The Scanning Microscope and Leeuwenhoek’s Legacy
RMS Summer Studentship Reports 2023
The RMS Mentoring and Application Scheme: Nurturing Microscopists across the world
Reflections on the RMS Diploma: Study, Mentorship, and Oversight


The Scanning Microscope and Leeuwenhoek’s Legacy
RMS Summer Studentship Reports 2023
The RMS Mentoring and Application Scheme: Nurturing Microscopists across the world
Reflections on the RMS Diploma: Study, Mentorship, and Oversight



The all-new JIB-PS500i from JEOL delivers fine milling capabilities essential for fast, high quality lamella preparation. This multi-purpose FIB-SEM enables high throughput sample preparation, high resolution STEM-in-SEM imaging, and analytical analysis.
Robust Workflow for Large Samples
Featuring a large specimen chamber with easy-access door and large high-tilt stage, the JIB-PS500i offers a truly flexible workflow. Transfer from the FIB-SEM to the TEM is seamless with TEM-Linkage, JEOL’s double-tilt cartridge.




4
16
Lakshana

infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.
infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK
Editor
Owen Morton
Tel. + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Laura Fumagalli, University of Manchester, UK
Myfanwy Adams, John Innes Centre, Norwich, UK
Maadhav Kothari, Zeiss Microscopy, UK
Hilary Sandig, Cancer Research, UK
Trevor Almeida, University of Glasgow, UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2024 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by Creative Design. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.
Dear Readers,
Happy New Year! I hope the first months of 2024 have treated you well – whether you’ve been experiencing the thick of winter in the northern hemisphere or something a little warmer in the south. It is a pleasure to bring to you the first issue of 2024.
This issue marks our first anniversary of infocus being a fully online publication. It has been a year of learning and new adventures for us all in the infocus team. We hope to continue bringing you the best possible range of content throughout 2024. Speaking of learning curves, we have a great piece on the RMS Diploma - a flexible, portfolio-based qualification, designed to be similar to a Masters degree.We get to hear three different perspectives on the programme – from the Diploma candidate, the mentor and from the Chair of the RMS Qualifications committee.
Another important strand of the RMS’s educational activities is the annual Summer Studentship scheme for undergraduates in their second year of university study.The students carry out projects involving microscopy or image analysis in physical or life sciences, submitting a report at the end. In this issue, we are delighted to publish all five reports from the ‘class of 2023’. Mohamed Ghali (University of Nottingham) details his project on the mechanical meniscus correction in phase contrast microscopy; Hio U Lao (Strathclyde University) describes the use of Raman scattering microscopy to study the uptake and distribution of Bruton’s tyrosine kinase inhibitors; Rebecca Harry (University of Oxford) details her project using microtubule depolymerisation assay to study BRCA2 and MCAK interactions; Miriam Czech (King’s College London) reports on her project evaluating different metrics used in deep learning for microscopy; and Lakshana Baheerathan (Durham University) describes her use of high-resolution scanning electron microscopy to image the LINC complex at the nuclear envelope. It is always fantastic to see young people getting involved in microscopy.
A couple of years ago, the RMS launched its Mentoring Working Group with the aim of helping scientists develop new skills and receive specialist support towards career development. In a further education-themed article, some of our current mentees and mentors provide their feedback and describe their experiences.
Finally, anyone with an interest in the history of microscopy is sure to be captivated by the latest article from RMS Honorary Fellow Brian Ford, who brings us an amazing story on the use of scanning electron microscopy to study and characterise Leeuwenhoek’s microscopes, 300 years after his death.
I hope you enjoy everything this issue has to offer, and look forward to seeing as many of you as possible at elmi2024 in June – or one of our other RMS events later this year!
Slàinte!
Leandro Lemgruber

A house clearance in East Anglia in December 2023 revealed this little microscope, which proved to have been hand-made by the Dutch pioneer Antony van Leeuwenhoek, some 350 years ago. Find out more by reading Professor Brian Ford’s fascinating article on p4.
electron microscope (SEM) to examine them in detail4. The bulk was left untouched, so that future analysts could return to them for future study, uncontaminated by the modern world.
There was a surge of public interest following the international publicity given to my discoveries, and an unknown Leeuwenhoek microscope was taken to the Boerhaave Museum in Holland. Someone had realised what it might be. This was not just a fluke, for the interest continues – and others are still coming to light to this day5. In December 2023 yet another came to light. It was found during a
After some 350 years, newly discovered Leeuwenhoek microscopes are emerging. Two have now been subject to examination with the scanning electron microscope (SEM). Their maker, Antony van Leeuwenhoek, was the first microbiologist in history. He died 300 years ago, having shown us protozoa, bacteria, and spermatozoa for the first time. His work on plant anatomy was remarkably accurate, and he did much to dispel the dogma of spontaneous generation. I found his source of inspiration in Robert Hooke’s great work Micrographia, which was published in 1665 and was the talking point of London society when Leeuwenhoek made a visit to England the following year1. Hidden in the unnumbered pages of the preface to Micrographia, and never cited by other scholars, I found that Hooke had described in detail how to make what we now call a Leeuwenhoek microscope2. Not only that, but the first specimens Leeuwenhoek sent to the Royal Society (the publishers of Micrographia) had been described by Hooke, and in the same order3. After my discovery of Leeuwenhoek’s original specimens, the Royal Society allowed me to take small portions of each one to Cardiff University, where I am a Fellow, so I could use the Cambridge Stereoscan scanning

house clearance in East Anglia and was submitted to Christie’s auction house in London, who had sold one of the known Leeuwenhoek Microscopes in 2009 for close to half a million dollars6. This one didn’t make the mark; it cost the unknown Californian purchaser a mere £175,000.
Mr James Hyslop, head of science and natural history at Christie’s, spent many years at the Whipple Museum for the History of Science in Cambridge, and fully supported my proposal for scientific investigation, by examining the microscope in detail. He kindly agreed to bring it to the Cavendish Laboratory at Cambridge University for the day, so that I could scrutinise it under the SEM7
This was the second Leeuwenhoek microscope I had analysed in this way. The first was a brass instrument found in the Netherlands in landfill mud dredged from a canal in Delft, Leeuwenhoek’s home town. The reaction of the Boerhaave Museum (who have several Leeuwenhoek microscopes in their collections) was that this newly discovered brass microscope was one of their replicas that had been carelessly discarded. Placed side-by-side with a known copy, they clearly have much in common8
There was less doubt expressed over the silver instrument; nobody ever created replicas of Leeuwenhoek’s silver microscopes, due to the value of the materials. The way authenticity has always been established is based on provenance or personal judgement. For most of the surviving Leeuwenhoek microscopes there is limited documentation, and most lack a link back to Leeuwenhoek’s time. I set out to establish a protocol that would allow for objective authentication. The SEM allows us to discern how these little instruments were made, and that provides the evidence we need.
Low-power SEM is an unusual technique to apply to antique instrumentation. It combines images that have the astonishingly high resolution we expect

from electron microscopy, with the clarity we find when secondary electrons from a rasterised scan are correlated to generate an image. The reductionist aim with electron microscopy has long been to obtain images of greater magnification and higher resolution, though I have shown that submicron resolution in combination with low power magnification confers unforeseen benefits9 .
Although large-chamber SEMs are available for industrial applications, the specimen chamber of a conventional scanning electron microscope imposes restrictions on the area that can be scanned. Images captured in this case, using the Hitachi S-3400N SEM at the Cavendish Laboratory, University of Cambridge, measured ~1-2 mm so imaging a Leeuwenhoek instrument can involve the capture of ~100 separate files. Changes in focus or positioning can lead to minor perturbations in contrast, brightness, proportion or dimensionality, so assembling an image can require much manual adjustment from frame to frame. Once completed, the appearance of these diminutive instruments is gratifyingly detailed and allows us to ascertain
methods of manufacture and assembly. The greatly increased resolution gives the unique insights we need to establish authenticity.
Replica microscopes are always cut with industrial dies, often oversized in order to imitate a handmade screw. Leeuwenhoek did not have such sophisticated equipment, and the threads of his screws were rolled. This method of manufacture displaces metal, rather than removing it. The SEM reveals the characteristic signs, notably the tendency for the crest of the thread to be grooved. Detritus within the root of the thread is further indicative of its provenance.
The same procedure was used in creating the newly-discovered silver microscope. Evidence of production can be discerned on the specimen pin, which features a softly faceted profile due to its being hand-forged. A replica pin would have been turned on a lathe, and the SEM will immediately display the characteristic signs of tooling.
Leeuwenhoek clearly wished his microscopes to be functional, rather than perfectly finished. Sometimes he decorated the main handle with

three perforations, and the edge profile of each of his microscope body plates is always polished and rounded, whereas the brass sheet of the replicas is always cut with shears, and has a sharp, angular cross-section. Similarly, the perforated handles Leeuwenhoek produced are thin and seem neatly punched and polished, whereas the replica handles are thicker, and are conspicuously machined with a countersunk drill-bit. Even though Leeuwenhoek took pains to add this decorative feature, he made little attempt to ensure consistency of design – no two are the same – and bilateral symmetry was never his aim. One of the characteristics of his microscopes is their lightness. I found that the original brass microscope, for example, weighs a mere 6.24 g, whereas the mass of the Boerhaave Museum replica, with which some confused it, weighs 14.71 g. Clearly Leeuwenhoek conserved his metals.
For more than two centuries it was accepted that no further examples of Leeuwenhoek microscopes would ever be found. When Haaxman wrote his

pioneering biography in 187510, the instruments were mentioned in the past tense, as if all were then known. The microscopes in Leeuwenhoek's possession that were left at his death were auctioned in 1747, after his daughter Maria had died. That was believed to be the last opportunity for further unknown examples to emerge. Dobell’s master-work, published in 1932 to mark the tercentenary of Leeuwenhoek’s birth, speaks of the remaining microscopes with no hint that any more might yet be found11 .

Since then, four previously unknown examples of his microscopes have emerged, three of which had passed through my hands. No scholar could have anticipated such a series of revelations. Clearly, unlikely as it seems, there may be others yet to emerge.
It proved to be the SEM that provided me with the means to establish a protocol by which these finds could be objectively authenticated. It would now be instructive to apply these findings to the remaining microscopes attributed to Leeuwenhoek, for the provenance of several is often weak, and their

genuineness has been asserted by subjective opinion, rather than scientific facts. When I first learned of Antony van Leeuwenhoek when a schoolboy, there were nine known microscopes associated with his name.Today there are 13. But, were we to scrutinise them all with the guidance of the objective criteria that I have ascertained, several of those will prove to be copies or forgeries. Perhaps, at the end of all these proposed investigations, there may still be nine authentic Leeuwenhoek microscopes after all.
The author is grateful to Professor Richard Langford at the Cavendish Labortory, and to JJ Rickard and Eric Tapley for technical assistance. The Royal Society generously funded part of this research, published some of the results, and presented the findings both at their Conversazione and Soirée.
1: Robert Hooke, Micrographia, or some Physiological Descriptions of Minute Bodies, London, Royal Society: Martyn & Allestry, 1665.
2: Brian J Ford, What were the Missing Leeuwenhoek Microscopes really Like?
Proceedings of the Royal Microscopical Society, 18 (2): 118-124, 1983.
3: Brian J Ford, The van Leeuwenhoek Specimens, Notes & Records of the Royal Society 36 (1): 3759, 1981.
4: Brian J Ford, Bacteria and Cells of Human Origin on van Leeuwenhoek’s Sections of 1674 [leading paper], Transactions of the American Microscopical Society, 101 (1): 1-9, 1982.
5: Dean Golemis, Editorial: Another Leeuwenhoek Microscope Comes to Light, The Microscope, 70 (3): ii, 2023 https://doi.org/10.59082/ KEAI2623
6: Gar y Laughlin, Rare Leeuwenhoek bids for history, The Microscope 57 (1): ii, 2009. https://www.mccroneinstitute.org/uploads/ Editorial_57-1_2009-1477083872.pdf
7: Brian J Ford, The search for authenticity, Leeuwenhoek microscopes under the SEM, Microscopy and Analysis, 70: 15-17, 2024.
8: Brian J Ford, Recording Three Leeuwenhoek Microscopes, InFocus, Proceedings of the Royal Microscopical Society 40: 30-43, 2015.
9: Brian J Ford, New protocol for old microscopes, Laboratory News: 20-21, 2015 https://www.brianjford.com/w-1507-labnewsavl.pdf
10: PJ Haaxman Antony van Leeuwenhoek, de ontdekker der infusorian. Leiden: SC van Doesburgh, 1875.
11: Clifford Dobell, Antony van Leeuwenhoek and his ‘little animals’. London: John Bale, Sons & Danielsson, 1932. Author
Email: brianjford@cardiff.ac.uk
Brian J Ford has studied Leeuwenhoek for over forty years. He discovered his original specimens hidden in the archives of the Royal Society, investigated how they were prepared, and was the first to take micrographs of a Leeuwenhoek section through an original microscope. Professor Ford has published many hundreds of papers on microscopy, including 250 publications on Leeuwenhoek alone, and many of his books have been devoted to the microscope. He is a Fellow of Cardiff University, former Fellow of the Open University and Visiting Professor of Leicester University, and he is currently based at Cambridge.

We are very pleased to continue offering a range of ‘in-person’ and virtual events this year, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend. The following information was correct at the time of publication but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates.
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
5 Virtual International Microscopy Lecture Series - Dr Harald Hess, Online (Multi society event)
26 – 28 AFM & SPM 2024, Durham, UK
April
25 GW4 Cytomics 2024, Exeter, UK (RMS sponsored event)
29 – 30 EBSD 2024, Glasgow, UK
June
4 – 7 elmi2024, Liverpool, UK (RMS-hosted event)
July
8 – 9 Light Microscopy Summer School 2024 York, UK
10 – 11
Getting the most from your Confocal Course 2024, York, UK
15 – 19
Electron Microscopy Summer School 2024 Leeds, UK
17 Laboratory-based X-ray Phase Contrast Imaging Workshop, London, UK (RMS-hosted event)
August
4 – 9 Strathclyde Optical Microscopy Course 2024,University of Strathclyde, UK (RMS-sponsored event)
September
2 – 6 Flow Cytometry Course 2024 York, UK
March
26 – 28 flowcytometryUK 2025, Newcastle, UK
June / July
30 June – 3 July mmc2025: Microscience Microscopy Congress 2025, Manchester, UK
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
EBSD 2024
also exciting emerging applications of EBSD to the biological sciences. Talks will likely include 29 - 30 April, Glasgow, UK state-of-the-art developments in instrumentation Scientific organiser: Luke Daly, University of and software, new techniques, as well as a variety Glasgow of applications and uses of EBSD, transmission Kikuchi diffraction (TKD), electron channelling The EBSD 2024 meeting will be held in person contrast imaging (ECCI), and related microscopy in Glasgow at the Mazumdar-Shaw Advanced modalities. Research Centre, on the 29 and 30 April 2024.
As part of this series, we continue to be excited
This Annual UK-based EBSD meeting is an to hear from those who use these techniques to excellent opportunity for the multidisciplinary further our understanding of applied science and EBSD community to meet and share the newest engineering challenges, as well as industrial, energy developments and applications of EBSD, and and environmentalchallenges (including the use EBSD-related techniques, that are used to study of EBSD data in Industry 4.0 and for the energy materials across geoscience, materials science transition). and engineering, and physical science. There are
elmi2024
4 - 7 June 2024, Liverpool
The European Light Microscopy Initiative was created in 2001 to establish a unique communication network between European scientists working in the field of light microscopy and the manufacturers of their equipment. Its aim is to promote the quickly developing field of light microscopy as a fundamental research tool for

the life sciences and to strengthen the channels of communication between researchers, core facilities and industry.
The annual meeting, which has been running for two decades at various venues across Europe, has an excellent reputation within the microscopy community, making this meeting a key event in the calendar of hundreds of scientists and developers. The strength of this meeting lies in the mixture of scientific lectures on state-of-the-art, high-end microscopy combined with “hands-on” workshops and exhibition of the latest technology, organised by the leading companies in the field.

