




















infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.
infocus Magazine
37/38 St Clements Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760 Email: infocus@rms.org.uk Website: www.infocus.org.uk Scientific Editor Leandro Lemgruber, University of Glasgow, UK Editor
Owen Morton Tel + (0)1865 254763, Email: editor@infocus.org.uk
Susan Cox, King’s College, London, UK Rebecca Higginson, Loughborough University, UK Emily Eden, University College London, UK
Laura Fumagalli, University of Manchester, UK Rhiannon Heard, University of Oxford, UK Maadhav Kothari, Zeiss Microscopy, UK Hilary Sandig, Cancer Research, UK Trevor Almeida, University of Glasgow, UK
Advertising
Email: advertising@infocus.org.uk ISSN: 1750-4740
© 2022 Royal Microscopical Society infocus is published four times per year by the RMS. Designed and produced by The ImageWorks. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.
We come to the last issue the year – and in fact, the last issue of infocus in print, as our magazine will be wholly digital from next year. I’m sure that several readers will miss receiving the issue through the mail or reading its contents while attending an RMS meeting. But going online will also open up new opportunities for our readers and our community, with content that can be explored in new ways. An exciting era is in front of us and we at infocus are working to bring you the best possible online experience.
As we aim for the future, we cannot forget our past, and our origins. In his absorbing article, Brian J Ford tells us about the life of Antony van Leeuwenhoek, founder of microbiology and a fine microscope builder. Brian describes his excitement at discovering original samples from Leeuwenhoek at the Royal Society archive, and how he imaged them using an original Leeuwenhoek microscope. Speaking of imaging and technical advances, we also have a great piece from Isabel Goodhand on optical filters and how LED illumination opened new possibilities of their use.
Looking towards the future (and perhaps the next generation of microscopists), we present a very interesting piece from Flavia Moreira-Leite, in which she relates her experience (and the pitfalls and challenges) of using transmission electron microscopy in outreach programmes with school students. It is a great guide for anyone interested in setting up similar projects with their local schools.
Also serving the next generation of microscopists is RMS volunteer Pete Banks, who kindly agreed to be interviewed for this issue. Pete has been working for several years alongside RMS members Chris Hammond and Peter Evennett to raise funds for RMS Outreach activities through selling donated microscopes. We can learn so much from Pete and his dedication to our microscopical community.
Finally, we celebrate the achievements of the 2022 RMS award-winners. These awards cover all areas of microscopy, imaging and cytometry, recognising scientific achievement as well as the efforts of those who go above and beyond for our society. Congratulations to them all!

I hope you enjoy this Christmas issue while perhaps treating yourself to some mulled wine and mince pies - or some other festive combination! I take this opportunity to wish you all a Happy Holidays and the warmest wishes from the infocus team for a great 2023.
COVER IMAGE: Number Plate.
By Karl Deckart. Nikon Metallurgical Microscope OPTIPHOT, magnification x50. Karl used reflected light to capture this image of a car number plate. Find out more about his technique on p23.
Friday 26 August 2023 marks the tercentenary of the death of Antony van Leeuwenhoek, the founder of microbiology. After three centuries, you might think that everything about his life and work has long since been discovered, but mysteries remain. Even his name is controversial: he was christened Thonis, not Antony, and many people still spell his name Antoni though Leeuwenhoek’s biographer Clifford Dobell pointed out in 1932 that the spelling in Dutch Antonij can be rendered only as Antony in English. 1 American scholars invented Anton, a convention unknown in Europe, and Antonie – not a name he’d have recognised – occurs in numerous standard sources, including Wikipedia, the Science Museum, and even Encyclopaedia Britannica. 2 It is said that Leeuwenhoek himself decided to add ‘van’ to give his name higher social status, though his uncle was using van Leeuwenhoek as the family surname back in 1628.
Ten single-lensed microscopes were associated with his name. One was acquired by an anonymous biotech organisation and has disappeared without trace, while another is surrounded by suspicions of double-dealing. Provenance of some remains a problem and not all those claimed by the Dutch were produced by Leeuwenhoek. Meanwhile I had the remarkable experience of identifying two previously unknown examples within a single year, bringing the total to twelve. 3
One of the greatest mysteries was how
Leeuwenhoek prepared his specimens. Brian Bracegirdle had summarised the consensus in 1978, reporting that early microscope specimens were: ‘prepared with little finesse.’ He added: ‘The first microscopists ... paid little attention to their specimens. No preparations from the seventeenth century have survived.’ 4
It was an extraordinary revelation to discover in 1981 that specimens prepared by Leeuwenhoek had remained untouched in the archives of the Royal Society since the 1600s. The Society’s president Sir Andrew Huxley had suggested that I peruse the
letters that Leeuwenhoek had sent to London and I’d envisaged discovering contemporaneous particulates (fungal spores perhaps, or a hair from his wig). Turning the pages of his letters and finding his original specimen packets with their contents intact made international news, and has given scholars a unique insight into his meticulous techniques. 5
Figure 2. Leeuwenhoek’s final mailing of specimens in 1686 consisted of three dried algal films. A correspondent in Courland had sent him what was claimed to be ‘heavenly paper’ dropped from the sky, but Leeuwenhoek used his microscopical skills to prove it was plant material from a dried pond.

I ensured that the bulk of each specimen remained untouched, so that future analysts could have access to material uncontaminated by the present day. I took small samples to the Netherlands to perform a unique experiment – for the first time since Leeuwenhoek, his own specimens would be imaged through an original microscope. The obvious choice was the most powerful instrument, the brass microscope at the University of Utrecht. Their director, Peter-Hans Kylstra, was excited by my proposal and a small technical team was assembled to assist in the demonstration. Over lunch I sketched on a paper napkin the modification that should be manufactured to fit my Olympus camera and the Leeuwenhoek microscope onto a sturdy microscope stand.The device was produced within the hour by their workshop, allowing the diminutive instrument to lie unencumbered on a perforated plastic plate. I admitted no other lens between the specimen, the microscope and the

film itself so that the image we obtained was not compromised by any extraneous optics.
Very few micrographs were taken before the microscope was safely returned to its box. These unique artefacts are delicate and should clearly have no more handling than is unavoidable. In any event, once we had images to prove the point, there was little to be gained by subjecting further seventeenthcentury microscopes to the same procedures. We could observe what Leeuwenhoek had seen

of his own specimens. This was an unprecedented demonstration and one of the key revelations in the history of science.
The samples were then mounted on aluminium stubs and gold-sputtered for the scanning electron microscope (SEM) at Cardiff University. I was eventually able to locate precisely the same areas of Leeuwenhoek’s sections that I had observed under the original microscope in Holland. There were
Figure 4. Found buried in mud from a Delft canal, this Leeuwenhoek microscope reveals many important details of the manufacturing methods of the seventeenth century. It was subsequently analysed under a scanning electron microscope

Figure 5. As an afterthought, the author prepared a finger-prick preparation of his blood on a coverslip and imaged it through the Utrecht lens. Not only are the erythrocytes vividly displayed, but the lobed nucleus of a granulocyte is resolved (top right).The lens could image objects down to 0.8 μm.
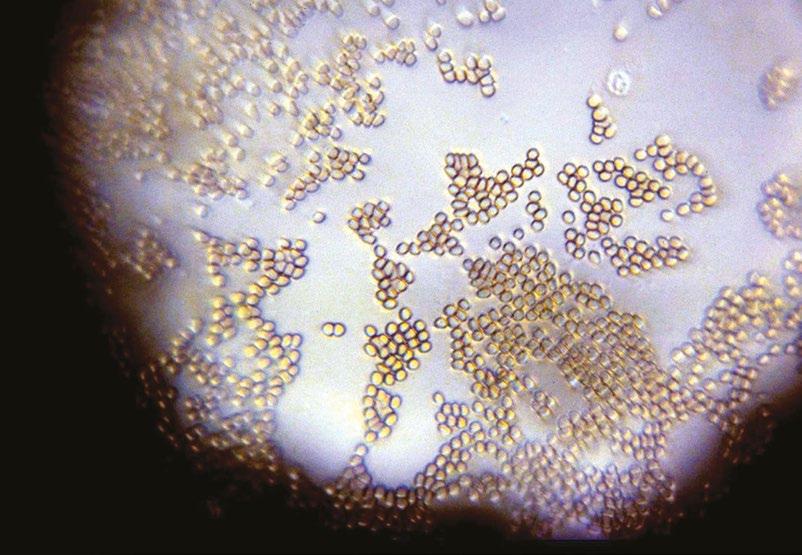
other traces that I found on the sections including presumptive blood cells (Leeuwenhoek used his shaving razor to cut the sections) and remains of the house-mite Tyrophagus about which he often complained.
One question remained – just how good were the best single lenses? Horace Dall was the man who knew. He was an authority on optics, and had ground a tiny lens of the type Leeuwenhoek used, using the mineral spinel rather than glass. Its refractive index (RI) is 1.7, compared with glass at 1.52, and has lower dispersion so chromatic aberration is reduced. Dall had mounted it into a pocket microscope little larger than an After Eight mint. I photographed numerous specimens using this instrument. Its specifications were the highest you could obtain with a single lens, and nothing Leeuwenhoek manufactured could have given images of such quality, but it usefully illustrates the extremes to which a single-lens microscope could aspire.
The results were remarkable: I had already shown that a microscope attributed to Leeuwenhoek could provide images of blood cells with a clarity that compared favourably with a modern compound instrument, and the images obtained with Horace Dall’s spinel microscope would be acceptable for most purposes. The points were proven once and for all. Contrary to conventional scholarly opinion, Leeuwenhoek was a superb microtomist, single-lensed microscopes could
Figure 6. Horace Dall was an experienced optical specialist based in Luton. He ground his lens from spinel, a mineral with an NI of 1.7 and low dispersion. The result is approximately 1 mm in diameter and magnifies 400x. Dall constructed this small pocket microscope which created impressive images.

Figure 7. Celebrating
for the first time
success
an
Leeuwenhoek’s
microscope
Utrecht:
Frederik, Jaap Stolp, Pieter Smiesing and the author. Karel Snethlage also assisted in the delicate procedure which proved to be a major landmark. The research on the specimens was reported internationally by the press and broadcast media, and in the major scientific journals.

provide images far better than was believed, and the ability of the pioneering microscopists to observe fine detail exceeds what the standard accounts had claimed.
With the generous support of Professor Richard Langford I carried out an extensive programme of scanning electron microscopy of a Leeuwenhoek microscope at the Cavendish Laboratory of Cambridge University. We can, for the first time, observe how it was made, and I have shown that the SEM offers an unprecedented method of characterising such antique artefacts. 6 Antony van Leeuwenhoek was a consummate professional, far from the amateur dilettante that has often been stated. To him we owe the birth of modern microscopy, and following in his footsteps by reprising some of his best work has been the challenge of a lifetime. The legacy it bequeaths to the history of the microscope will live on. Perhaps future historians will celebrate the achievements of that great Dutch pioneer more than we have in the past.
1 Clifford Dobell, Antony van Leeuwenhoek and his ‘Little Animals’, New York: Harcourt, Brace and company, 1932.
2 Brian J Ford, The Leeuwenhoek Legacy, Bristol: Biopress, and London: Farrand Press, 1991.
3 Brian J Ford, Recording Three Leeuwenhoek
Microscopes, InFocus, Proceedings of the Royal Microscopical Society 40: 30-43, December 2015.
4 Brian Bracegirdle, A History of Microtechnique: The Evolution of the Microtome and the Development of Tissue Preparations, London: Heinemann Educational Publishers, 1978.
5 Brian J Ford, The van Leeuwenhoek Specimens, Notes & Records of the Royal Society, 36 (1): 3759, 1981.
6 Brian J Ford, Genuine or copy? Novel methods of authenticating new Leeuwenhoek microscopes, Microscopy and Microanalysis, Digital image analysis supplement: S9-S13, January 2016.

Correspondence: Rothay House, Mayfield Road, Eastrea, Cambridge PE7 2AY.
Email: brianjford@cardiff.ac.uk
Brian J Ford is a leading Leeuwenhoek scholar and has published many books on microscopy. His research papers have appeared in publications including Nature, the British Medical Journal, Cell, and Scientific American. He joined the RMS over fifty years ago and was recently elected an Honorary Fellow of the Society. He has often appeared on radio and television programmes. Professor Ford lectures around the world and is a Fellow of Cardiff University. He was recently awarded the Ernst Abbe Medal by the New York Microscopical Society and was an award-winner in the 2022 Nikon Small World competition.










We are very pleased to be making a return to in-person events this year, although we will continue to organise a number of virtual events where appropriate, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend.
The following information was correct at the time infocus went to print but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates. Our online calendar includes all the details about forthcoming talks in the International Microscopy Lecture Series – a joint, online initiative established between the RMS, and a number of international societies. You can also sign up for the popular Imaging ONEWORLD talks covering all aspects of microscopy and imaging. These take place on Mondays at 1pm (GMT).
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
–
Botanical Microscopy Meeting 2023 –Norwich, UK (RMS-sponsored event)
–
Spring School in Electron Microscopy 2023 – Leeds, UK
EM-UK 2023
5 – 6 January, London, UK
Scientific Organisers: Dr Alex Ball, Natural History Museum; Professor Pippa Hawes, The Pirbright Institute; Professor Paul Verkade, University of Bristol
The EM-UK community meetings are designed to be an open forum for discussion of the latest
Flow Facilities Meeting 2023
5 – 6 January, London, UK
Scientific Organiser: Mr Derek Davies, The Francis Crick Institute; Dr Peter O’Toole, University of York
As in previous years, the meeting is aimed at all those who run or work in a Flow Facility and seeks to address common subject matters,
developments and challenges in the field, suitable for both academic and commercial microscopists. The meeting will include Techno Bites, talks and discussions about training.
This meeting is free to attend and will take place across the afternoons of the 5 and 6 January 2023.
www.rms.org.uk
themes, and circumstances within the community. We will also focus on new and emerging technologies as well as operational aspects of running, and working in, a core. We will include presentations from current core facilities (Crib talks) and also from our industry colleagues (Techno bites).
www.rms.org.uk
2023
9 – 10 January, Cambridge, UK
Scientific Organiser: Dr Peter O’Toole, University of York; Dr Alex Sossick, University of Cambridge
From very humble beginnings, we have grown to a much more significant and influential community of facility managers. Numbers of attendees have grown 10-fold since the first meeting in 2006 as more and more facilities have opened. We now represent one of the best organised facility groupings in the UK if not
indeed the world.
Following on from previous years, you can expect to find out more on the latest developments in UK Bioimaging and how we can feed into wider international groups that are starting up. We will also discuss some of the basic elements (funding, impact measures) of running a core facility as well as the latest technological and application developments that affect ourselves and our users. Once again, we will include Crib talks from attendees.
www.rms.org.uk
13 – 17 February, Nottingham, UK
This course consists of two and half days, broken into a morning and afternoon lecture sessions, covering the theory of the techniques. Days four
mmc2023 (incorporating EMAG 2023)
4 – 6 July, Manchester, UK
Scientific Organisers: Make sure your diary includes mmc2023! One of the biggest events of its kind in Europe, mmc2023 will bring you the very best in microscopy, imaging and cytometry from across the globe. With six parallel conference sessions, a world-class exhibition, workshops, satellite meetings, an international Imaging Competition and more, it’s simply the place to be for anyone who uses a microscope for work, study or pleasure.
Back at the superb Manchester Central conference centre for the first time since 2019,
and five (Thursday and Friday) will be filled with practical demo / Q&A sessions to explore the practical points of the techniques / approaches with the delegates in person, should they wish to come to Nottingham. www.rms.org.uk
the event will be taking place fully in person, providing the perfect opportunity for the scientific community to come together, make connections and share their research. As always, many of the leading companies in microscopy and imaging will be on hand to demonstrate the very latest equipment and technology at the exhibition, which is returning to Manchester from 4-6 July.
As with the previous events, you can expect a huge and varied scientific conference alongside Europe’s largest free microscopy and imaging exhibition filled with a huge number of free training workshops. www.rms.org.uk




Iwas very excited to attend an international conference and visit the beautiful city of Prague for the first time. The 16th International Congress of Histochemistry and Cytochemistry (ICHC) organised by the Society for Histochemistry was held on the 28th - 31st August 2022. The conference had been postponed twice as a result of the pandemic and for me, as I am due to submit my thesis in the next month, this was my final opportunity to experience an international conference during my PhD. I was attending on behalf of the Royal Microscopical Society who had nominated me as their “Young Histochemist”.
The ICHC brought together microscopists and histochemists to share ideas and gain insight into the exciting research going on across the globe. The conference had a packed schedule filled with a variety of different talks, poster sessions and social events. I came eager to expand my knowledge on how researchers across different fields make use
of microscopical techniques and I was impressed with the wide range of subject areas covered. This included areas less familiar to me, such as a fascinating talk by Dr.Ciler Celik Ozenci on the “Effects of maternal circadian rhythm disruption on maternal, fetal, placental, and offspring health”, where her lab had used Raman spectroscopy to track chemical signatures of wild-type and chronodisrupted embryos. Sessions covered topics such as cancer biology and more technical sessions focussing on sample preparation.

My PhD work focuses on membrane trafficking and I have used a wide range of microscopical techniques including Förster Resonance Energy Transfer (FRET) to explore protein-protein interactions and superresolution microscopy such as structured illumination microscopy (SIM) for localisation studies. I was drawn to one session that had a focus on “the structure of the cell” with a focus on how the actin cytoskeleton is regulated. Dr. Margarida Barrosos gave a talk about her lab’s work using FRET with Fluorescent Lifetime Imaging (FLIM) in the context of membrane trafficking. FRET has proven to be a very useful tool to me during my PhD, helping me identify interacting proteins when biochemical techniques proved unsuitable.
In addition to attending talks and the poster session, I had the wonderful opportunity to give a talk before I collected my Young Histochemist award. I spoke about the various ways I have used microscopy during my PhD such as using live-cell time-lapse microscopy without compromising on fluorophore bleaching. It was a privilege to present my work to the global microscopy community and receive feedback, which is especially useful with my viva looming in the near future! I also made sure I had
time to explore Prague, where I enjoyed wandering around the peaceful Royal Gardens and the historic Old Town. On the second evening the conference had organised a beer party at the Strahov Monastery.

