






As 2025 comes to a close, we bring you the 37th issue of PIE. This issue presents, first of all, the “heavyweights” in the vitreoretinal space such as how both clinical trial data and real-world evidence of aflibercept 8 mg are supporting longer treatment intervals for neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) patients; how to balance the retinal effects of GLP-1 drugs; exploring the promising role of ERG in predicting vision-threatening diabetic retinopathy (DR); how widefield OCT-A is transforming our understanding of DR; and the practical ways to make sustainability simple in the retinal space.
In addition, we are revisiting equally important topics… including, image biomarkers in exudative AMD, how to tackle diabetic eye disease beyond anti-VEGF monotherapy and the emerging approaches that target the neurodegenerative core in MacTel.
We also bring you a piece of the evolving science and strategy of endophthalmitis management and how inherited retinal disease (IRD) therapies are moving from lab to clinic at unprecedented speed…because why not? Endophthalmitis and IRDs are, after all, topics closest to the heart of the vitreoretina subspecialty.

Beyond these clinical deep dives, the Women in Ophthalmology (WIO) spotlight is on a phenomenal woman— Dr. Maria Berrocal—who blends wisdom, world-class science and a Caribbean spirit to keep retina care grounded and always moving forward.
We also got a unique look at the DOG (Deutsche Ophthalmologische Gesellschaft) [a.k.a. German Society of Ophthalmology] congress experience through the eyes of the first Filipino invited to speak at an all-German ophthalmology event—our regular contributor, and always a trailblazer, Dr. Perfecto Cagampang III. Finally, this issue, of course, will not be complete without coverage from major meetings this year: EURETINA (Paris) and AAO (Orlando), so kindly check those out too in the Conference Highlights section.
Most importantly, we are bringing PIE 37 to the 18th Congress of the Asia-Pacific Vitreo-Retina Society (APVRS 2025) in Manila, Philippines on December 12 to 14. Limited copies are available at the Media MICE booth #M5-6.
The Cover Story (page 24) on how real-world evidence is reshaping ophthalmology, offering practitioners insights that randomized trials alone cannot, featuring Prof. Nicole Eter (Germany), a powerhouse in ophthalmology, is definitely a must read. Prof. Eter shared valuable insights from her combined clinical expertise, and cutting edge research and leadership. As former president of the DOG and current chair of the Association of Ophthalmological Chairholders, Prof. Eter continues to shape the future of eye care in Germany.
See you soon, Pilipinas! Mabuhay.

Sincerely,
Gloria D. Gamat
Chief Editor
| PIE, CAKE and COOKIE
Head, Editorial Department | Media MICE

Dr. Alay S. Banker
Banker’s Retina Clinic and Laser Centre Ahmedabad, India alay.banker@gmail.com

Dr. Arshad Khanani
Sierra Eye Associates; University of Nevada, Reno School of Medicine Nevada, USA arshad.khanani@gmail.com

Dr. Barbara Parolini
Eyecare Clinic Milan, Italy parolinibarbara@gmail.com

Prof. Gemmy Cheung
Singapore National Eye Centre (SNEC) Singapore gemmy.cheung.c.m@singhealth.com.sg

Dr. Hudson Nakamura
Bank of Goias Eye Foundation Goiânia, Brazil hudson.nakamura@gmail.com

Dr. Veeral Sheth
University Retina and Macula Associates; University of Illinois at Chicago, USA vsheth@gmail.com



Beyond
How
Functional Red Flags ERG’s role in predicting vision-threatening DR
When
Seeing is Believing?
Reassessing imaging biomarkers in exudative AMD
Eye Spy Infection
The science and strategy of endophthalmitis management
From Rare to Repair
Inherited
Beyond the Vessels
Targeting the neurodegenerative core in MacTel


Of Evidence, Empathy and Excellence
Dr. María Berrocal blends wisdom, world-class science and a Caribbean spirit to keep retina care grounded and always moving forward
Panoramic Precision
How widefield OCT-A is expanding the view of diabetic retinopathy

The DOG Experience
What started as a simple case submission turned into a DOG debut, a scholarship surprise and a proud first for Philippine ophthalmology.
The Slow Burn of Dry AMD
This dry AMD session outlined hidden risks, trial bottlenecks and the growing promise of precision.
How to Move From Hype to Practice With AI in Retina Care
Can VR make vision tests easier, or chatbots lighten clinic load? EURETINA 2025 offered bold answers.
Breaking the Injection Habit
Smarter retinal drug delivery unveiled at EURETINA 2025
Sheets, Chips & Switches
Practical paths to vision restoration





How clinical trial data and real-world evidence support longer treatment intervals for nAMD and DME patients
By Brooke Herron
At the 25th Congress of the European Society of Retina Specialists (EURETINA 2025), experts gathered to discuss what every ophthalmologist really wants to know: Does aflibercept 8 mg perform as impressively in real life as it does in clinical trials?
Clinical trial data shows that aflibercept 8 mg delivers faster, longer and better drying1-8 than aflibercept 2 mg, while also extending durability1,2 and sustaining meaningful vision gains1,2 for eligible patients with neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) patients.
But how does that translate once you leave the pages of a trial report and step into the clinic?
During the Day 2 symposium chaired by Miss Clare Bailey, Bristol Eye Hospital (UK), From Data to Decisions: What Do We See in the Real World?, Prof. Andrew Chang, Sydney Eye Hospital (Australia); Dr. John Kitchens, Retina Associates of Kentucky (United States); and Dr. Aude Ambresin, Swiss Visio Montchoisi Private Retina Center (Switzerland) explored just that, weaving together clinical trial evidence and real-world patient cases.
Aflibercept’s multi-targeted approach and unique mechanism of action has been shaping retinal care for more than a decade. Now, aflibercept 8 mg is stepping up as a potential answer to some of the field’s biggest unmet needs9, said Prof. Chang. Compared with aflibercept 2 mg, the 8 mg formulation delivers a four-fold higher molar dose, has a 34% slower ocular clearance and offers VEGF suppression lasting six weeks or longer.10
Dr. Kitchens pointed out that the PULSAR and PHOTON extension studies show how aflibercept 8 mg can help reduce treatment burden across the board, regardless of a patient’s prior injection schedule.3,4
Both studies observed that aflibercept 8 mg has a comparable safety profile and similar visual outcomes as the 2 mg formulation for patients with nAMD and DME, while stretching the intervals and improving outcomes related to drying. By week 156, four in 10 patients with nAMD and five in 10 with DME had reached treatment intervals of five months or more. Some needed as few as two injections in the third year, while vision gains held steady through the year.3,4
The Phase II CANDELA study added another layer of evidence. Compared with aflibercept 2 mg, the higher dose delivered numerically greater improvements in anatomical and visual outcomes. That included a high proportion of eyes achieving fluid-free status in the central subfield [50.9% (n = 27) vs 34.0% (n = 18)] and stronger best corrected visual acuity (BCVA) gains [+7.9 (1.5) vs +5.1 (1.5) letters].11
“CANDELA showed that aflibercept 8 mg dramatically reduced (95%) intraretinal fluid (IRF) in patients with nAMD at Week 44,” said Prof. Michael Stewart (United States), during the Evolving Practice in Retinal Care: The Journey From Lab to Patient symposium on Day 1 of EURETINA 2025. This matters because a reduction in fluid
biomarkers is linked to improved anatomical stability for the patient and improved durability with confidence for physician, patient and caregiver.
The CUREOS Research Network put aflibercept 8 mg to the test outside of the clinical trial bubble, evaluating its early real-world efficacy and safety profile in 44 treatment-naive and 164 previously treated eyes across six Australian sites. According to Prof. Chang, CUREOS showed what the trials hinted at: aflibercept 8 mg delivers visual and anatomical improvements, supports extended treatment intervals and maintains an acceptable safety profile in patients with nAMD.12
“After initiation of aflibercept 8 mg, treatment-naive patients had a 10-letter gain by month 6 which parallels what we saw in PULSAR,” said Prof. Chang. “We started to extend the interval almost after the first injection and by month 6, about 40% of patients were treated at 10 weeks or longer.”
Eyes switched to aflibercept 8mg from other anti-VEGFs also experienced extended treatment intervals without compromising treatment outcomes. As Prof. Chang noted, “About two-thirds that were switched from another anti-VEGF to aflibercept 8 mg were being treated at longer intervals by month 6 without compromising treatment outcomes.”
Currently, aflibercept 8 mg is the only intravitreal agent approved for intervals as long as six months, with just three loading doses for eligible nAMD and DME patients. For Prof. Chang, this durability is more than a convenience. It could ease the load for patients, caregivers and physicians alike.
Adding another layer, Miss Clare Bailey of Bristol Eye Hospital (United Kingdom) presented results from SPECTRUM, a real-world observational study exploring fluid outcomes with aflibercept 8 mg in nAMD. According to SPECTRUM cohort data from Bristol Eye
Hospital, patients achieved early drying, with two-thirds showing no IRF or subretinal fluid (SRF) just eight weeks after their third loading dose. Impressively, one-third of patients were holding steady at Q16.13
These studies show the potential of aflibercept 8 mg to “shift the curve” for patients and physicians in everyday practice.
Simply put, “shifting the curve” means helping eligible nAMD and DME patients—often those with a higher treatment burden and frequent injections—move to extended regimens that require fewer injections. That translates to fewer clinic visits for patients and more breathing room in physicians’ schedules.
Prof. Chang, Dr. Kitchens and Dr. Ambresin shared outcomes from several real-world patients treated with aflibercept 8 mg, illustrating how they’ve managed to “shift the curve” in their clinics. Here’s a look at a few examples.
• Previously treated patient with nAMD (PCV)
• BL visit: One week after treatment with faricimab 6 mg
• Two weeks after faricimab 6 mg injection, patient switched to aflibercept 8 mg, then was extended to Q4 → Q7 → Q8, with Q10 planned
• At week 26, BCVA was 80 ETDRS letters (+34 from BL) and CST was 206 µm (-453 µm from BL)
“This patient was referred after a macular hemorrhage. We injected her with 8 mg aflibercept because of the initial drying effect. Following the switch to aflibercept 8 mg, this patient achieved rapid and meaningful vision improvement and resolution of hemorrhage that was



maintained over 8 months,” said Prof. Chang.
65-year-old female: Shift to Q10
• Treatment-naive patient with bilateral DME
• BL visit: Initiated on aflibercept 8 mg
• Three loading doses (Q4) + one additional Q4 injection → moved to Q8 at week 12, maintained until week 44, with Q10 planned.
• At week 44: CST was 259 µm (-241 um from BL) OD, 257 µm (-247 µm from BL) OS; BCVA 80 ETDRS letters (+10 from BL) OD and 70 ETDRS letters (+20 from BL) OS
“Following the switch to aflibercept 8 mg, this patient achieved vision and anatomic improvements with interval extensions in both eyes. The patient will continue on aflibercept 8 mg Q8 for two more visits before extending to Q10,” said Dr. Kitchens.
93-year-old female: Shift to Q16
• Treatment-naive patient with nAMD
• BL: Initiated on aflibercept 8 mg
• Three loading doses (Q4) → extended directly to Q16, maintained through week 44.
• After five injections: BCVA 70 ETDRS letters (+10 from BL); CST 165 µm (-105 µm from BL)
management, said Dr. Ambresin. One such tool is Bayer’s RetinAI Discovery, an image and data management platform powered by AI models that aggregate large datasets and deliver insights into patient outcomes.14
Dr. Ambresin explained that RetinAI Discovery can analyze data to identify biomarkers and quantify retinal fluid, extracting highresolution OCT features including fluid volume (e.g., IRF, SRF and PED) and area (e.g., HRF, RPE loss).
She then detailed real-world outcomes from a retrospective analysis conducted with RetinAI Discovery. The study included 99 nAMD patients who switched from faricimab 6 mg to aflibercept 8 mg and were evaluated using more than 2,000 OCT acquisitions from the US-based Retina Consultants of America RWE database.15
The analysis revealed that nAMD patients who switched to aflibercept 8 mg had numerically longer treatment intervals while achieving fluid reduction comparable to those achieved with faricimab 6 mg. Fluid control was maintained across both treatments, with aflibercept 8 mg offering the advantage of extended intervals.15
Prof. Stewart, who also shared this study during his Day 1 presentation, added that patient intervals were extended once they were switched to aflibercept 8 mg.
1. Lanzetta P, Korobelnik JF, Heier JS, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, doublemasked, non-inferiority, phase 3 trial. Lancet. 2024;403(10432):1141-1152.
2. Brown DM, Boyer DS, Do DV, et al; PHOTON Investigators. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, doublemasked, non-inferiority, phase 2/3 trial. Lancet. 2024;403(10432):1153-1163.
3. Wong TY. Three-year outcomes of aflibercept 8 mg in nAMD: safety and efficacy results from the PULSAR extension study. Presented at Angiogenesis, Exudation, and Degeneration 2025 Virtual Edition; 8 February 2025, Bascom Palmer Eye Institute, University of Miami School of Medicine, Miami, FL, USA.
4. Brown D. Aflibercept 8 mg in diabetic macular edema: 156-week results from the PHOTON Extension study. Presented at The Macula Society 48th Annual Meeting, 12–15 February 2025, Charlotte Harbor, Florida, USA.
5. Spitzer M. ARVO 2023. 23–27 April 2023. New Orleans, USA.
6. Do DV. Angiogenesis 2025. 8 February 2025. Virtual.
7. Brown DM. Macula Society 2025. 12–15 February 2025. Charlotte Harbor, USA.
8. Khurana RN. Macula Society 2024. 7–10 February 2024. Palm Springs, USA.
9. Korobelnik JF, Lanzetta P, Wykoff CC, et al. Sustained disease control with aflibercept 8 mg: a new benchmark in the management of retinal neovascular diseases. Eye (Lond). 2024;38(17):3218-3221.
10. Kaiser PK, Turner KC, Bihorel S, et al. Population pharmacokinetic modeling and simulation of ocular clearance for aflibercept 8 mg and 2 mg and association with durability of effect. Invest. Ophthalmol. Vis. Sci. 2024;65(7):3154.
11. Wykoff CC, Brown DM, Reed K.Effect of High-Dose Intravitreal Aflibercept, 8 mg, in Patients With Neovascular AgeRelated Macular Degeneration: The Phase 2 CANDELA Randomized Clinical Trial. JAMA Ophthalmol. 2023;141(9):834-842.
12. Chang A, Phan L, Chin J, et al. Early realworld efficacy and safety of aflibercept 8mg: audit report from the CUREOS Research Network. Presented at the 25th European Society of Retina Specialists (EURETINA) Congress, Paris, France, 4–7 September 2025.
Dr. Ambresin noted this patient remained stable on Q16, while Prof. Chang suggested treating-and extending further to 20 weeks, with continued follow-up.
Digital technologies like artificial intelligence (AI) are fast becoming valuable allies in retinal disease
Taken together—clinical trial evidence, real-world studies and patient cases—the findings suggest that aflibercept 8 mg not only makes a compelling first choice for treatment-naive patients but also may help increase clinic capacity and streamline real world practice.
The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event.
13. Bailey C, Konidaris V, Lange C, et al. SPECTRUM: Early clinical outcomes in the first global real-world study of aflibercept 8 mg in patients with treatmentnaive neovascular age-related macular degeneration. Presented at the 25th European Society of Retina Specialists (EURETINA) Congress, Paris, France, 4–7 September 2025.
14. RetinAI Discovery®. Available at: https:// www.retinai.com/solutions/clinics-hospitals. Accessed: August 2025.
15. Zhang X, et al. EURETINA 2025. 4–7 September 2025. Paris, France.
Disclaimer: This article and the contents herein have been sponsored by Bayer for educational purposes.
PP-EYL_8mg-ALL-0420-1 | November 2025




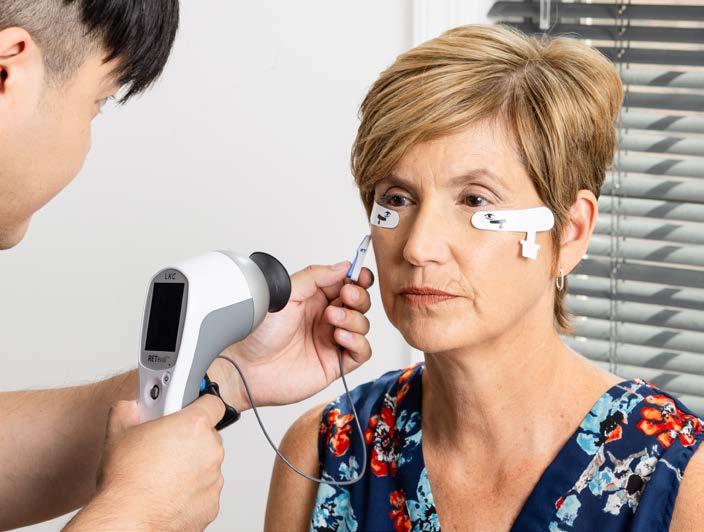
Sponsored by LKC Technologies
Vessels tell part of the story, but ERG spills the secrets. From uncovering preclinical dysfunction to predicting who’s headed for trouble, functional testing is stepping into the spotlight of DR management.
Diabetic retinopathy (DR) may be the quietest troublemaker in eye care, often advancing long before structural changes show up on imaging. By the time vascular abnormalities appear, the retinal dysfunction behind them may already be permanent.
Functional testing with electroretinography (ERG), including the handheld RETeval™ (LKC Technologies; Maryland, USA) device, offers a way to catch this dysfunction sooner.1 As a portable, non-mydriatic ERG, RETeval delivers rapid, objective insight into retinal
function without dilation. Growing evidence suggests that ERG can reveal preclinical changes and predict progression, positioning it as a potentially pivotal tool in the early detection and management of DR.
Associate Professor Dr. Dan Cao from Guangdong Provincial People’s Hospital (Guangzhou, China) described ERG as a powerful earlywarning system for DR. Dr. Cao and her team have spent more than 15 years studying the disease. Their early work with optical coherence tomography angiography (OCTA) uncovered microaneurysms in patients whose fundus exams still appeared normal. This led them to ask whether visual function could detect trouble brewing even earlier. “We wanted to see if ERG changes can precede the obvious fundus changes,” she said.
Initially, they combined OCTA with standard ERG to evaluate a large cohort of diabetic patients.2 But the traditional five-step ERG protocol proved too time-consuming. Transitioning to the RETeval DR protocol allowed them to capture implicit time and other key indices in



