mmc2023 (incorporating EMAG 2023): Review and Picture Special!
Light Under the Microscope
Allison Winton: A personal Reflection on nearly 40 years at the RMS


mmc2023 (incorporating EMAG 2023): Review and Picture Special!
Light Under the Microscope
Allison Winton: A personal Reflection on nearly 40 years at the RMS

The all-new JIB-PS500i from JEOL delivers fine milling capabilities essential for fast, high quality lamella preparation. This multi-purpose FIB-SEM enables high throughput sample preparation, high resolution STEM-in-SEM imaging, and analytical analysis.
Robust Workflow for Large Samples

Featuring a large specimen chamber with easy-access door and large high-tilt stage, the JIB-PS500i offers a truly flexible workflow. Transfer from the FIB-SEM to the TEM is seamless with TEM-Linkage, JEOL’s double-tilt cartridge.

infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.
infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK
Editor
Owen Morton
Tel + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Laura Fumagalli, University of Manchester, UK
Myfanwy Adams, John Innes Centre, Norwich, UK
Maadhav Kothari, Zeiss Microscopy, UK
Hilary Sandig, Cancer Research, UK
Trevor Almeida, University of Glasgow, UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2023 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by Creative Design. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.

Well, here we are, another summer has passed (for those of us in the northern hemisphere at least), the holidays are over, and it is time for our September issue of infocus!
An undisputed highlight for me (and, I suspect, for many of our readers) of the last few months was the fantastic mmc2023 Congress which took place in Manchester, UK, from 4 – 6 July.
It was great to be back – in person - at this essential event for the microscopy, imaging and flow cytometry communities. It was wonderful to see old friends, meet new people and talk with our corporate partners about the future in our field. I also had fantastic discussions regarding ideas for future infocus content. As always, we rely on your contributions, so if you would like to write something for the magazine – or come forward with an idea, don’t hesitate to get in touch! Feel free to approach me or any of our editorial board members, either through email or in any meeting that we are attending.
As you might expect, our September issue features a colourful ‘picture special’ capturing some of the best action from mmc2023, including personal reflections kindly submitted by delegates and other reports from the Congress. If you were there at Manchester Central, perhaps you can spot yourself in one of our official photos?
Also in this issue, amongst our range of regular features, long-standing contributor Winston Ingram puts ‘light itself’ under the microscope in his latest project, with some eye-catching results…
Finally, I would like to thank retiring RMS Chief Executive Allison Winton for contributing a wonderful piece about her 39-year career at the Society. Allison has become synonymous with the RMS over the years, and will be well known to many of our readers. On behalf of the infocus team, I send my very best wishes to you, Allison; I hope you enjoy a long, happy and richly deserved retirement! I would also like to extend a very warm welcome to our new Chief Executive, Sali Davis, about whom you can read more in this issue. Slàinte!
This image, by Suwarna Mahajan, was shortlisted in the Light Microscopy (Physical Sciences) category of the 2023 RMS International Scientific Imaging Competition. Salicylic acid crystals are formed from evaporation of Iso-Propyl alcohol from mixture. These are white, fluffy and needle shaped crystals. Instrument: ZEISS A2m; Magnification: x20; Mode: Reflected light plane polarised.

Well, that was fun, wasn’t it?
With the dust now settled on mmc2023 (incorporating EMAG 2023), we take a look back at what proved to be a fantastic few days in Manchester, celebrating the very best in microscopy, imaging and flow cytometry. Perhaps you can spot yourself in one of our official event photos!


mmc2023 saw a record-breaking 1,270 attendees, plus exhibitors, descend on Manchester Central as the Congress series returned to the iconic UK venue for the first time in four years.
The Royal Microscopical Society would like to thank everyone who attended its flagship event – the exhibitors, delegates, volunteers and everyone else who helped make the Congress such a special occasion for the microscopy, imaging and flow cytometry communities.

Over three days (four, including our Monday meetings), this international event combined a heady mix of vibrant conference sessions (36 in total – including EMAG 2023 sessions) covering all the latest techniques and applications in microscopy – with a world-class exhibition showcasing the very latest products and technology from some of the leading companies in the field.
The event incorporated pre-Congress meetings and workshops – including an Early Career Symposium and the BioImaging UK meeting – as well as free-to-attend company workshops throughout the exhibition. This year’s mmc also featured an expanded Learning Zone with its own lecture theatre, a dazzling exhibition of images shortlisted in our International Scientific Imaging Competition, and a series of vibrant and engaging poster sessions.
The RMS held its 2023 Annual General Meeting (as
well as AGMs for each of the Society’s Scientific Section Committees) during the Congress, which featured a number of award presentations for previously announced winners – plus some new recipients (see below). There were new Honorary Fellowships, awards for Scientific Achievement, Outreach and Education, service to the RMS and many more – including our poster prize-winners.
We would like to congratulate all our award-winners and once again, thank you to everyone who attended this hugely important event for the microscopy, imaging and flow cytometry communities
We hope to see you again at mmc2025 – and in the meantime, we hope you enjoy the following pages, as we revisit some of the action from this year’s Congress.
www.mmc_series.org.uk
Before the main Conference and Exhibition even began, mmc2023 warmed up on Monday 3 July with fully-booked Pre-Congress Workshops on image processing and analysis (‘ImageJ’ and ‘python’ programming), and two AFM & SPM-themed workshops. There was also the BioImagingUK meeting – bringing the UK Bioimaging community together to discuss priorities and strategies in national infrastructure, technology development, training, careers and ways to share knowledge across different disciplines.
The eagerly anticipated Early Career symposium also took place on the Monday afternoon. This was an interdisciplinary event aimed at students, postdocs and early career professionals working in the field of microscopy. The meeting served both as a networking opportunity ahead of the main conference, and an opportunity for attendees to showcase their research with peers.
A highlight of the meeting was the Early Career Award competition – with the best talk chosen on the day by a panel of judges. The prize went to Alex Johnson of the Institute of Science and Technology, Austria (Read Alex's report on p66).




With no fewer than 36 sessions taking place across six parallel ‘streams’ over three days, the conference delivered its usual breadth and depth of microscopy techniques and applications across the sciences. With standing room only, and a queue of delegates stretching out of the conference room entrance, the first Plenary talk delivered by Judith Klumperman gave a sure indication of attendance levels - and sizeable audiences were maintained throughout.
We would like to thank all our speakers and presenters for making mmc2023 a conference to remember – especially our brilliant cast of plenary speakers, who each brought their unique insights and expertise to the conference platform. Our
thanks go to Professor Klumperman (University Medical Center Utrecht, Netherlands), Professor Joerg Bewersdorf (Yale University, USA), Professor Amanda Petford-Long (Argonne National Laboratory, USA) and Professor Philip Withers (University of Manchester/Henry Royce Institute, UK)


We would also like to thank Dr Erin Tranfield (Instituto Gulbenkian de Ciência), who delivered mmc’s first Equity, Diversity, Inclusion and Accessibility (EDIA)-themed Plenary. Titled ‘Surviving a life-changing accident and relearning how to be a scientist’, Erin’s thought-provoking talk explored what it means to be a scientist with a disability, and was free and open to all attendees.




The mmc2023 exhibition featured more than 80 exhibitors, including many of the leading companies in the field. Beneath the grand arches of Manchester Central, they showcased the very latest products and technology, providing expert advice, product demonstrations and free workshops throughout the event. A very big thank you to all our exhibitors
We would also like to thank all our exhibitors who took part in the mmc2023 ‘Passport Competition’ – in which visitors used the Congress App to ‘collect’ a ‘full-house’ of QR codes from participating stands. A random prize-draw was carried out after
the exhibition closed and five lucky winners each received a £100 Amazon voucher. Our winners were as follows:
• Berardo Manuel Sanchez Tafolla
• Chloe Cooper
• Kseniia Bondarenko
• Rebecca Gascoyne
• Zeeshan Mughal
Many thanks to Prior Scientific , Thermo Fisher Scientific , Nikon UK, Cairn Research Ltd, and Greiner Bio-One for providing the prizes. Find out more about all our exhibitors.





The Learning Zone brought something a bit different to this year’s Congress, with a programme of daily talks, expert advice and eye-catching, historical displays.

An ever-popular - and free - feature of mmc, the Learning Zone provided the opportunity to learn more about the many facets of modern microscopy – including a daily programme of free introductory lectures at its dedicated seminar theatre. Visitors
also had access to a range of microscopes demonstrating the fundamentals of microscopy. For the history buffs, this year’s display collection included a Cooke, Troughton and Simms (CTS) microscope from the 1950s - very similar to the one used by Rosalind Franklin, the world-famous British chemist and X-ray crystallographer.The McCormick Collection of replica antique microscopes spanning hundreds of years of microscopy, and a collection of antique slides recently donated to the RMS were also on display.




o To extend the spectral sensitivity of CMOS and CCDs to electron detection for EBSD imaging and diffraction applications.
o P22 and P43 coatings for TEM / SEM with custom coatings options: ITO and Aluminium base and top coatings.


o Lens and fibre optic coupling options available.
o The highest resolution and imaging quality on the market.
This year’s Scientific Imaging Competition received more than 220 submissions from across the globe, spanning six different categories (Light Microscopy – Life Sciences; Light Microscopy – Physical Sciences; Electron Microscopy – Life Sciences; Electron Microscopy – Physical Sciences; AFM & SPM; Short Video Category). Our team of judges whittled these down to a shortlist of around 50 images,

which were displayed in the foyer at Manchester Central throughout mmc2023.
A well-attended prize-giving ceremony saw the winners revealed by RMS History Committee Chair, Dr John Hutchison Hon FRMS – who has been involved on the judging panel for many years. We were delighted that some of the winners were able to attend mmc2023 and receive their prizes in person (pictured opposite and overleaf). Read John's report on p64.
1st Prize Light Microscopy (Life Sciences)

2nd Prize Electron Microscopy (Physical Sciences)


 1st Prize AFM & SPM
Thomas Hackl, TU Wien / Automation and Control Institute (ACIN), receives 1st Prize in the AFM & SPM category from Professor Michelle Peckham, Editor of the Journal of Microscopy, which sponsored the award.
2nd Prize AFM & SPM
1st Prize AFM & SPM
Thomas Hackl, TU Wien / Automation and Control Institute (ACIN), receives 1st Prize in the AFM & SPM category from Professor Michelle Peckham, Editor of the Journal of Microscopy, which sponsored the award.
2nd Prize AFM & SPM
 1st Prize Short Video (Physical Sciences)
1st Prize Short Video (Physical Sciences)
The following images were announced as the winners and runners-up:

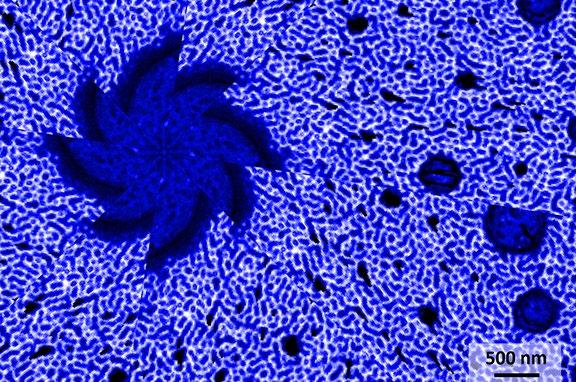 1st: Thomas Hackl, TU Wien / Automation and Control Institute (ACIN). Particle or Wave? Kelvin Probe Force Microscopy image of a flat and charged Poly(methyl methacrylate) (PMMA) surface. The potential distribution represents the double slit experiment, which is generated via a separate AFM charge writing scan (20x20μm).
1st: Thomas Hackl, TU Wien / Automation and Control Institute (ACIN). Particle or Wave? Kelvin Probe Force Microscopy image of a flat and charged Poly(methyl methacrylate) (PMMA) surface. The potential distribution represents the double slit experiment, which is generated via a separate AFM charge writing scan (20x20μm).

 1st: Josef Spacek, Professor of Pathology, Charles University Hospital, Hradec Kralove, Czechia. A look inside the pyramidal cell of the cerebral cortex 3D reconstruction, serial electron microscopy. Orig. magn. 6000x.
1st: Josef Spacek, Professor of Pathology, Charles University Hospital, Hradec Kralove, Czechia. A look inside the pyramidal cell of the cerebral cortex 3D reconstruction, serial electron microscopy. Orig. magn. 6000x.
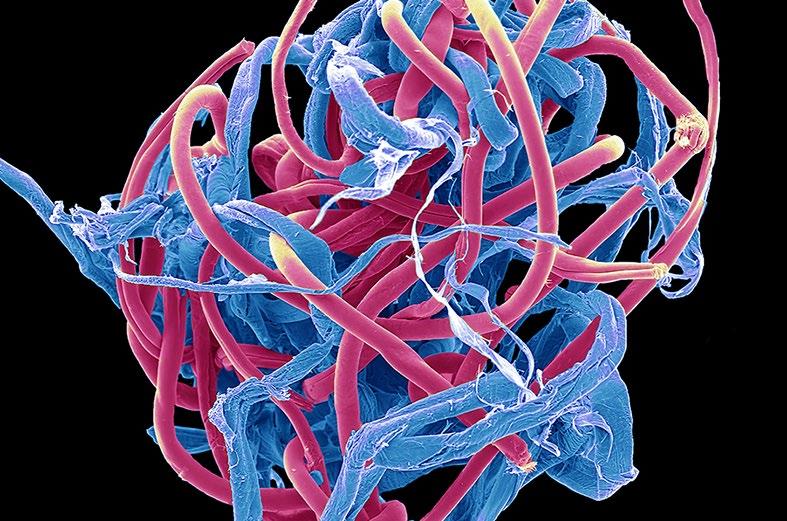 1st: Steve Gschmeissner. Plastic pollution. Coloured scanning electron micrograph of a microplastic bobble from a poly-cotton garment.
1st: Steve Gschmeissner. Plastic pollution. Coloured scanning electron micrograph of a microplastic bobble from a poly-cotton garment.
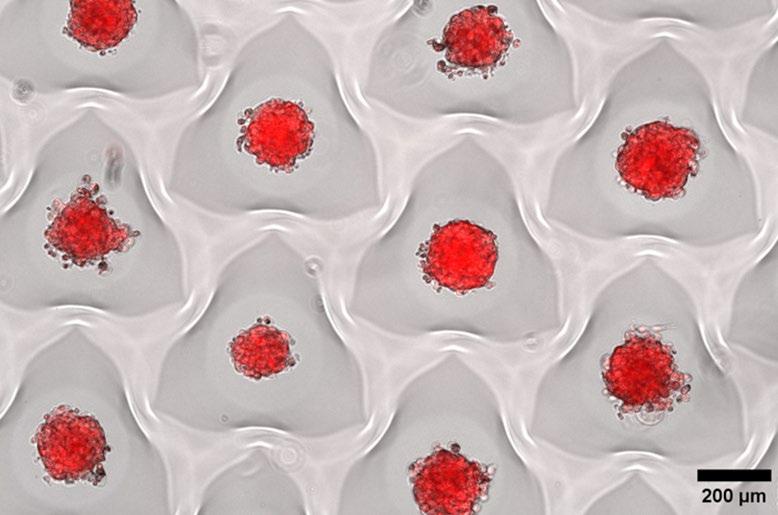
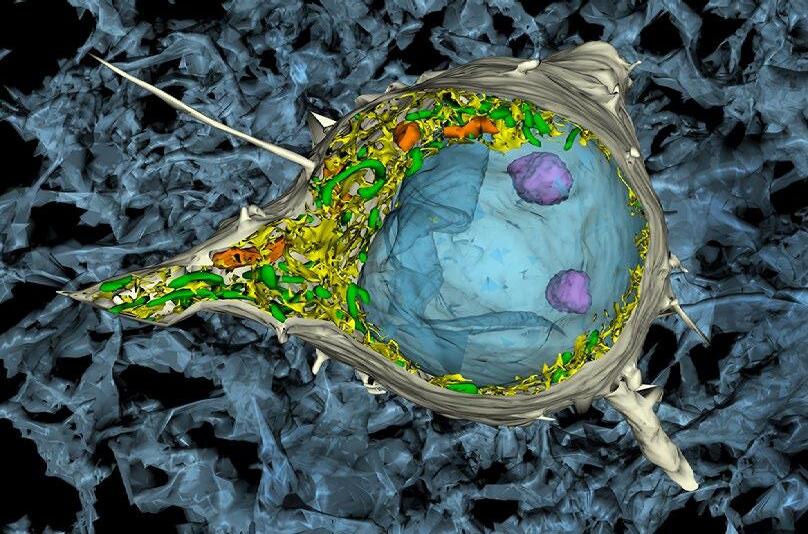
 1st: Vitoria Murakami Olyntho, McMaster University. Intestinal villi of a food-allergic mouse. Image depicting the intestinal villi of a mouse allergic to the egg protein, ovalbumin, upon secondary allergen exposure. The sample was stained with flourescent-labelled antibodies.
1st: Vitoria Murakami Olyntho, McMaster University. Intestinal villi of a food-allergic mouse. Image depicting the intestinal villi of a mouse allergic to the egg protein, ovalbumin, upon secondary allergen exposure. The sample was stained with flourescent-labelled antibodies.