I had a leisurely late summer evening walk up the hill to the monastery, from which I was able to get a feel for the city and appreciate its expanse.
I would like to thank the Royal Microscopy Society for contributing to the funding of this trip and the Society for Histochemistry for organising a brilliant conference. I would also like to thank Peter O’Toole and colleagues at the technology facility at the University of York for their expertise and training over the years. Particularly Grant Calder, Karen Hogg and Graeme Park who I have worked closely with during the course of my PhD.
Katherine Paine University of YorkYou can read the latest Early View papers online at www.journalofmicroscopy.org

Low-energy electron microscopy intensity-voltage data –factorisation, sparse sampling, and classification
F. Masia, W. Langbein, S. Fischer, J.-O. Krisponeit, J. Falta
Low-energy electron microscopy (LEEM) taken as intensity-voltage (I-V) curves provides hyperspectral images of surfaces, which can be used to identify the surface type, but are difficult to analyze. Here, we demonstrate the use of an algorithm for factorising the data into spectra and concentrations of characteristic components () for identifying distinct physical surface phases. Importantly, is an unsupervised and fast algorithm. As example data we use experiments on the growth of praseodymium oxide or ruthenium oxide on ruthenium single crystal substrates, both featuring a complex distribution of coexisting surface components, varying in both chemical composition and crystallographic structure. With the factorisation result a sparse sampling method is demonstrated, reducing the measurement time by 1-2 orders of magnitude, relevant for dynamic surface studies. The concentrations are providing the features for a support vector machine (SVM) based supervised classification of the surface types. Here, specific surface regions which have been identified structurally, via their diffraction pattern, as well as
chemically by complementary spectro-microscopic techniques, are used as training sets. A reliable classification is demonstrated on both exemplary LEEM I-V datasets.
A perspective of fluorescence microscopy for cellular structural biology with EGFR as witness M. L. Martin-Fernandez
The epidermal growth factor receptor (EGFR) is a poster child for the understanding of receptor behaviour, and of paramount importance to cell function and human health. Cloned almost forty years ago, the interest in EGFR’s structure/function relationships remains unabated, not least because changes in oncogenic EGFR mutants are key drivers of the formation of lung and brain tumours. The structure of the assemblies formed by EGFR have been comprehensibly investigated by techniques such as high-resolution X-ray crystallography, NMR, and all-atom molecular dynamics (MD) simulations. However, the complexity embedded in the portfolio of EGFR states that are only possible in the physiological environment of cells has often proved refractory to cell-free structural methods. Conversely, some key inroads made by quantitative fluorescence microscopy and super-resolution have depended on
exploiting the wealth of structures available. Here, a brief personal perspective is provided on how quantitative fluorescence microscopy and super-resolution methods have cross-fertilised with cell-free-derived EGFR structural information. I primarily discuss areas in which my research group has made a contribution to fill gaps in EGFR’s cellular structural biology and towards developing new tools to investigate macromolecular assemblies in cells.
Advancement and challenges in sample preparation for Atomic Force Microscopy and Infrared Microscopy for wood-based materials


rely on cutting and grinding the materials under test, including sensitive wood substrates. To investigate cross-sections and different layer topologies, characterisation techniques such as Atomic Force Microscopy (AFM) and Infrared (IR) microscopy are of potential interest. However, a huge limitation is that current sample preparation methods lead to smeared coatings on the sample cross-sections and high surface roughness. Hence, these methods are not applicable for the sample preparation in measuring AFM and IR microscopy. Therefore, new preparation techniques need to be developed accordingly. This article presents a new approach towards coated wood-based sample preparation including embedding processes to use those samples for AFM and IR microscopy technologies. The proposed method has been evaluated by obtaining AFM, IR, and microscopy measurements of more than 4 different wood-based samples such as (i) raw paper, (ii) impregnated paper, (iii) melamine-coated chipboards and (iv) mediumdensity fiberboards. The investigation results showed a significant improvement in sample preparation, as well as clear chemical and physical characterisation over whole sample construction, including coating layers, for wood-based materials.
Quantitative analysis of backscattered-electron contrast in scanning electron microscopy
Martin Čalkovský, Erich Müller, Dagmar Gerthsen
Wood-based materials such as composites or laminates play an important role in today’s furniture industry, especially in manufacturing high-quality kitchen and dining room furniture. One important aspect after fabrication is the investigation of these materials to derive quality metrics such as surface stain and scuff resistance. Current sample preparation methods are mostly straightforward and
Backscattered-electron scanning electron microscopy (BSE-SEM) imaging is a valuable technique for materials characterisation because it provides information about the homogeneity of the material in the analysed specimen and is therefore an important technique in modern electron microscopy. However, the information contained in BSE-SEM images is up to now rarely quantitatively evaluated. The main challenge of quantitative BSE-SEM imaging is to relate
the measured BSE intensity to the backscattering coefficient η and the (average) atomic number Z to derive chemical information from the BSE-SEM image. We propose a quantitative BSE-SEM method, which is based on the comparison of Monte–Carlo (MC) simulated and measured BSE intensities acquired from wedge-shaped electron-transparent specimens with known thickness profile. The new method also includes measures to improve and validate the agreement of the MC simulations with experimental data. Two different challenging samples (ZnS/ Zn(OxS1–x)/ZnO/Si-multilayer and PTB7/PC71BMmultilayer systems) are quantitatively analysed, which demonstrates the validity of the proposed method and emphasises the importance of realistic MC simulations for quantitative BSE-SEM analysis. Moreover, MC simulations can be used to optimise the imaging parameters (electron energy, detectionangle range) in advance to avoid tedious experimental trial and error optimisation. Under optimised imaging conditions pre-determined by MC simulations, the BSE-SEM technique is capable of distinguishing materials with small composition differences.
Scanning electron microscopy has been a powerful technique to investigate the structural and chemical properties of multiphase materials on micro and nanoscale due to its high-resolution capabilities. One of the main outcomes of the SEM-based analysis is the calculation of the fractions of material components constituting the multiphase material by means of the segmentation of their back scattered electron SEM images. In order to segment multiphase images, Gaussian mixture models (GMMs) are commonly used based on the deconvolution of the image pixel histogram. Despite its extensive use, the accuracy of GMM predictions has not been validated yet. In this paper, we proceed to a systematic study of the
evaluation of the accuracy and the limitations of the GMM method when applied to the segmentation of a four-phase material. To this end, first, we build a modelling framework and propose an index to quantify the accuracy of GMM predictions for all phases.Then we apply this framework to calculate the impact of collective parameters of image histogram on the accuracy of GMM predictions. Finally, some rules of thumb are concluded to guide SEM users about the suitability of using GMM for the segmentation of their SEM images based only on the inspection of the image histogram. A suitable histogram for GMM is a histogram with number of peaks equal to the number of Gaussian components, and if that is not the case, kurtosis and skewness should be smaller than 2.35 and 0.1, respectively.

Wear mechanisms description in nanoscale by SEM/TEM of multilayer Zr/ZrN coatings in dependence on phases ratio
L. Major, J. M. Lackner, M. Kot, R. Major, M. Dyner, B. Major
As a result of loading with an external force during the wear process, coating deforms uniformly. After a certain limit load is exceeded, coating deformation is localised through the formation of the so-called

Segmentation of SEM images of multiphase materials: When Gaussian mixture models are accurate?
Manolis Chatzigeorgiou, Michalis Vrigkas, Margarita Beazi-Katsioti, Marios Katsiotis, Nikos Boukos, Vassilios Constantoudis
shear bands. It has been showed experimentally the process of shear bands formation. The microstructural characterisation before and after the mechanical tests was performed using scanning and transmission electron microscopy (SEM and TEM) on cross-sections of the samples. The analysis indicated that in the case of multilayer coatings where the ratio of the metallic to the ceramic phase is 1:1, the shear bands are formed at an angle of 45°. With a greater proportion of the ceramic phase to metallic (ratio 1:2), the shear band changed the shear angle from ∼45° to ∼90°.
Mechanical in situ tests were carried out in the chambers of SEM and TEM. The scratch tests in the SEM were done with the simultaneous observation of the phenomena occurring on the surface of the tested materials showed that at a scratch force of 0.04 N, the additional outer a-C:H layer was damaged, which was shown in the form of a fault in the force–displacement diagram, and in the form of splits visible in the SEM image. However, the application of this additional layer had a positive effect on the wear mechanism of the entire coating structure. The test also indicated that in the case of coatings with phases ratio 1:2 and 1:4 (metallic to ceramic), the characteristics of the brittle material were demonstrated, unlike the coating with a 1:1 phase ratio, where plastic properties predominated. However, for the 1:2 phase ratio coating, the chip was more ductile than for the chip formed when testing a 1:4 phase ratio coating. For in situ mechanical testing in the TEM, a straining holder was

used.The test showed that the shear band angle for a 1:1 ratio coating has changed from 45° to 90° due to the different direction of force interaction.




The November 2022 issue of the Journal of Microscopy celebrates a former General of the Journal, Professor Tony Wilson. Tony, a fellow of Hertford College, University of Oxford, served as a Scientific Editor from 1994 to 1999 before becoming General Editor for 16 years, from 1999 to 2015. Tony also served as Executive Honorary Secretary of the Royal Microscopical Society from 2001 to 2010 and President from 2010 to 2013.
The current Editor of the Journal, Professor Michelle Peckham, commented: “Tony is a leading researcher in microscopy and applied optics, and well known for his pioneering work in confocal microscopy, and has made important contributions to the Royal Microscopical Society and the Journal of Microscopy. It is a real pleasure to publish this Festschrift to celebrate Tony’s career and many contributions.”
The festschrift features the following papers and an introduction by Professor Martin Booth, University of Oxford, reflecting on Tony’s career:
• Real-Time Optical Vascular Imaging, a new method for the diagnosis and monitoring of oral diseases. BASTOS, P., CARPENTIER, G., PATEL, V., PAPY-GARCIA, D., WATSON, T. and COOK, R.
• Perspective of fibre-optical microendoscopy with microlenses. Wang, B, Zhang, Q, Chen, X, Luan, H, Gu, M.
• Sensitivity of remote focusing micro- scopes
to magnification mismatch. Mohanan, S, Corbett, AD.
• Wavefront-sensorless adaptive optics with a laser-free spinning disk confocal microscope. Hussain, SA, Kubo, T, Hall, N, et al.
• Using saturated absorption for superresolution laser scanning transmission microscopy. Nishida, K, Sato, H, Oketani, R, Mochizuki, K, Temma, K, Kumamoto,Y, Tanaka, H, & Fujita, K.
• Robust deep learning optical autofocus system applied to automated multiwell plate single molecule localization microscopy. Lightley, J., Görlitz, F., Kumar, S. et al.
• Designing chromatic optical retarder stacks for segmented next-generation easySTED phase plates. Engelhardt, J., Ellerhoff, B., Gürth, C. -M., Sahl, S. J., & Hell, S. W.
View the issue at www.journalofmicroscopy. org.uk.

The Journal of Microscopy is the oldest journal dedicated to the science of microscopy and the only peer-reviewed publication of the Royal Microscopical Society.
The Journal publishes papers in physical and biological sciences in which the development of imaging methods using light, electrons, x-rays, and so on, as well as atomic force and near field techniques are a key focus of the research. Research papers covering optical theory, spectroscopy, novel specimen preparation, manipulation methods, image data processing and analysis are also welcome, as are research papers in which state-of-the-art imaging approaches are a key aspect of the research findings.
The Journal of Microscopy offers authors a hybrid open access option. The benefits include:
• High visibility all articles are immediately, freely available online
• Easy compliance with open access mandates with Creative Commons licenses
• Reuse and immediate deposit of final article in any website or repository
• Copyright retention – You retain the copyright for your article at all times
• Automatic export to PubMed Central/Europe PubMed Central (PMC) when appropriate
• High-quality and authoritative publishing standards with rigorous peer-review
The Journal publisher, Wiley, has several agreements in place with institutions and funders to help authors publish open access and ensure compliance with open access policies. Authors from an institution affiliated with an open access deal may publish primary research and review articles, open access, at no charge to the author.
We have published three themed issues in 2022papers from the 2019 and 2020 ToScA meetings; papers from the 18th Euroseminar on Microscopy Applied to Building Materials and a Festschrift for the Former General Editor of the Journal Professor Tony Wilson. There are plans for further themed issues in late 2022 and 2023, including “A Lens on the Future” featuring papers by early career researchers, “Women in Microscopy”, “Microscopy Core Facility Management”, “Ptychography” and “Botanical Microscopy”.
We’d love to hear from you if you have any ideas for a special issue on a particular topic or arising from a meeting!
The popular invited review series is continuing in the Journal of Microscopy and in 2022, we published three Invited Reviews:
and advances in optical 3D mesoscale imaging, Sebastian Munck, Christopher Cawthorne, Abril Escamilla-Ayala, Axelle Kerstens, Sergio Gabarre, Katrina Wesencraft, Eliana Battistella, Rebecca Craig, Emmanue; G. Reynaud, Jim Swoger, Gail McConnell
Quantitative analysis of 3D cellular geometry and modelling of the Arabidopsis embryo, Saiko Yoshida, Dolf Weijers
How
for volume electron microscopy, Arent J. Kievits, Ryan Lane, Elizabeth C. Carroll, Jacob P. Hoogenboom
We are delighted to announce our new Editorial Board members who have joined us in 2022:
Tonni Grube Andersen, Max Planck Institute for Plant Breeding Research, Germany
Emmanuelle Bayer, CNRS - Université Bordeaux, France
Laura Clark, University of Leeds, UK
Martin Booth, University of Oxford, UK
Matthieu Bugnet, CNRS – University of Lyon, France
Rik Drummond-Brydson, University of Leeds, UK
Patricia Goggin, University of Southampton, UK
Markus Gräfe, University of Jena, Germany
Izzy Jayasinghe, The University of Sheffield, UK
Leandro Lemgruber Soares, University of Glasgow, UK
Fabio Nudelman, University of Edinburgh, UK
Chris Parmenter, University of Nottingham, UK
Jessica Valli, Heriot-Watt University, UK
Paul Verkade, University of Bristol, UK Tom Vettenburg, University of Dundee, UK
General Editor
Professor Michelle Peckham, University of Leeds, UK
Scientific Editors
Dr Kurt Anderson, The Francis Crick Institute, London, UK
Dr Bert Hecht, University of Würzburg, Germany
Professor Carolyn Larabell, University of California, San Francisco, USA
Dr Richard Leapman, NIH, Bethesda, USA
Professor Jian Liu, Harbin Institute of Technology, China
Professor, Gail McConnell, University of Strathclyde, Glasgow, UK
Professor Pete Nellist, University of Oxford, UK Dr Ulla Neumann, Max Planck Institute for Plant Breeding Research, Köln, Germany
Professor Jens Randel Nyengaard,Aarhus University, Denmark
Professor Mark Rainforth, The University of Sheffield, UK
For more information about the Journal of Microscopy, please contact the Editorial Office Manager, Jill Hobbs, journaladmin@rms.org.uk
www.journalofmicroscopy.org.uk

innovations in methodology offer new prospects

Regular infocus contributor Karl Deckart has recently been exploring images captured by reflected light. Here he explains the background behind this issue’s striking cover image.
Karl says: “Licence plates should always be easy to read, especially in the dark! Therefore, many surfaces have been tested for their reflective properties and have found out that light surfaces reflect quite well, but not yet well enough. Special prisms reflect even better, but for licence plates and projection screens, sphere-coated backgrounds are best:
The principle of a pearl screen differs from other projection screens in that the surface structure contains tiny glass spheres (0.04 / 40 µ). According to the properties of full spheres, each transparent sphere produces a total reflection. That is, most of the projected light is reflected in the same direction from which it comes. The angle of reflection of the reflected light is thus the same as that of the incident light. An incident light microscope shows this best because the optical axes of the illumination beam and the reflection beam are congruent. However, the two are opposite in direction. The same principle is also used in the projection of an image on a pearl screen and also on license plates and particularly reflective traffic signs. Because this total reflection of the tiny spheres also totally reflects each colour, one can enjoy a projected

image in its natural colours.
In a reflected light microscope, the illumination beam path to the object is congruent with the beam path going to the sensor chip due to its reflection - however, because their directions are opposite, it is possible to optionally place colour filters in one of the two beam paths, the colour of which is particularly emphasised by the total reflection in the miniature glass sphere.
In both constellations the optical conditions are the same, but the clear glass spheres in the pearl canvas are on a movable background and on the licence plate on a fixed one.”
See more of Karl’s reflected light images here: https://mikromakro.de/index.php/ galerie/mikro-auflicht

The RMS is delighted to announce the winners of its full range of awards giving formal recognition to individuals making a special contribution across microscopy, cytometry and imaging.
The Society offers a wide range of award opportunities covering scientific achievement, education and outreach, technical support and more. Each year the RMS invites applications from across the globe, to ensure those making a real difference within the microscopy community receive the recognition they deserve.
RMS President, Professor Grace Burke said: “It is always a privilege to announce our award-winners and give formal recognition to the work of some of our leaders in microscopy. My warmest congratulations to all of the 2022 winners. It has been a pleasure to read about their impressive achievements and important contributions to the microscopy community and beyond.”
Recognising exceptional voluntary contributions to the work of the RMS.

Lynne has been a Fellow of the Royal Microscopical Society since 1987. Lynne worked at The Rank Research Centre at High Wycombe before joining at Agar Scientific (formerly Agar Aids), where she spent the majority of her career – firstly as Customer Liaison Officer, then Sales Director, then as Managing Director, before retiring in 2015.
Lynne served two terms as Honorary Treasurer of the RMS, firstly from 1995 to 2005, and a second term from 2014 to 2020. She has also been a member of the Corporate Advisory Board from 1992 to 2020. Since coming to the end of her term as Treasurer in 2020, she has continued her involvement by providing a smooth transition to Rod Shipley, the incoming Treasurer, as well as being invited to attend meetings of the Executive and Council. Lynne remains on the History Committee of the Society.

Lynne was a strong supporter of the RMS and frequently represented Agar Scientific at the MICRO exhibitions, and from 2002, the Microscience Exhibitions. While Treasurer, Lynne supported the RMS in the move to running the Microscience Exhibition at the ExCeL Centre, which was a major step for the Society.
Over many years, Lynne has devoted an enormous amount of time freely to the RMS and has made exceptional contributions to the Society, helping it grow and progress. She has been a tremendous asset and a source of so much knowledge and guidance to members of the Council as well as those working in the RMS office.
Debbie obtained a PhD in physics at the Cavendish Laboratory, Cambridge University, and in 1999 was awarded a Royal Society Dorothy Hodgkin Research Fellowship to continue investigating electron emission and charge-related phenomena in insulating materials and the stability of hydrated specimens in the environmental SEM (ESEM).
More recently, Debbie worked on new applications and methodologies for focused ion beam scanning electron microscopy (FIB SEM). She is interested in all kinds of electron microscopy, and is the author of a recent RMS-Wiley book entitled Principles and Practice of Variable Pressure/Environmental Scanning Electron Microscopy (VPESEM).
Debbie has been a member of the RMS Executive Committee since 2005, and a
member of the Society since 2003. As International Secretary she established and maintained relationships between the RMS and international Societies, companies and universities, as well as actively participating in the strategic planning of the Society.
Debbie has also served as RMS Vice-President and Honorary Secretary Science (Physical). In the latter role, Debbie oversaw the transition of the Microscience Congress to the Microscience Microscopy Congress that we all know so well today. One of Debbie’s greatest achievements was chairing the European Microscopy Congress in Manchester in 2012 – a fantastic event and major coup for the Society.
Debbie has given so much of her time freely to the RMS over very many years and has made exceptional contributions to the Society through her work. She has helped the RMS grow and progress, bringing her calm manner, knowledge and ability to see the bigger picture to many meetings of the Executive and Council, as well as a superb range of RMS t-shirts and other items.
Steve is an outstanding Principal Clinical Scientist, who heads up the Immunophenotyping Laboratory at the University Hospital of Wales, Cardiff. The laboratory provides a clinical flow cytometry service to most Health Boards in Wales.
He has participated in, and later organised, the Clinical Module of the Annual RMS Flow Cytometry Course for more than two decades, and in recent years, has also run the Virtual RMS Clinical Flow course. Both have been hugely successful and of benefit to thousands of delegates over the years.
Steve has regularly chaired many other educational sessions and best practice workshops. He is a founding member of UK multicolour immunophenotyping (MIG) group and co-authored national guidelines for the use of multicolour flow cytometry in the diagnosis of haematological neoplasms. He is also a founding member of PNH diagnostics interest group, which established and validated a consensus protocol for PNH flow cytometry.
Steve has also been actively involved in working groups to improve diagnosis of myeloma, leukaemia and lymphoma – as well as working to improve standardisation of flow cytometry within Europe through the Harmonemia initiative.