under five minutes, prompting the shift to the handheld ERG device.
Their findings showed that in the preclinical stage of DR—before any visible microaneurysms or retinal hemorrhages—patients already exhibited prolonged implicit time and reduced ERG responses.
“Additionally, when we looked at patients with different stages of non-proliferative diabetic retinopathy [NPDR], we found that once the disease progressed beyond moderate NPDR, the ERG implicit time was more sensitive than OCTA in detecting the progression of diabetic retinopathy,” she said.
In practice, Dr. Cao sees handheld ERG guiding earlier intervention. “I think if we perform the hand-held ERG test in patients every time they come to our clinic, and we see the score increasing or the implicit time prolonged—other than the retinal hemorrhage or macular edema—it indicates that the patient’s retinal function is declining,” she explained. “This allows us to intervene earlier, potentially performing laser treatment or administering anti-VEGF therapy before the disease reaches the end stage.”
Dr. Mitchell Brigell, consultant at Opus Genetics, Inc. (North Carolina,
USA) echoed Dr. Cao’s sentiment and emphasized ERG’s predictive value in managing DR.
In a study published in Ophthalmology Science3 on June 2025, Dr. Brigell and colleagues analyzed data from a clinical trial that was initially designed to test a drug aimed at preventing DR progression. Although the drug was ineffective, the dataset was a gold mine.
Participants with good vision and mild to severe NPDR underwent baseline ultra-widefield fluorescein angiography (FA), OCTA and RETeval ERG procedures. The researchers then assessed which measurements best predicted progression to visionthreatening diabetic macular edema (DME) or proliferative DR over the course of one year.
“We were surprised and happy to see that the ERG was the most predictive of who was going to progress to vision-threatening complications. The effect was fairly large: if the ERG was positive (meaning it showed an abnormal score) the likelihood of progression over the year was 73%,” he said. “Whereas if the ERG was negative, it was only 27%. Although some characteristics of the angiography and OCTA images were also statistically significant, this shows that the ERG has a strong predictive value.”
The reason, Dr. Brigell noted, is because it’s becoming much clearer that DR is more than just a vascular disease. “There’s a neurodegeneration component related to hyperglycemia and abnormal retinal metabolism. With imaging modalities you’re just looking at the vasculature. With the ERG, you’re actually looking at the function of the neural retina.”
“ERG should be used along with imaging technologies to better manage the care of patients with diabetic retinopathy.”
-
Dr. Mitchell Brigell
These insights directly inform risk stratification and treatment planning for patients with DR. ERG can help determine which patients with otherwise good vision are at high risk for progression and require more frequent monitoring. It also could impact decisions regarding which patients would benefit from early preventative treatment intervention. “If you could be better at predicting which patients are at high risk to progress, you can then offer them these treatment options with more assuredness that it’s going to help them,” he explained.
Referring to a 2020 Translational Vision Science & Technology study4, he added that combining ERG with the Diabetic Retinopathy Severity Scale (DRSS) “gives much more predictive value than either one alone.” Patients who appear structurally severe but have a negative ERG are often low risk and do not require frequent visits, whereas those with mild or moderate DR with a positive ERG warrant closer monitoring.
Dr. Brigell emphasized that the value of ERG lies in complementing—not replacing—structural imaging. “If you add ERG to your screening tests, you can better see who’s going
to benefit from interventions and better predict who you should follow more closely or intervene earlier to prevent progression,” he noted. “ERG should be used along with imaging technologies to better manage the care of patients with diabetic retinopathy.”
RETeval in the clinic
Electrophysiology, once highly regarded in the 1990s, gradually fell out of routine practice as traditional systems were cumbersome, timeconsuming, uncomfortable and largely confined to specialized teaching hospitals with long waiting lists. In contrast, RETeval ERG is portable, user- and patient-friendly, efficient and provides fast results.
“The RETeval device has transformed patient management and disease diagnosis,” emphasized Prof. Paulo Eduardo Stanga, ophthalmology professor at the University College London and founder of The Retina Clinic-London Ophthalmic Institute (London, UK).
“The RETeval device has transformed patient management and disease diagnosis.”
- Prof. Paulo Eduardo Stanga
“On an everyday basis, my patient can have electrophysiology—whether to diagnose an inherited retinal dystrophy or follow up ischemic changes in DR. In five minutes, we get results from both eyes and can objectively follow the stability or progression of retinal perfusion, stroke or retinal ischemia,” he said.
According to Prof. Stanga, RETeval is fundamental to multimodal assessment, adding rapid, objective electrophysiology to imaging and OCT without disrupting clinic flow. This enables comprehensive diagnosis at first consultation.

Contributors
“RETeval streamlines care, eliminating repeated testing and multiple follow-ups…thus reducing system burden, and giving patients peace of mind. RETeval can directly change management,” shared Prof. Stanga. For example, detecting ischemia in a diabetic patient can immediately indicate further investigation—either with ultra-widefield OCTA or invasive fundus fluorescein angiography. ERG also helps confirm diagnoses in uveitis and inherited retinal dystrophies, guiding treatment decisions, clinical trial eligibility and support gene therapy.
Based in the vibrant city of Guangzhou, Dr. Dan Cao serves as an assistant professor at the Guangdong Eye Institute within Guangdong Provincial People’s Hospital, one of China’s leading hubs for ophthalmic innovation. An experienced vitreoretinal and glaucoma specialist, Dr. Cao is known for tackling some of the most complex clinical challenges in the posterior segment. Whether managing advanced retinal disease or navigating the intricacies of refractory glaucoma, she brings deep subspecialty expertise and a calm, steady hand to every case.
caodan@gdph.org.cn

In a nutshell, RETeval enables rapid, non-invasive functional assessment, complements imaging, guides early intervention and streamlines DR management. As Prof. Stanga advised, clinicians should be open to trying RETeval: “They’ll be very pleasantly surprised by how quick and easy it is to do the test and get the results, how cost-effective it can be, and how much it is going to support their clinics on a day-to-day basis.”
With more than 15 years shaping clinical strategy for therapies targeting some of the world’s most common eye diseases, Dr. Mitchell Brigell brings deep experience to Opus Genetics. Before joining the company, Dr. Brigell served as vice president of Clinical Development at Aerpio Pharmaceuticals, where he steered a pipeline of novel small molecules and antibodies for DR, glaucoma, wet AMD and DME. He currently consults for a number of biotechs. Dr. Brigell began his career in academic medicine, spending more than a decade at Loyola University and the University of Chicago investigating electrophysical and behavioral changes in retinal and optic nerve disease. A prolific contributor to the field, he has published over 70 papers, is a fellow of ARVO and serves on the editorial boards of Translational Vision Science and Technology and Documenta Ophthalmologica
mgbrigell@gmail.com

References
1. Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019;103(12):1747-1752.
2. Zeng Y, Cao D, Yang D, et al. Retinal vasculature-function correlation in non-proliferative diabetic retinopathy. Doc Ophthalmol. 2020;140(2):129-138.
3. Davis CQ, Waheed NK, Brigell M. Predicting Progression to Vision-Threatening Complications in Diabetic Retinopathy. Ophthalmol Sci. 2025;5(6):100859.
4. Brigell MG, Chiang B, Maa AY, Davis CQ. Enhancing Risk Assessment in Patients with Diabetic Retinopathy by Combining Measures of Retinal Function and Structure. Transl Vis Sci Technol. 2020;9(9):40. A version of this article was first published on piemagazine.org
Prof. Paulo Eduardo Stanga is the founder and Chief Medical Officer of The Retina Clinic London, and a professor at the UCL Institute of Ophthalmology. With more than 30 years of experience in ophthalmology—and a focus on medical and surgical retina, including macular degeneration, diabetic retinopathy, vitreous floaters, cataracts, retinal laser, new therapies in R&D, surgical technology, advanced imaging and clinical studies—Prof. Stanga is dedicated to advancing eye care and transforming patients’ lives through pioneering work in the field. The Retina Clinic London is committed to providing bespoke, patient-centered eyecare. p.stanga@theretinacliniclondon.com






By Kendra Bruning
Rapid metabolic change can tip the retina off balance before it finds its rhythm.
In medicine, every breakthrough comes with a backswing. The harder we push the pendulum toward progress, the more forcefully it can swing back. Nowhere is that motion more literal than in the story of glucagon-like peptide-1 (GLP-1) receptor agonists: the drugs that reshaped diabetes care and weight management in one sweeping arc.
For the rest of the body, the benefits are easy to see with steadier glucose, lower cardiovascular risk and smaller waistlines. But in the eye, that rapid correction can feel like whiplash. Reports of retinopathy “worsening,” rare optic-nerve events and even new experiments in neuroprotection have turned the retina into a reflection of the wider metabolic swing.
To separate rhythm from recoil, Prof. Rafael Simó (Spain) has spent years charting how systemic change reverberates through the retina. His message is one of balance. The danger isn’t the drug, it’s actually the speed of control.
As Prof. Simó explained in his EURETINA 2025 lecture, the eye sometimes protests when the body’s chemistry changes too fast.1 His work (and a growing body of research) suggests it isn’t the molecule that harms, but the velocity of change.
Early worsening of diabetic retinopathy (DR) reminds clinicians that even improvement can have
consequences. It occurs when a rapid fall in glycated hemoglobin (HbA₁c) produces transient vascular stress in the retina, particularly when fragile neovessels already exist. Risk appears when HbA₁c drops by more than 1.5% in three months or 2% in six months.2
Yet real-world evidence tempers this fear. In a large Spanish population study, rapid HbA₁c reduction did not correlate with progression of mild or moderate non-proliferative diabetic retinopathy (NPDR), confirming that glucose optimization should not be delayed in early disease.2 The risk emerges mainly in advanced stages, where fragile vasculature cannot adapt to sudden metabolic change.
Across major cardiovascular-outcome trials, the pattern is consistent.
A meta-analysis of five GLP-1RA studies found that each percentagepoint decrease in HbA₁c increased short-term retinopathy risk, with no specific drug effect.3 At the Cleveland Clinic’s Cole Eye Institute, GLP-1RA users showed no higher odds of DR progression than matched sodiumglucose cotransporter 2 (SGLT-2) inhibitor users.4
Even the latest dual agonist, tirzepatide, follows the same rhythm. In a matched cohort, investigators observed higher odds of incident proliferative DR in those with preexisting disease, but lower odds of new-onset DR in those without baseline retinopathy.5 The signal, once again, reflects velocity rather than toxicity.
Prof. Simó’s practical advice is to document baseline retinopathy and titrate gradually in advanced cases, but not to hold back therapy in early disease. “Clinicians should not be afraid to optimize blood glucose in a relatively short period in subjects with mild or moderate nonproliferative diabetic retinopathy,” he emphasized.
The picture we’re seeing now is one of careful adjustment rather than caution. The retina may flinch when the pendulum of control swings too fast, but in most eyes, it quickly regains its balance.
The counter-swing toward protection
Every pendulum that swings too far eventually reverses course. For GLP-1 receptor agonists, that reversal has taken an unexpected direction: from suspected harm to potential protection.
In Prof. Simó’s laboratory, the very molecules once blamed for worsening retinopathy are showing signs of safeguarding the retina when given by a different route. In preclinical studies, GLP-1 analogues delivered as eyedrops reached the posterior segment within minutes, bypassing systemic metabolism entirely.
In diabetic animal models, topical GLP-1 receptor agonists prevented retinal neurodegeneration, curbing glial activation and preserving neuronal architecture.6 When semaglutide was administered topically, it went further by blocking NF-κB, which mediated inflammation, reducing interleukin-1 beta (IL-1β), IL-6 and IL-18, and the adhesion molecule ICAM-1, and preventing vascular leakage without altering blood glucose.7
Prof. Simó reported trans-scleral absorption with retinal drug levels detectable by 15 minutes. Complementary preclinical studies confirmed elevated retinal GLP-1 levels at one to two hours postdose and improved ganglion-cell function through enhanced gammaaminobutyric acid (GABA) release— effects abolished when GLP-1 or GABA-A receptors were blocked.8
“GLP-1 receptor agonists administered by the topical route
exert beneficial effects,” Prof. Simó told the EURETINA audience. Taken together, the data suggest the pendulum has swung toward equilibrium. What once unsettled the retina through rapid systemic change may, in time, become a direct shield against neurodegeneration.
Every pendulum has its outer edge, and for GLP-1–based drugs, that edge is defined by the optic nerve. Recent reports have linked semaglutide and tirzepatide to non-arteritic anterior ischemic optic neuropathy (NAION), a sudden, usually unilateral ischemic event affecting the optic nerve head.
In a JAMA Network Open study of 159,398 matched patients, GLP-1RA use was associated with a small but significant uptick in optic-nerve disorders, including NAION (35 versus 19 cases) over two years.9 Despite the relative increase, the absolute risk remains extremely low.
A separate 2025 analysis reported a 24.2% reduction in progression to blindness and fewer sight-threatening complications overall, even among patients with pre-existing DR.10 Together, these findings point toward balance and awareness, not alarm.
A meta-analysis of ten studies found a pooled hazard ratio (HR) of 2.62 for NAION, largely driven by semaglutide, which accounted for ~86% of reported cases. The signal became significant
References
1. Simó R. Diabetes and weight loss drugs: Retinal effects and their clinical relevance. Lecture at EURETINA 2025. Paris, France. September 7, 2025.
2. Simó R, Franch-Nadal J, Vlacho B, et al. Rapid reduction of HbA1c and early worsening of diabetic retinopathy: A real-world population-based study in subjects with type 2 diabetes. Diabetes Care. 2023;46(9):1633-1639.
3. Bethel MA, Diaz R, Castellana N, et al. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: A meta-analysis and meta-regression. Diabetes Care. 2021;44(1):290-296.
4. Joo JH, Sharma N, Shaia J, et al. The effect of glucagon-like peptide-1 receptor agonists on diabetic retinopathy at a tertiary care center. Ophthalmol Sci. 2024;4(6):100547.
5. Buckley AJ, Tan GD, Gruszka-Goh M, et al. Early worsening of diabetic retinopathy in individuals with type 2 diabetes treated with tirzepatide: A real-world cohort study. Diabetologia. 2025;68(9):2069-2076.
6. Hernández C, Bogdanov P, Corraliza L, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172-187.
only after two years of continuous exposure, suggesting a timedependent rather than immediate risk.11
Baseline risk matters too. Diabetes alone increases NAION incidence by roughly 50%, and common comorbidities like hypertension, obesity, sleep apnea and “disc-at-risk” anatomy add to that vulnerability.2
Prof. Simó recommends proportion over panic. “To avoid semaglutide based only on concerns regarding the risk of NAION might not be justified,” he said.1 For patients over 60 with vascular disease, neuropathy or sleep apnea, a cup-to-disc ratio under 0.3 can help flag higher risk. For most, though, GLP-1 drugs are still both safe and systemically beneficial.
The broader message for ophthalmologists is one of calibration. Rapid HbA₁c reduction does not appear to accelerate mild or moderate NPDR2, and any small rise in early retinopathy risk is outweighed by fewer blindness cases overall.10 In advanced disease, the approach is simply slower: document baseline DR, titrate doses gradually and monitor more closely when vasculature is fragile.
At the same time, the pendulum’s counter-swing is hopeful. GLP-1 eyedrops and dipeptidyl peptidase-4 (DPP-4) inhibitors have shown neuroprotective effects in preclinical models,
preserving ganglion cells and curbing glial activation.8,12 What began as a metabolic therapy may yet become a tool for protecting the eye itself: a balance between systemic success and retinal resilience.
Medicine is a study in motion. GLP-1 receptor agonists may shift the rhythm at first, but with care, their motion tends toward protection. The eye, like the body, adapts best when change happens at a manageable pace.
With steady treatment, consistent screening and growing insight into ocular effects, clinicians can keep both systems in sync. Balance isn’t about freezing the pendulum, it’s about keeping time with its swing.
Prof. Rafael Simo’s insights in this article are based on his Day 4 presentation at EURETINA 2025 Paris, titled “Diabetes and Weight Loss Drugs: Retinal Effects and Their Clinical Relevance”.

7. Simó R, Bogdanov P, Ramos H, et al. Effects of the topical administration of semaglutide on retinal neuroinflammation and vascular leakage in experimental diabetes. Biomedicines. 2021;9(8):926.
8. Shao YQ, Wang YC, Wang L, et al. Topical administration of GLP-1 eyedrops improves retinal ganglion cell function by facilitating presynaptic GABA release in early experimental diabetes. Neural Regen Res. 2026;21(2):800-810.
9. Wang L, Volkow ND, Kaelber DC, Xu R. Semaglutide or tirzepatide and optic nerve and visual pathway disorders in type 2 diabetes. JAMA Netw Open. 2025;8(8):e2526327.
10. Ramsey DJ, Makwana B, Dani SS, et al. GLP-1 receptor agonists and sight-threatening ophthalmic complications in patients with type 2 diabetes. JAMA Netw Open. 2025;8(8):e2526321.
11. Chen KY, Chan HC, Chan CM. Does semaglutide increase the risk of non-arteritic anterior ischemic optic neuropathy? A systematic review and meta-analysis of emerging evidence. Asia Pac J Ophthalmol (Phila). 2025 Sep 15:100245.
12. Simó R, Ramos H, García-Ramírez M, Hernández C. Effect of sitagliptin on diabetes-induced hyperpermeability of blood-retinal barrier components. Eye (Lond). 2025 Aug;39(12):24852486.
Simó has dedicated his career to illuminating the complex connections between diabetes, metabolism and human health. As chair of the Division of Endocrinology and Nutrition at Vall d’Hebron University Hospital and director of the Diabetes and Metabolism Research Unit at Vall d’Hebron Research Institute (VHIR), he leads one of Spain’s most dynamic research teams.
A professor of Medicine and Endocrinology at the Autonomous University of Barcelona and deputy director of Clinical Research at VHIR, Dr. Simó’s influence extends from the classroom to the clinic. His prolific output—over 400 peerreviewed articles and a Hirsch index of 50—speaks to his impact on global diabetes research.
Recognized with multiple national awards, including the Rodríguez Miñón, Dr. Josep Trueta, and Spanish Society of Endocrinology Awards, Dr. Simó continues to push the boundaries of biomedicine, bridging discovery and patient care with precision, passion and purpose. rafael.simo@vhir.org