8 – 9 July, York, UK
Scientific organiser: Peter O’Toole, University of York
The Light Microscopy Summer School is a two day course held at the University of York covering the principles of light microscopy. Participants are also trained in practical issues surrounding light microscopy. After introductory presentations, the
10 – 11 July, York, UK
Scientific organiser: Peter O’Toole, University of York
This two-day, annual confocal course utilises many different sample types and fluorescent probes (DNA stains, classic antibody labels and fluorescent proteins) which are chosen to best demonstrate particular problems and techniques. Focus is always on the techniques they enable
15 – 19 July, Leeds, UK
Scientific organisers: Louie Aspinall, Nicole Hondow, Rik Brydson; University of Leeds
course is taught predominantly through hands-on practical sessions. The course is suitable for both novices and more experienced users wanting to gain a greater understanding of the microscope and feedback every year is always fantastic. Students usually come from a range of backgrounds, within both research and commercial organisations. All benefited greatly from the course and left with increased understanding and skills. The course is immediately followed by a two-day, hands-on Confocal Course (see below).
and the problems they generate, which will be applicable to any sample types. The two days consist of short tutorials followed by hands-on practice.
Day 1 takes participants through the basic principles of confocal microscopy and then trains them, through hands-on practice, how to configure and image multicolour, multidimensional samples using a confocal microscope.
Day 2 builds on the experience of Day 1 and enables participants to try FRAP and spectral profiling.
The Electron Microscopy Summer School aims to provide a basic training in both the theory and practice of scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The course covers imaging, diffraction and chemical microanalysis as well as the highly important area of sample preparation.




Accelerate tissue imaging and extract contextual information unbelievably fast – even with multicolor acquisition.
> Increase your slide scanning speed
> Perform fast and efficient multicolor acquisition
> Evaluate more markers with less photobleaching
> Reduce the time to optimal results with THUNDER Live
“Facility management: Impact, facility operation, user training and image data”
During the week of 2124 February, 2023 Global BioImaging (GBI) and the Mexican National Laboratory for Advanced Microscopy (LNMA) organised a workshop titled “Facility management: Impact, facility operation, user training and image data” in Cuernavaca (México).
The workshop has gathered facility staff and bioimaging experts from Latin America, USA, Canada, Africa, Asia, Europe, and Australia. Several tens of countries were represented!
GBI (https://globalbioimaging.org/) is “an international network of imaging infrastructures and communities” that support training and job shadowing between facilities around the globe.
The LNMA (https://lnma.unam.mx/) is a network

of affiliated laboratories developing and offering advanced imaging techniques to academic and industrial partners across Mexico. It is supported by the distributed national university of Mexico (UNAM) and the CONACYT research council.
During the workshop, participants were taught how to measure the impact of facilities, identifying key performance indicators that align well with the values of the institution, and socio-economic indicators to evaluate the effects on the community, in terms of contribution to the overall education and technology development.
The intricate world of facility management was delineated, helping the participants to understand better how to manage microscopes, budget for the facility, offer efficient training, and deal with user expectations. The lessons on leadership and management held by Claire Brown, Phil Hockberger and many other trainers were invaluable.



In between training sessions we had the opportunity experience during the many group activities and to listen to seminars held by local speakers who are talks that a few of us were invited to do about how working to improve the education system across our facilities work. the country.
After the workshop I could finally meet the Particularly impressive is the effort of UNAM postdoc that I helped recruit during one of our university to implement ISO quality standards collaborations with the research group led by Adán across 3,000 research labs that host half a million Guerrero at the LNMA. This is a sign that “what we students in the country. This mission aims to do always has an impact”, as we learnt during the workshop. provide every student with the same teaching, guaranteeing the quality of research methods and The workshop took place in Cuernavaca, state of reliable standards for pharmaceutical research. The Morelos, which is in the centre of the country and quality management system has been adopted by located close to many marvellous world heritage 33 faculties and institutes and relies on 150 internal sites such as Xochicalco pyramids, the systems of auditors and 500 academics specialised in quality canals in Xochimilco and the world’s largest system control (seminar held by Monica Gutierrez, UNAM). of caverns that is the Cacahuamilpa national park.
A further initiative - the “Mexican Bioimaging I would like to thank the Royal Microscopical Workshops” - is aiming to reverse the concept of Society and the Francis Crick Institute for allowing centralised knowledge, by organising microscopy me to attend this Global BioImaging workshop, and image analysis workshops across the country, which was very well organised by the local host contributing with delocalised education to beat Adan Guerrero (IBT, LNMA) with the support of all social inequalities (Diego Delgado-Álvarez). the wonderful members of the LNMA lab.
Personally, taking part in the workshop has been
an enriching experience, allowing me to network
D’Antuono
Principal Microscopist and Image Analyst with many colleagues working in imaging facilities.
The Francis Crick Institute, London, UK I could get their perspective and learn from their
Student: Lakshana Baheerathan
Supervisor: Professor Martin Goldberg
Project location: Durham University, Imaging Facility
The cytoskeleton is anchored to the nucleus (specifically, the nuclear lamina) via the versatile connectors collectively named as the LINC complex. LINC consists of inner nuclear membrane proteins (SUN1/2), which bind to the nuclear lamina and chromatin, and link, via their C-terminal SUN domain in the nuclear envelope (NE) lumen, to the C-terminal KASH domain of outer nuclear membrane nesprins. Interactions between the various SUN and KASH pairs provides a direct physical link between the cytoskeleton and nucleoskeleton and LINC plays a crucial role in the bio mechanical properties of the nucleus (such as stiffness, mechanotransduction, chromosome positioning). Consequently, it may have critical roles in controlling nuclear deformability and integrity during cell migration, with implications for metastasis and genome instability. Depending on the isoform of nesprins, they directly or indirectly link to cytoskeletal filaments. For example, Nesprin-1 and -2 giant isoforms tether F-actin directly through their N-terminal actin binding domain.Thus, nesprincytoskeleton complexes, LINC complex, form a 3-D filamentous network that covers the NE exterior. Current models portray the LINC complex as simple anchorage points at the nucleus for the
cytoskeleton which radiates out from the nucleus. On the other hand, the LINC complex could also form a cage around the nucleus which attaches to the cytoskeleton. This implies, for instance, that mechano-sensing, via the cytoskeleton stretches and results in the tensioning of NE and connected chromatin broadly, rather than in a localised area. As opposed to the point anchorage structure, this would greatly contribute to the biomechanical properties of the nucleus and influence its deformability during restricted migration.
There is indirect evidence using immunofluorescence microscope and F-actin binding assays that suggest that nesprins could form similar structures at the NE. However, such a structure has not been directly imaged and this is the aim of the project.
Nesprin 2 Giant molecules are 800kD in size with a distinct extended structure and previously established extraction and fixation (aldehydes and tannic acid, OsO4) protocols, followed by critical point drying and sputter coating with ultra-fine platinum allows them to be imaged. However, the main challenge was to expose the nuclear surface enough while still retaining the cytoskeletal

attachments in order to image and interpret the hypothesised Nesprin 2 Giant network.
Initially, we used a standard cytoskeletal SEM sample preparation on silicon chips followed by coating with 3nm of platinum and a simple dry-fracture process which exposes the nuclear surface to varying degrees. We optimised this protocol by testing a variety of extraction detergents and incubation periods and found that 0.75% saponin extraction buffer with an incubation period of 20 minutes best exposed the layers of the nuclear membrane while retaining cytoskeletal filamentous attachments. However, upon fracturing with an adhesive we found that the nuclei and often the entire cell is completely removed, leaving only cell remnants. We

also tried different drying methods such as critical point drying and HMDS and found that the latter was much better for whole cell morphological visualisation. The optimum method to access the nuclear surface and reduce preparation artefacts was to cryo-section saponin-detergent extracted cells.
Furthermore, we used a gelatin-based postembedding immunogold labelling SEM protocol of ultrathin thawed cryo-sections, in accordance with the Tokoyasu method. Well characterised (primary) antibodies to N- and C-terminal ends of Nesprin 2 Giants (and secondary labelling with 10-nm colloidal gold) were used to elucidate doubts about the identity of structures that persistently appear

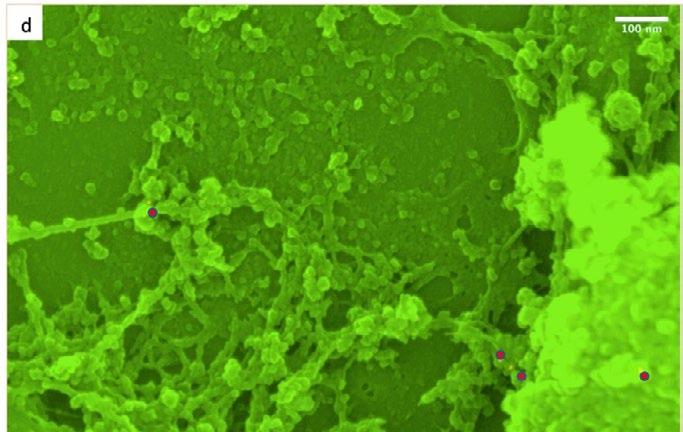


on the outer nuclear surface. Labelled sections at the nuclear envelope. However, optimisation of were coated with 3nm chromium and were the protocol involving improvements in antibody examined in the SEM. Images from secondary and selection line and dilution must be made for more backscattered images are acquired simultaneously specific and reliable binding. and are superimposed to show the location of gold Imaging at higher magnification also reveals a (labelling the protein of interest) on the secondary parallel and highly organised network of filaments image. (with a diameter ranging 10-15nm) that line the
nucleoplasmic side of the inner nuclear membrane. Based on previous literature, I hypothesise that this Our results provide preliminary evidence that network is the nuclear lamina. The network can be combines both the point anchorage and cage described as a sort of railroad that lies directly on structure model of the LINC complex at the nuclear the inner nuclear membrane and imaging at higher envelope. Images depict a large protein or group magnification revealed that heterochromatin and of proteins that are regularly spaced at intervals nucleosomes are associated or somehow bound in ranging from 150 – 200nm. I hypothesise that these a way to this network. This structure is similar to protein mounds act as an anchor that connects the those previously found in research associated with cytoplasmic cytoskeletal architecture directly to the identifying a nucleoskeleton. Imaging also revealed outer nuclear membrane (ONM). Spreading from that the filaments that spread over the surface of this point are filaments with diameters comparable the ONE forming a cage make direct contact with to actin and intermediate filaments, but short, finer the ONE and pass through the nuclear envelope fibres have also been visualised. I believe that the and embed in the inner nuclear membrane. protein anchor could act as a potential nucleation site that allows the extension of these filaments.The
filaments spread over the surface of the ONE and I learnt a vast array of sample preparation appear to connect to neighbouring protein mounds. techniques including cell culture, cryosectioning, Immunogold labelling revealed that the hypothesised glass knives making and critical point drying. While protein anchor are (or contain) Nesprin 2 Giants. anxious at first, I became much more confident in Immunogold SEM examination revealed that my abilities and understood the importance of not pAbK1 Nesprin-2-specific antibodies recognise putting too much pressure on the experiments both nuclear and cytoplasmic Nesprin-2 proteins themselves. Working primarily with scanning
electron microscopy, I gained a deeper appreciation for the physics underlying the principle of the SEM and learnt how to adapt the sample preparation and imaging techniques. To balance the resolution and magnification with sample contamination I tried out different sectioning and fracturing techniques and used various accelerating voltages.
I really enjoyed the freedom of designing and carrying out experiments independently and learning and adapting as I went along. I also had an opportunity to present and discuss my ideas during different stages of the studentship, and this allowed me to critically analyse and produce original ideas that directed and fuelled subsequent stages of the project.
Prior to this summer, my research interest tended to mostly lie around biochemical analysis, but this summer studentship has given me the time and experience to confirm my interest in cell biology research. While I have always appreciated the quantitative side of research, the freedom to critically interpret visual data and be creative with it is incredibly exciting as it fuels further research.

This is the first independent project that I have conducted and it has given me both technical and transferable skills that I hope to use as I progress in the world of research.
Following this summer, I am conducting a research project focusing on the biosynthesis of collagen and will be using confocal and immunofluorescence microscopy and live cell imaging. Additionally, I am keen on undertaking a master course targeted on understanding molecular and cellular signalling processes and aim to incorporate microscopy as a significant component of the project.
Student: Mohamed Y. Ghali
Supervisors: Kevin F. Webb, Optics & Photonics Research Group; Steve Greedy, George Green; Institute for Electromagnetics Research
Project Location: Electrical & Electronic Engineering, University of Nottingham
Imaging living cell samples is challenging due to the meniscus formed by immersing liquid at container walls. The meniscus distorts the passage of illumination, creating artefacts in transmitted light microscopy. This is especially a problem with phase contrast microscopy1, where the illuminating annulus must match the phase ring to produce phase contrast (as in Figure 1).
The effect of this meniscus distortion can be captured with an image of the microscope back focal plane (Figure 1, insets), which quantifies the misalignment between the phase ring (dark ring) and the illuminating annulus (bright ring). This mismatch can affect the XY-position, diameter, and circularity of the illumination (inset, Figure 1B).
This project aimed to physically correct this misalignment using a mechanical actuator (multi-
axis robot arm, Figure 2) to physically reposition the illuminated annulus in space, utilising the condenserfree illumination regime developed by Webb 2
The approach taken is described in the flowchart in Figure 3, where image processing (OpenCV) was used to locate and measure dark and bright ellipses in images of the back focal plane. Geometric parameters describing the distortion (Figure 4) were determined in real time, in order to guide the


motion of a multi-axis robot arm (WidowX-200, Trossen Robotics, IL) controlled by an OpenCM9.04 microcontroller3, which held a 100mm diameter white COB LED ring (eBay). This functioned as the condenser-free phase contrast illuminator for a Leitz Diavert inverted microscope.
A Raspberry Pi 4B single-board computer, equipped with v2.1 Pi camera, was focussed on the objective back focal plane using a phase telescope. OpenCV used a thresholded binary image of the back focal plane to fit an ellipse to the illuminating ring pixels. This was then compared to the hardwired phase ring within the objective to determine the relevant corrections (XY-translation, eccentricity, and inclination) to correct the size, shape, and position of the illuminating ring to again superimpose with the phase ring. Figure 4 shows the output
of the image processing algorithm, showing ring misalignment caused by a water droplet. Figure 4b shows the correction factors required to superimpose the illumination and phase rings.These corrections were sent via UART data interlink to physically reposition (in XYZ) and incline the ring in order to achieve the required correction via the robot arm (Figure 1). The project will now be furthered through my capstone final year individual BEng project in Electrical & Electronic Engineering.
Prior to this project, I had used microscopes a few times during biology experiments in school, where usage comprised blindly following instructions with very little understanding. During this project, my knowledge about optics and microscopy was vastly expanded. I gained familiarity with various microscopical tools for phase contrast microscopy in particular, including phase telescopes and the relationships between image and back focal planes. Further skills which are vital to any EEE engineer were also learned/strengthened, including 3D modelling/prototyping, image processing, UART communications, and 3D kinematic control architectures.
Whilst completing the final year of my BEng degree, my aim is now to look for support for a PhD programme (ideally in the UK), with the goal of an academic or research career. Prior to this summer studentship, my PhD ideas revolved around any subjects utilising IoT technology and/ or autonomous vehicles. This summer project catalysed a new interest in further specialisation on applications involving optics and/or microscopy, perhaps using information extracted through microscopy to control different parameters (temperature, humidity etc.) in an incubator, or to


1. Hofmeister, A., Thalhammer, G., Ritsch-Marte, M., & Jesacher, A. “Adaptive illumination for optimal image quality in phase contrast microscopy”. Optics Communications, 459, 124972, 2020, doi: doi:10.1016/j. optcom.2019.124972.
2. Webb, K.F., “Condenser-free contrast methods for transmitted-light microscopy”. Journal of Microscopy, 257: 8–22, 2015, doi:10.1111/ jmi.12181.
guide autonomous vehicles transporting biological cargo to optimise their travel (slow down; take a break; open windows etc.) to cater for their biological cargo.
3. ‘WidowX 200’, WidowX 200 Robot Arm. Accessed: Sep. 26, 2023. [Online]. Available: https://www.trossenrobotics.com/widowx200-robot-arm.aspx

