wound Centre, Istanbul, Turkey
Eugenia Gospodarek 0000-0003-0334-7520
Katedra Mikrobiologii, Collegium Medicum w Bydgoszczy, Uniwersytet Mikołaja Kopernika w Toruniu, Polska

Wydawnictwo Moc Media
ul. Mokra 2/51
03-562 Warszawa, Polska e-mail: biuro@mocmedia.eu
na prośbę Polskiego Towarzystwa Leczenia Ran | Polish Wound Management Association
Finn Gottrup
University of Southern Denmark, Copenhagen Wound Healing Centre, Bispebjerg University Hospital, Copenhagen, Denmark
Tomasz Grzela 0000-0001-8519-4868
Katedra i Zakład Histologii i Embriologii, Centrum Biostruktury, Warszawski Uniwersytet Medyczny, Polska
Tomasz Karpiński 0000-0001-6599-9204
Katedra i Zakład Mikrobiologii Lekarskiej, Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu, Polska
Anna Korzon-Burakowska 0000-0002-5655-1798
Katedra Nadciśnienia Tętniczego i Diabetologii, Gdański Uniwersytet Medyczny, Polska
Maria Kózka 0000-0002-5165-6929
Instytut Pielęgniarstwa i Położnictwa, Uniwersytet Jagielloński Collegium Medicum w Krakowie, Polska
Christine J. Moffatt 0000-0002-2436-0129
Centre for Research and Implementation of Clinical Practice, London, United Kingdom Nottingham University Hospitals, NHS Trust, Nottingham, United Kingdom
Paulina Mościcka 0000-0002-0128-5533
Katedra Pielęgniarstwa Zabiegowego, Wydział Nauk o Zdrowiu, Collegium Medicum w Bydgoszczy, Uniwersytet Mikołaja Kopernika w Toruniu, Polska
Harikrishna K.R. Nair
Wound Care Unit, Department of Internal Medicine, Specialist Complex and Ambulatory Care Centre (SCACC), Hospital Kuala Lumpur, Kuala Lumpur, Malaysia
Andrea Pokorná 0000-0002-1305-6455
Department of Nursing and Midwifery, Faculty of Medicine, Masaryk University, Brno, Czech Republic
Angelo Scuderi
Sao Paulo, Brasil
Anna Sobieszek-Kundro 0000-0002-8938-5263
Oddział Dermatologiczny, Wojewódzki Szpital Zespolony w Elblągu, Polska
Anna Spannbauer 0000-0002-7745-5754
Klinika Chirurgii, Wydział Nauk o Zdrowiu, Instytut Fizjoterapii, Uniwersytet Jagielloński Collegium Medicum w Krakowie, Polska
Elżbieta Tomaszewska
Oddział Opieki Paliatywnej, Hospicjum Palium w Poznaniu, Polska
Hakan Uncu
Division of Vascular Surgery, Department of General Surgery, Ankara University School of Medicine, Ankara, Turkey
Tomasz Urbanek 0000-0002-5044-9186
Katedra i Klinika Chirurgii Ogólnej, Naczyń, Angiologii i Flebologii, Śląski Uniwersytet
Medyczny w Katowicach, Polska
Peter Vowden 0000-0002-7068-6859
Faculty of Life Sciences, University of Bradford, and Honorary Consultant Vascular Surgeon, Bradford Royal Infirmary, Bradford, United Kingdom
Oficjalne czasopismo naukowe Polskiego Towarzystwa Leczenia Ran członka Europejskiego Towarzystwa Leczenia Ran www.pjwm.mocmedia.eu
Dostęp do artykułów publikowanych w czasopiśmie jest otwarty Wersja papierowa czasopisma jest wersją referencyjną

CEO Moc Media
Sylwia Chrabałowska
e-mail: s.chrabalowska@mocmedia.eu
Sekretarz Redakcji Moc Media
Monika Szymor
e-mail: m.szymor@mocmedia.eu
Dział Prenumerat Czasopism journals@mocmedia.eu
Dyrektor Biura Reklamy Crockett Media
Marta Orzełowska
e-mail: morzelowska@crockettmedia.pl

GUIDELINES
46 Treatment of nipple wounds during breastfeeding. Position of the Center for Lactation Science and the Polish Wound Management Association
Monika Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewicz-Rakowska, Monika Aleksy-Polipowska, Natalia Żółtowska, Roksana Kulińska, Debora Barbay
Annex 1. General principles of wound assessment
Annex 2. List of lavaseptics, antiseptics, and specialized dressings
ARTYKUŁ ORYGINALNY | ORIGINAL PAPER
80 Ekspozycja na materiał potencjalnie zakaźny i profilaktyka poekspozycyjna
Exposure to potentially infectious material and post-exposure prophylaxis
Katarzyna Cierzniakowska, Daria Kaniewska, Elżbieta Kozłowska, Aleksandra Popow
SPRAWOZDANIE | REPORT
88 Sprawozdanie z Warsztatów „Rozwój zaawansowanych kompetencji w leczeniu ran” realizowanych podczas II Międzynarodowej Konferencji Naukowo-Szkoleniowej
Beata Wieczorek-Wójcik, Paulina Mościcka, Katarzyna Kowalska, Katarzyna Cierzniakowska
Od Redakcji
tegoroczny drugi numer „Leczenia Ran” ma dla mnie szczególne znaczenie.
Po pierwsze, na dorocznym zjeździe EWMA w Barcelonie, w którym uczestniczyło ponad 5ooo osób, dr hab. n. med. Justyna Cwajda-Białasik z Bydgoszczy, po pięknej prezentacji zatytułowaniej Results of a pilot study of diabetes-related foot disease (DFD) screening in an AT-risk group, otrzymała nagrodę za najlepszy abstrakt roku (Best Abstract of the Year Prize). Praca ta powstała przy współudziale dr hab. n. med. Pauliny Mościckiej i pod kierownictwem prof. Marii Szewczyk w Katedrze Pielęgniarstwa Zabiegowego Collegium Medicum im. L. Rydygiera UMK i Poradni Leczenia Ran Przewlekłych Szpitala Uniwersyteckiego nr 1 w Bydgoszczy. To ogromne wyróżnienie i ukoronowanie wieloletniej pracy naukowej całego zespołu prof. Marii Szewczyk, która w 2000 r. rozpoczęła piękną przygodę z leczeniem ran przewlekłych, tworząc bardzo dobry ośrodek i wychowując kolejne pokolenie osób zainteresowanych tą dziedziną medycyny. Niedawno zatwierdzone dwa tytuły doktora habilitowanego dla dr Pauliny Mościckiej i dr Justyny Cwajda-Białasik są nie tylko potwierdzeniem właściwego procesu kształcenia i prowadzenia badań naukowych, lecz także znakomitym przykładem dla innych szybko rozwijających się ośrodków leczenia ran w Polsce, w jaki sposób łączyć i rozwijać te wszystkie elementy dla dobra chorego z raną przewleką.
Serdeczne gratulacje! Po drugie, numer ten jest ostatnim dla mnie w roli redaktora naczelnego. Podjąłem decyzję o zakończeniu współpracy z czasopismem „Leczenie Ran”. Chciałbym tym ciekawym, drugim tegorocznym, numerem podziękować wszystkim Państwu za miłą współpracę przez ostatnich blisko 18 lat. Stanowisko redaktora naczelnego przez te wszystkie lata było dla mnie ogromnym wyzwaniem polegającym nie tylko na kompletowaniu poszczególnych numerów, zapraszaniu do współpracy i pisania ciekawych artykułów,

ale i do odpowiednich relacji z wydawcami, których w czasie mojej kadencji aż czterokrotnie musieliśmy zmieniać.
Chciałbym serdecznie podziękować wszystkim członkom Rady Naukowej „Leczenia Ran” za wsparcie i pomoc w pozyskiwaniu prac oraz redagowaniu naszego czasopisma. Szczególne podziękowania kieruję do dr n. med. Katarzyny Cierzniakowskiej, sekretarz Redakcji, która nie tylko wykazywała się ogromną cierpliwością i wytrwałością w poprawianiu prac przychodzących do druku, ale i w kontaktowaniu się z autorami prac i mobilizowaniu recenzentów do dotrzymywania terminów ich recenzji.
Osobne podziękowania składam wydawnictwu
Moc Media i osobiście red. Sylwii Chrabałowskiej, której pomysł umiędzynarodowienia czasopisma zyskał moją aprobatę i okazał się przysłowiowym „strzałem w dziesiątkę”. Udało się nam pozyskać autorów z innych krajów i spowodować, że język angielski publikacji zagościł na łamach „Leczenia Ran” już na stałe, co pozwoliło na większą rozpoznawalność czasopisma na świecie.
Kończąc swoją, jakże ciekawą i zarazem wymagającą wiele wysiłku kadencję, chciałbym następnemu redaktorowi naczelnemu „Leczenia Ran” życzyć, aby zapoczątkowane zmiany udało się kontynuować, w tym działania o wprowadzenie czasopisma do nowych baz indeksacyjnych.
Wszystkim czytelnikom dziękuję za zainteresowanie naszym czasopismem i życzę, aby zawsze przekazywało ono najlepszą i najbardziej aktualną wiedzę o leczeniu ran w naszym kraju i na świecie.
To była wielka przyjemność służyć Państwu.
W imieniu Komitetu Redakcyjnego i Rady Naukowej „Leczenia Ran | Polish Journal of Wound Management” prof. Arkadiusz Jawień redaktor naczelny
From the Editor
This year’s second “Leczenie Ran” issue is significant to me.
Firstly, at the annual EWMA conference in Barcelona, attended by over 5,000 people, Dr. Justyna Cwajda-Białasik from Bydgoszcz, after a beautiful presentation entitled Results of a pilot study of diabetes-related foot disease (DFD) screening in an AT-risk group, received the Justyna Cwajda-Białasik from Bydgoszcz, after a beautiful presentation entitled Results of a pilot study of diabetes-related foot disease (DFD) screening in an AT-risk group, received the award for the best abstract of the year (Best Abstract of the Year Prize). This work was done in collaboration with Dr Paulina Mościcka, MD, PhD, and under the supervision of Prof. Maria Szewczyk at the Department of Surgical Nursing of the L. Rydygier Collegium Medicum of the Nicolaus Copernicus University and the Chronic Wound Treatment Clinic of University Hospital No. 1 in Bydgoszcz. This is a huge distinction and the crowning achievement of many years of scientific work by the entire team of Prof. Maria Szewczyk, who began her excellent adventure with chronic wound treatment in 2000, creating an outstanding centre and educating the next generation of people interested in this field of medicine. The recently approved two habilitation degrees for Dr Paulina Mościcka and Dr Justyna Cwajda-Białasik are not only a confirmation of the proper process of education and scientific research but also an excellent example for other rapidly developing wound treatment centres in Poland of how to combine and create all these elements for the benefit of patients with chronic wounds.
Congratulations!
Secondly, this issue is my last as Editor-in-Chief. I have decided to end my collaboration with the journal “Leczenie Ran” | “Polish Journal of Wound Management”.
I would like to use this interesting second issue of the year to thank you all for your kind cooperation

over the past nearly 18 years. The position of editor-in-chief has been a massive challenge for me over the years, involving putting together individual issues, inviting contributors, writing interesting articles, and maintaining good relations with publishers, which we had to change four times during my tenure.
I would like to sincerely thank all members of the Scientific Council of “Leczenie Ran” | “Polish Journal of Wound Management” for their support and assistance in obtaining articles and editing our journal. Special thanks are due to Dr. Katarzyna Cierzniakowska, MD, Editorial Secretary, who demonstrated great patience and perseverance in proofreading the submitted articles for publication, contacting the authors, and motivating the reviewers to meet their deadlines.
I would also like to thank the publishing house Moc Media and personal editor Sylwia Chrabałowska, whose idea of internationalizing the journal won my approval and proved a proverbial “bull’s eye”. We have attracted authors from other countries and made English a permanent feature of “Leczenie Ran” | “Polish Journal of Wound Management”, dramatically increasing the journal’s international recognition.
As I conclude my interesting and demanding term of office, I wish the next Editor-in-Chief of Leczenie Ran every success in continuing the changes that have been initiated, including efforts to introduce the journal to new indexing databases.
I would like to thank all readers for their interest in our journal and hope it will always provide the best and most up-to-date knowledge on wound treatment in our country and worldwide.
It has been a great pleasure to serve you.
On behalf of the Editorial Committee and Scientific Council of the “Leczenie Ran | Polish Journal of Wound Management” Prof. Arkadiusz Jawień Editor-in-Chief
GRUDZIEŃ
18.01.2025 | Łódź
Warsztaty PTLR
Cukrzycowe
owrzodzenia stópprofilaktyka i leczenie
8.03.2025 | Bydgoszcz
Warsztaty PTLR
Rany w praktyce dermatologicznej

12.04.2025 | Kraków
Warsztaty PTLR
Rany w ginekologii, położnictwie i neonatologii
5-7.06.2025 | Łochów k. Warszawy Kongres
Naukowo-Szkoleniowy Polskiego Towarzystwa Leczenia Ran
13.09.2025 | Poznań
Warsztaty PTLR
Leczenie ran w opiece paliatywnej i opiece długoterminowej
25.10.2025 | Katowice
Warsztaty PTLR Owrzodzenia goleni
15.11.2025 | Gdynia
Warsztaty PTLR Rany w POZ
6.12.2025 | Warszawa
Międzywojewódzka Konferencja Polskiego Towarzystwa Leczenia Ran
















oczyszczanie nawilżanie dezynfekcja ran hipoalergiczny od 1. dnia życia
Wytyczne
LECZENIE RAN 2025; 22 (2): 46–79
DOI: 10.60075/lr.v22i2.103

Monika Żukowska-Rubik1,2, Magdalena Nehring-Gugulska1,3, Beata Mrozikiewicz-Rakowska4, Monika Aleksy-Polipowska5,6, Natalia Żółtowska7, Roksana Kulińska8, Debora Barbay1,9
1 Centrum Nauki o Laktacji
2 Centrum Medyczne Żelazna
3 Klinika Zaburzeń Laktacji Centrum Medycznego Warszawskiego Uniwersytetu Medycznego
4 Klinika Endokrynologii Centrum Medycznego Kształcenia
Podyplomowego
5 Podmiot Leczniczy Zakonu oo. Kamilianów, Oddział Medycyny Paliatywnej, Szpital Św. Kamila, Tarnowskie Góry.
Bolesność i rany brodawek sutkowych to częsty problem w okresie laktacji, zwłaszcza w pierwszych tygodniach po porodzie. Stany te wiążą się z ryzykiem zapalenia piersi, słabego przyrostu masy ciała noworodka, dokarmiania mlekiem modyfikowanym oraz przedwczesnego przerwania karmienia piersią. Podstawowe znaczenie ma profilaktyka i wczesne rozpoznawanie tych stanów, są to zadania personelu medycznego sprawującego opiekę nad matką i niemowlęciem. Najczęstszą przyczyną jest zbyt płytkie uchwycenie piersi przez dziecko, dlatego w zapobieganiu i leczeniu uszkodzeń brodawek kluczowa jest poprawa techniki karmienia. Przy prawidłowym postępowaniu gojenie następuje szybko. Jednak w przypadku trudno gojących się czy zakażonych ran brodawek należy niezwłocznie podjąć działania diagnostyczne i lecznicze.
W stanowisku Centrum Nauki o Laktacji (CNoL) oraz Polskiego Towarzystwa Leczenia Ran (PTLR) zaprezentowano w praktyczny sposób zasady leczenia ran brodawek w okresie laktacji z uwzględnieniem ich odrębności w stosunku do ran o innej etiologii i w innych lokalizacjach. Omówiono


6 Małopolska Uczelnia Państwowa im. Rotmistrza Witolda Pileckiego Instytut Nauk o Zdrowiu, Oświęcim
7 NZOZ „Lekarz Rodziny” Aneta Wojno, Mariusz Wojno s.c., Sanniki
8 Centrum Zdrowej Skóry Saska Skin
9 Oddział Patologii Noworodka i Niemowlęcia, Szpital Dziecięcy im. prof. dr. med. J. Bogdanowicza, Samodzielny Publiczny Zakład Opieki Zdrowotnej
Adres do korespondencji
Monika Żukowska-Rubik, FTK/Centrum Nauki o Laktacji, ul. Bobrowiecka 9, 00-728 Warszawa, e-mail: mzr@kobiety.med.pl
Nadesłano: 15.05.2025; Zaakceptowano: 24.05.2025
zagadnienia związane z rozwojem zakażenia w ranie, strategię TIMERS, szczegółowo zasady postępowania podczas laktacji, których wdrożenie jest niezbędne, by proces gojenia mógł przebiegać optymalnie.
W opracowaniu zaproponowano podział ran na 4 kategorie: niezakażone, zagrożone zakażeniem, zakażone, szerzenie się zakażenia z rany na okoliczne tkanki. Opisując poszczególne kategorie ran, wskazano odpowiednie środki lecznicze w postaci lawaseptyków, antyseptyków i opatrunków specjalistycznych. Omówiono wskazania do antybiotykoterapii, technikę pobierania wymazu z rany oraz najczęstsze błędy popełniane w leczeniu ran brodawek.
Opisane zasady leczenia ran brodawek są oparte na światowych i polskich konsensusach w zakresie leczenia ran i wieloletnich, unikalnych doświadczeniach polskich doradców laktacyjnych.
Słowa kluczowe: karmienie piersią, bolesne brodawki, uszkodzone brodawki, rana brodawki, zakażenie rany, antyseptyki.
Materiał przeznaczony jest wyłącznie dla profesjonalistów wykonujących zawody medyczne ze wskazaniem na lekarzy, położne, pielęgniarki sprawujące opiekę nad kobietami i ich dziećmi w okresie laktacji. Będzie szczególnie przydatny dla specjalistów ds. laktacji.
Bolesność i rany brodawek to częsty problem matek karmiących. W badaniach pochodzących z różnych krajów aż 62–95% kobiet zgłaszało dolegliwości o takim charakterze wcześnie po porodzie [1]. Największą częstość występowania bólu obserwuje się w 1. tygodniu po porodzie, jego nasilenie u większości kobiet zmniejsza się do łagodnego w ciągu 7–10 dni [2]. Ponad połowa matek z bolesnością brodawek ma również widoczne rany brodawek lub inne zmiany skórne [3]. Na podstawie liczby urodzeń w 2024 r. (252 tys.) oraz faktu, że 97% kobiet w Polsce zaczyna karmić piersią [4], a 30% z nich ma rany brodawek, można oszacować, że ponad 73 tys. matek karmiących borykało się z problemem ran brodawek.
Kobiety z uszkodzonymi brodawkami w badaniu Coca i wsp. oceniały nasilenie bólu przeciętnie na 6,2 pkt w 1. tygodniu i 5,8 pkt w późniejszym okresie, kobiety bez widocznych uszkodzeń natomiast przeciętnie na 2,7 pkt [w numerycznej skali oceny bólu (NRS) lub innej skali z zakresem 0–10 pkt, gdzie ból łagodny oceniano jako 1–3, umiarkowany 4–6, a silny 7–10 pkt) [5].
Bolesność brodawek jest jedną z głównych przyczyn podawania mleka modyfikowanego i przedwczesnego zakończenia karmienia. W USA wśród matek, które zrezygnowały z karmienia w 1. miesiącu, 1/3 jako przyczynę podała ból lub rany brodawek [3]. Rany brodawek są też czynnikiem ryzyka zapalenia piersi [6]. Dolegliwości odczuwane przez matki wielokrotnie w ciągu dnia w związku z przystawianiem dziecka do piersi mają wpływ na jakość karmienia, aktywność, nastrój, sen matki i zwiększają ryzyko depresji. Poprzez zaburzenie odruchu oksytocynowego mogą powodować ograniczenie poboru mleka przez noworodka [7].
Ze względu na wszystkie poważne konsekwencje omawianych zagadnień potrzebna jest profilaktyka realizowana poprzez edukację matek po porodzie co do techniki karmienia oraz wczesne wykrywanie uszkodzeń i wdrażanie prawidłowego leczenia. Leży to w zakresie obowiązków wszystkich specjalistów opiekujących się matką i dzieckiem – położnych, pielęgniarek, lekarzy i doradców laktacyjnych.
Odrębności ran brodawek sutkowych w porównaniu z powszechnie występującymi trudno gojącymi się ranami
Rana to przerwanie ciągłości skóry lub skóry i tkanek pod nią położonych. Rana brodawki sutkowej lub otoczki powstaje zazwyczaj na
skutek urazu mechanicznego (zaciskanie, pociąganie, ocieranie, zasysanie) wynikającego z nieprawidłowego ssania piersi lub niewłaściwego używania laktatora. Rany kąsane powstają w wyniku ugryzienia przez ząbkujące niemowlę.
Rany brodawek cechuje szereg odrębności w porównaniu z ranami o innej etiologii i lokalizacjach. Występują one u młodych, na ogół zdrowych kobiet, w dobrze ukrwionej i unerwionej okolicy. Mimo swoich zwykle niewielkich, kilku- czy kilkunastomilimetrowych rozmiarów, powodują duże dolegliwości bólowe, a ich gojenie utrudnione jest przez regularne karmienie dziecka lub odciąganie pokarmu ze zranionej piersi. Stosowane opatrunki muszą być zdejmowane co kilka godzin na czas karmienia, a środki lecznicze usuwane z powierzchni skóry, tak aby podanie piersi było bezpieczne dla dziecka. Nie jest możliwe zastosowanie substancji lepkich, trudnych do spłukania.
Środowisko rany brodawki jest unikalne ze względu na to, że na jego kształtowanie ma wpływ mikrobiom skóry brodawki i przewodów mlecznych, właściwości pokarmu kobiecego oraz mikrobiom jamy ustnej dziecka i kontakt ze śliną. Odkryto też zależność między mikrobiotą jelita matki a jej pokarmem (droga jelitowo-piersiowa) [8]. Flora skóry brodawki jest utworzona przede wszystkim z bakterii (komensalnych, symbiotycznych, probiotycznych), w niewielkim stopniu grzybów i wirusów i jest zintegrowana z obroną immunologiczną gospodarza. Co ciekawe, flora ta różni się od flory mleka i stolca dziecka. Dominują bakterie z grupy Staphylococcus, Streptococcus, ale są też obecne Enterobacteriaceae, Corynebacterium , Propionibacterium , Bacteroides i inne [9].
Każde uszkodzenie naskórka czy skóry właściwej brodawki (również mikrouszkodzenia) wywołuje niekorzystne zmiany w składzie mikrobioty, co osłabia barierę skórną i stwarza warunki do kolonizacji przez drobnoustroje w różnym stopniu nasilenia.
Na brodawce, jako ujściu gruczołu skórnego, znajdują się bakterie komensalne w wyższym stężeniu niż w innych miejscach skóry piersi, więc kolonizacja rany następuje szybko. Pokarm matki oraz jego mikrobiom są ważnym czynnikiem ochronnym [3, 10].
W pokarmie znajduje się szereg składników o działaniu przeciwzapalnym, immunomodulującym, hamującym namnażanie drobnoustrojów i wspomagającym gojenie, takich jak interleukiny, laktoferyna, laktoadhezyna, lizozym, SIgA (wydzielnicza IgA),
antyoksydanty, czynniki wzrostu, interferon, witamina A, kwasy omega-3 i omega-6, mucyna i wiele innych [11, 12]. Ponieważ brodawka jest stale omywana pokarmem o silnych właściwościach bakteriostatycznych, to zlokalizowanym tam bakteriom jest trudniej się namnażać.
Postawiono hipotezę, że histatyny (peptydy o małej masie cząsteczkowej wydzielane do śliny u ssaków) obecne w ślinie dziecka mogą wspierać odbudowę naskórka przez pobudzanie migracji komórek naskórka, fibroblastów, angiogenezy i dodatkowo niszczyć bakterie [13]. Warto dodać, że karmienie z poranionej piersi, nawet jeśli rana jest zakażona, nie niesie ryzyka dla karmionego piersią niemowlęcia.
Przyczyny i objawy ran brodawek Najczęstszymi przyczynami bolesności i uszkodzeń brodawek są płytkie uchwycenie piersi, nieprawidłowe ssanie lub zaciskanie piersi dziąsłami, które zwykle spowodowane są niewłaściwą pozycją matki lub noworodka do karmienia lub nieprawidłowym sposobem przystawiania do piersi [14, 15]. Do uszkodzeń brodawek dochodzi często w nawale mlecznym lub obrzęku piersi z powodu trudności w przystawianiu spowodowanych obrzmieniem otoczki [14]. Niektóre niemowlęta generują zbyt wysokie podciśnienie, zarówno bazowe, jak i szczytowe, u ich matek częściej obserwowano bolesność [16]. Skrócone wędzidełko języka rzadko bywa przyczyną bólu brodawek wbrew panującej modzie na rozpoznawanie ankyloglosji Problem skrócenia wędzidełka dotyczy zaledwie 7% niemowląt [17], problemy z karmieniem zaś niespełna 50% tych dzieci [18]. Geddes badała grupę niemowląt z ankyloglosją, które wytwarzały zbyt niskie lub zbyt wysokie podciśnienie albo nadmiernie kompresowały brodawkę, jednak pobór pokarmu był wystarczający, a matki nie zgłaszały bolesności. Przyczyny uszkodzeń brodawek sutkowych zebrano w tabeli 1. Uszkodzenia są zwykle zlokalizowane na szczycie brodawek, czasem szczeliny i rozpadliny widoczne są
Tabela 1. Przyczyny uszkodzeń brodawek sutkowych [19]
Przyczyny uszkodzeń po stronie matki
Nieprawidłowa pozycja podczas karmienia, nieprawidłowe przystawienie dziecka
Napięta/obrzmiała otoczka w nawale lub obrzęku piersi
Płaskie, wklęsłe brodawki, inne trudne do chwytania warianty
Nieprawidłowe odłączanie dziecka od piersi
Niewłaściwy laktator (brak certyfikatu wyrobu medycznego)
lub niewłaściwie używany laktator (za duża siła ssania, za długie sesje, niewłaściwie dobrany rozmiar lejka)
na trzonie brodawki, u jej podstawy lub na otoczce. Mają różną głębokość i rozległość, mogą obejmować tylko naskórek oraz sięgać do głębszych warstw skóry. W tabeli 2 zaprezentowano rodzaje zmian skórnych obserwowanych w związku z uszkodzeniami brodawek sutkowych [20–22]. U każdej matki zwykle wyglądają inaczej, jednak Woolridge [23] zaobserwował pewne podobieństwa i zaproponował podział uszkodzeń brodawek na podstawie mechanizmu ich powstawania:
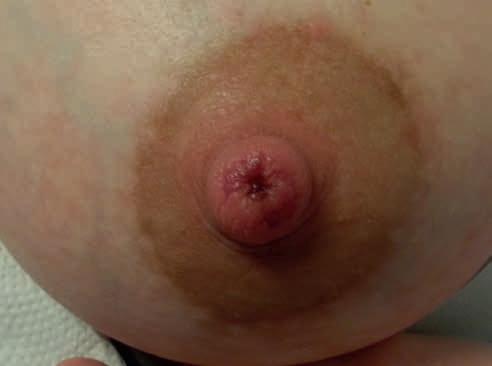
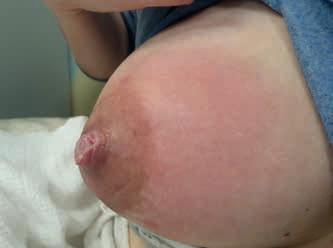
• uszkodzenie z kompresji – spłaszczenie brodawki widoczne po wyjęciu piersi z jamy ustnej dziecka, podłużne rany szczytu brodawki, szczeliny, strupy, określane jako pręgi pozycyjne, spowodowane niewłaściwym ułożeniem kompleksu brodawka–otoczka w jamie ustnej dziecka (ryc. 1 i 2);
• uszkodzenie z zasysania – uszkodzenia szczytu brodawki objawiające się jako zaczerwienienie,

Rycina 1. Spłaszczenie brodawki w mechanizmie kompresji widoczne po karmieniu (fot. M. Żukowska-Rubik).
Przyczyny uszkodzeń po stronie dziecka
Płytkie uchwycenie piersi podczas ssania
Nieprawidłowości anatomiczne jamy ustnej (skrócone wędzidełko języka, wysoko wysklepione podniebienie, mocno cofnięta żuchwa)
Zaburzenia czynnościowe funkcji ssania (nieprawidłowo wyrażone odruchowe reakcje oralne – nadmierne zasysanie, kąsanie, wygórowany odruch wymiotny), kompensacje)
Nieprawidłowe napięcie mięśniowe, asymetria, kręcz szyi
Wiercenie, rozglądanie, rozpraszanie – starsze niemowlęta
Ugryzienie piersi przez ząbkujące niemowlę
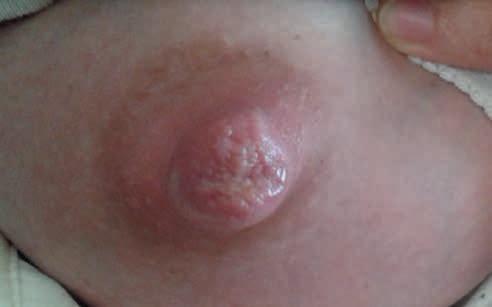
pęcherzyki, wybroczyny, pęknięcia o ułożeniu gwiazdowatym na szczycie brodawki, spowodowane generowaniem dużego podciśnienia w jamie ustnej dziecka (częściej obserwowane u matek z niedoborem pokarmu, upośledzonym wypływem mleka, z płaskimi lub wklęsłymi brodawkami; ryc 3).
Leczenie bolesności i ran brodawek sutkowych – przegląd bieżącej literatury Ze względu na potrzebę ochrony matek i niemowląt przed powikłaniami w postaci zapalenia piersi [6] czy przed przedwczesnym przerywaniem karmienia piersią skuteczne leczenie bolesności i ran brodawek stało się przedmiotem zainteresowania klinicystów i badaczy. W dotychczas opublikowanych badaniach oceniano skuteczność różnych metod. Bolesność i uszkodzenia traktowane są w badaniach na ogół łącznie pod określeniem sore nipples, tymczasem kaliber problemu zasadniczo się różni i wielu autorów postuluje, by precyzyjniej definiować badane grupy [5].
Technika karmienia i edukacja
Brodawka sutkowa w czasie ssania piersi przez dziecko znajduje się głęboko w jego jamie ustnej, szczyt brodawki sięga prawie do granicy podniebienia twardego i miękkiego (tzw. punktu HSPJ – hard and soft pallate junction), przeciętnie 6 mm od HSPJ. Tak ułożona brodawka nie ulega nadmiernej kompresji i dociskaniu do kostnej części podniebienia, jednocześnie wyzwala odruch ssania poprzez bodźcowanie zakończeń nerwowych umiejscowionych w tej okolicy. Szczyt brodawki, łącznie z końcowym odcinkiem podniebienia twardego i tylną częścią języka, uczestniczy w tworzeniu podciśnienia niezbędnego do pobierania mleka z piersi [24].
W niedawno opublikowanym przeglądzie systematycznym stwierdzono, że karmienie biologiczne

