From animalcules to biodiversity: microscopy putting its stamp on the world and its role in science education
Autofluorescence in Focus: The Hidden Light of Biological Samples
The Oxford-ZEISS Centre of Excellence at the Kennedy Institute of Rheumatology and the Institute of Developmental and Regenerative Medicine, Oxford

Plus...News, Calendar, Reviews, Reports
1 ISSUE 74 JUNE 2024 ISSUE 74 JUNE 2024





Robust Workflow for High Quality TEM Sample Preparation

Atomic Resolution STEM and TEM Sample Preparation
The all-new JIB-PS500i from JEOL delivers fine milling capabilities essential for fast, high quality lamella preparation. This multi-purpose FIB-SEM enables high throughput sample preparation, high resolution STEM-in-SEM imaging, and analytical analysis.
Robust Workflow for High Quality TEM Sample Preparation
Atomic Resolution STEM and TEM Sample Preparation
Robust Workflow for Large Samples
The all-new JIB-PS500i from JEOL delivers fine milling capabilities essential for fast, high quality lamella preparation. This multi-purpose FIB-SEM enables high throughput sample preparation, high resolution STEM-in-SEM imaging, and analytical analysis.
Featuring a large specimen chamber with easy-access door and large high-tilt stage, the JIB-PS500i offers a truly flexible workflow. Transfer from the FIB-SEM to the TEM is seamless with TEM-Linkage, JEOL’s double-tilt cartridge.
Robust Workflow for Large Samples
Featuring a large specimen chamber with easy-access door and large high-tilt stage, the JIB-PS500i offers a truly flexible workflow. Transfer from the FIB-SEM to the TEM is seamless with TEM-Linkage, JEOL’s double-tilt cartridge.








2 ISSUE 74 JUNE 2024
Josie
1 contents features 4 From animalcules to biodiversity: microscopy putting
stamp
the
its role in science education Joel Cohen 42 Autofluorescence in Focus: The Hidden Light of Biological Samples
its
on
world and
Guzman
Jake Jones 52 The
Oxford
Rose De
and
Oxford-ZEISS Centre of Excellence at the Kennedy Institute of Rheumatology and the Institute of Developmental and Regenerative Medicine,
Eade 73 Restoring the quality of electron microscope images through environmental monitoring
Consulting regulars 28 Calendar 36 Journal of Microscopy 50 New Member Welcome 64 Office News 80 Company News 84 New Products reports and other features 32 elmi2024: Welcome to Liverpool! 70 Focus on Microscopy 2024, Genoa, Italy 72 RMS Prize-Winner at Actin 2023: Simona Buracco 78 Technical Specialist Job Shadowing Scheme: A report from the RMS and BioImagingUK
Spicer

infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.
infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK
Editor
Owen Morton
Tel. + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Laura Fumagalli, University of Manchester, UK
Myfanwy Adams, John Innes Centre, Norwich, UK
Maadhav Kothari, Zeiss Microscopy, UK
Hilary Sandig, Cancer Research, UK
Trevor Almeida, University of Glasgow, UK
Mark Rigby, Nikon UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2024 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by Creative Design. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.

RoyalMicroscopicalSociety @RoyalMicroSoc @RoyalMicroSoc
MAGAZINE
FROM THE SCIENTIFIC EDITOR
Dear Readers,
It is a great pleasure to bring to you our second issue of 2024.
For our readers in the northern hemisphere, this time of year marks the arrival of summer (occasionally in Scotland!), with parks back to having green colours and people spending more time enjoying the outdoors. Hopefully the weather will be kind to us in Liverpool for elmi2024 (The European Light Microscopy Initiative) – which will be in full flow when this issue goes live! The organisers (and the RMS staff) have certainly put a lot of effort into making this an event to remember for years to come – and I’m sure that will be the case, whatever the weather. If you are joining us in Liverpool, I hope you really enjoy the scientific programme, exhibition and company workshops. The workshops are a key feature of elmi, and it’s always great to get a sneak preview of the latest technologies.
Speaking of technologies and microscopy, we have a fantastic piece from Joel Cohen from the RMS Outreach and Education Committee. He takes us on a journey through time, looking at the microscopists and microscopical images depicted on postage stamps across the world. It’s a real tour de force of cataloguing and well worth taking the time to read.
Elsewhere, Rose de Guzman and Jake Jones present a nice piece on methods to manage autofluorescence – an unwanted phenomenon in microscopy (most of the time).We also have an interesting article from the company Spicer, on restoring the quality of electron microscope images through environmental monitoring.
Partnerships between companies and academia are vital in our community. Here, Josie Eade describes a new, joint venture between Zeiss and University of Oxford: the Oxford-Zeiss Centre of Excellence. This new centre, opened in February 2024, brings together unique expertise from Zeiss and the groundbreaking research at the university.
I really hope you enjoy reading all of the content in this issue, and look forward to catching up with many of you at elmi2024!
Slàinte!
Leandro Lemgruber

COVER IMAGE: Microscopy is Everywhere, by Joel Cohen. Microscopy and microscopists have often featured on postage stamps across the world and throughout history. RMS Outreach and Education Committee member Joel Cohen takes a comprehensive look on p4.
3

4 ISSUE 74 JUNE 2024

From animalcules to biodiversity: microscopy putting its stamp on the world and its role in science education Joel I. Cohen, PhD Visiting scholar NSOE (Nicholas School of the Environment, Duke University); RMS Outreach and Education Committee. Joel.Cohen@Duke.edu 5
Abstract and Introduction
How is it one comes to know of life? For over 350 years, the microscope has been a companion on this journey. The microscope’s origin at various times and places meant it was used widely, and once perfected, it helped overcome our ignorance of disease. It opened previously unseen worlds in a drop of water. It laid the foundation for cell biology. More recently, it has shown value in studies of biodiversity. However, the microscope’s centrality in biology is not in keeping with its diminishing role in education, where images from the microscope are predominantly seen through on-screen applications rather than through a lens.
These observations led to this study’s research question: Do achievements, such as presented here and obtained through microscopy, warrant increased investments for education and career development?
To address this question, this paper examines the relevance of multiple points of invention leading up to today’s microscopes, followed by illustrative applications as they were used by five scientists. Concluding the section on applications shows microscopy entering the world of art. Postage stamps, or philatelic issuances, highlight and illustrate the issues and diversity found in the text, with over twenty-five countries represented, demonstrating the broad applicability and accessibility of the microscope.
The evidence provided demonstrates the centrality of microscopy across a range of diverse careers. To supply capable individuals for such careers, biology education should ensure that its curriculum allows time for theory and practice of microscopy. Lessons and labs should not only be used to expose students to the scientific value of microscopy, but also for applied studies of biodiversity, as an example. Finally, As seen in multicultural science classes, students can master the microscope regardless of gender, culture or abilities. Such use serves as a built-in tool for addressing diversity, equity, and inclusion in the classroom.
Part I. Overview, Background and Organisation Prologue
For those of us living today, a journey to the universe, moon and planets remains something of a dream. However, if one can don a wetsuit and scuba gear,

Figure 1. Giant maxi card announcing the first day of issue, 1989, with special cancellation in honour of the 150th anniversary of the Royal Microscopical Society. Signed by image creator, Roger Stewart, whose photograph shows a macrophage with its arms encircling foreign bacteria.
6 ISSUE 74 JUNE 2024

then diving to see coral reefs brings us to a place far different from our own terrestrial home, but one can reach it. Yet, for many others, that world is out of their reach as well. But, what of the world around us, the one visible to the naked eye? This world is within our reach, the one where seasons change, migrations come and go, species pass before our eyes, many of which we can reach out and touch. But still, for many, even when immersed in its study, nature can be removed and seem far away.
This brings us to the world featured in this paper, complete with creatures quite foreign in appearance and having life cycles all their own. However, despite these differences between our world and theirs, it is a world within our grasp. With the aid of but a single instrument, we can venture into vernal pools, tide pools, rivers or streams, and study organisms previously seen only as drawings in textbooks. This singular combination of lens, light and specimen makes up the microscope, and it can reveal a universe that does not require oxygen tanks or space suits; no rocket engines or submersibles needed.
Instead, it requires a modest, three-foot square space upon the dining table, from which all that is
scientific can be removed when the in-laws come for dinner. It requires time and attention, and your skills of observation - even though at first you couldn’t tell the difference between the leg of an ant and a cracked slide. So, just what might be waiting for you?
One can still see organisms that Van Leeuwenhoek first saw in 1674 (Pearle et al. 2010; Ford 2007). These fresh and saltwater microorganisms are still here to explore and conserve, to reflect on how life differs in size, complexity, and habitat. It is in the spirit of this world and its wonderment, as professed by Rachel Carson, that this paper was prepared. “A lens-aided view into a patch of moss reveals a dense tropical jungle, in which insects large as tigers prowl amid strangely formed, luxuriant trees” (Carson 1937). With microscope in hand, you have a frontrow seat to your own discoveries, and when found, the enjoyment of what was accomplished and learned about the world.
Educators arise and bring back the microscopes
With the ongoing and ever-rapid increase in biological and molecular technologies, and their
7
Figure 2. Israeli stamp (2019) depicting “science-oriented youth,” showing a microscope among the many technologies and ideas science students are exploring.
incorporation into STEM education (Figure 2), is there still a time and place for microscopic studies? To begin, let’s consider reasons why ensuring time for students to prepare and observe specimens directly through microscopes should remain a vital part of instruction (Wellner 2021).
First, educators should encourage students to take a firsthand look at life through the microscope, and explain how it can open doors of perception not possible otherwise. Such labs help students learn how to focus on a given specimen and compile their observations. By extension, the presence of cultured, living organisms can lead directly into a unit embracing each of the kingdoms (Cohen 2020) as seen through the microscope.
Second, understanding the rise of complex life forms can be facilitated by the microscope. Here, fixed specimens can be used to demonstrate the rise of complexity which follows the transition from prokaryote to eukaryote. In these early lessons, students can also observe asexual means of reproduction as found in many protists.
Third, discussing the history and evolution of the microscope itself can give students insight into how ideas are conceptualised, as well as what happens when different individuals designed and constructed the microscope around the same time.
Fourth, the microscope has proven to be ‘gender neutral’, meaning that both men and women and of various ethnic/cultural groups have all made important discoveries. The fifth “positive attribute” is that because of this type of success, students can also achieve a level of competence and discovery, which then allows them to become mentors and teach others, student to student. In addition, this factor makes microscopy a science with a message of diversity, equity, and inclusion, as attested by the images that follow.
Research Study Question
The general and guiding question for this study was: Do past achievements obtained through microscopy,
such as those to be found in this paper, warrant continued investments to provide for education and future careers?
Given this question, is it clear that microscopes are not just tools from the past, but also part of the present and future? In fact, the sixth benefit comes from the facts and examples collected for this study. They demonstrate new uses of microscopy, such as in forensics, infectious disease, and agriculture. Stamps and microphotographs taken using other innovative techniques are also represented.
How will examples and evidence be presented?
The surprising images from the work of van Leeuwenhoek (1632-1723; Ford 2007) and Robert Hooke (1635-1703) continue to amaze us today, as evidenced by Figures 3 to 8. These early pioneers, who realised the wonder and power of magnification, took what could be done by a drop of water and made this power appear whenever one gazed through a ground glass lens made by their own hands. Once others understood how such magnification could be obtained, the size, shape, and particular utility of what eventually became the microscope increased exponentially, with specific manufacturers labelling their work in a proprietary manner.
As microscopes became more specialised, their utility found its way into every aspect of science and engineering around the world. This study provides a topical survey of this diversity and reinforces the fact that, far from reaching an endpoint, the microscope and new innovative techniques continue to advance. These pages contain numerous philatelic examples across the scientific spectrum and will be illustrated and further discussed using the five categories listed below:
• Historical developments of the microscope conveyed on postage stamps
• Medical, scientific, and agri-food discoveries
• Biodiversity applications
8 ISSUE 74 JUNE 2024
• Scientists who used microscopes for their research, and
• Images taken from an array of topics and scopes
It is hoped that these pages will provide encouragement for forthcoming students and scientists to continue such explorations and careers.
Part II. How the Microscope Got Its Start
For a good many centuries, medical practitioners were plagued by misconceptions and enemies so small that they remained unseen. This invisibility led to a plethora of theories and practices, which, while well-intended, did little good, or could even create a great deal of harm. However, as microscopes came into use, what had remained invisible to the unaided eye was now recognisable. These instruments gave doctors a look into a patient’s blood as never before possible. No longer were doctors fooled or misguided. Wherever a microscope appeared, the identity of the causative organism of a major health concern at the time gradually became recognisable.

Finally, the seeming invisibility of these organisms had been overcome. Next came calls to perfect the microscope, and many private enterprises took up the challenge. The better the quality of the microscope, the more assured doctors and others were as to correctly identifying what they saw, many doing so for the first time. But how did all of this come about? What turned human interest to the lens, and not only that, but to the compound effect of multiple lenses as well? First, attempts were made to imitate the effect of magnification seen when staring through a drop of water on a leaf, as

one example. It was not long after making this observation that many groups from around the world began making their own version of what became known as “microscopes”. Here, those already versed in the art of grinding lenses to bend and refract light, now found that instead of building lenses to observe the distant galaxies (hence “tele”), they could just as well make lenses to visualise the smallest pieces of life (or “micro.”)
It all began following the remarkable applications and observations of what are often considered the world’s first such instruments, with one marking the starting point of the “compound microscope,” so called because it compounds the additive “eye power” of one lens near to the object, while the second lens is some distance away, carefully adjusted to give that second boost of “eye power” to the viewer. This microscope, the first of its kind, was described by others, but it is still attributed to the brothers Jansen of the Netherlands in 1590 (Gardner 1972; p.188; chronology). With that development, the word was out (Clay and Court 1975), and by the middle of the 17th century, several other designers produced their own version of the compound microscope (Clay and Court 1975), enabling further investigations of the microbial world. The next advancements came from two individuals, one in England and one in The Netherlands. The Englishman, Robert Hooke, was soon to publish his Micrographia (1665).
Meanwhile, in the Netherlands, Antony van Leeuwenhoek designed and used an ingenious onelens scope, which revealed to him the wondrous life that could be seen, among other places, in a drop of pond water (1674-1677). Rather than attempt a
9
Figure 3. Netherlands. Stamp issued 1937. The image closely resembles a portrait of Leeuwenhoek by Verkolje from 1686.
Figure 4. Grenada. Stamp was issued in 2000. Portrait of Leeuwenhoek and his microscope. Selected as part of the Millennium 2000 series.






10 ISSUE 74 JUNE 2024
Figure 5. Antigua and Barbuda. Issued in 1992. An original Leeuwenhoek microscope, held at Utrecht University Library, Netherlands.
Figure 6. Republic of Djibouti stamp commemorating Robert Hooke.
Figure 7. Grenada. Robert Hooke’s iconic flea etching. Stamp issued in 2000.
Figure 8. A photo blow-up of Hooke’s flea is hung in a special exhibit on Natural History at the St. Louis Museum of Science.
Figure 9. German postage. Issued in 1981. One of eight historic optical instruments made in Germany. Caption reads: binokulamikroskop um 1860. (Bifocal microscope).
Figure 10. German postage. Issued in 1981. One of eight historic optical instruments made in Germany. Caption reads: mikroskop 1790. (Monofocal).

book, Leeuwenhoek reported his findings through a series of letters to the Royal Society of London, which incorporated itself in 1662. Unfortunately, Leeuwenhoek’s images were not accepted until Robert Hooke was able to view them himself, and later show them to the Society (Snyder 2023).

By the time Robert Brown (1773-1858), a Scottish botanist, was appointed as the first Keeper of the Botanical Department at the British Museum, microscopy was so widespread that it had become fashionable (Allen 1976). Brown’s greatest love and specialty was plant taxonomy, and his skills with the microscope

enabled observations of cells and of what he called the nucleus. Brown became one of those contacted by Charles Darwin for the provision of expert advice, for which Brown accommodated the young naturalist before and after his voyage aboard the HMS Beagle (See details in Part IV).
Part III. Scientific Discoveries from Seeing the Unseen

Part III contains stamps grouped together to illustrate how microscopes provided the means to find and determine actual disease-causing organisms. These applications accomplished two things; first, they could identify and make certain which pathogen was present in the patient, and by doing so, were able to ensure the correct treatment was applied. By using the microscope in this manner (experimentation, observation and then diagnosis) it was possible to identify and treat smallpox, various pulmonary infections, tuberculosis, and ensuring that the correct pathogen was treated. In addition, there are a series of stamps for engraving, biodiversity, malaria and smallpox campaigns, cancer campaigns, agricultural research and diagnosis, livestock disease diagnosis, healthy meals, and the World Health
11
Figure 11. Canada. One of a set of four stamps issued in honor of Canada Day, 1988. Image of an electron microscope dated 1938.
Figure 12. Exterior view of drum microscope with its case and rare Victorian era slide. Authors collection.
Figure 13. German Democratic Republic (GDR), known as East Germany: 1949 to 1990. Stamps were issued in August 1980. Microscopes made by Carl Zeiss, from 1710-1873. All in museums.
Figure 14. Author’s microscope, made in England; compound microscope design with a flexible mirror and two lenses. Used in the Colonies.

Organisation.










12 ISSUE 74 JUNE 2024
Figure 15. Harvey Wiley with a microscope, in honour of the U.S. 50th anniversary of the pure food and drug laws.
Figure 16. 1981 issue, Republic of South Africa, recognising the 50th year celebration for the National Cancer Society.
Figure 17. Israel. Stamps for the Fight Cancer campaign, also featuring a microscope.
Figure 18. US Postage stamps ‘crusade against cancer’, showing a microscope as an image to encourage early detection.
Figure 19. Korean stamp (1971) to encourage overall healthy meals and to see an expert.
Figure 20. 1968 Pitcairn Island se-tenant pair, saluting the World Health Organisation. Microscope being used diagnostically.
Figure 21. India, 2006 stamp commemorating 100 years of service from the ICAR, Indian Council for Agricultural Research.
Figure 22. USA 1996. George W. Carver, included in “Celebrate the Century” for the 1910s, is an honour for him and his work with a microscope.
Figure 23. Chad, 1972. Farcha Veterinary and Zoological Research Lab.
Figure 24. Canada. 1957-58: International Geophysical Year. Microscopes are seen as central to discoveries.
Figure 25. Ghana issued a stamp in 1967, honouring the use of the microscope at the Cocoa Research Institute and its 25th Anniversary.







Part IV. Career Beginnings - Five Pioneers and Their Microscopic Worlds
Spend considerable time with a microscope and a partnership develops between humans and technology. While modern microscopes may appear much the same, scientists still find their own personal niche in where to work, and how to work. When it comes to revealing more of life’s seemingly endless forms, we turn to the microscope, and now, we can do so for artistry as well as taxonomy or anatomy. To illustrate these partnerships, five individuals were selected, from beginner status to those formally
13
Figure 26. Turkey, 1967. Se-tenant format for large and small animal vets.
Figure 27. Germany. 1971. Material science research using microscopes since concept.
Figure 28. Bahamas. A quartet of stamps commemorating progress in the fight against lung diseases.
Figure 29. Monaco. The application of microscopy to engraving and printing.
Figure 30. Mali. Microscopes are most recently playing a significant role in biodiversity collection, examination, and naming. Here, insects are being examined from a collection made by a boy.
Figure 31. Nigeria. Souvenir sheet with four stamps, each attesting to the importance of tropical medicine.
Figure 32. Germany. 2000. Celebrating the 100th anniversary of the Bernhard Nocht Institute for Tropical Medicine.
instructed in the modern microscope. Each person’s accomplishments are described here as undertaken with the aid of the microscope.
The scientists selected for this section are arranged in chronological order by date of birth, beginning with Charles Darwin, and his recognition of the need for and utility of a microscope on his voyage aboard the HMS Beagle, as well as later studies at Down House. Second, comes Mary Ward, who had minimal opportunities for formal schooling, but nonetheless, generated scientific illustrations of the worlds she saw through the microscope, proving the power of connection between observation, detailed illustrations, and success as a woman author.
The third individual is Edmond Locard. He is responsible for bringing the microscope into the forensics laboratory, and thus beginning scientificbased diagnostics. Then comes Dr. Barbara McClintock, who won a Nobel Prize for her work on transportable elements in maize. Her microscope is now stored at the Smithsonian Institute’s National Museum of American History, where this author was allowed to come for notes and observation.
The fifth individual is Rachel Carson, best known for her environmental writings, but equally a scholar of the sea and an important marine biologist and environmentalist. Her later works could read like warning signs to civilisation. But even at the end of her life, while ill from cancer, one could still find and share in the joy from her writings of the minute creatures she gathered and had seen in her stroll down from her cabin home in Maine to the rocky tide pools at the ocean’s edge.
CHARLES DARWIN (18091882)
Charles Darwin immersed himself in an extensive network of colleagues, ranging from pigeon breeders, to fellow students, and even college professors. While at Cambridge he befriended Mr. John M. Herbert, who so much appreciated what Darwin taught him on their excursions together that he made a gift of appreciation to mark their friendship. It was a Coddington’s Microscope, which Darwin said was a “most magnificent gift,” (Desmond and Moore 1991), especially as it would contain a “Coddington lens,” ground around the equator of the glass (Clay and Court 1975). It perfectly suited Darwin’s fascination with insects and his desire to work fast on specimens from the field.

