A Novel Horizontally Mounted Confocal Microscope Provides New Insights into Plant Development
Fake honey, Machine Learning and Microscopy
RMS Summer Studentship Reports 2021 (Part 2)
Nanoscale imaging of proteins’ filament assembly on membrane in rebuilt cell membrane
The Human Brain
1 ISSUE 66 JUNE 2022 Plus...News, Calendar, Reviews, Reports ISSUE 66 JUNE 2022
LOOK
E xpand the color capabilities for your cell analysis

At Sony, we strive to bring you the latest innovations and deliver the highest quality flow cytometers to accelerate your research. Our ID7000™ Spectral Cell Analyzer offers the most expanded performance yet, providing state-of-the-art, streamlined multicolor cell analysis to detect even the dimmest fluorochromes and rare cell populations.
The ID7000 incorporates our cutting-edge spectral technology to enable unrivaled flexibility in high parameter flow cytometry. Designed for ease of use at every step, with an intuitive interface and support for walkaway operation, it enables you to run your multicolor experiments with complete confidence of obtaining the best quality data from your cells.
For all your discovery research requiring high parameter data, the ID7000 delivers.

Take a sneak peek at the ID7000 system. Sign up for your virtual product tour at: www.sonybiotechnology.com/id7000 © 2022 Sony Biotechnology Inc. All rights reserved. Sony, the Sony logo, and ID7000 are trademarks of Sony Corporation. For Research Use Only. Not for use in diagnostic or therapeutic procedures. The ID7000 is classified as a Class 1 laser product. 9.15.061621.0 INSIDE WITH A VIRTUAL TOUR! Details below
4 A Novel Horizontally Mounted Confocal Microscope Provides New Insights into Plant Development
Suruchi Roychoudhry
12 Fake honey, Machine Learning and Microscopy
Alexis Gkantiragas
26 RMS Summer Studentship Reports 2021 (Part 2)
Jade Manning, Jack Pearce, Mostin Hu, Chiara Pillen
44 Nanoscale imaging of proteins’ filament assembly on membrane in rebuilt cell membrane
Fouzia Bano, Gerard Castro-Linares, Agata Szuba
64 The Human Brain
Winston Ingram
1 contents regulars 16 Calendar 22 Journal of Microscopy 52 New Member Welcome 54 Office News 82 Company News 88 New Products reports and other features 14 Virtual EBSD 2022 20 Applied Bioinformatics in Life Sciences (4th edition), Leuven, Belgium 36 37-year-old mystery is solved as ‘long-lost’ Fellow rejoins RMS! 70 Sun Protection Conference, London 2021 – UV imaging and microscopy talk 72 2nd Joint Meeting MSI and SMS, Galway, Ireland 76 Rising possibilities of quantitative phase imaging (Zuzana Nováková,Telight) 96 Meet the Staff: Katie Reynolds & Jess Cole features
infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.

infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK
Editor
Owen Morton
Tel + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Emily Eden, University College London, UK
Laura Fumagalli, University of Manchester, UK
Rhiannon Heard, University of Oxford, UK
Maadhav Kothari, University of Cranfield and Rolls Royce, UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2022 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by The ImageWorks. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.
MAGAZINE
RoyalMicroscopicalSociety @RoyalMicroSoc @RoyalMicroSoc
FROM THE SCIENTIFIC EDITOR
Dear Readers,
It is a great pleasure to bring to you our second issue of 2022. For our readers in the northern hemisphere, the month of June represents the arrival of summer (not so much here in Scotland!), with flowers in full bloom around us. Perfect timing then, for a honey-catching feature provided by Alexis Gkantiragas. He tells us about the use of Machine Learning to characterise pollen and use this to determine the origin of honey.The onset of summer might also prompt some of us to do a little spring cleaning around the house, checking old boxes – perhaps from times immemorial. In this issue we find out how the discovery of an old letter sparked a search through the RMS Proceedings (as infocus was previously known) archives and the welcoming back of a long-lost RMS fellow.
We also have interesting pieces on cellular and organismal levels. Suruchi Roychoudhry describes a special collaboration between academia and industry to provide a horizontally mounted microscope to study plant growth. Fouzia Bano and colleagues present us with a great piece on the use of Atomic Force Microscopy and its use in cell membrane. Meanwhile Winston Ingram describes his work using experimental microscopy techniques to take a fresh look at the circuitry of the human brain.
We continue with reports submitted by students who carried out RMS Summer studentship projects during 2021. Jade Manning tells us about her work on the ultrastructure of spheroids models for cancer tumours; Jack Pearce reports on his internship at Diamond Light Source, which involved the use of x-rays to study archaeological samples; Chiara Pillen describes her work at University of Sheffield on analysing nanodiamonds as probes for super-resolution microscopy; and Mostin Hu writes about her investigation into the endomembrane response of host cells, when infected by the group of parasites known as microsporidia. These studentships are a fantastic way to foster a new generation of microscopists.Thanks to all the students for your reports (some were published in issue 65) and I hope we continue to come across your work in the future.
As physical meetings start to happen again, I do look forward to seeing many friends and colleagues at meetings and congresses. And please feel free to approach me, RMS staff or any board member if you would like to discuss how you can contribute to infocus
Lastly, I would like to thank Ian Titley. He has been a fantastic board member of infocus, representing the Flow Cytometry Section. Ian is stepping down from this role and I take this opportunity to wish him all the best. Slàinte!
Leandro Lemgruber
Leandro Lemgruber
COVER IMAGE: Bindweed, by Mike Gibson
Leaf peel from bindweed, showing epidermal cells, including stomata. A combination of Rheinberg coloured filters and oblique (offset) illumination techniques was used with a phase condenser on a Swiss Wild M20 research microscope, coupled to an electronic 5 megapixel USB photo eyepiece.

3
A Novel Horizontally Mounted Confocal Microscope Provides New Insights into Plant Development
Dr Suruchi Roychoudhry, Research Fellow, University of Leeds S.Roychoudhry@leeds.ac.uk
One of the greatest challenges we face in the 21st century is to sustainably feed nine to ten billion people by the year 2050, while simultaneously attempting to reduce the environmental impact of food production. Some options that have been proposed to address these challenges include closing the yield gap (i.e making the difference between attempted and attained yields smaller) and increasing the production potential of crops, largely through the use of new technologies and investing in research in the plant sciences field (Smith and Gregory, 2013). Thus, a crucial overarching aim of the plant sciences is the ability to ‘futureproof’ crops, i.e. use a combination of molecular breeding and genetic techniques to generate elite crop varieties that are able to withstand environmental stresses such as increased CO2 levels as well as water and nutrient scarcity, factors which strongly constrain plant growth in soil (Kissoudis, 2016).
While overall plant architecture both above and below ground, is largely determined by the number and length of secondary (or lateral) organs (Reinhardt and Kuhlemier, 2002), recent studies have demonstrated that the angle at which these lateral organs grow at is also a crucial determinant of plant architecture, and ultimately overall plant fitness (Uga et al 2015). This is because, nutrients such as water, nitrates and phosphates are most often heterogeneously distributed within the soil
strata. For example, nitrates are leached out of soil by precipitation and tend to accumulate within deeper soil layers. Thus, modulation of root growth angle to generate deeper rooting crop species is likely to be a desirable trait, particularly in nitrate deficient soils (Uga et al, 2015). Additionally, since recent research has shown that use of nitrogen fertilisers contributes significantly to global warming, enhancing the ability of plants to take up nitrates from soils more efficiently is also an
4 ISSUE 66 JUNE 2022
important aspect of reducing CO2 emissions and hence, global warming (Tian et al, 2020).
Considering the importance of root (and shoot) growth angle in determining nutrient acquisition efficiency, and ultimately plant fitness and yield, it is not surprising that a number of researchers have begun to investigate the mechanisms by which oblique growth patterns in flowering'bngn dfl...e4 plant organs are regulated, with particular focus on the molecular genetic pathways, with the aim of identifying specific genes that may be targeted to engineer crops with deeper or shallower root and shoot growth angles. While much about these mechanisms remains unknown, using the model plant Arabidopsis thaliana, it has been convincingly demonstrated that the plant hormone auxin, often described as the ‘master regulator’ of plant development controls nonvertical/ oblique growth patterns in higher plant organs (Roychoudhry et al, 2013; Ruiz-Rosquete et al, 2013).
Recently, a broad overall consensus has emerged relating to the molecular mechanisms of regulation of nonvertical growth in plant organs. In contrast to the main parent body of the plant, both, lateral roots and shoots are growing at an angle to the gravity vector. Thus, it is clear that gravity, which broadly acts to ensure that plant roots grow vertically downward and shoots grow vertically upward, is not acting upon lateral organs in the
same manner as the parent axis (reviewed in Roychoudhry and Kepinski, 2015). This has led to several research groups, to further investigate one of the most fundamental processes of plant development – gravitropism. Gravity is the most constant force acting on plants, and the ability of plants to respond to gravity i.e. broadly, for shoots to grow upwards and roots to grow downwards, is known as gravitropism. Because the direction and magnitude of gravity are almost constant on the face of the earth, gravitropism can be regarded as a mechanism of posture control, triggered by sensing the tilt of organs relative to the direction of gravity.
In the model plant A. thaliana (and indeed in most multicellular plants), gravity is sensed in specialised cells along the shoot axis and in the root tip known as statocytes. These cells contain dense starch-filled granules called “amyloplasts’ which sediment in the direction of gravity, a process that was observed as early as 1900 (Haberlandt, 1900). Thus, when a plant organ, for example, a root is rotated by 90o so as to be placed perpendicular to the earth’s surface, gravity ensures that within a few minutes, the amyloplasts sediment towards the new lower side of the root. The sedimentation of these amyloplasts then triggers a signaling cascade that, within a few minutes (reviewed in

5
Morita, 2010; Su et al, 2017), begins with the repolarisation of a set of membrane proteins, known as PIN-FORMED, or PIN proteins towards the lower side of the gravity-
Figure 1: Early molecular events of plant gravitropism (Adapted from Sato et al, 2015). When a vertically growing Arabidopsis seedling (left) is reoriented by 90o and placed perpendicular to the gravity field, this triggers the sedimentation of dense starch filled amyloplasts towards the new physical bottom of the gravity sensing cells in the root tip. The physical signal of amyloplast sedimentation leads to the generation of a biochemical signal that is responsible for repolarisation of the PIN auxin efflux transporter proteins towards the bottom of the gravity sensing cells. The polarisation of PIN proteins leads to the accumulation of auxin towards the bottom of the graviresponding root, which is a key step in the ability of plant organs to respond to gravity.
sensing cells, that are now in contact with the amyloplasts (See Figure 1). Because PIN proteins function as transporters of the plant hormone auxin, this process in turn ensures that the auxin is preferentially transported along the lower side of the root. Remarkably, auxin has the ability to inhibit cellular elongation in roots. This means that as auxin accumulates on the lower side of a gravistimulated root, the cells on this side cease to elongate, while those on the upper side continue to
do so.This resulting asymmetry in auxin distribution and ultimately cellular elongation results in the root ‘correcting’ its direction of growth to grow along the gravity vector again.
Although the overall process of gravitropism is so fundamental to regulating overall plant architecture, surprisingly many aspects of the molecular mechanisms underpinning both gravitational sensing and response in plant organs remain poorly understood. In Arabidopsis roots, particularly, it is



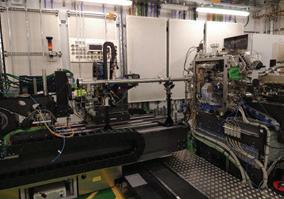
At ZEISS we recognise that microscopes designed for a production line, even when they are highly modular and configurable, may not always be perfectly adapted for observing all of nature’s biological processes. Our Special Customer Solutions (SCS) team focus on designing and developing bespoke microscopy solutions for more challenging applications. In Prof. Kepinski’s case when studying plant root growth the challenge is to keep the plant in its natural vertical orientation so that the it exhibits a typical geotrophic response. A typical upright or inverted microscope is therefore not appropriate for observing this growth.The challenge was therefore to rotate the microscope stand with attached laser scanning confocal microscope so it has a horizontal optical axis. The concept to adapt the microscope to the sample’s “natural” conditions rather than the other way round was both equally interesting and challenging for our SCS and local application support teams.
The initial on-site installation was completed as normal for an LSM800. Then the microscope was tilted and secured on its back using special mounts designed by a collaborative group in Vienna.
Integrating the control of the motorised rotating stage was performed by our colleagues in software development. This allowed the angle of the observed plants to be fully controllable by the software and macros; and with some trigonometry, the stage position is adapted so the root is still in the centre of the field of view while rotating.
The ZEISS customer Service team on the design and installation of the new horizontally mounted LSM800 at the University of Leeds.
6 ISSUE 66 JUNE 2022
Figure 2. Confocal images of GFP and YFP markers of amyloplasts (left), ER (middle) and PIN proteins (right) in 5-day-old Arabidopsis seedling root tips taken at 40X magnification with the LSM800 horizontal microscope. The seedlings were grown in chambered slides (Labtek) and counterstained with propidium iodide prior to imaging. For imaging the PIN protein GFP marker, a series of Z stacks were taken through the root, and compiled to create a ‘sum of stacks’ 3D projection in Fiji.
ER PIN
Amyloplast
The horizontal microscope configuration was also an issue for the XY stage at first, as gravity was pulling it down under the weight of the stage and insert. A temporary workaround involving pulleys and weights allowed the system to work until ASI provided a stage with a different gear pitch strong enough to work against gravity and solve the issue. Although this was a technically challenging project, we are extremely pleased to have worked closely with the University of Leeds and ultimately delivered on a bespoke microscopy solution that focuses on the sample in its most natural state. ZEISS hope that the horizontal LSM800 will unlock many new opportunities at the University of Leeds to study new areas of plant biology that were previously impossible. The ZEISS Customer Service team on the challenges of installing the bespoke motorised rotating stage for the horizontally mounted LSM800 at the University of Leeds.
not clear how the first physical signal of amyloplast sedimentation triggers subsequent biochemical pathways leading to PIN repolarisation and auxin redistribution. Furthermore, plants also have the ability to mount a gravitational response that is proportional to the angle of stimulation, according to the so called ‘Sine Law’, first described by von Sachs in the 1800s. Thus, a root that is reoriented by 900 bends downward in the direction of gravity at a faster rate than a root that is reoriented by a smaller angle, such as 45o. (Sachs J., 1882).
Amongst the several biochemical and physiological tools that researchers have employed to answer these questions, real-time live cell imaging provides a powerful strategy to elucidate the spatiotemporal processes of gravity sensing and response in situ. These approaches have been aided by the generation of several stable transgenic plant lines by multiple research groups over the years, containing fluorescent markers for different organelles involved in the gravitropic response (Friml et al, 2003; Nelson et al, 2007; Geldner et al, 2009, see Figure 2). As some examples,YFP tagged lines for the starch-filled statoliths, as well as the PIN membrane proteins have been utilised to visualise sedimentation kinetics of statoliths and PIN repolarisation in response to gravity and have provided valuable insights into the temporal resolution of these steps. However, a key limitation of all these studies has been the inability to perform dynamic, live cell confocal
(or indeed super resolution) imaging on vertically growing plant roots, or those reoriented within the X-Y plane. These challenges have been difficult to overcome, due to the horizontal sample positioning set up of confocal (and other) microscopes, as well as the continuous displacement of the root tip as it undergoes the gravitropic response. Thus, previous work on the kinetics of gravi-dependent organelle repositioning has involved the fixation of Arabidopsis root samples prior to imaging, usually using a 4% paraformaldehyde solution. More recently, advanced techniques for sample fixation such as ClearSee (Kurihara et al, 2015) have been developed. While fixation preserves the orientation of the sample with respect to gravity, the overall process is still lengthy, time consuming and often quenches the fluorescent signal. Our innovative solution to overcome all these technical challenges at the University of Leeds was the installation of a novel horizontally mounted confocal microscope with a rotating vertical stage.
Initially funded through a BBSRC equipment alert in 2016, the setup is currently located within the Bioimaging Suite at the Faculty of Biological Sciences at the University of Leeds. Briefly, it consists of an LSM800 confocal microscope (with Airyscan, ZEISS) that has simply been rotated by 90o so that it is mounted horizontally on its back. The microscope is secured in this horizontal position using special metal mounts that were specifically designed for
7
this purpose by our collaborators, the Friml group at the Institute of Science and Technology (IST), in Vienna, Austria. This configuration preserves access to all the features of the microscope, while now having the additional capacity of being able to hold plants vertically in their native states. Plant samples are grown vertically on commercially available ‘Labtek” chambered slides between the cover slip and the addition of a thin layer of plant growth medium (See Figure 3).
Additionally, the microscope setup also contains a specially engineered bespoke rotating stage with the capacity of holding a range of different types of slides, so that plants (or indeed other samples) can be reorientated at any desired angles ranging from 0-360o, as well as customised LED lighting to carry out experiments related to phototropic (i.e. the movement of plants in response to directional light) responses. Such an approach was first developed by the lab of Prof. Jiri Friml at IST, using an LSM700. However, the newly mounted LSM800 at Leeds is approximately 20 times more sensitive than this system, and the inclusion of Airyscan has enabled up to 1.7x increased resolution in all three axes, making this one of the most advanced microscopes of its kind anywhere in the world. Another advantage of using this system is that the chambered slides can be mounted onto the rotating stage of the microscope directly, without any additional sample preparation. This process has the added benefit of
zero perturbation of the samples prior to imaging.



In addition to all this, the ZEISS team have further developed a customised software that enables tracking of the root tip for up to 24 hours. This means that researchers can set up their reorientation experiment with, for example, a number of Arabidopsis roots expressing different fluorescent markers and create time lapse image series at high resolution with minimal input.
In order to generate longer time lapse films at magnifications of 40X and 60X however, an added challenge remained initially. Typically, the use of these oil immersion lenses requires the placement of a drop of oil either on the lens surface, or the sample. While this is easily achieved with a typical confocal microscope, placement of oil on the lens of the horizontally mounted LSM800 caused it to drip downwards off the lens. After much trial (and error!) we found that the use of ultrasound gel, of the kind used generally for sonography was a good substitute for oil. The viscosity of the gel ensures that the slide can be coated with a layer of it for imaging at higher magnification with no issues.
This cutting-edge imaging system has already provided invaluable novel insights into the molecular processes of root gravitropism in the model plant A. thaliana. For example, we have discovered that contrary to previous data, where amyloplast sedimentation in response to gravity was
8 ISSUE 66 JUNE 2022
Figure 3. Front (left panel) and side (middle panel) views of the horizontally mounted Zeiss LSM 800 confocal microscope located within the Bioimaging suite at the University of Leeds. The asterisk (*) denotes the specially engineered metal mount to support the microscope in its horizontal position. The red lines (middle panel) show an inset of the motarised rotating stage (right panel) holding a chambered slide containing vertically grown 5-day-old Arabidopsis seedlings.
Front view
Motorised Rotating Stage
Side view
thought to be a fairly rapid process, the spreading of amyloplasts over the plasma membrane at the new physical bottom of the gravity sensing statocytes can take up to 20 mins. Further, confirming previous studies, we have found that the sedimenting amyloplasts are able to mechanically deform the ER network overlying the lower plasma membrane, and interestingly, that ER structure is restored after 15-20 mins post statolith sedimentation. Finally, we have started to make inroads into elucidating the molecular mechanisms of angle dependent gravitropic response in Arabidopsis roots, by demonstrating that the percentage of PIN proteins that polarise to the lower membrane of gravitysensing cells post reorientation, are proportional to the angle of reorientation.
Taken together, this novel ZEISS LSM800 horizontally mounted microscope has a range of potential benefits and beneficiaries. Besides allowing plants to be imaged in their native vertically growing states with such detail for the first time, this imaging set up has already dramatically enhanced research output at the University of Leeds, where it is located, allowing researchers to develop new skills in high resolution live cell imaging, automation methods and image analysis. Secondly, we envisage that the quality of plant sciences research within the UK will be significantly enhanced. This will subsequently lead to increased economic competitiveness of the UK as well as foster cutting edge interdisciplinary research - not only within the academic field but also within public and private sectors. Finally, in the long term, our findings will be crucial to the understanding of plant gravitropism and eventually the regulation of plant architecture, the modification of which provides an extremely attractive target in the quest for food security in the face of global warming and a rapidly expanding population.
For more information on usage and access to the ZEISS LSM800 horizontal microscope please contact bioimaging-facs@leeds.ac.uk
References:
Friml J. et al. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153
Geldner, N., Dénervaud-Tendon, V., Hyman, D. L., Mayer, U., Stierhof, Y. D., & Chory, J. (2009). Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. The Plant journal : for cell and molecular biology, 59(1), 169–178. https://doi.org/10.1111/j.1365-313X.2009.03851.x
Gilroy S. (2013). Plant Tropisms. Current Biology, 18 (7): 275-277
Haberlandt, G. (1900). Ueber die perzeption des geotropischen reizes. Ber Dtsch Bot Ges 18, 261–272
Kissoudis C, van De Weil C, Visser RGF, ven der Linden G. (2016). Future-Proof crops: Challenges and strategies for climate resilience improvement. Current Opinion in Plant Biology 30:47-56
Kurihara D, Mizuta Y, Sato Y, Higashiyama T. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development. 2015 Dec 1;142(23):4168-79. doi: 10.1242/dev.127613.
Epub 2015 Oct 22. PMID: 26493404; PMCID: PMC4712841.
Morita M.T. (2010). Directional gravity sensing in gravitropism. Annual Reviews in Plant Biology, 61: 705-720
Nelson, B.K., Cai, X. and Nebenfuhr, A. (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136.
Reinhardt, D., & Kuhlemeier, C. (2002). Plant architecture. EMBO reports, 3(9), 846–851. https:// doi.org/10.1093/embo-reports/kvf177
Sato, E. M., Hijazi, H., Bennett, M. J., Vissenberg, K., & Swarup, R. (2015). New insights into root gravitropic signalling. Journal of experimental botany, 66(8), 2155–2165. https://doi.org/10.1093/jxb/eru515
Sachs J (1882) Über orthotrope und plagiotrope pflan- zenteile. Arb Bot Inst Würzburg 2:226–284.
9
Smith P. and Gregory P.J. (2013). Climate change and sustainable food production. 72: 21-28
Su. S, Gibbs NM, Jancewicz AL, Masson PJ (2017). Molecular mechanisms of root gravitropism. Current Biology 17(27): 964-972
Tian, H., Xu, R., Canadell, J.G. et al. (2020). A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256
Suruchi Roychoudhry

Uga, Y., Kitomi, Y., Ishikawa, S., & Yano, M. (2015). Genetic improvement for root growth angle to enhance crop production. Breeding science, 65(2), 111–119. https://doi.org/10.1270/jsbbs.65.111
Zádníková P, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137:607–617.
Suruchi Roychoudhry (she/her) is a Senior Research Fellow in Plant Developmental Biology currently working within the Centre for Plant Sciences at the University of Leeds. Her PhD work uncovered a novel role of the plant hormone auxin in regulating a critical aspect of plant architecture – oblique growth angles in secondary organs. She then did a short postdoc at the University of Chicago, USA investigating innate immunity in plants through characterisation of protein-protein interactions in planta with a heavy focus on bioimaging. Through this work, she discovered a newfound special interest in live cell imaging and image analysis, techniques that are especially useful for her current work on understanding the molecular basis of plant gravitropism. In her spare time she enjoys sharing her research with different audiences and the general public in the form of articles, seminars, outreach events and through her blog ‘Plantasia’ (https://www.plantasia.online).
10 ISSUE 66 JUNE 2022

Fake honey, Machine Learning and Microscopy
By Alexis Gkantiragas
At a conference I bumped into an eccentric fellow student who was toying with the idea of developing a new method for honey authentication. His background was in machine learning, but he had little microscopy or bee research experience and so I offered to help. Honey is one of the world’s most faked products, and one can theoretically identify the origin of the honey from the morphology and size of the pollen in the honey.
Since current methods are either ineffective or prohibitively expensive, and faked honey harms both beekeepers and bees, an authentication tool that was affordable but effective would have tangible benefits. We also came to the realisation that it could have practical benefits in bee research and environmental monitoring. Indeed, when I reached out to a former supervisor who works on both honeybees and bumblebees, it transpired that all pollen-based research in bee research was being done manually.
Unfortunately, my supervisor at the time did not want me using the lab’s microscopes for the proposed project as this was not covered by the grants. So, we began by using my microscope that I had left over from my school days and mounting a smartphone camera to its eyepiece. Sadly, this promptly broke and we had to get a second one. We grabbed honey from various shops and supermarkets. The use of affordable equipment was crucial since current methods for honey authentication are prohibitively expensive for all but the largest scale beekeepers. We wanted to make a tool that could be used by any beekeeper with basic competency and around £100 spare to spend on a microscope.
I managed to set up a very makeshift microscope rig in my bedroom and began scanning slides of honey (figure 1). By appropriately compressing the cover slip onto the honey I was able to view the pollen in roughly a single focal plane. Amusingly, some of the samples tested had no pollen visible whatsoever due to ultrafiltration of the honey prior to its sale.
The initial work, which was later presented at NeurIPS1 (Conference on Neural Information