Rycina 2. Pręga pozycyjna, drobne uszkodzenia naskórka na jej przebiegu (fot. M. Żukowska-Rubik).
3. Strupek na szczycie (fot. M. Żukowska-Rubik).
(ciało matki w odchyleniu, dziecko ułożone na jej brzuchu samo inicjuje chwytanie piersi) w porównaniu z tradycyjnymi pozycjami (krzyżowa, klasyczna, spod pachy, na boku) zmniejszało ryzyko bolesności i urazów brodawek, pracy zarzucono jednak słabą jakość włączonych badań [25]. Bashiri nie zaobserwowała różnicy pomiędzy pozycją biologiczną a klasyczną w odczuciach matek i komforcie karmienia [26].
Tabela 2. Rodzaje zmian skórnych obserwowanych w związku z uszkodzeniami brodawek sutkowych [20–22]
Otarcie Niewielka rana powstała na skutek starcia naskórka (patrz ryc. 12A)
Nadżerka Ubytek naskórka nieprzekraczający błony podstawnej, ustępuje bez pozostawienia blizny (patrz ryc. 13A)
Szczelina, rozpadlina
Linijne pęknięcie naskórka (szczelina) lub naskórka i skóry właściwej (rozpadlina – ustępuje z pozostawieniem blizny) (patrz ryc. 12B, 14A)
Owrzodzenie Ubytek skóry przekraczający błonę podstawną, ustępuje z pozostawieniem blizny (patrz ryc. 14B)
Strup Powstaje wskutek zasychania płynu wysiękowego, treści ropnej lub krwi na powierzchni pęcherzyków i pęcherzy, nadżerek, owrzodzeń albo ran (ryc. 3)
Blizna Zmiana skórna będąca najczęściej następstwem uszkodzenia skóry właściwej i zastąpieniem ubytku tkanką łączną włóknistą (ryc. 4)
Dodatkowo w opisie zmian skórnych należy odnieść się do obecności wysięku, tj. jaki ma charakter – treść surowicza, ropna i z jakiej zmiany pochodzi, np. nadżerka sącząca treścią surowiczą
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
W innej pracy stwierdzono większe ryzyko uszkodzeń w przypadku nieprawidłowego ułożenia twarzy niemowlęcia przy piersi (brak przylegania nosa, brody i obu policzków) oraz w pozycjach krzyżowej i spod pachy w porównaniu z pozycją klasyczną i leżącą [15]. Autorzy stawiają hipotezę, że chwytanie dziecka za anatomiczne struktury głowy i szyi ogranicza swobodę ruchów kręgosłupa szyjnego i funkcję stabilizującą więzadła karkowego, utrudniając dziecku dotykanie, wąchanie i smakowanie w celu zlokalizowania brodawki sutkowej. Możliwe, że powoduje to również zbyt płytkie uchwycenie piersi, niesprawność żuchwy i tarcie brodawki przez język o podniebienie twarde.
Ostatnio przedstawiono ciekawą koncepcję niezgodnych wektorów – jamy ustnej dziecka i osi piersi, związaną z nieprawidłową techniką karmienia. Zgodnie z nią nierównomiernie rozłożone siły na powierzchni brodawki powodują napinanie i pociąganie skóry, w wyniku czego dochodzi do wydzielania cytokin, histaminy, mikrouszkodzeń naczyń krwionośnych, a w konsekwencji uruchomienia reakcji zapalnej z obrzękiem w śródmiąższu, podrażnieniem zakończeń nerwowych i pojawieniem bólu. Powtarzający się uraz prowadzi do powstania uszkodzeń (ryc. 5, 6) [3].
Edukacja matek, instruktaż techniki vs. różne środki wspomagające gojenie
Przegląd systematyczny Dennis 2014 [2] obejmował 4 badania o dobrej jakości metodologicznej z udziałem 656 kobiet, w którym sprawdzano skuteczność uśmierzania bólu brodawek przez stosowanie własnego pokarmu, maści trójskładnikowej na brodawki (mupirocyna, mikonazol, betametazon), lanoliny, muszli ochronnych oraz opatrunków hydrożelowych. Wszystkie matki w badaniach objętych metaanalizą otrzymały instruktaż, jak przystawiać dziecko jako element rutynowej opieki. Wyniki sugerują, że brak interwencji lub stosowanie tylko odciągniętego mleka matki może być równie lub bardziej korzystne w krótkotrwałym odczuwaniu bólu brodawek niż pozostałe sposoby. Jednym z ważnych ustaleń tego przeglądu jest to, że niezależnie od zastosowanego leczenia, u większości kobiet ból brodawek sutkowych zmniejszył się do łagodnego poziomu po 7–10 dniach po porodzie i o tym trzeba informować matki. W kolejnym dużym przeglądzie Niazi i wsp. oceniali wiele interwencji podejmowanych w uszkodzeniu brodawek [27]. Podstawowym działaniem podjętym u pacjentek była również korekta pozycji do karmienia.
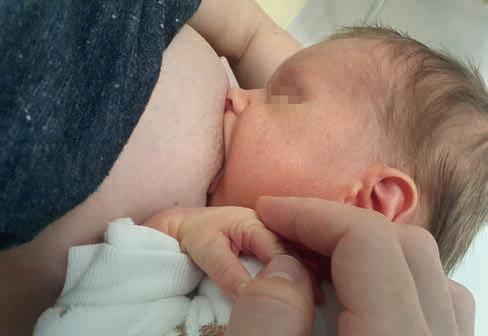
Rycina 5. Prawidłowy, szeroki chwyt piersi, dolna warga wywinięta, górna w pozycji neutralnej, szeroki kąt między wargami, nos i broda w kontakcie z piersią (fot. M. Żukowska-Rubik).

Rycina 6. Nieprawidłowe, płytkie ssanie piersi – zaciśnięty kącik ust, kąt warg zaledwie około 90 stopni, niewielka część otoczki objęta ustami (fot. M. Żukowska-Rubik).
Miejscowe środki farmakologiczne zmniejszające dolegliwości bólowe i inne metody
Lanolina
Bardzo popularnym środkiem zalecanym matkom jest hipoalergiczna lanolina, oczyszczona z alkoholi i pozostałości pestycydów. Uważa się, że preparat wytwarza bakteriostatyczną barierę, utrzymuje wilgotne środowisko i przyśpiesza gojenie. Jednakże w przeprowadzonym przez Jackson i Dennis badaniu z randomizacją z 2017 r. nie wykazano istotnej skuteczności lanoliny w łagodzeniu bólu brodawek z widocznym urazem brodawki we wczesnym okresie po porodzie ani też wpływu na czas karmienia piersią w porównaniu z rutynową opieką oferowaną matkom w szpitalu po porodzie. Natomiast matki stosujące lanolinę zgłaszały większą satysfakcję z leczenia [1]. W innej pracy z 2017 r. Vieira i wsp. stwierdzili, że zastosowanie na brodawki pokarmu matki i muszli ochronnych było skuteczniejsze od lanoliny w łagodzeniu bolesności [28]. Zastosowanie lanoliny na ranę budzi obawy w związku z ryzykiem wywołania alergii kontaktowej. Preparaty lanoliny przeznaczone do smarowania brodawek są oczyszczane z alkoholi, co zmniejsza ryzyko podrażnienia, niemniej jednak nie powinny być stosowane na uszkodzoną skórę ani też u pacjentek z atopowym zapaleniem skóry (AZS) [29]. Większość popularnych maści i kremów pielęgnacyjnych do stosowania na brodawki w czasie laktacji nie została przebadana pod względem skuteczności w łagodzeniu bolesności.
Różne metody stosowane w leczeniu bolesności i uszkodzeń brodawek
Wśród prac objętych przeglądem systematycznym przez Niazi i wsp. w 2018 r. [28] dwa badania dotyczyły wpływu mięty na pęknięcie brodawki sutkowej i ból, miały one akceptowalny projekt i walidację, w obu wykazano skuteczność mentolu w postaci żelu lub olejku. Badanie RCT Lavergne [30] potwierdziło skuteczność stosowania kompresów z ciepłej wody lub esencji herbacianej w łagodzeniu bólu brodawek związanego z podrażnieniem i uszkodzeniem wcześnie po porodzie (kompresy stosowano 4 razy dziennie po 15 minut przez 5 dni). Ze względu na niewielkie liczebnie grupy biorące udział w powyższych badaniach nie można było wydać silnych rekomendacji w zakresie ich stosowania.
Stosowanie lasera niskoenergetycznego miało znaczący wpływ na łagodzenie bólu brodawek sutkowych, ale jego wysoki koszt sprawia, że powszechne korzystanie z tej terapii jest utrudnione. Warto dodać,
że poza wcześniej wymienionymi, testowano inne sposoby leczenia ran brodawek, które często mają specyfikę regionalną. Przykładowo można znaleźć doniesienia nt. wykorzystania oliwy extra virgin, ekstraktu z kurkumy, krwawnika pospolitego, miodu, aloesu, mieszanki olejów sezamowego, makadamia, morelowego, kremu z portulaki pospolitej, a nawet fototerapii w zakresie wykorzystania 360–1000. Z badań tych trudno wyciągnąć jednoznaczne wnioski, co jest najskuteczniejsze, a wyniki czasem są sprzeczne.
Mechaniczne sposoby zapobiegania i leczenia bolesności i uszkodzeń brodawek sutkowych
Muszle ochronne Niekiedy korzystne jest zakładanie po karmieniach muszli ochronnych, które chronią brodawki przed kontaktem z wkładką laktacyjną czy bielizną. Biustonosz musi być wtedy nieco luźniejszy, by muszla nie uciskała piersi. Należy jednak pamiętać, że środowisko w muszli jest wilgotne i ciągłe noszenie jej prowadzi do maceracji naskórka (w wyniku długotrwałego działania wilgoci dochodzi do rozmiękczenia i oddzielania się naskórka). Wyniki badań co do skuteczności muszli są niejednoznaczne (ryc. 7).
Nakładki ochronne (kapturki)
Kapturek, czyli nakładka ochronna zakładana do karmienia u niektórych matek, ogranicza dolegliwości bólowe. Jeśli nie udaje się zredukować bólu poprawą techniki karmienia lub zmniejszeniem napięcia otoczki, można zastosować kapturek na określony czas, szczególnie w przypadku silnego zasysania (dużego podciśnienia w jamie ustnej) przez noworodka pomimo głębokiego przystawienia [16, 31]. Założenie kapturka można rozważyć także w anatomicznych

Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
i czynnościowych zaburzeniach funkcji ssania, które nie podlegają natychmiastowej poprawie, i trudno jest uzyskać postęp w gojeniu rany brodawki.
Oceniano wpływ kapturków na pobieranie pokarmu. Wyniki tych badań nie są jednoznaczne, więc przebieg karmienia i efektywność ssania w czasie stosowania nakładki muszą być ściśle monitorowane [30].
Wykazano, że jeśli po kapturek sięgają matki bez problemów w karmieniu piersią, to może to mieć niekorzystny wpływ na pobór pokarmu i czas ssania odżywczego, a więc kapturek może być zalecany wyłącznie po uważnym wyważeniu wskazań [31]. Stosowanie kapturków wiązało się ze skróceniem czasu karmienia piersią, ale nie wtedy, gdy było nadzorowane przez osoby specjalizujące się w poradnictwie laktacyjnym [33, 34].
Kapturek należy myć detergentem po każdym użyciu i raz dziennie przeprowadzić dezynfekcję termiczną przez zanurzenie na 5–10 minut w wodzie o temp. 90–100°C lub w specjalnej torebce do dezynfekcji termicznej w kuchence mikrofalowej. Należy pamiętać, że brak higieny kapturka i innych sprzętów mających kontakt ze skórą brodawki zwiększa ryzyko zakażenia ran (ryc. 8).
Konsensusy dotyczące leczenia ran w kontekście ran brodawek sutkowych
W przypadku trudno gojących się czy zakażonych ran brodawek poprawa techniki karmienia, smarowanie pokarmem czy inne z opisanych powyżej metod nie będą wystarczające. Tymczasem trudno jest znaleźć doniesienia o leczeniu takich ran w oparciu o nowoczesne zasady przyjęte w licznych konsensusach dotyczących leczenia ran w chirurgii czy dermatologii, gdzie omawia się zastosowanie lawaseptyków klasycznych, lawaseptyków z zawartością surfaktantu, antyseptyków czy opatrunków