However, when it came to the voyage that lay ahead of him, he consulted with a key individual from the British Museum, this time. This was Robert Brown, the senior botanist at the Museum. It was Brown who introduced the young naturalist to the Bank’s single lens scope (pictured, Figures 33 and 34). It

14 ISSUE 74 JUNE 2024
Figure 33. Darwin’s microscope aboard the Beagle. It is self-contained in a box. A “simple” scope, with only one ground lens. Falkland Islands, 1982.
Figure 34. Stamps on the left and right show Darwin using the microscope while on the Beagle. These are from Mozambique, 1982.

served Darwin well and was easy to fold into its box, thus affording more cabin room when needed while aboard the cramped officers' cabins on the Beagle. Upon return, he used a compound scope, as seen in Figure 35.
Darwin wrote in his collected letters, “During this time I saw a good deal of Robert Brown; I often used to call and sit with him during his breakfast on Sunday mornings, and he poured forth a rich treasure of curious observations and acute remarks (Darwin 1898). On one occasion he asked me to look through a microscope and describe what I saw. This I did, and I believe now that it was the marvellous currents of protoplasm in some vegetable cell. I then asked him what I had seen; but he answered me, “That’s my little secret.”
MARY KING WARD (1827 –1869)
Such was the success which followed Mary King Ward’s adventures with the microscope and her detailed illustrations, that 250 copies of her book on microscopy (Sketches with the Microscope), sold almost immediately. As if to show readers all that was possible in a context they could relate to, Mary Ward conducted all her investigations, drawings and completed her book on microscopy



15
Figure 35. A Togo stamp from 2012 shows one of Darwin’s microscopes at home. Stamp honours 130th anniversary of Darwin’s death.
Figure 36. A recent edition of Ward’s work contains the two illustrations below (Figures 37 and 38).
Figure 37. Ward’s microscope. Made by Hull’s lab.
Figure 38. Hair samples through the microscope and hand-drawn by Mary Ward. Please see Acknowledgements for full information on Ward.
from home. She was the first woman to have such a publication, and the book’s design, illustrations, and text made it an instant hit.
In London, a professional publisher (Groombridge and Sons) retained a copy of her book, and he proposed to print it as a hardbound book under the title, “A World of Wonders Revealed by the Microscope,” which also did remarkably well. And this was at a time when the writing and production of a solo publication by a woman, was practically an impossibility (Cohen 2022).
The detail apparent in her hand-drawn illustrations is indicative of what comes from careful examination and subsequent observations of each specimen. In so doing, she became a scientist at home, keeping meticulous records of her investigations while teaching herself the art of sketching what she observed through the lens.
A recently produced copy of the book (Figure 36), done with expert care and attention, is based on Ward’s 1857 publication, “Sketches with the Microscope in a Letter to a Friend.” Her observations were made while using a good quality Ross microscope her father bought (Figure 37). This microscope was so important to her that she included her own drawing of it in the book.
Ward died suddenly and tragically in an automobile accident, something unheard of at the time. However, in her 42 years, much was accomplished. The letter, written to her friend Emily, begins by stating, “You have expressed a wish to receive tidings from the world of wonders that surrounds us, and which is revealed only by the microscope” (Ward 1857).
This sense of wonderment was a guiding theme throughout her work. Over time, this grew ever more diverse. For example, one week she found herself working on the wings of the butterfly, dragonfly, and flying beetle (including detailed drawings of the scale patterns on the butterfly wings). Then, hair — from all kinds of animals (Figure 38), each carefully drawn with magnification recorded.

EDMOND LOCARD, ESQUIRE, AND M.D. (1877-1966)
Forensic science owes its birth to two men working in the city of Lyon. Alecsandre Lacasagne and his best student, Edmund Locard, set up the world’s first forensic science laboratory in the Lyon courthouse. When they started, this branch of science was unheard of among detectives. Soon, however, forensic anthropology (examination of bodies and bones after death), forensic ballistics (study of guns and bullets used in crimes) blood spatter analysis, trace evidence (dust, hair, fibres) developed, with the microscope playing a central role.
Originally, detectives thought of their work as limited to one person. Deliberately following in the footsteps of Sir Arthur Conan Doyle’s creation, Sherlock Holmes, it seemed as if Edmond Locard
16 ISSUE 74 JUNE 2024
Figure 39. Edmond Locard. Stamp commemorating the 60th anniversary of Locard’s death. The French stamps are mounted on a Giant Maxi Card, along with a brief biography.
might have been a model for Holmes, rather than the other way around. Locard’s most ardent adherents were very familiar with Holmes’s use of deductive reasoning and his skills in the branches of science that would most aid his consulting detective persona.
The microscope was a key part of this duality. Like Holmes, Locard used every source of evidence he could muster, often relying on things the regular police force failed to consider or simply dismissed. Much of this inborn commitment to evidence was summarised in his famous precept, to be called, “Locard’s Exchange Principle,” stating, “When two things come into contact, each object will leave trace amounts of itself on the other,” (Artieres 2016).
BARBARA MCCLINTOCK (1902-1992): Nobel Prize Recipient


Barbara McClintock was honoured by a United States commemorative stamp in 2005 for her scientific achievements leading to the Nobel Prize in 1983 (Figure 41) and by Sweden in 1989 (Figure 42). When finally awarded, coming after years of


17
Figure 40. The Bausch & Lomb Optical Co. microscope used by Dr. McClintock in her cytogenetic research on selected maize varieties.
Figure 41. USA. Barbara McClintock’s stamp was issued in 2005, as one in a group of four American Scientists.
Figure 42. A Swedish entry into the world of molecular genetics, picturing Barbara McClintock with an ear of corn segregating kernels behind her.
Figure 43. Maize ears that were used in Dr. McClintock’s research at Cold Spring Harbor. Studying kernel colour transmission enabled her to eventually explain the jumping genes.

disbelief in her chromosomal explanations and data, it was, and still is the only ‘solo’ medal in Medicine or Physiology to have been won by a woman (Bjerklie 2018).
The Bausch & Lomb Optical Co. microscope used by McClintock in her cytogenetic research on selected maize varieties is seen in Figure 40. It is monocular,


winners in this early photo.
with a mechanical revolving stage specifically designed for photomicrography. It currently has a 40X objective, with a set of achromatic lenses held separately. McClintock preferred the single objective because it offered a direct line of light available for photographing chromosomes. This work laid the foundation for what would become a Nobel Prize.
Shown in Figure 43 are actual corn selections tabulated for effects from “jumping genes,” as they were known then, with Figure 44 showing resultant colours. For close examination of such kernels and other material, a dissecting scope was used (Figure 45). Finally, when corn tests and line development were required, Dr. McClintock became part of the pollinating crew in Ithaca, NY (figure 46).
RACHEL CARSON, MARINE BIOLOGIST AND ENVIRONMENTALIST (19071964)
Long before Rachel Carson turned her attention to environmental concerns (Carson 1962; Figure 47), she wrote about the ocean as “the home of living things so small that your two hands might scoop up as many of them as there are stars in the Milky Way. And it is because of the flowering astronomical numbers of these diminutive plants, known as
18 ISSUE 74 JUNE 2024
Figure 44. This 2006 US stamp features the same type of coloration shown in corn as the previous figure.
Figure 45. The second of Barbara McClintock’s microscopes. This is a dissecting scope, used to study whole ears of corn and kernel properties.
Figure 46. The pollinating crew at Cornell and Cold Spring Harbor. Photo courtesy of Dr. Walton C. Galinat. Two eventual Nobel Prize


diatoms, that the surface waters of the ocean are boundless pastures” (Carson 1937). One can just imagine Carson sitting in her wood-paneled study in Maine, light pouring through the three windows, and a slide of diatoms gathered from below on the water’s edge (Figure 48).
Best known for her environmental literature that came late in her painfully short life, Carson’s beginnings were rooted in the sea. A reflection of her hallmark “narrative scientific” style is seen in the previous paragraph, and through such, Carson imbued life into the organisms she studied, making
19
Figure 47. Stamp itself issued to commemorate the first Earth Day, which many attribute to Rachel Carson’s book, Silent Spring (published in 1962).
Figure 48. A ‘Notable People’ card, this one for those who lived during the period of 1950–1974. Carson is listed on the card as a “writer, biologist and environmentalist.” Always shown with her microscope.



20 ISSUE 74 JUNE 2024
Figure 49. Rachel Carson is pictured among sixteen events/personalities considered to be Environmental Heroes of the 20th Century. Stamps issued in 1999 by Palau.
Figure 50. In addition to her international prominence, Carson was also featured on this US stamp, issued in 1981.
Figure 51. The stamp featured on the first-day cover, signed by its designer, Ward Brackett, which placed Rachel Carson within the Great Americans Stamp Series (Cohen 2019).
Other scientists advancing our understanding of microscopy





them understandable to those reading her books. Such skilled writing was enlivened by the careful illustrations provided by wildlife artist, Bob Hines. Together, they were able to bring organisms from the ocean depths and make them seem real in one’s living room, tackling one after the other, from microbes to whales.
The significance of her work is still being felt, as shown by commemorations from other countries (Figure 49), and in the USA (Figures 50 and 51).
Part V. Microscopic images – an everexpanding repertoire
THE ROYAL MICROSCOPICAL SOCIETY.
In 1989, a set of stamps (Figure 57 overleaf) was issued with four carefully selected images based on
21
Figure 52. Edward Jenner, UK.
Figure 53. Louis Pasteur, France.
Figure 54. Robert Koch, Germany.
Figure 55. Albert Schweitzer, Hungary.
Figure 56. South Africa Mail, issued in 1991: South African Scientists. A four-value set featuring the microscope, Sir Arnold Theiler (a veterinarian), microscope and bacteria on the stamp.

57. Issued in 1989, Royal Mail salutes the 150th Anniversary of the Royal Microscopical Society (RMS; Hutchison 2023). For this occasion, four stamps were issued along with their respective magnifications.
CUTTING EDGE IMAGERY FROM USPS – LIFE MAGNIFIED. The souvenir sheet (below) is from the U.S. Postal Service, and was designed by Tagide de Carvalho (see caption for details).

58. Tagide
is the microscopist and artist behind the scenes of the images captured here. She is the director of the Keith R.
in UMBC’s College of Natural and Mathematical Sciences. As with others seeking such expressions, she has combined her artistic work with skills learned over the years at the lab bench. Her work has now been recognised nationwide. The stamps begin with red blood cells, and then progress to the end with an oak leaf surface.
22 ISSUE 74 JUNE 2024
Figure
Figure
de Carvalho
Porter Imaging Facility
IMAGERY: SET OF ARTFUL MICROSCOPICAL STAMPS FROM GREECE. These stamps and images come from the microscopy of Maria Lambropoulou of Greece (see caption for details).

Figure 59. A set of five stamps that were issued by Greek Post in 2018 and stemmed from the microscope and imagination of Dr. Maria Lambropoulou, who is a pathologist and professor at the Democritus University Medical School. Stamp A is tissue seen as flowers; B is tissue seen as butterflies; C is in the shape of a heart; D shows a beautiful, primitive deer; while E presents a wreath formation. Unfortunately, no further information was provided on the source of the tissues and organs being examined.
observations from the microscope.They include the snowflake, house fly, blood cells of multiple types, and a microchip, the full details of each can be found in the article. by Evennett (1989). When it was introduced, it came with a presentation packet and complete set of first day covers.
Epilogue
This paper brought together images of microscopy from over twenty countries, showing the importance of the research so illustrated at the local, national,
Note that the two issuances that follow overleaf (Figures 58 and 59), one from the USPS and one from Greece, Hellenic Post, have been produced by women who are skilled in both microscopy and in the art behind their final productions.
23
A B C D E



and international level. Such work can be inspiring and lead to new skill acquisition in school classes and professional positions, as well as student to student teaching (Fig 60). In addition, these stamps attest to the use of the microscope across gender, ethnic, and cultural backgrounds, making it one approach to technology in the context of diversity, equity, and
inclusion. Applications of microscopy have grown along with new innovations, and users are seeking out such things as art in nature, and how to see and appreciate it through the microscope (Figure 59) as well as traditional roles – for example, attempts to control malaria (figure 61). It remains an eye on the world (Figure 62) that continues to open the doors of perception, offering one way to find what is most probably a humble beginning to all that life on earth has to offer.
Joel I. Cohen Member, RMS Outreach and Education Committee, and Visiting Scholar, Nicholas School
of the Environment, Duke University –joel.cohen@duke.edu
January 26, 2024
24 ISSUE 74 JUNE 2024
Figure 60. Set of six student activities from China, 1979. The stamp second from right shows students working together to learn and explore the microscope.
Figure 61. Microscopes are pictured on each of the two UN stamps: the 25th UN Anniversary, and for control of Malaria.
Figure 62. "gift stamp" for the holidays, their catalogue, done with UNESCO. including the microscope in
Acknowledgements
The author is indebted to several people and institutes in conducting research and consultation for its eventual publication.
Smithsonian Institution’s National Museum of American History
Diane Wendt, Curator Medical and Science
For arranging the Museum’s artifacts on Barbara McClintock, including her microscope, books, and lab apparatus
Offaly Historical and Archaeological Society
Michael Byrne, Secretary Tullamore, Ireland
Providing permission to reprint images from the Society’s publications
Steven Altman, Literature Judge, The Specialist United States Stamp Society
Reading early draft and extending suggestions on revision
Royal Microscopical Society
Sali Davis, Chief Executive
37/38 St Clements, Oxford, OX4 1AJ, UK
For encouragement and reading prior versions of article
References
Allen, D.E. 1976. The Naturalist in Britain. A Social History. Princeton University Press. 270 pages.
Artieres, P. 2016. Edmond Locard. Phil Post/21 16 517. Collection Historique du Timbre-Poste Français.
Carson, R. 1962. Silent Spring. Houghton Mifflin Company, Boston. 368 pages. Fourth Printing.
Carson, R. 1937. Undersea. Atlantic Monthly. 78:5567.
Clay, R.S. and T.H. Court. 1975 (edition). The History of the Microscope. The Holland Press, London, UK. 266 pages.
Cohen, J.I. 2022. Incorporating lessons from women naturalists to support biodiversity education and under-represented students. SN Soc Sci 2, 41 (2022). https://doi.org/10.1007/s43545022-00333-8
https://www.researchgate.net/ publication/359887798_Incorporating_lessons_ from_women_naturalists_to_support_ biodiversity_education_and_under-represented_ students
Cohen, J.I. 2021. The Pursuit of Meaning –Placing Biodiversity and Biography at the Center of Biology. Journal of Education, Pages 1-9 August 2021. doi.org/10.1177/00220574211026890
https://journals.sagepub.com/ doi/10.1177/00220574211026890
Cohen, J.I. 2020. Applications of microscopy in science education: gifted youth, public school, and the next generation science standards (NGSS). Journal of Biological Education. Pages 1-10. https:// doi.org/10.1080/00219266.2020.1720772
https://www.tandfonline.com/eprint/ QQFKD5DCDITXGZVNYWKN/full?targ et=10.1080/00219266.2020.1720772
Cohen, J.I. 2019. Rachel Carson and the Great American Series. U.S. Specialist: 90(3): 121-132.
Darwin, C. 1898. Life and Letters of Charles Darwin. Edited by: Francis Darwin. D. Appleton and Company. New York.
Desmond, A. and J. Moore. 1991. Darwin. The life of a Tormented Evolutionist. W.W. Norton and Company, New York and London. 808 pages.
Evennett, P. 1989. The Royal Microscopical Society Stamps. Proceedings of the RMS 24(4): 231-237.
Ford, B. 2007. Antony van Leeuwenhoek, The Discoverer of Bacteria. Pages 104-110 In, The Great Naturalists (R. Huxley, Editor). Thames and Hudson, UK.
Gardner, E.J. 1972. The History of Biology. Third Edition. Burgess Publishing Company, MN. 464 pages.
Hansen, S. 2023. The new “Life Magnified” USPS stamp series features Tagide deCarvalho’s images of microscopic life. UMBC New: January 19th . https://umbc.edu/stories/microscopic-life-stamps/ Harris, H.E. 2019. US/BNA Postage Stamp Catalog. Whitman Publishing, AL. 405 pages.
Hooke, R. 1665. Micrographia. Facsimile edition (Dover Publications, 2003)
25
Hutchison, J. 2023. RMS on show at the Royal Society. infocus 72: 4-8.
Pearle, P., B. Collett, K. Bart, D. Bilderback, and D. Newman. 2010. “What Brown saw and you can too.” Biological Sciences Faculty Publication. P.330. https://scholarworks.umt.edu/biosci_pubs/330
Reiser, F.W. 2021 April. Women Devotees of Nineteenth Century Microscopy. Searching an Invisible World for Its Tiniest Thing. A webpage and exhibit exploring the public’s captivation with microscopy during the nineteenth-century.
Schulze, F. 2011. Stamps and Microscopes. Micscape Magazine: October 2011 issue. http://www.microscopy-uk.org.uk/mag/artoct11/fsStamps-and-Microscopes.pdf
Snyder, L.J. 2023. A Kingdom of Little Animals. The American Scholar, June: 2023. 17 pages.
Stampboards.com. 2024. Stamps and covers featuring microscopes/microscopists. https://www. stampboards.com/viewtopic.php?t=95744
Pearle, P.; B. Collett; K. Bart; D. Bilderback; D. Newman; S. Samuels. 2010. What Brown saw and you can too. Am. J. Phys. 78, 1278–1289. https://doi. org/10.1119/1.3475685
Vantanoglu-Lutz, E.E. and A.D. Ataman. 2016. Medicine in philately: Antoni van Leeuwenhoek, the father of microscope. Turkish Journal of Biochemistry 41(1):58-62.
Ward, M. 2019. Sketches with the Microscope. A reproduction with essays. Printed by Brosna Press for Offaly Historical and Archeological Society, Ireland. 56 pages.
Walker, D. 2016. A gallery of postage stamps and postal stationery showing aspects of Robert Hooke’s and Antoni van Leeuwenhoek’s work. Microscopy UK Front Page.
Wellner, K. 2021. Guest Commentary: Focusing on the microscope: a tool to enhance critical thinking. American Biology Teacher: p. 495. DOI: https://doi. org/10.1525/abt.2021.83.8.495
Royal Microscopical Society
26 ISSUE 74 JUNE 2024
the
The offices of the Royal Microscopical Society are at: 37/38 St Clements, Oxford, OX4 1AJ, UK Tel: +44 (0) 1865 254760 For general enquiries email info@rms.org.uk For information about meetings and courses email events@rms.org.uk For membership enquiries email membership@rms.org.uk www.rms.org.uk
Contacting

