
The RGS Medical Magazine
Produced by Menasha Herath and Elizabeth Hodgson.
A note from the editors
Our names are Elizabeth and Menasha and we are currently students in RGS Sixth Form reaching the end of Lower Sixth. We have been able to get involved with a lot of Medicine related things here at Reigate Grammar, particularly Medical Discussion Club, but we wanted something different to be implemented, to allow everyone to get involved in something medicine related, no matter their age (or even whether they have an interest in medicine). We created this magazine to allow your voices to be heard, to give a chance to all of you to showcase your interests. We hope you enjoy reading through the submissions we received this term, as much as we enjoyed them, and if your work has been published in here, a huge congratulations to you! If you weren’t able to get involved this time, but would like to write something for our next issue which will hopefully be published some time during the first term of the next academic year, please write to us (19Hodgeli@reigategrammar.org/19MHeramen@reigategrammar.org)
we are always happy to receive your work. Enjoy!
The Peculiar Case of H.M.
Written by: Elizabeth Hodgson
Henry Gustav Molaison, more commonly referred to simply as “H.M” was a patient of the 1950s who had suffered from epileptic seizures which had manifested during his childhood. These seizures were thought to be the result of an accident he had at age 7, where he was knocked down by a bicycle. From the age of 10, he began to suffer minor seizures, and as he progressed into adolescence these seizures worsened and became more major.1 H.M. did work for some time on the production line, taking high doses of anticonvulsant medication in an attempt to pacify his seizures, but eventually became too incapacitated to work. At age 27, in 1953, Molaison was entirely unable to work or lead a normal life, and so agreed to undergo experimental surgery which involved removing parts of his brain which were thought to be responsible for his untameable seizures.2 This procedure was to be carried out by a Neurosurgeon from Hartford, Connecticut, named William Scoville.1 The outline of the procedure involved removing the front temporal lobes of the brain (a lobectomy) as this was the region surmised to be responsible for the seizures.3 Contrary to what we know about the brain today, in 1953 the brain, and procedures relating to it, were not well understood. Nowadays, procedures like this would be carried out with higher levels of integrity and care, but this was still early days in operations on the brain. Scoville had tested this procedure on previous patients suffering from psychosis, to reasonable success, hence he felt it was fit for H.M. He writes in his reflection in 1957 that he “probably destroyed…the uncus and amygdala” whilst removing 8cm of brain tissue from the anterior two thirds of the hippocampus.4 (see figure 1 for a simplification of the procedure carried out on H.M.’s brain, and what was removed).5

Figure 1: The procedure which removed the majority of H.M.’s hippocampus, as well as parts of his amygdala and entorhinal cortex.
As we know today, the hippocampus is vital when it comes to memory. Its main role is to convert short-term memories into long term memories, and it does this by organizing, storing and retrieving memories from within your brain. It also plays a part in learning, for example retaining information about your environment (spatial memory), making you aware of what’s around you, as well as verbal memory, so that you remember what to say in certain scenarios, such as when you are registered at the beginning of a lesson.6 Your hippocampus will also be a crucial component in helping you to retain information that you have learnt about in this article!
As you can imagine therefore, the removal of Molaison’s hippocampus (and corresponding regions of the brain) stripped him of these abilities entirely. This is a condition now known as anterograde amnesia, the total inability to form new memories7. At this point in time, the discoveries this case induced for neurology, and the understanding of the brain were only just beginning. With an absence of brain scanning technology, it was difficult to assess what was going on within the brain of H.M. but to his family, and more specifically his mother, it was evident he had become a changed person. H.M. forgot daily events almost as fast as they occurred, underestimated his own age, and apologised to people he had only just met for forgetting their names.1 While previously it was not known to any large extent what role the hippocampus (the region of H.M.’s brain removed) played in cerebral function, it now became glaringly obvious.