8. Różne rodzaje i rozmiary kapturków (fot. M. Żukowska-Rubik).
specjalistycznych [35–38]. Stanowiska ekspertów dotyczą wprawdzie innego rodzaju ran niż rany brodawek, a więc trudno gojących ran i owrzodzeń w przebiegu cukrzycowej choroby stóp, owrzodzeń goleni, odleżyn, u osób zwykle w podeszłym wieku z licznymi przewlekłymi obciążeniami zdrowotnymi. Jednak działanie lawaseptyków czy antyseptyków jest uniwersalne. Środki te są od kilkunastu lat wykorzystywane w Polsce przez doradców laktacyjnych do leczenia ran brodawek, w obserwacjach klinicznych z dobrym rezultatem, a Centrum Nauki o Laktacji uwzględnia je w nauczanych protokołach [39].
Rany trudno gojące się
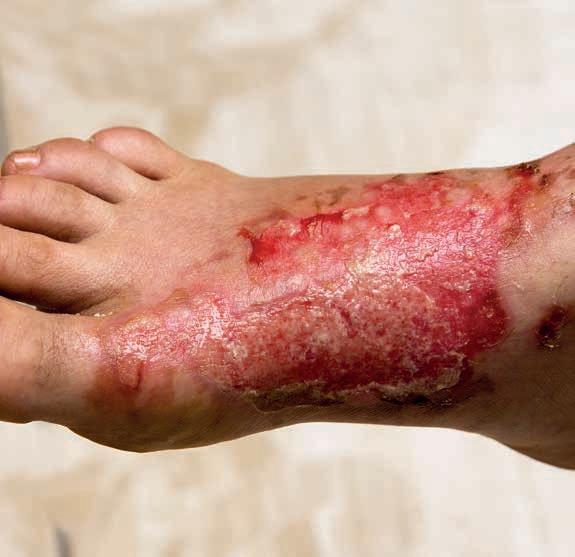
Do niedawna używano określenia rany przewlekłe, czyli takie, które nie przechodzą przez fazy gojenia w sposób uporządkowany i terminowy, a proces ich gojenia przekracza 6–8 tygodni (w różnych pracach od 4 tygodni do 3 miesięcy) [38]. Obecnie przyjmuje się nową definicję wg Murphy, gdzie zamiast określenia rana przewlekła wprowadzono termin „rana trudno gojąca się”. Każda rana może być zaliczona do tej grupy, niezależnie od typu i etiologii. Są to rany, w przypadku których istnieją czynniki mogące upośledzać gojenie, nieodpowiadające na standardowe leczenie. Udokumentowano, że prawie wszystkie zawierają biofilm (ryc. 9). Kluczowym momentem zakwalifikowania ich jako trudno gojące się jest 3. dzień, w którym rana wykazuje zwiększony wysięk, włóknik w dnie rany i zwiększa się jej rozmiar, pomimo stosowanego leczenia [39].
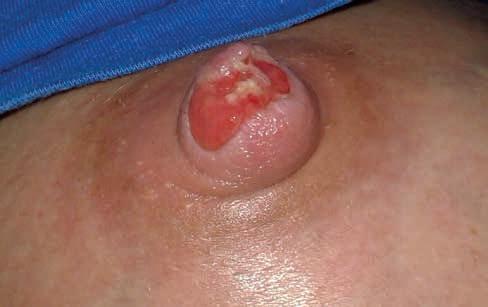
Rycina 9. Biofilm pokrywający rozległą ranę brodawki, obrzęk brzegów rany i całej brodawki, zwiększona ilość wysięku.
Źródło: Żukowska-Rubik M., Nehring-Gugulska M. Patologia piersi i brodawek podczas laktacji. W: Żukowska-Rubik M., Godyń-Myśliwy Z., Nehring-Gugulska M. Atlas fizjologii i patologii laktacji. Wyd. 1. Medycyna Praktyczna, Krakow 2025, za zgodą
BIOFILM to złożony wielogatunkowy ekosystem drobnoustrojów zamkniętych w zewnątrzkomórkowej substancji polimerowej – matrix. Powoduje on subkliniczne miejscowe zakażenie rany. Wewnątrz biofilmu bakterie wytwarzają środowisko odmienne metabolicznie i immunologicznie, są oporne na działanie antybiotyków i antyseptyków. Biofilm ma zdolność do odbudowywania się w ciągu kilkunastu godzin, po 48–72 godzinach osiąga dojrzałość. O tworzeniu się biofilmu świadczy duża ilość martwej tkanki w ranie, połyskliwość (szklistość), powłoka fibrynowa, zwiększony wysięk, nieprzyjemny zapach [36]. Działania przeciwdziałające namnażaniu biofilmu polegają na mechanicznym usuwaniu struktury matrycy polimerowej oraz niedopuszczaniu do odbudowy postaci dojrzałej (ryc. 9).
W obserwacjach klinicznych typowe dla pierwszych dni po porodzie niewielkie rany brodawek po poprawie techniki karmienia goją się szybko, w ciągu kilku dni. Sprzyjają temu bakteriostatyczne właściwości mleka matki, szczególnie bogatego w czynniki bioaktywne w pierwszych dobach po porodzie. W niewielkim badaniu przeprowadzonym w 2018 r. przez Nehring-Gugulską i wsp. przeciętnie czas gojenia wynosił 4,15 dnia, a ustąpienie bolesności następowało po średnio 5,86 dnia [39]. W praktyce
klinicznej doradcy laktacyjni często mają do czynienia z ranami brodawek, które należą do trudno gojących się. Dotyczy to zwykle ran rozległych, głębokich, które też łatwiej ulegają zakażeniom. Gojenie takich ran trwa zwykle 2–3 tygodnie, ale czasem nawet kilka miesięcy. Warto pamiętać, że czynniki, które doprowadziły do uszkodzenia brodawki, mogą też upośledzać jej gojenie, np. zaburzenia funkcji ssania.
Rozwój zakażenia w ranie
W ranach brodawek może rozwijać się zakażenie w kontakcie z drobnoustrojami bytującymi na skórze, przenoszonymi na rękach matki czy personelu medycznego oraz obecnymi w jamie ustnej dziecka. Najczęstsze patogeny to gronkowce, głównie metycylinowrażliwe, rzadko metycylinooporne, enterokoki, Escherichia coli i inne pałeczki Gram-ujemne, paciorkowce [41]. Zakażenie może szerzyć się na sąsiadujące tkanki, w tym skórę piersi, tkankę podskórną i gruczoł piersiowy, musi więc być szybko rozpoznane i leczone. Zakażenie poprzedzone jest przez wcześniejsze fazy interakcji między bakteriami i gospodarzem, a wiec kontaminacją i kolonizacją (tab. 3).
Objawy zakażenia rany brodawki: znaczne nasilenie dolegliwości bólowych, zaczerwienienie, obrzęk brodawki, ucieplenie, obrzęk brzegów rany, wysięk – często ropny, obecność martwiczej tkanki [42], brak postępu gojenia,
Tabela 3. Stadia rozwoju infekcji w ranie brodawki na podstawie wytycznych PTLR [36, 43]
Kontaminacja
Kolonizacja
drobnoustroje obecne w ranie, proliferacja patogenów (–), odpowiedź immunologiczna gospodarza (–), opóźnienie gojenia rany (–)
rana bez obrzęku i rumienia brodawki, bez obrzęku brzegów rany, bez sączenia, goi się w typowym czasie
drobnoustroje obecne w ranie, proliferacja patogenów (+), odpowiedź immunologiczna gospodarza (–), opóźnienie gojenia rany (–) jw.
Zakażenie miejscowe utajone proliferacja patogenów (+++), odpowiedź immunologiczna gospodarza (–), opóźnienie gojenia rany (+)
Zakażenie miejscowe jawne
proliferacja patogenów (+++), odpowiedź immunologiczna gospodarza (+), opóźnienie gojenia rany (+)
Zakażenie rozprzestrzeniające się dalsza proliferacja patogenów, jw.
Szerzenie zakażenia na otaczające tkanki z reakcją ogólnoustrojową
pogorszenie stanu ogólnego, gorączka, leukocytoza, wzrost stężenia białka C-reaktywnego i prokalcytoniny
krwawienie, zwiększający się wysięk brak postępu gojenia
nasilenie bólu, obrzęk, rumień brodawki, obrzęk brzegów rany, ucieplenie okolicy rany, wysięk z rany, nieprzyjemny zapach brak postępu gojenia
rozszerzający się rumień i obrzęk wokół rany > 2 cm
ból, obrzęk, rumień, ucieplenie piersi lub jej fragmentu do różnicowania: zakażenie skóry i tkanki podskórnej – róża, tkanki podskórnej – cellulitis [44], zakażenie struktur gruczołu piersiowego [45]
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
mimo że nie ma zastrzeżeń do techniki karmienia czy funkcji ssania u noworodka.
Postępowanie z raną
Na potrzeby opisu sposobów postępowania z raną wykorzystywana jest strategia TIMERS. Każda z liter powyższego akronimu oznacza kolejną składową. Poniżej rozwinięto poszczególne składowe strategii TIMERS z komentarzem dotyczącym ran brodawek.
T – tissue debridement – oczyszczenie rany
I – infection and inflammation control – kontrola infekcji i rozwoju procesu zapalnego
W celu kontroli infekcji i rozwoju procesu zapalnego stosuje się antyseptyki oraz opatrunki zawierające substancje przeciwbakteryjne. Niekiedy są wskazania do podania antybiotyku ogólnoustrojowo. Antyseptykami nazywa się różne substancje o właściwościach antyseptycznych, niektóre mają rejestrację jako leki, inne jako wyroby medyczne. Antyseptyki wykazują działanie bójcze. Istotne jest stosowanie antyseptyków do czasu utrzymywania się klinicznych cech zakażenia miejscowego, należy je odstawić, gdy ustępują objawy zakażenia i rana zaczyna się goić.
Rany brodawek – komentarz: Dobór preparatów zależnie od kategorii rany i ich rodzaje omówiono poniżej.
Funkcję opatrunku może pełnić preparat z zawartością substancji antyseptycznej w postaci żelu, który może pozostawać na ranie do kolejnego karmienia. Żel nakłada się cienką warstwą, wskazane jest nałożenie opatrunku wtórnego w postaci np. jałowego gazika.
Lawaseptyki i antyseptyki omówiono szczegółowo w Aneksie 2.
M – moisture balance – utrzymanie równowagi wilgotności w ranie
Opatrunki specjalistyczne charakteryzują się różnymi właściwościami, między innymi zapewniają wilgotne środowisko, które sprzyja proliferacji i migracji komórek naskórka [47], niektóre opatrunki mają cechy absorbujące wysięk i ich zadaniem jest zmniejszenie ryzyka maceracji skóry wokół rany; inne zawierają substancje przeciwbakteryjne.
Ze względu na budowę można je podzielić na siateczkowe, hydrożelowe, hydrokoloidowe, hydrowłókniste, piankowe, z warstwą kontaktową oraz hydrożele z substancjami przeciwbakteryjnymi.
Zastosowanie znajdują lawaseptyki, czyli preparaty służące do przemywania rany i usuwania zanieczyszczeń fizycznych, chemicznych i biologicznych. Regularne oczyszczanie rany jest jednym z warunków koniecznych do utrzymania prawidłowego, niepowikłanego gojenia. Wśród lawaseptyków klasycznych znajdują się płyn Ringera oraz 0,9-proc. roztwór NaCl. Według najnowszych badań 0,9-proc. roztwór NaCl może powodować nasilenie proliferacji komórek mezotelialnych, zaburzenia układu fibrynolizy oraz spadek aktywności interleukiny 6, a tym samym wpływać niekorzystnie na regenerację tkanek, dlatego należy wybierać inne metody. Z tego powodu coraz częściej odchodzi się od regularnego stosowania 0,9-proc. NaCl do opisywanych procedur. Idealny lawaseptyk powinien zawierać surfaktant, czyli środek powierzchniowo czynny, który przez obniżenie napięcia powierzchniowego wpływa m.in. na destabilizację biofilmu. Do substancji tych należą np. betaina, etyloheksylogliceryna, poloksamer. Lawaseptyki mogą zawierać również substancje antyseptyczne, takie jak np. dichlorowodorek oktenidyny, w niższym stężeniu niż zawarte w antyseptykach. Podchloryny (podchloryn sodu i kwas podchlorawy) są również stosowane w lawaseptyce, choć nie zawierają surfaktantu. Preparaty mogą mieć postać płynu do przemywania lub żelu. Substancje te powinny charakteryzować się szerokim spektrum aktywności, dobrą penetracją przez struktury biofilmowe i nie mogą indukować rozwoju oporności bakterii. Rany brodawek – komentarz: Rany brodawek mogą być mechanicznie oczyszczane strumieniem lawaseptyku (rozprysk, spłukanie), przez przetarcie zwilżonym lawaseptykiem sterylnym kompresem z gazy lub opatrunkiem przeznaczonym specjalnie do oczyszczania rany (produkt zbudowany z włókien monofilamentowych). W przypadku obecności w ranie włóknika, przyschniętego wysięku, biofilmu, strupa korzystne będzie zastosowanie gazika zwilżonego lawaseptykiem lub lawaseptyku w postaci żelu (czas aplikacji wg zaleceń producenta) w celu nawilżenia, rozmiękczenia powłoki, a następnie delikatne oczyszczenie gazikiem. Należy pamiętać, by wszystkie zabiegi były wykonywane delikatnie ze względu na bogate unerwienie czuciowe brodawki. Gao i wsp. opisali chirurgiczną metodę oczyszczania przewlekających się uszkodzeń, nawarstwień keratotycznych i zmian martwiczych z powierzchni brodawki u kobiet karmiących piersią. Metodę zastosowano, gdy nie następowała poprawa po 2 tygodniach po zastosowaniu innych metod leczenia. Po oczyszczeniu skóry u około połowy pacjentek w ciągu tygodnia następowało wyleczenie uszkodzeń i ustąpienie bólu, istotnie częściej niż w grupie kontrolnej [46]. Nie ma doniesień nt. innych sposobów oczyszczania ran brodawek – enzymatycznych czy terapii podciśnieniowej.
Rany brodawek – komentarz: Wilgotne środowisko gojenia rany brodawki poza wspomnianymi korzyściami zmniejsza dolegliwości bólowe przy karmieniu przez utrzymanie elastyczności i nawilżenia skóry brodawki, chroni również przed tworzeniem strupa, który przy kolejnym karmieniu jest naruszany, co powoduje dodatkowy ból i uszkadza gojącą się powierzchnię. Może być korzystny, gdy matka zgłasza silny ból lub rana ma tendencję do przysychania, przykleja się do wkładki laktacyjnej, bielizny. W Aneksie 2 omówiono opatrunki zarejestrowane przez producentów do stosowania na brodawki w okresie laktacji (tab. I) oraz inne rodzaje opatrunków specjalistycznych, które mogą znaleźć zastosowanie w leczeniu ran brodawek po rozważeniu korzyści i ryzyka (Aneks 2). Opatrunki hydrokoloidowe i hydrowłókniste mocno przylegają do rany i nie nadają się do stosowania na rany brodawek.
E – edges, epidermization stymulatio n – ochrona brzegów rany i stymulacja naskórkowania
W celu ochrony skóry przed maceracją, alergią kontaktową, podrażnieniem zaleca się delikatne preparaty myjące o pH lekko kwaśnym, hipoalergiczne substancje nawilżające, wazelinę na skórę wokół rany, opatrunki aktywne, parafinę, substancje pobudzające naskórkowanie oraz przeciwzapalne.
Rany brodawek– komentarz: Rany brodawek mają na tyle małe rozmiary, że aplikowanie innych środków na ranę, a innych na jej brzegi nie jest możliwe.
R – regeneracja – wsparcie procesów regeneracyjnych i naprawczych
Rany brodawek– komentarz: Jeśli nie jest to bezwzględnie konieczne, nie stosuje się dodatkowo preparatów z tej grupy. Ze względu na dobre ukrwienie brodawki, w momencie kiedy stworzone są odpowiednie warunki do gojenia rany przez jej oczyszczanie, zapewnienie wilgotnego środowiska i kontrolowanie infekcji, rana zaczyna się goić. Dodatkowy preparat jest kolejnym wydatkiem i wymaga zmywania, co jest kłopotliwe.
S – uwzględnienie czynników społecznych dotyczących pacjenta i rodziny
Opatrunki specjalistyczne
Dostępnych jest wiele rodzajów opatrunków specjalistycznych do ran niezakażonych i zakażonych, w tym różne opatrunki przeznaczone do stosowania na brodawki sutkowe. Wśród opatrunków o działaniu
przeciwbakteryjnym chętnie wykorzystywane w leczeniu ran trudno gojących są opatrunki z zawartością jonów srebra. Nie ma natomiast jednoznacznych badań ich skuteczności w zastosowaniu na brodawki u matek w okresie laktacji i bezpieczeństwa dla niemowlęcia. Jedno badanie dotyczy srebrnych nakładek na brodawki, które znacznie redukowały dolegliwości bólowe i przyspieszały gojenie w 7. i 15. dobie po zastosowaniu w porównaniu ze standardową pielęgnacją ran brodawek (dobra technika karmienia, prawidłowa higiena, pokarm na brodawki) [48]. Hale opisuje stosowanie preparatów zawierających srebro miejscowo, ale nie na brodawki sutkowe w okresie karmienia [49]. W badaniach in vitro stwierdzono, że nanocząsteczki srebra słabo wchłaniają się ze skóry, ze skóry nieuszkodzonej 0,46 ng/cm2, z uszkodzonej 2,32 ng/cm2. Nie wiadomo, czy cząsteczki srebra wchłaniają się z błon śluzowych, jeśli dziecko ssie pierś leczoną preparatem, oraz na ile skuteczne byłoby oczyszczenie powierzchni brodawki przed karmieniem. Badania na zwierzętach wykazują, że srebro koloidalne może przenikać do mleka matki. Mimo że jony srebra zawarte w sulfadiazynie w postaci kremu nie wchłaniają się ze skóry, nie wiadomo, na jaką jego ilość jest narażone dziecko ssące pierś. Preparaty sulfadiazyny srebra nie są zalecane w leczeniu ran przez PTLR.
Nie ma jednoznacznych danych dotyczących stosowania miejscowego preparatów ze srebrem oraz srebrem koloidalnym u kobiet karmiących. Z drugiej strony biodostępność srebra przy podaniu doustnym nie przekracza 10%, a toksyczność srebra występuje przy znacznej ekspozycji przez długi czas przekraczającej zdolności wątroby do usuwania pierwiastka. Przypadki argyrii opisano po spożyciu kilku gramów pierwiastka. Wydaje się, że ekspozycja niemowlęcia w przypadku leczenia ran brodawek opatrunkiem czy preparatem z zawartością srebra byłaby niewielka, ale brak danych nakazuje ostrożność.
Wśród opatrunków o działaniu przeciwdrobnoustrojowym znajdują się te z zawartością miodu Manuka. Ich zastosowanie nie było dotychczas badane w procesie gojenia ran brodawek. W badaniu in vitro zaobserwowano działanie cytotoksyczne na keratynocyty i skórne fibroblasty ludzkie [50]. Miodu nie powinny spożywać niemowlęta do 12. miesiąca życia. Brak danych nt. bezpieczeństwa i skuteczności u matki karmiącej nakazuje ostrożność.
Antybiotyki do stosowania miejscowego Zgodnie z aktualną wiedzą o mikrobiologii ran nie powinno się stosować miejscowo antybiotyków,
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
jednak w wybranych sytuacjach klinicznych jest to dopuszczalne. Antybiotyki stosowane miejscowo są wykorzystywane w leczeniu miejscowych zakażeń skóry, zwłaszcza na podłożu wyprysku czy atopowego zapalenia skóry [51, 52], w okulistyce, a także w profilaktyce zakażeń ran chirurgicznych [53]. Najczęstsze zastosowanie znajdują mupirocyna, kwas fusydowy (wykazuje aktywność w głębszych warstwach skóry), antybiotyki z grupy aminoglikozydów i inne. Retamapulina, ozenoksacyna są rekomendowane w zakażeniach szczepami MRSA 52]. W praktyce lekarzy doradców laktacyjnych antybiotyki miejscowe są stosowane do leczenia trudno gojących się ran brodawek, w obserwacjach z dobrym rezultatem, ale brak badań potwierdzających ich skuteczność w tym wskazaniu [54]. W niewielkiej obserwacji 29 pacjentek z badania CNoL zastosowano antybiotyki miejscowo tylko w przypadku zakażonych ran o dużej rozległości i osiągnięto lepszy efekt leczniczy niż w grupie stosującej opatrunki bez substancji antybakteryjnej, jednak próba jest zbyt mała, by wyciągać pewne wnioski [39]. Jeśli pomimo prawidłowego zastosowania lawaseptyków, antyseptyków czy opatrunków z substancją przeciwbakteryjną nie ma postępu w leczeniu rany, może być uzasadnione zastosowanie antybiotyku miejscowego zgodnie z uzyskanym antybiogramem. Jest to ostatni krok przed antybiotykoterapią ogólną, którą rezerwuje się dla przypadków szczególnie opornych w leczeniu miejscowym lub rozszerzania się zakażenia z rany na okoliczne tkanki piersi. Wydaje się, że leczenie miejscowego zakażenia niewielkiej rany ogólnym antybiotykiem jest zbyt agresywne, zważając na fakt karmienia piersią niemowlęcia i zasadę pierwszeństwa preparatów działających miejscowo w farmakoterapii kobiet karmiących [55]. Natomiast nie powinno się stosować antybiotyków miejscowo w rutynowym postępowaniu jako leczenia I rzutu, bez wskazań wynikających z antybiogramu. Stosowanie środków przeciwbakteryjnych i przeciwgrzybiczych, zwłaszcza dostępnych w aptekach bez recepty, bez wskazań przedłuża proces gojenia rany i przysparza cierpienia matkom.
Substancje niezalecane do leczenia ran brodawek:
• powidon jodu (wchłanianie z rany, kumulacja w pokarmie),
• chlorheksydyna (cytotoksyczność),
• balsam peruwiański,
• woda utleniona (działa krótko, nie eliminuje drobnoustrojów),
• nadmanganian potasu (cytotoksyczność),
• rivanol (przedłuża żywotność Pseodomonas aeruginosa, działanie kancerogenne),
• anestezyna,
• sulfadiazyna srebra (niewystarczająca aktywność przeciwdrobnoustrojowa),
• glikokortykosteroidy (działanie opóźniające gojenie; część preparatów do leczenia zmian skórnych je zawiera, ale ich przeznaczenie zgodnie z charakterystyką produktu leczniczego jest inne).
Najczęstsze błędy w leczeniu ran brodawek:
• brak poprawy techniki karmienia,
• brak zadbania o chwytny kompleks brodawka––otoczka,
• brak sprawdzenia poprawności stosowania akcesoriów laktacyjnych,
• brak właściwej higieny rąk, akcesoriów do karmienia lub odciągania mleka,
• zapewnianie, że „samo przejdzie”,
• zbyt długie wietrzenie brodawek po karmieniach,
• brak rozpoznania rany zagrożonej zakażeniem lub zakażonej,
• brak leczenia antyseptycznego w ww. ranach,
• kontynuowanie tego samego leczenia przy braku postępu w odpowiednim czasie,
• brak wymazu z antybiogramem i leczenie niecelowane,
• stosowanie opatrunków żelowych, hydrożelowych na zakażone rany,
• stosowanie lanoliny i innych środków pielęgnacyjnych (np. maści z witaminami) w ranach zakażonych (brak uzasadnienia, brak celowanego leczenia),
• stosowanie w leczeniu ran preparatów steroidowych (opóźnianie gojenia),
• stosowanie preparatów przeciwgrzybiczych (brak uzasadnienia, brak celowanego leczenia),
• stosowanie antybiotyków miejscowych OTC.
Protokół postępowania w ranach brodawek
Porada laktacyjna
Wywiad W trakcie wywiadu należy ustalić, od kiedy obecne są uszkodzenia, czy bolesność ustępuje, czy się nasiliła, ocenić natężenie bólu w NRS, zapytać, w jakich okolicznościach ból jest najdotkliwszy, czy matka obserwowała sączenie ropnej wydzieliny, co zastosowała w leczeniu
do tej pory i jakie to przyniosło efekty. Istotna jest również informacja o dotychczasowej pielęgnacji, higienie piersi, rąk i akcesoriów laktacyjnych. Brak postępu gojenia może wynikać z niestosowania się do zaleceń. Należy zebrać informacje nt. stanu zdrowia matki i dziecka, stosowania antybiotyków w ostatnim czasie, obecności zmian skórnych w innych lokalizacjach (czynniki zwiększające ryzyko infekcji rany zamieszczono w ramce).
Czynniki zwiększające ryzyko infekcji rany:
• wiek powyżej 35 lat,
• niedobory odporności,
• cukrzyca,
• choroby przewlekłe (niewydolność nerek, niewydolność krążenia),
• niedożywienie, otyłość,
• niezdrowy styl życia, palenie tytoniu,
• leki (glikokortykosteroidy, insulina, leki cytostatyczne, immunosupresyjne).
Badanie dziecka
W badaniu dziecka należy zwrócić szczególną uwagę na napięcie mięśniowe, symetrię twarzy, ciała, obecność zmian skórnych, przyrost masy ciała, ocenić budowę anatomiczną jamy ustnej, stan śluzówek, ruchomość języka, funkcję ssania.
Badanie piersi
Badając piersi, ocenia się zabarwienie skóry, spoistość tkanki piersi, obecność objawów zapalenia gruczołu lub skóry i tkanki podskórnej oraz węzły chłonne pachowe. Należy ocenić kształt brodawek, elastyczność kompleksu brodawka–otoczka. Badanie piersi służy również ocenie stanu brodawek, wielkości i głębokości rany, połyskliwości, obrzęku brzegów rany, obecności rumienia i obrzęku całej brodawki, szerzenia się rumienia i obrzęku wokół brodawki lub przeciwnie obserwacji oznak gojenia (Aneks 1). Jeśli są ku temu wskazania, należy pobrać wymaz z rany.
Ocena aktu karmienia
Kluczowym elementem niezbędnym do ustalenia rozpoznania jest obserwacja aktu karmienia, najczęściej z poprawą pozycji matki czy dziecka i sposobu przystawienia do piersi. Doświadczenie pokazuje, że kobiety są w stanie karmić dziecko nawet z ranami sporych rozmiarów, dopóki odczuwalność bólu jest do zniesienia (7–8/10 pkt). Często korekty techniki są drobne, ale istotnie poprawiają sposób uchwycenia piersi. Nawet rozległa rana może nie być odczuwalna
przez matkę w czasie karmienia, jeśli brodawka znajdzie się odpowiednio głęboko w jamie ustnej dziecka. Poradę laktacyjną przeprowadza odpowiednio przeszkolona osoba, do której należy skierować matkę i dziecko (doradca laktacyjny – lekarz, położna, pielęgniarka). Na podstawie wywiadu i badań jest ustalane rozpoznanie i dobierane odpowiednie postępowanie lecznicze.
Leczenie przyczynowe Podstawowe znaczenie w leczeniu bolesności i uszkodzeń brodawek ma wyeliminowanie ich przyczyny [56].
Z niewielkiego badania obserwacyjnego, które było przeprowadzone przez CNoL w 2018 r., wynika, że ranom brodawek towarzyszył ból o średniej sile 7,5/10, u 90% matek konieczna była korekta techniki karmienia, która przyniosła ulgę w bólu. Ponownego instruktażu podczas wizyty kontrolnej wymagało 10% matek. Rany brodawek leczone zgodnie z zastosowanym protokołem goiły się w ciągu 4,15 dnia, a ból znikał całkowicie po niespełna 6 dobach [39].
Poprawa techniki karmienia to podstawowe działanie, dzięki któremu większość uszkodzeń ma szansę wygoić się w krótkim czasie bez potrzeby korzystania z dodatkowych metod.
Pozycja do karmienia powinna być wygodna i stabilna dla obojga, dziecko przytulone do ciała matki, główka powinna być w lekkim odchyleniu, rączki obejmować pierś z obu stron, a broda oprzeć się na piersi. Wyjściowo brodawka znajduje się na wysokości nosa, jest lekko uniesiona, skierowana w stronę podniebienia, a w momencie, gdy dziecko odchyla główkę i otwiera szeroko usta, kieruje się ją na podniebienie. W pozycjach w odchyleniu, gdy dziecko leży na matce, może samo wykonać ruch główką do przodu i uchwycić pierś, w pozostałych pozycjach matka delikatnie przytula dziecko do siebie w momencie szerokiego otwarcia ust (ryc. 10, 11).
Jeśli otoczka jest twarda, przepełniona pokarmem, należy zaproponować matce odciągnięcie ręcznie niewielkiej ilości pokarmu w celu zmniejszenia napięcia otoczki. Przepełnione piersi i napięta otoczka to jest problem przede wszystkim okresu pierwszych tygodni po porodzie, ale może występować u matek z nadprodukcją w dowolnym okresie laktacji. W obrzęku piersi i otoczki stosuje się technikę RPS (reverse pressure softening) – ręcznego odprowadzania płynu przesiękowego zbierającego się pod otoczką. Kompleks brodawka–otoczka ma być podatny do formowania się w jamie ustnej dziecka. Instruktaż prawidłowej techniki karmienia, jak i odciągania
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
ręcznego matki powinny uzyskać w czasie pobytu na oddziale. Jest to obowiązkiem personelu szpitala zgodnie z zapisami Standardu Organizacyjnego Opieki Okołoporodowej. Jednak wiele matek nadal potrzebuje pomocy po opuszczeniu szpitala. Korzystają z pomocy położnych POZ, ale jeśli jest ona niewystarczająca, szukają profesjonalnych doradców laktacyjnych [57].
Dla noworodków z nieprawidłowościami w obrębie jamy ustnej będzie wskazane wykorzystanie specjalnych pozycji do karmienia, technik manualnego wspomagania, korzystna może być współpraca z logopedą wczesnej interwencji. W przypadku zaburzeń funkcji ssania anatomicznych lub czynnościowych można rozważyć wykorzystanie kapturka. Restrykcyjne wędzidełko języka powinno być niezwłocznie podcięte. W przypadku asymetrii i zaburzeń napięcia mięśniowego należy zalecić rodzicom prawidłowe zasady pielęgnacji niemowlęcia, a w niektórych sytuacjach konsultację neurologa lub fizjoterapeuty.
U matek odciągających mleko laktatorami niezbędne jest sprawdzenie siły odciągania i wielkości lejka, zważając na to, że część laktatorów dostępnych na rynku nie ma rejestracji jako wyroby medyczne i może mieć niekontrolowaną siłę ssania, część nie ma wymiennych lejków, u części matek nikt nie pomógł dobrać lejka do rodzaju brodawki (średnica, wyciągliwość). Bywa, że rozmiar brodawki może zmienić się po pewnym okresie odciągania. Niektóre rodzaje laktatorów, tzw. muszlowych, mają nieprzezroczyste muszle i matka nie ma dostatecznej kontroli nad ułożeniem brodawki. Warto też zapytać matkę, jak zapewnia higienę sprzętu, i skorygować błędy. W trakcie leczenia ran z kategorii 2, 3 i 4 wskazana jest podwyższona higiena sprzętu [58].
Nieodłącznym elementem porady jest wspieranie matki, zrozumienie dla jej skarg, docenianie zaangażowania w karmienie piersią, dodawanie otuchy, by wytrwała trudny czas leczenia ran.
Leczenie – wspomaganie gojenia, ochrona przed zakażeniem, leczenie zakażenia
W dniu pierwszej oceny należy się zastanowić, z jaką raną mamy do czynienia w zależności od długości wywiadu, nasilenia objawów miejscowych lub miejscowych i ogólnoustrojowych, zastosowanego dotychczas leczenia bądź jego braku
Zalecenia dla matki muszą być proste i możliwe do wykonywania wielokrotnie w ciągu dnia – zgodnie z rytmem karmień. Karmienie wiąże się z koniecznością usuwania naniesionych substancji (co jest
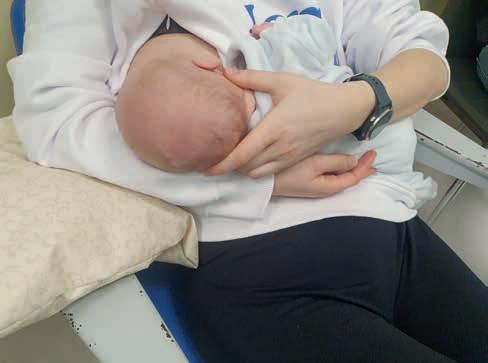
Rycina 10. Prawidłowa pozycja do karmienia – matka w pozycji odchylonej, niemowlę przytulone, ustabilizowane na brzuchu mamy, rączki wokół piersi, główka lekko odchylona (fot. M. Żukowska-Rubik).
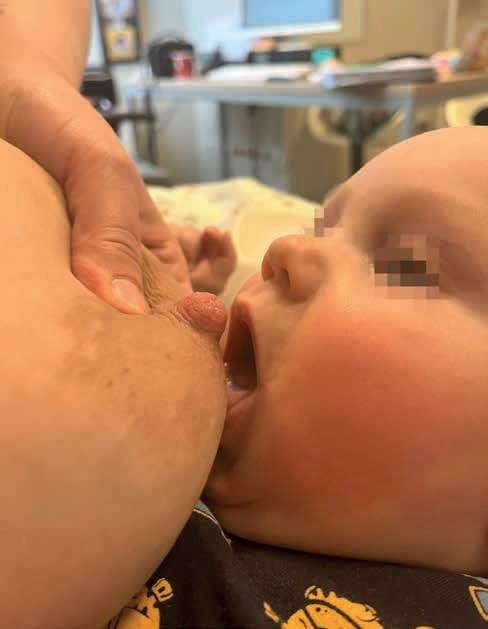
Rycina 11. Przystawienie asymetryczne (fot. M. Nehring-Gugulska).
kłopotliwe i bolesne, a dotyczy to większości preparatów czy opatrunków) i ponownego aplikowania po karmieniu. Ponadto nie są one tanie, muszą być finansowo dostępne dla pacjentki, a potrzebne są zwykle nie dłużej niż przez 1–3 tygodnie. Należy więc unikać nadmiernej ilości preparatów. Opatrunki dla kobiet karmiących nie podlegają refundacji, ponieważ rany brodawek nie spełniają kryteriów rany przewlekłej w rozumieniu NFZ.
Leczenie ran brodawek piersiowych w okresie karmienia piersią
Tabela 4. Kategorie ran brodawek, postępowanie i dobór środków leczniczych. Opracowanie: M. Żukowska-Rubik, M. Nehring-Gugulska
Rana brodawki – oceń kategorię rany
Kategoria 1. Niezakażone
Rany:
• powierzchowne (otarcia naskórka, szczeliny, nadżerki)
• niewielkich rozmiarów – do kilku milimetrów
• świeżo powstałe (kontaminacja) (ryc. 12A–C)
Kategoria 2. Zagrożone zakażeniem
2A.
• każda rana u kobiety z czynnikami ryzyka
• rany rozległe zajmujące ponad połowę powierzchni brodawki, rany na trzonie i u podstawy brodawki
• rany głębokie – owrzodzenia, rozpadliny (kolonizacja)
2B.
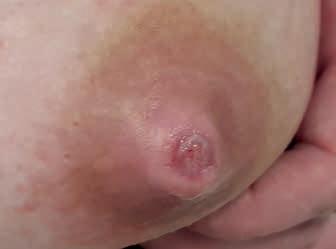
• rany trudno gojące się – pojawiają się niepokojące objawy – wysięk, włóknik, rana powiększa się lub brak oznak gojenia w ciągu 4 dni obserwacji (ryc. 13A–C) (narasta kolonizacja)
Kategoria 3. Zakażone
• znaczne nasilenie dolegliwości bólowych, zaczerwienienie, obrzęk, ucieplenie brodawki, obrzęk brzegów rany, wysięk – często ropny, obecność martwiczej tkanki [42]
• brak postępu gojenia, mimo że nie ma zastrzeżeń do techniki karmienia czy funkcji ssania u noworodka (ryc. 14A–C)
Postępowanie
• zalecić smarowanie pokarmem matki po karmieniach, osuszenie przez kilkanaście sekund
• dodatkowo w razie potrzeby opatrunki bez substancji przeciwbakteryjnej pomiędzy karmieniami (opatrunki żelowe, hydrożelowe) lub ciepłe mokre kompresy z przegotowanej wody (4 razy dziennie po 15 minut przez 5 dni) [30] po karmieniach – utrzymanie wilgotnego środowiska gojenia, zmniejszenie dolegliwości bólowych
• w celu dekolonizacji, zapobieganiu tworzeniu biofilmu i do oczyszczania stosować lawaseptyk z dodatkiem surfaktantu – wypłukanie rany lub okład z gazika zwilżonego preparatem po każdym karmieniu (czas utrzymania na ranie zalecany przez producenta a)
• opatrunki bez substancji przeciwbakteryjnej w celu zapewnienia prawidłowej wilgotności w ranie pomiędzy karmieniami
• w przypadku rany z cechami trudno gojącej się
• stosować lawaseptyk z dodatkiem surfaktantu i substancję antyseptycznąe
• do rozważenia opatrunki z substancją przeciwbakteryjną
• do rozważenia pobranie wymazu
• pobrać wymaz z rany brodawki
• w celu usuwania biofilmu oraz do oczyszczania stosować lawaseptyk z dodatkiem surfaktantu i substancję antyseptycznąC – wypłukanie rany lub okład z gazika zwilżonego preparatem po każdym karmieniu (czas utrzymania na ranie zalecany przez producenta a)
– raz dziennie, przecierając ranę za pomocą zwilżonego gazika, należy usuwać obfity wysięk, biofilm, oddzielające się strupy czy włóknik (wcześniej nawilżyć ranę)
• stosować opatrunki z substancją przeciwbakteryjną, można wykorzystać preparat w postaci żelub do aplikacji po karmieniach minimum 3-4 razy dziennie lub po każdym karmieniuc
• w razie maceracji skóry ograniczyć ilość lub czas aplikacji
• dobrać opatrunek odpowiednio do ilości wysięku
Monitorowanie leczenia
Czas obserwacji: w ciągu 4 dni powinny pojawić się oznaki gojenia* Poprawa/rana goi się: kontynuować powyższe leczenie do pełnego wygojenia Brak poprawy/rana nie goi się: zweryfikować, czy zalecenia zostały poprawnie zastosowane, rozważyć zmianę kategorii rany
Czas obserwacji: w ciągu 4 dni powinny pojawić się oznaki gojenia* Poprawa/rana goi się: kolejna kontrola za 4 dni, kontynuować leczenie do czasu wyraźnej poprawy w gojeniu, wtedy można odstawić substancję antyseptyczną, utrzymać opatrunek zapewniający wilgotne środowisko gojenia Brak poprawy/rana nie goi się: zweryfikować, czy zalecenia zostały poprawnie zastosowane, rozważyć zmianę kategorii
Czas obserwacji: 4 dni
Poprawa: kolejna kontrola za 4 dni, kontynuować leczenie do momentu opanowania zakażenia**, uzyskania wyraźnej poprawy w gojeniu*, wtedy można zaprzestać stosowania substancji antyseptycznej zastosować opatrunek zapewniający wilgotne środowisko gojenia
Brak poprawy/obecne objawy zakażenia/ rana nie goi się:
• zweryfikować, czy zalecenia zostały poprawnie zastosowane
• kontynuować lawaseptyk z surfaktantem i substancję antyseptyczną
• rozważyć antybiotyk miejscowo zgodnie z antybiogramem na 5–7 dnid
Poprawa/ zakażenie opanowane/ rana goi się: odstawić substancję antyseptyczną, zakończyć antybiotyk miejscowy, zastosować opatrunek zapewniający wilgotne środowisko do czasu pełnego wygojenia
Brak poprawy/obecne objawy zakażenia/ rana nie goi się:
• kontynuować lawaseptyk z surfaktantem i substancję antyseptyczną***, opatrunki z substancją przeciwbakteryjną
• zastosować antybiotyk ogólnoustrojowo zgodnie z antybiogramem
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
Table 4. Categories of nipple wounds, management, and selection of medicinal agents (cont.)
Rana brodawki – oceń kategorię rany
Kategoria 4. Szerzenie się zakażenia na okoliczne tkanki
• obrzęk i rumień wokół brodawki (na otoczce) > 2 cm
• róża, cellulitis, zakażone zapalenie gruczołu piersiowego
• cechy infekcji ogólnoustrojowej (gorączka, dreszcze, uczucie rozbicia, podwyższone wskaźniki stanu zapalnego w badaniach laboratoryjnych) (ryc. 15A–B)
Postępowanie
• leczenie miejscowe jak w kategorii 3 (z wyjątkiem miejscowego antybiotyku)
• jeśli był wcześniej wykonany wymaz z rany – antybiotyk ogólnoustrojowo na podstawie antybiogramu
• jeśli nie było wymazu – pobrać, podać ogólnoustrojowo antybiotyk dobrany empirycznie
Monitorowanie leczenia
Czas obserwacji: poprawa powinna nastąpić w ciągu 3 dni leczenia antybiotykiem
Poprawa/ustąpienie objawów ogólnoustrojowych
Poprawa stanu miejscowego: kontynuacja leczenia antybiotykiem zgodnie z przyjętymi zasadami, kontynuacja leczenia miejscowego do uzyskania wyraźnej poprawy w gojeniu rany*
Brak poprawy:
• weryfikacja leczenia zależnie od sytuacji:
• w przypadku zapalenia gruczołu piersiowego pobrać pokarm na posiew
• jeśli dostępny wynik antybiogramu do wymazu z rany – zmienić antybiotyk dobrany empirycznie na dostosowany do wyniku antybiogramu
• jeśli brak antybiogramu, zlecić antybiotyk o szerszym spektrum działania
* Oznaki gojenia – zmniejszanie nasilenia bólu, zmniejszenie wielkości i głębokości rany, pokrywanie się naskórkiem lub ziarniną, zamykanie rany od jej brzegów.
** Opanowanie zakażenia – zmniejszanie nasilenia bólu, ograniczenie ilości wysięku, ustępowanie obrzęku brzegów rany, obrzęku i rumienia brodawki.
*** Brak poprawy w leczeniu antyseptykiem w ciągu 2 tygodni wymaga bezwzględnie weryfikacji.
a Czas utrzymania na ranie zalecany przez producenta – należy zapoznać się z odpowiednią dokumentacją: w przypadku leków ChPL (charakterystyka produktu leczniczego), w przypadku wyrobów medycznych z IFU (instrukcja użytkowania wyrobu medycznego) (tab. II, Aneks 2).
b Opatrunki pierwotne są aplikowane bezpośrednio na ranę. Niektóre wymagają położenia opatrunku wtórnego, którym może być jałowy gazik. Niekiedy warto rozważyć dołożenie opatrunku wtórnego wchłaniającego wysięk zależnie od jego ilości (np. opatrunek piankowy). Niektóre opatrunki mogą pełnić rolę zarówno pierwotnych, jak i wtórnych, np. opatrunki piankowe.
c Preparaty i opatrunki należy zmywać przed kolejnym karmieniem, wyjątkiem są substancje antyseptyczne bez efektu rezydualnego; w ulotce należy sprawdzić czy opatrunki zarejestrowane przez producentów do stosowania na brodawki sutkowe wymagają zmywania.
d Nie zaleca się rutynowego leczenia ran antybiotykami miejscowymi, wskazania są ograniczone do sytuacji opisanych w artykule. Podany czas, zgodnie z doświadczeniem, jest wystarczający do leczenia miejscowym antybiotykiem rany brodawki, zgodny z ChPL preparatów (zwykle 5–7 do 10 dni). Dłuższe leczenie bez oznak poprawy jest nieuzasadnione.
e Substancje antyseptyczne rekomendowane zależnie od sytuacji klinicznej.
I rzut
rany zagrożone infekcją
II rzut
poliheksanidyna (0,02%, 0,04%, 0,1%), oktenidyna 0,05% oktenidyna/fenoksyetanol 0,1%, podchloryn rany zakażone
oktenidyna/fenoksyetanol 0,1% octenidyna 0,05%, poliheksanidyna
Leczenie ran brodawek piersiowych w okresie karmienia
B C
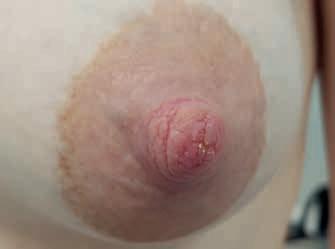
Rycina 12. Kategoria 1 ran: A) otarcie naskórka; B) szczelina na szczycie brodawki (9–13); C) Gojąca się szczelina w bruździe brodawki (w kierunku 5–11), bez sączenia, obrzęku brzegów ranki (fot. M. Żukowska-Rubik).