Thanks and acknowledgement go to Dept of Earth Sciences, University of Cambridge
Calendar
We are very pleased to continue offering a range of ‘in-person’ and virtual events this year, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend.
The following information was correct at the time infocus went to print but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates.
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
2024
June
4 – 7 elmi2024, Liverpool, UK (RMS-hosted event)
12 Expansion Microscopy User Group Meeting - Canada Hosted - June 2024 (Online)
July
8 – 9 Light Microscopy Summer School 2024, York, UK
10 – 11 Getting the most from your Confocal Course 2024, York, UK
15 – 19 Electron Microscopy Summer School 2024, Leeds, UK
17 Laboratory-based X-ray Phase Contrast Imaging Workshop, London, UK (RMS hosted event)
28 July – 1 August Microscopy & Microanalysis 2024, Cleveland, Ohio USA (RMS Exhibiting at event)
August
4 – 9 Strathclyde Optical Microscopy Course 2024, University of Strathclyde, UK
(RMS-sponsored event)
25 – 30 emc2024 Copenhagen, Denmark (RMS exhibiting at event)
September
2 – 6 Flow Cytometry Course 2024, York, UK
October
2 Microscopy: Advances, Innovation, Impact 2024 - incorporating the RMS AGM & Section AGMs, London, UK
November
11 – 12 Frontiers in Bioimaging 2024 Oxford, UK
19 – 20 Frontiers in Physical Imaging 2024, London, UK
2025
March
26 – 28 flowcytometryUK 2025, Newcastle, UK
June / July
30 June – 3 July
mmc2025: Microscience Microscopy Congress 2025, Manchester, UK
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
28 ISSUE 74 JUNE 2024
Featured RMS events
elmi2024
4 - 7 June 2024, Liverpool

The European Light Microscopy Initiative was created in 2001 to establish a unique communication network between European scientists working in the field of light microscopy and the manufacturers of their equipment. Its aim is to promote the quickly developing field of light microscopy as a fundamental research tool for the life sciences
and to strengthen the channels of communication between researchers, core facilities and industry. The annual meeting, which has been running for two decades at various venues across Europe, has an excellent reputation within the microscopy community, making this meeting a key event in the calendar of hundreds of scientists and developers. The strength of this meeting lies in the mixture of scientific lectures on state-of-the-art, high-end microscopy combined with “hands-on” workshops and exhibition of the latest technology, organised by the leading companies in the field.
(See page ** for more details)

Light Microscopy Summer School 2024
8 – 9 July, York, UK
Scientific organiser: Peter O’Toole, University of York
The Light Microscopy Summer School is a two day course held at the University of York covering the principles of light microscopy. Participants are also trained in practical issues surrounding light microscopy. After introductory presentations, the course is taught predominantly
Getting the most from your Confocal Course 2024
10 – 11 July, York, UK
Scientific organiser: Peter O’Toole, University of York
This two-day, annual confocal course utilises many different sample types and fluorescent probes (DNA stains, classic antibody labels and fluorescent proteins) which are chosen to best demonstrate particular problems and techniques. Focus is always on the techniques they enable and the problems they generate, which will be
through hands-on practical sessions. The course is suitable for both novices and more experienced users wanting to gain a greater understanding of the microscope and feedback every year is always fantastic. Students usually come from a range of backgrounds, within both research and commercial organisations. All benefited greatly from the course and left with increased understanding and skills. The course is immediately followed by a two-day, hands-on Confocal Course (see below).
applicable to any sample types. The two days consist of short tutorials followed by hands-on practice.
Day 1 takes participants through the basic principles of confocal microscopy and then trains them, through hands-on practice, how to configure and image multicolour, multidimensional samples using a confocal microscope.
Day 2 builds on the experience of Day 1 and enables participants to try FRAP and spectral profiling.
29
Electron Microscopy Summer School 2024
15 – 19 July, Leeds, UK
Scientific organisers: Louie Aspinall, Nicole Hondow, Rik Brydson; University of Leeds
Frontiers in Bioimaging
11 – 12 November, Oxford, UK
Scientific organisers: Anjali Kusumbe, University of Oxford; Kurt Anderson, The Francis Crick Institute; Stefania Marcotti, King’s College London
Frontiers in Bioimaging 2024will focus on the latest developments in optical and electron microscopy as well as image analysis. Sessions
The Electron Microscopy Summer School aims to provide a basic training in both the theory and practice of scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The course covers imaging, diffraction and chemical microanalysis as well as the highly important area of sample preparation.
will cover novel technical developments and applications of these microscopy-based approaches to key cell and molecular biology questions with an overarching aim to bring insights on how they participate in our understanding of human health and disease. We aim to provide an environment where early-careers and established researchers can meet and engage with a broad range of imaging approaches and to make valuable contacts with leading groups in the field.

Frontiers in Physical Imaging 2024
19 – 20 November, London, UK
Scientific organisers: Alex Ball, Natural History Museum; Asa Barber, London South Bank University; Thomas Walther, University of Sheffield
The meeting ‘Frontiers in Physical Imaging’ aims at providing a forum to discuss state-of-the-art imaging and spectroscopy techniques applied to the characterization of materials.
Scientific presentations of novel results and educational tutorials to train young scientists in methodological know-how are both highly welcome.
The inaugural meeting planned in London on 19 & 20 November 2024 will consist of four sessions centred on the broad area of in-situ imaging:
• pigment analysis in cultural heritage (Alex Ball, NHM London),
• in-situ mechanical testing in SEM & TEM (Asa Barber, City University London),
• liquid cell TEM imaging of biomaterials (Roland Kröger, York),
• radiation damage in analytical STEM (Thomas Walther, Sheffield)
30 ISSUE 74 JUNE 2024



Discover our latest electron microscopy innovations
If you’re planning to attend the European Microscopy Congress in Copenhagen, be sure to visit Thermo Fisher Scientific in booth C13 to learn about all our latest EM innovations for life sciences and materials science and see our instruments and software in action.
Join us for:
On-site demonstrations of our new high-resolution SEM with EDS and EBSD for materials science as well as volume EM and cryo-EM for life sciences
Dedicated lunchtime symposiums of the first advanced analytical (S)TEM for materials science and the latest development in cryo-EM for life sciences
Preconference workshops unveiling our state-of-the-art EM duo for materials science and highlighting the workflows and tools for life sciences volume EM imaging
Visit Thermo Fisher Scientific at
Congress 2024 For research use only. Not for use in diagnostic procedures. For current certifications, visit thermofisher.com/certifications © 2024 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. AD081-EN-04-2024 Learn more at thermofisher.com/EMC
European Microscopy
elmi2024: Welcome to Liverpool!
The publication of our June issue of infocus coincides with elmi2024, taking place in the wonderful city of Liverpool, UK. If you are among the 600+ attendees currently taking part in this great event, we hope you are enjoying yourself!
The European Light Microscopy Initiative – to give it its full name – was created in 2001 to establish a unique communication network between European scientists working in the field of light microscopy and the manufacturers of their equipment. It has become a hugely important fixture in the calendar of hundreds of scientists and developers.
The meeting is well known for its blend of scientific lectures on state-of-the-art, high-end microscopy combined with “hands-on” workshops and largescale exhibition of the latest technology. The RMS is proud to be hosting elmi2024, and we hope this year’s instalment of the meeting series will live long in the memory of everyone attending in Liverpool.
Ahead of the event, RMS Chief Executive Sali Davis said: “We would like to say a big ‘thank you’ to everyone who has registered for this fantastic event. There is clearly a huge buzz around elmi2024 - as evidenced by the speed at which tickets sold out in the first few months of the year.
“We are currently finalising our preparations for what promises to be one of the best ‘elmis’ ever and look forward to welcoming all our attendees to

32 ISSUE 74 JUNE 2024 REPORT

ACC Liverpool – a fantastic venue - in June.”
elmi2024 includes:
• a blockbuster meeting programme covering all the latest techniques, applications and technology. Topics include: New Technologies, Imaging Across Scales, Super-resolution and Nanoscale Imaging, The AI Revolution, The Science of Tomorrow Today, and Multimodal Imaging.
• a large number companies showcasing their latest technology and running workshops timetabled outside of the main meeting programme.
• an accompanying exhibition in the purpose-built hall alongside posters, food and drink. Find out more about all our exhibitors
• a community workshop space at the heart of the exhibition, with many groups hosting meetings and running workshops
• an event dinner with networking at the Rum Warehouse.
• the ever-popular ‘academy versus industry’ football match
View from our Early Career ‘mentee’ organisers
Ahead of the event, we caught up with two of our Early Career Representatives on the organising Committee, to find out more about their roles and what they are most looking forward to at elmi2024.
Dr Liam Rooney (RMS Early Career Committee Chair)
After a few years in the organising, elmi2024 is just around the corner and we can’t wait to welcome you to Liverpool! I’m a postdoctoral researcher based at the University of Strathclyde, current Chair of the RMS Early Career Committee, and one of the early career organisers of elmi2024, together with Joelle

33
Goulding, Siân Culley and Jessica Valli. We’ve been working with a team of established mentoring organisers to bring Europe’s largest gathering of light microscopists to the UK for what’s shaped up to be a week of excellent talks, commercial and community workshops, and networking.

The four ECR organisers were approached in 2022 to gear elmi2024 towards amplifying the work of early career microscopists as part of a flagship international congress. Gail McConnell (University of Strathclyde) and I presented the bid for the UK to host elmi while we were at the 2022 meeting in Turku, Finland. Liverpool was chosen as the host city and the organising committee have put together a great programme of events spanning a range of topics across the discipline of light microscopy.
Perhaps I’m biased, but I’m really looking forward to our dedicated Early Career Researchers Session; The Science of Tomorrow, Today. It’s looking to be a truly cross-disciplinary session and will capture the latest innovations being led by ECRs across the field. The session will cover areas from multi-scale imaging, label-free techniques, and image analysis. The Imaging Across Scales Session comes in at a close second; covering methods which permit imaging over greater scales in both space and time, with higher resolution and greater depth penetration.
A major highlight of elmi is always the workshops – it brings a totally different conference environment to most other meetings and allows delegates to engage with new techniques and instrumentation. elmi2024 will see commercial and community workshops, the latter having had delegates submit proposals to host six bespoke community-led workshops over the week. The vibrant programme and workshops offered at elmi2024 provide something for every light microscopist and I’m looking forward to seeing everyone in Liverpool this June!
Dr Joëlle Goulding
(RMS Light Microscopy Committee & Professional Development and Training Focussed Interest Group)
I am a senior research fellow in advanced microscopy within COMPARE (Centre of Membrane Proteins and Receptors) based in the School of Life Sciences at the University of Nottingham. I specialise in the application of advanced light microscopy to explore the function and organisation of membrane proteins. In particular my research aims to study physiologically relevant biological systems which more closely model real-life but are typically difficult to image!
I’ve been in this role now for seven years, and can happily say my main focus and my passion is microscopy - but before this, microscopy had simply been a technique to apply to a biological question. As I was making this career change I was fortunate to attend a couple of microscopyfocussed conferences; mmc2017 and elmi2018 (in Dublin). Suddenly I was immersed in a diverse, friendly community whose passion I now share. I really enjoyed the intimate but diverse nature of elmi, from the famous core-facility football match, to the specialised workshops and the single stream of research talks. On the first day I only knew a few faces but these faces introduced me to more, and steadily my network grew! Off the back of these conferences I have forged collaborative links, been invited to join committees and have established myself as part of this community both in the UK and internationally.
I am thrilled to be part of the organising committee of elmi2024, having a chance to put my influence on the choice of sessions and speakers. I’m really excited to meet up with everyone in June and I hope we can inspire more newcomers to join and build our community further!
Be sure to read the full report and picture special on elmi2024 in our September issue of infocus!



34 ISSUE 74 JUNE 2024

Ÿ Easy to use
Ÿ Reliable GLC production in 3 minutes
Ÿ On standard TEM grids
Ÿ GLC density:10-100 / µm
2
Ÿ Aqueous buffers or pure water
Ÿ Graphene included



The Naiad-1 is VitroTEM’s benchtop device for automated GLC fabrication. Naiad-1 fully automates the preparation and handling of graphene, delivering efficient and reliable sample production. Easy-to-use software guides the user through the GLC assembly process. Naiad-1 makes the use of GLCs easy and attainable for any laboratory.
Naiad-1
Graphene liquid cells at a click
info@vitrotem.com www.vitrotem.com

Microscopy Journal of
The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences.
You can read the latest Early View papers online at www.journalofmicroscopy.org
They include:
THEMED ISSUE ARTICLE
Open Access
Recognising the importance and impact of Imaging Scientists: Global guidelines for establishing career paths within core facilities
Graham D. Wright, Kerry A. Thompson, Yara Reis, Johanna Bischof, Philip Edward Hockberger, Michelle S. Itano, Lisa Yen, Stephen Taiye Adelodun, Nikki Bialy, Claire M. Brown, Linda Chaabane, Teng-Leong Chew, Andrew I.
Chitty, Fabrice P. Cordelières, Mariana De Niz, Jan Ellenberg, Lize Engelbrecht, Eunice Fabian-Morales, Elnaz Fazeli, Julia FernandezRodriguez, Elisa Ferrando-May, Georgina Fletcher, Graham John Galloway, Adan Guerrero, Jander Matos Guimarães, Caron A. Jacobs, Sachintha Jayasinghe , Eleanor Kable, Gregory T Kitten, Shinya Komoto, Xiaoxiao Ma, Jéssica Araújo Marques, Bryan A. Millis, Kildare Miranda, Peter JohnO’Toole, Sunday Yinka Olatunji, Federica Paina, Cora Noemi Pollak, Clara Prats, Joanna W. Pylvänäinen, Mai
Atef Rahmoon, Michael A. Reiche, James Douglas Riches, Andres Hugo Rossi, Jean Salamero, Caroline Thiriet, Stefan Terjung, Aldenora dos Santos Vasconcelos, Antje Keppler
In the exciting world of scientific research, imaging core facilities are essential hubs where scientists use advanced technologies to conduct experiments and uncover fascinating discoveries. What makes these facilities remarkable is that multiple scientists can access and utilise a variety of instruments for a wide range of multidisciplinary research projects, fostering collaboration and innovation. At the forefront of this scientific adventure are Imaging Scientists,experts who play a crucial role in planning experiments, preparing materials, adapting and acquiring technologies, collecting data, training and supporting researchers, analysing images and forming conclusions. Despite their pivotal contributions, there are challenges in recognising the importance of Imaging Scientists and ensuring they have ample opportunities to advance in their careers. These challenges include a mismatch between the typical academic career path and the unique roles and responsibilities of Imaging Scientists, a lack of widespread understanding of their value plus financial constraints, insufficient training opportunities, and difficulties in attracting and retaining talented individuals.To address these issues, Global BioImaging (GBI;www.globalbioimaging.org) has brought together Imaging Scientists from around the world to develop a generally applicable set of recommendations in three key areas: highlighting the significance and value of Imaging Scientists, making it easier to recruit and retain them, and supporting their ongoing learning and professional growth. A notable concept is to
36 ISSUE 74 JUNE 2024


reimagine the traditional separation between academic roles and technical support roles. GBI envisions that these recommendations will not only benefit imaging facilities but also prove valuable for research institutions housing diverse technologies organised into core facilities. Recognising the diverse nature of research performing institutions globally, the GBI community sees this guide as a starting point that will initiate dialogue and instigate change, which should be periodically updated as the needs of Imaging Scientists change. This initial version lays a solid foundation for future enhancements, contributing to the acknowledgement and support of the invaluable work done by Imaging Scientists on a global scale.
THEMED ISSUE ARTICLE
Open Access
Characterisation and correction of polarisation effects in fluorescently labelled fibres
Nandini Aggarwal, Richard Marsh, Stefania Marcotti, Tanya J Shaw, Brian Stramer, Susan Cox, Siân Culley
Many biological structures take the form of fibres and filaments, and quantitative analysis of fibre organisation is important for understanding their functions in both normal physiological conditions and disease. In order to visualise these structures,
fibres can be fluorescently labelled and imaged, with specialised image analysis methods available for quantifying the degree and strength of fibre alignment. Here we show that fluorescently labelled fibres can display polarised emission, with the strength of this effect varying depending on structure and fluorophore identity. This can bias automated analysis of fibre alignment and mask the true underlying structural organisation. We present a method for quantifying and correcting these polarisation effects without requiring polarisation-resolved microscopy and demonstrate its efficacy when applied to images of fluorescently labelled collagen gels, allowing for more reliable characterisation of fibre microarchitecture.
ORIGINAL ARTICLE
Open Access
Analysis of microscopy techniques to measure segregation in continuouscast steel slabs
Araf Al Rafi, Begoña Santillana, Renfei Feng, Brian G. Thomas, André B. Phillion
The accurate characterisation of centreline segregation requires precise measurements of composition variations over large length scales (10−1 m) across the centreline of the cast product, while having high resolution, sufficient to quantify the significant composition variations between dendrites due to microsegregation at very small length scales (10−5m). This study investigates the potential of a novel microscopy technique, named Synchrotron Micro X-ray Flurorescence (SMXRF), to generate large-scale high-resolution segregation maps from a steel sample taken from a thin slab caster.Two methods, Point Analysis and Regression Analysis, are proposed for SMXRF data calibration. By comparing with the traditional Laser-Induced Breakdown Spectroscopy (LIBS), and Electron Probe Micro Analyser (EPMA) techniques, we show that SMXRF is successful in
37


characterisation of centreline segregation. Over large areas (e.g. 12 × 16 mm2) and at high resolution (10–50 µm pixel size) various techniques yield comparable outcomes in terms of composition maps and solute profiles.The findings also highlight the importance of both high spatial resolution and large field of view to have a quantitative, accurate, and efficient measurement tool to investigate segregation phenomena.
ORIGINAL ARTICLE
Single-shot differential phase contrast microscopy using ringshaped polarisation multiplexing illumination
Shengping Wang, Yifu Ma, Mengyuan Xie, Manhong Yao, Zibang Zhang, Jingang Zhong
We propose a differential phase contrast microscopy that enables single-shot phase imaging for unstained biological samples. The proposed approach employs a ring-shaped LED array for polarisation multiplexing illumination and a polarisation camera for image acquisition. As such, multiple images of different polarisation angles can be simultaneously captured with a single shot. Through polarisation demultiplexing, the sample phase can therefore be recovered from the single-shot measurement. Both simulations and experiments demonstrate the effectiveness of the approach. We also demonstrate that ring-shaped illumination enables higher contrast and lower-distortion imaging results than disk-shaped
illumination does.The proposed single-shot approach potentially enables phase contrast imaging for live cell samples in vitro.
THEMED ISSUE ARTICLE
Open Access
Tales from the crick: The art of demo
Matthew J. Renshaw, Camille Charoy
Equipment demonstrations (demos) play an important role in the evaluation of new systems. As well as the excitement of exploring emerging technologies, a well-organised demo can help guide procurement decisions and support funding applications. However, it is easy to underestimate the substantial effort required both before and following the demo to maximise its potential impact. Here, we discuss how our approach to demos at the Crick Advanced Light Microscopy Science and Technology Platform (CALM-STP) has evolved over the last few years, emphasising the importance of a documented approach that combines quantitative with qualitative comparisons and engages with your user base in order to build up support for any potential system purchase.
THEMED ISSUE ARTICLE
Open Access
Modulated illumination microscopy: Application perspectives in nuclear nanostructure analysis
Christoph Cremer, Florian Schock, Antonio Virgilio
Failla, Udo Birk
The structure of the cell nucleus of higher organisms has become a major topic of advanced light microscopy. So far, a variety of methods have been applied, including confocal laser scanning fluorescence microscopy, 4Pi, STED and localisation microscopy approaches, as well as different types of patterned illumination microscopy, modulated either laterally (in the object plane) or axially (along the optical axis). Based on our experience, we discuss here some application perspectives of Modulated Illumination Microscopy (MIM) and its combination with single-molecule localisation microscopy (SMLM).
38 ISSUE 74 JUNE 2024

For example, spatially modulated illumination microscopy/SMI (illumination modulation along the optical axis) has been used to determine the axial extension (size) of small, optically isolated fluorescent objects between ≤ 200 nm and ≥ 40 nm diameter with a precision down to the few nm range; it also allows the axial positioning of such structures down to the 1 nm scale; combined with laterally structured illumination/SIM, a 3D localisation precision of ≤1 nm is expected using fluorescence yields typical for SMLM applications. Together with the nanosizing capability of SMI, this can be used to analyse macromolecular nuclear complexes with a resolution approaching that of cryoelectron microscopy.
ORIGINAL ARTICLE
Open Access
Studying crystallisation processes using electron microscopy: The importance of sample preparation
We present a comparison of common electron microscopy sample preparation methods for studying crystallisation processes from solution using both scanning and transmission electron microscopy (SEM and TEM). We focus on two widely studied inorganic systems: calcium sulphate, gypsum (CaSO4·2H2O) and calcium carbonate (CaCO3). We find significant differences in crystallisation kinetics and polymorph selection between the different sample preparation methods, which indicate that drying and chemical quenching can induce severe artefacts that are capable of masking the true native state of the crystallising solution. Overall, these results highlight


the importance of cryogenic (cryo)-quenching crystallising solutions and the use of full cryo-TEM as the most reliable method for studying the early stages of crystallisation.
Submit to the Journal of Microscopy
1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints

Journal of Microscopy App Available for iPhone and Android
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
Submit online at https://mc.manuscriptcentral.com/jmi
View the Guidelines for Authors and full submission details online at: www.journalofmicroscopy.org
39

Journal of Microscopy: Special Issues

19th Euroseminar on Microscopy Applied to Building Materials (EMABM) 2024
The Journal is pleased to announce that the May 2024 issue is a special issue dedicated to the 19th Euroseminar on Microscopy Applied to Building Materials (EMABM) 2024 meeting which took place 12-15 May 2024.
The issue has been guest edited by Dr Alexander Wetzel from the Universität Kassel, Germany, and features 18 papers.
The issue is available to view here: https:// onlinelibrary.wiley.com/toc/13652818/2024/294/2
Light Microscopy Core Facility Management special issue
The Journal is pleased to announce the publication of the Light Microscopy Core Facility Management special issue, which was published as the June 2024 volume.
This special edition has been guest edited by Dr. Sebastian Munck (KU Leuven, Neuroscience Department, Leuven, Belgium & VIB BioImaging Core, Leuven, Belgium) and Dr. Kurt I. Anderson (The Francis Crick Institute, London, UK).
The featured cover image is an artist’s depiction of the topic of this issue, namely Light Microscopy Core Facility Management. The image depicts a Möbius strip, a one-sided object and a symbol of infinity, with the words “science” and “service” engraved. The iconic vertex of the half-twist separates the two engraved words. Encapsulated in a single object, this conceptualisation symbolises the dual nature of core facilities—advancing science and providing service.
Additionally, the Möbius strip appears to be made from a translucent material such as glass. With a beam of light passing through the ribbon, this object is also reminiscent of an objective lens, which is at the heart of light microscopy.
The ribbon and the beam are set in front of a canvas, which represents a digital image with different fluorescent entities in blue and red showing up in the background.