Guest
Extended


Name of Student: Miriam Czech
Supervisor: Susan Cox, Siân Culley
Project location: Randall Centre for Cell & Molecular Biophysics, King’s College London
Fluorescence microscopy is an essential tool in cell biology as it allows scientists to look at what is inside of a cell and easily see the organisation of organelle by labelling different organelles selectively. One of this technique’s major limitations is that the light needed to image a live sample can also damage it, if applied at too high an intensity or for too long. This leaves the researcher needing to choose between a limited duration of imaging or image artefacts introduced by photo-damage. A possible solution is imaging at a low laser intensity and later computationally restoring acquired images (Weigert et al., 2018). My project focused on investigating two state-of-the art deep learning methods used for image restoration in microscopy. The two learning networks were trained on synthetic images. Their performance was evaluated against parameters of the laser intensity of imaging and the structural content density in terms of four restoration quality assessment metrics that are conventionally used for assessing denoising methods. The performance of the networks at background and foreground prediction was also compared. Finally, a comparison to the simplest denoising technique of applying a Gaussian blur was made.
The project’s aim was to better understand two popular denoising networks, CARE (Weigert et al., 2018) and Noise2Void (Krull et al., 2019) and to test the appropriateness of four quality assessment metrics conventionally used in training and
evaluation of image restoration networks used for enhancing the quality of microscopy images.
To achieve the aim, the two architectures were examined against images with different amounts of structure, and different peak signal values.
After training a network, its predictions were compared with their source ground truth in terms of the conventionally used metrics - Normalised Root Mean Squared Error (NRMSE), Peak Signal to Noise Ratio (PSNR), Structural Similarity Index (SSIM) and Multi-Scale Structural Similarity Index (MS-SSIM). The networks were also benchmarked against the simplest possible denoising technique of a Gaussian blur. Furthermore, the analysis was complemented by looking at the pixel values’ histograms and qualitative inspection of chosen pairs of images with inspection ImageJ tools.
To easily obtain an adequate volume of training and evaluation datasets with a wide, fine-grained, and systematically defined spectrum of parameters’ values, synthetic images were generated. For each parameter, three distinct values were chosen for training and 20, within the same range, for evaluation. Poisson noise was applied to simulate the sort of noise expected in low power intensity microscopy images.
Surprisingly, CARE did not outperform the technique of Gaussian blur (GB) for most parameter

combinations. Its performance was especially poor structural content combinations. for low structural content density and low signal.
An N2V network was trained with images with a It was hypothesised this stems from an intensity Gaussian background. According to NRMSE, PSNR correction the algorithm applies to deal with pixels and SSIM, on average it did not outperform GB for of very low and high intensity. These are returned only a minority of parameter combinations with a by the camera because of a Gaussian laser beam low structural content and low signal peak value. profile - an artefact caused by most common lasers.
In terms of MS-SSIM, N2V outperformed GB in To investigate this, a large background Gaussian most cases, failing for high density, low signal values was added leading to better performance for all combinations. Its restoration improved images for all parameter combinations according to NRMSE, and combinations in terms of NRMSE, PSNR and MS- improving most combinations according to other SSIM and SSIM. metrics. To understand the impact of altering the structure on the metrics, the impact of changing
The background was predicted less successfully than the number of blobs in the model was evaluated for the foreground by the N2V network. However, the each metric (see Figure 1). maximum discrepancy between the two was much smaller than for CARE. In terms of NRMSE, N2V The performance of the network architecture proved to be influenced by the diversity in the seems to have a more consistent performance training data. Another network was trained on across different levels of structural content and a narrower spectrum of signal values and the a more balanced one between background and performance was improved, except for low foreground, whereas CARE seems to do better than
N2V with high structural content and high signal value.
From a visual inspection we found that the errors in the background are unlikely to be spotted by eye. Since in microscopy the background pixels usually dominate, the overall metric scores get dominated by the background error. Hence, the overall image metric scores are possibly not reflective of the actual quality of restoration if the accuracy of the foreground is the primary consideration. Thus, this study shows the need to better understand how the restoration technology underpinning evaluation metrics works for fluorescence microscopy images.
I really enjoyed learning to use the PyTorch framework and figuring out how to do operations on tensors to achieve high code efficiency when operating on large volumes of data. Discussing with my supervisors the possible explanations for the results we would get was extremely developing and exciting. I also loved going to my host lab’s weekly lab meetings and listening about what lab members were working on at the time.
I learned about the basic terms relating to neural network training and performance evaluation, how to use TensorFlow’s TensorBoard to help with improving the quality of training and ways of identifying whether a network is undertrained or overtrained. Moreover, I learned the basic premises of neural networks such as the gradient descent algorithm, the fundamental structural elements of a neural network and about the U-net convolutional neural network architecture in more detail. Participating in the weekly lab meetings and the discussions with my supervisors taught me about the current limitations of microscopy as physical science and the applicability of computational techniques as possible ways forward and types of image restoration assessment methods.
The project allowed me to explore the mathematical
premises of neural networks in more detail and motivated me to dive deeper into how neural network architectures are designed. I also got very excited about developing further my ability to use the PyTorch framework. I had not known much about computer vision and the internship made me excited for some potential related projects in the future.
I was also able to witness the day-to-day life of a lab researcher and was especially captivated with how much autonomy there is in the work and to what a large extent it seems to be dictated by where their curiosity takes them. My internship experience added to arguments in favour of pursuing a PhD someday.
1. Krull, A., Buchholz,T.-O. and Jug, F. (2019) ‘Noise2void - learning denoising from single noisy images’, 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) [Preprint]. doi:10.1109/cvpr.2019.00223.
2. Weigert, M. et al. (2018) Content-aware image restoration: Pushing the limits of fluorescence microscopy, Nature News.Available at: https:// www.nature.com/articles/s41592-018-0216-7 (Accessed: 16 October 2023).

Student: Hio U Lao Kathleen
Supervisor: Dr William Tipping, Professor Duncan Graham
Where the project took place: University of Strathclyde, Glasgow
Supported by an RMS Summer Studentship award, I spent time in the lab of Professor Duncan Graham at the University of Strathclyde to study the uptake and distribution of Bruton’s tyrosine kinase (Btk) inhibitors, ibrutinib, acalabrutinib and ibrutinib-yne using a combination of Raman and stimulated Raman scattering microscopy. Raman imaging techniques are beginning to shed new insight into drug localisation and drugcell interactions without the use of exogenous tags and labels. We sought to detect Btk inhibitors at their site of action in the cellular environment to detect differences in uptake concentration and localisation in a panel of cell lines expressing different levels of Btk. We also investigated the impact of Btk inhibition on cellular lipid metabolism.
Lab Summary:
Bruton’s tyrosine kinase (BTK) was known to be highly expressed in B cells, and some breast and prostate cancer cell models. It is a crucial mediator in cellular function and is involved in B cell proliferation. Here, we used Raman microscopy and stimulated Raman scattering (SRS) microscopy to visualise the cellular uptake of Btk inhibitors (Btki) in cancerous cell lines. We selected cell lines which express low levels of Btk including the ovarian cancer cell model HeLa and the hepatocellular carcinoma HepG2 cells, whilst the prostate cancer model PC3 and chronic myeloid leukaemia cell line K562 were used because these cell lines express moderate levels of Btk. Three Btk inhibitors were selected: ibrutinib (first generation) and acalbrutinib
(second generation) are approved Btk inhibitors, whilst with ibrutinib-yne contains a phenylacetylene motif that provides access for click chemistry applications. We sought to determine the uptake and localisation of these molecules across the panel of cell lines together with detection of ibrutinibyne using the alkyne tag as a marker in the Raman spectrum.
Various concentrations of Btki were treated on cell lines for 4hr, 18hr and 24hr. However, no alkyne peak was observed in both Raman mapping and SRS results of cell lines that express low levels of Btk (HeLa and HepG2). Lipid droplet analysis showed that in the prostate cancer cells, PC3 resulted in higher percentage of lipid droplets
than compared to the prostate healthy, PNT2 cells. Following treatment with Btk inhibitors, we detected a reduction in lipid droplets after drug treatment which we predicted was related to the cytotoxicity of drug effects. To test this, cell viability assays were performed using AlamarBlue which showed Ibrutinib induced a higher cytotoxicity effect compared to Acalabrutinib, which correlated to a greater lipid droplet reduction in these cells. In K562 cells, we were able to detect the localisation of ibrutinib-yne using the Raman signal of the alkyne group at 2107 cm-1 indicative of the alkyne vibration. The signal was concentrated in small punctate in the cell cytoplasm which we propose could be the lysosome, which is an organelle used for the degradation of waste products in the cell. Studies are currently ongoing to identify the nature of this interaction using a multiphoton Raman and fluorescence imaging approach.
In conclusion, although low Btk-expressed cells were not able to be screened using Raman microscopy, our further approach is to work on screening high BTK expression cells using Ibrutinib-yne.
We focused on treatments using Btk inhibitors Ibrutinib (Ib), Ibrutinib-yne (Ibyne) and Acalabrutinib (Acal) on cell lines that consist of relatively low Btk expression (HeLa and HepG2) together with cell lines that expressed moderate Btk levels (PC3 and K562). The presence of alkyne moiety within the

structure of ibrutinib-yne allowed label-free imaging of the drug uptake using Raman microscopy and SRS microscopy.
Human cancerous cell lines (HeLa, HepG2, PC3 and K562) and normal cell line PNT2 were split and cultured in DMEM and RPMI respectively. Cells were treated with concentrations of 2-50 μM for a range of timepoints (1hr, 4hr, 18hr, 24hr) and were incubated at 37°C with 5% of CO2. Overnight serum starvation (-FBS) was also performed in all cell lines.
Cells were cultured and incubated overnight with excess PBS filled in the outer wells. HeLa cell lines were treated with Ib and Acal in concentrations ranging from 100 μM to 0.03 μM for 72hr. PC3 and K562 cell lines were treated with Ib, Acal and Ibyne with the same concentration range and time. 10 μl of Alamar Blue was used in staining for analysis following 4hr incubation in each well.
Raman spectra and mapping were obtained at 532 nm using the Renishaw microscope, with a 60x objective lens, 1 μm step size in x and y coordinates, 5s acquisition time, 36 mW laser power and 1500cm-1 for the spectral centre.
For SRS microscopy images were captured at


2930cm-1

proteins and lipids. Alkyne images were acquired at 2107 cm-1. To do so, the pump and Stokes laser powers were set to 0.15 W and 0.3 W respectively. The image acquisition parameters were as follows: 410 v gain, 10Hz acquisition rate, across a 512 × 512 frame.
Raman mapping was first performed in HeLa cells which showed that label-free ratiometric imaging of the protein, lipid and DNA content was possible (Figure 1). The cellular lipid content was detected at 1440 cm-1/1656 cm-1 (CH2/amide-1), nucleic acid contents at 1340 cm-1/1656 cm-1 (C-N mode/amide-1), and protein content using the phenylalanine stretch at 1003 cm-1/ 1656 cm-1 (phe/ amide-1). The ratiometric imaging revealed a labelfree method for cellular visualisation based on the vibrational spectrum.
In cells treated with Btk inhibitors, a reduction in cellular lipids was observed at 1440 cm-1. We
believe this may be due to toxicity causing the cells to use stored lipids for energy demands. Additional Raman mapping was performed on CaF2 plates which showed a reduced spectral background. We then elected to use SRS microscopy to visualise the prostate cell lines due to the faster image acquisition rates for SRS microscopy compared to Raman microscopy. SRS microscopy showed that the PC3 cells had more lipid droplets than the PNT2 prostate healthy cells when treated with ibrutinib (5 μM, 4 h) because an increase in the signal at 2851 cm-1 (CH2 stretch, Figure 2) was observed.
K562 cells which express high levels of BtK were treated with ibrutinib-yne (5 μM, 4 h) and SRS revealed an alkyne signal was detected at 2107 cm-1 indicative of the alkyne stretching frequency (Figure 3). The alkyne signal was not detected in K562 cells treated with ibrutinib (5 μM, 4 h) as expected. However, in cells that express low levels of Btk, we found that Raman microscopy might not be sensitive enough for detecting alkyne signals following treatment with ibrutinib-yne. Future studies will

investigate the quantification and localisation of ibrutinib-yne in real-time to identify mechanisms of drug uptake and retention.
Reflection:
Throughout the studentship, the research project has been more challenging and inspiring than I initially anticipated. Engaging in the planning and conducting of experiments has helped me to further develop a more flexible and comprehensive mindset. Through working both independently and collaboratively, I have not only strengthened my practical skills and reinforced my knowledge, but I have also learnt how to work and communicate efficiently with others. I particularly enjoyed the practical side of this project, from learning about cell splitting and culturing to treatments and operation of the Raman and SRS equipment. It has been an incredibly fascinating experience for me thus far. Furthermore, I was also delighted to have the opportunity to attend an imaging conference, where I had the chance to understand some of the cutting-edge research being carried out by students
and professors across Scotland. In conclusion, the experiences and skills I have acquired throughout this studentship have provided me with the motivation to work more independently, with great efficiency and attention to detail in my upcoming industrial placement. I am very grateful to the RMS and the department of Chemistry for this valuable opportunity.

Student: Rebecca Harry, University of Oxford
Supervisor: Professor Claire Friel, University of Nottingham
Project Location: University of Nottingham, School of Life Sciences, Friel Lab
Lay Summary
Eukaryotic cells have a cytoskeletal structure which helps to maintain shape and organise components of the cell. The cytoskeleton provides mechanical support that enables cells to carry out essential functions like division and movement. The key components of the cytoskeleton are microtubules, actin filaments, and intermediate filaments. This project explored how microtubules are built up and broken down dynamically inside eukaryotic cells, contributing to the constantly changing cellular environment and helping with cell division.
Kinesins are biological motor proteins that work in an ATP-dependent manner, working in cells to transport molecules along microtubules. The kinesin-13 family drive depolymerisation (breakdown) of the microtubules, contributing to the regulation of these fibres in the cell. In this project, I generated microtubules in vitro with rhodamine labelled tubulin and then followed their depolymerisation when MCAK (a kinesin-13 family protein) was added. This data was then analysed to calculate the rate at which the microtubules were depolymerised. This allowed


comparison between MCAK alone and MCAK in depolymerisation assay to help gain understanding the presence of various fragments of BRCA2, that of how interactions that have been detected have been shown to bind to MCAK, on the rate between MCAK and BRCA2 affect the microtubule of microtubule depolymerisation. This work allows depolymerising activity of MCAK. This has us to understand more about a recently discovered possible therapeutic implications due to the role interaction between MCAK and BRCA2 which of microtubules in cell division and BRCA2’s well could have therapeutic importance due to the role known role as an oncogene. of microtubules in cell division.
Project Aim
Using a depolymerisation assay allowed us to follow The aim of this project was to conduct a the process of microtubule depolymerisation by

MCAK. To set this up, microtubules were grown in vitro with rhodamine labelled tubulin and then imaged using a high-resolution light microscope. In order to conduct a depolymerisation assay, channels were engineered between two coverslips, and the channel then filled with anti-tubulin antibodies. This allowed us to fix microtubules in the channels so that they could be imaged in the same position throughout the experiment. Next, MCAK was added to channel at a set timepoint and then the process of depolymerisation captured via timelapse capture. Once a baseline rate for MCAK activity had been established, we could then test the effect of each of the BRCA2 peptides on the activity of MCAK.
From completing depolymerisation assays with
1) MCAK, 2) MCAK plus a BRCA2 peptide, and 3) MCAK plus a phosphorylated BRCA2 peptide, we observed no significant effect of either BRCA2 peptide on the ability of MCAK to depolymerise microtubules (Figure 1 and Figure 2). These data indicate that binding of these BRCA2 peptides to MCAK does not impact microtubule depolymerisation and that we should look elsewhere for the functional impact of the observed interaction between BRCA2 and MCAK.
During this project I learnt how to grow microtubules in the lab so that they could be visualised and analysed via microscopy. I also learnt the importance of kinesins in depolymerisation in addition to their more traditional role as transporters along microtubules, moving key components around the cell. I also learnt more about how microscopy can be essential for tracking processes in the cell over a time period as well as for single frames which visualise just one moment. In particular, I enjoyed the process of starting from tubulin subunits and building up to a working assay with microtubules that could then be used to assess protein activity.
I had not previously thought of microscopy as an essential technique for in vitro protein study, so I was appreciative of the diversity in which it can be used to study biological processes.
This project was my first real experience with working in a lab on a set project, due to various limitations that were in place due to Covid-19 earlier in my degree. I enjoyed working in a lab, with the opportunity to work independently but still with the support of my supervisor, Professor Claire Friel. After completing this project, I will be researching PhD programmes going forward, as well as completing my Masters this coming year. Moving forward, I will continue to view microscopy as a key tool for the biochemist, useful in both in vitro and in vivo processes, as well as static and dynamic situations. I will be incorporating microscopy into my experiments investigating X chromosome silencing, using immunofluorescence to examine efficiency of the process under different conditions. I am extremely grateful to the Royal Microscopical Society for enabling me to complete this valuable experience and supporting my interest in the Friel group’s work.