By April Ingram
Think we’re seeing exudative AMD clearly? Maybe not.
At EURETINA 2025, Dr. Steffen Schmitz-Valckenberg put our favorite imaging biomarkers under the microscope, challenging clinicians to zoom out, rethink what really matters and decide whether it’s time to refocus our view on what “seeing is believing” truly means.
As clinician-researchers, our days are split between two demanding callings: providing the best possible care for our patients and pushing the boundaries of what that care can become.
Anyone who has ever developed a research protocol knows how crucial it is to get every element right, from measuring the right things to defining outcomes that are scientifically sound and ensuring they truly matter for patients. The secret ingredient often lies in the biomarkers, those measurable indicators that reflect normal biological processes, disease states or response to therapy.
Thanks to leaps in imaging technology and our ever-deepening understanding of retinal structure
and pathology, we now have more potential biomarkers than ever before. At EURETINA 2025, Dr. Steffen Schmitz-Valckenberg (USA) posed a thought-provoking question that many of us have quietly wondered, “Are we evaluating the right imaging biomarkers?”1
And if anyone is qualified to explore that question, it’s him. A professor of Ophthalmology and Visual Sciences at the University of Utah and cofounder and director of the Grading of Digital Fundus Examination Reading Center at the University of Bonn, Germany, Dr. Schmitz-Valckenberg has helped pioneer high-resolution retinal and fundus autofluorescence imaging techniques that have shaped modern retinal research around the world.
Dr. Schmitz-Valckenberg began by framing the current landscape of exudative macular neovascularization (MNV)—the culprit behind irreversible visual impairment in individuals with age-related macular degeneration (AMD). The advent of anti-vascular endothelial growth factor (VEGF) therapy has dramatically reduced the number of patients experiencing severe vision loss. However, as most of us in clinic can attest, it comes at a price: an enormous assessment and treatment burden to sustain visual function.
Even with regular injections and vigilant follow-ups, not every patient hits the jackpot. Roughly 15% of eyes fall into the non- or suboptimalresponder category.2 That variability underscores why accurate imaging— and choosing the right biomarkers— matters more than ever.
Multimodal retinal imaging remains central to diagnosis, treatment monitoring and detecting complications. It also forms the foundation of numerous clinical endpoints. As Dr. SchmitzValckenberg explained, “When considering multimodal imaging in the context of MNV there are several practical considerations, such as the high volume of patients, as well as effective end analysis.”
To answer whether we are truly evaluating the right imaging biomarkers, he dissected the question from several angles…
The art of differential diagnosis
“Not every macular exudation observed in an individual over the age of 55 is attributable to AMD,” Dr. Schmitz-Valckenberg noted, adding that most patients with exudative MNV secondary to AMD are over 70 years old. With a long list of possible differential diagnoses, multimodal imaging often holds the key to getting it right.
He referenced findings from the ORCA study3, which showed that “the baseline diagnosis of the treating ophthalmologist was confirmed by the reading centers in eyes with neovascular AMD in 82.3%,
or approximately four out of five physicians.”
Next, Dr. Schmitz-Valckenberg explored how genetics intertwines with imaging. Two main AMDassociated genetic loci—CFH on chromosome 1 and ARMS2/HTRA1 on chromosome 10—significantly influence both the risk and the subtype of MNV.4
“Prior association of well-established imaging markers with age, genetic group and MNV subtype suggest that most imaging markers are rather unspecific when it comes to the signs of exudation, until later in the disease progression,” he explained.
Early detection is where imaging earns its keep. Dr. SchmitzValckenberg questioned the minimal required B-scan density and compared the sensitivity of detecting intra- and subretinal fluid changes at 240 versus 120 microns, demonstrating just how easily lesions can be missed. “This is critical to disease detection and timely initiation of treatment,” he emphasized.
But, as he cautioned, not every subretinal hyporeflective space equates to fluid secondary to MNV. To illustrate, he shared seven years of longitudinal imaging from an asymptomatic 59-year-old woman whose subretinal changes resolved without treatment—a benign evolution of subretinal changes.
With an ever-growing number of patients under long-term monitoring and anti-VEGF treatment, the conversation inevitably turns to chronic management. “There are a high number of patients with remarkably good to moderate vision,” Dr. Schmitz-Valckenberg explained. “But when there are complaints from patients associated with losing vision over time, often, this means that atrophy or fibrosis has developed.”
That’s when en face imaging and autofluorescence come to the forefront. Identifying and visualizing fibrosis can be tricky, and once it’s present, there are no targeted therapies yet (though research continues).
He also highlighted the work of Prof. Cheung and colleagues, “that describe how it is important to look at the integrity of the outer neurosensory retina, preservation of the ELM and level of fibrosis relative to the RPE” as key indicators of preserved vision.5
At the end of the day, imaging alone doesn’t tell the full story. “Visual function is about more than visual acuity and there is a need for more sensitive tools,” Dr. SchmitzValckenberg reminded the audience.
He shared how artificial intelligence can estimate retinal function from imaging data, introducing the term inferred sensitivity to describe this approach.6 “This can be used as a quasi function surrogate endpoint in future clinical trials… providing more precise evaluation of treatment effects that goes beyond BCVA [best-corrected visual acuity] testing.”
Dr. Steffen Schmitz-Valckenberg’s insights in this article are based on his Day 3 presentation at EURETINA 2025 Paris, titled “Exudative AMD: Are we evaluating the right imaging biomarkers?”.
1. Schmitz-Valckenberg S. Exudative AMD: Are we evaluating the right imaging biomarkers? Lecture at EURETINA 2025. Paris, France. September 6, 2025.
2. Finger RP, Wickremasinghe SS, Baird PN, et al. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59(1):1-18.
3. Brinkmann CK, Chang P, Schmitz-Valckenberg S, et al. Baseline diagnostics and initial treatment decision for anti-vascular endothelial growth factor treatment in retinal diseases: Comparison between results by study physician and reading centers (ORCA/OCEAN study). Ophthalmologe. 2019;116(8):753-765.
4. Solinsky B, Schmitz Valckenberg S, Zouache MA, et al. Clinical manifestation of macular neovascularization in age-related macular degeneration among individuals homozygous for risk alleles on chromosome 1 (CFH-CFHR5), on chromosome 10 (ARMS2/HTRA1) or both. IOVS. 2024;65(7):2788.
5. Cheung GCM, Grewal DS, Teo KYC, et al. The evolution of fibrosis and atrophy and their relationship with visual outcomes in Asian persons with neovascular age-related macular degeneration. Ophthalmol Retina. 2019;3(12):1045-1055.
6. von der Emde L, Pfau M, Schmitz-Valckenberg S, et al. AI-based structure-function correlation in age-related macular degeneration. Eye (Lond). 2021;35(8):2110-2118.

As Dr. Schmitz-Valckenberg wrapped up his lecture, he circled back to his opening question: are we truly evaluating the right imaging biomarkers? The answer, it seems, lies in embracing the interplay of multiple imaging modalities, coupled with genetic and functional insights.
“Further insights into disease processes can be obtained when multimodal imaging is utilized in combination with other findings, particularly those related to genetics and visual function,” he concluded.
In other words, it’s not just about what we see. It’s about how we see it…and how well that vision aligns with the reality of our patients’ outcomes.
Dr. Steffen SchmitzValckenberg is a leading specialist in macular and retinal diseases, and he’s internationally recognized for his expertise in highresolution retinal imaging.
Before joining the University of Utah’s Moran Eye Center, Dr. Schmitz-Valckenberg co-founded and directed the Grading of Digital Fundus Examination Reading Center at the University of Bonn, Germany, where his pioneering work in fundus autofluorescence imaging helped chart the stages of AMD progression. His imaging maps are now used by researchers around the world to track treatment outcomes in clinical trials.
At Moran, he directs a state-of-theart ophthalmic image reading center central to the Sharon Eccles Steele Center for Translational Medicine, bringing new AMD therapies from the lab to patients.
With over 160 publications and recognition among The Ophthalmologist’s “Top 50 Rising Stars,” Dr. Schmitz-Valckenberg is helping the world see the future of retina care more clearly.
steffen.schmitz-valckenberg@ukbonn.de



By Hazlin Hassan
Illustration: Orla/Shutterstock.com
Once an unpredictable foe lurking behind the microscope, endophthalmitis is now being challenged by smarter prevention strategies, molecular diagnostics and modern surgical finesse.
By all accounts, few words strike more fear into a vitreoretinal surgeon’s heart than endophthalmitis. This rare yet devastating infection of intraocular tissues remains one of the field’s most dreaded complications.
“The primary risk factors for endophthalmitis after cataract or vitreoretinal surgery include wound leak, posterior capsule violation, vitreous prolapse and prolonged surgical time,” said Dr. Landon Rohowetz (USA), a vitreoretinal surgeon at the Bascom Palmer Eye Institute.
“Identifying and addressing these complications intraoperatively significantly reduces the risk of developing endophthalmitis,” he added.
In other words, prevention begins before the first drop of povidoneiodine hits the ocular surface. From meticulous draping to managing pre-existing conditions, every detail counts in closing the door on endophthalmitis before it even knocks.
Sterile starts and smart prevention
When it comes to infection control, there’s nothing glamorous about
sterile technique, but it remains the most powerful weapon in a vitreoretinal surgeon’s arsenal. “Preoperative preventive measures— including meticulous sterile technique and draping of the eye, preparation of the ocular surface with povidone-iodine and treatment of meibomian gland disease—are particularly important,” Dr. Rohowetz explained.
The pre-op checklist might sound old-school, but it is supported by decades of evidence.
Data from a large retrospective study of 68,323 intraocular surgeries conducted at a single center from 1990 to 2009 suggest that generous preoperative application of povidone–iodine may reduce the risk of endophthalmitis.1
The European Society of Cataract and Refractive Surgery (ESCRS) recommends using 5% to 10% povidone-iodine prior to cataract surgery, while the American Academy of Ophthalmology (AAO) recommends 5%.2 So, whether you’re in Barcelona or Boston, the message is: iodine works.
Equally important is recognizing patients who walk in with preloaded risks. “Those with blepharitis, previous intraocular trauma,
diabetes mellitus and other forms of immunosuppression” are especially vulnerable, Dr. Rohowetz noted. Proper preoperative management of these systemic and ocular conditions can make all the difference in keeping the postoperative course uneventful.
And what about the perennial debate surrounding prophylactic intracameral antibiotics? Opinions are divided. “The role of prophylactic intracameral antibiotics, while used in many parts of the world, is unclear,” Dr. Rohowetz said.
The landmark 2007 ESCRS trial demonstrated that intracameral cefuroxime could cut the risk of postoperative endophthalmitis.3 Yet, the findings have not achieved universal adoption, particularly in the United States, where variations in drug availability, regulatory approvals and workflow preferences continue to shape clinical decisions.
Despite our technological advances, diagnosing endophthalmitis can still feel a bit like detective work in the dark. Traditional cultures—long considered the gold standard— fail to yield results in up to 70% of suspected cases.4 That leaves clinicians treating empirically and often guessing at the invisible culprit.
Enter molecular diagnostics, a 21st-century upgrade to an ageold problem. Techniques such as polymerase chain reaction (PCR) and next-generation sequencing (NGS) can identify pathogens more rapidly and in more cases than conventional cultures.5
These methods, Dr. Rohowetz said, have “expanded our ability to identify causative organisms in cases of culture-negative endophthalmitis.”
However, these molecular marvels don’t come cheap. “A primary challenge to implementing these technologies is their associated costs, as most practices and small laboratories do not have the resources necessary to expend on these technologies,” he noted. As a result, they remain largely confined to major tertiary academic centers.
Even where resources abound, the jury’s still out on whether faster
and broader microbial detection translates to better outcomes. “It is unclear if their capacity for microbiologic diagnosis impacts management strategies and treatment outcomes,” Dr. Rohowetz cautioned. Before they become routine, “these factors must be addressed.”
Still, as the technology matures and costs decline, it’s not hard to imagine a future where a PCR panel could become as routine as a slit-lamp exam.
To understand how far we’ve come, it helps to recall how it all began. In the 1970s, when Dr. Gholam Peyman introduced the concept of intravitreal antibiotics in rabbit models, leading to the “tap and inject” era. Soon after, Dr. Robert Machemer and his colleagues pioneered pars plana vitrectomy (PPV), adding another powerful tool in the ophthalmic arsenal.
The Endophthalmitis Vitrectomy Study (EVS) of the late 1980s and early 1990s helped define the standard approach, finding no clear benefit of vitrectomy over intravitreal antibiotics for post-cataract cases, with visual acuity better than light perception. For decades, these guidelines served as gospel.
But medicine, like technology, doesn’t stand still. “In light of these advancements, some have expanded the role of PPV in the treatment of endophthalmitis,” said Dr. Rohowetz. With today’s small-gauge vitrectomy systems and modern phacoemulsification, what was once a high risk procedure has become faster, safer and more refined.
Modern decision-making is also more nuanced. “Some authors have instituted more nuanced approaches to decision-making, relying on a combination of objective measures including fundus visualization, corneal transparency and the presence of a hypopyon when determining the optimal initial management approach,” he explained.
Still, Dr. Rohowetz noted, “convincing evidence for either approach is limited and the decision to perform vitrectomy should take into account disease severity on presentation, response to initial intravitreal antibiotic therapy and suspected organism virulence.
It seems the old “tap and inject” versus “cut and clear” debate still has a few rounds left to play out.
Where does the field go from here? According to Dr. Rohowetz, “promising research directions include the development of advanced diagnostic techniques to allow the rapid identification of organisms to potentially enable more targeted therapeutic approaches, expansion of the role of intracameral antibiotics and sustained release antimicrobial materials, and continued refinement of vitreoretinal and cataract surgery techniques.”
One particularly exciting avenue lies in novel drug delivery systems. Innovations such as drug-eluting intraocular lenses, hydrogels and nanoparticles are being engineered to overcome the eye’s formidable barriers: corneal, conjunctival and blood-ocular alike. These systems could prolong drug residence time, improve permeability and solubility, and reduce toxicity, paving the way for more effective and sustained prophylaxis.5
reactive approach to a predictive, precision-guided model. With molecular tools illuminating the unseen, smarter prophylactic strategies taking root, and surgical advances continuing apace, the fight against endophthalmitis is moving beyond the microscope…and perhaps, finally, beyond fear.
1. Gess AJ. Support for generous iodine use to reduce postop endophthalmitis risk. American Academy of Ophthalmology. February 18, 2015. Available at: https://www.aao.org/education/editors-choice/ povidone-iodone-may-be-key-to-decreasing-postopen. Accessed on October 26, 2025.
2. Garcia O’Farrill N, Abi Karam M, Villegas VM, et al. New approaches to overcoming antimicrobial resistance in endophthalmitis. Pharmaceuticals. 2024;17(3):321.
3. Barry P, Seal DV, Gettinby G, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32(3):407-410.
4. Naik P, Gandhi J, Joseph J. Recent advances and ongoing challenges in the diagnosis of culture negative endophthalmitis. Semin Ophthalmol. 2022;38(1):92-98.
5. Mahaling B, Baruah N, Dinabandhu A. Drug delivery systems for infectious eye diseases: Advancements and prospects. Journal of Nanotheranostics. 2024;5(4):133-166.
6. Harley O, Amelia YS, Gustianty E, et al. Controversies in the management of endophthalmitis: A 5-year retrospective cohort study. J Ophthalmic Inflamm Infect. 2025;15(1):28.

For a condition that occurs in only 0.03% to 0.7% of surgeries, endophthalmitis casts a long shadow.6 Each case is a stark reminder that even in the era of femtosecond lasers and microincisions, infection remains a formidable adversary. Delays in diagnosis or treatment can mean permanent vision loss, underscoring why vigilance and innovation must go hand in hand.
The next decade will likely redefine what infection control means in ophthalmology, shifting from a
Dr. Landon Rohowetz is a vitreoretinal surgeon at the world-renowned Bascom Palmer Eye Institute. He completed both his ophthalmology residency and fellowship there, earning the Heed Ophthalmic Foundation Award as a resident and the Michels Fellowship Foundation Award as a fellow.
A proud member of Phi Beta Kappa and Alpha Omega Alpha, Dr. Rohowetz has built his career around precision, curiosity and an unwavering commitment to patient care. His clinical and research passions include endophthalmitis, retinal detachment, diabetic retinopathy, ocular trauma and refining vitreoretinal surgical techniques.
With more than 50 peer-reviewed publications and regular appearances on the international conference circuit, he’s not only advancing surgical science but also shaping the future of retina care—one presentation, one paper and one repaired retina at a time.
ljr108@miami.edu
By Hazlin Hassan
Anti-VEGF therapies changed the game, but the game’s moving on. As new contenders—from eye drops to gene editing—step onto the field, diabetic eye disease treatment is evolving fast. Here’s what’s next in the race to outsmart retinal damage.

When you think of turning back the clock on vision loss in people with diabetes, the spotlight usually falls on the heavyweights: vascular endothelial growth factor (VEGF) inhibitors. They’ve long dominated the fight against retinal diseases such as diabetic retinopathy (DR) and diabetic macular edema (DME). But the story doesn’t end there.
A new generation of treatments— from simple eye drops to gene therapies—is stepping up to the plate. Let’s see what’s making headlines in the evolving world of diabetic eye care.
The arrival of VEGF inhibitors like aflibercept, ranibizumab and bevacizumab in the early 2000s marked a seismic shift in retinal disease management.
“Anti-VEGF alone is insufficient for nearly 50% of people with diabetic macular edema, so nonVEGF pathways need to be evaluated.”
The Phase IIb CLARITY trial, led by Prof. Sobha Sivaprasad (UK) and published in 2017, demonstrated that anti-VEGF therapy could outperform pan-retinal photocoagulation in proliferative diabetic retinopathy.1
But here’s the catch: anti-VEGF therapy itself isn’t cutting it for everyone. Data shows that nearly half of patients with DME fail to achieve optimal results with VEGF inhibition alone.
In fact, patients treated with aflibercept, bevacizumab or ranibizumab still had persistent DME in 44% to 68% of cases at two years.2
“Anti-VEGF alone is insufficient for nearly 50% of people with diabetic macular edema, so non-VEGF pathways need to be evaluated,” said Prof. Sivaprasad. The conclusion? It is high time to look beyond VEGF inhibition.
Dexamethasone eye drops. Meet OCS-01, a high-concentration (15 mg/ml) dexamethasone eye drop from Oculis (Zug, Switzerland). It could become the first noninvasive treatment for DME, offering convenient early-stage management and compatibility with other therapies in later stages.3
Stage 1 of the Phase III DIAMOND trial, involving 148 patients, has been completed. The company reported a “significant increase in visual acuity… and a reduction of macular edema, all with robust statistical significance,” compared to Phase II data. Topline results are expected in Q2 2026.3
Angiopoietin-2 (Ang-2). A key player in DME pathophysiology, Ang-2 influences vascular stability and inflammation. Six Phase III trials comparing faricimab (6 mg)—a dual Ang-2/VEGF-A bispecific antibody— with aflibercept suggest that dualpathway inhibition offers superior disease control, managing both neovascularization and vascular leakage.4
“Based on the evidence…earlier treatment with a dual pathway inhibitor has the potential to improve long-term patient outcomes,” noted Chaudhary et al. in a 2024 paper.4
Imagine fewer injections, fewer visits and a steadier course of treatment.
Earlier in 2025, Roche’s (Basel, Switzerland) Susvimo (ranibizumab implant, 100 mg/mL) became the first FDA-approved continuous delivery treatment for DME, offering a much-needed reprieve from monthly appointments.5
“Advances in nucleic acid therapeutics like siRNA, miRNA and CRISPR/Cas9 have shown efficacy in preclinical models, reducing VEGF levels and neovascularization.”
The approval was based on the Phase III PAGODA study, where patients refilled every six months achieved non-inferior visual gains (9.6 eye chart letters) compared with monthly ranibizumab injections (9.4 letters).5
Meanwhile, gene therapy is carving out its place in the retina realm.
“Advances in nucleic acid therapeutics like siRNA, miRNA and CRISPR/Cas9 have shown efficacy in preclinical models, reducing VEGF levels and neovascularization,” wrote Mengistie Diress et al. in a 2025 paper.6
AAV-based vectors such as RGX-314 (Regenxbio; Maryland, USA) and ADVM-022 (Adverum
Biotechnologies; California, USA) promise long-term anti-VEGF expression, while newer non-viral lipid-polymer systems improve stability and targeting. However, safety, complexity and vector limitations remain key challenges.
The race is on to refine these technologies into durable, safe solutions that match the intricate dynamics of DR.
When all is said and done, what works in a clinical trial can often hit snags in daily practice.
Patient visits get delayed, comorbidities interfere and some hard-hit populations may not be captured at all.
“Less treatment burden is key to successful treatment,” Prof. Sivaprasad noted. “If drugs can be delivered every six months, it would be a great relief to patients, caregivers and the health system as a whole.”
These factors are central in why the next wave of therapies is so important. They must reduce burden, increase durability and fit real-world patient lives, not just ideal trial conditions.
Cost, limited availability, uncertain long-term safety, and the need for robust head-tohead evidence against current first-line therapies all pose barriers to widespread adoption, said Prof. Sivaprasad.
Given that VEGF inhibition alone fails to deliver ideal outcomes for many, combination strategies are increasingly compelling.
If you ask what moves the needle most? Prof. Sivaprasad did not hesitate, saying, “Trials on combination therapies that test vision outcomes and durability.”
Simply put, the next big leap will not come from yet another individual drug. It will come from smart combinations, longer-lasting therapies, fewer clinic visits and wider reach.
The question has shifted from “what blocks VEGF best?” to “what else can we do?”
The next revolution in diabetic eye care won’t hinge on a single drug, but on how cleverly these therapies are combined, delivered and adapted to real-world needs. Because in the world of vision care, teamwork— molecular or otherwise—just might make the dream work.