Throughout his career Steve has remained at the forefront of clinical flow cytometry by consistently reviewing and evolving the needs of his service and patients. He has had an active role in clinical trials and is seen as an expert in several fields including lymph node flow cytometry, where Cardiff has a reputation as a reference centre.
By sharing his expertise over many years, Steve has enabling countless others to make advances and developments in clinical cytometry. His contributions have been appreciated nationally and internationally, and have significantly enhanced the profile of the RMS.
Recognising the critical contributions to microscopy research, technique development or education through this award to a scientist, engineer or laboratory research staff.
Ms Xiangli Zhong, Senior Experimental Officer in the School of Materials, University of Manchester
Xiangli has developed a long track-record of outstanding contributions to the microscopy community for many years, and her exemplary engagement in RMS activities have had a continuous impact on microscopy research and the microscopy community.
As leader of the RMS FIB- Focused Interest Group and the UK FIB & EM Preparation User Group since 2013, she has been engaged in numerous activities to promote the good use and expansion of focused ion beam (FIB) facilities in the UK.
She has successfully organised multiple sessions and workshops on volume microscopy and FIB related-sessions at the mmc congresses and chaired multiple FIB related-sessions at other national and international conferences. She has also served for several years as an active member of the RMS Engineering and Physical Sciences Committee
As Coordinator of the University of Manchester Ion Beam Tech Forum, she has developed a vital role to shape the FIB and scanning electron microscopy facilities by e.g. spearheading remote operation of FIB.This was essential for continued operation during the COVID lockdowns, and has paved the way for future FIB usage schemes and the upcoming purchase of cryo-FIB equipment at Manchester.
Her rich experience and dedication to EM management, training and promotion of user communication is exemplified by her initiation and implementation of much needed Standard Operation Procedures (SOP’s) in training and application.
Xiangli promotes the use of ‘classical’ Materials Science sample preparation and characterisation techniques in the Life Sciences, which is further underpinned by collaborations across the Physical Sciences and Life Science boundary.
Xiangli has authored and co-authored more than 60 peer reviewed publications in key and high-impact-factor journals - many of them of specific interest for the larger scientific community.
Celebrating outstanding scientific achievements across all areas of microscopy and flow cytometry. Selected by each RMS Science Section.
Dr Erin Tranfield, Head of the Electron Microscopy Facility at the Instituto Gulbenkian de Ciência in Oeiras, Portugal

Erin receives the award in recognition of her research and development in advanced electron microscopy, and additionally, for the creation of the TechEM Seminar Series during the COVID-19 pandemic, enabling much-needed networking, training and development opportunities for the global EM Community.
Erin completed her PhD in 2007 at the University of British Columbia in Canada and conducted postdoctoral research at the NASA Ames Research Centre in the USA studying lunar dust, and in the Cell Biology and Biophysics Lab at EMBL in Heidelberg studying the frog spindle using electron tomography.
Specialising in electron tomography, cryofixation and correlative light and electron microscopy, Erin has supported numerous researchers with advanced imaging in fields as diverse as cell biology, pathogens, neuroscience and cancer. Erin is also active in multiple learned societies, networks, conferences and training courses.
During the first lockdowns of the COVID-19 pandemic, Erin realised that much of the international EM community was operating in isolation, whether working from home, responding to the needs of researchers working on SARS-CoV-2, or working on experiments that could not be stopped despite the pandemic.
She quickly established the TechEM online seminar series, with the idea of inviting EM specialists to give talks about their real hands-on experience of EM techniques and technologies.The seminars filled a crucial gap in the provision of networking and training for the global EM community, and the seminar series attendance quickly grew, with many of the talks attracting around 100 people.
Through the TechEM seminar series, Erin has made a huge impact on the EM community, creating international networks and transfer of knowledge between different disciplines that has, and will, reach beyond the pandemic.
Christian has made outstanding scientific achievements applying or developing new forms of light microscopy.

Among his many achievements, he has developed a new kind of optical microscopy technique to investigate molecular dynamics of lipids in the plasma membrane of living cells - with unprecedented spatial resolution. He has applied FluorescenceCorrelation-Spectroscopy (FCS) on a super-resolution Stimulated-EmissionDepletion (STED) microscope, which enabled the disclosure of anomalous molecular diffusion dynamics previously hidden by the limited spatial resolution when FCS is employed on conventional diffraction-limited microscopes.
A unique feature of the STED microscope is that its observation spot (and thus spatial resolution) can be tuned by the intensity of the additional STED laser of that microscope. By probing the diffusion characteristics of molecules for different STED laser powers (i.e. different observation spot sizes) it is possible to decipher the diffusion mode of the molecules under study, revealing hindrances with molecular scale resolution. In recent years Christian has significantly improved this STED-FCS approach, now allowing the simultaneous observation of diffusion modes at different spatial positions.This has led him to a series of novel discoveries on the diffusion and interaction characteristics of lipids in the plasma membrane of living cells.
Christian has published many important articles in high-ranked journals (h-index 79) and has appeared as an invited and keynote speaker at multiple international conferences. In addition, his position as the scientific director of the Wolfson Imaging Centre Oxford, has brought him recognition for driving such novel advanced (super-resolution) microscopes into application and making them accessible to lessexperiences users.
Christian’s achievements in applying or developing new forms of light microscopy are outstanding, as are the discoveries he has been able to make as a result this work.
Dr Natalie Reznikov, Assistant Professor, Department of Bioengineering, McGill University, Canada.
Natalie is an extraordinarily vibrant scientist in the area of electron microscopy and X-ray analysis of biological materials, including the use of focused ion beam in conjunction with X-ray tomography, scanning electron microscopy and transmission electron microscopy.
She has made important contributions for the 3D characterisation of biomineralising systems, in particular bone. This has led to several highly cited publications. Her

publication record includes a book chapter and 30 peer-reviewed research papers in high impact journals such as Science, Nature Reviews Materials and Acta Biomaterialia. Since August 2020, she has been Assistant Professor in Bioengineering at the McGill University in Montreal, establishing the use of advanced X-ray and electron imaging for the analysis of biomineralizing systems. Her key contributions include:
• Development of a high-resolution 3D-mapping algorithm for anisotropy and volume fraction of inhomogeneous solids using machine learning for large scale 3D data segmentation.
• Application of Network topology to the quantitative characterisation of trabecular bone leading to a topological blueprint of trabecular bone in the joints or in the spine.
• Identification of the fractal-like structure of bone, resulting in the assembly of bone components into nested, helicoidal patterns which explains the paradoxical combination of the simultaneous stiffness and toughness of bone.
• Discovery of order and chaos in bone, showing that about 25% of the collagen fibrils are haphazardly organized into a chaotic, disordered phase that intercalates with the predominant ordered arrays
• Natalie’s scientific contributions are truly outstanding, as are her contributions to the interdisciplinary character of the microscopy community. She is an exceptionally gifted team player and an effective network builder who strongly contributes to the interdisciplinary character of the microscopy community.
Dr Alice Pyne, Senior Lecturer & UKRI Future Leaders Fellow, Department of Materials Science and Engineering, University of Sheffield, UK
Alice is an exceptional microscopist who has worked closely with industry to develop new atomic force microscopy methods, capable of routinely resolving the DNA double helix on individual molecules.

Alice has been an independent fellow since 2017, firstly at UCL, and at University of Sheffield from 2019. Now a Senior Lecturer, her pioneering studies include unique time-resolved imaging of DNA at sub-molecular scale, showing DNA molecules twisting and ‘dancing’ in ways that had not previously been accessible (Nature Communications, 2021).
She has worked on technological improvements in collaboration with industry (Bruker), and to make them available to the field, in particular on AFM probes for high resolution imaging. Building on her work, major and minor groove resolution on DNA has become a benchmark in the field for resolution. More recently she has pioneered approaches for quantitative and automated analysis of AFM images of single molecules (Methods, 2021).
These efforts are furthered by her commitment to open science and open data. An important feature of this work for the AFM community has been her championing of an international effort to provide quantitative tools for analysis in AFM including
leading the inauguration of a regular RMS conference on ‘Data analysis in AFM’.
Alice is the acknowledged leading light in the field of high-resolution imaging of DNA and DNA protein interactions, and has also been instrumental in steering the community towards a more integrated and collegiate approach to AFM image analysis.”

Anjali is an outstanding, internationally recognised scientist. Since establishing her independent group with an MRC Career development fellowship and an ERC starting grant at the University of Oxford, she has employed state-of-the-art imaging methods to understand age-related changes in multiple organs.
Her cutting-edge tissue imaging approaches have provided novel insights into the vascular regulation of tissue ageing. Anjali has been proactive in sharing her protocols and imaging tools with the scientific community and has also made 3D tissue maps publicly available through freely accessible open resource databases.
As the Scientific Lead for the Wolfson Imaging Centre in Oxford, Anjali has been instrumental in setting up light-sheet imaging, and has also developed new, ultrafast methods for whole-organ imaging and clearing bones.
Her works have gained wide recognition in the field, as evidenced by their extensive citations and the steady stream of invitations which she receives to speak at prestigious scientific conferences.
She has supervised and mentored students and postdoctoral trainees, and is also active in scientific outreach, participating in science festivals and attracting women to STEM subjects.
Anjali’s achievements in applying microscopy to life sciences are outstanding.Through her publications, mentorship and scientific citizenship, she has made an enormous contribution to the scientific community.
Chris is currently the Flow Cytometry Facility Deputy Manager at The Babraham Institute. In this role he demonstrates expertise in cell sorting, experiment design, data analysis, and flow cytometry analysis. Previously he ran an efficient and reliable flow cytometric service at EMBL, Rome, at the Epigenetics and Neurobiology Unit. Here, he provided instrumentation, education, and expertise for all flow cytometry and cell sorting needs.

Chris has made, and continues to make, a significant contribution to the discipline of flow cytometry in terms of his academic output, professional affiliations, teaching activities and freeware-based codes for cytometry analysis.
Prior to working in flow cytometry, Christopher was involved in the RAGULA clinical trial at King’s College London and worked as a microbiologist at GSK and Findus UK. Chris willingly and enthusiastically contributes to the flow cytometry community as an active member of ISAC and is currently a member of the ISAC Emerging Leader programme.
He has established a course to teach R flow cytometry data analysis to nonbioinformaticians, which can be found on the facilities GitHub page. He also sits on workshop forum panels at meetings and conferences and is an active participant in discussions.
Over several years now, Chris has made an immense contribution to flow cytometry. His technical expertise, academic contributions, and role as an educator have been invaluable to his colleagues.
Katherine Paine, PhD student, University of York, UK
Katherine, who began her PhD in 2018, has brought a number of novel approaches in imaging and cytometry to her studies on the regulation of cell surface membrane proteins.
Cell surface membrane proteins perform diverse and critical functions and are spatially and temporally regulated by membrane trafficking pathways. These trafficking pathways are evolutionary conserved from yeast to humans. Based at Chris Macdonald’s lab at the University of York, Katherine has been using yeast as a model organism to study these pathways.
It became clear from Katherine’s initial studies that although standard confocal microscopy could be used to visualise some of the processes she was interested in, there were also limitations. She then helped optimise a suite of imaging and cytometry approaches to study surface proteins. This includes Airyscan2, structured illumination (SIM) and photoactivated localisation microscopy (PALM); all of which can be coupled to bespoke microfluidic exchange systems.
Katherine is also in the process of optimising a high throughput method to measure Förster resonance energy transfer (FRET) in yeast using robotics and flow cytometry.
Not only have Katherine’s approaches allowed her to discover novel mechanisms related to surface protein trafficking in yeast, but these methods can now be used by others in the field in the future.

Pete is best known for his creation and development of the software QuPath. In the crowded scene of bioimage analysis software, QuPath stands out as a fundamental cornerstone. Though initially designed as a tool for quantitative pathology, it is being widely used in many microscopy areas due to its versatility and capabilities to handle large imaging data.
QuPath is special because it is open-source and is also developed to an exceptionally professional standard. It makes the exploration and analysis of extremely large images effortless, boasting a google maps style zoom functionality. It is also highly versatile with many forms of analysis, utilising the best of signal processing, machine learning, and data visualisation techniques. In addition, Pete has written a book on bioimage analysis, and developed many online resources for QuPath.
Pete deserves special praise for his work developing QuPath – which really does stand out as an exceptional piece of analysis software. He has been quietly contributing to bioimage analysis for many years at an exceptional standard without being formally recognised, and is thoroughly deserving of this award.
Robert is an extremely active member of the computational image analysis community. His contributions to the DAIM community are vast, including software development, public engagement through his popular YouTube channel, and an incredible input to the image.sc forum.

In the last few years Robert has brought GPU processing to ImageJ / Fiji with Clij, Clij2 and ClijX plugins. This has brought huge speed improvements to many image analysis workflows. Together with his collaborators, he is expanding GPU processing to multi language support with clEsperanto – providing a common language to bring together programmers from different backgrounds.
Meanwhile Robert’s presence on image.sc is immense. He has replied more than 2,500 times to user posts and is among the ‘most appreciated’ members of the community.
Robert’s you tube channel, developed during the covid pandemic, is also a fantastic community resource. More than 2,000 subscribers have benefitted from his comprehensive and accessible lecture series on Fiji, general Bio Image Analysis, and Clij and Clesperanto.
Robert has become a hugely well-known and respected figure within the image analysis community. Through his achievements as a software developer, and commitment to sharing his knowledge with others, he has made a unique impact.

Celebrating and marking outstanding scientific achievements in any area of microscopy or flow cytometry for established, mid career researchers

(NIST), USA.
Leader,Andrea is an exceptional scientist whose work has contributed to overcoming one of the main shortcomings of atomic force microscopy (AFM) – its inability to conclusively identify chemical species in the nanoscale resolution maps the AFM provides.
He was one of the first scientists to appreciate the potential of combining the infrared spectroscopy – the gold standard for chemical and materials identification – with the nanoscale resolution AFM.
Andrea’s developments in nanoscale microscopy include the photothermal induced resonance (PTIR) and scanning thermal infrared microscopy (STIRM) techniques. Particularly the introduction of ground-breaking optomechanical nanophotonic probes by his group, has allowed increasing the throughput of the chemically sensitive PTIR method by ≈500,000 times and has enabled concurrent measurement of the sample’s thermal conductivity.
The research group he established in the National Institutes of Standards and Technology in 2010, became a world leader in chemically sensitive AFM. It has made large impact across material science and biotechnology, bringing in multiple international collaborations and exploiting the unique capabilities unlocked by the novel microscopy methodology developed by Andrea.
Andrea’s research led to new understanding of the fundamental properties of perovskites solar cells, metal organic frameworks, and plasmonic materials and has identified and greatly reduced nanoscale contaminants hindering the performance of 2D materials heterostructures, among others. He also contributed to the development of new nanoparticle sensors for detection and treatment of cancer, and to the understanding of proteins folding into linear structures linked to diseases such as Alzheimer’s.
Andrea has been prolific in both the development of novel scanning probe methodologies, and in their use to address fundamental scientific and applied questions.
Roland has been a European Light Microscopy Initiative (ELMI) member since 2004, co-founder and member of the Steering Board of German BioImaging - Society for Microscopy and Image Analysis (GerBI-GMB), founder of the Three-Nationale network “MIAP” of Imaging Facilities in the upper Rhine valley, and in his role as head of the Life Imaging Centre in Freiburg, Germany, for 20 years.
Throughout this time, he has established collaborations with other academic groups, Standard bodies (ISO and DIN), and industrial partners to help drive the field towards better Standardisation and Quality Control. He joined in 2015 as a contributing member of the DIN Standards Committee (NA 027-01-04 AA Microscopes) and the International Standard Organization (ISO - 172/SC 5 Microscopes and Endoscopes).

Roland was instrumental in bringing together many of these contacts upon the release of the Confocal ISO 21073:2019 Standard in an effort to define standard methodologies for reporting these Quality Control parameters. This led to the establishment of QUAREP-LiMi (Quality Assessment and Reproducibility for Instruments & Images in Light Microscopy) in 2020, which Roland runs and has enlarged to become a global initiative to achieve a community-driven, and agreed, methodology for maintaining light microscopes.
Although still in its infancy, QUAREP has garnered more than 450 members worldwide and has published two important White Papers outlining the need for Quality Control in Light Microscopy, as well as being near to finalising several Working Group outputs aimed at defining methodologies for capturing QC data.
This work has been instrumental in improving public and scientific confidence in published light microscopy data through improved reproducibility and consistency.
Lothar has an outstanding track record of combining advanced optical imaging method development with biology application, particularly for the study of spatiotemporal genome organisation.
As a postdoctoral researcher and co-investigator, he pioneered the biological application of super-resolution three-dimensional structured illumination microscopy (3D-SIM) and led the development of novel optical imaging methods to interrogate the mammalian nucleus. This was a major development, as in 2008 other superresolution methods such as STED and SMLM were still two-colour and 2D only, while Lothar’s work pushed SIM to three colours in 3D.

He is actively involved in the development of super-resolution labelling tools, software for imaging data quality assessment (SIMcheck), and protocols for the best practices in super-resolution imaging.
Lothar has also been focussing on exploring principles of higher-order chromatin organisation and the underlying interplay of biophysical forces and epigenetic mechanisms that regulate genome gene expression, DNA replication and repair in mammalian cells.
Since 2011, Lothar has been leading a research team as part of the Micron Oxford Advanced Bioimaging Unit (www.micronoxford.com), funded by a Wellcome Trust Strategic Award to provide bespoke optical imaging solutions and access to high-end microscopy equipment for the Oxford research community.
He has also been responsible for the conceptualisation and running of practical courses, lectures, and seminars for undergraduate students in various formats.

Hari has been phenomenally successful in devising methods that revolutionise our ability to interrogate living systems by circumventing difficulties in optical microscopy relating to phototoxicity, penetration depth, and spatiotemporal resolution.
His inventions have been published in Nature, eLife, Nature Methods, Nature Biotechnology, PNAS, etc. and commercialised by a number of manufacturers.
Hari has also created novel computational methods to “untwist” images of embryos, enabling detailed analyses of their complex dynamics and accelerating his work to create the first 4D atlas of animal neurodevelopment. He has developed computational methods aimed at combating the ‘data deluge’ that accompanies high speed imaging, alleviating the bottleneck from image acquisition to biological insight. His ongoing research seeks to further enhance the extraction of information from limited fluorescence budgets by incorporating artificial intelligence, with his most recent methods merging multi-view imaging, super-resolution imaging, and deep learning to significantly outperform standard confocal imaging techniques.
Finally, Hari prioritises broad dissemination and application of his methods to outstanding biological problems. Among the many examples of this, his methods have elucidated the organisation of proteins within the bacterial spore coat; demonstrated that Drosophila germ granules are composed of homotypic mRNA clusters; and dissected the mechanism of cortical granule trafficking for post-fertilisation exocytosis.

In his current role, Wim administers a pool of eight transmission electron microscopes, training users, trouble-shooting equipment and running EM projects for visitors.
He has set up extensive software automation for high-throughput, high-quality electron microscopy data acquisition, and developed dose symmetric tilt scheme for high-resolution sub-tomogram averaging. Wim has also co-authored several high-impact scientific publications in Nature, Science and Cell.
Wim has had a huge impact on the field of cryo-electron tomography and cryoEM more generally, using his engineering background to design and implement Cryoelectron tomography workflows which have been widely adopted in the field and used globally.
Wim pioneered the use of dose symmetric acquisition schemes, colloquially and commonly known as ‘Hagen scheme’. These data acquisition schemes are the foundation for a growth in subtomogram averaging projects, as well as an improvement in their resolution.
More broadly, Wim is a well-known cryoEM facility manager who has driven and supported a wide range of methods development work which has benefitted the cryoEM community, including detector benchmarking and single particle cryoEM acquisition schemes.