This image, which was shortlisted in the AFM & SPM category of the 2023 RMS Scientific Imaging Competition, shows atomically resolved crystal structure of MoS2 surface - clearly identifying a missing sulfur atom. This image was obtained via conductive atomic force microscopy under ambient conditions.
Image credit: Saima Aktar Sumaiya, Mehmet Baykara (University of California, Merced; Columbia University).

Also shortlisted in the AFM & SPM category of last year’s imaging competition was this 3D height visualisation of the internal peptidoglycan structure of a Staphylococcus aureus mutant. It shows individual glycan strands, taken with AFM in liquid. The colour scales correspond to the height data (lower – green; higher - blue).
Image credit: Dr Laia Pasquina Lemonche (University of Sheffield).

Shortlisted in the Electron Microscopy (Physical Sciences) category of the RMS Scientific Imaging Competition, this is a coloured scanning electron micrograph of particles from a silver roller ball ink pen in their carrier (pink) on newspaper.
Image credit: Steve Gschmeissner.

Sub-units
Shortlisted in the Electron Microscopy (Physical Sciences) category of the RMS Scientific Imaging Competition, this is an image from thin films of manganese dioxide, developed for confection of flexible polimeric structures. The acquisition was performed by scanning electron microscopy (JEOL- JSM IT500 HR) in the Multi-user Center for Analysis of Biomedical Phenomena (CMABio-UEA) located in the middle of the Amazon rainforest, in the north of Brazil.
Image credit: Jessica Araujo Marques and Jander Matos Guimarães (Universidade do Estado do Amazonas).

The RMS is very proud to be hosting elmi2024, taking place in Liverpool, UK, from 4 - 7 June.
With a blockbuster scientific programme , worldclass exhibition, ‘hands-on’ workshops and more, the European Light Microscopy Initiative (elmi) has become regarded as an essential event for the Light Microscopy community.
‘Early bird’ booking rates are available until 9 Aprilproviding a discount of more than 20% on the cost of a standard ticket, and 30% off for students. Find out more about registration rates and book now!

The European Light Microscopy Initiative (elmi) was created in 2001 to establish a unique communication network between European scientists working in the field of light microscopy and the manufacturers of their equipment. Its aim is to promote the quickly developing field of light microscopy as a fundamental research tool for the life sciences and to strengthen the channels of communication between researchers, core facilities and industry. The event has been running for two decades at various venues across Europe, and has an excellent reputation within the microscopy community, making it a key fixture in the calendar of hundreds of scientists and developers.
The strength of this meeting lies in the mixture of scientific lectures on state-of-the-art, high-end microscopy, combined with ‘hands-on’ workshops and an exhibition of the latest technology, organised by the leading companies in the field.
elmi2024 is being held at ACC Liverpool, a purposebuilt arena and convention centre in the heart of
the iconic city, on the King’s Dock.
The venue has great transport links as well as being walking distance from a large number of hotels, restaurants, shops and bars.
Liverpool is one of the UK's best loved cities with a rich, cultural heritage, a wide range of attractions and buzzing nightlife.
With the conference venue just a short walk from the bustling waterfront and commercial centre, there is no shortage of things to see and do during your stay.
• A blockbuster meeting programme covering all the latest techniques, applications and technology. Topics include: New Technologies, Imaging Across Scales, Super-resolution and Nanoscale Imaging, The AI Revolution, The Science of Tomorrow Today, and Multimodal Imaging.
• A wide range of companies showcasing their latest technology and running workshops timetabled outside of the main meeting programme.
• An accompanying exhibition in the purposebuilt hall alongside posters, food and drink;
• A community workshop space at the heart of the exhibition, with many groups hosting meetings and running workshops.
• An event dinner with networking at the Rum Warehouse.
https://www.elmi2024.org/

The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences.
You can read the latest Early View papers online at www.journalofmicroscopy.org
They include:
Multimodal optical mesoscopy reveals the quantity and spatial distribution of Gram-positive biofilms in ex vivo tonsils
Megan Clapperton,Tash Kunanandam, Catalina D. Florea, Catriona M. Douglas , Gail McConnell
Biofilms, which are communities of bacteria known to be more resistant to antibiotics than normal (planktonic) bacteria, are thought to play a role in diseases of the tonsil. Biofilms are difficult to study in patient tissue due to limits in standard microscopes, which compromise either on the tissue volume that can be studied or the amount of detail in the image. We have used the Mesolens, a novel microscope lens, to visualise bacteria and biofilms in unusually large volumes of freshly excised tonsils from a local children’s hospital following tonsillectomy. Using this method, we have shown that biofilms were present in all diseased tonsils on both the surface and the interior of the tonsil.This new way of studying diseases in human tissue may prove useful in understanding the role of biofilms in other diseases and infections.
ORIGINAL ARTICLE (Open Access)
An automated slide scanning system for membrane filter imaging in diagnosis of urogenital schistosomiasis
Prosper Oyibo,Tope Agbana, Lisette van Lieshout,Wellington Oyibo, Jan-Carel Diehl, Gleb Vdovine
Traditionally, automated slide scanning involves capturing a rectangular grid of field-of-view (FoV) images which can be stitched together to create whole slide images, while the autofocusing algorithm captures a focal stack of images to determine the best in-focus image. However, these methods can be time-consuming due to the need for X-, Yand Z-axis movements of the digital microscope while capturing multiple FoV images. In this paper, we propose a solution to minimise these redundancies by presenting an optimal procedure for automated slide scanning of circular membrane filters on a glass slide. We achieve this by following an optimal path in the sample plane, ensuring that only FoVs overlapping the filter membrane are captured. To capture the best in-focus FoV image, we utilise a hillclimbing approach that tracks the peak of the mean of Gaussian gradient of the captured FoVs images along the Z-axis. We implemented this procedure to optimise the efficiency of the Schistoscope, an automated digital microscope developed to diagnose urogenital schistosomiasis by imaging Schistosoma haematobium eggs on 13 or 25 mm membrane filters. Our improved method reduces the automated slide scanning time by 63.18% and 72.52% for the respective filter sizes. This advancement greatly supports the practicality of the Schistoscope in large-scale schistosomiasis monitoring and evaluation programs in endemic regions. This will save time, resources and also accelerate generation of data that is critical in achieving the targets for schistosomiasis elimination.


THEMED ISSUE ARTICLE (Open Access)
A rapid freezing method to determine tissue layer thickness in drought-stressed leaves
Maryam Alsadat Zekri, Carina Leimhofer, Nicole Drexler, Ingeborg Lang
Plants have been affected by water stress ever since they settled on dry land. In severe and persisting drought, plant leaves are wilting. However, a documentation at the anatomical level of the minute changes that occur before wilting is challenging. On the other hand, understanding the anatomical alteration in plant leaves with respect to water stress provides a stronger basis to study molecular and submolecular processes through which plants enhance drought tolerance. In this work, we applied an affordable method to visualise mesophyll layers of Arabidopsis thaliana cell lines without preparation steps that would alter the volume of the cells. We rapidly plunge-froze the leaves in liquid nitrogen, cut them while in the N2 bath, and immediately imaged
the mesophyll cross sections in a scanning electron microscope.We applied a reduction of watering from 60 to 40 to 20 mL per day and investigated two time points, 7 and 12 days, respectively. Interestingly, the overall thickness of leaves increased in water stress conditions. Our results showed that the palisade and spongy layers behaved differently under varying watering regimes. Moreover, the results showed that this method can be used to image leaf sections after drought stress without the risk of artefacts or swelling caused by contact to liquids as during chemical fixation.
ORIGINAL ARTICLE (Open Access)
The use of fluorescence lifetime imaging (FLIM) for in situ microbial detection in complex mineral substrates
Yekaterina Chmykh, Jay L. Nadeau
The standard method of bacterial enumeration is to label the cells with a fluorescent dye and count them under high-power fluorescence microscopy. However, this can be difficult when the cells are embedded in soil and rock due to fluorescence from the surrounding minerals and dye binding to ambiguous features of the substrate. The use of fluorescence lifetime imaging (FLIM) can disambiguate these signals and allow for improved detection of bacteria in environmental samples.
ORIGINAL ARTICLE
A multiangle polarised imaging-based method for thin section segmentation
Yan Chen,Yu Yi,Yongfang Dai, Xiangchao Shi
The most crucial task of petroleum geology is to explore oil and gas reservoirs in the deep underground. As one of the analysis techniques in petroleum geological research, rock thin section identification


includes particle segmentation, which is one of the key steps. A conventional sandstone thin section image typically contains hundreds of mineral particles with blurred boundaries and complex microstructures inside the particles. Moreover, the complex lithology and low porosity of tight sandstone make traditional image segmentation methods unsuitable for solving the complex thin section segmentation problems. This paper combines petrology and image processing technologies. First, polarised sequence images are aligned, and then the images are transformed to the HSV colour space to extract pores. Second, particles are extracted according to their extinction characteristics. Last, a concavity and corner detection matching method is used to process the extracted particles, thereby completing the segmentation of sandstone thin section images. The experimental results show that our proposed method can more
accurately fit the boundaries of mineral particles in sandstone images than existing image segmentation methods. Additionally, when applied in actual production scenarios, our method exhibits excellent performance, greatly improving thin section identification efficiency and significantly assisting experts identification.
Atomically resolved a novel, compact and stiff scanning tunnelling microscopy in cryogen-free superconducting magnet
Behnam Esmaeilzadeh, Muhammad
Touqeer, Liu Junwei, Shaofeng
Zheng, Tao Geng,Yubin Hou, Qingyou
Lu
We present the design and performance of a novel scanning tunnelling microscope (STM) operating in a cryogen-free superconducting magnet. Our home-built STM head is compact (51.5 mm long mm in diameter) and has a single arm that provides complete openness in the scanning area between the tip and sample. The STM head consists of two piezoelectric tubes (PTs), a piezoelectric scanning tube (PST) mounted on a well-polished zirconia shaft, and a large PT housed in a sapphire tube called the motor tube. The main body of the STM head is made of tantalum. In this design, we fixed the sapphire tube to the frame with screws so that the tube’s position can be changed quickly. To analyse the stiffness of the STM head unit, we identified the lowest eigenfrequencies with 3 and 4 kHz in the bending modes, 8 kHz in a torsional mode, and 9 kHz in a longitudinal mode by finite element analysis, and also measured the low drift rates in the X–Y plane and in the Z direction. The high performance of the home-built STM was demonstrated by images of the hexagonal graphite lattice at 300 K and in a sweeping magnetic field from 0 T to 9 T. Our results confirm the high stability, vibration resistance, insensitivity

to high magnetic fields and the application potential of our newly developed STM for the investigation of low-frequency systems with high static support stiffness in physics, chemistry, material and biological sciences.
Comparison of holotomographic microscopy and coherencecontrolled holographic microscopy
Vera Chvalova, Tomas Vomastek, Tomas GrouslQuantitative phase imaging (QPI) is a powerful tool for label-free visualisation of living cells. Here, we compare two QPI microscopes – the Telight Q-Phase microscope and the Nanolive 3D Cell Explorerfluo microscope. Both systems provide unbiased information about cell morphology, such as individual cell dry mass, perimeter and area. The Q-Phase microscope uses artefact-free, coherence-controlled holographic imaging technology to visualise cells in real time with minimal phototoxicity. The 3D Cell Explorer-fluo employs laser-based holotomography to reconstruct 3D images of living cells, visualising their internal structures and dynamics. Here, we analysed the strengths and limitations of both microscopes when examining two morphologically distinct cell lines – the cuboidal epithelial MDCK cells which form multicellular clusters and solitary growing Rat2 fibroblasts. We focus mainly on the ability of the devices to generate images suitable for single-cell segmentation by the built-in software, and we discuss the segmentation results and quantitative data generated from the segmented images. We


show that both microscopes offer slightly different advantages, and the choice between them depends on the specific requirements and goals of the user.

1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints
Available for iPhone and Android
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
Submit online at https://mc.manuscriptcentral.com/jmi
View the Guidelines for Authors and full submission details online at:
www.journalofmicroscopy.org
The 18th International Conference IEEE Nanotechnology Materials and Devices Conference (NMDC 2023) was held in the pleasant environment of the ancient city of Paestum, Salerno, Italy, on October 22-25, 2023. Paestum is a place of exceptional artistic and natural beauty, with wonderful sea beaches as pristine as a bioreserve, nearly untouched by pollution caused by the present day public transportation system. It is known especially for the ruins of Paestum, a UNESCO heritage site famous for its three ancient Greek temples in the Doric order dating from about 550 to 450 BC . NMDC is a flagship conference series of the IEEE Nanotechnology Council (NTC), focusing on research advances in the fields of nanoscience and nanotechnology. The conference offered a great opportunity for researchers working on various aspects of nano-materials and devices, worldwide, to showcase their research on such a big platform. An important pre-conference event – ‘2023 IEEE NTC Forum on Nanomechanics and Machine Learning’ on the utilization of Machine Learning in science and technology, very relevant to today’s research, was also held on 21-22 October 2023 at the same venue.
As for the NMDC statistics, the conference witnessed 351 attendees from 39 countries. There were eight plenary, 48 keynote, 146 invited, 113 contributed talks and 36 poster presentations in six parallel sessions. There were four special sessions on Nanoporous materials, Neuromorphic & unconventional computing, 2D and 3D printed electronics and Nano-enabled Icephobicity. The conference started off with an excellent plenary talk by professor X. Jiang from NC State University on Micro/Nanotechnology Enabled Intravascular Ultrasound Imaging and Therapy. A total of 113
full papers were accepted for publication in IEEE Xplore with a special issue on IEEE NMDC 2023 in the journal of IEEE Transactions on Nanotechnology (TNANO).
The most interesting sessions for me were the use of machine learning in the fabrication and characterisation of materials and on the structure-property relationship derivations from advanced electron microscopy (TEM and SEM) of materials. It was quite fascinating to know that through machine-learning-based techniques the evaluation of the complex physical properties of