Aside from the anterograde amnesia, which manifested after his surgery, it is noted that H.M. also suffered from another type of amnesia called retrograde amnesia.2 Retrograde amnesia describes the inability to recall memories which were formed before the event that caused the amnesia (in H.M.’s case this would be the lobectomy), however current research theorizes that this variant of amnesia was not actually caused by the removal of his hippocampus, but rather from a combination of the high dosage of antiepileptic drugs he was taking before the surgery, as well as his frequent seizures which would have done their damage to his brain.
Despite suffering from these two types of amnesia, severely impairing H.M.’s ability to retain information and learn typically, researchers studying him discovered no apparent loss to his ability to learn and adapt to tasks At first, when H.M. awoke after his procedure, and the damage to his brain became apparent, it was thought that the hippocampus (the region of his brain removed), must be entirely responsible for memory, and the ability to remember. However, it is not that simple. Broadly speaking, there are two distinct types of memory; explicit (conscious) memory and implicit (nonconscious) memory. Your explicit memory would be, for example, recalling phone numbers, remembering important event dates, as well as birthdays and names of
8
people you meet Contrastingly, implicit memory might be something like brushing your teeth, riding a bicycle, or navigating your way through your neighbourhood or familiar place.
As suspected from what we already know about H.M. who struggled to navigate new and unfamiliar tasks, the hippocampus (the region of his brain which was removed) predominantly controls explicit memory. The peculiar thing about Molaison which confirmed this hypothesis, was that while unable to recollect new facts and details which he was told, he was able to acquire new physical skills taught to him, without any memory of learning how to do these tasks. Thereby these physical skills he was taught formed part of his unaffected and fully functional implicit memory.9
In a 1962 case study on H.M., practicing neuropsychologist Brenda Milner asked H.M. to replicate a drawing of a star, whilst only being able to see the mirror-image version of the star and his hand in the mirror. (see figure 2)

This was a complex task, and Molaison did not manage to complete it right away, however what Milner observed was that each time he was asked to carry out the task, he would get better. His precision would improve, along with his accuracy, and the time it took him to complete the replica decreased too. The peculiar thing being, that he did
Figure 2: The task which Brenda Milner assigned to H.M. during her study of him
not remember the template of the star, or even having been asked to draw the star before. His ability to do the skill was improving, yet he had no recollection of having done it before.
What H.M. taught neurologists and neuropsychologists alike therefore, was that implicit memory was not localised in the hippocampus, as H.M. without a hippocampus, was still capable of acquiring new skills. Molaison died on December 2, 2008 of respiratory failure, at age 82. His body post-mortem was further used in research and medical discovery, and if you are interested in learning more about this, see below.
Further research has shown that the control centres for implicit memory are instead located in the basal ganglia and cerebellum. If you are interested in learning more about implicit memory, see below.
Further reading
- The importance of HM in neurological discovery: H. M.’s Medial Temporal Lobe Lesion: Findings from Magnetic Resonance Imaging - PMC
- The contributions of H.M. post-mortem: Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction | Nature Communications
- Implicit memory: Where are memories stored in the brain? - Queensland Brain Institute - University of Queensland
References
1. The Legacy of Patient H.M. for Neuroscience - PMC
2. Patient H.M. Case Study In Psychology: Henry Gustav Molaison
3. Childhood Epilepsy: The Brain (where seizures occur)
4. Scoville and Milner (1957) | Reference Library | Psychology | tutor2u
5. Scoville & Milner AO1 AO3 - PSYCHOLOGY WIZARD
6. Hippocampus: What It Is, Function, Location & Damage
7. Profound Retroactive Interference in Anterograde Amnesia: What interferes? - PMC
8. Implicit vs. Explicit Memories
9. Neurology through history: What patient H.M. taught us about the secrets of the… | MedLink Neurology
Insight Into Neurological Disorders: Parkinson’s
disease
Written by: Savannah Nsofor
What is Parkinson’s disease?
