




A cautionary tale for clinicians who forget self-care.
Ihave a brother-in-law who, until a stroke hit him a couple of years ago, could best be described in two words: a phenomenon. He could have been a Steve Jobs-like CEO, as he had the ability to create a Jobsian reality distortion field around him. He could have been a highly successful politician, with his wit, rhetorical skills and, despite being German, almost British-privateboarding-school levels of ability to ingratiate and surround himself with those who had money, power and influence.
Given my political opinions are very different from his, it’s fortunate that the career he pursued was that of a priest, not a politiker. But it’s a tragedy to see a man in his early fifties diminished because he chose not to take hydrochlorothiazide once a day. High blood pressure gives you the feeling of POWER! Diuretics rob you of that and mean you spend most of the morning peeing; it’s just the blood vessels that are slowly paying the price.
There’s a reason why religions rely on storytelling rather than reasoned, evidenced-based essays on why the sheep should obey the shepherd: it’s the most effective form of communication. These stories easily sink into your brain, and they easily pop up again later. (They’re also a wonderful way of sneaking fallacies unchallenged into people’s brains, but that’s another story…) And so, the Parable of Pater G has been sobering enough for me to pay attention to my own health a bit better and, I hope, for you too.
Physicians, in my experience, are either the absolute best of all people in terms of taking care of their

Photo: lassedesignen/Shutterstock.com
health… or the absolute worst. I understand: the job is hugely demanding. Surgical microscopes aren’t designed for ergonomics. Clinics don’t equal cardio. Long days don’t permit long sessions in the gym.
Further, middle-age equals the onset of aches and pains, doesn’t it? That’s to be expected—and therefore ignored—right? Writing this, I’m thinking: I work in an eye clinic. When was the last time I had my eyes checked? In 2018, when I needed to apply for a Swiss driving license. Oops.
My point here is this. Every doctor has had to work hard to get where they are. At school to get the grades. At medical school and as a resident as a rite of passage. In their institutions and practices to succeed and make a decent living (and to pay those medical school loans back). By the time you’re hitting the presbyopia, pains and occasional palpitations of middle-age, you’re at the top of your game. You owe it to yourselves to make sure you stay there for as long as possible—and in an ideal world, society should thank you for it too. Amen.

Cheers,
Mark Hillen, PhD Director of Communications ELZA Institute, Zurich, Switzerland Editor-At-Large | CAKE






Dr. Harvey S. Uy
University of the Philippines; Peregrine Eye and Laser Institute, Manila, Philippines harveyuy@gmail.com

Dr. William B. Trattler
Center For Excellence In Eye Care Miami, Florida, USA
wtrattler@gmail.com

Prof. Burkhard Dick
University Eye Hospital Bochum Bochum, Germany
burkhard.Dick@kk-bochum.de

Dr. Francis Mah
Scripps Clinic Medical Group La Jolla, California, USA
Mah.Francis@scrippshealth.org

Dr. Cathleen McCabe
The Eye Associates Sarasota, Florida, USA
cmccabe13@hotmail.com

Prof. Dr. Sorcha Ní Dhubhghaill Brussels University Hospital (UZ Brussel) Brussels, Belgium
nidhubhs@gmail.com

Matt Young CEO & Publisher
Gloria D. Gamat Chief Editor
Diana Truong Associate Editor
Maricel Salvador Graphic Designer Writers
April Ingram
Chow-Ee-Tan
Elif Uslu
Hazlin Hassan
Kendra Bruning
Contributor
Luis Diaz-Santana
Matt Herman Head of Content Strategy
Hannah Nguyen COO
Travis Plage CFO
Ruchi Ranga Society Relations & Conference Manager
International Business Development
Brandon Winkeler
Robert Anderson
Sven Mehlitz


Please refer to relevant products Instructions for Use for complete list of indications, contraindications and warnings. *When compared with Constellation and Centurion Vision Systems. Based on bench testing.
1. Alcon Data on File, 2024. [REF-24644];
2. Alcon Data on File, 2024. [REF-24379]
3. Alcon Data on File, 2024. [REF-24576]; 4. Alcon Data on File, 2024. [REF-24615]
This product is CE marked and commercially available in many markets including but not limited to Australia, Brazil, Europe, Japan, United States (FDA): however,regulatory approval is still pending in select regions. Eye care professionals should consult their local regulatory authority or company representative for availability and approved indications.



A new generation of handheld devices is making cataract surgery viable where phaco machines can’t go.
By Kendra Bruning
Cataract may be an old adversary, but in many parts of the world, it still wins far too often.
A new wave of technical wizardry and innovation, however, may change that. Modern phacoemulsification has transformed cataract surgery into a high-precision art, but it still remains out of reach for millions— blocked not by medicine, but by machinery, infrastructure and economics.
Elsa, a 41-year-old mother of four, was one of them. Blind for years, she arrived at a regional hospital near the Tanzania-Mozambique border with her baby, whom she had never seen, on her hip. The day after her cataract surgery, she was walking unaided, smiling and helping other patients.
“She had her confidence back,” said Dr. Susan MacDonald, co-founder of Eye Corps, the outreach organization that helped guide Elsa’s care. “It’s not just her life that changed—it’s the lives of her children.”
Stories like Elsa’s give a glimpse not only into a critical gap in eye care delivery, but also the growing push to close it. And at the center of this push lies an emerging generation of portable, console-free devices designed to bring cataract surgery to places where traditional phaco can’t go.
In many parts of the world, the tools of modern cataract surgery simply don’t fit the environment.
“We lose our electricity,” said Dr. MacDonald of EyeCorps’ work in Tanzania. “We always have a backup generator.”
Electricity is just one among many resources that become scarce when eye care moves beyond major population centers. Engineers are scant. Consumables can be costly or impossible to restock. The list goes on.
Dr. MacDonald’s team now travels with more than a dozen microscopes and slit lamps, each selected for portability and durability. “If something breaks, we need to ask: Can we repair it? Do we have the parts? The tools?”
In this landscape, every piece of equipment must earn its place through performance, portability, repairability and cost-effectiveness. High-tech phaco systems, with their consoles, foot pedals and single-use packs, often don’t make the cut.
This can lead to a compounding cycle where cataracts that aren’t treated early progress into more challenging surgeries, which require more sophisticated and unwieldy equipment to remove.
“These cataracts are ten times worse than anything I see in the U.S.,” said Dr. MacDonald.
When the environment limits what technology can be used, surgeons must adapt, rethinking both logistics and the very tools of surgery itself.
If phacoemulsification is the gold standard, manual small-incision cataract surgeries (MSICS) is the workhorse, quietly restoring sight where phaco machines can’t tread.
But a new category of devices is beginning to take shape in the space between: compact, handheld lens extraction systems that trade complexity for adaptability.
“Cataract surgery, unplugged,” as Dr. MacDonald puts it.
Rather than hauling in an entire OR, these devices promise safe, efficient cataract removal in mobile units
and regional hospitals, even where electricity is intermittent and supply chains unpredictable.
“Keeping it simple has allowed us to get excellent results and train surgeons who can then perform surgery on their own, in their own communities,” she added.
Sometimes, the right tool is the one that can travel.
“Keeping it simple has allowed us to get excellent results and train surgeons who can then perform surgery on their own, in their own communities.”
- Dr. Susan MacDonald
Portable innovation in progress
While the category of console-free extractors is still emerging, a handful of portable devices are beginning to push cataract surgery beyond the OR. Among these is the ZEISS MICOR 700 (Carl Zeiss Meditec, Jena, Germany), which reimagines traditional lens extraction.
Instead of standard phacoemulsification, it uses sonic mechanical oscillation to remove the lens, eliminating cavitation and heat in the eye. It also replaces the foot pedal with fingertip control and utilizes a gravity-fed irrigation system with 25 to 50cc of BSS per case.
"I love the concept," said Dr. Guillermo Amescua, who uses the device in dual-room workflows. Dr. Amescua is a professor of Clinical Ophthalmology at the University of Miami Miller School of Medicine's Bascom Palmer Eye Institute (Florida, USA). “You open the pack, everything’s sterile and it’s good to go. There’s less plastic than a typical phaco pack and the setup is fast.”
Early data are promising. In one study of 665 cases, the capsular tear rate was 0.45% across a range of nuclear
densities. Another found that surgical time improved with experience, and visual outcomes were comparable to standard phaco.1-2
While not suited for mature or leathery nuclei, handheld extractors may be ideal for pediatric cases, refractive lens exchange or settings where phaco is logistically impractical.
Other contenders include:
• Oertli (Berneck, Switzerland) CataRhex 3: a highly portable phaco platform with ab interno MIGS capabilities
• Mynosys (California, USA) Zepto: not a full extraction system, but delivers laser-free, portable anterior capsulotomy For patients like Elsa, these devices may be the bridge to restored vision.
“You open the pack, everything’s sterile and it’s good to go. There’s less plastic than a typical phaco pack and the setup is fast.”
- Dr. Guillermo Amescua
Where handheld solutions shine…and stall
Not every surgery needs a symphony of machines. In some places, a reliable instrument, a steady hand and a bit of shade from the midday sun are what’s available—and what must suffice.
That’s where handheld, console-free devices may prove most valuable. Rural hospitals with patchy power, in-office procedures in emerging markets and mission-based outreach programs all stand to benefit from a simplified, power-light tool.
However, these machines aren’t a panacea. “Handheld phaco devices would be challenged to do the harder, more mature cataracts,” said Dr. Ronald Yeoh (medical director and senior consultant ophthalmic
surgeon at Eye & Retina Surgeons, Singapore), noting that both portable phaco systems and MSICS will likely remain the go-to solutions in outreach and mission work.
Beyond that, safe surgery requires structure. “You cannot perform cataract surgery safely without some OR infrastructure wherever you happen to be,” Dr. Yeoh noted, reflecting on a recent outreach camp in Bhutan. Logistics remain the hardest part, from transporting supplies to ensuring the right IOL inventory makes it up the mountain.
Dr. MacDonald, who has tested handheld systems in Tanzania, sees promise but warns of a common pitfall. “The technology is there, but without a structured program to teach it, adoption will stall,” she said. “We need to invest in training the trainers. That’s what creates sustainability.”
Despite their promise, handheld cataract tools remain largely on the sidelines of surgical practice—even in the places where they could do the most good. It’s not because they lack potential, but because the system around them isn’t built for them.
Part of the answer lies in access. Some devices remain available only in select markets, and global expansion has been cautious. “It’s [MICOR 700] only in the U.S. right now,” noted Dr. Amescua. “They’re slowly training more reps and surgeons, but it takes time.”
Cost is another obstacle. Even simplified tools must prove affordable at scale in regions where the average income may not cover a single intraocular lens. For smaller companies, the financial risk of developing outreach-focused technology can be hard to justify.
There’s also inertia. Surgeons trained on phaco may see little incentive to switch, especially in the absence of strong data, reimbursement pathways or institutional support. And in low-resource settings, cost and training remain formidable hurdles.
“Technology alone isn’t enough,” said Dr. MacDonald. “We need structured programs that teach it, maintain it and adapt it to local needs.”