nanostructured materials could be done with only a fractional computational cost of conventional DFTbased solutions, cutting down the time taken from months to tens of hours. In a talk by Dr. R. Arenal it was exciting to know about improved capabilities of TEM combining the imaging and spectroscopy for studying the atomic structure and the optoelectronic properties of different inorganic 1D and 2D nanostructures. In his talk, he presented his work on an in-situ thermal reduction of Graphene Oxide. On the other hand, M. Zaghloul discussed an implementation of dynamical imaging of MEMS by time-resolved scanning electron microscopy in his talk. He showed that obtaining stroboscopic movies of MEMS resonator by sequential recording of secondary electron signals is possible. Apart from
these, there was always something interesting to learn in the ongoing parallel sessions throughout the conference.
I am grateful to the conference committee for selecting my paper “Direct Synthesis of Silicon Wires on a polymer substrate without the substrate heating using Hot-Wire-Enabled VLS method” for oral presentation. One of widely used bottomup methods of synthesis of Silicon wires (SiWs), the Vapor-liquid-solid (VLS) method, essentially poses limitations for their direct integration on low operating temperature (~150 °C) polymeric substrates such as Polyethylene Naphthalate (PEN) substrate. In the presented work we were able to show that direct integration of Si wires is feasible even without substrate heating via Hot-WireEnabled VLS method, a variation of conventional VLS method, in which the heated filament (the Hot-wire) plays a critical role in enabling the synthesis of the wires at sufficiently low substrate temperatures. In this way, direct integration of SiWs can be done virtually on any substrate. This work opens up avenues for replicating existing SiWsbased devices on low-melting-point polymeric substrates in a facile and cost-effective manner. My work was well received and appreciated by many researchers. A full paper of the presented work can soon be referred in IEEE Xplore.
On the social front, there were four events including three conference dinners and a tour to


the Archaeological Park of Paestum. These events provided excellent opportunities to interact informally with researchers from across the world one of the best ways of networking. One event worth mentioning here is ‘Young Professional Social Event’ in which a few senior researchers were also present to guide doctoral students on career prospects in research in Europe. I benefitted immensely from this event.
I was overwhelmed by the great atmosphere of cordiality and hospitality showed by the conference organisers. IEEE NMDC 2023 covered a broad spectrum of topics related to nanoscience and technology, from ‘Application of nanotechnology in medical sciences (nano-biomedicine)’ to ‘Nanotechnology ethics’ with an extra 2-day forum
on the use of machine learning in nanotechnology. The tour to the archaeological site quenched my long-standing thirst to know about ancient Greek and Roman cultures. The conference was successful in serving its purpose of providing a platform for international exposure to scholars’ research work, opportunities for networking and information about career prospects in research in Europe.
I would like to thank the Royal Microscopical Society and Indian Institute of Technology Bombay India for the travel grant, without which it would have not been possible for me to attend this conference.
Nitin Arya Indian Institute of Technology Bombay, IndiaThe Royal Microscopical Society would like to welcome our new members who have joined us in the last three months. We hope they enjoy a long and rewarding membership with the RMS.
Mr Jagen Burke
Dr Bev Goward
Dr Kevin Conway
Lisa Pang
John Maddock
Mx Arman Yousefi
Dr Michael Macey
Mr Warren Hatch
Dr Mark Willett
Mr Stuart King
Dr Wanrong Geng
Alan Marron
Ms Rebecca Betts
Miss Katie Sharrocks
Mr Ishfaq Mir
Dr Liene Spruzeniece
Dr Konstantinos Kalyviotis
Dr Samuel Johnston
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk


 Dr Peter O'Toole.
Dr Peter O'Toole.
Dear Readers,
It feels like time has really flown since our December issue of infocus - despite (or perhaps the result of) so much having taken place.
During January and February, we had three highly successful events in the form of UK Light Microscopy Facilities Meeting, Flow Facilities Meeting, and Electron Microscopy UK & Ireland (EM-UKI). All these meetings were geared towards helping microscopists in their day jobs, and certainly made for a highimpact start to the year, with each attracting around 100 to over 180 attendees. I was fortunate to be able to attend all three of these, and the community spirit and networking was second-to-none. That’s a huge credit to both the local organisers and the attendees themselves.
Our new Chief Executive Sali Davies has been with us now for several months, and it is great to see the impact she is making. There is a real sense of energy and excitement around our activities, and I’m really looking forward to our next round of committee meetings. I think we will see the Society evolving at pace over the next couple of years, and as always, we would love to hear from any members who have any ideas or thoughts about how the RMS can be active in supporting microscopy. It’s important to emphasise that this is your Society, and – as has been the case since 1839 – its work is entirely dependent on the input from its membership.
Our biggest event on this year's horizon is, of course, elmi2024, taking place in Liverpool from 4 – 7 June. We are very honoured to have been tasked with delivering this year’s instalment of the meeting series, which has been staged at locations across Europe since 2001. With a likely attendance of more than 400, and a buzzing atmosphere, this promises to be a truly special occasion. The venue - ACC Liverpool - is a purpose-built convention centre in the heart of the city on the King's Dock. It looks fantastic, and I can’t wait to see all the preparations coming to fruition in early June.
We also have a wide range of other events lined up throughout 2024, and I would urge readers to visit our event calendar to see what’s on offer; we hope there is something for everyone.The RMS is a very inclusive society, and our activities cover all aspects and branches of microscopy and cytometry – plus many closely associated techniques and disciplines such as mass spectroscopy and X-ray imaging.The RMS is always keen to bring new technologies under its umbrella and to extend its support for the communities using them.
I look forward to seeing you at elmi2024 – or perhaps one of our other events later this year.
Best wishes,
Dr Peter O’Toole, RMS PresidentWe are delighted to announce Professor David Stephens as the latest recipient of the RMS Scientific Achievement Award.
David has been a group leader in the School of Biochemistry at University of Bristol since 2001. He is an outstanding cell biologist who uses advanced light microscopy and electron microscopy to study the secretory pathway. His work spans over

30 years of excellence in research with over 100 publications.
In 2005 he made the key finding that membranes of the early secretory pathway can be linked to microtubules and motor proteins for subsequent organisation and movement to the Golgi apparatus. He has also made many contributions to the understanding of cilia biology, including work identifying the subunit composition and role of the dynein-2 motor protein complex in the formation and maintenance of primary cilia.
In 2018 his work, David provided an important insight into the process of procollagen trafficking, revealing a short-loop pathway from the ER to the Golgi, without the use of large carriers. Further work has defined roles for early secretory pathway proteins, including giantin and TANGO1, in the processing and secretion of procollagen. David continues to publish in these areas with some exciting new publications coming up in 2024.
As well as driving his own successful research group, David has been a collaborator on many research projects, generously giving of his time and mentorship to support the cell biology community.
During his research career he has held funding from UKRI and The Wellcome Trust, with a significant amount dedicated to the provision of advanced microscopy platforms. Microscopy has always underpinned David’s research and he was an enthusiastic member of the Royal Microscopical Society Life Sciences Section for several years, supporting meetings including the Microscience Microscopy Congress (mmc).
David has been an active member of the British Society for Cell Biology and the British Society for Matrix Biology and is a Fellow of the Royal Society of Biology. He has also served as a highly regarded editor with the Journal of Cell Science since 2015. David has also invested considerable time as a grant panel member in the UK and internationally, as well as serving on the Council of UKRI-BBSRC.
The Editors of the Journal of Microscopy are pleased to announce the winners of the inaugural ‘Best Paper’ awards for Early Career Researchers in 2023.
Each prize was £200 and was judged by General Editor Professor Michelle Peckham, Deputy Editor Professor Pete Nellist and our team of Scientific Editors.
For life sciences, Dr Charlotte Pain has been awarded ‘Best Paper’ for the paper intER-ACTINg:
The structure and dynamics of ER and actin are interlinked. Charlotte is a post-doctoral researcher in the department of Biological and Medical Sciences at Oxford Brookes University.
For physical sciences, Dr Alex W. Robinson has been awarded ‘Best Paper’ for the paper Towards real-time STEM simulations through targeted subsampling strategies. Alex is a post-doctoral research associate at the University of Liverpool, and Research Lead at SenseAI innovations.

Scientific Editor Dr Ulla Neumann said: “The study by Charlotte Pain and colleagues beautifully shows the intricate relationship between the actin cytoskeleton and the endoplasmic reticulum in plants. Highquality confocal microscopy combined with solid image analysis and data quantification clearly show that changing one of the two partners in the plant ER-actin relationship inevitably alters the other. It also makes the point that the choice of a specific actin marker is crucial when it comes to studying organelle structure and dynamics. Congratulations to Charlotte and her
colleagues for winning the ERC Best Paper prize in the Life Sciences.”
Professor Peckham added: “This is comprehensive study that demonstrates the strong interaction between the actin cytoskeleton and the endoplasmic reticulum (ER) in plants. The images are great, have been expertly quantified, and the work additionally demonstrates how choice of fluorescent probe for actin and ER in important in avoiding off target effects.”
Scientific Editor Professor Mark Rainforth said: “Scanning transmission electron microscopy images can be complex to interpret at the atomic scale as the contrast is sensitive to many variables such as sample thickness, composition, the presence of defects, and of course, aberrations. This paper took a new approach where the manner in which the signal was sampled was changed, providing a more accurate and efficient method for STEM simulations. The paper won because of the novelty and quality of the work undertaken.”

Professor Nellist added:“This paper brings together simulations of scanning transmission electron microscope (STEM) images with the emerging field of compressive sensing. Simulations of images are a vital tool to allow the inversion of STEM data to meaningful, quantitative measurements. These simulations are, however, computationally demanding which can limit the range of models and parameters that can be explored. Compressive sensing has been used for in-painting data from sub-sampled experimental data, but the innovation in this paper is its use for sub-sampling of modelling parameters in simulations with the aim of making simulations real time.”
www.journalofmicroscopy.org.uk
Applications for the RMS’s fantastic Summer Studentship scheme are now being invited.
Up to six studentships of £2,000 are offered to undergraduates every year, split evenly between physical sciences, biological sciences and interdisciplinary projects.
Applications for our Summer Studentships must include a significant microscopy component and should be submitted by a suitable host academic on behalf of a student.
The deadline for applications is 31 March 2024.
The Studentship is offered on the understanding that a 500-word project report is completed by the student by the end of the period of study and submitted to the RMS. Students will also be asked to do a two-to-five-minute recording briefly talking about their experience for the RMS YouTube Channel.
Students who are in their first or final year are not eligible. The person making the application must be a Member or Fellow of the Royal Microscopical Society.
Find out more and apply

The Journal of Microscopy is pleased to announce a new special issue featuring papers on “Microscopy and Infectious Diseases (host-pathogen interaction and pathogens structure)”.
The special issue will be guest edited by Dr Leandro Lemgruber, Head of the Cellular Analysis Facility, University of Glasgow, UK, and Mariana De Niz, The Feinberg School of Medicine, Northwestern University, USA.
We welcome submissions for this issue and papers can be Reviews, Methods and Protocols or Primary Research articles.
Read our guidelines for authors Deadline: September 30th, 2024
If you have any questions, please contact Editorial Office Manager Jill Hobbs, journaladmin@rms.org. uk

RMS now administering membership for Association of Clinical Electron Microscopists (ACEM)
The RMS is delighted to be providing membership administration support for the Association of Clinical Electron Microscopists (ACEM).
The Society has taken on responsibility for maintaining membership records, receiving funds and issuing renewals. It will also process new
membership applications - following confirmation of membership by the ACEM committee. New and ‘lapsed’ members can apply for membership via the website at www.acem.org.uk/membership.
The ACEM membership fee is now £35 including a nominal RMS administration fee. To renew your ACEM membership, please contact Debbie Hunt.
Chief Executive Sali Davis joins line-up of speakers at ITSHER Scientific Conference (7-8 March, London and Dublin)
The RMS is proud to support the upcoming ITSHER 2024 conference, part of the Women in STEMM Summit 2024.

The groundbreaking event will bring universities and businesses in the UK and Ireland together to celebrate science, research and innovations by women across all disciplines in STEMM.
RMS Chief Executive Sali Davis will join an exciting cast of speakers at the dynamic, interdisciplinary conference, taking place simultaneously at the Institute of Physics (IOP), London, and Trinity College Dublin (TCD), Dublin. There will also

be the opportunity to watch the sessions and panel discussions interchangeably through live stream. The event has been organised by Professor Anna Baldycheva (Exeter University) who is also Chair of the RMS Engineering, Physical & Material Sciences Committee.
The scientific programme is carefully curated to offer a blend of powerful talks and enlightening poster presentations, showcasing the incredible research and innovations spearheaded by female scientists and engineers across the UK and Ireland. Topics include all STEMM disciplines: Physics, Engineering, Mathematics, Computer Science, Health Sciences, Life Sciences, and Material Science. Moreover, the programme is enriched by special sessions and panel discussions devoted to career development in academia and industry for female professionals, as well as Innovation Sessions with the opportunity to receive feedback from industry practitioners.
Find out more and register
The RMS would like to give special thanks to Dr Alex Ball and Dr Alex Sossick for hosting a special staff visit to the Natural History Museum in London on 1 February.

The team was given a ‘behind-the-scenes’ tour to take a look at some of the fascinating science and microscopy taking place at the iconic venue – where both ‘Alexes’ are currently based.


From scanning microscopy and 3-D modelling, to conservation and fossil preparation, staff were given a taste of the wide-ranging research currently being undertaken.
There was even time to view some of the museum’s extensive hoard of animal specimens stored in alcohol in the Museum’s spirit collection. A visit to the Wildlife Photographer of the Year exhibition capped off a memorable away-day.
Once again, our thanks to Alex and Alex, for taking time out from their busy schedule to make this possible.
The RMS mentoring scheme is unique as it is crafted by microscopists for microscopists. Indeed, this is the reason why it came about in the first place, as we aimed to address the distinctive needs of individuals in this specialised field. Originating from conversations among Joelle, Scott, Georgina, Paul, and Alex, aka “the Mentoring Working Group”, we recognised the lack of microscopist-focused guidance. Given the niche nature of our roles, seeking career or specialist advice often means looking beyond our immediate workplace or even country, and building such networks can be challenging.
So, in 2022 and 2023 we conducted a pilot of the mentoring scheme. This provisional programme encompassed 1) personal mentoring for soft skills and 2) application mentoring pairing microscopists with specific instrument or software expertise. Drawing on their professional connections, the Mentoring Working Group matched together eight pairs, catering to early career as well as more established microscopists that were looking for a range of diverse goals, from transitioning to industry to developing core facility management skills. The feedback was very positive, with some examples overleaf…
“It’s been great so far. I am very lucky to have a mentor that is passionate about helping me. She is a brilliant listener and gives me advice that I don’t get from academic supervisors. The insight into industry has been super.”
“Just thanks! It’s been very good so far!”
“Early days, but I’m optimistic about the insights and interest the mentor has shown”
Buoyed by the pilot’s success, we officially launched the scheme in July 2023, with plans for biannual pairing rounds. Applicants are asked to provide detailed information about their mentoring needs, job role, and career stage, with location details being non-restrictive, as most interactions occur virtually. The working group diligently reviews applications, aiming to find the ideal mentor either from our growing database or the wider microscopy community.
The mentorship experience is rewarding for both parties, fostering confidence, career growth, and transferable skills like leadership, empathy, networking, and communication. We encourage more mentors to join our database by completing the online application form. Once pairs are matched, we introduce them via email along with some mentoring resources kindly provided by the Francis Crick Institute. While the pairings are initially designed for six-to-12 months, there’s no strict limit, allowing connections to flourish as long as both parties desire. Periodic check-ins and feedback questionnaires contribute to continuous improvement.
Our first cohort, initiated from the July-August 2023 call, is now 4-6 months into their pairing. The response to the inaugural call surpassed our expectations, with numerous applicants
and successful pairings, including several on the application coaching stream. Applicants hail from various corners of the globe, expanding our network of both mentees and mentors.
Below, some of our pilot cohort reflect on their experiences of mentoring, what they have learned and advice for anyone thinking of getting involved themselves:

Charlotte
Roslin
Why did you decide to get involved?
As a scientist with a fascination for microscopy and the recent technical and methodical developments in bio-imaging, it seemed a perfect fit for me to get involved with a fantastic society like the RMS, and also be able to receive advice and help from experts in this field, to help me develop in my research career.
Can you describe what you have been doing and your experience so far?
So far I have been having 1:1 meetings with my mentor to gain general advice about improving my skills in microscopy, and also guidance while attempting advanced imaging techniques such as Correlative Light and Electron Microscopy (CLEM).
How do you feel you have benefitted from taking part?
Being able to ask questions (both complex and simple!) to gain a broader understanding of the field, the different techniques and different instruments available to me, has been very useful.Also just having a second opinion on my images/figures/experimental design has been very useful indeed, and helped me develop better approaches to answer my biological question.
What have you learned that will be most useful to you going forward?
Imaging and the preparation steps are often (but not always) a multi-step process. Always have extra samples, extra images, extra everything (!) to make sure that by the end you have useful, representative and reproducible data to take forward to analysis.
Why do you think the RMS scheme is important for the microscopy community?
I think microscopy can seem exciting but very daunting to get into – so having experts and a welcoming community like the RMS ready and available to help support those wanting mentoring is a very valuable resource, and one I’m very happy
to be part of.
Is there any advice you would give for anyone thinking of becoming a mentor / mentee?
Mentees: Ask all the questions you feel you need – a great part of this partnership is just getting a more well-rounded understanding of microscopy.
Mentors: Mentees often have gaps in their knowledge that even they are sometimes not aware of. Sometimes we need to go back to the basics to build up a better understanding of the techniques we’re learning/attempting. Clarity and sometimes step-by-step methods (if relevant) are really valuable to us!