Parkinson’s is a progressive neurological disorder that affects voluntary movement in the body, causing tremors, slow movement, and muscle stiffness. Its cause remains unclear; however, researchers believe that the disease affects the brain’s dopamine system. Parkinson's is a multifactorial disease, as the loss of nerve cells in a brain region called the substantia nigra (which leads to a reduction in the neurotransmitter dopamine) is onset from a variety of factors, including environmental and genetic. It typically affects people over 60, though early-onset cases can occur.
Symptoms
As Parkinson’s is a progressive disorder, symptoms develop gradually. This could be a barely noticeable tremor in the hand or jaw. As the condition progresses, you begin to lose automatic movements that are usually performed without conscious effort. Facial expressions start to dwindle, arms may not swing while you walk, and your speech may become soft or slurred.
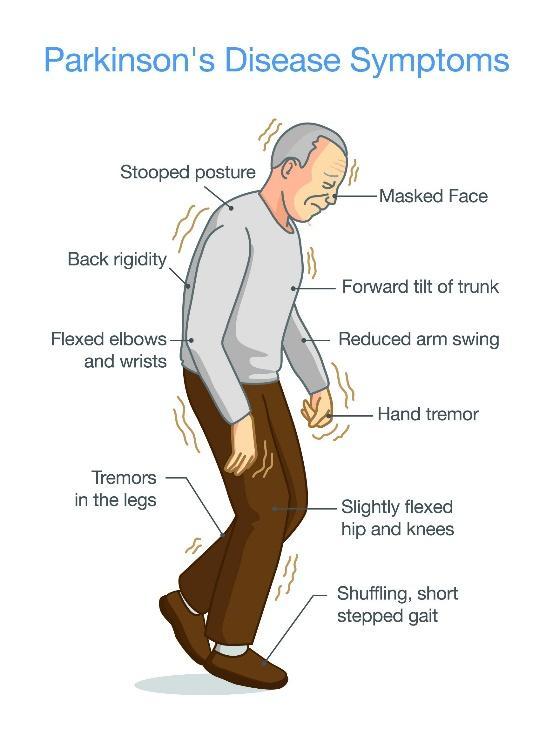
Another key symptom of Parkinson’s is bradykinesia (slowed movement). This makes daily tasks, such as getting dressed, difficult. In some cases, blinking becomes difficult.
Causes and Risk Factors
The exact causes of Parkinson’s are unknown. However, the main region of the brain associated with the disease is the substantia nigra which sits in the middle, in the basal ganglia at the top of the brain stem. There are 2 substantia nigra, one in each hemisphere. Often, one side experiences loss of cells before the other, leading to symptoms beginning and remaining worse on one side of the body than the other.
Parkinson’s may also involve norepinephrine, a neurotransmitter linked to mood regulation. A lack of norepinephrine contributes to the motor and nonmotor symptoms of the disease, with common symptoms including: depression, disrupted sleep and cognitive impairments.
Genes and environmental factors also influence the disease. Although most cases of Parkinson’s are sporadic and not inherited, some genetic changes have been linked; however, these are rare unless family members have had the disease. Environmentally, exposure to toxins may increase your risk of Parkinson’s. An example is MPTP, which is a substance found in illegal drugs and is sometimes sold illegally as ‘synthetic heroin’. More common environmental factors include well water used for drinking and pesticides. But no single environmental factor has proved to be a direct cause.
Changes in brain cells
Loss of brain cells is the main structural difference that underpins Parkinson’s. Research has found that a specific form of a protein called alpha synuclein is causing issues. When their tertiary structure (3D structure) becomes damaged, they form toxic clumps that impair cell function. In Parkinson's, Alpha-synuclein can clump together, forming Lewy bodies that damage cells. This protein is thought to spread between neurons, contributing to the disease’s progression. This could explain why Parkinson’s impacts multiple brain areas, and why many symptoms are linked to the condition.