The device may be ready. The system around it often isn’t. But it can be built.
“You cannot perform cataract surgery safely without some OR infrastructure wherever you happen to be.”
- Dr. Ronald Yeoh
The next chapter in portable cataract care won’t be written by technology alone. It will require partnerships between industry and NGOs, between engineers and outreach teams, between innovation and context.

Dr. Susan MacDonald is the co-founder, chief executive officer and executive director of EyeCorps, a nonprofit in subSaharan Africa. Within five years, Eye Corps has grown from a small idea to a well-respected model of eye care provided by incountry professionals. Under Dr. MacDonald’s leadership, Eye Corps has grown to three sites in regional hospitals located in Kilimanjaro, Lindi and Ruvuma regions of Tanzania. She is a board-certified ophthalmologist. She received her medical degree from the University of Massachusetts Medical School (USA) and trained in two specialties: Internal Medicine at Brown University (USA) and Ophthalmology Residency at the University of Utah (USA).
susan@eyecorps.org
Trials in low- and middle-income countries, not just academic centers, will be key to proving real-world value. Hybrid models that combine reusable and disposable components could cut costs, while public-private collaborations may unlock new funding pathways.
Dr. MacDonald’s wish list is simple: “Equipment and education,” she said without hesitation. “And investment in local leadership. These countries aren’t just recipients—they’re emerging ophthalmic markets. Any company that sees that now will be a leader in the future.”
For her, every tool and partnership ultimately points back to a single outcome: a mother like Elsa, seeing her child for the first time and regaining the independence to guide others the very next day.
Phaco will remain the gold standard. But in places where the power cuts out and the sterilizer sits idle,

Dr. Ronald Yeoh is the medical director and founding member of Eye & Retina Surgeons in Singapore. He is the immediate past-president of the APACRS. Dr. Yeoh is also on the faculty of Singapore National Eye Centre where he is a member of the Cataract Subspecialty Group. He received his medical degree from St. Bartholomew’s Hospital (London) and did his ophthalmic residency at St. Thomas’ Hospital (London), followed by a surgical vitreoretinal fellowship at Moorfields Eye Hospital (London). Having been one of the pioneers in phacoemulsification in Singapore, he ran a highly-rated basic phaco course at the ASCRS annual meeting every year from 2000 to 2012.
ersryeoh@gmail.com
a pocket-sized alternative could change the surgical map.
For handheld solutions to succeed, they must do more than travel well. They must be teachable, sustainable and trusted, wherever the surgery takes place.
1. Ianchulev T, Yeu E, Hu EH, et al. First in-human clinical performance of a new noncavitating handheld lensectomy system in 665 consecutive cataract surgeries. J Cataract Refract Surg. 2024;50(7):693–697.
2. Beniz LA, Chatzea MS, Zarei-Ghanavati S, et al. Finger-controlled nonultrasonic lens extractor. J Cataract Refract Surg. 2025;51(1):60–65.
A version of this article was first published on cakemagazine.org.

Dr. Guillermo Amescua is an ophthalmologist in Florida (USA) and is affiliated with multiple hospitals in the area, including Bascom Palmer Eye Institute-Anne Bates Leach Eye Hospital and University of Miami Hospital and Clinics. He received his medical degree from Monterrey Institute of Technology (Mexico), completed his residency training at the University of Pittsburgh (USA), followed by cornea and uveitis training at the Bascom Palmer Eye Institute. He specializes in anterior segment related, cataract related, cornea and external disease, and uveitis.
GAmescua@med.miami.edu


Combining the proven power of Scheimpflug imaging with the precision of ultra high-resolution OCT enables the detection of previously unseen corneal pathologies with unprecedented clarity.
Diagnose earlier. Treat smarter. Care deeper.
TEAR FILM
EPITHELIUM
BOWMAN’S LAYER
STROMAL LAYER

Ambrósio Jr.

The Pentacam® Cornea OCT can increase the confidence that your diagnosis is correct.
ESCRS 2025 | Booth C2.017
Lunch Symposium I Room A3 Sat, 13 September 2025, 13-14 h
Integrating key modalities for a complete refractive work-up
The availability of products and features may vary by country.

From VR-style IOL tryons to lenses that can be adjusted after surgery, new tools are helping surgeons turn patient wish lists into reality—and sidestep postop letdowns.
Some patients walk into cataract surgery expecting an instant “HD upgrade” for their eyes, but then leave feeling like they got standard definition. Despite the procedure’s stellar success rate, disappointment still pops up, usually because the vision in their head doesn't match the vision in real life.
For ophthalmologists, this expectation gap is all-too familiar.
“Cataract surgery is one of the procedures leading to the highest levels of satisfaction in patients,” said Dr. Susana Marcos, an ophthalmology professor at the University of Rochester (USA). “However, a reason leading to dissatisfaction is not meeting expectations regarding postoperative vision.”
That disappointment can take many forms: blurry distance vision, still needing reading glasses or glare and haloes that make night driving tricky.
“For example, not achieving spectacle independence if the patient thought that they would not need glasses at near. Another reason for dissatisfaction is not achieving full, crisp vision at distance with a multifocal IOL,” Dr. Marcos explained.
The letdown hits even harder for patients who chose premium intraocular lenses (IOLs) like multifocals or extended depth of focus (EDOF) ones. Not only have they paid extra, but they also expect visual perfection.

By Hazlin Hassan
Research backs up these clinical observations. In one study of 4,335 patients, 27% of those with low preintervention visual acuity (VA)—even after gaining more than 0.4 in VA— still reported being unsatisfied.1
The takeaway: patient satisfaction isn’t just tied to clinical outcomes. It’s heavily influenced by what patients expect their visual improvement to feel like in daily life.
Even with thorough counseling, it can be hard for patients to truly imagine what “slight halos” or “some residual astigmatism” will mean behind the wheel or on their phone screen. That’s where a new wave of technology is stepping in, giving patients the chance to “test drive” their vision before making a permanent choice.
Enter IOL simulation devices like the SimVis Gekko, developed by 2EyesVision (Madrid, Spain) from research led by Dr. Marcos and her team. This wearable headset allows patients to experience the real world through binocular simulations of different IOL types, essentially giving them a preview of sight after surgery. Instead of just explaining optical trade-offs, clinicians can now show them.
“There is strong evidence that practices using visual simulators such as the SimVis Gekko on
a regular basis have seen an improvement in vision satisfaction and a reduction in post-operative surprises,” said Dr. Marcos, who is also co-founder of 2Eyes Vision. “Patients are not only told what to expect but actually see the real world binocularly through the simulated lens and compare across different options.”
A 2025 clinical study by Henriques et al. at Centro Hospital Universitário de Coimbra (Portugal) evaluated 51 patients using the SimVis Gekko. Participants compared simulations of a monofocal IOL, a diffractive trifocal and an EDOF lens. After “trying them on,” 86% chose a premium IOL—51% selected trifocals, 35% opted for EDOF—and only 14% went with monofocals. Moreover, 92% described the simulation as “very useful.”2
Here’s how it works: the clinician aligns the simulator headset and, using a Bluetooth-connected app, sends signals to present different lens types in each eye. Patients then view real-world tasks—reading cards, eye charts or objects around the room—and give feedback on clarity and comfort at various distances.
“The consultation typically begins with the clinician explaining the concept of multifocality,” said Dr. Marcos. “The clinician evaluates visual performance and asks the patient to assess perceived visual quality at different distances, to determine subjective comfort and quality of vision with each correction.”
Crucially, studies show the simulator closely mimics real optical effects, including halos. Research from Vinas et al., Zaytouny et al., and Dorronsoro et al. found a strong correlation between simulated pre-op defocus curves and actual post-op results.3-5
“Patients love the possibility of experiencing prospective vision postsurgery and it relieves uncertainty of the outcomes,” Dr. Marcos said. “Some patients are referred to clinics with the simulator after having had surgery in one eye, wanting to optimize the choice for the second. Their reaction is that they wished to have been able to do the simulation earlier.”
For patients who want even more flexibility, the Light Adjustable Lens (LAL; RxSight, California, USA) provides a unique post-op advantage.
FDA-approved in 2017, the LAL is currently the only IOL that can be adjusted after implantation, letting surgeons fine-tune vision based on real-world patient feedback.
“LAL offers a unique opportunity to refine the refractive outcome postoperatively and allow patients to decide what focal point they want for each eye after surgery,” said Dr. Sumitra Khandelwal, professor of ophthalmology at Baylor College of Medicine (USA).
Here’s how it works: The LAL is a monofocal lens, so it doesn’t correct astigmatism at the time of surgery. About three weeks post-op, patients return for a light-based treatment that reshapes the lens. At this stage, the surgeon can determine how much sphere and astigmatism correction is needed, and even trial different levels of monovision to gauge patient tolerance.
“This is excellent for patients who have never tried monovision before but would like to have a range of vision,” Dr. Khandelwal said. “We can trial monovision with several powers for the near eye to determine how much near vision they want but can still tolerate the difference between the eyes.”
The LAL has proven especially useful in complex cases, such as patients with prior myopic LASIK, short axial lengths or unusual keratometry.

“The outcomes have been excellent, especially for eyes that have undergone prior myopic LASIK or have a tendency for more refractive error,” she said. “The key is to make sure the patient is easily refractable in the clinic. Any problems with the cornea or tear film can make locking challenging post-op.”
As cataract technology evolves, tools like IOL simulation and adjustable lenses are giving patients more say in their own visual outcomes. “Definitely those patients considering a multifocal or EDOF IOL or binocular combinations like mini-monovision, benefit from simulation,” said Dr. Marcos. “It is helpful for hesitant patients and also for surgeons as the optimal way to manage expectations.”
As patients become more informed, the demand for personalized, previewable vision will only continue to grow. From IOL simulations to tweakable tech, cataract surgery is entering a new era. One where patients don’t just see the future, they can try it on for size first. And if all goes well, the only surprise left is just how clear the world can look.
Dr. Susana Marcos is currently the David R Williams Director of the Center for Visual Science, Nicholas George Professor of Optics at the Institute of Optics and Professor of Ophthalmology at the Flaum Eye Institute, University of Rochester (New York, USA). She is the former Director of the Institute of Optics at the National Research Council in Spain. Dr. Marcos obtained her Ph.D. in Physics at the University of Salamanca (Spain) and was a Fulbright and Human Frontier Postdoctoral Fellow at the Schepens Eye Research Institute of Harvard University (USA). She is a leading researcher in visual optics, having pioneered multiple technologies of eye optical imaging diagnostics and treatments, including novel IOL designs. She has published more than 200 highly cited publications and is a co-inventor of 28 patents. She is co-founder of 2EyesVision, a company that developed SimVis, a visual simulator of presbyopic corrections. She is a Fellow of Optica, European Optical Society and the Association for Research in Vision and Ophthalmology. Her work has been recognized with numerous awards.
marcos2@ur.rochester.edu