12 ISSUE 66 JUNE 2022
Figure 1. The makeshift rig.
Processing Systems - formerly NIPS), the largest machine learning conference, was done using a ‘supervised’ approach. In practice, this consisted of my spending hours in front of a laptop painstakingly segmenting (drawing boxes around) and labelling the pollen. I attempted (unsuccessfully) to convince my university to fund my travel to the conference, since only one person would be funded by the conference itself. They expressed confusion as to how and why a biochemistry student had managed to get his work into a machine learning conference. After the work was presented, we were baffled to receive media coverage from FastCompany and Techxplore.
For the next set of work, we set up two microscopes (we bought another so we could work simultaneously) in my family’s caravan, since my new room lacked a desk or space to put one. The algorithm used for this segment of work was unsupervised - meaning that it classified the pollen into groups without human input (figure 2). From the initial data we were able to deduce that it achieved roughly family level classification. After we presented our research again at the International Conference on Machine Learning (ICML)2, the World Bee Project CIC kindly gave us a modest grant to continue our work.
Wanting to refine our work further and benchmark to human classification we came upon the realisation that we needed to be able to measure the dimensions of the pollen accurately. Unfortunately, the cheap microscopes we had bought did not have integrated eyepiece graticules and we realised that to require users to have an eyepiece graticule would make the technology prohibitively expensive for many beekeepers. Thus, we developed an opensource programme which calibrated the microscope from a stage micrometre alone3. Surprisingly this was novel, and we hope that it will increase access to microscopic techniques more broadly. Next, we plan to apply this sizing technology alongside our pollen identification and classification tools.
It has surprised me just how accessible the intersection between light microscopy and machinelearning is and how viable it is to do actual novel and impactful research with little-to-no funding. I think it is quite plausible there are similar gaps and opportunities for relatively cheap, proof-of-concept work in other areas waiting to be addressed.
References
1. He, P., Gkantiragas, A. & Glowacki, G. Honey authentication with machine learning augmented bright-field microscopy. arXiv (2018).
2. He, P., Glowacki, G. & Gkantiragas, A. Unsupervised Representations of Pollen in Bright-Field Microscopy. in international conference on machine learning (2019). doi:1901.00516.
3. Glowacki, G., Gkantiragas, A., Brooke, B.-H., Mihalik, D. & He, P. Development of an open source program to identify the size of objects using bright-field microscopy - an automated calibration tool. bioRxiv (2021).
13
Figure 2. Unsupervised clustering of pollen types. From He et al., 2019.
Virtual EBSD 2022
Connecting Materials Science, Geology and Biology
Authors: Martin Heller (RWTH Aachen University & University of British Columbia), Ruth Birch (Imperial College London)
This year’s annual Electron Backscatter Diffraction (EBSD) conference, hosted by the RMS, was held virtually for the second time in April due to the ongoing pandemic. EBSD is used in many fields from Materials Science to Geology to Biology, all of which were present at the conference. With attendees from a wide range of countries, the two-day conference consisted of four oral sessions, one poster session and a hands-on electron microscopy workshop hosted by Carl Zeiss AG.
By means of EBSD, crystal phase and orientation data can be collected from well-prepared surfaces of crystalline materials. More detailed analysis of the diffraction patterns or in combination with other characterisation methods like X-ray diffraction, cathodoluminescence or Raman spectroscopy can provide further insights into the microstructure. As this conference has shown, it is always impressive how much we can learn about our materials through EBSD and how much untapped potential there still is in this method.
Hereafter, we would like to highlight three great and informative talks in more detail. First, the presentation by Dr Christiane Rößler et al. on Sample preparation and EBSD-EDS characterization of Portland cement clinker from the Bauhaus University Weimar, Germany. EBSD demands a high surface finish which is difficult to achieve for the water-reactive, low-symmetry silicate clinker phases contained in Portland cement clinker. However, Dr Rößler and team not only succeeded with the preparation but also showed interesting first insights into the correlation between phase, chemical composition, and grain size of this
material. Second, we would like to highlight the interactive presentation by Dr Lionel Germain et al. on Processing of EBSD data by Deep Learning Networks for Phase Segmentation: an Experience Feedback from the Université de Lorraine, France. He and his group tried to approach the well-known difficulties with (subjective) quantitative steel phase segmentation by EBSD and a deep learning network approach. The subjectivity of the usual, manual selection approach was emphasised by allowing the audience to categorise various microstructures, which was a fun way to participate in the talk. Finally, we would like to highlight the talk by Maggie Cusack (President of Munster Technological University, Ireland) et al. on Understanding Biominerals: as easy as EBSD. Among other things, she impressively showed how increasing acidification (related to climate change) changes the material properties of mussels through an inherent reduction of crystallographic orientation control by EBSD measurements. The schedule was busy, so there was only time for one or two questions after each talk, but the virtual format offered other ways for people to connect and follow-up with ideas using the Zoom chat and a specially set up Slack channel.
14 ISSUE 66 JUNE 2022
This year, the poster sessions were organised using the Zoom breakout room tool. One room was created for each poster and the audience could move between rooms to ask questions, discuss the results, or just listen to what was currently going on in that breakout room, which led to a lively exchange of ideas. The winners of this year’s poster award are Elisabetta Mariani, University of Liverpool, for her group’s poster on Hydrothermal venting along an active normal fault in fast spreading oceanic crust and Ning Fang, Imperial College London for her group’s poster on Optimising broad ion beam polishing of zircaloy-4 for electron backscatter diffraction analysis. As so often, this poster session was a good opportunity for in depth discussion, and the format allowed for an intense but productive exchange of ideas.
In addition to sponsoring, companies such as EDAX, Gatan, and Oxford Instruments also participated by providing small techno bites at the end of each session, and in some cases even contributed with scientific presentations, nicely demonstrating the link between science and industry and possible future career paths for all attendees.

While there are many advantages to an in-person meeting, the virtual format has many distinct advantages for smaller topical conferences. Careful scheduling enabled a wide range of people to participate and engage, including scientists of all career stages from Canada, USA, Germany, UK, Ireland, Denmark, France, Argentina, India, Poland, and Netherlands. Furthermore, people with different caring responsibilities and other priorities could balance the half day sessions into their busy schedules. In the future, a hybrid format could combine the advantages of both formats.
In summary, the conference was a great enrichment for our daily scientific work. The broad range of scientific disciplines and the international visibility of the conference should be emphasised, potentially promoting scientific creativity and cooperation between different disciplines. A big thank you goes to the RMS for organising this annual conference as well as to the scientific organisers Dr Ben Britton (University of British Columbia and Imperial College London), Dr Katherina Marquardt (Imperial College London) and Dr João Quinta da Fonseca (University of Manchester).

15
Poster prize-winner Ning Fang.
Poster prize-winner Elisabetta Mariani.
Calendar
We are very pleased to be making a return to in-person events this year, as we emerge from the Covid-19 pandemic, although we will continue to organise a number of virtual events.
The following information was correct at the time infocus went to print but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates. Our online calendar includes all the details about forthcoming talks in the International Microscopy Lecture Series – a joint, online initiative established between the RMS, and a number of international societies. You can also sign up for the popular Imaging ONEWORLD talks covering all aspects of microscopy and imaging. These take place on Mondays at 1pm (BST).
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
2022
June
6 – 10 Cryo-Electron Microscopy Course 2022 – Harpenden, UK
7 – 10 elmi2022 - Logomo, Turku, Finland (Non-RMS Event)
15 – 17 Virtual ESRIC Summer School 2022 & 20 – 22
July
4 – 6 Virtual RMS AFM & SPM Meeting 2022
5 – 6 Frontiers in BioImaging 2022 –Birmingham, UK
5 – 7 EMAG 2022 - Multidimensional Electron Microscopy (Non-RMS Event)
13 – 15
Challenges in biological cryo electron microscopy: Faraday Discussion (Non-RMS Event)
18 – 19 South-West Electron Microscopy, Plymouth, UK (Non-RMS Event) 20 –22 FlowcytometryUK 2022 – Birmingham, UK
31 – 4 Microscopy & Microanalysis 2022, Portland, August Oregon, USA (Non-RMS Event)
August
22 – 26 ELMINA2022, Belgrade, Serbia (Non-RMS Event)
28 – 31 CHC 2022, Prague, Czech Republic (Non-RMS Event)
September
11 – 15 Abercrombie Meeting 2022 – Oxford, UK
12 – 16 Flow Cytometry Course 2022 – York, UK
29 Microscopy: Advances, Innovation, Impact 2022 – incorporating RMS AGM & Section AGMs – London UK
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
16 ISSUE 66 JUNE 2022
Featured RMS events
Virtual RMS AFM & SPM Meeting 2022
4 – 6 July, Online & Hybrid (Sheffield)
Scientific Organisers: Professor Jamie Hobbs & Dr Alice Pyne, University of Sheffield
AFM&SPM 2022 replaces the biennial UKSPM series of conferences. This year the meeting will run over three days and be fully virtual on the first and last day with the option to attend a hybrid in-person meeting on the middle day. We hope this compromise approach will provide an opportunity for the UK community to come together while maintaining the international feel for the meeting that the fully virtual AFM&SPM 2020 had, and avoid the travel issues that remain
Frontiers in BioImaging 2022
5 – 6 July, Birmingham, UK
Scientific Organisers: Dr Joelle Goulding, University of Nottingham; Dr Martin Jones, Francis Crick Institute; Dr Deirdre Kavanagh, University of Oxford, Dr Leandro Lemgruber, University of Glasgow; Dr Ferran Valderrama, St George’s University of London
Frontiers in Bioimaging 2022 will focus on the latest developments in optical and electron microscopy as well as image analysis. Sessions will cover novel technical developments and applications of these microscopy-based
inevitable at the moment.
We have an exciting programme of invited speakers and contributed talks, posters and flash presentations from across all aspects of scanning probe microscopy, from biological imaging, through nano infra-red spectroscopy to UHV non-contact AFM. There will be a strong focus on methods with “nuts-and-bolts” talks to complement some of the lectures, break-out rooms for speakers to provide an opportunity to ask in-depth questions, and panel meetings where developments in the field can be discussed. We very much look forward to meeting you.
www.rms.org.uk
approaches to key cell and molecular biology questions with an overarching aim to bring insights on how they participate in our understanding of human health and disease. We aim to provide an environment in which earlycareer and established researchers can meet and engage with a broad range of imaging approaches and make valuable contacts with leading groups in the field.
This event will also have opportunities for companies to exhibit and sponsor.
www.rms.org.uk
17
FlowcytometryUK 2022
20 – 22 July, Birmingham, UK
Scientific Organisers: Mr Derek Davies, The Francis Crick Institute; Dr Rachael Walker, Babraham Institute
This meeting will consist of themed plenary sessions with talks from invited speakers. There will also be parallel scientific workshops organised by members of the cytometry community and parallel commercial workshops.

There will be a large exhibition and the opportunity to network with flow and image cytometrists from all over Europe and beyond. The meeting will highlight advances in flow and image instrumentation, high content screening, cancer and stem cell biology, applications of clinical cytometry and the development of novel probes and approaches in many areas of biomedical research.
www.rms.org.uk
Abercrombie Meeting 2022
11 – 15 September, Oxford, UK
Scientific Organisers: Dr Brian Stramer, King’s College London; Dr Gaudenz Danuser, UT Southwestern Medical Center, Dallas; Professor Ewa Paluch, University of Cambridge
The series of Abercrombie meetings have been held since the death of Michael Abercrombie
in 1979. Michael was a pioneer in the field of investigating cell behaviour using timelapse microscopy. Abercrombie meetings are held only every five years and therefore offer an excellent opportunity to review the major advances in our understanding of cell motility and look to the new emerging concepts in the field.
www.rms.org.uk
18 ISSUE 66 JUNE 2022







RAMAN MICROSCOPE LabRAM Soleil www.horiba.com/labramsoleil Explore the future of your application Ultrafast Imaging Automation Intuitive Software Confocal Raman Imaging microscope. New and smart imaging capabilities let you map up to 100x faster, with confidence. Robust high performance optical design, coupled with advanced automation, including ultrafast Auto-alignment and objective recognition, offers ultimate reliability and stability. Check out performance levels: Pharmaceutical tablet Getting There Faster! enquiries.uk@horiba.com Request a demo:
Applied Bioinformatics in Life Sciences (4th edition)
Leuven (Belgium) 10-11 March 2022

The 4th edition of Applied Bioinformatics in Life Sciences (ABLS22), organised by VIB, took place in Leuven last March.
VIB is a life sciences research institute that includes laboratories and facilities distributed across different cities of the Flanders (Belgium).
VIB Conference Series (www.vibconferences.be), a program curated by a dedicated organisation team, is aimed at connecting researchers at international level. The past events, from 2014 to date, included a wide variety of topics such as immunology, cancer, neurosciences etc.
ABLS22 took place in the fascinating urban
landscape of Leuven, a university city since 1425.The city hosts several ancient and modern constructions and monuments, amongst which is certainly worth of mention the University Library, a neoRenaissance style building that has been destroyed and reconstructed twice (in WWI and WWII).
Leuven is a vibrant city enriched by the presence of thousands of students and probably as many bikes flowing through the streets and the numerous bike lanes that run around the city.
After almost two years of remote work, meeting and networking, the possibility to join a large group of international researchers, students, and facilities staff, has been very motivating. During the conference I had the opportunity to discuss my work in the field of microscopy and image analysis, and present my poster on 3D image segmentation

20 ISSUE 66 JUNE 2022
REPORT
University Library – Leuven.
‘De ontvoering van Europa’ – statue in front of the ‘Provinciehuis’ building.
and parent-child relation on microscopy data sets with napari. The in-person conference, beyond virtual talks, was a valuable chance to promote my plugin that I recently developed as open-source software. Hopefully, other microscopists and image analysists may adopt it or criticise, copy, and/or ameliorate my code, pushing forward the field of open-source bioimage analysis.
The conference was articulated in four different sessions addressing the most recent developments of bioinformatics applied to: image analysis, single cell analysis, proteomics and metabolomics, and data integration. The topics included novel applications of machine and deep learning to image segmentation (Anna Kreshuk, Cell Biology and Biophysics, EMBL Heidelberg, DE) and restoration (Florian Jug, Computational biology, Fondazione Human Technopole, Milan, IT), as well as to multi-
omics and spatially resolved data. There have been interesting talks about the adoption of the FAIR principles for data management, and a special focus on the available technology to deal with Big Data and collaborative analysis. Moreover, several talks were delivered by facility staff, showing how scientists working in research supporting roles can develop cutting-edge technology and offer it as a service in the fields of light/electron microscopy, single cell analysis and data management.
ABLS22 saw the presence of 25 notable speakers, 64 presented posters and an in-person audience of several hundreds. I would like to thank the Royal Microscopical Society and the Francis Crick Institute for supporting me in attending ABLS22.


Rocco D’Antuono, Light Microscopy STP, The Francis Crick Institute, London, UK
21
Poster session at ABLS22 conference.
'Horror Vacui' graffiti - Leuven.
Microscopy Journal of
The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences. You
Challenges and advances in optical 3D mesoscale imaging

Sebastian Munck, Christopher Cawthorne, Abril Escamilla-Ayala, Axelle Kerstens, Sergio Gabarre, Katrina Wesencraft, Eliana Battistella, Rebecca Craig, Emmanuel G. Reynaud, Jim Swoger, Gail McConnell
Optical mesoscale imaging is a rapidly developing field that allows the visualisation of larger samples than is possible with standard light microscopy, and fills a gap between cell and organism resolution. It spans from advanced fluorescence imaging of micrometric cell clusters to centimetre-size complete organisms. However, with larger volume specimens, new problems arise. Imaging deeper into tissues at high resolution poses challenges ranging from optical distortions to shadowing from opaque structures. This manuscript discusses the latest developments in mesoscale imaging and highlights limitations, namely labeling, clearing, absorption, scattering, and also sample handling. We then focus on approaches that seek to turn mesoscale imaging into a more quantitative technique, analogous to quantitative tomography in medical imaging, highlighting a future role for digital and physical phantoms as well as artificial intelligence.
Carbon-film-based Zernike phase plates with smooth thickness gradient for phase-contrast transmission electron microscopy with reduced fringing artifacts
M. Obermair, S. Hettler, M. Dries, M. Hugenschmidt, R. Spiecker, D. Gerthsen
Phase plates (PPs) in transmission electron microscopy (TEM) improve the contrast of weakly scattering objects under in-focus imaging conditions. A well-established PP type is the Zernike (Z)PP, which consists of a thin amorphous carbon (aC) film with a micro-scaled hole in the centre. The mean inner potential of the aC film is exploited to shift the phase of the scattered electrons while the unscattered electrons in the zero-order beam propagate through the hole and remain unaffected. However, the abrupt thickness increase at the hole edge induces an abrupt change of the phase-shift distribution and leads to fringing, i.e., intensity oscillations around imaged objects, in TEM images. In this work, we have used focused-ion-beam milling to fabricate ZPPs with abrupt and graded thickness profiles around the center hole. Depending on the thickness gradient and inner hole radius, graded-ZPP-TEM images of an aC/vacuum interface and bundles of carbon nanotubes (CNTs) show strongly reduced fringing. Image simulations were performed with ZPP-phase-
22 ISSUE 66 JUNE 2022
online at
can read the latest Early View papers
www.journalofmicroscopy.org They include:
shift distributions derived from measured thickness profiles of graded ZPPs, which show good agreement with the experimental images.
• Fringing artifacts, i.e. intensity oscillations around imaged objects, are strongly reduced for Zernike phase plates with a graded thickness profile around the center hole.
• Focused-ion-beam milling is used to fabricate graded Zernike phase plates with specific inner hole radius and thickness gradients.
• The phase-shift distribution is obtained from measured thickness profiles around the centre hole.
• Image simulations based on experimentally measured thickness/phase-shift distributions show good agreement with experimental Zernike phase-plate TEM images.
The effects of measurement parameters on the cancerous cell nucleus characterisation by atomic force microscopy in vitro

Jiajing Zhu, Yanling Tian, Jin Yan, Jing Hu, Zuobin Wang, Xianping Liu
Cancer is now responsible for the leading cause of death worldwide. It is noteworthy that lung cancer has been recognised as the highest incidence (11.6%) and mortality (18.4%) for combined sexes among a variety of cancer diseases. Therefore, it is of great value to investigate the mechanical properties of lung cancerous cells for early diagnosis. This paper focus on the influence of measurement parameters on the measured central Young’s moduli of single live A549 cell in vitro based on the force spectroscopy mode of atomic force microscopy (AFM). The effects of the measurement parameters on the measured central Young’s moduli were analysed by fitting the force–depth curves utilising the Sneddon model. The results revealed that the Young’s moduli of A549 cells increased with the larger indentation force, higher
indentation speed, less retraction time, deeper Z length and lower purity percentage of serum. The Young’s moduli of cells increased first and then decreased with the increasing dwell time. Hence, this research may have potential significance to provide reference for the standardised detection of a single cancerous cell in vitro using AFM methodologies.
Investigation of trapped charges profile for an irradiated insulated material
Ali S. Mahdi, Hassan N. Al-Obaidi, Huda
K. Husien
The process of examining and analysing insulating materials using a scanning electron microscope usually accompanied by an important phenomenon called the mirror effect or charging effects. Such effects arise due to the ability of insulators to trapping charges at the sample surface for a period. The accumulation of charges leads to creating an electric potential that may be strong enough to deflect incident electrons in the same way a convex mirror scatters light. The created potential depends mainly on the charge amount, charges accumulation profile and the way by which the charges arranging themselves. Present work aims at exploring the influences of the charges distribution profile and their arrangements.
In order to achieve such a goal, the samplesurface potential has theoretically formulated to include various shapes of the accumulated charges. Thereafter, the correspondence expression of the mirror plot curve is defined to link the geometrical distribution of charges. The resultant formula for the surface potential and mirror plot showed that the point charge approximation is a special case of the presented model.
The formula of mirror-plot curve has put forward to be a detection tool for the actual build-up form that the electrons accumulating might take on the
23
insulator surface. Simulation results have shown that the presented procedure could be adopted to search for the optimum distribution profile that may meet an experimental data. It is found that the most probable profile that accumulated electrons might form is the semi-hemispheric one. The surface of this profile is generally an ellipsoid of a variant axis rather a flat one. Results also reveal that, all the multipole-moment types could be formed for any shape of accumulation, but their weightiness progressively decreases whenever the pole-number increases. Furthermore, the configurations that trapped electrons arrange themselves within each distribution profile can be traced with the variation of scanning potential.

Visualising the effect of freezing on the vascular system of wheat in three dimensions by in-block imaging of dye-infiltrated plants

David Livingston, Tan Tuong, Ripley
Tisdale, Rich Zobel
Infrared thermography has shown after roots of grasses freeze, ice spreads into the crown and then acropetally into leaves initially through vascular bundles. Leaves freeze singly with the oldest leaves freezing first and the youngest freezing later.Visualising the vascular system in its native 3-dimensional state will help in the understanding of this freezing process. A 2cm section of the crown that had been infiltrated with aniline blue was embedded in paraffin and sectioned with a microtome. A photograph of the surface of the tissue in the paraffin block was taken after the microtome blade removed each 20 μm section. Two hundred to 300 images were imported into Adobe After Effects and a 3D volume of the region infiltrated by aniline blue dye was constructed. The reconstruction revealed that roots fed into what is functionally a region inside the crown that could act as a reservoir from which all the leaves are able to draw water.When a single root was fed dye solution, the entire region filled with dye and the vascular bundles of every leaf took up the dye; this indicated that the vascular system of roots was not paired with individual leaves. Fluorescence microscopy suggested the edge of the reservoir might be composed of phenolic compounds. When plants were
frozen, the edges of the reservoir became leaky and dye solution spread into the mesophyll outside the reservoir. The significance of this change with regard to freezing tolerance is not known at this time.
Thermal cameras that allow visualisation of water freezing in plants have shown that in crops like wheat, oats and barley, ice forms first at the bottom of the plant and then moves upwards into leaves through water conducting channels. Leaves freeze one at a time with the oldest leaves freezing first and then younger ones further up the stem freeze later. To better understand why plants freeze like this, we reconstructed a 3-dimensional view of the water conducting channels. After placing the roots of a wheat plant in a blue dye and allowing it to pull the dye upwards into leaves, we took a part of the stem just above the roots and embedded it in paraffin. We used a microtome to slice a thin layer of the paraffin containing the plant and then photographed the surface after each layer was removed. After taking about 300 images, we used Adobe After Effects software to re-construct the plant with the water conducting system in three dimensions. The 3D reconstruction showed that roots fed into a roughly spherical area at the bottom of the stem that could act as a kind of tank or reservoir from which the leaves pull up water. When we put just one root in dye, the entire reservoir filled up and the water conducting channels in every leaf took up the dye. This indicates that the water channels in roots were not directly connected to specific leaves as we had thought. When plants were
24 ISSUE 66 JUNE 2022
frozen, the dye leaked out of the reservoir and spread into cells outside. Research is continuing to understand the significance of this change during freezing. It is possible that information about this effect can be used to help breeders develop more winter-hardy crop plants.
Individual fibre separation in 3D fibrous materials imaged by X-ray tomography

Dorian Depriester, Sabine Rolland du Roscoat, Laurent Orgéas, Christian Geindreau, Benjamin Levrard, Florian Brémond
Modelling the physical behaviour of fibrous materials still remains a great challenge because it requires to evaluate the inner structure of the different phases at the phase scale (fibre or matrix) and the at constituent scale (fibre). X-ray computed tomography (CT) imaging can help to characterise and to model these structures, since it allows separating the phases, based on the grey level of CT scans. However, once the fibrous phase has been isolated, automatically separating the fibres from each other is still very challenging. This work aims at proposing a method which allows separating the fibres and localizing the fibre–fibre contacts for various fibres geometries, that is: straight or woven fibres, with circular or noncircular cross sections, in a way that is independent of the fibres orientations. This method uses the local orientation of the structure formed by the fibrous phase and then introduces the misorientation angle. The threshold of this angle is the only parameter
required to separate the fibres.This paper investigates the efficiency of the proposed algorithm in various conditions, for instance by changing the image resolution or the fibre tortuosity on synthetic images. Finally, the proposed algorithm is applied to real images or samples made up of synthetic solid fibres.
Submit to the Journal of Microscopy
1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints
Journal of Microscopy App Available for


iPhone and Android
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
Submit online at https://mc.manuscriptcentral.com/jmi
View the Guidelines for Authors and full submission details online at:
www.journalofmicroscopy.org

25
3D Nanoanalysis of the Tumour Microenvironment: Subcellular Ultrastructure of Model Systems

Student: Jade Manning
Supervisor: Dr Kenton Arkill
Project location: University of Nottingham across the Nanoscale and Microscale Research Centre and The Biodiscovery Institute
Many cancer tumours have a hypoxic core, I wanted to determine ultrastructural differences of the tumour regions using a 3D cell culture model. When cells lack oxygen, they metabolise differently via hypoxia inducible factors (HIF). I therefore compared non-hypoxic outer regions and hypoxic core regions of the spheroid culture model of HIF-1 or HIF2 knockouts using resin embedded sections and transmission electron microscopy.
Aim
The aim of my project was to look at the subcellular structural changes in different hypoxia inducible factor (HIF) knockouts.
Addressing the aim
I used resin-embedded spheroid knockouts that
were stained with osmium and uranyl acetate, they were grown by Luke Thornton and sectioned by Denise McLean. Using these spheroids, I used a transmission electron microscope (TEM; Tecnai-12), to image cross-sections through the core of the spheroids.
26 ISSUE 66 JUNE 2022
2021 SUMMER
STUDENTSHIP REPORT
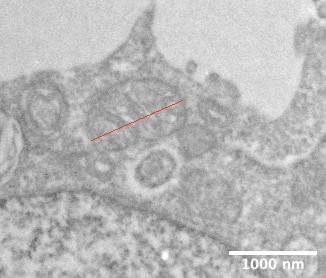
Figure 1. Transmission electron microscopy overviews of the control, HIF-1 and HIF-2 HCT116 spheroid knockouts. I have marked regions considered the hypoxic core and outer regions used for the pilot analysis.
Firstly, I imaged the full diameter of the spheroids, at a microscope nominal magnification of 1050x. Once I had the images, usually seven at this magnification, I used Fiji software to edit the images creating a stack allowing me to piece them into one montage on MosaicJ, a plugin for Fiji (Figure 1). I also took images at higher magnification at the spheroids to allow me to observe and quantify changes in the mitochondria and other organelles.
Next, I worked out the proportion of cells in the different regions of the spheroids. I used the cell counter plugin in Fiji. I determined if cells were alive, at time of fixation, depending on if their overall appearance had the characteristics of alive cells or necrotic cells. I used the results to analyse the proportion of cells in the spheroids and the ratio of proportion of cells in the core compared to the outer regions.
Finally, I analysed the length of the mitochondria using the high magnification images taken at different regions of the spheroids. I worked out the length of a large majority of the mitochondria in each of the knockouts to see if this influenced the sectioned size of mitochondria as an indicator of preferred orientation of the cells.
What did I find out?
I used the areas marked ‘outer regions’ and ‘hypoxic core’ in figure 2 to perform regional analysis on the necrotic proportion of cells and the spatial organisation of the core.
With these micrographs I was able to perform analysis on the spheroids structure between the regions. Firstly, to determine the ratio of cells to total micrograph area I used both filtering then thresholding to segment the cells and the intercellular space (Figure 2). I expected in the hypoxic core of the spheroid there to be a smaller area taken of cells, from many dying due to hypoxia, than the outer regions.