Diagnosis and treatment
Currently, no single test can definitively diagnose Parkinson’s. Neurologists use physical exams, medical history, and brain scans, e.g. MRIs to diagnose Parkinson’s conditions.
A DAT scan can help confirm the diagnosis. A DAT scan (dopamine transporter scan) is a type of SPECT (single-photon emission computed tomography) scan. This visualises the dopamine transporters in the brain, specifically in a region called the striatum.
There is also an alpha-synuclein test, which detects Parkinson’s before symptoms begin. The protein can be tested for in the skin or spinal fluid. The alpha-synuclein seed amplification assay correctly identified people with Parkinson’s 87.7% of the time in a study conducted in 2023 with over 1000 participants.
There is no cure for Parkinson’s. However, medicines can help manage the condition, and surgery is an option when medicine is unsuccessful. Medicines work by increasing the concentration of dopamine at the synapse. The most effective of these is Carbidopa-levodopa. Levodopa is a natural chemical that passes into the brain and becomes dopamine. It is combined with Carbidopa, which helps reduce side effects.
Surgery includes deep brain stimulation, which involves putting electrodes within the brain that are connected to a pacemaker-like device that is inserted under the skin on the chest. Electrodes send impulses to the brain, reducing symptoms. Deep brain stimulation may have long-term benefits, but it does not keep Parkinson’s from getting worse.
The future of Parkinson’s
New Parkinson’s research focuses on early detection and disease modification. Researchers aim to treat symptoms by identifying biomarkers and developing targeted biological therapies, which could help slow or even prevent the progression of the disease.
If any of the topics in this article have affected you, several online charities provide support, including Parkinson’s UK. Alternatively, speak to your form tutor, head of year, or the wellbeing centre, who are always happy to help.
Brain Vs AI : What makes our thinking so special?
Written by: Charvi Ramakrishnan
In the age of smart assistants, self-driving cars, and AI-generated art, it's easy to think that artificial intelligence (AI) is catching up to the human brain. But despite all the amazing things AI can do, our brains are still the most powerful and mysterious "machines" in existence. So, what exactly makes the human brain so unique, and how does it compare to artificial intelligence?
The Power of the Brain

The human brain is made up of around 86 billion neurons, each connecting to thousands of others in a massive web of communication. This biological network controls everything we do from breathing and walking to dreaming, creating, feeling, and thinking. Unlike computers, our brains aren't programmed; they learn through experience, emotion, and social interaction.
What really sets the brain apart is its flexibility and consciousness. While AI can be trained to recognize patterns or play chess at a world-class level, it doesn't understand what it’s doing. It has no feelings, no sense of self, and no awareness. The human brain, on the other hand, can imagine the future, reflect on the past, feel empathy, and make decisions based on complex emotions and values.
How AI Works
Artificial intelligence, especially machine learning, tries to copy how humans think but in a very narrow way. AI systems learn by analysing huge amounts of data and finding patterns. For example, an AI can learn to recognize cats in photos by processing thousands of images labelled “cat.” But if you give it a slightly unusual photo say, a
cartoon cat or a cat in a costume it might get confused. That’s because AI lacks common sense, something humans develop naturally over time.
AI also can’t truly understand context. It can translate languages or write essays, but it doesn’t know what words mean the way people do. It processes language like a puzzle, not like a conversation.
Psychology and the Brain
Psychology is the science of behaviour and the mind. It helps us understand how people think, feel, and act. Psychologists study everything from memory and learning to emotions, dreams, and mental health. What they’ve learned about the brain helps shape everything from education to therapy to technology. One fascinating thing psychologists and neuroscientists have found is that the brain is like plastic it can change and grow over time. This ability, called, neuroplasticity means we can adapt to new challenges, recover from injuries, and continue learning throughout our lives. That’s something no AI can match once an AI model is trained, it stays the same unless reprogrammed.
The Future: Brain and AI Together?