1. Garcia-Gutierrez S, Quintana JM, Aguire U, Barrio I, Hayas CL, Gonzalez N. Impact of clinical and patient-reported outcomes on patient satisfaction with cataract extraction. Health Expect. 2012;17(6):765-775.
2. Henriques JFS, Queiros T, Coimbra R, Lobo CL, Murta JCN. Impact of preoperative seethrough visual simulation in cataract surgery of presbyopia-correcting intraocular lenses. J Cataract Refract Surg. 2025;51(7):570-577.
3. Vinas M, Aissati S, Romera M, et al. Pre-operative simulation of post-operative multifocal vision. Biomed Opt Express. 2019;10(11):5801–5817.
4. Zaytouny A, Siso-Fuertes I, Barcala X, Slides L, Dorronsoro C, Marcos S. Clinical validation of simulated multifocal intraocular lenses. Invest Ophthalmol Vis Sci. 2023;64(8):5421.
5. Dorronsoro C, Rodriguez-Lopez V, Papadogiannis P, et al. Subjective evaluation of halo size produced by multifocal intraocular lenses. Invest Ophthalmol Vis Sci. 2025;66(8):5387.
Dr. Sumitra Khandelwal is a professor at the Cullen Eye Institute, Baylor College of Medicine, where she specializes in cornea, cataract and refractive surgery. She also serves as the medical director for the Lions Eye Bank of Texas. Dr. Khandelwal has authored numerous peerreviewed papers focusing on optimization of cataract and cornea surgery outcomes. In addition, she is a co-editor for the book Cornea Crosslinking and contributed to several projects on diagnostic and treatment options for keratoconus. Dr. Khandelwal is currently president of Cedars Aspens Society and serves on the ASCRS Cornea Committee and Membership Committee, as well as on the cataract subcommittee for AAO. In her spare time, she enjoys spending time with her husband and three kids.
Sumitra.Khandelwal@bcm.edu
Forget lasers. This technique tweaks the cornea with nothing more than a zap and a lens.
LASIK may one day have competition from a technique that skips the scalpel and sidesteps the laser. Researchers who presented at the American Chemical Society (ACS) Fall 2025 meeting are exploring a surgery-free way to reshape the cornea using electrochemistry, and early results suggest it could be a game-changer for vision correction.
The method, known as electromechanical reshaping (EMR), is the brainchild of scientists at Occidental College (USA) and the University of California, Irvine (USA). In ex vivo studies with rabbit eyes, EMR successfully altered corneal curvature while maintaining transparency and preserving cell health.1
Instead of cutting or ablating tissue, EMR harnesses controlled electrical pulses delivered through a custom platinum contact lens. The pulses cause temporary pH shifts that loosen ionic bonds in the corneal stroma, making it malleable. Once the stimulus stops, the pH normalizes, and the cornea “locks in” its new shape.1
To make this possible, the team press-molded platinum-foil disks onto 3D-printed hemispheres, creating reshaping lenses of varying focal lengths. During treatment, the lens is placed on the cornea, submerged in physiological solution, and exposed to a precisely timed current, typically for about a minute.1
Optical coherence tomography (OCT) confirmed accurate
reshaping, while second-harmonic generation microscopy showed the cornea’s collagen structure remained intact. Cell viability tests revealed only minimal effects on stromal keratocytes, with changes largely confined to the epithelial layer.2
Importantly, the researchers achieved controlled refractive changes across a range of powers, all with equipment far less complex than laser platforms. This raises the possibility of a more cost-effective and accessible alternative.2
To push precision further, the team tested “half-moon” lenses in which only part of the surface was electrochemically active. The results demonstrated EMR’s ability to selectively target reshaping zones while maintaining overall tissue integrity.2
Unlike LASIK and other laserbased procedures, EMR does not permanently remove corneal tissue or compromise the biomechanical strength of the cornea. This could make it particularly valuable for patients with thin corneas who are not candidates for traditional laser surgery.1
The technique also shows promise in maintaining corneal transparency and cellular viability, crucial factors for long-term success in vision correction procedures. The researchers noted that by carefully controlling the treatment parameters, they could minimize potential damage to the corneal tissue.1
Of course, a clever concept in the lab still has miles to go before reaching the clinic. The researchers stress the need for extensive animal
studies and eventual human trials. Key questions include long-term stability, the effects of blinking and eye movement, optimal treatment parameters and safety across different patient groups.1
"There's a long road between what we've done and the clinic," lead researcher Prof. Michael Hill noted in a statement. "But, if we get there, this technique is widely applicable, vastly cheaper and potentially even reversible."
If EMR fulfills its promise, it could shift the landscape of refractive surgery: simpler tools, lower cost and reduced invasiveness—topped off with the intriguing possibility of reversibility. Accessibility in resource-limited settings could be one of its greatest impacts.
For now, funding efforts are underway to advance EMR toward clinical translation, and LASIK remains unchallenged. But this “nocut, no-laser” contender may just give the reigning champ something to blink about.
1. American Chemical Society. Forget LASIK: Safer, cheaper vision correction could be coming soon. ScienceDaily. August 18, 2025. Available at: https://www.sciencedaily.com/ releases/2025/08/250818102941.htm. Accessed on August 19, 2025.
2. Stokolosa AM, Wong BJF, Hill MG, et al. Electromechanical cornea reshaping for refractive vision therapy. ACS Biomater Sci Eng. 2023;9(2):595-600.
Editor’s Note:



Sponsored by Visionix
Why settle for a diagnostic snapshot when you could have the full gallery? The Optovue Solix OCT/OCT-A offers highdef imaging, fast scans and smart analytics, giving you more than just a pretty picture. It’s time to see the whole story.
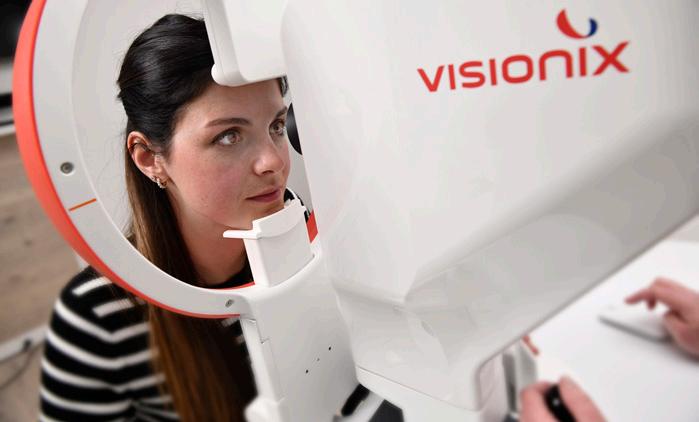
In photography, there’s a clear line between a quick smartphone snap and an image captured with a professional camera: the depth, the crisp detail, the ability to capture what most eyes miss. The same goes for eye care diagnostics. The Optovue Solix (Visionix; Normandy, France) delivers that same high-definition clarity, bringing professional-grade imaging straight into your practice.
The device marks a meaningful step forward in spectral-domain OCT (SD-OCT) and OCT angiography (OCT-A). High-resolution images? Check. Lightning-fast scans? Check. A broader field of view? Also check. This isn’t a dramatic reinvention, but it is a well-calibrated refinement that gives you more information with less effort.
“With the Optovue Solix, I moved from a lower density radial ring pattern to a very high density Q pattern,” said Dr. Robert Rothstein, founder of Eyenamics NY (USA).
“In addition, there’s deep learning segmentation and improved artifact removal. This gave me better repeatability, reproducibility and more trustworthy trend data,” he noted. “I make decisions with greater confidence and fewer rescans…it’s almost three times the improvement.”
The Optovue Solix's 120,000 A-scans per second speed helps deliver detailed images of the retina, choroid and anterior segment, without the motion artifacts that used to haunt OCT scans. The
result? Fewer rescans, better efficiency and clearer images you can count on.
The system’s 12 mm by 12 mm widefield en face OCT gives you a panoramic view of the retinal and choroidal vasculature. With it, you’re no longer boxed into the central macula. Now, you can assess the periphery with the same clarity a wideangle lens brings to a landscape shot.
"Recently, I saw a patient who had pseudotumor cerebri, but I also suspected posterior choroiditis. You could see on the OCT and fundus photos evidence of multifocal choroiditis,” Dr. Rothstein recalled.
“The Optovue Solix can actually look at abnormalities of the retina found on fundus photography, especially on wide field fundus photography, and then see a cross section of it on the OCT. The optic nerve scans are now 6 mm by 6 mm, so you have a higher density and less interpolation."
The Optovue Solix's AngioVue® technology gives you a dyefree, non-invasive look at microvasculature, while AngioAnalytics™ translates those visuals into hard numbers (like vessel density, flow area and the foveal avascular zone). The combination delivers both visual and quantitative insights to support more informed decision-making.
And this visual versatility extends beyond the retina. The Optovue Solix handles anterior segment imaging with the same precision. Corneal epithelial, stromal and pachymetry maps help inform keratoconus management and refractive surgery planning. Its anterior chamber angle assessment adds yet another layer, visualizing angle structures and measuring them with the clarity of a macro lens capturing every fine edge.
In today's busy practice environment, every second counts. With rapid scanning speeds and smart automation, the Optovue Solix helps eye care pros stay efficient without cutting corners on image quality.
Thanks to its built-in Motion Correction Technology (MCT), the system automatically smooths out patient movement (yes, even the fidgety ones), minimizing the need for frustrating rescans. And with FastTrac™ eye-tracking, it keeps the image crisp by adjusting for eye motion mid-scan.
"The Optovue Solix is faster and requires fewer repeats. It certainly cuts back chair time, so we could see more patients,” said Dr. Rothstein. “The Optovue Solix now has 120,000 A-scans versus Avanti, which was 60,000. In terms of workflow, it's easier to see patients faster and it's certainly more reliable testing."
Think of it like a high-end camera with image stabilization and autofocus. You’ll capture what you need, fast and sharp, right from the first click.
Like a seasoned photographer balancing lighting, composition and timing, the Optovue Solix takes a similarly thoughtful approach to glaucoma management, offering a big-picture view with sharp attention to detail. By combining structural and vascular measurements, it helps clinicians see more of the disease process…and see it earlier.
"With the Optovue Solix, you can now really correlate RNFL and ganglion cell loss with vessel density loss. We now have another indicator, another biomarker, so to speak, to follow when monitoring glaucoma patients,” said Dr. Rothstein.
“The Optovue Solix is actually much more sensitive in picking up changes that might not have been picked up on the Avanti. You can pick up changes in the RNFL and the ganglion cell complex, and you might be able to pick up earlier pathology in a patient with suspected optic neuropathy, in particular. Or in glaucoma patients, you might pick up earlier progression."
And then there’s the Hood Report. This feature overlays OCT data with visual field results, making it easier to connect the dots between
what you see and what the patient experiences. Think of it as the diagnostic version of a photo essay that reveals not just the headline image, but the full narrative.
Investing in a system like the Optovue Solix isn’t just about upgrading your tech, it’s about leveling up your entire practice. While the clinical perks are clear, the financial benefits are worth a closer look, too.
With its all-in-one imaging capabilities, the Optovue Solix can help you consolidate testing, streamline workflows and potentially boost reimbursement. It’s a win-win: fewer tests for patients, more efficient throughput for you. And when you’re spotting disease earlier and managing it more effectively? That translates to better outcomes, happier patients and maybe even a few glowing reviews.
tips straight from the surgeons who use the Optovue Solix day in, day out? Swing by the Visionix booth (Stand B4.116) at ESCRS on September 13 and 14, 2025. Join the experts for lively, hands-on booth talks packed with insights, real-world cases and clever ways to elevate your diagnostics. Discover how bringing true anterior OCT to Visionix’s renowned posterior technologies can help you see everything, crystal clear.