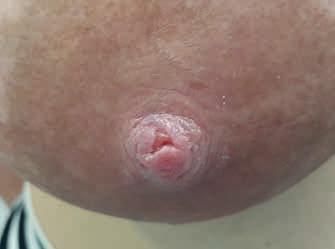
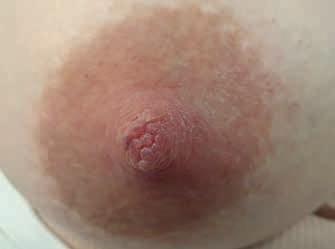
Rycina 13. Kategoria 2 ran: A) nadżerki na szczycie; B) szczelina okalająca ponad połowę obwodu brodawki u podstawy, niewielki obrzęk i maceracja brzegów rany, na szczycie otarcie; C) rana niewielkich rozmiarów, ale przekraczająca głębokość naskórka, bez cech zakażenia (fot. M. Żukowska-Rubik).
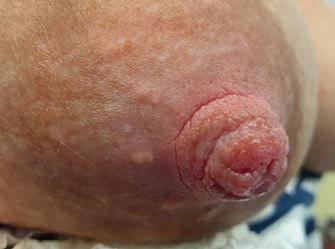
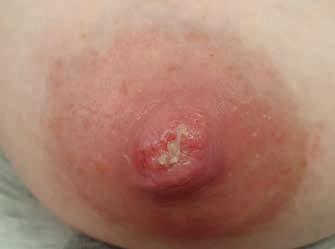
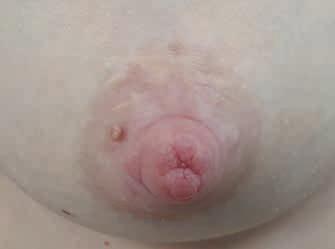
Rycina 14. Kategoria 3 ran: A) rozpadlina na szczycie (dolna część szczytu brodawki) i wokół trzonu; B) owrzodzenie na szczycie, pokryte oddzielającym się naskórkiem; C) rozległa rana szczytu brodawki, pokryta częściowo włóknikiem, sączenie ropnej wydzieliny, mocno obrzęknięte brzegi rany i cała brodawka, rana nie goi się od 2 tygodni (fot. M. Żukowska-Rubik).

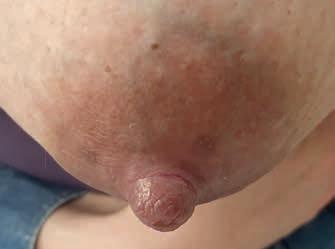
Rycina 15. Kategoria 4 ran: A) cellulitis – zapalenie skóry i tkanki podskórnej piersi, na brodawce zakażona głęboka rana, obrzęk, zaczerwienienie brodawki, sączenie ropnej wydzieliny; B) ropień jako powikłanie nieleczonej zakażonej rany brodawki (fot. M. Żukowska-Rubik).
Monika Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
Tabela 5. Zestawienie preparatów z zawartością substancji antyseptycznej oraz ich właściwości przeciwdrobnoustrojowych
Właściwości
Wymagany czas kontaktu /czas udowodniony w badaniach in vitro
Dichlorowodorek oktenidyny 0,05%+ etyloheksylogliceryna Poliheksanidyna + betaina Poliheksanidyna + poloksamer
Około 1–5 minut/60 sekund
15 minut/5–15 minut
15 minut/5–15 minut
Podchloryny [58–61]
Dichlorowodorek oktenidyny 0,1%+ fenoksyetanol 2%
15 minut/5–15 minut 1 minuta/30 sekund
Efekt rezydualny tak tak tak nie tak
Sterylność tak tak nie nie nie
Łączenie z opatrunkami zawierającymi cząsteczki srebra możliwe możliwe możliwe możliwe możliwe
Tworzenie szczepów opornych nie nie nie nie nie
Status wyrób medyczny wyrób medyczny wyrób medyczny wyrób medyczny lek
Inhibicja gojenia nie nie nie nie nie
Rana głęboka, bez drenażu, oko [34] nie nie nie tak nie
Potencjalnie większe ryzyko uczuleń nie
Efekt rezydualny – substancja czynna pozostaje na skórze, te specyfiki powinny być spłukane/usunięte z powierzchni brodawki przed karmieniem/ odciąganiem.
Wśród substancji antyseptycznych jako leki są zarejestrowane: Octenisept (dichlorowodorek oktenidyny), Braunol i Betadyna (PVP-J) (preparaty zawierające PVP-J nie są zalecane w czasie laktacji). Pozostałe preparaty mają status wyrobu medycznego (patrz Aneks 2).
Tabela 6. Technika pobrania wymazu z rany brodawki [63, 64]
Technika pobrania (ryc. 16) Komentarz
1. Przygotuj narzędzia:
• jałowe podłoże transportowe
• jałowe gaziki
• ampułka soli fizjologicznej
• jałowe rękawiczki oraz skierowanie na wymaz w kierunku flory tlenowej i w niektórych przypadkach także beztlenowej (przetoka, kieszeń, rana po ugryzieniu)
2. Przemyj obficie ranę solą fizjologiczną; przetrzyj ranę gazikiem nasączonym solą fizjologiczną, jeżeli jest pokryta wysiękiem, biofilmem, strupami
3. Oceń ranę
4. Pobierz wymaz
Technika Levine’a – należy obracać wymazówkę w dnie rany, wywołując odpowiedni nacisk w celu wyciśnięcia płynu z łożyska rany, wymazówka jest ustawiona prostopadle do powierzchni rany
5. Umieść wymazówkę w podłożu transportowym, opisz, przekaż ze skierowaniem do analizy
Uwagi
• Wymazy pobiera się przed karmieniem
• oczyszczenie rany z wysięku
• usunięcie z powierzchni maści, kremów, żeli stosowanych przez pacjentkę
• zmniejszenie ryzyka pobrania flory zanieczyszczającej ranę
• nie zaleca się przemywania skóry środkiem odkażającym przed pobraniem, powoduje to uzyskanie wyników fałszywie ujemnych
• jeśli jest sucha, zwilż wymazówkę solą fizjologiczną, jeśli jest mokra, użyj suchej wymazówki
• należy unikać dotykania wymazówką otaczającej skóry
• jeśli występuje przetoka lub kieszeń – pobrać materiał także z tego miejsca
• Do analizy mikrobiologicznej nie zaleca się pobierania: ropy, wydzieliny po zdjęciu opatrunku, śluzu z dna rany, strupa, materiału po zastosowaniu antyseptyku [43]
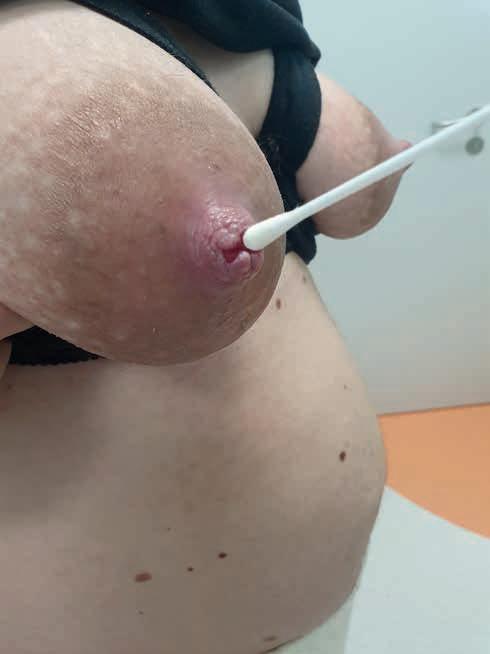
Rycina 16. Pobieranie wymazu z rany metodą Lewina – wymazówka ustawiona prostopadle do rany, ruch rolujący z lekkim uciskiem dna rany (fot. M. Żukowska-Rubik).

Rycina 17. Rana kąsana brodawki (fot. M. Nehring-Gugulska).
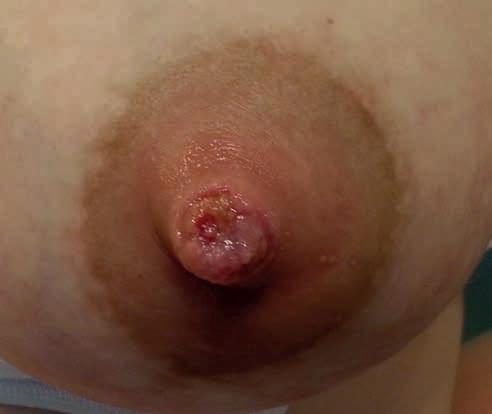
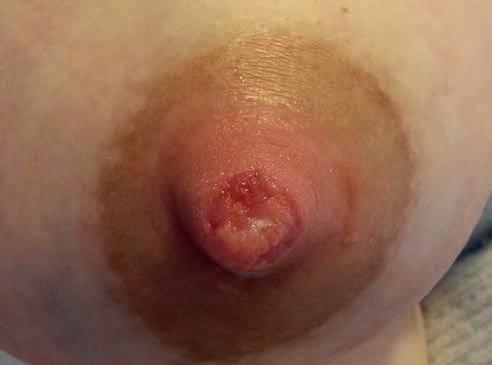
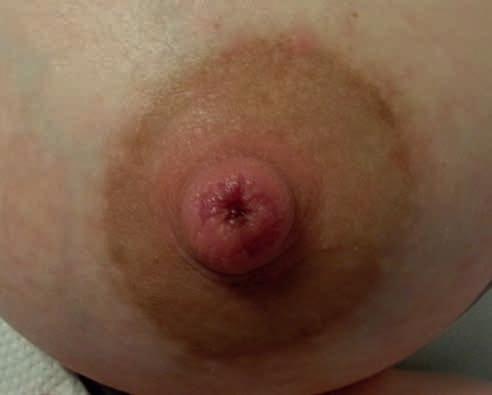
Rycina 18. Przykładowe gojenie. A) Przebieg gojenia rany zakażonej po zastosowaniu lawaseptyku z surfaktantem i i antyseptyku. Rozległa rana brodawki obejmująca cały szczyt i głęboka w centrum, obrzęk brzegów rany, wysięk surowiczo-ropny obserwowany na wkładkach laktacyjnych. B) Po tygodniu rana zaczyna wypłaszczać się, pokrywać się naskórkiem, mniejszy wysięk i obrzęk brzegów. C) Po kolejnym tygodniu w centrum brodawki cienki strup, młody naskórek pokrywa brodawkę wokół (fot. M. Żukowska-Rubik).
Table 7. Techniki pobierania wymazów [63, 64]
Technika Opis Komentarz
Metoda Levine’a
Technika Z (zig-zag)
Należy obracać wymazówkę w ranie, wywołując odpowiedni nacisk w celu wyciśnięcia płynu z łożyska rany, wymazówka jest ustawiona prostopadle do powierzchni rany
Należy wykonać ruch zygzakowaty wymazówką po zmianach skórnych; jednocześnie należy rotować/obracać wymazówkę między palcami
Istotne jest, żeby postęp leczenia monitorować w krótkich odstępach czasu i zmieniać strategię w razie potrzeby. Dobór leczenia w zależności od kategorii rany opisano w tabeli 4. W leczeniu ran brodawek rzadko zachodzi konieczność podania antybiotyku ogólnoustrojowo, w ramce wymieniono wskazania. Optymalnym postępowaniem jest wybór zgodnie z antybiogramem uzyskanym na podstawie posiewu z rany. W szczególnych sytuacjach może być konieczne rozpoczęcie leczenia empirycznego – stosuje się wówczas cefalosporyny I generacji, ponieważ najczęstszym patogenem wywołującym zakażenie są gronkowce złociste MSSA. Należy pamiętać, że środki lecznicze zadziałają łącznie z interwencjami opisanymi wcześniej niwelującymi czynnik przyczynowy powstania uszkodzenia.
Sytuacje wymagające zastosowania antybiotyku ogólnoustrojowo w zakażonych ranach brodawek:
• brak poprawy pomimo prawidłowego przeprowadzenia wszystkich etapów leczenia miejscowego,
• szerzenie się zakażenia na otoczkę,
• szerzenie się zakażenia na skórę piersi, tkankę podskórną, tkankę gruczołową,
• objawy zakażenia ogólnoustrojowego.
W tabeli 5 zestawiono substancje o działaniu antyseptycznym i ich charakterystyki. W tabelach 6 i 7 opisano technikę pobierania wymazu z rany brodawki i zmian skórnych. Wskazania do pobrania wymazu z rany brodawki lub skóry brodawki i otoczki oraz do pobrania pokarmu na posiew w zapaleniu gruczołu piersiowego zamieszczono w ramkach.
Ugryzienie przez ząbkujące niemowlę
Szybkiego wdrożenia leczenia wymagają ugryzienia brodawki czy otoczki przez ząbkujące niemowlę.
• Zalecana (wyższa skuteczność w uzyskaniu właściwego posiewu)
• Bardziej bolesna dla pacjenta
• Technika lepiej tolerowana przez pacjentów
• Zalecana podczas pobierania wymazu ze skóry brodawki i otoczki
• Niezalecana do ran brodawek ze względu na wyższe ryzyko zanieczyszczenia pobranego materiału bakteriami z brzegów rany
Postępuje się jak w ramach kategorii 3. – należy pobrać wymaz, zalecić przemywanie rany, stosować opatrunek z substancją przeciwbakteryjną i w przypadku braku gojenia w ciągu kilku dni podać ogólnie antybiotyk o szerokim spektrum lub wg antybiogramu. Substancjami antyseptycznymi z wyboru u matki karmiącej z raną kąsaną są podchloryn sodu i kwas podchlorawy, które można wykorzystać do irygacji rany (jest to jednocześnie działanie lawaseptyczne). Podchloryny w niskich stężeniach łączą wysoką skuteczność przeciwdrobnoustrojową z bezpieczeństwem stosowania, wykazują brak działania cytotoksycznego dla zdrowych tkanek. Podchloryny ponadto wykazują właściwości przeciwzapalne, przeciwświądowe, mają obojętny odczyn (ryc. 17) [59, 60, 62].
Na rycinie 18 przedstawiono przykład gojenia się zakażonej rany brodawki.
Wskazania do pobrania wymazu z rany brodawki lub skóry brodawki i otoczki obejmują [58]:
• objawy zakażenia rany,
• rany trudno gojące się,
• utrzymujący się ból brodawek mimo poprawy techniki karmienia,
• zmiany na skórze brodawki lub otoczki w postaci rumienia, sączenia, łuszczenia, pękania.
Wskazania do pobrania pokarmu na posiew w zapaleniu gruczołu piersiowego:
• brak poprawy po 3 dobach leczenia antybiotykiem empirycznym,
• zapalenie o ciężkim, septycznym przebiegu,
• podejrzenie zakażenia szpitalnego,
• nawrót zapalenia (objawy zapalenia w tej samej piersi do 3 miesięcy od poprzedniego epizodu),
• podostre zapalenie piersi,
• uczulenie pacjentki na liczne antybiotyki.
Zastosowanie środków leczniczych a karmienie Środki lecznicze stosowane na skórę brodawki powinno się nakładać po nakarmieniu dziecka. Kremy lub maści z antybiotykiem należy nakładać przed dłuższymi przerwami w karmieniu, np. nocną, przed wyjściem na spacer. Antyseptyki bez efektu rezydualnego nie wymagają zmywania przed karmieniem, pozostałe preparaty należy spłukać przegotowaną wodą. Do oczyszczenia piersi przed karmieniem z preparatu w postaci kremu lub maści należy użyć odciągniętego mleka matki (wyciśniętego na gazik) lub środka o lekko kwaśnym pH. Podobnie powierzchnia brodawki powinna być oczyszczona po zdjęciu opatrunku, by minimalizować narażenie niemowlęcia na środki lecznicze i np. oleje mineralne użyte jako podłoże [65, 66].
Zasady higieny
W czasie karmienia piersią, a w szczególności, kiedy brodawki są poranione należy przestrzegać zasad higieny:
• mycie rąk przed karmieniem;
• mycie rąk przed opatrywaniem rany;
• mycie piersi przy okazji toalety całego ciała (nie rzadziej niż raz dziennie) wodą i delikatnym mydłem lub optymalnie środkiem o lekko kwaśnym pH (syndet) [67], do mycia piersi nie zaleca się mydeł lub preparatów z zawartością substancji przeciwbakteryjnych, nie zaleca się też mycia piersi przed każdym karmieniem;
• mycie i dezynfekcja termiczna akcesoriów mających kontakt z piersią (nakładki, elementy laktatora) raz dziennie, a w ranach kategorii 2, 3 i 4 – po każdym użyciu (podwyższona higiena sprzętu laktacyjnego). Dezynfekcję termiczną wykonuje się przez zanurzenie sprzętów na 5–10 minut w wodzie o temp. 90–100°C;
• częsta wymiana wkładek laktacyjnych;
• dezynfekcja rąk przez personel przed kontaktem z matką lub dzieckiem;
• higiena rąk członków rodziny przed kontaktem z matką, dzieckiem, sprzętem laktacyjnym.
Ograniczanie dolegliwości bólowych
Matce należy zaproponować na krótki czas leki przeciwbólowe, wybierając najbezpieczniejsze w okresie laktacji (klasyfikacja L1-2 wg Hale) – paracetamol lub niesteroidowe leki przeciwzapalne (ibuprofen, ketoprofen, diklofenak). Metodą łagodzenia bólu jest też ręczne odciągnięcie mleka przed podaniem piersi w celu uruchomienia odruchu wypływu pokarmu i uzyskania działania przeciwbólowego oksytocyny w ośrodkowym układzie nerwowym
(OUN). Podstawowe znaczenie ma poprawa techniki karmienia.
Przerwanie karmienia na pewien czas
Karmienie piersią jest bezpieczne dla dziecka, może jednak być zbyt bolesne dla matki. W tej sytuacji można rozważyć czasowe przerwanie karmienia na kilka czy kilkanaście godzin, czasem kilka dni, regularne odciąganie pokarmu i podawanie go dziecku, do uzyskania podgojenia ran, pod warunkiem że odciąganie jest mniej bolesne niż karmienie, nie narusza rany i zapewnia skuteczne opróżnianie piersi.
Ogólna kondycja matki
Szczególną uwagę należy zwrócić na kobiety z obciążonym wywiadem, u których gojenie może być upośledzone, a ryzyko zakażenia jest większe niż w zdrowej populacji (patrz czynniki zwiększające ryzyko infekcji rany wymienione w ramce). Oporne gojenie się rany jest wskazaniem do oceny ogólnego stanu zdrowia matki i wykonania badań dodatkowych, np. w celu wykluczenia niedokrwistości czy nieprawidłowej glikemii. Konieczna jest również ocena odżywiania się matki, ponieważ około połowa polskich matek stosuje diety eliminacyjne w czasie karmienia dziecka. Najczęściej unikają nabiału, w tym jaj, ale też wielu innych produktów będących źródłem energii, białka i witamin, chcąc rzekomo chronić dziecko przed kolką, alergią i in. Taka niedoborowa dieta może wpływać na opóźnianie gojenia [68]. Niedożywienie zwiększa ryzyko zakażenia rany. Zgodnie z zaleceniami pacjent zmagający się z raną przewlekłą powinien otrzymać 30–35 kcal/kg mc./dobę (nie mniej niż 1800 kcal) oraz odpowiednią ilości białka (1,5–2,5 g/ kg mc.), węglowodanów (55–60%) i tłuszczy (20–25%) [45].
Podsumowanie zasad leczenia ran brodawek u matek karmiących:
• korekta techniki karmienia, zmniejszenie napięcia otoczki przed przystawieniem dziecka (jeśli wskazane),
• odpowiednie interwencje dotyczące usprawnienia funkcji ssania u dziecka, jeśli są wskazania (konsultacje – doradca/konsultant laktacyjny, neurologopeda, fizjoterapeuta, frenotomia),
• dobór/korekta rozmiaru lejka i siły laktatora w celu zapewnienia komfortu matki podczas odciągania mleka,
• higiena rąk, piersi i podwyższona higiena sprzętu laktacyjnego,
Żukowska-Rubik, Magdalena Nehring-Gugulska, Beata Mrozikiewcz-Rakowska i wsp.
• zastosowanie lawaseptyków, substancji antyseptycznych, opatrunków specjalistycznych, antybiotyków – zależnie od kategorii rany,
• leczenie przeciwbólowe, w wyjątkowych sytuacjach przerwa w karmieniu na 1–2 doby (w tym czasie odciąganie mleka i podawanie go dziecku),
• prawidłowe odżywianie matki.
ZADANIA – JAKIE LECZENIE ZASTOSUJESZ?
• Zadanie 1. (ryc. 19): brak postępu gojenia rany od 3 tygodni, u podstawy brodawki szczelina obejmująca większość obwodu brodawki, o zróżnicowanej głębokości, z sączeniem wysięku, silny ból, matka bliska rezygnacji z karmienia piersią. Dotychczas smarowała ranę pokarmem po karmieniach, stosowała kompresy hydrożelowe. Technika karmienia jest prawidłowa.
• Zadanie 2. (ryc. 20): głęboka rana od miesiąca obejmująca szczyt i boki brodawki, pokryta biofilmem, obrzęk brzegów rany, sączenie wysięku, obrzęk brodawki, ból. Matka karmi piersią kilka razy dziennie, ale karmienie jest bardzo bolesne, więc częściej odciąga pokarm. Dotychczas zalecano jej kremy pielęgnacyjne, muszle ochronne, stosowała też antyseptyk przez kilka dni.
Różnicowanie zmian zlokalizowanych na brodawkach sutkowych
U karmiącej matki zazwyczaj występują uszkodzenia związane z nieprawidłową techniką karmienia lub odciągania pokarmu laktatorem. Jednak zmiany na brodawkach mogą być też wynikiem choroby ogólnoustrojowej np. wirusowej (np. Herpes simplex, Herpes zoster, Varicella zoster), bakteryjnej (np. kiła, rzeżączka), układowej (np. łuszczyca, rybia łuska, wyprysk, AZS). Należy zachować czujność onkologiczną w przypadku zmian nie poddających się leczeniu w oczekiwanym czasie i skierować pacjentkę na diagnostykę (np. rak Pageta). Na rycinie 21 przedstawiono informacje istotne w postępowaniu w bolesności i ranach brodawek. Algorytm uwzględnia zwięźle inne problemy występujące w okresie laktacji, istotne w różnicowaniu przyczyn bolesności brodawek.
Podsumowanie
Rany brodawek to często pojawiający się problem, zwłaszcza wcześnie po porodzie, i jedna z głównych przyczyn przedwczesnej rezygnacji matek z karmienia piersią. Znajomość podstawowych zasad leczenia ran brodawek jest niezbędna wszystkim pracownikom ochrony zdrowia, którzy opiekują się matką lub niemowlęciem w okresie laktacji na różnych poziomach systemu opieki i różnych stanowiskach, a więc położnym, pielęgniarkom, doradcom laktacyjnym,
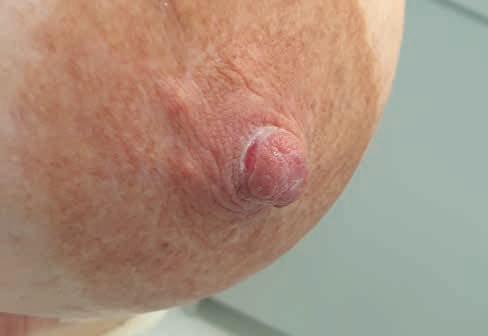
Rycina 19. Zadanie 1 – jakie leczenie: rana trudno gojąca się u podstawy brodawki, obejmująca większość obwodu brodawki, o zróżnicowanej głębokości, z sączeniem wysięku, brak postępu gojenia od 3 tygodni (fot. M. Żukowska-Rubik).