During his career, Ardan has been working in different fields of microscopy and over time he has been at the forefront of demonstrating the importance of data in microscopy.
Ardan has made huge strides in the promotion and development of tools for the curation and public availability of microscopy data. He has been working at EMBLEBI since 2011 and there, he has been one of the driving forces behind a number of microscopy databases including EMPIAR, an invaluable tool for the structural and cellular biology fields.
EMPIAR has been instrumental in supporting software and general methods development, in enabling validation of cryo-EM structures, and in providing data for community challenges, teaching, training and software and data-processing tutorials. The scope of EMPIAR has gradually expanded and now incorporates archiving support for various kinds of volume EM and X-ray imaging data.
Ardan is also one of the four people who, in 2019, started the volume EM Community Initiative in the UK that has now grown into a vibrant international community effort.
He has further played a role in the early stages of the CCP-EM project and was one of the thought-leaders behind the establishment of the BioImage Archive at EMBL-EBI.
Ardan has been one of the driving forces in demonstrating the importance and power of data in the field of electron microscopy. He has not only done this through his scientific publications but importantly, by bringing together different imaging communities through his high levels of engagement.
Celebrating a substantial contribution to the field of education, or to outreach and public engagement, over the course of someone’s career.
Dr Elisabeth Bik, Science Consultant, Harbers Bik LLC
Through her work in identifying and querying images with errors published in research papers, Elisabeth has literally made thousands of significant and important contributions to the field of education in microscopy. Moreover, she has taken a unique approach to communicating her findings. While other ‘image sleuths’ report their work directly to either journals or institutions, Elisabeth has developed a vast, worldwide online profile (with over 131k followers on Twitter alone), and she reports her findings virtually every day on Twitter and other online forums.
Readers are invited to identify and discuss areas of an image that may have been manipulated in an unclear or potentially nefarious manner. This engages both the lay and scientific communities to spot regions that have been altered in a way that changes the research conclusions.
She awards an ‘emoji trophy’ for those who are first to spot some or all of the manipulations; this electronic reward is now a much-coveted badge of honour for microscopists using Twitter.
The educational value of this structured public assessment of images cannot be underestimated. It teaches researchers at all career stages how to spot duplications and manipulations, and invites and educates all microscopists to think critically about published image data.
Elisabeth has reported more than 6,200 papers for issues with image duplication or other concerns and, at the time of writing, her discoveries have led to 905 retractions, 122 expressions of concern, and 955 corrections/errata. The work and social media reach of Dr Bik means that suspect images can be discussed in a public forum, and empowers readers to decide for themselves whether or not to trust data.
In 2019 Elisabeth founded a blog series dedicated to research integrity and misconduct (Science Integrity Digest) and has made over 5,000 posts on PubPeer. The same year, she committed to pursuing image integrity work full-time, free of charge for at least 12 months.
Such is the demand for her work, Elisabeth has continued beyond this initial year, even though she has faced harassment for reporting images with obvious data manipulation. Her tireless work has inspired others to publicly post their image misconduct findings and encouraged researchers to improve peer-review practices.
Elisabeth is truly dedicated to ensuring good practice and standards relating to microscopy and communication of image data. Her work is of relevance and interest to anyone who acquires digital images with a microscope of any kind.
Established in 1982 to honour the work of Professor AGE Pearse. A prestigious award recognising significant contributions to histochemistry and life sciences either through the development of a new technique or through the application of existing methods.

Michael has had a long and illustrious career in mechanobiological research. He obtained his PhD while working in the laboratory of Sunney Chan at Caltech using NMR to probe membrane composition. He then joined the laboratory of Jonathan Singer where he made important discoveries on the bilayer couple’s role in shaping cells and interactions between the cytoskeleton and the cell membrane, particularly linking actomyosins to spectrin.
Michael started his research group at University of Connecticut in Farmington before moving to Washington University School of Medicine in St Louis. From 1990 to 2000 he was Chair of Cell Biology at Duke University Medical School, and then became William Keenan Professor of Cell Biology at Columbia University, where he headed a program in nanomedicine.
While working in this role, Michael founded the Mechanobiology Institute at the National University of Singapore where he was director for 10 years until 2019. He is currently Robert A Welch Distinguished University Chair in Chemistry at University of Texas Medical Branch in Galveston.

Michael is particularly renowned for pioneering in vitro motility assays for myosin which led to the discovery of kinesin, for which he, together with James Spudich and Ronald Vale, were awarded the Lasker Prize in 2012. The movies of microtubule movement driven by the motor proteins dynein and kinesin are a fundamental part of any cell biology course.
These landmark achievements led to understanding of the mechanisms by which cells move their subcellular components and generate force to facilitate biological processes including muscle contraction, heartbeat, cell migration in development, cell division, and orderly delivery of molecular components to the necessary parts of each cell. Latterly his research has demonstrated that the rigidity of the cell matrix dramatically affects matrix cytoskeleton linkages. He has also identified a rigidity sensor found to act as a tumour suppressor in cancer cells. It is his interest in the mechanical forces generated by the motors that has highlighted the importance of mechanical parameters in biology, i.e. Mechanobiology, a field in which he has been a seminal leader.
Michael has received multiple awards and, since 2013, has appeared in the list of 20 most influential medical researchers alive today. He has 357 publications, has been cited more than 66,000 times, and is senior author on 11 Cell Papers, 10 Nature Papers and six Science Papers.With his most recent paper in Science Advances 2022, Michael’s work continues to lead fundamental understanding of motor functions at the nanometer level to the benefit of human health.
Submit your abstract for an oral
presentation at mmc2023 incorporating EMAG 2023 and join an impressive line-up of speakers to highlight and demonstrate the very best in microscopy techniques and applications
The Microscience Microscopy Congress (mmc) is back with a bang in 2023! One of the biggest events of its kind in Europe, mmc2023 incorporating EMAG 2023 will bring you the very best in microscopy, imaging and cytometry from across the globe. With six parallel conference sessions, a world-class exhibition, workshops, satellite meetings, an international Imaging Competition and more, it’s simply the place to be for anyone who uses a microscope for work, study or pleasure.
Back at the superb Manchester Central conference centre for the first time since 2019, the event will be taking place fully in person, providing the perfect opportunity for the scientific community to come together, make connections and share their research. As always, many of the leading companies in microscopy and imaging will be on hand to demonstrate the very latest equipment and technology at the exhibition.
We welcome abstract submissions from all areas of microscopy, cytometry and imaging, and from people at any stage in their career.

For more information about conference sessions at mmc2023, including session descriptions, keywords, audience and invited speakers go to www.mmc-series.org.uk/conference-sessions
• AFM in Life Sciences
• Nanoscale Probing of Physical Properties via AFM & SPM
• Nanoscale Science of Materials for Energy Storage and Generation
• Atomic and Molecular Resolution Phenomena via AFM, STM and Scanning Probes

• Multimodal Microscopy
• Advances in label-free Imaging
• Optical Imaging of Fast Dynamic Processes
• Using FLIM and FCS to Determine Interactions and Dynamics
• Spatial and Imaging Cytometry
• Artificial Intelligence
• FIB Applications & EM Sample Prep Techniques in Biological Sciences
• Public Health: the Impact of Microscopy
• Microscopy to Modelling
• Sustainability and Carbon Net Zero
• FIB Applications & EM Sample Prep Techniques in Physical Sciences
• Microbial Imaging
• Clinical Diagnostic Imaging
• Imaging Biomechanics
• Reproducibility of Data Analysis at Scale
• Mass Spectrometry Imaging Across Length Scales in Life and Physical Sciences - Providing Atomic and Molecular Information
• Archaeology
• Cryo-Electron Microscopy
• Correlative and Multimodal X-ray Microscopy
• Multiscale and Correlative Microscopy Approaches to Microanalysis and Spectroscopy
• QUAREP-LiMi: an International Collaboration for Microscope Quality Control
Instrumentation and Quantitative
• Advanced EM, electron crystallography and diffraction studies
• 4D-STEM, phase-sensitive, ptychography and Lorentz techniques
• Advances in (S)TEM instrumentation and techniques
• Advances in SEM (CL, EBSD, spectroscopy, STEM, etc)
• Spectroscopy and spectrum imaging
• Correlative microscopy
• Tomography and three-dimensional electron imaging
• In situ and dynamic microscopy techniques and development
• Low Dose/Low Voltage Imaging and analysis
• Advances in cryogenic TEM and cryogenic sample preparation
• Focused Ion Beam techniques and advances (including FEBID and sample preparation)
• Detector/Camera Technologies and New Instrumentation
• Automated Control, Advanced Data Processing and Modelling Techniques
• ‘Smart’ collection, deep/machine learning and advanced processing of large datasets Materials
• Structural materials, composites and metallurgy

• Functional semiconductor, oxide and advanced materials
• Natural materials, geological materials and mineralogy
• Catalytic materials and supports
• Low-dimensional materials and nanomaterials
• Energy materials (storage, generation, efficiency, etc)
• Biological Materials, biomaterials, soft matter, polymers and composites

• Surface imaging and modification
• Microscopy of thin films, interfaces and heterostructures
• High resolution chemical and structural analysis

July 31st - August 4th, 2022
In August 2022 the first in-person Microscopy and Microanalysis conference was held since the pandemic hit in 2020. Organised by the Microscopy Society of America (MSA) and Microanalysis Society (MAS), the 2022 conference was hosted in the extraordinarily designed Oregon Convention Centre in Portland. A truly interdisciplinary meeting, the conference was a wonderful opportunity for researchers to present, discuss and learn about all the new and exciting research in the different areas of microscopy and their applications.

With its abundance of local breweries and countless restaurants with a variety of international cuisine, Portland proved to be a well-suited city for the range of social events organised throughout the week. The city itself was beautifully surrounded by lush trees and I received a stunning view of Mount Hood as I flew in. The conference of course had some adjustments in comparison to a prepandemic world. This included a vaccination check on arrival and mask-wearing throughout different activities. These however added a sense of security to the week and over all made it a more enjoyable experience.
The conference was kicked off by two interesting plenary talks. James Rea’s interactive talk from the Alan Alda Center for Communicating Science
proved to be quite relevant to today’s time with the title ‘Science is complex. It shouldn’t be exclusive.’ While Dr. Wendy Garrett from Harvard T.H. Chan School of Public Health’s talk was extremely thought provoking and engaging. This initial session also included an award ceremony where I received the M&M 2022 Eric Samuel Memorial Scholarship Award. I was delighted to have received an award for my upcoming presentation, and it was exciting to have gained this recognition of my work.
Each day included an abundance of presentations and a poster session from the different symposia which comprised of analytical, biological and physical sciences. The cross-cutting symposia was a new addition to this year’s conference. It proved to be a great success including many fascinating talks not limited to discussing microscopy infrastructures, artificial intelligence, and automation. One talk I found particularly interesting was from Clemens Mangler (University of Vienna) who presented work on a novel UHV transfer system.
I was lucky enough to be selected to present both a poster and talk which coincided on the last day of the conference. My poster was entitled ‘Benchmarking the Performance of a New Photoelectron Source’. The poster session was a great opportunity to have productive and in-depth conversations about my research. My talk ‘Retrofitting a Photoelectron Source: Improving
Resolution & Functionality’ took place in the afternoon. I discussed the latest results from our prototype photoelectron source in the context of potentially improving low voltage imaging. I was asked several interesting questions on my research and was delighted with the reception the talk received.
Throughout the week there were many social events to further get to know other researchers. The president’s reception in the Portland art gallery was a particular highlight. There was also the MSA organised meal with a mentor and a ‘women in microscopy’ breakfast hosted by Thermofisher. It included a brilliant talk by Dr. Karren More (Oak Ridge National Laboratory) about her career journey and some advice from her experiences. It was really encouraging to see such a diversity in researchers at this year’s conference.

I would like to sincerely thank the Royal Microscopical Society, MSA, MAS, the Trinity Trust, the Royal Society and Trinity College Dublin for their travel grants which allowed me to attend this conference.
Frances Quigley School of Physics, Trinity College Dublin, Ireland
Microscopy experts are well versed in the importance of carefully matching the fluorophore selection, optical filters and light source for fluorescence microscopy experiments. However, the advancement of LED illumination technology for widefield fluorescence has introduced new and sometimes surprising opportunities for high-speed, high-contrast multilabel imaging. Below we cover several options which range in complexity, cost and performance – and also provide advice to keep in mind when using optical filters with LED illumination.
Imaging a single fluorophore is relatively simple, requiring a single filter cube housed in the microscope. But when it comes to capturing multiple fluorophores in a single experiment, choosing an approach requires extra consideration to balance speed, contrast and budget.
Single-band filters – Multiple single-band filter cubes can be housed in a motorised microscope filter cube turret, which is ideal for high-contrast imaging since each cube contains filter sets specific to each fluorophore. However, switching between cubes is slow, limiting dynamic observations of multi-labelled samples to the order of seconds.
Multi-band filters – If temporal resolution takes priority over contrast, using multi-band filters enables simultaneous imaging of multiple fluorophores by selectively transmitting or blocking multiple wavelengths. The drawback here is that full multi-band sets can lead to reduced contrast from
Single-band filters
Single-band filters.
Multi-band filters.
spectral bleed-through (also known as crosstalk). Improving contrast through reduced bleed-through is possible by combining single-band and multi-band filters in Pinkel and Sedat configurations – and a modern twist on these setups can reach impressive levels of speed and contrast.
Pinkel filters.
Pinkel filters – This configuration combines single-band excitation filters with a multi-band dichroic mirror and multi-band emission filter. Instead of housing the single-band filters in a highspeed filter wheel, LED illumination systems which allow the single-band filters to be housed in the light source itself (in front of each LED), provide an even faster and efficient alternative. Here, speed is now determined by LED channel switching, which can be under 7 µs. Taking this one step further, a manual microscope can be transformed into a costeffective automated imaging system by employing ‘sequence runner’ functionality, which makes use of a single TTL output available on many cameras, and cycles through user-defined illumination sequences. While this setup is ideal in terms of speed and budget, the presence of a multi-band emission filter can risk some bleed-through and a reduction in contrast due to the broad spectral nature of fluorophore emissions.
Sedat filters – To avoid the risk of bleedthrough, a Sedat filter configuration uses a similar setup to the Pinkel, but maximises contrast by using single-band emission filters. To avoid filter wheels in the emission path, another clever trick is to incorporate an image splitter into the emission light path. These split the emission light path into up to four images displayed on different areas of the camera chip (or different camera in the case of a camera splitter), and house the single-band emission filters. While this setup raises the price point compared to the Pinkel configuration, it combines the high contrast of a Sedat filter set with <7 µs LED switching speeds.


Check your filters when upgrading: Historically, filters were designed around the irradiance peaks of mercury and metal halide lamps. When used with LED illumination systems, the
poor matching of excitation filters can reduce the irradiance of excitation light and therefore signal, which can compromise image quality. Upgrading a light source requires a rethink of optical filters, and LED-compatible filters are now available that match the spectra of LEDs and popular fluorophores.
LEDs require excitation filters: Even when exciting a fluorophore using only the corresponding LED, each LED spectrum has a ‘tail’ which can contribute to background noise if not blocked using an excitation filter.
Utilise individual LEDs to enhance contrast: No optical filter provides a complete block to unwanted light. White light illumination will therefore result in higher background (and therefore lower contrast) when compared to utilising LED illumination technology featuring individual channel control.
LEDs differ from lasers: Each LED has a broader spectrum compared to a laser, and unlike lasers can be tailored using optical filters to excite a broader range of fluorophores.

Make use of online tools: Analysing spectral profiles to optimise a setup can be complex and time-consuming, but online tools can help simplify
the task, or avoid it entirely. For example, FPbase provides clear and side-by-side visualisation of fluorophores, filters, light sources and detectors.1

Filter manufacturers also have their own visualisation tools, and the CoolLED Filter Finder Tool bypasses all analysis to provide a simple list of filters optimised for different LED wavelengths.
For specific optical filter requirements: Filter manufacturers do not list all optical filters on their websites, and are likely to have a filter set to suit your individual requirements if you contact them to ask.

LED illumination technology is progressing all the time, and the available control options are now a world away from traditional lamps. Investing the time to ensure all components in the light path work together to achieve the best performance is crucial, but isn’t always simple – so please contact us at CoolLED if you have any questions or would like further advice.
Lambert, TJ (2019) FPbase: a community-editable fluorescent protein database. Nature Methods. 16, 277–278. doi: 10.1038/s41592-019-0352-8











The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months.We hope they enjoy a long and rewarding membership with the RMS.
Dr Martin Salter
Mr Gaurav Chand
Mr Cheng Zhao
Mr Christopher Bearcroft
Miss Shravani Maral
Miss Kay Polland
Dr Padmaja Parameswaran Nampi/Padmaja Vasudev
Dr Anna Bajur George Stonadge Dr Raif YuecelIf you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk
View p94 for the latest information on membership rates and benefits.
Tell Us About You?
I am a predoctoral researcher in the Department of Microbiology at the University of Granada. My area is within environmental microbiology, specifically in the study of microbial interactions with heavy metals such as selenium. My main objective is to discover the molecular mechanisms of selenium detoxification by the strain Stenotrophomonas bentonitica BII-R7.

Why did you become a member of the RMS?
To keep myself informed of all the possible courses, congresses and
benefits of the magazines that it offers. In this way, I would like to evolve in my scientific career and continue training more and more.
The RMS provides a wide range of possibilities in the scientific field, from training and improvement courses in scientific techniques to the possibility of certain advantages in some journals of interest for research growth.

Name
Ruhila S.
Tell Us About You?
I’m a third year undergraduate student in an integrated BS-MS programme I have a keen and abiding interest in genetics from both a wet-lab and theoretical standpoint. Microscopy is of course a cornerstone of the experimental methods of our field, and the theoretical principles are a rich vein of never-ending wonder to me as well.
Why did you become a member of the RMS?
The RMS as a society is the perfect melting pot for my interests, where I expect to find my place in academia and grow as a researcher and an academic. I intend to focus on practical, possibly industrial contexts for human genetics,

membership seemed perfect to me.
How do you feel being an RMS member benefits you?

I believe that membership opens a wide range of career development opportunities. I also hope to be awarded an internship studentship to further my career in a concrete manner. This is apart from other tangible benefits including being a part of such a vibrant community and keeping up with the latest research.
Tell Us About You?
I received my MSc from Norwegian University of Science and Technology in September 2022, and am currently working towards a PhD in the Ultramicroscopy group at Trinity College Dublin.
Because I work with transmission electron microscopy. I work on developing a method for ultra low-dose STEM-DPC imaging with the aim of being able to image and characterise electron beam-sensitive materials in the TEM.
A breathtaking display with a microscopical twist captured the imagination of shoppers in Bristol (UK) this summer.

‘Overstory’, conceived by contemporary artists Ivan Morison and Heather Peak, consisted of a pair of pleated structures suspended over a Bristol street, giving passers-by a view into the microscopic world of trees.
The installations created a geometric, zig-zag profile in the sky, with brightly coloured micrographs depicting the cellular systems of an Oak and Lime tree. Both species were selected to represent prominent, real-life trees growing in Bristol, each in some way encapsulating the city’s history. The aim was to highlight the importance of urban tree cover and encourage the public to discover the diversity of native trees around Bristol’s local parks, wildlife reserves and older neighbourhoods.
The lime tree specimen (main image, previous page) was captured by RMS Early Career Committee Chair, Dr Liam Rooney, using the Mesolens - a giant microscope designed for high-resolution imaging
of large specimens, based at The University of Strathclyde’s Mesolab.

Liam said: “This Tilia specimen demonstrates the complex organisation of cells arranged into specialised layers of tissues. Its tiny holes are sections of long tubes that transport nutrients and water along the stem of the plant”.
Mesolab founder and Chair of the RMS Light Microscopy Section, Professor Gail McConnell added: “We are using the Mesolens to image many different kinds of biological specimens including plants, bacteria, and fungi; the bright features of the images are from naturally occurring molecules present in the tissues”.
The digital image of the oak (featured in page opposite) was produced by Alicia Musson, Collections Assistant at the Royal Botanic Gardens, Kew. The cross section of wood was imaged with brightfield illumination using a ZEISS Axio Scan Z1 microscope.

Co-creator of the artwork, Ivan Morison said: “The concept was to take two microscopic images and blow them up so that you have them above people’s heads, acting like a canopy. So we’re underneath, looking up at something that in reality is hundreds of times bigger than it should be. And it starts to translate into something quite colourful and playful.
“Trees are an amazing way to corridor a city or a place – just to move through it and think about the history of the place and what it stands for, and Bristol certainly has that. It has some incredibly old trees.”
The fabric design combined the vibrant organic shapes of the microscopic imagery with sharp, repeating folded lines, delivering a constantly changing view from beneath. Each structure was suspended between buildings either side of the street using a system of cables.

Ivan said: “Although the structure looked very geometric, it actually mirrored a lot of the structural characteristics of natural forms. With something like this, you don’t really know what they are going to look like until they’re up. There was a bit more
movement and they were far more alive than we thought they would be.There was a bit more flutter to them than we had imagined, which gave them a gentleness – a bit like a butterfly.”
The artwork remained suspended for 12 days in late August, becoming an attractive talking point for shoppers and other passers-by. Throughout the exhibition, representatives of the National History Consortium, which commissioned the work, were on hand to discuss its activities with the public –including ongoing work to increase the amount of tree cover in Bristol.
Ivan said: “The message to people was that there is such a wealth of trees in the city, and so much to be got from them, if you take more notice of them day-to-day.”
OVERSTORY was produced by PONY and commissioned with The Natural History Consortium The project was delivered as one of the activities under the City Centre and High Streets Recovery and Renewal programme, funded by Bristol City Council and the West of England Combined Authority’s Love Our High Streets project.