There is consistent difference by observation, even in this n=2 pilot, in the number of non-necrotic cells in the core compared to the outer regions (Data not shown). The non-cellular space was significantly less in the core for all groups (Figure 3) and indication of changes in adhesion. Further, there was a trend that in the knockouts there was more adhesion between the cells in the outer regions compared to cells in the hypoxic core. As a significance is seen we will continue to look at this with a larger sample size. I also tried normalising to the outer region (figure 4), which may with

27
Figure 2. Shows the process of taking a micrograph and converting it to a binary mask. The mask is used to work out the percentage of area that is cells using thresholding. Sample demonstrated is from the outer region of the control spheroid.
Figure 3. Proportion of spheroid cellular content is higher in the outer region. The area that is cell as a proportion of the total area for the different spheroid groups.
increased sample size turn out to be a more robust measure, but here there was no significance. Using the higher magnification micrographs, I also compared the length of the mitochondria in each of the spheroid knockout types (Figure 5). As expected, (as the mitochondria are sectioned tubes) I did not see any significance between spheroids or groups indicating there was no difference in cellular orientation/alignment induced by knockout.

From this initial pilot study, I can continue the analysis on a complete data set and look specifically for intercellular connections, quantify cristae and likely changes in the ER. Further, we also high pressure froze the samples for mass spectrometry imagining (3D-OrbiSIMS) so watch this space!

What did I learn?
I learnt to resin embed my spheroids (post osmium steps). I was trained (using autophagosomes for a
related project) to use the Tecnai-12 independently by Dr Julie Watts. I also was able to spend some time on a scanning electron microscope (Zeiss 550 crossbeam) in transmission mode, with Dr Jacqueline Hicks. From this I was able to use the images for comparative microscopy, looking especially at the resolution (or not) of double membranes in the nucleus. I had a great time and managed to join in with many of the general processes within the nanoscale and microscale research centre.
I also had an opportunity to present my work to others at different stages of the studentship. This allowed me to grow my confidence in presenting, particularly with this type of data.
How has this affected my long-term goals
From undertaking this summer studentship, I was able to confirm that I would like to have a future career which includes imaging on a nanoscale. Halfway through this placement I decided to transfer from BSc to MSci version of my degree, This summer studentship was key in securing me a place on the MSci, as I gained both lab experience and became confident that I would enjoy the extra year of the course. The MSci includes a nine-month placement in either academia or industry, I am hoping to have a placement which has multiple microscopy elements.
28 ISSUE 66 JUNE 2022
Figure 4. Portion of hypoxic core cellular content normalised to the outer region shows no significance with only 2 spheroids per group.
Figure 5. A high magnification image of a mitochondrion. The red line shows how I measured each single mitochondrion for analysis.
2021 SUMMER STUDENTSHIP REPORT
Figure 6. Me next to the Tecnai-12, which I used for my imaging.
Synchrotron X-ray
Nanoprobe Analysis of Archaeological Samples: A Feasibility Study
Student: Jack Pearce
Supervisor: Julia Parker (Diamond Light Source) with Anita Radini
(University of York)
Project location: Diamond Light Source and University of York
The summer studentship carried out jointly with Diamond Light Source and the University of York aimed to explore and test the use of synchrotron X-ray Nanoprobe analyses for the study of archaeological remains.
Introduction
Diamond Light Source is the UK’s national synchrotron facility. Synchrotrons accelerate electrons in a storage ring where they emit radiation (from UV to X-rays wavelengths). Each beamline uses a specialised setup, energy range, resolution and technique to analyse the internal makeup of a sample, for example X-ray diffraction (XRD) or spectroscopy. Beamline I14 is a Hard X-ray Nanoprobe beamline using X-ray fluorescence (XRF), diffraction and imaging techniques for mapping elemental composition, and structural variations with 50nm resolution [3].
Analytical techniques used in archaeology and bioarchaeology include XRD, Raman spectroscopy and electron microscopy to study various aspects of historical samples. XRF has been used in various other studies in archaeology such as by analysing pottery fragments to study their composition [1], artefacts such as historic astrolabe [2] and to assist in criminal investigations through the analysis of dental resins [4]. Each of these techniques has its own unique set of advantages and disadvantages, including ease of sample preparation, achievable resolution, and field of view. In this work we have used nano- XRF in order to give a high resolution map of the elemental composition of the samples, and aimed to test the feasibility of preparing suitable samples, their integrity during X-ray exposure and develop knowledge of methods for subsequent data processing and analysis
Methods
The samples were prepared for analysis using a gallium FIB, milled to a size of 20 x 10 x 0.5 microns mounted onto Copper Onmiprobe grids. The thickness of the samples was chosen to give a good balance between penetration of the X-rays, signal strength and not to compromise the resolution of the X-ray probe.
29
B A
Figure 1. The I14 beamline experimental hutch showing the endstation where the sample was mounted (A) and detector (B).
Grids were mounted on beamline sample holders and placed in the endstation (see Figure 1) and scanned through the focussed 12 and 15 keV X-ray beam with 50 nm steps and a 0.015 sec/pt dwell time. These conditions provided a high-resolution image

Conclusions
In conclusion, the nanoprobe XRF mapping has proven to be highly effective in the analysis of the specimens. The technique can provide an insight into the nanoscale elemental composition of the specimens. With these irreplaceable samples, preservation is key and as the technique is virtually non-destructive, it allows minimal damage to occur to the samples which aids in the preservation and further study of them.

of the samples. Data were analysed using PyMCA to extract individual elemental maps.
Results
Data from the archaeological sample tested are not presented here as they will be published elsewhere, however, the samples were successfully able to be FIB milled and analysed to extract meaningful nanoscale elemental composition maps. Instead, the results from some calibration samples are shown in order to demonstrate the application of the technique. The data from XRF can be viewed as a map of the sample as shown in Figure 2. This clearly shows the outline of the logo where the tungsten is located. Figure 3 shows an example of XRF data plotted in a spectra. From this spectra the fluorescence line for the elements Ti and Cr are visible, from this the composition of the sample can be observed.
The experience at Diamond allowed me the opportunity to visit a dedicated research facility to collect data which provided me with a valuable insight into real life microscopy experiments. I thoroughly enjoyed my time visiting Diamond and every day was filled with the chance to get real life data on state of the art equipment going towards the group’s research. I was privileged to work with a great research team on an incredibly interesting piece of research and I am hoping to go on to study a PhD using microscopy techniques, such as electron microscopy, so I can work in further research groups.
References
1. Morphology of ancient potteries using X-ray diffraciotn analysis and X-ray fluorescence in Sistan Plain, Eastern Iran., P. Vahid et al., . (2017) Mediterranean Archaeology and Archaeometry, 17(2), 175-186.
2. Synchrotron X-ray diffraction and fluorescence study of the astrolabe. M Notis et al.,(2013) Appl. Phys. A 111, 129–134.

3. The Hard X-ray Nanoprobe Beamline at Diamond Light Source PD Quinn et al., (2021) Journal of Synchrotron Radiation 28(3) 1006013.
4. Analytical Survey of Restorative Resins by SEM/ EDS and XRF: Databases for Forensic Purposes, MA Bush et al., (2008), Journal of Forensic Sciences, 53, 419-425.
Acknowledgements
Data were collected at Diamond Light Source, Beamline I14 under proposal number MG27407
30 ISSUE 66 JUNE 2022
Figure 2. W la map from a 1micron thick W tungsten patterned calibration chart.
Figure 3. Example nano-XRF spectra from a sample containing Chromium and Titanium with the fluorescence lines labelled.
Endomembrane interactions in microsporidian infection
Student: Mostin Hu
Supervisor: Dr John Lucocq
Project location: School of Medicine, University of St Andrews
Lay summary:
Microsporidia is a group of intracellular eukaryote parasites which require a host cell to survive and reproduce. For most people these organisms do not cause harm, but infection in people with a compromised immune system (for example, due to chemotherapy or HIV-AIDS) can cause serious illness and even death. We previously identified close association of host membranes with microsporidia and speculated that these membranes help wall off and protect the parasite. This project used microscopical techniques and knockdown of host proteins to characterise these membranes and potential mechanisms.
Project Aims:
Previous research in the Lucocq lab demonstrated a host-endomembrane response is triggered by microsporidia-infected cells. This project aimed to investigate which membranes of the are recruited and whether this response is specific for proteins of the organism. By suppression of host cell components, we aimed to elucidate whether this response was protective to the host or beneficial for the parasites.
What I learned:
I am so incredibly thankful to have been given this summer studentship as it allowed me to not only develop key laboratory skills, but it also allowed me to learn and practice the qualitative and quantitative analysis of microscopy images. I was able to refine key research and laboratory techniques such as sterile cell culture, Western blotting, IF staining and how to choose antibodies against the proteins of interest using known data available from other similar species and performing sequence alignment. Working primarily with immunofluorescence microscopy, I learned how to obtain images using systematic
uniform random (SUR) sampling, a method which allows for the non-biased acquisition of images imperative for quantification.
How this project has affected my long-term goals:
This summer studentship has offered me the time and experience to confirm my interest in cell biology research. As a medical student, I found that research is often portrayed to be a separate career path from clinical medicine; however, I have grown to realise that both fields are highly complementary to one another. Crucially, I have been able to appreciate the many transferable skills that can be learned through research which will hopefully make me a better clinician. The skills of inquiry, creative thinking, hypothesis testing, pattern recognition, and critical analysis of data which are practiced daily in research are equally important in clinical practice.
I am very keen to undertake a PhD during my medical training in the field of host-pathogen interactions, an interest I developed through this studentship and hope to use the microscopy techniques I learned this summer in my future research.

31
Investigating the Use of Fluorescent Nanodiamonds as Imaging Probes for Super-Resolution Microscopy
Student: Chiara Pillen
Supervisor: Dr Izzy Jayasinghe
Project location: Department of Molecular Biology and Biotechnology, University of Sheffield
Lay Summary:
Microscopy is a scientific technique that allows us to look at the building blocks of life. One of the biggest challenges in the field is that the components of cells intrinsically lack colour, so to be able to visualise different parts of the cell, common practice is to tag them with labels. This labels the structures we are interested in with a fluorescent tag, which glows in the dark and can be seen easily through
the microscope. Using this strategy, we can now see components as small as individual molecules such as proteins and DNA! However, there is a big stumbling block - these traditional fluorescent dyes are not durable, and during imaging they can degrade and reach a state where they no longer glow in the dark. This means that images must be taken very quickly and in the dark to protect samples from exposure to light and degradation, limiting the quality of the images obtained. In this project I explored strategies to use tiny diamonds (nanodiamonds) as alternatives to fluorescent dyes – as diamonds are indestructible, they can glow permanently. I developed nanodiamond tags to label certain parts of the cell, using two different types of super resolution microscopy. I focused on labelling a structure called the nuclear pore complex, a protein channel that makes up the main gateways to the nucleus (the brain of the cell), and successfully obtained images showing the location of these channels.
Introduction:
The field of super-resolution microscopy has grown rapidly over the last few years due to the ability to take images that bypass the diffraction limit - a development recognised by the Nobel prize in Chemistry in 2014. A key bottleneck preventing
32 ISSUE 66 JUNE 2022
2021
SUMMER STUDENTSHIP REPORT
Figure 1. HEK 293T cell imaged using an Airyscan Confocal Microscope, illustrating the phenomenon of photobleaching. The outer part of the image was taken using a low-powered laser, however the dim square in the centre was imaged using a very powerful laser to try to achieve a better image, so became photobleached. The red channel shows SERCA dyed with Alexa Fluor 488, and the cyan channel shows the nuclear pore complex (Nup-98) dyed with Janelia Fluor 549.
further utilisation of these techniques, however, is the durability of the fluorescent dyes that are used to tag cell structures. Unfortunately, traditional aromatic dyes are susceptible to photobleaching under intense laser light, so degrade quickly during imaging, as seen in Figure 1. A more durable alternative to these are nanoparticles such as carbon dots, quantum dots, and fluorescent nanodiamonds (FNDs). FNDs are ideal for this purpose as they undergo zero photobleaching, are not toxic to the cells, and can be surface functionalised to adapt to various applications. Using FNDs for super-resolution imaging combines the advantages of such a high resolution with an unlimited imaging duration. This project focuses on assessing the viability of a FND conjugation protocol for this brand-new optical super-resolution modality known as self-activated nanodiamond-based Stochastic Optical Reconstruction Microscopy (sandSTORM). The current gap in this field is the limited understanding of the chemistries that allow nanodiamonds to be conjugated to molecular probes such as antibodies, which is something I investigated over the course of this project.
Aim:
The aim of this project was to trial a protocol for the N-Hydroxysuccinimide (NHS)-ester based conjugation of FNDs and benchmark this against an NHS-ester based conjugation of an aromatic fluorophore, Janelia 549, then to assess the efficacy
of this conjugate as a probe for performing highresolution (Airyscan Confocal Microscopy) and super-resolution (sandSTORM) imaging of molecular targets in cells, namely the nuclear pores and markers of the endoplasmic reticulum.
Methods and Approach:
We conjugated FNDs to secondary antibodies, ensuring minimal clumping by using a bath sonicator, as well as conjugating Janelia Fluor 549 to secondary antibodies as a control probe. The conjugation efficiency was quantified by measuring the degree of labelling obtained using a nanodrop spectrophotometer, and the protocol was altered to result in improved labelling efficiencies.We then used these optimised conjugates as secondary antibodies to label various cell structures in human embryonic kidney (HEK 293T) cells including: the nuclear pore complex sub-unit Nup-98, an ionic pump on the endoplasmic reticulum called the sarcoplasmicendoplasmic reticulum calcium ATPase (SERCA), a peptide target in the endoplasmic reticulum called KDEL, actin anchor α-Actinin, microtubule monomer Tubulin, the membrane protein Cav-3, and a giant calcium channel called the ryanodine receptor RyR-1.We then used these samples for imaging using a Zeiss Airyscan confocal microscope and a Nikon n-STORM microscope.
Key Findings:
To optimise the conjugation protocol for Janelia 549, various methods were tested including altering the concentration of reagents, the length of the incubation period, the temperature during incubation and the method of mixing. As shown in Figure 2 the limiting factor for achieving the desired degree of labelling appeared to be the method of mixing during incubation. A degree of labelling of 10.015 was achieved for the Anti-mouse Janelia Fluor 549 conjugate, after the protocol was optimised. Only one nanodiamond conjugation experiment was successful, due to clumping of the nanoparticles and degradation of reagents. The batch that succeeded

33
Figure 2. Graph showing the effect of changing the method used to mix the sample during incubation, on the degree of labelling achieved.
had a degree of labelling of 0.918. This was very close to the value of 1 degree of labelling that was expected, due to the steric hindrance caused by the large size of the nanoparticle (100 nm) binding to the much smaller antibody (~10 nm) preventing further labelling.
The ratio of the concentration of FNDs to antibodies was calculated to be 1666:1, far larger than that for the Janelia 549 conjugations which was 10:1. The scope, and short timescale, of this project unfortunately did not give me time to tweak the protocol, but if more time was available, I would have adjusted this ratio to be 10:1 instead, and assessed if this affected the resulting degree of labelling.
To decide which target would be best to showcase the FND probe, many targets were tested using different commercial dyes, to identify which had the most unique, identifiable, and clear distribution in HEK 293T cells.
This method allowed a direct comparison of the staining of the nuclear pore complex with different types of probes across two different imaging modalities, as seen in Figure 3.

In each image we can see punctate labelling, of similar density, on the nuclear envelope. The FND labelling is of the same high quality to the established label, Janelia Fluor 549. Unlike Janelia Fluor 549, which is
significantly less effective for STORM imaging, the FND probe is an effective dye for both confocal and STORM imaging. Another benefit of using FNDs instead of traditional dyes is that during STORM imaging the sample does not need to be immersed in a reducing environment to ensure oxygen depletion. This makes the imaging process simpler, faster, and more accessible for portable microscopes - for applications from monitoring marine environments in situ, to diagnostics. In conclusion, the FND probes are very versatile dyes for super resolution microscopy.
What did you learn from participating in this project?
I really enjoyed the practical elements of the project and being immersed in the laboratory work was exciting. I learnt a great deal about cell culture and using sterile techniques – and as a chemistry student this was all new to me, so was a steep learning curve! I enjoyed the freedom of being able to design and carry out my own experiments, tweaking them as I went along. I also learnt patience and resilience when things didn’t work out as I expected them to, whether that be reagents being delayed at customs or cell culture batches failing. I learnt how to use an Airyscan confocal microscope proficiently and how to analyse the images obtained in ImageJ, and I even got the chance to use an n-STORM microscope on one occasion. During the project I also attended
34 ISSUE 66 JUNE 2022
Figure 3. Table comparing images taken of the nuclear pore complex dyed with FNDs and Janelia Fluor 549, taken on a Zeiss Airyscan confocal microscope and on a Nikon nSTORM microscope. The scale bars show 1 µm for the left and middle columns, and 200 nm for the right column.
the Microscience Microscopy Congress (MMC) conference and joined their virtual super-resolution workshop. This was the first conference I have attended; it was eye opening to see such a breadth of research presented and was a great introduction to the field of super-resolution microscopy. Despite the limitations that COVID-19 restrictions placed on the project, something that really stood out to me was the community aspect, which is so important to the research process. Working as part of a such a cutting-edge research group and being part of such a thriving scientific community in the department of molecular biology and biotechnology was an amazing opportunity, which I am very grateful for.
How has this project affected your long-term goals?
I really enjoyed the microscopy component of the project – having such visual feedback on experiments is very exciting. My long-term goals before I started the project were to continue with research in inorganic chemistry, either by doing a PhD or going into industry. However, after completing the project,
they have shifted slightly, as I would love to be involved with research that incorporates microscopy in the future. I am interested in research which combines material science with imaging, for example in crystallography. In addition to this, the technical aspect of the microscopes, such as understanding the alignment of the lasers intrigued me, as well as the biological aspects which I hadn’t had much previous exposure to – cell culture was a very new field for me, and I would like to continue to develop my skills in this area. This project has opened up a whole host of potential career avenues that I am excited to explore further.

35
Contacting the
Microscopical Society The offices of the Royal Microscopical Society are at: 37/38 St Clements, Oxford, OX4 1AJ, UK Tel: +44 (0) 1865 254760 For general enquiries email info@rms.org.uk For information about meetings and courses email events@rms.org.uk For membership enquiries email membership@rms.org.uk www.rms.org.uk
Photograph of me in the lab.
Royal
37-year-old mystery is solved as ‘long-lost’ Fellow rejoins RMS!
Chance discovery of letter reunites former UCL lecturer with Society

As Ferey Faridian rummaged through a box of documents at his home in California, he came across an intriguing old letter.
The 65-year-old’s interest was piqued for three reasons: firstly, because the missive appeared to relate to his much-cherished time as a young academic at University College London in the 1980s, and secondly, because he suddenly recalled receiving a letter from the RMS at a scientific meeting around that time. The greatest intrigue of all, however, arose from the fact that the top half of the paper – and therefore almost all the letter’s content - was missing.
Determined to get to the bottom of the matter, Ferey contacted RMS Chief Executive Allison Winton, emailing her a scan of the remaining portion of the letter (pictured, opposite).
Ferey explains: “As a microscopist, I had the privilege of working with some of the pioneers of a new form of microscopy at University College London and Stanford University. My research culminated in the development of the first gas coupled acoustic microscope under the auspices of a beloved mentor, the late professor Sir Eric Ash, former rector of Imperial College.
“When I found the document, I remembered there was an international conference back in 1984 hosted in London, and prof. Andrew Briggs [Hon FRMS] was present and had given Eric and myself this letter on behalf of the RMS. They must have thought the work we were doing was new and interesting and wanted to recognise that formally.”
Picking up the investigative trail, Allison consulted a
volume of the RMS Proceedings from January 1985, and Ferey’s hunch was proven correct.
She says:“Looking at the last few lines on the bottom half of the letter, I guessed that this was a letter confirming Ferey’s election as a Fellow of the RMS – and sure enough, his name was right there, listed under ‘Elections to Fellowship’. He had obviously let his membership lapse, but I told him it’s never too late to rejoin the Society!”
Ferey duly reinstated his membership, bringing a close to the mystery, and reigniting his pride at belonging to the world’s oldest society dedicated to Microscopy and Imaging.
He says: “I was only 28 at the time of the letter, and so I was very honoured, but perhaps I didn’t fully
36 ISSUE 66 JUNE 2022
A young Ferey, pictured in the early 1980s.
understand this enough to look after my letter and membership! I had completely forgotten about it, so it was really nice to kind of pin down where this came from, and to know that I had actually been elected to the Society. It was a very pleasant experience.”
Ferey arrived in the UK from his native Iran as a teenager in the mid 1970s. His initial plan was to study here for his degree and return home, but as the Iranian revolution erupted mid-stride of his master’s degree at UCL in London, he pivoted to academic research, starting his PhD in 1980, and going on to become one of the youngest members of UCL’s Faculty of Engineering, as a lecturer, by 1986. By this point he had also gained UK citizenship, married his “English rose”, and started a family.