Instead of seeing AI as competition, many scientists believe the best future is one where the brain and AI work together. AI can help doctors diagnose diseases, assist people with disabilities, or even enhance how we learn. At the same time, studying the brain helps us build better, more human-like AI systems. But no matter how advanced technology gets, the human brain remains unique. It’s not just a processor it’s the source of our creativity, emotions, and individuality. It's what allows us to love, dream, question, and grow.
Final Thoughts
Artificial intelligence is smart but your brain is alive. It’s adaptable, emotional, selfaware, and deeply connected to your body and your experiences. Psychology helps us understand the mind’s complexity, while AI gives us new tools and challenges. In the end, it's not about man Vs machine, but how both can evolve side by side. So, the next time your phone auto-corrects your text or recommends a song, remember it’s impressive, but it’s no match for the human brain that made it all possible.
Further reading
- Further analysis and debate on the use of AI in Medicine: https://bjgp.org/content/bjgp/68/668/143.full.pdf
- Opinion on whether or not AI can transform Medicine: https://www.cam.ac.uk/opinion-ai-and-health-and-medicine
- https://www.england.nhs.uk/long-read/artificial-intelligence-ai-and-machinelearning/

Can Artificial Intelligence (AI) Transform Radiology?
Written by: Aabish Khan
Imagine this: an AI system scans your chest X-ray, flags a tumour before your radiologist even sees it, and sends an alert to your doctor. This is all within seconds. It sounds like science fiction, but this is rapidly becoming reality. Artificial Intelligence (AI) is a technology that allows computers and machines to carry out cognitive functions we usually associate with human minds, such as decision making, problem solving, creativity and autonomy.
Radiology is a branch of medicine that uses imaging technology for diagnosis and stands at the forefront of AI in healthcare. With the growing demand of diagnostics in the NHS, AI offers remarkable potential to assist radiologists. Though, despite its promise, integrating AI in radiology raises ethical, technical, and practical concerns that must be carefully addressed to ensure effective and responsible implementation
A significant way AI can transform radiology is by enhanced image analysis. Machine Learning is when computers learn data without explicit programming and identifying patterns from examples. Deep Learning is advanced version of Machine Learning using neural networks and mimics the brain’s structure to identify complex patterns in a large data set. AI algorithms using Deep Learning can detect abnormalities like tumours with precision and speed. This can help reduce diagnostic errors, assist early detection and calm the workload of overburdened radiologists.

In the 2022 Landmark study an AI model outperformed human radiologists in detecting cancer in screening mammograms (scan to detect breast cancer) The diagnostic accuracy without AI was 70% and with AI was 85%.
Despite these advantages, there are ethical issues that surround the integration of AI in radiology.
One concern is the potential of bias in algorithms. If training data is imbalanced and unrepresentative, AI may underperform, leading to unequal healthcare outcomes. Another problem is transparency as most AI models function as black boxes This means humans have little insight to the decisions it makes and cannot understand how it works. How much can we really trust AI if that is the case? Who would be responsible
if an AI system makes a mistake? This can lead to the preservation of patient privacy, this is concerning as radiology consist of sensitive imaging data to train algorithms. Significant barriers can also be posed by technical limitations.
AI may be able to perform well in a training environment, but in a real-world clinical setting, it could fail. AI systems must be robust enough to handle this much variability without compromising accuracy. Data availability is also an issue as large, high-quality datasets are essential for training an effective model that we want to be successful, Though, access to this data can be limited due to privacy policies and inconsistent data standards. Implementation challenges especially affect AI adoption in radiology. Integrating AI systems and tools into healthcare requires large investments in technology and training. Radiologists have to be trained, not only to use the AI systems but also critically evaluate the outputs and understand the algorithms.
To overcome and navigate these challenges, a balanced approach to AI integration is necessary. Regulatory frameworks must be developed to protect rights and enhance safety. AI systems should train in diverse clinical settings before they are deployed and Stakeholders should work together to create guideline and ensure AI is responsible when in use for diagnostics.