 1st: Cagri Yalcin. Crystallization Scene of Magnesium Nitrate dissolved in water and ethanol. Half teaspoon of magnesium nitrate is dissolved in 30ml of water and ethanol. Two drops are spread on a slide which is heated and then put on an icy surface to cool down.
1st: Cagri Yalcin. Crystallization Scene of Magnesium Nitrate dissolved in water and ethanol. Half teaspoon of magnesium nitrate is dissolved in 30ml of water and ethanol. Two drops are spread on a slide which is heated and then put on an icy surface to cool down.
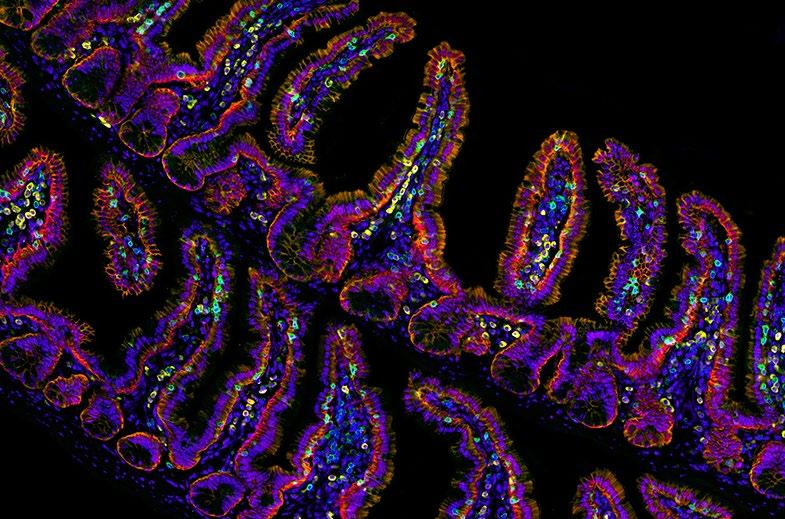
1st Life Sciences: Annalisa Bellandi, Faulkner Laboratory, John Innes Centre, Norwich, UK. A small plant with mighty signals - plants transmit wounding information to distal leaves. There is a universe of dynamic signals inside plants - when a plant is wounded, long-distance calcium signals are transmitted quickly from the wounded leaf to the others!
Find out more about all these winning images and videos
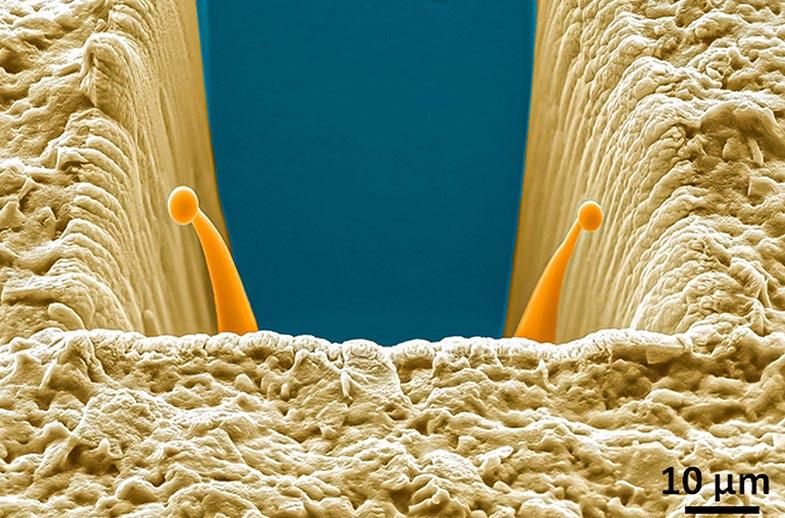
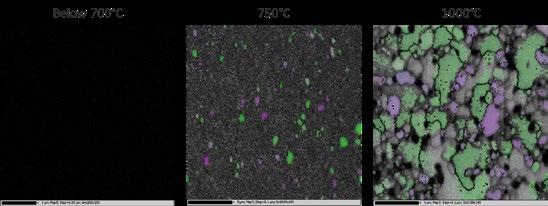
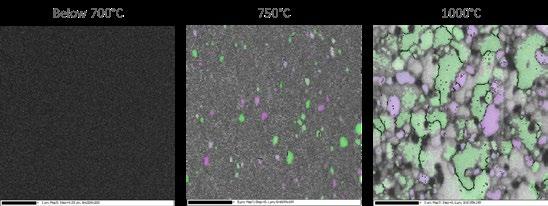
 1st Physical Sciences: Albert D. Smith, TESCAN-UK. Wave of Relief. Iron diffusing into titanium at 850°C over 24 hours as observed in an SEM with a 60μm view field.
1st Physical Sciences: Albert D. Smith, TESCAN-UK. Wave of Relief. Iron diffusing into titanium at 850°C over 24 hours as observed in an SEM with a 60μm view field.

Among the many announcements at the RMS Annual General Meeting, which took place on Tuesday 4th, was the appointment of Dr Peter O’Toole as RMS President. Professor Grace Burke was on hand to pass on the President's official medallion of office.
Pete said: “I have always been immensely proud to be a part of this wonderful Society, and it is a huge honour to serve as RMS President. I look forward to working alongside all our members, volunteers, and staff, as we look to continue to evolve and develop further to continue supporting the science of microscopy, imaging, analysis and flow cytometry communities.”
Read more on page 72.
Fittingly, Pete’s first duty as RMS President was to announce a surprise Honorary Fellowship for Grace.


Throughout her remarkable career in the US and UK, Grace has made an enormous contribution to microscopy and Materials Science, and has become
a hugely influential figure in both industry and academia. She is also the first person to have served both as President of the Microscopy Society of America (MSA) and the RMS.
You can read more about Grace’s career and achievements on page 76.
…Sometimes a picture really does say it all!

Also joining the RMS Honorary Fellowship was Professor Rafal Dunin-Borkowski – a worldleading Electron microscopist who has made major contributions to a wide range of electron
microscopy techniques and applications.
Rafal was recognised not only for his groundbreaking research, but for his vision and leadership as Co-Director of the Ernst Ruska-Centre for Microscopy and Spectroscopy with Electrons (ERC) at Forschungszentrum Jülich.
Read more on page 78.
Another highly popular award recipient was RMS stalwart Jeremy Sanderson, who received the President’s Award in recognition of his exceptional contributions to the work of the Society.

Jeremy has been involved with the RMS for 35 years – notably as a prominent teacher on the longrunning RMS Light Microscopy courses.
He is also well known as an author on microscopy; his latest book, a comprehensive guide titled Understanding Light Microscopy, was published in 2019 as part of the RMS Wiley Book series.
Read more on page 79.
The RMS bade an emotional farewell to retiring Chief Executive Allison Winton, who has dedicated an incredible 39 years of service to the RMS as a member of staff.

It was revealed that a new RMS award has been established in Allison’s name; the ‘Winton Prize’ will be awarded in future to those who have made outstanding, sustained contributions to community engagement and collaboration in microscopy and RMS-related activities. The announcement sparked a spontaneous and prolonged standing ovation for Allison.
You can read more about Allison’s extraordinary career at the RMS on page 82.
mmc2023 provided the perfect stage for many previously-announced RMS award-winners to receive their awards in person, and gain public recognition from the microscopy, imaging and cytometry communities.


The four-year gap since the last mmc, and a reduced number of in-person RMS events since 2019 due to Covid, meant a large number of recipients had yet to receive their awards. Happily, we were able to put that right in Manchester for many of our recipients, and we would like, once again, to congratulate them on their achievements.

 Dr Alice Pyne (right), receiving the RMS AFM & SPM Award.
Dr Andrea Centrone (left) receiving the RMS Scientific Achievement Award.
Dr Ardan Patwardhan (right), receiving the RMS Scientific Achievement Award.
Dr Alice Pyne (right), receiving the RMS AFM & SPM Award.
Dr Andrea Centrone (left) receiving the RMS Scientific Achievement Award.
Dr Ardan Patwardhan (right), receiving the RMS Scientific Achievement Award.


 Craig Holliday (right), receiving the RMS Diploma from Professor Susan Brooks (centre) and Dr Kerry Thompson.
Dr Elisabeth Bik (left), receiving the RMS Chris Hawes Award for Outreach and Education.
Craig Holliday (right), receiving the RMS Diploma from Professor Susan Brooks (centre) and Dr Kerry Thompson.
Dr Elisabeth Bik (left), receiving the RMS Chris Hawes Award for Outreach and Education.

 Dr Hari Shroff (right), receiving the RMS Scientific Achievement Award.
Dr Hari Shroff (right), receiving the RMS Scientific Achievement Award.


 Professor Michael Sheetz (right), receiving the RMS Pearse Prize.
Professor Michael Sheetz (right), receiving the RMS Pearse Prize.


 Dr Peter Bankhead (right), receiving the RMS Data Analysis in Imaging (DAIM) Award.
Wim Hagen (left), receiving the RMS Scientific Achievement Award.
Dr Peter Bankhead (right), receiving the RMS Data Analysis in Imaging (DAIM) Award.
Wim Hagen (left), receiving the RMS Scientific Achievement Award.

 Dr Robert Haase (left), receiving the RMS Data Analysis in Imaging (DAIM) Award.
Dr Robert Haase (left), receiving the RMS Data Analysis in Imaging (DAIM) Award.
We were particularly delighted to welcome RMS Honorary Fellows, Professors Mervyn Miles and

Alan Craven to receive their awards in person. Mervyn’s Hon Fellowship was announced in 2019 for his pioneering work in AFM & SPM – particularly in its development and application to biological systems. Alan was appointed an Honorary Fellow in 2021 in recognition of his seminal contributions to the development of instrumentation and techniques – including the STEM-EELS technique.
 Professor Mervyn Miles (right), receiving his RMS Honorary Fellowship.
Professor Mervyn Miles (right), receiving his RMS Honorary Fellowship.
Among our many award-recipients at mmc2023 were the poster prize-winners, covering a wide range of microscopy techniques across the sciences. Each day of the event featured vibrant poster sessions, with presenters engaging in absorbing discussions with visitors in the main exhibition hall.
After some very difficult deliberations, the judges picked their winners on the final day of mmc2023. The RMS would like to congratulate all our winners and runners-up, as follows:
1st Prize - Microbial Imaging Session
Helena Maier, The Pirbright Institute
Cross virus comparison of coronavirus proteins involved in replication organelle formation
1st Prize - Public Health Session
Patrick Phillips, Diamond Light Source. The Pirbright Institute. Oxford University
Correlative cryo-bioimaging to study coronavirus replication organelles
1st Prize - Frontiers in BioImaging
Edward Ward, University of Cambridge
Combining machine learning with interferometric structured illumination microscopy for the imaging of dynamic process in three-dimensions
2nd Prize - Frontiers in BioImaging
Lydia Daly, King’s College London. Queen Mary, University of London
Studying the kinetochore corona with super-resolution and fast lattice-light sheet imaging
AFM & SPM, Physical Sciences
Pieter Keenan, University of Bath
Passive identification of adsorbate single bomobenzene molecules on atomically resolved Si(111)-7×7 surface by I-z spectroscopy at room temperature
AFM & SPM, Life Sciences
William Trewby, Durham University
Direct, nano-rheological studies of in-plane lipid dynamics in model and native membranes
1st Prize - mmc2023
Mahesh Uttamlal, Glasgow Caledonian University
Microscopic analysis of new and historic cotton fibre cross-sections
Runner Up - mmc2023
Patrick Phillips, Diamond Light Source. The Pirbright Institute. Oxford University
Correlative cryo-bioimaging to study coronavirus replication organelles
Runner Up - mmc2023
Alex Robinson, University of Liverpool
Advances in Probe Subsampling for 4D-STEM
1st Prize - New & Emerging Concepts
Florian Steiner, Ludwig-Maximilians-University Munich
Quantum optics meets microscopy – An ultra-sensitive resonator microscope for nano- and life sciences
2nd Prize - New & Emerging Concepts
Mollie Brown, University of Strathclyde
Obtaining super-resolved images at the mesoscale through Super-Resolution Radial Fluctuations
EMAG - Best Student Posters
Seojin Kim, University of St Andrews
Microscopic characterisation of Ni nanoparticles exsolution in La0.4CaxSr0.4-xNi0.06Ti0.94O3-d perovskite oxides
Atul Atul, University of Groningen
Resolving phase coexistence in VO2 thin films by real space imaging of oxygen atoms
Ella Kitching, Cardiff University
Investigation of the capabilities of iDPC tomography to image CeO2 nanoparticles
EMAG Best Flash Talk
Frances Quigley, Trinity College Dublin. Advanced Microscopy Laboratory, Centre for Research on Adaptive Nanostructures and Nanodevices (CRANN), Dublin
Developments in designing a retrofittable photoelectron source for low voltage electron microscopy
EMAG Best Talks
Zhiquan Kho, University of Manchester
Understanding multi-phase nano-scale structural complexity of reacted SiC coating of TRISO particle using STEM and unsupervised clustering
John Scott, Queen’s University Belfast
A Multiscale Study of Ferroelastic Domains as a Function of Aspect Ratio
Andreas Körner, Helmholtz Institute Erlangen-Nürnberg for Renewable Energy
What radiation does to water: the case of acidity under electron and X-ray exposure
Some of our prize-winners are pictured below









After collecting my badge, I first strolled around the RMS imaging competition exhibition while waiting for the conference to begin. I was astounded by the entries, as the exhibition was full of images which would not have been out of place in an art gallery.
I was delighted to present my easy-to-use Pythonbased image processing package, SimpliPyTEM, to the Early Careers Symposium. Many attendees seemed to agree that although simple, the package could make life much easier for many microscopists, and that sharing well-documented workflows was highly valuable.
The diversity of the projects presented at the mmc was astounding, and the whole week was an
incredible opportunity to catch up with old friends, make new friends and learn about the newest and most exciting developments in the world of microscopy. I loved learning what the rest of the microscopy world is up to. Thanks to the RMS for providing financial support which made it possible for me to attend, and to all the organisers and sponsors who made this incredible event possible; it was truly a highlight of my year.
mmc2023 was an excellent platform for sharing knowledge, presenting ground-breaking research, and discussing the latest advancements in microscopy techniques and applications.

“An excellent platform for sharing knowledge”
I was impressed by the display of leading microscope manufacturers, imaging software developers, microscopy accessory suppliers, and related technology providers. I got the chance to listen to the introductions and workshops from the leading microscope manufacturers and suppliers like Nikon, Zeiss, and Olympus, and the providers of specialised microscopy accessories and sample preparation tools, such as Electron Microscopy Sciences, Gatan, and Agar Scientific. I was particularly interested in the techniques that were useful in my research field - microscopy study on battery materials.
I would like to thank the Royal Microscopical Society for the financial support that made it possible for me to attend this conference. I believe this experience can significantly contribute to my professional development, knowledge expansion, and networking

opportunities.
My work at the CRUK Beatson Institute for Cancer Research in Glasgow meant that I went to mmc with a focus on life sciences, especially fluorescence and confocal microscopy, histology and high-throughput imaging. I presented a poster on the third day of the conference, showcasing our core facility and the many types of tissue imaging, multiplexing and analysis we perform.
“I will definitely be attending mmc2025”


I am also a committee member of the Scottish Microscopy Society (SMS), and we had our own stand to advertise our upcoming symposium and our first ever Scottish Microscopist of the Year competition. Our stall was a great way to meet new people from all over the UK and further afield, and the free Irn Bru and Tunnocks caramel wafers we supplied did not last long!
There were many social events at mmc, with opportunities during the exhibition, poster sessions and evenings. Manchester is a wonderful city with plenty of restaurants, pubs and bars (all of which I remember well from my time there as a PhD student). Overall mmc was an excellent experience, allowing me to network with old and new colleagues and friends. I look forward to ELMI and EMC in 2024, and will definitely be attending mmc in 2025!
Andreas Bruckbauer, CRUK Cambridge Institute, University of Cambridge, UKAttending mmc2023 has provided me with immense value for my professional development and career in electron microscopy. As one of the largest microscopy conferences in the world, mmc2023 offers me exceptional opportunities to learn about the latest research and technological advances in the field. I also had opportunities to interact with many leading experts from academia and industry to allow me to gain insights on cutting-edge techniques and applications of electron microscopy. I also met peers facing similar research challenges and share experiences on techniques and problemsolving approaches. These connections can lead to future collaborations and knowledge-sharing with researchers in my field. Additionally, I can use this opportunity to connect with potential mentors and advisers to further my professional development.
The Microscience Microscopy Congress has everything you wish for: interesting workshops, great scientific program, RMS award presentations, large microscopy exhibition, learning zone, imaging competition and evening networking events! And we can now enjoy all this in person without COVID restrictions.
The large exhibition hall in the centre of Manchester, with good traffic connections and plenty of hotels nearby, is an ideal location for an event of this scale, and the place was buzzing with excitement. With so many parallel sessions and the large variety of technologies on display, I can only give a snapshot of my personal experience of mmc2023.
Many thanks to the organisers and all the participants who mad mmc2023 such an enjoyable experience.
Wednesday was the busiest day for me, as it included giving my flash talk entitled ‘Developments in designing a retrofittable photoelectron source for low voltage electron microscopy’. The talk appeared to be received well and I later had the opportunity to discuss this work with many different researchers at the poster session in the main exhibition hall. The day however didn’t end there, with Revolucion de Cuba booked for the EMAG networking event in the evening. The buffet food provided was delicious and it was great to catch up with other EMAG members.
A surprising highlight of the week was seeing Dr Mckee’s talk on the avian eggshell membrane
“The place was buzzing with excitement”
“Exceptional opportunities to learn”
“Great to catch up with other EMAG members”
interface. While not at all in my area of research this talk proved to be extremely interesting and informative, including giving tips on the best way to store eggs when shopping.
The end of the conference involved the prize giving for the student talks and posters. I was delighted to have been awarded a prize for best flash talk. It was a great way to end the conference which was extremely informative and allowed me to have some interesting discussions with some exceptional researchers.
I presented a poster, entitled “Atomic resolution electron imaging of grain boundaries and intragrain between interfaces in perovskite semiconductors” in the poster sessions. The poster was well received and resulted in several interesting discussions. The location and distribution of dopants, impurities, and defects within the perovskite layer and at the interfaces with other layers in perovskite solar cells are well investigated through aberration-corrected scanning transmission electron microscopy. This opportunity allowed me to receive constructive feedback from experts in the field, further refining my work and validating its significance.
For me, the absolute best thing about mmc2023 was seeing my international colleagues in real life for the first time in four years.
During the conference, I enjoyed the atmosphere listening to talks, and was pleased to see a large audience during my contributed talk in the multimodal imaging session. A large amount of my time was also spent at the exhibition hall, catching up with new technology and old friends, as well as making new connections. As usual, many exhibitors had brought impressive pieces of demo equipment, and I will be sure to follow up on many of the latest developments with my local vendors.
On the social side, the in-person poster sessions were a definite highlight - overall the atmosphere, quality of research and enthusiasm of the presenters all contributed to a delightful experience. The second poster session was followed by the networking dinners, one for each - EMAG, AFM/SPM and Frontiers in Bioimaging. I attended the Frontiers dinner at Fazenda, which was still at full swing when I retired well past my bedtime at 10:15pm.
One talk that stood out for me was that of Dr Peter Bankhead during the engaging Artificial Intelligence session. Peter’s 2014 book Analyzing fluorescence microscopy images with ImageJ helped me immensely as a novice PhD student with no knowledge of image analysis, and it was fascinating to see how he went on to combine image analysis with machine learning in the open-source software, QuPath.
In addition to the posters, plenary lectures, and themed sessions, delegates were given the opportunity to talk with over 80 exhibitors at the mmc2023 exhibition. On show was the most cuttingedge technologies available for microscopy, and my imagination ran wild thinking about what could be achieved with some of the equipment there.
Liisa Hirvonen, Centre for Microscopy, Characterisation and Analysis (CMCA). The University of Western Australia
“Poster sessions were a definite highlight”
Xinjuan Li, Department of Materials Science and Metallurgy, University of Cambridge, UK
“Constructive feedback from experts in the field”
“My imagination ran wild”
Introducing Unity, the world’s first combined Backscattered Electron and X-ray (BEX) imaging detector. Accelerate your journey to scientific discovery with instant microstructural and chemical images, acquired simultaneously with the Unity detector.