Prof. Sobha Sivaprasad is one of the brightest minds shaping the future of retinal research. As a consultant ophthalmologist at Moorfields Eye Hospital and professor of Retinal Clinical Research at University College London, she stands at the crossroads of science, innovation and patient care.
1. Sivaprasad S, Prevost AT, Vasconcelos JC, et al; CLARITY Study Group. Clinical efficacy of intravitreal aflibercept vs panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, Phase 2b, noninferiority trial. Lancet. 2017;389(10085):2193–2203.
2. Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257–269.
3. Diabetic Macular Edema (DME). Oculis. 2025. Available at: https://oculis.com/our-areas-of-focus/diabetic-macularedema/. Accessed on October 24, 2025.
4. Chaudhary V, Mar F, Amador MJ, et al. Emerging clinical evidence of a dual role for Ang-2 and VEGF-A blockade with faricimab in retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2025;263:1239–1247.
5. FDA approves Roche’s Susvimo as the first and only continuous delivery treatment for the leading cause of diabetes-related blindness. Roche. February 4, 2025. Available at: https://www.roche.com/media/releases/medcor-2025-02-04. Accessed on October 24, 2025.
6. Diress M, Ionescu CM, Foster T, et al. Toward a new frontier in diabetic retinopathy treatment: A synergistic approach using gene therapy and nanotechnology. Journal of Drug Delivery Science and Technology. 2025;111:107172.
She directs the Moorfields Clinical Research Facility and leads the Vascular Theme at the Moorfields Biomedical Research Centre, where her work focuses on clinical trials, imaging and risk prediction in AMD and retinal vascular diseases.
With over 600 peer-reviewed publications to her name, Prof. Sivaprasad’s research has left an indelible mark on global ophthalmology. Beyond the lab, she also shapes the field through her editorial leadership as editor-in-chief of Eye since 2018, and by launching Eye Open, the Royal College of Ophthalmologists’ second journal. sobha.sivaprasad@nhs.net
This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
By Kendra Bruning
From genes to dreams of sight restored, IRD therapies are shifting from the lab to the clinic at record speed.
On Day 3 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025) in Paris, the Grand Amphitheater lit up with innovation as the Current Status of Innovative Therapies for Inherited Retinal Disorders session unfolded under the guidance of Prof. Dr. Isabelle Audo (France) and Dr. Bart LeRoy (Belgium).
Eight speakers charted a course through gene replacement and editing, CRISPR and antisense oligonucleotides, cell therapy, optogenetics and gene-agnostic pipelines. The discussion mixed technical tenacity with clinical imagination, balancing delivery routes, trial design and patient selection. The result was a Parisian tableau of progress, where IRDs look not only inheritable, but increasingly interruptible.
Prof. Dominik Fischer (UK) opened the session with pragmatic advice on delivering gene therapy for RPE65mediated IRDs. Real-world data, including the PERCEIVE study, show only modest gains in visual acuity overall, though younger patients often see greater benefit, especially in light sensitivity.
“From the PERCEIVE study, the realworld evidence suggests that there
is not going to be a significant change of your best-corrected visual acuity overall,” he noted. “However, that does depend on the age and, of course, the disease stage in which you start treating.”
Surgical nuance mattered, like slow subretinal injection, careful cannula placement and a virus lavage help reduce spillover and inflammation. Still, side effects like gene therapyassociated uveitis, atrophy and steroid-induced ocular hypertension still call for close monitoring.
Prof. Fischer’s takeaway was to choose patients wisely, set realistic expectations and never skip the fluidair exchange.
Prof. Dr. Robert MacLaren (UK) presented compelling results from RPGR gene therapy in X-linked retinitis pigmentosa (XLRP), a condition he described as one of the most common and severe IRDs.
Subretinal delivery of a codonoptimized full-length RPGR transgene produced clear anatomical recovery, including reappearance of the external limiting membrane and thickening of photoreceptor layers, together with functional gains in retinal sensitivity. In some patients, improvement was visible within three months of treatment.
“The anatomical reversal we’ve seen in RPGR gene therapy is an incredibly important sign,” Prof. Dr. MacLaren noted, “and much more objective than functional tests.” Importantly, no evidence suggested that proximal RPGR mutations impaired outcomes. His conclusion was that, for the first time, reversal of retinal degeneration in XLRP appears not only possible but measurable.
Dr. Jasmina Kapetanovic (UK) offered a frank look at gene therapy for choroideremia, an X-linked degeneration caused by REP1 deficiency. In the multicenter STELLAR and REGENERATE trials, treatment proved safe but failed to deliver statistically significant gains in visual acuity, though trends favored earlier intervention. Patients with preserved autofluorescence and central retinal structure responded best, and microperimetry proved more sensitive than BCVA as an endpoint.
“What I would like to draw attention to here is how thin and fragile that retina is in choroideremia,” Dr. Kapetanovic reminded the audience. “And it’s up to us surgeons to detach this retina safely without further iatrogenic damage.”
She also pointed to advances on the horizon, including optogenetic therapies and robotic subretinal delivery, both of which may help overcome current biological and surgical obstacles.
Dr. Bart LeRoy (Belgium) tackled one of the toughest barriers in inherited retinal disease (IRD) gene therapy: what to do when the target gene is simply too big for an adeno-associated virus (AAV) vector. For oversized genes like CEP290, antisense oligonucleotides (AONs) provide a vector-free workaround by correcting splicing errors with short RNA molecules, delivered intravitreally and dosed repeatedly.
“The capacity of AAV vectors is limited,” Dr. LeRoy explained. “It’s like a little delivery can[ister] that can only deliver so much. So there needs to be technology that is able to be used for large genes.”
Other approaches on the table include dual-vector approaches that split genes such as MYO7A and ABCA4, along with RNA transsplicing, which repairs faulty mRNA directly. Together, these strategies are pushing the therapeutic horizon for patients previously considered untreatable.
Dr. Mark Pennesi (USA) presented long-term results from the BRILLIANCE trial, the first in vivo CRISPR/Cas9 therapy tested in humans, targeting CEP290-related Leber congenital amaurosis (LCA). Patients with this severe retinal dystrophy often retain structure despite profound vision loss, making them prime candidates for editing. EDIT-101, delivered subretinally via AAV5, removes the intronic mutation that disrupts protein function.
At one year, four of 14 patients gained ≥ 0.3 logMAR in visual acuity, six improved on full-field sensitivity testing and four performed better in mobility navigation. “We do see functional improvement with EDIT101,” Dr. Pennesi told the audience, “with nearly 80% of patients showing gains in at least one metric.” Benefits persisted up to four years in most responders, bringing home the durable potential of gene editing in IRDs.
Prof. Dr. Isabelle Audo (France) featured gene-agnostic therapies as a way to tackle the vast heterogeneity of IRDs. Instead of correcting one mutation, these strategies target universal processes to preserve or restore vision.
“The rationale for gene-agnostic therapy is the clinical, but also, and more importantly, the genetic heterogeneity of IRDs,” she explained. “The idea is then not to
correct a mutation or a gene, but to act through universal phenomenon.”
For neuroprotection, she pointed to NXNL1-based therapies such as SPPN06 in the ProDigi trial, which enhance glucose metabolism and counter oxidative stress. AAVdelivered NR2E3 (Ocugen) has also shown early signals of efficacy.
In advanced degeneration, optogenetics hope to repurpose ganglion cells with channelrhodopsin, while GRK1 transfer (SPPN20) seeks to revive dormant cones. Though early, these therapies promise new options for patients beyond genespecific solutions
Prof. Lyndon da Cruz (UK) outlined progress in cell-based therapies for IRDs and age-related macular degeneration (AMD). The concept is that when retinal cells are lost, replacing them may restore function.
Researchers are exploring sources including embryonic stem cells, induced pluripotent stem cells (iPSCs) and adult progenitors to engineer retinal pigment epithelium (RPE) sheets, suspensions and even retinal organoids.
Delivery is still a surgical challenge, with trials testing subretinal injection, patch implantation and robotic assistance. “Since 2012, more than 100 patients have received some form of published stem cell transplantation,” Prof. da Cruz reported. “There’s no major safety signal in terms of tumor and immune reaction.”
Early results suggest persistence and safety, though efficacy remains limited. Even so, as techniques mature, cell therapy is inching closer to clinical reality.
Dr. Kanmin Xue (UK) closed the session with a reminder that as gene therapy moves from rare IRDs into common conditions like AMD and diabetic retinopathy (DR), inflammation management must
move to the forefront. Gene therapyassociated uveitis (GTAU) varies by delivery route, with intravitreal injections often triggering anterior chamber reactions and subretinal approaches producing localized infiltrates
“Gene therapy is becoming more common,” Dr. Xue said, “and initially we started offering rare diseases such as IRDs, but now several clinical trials are leading to advanced stages for developing similar treatments for more common diseases such as AMD and DR. Therefore, controlling inflammation has become much more relevant clinically.”
Strategies under study include improved vector design, personalized immunosuppression and suprachoroidal delivery. Proactive monitoring, tailored steroid regimens and trial endpoints that balance safety with efficacy will be key as the field scales up.
This session unveiled a field in full stride, powered by deeper genetic insight, smarter delivery systems and growing clinical experience. Gene replacement is entering real-world practice, while editing, agnostic and optogenetic strategies push into new territory. Cell-based approaches are edging closer to viability and surgical finesse is steadily improving.
Challenges like inflammation, delivery limits and trial design linger, but the collective momentum is undeniable. Therapies are maturing, patients are being enrolled earlier and conversations with regulators are happening. For conditions once thought irreversible, the pipeline now carries more than proof of concept. It carries proof of possibility.
The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. A version of this article was first published on piemagazine.org

By Tan Sher Lynn
After years of firing blanks at MacTel, researchers may have finally hit their target…by aiming at the neurons instead of the vessels. Could CNTF therapy be the game changer the field’s been waiting for?
Macular telangiectasia type 2 (MacTel) has long been the unsolved puzzle on the retina specialist’s board. As Prof. Frank Holz (Germany) quipped during his talk at EURETINA 2025,1 “There have been several attempts to tackle this particular disease…whenever we see a leakage, then there seems to be a golden bullet to shut.” Sadly, that “golden bullet” usually ricocheted.
Over the years, nearly every therapy imaginable was fired at MacTel: laser photocoagulation, microthermal laser, photodynamic therapy,
steroids, indocyanine green (ICG), even photothrombosis. “You name it,” said Prof. Holz, “The list is really long, but we must say that this was not changing the outcomes in patients in many respects.”
When it comes to nutrition, the story wasn’t much rosier. Macular pigment carotenoids such as lutein and zeaxanthin—mainstays in age-related macular degeneration (AMD)—have been considered for MacTel due to their antioxidant and blue-light filtering properties. But the evidence remains underwhelming.2
While a diet rich in carotenoids is generally sound advice for retinal health, there’s little to suggest it halts MacTel progression. For now carotenoids remains supportive, not transformative.2
The introduction of anti-vascular endothelial growth factor (anti-VEGF) agents sparked hope. Referencing work by Peter Issa and colleagues,3 Prof. Holz noted that early imaging showed “a significant amount of hypofluorescence that either largely resolved or improved after several months of treatment with ranibizumab.” Yet the improvement proved fleeting.
“When the therapy was terminated after 12 months, there was a massive rebound,” he recalled. “It looked the same as before, so there was no long-lasting effect and no functional benefit.”
Worse still, there was evidence of harm. “There was no functional benefit for anti-vascular endothelial growth factor treatment whatsoever,” Prof. Holz stated. Optical coherence tomography (OCT) revealed retinal thinning, not a good sign in this context. “Treated eyes…had faster loss of photoreceptors. They had worse visual acuity,” he added.
His takeaway was firm. “The key message is: don’t use anti-VEGF therapy in the non-proliferative stage of MacTel. Unless there is rapid proliferation involving intraretinal or subretinal neovascularization, in which case, anti-VEGF treatment may be worthwhile,” he emphasized.
The game changer in MacTel research was the shift from thinking vascular to thinking neural.
What was once considered a vascular oddity is now recognized as primarily a neurodegenerative disease. As Prof. Holz explained, neuronal damage occurs first, and the vascular changes follow as secondary effects.
This insight refocused attention on neuroprotection—specifically, the ciliary neurotrophic factor (CNTF), a protein that supports photoreceptor survival. “Preclinical models have shown that photoreceptors could be rescued by injecting into the eye the
CNTF factor,” said Prof. Holz. These findings paved the way for clinical application.
Enter Neurotech Pharmaceuticals’ (Rhode Island, USA) NT-501 (Encelto), an encapsulated cell therapy implant that delivers CNTF directly into the eye. Acting as a self-sustaining reservoir, it continuously produces CNTF over time, offering sustained neuroprotective effects. Impressively, explanted human devices have shown CNTF production lasting up to 14 years, according to Prof. Holz.
The surgical procedure is straightforward. The tiny device is implanted in the vitreous and sutured to the sclera, maintaining steady intraocular CNTF levels without the need for repeated injections.
To secure regulatory approval, strong evidence was essential. Prof. Holz shared data from two parallel Phase III trials (NTMT03A and NTMT03B) conducted across 47 international sites. Participants received either the Encelto implant or underwent sham surgery, with investigators masked to the assignments.4
Eligible patients were aged 21 to 80, each with an ellipsoid zone (EZ) break of moderate size—“not too small” to risk spontaneous regression and “not too large” to remain treatable.4
The primary endpoint was the change in EZ area loss from baseline to month 24, a structural marker accepted by regulators.4 “The primary endpoint was chosen at two years, and not 12 months, due to the slow progression of the disease,” said Prof. Holz.
Secondary endpoints included changes in retinal sensitivity within the EZ break and monocular reading speed, a functional marker of vision.4
The top-line results were impressive. At 24 months, the primary endpoint was met in both trials, with a 54% and 36% reduction in EZ area loss, respectively.4 “Both studies showed a similar effect on function and structured outcome,” said Prof. Holz.
“Treatment with NT-501 versus sham preserved more photoreceptors through two years in both Phase III clinical trials. Overall, the results do demonstrate that the therapy works. Encapsulated cell therapy is effective and found to be generally safe and well tolerated.”
Retinal sensitivity preservation was mixed—significant in NTMT03A, not in NTMT03B—but functional vision outcomes were more consistent.4 “Patients treated could read faster in the end compared to baseline and compared to the sham-treated eye,” he noted.
Safety outcomes were reassuring, with no significant differences in visual acuity loss or treatment-emergent serious adverse events between the NT-501 and sham groups.4
For years, MacTel has left clinicians with little more than crossed fingers and cautious monitoring. Now, with advances in imaging, a deeper understanding of its neurodegenerative roots, and the dawn of CNTF-based therapy, a new era is beginning.
If the momentum continues, the field may soon move from “managing decline” to “preserving function”— and perhaps, one day, to “restoring vision.” That’s not just progress. For MacTel patients and retina specialists alike, it’s a long-overdue glimmer of hope.
Prof. Frank G. Holz’s insights in this article are based on his Day 3 presentation at an EURETINA 2025 Paris session on MacTel Type 2, titled “Current and Upcoming Therapies”.

