Nature’s smallest glass-houses visible from space
Prismatic contrast enhancement microscopy (PACEM)

mmc2023 (incorporating EMAG 2023): Book now!
The importance of Industrial-Academic relationships
– Johnson Matthey

Nature’s smallest glass-houses visible from space
Prismatic contrast enhancement microscopy (PACEM)

mmc2023 (incorporating EMAG 2023): Book now!
The importance of Industrial-Academic relationships
– Johnson Matthey

The all-new JIB-PS500i from JEOL delivers fine milling capabilities essential for fast, high quality lamella preparation. This multi-purpose FIB-SEM enables high throughput sample preparation, high resolution STEM-in-SEM imaging, and analytical analysis.

Robust Workflow for Large Samples
Featuring a large specimen chamber with easy-access door and large high-tilt stage, the JIB-PS500i offers a truly flexible workflow. Transfer from the FIB-SEM to the TEM is seamless with TEM-Linkage, JEOL’s double-tilt cartridge.


infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.

infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK Editor
Owen Morton
Tel + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Laura Fumagalli, University of Manchester, UK
Myfanwy Adams, John Innes Centre, Norwich, UK
Maadhav Kothari, Zeiss Microscopy, UK
Hilary Sandig, Cancer Research, UK
Trevor Almeida, University of Glasgow, UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2023 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by The ImageWorks. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.
Dear Readers,
It is a great pleasure to bring to you our second issue of 2023.
For our readers in the northern hemisphere, June represents the arrival of summer (not so much in Scotland!), with parks and forests around us back to green colours. Perfect timing then, for a plant-based article from John Hutchison, which serves as a follow-up piece to ‘Bugs up Close’ (issue 69, March 2023). This time John examines an antique set of diatom slides recently donated to the RMS, capturing some of their magnificence – and the unparalleled skill of the slide mounters – in the process. Diatoms were often used as test specimens for 19th Century microscopes, as improved resolving power and new innovations opened up new possibilities.
Speaking of new innovations – and moving from the past to the present - we have a fascinating piece from Shiraz Kaderuppan on an imaging technique to enhance contrast in a brightfield microscope. Meanwhile, a new venture promoting interactions between academia and industry is the subject of an absorbing article by Aakash Varambhia and colleagues. This is a particularly topical subject in microscopy, and something very much on the RMS radar; the open innovation programme outlined by the authors is sure to be of particular interest to early career researchers.
In a few weeks we will be making a very happy return to Manchester for mmc2023 (4 – 6 July).This fantastic international Congress (one of the biggest of its kind in Europe) will be taking place in person for the first time in four years. You can read more about everything on offer at mmc2023 in this issue - from the world-class (and free!) exhibition, workshops and satellite meetings, to the amazing conference programme covering the full spectrum of imaging techniques and applications. If you haven’t done so already, there’s still time to book your ticket and share in this one-of-a-kind experience for the international microscopy, imaging and flow cytometry community. I will also be available to chat about infocus Magazine and discuss any ideas you may have for new content!
Please feel free to approach me at any point during mmc2023 if you would like to discuss how you can contribute to infocus
Slàinte!
COVER IMAGE: Biofilm or solar flares?, by Beatrice Bottura, University of Strathclyde

E. coli biofilm grown on a minimal medium agar substrate. The internal network of channels help transport nutrients from the environment towards the centre of the biofilm.
Equipment used: Mesolens (confocal laser scanning mesoscope), magnification 4x, N.A. 0.47.

It is well-known that algae grows wherever there is water, both fresh and salty, sometimes causing serious problems. It is formed by tiny, unicellular organisms known as diatoms. These were first identified in 1703 in a report in the Philosophical Transactions of the Royal Society, although the name of the finder is surprisingly, unknown. Observing with a simple microscope the roots of pond weed, he "saw adhering to them (and sometimes separate in the water) many pretty branches, compos'd of rectangular oblongs and exact squares." This description is our first written record of diatoms in the scientific community.
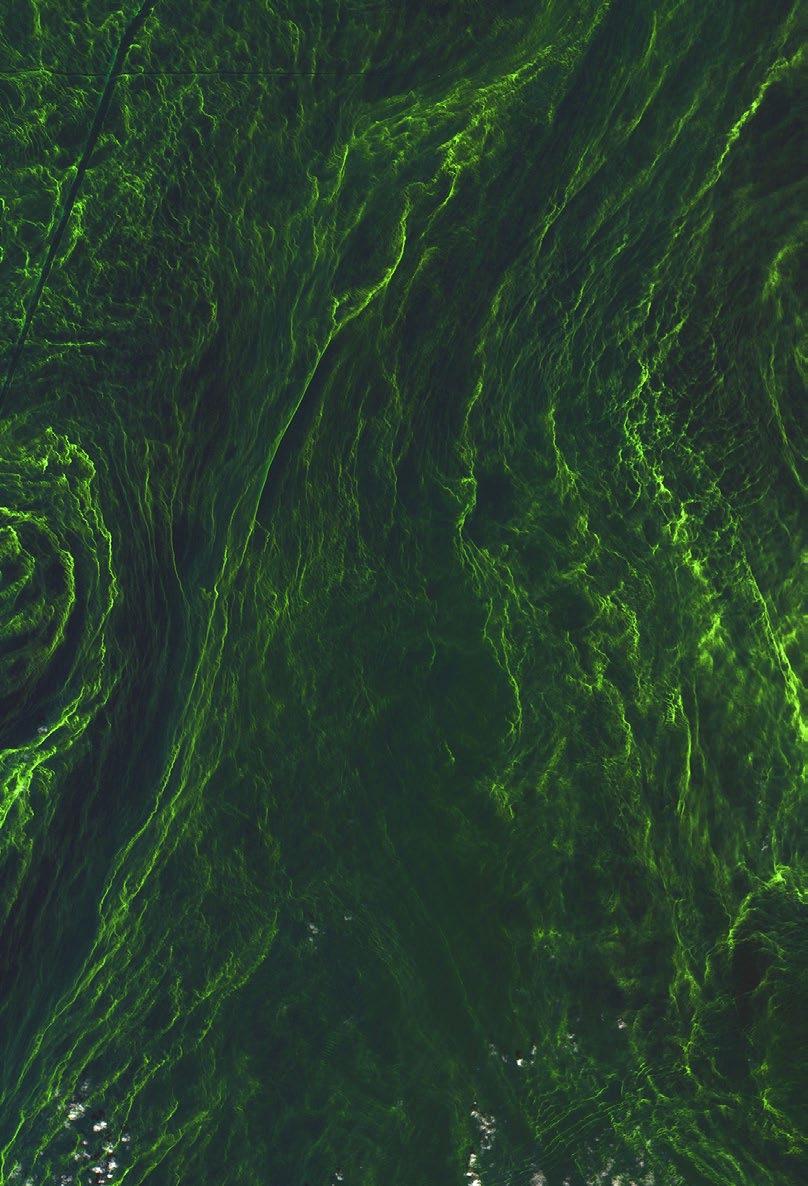
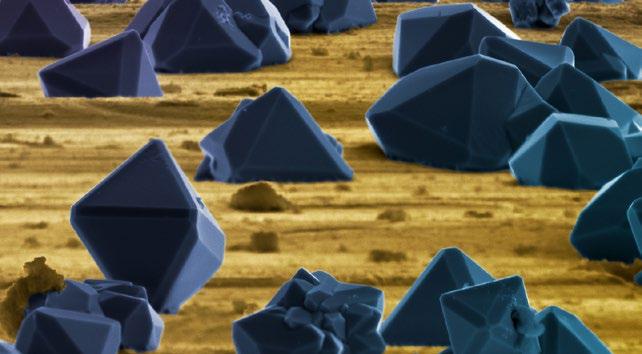
In 1844, Friedrich Kutzing published a monograph in which he classified all diatoms as algae. The close of the 19th century left us with a huge collection of diatom types obtained on a worldwide basis. Diatoms were also among the first specimens in which the details of cell division (i.e. mitosis) were examined. The exquisite drawings of diatom mitosis by Robert Lauterborn, published in 1896, illustrate this. Diatoms exist in huge numbers, and when conditions are ‘just right’, they can even form ‘algal blooms’ on the surfaces of lakes or oceans that are visible from space (Figure 1)! Being toxic, these cause serious environmental problems, and are harmful to wildlife. ‘Blue-green algae’ deposits are more familiar in the UK, when they affect lakes and riverbanks.
On the other hand, diatoms are in fact also beneficial in that they absorb CO2 and actually produce much of the oxygen (over 20%) that we breathe – more than all the world’s rainforests.They are also important contributors in the food chain for invertebrates and fish. They live relatively close to the surface and banks or shores of water bodies
– both fresh and salt - and even moist earth, where there is light for photosynthesis.
Being the only organisms that produce them, diatoms are interesting for their silicaceous (i.e. glass) outer skeletons. These are highly porous structures that enclose the organism and are remarkably regular. They were given the name “frustules” (little pieces), but this rather ugly word’s similarity to an unpleasant skin ailment perhaps led to the ‘diatom’ name being used for both the organism and also its glass cage. The many thousands of different diatom species occur in two types: those with cylindrical or prismatic skeletons displaying radial symmetry and those with bilateral symmetry, being elongated and often cigar-shaped, called pennates.
The shells all have two valves, and reproduction takes place by their splitting apart.
Being silica, the shells of diatoms survive after the cell dies, and accumulate in huge numbers on lakeand sea-beds, forming a fine sediment, ‘diatomite’.
Fossilised deposits are also known. Being hard, very fine-grained and highly porous, this material finds widespread uses, as toothpaste additive, or tooth powder, being marketed as “Sozodol” in America in the 1880s, metal polishes, facial cleansing cream and many others. As ‘kieselguhr’, it is also widely used

beer filtration and as the stabilising ingredient in dynamite. Highly pure diatomaceous earth can also be taken as a food supplement, and at the other end, it is also sold as cat litter. Diatoms are now also used by specialist, forensic pathologists, their occurrence and identification in a corpse’s lung tissue and

other organs indicating death by drowning, and possibly even – depending on the species of diatom identified - where that drowning occurred.
At this point you might well be wondering just what relevance all this has to popular microscopy? Well, just read on………
In the mid-1800s (the early days of the RMS), diatoms became popular objects for the growing fashion for examining novel materials, particularly as microscopes became more widely available. As optical resolving power improved, diatoms often were used as resolution test specimens. Elaborate protocols were developed for removing the organic material and other debris from the diatoms and mounting them in suitable arrangements to satisfy the rapidly developing market for novel and interesting microscope slides. This usually involved boiling the diatoms in sulphuric or nitric acid before washing and drying.

As an indication of the superb skills of the mounters, consider that the largest diatoms are barely visible to the naked eye, most being much smaller, ranging from ~2 to ~200 microns in size. Once cleaned and dried, they had to be carefully placed in position on a slide using a very fine filament or hair (often a

pig’s eyelash!), then fixed in position in resin, usually Canada Balsam, before the cover slip was placed on top. Alternatively, they could be laid on balsam on the underside of a cover slip which was then placed on the slide. A small, coloured circular marker was then frequently positioned to help the microscopist to locate the diatoms – as shown in Figure 2
In response to the growing popularity and demand for diatom slides, several individuals both in the UK and in Europe rose to the forefront of this business. Among them was William A Firth, whose family moved with its bleachworks from Barnsley to Belfast in the 1860s, from where he developed his own trade in diatom slides. He advertised widely for new samples from overseas (Figure 3) and all of his diatom slides include not only the species’ names, but also the exact location where they were found (see Figure 2).
By the 1890s, Firth had developed superb skill in preparing microscope slides of diatoms. The 1893 Proceedings of the Belfast Naturalists’ Field Club gave an award:
“... to William A. Firth for his very superior slides of grouped diatoms, a set remarkable for the amount of manipulated skill displayed in each separate slide, as well as in the taste and general excellence of the entire set.”
In 1897, Firth’s expanding business placed an advertisement in the ‘For Sale’ section of an issue of The English Mechanic and World of Science (Figure 4). It is not clear how long before that date he had begun to sell significant numbers of his slides.
Dr Edmund Spitta, a well-known Victorian expert in photomicrography, wrote in 1899:
“We know of no mounter of diatoms in the United Kingdom that can surpass Mr. Firth, of Belfast, and few that can equal him....”
Praise indeed!
Our recently acquired collection of slides (see infocus issue 69, March 2023) includes over fifty
Some of Firth’s slides contain only a single diatom; Figure 5 shows an example of the species Triceratium Bergonii, from Sendai.
The slide was mounted in 1896. The diatom is located at the centre of the black marker ring.

Some individuals prepared mounts with two identical specimens carefully placed side-by-side, as in Figure 6 – probably also by Firth, although the slide is anonymous.
A particularly nice example of several diatoms of a single species is shown in Figures 7a-c.

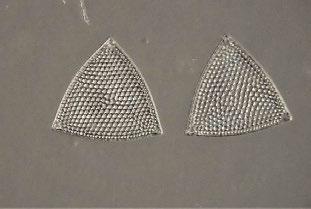

The slide labels indicate the species, and bear the signature “DG”.This signature appears on many slides

by other mounters such as Firth, but otherwise he is unknown. Perhaps he either provided the diatoms or else sold his own mounted slides through the more well-known names.
Examples of several identical diatoms are shown in Figures 8 and 9.


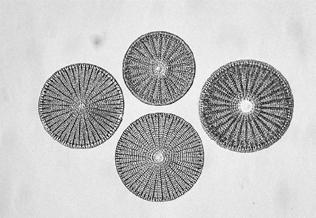
Firth later taught John Long, a schoolmaster based in Bradford, the skills required to mount good diatom slides. Examples of his work are shown in Figures 10 and 11.



As demand for fancy layouts of diatoms grew, so the best mounters created ever-more elaborate arrangements, ranging from relatively simple groupings of one (Figures 8 - 11) or several different
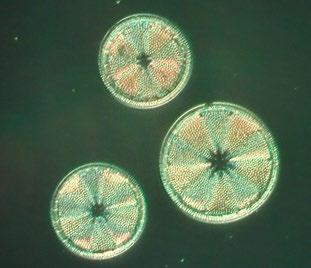
species (Figures 12 – 14) to others containing over 100 diatoms in geometrical patterns (Figure 15).

Eduard Thum (1847 – 1926) of Leipzig, with his own self-styled ‘Microscopy Institute', was another of the foremost preparers of diatom slides of his time. His

mounted slides range from simple arrangements of diatoms, to elaborate layouts that are keenly sought-after by collectors today. Our recently acquired collection includes several of his slides, and an example is shown in Figure 16.
When mounting narrow, pennate diatoms having very low contrast Thum adopted a trick of positioning larger, cylindrical specimens on either side of the main items of interest as a kind of ‘navigational aid’ for the microscopist. A nice


example of this is shown in Figure 16a.
Being thicker than the main subjects, these markers appear much darker, but oblique illumination can be used to enhance their contrast, and also that of the pennates, as shown in Figure 16b.
Mounters also prepared slides on which single species were simply ‘strewn’ onto a slide before being fixed. These often produce interesting effects, particularly when the ‘strews’ are viewed by
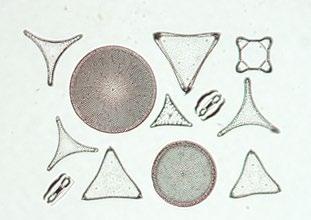
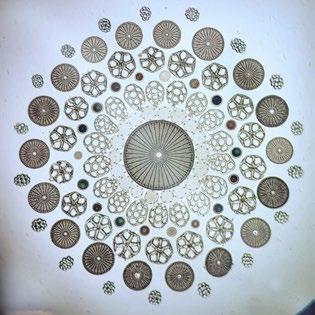


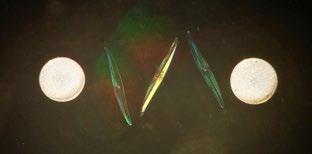
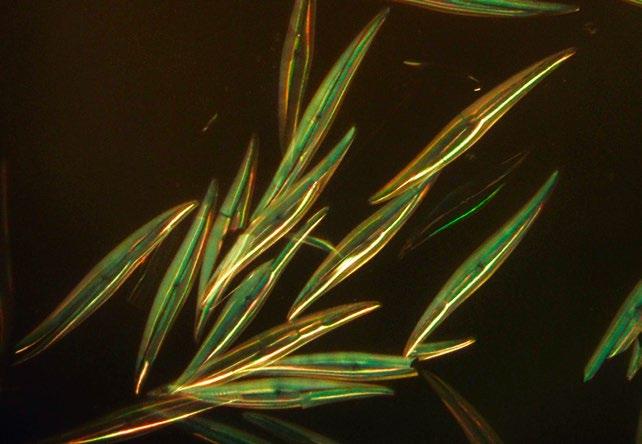
Figure 18. Thum’s Algensucher microscope. oblique illumination. Thun’s ‘strews’ are well known examples, as shown in Figure 17.