Aserene mid-September day in Oxford marked the beginning of the 9th Abercrombie meeting, hosted by the Royal Microscopical Society (RMS). This renowned gathering – named after the pioneering cell biologist Michael Abercrombie – has been held every five years since 1979, providing a space for the scientific community to engage
with the most recent developments in cell motility. Having started my PhD in 2020 during a global pandemic, my doctoral experience has thus far been anything but conventional. I was therefore looking forward to meeting with fellow scientists in person, and was grateful to be given the opportunity to present a 90-second flash talk and accompanying poster for the first time.
Over the two-day meeting, talks were given that fell under three main categories: Evolution, Mechanisms and Mechanics, and Development, Physiology and Disease. Laura Machesky delivered the opening keynote, discussing the role of CYRI proteins in cellular decision-making between nutrient uptake and migration, and its possible implication in cancer progression. Many of the talks provided enthralling insights into unfamiliar territory for me, including Raymond Goldstein’s talk on the driving forces for evolutionary transition to multicellularity and Daniel Cohen’s multi-disciplinary work on understanding and controlling collective cell migration. My favourite talk of the meeting was given by Danijela Matic Vignjevic, discussing how cancer associated fibroblasts (CAFs) actively modulate mechano-transduction. Using capsular cell technology, her group beautifully demonstrated that both confinement of cancer cells by CAFs and pressure build-up from proliferation triggers encapsulation, and important considerations for its possible effect on cancer therapy were raised.
Flash talks were interspersed between talks, followed by poster sessions in the evenings. With around 60 delegates and 35 posters, the poster sessions provided an intimate environment that nurtured active participation and discussion amongst all present. One particularly memorable interactive
poster included historic videos of phase contrast microscopy and paid homage to its history and significance within the biological science community. Another displayed stunning live cell imaging of microtubule dynamics in migrating amoeboid cells. I presented a poster titled “Biophysical regulation of EGFR signalling in tumour cells” and was pleased to have many insightful discussions about my work with fellow students, senior academics and sponsor representatives. A few discussions in particular provided invaluable feedback that helped me to shape future experiments on return to the lab.
I extend my gratitude to the RMS for providing me with a travel grant that enabled me to attend. I would also like to thank Ewa Paluch, Gaudenz Danuser and Brian Stramer for their efforts in organising this meeting – a most memorable and engaging event that has no doubt inspired the newest cohort of cell motility enthusiasts.
Aneesa ShaikhRandall Centre for Cell and Molecular Biophysics

King’s College London


As we approach the end of 2022, the RMS can reflect on a highly eventful year in which we made a very welcome return to in-person meetings, and also launched some exciting new initiatives aimed at
supporting RMS members and the wider microscopy community.
The few months since our last issue of infocus have been particularly busy, with a number of regular RMS courses and meetings taking place. These included the York-based Flow Cytometry Course 2022, and Abercrombie Meeting 2022, both of which occurred in September. The latter event, held in Oxford, was the latest instalment in a series of meetings held since the death in 1979 of Michael
I hope the month of December finds you well, and that you are looking forward to the festive season and the promise of exciting new beginnings in 2023.
Abercrombie - a pioneer in the field of investigating cell behaviour using timelapse microscopy.
It was a pleasure to see so many faces – both in person and those joining us online – at our Annual General Meeting, which was held in late September as part of Microscopy: Advances, Innovation, Impact 2022. We enjoyed some fantastic, live-streamed talks throughout the day and took the opportunity to catch up with many friends and colleagues. To coincide with this event, we also announced the winners of the 2022 RMS Awards. Covering all aspects of microscopy, cytometry and imaging, these awards recognise the achievements of some of the leading figures within our community. On a personal note, it was my great pleasure to present, in person, several of these awards to some of this year’s and last year’s winners, as well as finally honouring our 2020 Honorary Fellows Professor David B. Williams (The Ohio State University) and Professor C. Barry Carter (University of Connecticut/Sandia National Laboratory), and our 2022 Honorary Fellow Professor Edward D. Boyes (University of York).You can read more about all our latest awards on p***.
In October, we were delighted to support our partners Canada BioImaging (CBI) and BioImaging North America (BINA) in hosting the Expansion Microscopy User Group Meeting. This is a very important online forum that enables researchers to share their experiences and facilitate rapid uptake of this emerging technology. Indeed, the benefits of hosting virtual events – in terms of increased accessibility and inclusivity – are now well known, and as such, we will continue to deliver online and hybrid events where appropriate.
Among our ongoing online offerings, the International Microscopy Lecture Series is back with a bang, with Emeritus CNRS Research Director, Professor Christian Colliex due to give a talk this month. Further instalments are planned for 2023, as we continue to collaborate with our partners; The Microscopical Society of Canada, The Israel Society for Microscopy, and the Microscopy and Microanalysis Society of Brazil.
The series also continues to be supported by the International Federation of Societies for Microscopy (IFSM). Meanwhile, the highly successful Imaging ONEWORLD series of talks continues to attract online audiences on Monday afternoons. This RMShosted series has now been running for more than two years, having been initially established as a means of promoting science communication during Covid.
As I mentioned in my opening remarks, 2022 has been a year in which the RMS has taken forward a number of exciting new initiatives. Our previous issue brought news of how the Society became proud signatories of the Technicians Commitment, and also launched a pilot mentoring scheme to support RMS members at all career stages.
Following on from these projects, and as part of the Society’s commitment to being a welcoming and inclusive organisation, we have also now launched our Equality, Diversity, Inclusivity and Accessibility (EDI&A) Policy. This stems from a piece of work carried out by our recently-formed EDI&A Group, which was set up to examine the RMS’s existing policies and to review all its activities. A working document is now available on our website, and sets out how the RMS is seeking to improve accessiblity, inclusivity and diversity in all its activities. We recognise that there is still much work to do, and we welcome discussion and scrutiny of all our policies and actions. Find out more and read the working document here: https://www.rms.org.uk/ community/edi-a-group.html
Looking ahead to next year, I would be remiss if I failed to point out that we will be returning once again to Manchester Central for mmc2023! This will be our first in-person mmc since 2019, and I can already sense the excitement and anticipation building. I hope as many of you as possible will be able to join us for what promises to be a truly memorable and top-tier scientific event.
Wishing you a wonderful Christmas and a Happy New Year filled with peace and joy!
Professor Grace Burke, RMS PresidentThe RMS Annual General Meetings took place in September as part of the fifth one-day meeting in the Microscopy: Advances, Innovation, Impact series. The event was held at the Royal Society of Chemistry’s Burlington House in London, with around 70 attendees, and more than 40 others watching a live-stream of the event online.
As well as all the updates from the General AGM and a number of Section AGMs, the day featured a series of well-received short talks, and the presentation of several RMS awards, from both this year and those announced in previous years. These included


Honorary Fellowships presented to Professors Barry Carter, David Williams and Ed Boyes. Professor Boyes also delivered a talk titled ‘Maverick Microscopy: Past, Present and Future’. The event was rounded off with a drinks reception, enabling friends and colleagues to catch up, in many cases, for the first time since the onset of the Covid pandemic.
The RMS AGMs are open to all members and nonmembers alike, providing an important opportunity to reflect on the year and plan for the future. The live-streaming of this year’s event was part of the Society’s ongoing efforts to be as open, accessible and inclusive as possible in its activities.







 Georgina Fletcher, BioImagingUK Project Officer and UK Node Manager
Georgina Fletcher, BioImagingUK Project Officer and UK Node Manager
Just as infocus was going to press, the UK and wider European bioimaging community received some exciting news that the UK are confirmed as hosts to a EuroBioImaging Node! EuroBioImaging is a European Research Infrastructure Consortium that offers open access to state-of-the-art bioimaging techniques. Operating as a legal entity since 2019, there are now 17 countries, 35 Nodes and 173 facilities that make up this landmark research infrastructure. The UK, along with Slovenia, are the latest countries to offer access to users from across the world.

The distributed UK Node has sites at King’s College London (which also acts as administrative host), The Francis Crick Institute, Edinburgh Super Resolution Imaging Consortium, OCTOPUS - Harwell Research Complex, Oxford Brookes, York, and Liverpool Universities.These seven sites will make a wide range of advanced technologies available to the community, including: correlative, multimodal, intravital, highcontent and super-resolution imaging platforms. Many of the instruments are bespoke and/or require specific support for acquisition or analysis pipelines, available from the local technical support teams within these world-class research environments.
The technologies on offer at the UK Node can be applied to fundamental and translational research projects to enable imaging at molecular to cellular resolutions in single cells through to 3D in vitro
Confocal image from cells showing mitochondria labelled with mitotracker dye (blue-green) and epidermal growth factor receptor (EGFR) labelled with Alexa 647N (red). The lower image shows an overlay of a MINFLUX image of EGFR with the confocal mitochondria image. For resolution comparison, a confocal EGFR image is also shown. Courtesy of Chris Tynan, CLF Octopus Facility.

models and whole organisms.Support will be provided for the entire experimental pipeline including (where appropriate) initial user consultation, experimental planning, full hands-on training, assistance with sample preparation, gathering the highest quality imaging data and data analysis.
Any researcher from academia or industry can apply
to access the services at these sites whenever they have a project requiring these imaging technologies and expertise and do not have the relevant support to perform the experiments at their home institute. The application process involves an initial consultation between the prospective user and the Node site or Euro-BioImaging team, followed by completion of a request form, scientific and technical checks by the relevant site and granting of access once approved.
Euro-BioImaging is a publicly funded, non-profit research infrastructure. Hence, the costs to access Euro-BioImaging Nodes are kept to a minimum, with the sole aim of charging users only to support the effective running of the infrastructure. To access Euro-BioImaging technologies, users may also need some financial support for travel and accommodation expenses. A number of funding opportunities exist to help with this, and further
details can be found on the website link (below).
The UK’s successful application to Euro-BioImaging was overseen by UKRI and BioImagingUK, the community network that is supported by funding from UKRI-BBSRC in partnership with the RMS. BioimagingUK Lead, Professor Maddy Parsons said: “We are very grateful for the support of all partners in driving this exciting initiative forward. Being part of this important international infrastructure will enable broader impact to be delivered from our world-leading bioimaging facilities to the community and encourage greater collaboration and training in this rapidly growing area. We are really looking forward to facilitating excellent new scientific discoveries at our new UK Node sites and working closely with Euro-BioImaging and UKRI to potentially expand our offering in future”.
Please visit www.eurobioimaging.eu for more details and how to apply.
Join an impressive line-up of speakers at mmc2023 incorporating EMAG 2023
Abstract submission for oral and poster presentations is officially open for Microscience Microscopy Congress 2023 (incorporating EMAG 2023).
One of the biggest events of its kind in Europe, mmc2023 brings the very best in microscopy, imaging and cytometry from across the globe.

As anticipation starts to build ahead of the RMS’s flagship event, now is the time to join our impressive line-up of speakers covering all the latest techniques and applications in microscopy.
With six parallel conference sessions, a worldclass exhibition, workshops, satellite meetings, an international Imaging Competition and more, it’s simply the place to be for anyone who uses a microscope for work, study or pleasure.
Back at the superb Manchester Central conference centre for the first time since 2019, the event will be taking place fully in person, providing the perfect opportunity for the scientific community to come together, make connections and share their research.
As always, many of the leading companies in microscopy and imaging will be on hand to demonstrate the very latest equipment and technology at the exhibition.
Find out more and submit your abstract here: https://www.mmc-series.org.uk/

A Working Document setting out how the RMS is striving to become a more inclusive Society is now available to download at https://www.rms.org.uk/ community/edi-a-group.html
This has been produced by a working group recently set up to establish an Equity, Diversity, Inclusion & Accessibility (EDI&A) policy for the Society, and to review all its activities.
The RMS is committed to being a welcoming, inclusive Society and encourages diversity across all activities and in the membership of its committees and groups.
The Society welcomes discussion and scrutiny of its proposals and aims to be transparent and open in all its policies and actions. Suggestions can be made on the EDI&A webpage, and questions from the community are also welcome.
If you are interested in writing a book on an aspect of microscopy or its applications, the RMS Wiley Book Series would like to hear from you!
This book series aims to cover all aspects of microscopy, from compositional mapping, spectroscopy and image analysis embracing applications in chemistry, life sciences, physics,
material sciences, technology and engineering. The series encompasses books for active professionals, researchers at the cutting-edge of techniques and applications as well as advanced textbooks to introduce key methodology and topics to students.
For more information and to submit your book idea, please go to www.rms.org.uk/books
A very warm welcome to Dr Hilary Sandig and Dr Trevor Almeida, who both joined the infocus Editorial Board recently.
Hilary, who sits on the RMS Flow Cytometry Section, is currently a group leader at Cancer Research UK’s Therapeutic Discovery Laboratories (CRUK-TDL), where flow cytometry is used to progress immuno-oncology drug projects. After completing a PhD at Imperial College London, and several postdoctoral positions, she moved to Medimmune to support the use of flow cytometry across functions. This led her to a position at Autolus, again with a focus on flow. The technology has been central to her career as an immunologist in academia and industry.
Trevor, who is a member of the RMS Engineering, Physical & Material Sciences Section, is a Lecturer in the Materials and Condensed Matter Physics Group at the University of Glasgow. He obtained his PhD in Material Science at the University of Nottingham, focusing on the transmission electron microscopy (TEM) of magnetic nanoparticles. His research
evolved to investigate a range of nanomagnetic processes by combining in-situ TEM methods with Lorentz microscopy techniques. Trevor’s primary interests include the functional magnetism within 3D nanostructures, nanoelectronics, magnetotactic bacteria, meteorites, minerals, nanoparticles and thin films.

infocus Scientific Editor Leandro Lemgruber said: “It’s great to have both Hilary and Trevor joining the team. We always try to ensure that our magazine covers the full spectrum of microscopy, imaging and cytometry, so it’s important to have representation from as a wide a range of scientific perspectives as possible. Both Hilary and Trevor will certainly help us achieve that aim, and I’d like to formally welcome them to the team.”
A very warm welcome to Alessandra Reni, who recently joined the RMS Events team.

Alessandra, who hails from Ancona on Italy’s Adriatic Coast, will help plan and deliver the wide range of meetings, courses and conferences offered by the Society.
Alessandra came to live in Oxford eight years ago, having gained experience working in the events industry in Milan and Verona. Impressively, she also played basketball

to a professional level before moving to the UK. She completed an Events Management course at Oxford Brookes University, before working in a number of roles including Host Manager at the Randolph Hotel.
Alessandra said: “I have really enjoyed getting to know everyone at the RMS, and I love the working environment here. It’s a very different role for me, so I’m really looking forward to learning more about what everyone does, and benefitting from the experience of my colleagues.”
In her spare time Alessandra enjoys travelling, painting and meeting new people. She is also passionate about food and loves going out to restaurants.

Pete Banks is not a man to rest on his laurels – or more specifically, to put his feet up in retirement. Over the past 10 years or so, he has become one of the RMS’s most important volunteers, raising crucial funds for its outreach activities through the sale of microscopes and other equipment which has been kindly donated to the Society.
With no previous experience in microscopy, and looking for a new hobby, Pete joined The Leeds Microscopical Society (LMS) – which just so happened to meet at a church hall within walking distance of his home. It was here that he met up with long-serving RMS members Chris Hammond and Peter Evennett, who had already been fundraising for many years, selling instruments at table-top microscopy fairs and other events.
Pete says: “I wondered along to see what it was all about and met such interesting people, so I kept going back. Once or twice I even took my wife and daughters along to listen to the guest speakers. We occasionally had fascinating talks from the famous forensic pathologist, the late Professor Michael Green who was an LMS member. Chatting with him after, I discovered we were both members of the same motoring club and I persuaded him to repeat his talks for the motoring club as they were so interesting.
“I became aware that Peter and Chris were there, occasionally putting out a table and selling all the donated RMS equipment. I bought my first Pete Banks.
microscope from them and still use it today. Peter, Chris and other members of the club were more than willing to show me how to use it. They also encouraged me to attend the various microscope fairs and search the sales tables for that elusive accessory for my microscope. I quickly learnt that with microscopy you always have a need for that elusive accessory! Peter and Chris were both very enthusiastic and dedicated with their project to get microscopes into schools, and that enthusiasm undoubtedly rubbed off on me, so I offered to help out.”
Pete’s involvement has grown since Peter moved to Derbyshire a couple of years ago. He says: “The distance made it impossible for Peter and Chris to work as a team, so I offered to transport the goods if Chris continued with receipt and checking over of the stock.”
Although the microscope fairs have continued to provide a useful sales outlet, Pete has been able to expand the operation through his own advertising efforts.

He says: “At the fairs, most people have already got three of every type of microscope and tell you they daren’t go home with yet another one! They need a lot of persuading to buy more and when they do it’s going to have to be at a really competitive price. I was aware that if we went out into the big, wide world with some donations, we would sell at far better prices. You have to find the person out there that really wants the exact microscope you’re selling and hasn’t got one already – and I can do that.
“If you advertise a microscope, and it is just the thing that they want, then they will travel for it. We never offer packing and posting due to the many problems that can bring. I advertise equipment on various sales platforms and local blogs and my daughter occasionally advertises for me on the social media platforms that she uses.”
He adds: “We still like to give the supporters first pick though. These are the club members who actively support the microscope community and attend club events and fairs. Some travel quite long
distances to engage, and the social aspect is evident. We may have that elusive item waiting for them too!”
Pete spent his entire career working in the defence industry, initially learning his trade working on Royal Naval warships at bases in Singapore and his native Portsmouth, before moving into a contract management role overseeing government contracts placed with firms across the north of England. It was this job that brought him to Leeds around 45 years ago.
“I ended my career as an Engineering Authority in RAF Support Command”, Pete explains. “I was responsible for the through-life support of equipment required by the three services to keep military aircraft flying. That has given me a lot of experience in buying, maintaining and disposing of technical equipment. I took early retirement at 56 and realised that I had a lot of free time on my hands. Although living there, I didn’t know Leeds or the people living around me that well because I was always away with work. I was really shocked to learn quite recently that my daughters never really knew what I did for a living – dad just went to work on Monday and came home on Friday.”
After retiring, Pete sought out several ‘hobbies’ including helping to run elections for the local council and rental property management. Above all though, he wanted to involve himself in his community and get to know people from further afield. It turns out that microscopes and microscopy have provided the perfect means of doing just that. And of course, Pete’s professional background has given him both the practical skills to turn his hand to fixing the instruments and other bits of kit, as well as the sales know-how to market them.
He says: “I really enjoy meeting interesting people coming to my door and talking to them. A recent microscope that I sold was a top spec model suitable for advanced photography and the buyer travelled some 240 miles to pick it up. He turned out to be an amateur who studied and photographed everything
that lives in the mud on the foreshore. He wanted to know how good this microscope would be for his project and I said: ‘I don’t think you’re going to be disappointed’. But then I declared that I’m only the seller - not an expert on photography through a microscope - so on the day of pick up, Chris joined us and gave the chap a couple of hours’ tuition on how to use the microscope, and he was well happy.”
Pete’s most recent sale was to a buyer from London who travelled to Leeds to pick up a Leitz Microscope and a few accessories. He turned out to be a user and collector of all things ‘Leitz’, and was extremely knowledgeable about the brand - to the point that he could tell a Leitz microscope’s year of manufacture by the shade of grey paint finish.
Pete says: “He asked for first refusal on anything ‘Leitz’ coming along in the future, and within 24 hours of his visit I was able to pass his phone number on to a club member who was having trouble with his Leitz microscope. I find this type of ‘networking’ and meeting people very beneficial in learning about microscopes and what they get used for, as well as being able to promote all things ‘microscopical’ like the various clubs and RMS Outreach work. For instance, If I get an enquiry from a schoolteacher looking to buy microscopes, I’ll point them in the direction of the Microscope Activity Kits, that they can borrow for free.”
One thing Pete is keen to emphasise is his amazement at the generosity of the donors supporting RMS Outreach. At the moment he has everything from a research grade Zeiss Imaging Microscope to an antique Vickers Photoplan with a large plate camera mounted on the top.
He says: “The Zeiss is going to test my ability to find it a home due to its specialisation and my limited knowledge of the laboratory market but I’ll relish the opportunity trying. The Vickers? Much easier. No microscopist is going to want that, but people are interested in the Vickers already as they want to ‘up cycle’ it.”
Pete elaborates: “That’s a market I stumbled on by
accident. We once had six old microscopes that were rusty and beyond repair, and I joked with Chris that they would be more useful in my old job and make good boat anchors. They were put aside to be dropped off at the metal recyclers when passing. However, I came around to thinking that I could dodge a trip in the car and that someone may just well buy them for the odd spare part, so
I placed an advert at twenty pounds each. That turned out to be the fastest microscope sale that I have ever made. I was completely overwhelmed by ‘up cyclers’ – those artistic people who like to turn odd things into table lamps and the like. Outreach quickly had £120 in the kitty that I hadn’t planned for.”