"Where the OCT can become a bottleneck, when it's easier to see these patients and scan them with more reliability, we can increase the volume. Patients can be satisfied because there's less chair time,” said Dr. Rothstein. “Regarding reimbursements, the OCT-A is now being reimbursed at a higher fee [in the United States], and that's certainly a gain for the practice."
Think of it like this: a professional photographer doesn’t settle for a smartphone snap. They invest in gear that captures every nuance. The Optovue Solix is that highperformance camera for your clinic, delivering crystal-clear, wide-angle views paired with powerful analysis tools that help you catch what others might miss.
In a field where details matter, the Optovue Solix OCT/OCT-A doesn’t just help you diagnose, it helps you tell the whole story of ocular health, one expertly captured scan at a time.
Curious about the newest Optovue Solix innovations in action? Want
Dr. Robert Rothstein is the founder of Eyenamics NY (USA) and a board-certified ophthalmologist specializing in glaucoma, neuro-ophthalmology and cataract surgery. A native New Yorker, he graduated from the combined BA/MD program at Brooklyn College and SUNY Downstate Medical Center, where he also completed his ophthalmology residency. He went on to pursue fellowships in glaucoma at New York Eye and Ear Infirmary under Drs. Robert Ritch and Jeffrey Liebmann, and in neuro-ophthalmology at SUNY Downstate under Dr. Arthur H. Wolintz.
Dr. Rothstein is an attending surgeon at New York Eye and Ear Infirmary and has trained both ophthalmology residents and optometry students. He is a member of the New York Glaucoma Society, American Glaucoma Society, North American Neuro-Ophthalmology Society and the American Academy of Ophthalmology.
With more than 20 years of experience, Dr. Rothstein brings cutting-edge expertise in glaucoma and neuro-ophthalmology, coordinating care with specialists across multiple disciplines. He also offers advanced cataract surgery, including femtosecond laser–assisted techniques and premium intraocular lenses.
Robert.Rothstein@eyenamicsny.com

You’ve spotted a fix for a daily clinical headache and maybe even built a prototype. But now what? Before you dive headfirst into the startup world, here’s a smart, no-fluff guide to turning your idea into a real, fundable, scalable solution.
By Luis Diaz-Santana
As a clinician, you regularly come across problems that slow you down or compromise care. Sometimes, the fix seems obvious. You wonder why no one has built it yet, and whether you should.
And so you start mapping out an alternative. You spot a business opportunity. Maybe it’s a better way to take notes, a tool to screen patients faster or an improvement to a surgical procedure. An approach that would not only help you but others too.
The path from idea to product is rarely simple. But you already have a major advantage:
you’re addressing a real problem. You might have already built a first prototype and tested it with a few peers.
So, what’s next?
The hardest questions are often the ones asked first. How long will it take? How much will it cost? What needs to happen, and in what order? There’s no universal formula, but there is a structured way to find out.
This article introduces a framework I use with early-stage entrepreneurs to create a realistic plan and reduce uncertainty. It breaks the challenge into three core areas:
• The product
• The organization
• The market and ecosystem
Let’s start with the part you’re probably most comfortable with: the product. You’ve designed it to solve a specific clinical problem, and you likely know how it fits into current workflows. It may even improve them. But there are two additional aspects you’ll need to consider.
First, integration with existing clinical systems—such as patient records, diagnostics or imaging platforms. You don’t need a detailed integration plan yet, but begin mapping how your product connects with what’s already in use. How will data flow between your tool and existing systems? What steps might your product streamline or replace? Thinking this through early will clarify your integration needs.
Second, the regulatory pathway. Will your product be classified as a medical device? If so, which regulatory body will you need to engage first, and what clinical or technical claims will you need to support?
These layers—product design, integration and regulation—don’t exist in isolation. Each will be shaped by the broader business plan you’re building. A regulatory strategy that works in one region may not apply in another. Likewise, system integration needs will vary by setting.

Start by noting what you already know, and, just as importantly, the assumptions you’re making. You don’t need all the answers upfront, but you do need to connect the dots and identify the gaps. Once you’ve worked through the full framework, you can return to these choices with greater clarity.
With the product taking shape, the next step is to define the organization that will bring it to life. A key part of this is your revenue model. This is something that must be addressed early as it influences everything: your team, partnerships, regulatory path and more.
To get this right, go beyond patients and clinicians. Speak with procurement officers, practice owners and potential distributors. These are the people who ultimately decide what gets adopted and paid for.
Here’s why this matters: a premium wellness product might require concierge-level partnerships with spas or retailers. But if it’s prescribed by doctors and sold through pharmacies, you’ll need buy-in from clinicians and agreements with pharmacy chains. Each path leads to a different structure, different processes and different partners. While this example shows two very different models, simple variations in more similar models may also affect your business structure. This is an important step, so consider it carefully.
Once you’ve outlined a few viable revenue models, you can start identifying the capabilities your company needs. Who does what, and when? Early on, you’ll likely rely on external help. That’s normal. What’s important is understanding what needs to remain in-house, what can be outsourced and how that balance will evolve over time.
You should also define your core processes. Some are obvious, like your regulatory and clinical strategy. Others are just as vital: business development, scientific positioning and customer support. These foundational choices shape how investors assess your team and how potential partners judge your credibility.
Now, look outward. How will clinicians, patients and the wider health system experience your product? Together, your product and revenue model will shape how the solution is delivered and supported. That’s where the service model and partnerships come in. And this is often where early-stage clinical entrepreneurs encounter unexpected friction.
Your service model includes everything around the
product: training, onboarding, troubleshooting, follow-up and even long-term monitoring. Understanding what your users expect, and what your product requires, is critical. Done well, it adds value. Done poorly, it becomes a barrier to adoption.
Strategic partnerships are essential for scaling. You may need partners for distribution, data integration, training, support or reimbursement. The right partner can unlock rapid growth. The wrong one can block it.
Overlaying all of this are two often-overlooked elements: brand and customer engagement. Your brand communicates what you do and why people should trust you. A logo or design helps users associate that trust with your product.
pitches, grant proposals and partnerships all become easier when your plan has structure.
Most importantly, you’ll know what kind of help you need, and where others can meaningfully contribute. With clarity and direction, asking for support becomes more than a request. It becomes an invitation to join something real.

But customer engagement doesn’t end at the point of sale. It’s what keeps users informed, supported and loyal, whether you’re building a B2B or B2C company. Engagement must be intentional and systematic. The choices you make here—how your product is positioned, delivered and supported—flow backward through the framework. They add another layer to shape your team, processes, partnerships and even how you’re perceived in the market.
Service, partnerships, brand and customer engagement are not addons. They are central to your success.
This journey is not one to take alone. Surround yourself with people who bring diverse perspectives, whether it’s deep experience, complementary skills or simply a different way of looking at the problem. The questions we’ve explored can’t all be answered in isolation. Some of the most innovative solutions emerge when clinicians, technologists, business thinkers and users cocreate together.
Once you’ve worked through the three layers—product, organization and market—you’ll be in a strong position to move forward. Investor
Luis Diaz-Santana is passionate about efficiently harnessing innovation to address the challenge of caring for a growing and aging global population. With over 25 years of experience, he brings a wealth of expertise in developing complex instrumentation and products in ophthalmology and eye care, providing clients with tailored solutions and industry insights.
Working at the forefront of ophthalmic innovation, Luis has influential connections across global eye care and ophthalmology. He founded LDSH Strategy in 2023 and previously led ophthalmology innovation at Cambridge Consultants in the UK, focusing on developing groundbreaking devices to meet pressing unmet needs.
In recent years, Luis has helped global eye care companies and start-ups navigate the complex innovation landscape in areas such as intraocular lenses, surgical robotics and drug delivery. He brings a unique combination of technical skills, market understanding and insights into human-technology interactions.
Luis holds a Ph.D. in ophthalmic optics from Imperial College and was a pioneer in aberrometry and high-resolution retinal imaging.
luis@ldshstrategy.com

Presbyopia may be inevitable, but settling for reading glasses isn’t. With the PC-IOL market expanding and evolving, ECPs face a growing menu of lens technologies, patient personalities and visual priorities. From neuroadaptation hurdles to futuristic fluid optics, here’s what the experts are eyeing—and why personalization is still the sharpest tool in your surgical kit.
By April Ingram
Aging may come with wisdom, but it also brings the inevitable—like presbyopia. For even the most diligent accommodative exercisers among us, there’s a moment when a restaurant menu or smartphone screen stubbornly refuses to come into focus, regardless of arm length. Does that mean it’s time to surrender to bifocals and embrace the “granny glasses” stereotype? Not necessarily. Thanks to the evolution of presbyopia-correcting intraocular lenses (PC-IOLs), the outlook is bright for aging eyes, even up close.
PC-IOLs, implanted during cataract surgery or refractive lens exchange, are designed to address the visual limitations at near and
intermediate distances, compared to the traditional distance target achieved with monofocal IOLs. This allows patients to eliminate or reduce their dependence on spectacle lenses.
Current PC-IOLs fall into three main categories:
• Multifocal IOLs use concentric diffractive rings to create multiple focal points, typically for near and distance vision.
• Accommodative IOLs mimic the natural lens by shifting position or changing shape.
• EDOF IOLs offer a smoother visual experience, delivering continuous range of focus through a single zone using diffractive or non-diffractive optics.
Over the past decade, PC-IOLs have gained significant traction. According to the European Society of Cataract & Refractive Surgeons (ESCRS) clinical trends survey, multifocal/trifocal and EDOF lenses now represent 42% and 40% of presbyopia-correction IOL usage, respectively.1