As an aid to finding, and hopefully identifying diatoms in the field, Thum also developed a simple, but remarkably effective, miniature, compound microscope which he called the “Algensucher” (lit. “algae seeker”), shown in Figure 18. He advertised this device around 1880, at a selling price of 6 DM. Our slide collection includes one of these unusual instruments. We note that it used slides which, being 5 x 1.7 cm in size, are much smaller than the standard ‘RMS’ 3 x 1-inch ones.
Johan Möller, of Holstein, was also well-known for his diatom slides. He exhibited them at the St. Petersburg Exhibition of 1869, where they were apparently awarded Gold Medals. An example is shown in Figure 19.
Möller also produced novel diatom slides which included microphotographs – precise arrays of spaces along with species’ names which he printed onto slides. He then managed to position the correct diatom in each of the spaces, before fixing everything in place with balsam.
In 1874, ‘The Quarterly Journal of Science’ reported: “Herr Möller has introduced a very ingenious modification of his celebrated Diatomacean typenplatte … The new arrangement consists of a photograph about 4 millimetres square, of eighty circles, ten in a longitudinal and eight in a vertical direction; beneath each circle is the name of the object and its author, and in the centre of each of these circles is a diatom, and in many cases two are mounted in order to show front and side views. The whole collection independently of its great value to the student of Diatomaceae is a marvel of manipulative skill.”
Our collection contains one of Möller’s ‘Typen Platten’ slides, containing 80 individual diatoms (Figure 20).


To leave the viewer in no doubt about the mounter, Möller even included his name and institute along with a diatom in the corner of the grid, shown in Figure 23.
‘Typen-Platten’ slides of this sort are quite rare, and keenly sought after. In addition to the 80-diatom slide shown here, examples containing 400 and even 1715 diatoms are known, the latter of which was prepared for the Emperor of Brazil in 1890.
Diatoms are still studied widely, with an International Society for Diatom Research. And for enthusiasts

there is a fascinating magazine “The Amateur Diatomist”, published regularly. Superb, modern slides with selected diatoms are still sold by e.g. The Diatom Shop, and the late Klaus Kemp continued producing amazing arrangements of diatoms and foraminifera until his death in 2022.



My brief foray into the microscopic world of diatoms has revealed some truly astonishing structures, preserved in all their beauty by the amazing skills of the mounters who didn’t have the benefits of modern microscopes and micro-manipulators. If you would like to see some of these wonders for yourself, call in at the RMS Learning Zone during mmc2023 in July – we will look forward to meeting you. Meanwhile next time you step in a puddle, or visit a rock-pool by the beach, just pause a moment to think of all the tiny glass cages lurking beneath the surface!
John Hutchison Hon FRMSThis was my first time attending an RMS International Botanical Microscopy Meeting, and I had been looking forward to it well in advance. My research predominantly uses confocal microscopy to investigate mycorrhizal symbiosis in rice, so a whole conference entirely dedicated to plant microscopy (sparsely represented at other conferences I had attended) seemed too good to be true. And as an added bonus, this 12th Meeting was hosted at the John Innes Centre, Norwich, so just a short trainride away and an opportunity to visit the renowned plant science hub of the Norwich research park.
The programme kicked off with a fascinating plenary from Enrico Coen, including stunning images, multiple microscopy techniques, mathematical modelling, Bladderwort, and synchronised swimming (analogies for cell behaviour during tissue development, of course). Other than the swimming, many aspects of Enrico’s broad-ranging talk turned out to be recurring themes of the conference, such as:
Diverse microscopy techniques – live-cell confocal microscopy, scanning electron microscopy, electron tomography, super-resolution 3D-SIM, synchrotron XRF, NanoSIMS, X-ray microscopy… the list goes on. As well as the sheer number of techniques, it was interesting to see how many researchers are using multiple complementary techniques, for example X-ray microscopy to find regions of interest or sample preparation problems for subsequent electron microscopy (Duncan et al.,
2022), and correlative light and electron microscopy to locate ‘rare’ events or regions of interest before revealing corresponding cell ultrastructure with TEM (Su et al., 2023). I look forward to seeing the expansion of these techniques in the plant science world, and using them in my own research.
Mathematical modelling – the use of modelling alongside microscopy featured heavily in the conference, both generation of models to probe cellular mechanisms underlying microscopical observations, and validating model outcomes with imaging (Coen & Cosgrove, 2023; Bellandi et al., 2022; Fozard et al., 2023). These talks certainly persuaded me of how fruitful collaborations with computational modellers can be.
Not just Arabidopsis – there is a perception that, like a lot of plant research, Arabidopsis is the go-to model for cell-biological and microscopy studies. The research presented at this conference suggested otherwise: from mosses to Mimosa and cereal crops to carnivorous plants, microscopical investigation of at least 38 plant species was reported, as well as their interacting microbes (both beneficial and pathogenic) (Duncan et al., 2022; Eseola et al., 2021; Ivesic et al., 2023; Lace et al., 2023; Sleboda et al., 2022). In each case the unique imaging challenges of the system were overcome, proving the potential of microscopy in applied plant research, e.g. agriculture, conservation and remediation.
Live imaging – from the scale of individual chromosomes and golgi bodies up to calcium waves and leaf movements, many talks included live and time-lapse imaging (Bellandi et al., 2022; Eseola et al., 2021). Both the resolution achieved in these studies as well as the length of time over which plants and
microbes were imaged were truly impressive. This bodes well for more non-invasive, long-term, in-situ imaging to capture true cellular and tissue dynamics going forward. I certainly gained lots of motivation for my own efforts in this area, having seen so much success with other difficult imaging systems.
Alongside the talks, the Botanical Microscopy Meeting also included a bustling poster session, guided tours of the John Innes Centre microscopy facilities and rare books collection, and a lovely conference dinner in Norwich. There was ample time in the lunch and tea breaks for discussing and engaging with the very welcoming plantmicroscopist community, and I left the conference full of inspiration and ideas of microscopy techniques to try and questions to pursue. The only negative from my point of view: we have to wait another four years until the next conference!
Thank you to the organisers from the Royal Microscopical Society, John Innes Centre and organising committee for such a well-planned and well-rounded conference. Thank you also to the sponsors, particularly New Phytologist for the Early Career Researcher grant for attendance, and the Journal of Microscopy for sponsoring the prizes.

Bellandi A, Papp D, Breakspear A, Joyce J, Johnston MG, de Keijzer J, Raven EC, Ohtsu M, Vincent TR, Miller AJ, et al. 2022. Diffusion and bulk flow of amino acids mediate calcium waves in plants. Science Advances 8: eabo6693.
Coen E, Cosgrove DJ 2023. The mechanics of plant
Duncan KE, Czymmek KJ, Jiang N, Thies AC, Topp CN 2022. X-ray microscopy enables multiscale high-resolution 3D imaging of plant cells, tissues, and organs. Plant Physiology 188: 831–845.
Eseola AB, Ryder LS, Osés-Ruiz M, Findlay K, Yan X, Cruz-Mireles N, Molinari C, GarduñoRosales M, Talbot NJ 2021. Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genetics and Biology 154: 103562.
Fozard JA, Morgan C, Howard M 2023
Coarsening dynamics can explain meiotic crossover patterning in both the presence and absence of the synaptonemal complex. eLife 12: e79408.
Ivesic C, Krammer S, Koller-Peroutka M, Laarouchi A, Gruber D, Lang I, Lichtscheidl IK, Adlassnig W 2023. Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants 12: 341.
Lace B, Su C, Invernot Perez D, RodriguezFranco M, Vernié T, Batzenschlager M, Egli S, Liu C-W, Ott T 2023. RPG acts as a central determinant for infectosome formation and cellular polarization during intracellular rhizobial infections. eLife 12: e80741.
Sleboda DA, Geitmann A, Sharif-Naeini
R 2022. Multiscale structural control of hydraulic bending in the sensitive plant Mimosa pudica. BioxRv: 2022.02.28.482281.
Su C, Zhang G, Rodriguez-Franco M, Hinnenberg R, Wietschorke J, Liang P, Yang W, Uhler L, Li X, Ott T 2023. Transcellular progression of infection threads in Medicago truncatula roots is associated with locally confined cell wall modifications. Current Biology 33: 533-542.e5.
We are very pleased to continue offering a range of ‘in-person’ and virtual events this year, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend.
The following information was correct at the time of publication but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates.
Our online calendar includes all the details about forthcoming talks in the International Microscopy Focus Lecture Series – a joint, online initiative established between the RMS, and a number of international societies.
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
2023
June
14 Expansion Microscopy User Group Meeting - Canada Hosted - (Online)
July
4 – 6 mmc2023: Microscience Microcopy Congress 2023 (incorporating EMAG 2023) - Manchester, UK
7 Super-resolution workshop - Leeds, UK
10 – 11 Light Microscopy Summer School 2023York, UK
12 – 13
Getting the most from your Confocal Course 2023 - York, UK
17 – 21 ESRIC Super-resolution Summer School 2023 - Virtual, UK
23 – 27
Microscopy and Microanalysis 2023
(M&M 2023) - Minneapolis MN, USA
(Non-RMS Event)
September
4 – 8 Flow Cytometry Course 2023 - York, UK
5 – 8 Adhesion and migration in disease: Translational and therapeutic opportunities - Warwick, UK
October
9 – 10 Facilities Management Training Course - York, UK
9 – 13 All Things Cryo - Nottingham, UK
November 16 flowcytometryUK 2023Cambridge, UK
2024
June 4 – 7 elmi2024 - Liverpool, UK
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
mmc2023 (incorporating EMAG 2023)
4 – 6 July, Manchester, UK
Make sure your diary includes mmc2023! One of the biggest events of its kind in Europe, mmc2023 will bring you the very best in microscopy, imaging and cytometry from across the globe. With six parallel conference sessions, a worldclass exhibition, workshops, satellite meetings, an international Imaging Competition and more, it’s simply the place to be for anyone who uses a microscope for work, study or pleasure.

Back at the superb Manchester Central conference centre for the first time since 2019,
the event will be taking place fully in person, providing the perfect opportunity for the scientific community to come together, make connections and share their research. As always, many of the leading companies in microscopy and imaging will be on hand to demonstrate the very latest equipment and technology at the exhibition, which is returning to Manchester from 4-6 July.
As with the previous events, you can expect a huge and varied scientific conference alongside Europe’s largest free microscopy and imaging exhibition filled with a huge number of free training workshops.
10 – 11 July, York, UK
Scientific organiser: Peter O’Toole, RMS Vice President, University of York
The Light Microscopy Summer School is an annual, two-day course held at the University of York covering the principles of light microscopy. Participants are also trained in practical issues surrounding light microscopy. After introductory
presentations, the course is taught predominantly through hands-on practical sessions.The course is suitable for both novices and more experienced users wanting to gain a greater understanding of the microscope and feedback every year is always fantastic. Students usually come from a range of backgrounds, within both research and commercial organisations. All benefited greatly from the Course and left with increased understanding and skills.

12 – 13 July, York, UK
Scientific organiser: Peter O’Toole, RMS Vice President, University of York
This two-day, annual confocal course utilises many different sample types and fluorescent probes (DNA stains, classic antibody labels and fluorescent proteins) which are chosen to best demonstrate particular problems and techniques. Focus is always on the techniques they enable
and the problems they generate, which will be applicable to any sample types. The two days consist of short tutorials followed by hands-on practice.
Day 1 takes participants through the basic principles of confocal microscopy and then trains them, through hands-on practice, how to configure and image multicolour, multidimensional samples using a confocal microscope.
Day 2 builds on the experience of Day 1 and enables participants to try FRAP and spectral profiling.
4 – 8 September, York, UK
Scientific organisers: Dan Payne, Haematological Diagnostic Service (HMDS), Leeds; Derek Davies, The Francis Crick Institute; Karen Hogg, RMS Flow Cytometry Section Chair, University of York; Peter O’Toole, RMS Vice President, University of York
This Flow Cytometry Course is aimed at both clinical applications and applications in cell biology, with the common fundamentals covered on Day 1 and 2. The course then splits into clinical applications and applications in cell biology streams, from practical demonstrations to lectures highlighting not just the applications, but best practise as well.
The course is constructed as a set of three modules. You can elect to attend the course from between two to five days, depending on the modules selected.
The modules consist of lectures interspersed with sessions in the laboratory. It is anticipated that instruments from two manufacturers will be available for practical work.
This course is open to all and is suitable for those who are relatively new to flow cytometry and who wish to expand their experience with applications and specific analysis.
A trade exhibition will be held at this event on Wednesday 6 September, if you are interested in exhibiting please contact Jess Cole.
trusted by thousands of customers globally to deliver excellent high quality images for a wide range of magnification applications, in an easy to use ergonomic system.

Mantis gives you a unique package of advantages to your stereo microscopy including a large high quality optical stereo image, ergonomic eyepiece-less design for user comfort and productivity, 5 different ways to illuminate your subject to perfectly control your view, digital imaging for collaboration, 3 model range versatility, and suitability for a wide range of applications.
To meet Mantis for yourself, and to see what you’ve been missing, search Vision Engineering Mantis, scan the QR code or call us on 01483 248300. www.visioneng.com

 Shiraz S Kaderuppan
Shiraz S Kaderuppan
Introduction
Brightfield microscopy has been a viable observation technique for cytology and histological analysis employing stained cells and tissue sections mounted on a microscope slide. Many live cells (including phytoplankton and zooplankton) are generally transparent, preventing their clear observation using traditional brightfield microscopy. In this respect, other imaging modalities (such as darkfield [1], phase contrast [2] and DIC [3] microscopies) were developed, allowing the user to gain an insight into unstained living tissue (and cells) for image/video acquisition and observation of dynamic processes occurring real-time in vivo. However, most of these techniques may require additional accessories or even a total revamp of the entire microscope setup, if the said microscope is unable to support these techniques. In this regard, this article proposes an innovative imaging technique (termed PACEM) developed to circumvent these issues, as described in the following sections.
A trapezoidal prism was placed above a half-wave plate mounted in a rotating filter holder, which was in turn localized above a thin circular polarizing film. Another (fixed) half-wave plate was positioned above the first trapezoidal prism, and a second tilting trapezoidal prism being sited above this halfwave plate; the two components being subtended by a gap to allow for insertion of the specimen
slide containing the specimen sandwiched between a coverslip and the slide. The entire assembly was subsequently placed on a microscope stage for image acquisition. A second circular polariser (analyser) was also positioned into the optical train (above the microscope objective) for inspection of the specimen using circularly polarised light microscopy [4]. Figure 1 depicts the setup and the schematic for the light path established through the employment of this setup.

Variability in the optical path lengths (OPL) of the polarised light waves was thus achieved by tilting the prism below the slide (via a screw) and the images acquired compared against those without the use of this setup.The following Figure 2 shows the assembly mounted on the stage of an upright microscope (a Leica DM4000M), with a microscope slide inserted into it for diascopic observation under the eyepieces.
A couple of commercially prepared permanent slides of chemically clarified samples (Paramecium sp. and Stentor sp.) were imaged under the currently described setup, and the results portrayed in figures 3 and 4.

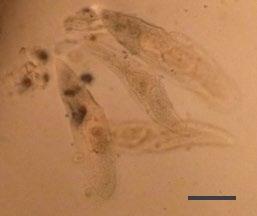
From Figures 3 and 4, it is evident that the optical setup of the assembly substantially aids in improving image contrast and apparent resolution without the need for specimen fixation and staining, observed real time with a substantially high resolution in vivo. Moreover, the assembly would also prove to be a highly useful addition for the optical microscopist toolbox, both for amateur and corporate microimaging applications.
The afore-described assembly has proven to be a highly useful accessory for both the professional microscopist and hobbyist. The images gleaned through the use of the accessory exemplify improved clarity manifested through an enhancement of image contrast coupled with increased lateral & axial resolution.