We are very pleased to continue offering a range of ‘in-person’ and virtual events this year, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend. The following information was correct at the time of publication but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates. Our online calendar includes all the details about forthcoming talks in the International Microscopy Focus Lecture Series – a joint, online initiative established between the RMS, and a number of international societies.
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
2023
September
26 Advanced Reconstruction Toolbox: Leveraging AI to Solve X-ray Imaging Challenges on ZEISS Xradia Platforms (Online, RMS-hosted event)
26 - 27 Nanoscale In Situ Microscopy Workshop 2023, Diamond Light Source (RMS-sponsored event)
October
4 Expansion Microscopy User Group Meeting - Canada Hosted, (Online)
9 – 10 Facilities Management Training Course - York, UK
9 – 13 All Things Cryo - Nottingham, UK
November
16 flowcytometryUK 2023Cambridge, UK
December
4 - 5 Virtual European Flow Core Meeting 2023 (Online)
2024
January
4 – 5 UK Light Microscopy Facility Meeting 2024, Edinburgh, UK
8 – 9 Flow Facilities Meeting 2024 , Birmingham, UK
February
8 – 9 EM-UKI 2024 (Electron Microscopy UK and Ireland), York, UK
March
25 – 28 AFM SPM 2024, Durham, UK
June
4 – 7 elmi2024, Liverpool, UK (RMS-hosted event)
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
9 – 13 October, Nottingham
Scientific organiser: Chris Parmenter, University of Nottingham
This course consists of two and half days, broken into morning and afternoon virtual lecture sessions, covering the theory of the techniques.
9 – 10 October, York
Scientific organisers: Jemima Burden, University College London; Derek Davies, The Francis Crick Institute; Joanne Marrison, University of York; Peter O’Toole, University of York; Alex Sossick, Natural History Museum
This is an intensive in-person course equipped
16 November, Cambridge
Scientific organisers: Derek Davies, The Francis Crick Institute; Rachael Walker, Babraham Institute
The flowcytometryUK 2023 Meeting will take place in person and will highlight the diverse
2023
4 – 5 December, Online
Scientific organisers: Charlotte Petersen, Aarhus University; Derek Davies, The Francis Crick Institute; Peter O’Toole, RMS President, York University; Desiree Kunkel, BIH at Charité; Marta Monteiro, Instituto Gulbenkian de Ciência; Marjolijn Hameetman, Leiden University Medical Center
This will be the first meeting of what will hopefully become a new tradition - an annual event to meet with fellow flow core colleagues from all over Europe. The meeting is aimed at
Days 4 and 5 (Thursday and Friday) will be filled with practical demonstrations / Q&A sessions to explore the practical points of the techniques / approaches with the delegates in person, should they wish to come to Nottingham. This course is designed to be suitable for anyone that wants to explore cryogenic microscopy methods to support their research and this could apply to life sciences, material sciences and beyond.
to give you the basic knowledge to enable you to run a core facility effectively. There will be tips and tricks throughout from experienced experts in the area. It is geared towards all levels, from those experienced in running a core lab to those looking to move in to this field as a career.
This course will take place the University of York, UK.
areas in which cytometry is a vital resource. There will be scientific presentations from a number of speakers covering immunology, marine biology, extracellular vesicles and other important research areas. Talks will be interspersed with commercial flash presentations. Sponsors will allow delegates to have access to information on recent developments in the field.
all those that run or work in a Flow Cytometry Core Facility and seeks to address common subject matters, themes, and circumstances within the European community. We will focus on new and emerging technologies as well as operational aspects of running, and working in, a core. We will include talks about national cytometry societies, presentations from current core facilities (Crib talks) and also from our industry colleagues (Technobites). The meeting will run over two days, from lunchtime to lunchtime (04 and 05 December 2023). Although in a virtual format, there will be ample time in the programme for discussion and questions from the community. The meeting will be ‘live’ and will not be recorded, it will be unavailable online afterwards.
The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences.
online at www.journalofmicroscopy.org
They include:
Dehydration as alternative sample preparation for soft X-ray tomography
Anthoula Chatzimpinou, Charlotta Funaya, David Rogers, Stephen O’Connor, Sergey Kapishnikov, Paul Sheridan, Kenneth Fahy, Venera Weinhardt
Soft X-ray tomography (SXT) is an imaging technique that allows to see the internal structures of cells without the need for special treatments like fixation or staining. Typically, SXT imaging involves freezing and imaging cells at very low temperatures. However, since many labs lack the necessary equipment, we explored whether SXT imaging could be done on dry samples instead. We compared
different dehydration methods and found that critical point drying (CPD) was the most promising for SXT imaging. CPD-dried cells showed high structural integrity, although they absorbed more X-rays than hydrated cells, demonstrating that CPD-dried sample preparation is a viable alternative for SXT imaging.
Rebecca M. Williams, Jordana C. Bloom, Cara M. Robertus, Andrew K. Recknagel, David Putnam, John C. Schimenti, Warren R. Zipfel
Lightsheet microscopy offers an ideal method for imaging of large (mm–cm scale) biological tissues rendered transparent via optical clearing protocols. However the diversity of clearing technologies and tissue types, and how these are adapted to the microscope can make tissue mounting complicated and somewhat irreproducible. Tissue preparation for imaging can involve glues and or equilibration in a variety of expensive and/or proprietary formulations. Here we present practical advice for mounting and capping cleared tissues in optical cuvettes for macroscopic imaging, providing a standardised 3D cell that can be imaged routinely and relatively inexpensively. We show that acrylic cuvettes cause minimal spherical aberration with objective numerical
apertures less than 0.65. Furthermore, we describe methods for aligning and assessing the light sheets, discriminating fluorescence from autofluorescence, identifying chromatic artefacts due to differential scattering and removing streak artefacts such that they do not confound downstream 3D object segmentation analyses, with mouse embryo, liver and heart imaging as demonstrated examples.
Lanjiao Liu, Mingxin Chen, Yifan Gao, Liguo Tian, Wenxiao Zhang, Zuobin Wang
Many diseases are related to changes in the biomechanical properties of cells; their study can provide a theoretical basis for drug screening and can explain the internal working of living cells. In this study, the biomechanical properties of nephrocytes (VERO cells), hepatocytes (HL-7702 cells), and hepatoma cells (SMCC-7721 cells) in culture were detected by atomic force microscopy (AFM) to analyse the side effects of colchicine at different concentrations (0.1 μg/mL (A) and 0.2 μg/mL (B)) at the nanoscale for 2, 4 and 6 h. Compared with the corresponding control cells, the damage to the treated cells increased in a dose-dependent manner.Among normal cells, the injury of nephrocytes (VERO cells) was markedly worse than that of hepatocytes (HL-7702 cells) in both colchicine solutions A and B. Based on the analyses of biomechanical properties, the colchicine solution reduced the rate of division and inhibited metastasis of SMCC-7721 cells. By comparing these two concentrations, we found that the anticancer effect of colchicine solution A was greater than that of solution B. Studying the mechanical properties of biological cells can help understand the mechanism of drug action at the molecular level and provide a
theoretical basis for preventing the emergence and diagnosis of diseases at the nanoscale.

Mohammad Abushad, Swaleha Naseem, Mohammad Arshad, Adil Shafi, Mohammad Zain Khan, Azizurrahaman Ansari, Vishal Kumar Chakradhary, Fouran Singh, Shahid Husain, Wasi Khan
In the present study, nanoparticles of TiO2 and Crdoped TiO2 have been synthesised by a cost-effective acid-modified sol-gel process. The effect of Cr doping on the microstructure, thermal, magnetic and photocatalytic properties of TiO2 were explored in detail. The transmission electron microscopy (TEM) images exhibit the presence of elongated nanoparticles with an average size of 10 nm. Field emission scanning electron microscopy (FESEM) was used to study the surface morphology of the synthesised materials, which revealed nonuniform morphologies and less aggregation of the particles in the Cr-doped sample. Energy dispersive x-ray spectroscopy (EDS) confirms the elemental compositions with the appropriate stoichiometry of the elements. Raman spectra ensure the phase purity of the materials and also a blue shift with the incorporation of Cr ions in TiO2. X-ray photoelectron spectra (XPS) predict the chemical state of the elements and oxygen vacancies in the prepared samples. The magnetic nature of all the synthesised samples was examined through the vibrating sample magnetometer (VSM) and revealed weak ferromagnetic behaviour of the samples. These results signify that the oxygen vacancies and defects play a crucial role in developing the ferromagnetic nature of oxide semiconductors. The differential thermal analysis (DTA) shows the structural phase transition at ∼630°C.The photocatalytic performance
of the prepared samples was studied for the degradation of methylene blue (MB) dye under irradiation of visible light. A higher photocatalytic efficiency was found for the 20% of Cr-doped TiO2. These studies propose that the appropriate incorporation of Cr ions makes TiO2 very efficient for visible lightdriven photocatalysts required for applications in wastewater treatment.

Xinglin Li, Xiangcheng Li, Tao Wu, Chenglong Lv, Canying Cai
Electron backscatter diffraction (EBSD) can be employed to determine crystal structures but has not been used alone to identify defects at the atom scale due to the lack of understanding of the EBSD patterns generated by various structure defects. In the present work, the EBSD patterns of FCC-Fe with 9-layer, 6-layer and 3-layer twin structures are simulated, respectively, using the revised real space (RRS) method and compared with the counterpart of perfect crystals. Our results show that when the electron beam is incident along a direction parallel to the twin plane, the pattern appears symmetrical with respect to the corresponding Kikuchi band of the twin plane, and the diffraction details within the Kikuchi band also exhibit symmetry with respect to the middle line of the Kikuchi band. Moreover, the overall clarity of the patterns decreases, and the pattern becomes more blurred with increasing the distance from the Kikuchi band corresponding to the twin plane. By contrast, the incident electron beam along the direction perpendicular to the twin plane
results in diffraction superposition of the matrix region and the shear region, which shows two-fold rotational symmetry with respect to the Kikuchi pole corresponding to the normal to the twin plane. In addition, some extra Kikuchi bands appear in the EBSD patterns due to the long-period structures of the multilayer twins. As the number of multilayer twins decreases, the number of extra Kikuchi bands decreases and the area of the blurring pattern increases. The correlation between twin structures and EBSD patterns provides theoretical insights for identifying twin structures by the EBSD technique.

Siân Culley, Alicia Cuber Caballero, Jemima J Burden, Virginie Uhlmann
Images are at the core of most modern biological experiments and are used as a major source of quantitative information. Numerous algorithms are available to process images and make them more amenable to be measured. Yet the nature of the quantitative output that is useful for a given biological experiment is uniquely dependent upon the question being investigated. Here, we discuss the three main types of information that can be extracted from microscopy data: intensity, morphology, and object counts or categorical labels. For each, we describe where they come from, how they can be measured, and what may affect the relevance of these measurements in downstream data analysis. Acknowledging that what makes a measurement ‘good’ is ultimately down to the biological question being investigated, this review aims at providing readers with a toolkit to challenge how they quantify their own data and be critical of conclusions drawn from quantitative bioimage analysis experiments.
Fan Peng, Jimei Zhang, Ziwei Liu, Xuemei Song, Wei Zheng, Yi Zeng
Commercial electron backscatter diffraction (EBSD) systems generally use interplanar angle matching for pattern indexing, and thus, they are unable to distinguish between some similar phases with close interplanar angles, such as Al and Si. The interplanar spacing is more diagnostic but generally difficult to apply in pattern indexing because it lacks precision. In this study, we proposed an efficient approach for accurately measuring interplanar spacing by correcting the reciprocal-lattice vector (RLV). The phase discrimination of Al and Si was performed by interplanar spacing matching. The Kikuchi bands were identified automatically by the self-developed method using pattern rotation combined with grey gradient recognition without the help of human eyes. The reliable RLV relationship was extracted by accurately drawing reciprocal-lattice vectors. The lengths of RLVs were corrected, and then the RLVs were used for evaluating lattice spacing. The results of five Kikuchi patterns with different clarity showed that this new method reduced the average error of interplanar spacings by 50.611% and achieved an average accuracy of 1.644% for lattice spacing calculation. The method could distinguish structures with a difference in lattice spacing of at least 3.3%. This method was also effective for fuzzy patterns and partially missing Kikuchi bands and might be used as a new strategy for improving the calculation accuracy of lattice spacing for fuzzy patterns. The method did not have additional requirements concerning the number of detected Kikuchi bands and poles. The accuracy of lattice spacing could be effectively improved by correcting the RLVs based on routine pattern recognition. This method might be used as
an auxiliary approach to differentiate between similar phases and is well-adapted to the existing commercial EBSD system.

1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
www.journalofmicroscopy.org


The Journal of Microscopy is published by the Royal Microscopical Society on our behalf by Wiley. The Journal is critical to the Society as almost all of the income it generates comes back into the Society and supports many of our activities. It is the oldest journal dedicated to the science of microscopy. It was first published as ‘The Microscopic Journal and Structural Record’ in 1841, shortly after the Society was first formed (in 1839). It then became ‘the Transactions of the Microscopical Society of London’, then ‘the Quarterly Journal of Microscopical Sciences, and finally, the Journal of Microscopy in 1878. All of these issues are available online in the back-catalogue, and are a fascinating read, including articles from some very famous microscopists such as Ernst Abbe, who published several articles in the journal, and was made an honorary fellow by the Society. Interestingly, even then, part of the aim of publishing the Journal was to provide a source of income for the society, over and above subscriptions from members, and of course, this is still true today.
Today, the Journal faces several problems. First, is the perceived problem of its impact factor, which has been gradually increasing and is now 2.0 (in 2022). The impact factor is based on the average number of citations per paper in the past 2 years and was originally used by librarians to help decide which journals to subscribe to. It then became used as a sign of quality; the higher the impact factor of the journal that a paper is published in, then the better it must be. However, this is not correct. Even in journals that have a high impact factor, some papers are barely cited, and occasionally some are not cited at all. Thus, the simple fact of publishing in a high impact factor journal does not mean a specific paper is in itself very high in quality or has a
strong impact on the field.
Importantly, the use of the impact factor to judge a paper’s worth has now been largely discredited. Just over 10 years ago, the scientific community came together to sign DORA, the San Francisco Declaration on Research Assessment, which you can read here: https://sfdora.org/read/. One of the key general recommendations of that declaration was not to use Journal Impact Factors to assess the merit of research articles as well as the contribution of individual scientists in hiring, promotion, grants, fellowships etc. Those recommendations are largely being taken up worldwide. For example, all in the UK, all UKRI grant committees are explicitly told not to use impact factors to judge the outputs of
the applicants. This should be reassuring to early career researchers, and yet, many people still consider impact factor when choosing where to publish.

I think we need to think more about whether the journal is a good place to publish your paper, rather than simply focus on impact factor. The Journal of Microscopy is a good place, because all of the editors (Fig. 1) and the editorial board are all expert microscopists. This means your paper has the best chance of being expertly reviewed by expert microscopists! And then, once it is published, it is published alongside many other articles that focus on microscopy, so it is in a good place in terms of visibility. Finally, the journal should be the natural home for papers focussed on microscopy, and specifically those from members of the Society as well as microscopists generally. This was one of the drivers for starting up the Journal many years ago, to provide a place where the papers are focussed

on the topic of microscopy, and it is still one of its main goals today.
The second problem that faces the journal and the Society is open access. Open access is a great venture, in that all papers will eventually be free to read, without needing a subscription to the journal. Many grant funding agencies now expect that you publish your work as ‘open access’. The Journal of Microscopy currently publishes articles that are either open access and free to read, on the payment of an APC (article processing charge), or published at no cost to the authors, but not freely available to read for 12 months, and can only be read if you, or your institution has a subscription to the Journal. However, did you know that if you want or need to publish your paper ‘open access’ you can do this, without incurring any costs, if your institution has signed up to the Wiley Transformational deal? Many institutions across the UK (Jisc institutions), Europe, and many other countries have done this. Under
this deal, not only can you read the journal for free, but you can also publish open access papers with no cost to you individually. The subscription deal includes APC costs for a certain number of papers per institution. Currently, the Journal of Microscopy has a really broad reach; over 9000 institutions across the world have access to the journal via subscription. Articles published in 2022 were read 789 million times over 252 countries and territories. However, papers do tend to be read more widely if they are open access, and you can do this! You can find out more about this and your eligibility here: (and see more details below)
https://onlinelibrary.wiley.com/page/ journal/13652818/homepage/open-access
Eventually, the Journal of Microscopy will switch to being solely open access. This is great, scientifically, but it gives the Society yet another problem. When this happens, the amount of money that the Society will receive per article for each paper published, will drop by well over 50%, severely impacting the income that the society receives from publishing the Journal, and thus likely to affect our ability to support the wide range of activities that we do currently. We are not alone, in that this is a problem for all journals published by scholarly societies. The only way we can start to make up this deficit is by publishing more papers. Currently, we publish about 80 papers a year, in 12 issues. We encourage you to submit your papers to the journal, to help us increase the number of papers we publish, and we also encourage any ideas for special themed issues.
To summarise, the Journal of Microscopy is the oldest journal dedicated to the science of microscopy and the only peer-reviewed publication of the Royal Microscopical Society. It publishes papers that report on the very latest developments in microscopy such as advances in microscopy techniques or novel areas of application. The scope covers research in the physical and biological sciences and covers imaging methods using light, electrons, X-rays and other radiations as well as
atomic force and near field techniques. It publishes high quality papers, reviewed by experts, and should be your first journal of choice for all things in microscopy! So, please do consider us when you are next putting your paper together, and if you are uncertain as to whether it is a good fit or not, please do email us and ask!
Prof. Michelle Peckham General Editor of the Journal of Microscopy m.peckham@leeds.ac.ukThe Journal publisher, Wiley, has several agreements in place with institutions and funders to help authors publish open access and ensure compliance with open access policies. Authors from an institution affiliated with an open access deal may publish primary research and review articles open access at no charge to the author.
The Journal of Microscopy offers authors a hybrid open access option. The benefits include:
• High visibility all articles are immediately, freely available online
• Easy compliance with open access mandates with Creative Commons licenses
• Reuse and immediate deposit of final article in any website or repository
• Copyright retention – You retain the copyright for your article at all times
• Automatic export to PubMed Central/Europe PubMed Central (PMC) when appropriate
• High-quality and authoritative publishing standards with rigorous peer-review
Consortia included in these agreements include:
• Jisc institutions in the UK;
• Projekt DEAL institutions in Germany;
• Dutch Universities/UMCs (VSNU);
• Austrian Academic Library Consortium (KEMÖ);
• Irish institutions in Wiley’s IReL agreement;
• Swedish Bibsam institutions;
• FinELib institutions;
• Conferenza dei Rettori delle Universita Italiane (CRUI);
• Hungarian Electronic Information Service Programme (EISZ) institutions;
• Norwegian institution participating in the Unit agreement;
• Conference of Rectors of Universities (CRUE) and the Higher Council for Scientific Research (CSIC) in Spain;
• Consortium of Swiss Academic Libraries (CSAL).
Please visit Wiley Author Services for more
information and to check if your institution is participating:
Automatic Article Publication Charge waivers and discounts will be given to authors from countries on the Waivers and Discounts List. Authors should submit a waiver or discount request during the submission of their article.
Please contact Editorial Office Manager Jill Hobbs for further information.
This special issue was co-edited by Michelle Peckham (University of Leeds), Ulla Neumann (Max Planck Institute for Plant Breeding Research, Köln, Germany) and Siân Culley (KCL). Together we solicited papers from a broad range of women involved in microscopy with the aim of celebrating their achievements and contributions to microscopy. This has culminated in Part 1 of Women in Microscopy, which has now been published online . We hope to publish Part 2 shortly.
To find out more about this special issue, please read the introduction here: https://onlinelibrary. wiley.com/doi/10.1111/jmi.13207
It is a lovely mix of articles, with advice on careers from Rita Strack, editor of Nature Methods, an account of the election of women as fellows to the Royal Microscopical Society, original papers on advances in microscopy, and reviews that highlight advances in microscopy across a range of topics. I encourage you to read this special issue, and we look forward to part 2!
The issue (July 2023) features the following papers:
A bird’s eye view on microscopy
Rita Strack
The election of women as Fellows of the Royal Microscopical Club
Pam Hamer
Multi-foci parallelised RESOLFT nanoscopy in an extended field-ofview
Xavier Casas Moreno, Francesca
Pennacchietti, Guillaume Minet, Martina Damenti, Dirk Ollech, Federico Barabas, Ilaria Testa
A method for reproducible highresolution imaging of 3D cancer cell spheroids
Thomas A. Phillips, Valeria
Caprettini, Nandini Aggarwal, Stefania
Marcotti, Rob Tetley, Yanlan Mao, Tanya Shaw, Ciro Chiappini, Maddy Parsons, Susan Cox
Non-fitting FLIM-FRET facilitates analysis of protein interactions in live zebrafish embryos
Julia M. T Auer, Laura C Murphy, Dong Xiao, David U. Li, Ann P Wheeler
In-depth polarisation resolved SHG microscopy in biological tissues using iterative wavefront optimisation
Dmitry Nuzhdin, Emily G. Pendleton, Eleanor B. Munger, Luke J. Mortensen, Sophie Brasselet
A perspective of fluorescence microscopy for cellular structural biology with EGFR as witness

M. L. Martin-Fernandez
Seeing clearly – Plant anatomy through Katherine Esau’s microscopy lens
Anja Geitmann
intER-ACTINg: The structure and dynamics of ER and actin are interlinked
Charlotte Pain, Frances Tolmie, Stefan Wojcik, Pengwei Wang, Verena Kriechbaumer
Overcoming the challenges of preserving lipid-rich Cannabis sativa L. glandular trichomes for transmission electron microscopy
Samuel J. Livingston, Eva Yi Chou, Teagen
D. Quilichini, Jonathan E. Page, A. Lacey Samuels
Microscopical palynology: Birch woodland expansion and species hybridisation coincide with periods of climate warming during the Holocene epoch in Iceland
Kesara Anamthawat-Jónsson, Lilja
Karlsdóttir, Ægir Thór Thórsson, Margrét Hallsdóttir


• Reliable, reproducible, and convenient
• Best temperature stability (+/ 0.1 °C)

• Most cost effective solution on the market


Based on extensive experience with in situ high temperature applications, the operation of the HTME 1000 is safe and reliable. The EBSD specific thermal shielding and the active cooling system are optimized for the best image quality and detector protection.