Yet adoption is not without challenges. The most frequently cited barrier is cost to patients, which can exceed reimbursement thresholds. Beyond cost, clinicians must carefully assess patient lifestyle, ocular health and personality, as well as manage expectations around potential side effects like glare and halos. Indeed, over half of clinicians in the ESCRS survey voiced concerns about nighttime visual quality following PC-IOL implantation.1
At the heart of every PC-IOL procedure is a decision that can define a patient’s visual outcome: which lens to choose. To bring our readers deeper clinical insight, we caught up with two respected clinician-researchers to hear how they approach lens selection, manage patient expectations, assess real-world outcomes and where the PC-IOL space is headed next. Let’s dive in!
Prof. Vito Romano, who teaches at the University of Brescia in Italy, explained that his approach to PC-IOL selection has evolved over time. “Initially, I favored multifocal lenses, but with the advent of EDOF technology, I have embraced a more patientcentered, customized approach,” he said. “Today, EDOF lenses (especially non-diffractive ones) are my preferred option for patients prioritizing contrast sensitivity, driving at night, or who have even subtle ocular surface irregularities.”
He added, “However, modern trifocal IOLs still represent a strong solution for highly motivated patients with excellent ocular health and a strong desire for near vision independence.”
Dr. Mayank Nanavaty, a cataract, cornea and refractive surgery consultant at Sussex Eye Hospital (UK), also weighed in. He has more than a decade of experience with presbyopia-correcting IOLs, and his approach to lens choice is similarly individualized. “It is very patient specific and based on several criteria,” he shared.
So, what makes someone a good candidate for a PC-IOL? Can anyone who wants the convenience (and can afford it), just go for it? Not exactly. As it turns out, selecting the right lens involves a mix of clinical factors and personal preferences.
Dr. Nanavaty walked us through his approach. “The first thing I want to know is what their visual needs are, their expectations and their lifestyle,” he said. From there, he moves on to a full eye exam. “I would do a thorough examination of the entire eye, including assessment of topography and OCT scans,” he added.
Then comes the crucial conversation about side effects.
“I would explain the side effects of glare and halos with some diffractive multifocal IOLs and also explain the concept of mini monovision with some refractive EDOF IOLs,” he noted.
Once everything’s on the table, Dr. Nanavaty helps patients weigh their options. “Then based on their occupation [visual needs] and their personalities, after ensuring that they have understood the options and their risks and benefits clearly, I would encourage them to make a choice between the lenses,” he explained.
Prof. Romano follows a similar path, and he’s even helped write the playbook. He and the ESASO Study Group recently published guidance on how to match the right PC-IOL with the right patient.2 “Our recent Delphi consensus highlighted key parameters that should guide preoperative evaluation: ocular surface health, pupil size dynamics, corneal higher-order aberrations, macular and optic nerve status, and lifestyle factors such as professional demands and hobbies,” he explained.
For Prof. Romano, it’s all about giving patients the full picture and keeping them part of the process. “I take the time to educate patients using a transparent, shareddecision model. We have strong consensus that age, habits and patient motivation are among the most important criteria in selecting a presbyopia-correcting IOL.”
And he’s careful to keep expectations grounded. “Critically, I always temper enthusiasm by reinforcing that no IOL is ‘perfect,’ and mild visual compromises, especially under scotopic conditions, may occur,” Prof. Romano added.
The “well-researched” patient
Across all fields of medicine, the patient landscape is shifting. With unprecedented access to both credible and questionable


information, patients are arriving at consultations more informed—and sometimes more opinionated—than ever. This can mean that they show up having already chosen the lens they think is best for them.
So how do clinicians manage these well-researched patients?
“These patients can be both an opportunity and a challenge,” said Prof. Romano. “More patients come in with expectations based on marketing or anecdotal reports. My approach is to re-center the conversation around personalized suitability. I explain why some lenses that work well for others might not suit their ocular profile or visual goals.”
He stressed that managing expectations, and reframing the conversation, is crucial. Informed consent becomes not just a legal step but a process of mutual understanding.
Dr. Nanavaty echoed this point. “The majority of the patients attend the private clinic due to 'word of mouth' and so they already have an idea about the outcomes of the person they know, who has referred them to us. But, as a general rule, I go through all the eligible options with the patient in detail so that they can make an informed choice.”
When patients walk in with strong expectations, the consultation becomes more than a clinical assessment. It becomes an educational dialogue. Taking the time for these deeper conversations may be more demanding, but ultimately, it’s key to guiding patients toward the right decision for their individual needs.
You can count on seeing researchers and IOL designers at every ophthalmology conference, ready to showcase the latest advancements in PC-IOL technologies and often backed by a growing volume
of outcomes data aimed at demonstrating improvements in visual performance and patient satisfaction.
With more presbyopia-correcting IOLs moving through development and hitting the market, the question becomes: what kind of data really influences clinical decision-making? We asked our experts what evidence they find most impactful when considering new or alternative PC-IOLs in their own practices.
Naturally, the gold standard remains long-term data from large patient cohorts. As Dr. Nanavaty explained, “Any prospective study on the outcomes of the IOL, at least three months post-surgery is useful. Systematic reviews or metaanalyses of these are even better to get a comparative idea on the outcomes.”
Prof. Romano agreed that controlled studies are critical, but added that real-world performance data is just as valuable. “I prioritize comparative, peer-reviewed studies that combine defocus curves with validated patient-reported outcome measures,” he explained. “However, I’m also attentive to real-world performance, including surgeon-tosurgeon variability, neuroadaptation profiles and explantation rates.”
Today’s PC-IOL market is dominated by a handful of key players: PanOptix and Vivity (Alcon; Geneva, Switzerland), TECNIS Synergy and Symfony EDOF (J&J Vision; Florida, USA), RayOne Trifocal and Galaxy (Rayner; Worthing, UK), AT LISA (Carl Zeiss Meditec AG; Jena, Germany) and FineVision (Bausch + Lomb; Laval, Canada). But what kind of real-world outcomes are patients seeing with these leading lenses?
To find out, Jinyu Li and colleagues from Binzhou Medical University (Shandong Province, China) recently conducted a meta-analysis of 28 randomized controlled
trials assessing the efficacy and safety of various PC-IOLs.3 The findings? As expected, trifocal IOLs outperformed monofocals when it came to uncorrected visual acuity, and both trifocal and EDOF lenses showed advantages at intermediate distances.
One of the most meaningful outcomes for patients is spectacle independence, and the results were impressive. In studies using the AT LISA tri 839MP, 97% of patients were spectacle independent at distance and 80.7% at intermediate. For the AcrySof IQ PanOptix, 83% achieved spectacle independence at near. The authors echoed what our expert contributors also emphasized: successful IOL selection depends on matching lens characteristics with individual patient needs.3
Of course, visual freedom isn’t without trade-offs. Halos and glare continue to be pain points for some patients, largely due to the diffractive design that splits incoming light across multiple focal points. This design challenge, paired with variability in neuroadaptation, can lead to dissatisfaction. “Glare, halos and dysphotopsia still remains a challenge with multifocal IOLs due to the diffractive design,” said Dr. Nanavaty.
Beyond visual side effects, there are still broader design gaps to address. As Dr. Romano pointed out, “We still lack a truly accommodating lens with dynamic, physiological response. Moreover, patient selection remains a challenge, and predicting neuroadaptation and satisfaction is not straightforward. Managing patients with borderline parameters, such as high myopia, irregular astigmatism or previous refractive surgery, remains an area of active learning.”
There’s a wave of innovation coming down the pipeline in presbyopiacorrecting IOLs, with several promising designs and technologies aiming to sharpen vision at all
distances while cutting down on the visual disturbances that can frustrate patients.
Some of the most talked-about advances include modular IOLs like those from LensGen (California, USA), which uses a multi-component design that may one day make optic exchange more straightforward. Others, such as RxSight’s (California, USA) Light Adjustable Lens, enable postoperative power adjustments through targeted ultraviolet irradiation, pushing us closer to more precise refractive outcomes.
The holy grail, of course, is an IOL that restores accommodation to the level of a youthful natural lens. While we’re not quite there yet, we’re edging closer. At ASCRS 2025, buzz surrounded OmniVu (Atia Vision; California, USA), an IOL featuring a front optic set for emmetropia and a fluid-filled base designed to mimic the mechanics of natural accommodation. In the same space, Alcon’s FluidVision lens, built with a hydrophobic acrylic shell and filled with index-matched silicone oil, has shown the ability to dynamically change shape, offering around 2.00 D of accommodation. Meanwhile, JelliSee (Virginia, USA) is developing a monofocal lens that reportedly provides up to 7.00 D of accommodation.
So what are our experts hoping to see in the next generation of PC-IOLs? Unsurprisingly, their top wish is for optics that can offer more individualized predictability while reducing or eliminating common visual disturbances like halos and glare. Dr. Nanavaty shared his outlook, saying, “There will be a time in the future where outcomes can be fully and accurately predicted for each patient with customizable IOLs and least dysphotopsia.”
Prof. Romano also sees a future built on personalization and smarter design. “The future, I think, lies in
greater personalization, reversibility and hybrid optical profiles,” he predicted. “We are likely to see improved light-adjustable and programmable optics, better integration between preoperative diagnostics and AI-powered IOL selection tools, broader platform compatibility across optic designs, and smarter ways to measure and predict patient satisfaction, including psychometric profiling.”
But Prof. Romano also points to a more immediate need: clearer communication. “I believe we need a unified nomenclature. The terminology [EDOF, multifocal, hybrid] remains confusing, both for surgeons and patients. Establishing clear, shared definitions would greatly improve communication and training,” he noted.

Prof. Vito Romano is a professor of Ophthalmology at the University of Brescia, Italy, where he leads clinical research focused on the cornea and ocular surface disease. He is internationally recognized for his contributions to corneal imaging, refractive cataract surgery and surgical education. Passionate about translating evidence-based innovation into everyday clinical care, Prof. Romano continues to shape the future of ophthalmology through both research and practice.
vito.romano@unibs.it

If there’s one thing the future of PC-IOLs doesn’t need, it’s more guesswork. The direction is clear: smarter lens design, more customization and sharper conversations between surgeon and patient. With the right match and managed expectations, presbyopic patients can trade their reading glasses for visual freedom…and you just might get to play matchmaker.
1. Supplement: ESCRS Clinical Trends Survey 2023 Results. ESCRS. September 2024. Available at: https://www.escrs.org/channels/ eurotimes-articles/supplementescrs-clinicaltrends-survey-2023-results/. Accessed on July 28, 2025.
2. Romano V, Madrid-Costa D, Alfonso JF, et al. Recommendation for Presbyopia-Correcting Intraocular Lenses: A Delphi Consensus Statement by the ESASO Study Group. Am J Ophthalmol. 2023;253:169-180.
3. Li J, Sun B, Zhang Y, et al. Comparative efficacy and safety of all kinds of intraocular lenses in presbyopia-correcting cataract surgery: A systematic review and metaanalysis. BMC Ophthalmol. 2024;24(1):172.
Dr. Mayank Nanavaty is a consultant in cataract, cornea and refractive surgery at Sussex Eye Hospital, University Hospitals Sussex NHS Foundation Trust. He also serves as an honorary senior clinical lecturer at Brighton & Sussex Medical School. His clinical interests include medical and surgical cornea, external eye diseases, cataract and refractive surgery. At Sussex Eye Hospital, he leads the cataract, corneal crosslinking, contact lens and research departments.
Dr. Nanavaty has secured numerous research grants from esteemed institutions such as the ESCRS and NIHR, as well as industry partners, to support clinical research within the NHS. His research focuses on astigmatism, wavefront aberrations, ectatic corneal disorders, endothelial disease, lamellar corneal transplants, visual quality in pseudophakia, cataract surgical techniques, intraocular lenses and posterior capsule opacification.
An active academic contributor, he has published extensively in highimpact, peer reviewed journals. He has also delivered over 300 presentations at national and international conferences.
mayank.nanavaty@nhs.net
This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.