It would be noteworthy to mention that the proposed approach (PACEM) may resemble some previous techniques {such as DIC [3] or JaminLebedeff interference (JLI) [5] microscopy}, but it (i.e. PACEM) differs quite fundamentally from these techniques (DIC or JLI) as follows:
DIC employs interference between polarised light rays which have been split into their component O- (& E-) rays and which are shifted by a very small distance (shear) in the specimen plane [3] to give a 3D effect, while the proposed method (PACEM) employs polarised light rays which are shifted by a large distance to recombine and provide the final image in the image plane.
JLI involves splitting the polarised light into its component O- (& E-) rays, which are then differentially phase-shifted upon passing through the ½λ plate as well as the specimen [5]. In contrast, PACEM does not separate the O- (& E-) vectors of the polarised light wave, when it is transmitted through the beam-splitter.
1. W. Chambers, T. J. Fellers and M. W. Davidson, “Darkfield Illumination,” Nikon Instruments Inc, [Online]. Available: https:// www.microscopyu.com/techniques/ stereomicroscopy/darkfield-illumination.


[Accessed 4 7 2021].
2. D. B. Murphy, R. Oldfield, S. Schwartz and M. W. Davidson, “Introduction to Phase Contrast Microscopy,” Nikon - MicroscopyU, [Online]. Available: https://www.microscopyu.com/ techniques/phase-contrast/introduction-tophase-contrast-microscopy. [Accessed 24 Apr 2019].
3. C. R. Bagnell, Jr., “Chapter 11 - Differential Interference Contrast Microscopy,” 2012. [Online]. Available: https://www.med.unc.edu/ microscopy/files/2018/06/lm-ch-11-dic.pdf.
[Accessed 2 11 2019].
4. P. C. Robinson and M. W. Davidson, “Polarized Light Microscopy,” Nikon Instruments
Inc., [Online]. Available: https://www. microscopyu.com/techniques/polarizedlight/polarized-light-microscopy. [Accessed 4 7 2021].
5. D. G. Stavenga and B. D. Wilts. (2019). “Measuring the refractive index dispersion of (un)pigmented biological tissues by Jamin-Lebedeff interference microscopy,” AIP Advances 9, 085107. doi: 10.1063/1.5113485
Shiraz S Kaderuppan
An avid optical microscopist, Shiraz enjoys developing new imaging techniques for optical microscopical imaging, as well as potentially reviving nowdefunct antiquated approaches in this domain. In the same light, Shiraz also enjoys exploring the use of computational methods/approaches to facilitate the accomplishment of such imaging technologies.

Iwish to thank the Royal Microscopical Society for awarding me the “Best Imaging in a Talk” prize at the Actin Meeting 2022 for my talk about the swimming migration of Drosophila adipocytes.

Many cells inside our body have the ability to migrate. This is important not just for the development of various tissues and organs but also has a detrimental effect in many diseases such as cancer metastasis. In the Franz lab we are interested in swimming cell migration, an extreme mode of adhesion-independent cell migration. It allows a cell to migrate inside a liquid without holding on to other tissues. We use Drosophila adipocytes, also known as fat body cells (FBCs), as a model system to study this ill-defined migration mode. FBCs are dissociated giant cells that actively move by swimming migration in the pupal hemolymph, the
propagated toward the FBC rear (as shown in the left image). The FBC in the image is expressing LifeActGFP (F-actin) and NLSmCherry (nucleus). These actin waves are associated with actomyosin contraction.

body fluid of the fly. One function of FBC migration is to allow FBCs to reach epithelial wounds to promote wound healing and fight infection.
Like other migratory cells, FBCs need to generate internal forces to swim. For this purpose, actin waves are produced in the FBCs cortex and
One of the goals in our lab is to explore the mechanism underlying the swimming migration of FBCs in more detail. To address this, we use live imaging and automated cell tracking to follow FBC migration and combine this with genetic screening to test various candidate proteins for their involvement in cell migration. Moreover, we use confocal microscopy and live imaging to assess FBC polarity, actin and microtubule organisation and actomyosin contraction during swimming migration.
The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences.
online at www.journalofmicroscopy.org
They include:
Diversity under the microscope: lessons for building belonging in interdisciplinary spaces from the Women in Imaging + Industry bootcamp
Meagan Esbin, Lena Blackmon
As scientific projects and labs benefit from increasingly interdisciplinary expertise, students and trainees find themselves navigating a myriad of academic spaces, each with its own workplace culture and demographics.A clear example is the interdisciplinary field of optics and biological microscopy which bridges biology, physics, and engineering. While Biology PhDs are now >50% women, men in physics and engineering fields still significantly outnumber women, resulting in an imbalance of gender representation among microscopists and other “tool innovators” in the interdisciplinary field of biological microscopy and biomedical optics. In addition to the cultural and cognitive whiplash that results from disparate representation between fields such as Biology, Engineering, and Physics, indifference from institutional leaders to implement equityfocused initiatives further contributes to cultures of exclusion, rather than belonging, for women. Here we elaborate on the motivation, structure, and outcomes of building a specific affinity-based bootcamp as an
intervention to create an inclusive, welcoming learning environment for women in optics. Considering the presence of nonbinary, trans, and other gender minoritised scientists, we recognize that women are not the only gender group underrepresented in biological microscopy and biomedical optics; still, we focus our attention on women in this specific intervention to improve gender parity in biological microscopy and biomedical optics. We hope that these strategies exemplify concrete paths forward for increasing belonging in interdisciplinary fields, a key step towards improving and diversifying graduate education.
Visualisation of calcium oxalate crystal macropatterns in plant leaves using an improved fast preparation method
Hans-Jürgen Ensikat, Mahdieh
Malekhosseini, Jes Rust, Maximilian Weigend
Leaves of the majority of plants contain calcium oxalate (CaOx) crystals or druses which often occur in spectacular distribution patterns. Numerous studies on CaOx in plant tissues across many different plant groups have been published, since it can be visualised readily under a light microscope (LM). However,
there is surprisingly limited knowledge on the actual, precise distribution of CaOx in the leaves of quite ordinary plants such as common native and exotic trees. Traditional sample preparation for the documentation of the distribution of CaOx crystals in a given sample – including overall distribution –requires time-consuming clearing procedures. Here we present a refined fast preparation method to visualise the overall CaOx complement in a sample: The plant material is ashed and the ash viewed under the polarising microscope. This is a rapid method which overcomes many shortcomings of other methods and permits the visualisation of the entire CaOx content in most leaf samples. Pros and cons in comparison with the conventional clearing technique are discussed. Further aspects for CaOx investigations by micro-CT and scanning electron microscopy are discussed.
Investigation of Rhodnius
Gabriela Sena, Ademir X. da Silva, L.
P. Nogueira, M. V. Colaço, Brian
Mestcher, G. Fidalgo, A. Pickler, P. Azambuja, M. S. Gonzalez, D. P Mattos, Regina.Cely. Barroso
In the last years, microtomography has proved to be a powerful technique on insects’ studies, allowing a detailed view of the structures’ internal with a high resolution. One of the most important advantages of the use of microtomography in these studies is the fact that the dissection is not necessary, which decreases considerably the number of samples used on the insects’ research. Some insects are used constantly in studies about morphology, metamorphosis, and reproduction, because they work as a model for others, and Rhodnius prolixus is one of the most studied in this group.This insect is also one of the main insect vectors of Chagas disease that kills around 12,000 people every year in Latin America. Some studies using laboratory microtomography conventional scanners combining with the correct staining methods have proved that it could be a powerful tool in biological research, allowing the visualisation of low-density tissues. The main goal of the present work was to use staining protocols to study Rhodnius prolixus with laboratory microtomography conventional scanners. The experiments were carried out at the imaging lab in the Theoretical Biology Department, University of Vienna, using an Xradia MicroXCT and at the University of Oslo, using a Skyscan 2211.
ORIGINAL ARTICLE
Seven-layer analysis model of an optical waveguide excitation fluorescence microscopy

Yuan-Jie Long, Guo-Fang Fan, Yan-Jun
Hu, Xin-Gang Dai, Hong-Ru Zhang, Shi Li, Gao-Shan Jing, Da-Lin Wu, Yuan Li
In this paper, an optical waveguide evanescent field fluorescence microscopy is studied. Based on Maxwell’s equation, a seven-layer theoretical analysis model is developed for the evaluation of an optical waveguide excitation fluorescence microscopy. The optical waveguide excitation fluorescence microscopy

structure is systematically and comprehensively analysed at the wavelengths of 488, 532 and 646 nm for fluorescent dyes. The analysis results provide some useful suggestions, which will be beneficial to the research of an optical waveguide evanescent field fluorescence microscopy.
ORIGINAL ARTICLE
Estimating microstructural feature distributions from image data using a Bayesian framework
Noah Wade, Lori Graham-Brady
Many microstructural characterisations methods collect data on a regular pixelised grid. This method of discretisation introduces a form of measurement error which can be shown to be proportional to the resolution at which they are collected. Intuitively, measurements made from low-resolution data are associated with higher error, but quantification of this error is typically not performed. This is reflected in international standards for measurements of
component is sufficiently resolved. In this work, a new method for quantifying the relative uncertainty of such pixelised measurements is presented. Using a Bayesian framework and simulated data collection on features collected from a Voronoi tessellation, the distribution of true geometric properties given a particular set of measurements is computed. This conditional feature distribution provides a quantitative estimate for the relative uncertainty associated with measurements made at difference resolutions. The approach is applied to measurements of size, aspect ratio and perimeter of given microstructural components. Size distributions are shown to be the least sensitive to sampling resolution, and evidence is presented which shows that the international standards provide an overly conservative minimum resolution for grain size measurement in microstructures represented by a Voronoi tessellation.
ORIGINAL ARTICLE
The microstructure and thermal stability of the two-phase amorphous melt-spun alloys ejected from a double-chamber crucible
grain size, which only provide a recommended minimum number of sample points per microstructural component to ensure each
This work presents the microstructure and properties of two-phase amorphous melt-spun alloys ejected from the crucible with partition between liquids. The microstructure was studied by scanning electron microscopy and transmission electron


microscopy and the phase composition was studied by X-ray diffraction. The thermal stability of the alloys was determined using differential scanning calorimetry. The microstructure study proves that the composite alloys are heterogeneous because of the existence of the two amorphous phases obtained due to the use of a partition between the liquids.This microstructure correlates with complex thermal characteristics not found in homogeneous alloys of the same nominal composition.The layered structure of these composites influences the formation of fractures during tensile tests.
Combining atomic force microscopy with complementary techniques for multidimensional single-cell analysis
The advent of atomic force microscopy (AFM) provides an amazing instrument for characterising the structures and properties of living biological systems under aqueous conditions with unprecedented spatiotemporal resolution. In addition to its own unique capabilities for applications in life sciences, AFM is highly compatible and has been widely integrated with various complementary techniques to simultaneously sense the multidimensional (biological, chemical and physical) properties of biological systems, offering novel possibilities for comprehensively revealing the underlying mechanisms guiding life activities particularly in the studies of single cells. Herein, typical combinations of AFM and complementary techniques (including optical microscopy, ultrasound, infrared spectroscopy, Raman
spectroscopy, fluidic force microscopy and traction force microscopy) and their applications in single-cell analysis are reviewed.The future perspectives are also provided.


1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
Our warmest congratulations go to Alfonso Schmidt, who becomes the latest recipient of the RMS Diploma!
Alfonso manages the Histology and Bioimaging facility at the Hugh Green Cytometry Centre in Wellington, New Zealand. The facility uses light microscopy technology to understand the immune system in diseases such as cancer, infectious diseases, allergies and inflammatory diseases.
Alfonso kindly answered some quick-fire questions about his experience completing the portfolio-based qualification, which is suitable for those using microscopy or cytometry as part of their career, and designed to complement the candidate’s current employment.

How did you hear about the RMS Diploma?
I found out about the Diploma programme by checking the RMS website.
Why did you decide to do the RMS Diploma?
I was looking for an opportunity to increase my knowledge in the microscopical field. The RMS diploma seemed the perfect opportunity for expertise accreditation, mentorship, and professional collaboration.
What would you say the diploma offers that other courses / qualifications don’t?
The RMS diploma programme is very well designed for professionals already working in the microscopical field. With a flexible study portfolio and based in the workplace, it provides the perfect conditions for professional development.
What area of microscopy / science did you study and why?
The main project during the diploma was the development of a three-dimensional immunofluorescent staining protocol in skin. The project will provide very useful guidance to develop visualisation models to understand allergic immune reaction in the skin.
How will your research be of benefit to other microscopists / scientists?
The project “Skin whole-mount immunofluorescent staining protocol, 3D visualisation and spatial image analysis” will be a very beneficial procedure for the research we are doing at the Malaghan Institute of Medical Research and the scientific community, especially in the understanding of the interaction of the immune cells associated with the skin layers and the structures.
How do you think gaining the diploma will help you in your current role and future career?
The Diploma is a specialisation opportunity that is not often available in mainstream curriculum. Being granted with a Diploma degree in Microscopy from RMS is a huge asset for my career as a specialist in the field.
Will you be continuing to build on / develop the work / area of study from your diploma?
As part of my role in the facility we are constantly developing new protocols and innovative approaches to improve the research at the Malaghan Institute of Medical Research. I am confident I will keep developing new techniques, especially in the field of three-dimensional histology.
What was the most challenging aspect of the diploma, and why?
Being based in Wellington, New Zealand, was challenging in terms of attending meetings and courses at very un-conventional hours, but definitely worth the effort.
What did you enjoy the most about the course?
I enjoyed very much the opportunity to be involved in a self-driven project and the technical essay in relation to laser scanning confocal microscope quality assessment. I also enjoyed the regular meetings with my mentor, Graham Wright, talking about the project, professional opportunities, and life.
What’s the best piece of advice you could give to someone considering doing the diploma?
I think it is important to consider that the diploma requires extra workload, in addition to the busy life working in a microscopy facility. However, this is a fully recommendable experience for facility staff with a hunger to learn new skills and to be involved in the Royal Microscopical Society.
Find out more about the RMS Diploma