He recalls: “I was a member of a prestigious lab - the ‘904’ - and I loved it. We were on the ninth floor of a building in Torrington Place and doing sub-micron microscopy in a building that moved by several centimetres every time the wind blew! It was a wonderful place, and the lab had many good people in it.
“Eric Ash was a strict but wonderful mentor. He had
been working on focusing techniques for electron beams and holography under Denis Gabor, a Nobel laureate, so he expected monk-like dedication from his students. Gradually he went into surface wave acoustics. By my time, we were working on GHz acoustic microscopy. The researchers at Stanford had picked it up, and Eric had done his rounds there, and we were all trying to put together variants of a microscope that would ‘see’ with sound at resolutions comparable to optical microscopes.
“The teams at UCL and Stanford were the two leading teams commercialising the technology, so we became more and more known for our multi-modal imaging and pioneering microscopy techniques. The one I worked on was unique because the sound beam had to go through a column of gas, and when you do that, you have orders of magnitude more difficulty in reaching your object, but much better resolutions. It was the first gas acoustic microscope and we had to develop a perfectly polished 10 mm radius lens, the smallest lens ever produced at the time, in precisely oriented and cut to a minute of arc in anisotropic sapphire crystals.
37
The remaining portion of the letter from 1984, which bears the signatures of former RMS President, Professor Archie Howie Hon FRMS, and the Society's Administrator at the time, Colonel Fleming.
“In 1986 we thought that Cal Quate and Eric Ash might get the Nobel Prize for acoustic microscopy, but once the scanning tunnelling microscope came along, that was it – and of course that year the Nobel prize in Physics was shared between Ruska, Binning and Roher for what became the precursor of the atomic force microscope. One of my earlier advisers, prof. Kumar Wickramasinghe, who had just returned from Stanford, went on to join that team at IBM.”
The commercial world soon came calling and by the late 1980s, Ferey had taken up a role at Schlumberger, leading an accelerated product development SWAT team at Weston-Solartron, working with "great people" at Enfield, Farnborough, Rolls Royce and the Royal Aircraft Establishment (fascinatingly, one of the products the team developed during this time is used to this day on NATO’s Euro-Fighter aircraft).
Ferey says:“I felt so lucky to be doing what I was doing in academia, but I remember at the time London was expensive on my lecturer’s salary. I suppose I sold my academic soul, but in the end, when someone in a Global 500 company says they will double your salary, it’s very difficult to turn that down.”
In 1990 Ferey moved into Management Consultancy at Sagentia, a technology and business consulting and venture group, in Cambridge. He says: “This was a truly amazing company that oozed with innovation and afforded me the mentorship of the late Gordon Edge of the Cambridge phenomenon fame.” Ferey represented the company and high-tech SMEs on the UK’s Parliamentary and Scientific committee while his international assignments gradually morphed from technology management to change management, corporate strategy and M&As in twelve countries.
By 1996 he had moved to Boston and within a year to Los Angeles, California, into the world of Fortune 100 consulting and middle-market investment banking. Having gained several years’ experience at the sharp end of corporate strategy and M&As, Ferey worked for more than 10 years in numerous investment banking and principle investing roles in private equity and venture capital, before serving as a CEO or Board
Member in a number of Silicon Valley and SoCal hitech companies. Now on four boards, he is Managing Director and Partner of Newport LLC, a national CEO advisory firm in the US, assisting founders of middle market and growth companies, and the funds investing in them, with value acceleration, M&As and exits. He is also a principal advisor at LARTA - a non-profit organisation which helps entrepreneurs secure seed funding from government agencies to commercialise cutting-edge technologies.

Ferey says: “I absolutely love my job, and my background in imaging and microscopy has certainly helped me to stay sharp. It means that whenever there is a company looking for a board member, and they have some really sophisticated technology, it obviously helps when they realise I have a deep understanding of the technology.”
Reflecting on his time in academia and being at the forefront of developments in microwaves, optics, acoustics, imaging and microscopy, he adds: “I feel very lucky to have been close to such advanced research at UCL and Stanford, and after all these years, it really feels like a crowning achievement to discover the intriguing half of the RMS letter, like a message in a bottle, and then to decipher what the other half of it meant!”
Owen Morton
38 ISSUE 66 JUNE 2022
Ferey as he is today.
High resolution, low noise, deep cooled, digital SWIR camera
Raptor Photonics Ninox 640 SU is a vacuum cooled InGaAs based camera with 640×512 sensor with a 15µm x 15µm pixel pitch for the highest spatial resolution. The Ninox 640 SU is vacuum cooled to -80°C for ultra-long exposures of up to 5 minutes. Its ultra-low dark current and read-noise result in the best scientific SWIR camera on the market today. It is the perfect camera for staring applications in SWIR wavelengths (from 900nm – 1700nm) including NIR-II In-Vivo Imaging and Fluorescence Imaging.

■ Vacuum cooled to -80°C Enables ultra-long exposure times
■ Ultra-low dark current and read-noise
■ 15µm x 15µm pixel pitch enables highest spatial resolution



■ PentaVac Vacuum Technology Guaranteed protection and integrity of sensor
Raptor Photonics UK/Ireland Distributor: Quantum Design UK and Ireland Ltd, 1 Mole Business Park, Leatherhead, Surrey KT22 7BA
Tel: +44 (0)1372 378822
Email: info@qd-uki.co.uk
qd-uki.co.uk/imaging-cameras to see the full range of Raptor Photonics EMCCD and SWIR cameras suitable for low light level imaging
Visit
Live Cell Imaging In-Vivo Fluorescence Imaging Chemiluminescence
Raptor Ninox 640 SU SWIR Camera

40 ISSUE 66 JUNE 2022 Congratulations Craig Holliday, on completing the
Diploma!
RMS
Suitable for those using microscopy or cytometry as part of their career, the RMS Diploma is attained via a flexible portfolio-based course of study that is designed by the candidate with the assistance of their line-manager, and with input from existing Fellows of the Society.
This approach ensures that the study is both challenging and rewarding whilst fitting with, and complementing, the candidate’s existing employment. It is estimated that it will take around two years to successfully complete a project. Here, Craig sheds some light on his experience undertaking the RMS Diploma, and why the course can be a great option for those working in microscopy.
How did you hear about the RMS Diploma?
My supervisor at Innovia introduced the idea of the diploma to me shortly after I joined the microscopy department there. He is a long-standing member of the RMS who had himself completed an RMS qualification a number of years ago, and had therefore seen the benefits of combining our day-to-day work with this kind of project.
Why did you decide to do the RMS Diploma?
It wasn’t an immediate decision for me, as I was keen to get some experience in the field before undertaking a formal project. After a few years I began to see how the work I was doing in the department would fit a diploma though, particularly with the AFM capability I was developing in the lab. It felt like a way of not only gaining a recognised qualification for the work I was doing but also an opportunity to raise the profile of the research within the company.
What would you say the diploma offers that other courses / qualifications don’t?
For me I think it’s the opportunity to fit a qualification into the day job. The subject matter I chose for my
project was something I would have been working on at Innovia anyway, and having the flexibility to build a qualification into that was perfect. It also provided me with a bit more drive and focus to persist with things as I could see a tangible goal at the end. It also provided with access to academic experts within the RMS whose expertise I wouldn’t have been able to engage if I hadn’t registered for the course.
What area of microscopy / science did you study and why?
My project was predominantly based around Atomic Force Microscopy. This was a technique that had been introduced into the department a number of years before I joined but we hadn’t really had the time or opportunity to make the most of what it could offer. The lateral and vertical resolution of the technique was perfect for imaging nanoscale features on polymers, and the additional capability of differentiating materials by property again seemed ideal for the multi-polymer systems we were working with. Although we’d had some joy previously, imaging was always inconsistent and I needed to develop a better understanding of AFM to progress the work we were doing.
Modern AFMs are generally well automated so the fundamental understanding can be overlooked. With input from my academic mentor, I was able to better understand what parameters needed to be adjusted and why they were important to image the polymer systems I was working with.
How will your research be of benefit to other microscopists / scientists?
The subject matter I undertook was very specific to Innovia films so probably doesn’t hold much relevance for the wider community. However, I think the development work that was undertaken was invaluable to developing the capability of AFM within the company and demonstrating the benefits that the technique can offer in an industrial setting. I hope this work will be used at Innovia to continue imaging
41
Many congratulations to Craig Holliday (formerly of Innovia, now at the National Nuclear Laboratory), who is the latest RMS member to complete the Society’s Diploma qualification.
developmental polymer systems for new coating products.
How do you think gaining the diploma will help you in your current role and future career?
Since starting the diploma I’ve left Innovia Films and joined the National Nuclear Laboratory. Whilst my new role hasn’t been directly related to microscopy, I found the process of revisiting academic research and project write-up invaluable in my new role. It’s been quite a few years (more than I care to remember) since I graduated and therefore the process of sourcing references and citing them in my own work was something I was very out of practice with. Re-visiting this for the diploma certainly helped me develop my own research and reporting at NNL.
What was the most challenging aspect of the diploma, and why?
Although the technical aspects of method developing were difficult (as with most things where AFM is concerned), I found the most challenging aspect was the portfolio write up in general. It’s something that can’t be rushed and needs to be thoroughly researched and properly referenced. Finding the time (and energy) to do this with a young family and new job was very challenging, especially through periods of lockdown. Ultimately though, I’m happy with the finished article and the reward of a recognised diploma makes it all worthwhile.
What did you enjoy the most about the course?
I’d have to say it was the interaction with other RMS members at the mmc congress and meetings. I was lucky enough to attend the light microscopy summer school way back in 2012 and have visited the mmc exhibition a number of times since then, so I’ve been aware of the networking opportunities membership provides and I think it’s these opportunities that really stand out to me.
What’s the best piece of advice you could give to someone considering doing the diploma?
Be prepared! The write-up process was more difficult and time consuming than I imagined. Even though I
was fitting the diploma around my work, you will still need to give up a sizeable chunk of your own time to get through. At times I found it disheartening trying to generate interest in my project within the company but knowing I was working towards a goal really helped keep me motivated and the sense of achievement I’ve gained from seeing it through has really made it all worthwhile!
“I have had the great privilege of training Craig at work and it has been marvellous to see him develop so well in all aspects of microscopy and especially in AFM. Without the support and training that the RMS has given both of us, none of this would have been possible. I wish the RMS all the best in its support of microscopists throughout the microscopy community in the future.”
Chris Walford, Craig’s Supervisor at Innovia during his Diploma
“I have been very impressed by Craig's growth and perseverance through this project. Despite changes at work and the impact of the pandemic, he pressed on and completed an impressive piece of work, applying atomic force microscopy to characterise industrial thin film coatings. In the process, he has expanded his knowledge of microscopy and deepened his understanding of scanning probe techniques, benefitting from the RMS courses and his own reading. He is thoroughly deserving of the RMS diploma.”
To find out more about the RMS Diploma visit www.rms.org.uk
42 ISSUE 66 JUNE 2022
Craig’s Mentor Neil Wilson

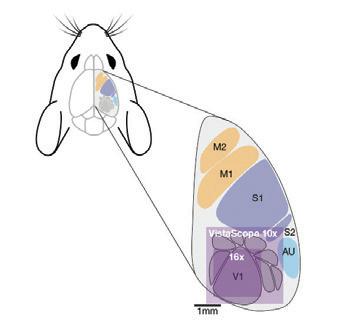
Nanoscale imaging of proteins’ filament assembly on membrane in rebuilt cell membrane
Fouzia Bano1, Gerard Castro-Linares2, Agata Szuba3
1Department of Clinical Microbiology & Wallenberg Centre for Molecular Medicine, Umeå University, 901 87, Umeå, Sweden
2Department of Bionanoscience, Kavli Institute of Nanoscience Delft, Delft University of Technology, 2629 HZ Delft, The Netherlands
3School of Electronic and Electrical Engineering, University of Leeds, LS2 9JT, Leeds, United Kingdom
Atomic force microscopy (AFM) is a multipurpose technique that can simultaneously produce a high-resolution 3D image while also obtaining mechanical information of samples on surfaces. These abilities make AFM a very useful technique for studying filamentous membrane-bound proteins in their native environment with minimal sample preparation. Here we report how we used AFM together with other imaging methods to investigate a biomimetic cell membrane. Specifically, we studied how septin proteins, which are part of the cell’s cytoskeleton, bind and polymerise on flat supported lipid bilayers. With AFM, single filaments and their substructures were visualised which were not resolvable via fluorescence microscopy, while also obtaining height and mechanical stability information. Taken together, this multi-technique study showed that septin form ordered arrays of single and paired filaments on lipid membranes that might mechanically support cells.
Introduction:
Protein polymerisation on the cell membrane is crucial for multiple cellular processes including cell motility, cell signaling, and intracellular transport. Sometimes this leads to disease, for instance in the case of amyloid fibers leading to Alzheimer’s disease. These processes are often very complex and require the presence of multiple accessory proteins,
but the basic mechanisms always involve creating forces leading to plasma membrane remodeling. The polymerisation dynamics of proteins and the nature of their association with lipid membranes is vital to understand the mechanisms leading to complex membrane shape changes. Here, we focus on the characterisation of septins, proteins involved in diverse cellular mechanisms including cell
44 ISSUE 66 JUNE 2022
migration and cell division. Septins are filamentous proteins that have been recently recognised as a fourth element of the cell’s cytoskeleton, a filamentous network that provides cells with shape and mechanical strength. Of the four cytoskeletal filament systems, the septin cytoskeleton is the only one interacting directly with the plasma membrane.
In the course of our recent journey to understand the process of septin self-assembly reported in elife, we used one of the most powerful nanoscale
imaging tools – AFM. It is an exceptional imaging tool since it allows imaging of biomolecular systems in their native form, in liquid, and without the requirement of labelling. AFM has been successfully used for imaging of other filamentous proteins assembled in the bulk (e.g. amyloid fibers, collagen, fibrin). However, we quickly realised that AFM, in parallel with other imaging techniques, also is a unique tool to examine septin organisation on lipid membranes. This multi-technique approach
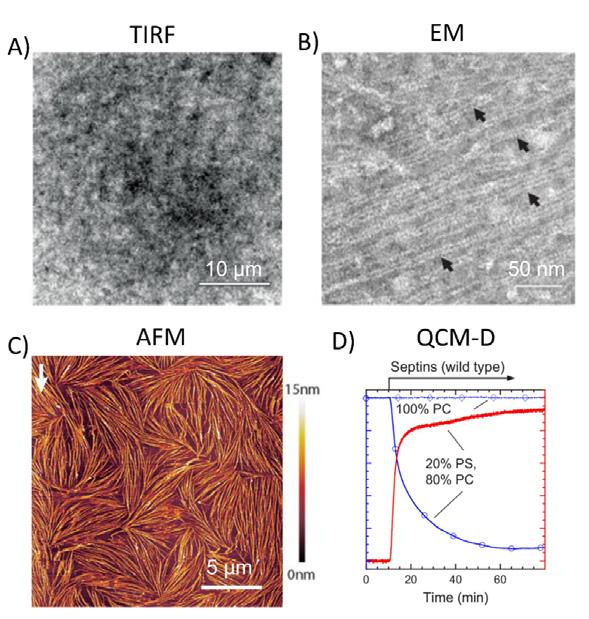
45
Figure 1. A multi-technique approach is needed for comprehensive understanding of fibrous protein assembly on membranes. An example is shown for septin filament assembly on a reconstituted membrane containing 20% negatively charged lipids. PS = Phosphatidylserine and PC = Phosphatidylcholine, a zwitterionic phospholipid. This figure is adapted from (Szuba et al. 2021). Each imaging technique (TIRF (A), EM (B) and AFM (C)) provided unique information about the organisation of the septin filaments on the membrane in steady state, whereas QCM-D (D) revealed the kinetics of the binding and assembly process. Black arrows in (B) indicate examples of individual paired septin filaments and white arrow in (C) show the direction of slow scan axis.
provided a comprehensive understanding of how septin proteins bind and self-assemble on lipid membranes. In this feature article, we will mainly focus on the role of AFM and discuss how AFM advanced our understanding of septin assembly on lipid membranes while at the same time providing information about septin filament mechanics.
A multi-technique approach for comprehensive understanding of fibrous protein assembly on membrane:
Self-assembly of proteins can lead to highly ordered structures such as filaments, virus capsids, 2D lattices, or less ordered and irreversible aggregates. Considering the highly complex and dynamic nature of such processes, knowledge about both the structural properties of individual or monomeric proteins and how the individual components are arranged relative to each other is needed. Septins provide an interesting example of a filament-forming protein whose assembly is tuned by membrane binding. Septins are a family of eukaryotic cytoskeletal proteins involved in
important cell processes in animal cells such as cell division, vesicle trafficking, pathogen-host interactions, and regulation of cell surface rigidity. Septin monomers form hetero-oligomers that act as building blocks for their polymerisation into filaments and higher order structures. These structures are able to interact in turn with vital cell components such as the cell membrane, actin filaments, and microtubules (Mostowy and Cossart 2012).
We have recently investigated the interactions of recombinant fly septins with model lipid biomembranes using a set of techniques in a cell-free setting. Using optical microscopy, in our case total internal reflection fluorescence (TIRF) microscopy, we could observe that septins bind to membranes containing anionic lipids and form dense layers (Figure 1A, septin bulk concentration = 500 nM). However, due to the limitations of diffractionlimited optical microscopy, we were unable to resolve individual filaments of septins within these layers even at low bulk concentration of septin (50 nM, Figure 3A).Alongside TIRF, we used transmission electron microscope (TEM), which taught us that the septins arrange themselves in paired filaments
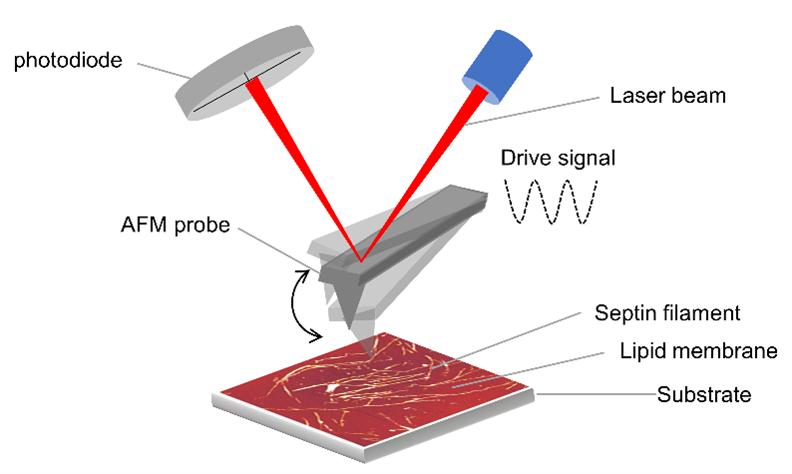
46 ISSUE 66 JUNE 2022
Figure 2. Schematic representation (not to scale) of imaging septins bound to a lipid membrane supported on a SiO2 substrate with the AFM operating in PeakForce Tapping mode. In this mode, the AFM probe is oscillated with a drive frequency that is much lower than its resonance frequency, which permits imaging of the surface with intermittent contact as well as with low imaging force.
(Figure 1B, septin bulk concentration = 65 nM). However,TEM operates in vacuum on dried samples and does not provide any direct information about the thickness of the septin layer or its mechanical properties. To gain further insights into septin’s filament organisation and physical properties, we used AFM, which enabled us to record structural data at various length scales or scan sizes, (Figure 1C and Figure 3, septin bulk concentration = 60 nM) under physiological conditions, and at various experimental conditions (Figure 4) without the requirement of fluorescent labelling. Besides structural data, AFM also provided information about the mechanical properties of the septin filaments on the membranes – a unique feature of this imaging tool. We finally complemented all this information with quartz crystal microbalance with dissipation monitoring (QCM-D) experiments, which gave information about the kinetics of septin adsorption to the membrane (Figure 1D, septin bulk concentration = 60 nM). In the following sections we will describe, within the context of this septin-membrane study, how AFM is an ideal tool to obtain a whole range of information ranging from structural to mechanical.
AFM as an ideal imaging tool to study septins on lipid membrane
AFM is a powerful and multipurpose imaging technique that belongs to the family of scanning probe microscopies. It is powerful because it can create an image of scan sizes spanning from a few 100 nm to tens of micrometres in the XY direction (depending on the application or purpose of imaging) with nanometre scale information in height (or Z direction) without the requirement of sample labelling. It is multipurpose because it not only provides information about the sample topography (size and height) but also about different material properties such as mechanical (Szuba et al. 2021), electrical (Nonnenmacher et al. 1991), conductive (Murrell et al. 1993), and magnetic (Martin and Wickramasinghe 1987), to name a few.
Other unique features of AFM are that (i) it is easy to work with AFM samples due to their large size (silica wafer with total surface area of ~1x1 cm2 vs. ~3 mm EM grid), (ii) the sample time preparation is reduced considering no requirement for labelling step, and (iii) it can be operated in both vacuum and ambient conditions like air, water, and physiological buffer.
The working principle of AFM is rather simple. It is based on “feeling” the surface by a cantilever with a sharp tip on its free end to scan over the surface, equivalent to the tactile reading of braille. AFM images are three-dimensional (XYZ) maps of the surface topographic features. AFM can be operated in three classical imaging modes depending on how closely the tip is interacting with the surface. These modes are contact, intermittent (also known as tapping) contact and non-contact.
Contact mode offers high spatial resolution due to direct contact, hence higher lateral interaction forces. However, it is not useful when the sample is not laterally stable and can result in disruption of the sample due to interactions with the tip, which we found was the case for septin filaments on a lipid membrane. Tapping mode, in which the tip is intermittently tapping the sample during the imaging, is more suitable for soft or fragile samples. However, damping effects of the fluid together with cantilever oscillation immersed in a fluid cell can cause a change to the free amplitude of oscillation of the cantilever, which can result in a change in the applied forces during imaging. This change in applied force can fluctuate considerably and can result in a loss of resolution and damage to the tip or to the sample. In the past decade, the AFM community has witnessed the development of alternative dynamic modes that allow the users to collect “quantitative nanomechanical mapping” data of materials like polymers (Sheiko and Magonov 2012) and live cells (JPK 2011, Dokukin and Sokolov 2017). One such mode is known as “PeakForce Tapping™” (Pittenger et al. 2010) (Figure 2). By controlling the imaging force with high precision, this dynamic
47
mode offers high stability of applied forces during imaging. This high stability has been achieved by the way the feedback loop is set up to control the tipsample interactions. The applied force, also known as “peak force”, is calculated from a pixel-wise force-distance curve, which is used as an input to regulate the z-piezo position for maintaining the tip-sample interaction at a certain set point or a force. This allows the users to image the sample at well-defined imaging forces, which prevents causing any major damage to the sample or to the tip. Some examples of challenging samples that were successfully imaged by this dynamic mode are DNA on mica (Pyne et al. 2014), Snf7 proteins on a lipid bilayer (Chiaruttini et al. 2015) and our own work on membrane-bound septin filaments (Szuba et al. 2021). Next, we highlight how this mode has enabled us to understand the structural arrangement of septin filaments on lipid membranes.
AFM is blind but it can ‘see’ at high spatial resolution
Historically, when it comes to image the surface at micrometer scale at similar experimental conditions, AFM can outperform conventional
optical microscopy methods such as TIRF and confocal microscopy due to its ability to ‘feel’ the sample without the requirement of fluorescent labelling by a blind sharp tip. For instance, Chiaruttini et al in 2015 used AFM (PeakForce Tapping mode in liquid) to resolve the molecular structure of Snf7 patches on membranes for understanding how the polymerisation of ESCRT-III filaments could drive membrane curvature. (Major component of ESCRT-III) spirals on the surface of a lipid bilayer" by "proteins on a lipid bilayer. In their study, the authors have first reported about micrometer size patches observed by TIRF imaging, which were later resolved to be packed arrays of Snf7 circular assemblies on membranes when imaged by AFM. Furthermore, AFM also revealed that each assembly was formed by concentric circle-like structures from a single Snf7 spiraling filament. On a similar note, in our TIRF data, we found that septins were absorbed to anionic lipid bilayer but failed to provide any details due to limited resolution (Figure 3A and 1A). In contrast, with AFM nanoscale imaging, we discovered that septins formed either individual or bundled filaments, depending on the septin concentration in solution (Figure 3B and 4).
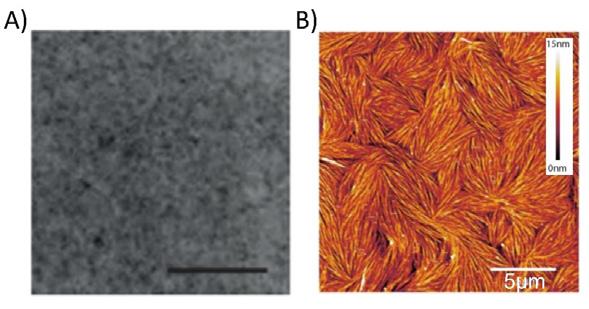
48 ISSUE 66 JUNE 2022
Figure 3. AFM imaging reveals what TIRF cannot about septin assembly on membrane: Purified fly septin hexamers which were deposited on a glass-supported lipid bilayer composed of 80% of net-neutral and 20% of anionic lipids and imaged using TIRF (A) and AFM (B). It is immediately clear that AFM provides a more detailed information about septin’ filaments when imaged at comparable scan sizes of TIRF and at nearly similar septins bulk concentration (~50 nM). This figure is modified from (Szuba et al. 2021).
Beyond resolving filament/bundle forms (Figure 4A and 4B) with high resolution AFM, we also learned that septin filaments form dense arrays at high concentration of septins in solution (Figure 3B and 4C). These examples highlight how AFM can ‘feel’ (or image) better in comparison to commonly used fluorescence imaging techniques by avoiding the use of labelling tags. Moreover, these examples also demonstrate how, by taking advantage of imaging by a blind sharp tip, AFM can resolve the structural details of biomolecular assemblies under physiological conditions.
AFM provides more than just topographic features
Beyond topographic mapping, AFM imaging provides information about height and width of surface features. Moreover, it can yield information about the binding stability of the biomolecules to the underlying surface through consecutive imaging of the same surface provided that (i) there are no strong tip-sample interactions, and (ii) imaging is acquired with minimum or low imaging force.These conditions are essential because if the tip-surface
interactions are too strong, molecules can either stick to the tip or can be easily moved around by the tip, even when imaging is acquired in the gentlest available imaging mode. If imaging forces are too high, the tip can completely displace molecules from the imaging frame and can distort the sample. We took advantage of these AFM abilities in our recent study and have gained additional information about septins.We observed that individual filaments have an average height of 5 nm (Figure 4, left), which increased to an average height of 13 nm when septins were attached as bundles (Figure 4, right). Even more interestingly, we found those individual filaments to be more sensitive to the touch of the AFM tip as compared to bundled filaments: specifically, individual filaments were brittle and appeared as “jittering lines” (white arrows, Figure 4, centre), whereas bundle filaments remained stably bound to the membrane (purple arrows, Figure 4, center).
On top of gaining information about molecular height and binding stability of attached molecules, AFM imaging can also provide quantitative information