A visionary surgeon and mother of four, Dr. Noor Aniah Azmi pairs medical precision with heartfelt compassion to inspire lives—both in and beyond the clinic.
By Chow Ee-Tan
n a field driven by optics, Dr. Noor Aniah Azmi (Malaysia) offers a different kind of clarity. One that blends surgical finesse with soulcentered leadership. As medical director of Ikonik Eye Specialist Centre in Malaysia and a leading cataract and refractive surgeon, she moves with intention through operating theaters, parenting duties and the digital public square. Her work is grounded in technical mastery but powered by meaning.
Whether she’s implanting a phakic IOL, mentoring young surgeons or guiding women in healthcare via social media, Dr. Aniah’s message is consistent: lead with purpose, serve with heart.
Dr. Aniah’s path to ophthalmology didn’t begin in a lecture hall. On just her third day as a medical intern, she assisted in fitting a prosthetic eye for a 75-year-old man from a local nursing home. Though medically routine, the encounter left a lasting emotional imprint.
“At first, I wondered, ‘what’s the point?’ It wouldn’t restore his sight,” she recalls. But as the man looked into the mirror, a radiant smile bloomed across his face. “His posture straightened. He walked out with new confidence. That moment showed me that even small acts in
ophthalmology can restore dignity and self-worth.”
Later, undergoing LASIK herself deepened her appreciation for vision correction. “Being able to wake up and see the world without glasses is liberating,” she shares. “And I wanted others to feel that same freedom.”
Today, Dr. Aniah treats a wide spectrum of patients, from high myopes to those seeking independence from presbyopia. She’s witnessed firsthand how restored vision renews confidence and uplifts lives. “What we do may seem clinical,” she reflects, “but every case has a soul. We must treat both the eye and the spirit.”
Before leading one of Malaysia’s premier eye centers, Dr. Aniah spent years in academia. She earned her MBBCh from Cairo University in 2010, followed by a doctorate in ophthalmology from Universiti Kebangsaan Malaysia (UKM) and a UK postgraduate diploma in cataract and refractive surgery. Her academic tenure at Universiti Putra Malaysia (UPM) allowed her to immerse in research, clinical service and mentorship.
While she found purpose in teaching and research, she felt pulled toward more immediate, hands-on impact.
In 2021, she made the leap to private practice, becoming medical director at Ikonik Eye Specialist Centre. It was a bold pivot from structured academia to the fast-paced, multifaceted demands of surgery and entrepreneurship.
The transition broadened her scope beyond clinical care to business operations, patient engagement, branding and team leadership. “In academia, you train minds. In private practice, you serve hearts and build systems that allow you to scale that service,” she explains.
At Ikonik, Dr. Aniah introduced advanced procedures such as phakic intraocular lenses (IOLs), laser vision correction and multifocal IOL implantation, delivering state-of-theart solutions with a personal touch. She continues to teach through public health seminars, internal staff training and national conferences.
Among the many surgeries Dr. Aniah has performed, one stands apart: operating on her own husband. Already pseudophakic from a previous cataract surgery, he sought her help for presbyopia. She recommended a piggyback multifocal IOL, which required both technical precision and emotional poise.
“It tested my patience, accuracy and focus in a new way,” she admits. “I had to hold both my role as a wife and as a surgeon, each with care.”
Postoperatively, he experienced night dysphotopsia and underwent a period of neuroadaptation, giving Dr. Aniah new empathy for patients adjusting to visual changes.
“Seeing my husband through his recovery revealed to me that healing is a shared journey,” she says. “It taught me that the trust we place in our surgeons is sacred. And it reminded me how every patient, no matter how experienced we are, deserves time, explanation and presence.”
As a mother of four, Dr. Aniah juggles caregiving, clinical leadership and public advocacy—an ongoing
challenge she approaches with discipline, support and intention.
“Private practice has been overwhelming lately,” she confides. “Some days feel like a blur—grief, deadlines, clinic days, parent-teacher meetings, laundry…it all piles up.” Rather than chasing perfect balance, Dr. Aniah strives for ‘purposeful harmony’: setting clear priorities, protecting her energy and cultivating sustainable routines.
Her day begins with prayer, gratitude journaling and mindfulness. Physical movement—Pilates, yoga or core training—is non-negotiable. “If I skip movement, I feel off. Exercise helps me stay calm, focused and ready to serve,” she explains.
Dr. Aniah is also intentional with her time. “At work, I aim for efficiency and focus. At home, I strive to be present and joyful. And I say no to anything that doesn’t align with our family’s vision,” she notes. That same mindset shapes her leadership, rooted in empathy and purposedriven care.
Dr. Aniah’s early life was steeped in spirituality, compassion and a deep commitment to growth. Her mother, also an ophthalmologist, served as a formative role model. She recalls how car rides were soundtracked by religious cassette tapes—subtle moments that instilled lifelong values.
“My parents taught me to think well, speak kindly and live with sincerity,” she reflects. “I believe their examples built the foundation for my career, motherhood and leadership. My father’s quiet support and my mother's strength have continued to shape how I engage with the world.”
And these values now guide her clinical approach, especially in times of uncertainty.
“I never make decisions from fear or ego. When faced with complications, I pause, ground myself and reconnect with my intention to heal. Kindness is a force—it calms anxious patients,
builds trust and opens the way for true healing,” says Dr. Aniah.
In today’s `noisy’ digital world, Dr. Aniah carves out space for calm and clarity. Through Instagram (@draniah) and TikTok (@ draniahazmi), she shares thoughtful content on wellness, mental focus and motherhood, offering encouragement to women in healthcare and beyond.
“I believe women don’t need others to change for them to thrive. They just need systems, support and clarity within. That’s where transformation begins,” she explains.
Her message resonates widely. From television to TikTok, her advocacy has inspired countless followers who see her as a model of authenticity and balance.
As a mentor and leader, she is deeply committed to nurturing the next generation of refractive surgeons. Not just technically, but emotionally and ethically.
“Through my clinical work and my posts on social media, I aim to spark a ripple of kindness, empowerment and intentional living—leaving behind a brighter world, one act of love and meaningful change at a time,” she smiles.
Indeed, Dr. Noor Aniah Azmi embodies a vision of modern medicine where skill meets soul, and where the future of eye care is guided just as much by heart as by hand.

At its core, her message is about intentional living: building a life rooted in purpose, even amid chaos. The feedback she receives from patients and young professionals alike reflects a shared appreciation for her values of joy, simplicity and self-discipline.
“I believe women don’t need others to change for them to thrive. They just need systems, support and clarity within. That’s where transformation begins.”
When asked about the legacy she hopes to leave, Dr. Aniah begins with her children. “I would like to see them pursue their dreams with courage, compassion and selflessness. I hope they live with intention, give with open hearts, and believe that the impossible is always within reach.”
Dr. Noor Aniah Azmi is an inspiring cataract and refractive surgeon, medical director of Malaysia's Ikonik Eye Specialist Centre and a passionate advocate for women's empowerment in medicine. With degrees from Cairo University, the National University of Malaysia, and a postgraduate diploma from the UK, she began her career in academia at Universiti Putra Malaysia before transitioning to private practice in 2021. Since then, she’s embraced advanced techniques like phakic IOLs and laser vision correction, all while raising four children.
Driven by the life-changing impact of vision restoration, Dr. Aniah combines surgical precision with deep compassion. She shares wellness tips and career insights on Instagram (@draniah) and TikTok (@draniahazmi), inspiring working mothers around the world.
A mother, mentor and modern leader, she embodies the balance of clarity, kindness and purpose, proving that ambition and family joy can thrive side by side.
draniah@ikonik.com.my