The 67th Biophysical Society Annual Meeting (affectionately named Biophysics for short), was held this year in San Diego. This absolute monster conferencetypically comprising eight parallel sessions at any given time, and running 8am6pm for a grand total of five days (Saturday through Wednesday) - is the kind where you wish you could duplicate yourself so as to not miss out on all the great science on offer. I am glad to have received some very wise advice beforehand - “marathon not a sprint”, ensuring a sustainable Biophysics experience.
which I watched on the plane over. The convention centre sits directly next to the bay, meaning the brief respites between talks could be spent with a coastal breeze and a pleasant view of shipyard and the Coronado bridge.
Networking began on the plane, a considerable fraction of which appeared to be filled by Biophysics attendees – clear from the number of PowerPoints visible from the aisle, with conference-goers making last minute changes to their talks. With over 5,000 scientists rumoured to be attending, one can only imagine how many tacos were consumed by Biophysical fanatics over the course of the five days in the ‘Birthplace of California’.
On my first day I started acclimatising to the Californian sun at San Diego Zoo, in awe of the incredible polar bears. My accommodation near Little Italy, roughly a mile away from the venue, gave me a grand total of 12 blocks of sidewalk on which to gather myself (and fill myself with tasty eats), ready for the onslaught of science. The venue itself, San Diego convention centre, akin to a plane hangar in size, gave me flashbacks to Top Gun Maverick
This was my first international conference as a postdoc (I last attended SMLMS 2018 in pretty Delft as a third-year PhD student). My goals for the conference were to take in talks from a variety of research areas which could inspire my own fellowship goals, and to also network with some of the 150 companies in the exhibit hall to get a better sense of life outside of academia.
It would be impossible to summarise the sheer mass of talks across the entire conference given the wealth of sessions, but I attended stimulating sessions relating to super-resolution microscopy approaches (including innovations in dSTORM and PAINT and MINFLUX), smooth and cardiac muscle, and voltage gated ion channels. On Saturday there was a range of subgroups specialising in focused areas; I took my place in the group focused on ‘Nanoscale Approaches to Biology’. On Monday evening we were treated to the plenary 2023 Biophysical Society Lecture, delivered by 2021 Nobel Prize Laureate Ardem Patapoutian, on molecules that sense touch.
As an expansion microscopy user of seven years,
I was delighted to see so many researchers presenting work featuring the hydrogel-based super-resolution imaging technique, considerably more than I’ve observed at previous conferences. Interacting with these other expansion users was definitely one of the most satisfying aspects of the conference.
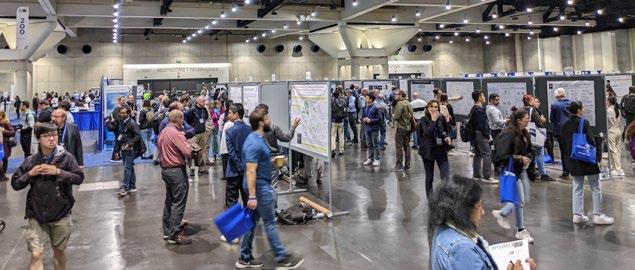
Poster sessions generated a palpable buzz in the exhibit hall. I thoroughly enjoyed the opportunity to present my poster on the Monday afternoon. In my poster, titled ‘Diverse labelling of cellular compartments with NHS esters in expansion microscopy’, I presented new insights into the use of amine-reactive ester forms of fluorescent dyes as nondescript stains to visualise overall cellular or tissue architecture. We have characterised a palette of dye esters with a range of physical properties (particularly hydrophobicities) which yields labelling of different cellular compartments in the broadly used human cell model HeLa. We demonstrated their utility as counterstains that can be combined with traditional fluorescent probes such as antibodies for both diffraction-limited, and ExM imaging. Crucially, our observations highlight a number of key considerations with regards to multiplexed labelling with fluorescent dye esters, providing a roadmap for adopting and validating the structures that they stain. The work is now available as a pre-print publication which you can read for yourself at: https://www.biorxiv.org/content/10.110 1/2023.02.21.529394v1
Aside from the scientific talks, career workshops (delivered by the charismatic figures of Andrew
Green and Alaina Levine) were a highlight, focusing on a range of topics including leveraging Linkedin, the postdoc experience, and identifying career options beyond academia.
Networking feels like the main draw to a whopping conference like Biophysics. I enjoyed reconnecting with old colleagues as well as meeting so many new faces. On Tuesday evening I made it to the dinner meetup, where Alaina Levine (representing Networking for Nerds) led a horde of attendees over to Hard Rock hotel’s own restaurant, much to the surprise of the waiting staff.
My final Biophysics impressions? With such a mindboggling quantity of science across a broad range of disciplines, I leave feeling inspired with a lot of food for thought - not just in the form of tacos - applicable to my research interests and careers goals. The Biophysics community feels so vibrant; I really hope this isn’t my last!
The final conference night I took a ferry over to Coronado island for a beach sunset and following the conference finale I road-tripped up towards the desert in Palm Springs, being lucky enough to experience a rare California snowstorm en route to Los Angeles.
I would like to deeply thank the Royal Microscopical Society (RMS) for providing funding which has enabled me to attend, and I’d also like to thank the research funders - UK Research and Innovation (UKRI) and Integrated Biological Imaging Network (IBIN).
The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months. We hope they enjoy a long and rewarding membership with the RMS.
Dr Helena Coker
Mr Ilias-Panagiotis Oikonomou
Yucheng Hu
Miss Ellie Singh
Dr Elise Darmon
Dr Judy Kim
Miss Lauren Toms
Ms Yvonne Chart
Mrs Kristina Buch
Dr Sarah Keary
Dr Henry Banks
Dr Ramneek Johal
Dr Patrizia Canton
Dr Julio Spadotto
Ms An Mei Daniels
Associate Professor Michela
Relucenti
Mr Samuel Davis
Dr Imran Rahman
Professor Martin R. Lee
Dr Guilherme Costa
Ms Kyjana Barnett
Ms Niloufar Nikkhah Bahrami
Dr Elizabeth Williams
Dr Yoshie Murooka
Dr Alistair Siebert
Dr Saumitra Dey Choudhury
Ms Ieva Ragaisyte
Miss Georgia Osborn
Mr Sanket Jugade
Miss Ella Kitching
Dr Elisabeth Kugler
Mr Subhajit Dutta
Dr Christopher Allen
Mr Pieter Keenan
Dr Jubina Balan Venghateri
Ms Amy Hassett
Ms Christina Boukouvala
Dr Judith Lutton
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk
Name
Aarti Sagar Patankar
Tell Us About You?
Currently pursuing Bachelors degree in Microbiology. I have a keen interest in Genetics. I would like to study CRISPR Cas9 system and its applications. I’m an enthusiastic individual passionate to improve my understanding of the subject. I’m also looking for opportunities in the field of Microbiology and Genetics and hoping to contribute to the Society by doing extensive research.
Why did you become a member of the RMS?
Becoming a member of RMS will give me insights and keep me updated with ongoing research and developments. I am very excited to be a part of this esteemed society.
How do you feel being an RMS member benefits you?
RMS will help me to grow in academia, as a researcher, and to broaden my perspective. It will open the doors to new opportunities.

Name
Mantu LalTell Us About You?
I am currently pursuing a Masters in Molecular and Human Genetics from India. I am very interested in cell functioning and epigenetics and how they relate to cancer development, especially in leukemia and other haematological cancers.

Why did you become a member of the RMS?
I became a member because I feel that RMS gives me a unique perspective on the global happenings in the field of microscopy. I am very intrigued by FACS and would love to know more as well as grasp a deeper understanding of its concepts, and I think RMS is the perfect platform for doing so.
How do you feel being an RMS member benefits you?
RMS conducts a lot of conferences and events worldwide that allow early career researchers like me get exposure to the latest techniques and get in touch with leading researchers in the field of microscopy. I hope I can attend these events and gain the knowledge that I can apply to my own research in the future.
Name
Soudnya Sunil KadamTell Us About You?
I am an undergraduate student in TYBSC Industrial Microbiology and an enthusiastic researcher keen to be updated about new things.
Why did you become a member of the RMS?
To stay up to date with knowledge and discoveries regarding microscopy.
How do you feel being an RMS member benefits you?
It benefits me by expanding my knowledge, capabilities and interests towards research.
Name
Riccardo Di Frenna
Tell Us About You?
I am an Undergraduate student of Biomedical Science who would like to explore the microscopy world and imaging methods because I find them fascinating.
Why did you become a member of the RMS?
To find new opportunities, training and knowledge.
How do you feel being an RMS member benefits you?
I feel very proud.

www.mmc-series.org.uk
We are now just weeks away from mmc2023 (incorporating EMAG 2023) kicking off in the wonderful and vibrant city of Manchester, UK, and the excitement is well and truly building ahead of the RMS’s flagship event.
The Microscience Microscopy Congress (mmc) is renowned as one of the biggest and best international events in microscopy, imaging, and flow cytometry - bringing together hundreds of people who use microscopes for work, study and pleasure. Alongside a huge, three-day conference, the event boasts a world-class exhibition, showcasing the very best in microscopy for research and industry visitors. There is also a wide range of workshops, satellite meetings, social networking opportunities and more.
After a Covid-enforced, four-year gap since the last in-person mmc, the Congress will be taking place once again at the superb Manchester Central Convention Complex, from 4 – 6 July. This year’s Congress is shaping up to be one of the best ever. A record number of abstracts (more than 400) have been submitted, and the event will also include a new and improved RMS Learning Zone, plus a multi-category, International Scientific Imaging Competition.
Registration is still open, so now is the time to book your place at this fantastic event for the microscopy, imaging and flow cytometry community.
Up to 80 companies – including many of the biggest names in microscopy and imaging – will be on hand to showcase their products and give practical demonstrations. The exhibition also provides important exposure for a number of smaller companies keen to share their latest technological developments.
Find out more about all our exhibitors by checking out the latest exhibitor list and exhibition floorplan.
The exhibition runs for three days alongside the conference, and is completely FREE for anyone to attend. Visitors can register in advance, or simply turn up and register for an exhibition-only ticket, giving them access to everything on show – including live demonstrations, expert advice, and company workshops all under one roof. Free access to all the poster sessions, Scientific Imaging Competition and everything that the RMS Learning Zone has to offer, is also included.
mmc2023 Exhibition








Meanwhile the conference itself consists of six parallel streams, with no fewer than 36 sessions covering every aspect of microscopy, imaging and flow cytometry, including recent and emerging applications. The blockbuster programme will cover the full range of latest techniques, applications and hottest emerging topics – plus an incredible cast of speakers and supporting poster sessions. The conference will also incorporate EMAG 2023, organised by the Institute of Physics’s Electron Microscopy and Analysis Group (EMAG).
In addition to the academic content, the programme includes a number of sessions sure to be of interest to a wide range of scientific industries. Check out the full conference programme.
Our line-up of Plenary speakers for mmc2023 features some of the leading figures in microscopy and imaging from across the globe. Their talks are sure to light up the conference platform, and we are delighted to welcome them all to Manchester Central.
Yale University, USA
degree (Dipl. Phys., 1998) and his doctoral degree in physics (Dr. rer. nat., 2002) training with Dr. Stefan W. Hell at the Max Planck Institute for Biophysical Chemistry in Goettingen, Germany. After 4 years at The Jackson Laboratory in Bar Harbor, Maine, he relocated his research group to Yale University in 2009. An optical physicist/biophysicist by training, Dr. Bewersdorf has been a long-time contributor to the field of super-resolution light microscopy development and the application of these techniques to cell biological questions.

University Medical Center Utrecht, Netherlands
title:
Talk title:
Joerg Bewersdorf is the Harvey and Kate Cushing Professor of Cell Biology and Professor of Biomedical Engineering and of Physics at Yale University. He received his Master’s
Judith Klumperman has been professor of Cell Biology at the University Medical Centre Utrecht in The Netherlands since 2001. Her laboratory focuses on understanding membrane trafficking in health and disease, with focus on the endolysosomal system. Judith is expert in electron microscopy and combines molecular and biochemical approaches with advanced light and electron microscopy. Her lab is widely recognised as expertise centre for electron microscopy (EM), especially immuno-EM and correlative microscopy (CLEM). By CLEM, molecular, dynamic and functional information from light or live cell microscopy is directly correlated to EM images. Judith’s current research focuses on the role of tethering complexes in lysosome biogenesis and the role of lysosomes in cancer and neurodegenerative diseases. Judith is Chair of the national Netherlands Electron Microscopy Infrastructure (https://nemi. microscopie.nl/).


Argonne National Laboratory, USA
Talk title: Exploring the local behaviour of functional nanostructures using transmission electron
Amanda Petford-Long is an Argonne Distinguished Fellow in the Materials Science Division (MSD) at Argonne National Laboratory in Chicago. In addition to her own research programme, she serves as the Division Director of MSD and leads Argonne’s Microelectronics strategy development group. She has a D.Phil (PhD) in Materials Science from the University of Oxford and a BSc in Physics from University College, London. She moved to Argonne in 2005 from the University of Oxford where she was a full professor in the Materials Department. Her research focuses on nanomaterials and a particular emphasis is on magnetic and resistiveswitching nanostructures with potential applications in information storage technology, and on the use of in-situ TEM. She has published over 350 scientific papers. She is a Fellow of the Royal Academy of Engineering, the Royal Microscopical Society, and the American Physical Society and is a Professor in the Materials Science and Engineering Department at Northwestern University.
University of Manchester/Henry Royce Institute, UK
Talk title: Correlative 3D
Philip Withers is the first Regius Professor of Materials at the University of Manchester and

Chief Scientist of the Henry Royce Institute for Advanced Materials. The Royce brings together the universities of Manchester, Leeds, Sheffield, Oxford, Cambridge, Cranfield, Strathclyde and Imperial College, NNL and UKAEA to support the accelerated design of new materials and a better understanding of existing ones. He has pioneered the use of X-ray CT and electron microscopy to undertake correlative multiscale, multimodal and time-lapse characterisation. In this approach he employs advanced techniques to follow the behaviour of engineering and natural materials often in 3D in operando. In 2008 he set up the Henry Moseley X-ray Imaging Facility, one of the most extensive suites of X-ray Imaging facilities in the world with a special focus on in situ time lapse 3D X-ray imaging and now part of a National Research Facility for Lab. X-ray CT. In 2014, the Facility was awarded the Queen’s Anniversary Prize.

Instituto Gulbenkian de Ciência, Portugal
Talk title: Surviving a life changing accident and relearning how to be a scientist
Erin Tranfield obtained her PhD at the University of British Columbia (Canada), did a postdoc at NASA Ames Research Center (USA) and another at the European Molecular Biology Laboratory (EMBL-HD, Germany). In 2013, she moved to the Instituto Gulbenkian de Ciência to build a biological electron microscopy facility. Today, Erin and her dedicated team support the research of Portuguese-based scientists, aiming to answer a diverse array of biological and material
science questions. Erin has more than 20 years of biological electron microscopy experience with expertise in room temperature EM, cryoimmobilisation, electron tomography, and CLEM. She is the President of the Portuguese Microscopy Society, the co-chair of the ESA Topical Team on Celestial Dust Toxicity, a member of the EMBL Alumni Board, part of numerous evaluation panels and she recently joined the Editorial Board of Wiley Analytical Science. In 2020 Erin founded the TechEM Seminar Series which aims to bring advanced technical seminars to EM Facility staff all over Europe and Asia. Erin received the 2023 Alan Agar Award for Electron Microscopy from the Royal Microscopy Society.

The new mmc2023 App is a great tool to help you navigate your way through the Congress. You can create a personal schedule of the talks and workshops you want to catch, take notes and
The congress app is hosted by Engagefully, just search for them in your app store.
To mark the opening of mmc2023, an informal BioImagingUK / Early Career Networking Reception will take place in the foyer of Manchester Central from 6pm onwards on Monday 3 July. This is free to attend.
From the Tuesday onwards, once the conference talks have finished for the afternoon, the daily poster sessions allow you to browse the mmc2023 Poster Village and discuss the research with the authors. The mmc2023 Poster Village will house over 100 posters incorporating a wide range of microscopy techniques in both life and physical science. Poster sessions are free to attend and open to conference and exhibition visitors alike. There is no need to book in advance, just turn up and register.





On Wednesday 5 July, a choice of three evening networking events at Manchester restaurants are available to book with your conference ticket.These have been organised for EMAG delegates, Frontiers in Bioimaging and the AFM & SPM scientific communities.
mmc2023 (incorporating EMAG 2023) will bring together a number of smaller meetings and workshops, allowing you to meet with colleagues working in your field as well as with crossdisciplinary peers, all at the same event.

If you have not already done so, you can add these to your booking when registering for your main Congress ticket:
• Pre-Conference Workshops (including EMAG and ImageJ)Monday 3 July
• BioImagingUK MeetingMonday 3 July
• Early Career SymposiumMonday 3 July
• Super-Resolution WorkshopFriday 7 July (this workshop needs to be booked separately)
An ever-popular fixture at mmc, the RMS Scientific Imaging Competition will be running once again during this year’s Congress. A shortlist of the best submitted images will be on display throughout the
event, providing an artistic backdrop to proceedings – and some essential viewing for all our visitors.
The competition features no fewer than seven scientific categories, including a ‘short video’ category for the very best moving images. Winners and runners-up in each category will be announced during the Congress, with the judges making their final deliberations at Manchester Central.
The 2023 RMS Scientific Imaging Competition
The RMS is bringing a fresh twist to its mmc2023 Learning Zone, with lunchtime lectures, workshops and panel discussions. Our experts will, as ever, be on hand to share their knowledge with visitors and provide tutorials covering a range of fundamental microscopy techniques.
Meanwhile, don’t forget to take a look at the Society’s fabulous collection of replica antique microscopes from the McCormick Collection.These eye-catching instruments have been manufactured to the absolute letter of the original specifications –dating back, in some cases, to the 1600s.
Also on display will be a recently donated collection of beautiful Victorian slides – and the original microscope used to view them. These slides are the subject of two infocus articles by RMS History Committee Chair Dr John Hutchison Hon FRMS, the latest of which appears in this issue!
If you haven’t already booked your hotel room, you need to get your skates on! Accommodation is not included with registration for mmc2023 but there are a huge number of hotels located just a short walk away from Manchester Central.
Visit our mmc2023 accommodation page for more information and a list of guide prices. There may still be time to take advantage of exclusive rates offered to conference delegates and exhibitors - though bookings will be subject to availability and prices may now vary.