49
Figure 4. Height measurement of septin filaments and arrays thereof at various septin concentrations measured by AFM. Despite imaging with low force, single filaments were easily disturbed by the AFM tip (white arrows), while bundled filaments were more stable and did not change their positions (purple arrows). The figure is modified from (Szuba et al. 2021).
about adhesion energy, elastic modulus, dissipation, and deformation. This is collectively known as nanomechanical mapping and can be performed by operating the AFM in modes commonly known as amplitude modulation, QI (Quantitative Imaging introduced by JPK) and an extended version of the PeakForce tapping mode called PeakForce QNM. The basic idea behind these advanced modes is (i) collection of force-distance curves at each pixel during imaging (or within an individual oscillatory cycle of the probe) and (ii) rapid fitting of these curves (approach curves) using models like the Hertz, Derjaguin-Muller-Toporov (DMT), or Johnson-Kendall-Roberts (JKR) models to calculate the adhesion energy and elastic modules. This allows the users to gain detailed information about the nanoscale mechanical properties of the sample - an investigation yet to be performed for septins.
Closing remarks
AFM is one of the most versatile imaging tools to study biological molecules because it can provide information about their morphology, assembly, and height, as well as mechanics, all at high spatial resolution. Additionally, its comparatively easy sample preparation, the ability to image under hydrated conditions, and the possibility to operate in various imaging modes make AFM an attractive technique in biology that can be applied both to living cells and to reconstituted systems.
In our recent study, by complementing AFM with fluorescence microscopy and TEM, we obtained quantitative insights into membrane-templated septin assembly (filaments and bundle formation) and showed that bundling protects septin filaments from mechanical damage. In follow-up work investigating nanomechanical mapping of septin filaments by AFM, we aim to understand the role of septins in biological processes like cell division and regulation of cell surface rigidity.
References
Chiaruttini, N., et al. (2015). “Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation.” Cell 163(4): 866-879.
Dokukin, M. E. and I. Sokolov (2017). “Nanoscale compositional mapping of cells, tissues, and polymers with ringing mode of atomic force microscopy.” Scientific Reports 7(1): 11828.
JPK. (2011). “JPK Technical Note, QI™ mode –Quantitative Imaging with the NanoWizard®3 AFM.”, from https://www.jpk.com/app-technotesimg/AFM/pdf/jpk-tech-quantitative-imaging.14-1. pdf.
Martin, Y. and H. K. Wickramasinghe (1987). “Magnetic imaging by ‘‘force microscopy’’ with 1000 Å resolution.” Applied Physics Letters 50(20): 1455-1457.
Mostowy, S. and P. Cossart (2012). “Septins: the fourth component of the cytoskeleton.” Nature Reviews Molecular Cell Biology 13(3): 183-194.
Murrell, M. P., et al. (1993). “Spatially resolved electrical measurements of SiO2 gate oxides using atomic force microscopy.” Applied Physics Letters 62(7): 786-788.
Nonnenmacher, M., et al. (1991). “Kelvin probe force microscopy.” Applied Physics Letters 58(25): 2921-2923.
Pittenger, B., et al. (2010). Quantitative mechanical property mapping at the nanoscale with PeakForce QNM, Burker Application Note No. 128.
Pyne, A., et al. (2014). “Single-Molecule Reconstruction of Oligonucleotide Secondary Structure by Atomic Force Microscopy.” Small 10(16): 3257-3261.
Sheiko, S. S. and S. N. Magonov (2012). Scanning Probe Microscopy of Polymers. Polymer Science: A Comprehensive Reference. K. Matyjaszewski and M. Möller, Elsevier Science. 2: 559-600.
Szuba, A., et al. (2021). “Membrane binding controls ordered self-assembly of animal septins.” Elife 10.
50 ISSUE 66 JUNE 2022
Dr Fouzia Bano Senior Research Engineer, Umeå University

Fouzia has a MS in Physics, a PhD in Statistical and Biological Physics, and two postdocs in single molecule biophysics. Fouzia has worked in diverse research fields ranging from nanoscale surface chemistry, glycobiology and virology during her more than 12 years’ research experience. Fouzia now works in the department of clinical microbiology at Umeå university as a senior research engineer to characterise the virusesglycosaminoglycan interactions to elucidate their role in virus entry of the cell.
Gerard Castro-Linares
Gerard Castro-Linares is a PhD candidate at the Delft university of Technology, interested in cell division and synthetic biology. He is studying the interactions of septins with the cell membrane and the actin and microtubule cytoskeletons to understand the roles of septins in cell division and to explore their potential for a synthetic cell division mechanism.

Submit to infocus
You provide the text and images and we take care of the rest. It’s the ideal way to share your work with the microscopical community. Full submission information and guidelines are available at www.infocus.org.uk.
To submit an idea or if you have any questions about the process please email the Editor (editor@infocus.org.uk)

infocus welcomes submissions of articles of general interest to microscopists.
New Member Welcome
The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months.We hope they enjoy a long and rewarding membership with the RMS.
Mr Alan Stokes
Ms Alex Dickinson-Lomas
Dr Stephen Hoskins CBiol. FRSB
FLS
Mr Avishek Roy
Mr Philip Reilly
Dr Adam Wollman
Dr Matteo Allegretti
Dr Sourav Bhattacharjee
Peter Thomason
Miss Lauren Woodburn
Mrs Michelle Billington
Dr Artem Smirnov
Dr Kirti Prakash
Ms Meryl Attrill
Miss Katherine Paine
Dr Marcus Yio
Dr Matt Bilton
Dr Srinjan Basu

Dr Yoshishige Tsuchiya
Miss Maike Steindel
Dr Nuno Oliveira
Dr Anjali Kusumbe
Dr Subash Rai
Miss Tayla Shakespeare
Mr Manohar Reddy Esukapalli
Ms Aasiya Lakhi
Dr Peixun Zhou
Professor Johanna Ivaska
Miss Khrievono Kikhi
Dr Bob Gooday
Dr Colin Shaw
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – membership@rms.org.uk. Application forms are available to download at www.rms.org.uk/ membership.
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website you can register your details to gain access, please remember to use the email address that is linked to your membership. If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk
New Corporate Member
Sony Europe BV is dedicated to helping the scientific community, researchers, laboratory professionals, and institutions achieve the best scientific results possible. By leveraging Sony’s comprehensive expertise in electronics innovation and design and with our technological assets we are accelerating development of
Member Profiles
Name
Aaran Vijayakumaran
Tell Us About You?
Currently a PhD candidate at the MRC Toxicology Unit, University of Cambridge, with a focus on airway biology using advanced imaging techniques at a nanoscale in the Mennella group. Prior, I attended the University of Nottingham, where I achieved a 1st Class Honours in Medical Physiology, and then went onto King's College London to study a Masters of Research in Translational Cancer Medicine, where I achieved a Distinction and undertook research in Molecular Oncology
next-generation cell analysis systems. We bring a unique perspective to science’s high-level instrumentation and are creating innovative products to address our customers' challenges.
and Nanomedicine.
Why did you become a member of the RMS?
We use super resolution microscopy and electron microscopy techniques to certain biological systems. Being a member of the RMS will be very beneficial for my research, and will allow me to network with other microscopy enthusiasts.
How do you feel being an RMS member benefits you?
It would be great to meet experienced researchers and learn from a variety of people through conferences and seminars. I cannot wait to reap the benefits which I know will expand my knowledge and help my overall research in the future!

52 ISSUE 66
JUNE 2022
Name
Dr Vijayshankar Asokan
Tell Us About You?
I completed my PhD from the University of Bergen, Norway, and postdoc research activities from Zhejiang University, China, and Chalmers University, Sweden. I’m currently working as research fellow at Limerick University, Ireland. For the past 14 years, I have been working with
Name Hannah Baird
Tell Us About You?
I am a biophysics PhD student at Cardiff University, researching the structure and function of pores formed in bacterial membranes by antimicrobial peptides at a single-molecule level, using physical techniques such as TIRF and FRET microscopy.

Why did you become a member of the RMS?
Although I have some general
Name Avishek Roy
Tell Us About You?
I am a final year PhD student in the Department of physiology, All India Institute of Medical Sciences, New Delhi. I work on animal model of sporadic Alzheimer's disease. My research interest is on learning and memory impairment with age. My PhD work is on deciphering the role of low frequency magnetic field stimulation on streptozotocin-induced rat model of Alzheimer's disease.


Name Binney Sharma

Tell Us About You?
I Just submitted my PhD thesis which involves the use of electron microscopic techniques for the identification of Neuromuscular junction. But for me, it’s very hard to identify the NMJ by
electron microscopy for various materials analyses. Currently working with in-situ high resolution imaging of engineering alloy materials.
Why did you become a member of the RMS?
To get connected with the microscopy society, and engage with RMS activities to explore microscopy research.
knowledge about different types and applications of microscopy, my experience is limited. Since microscopy is such an important part of my research, I became a member of the RMS to increase and improve the breadth and depth of my microscopical knowledge.
How do you feel being an RMS member benefits you?
I hope that, through events and the RMS community, I will be able to broaden my research network and learn about cutting-edge microscopy techniques, which will allow me to better apply myself to my own research.
Why did you become a member of the RMS?
I wanted to connect with the members of the society to get more visibility and discuss about their findings and experience in microscopy of different types. Furthermore, a long-term goal of my membership is to get involved / participate in different workshops or symposiums in order to get in-depth knowledge on the known techniques and to update myself with upcoming advancements.
electron microscopy. I need inputs from various scientists regarding the NMJ identification and many more.
How do you feel being an RMS member benefits you?
It helps in my thesis and other research related problems.

53
From the RMS President

It may be a well-worn cliché, but time really does seem to be flying this year. This is especially true for the RMS as there continues to be lots of activities underway and planned for 2022 and beyond. Since our last issue of infocus, our events have come thick and fast, with Virtual Microscopy
Characterisation of Organic-Inorganic Interfaces 2022 that attracted an audience of 235 attendees over two days in March, and our fully-booked Virtual Flow Cytometry Data Analysis Course Spring 2022, which was held soon after. This was swiftly followed by the highly successful and well-attended Virtual
54 ISSUE 66 JUNE 2022
NEWS
EBSD (Electron Back Scatter Diffraction Meeting) 2022, which had over 100 attendees logging in over two afternoons in April. This important annual meeting is a key opportunity to share new developments and applications in EBSD. This year’s meeting featured absorbing presentations, commercial talks and poster sessions, once again stimulating some important discussions and, no doubt, future collaborations.
In addition to all of the meetings and courses, the International Microscopy Lecture Series – a multi-society collaboration – continues to bring online talks from some of the biggest names in microscopy. World-renowned biologist Benjamin Geiger entertained us in March with A microscopymediated view of the social life of living cells, and Nobelprize-winner Dan Schechtman lectured on The Role of TEM in the Discovery of Quasi-Periodic Materials the following month. If you missed either of these talks first time around – or any of the previous IMLS presentations, you can watch them at your leisure by visiting the RMS YouTube channel.
Looking ahead to the summer, we are eagerly awaiting a further flurry of great meetings, courses and conferences. Our Cryo Electron Microscopy Course 2022 takes place in Harpenden from 6 – 10 June, marking a welcome return to in-person RMS events. It has definitely been a long time coming, and I am certain that everyone taking part in this course will be delighted finally to get together in one place without Zoom.
However, as we have learned over the last two years, online meetings have some important advantages in terms of greater accessibility, and we will continue to take advantage of this format for some events, where appropriate. As such, the Virtual AFM & SPM Meeting 2022 will be held in early July, alongside the ever-popular Frontiers in Bioimaging 2022, which will take place at Birmingham’s Edgbaston Park Hotel. This latter event is renowned for providing an environment where early-career and established researchers
can meet and engage with a broad range of imaging approaches, making valuable contacts with leading groups in the field.
Rounding things off in July will be FlowCytometryUK (20-22 July - also in Birmingham), featuring themed plenary sessions, scientific and commercial workshops, and a large exhibition. As always, this is a great opportunity to network with flow and image cytometrists from all over Europe and beyond.
Meanwhile, a range of exciting projects are due to be carried out by the recipients of this year’s RMS Summer Studentship grants. This year, three students have been successful in securing up to £2,000 to help cover lab costs and living expenses while they undertake scientific projects with a strong microscopy element. Each student will produce a report for infocus, and I am delighted to see four such reports from last year’s recipients included in this issue!
We are also pleased to announce the launch of a pilot mentorship scheme in July, aimed at supporting all RMS members at every career level (junior or senior, scientific and technical). The scheme will involve peer-to-peer support to tackle soft skill development, as well as ‘hard/technical’ skills. This is a very exciting new RMS initiative, about which you can also read more in this issue (overleaf, p56).
In terms of major upcoming events, preparations are already underway for our flagship event, Microscience Microscopy Congress 2023 (mmc2023) incorporating EMAG, which will be held in Manchester, UK, from 3 – 6 July 2023. Please save the date for what is shaping up to be another fantastic international event!
I also wish to express my thanks to all of our RMS Staff and our RMS members who have been working so hard to provide such a wonderful programme of events. Finally, I hope you all have a wonderful and safe summer, and my very best wishes to you all!
Professor Grace Burke, RMS President
55
RMS set to launch exciting new Mentoring Scheme
We are pleased to announce that the RMS is launching a pilot mentorship scheme in July 2022 with the aim of supporting all RMS members at every career level (junior or senior, scientific and technical).
Background
As part of the RMS Professional Development and Training Focussed Interest Group (PTD-FIG), the mentoring working group have spent the last two years discussing and formulating a mentoring scheme aimed at RMS members in order to support career development and the unique microscopy roles we hold. The impetus to design an RMS scheme came from the experience of three members of the working group who themselves had searched out these mentorship relationships to forward career and technique goals. Scott Dillon was in conversation with Paul Verkade over his experience in core facility management, whereas Joëlle Goulding had searched out a contact in order to help her with fluorescence lifetime imaging (FLIM). Whilst a variety of mentorship schemes exist within home institutions and workplaces, the pool of mentors that specialise in microscopy-based skills can be quite limited. In addition, the unique position of facility scientists and managers means that mentors able to provide guidance on career development and day-to-day running of a facility are often lacking entirely. As such the RMS is uniquely placed to facilitate a scheme for microscopists, by microscopists.
It was clear that two different tracks of the RMS Mentoring Scheme were of interest - one to tackle soft skill development (personal mentoring) and the other for hard/technical skills (application coaching). Both are aimed at peer-to-peer support, importantly not replacing project supervisory roles nor the invaluable relationships we can foster with commercial partners. It is hoped that both mentees and mentors can benefit from the
relationship, finding it rewarding and useful for career development.
Personal Mentoring track
Personal mentoring targets soft skills and career development through regular discussion with a senior member of the microscopy community. Often core facility scientists and academic microscopists work in small teams and may only have access to a very limited number of colleagues to learn from. Furthermore, opportunities for career development locally can sometimes be narrow and vary between institutions. While direct supervisory relationships are important, many individuals may benefit from guidance from colleagues with alternative skills and experience which apply to them specifically, or simply from those with an outside perspective. These mentoring relationships are therefore geared towards building the mentee’s professional networks and to providing guidance towards career development.
Mentoring pairs are matched depending on relevant skills and experience and based on areas the mentees have identified as important to their professional advancement. They are encouraged to meet once a month for a period of six months initially, and to set out tangible goals they would like to work towards. These relationships can be short to achieve a specific goal, or act as a foundation for a longer relationship outside the scheme.
Case study - personal mentoring Scott & Paul
During the first pandemic lockdown, Scott Dillon joined the Cambridge Stem Cell Institute at the
56 ISSUE 66 JUNE 2022 NEWS
University of Cambridge as an Electron Microscopy Applications Scientist within the imaging core facility. As the only electron microscopist in the facility, Scott was responsible for establishing these techniques in the institute and getting the new microscopes on their feet.

He says: “Electron microscopy was brand new to the Stem Cell Institute and so there wasn’t much local expertise available to help me with setting up the facility and in developing training resources for users. Given pandemic restrictions it was even quite difficult to make contacts with other facilities in Cambridge. I was also completely new to the world of core facility management and didn’t have any contacts in the wider biological electron microscopy community who I could source advice from. I particularly needed guidance on the intricacies of handling procurement of microscopes and other equipment.”
Scott decided to reach out to a senior colleague outside his university who would be able to give an experienced and independent viewpoint on how to develop his facility and build his contacts.
He says: “Paul (Verkade) was fantastic help and was
able to give me real practical input on the challenges of establishing a new facility. He also was able to introduce me to many new colleagues and got me involved in work with EM-UK. Our meetings were very informal and scheduled whenever we both had the time.”
Paul Verkade is a Professor of Bioimaging based at the University of Bristol who has set up and run a number of EM facilities. “I was initially surprised and honoured that Scott approached me to form a mentoring pair”, he says. “For me it was a great opportunity to share the experience I had gained over the years but also to reflect on how I had developed over that time.
“What did I learn? - the mentoring scheme provides a chance to pass on the knowledge acquired, how to deal with users, microscope manufacturers, and management and share good and bad experiences. We all have to start at some point and I know I would have loved to have had a mentoring scheme when I started out.”
Application Coaching track
Application coaching is aimed at providing technical advice and instruction on specific instrumentation and software. Setting up new instrumentation or new applications for instrumentation already in use can often be challenging and time-consuming. Often, we receive comprehensive training on a microscope from the supplier, however, the day-to-day use and specifics can differ dramatically depending on the research question being applied. By pairing up expert microscopists who are seeking and who are able to provide insight into these applications it is hoped that time can be used more effectively, data can be more reliably collected and new networks built.
Mentoring pairs will be matched on specific instrument or application expertise.The relationship can be quite short - a couple of communications in order to troubleshoot an application for instance - but equally, a longer-term collaboration may be
57
Scott Dillon.
established outside of the scheme. The coaching is not aimed at students learning an application for their direction of study, but rather, at scientists who already employ microscopy in their work, and may need help with a particular application, instrumentation or software.
Case Study - application coaching Joëlle & Beccy
Joëlle Goulding is a research fellow in advanced microscopy based at the University of Nottingham. She specialises, researches and trains in the field of fluorescence fluctuation spectroscopy.

She says: “We’d recently purchased and set up a new microscope, the Picoquant MicroTime 200, primarily to further our fluorescence correlation spectroscopy (FCS). This microscope has pulsed lasers and so we could also extract data on fluorophore lifetime and deliver fluorescence lifetime imaging (FLIM). This was the first time I had ever done FLIM and following my initial training from the microscope supplier I had started to collect data as I taught myself. I was happy with the experimental workflow but was unsure on the effect of laser power, the importance of collection time vs. photon number and how important each of these were to the data. I wanted to be able to train others in this technique but didn’t have a huge amount of time to spend optimising. I decided to contact Beccy as I knew she had worked with FLIM in the past and also used a picoquant microscope so was familiar with its use. I emailed Beccy who was really helpful and I think we probably exchanged a
few emails over four months in total. I sent her some data I’d collected and she was able to advise me on its interpretation and perhaps a few parameters to change. When I had time I tried these out and sent her my new and improved data-set. I didn’t expect Beccy to have all the answers but our to-and-fro email conversation gave me confidence in the data I was producing.”
Rebecca Saleeb is a PDRA based at the University of Edinburgh. Whilst her current research into the detection of neurodegenerative disease biomarkers uses mainly single-molecule imaging approaches, she spent her PhD optimising and developing tools for FLIM.
She says: “A large portion of my doctoral research used a Leica SP5 with Picoquant’s Picoharp 300 TCSPC module to achieve “high-resolution” singlepixel FRET detection. I was fortunate to be exposed to fantastic training opportunities and support from industry application specialists. However, my research demanded higher photon statistics than most standard approaches, necessitating careful validation of what was minimally required and the possible cost this may incur on data integrity. When Joëlle reached out to me, it was a genuine pleasure to be able to chat to someone asking many of the questions I did and share some of the expertise I had built up. Often these optimisations go unpublished, so it was a nice outlet to share what I had learned and to discuss the technique candidly. It was also a low-pressure interaction that didn’t demand an immediate response, allowing me to help when I could and to take the time to properly mull over Joëlle’s questions.”
Pilot
For its first year the RMS mentoring scheme will run as a pilot, setting up a limited number of pairings comprising both personal mentoring and application coaching tracks. These pairs will be facilitated and supported by the RMS and the mentoring working group for a fixed period. Pairing aims and outcomes will be collected by a survey at six and 12 months
58 ISSUE 66 JUNE 2022 NEWS
Joëlle Goulding.
in order to judge the success of the scheme and gather feedback for future implementation. If you are interested in being a mentee or a mentor you can find more details at www.rms.org.uk/mentoring
Please note that selecting one option (mentor or mentee) will not exclude you from the other.
Applications are open from July to the end of September 2022.
The Mentoring Working Group - Joëlle Goulding, Scott Dillon, Georgina Fletcher, Alex Sossick and Paul Verkade
Georgina Fletcher
2022 Summer Studentships announced!

We are pleased to announce that three students have been successful in applying for an RMS Summer Studentship to take place later this year.
Each student will receive up to £2000 to help cover laboratory and living costs during their projects, and complete a report on their work which will be published in infocus Magazine.They will also record a short video talking about their experiences for the Society’s YouTube channel.
Many congratulations to this year’s successful applicants, who are as follows:
• Ben Watson - currently in the 2nd year of an MPhys Physics degree at the Department of Physics, University of Strathclyde. Supervisor - Dr Liam Rooney
• Hale-Seda Radoykova - Currently in her third year of an MSci in Biological Sciences: Computational Biology. Supervisor - Dr Sian Culley
About RMS Summer Studentships
Up to six studentships are available every year, split evenly between physical sciences, biological sciences and interdisciplinary projects.
Applications for our Summer Studentships must include a significant microscopy component and should be submitted by a suitable host academic on behalf of the student. Students who are in their first or final year are not eligible. The Studentship is offered on the understanding that a 500 word project report is completed by the student at the end of the period of study.
Find out more at www.rms.org.uk
59
• Catherine Read - Currently in the second year of an MSci in Natural Sciences. Supervisor - Dr Alex Payne-Dwyer
Winner of 2022 RMS Early Career Award announced!
The RMS is delighted to announce the 2022 winner of the Early Career Award as Katherine Paine, of the University of York.
Katherine, who began her PhD in 2018 at Chris MacDonald’s laboratory, was chosen in recognition of the novel approaches in imaging and cytometry she has brought to her studies on the regulation of cell surface membrane proteins.
She will receive a £100 cash prize and an invitation to deliver a keynote presentation at Microscopy: Advances, Innovation, Impact 2022 – an event which includes the RMS Annual General Meetings.
RMS Early Career Committee Chair, Liam Rooney said: “Not only have Katherine’s approaches allowed her to discover novel mechanisms related to surface protein trafficking in yeast, but these methods can now be used by others in the field in the future. It is a privilege to commend her achievements with this award.”
More about Katherine’s work
Cell surface membrane proteins perform diverse and critical functions and are spatially and temporally regulated by membrane trafficking pathways. These trafficking pathways are evolutionary conserved from yeast to humans. MacDonald lab uses yeast as a model organism to study these pathways.
It became clear from Katherine’s initial studies that although standard confocal microscopy could be used to visualise some of the processes she was interested in, there were also limitations. She then helped optimise a suite of imaging and cytometry approaches to study surface proteins. This includes Airyscan2, structured illumination (SIM) and photoactivated localisation microscopy (PALM); all of which can be coupled to bespoke microfluidic exchange systems.
Katherine is also in the process of optimising a high throughput method to measure Förster resonance energy transfer (FRET) in yeast using robotics and flow cytometry.

60 ISSUE 66 JUNE 2022 NEWS
Congratulations to Katherine Paine, of the University of York
Katherine Paine.
Steve Couzens leaves RMS Flow Cytometry Course after 20 years
A huge ‘thank you’ and our warmest wishes go to Steve Couzens, who has stepped down as an organiser of the York-based RMS Flow Cytometry Course ahead of his planned retirement.
Steve, who works at the Haematology Department of the University Hospital of Wales, Cardiff, has been involved as both a participant and organiser of the course’s Clinical Module for around 20 years. He is planning to enter retirement next year, which means that the virtual course held in March was his last.