Sometimes Pete receives a batch of surplus microscopes from a school or college that has recently upgraded its kit. These are usually the sort of instruments that are perfect for youngsters to get started in microscopy – opening up a further market for Pete.
He says: “I will have a queue of people up my drive taking them away, sometimes two or three at a time for £25-£30 a ‘scope – and it is parents and grandparents buying them for their youngsters. I’ll service the microscope to make sure they can see something through it and offer it for sale with a few slides and cover slips to get them going.
“I will spend time telling them how to get their youngsters interested, show them video clips of diatoms and tardigrades on my phone. I’ll advise them to buy a turkey baster in the pound shop, collect a few jam jars together and then send them on their way to go pond-dipping and tardigrade hunting. I’m sure some parents and grandparents start dabbling with the microscope and the youngsters don’t get a look-in! The other thing I’m able to do is let them know that they live close to a microscopy club and when the next microscope fair is. We do get the odd one or two join that way.”
For the more ‘basic’ microscopes, there’s a huge variety of customers, all with their own specific needs – and stories to tell.
Pete says: “I’ve had a few beekeepers looking for verroa mites and a few fish keepers who want to look for parasites on their koi carp. Once a lass running a hedgehog sanctuary came along saying: ‘I’m wanting to look at hedgehog poo’. I was told that every hedgehog in the world is full of parasites and they’ve become resistant to the medicines. Someone else came along and said they were looking at pigeon poo! I’m learning something about microscopes all the time!
“Then there was a chap from the local fly-fishing club. They had put up nets along the riverbank and wanted a microscope to determine the type and sex
of the insects that they were catching. As a recent convert to fly fishing, I’m out there in the garage until 11 o’clock at night showing him the microscope and talking fish. The party was broken up when he had a phone call from his wife asking: ‘Are you coming home for your tea tonight?’ He replies: ‘Yeah, I meant to, but this guy is into microscopes and flyfishing!’
“He offered to make me a member of the club, and I’d have loved to, but advised that I don’t really want to be waist-deep in cold water at my age. My kind of fly-fishing is the kind where you stand on the bank!”
Now aged 74, there’s no sign of Pete slowing down, and his sales operation is ticking over as strongly as ever - with ongoing assistance from Chris. He does wonder, though, who might take on the job when he eventually decides to wind down. Perhaps he will need to draft in an apprentice?
“I have thought about what might happen in the future, because I can’t go on forever”, he says.“But I’ll do it for as long as I can. It’s a hobby really. I was just looking for something to do. I don’t sit and watch the telly very often and prefer it that way. My wife will be watching the telly and I will be interrogating Google about microscopes.
“I try to be pretty active, and as I get older it gets harder. I took up gardening during Covid lockdown, so it’s now looking like ‘The Good Life’ in my garden with vegetables growing everywhere. I have done a lot of things since I retired, and for me it was always about meeting members of the community that I lived around but didn’t know, because I was never there when I was working. I have succeeded in meeting a lot of interesting people through all the different things I have done but the microscope world has certainly ticked all the right boxes for me.”
Many thanks, Pete, for speaking to infocus, and for all your efforts helping to raise funds for RMS Outreach projects.
Owen MortonDense particles inside a rock core can be positively identified as gold, by looking at the spectral signature of these particles. The K-edge for gold is a unique determination tool, that can be detected at any point inside a sample using SPECTRAL CT.
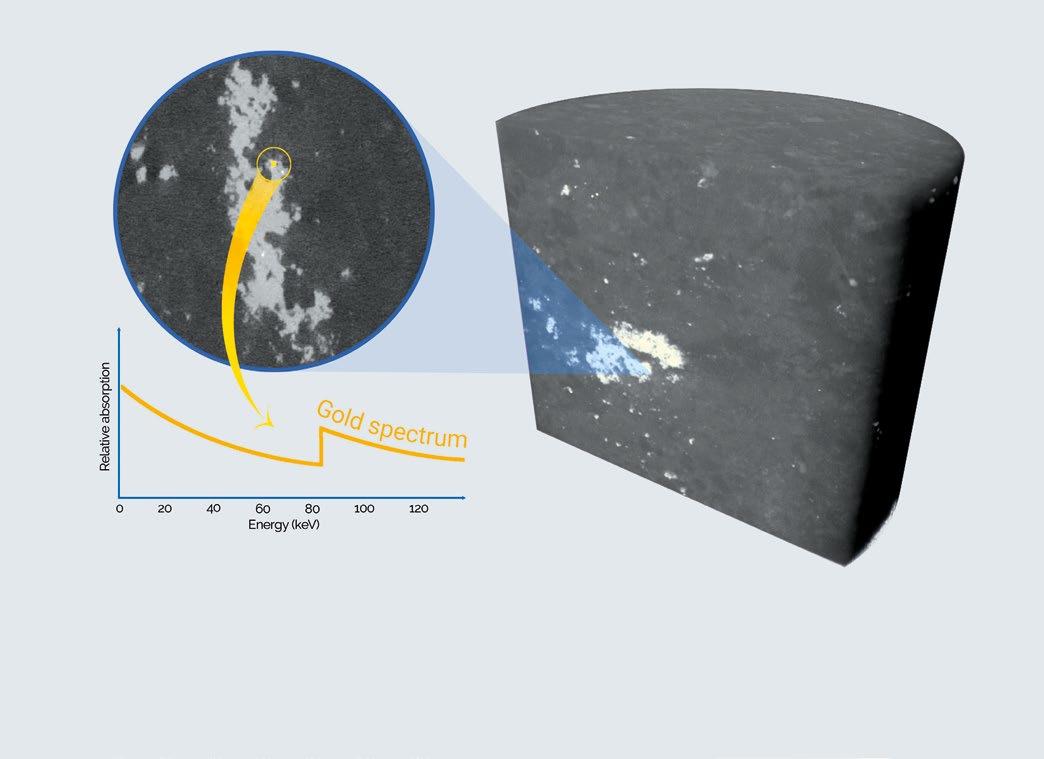
TESCAN revolutionizes micro-CT scanning with unique, analytical capabilities. SPECTRAL CT enables absolute elemental identification in unknown samples using K-edge detection, it offers TrueContrast™ for challenging material differentiation and absolute determination of concentrations and densities.
Dr. Flavia Moreira-Leite, Bioimaging Manager, Oxford Brookes Centre for Bioimaging, Oxford Brookes University, Oxford, UK.
Thin-section transmission electron microscopy (TEM) of cells and tissues is not the technique that comes to mind when electron microscopy (EM) enthusiasts itch for outreach. Conventional scanning EM is far easier to love, and easier to do, but does that mean looking at cells down the TEM cannot inspire the public? In this article, I describe my experience of trying to make the ultrastructural world reach out of the lab, by running a live TEM webinar targeting secondary school students. Jumping on the bandwagon of virtual interactivity with TEM had its challenges. However, it allowed us to showcase this amazing microscope to a wider audience than we had done before in a single session, taking the intracellular world directly into classrooms and family homes.
Electron microscopy outreach is focused on scanning electron microscopy (SEM). Conventional (secondary electron imaging) SEM is easy to understand and makes anything look awesome. In the SEM, large specimens - such as whole insects or an anther packed full of pollen - can be visualised in
their entirety at magnifications low enough to make the context of imaging immediately clear. Then, a quick zoom into intricate surface details offers a ‘wow factor’ to any audience. At the Centre for Bioimaging, as in other EM labs across the world, we use the SEM in outreach and in other events for

the general public, for guaranteed success.
TEM probably sits on the opposite end of the outreach spectrum relative to SEM. It is harder for the public to understand the context of TEM images, given the huge difference in scale between them and what can be seen with the naked eye. Harder than coming to grips with the scale is making sense of the context in a thin-section of tissue, which represents a very limited slice of the whole ‘story’. Can we really expect the general public to appreciate looking at tissue sections down the TEM?
I believe that thin-section TEM can indeed be aweinspiring to young people and adults beyond the lab. When visitors come to the Centre for Bioimaging,
I can see the excitement in their eyes when I show them our TEM and the images it produces. With its amazing resolution and imposing looks, the TEM has a very distinctive ‘wow factor’: it shows that Biology has order and beauty in a scale so inconceivably small, in pictures that are worth a thousand words. Yes, it is harder to understand than SEM, but thinsection TEM can certainly amaze the public in its own way.

Before the pandemic made face-to-face outreach harder, we used to run electron microscopy sessions to small groups of secondary school students.These sessions included not just SEM but also thin-section TEM. I always felt that it was important to give a short explanation on sample preparation before showing TEM imaging of sections, to give students an idea of how we go from a whole organism or piece of tissue to minuscule slices showing only a few nanometres of a limited number of cells. Typically, we did thin-section TEM with secondary (not primary) school groups, because at this stage students have started to learn cell biology and are, thus, more likely to appreciate looking at the cellular ultrastructure directly. They will have seen some textbook TEM images and are often able to recognise organelles and other cell structures.
Aside from the small group sessions, the Centre for Bioimaging also ran larger scale outreach at the Oxford Brookes Science Bazaar, a one-day festival showcasing research from across the University to the local community. The last Science Bazaar was in February 2020, and we now think of this very busy face-to-face event with nostalgia, as it happened just before socialising was banned by the first lockdown. Typically, we used light microscopes only at the Science Bazaar, because the event was held outside the labs and none of our electron microscopes are really portable. However, in 2021, Science Bazaar activities resumed with a series of virtual sessions (https://www.brookes.ac.uk/science-bazaar/), which enabled us to show the electron microscopes in action to a wider audience. In fact, our virtual SEM session inaugurated the Science Bazaar virtual
activities in September 2021, by allowing families at home to choose one of our typical samples (insects, pollen and unicellular parasites) to look at under the SEM.
As a TEM lover, I seized the opportunity to show my favourite microscope to a larger audience, and maybe inspire the next generation of cell biologists. In collaboration with the Science Bazaar organiser Dr. Ellie-May Beaman, I planned a live TEM webinar for secondary schools mimicking the face-to-face sessions we used to run before the pandemic. Ellie sought advice from secondary school science teachers on the format, and then we scheduled a one-hour session to coincide with the 2022 UK Science week, when students were likely to be focusing on science activities during and after school. Advertising was focused on secondary schools, although anyone could register for free and join in. The session was advertised to 66 secondary schools (in the Oxfordshire, Berkshire, Buckinghamshire and the London area), and we had 59 registrants, including both individuals and schools, with 40 registrants attending on the day.
As with face-to-face sessions, I felt that explaining TEM sample preparation (albeit in very basic terms)
was essential, if a little daunting in a virtual format. I had a carefully orchestrated plan of what to show for each of the basic steps (of fixation, staining, embedding and sectioning), with one or two objects - such as sample blocks, TEM grids and a diamond knife - to illustrate each step, as well as our home-made sectioning video ready to play at the correct time. Given the small scale of TEM sample preparation details, I used a stereoscope linked to a screen to show small objects magnified. I felt that this was important in a virtual session, where viewers would not be able to get closer to objects to have a better look at them, while webcams struggle to focus on small objects brought too close to them. On a bench next to the TEM, we set up all the necessary equipment to explain sample preparation, and we planned carefully how we were going to move the camera around the room during the session. My colleague Ed Rea helped with the logistics on the background, which was essential for the session to run smoothly. We fitted the webcam to a tripod and Ed marked the tripod positions precisely on the floor, in advance of the session, to help reposition the camera with minimal delay. After showing sample processing, I felt that it was important to show how we insert samples into the

TEM, with the fancy airlock system that prevents vacuum from breaking during sample insertion. While we presented the camera’s live capture screen directly to the audience, the webcam showed me turning dials and pressing buttons on the TEM, to give the audience a sense of just how cool it is to operate this microscope, but also to make them feel as if they were in the room with us.
I have to admit that, in the last minute, I realised I had not planned an important aspect of my outreach session: how to encourage audience participation! Just before the session started, I had the idea of pointing at organelles and structures on the screen and asking the audience to tell me what they were. I gave the audience 10 seconds to answer each question, and Ed followed the chat and relayed the answers to me. It was not a terribly creative approach, but it worked well and interaction was great, with over half of those attending the session posting answers to questions. Considering how tricky it can be to interact with the audience on a webinar format, the ‘Q&A’ strategy showed me that some aspiring scientists were hanging on my every word on the other side of the line!
Overall, the experience of hosting a virtual TEM session was certainly very positive and those
interested can now watch a (slightly edited) video of our live TEM webinar on the Science Bazaar website (https://brookes.cloud.panopto. eu/Panopto/Pages/Viewer.aspx?id=0ca9be39-70ce4543-a262-ae8a00df3935). Despite the success of this first experience, I recognise that there is room for improvement. On the technical side, I regret not having used a lapel microphone for clearer and more consistent sound, and I should have been more mindful of a lag between camera movement and updating of the live broadcast (a problem that could be specific to our system). On the session planning side, sample preparation details could be further simplified (particularly for a younger audience), and I hope to be more creative with interactivity on our next TEM virtual session, which is scheduled for the autumn of 2022 (follow us @OBCBioimaging for details of future TEM webinars). I would like to experiment with showing different samples, including negative staining TEM of viral particles, which is much easier to understand than thin-sections (as well as sadly topical). We hope that our experience will inspire other EM labs to do more outreach and public engagement with their TEMs, sharing with the public the excitement of looking directly into the nanoscale world.
 Dr Flavia Moreira-Leite
Dr Flavia Moreira-Leite
Market leader in temperature and environmentalcontrolled microscopy, Linkam Scientific Instruments, was represented at this year’s Advanced Materials Show (AMS) by global leader in microscopy and materials characterisation, McCrone Microscopes & Accessories. McCrone Microscopes & Accessories presented a range of Linkam’s leading temperature and environment-controlled stages at this year’s AMS 2022. The Linkam Modular Force Stage (MFS) is designed to gain a better understanding of a material’s behaviour under different temperature, mechanical stress and environmental conditions. The system offers advanced sensitivity, high resolution and a modular concept for customised experiment design and control.
Also showcased was the Linkam THMS600, one of the industry’s most widely used heating and freezing microscope stages. It is suited for use in applications that require high stability, high heating/freezing rates and 0.01°C accuracy. With a temperature range of -195°C to 600°C, samples are quickly characterised by rapid and accurate heating to the required temperature at a rate of up to 150°C per minute, with minimal overshoot for close examination of sample changes.

The Linkam FDCS196 Freeze Drying Cryo System enables the rapid, accurate and highly detailed
investigation of the freeze-dried structure of complex samples. Both stage pressure and temperature can be accurately controlled and programmed to simulate industrial procedures and determine ideal drying parameters.
The Linkam PE120 Peltier system offers a highly accessible solution for a wide range of temperaturecontrolled analyses. This easy-to-use, cryo-free, thermoelectrically cooled stage accurately controls the temperature of microscope slides to +/-0.1°C from -25°C to 120°C in a cost-effective system to handle a variety of applications.
Ross Browne, managing director at Linkam commented: “An in-depth understanding of the performance of materials – especially new and emerging materials – helps uncover their potential to improve human life. As scientists continue to seek new ways to tackle major global issues, such as delivering sustainable energy to address climate change and bio-engineering to improve human healthcare, characterising the physical properties of advanced materials is more important than ever before. We are delighted that McCrone represented our latest product offer at the AMS conference this year.”
www.linkam.co.uk
The Archaeology Department of the University of Liverpool has carried out two ground-breaking
VHX-7000 was used to gather information on the different kinds of corrosion found on the mirrors. Elizabeth Thomas (Archaeology PhD student at the University of Liverpool) felt it was important to see the different layers in the mirrors and how they were made, which was not possible using a scanning electron microscope (SEM) where image delivery was in grayscale.

In addition, the digital microscope made stitching images a straightforward process, whereas an SEM would require image stitching to be carried out manually using separate software. The VHX-7000 also eliminated the need for preparing samples – an SEM requires them to be secured on to a stand, as well as being fixed, hydrated, embedded in resin etc. –while the measurement capabilities of the KEYENCE system saved Elizabeth a considerable amount of time as the sample can just be placed on the stage.
projects that have improved our understanding of how human culture developed in ancient times. Playing a key role in that research has been the KEYENCE VHX-7000 Series Digital Microscope.The
“There are many advantages to using the VHX-7000,” says Elizabeth, “and it represents a massive jump in functionality from any previous digital microscopes I’ve used. For example, the flexibility of being able to move the unit’s fully integrated head around a sample is invaluable. It means that just by tilting the head, it’s possible to create multiple views of the same sample.”

www.keyence.co.uk
Photon Lines is pleased to announce a new distribution partnership with Austrian Company, Prospective Instruments. Prospective’s MPXseries is the world’s first multimodal multi-photon microscope that combines ease of use with configuration flexibility. These instruments offer the following integrated modalities: 2-PFE, 3-PFE, SHG, THG, & widefield fluorescence, and they include Prospective Instruments’ own femtosecond laser, allowing true turn-key operation. The manufacturer states that they have achieved their primary aims to create an instrument which is compact, rugged and robust, with highly integrated optics and electronics.
For more information on the MPX-SERIES, contact Photon Lines Ltd at info-uk@photonlines.com, or call +44 (0)333 2427905.
www.photonlines.co.uk
Nobel Laureates feature in many of the interviews with microscopy pioneers published on Wiley Analytical Science website. Here’s a glimpse of what you can read about.
Becoming a Nobel Laureate is rarely straightforward, as Rebecca Pool from Wiley Analytical Science learnt when she interviewed Richard Henderson, Eric Betzig and Stefan Hell.
Long before Scottish molecular biologist, Richard Henderson, won his 2017 Nobel Prize in Chemistry for developing cryo-electron microscopy, he nearly didn’t make it to university.
As the only student in his class to fail French, a future in higher education looked, at the time, somewhat tenuous. “Fortunately another girl had failed her Higher exam, so the two of us spent an entire year learning French with one teacher,” Henderson told Wiley Analytical Science.“I scraped a pass but at least knew I was then allowed to go to university.”
Decades later, Eric Betzig, was growing weary of research. It was 1994, and while the then Bell Labs researcher had just worked out how to resolve single molecules using low temperature scanning tunnelling microscopy with his best friend Harald Hess, he couldn’t see how near-field microscopy would ever have the resolution of an electron microscope.
Betzig took time out to work for his father at the Ann Arbor machines company, spent several years mulling over molecular optical imaging strategies, and then quickly leapt back onto the academic circuit to build the first super-resolution photoactivated localization microscope (PALM) prototype - in Hess’s home as it turned out. A fruitful collaboration with cellular biologist, Jennifer Lippincott-Schwarz, was to prove the technology.As the Nobel Laureate pointed out to WAS: “Many people would have blown off these guys who were ten years from having written a paper and talking crazy talk... But Jennifer said the microscope sounded great.”
Meanwhile, Stefan Hell was to spend many years convincing his scientific peers that his unconventional approach to breaking the diffraction barrier in farfield fluorescence microscopy – via stimulation emission depletion - actually worked. As the 2014
Nobel prize winner revealed in his interview, ‘The Nobel Laureate nobody believed’: “Laboratories across the US and Europe had funds to build nearfield optical microscopes but I was seen as this funny guy claiming these efforts were ill-fated as superresolution microscopy should be done in the far-field instead.”
These are but a few examples of the unexpected insights that WAS microscopy profiles have provided over the last few years. Along the way, exRMS President, Michelle Peckham, has told of her passion for microscopy, while structural biologist, Jason McLellan, has explained how he resolved the structure of the SARS-CoV-2 virus and helped to developed a Covid-19 vaccine so remarkably quickly.
But perhaps comment from planetary scientist, Natasha Stephen, who hunts meteorites while running a multi-million pound electron microscopy facility, best sums up the circuitous, and often, unexpected, routes microscopy can take you: “All of my opportunities have been very random and certainly not from traditional paths.”
Scan the QR code or access Wiley Analytical Science via https://bit.ly/Minds-in-Microscopy to read the complete article collection.