Alternatively you can telephone the Reservation Highway helpdesk on +44 (0) 1423 525577 or email admin@reservation-highway.co.uk. You can also complete a downloadable booking form for mmc2023 if you prefer.
The RMS is committed to reducing waste, and making all of our activities more environmentally friendly and sustainable. This is especially important to us at our flagship event, mmc2023 (incorporating EMAG 2023).
We have already made a number of improvements to the way we operate at the RMS. If you would like
to hear more about our environmental activities, you can view the RMS Sustainability Statement
Here are a few ways in which the Society is delivering more sustainable events – including mmc2023:
• Promoting the use of public transport
• Choosing a sustainable venue, with excellent transport links - Manchester Central Sustainability Strategy
• Not printing conference material but making it accessible online and through the conference app
• Reusing existing event materials like pens and notepads
• Where possible, using local suppliers
• Including vegan options and sustainable catering
Meet those who have shaped and changed the field of microscopy, imaging and flow cytometry, in both life and physical sciences.
Network with mentors, professors and students –all of whom share your passion for microscopy and imaging.
Seize the opportunity to forge relationships with
potential future colleagues and collaborative partners for your research.
1. Dress the part. Attire for the congress is business casual.
2. Attend the sessions that interest you. Sadly, there just won’t be time to see and do everything, so focus on the things you are most passionate about, rather than trying to attend every session.
3. Check out the lectures and handson equipment in the RMS Learning Zone. These are great ways to learn more and improve your skills.


4. Socialise! Attend social events. Quite simply, this is the best way to network and get to know the people you ought to stay in touch with.
5. Check out the programme online in advance. Plan which sessions you wish to attend and familiarise yourself with their general location.
6. Wear comfortable shoes. You’ll probably be on your feet a fair bit, so don’t let them get sore – a sure-fire way to ruin any day!
7. Remember to visit the Exhibition, Workshops, Scientific Imaging Competition Display and Poster Village. Free refreshments are available in the Exhibition all day!
8. Download the app. It’s a mobile and fast way to view the programme while you are attending sessions.
9. Find time to get out and about. Explore the amazing city of Manchester. The birthplace of the industrial revolution boasts great historical and cultural attractions, as well as shops, restaurants and nightlife.
10. Book your hotel – if you haven’t already! Make booking easy and book your hotel room via the hotel booking agency on the mmc2023 website.
11. Share your experience via Facebook, LinkedIn, and Twitter and use the event hashtag: #mmc2023UK
Registration is still open, and all the information on rates, accommodation and transport can be found on the official mmc-series website
We look forward to seeing you there!


I hope you are enjoying our latest issue of infocus, and that the three months since our last edition have treated you well.
For most of our members, the month of June will mark the onset of summer, and the chance to enjoy some warmer weather (and looking forward to a well-earned holiday, perhaps!). The start of summer in an ‘mmc’ year is also an exciting time for everyone associated with the RMS, as we finalise details ahead of our international flagship event at Manchester Central.
It gives me such great pleasure to say that mmc2023 is on track to be a tremendous, international event for our microscopy and microanalysis community. The Scientific programme is as rich and diverse as ever, with a great line-up of speakers and hundreds of abstracts submitted. Meanwhile, I have no doubt our exhibitors are relishing the opportunity to bring the very best in microscopy and imaging to the first ‘in-person’ mmc since 2019. As the old adage goes, absence makes the heart grow fonder, and I suspect many of you will sense, as I do, something extra special about this year’s event. There is still time to register, so please check out the official mmc-series website and find out more about what lies in store for attendees! Whether you are a seasoned mmc participant or a potential first-time delegate, you are sure to be at home at this year’s Congress if microscopy, imaging, microanalysis or flow cytometry is your passion.
Amid all the excitement, it is easy to overlook what has been a very busy time in the ‘regular’ RMS events calendar these last few months. We kicked off in March with Virtual Flow Cytometry Data Analysis Course Spring 2023, which took place over five days, including an optional ‘clinical’ programme. This was followed in April by Botanical Microscopy Meeting 2023 at the John Innes Centre, Norwich. The ‘Botanical’ series of meetings constitutes a rich and long-running RMS tradition, dating back to the 1960s and covering all
aspects of bioimaging relating to modern plant cell biology. Long may it continue!
Also in April (and just 60 miles away at Robinson College, Cambridge) attendees at the wellestablished and international Microscopy of Semi-Conducting Materials conference enjoyed an excellent and timely scientific programme covering all the latest advances in the field of electronic materials/devices – utilising state-of-the-art TEM/STEM techniques, in situ microscopy and a variety of other characterisation techniques to understand the behaviour of defects in electronic materials and devices. This premier international conference has long been a ‘staple’ in the semiconductor materials research and development field.
Another RMS ‘double-bill’ took place at Leeds University in April, with a well-attended Spring School in Electron Microscopy 2023, and EBSD 2023, the latter making a most welcome return as an ‘in-person’ meeting. Interspersed with these well-established RMS events, the Society continued to support the Expansion Microscopy User Group meetings, hosted in both Canada and Australia – a new venture about which I was delighted to learn more in our last issue of infocus. One of our other great multi-Society collaborations, the International Microscopy Lecture Series, also had another magnificent event in April with a tremendous presentation by former RMS President and RMS Honorary Fellow Professor Archie Howie, FRS.
We move from Spring to Summer having announced the recipients of the annual RMS Summer Studentships. This year, up to £2,000 will be awarded to support six undergraduates undertaking microscopy-related projects over the summer. This is just one of a number of ways in which the Society endeavours to support the next generation of microscopists – but it is an important scheme nonetheless, and I look forward to reading about the achievements of all our students later in the year.
Finally, as this is my last infocus letter to you, I wish to express my very great and sincere thanks to you all for your much appreciated support: our fantastic RMS volunteers who devote their efforts to continuing to make the Royal Microscopical Society such a great organisation, RMS Council, Sections, Committees and Focused Interest Groups for their hard work and support, and my friends and colleagues on the Executive Committee over my time as President. Last, but by no means least, I express my deepfelt gratitude to our outstanding RMS Staff – Allison Winton, Victoria Masters, Adam Clay, Jade Sturdy, Kate Wooding, Katie Reynolds, Alessandra Reni, Jess Cole, Dawn Hopkins, Nick Cameron, Debbie Hunt, Georgina Fletcher, Lucy Ridler, Jill Hobbs, Owen Morton and Tracey Clay. Their dedication and hard work are so integral to the success of our tremendous society.
We have experienced a rather tumultuous period (i.e. the pandemic), and our amazing staff have risen to the unexpected challenges so that the RMS was able to continue operations and programs as we entered the world of virtual meetings and events –including our AGM. We are all most grateful for their extraordinary efforts. As a long-time RMS member, I have been deeply humbled and honoured to serve as your President. The RMS has a special place as the oldest microscopy society in the world, with an amazing history. As we will be approaching the 200th Anniversary of the RMS in another 16 years, I am confident that our younger members and Staff will carry on the great traditions of the Society –and I can only imagine what wonderful celebrations will occur. And I welcome our incoming President, Dr Peter O’Toole, and wish him great success as I know he will do a magnificent job leading RMS.
I trust that I will see many of you in person at mmc2023 – or elsewhere in the microscopy community before too long!
My warmest wishes and sincere thanks to you all,
Professor Grace Burke, RMS PresidentRMS staff gathered for a special lunch to bid a fond (and emotional) farewell to their long-standing colleague Chloe Goode.
Chloe has been part of the team since 2011, working closely with exhibitors and corporate partners, and ensuring the smooth running of countless RMS events.
A hugely popular member of the RMS team, Chloe has worked on many high-profile events including emc2012, ELMI and the mmc exhibitions. In recent years she took on responsibility for the Society’s engagement with its many Corporate Members across the globe.

We would like to put on record our thanks to Chloe for all her efforts over the last 12 years, and wish her the very best of luck in her next steps.
From everyone at the RMS: We’ll miss you Chloe!
anywhere in Europe, the Congress is a must for anyone who uses microscopy for work, study or pleasure.
Tickets are still available for all three days of the Conference, and the exhibition is completely FREE to attend.
With just a few weeks to go before the start of mmc2023 in Manchester, UK, now is the time to register for this superb international event for the microscopy, imaging and flow cytometry community.

One of the biggest and best events of its kind
Find out more about everything mmc2023 has to offer in our special preview on p36. You can also visit the official mmc-series website to book your ticket and view the full conference programme. Register today!
Congratulations to our poster prize-winners at the Microscopy of Semi-Conducting Materials conference in Cambridge.
Around 20 posters and more than 30 oral presentations featured during the event, which took place at Robinson College Cambridge, from 3 – 6 April.
First prize in the poster competition went to Samba Ndiaye, of the University of Rouen, Normandy, for his poster titled Atom Probe analysis of Ge layers with high Sn and Sb content introduced by pulsed laser melting. The runner-up prize went to Ruben Bueno, of the Max-Planck-Institut für


Eisenforschung GmbH. His poster was titled: HRSTEM and APT studies of grain boundary phase transitions to tune the transport properties of NbFeSb Half-Heusler semiconductors for thermoelectric applications.
The conference took in four action packed days, with a wide range of illuminating talks covering the latest advances in the study of the structural and electronic properties of semiconducting materials.
There was also a well-attended conference dinner – and even some ‘down time’ for a spot of afternoon punting along the river!
Congratulations to this year’s successful applicants for an RMS Summer Studentship.
Each year, six RMS studentships of up to £2,000 are available to support undergraduates carrying out microscopy-based projects over the summer. The projects are split evenly between physical sciences,
Caitlin Piper, BSc Biomedical Science, 2nd Year
Sarah Quinn, BSc Biomedical Science, 2nd Year
Supervisor: Dr Ryan Delaney
Miriam Czech, BSc Computer Science (Artificial Intelligence), 2nd Year
Supervisor: Dr Susan Cox
Rebecca Harry, Biochemistry (Molecular and Cellular) – Mbiochem, 3rd Year
Supervisor: Assistant Professor Claire Friel
Lakshana Baheerathan, BSc in Biological Sciences, 2nd Year
Supervisor: Professor Martin Goldberg
Hio U Lao (Kathleen), MChem Forensic and Analytical Chemistry, 3rd year
Supervisor: Dr William Tipping
Mohamed Ghali, MEng Electrical and Computer Engineering, 2nd Year
Supervisor: Dr Kevin Webb
biological sciences and interdisciplinary projects.
The students produce a written report about their work for infocus and a short video documenting their experiences for the RMS YouTube channel.
Congratulations to the following students, and good luck with your microscopy projects later this summer!
Queen’s University Belfast
A comparison of microscopy and image analysis approaches to elucidate structural information in biological samples.
King’s College London Evaluating deep learning methods for localisation microscopy
University of Nottingham Using total internal reflection fluorescence (TIRF) microscopy to quantify the impact of a novel microtubule growth inhibiting peptide on microtubule dynamics
Durham University Imaging the structural organisation of the LINC complex cage at the nuclear envelope – high resolution scanning electron microscopy and 3D SIM
University of Strathclyde
Imaging Bruton’s Tyrosine Kinase Inhibitors in Cancer Cell Models using Stimulated Raman Scattering Microscopy
University of Nottingham
Delivering an image-processing plugin (C++) capable of instructing a closedloop meniscus-correction system to dynamically realign a microscope imaging system in the face of meniscus artefact to allow wall-to-wall imaging within multiwell plates.

The RMS would like to congratulate Jen McGaley of the University of Cambridge, who received both the Chris Hawes Poster Prize Award and the Image Competition Prize at Botanical Microscopy Meeting 2023 - held at the John Innes Centre.
Jen’s winning poster was titled Spatiotemporal dynamics of nutrient exchange during arbuscular mycorrhizal symbiosis, while her winning image,
titled Live view of a concealed symbiosis, revealed the intricacies of an arbuscule inside a living rice root.
Congratulations also go to Ana Romina Fox of UCLouvain, who was the runner-up in the Poster Competition, with her poster titled PIP aquaporin interactions at the reticulum endoplasmic-plasma membrane interphase.



The RMS team recently welcomed the arrival of PhD students Jo Cull and Zoe Barr, who are both carrying out internships at the Society.
Jo has been co-ordinating preparations for the RMS
Meanwhile Zoe has been tasked with writing an impact report to gather the success stories of the Hitachi SEM Outreach scheme – an RMS-backed initiative enabling schoolchildren to carry out projects with a portable electron microscope. She will also be helping out at mmc2023 next month.

Zoe’s PhD is a joint project between the University of St Andrews and the James Hutton Institute. Her research focuses on plasmodesmata, the nanochannels that span the cell wall in plant cells and allow for intercellular connectivity. She particularly enjoys confocal microscopy working with immunostaining and fluorescent protein fusions.
Zoe said: “I was excited to come to the RMS because within the society I have the opportunity to work with people who focus on events, outreach, publishing and more. I’m also looking forward to mmc of course!”
cancerous and normal, to help understand the control and functional significance of O-linked glycosylation. She is employing a wide variety of techniques including confocal microscopy, light microscopy and flow cytometry.
She said: “It’s really nice to see the other side of the coin by working with the society. It has been really exciting to learn about the events and conferences the RMS organises, working with industry and developing skills outside of the lab.”
The RMS will be holding its full range of Annual General Meetings (AGMs) at mmc2023 – including the main RMS AGM and meetings for each of its Scientific Committees (Sections).

These meetings are open to both members and non-members attending mmc2023, including conference delegates, exhibition visitors and exhibitors. Anyone wishing to attend specifically for the AGMs can do so with their conference or FREE exhibition registration.
Details of each of the meetings, including the relevant meeting rooms at Manchester Central, are as follows:
Monday 3 July:
The Early Career Committee (ECC) AGM will be
held at 3:05pm in Central 5,6 & 7, as part of the Early Career Symposium (3-6PM)
Tuesday 4 July: RMS AGM and Outreach & Education Committee AGM will be held at 6pm in Charter 1
Wednesday 5 July: AFM & SPM Section AGM at 12:15pm in Central 5,6 & 7.
Electron Microscopy Section and Engineering Physical and Material Sciences Section AGMs at 4:45pm in Workshop 1.
Data Analysis in Microscopy (DAIM) Section, Light Microscopy Section and Life Sciences
Section AGMs at 4:45pm in Workshop 2.
The Facility Line is a truly cutting-edge microscope from the inventors of STED nanoscopy, Nobel prize winning team, Abberior Instruments.
The lasers and optics are permanently aligned and ready to use so you can spend less time getting things set up and running. Truly turnkey multiphoton microscopy.
LINCAM
Photon detection and counting camera that is perfect for FLIM and Light Sheet Microscopy applications.


PICOSECOND PULSED LASER
Universal diode lasers with pulsed and continuous wave operation.

LIGHTHUB-ULTRA®
Plug&Play Laser Light Engines with up to 7 userupgradable wavelengths and 2 fibre outputs.

s
Highest 26 MPix resolution cooled SCMOS camera with true global shutter.