Rycina 20. Zadanie 2 – jakie leczenie: głęboka rana obejmująca szczyt i boki brodawki, pokryta biofilmem, obrzęk brzegów rany, sączenie wysięku, obrzęk brodawki (fot. M. Nehring-Gugulska).
Źródło: Żukowska-Rubik M., Nehring-Gugulska M. Patologia piersi i brodawek podczas laktacji. W: Żukowska-Rubik M., Godyń-Myśliwy Z., Nehring- Gugulska M. Atlas fizjologii i patologii laktacji. Wyd. 1. Medycyna Praktyczna, Krakow 2025, za zgodą.
ginekologom, neonatologom, pediatrom i lekarzom rodzinnym. Wiedza i współpraca tych specjalistów służy zdrowiu publicznemu. Podstawowe znaczenie w leczeniu ran ma poprawa techniki karmienia i zadbanie o podatny kompleks brodawka–otoczka, tak by umożliwić dziecku głębokie uchwycenie piersi. Istotne jest częste monitorowanie postępu gojenia rany, by w porę zareagować na objawy pojawiającego się zakażenia. W opracowaniu autorzy zwracają uwagę, kiedy do leczenia ran w zależności od ich kategorii można wykorzystywać matczyny pokarm, nowoczesne lawaseptyki, substancje antyseptyczne czy opatrunki specjalistyczne, biorąc pod uwagę szczególną lokalizację tych ran i kontakt jamy ustnej dziecka z leczoną piersią.
zależnie od przyczyny i stanu brodawek:
Poprawa techniki karmienia, chwytności kompleksu brodawka–otoczka, funkcji ssania, sposobu odciągania Do rozważenia: leki przeciwbólowe, muszle ochronne, kapturki, przerwanie karmienia/odciąganie przez pewien czas
Wymaz z rany/zmian na skórze otoczki i brodawki –przy podejrzeniu zakażenia
ROZPOZNANIE
ZAPYTAJ: od kiedy występują ból lub uszkodzenia, nasilenie bólu, związek z karmieniem, higiena piersi, dotychczasowe leczenie; zależnie od sytuacji –pytania nakierowane na specyficzny problem, np. odciąganie, ząbkowanie
Skieruj pacjentkę do lekarza –leczenie rany zgodnie z antybiogramem, leczenie chorób skóry, innych problemów Skieruj dziecko do specjalisty , jeśli są wskazania (pediatra, neurolog, neurologopeda, fizjoterapeuta, inni)
Monitoruj przebieg karmienia, przyrosty masy ciała dziecka
Żółtawa grudka na brodawce –rozważ: czop zapalny, czop zastoinowy, –Leczenie przyczynowe torbiel łojowa, gruczolak łojowy –Leczenie chirurgiczne Zmiana jednostronna –nadżerka, sączenie, strup, łuszczenie + długi wywiad, brak poprawy po leczeniu –pomyśl o raku Pageta –skieruj do specjalisty * mycie piersi delikatnym mydłem w czasie toalety całego ciała, optymalnie środkiem myjącym o pH lekko kwaśnym, mycie rąk przed karmieniem, personel –czyste i zdezynfekowane ręce; ** smarowanie pokarmem po karmieniach, ewentualnie specyfikami przeznaczonymi do stosowania na brodawki w czasie laktacji *** podwyższona higiena sprzętów –dezynfekcja termiczna przed i po każdym użyciu
Zmiany rumieniowe, sączące, łuszczące na brodawkach i otoczkach –rozważ: wyprysk, zakażenie bakteryjne, mieszane, grzybicze, łuszczyca, inne, podrażnienie skóry przez nieprawidłową pielęgnację –Leczenie przyczynowe Pęcherzyki na skórze brodawki/otoczki –rozważ: silne zasysanie, ocieranie brodawki, zakażenie skóry –opryszczka, półpasiec, liszajec –Leczenie przyczynowe
ZBADAJ: Dziecko: nieprawidłowe napięcie mm., asymetria? Zakażenie skóry? Jama ustna –nieprawidłowa anatomia, funkcja? Zmiany na śluzówkach?
Matkę: wygląd zmian na brodawkach/otoczkach, obecność objawów zakażenia rany? kształt i elastyczność kompleksu brodawka–otoczka
Karmienie: nieprawidłowa technika, uchwycenie? wciąganie brodawki? spłycanie, zaciskanie, szarpanie?
Brodawki bolesne bez uszkodzeń –rozważ: nadwrażliwość fizjologiczna –standardowa higiena*, neutralna pielęgnacja** objaw Raynauda –leczenie przyczynowe –suche ciepło, Ca-blokery
Brodawki uszkodzone bez objawów zakażenia –standardowa higiena*, pokarm, ewentualnie mokre, ciepłe kompresy, opatrunki żelowe, hydrożelowe; rany zagrożone zakażeniem –do przemywania po karmieniach lawaseptyk z surfaktantem, do rozważenia antyseptyk i opatrunki specjalistyczne z substancją przeciwbakteryjną, podwyższona higiena sprzętów***
Grzybica Pleśniawki Rumień pieluchowy Antybiotyki Leki immunosupresyjne Cukrzyca
Dysbioza Uszkodzenia brodawek po porodzie Antybiotyki Nieprawidłowa pielęgnacja
Czop zapalny Zmiana jednostronna Białożółta plama/grudka na brodawce Ból kłujący, drążący
Wyprysk Środki czystości Kosmetyki Wkładki Leki i nowe pokarmy u dziecka
Brodawki uszkodzone, obecne objawy zakażenia –standardowa higiena*, podwyższona higiena sprzętów*** + przemywanie ran po karmieniach lawaseptykiem z surfaktantem, antyseptyk, opatrunki specjalistyczne z substancją przeciwbakteryjną, w szczególnie trudnych przypadkach antybiotyk miejscowy zgodnie z antybiogramem, jeśli zakażenie rozszerza się –antybiotyk ogólnie
Blednięcie/objaw Raynauda Blednięcie, trzyfazowa zmiana zabarwienia Reakcja na zimno Skłonność do marznięcia palców Leki zwężające naczynia krw.
Choroby matki Palenie papierosów
Odciąganie Siła ssania Ułożenie piersi w lejku Szerokość lejka Czas sesji Higiena sprzętu
PROBLEM
NA SPECYFICZNY
NAKIEROWANE
PYTANIA
Rycina 21. Algorytm –bolesność i uszkodzenia brodawek –różnicowanie i postępowanie. Opracowanie: dr n. med. Monika Żukowska-Rubik
• Zadanie 1. (ryc. 19.) – rana kategorii 2B, rana trudno gojąca się, należy zalecić:
• pobranie wymazu z rany,
• opcja 1. – przemywanie po karmieniach lawaseptykiem z zawartością surfaktantu i antyseptyku, pomiędzy karmieniami opatrunek piankowy,
• opcja 2. – żel z surfaktantem i antyseptykiem do nakładania cienką warstwą po karmieniach i pozostawienie do następnego karmienia,
• leki przeciwbólowe,
• higienę,
• kontrolę za 4 dni.
• Zadanie 2. (ryc. 20) rana kategorii 3, należy zalecić:
• poprawę techniki karmienia,
• pobranie wymazu z rany,
• oczyszczanie rany lawaseptykiem z surfaktantem po karmieniu/odciąganiu optymalnie w postaci przymoczka z gazika nasączonego płynem, antyseptyk optymalnie I rzutu do ran zakażonych,
• oczyszczanie powierzchni rany z biofilmu raz dziennie,
• pomiędzy karmieniami opatrunki z zawartością substancji przeciwbakteryjnej i kontrolą wysięku,
• leki przeciwbólowe,
• higienę (podwyższona higiena sprzętu do odciągania),
• kontrolę za 4 dni.
Oświadczenia
Beata Mrozikiewicz-Rakowska – konsultacje i wykłady dla firm: Convatec, Hartmann, Schulke, Smith@ Nephew, Urgo.
Monika Aleksy-Polipowska – konsultacje i wykłady dla firm: ConvaTec, Verco, Schulke, Urgo. Pozostałe autorki nie zgłaszają konfliktu interesów. Praca nie uzyskała finansowania zewnętrznego. Zgoda Komisji Bioetycznej nie była wymagana.
Piśmiennictwo
1. Jackson KT, Dennis CL. Lanolin for the treatment of nipple pain in breastfeeding women: a randomized controlled trial. Matern Child Nutr 2017; 13: e12357. DOI: 10.1111/mcn.12357.
2. Dennis CL, Jackson K, Watson J. Interventions for treating painful nipples among breastfeeding women. Cochrane Database Syst Rev 2014; (12): CD007366. DOI: 10.1002/14651858.CD007366.pub2.
3. Douglas P. Re-thinking lactation-related nipple pain and damage. Womens Health 2022; 18: 17455057221087864. DOI: 10.1177/ 17455057221087865.
4. Królak-Olejnik B, Błasiak I, Szczygieł A. Promotion of breastfeeding in Poland: the current situation. J Int Med Res 2017; 45: 1976–1984. DOI: 10.1177/0300060517720318.
5. Coca KP, Amir LH, Alves MDR da S i wsp. Measurement tools and intensity of nipple pain among women with or without damaged nipples: A quantitative systematic review. J Adv Nurs 2019; 75: 1162–1172. DOI: 10.1111/jan.13908.
6. Wilson E, Woodd SL, Benova L. Incidence of and Risk Factors for Lactational Mastitis: A Systematic Review. J Hum Lact Off J Int Lact Consult Assoc 2020; 36: 673–686. DOI: 10.1177/0890334420907898.
7. McClellan HL, Hepworth AR, Kent JC i wsp. Breastfeeding frequency, milk volume, and duration in mother-infant dyads with persistent nipple pain. Breastfeed Med Off J Acad Breastfeed Med 2012; 7: 275–281. DOI: 10.1089/bfm.2011.0117.
8. Fernández L, Langa S, Martín V i wsp. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013; 69: 1–10. DOI: 10.1016/j.phrs.2012.09.001.
9. Pannaraj PS, Li F, Cerini C i wsp. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr 2017; 171: 647–654. DOI: 10.1001/jamapediatrics.2017.0378.
10. Kim SY, Yi DY. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp Mol Med 2020; 52: 1288–1297. DOI: 10.1038/s12276-020-0470-5.
11. Quitadamo PA, Comegna L, Cristalli P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic From COVID-19. Front Public Health 2020; 8: 589736. DOI: 10.3389/fpubh.2020.589736.
12. Asena L, Suveren EH, Karabay G, Dursun Altinors D. Human Breast Milk Drops Promote Corneal Epithelial Wound Healing. Curr Eye Res 2017; 42: 506–512. DOI: 10.1080/02713683.2016.1223318.
13. Pan L, Zhang X, Gao Q. Effects and mechanisms of histatins as novel skin wound-healing agents. J Tissue Viability 2021; 30: 190–195. DOI: 10.1016/j.jtv.2021.01.005.
14. Santos KJ da S, Santana GS, Vieira T de O i wsp. Prevalence and factors associated with cracked nipples in the first month postpartum. BMC Pregnancy Childbirth 2016; 16: 209. DOI: 10.1186/ s12884-016-0999-4.
15. Thompson R, Kruske S, Barclay L i wsp. Potential predictors of nipple trauma from an in-home breastfeeding programme: A cross-sectional study. Women Birth J Aust Coll Midwives 2016; 29: 336–344. DOI: 10.1016/j.wombi.2016.01.002.
16. McClellan H, Geddes D, Kent J i wsp. Infants of mothers with persistent nipple pain exert strong sucking vacuums. Acta Paediatr Oslo Nor 1992 2008; 97: 1205-1209. DOI: 10.1111/j.1651-2227.2008.00882.x.
17. Cruz PV, Souza-Oliveira AC, Notaro SQ i wsp. Prevalence of ankyloglossia according to different assessment tools: A meta-analysis. J Am Dent Assoc 1939 2022; 153: 1026–1040.e31. DOI: 10.1016/j. adaj.2022.07.011.
18. Cordray H, Mahendran GN, Tey CS i wsp. Severity and prevalence of ankyloglossia-associated breastfeeding symptoms: A systematic review and meta-analysis. Acta Paediatr 2023; 112: 347–357. DOI: 10.1111/apa.16609.
19. Żukowska-Rubik M. Bolesność brodawek. W: Nehring-Gugulska M, Żukowska-Rubik M, Pi etkiewicz A (red.). Karmienie piersią w teorii i praktyce. Medycyna Praktyczna, FTK, Centrum Nauki o Laktacji, Kraków, Warszawa 2017; 209–229.
20. High WA, Tomasini CF, Argenziano G, Zalaudek I. Podstawowe zasady w dermatologii. W: Bolognia JL, Schaffer JV, Cerroni L (red.). Der matologia. T. I. Wyd. Med. MediPage, Warszawa 2022; 3–6.
21. Burgdorf WHC, Plewig G, Wolff HH, Landthaler M. Dermatologia Braun-Falco. Gliński W, Czarnecka-Operacz M, Krasowska DD i wsp. (red.). V. I. Czelej, Lublin 2017; 9–14.
22. Waśkiel-Burnat A, Sar-Pomian M. Diagnostyka chorób skóry. W: Rudnicka L, Olszewska M, Rakowska A, Sar-Pomian M. (red.). Współczesna dermatologia. T. 1. PZWL, Warszawa 2024; 33–36.
23. Woolridge MW. Aetiology of sore nipples. Midwifery 1986; 2: 172–176. DOI: 10.1016/s0266-6138(86)80042-0.
24. Geddes DT, Kent JC, Mitoulas LR, Hartmann PE. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum Dev 2008; 84: 471–477. DOI: 10.1016/j.earlhumdev.2007.12.008.
25. Wang Z, Liu Q, Min L, Mao X. The effectiveness of the laid-back position on lactation-related nipple problems and comfort: a meta-analysis. BMC Pregnancy Childbirth 2021; 21: 248. DOI: 10.1186/ s12884-021-03714-8.
26. Bashiri A, Amiri-Farahani L, Salehiniya H, Pezaro S. Comparing the effects of breastfeeding in the laid-back and cradle position upon the experiences of primiparous women: a parallel randomized clinical trial. Trials 2023; 24: 109. DOI: 10.1186/s13063-023-07143-0.
27. Niazi A, Rahimi VB, Soheili-Far S i wsp. A Systematic Review on Prevention and Treatment of Nipple Pain and Fissure: Are They Curable? J Pharmacopuncture 2018; 21: 139–150. DOI: 10.3831/ KPI.2018.21.017.
28. Vieira F, Mota DDCF, Castral TC i wsp. Effects of Anhydrous Lanolin versus Breast Milk Combined with a Breast Shell for the Treatment of Nipple Trauma and Pain During Breastfeeding: A Randomized Clinical Trial. J Midwifery Womens Health 2017; 62: 572–579. DOI: 10.1111/jmwh.12644.
29. Lis K. Hypersensitivity to Lanolin: An Old-New Problem. Life Basel Switz 2024; 14: 1553. DOI: 10.3390/life14121553.
30. Lavergne NA. Does application of tea bags to sore nipples while breastfeeding provide effective relief? J Obstet Gynecol Neonatal Nurs 1997; 26: 53–58. DOI: 10.1111/j.1552-6909.1997.tb01507.x.
31. Coentro VS, Perrella SL, Lai CT i wsp. Nipple shield use does not impact sucking dynamics in breastfeeding infants of mothers with nipple pain. Eur J Pediatr 2021; 180: 1537–1543. DOI: 10.1007/ s00431-020-03901-3.
32. Coentro VS, Perrella SL, Lai CT i wsp. Impact of Nipple Shield Use on Milk Transfer and Maternal Nipple Pain. Breastfeed Med 2021; 16: 222–229. DOI: 10.1089/bfm.2020.0110.
33. Kronborg H, Foverskov E, Nilsson I, Maastrup R. Why do mothers use nipple shields and how does this influence duration of exclusive breastfeeding? Matern Child Nutr 2017; 13: e12251. DOI: 10.1111/ mcn.12251
34. Ekström A, Abrahamsson H, Eriksson RM, Mårtensson BL. Women’s use of nipple shields – Their influence on breastfeeding duration after a process-oriented education for health professionals. Breastfeed Med Off J Acad Breastfeed Med 2014; 9: 458–466. DOI: 10.1089/ bfm.2014.0026.
35. Kramer A, Dissemond J, Kim S i wsp. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol Physiol 2018; 31: 28–58. DOI: 10.1159/000481545
36. Sopata M, Jawień A, Mrozikiewicz-Rakowska B i wsp. Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych – przegląd dostępnych substancji przeciw drobnoustrojowych stosowanych w leczeniu ran. Zalecenia Polskiego Towarzystwa Leczenia Ran. Leczenie Ran 2020; 17: 1–21. DOI: 10.5114/lr.2020.96820.
37. Babalska ZŁ, Korbecka-Paczkowska M, Karpiński TM. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharm Basel Switz 2021; 14: 1253. DOI: 10.3390/ ph14121253.
38. Barrigah-Benissan K, Ory J, Sotto A i wsp. Antiseptic Agents for Chronic Wounds: A Systematic Review. Antibiot Basel Switz 2022; 11: 350. DOI: 10.3390/antibiotics11030350.
39. Nehring-Gugulska M, Nadratowska I, Fajdek J i wsp. Czy to przełom w leczeniu ran brodawek? Prezentacja wyników badania. Mater Konf XI Zjazd CNoL. Opublikowano online 2018.
40. Sopata M, Mrozikiewicz-Rakowska B, Jawień A. Stanowisko Polskiego Towarzystwa Leczenia Ran – postępowanie przeciwdrobnoustrojowe w ranie skolonizowanej, z cechami infekcji i zagrożonej infekcją w erze antybiotykoodporności. Leczenie Ran 2023; 20: 125–141. DOI: 10.60075/lr.v20i4.59.
41. Kulińska R. Różnicowanie zmian skórnych u matek karmiących. XVI Zjazd Centrum Nauki o Laktacji Warszawa. Published online 2023.
42. McClellan HL, Hepworth AR, Garbin CP i wsp. Nipple pain during breastfeeding with or without visible trauma. J Hum Lact Off J Int Lact Consult Assoc 2012; 28: 511–521. DOI: 10.1177/0890334412444464.
43. Sopata M, Mrozikiewicz-Rakowska B, Jawień A i wsp. Uaktualnienie dokumentu: Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych – przegląd dostępnych substancji przeciwdrobnoustrojowych stosowanych w leczeniu ran. Zalecenia PTLR 2020. Leczenie Ran 2023; 20: 125–141 DOI: 10.60075/lr.v20i4.59.
44. Baran A, Flisiak I. Choroby infekcyjne skórne. W: Rudnicka L, Olszewska M, Rakowska A, Sar-Pomian M (red.). Współczesna dermatologia. T. 1. PZWL, Warszawa 2024; 114–116.
45. Nehring-Gugulska M, Kowol-Trela K. Schorzenia brodawek sutkowych w okresie laktacji. Ginekol Po Dyplomie. Online 2022.
46. Gao H, Wang J, Ding S i wsp. A retrospective analysis of debridement in the treatment of chronic injury of lactating nipples. Sci Rep 2021; 11: 3625. DOI: 10.1038/s41598-021-83172-6.
47. Cable B, Stewart M, Davis J. Nipple wound care: a new approach to an old problem. J Hum Lact Off J Int Lact Consult Assoc 1997; 13: 313–318. DOI:10.1177/089033449701300417.
48. Marrazzu A, Sanna MG, Dessole F i wsp. Evaluation of the effectiveness of a silver-impregnated medical cap for topical treatment of nipple fissure of breastfeeding mothers. Breastfeed Med Off J Acad Breastfeed Med 2015; 10: 232–238. DOI: 10.1089/bfm. 2014.0177.
49. Hale TW, Krytsch K. Hale’s Medications & Mothers’ Milk 2023 Twentieth Edition. 2023. Dostępne na: https://www.springerpub. com/hale-s-medications-mothers-milk-2023-9780826160638.html (Access: 13.07.2024).
50. Yabes JM, White BK, Murray CK i wsp. In Vitro activity of Manuka Honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med Mycol 2017; 55: 334–343. DOI: 10.1093/mmy/myw070.
51. Bonamonte D, Belloni Fortina A, Neri L, Patrizi A. Fusidic acid in skin infections and infected atopic eczema. G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr 2014; 149: 453–459.
52. Galindo E, Hebert AA. A comparative review of current topical antibiotics for impetigo. Expert Opin Drug Saf 2021; 20: 677–683. DOI: 10.1080/14740338.2021.1902502.
53. Heal CF, Banks JL, Lepper PD i wsp. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst Rev 2016; 11: CD011426. DOI: 10.1002/14651858. CD011426.pub2.
54. Livingstone V, Stringer LJ. The treatment of Staphyloccocus aureus infected sore nipples: a randomized comparative study. J Hum Lact Off J Int Lact Consult Assoc 1999; 15: 241–246. DOI: 10.1177/089033449901500315.
55. Livingstone VH, Willis CE, Berkowitz J. Staphylococcus aureus and sore nipples. Can Fam Physician Med Fam Can 1996; 42: 654–659.
56. Kent JC, Ashton E, Hardwick CM i wsp. Nipple Pain in Breastfeeding Mothers: Incidence, Causes and Treatments. Int J Environ Res Public Health 2015; 12: 12247–12263. DOI: 10.3390/ijerph121012247.
57. Czy Polska jest krajem przyjaznym matce karmiącej? Wizyty położnej podstawowej opieki zdrowotnej i u ginekologa-położnika. Raport z badania ankietowego 2017. Available at: http://cnol. kobiety.med. pl/wp-content/uploads/2019/01/raport_2017.pdf (Access: 13.03.2019).
58. Żukowska-Rubik M. Bolesność brodawek. W: Nehring-Gugulska M, Żukowska-Rubik M, Pietkiewicz A (red.). Karmienie piersią w teorii i praktyce. Medycyna Praktyczna, FTK, Centrum Nauki o Laktacji, Kraków, Warszawa 2017; 209–229.
59. Draelos ZD. Antipruritic hydrogel for the treatment of atopic dermatitis: an open-label pilot study. Cutis 2012; 90: 97–102.
60. Gold MH, Andriessen A, Dayan SH i wsp. Hypochlorous acid gel technology – Its impact on postprocedure treatment and scar prevention. J Cosmet Dermatol 2017; 16: 162–167. DOI:10.1111/jocd.12330.
61. Raport Br-0197-22 Mikrodacyn 60 ® Wound Care. Narodowy Instytut Leków z 31.08.2022.
62. Gold M, Andriessen A, Bhatia A. Podawany miejscowo stabilizowany kwas podchlorawy: Przyszły złoty standard w leczeniu ran i blizn w zabiegach dermatologicznych i chirurgii plastycznej. J Cosmet Dermatol 2020; 19: 270–277. DOI: 10.1111/jocd.13280.
63. University of North Carolina School of Medicine – Lactation culture protocole. Published online 2018.
64. Wound Infection in Clinical Practice. Principles of best practice.
65. Lactmed. Available at: https://www.ncbi.nlm.nih.gov/books/ NBK501885/.
66. Concin N, Hofstetter G, Plattner B i wsp. Mineral oil paraffins in human body fat and milk. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 2008; 46: 544–552. DOI:10.1016/j.fct.2007.08.036
67. Mijaljica D, Spada F, Harrison IP. Skin Cleansing without or with Compromise: Soaps and Syndets. Mol Basel Switz 2022; 27: 2010. DOI:10.3390/molecules27062010.
68. Nehring-Gugulska M, Bębenek D, Krauze A i wsp. Stosowanie diety eliminacyjnej przez polskie matki karmiące piersią w świetle aktualnej wiedzy. Pediatr Po Dyplomie 2023; 6: 57–65.
69. Augustin M, Herberger K, Wille A, Twarock S. Impact of human wound exudate on the bactericidal efficacy of commercial antiseptic products. J Wound Care 2023; 32: 422–427. DOI: 10.12968/ jowc.2023.32.7.422.
70. Radischat N, Augustin M, Herberger K i wsp. Influence of human wound exudate on the bactericidal efficacy of antiseptic agents in quantitative suspension tests on the basis of European Standards (DIN EN 13727). Int Wound J 2020; 17: 781–789. DOI: 10.1111/iwj.13336.
Ocena rany
Trójkąt oceny rany to podstawowe narzędzie, przydatne zwłaszcza dla początkujących, zaczynających się zajmować leczeniem ran. Oceniamy następująco:
• łożysko rany,
• brzegi rany,
• otaczającą skórę.
Łożysko rany
Brzegi rany
Rycina I. Trójkąt oceny rany.
Ocena łożyska rany
1. Rodzaj tkanki.
2. Wysięk.
3. Infekcja.
Ocena brzegów rany
1. Maceracja.
2. Wysuszenie.
3. Kieszeniowanie.
Ocena otaczającej skóry
1. Maceracja.
2. Przeczos.
3. Sucha skóra.
4. Hiperkeratoza.
5. Stwardnienia.
6. Zmiany wypryskowe.
1. Rodzaj tkanki
• Nekrotyczna.
• Martwica rozpływna.
• Ziarninująca.
• Naskórkująca.
2. Wysięk
Otaczająca skóra
• Poziom: brak; mały; średni; duży.
• Rodzaj: wodnisty; gęsty; przejrzysty; mętny; ropny; barwy różowej/czerwonej.
3. Infekcja
• Miejscowa: wzmożony ból; rumień; obrzęk; miejscowo podwyższona temperatura; wzmożony wysięk; krucha ziarnina; przykry zapach; kieszeniowanie.
• Rozprzestrzeniająca się/systemowa: silny rumień; gorączka; ropień/ropa; rozejście się rany; zapalenie tkanki łącznej; ogólne osłabienie; leukocytoza; zapalenie naczyń chłonnych.
Słownik pojęć
• Hiperkeratoza – nadmierne rogowacenie naskórka.
• Infekcja – obecność bakterii i innych mikroorganizmów w ilości na tyle dużej, że prowadzą one do uszkodzenia tkanek i utrudnienia procesu gojenia. Kliniczne objawy zakażenia mogą nie występować u pacjentów z obniżoną odpornością, zaburzeniami perfuzji lub raną przewlekłą.
• Kieszeniowanie – tkanka naskórkowa nabudowująca się w głąb rany, a niepokrywająca jej całej powierzchni. Może pojawić się w przypadku ran o podłożu zapalnym, w tym nowotworowym, i może skutkować utrudnionym gojeniem, o ile nie zostaną przedsięwzięte odpowiednie działania.
• Maceracja – proces, w trakcie którego dochodzi do zmiękczenia tkanek brzegu rany wskutek długotrwałego wystawienia na działanie wilgoci wysięku z rany. Zmieniona tkanka jest zazwyczaj koloru białego.
• Martwica rozpływna – żółta, włóknista tkanka składająca się z włókien, ropy i materiału białkowego.
• Mobilizacja zapalna – rozpad tkanek lub owrzodzenie rozprzestrzeniające się pod brzegiem rany, w wyniku czego rana ma większą powierzchnię u podstawy niż na poziomie skóry.
• Naskórkująca – różowa tkanka na końcowym etapie gojenia, gdy na powierzchni rany pojawiają się komórki naskórka.
• Przeczos – stan wywołany wielokrotnym uszkodzeniem powierzchni skóry wskutek urazów, np. drapania, otarcia.
• Stwardnienia – zgrubiała i stwardniała skóra lub tkanka miękka, szczególnie w okolicy poddanej wcześniej tarciu lub naciskowi.
• Tkanka nekrotyczna (tkanka martwa) –czarna, martwa tkanka, zawierająca martwe komórki oraz resztki tkanek powstałe w wyniku podziału umierających komórek.
• Wysięk – płyn zapalny wypływający z rany – w prawidłowo przebiegającym procesie gojenia ilość wysięku zwiększa się na etapie zapalnym, tym samym oczyszczając ranę i utrzymując prawidłowy poziom
jej nawilżenia, co tym samym przyspiesza gojenie. W przypadku ran przewlekłych wysięk ma inny skład biochemiczny, co prowadzi do rozpadu struktur białkowych w ranie i dalszego rozpadu tkanek.
• Wysuszenie – niski poziom nawilżenia, który uniemożliwia tworzenie i przemieszczanie się komórek koniecznych do budowy nowej tkanki.
ran brodawek piersiowych w okresie karmienia piersią
Komórki keratynowe stają się płaskie i łuszczące. Skóra staje się szorstka w dotyku i łuszczy się.
• Ziarninująca – nowa, czerwona tkanka oraz naczynia włosowate powstające na powierzchni rany podczas procesu gojenia
• Zmiany wypryskowe – stan zapalny objawiający się swędzeniem, zaczerwienieniem oraz wysypką.
Lawaseptyki, czyli wyroby medyczne sterylne lub niesterylne zalicza się do produktów do pielęgnacji, oczyszczania, mycia i płukania skóry czy błon śluzowych. Stosowane są do oczyszczania ran czy usunięcia włóknika, skrzepniętych wydzielin, strupów. Powinny być stosowane zgodnie z zaleceniami, również przed zastosowaniem właściwego antyseptyku.
Lawaseptyki możemy podzielić na te zawierające lub niezawierające surfaktantu, czyli substancji ułatwiającej oczyszczanie poprzez zmniejszenie napięcia powierzchniowego wydzielin czy biofilmu bakteryjnego na powierzchni rany. Niektóre z lawaseptyków zawierają również substancję przeciwdrobnoustrojową w mniejszym stężeniu niż w antyseptyku. Lawaseptyki mogą występować w postaci płynu lub żelu.
Do oczyszczania ran nie zaleca się wody z mydłem ze względu na wysokie niefizjologiczne pH mydła powodujące napięcie i wysuszenie tkanek, osłabienie bariery ochronnej skóry i opóźnianie procesu gojenia.
Wymienione poniżej preparaty i opatrunki specjalistyczne są dostępne w sprzedaży i scharakteryzowane na podstawie IFU oraz ChPL zgodnie z danymi dostępnymi w momencie oddawania publikacji do druku.
Grupa 1. Lawaseptyki niezawierające surfaktantu
NaCl 0,9%
• Postać: roztwór.
• Sterylność zależy od producenta i formy.
• Uwagi: w literaturze pojawiają się doniesienia naukowe i drażniącym działaniu roztworu NaCl o stężeniu 0,9% na tkanki.
• Sugerowane zastosowanie: do krótkotrwałego opłukiwania brodawki sutkowej
Płyn Ringera
• Postać: roztwór.
• Sterylność zależy od producenta i formy.
• Uwagi: brak.
• Sugerowane zastosowanie: do opłukiwania brodawki sutkowej.
Roztwory zawierające w składzie niskie stężenia kwasu podchlorawego lub podchlorynu sodu Udowodnione jest przeciwzapalne działanie roztworów podchlorynów, stosowane w dermatologii np. w AZS. Literatura wskazuje na właściwości zmniejszenia obciążenia mikrobiologicznego z powierzchni tkanki na skutek płukania podchlorynami. Wyniki badań na temat działania przeciwdrobnoustrojowego są sprzeczne ze względu na brak efektu rezydualnego podchlorynów, które ulegają rozpadowi i dezaktywacji. Z drugiej strony dzięki temu wykazują one wysoki profil bezpieczeństwa. Podchloryny są substancjami o wysokim potencjale utleniającym, co jest głównym mechanizmem niszczącym struktury drobnoustrojów. Komórki ludzkie nie są wrażliwe na działanie niskich stężeń rzędu 40/50 ppm, ponieważ są wyposażone w naturalny mechanizm detoksykacji. Jest to wynikiem naturalnego procesu produkcji HOCl podczas procesu fagocytozy.
Microdacyn
• Postać: roztwór. Wyrób medyczny.
• Sterylność: brak, producent zapewnia czystość mikrobiologiczną (dostępne oświadczenie).
• Przeciwwskazania i środki bezpieczeństwa (IFU). nadwrażliwość na którykolwiek ze składników preparatu. Nie należy połykać.
• Uwagi: producent zaleca czas działania – 15 min.
• Sugerowane zastosowanie – do opłukiwania rany brodawki sutkowej.
Granudacyn
• Postać: roztwór. Wyrób medyczny.
• Sterylność: brak, producent zapewnia czystość mikrobiologiczną.
• Przeciwwskazania i srodki bezpieczeństwa (IFU: nadwrażliwość na którykolwiek ze składników preparatu. Nie należy połykać.
• Uwagi: producent zaleca czas działania – 15 min.
• Sugerowane zastosowanie: do opłukiwania rany brodawki sutkowej.
Granusept
• Postać: roztwór. Wyrób medyczny.
• Sterylność: brak, producent zapewnia czystość mikrobiologiczną.
• Przeciwwskazania i środki bezpieczeństwa (IFU): nadwrażliwość na którykolwiek ze składników preparatu. Nie należy połykać.
• Uwagi. Producent zaleca czas działania – 15 min.
• Sugerowane zastosowanie: do opłukiwania rany brodawki sutkowej.
Aquitox D
• Postać: roztwór. Wyrób medyczny.
• Sterylność: brak, producent zapewnia czystość mikrobiologiczną.
• Przeciwwskazania i srodki bezpieczeństwa (IFU). Nadwrażliwość na którykolwiek ze składników preparatu. Nie należy połykać.
• Uwagi: producent zaleca czas działania – 2 min.
• Sugerowane zastosowanie: do opłukiwania rany brodawki sutkowej.
Grupa 2. Lawaseptyki zawierające surfaktant
Octenilin® Roztwór do przemywania ran
• Postać: roztwór do przemywania.
• Skład: oktenidyna, etyloheksyloglireryna, glycerol.
• Surfaktant etyloheksylogliceryna.
• Sterylność: tak.
• Działanie bójcze.
• Wyrób medyczny. Literatura wskazuje, iż tożsame stężenie oktenidyny, jakie jest w wyrobie medycznym Octenilin działa bójczo (Czasy ekspozycji wymagane do redukcji 5 log poziomu bakterii) wobec drobnoustrojów Gram-dodatnich i Gram-ujemnych już w nawet w 15 sekundzie (inkubacja z wysiękiem).
• Przeciwwskazania i środki bezpieczeństwa (IFU): środek nie drażni, nie uczula, jego zastosowanie jest bezbolesne, nie jest toksyczny dla tkanek i nie ogranicza procesu ziarninowania oraz odbudowy nabłonka. Dobra tolerancja tkankowa roztworu. Roztwór do przemywania ran Octenilin® dzięki zawartości surfaktantu zmniejsza napięcie
powierzchniowe rany, posiada bardzo dobre właściwości nawilżające i oczyszczające powierzchnię tkanki, także w trudno dostępnych miejscach, takich jak rozpadliny, pęknięcia oraz wnętrze kieszeni rany. Tylko do zastosowania zewnętrznego na rany skóry. Interakcje: Nie stosować w połączeniu z anionowymi związkami powierzchniowo czynnymi lub innymi oczyszczającymi rany mydłami, maściami, olejkami, enzymami itp., ponieważ może to prowadzić do niekorzystnego wpływu na konserwację. Nie stosować w połączeniu z PVP-jodem, ponieważ może to prowadzić do przebarwień i ograniczenia działania antyseptycznego PVP-jodu. W przypadku stwierdzonego uczulenia lub podejrzenia uczulenia na jeden lub kilka składników nie wolno stosować produktu Octenilin® Roztwór do przemywania ran. W razie wątpliwości należy skonsultować się z lekarzem. W celu zapobiegania możliwym uszkodzeniom tkanek nie wolno stosować produktu Octenilin® Roztwór do przemywania ran na chrząstce szklistej, do oczu, w uszach, nosie, pęcherzu moczowym i jamie brzusznej! Nie stosować do infuzji ani do wstrzyknięć! Nie połykać. W celu uniknięcia możliwości uszkodzenia tkanek, nie wolno wstrzykiwać lub wprowadzać produktu do tkanki pod ciśnieniem. W każdym przypadku należy zapewnić odpowiedni odpływ z jam rany.
• Uwagi: oktenidyna nie wchłania się do krwioobiegu, bardzo niskie ryzyko obecności w mleku matki. Efekt rezydualny. Czas działania – 1 min.
• Sugerowane zastosowanie: do opłukiwania rany brodawki sutkowe, w celu jej oczyszczania i działania zmniejszającego obciążenie mikrobiologiczne. Ze względu na efekt rezydualny zalecane spłukanie przed karmieniem dziecka.
Prontosan ® Roztwór do irygacji ran
• IFU 22.04.2025 – Ciąża i okres laktacji. Nie ma dowodów na działanie mutagenne lub toksyczne dla zarodków związane ze składnikami tego produktu. Ze względu na brak odpowiednich badań klinicznych i doświadczenia klinicznego nie należy stosować roztworu do irygacji ran Prontosan u kobiet w ciąży i karmiących piersią.
SutriSept® Płyn na rany
• Postać: płyn do przemywania.
• Skład: woda, poloksamer 188, poliheksametylen biguanidu (poliheksanid, PHMB) 0,1%.
• Surfaktant poloksamer 188.
• Sterylność: nie.
• Działanie bójcze. Wyrób medyczny.
• Przeciwwskazania i środki bezpieczeństwa (IFU): poliheksanid nie wchłania się do krążenia ogólnoustrojowego, prawdopodobieństwo jego wydzielania do mleka matki karmiącej jest niemal zerowe. Ze względu na brak badań klinicznych i doświadczenia w stosowaniu wyrobu u kobiet w ciąży i karmiących piersią, SutriSept® Płyn na rany należy stosować zachowaniem ostrożności, po przeprowadzeniu oceny medycznej.
• Noworodki i niemowlęta: ze względu na niewystarczające dane kliniczne u noworodków i niemowląt SutriSept® Płyn na rany należy stosować wyłącznie pod ścisłym nadzorem lekarskim.
• Dotychczas brak jest doniesień dotyczących działań niepożądanych wyrobu SutriSept® Płyn na rany, ale zgłoszono rzadkie przypadki (częstość występowania poniżej 1 na 10 000) wstrząsu anafilaktycznego wywołanego przez poliheksanid. SutriSept® Płyn na rany może powodować reakcje uczuleniowe, takie jak świąd (pokrzywka) i wysypka. Nie stosować wyrobu u osób wykazujących nadwrażliwość na tę substancję. W bardzo rzadkich przypadkach po zastosowaniu płynu SutriSept® może wystąpić łagodne uczucie pieczenia, które jednak ustępuje w ciągu kilku minut.
• SutriSept® Płyn na rany wykazuje kompatybilność ze wszystkimi nowoczesnymi opatrunkami na rany.
• Nie należy stosować wyrobu SutriSept® Płyn na rany w następujących przypadkach: nie jest odpowiedni do stosowania na chrząstkę szklistą i w przypadku aseptycznych operacji stawów, w uchu środkowym i wewnętrznym, nie można go aplikować do oczu, na chrząstkę szklistą. W obrębie ośrodkowego układu nerwowego i opon mózgowych. Jeżeli jest stosowany razem z anionowymi środkami powierzchniowo czynnymi, mydłami, kremami, olejkami, enzymami substancje te należy dokładnie usunąć z powierzchni rany przed zastosowaniem wyrobu.
• Wyłącznie do użytku zewnętrznego na skórę.
• Wyrobu SutriSept® Płyn na rany nie wolno podawać we wlewach dożylnych ani iniekcjach.
• Uwagi: PHMB nie wchłania się do krwioobiegu, mało prawdopodobieństwo do przedostania się do mleka matki. Efekt rezydualny. Czas zastosowania – 15 min.
• Sugerowane zastosowanie: do opłukiwania rany brodawki sutkowej w celu jej oczyszczania i działania zmniejszającego obciążenie mikrobiologiczne. Ze względu na efekt rezydualny sugerowane spłukanie.
Grupa 3. Hydrożel o działaniu przeciwdrobnoustrojowym i przeciwzapalnym
• Stosować jako opatrunek o działaniu nawilżającym.
• Wyrób medyczny.
• Przeciwwskazania i środki bezpieczeństwa – takie same jak w przypadku roztworów.
• Uwaga: preparaty do stosowania zewnętrznego, tylko pod kontrolą lekarza lub doradcy laktacyjnego! Brak badań u kobiet w trakcie laktacji. Stosować na brodawkę sutkową po karmieniach. Preparat może pozostawać na powierzchni rany aż do następnej zmiany opatrunku. Przed karmieniem dziecka należy środek dokładnie zmyć z brodawki sutkowej, żele upłynniają się w kontakcie z wodą.
• Aquitox 0,03%
• Granudacyn 0,004%
• Microdacyn Hydrogel 0,006%
• Octenilin żel 0,05%
• Sutrisept żel 0,1%
W % podano stężenie substancji antyseptycznej.
Octenisept ® Spray
• Zawiera surfaktant, betaina.
• Skład oktenidyna 0,1%, fenoksyetanol 2%, betaina
• Produkt leczniczy do dezynfekcji ran, błon śluzowych i skóry
• Wskazanie – do ran powierzchownych, w przypadku cech infekcji i podejrzenia kolonizacji rany szczepami wielolekoopornymi.
• Czas działania – 1 min.
• Stosować jako rozprysk, rozmaz lub przymoczka do 5 min.
• Powszechnie stosowana od owrzodzeń po rany ostre.
• Postać: od płynu po żel po emulsje myjące, opatrunki.
• Oktenidyna posiada wysoki profil bezpieczeństwa, może być stosowana od 1. dnia życia.
• Wykazuje szerokie spektrum bójcze od wielolekoopornych bakterii Gram-dodatnich, Gram-ujemnych, po grzyby i drożdze.
• W porównaniu z innymi substancjami ma skuteczne działanie bójcze przy krótkim czasie kontaktu, niskim stężeniu, przy obciążeniu białkowym (wysięk, krew) 30 sekund.
• Posiada przeciwzapalne i immunomodulacyjne właściwości wpływające na proces gojenia.
• Wykazuje efekt rezydualny.
Powidon jodu (źródło ChPL Braunol 7,5%, roztwór na skórę)
• Nie zawiera surfaktantu.
• Produkt leczniczy.
• W okresie ciąży i karmienia piersią produkt Braunol, podobnie jak wszystkie produkty zawierające jod, powinien być stosowany tylko według ścisłych wskazań, a jego użycie powinno być bardzo ograniczone. Po zastosowaniu produktu zaleca się przeprowadzenie oceny funkcji gruczołu tarczowego dziecka. W przypadku stwierdzenia niedoczynności tarczycy należy podjąć wczesne leczenie hormonalne aż do przywrócenia normalnego działania tarczycy. Podczas karmienia piersią należy zachować ostrożność, aby nie dopuścić do przypadkowego przedostania się produktu Braunol do ust dziecka z poddanych leczeniu części ciała matki.
• Przeciwwskazania do stosowania PVP-I obejmują uczulenie na substancję czynną, wole nadczynne, chorobę Hashimoto, choroba Duhringa, a także płukanie jamy otrzewnej.
• Przenika do mleka matki i stężenie jodków w mleku wzrasta w większym stopniu niż w surowicy krwi.
• Antyseptyki zawierające powidon jodowany nie powinny być stosowane w trakcie ciąży i okresie karmienia piersią.
OPATRUNKI SPECJALISTYCZNE
Opatrunki specjalistyczne, których zastosowanie można rozważyć w ranach brodawek sutkowych po uwzględnieniu korzyści i ryzyka
Opatrunki specjalistyczne należą do wyrobów medycznych. Należy je używać z zgodnie z instrukcją używania, pod kontrolą lekarza, pielęgniarki, położnej lub doradcy laktacyjnego.
ZALECENIE po każdym usunięciu opatrunku ranę i skórę należy dokładnie zmyć. Według standardowego protokołu przygotowania piersi do karmienia.
Należy pamiętać, że rany brodawek sutkowych w przebiegu laktacji cechują się odmiennością w odniesieniu do cech innych ran trudno gojących się. Dobierając do nich opatrunek specjalistyczny, należy mieć tego świadomość! Wśród korzyści zastosowania opatrunków znajduje się tworzenie wilgotnego środowiska gojenia, wchłanianie wysięku, działanie przeciwdrobnoustrojowe, zabezpieczenie rany przed kontaktem ze środowiskiem zewnętrznym. Wśród utrudnień należy
• Z powodu potencjalnych działań niepożądanych niezalecany do stosowania w przypadku ran brodawek sutkowych.
Powidon jodu (źródło ChPL BETADINE, 100 mg/ml, roztwór na skórę)
• Nie zawiera surfaktantu.
• Produkt leczniczy.
• Stosowanie produktu po drugim miesiącu ciąży i w czasie karmienia piersią jest dozwolony jedynie w przypadku dokładnej diagnozy i bezwzględnych (!) wskazań do zastosowania produktu. Konieczne jest w takich przypadkach monitorowanie czynności tarczycy u matki i dziecka. Stosowanie leku należy ograniczyć do krótkiego czasu.
• Jod przenika przez łożysko i do mleka matki. Ponadto jod osiąga w mleku matki większe stężenia niż w surowicy. Jodowany powidon może indukować przemijającą niedoczynność tarczycy u noworodka.
• Należy bezwzględnie chronić niemowlę przed połknięciem produktu!
• Powidon jodowany nie powinien być stosowany w trakcie ciąży i okresie karmienia piersią.
• Z powodu potencjalnych działań niepożądanych niezalecany do stosowania w przypadku ran brodawek sutkowych.
uwzględnić konieczność częstego zdejmowania opatrunków na karmienie i zmywania ich pozostałości z powierzchni brodawki, co może naruszać gojące się tkanki i sprawia ból pacjentce. Tak więc każdorazowo należy rozważyć korzyści i ryzyko.
W Aneksie uwzględnione zostały przykładowe opatrunki dostępne w momencie oddawania publikacji do druku
Grupa 1. Sterylny opatrunek hydrożelowy
• Opatrunek wskazany do leczenia między innymi „otarć i innych uszkodzeń naskórka”
• Opatrunek hydrożelowy (Aqua-Gel®) należy do grupy opatrunków specjalistycznych, zbudowany w 91,5% z wody jakości farmakopealnej oraz naturalnych i syntetycznych polimerów połączonych trwale między sobą za pomocą wiązki elektronów. Polimery te tworzą sieć, która zapewnia stabilną postać opatrunku.
Tabela I. Podział i rodzaje opatrunków zarejestrowanych przez producentów do stosowania w bolesności i ranach brodawek sutkowych
Marka Nazwa Skład Status wyrobu medycznego
Wkładki i opatrunki żelowe
Ardo Kompresy Ardo Care Woda, glikol pentylenowy, guma ksantanowa, betaina, hialuronian sodu, anisat sodu, kwas mlekowy
Multi-Mam Multi-Mam Kompresy
Ekstrakt z liści aloesu (kompleks 2QR: opatentowane bioaktywne polisacharydy), woda, gliceryna, guma ksantanowa, kwas cytrynowy, glikol kaprylowy, benzoesan sodu, sorbinian potasu, wodorotlenek sodu
Wkładki i opatrunki hydrożelowe
Canpol babies Kojące wkładki hydrożelowe na piersi z lanoliną Woda, glikol, polimer, lanolina
Chicco
Hydrożelowy kompres na brodawki –aktualnie niedostępny w sprzedaży
Lansinoh SOOTHIES® żelowe kompresy chłodzące
Poliester, woda, glicerol, polimer
Tak
Tak
Tak
Tak
Gliceryna roślinna, polimer, woda. Tak
Medela Wkładki hydrożelowe Woda, glicerol, polimer (AMPs sodu) Tak Opatrunki piankowe
Ferris Mfg. Corp. Nursicare
Matryca poliuretanowa, gliceryna, substancja powierzchniowo czynna: surfaktant f-68; absorbent: kopolimer skrobi
• Opatrunek dzięki hydrożelowej budowie ma działanie kojące i uśmierzające ból, co poprawia komfort pacjenta.
• Wspomaga autolityczne oczyszczanie rany.
• Opatrunek jest hipoalergiczny i nie powoduje uczuleń. Składniki opatrunku są całkowicie biozgodne.
• Transparentność opatrunku jest atutem w procesie leczenia. Stała obserwacja rany pozwala wychwycić wszelkie niekorzystne zmiany.
• Ważne: przy długotrwałym stosowaniu sterylnego opatrunku hydrożelowego może wystąpić maceracja skóry wokół rany.
• Częstość zmiany opatrunku zależy od ilości wysięku z rany.
• Podczas zmiany opatrunku, należy przepłukać ranę np. preparatem przeznaczonym do dekontaminacji ran, producent zaleca np. Microdacyn płyn.
Grupa 2. Opatrunki
Główne cechy:
• Brak substancji przeciwdrobnoustrojowej.
• Sterylne.
• Chronią ranę przed wyschnięciem, jednocześnie umożliwiając swobodny transfer wysięku do opatrunku wtórnego.
• Konieczność zastosowania opatrunku wtórnego, w postaci sterylnego kompresu gazowego,
Tak
opatrunku wysokochłonnego, pianki poliuretanowej i umocowania za pomocą przylepca lub bielizny.
• Opatrunki tworzą oddychającą barierę ochronną.
• Umożliwiają swobodny przepływ wysięku do opatrunku wtórnego.
• Zastosowanie do ran powierzchniowych bez cech zakażenia!
Opatrunek siatkowy impregnowany neutralną maścią
• Opatrunek w postaci siateczki nie zawiera substancji czynnych. (GRASSOLIND).
Opatrunek siatkowy impregnowany parafiną
• Opatrunek parafinowy. Stworzony z jałowej gazy pokrytej białą parafiną. Opatrunek jest kompatybilny z maściami, także zawierającymi antybiotyk. Opatrunek został wykonany z wysokiej jakości gazy bawełnianej, która zapewnia skuteczną ochronę rany. (JELONET).
• Opatrunek z gazy nasączony parafiną. Utrzymuje prawidłową wilgotności rany, zapobiega jej wysuszaniu i maceracji skóry na obrzeżach rany. Neutralne właściwości parafiny pozwalają na stosowanie opatrunku w połączeniu z innymi preparatami zawierającymi substancje lecznicze lub środki antyseptyczne. (PARAFFINET).
Opatrunek siatkowy o różnej budowie
• Opatrunek z cienkiej hydrofobowej siateczki tiulowej, impregnowanej neutralną maścią, niezawierającą składników czynnych i uczulających. Zapewnia dobrą wentylację. (ATRAUMAN).
• Opatrunek zbudowany z gładkiej dzianiny wiskozowej impregnowanej emulsją oleisto-wodną, o wielkości oczek 1 × 1 mm, co zapobiega wrastaniu tkanki ziarninującej. Jest opatrunkiem kontaktowym o działaniu nawilżająco-natłuszczającym i zapobiega przywieraniu do powierzchni rany. (ADAPTIC).
• Opatrunek żelowy w postaci siatki tiulowej z włókien poliestrowych zaimpregnowany masą powłoki składającą się z matrycy polimerowej. Jest to masa mocująca dająca się elastycznie kształtować. Dodatkowymi składnikami są wazelina i hydrokoloid. (LOMATUELL PRO).
• Opatrunek nieprzywierający zbudowany z siatki tiulowej, impregnowanej macierzą polimerową zawierającą tłuszcz oraz hydrokoloid. Cząsteczki hydrokoloidu w kontakcie z cieczami tworzą żel, który wraz z wazeliną tworzy warstwę amfflową (hydroflowolipoflową). Wspomaga to gojenie się rany. Ponadto to zapobiega to przywieraniu do rany. Opatrunki są sterylnie pakowane pojedynczo. (LOMATUELL® PRO).
Opatrunki w postaci siatki z zawartością substancji przeciwdrobnoustrojowej
• Zawierają substancję przeciwdrobnoustrojową, głównie cząsteczki srebra.
• Zalecane do ran z cechami zakażenia lub zagrożonych zakażeniem.
• Forma siatki pozwala na przycinanie opatrunku w celu dopasowania go do wymiarów rany, zapewniając oszczędne stosowanie opatrunku i ograniczając ryzyko maceracji skóry otaczającej ranę.
• + jak w grupie 1.
Opatrunek antybakteryjny z neutralną maścią zawierający srebro metaliczne
• Opatrunek o niskiej cytotoksyczności ze srebrem metalicznym na rany zakażone i objęte ryzykiem zakażenia. Zalecany jest jako uzupełnienie leczenia ran zainfekowanych lub narażonych na zanieczyszczenie.
• Producent na podstawie badań rekomenduje do stosowania przeciwko bakteriom Gram-ujemnym, Gram-dodatnim, w tym przeciwko szczepom
Staphylococcus aureus, Staphylococcus aureus (oporny na metycylinę), Staphylococcus epidermidis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis
• Materiał nośny opatrunku składa się z hydrofobowej siatki poliamidowej impregnowanej maścią zawierającą kwasy tłuszczowe pochodzenia roślinnego. Maść pielęgnuje brzegi rany i zapewnia ich elastyczność. Opatrunek specjalistyczny nie zawiera wazeliny lub innych składników na bazie parafiny, co zapobiega przywieraniu opatrunku do rany i tym samym umożliwia jego bezbolesne zdejmowanie. (ATRAUMAN AG).
Opatrunek antybakteryjny impregnowany solami srebra, zbudowany w technologii lipidowo-koloidowej
• Opatrunek impregnowany solami srebra. Opatrunek zawiera matrycę gojącą ze srebrem, wykonaną z siateczki poliestrowej impregnowanej cząsteczkami hydrokoloidu (karboksymetylocelulozy), wazeliny, polimerów kohezyjnych i soli srebra.
• Skuteczność bójcza w kierunku bakterii (Pseudomonas aeruginosa, Staphylococcus aureus).
• Może być stosowany w ranach głębokich, szczelinowych.
• Jest wskazany do leczenia ran z ryzykiem lub objawami miejscowego zakażenia bakteryjnego, z niewielką ilością wysięku. (UrgoTul Ag/Silver).
Opatrunek ze srebrem nanokrystalicznym
• Wykazuje działanie bakteriobójcze. Opatrunek przeznaczony jest do zaopatrywania ran powierzchniowych, trudno gojących się z tendencją do wysięku oraz ran znajdujących się w miejscach trudnych do opatrzenia.
• Zastosowana powłoka z nanokrystalicznego srebra uwalnia jony srebra. Polietylenowa siatka o dużej gęstości splotu. Słabe przyleganie ułatwia usunięcie opatrunku.
• Wskazany na zainfekowane oraz narażone na infekcje płytkie i głębokie rany. (ACTICOAT FLEX 3).
Opatrunek ze srebra jonowego (1,2%), zbudowany w technologii Hydrofiber™. Opatrunek antybiofilmowy –kwas etylenodiaminotetraoctowy (EDTA) i chlorek benzetoniowy (BEC)
• Zbudowany jest z dwóch warstw, wykonanych z włókien karboksymetylocelulozy sodowej, wzmocnionych przeszyciami. Do jego stworzenia wykorzystano technologię Więcej niż srebro™ oraz technologię Hydrofiber™.
• Zalecany do ran z wysiękiem umiarkowanym i/lub obfitym i wytworzonym biofilmem, nie zaleca się do ran bez wysięku.
• W zatknięciu z wysiękiem tworzony jest spójny żel. Pozwala to na stworzenie idealnego środowiska wspomagającego proces gojenia rany. Dzięki technologii Hydrofiber™ opatrunek zamyka nadmiar wysięku, bakterie i biofilm w swojej strukturze.
• W przypadku głębokiej rany ranę należy wypełnić opatrunkiem jedynie do 80%. Mają na to wpływ właściwości opatrunku, który w kontakcie z wysiękiem początkowo lekko obkurcza się w trakcie żelowania, a następnie zwiększa objętość i wypełnia pustą przestrzeń.
• Wymaga zastosowania opatrunku wtórnego. (AQUACEL AG +EXTRA).
Grupa 3. Opatrunki piankowe bez substancji przeciwdrobnoustrojowej
Cechy
• Umożliwiają skórze oddychanie.
• Chronią ranę przed wyschnięciem, pochłaniają wysięk w różnych mechanizmach – zależy od budowy opatrunku.
• Zastosowanie do ran powierzchniowych, bez cech zakażenia!
• Wersja – przylepna i nieprzylepna. NIEPRZYLEPNE nie można ciąć.
• Jako opatrunki pierwotne (do ran płytkich, typu otarcie) i wtórne, jako zabezpieczenie np. opatrunku z substancją bójczą w postaci siatki.
• Wszystkie opatrunki piankowe mają cechy amortyzujące.
• Są wodoodporne, przezroczyste i elastyczne, co zwiększa komfort ich użytkowania.
• Nie przywierają do rany, dzięki czemu zmiana opatrunku jest bezbolesna.
• Zabezpieczają ranę przed przedostaniem się do niej bakterii i drobnoustrojów.
• Dbają o utrzymanie środowiska optymalnego do gojenia się rany.
Wielowarstwowy opatrunek piankowy na rany z wysiękiem
• Opatrunek piankowy z warstwą kontaktową wykonaną w technologii Hydrofiber™. Dzięki właściwościom chłonnym i zastosowaniu silikonowych przylepców opatrunek świetnie się sprawdza w terapii ran o różnej etiologii.
• Zbudowany jest z wodoodpornej zewnętrznej błony poliuretanowej o wysokiej paroprzepuszczalności, miękkiej warstwy absorbującej pianki,
warstwy kontaktowej z raną wykonanej w technologii Hydrofiber™ i delikatnego silikonowego przylepca w wersji przylepnej.
• Budowa zewnętrznej warstwy błony poliuretanowej sprawia, że jest to opatrunek wodoodporny, dzięki czemu chroni on ranę przed wirusami i bakteriami, a jednocześnie pozwala pacjentowi na mycie się bez konieczności zdejmowania opatrunku.
• Dzięki zewnętrznej błonie poliuretanowej w środowisku rany zachowany jest odpowiedni poziom wilgoci, która będąc w nadmiarze, zostaje odparowana.
• Opatrunek wchłania i zatrzymuje w swojej strukturze wysięk, dzięki czemu zmniejsza ryzyko maceracji skóry.
• Właściwości technologii Hydrofiber™ zapewniają ponadto zmniejszenie poziomu aktywnych metaloproteinaz w ranach przewlekłych i stymulowanie angiogenezy. Wpływa to bardzo korzystnie na proces gojenia się ran. (AQUACEL FOAM).
Opatrunek piankowy
• Opatrunek zbudowany z trójwarstwowej konstrukcji składającej się z:
– perforowanej warstwy stykającej się z raną pozwala nawet lepkiemu wysiękowi przejść do opatrunku;
– hydrocellularnego rdzenia, który pochłania i zatrzymuje ciecz w swojej mikroskopijnej strukturze; – oddychającej zewnętrznej powierzchni opatrunku, która umożliwia odparowanie nadmiaru wilgoci z opatrunku, a jednocześnie jest ciasną barierą dla bakterii i ciał obcych z zewnątrz.
• Opatrunek jest wskazany do wchłaniania wysięku i leczenia ran o częściowej lub pełnej grubości.
• Nie należy stosować opatrunków ze środkami utleniającymi, takimi jak roztwory podchlorynu lub nadtlenkiem wodoru, ponieważ mogą one rozkładać absorbujący hydrokomórkowy składnik opatrunku. (ALLEVYN).
Opatrunek piankowy z warstwą kontaktową miękkiego silikonu Safetac®
• Jest miękki i dopasowuje się do otaczającej go skóry.
• Opatrunek posiada oryginalną, minimalizującą ból warstwę kontaktową z miękkiego silikonu Safetac®. W badaniach klinicznych wykazano, że opatrunki z technologią Safetac® minimalizują uszkodzenia rany i skóry podczas zdejmowania opatrunku. Uszczelniając brzegi rany, pomagają zapobiegać maceracji. Ponieważ nie uszkadzają rany i skóry, ból podczas zmiany opatrunku jest
zminimalizowany. Na rany płytkie, z małym do umiarkowanego wysiękiem. (MEPILEX).
Opatrunek piankowy na rany z małym wysiękiem składający się z 3 warstw
• Opatrunek zbudowany z warstwy zewnętrznej poliuretanowej, która jest wodoszczelna i zapewnia skuteczną ochronę rany przed bakteriami i wirusami, a jednocześnie umożliwia optymalne odparowywanie wilgoci ze środowiska rany. Warstwa środkowa ma postać cienkiej, elastycznej i miękkiej pianki, która absorbuje wysięk, a jednocześnie utrzymuje w ranie wilgotne środowisko, które sprzyja gojeniu. Przylepna warstwa kontaktowa zbudowana jest z silikonu. Jest delikatna i przyjazna dla skóry, umożliwia bezpieczną i bezbolesną dla pacjenta aplikację opatrunku, a także jego zmianę i przesuwanie.
• Dzięki elastycznej i miękkiej strukturze łatwo dopasowuje się do różnych powierzchni ciała. Opatrunek skutecznie chroni, zabezpiecza i pielęgnuje rany z małym wysiękiem. (Foam Lite™ ConvaTec).
Opatrunek piankowy z silikonem Kliniderm ®
• Miękki, dopasowujący się, chłonny opatrunek z pianki poliuretanowej z silikonową warstwą kontaktową. Warstwa ta chroni otaczającą skórę i zmniejsza ból przy zmianie opatrunku. Wysięk zatrzymywany jest wewnątrz opatrunku, a zewnętrzna warstwa pozwala na swobodną wymianę gazową.
• Opatrunek nadaje się do ran powierzchniowych. (Kliniderm Foam Silicone).
Opatrunek piankowy z warstwą kontaktową wykonany w technologii TLC
• Opatrunek kontaktowy, przeznaczony do ran czystych, z niewielką ilością wysięku, szczególnie w stadium ziarninowania i epitelializacji. (UrgoTul Absorb).
Grupa 4. Opatrunki piankowe z zawartością substancji przeciwdrobnoustrojowej
Opatrunek wykonany z poliuretanu z zawartością 0,5% PHMB i 0,1% B-pantenolu
• Opatrunek składa się z rdzenia piankowego o strukturze otwartej. Rdzeń z pianki pochłania i zatrzymuje wysięk, zmniejszając macerację skóry dookoła rany. Dodatek przeciwdrobnoustrojowego PHMB (biguanid poliheksametylenowy) ogranicza i zapobiega rozwojowi bakterii w ranie
i opatrunku. B-pantenol działa nawilżająco i poprawia nawilżenie skóry. Ponadto opatrunek posiada wierzchnią warstwę z oddychającego poliuretanu, która umożliwia przenikanie wilgoci od wewnątrz oraz jest odporna na działanie bakterii i wody z zewnątrz, dzięki czemu utrzymuje wilgotne środowisko rany.
• Może być stosowany jako opatrunek pierwotny lub wtórny. (KLINIDERM FOAM PHMB).
Opatrunek przeciwbakteryjny piankowy z silikonowej warstwie kontaktowej Safetac®
• Przeciwbakteryjny opatrunek piankowy z małym i średnim wysiękiem.
• Opatrunek delikatnie przylega do skóry dzięki unikalnej silikonowej warstwie kontaktowej Safetac® zmniejszającej ból. W ten sposób ból odczuwany przez pacjentów podczas zmiany opatrunku jest znacznie mniejszy.
• Wykazuje szybkie i długotrwałe działanie przeciwbakteryjne.
• Badania in vitro wykazały, że opatrunek piankowy ze srebrem jest w stanie inaktywować patogeny z rany (bakterie i grzyby) w ciągu 30 min. Zgodnie z międzynarodowym konsensusem, działania przeciwbakteryjne są niezbędne i kluczowe, by zmniejszyć zanieczyszczenie biologiczne w zakażonej ranie. Opatrunek działa jak bariera przeciwbakteryjna przy wysokim ryzyku infekcji lub ponownej infekcji. (Mepilex Ag).
Wielowarstwowy opatrunek piankowy z jonami srebra
• Opatrunek wskazany na rany płytkie, zakażone lub z podejrzeniem zakażenia, z umiarkowanym lub obfitym wysiękiem.
• Opatrunek może być stosowany jako opatrunek pierwotny lub wtórny.
• Opatrunek, który dzięki obecności jonów srebra wykazuje skuteczność przeciwko szerokiemu spektrum drobnoustrojów, m.in. MRSA, VRE oraz innych szczepów antybiotykoopornych.
• Dzięki wykorzystaniu technologii Hydrofiber opatrunek może być stosowany również na rany obficie sączące. W kontakcie z wysiękiem z rany warstwa kontaktowa opatrunku zmienia się w postać spoistego żelu, dzięki czemu dokładnie dopasowuje się do powierzchni rany i wypełnia puste miejsca, w których mogą namnażać się bakterie.
• Opatrunek wspomaga również usuwanie tkanki martwiczej z rany (oczyszczenie autolityczne), nie uszkadzając przy tym zdrowych tkanek. Zmniejsza również ryzyko zakażenia krzyżowego
podczas zmiany opatrunku, ponieważ zatrzymuje wysięk i jego szkodliwe komponenty w opatrunku. (AQUACEL® Ag Foam).
Definicja wyrobu medycznego zgodnie z ustawą o wyrobach medycznych
Zgodnie z art. 2 ust. 1 Ustawy z dnia 7 kwietnia 2022 r. o wyrobach medycznych (Dz.U. 2022 poz. 974): Wyrób medyczny to narzędzie, aparat, urządzenie, oprogramowanie, implant, odczynnik, materiał lub inny artykuł, przeznaczony przez producenta do stosowania u ludzi w celach medycznych, takich jak:
• diagnozowanie, zapobieganie, monitorowanie, leczenie lub łagodzenie choroby,
• diagnozowanie, monitorowanie, leczenie, łagodzenie lub kompensowanie urazu lub niepełnosprawności,
• badanie, zastępowanie lub modyfikowanie budowy anatomicznej albo procesu fizjologicznego,
• kontrolowanie poczęć.
Dodatkowo:
• Wyrób nie działa zasadniczo poprzez środki farmakologiczne, immunologiczne ani metaboliczne, ale jego działanie może być wspomagane takimi środkami.
Różnice między wyrobem medycznym a lekiem
Definicja i działanie
• Wyrób medyczny działa mechanicznie, fizycznie lub elektronicznie, np. implanty, strzykawki, aparatura diagnostyczna, opatrunki. Może zawierać substancje farmakologiczne, ale nie one są głównym mechanizmem działania.
• Lek działa na organizm poprzez środki farmakologiczne, immunologiczne lub metaboliczne, np. antybiotyki, szczepionki, środki przeciwbólowe.
Regulacje prawne
• Wyroby medyczne podlegają Rozporządzeniu UE 2017/745 (MDR)
• Leki są regulowane przez Prawo farmaceutyczne oraz Rozporządzenie UE 2001/83/WE. Ocena i dopuszczenie do obrotu
• Wyroby medyczne wymagają oceny zgodności i certyfikacji CE często przy udziale jednostki notyfikowanej.
• Leki muszą przejść badania kliniczne i uzyskać pozwolenie, np. od EMA (Europejska Agencja Leków) lub URPL (Urząd Rejestracji Produktów Leczniczych w Polsce)
• Podsumowując: wyrób medyczny to nie lek, choć niektóre produkty (np. inhalatory z lekiem) mogą łączyć cechy obu kategorii.
• Definicja produktu biobójczego znajduje się w Ustawie z dnia 9 października 2015 r. o produktach biobójczych (Dz.U. 2022 poz. 2178).
Definicja produktu biobójczego
Zgodnie z art. 2 ust. 1 pkt 1 tej ustawy:
• Produkt biobójczy to „substancja, mieszanina lub wyrób, w postaci, w jakiej jest dostarczany użytkownikowi, składający się z jednej lub większej liczby substancji czynnych, zawierający je lub wytwarzający je w celu niszczenia, odstraszania, unieszkodliwiania organizmów szkodliwych, zapobiegania ich działaniu lub ich zwalczania w inny sposób niż działanie czysto fizyczne lub mechaniczne”.
• Definicja ta jest zgodna z unijnym Rozporządzeniem (UE) nr 528/2012 i stanowi jego implementację w prawie krajowym.
Tabela II. Różnice między lekiem, wyrobem medycznym a produktem biobójczym
Cecha Lek Wyrób medyczny Produkt biobójczy Cel stosowania
Mechanizm działania
Przykłady
Regulacje prawne
Leczenie, zapobieganie chorobom, diagnozowanie lub modyfikacja funkcji organizmu
Środki farmakologiczne, immunologiczne lub metaboliczne, wpływające na organizm człowieka
Antybiotyki, szczepionki, środki przeciwbólowe
Prawo farmaceutyczne (Dz.U. 2022 poz. 2301)
Diagnozowanie, zapobieganie, monitorowanie, leczenie lub łagodzenie chorób i urazów Niszczenie, odstraszanie, unieszkodliwianie organizmów szkodliwych
Działanie fizyczne, mechaniczne lub elektroniczne (ew. wspomagane farmakologicznie)
Strzykawki, implanty, tomografy, soczewki kontaktowe, oprogramowanie medyczne
Ustawa z dnia 7 kwietnia 2022 r. o wyrobach medycznych (Dz.U. 2022 poz. 974)
Działanie chemiczne lub biologiczne przeciwko mikroorganizmom (np. bakteriom, wirusom, grzybom)
Środki dezynfekujące, środki owadobójcze, preparaty przeciw pleśni
Ustawa z dnia 9 października 2015 r. o produktach biobójczych (Dz.U. 2022 poz. 2178)
Praca oryginalna | Original paper
LECZENIE RAN 2025; 22 (2): 80–87
DOI: 10.60075/lr.v22i2.100
Ekspozycja na materiał potencjalnie zakaźny
Katarzyna Cierzniakowska1,2, Daria Kaniewska1,3, Elżbieta Kozłowska1,2, Aleksandra Popow1
1 Katedra Pielęgniarstwa Zabiegowego, Wydział Nauk o Zdrowiu, Collegium Medicum w Bydgoszczy, UMK w Toruniu
2 Klinika Chirurgii Ogólnej i Małoinwazyjnej, Szpital Uniwersytecki nr 2 im. dr J. Biziela w Bydgoszczy
3 Oddział Pediatrii, Pneumonologii i Alergologii, Wojewódzki Szpital Dziecięcy im. Józefa Brudzińskiego w Bydgoszczy
Streszczenie
Wstęp: Ekspozycja na materiał potencjalnie zakaźny umożliwia transmisję zakażenia i w sposób szczególny podnosi ryzyko poważnych konsekwencji zdrowotnych pracowników medycznych.
Cel pracy: Ocena zakresu występowania ekspozycji zawodowej i zastosowania profilaktyki poekspozycyjnej.
Materiał i metody: W badaniu wzięło udział 229 pielęgniarek i 13 pielęgniarzy. Zastosowano metodę sondażu diagnostycznego z wykorzystaniem anonimowej ankiety własnego autorstwa, które przeprowadzono drogą elektroniczną. Wyniki: Ekspozycja zawodowa wystąpiła u 53,7% badanych. Do ekspozycji dochodziło ze zbliżoną częstotliwością podczas pobierania krwi (28,5%), podawania leków (26,2%) oraz wykonywania innych czynności terapeutyczno-pielęgnacyjnych 23 (20%). Nie występowały różnice statystyczne względem poszczególnych przyczyn ( p > 0,05). W przypadku 8,2% badanych do ekspozycji przyczynił się brak bezpiecznego sprzętu medycznego. Istotnie statystycznie częściej do ekspozycji przyczyniało się pobudzenie psychoruchowe chorych (26,9%) i brak ostrożności (24,6%). Nie obserwowano związku pomiędzy występowaniem ekspozycji, a stażem, wykształceniem, miejscem pracy, systemem pracy czy formą zatrudnienia (p>0,05). Czynności ryzykownych nie podejmowało w badanej grupie jedynie 45 osób (18,6%). Zgłaszalność ekspozycji wynosiła 80,8%.
Wnioski:
• Ponad połowa badanych doznała ekspozycji zawodowej.
• Najczęstsze procedury podczas których dochodziło do ekspozycji zawodowej ze zbliżoną częstotliwością, to: pobieranie krwi, podawanie leków oraz czynności pielęgnacyjne.
• Jako najczęstsze przyczyny ekspozycji badani podawali pobudzenie psychoruchowe chorych lub nieadekwatną reakcję na wykonywaną u nich czynność zabiegową.