In summary, these trials mark the first real success in changing the natural course of MacTel. “Treatment with NT-501 versus sham preserved more photoreceptors through two years in both Phase III clinical trials. Overall, the results do demonstrate that the therapy works. Encapsulated cell therapy is effective and found to be generally safe and well tolerated,” Prof. Holz concluded.
1. Holz F. MacTel Type 2: Bridging Diagnosis and Emerging Therapies. Lecture at EURETINA 2025. Paris, France. September 6, 2025.
2. Richer S, Stoumbos VD, Bowling N, et al. The emerging roles of the macular pigment carotenoids lutein and zeaxanthin. Prog Retin Eye Res. 2021;81:100897.
3. Pauleikhoff L, Heeren TFC, Issa PC, et al. Fundus autofluorescence imaging in macular telangiectasia type 2: MacTel Study report number 9. Am J Ophthalmol. 2021;228:27-34.
4. Chew EY, Gillies M, Jaffe GJ, et al. Cell-based ciliary neurotrophic factor therapy for macular telangiectasia type 2. NEJM Evidence. 2025;4(8):EVIDoa2400481.
Prof. Frank Holz is professor and chair of Ophthalmology at the University of Bonn, Germany, and one of Europe’s leading minds in retinal research. After training at the University of Heidelberg, he honed his skills at London’s Moorfields Eye Hospital under the legendary Prof. Alan Bird.
A visionary in both clinic and lab, Prof. Holz co-founded the German Research Foundation Priority Program for AMD and the GRADE Reading Center in Bonn, shaping how retinal diseases are studied and understood.
A member of just about every esteemed retina society you can name—including EURETINA, the Macula Society, the Club Jules Gonin and the Gass Club—he also serves as Editor-in-Chief of Der Ophthalmologe and an ever-demanded journal reviewer.
With over 400 peer-reviewed papers and several textbooks to his name, his work spans surgical and medical retina, retinal imaging and the molecular mysteries behind macular and retinal disease.
frank.holz@ukbonn.de

By Diana Truong
Real patients, real patterns, real insights. Real-world evidence is here to show how treatments actually play out in the clinic. From massive registries to daily practice patterns, discover how RWE is giving ophthalmologists the insights RCTs can’t.
In a world where Netflix knows your next binge before you do and Spotify can predict your mood with a playlist, it feels a little oldfashioned that medicine still leans so heavily on tightly controlled clinical trials…especially when those studies often leave out the kinds of patients ophthalmologists actually see every day.
Just as streaming platforms reshaped how we consume entertainment by tapping into real-world user data, real-world evidence (RWE) is now set to reshape how we practice ophthalmology. But should it?
Let’s take a closer look at the realm of real-world evidence in eye care, where the rubber of research meets the road of clinical reality.
So, what exactly is realworld evidence?
Before we can decide if real-world evidence deserves a starring role in ophthalmology, it helps to know exactly what we’re talking about. RWE refers to clinical evidence gathered from analyzing real-world data (RWD). In other words, information collected outside the structured environment of randomized controlled trials (RCTs).
"A couple of years ago, we started a real-world registry in Germany,” said Prof. Nicole Eter (Germany) at her EURETINA 2025 lecture.1 “It's not only about the retina, it comprises ophthalmology in all the different fields."

According to Prof. Eter, that registry, named Oregis, now holds data from over two million patients, offering a treasure trove of insight with some jaw-dropping numbers:1
• 12.3 million visual acuity assessments
• 2.6 million procedures
• 9.5 million prescribed medications
• 7.2 million diagnoses
• 13.8 million consultation
This real-world data comes from an array of everyday sources: electronic health records (EHRs), disease registries, insurance claims, patient-reported outcomes, wearable devices, smartphone apps and even social media platforms.1
The key takeaway? Unlike clinical trials, which operate in controlled conditions, this data reflects what’s actually happening in real clinics, with real patients, in the beautifully messy world of real life.
Why everyone’s talking about real-world evidence
Traditional RCTs may be the gold standard of research, but let’s be honest, they don’t always sparkle in real life. They’re precise, polished and painstakingly controlled…and that control comes at a cost. Enter RWE, stepping into the scene to fill in the gaps that RCTs can’t quite cover.
Here’s why RWE is gaining traction2:
• Limited generalizability. RCTs tend to exclude the kinds of patients ophthalmologists actually see in clinic: those with comorbidities, older adults, pregnant women and others who don’t fit the textbook profile.
• Cost and time efficiency. Running an RCT can take years (and a small fortune). RWE, on the other hand, can be gathered faster and far more affordably.
• Practical clinical insights. Realworld data show treatments hold up when life gets out of hand:
when patients skip doses, miss follow-ups or juggle multiple conditions.
• Long-term outcomes. RWE can follow patients over extended periods, painting a more complete picture of how treatments perform over time.
• Rare conditions. For uncommon ophthalmic diseases, where it’s nearly impossible to recruit enough participants for a traditional trial, RWE can be the key to unlocking new understanding.
"We are just in the beginning of analyzing our data, and this data grows every day as we have more centers to contribute,” Prof. Eter pointed out. “Comparing your own data to the rest of the registry data may enable you to preserve or change your treatment regimen and then, of course, get better."
In short, RWE isn’t here to replace traditional trials. It’s here to make them smarter, faster and a whole lot more reflective of the real world.
“We are just in the beginning of analyzing our data, and this data grows every day as we have more centers to contribute.”
- Prof. Nicole Eter
So, what happens when all this data actually hits the clinic? According to Prof. Eter, Oregis offers a glimpse into how RWE is changing the ophthalmic game, starting with neovascular age-related macular degeneration (nAMD).
In its first year of tracking treatment patterns, the registry found that patients attended an average of 9.4 appointments, effectively addressing long-standing concerns
about undertreatment back in the early days of intravitreal injections.1
The data also shed light on real-world treatment intervals. “The interval between the intravitreal injections [is] basically 28 days. So this load up of monthly injections appears to be really efficient in the real world,” Prof. Eter noted. “And then there is an average of 42 days for the next three injections."
And Germany isn’t the only country getting in on the RWE action. Around the world, ophthalmology registries are showing just how powerful realworld data can be:
• The IRIS Registry. The American Academy of Ophthalmology's IRIS (Intelligent Research in Sight) Registry has mined RWE to explore everything from cataract surgery outcomes to rare diseases, like identifying 27,339 eyes with giant cell arteritis to determine whether the condition’s incidence varied by season (spoiler: it didn’t).3
• IIH Registries. For idiopathic intracranial hypertension, multiple registries have helped reveal prognostic factors, track disease behavior across patient subgroups and assess the safety of medications during pregnancy.4
• MSBase Registry. This international database provided valuable real-world insights, showing that acute treatment improved visual outcomes and reduced the risk of progression to MS in patients with acute optic neuritis.5
The streaming service approach
If Netflix has taught us anything, it’s that one size definitely doesn’t fit all. Just as the platform tailors recommendations to your viewing habits (true crime binge, anyone?), RWE reminds us that not every patient with the same diagnosis should receive the exact same treatment.
The German registry data on AMD treatment switching drives that point home. “Here you can see the total number of patients who underwent a switch of intravitreal agents,” Prof. Eter noted. “So that was over
6,000, and more than 1,000 patients switched two times."
That’s not just an interesting statistic. It’s a window into how realworld practice actually unfolds. In the regimented world of traditional clinical trials, switching therapies midstream is often a no-go. But in real life? It happens all the time. And RWE captures those nuances beautifully, showing clinicians what truly works for individual patients, not just the average one.
Before we toss our beloved RCTs out the window and declare RWE the new ruler of research, let’s remember: even Netflix occasionally suggests a show we’d never watch. RWE, for all its promise, comes with its own set of quirks and complications.
Here are some of the main challenges that can throw off the algorithm:2
• Data quality and standardization. RWD isn’t collected under the same tightly controlled conditions as RCTs. That means inconsistent entries and messy datasets can sometimes muddy the picture.
• Selection bias and confounding factors. Without randomization, results can easily be skewed by who gets included and who doesn’t.
• Missing data. Incomplete records are the bane of RWE. Some variables just aren’t consistently tracked across every patient.
• Privacy concerns. Patient data is powerful, but protecting it requires airtight security and ethical oversight.
• Interoperability issues. Integrating data from different systems and formats can feel a bit like trying to get Spotify and Apple Music to share playlists.
As Kodra et al.6 puts it, “The quality of data in rare disease registries may be compromised by several factors, including the low prevalence of disease which can limit statistical
power and reliability of the data, and variability in data collection influencing the accuracy of the information gathered."
So, while RWE gives us a bigger, more dynamic picture of clinical life, it’s not without its fuzzy pixels.
It doesn’t have to be a cage match between RWE and RCTs. In fact, most experts agree the smartest approach is to use both…kind of like checking both Netflix’s “Top Picks for You” and the critics’ reviews before committing to a two-hour movie.
“We should use all the tools in our tool chest and use knowledge from all sources in an iterative and synergetic fashion,” Johnson & Johnson Chief Medical Officer Joanne Waldstreicher said in Examining the Impact of RealWorld Evidence on Medical Product Development 7
So what does this partnership actually look like in practice? Think of it as research teamwork:8
• RWE to inspire, RCTs to confirm. Use RWD to generate hypotheses that can later be tested in controlled trials.
• RCTs for proof, RWE for perspective. Let RCTs establish efficacy, turn to RWE to see how those results hold up in broader, messier real-life populations.
• Long-term safety checks. After a treatment wins approval through RCTs, RWE can keep tabs on how it performs over time.
• When RCTs can’t cut it. For rare conditions or emergency settings where traditional trials aren’t feasible, RWE can fill critical knowledge gaps.
In the end, it’s not about choosing sides, it’s about blending the precision of RCTs with the practicality of RWE to create a clearer, more complete picture of patient care.
Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are starting to give RWE a
serious seat at the table. While RCTs remain the gold standard for proving efficacy, regulators increasingly recognize that RWE offers invaluable complementary insights, especially when it comes to safety monitoring and understanding how treatments perform outside the tidy world of clinical trials.9
Regulatory acceptance of RWE is steadily evolving, with some agencies now leveraging it to9:
• Accelerate approval for certain treatments
• Extend approved indications to broader patient populations
• Add safety information to drug labels
• Monitor post-marketing safety and outcomes
For eye care professionals interested in incorporating RWE into their practice, here are some practical steps8:
1. Participate in registries. Contributing to initiatives like IRIS doesn’t just help researchers. It helps build the evidence base that will guide future practice.
2. Compare your outcomes. As Prof. Eter noted, looking at your own data alongside registry results can highlight patterns in your treatment approach and reveal opportunities for improvement.
3. Stay informed. Keep up with publications and studies that use RWE in your specialty area. Knowledge is power, after all.
4. Combine evidence sources. Don’t ditch RCTs. They provide essential efficacy data. Instead, let RWE complement RCT findings for a fuller picture of patient care.
5. Discuss with patients. Sharing relevant RWE with patients can make your consultations more personalized, especially when RWD better reflects their individual circumstances than traditional trials.
Just as streaming services haven't completely replaced traditional television—but have definitely changed how we consume content—real-world evidence won't replace RCTs, but it will transform how we generate and apply evidence in ophthalmology.
The real question isn't whether RWE should influence our practice, but rather how to blend
it with other forms of evidence to deliver the best patient care. As registries like Oregis show, RWE offers insights that complement and extend what RCTs teach us.
The goal isn’t to pick sides between trial types. Instead, it’s about using all available data, from tightly controlled studies to messy, realworld practice, to make smarter, patient-centered decisions. Just like consulting both critics’ reviews and viewer ratings before choosing your next binge-worthy show, combining RCTs and RWE gives clinicians the clearest picture for clinical decisionmaking.
The data is out there – it's up to us to stream it wisely.
Prof. Nicole Eter’s insights in this article are based on her Day 3 presentation at EURETINA 2025 Paris, titled “Recent Advances in the Management of AMD That Are Changing Our Clinical Practice”.

References
1. Eter N. Should Real-World Evidence Change Our Practice? Lecture at EURETINA 2025. Paris, France. September 6, 2025.
2. Costa V, Custodio MG, Gefen E, Fregni F. The relevance of the real-world evidence in research, clinical and regulatory decision making. Front Public Health. 2025;13:1512429.
3. Wladis EJ, Ata A, Li C, et al. The impact of month and season on the incidence of giant cell arteritis: an Intelligent Research in Sight (IRIS) Registry analysis. Graefes Arch Clin Exp Ophthalmol. 2024;262(2):609-614.
4. Thaller M, Homer V, Mollan SP, Sinclair AJ. Disease course and long-term outcomes in pregnant women with idiopathic intracranial hypertension: The IHH Prospective Maternal Health Study. Neurology. 2023;100(15):e1598-e1610.
5. Kenney R, Liu M, Patil S, et al; MSBase Study Group. Long-term outcomes in patients presenting with optic neuritis: Analyses of the MSBase registry. J Neurol Sci. 2021;430:118067.
6. Kodra Y, de la Paz MP, Coi A, et al. Data quality in rare disease registries. Adv Exp Med Biol. 2017;1031:149164.
7. National Academies of Sciences, Engineering, and Medicine. 2019. Examining the Impact of Real-World Evidence on Medical Product Development: Proceedings of a Workshop Series. Washington, DC: The National Academies Press.
8. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763-1774.
9. Alipour-Haris G, Liu X, Acha V, Winterstein AG, Burcu M. Real-world evidence to support regulatory submissions: A landscape review and assessment of use cases. Clin Transl Sci. 2024;17(8):e13903.
Prof. Nicole Eter is a powerhouse in ophthalmology, combining clinical expertise, cutting-edge research and leadership. A graduate of the University of Bonn, she completed her residency there before embarking on a career that spans both the lab bench and the boardroom. From 2017 to 2018, she served as president of the German Society of Ophthalmology and now chairs the Association of Ophthalmological Chairholders, helping shape the future of eye care in Germany.
Her research focuses on age-related macular degeneration, anti-angiogenic therapies, molecular imaging and nanotechnology in ophthalmology. In 2018, she founded the German Register for Health Care Research in Ophthalmology (OREGIS), bridging science and real-world patient care.
A prolific author and speaker, Prof. Eter has published over 200 papers, contributed six book chapters and delivered more than 500 presentations worldwide. Her work is driven by one goal: turning scientific insight into better vision for patients everywhere.
nicole.eter@ukmuenster.de
This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.



By Chow Ee-Tan
Laser-focused on what works and fearless in challenging trends, Dr. María Berrocal blends wisdom, world-class science and a Caribbean spirit to keep retina care grounded and always moving forward.
From San Juan to international symposium spotlights, Dr. María Berrocal (Puerto Rico) embodies not just excellence in retina, but resilience, gratitude and grace. Her journey is a testament to wisdom passed down, shared generously and carried forward…one act of kindness at a time.
Ophthalmology may not technically run in her DNA, but inspiration certainly does. Her father, the late Dr. José Berrocal, was the first retina specialist in Puerto Rico and a pioneer throughout the Caribbean.
“He performed the first scleral buckle in the Dominican Republic,” she recalled, still full of admiration. “Teaching was central to his life. He chaired the Department of Ophthalmology in Puerto Rico and devoted his life to his patients and students.”
Growing up surrounded by rounds, conferences and retina legends from around the world, Dr. Berrocal absorbed excellence as a way of life.
“I worked in his office, rounded with him on weekends and met retina specialists from all over the world. His passion for ophthalmology and teaching shaped my values,” she
said. “I’ve tried to emulate that same dedication, and I’m proud that my children have followed in his footsteps.”
Dr. Berrocal’s medical path led her through the University of Illinois, the University of Puerto Rico (where she earned the Best Surgeon Award) and vitreoretinal fellowships at both Cornell and Bascom Palmer Eye Institute. A brilliant career anywhere was hers for the taking, but her compass pointed home.
“My father and I were very close, and I wanted to work alongside him,” she recalled. “I also felt a strong commitment to serve the people of Puerto Rico, where diabetes and its complications are so prevalent. I knew I could make the greatest impact there.”
That decision anchored her life’s mission: to honor a legacy through innovation, compassion and the mentorship of generations to come.
The many hats of a single mission
Today, as CEO of Drs. Berrocal and Associates in San Juan and an Associate Professor at the University of Puerto Rico, Dr. Berrocal simultaneously leads, teaches, performs surgery, researches
and advocates. But don’t call it “balancing.”
“I don’t like the word ‘balance,’” she said with a smile. “I don’t think of my work as something to balance. I simply love what I do. Teaching, traveling, seeing patients and performing surgery are part of my life’s mission.”
That unity carries into her home as well.
Dr. Berrocal’s husband, an oncologist, shares her devotion to patients. Her daughter helps run the practice as COO. And her son—soon to return after completing his retina fellowship—will extend the family tradition. “Our shared purpose makes everything flow naturally,” she said. “It’s not work-life balance; it’s one life, lived with purpose.”
Research with a real-world pulse
With more than 180 scientific publications, Dr. Berrocal’s research is prolific yet deeply grounded in patient reality.
“Through PACORES, the PanAmerican Collaborative Retina Study Group, we’ve conducted multicenter studies across Latin America,” she explained. “This is essential real-world research that reflects our region’s genetic, ethnic and environmental diversity.”
Dr. Berrocal’s focus on diabetic retinopathy, one of the leading causes of blindness worldwide, reveals both scientific precision and social awareness.
“The diabetes explosion stems largely from obesity and poor nutrition,” she explained. “GLP-1 inhibitors and awareness of ultra-processed foods may help address the root causes, but poverty and limited access to care remain systemic issues that require policy solutions.”
Dr. Berrocal sees prevention leading the future: long-acting drugs, gene therapy and multi-target approaches that intervene earlier and more effectively.
“A patient who lives 12 hours from an ophthalmologist needs a different strategy than one with immediate
access to care,” she said. “We must tailor treatments to each patient’s reality, otherwise, the best science will still fail in the real world.”
At EURETINA 2025, Dr. Berrocal delivered the prestigious Ingrid Kreissig Award Lecture. Titled Don’t Throw Away Wisdom, it served as both a warning and a wake-up call.
“We often embrace new treatments as ‘modern’ without carefully examining the evidence,” she explained. “Minimal scleral buckling—segmental or radial, no drainage—for phakic rhegmatogenous detachments provides better visual outcomes and fewer complications than vitrectomy. Yet many have abandoned it.”
Her perspective extends to proliferative diabetic retinopathy, where she reinforces that pan-retinal photocoagulation remains the gold standard.
“The treatment burden and cost are unsustainable,” she cautioned. “Patients lost to follow-up after anti-VEGF monotherapy often suffer devastating complications that could have been avoided with laser treatment. This is a global problem, as injections are mistakenly viewed as simpler alternatives.”
Her advocacy is steadfast: evidence first, marketing never. She also pointed out that higher mortality signals in diabetics treated with monthly anti-VEGF reinforce the need to scrutinize real-world outcomes.
Among Dr. Berrocal’s many international accolades—including the American Academy of Ophthalmology Lifetime Achievement Award, the American Society of Retinal Specialists Founders Award and the Stanley Chang Lectureship— one holds special meaning: the Kreissig Award Lecture.
“I trained with Harvey Lincoff, who pioneered minimal surgery with Ingrid Kreissig, a true role model and one of the first women to chair an ophthalmology department,” she shared. “Coming from a small
Caribbean island, as a Hispanic woman, to be recognized by the European retina community for continuing that legacy was profoundly humbling.”
As one of the most respected female leaders in retina, Dr. Berrocal continues to mentor and inspire young women in ophthalmology worldwide. Her message is one of empowerment rooted in grace. A reminder that leadership doesn’t require imitation, but authenticity.
She remembered what it felt like to be one of few women in a maledominated specialty. “Be yourself. Don’t try to be like men,” she said. “Women bring unique strengths to medicine: empathy, listening and a holistic concern for patients’ lives.”
Dr. Berrocal noted that studies show better outcomes for patients of female physicians, evidence that communication and understanding are powerful tools, not soft skills.
“Overcome imposter syndrome,” she advised. “Women who reach this level have often had to be more qualified than their male peers. Own it. Be proud, confident and kind.”
In the coming years, she hopes to contribute more to global training efforts.
“My experience with ORBIS in Bangladesh was one of the most fulfilling of my life—teaching local retina specialists, many of them women, and adapting therapies to their realities was deeply inspiring,” she recalled. “True progress means understanding local needs, not exporting one-size-fits-all solutions. I hope to continue sharing knowledge with colleagues working under challenging conditions.”
Her greatest legacy, however, remains rooted close to home.
“I’m grateful every day for my health after surviving cancer, for my family and for the privilege of doing what I love,” she said. “My son will soon join me in practice. My daughter continues to manage the clinic. And my sister—also a retina specialist— practices in Miami. Our family’s life work continues, and hopefully, we will make life better for others.”