The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months. We hope they enjoy a long and rewarding membership with the RMS.
Dr Alice Macente
Ms Oi Ting Bernadette Kwok
Mr Harrison Greenwood
Mr Viktor Ellingsson
Dr Matthew Piggott
Mr Michael Large
Mr Philip Hubbard
Shaoxia Chen
Miss Hannah Anderson
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk
Name
Ajit Roy
Tell Us About You?
Ajit is a master’s degree student in Optics and Photonics as a part of Erasmus Mundus Joint Master Degree euroPHOTONiCS programme.
He earned his bachelor's degree in Optics and Optoelectronics Engineering from the Department of Applied Optics and Photonics, University of Calcutta (India).

He has a keen interest in Fourier Optics, Microscopy and Ultrafast Photonics.
Why did you become a member of the RMS?
I am an Optics and Photonics master’s student with a great interest in light Microscopy. Currently I'm building the world's latest 6th generation Single Molecule Localisation Microscope in close collaboration with EMBL Imaging Centre
and EMBL Cell Biology Biophysics Unit (Ries Lab). I want to continue in this special field of science and want to be a major contributor to the field of Optical microscopy and Bioimaging. Becoming a part of this society is a primary step towards my greater goal.
How do you feel being an RMS member benefits you?
I feel amazed and overwhelmed. I love microscopy and it was my dream to be a part of this enriched, well-known, old society.


www.agilent.com
As a global leader in analytical and clinical laboratory technologies, Agilent is committed to helping our scientists bring great science to life. Leveraging more than 50 years of analytical and clinical laboratory expertise, we deliver trusted answers to our customers’ most critical questions and challenges. We passionately help scientists and clinicians make the smallest everyday advancements and life-changing discoveries that advance the quality of life.
Alemnis AG
www.alemnis.com

Alemnis AG is a Swiss pioneer in the field of insitu micro- and nanomechanical property testing with 15 years of experience. At the core of Alemnis instruments lies the ambition to push the boundaries of testing in extreme environments and at extreme timescales. Furthermore, Alemnis AG revolutionizes in-situ micromechanical property testing with their unparalleled True Displacement Control, provide researchers with an exceptional level of control and precision at the nm and μN range.
Delmic B.V.
https://www.delmic.com/en/
Delmic is a passionate
high-tech company based in Delft, Netherlands that develops powerful and user-friendly solutions for electron microscopy, bringing researchers and organizations closer to research insights across diverse application fields. We are driven to create a greener and healthier future by making microscopy an easy and accessible technique through automation of the entire workflow.
Stratocore www.stratocore.com
Research management delivered. Globally. Stratocore is the leading enterprise software company focused on helping research institutions better manage scientific resources. Stratocore delivers a powerful, continuously supported and refined core facility management solution that is globally deployed at over 200 research organizations around the world. Stratocore increases core facility efficiency and enhances the overall strategic management of institutions’ research enterprises.

www.vitrotem.com
Vitrotem develops and sells userfriendly systems for the fabrication Graphene Liquid Cells (GLCs) that enable researchers to study (biological) materials and processes in liquid at unprecedented resolution in standard electron microscope. Vitrotem was founded in 2019 as a spin-off of the University of Leiden, the Netherlands. The company is located at Sciencepark Amsterdam and has a satellite lab in Leiden.

An ever-popular feature linked to our flagship mmc series of conferences is the RMS Scientific Imaging Competition. This has developed from the Micrograph Competition which has been running for over 50 years. I well remember being awarded first prize for a lattice image of a complex oxide crystal, several decades ago…..!

In those days, monochrome micrographs were usually enlarged to about 10x8 inch prints, mounted on stiff card and posted in to the RMS office in central Oxford. Now that digital photography has all-but replaced negatives and prints, images can now be submitted online, with entries to the 2023
competition coming from more than 30 different countries, including the United States, India, South Africa and Australia. With the RMS including all areas of microscopy, we were delighted to receive over 200 images or video clips, almost all of which showed a very high standard of presentation. One of
the aims behind the competition is to promote the presentation of scientific images at the highest level, demonstrating practical skill in recording the image, as well as careful processing where appropriate.
The judging panel, consisting of Joelle Goulding, Pippa Hawes, Alice Pyne and myself, reviewed all the submissions separately, before agreeing on a short-list from each category, which was selected for displaying at mmc prior to the final judging. The images were on display throughout mmc, in the entrance foyer. Selecting the short-listed entries was a very tough call, as nearly all images would have been worthy winners in their own right. As well as considering the underlying science behind each image, and whether an image “told a story”, we also looked for that elusive “wow factor”, which caught the viewer’s eye. The use of common image processing software was used very effectively in
many images, although some authors produced rather garish colour “enhancements” which can occasionally detract from the image itself. Some very effective images were actually reproduced in grey-scale without any additional, false colour.
Authors of the winning entries (shown on pages 20-25) all received certificates and very attractive prizes, including computer tablets and portable speakers.
We look forward to seeing your entries in two years’ time at mmc2025!
Finally, a word of thanks to the office staff Lucy Ridler and Owen Morton who worked behind the scenes in collating all the entries and setting up the eye-catching display of images.
Find out more about all the winning entries from the 2023 RMS Scientific Imaging Competition.
 John Hutchison
John Hutchison
Current affiliation: Center for Anatomy and Cell Biology, Medical University of Vienna, Austria
Providing a platform for early career researchers to showcase and integrate their research into the scientific community is fundamental to maintaining and developing a healthy research environment. So, it was fantastic to attend the recent RMS Early Career Symposium at mmc2023 to see this very thing in action - early career researchers promoting their research contributions, networking, and hearing the career experiences of fellow/senior researchers. It was even more fantastic to attend the symposium as a nominee of the RMS Early Career Award!
The symposium specifically focuses on research in the field of microscopy, so hosted a wide range of research topics with a strong interdisciplinary theme throughout. It opened with an invited talk by Dr. Judy Kim (Rosalind Franklin Institute, UK) who not only spoke about her work using electron ptychography
to investigate nanostructures within cells, but also shared insights into how she balanced career progression while starting a family. Next, it was the selected abstract talks from students, postdocs and early career professionals. These included examples of applying state-of-the-art imaging and analysis tools to investigate biological processes; such as the function of glycosylation in cell motility (Joanna Cull [Oxford Brookes University, UK]), stem cell renewal (Aishwarya Sivakumar [University of Edinburgh, UK]) and a novel analysis package to quantify electron microscopy images (SimpliPyTEM) (Gabriel Ing [University College London, UK]). A highlight was the presentation of Jay Christopher (University of Strathclyde, UK) who shared his work on 3D printing lenses for fluorescence microscopy – it will be extremely interesting to see how these printing approaches can reduce cost barriers and
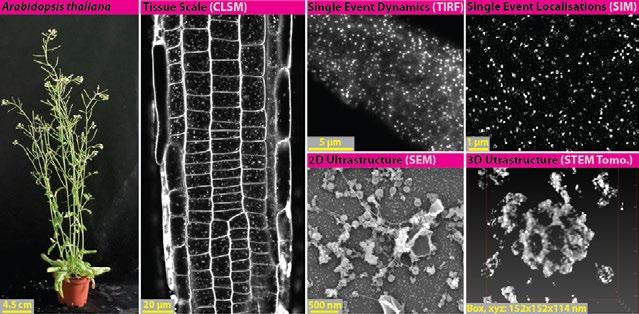
Figure 1. Multiscale quantitative imaging of clathrin-mediated endocytosis in the plant model Arabidopsis thaliana. Confocal imaging can be used to examine the overall efficiency of endocytosis using lipid dye markers like FM4-64 at the tissue scale. TIRF and SIM provide live high and super resolution imaging of single events of endocytosis in intact plant samples, which can define endocytic dynamics and provide precise protein localizations. SEM and STEM can be used to examine the ultrastructure of endocytic events, and further combined with tomography to visualise endocytosis in three dimensions.
impact custom optical designs.
Next, it was the RMS Early Career Award competition, which seeks to recognise the achievements and contributions to the imaging community of an early career scientist. It takes the form of a five-minute presentation where nominees are judged on the impact, complexity, and novelty of their research. Mart Last (Leiden University Medical Center, NL) presented his research pioneering correlated light and electron microscopy using super-resolution light microscopy and cryogenic electron microscopy (cryo-CLEM), and Lara Dresser (University of York, UK) demonstrated how to use FRET and model membrane vesicles to understand the biophysics of membrane fusion events. Finally, I presented my work investigating endocytosis in the plant model Arabidopsis

While clathrin-mediated endocytosis (CME) is an essential eukaryotic process, surprisingly, how it
functions in plants is poorly understood. A major reason for this has been the lack of tools to visualise and quantify CME in plants at appropriate resolutions. This is because plants are an ‘unfriendly’ sample for many microscopy approaches; for example, cell walls produce many refractive index mismatches and light scattering effects, and the extreme turgor pressure in plant cells has hampered the preservation of CME structures in traditional electron microscopy protocols. Therefore, to begin investigating planta CME, we had to overcome these optical and sample preparation challenges. By working with an amazing team of supervisors, colleagues and collaborators, I developed a range of quantitative tools to directly image CME in plant samples at multiple scales: automated unbiased analysis pipelines paired with (i) confocal provides analysis of tissue scale efficiency of CME, (ii) total internal reflection fluorescence microscopy (TIRF-M) and structured illumination microscopy (SIM) provide temporal dynamics
and precise localisations of proteins at live single events, (iii) scanning electron microscope (SEM) and scanning transmission electron microscopy (STEM) tomography of metal replicated unroofed plant cells provide the high-throughput and 3D analysis of endocytic events at the ultrastructural resolutions (Fig. 1). Using these tools, we have been able to significantly update our working model of plant CME and uncover that plant endocytosis is evolutionarily unique from other eukaryotic model systems. For example, we found that despite the high turgor in plants, plant CME is independent of actin [1], and that it is in fact a plant-specific complex (absent in yeast and mammals) that mediates endocytic membrane bending [2]. To aid the progression of plant CME investigation, and provide the community with a standardised toolkit which allows the direct comparison of data generated from different labs, I have published the full protocols, analysis codes and example data for these techniques as open access resource publications [3, 4]. I am honored to say that the judges presented me with the award for this work (Fig.2)!
I would like to again express my gratitude to those who have supported me throughout my career. I
have been extremely lucky that people have trusted in my research vision, and I’ve found willing and amazingly talented interdisciplinary collaborators to enable them. Special mentions include Dr. Christien Merrifield (CNRS, FR), Dr. Grégory Vert (CNRS, FR) and Prof. Jiří Friml (Institute of Science and Technology Austria).
1. Narasimhan, M., et al., Evolutionarily unique mechanistic framework of clathrin-mediated endocytosis in plants. Elife, 2020. 9
2. Johnson, A., et al., The TPLATE complex mediates membrane bending during plant clathrin-mediated endocytosis. Proc Natl Acad Sci U S A, 2021. 118(51).
3. Johnson, A., et al., Experimental toolbox for quantitative evaluation of clathrin-mediated endocytosis in the plant model Arabidopsis. J Cell Sci, 2020. 133(15).
4. Johnson, A., et al., Three-dimensional visualization of planta clathrin-coated vesicles at ultrastructural resolution. Mol Plant, 2022. 15(10): p. 15331542.
For general enquiries email info@rms.org.uk For information about meetings and courses email events@rms.org.uk For membership enquiries email membership@rms.org.uk www.rms.org.uk
I’m a third-year PhD student at Oxford Brookes University on the Oxford Interdisciplinary Bioscience Doctoral Training Programme, and my internship at the RMS involved coordinating the design and delivery of the free-to-attend Learning Zone at Microscience Microscopy Congress 2023 (mmc2023), 4-6th July 2023.
The aim of the Learning Zone is to provide core microscopy knowledge and provide early career researchers with advice and tools to help them progress. Working alongside Andrew Scott, the design process involved planning of audio/visual, power, signage, and furniture requirements. It also involved liaising with industry to arrange sponsorship through the loan of microscopes that were used in the Learning Zone for demonstrations. It was a great experience to communicate with industry and get that experience, as it can be hard to make those connections working in an academic lab. It was a great insight into how industry works!
I worked alongside Kerry Thompson and the Outreach and Education Committee to create and deliver a programme of 25 lectures. These were based on three themes: 1) Career and Professional
Development, 2) Open-source hardware and software and 3) Techniques, image analysis and practice. Two workshops were delivered around the theme of open-source hardware and software. The first workshop was an introduction to image analysis in three popular free platforms: FIJI, napari and Jupyter notebooks. The second workshop showcased five open-source hardware projects in microscopy which included EnderScope, UC2 and OpenFlexure. It also included a demonstration from the Natural History Museum about the use of 3D printed models and ‘Museum in a box’ in outreach and engagement for the visually impaired. Both workshops on open-source software and hardware had a record attendance for the Learning Zone. This was a great insight into how conference programmes are put together! I also liaised with the Historical Committee to showcase their collection of rare historical McCormick collection of microscopes and slides on the Learning Zone.
I thoroughly enjoyed my internship at the RMS and have learnt so many invaluable skills – I was given so many opportunities to develop and learn. Being able to attend mmc2023 and see the delivery of the Learning Zone was very rewarding. It was great to interact with so many different people and disciplines and I will miss the teamwork at the RMS!


Dear Readers,
It is my great pleasure to be writing my first piece for infocus Magazine as RMS President. It is a tremendous honour and a privilege to have been chosen to lead this wonderful Society, and I feel humbled that my colleagues on the RMS Council have put their faith in me. I would also like to pay tribute to my predecessor, Professor Grace Burke, who led the society so well for four years and, I am glad to say, will continue to provide her support and wisdom as Vice President. I hope to build on everything that has been achieved under Grace’s leadership – and all others who have held this privileged position before me.
For those who don’t know me, I have a broad background in microscopy – my lab at The University
of York employs both light and electron microscopy, as well as an array of flow cytometers and other assorted instruments. I have been involved in microscopy and cytometry since the start of my PhD (I won’t say how long ago!), and while my current lab deals mostly with life sciences samples, we still have a significant number of materials projects running through it. I have been involved with the RMS since the early ‘Noughties’ – serving as a science section Committee Member, then as Committee Chair, and then as part of the Society’s Executive Committee.
While it is still so fresh in the memory, I couldn’t possibly write this entry without commenting on the tremendous success of mmc2023, which saw the biggest attendance in its history. It was great to

catch up with so many friends and colleagues and to enjoy all the amazing science on show – in both the conference rooms and exhibition hall. I would like to pay special tribute to the efforts of the Scientific Organisers and RMS Office Staff, who pulled out all the stops to put on the biggest – and perhaps best ever – mmc.
Since taking on the Presidency, I have been thinking a lot about the bigger picture where microscopy is concerned, and how important it is to the world. It strikes me that we can sometimes lose sight of this when we are so focused on our own particular areas within academia or industry. But the fact is that almost everything around us has, at some point, gone under a microscope. From the jet engines on our aeroplanes that fly us safely, to the medicines we are prescribed; from ensuring the quality of our water to the food we eat - microscopy is involved somewhere along the line.
This is why an international organisation such as the RMS, and its role in bringing scientific communities together, is so important. Science cannot – and does not – operate in a vacuum. Collaborations must be fostered; knowledge must be harnessed and disseminated. I wonder how many crucial conversations have taken place at RMS meetings and conferences over the years for instance? Or put another way, how many new discoveries and technological advances might not have materialised without such opportunities to engage and share? I don’t think it is overstating the case to say that the work we do here at the RMS is, in its own way, profoundly important in helping the world move forward.
Speaking of RMS meetings and other events, a number of other highly successful events have taken place this summer, including our Light Microscopy and Confocal Courses, both returning for the first time since Covid, with excellent numbers and great feedback. Hot on the heels of mmc2023 was our Super-Resolution Workshop, which was superbly well attended – showing the growth of interest in this ever-developing area.
On the publications front, the Journal of Microscopy
is very much on the ‘up’, with an improved ‘impact factor’. This is a great endorsement of the work of the Journal Editors, and those who have submitted, reviewed and otherwise contributed to its increasing success. You can read more about the Journal on page 52.
Looking ahead, it is really exciting to see our programme is full of different activities – ranging from our annual courses and meetings such as the Flow Cytometry Course in early September, to the new and more niche areas such as Adhesion and Migration Meeting, taking place in Warwick. In addition, the RMS continues to offer its assistance in hosting events for others – including corporate members. One such event – a webinar hosted on behalf of Zeiss – is taking place in late September. We will be looking to take on more of these events, while continuing to support other initiatives – such as FlowCytometryUK, which we are preparing to host in November.
Before signing off, I would like to pay special tribute to our long-standing Chief Executive and RMS staff member Allison Winton, who is retiring after almost 40 years at the Society. As you will read elsewhere in this issue, Allison’s knowledge of the RMS and its activities over the years is second to none, and she has certainly been an invaluable mentor for me personally, throughout my time here.
While we will miss Allison greatly, it is – more importantly - an exciting time for her, as she embarks on a new chapter in her life, and I look forward to regular updates on her activities!
It is also an exciting moment for the RMS, as we welcome our new Chief Executive, Sali Davis. It was great to get to know Sali at mmc2023, and her obvious enthusiasm for the science world. Sali has a wealth of knowledge and experience within the charitable sector, and I’m really looking forward to working with her. I am sure she will provide great stewardship for the Society as we move forward.
My very best wishes to you all.
Dr Peter O’Toole, RMS PresidentThe RMS is delighted to welcome Dr Peter O’Toole as the Society’s new President, following its Annual General Meeting at mmc2023 in Manchester.
Peter, who is Director of the Technology Facility and heads the Imaging and Cytometry Labs at the University of York, takes over the reins from outgoing President Professor Grace Burke, who has led the Society since 2019.
Peter, who was previously RMS Vice President, has a long and distinguished association with the Society. Among his many contributions to RMS activities, he is perhaps best known for his extensive involvement with teaching microscopy and flow cytometry - including both the RMS Light Microscopy Summer School and the RMS Practical Flow Cytometry courses - as well as being one of the pioneers of the influential Light Microscopy and Flow Cytometry Facilities Meetings that have helped these communities come together.
In his role at York, Peter oversees both the Imaging and Cytometry labs, featuring an array of confocal microscopes, flow cytometers and electron microscopes. He collaborates with many leading microscopy and cytometry companies, and his group also provides research support and consultancy to many academics and commercial organisations. His research is currently focused on both technology and method development of novel probes and imaging modalities.
Peter said: “I have always been immensely proud to be a part of this wonderful Society, and it is a huge honour to serve as RMS President. I look forward to working alongside all our members, volunteers, and staff, as we look to continue to evolve and develop further to continue supporting the science of microscopy, imaging, analysis and flow cytometry communities.”
He added: “I am fortunate to be following on from Grace Burke, who has shown great leadership and wisdom in guiding the RMS over these last few years – especially as these have been a particularly challenging period for the scientific community during the Covid pandemic. Following Grace is no easy task, but I am thankful that she will remain as a Vice-President to help support.”
Grace, who is Laboratory Fellow at Idaho National Laboratory, USA, and Professor EmeritusUniversity of Manchester said: “It has been a great honour to serve as RMS President and I would like to thank everyone at the Society for their incredible support over the past four years. I have truly enjoyed my time in the role, and take great pride in everything we have achieved – and continue to achieve - as an international Society (founded in 1839!) in support of all forms of microscopy and related techniques.”
She added: “I am delighted to pass on the baton to Peter O’Toole, who I have come to know well as a colleague and friend. Pete is a great asset to the RMS and the wider microscopy community; the Society is undoubtedly in safe hands under his leadership.”