Steve said: “I will miss the camaraderie, the networking, the fantastic venue, the food and especially the trips down the Charles XII. Teaching has also meant that I learned new stuff and broadened my horizons, which has been very satisfying, and I am pleased that I have been able to bring that added perspective back to my own lab. ”
He added: “The Course has evolved considerably over the years and most of that positive change has been due to the involvement of Dave (Bloxham) and Dan (Payne). I am pleased to say that Dan has volunteered to be the clinical organiser moving forward. I wish you all the very best for the future. It has been a true pleasure and I have no doubt that the Course will continue to go from strength to strength.”
RMS Vice President Peter O’Toole was joined by Flow Cytometry Section representatives Derek Davies and Karen Hogg in paying tribute to Steve’s efforts over the years.
They said: “It has been a pleasure and an honour to work with Steve with the RMS Flow Cytometry Course. His calm, jovial, open nature was appreciated by everyone he met. Feedback from the students was always outstanding, much of this thanks to his keenness to share his expertise with all and help everyone in the world of clinical flow cytometry.
From a personal perspective, we will also miss him being around as a friend during these courses and he will be badly missed by all.”
61
Steve Couzens.
New starter: Nick Cameron
A very warm welcome to new starter Nick Cameron, who joins the Society as Sponsorship and Events Assistant.
Nick will be working closely with Corporate Members and other partners, delivering sponsorship packages for RMS events and ensuring our sponsors are able to get the most out of supporting the Society.
Nick comes from an events background, and formerly worked in conferencing and events at the Kassam Stadium in Oxford.

He said: “It’s been great meeting my new colleagues and learning more about the RMS
and the important work it does. I’m also looking forward to working with the many companies who support the Society’s activities.”
Interested in joining an RMS Committee?
Help the RMS remain at the forefront of advancements and developments in microscopy

If you or a colleague are interested in joining one of our Science Sections or other committees which advise the RMS, we would like to hear from you!
The RMS has seven Science Sections covering the main branches of microscopy, plus an Outreach and Education Committee, an Early Career Committee and a History Committee. In addition, all the
Society's activities are overseen by the RMS Council.
The RMS is committed to being a welcoming, inclusive Society and encourages diversity across all activities and in the membership of our committees and groups.
If you would like information on how to join an RMS committee or about our committees' activities, please email our Chief Executive, Allison Winton allison@rms.org.uk
RMS Annual General Meetings
Please note the below dates for all RMS Annual General Meetings (AGMs) in 2022:
62 ISSUE 66 JUNE 2022 NEWS
Committee AGM Date Life Sciences 5 July (During Frontiers in Bioimaging in Birmingham) Light Microscopy 5 July (During Frontiers in Bioimaging in Birmingham) Flow Cytometry 21 July (During FlowCytometryUK in Birmingham) Main RMS Annual General Meeting 29 September – One day meeting in London Early Career Committee 29 September AFM &SPM 29 September Electron Microscopy 29 September Outreach 29 September Engineering and Physical Sciences 29 September Data Analysis and Imaging 29 September
Step into the Microhub era. Mica. The world‘s first Microhub.

Mica. This changes everything.
> Access for all
> No constraints
> Radically simplified workflows

64 ISSUE 66 JUNE 2022
The Human Brain
infocus contributor and Fellow of the RMS Winston Ingram recently turned his attention to the circuitry of the human brain. Using a series of original MRI scans, Winston sought to enhance the detail contained within them using a combination of experimental microscopy techniques.
65
Winston Ingram
Winston writes:
If we look at the human brain as a computer, it must have many electrical circuits and microcircuits. There is clearly much to learn in terms of identifying the various components, voltages, and how they travel to the different parts of the body to allow us to move, think, reason, learn and complete the many functions of the human mind and body. When these voltages go wrong, owing to mental or physical problems, might it one day be possible to find a way that is simple and not invasive to correct the voltages and restore them to their original values?
With a lot of experimentation I have found a way to show up the inner parts of the human brain.Though it may take many years of research by experts in the different fields we wish to treat, I hope I have opened the door with my experimental work to show the circuits and microcircuits in great detail.
These images were achieved using monochrome MRI scans. I first took the sheets and cut the brain images into small samples in order to get them under my microscope. I used my stereo microscope which I have modified to enable all the techniques – Fluorescence, Polarisation, Bright Field, Dark Field and Phase Contrast.This allows for mixing – e.g. Fluorescence with Phase, Polarisation with Fluorescence, among other combinations. I modified and built power supplies to enable upper and lower lighting to be used together. I then combined multiple microscopy techniques to colour, and also increase focus, contrast and depth of field. I mixed Polarisation with Fluorescence, Phase Contrast with fluorescence, some with Dark field, and in some cases, three techniques together. –e.g. Fluoresecence, Polarisation and Dark Field. The work was completed in Photoshop CS3. This approach allows many colour variations and lighting, bringing out more detail than was previously visible in the originals.


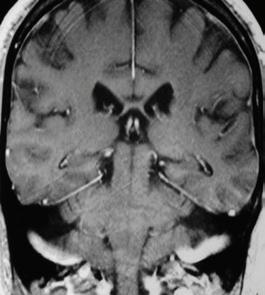
66 ISSUE 66 JUNE 2022
a
Figure 1. Original frontal brain scan (a) alongside new versions (b) and (c)
b c


67
Figure 2. Hypothalamus original scan (a) alongside a new version (b)
a b
Figure 3. Thalamus original scan (a) alongside a new interpretation (b)
a
b






68 ISSUE 66 JUNE 2022
Thalamus and hippocampus
Prefrontal cortex and cingulate gyrus
Hypothalamus and mid-brain
Clusters of brain stem neurons, excitatory and reticular formation
Clusters of brain stem neurons
Cerebral white matter
About the author
Winston Ingram was born in 1940, and from an early age developed an interest in Science and Photography.


He went into private practice in 1978, working freelance for various companies, and teaching scientific, medical and technical photography. He retired in 2002 and started producing books using photo microscopy as an art form. He likes to explore different techniques with the microscope, and also mix the various facilities available.

69
Brain texture, cerebral cortex
Brain circuit overview (1)
Brain circuit overview (2)
Sun Protection Conference, London 2021 – UV imaging and microscopy talk

In November 2021, I was fortunate enough to present some of my imaging work at the 15th Sun Protection Conference held at the Royal College of Physicians, London. This conference is held every two years, and brings together researchers from around the world to discuss different aspects of skin and sun protection.
It was a great conference location and there was a lot of lively discussion about the work being shared. My talk was on the role that Ultraviolet (UV) imaging can play in helping to understand the behaviour of topical sun protection products and skin biology. As well as covering general aspects of UV photography, I talked about a new application I have recently been experimenting with – UV transmission microscopy of sunscreens – and shared some initial images taken using it. I ended up basing my microscope on an Olympus BHB which has been extensively modified (the development of which was covered in the December 2021 issue of infocus Magazine). An image from the conference is shown in Figure 1.
I started the microscopy section by saying that rather than UV transmission microscopy being a new thing, it had been around for over 100 years, and was originally done with the goal of improving resolution by using shorter wavelengths of light. Despite the obvious application for the imaging of sunscreens and sunscreen ingredients, given their
UV absorption, it has not however been reported for use on that. This was what sparked my interest, as changing the wavelength of UV light being used would enable me to look for different UV absorbing actives in a sunscreen due to their different optical properties.
The talk was well received and I won an award for it. The award was from the International Journal of Cosmetic Science (IJCS), and was based on the originality of the work, contribution to the knowledge of the field and quality of the presentation. As a result I get to submit a paper to the IJCS, which, if accepted, will be published Open Access without me needing to cover the fees. As the UV microscopy work was the most novel part of my presentation, the paper will be on the UV microscopy of sunscreens.
Jonathan Crowther, JMC Scientific Consulting Ltd
Editor’s note: The online version of Jonathan’s article from infocus December 2021 now includes higher resolution images. RMS members can view this, along with all our other latest content, at www.rms.org.uk
70 ISSUE 66 JUNE 2022
REPORT
Figure 1. Jonathan in action at the Sun Protection conference.


TESCAN UniTOM HR The first micro-CT system to provide sub-micron spatial resolution and high temporal resolution dynamic CT in a single, highly versatile system. What can you do with TESCAN UniTOM HR? Contact us today to find out: www.tescan.com Wood sample scanned at 280 nm voxel sizevessels are color-coded to thickness.
2nd Joint Meeting
Microscopy Society of Ireland (MSI) and Scottish Microscopy Society (SMS)

6 - 8 April 2022
Galway Bay Hotel, Salthill, Galway, Ireland
The 2nd Joint Meeting of the MSI and SMS was held at the Galway Bay Hotel in beautiful Salthill, Galway on the west coast of
Ireland. Session topics from invited speakers and short talks integrated research from across life and materials sciences, instrument development
72 ISSUE 66 JUNE 2022
REPORT
and image analysis, alongside panels specifically discussing issues relating to early career and community development. The meeting was opened with a welcome by co-chairs of the symposium Dr Kerry Thompson (MSI) and Dr Charlotte Buckley (SMS) and was followed by the first session of the conference, the Early Career Session. Flash Talks from early career researchers (ECRs) set the scene for a fantastic poster session and gave an exciting overview of the microscopyrelated research happening across Ireland and Scotland. A Q&A panel discussion followed, giving ECRs the chance to ask questions and learn more about specific career pathways and development, both within and outside academia. Later that evening, a table quiz was held in O’Reilly’s Bar in Salthill. The walk from the conference venue to the bar was short but with
the gale force winds jostling them along, conference delegates quickly began to understand why the west coast of Ireland is called the ‘Wild Atlantic Way’!
Day Two of the conference began with Session 2: Instrument Development & Image Analysis. This was a fantastic session with topics ranging from how to image beating zebrafish hearts in vivo (Dr Jonathon Taylor, University of Glasgow), to how to build a microscope without knowing how to build a microscope (Ms Niamh Burke, University College Dublin), to what it is like to work as a Core Facility Image Analyst (Dr David Barry, Francis Crick Institute). The third session of the meeting focussed on Biological and Life Sciences, hearing talks from abstract submissions, which showed the high standard of research taking place across the different universities. This was rounded off by a fascinating presentation by keynote speaker Prof Garry Duffy (National University of Ireland, Galway) in which he described how advanced imaging tools contribute to helping researchers understand and overcome

73
the foreign body response to medical devices. The final session of the day focussed on Community Development. Invited speaker Dr Caron Jacobs (University of Cape Town, South Africa) joined via zoom and spoke about the development of the microscopy community in Africa. This was followed by a panel discussion led by MSI president Prof Lewys Jones (Trinity College Dublin). Panellists included representatives from various funding bodies and discussion focussed around how these bodies can best support the microscopy community and essential research infrastructure. Day Two concluded with a fantastic poster session and gala dinner.
The final day of the meeting began with Session 5: Materials and a brilliant talk by invited speaker Dr Raymond McQuaid (Queen’s University Belfast) about how Scanning Probe Microscopy can be applied to understanding functional properties of materials at the nanoscale. The last session of the conference, Session 6: Correlative & Multimodal Imaging, included a talk by invited speaker Prof Paul Verkade (University of Bristol) about the
many ways to do CLEM (correlative light and electron microscopy) and concluded with the final keynote speaker of the meeting, Dr Anna SartoriRupp (Institut Pasteur, France). She described how cryo-correlative light and electron tomography can be applied to visualise intact cells in 3D with nanometre scale resolution.
There was a fantastic trade exhibition held in the lovely conservatory of the hotel from our conference sponsors, who enabled the registration fees to be kept incredibly low. Trade representatives not only showed their new technology and gave fantastic technobite talks during the session, but happily discussed career pathways with early career delegates.
Overall, the meeting was a huge success with a great turnout by microscopists based in Ireland and Scotland. Having been starved of in-person meetings for two years, the organisers wanted to focus on networking and in-person discussion, which the meeting definitely delivered on. A diverse range of exciting research topics and lively panel discussions

74 ISSUE 66 JUNE 2022
led to a vibrant and fascinating meeting which will hopefully result in many future collaborations.
To learn more about the Irish and Scottish Microscopy Societies, or if you are interested in joining either, visit the following websites.

Microscopy Society of Ireland: www.microscopy.ie
Scottish Microscopy Society: www. scottishmicroscopygroup.org.uk
Conference Prize Winners
Flash Talk & Presentation prizes
Laura Gambini (Trinity College Dublin) sponsored by the RMS Early Career Committee
Maurice O’Mara (University College Dublin) sponsored by the RMS Early Career Committee
Eoin Moynihan (University of Limerick) sponsored by Ibidi
Louise Colfer (Tyndall National Institute) sponsored by ThermoFisher/FEI
Patrick McBean (Trinity College Dublin) sponsored by the Chan Zuckerberg Initiative
Niamh Burke (University College Dublin) sponsored by ThermoFisher/FEI
Poster Prizes
Rachel Beatty (National University of Ireland Galway) sponsored by Mason Technology
Cameron O’Byrne (Trinity College Dublin) sponsored by the RMS Early Career Committee
Travel awards
Amelie Sobczak (University of St Andrews) sponsored by the RMS Early Career Committee
Pietro Esposito (University of St Andrews) sponsored by the RMS Early Career Committee
Lucia Hughes (Trinity College Dublin) sponsored by JEOL
Sarah Keary (The Institute of Photonic Sciences, Spain) sponsored by Focal Plane
75
Rising possibilities of quantitative phase imaging

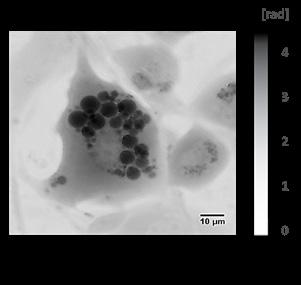 Zuzana Nováková, Application Specialist at Telight.
Zuzana Nováková, Application Specialist at Telight.
Quantitative phase imaging (QPI) technology is on the rise. Several main factors have contributed to its popularity. Live-cell imaging is the key approach to cell analysis in both primary and applied research. QPI enables researchers to perform long-term experiments on live cells without altering their nature thanks to its label-free approach to analysis. Despite the ongoing progress in the fluorescence microscopy (the development of less toxic and more stable fluorophores, low-power lasers and more sensitive detectors), label-free imaging remains crucial when monitoring cells in an undisturbed environment - for example, in drug testing studies or studies of differentiation.
Obtaining quantitative data on cell morphology and dynamics is critical. Compared to routinely used labelfree tools for cell visualisation like phase contrast or differential interference contrast, the QPI provides direct information on various cell parameters such as dry mass, motility, area, or cell density. QPI is also a suitable method for cell population analysis because it can capture rare cell events and detect unique
cell phenotypes non-invasively and quantitatively, therefore reliably and precisely. Let us look at several fields where QPI has a strong impact already.
The importance of QPI in current research
Cancer research
One of the cancer cell hallmarks is its ability to
76 ISSUE 66 JUNE 2022
Examples of cell parameters obtained from the QPI method
1. Cell dry mass
2. Area
3. Perimeter
4. Circularity
5. Density
6. Growth speed
7. Speed
8. Euclidean distance
9. Meandering index
10. Directionality
11. Trajectory length
Morphology
Motility
avoid programmed cell death. Understanding this phenomenon is therefore one of the main focuses of cancer research and correct cell death type classification is crucial. Predominant types of cell death can be detected by flow cytometry. Nevertheless, the absence of cellular morphology analysis may lead to the misclassification of cell death type. QPI can overcome this phenomenon, moreover, without the use of fluorescent labels. Researchers from Masaryk University (Brno, Czech Republic) have been using QPI intensively for studies on cell death. Recently, they managed to predict the cell death timing using a long-short term memory neuronal network and classify the cell death type as apoptotic or lytic. Importantly, the model was trained solely on QPI data and achieved 75% accuracy [1].
Millions of people are treated with cytostatics every year. The success of the therapy can be easily ruined by cancer cells gaining resistance. To overcome this,
scientists are exploring nanomaterials as possible drug carriers. For example, the research team from the Center for Advanced Functional Nanorobots found out that nanomaterial carrying the cytostatic doxorubicin significantly enhanced the drug intake and affected positively the cell behavior. Based on QPI data, they describe how both proliferation and motility of drug-resistant cancer cells decreased [2].

Mechanical cell properties
Retrieving the information about the transmitted light wave phase can be used not only for the quantification of cell dry mass, but the obtained phase values can be used to evaluate the mechanical properties of cells as well. The golden standard in mechanophenotypisation studies is atomic force microscopy. However, it is based on direct contact of the cantilever and cell which can lead to stress and possibly affect the results. QPI, as a non-
77
QPI of human embryonic stem cells.
Timelapse of protein condensate.
invasive method, opens new possibilities for cellfriendly evaluation of viscoelastic properties like cell stiffness [3].
Biomolecular condensates
The research team from MPI in Dresden (Germany) used QPI for the analysis of biomolecular condensates [4]. These membrane-less and proteinrich cell compartments have been shown to play an important role in biochemical cellular processes. However, their composition, that crucially affects the phase separation mechanism, remains less understood. McCall and colleagues found out that using QPI, the protein concentration and condensate temperature-dependent shape can be determined more precisely and more efficiently when compared with traditionally used methods like fluorescence microscopy or optical diffraction tomography.
AI and QPI
QPI output is an image that contains quantitative information about a sample, which makes it well-suited for subsequent automated analysis. Furthermore, the high contrast and uniform quality of QPI data suit well with the ongoing development of segmentation algorithms and machine learning applications [5].
Want to know what QPI can do for your research?
Companies like Telight offer professional consultation on QPI and its applicability in specific research. If you have ever struggled with phototoxicity, disturbances during staining or cell segmentation, struggle no more. Assisted sample analysis can be

conducted in several places around Europe through the EuroBioimaging platform or via www.telight.eu

[1] Vicar, T., Raudenska, M., Gumulec, J., & Balvan, J. (2020). The Quantitative-Phase Dynamics of Apoptosis and Lytic Cell Death. Scientific Reports, 10(1), 1–12. https://doi.org/10.1038/s41598-02058474-w
[2] Fojtů, M., Balvan, J.,Vičar, T., Polanská, H. H., Peltanová, B., Matějková, S., Raudenská, M., Šturala, J., Mayorga-Burrezo, P., Masařík, M., & Pumera, M. (2021). Silicane Derivative Increases Doxorubicin Efficacy in an Ovarian Carcinoma Mouse Model: Fighting Drug Resistance. ACS Applied Materials & Interfaces, acsami.0c20458. https://doi.org/10.1021/ ACSAMI.0C20458
[3] Vicar, T., Chmelik, J., Navratil, J., Kolar, R., Chmelikova, L., Cmiel,V., Jagos, J., Provaznik, I., Masarik, M., & Gumulec, J. (2022). Cancer cell viscoelasticity measurement by quantitative phase and flow stress induction. Biophysical Journal, 0(0). https://doi.org/10.1016/J.BPJ.2022.04.002
[4] McCall, P. M., Kim, K., Fritsch, A. W., IglesiasArtola, J. M., Jawerth, L. M., Wang, J., Ruer, M., Peychl, J., Poznyakovskiy, A., Guck, J., Alberti, S., Hyman, A. A., & Brugués, J. (2020). Quantitative phase microscopy enables precise and efficient determination of biomolecular condensate composition. BioRxiv, .(.), 2020.10.25.352823. https:// doi.org/10.1101/2020.10.25.352823
[5] Jo,Y. J., Cho, H., Lee, S.Y., Choi, G., Kim, G., Min, H. S., & Park,Y. K. (2018). Quantitative Phase Imaging and Artificial Intelligence: A Review. IEEE Journal of Selected Topics in Quantum Electronics, 25(1). https:// doi.org/10.1109/JSTQE.2018.2859234
78 ISSUE 66 JUNE 2022
Society saddened to learn of death of Dr Michael Ormerod Hon FRMS
Honorary Fellow made enormous contribution to Flow Cytometry

The RMS was deeply saddened to learn of the death of Dr Michael (Mike) Ormerod Hon FRMS, in late April.
Mike made a huge contribution - not only to the UK cytometry community, but also to worldwide education in cytometry in a career spanning more than 35 years.
He worked for many years at the Institute of Cancer Research in Sutton, Surrey, where he headed the flow facility until he left to become an independent consultant in flow cytometry.
In 1985 he co-founded the first informal gathering of flow cytometrists - the London Flow Club, and two years later was elected to the first Flow Cytometry Committee of the RMS. The sectionwhich has thrived ever since - only came about due to the efforts of Mike and his fellow enthusiasts.
Mike set up and ran the annual RMS Flow Cytometry Course for many years, and taught on several
international courses including in India (where he was born), Uruguay, South Africa, Egypt and Iran. For 15 years, he also ran the world's only distance learning course on Flow Cytometry for the Virtual School of Biomedical Sciences, University of Ulster.
Mike wrote several books, including 'Flow Cytometry: A Practical Approach' - a familiar sight on the shelves of many a flow cytometry laboratory – and ‘Flow Cytometry: A basic Introduction’ which is still used on the RMS course today.
In 2015 he was awarded the Honorary Fellowship of the RMS in recognition of his contributions to both the UK cytometry community and worldwide education.
Mike's contribution to the world of flow cytometry, his passion, friendliness and support given throughout his career leaves a legacy of thankful flow cytometrists that ensures that he will not be forgotten.
The RMS offers its sincere condolences to Mike's family and friends at this time.

79
Mike receiving his RMS Honorary Fellowship from former President Pete Nellist, in early 2016.
Professor A.G. (Tony) Cullis, Hon FRMS (1946 - 2021)


Tony Cullis was born in Worcester on 16th January 1946. He studied at Oxford University’s Wadham College and the Department of Metallurgy (now Materials), gaining his BA degree in chemistry in 1968 before going on to complete his doctorate in the late Roger Booker’s Semiconductor Group in 1972. This involved building a novel Molecular Beam Epitaxy instrument with which he grew germanium islands on silicon substrates, characterising them with both transmission and the newly developed scanning electron microscopes. During his student years at Oxford Tony impressed his fellow students not only with his quiet, hard work but also by (nearly) always wearing a bow-tie and driving an MGB sports car. Tony then went to continue his research in New Jersey from 1972-75, at the Bell Telephone Laboratory (“Bell Labs”) where he quickly made his mark by using TEM to solve some real and pressing problems - rather than doing esoteric research!
Returning to his native Worcestershire, Tony moved to a new position at the Royal Signals and Radar Establishment (RSRE, later DRA and DERA) in Malvern where he led a very active electron
microscopy effort, again studying semiconductors and electronic device materials. The electron microscope suite that he built up included a 400 kV (kilovolt) instrument (a JEOL 4000EX) – a large installation with a superstructure crane used for lifting the huge high voltage generator tank and the electron gun chamber.This created a problem in that the room chosen for the installation had a rather low ceiling……. But nothing daunted Tony – he foresaw the problem and arranged for a large hole to be cut in the ceiling to accommodate the crane! At that time he and one of us (John) were both running these instruments and our shared experiences proved very valuable when we encountered difficulties. Tony stayed at RSRE for more than 20 years.
1979 saw the start of a series of meetings for which Tony will long be remembered. He set up a conference with the theme “Microscopy of
80 ISSUE 66 JUNE 2022 In Memoriam
Tony receiving the Honorary Fellowship of the RMS in October 2016.
Semiconducting Materials (MSM)” which would go on to run in odd years in Oxford, and later also in Cambridge. With administrative support provided alternately by the RMS and the Institute of Physics, these three-day events attracted a global network of enthusiastic microscopists, including several delegates from the former Soviet Union for whom these visits were their first to the West. Permanent records of the meetings were published, containing papers of all contributions, these being peer-reviewed during the meeting and comments/recommendations given to the authors before they left. The success of these volumes, produced by IoP Publishing and later by Springer, was due to Tony’s superb organisational and editing skills, along with a very fine eye for detail – no misprint went un-noticed! We experienced these skills ourselves when we worked alongside Tony as co-chairmen for several of the conferences. When a problem or crisis arose – as sometimes happened – Tony would calmly deal with it, or else knew who could. This included an emergency when an attendant had mistaken some external medication for his toothpaste so an ambulance had to be called, and another case where a delegate found a waterdamaged intermediate ceiling had collapsed onto his bed and suitcase. One characteristic of these meetings has always been quizzes after the formal conference dinners in hall, combining tests of general knowledge with creative problem solving, and the evenings usually ended up in the college bars. It was on this occasion the younger of us (Thomas)
actually met Tony for the first time when attending the 1993 conference as an undergraduate student, not knowing then that Tony would a year later become his co-advisor on a PhD project led by Colin Humphreys at Cambridge University.
Tony published a lot with only a small number of collaborators, resulting in many highly cited original articles that paved the way in understanding materials growth, which earned him a number of honours, including a fellowship of the Royal Society in July 2014 and an Honorary Fellowship of the RMS in October 2016.