Confocal Technician, University College London
Why did you decide to get involved?
I felt that my career wasn’t progressing at all, and I didn’t know what to do about it. I tried the mentoring programme inside my institution, but
being in a microscope core facility is a very niche we couldn’t connect, and I felt she didn’t understand set of skills that is difficult to understand for an what I did. outsider. I saw the mentoring program in the RMS
Is there any advice you would give to and I thought it would be a great opportunity to anyone thinking of becoming a mentor/ have input from someone senior in the field. mentee?
Can you describe what you have been doing and
My experience is only as a mentee, and I will your experience so far? We have met using Teams recommend anybody thinking about going for it. several times. I am hoping we can catch up in I would recommend to anybody more senior to person soon. It was very informal, we discussed become a mentor, as the community needs as much my progression and what I want to achieve in the input as possible. future, and my mentor gave me ideas to work with.
How do you feel you have benefitted from taking part?
I have learnt a lot from my mentor. I discovered some paths I didn’t think about or even didn’t know existed. During that time, I applied and changed jobs.
I was part of the Herschel program too.
I have also benefitted from having an external point of view, the input is very useful.
I also saw photos of puppies, that was a bonus.
What have you learned that will be most useful to you going forward?
We talked about different opportunities for learning new skills that will be useful for career progression (e.g., apprenticeship). Also, we discussed about group dynamics, how to navigate those dynamics, etc. He is in different working groups, so it was very useful to talk to him about that.
 Peter O’Toole – Mentor
Peter O’Toole – Mentor
Why do you think the RMS scheme RMS President, Head of Imaging and is important for the microscopy Cytometry Labs, University of York community?
It allows contacting and connecting with people
Why did you decide to get involved?
The simple answer is that I was asked to help! –with a career more related to yours. There are but mentoring was something I had already been other mentoring opportunities schemes out there doing with a few people informally, as they took (in the workplace, other professional bodies), but on new roles. I had previously mentored people I I found them very general, and our professional had known from conferences who contacted me profile is quite different. afterwards for advice. I have also mentored PhD
As I mentioned, I tried the one offered at my and post-doc students who kept in touch after previous institution. The mentor was very nice, but continuing their careers.
Can you describe what you have been
does take time to grow into the role, and there is doing and your experience so far? obviously the time commitment in general. But it can be very rewarding, and the self-reflection that Having joined the programme as a mentor, I comes from taking part is really important. From a suddenly had multiple requests for mentorship moral perspective, I also think it is a good thing to overnight ! Sometimes I’m giving help and technical give something back, and to help your community advice as a scientist - on what works best from a in general. science perspective; at other times it’s about the intricacies of running a facility – from charging and HR procurement to communication with users. At that level, it is not just about microscopy, and it can Paul Verkade: Mentor involve people from outside the microscopy world. Professor of Bioimaging, University of How do you feel you have benefitted Bristol from taking part?
The meetings give the mentees a lot of enthusiasm,
Why did you decide to get involved?
Before we even discussed setting up the scheme, I and when you hear that they feel really motivated had been contacted by Scott Dillon who was setting and itching to get back and put some of the ideas up a new facility and asked if I wanted to mentor you have been talking about into practice, that is him in that process - dealing with users, career very rewarding. progression and those kind of issues. That was a There’s an element of worry in case you give the very useful experience for both of us and it was wrong advice to someone – but overall it is a really then only natural to be engaged when it grew into positive process which can help both the mentor the RMS scheme. and mentee to come up with new ways of working.
What have you learned that will be most useful to you going forward?
Every time you mentor someone, you inevitably reflect on what you are doing yourself. Mentees will ask difficult questions and challenge your thinking, and you question yourself as to whether you are still enacting best practices. Working practices are always evolving, so that process of self-reflection is a very healthy and helpful thing.
Why do you think the RMS scheme is important for the microscopy

Can you describe what you have been community? doing and your experience so far?
I think that sometimes people don’t have the We meet up every now and then, mainly depending confidence to ask for help - or to know who to ask. on the need, and we have a chat about questions That is why having a formal mentoring programme the mentee has and I try to share my thoughts and is so important – because it really empowers people experiences from my career. It depends what kind to come forward and removes that barrier. of mentorship it is, “soft skills” or more technical. For anyone thinking of becoming a mentor, it With the soft skills you always have to remember
that these are my own experiences and don’t always translate one-to-one to another situation.
How do you feel you have benefitted from taking part?
For me, personally, it has been a very useful exercise in reflecting on my own career - the decisions I have made, how I have learned from my experiences, why am I in the position I am now (that sounds quite grand!).
What have you learned that will be most useful to you going forward?
That the microscopy community is a very friendly and open community and because of that, it is very easy to discuss and engage with other people. It is my second family.
Why do you think the RMS scheme is important for the microscopy community?
We can learn so much from each other and we should not let that know-how go to waste. Is there any advice you would give for anyone thinking of becoming a mentor / mentee?
Everyone has been in the situation where they started something and were not sure how to tackle this. Most likely you will have thought, who can I ask? Maybe there was someone, maybe there wasn’t. Both are the perfect reason to get involved, because there are a lot of microscopists out there very willing to share their experience.
Find out more about the RMS Mentoring and Application scheme
Submit to infocus infocus welcomes submissions of articles of general interest to microscopists.
You provide the text and images and we take care of the rest. It’s the ideal way to share your work with the microscopical community.
Full submission information and guidelines are available at www.infocus.org.uk
To submit an idea or if you have any questions about the process please email the Editor (editor@infocus.org.uk

2023 was an exciting year for FocalPlane, our online microscopy community site. We’ve seen upgrades to the site, new contributors and fresh content from our regulars! The number of people using and contributing to the site is also growing, as we continue to reach out to the global community of imaging scientists, engineers, bioimage analysts and cell biologists.
We recently implemented two main upgrades to the site: a new partnership with MicroscopyDB, and a new look for the site thanks to our image competition finalists. The partnership with MicroscopyDB means that our events calendar and jobs board are now powered by MicroscopyDB, making the calendar and board essential viewing for planning your conference travel or finding for a new position. On the FocalPlane homepage, we have released a series of banner images which now appear alongside our characteristic lens apertures. We thank Marie-Charlotte Domart & Chris Peddie, Nick Gatford, Oona Paavolainen, Rebecca Simkin and Till Stephan for sharing their images with us.
2023 was also a busy year for our FocalPlane correspondents, Mariana de Niz and Mai Rahmoon.
Mariana continued her excellent ‘Latin American Microscopists’ series, while also starting a ‘Toward Global Access’ series with Constadina Arvanitis. Mai

has been introducing us to imaging communities through interviews with their founders. We look forward to continuing to work with Mariana and Mai, and we are excited to announce our new team of correspondents, Daniel Doucet, Subhajit Dutta and Greg Redpath. Visit our website to read our announcement post introducing the three of them and find out what topics they will be writing about for FocalPlane.
We’ve also been busy planning some new initiatives for 2024 and hope to announce these soon. The easiest way to stay up to date with the latest news from FocalPlane is by getting our weekly digest or our quarterly newsletter delivered straight to your inbox. Simply register with the site and select the appropriate box.
Finally, we would like to thank all our readers, subscribers, and most of all, our contributors in 2023. Remember, FocalPlane is your site. Once registered, you are free to post, and if you have suggestions or feedback, you can contact the team at focalplane@biologists.com.
https://focalplane.biologists.com

Labtech International has been appointed as exclusive UK distributor of Tomocube, the innovative manufacturer of cutting-edge label-free 4D Holotomography systems. Holotomography utilises 360° quantitative refractive index measurement to build 3D holograms with high resolution and temporal specificity while minimising cellular stress.
The Tomocube range includes the HT series for live cell imaging, the HT-X1 series for higher throughput using multiwell plates, and analysis software to unleash the full potential of Holotomography.
In addition, Labtech has been appointed as one of the UK distributors for Nikon’s range of life science microscopes. Nikon is a leader in microscopebased imaging technologies for the life sciences and Labtech is delighted to be working alongside their team of UK specialists. We can supply and support your imaging needs, especially within the private sector.
https://www.labtech.com
TDK-Lambda UK, a major manufacturer of power supplies to markets such as medical and industrial, is taking advantage of a newly installed KEYENCE VHX 970F high accuracy digital microscope to drive the company’s inspection and quality function. With such a reputable global brand, there is no margin for failures.

Part of Japanese multinational electronics giant TDK Corporation, TDK-Lambda UK is Britain’s largest designer and manufacturer of both standard and configurable AC-DC and DCDC power supplies. The company’s 350-employee facility in Ilfracombe is home to its manufacturing, new product development, sales and service functions. A key part of delivering business success at TDK-Lambda UK is component engineering.
“As a department, we’re responsible for component analysis; looking at alternative or new components that we test for robustness and conformity to data sheet specifications,” explains Component Engineering Manager Dan Massey. “We also look after supplier quality from our contract
manufacturers, while further responsibilities include goods inwards, sample inspection and compliance with RoHS.”
TDK-Lambda UK’s previous KEYENCE microscope served the company well for around 15 years but it upgraded to keep pace with the latest innovations and improvements.
“We saw the KEYENCE VHX970F high-accuracy digital microscope and knew it would meet our requirements,” says Massey. “Also, staying with KEYENCE meant we could continue using our existing lenses. It was an easy choice.”
As an option, the company invested in a digitally controlled Z-axis for the VHX-970F that it did not have previously.
“It allows us to automate the focus, assisting ease of use,” he says. “Supported by the microscope’s impressive depth of field we can also use the digitally controlled Z-axis to take multiple images from different height perceptions if we have a particularly awkward or complex component to inspect.”
www.keyence.co.uk/TDK
The EasyScan 2 system with its compact design had been a very popular instrument until Nanosurf discontinued its production back in 2013. Now, 10 years later, we are facing more and more challenges servicing these instruments due to the lack of availability of spare parts. Nanosurf therefore decided to officially end the service for the Easyscan 2 AFM system.
Nevertheless, we strive to provide our customers with as much support as possible. You may still

contact us in case of service requirements, and we will evaluate on a case-by-case basis if service of the device is still possible.
We recommend to EasyScan 2 AFM users to consider an upgrade to FlexAFM, one of the most established research AFMs available. The scan head recently received a design update that provides additional benefits:
• Footprint now matches DriveAFM: the result is a shared accessories universe across systems.
• Larger number of functional accessories.
• Improved top/side-view camera.
• FlexAFM can be run with the high-end CX controller, bringing you improved performance.
www.nanosurf.com
Queensgate, a brand of Prior Scientific, has won funding from Innovate UK, the UK’s innovation agency, for a fourth nanopositioning research project as part of Analysis for Innovators (A4I) program (Round 11).
This will be the fourth project Queensgate has won funding for through A4I, and once again, they will be working with the dimensional nano and sub-nano-metrology team at the National Physical Laboratory (NPL). This project will qualify the performance of Queensgate’s velocity control and special correction capabilities when applied to multi-directional motion profiles.

As Queensgate’s product manager, Craig Goodman, explained, ‘Our aim is to provide improved performance for atomic force microscopy, nanolithography and fast optical alignment systems.’ Queensgate has already developed a number of unique capabilities such as velocity control and spatial correction which allow very high-speed raster scanning with high precision. This project will help the company to refine these capabilities further.
Previous projects that Queensgate has partnered with NPL on include Spatial positioning correction for multi-axis nanopositioning stages; Improved linearisation of single-axis piezo nanopositioning stages; and developing a mathematical model for multi-axis control.
Innovate UK has released a case study video about the first, completed, project: Improving micron scale accuracy
www.prior.com
www.nanopositioning.com
We are thrilled to unveil our new, expanded headquarters at Keele University Science and Innovation Park. Our state-of-the-art facilities now include a production lab and spacious offices, allowing us to take our services to the next level.
Since relocating to Keele’s campus, our dedicated team has expanded, and our capabilities have multiplied. This means we can better serve our valued customers. But we’re not stopping there.
With larger office and laboratory spaces, EM Resolutions is now even more equipped to provide top-quality accessories to as many researchers as possible. Whether you’re a seasoned scientist or a curious newcomer, our expertise and commitment remain unwavering.
We extend our heartfelt gratitude to our loyal customers and the Science and Innovation Park. Your support has been instrumental in our journey.

For those who haven’t yet collaborated with us, allow us to introduce ourselves: EM Resolutions is a leading UK supplier specialising in TEM Microscopy accessories. Our focus includes the manufacture and supply of support films, and we are proud to be the sole UK distributor of UltrAuFoil®, meticulously crafted by Quantifoil®.
Join us as we continue to empower scientific discovery. Together, we’re shaping the future—one support film at a time.
www.emresolutions.com
Oxford Instruments, a leading provider of high technology products and services to industry and scientific research communities, announces the acquisition of First Light Imaging SAS (“First Light”), a scientific camera specialist.
Based near Aix-en-Provence, France, and founded in 2011, First Light specialises in the design and manufacture of high-speed, low-noise scientific cameras for infrared and visible imaging, with applications in astronomy and life sciences. It will become part of Oxford Instruments Andor (“Andor”), with a common global customer base spanning leading academic institutions and commercial companies. Its cameras are highly complementary to Andor’s existing scientific camera portfolio, with the acquisition providing opportunities to enhance both companies’ offerings and to expand into adjacent markets.

Richard Tyson, Chief Executive Officer, Oxford Instruments plc, said: “We are delighted to welcome the talented First Light team to Oxford Instruments. Like us, they have a reputation and track record for quality, innovation and scientific expertise, and their leading range of products dovetails well with our scientific camera portfolio. Joining forces will significantly enhance our world-class camera offering to our combined customer base and drive First Light’s next phase of growth as part of the wider Group.
“Our expanded portfolio will help us accelerate our customers’ roadmaps in the life science market and in physical sciences, a long-standing area of strength for Oxford Instruments.”
Under the terms of the acquisition, Oxford Instruments is paying an initial cash consideration of €15.7m, on a cash-free, debt-free basis, with a further €3m cash consideration conditional on First Light’s trading performance over a 12-month period following completion. First Light’s estimated revenue for the 12 months to 31 December 2023 is €8.0m (£7.0m), with return on sales below the average for the Group.
David Boutolleau, co-founder of First Light, said: “Joining Oxford Instruments and Andor is a real opportunity for First Light Imaging. After 12 years building First Light as a successful independent business, it became clear that to take the business to the next stage of its development, we would need industrial and commercial support to reinforce our manufacturing, technology and routes to market.
“Our acquisition by Oxford Instruments Andor accelerates our growth and secures our future for our customers, partners and employees.”
www.oxinst.com
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.