Adres do korespondencji
Katarzyna Cierzniakowska, Katedra Pielęgniarstwa Zabiegowego, Wydział Nauk o Zdrowiu Collegium Medicum w Bydgoszczy, ul. Łukasiewicza 1, 85-821 Bydgoszcz, e-mail:
Nadesłano: 25.05.2025; Zaakceptowano: 1.06.2025
Abstract
Introduction: Exposure to potentially infectious material enables the transmission of infection and particularly increases the risk of serious health consequences for medical workers.
Aim of the study: Assessment of the scope of occupational exposure and the use of post-exposure prophylaxis.
Material and methods: The study involved 229 nurses and 13 male nurses. The diagnostic survey method was used using an anonymous self-authored questionnaire, which was conducted electronically.
Results: Occupational exposure occurred in 53.7% of the respondents. Exposure occurred with similar frequency during blood collection (28.5%), drug administration (26.2%) and performing other therapeutic and nursing activities 23 (20%). There were no statistical differences in terms of individual causes ( p > 0.05). In the case of 8.2% of the respondents, the lack of safe medical equipment contributed to the exposure. Psychomotor agitation of patients (26.9%) and lack of caution (24.6%) contributed to the exposure significantly more often. There was no relationship between the occurrence of exposure and seniority, education, place of work, work system or employment form (p>0.05). Only 45 people (18.6%) did not undertake risky activities in the study group. The reporting of exposure was 80.8%.
Conclusions:
• More than half of the respondents experienced occupational exposure.
• The most common procedures during which occupational exposure occurred with similar frequency were: blood collection, medication administration and nursing activities.
• The most common causes of exposure were psychomotor agitation of patients or inadequate reaction to the surgical procedure performed on them.
• Ryzyko ekspozycji zawodowej dotyczy całej grupy zawodowej pielęgniarek i pielęgniarzy niezależnie od ich poziomu wykształcenia, stażu pracy, miejsca pracy, sposobu zatrudnienia czy systemu pracy.
• Tylko, niespełna co piąta ankietowana osoba wskazała, że nie podejmuje działań ryzykownych uznawanych za potencjalne czynniki prowadzące do ekspozycji. Słowa kluczowe: ekspozycja zawodowa, materiał zakaźny, profilaktyka.
Ekspozycja na materiał potencjalnie zakaźny oznacza narażenie na krew lub inny potencjalnie zakaźny materiał biologiczny w czasie wykonywania obowiązków zawodowych przy udzielaniu świadczeń zdrowotnych. Do ekspozycji zawodowej dochodzi w wyniku zranienia skóry ostrym skażonym narzędziem, kontaktu błon śluzowych, uszkodzonej lub nieuszkodzonej skóry z materiałem pochodzącym od pacjenta, ugryzienia [1, 2].
Narażenie pracowników medycznych na materiał biologiczny pochodzący od pacjenta może następować, zarówno poprzez bezpośredni kontakt z chorymi (drogą kropelkowa) bądź kontakt z narzędziami i sprzętem zanieczyszczonym krwią lub innymi wydalinami chorego (drogą krwiopochodną) [3].
Ekspozycja na materiał potencjalnie zakaźny umożliwia transmisję zakażenia i w sposób szczególny podnosi ryzyko poważnych konsekwencji zdrowotnych, dlatego też konieczne jest rozważenie profilaktyki poekspozycyjnej.
Czynniki etiologiczne przenoszone drogą krwi Wykonywanie zadań związanych z dużym ryzykiem przerwania ciągłości skóry, takich jak: udział w zabiegach chirurgicznych, opatrywanie ran, pobieranie krwi, wykonywanie iniekcji, intubacji, badań endoskopowych, charakteryzuje się wysokim prawdopodobieństwem zakażenia wirusami przenoszonymi droga krwiopochodną. W grupie zawodów medycznych najwyższe ryzyko ekspozycji występuje wśród pielęgniarek i lekarzy [4].
Przyczynę zakażeń wynikających z narażenia na kontakt z krwią i płynami ustrojowymi może stanowić ponad 30 różnych patogenów. Wśród nich, największe zagrożenie stanowi ekspozycja na wirusy hepatotropowe. Wirus zapalenia wątroby typu B (hepatitis B virus – HBV), wirus zapalenia wątroby typu C (hepatitis C virus – HCV) i ludzki wirus niedoboru odporności (human immunodeficiency virus – HIV), powodują najwyższy odsetek zakażeń
• The risk of occupational exposure applies to the entire professional group of nurses and male nurses regardless of their level of education, seniority, place of work, employment method or work system.
• Only less than every fifth person surveyed indicated that they did not undertake risky activities considered to be potential factors leading to exposure.
Key words: occupational exposure, infectious material, prevention.
rozpoznawanych wśród personelu medycznego. Wskaźniki zachorowań wśród personelu medycznego na świecie powstające na skutek swoistego rodzaju ekspozycji zawodowych podawane przez Światową Organizację Zdrowia (World Health Organization – WHO) (2003 r.) wynoszą odpowiednio: 37,6% zachorowań na wirusowe zapalenie wątroby typu B (WZW B), 39% na wirusowe zapalenie wątroby typu C (WZW C) oraz 4,4% zakażeń HIV, co jest konsekwencją rozpowszechnienia tych wirusów w populacji [1, 4–6].
Analiza wielu badań dotyczących wszystkich aspektów zapalenia wątroby typu B u pracowników ochrony zdrowia opublikowanych od 2017 r. do kwietnia 2023 r, ujawniła duże zróżnicowanie częstości występowania zakażenia od 0,6% w Europie do > 8,7% w Afryce, prawie zawsze w powiązaniu z bardzo niskim wskaźnikiem szczepień. Wiedza i poglądy pracowników medycznych na temat WZW B i szczepienia przeciwko WZW B determinują ich status szczepień oraz ogólne postawy i praktyki dotyczące zakażenia WZW B [7].
Szczepienie przeciw WZW B w Polsce jest szczepieniem obowiązkowym dla noworodków od 1994 roku.
Czynniki etiologiczne przenoszone drogą kropelkową
Pracownicy medyczni, obok ryzyka zakażeń krwiopochodnych, są również narażeni na wysokie ryzyko zakażeń układu oddechowego. Ze względu na łatwość zakażenia drogą wziewną, kontakt z chorymi na grypę [wirusy grypy typu A, B i C (Orthomyxoviridae)] czy gruźlicę [prątki gruźlicy (Mycobakterium tuberculosis)] stanowi poważne zagrożenie dla personelu medycznego. Najlepszym sposobem prewencji zachorowań jest stosowanie środków ochrony osobistej oraz szczepienia ochronne [3].
W świetle badań, za procedury medyczne, które prawdopodobnie generują aerozole i kropelki jako źródło patogenów układu oddechowego i tym samym stwarzają zwiększone ryzyko przeniesienia infekcji
Beata Wieczorek-Wójcik, Paulina Mościck,a Katarzyna Kowalska, Katarzyna Cierzniakowska
na personel, uznano intubację tchawicy, wentylację nieinwazyjną, tracheotomię i wentylację manualną przed intubacją. W przypadku innych procedur stymulujących kaszel i sprzyjających powstawaniu aerozoli, takich jak: odsysanie płynów ustrojowych, bronchoskopia, nebulizacja, tlenoterapia, defibrylacja, uciskanie klatki piersiowej, zakładanie sondy nosowo-żołądkowej i pobieranie plwociny, ryzyko przenoszenia przez nie infekcji nie zostało udowodnione [8].
Podczas czterotygodniowej obserwacji oraz na podstawie pobieranych z nosogardzieli wymazów od personelu medycznego w czterech chińskich szpitalach (223 osoby z klinik gorączkowych oraz oddziałów pulmonologicznych, pediatrycznych, ratunkowych/intensywnych) u 88% zidentyfikowano kolonizację bakteryjną, a u 22% z nich stwierdzono współzakażenie wirusem [9].
Leczenie ran a ryzyko ekspozycji
Zaopatrywanie ran – zarówno ostrych (szycie ran pourazowych, usuwanie szwów), jak i ran trudno gojących się (konieczność oczyszczania z tkanek martwiczych) – wiąże się z używaniem ostrych narzędzi, co stanowi potencjalne ryzyko ekspozycji zawodowej.
Z uwagi na to, że nie każdy chory hospitalizowany bądź leczony ambulatoryjnie ma określany profil serologiczny, zawsze ludzki materiał biologiczny należy traktować jako potencjalnie zakaźny. Szacuje się, że w Polsce liczba zakażonych HIV może sięgać 35 tys. osób, a zakażonych wirusem zapalenia wątroby typu C 150–200 tys. osób. Często, bezobjawowy przebieg choroby sprawia, że nawet 90% z tych osób może nie być świadoma zakażenia [1, 10, 11].
Tran i wsp. podkreślają, że brakuje dowodów naukowych dotyczących ryzyka i mechanizmów przenoszenia drobnoustrojów w wytwarzanych aerozolach z dróg oddechowych chorego oraz trudno stwierdzić, czy pracownicy ochrony zdrowia opiekujący się pacjentami poddawanymi procedurom generującym aerozol są bardziej narażeni na zarażenie się chorobami w porównaniu z pracownikami ochrony zdrowia opiekującymi się pacjentami niepoddawanymi takim procedurom [8].
W badaniach mikrobiologicznych ran trudno gojących się, a w szczególności owrzodzeń żylnych goleni, najczęściej izolowane są: Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter cloacae complex, Enterococcus faecalis i Klebsiella oxytoca [12, 13]. Prawdopodobnie, wysoki stopień narażenia na materiał biologiczny występuje również podczas mechanicznego oczyszczania ran zakażonych. Płukanie rany,
sonoterapia, VersaJet, czy inne sposoby opracowania rany, podczas których powstaje aerozol, poprzez kontakt z błoną śluzową dróg oddechowych lub spojówek, mogą prowadzić do infekcji u personelu medycznego. Niestety, nie ma badań w zakresie ryzyka narażenia i zachorowań w grupie pielęgniarek i lekarzy zajmujących się leczeniem ran.
Profilaktyka
Obszary działania w przypadku pracowników sektora medycznego, którzy są narażeni na wpływ czynników biologicznych z zakresu wczesnej profilaktyki, dotyczą rozwiązań technicznych, organizacyjnych i prawnych. Ryzyko ekspozycji obniżają między innymi: przygotowanie pomieszczeń do pracy, klimatyzacja, dezynfekcja powietrza, dezynfekcja i sterylizacja narzędzi lub sprzętu, stosowanie jednorazowego sprzętu medycznego, zaopatrzenie pracowników w środki zabezpieczenia indywidualnego, zmniejszenie długości czasu pracy, przestrzeganie zarządzeń i rozporządzeń, standardowych postępowań poekspozycyjnych, szczepienia obowiązkowe i zalecane oraz szkolenia z zakresu zagrożeń zawodowych i zasad BHP [14–18].
Cel pracy
Celem pracy była ocena zakresu występowania ekspozycji zawodowej i zastosowania profilaktyki poekspozycyjnej.
Materiał i metody
Badanie zostało przeprowadzone w wersji elektronicznej w okresie od lutego do kwietnia 2023 r. Respondentów poinformowano na wielu forach internetowych, grupach zrzeszających pielęgniarki i pielęgniarzy, głównie poprzez portal Facebook. com. Aby zapewnić anonimowość, kwestionariusz umieszczono na odrębnej stronie WWW, niewymagającej logowania i podawania danych personalnych. Przystąpienie do badania było dobrowolne. Ankieta składa się z 19 pytań zamkniętych i podzielona została na dwie części. Część pierwsza składa się z ogólnych pytań określających ankietowanego oraz jego doświadczenia związane z możliwością narażenia na ekspozycję zawodową. Część druga skierowana była do osób, które kiedykolwiek doświadczyły ekspozycji zawodowej.
Charakterystyka osób badanych
W ankiecie łącznie udział wzięły 242 osoby, w tym 229 kobiet (94,6%) oraz 13 mężczyzn (5,4%). Średni wiek ankietowanych wynosił 37,4 roku (min. 22, maks.
65, mediana 35 lat). Wśród ankietowanych 13 osób (5,4%) posiadało wykształcenie średnie, 139 (57,4%) wykształcenie licencjackie, 56 (23,1%) magisterskie, a 34 (14,1%) posiadało dodatkowo specjalizację.
Średni staż pracy w zawodzie wynosił 14,18 roku (min. 4 miesiące, maks. 42 lata, mediana 10 lat). Wśród ankietowanych przeważającą większość stanowiły osoby zatrudnione na podstawie umowy o pracę. Tylko 22 osoby były zatrudnione w formie umowy zlecenie lub umowy cywilno-prawnej.
Przeważająca część (33,1%) ankietowanych pracuje na oddziałach zachowawczych, 64 osoby (26,4%) pracują na oddziałach zabiegowych, w przychodniach lub pracowniach 34 osoby (14%), a pozostałe 64 osoby (26,4%) w innych miejscach, w tym na bloku operacyjnym, oddziale ratunkowym, intensywnej terapii.
Jeżeli chodzi o system pracy, to zdecydowana większość respondentów pracuje w systemie dwuzmianowym (79,8% odpowiedzi).
Analiza statystyczna
Dane opracowano za pomocą metod statystycznych przy użyciu programu Excel i programu IBM SPSS Statistics 28. Jako poziom istotności statystycznej przyjęto p≤0,05.
Standardy bioetyczne
Na prowadzenie badań uzyskano zgodę Komisji Bioetycznej przy Collegium Medicum w Bydgoszczy (KB 528/2022)
Wyniki
Szkolenia związane z ekspozycją zawodową
Wśród ankietowanych 55 osób (22,7%) nie uczestniczyło w szkoleniach dotyczących ekspozycji zawodowej, w tym 53 (21,9%) z nich nie spotkało się z takimi szkoleniami, a dwie (0,8%) nie przejawiały zainteresowania tego typu szkoleniami. Pozostałe 187 osób (77,3%) uczestniczyło w tego typu szkoleniach, przy czym 82 (43,85%) tylko w dniu rozpoczęcia pracy, 86 (45,99%) uczestniczy w tego typu szkoleniach systematycznie, a 19 (10,16%) poza szkoleniami uzupełnia jeszcze wiedzę w ramach samokształcenia.
Zranienia bez ekspozycji na materiał zakaźny
Wśród ankietowanych 181 osób (74,8%) przyznało, że w czasie wykonywania czynności zawodowych doszło u nich do zranienia bez ekspozycji na materiał zakaźny, przy czym u dwóch osób (0,8%) było to jednokrotne zranienie, u 146 (60,3%) takie zranienia zdarzają się sporadycznie, rzadziej niż raz w roku,
a u 33 (13,6%) wielokrotnie, kilka razy w roku. Wśród pozostałych ankietowanych, 29 (12,0%) nie przypomina sobie takiego zdarzenia, a 32 (13,2%) nigdy się nie zraniły w czasie wykonywania czynności zawodowych. Do zranienia tego typu najczęściej dochodziło podczas otwierania ampułki (46,7%), posługiwania się nie zainfekowanym skalpelem lub innym ostrym narzędziem (20,6%) lub podczas przygotowywania roztworów leków (9,5%).
Ekspozycja zawodowa
W odniesieniu do ekspozycji na materiał zakaźny w trakcie wykonywania czynności zawodowych, wystąpiła ona u 130 osób (53,7%). Przy czym u jednej osoby (0,8%) było to jedno zdarzenie, u dwóch (1,5%) sytuacja taka wystąpiła dwukrotnie, 119 (91,5%) przyznało, że sytuacje takie występują sporadycznie, rzadziej niż raz w roku, a 8 (6,15%), że wielokrotnie, nawet kilka razy w roku. Ponadto 47 ankietowanych (19,4%) uznało, że sytuacja taka u nich nigdy nie wystąpiła, a 65 (26,8%) nie przypomina sobie takiej sytuacji.
Narażenie na ekspozycję zawodową potęguje wykonywanie czynności w stresie, w pospiechu, niezgodnie z zasadami czy z przepisami bezpieczeństwa i higieny pracy (BHP). Tylko 45 ankietowanych (18,6%) uznało, że nigdy nie wykonywali oni czynności ryzykownych w trakcie pracy. Wśród pozostałych, 85 osób (35,1%) nakładało nasadkę na zainfekowaną igłę, 62 (25,6%) nie zwracało uwagi na przepełnione pojemniki przeznaczone do wyrzucania ostrych odpadów, 25 (10,3%) niewłaściwie zabezpieczyło ostre przedmioty podczas wykonywania zabiegów medycznych, 22 (9,1%) wykonywało czynności pielęgnacyjne / zabiegowe bez należytego zastosowania środków ochronnych, a 3 (1,2%) zaniedbały przepisy BHP. Kolejne pytanie dotyczyło zgłaszania ekspozycji: Czy jeżeli doszłoby u Pani / Pana lub innej osoby obecnej na dyżurze do zranienia / ekspozycji, zgłosiłaby Pani / zgłosiłby Pan tą sytuację bezpośredniemu przełożonemu / pielęgniarce epidemiologicznej / do działu BHP?. W odpowiedziach 192 osoby (79,3%) na pewno taką sytuację za każdym razem zgłosiłyby przełożonemu lub innej odpowiedniej osobie. Natomiast 46 osób (15,8%) takiej sytuacji by nie zgłosiło, przy czym 20 z nich (8,3%) uznało, że procedura poekspozycyjna zawiera zbyt wiele formalności do realizacji, 24 (5,8%) jest zdania że jest to błahe zdarzenie bez konsekwencji, 5 osób (2,1%) wstydziłoby się tego, 3 (1,2%) nie mają w pracy tego typu procedur, a po dwie osoby (0,8%) odpowiedziały, że w ich pracy zdarzenia takie nie podlegają rejestracji oraz że nie miało świadomości,
Beata Wieczorek-Wójcik, Paulina Mościck,a Katarzyna Kowalska, Katarzyna Cierzniakowska
że należy zgłosić gdzieś tego typu zdarzenie. Żadnej odpowiedzi nie zakreśliły 4 osoby.
Dalsza część ankiety przeznaczona była dla osób, które kiedykolwiek doświadczyły ekspozycji zawodowej (130 osób).
Do ekspozycji najczęściej dochodziło podczas pobrania krwi (28,5%), podawania leków (26,2%), podczas czynności terapeutyczno-pielęgnacyjnych (20%), posługiwania się skalpelem lub innym ostrym narzędziem (14,6%) lub też podczas czynności porządkowania stanowiska pracy (10,8%). Ekspozycja miała miejsce w 70 przypadkach (53,8%) przy łóżku chorego, w 45 przypadkach (33,1%) w gabinecie zabiegowym lub opatrunkowym, w 15 (11,5%) na sali operacyjnej lub porodowej, a w 2 przypadkach (1,5%) w karetce. Zdecydowana większość osób, które uległy ekspozycji (80,8%) zgłosiła ten fakt bezpośredniemu przełożonemu lub pielęgniarce epidemiologicznej i wdrożyła we właściwy sposób procedurę poekspozycyjną. Pozostałe nie poinformowały o ekspozycji, ponieważ (według częstości odpowiedzi): procedura poekspozycyjna zawiera zbyt wiele formalności do realizacji; miały pewność, że po ekspozycji nie ulegną zakażeniu; nie było na to czasu; nie miały świadomości, że trzeba zgłaszać takie zdarzenia; obawiały się wyników badań serologicznych; wstydziły się, że doszło do takiego zdarzenia.
W procesie badawczym przyjęto hipotezę, że do zwiększonego ryzyka ekspozycji może przyczynić się brak wyposażenia stanowisk pracy w odpowiedni sprzęt. Jednak okazało się, iż na brak bezpiecznego sprzętu medycznego zmniejszającego ryzyko ekspozycji wskazało 8,2% badanych, którzy ulegli ekspozycji. Istotnie częściej w porównaniu z tym czynnikiem ryzyka ankietowani zgłaszali jako przyczynę ekspozycji: pobudzenie psychoruchowe chorego lub jego nieadekwatną reakcję na wykonywaną czynność (26,9%; Z-test, p < 0,001), brak ostrożności i zlekceważenie zasad bezpieczeństwa (24,6%; Z-test, p < 0,001), zmęczenie i brak koncentracji (17,9%; Z-test, p = 0,018). Ponadto 12,7% badanych wskazało, że do ekspozycji przyczyniły się inne osoby (np. pozostawiono ostre niezabezpieczone narzędzie, doszło do popchnięcia w trakcie czynności, nastąpił nieoczekiwany zbieg okoliczności,
zaskoczenie). Nieco rzadziej ekspozycja nastąpiła w wyniku wykonywania pracy w stresujących warunkach np. podczas reanimacji (9,7%).
Analiza szczegółowa danych nie potwierdziła zależności pomiędzy ekspozycją zawodową a wykształceniem badanych (test test χ 2 = 0,437, p = 0,933), formą zatrudnienia (test χ 2 = 2,319, p = 0,677) czy systemem pracy (test χ 2 = 0,680, p = 0,410). Największa liczba badanych doznających ekspozycji zawodowej pracowała na oddziałach zabiegowych, a nieco mniejsza, na bloku operacyjnym, na oddziałach ratunkowych i na oddziale intensywnej terapii. W odniesieniu do pozostałych miejsc pracy (oddziały zachowawcze i przychodnie) różnica w występowaniu ekspozycji nie była istotna statystycznie (test χ 2 = 0,680, p = 0,410). Nie potwierdziło się również założenie, że osoby z dłuższym stażem pracy, z racji swojego doświadczenia, rzadziej ulegają ekspozycji niż osoby z krótszym czasem. Dane dowiodły braku istotnej zależności w stażu pracy pomiędzy osobami, które uległy ekspozycji, i pomiędzy pozostałymi ankietowanymi (test U Manna-Whtneya, p = 0,353) (tab. 1).
Ryzyko transmisji zakażeń po ekspozycji zawodowej wg Centers for Disease Control and Prevention (CDC) wynosi 6–30% dla HBV, 0–7% dla HCV i < 0,3% dla HIV. Ekspozycja zawodowa, a właściwie jej potencjalne skutki zdrowotne, stanowią ważny współczesny problem wśród personelu medycznego na całym świecie. Oprócz zagrażających życiu skutków wywoływanych przez te patogeny, zaobserwowano znaczący wzrost strachu i lęku wśród pracowników ochrony zdrowia oraz wzrost obciążenia finansowego systemu opieki zdrowotnej z powodu nadzoru i profilaktyki poekspozycyjnej [19].
Niemal 75% badanych przyznało, że w czasie wykonywania czynności zawodowych doszło u nich do zranienia bez ekspozycji na materiał zakaźny. Z kolei u ponad połowy badanych w trakcie wykonywania pracy doszło u ekspozycji na materiał zakaźny. Wyniki te nie odbiegają od innych badań prowadzonych w Polsce [20] i w innych krajach [21–23]. Wiele doniesień wskazuje, że ekspozycji najczęściej ulegają pielęgniarki, głównie poprzez ukłucie igłą [5, 19, 24–26].
Tabela 1. Statystyki opisowe stażu pracy wśród ankietowanych, którzy ulegli ekspozycji oraz wśród pozostałych ankietowanych
Ekspozycja N Średnia Mediana SD Min Maks p Staż pracy w zawodzie [lata]:
W związku z tak wysokim narażeniem personelu na zranienie, należy dążyć do zwiększenia dostępności do sprzętu bezpiecznego, posiadającego zabezpieczenia przed zakłuciem. Stosowanie urządzeń zaprojektowanych pod kątem bezpieczeństwa w połączeniu z odpowiednimi programami szkoleniowymi oraz zaangażowaniem kierownictwa szpitala i personelu medycznego w dobór urządzeń zabezpieczających, może doprowadzić do zmniejszenia częstości występowania urazów przezskórnych nawet o 50% [27, 28]. Dyrektywy unijne i krajowe przepisy regulują konieczność zastosowania bezpiecznego sprzętu medycznego ograniczającego ryzyko ekspozycji zawodowej [29]. Jednak to nie wystarczy. Niezbędne są szkolenia w zakresie wykorzystania bezpiecznych igieł, kaniul, cewników, urządzeń do próżniowego pobierania krwi i innych ostrych narzędzi, ponieważ nawet 30% urazów czy zakłuć z udziałem sprzętu bezpiecznego było spowodowane ich niewłaściwym użyciem [30].
Dulon i wsp. podają, że szycie i zabiegi chirurgiczne były związane z 27,6% wszystkich ekspozycji zawodowych w badanych szpitalach, przy czym w niewielu sytuacjach wykorzystywano bezpieczny sprzęt medyczny (z konstrukcją zabezpieczającą przed zranieniem). W gabinetach lekarskich używano sprzętu bezpiecznego średnio do co trzeciej procedury zabiegowej [31]. Również w polskich szpitalach większość zgłoszonych przypadków urazów miała miejsce podczas zabiegów chirurgicznych, takich jak szycie i używanie skalpeli, do których nie ma dostępnego mechanizmu zapobiegania urazom [28]. Istotnym elementem ochrony przed ekspozycją błon śluzowych jest stosowanie masek i okularów ochronnych. Dzieje się tak również podczas płukania ran, nacinania ropni, pobierania krwi czy innych zabiegów gdzie teoretycznie można spodziewać się rozprysku wydzieliny na twarz. Kaur i wsp. opisali obrażenia, które wystąpiły z powodu braku środków ostrożności w zakresie ochrony twarzy. Wystąpiły one głównie na oddziale intensywnej opieki medycznej (16%), w obszarach angiografii, endoskopii i radiologii (13%) i na sali operacyjnej (11%) [30]. Wśród pracowników medycznych dochodzi do lekceważenia ryzyka skażenia oczu. Okazuje się również, że niektórzy nie używają środków ochrony osobistej, ponieważ nikt tego nie robi, a oni sami boją się, że staną się obiektem drwin [32].
Ekspozycja zawodowa jest zjawiskiem często występującym w pracy personelu medycznego i wydaje się, że stanowi mocno niedoceniany problem. Prawdopodobnie nigdy nie uda się jej wyeliminować, ale zastosowanie mechanizmów ochronnych,
przestrzeganie procedur i eliminowanie innych czynników ryzyka, takich jak: stres, presja czasu, przeciążenie, nieostrożność i rozproszenie uwagi, niewłaściwa lub wykonana z opóźnieniem utylizacja przedmiotu może przyczynić się do zmniejszenia liczby urazów, a tym samym do zmniejszenia konsekwencji dotyczących zdrowia i aspektów finansowych. Obrażenia możliwe do uniknięcia mieszczą się w przedziale 30,9–55,2% [30]. W prezentowanych wynikach badań co trzecia osoba przyznała się do zachowań ryzykownych (np. nakładanie nasadki na używaną igłę, brak wymiany przepełnionych pojemników na ostre narzędzia bądź świadome niestosowanie środków ochrony osobistej).
Ponad 80% badanych zgłosiło fakt ekspozycji i przystąpiło do realizacji procedury poekspozycyjnej. Ten dość wysoki odsetek należy ocenić pozytywnie, ponieważ przywoływane w piśmiennictwie badania podają często zgłaszalność na niskim poziomie: ok. 40% [33, 34], ok. 60% [35], ale są również polskie badania wskazujące na zgłaszalność sięgającą 90% [36]. Brak zgłoszenia ekspozycji lub późne zgłoszenie może wynikać z braku czasu i możliwości zgłoszenia ekspozycji podczas pracy na zmianie popołudniowej, wstydu po urazie powstałym z własnej winy pracownika [37], z niedoborów kadrowych [38, 39], braku doświadczenia [39] czy bagatelizowania wyniku serologicznego [33].
W prezentowanym badaniu nie zaobserwowano istotnych zależności między wiekiem, stażem pracy, wykształceniem czy rodzajem zatrudnienia a skalą występowania zjawiska ekspozycji zawodowej. Z kolei wg analizy Gregorowicz-Warpas [35] u pielęgniarek z wieloletnim doświadczeniem zawodowym (≥ 50 lat) zdecydowanie częściej występowały zakłucia i zranienia (70%) niż w przypadku osób młodszych w wieku 22–25 lat z krótszym stażem pracy. Autorka przyczynę tego problemu dostrzega w braku znajomości procedur oraz traktowaniu ekspozycji jako nieunikniony element pracy [35]. Dane ze szpitali w centralnej Polsce wskazują, że urazom najczęściej ulegali pracownicy powyżej 40. roku życia, na zmianach porannych od 7:00 do 13:00. Na oddziałach chirurgicznych odnotowano dwa razy więcej zakłuć igłą i ostrymi narzędziami niż na oddziałach niechirurgicznych, ale różnice te nie były istotne [40]. Z doniesień z jednego ze szpitali w Hiszpanii wynika, że wyższe ryzyko wystąpienia wielokrotnych wypadków o ryzyku zakażenia materiałem biologicznym zaobserwowano u kobiet, zatrudnionych na umowę o pracę na czas nieokreślony, pracujących w obszarze chirurgicznym i były to osoby z dłuższym stażem pracy [37]. Ciekawe
Beata Wieczorek-Wójcik, Paulina Mościck,a Katarzyna Kowalska, Katarzyna Cierzniakowska
obserwacje poczynili Santos i wsp., wnioskując, że wyższe ryzyko ekspozycji dotyczyło pracowników samotnych (brak partnera, p = 0,010), ze stażem pracy w szpitalu wynoszącym 6–15 lat ( p = 0,007) lub ≥ 16 lat ( p = 0,013). Natomiast z niższym ryzykiem wypadków w grupie lekarzy wiązał się wiek między 30. a 39. rokiem życia ( p = 0,022), posiadanie ≥ 1 dziecka ( p = 0,030) i świadomość zasad powiadamiania o narażeniu w szpitalu ( p = 0,023) [41].
W celu uniknięcia ekspozycji zawodowej oraz zwiększenia wiedzy personelu na temat procedury postępowania poekspozycyjnego konieczne jest przeprowadzanie regularnych szkoleń. Okazuje się, że większość pracowników medycznych zmienia swoje zachowania pod wpływem świadomości opieki nad pacjentem zakażonym. Jednakże niepokojący wydaje się brak świadomości, że każdy chory powinien być traktowany jako potencjalne źródło zakażenia [35].
Wnioski
• Ponad połowa badanych doznała ekspozycji zawodowej.
• Najczęstsze procedury, podczas których dochodziło do ekspozycji zawodowej ze zbliżoną częstotliwością, to: pobieranie krwi, podawanie leków oraz czynności pielęgnacyjne.
• Jako najczęstsze przyczyny ekspozycji badani podawali pobudzenie psychoruchowe chorych lub nieadekwatną reakcję na wykonywaną u nich czynność zabiegową.
• Ryzyko ekspozycji zawodowej dotyczy całej grupy zawodowej pielęgniarek i pielęgniarzy niezależnie od ich poziomu wykształcenia, stażu pracy, miejsca pracy, sposobu zatrudnienia czy systemu pracy.
• Tylko niespełna co piąta ankietowana osoba wskazała, że nie podejmuje działań ryzykownych uznawanych za potencjalne czynniki prowadzące do ekspozycji.
Oświadczenia
Autorki deklarują brak konfliktu interesów. Praca nie uzyskała finansowania zewnętrznego. Na prowadzenie badań uzyskano zgodę Komisji Bioetycznej przy Collegium Medicum w Bydgoszczy (KB 528/2022).
Piśmiennictwo
1. Tomaszewska K, Kisiel R. Biological hazards at the nurse’s workplace. Journal of Education, Health and Sport 2023; 13: 213–220. DOI 10.12775/JEHS.2023.13.04.024.
2. Ekspozycja zawodowa w ochronie zdrowia. Dostępne na: https://www.nik.gov.pl/kontrole/wyniki-kontroli-nik/pobierz,
NIK-P-20-061-ekspozycja-zawodowa-w-ochronie-zdrowia,typ,kk. pdf (dostęp: 10.04.2025).
3. Prażak Z, Kowalska M. Biological factors in occupational environment of nurses and possibilities of reducing exposure. Hygeia Public Health 2017; 52: 111–118.
4. Salasa M, Goździalska A. Ocena stanu wiedzy dotyczącej zachorowalności na ostre zapalenie wątroby typu B i C wśród personelu medycznego. Państwo i Społeczeństwo 2015; 15: 23–26. https://www. panstwoispoleczenstwo.pl/numery/2015-3/panstwo-i-spoleczenstwo-2015-nr3-salasa-gozdzialska.pdf (dostęp 14.02.2019).
5. Różańska A, Szczypta A, Baran M i wsp. Healthcare workers’ occupational exposure to bloodborne pathogens: a 5-year observation in selected hospitals of the Małopolska province. Int J Occup Med Environ Health 2014; 27: 747–756. DOI: 10.2478/s13382-014-0307-3.
6. Czapla S. Stan wiedzy pielęgniarek na temat profilaktyki zakażeń krwiopochodnych oraz postępowania po ekspozycji na potencjalnie zakaźny materiał biologiczny. Piel Zdr Publ 2020; 10: 115–121. DOI:10.17219/pzp/113296
7. Nikolopoulou GB, Tzoutzas I, Tsakris A, Maltezou HC. Hepatitis B in Healthcare Personnel: An Update on the Global Landscape. Viruses 2023; 15: 2454. DOI: 10.3390/v15122454.
8. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. DOI: 10.1371/journal.pone.0035797.
9. Raina MacIntyre C, Chughtai AA, Zhang Y i wsp. Viral and bacterial upper respiratory tract infection in hospital health care workers over time and association with symptoms. BMC Infect Dis 2017; 17: 553. DOI: 10.1186/s12879-017-2649-5.
10. https://www.gov.pl/web/psse-lobez/hcv
11. Sierpińska L. Ryzyko zakażenia wirusem HCV w przypadku ekspozycji zawodowej pracy pielęgniarek i położnych. Aspekty Zdrowia i Choroby 2017; 2: 7–17.
12. Leończyk-Spórna M, Czarnecka D, Sobieszek-Kundro A i wsp. Analysis of the microbial flora of lower limb ulcers in patients hospitalized in the Department of Dermatology of the Provincial Integrated Hospital in Elbląg in 2022. Leczenie Ran 2023; 20: 96–102. DOI: 10.60075/ lr.v20i3.44.
13. Kruszewska K, Wesolowska-Gorniak K, Czarkowska-Paczek B. Venous leg ulcer healing time is increased with each subsequent bacterial strain identified in the ulcer. A retrospective study. Phlebology 2021; 36: 275–282. DOI: 10.1177/0268355520961945.
14. Prochota B. Acute problem” – what to do following occupational exposure. Współczesne Pielęgniarstwo i Ochrona Zdrowia 2026; 5: 35–37.
15. Budnik-Szymoniuk M, Kujawa W, Pluta A, Basińska-Drozd H. Zakłucia, skaleczenia, zranienia – zapobieganie ekspozycji zawodowej w rok po wejściu w życie uregulowań prawnych – spostrzeżenia. Zakażenia 2014; 5: 62–70.
16. Szczeniowski A, Gańczak M. Implementacja przepisów regulujących zapobieganie ekspozycji zawodowej na patogeny krwiopochodne z perspektywy Polski, jako kraju Unii Europejskiej. Med Pracy 2011; 62: 57–66.
17. Rożkiewicz D. Niedoceniane potencjalne źródła zakażeń szpitalnych. Zakażenia 2014; 4: 65–70.
18. Rybacki M. Opieka profilaktyczna nad narażonymi na zakażenia krwiopochodne. Medycyna i higiena pracy. Walusiak-Skorupa J. CKP, Warszawa 2011; 159–166.
19. Erturk Sengel B, Tukenmez Tigen E, Bilgin H i wsp. Occupation-Related Injuries Among Healthcare Workers: Incidence, Risk Groups, and the Effect of Training. Cureus 2021; 13: e14318. DOI: 10.7759/ cureus.14318.
20. Jończyk A, Szczypta A, Talaga-Ćwiertnia K. Injuries as exposure events in providing medical services by nursing staff. Przegl Epidemiol 2018; 72: 371–381
21. Akyol AD, Kargin C. Needle Stick and Sharp Injuries among Nurses. Global Journal of Nursing & Forensic 2016; 1: 1–5.
22. Wahyu A, Rostati, Nasir S Multivariate Analysis of the Occurrence of Needlestick Injuries on the Nurses at X Hospital in Makassar.
Indian Journal of Public Health Research & Development 2018, 9: 1382.
23. Memish ZA, Assiri AM, Eldalatony MM, Hathout HM. Benchmarking of percutaneous injuries at the Ministry of Health hospitals of Saudi Arabia in comparison with the United States hospitals participating in Exposure Prevention Information Network (EPINet™). Int J Occup Environ Med 2015; 6: 26–33. DOI: 10.15171/ijoem.2015.467.
24. Garus-Pakowska A, Górajski M, Szatko F. Did legal regulations change the reporting frequency of sharp injuries of medical personnel? Study from 36 hospitals in Łódź Province, Poland. Int J Occup Med Environ Health 2018; 31: 37–46. DOI: 10.13075/ijomeh.1896.01045.
25. Ji Y, Huang J, Jiang G i wsp. Investigation of the occupational exposure to blood-borne pathogens of staff at a third-class specialist hospital in 2015-2018: a retrospective study. Sci Rep 2022; 12: 1498. DOI: 10.1038/s41598-022-05436-z.
26. Saadeh R, Khairallah K, Abozeid H i wsp. Needle Stick and Sharp Injuries Among Healthcare Workers: A retrospective six-year study. Sultan Qaboos Univ Med J 2020; 20: e54–e62. DOI: 10.18295/ squmj.2020.20.01.008.
27. Ottino MC, Argentero A, Argentero PA i wsp. Needlestick prevention devices: data from hospital surveillance in Piedmont, Italy – comprehensive analysis on needlestick injuries between healthcare workers after the introduction of safety devices. BMJ Open 2019; 9: e030576. DOI: 10.1136/bmjopen-2019-030576.
28. Szczypta A, Różańska A, Siewierska M i wsp. Did safety-engineered device implementation contribute to reducing the risk of needlestick and sharps injuries? Retrospective investigation of 20 years of observation in a specialist tertiary referral hospital. Int J Occup Med Environ Health 2024; 37: 234–243. DOI: 10.13075/ijomeh.1896.02308.
29. Goniewicz M, Włoszczak-Szubzda A, Niemcewicz M i wsp. Injuries caused by sharp instruments among healthcare workers--international and Polish perspectives. Ann Agric Environ Med. 2012; 19: 523–527.
30. Kaur M, Mohr S, Andersen G, Kuhnigk O. Needlestick and sharps injuries at a German university hospital: epidemiology, causes and preventive potential – a descriptive analysis. Int J Occup Med Environ Health 2022; 35: 497–507. DOI: 10.13075/ijomeh.1896.01854.
31. Dulon M, Stranzinger J, Wendeler D, Nienhaus A. Causes of Needlestick and Sharps Injuries When Using Devices with and without Safety Features. Int J Environ Res Public Health 2020; 17: 8721. DOI: 10.3390/ijerph17238721.
32. Wicker S, Wutzler S, Schachtrupp A i wsp. Arbeitsbedingte Blutexpositionen in der Polytraumaversorgung [Occupational exposure to blood in multiple trauma care]. Anaesthesist 2015; 64: 33–38. DOI: 10.1007/s00101-014-2401-0.
33. Behzadmehr R, Balouchi A, Hesaraki M i wsp. Prevalence and causes of unreported needle stick injuries among health care workers: a systematic review and meta-analysis. Rev Environ Health 2021; 38: 111–123. DOI: 10.1515/reveh-2021-0148.
34. Kasatpibal N, Whitney JD, Katechanok S i wsp. Practices and impacts post-exposure to blood and body fluid in operating room nurses: A cross-sectional study. Int J Nurs Stud 2016; 57: 39–47. DOI: 10.1016/j. ijnurstu.2016.01.010.
35. Gregorowicz-Warpas D. Ekspozycje zawodowe pracowników opieki zdrowotnej. Zgłaszanie i realizacja zaleceń poekspozycyjnych. Zakażenia XXI Wieku 2019; 2: 187–192.
36. Garus-Pakowska A, Szatko F Ekspozycje przezskórne personelu medycznego. Medycyna Pracy 2011; 62: 473–480
37. Tejada-Pérez JJ, Herrera-Burgos MR, Parrón-Carreño T, Alarcón-Rodríguez R. Biohazard Accidents, Harmful Elements to the Wellness of Healthcare Workers, and Their Risk Factors. Int J Environ Res Public Health 2022; 19: 13214. DOI: 10.3390/ijerph192013214.
38. Clarke SP, Sloane DM, Aiken LH. Effects of hospital staffing and organizational climate on needlestick injuries to nurses. Am J Public Health 2002; 92: 1115–1119. DOI: 10.2105/ajph.92.7.1115.
39. Patrician PA, Pryor E, Fridman M, Loan L. Needlestick injuries among nursing staff: association with shift-level staffing. Am J Infect Control 2011; 39: 477–482. DOI: 10.1016/j.ajic.2010.10.017.
40. Garus-Pakowska A, Ulrichs M, Gaszyńska E. Circumstances and Structure of Occupational Sharp Injuries among Healthcare Workers of a Selected Hospital in Central Poland. Int J Environ Res Public Health 2018; 15: 1722. DOI: 10.3390/ijerph15081722.
41. Santos LF, Gonçalves GKN, Sanches SRA, Clemente WT. Factors associated with accidents involving biological material among health professionals. Rev Bras Med Trab 2024; 21: e2022994. DOI: 10.47626/1679-4435-2022-994.
Sprawozdanie | Report
LECZENIE RAN 2025; 22 (2): 88–90