Linkam Scientific Instruments has opened the doors at its new UK premises in Surrey on its 40th anniversary in temperature-controlled microscopy. The move stems from the company’s unprecedented growth in recent years, particularly in customdesigned solutions to support scientific research in precision temperature- and humidity-controlled environments. Linkam has gone from strength to strength, with the new site expansion featuring a state-of-the-art interactive demonstration suite where customers and prospective customers can get hands on with the product range.
The new site consolidates the entire Linkam production function, including raw materials, inventory, machine tooling and manufacturing, facilitating cross-functional team working, improving collaboration across the business and delivering significant productivity gains.
As part of the expansion and to meet growing customer demand, Linkam has also ramped up the team across all areas of the business.
To boost the business development drive, Clara Ko joins as sales and marketing manager. Clara has a BSc in Biology and an MBA from the University of London, and has worked for a variety of multinational scientific companies in Asia-Pacific, Europe, and North America. She has also worked for leading microscopy and spectroscopy companies in bioscience, chemical analysis and material science, especially in X-ray fluorescence, ICP/Optical
emission, and thermal analysis. Clara will be heading up the sales and marketing team, and aims to step up the services Linkam provides to customers and distributors worldwide.
An applications scientist will soon be joining the team to improve Linkam’s technical and application capabilities and further improve customer support.
Ross Browne, Managing Director at Linkam commented: “Sustained business growth in recent years has taken our business to the next phase in its development. We have been successfully supporting researchers in the materials science and life science sectors for 40 years and are proud to mark this major milestone with our new state-ofthe-art premises, located five miles from Gatwick International Airport. We now have a site that offers an improved customer experience, showcases our innovative scientific instrument range, and brings together our whole UK team in a modern and efficient working environment – while offering space to expand in the future as we continue to develop our technology and our team.”
www.linkam.co.uk

Koso Corporation (677-1, Kakeshita, Iwata City, Shizuoka Prefecture, Japan) is a subsidiary of Hamamatsu Photonics, which manufactures electron tube light sources. Koso Corporation has been constructing a new factory Building No. 2 to boost production capacity. The construction of this new factory is now complete and its operations will start in April, 2023.The completion ceremony for the new factory building was held on Monday, August 8, 2022.
www.hamamatsu.co.uk
completed

This KEYENCE digital microscope is invaluable to Public Analyst Scientific Services, as it helps with the production of detailed images of foreign bodies. These images are often included in sample reports where a clear and detailed image is important for end clients.
up and use on a daily basis, whilst not compromising the microscope’s overall capabilities.
Other benefits of the VHX Series include realtime depth composition achieved without the need to actually adjust the focus of the device. The microscope delivers images of unprecedented clarity and texture, thanks to a high resolution HDR offering 256 times the information of a normal eight-bit digital image. Balancing out brightness levels and improving contrast simultaneously is key to delivering excellent viewing results.
“The measurement features also help provide more information that could not be obtained easily otherwise” says Downie. “For example, a radius of curvature of a fragment of glass allows us to estimate the size of the object the fragment has come from.”
“After consideration, we decided to acquire a VHX Series Digital Microscope from KEYENCE” says Emma Downie, a public analyst at Public Analyst Scientific Services. “This digital microscope was a new acquisition for us, and we selected this product primarily for its comprehensive imaging capabilities.” The VHX Series Digital Microscope is very easy to set
The engineers at KEYENCE are always ready to solve issues and answer technical questions about the company’s products. The VHX Series Digital Microscope is often seen as a strategic acquisition –a game changer, in other words. www.keyence.co.uk
Teledyne Princeton Instruments, part of Teledyne’s Vision Solutions Group, announced today that its COSMOS camera was recognised among the best by the 2022 Laser Focus World Innovators Awards. An esteemed and experienced panel of judges from the optics and photonics community recognised Teledyne Princeton Instruments as a Gold honoree.
“On behalf of the Laser Focus World Innovators Awards, I would like to congratulate Teledyne Princeton Instruments on their Gold-level honoree status,” said Laser Focus Word Editor-in-Chief Peter Fretty. “This competitive programme allows Laser Focus World to celebrate and recognise the most innovative products impacting the photonics community this year.”
The deeply cooled COSMOS camera, supercharged by the next generation of Teledyne’s LACera™ (large area CMOS image sensor technology), allows astronomers to peer deep into the universe. The

camera provides unprecedented performance— up to 81 mm x 81 mm imaging area, back side illuminated (BSI) technology, sub electron read noise and >90% peak quantum efficiency.
The CMOS camera is designed for mission-critical operations and delivers performance required by cutting-edge astronomy applications such as low orbital object tracking, exoplanet characterisation, time-domain astronomy, and more. www.teledyne.com
As part of the Graz-µCT consortium, Graz University of Technology has purchased two new TESCAN micro-CT systems that will be used to non-destructively observe and measure structural changes inside materials under real operating conditions.
TESCAN ORSAY HOLDING a.s. announce the installation of two new micro-CT systems at Graz University of Technology: the TESCAN UniTOM HR and UniTOM XL. These are the first micro-CT systems to be installed in Austria-bringing the power of dynamic micro-CT to Austrian researchers for the first time. The devices open up a window into materials of all kinds at the micrometre scale without destroying the material samples.
TESCAN’s UniTOM HR is the first dynamic microCT system to offer sub-micron resolution 3D nondestructive imaging and high temporal resolution for uninterrupted 4D dynamic CT experiments. It provides the extra step in resolution needed to fully understand the behaviour of complex materials, as the smallest features often play a crucial role in material performance. TESCAN’s UniTOM XL is a multi-scale imaging CT solution that features
volume of interest scanning (VOIS) capabilities and is designed to accommodate a wide range of sample sizes and types.
The two new highly innovative micro-CT devices are housed in the basement of the Physics building of Graz University of Technology (TU Graz) at Campus Neue Technik. They were purchased by three Graz universities as part of the Graz-µCT consortium with financial support from an R&D infrastructure grant from the Austrian Research Promotion Agency FFG. The aim of the purchase is to enhance research at the universities with in-situ experiments in the fields of medicine, pharmaceutics, geology, metallurgy, and pulp and paper technology. The systems will also be available, upon request, to external universities and non-university research institutions, which is unique in Austria.
TU Graz Rector, Harald Kainz, states, “I’m proud that we have the opportunity to use such highly innovative research equipment at TU Graz. This novel method of examining materials without destroying the samples in the process heralds a new era in materials research.”
www.tescan.com
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk. The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment.The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
Not only does the VHX-7000N have the highest resolution in the history of microscopes, but it is capable of delivering images that rival a Scanning Electron Microscope (SEM).This can all be performed by novice users. Below, are examples of how the KEYENCE VHX Series has been utilised in various industries.


One significant issue for many R&D professionals working in this sector is that existing microscopes tend not to possess the depth of field required to generate the extremely clear image that they require. Also, microscopes will often need to be connected to a separate camera and to external software in order to process the images and communicate the results. In addition, R&D staff usually have to carry out preparation work before using several different microscopes – a 2D measurement microscope, a scanning electron microscope (SEM) and others. This preparation can be hugely time consuming and switching between microscopes can present logistical problems if the various microscopes are used by different teams in separate rooms.
The all-in-one VHX-7000 obviates the need to buy several different microscopes and is highly costeffective when compared to an SEM. Also, the unit can be upgraded and customised, representing an investment in the future. Different stages, stands and optional features such as the motorisation of axes and a range of optical lenses can be added to the base model depending on the user’s requirements. As the only 4K digital microscope in the world,
the VHX-7000 is particularly suited to the semiconductor market and surpasses traditional lensbased microscopes that rely on eyepieces. As well as its superior magnification of minute features (from 20x to 6000x), the KEYENCE unit has the ability to subtly shift the finely-tuned XY motorised stage automatically to increase resolution by 33%.
Says Claire Lillywhite, Applications Engineer, Metrology & Microscopy Divisions at KEYENCE: “Whether looking to do checks on wafer bonding and packaging or carry out non-destructive final testing or a range of other R&D inspections, the semiconductor industry now has a solution to the many issues that have bedevilled it in the past.” www.keyence.co.uk
Microscopes in augmented reality, Telight is bringing a new innovative way how to experience and examine their Quantitative Phase Microscope Q-Phase to conferences or at home. A one-to-one model was created and transferred onto the proprietary app. At the same time, Telight managed to reduce its CO2 impact, as their models in AR weigh nothing.
www.telight.eu/
KEYENCE have established themselves as a fundamental player in the next era of digital microscopy by releasing the VHX-7000N (5th Generation), the world’s first 4K Ultra-High Accuracy Digital Microscope
Queensgate, a brand of Prior Scientific, has announced the launch of the WP-Z-120A Wafer Stage. Millisecond response times and high bandwidth make the WP-Z-120A wafer positioning system ideal for applications where high throughput is essential. The Wafer Stage can be used in wafer inspection with an optical microscope such as the Prior Scientific OpenStand microscope or an OEM version using various optical or other inspection techniques.
The stage is designed to hold 300 mm (12”) wafers and delivers an exceptional performance with a load capacity of up to 8 kg, suitable for even very heavy wafer chucks. The stage incorporates capacitive positioning sensors to provide nanometer displacement measurement and precision closedloop feedback. Flexure guidance offers friction-free, reliable motion to more than 120 µm in closed-loop mode and achieves fast response and settle times with Queensgate’s digital closed-loop controllers.
“We are pleased to introduce WP-Z120A Wafer Scanner to the market. We wanted to design a wafer scanner that can carry a high load but achieves market-leading fast response and settle times,” said Alison Raby, Business Development Manager for Queensgate, “The Wafer Scanner was designed with OEM customers in mind and can be adapted to new custom versions easily.”
WP-Z-120A Wafer Stage can be customised for different environments and specifications. It is vacuum (HV) compatible and ideal for a chamber in an electron microscope. Ultra-high vacuum (UHV) and UHV-RAD radiation-hard versions are also available. A higher load custom version can be used for up to 14 kg loads and a tip-tilt version for platform levelling and scanning is available. An extended range of 160 µm and a smaller size for 200 mm wafers are possible upon request.
www.prior.com
Safematic has further expanded the capabilities of their popular CCU-010 coater range with the introduction of a large chamber coater. Specimens up to 6” (150mm) can be accommodated and additional holders for a 4” wafer or for multiple smaller specimens can be fitted.
For existing CCU-010 users there is a simple upgrade to large chamber specification. The Safematic CCU-010 range is available in high vacuum and standard vacuum formats. By selecting preferred sputtering and/or carbon coating head modules, the high vacuum system is suitable for SEM sputter coating

applications using oxidising metals such as Cr, Ir and Al and non-oxidising metals, such as gold and platinum. It also has an impressive high vacuum performance which ensures carbon coating quality that is optimal for both SEM and TEM applications.
A stand-out highlight of the carbon evaporation module is a unique (patented) carbon fibre spooling system. This allows up to 50 carbon coatings to be carried out without the need to change the carbon source - a major advantage over old-style, labour intensive “single shot” carbon coating systems.
To ensure precise control of coating thickness, all safematic coaters come as standard with a terminating film thickness monitor.
Sussex-based Labtech International offer the full range of Safematic coaters in the UK and Ireland.
www.labtech-em.com
Hamamatsu Photonics announces the release of a new updated version of the NanoZoomer S60, an automated system capable of scanning up to 60 glass slides. The NanoZoomer S60v2MD Slide scanner system (C16600-21MDEU) is the latest addition to the NanoZoomer series of whole-slide scanners, which support clinical diagnosis.
The latest version not only scans faster compared to the previous model in 40x mode, but it also offers exciting new features. Three cassette slots can be used interchangeably for standard and mega-sized slides* and it includes selectable, fully automated and semi-automated scanning modes. Customers can still expect the convenience of the operational software to help them create scan profiles and perform quality checks on images. Additionally, the S60v2MD complies with IVDR, making it ideal for our clinical customers.
The NanoZoomer series is a family of whole slide scanners that convert glass slides into high-resolution digital data by high-speed scanning. Scanned data can be viewed on a PC monitor by using the dedicated viewer software, including a patented navigation map

delivering a viewing environment just as if operating a microscope. The image acquisition software (NZAcquireMD) and the image viewing software (NZViewMD) allow users to easily create, view and perform quality-checks on whole slide images.
Behind the hardware and software, clinical customers will find trained specialists supporting them through the adoption of new workflows. In fact, trainings and technical support are in place to ensure a smooth transition whether it is integrating a new scanner model into your workflow or changing from manual to digital pathology.
Committed to the clinical market for over 15 years, Hamamatsu Photonics is driven to offer the highest quality solutions while complying with the latest medical regulations. Known for our market-leading photonics technology, we integrate our knowhow into the NanoZoomer Slide scanner systems providing a stamp of reliability.
www.hamamatsu.co.uk
Launched earlier this year NuNano’s silicon nitride SENSE probes have been delivering great results for our customers. Sub-nm resolution was achieved on a 2D protein crystal array with SENSE 70 in Peak Force Tapping mode, demonstrating they work well in modes for nanomechanics.
SENSE probes also allow AFM imaging of soft samples using contact mode and AC mode in air or liquid, with guaranteed tip sharpness and minimal variation in mechanical properties. Using Scanning Electron Microscopy (SEM) we perform rigorous inspection of all NuNano probes, ensuring reliable imaging every time.
Dr Peng Bao & Kyle Phillips, University of Liverpool say: “We have tested the new NuNano SENSE 70 tips for the imaging of 2D protein arrays in aqueous environments. The performance of these tips is outstanding. We can get sub-nm resolution images routinely!”
The SENSE probes feature a sharp silicon tip integrated onto a silicon nitride cantilever with gold reflective backside coating. They include the SENSE 20 (0.07 N/m, 20 kHz) and SENSE 70 (0.4 N/m, 70
kHz) probes and are suitable for imaging soft samples due to their sensitivity, minimising sample damage.

Dr James Vicary, Managing Director at NuNano says: “We’re pleased with the reception our SENSE probes have received from around the world. Being able to support our customers through the provision of silicon nitride probes has been a key driver for us in the continuing expansion of our range of probes.”
NuNano is a UK-based SME specialising in the design and manufacture of AFM probes. The SENSE tipped silicon nitride probes form part of NuNano’s existing range of conductive, silicon and tipless silicon nitride probes currently available.
www.nunano.com

Andor Technology, an Oxford Instruments company and a world leader in scientific imaging solutions, has announced the launch of two new scientific CMOS cameras, specifically designed for life science researchers.These new product introductions further strengthen Andor’s broad portfolio of cameras and microscopy systems, while the company also offers market-leading image analysis software with Imaris.
ZL41 Cell is the next generation in the highly successful Zyla sCMOS series. With ZL41 Cell, it is possible to transform a normal fluorescence microscope into a super-resolution microscope using our exclusive SRRF-Stream+ technology. ZL41 Cell offers remarkable flexibility, as well as superb sensitivity, speed and resolution, making it ideal for those that are upgrading their imaging system, or for those that have specific application needs such as live-cell imaging, or Single Molecule Localisation Microscopy.
Dr. Alan Mullan, Andor’s Product Manager for Microscopy Cameras, commented: “The combination of the ZL41 Cell and SRRF-Stream+ opens up superresolution capabilities to a much wider range of researchers than ever before, and at a much lower price point. You can use your existing labelling
protocols, and normal fluorescence microscope to obtain highly detailed and vibrant images in real-time. Therefore, super-resolution to 100 nm becomes accessible even to those that previously considered the technique to be difficult or costly.”
The Sona-6 back-illuminated sCMOS has been enhanced to push the limits of detection further, even for the most challenging, light-starved imaging applications such as live-cell confocal and single molecule studies. The noise floor has been reduced to ≤1.0e-, which, when combined with the 95% Quantum Efficiency and lowest dark current, allows detection of the weakest signals. This means exposures and phototoxicity are reduced, maintaining cell physiology, for fast and gentle imaging of delicate cells over longer durations.
A new High-Speed mode has been implemented to meet the needs of fast imaging applications like calcium and ion imaging, FRET and blood flow studies. By exploiting a 2-lane CoaXPress connection, 135 fps of sustained and stable high-speed operation is now possible. Further optimisations have enhanced image quality for even clearer and more vibrant images.
www.andor.oxinst.com

Quantum Design (QD) is very proud to announce the culmination of almost a decade of research into the area of correlative microscopy. The FusionScope is an innovative correlative microscope that combines the power of AFM with the benefits of SEM imaging. Designed from the ground up to seamlessly integrate these two powerful techniques, the FusionScope offers benefits of use and design previously unseen in this field.
“I love exciting technology and the FusionScope is a perfect example of that,” stated Chris Schwalb, COO of QD Microscopy. “The seamless combination of two of the most powerful microscopy techniques – AFM and SEM – enables the user completely new insights into the micro- and nanoworld.”
FusionScope takes advantage of an innovative shared coordinate system that automatically aligns both AFM and SEM operations for measurements and sample positioning. This removes burdens often found in other systems, such as having to move the sample from one system to another or having to switch between different controller software interfaces.
Through this integrated shared mapping, FusionScope allows you to easily identify your area of interest, measure your sample, and combine your imaging data in real time.
“The ability to scan and image across differing magnification scales in the FusionScope is the system’s major enabling attribute,” said Stefano Spagna, CTO of Quantum Design. “It allows smooth image transitions between millimeter, micron, and sub-nanometer scales, allowing you to see new correspondences in your data from specific sample areas.”
Researchers will be able to use the FusionScope software to interactively overlay AFM imaging data onto SEM images while operating the microscope in real time, allowing them to create stunning 2D and 3D visualisations with nanoscale resolution. In addition, FusionScope’s software provides automation for most routine functions within an intuitive and customisable user interface, and intelligent data handling organizes all your AFM and SEM data for easy access in the future. www.qd-uki.co.uk
CoolLED has launched its new pE-400 Series Illumination Systems where sophisticated control combines with powerful illumination for costeffective fluorescence microscopy, from routine to advanced applications.