For more information and to see our full Advanced Light Microscopy range please visit our website
 CMOS CAMERA
Abberior Instruments Facility Line
Prospective Instruments MPX-series
CMOS CAMERA
Abberior Instruments Facility Line
Prospective Instruments MPX-series

The following tributes to the late Jeremy Pickett-Heaps and Larry Fowke celebrate their pioneering contributions to botanical microscopy and plant cell biology. They were born in 1940 and 1941, in Bombay and Toronto, and gained their PhDs in Cambridge and Ottawa in the 1960s. After they had published their early researches they serendipitously joined forces in Canberra, in the Australian National University’s then new Research School of Biological Science.
In those heady days, with improved instruments and specimen preparation procedures, electron microscopy of plant cells had the potential to reveal something new and exciting in almost everything that was examined. So it was in Canberra, where they collaborated on a project that was new to both of them: in the brief period 1968-9 they found key ultrastructural evidence for the evolutionary pathway from green algae to “higher” plants, a seminal advance for plant science.
Thereafter their studies and locations diverged, though their mutual respect and friendship continued throughout their illustrious careers in Saskatoon, Boulder, and Melbourne. They were held in high esteem for their publications and presentations, always advancing knowledge, always with beautiful, informative micrographs.
Sadly, both fell victim to Parkinson’s disease in retirement. They passed away within a year and a half of one another, in 2021 and 2022.
Jeremy David Pickett-Heaps FAA, FRS died on 11 April 2021 aged 80. He was born to Australian parents in Bombay, India in 1940 and educated at Geelong Grammar School in Victoria, Australia, and Clare College, Cambridge, obtaining his PhD under D H Northcote FRS in 1965. He then joined The John Curtin School of Medicine in the Australian National University (ANU) and soon transferred to ANU’s new Research School of Biological Science, before becoming Professor in the Department of Molecular, Cellular, and Developmental Biology at the University of Colorado, Boulder (1970-1988).
He returned to Australia as Professor of Botany in the University of Melbourne and stayed there until his retirement in 2002. He was elected as a Fellow

of the Australian Academy of Science in 1992 and a Fellow of the Royal Society in 1995.
His outstanding record of fundamental discoveries
started during his PhD, which was ostensibly to study biosynthesis of plant cell walls and soon turned to electron microscopy. He pioneered the use of autoradiography at the electron microscope level and was the first to demonstrate the role of the Golgi apparatus in processing and delivering polysaccharides. At the same time he discovered, documented and named the “Pre-prophase Band” of microtubules, with its still mysterious property of predicting the site and plane of division in plant cells, a crucial aspect of morphogenesis. He wrote a wonderful account of these early adventures in botanical microscopy in his final publication (The Plant Journal 75, 189-201, 2013).
In ANU he and his Canadian colleague Larry Fowke embarked on comprehensive studies of ultrastructure and cell division in green algae, leading to a new understanding of the evolution of the land plants, summarised in his classic book “Green Algae” (Sinauer, 1975). It was a landmark contribution in the plant sciences, now confirmed and fleshed out by DNA sequencing. The same programme led him to his concept of the “microtubule organising centre”, thus opening a new area of research for animal as well as plant cell biologists. In the USA he turned to the mechanism of mitosis and the interaction of microtubules with chromosomes, exploiting the unique mitotic spindle organisation of diatoms, which gave new insights into microtubule dynamics and force generation. He was honoured with the lifetime “Award of Excellence” from the Phycological Society of America in 2008 for this and his many other studies of algae, and in further recognition several of his colleagues and friends named a most unusual green alga after him, Microrhizoidea pickettheapsiorum. A comprehensive account of his research achievements will appear in Biographical Memoirs of Fellows of the Royal Society.
He was a supremely skilled microscopist and had a keen and perceptive eye for the beauty of nature at all levels. In his later work he sought to communicate it through production of a series of
magnificent Laser disc and DVD presentations filled with fascinating movies of microscopic organisms and their biology, never before available to students or the general public. In this he was aided by his wife Julianne, who did much of the editing and running of the company they founded, “Cytographics”. In retirement that activity extended to beautiful depictions of native bird behaviour, obtained by patient filming in habitats near their home in Mallacoota, despite the depredations of Parkinson’s Disease, an affliction that marred his last 18 years. Many of his movies are available on YouTube.
Jeremy’s other great passions in life, besides family, included surfing and gliding when he was younger, teaching himself boogie woogie piano (he often entertained at home and scientific meetings), and skiing during his years in Colorado.
An inspiration to many under- and post-graduates in Melbourne and elsewhere, he is greatly missed by his friends, colleagues and former students. His unmatched enthusiasm and curiosity along with his wit and good humour will always be with us.
Plant Science Division, Australian National University

Richard Wetherbee
Bioscience Division, University of Melbourne
Professor Emeritus Larry C. Fowke of the Biology Department, University of Saskatchewan (U of S), Saskatoon, passed away on December 13, 2022 after succumbing to Parkinson’s disease. Larry was born in Toronto, Ontario, on June 6, 1941, grew up in Saskatoon where he went to high school, and did his B.Sc. Honours at the U of S in 1963. He received his PhD from Carleton University in Ottawa in 1968, and then went to Australia on an NRC postdoctoral
fellowship, after which he joined the U of S as Assistant Professor in 1970. He moved through the ranks quickly and was promoted to full Professor in 1979. He served the Biology Department as Assistant Head, from 1992-94, and as Head from 1994-2000. For his service to the Department Larry was honoured with the Rawson Professorship from 2002 to 2005.
Larry was a nationally and internationally renowned plant cell biologist; his research interests ranged from plant protoplasts, plant tissue culture of somatic and microspore embryogenesis especially of trees, endocytosis in plant cells and control of plant cell division. He was an author and co-author of over 120 research publications, several book chapters and conference proceedings, he co-edited a book on Plant Protoplasts, and wrote a book titled, “Cells are Life”, which he completed during his illness and was published in 2021. He was invited to several national and international conferences and to various institutions around the world to present his research and remains one of the most highly cited researchers in his field of work. For his research on somatic embryos of conifer trees, he and his co-workers hold five patents in USA, New Zealand and Canada. He was also an Associate Editor of Canadian Journal of Botany, Cell Biology International, Plant Cell Reports and Protoplasma. For his tremendous research contributions and

accomplishments, Larry received several awards including, Distinguished Researcher award in 1998, an Earned D. Sc. in 2006, and Award of Innovation from the U of S in 2008. He was elected as a Fellow of the Royal Society of Canada in 2009 for his sustained and exemplary lifetime research contributions to the field of Plant Cell Biology.
Larry was also a passionate teacher and was highly respected by his students for his well-organised lectures with high quality imaging, novel models, and a sprinkling of humour. He treated students with respect and made himself available freely to help them with their problems. He was duly recognised for his superior teaching and was nominated three times for Teaching Excellence award. Larry also supervised research of many graduate students and postdoctoral fellows who now hold various positions, in their own right, with academia, industry and research centres worldwide.
Above all, Larry was a very fine human being, a kind, compassionate and gentle soul, a great friend and mentor to many, and had a wonderful sense of humour participating in departmental skits and telling jokes to friends and family. He was also a gifted photographer and many of his pictures appear in books and periodicals, and they adorn the walls of many friends’ homes. He was also a great musician; he played saxophone for the Saskatoon Community band, and during his many visits to Australia learned to play the didgeridoo, which he loved playing at gatherings and sometimes to students in his class. He also loved exploring Northern Saskatchewan on canoe trips, and enjoyed surfing at beaches in Australia.
Larry is survived by his wife Lynne Fowke (Turner) of over 60 years, son Vernon (Sylvie), daughters Christine (Shawn) and Jocelyn (Jason), and grandchildren, Connor, Victoria, Nathan, Noah, Annika, Jacob and Jeremy. He will be sorely missed by his family and friends, and plant biologists and plant cell biologists in Canada and around the world.
 Vipen Sawhney, Biology Department, University of Saskatchewan
Vipen Sawhney, Biology Department, University of Saskatchewan
Johnson Matthey Technology Centre, Blounts Court Road, Sonning Common, Reading RG4 9N
For early career researchers, support from an industrial partner can go a long way in laying the groundwork required for an established career with long-standing industrial collaborations. Industrial support can be available in many forms ranging from mentorship and guidance to support in obtaining funding from a research council. These are some of the many ways industry can help and is of mutual benefit to both parties. In this article we will be showcasing some examples of support companies such as Johnson Matthey (JM) provide by highlighting JM’s academic open innovation programme. Our intention is to spread awareness of the type of support RMS early career researchers can tap into for future collaborations and support.
Johnson Matthey is a global leader in sustainable technologies that enable a cleaner and healthier world. The company applies cutting-edge science with a strategy focussed on catalysing the net zero transition. To achieve this vision, JM has identified that four essential transitions are required 1) driving down transport emissions, 2) transforming our energy systems 3) decarbonising chemicals production and 4) creating a truly circular economy.
The challenges faced within these areas are complex and require developing innovative solutions through collaboration. As the world faces the challenges
of climate change and resource scarcity, it will be cutting edge research in close collaboration with academic institutions, industrial partners and startups that will allow us to rise to address these tough challenges.
Delivering successful ideas can take time and JM takes a long-term view by working with over 40 leading academic institutions in the UK and mainland Europe. JM manages its research and development of academic collaborations through its open innovation department by coordinating with different departments in the company and provides a clear point of contact into JM, Figure 1. The team can be contacted at https://matthey.com/scienceand-innovation/collaboration
For early career researchers in the RMS, JM’s Open Innovation department interacts externally in the following ways:
• A point of contact for experts in metal chemistry, catalysis and process engineering
• Advice on joint grant applications in areas of interest to JM
• Consideration for time on high-end advanced characterisation equipment
• Letters of support for research projects which are aligned with JM’s R&D strategy

PhD projects are an important training mechanism for early-stage researchers who carry out research in academically challenging areas of science. In addition to the educational aspects of PhDs they are an important mechanism for industry to explore new areas of science and develop new technical capabilities. From JM’s perspective, they provide valuable insight into fundamental scientific
discoveries which directly impact research and development activities. In turn, the university or research institution gains an understanding of realworld applications to apply their research to.
The PhD student or early-career researcher builds scientific capability in an area of interest to JM, often with time spent using high-end scientific equipment in a JM laboratory. Parallel to this, JM also provides training sessions in modern business ethics, networking and industrial awareness, all of which aim to prepare the researcher for their future career.
Readers of this magazine may be interested in JM’s advanced characterisation and modelling groups which use X-ray tomography and electron microscopy (FIB-SEM and TEM) to study materials all the way down to the atomic scale during their operation. Close collaboration with modelling scientists accelerates the materials discovery process, enabling iterative refinement of models and ultimately the synthesis of promising candidate materials. Projects investigating the relationship between chemical and physical properties of JM materials and their behaviour are of high interest. Using this insight, JM scientists and engineers can make changes to optimise the performance of the

material for specific applications. Figure 2 showcases all the core capabilities which can also be accessed here: https://matthey.com/science-and-innovation/ core-capabilities
For context, an example of how such partnerships could work over the long term is the establishment of the solid-state Nuclear Magnetic Resonance (NMR) team. In 2002 JM began funding PhD studentships at the University of Warwick to gain an understanding of how NMR could be used for characterising molecular environments. In 2009, the technique was transferred into JM through a knowledge transfer partnership with continued academic support provided through PhD studentships and the company procured a 400MHz solid-state NMR to study catalysts and mixed oxide materials with a throughput of ~600 samples/year. Following this success, the company invested in a second higher field NMR with improved resolution and sensitivity with better capability for organics and polymers whilst also continuing academic collaboration with Warwick University. This investment with additional solid-state NMR spectroscopists increased sample throughput to 1000 per annum and has produced 20 publications. This example demonstrates JM’s commitment to collaborative research aligned to the company’s strategies. It all started with a PhD studentship and evolved into a world-class solidstate NMR facility.
There are a number of studentships with Oxford University Materials department with one project spanning five generations of PhD students concentrating on number-based atomic resolution imaging with compositions data from X-ray and electron energy loss spectroscopy. After all, catalysis
is an atomic process, and being able to access 3D atomic information of a nanoparticle was crucial in better understanding of Oxygen binding mechanism during catalysis, leading to the development of better materials and processes.
From academic interactions spanning over a decade at the University of Oxford, there was clear justification for JM to acquire an aberration corrected electron microscope. Through a partnership with Diamond Lightsource Ltd the JM TEM (JEOL ARM CF200) was situated as part of the ePSIC facility in Diamond in 2016. This has led to many valuable collaborations with Diamond beamlines as well as the neutron source and central laser facility in helping JM interrogate its materials with multiple beams (X-rays, neutrons, electrons and lasers). JM is proud to be a proactive industrial partner on the Harwell campus.
Both the NMR and TEM examples showcase how JM’s approach to collaboration by working closely with academic departments with a long-term view to research and potentially transfer the technology and learnings in-house for commercial applications.
PGM metals are essential components for many current and future technologies but access to this valuable resource can often present a barrier to beginning or continuing research. Through this scheme, JM invites academic and research groups to apply for research-quantities of PGM, which can be used in any field to further their understanding and application. The award scheme is open for application from any university or research institution globally and can be contacted at https:// matthey.com/science-and-innovation/collaboration/ jm-platinum-group-metal-award-scheme
JM also has a peer review journal – the Johnson Matthey Technology Review – that is open access
which publishes articles, reviews and short reports on science driving down transport emissions, transforming energy systems, decarbonising chemicals production and creating a circular economy. The journal has wide international coverage within industry with a high readership. Academics interested in promoting their research within wider industry networks and its applications are welcome to submit an article at https:// technology.matthey.com/
Through its Open Innovation department, JM aims to facilitate and simplify the process of setting up industry-academia collaborations. JM is keen to encourage research into future applications focusing on clean air, clean energy, and efficient use of the planet’s natural resources. It is with this vision in mind that the Open Innovation department was set up to facilitate external collaborations in strategically important areas such as these.
infocus welcomes submissions of articles of general interest to microscopists.

You provide the text and images and we take care of the rest. It’s the ideal way to share your work with the microscopical community.
Full submission information and guidelines are available at www.infocus.org.uk.
To submit an idea or if you have any questions about the process please email the Editor (editor@infocus.org.uk)

















Linkam Scientific Instruments has launched a new application note that investigates mineral precipitates, which contain microscopic inclusions of the ancient fluids from which they formed. These inclusions are, essentially, miniature timecapsules that can unlock clues into the conversion process of carbon dioxide (CO2) into minerals for permanent sequestration, which could suggest a potential solution to current environmental concerns.

Spotlighting the Linkam THMS600 and TS1400XY stages, the new application note discusses how researchers at The Arctic University of Norway used Linkam systems to analyse 500 million-yearold granitic pegmatite from the Rio Grande do Norte region of Brazil.The goal of the research was to understand the fluid inclusions present within the samples and compare them with fluid inclusions
in mineral samples from Cu-Au ore deposits from the Republic of North Macedonia.
Andrew Davies, Applications Scientist at Linkam, describes the importance of the research:
“The Linkam THMS600 stage was critical in ensuring the reliability of the data obtained, as it allowed the scientists to exert precise temperature control over the samples and create a detailed picture of the mineral-CO2 reaction. These investigations allow us to better understand the geological processes taking place within the minerals of fluid inclusions, which also allows us to gain deeper insight into the conversion of CO2 into minerals for sequestration. Overall, the insights provided by the case study are particularly useful in providing a potential solution to address current environmental concerns.”
www.linkam.co.uk
FocalPlane, the online community site for microscopists, is the place to share your latest research and images in a more informal way than a traditional publication. FocalPlane is your site – once registered you are free to post. We aim to bring together all members of community, including chemists, optical engineers, bioimage analysts, biologists, microscopy facility staff and technicians, by highlighting research and tools in accessible blog posts.

Our most popular category is our ‘How to’ section, with new blog posts going up regularly.
Recent posts include a series on bioimage analysis from Mara Lampert, a post on ‘any immersion’ microscopy from Alfred Millett-Sikking and the story behind Fabian Voigt’s research on the Schmidt
objective, which takes inspiration from telescopes and scallops.
Another popular series on FocalPlane is our ‘Latin American Microscopists’ interviews from Mariana De Niz. So far, Mariana has interviewed scientists from Brazil, Uruguay, Argentina, Chile, Paraguay and Bolivia as she makes her way around Latin America. Look out for the series on focalplane.biologists.com.
Finally, we are excited to launch our 2023 image competition. We are looking for striking images that will be showcased on our banner on the front page of FocalPlane. In addition, the winning image will be featured on the cover of Journal of Cell Science. Check out our post on FocalPlane for more details!
To stay up to date with the latest posts on FocalPlane, register to receive our weekly digest or our quarterly newsletter.
www.focalplane
Martyn joins us from Cairn Research Ltd where he was previously responsible for delivery of advanced imaging systems for microscopy. Over the last 20 years he has built a career in the design and delivery of fluorescence measurement instrumentation for biomedical and life science research applications. Initially training in Physics before completing his PhD in Physiology, Martyn spent several years working as a postdoctoral scientist in medical research before moving into fluorescence instrument development. Over time this evolved to the configuration of complex high speed microscopy imaging systems and provision of associated technical backup.
An advocate of providing strong customer support to deliver advanced imaging capabilities for scientific research and beyond, we are looking forward to strengthening the Photon Lines UK team with his expertise.
Martyn said:“I am delighted to be taking up the reins of Photon Lines Ltd and look forward to building on the company successes to date in both the Physical and Life Sciences.This is an exciting time for Photon Lines with pioneering instrumentation core to the product line-up. Growing the team to effectively deliver and support these technologies to UK labs is a challenge I will relish.”
David Gibson, former Managing Director of Photon Lines stated: “I have known Martyn for a number of years and have been impressed by the work he and his colleagues at Cairn Research have been doing. It is a great pleasure to welcome him on board at Photon Lines Ltd, I will look forward to seeing the company grow and develop under his stewardship”.

About Photon Lines: Photon Lines is a value-added distributor of photonics equipment, including a wide range of scientific lasers, scientific cameras and sophisticated optical components, as well as high end fluorescence microscopy solutions. We have been serving the UK and Ireland in industry, academia, and research organisations for 20 years, having spun out from our French parent company, with headquarters in Rennes, Brittany.
www.photonlines.co.uk
Telight has won a public tender announced by the University of Warwick, which has chosen its special holographic microscope Q-Phase for its research facility. One of the first users will work with Q-Phase to analyse cell shapes and motility.