Her service extends far beyond the clinic, from scholarships for cancer survivors to educational support programs and contributions to drug rehabilitation initiatives in San Juan.
Privilege, she said, demands responsibility. “Being born into a supportive family, in a society that values education, is an immense privilege,” she noted. “I remind my children to stay humble and grateful, because many with equal or greater talent never get the same chance.”
Looking ahead, Dr. Berrocal envisions a retina field transformed by more targeted, longer-acting therapies and the expanding use of gene therapy. Surgically, she anticipates continued evolution with smaller-gauge instrumentation and digital visualization.
Dr. Maria Berrocal is a force of nature in retina care: equal parts surgeon, innovator and community champion. Based in San Juan, Puerto Rico, she leads Drs. Berrocal and Associates while training future eye specialists as an Associate Professor at the University of Puerto Rico. A standout from the very start, she completed her ophthalmology training there, earning the Best Surgeon Award, before refining her surgical and medical retina expertise at New York Hospital/ Cornell University and Bascom Palmer Eye Institute as a Heed Fellow under Dr. J. Donald Gass.
Her research sharpens the tools of vitreoretinal surgery, particularly for diabetic retinopathy, and she has contributed more than 180 publications to the field. Her achievements have earned her the AAO Lifetime and Senior Achievement Awards, the ASRS Senior Honor Award, the prestigious Euretina Ingrid Kreissig Lecture Award and a lengthy list of additional accolades. Beyond the clinic and the podium, Dr. Berrocal invests deeply in her community, supporting education, rehabilitation and mentorship throughout Puerto Rico.
mhberrocal@drsberrocal.com

How widefield OCT-A is expanding the view of diabetic retinopathy

By Kendra Bruning
Once limited to the macula, OCT angiography is widening its gaze to reveal ischemia, neovascularization and subtle signs of progression before they reach the center. With sharper detail and broader scope, the technology is changing how clinicians map, monitor and manage diabetic eye disease.
Sometimes progress begins by stepping back. For decades, diabetic retinopathy (DR) was mapped through a tight, maculacentered view that left much of the retina out of frame.
Now, with widefield optical coherence tomography angiography (OCT-A), clinicians are seeing the full circulation: the distant capillaries, the pockets of ischemia, the earliest neovascular sprouts. What once looked stable from the center is now revealed as dynamic and evolving at the periphery.
Diabetic retinopathy used to be studied through a keyhole. Now, the lens has widened and the view keeps getting bigger.
The earliest stress signals often appear in the retinal periphery, where areas of non-perfusion forecast later macular decline.1 Widefield OCT-A pulls those distant regions into the picture, charting microvascular change that used to slip beyond the frame.2
“Color fundus imaging is our gold standard for monitoring disease stages. It is quick, it is noninvasive and we can use it to stage disease severity,” said Dr. Andreas Pollreisz (Austria) during his EURETINA 2025 lecture.2 “But on OCT angiography, you see much more detail because
there is no leakage, and you can visualize the swollen capillaries at high lateral resolution.”
The secret lies in motion contrast. OCT-A captures multiple scans within milliseconds at the same retinal spot, then calculates the signal differences that trace blood flow.3 The result is a dye-free, depth-resolved map of circulation that can expose early remodeling invisible on fundus imaging.
Expanding the field still brings tradeoffs. “So far, we have a limited field of view,” explained Dr. Pollreisz. “When we go beyond the posterior pole, we face certain compromises. A wider capture needs longer acquisition time, which increases motion artifacts, and there are trade-offs in image quality and resolution. All of these factors need to be considered together.” Even so, 65° single-capture and 24 × 20 mm² systems already reach far beyond the macula, and research prototypes keep stretching toward true ultra-widefield coverage.4,5
Fluorescein angiography (FA) still draws the roadmap, but OCT-A offers the return trip: quicker, cleaner and needle-free. Use fluorescein to lay out the map and use widefield OCT-A to revisit it often, with more layers and less friction.
The periphery often writes the plot. Ultra-widefield FA first showed that
retinal non-perfusion tracks with worsening disease and treatment need.1 Widefield OCT-A now measures the same story without dye, using an ischemic index (the non-perfused area divided by total area) to turn pictures into prognostics.
“Retinal non-perfusion in the periphery is significantly associated with the stage of diabetic retinopathy,” Dr. Pollreisz noted. “Higher stages need more extensive non-perfusion.”
Eyes with predominant peripheral lesions show a higher ischemic index and are more likely to develop macular edema (ME) or require antiVEGF or panretinal photocoagulation (PRP).6 For clinicians, that means these patients need tighter followup and earlier intervention. The periphery isn’t the background. It’s the plot twist that tells you what happens next.
Some lesions look alike in a snapshot but tell very different stories over time. OCT-A can separate the characters. “A very useful application for quick and easy, non-invasive imaging is using OCT angiography to differentiate between intraretinal microvascular abnormalities [IrMA] and neovascularization,” said Dr. Pollreisz. “When flow extends into the vitreous, that’s neovascularization. If it stays below the internal limiting membrane, it’s IrMA.”
The “ILM [internal limiting membrane]-breach” rule developed in 2020, when researchers showed that OCT-A could objectively flag whether vessels crossed that invisible boundary.7 This turned a subjective call into a clear diagnostic
line, giving clinicians an objective, reproducible way to classify vascular change.
Longitudinal OCT-A adds motion to the picture, showing neovascular regression after antiVEGF, quiescence after PRP and architectural repair post-surgery.8 Think of it less as a still life, more as a live feed of the retina’s recovery arc.
Some of the most telling features are invisible to the naked eye. Widefield OCT-A has revealed subclinical neovascularization, those delicate proliferations that slip past ophthalmoscopy and fundus photography. In one study, graders detected such hidden lesions in 19% of eyes graded as non-proliferative by conventional criteria.9
Coverage is everything. A 65° singlecapture system demonstrated 95% sensitivity for detecting proliferative DR, showing that peripheral reach is crucial for reliable diagnosis.10 When those hidden lesions appear, they often coincide with mid-peripheral ischemia—an early warning that the disease is poised to advance.2
In practical terms, OCT-A doesn’t just widen the frame. It stretches the timeline, letting clinicians preview where the disease is heading rather than just where it’s been.
References
1. Silva PS, Marcus DM, Liu D, et al. Association of ultra-widefield fluorescein angiography–identified retinal nonperfusion and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140(10):936-945.
2. Pollreisz A. OCT-A Widefield Imaging: Must have or nice to have? Lecture at EURETINA 2025. Paris, France. September 7, 2025.
3. Sun Z, Yang D, Tang Z, et al. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye (Lond). 2021;35(1):149-161.
4. Zeng QZ, Li SY, Yao YO, et al. Comparison of 24×20 mm² swept-source OCTA and fluorescein angiography for the evaluation of lesions in diabetic retinopathy. Int J Ophthalmol. 2022;15(11):17981805.
5. Xia F, Hua R. The latest updates in swept-source optical coherence tomography angiography. Diagnostics. 2024;14(1):47.
6. Stino H, Huber KL, Niederleithner M, et al. Association of diabetic lesions and retinal nonperfusion using widefield multimodal imaging. Ophthalmol Ret. 2023;7(12):1042-1050.
7. Arya M, Sorour O, Chaudhri J, et al. Distinguishing intraretinal microvascular abnormalities from retinal neovascularization using optical coherence tomography angiography. Retina. 2020;40(9):16861695.
For all its panoramic power, OCT-A still works best with a travel companion. “Fluorescein angiography is still necessary when we need to visualize the full retinal extent, which we cannot always achieve with widefield OCT angiography,” Dr. Pollreisz said. “We usually perform a baseline FA that guides us to suspicious lesions we can then follow with OCT-A.”
That tandem approach mirrors the American Society of Retina Specialists (ASRS) 2024 guidelines, which frame multimodal imaging as the new standard.11 Fluorescein offers the grand map and leakage timeline, whereas OCT-A supplies repeatable, depth-resolved detail.12 Reducing dye exposure also spares patients from the small but real risk of allergic reactions to fluorescein.13
It’s less a rivalry and more a relay in which fluorescein draws the map and OCT-A updates it in real time. And because OCT-A is fast, dye-free and endlessly repeatable, it turns followup visits into ongoing surveillance rather than staged events. The multimodal strategy of one map and many revisits mirrors how retina specialists work today: focused, efficient and using each tool for what it does best.
Each generation of widefield OCT-A pushes the edge outward while cutting acquisition time and motion blur. What began as a research luxury is fast becoming standard practice.
Fluorescein may keep its compass role, but OCT-A provides the panorama, the wide shot that gives every close-up its context. The view will continue to widen, but the goal isn’t just a bigger picture. It’s a sharper one, where every corner of the retina contributes to understanding the whole. In the evolving story of diabetic retinopathy, the view is finally big enough to match the disease.
Dr. Andreas Pollreisz’s insights in this article are based on his Day 4 presentation at EURETINA 2025 Paris, titled “OCT-A Widefield Imaging: Must Have or Nice to Have?”.

8. Russell JF, Shi Y, Hinkle JW, et al. Longitudinal wide field swept source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Ret. 2019;3(4):350-361.
9. Tsuboi K, Mazloumi M, Guo Y, et al. Utility of en face OCT for the detection of clinically unsuspected retinal neovascularization in patients with diabetic retinopathy. Ophthalmol Ret. 2023;7(8):683-691.
10. Stino H, Niederleithner M, Iby J, et al. Detection of diabetic neovascularisation using single-capture 65°-widefield optical coherence tomography angiography. Br J Ophthalmol. 2024;108(1):91-97.
11. Ramakrishnan MS, Kovach JL, Wykoff CC, et al. American Society of Retina Specialists Clinical Practice Guidelines on Multimodal Imaging for Retinal Disease. J Vitreoretin Dis. 2024;8(3):234-246.
12. Parravano M, Cennamo G, Di Antonio L, et al. Multimodal imaging in diabetic retinopathy and macular edema: An update about biomarkers. Surv Ophthalmol. 2024;69(6):893-904.
13. Kornblau IS, El-Annan JF. Adverse reactions to fluorescein angiography: A comprehensive review of the literature. Surv Ophthalmol. 2019;64(5):679693.
Dr. Andreas Pollreisz is a specialist in ophthalmology at the Medical University of Vienna, his clinical expertise spans age-related macular degeneration, diabetic retinopathy and vitreoretinal surgery. But step into his research world, and you’ll find him pushing imaging technology to its limits.
Dr. Pollreisz’s work dives deep into the power of retinal imaging— from OCT and OCT-angiography to adaptive optics systems that reveal the eye’s hidden microcosm. Beyond imaging, he investigates the pathobiology of the retinal pigment epithelium and the molecular pathways driving retinal angiogenesis.
Having honed his scientific skills at Columbia University’s Division of Surgical Sciences in New York, Dr. Pollreisz now leads the Translational Ophthalmology Research Group and serves as deputy leader of the Vienna Clinical Trial Center.
andreas.pollreisz@meduniwien.ac.at



By Diana Truong
Think sustainability can’t mix with retina care? Think again. These clever tweaks prove you can cut waste, save cash and keep patient safety on beat.
In a world where “reduce, reuse, recycle” rules the eco-conscious crowd, the medical field—and particularly ophthalmology—is finally spinning the same tune. Think of retina specialists as DJs of sustainability, remixing their clinical routines for a greener future without missing a beat in patient care.
Medicine’s inconvenient truth
“The climate crisis is a great health threat of the 21st century,” warned Dr. Johannes Birtel (Germany) during his EURETINA 2025 lecture1, noting that “about 5% of all global emissions are due to the healthcare sector.” It’s a stark reminder that while retina specialists work in one of the most resource-intensive corners of medicine, they also hold tremendous potential to drive meaningful change.
And the data backs it up. Recent research shows that eye surgeons are becoming increasingly aware of their environmental footprint, and many are ready to do something about it.2
The same could be said on the patient end. When those in injection clinics were surveyed, “patients really think that climate change is a significant issue and should be
addressed by ophthalmologists,” Dr. Birtel pointed out.
Just like how a subtle bass line can completely change the mood of a song, a few simple tweaks in your practice can make a big impact on sustainability. Dr. Birtel offered several easy ways to go greener that don’t require reinventing the wheel:
Power down the OR. “You can reduce the ventilation of the OR…30 minutes before surgery, which actually offers as [good] infection control as 24-hour ventilation,” said Dr. Birtel. In other words, same safety, far less energy wasted.
Water conservation. Evidence shows that “alkaline solutions are actually superior to surgical scrub brush,” Dr. Birtel noted, allowing surgeons to “save quite a lot of water, about 50 liters…for surgery.” Multiply that by 15 cataract cases a day, and surgeons can be saving the equivalent of “drinking water for a whole year in just one theater.” Not bad for a simple switch.
Major ophthalmology organizations like the American Academy of Ophthalmology (AAO) recommend that “eye drops should be used in multiple patients,
also multiple dose containers, if actually proper guidelines are following this regard.” Medications should also be used until they expire. And if it’s appropriate, patients can take them home.3 Less waste, more sense.
For retina specialists, intravitreal injections are where sustainability can really hit its stride. With roughly 1.5 million injections performed every year in Germany alone—and even more across the United States—the environmental footprint adds up fast.4
Dr. Birtel’s research revealed that injection sets can range in weight from 90 grams to 280 grams. “Most of the material was plastic-based. So [with] 1,000,000 injections, [you create] around 165 tons of waste per year, which is equivalent to 302 tons of CO2,” he explained, showing that small procedural choices can have massive environmental echoes.
The Oxford Eye Hospital (UK) offers a smart blueprint for easing into more sustainable injection practices, one small change at a time.
“It was quite clever [how] a small reduction over time was performed,” Dr. Birtel said. “In 2017, topical antiemetic eye drops were omitted. Then in 2020, the drape was optional. In 2022, the lid speculum became optional. And now in 2024, injection packs have been omitted.”
By phasing in these changes gradually, the hospital could closely track outcomes, and the results speak volumes. Despite the scaledback supplies, there was no rise in endophthalmitis rates, not to mention the environmental and financial payoffs (more on that later).5
When it comes to intravitreal injections, Dr. Birtel argued that we may be overcomplicating things. His research shows that a more minimalist, material-sparing approach can slash injection set waste by a whopping 99%, all without sacrificing patient safety.4
So, what does this pared-down process look like? It’s surprisingly simple: just a topical anesthetic followed by povidone iodine drops in the conjunctival sac.
Many injectors skip the lid speculum altogether, gently retracting the eyelids with their fingers instead. Even the injection site can be marked with the needle cap, no fancy calipers required.
Sustainability extends outside of the injection room. Dr. Birtel encouraged a rethink of standard care, saying, “We should also ask…’Do we have to do all the checkups and followup examinations before and after surgery?’”
His research uncovered major variations in perioperative routines for cataract surgery, suggesting that there’s room to “streamline not only for cataract surgery, but also for retinal surgeons…to see how we can really focus on things which are important.” In other words, less busywork, more meaningful care.
And then there’s the hidden culprit: travel. Patient transport adds up to a hefty chunk of emissions, contributing to an estimated 10.49 kg CO2eq per injection—the single largest contributor to the injection carbon footprint.6 As Dr. Birtel noted, novel therapeutic agents that reduce treatment frequency could go a long way toward shrinking that number.
For those ready to embrace sustainability in retina care, here’s a comprehensive playlist of changes to consider:
1. Optimize ventilation in operating rooms
2. Use alcohol-based hand rubs instead of water-based surgical scrubs
3. Reduce drug waste by using multidose containers appropriately
4. Minimize packaging waste by reviewing what’s truly necessary in surgical packs
5. Increase efficiency in patient care by reducing unnecessary visits
6. Consider same-day bilateral injections when appropriate
7. Eliminate topical antibiotics for intravitreal injections, as evidence suggests they’re ineffective in preventing endophthalmitis7
8. Adopt a minimalist approach to injection supplies
9. Evaluate the environmental impact when choosing between anti-VEGF agents
Perhaps the most compelling argument for sustainability is also the simplest: it saves money. As Dr. Birtel puts it, “If you go for a sustainability approach, it can often also save a lot.”
Take the Oxford Eye Hospital’s sustainable injection protocol, for example. It’s estimated to save £150,000 to £180,000 a year while cutting more than 3,000 kg of waste annually. That’s cash and carbon saved in one go, plus resources that can be reinvested into better patient care or new services.
The sustainable future of retina care
Like a DJ who knows just when to drop the beat, the timing for sustainability in retina care couldn’t be more perfect. The climate crisis is calling for action, and ophthalmologists are in a prime position to make changes that benefit both their patients and the planet.
When it comes to remixing your practice, start with the easy tracks first: tweak your OR ventilation, save some water and cut down on drug waste. Once you’ve got the rhythm, move on to the headliners: revamping injection protocols or rethinking perioperative care.
The data speaks volumes. Sustainable practices in retina care can dramatically reduce environmental impact without sacrificing safety or outcomes. And just like a killer remix can breathe new life into a classic tune, these small shifts can energize your practice while helping to create a healthier world.
So, grab your headphones, cue up that sustainability track and start remixing your retina routine. The planet—and your bottom line—will be dancing in gratitude.
Dr. Johannes Birtel’s insights in this article are based on his Day 3 presentation at a session on sustainability in retinal health care at EURETINA 2025 Paris, titled “Sustainable Options Made Easy”.
1. Birtel J. Sustainable Options Made Easy. Lecture at EURETINA 2025. Paris, France. September 6, 2025.
2. Nanegrungsunk O, Kunavisarut P. Toward a greener vision: A review on advancing sustainability in ophthalmology. Asia-Pacific Journal of Ophthalmology. 2025;14(2):100182.
3. Palmer D, Tauber J, Lee AG, et al. Sustainability and ophthalmology. AAO. October 6, 2025. Available at: https://eyewiki.org/Sustainability_and_ Ophthalmology. Accessed on October 7, 2025.
4. Birtel J, Hammer M, Feltgen N, et al. Intravitreal injections: Improving sustainability by reducing clinical waste. Klin Monbl Augenheilkd. 2024;241(10):1156-1162.
5. Ong AY, Buckley TMW, Birtel J, de Silva SR, Stone N, Issa PC. Towards greener intravitreal injections: The Oxford Eye Hospital experience. Eye. 2025;39:2489-2491.
6. Birtel J, Schellstede A, Pauleikhoff LJB, Spitzer MS, Yang-Seeger D. Travel-associated emissions of intravitreal injections. IOVS. 2025;66(8):4119.
7. Hunyor AP, Merani R, Darbar A, Korobelnik JF, Lanzetta P, Okada AA. Topical antibiotics and intravitreal injections. Acta Ophthalmol. 2018;96(5):435-441.