In conclusion, AI has the potential to transform radiology by improving diagnostic accuracy, efficiency and reducing workload. Although, it can only be successful if ethical, technical and implementation challenges are addressed. Instead of replacing radiologists, AI should be viewed as an enhancing tool that can work to aid radiologists and provide safer and faster care in this new age of medicine.

Mercy or Murder: Euthanasia and the Right to Die
Written by: Shreeyana Rajbahak
What does it mean to have a ‘good death’? As medicine extends life far beyond what once seemed possible, we now face a difficult question: should people have the right to choose when and how they die?
Euthanasia: the act of deliberately ending a life to relieve suffering - is one of the most controversial topics in modern medical ethics. It challenges our beliefs on autonomy, dignity, suffering, and what it means to live and die well.
Types of Euthanasia
Voluntary: The patient gives informed consent to end their life.
Involuntary: Performed without the patient's consent (often considered murder).
Assisted suicide: A doctor provides the means for death, usually through prescribed medication.
Passive euthanasia: Withholding or withdrawing treatment that prolongs life (such as turning off a life-support machine).
Each type comes with unique ethical, legal, and medical complications, and different countries respond to them in different ways.
Global Approaches to Euthanasia:








Netherlands & Belgium: Pioneers of legal euthanasia. Both allow voluntary euthanasia and assisted suicide under strict regulations. Even some cases involving psychological suffering are permitted.
Switzerland: Assisted suicide is legal, but euthanasia (where someone else performs the act) is not. Notably, Swiss clinics like Dignitas attract patients from abroad.
Canada: Medical Assistance in Dying (MAID) is legal for adults with grievous and irremediable conditions. A controversial expansion to include mental health conditions is under debate.
United States: Only a handful of states (e.g. Oregon, Washington, California) permit physician-assisted suicide. Euthanasia itself remains illegal nationwide.
UK: Both euthanasia and assisted suicide are illegal. Patients can refuse treatment, but doctors cannot take active steps to end life. High-profile cases (like Tony Nicklinson's) have reignited public debate.
Japan & India: Euthanasia is not formally legal, but courts have occasionally accepted passive euthanasia under strict guidelines.
The Case For Legalisation
Autonomy: People should have the right to decide what happens to their own bodies, including when they are in unrelenting pain.
Compassion: Why prolong suffering when death is inevitable and quality of life is severely diminished?
Dignity in death: Some argue that choosing the time and manner of death can preserve a person’s dignity and sense of control.
Resource allocation: Critics point out that limited healthcare resources are stretched by keeping terminally ill patients alive through invasive, often futile treatment.
The Case Against Legalisation
Risk of quiet coercion: There are fears that legalising euthanasia could lead to abuse or pressure on vulnerable groups (the elderly, disabled, or mentally ill) to "choose" death.
Medical ethics: Many doctors argue that taking life contradicts the Hippocratic Oath and the role of healers.
Palliative care: Critics argue we should invest more in pain relief and hospice care instead of ending lives.
The Grey Areas
Religious beliefs: Many faiths view life as sacred and death as something only a higher power can determine.
The Grey Areas…
• Can a person with severe depression request euthanasia?
• Should someone be allowed to die if they have dementia and cannot consent later?
• Can we ethically allow children with terminal conditions to request euthanasia?
In Conclusion
Whether one supports or opposes euthanasia, the debate forces us to ask: what kind of death would we want for ourselves and those we care about? And how far should society go to make that possible?