40 ISSUE 74 JUNE 2024
The cover was created by Christof De Bo from VIB Technologies.
The papers in this issue include:
Setting up a light microscopy core facility: Facility design
Timo Zimmermann
Strategies for Selecting and Managing Equipment in a Light Microscopy Facility
Kurt Anderson
Staying on track – Keeping things running in a high-end scientific imaging core facility
Oliver Renaud et al
Improving light microscopy training routines with evidence-based education
Gabriela Imreh, Jianjiang Hu, Sylvie Le Guyader
Tales from the crick: The art of demo
Matthew J. Renshaw, Camille Charoy
Innovating in a bioimaging core through instrument development
Sebastian Munck, Christof De Bo, Christopher Cawthorne, Julien Colombelli
Challenges and opportunities for bioimage analysis core-facilities
Johannes Richard Soltwedel, Robert Haase
A practical guide to bioimaging research data management in core facilities
Christian Schmidt et al
A perspective into full cost recovery within a core facility/shared resource lab
Peter J. O’Toole, Joanne L. Marrison
‘Branded’ microscopy core facilities –Mutually beneficial partnerships between academia and industry
Joshua Z. Rappoport
The challenges and opportunities of open-access microscopy facilities
Heather N. Cartwright, Chad M. Hobson,Teng-Leong
Chew, Michael A. Reiche, Jesse S. Aaron
Recognising the importance and impact of Imaging Scientists: Global guidelines
for establishing career paths within core facilities
Graham D. Wright et al
Future proofing core facilities with a seven-pillar model
Erin M. Tranfield, Saskia Lippens
Building momentum through Networks: Bioimaging across the Americas
Mariana De Niz et al
More than just ‘added value’: The perils of not establishing shared core facilities in resource-constrained communities
Mai A. Rahmoon, Chad M. Hobson, Jesse S. Aaron, Harikrushnan Balasubramanian, Teng-Leong Chew
The full issue is available to view here: https:// onlinelibrary.wiley.com/toc/13652818/2024/294/3
Call for Papers for new Special Issue
Microscopy and Infectious Diseases (host-pathogen interaction and pathogens structure)
The Journal of Microscopy is pleased to announce a new special issue featuring papers on “Microscopy and Infectious Diseases (host-pathogen interaction and pathogens structure)”.
The special issue will be guest edited by Dr Leandro Lemgruber, Head of the Cellular Analysis Facility, University of Glasgow, UK, and Mariana De Niz, The Feinberg School of Medicine, Northwestern University, USA.
We welcome submissions for this issue and papers can be reviews, Methods and Protocols or primary research articles.
Read our guidelines for authors
Deadline: September 30th, 2024
If you have any questions, please contact Editorial Office Manager Jill Hobbs, journaladmin@rms.org. uk
https://www.rms.org.uk/resource/ journal-of-microscopy-call-for-papersfor-new-special-issue.html
41
Autofluorescence in Focus: The Hidden Light of Biological Samples
Authors: Dr Rose De Guzman and Dr Jake Jones
Since its inception, fluorescence imaging has revolutionised biological research, providing an invaluable tool for scientists to explore and visualise cellular structures and processes at a microscopic scale.This article focuses on what happens when the sample of interest emanates its own fluorescence, causing an autofluorescence background, and explores methods to manage unwanted autofluorescence in your imaging experiments.
Shedding Light on Fluorescence Imaging
The sun emits a broad-spectrum electromagnetic radiation, where the typical human eye can detect wavelengths from approximately 400 nanometers (nm; violet) to 700 nm (red), which corresponds to the visible light spectrum. Beyond the visible light spectrum includes ultraviolet B (UVB; 290–320 nm), ultraviolet A2 (UVA2; 320–340 nm), ultraviolet A1 (UVA1; 340–400 nm), and infrared (IR; 700–1000 nm) radiations (Gasparro et al. 1998).
The excitation of a molecule by ultraviolet or visible light photons is a phenomenon called photoluminescence, which is then divided into two categories: fluorescence and phosphorescence. As depicted in a Jablonski diagram for organic molecules, a molecule’s absorption of a photon triggers a series of photophysical events: vibrational relaxation (energy loss in the absence of light emission), fluorescence, intersystem crossing (from a singlet state to triplet state), and phosphorescence.
Fluorescence is the absorption of light at a specific wavelength (λEX) and subsequent emission of light at a specific, longer wavelength (λEM). Phosphorescence occurs similarly to fluorescence, but the process has a longer duration between the absorption of energy and emission of light.
Fluorescence imaging has become a crucial research tool used for its high sensitivity and non-invasiveness (Ladokhin et al. 2000). As Lakowicz (2006) details, fluorescence imaging detects fluorescence emission from fluorophores, which are chemical compounds that re-emit light when excited. Fluorescence imaging instruments, including microscopes and flow cytometers, have lasers that produce light at specific wavelengths to excite fluorophores and have sensors that detect light emitted by fluorophores.
If you have ever used a fluorescence microscope to image biological samples, then you might have noticed a faint, omnipresent background signal
42 ISSUE 74 JUNE 2024

1. Modern strategies use near-infrared (NIR) excitation light with labels excitable over 700 nm (e.g., Alexa Fluor 750) to avoid excitation and emission ranges of common autofluorescence sources.
permeating the tissue.The signal can vary in intensity across different structures and will likely exhibit changes in intensity depending on the wavelength of your excitation source.
However, before you check protocols and notes for faulty staining techniques, consider the biological composition of your sample. Is the sample cellularised? Is the sample pigmented? Is there a significant number of structural proteins present? If you answered “yes” to any of these questions, then the signal you are seeing might not be as foreign as you think. Some, if not all that background, emanates naturally as autofluorescence.
Understanding Nature’s Own Fluorescence
Autofluorescence is the intrinsic cellular fluorescence of prokaryotic and eukaryotic cells induced by endogenous structural components and metabolites exhibiting the ability to fluoresce and encompass most of the visible light spectrum. In a review by Berezin and Achilefu (2010), the earliest report of autofluorescence was from a blue opalescence of a Mexican wood extract in Europe (Acuña and Amat-Guerri 2007), where the fluorescence origin was later confirmed to be the
product of flavonoid oxidation (Acuña et al. 2009).
As chronicled by Harvey (1926), the invention of the fluorescence microscope in the early 1900s enabled the study of cellular and tissue autofluorescence.
Much like the engineered fluorophores used in stains and dyes, autofluorescent molecules are typically comprised of polycyclic hydrocarbons with delocalised electrons that can be excited by incoming photons. Autofluorescent molecules show resistance to efficient vibrational relaxation after being stimulated by incoming light. As a result, the excess energy is emitted as a new photon with lower energy and a higher wavelength than the excited photon.
A few endogenous molecules, like chitin, are known to autofluoresce vibrantly under ultraviolet stimulation. However, many autofluorophores are less likely to be excited by incoming photons compared to their engineered counterparts that scientists are more accustomed to seeing in the laboratory. The concentration of engineered contrast agents can likewise be adjusted in a benchtop staining protocol to restrict or boost a fluorescence signal.
Still, natural autofluorescence is always limited
43
Figure
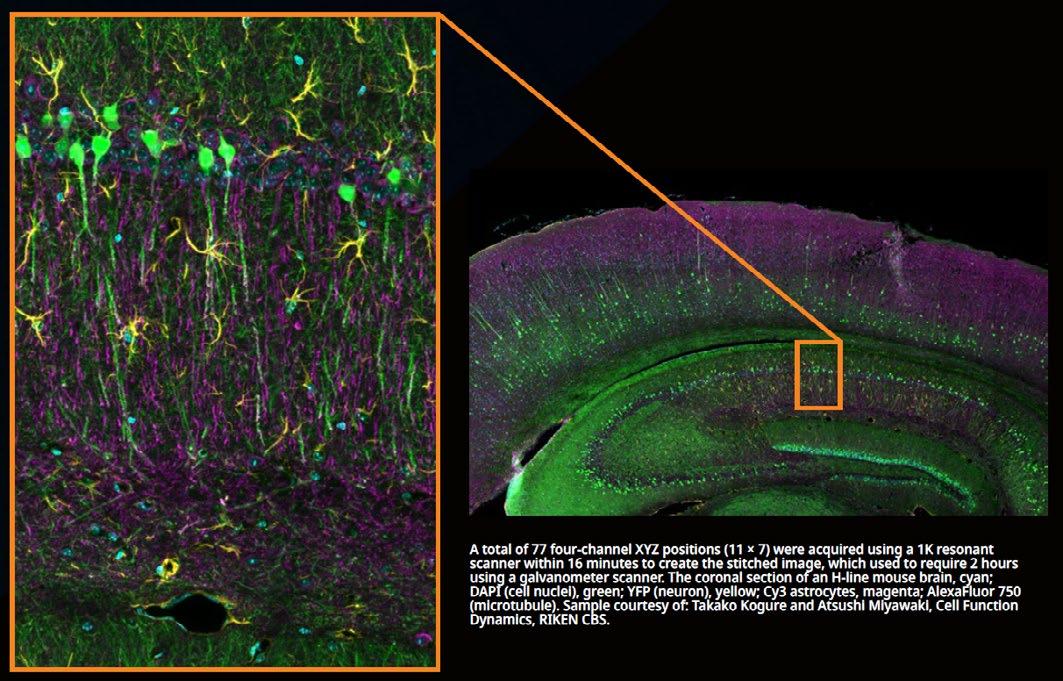
to biological concentrations. Autofluorescent molecules also have distinct excitation windows, although most autofluorescence is excitable in the UV-green range and yields broad excitation spectra. Combined, these factors make autofluorescence much more common to encounter when using imaging systems with dedicated fluorescencestimulating light sources and photon detectors more sensitive than the naked eye.
Sources of Autofluorescence in Life Science Research
Autofluorescence has been considered problematic in fluorescence imaging as it increases interference with fluorescence measurements (Billinton and Knight 2001; Cordina et al. 2018; Jun et al. 2017; Spitzer et al. 2011). To properly accommodate for the presence of autofluorescence in research imaging experiments, consider the endogenous fluorophores of your samples, including coenzymes of key enzymes in redox reactions, extracellular fibrous proteins, and functional proteins. Below are several endogenous fluorophores that researchers commonly encounter in the laboratory.
1. Nicotinamide adenine dinucleotide
Reduced nicotinamide adenine dinucleotide (NADH) is a metabolic coenzyme and an electron donor for reductive biosynthesis found throughout the cytoplasm, where it serves as a critical component in glycolysis and the pentose phosphate pathway.
The fluorescence characteristics of NADH have been investigated in detecting and monitoring metabolic changes without the introduction of exogenous labels and dyes (Cannon et al. 2021; Chance et al. 1962; Georgakoudi et al. 2002). Chance et al. (1979) demonstrated that live tissues illuminated with UV light emit blue fluorescence, arising from mitochondrial NADH.
When NADH is phosphorylated (NADPH, nicotinamide adenine dinucleotide phosphate), fluorescence arises from the nicotinamide ring of NADPH (De Ruyck, 2007; Patterson et al. 2000). Although spectrally identical to NADH in its unbound form, NADPH is biochemically and
44 ISSUE 74 JUNE 2024
λEM ≈
(λEX ≈ 320–380 nm,
420–480 nm)
Figure 2. Coronal section of an H-line mouse brain taken with a confocal laser scanning microscope with near-infrared (NIR) capabilities.
functionally distinct (Cannon et al., 2021; Visser and van Hoek, 1981). The indistinguishable fluorescence of NADH and NADPH, therefore, is commonly called NAD(P)H emission (Galeotti et al. 1970).
Being a crucial component of cellular metabolism means that NAD(P)H autofluorescence is present in almost all living cells. To perform its metabolic functions, the molecule exists naturally in an oxidised (NAD+) and reduced (NAD(P)H) state. However, only NAD(P)H is fluorescenceproducing. The oxidised form NAD+ absorbs at a much shorter wavelength (~260 nm) and does not fluoresce (Berezin and Achilefu 2010). The reduced form, therefore, is widely used in fluorescence imaging.
2. Flavins (λEX ≈ 380–490 nm, λEM ≈ 520–560 nm)
Commonly found in the form of flavin adenine dinucleotide (FAD), this metabolic coenzyme plays important roles in the tricarboxylic acid cycle and electron transport chain (Chance et al. 1962; Chance et al. 1979). FAD is localised primarily in the mitochondria where active metabolic processes produce hotspots of the autofluorescent signal. Some researchers prefer to refer to autofluorescence from flavins as “flavoprotein fluorescence” as FAD is functionally bound to protein complexes in the mitochondria like succinate dehydrogenase. Unlike NAD(P)H, which is fluorescent in the reduced state, flavins are fluorescent in the oxidised state and not fluorescent in the reduced state (Chance et al. 1979; Deyl et al. 1980).
3. Collagen (λEX ≈ 270 nm, λEM ≈ 390 nm)
Collagen is a key mechanical protein that can be assembled to create various supporting structural matrices for most tissues. It can be encountered in the dermis of skin, the extracellular matrix of internal organs, and surrounding vasculature. It comprises tendons, ligaments, hair, and fingernails. While collagen is seldom seen in cell cultures, researchers using in vivo samples or whole tissues
for their imaging experiments will find collagen unavoidable (Georgakoudi et al. 2002).
4. Elastin (λ
Another prominent extracellular matrix (ECM) protein, elastin is often interspersed with collagen to give extracellular matrices more mechanical extensibility. Elastin is densely located around vasculature and experiences frequent elastic deformation to deal with changes in blood pressure. It is also found in the skin where pliability is necessary to complement movement from underlying bones and muscles. Once again, researchers imaging in vivo samples or whole tissues must be aware of the autofluorescence caused by structural proteins like elastin (Deyl et al. 1980).
5. Lipofuscin (λEX ≈ 345–490 nm, λEM ≈ 460–670 nm)
A muddled autofluorescent molecule, lipofuscin can be found comprising small yet vibrant structures in the fluorescence spectra of neurons, glial cells, skeletal muscle cells, and cardiac muscle cells, among others. Lipofuscin can be recognised in both cell cultures and whole tissues. It has been noted to become progressively more apparent as a sample experiences biological aging. While the name may imply that a lipid or a lipoprotein is responsible, the autofluorescence signal from lipofuscin has been attributed to an amalgamation of proteins, carbohydrates, and lipids (Billinton and Knight 2001).
6. Tryptophan (λEX ≈ 280 nm, λEM ≈ 350 nm)
Proteins, due to the presence of aromatic amino acid constituents, exhibit intrinsic fluorescence that has enabled the study of protein conformational changes (Lakowics 1999). An essential amino acid and a required precursor to signaling molecules like serotonin and melatonin that can be encountered in neurological research, tryptophan is a staple component of protein biosynthesis and has been the primary contributor to intrinsic protein
45
≈
EM ≈
EX
350–450 nm, λ
420–520 nm)
fluorescence (Lippitz et al. 2002). Its omnipresence cannot be understated, as tryptophan residues can be found in most folded proteins, and its autofluorescence signal subsequently permeates cells and tissues alike.
As its structural chemistry is tied into protein conformations, tryptophan autofluorescence has been observed to change in wavelength and intensity as a function of changes to protein structure and binding status. The indole group of tryptophan is the dominant source of UV absorbance at ~280 nm and emission at ~350 in proteins (Ghisaidoobe and Chung 2014;Teale 1957).The fluorescence emission maximum (λEM) and intensity of tryptophan are sensitive to the polarity of its local environment (Ghisaidoobe and Chung 2014).
7.
Melanin (λEX ≈ 340–400 nm, λEM ≈ 360–560 nm)
Melanin is a diverse group of pigments produced by melanocytes. Specifically, eumelanin and pheomelanin are black and yellow-brown pigments, respectively, that contribute to the coloration of skin, hair, and eyes (Wakamatsu and Ito 2002). Produced by cells in the basal epidermis of skin, melanin functions as a photoprotective molecule preventing the sun’s UV light from damaging the valuable proteins and DNA in outwardly facing skin cells.
Unless you are culturing melanocytes directly, melanin requires the most consideration for experiments that image through the skin as its natural concentration and distribution can vary even within the same samples (Gallas and Eisner 1987).
Fluorescence from Sample Preparation
The above sources radiate photons naturally from biological tissues. Many researchers also encounter fluorescence from non-biological components or from reagents required to prepare samples in the lab.
For example, the plastic bottoms in petri dishes,
well plates, and cell culture flasks can fluoresce brightly and over a broad spectrum.We recommend using glass bottom or specifically nonfluorescent polymer containers whenever you need to perform fluorescence imaging of biological samples. Phenol red, a common additive to cell culture media, can also significantly increase background fluorescence when imaging live cells and interferes with the observation of certain fluorophores. This can be easily avoided by using a medium lacking phenol red as an alternative before beginning any imaging experiments. A phenol red-free medium also permits a lower amount of illumination required for fluorophores of interest.
Paper labels or stickers can cause similar problems, as paper is highly fluorescent. When using paper labels on containers or slides, keep the labels out of the way of samples that you plan to image.
Finally, aldehyde fixatives are commonly used in dying or staining protocols. Reagents such as glutaraldehyde or formaldehyde react with proteins to create fluorescent crosslinks throughout cells and tissues. Replace them with non-aldehyde fixatives to prevent an accumulation of unintended fluorescence signals.
While these examples are not naturally occurring sources of autofluorescence, understanding their origin and impact can prevent unwanted effects on image data.
Methods to Manage Unwanted Autofluorescence
So far, we have established several endogenous fluorophores and where to find them. Now, a question arises: How can you manage these signals before starting an imaging experiment? Here are several methods to manage unwanted autofluorescence:
1. Careful selection of stains and filters
In simple tissues or monocellular cultures, carefully choose the excitation and emission spectra of
46 ISSUE 74 JUNE 2024
commercial stains and find accompanying narrow filters that avoid major autofluorescence peaks. Doing so can boost your signal-to-noise ratio.
2. Bioluminescence imaging
Techniques such as bioluminescence imaging completely remove the chance for autofluorophores to contribute signal to your collected data. Luminescence experiments do not require excitation light for the chemiluminescent reactions to produce emission photons, leaving autofluorophores unstimulated.
3. Confocal or multiphoton microscopy systems
If flexibility is allowed, investigate if alternate imaging modalities might work for your experiments. For thick samples or tissues, confocal or multiphoton microscopy systems can minimise the overall autofluorescence contribution. They do so by eliminating the collection of out-of-focus light or limiting excitation to the focal plane, respectively.
4. Near-infrared imaging
If your protocol allows for it, choose high quantum efficiency stains or experiment with increased concentrations of contrast agent. This can also boost the signal, albeit at a higher cost. Modern strategies use near-infrared (NIR) excitation light with labels excitable over 700 nm (e.g., Cy7 or Alexa Fluor 750) to avoid excitation and emission ranges of frequently encountered autofluorophores.
As shown in Figure 1, NIR range applies to a wide spectrum of wavelengths.The typical human eye can detect wavelengths from 350 nm to 740 nm, which corresponds to the violet to red rainbow. Beyond 740 nm is the infrared spectrum—exceeding what the human eye can detect. Confocal systems rely on the visible spectrum of light between 350 nm to 750 nm, whereas multiphoton systems rely on the spectrum of light between 700 nm to 1300 nm. As technologies continue to develop, we can now expand the spectrum of light detected by
confocal microscopy and start taking advantage of wavelengths from 750 nm to almost 900 nm. NIR imaging capabilities can enhance the performance of a confocal microscope by providing reduced autofluorescence.
NIR imaging enables users to avoid autofluorescence in their sample as most autofluorescence occurs in the visible spectrum, particularly in the blue and green wavelengths. Figure 2 showcases the results of using a confocal laser scanning microscope, which offers greater multiplexing capabilities by extending the excitation and detection spectral profile.
In tissues, most of the autofluorescence that you will see showing up in your samples comes from compounds, such as flavins and nucleotides, especially NAD(P)H. This autofluorescence shows up mostly between 400 and 550 nm, which conflicts with some of the most common fluorophores used in microscopy, such as DAPI (4',6-diamidino-2phenylindole) and GFP (green fluorescent protein). By shifting the dyes toward the red end of the spectrum, we can easily avoid the problem windows of autofluorescence.
When working with plant samples, we encounter further difficulty with multiple autofluorescence peaks. Chlorophyll has multiple excitation maxima across the visible range. Being able to take advantage of the optical window past 700 nm provides feasibility to avoid autofluorescence peaks.
5. Post-acquisition image processing
When autofluorescence signal contribution cannot be mitigated before imaging, post-acquisition image processing techniques, such as spectral demixing or background subtraction, can be effective alternatives. These computational techniques require researchers to tease out the endogenous fluorophores in their sample.
Conclusion
Fluorescence imaging, a transformative tool in biological research, offers a window into the
47
microscopic world, revealing the intricate details of cellular structures and processes. As technology evolves and our understanding deepens, we continue to uncover the hidden nuances of fluorescence imaging, turning challenges into opportunities for discovery.
Here we provided several common endogenous fluorophores that researchers commonly encounter in the laboratory. Knowing the common sources of endogenous fluorophores and ways of managing unwanted autofluorescence enables the optimal acquisition of data and continues to advance biological research and clinical assessments through fluorescence imaging.
References
1. Acuña, A.U. and Amat-Guerri, F. 2007. “Early History of Solution Fluorescence: The Lignum Nephriticum of Nicolás Monardes.” In Fluorescence of Supermolecules, Polymers, and Nanosystems, 3–20. Berlin, Heidelberg: Springer Berlin Heidelberg.
2. Acuña, A.U., Amat-Guerri, F., Morcillo, P., Liras, M., and Rodríguez, B. 2009. “Structure and Formation of the Fluorescent Compound of Lignum Nephriticum.” Organic Letters, 11(14), 3020–3023.
3. Berezin, M.Y. and Achilefu, S. 2010. “Fluorescence Lifetime Measurements and Biological Imaging.” Chemical Reviews, 110(5), 2641–2684.
4. Billinton, N. and Knight, A.W. 2001. “Seeing the Wood through the Trees: A Review of Techniques for Distinguishing Green Fluorescent Protein from Endogenous Autofluorescence.” Analytical Biochemistry, 291(2), 175–197.
5. Cannon, T.M., Lagarto, J.L., Dyer, B.T., Garcia, E., Kelly, D.J., Peters, N.S., Lyon, A.R., French, P.M., and Dunsby, C. 2021. “Characterization of NADH Fluorescence Properties under One-Photon Excitation with Respect to Temperature, Ph, and Binding to Lactate Dehydrogenase.” OSA Continuum, 4(5), 1610–1625.
6. Chance, B., Cohen, P., Jobsis, F., and Schoener, B. 1962. “Intracellular Oxidation-Reduction States In Vivo.” Science, 137(3529), 499–508.
7. Cordina, N.M., Sayyadi, N., Parker, L.M., Everest-Dass, A., Brown, L.J., and Packer, N.H. 2018. “Reduced Background Autofluorescence for Cell Imaging Using Nanodiamonds and Lanthanide Chelates.” Scientific Reports, 8(1), 4521.
8. De Ruyck, J., Famerée, M., Wouters, J., Perpète, E.A., Preat, J., and Jacquemin, D. 2007. “Towards the Understanding of the Absorption Spectra of NAD (P) H/NAD (P)+ as a Common Indicator of Dehydrogenase Enzymatic Activity.” Chemical Physics Letters, 450(1-3), 119–122.
9. Galeotti, T., Van Rossum, G.D., Mayer, D., and Chance, B. 1970.“Fluorescence Studies of NAD (P) H Binding in Intact Cells.” Hoppe-seyler’s Zeitschrift fur Physiologische Chemie, 351(3), 274–275.
10. Gasparro, F.P., Mitchnick, M. and Nash, J.F. 1998. “A Review of Sunscreen Safety and Efficacy.” Photochemistry and Photobiology, 68(3), 243–256.
11. Geisler, A.N., Austin, E., Nguyen, J., Hamzavi, I., Jagdeo, J., and Lim, H.W. 2021. “Visible Light. Part II: Photoprotection against Visible and Ultraviolet Light.” Journal of the American Academy of Dermatology, 84(5), 1233–1244.
12. Georgakoudi, I., Jacobson, B.C., Muller, M.G., Sheets, E.E., Badizadegan, K., Carr-Locke, D.L., Crum, C.P., Boone, C.W., Dasari, R.R.,Van Dam, J., and Feld, M.S. 2002.“NAD (P) H and Collagen as In Vivo Quantitative Fluorescent Biomarkers of Epithelial Precancerous Changes.” Cancer Research, 62(3), 682–687.
13. Ghisaidoobe, A.B. and Chung, S.J. 2014. “Intrinsic Tryptophan Fluorescence in the Detection and Analysis of Proteins: A Focus on Förster Resonance Energy Transfer Techniques.” International Journal of Molecular Sciences, 15(12), 22518–22538.
14. Harvey, E.N. 1926. “Bioluminescence and Fluorescence in the Living World.” American
48 ISSUE 74 JUNE 2024
Journal of Physiology-Legacy Content, 77(3), 555–561.
15. Jun, Y.W., Kim, H.R., Reo, Y.J., Dai, M., and Ahn, K.H. 2017. “Addressing the Autofluorescence Issue in Deep Tissue Imaging by Two-Photon Microscopy: The Significance of Far-Red Emitting Dyes.” Chemical Science, 8(11), 7696–7704.
16. Ladokhin, A.S., Jayasinghe, S., and White, S.H. 2000. “How to Measure and Analyze Tryptophan Fluorescence in Membranes Properly, and Why Bother?” Analytical Biochemistry, 285(2), 235–245.
17. Lakowicz, J.R. ed. 2006. Principles of Fluorescence Spectroscopy. Boston, MA: Springer US.
18. Lippitz, M., Erker,W., Decker, H.,Van Holde, K.E., and Basche, T. 2002. “Two-Photon Excitation Microscopy of Tryptophan-Containing Proteins.” Proceedings of the National Academy of Sciences, 99(5), 2772–2777.
19. Patterson, G.H., Knobel, S.M., Arkhammar, P.,
Thastrup, O., and Piston, D.W. 2000.“Separation of the Glucose-Stimulated Cytoplasmic and Mitochondrial NAD (P) H Responses in Pancreatic Islet Β Cells.” Proceedings of the National Academy of Sciences, 97(10), 5203–5207.
20. Spitzer, N., Sammons, G.S. and Price, E.M. 2011. “Autofluorescent Cells in Rat Brain Can Be Convincing Impostors in Green Fluorescent Reporter Studies.” Journal of Neuroscience Methods, 197(1), 48–55.
21. Teale, F.W.J. and Weber, G. 1957. “Ultraviolet Fluorescence of the Aromatic Amino Acids.” Biochemical Journal, 65(3), 476.
22. Visser, A.J.W.G. and Hoek, A.V. 1981. “The Fluorescence Decay of Reduced Nicotinamides in Aqueous Solution after Excitation with a UV‐Mode Locked Ar Ion Laser.” Photochemistry and Photobiology, 33(1), 35–40.
23. Wakamatsu, K. and Ito, S. 2002. “Advanced Chemical Methods in Melanin Determination.” Pigment Cell Research, 15(3), 174–183.
Could you be an RMS Event
Help us stay at the forefront of microscopy, imaging and flow cytometry
The RMS is looking for new scientific organisers to lead its busy schedule of events in support of microscopy, imaging and flow cytometry. The Society hosts a wide range of events throughout the year - from workshops and courses to meetings and large-scale conferences. As an organiser, you would be responsible for the scientific content of the event, choosing speakers and topics. We provide full administrative support and we are open to new ideas.
Anyone interested should contact RMS Events Director, Victoria Masters (victoria@rms.org.uk) for more information