NuNano, the high-quality atomic force microscopy (AFM) probe manufacturer, is delighted to announce that their conductive AFM probe range, SPARK, are now available on the Oxford Instruments eStore.
The addition of SPARKs to the eStore reflects the expanding global distribution partnership with Oxford Instruments Asylum Research (Santa Barbara, US). The integration of SPARKs broadens the product offering, ensuring customers achieve reliable imaging every time when using NuNano’s
range of AFM probes alongside Oxford Instruments Asylum Research AFM instruments.
“Nano-electrical measurements are becoming increasingly important for materials characterisation, particularly for those materials being incorporated into a new generation of electronic devices” said James Vicary, NuNano Co-founder & Managing Director.
The SPARK range of conductive AFM probes are designed for electrical AFM measurements, such as Kelvin Probe Force Microscopy, Piezo-response Force Microscopy and Conductive AFM. Attention to quality and reliability, guarantees tip radius, while the symmetric tip profile ensures measurement precision across a wide range of nano-electrical applications.
Manufactured with the tightest dimensional tolerances in the market, SPARK conductive AFM probes offer minimal variation in spring constant and resonant frequency, setting the standard in probe reliability and performance. The SPARK series joins NuNano’s silicon AFM probe collection (SCOUT) in the Oxford Instruments eStore and direct from their regional offices.
www.nunano.com
The new Kleindiek Nanotechnik LT14030 substage is designed for orthogonal positioning in atmosphere, SEM/FIB and UHV. The LT14030 replaces the previous model LT12830 and features greater stability and drift performance. Its primary use is in SEM and FIB-SEM to enhance the accuracy and functionality of standard microscope stages. It is an economical alternative to laser interferometer stages. The LT14030 uses two positional encoders per axis for automatic yaw error compensation and has an option for interferometers, should that extremely high degree
of precision be required, after all.

Applications include eBeam lithography, memory cell and particle counting, tensile measurement, forensic analysis (e.g. GSR), metrology and failure analysis.
Sussex-based Labtech International offer the LT14030 and a full range of Kleindiek instruments in the UK and Ireland.
https://www.labtech/em.com
Teledyne will showcase their newest products and solutions at SPIE’s Photonics West exhibition taking place January 30 – February 1st, 2024, in San Francisco, California.
Visitors to the Teledyne booth #327 can expect representation from the businesses within its Imaging group, including Teledyne DALSA, e2v, FLIR, Acton Optics, Judson, Lumenera, Photometrics, and Princeton Instruments.
New products introduced include:
Teledyne e2v announces OnyxMax™, the next generation of its popular Onyx 1.3M low light CMOS image sensor. This new sensor has been designed for extremely low light conditions, down to 1 mLux. The combination of sensitivity and image resolution increases its range, allowing even small objects to be detected in harsh conditions. This makes OnyxMax ideal for a wide range of applications including science, defense, traffic
cameras, broadcast, surveillance, border control and astronomy.
Teledyne Photometrics releases the Retiga E9 camera that can capture exposures from tens of microseconds to tens of minutes, delivering detailed, high-resolution images without extraneous noise. With its stacked CMOS sensor technology developed by Sony, the E9’s square pixel array with 3.76 μm pixels and high dynamic range (>80 dB) combine to create a sensitive, versatile, and costeffective low noise camera ready for integration or benchtop use.
Teledyne FLIR IIS introduces the new Dragonfly® S USB3 series designed to accelerate the inception stage of imaging application development. The Dragonfly S series addresses the essential need of a modular, compact, and lightweight camera for atscale manufacturing, volume-based applications, and multi-camera systems.
www.teledyne.com

The mining industry is constantly searching for innovative and efficient technologies to gain deeper insights into the composition and structure of geological materials. One technology that has emerged as a game-changer is Micro X-ray Fluorescence (MicroXRF) Spectrometry. This stateof-the-art method, derived from X-ray fluorescence spectroscopy, enables the analysis of geological specimens at a micro-scale level. By irradiating a specimen with a finely focused X-ray beam, the atoms within the sample emit characteristic secondary X-rays, which can be used to determine the sample’s elemental composition. With the exceptional precision of MicroXRF instruments, elemental maps of thin section and core samples can be obtained, with each pixel, down to 5μm diameter, containing its own unique elemental spectrum. This high-resolution mapping capability allows for further phase analysis, which is crucial in understanding the mineralogy of the samples. MicroXRF is revolutionising mineral exploration, resource management, and quality control in the mining industry.
MicroXRF possesses a notable advantage in its non-destructive and non-invasive nature. This characteristic ensures the preservation of sample integrity and allows for consecutive analyses
without any harm or alteration to the specimen. Real-time analysis and immediate results provided by MicroXRF are of utmost importance in timesensitive operations within the mining industry. Mineral exploration stands as a significant application of MicroXRF in the mining field. It facilitates enhanced geochemical analysis, enabling geologists to accurately identify potential mineral deposits. For instance, mineral alteration zones often encompass ore bodies, containing specific elements that may indicate the presence of valuable resources. By employing MicroXRF, explorers can detect these altered mineral phases and subsequently improve their predictive models for the presence of ore.
MicroXRF is an essential tool in resource management, particularly in the control of ore grade. This advanced technology enables the rapid and precise determination of element concentrations in ores, including both valuable elements such as gold, copper, and nickel, as well as potentially harmful elements like arsenic, lead, or mercury. By utilising this valuable information, mining companies can effectively plan their extraction and processing operations while also mitigating potential environmental risks.
www.qd-uki.co.uk

Excelitas Technologies Corp., a leading industrial technology manufacturer focused on delivering innovative, market-driven photonic solutions, announces the X-Cite XYLISTM II Broad Spectrum LED Illumination System. A replacement for the current X-Cite XYLIS, XYLIS II offers several improvements, including significantly quieter operation and increased optical output, with the broadest spectrum available in a white light LED for fluorescence microscopy applications.
The introduction of XYLIS II comes as Excelitas Technologies celebrates the 20-year anniversary of the X-Cite product brand, which has grown to be the industry standard in fluorescence illumination.
performance. XYLIS II uses the same speedDIAL, light guides, adaptors and command set as the original XYLIS, allowing for a seamless transition between the two systems.
XYLIS II features include:

• Whisper quiet operation
• Broad spectrum, UV and visible
• Light guide coupled, optimised for 3mm
• Power levels comparable to (or better than) the current X-Cite XYLIS
• Control – manual (via speedDIAL), TTL, USB
Rivaling traditional arc lamps for brightness, XYLIS II is ideal for both compound and stereomicroscopes. It incorporates Excelitas’ patented, award-winning LaserLED Hybrid Drive® technology to overcome the LED green gap from the 540nm to 590nm region of the spectrum, where LED-only systems are significantly challenged. The powerful output and broad DAPI to Cy7 spectral range of XYLIS II offers researchers and microscope lab managers the benefits of LEDs without compromising on price, flexibility or
“Excelitas is proud of our long-standing commitment to delivering X-Cite Lamp and LED light sources for fluorescence microscopy, offering exceptional stability and superior illumination uniformity to meet the unique requirements of our customers,” said Michelle Gal, Senior Product Manager, X-Cite at Excelitas. “XYLIS II builds upon the success of our 20-year X-Cite heritage and addresses the feedback we’ve received from our valued customers. With optical output improvements across its broad spectrum and sound levels 10dB quieter than the original XYLIS, the only thing customers will notice about XYLIS II is its great performance.”
https://www.excelitas.com/product/xcite-xylis-ii-broad-spectrum-ledillumination-system
Oxford Instruments - Andor has released Imaris 10.1, the latest version of its market-leading AI microscopy image analysis software. Imaris 10.1 integrates AI segmentation tools into its main image analysis workflows, improving ease of use and providing better and more versatile segmentation for all researchers across life science applications.
In Imaris 10.1, a native, trainable AI pixel classifier is an integral part of its big-data-capable Surface segmentation model. Using the Imaris AI pixel classifier simplifies and improves the segmentation and object detection in challenging fluorescent datasets. In addition, it opens doors for 3D Scanning Electron Microscopy (SEM) segmentation and shape recognition.

Crucially, the AI pixel classifier can be used by all researchers, regardless of their experience. Users can train and compute the results with the classifier on their local machine by painting the data with the efficient and modern smart brush tool. Training and the results preview are extremely fast and interactive, improving the user experience and saving researchers’ time.
The pixel classifier can be trained and utilised on 2D, 3D multi-channel and time-lapse datasets and used in a batch mode to analyse more samples of similar type.
Anna Paszulewicz, Software Manager for Oxford Instruments - Andor, says: “Imaris 10.1 AI pixel classifier delivers a whole new experience of interactivity and speed. Together with Imaris’ native shape and distance measurements, it’s an amazing analysis and 3D visualisation tool for a wide range of research projects.”
Imaris’ goal is to bring researchers the most comprehensive visualisation and analysis software for 3D/4D and time-lapse microscopic images. It’s notable that Imaris is inclusive for all available microscopy file formats and provides seamless conversion to native IMS, using Bio-Formats.
Users can download a 10-day free trial of Imaris to explore the software first-hand.
https://www.oxinst.com/
Phasefocus label-free technology is breaking new ground with the development of its latest product, the Neurite Outgrowth assay. This pioneering new assay will aid researchers in both developmental studies investigating processes involved in the initial organisation of neuronal networks as well as the study of neuronal pathologies, such as degenerative diseases or neurotoxicity without the potential harmful effects of dyes and labels.
Livecyte revolutionises neurite outgrowth assays by producing high contrast, label-free Quantitative Phase Images (QPI) that can be automatically analysed to track cell bodies, and neurite characteristics (e.g. length and branching) during the development of an in vitro network. This enables researchers to quickly obtain metrics on their neurite outgrowth assays, without the requirement of costly fluorescent reagents which can impact the behaviour of their cells or by laborious manual analysis. By segmenting and tracking cells alongside a unique skeleton profile, Livecyte automatically quantifies neurite outgrowth metrics per cell and enables the tracking of cellular migration and organisation of cells during initial network formation, revealing trends that would have been overlooked by looking solely at population-based metrics. This cutting-edge technology leads to an increase in the physiological relevance of data
collected more accurately recapitulating in vivo neuronal cell behaviour in vitro and ultimately improving drug development efficiency.
Key Advantages of Livecyte Neurite Outgrowth Assay:
• Automated, accurate neurite detection labelfree: Livecyte employs advanced algorithms to automatically segment and skeletonise neuronal cells allowing streamlined network measurement.
• The Neuronal cell dashboard presents a wealth of metrics based on neurite characteristics, offering an invaluable depth of insight into the effect of mediators on neurite outgrowth.
• Livecyte’s ability to independently quantify neurite growth on a per cell basis eliminates any seeding density dependant differences and providing a more profound understanding of neuronal cell behaviour.
• Single-cell analysis of migration and morphology metrics alongside neurite outgrowth provides a more comprehensive way of understanding early neuronal cell behaviour.
• All this is achieved without the need to fluorescently label neuronal cells which alters their natural behaviour.
https://www.phasefocus.com/ applications/neurite-outgrowth

Teledyne Photometrics, a part of Teledyne Technologies, announces the release of the Retiga E9 camera that can capture exposures from tens of microseconds to tens of minutes, delivering detailed, high-resolution images without extraneous noise. With its stacked CMOS sensor technology developed by Sony, the E9’s square pixel array with 3.76 μm pixels and high dynamic range (>80 dB) combine to create a sensitive, versatile, and costeffective low noise camera ready for integration or benchtop use.
With a combination of low readout noise, high quantum efficiency, small pixel size, and large full well capacity the Retiga E9 is an ideal solution for imaging low-light signals for the extended periods of time required in gel documentation, DNA and RNA sequencing, qPCR, fluorescence/phosphorescence imaging, and general microscopy applications. The 9-megapixel, 3k x 3k array matches the field of view of many scientific imaging instruments, microscopes, and other C-mount-based optical systems.
The Retiga E platform, using the Teledyne Photometrics Citadel Sensor Chamber, isolates the sensor from the environment and allows air cooling down to below -20° C. The platform is designed for easy device integration into instrumentation, with a compact form factor, USB 3.2 10Gbs data communication, and long camera lifetime.
“The Retiga E platform outperforms many scientific CMOS cameras available today. The Retiga E9, with smaller pixels, captures better resolution at low magnification. Additionally, the dynamic range of Retiga E cameras is better than most scientific CMOS cameras, even when compared to those with larger pixels. And most scientific CMOS cameras are limited to seconds of exposure due to dark current, not the tens of minutes possible with the Retiga E9. This makes the Retiga E9 one of the most flexible and powerful cooled CMOS cameras available today,” states Product Manager Phil Allen, PhD.
www.teledyne.com

The DP75 digital microscope camera breaks new ground in microscopic imaging. Users can capture brightfield or wide-wavelength fluorescence images using a single camera, eliminating the inconvenience of switching between multiple cameras.
Designed for a variety of challenging life science and industrial applications, the DP75 camera empowers researchers and inspectors to capture highresolution images from brightfield to fluorescence, even in the near-infrared (NIR) range.This innovative camera simplifies the microscopy imaging process, enabling users to concentrate more on their work.
The high-sensitivity cooled CMOS sensor makes it easier than ever to capture sharp, low-noise fluorescence images. With a fast frame rate of 60 fps at full HD and 22 fps at over 4K resolution, the camera provides smooth, fast, live images for easy framing and comfortable live observation.
Whether inspecting the intricate structures of a cell or the surface of an industrial material, the DP75 camera provides outstanding image quality and ease of use. The camera’s advanced multiple-axis color correction technology helps ensure true-to-life color reproduction, making your images as vivid as looking through the microscope oculars. In addition, its smart AI-based scene detection automatically recognizes the observation method and adjusts the appropriate imaging parameters, which allows for easy capture of high-quality images, no matter the observation method.
Quantitative data collection is easy with the DP75 camera’s linear mode, and it enables a relative intensity evaluation of fluorescence expressions on a sample. Moreover, the camera enables you to easily overlay fluorescence and brightfield images

with pixel precision so that you can precisely identify the locations of fluorescent expression with the morphology of your specimen. For industrial applications, the camera’s live high dynamic range (HDR) feature provides high-fidelity images, capturing textures, flaws and previously undetectable defects with outstanding clarity.
The DP75 camera is both powerful and flexible. With compatibility via USB 3.1 Gen2, this camera works with most PCs, making it an ideal upgrade for any existing system. Its wide field of view capabilities further ensure fast and efficient research and inspections.
www.olympus-lifescience.com/camera/ color/dp75/
Queensgate’s NanoScan SP800 nanopositioning sample scanner offers high-accuracy positioning performance for longer ranges.
Queensgate has added a new model to its range of piezo-driven sample positioners. The SP800 offers 800 μm of travel (closed loop) with a resolution of 1.2 nm and a step settle time of under 10 ms. It can cope with up to 500 g loads, with higher load capacity available on request.
As with the SP400 and SP600 models, the SP800 is compatible with all Prior Scientific motorized microscope stages and components as well as other leading microscope brands.
Coupled with the Queensgate NPC-D-6110 digital controller, the system is easy to use and quick to set up using standard 0-10 V analog input and output. The digital control technology features variable acceleration/deceleration algorithms which allow exceptionally fine control of stage movements.
Thanks to this, the NanoScan SP400/SP600/
SP800 has the fastest step settle and recovery time between Z stacks to offer market-leading resolution.
The SP800 has a slim profile to enable easy access for illumination of the sample area. A wide variety of sample insert plates are available, including full microtiter plates, making it suitable for a range of applications in life sciences and materials:
• Optical sectioning producing 3D images
• Live cell imaging
• Autofocus systems for time-lapse imaging
• High content screening
• Surface analysis
• Wafer inspection
https://www.nanopositioning.com/ product/nanoscan-sp400-sp600-sp800piezo-sample-positioner-scanner

If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
If you are an RMS Member using microscopy or cytometry as part of your career, you can apply to study for an RMS Diploma - a flexible, portfolio-based, international qualification designed to complement and fit around your current employment.
The Diploma is intended to be of a similar standard to a Masters degree. Projects are designed by the candidate with the assistance of their line-manager, and mentorship from existing Fellows of the Society. This approach ensures that the study is both challenging and rewarding whilst complementing the candidate’s existing employment.
Here, we hear three different perspectives on the RMS Diploma, from some of those currently involved in the initiative.
Current Diploma student Tim Young is a Laboratory Supervisor for the Lehner Group, CITIID, Jeffery Cheah Biomedical Centre, Cambridge Biomedical Campus
In 2021, whilst still under COVID restrictions and things were quiet in the lab, I had time to think, plan, experiment and write my own project whilst undertaking all of my regular laboratory management duties. I was reading microscopy papers alongside almost unlimited access to the unit’s Zeiss 880 LSM. I was able to make great progress whilst increasing my confocal competency and producing some satisfying images too.
When I read about the RMS diploma on their
website, it seemed too good to be true; I could carry on developing my project, without reducing my paid hours and for a fraction of the price of a Masters degree. Furthermore, with my son starting school soon, the flexibility offered by the diploma was perfect for this stage in my life.
However, this was not to last long; after six months my PI moved his lab to Germany and I was unable to follow. I found another position at Cambridge University, and agreed to join as their Lab Manager with the condition that I could continue my diploma. Although my manager is supportive of my studies, the balance between my paid duties and my diploma has been much more challenging to maintain. I would like to share some of my insights from these last two years for anyone considering an RMS diploma.