W dniach 28–29 kwietnia 2025 r. w Warszawie odbyła się II Międzynarodowa Konferencja Naukowo-Szkoleniowa „Zaawansowana praktyka pielęgniarska i położnicza w Polsce jako odpowiedź na potrzeby zdrowotne pacjentów” zorganizowana przez Naczelną Izbę Pielęgniarek i Położnych, Polskie Towarzystwo Pielęgniarskie i Polskie Towarzystwo Położnych.
Bogaty program konferencji obejmował sesje wykładowe, panele kliniczne, panele dyskusyjne i warsztaty prowadzone przez ekspertów medycyny, pielęgniarstwa, przedstawicieli agend i instytucji rządowych, menedżerów oraz przedstawicieli organizacji pacjentów. Były to dwa dni intensywnych dyskusji na temat przyszłości pielęgniarstwa i położnictwa w Polsce, poszerzania obowiązujących i nowych rozwiązań kompetencyjnych pielęgniarek i położnych oraz wyzwań stojących przed systemem opieki zdrowotnej.
Poruszane zagadnienia dotyczące rozwoju zaawansowanej praktyki pielęgniarskiej (Advanced Practice Nursing – APN) i zaawansowanej praktyki położniczej (Advanced Practice Midwife – APM) wzbudzały ogromne zainteresowanie uczestników konferencji, a emocjonujące dyskusje były kontynuowane podczas przerw w obradach.
W pierwszej sesji dominowała tematyka związana z zaawansowaną praktyką pielęgniarską postrzeganą jako zmiana kompetencyjna, systemowa i kulturowa.
W kolejnej sesji zaprezentowane zostały przykłady dobrych praktyk klinicznych w kierunku APN w wybranych dziedzinach, w tym: w zaawansowanej praktyce położnej, w dziedzinie pielęgniarstwa diabetologicznego, w dziedzinie kardiologii i pielęgniarstwa kardiologicznego oraz w opiece i leczeniu pacjentów ze stomią.
W pozostałych panelach prelegenci przedstawiali kierunki działań dla usankcjonowania APN
w ustawodawstwie i systemie opieki zdrowotnej, kontynuowano merytoryczne dyskusje w odpowiedzi na pytanie, czy wdrożenie APN oraz APM poprawi dostępność i jakość opieki dla wybranych grup odbiorców, oraz zostały wskazane propozycje systemu uzyskiwania uprawnień APN w Polsce.
Drugi dzień konferencji w całości był poświęcony warsztatom specjalistycznym dotyczącym APN w różnych obszarach pracy pielęgniarek. Były to warsztaty poświęcone żywieniu klinicznemu w praktyce pielęgniarskiej, kierunkom rozwoju zaawansowanej praktyki w POZ, w opiece długoterminowej, w neonatologii, w opiece paliatywnej, w opiece diabetologicznej, w anestezjologii, w położnictwie i w leczeniu ran. Największą frekwencją cieszyły się zajęcia dotyczące wykorzystania Chatu GPT w praktyce pielęgniarskiej i położniczej, jako nowe możliwości wspierania pracy zespołów interdyscyplinarnych. Rozwój zaawansowanych kompetencji w leczeniu ran (ryc. 1), był jednym z ostatnich warsztatów w programie konferencji, a mimo to zgromadził bardzo dużą grupę pielęgniarek i położnych zainteresowanych tą tematyką. Warsztat prowadziły: dr Beata Wieczorek-Wójcik, dr Katarzyna Cierzniakowska, dr hab. Paulina Mościcka i mgr Katarzyna Kowalska (ryc. 2). Celem spotkania była prezentacja założeń pilotażu dla zaawansowanych kompetencji pielęgniarki w leczeniu ran, wskazanie propozycji szerokospektralnych uprawnień niezbędnych do całościowej opieki nad chorym z raną oraz uzyskanie od uczestników opinii dotyczących decyzji klinicznych i propozycji kompetencji APN w leczeniu ran. Wypracowane przez Zespół, ogólne założenia pilotażu APN w zakresie leczenia ran i przetok były w ubiegłym roku opublikowane w na łamach „Leczenia Ran” (Wieczorek-Wójcik B, Kowalska K, Leyk-Kolańczak M i wsp. Działania na rzecz rozwoju pielęgniarki zaawansowanej
praktyki w Polsce – projekt pilotażu APN w zakresie leczenia ran i przetok. Leczenie Ran 2024; 21: 92–97). Projekt zawierający kluczowe dla APN uprawnienia w prowadzeniu procesu diagnostycznego i terapeutycznego u chorych z ranami, wzbogacony o opisy przypadków, ryciny i filmy spotkał się z pozytywnym odbiorem uczestników konferencji. Podkreślano wielokrotnie znaczenie rozszerzonych kompetencji APN w leczeniu ran w kontekście korzyści dla chorych, u których efektywne leczenie ran przyspiesza proces powrotu do zdrowia, łagodzi dolegliwości bólowe, obniża ryzyko izolacji społecznej, poprawia stan psychiczny, poprawia jakość życia, a nierzadko umożliwia powrót do pracy i innych aktywności. Moderatorki warsztatu zwróciły również uwagę na fakt, że szersze uprawnienia dla pielęgniarek oznaczają większe i szybsze możliwości dostępu chorych do opieki zdrowotnej, objęcie ich opieką holistyczną, uwolnienie zasobów medycznych lekarzy specjalistów, którzy w tym czasie mogą realizować bardziej skomplikowane działania medyczne i zabiegowe, a w konsekwencji obniżenie kosztów opieki.
APN w leczeniu ran opiekuje się chorymi w każdym wieku i w różnych miejscach świadczenia opieki. Autonomia pielęgniarki zaawansowanej praktyki będzie przejawiać się w AOS, POZ, opiece długoterminowej i hospicyjnej, w opiece doraźnej oraz w ramach konsultacji międzyoddziałowych w szpitalu. Kontynuacja prac nad modelem zaawansowanej praktyki pielęgniarskiej w Polsce powinna być prowadzona w oparciu o konsensus środowisk medycznych, we współpracy z instytucjami rządowymi, Ministerstwem Zdrowia oraz Narodowym Funduszem Zdrowia, tak by właściwa opieka i pomoc w leczeniu ran docierała do chorego na czas. W ostatnim etapie warsztatu, w dyskusji, głos zajmowali uczestnicy. Zgłoszono m.in. postulat, aby