Royal Microscopical Society 37/38 St Clements Oxford, OX4 1AJ www.rms.org.uk Registered Charity: 241990 @royalmicrosoc
Organiser?
New Member Welcome
The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months. We hope they enjoy a long and rewarding membership with the RMS.
Mr Zhiyang Zhong
Mr Alon Hannuna
Dr Susana Rams
Dr Megan Taggart
Miss Katie Beirns
Mr Joseph Fihosy
Dr Salime Can
Dr Shihao Wang
Dr Sonya James
Dr Triana Amen
Mr Raman van Wee
Dr Lisa-Maria Needham
Miss Mollie Stefanek
Dr Clodomiro Cafolla
Dr Giuseppe Di Caprio
Mr Rohan Rama Krishnan
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk
Member Profiles
Name Giuseppe Di Caprio
Tell Us About You?
I am currently appointed as Associate Professor (Senior Lecturer) at the University of Strathclyde. I am an interdisciplinary biophysicist interested in Microscopy, Quantitative Bioimage Analysis, Organs-On-Chip and Machine Learning. I worked for 11 years at Harvard University, where I specialised in developing advanced microscopy systems for imaging flow cytometry, and I implemented computational solutions to quantify biological data from 4D fluorescenceand 3D electron -microscopy.
Why did you become a member of the RMS?
I'm passionate about microscopy and wanted to join a community of professionals and enthusiasts who share similar interests.
How do you feel being an RMS member benefits you?
I hope that being part of the society will provide access to valuable resources, networking opportunities, and the latest developments in the field of microscopy.

50 ISSUE 74 JUNE 2024
Name Rohan Rama Krishnan
Tell Us About You?
I'm a postgraduate in Food Science and Biotechnology at the University of Leeds, originally from Bangalore, India. My passion for food microbiology drives my studies, focusing on the microscopic aspects of food safety and innovation. I'm excited about using biotechnology to enhance our food systems.
Why did you become a member of the RMS?
To further my professional development and stay abreast of the latest advancements in microscopy. Joining RMS has opened up numerous opportunities for networking with other professionals and experts in the field, which is invaluable for collaborative and career opportunities. The society provides access to a range of exclusive resources including cuttingedge research papers and newsletters that keep me updated on the latest developments. Additionally, attending conferences and events organised by RMS not only enriches my knowledge but also allows me to share my work with peers. Moreover, being part of this society enables me to contribute to the advancement of microscopy, participating in committees and

Name Harjodh Rai
Tell Us About You?
I am a freelance researcher on Upwork and a tutor at Find Tutors, with a strong passion for Biochemistry and scientific research.
I am currently pursuing a Biochemistry degree at Queen Mary University of London, with a year in industry/research, and expect to graduate in 2027.
Why did you become a member of the RMS?
Membership of the RMS aligns perfectly with my interest in exploring the complexities of biology and related science. It provides a platform to interact with experts in microscopy, access cutting-edge research, and contribute to the advancement of the field. Through membership I can enhance my understanding of microscopy techniques and applications,
discussions that shape the future of our field.
How do you feel being an RMS member benefits you?
Being a member of the Royal Microscopical Society (RMS) offers several significant benefits that align well with my career goals and personal growth within the field of microscopy. The professional development opportunities provided through RMS workshops and training sessions are crucial for staying current with the latest techniques and technologies. This knowledge not only enhances my skills but also increases my value as a professional. In addition, access to RMS resources, including specialised publications and exclusive research, enriches my understanding and keeps me informed about the forefront of microscopic science. This continuous influx of information is essential for my research and helps in formulating new ideas or improving existing projects. Participating in conferences and other RMS events also provides a platform to present my work, receive feedback from peers, and engage with cuttingedge research from around the world.

enhance my skills and knowledge. In addition, community involvement provides opportunities for leadership, mentorship and recognition, which support my career aspirations in scientific research and discovery. Specifically, joining the community is an important step in achieving my goals and furthering my interest in the exciting world of microscopy.
How do you feel being an RMS member benefits you?
Being a member of the Royal Society of Microscopy gives me access to vibrant expertise, resources and opportunities essential to growing my career. Interacting with fellow members allows for valuable networking, collaboration on research projects, and exposure to the latest developments in microscopy. The society’s educational materials will give me insight into the latest techniques and applications, enhancing my skills and competencies in the field. Additionally, community involvement provides opportunities for leadership, mentorship, and professional exposure, strengthens my credentials, and sets me up for success in scientific research and discovery.
51
The Oxford-ZEISS Centre of Excellence at the Kennedy Institute of Rheumatology and the Institute of Developmental and Regenerative Medicine, Oxford
Josie Eade, NDORMS, University of Oxford
The Oxford-ZEISS Centre of Excellence (CoE) at the University of Oxford is an imaging centre with an eye on the future. It’s been built on a concept that successfully brings together academia and industry partners to not only provide state-of-the-art technology, but also give researchers access to the unique expertise of ZEISS engineers in their research and development team. Creating a melting pot for new imaging ideas, the centre’s ground-breaking technologies allow researchers to observe scientific processes in increasingly remarkable ways to address novel biological research questions. The centre celebrated its official opening in February 2024.


52 ISSUE 74 JUNE 2024
The Kennedy Institute, University of Oxford.
The Institute of Developmental and Regenerative Medicine (IDRM) Oxford.

The Oxford-ZEISS CoE is a strategic partnership between the Kennedy Institute of Rheumatology (KIR), the Institute of Developmental and Regenerative Medicine (IDRM), and Carl ZEISS AG (ZEISS), a leader in the field of optics and optoelectronics. The KIR and IDRM sit together on the Old Road Campus at the heart of the University of Oxford’s biomedical research. They have broad
research interests across immunology, neurology, embryology, cardiology, and oncology. Their goal is to study the key biological processes that underpin normal healthy development, and where these may go wrong in diseases such as cancer, cardiovascular disease, neuro-degenerative diseases, or conditions related to inflammation or tissue repair.
Although it was officially opened by the University
53
ZEISS LSM 980 confocal.

of Oxford’s Vice-Chancellor Professor Irene Tracey on 21 February 2024, the centre has been building up its microscopy capabilities for the last three years. In 2021, it became the first place in the UK or Europe to install a ZEISS Lattice Lightsheet 7, two years before anywhere else. The microscope
was later equipped with two high-speed cameras for simultaneous two-colour high-speed acquisition. These sit alongside a multi-photon microscope for powerful deep imaging, Airyscan confocals for imaging across the scales from sub-cellular structures to the large tissue tile regions, and a
54 ISSUE 74 JUNE 2024
ZEISS Lattice Lightsheet 7.

high throughput slide scanner, to ensure that the Centre provides a whole suite of light microscopy. It offers its service to researchers around the University for a diverse range of applications from mitochondrial morphology at a sub-cellular scale, to the development of whole organisms, and is actively looking for additional partners to come into the fold. A team of experts has been appointed to support the widest range of applications for microscopy and analysis.
The Oxford-ZEISS CoE is the brainchild of Marco Fritzsche, Professor of Biophysical Immunology at the KIR, and Scientific Director of the centre. Having studied physics and mathematics in
Germany, he went on to study Experimental Physics and Biology at University College London. While conducting postdoctoral research at the University of Oxford, Marco met physicist and Nobel Laureate Eric Betzig who developed the Lattice Lightsheet microscope at the Janelia Farm Research Campus in the USA. Marco became fascinated by the Lattice Lightsheet microscope technology and began a long-term collaboration with the Janelia Research Campus. Speaking about the technology, Marco said: “Because of the minimally invasive Lattice Lightsheet we can study cells for long periods of time, gaining an understanding of their history, studying individual interactions of the cell within the biologically designed environment, and we can
55
The winner of the first Oxford-ZEISS CoE Image competition. Mitochondrial moon phases by Robert Mitchell imaged on ZEISS ELYRA 7 with lattice SIM. Mitochondria are highly dynamic organelles which cannot reorganise spatially, but are able to change their morphology through fission and fusion events. This image shows the dynamic nature of mitochondrial morphology reminiscent of the lunar cycle.
change the microenvironment to analyse the cells’ response. I like to call it the ZEISS Lattice Lightsheet revolution and I think that the lattice will replace confocal microscopes one day.”
Development leaps from disruptive technology
While many imaging centres provide cutting-edge technology, the Oxford-ZEISS CoE has taken a powerful step forward by bringing together Oxford scientists with the ZEISS research and development team. This partnership creates powerful new technologies developed alongside the research questions they are designed to solve.
“What this delivers”, says Marco, “is the freedom to raise questions relating to our areas of biological study that may challenge current microscopy capabilities. The idea is to create leap changes in the biomedical field by disruptive technology and close the gap between scientific needs and technological developments.”
Dr Bernhard Zimmermann, Head of Life Sciences at ZEISS Research Microscopy Solutions explained: “Developing our solutions in line with the rapidly changing demands of the scientific community continues to be vital for our success. Strong collaborations with leading-edge academic partners are key for reaching this goal. Thus, extending and reinforcing our interactions with the world class research teams at Oxford through the OxfordZEISS CoE is putting us in an excellent position to develop new technologies that are tailored to support new and upcoming application demands.”
Breaking the boundaries (imaging in four dimensions)
The Lattice Lightsheet microscope was developed to overcome one of the greatest challenges in imaging live cells: to observe them without light affecting their behaviour or damaging the sample.
Lightsheet microscopy uses a plane of light to illuminate a very thin slice of a sample. In comparison
to standard confocal laser-scanning microscopy, lightsheet microscopy causes less photodamage to cells while gathering the same amount of data. Multiple planes can be used to render an image in four dimensions: three physical dimensions and time. Lightsheet microscopy can be used to image whole structures like organoids and whole organisms.
Lattice Lightsheet microscopy goes a step further, using successive planes of light in a ‘lattice’ shape to generate a series of images rendered in three dimensions and over time. It benefits from the same gentle illumination of lightsheet microscopy which minimises photobleaching. The technology enables researchers to watch biology live, as it unfolds over time by capturing previously unseen dynamic biological phenomena in three dimensions, in multiple colours, and for multiple hours per experiment.
As an example, Professor Paul Riley, Director of the IDRM, together with Professors Shankar Srinivas and Professor Georg Hollaender have used the ZEISS Lattice Lightsheet to study the cellular dynamics of embryos during the development of their cardiovascular and immune systems, allowing unprecedented insights under physiological conditions.
If the Lattice Lightsheet is the star of the OxfordZEISS CoE, the confocal microscopes are what Dr Kseniya Korobchevskaya and Dr Helena Coker, the Advanced Facility Managers, call the ‘pillars’. Confocal microscopy is a widely used ‘workhorse gold standard’ technique, and the Centre is equipped with two of the latest fast ZEISS LSM 980 confocal microscopes. These systems offer a range of capabilities including enhanced-resolution imaging using Airyscan 2, as well as spectroscopy and life-time measurements for the F-techniques; FCS, FCCS, FLIM, FRET and FRAP. This allows users not only to take images with confocal optical sectioning but probe the dynamics of molecules within cells and tissues.
Fluorescence correlation spectroscopy (FCS) is a
56 ISSUE 74 JUNE 2024