The microscope is placed at Warwick Medical Centre, where it will be looked after by a group of experts from CAMDU (Computing and Advanced Microscopy Development Unit). We are excited to strengthen our position in the UK and will be present at Warwick for the conference Adhesion and Migration in Disease 2023 from 5th to 8th September.
www.telight.eu

It’s not just the exceptional performance and convenience of LEDs that make them a popular alternative to mercury or metal halide lamps. They are also significantly better for the environment –conserving energy, reducing waste and avoiding the use of toxic mercury. All in all, LEDs are the go-to sustainable option – but don’t just take our word for it!
environmental impact score, which is like an eco-nutrition label for laboratory equipment, providing clear, third-party information about the environmental impact of a product and its packaging.
By understanding the environmental impact of equipment, this helps labs make sustainable purchasing choices. By having a score, this also helps manufacturers like CoolLED focus on ways to reduce impact even further and become as sustainable as possible.The pE-400 Series scored 42 which is just the start of its sustainability journey.
The standard pE-400 and pE-400max Illumination Systems make up the pE-400 Series. Four intense LEDs offer broad spectral coverage from 365-635 nm for use with all common fluorophores ranging from DAPI through YFP to Cy5.
Now you can see exactly how CoolLED measures up with its new CoolLED four-wavelength pE400 Series, thanks to its My Green Lab ACT Label certification. ACT stands for Accountability, Consistency, and Transparency. It assigns an

While the LED configuration is identical, the difference comes down to control. The white light pE-400 Illumination System offers a modern lamp replacement, which is ideal for applications such as fluorescence screening or sample visualisation on confocal setups. For more demanding applications such as high-speed live cell imaging and optogenetics, the four-channel pE-400max features individual channel control and the ability to fit optional inline excitation filter holders. Cost-effective high-speed automation can also be achieved with the fourchannel Sequence Runner.
www.coolled.com
We installed a DriveAFM in Dr. Zuzana Gažová's research group at the Institute of Experimental Physics of the Slovak Academy of Science in Košice.
Amyloid fibrils is one of the subjects that the group around Dr. Andrea Antošová is focusing research on. Amyloid fibril formation is associated with the pathology of more than 50 diseases like Alzheimer´s and Parkinson´s disease. WaveMode is ideal for imaging the structure of these thin fibres at the
molecular level.
The WaveMode measurement shown took less than 60 seconds, for 500 x 500 pixels and using 10 kHz tapping rate.The fibrils were deposited on mica and dried. The DriveAFM will also be used to study the mechanical properties of living cells for cancer research by measuring high resolution force maps.
www.nanosurf.com
Linkam Scientific Instruments recently partnered with one of the largest universities in Stockholm, Sweden, to support the pioneering research of Dr. Benjamin Schmuck into the manufacture and characteristics of synthetic spider silk.
Major ampullate silk, which is highly extensible and as strong as steel whilst only a fraction as dense, has a myriad of potential applications, including robotics and medicine. Aside from its impressive functional properties, spider silk is also biodegradable and environmentally benign; its production could form a circular process aligned with the current drive for more eco-friendly manufacturing methods. To investigate this exciting material and more accurately understand its properties, Dr. Schmuck utilised a Linkam Modular Force Stage (MFS), collaborating with Linkam to develop customised grips to mount his samples.
The MFS allowed for the synthetic silk to be tested under different environmental conditions, advancing our understanding of its capabilities. Dr. Schmuck outlines the unique advantages offered by the Linkam MFS: “It enables us to stretch our fibres under different temperatures and humidities – and
the instrument makes this really easy to set up.With a regular instrument, we wouldn’t get the detailed microscopic insights that the MFS provides, and we certainly wouldn’t be able to change the conditions during the test.”

Although research into bioengineered spider silk is still in its infancy, advanced studies such as this illuminate the scope of its fibre and molecular strength. Dr Schmuck explains: “The strength and versatility of spider silk will dictate the success of its role in application. Thanks to the Linkam instrumentation, we’re well placed to determine those properties, and so ensure that spider silk fulfills its potential.”
www.linkam.co.uk

Our super-resolution imaging platform LiveCodim will be available at a practical course on superresolution imaging organised by EMBO in Prague from June 18th to 23rd. We will organise demo measurements and demonstrate how any widefield or confocal microscope can be turned into a super-resolution one for studying live cells with a resolution of up to 90 nanometers.

www.telight.eu
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
Linkam Scientific, has collaborated with Radiant Technologies, a major player in the design and manufacture of testing equipment for electrical systems, to modify its HFS600E-PB4 probe stage. The modifications enhance the usability of the stage for microsized electrical component temperature control applications, and will be implemented in existing stages later this year.
The combination of Linkam’s HFS600E-PB4 stage with Radiant test platforms has benefited Radiant’s customers for a number of years, allowing them to carry out analysis of macro-scaled electronic components including ferroelectric and piezoelectric materials across a temperature range from -196 to 600 °C.
However, when testing micro-sized electronic components, positioning the probe was previously challenging for Radiant’s customers, as it was locked into a holder that was magnetically secured into the chamber. Taking this feedback, Linkam worked on a new spring-loaded probe that resulted in a new positioner, allowing manual probe repositioning so users can fine-tune the position of the probes when testing small electronic components.
Clara Ko, Sales and Marketing Manager, Linkam, stated: “For Linkam, the fact that we have upgraded
the product line as a result of customer feedback like this really underlines the importance of this type of collaboration – it’s all about working with our customers to find solutions to practical problems.”
Linkam’s HFS probe systems are based upon the temperature control technology used in the THMS600 heating and freezing stages. Up to four positional probes can be attached to the electrical connectors within the sample chamber, enabling electrical tests to be carried out while changing the temperature inside a gas tight environment.The sample is placed on the heating element and the probes are moved manually to make contact at the appropriate points.
The connectors in the HFS600E-PB4 handle up to 300 V (with a future revision expected to handle 500 V), making it possible to execute electrical measurements of thin ferroelectric and piezoelectric capacitors (as well as bulk ceramic or single-crystal capacitors). Moreover, the Faraday cage effect resulting from the earth-grounding of the device, combined with an additional ceramic insulator, ensures that capacitance measurements can be made down to ultra-low values.

www.linkam.co.uk
The main changes to our holographic microscope include implementing new cameras - both for QPI and fluorescence, and improvements to the fluorescence module - to capture a broader range of fluorescent markers.

Another significant improvement is the implementation of a multiwell plate. Now, scientists can observe up to 24 separate experiments simultaneously.
www.telight.eu

The MiniQS is ideally suited to the budget-conscious user who also demands reproducible results in an easy-to-use instrument. Quorum Technologies has a long history of producing excellent and innovative coaters for the SEM market, with the MiniQS filling a gap in our coater product-line for the budgetconscious user.
The instrument has an automatic operation with minimal user intervention required, enabling the user to ‘set and go’. Its compact size is ideal for use with benchtop SEMs or those with limited space.
The MiniQS meets UK and European (CE) industry standards, and vacuum interlocks remove power from deposition source, preventing users being exposed to high voltage. The instrument comes
with a gold target as standard and can be issued with or without a pump.
www.quorumtech.com
Electron microscopy imaging techniques such as SEM, DualBeam and TEM are crucial to study the 2D and 3D morphology of battery components at different stages in the lifecycle.
Optimum sample preparation and sample transfer between the various instruments without compromising the integrity of air-sensitive samples like Lithium containing battery components is key.
To avoid degradation of the sample upon contact
with air, the Thermo Scientific™ Inert Gas Sample Transfer (IGST) Workflow is introduced. The Thermo Scientific CleanConnect™ Samples Transfer System with its argon environment ensures that the sample is well protected during the transfer to the microscope. The ergonomic and modular design of CleanConnect enables uncomplicated sample handling without modification to the glove box.
The Thermo Scientific™ CleanMill™ is a complete ion-beam based sample preparation solution to get the best possible surface for SEM characterisation. CleanMill is fully compatible with the CleanConnect IGST for air sensitive samples.
www.thermofisher.com

Vision Engineering, the world-leading provider of innovative inspection, metrology, and digital 3D visualisation solutions, today announced the launch of Mantis 3rd Gen, the latest addition to its bestselling and award-winning range of ergonomic optical stereo microscopes.
Mantis is in use in tens of thousands of R&D, manufacturing and analytical sites around the world. Mantis 3rd Gen incorporates the latest developments in optics, digital cameras and fully adjustable LED lighting, to keep Mantis at the forefront of stereo imaging.
Mantis is designed for precision engineering, electronic engineering, medical device manufacture, and a wide range of other applications that require high-quality images and superior ergonomics. It features a unique, patented, eyepiece-less design that delivers a large, high-quality optical stereo image directly into the user's eyes, making it more comfortable and easier to view than traditional microscopes.
Manipulative, rework and restoration tasks need stereo images, to allow easy hand to eye coordination and depth perception. Mantis 3rd Gen combines stereo optical images, with high resolution camera options for manipulation and recording.
Mantis 3rd Gen features long working distance and excellent depth perception, now with a choice of 3 magnifications, making it ideal for a wide range of applications. It also now comes as standard with five different ways to illuminate your subject, giving you the flexibility to adjust the lighting to get the perfect image for your needs.
In addition to its outstanding image quality and ergonomics, Mantis also features a powerful digital imaging system that allows you to capture, review, and share high-resolution images.This makes it easy to share your work with colleagues, document your findings, and train new employees.

"As our customers have told us for the last 28 years, Mantis is an ideal solution for anyone who needs to perform precise work with small objects," said Mark Curtis, Managing Director at Vision Engineering. "We invest substantial R&D time and effort in exploiting the opportunities that fast moving optic, digital and lighting technologies offer our dedicated customer base. Mantis 3rd Gen offers the best of both worlds: superior ergonomics and optical image quality, combined with the latest digital imaging technology."
www.visioneng.com/mantis

Wiley, one of the world’s largest publishers and a global leader in scientific research and careerconnected education, today announced a new software integration between Digital Surf’s Mountains® software for spectroscopic imaging and surface analysis and Wiley’s KnowItAll software for spectral analysis and data management. Users of Mountains® will now have the option to send extracted spectra from spectroscopic images (Raman and IR: FTIR, ATR, NIR) direct to Wiley’s KnowItAll software to analyse components, taking advantage of its powerful search, prediction and mixture analysis algorithms and vast spectroscopic data collections.
and surface analysis. We hope that this lends to an acceleration, broadening and overall improvement of characterisation across life sciences and physical sciences” said Graeme Whitley, Director, New Business Development at Wiley.
“We’re thrilled to be able to bring the power and wealth of KnowItAll’s spectral database to users of our Mountains® solutions for spectroscopy analysis” said Renata Lewandowska, Product Manager for Spectral Applications at Digital Surf. “The connection between the two platforms is another step towards our goal of providing a 360° dedicated solution for processing & combining images and other data from spectroscopic techniques.”
“Confidently analysing spectra extracted from spectroscopic images is easier now that users of Mountains® can effortlessly transfer spectra into KnowItAll for full spectrum search and mixture analysis.We can’t wait to see the outcomes of these efforts appear in the published results in microscopy
Wiley Science Solutions is home to the world’s largest spectroscopy collection, providing researchers with integrated software solutions and superior spectral and chemical data collections with the aim to improve the quality of analysis and identification, reduce errors, and increase productivity in the laboratory. This partnership underscores Wiley’s commitment to investing in technology-based solutions that support the important research and development taking place in government, university and commercial labs around the world.
www.wiley.com
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
infocus Magazine has a long and proud history – both as ‘infocus’ since 2006, and as ‘The Proceedings of the Royal Microscopical Society’ since 1966. In this new feature, we take a look back at some interesting snippets of content and notable milestones from past issues – and where better to start than two of the very first photographs to appear in the publication, and the poignant connection between them.
The first instalments of the 1966 ‘Proceedings’ feature just a handful of images – unsurprising, perhaps, given the relative difficulty (compared with recent decades), in taking, developing, submitting and reproducing good quality photographs of broadly contemporaneous events. Those depicting people are of historical figures, one of whom being Dr J.J. Woodward, an American Civil War surgeon and pioneering microscopist. A portrait image of Woodward is featured as part of an absorbing article in the first issue of 1966, written by former RMS President and Honorary Archivist, the late Gerard L’E Turner.
Woodward served as Assistant Surgeon to the Artillery during the early part of the Civil War, and later transferred to the Office of the Surgeon General in Washington, where he remained for the rest of his career. His major work concerned research and publications on war-related diseases, but he also became a renowned figure in the development of micrography. He was elected an Honorary Member of the RMS in 1875 and a collection of his micrographs remains in the Society’s archives to this day. Other career highlights (so to speak) included Woodward’s carrying out of autopsies on the murdered US president, Abraham Lincoln, and his assassin, John Wilkes Booth. Years later, he also attended to the mortally wounded James Garfield for more than two months, until the 20th president finally succumbed to his injuries. Ever

the devoted doctor, Woodward kept daily notes on Garfield’s condition and eventually published the official record of the post-mortem examination.
Another milestone in the history of the Proceedings arrived in 1967, with the publication of the first photograph of a serving RMS President. The image appears as part of a newly-conceived feature, titled ‘News and notes’ (perhaps best thought of as the original pre-cursor to today’s ‘Office News’ section), beneath which readers are informed:
“Under this heading, it is hoped to publish, from time to time, information of the activities of the Fellows of the

Society and of items of news which may be of interest to Fellows” .
There follows a photograph and brief description of newly appointed RMS President, Professor Robert Barer – who, incidentally, followed Prince Philip, Duke of Edinburgh, as RMS President. The text references some of Professor Barer’s scientific achievements: his election as an RMS Honorary Fellow in 1961, his extensive writings on microscopical subjects, his position as Chair of Human Biology and Anatomy at Sheffield University. The Society is “assured of excellent guidance during Robert Barer’s tenure of the Presidency”, we are told.
What ‘Proceedings’ readers – as well as some of Barer’s colleagues and students - may not have known at the time, was that he was also a distinguished serviceman who had received the Military Cross for his actions as a Medical Officer during the Second World War. Professor Barer died in 1989, and obituaries published in both the British Medical Journal (1989;299:318) and Journal of Anatomy (1990; 170; 203-205) document his remarkable actions during the latter stages of the conflict, as the Allies advanced across northern Germany following the Normandy landings.

The description of events in the latter publication serves both as a reminder of the horrors of war, and testimony to the courage and fortitude of ordinary civilians – like Professor Barer - called upon to do truly extraordinary things. An extract is reproduced here:
“The second world war had, of course, already started when he [Barer] qualified, and after a brief surgical post in East London he joined the RAMC. At first with a unit in England, his request to be near the action was granted when he was posted with the rank of Captain to the Guards Armoured Division where he was MO to the Welsh Guards. He was with them at the Normandy landing of June 1944 and at the German breakout at Falaise. At the Nijmegen Bridge (‘a bridge too far’) where
the action was severe he had a casualty clearing station under the carriageway of the bridge. When the Allies had entered Germany and were advancing towards Rotenberg, he was given orders from ADMS Guards Armoured Division to advance to take over from the Germans a hospital believed to contain allied wounded. In the furthermost forward thin-skinned vehicle and under accurate shell fire he rescued the crew of three burning tanks whilst on his way to the hospital. The reorganisations completed, he personally led, on his own initiative, the relief of the concentration camp at Sandbostel. This was achieved at the second attempt in the face of determined resistance and with very little cover. For these various actions he was awarded the Military Cross. He remained affected by the terrible scenes in that typhus-infected camp and by memories of the wounded at the Nijmegen Bridge.”
And so, from those earliest issues of the Proceedings, an unlikely symmetry emerges between two medical men who served their countries on the battlefield and witnessed the horrors of war; two men separated by a continent and the better part of a century – but united in their passion for microscopy.
 Owen Morton
Owen Morton
In April 2023, the annual Material Research Society (MRS) Spring Meeting was held in San Francisco across three venues bringing researchers from countries such as United States, South Korea and the UK to name a few. With California home to many of the world’s leading universities and big technical companies such as Apple and Google, it was an ideal location to host such a prestigious material science meeting.The meeting also coincided with the 50th anniversary of the foundation of the MRS. San Francisco is very sunny in April, which makes a very nice change if you are coming from Belfast after the winter months!
With over 59 symposium sessions running over five days, including a mixture of posters and talks, it was a great chance to learn about some of the exciting developments in the various material science fields. The exhibit also gave a chance to see some companies promoting their latest products to help with research. One of my personal favourite stalls was a company promoting IR spectrometry which gave out freebie bags with “Genius” spelt out using the periodic table elements. Not surprisingly, it was very popular among attendees at the conference!
I was fortunate enough to present my work on dislocation and strain in InGaAs Metamorphic lasers, which is currently under review for publication. Metamorphic lasers are a type of semiconductor laser whereby a buffer layer is grown between the substrate and cladding/ active regions of the laser, which reduces lattice mismatch between these layers. In turn this helps reduce the number of performance-inhibiting, nonradiative recombination centres. My talk was well received and got a few questions on the technique of Geometric Phase Analysis (GPA), a strain mapping technique used in scanning transmission electron microscopy. It was also such a confidencebooster to go out and promote my work at a big international meeting.