Rycina 1. Strona tytułowa prezentująca temat warsztatu.

Rycina 2. Moderatorki warsztatu: od lewej Paulina Mościcka, Katarzyna Kowalska, Katarzyna Cierzniakowska, Beata Wieczorek-Wójcik
do kompetencji APN należała również możliwość wystawiania zaświadczeń o zakończonym leczeniu rany w ramach leczenia że środków publicznych AOS czy POZ. Brak odpowiedniego finansowania porady w AOS (1,8 zł × 17 pkt.) nie daje możliwości zakupu odpowiednich materiałów medycznych za tą kwotę. Ponadto dyskusja dotyczyła również jakości kształcenia podyplomowego pielęgniarek na kursach specjalistycznych i szkoleniach specjalizacyjnych. Wskazano na różnice w kompetencjach pielęgniarek i położnych, chociażby w zakresie szycia ran. Położne również zgłaszały potrzebę aktualizacji programu kształcenia i zwiększenia kompetencji po ukończeniu kursu „Leczenie ran dla położnych”. Zauważono również konieczność modyfikacji wykazu leków, które pielęgniarka może stosować bez zlecenia lekarskiego.
Dzięki zaangażowaniu dużej grupy osób z pośród wszystkich uczestników warsztatu, udało się pozyskać drogą elektroniczną 71 opinii na temat proponowanych uprawnień APN. Odpowiedzi na pytania udzieliło 66 kobiet i 5 mężczyzn. Wiek ankietowanych zawierał się w przedziale 29–67 lat (średnia 47,62 roku, mediana 50). Staż pracy mieścił się pomiędzy 5 a 41 lat (średnia 23,44 roku, mediana 26). Tylko 2 osoby nie posiadały specjalizacji w żadnej z dziedzin pielęgniarstwa. Najwięcej było specjalistek w dziedzinie pielęgniarstwa chirurgicznego (18, 25%). Kadrę zarządzającą reprezentowało 29 osób (40,8%). Przygotowana na potrzeby ewaluacji warsztatów ankieta zawierała 7 pytań. Poniżej zamieszczono pytania i odsetek odpowiedzi.
• Czy uważasz, że wdrożenie APN w leczeniu ran poprawiłoby obecny stan opieki nad chorymi z ranami w Polsce? Tak: 70 (98,6%); Trudno powiedzieć: 1 (1,4%)
Beata Wieczorek-Wójcik, Paulina Mościck,a Katarzyna Kowalska, Katarzyna Cierzniakowska
• Czy zasadne jest poszerzenie kompetencji APN w leczeniu ran o drobne zabiegi w diagnostyce i zaopatrywaniu ran ostrych (szycie rany, nacięcie, pobieranie wycinków do badania)? Tak: 70 (98,6%); Trudno powiedzieć: 1 (1,4)%
• Czy uważasz, że APN w leczeniu ran powinna mieć kompetencje do zlecenia konsultacji anestezjologicznej w zakresie preskrypcji leków z grupy adiuwantów oraz II i III stopnia drabiny analgetycznej? Tak: 64 (90,1%); Nie: 3 (4,2%); Trudno powiedzieć: 4 (5,6%)
• Czy uważasz, że APN w leczeniu ran powinna mieć kompetencje do znieczulenia nasiękowego z wykorzystaniem lignokainy? Tak: 69 (97,2%); Trudno powiedzieć: 2 (2,8%)
• Czy uważasz, że APN w leczeniu ran powinna mieć prawo do opracowania planu leczenia ran u chorych, w tym również do podejmowania decyzji o odstąpieniu od leczenia i skierowaniu pacjenta do lekarza lub zakończeniu leczenia? Tak: 70 (98,6%); Trudno powiedzieć: 1 (1,4%)
• Czy uważasz, że podejmowanie decyzji klinicznych przez APN w leczeniu ran jest równoznaczne
z ponoszeniem odpowiedzialności za efekty końcowe leczenia rany u chorego? Tak: 66 (92,9%); Nie: 1 (1,4%); Trudno powiedzieć: 4 (5,6%)
• Czy uważasz, że jesteś gotowa/gotowy do wykorzystania poszerzonych kompetencji APN w leczeniu ran w swojej codziennej pracy? Tak: 50 (70,4%); Nie: 12 (16,9%); Trudno powiedzieć: 9 (12,7%)
„Model praktyki APN został wdrożony w wielu krajach na świecie, a jego zasadność potwierdzona w licznych badaniach naukowych jako działania mające wpływ na optymalizację opieki zdrowotnej oraz jej efektywność” (stanowisko Polskiego Towarzystwa Pielęgniarskiego). Konferencja była doskonałą przestrzenią do kontynuacji merytorycznych dyskusji dotyczących rozwoju APN i APM w Polsce. Organizatorom bardzo dziękujemy za przestrzeń do nauki i wymiany doświadczeń, a wszystkim uczestnikom za inspirujące spotkania i rozmowy.
Beata Wieczorek-Wójcik
Paulina Mościcka
Katarzyna Kowalska Katarzyna Cierzniakowska


Kwartalnik | Zeszyt 2 | Tom 22 | Rok 2025
Kwartalnik | Zeszyt 2 | Tom 20 | Rok 2023
ISSN: 1733-4101 eISSN: 1733-7607 MNiSW: 20 IC: 86,65
ISSN: 17334101 eISSN: 1733-7607 MNiE: 20 IC: 72,67

BEZPŁATNA E-PRENUMERATA !!!
ZAPISZ SIĘ DO NEWSLETTERA NA WWW.PTLR.ORG
ZGŁOŚ ARTYKUŁ DO PUBLIKACJI I ODBIERZ WEJŚCIÓWKĘ NA KONGRES PTLR 2026!
Autor artykułu, który zostanie opublikowany w Czasopiśmie Leczenie Ran, otrzyma bezpłatną wejściówkę na Kongres Naukowo-Szkoleniowy Polskiego Towarzystwa Leczenia Ran 2026!
Prace należy zgłosić przez internetowy system redakcyjny na stronie internetowej: czasopisma.mocmedia.eu



leczniczy

Produkt leczniczy