statistical microscopy method which uses a laser beam to study precisely how proteins diffuse and interact in cells. However, FCS typically uses a strong, narrow laser beam to focus on a single spot which can bleach fluorescent molecules. Dr Narain Karedla, a postdoctoral researcher at the KIR and a specialist in advanced microscopy, combined the ZEISS LSM 980 with scanning FCS to develop a technique which scans in a laser line across the cell surface rather than in a single spot. This technique reduces photobleaching while increasing the amount of data collected. Narain used this technique to study the interaction of cell surface and cytosolic proteins in a remarkably precise way.
“We can now estimate these molecular spatiotemporal organisation with previously unachieved sensitivity and accuracy”, said Narain.
Where dynamic imaging is not the goal, the centre also houses a ZEISS LSM 880 Non-Linear Optical (NLO) confocal, a multi-photon microscope for powerful deep tissue and intravital imaging.
The centre has a ZEISS Axioscan 7 automated slide scanner which can be used for static rather than live-cell imaging. It takes 100 different slides at a
time and can rapidly digitise samples in bright field and up to seven fluorescent channels.
“We leverage the ZEISS microscopy platform, especially the Axioscan, to acquire high-resolution whole-slide chromogenic and fluorescent images for tissue biology research”, said Ray Shih-Hsuan Mao, M.D., DPhil Student in the Nanchahal Group, KIR. “This enables comprehensive analysis of the tissue microenvironment, guiding therapeutic development. The platform facilitates unbiased image analysis and efficient protocol optimisation for advanced spatial technologies, such as spatial transcriptomics and multiplexed imaging.”
Most recently, the KIR won a UKRI Medical Research Council (MRC) grant in partnership with the Centre for Human Genetics to install a ZEISS ELYRA 7 with Lattice Structured Illumination Microscopy (SIM). Thanks to its sub-diffractionlimited spatial resolution, this fast, super-resolution microscope adds to the highly versatile imaging technology employed to answer the kind of research questions that are active in the health and disease space.
For DPhil student Robert Mitchell, the
57
Shown (from left to right): Dr Bernhard Zimmermann, Martin Fischer, Professor Irene Tracey, Professor Marco Fritzsche, Dr Leong Chew, Professor Paul Riley.
ELYRA 7 opened up new possibilities in his immunometabolism studies. “The ZEISS ELYRA 7 with Lattice SIM has enabled me to image mitochondrial networks in 3D with high resolution, making it possible to distinguish separate mitochondria which are packed tightly together in the small cytoplasmic space in B cells -something that had been a barrier for me even with Airyscan technology. It’s helping me to characterise how mitochondria in B cells change their morphology in response to different stimuli.”
Dr Jacky Ko, a Bioimage Specialist at the CoE added: “Complex biological processes are a puzzle for microscopes to unfold across multiple scales. Through the orchestration of multimodal machines at the ZEISS Centre, tiny details are gathered in one place to bring the full scientific picture out of the mist.”
Expertise and vision
Another strength of the centre is the quality of the team that supports researchers with a complete end-to-end service – providing training and support from imaging to data management and analysis.
“This core team is small, so the group’s approach to bringing new technologies to researchers and their diverse biological applications requires a certain element of adaptability”, said Marco. But the breadth of expertise and passions within the team are reinforced by teamwork and collaboration to help each other deliver excellence across disciplines.This approach works well for the researchers who find that the advice and guidance from the team provide quick and optimal solutions for their studies. If their requirements sit beyond the immediate capabilities of the centre, active collaboration between the Oxford and ZEISS R&D team, and technical modifications to ZEISS instruments, ensure needs are met that offer exciting possibilities for new scientific discovery.
KIR DPhil student Kaitlyn Purdie said: “The help that I’ve had from Kseniya, Helena and Jacky has
helped me understand more intricately how the microscopes actually work, and why specific methods of analysis are better than others, which has deepened my knowledge beyond just understanding what buttons to press. The expertise they offer exponentially improves the quality of the data I can collect and present.”
The Oxford-ZEISS CoE supports researchers from interdisciplinary centres where people have different levels of expertise. Some have never even seen a microscope while others are expert users. Bringing them all up to the same quality of expertise is seen as a challenge but it is a core goal of the centre that the visualisation of microscopy data is never a barrier to important scientific discoveries. It has connected to the Oxford microscopy community to deliver courses, and in the autumn of 2023, the centre ran its first Oxford-ZEISS Deep Tissue Imaging course.
David Grainger was one of 12 participants on the highly specialised, week-long course. Aimed at researchers with experience in microscopy but looking to accept more challenging tasks for their research, the group explored five imaging platforms, accumulating a grand total of 48 hours of imaging and 12 hours of image analysis. “The major benefit was being taught how different microscopes acquire their images enabling me to make an informed decision on the most appropriate technology for my tissue research question. It’s given me the confidence to do experiments that I previously would have deemed too challenging”, said David.
A huge success, and a learning curve also for the Oxford-ZEISS team, the course will be replicated again this year.
Staff profiles
Name: Marco Fritzsche
Position: Scientific Director
Qualifications: DPhil. Biophysics, MSci Theoretical Physics, BSc Math, BSc Physics
58 ISSUE 74 JUNE 2024

Background: Prof Fritzsche is the Scientific Director of the Oxford-ZEISS Centre of Excellence and leads the Biophysical Immunology Laboratory (www.bpi-oxford.com) between the Rosalind Franklin Institute and the Kennedy Institute of Rheumatology at the University of Oxford, UK. The BPI Laboratory aims to unravel the impact of biophysics and mechanobiology on the human immune response in health and disease. For this mission, the BPI lab develop custombuilt microscopy technology at the interface of biophysics and immunology. Prof Fritzsche holds a MSc in theoretical physics and conducted his PhD in experimental biophysics and cell-biology at the London Centre for Nanotechnology at the University College London, UK. He performed his Postdoctoral work at the University of Oxford in close collaboration with the Howard Hughes Medical Institute Janelia Farm, USA.
Favourite microscope: I think that Lattice Lightsheet will change the microscope landscape.
How do you relax in your spare time? I love walking cities, mostly London.
answering biological questions. I have led several development projects, which resulted in fully functioning bespoke imaging systems: Large Field of View Light Sheet, TIRF-SIM and Near IR Label Free Transient Absorption microscopes. I enjoy working together with biomedical researchers on exciting imaging projects, where my experience and expertise in fluorescent and super-resolution microscopy can contribute best.

Favourite microscope: ZEISS Airyscan Confocal
How do you relax in your spare time?
I love hiking, potholing, canoeing and all other outdoor activities. But as those are quite demanding on my time, on most days I have to limit myself to less fancy reading and knitting.
Name: Helena Coker
Position: Advanced Microscopy Manager
Name: Kseniya Korobchevskaya
Position: Advanced Microscopy Facility Manager
Qualifications: PhD
Background: I am an optical physicist by training. I did my PhD in the field of ultra-fast spectroscopy. However, I was always fascinated by biology and this motivated me to move to the field of fluorescent microscopy, where my optical skills can be applied
Qualifications: DPhil. Physical and Theoretical Chemistry, MSci. Natural Sciences
Background: My background is quite broad as I studied Natural Sciences (mostly chemistry and pharmacology) for my MSci, then completed a DPhil in Physical and Theoretical Chemistry. During my DPhil., I built and optimised an interferometric scattering (iSCAT) microscope for very fast tracking of diffusing particles - this is where my love
59

for optics began to drive my career. In 2018 I joined the University of Warwick as an Imaging Specialist in Lattice Lightsheet microscopy (LLSM). I moved back to Oxford in 2021 to manage the TIRF-SIM microscope and in 2023 I joined the microscopy facility full time.
Favourite microscope: ZEISS LLS7
How do you relax in your spare time? I am a ‘do-er’ inside and out of work so in my spare time I do a lot of art and crafts including dressmaking (I made my Inauguration dress!), cycling including racing on Zwift, and once I’ve tired myself out ‘doing’, I sit with my cat.
Name: Jacky Ka Long Ko
Position: Advanced Bioimage Analyst
Qualifications: MPhil/PhD in Imaging and Interventional Radiology
Background: I trained as a physicist, then redirected my research focus towards leveraging computational methods for biomedical imaging applications. Throughout my career, I have been deeply involved in medical image analysis, particularly in integrating artificial intelligence into the field. In 2017, I spearheaded the development of image-based surgical navigation systems with respiratory-gated robotic controls. Building upon this experience, my doctoral studies delved deeper
into cluster and GPU computing for neurovascular investigations, specifically exploring atherosclerosis treatments through hemodynamic simulations with AI-assisted modelling. In 2022, I brought my expertise to the Oxford-ZEISS CoE, where I introduced advanced computer vision techniques in bioimage research.
Favourite microscope: ZEISS Axioscan 7 Slide Scanner

How do you relax in your spare time? In my spare time, I indulge in my passion for photography, particularly exploring landscapes as I travel across cities and fields. Recently, I’ve delved into aerial photography using drones, which offers a thrilling opportunity to capture unseen perspectives of the world around me.
Case study
Case study – the building blocks of life itself
Every human body begins as a clump of cells. The fertilized egg undergoes dramatic changes in shape over the course of its development in the womb, during which we are transformed into something more recognisable as a developing foetus. The process is called morphogenesis and it’s incredibly dynamic. A myriad of distinct cell types have to be created and positioned correctly as the
60 ISSUE 74 JUNE 2024
basic plan of the body is laid down to produce a healthy embryo. An understanding of embryonic morphogenesis at the cellular and molecular level would advance our understanding of the fundamental process of tissue remodelling, and how this might go wrong in disease. Knowing the normal fate of cells is important not only for an understanding of development but also has implications for therapy in humans.
Shankar Srinivas, Professor of Developmental Biology at the IDRM, is an expert in morphogenesis.
Lattice Lightsheet technology at the ZEISS CoE has transformed his research. ‘Embryos are really very sensitive to imaging and so the Lattice Lightsheet is transformative to our research, as it preserves their viability during our experiments,’ he said. ‘It enables us to image at much higher spatial and temporal resolution than any other microscope that we’ve used before, allowing us to watch the cells in the embryos as they migrate and shape various features.’
Recent work from his group has characterised the migration of a group of cells in the embryo responsible for setting up the head-tail axis. Lattice Lightsheet imaging enabled Dr Shifaan Thowfeequ in Shankar’s team to characterise a mutant with a defect in this important migratory process (now accepted for publication in Developmental Cell). This advances our understanding not only of how the body forms, but also more broadly of how cells in the body migrate, a normal process that can be subverted in diseases such as cancer.
Shifaan has been able to observe cells during gastrulation, a very early process in embryonic development where cells diversify from a single layer into different cell types which migrate and begin to form the structures of the body. ‘This imaging technology has enabled us to understand how individual cells leave one cell layer, emerge into another layer, and start moving in a coordinated manner,’ explained Shankar.
While it’s still early days for the CoE, the centre has already enabled Shankar to do more ambitious

E5.5_AVE_migration: Single frame from a Lattice Lightsheet timelapse movie showing the migration of genetically-labelled anterior visceral endoderm cells (red) from the distal tip to the embryonicextraembryonic boundary of the 5.5 dpc mouse embryo (cyan) necessary for setting up the anterior-posterior axis of the body plan.
experiments and has helped his team to upskill through the centre’s commitment to training and development.

E6_mesoderm_migration: Single frame from a Lattice Lightsheet timelapse movie showing the emergence of nascent mesodermal cells (green) through the primitive streak and their subsequent migration towards the anterior midline of the embryo (red) where the heart forms.
61

Introducing the Nuclear Laboratory Technician’s (NLT) Group
David Williams, Bangor University
My name is David Williams and I’m the founder of the Nuclear Lab Technician’s (NLT) group. I’m a Senior Laboratory Technician for Bangor University and have been since April 2021. As a technician starting out in the nuclear sector, I had very little knowledge on how to operate a lab that handles radioactive material. With so many questions that needed answering, it was suggested that I should contact other technicians within the sector - and so, the idea for the NLT group was formed.
As technicians in the nuclear sector, we come across many challenges. These include general radiation protection, keeping abreast of the policies and legislation underpinning legal requirements, and understanding which equipment suits working with radioactive material. We also need to adopt the correct processes and personal protective equipment (PPE) required to minimise risks while working with radioactive material.
In September 2021 the group was officially founded with technicians from Bangor University, AWE, Diamond, Lancaster University, Leeds University, NNL, Manchester University, Sheffield University, and UKAEA. Monthly meetings were organised to discuss health and safety in nuclear labs, lab processes, and any specific issues raised by those taking part. A website was also created to help facilitate communication between technicians.
In November 2022, the NLT held its first conference at Bangor University. Suppliers such as Mirion Technologies, Sci Med Ltd, and Spectrographic sponsored and supported the event.The conference
enabled members to provide updates on what their institutions were up to, and provided suppliers with an opportunity to show what they had to offer. It also served as an important vehicle for networking between members and suppliers. In November 2023, the NLT held its first ‘glove box’ workshop, held in Manchester University. The event talked about setting up glove boxes for the handling of radioactive materials, and how to operate them.The event was also supported by MBRAUN, who gave a presentation on what they can offer and answered questions from the technicians present.
On the 24th and 25th of June 2024, the NLT will host its second conference at Bangor University. Currently the event will have the following suppliers and organisations involved through their kind sponsorship and attendance: Buehler, Conwy Mind, HTSL, MBRAUN, Mirion Technologies, NSAN, Phoenix Dosimetry, RMS, RSC, SLS, and Thermofisher. The conference will centre around promoting communication between technicians and suppliers, and showcasing what the suppliers have to offer. There will also be a workshop discussing the adaptation of equipment to deal with radioactive material. Alongside this, we are very pleased that Conwy Mind have accepted an invitation to talk about mental health in the workplace – a hugely important issue for all staff and their employers.
The ongoing aim of the NLT Group is to help technicians from all nuclear and radiochemical organisations, to support them if they have any issues, and to help improve health and safety within nuclear and radiochemical labs.
62 ISSUE 74 JUNE 2024











Diamond Knives www.labtech-em.com/em/em-home Tel: 01435 517000
• Unsurpassed sectioning quality and durability • Knives for all applications • Resharpening and exchange services • Available from Labtech-em
... innovative and practical tools for electron microscopists
TEM image courtesy of Steve Gschmeissner
From the RMS President
Dear Readers,
It has been such a busy few months at the RMS that it is hard to know where to start, but I would like to begin by reflecting on the fantastic news that the Society has been selected as the Professional Congress Organiser (PCO) for the International
Microscopy Congress (IMC) 21, taking place in Liverpool, UK in 2026.
This really is one of the biggest Microscopy events and it’s an honour to be part of the organising team tasked with putting on what we all hope will be a great show! We look forward to working closely with the scientific organising committee, the University of Liverpool and other partners - including the event venue, ACC Liverpool – as preparations gather pace over the next two years.

The RMS is always keen to reach out to new audiences and foster new connections across the whole of the scientific community. As such, I was delighted that we were among the exhibitors at the Advanced Materials Show at the NEC in Birmingham in May. Later this summer, we will also be attending M&M2024 – the premier microscopy event in the US – and emc2024, taking place in Copenhagen, Denmark. As a truly international Society, it is vital that we seek out opportunities to both strengthen and grow our connections across the globe.
At the time of publication, I will be in Liverpool for elmi2024 –a brilliant event with a great reputation, and an essential date in the diary for the Light Microscopy community. This is the sixth time the Society has overseen the meeting, and it is always both Dr Peter O'Toole.
NEWS
an honour and a privilege. This year we have done things a little differently, with the ‘seasoned’ scientific organisers acting as mentors for a team of Early Career representatives who have taken on responsibility for the scientific programme. You can hear more from two of them – Liam Rooney and Joelle Goulding – on p32. If you are here with me in Liverpool as you read this, I hope you are enjoying all the fantastic science, as well as catching up with friends and colleagues.
Our flagship publication, The Journal of Microscopy, continues to go from strength to strength, with a number of Special Issues recently published – and others in the pipeline. The ‘Core Facility Management’ Special Issue for June features some brilliant contributions from a wide range of authors, and should make essential reading for anyone interested in the latest issues in core facilities. It was a pleasure to have had a hand in a couple of the papers myself. If you have yet to discover what the JoM has to offer – for both readers and authors seeking to publish - I strongly urge you to find out more.
Looking ahead to the summer, I am really excited about being among the tutors on the RMS Light Microscopy, Confocal and Flow Cytometry courses. We also have the ever-popular Electron Microscopy Summer School taking place in Leeds in July. Education and training are absolutely key elements of our work as a Society – and on that note I should also mention our six Summer Studentships due to take place in the coming months, about which you can read more on p66.
Speaking of content in this issue, two articles in
particular caught my eye – for very different reasons. Back in February, I had the pleasure of being invited to say a few words at the opening ceremony for the Oxford-ZEISS Centre of Excellence at the Kennedy Institute of Rheumatology and the Institute of Developmental and Regenerative Medicine. It truly is a remarkable facility, and you can read more about what it has to offer on p52 . Meanwhile, pride of place on my work desk sits a framed copy of four RMS postage stamps - specially commissioned in 1989 to celebrate the Society’s 150th anniversary. You might spot these among the colourful images in Joel Cohen’s excellent article on how microscopy has been represented on postage stamps around the world! (p4).
Returning to our activities, the RMS is continuing to move forward at pace, in pursuit of new opportunities and initiatives. For example, through closer working with our corporate members and partners, we now offer our support in hosting courses and webinars on their behalf. It’s important to remember that the RMS represents the whole of the microscopy, imaging and Flow Cytometry community – supporting both the academic and corporate worlds.
Before signing off, I’d like to take this opportunity to thank our Chief Executive, Sali Davis, the RMS staff and all our dedicated volunteers for continuing to enable these activities in support of microscopy around the world. Finally, I would like to thank all of you, our members, for your ongoing support.
Best wishes,
Dr Peter O’Toole, RMS President
65
Summer Studentships 2024: Successful applicants revealed!
Six projects set to take place this summer with RMS funding support
We are pleased to announce that six students have been successful in applying for an RMS Summer Studentship to take place later this year.
The studentships are awarded each year to undergraduates undertaking projects involving a significant element of microscopy. The RMS aims to split the awards evenly between physical sciences, biological sciences and interdisciplinary projects.
Each student will receive up to £2,000 to help cover laboratory and living costs during their projects, and complete a report on their work which will be published in infocus Magazine. The students will also be asked to record a short video, briefly talking about their experience.

Ben Watson completed an RMS Summer Studentship at the University of Strathclyde in 2022.
Many congratulations to this year's successful applicants, who are as follows:
Student Supervisor Institution
Ash Eana
Elizabeth Barnett
Odell Wong
Daniel Hopper
James Wedlinscky
Guillaume Macneil
Gail McConnell
University of Strathclyde
Peter Thomason and Nikki Paul CRUK Scotland Institute
Saskia Bakker University of Warwick
Nicole Hondow University of Leeds
Richard Bowman University of Glasgow
Francesco Boselli Durham University
Complete the RMS Training Survey
Tell us about your training needs – and enter prize draw for £100 Amazon voucher!