Rob has led the Atomic Force Microscopy (AFM) facility at the University of Bristol’s School of Chemistry for the last 11 years, making it a highly respected hub for the technique. He has driven AFM as an interdisciplinary platform, collaborating across the Schools of Chemistry, Physics, Earth Sciences, Medical Sciences, and the Faculties of Life Sciences and Engineering.
Rob is a well-known and respected expert within the University and beyond, as a pointof-reference for AFM. He is renowned for his application of advanced force microscopy skills, his innovations, original project ideas and his thoroughly positive, ‘can-do’ spirit. His collaborations have resulted in over 80 publications, with 17 in Science and Nature journals, with an h-index of 32.
Rob has steered the addition of new capabilities within the AFM facility to broaden the relevance of the technique, and secured funding for a range of new projects and interdisciplinary collaborations, both local and international.

In addition to his extremely rich portfolio of skills in force microscopy, Rob has also been heavily involved in the development of new instrumentation. This has been influential in several areas including photothermal and photoradiative cantilever drives, and perhaps most significantly, through vertically-oriented probe (VOP) force microscopy. Rob designed and built the first high-speed noncontact VOP microscope, achieving remarkable
resolution imaging molecular ultra-structure. The unique balance of sensitivity and control in VOP microscopy was also essential in the world-first direct measurement of the spin-momentum of photons, a fundamental quantum force. Rob’s contributions were vital in the adaptation of vertical probe microscopy to measure this effect and prove the physical existence of the Belinfante momentum.
Rob’s experience across a range of fields at the nanoscale has not only enabled him to invent new techniques in applications such as the nano-templating of diamond, but also enriches the teaching environment at the University. In the Bristol Centre for Functional Nanomaterials, he has developed and supervised numerous interdisciplinary Masters projects where AFM forms the core in technological development or characterisation of unique materials. During the Covid lockdown, he developed innovative datamining projects for Masters students, with such success that these projects were successful to the point they are now a regular feature in the School syllabus.
Former RMS Vice President Susan Anderson said: “Rob’s achievements are seriously impressive, and he continues to go above and beyond his technical role in the AFM Facility at Bristol.
Through the application of his skills, innovations and collaborations, he is enriching both the learning of others, and the field in general. He is a highly deserving recipient of the Vice President's Award.”
The Royal Microscopical Society (RMS) is very pleased to announce the appointment of Sali Davis as its new Chief Executive, following the retirement of Allison Winton – who has served almost 40 years at the Society.
Sali, who comes from a legal background, joins the RMS having spent 14 years as the Chief Executive of Optometry Wales, a membership body representing all primary care Optometrists in Wales. Prior to this,
she worked for Deloitte and Touche LLP, running a practice review programme for Tax Partners on both a local and national basis. She has also worked as a Project Officer for the Royal College of GPs. Sali is a lay panel member for Social Care Wales sitting on Fitness to Practise panels for registrants in the Social Care profession. She is also a ‘Mentor Mum’, coaching new mothers who are returning to the workplace following maternity leave.

Sali said: “I am so excited to take on this new challenge, and to be joining such a highly respected international Society with a rich and unique history. Under Allison’s leadership the RMS has strengthened its reputation for the delivery of high quality events, publications and Outreach and Education activities. I hope to ensure her legacy is preserved, and to build on everything that has been achieved.”
She added: “Having attended mmc2023, I was able to see what a huge role the RMS plays on the international stage and how crucial it is to the scientific communities. Within the first few months of my role, I will be spending time immersing myself in the world of microscopy, working with our dedicated team and identifying opportunities for greater collaboration with stakeholders across the international stage and looking at what opportunities there are to really promote the work that we do.
“I am very much looking forward to meeting all committee chairs, attending committee meetings and first and foremost, supporting the team at RMS to help them do what they do best – serving our membership to the best of our combined abilities.”
Allison, who joined the RMS in 1984 as Secretary to the Administrator, is retiring having held the Chief Executive post since 2017, and the position of Events Director for five years prior to that.
During her decades of service, Allison has performed a wide variety of roles – from publications advertising and sponsorship, to organising outreach initiatives, exhibitions, courses and conferences. She has overseen the

delivery of countless major events, and has worked with no fewer than 18 different RMS Presidents. She said: “The RMS is a brilliant organisation and I feel very fortunate to have been a part of it for so many years. It is a bittersweet moment for me, but I feel the time is right to step down and move onto the next chapter in my life. Over the years I have worked with some amazing people and I would like to thank everyone involved at the RMS – past and present – for making this such a wonderful journey. I would also like to wish Sali every best wish for the future, and I hope that she will enjoy working for the Society as much as I have – it is such a special organisation, and I am sure it is in very good hands under her leadership.”
RMS President Dr Peter O’Toole said: “Allison has been an incredible support, ambassador and leader for the RMS for so many years. She has also been a great mentor for me personally – in terms of learning about the Society and how to navigate the charitable world. We will all miss her greatly, but it is also an exciting time for Allison, as she opens a new chapter in her life, and we wish her every happiness.
“This also opens up an exciting new chapter for the RMS, and I am delighted to welcome Sali Davis as our new Chief Executive. Sali has a vast amount of experience within the charitable sector and great enthusiasm for the scientific world. I’m really looking forward to working alongside her and the rest of the RMS team as we move forward together.”
The RMS was delighted to announce a surprise Honorary Fellowship for Professor Grace Burke at mmc2023.

Grace, who recently stepped down as RMS President having led the Society since 2019, learned the news during the RMS Annual General Meeting which took place at the event.
Grace has made seminal contributions to microscopy and Materials Science throughout her remarkable career, which has included long periods in both industry and academia, in the US and UK.
She took her Undergraduate degree in Metallurgical Engineering at the University of Pittsburgh in the USA, and then travelled to the UK to undertake a PhD in Metallurgy at Imperial College London
with Peter Swann (founder of the company Gatan), and subsequently F. J. (John) Humphreys, on the mechanism of stress corrosion cracking in austenitic stainless steel. Here, she pioneered the use of energy dispersive X-ray analysis using scanning transmission electron microscopy (TEM) to understand microstructural and microchemical changes that affect environment-sensitive behaviour of austenitic stainless steel.
After returning Stateside, Grace joined the US Steel Research Laboratory, where she worked in corrosion, then in physical metallurgy/ thermomechanical processing of steels, and led the use of analytical S/TEM. It was during this time that she became involved with Atom Probe FieldIon Microscopy to study a variety of precipitation/ segregation phenomena, eventually starting her research into irradiation embrittlement of nuclear reactor pressure vessel steels.
Following the demise of basic research at US Steel, Grace continued this research as a member of the Research faculty at the University of Pittsburgh, where she continued to work in irradiation damage and thermomechanical processing of steels. She moved to the Westinghouse Science and Technology Centre in 1987, and continued to work on a broad range of materials, including structural materials used in light water reactors. Grace eventually transferred to the (Westinghouse) Bettis Atomic Power Laboratory in Pittsburgh, where she continued her research into materials for nuclear power systems.
She became President of the Microscopy Society of America (MSA) in 2005, and in 2011, following her nearly 30-year career in the US nuclear industry, she accepted a position at the University of Manchester as Professor and Director of the Materials Performance Centre, where she also was appointed Director of the Electron Microscopy Centre (20122016).
Grace became RMS President in 2019, and is the only person to have served as President for both the RMS and MSA. In 2018 Grace received the President's Award of the MicroAnalysis Society, and in 2020 she won the Henry Clifton Sorby Award of the International Metallographic Society / ASM International. Most recently, in February 2023, Grace received the Charles S. Barrett Silver Medal of ASM International and presented the Barrett Lecture in Golden CO at the Rocky Mountain ASM Chapter.


Despite long periods in industry, Grace has over 250 publications, all of which have utilised advanced microscopy methods to elucidate key microstructureproperty-performance relationships, particularly in metals. In addition, she has made seminal contributions towards the correlation of Atom Probe Field-Ion Microscopy with analytical transmission electron microscopy for structural materials, and the use of in-situ microscopy methods for studying degradation phenomena in engineering materials.
The RMS staff team has said goodbye to two PhD students who have been carrying out internship projects as part of their courses.
We would like to say a huge ‘thank you’ to Joanna Cull of Oxford Brookes University, and Zoe Barr, of the University of St Andrews.
Jo co-ordinated preparations for the RMS Learning Zone at mmc2023 and was instrumental to its success during the Congress (read
more on page 69).
Meanwhile Zoe has written an impact report to gather the success stories of the Hitachi SEM Outreach scheme –an RMS-backed initiative enabling schoolchildren to carry out projects with a portable electron microscope. You can read more about Zoe’s work
and the project itself in our next issue of infocus!
We would like to wish both Jo and Zoe all the best in their future studies and careers.

Rafal is currently Co-Director of the Ernst RuskaCentre for Microscopy and Spectroscopy with Electrons (ER-C) at Forschungszentrum Jülich, which has come to be regarded as one of the world’s leading centres for electron microscopy research. He has facilitated many successful research proposals involving national and international collaborations and, together with his colleagues Joachim Mayer and Carsten Sachse, is leading the development of several unique instruments that combine highly sought-after sample handling and imaging capabilities.
The RMS is delighted to announce Professor Rafal E. Dunin-Borkowski as an RMS Honorary Fellow.
Rafal is universally-recognised as a worldleading electron microscopist, who has made major contributions to a wide range of electron microscopy techniques and applications.
Having obtained his M.A. in physics and PhD in materials science at the University of Cambridge, Rafal held postdoctoral and research scientist appointments in Cambridge, Arizona State University and Oxford, and a Royal Society University Research Fellowship at Cambridge, before his appointment as Founding Director of the Center for Nanoscopy at the Technical University of Denmark.
In the latter position, Rafal had responsibility for the design, construction and commissioning of the microscope building and its suite of state-of-the-art instruments, which included a leading facility for environmental TEM, and he supervised a large staff of research scientists and technicians to support its operation.
Rafal has made ground-breaking personal contributions to several fields involving quantitative electron microscopy, including electron holography of magnetic and electric fields, electron tomography, aberration-corrected electron microscopy, and instrumentation development. His research has impacted the fields of chemistry, geology and biology, as well as his core disciplines of physics and materials science.
Rafal is the author/co-author of over 500 peerreviewed publications, and his published works have been cited more than 22,000 times, with a Hirsch (‘h’) factor of 72. He has also authored/co-authored 27 book chapters and numerous review papers. Rafal is widely sought after as an invited speaker at many national and international conferences, and he continues to provide commendable service on important national and international committees and advisory boards across the globe.
RMS Vice President, Professor Grace Burke said: “Rafal is without doubt a world-leading electron microscopist who has not only made a series of groundbreaking personal contributions across multiple fields throughout his career, but also driven scientific discovery and innovation through his remarkable vision and inspiring leadership qualities. It is my very great pleasure to recognise him with our highest award - Honorary Fellowship of the RMS.”
The RMS is delighted to announce Jeremy Sanderson as a recipient of the RMS President’s Award – in recognition of his exceptional contribution to the work of the Society.

Jeremy, who received his award at mmc2023, is a well-known and highly respected member of the national and international microscopy communities. Over the past 35 years he has contributed directly and indirectly to the work of the RMS in many different ways.
Jeremy joined the RMS in 1988 and has been an actively engaged member of the Society ever since. His talent for microscopy was first recognised in 1991 when his Tech RMS project (a precursor to the RMS Diploma) was awarded a Student Medal in recognition of his outstanding examination paper and viva.
In 1994 he published his first microscopy handbook through the RMS (Biological Microtechnique), which focused on histological sample preparation for the microscopy enthusiast. More recently he has published ‘Understanding Light Microscopy’ (RMS/ Wiley 2019), a substantial addition to the literature of unusual breadth and depth.
In the early 1990s Jeremy began teaching the principles of light microscopy through the RMS LM
summer school in Leeds, and later York. He also became a stalwart of the confocal microscopy part of the Learning Zone at MMC, an essential feature of this flagship RMS event. Jeremy served on the technology-focused LM Section of the RMS from 2006-2009, and his dedication and support for the Society were further recognised by his appointment as a Trustee on the governing Council of the RMS from 2009 – 2012.
As a ‘lover of the microscope’, Jeremy embodies the spirit of the RMS in his professional life. He has worked as a histologist with the NHS in Oxford, as a core facility technician at the MPI-CBG in Dresden, and has directed light microscopy facilities at the University of Sheffield and the MRC unit Harwell.
RMS President Grace Burke said: “Over the years, Jeremy has dedicated a huge amount of his time and expertise to a wide range of RMS activities, especially through his invaluable teaching and writing on the subject of light microscopy. He is an enormously popular figure within the microscopy community –renowned both for the seriousness and dedication he brings to his work, and the lightness and good humour he shares with colleagues. It is a great pleasure to announce Jeremy as recipient of the RMS President’s Award.”
of Materials at the University of Oxford, UK, and in the Department of Applied and Engineering Physics at Cornell University, NY, where he contributed to the development of electron spectroscopy for the nanoscale characterisation of materials.
He subsequently moved to the National Institutes of Health (NIH) to develop methods based on scanning transmission electron microscopy and electron spectroscopy to determine the structure and chemical composition of cells and supramolecular assemblies. He was elected a Fellow of the Microscopy Society of America in 2011.

Richard has acted as a Scientific Editor of the Journal of Microscopy for many years, a member of the editorial boards of other microscopy and nanotechnology journals and has served on numerous national scientific advisory committees.
Congratulations to Dr Richard Leapman, who has also received the RMS President’s Award, in recognition of his outstanding contributions as a Scientific Editor for the Journal of Microscopy.
Richard, who is stepping down from the Journal of Microscopy Editorial Board having joined in 1999, received the award at the Microscopy and Microanalysis (M&M) 2023 event in Minneapolis.
Richard obtained B.A and M.A in Natural Sciences and a PhD from the University of Cambridge. He then trained as a postdoctoral fellow in the Department
RMS Executive Honorary Secretary and Journal of Microscopy Editor Michelle Peckham said: “For so many years, Richard has been a key member of the Journal team and contributed to the publication’s ongoing success. In addition to his work as a Scientific Editor, he has attended numerous meetings to promote the Journal – notably the Microscopy & Microanalysis events held in the United States. On behalf of the RMS, I would like to thank Richard for his hard work over the years and wish him well for the future.”

The UltraMicroscope Blaze is the only fully automated light sheet microscope for imaging large or multiple cleared samples at subcellular resolution. Our pioneering UltraMicroscope technology combined with cutting-edge light sheet optics ensures excellent data quality.

u miltenyibiotec.com
Miltenyi Biotec Ltd. | Almac House, Church Lane | Bisley, Surrey GU24 9DR | UK | Phone +44 1483 799 800 | Fax +44 1483 799 811 | macsuk@miltenyi.com | www.miltenyibiotec.com
Miltenyi Biotec provides products and services worldwide. Visit www.miltenyibiotec.com/local to find your nearest Miltenyi Biotec contact.
Unless otherwise specifically indicated, Miltenyi Biotec products and services are for research use only and not for therapeutic or diagnostic use. MACS and the Miltenyi Biotec logo are registered trademarks or trademarks of Miltenyi Biotec and/ or its affiliates in various countries worldwide. Copyright © 2020 Miltenyi Biotec and/ or its affiliates. All rights reserved.
It hardly seems possible, but RMS Chief Executive Allison Winton is retiring after more than 39 years of service at the Society. For so long the ‘face’ of the RMS, and having worked in numerous different roles over the decades, Allison has an unparalleled working knowledge of the Society, its achievements, and the personalities that have driven it forward throughout its recent history.
H M ex in Pe
ere she reflects on her remarkable career: y RMS story starts with two weeks of work perience at the Society during the Easter Holidays 1984. I was studying for an RSA Diploma for rsonal Assistants at Abingdon College of Further

Education, and the Head of Year suggested that I was exactly the ‘sort of person’ that would fit in well at the RMS (as Karen Collins was the following year!).

I arrived at the St Clements offices with the scruffy red door, and was introduced to Colonel Fleming and Ben the elderly Old
Job offer letter 1984: Allison still has the original copy of the job offer letter she received from RM Administrator Colonel Fleming in 1984.
tasks, and helped out at registration for the Microscopy of Catalysis Meeting which I think took place at the Department of Engineering at the University of Oxford, organised by Ed Boyes. I must have done okay during my work experience, because there was a vacancy for ‘Secretary to the Administrator’ and I was asked if I would like that role, subject to the agreement of the Executive Honorary Secretary, Gillian Bullock.
My first proper day was 1 July 1984, the week before MICRO 84, which was held at the Bloomsbury Crest Hotel and the School of Pharmacy in London. In the office there were no computers, emails or faxes, just electric typewriters! We were fully reliant on the post, of which we had two deliveries a day. I was told that on opening the post, never have your legs under the desk, in case of any letter or parcel bombs being sent to the RMS! The committee structure was very similar to how it is now, though the Flow Cytometry, AFM & SPM, DAIM and Early Career committees did not exist. The committee members all looked much older than they do now, but that may just be because I was so young then! We ran a full programme of events, the LM and EM Schools and other courses and conferences, plus a five-day MICRO Conference and Exhibition every other year.