Forget the hospital gowns and IV drips. Office-based cataract surgery is stepping out of the OR and into the clinic. But before you swap the big theater lights for a streamlined surgical suite, here’s what you need to know to keep safety, efficiency and patient experience in sharp focus.
By Hazlin Hassan
For decades, cataract surgery has been the quintessential operating room (OR) procedure: crisp gowns, bright theater lights, the familiar hum of hospital machinery. But increasingly, some ophthalmologists are trading all that for a more compact stage: the office-based surgical suite (OBS). The shift isn’t just about convenience. It’s about reimagining how, where and why cataract surgery gets done.
Outpatient facilities like ambulatory surgery centers (ASCs) have long
provided an alternative to hospitals, but OBS takes the concept a step further, moving surgery into the physician’s own workspace. Advocates say the benefits go beyond operational efficiency, touching everything from patient comfort to cost control. But detractors point to serious questions. Chief among them: safety.
This isn’t a leap to make on a whim. Moving from the OR to the office requires careful planning, robust infrastructure and a willingness to rethink workflows from the ground up. Let’s explore the motivations, the challenges and the roadmap for those contemplating this next frontier.
Control is the word that comes up most often. “Surgeons can control the patient experience, control costs, control scheduling and control technology,” said Dr. Lance Kugler, a pioneer in office-based surgery and CEO of Kugler Vision in Nebraska (USA). “Patients, in turn, benefit from streamlined care, familiar staff and environment, and shorter recovery times.”
The experience itself is notably different from the traditional OR. “Patients do not feel like they are in a medical facility having a major intervention. They can eat and drink before their procedure. They do not change into a gown or start an IV. Most of the usual signals that surgery is about to happen are not there,” Dr. Kugler explained. “It is much more of a LASIK-like experience, with less emotional stress.”
From a practice management perspective, OBS allows ophthalmologists to set their own schedules without jockeying for OR time, standardize processes across their team and reduce percase costs. For patients, it removes layers of formality and perceived intimidation.
For all the appeal, safety remains the unavoidable sticking point. Cataract surgery ranks sixth in intraservice work per unit time (IWPUT) among more than 3,000 surgical procedures, according to Dr. Frank Cotter of Vistar Eye Center (Virginia, USA). That
high procedural intensity demands readiness for unexpected challenges like iris prolapse, shallow chambers and sudden patient anxiety.
“Surgeons performing procedures requiring this level of intensity need anesthesia support to manage any problems that arise,” Dr. Cotter emphasized.
He also highlights structural differences between OBS suites and facilities like ASCs. “OBS suites are certified as offices. One example of the differences is that OBS suites are not required to have a backup generator. What happens if the power goes out mid-case?” he asked.
Still, proponents like Dr. Kugler point to strong evidence supporting OBS safety when implemented with rigor. His multicenter study of more than 21,000 office-based cases showed adverse event rates comparable to, or better than, those reported for traditional OR-based cataract surgery.1
Similarly a 2016 study—the largest U.S. investigation of office-based cataract surgery performed in minor procedure rooms—reported consistently excellent efficacy outcomes and a safety profile in line with ASC and hospital outpatient department (HOPD) performance.2
“Our study, and others since then, have demonstrated that the safety profile of office-based lens surgery either matches or exceeds the literature-reported values of adverse events documented for modern cataract surgery,” said Dr. Kugler.
The key takeaway? OBS can be safe, but only with the right infrastructure, protocols and clinical judgement.
Despite its name, office-based surgery requires a purpose-built environment. “OBS suites are full operating theaters specifically designed for the procedures performed in them, with the same or better ocular standards as those found in an ASC,” said Dr. Kugler. “They should follow the same ophthalmic safety standards, protocols, sterility and infection control as an ASC or HOPD.”
Specialist firms like iOR Partners stress the importance of designing a space that meets those requirements. This means HVAC systems with laminar airflow, appropriate lighting, sterile processing facilities and contingency systems for emergencies. The upfront investment can be significant, but so is the payoff in safety and efficacy.3
Transitioning to OBS changes staffing needs. Existing clinic teams may not have the surgical training required for sterile processing, circulating nurse duties or intraoperative support.
“We worked with iOR Partners to train our clinic staff for essential surgical roles,” said Dr. Kugler. “That includes pre/post-op care, sterile processing, procedure tech and circulator responsibilities.”
Anesthesia management is another area of debate. While OBS models rely on oral sedation only, Dr. Cotter warns against underestimating the value of professional anesthesia support.
“Depriving patients of the support offered by a nurse anesthetist is not good medicine. Fully informed patients would not choose to have surgery without anesthesia support,” he said.
Even if complication rates are low, having anesthesia expertise on hand provides a critical safety net. One that can be the difference between a smooth case and a crisis.
Switching to OBS is not about cutting corners. It’s about building a new, streamlined surgical environment that holds itself to the same standards as the OR. For practices considering the move, here’s a strategic sequence to follow:
1. Feasibility Assessment
Analyze patient volume, procedure mix and regulatory conditions. Consult advisors familiar with OBS regulations and reimbursement.
2. Space Planning
Design or retrofit a surgical suite with compliant HVAC, lighting, sterilization and safety systems, including emergency protocols.
3. Technology Procurement
Acquire ophthalmic surgical equipment, integrate with existing EMR systems and ensure seamless diagnostic-to-treatment workflows.
4. Staff Training
Retrain clinic staff or hire surgical personnel with the necessary skills. Establish standard operating procedures for all aspects of care, including sterility, sedation and emergencies.
5. Anesthesia Support
Define sedation protocols. Secure reliable anesthesia professionals for appropriate cases.
6. Accreditation & Licensing
Partner with recognized accrediting bodies to meet safety, sterility and insurance requirements.
7. Trial Run
satisfaction and more control over surgical delivery. And while the OR will always have its place, for some ophthalmologists the future might just be…down the hall.
1. Kugler LJ, Kapeles MJ, Durrie DS. Safety of office-based lens surgery: U.S. multicenter study. J Cataract Refract Surg. 2023;49(9):907-911.
2. Ianchulev T, Litoff D, Ellinger D, Stiverson K, Packer M. Office-Based Cataract Surgery: Population Health Outcomes Study of More than 21 000 Cases in the United States. Ophthalmology. 2016;123(4):723-728.
3. Key Considerations for Transitioning to an Office-Based Surgery Suite. iOR Partners. 2024. Available at: https://iorpartners.com/ blog/considerations-for-in-office-cataractsurgery. Accessed on August 11, 2025.

Conduct simulations or pilot cases with backup systems in place. Refine processes before scaling.
8. Launch & Monitor
Begin with low-risk cases, track outcomes rigorously and adjust protocols as needed.
Office-based cataract surgery isn’t the surgical Wild West. It’s more like opening a well-run boutique next to a bustling department store. Done right, it offers patients a calmer, more personalized experience while giving surgeons greater autonomy over their work.
Founder and CEO of Kugler Vision in the United States, Dr. Lance Kugler recognized early on that office-based surgery was the future of ophthalmology and embraced it wholeheartedly. His practice focuses exclusively on refractive surgery. He is involved in refractive surgery nationally and internationally through many organizations and serves as the North American Regional Chair for the World College of Refractive Surgery. His fellowship in refractive surgery is helping to train the next generation of refractive surgeons.

But autonomy brings responsibility. OBS success depends on replicating OR-level safety and sterility within a smaller footprint, supported by a team trained to handle the unexpected.
For forward-looking practices, the payoff can be significant: improved workflow, enhanced patient
Dr. Frank Cotter is the medical director at Roanoke Valley Center for Sight and an ophthalmologist at Vistar Eye Center in the United States, specializing in glaucoma and cataract surgery. He completed his Doctorate of Medicine at Georgetown University and went on to do his residency at Walter Reed Army Medical Center. He also undertook two fellowships with a focus on glaucoma at the University of Virginia, and the Medical College of Virginia.
fcotter@vistareye.com

Crafting clear vision isn’t always about a perfect match—it’s about the right mix. In the hands of today’s surgeons, IOL pairings are shaken, stirred and tailored to taste.
By Kendra Bruning
In refractive cataract surgery, symmetry isn’t always the secret to satisfaction. Like a master mixologist balancing bitter and sweet, today’s surgeons are blending intraocular lens (IOL) technologies to craft custom visual cocktails that are designed not for flavor, but for clarity, contrast and spectacle freedom.
Some eyes call for distance. Others demand near. And a growing number of surgeons are reaching for more than one bottle behind the bar.
“The goal is a customized plan that protects contrast where it is most vulnerable while preserving as much spectacle freedom as possible,” says Dr. J. Morgan Micheletti, partner and fellowship director at Berkeley Eye Center (Texas, USA).
With today’s trifocals and nondiffractive extended depth of focus (EDOF) platforms, surgeons now have a full array of options to mix and match to accommodate their patients' unique needs.
For patients with dry eyes, irregular corneas or demanding night vision needs, the standard recipe sometimes needs adjusting. That’s where optical mixology begins: a touch of EDOF in one eye, a splash of trifocal in the other. Like a wellbalanced cocktail, each component in a pairing brings out the best in the other, smoothing over potential drawbacks.
Among premium IOLs, bilateral trifocals are still considered the gold standard. Dr. Micheletti’s first choice is often two matched continuousrange-of-vision (trifocal) lenses, which he notes “deliver roughly 90% to 93% spectacle independence, as seen in multiple studies and metaanalyses.”
But even a well-aged single malt isn’t for every palate.
Dr. Micheletti steers away from trifocals when contrast sensitivity might be compromised, such as in patients with subtle postLASIK irregularities, mild epiretinal membrane or symptomatic dry eye. He also adjusts his approach for patients especially sensitive to dysphotopsias or those whose professions require superior night vision.
In these cases, he starts with a nondiffractive EDOF lens in the dominant eye and waits a week. At the followup, he asks one key question: "Are you satisfied with your near vision?" That answer determines the second lens. If the patient is content with near vision, the same lens is used in the second eye. If more near acuity is needed, he adds a trifocal. When the non-dominant eye has pathology, he simply reverses the order.
Recent data support this strategic mixing. A 2025 review in the Journal of Clinical Medicine found that mixing an EDOF lens with a bifocal or trifocal IOL can equal, or even outperform, bilateral trifocals at intermediate distances, while reducing visual disturbances.1
"Comparative studies suggest that a thoughtful mix-and-match strategy can achieve near acuity comparable
to bilateral trifocals while generating fewer dysphotopsias," Dr. Micheletti explains.
Whether he’s combining EDOF with trifocal optics or tailoring to a patient’s pathology, his guiding principle remains: protect contrast, preserve range.
Today’s IOL lineup reads like a wellstocked back bar: trifocals with high add power, smooth-sipping EDOF lenses and monofocals that play well with others.
“The biggest advance for mixing strategies has been the development of non-diffractive EDOF optics,” says Dr. Micheletti. These lenses—such as the Clareon Vivity (Alcon; Geneva, Switzerland), PureSee (Johnson & Johnson Vision; Florida, USA) and enVista Envy (Bausch + Lomb; Laval, Canada)--offer distance and intermediate clarity with a visual disturbance profile closer to monofocals, making them ideal for pairing with trifocals or other IOLs.
Meanwhile, trifocal platforms continue to improve. In Presbyopia Physician, Dr. Blake Williamson (USA) described excellent near acuity with the TECNIS Synergy (Johnson & Johnson Vision) in nondominant eyes, crediting its high add power and violet-light filter for achieving “best-in-class” results.2
He also found that pairing Synergy with an EDOF lens like the TECNIS Symfony OptiBlue (Johnson & Johnson Vision) gives patients the best of both worlds: improved contrast and reliable near vision. Other go-to combinations include Vivity with PanOptix (Alcon) and Symfony with ZLB00 (Johnson & Johnson Vision).3
These aren’t haphazard pairings. “In my practice,” said Dr. Williamson, “my happiest patients are those for whom I’ve mixed and matched different lens technologies.”2 Evidence backs him up. Customized IOL strategies can match or outperform bilateral monofocals
across visual parameters, with especially strong performance at the key 40 cm near reading distance.1
Like any good cocktail, not every mix suits every palate. "True red flags are uncommon because the hybrid concept is itself a compromise," notes Dr. Micheletti. Still, some patient profiles require closer scrutiny.
Low-to-moderate myopes used to crisp unaided near vision can be especially tricky. They often have high expectations and low tolerance for reduced near acuity. “Success often hinges not on the lens, but on the patient’s psychology,” Dr. Micheletti adds.
Dr. Cullen Ryburn (USA) agrees. While patients with previous monofocal IOLs or ocular pathology can still benefit from selective mixing, it takes honest counseling and careful planning.3 He’s seen success in amblyopia and irregular astigmatism cases using smallaperture lens or EDOF lenses in one eye and monofocal in the other.
Patient satisfaction often depends more on communication than complexity. "If the plan is set from the start—because of pathology or clear preference—the counseling is similar to bilateral trifocals," says Dr. Micheletti. When taking a staged approach, he spends extra time explaining the rationale.
thoughtfully blended approach offers surgeons another way to tailor outcomes to the individual in the chair.4
It's not about loyalty to any one method, Dr. Micheletti adds, it's about using “any combination that best serves the patient's ocular status and visual goals”.
As the era of optical mixology matures, success comes down to knowing what to mix and when to blend, as well as who will benefit most from a customized approach.
1. Naujokaitis T, Łabuz G, Khoramnia R, Auffarth GU. Review of mix-and-match approach and binocular intraocular lens systems. J Clin Med. 2025;14(12):4263.
2. Williamson BK. Mixing and matching IOLs: a new normal. Presbyopia Physician. 2022 Dec. Available from: https://www. presbyopiaphysician.com/issues/2022/ december/mixing-and-matching-iols-a-newnormal/. Accessed on July 25, 2025
3. Stodola E. Mixing and matching IOLs. EyeWorld. April 2023. Available from: https://www.eyeworld.org/2023/mixing-andmatching-iols/. Accessed on July 25, 2025.
4. Micheletti JM, Walter KA. The art of mixing and matching. CRST. 2022 Feb. Available from: https://crstoday.com/articles/feb-2022/ the-art-of-mixing-and-matching. Accessed on July 25, 2025.