At the end of 1995 Tony became professor at the University of Sheffield, leading the Semiconductor Materials & Devices group, a post from which he retired after 14 years due to health issues. He was instrumental in getting the first 200kV fieldemission electron microscope and the first 300kV double aberration corrected field-emission electron microscope in the UK. In the 1990s Tony used to drive a Rover Grand Vitesse with a three-litre engine and later, a Nissan Skyline with double-turbo, to get into which actually took some flexibility!
Tony sadly passed away on 9 December 2021 after a long battle with Parkinson’s disease which had led to his early retirement more than a decade earlier, when he and his wife Ruth had returned to Malvern.
 By John Hutchison and Thomas Walther
By John Hutchison and Thomas Walther
81
Tony (right) with the late Harry Kroto (left) and Alan Law, the Lord Mayor of Sheffield (centre) at the opening of the Kroto Centre for High Resolution Imaging and Analysis in Sheffield in June 2010.
Tony at a business dinner in Tokyo in November 2009, after the acceptance test for the 300kV microscope in the JEOL factory in Akishima, Tokyo.
Teledyne announces partnership with Center for Quantum Networks

Teledyne Princeton Instruments and Teledyne Photometrics are pleased to announce they are now an industrial partner in the Innovation Ecosystem of the Center for Quantum Networks (CQN), a National Science Foundation Engineering Research Center.
The Center for Quantum Networks (CQN) is taking on one of the greatest engineering challenges of the 21st century: to lay the technical and social foundations of the quantum internet. The quantum internet will require coordination of the quantum states of particles serving as computational bits between quantum computers, that is not present in the realms of classical physics.
Teledyne’s solutions have been used to clarify
quantum optical systems and develop the novel materials needed to connect networks of quantum computers. Stephen Fleming, Innovation Ecosystem Direct at CQN notes, “Teledyne's leadership in sensing and transmitting information in challenging environments makes it a perfect fit with the Center for Quantum Networks' mission to lay the technical and social foundations of the quantum internet.”
The overarching mission of the CQN Innovation Ecosystem is to spur new industries and a competitive marketplace of quantum service providers and application developers, laying the foundation for a socially responsible quantum internet. As part of the Innovation Ecosystem, Teledyne provides myriad products used in various aspects of quantum including sensing, computing, networking, as well as basic research. Related products include scientific CMOS, EMCCD (Electron Multiplying Charge Couple Device), and InGaAs detectors, as well as a variety of high-resolution spectrographs and spectrometers needed for material science research.
Teledyne Princeton Instruments and Teledyne Photometrics are part of Teledyne’s Vision Solutions group and are leaders in the design, manufacture, and deployment of digital imaging components for scientific research and applications of advanced technology.
www.teledyne.com
New DriveAFM applications
Last summer we had the pleasure to host the wellknown AFM veteran Sergei Magonov for a few weeks at our labs in Liestal to test the DriveAFM.
Since the early days of scanning probe microscopy, Sergei has contributed to the advancement of SPM in various roles, where he specialised in the investigation of polymer materials.
Having brought a variety of different polymer samples to Nanosurf, Sergei started imaging his samples with the DriveAFM from the first day of his visit, mainly using dynamic mode with CleanDrive
photothermal excitation - but he was also the first person outside Nanosurf to use WaveMode. During his stay Sergei collected a wealth of data that we have now compiled into three application notes:
• Characterization of Polyethylene with DriveAFM
• Exploring Nanoscale Organization of Normal Alkanes on HOPG Substrate with DriveAFM
• Studies of Self-Organisation of Semi-Fluorinated Alkanes on Different Substrates with DriveAFM
www.nanosurf.com
82 ISSUE 66 JUNE 2022
COMPANY NEWS
Management Change at Nanosurf
CEO and James Berwick will take on the role of COO. The newly created Chief Operating Officer role will heavily focus on the operational performance of the business, as the evolution of Nanosurf’s growthstory continues. The Board is convinced that the two managers, with their complementary skills, are an excellent successor team for Urs. Björn Pietzak’s role as head of the Industrial Solutions business will continue to play a key role in Nanosurf’s evolution. As a high-tech company in a very competitive environment, the board decided on Dominik for the CEO role because of his excellent understanding of technologies and market needs. These are the skills needed to provide outstanding AFM solutions in the future. Dominik has already kicked off the search for a suitable leader for our R&D - who will ensure Nanosurf will keep up the rate of developing world class solutions.
In the last 13 years, under the leadership of CEO Urs Matter, Nanosurf has undergone a huge phase of development and growth. Nanosurf’s board has been concerned about Urs’ succession plan. Urs took on the challenge of assembling an excellent leadership team by promoting employees to manage the company who will continue to drive Nanosurf along the path of a successful company.
The Nanosurf Board decided the following changes to the management structure:
As of June 1st, Dominik Ziegler will become the new

Furthermore, the board proposes that Urs Matter shall be elected as president at the general assembly on May 20th. Urs will be an active president and coach to Dominik and James. He will reserve sufficient time for Nanosurf to make the transition into the future smooth and ensure the company continues its highgrowth development. Lukas Howald, as president of Nanosurf for the past 16 years, will remain a member of the board, next to Markus Oswald, Jeff Jones, Giancarlo Rizzoli, and Dominik Brändlin.
www.nanosurf.com
Lapping and Polishing Solutions from Agar Scientific

Agar Scientific is pleased to have been appointed as the exclusive distributor for Lam Plan specimen preparation equipment in the UK.
Lam Plan are based in Gaillard, France and specialise in manufacturing Lapping and Polishing solutions with an extensive range of machines and consumables to fit almost any sample preparation application. Lam Plan design their equipment with quality in mind.
Agar Scientific supply the complete range of Lam Plan's grinders, polishers and presses, along with tailor-made consumables to help customers with their polishing, grinding, lapping and general sample preparation requirements. Uniquely, Lam Plan also maintain a fully purposed test lab where customer's samples can be evaluated, and suitable protocols developed for processing. This, combined with advice on which grinding and polishing materials to use, allows the very best results to be achieved from
their grinding and polishing machines.
A wide range of grinders, polishers and presses are available from Agar Scientific, including the Cutlam disc cutting machines, Smartlam grinder/polishers, Masterlam grinder/polishers and the Presslam mounting press.
A wide range of consumables are offered to be used alongside the Lam Plan machines, including polishing cloths and pads with either self-adhesive or magnetic fixings, Cameo Disc diamond polishing discs for rapid grinding of metallographic samples and a range of monocrystalline and polycrystalline diamond abrasive. Hot mounting resins have been developed and tested to suit a wide range of applications, as well as cold mounting resins as a solution for samples which do not withstand high pressures or temperatures.
www.agarscientific.com
83
Dominik Ziegler
Linkam surpasses 35,000 mentions in Google Scholar

Our customers work in a wide range of industries and research disciplines, and we are always excited to see published work that features one of our instruments. Now, we have more than 35,000 mentions in papers and articles indexed by Google scholar – and that’s not including papers that have not been digitised in the online library. That’s a lot of research!
Many of our standardised sample characterisation systems started out as an idea brought to us by a scientist either dissatisfied with what was available or looking to create something completely new. Therefore, their usefulness to other scientists has created extensive demand.
Recent published work featuring Linkam products has included:
1. A paper published by University of Petra, Jordan, describes for the first time the formation of room temperature therapeutic deep eutectic solvent (THEDES) of RIS, an antipsychotic drug that is used in the treatment of schizophrenia, mixed and manic states associated with bipolar disorder and irritability in children and adolescents with autism. Our FDCS196 stage was used in this study to develop a novel transdermal delivery system for risperidone.
2. Work by a team of researchers at Queen’s University Belfast and University College London reported on a newly developed
strategy for the manufacture of a high drug loaded amorphous solid dispersions (HDASD) using a hot-melt extrusion (HME) based platform. Using a Linkam THMS600 heating and cooling stage with T94 controller and LNP liquid nitrogen cooling system, the study was able to directly observe the temperature dependences of the X-ray scattering pattern for the crystalline drugs (IND, NPX and IBU) in the presence of a polymeric carrier.
3. Researchers from the University of Huddersfield’s Thermal Methods Research Unit (TMRU) combined differential scanning calorimetry (DSC) with thermomicroscopy to shed light on materials’ energy changes and optical features in this recent paper, using our Optical DSC450 System.
4. A study by Swansea University used a Linkam LTS420E-P attached to a novel ultra-sensitive external quantum efficiency system to characterise the photogeneration process of organic solar cells. They proved it is possible to achieve near-unity charge generation quantum yields in organic solar cells.
Those are just some of the many recent examples where our systems have helped researchers to make important breakthroughs.
www.linkam.co.uk
84 ISSUE 66 JUNE 2022
COMPANY NEWS
Speed, Image Quality and Versatility Bring Time-resolved Tomography to the Lab
at this number of publications, applications and possibilities of dynamic micro-CT, the ability to perform fast 4D experiments on a micro-CT system is something to consider.

Dynamic CT allows the analysis of samples under changing environmental conditions, such as mechanical stress or non-ambient temperature and humidity. Due to its non-destructive nature, it has been used for time-resolved studies ever since it was introduced. However, recent developments in features that work together to increase speed, image quality and system versatility make dynamic CT a viable technology for use in the lab, and not only at large synchrotron facilities, making lab-based timeresolved tomography dominant over synchrotronbased experiments in publication numbers. Looking
To be able to perform fast dynamic experiments, all components of a micro-CT system must work together in perfect harmony to get the job done. A high-power x-ray source, combined with fast detectors are needed to keep exposure times and therefore scan times short enough to keep up with the fastest of processes. Ample hardware connections for power and signals – preferably through a slip-ring connection enabling endless rotation of the experiment setup – and additional cable feedthroughs are needed to install complex in-situ setups inside the system. And reconstruction and visualisation software need to be able to handle data acquired over multiple hundreds of samples (or gantry) rotations. As no experiment can be perfectly predicted, ultimate flexibility in the begin- and endpoint of the reconstruction blocks needs to be in place, not only to capture the exact event of interest, but also to reduce overhead in time and storage.
www.tescan.com
Exploring ferroelectricity in layered materials using atomic force microscopy in vacuum
Atomic force microscopy (AFM) and its associated functional modes such as Kelvin probe force microscopy (KPFM) detect forces on the scale of 1 pN in order to measure the topography and functional properties of surfaces with nanometre scale resolution. Under ambient conditions however, damping of the cantilever and interactions with airborne contaminants adsorbed at interfaces degrade the sensitivity of AFM measurements. By utilising the Park NX-Hivac, a system which enables the performance of AFM measurements down to the 1x10-6 mbar pressure range, we demonstrate performance in topography and electrostatic modes which are closer to the intrinsic limit of scanning probe microscopy systems in a platform free from the laborious operating procedures and practical limitations of full ultra-high vacuum-based AFM.

In the full version of this article, link below, we study a system which has received significant recent attention from the layered materials research community; ferroelectric superlattices prepared by
the formation of parallel stacked interfaces. Taking such a parallel stacked boron nitride interface on graphene, we observe ferroelectric domains using both electrostatic force microscopy (EFM) and KPFM with improved sensitivity versus measurements performed under ambient conditions, exemplifying the advantages of performing electrostatic AFM measurements in vacuum.
Read the full article at: www.parksystems.com/ medias/nano-academy/articles.
www.parksystems.com
85
Linkam Scientific Instruments moves to new UK premises to support global expansion

Linkam Scientific, market leader in temperaturecontrolled microscopy, is excited to announce that it is expanding, and has moved to new premises. Bringing together the entire production function, including raw materials, inventory, machine tooling and manufacturing into an efficient, streamlined process, the new site has undergone a full fit out to
commented: “This move comes on the back of sustained business growth over the past few years. We now have a site that offers an improved customer experience, showcases our innovative scientific instrument range, and brings together our whole UK team – while offering space to expand in the future as we continue to develop our business to support key research in scientific and industrial markets.”
house the entire UK business. The premises features a state-of-the-art, interactive demonstration suite where customers and prospective customers can get hands on, to experience the products for themselves.
The business formerly occupied six separate buildings and the move to a single premises facilitates collaboration, with full cross-functional team working, and productivity gains. Linkam’s new site is conveniently located close to Gatwick airport and Salfords railway station in Surrey, with direct rail links to central London including St Pancras, for Eurostar services, and London Bridge.
Ross Browne, Managing Director at Linkam
Duncan Stacey, Director of Sales and Marketing added: “With the support of our partners and customers the business has grown steadily with new hires across production, marketing, sales, R&D and finance. Our new customer demonstration area and conference facilities allow us to present our global business, while offering our team a modern working environment to help us continue to attract the best in talent. We look forward to welcoming our customers and partners to our innovative new space.”
Linkam was founded in 1982 by Arnold and Louise Kamp and the company designs and manufactures temperature and environmental control stages, electronics and software that can be used in conjunction with light microscopes and a wide range of analytical techniques, including Raman, FTIR,WAX/ SAX and other X-ray techniques, to enable scientists to analyse and characterise samples.
www.linkam.co.uk
Linkam showcases key temperature-controlled stages at the Advanced Materials Show 2022
Market leader in temperature-controlled microscopy, Linkam, will be exhibiting at the Advanced Materials Show in Birmingham, UK, this year. Linkam will be showcasing two key systems from its range of sample characterisation solutions – its humidity control and electrical sample testing systems.
Earlier this year, Linkam announced the launch of its new product, the RHGen Relative Humidity (RH) controller. This new system offers humidity control between 3% and 95% RH, at temperatures from ambient to 85°C, with an upgraded RH sensor and improved connectors, providing accurate and reliable environmental control to a variety of Linkam temperature control stages and third-party chambers. Linkam will also be highlighting its electrical systems, which have been used to support the microelectronics and semiconductor industries for a number of years. These stages can be fitted with electrical connection or gold-tipped tungsten needle probes, which allow the measurement of the electrical properties of the sample and their change with temperature.These can
be combined with the RHGen to provide a complete picture of the electrical properties of a material under different temperature and humidity conditions.
Duncan Stacey, Sales and Marketing Director at Linkam, comments: “We are looking forward to demonstrating two of our most popular systems at this year’s Advanced Materials Show. Supporting scientists and researchers with their materials characterisation is at the heart of what we do, so we are looking forward to discussing their material characterisation requirements and the solutions we can offer.”
The Advanced Materials show is an annual event that will be taking place this year in NEC Birmingham, on the 29th-30th June. This free-to-attend exhibition is due to host over 300 exhibitors and covers materials innovation across a broad range of applications including aerospace, automotive, construction, defence, electronics, energy, medical, and renewables.
www.linkam.co.uk
86 ISSUE 66 JUNE 2022
COMPANY NEWS
Advances in Cryo-EM Studies Unravel Neurodegenerative Mysteries
Since being named Method of the Year by Nature in 2015, cryo-electron microscopy (cryo-EM) has catapulted the imaging of biomolecules into a new era.
In 2017, Jacques Dubochet, Joachim Frank, and Richard Henderson won The Nobel Prize for the development of cryo-EM. In 2020, the very first atomic-resolution cryo-EM structure of a protein determined by Thermo Fisher Scientific technology at only 1.2 Angstrom resolution dawned the cover
of Nature. The world was, for the first time, seeing individual atoms in all their glory by cryo-EM.
Now, less than two years later, scientists are using cryo-EM to unravel biological mysteries, including those surrounding some of the most impactful neurodegenerative diseases. In a series of recent studies, scientists from MRC Laboratory of Molecular Biology, Thermo Fisher Scientific, Tokyo Metropolitan Institute of Medical Science and other institutions examined how tau protein cease their usual protective function, aggregating to form large tangles that damage cells in the brains of patients with a class of neurodegenerative diseases called tauopathies.
Until now, a lack of model systems to generate the structures of tau filaments hampered efforts to uncover the molecular mechanisms that underlie tauopathies. Yet in March 2022, the team published a record-breaking 76 structures of recombinant tau filaments assembled in vitro that replicate the structures of filaments from Alzheimer’s disease and CTE. Their findings suggest molecular structures of tau tangles are key to understanding neurological diseases and will have large implications for diagnosing and treating these diseases in future.
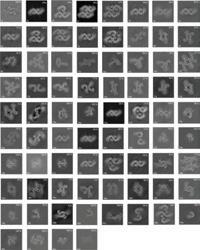
www.thermofisher.com
Telight focusing on artificial intelligence

Long-term experiments are one of the key fields of expertise for Q-Phase as the microscope can perform them in an automated way while collecting numerous data that are subsequently processed and analysed. Of course, we are not the only ones on the microscopy market that have to deal with excessive quantities of data, also other players on the market face these problems. This was one of the reasons why Telight decided to focus on creating of an artificial intelligence model.
Data analysis is very time-demanding and the artificial intelligence model can be much more efficient in this task. When used correctly, artificial intelligence can produce new results based on an analysis that would not be done in standard conditions by human researchers and thus move the research one step further.
It helps to save researchers´ time during repetitive procedures, while also reducing the error rate in results analysis to a certain extent. More specifically, we focus on pixel classification, image enhancement, cellular analysis in time, detection of abnormalities and other.
For the complicated tasks ahead, we have hired well qualified specialist Hadi Abdi Khojasteh to help us reach our goals.
www.telight.eu
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk.
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment.The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
87
Expert level Raman Images for Everyone with EasyImageTM
HORIBA, a global leader in measurement and analysis solutions for advanced research and industry, and the world leader in Raman microscopy and nanoscopy, is thrilled to announce the launch of EasyImageTM, a new imaging tool for Raman microscopy.
EasyImageTM is a new App within HORIBA’s well respected Labspec6TM Raman imaging software package. It provides guidance to new users, allowing them to quickly generate publication-ready results.
EasyImageTM flattens the learning curve for beginners in Raman imaging by assisting in the understanding of the five key steps required for chemical image generation:
• Sample focusing and area of interest selection
• Imaging parameter optimisation

• Spectral fingerprint selection
• Rapid measurement preview
• High quality chemical image collection using Raman spectroscopy
EasyImageTM includes intelligent spectral range and other acquisition parameter selection, automated (and manual) spectral display at multiple points on the sample – ensures optimal parameter selection, automated background removal, automated spectral identification (with optional KnowItAll database), automated chemometric loading generation and customisable automatic analysis report generation.
EasyImageTM embeds expert knowledge to minimise
training requirements and its image centric workflow makes it ideal for labs with many relatively inexperienced uses. It is also a great assistant for busy scientists as its workflow naturally brings the right data and important parameters to the attention of the user at the right time.
EasyImageTM complements the wide range of usability features included in the new LabRAM SoleilTM Raman microscope and can also be used with other HORIBA Raman microscopes. EasyImageTM provides benefits for almost all sample types including 2D materials, nano-materials, pharmaceuticals, tissue and cells and even photoluminescence and Raman analysis of semiconductors. “With LabRAM SoleilTM and EasyImageTM, prepare to enter a new era of automation and generate high- definition Raman images in just a few clicks”, said Adam Holland, Senior Raman Product Manager, HORIBA UK.
www.horiba.com
Hamamatsu Photonics announces the world’s first Quantum Cascade Laser (QCL) module with a tunable frequency range from 0.42 to 2 THz

Hamamatsu’s breakthrough was achieved by analysing the principle governing the generation of terahertz waves, which enhance the QCL’s output power and the configuration of the highly efficient external cavity, while using our advanced optical design technology.
This research results in generating narrow-band
terahertz waves all while switching the frequency of just one QCL module. This technology will help in polymer material identification as well as boost accuracy in quality evaluation for non-destructive inspection of drugs, foods, and semiconductors containing components that absorb terahertz waves. The QCL module using this technology will also prove to be a key innovative device for achieving future ultra-high-speed wireless communication.
The Optica Publishing Group in the electronic version of the scientific journal “Photonics Research” published these research results on the 22 February 2022. Part of this research was commissioned by the Japanese Ministry of Internal Affairs and Communications, as a “Strategic Information and Communications R&D Promotion Program (SCOPE)” (No. JP195006001).
www.hamamatsu.co.uk
88 ISSUE 66 JUNE 2022
NEW
PRODUCTS
Versatility of VHX-7000 Brings Academic and Commercial Benefits to Leading UK University
World-leading microscopic technology from KEYENCE is enabling a major UK research institution to carry out highly detailed observation, analysis and measurement on projects in fields ranging from microbiology and invertebrate biology to mycology, forensic science and geological thin section analysis. The University of Derby is a teaching and research institution dedicated primarily to undergraduate students, and researchers for Masters degrees and PhD’s. To aid students in their studies, the University has invested in a KEYENCE VHX7000 Digital Microscope, which combines high magnification and precision with versatility and ease of use.
The all-in-one microscope, boasting an impressive ultra-high accuracy 4K camera and monitor, is being employed across all levels of undergraduate degree teaching, where it provides a fully automated system for students to carry out their own sample observation and analysis in up to 6000x magnification.

In particular, it is seeing extensive service with final year undergraduate students during their independent study projects, where they work largely unsupervised on a research topic of their choice. Although these projects vary significantly in nature, many require the availability of a microscope and image capture
software, and the VHX-7000 combines both functions in a convenient and compact package.
One recent example involved a student who was seeking to analyse mucus flow rates from coral samples. Here, the time lapse photography and video features of the VHX-7000 proved invaluable, providing detailed high-resolution images.
Another project was for a geology PhD researcher working with microfossils.The multi-lighting capability of the VHX- 7000, as well as the depth composition and flexible stage and tilt features, allowed the capture of high-quality images on a nondestructive basis.
The VHX-7000 is even being used in commercial projects undertaken by the University. A local company approached the team with a request to use the VHX-7000 and scanning electron microscope to solve a problem with its production processes. The company was a producer of titanium aerospace fasteners, and a problem with the forging of these items was resulting from contamination in the process. The VHX-7000 captured highly detailed images of the contaminated areas and, in conjunction with the University’s electron microscope, ultimately enabled the company to identify where in the process this problem was occurring – and take appropriate remedial action.
www.keyence.co.uk
Empower superior two-photon imaging by optimising your laser beam
The Beam Conditioning Unit (BCU) optimises your laser beam and enables fast intensity control in your optical path between the laser and your multiphoton microscope, all in one stable and compact box.