Over the next few years, my knowledge of the Society grew, and I also became involved in the advertising, and assisting Peter Evennett with the production, of the Proceedings. Back in 1984 there were just eight full-time members of staff, plus a part-time bookkeeper. The office environment was much more formal then, with set routines – start at 9 am, coffee at 11 am, lunch at 1 pm, tea at 3 pm, and depart at 5 pm, and all instructions for events were written like military plans! We always referred to Colonel Fleming as Colonel Fleming, and Mr Baker, the retired bank manager who only worked on Wednesday, referred to each other in this formal style too. Colonel Fleming was very protective and supportive of all the staff in the office, and all members of RMS staff knew that if we ever needed help with anything he would be there for us.
In July 1988, just before the EUREM 88 event in York, Colonel Fleming left the Society. It was quite a tumultuous time for the Society, which led to contested elections to Council later that year. During this time, Kate Wooding (nee Wellby) and Dawn Hopkins (nee Hague) both left the Society to further their careers, only to return in 2015 to cover periods of maternity leave, and they are still working at the Society to this day. Judy, Karen and I kept the Society running from July to November 1988.

I was promoted to Exhibition Organiser in the

summer of 1988, and involved in the final stages of the EUREM 88 Exhibition at the University of York. This was a baptism of fire with an exhibition in a marquee on scaffolding, constructed over steps next


to the lake! To this day I remember hiring in heaters to dry the air in the exhibition after a particularly wet and windy typical August bank holiday weekend, to hopefully make it a better and less damp environment for setting up the microscopes. The walls of the marquee flapped in the wind, and the floor was a temporary structure made up of bouncy floorboards – a real challenge for setting up SEMs! For those who required liquid nitrogen, we just wheeled cannisters round the lake to the

exhibition on sack trollies – health and safety would not be impressed now! EUREM 88 was a very busy event with all accommodation in the colleges on campus, and a concert at the Minster followed by a reception at the racecourse. Prior to the event, on the abstract deadline date, we had four sacks of post delivered, which all contained abstracts in hard backed envelopes, which took about two days to open and catalogue! The congress dinner (to which the RMS staff were invited as guests) was at Castle Howard – this consisted of a very slow walk in a queue through various rooms, with drinks given to you in each room, but no food until the very end room! I do remember having to wake some attendees up who had fallen asleep in various places around the venue, just so that they could get the buses back to the University!
During the Autumn of 1988, although I was now working on the exhibitions and Proceedings advertising, I still attended all the committee meetings and took minutes. This gave me a good overall view of how the Society operated, which has been really useful to this day. A review into how the RMS was run was conducted by our auditors
at that time. They didn’t really have anything to add other than suggesting that the office needn’t be in Oxford, but could be anywhere in the UK! At the end of 1988, Paul Hirst was appointed as the new Administrator, and he used to refer to me as the “fount of all knowledge”! We were then busy working on the 150th Anniversary of the Societywe were also involved in the organisation of a sixmonth exhibition at the Science Museum in London (we ran a number of evening receptions where we used to serve cheese and chicken legs, and wine, of course, which Brian Bracegirdle used to buy earlier in the day and store in his office until we arrived!), a two-day meeting at the Royal Institution, followed by EMAG-MICRO 89 at the Royal National Hotel in Bloomsbury, with the conference sessions across the road at the Institute of Child Health. The Conference Dinner was at The Brewery in East London. The EMAG-MICRO exhibition was ‘an experience’, being situated in two large rooms, a marquee and along numerous corridors at the


Royal National Hotel, very unlike the professional exhibition venues we use for mmc nowadays. The organisation of the 150th anniversary year was an amazing experience to be involved in, and it was good to witness the Royal Mail RMS postage stamps being launched, as well as seeing Peter Evennett and

Dick Paden on the TV programme Blue Peter with microscopes on display. Producing the souvenir catalogue of the Science Museum Exhibition was a challenge, but fun too, with almost daily phone calls with Peter Evennett, and some of those at 7am in the morning in the office, when he was on holiday in Norway with his family. Soon after this we had our very first fax machine! It was very exciting to be able to fax a proof to someone in say, Australia, and have a reply on my return to the office the next day!
I was then involved in the organisation of MICROs at a variety of London hotels between 1990 – 2000. The Bloomsbury Crest Hotel was no longer suitable, so we tried the London West Hotel in Lillie Road as well as the Novotel in Hammersmith. I was talking about the Novotel experience with a number of exhibitors at mmc2023, and we all remembered it being like a multi-storey car park, with enormous pillars! I remember one year turning up to find that they had done some building work between me selling exhibition space and the day of the event. When one exhibitor was due to arrive to set up their stand, we had discovered that it was no longer possible to put their stand there, as the hotel had done some building work, reducing this area of exhibition space! We managed to find a solution
which the exhibitor was happy with, but that wasn’t a great experience to start the exhibition build with. During my time as Exhibition Organiser I worked very hard at developing a good working relationship with all the exhibiting companies, and understanding their needs and requirements. The Corporate Advisory Board (formerly the Trade Advisory Committee) was a great place to develop this relationship. I also started to attend other exhibitions as an exhibitor, which gave me a good insight into what it’s like to be an exhibitor too –e.g. 15 minutes for exhibition time during a one-day meeting should definitely NOT be done!

The Novotel in Hammersmith was becoming too small for MICRO, and at MICRO 2000 we had a waiting list of approximately 20 companies! It was time to find a new venue, and I was very much involved in the planning and move to ExCeL for the first MicroScience event in 2002. MicroScience was a new name, but still keeping the MICRO. We went for a new layout, having the exhibition in a diamond layout – this worked in that you nearly always walked different ways through the exhibition, but many people complained about getting lost! Moving to ExCeL brought us many new challenges; we had to order and pay for so many more things than we had at the London hotels, e.g. carpets in the exhibition, but this was a really exciting project to be involved with.

Paul retired in 2005, as did Judy Lewis (then Deputy Administrator) and Rob Flavin was recruited as the Executive Director. Rob came to the Society with many fresh and new ideas, and made many changes to the fabric of the office building. He and I went to Sapporo in September in 2006 to assist Debbie Stokes and John Hutchison with the bid to run the International Microscopy Congress (IMC) at ExCeL in September 2010. We were unlucky and came second to Brazil, but it was a tremendous opportunity to visit Japan and see an International
joining Rod Shipley (then at FEI) at crab restaurants, and going out with Pete Lander (then at JEOL) and others to a beer party followed by a karaoke evening – those who were there will remember it well, and I still have the photos!! Following on from going to IMC in 2006, I started to go to many other international meetings, and these included many M&M meetings in the US, Neuroscience in the US, EMC in Aachen and Lyon and IMC in Prague and Sydney. Such great opportunities to meet current and potential new members and exhibitors, and


International Society! 2007 was also our first involvement in the elmi meeting, which we ran in York with Peter O’Toole. Elmi is a very different event for us and finding workshop rooms is a challenge, but a good one! Since then we have organised elmi in Leuven and Dublin, a virtual elmi in 2022, and the RMS will be organising the 2024 elmi in Liverpool.
Rob left the Society in 2009 and started his own promotion company, CooperRepCo, and continued working with the Society. I was then promoted to Event Director, attending all committees and overseeing all RMS Events, with Karen Collins becoming the Administrator, and we were a great team, working together on overseeing the various aspects of the Society.
Our next challenge was emc2012! During emc2008 in Aachen, we had successfully won the bid to host emc2012, on behalf of the European Microscopy Society (EMS), in London in September 2012. We


had originally thought it would be great to follow on after the Olympics, but unfortunately the Olympics had tenancy agreements in place for all London venues until the end of September 2012, with no flexibility, even though the venues would not be used for our dates as the Olympics would be over by then. This started us on a mission to find an alternative venue, and I contacted all potential venues around the UK. Manchester Central could offer us the dates we needed, and on paper, it looked like they could offer us the space we needed as well. So Karen and I, accompanied

by Les Stump (the Honorary Treasurer) visited the venue and realised that it could be ideal! We then visited again with Debbie Stokes (emc2012 Chair) and representatives of the EMS, who all agreed. The whole office team then organised the whole of the emc2012 congress in Manchester, with the Congress Banquet at Manchester United, Old Trafford. It was an amazing event to be involved in, and to see it all run so successfully. I was in Manchester for 10 days before, during and at emc2012, and managed just four hours of sleep each night, as the adrenaline and stress involved in such a large event kept me awake. It was all worth it though!
After emc2012 had taken place, we realised that ExCeL didn’t really provide us with suitable lecture theatres close enough to the exhibition, and we realised that this was something we had at Manchester Central. After Council and CAB approval of moving to Manchester Central, we moved mmc (a new name from Microscience, but short for Microscience Microscopy Congress) to Manchester for mmc2014. This went well, and we then had further discussions about moving our congress to ‘odd’ years to avoid the EMC and IMC International Congresses, and talked to EMAG about joining us at mmc – this was all agreed, and the first mmc incorporating EMAG took place in Manchester in 2015. This then started the pattern of mmc every other year, during ‘odd’ years, and the successful organisation of mmc2017 and mmc2019. We’ve had many experiences at the Manchester event, with the staff at the venue being really helpful and friendly. I only had one instance where I had to complain and that was during the mmc2017 exhibition breakdown, where traffic would not let any of the exhibitors through to the loading bay when the exhibition closed. I went out to find out what was happening, and was told that traffic were waiting to hear from the organisers that they could let people through – that was the only time that I said in a firm voice, “I am the organiser – let them through!”

During 2016 Karen (Collins) decided to take early

retirement at the end of the year. I had discussions with Karen and members of the Executive Committee, and after giving a presentation to the Executive Committee with my thoughts and plans for the future, I was promoted to the position of Chief Executive from 1 January 2017. I really missed working with Karen, but fortunately Tor (Masters) and Adam (Clay) were appointed as Event and Finance Directors at the beginning of 2017, and we have developed excellent working relationships of support, bouncing ideas of each other, and delivering the very best we can for the Society. The first two years of being Chief Executive went well, with much of this time focussed on being the Professional Congress Organiser (PCO) for emc2020 in Copenhagen. This was a very busy time as we still had mmc2019 taking place, and we took on two additional members of staff to work solely on emc2020. All was looking great, with all plans coming together, and we held an exhibitor site visit at the Bella Center in Copenhagen in February 2020. We were hearing about Covid at that time, but were still planning with Klaus Qvortrup and the rest of the organising committee, for emc2020 to go ahead in August 2020. Then Covid started to affect us all…….
During the first national UK Lockdown, we went from being in the office on the Monday to everyone working remotely from the Wednesday. Fortunately, all our IT could handle this, and we just changed our working patterns to suit, with lots of Teams meetings etc. We were still hopeful that emc2020 would be able to go ahead, but were starting to worry that we would not get the attendance needed…We were unable to cancel the event because of the financial implications, but fortunately the Danish government decreed that no event with more than 1,000 people could take place until the September, so our financial loss was less than it could have been. It was so disappointing for everyone concerned, but we had no option. We did, however, run a virtual emc2020 student congress in the November which was well received. Early on in the pandemic we
realised that we would not be able to run any inperson events or committee meetings, and swiftly moved forward with Zoom committee meetings, as well as developing various online meetings, and ways of communication and keeping the community together. I would like to thank both Tor and Adam for developing these virtual meetings so well. During the height of the pandemic most of the RMS Staff were furloughed, and during this time, just Tor, Adam, Jill and I continued working. It was a strange time indeed, but great to develop the virtual meetings, which we continue to this day, but now we run a mix of different types of meetings – in-person, virtual and hybrid.
There was a gradual return to working in the office but there is more working from home now, as we all realise the benefits of not sitting in heavy traffic every day! We do still like to be in the office though, working with the rest of the brilliant RMS Team!

During my time at the RMS I have seen many changes in technology! As I mentioned earlier, when I first
correspondence (peach for the alphabetical file, white for the date file and green for the event file). Badges were made using a machine where we had to turn a wheel to print each letter of someone’s name and affiliation (not quite the mail merges we have today) which was very time consuming. These names then had to have the back bit pulled off and the name stuck on a paper badge, taking care to attach names in a straight line! Bas Ploem became involved with our first foray into computers, helping us to develop Lex, the first RMS Database. Bas spent many hours in the office, and always worked to the wire to get as much done before walking along St Clements to the bus stop to catch the bus to the airport! He did miss the bus a couple of times. Karen and I spent a very enjoyable week in Leiden, working in the University in the mornings finding out more about Lex, and then exploring The Netherlands in the afternoons – a great experience. Our very first email address was accessed on just the one computer in the office, with an address as part of Oxford University. We had to switch it on and dial up each time, and if we received an email we

were unable to forward it to anyone in the office to deal with and were unable to print it, so we used to write down what the email said by hand, and then pass the piece of paper over to the appropriate member of staff! Incredible to think that is what happened then!! The move to us all having our own laptops was an excellent development, as this meant we all still have access at events and at home.
The growth of Outreach activities has been great to see. The ‘A Microscope For Every School (AMFES) Scheme) which was introduced in the early 1990s, where the Society raised some money so that we could provide a partial refund for up to two recommended microscopes purchased for UK primary schools is still running today. The biggest change and development was the introduction of the Microscope Activity Kits, which were the brainchild of Susan Anderson, who led the development of these. This scheme has been absolutely amazing, and I’m so pleased at how successful the scheme has been and continues to be. Back in the early 1990s I spent some time travelling around the UK visiting various Science Centres and Museums, some of which was with Peter Evennett and Chris Hammond. We were trying to find a new home for the Buxton Micrarium displays, as the town council of Buxton wanted its pump room back….. Unfortunately we were unsuccessful finding a new home, but it was really interesting seeing what was available in the various museums. The Tabletop SEM Scheme, masterminded by Alex Ball and James Perkins (with Hitachi, IRIS and the NHM) has been an amazing scheme going out to many schools in the UK. I really hope that they are successful in obtaining funding to move this forward.
I am writing this after having the most successful mmc (and EMAG) in Manchester, since we organised the first congress there in 2014. We had more conference delegates and exhibition visitors attending, and there was a real ‘buzz’ at the whole event. I was especially touched and surprised to be awarded the very first Winton Award, and for once I was totally speechless, as well as fighting back the
emotional happy tears! Looking back at my years at the Society, I feel incredibly lucky to have worked with so many amazing people on so many things. When I took over the post of Chief Executive I said that I am the ‘caretaker’ of the Society, and that the Society will long outlive me! I wish my successor Sali Davis, every best wish for the future, and I hope that she will enjoy working for the Society as much as I have – it is such a special organisation.
I would like to thank the following ex-members of staff, and there are many more too (sorry to anyone I have missed):

Colonel Fleming, Judy Lewis, Karen Collins, Rob Flavin, Susan Robertson, Rachel Holborrow (nee Knibb), Liz Howe, Chloe Goode, Mel Reedman, Zu Dragnevska and Amanda Jarman.
I would particularly like to thank the current RMS staff team of Tor Masters, Adam Clay, Jade Sturdy, Dawn Hopkins, Kate Wooding, Debbie Hunt, Tracey Clay, Lucy Ridler, Owen Morton, Jill Hobbs, Georgina Fletcher, Katie Reynolds, Nick Cameron, Jess Cole and Alessandra Reni – for making the RMS such a great place to work at on a day-to-day basis – the current team is the best team ever and I’ll miss you all!
Over the years I have worked with some amazing members and committee members, and to name some who I would particularly like to thank over very many years – Peter Evennett, Chris Hammond, John Hutchison, Chris Kennedy, Les Stump, Jeremy Sanderson, Phil Robinson, Gillian Bullock, Lynne Joyce, Debbie Stokes, Tony Wilson, Mark Rainforth, Pete Nellist, Dick Paden, Susan Brooks and Ron Van Noorden, and those no longer with us – Savile Bradbury, Brian Bracegirdle, Nick Wrigley, Don Thomson, Nick Read, and, of course, Chris Hawes. You have all had such an impact on me.
I would like to thank all current members of Council, the Sections, FIGs, History Committee, the Corporate Advisory Board, and especially our current Executive Committee, who put so much of their own time into the Society. They will do a
great job at guiding the Society through the next few years - thank you Pete, Grace, Rik, Michelle, Rod, Maddy, Andy, Kerry and Susan.
The RMS is in a great place for the future, and will continue to grow despite the challenges of Open Access. I wish everyone involved with the Society all the very best for the future – such a special and
unique Society, and I feel privileged to have spent nearly 40 years working here.
Finally, I would like to thank my Mum and Dad for being there for me and for supporting me throughout my RMS career, my daughter Lauren (who feels like the RMS is part of her extended family), and of course, my partner Gary and his family. I am so lucky to have you all in my life.
During Allison’s 39 years at the Society, she has: worked with 18 RMS Presidents worked with 6 Honorary Treasurers (one of whom twice, as Lynne Joyce had two terms in office) worked with 6 Executive Honorary Secretaries
Been involved in the organisation of: 8 MICROs
1 Industrial and BioMedical MICRO in 1987
3 EUREM/EMC meetings (York, Manchester and almost Copenhagen), though EMC2020 was cancelled a few months before because of Covid
1 EMAG/MICRO in 1989
5 MicroSciences

6 mmc congresses (5 with EMAG)
Seen the publication of 468 issues of the Journal of Microscopy and 168 issues of the RMS Members’ Magazine, covering its transformation from the ‘Proceeding of the RMS’ into infocus in 2006, and then its recent transition to an online publication.
• The RMS staff mmc uniform is one thing that in my Chemistry and Physics O Levels! I’ve hasn’t changed much during Allison’s career! learnt so much while at the RMS though, and She says: “At MICROs in the 1980s, our wish that I had been enthused in science uniforms consisted of men’s striped blue and while at school. This is one of the reasons white shirts from M&S, which then changed why I think the RMS Outreach Programme to the current Boden stripy tops. The only is so important.” time we diverted away from stripes was at
• Finally, Allison has very many RMS ‘stories’ emc2012, when we wore something ‘red’!” from over the years, none of which, she tells
• Allison has very little formal scientific us, “can possibly be put into print!” education. She says: “I only gained grade C



 Allison (back, centre) joins the rest of the RMS team for a group photo in the Manchester Central foyer at mmc2023.
Allison strikes a colourful pose with Jade Sturdy (PA to the Chief Executive) during the exhibition ‘build’ ahead of mmc2023.
Allison (back, centre) joins the rest of the RMS team for a group photo in the Manchester Central foyer at mmc2023.
Allison strikes a colourful pose with Jade Sturdy (PA to the Chief Executive) during the exhibition ‘build’ ahead of mmc2023.
The RMS is committed to encouraging members to improve and develop their skills and understanding of microscopy; we believe qualifications play an important part in this.
If you are an RMS Member using microscopy or cytometry as part of your career, you can apply to study for an RMS Diploma - a flexible, portfoliobased qualification designed to complement and fit around your current employment.
The Diploma is intended to be of a similar standard to a Masters degree. Projects are designed by the candidate with the assistance of their line-manager, and with input from existing Fellows of the Society.
This approach ensures that the study is both challenging and rewarding whilst complementing the candidate’s existing employment.
We caught up with some of our recent diplomates to find out more about their experience of completing the course – including words of advice for anyone who might be interested in applying.
Many thanks to Jennifer Simpson, Craig Holliday and Alfonso Schmidt for giving up their time to share their thoughts on the RMS Diploma!
Watch the video on YouTube
Find out more about the RMS Diploma, including how to apply
Dendrite of a pyramidal nerve cell from cerebral cortex surrounded by a glial network. On numerous drumstick-like dendritic spines, red-marked synaptic contacts are visible. Synapses are sites of electrochemical transmission of information signals and together with dendritic spines represent important structures for learning and memory formation. The image is a 3D reconstruction resulted from serial electron microscopy. Scale cube = 0.1 μm per side. Equipment Used: computer-aided serial electron microscopy. Josef Spacek, emertus professor of pathology, Charles University Hospital, Hradec Kralove, Czechi.
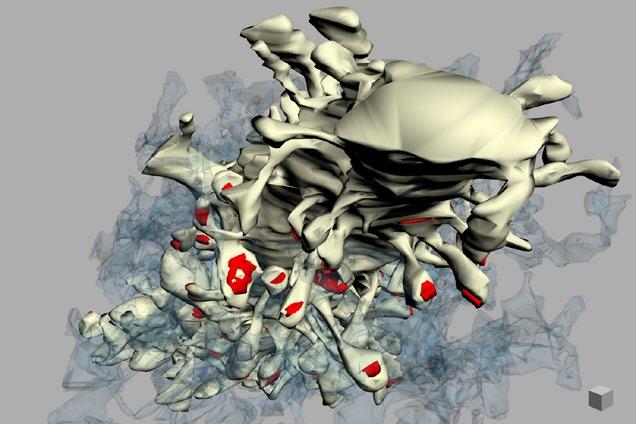
3D model of surface topography with an overlay of chemical composition on a Cobaltite sample generated with MountainsSpectral® software. Equipment Used: JEOL SEM IT700HR and JEOL EDS system + SMILE VIEW™ Map software powered by Mountains®. Digital Surf in collaboration with JEOL (France). Sample courtesy of Emmanuel Guilmeau (CRISMAT) and Jean-Claude Ménard (JEOL France).
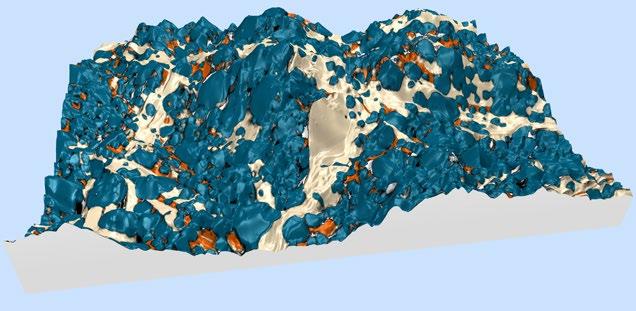
The FocalPlane Network is an international directory of researchers with microscopy expertise including developers, imaging scientists and bioimage analysts. The idea behind the FocalPlane Network is to facilitate promotion and networking as well as assisting those seeking conference speakers, committee members, reviewers or collaborators.