Dr. Johannes Birtel is a globetrotting ophthalmologist with a knack for turning complex eye science into real-world impact. Currently a consultant at the University Medical Center HamburgEppendorf in Germany, he has worn many hats—from postdoctoral researcher at Oxford’s Nuffield Laboratory to MBA graduate with summa cum laude honors. His training reads like a travel itinerary for the ambitious: Harvard, Zurich, Sydney, Tanzania, London… the list goes on.
A decorated researcher, Dr. Birtel has scooped up awards from Bayer, Novartis and multiple German foundations, reflecting his pioneering work in ophthalmology. Whether in the clinic or the lab, Dr. Birtel blends sharp clinical insight with a global perspective to keep the future of eye care bright.
j.birtel@uke.de


By Dr. Perfecto E.O.R. Cagampang III
What started as a simple case submission turned into a DOG debut, a scholarship surprise and a proud first for Philippine ophthalmology.
“Good Relations!” my professor once told me. That phrase echoed in my mind as I made my way to the 2025 German Ophthalmology Society (DOG) Congress at the Estrel in Berlin.
I first attended the DOG back in 2019, when I was still a guest researcher in Heidelberg under the auspices of Prof. Gerd Auffarth at the David J. Apple Laboratory, Department of Ophthalmology, Heidelberg University. Back then, I was merely a delegate. And, truthfully, more of a wide-eyed
spectator. My Deutsch was minimal, to say the least.
Fast forward several years (and three international ophthalmology fellows) later, I received quite the surprise: my application for the DOG Scholarship Grant had been approved. I had submitted two pieces of work: a talk titled Ophthalmic Care in the Philippines and a poster presentation on Sympathetic Ophthalmia Treatment: A Case Treated for Three Years.
I had simply rummaged through my collection of cases and previous
talks, not expecting much. Two months later, in May 2025, an email from Jutta landed in my inbox with wonderful news. My poster on sympathetic ophthalmia had been accepted for presentation. And not just for display. I’d also have to give a three-minute talk about it, followed by a two-minute Q&A.
Even more exciting, my lecture Ophthalmic Care in the Philippines would be part of the international ophthalmology session. I later gave it a more poetic title: Ophthalmology Across 7,641 Islands: The Philippines
The poster session took place on September 27, 2025—a personal milestone, as I became the first Filipino ophthalmologist invited to give a poster talk at the DOG. That was quickly followed by my presentation on Philippine ophthalmic care, a seven-minute talk with a lively three-minute Q&A. Another first: it was the debut of a Filipino speaker in an all-Germanspeaking meeting.
The third highlight came as an even greater honor: I was awarded the prestigious DOG Fellowship Grant, which included an all-expensepaid travel, accommodation and a two-week stipend for observership and shadowing in my chosen field. I selected Dresden University, only a
few hours by train from the Estrel in Berlin.
Dresden also held special meaning. It’s where my former Heidelberg colleague, Dr. Ramin Khoramnia, now serves as chief of Ophthalmology, and where another colleague and former international
fellow, Dr. Tadas Naujokaitis, is currently practicing.
In Berlin, I finally met the organizers who made my participation possible. It was a truly eye-opening experience, and one that left me eager to return to the DOG again soon.


Dr. Perfecto E.O.R. Cagampang III is a board-certified ophthalmologist and retina specialist. He earned his MD in 1996 and completed his ophthalmology residency in 2000, followed by a retina fellowship at the Eye Referral Center in Manila. In 2011, his passion for advancing retinal care took him to Europe as a scholar at the European Vitreo-Retina Society (EVRS) Training School.
Today, Dr. Cagampang is an active member, moderator and speaker at EVRS, while also leading the Scientific Program Committee at Manila Doctors Hospital. He plays a key role in curating top-tier educational programs for both his hospital and the Philippine Academy of Ophthalmology.
With numerous journal publications and a knack for bringing brilliant minds together, Dr. Cagampang continues to illuminate new horizons in vitreoretinal medicine.
pcagampangiii@yahoo.com
In a move that could reshape how IRDs are treated, two innovative companies are joining forces to make long-lasting vision therapies a reality.
PolyActiva (Victoria, Australia) and RareSight (California, USA) have announced a strategic collaboration to co-develop sustained-release therapies for rare pediatric retinal diseases, marking PolyActiva’s expansion into new ophthalmic indications beyond glaucoma.
“This collaboration represents an important step in PolyActiva’s growth as we expand our proprietary platform into new therapeutic areas,” said PolyActiva CEO and Board Director Jerry St. Peter in a news release. “Partnering with RareSight allows us to apply our PREZIA technology beyond glaucoma to address the urgent unmet needs of children living with inherited retinal disorders. This effort reflects our broader vision to build a diversified ophthalmic pipeline that delivers durable, targeted and potentially life-changing treatments for people who have long been overlooked.”
The partnership will harness PolyActiva’s biodegradable sustain-release implant platform and RareSight’s therapeutic
discovery capabilities to address inherited retinal diseases (IRDs) that affect children. The companies plan to develop long-acting pharmacologic therapies capable of maintaining intraocular drug delivery for extended duration, minimizing the need for repeated procedures.
“At RareSight, we are dedicated to preventing sight loss caused by rare pediatric eye diseases,” said RareSight CEO and Founder Carmen Caricchio. “By combining RareSight’s therapeutic discovery and development expertise with PolyActiva’s novel drug delivery platform, this collaboration represents a major step toward transforming how inherited eye diseases are treated. Together, we are advancing a new class of long-acting pharmacologic therapies to address early-onset vision loss, with the potential to change a child’s life and bring hope to generations to come.”
The two companies have not disclosed specific molecules or timelines for initial clinical evaluation, but early-stage formulation and feasibility studies are underway.
According to IRD specialist Dr. Sandeep Grover, “As a pioneer of the earliest
research in this field and caring for kids and their families for more than two decades, I am encouraged by novel therapeutic strategies that support our goal of helping patients maintain vision, function and independence as long as possible.”
The collaboration with RareSight expands PolyActiva’s clinical-stage ophthalmic portfolio, which includes PA5108, a new chemical entity and biodegradable ocular micro-implant developed for sustained intraocular pressure control of up to six months using the PREZIA platform. The Phase IIb U.S. study, involving about 75 patients across 12 sites, is currently evaluating the implant’s safety, tolerability and durability over time.
By extending this proprietary pro-drug technology into new therapeutic areas, including rare pediatric retinal diseases, the two companies aim to bring forward much-needed drug-based options for underserved patient groups.
A version of this article was first

By Kendra Bruning
This dry AMD session outlined hidden risks, trial bottlenecks and the growing promise of precision.

Photo: Fadaway Creative/Shutterstock.com
On Day 1 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025), the Nonexudative AMD symposium gathered five international experts who examined supplements, imaging biomarkers, structure-function mapping, AI models and the evolving treatment pipeline in meticulous detail.
A unifying current ran through the talks, framing geographic atrophy (GA) as layered, multifactorial and anything but uniform, demanding sharper endpoints, individualized risk assessment and earlier, smarter intervention.
What’s next in the dry AMD pipeline?
Dr. Jordi Monés (Spain) opened the age-related macular degeneration (AMD) session with a sober but forward-looking assessment of where the field stands on GA.
Despite recent approvals of complement inhibitors such as pegcetacoplan (Apellis Pharmaceuticals; Massachusetts, USA) and avacincaptad pegol (Astellas Pharma; Tokyo, Japan), progress in Europe has felt glacial, slowed by regulatory lag and trial design hurdles.
For Dr. Monés, the central issue is precision. GA is not a monolith but a spectrum of phenotypes that are sometimes fast, slow and resistant, and “lumping all patients into one category,” he warned, risks diluting meaningful results.
He emphasized the importance of phenotypic stratification and argued for incorporating structural biomarkers such as subretinal drusenoid deposits and photoreceptor thinning as enrichment tools and endpoints, especially in earlier pre-atrophic stages where intervention may be most effective. Adaptive trial designs that capture both structure and function will be key to unlocking progress.
“The disease is so different and the phenotypes are so different,” Dr. Monés said. “That is like if you were asking, ‘Are you going to treat skin cancer and pancreatic cancer with the same drug?’ No way, it’s probably a very different animal. So we will need to do different therapies to different patients and maybe combined therapies.”
The future, he concluded, lies in tailored, earlier intervention that shifts GA from “wait and watch” to “diagnose and intervene.”
Dr. Tiarnan Keenan (USA) revisited the AREDS story with a sharper lens, asking whether supplements might still have a role in GA. While past analyses suggested little impact on lesion growth, his re-examination of AREDS and AREDS2 told a more nuanced tale.
By applying a “growth-to-center” metric that tracked how quickly lesions encroach on the fovea, Dr. Keenan found signals that standard area-based measures had missed. Supplementation slowed GA’s advance toward central vision by roughly 35%, and when lutein and zeaxanthin were factored in, the risk of foveal involvement was cut by more than half.*
These effects were most apparent in extrafoveal lesions, where functional vision remains intact. “The area metric is blunt,” he noted. “It misses the functional relevance of where the lesion is progressing.”
Zinc and other components showed subtler, stage-dependent patterns, though none were definitive. Importantly, these insights emerged from secondary analyses, so confounding remains a concern.
“We need a new dedicated prospective trial looking specifically for this as part of the AREDS 3 program… even when people develop geographic atrophy and particularly in that extrafoveal case,” said Dr. Keenan, stressing the need for validation.
For now, supplements remain safe, inexpensive and accessible, and provide a modest but meaningful tool in an otherwise limited armamentarium.
“Best-corrected visual acuity alone is no longer enough.” With that, Dr. Stela Vujosevic (Italy) set the tone for her call to rethink endpoints in nonexudative AMD.
While OCT holds the fort as the backbone of diagnosis, she stressed that structure must be paired with function if trials are to reflect real-
world patient experience. “Structural imaging tells us what’s there,” she explained, “but functional tests tell us what the patient experiences.”
Dr. Vujosevic highlighted tools such as low-luminance visual acuity (LLVA), microperimetry, dark adaptometry and contrast sensitivity that are all validated in longitudinal studies like MACUSTAR. Their utility varies by disease stage: extrafoveal lesions align best with microperimetry and LLVA, while fovea-involving disease may still justify BCVA.
Importantly, evidence suggests functional decline often precedes structural change, meaning these assessments can flag vision loss earlier than imaging alone.
Dr. Vujosevic also pointed to OAKS trial data showing that microperimetry demonstrated preserved function with pegcetacoplan, illustrating how smarter endpoints can capture therapeutic benefits that BCVA misses. She concluded that “the composite endpoints of structure and function that are tailored to GA progression, patient needs would be ideal for upcoming clinical trials, allowing earlier intervention”.
For physicians, this means moving beyond lesion size to patientcentered measures that better guide care.
Prof. Robyn Guymer (Australia) shifted the spotlight to intermediate AMD—the gray zone where disease is present but its future course is unpredictable.
She argued that fundus-based classifications undersell the precision now possible with OCT, where biomarkers such as nascent GA, incomplete retinal pigment epithelium (RPE) and outer retinal atrophy (iRORA), and complete RPE and outer retinal atrophy (cRORA) are rewriting the risk playbook.
Hazard ratios climb as high as 78 for nascent GA, a stark measure of their predictive weight. Retinal layer changes, including early loss of the
ellipsoid zone and external limiting membrane, can also flag progression years before overt GA emerges.
Subretinal drusenoid deposits (SDDs) drew particular focus. “SDDs are not just a variant,” Prof. Guymer noted. “They represent a fundamentally different phenotype with faster progression and poorer outcomes.”
Differentiating SDDs from softer drusen is critical, not only for prognosis but also for eventual treatment matching. Her group has even released an AI-driven algorithm to standardize SDD detection across large datasets, an advance that could sharpen enrichment strategies in future trials.
“If you want to be highly specific in your predicting ability, then we’re really not very accurate. We’re less than 50% likely to predict who’s most likely to progress,” said Prof. Guymer, acknowledging current limitations.
Closing the session, Dr. Maximilian Pfau (Germany) reframed GA through a three-dimensional lens.
“It’s not a two-dimensional disease,” he declared, introducing the concept of GA as a funnel of photoreceptor degeneration extending far beyond the visible atrophic border. Using AI-powered OCT segmentation and deviation maps, his team tracked outer nuclear layer (ONL) thinning, a marker that predicts both future lesion growth and therapeutic response.
Patients with broader ONL loss at baseline progressed more quickly, yet paradoxically responded better to treatment. This convergence of risk and opportunity highlights the potential of phenotype-specific modifiers, such as subretinal drusenoid deposits, to guide therapy allocation and refine trial design.
Dr. Pfau likened this to treatment effect modification, well established in intermediate AMD and now reaching into GA.
Beyond anatomy, his team has built AI models capable of inferring functional vision directly from OCT, effectively bypassing the need for microperimetry. These sensitivity maps offer higher spatial resolution and greater reliability for longitudinal tracking.
As he explained, “AI-based inferred sensitivity can actually be used as a surrogate of microperimetry in real world clinics and it provides much better reliability and spatial resolution.”
For Dr. Pfau, AI is not an accessory but the engine powering smarter endpoints, earlier intervention and more personalized care.
While the silver bullet for GA is yet to be found, Thursday’s session showed how rapidly the landscape is being redrawn. From supplements and OCT biomarkers to functional testing, gene and mitochondrial strategies, and AI models that map disease in three dimensions, the toolbox is expanding.
The experts agreed that without sharper trial designs and better patient segmentation, even the strongest candidates risk falling short. With earlier detection, composite endpoints and precision strategies that match therapy to phenotype, nonexudative AMD may finally move from “untreatable” toward “manageable,” giving patients the real options that they’ve been waiting for.
* Keenan TDL, Agron E, Keane PA, Domalpally A, Chew EY. Oral antioxidant and lutein/zeaxanthin supplements slow geographic atrophy progression to the fovea in age-related macular degeneration. Ophthalmol. 2025;132(1):14-29.
The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. A version of this article was first published on piemagazine.org

By Elif Uslu
Can VR make vision tests easier, or chatbots lighten clinic load? EURETINA 2025 offered bold answers.

The second day of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025) put artificial intelligence not just under the microscope, but squarely in the hands of clinicians. In a buzzing session hall at the Palais des Congrès, AI was tested, debated and demystified.
With Prof. Dr. Martin Zinkernagel (Switzerland) and Prof. Pearse Keane (UK) chairing, the session sprinted from clinic letters to chatbots VR perimetry and intraoperative OCT, before closing on equity metrics that could change regulation itself.
Large language models (LLMs) have become part of everyday life,
but their clinical potential is only just being realized. Moorfields Eye Hospital’s Dr. Ariel Ong (United Kingdom) argued that the true promise lies in turning the “narrative chaos” of healthcare into structured insights.
Only a fraction of healthcare data exists in structured fields; most is trapped in clinic letters, referral notes and reports. Traditional natural language processing (NLP) required immense effort and often failed when faced with new documentation styles. By contrast, Dr. Ong explained, “LLMs can generalize to many tasks with little to no labeling and capture clinical context that’s more subtle.”
Her team is testing ways to extract meaningful clinical variables from millions of patient letters—
unlocking a resource for both care and research. She also pointed to the power of AI in clinical trials, where patient eligibility screening could be accelerated if models can explain their reasoning. As she put it, “The real challenge is not whether these tools can do something, but how—and whether—we adopt them responsibly.”
Dr. Ong’s closing emphasis on co-intelligence was the session’s leitmotif: empower researchers and clinicians, don’t replace them.
“The real challenge is not whether these tools can do something, but how—and whether— we adopt them responsibly.”
- Dr. Ariel Ong
While Dr. Ong focused on data extraction, Dr. Fares Antaki (Canada), vitreoretinal surgery fellow at Cleveland Clinic, asked whether chatbots might change the very rhythm of clinical practice. His answer: yes, but as an ally, not a rival.
Dr. Antaki highlighted two areas. First, workflow support: AI scribes listen to consultations, generate notes and produce simple patient summaries. Second, clinical triage: AI assistants are available 24/7 to answer urgent questions and redirect patients appropriately.
One of his most striking ideas was the introduction of ‘vibe coding’, where clinicians build apps without writing code. “You can use English to build software applications,” he said, demonstrating how a specialist could design a triage chatbot in minutes by conversing with an AI agent.
When asked whether he personally uses AI for medical help, Dr. Antaki didn’t hesitate: “All the time.” He added that, for him, ChatGPT remained the most engaging and effective model. His point was clear— AI is already woven into the clinician’s toolkit, but its safe integration
into official systems still requires validation and oversight.
For patients with advanced macular disease, central fixation is often impossible. So how do you test vision when patients can’t fixate? That’s the problem University of Bern PhD Candidate Marta Colmenar Herrera (Switzerland) tackled with her group’s work on AI-assisted fixation-agnostic perimetry in virtual reality.
Her team built a VR-based, gazecontingent suprathreshold perimetry for the central 10 degrees, with a large fixation cross for orientation even when the center is missing. “We track gaze continuously and present a stimulus related to the gaze,” she explained, allowing even those with unstable fixation to complete the exam.
Early studies with 26 eyes showed good agreement with standard imaging, and—crucially—patients tolerated the VR headset well. Ms. Colmenar Herrera noted that “most of them were happier doing the virtual reality test than the microperimetry,” even though the average age was around 80.
The roadmap is practical: add thresholding at the atrophy border, seed customized patterns from imaging and keep adapting to the patient rather than forcing the patient to adapt to the test. It’s AI as ergonomics.
Moving from clinic to theatre, Prof. Daniel Ting (Singapore) explored how AI is being integrated into vitreoretinal surgery. He broke the workflow into three phases: pre-op, intra-op and post-op.
Preoperatively, LLMs can help summarize complex referral histories or support patient counseling. Imaging algorithms can already detect conditions such as macular holes or retinal detachments. Intraoperatively, Prof. Ting sees major potential in pairing intraoperative OCT (iOCT) with AI segmentation to provide real-time feedback.
“If we could couple iOCT with AI segmentation algorithms to quantify how much gene therapy has already gone into the subretinal space, I think that would be ideal,” he said.
He also pointed to opportunities for automated surgical summaries that could save time after lengthy cases. As for robotics, Prof. Ting acknowledged their promise for tremor reduction and precision but dismissed full autonomy as decades away.
“If we could couple iOCT with AI segmentation algorithms to quantify how much gene therapy has already gone into the subretinal space, I think that would be ideal.”
- Prof. Daniel Ting
As for the perennial question—will robots do this on their own? Prof. Ting kept both feet on the ground. The frontier is tremor suppression, precision and safety prompts; full autonomy is far off. “I would be surprised maybe in 50 years… I’m not sure,” he admitted, underlining that augmentation, not replacement, remains the goal.
The final word came from Prof. Catherine Egan (United Kingdom), who tackled perhaps the most pressing question: Can AI be fair? First, she reminded the audience that bias is not theoretical: “People died because pulse oximeters overestimated blood oxygen levels in people with more pigmented skin.”
In ophthalmology, algorithms for diabetic retinopathy screening are being rolled out globally, but training datasets may not reflect the diversity of patient populations.
Prof. Egan’s team developed the Retinal Pigment Score (RPS), a continuous, image-derived measure
of retinal background pigmentation. “We sought to develop a metric that could describe datasets and be used as a variable to compare diagnostic algorithms,” she explained.
Their findings showed that while overall accuracy was similar across pigmentation levels, false-positive rates varied significantly between algorithms. That means some patients could face unnecessary anxiety or treatment depending on the system used.
Prof. Egan was unequivocal about the implications: “I’d like to see this become a regulatory requirement, and retina could potentially lead the way.” By insisting on transparency and fairness, she argued, ophthalmology can set the standard for other fields.
If there was one thesis running through the session, it was this: AI is transformative only when it is collaborative. Every speaker underscored the same truth in different ways—these tools work best not as replacements, but as partners. For Dr. Ong, that meant co-intelligence. For Dr. Antaki, it meant allyship in workflow. For Ms. Colmenar Herrera, ergonomics. For Prof. Ting, augmentation. For Dr. Egan, fairness.
The roadmap is clear. Keep humans in the loop. Validate relentlessly. Publish not just accuracy, but equity. Then ship the tools that give clinicians back time, patients back confidence and all of us a cleaner line of sight to better outcomes. AI may be rewriting the script of retina, but as this session showed, the authorship remains firmly human.
The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. A version of this article was first published on piemagazine.org