Like a signature cocktail, a successful IOL mix calls for precision, patience and a practiced hand.
“It’s like ordering surf and turf,” says Dr. Micheletti, quoting Dr. David Chang (USA). “You’re combining the strengths of both options, rather than potentially doubling down on the weaknesses of one.”
While bilateral trifocals remain the standard pour for many, a
Dr. J. Morgan Micheletti is a board-certified ophthalmologist and director of research at Berkeley Eye Center, where he specializes in advanced cataract, refractive and glaucoma surgery. He has performed over 10,000 procedures and serves as a medical advisor to more than 20 companies. A prolific innovator with six surgical device patents pending, Dr. Micheletti has received numerous awards, including the 2024 Outstanding Young Texas Ex Award and IIRSI Gold Medal. He also hosts The History of Eyecare podcast and serves on editorial boards for multiple ophthalmology publications.
morgan.micheletti@gmail.com

Photo: Kniazeff/Shutterstock.com
When cataracts collide with complex eyes, the strategy is survival. This ASCRS 2025 symposium served as the ultimate battle plan for tricky surgeries.
By Elif Uslu
Cataract surgery gets a lot trickier when other eye diseases join the party—and that’s exactly what the Cataract Crossover Symposium tackled on Day 3 at the 2025 Annual Meeting of the American Society of Cataract and Refractive Surgery (ASCRS 2025).
Moderated by a powerhouse trio— Drs. Cathleen McCabe (USA), Marjan Farid (USA) and Zaina Al-Mohtaseb (USA)—the session explored how to navigate cataracts when the patient brings along lid abnormalities, retinal disease, glaucoma, neuro-ophthalmic challenges or corneal disorders.
From pre-op planning to post-op curveballs, the session was packed
with practical pearls for tackling complex eyes
Prof. Dr. Seanna Grob (USA) turned the spotlight on an often-overlooked player: the eyelids. As she reminded the crowd, “The eyelids in orbit are really the protectors of the globe. If you don’t have the eyelids, the cornea will just kind of melt.”
From ptosis to facial nerve palsy and thyroid eye disease, she showed how periocular anatomy can quietly sabotage what looks like a straightforward cataract case. Even something seemingly
minor, like ptosis, can skew your pre-op measurements. “If ptosis is significant, it may actually affect your preoperative calculations,” Dr. Grob warned.
And post-op? Surgeons should stay alert. Ptosis after surgery isn’t rare— rates can range from under 5% to as high as 30%. Bottom line: success isn’t just about the lens; it’s about respecting the whole ocular surface system.
Patients with neuro-ophthalmic disease
Dr. Vivek Patel (USA) then brought the room to full attention as he unpacked one of cataract surgery’s trickiest terrains: patients with neuroophthalmic disease.
With his engaging style, Dr. Patel walked the audience through case after fascinating case, showing that not all vision loss is about the cataract—and why surgeons need to dig deeper.
First up: patients with optic nerve damage from multiple sclerosis or past optic neuritis. Even if the cataract is obvious, “OCT of the optic nerve and ganglion cell analysis
can be super helpful,” he advised. Thinning of the ganglion cell layer, particularly below 75 microns, can hint at permanent visual field loss, even if the lens looks like the main culprit.
Then came a show-stopping reminder: visual fields still matter. In one case, a seemingly ordinary cataract patient actually had a massive prolactinoma, caught only thanks to a suspicious visual field test and subtle ganglion cell layer changes.
Dr. Patel also tackled the much-debated question over cataract surgery’s increasing risk of ischemic optic neuropathy (ION). His take? Modern surgical techniques have significantly lowered that risk, but a careful conversation with patients, especially those with “disc-at-risk” anatomy, is still essential.
Glaucoma patients deserve more
Dr. Zarmeena Vendal (USA) didn’t hold back when diving into the complexities of cataract surgery in glaucoma patients. She first walked through her approach to lens selection: EDOF lenses like Alcon’s (Geneva, Switzerland) Vivity and Johnson & Johnson Vision’s (Florida, USA) TECNIS Symfony are strong choices for mild to moderate glaucoma cases, providing crisp distance and functional intermediate vision without sacrificing contrast sensitivity. She’s also a big fan of RxSight’s (California, USA) Light Adjustable Lenses (LAL) for patients with tricky biometry.
But for Dr. Vendal, mastering MIGS is no longer optional. “If we’re going to dabble in the world of advanced technology lenses, it behooves us to absolutely also be comfortable doing MIGS at the same time,” she noted.
With smart strategy, Dr. Vendal argued, we can give glaucoma patients freedom from glasses and
better control over their disease—all at once.
Watch out: retinal trouble can hide
When it comes to cataract surgery in patients with retinal disease, Dr. Mitul Mehta (USA) didn’t mince words: start with a pre-op OCT or prepare for trouble. “Sometimes the cataract is pretty bad and you can’t see subtle pathology,” he said, urging surgeons not to skip that crucial imaging step.
Dr. Mehta walked through cases where multifocal lenses were mistakenly implanted in eyes with hidden retinal problems like macular traction and epiretinal membranes— mistakes that could have been caught early with an OCT. “Multifocal lenses are a no-go zone for retinal pathology,” he noted.
For patients with stable mild disease, Dr. Mehta advocated for careful choices like EDOF lenses or LALs, depending on the individual eye. And when it comes to diabetic retinopathy, even without macular edema, pre-op anti-VEGF injections can be smart insurance.
Dr. Mehta’s advice? Show patients their OCTs, manage expectations upfront and don’t assume the retinal status just by slit lamp exam. “If you don’t look, you’re not going to know,” he said, reminding the audience that retinal diseases can sneak up silently, and post-op surprises are no fun for anyone.
Closing the symposium, Dr. Zeba Syed (USA) tackled cataract surgery when the cornea isn’t playing nice. “Corneal diseases do affect IOL selection. Cataract surgery has the potential to contribute to progression of preexisting corneal diseases…and of course, it limits visual outcomes and affects our counseling.”
Dr. Syed took the audience on a fastpaced tour through common corneal challenges, starting with dry eye syndrome, which she called a major culprit in bad biometry.
“We know that it affects the topography. It prevents precise keratometry readings for accurate IOL calculation,” she explained, emphasizing the risks when planning for premium lenses.
Dr. Syed also shared pearls on anterior basement membrane dystrophy (ABMD), stressing the importance of treating irregular astigmatism early—and waiting three months post-treatment before biometry.
For HSV keratitis, Dr. Syed recommends a three-month inactivity period before surgery and starting prophylactic antivirals. In keratoconus, she flagged the importance of stability checks and cautious use of toric lenses.
When tackling Fuchs’ dystrophy, she often pairs cataract surgery with DMEK and always plans for a hyperopic shift after endothelial transplants.
The future of cataract surgery is crossover
The Cataract Crossover Symposium wasn’t just another stop on the ASCRS 2025 schedule, it was a rallying cry for surgeons ready to raise the bar. Whether it’s lids, nerves, retinas or corneas, the old rules no longer apply. Today’s cataract surgery demands fearless planning, smarter tools and a new level of clinical swagger. If you’re not crossing specialties, you’re falling behind. The future is complex, and it’s already here.
Reporting for this story took place during the annual meeting of the American Society of Cataract and Refractive Surgery (ASCRS 2025) being held from 25-28 April in Los Angeles, California, United States. A version of this article was first published on cakemagazine.org.
Latest data shows TearCare delivers nearly two years of sustained dry eye relief after only two treatments.
The Phase III results from Sight Sciences’ (California, United States) Sahara randomized controlled trial are now in, and the results are telling.
Published in Optometry and Vision Science, the study reports that after just two TearCare treatments within the first five months, patients achieved clinically significant gains in tear stability, gland function and symptom relief that lasted close to two years, with most never needing another round.1
The Sahara trials at a glance
Sahara is one of the largest and most rigorous evaluations to date of a device-based therapy for meibomian gland dysfunction (MGD)-related dry eye. Conducted across 19 U.S. sites, the Phase III study followed patients with moderate-to-severe disease for over two years.
The trial unfolded in three acts. In Phase I, TearCare localized heat therapy with manual gland expression was compared with cyclosporine ophthalmic emulsion (Restasis; AbbVie, Illinois, United States). TearCare not only improved tear stability more effectively but also demonstrated non-inferior symptom relief.
In Phase II, patients originally assigned to cyclosporine crossed over to TearCare and experienced similar gains.
Phase III extended follow-up for patients who had received two TearCare treatments, assessing the durability of benefits over the long term.
At baseline, patients had unstable tears with an average tear breakup time (TBUT) of 4.4 seconds. After two TearCare sessions, TBUT improved to around seven seconds and remained significantly above baseline through 24 months. Meibomian gland secretion scores nearly tripled after the initial treatment and stayed elevated.1
On the Ocular Surface Disease Index (OSDI), patients improved by about 18 points after the second treatment and maintained a benefit of roughly 14 points above baseline at two years. This exceeds the threshold for clinically meaningful improvement and translates into noticeable relief in day-to-day life.1
Durable outcomes with minimal retreatment
Two-thirds of patients never required another TearCare session. Among those who did, the median time to retreatment was eight months. And even then, outcomes stayed significantly better than baseline.1
Over 19 months, adverse events were rare and unrelated to TearCare. No changes in visual acuity or intraocular pressure were reported.1
TearCare is a device-based therapy that delivers precisely controlled heat (45°C for 15 minutes) to the eyelids via SmartLids, followed by manual expression of the glands.
This approach directly targets the obstructed meibomian glands that drive evaporative dry eye disease.
Unlike warm compresses at home—which can be a hit-ormiss—TearCare is designed to offer precise temperature control and professional clearance of the glands, ensuring both consistency and efficacy.
The Sahara Phase III trial offers some of the strongest evidence to date that an office-based, devicedriven therapy can deliver sustained relief for patients with MGD-related dry eye.
For clinicians, the durability means fewer retreatments and a practical frontline option. For patients, it means nearly two years of meaningful comfort from only two treatment sessions.
Most importantly, TearCare’s performance suggests a shift in dry eye care: away from short-term symptom management and toward lasting restoration of gland function.
In short? TearCare may just be the device that helps turn dry eye treatment from a revolving door into a long-term solution.
Reference
1. Hovanesian JM, Ayres BD, Bloomenstein MR, et al. Durability of the TearCare treatment effect in subjects with dry eye disease: Stage 3 of the Sahara randomized controlled trial. Optometry and Vision Science. 2025;102(8):495-504.
Editor’s Note: A version of this article was first published on cookiemagazine.org