We aim to be an inclusive site that will help promote diversity in the microscopy community. Members provide information on their scientific field, microscopy area of expertise, place of work and career stage. They can also voluntarily provide details on aspects of diversity such as gender, race/ ethnicity, LGBTQ+ identity and disability status.
There are two options for using the Network: as a ‘user’ or as a ‘member’. As a ‘user’, you will have access to the database but your details will not appear on the site, while as a ‘member’, your details will be included for others to see and potentially contact you. If you are already registered on FocalPlane, request access to the Network on your profile page as either a ‘user’ or a ‘member’.
If you are not registered, you must do this first, and then request access. Once you are a user of the FocalPlane Network, you can contact other
members using the online contact form available in the member’s details (no email addresses are made public). If you want to become a member of the Network, request access to the Network and enter your details in your profile page. Your registration will then be manually approved to ensure the entry is valid and to avoid spam. The FocalPlane Network has been designed with data protection bestpractice in mind to ensure all personal information is secured.
We would like to thank the members of the community and our Scientific Advisory Board for their help and input in shaping the FocalPlane Network.We also want to thank Sian Culley and the RMS team behind the Microscopy Gender Equality Resource for their feedback. We recommend checking this resource if you are looking specifically for women in the field of microscopy, imaging and cytometry.
https://focalplane.biologists.com/ network/
https://focalplane.biologists.com/ network/?utm_source=infocus&utm_ medium=blog&utm_id=RMS
We are proud that we achieved 90 nm spatial resolution on the LiveCodim super-resolution system. We have taken part in a recent EMBO course organised in Prague at the Institute of Molecular Genetics (IMG) and here is something we were able to capture. Nucleopores in green and mitochondria in red, pushing the limits further. A small microscope add-on which can turn your widefield into a super-resolution platform.
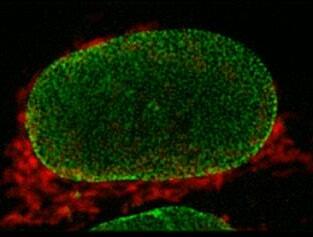
www.telight.eu
Over the years, KEYENCE has become a global leader in the development of measurement and quality assurance solutions. It was no surprise, therefore, to see medical aesthetics specialist GC Aesthetics™ turn to KEYENCE when the time came to acquire a new digital microscope. One particular requirement is that of surface finish on implants to assess various metrology parameters and surface topography features, patterns, consistency and uniformity.
Having in-house capability to produce high resolution images of surface topographies along with the necessary metrology parameter specific data on surface profiles gives GC Aesthetics™ the ability to produce test data very quickly, maintain quality and control,” says Mr Harvie.
Not only is speed to results of vital importance. So too is the number of samples GC Aesthetics™ can test, review and compare. This now gives the company an unprecedented ability to turn-around and produce high volumes of sample data on varied samples in days, and in some cases hours, compared to before.
Another key advantage of using a KEYENCE solution is the initial, and indeed ongoing, training. “It proved extremely helpful to GC Aesthetics™” said Mr Harvie.“KEYENCE has shown a high degree of understanding as to the needs of its customer; this was instrumental in delivering a satisfactory outcome with regard to complex testing needs.”
Techniques such as scanning electron microscopy (SEM) and digital microscopy were outsourced by GC Aesthetics™. According to Mr. Harvie, results turnaround time with outsourcing such tests was time consuming.

“We purchased and started operating a KEYENCE digital microscope, a product known to provide superior analysis thanks to clearer observations.
Months on from its acquisition, the KEYENCE digital microscope delivers high quality information and enables GC Aesthetics™ stakeholders to make informed choices. That’s a positive outcome for everyone involved.
www.keyence.co.uk/infocus/ gcaesthetics/202309
More than 6 in 10 people in the UK have some requirement for glasses, meaning the range of lens combinations required to suit all of their needs is almost infinite. Significant effort goes into the cutting, polishing and treatment of every lens. One of the UK’s leading manufacturers of bespoke lenses, and a partner of 20 years plus to many of the leading names in the dispensing optician sector, is Vision Labs Ltd.
offer this – combined with two-dimensional edge detection software which snaps to the lines of contrast meaning the risk of differences between users is eliminated, allows calibration tolerances as low as 0.1 microns.
Meanwhile, the glare removal feature and rapid 3D depth composition, combined with the VHX’s easyto-use of software, meant that wear on diamond cutting tools can be rapidly assessed to ensure they are replaced only when needed, creating cost savings for Vision Labs.
Perhaps the greatest benefit has come in the area of analysing coatings, where the VHX’s world first Optical Shadow Effect Mode allows these transparent features to be viewed more readily and so defects are identified more rapidly. The multilighting feature, allowing immediate change between ring, coaxial, polarised and transmitted lighting, also assists the clarity of view.
Anthony Woodhouse, Technical Manager of Vision Labs, explains: “Previously we assessed machine tools using an eye glass with a scale. However, this method has limitations, even for the highly experienced tool setters within our team.”
Because of these challenges, the management team at Vision Labs sought another solution, and it came in the form of a KEYENCE VHX-7000 digital microscope. The system’s high magnification and 4,000 pixel count – the world’s first microscope to

Anthony Woodhouse continues: “The KEYENCE microscope is easy to use and removes the issues associated with the previous visual testing. We had used other KEYENCE equipment with great success for a number of years and so we were already aware not just of the quality of their products, but the depth of support and training that would be provided.”
www.keyence.co.uk/infocus/ visionlabs/202309

Q-Phase was successfully installed and calibrated at the Medical Faculty of Warwick University. Instructions on how to control the microscope and subsequently analyse the obtained data using our software were provided to main users and responsible persons from the Computing and Advanced
Microscopy Development Unit (CAMDU). Telight is also a platinum sponsor at a conference that will take place in Warwick Adhesion and migration in disease 2023, we would be glad to talk to you about our machine.
www.telight.eu
As a hybrid manufacturer and distributor of key scientific technology, we at Quantum Design UK and Ireland are always looking to provide our customers the best technology for their research and development.
With a globally renowned service team backed by our central headquarters in San Diego and with offices stretching across to Europe, South America, Asia as well in the UK, our manufactured systems are some of the best and most unique in the world, with a very strong service support structure.
This benchmark is also how we create our supplier network and choose which manufacturers that we partner with and introduce into our respective territories. RHK Technology is one such manufacturer whom we are now in partnership with and distributing for here in the UK and Ireland.
RHKs surface science systems integrate only the best analytical and preparation instruments from only the top industry suppliers. With support from the best scientists, RHK continue to advance this technology further for supporting university, industry and government labs around the globe.
Founded in 1981 by Adam Kollin, President of RHK Technology, they bring over 30 years of experience in the design and manufacture of advanced UHV SPM instruments with their install base, currently
consisting of over 200 systems and 1200 controllers, continuing to grow. This install base includes the world’s first closed-cycle cryogen-free SPM in the form of their PanScan systems, to UHV SPM technology in the form of the Beetle family, to even their R10 SPM controller.
A combination of this experience and versatile history, along with positive comments from their existing global customer base which consists of the Tata Institute of Fundamental Research, Tsinghua University, Los Alamos National Laboratory and others, has contributed to QDUKI and RHK agreeing to work together to continue their support of global research and development activity.
“Together, QDUKI and RHK Technology will bring their key technology and combined experience to the UK and Ireland’s cryogenic and microscopy research community,” commented Dr Satyam Ladva, Technical Product Manager at QDUKI. He continued: “And aid in its development and growth, both supporting those already immersed in this area but also to aid those venturing into this field of research for the first time.”
www.qd-uki.co.uk
Experienced business leader Sam Crossley joins Vision Engineering as it navigates sustained growth across global markets.
Vision Engineering is pleased to announce the appointment of Sam Crossley as Managing Director, a role that will draw upon his extensive global leadership and financial experience.
Previously a member of the senior leadership team at Rotork, which employs over 3,700 employees in 39 countries and serving customers in over 170 countries, Sam has an in-depth market knowledge in oil & gas, petrochemical, mining and automation, specialising in engineered technical products including mechanicals seals, pumps, valves and fluid control sectors. Sam’s broad international experience includes steering company growth in Asia Pacific, Middle East, Russia, Europe, Africa and North America.
Says Sam: “What appealed most about joining the team at Vision Engineering was the idea of leading an innovative technology company with such a long and distinguished history. There are plenty of exciting opportunities to grow the business, and I will be focussing on these over the coming years. Vision Engineering has a well-deserved brand and reputation and I’m delighted to be leading it into its next phase of global growth.”
Mark Curtis, current Managing Director will continue to head the Board of Vision Engineering Group as Chief Executive Officer, focusing on strategic development and wider Group growth opportunities.

Vision Engineering is a world-renowned name in the design and manufacture of industry-leading microscopy, metrology and digital 3D visualisation solutions. Designed to help those working in a wide
range of sectors, they are recognised internationally for their clear focus on improving productivity for those who use magnification through superior ergonomics. “Our market leading technology, focus on ergonomics, and innovation are undoubtedly three main pillars that support the global reputation of our brand. In these areas our record is second to none.”
The many industries in which Vision Engineering’s solutions have become standard equipment include electronics, automotive, aerospace, medical device manufacture, biotech, telecom and cable. Its sales and tech support offices are located throughout North America, Europe and Asia, supported by a fully trained network of distributors.
www.visioneng.com
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
» Go faster: 500 fps full frame
» Image larger: 29 mm field of view
» Increase sensitivity: 0.7 e- read noise, 96% QE

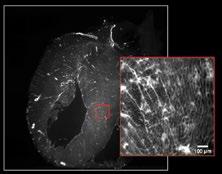
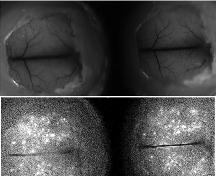
Click on the images to read the customer stories from the researchers
Learn more at www.teledynephotometrics.com or reach out to us at photometrics.info@teledyne.com


My idea for this project was to be able to control light emitted from different sources, and illuminate each subject with the desired colour.

I selected various different lights - halogen, torch, house lamp - and by shining them through different types of clear, plastic prisms (one for each photo), directed them on to the specimen stage of my microscope. I then used a variety of techniques - fluorescence, polarisation, brightfield and darkfield (sometimes in isolation, sometimes combined) - to photograph the results.
The main challenge was to get the correct intensity to illuminate the subject without washing it out. I experimented with different intensities until I got each subject illuminated with no ‘wash-out’.
The microscope used is a Stereo, with the facility to interchange colour cubes - a modification I made myself.
I hope you like the results!
For Figure 1, I used a darkfield condenser, so as to get a black background, with an external fibre optic light source. This technique was also used in Figures 2 and 3. I used a green card for Figure 4, and a red card in Figure 5.

A red light source was used for Figures 6 and 7, a blue filter in Figure 8, and red filter for photo 9. This enabled the overall colour for each photo.
Figures 1, 2 and 3 were taken using darkfield and the final six, with brightfield.

 Figure 2.
Figure 2.

 Figure 4.
Figure 4.
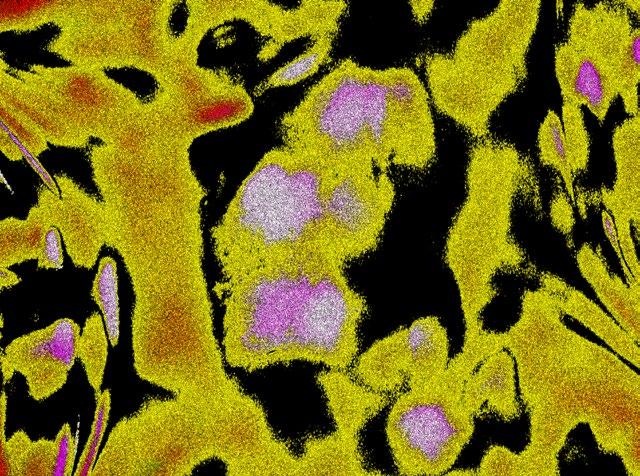
 Figure 6.
Figure 6.
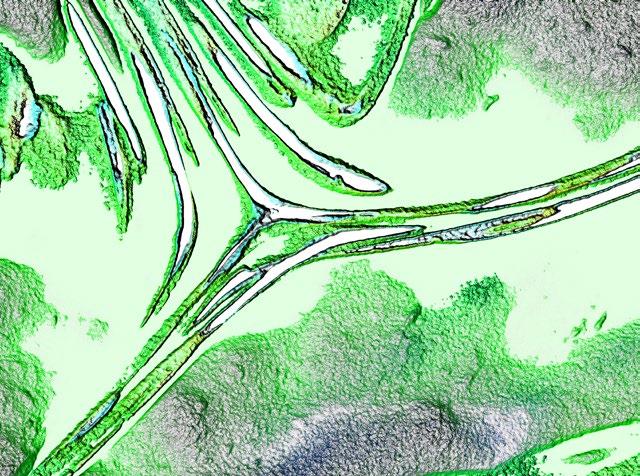
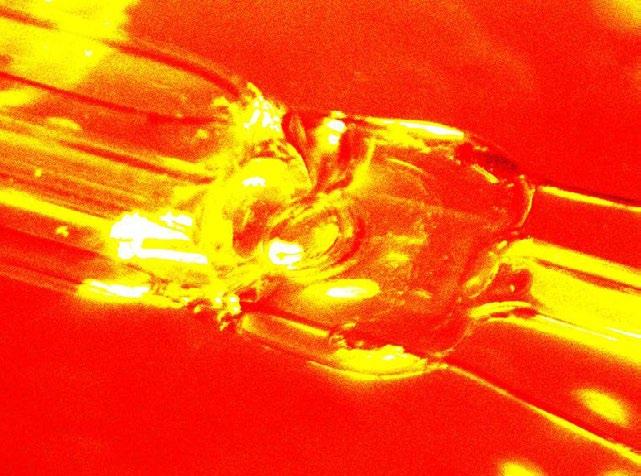 Figure 8.
Figure 8.
Winston Ingram was born in 1940, and from an early age developed an interest in Science and Photography.
He went into private practice in 1978, working freelance for various companies, and teaching scientific, medical and technical photography. He retired in 2002 and started producing books using photo microscopy as an art form. He likes to explore different techniques with the microscope, and also mix the various facilities available.