A new survey has been launched to help the RMS understand current demand for training opportunities and courses in microscopy, imaging and flow cytometry.
The Society is committed to providing the courses and schemes that best meet the needs of the international microscopy community – and is keen to identify where there may be gaps in current provision.
The survey only takes around 15 minutes to complete – and as an added incentive, respondents can enter a prize draw for the chance to win a £100 Amazon voucher!
The survey results will be strictly anonymised and treated with utmost confidence - in line with current GDPR. If you decide to provide your email address we will only contact you in accordance with your wishes.
The survey will remain open until the end of July and the results will be published on the RMS website later this year.
Find out more and complete the survey
66 ISSUE 74 JUNE 2024 NEWS
RMS proud to support Women in STEMM Summit 2024
Promoting gender equality and empowering women in STEMM fields
The RMS proudly supported the Women in STEMM Summit 2024 held at the Institute of Physics (IOP), London and Trinity College Dublin (Ireland).
The summit, which took place over the 7th and 8th March, brought together leading experts, professionals, and advocates in the field to address the challenges and opportunities facing women in microscopy across all STEMM Disciplines (Science, Technology, Engineering, Mathematics and Medicine).
The Summit was organised by Anna Baldycheva, Chair of the EPMS (Engineering, Physical & Material Sciences) Section at the RMS.
During the event, esteemed speakers from various backgrounds shared insights and initiatives aimed at promoting gender equality and empowering women in STEMM fields. Discussions ranged from fostering inclusive workplace environments to promoting mentorship programmes and advocating for policy changes to support women’s advancement in
STEMM careers.
On the second day of the summit - also International Women’s Day - RMS Chief Executive Sali Davis delivered an inspiring opening keynote talk, highlighting the society’s commitment to supporting women in STEMM.
She emphasised the importance of creating equal opportunities and a supportive ecosystem for women to thrive in scientific fields. Sali also outlined the RMS’s ongoing efforts to provide resources, mentorship, and networking opportunities for women scientists, aiming to bridge the gender gap in STEMM professions.
Throughout the summit, participants engaged in fruitful discussions, exchanged best practices, and forged valuable connections to further advance the cause of women in STEMM. The RMS is committed to promoting diversity, inclusion, and gender equality in the scientific community and looks forward to continuing its collaborative efforts to support women in STEMM.

67
The Women in STEMM summit took place in both Dublin and London, coinciding with International Women’s Day 2024. Organiser Anna Baldycheva is pictured addressing attendees.
RMS set to deliver International Microscopy Congress (IMC21)
‘Olympics of Microscopy’ coming to Liverpool in 2026
The RMS is proud to be taking on the role of Professional Congress Organiser (PCO) for the 21st International Microscopy Congress, taking place in Liverpool in 2026.
The Society will be providing full administrative support and organisational services for the event, which is overseen by the International Federation of Societies for Microscopy (IFSM) and has become known as the ‘Olympics of Microscopy’.
Held every four years since 1954 and attracting hundreds of delegates from across the globe, the Congress features world-renowned plenary speakers alongside an extensive trade exhibition with leading companies launching groundbreaking new instruments.
As planning takes shape over the coming two years, the RMS will be working closely with the scientific organising committee, the University of Liverpool
and other partners - including the event venue, ACC Liverpool.
RMS Chief Executive Sali Davis said: “This is wonderful news, and we are very honoured - and of course, excited - to be providing the organisational support for IMC21. The RMS has a wealth of experience in staging events for the global microscopy community, and we are really looking forward to working with all our partners as planning takes shape over the next couple of years. We can’t wait to see you in Liverpool in 2026!”
The successful bid to hold IMC21 was made by the Scientific Programme Committee, the University of Liverpool, Liverpool City Region and the RMS.
Save the date:
The 21st International Microscopy Congress (IMC21) is set to take place in Liverpool, UK, from 28 August - 05 September 2026.

68 ISSUE 74 JUNE 2024 NEWS
RMS join exhibitors at The Advanced Materials Show
Society makes first appearance at popular exhibition for materials scientists
and developers
The RMS joined a host of exhibitors at the NEC Birmingham for the Advanced Materials Show 2024 on 15 May.
The event brought together leaders in engineering, science and innovation to share knowledge and showcase materials with exceptional properties for engineering and electronic applications, along with the technologies used to develop them.
It was the RMS’s first appearance at the popular event, providing the opportunity to reach a new audience and promote the work of the Society.
Chief Executive Sali Davis was joined by Finance & Operations Director Adam Clay on the RMS stand, engaging with visitors about the benefits of RMS membership – and much more. The famous RMS ‘Wheel of Fortune’ also made the trip to Birmingham, offering the chance to win a modest prize!
Sali said: “This was a great event to be a part of, and a new venture for the RMS. It’s important to remember that Microscopy is a fundamental part of science as a whole – across both the academic and commercial sectors. We are always keen to reach out to new audiences and communities to promote our work and encourage new members.
She added: “It was particularly pleasing to meet so many Early Career Researchers and encourage them to publish their research in the Journal of Microscopy. I look forward to seeing some resulting papers being submitted!”
Find out more about the Advanced Materials Show

69
RMS Finance and Operations Director Adam Clay at the Advanced Materials Show.
Focus on Microscopy 2024
Genoa, Italy 24th –
27th March 2024
Focus on Microscopy is a conference which brings together a diverse range of scientists bound together by their dedication to the innovation and application of microscopes, imaging probes, and image analysis. This year saw the conference return to Genoa, Italy, after 20 years.
It is safe to say that a lot has happened in the field of microscopy since then!
Uniquely, this conference begins with a morning of tutorials which delegates can choose from. These provide an opportunity to introduce yourself to a new topic, or, in my case, revise some physics I hadn’t touched since my undergraduate days. This was a nice initiation into the conference, especially given I had just come from a meeting on cancer biology and needed to switch gears. Leaving the tutorial, I felt ready for what was to come.
Ideas and advances happen at this meeting, evidenced by Nobel Laureate Stefan Hell as he recalled that it was in Genoa 20 years earlier that he’d had his prize-winning idea for super-resolution microscopy. Stefan’s keynote presentation along with Colin Sheppard’s recount of 50 years of
confocal microscopy gave a real sense that we were all part of history in the making. The venue provided a symbolic backdrop for such a meeting: a renovated cotton warehouse in the old port of a bustling city with a real sense of working history.
Stefan and Colin’s talks were just two of many highlights at this conference, which included exciting new applications in polarisation microscopy from Sophie Brasselet at the Institute Fresnel, and rotating coherent scattering microscopy from the lab of Alexander Rohrbach at the University of Freiburg.
A theme that emerged was the emphasis on getting more from imaging. Traditionally this means quantitatively analysing images in various ways such as measuring intensity or object tracking. But this is now being taken further, by extracting data directly from probes or from the optical setup itself. The work of Brasselet and Rohrbach are prime examples: using the polarisation of fluorescence to quantify object directionality and using back scattered light to determine material properties, respectively.
Despite being a sizeable conference with multiple parallel sessions, a sense of community was

70 ISSUE 74 JUNE 2024 REPORT
never far away. This was my first time at Focus on Microscopy, and I went alone representing Bioimaging and Biophotonics at King’s College London – a potentially daunting prospect. However, I was presenting a poster and was lucky enough to be selected for a flash talk, which fast-tracked my introduction to everyone. I found plenty of interest in our work combining fast fluorescence lifetime imaging and super-resolution microscopy, a collaborative effort between the labs of Simon Ameer-Beg (King’s College London), Simon Poland (also King’s College London), and Robert Henderson (University of Edinburgh).
The poster session was well-coordinated and organised thematically which generated lots of local discussion. Since I was presenting a diverse portfolio which included elements of (FRET) probe design, new engineering (large lifetime-on-chip SPAD arrays), and imaging methods (such as PAINT single molecule localisation and swept laser array scanning) I also had a diverse range of visitors to my poster making the conversations even more interesting and the time fly by. But good Italian food was calling and some of the other flash talk presenters and I had arranged to go to dinner which was more than enough to pull me away at the end of the poster session.
As usual, the end of the conference was marked by a gala dinner, which was held in the grand Maritime Station, and ended the week in style. For once, this conference hosted its gala dinner on the final night and therefore could be enjoyed without the
prospect of needing to be back in presentations (and awake) the following morning.This opportunity wasn’t missed by those of us who went out to the local plazas after the meal had concluded.
I want to thank this year’s hosts, in particular Alberto Diaspro and Paolo Bianchini from Genoa for organising this conference and I look forward to attending in the future. Focus on Microscopy alternates between Europe and Asia: next year the conference is due to be held in Taiwan, and the hosts were clearly excited to be welcoming the community to their home country. I would highly recommend this conference whether you are new to microscopy and looking for a crash course in developments, or whether you are deeply involved and looking for new inspiration, feedback, and insightful discussion.
Speaking on behalf of the lab I am in, we are already saving up for the trip.
I would like to thank the Royal Microscopical Society, as well as the British Society for Cell Biology and the Company of Biologists for their financial support in attending this conference. The ongoing support of the RMS makes it possible for young academics to access exciting meetings such as this held across the world, and thereby actively contributes to the development of a rich and diverse new generation of microscopists.
Tommy Pallett
Postdoctoral Research Fellow Comprehensive Cancer Centre, King’s College London, UK

71
RMS Prize-Winner at Actin 2023: Simona Buracco
15 December 2023, Bristol, UK
We would like to thank the organisers of Actin 2023 for the opportunity to present our data on the regulation of actin polymerisation by the Scar/WAVE complex, and to the RMS for its generosity in sponsoring the award.

The Scar/WAVE complex is the main catalyst of pseudopod and lamellipodium formation during cell migration. It’s a large hetero-pentamer that is usually constricted in an autoinhibited structure that sequesters WAVE’s C-terminal VCA domain. According to current models, when a signal triggers its activation, the Scar/WAVE complex changes conformation and exposes the VCA domain to activate the Arp2/3 complex. This induces the generation of branched actin networks that form cell protrusions.
By a combination of genetic manipulation and microscopy techniques (we thank the BAIR Beatson Advances Imaging Resource for the support), we demonstrated in both B16-F1 mouse melanoma cells and the social amoeba Dictyostelium discoideum that Scar/WAVE without its VCA domain still induces
the formation of morphologically normal, actinrich protrusions (as shown in the super-resolution microscope image presented here), extending at comparable speeds despite a drastic reduction of Arp2/3 recruitment. However, the proline-rich regions in Scar/WAVE and Abi subunits are essential, though either is sufficient for the generation of actin protrusions in B16-F1 cells. We also showed that N-WASP can compensate for the absence of Scar/WAVE’s VCA domain and induce lamellipodia formation, but intact WAVE complex is still needed. Based on our data, we conclude that the Scar/ WAVE complex does more than activating Arp2/3, with proline-rich domains playing a central role to promote actin protrusions. This implies a broader function for the Scar/WAVE complex, bringing many actin regulating proteins together simultaneously as a lamellipodium-producing core.
Further work will be needed to investigate more in details the molecular mechanisms of these newly discovered functions of the Scar/WAVE complex and of its polyproline domains.

72 ISSUE 74 JUNE 2024 REPORT
Simona Buracco (left) receives the RMS Imaging Prize from Claire Wells.
Restoring the quality of electron microscope images through environmental monitoring
Spicer Consulting Limited
Scanning electron microscopes (SEMs) are popular imaging tools across a wide range of research and industrial applications, enabling clear view at nanometre scales due to their unparalleled magnification capabilities. Unfortunately, these powerful imaging tools are very sensitive to perturbations from magnetic fields, acoustic noise and vibrations. It is therefore necessary to monitor these parameters and keep disturbances below a critical level to ensure optimal image resolution. Until recently, continuous monitoring has been a significant challenge, but the development of purpose-made monitoring systems is now helping both the manufacturers and end users of SEMs to keep track of any changes in the instrument surroundings. This solution enables long term, uninterrupted readings to help quickly diagnose any reduction in image quality.
Sensitive to disturbances
The superior magnification offered by SEMs compared to optical microscopes results from use of electrons instead of light to probe a sample; these particles can have a much shorter wavelength, and are therefore better suited to viewing small features. The beam of electrons is focused on the specimen, interacting with atoms in the sample to produce signals that contain information about surface topography and composition. Seeing details at such a small scale requires the beam to
be perfectly positioned and focused, which can be compromised by external interferences from acoustic noise and vibrations, leading to blurred images. Additionally, since the electrons are charged particles, SEM performance is also greatly affected by external magnetic fields, which cause distortion of the images. SEM manufacturers therefore precisely map and specify the degree of interference that their instruments can handle before the image quality drops below the acceptable limit. End users of SEM often install special equipment to monitor and cancel
73

out magnetic fields, as these perturbations can have a significant impact on their work.
Electromagnetic interference
Electrons emitted by the source inside an SEM are accelerated through a column by an anode that generates an electric field. This electron beam is controlled by a number of apertures – as well as a magnetic lens in the middle of the column – to focus it onto the sample.1 Scanning coils produce an electromagnetic field that deflects the beam, moving it over the sample surface to generate an image (see Figure 1).
Electromagnetic interference is the most common problem in SEMs, as alternating magnetic fields can shift the direction of the electron beam, creating characteristic periodic deformations in the images.2 There are several ways that electromagnetic interference can cause this issue, but the two main mechanisms are:
1. By external magnetic fields penetrating the SEM column to directly affect/deflect the electron beam.
2. By inducing currents in the electronic circuits that are used to steer the beam to indirectly affect the beam.
In the first case, the magnitude of the resulting distortion will depend on factors such as the distance between the SEM and the sample, the
strength of the field, and the speed of the electrons; a higher field means the electrons will be subjected to a larger force, while the speed and distance will determine how long this force will be acting on them. However, if the distortion arises from disturbance of the scanning coils, it will no longer depend on the abovementioned parameters, and the effect will be more complex.2
Spotting the issue
Ensuring optimal performance of an SEM requires careful monitoring of the environment, both before and after installation. Purpose-made survey systems can be used to evaluate if a room is suitable to host such sensitive equipment, identifying vibrations and noise – for example, from nearby traffic or elevators – as well as magnetic fields that come from surrounding equipment or other less obvious sources, like heating or air conditioning systems. It is just as important to check for these perturbations once installation is complete, particularly for applications such as semiconductor manufacturing, where even small interferences can affect the quality and integrity of the devices produced. However, until recently, there was no technology available on the market that would allow uninterrupted, long term monitoring of magnetic fields, sounds, vibrations, temperature and humidity, meaning few SEM users had access to the relevant environmental data when an issue with imaging performance arose.
Seamless diagnostics
For most SEM users, delays caused by blurry or distorted images cost precious time – potentially halting the progress of research or leading to a drop in manufacturing productivity – making it essential to resolve imaging issues quickly and efficiently. Unfortunately, these issues can often take a long time to solve; when something is wrong with an SEM image, it can be hard to discern if the problem is internal –originating from faulty instrument components – or if some external disturbances are affecting electron beam focusing. For most SEM users, the first point of contact to resolve performance issues will be their SEM manufacturer or service organisation, who will send an engineer to investigate the problem. However, if no obvious fault can be found with the instrument, the manufacturer will then reach out to a magnetic field expert, as well as vibration and
74 ISSUE 74 JUNE 2024
Figure 1: Schematic diagram of an SEM.

acoustic protection suppliers, to help them diagnose what is wrong, which results in a lengthy process and multiple site visits.
Continuous environmental monitoring provides a convenient solution to help eliminate this drawn out diagnostic process. If a problem with SEM performance occurs, users can quickly and easily review the magnetic field, acoustic, vibration, temperature and humidity data – organised into datestamped files and viewed using bespoke software – from the time of image quality loss, immediately identifying if the problem is the result of an issue with the instrument itself or an external factor. This data can even be supplied to relevant external parties –equipment manufacturers or service organisations – to allow more effective diagnosis of instrument problems without visiting the site, significantly reducing the downtime and ensuring any necessary on-site interventions are as efficient as possible.
Continuous monitoring also provides the option to instigate an early warning system if environmental parameters stray outside the intended instrument operating specifications, which could be essential to avoid unnecessary downtime or product loss in manufacturing or QC environments, such as during semiconductor production. This warning system can be either visual – a traffic light tool that is green when the environmental parameters are within the user-specified range, and turns red when an issue is spotted – or electronic, sending an email notification to the user with a short summary of the situation. This technology will also be of great
value for SEM manufacturers, as they can easily check whether issues with their instruments are caused by hardware problems or perturbations in the surroundings, something that can be equally useful during instrument assembly.
Cancelling out external magnetic fields
It is hard to avoid magnetic fields in most cases, but active magnetic field cancelling systems – that detect the perturbations and react to them – can be used to mitigate their detrimental effect on SEMs.A common set-up includes a magnetic field control unit, one or more magnetic field sensors, and 3-axis cables that can cancel out the detected fields.
Figure 3 illustrates a basic cable set-up, with the cables situated around the SEM along three orthogonal planes. This makes it possible to create a magnetic

75
Figure 2. A long-term monitoring system can be used to continuously check for perturbations, including magnetic fields, vibrations and acoustic noise.
Figure 3. Basic cable set-up for a magnetic field cancelling system.
field in the opposite direction of any perturbations detected. However, there are other ways to position the cables, and each configuration has its advantages regarding complexity, cost and performance.3 Using a magnetic field cancelling system helps to keep the magnetic fields around the SEM column static, and can have a great effect on the instrument’s resolution, as can be seen in Figure 4.
Summary
Blurry or distorted SEM images can cause a variety of issues for researchers and engineers alike. Fortunately, SEM users now have access to a dedicated environmental monitoring solution that can help pinpoint whether a problem is caused by internal factors, or is a result of external interferences.
– such as the SC24 Magnetic Field Cancelling System (Spicer Consulting) – giving SEM operators full confidence that their instruments are operating under appropriate conditions.
References
1. ‘Measurement of Magnetic Field Distorting the Electron Beam Direction in Scanning Electron Microscope’, Mariusz Pluska, Łukasz Oskwarek, Remigiusz J. Rak, and Andrzej Czerwinski, Transactions on Instrumentation and Measurement,Vol. 58, No. 1, January 2009
2. ‘Separation of image-distortion sources and magnetic-field measurement in scanning electron microscope (SEM)’, Mariusz Płuska, Andrzej Czerwinski, Jacek Ratajczak, Jerzy Katcki, Łukasz Oskwarek and Remigiusz Rak, Micron, Vol.40, Pages 46-59, 2009
3. https://siliconsemiconductor.net/article/115520/ Eliminating_EMI_helps_ensure_accurate_test_ measurement
For more information about the SC28 monitoring system go to: www.spicerconsulting.com/sc28monitoring-system
For more information about the SC24 Magnetic field cancelling system go to: www.spicerconsulting.com/ sc24-magnetic-field-cancelling-system
About Spicer Consulting Limited
The new SC28 monitoring system (Spicer Consulting) can track magnetic fields, acoustic noise and vibrations – as well as temperature and humidity – to provide SEM users with the environmental data essential for timely and efficient diagnosis of imaging performance issues. This system can even notify the user when these parameters are beyond the allowed limits, saving precious time. Magnetic fields can be cancelled out by purpose made technology
Spicer Consulting magnetic field cancelling systems protect electron beam instruments – including scanning electron microscopes (SEM), transmission electron microscopes (TEM), electron beam lithography tools and SEM-based metrology and inspection tools – in the world’s leading laboratories, universities and semiconductor manufacturing plants, as well as in the test facilities of electron and ion beam equipment manufacturers. Its magnetic field, vibration and acoustic analysis systems have been adopted as standard equipment for conducting site surveys by leading equipment manufacturers. Spicer Consulting is located in Stewartby, Bedfordshire, within the United Kingdom’s golden triangle of elite universities in London, Cambridge and Oxford.
For more information, visit www.spicerconsulting.com
76 ISSUE 74 JUNE 2024
Figure 4. Use of magnetic field cancelling system has a great impact on the resolution of the EM.
Environmental Isolation Solutions



Long-term Monitoring Systems




Surveying Systems Acoustic
Magnetic Field Cancelling Systems


Enclosures




Call +44 (0)1354 669899 Visit www.cntech.co.uk
Passive Vibration Isolation Negative Vibration Isolation Active Vibration Isolation
Isolation
Technical Specialist Job Shadowing Scheme:
A report from the RMS and BioImagingUK
The RMS and BioimagingUK are delighted to report the results of a jobshadowing pilot scheme which enabled three scientists to gain experience of technical specialist roles at UK core facilities.
The scientists spent up to five days ‘on the job’ at their host institutions, shadowing expert staff and gaining valuable hands-on experience.
The scheme was largely funded by the Technician Commitment Collaboration Fund 2023 – to which the RMS and BioimagingUK were granted access as joint signatories of the Technician Commitment.