The laser beam conditioning is taken care of, so you can focus on the imaging due to the straight out the box launch optics solution the BCU offers.
The BCU’s compact design shortens your table launch path, allowing more room on your table to grow your experiments within the same set-up area.
The BCU+ is built for working with advanced
microscopes with high GDD, such as the Scientifica VistaScope. It includes all the functionality of the BCU, plus a prism prechirper designed to cover the GDD precompensation range required by modern microscopes. The result is brighter images for a given average laser power – improving data while protecting the sample.
Save precious funds as both the BCU and the BCU+ can be integrated with any multiphoton system, giving you the freedom to use it with your existing equipment in the lab.
www.scientifica.uk.com
89
Nanosurf announces WaveMode, a new AFM imaging mode launched at the 2022 Biophysical Society meeting

mode is based on CleanDrive, Nanosurf’s exclusive photothermal actuation of the cantilever; CleanDrive provides stable, low drift, and high signal to noise cantilever tunes that are insensitive to changes in the environment. Providing key advantages in both liquid and air environments, WaveMode enables a faster workflow and improved results for AFM imaging in both the life science and materials science applications.
WaveMode is the fastest force curve-based imaging mode with application to all samples and all environments and is available exclusively on the DriveAFM. It represents the first commercially available off-resonance mode that can use photothermal actuation of the cantilever – instead of the traditional piezoacoustic actuation – to enable fast, stable, and gentle imaging. This new mode continues the tradition of Nanosurf’s 25-year history of innovation in scanning probe microscopy.
WaveMode offers users of all experience levels and backgrounds many advantages, including fast imaging rates, no cantilever tuning, and a fully automated laser and photodetector alignment. This new imaging
The DriveAFM was released in 2020 and is Nanosurf’s flagship AFM platform based on a tip-scanning design combining atomic resolution, imaging capability from the atomic scale through 100 mm, full motorisation, and fast scanning. With innovations in scanner design and optical beam path engineering, the DriveAFM offers superior research performance.
Nanosurf was founded in 1997 in Liestal, Switzerland, and has since become one of the most trusted and established AFM brands in the market today. While Nanosurf continues to develop and manufacture AFMs at its Swiss headquarters, it is a global company with direct sales, service and support operations in China, Germany, India, Singapore, the UK and US.
www.nanosurf.com
LC35 Digital Microscope Camera Delivers an Outstanding Value for Standard Brightfield Imaging
The LC35 microscope camera makes capturing quality brightfield images affordable. With a 3.5-megapixel CMOS sensor and versatile exposure times and resolution modes, the camera’s provides quality brightfield images in a costeffective package.
Flexible for use with a wide range of samples under different observation conditions, the LC35 camera delivers the detail needed for routine imaging, inspection and documentation. The frame rate of up to 40 frames per second (fps) makes navigating around the sample fast to achieve precise focusing and fast image capture.
The camera integrates seamlessly with Olympus life science and industrial imaging systems, including full microscope set up assistance, software support

and optical compatibility. For basic imaging and documentation, customers can use our free LCmicro software, while advanced image analysis is possible using cellSens™ software for life science and OLYMPUS Stream™ and PRECiV™ software for industrial applications.
Installing the camera is simple—a single USB 3.1 cord provides power and a fast connection to your PC or laptop. The camera easily mounts to any light microscope using different C-mount adaptors. Using the camera is just as easy. With only a little training, operators can use the camera to its full potential, including interactive measurements, commenting on images, archiving and creating reports.
www.EvidentScientific.com
90 ISSUE 66 JUNE 2022 NEW PRODUCTS
72DL PLUS™ Thickness Gauge Measures Ultra-Thin Layers and at High Speed
The new 72DL PLUS™ ultrasonic thickness gauge delivers precision thickness measurements at high speed in a portable, easy-to-use device. With fast scanning, advanced algorithms and Olympus’ lowest-ever minimum thickness capability, the gauge can measure the thickness of very thin layers for challenging applications across industries.
Whether used as a paint thickness gauge, coating thickness gauge or material thickness gauge, the 72DL PLUS instrument delivers reliable, lab-quality measurements to maximise productivity and throughput on the production floor.
Capable of displaying up to six layers at once for multilayer coatings, paint, plastics and other materials, the gauge includes a full-colour touch screen and five measurement layouts so thickness changes can be accurately tracked and visualised. Guided workflow makes it easier to create and save an application setup. For routine applications, inspectors can store and recall settings to simplify device setup before each inspection.
Built-in data logging and onboard file management provide streamlined thickness data collection and processing, while the PC Interface Application includes intuitive tools to review and manage data for multiple devices and parts. Connected and cloud capable for Industry 4.0 practices, the gauge supports wireless LAN, Bluetooth®, and USB connectivity and integrates into the Olympus Scientific Cloud™ (OSC).
Built for industrial environments, the gauge can be used indoors and outdoors, on a work surface, or
using a four-point chest harness or shoulder strap. The gauge meets military drop test standards (MILSTD-810G) to protect against accidental drops or impacts, is designed to meet IP65 requirements to protect against dust and moisture, and takes reliable measurements in hot and cold climates with an impressive operating temperature range of –10 °C to 50 °C (14 °F to 122 °F).
72DL PLUS thickness gauges are available in Standard and High-Frequency models. The High-Frequency model can drive transducer frequencies up to 125 MHz to measure ultra-thin materials—including multilayer paint, plastics, metals and coatings—and simultaneously display the thickness of up to six layers. The 72DL PLUS gauge provides up to 2 kHz measurement speeds, a 60 Hz display update, and clean signals for fast, accurate measurements.
www.olympus-ims.com
Micro electron diffraction: using cryo-electron microscopy to find molecular structure from nanocrystals

MicroED is a cryo-EM technique that can quickly determine high-resolution structures of small molecules and proteins. Atomic details can be extracted from individual nanocrystals (<200 nm in size), even in a heterogeneous mixture.
a crystal with electrons is much stronger than it is with X-rays. Data collection is completed in only a few minutes and 3D structures can be determined at atomic resolution. If crystals are initially too large, focused ion beam (FIB) milling can be used to thin the frozen crystal sample into a lamellae.
MicroED data is acquired on a cryo-transmission electron microscope (cryo-TEM) using electrons as the incident beam. Since MicroED is a diffraction technique, it requires crystalline samples, much like X-ray crystallography. However, much smaller crystals are required for MicroED because the interaction of
MicroED is becoming increasingly popular in drug discovery as it can be used to determine the structure of protein-drug complexes and small molecules. Critically, MicroED alleviates the need large crystals, a significant benefit in the fast-paced pharmaceutical industry. For small molecules, this means that structures can be obtained directly from femtograms of powder within minutes.
www.thermofisher.com
91
NEW PRODUCTS
Keyence LM Series Delivers Breakthrough Measurement Accuracy Alongside Unrivalled Ease Of Use
Boasting accuracy to ±0.7μm, the LM Series combines the ease of use associated with the Keyence range with the breakthrough precision demanded across an ever-broader range of applications. Consistent sub-micron accuracy and repeatability is guaranteed through automated focusing and part positioning, making it ideal for applications with very tight tolerances in sectors from medical and electronics to high-precision metal manufacturing.
As with other Keyence systems, the LM Series is easy and rapid to program with intuitive on-screen help always available, and it can be operated with just one button. Items do not need to be precisely positioned, while rapid stage movement and a large field of view for a precision measurement system deliver optimum speed as fewer images are needed compared with alternative systems. The auto-focus function ensures accurate focus irrespective of operator experience.

Up to 300 dimensions can be measured at a time, while the LM Series can measure up to 100 individual parts simultaneously. A convenient integral coaxial light offers a broad variety of lighting options to provide optimised stable edge detection, whatever the material of the item being measured. While more typically used in laboratory environments, the system is sufficiently robust to withstand the rigours of the shopfloor environment with its lowvibration stage.
An integral highmagnification lens with Z-focus positioning allows for highly accurate non-contact height and depth measurement for areas as small as 20 × 20 μm. Additionally, plane elements can be created which are independent from the stage and overall part slope, with flatness and interplane angle measurements easily added to programs. Suitable applications are plentiful and range from prototype and first-off part inspection, to in-process sample and part inspection; and pre-shipping and incoming goods inspections.

Ailsa Morrison, Applications Engineer for the Metrology Division at Keyence, explained: “Ever tighter tolerances are a defining characteristic of modern manufacturing and through the LM Series, Keyence is offering a rapid and reliable means to verify part dimensions to ever greater levels of accuracy. Alongside that, it really is a system that anyone, anywhere, can use – thanks to a range of automated and intuitive functions.”
www.keyence.co.uk
Hamamatsu Photonics has developed the world’s first THz image intensifier. Capable of real-time non-destructive imaging, it is promising for foreign matter inspection of food products and body scanning
Hamamatsu Photonics has developed the world’s first terahertz image intensifier (THz image intensifier or simply THz-I.I.) by leveraging its imaging technology fostered over many years. This THz-I.I. has high resolution and fast response which allows for realtime imaging of terahertz wave (*) pulses transmitted through or reflected from target objects.
This THz-I.I. was unveiled at “The 69th JSAP (Japan Society of Applied Physics) Spring Meeting” held at the Sagamihara Campus of Aoyama Gakuin University (in Sagamihara City, Kanagawa Prefecture, Japan) for 5 days from Tuesday, March 22 to Saturday, March 26.
*Terahertz waves are electromagnetic waves near a frequency of 1 THz and have the properties of both light and radio waves.
www.hamamatsu.co.uk
92 ISSUE 66 JUNE 2022
TESCAN’s Unique Lift-out Solution Enables Highest Quality Lamella from Complex Materials
The success of TEM/STEM characterisation is directly connected to the sample’s overall quality. However, preparing lamella from novel materials, such as soft and hard phases, varied feature orientations or hallow structures, using conventional “top down” FIB-SEM preparation process often results in unsatisfactory quality.These types of samples exhibit uneven polishing, scratches on the surface and similar damage.

Sample thickness is not the only parameter that must be considered for these types of materials. Other techniques, such as inverted TEM specimen or planar lamella preparation, are often needed. Using these techniques, the TEM specimen can be manipulated to the preferred polishing direction, which mitigates artifact creation.
TESCAN FIB-SEMs provide the ability to change the sample geometry freely during the preparation process. Their patented hardware design integrates a nanomanipulator with a rotating tip, positioned below the FIB to enable easy and intuitive geometrical

adjustment of the TEM specimen. Allowing adjustments such as 90° rotation in X to facilitate planar lamella preparation or 180° rotation in the Y axis for preparing inverted lamella, is critical for many samples. All these advanced adjustments are intuitive to execute because they follow the routine for conventional TEM prep, without introducing complexities like special hardware or additional steps to the process.
TEM prep challenges that are addressed with this unique, below-the-FIB position of the nanomanipulator are described in one of TESCAN’s webinars, “Achieving the Highest Quality TEM Lamella from Complex Materials Using a Unique Lift Out Solution”.
www.tescan.com
Next Generation in Lattice Light-Sheet Microscopy
ZEISS releases the next generation of ZEISS Lattice Lightsheet 7. The microscope system was introduced to the market in December 2020 and opened up a new way for researchers to explore the dynamics of life at subcellular resolution. It was the first commercial, easy-to-use implementation of the lattice light-sheet technology known for enabling sample-preserving, long-term imaging of living cells. The new generation of ZEISS Lattice Lightsheet 7 now provides a number of improvements to the life science research community that will substantially advance live cell experiments.
Equipped with a more powerful camera, the Hamamatsu ORCA-Fusion, the microscope now enables imaging just above the noise level, making lattice light-sheet microscopy even gentler to lightsensitive samples. At the lowest photon count rates, the system provides both an unprecedented uniform background and a sufficiently strong signal to distinguish the finest subcellular structures, while protecting the sample from photodamage and bleaching over hours and days of constant imaging.
As requested by the research community, the system can now be equipped with two Hamamatsu ORCA-Fusion cameras. This increases the imaging speed and significantly improves multicolour fluorescence experiments.
Two cameras double the temporal resolution at which data is acquired. Researchers can watch delicate processes like mitosis without missing the finest details or the fastest events. The innovative design of the excitation beam path allows simultaneous excitation of the sample with multiple laser lines. Combined with two cameras, this enables truly simultaneous imaging of two channels – without any time delay. A dual-camera setup also minimises crosstalk and achieves cleanest results without compromising speed.
The study of dynamic processes in living cells benefits from faster and simultaneous multichannel imaging. Additionally, this new generation of ZEISS Lattice Lightsheet 7 offers, in particular, new possibilities for ratiometric experiments and the investigation of structural co-localisation.
www.zeiss.com
93
HORIBA and Digital Surf partner to launch graphYX software range
World leader in Raman microscopy and nanoscopy, HORIBA Scientific and Digital Surf, creator of the Mountains® software platform for image and surface analysis in microscopy and metrology, today announced the release of graphYX, a new software range for users of HORIBA's Raman spectroscopy solutions, comprising two product levels: graphYX and graphYX-3D.
graphYX, powered by Mountains® technology, is an app included in HORIBA’s LabSpec 6 software suite that allows users to highlight features of their samples by combining multimodal images obtained from SEM, Raman, CL, AFM, NanoRaman, EDX, EBSD, FTIR and other techniques. It will be delivered as standard on instruments such as the HORIBA AFM-Raman and nanoGPS navYX.
graphYX software, when combined with nanoGPS navYX, provides a complete solution for quickly relocating points of interest and overlapping map data on the sample surface. nanoGPS navYX is a multimodal and multiscale solution that facilitates sample study and collaboration between researchers using different analytical tools at different locations.
graphYX users will benefit from the following features:
• Colocalisation tools allowing the correlative analysis of data from several sources or several datasets from the same instrument (these can be
from multiple users and multiple labs):
o from a single instrument: study sample kinetics, monitor evolution over time, overlap data from more than two modalities (Raman, photocurrent, epifluorescence, darkfield etc.), optimise palette, contrast and brightness of the various components of multivariate analysis;
o from multiple instruments: correlate optical microscope images with SEM images, adjust orientation, scale and size of images generated by SEM, AFM, and optical microscopes;
• Quick enhancement and correction of images and chemical maps.
• Interactive document layout and workflow allowing users to track and modify each individual analysis step at any time.
• Compatible with multiple types of HORIBA analysers.
• Combine graphYX with nanoGPS navYX to quickly relocate points of interest and overlapping map areas on samples.
• graphYX-3D adds 3D topographic image rendering for techniques such as AFM and AFMRaman.
www.HORIBA.com

94 ISSUE 66 JUNE 2022
www.digitalsurf.com NEW PRODUCTS
TESCAN and Henry Royce Institute Collaborate on New In-situ Thermomechanical Testing System
TESCAN ORSAY HOLDING, Henry Royce Institute and NewTec Scientific are collaborating in the development of a fully integrated, automated insitu thermomechanical testing system. The TANIST (TESCAN And NewTec In-Situ Testing) sStation is custom designed to allow thermal and mechanical experiments with simultaneous microstructural observations.The system is automated to run 24/7 for measurements to take place between smaller load/ temperature steps over a larger range than previously possible. Users have the ability to setup automated collected maps for high resolution imaging over a large area.
“Traditionally in-situ testing was done manually; users were not able to run the system overnight without an operator and the experiments were prone to user error,” said Albert Smith, R&D applications engineer, TESCAN UK. “Automated in situ testing allows for efficient use of instrument time, with the ability to collect three times the number of acquisition steps as traditional testing.”
The TANIST is based on a TESCAN CLARA field emission gun (FEG) scanning electron microscope (SEM). Combining the platform microscope with a NewTec MT1000 deformation stage, it is possible to automatically observe material deformation at an unprecedented spatial and temporal resolution.
The project is currently focused on integrating the full automation of EBSD in collaboration with Oxford Instruments. Once this milestone has been met, the humidity-controlled gas injection and cryogenic testing functionality will be implemented with the system. Further refinements and materials research with partners at The University of Manchester are ongoing.

The first two systems haves been installed at the University of Manchester and the system is available from TESCAN as an integrated solution.
www.tescan.com
95
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk. The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
Meet the Staff: Katie Reynolds and Jess Cole

Our Meet the Staff feature continues this issue with a doubleheader, as we find out more about two popular members of the team – Events Organiser Katie Reynolds and Jess Cole, who provides Team Support and much more besides.
Here we go…
Katie, who grew up in Oxfordshire, has been part of the team since 2019. She’s an events organiser, essentially handling the logistics and administration of a range of meetings, courses and conferences in the RMS calendar. Much of her working day is spent liaising with scientific organisers, speakers and venues ahead of events - and then making sure they go off (mostly) without a hitch.
“For an in-person meeting I will be talking to the venues and arranging all of the catering and the seating, and the real ‘nitty-gritty’ things like how many tables we are going to need”, she says. “I also have a lot of contact with speakers, to make sure they know what they need to prepare for the event.”
What does Katie find most rewarding about the role?
She says: “It is always nice at the end of an event when the organisers thank you personally, and it has gone well and everyone leaves happy. It’s great when that happens and it makes me feel like, ‘yay, I did that’! I also really like everyone that I work with, and it is a privilege to be with such nice people. There is such a nice culture here, where everyone mucks in with all the little jobs that need doing.”
Jess, a proud Bristolian who has been at the Society for around five years, provides administrative support for all her colleagues – covering finance tasks, events support and more. She oversees the delivery and return of the Microscope Activity Kits (MAKs) for primary schools and also manages the main RMS email inbox – making her the first point of contact for a huge range of different queries and requests.
For Jess, the opportunity to help colleagues engaged in many different tasks is its own reward. She says: “It means that I have had some sort of input into almost everything that happens in the office. It can be difficult to prioritise everything that needs doing, but luckily,
having four children means I have a good multi-tasking brain!”
She adds: “I love the people I work with – we are all from different backgrounds and lead different lifestyles, but we are such great colleagues. Also the work / life balance that being able to work flexibly at the RMS has given me, has been incredible.”
And what do they find most challenging?
Katie says: “I think it’s when you have organisers who want one thing, and speakers who have their own requirements, which means it can sometimes be difficult to accommodate everyone’s expectations. So it’s about trying to find the middle ground and making everybody as happy as possible.
“I don’t mind the pressure of having to meet deadlines, because there are always lots of different deadlines for one event – so you’re always keeping busy. But if you’re the kind of person who gets stressed out at having to meet deadlines, then you probably wouldn’t want to do this job.”
As her colleagues will know, a large portion of Jess’s time is spent chasing up the various courier companies who distribute and return the MAKs for primary schools. Whether it’s the wrong address, rush-hour traffic, or a complete no-show, Jess will be on a phone somewhere, trying to save the day.
“It is just a generic courier problem”, she says. “I sent one to the Natural History Museum the other day, and he was driving around London telling me that he couldn’t find it. I mean, I’m sorry, but it’s the Natural History Museum. That building is Jess Cole
96 ISSUE 66 JUNE 2022
absolutely massive. Are you telling me that you can’t find the Natural History Museum?! I’ve heard every excuse in the book!”
Katie’s arrival at the RMS was something of a baptism of fire, as she took on the role just three weeks before mmc2019 in Manchester. Just a few months later, the Covid-19 pandemic hit, which means her time at the Society has been largely defined by its impacts.
“I had only done one or two in-person events before we went into lockdown”, she says. “Since then I have become really comfortable with doing virtual events, so now that I’m starting to do in-person events again, I’m having to relearn everything. In many ways it’s like starting the job all over again.”
While they are both glad to see the return of in-person events, Katie and Jess agree that the virtual format has worked well since Covid, providing easier access for many people, including a more international audience.

Jess adds: “Being virtual we can offer ourselves out across the world. We have also been able to give opportunities to speakers from so many different countries, which has made us more inclusive as well as more sustainable.”
What do Jess and Katie think are the most important
aspects of the Society’s work in promoting microscopy?
Katie says: “The RMS contributes to all sorts of research and it’s kind of cool to know that people are talking to each other and research is being furthered because of our events. It was particularly interesting during Covid, to hear talks about how microscopes were being used to understand more about the virus. I just hadn’t appreciated how much microscopes are used and how varied they can be.”
She added: “I also really like what we do with the microscope activity kits – getting kids and especially girls into STEM, which is something I’m really passionate about.”
Jess agrees, saying: “It’s really rewarding to be able to reach out to primary schools. Being a mum, I can see how difficult it is for schools to get funding for this sort of thing. When I went to school, I would have loved to have had an opportunity like that, and the fact that we can provide these kits and it’s completely free, is amazing.”
As it happens, Jess grew up wanting to become a forensic scientist, and is still fascinated by TV dramas such as CSI and Silent Witness.
She says: “When I was nine years old I was adamant
97
Katie and Jess
that I wanted to be a scene-of-crime officer. It sounds a bit weird, but I used to imagine that one of my Barbie dolls had been murdered, and then I would try and ‘work out’ how it had happened.
“Unfortunately I just didn’t have the academic ability to become a scientist. But I’m still really passionate about it, and it makes me feel really good when I’m sending these microscopes out to schools.”
Jess worked for several years as an anti-social behaviour officer for a housing organisation. In this role she responded to all sorts of complaints and crisis situations, resolving conflicts and liaising with emergency and social care services. Her colleagues will no doubt agree that she has the perfect, no-nonsense temperament for such a role.
Jess recalls: “I used to deal with some awful stuffcomplaints about smoking drugs, domestic incidents, or people just chucking a load of rubbish into next door’s garden.”
Before she came to the RMS, Katie worked as a fundraiser for another charity, but prior to that, harboured dreams of becoming a dancer – something she did from the age of five until she went to university. She says: “I used to do four out of five nights in the week, at an after school club in Wantage. I did ballet,
jazz, tap and freestyle, and I absolutely loved it. I was even going to apply to study dance at university but changed my mind and did English Literature and Media Communications instead.”
In her spare time Katie is a proud Girl Guide Leader, having progressed through the organisation as a child and then volunteered as a young adult.

She says: “When I was a kid I was really quite shy and anxious, and I think my time at Guides really helped. The leaders that I had did a lot to build my confidence, so I’m really happy to be able to give something back now. I now run the same unit I was in as a kid, so it’s a really nice feeling.”
She has even introduced her young charges to the world of microscopy by taking a MAK along to one of the sessions. “They were getting little leaves and twigs from a park across the road and water from the brook – anything they could find – and messing about putting everything under the microscope.”
In her spare time Katie enjoys the music of Korean ‘K-pop’ sensations BTS – so much so, in fact, that she is currently attempting to learn the language via a mobile phone app, to find out what her heroes are singing about. She says: “I’m terrible at languages and failed French at school. So there is a battle going on between my determination to learn a language and my ability to do it.You’ll have to come back in a year and see if I have learned anything.”
Katie also enjoys reading fantasy novels and has recently become a follower of Formula One racing –for which she blames the influence of her former RMS colleague and renowned petrol-head, Hallie Martin. Meanwhile Jess’s burning passion is the music of Freddie Mercury, Rod Stewart, and other luminaries from the world of classic rock. She also has a bucket list of sporting events and venues she is hoping to tick off during her lifetime. She explains: “I like watching sport, but I’m more into the atmosphere that it creates. So I want to spend all day at a cricket test match, or have a pint of Guinness watching the rugby in Ireland. I’d also be just as happy going to watch a basketball game in the US. Most of the time though, I’m just a busy mum. In my spare time the dog gets walked.”
Our thanks to both Jess and Katie for going under the microscope in this issue!
98 ISSUE 66 JUNE 2022 NEWS
Owen Morton
Katie Reynolds
6 -7 OCTOBER 20 22
Conference Topics
• Emerging Nanomaterials for Advanced Technologies
• Functional Surfaces
• Advanced Techniques and Automation in SPM
• Overcoming barriers in AFM
• Correlative Microscopy
Keynote Speakers
• Prof. Sergei V. Kalinin
University of Tennessee, USA
• Prof. Dr. Lukas Eng Technical University Dresden, Germany
• Dr. Bizan Balzer
University of Freiburg, Germany
• Dr. Deepak Venkateshvaran
University of Cambridge, United Kingdom
• Stanislas Rohart
CNRS/Université Paris-Saclay, France
• Dr. Tobias Cramer
University of Bologna, Italy
• Dr. James Kerfoot
Park Systems, United Kingdom

SPM Methods
• Nanomechanical and electrical characterization

• Characterization of soft materials in liquid environment
• Advanced imaging
• High resolution imaging
• Automation in AFM
• Dr. Nilanthy Balakrishnan Keele University, United Kingdom
• Prof. Brian Rodriguez
University College Dublin, Ireland
• Dr. Ilka Hermes
Leibniz Institute of Polymer Research
Dresden, Germany
• Dr. Sebastian Schmitt
Helmholtz Centre for Materials and Energy, Germany
• Thomas Modes
Fraunhofer Institute for Organic Electronics, Germany
Website I Registration I Abstract Submission: www.nanoscientificforum.com

Contact: info@nanoscientificforum.com
REGISTER for #NSFE2022 TODAY

www.nanoscientificforum.com

Submit your Abstract at #NSFE2022 and win up to €500 AFM Scholarship!

Deadline: June 30, 2022
SUPPORTED BY
Submission Guidelines
infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS. infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more.
infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms. org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.uk
Article Text
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
Images / Figures
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
100 ISSUE 66 JUNE 2022
• Total number of images/figures/tables should not exceed 15 including tables.
Proofs
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Offprints
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Copyright
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).
101
One column/half page width, 65.5mm
Figure 1. Width of figure or table confined to one column.
Figure 2. Width of figure or table spanning full width of page.
Two column/full page width, 135mm
Choose the Right SEM for Your Lab
Choose the Right SEM for Your Lab
Choose the Right SEM for Your Lab

JEOL Intelligent SEM technology offers the best solutions for your imaging and analysis needs. All JEOL SEMs feature advanced electron optics combined with hardware and software algorithms to provide exceptional ease of use and streamlined workflow:
JEOL Intelligent SEM technology offers the best solutions for your imaging and analysis needs. All JEOL SEMs feature advanced electron optics combined with hardware and software algorithms to provide exceptional ease of use and streamlined workflow:
•Automated alignment, focusing and stigmation
JEOL Intelligent SEM technology offers the best solutions for your imaging and analysis needs. All JEOL SEMs feature advanced electron optics combined with hardware and software algorithms to provide exceptional ease of use and streamlined workflow:
• Seamless navigation from optical to SEM image
• Embedded chemical composition analysis
•Automated alignment, focusing and stigmation
• Multi-area automated acquisition (mosaic)
• Seamless navigation from optical to SEM image















• Unparallel service and applications support
•Automated alignment, focusing and stigmation • Seamless navigation from optical to SEM image





• Multi-area automated acquisition (mosaic)
• Embedded chemical composition analysis
• Unparallel service and applications support
• Embedded chemical composition analysis • Multi-area automated acquisition (mosaic)

• Unparallel service and applications support


100um 10um 100nm 10nm
Tabletop W- Multipurpose FEG UHR FEG $ $$$ 100nm/pix 10nm/pix 5nm/pix 2nm/pix
100um 10um 1um 100nm 10nm
Tabletop W-SEM Multipurpose FEG UHR FEG $ $$$ 100nm/pix 10nm/pix 5nm/pix 2nm/pix
100um 10um 1um 100nm 10nm
Tabletop W-SEM Multipurpose FEG UHR FEG $ $$$ 100nm/pix 10nm/pix 5nm/pix 2nm/pix
@JEOLEUROPE















































































































 Zuzana Nováková, Application Specialist at Telight.
Zuzana Nováková, Application Specialist at Telight.








 By John Hutchison and Thomas Walther
By John Hutchison and Thomas Walther






















