HORIBA has further expanded its XGT-9000 series product line with the release of the two new microXRF (X-ray fluorescence) analysers: the XGT-9000 Pro and the XGT-9000 Expert X-ray Analytical Microscopes.
The XGT-9000 Series is ideal for a broad range of fields, including fuel cells, lithium-ion batteries, pharmaceutical, and other industrial fields to detect foreign mater and develop new materials. It can also be used in astrogeology, archaeology, forensics, and other research fields to perform non-destructive analyses on precious samples. The XGT-9000 Series has also been used during the initial analysis of sand and gravel collected from the Ryugu asteroid by the Explorer “Hayabusa2”.
The new XGT-9000 Pro and XGT-9000 Expert incorporate improved detection systems and HORIBA’s patented pulse processing algorithm to achieve significantly faster analysis. Additionally, the XGT-9000 Expert realises the world’s first light element analysis (down to boron) in a benchtop energy dispersive micro-XRF analyser. Supersensitive analysis of light elements, such as carbon (C), nitrogen (N), and oxygen (O) allows the analyses of oxides, nitrides, organic matter, and other materials by a single micro-XRF analyser
without the use of several different instruments.
These new capabilities enable time reductions and efficiencies of material analysis, both in terms of quality control and R&D processes in material manufacturers and a broad range of other industries.
With the increasing importance of quality control in manufacturing process, the detection of tens of microns sized (1micron = 0.001mm) contaminants or foreign mater, which are difficult to be visually recognised, has been a challenge. Such a microscopic contaminant can result in lower performance and other serious problems. That is why micro-XRF analysers are becoming more essential than ever before.
While micro-XRF analysers are powerful for the analysis of inorganic materials and metals, highsensitivity analysis of light elements below sodium (Na) has generally been difficult for them up until now. The analysis of nitrides and organic matter had required a different type of analyser. The ability of the XGT-9000 Expert to achieve highsensitivity analyses of light elements such as carbon (C), nitrogen (N), and oxygen (O), allows a single instrument to provide a way to analyse not only metals, but also oxides, nitrides and organic matter.
https://horiba.link/xgt-9000

It’s highly accessible and user-friendly, rapidly processing samples to provide reliable data on morphology and elemental composition in just two minutes.
While SEM has significantly improved recently, it remains a complex technique. Axia ChemiSEM simplifies without compromising functionality and flexibility. Continuous EDS provides live elemental data updates and the toggle on/off feature isolates samples areas of interest easily.
Axia ChemiSEM introduces a new SEM-EDS workflow for acquiring, processing and presenting compositional sample information. It applies to diverse fields, including:
• Process and quality control
• Failure analysis
• Materials science
• Glass fibre-reinforced polymers
• Composite materials
• Battery materials
• Aerospace materials development
Its low energy capability makes Axia ChemiSEM an ideal choice for polymer analysis, operating at 200v and featuring beam declaration for enhanced imaging. A unique capability is our ability to operate at low voltages as low as 200V, which combined with beam declaration for enhanced imaging makes it the ideal choice for polymer analysis.
Axia ChemiSEM offers an integrated user guide, real-time compositional imaging, and alignmentfree operation. It simplifies workflows, saves time, and enhances laboratory sustainability without compromising space. The Axia ChemiSEM system is capable of accommodating samples weighing up to 10kg. It allows for precise x-y movement, offering versatility in sample positioning. Its open door design facilitates easy loading of large and awkward samples, allowing seamless handling of various sample types without sacrificing efficiency.
The SEM device delivers high-quality imaging performance to meet diverse application needs. It can handle a wide range of samples, including insulating items, with its Low Vac operation. The advanced beam scanning capabilities ensure highfidelity imaging, providing detailed and accurate analysis results. The Axia ChemiSEM system provides access to a range of application software options. It supports advanced system automation through software like Thermo Scientific Maps. Thermo Scientific AutoScript also allows users to tailor the system to meet their requirements. For consistent, high-quality performance compared to a benchtop, look no further than Axia ChemiSEM.
https://blue-scientific.com/product/
Many of those tasked with checking for defects in materials are hampered by having to rely on relatively basic microscopes, whereby the operator is looking top-down on to a sample, which makes it very difficult to determine whether the problem is a dent, a scratch or a raised section.
Another issue for inspectors is that the vast majority of microscopes used in defect analysis on materials provide low magnification observation, making it difficult to check grain size or carry out particle analysis of the structure when working with composites. It’s also impossible to share in real-time any data gathered during defect analysis with colleagues or departments on other sites or in other parts of the world.
Enter the VHX-7000 from KEYENCE, the world’s first 4K ultra-high accuracy digital microscope. It offers magnifications from 20x to 6000x and has the ability to subtly shift the finely-tuned XY motorised stage. It also has a handheld option for all-round inspection of larger parts.
A key differentiating factor of this digital microscope is its software. Based around a Windows 10 PC
with a large screen, the unit has an intuitive user interface, sub-menus and a step-by-step guide that means even a novice can carry out detailed material analysis. Thanks to a built-in 1TB hard drive that enables ultra-high resolution images to be saved, the operator can share them over a network or via a USB drive. Detailed reports can also be produced automatically in Excel format.
A particularly powerful (and world-first) feature of the software includes Optical Shadow Effect Mode which uses illumination at multiple angles to identify defects that would not be visible to traditional microscopes. At the same time, all analysis can be carried out in 3D (although a two-dimensional function is available) which is simply not possible with basic microscopes.
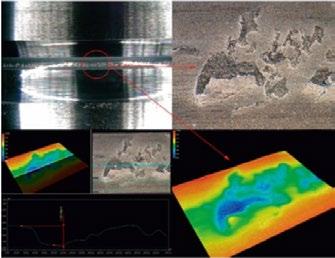
Also, the VHX-7000 is customisable, with interchangeable parts, so that customers have complete flexibility when building a system specification around their particular application.
www.keyence.co.uk/infocus/
HORIBA UK has introduced nanoGPS navYXTM, a combination of HORIBA’s patented nanoGPS tag and navYX software, that makes it easier and faster than ever before for researchers to get more data from their samples.
Collaborative Correlative Microscopy allows the combination of measurement modalities to enable an enhanced understanding of a sample. Until now, relocating specific areas of interest after a sample has been moved between instruments, has taken a long time. Now, with the nanoGPS navYX, researchers can relocate these interesting regions in just a few seconds.
The nanoGPS navYX uses HORIBA’s patented coordinate transfer tag technology to simply and seamlessly locate the same points of interest on a sample when it is transferred between different microscopy modalities and instruments. Researchers simply attach a small, patterned tag near to the sample or substrate, and the embedded position-sensing technology allows both absolute XY coordinates and angular orientation to be automatically converted, ensuring the same point of interest can be re-localised to within a few

microns. The tags are multimodal and multiscale and can be used with various instruments, including Raman microscopes, scanning electron microscopes, cathodoluminescence and upright optical microscopes.
Delivering enhanced accuracy, nanoGPS navYX automatically accounts for sample rotation and magnification differences, and enables the overlay of data from each modality to get additional information on specific zones of interest. To combine micro-molecular analysis with other more conventional characterisation techniques, collaboration between specialists in different labs is common, and transferring the sample from one technique to another has always been problematic. With nanoGPS navYX, there is no risk of confusion or misunderstanding when samples are sent to other laboratories. nanoGPS navYX tags do not age and can be easily fixed permanently to your samples using carbon tape, and as each tag is only 2 mm x 2 mm, they can be used with different samples and substrates in materials, life and environmental sciences research.
https://horiba.link/nanogps
LED illumination has revolutionised widefield fluorescence, especially with the ability to control the light source through popular imaging software programs.
USB connectivity features on many CoolLED Illumination Systems, allowing the efficient control of illumination settings when coupled with imaging software support. Whether acquiring images in live mode or performing a complex time-lapse experiment, the ability to program, save and reload LED selection and intensity settings provides both user efficiency and experimental precision.
Choose from a wide range of LED Illumination Systems, including the eight-channel pE-800 Series for lightning-fast fluorescence microscopy and supercharged calcium imaging which can be controlled through Evident cellSens Dimension, Nikon NIS Elements, μManager and more.
Combining sophisticated control with powerful illumination, the new pE-400 Series is ideal for costeffective fluorescence microscopy, from routine to
advanced applications. Both the advanced pE-400max and white light pE-400 offer USB connectivity and support in μManager, and are set to be integrated into more programs soon.
For everyday fluorescence applications, the popular pE-300white and pE-300ultra are supported in all the major software programs, including both Evident cellSens Standard and Dimension, Nikon NIS Elements and Leica LAS X.
Control is where CoolLED LED Illumination Systems come into their own, and software is just one method. The pE-800 Series and pE-400max also include the intuitive CoolLED LightBridge virtual control pod to complement imaging software. Moreover, the pE-300 Series and pE-400 Series include bench-top control pods for simple manual control, while several models offer TTL for highspeed live cell imaging.
www.coolled.com/support/imagingsoftware
Japan Electron Optics Laboratory Co., Ltd (“JEOL”), a leading manufacturer of semiconductor, industrial, scientific, metrology, and medical equipment, came to Scintacor Ltd with hopes of finding the ideal phosphor screen to detect electrons with high spatial accuracy to support products within their TEM segment. JEOL has always been at the forefront of SEM/TEM development, bringing the highest performance and quality products to the market. It was essential to partner with a true expert in phosphor coating in order to achieve the performance levels expected on the JEOL CMOS camera.
With a high level of collaboration between the company’s development teams, the project resulted in the launch of SightSKY1 - a high sensitivity, low noise, fiber coupling CMOS camera used with Transmission Electron Microscopes (“TEM”). With Scintacor’s phosphor coating, we were able to optimise both the sensitivity and the resolution of the camera whilst keep a low noise level, allowing SightSKY to bring a new level of performance to the TEM camera market.
“Scintacor’s knowledge and development capabilities were exactly what we needed to make this project a success,” said Dr Yuji Konyuba, a member of JEOL’s EM R&D Department. “The phosphor screen for electron detection, that was developed, exceeded our initial project expectations, bringing the camera even greater sensitivity than what was
first anticipated.”
Transmission Electron Microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a biological specimen or sample to produce a high-resolution, magnified image. These extremely powerful microscopes allow for visualizing material structures and properties at resolutions better than 1 nanometre. Used in a variety of fields, such as life sciences, nanotechnology, biological and material research, forensic analysis, and gemology and metallurgy, TEMs provide topographical, morphological, compositional, and crystalline information about the sample. Scintacor’s TEM screens convert the electron energy to detectable light for image sensing.
Andrew Lee, CTO at Scintacor remarks, “It has been a great opportunity to work with JEOL on this particular development project. In close collaboration between Scintacor and JEOL, engineers tested several potential technical options and together we determined the best process and phosphor required for JEOL’s demanding and innovative design.”
www.scintacor.com
https://www.jeol.com/products/ scientific/tem/SightSKY_Camera.php

HORIBA, leader in Raman microscopy, announces the new LabRAM Odyssey Semiconductor system, a Raman/Photoluminescence microscope based on the best-selling high resolution LabRAM HR Evolution confocal Raman microscope and equipped with a sample mounting stage to handle the common wafer size of 300mm.
As compound semiconductors are becoming more complex with a higher number of elements, uniformity assessment on blanket wafers is essential for high quality devices and high yield. The LabRAM Odyssey Semiconductor system will help process engineers qualify the different process steps in a
The LabRAM Odyssey Semiconductor system includes a 300 mm × 300 mm automated sample stage and an automated objective turret, enabling the acquisition of maps of full wafers of diameter up of 300mm. In addition, the DuoScan imaging function permits both variable size laser macrospot for full wafer maps and high spatial submicron step scanning for small area maps. The range of available excitation lasers, combined with a wide range of spectral detection, from deep UV to near IR, makes the LabRAM Odyssey Semiconductor system a two-in-one Raman and Photoluminescence spectroscopy tool. The “Tilt at midway” autofocus function overcomes possible sample/holder tilt and ensures reliability in uniformity response.
As Raman and Photoluminescence characterisation is moving from the lab to the fab for the emerging 2D materials-based devices, the LabRAM Odyssey Semiconductor system is the perfect tool for metrology technical managers.
Dr Adam Holland, Senior Raman and micro PhotoLuminescence Product Manager, HORIBA
timely manner and with a high level of confidence. With a high spatial resolution mode, the LabRAM Odyssey Semiconductor offers the capability to detect and identify defects and submicron inhomogeneities to understand and give insights about their origin.
UK said: “The LabRAM Odyssey Semiconductor is an ideal tool for compound semiconductor photoluminescence and Raman characterisation, providing both full wafer mapping for uniformity testing and micron level spatial resolution data for device development and failure analysis on the same tool. It is a game changer for both R&D and low volume production QC.”
https://horiba.link/labram-odysseysemiconductor
Digital Surf announced the release of the tenth major version of the company’s renowned Mountains® software analysis platform for surface metrology & microscopy, trusted by 50+ leading instrument manufacturers and 22,000+ users. Version 10 highlights include:
A new product family for users of light microscopes, MountainsImage®, providing a complete toolkit for pre-processing and analysing image data. The new line includes tools for enhancement, measurement, luminance histograms, colour inversion, conversion to monochrome, particle analysis including colour segmentation, fibre analysis and image contour analysis.
A new job-specific Lens Analysis module for the study of aspheric optical surfaces and profiles in applications from many industries including imaging systems, sensors and lasers.
The improvement of features for Automation, key in today’s fast-paced industrial and research environments. In particular a new Automation tab now groups together Mountains® productivity functions such as document templates for processing of large batches of measured data, userdefined analysis recipes (known as Minidocs) and customisation options allowing customers to work faster.
CAD compare on Shell data (freeform surfaces): users can now compare measured Shell data with CAD models and access full surface texture analysis on this kind of dataset.
MountainsSpectral® Analyze for users working with spectra only, including automatic peak detection and fitting and parameter map generation.
The recently-released Fiber analysis tool has been extended to all product families including those designed for topography analysis.
“In the ever-evolving surface metrology and microscopy industries, Digital Surf remains dedicated to building the best and most up-to-date surface imaging and analysis software” said Christophe Mignot, Digital Surf CEO. “Version 10 is our most comprehensive product release yet, bridging all the remaining gaps between the various datatypes and instrument technologies we cater for: topography analysis, image analysis and spectroscopy analysis. We are very excited to deliver Mountains® 10 to our users and receive their feedback.”
www.digitalsurf.com/news/pressrelease-digital-surf-unveils-mountains10-the-most-comprehensive-versionto-date/
Vision Engineering, the world-leading provider of innovative inspection, metrology, and digital 3D visualisation solutions, today announced the launch of Mantis 3rd Gen, the latest addition to its bestselling and award-winning range of ergonomic optical stereo microscopes.

Mantis is in use in tens of thousands of R&D, manufacturing and analytical sites around the world. Mantis 3rd Gen incorporates the latest developments in optics, digital cameras and fully adjustable LED lighting, to keep Mantis at the forefront of stereo imaging.
Mantis is designed for precision engineering, electronic engineering, medical device manufacture, and a wide range of other applications that require high-quality images and superior ergonomics. It features a unique, patented, eyepiece-less design that delivers a large, high-quality optical stereo image directly into the user’s eyes, making it more comfortable and easier to view than traditional microscopes.
Manipulative, rework and restoration tasks need stereo images, to allow easy hand to eye coordination and depth perception. Mantis 3rd Gen combines stereo optical images, with high resolution camera options for manipulation and recording.
Mantis 3rd Gen features long working distance and excellent depth perception, now with a choice of 3 magnifications, making it ideal for a wide range of applications. It also now comes as standard with five different ways to illuminate your subject, giving you the flexibility to adjust the lighting to get the perfect image for your needs.
In addition to its outstanding image quality and ergonomics, Mantis also features a powerful digital imaging system that allows you to capture, review, and share high-resolution images. This makes it easy to share your work with colleagues, document your findings, and train new employees. Substantial R&D has resulted in a new range of stand options, to allow flexibility, stability and reduced footprint.
“As our customers have told us for the last 28 years, Mantis is an ideal solution for anyone who needs to perform precise work with small objects,” said Mark Curtis, Managing Director at VisionEngineering. “We invest substantial R&D time and effort in exploiting the opportunities that fast moving optic, digital and lighting technologies offer our dedicated customer base. Mantis 3rd Gen offers the best of both worlds: superior ergonomics and optical image quality, combined with the latest digital imaging technology.”
www.visioneng.com/mantis
Oxford Instruments today launches the world’s first imaging detector to combine Backscattered Electron and X-ray imaging in a single technique – BEX. Unity enables researchers, scientists, and students to achieve instant visual and compositional sample analysis simultaneously in the Scanning Electron Microscope (SEM).
Traditionally, determining the elemental makeup and composition of materials in the SEM has been a repetitive, laborious process. This involves acquiring black-and-white electron images based on the secondary electron and backscattered electron signals. X-ray signals are then used to determine chemical composition. This is time-consuming, especially because users must replicate this process repeatedly until anomalies or areas of interest are located. However, with Oxford Instruments’ Unity detector and innovative BEX technique, BSE and X-ray signals are captured simultaneously, allowing users to instantly observe the microstructure and chemistry of samples in full-colour, high-resolution images.
The game-changing Unity detector combines the topographic, crystallographic, atomic number and elemental information in an immediate highresolution visual output. Users can effortlessly navigate around the sample, making sophisticated
sample analysis simpler and faster than ever before. The Oxford Instruments team has spent six years developing the Unity detector, revolutionising the workflow for sample analysis. The unprecedented speed of the Unity detector provides accurate, high-resolution chemical mapping of whole samples in just minutes, allowing users to quickly identify features and regions that warrant further investigation. By having this valuable cartographic data at their disposal before detailed examination, users can swiftly transition from initial instincts to scientific insights, opening inspiring new research directions.
Dr Haithem Mansour, product manager at Oxford Instruments, said: “Our Unity detector and BEX technique are truly ground-breaking. We’re not only making complex sample analysis quicker and easier, but we’re also helping ensure the process is more robust and accurate, and giving users more time to focus on specific areas of their sample that demand their attention. Unparalleled in the market today, the powerful combination of hardware and software within the Unity detector brings new levels of certainty, reliability, and pace to the scientific community, accelerating the journey to scientific discovery.”
nano.oxinst.com
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
ISAC, the International Society for the Advancement of Cytometry, held its most recent annual Congress at the Palais de Congress in Montreal, Canada. The meeting attracted around 2,000 delegates including students, academics and exhibitors. As usual this was the time to hear about the latest developments in flow cytometric hardware, software, and applications. And, as at all previous congresses, the quality of talks and posters was extremely high. ISAC has several development programmes –Scholars, Shared Resource Laboratory Emerging Leaders and Innovators, and it was great to see those who had been on the schemes for a while returning to CYTO to present their work.
The first day included 15 tutorials on such diverse subjects as career development, training, data analysis, cell sorting, and setting up your own startup company. A new introduction this year was an open-ticketed Presidents reception where all
profits were split between three ISAC civic mission initiatives – CYTO Youth, Instruments for Science and Live Education. This meant that all attendees at the conference were able to mingle with the ISAC councillors and members of various committees within the society. First-time attendees also had their own session where more experienced attendees could offer advice on how to get the most out of what is a large and vibrant meeting.
Another key aspect of this meeting is the exhibition space and 77 companies were represented showcasing their new wares. Companies also gave commercial tutorials over lunches enabling them to describe their new equipment, reagents or software. Time in the exhibition hall is always worthwhile, even for those who have no financial authority. Cytometry is a very welcoming and altruistic field and people can’t wait to tell you about their latest discovery or newest piece of kit!
The prestigious Hooke lecture was given by Anthony Hyman from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden. He spoke on the fascinating subject of biomolecular

condensates. Other plenary sessions featured views on the state of the art in cytometry. The two that stood out for me were given by the RMS’s own Peter O’Toole (University of York, UK), who spoke on extracting data from label-free images, and Ryan Brinkman (Vancouver, Canada), who described a crowd-sourced gaming approach to data analysis. Some of the recent developments including full spectrum cytometry, microfluidic sorting devices, standardisation, clinical biomarkers, and extracellular vesicles were well represented in the parallel sessions which ran every day.
For the past few Congresses, ISAC has run CYTO Youth – this is an outreach programme aimed at teachers and students of all ages and is designed to highlight that a career in STEM is an attractive option. As CYTO is held at different venues every year, this enables that message to be spread globally and many of the attendees at the Congress itself contribute to this day of learning.
What were the other highlights? CYTO Innovation is a relatively new part of the meeting with the aim of showcasing start-up companies in the field.These companies can pitch their ideas as well as receive feedback. It is a competitive process and the winner this year was Spectradyne who have developed a technology to accurately measure nanoparticles
The posters always provide a good means of interacting with other delegates and seeing new approaches, and two live sessions were held where authors had the opportunity to describe their work in more detail.The meeting was rounded off with the

presentations event – ISAC rewards its members with several long-service recognition awards as well as awards for Best Posters and Outstanding Students – and a closing ceremony.
CYTO would not be the same without the extensive social activities. Montreal is a very vibrant city and many groups got together in the evenings to visit restaurants and bars, renew acquaintances and make new friendships. In particular there was excitement over the venue for next year’s Congress which will be in Edinburgh in early May 2024.
Derek Davies, The Francis Crick Institute, London

Rachael Walker, Babraham Institute, Cambridge
Both authors had presentations and posters at this meeting and we gratefully acknowledge the RMS for its support in making it possible to attend the Congress.
infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS. infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more.
infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms.org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.ukj
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
• Total number of images/figures/tables should not exceed 15 including tables.
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).
Amplify the power of flow cytometry with innovative technology—cells can finally be sorted with spatial and morphological insights on the first real-time imaging, spectral flow cytometer. The BD FACSDiscoverTM S8 Cell Sorter with BD CellViewTM Image Technology and BD SpectralFXTM Technology empowers researchers to study cells that were previously impossible to identify or isolate. Be part of the next era of breakthroughs enabled by these advanced technologies.
Put discovery in view at bdbiosciences.com