By Diana Truong
Suprachoroidal shortcuts, refillable implants and even robots: pick your retinal route.
If intravitreal injections were a dinner party guest, they’d be the one who overstays their welcome. Necessary? Maybe…but exhausting for everyone.
On Day 2 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025), one symposium tackled that very problem, spotlighting ways to lighten the load for patients and clinicians alike. From refillable implants to creative routes through the eye’s hidden spaces, drug delivery is getting smarter, slicker and just a little more futuristic.
Prof. Aude Couturier (France) unveiled the kind of data every retina specialist dreams of: five years of durability from the Port Delivery System (Roche, Basel, Switzerland), the surgically implanted reservoir that slowly releases ranibizumab.
“We now have five years of clinical data on the PDS for nAMD [neovascular age-related macular degeneration] from the Archway and Portal trials, along with two years of data for DME [diabetic macular edema] and DR [diabetic retinopathy] from the Pagoda and Pavilion trials,” she reported.
The takeaway? Refills stretched to six or nine months with hardly anyone needing a top-up in between. Specifically, 98% of nAMD patients, 96% of DME patients and 100% of DR patients made it
through their first interval without supplemental treatment.
Visual acuity stayed steady too, with more than half of nAMD patients maintaining 20/40 vision or better throughout.
Safety also matured with technique. Vitreous hemorrhage rates fell from nearly 50% in early trial days to just over 5% after adding laser photocoagulation to the choroid. Endophthalmitis dropped to under 1% with improved Tenon’s capsule dissection.
Looking ahead, Prof. Couturier pointed to new molecules like zifibancimig (DutaFab targeting Ang-2 and VEGF-A; Roche), currently in the BURGUNDY Phase I/II study, as potential partners for the PDS platform.
Delivering therapies to the subretinal space isn’t exactly a walk in the park. It’s more like threading a needle while riding a unicycle.
That was the picture painted by Dr. Fanny Nerinckx (Belgium),
who spotlighted both the pitfalls and progress of the transvitreal route, the go-to approach for gene therapies, stem cells and subretinal implants.
The old-fashioned way (manual subretinal injections) comes with plenty of headaches: tremor-induced retinotomies that stretch wider than intended, misplaced needles, shearing forces and the frustrating loss of precious payload to reflux.
“Studies have shown that up to 60% of the injected volume may reflux into the vitreous cavity,” Dr. Nerinckx said, underscoring just how leaky and inefficient the process can be.
Luckily, technology is starting to catch up. Automated injection systems, like the FDA-approved INCIO microinjection system (DORC Global, Zuidland, The Netherlands), now regulate pressure with the kind of consistency no human hand can guarantee.
Meanwhile, robotics is moving from science fiction to OR reality. “We performed a first-in-human clinical trial using the Acusurgical (Montpellier, France) Luca robot that allows bimanual surgery,” she reported. “When we think about robots, we think we have to freeze the instrument. But when performing a large bleb, you have to be dynamic and lift the subretinal needle as the bleb enlarges.”
And while the tools are getting sharper, so is awareness of what happens after injection. Dr. Nerinckx stressed the need to monitor for inflammation and toxicity, pointing to recent findings on gene therapyassociated uveitis.1 “It is important to know because we have to give immunomodulating treatment to the patients pre- and postoperatively,” she said.

Subretinal injections may look straightforward on paper (poke, inject, done), but anyone who’s tried knows the bleb has a mind of its own.
Prof. David Steel (UK) broke down the fluid dynamics of bleb formation with the precision of an engineer and the pragmatism of a
surgeon who’s seen reflux ruin a good day.
“Retinal stress in a bleb is greater at its apex, which is typically near the retinotomy,” Prof. Steel explained.
“If you inject an area of thin retina, you’re likely to have high stress at the injection point, which will stretch the injection point and lead to more reflux.”
He advised keeping intraocular pressure (IOP) low during injection, dialing the flow rate down to a gentle one to three microliters per second, and resisting the urge to go all-in with one massive bleb.
“If you spread the dose across multiple blebs, you not only reduce the height by 25% but also increase the area by 50% compared to one bleb,” he noted.
And when it comes to the fovea, Prof. Steel’s advice was clear: keep your distance. “You want the fovea to be in the most distal part of the bleb, not anywhere near the apex, to reduce stress on the fovea as it detaches.”
If the vitreous cavity is the main stage, the suprachoroidal space is like the VIP backstage lounge: harder to get into, but once you’re there, the access is unbeatable. Both Prof. Dominik Fischer (UK) and Prof. Siegfried Priglinger (Germany) pulled back the curtain on this narrow but promising therapeutic corridor between the sclera and choroid.
“Three arguments are put forward for suprachoroidal delivery,” Prof. Fischer explained. “First, if you could fill the suprachoroidal space completely, you could potentially reach 100% of the retina. Second, it’s an office-based procedure that avoids vitrectomy. Third, its proximity to the outer retina minimizes effects on the anterior segment.”
That kind of access comes with a growing toolbox: purpose-built microneedles with plastic stops for safe, office-based injections; transscleral injector systems in both micro- and macroneedle flavors;
suprachoroidal catheterization; and even open surgical routes for trickier cases.
Prof. Priglinger’s team has gone one step further, successfully parking Ozurdex (AbbVie, Illinois, USA) implants in the suprachoroidal space for patients at high risk of anterior chamber migration—an elegant workaround for a tricky complication.
The safety record so far is encouraging. Across trial patients, there were no lens injuries, suprachoroidal hemorrhages or endophthalmitis, and just one case of retinal detachment.2
Better yet, Prof. Priglinger reported real-world gains: “In 72% of patients, we saw a reduction of central retinal thickness by 35%, and in responder patients, we saw a significant improvement in visual acuity,” he said.
Still, questions remain. How long will therapies last in this space, especially gene vectors that prefer a permanent address? Will filling the suprachoroidal space actually deliver the holy grail of 100% retinal coverage? Or will biology remind us who’s boss?
“Suprachoroidal injections are a welcome tool in our arsenal for retinal drug delivery, but [their] pros and cons must be considered in the context of which condition you treat, where you target and what is the payload,” Prof. Fischer concluded.
Who says you need a scleral buckle to fix a retinal tear? Certainly not Prof. Ehab El Rayes (Egypt), who is pushing the boundaries of retinal detachment repair with a decidedly less invasive route: transconjunctival suprachoroidal buckling.
The concept is as elegant as it is disruptive. Instead of sewing, tacking or otherwise wrangling the sclera, his approach uses a new device with a rounded, atraumatic needle tip to slip filler directly into the suprachoroidal space, all through the conjunctiva.
The filler of choice? Hyaluronic acid, which lingers a comfortable 12 to 14 months in the suprachoroidal pocket. He purported that, in a randomized trial, this method delivered a 91% single-injection success rate for repairing detachments, which certainly puts it in the “worth paying attention to” category.
“You don’t need a sclera to close a retinal tear,” Prof. El Rayes quipped, distilling the philosophy behind the technique.
He added that the procedure is not just minimally invasive but refreshingly straightforward. “It could be done using just an indirect ophthalmoscope, and it’s potentially an office-based procedure that avoids the problems of scleral buckling and vitrectomy,” Prof. El Rayes concluded.
What emerged from this session is a field in motion: retinal drug delivery is shedding some of its needles-andrepeats monotony and embracing smarter, longer-lasting and less invasive options.
Whether it’s a refillable quietly doing its job for half a decade, a robotguided bleb placed with surgeon-like finesse, or a microneedle tapping into the eye’s hidden highway, the future looks less like a treadmill of injections and more like a menu of tailored solutions.
1. Purdy R, John M, Nerinckx F, et al. Gene therapy-associated uveitis (GTAU): Understanding and mitigating the adverse immune response in retinal gene therapy. Prog Retin Eye Res. 2025;106:101354.
2. Asani B, Kruse F, Priglinger SG, et al. Suprachoroidal implantation of corticosteroid slow release implants for the treatment of cystoid macular edema. Scientific Reports. 2025;15:20166
The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. A version of this article was first published on piemagazine.org

By Kendra Bruning
From living RPE monolayers to wireless implants and geneagnostic optogenetics, Monday’s ARVO-cosponsored symposium focused on therapies built to restore usable vision, not just slow the slide.

Orlando trades on sunshine and spectacle, and this program brought both to the retina. In the Therapeutic Approaches to Vision Restoration symposium, chair Dr. Wei Li (USA) convened four teams advancing different routes to the same goal: rebuilding the RPE with living monolayers, converting images to current with a photovoltaic chip, sensitizing surviving retinal circuits with optogenetics and replacing lost photoreceptors with self-powered nanowire arrays.
Covering both living tissues and engineered hardware, the session felt more Space Coast than theme park, pairing careful surgical playbooks with maturing safety data and patient-level gains that suggest these ideas are leaving the launchpad and taking flight, one carefully chosen eye at a time.
Starting the session, Dr. Amir H. Kashani (USA) walked us through the long road from early autologous
retinal pigment epithelium (RPE) grafts that triggered proliferative vitreoretinopathy (PVR) to today’s engineered monolayers on defined scaffolds for dry age-related macular degeneration (AMD). He noted that intravitreal cell suspensions were not effective. Subretinal suspensions produced scattered pigment, which led teams to pivot toward true sheets and more controlled delivery. “This truly requires cell-based therapy to restore structure and hopefully function,” he said.
Modern trials place differentiated RPE as a continuous layer in a hydrodissected pocket under intraoperative OCT guidance.1,2 Safety has been steady, with no tumor signal, low inflammation and good implant stability. In a California series, surgical refinements reduced early hemorrhage and vision trends were encouraging: “Of 15 patients, up to 27% improved by at least one line of visual acuity at three years; 60% had stable or improved vision, while unimplanted eyes declined as expected.”1
Donor eye pathology two years postimplantation showed pigmented, polarized RPE expressing RPE65 with rhodopsin co-localization, a sign that phagocytic function may be present. Next-generation work uses a biodegradable Poly(lactic-coglycolic acid) (PLGA) scaffold to host a single-cell monolayer and targets geographic atrophy (GA) borders where photoreceptors remain. The field still chases perfect coverage and durability, but the direction is set. Build the layer, keep it alive, let the retina do the rest.
Dr. Ralf Hornig (Germany) presented PRIMA, a subretinal photovoltaic implant for central vision loss from GA. Smart glasses capture the scene, an on-body processor prepares the signal and near-infrared light projects it onto a 2 × 2 mm chip that is 30 µm thick. The chip holds 378 pixels, each a small solar cell that stimulates inner retinal neurons. As he summarized, “Unlike previous visual prostheses, PRIMA provides shape perception— patients draw and recognize letters as expected.”
The surgical steps will be familiar to vitreoretinal teams: create a
subretinal bleb, deliver the implant through a retinotomy, flatten with perfluorocarbon and position the device before gas. Rehabilitation begins around week four. In the PRIMAVERA pivotal study of 38 eyes, peripheral native vision stayed stable while central function improved with the implant.3 “Mean visual acuity improvement at 12 months was 25.5 letters; one patient gained 59 letters.” Many participants now use the system at home for labels, manuals, cards and low-vision books. Safety findings tracked mainly to surgery rather than to the device
Next steps include tighter pixel spacing, a wider field and slimmer glasses with an integrated projector. He advised moving forward in a practical direction by keeping surgery familiar, maintaining structured rehab and continuously refining the hardware.
Dr. Samarendra Mohanty (USA) and Dr. Stephen Tsang (USA) pitched a gene-agnostic optogenetic strategy for advanced retinitis pigmentosa (RP) and Stargardt disease. Nanoscope’s multi-characteristic opsin (MCO) platform uses an adenoassociated virus (AAV) vector to transduce more than 70% of bipolar cells with a broad-spectrum opsin. The response spans 400–700 nm with four orders of dynamic range, so patients do not need high-intensity light or scanning systems. As Dr. Mohanty explained, “Our goal is a disease-agnostic and gene-agnostic optogenetic therapy,” a practical way to step around the genetic diversity of inherited retinal diseases (IRDs).
Targeting bipolar cells preserves upstream retinal processing and increases potential “pixel count” compared with ganglion-cell approaches. Early studies back the choice. In a dose-ranging phase 1–2 trial for RP, the higher dose produced about a half-logMAR gain and informed a phase 2b design that reached significance at weeks 52 and 76, with durability out to three years and a clean safety profile.4,5
Dr. Tsang shared data from a six-patient phase 1–2 Stargardt
cohort, where macula-predominant phenotypes appeared to respond best. Several eyes gained doubledigit ETDRS letters and selected patients reached five lines with a single intravitreal injection. As he noted, “Some achieved up to five lines improvement in acuity—about 32 ETDRS letters—with a single intravitreal injection.”6
Presenting work from Dr. Y. Zhang, Dr. Chunhui Jiang (China) described a subretinal nanowire array that behaves like a dense bed of artificial photoreceptors. The device uses gold–titanium dioxide nanowires about 1 nm in diameter and 2.5 µm long, packed at a higher density than native photoreceptors. The design generates current directly in response to light, so there is no belt pack, cable or goggles. As Dr. Jiang put it, “The arrays are self powered, with no external power or goggles required.”
In macaques, implants were stable at one year without detachment or proliferative responses, and animals completed dim-light tasks. Early human data from a Chinabased study add cautious optimism. Two long-blind patients received a 2 × 2 mm, 200 µm thick chip using standard subretinal delivery. Postoperative inflammation was mild and transient. Imaging confirmed stable placement, steady intraocular pressure and resolution of early edema by month three.7
Functionally, both patients improved from no light perception to consistent stimulus detection with a blue-leaning response profile. They followed floor cues in navigation tasks and screen-based discrimination improved over days. “Patient 1 reached 100% accuracy on shape and direction detection within days; Patient 2 improved from 30% to 100% over a week.” Daily-life gains varied, but the signal supports continued enrollment as the team refines materials and surgical steps.
What unfolded Monday morning in Orlando felt less like a gadget
parade and more like a blueprint for rebuilding vision. Stem cell sheets that behave like RPE, photovoltaic chips that sketch letters, optogenetic switches that recruit bipolar cells and self-powered arrays that remove the need for external gear, all pointed in the same direction.
The hurdles are real in manufacturing, durability, rehabilitation and equitable access. Yet the signal is getting stronger across models, trials and early patient stories. Florida knows a good launch when it sees one. After this morning, it is hard not to believe that some of these approaches are already clearing the tower.
1. Humayun MS, Clegg DO, Dayan MS, et al. Longterm follow-up of a phase 1/2a clinical trial of a stem cell-derived bioengineered retinal pigment epithelium implant for geographic atrophy. Ophthalmology. 2024;131(6):682-691.
2. Kashani AH, Lebkowski JS, Rahhal FM, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10(10):13.
3. Holz FG, Le Mer Y, Muqit MMK, et al. Subretinal photovoltaic implant to restore vision in geographic atrophy due to AMD. N Engl J Med. 2025 Oct 20.
4. Nanoscope Therapeutics. Nanoscope Therapeutics announces positive top-line results from randomized controlled trial of MCO-010 for retinitis pigmentosa. Available from: https://nanostherapeutics. com/2024/03/26/nanoscope-therapeuticsannounces-top-line-results-from-ph2-trial-of-mco010-for-retinitis-pigmentosa/ Accessed on October 21, 2025.
5. Nanoscope Therapeutics. Nanoscope presented positive 2-year randomized, controlled trial results of MCO-010 for retinitis pigmentosa. https:// nanostherapeutics.com/2024/10/31/nanoscopepresented-positive-2-year-randomized-controlledtrial-results-of-mco-010-for-retinitis-pigmentosa/ Accessed on October 21, 2025.
6. Lam BL, Zak V, Gonzalez VH, et al. Safety and efficacy of MCO-010 optogenetic therapy in patients with Stargardt disease in USA (STARLIGHT): an open-label multi-center Ph2 trial. EClinicalMedicine. 2025;87:103430.
7. Yang R, Zhao P, Wang L, et al. Assessment of visual function in blind mice and monkeys with subretinally implanted nanowire arrays as artificial photoreceptors. Nat Biomed Eng. 2024;8(8):10181039.
The American Academy of Ophthalmology Annual Meeting 2025 (AAO 2025) was held October 17-20, 2025, in Orlando, Florida. Reporting for this story took place during the event. A version of this article was first published on piemagazine.org