Further reading:
- How could assisted dying laws change across the UK? - BBC News (more on assisted dying and its changing legality)
- Why active euthanasia and physician assisted suicide should be legalised: If death is in a patient's best interest then death constitutes a moral good - PMC (opinion on why assisted dying should be legalised)
- Six reasons we need an assisted dying law (Humanists approach to the topic)
- Eight Reasons Why We Must Not Legalise ‘Assisted Dying’ | Anscombe Bioethics (ethical reasons why assisted dying should not be legalised)
- Why We Should Not Legalize Euthanasia (Catholic approach)
From Printer to Patient: Exploring the Three-Dimensional (3D) Bioprinting Process and How It Is Transforming Modern Medicine
Written by: Chivali Vohra
Three-dimensional (3D) bioprinting stands as one of the most promising developments in biomedical engineering today. This innovative technology involves printing biological structures that replicate human tissues and organs, with the eventual aim of producing completely functional, transplantable organs. It holds huge possibilities for the future of healthcare, especially since it can tackle the severe shortage of organs for transplantation.
In simple terms, 3D bioprinting is a specialised type of additive manufacturing. The process begins with a medical imaging such as Computed Topography (CT) or Magnetic Resonance Imaging (MRI) to identify the specialised tissue to be printed This image allows the bioprinter to precisely print layers of ‘bioink’ . Bioink is a mixture of living cells; often these are stem cells which have been harvested from a patient, in order to minimise the risk of rejection. These cells are printed onto a 3D supportive structure that mirrors the natural environment of the cells and helps them grow. This is known as a ‘biomaterial scaffold’
Not only does the biomaterial scaffold provide critical physical support but it also influences cell behaviour, such as attachment, proliferation and differentiation. Often, these scaffolds are designed to be biocompatible; made from both synthetic and natural materials. Synthetic materials allow for controllable degradation rates and higher mechanical strengths whereas natural materials do not release toxic compounds during biodegradation and vividly create the right environment for attachment, growth and differentiation. Materials are chosen specifically for their biodegradability, allowing them to break down naturally as the tissue develops.

Image depicting a human heart undergoing bioprinting
Currently, there is a global organ donor shortage which compromises the care for patients awaiting transplants. It’s tragic that patients with conditions like cystic fibrosis and heart defects continue to die prematurely, despite the fact that a transplant could have saved them 3D bioprinting has the potential to revolutionise healthcare by addressing this crisis by offering the ability to readily print organs and keep up with the demand. This can drastically reduce waiting times and save lives. Furthermore, by using a patient’s own cells, the risk of rejection is lowered, eliminating the need for lifelong immunosuppressant drugs, which often come with harmful side effects and economic burden
Currently, there is a global organ donor shortage which compromises the care for patients awaiting transplants. It’s tragic that patients with conditions like cystic fibrosis and heart defects continue to die prematurely, despite the fact that a transplant could have saved them 3D bioprinting has the potential to revolutionise healthcare by addressing this crisis by offering the ability to readily print organs and keep up with the demand This can drastically reduce waiting times and save lives. Furthermore, by using a patient’s own cells, the risk of rejection is lowered, eliminating the need for lifelong immunosuppressant drugs, which often come with harmful side effects and economic burden
Despite the positives, bioprinting faces a number of challenges before it can be used worldwide. One of the biggest challenges is that printed organs often fail to develop the complex networks of blood vessels – a process known as ‘vascularisation’ – which is essential for long term survival. Printed organs can’t survive without blood vessels, which are vital for delivering oxygen and nutrients to cells. Without this internal network, waste builds up inside the tissue, causing cells to become toxic and die, making vascularisation one of the greatest challenges in 3D bioprinting. Secondly, due to being a recent concept, high production costs and slow printing speeds make it difficult for everyday hospital use Ethically, 3D bioprinting raises important questions about the creation and ownership of human tissues. These concerns also extend to health equality, as there is a risk that bio printed organs could become a luxury reserved for the wealthy – creating a ‘designer organ’ market that excludes those who need them most.