• Manual control pod, global BNC for TTL, and USB (Type B) for operation in third-party imaging software.
• <10 µs TTL triggering for high temporal resolution.
For more demanding applications such as high-speed live cell imaging and optogenetics, the four-channel pE-400max also includes:
• On/off and irradiance control of individual channels.
• Ability to fit optional inline excitation filter holders, removing mechanical latency of filter wheels for high-speed multi-channel experiments.
• 4-channel Sequence Runner, transforming a manual microscope into a cost-effective automated imaging system by triggering illumination sequences with a camera’s TTL output.
The standard pE-400 and pE-400max Illumination Systems make up the pE-400 Series. Four intense LED wavelengths offer broad spectral coverage from 365-635 nm for use with all common fluorophores ranging from DAPI through YFP to Cy5.
While the LED configuration is identical, the difference comes down to control. The white light pE-400 Illumination System offers a modern lamp replacement, which is ideal for applications such as fluorescence screening or sample visualisation on confocal setups, with features including:
Supporting laboratories in achieving their sustainability goals, the pE-400 Series eliminates toxic mercury, cutting energy consumption and utilising 80% recycled aluminium. Stable and robust performance with near-silent operation come as standard, and liquid light guide or direct fit delivery ensure compatibility with most major microscope models. The Illumination Systems are also supported by CoolLED’s world-renowned customer service and a 36-month warranty.
www.coolled.com
Researchers at Telight were able to achieve 90 nm spatial resolution on the LiveCodim super-resolution system.The LiveCodim, developed by BioAxial part of Telight, is an add-on device designed for fluorescence microscopy. It employs structured illumination light patterns that are scanned across a sample to probe scales below the diffraction limit. LiveCodim specialises in the imaging of live cells over long-term experiments.
www.telight.eu
its market-leading photonics technology, built and integrated its knowhow into the NanoZoomer Slide scanner system providing a stamp of reliability.
The NanoZoomer S360MD Slide scanner system (C13220-21MDEU) is no different, also offering excellent image quality and high-speed scanning ideal to support primary diagnosis applications. The image acquisition software (NZAcquireMD) and the image viewing software (NZViewMD) allow users to easily create, view and perform quality-checks on whole slide images.
The EU IVD regulation is challenging medical equipment manufacturers to review and update their instruments to address concerns amidst patient safety and transparency by May 2022. Hamamatsu Photonics can now provide assurance that its solution and services respond to the latest regulations.
Committed to the clinical market for now seven years, Hamamatsu Photonics is driven to offer the highest quality solutions while complying with the latest medical regulations. The Company, known for
Behind the hardware and software, clinical customers will find trained specialists supporting them through the adoption of new workflows. In fact, trainings and technical support are in place to ensure a smooth transition whether it is integrating a new scanner model into your workflow, or changing from manual to digital pathology.
Please contact Hamamatsu Photonics for any questions concerning the NanoZoomer S360MD Slide scanner system (C13220-21MDEU) or concerns around the IVDR transition. The NanoZoomer specialists’ team will be able to support.

www.hamamatsu.co.uk
Laser 2000 is excited to offer Spark Lasers’ new generation ALCOR 920, with increased specifications.
ALCOR 920 offers unmatched laser parameters to achieve ultimate imaging performance thanks to HPC® Technology (High Pulse Contrast). HPC® increases average power while cleaning pulses.
ALCOR 920 enables optimal brightness in your microscope thanks to:
• Increased average power for two-photon fluorescence: 2.5 W for ALCOR 920-2 / 1.5 W for ALCOR 920-1
• Short pulses (<100 fs)
• Clean pulses with virtually no power outside the main pulse, enabled by HPC® technology (High Pulse Contrast)
ALCOR 920 offers the highest peak power on the market, leading to optimal brightness in your images. Get in touch to find out more.
www.photonics.laser2000.co.uk/ALCOR
Prior Scientific is pleased to introduce its newest automated slide loader, the SL160. This 160 slide capacity loader combines reliability, easy set up, and high capacity to provide automated slide loading to a wide variety of existing upright microscopes or with the use of Prior’s OpenStand microscope. This SL160 will be the standard for OEMs and system integrators and allow for precise and safe loading of slides for the pathology, cytology, and screening markets.

Prior Scientific designed this system from the ground up to be the safest and most reliable slide loader on the market. Each precious slide is simply and securely mounted in the custom four slide holder. Mounted within the four slide holder, slides remain safe avoiding all contact with moving parts of the loader. Reliability is unmatched with millions of slides
loaded and unloaded in extensive testing. “The new SL160 Slide Loader is the culmination of years of testing and redesign to achieve the highest standards in the industry”, reports Thomas Freda, CEO of Prior Scientific. Freda adds, “Full 15 by 15 millimeter areas of each slide under a 20x magnification objective can be completed in as little as 60 seconds”.
After placing hundreds of units in the field over two decades with previous loader designs, Prior Scientific is utilising and leveraging that expertise in our newly designed loader, the SL160. The system has far fewer moving parts, can be set up in as little as 30 minutes and with several integrated sensing systems, your slides are truly handled properly with the utmost care. By loading four slides at a time, the SL160 allows for much higher overall throughput of scanning. The optional preview station with integral illuminator is a scan time saver and used to pre-determine areas of interest. Prior’s proven and reliable ProScan system controls the loader, scanning stage, and the optional OpenStand automated microscopy platform for a one controller, multi-axis solution to simplify software integration.
www.prior.com
Linkam Scientific Instruments has launched a new software, NEXUS, revolutionising the way Linkam stages are used. It is used to precisely control and record all aspects of heating, cooling, vacuum, humidity, and other environmental conditions in a Linkam stage with optional optical measurement capabilities.
NEXUS Core is the main NEXUS platform that introduces a range of new specifications to enable a greater degree of experiment control. Users can easily monitor the status of their experiment with the live display feature, quickly creating observations and adjustments that alter the data collection process. The upgraded software ensures precise synchronisation between samples through the live status display in parallel with a real-time temperature chart.
The new optional NEXUS Image Capture Module facilitates automatic image capture at specific points during an experiment. Each image can be stamped with current temperature and other parameters to make offline analysis a straightforward process. Rewind and review capabilities offer a highly detailed analysis and can be used during live data recording.
Other optional modules build upon the strong foundation of features available in NEXUS Core. These include:
• Extended Measurements Module – fully calibrated measurements, annotation tools, and the ability to burn measurements and annotations into the image for quick analysis.
• Reporting Module – automates the export of charts and images into a user customisable Microsoft Word document.
• TASC Module – Thermal Analysis by Surface Characterisation (TASC) measures multiple microscopic locations across the sample, making it ideal for studying sample inhomogeneity.
• 21CFR11 Module – the compliance module for NEXUS provides simple access to control requirements and enables audit trail creation.
Clara Ko, Sales and Marketing Manager at Linkam commented: “With the increasing focus on automation making it critical for researchers to stay up to date with modern, sustainable experimentation techniques, our new software extends the existing functionalities of Linkam stages to keep users at the cutting edge of technology. Automated, realtime measurements are crucial to the digitisation of experiments. The new NEXUS software release supports our most popular stage families, with new stages being added on an ongoing basis.”
www.linkam.co.uk
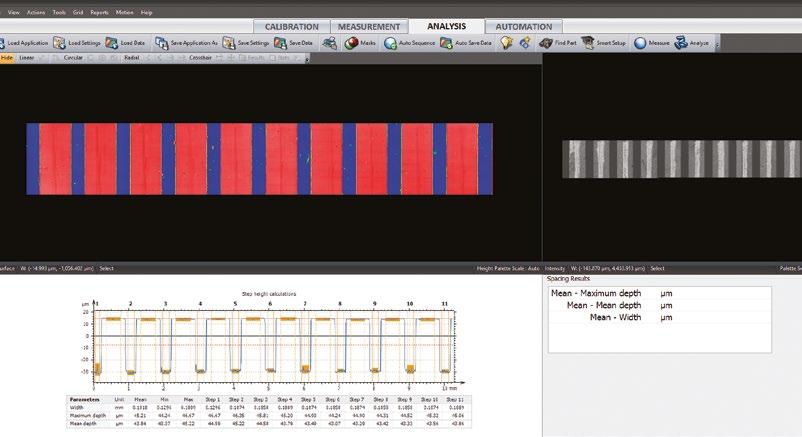
A global leader in the design and manufacture of advanced optical metrology systems — Zygo Corporation — and Digital Surf, creator of the Mountains® software platform for image and surface analysis in microscopy and metrology, today announced the release of Mx™ with Mountains® Advanced Contour. This is a new software package for users of Zygo Corporation’s range of optical profilers.
Michael Schmidt, Market Development Manager for industrial metrology at ZYGO, says, “ZYGO™ Mx™ software powers complete system control, data acquisition and analysis, including rendering interactive 3D maps, quantitative topography data, intuitive navigation, and built-in SPC for our industry-leading 3D non-contact optical products. Mx™ metrology software is already well known throughout the industry for its robust measurements, flexible analyses, intuitive user interface, and easy configuration of automated measurement sequences. The addition of Mountains® Advanced Contour will enable customers to use the profile slices captured in Mx™ and calculate critical dimensions (CD) geometries such as 2D angles, profile circles, step heights, feature
sizes, diameters, and distances. As products and components become smaller and smaller, such CD specifications become tighter. Combining ZYGO’s coherence scanning interferometry (Z-CSI) powered by Mx™ and Mountains® Advanced Contour module creates a flexible and repeatable solution and serves the requirements of our customers dealing with tight CD specs.”
Christophe Mignot, CEO at Digital Surf, says, “We are excited about our collaboration with ZYGO, a company with a long and highly respected history in the optical metrology sector. Digital Surf has been devoted to developing premium software solutions for over 30 years, and Mountains® has today become the industry standard in surface and image analysis for engineers; at the core of Mountains® is micro-topography, the science of studying surface texture and form in 3D at the microscopic scale. The Advanced Contour Analysis Module, which we have added to ZYGO’s Mx™ 9.1 release, provides advanced geometric dimensioning and tolerancing of contour profiles and horizontal contours extracted from images and surfaces.”
www.digitalsurf.com
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk. The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.



Following a recent review, the RMS is widening the accessibility of the benefits of membership by introducing some new categories for 2023. All members will receive the same benefits of membership, but the cost is configured as to be appropriate to career stage. We hope this will enable all our members to benefit equally and that it will encourage sustained membership for mutual benefit.
Undergraduate student member
Doctoral student member*
Any Undergraduate or Masters student enrolled in full or part time undergraduate study anywhere in the world Free
Any student enrolled in full or part time doctoral research anywhere in the world £15
Early career* Within first 5 years of employment (not including career breaks) £30
Ordinary member* Full, standard cost membership £80
Fellow* After three years’ continuous ordinary membership, you are eligible to become a Fellow of the Society and display the postnominals FRMS. Fellowship is subject to meeting set criteria. Fellows are also able to vote at Society meetings and can themselves be elected to the Society’s Sections and Council.
£80
Emeritus* Retired members £30
Honorary Fellow By invitation for people excelling in the field Free
* 50% reduced rate for economically challenged regions as defined by the OECD https://www.oecd.org/dac/financing-sustainable-development/development-finance-standards/DAC-List-ODARecipients-for-reporting-2021-flows.pdf
• Discounted registration fees for RMS meetings, courses and conferences, with great opportunities to network at the events.
• Access to an annual bursary fund to support your attendance at domestic and international conferences.
• Access to apply for one of our Summer Studentships.
• Opportunities to contribute to Outreach and Public Engagement events and funding support for your microscope-related outreach events. Your membership also supports the RMS Engagement and Outreach programme including the Microscope Activity Kit scheme providing free microscopes and science activities for primary Schools in the UK.
• Opportunities for Continuing Professional Development via the RMS Diploma.
• Opportunities to contribute to shaping the future of the field as part of a respected voice with policymakers, research councils, related societies and government.
• Free subscription to Infocus magazine - the quarterly RMS publication. Articles include microscopy techniques and developments, events calendar, news, event reports, book reviews and new product information.
• Discounted subscription to the Journal of Microscopy, a leading multidisciplinary journal.
• Discounts on purchases made online at wiley. com, publishers of academic and educational books and journals.
• Automatic membership of the European Microscopy Society and the additional benefits this entails
To improve your microscope working experience call 01483 927193. Find out more www.visioneng.com




In this issue we catch up with Dr Georgina Fletcher, who joined the RMS in early 2020 in order to co-ordinate the flourishing BioImagingUK community network - a dynamic forum open to any UK-based imaging scientist working in Life Sciences.

Established as a grassroots organisation more than 10 years ago, BioImagingUK is currently jointly funded by the RMS and UKRI-BBSRC. Among its many aims, the network seeks to improve training and career development for imaging scientists, maintain an overview of UK strategy in terms of infrastructure and new technology, and ultimately ensure the UK is well placed to continue being at the forefront of imaging science. New ‘members’ simply sign up to a mailing list and can put forward ideas, highlight events, and advance new projects.
Georgina, who has 20 years’ experience working and studying in bioimaging, provides the organisational backbone for the operation – developing proposals, organising meetings, writing up documents and generally working with members of the community to bring ideas to fruition.
She explains: “In terms of organisation, it is very much a community-driven thing. So if people want to do something or make something happen, they can suggest it and I will work with them and provide administrative support. I post all the updates and the latest information, and members can also post their events and questions. The email archives are public, which means our work is open to view and you can really get a feel for all the things the network is doing.”
“Everybody is involved for a slightly different reason, and the aims of the community are quite varied. It gives things an interesting twist and means my job can be extremely varied from one day to the next. For example, we were built from a community of light and electron microscopists and since then, we have grown to include X-Ray imaging, pre-clinical
Georgina Fletcher.imaging and data science. We are also aiming to engage with the medical imaging community - where there hasn’t traditionally been too much cross-over, but in the future it would be very beneficial to join these communities up, especially as we aim to go ‘from protein to patient’ and image across those very different scales.
She adds: “I really enjoy the reward from feeling that you have helped somebody, or a group of people – and you have made a difference.”
Just one small example of this relates to a recent public art installation in Bristol (about which you can read more on p53). When the artists involved were seeking micrographs of tree roots upon which to base their project, it was through BioimagingUK –and Georgina – that they were able to track down just the very thing they were after.
In terms of the network’s ‘core business’, two major successes this year were the establishment of a UK Node in Euro-BioImaging (read more on p64) and the completion of a second round of Business Interaction Vouchers.The voucher scheme invests in partnerships between academic researchers and industry –supporting new discoveries, the development of imaging applications and training.
Georgina says:“We were able to secure more funding from UKRI-BBSRC by showcasing flash talks of three successful projects from the first round of vouchers in 2019/2020 at our virtual pre-congress MMC meeting last year. That was really rewarding as they clearly thought it was a fantastic scheme to re-fund it for a second time.”

What does Georgina find most challenging about her role?
“It’s important to be mindful that the people I work with are volunteering their time to help the community”, she says. “Sometimes they are very busy, and there are limits to what can be achieved. It’s sometimes about getting to know when people do have time, and when we might need to pick something up again a few months later.”
Having joined the RMS team shortly before the onset of the Covid pandemic, the recent months have provided a welcome opportunity for Georgina to travel to meetings and engage with colleagues – many of whom were previously only known to her via a computer screen.
She says: “I started in January, so we had about three months before everything began to stop happening, and thankfully I had at least been to two in-person meetings, so I got to meet a lot of people at those.
“In some ways the lockdowns had a positive effect because it meant people were at home more, but still wanted to contribute to the community. It meant I could start to get people together who wouldn’t necessarily have had the time or been able to travel, so those two years were actually really productive. Eventually people got so used to doing Zoom and
Teams, it made organising meetings a breeze.
“I work from home and it is quite a solitary job despite the nature of what I do. So it has been really nice to be able to go to meetings again recently, and see people face to face. There is just no substitute for that.”
With the best part of three years working at the RMS now under her belt, what are Georgina’s overall impressions of the Society, and what does she like best about working here?
“I really think everyone who works for the RMS –either staff or members – are really great to work with, and helpful and generous with their time”, she says. “It is a privilege to be able to learn about all the fantastic microscopy techniques and see the amazing images people produce on a daily basis that get us closer to understanding the fascinating mysteries of cells and tissues.”
She adds: “I’m coming from a biological science background, but what is really special about the RMS is that they also have physical and engineering sciences, and materials sciences, so it is a door to another world for me, and it is special to be able to have that every day.
“The RMS is global, but with my Bioimaging UK hat on, we are very lucky to be based in the UK, here in Oxford. I think it helps us bring people together across the science communities and gives the UK an
edge in terms of being able to link people up easily. So much in science comes from that ‘soft side’ of getting to know people, and being able to contact them and discuss ideas. So it’s a real asset to the UK as well as internationally.”
Georgina is also keen to stress the importance of the RMS’s efforts to promote a healthy gender balance on its committees, and in terms of representation at meetings and conferences. This includes the online gender equality database established by RMS Light Microscopy Committee member Sian Culley.
Georgina says: “I’m very proud to work for an organisation that traditionally, probably came from a very male orientated background, but you wouldn’t know that from looking at the RMS today.That is very progressive and inspiring.”
Prior to joining the RMS, Georgina worked and studied in the Life Sciences for around 20 years. She has a PhD from King’s College London, and worked in a number of post-doc positions in Germany and London.
She says:“I was always interested in nature throughout my childhood, and it was when I first found out about
the human genome project that I decided I wanted to do human genetics as an undergraduate. But during that time, I had lectures on developmental biology, about how a single cell becomes a full organism, and that was fascinating. So for my PhD I studied cell migration during gastrulation – a process which has been described as the single most important event in your lifetime.”
After two decades conducting experiments of one sort or another, Georgina started to become more interested in the organisation and management side of working in the lab – and also organising scientific talks. When her London-based supervisor moved to Canberra, she stayed behind and began looking for something new along these lines – just as the BioImaging UK role cropped up.
She says: “Having worked with microscopes for many years and enjoyed the time getting fluorescent images off a confocal microscope, I knew this job was going to be really interesting and enable me to learn more from a technological point of view. It was a good fit for me, with my skill set.”
But what alternative career might Georgina have
pursued, had the world of science not come calling?
She says: “As a child I always wanted to work with animals, but I didn’t think it would end up being fruit flies and frogs in a lab setting – that was a bit of a surprise!

“I really enjoy watching ‘Scandinavian noir’ TV shows – detective shows, or that kind of thing in books. So if I combine my twin passions of animals and solving puzzles I could have been a dog handler in the police or something like that!
“I tended to always have a cat when I was growing up, and now I have adopted two kittens. I would like to have many more animals, but my circumstances don’t allow it at the moment.”
Another of Georgina’s passions is singing in a local choir near her home in Tottenham. She’s been doing this for around the last seven years, and has found it to be a great way of getting to know people in her community. It has also provided an unexpected brush with fame…
“They had asked different choirs to sing before the start of every game and I don’t know how we got asked to do it. Being in a choir is a great way to meet people who you wouldn’t normally meet. There is just something nice about singing together and that sense of achievement when you have produced something that sounds good.
“I live in Tottenham, which is a very mixed area of London – we have probably got every nationality on Earth! I like the fact that we have lots of diversity in the choir, as it reflects the community.”
Georgina was also involved in setting up a Friends group for her local park – Bruce Castle Park Grounds in Tottenham. She says: “We put on openair theatre and live music events in the park and organise monthly litter picks, with home-made cake for volunteers to eat afterwards! We have some excellent bakers in our group and that, plus a nice cup of tea, is a very effective social glue! It has been a really good way to get more connected and meet our local neighbours. In London that is quite rare but really important I think. It makes you feel part of where you live.”
Among Georgina’s other passions are yoga, bikeriding and Manchester City F.C. - the club she has supported for around 25 years. And if the football’s on, you might find her enjoying a pint with friends at her local community-owned pub on a Sunday afternoon.
She says: “Our high point was being asked to sing at Twickenham for the Rugby World Cup. We sang the national anthems before the England – Australia game. We were down on the pitch, really close to these giant rugby players and the atmosphere was incredible. Then we were up in the stands and saw a few famous people sitting in our section. Rio Ferdinand was there – he was in our lift going up to the stand.
She says: “I grew up in Ramsbottom in Greater Manchester and moved out when I first went to University. Despite not having lived there since 1996, to the wonder of my family and friends, my northern accent seems to get stronger every year!”
Many thanks to Georgina for speaking to infocus! BioImagingUK sign up page: https://www. jiscmail.ac.uk/cgi-bin/webadmin?A0=UK-EUROBIOIMAGING-PROJECT
RMS website page: https://www.rms.org.uk/ community/networks-affiliates/bioimaginguk-network.html
infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS.
infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more.
infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms. org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.uk
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
• Total number of images/figures/tables should not exceed 15 including tables.
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
One column/half page width, 65.5mm
Figure 1. Width of figure or table confined to one column.
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).
Two column/full page width, 135mm
Figure 2. Width of figure or table spanning full width of page.
Achieve outstanding Scanning Electron Microscope results and polish pristine SEM samples right from your tabletop. JEOL’s NeoScope benchtop SEM stands apart with high resolution imaging and analysis, Live 3D, Zeromag navigation, automated montage, high and low vacuum modes and Live EDS. JEOL’s broad ion beam milling systems reliably deliver fast, clean cross sections and surfaces. Choose our 2-in-one high speed milling and coating system or our cryo-CP with air-isolation for sensitive samples.
Let us help you set your lab table with the right tools. Learn more at www.jeol.com