‘Shadowee’: Jessica Parker, PhD Student at Northumbria University
Host and Institution: John Le Quesne and Nigel Jamieson, CRUK Beatson Institute & University of Glasgow
Jessica learned about applying spatial biology imaging workflows to organoids in the Deep Phenotyping Facility and the Jamieson Spatial Laboratory.
This included:
• Sample optimisation and preparation,
• Operation of key platforms/equipment
• Use of key specialist software
• Troubleshooting
• Quality control
• Data handling, analysis and storage
Jess said: “This was a valuable experience which provided the chance to observe and gain hands-on experience of various established workflows that I had not previously encountered. It also equipped me with new technical skills and insights.
“From this visit, I have gained a broader understanding of spatial biology, with skills that I can take forward in my career progression. It has also highlighted the importance of collaborative efforts between cross-institutional research groups and facilities in developing and optimising established workflows into new areas of research. I would like to thank the RMS and BioImagingUK for funding my placement, the core facility for hosting me and to 3RsLabPal for their support.”
‘Shadowee’: Thomas Dunnett, Experimental Officer at Surrey University
Host and Institution: Wayne Lam, Brunel University
Thomas learned about material characterisation
78 ISSUE 74 JUNE 2024 REPORREPORTT
Jess Parker.

research in the Experimental Techniques Centre with a view to transferring some of these practices to the University of Surrey.
His job-shadowing role included:
• Exploring different technologies such as Electron Microscopy (SEM and TEM), X-Ray Analysis (XRD and XRF) and Optical Spectroscopy (UV-vis, FTIR and Raman)
• Exchanges of experience with a view to collaborating on strategic infrastructure/research grants in the future
• Learning about recognition and attribution
Thomas said:“My visit to the Experimental Techniques Centre at Brunel University London, through the Technical Specialist Job Shadowing Scheme, was truly enlightening. It provided me with invaluable insights into advanced material characterisation techniques and operational management, fostering my professional growth at the University of Surrey and paving the way for exciting collaborative opportunities.”
‘Shadowee’: Stefania Marcotti, Post-doc at King’s College London
Host and Institution: David Barry, Francis Crick Institute
Stefania learned from a world-leading image analysis facility how bioimage analysis support is performed on a day-to-day basis, with the intention to transfer these practices to a brand-new facility at King’s.
This included:
• How user support works in practice and how to handle initial consultations with users
• How time/resources are allocated on a project
• How to give feedback on data acquisition for analysis
• How data management is performed
Stefania said: “I found my shadowing week at the Crick an incredible learning experience. Being able to observe how more experienced image analysts offer user support and deal with the day-to-day running of the service has given me useful insights

for my career development. Moreover, the time spent working with Dave and the team has also strengthened the collaboration between our two institutions, and various image analysis projects and ideas are currently being brought forward as a result. Everyone in the hosting institution has been incredibly welcoming and made me feel part of the team, and the whole process from the initial application to the actual work experience has been smooth and seamless. Thank you to everyone involved in making this happen!”
Find out more
RMS and BioImagingUK will be supporting annual Job Shadowing schemes for life and physical science for the next three years, with the next round of applications opening this summer! https://www.rms.org.uk/opportunities/professionaldevelopment/job-shadowing.html
79
Thomas Dunnett.
Stefania and David.
Telight has won a media award!
The expert jury selected the Brno microscopy company Telight as one of the best 10 Innovators of 2024. The competition, regularly organised by a prestigious media Hospodářské noviny, emphasised Telight’s innovation and revolution in superresolution microscopy and quantitative phase imaging, which can contribute to the widespread use of these technologies.
www.telight.eu

Discover the possibilities with EM Resolutions and SiMPore – where science meets innovation
EM Resolutions, leading provider of high-quality electron microscopy consumable and accessories, is thrilled to announce the continuation and expansion of its successful distribution agreement with SiMPore Inc.; a leader in silicon-based membrane technology. This partnership reaffirms a commitment to bringing innovative solutions to researchers and industry professionals worldwide.

Under the renewed agreement, EM Resolutions will continue to distribute SiMPore’s cutting-edge products, including their renowned Silicon-based TEM & Cryo-EM Grids. The expansion of the agreement will also see EM Resolutions distributing a wider range of SiMPore’s products, catering to the diverse needs of the wider scientific community.
SiMPore’s products are founded on the latest science and designed with best practices and patented technologies. Their silicon membranes allow for more exact analysis and superior data collection, overcoming traditional limitations
in sample preparation and EV detection. This partnership ensures that these state-of-the-art products are more accessible than ever before.
Whilst EM Resolutions is known for a focus on electron microscopy with specialism in producing high-quality TEM support films, they are also eager to highlight SiMPore’s extensive range of additional products. These include:
• X-Ray Windows: Ideal substrates for X-Ray microscopy and spectroscopy, compatible with a range of vacuum and temperature conditions.
• Porous Membrane Chips: Nanoporous, microporous, and microslit membrane chips, available in a number of cut-off sizes.
• Microplastic Filters: Capture and analyse microplastics on the same substrate with optical, infrared, or Raman microscopy.
• Cultureware: Unique single-cell spheroids with MicroBubble Arrays and barrier cell models with exceptional resolution with µSiM-CV.
• Nanoparticle Isolators: Nanomembrane-enabled nanoparticle isolation and analysis.
To explore the full range of SiMPore’s products, visit SiMPore’s product page and enhance the quality of your data and the efficiency of your research. www.emresolutions.com
80 ISSUE 74 JUNE 2024
COMPANY NEWS
New £1,000 Training Grants available from Journal of Cell Science and FocalPlane
Microscopy Training Grants
Supporting microscopy training for early-career researchers in cell biology
Apply for a grant for up to £1,000
Application dates:
7 June 2024
7 September 2024
22 November 2024
biologists.com/grants/jcs-focalplane-training-grants

We are excited to launch our new Training Grants together with the Journal of Cell Science (journals. biologists.com/jcs). Early-career researchers in cell biology can apply for grants of up to £1,000 to support their attendance at microscopy courses, including bioimage analysis courses. These training grants can be used to cover the registration fees, accommodation and/or travel costs associated with attending such a course and are designed to support those who wouldn’t otherwise have sufficient funding to attend.
The deadline for our first round of applications is 7 June 2024 and we’ll have two further rounds of funding this year. You can find our application handbook and FAQs at biologists.com/grants/ jcs-focalplane-training-grants, and if you have any further questions, you can contact us at focalplane@ biologists.com.
www.biologists.com
Learn about LED Illumination at elmi
Get ready for one of the highlights on this year’s microscopy calendar! At elmi in Liverpool, you’ll have the chance to see a range of our powerful and controllable LED Illumination Systems. Whether you’re a facility manager or researcher, at CoolLED we’ve got you covered whatever your fluorescence needs:
Versatile wavelengths for imaging facilities – the pE4000 includes 16 LEDs spread across four channels, ensuring there’s always a perfect combination for your experiment.
Routine to advanced fluorescence – the latest pE400 Series has four LEDs, covering DAPI and YFP through to Cy5, with a few extra useful features to get the most information from your fluorescence samples.
High-speed live cell applications – elevate your research with the pE-800, offering eight individually
controllable LEDs and lightning-fast TTL triggering speeds of <7 µs.
It’s not all about the products on show; we’ve also got some exciting news about our imaging competition with a difference. If you’ve found an Image in an Image, make sure you stop by the CoolLED to see what it’s all about!
www.coolled.com

81
Telight strengthens position in the UK

We have a new sales representative, Huw Thomas, that has recently joined our team to provide a direct support to our customers in the UK. Huw has over 30 years’ experience in the life sciences, biotechnology and pharma markets in sales roles, sales management, product management and at Director level across the UK, Europe and Globally.
His career includes being awarded the Global Presidents Award for his business development in the field of confocal microscopy and imaging and he has significantly grown business he has been involved with. Feel free to connect with him, to discover the future of live cell imaging.
www.telight.eu
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
Submit to infocus
infocus welcomes submissions of articles of general interest to microscopists.
You provide the text and images and we take care of the rest. It’s the ideal way to share your work with the microscopical community.
Full submission information and guidelines are available at www.infocus.org.uk. To submit an idea or if you have any questions about the process please email the Editor (editor@infocus.org.uk)
82 ISSUE 74 JUNE 2024 COMPANY NEWS
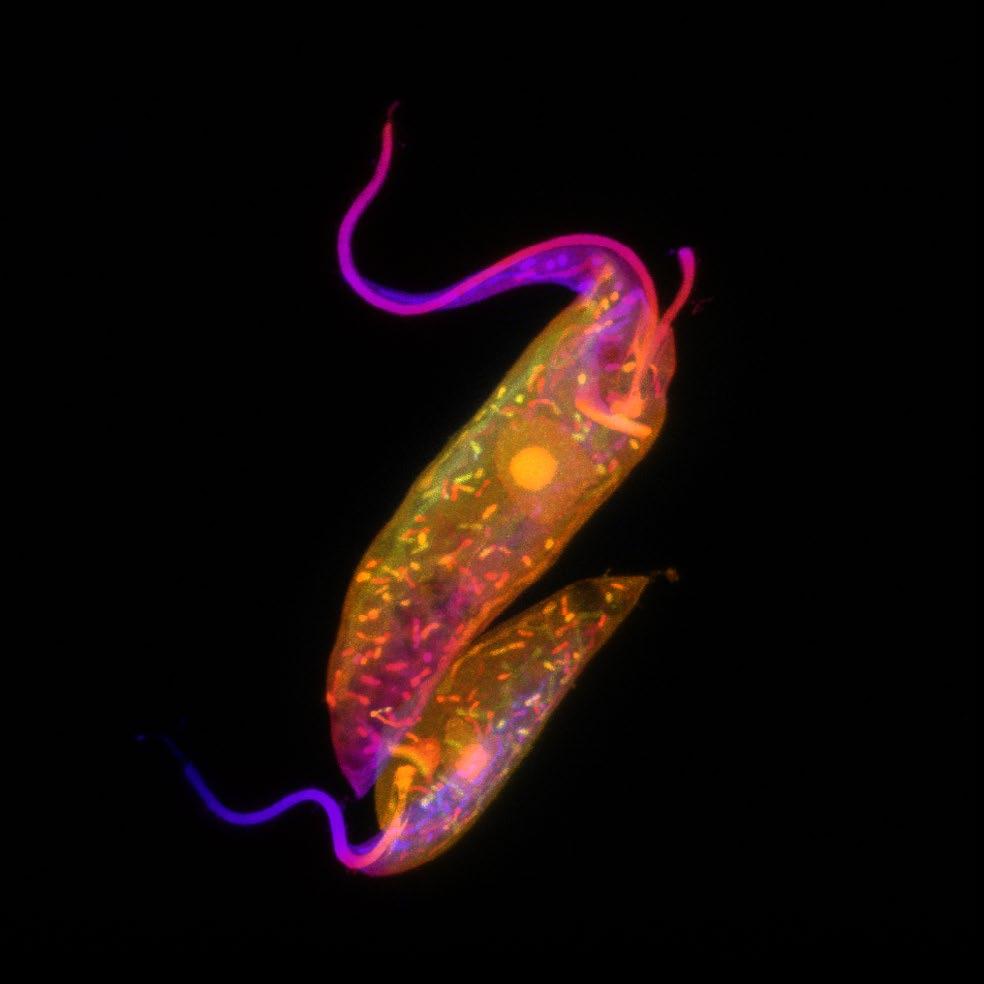
Expanded
Trypanosoma cruzi in
rainbow colours
This image, which was shortlisted in the 2023 RMS Scientific Imaging Competition (Life Sciences, Light Microscopy category), shows two epimastigote stage of Trypanosoma cruzi, the pathogen of Chagas disease. Chagas disease is a Neglected Tropical Disease, endemic in Latin America. The parasite’s proteins have been stained with NHS-ester conjugated to a fluorophore and edited so that the colour represents the 3D volume of the cell.
Image by Victoria Alonso, IBR-CONICET-UNR
NEW PRODUCTS
Livecyte gives single-cell insight into Immunotherapy with the latest T cell killing assay analysis platform
Phasefocus, a pioneer in cutting-edge life science solutions, has launched a revolutionary new product, the Livecyte T-Cell Killing Assay. This ground-breaking new assay addresses key challenges faced by researchers in investigating the efficacy and cytotoxicity of engineered T-cells in killing target cancers.
By offering unparalleled insights at the single-cell level, Livecyte empowers researchers to unlock new frontiers in cancer therapy development and evaluation. Current live-cell imaging assays focus on population-level analysis and target cell death without exploring the effector interactions leading to death. Consequently, the gap between in-vitro and in-vivo T-cell therapy performance remains a significant challenge, hindering the development of robust and reliable T-cell therapies.
Livecyte revolutionises T-cell killing assays by introducing advanced segmentation and tracking algorithms, enabling the automatic segmentation of target cells and label-free effector T-cells. Through this cutting-edge technology, Livecyte accurately tracks target cells over time, creating unique interaction profiles from the beginning of the experiment until target cell death.
Livecyte’s T-Cell Dashboard view compiles comprehensive metrics based on these
interaction profiles, providing an array of information that summarises T-cell cytotoxicity and target cell recognition. Researchers gain a deeper understanding of T-cell-target cell avidity independently from attack potency, yielding greater depth of insight into how T-cells find and kill their targets.
Key Advantages of Livecyte T-Cell Killing Assay:
• Invaluable Depth of Insight: Livecyte employs advanced algorithms to automatically segment and track target cells, eliminating the need for manual, error-prone analysis.
• The Livecyte T-Cell Dashboard presents comprehensive metrics based on interaction profiles, offering a wealth of data on T-cell cytotoxicity and target cell recognition.
• Livecyte’s ability to independently quantify target cell death and T-cell interaction history at the single cell level, provides a profound understanding of T-cell behaviour leading to killing.
• All this is achieved without the need to fluorescently label T-cells which could alter their natural behaviour.
https://www.phasefocus.com/ applications/t-cell-killing-assay

84 ISSUE 74 JUNE 2024
New version of cell imaging software SophiQ

We’re excited to announce the release of Telight’s latest software update, SophiQ 10, which enhances advanced cell imaging and analysis capabilities. This update has been eagerly embraced by our user community, reflecting our commitment to integrating their valuable feedback. One key advantage of partnering with Telight is our responsiveness to the specific needs of researchers, allowing for tailored modifications to our software and hardware.
In addition to standard features that measure various
cell parameters — such as dry mass, cell growth, and cell motility — we have significantly improved our automatic cell segmentation capabilities while keeping manual adjustment options offering greater precision and flexibility. Many users have asked us for the implementation of well plates, so our microscope Q-Phase now supports up to 24 well plates. For an insightful comparison of Q-Phase with other microscopes using similar technology, have a look at the following article .
www.telight.eu
85
Introducing the InverTau™ Fluorescence Lifetime Imaging Platform: HORIBA’s cutting-edge solution for advanced fluorescence lifetime microscopy
HORIBA is thrilled to unveil the new InverTau™ platform, revolutionising Fluorescence Lifetime Imaging (FLIM) with its innovative design and unparalleled performance. With decades of expertise in single photon counting technology, HORIBA’s new platform offers researchers an affordable yet powerful laser scanning confocal solution for FLIM applications. Engineered to seamlessly integrate with inverted fluorescence microscopes, the fully software-controlled InverTau™ can be effortlessly attached to the side port of existing setups, providing researchers with a versatile and adaptable imaging solution.
Key features of the InverTau™ platform include:
• Seamless integration of pulsed laser sources and single photon counting detectors
• Compatibility with the award-winning FLIMera widefield camera, for video-rate FLIM acquisitions
• Lifetime acquisitions ranging from 50 ps to seconds, catering to a wide range of needs
• Fully computer-controlled confocal optics, streamlining setup, and maximising productivity
• Intuitive and automated EzTime Image software for easy control, acquisition, and analysis.
• Optimised for touchscreen devices, enhancing user experience and promoting accessibility.
Combining advanced technology with user-friendly software and exceptional support, the InverTau™ empowers researchers within life science and cell biology. Couple with existing microscopes or acquire as part of a full HORIBA platform, the InverTau™ combines flexibility with customisation to suit specific needs.
https://www.horiba.com/int/ scientific/products/detail/action/ show/Product/invertau-6495/?utm_ source=constant-contact&utm_ medium=newsletter&utm_ campaign=huk-sci-invertau2024&utm_term=infocus%20 magazine&utm_content=email

86 ISSUE 74 JUNE 2024 NEW PRODUCTS
Building upon the world’s first quantitative CMOS camera, Hamamatsu Photonics launches enhanced new model of ORCA®-Quest

Hamamatsu Photonics, a leader in the creation of scientific cameras, proudly releases the latest addition to its prestigious ORCA series: the ORCA-Quest 2. Building upon the success of its predecessor, the ORCA-Quest, the world’s first quantitative CMOS camera (qCMOS), this new version offers exceptional performance and versatility. Designed to meet the evolving needs of researchers in physics and life sciences, as well as innovative startups and established imaging solution providers, particularly in microscopy and quantum computing, the ORCA-Quest 2 includes the following key features:
ORCA-Quest 2- C15550-22UP
• Extreme low-noise performance at video rate speeds: With a remarkable 0.30 electrons rms and an elevated speed of 25 fps at full resolution (9.4MP), researchers can capture clear, highquality images even in extreme low-light conditions.
• Enhanced UV quantum efficiency: Compared to its predecessor, the quantum efficiency in the UV
has been greatly improved – up to 50% @300nm.
• High-resolution imaging: Features a backilluminated sensor structure and trench structure in one-by-one pixels for reduced crosstalk, for exceptional resolution and image clarity.
• Photon number resolving output: The new model remains the only CMOS camera on the market that enables precise quantification of photoelectrons.
Moreover, compared to EM-CCD cameras, it offers higher data rates and lower latency, making it the preferred choice for demanding applications that require rapid data acquisition.
Applications include:
• Ion and atom imaging for quantum technologies
• Quantum imaging / single photon imaging
• Super-resolution microscopy
• Bioluminescence imaging
• Spinning disk confocal microscopy
“As with the previous ORCA-Quest model, the Quest 2 offers the lowest readout noise on the CMOS market, yet with multiple improvements, providing substantial benefits across various scientific disciplines, especially those with leading-edge requirements”, said Michael Kehr, Camera Group Leader at Hamamatsu Photonics. “At Hamamatsu Photonics, we do not rely solely on our unique technology like qCMOS but listen to customers’ feedback and suggestions to continuously develop and produce even better results.We are committed to constantly pushing the boundaries of what is possible to assist the most innovative researchers in excelling in their work.”
ORCA-Quest qCMOS camera C1555022UP | Hamamatsu Photonics
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
87
Submission Guidelines
infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS. infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more. infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms.org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.ukj
Article Text
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
Images / Figures
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
88 ISSUE 74 JUNE 2024
• Total number of images/figures/tables should not exceed 15 including tables.
Proofs
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Offprints
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Copyright
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
One column/half page width, 65.5mm
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).
89
Figure 1. Width of figure or table confined to one column.
Figure 2. Width of figure or table spanning full width of page.
Two column/full page width, 135mm










































































































































































