Given the breadth of subjects being presented, I attended a mixture of talks to learn about other
fields and some which were relatable to my field of study – an example being a talk about obtaining bandgaps in batteries using low loss electron energy loss spectroscopy (EELS). As well as being an interesting presentation, this talk helped me a great deal in terms of my current work involving obtaining bandgaps using EELS. It was good to briefly talk with the presenter about how they went about the analysis.
Outside my main research area, I attended presentations given by a few of my colleagues from Queen’s University Belfast who were investigating ferroelectrics using Atomic Force Microscopy. The conference also gave me the opportunity to catch up with a few people I knew from my undergraduate degree and see their presentations. One of them discussed the synthesis of conjugate
polymers for solar cells, and another presented contrasting agents for Magnetic Resonance imaging, part of which involved in-situ transmission electron microscopy measurements.
The conference also had many featured talks from distinguished speakers. I was able to attend the featured talk ‘Designing Inorganic Nanomaterials for Energy and Soft-Electronics Applications’ presented by Taeghwan Hyeon, one of the most cited chemists in the last decade. One particular section that stood out for me was about Silver/ Gold mesh that can help treat heart failure, one of the biggest causes of mortality in the US. Although this is just one example, I think this demonstrates the importance that material science can have in the world. The talk overall was very informative and covered other areas such as wearable electronics and electrocatalysis.
Outside of the conference I also had the opportunity to do tourist sightseeing. I was able to walk across the golden gate bridge, visit the palace of fine arts and do a tour around Alcatraz. The tour encompassed walking around the Island, seeing many parts such as the prisoner cells, the watchtowers and reminders from the American Indian occupation protest of Alcatraz during the late 1960s. The steep terrain around San Francisco

also provided a great way to walk off some very nice food options that were on offer!
In conclusion, I had a great time at MRS Spring 2023, and greatly appreciate the travel bursary from the RMS to help fund my attendance. I am also grateful to Queen’s University Belfast for help in covering expenses and funding. Finally, I would like to thank the conference organisers for giving me the opportunity to present at a major international conference, and I would recommend the MRS Spring Meeting for any readers whose research is based in material science.

Nicholas Stephen Centre for Quantum Technologies
Queen’s University Belfast, UK
The RMS Digital Calendar for 2023 featured some great images over the last three months, showcasing an abundance of technical skill and artistic flair.

Each month you can download a new image as your computer wallpaper or desktop.
Or if you’d prefer you can download:
• an A1 poster of all 12 months, or
• an A4 print version, one month per page
All the images used were submitted to our annual calendar image competition. Congratulations to the winners!
MgSO4 crystals (recrystallized from Epsom Salt) Viewed under a total magnification of 100X using diascopic C-Pol with variable compensation. Equipment Used: Leica DM4000M microscope with Xiaomi Mi A3 camera. Shiraz S/O Kaderuppan.
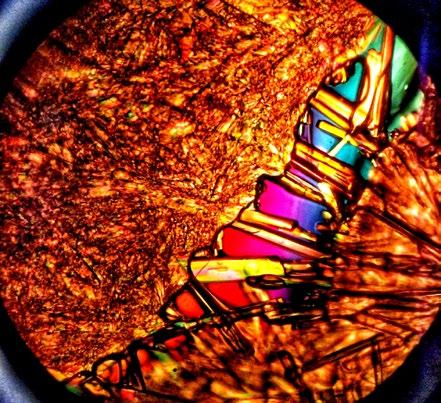
Jumping Spider
Side view of a jumping spider. Equipment Used: Dead, dried black spider was prepared for light sheet microscopy. Data acquisition based on autofluorescence alone using UltraMicroscope Blaze™. Rendering was done using Imaris. Simon F. Merz, LaVision BioTec, a Miltenyi Biotec company. Image Credit: Lea Bornemann, Tobias Jarzemski, Simon F. Merz.

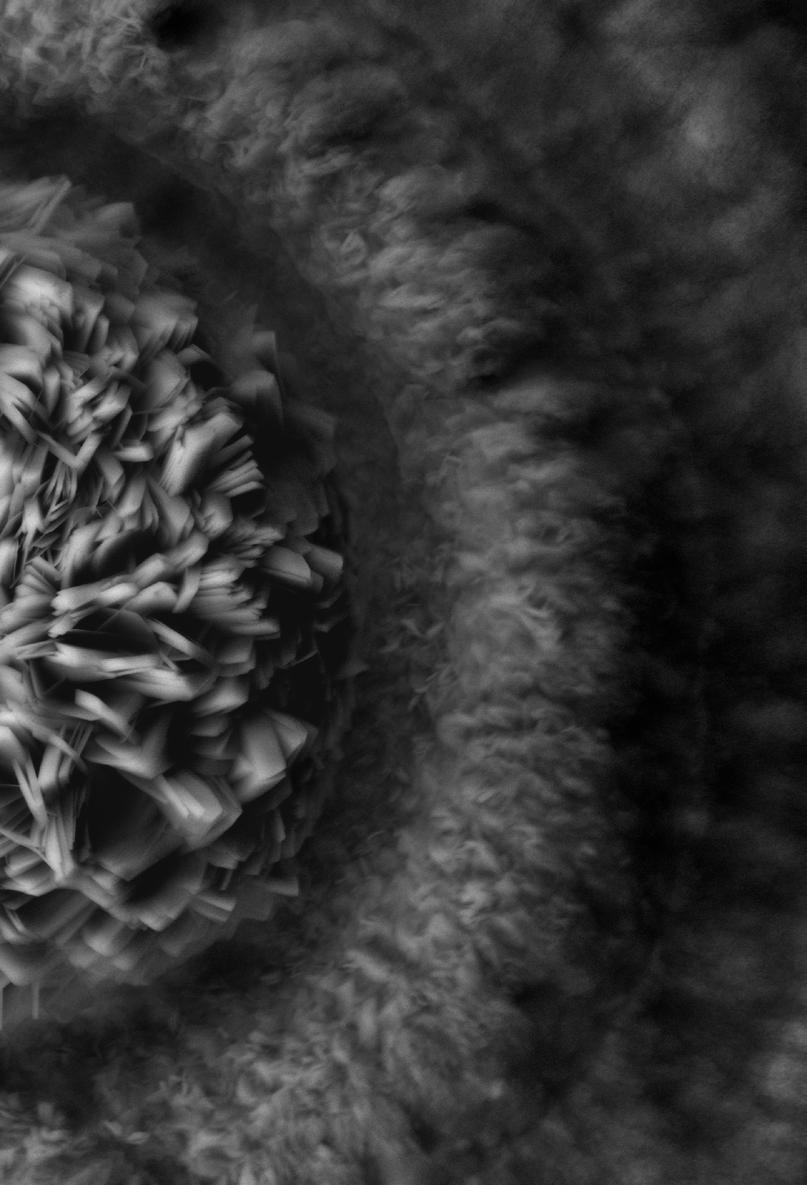

Prof. Nick D. Read (1954-2020) revolutionised our understanding of the filamentous fungal lifestyle. With his research teams at the University of Bristol (1978-1985), the University of Edinburgh (1985-2013) and the University of Manchester (2013-2020) he pioneered the use of fluorescent dyes in fungi and repeatedly raised the standards of advanced live-cell imaging microscopy. With their dedicated work, the Nick Read Labs revealed and explained
fascinating details of fungal cell biology with unparalleled beauty and expertise.
Nick’s tremendous enthusiasm, drive and vision paired with knowledge and innovative ideas made him an exemplary inspiration for the global community of fungal biologists and his many group members throughout the years. Along with his scientific expertise came fellowship and friendship, prompting some to declare the Nick Read Lab as “an extended family”.
To commemorate the life and work of this exceptional fungal cell biologist and, at the same
time, recognise outstanding achievements in the field of live-cell imaging of filamentous fungi, the Nick D. Read Imaging Award was initiated.
and by the attendees of the Neurospora Satellite Workshop taking place on Sunday March 5th 2023 just before the main conference.
Congratulations to the winners as follows:
Nick
Thanks are due to the ECFG16 organisers for providing a stage to premiere this award and to The British Mycological Society, The Royal Microscopical Society and inncellys GmbH for sponsoring.On this occasion, the three prize-winners were selected from

1st Prize: Lucas Well, Institut für Genetik, Technische Universität Braunschweig
2nd Prize: Marisela Garduno-Rosales, Departamento de microbiologia, Cicese Ensenada Mexico
3rd Prize: Luis Larrondo, Departamento de Genética Molecular y Microbiología, Pontificia Universidad Católica de Chile

There are plans in place to continue this award in the future. Thus, we anticipate to put the Nick D. Read Imaging Award on an even bigger stage at ECFG17 in Dublin in 2025 and beyond.
Alex Lichius, CEO inncellys GmbH
In this issue, we catch up with one of the RMS’s newest recruits, Alessandra Reni, to find out how she’s been settling into her role as Events Assistant. We also find out about Alessandra’s former life as a professional basketball player (yes, that’s correct), tales from backstage at Milan Fashion Week, and, most importantly of all, some cooking tips from the ‘Queen of Carbonara’!
Let’s kick off by asking Alessandra to describe what her role involves.
She says: “I’m responsible for supporting the events team, as well as organising some of the events myself. I’m always trying to help out, and I’m always ready to learn new things. I’m in contact with the scientific organisers – so, talking with them throughout the whole process from start to finish - and creating the scientific programme online. I also post information on our website about other events being run by external organisations.”
Having joined the Society mid-way through 2022, it has been a busy first year for Alessandra – finding her feet amid a steady stream of events, learning the ropes and building relationships across the RMS community. How has she found things?
Alessandra says: “I have been growing into the role, little by little. I love doing events – especially face-toface meetings. Being able to bring people together and celebrating when people spend time together and create those bonds is really great. I also really enjoy the opportunity to travel to different events around the UK, also meeting people from abroad. You are always in contact with different cultures, people, and different points of view.”
What does Alessandra like best about working for the RMS, and what are the biggest challenges?
“I really love the environment here, and the way people treat each other with respect. Everyone has been so helpful since I joined, and I really appreciate that.
“There’s obviously a bit of pressure when you want to make everything work for an event, and it’s sometimes challenging to keep everyone happy. You talk with so many different people – sponsors, scientific organisers, invited speakers, and everyone has their point of view – so just trying to make everybody happy is probably the most challenging part. Usually, it is not a big issue though.”
The LM Meeting in January was Alessandra’s first RMS event, as well as a first for the scientific
organisers involved. As such, she was particularly pleased – and relieved – to receive such positive feedback following the meeting. Since then, she has gone from strength to strength, and is now looking ahead to her first mmc in Manchester next month. She says: “I feel like every single event has given me something different. For instance, I went to the Natural History Museum to help with EM-UK. I had never been to the museum before, and we had an opportunity to spend some time walking around on the last day. It was a really inspiring environment, because you are surrounded by history and research.”
Alessandra adds: “One of the best things is how the Society provides opportunities for people from around the world to connect with each other – especially connecting the older and younger generations at these events, so that they can learn from each other.
“At every event I see lots of young students and early career researchers, which is a really good thing for the future of the RMS and microscopy in general of course.”
Prior to joining the RMS towards the end of 2022, Alessandra’s education and early career took her back and forth between Italy and the UK, including various roles in fashion events and the hospitality industry – as well as a degree in Events Management at Oxford Brookes University.
She explains: “When I finished high school, I bought a one-way ticket to London because I wanted to go and learn English. I had found a professional course in fashion events in Verona, but I already had the ticket, so the director told me to go to Oxford where a colleague was working in the fashion industry – and then I could come back to do the course. She was recruiting a group of fashion models, so I worked as an assistant, in contact with all the models and the agencies. I did three months over here and then went back to Italy. I did the professional qualification at Verona for a year, and during that time I worked at the Milan Fashion Week and many other places supporting the events team.”
Well, that’s pretty impressive. There can’t be many RMS staff – or members for that matter - who can boast Milan Fashion Week on their CV. Tell us more!

“I was working backstage”, says Alessandra, “helping all the models to get dressed and moving all the things around, and moving all the dresses and the shoes and supporting everything happening backstage. Everything moves really fast and there is a little bit of tension.

“There was always an after-party, but I didn’t really meet any famous models. The bigger personalities were always the people working behind the scenes – the stylists and the make-up artists, they were the real ‘divas’!”
Alessandra says: “I wanted to become a fashion stylist when I was a child. I was always drawing dresses every day. My grandmother was a seamstress but she never wanted to teach me how to do it, because she didn’t want me to follow in her footsteps.
“After that, I wanted to become an archaeologist, and then a tornado researcher – chasing tornadoes, like in the film Twister. My dad told me I was crazy and that I was watching too many movies.”
couldn’t be released into the wild. I know it was the right thing to do, but it was so painful, and I cried so much. I still feel it now.”
Growing up, Alessandra’s greatest passion, however, was playing sports – especially basketball. So much so, that she played the sport professionally during her late teens, in what was effectively the second national division in Italy.

She says: “I did tennis, swimming and horse-riding too, but when I got to secondary school my parents said I had to pick one, and I chose basketball. I had an amazing time playing in the local team with my friends, and we got really good. We were the best team in our age group and even at the age of 15 I was training three-to-five hours a day.
“Things started to get serious and when I was 18, I moved from the girls’ team to the professional team, playing in the national league A-2. It was my last year, because in a ‘small’ sport, unless you are the very best – like, European standard - it’s going to be hard to earn any sort of decent salary. I was good, but probably not going to make it to that level, and I wanted to be able to have time to do other things.”
Speaking of the big screen, Alessandra’s childhood love of animals once led to scenes reminiscent of a famous Alfred Hitchcock movie at her family home.
She explains: “We had more than 80 birds at our house. I wanted to get a dog but my parents refused. Instead, my dad bought me two little birds. From there on, we got more and more until we had a giant cage full of them in the garden. Sometimes I would bring them into the house and let them fly free down the corridors, and my mum was going crazy. Eventually we had so many that we had to give them away to an animal sanctuary – because they
Although she has abandoned the basketball ‘big time’, Alessandra still enjoys the odd game – just messing around on the court with friends. She would like to join a local team, but with the sport enjoying less popularity here in the UK, she has yet to find one, unfortunately.
All of which leaves plenty of spare time for Alessandra’s other enduring passion – food.
She says: “I like all food, but of course, coming from Italy, I love Italian food. In Italy food is like a religion, and the passion that people put into the way they make everything, and the ingredients; it just makes it taste better, and healthy too.”
A dab-hand in the kitchen, Alessandra has become
known in certain circles as the ‘Queen of Carbonara’, such is her authentic mastery of the Roman classic (one egg yolk per person, plus one whole one at the end – take note people!). Her Spanish partner Guillermo once fell into the trap of serving up an erroneous version of the dish, and was sent packing in no uncertain terms. “There was double cream all
over it”, Alessandra recalls, with a solemn shake of the head. “I told him, that is not a Carbonara.” It all ended well, though, and Alessandra now cooks her version on a regular basis, with Guillermo only too happy (and too wise, presumably) to defer to her culinary prowess.
When she is not cooking up a storm (or chasing one, for that matter), Alessandra enjoys going out to restaurants in and around Oxford.

She says: “I love eating out, and I have even been to a few Michelin-Star restaurants on special occasions. I’m more of a person who enjoys experiences – you don’t really need the material things, but you will never forget the taste of an amazing meal.”
And on this philosophical and mouth-watering note, our chat comes to a close. Many thanks for taking part, Alessandra!

infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS. infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more.
infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms.org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.ukj
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
• Total number of images/figures/tables should not exceed 15 including tables.
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).