The most crucial aspect to consider is passion. Whilst you are supported during the diploma, ultimately you are the driving force behind it and without a passion for microscopy it is not worth pursuing.
Does your job and your life circumstances allow time for study? Passion is key but without time in your week to fit in some reading, writing and (ideally) microscopy it will be stressful and a struggle from day one. Most employers support professional development amongst their staff, especially in academia, and if you are considering a diploma, your Line Manager or PI should be your first stop. You may be able to negotiate some reduced duties or set aside half a day for dedication to your studies. For me, it began with a casual conversation, but such topics can be taken to HR or senior management for more serious talks.
It is advisable to know exactly what you are asking for in advance and to remember to display the passion for it; you don’t want to meet resistance at the first meeting. Have a plan as to how you can fit the diploma around your current workload and how it can complement the lab or facility’s remit.
Although I negotiated to continue my diploma in my new role, it was under the condition that my managerial duties must always take priority. This may be helpful to emphasise to your employer as assurance that your regular duties will not be neglected. However, it does come with its own
issues, as my workload, like many laboratories, is variable and can drastically increase at little or no warning.This summer, I made the difficult to decision to stop all diploma activities during a particularly busy period in the lab. It was the right decision as the alternative could make all situations worse.
Thankfully, the RMS team understand that diploma students are usually full-time workers, not full-time students and that there are external responsibilities. Because of this they are realistic about expectations, and you should be too; if you are devoting a halfday a week to your diploma, do not expect the same experimental output as the full-time PhD student working alongside you. This is why the RMS are generous and flexible with their diploma requirements allowing between two and five years with ‘regular’ circumstances.
A particular difficulty I have experienced is starting and maintaining longer-term experiments. My work with stem cells proved challenging due to the regular passaging requirements which I was unable to continually maintain due to lab management work taking priority.
Planning for such circumstances is difficult alongside a variable and uncertain workload. Here a supportive co-worker is invaluable: someone who can cover your work at short notice, just to keep that experiment going or the cells alive. If this isn’t available, then you may need to re-think your experimental plan. For example, factor in pauses where you can stop, as needed, and re-start when things settle down.
As the saying goes, ‘make hay while the sun shines’. When your workload allows be prepared to re-start experiments quickly to fully utilise quieter periods. Make meticulous notes detailing all experimental protocols and results. These proved invaluable to me after months of unrelated work.
Have clear objectives. These can be laid out and discussed at your progress meetings and should be reviewed regularly with your mentor.
Balancing work, study and life will be a challenge, so it would be foolish to pretend it won’t be. But if
you go in with realistic expectations of yourself, a supportive manager and most importantly, passion, you will give yourself the best chance.
Dr Joëlle Goulding (FRMS) is based at the Centre of Membrane Proteins and Receptors (COMPARE), University of Nottingham, UK.
Over the last few years I have become heavily involved with mentoring, something I had never really considered before. Part of this was because I thought that I didn’t have enough experience to pass on. To me the benefit of having a mentor is clear – someone to bounce ideas off, gain advice over tricky decisions and to be a sounding board. I didn’t have the confidence to offer this. What we often overlook, however, is how much experience - both in work and life - that we have amassed. When I stepped back to think about it, I realised I have experienced a number of job roles, written dissertations and given talks, survived fixed-term contracts and maternity leave which then led on to juggling childcare with a career. I have also gained expertise in a number of different imaging modalities, worked up and troubleshot protocols, and pursued collaborative and independent research interests. In the end it is all this experience together that actually makes me a very useful mentor for a diploma student …. Hopefully!

I am involved in the running of both my faculty mentoring scheme at the University of Nottingham and also the mentoring scheme which is offered by the RMS. Whilst both schemes are largely focussed on career and professional development, their angles and method of working are slightly different. And again the mentorship as part of the Diploma has its own slant. As a Diploma mentor your main purpose is to support the Diploma student through their passage of work. This does not mean directing their project as a supervisor would, or indeed, judging the work as an examiner. You are also the contact between the mentee and the organising body, the RMS. Whilst representing the RMS in this fashion, you still have an outside view on the programme of study and hopefully a bigger picture view on how the diploma in the long run could influence the student’s career.
My own role as a Diploma mentor has varied, this was my first time in this role and a bit of a steep learning curve into what was expected. This wasn’t an unpleasant curve but there is a level of responsibility about knowing what is expected of the student and guiding them in some of the course credits, such as the technical essay and identifying a suitable training course. For this, luckily, I could draw on my experience, and through working with the mentee, realise I DID have the experience to pass on. Hopefully my mentee hasn’t suffered, but if I take on the role again I will be a lot more prepared! What I didn’t realise was the vested interest that I developed in their career, being keen to make them aware of opportunities, conferences, societies and options that I, in their place, would have found invaluable. Your relationship has been set-up purely for this period of study, however this is likely to be a connection, a friendship that will continue.
Susan, who is based at Oxford Brookes University, is Chair of the RMS Qualifications Committee which meets twice a year to discuss current diploma candidates’ progress reports.
Individuals who undertake the RMS Diploma always report very positively on the experience.
When we have asked them “what has the Diploma done for you professionally and personally?” we receive responses such as “(it has)..showcased my enthusiasm for microscopy to future employers… (I’ve made) invaluable contacts within the community”; they say that it’s been “professionally .. a great experience”, and that it has brought them ‘’professional recognition” and “credibility amongst my peers”.
So, what is the Diploma, and who is it for? The Diploma is an international programme – we have Diploma candidates worldwide, including from Germany, Switzerland, Portugal, Australia and New Zealand – and, of course, from all parts of the UK. They are based in Universities, research institutes, industry and schools.
Typically, those undertaking a Diploma are working in a role where microscopy or cytometry is a major part of their employment and they wish to develop their professional competence, challenge themselves to learn new things, and develop additional skills. There is also the desire to receive formal, professional recognition for their expertise within their specialism. It is considered to be of equivalent status to a masters level qualification.
The Diploma is a bespoke, portfolio-based qualification that flexibly fits around, and complements, your current professional role. During your Diploma programme, which takes a minimum of two years but can be extended (depending on your work commitments), you undertake a workplace-based practical project, based in your employment, and designed by yourself with the assistance of your line manager and with expert support from the RMS. You then present a report on your project and a technical essay demonstrating your knowledge and understanding of the microscopy / cytology techniques and approaches you have used. In addition, you must attend a course supporting you to further develop your practical
expertise, a scientific meeting or conference, and also undertake an outreach or public engagement activity that promotes microscopy. The programme is designed to be challenging, rewarding and fitting with, and complementing, your existing employment. Your final portfolio will demonstrate a range of skills and capabilities; not just technical, but also your ability to communicate these to both specialist and non-specialist audiences, and your commitment to continued professional development.
Undertaking the Diploma is therefore a clear demonstration of your commitment to improving your skills and to promoting awareness of microscopy to a wider audience. You will improve your existing skills - and acquire new ones - that will benefit your career and your employer for many years to come. During your study, you will have the support of a dedicated RMS mentor. The Society is currently pursuing a path to become licensed by the Science Council to award Chartered Scientist. When this is in place, a Diploma should provide a pathway to Chartered Scientist. As one of our Diploma candidates said: “(this was)….an incredible opportunity to advance in my career as a microscopist”.

Find out more about the Diploma at http://www. rms.org.uk/study-read/rms-diploma.html/ or contact Kate Wooding kate@rms.org.uk to discuss your ideas for a Diploma project.
We are very pleased to welcome five new candidates onto the RMS Diploma programme, and wish them all the very best of luck as they progress their projects.
The RMS Diploma is a flexible, portfolio-based qualification designed to complement the candidate’s existing employment. Projects are designed by the candidate, with assistance from their line-manager and input from existing Fellows of the Society.
Here is a run-down of our latest candidates, and their chosen projects:
Samuel Davis, Loughborough University, UK
Project: Electron Microscopy Study of Precipitates in Energy Generation Steels
Using a multimodal approach to compare precipitate imaging techniques of power generation steels. Both

Scanning Electron and Focused Ion Beam (FIB) systems will be used to collect images, employing a combination of modern detectors, and optimised operating parameters for each system. These will be compared to benchmark examples cited in the literature with a view to improving upon the currently referenced standards.
Susan Duncan, John Innes Centre, UK
Project: Development of Quantitative Single Molecule RNA Imaging in Plant Crop
Species
Quantitative single molecule RNA imaging is highly complementary to next generation RNA sequencing approaches. Although lower throughput, imaging has an advantage over sequencing as it reveals subcellular RNA localisation patterns that can have significant consequences for gene regulation.

Despite advances in RNA imaging in many other model organisms, for plant biology this approach is currently limited to a few tissues in the model plant Arabidopsis thaliana. The project proposed for this Diploma aims to expand this imaging approach to a wider range of tissues in commercially important crop plants including wheat, sugar beet and oil seed rape.
Project: Investigation of the dynamic localisation and dimerisation of ATM kinase
Ataxia-Telangiectasia Mutated (ATM) is the central regulator of the DNA damage response in cells.ATM is mainly located in the nucleus or cytosol but has also been found at synapses – and the significance of this is unknown. It is known that ATM exists both in a monomeric and dimeric form but is unknown what significance the localisation and dimerisation

has. Using confocal and super-resolution microscopy, the localisation and dimerisation status of ATM will be observed before and after DNA damage in neuronal and non-neuronal cells, to observe its pattern of behaviour and speculate as to its significance in the DNA damage response.

In the manufacture of red blood cells (RBCs), it is crucial to track the maturation of cells in the bioreactor from a hematopoietic progenitor cell to a mature RBC. This can be achieved by fluorescent staining of maturation markers. Using imaging cytometry, we can collect the fluorescent signal of cells along with their corresponding brightfield image that captures morphological changes of maturing RBCs. My study aims to investigate if we can apply deep learning tools to our imaging cytometry data, so we can characterise the stage of maturation of manufactured RBCs in a label-free manner.
The study aims to develop and validate a new approach in microscopy for the rapid and non-destructive analysis of fresh tumor biopsy samples.With this method I’m planning to address a longstanding need in pathology for real-time, on-site biopsy examination while preserving the tissue for further analysis. This could expedite the initiation of targeted therapy and reduce the need for repeat sampling due to insufficient material.


4 - 7 June 2024, Liverpool, UK
The event will encompass:
• a blockbuster meeting programme covering all the latest techniques, applications and technology. Topics include: New Technologies, Imaging Across Scales, Super-resolution and Nanoscale Imaging, The AI Revolution, The Science of Tomorrow Today, and Multimodal Imaging;
• a wide range of companies showcasing their latest technology and running workshops timetabled outside of the main meeting programme;
• an accompanying exhibition in the purpose-built hall alongside posters, food and drink;
• a community workshop space at the heart of the exhibition, with many groups hosting meetings and running workshops;
• an event dinner with networking at the Rum Warehouse.
Find out more at www.elmi2024.org


Royal Microscopical Society
37/38 St Clements
Oxford, OX4 1AJ
www.rms.org.uk
Registered Charity: 241990


The annual Materials Research Society (MRS) Fall conference was held in Boston at the Hynes Convention Centre from 27 November to 1 December 2023.
As the plane from Dublin descended into Logan airport and the Prudential Centre came into clear view, the excitement for the upcoming MRS conference took over! As one of the largest materials science conferences in the world, with regular attendances of over 5000 people, it was sure to be packed with amazing talks and posters.
Given the recent funding rounds on both side of the Atlantic for more research into the topic, it was obviously clear that the theme of this year, at least in the physical sciences, was quantum-based materials and devices. As a microscopist (and with this being an in-house microscopy journal), there was something for everyone here. I attended the EELS and 4DSTEM sessions, and was inspired by the cutting-edge materials microscopy being performed, especially on quantum phenomena and materials such as the Rashba effect and topological insulators.
The conference also had a series of excellent plenary talks which I had the privilege of attending. These included the recent Nobel Prize in Chemistry winner, Moungi Bawendi of MIT, and Takashi Taniguchi of NIMS, Japan - the go-to guy for excellent quality hBN crystals. It was fascinating to see their academic career journey and helped to emphasise the point that since there is no easy, straight-line path to success, you should make your own path!
I presented my works on “Structural Characterisation of BaZrS(3-y)Sey Perovskite Thin Films via Scanning Transmission Electron

Microscopy” and “Thermoelectric StructureProperty Relationship Establishment in TlGaSe2” as oral presentations at the end of the week. Despite being tired from such a long but engaging week, the talks went very well, and I had a number of interesting discussions with people from various fields in materials science about my work, which was quite rewarding!
As I am within the last few weeks of my PhD, MRS Fall 2023 was my last conference as a PhD student, and I am delighted that it was! Having spent some time in MIT last year, it was lovely to catch up with old mates and colleagues, as well as make new ones. I am very grateful to the RMS for the financial assistance to enable me to attend this conference.
Tigran SimonianSchool of Chemistry & Advanced Microscopy Lab, Trinity College Dublin, Ireland

Many long-standing RMS members will recall that 1989 was a celebratory year for the Society, marking 150 years since its inception. Later this year, we’ll be looking back at some of the anniversary events that took place – as documented by The Proceedings – but for now, our attentions turn to an unusual pair of photographs from Volume 24 of the Proceedings, published in January 1989.
The issue includes a list of speakers due to give talks later that year, at – as it happens – a special meeting to mark the aforementioned anniversary. Those speakers included Dr Hans Tanke, of the University of Leiden, Netherlands. However, his biography photo appears in double, giving - at first glance - the impression of an unlikely production error.

This was no error, but rather, an inventive piece of editing from Proceedings editor, Peter Evennett.
(You can read a fascinating interview with Peter, which appeared in our June 2021 issue.)
As it happens, the presentation of the photographs was linked to Dr Tanke’s decision to submit a pair of images, apparently taken at a photo booth. A footnote beneath Dr Tanke’s listing directs readers to a later page to seek the explanation (see image above).
To round the story off, infocus managed to get in touch with (the now) Professor Tanke, who retired as Head of Department at Leiden in 2017. He was kind enough to share some memories of his involvement in the 1989 anniversary events – and, it turns out, he even remembers the photographs from 35 years ago!
He said: “I do remember the 150th celebration of the RMS. It was a privilege to lecture there instead of Professor Johan (Bas) Ploem, former president of the RMS, who was originally invited. I remember the famous lecture theatre, in which I was told, Isaac Newton had presented his work years ago.
“The speaker after me was Dr Weibel, who explained his theory of stereologic measurements. During his


he threw a handful of matches on the wooden floor and invited the participants on the first row to count the number of crossings with the structure of the wooden floor: of course his prediction of the outcome was right!”
On the subject of the photographs, he added: “Indeed, they were taken at a kiosk, since the real digital world was a little ahead. I have never realised though, that they represent stereo pairs. My wife - whom I have been married to for 48 yearsliked them, and said “in those days, you looked much more...... (you may fill it in!).”

…Well, whatever she meant, here’s a lovely, recent photo of Professor Tanke to bring us up to date: Hans
infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS.
infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more.
infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms.org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.ukj
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
• Total number of images/figures/tables should not exceed 15 including tables.
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).