To conclude, 3D bioprinting is actively reshaping the landscape of modern medicine. It is pointing towards a future where healthcare may be safer, faster and more personalised. Currently, it is being used on a small scale, such as printing cartilage for joint repair and patches for damaged heart tissue. It is pushing medical limits by being more patient-specific, efficient, and less reliant on limited biological resources. Therefore, holding the potential for a true revolution in medical treatment and disease management. Looking into the future this technology could result in a world where organs can be printed to meet the large demand in an era with increased life expectancy and organ shortages.
Further reading:
- Bioethical and Legal Issues in 3D Bioprinting - PMC (more on ethical and legal issues associated with bioprinting)
- 3D Printed Organs: How, Why and When (the future of bioprinting)
- A Bioprinting Breakthrough Could Lead to 3D-Printed Blood Vessels (current news in the field of bioprinting)
Aortic Aneurysms and Their Treatment
Written by: Menasha Herath
What is an Aortic Aneurysm?
An aortic aneurysm is a life threatening vascular condition, an abnormal bulge or ballooning of the aorta - one of the most important arteries in our body carrying oxygenated blood to vital organs. If left untreated aortic aneurysms can rupture and lead to internal bleeding and death. The aorta is our body’s powerhouse, and main blood vessel, any structural damage or weakness to this major artery can lead to detrimental consequences. As aortic aneurysms are often left unnoticed with no symptoms, the silent nature of this condition combined with its severity makes early detection and effective treatment crucial.
There are two types of aortic aneurysms: abdominal aortic aneurysms (AAA) and thoracic aortic aneurysms (TAA).
AAA’s are located in the abdomen/stomach area, while TAA’s are located in the chest.

showing the two different types of Aneurysm
Image
When Aortic Aneurysms Require Surgery
The primary objective when treating aortic aneurysms is to ensure that rupture and growth of the aneurysm is prevented as the outcomes of aneurysm rupture can be life threatening. Therefore, surgeons must consider a range of factors to provide the best possible treatment for their patient.
The National Institute for Health and Care Excellence (NICE) recommends that surgical repair should be considered when an asymptomatic aneurysm reaches 5.5 centimetres (5cm in women) or larger in diameter, or if it is larger than 4.0 cm and grows more than one centimetre within a year's period, due to the increased risk of rupture beyond this threshold.
Further symptoms such as back or abdominal pain, or evidence of leak or impending rupture also prompts surgical consideration Similarly for TAA’s surgical repair should be considered when the aneurysm is between 5.5-6.5 centimetres, however surgery is often sooner for individuals with genetic conditions like Marfan syndrome1 and patients with underlying health conditions, or a family history of aortic aneurysms which can make them more susceptible to aneurysm formation and rupture
As of today, there are two methods of treatment for aortic aneurysms:
• Open Surgical Repair (OSR)
• Endovascular Procedures (EVAR).
Open Surgical Repair
OSR is the more traditional method, a major surgery which requires direct access to the damaged aorta via a large incision down the middle of the chest where the damaged part of the aorta is replaced with a synthetic graft. This procedure is highly invasive and is associated with long, demanding recovery and extended hospital stays. However, it has been the gold standard for decades as a result of its long-lasting durability and low reintervention rates. Recent advancements in medical technology have led to the introduction of EVAR, a more minimally invasive approach, which is continuing to increase in popularity and be endorsed by vascular surgeons worldwide
Endovascular Procedures
EVAR involves accessing the damaged aorta via catheters through small incisions in the groin and the swollen artery is relined using a series of covered stents Although EVAR is a less invasive procedure, has a shorter recovery time and reduced operative time, there are concerns regarding its patient suitability and long-term durability which has led to growing debate within the medical community about whether EVAR should fully replace OSR.

This figure depicts both OSR (A) and EVAR (B)
Further reading:
- Editor's Choice – Endovascular vs. Open Repair for Abdominal Aortic Aneurysm: Systematic Review and Meta-analysis of Updated Peri-operative and Long Term Data of Randomised Controlled Trials - European Journal of Vascular and Endovascular Surgery (more on the successes of OSR vs EVAR)
- A 35 Year History of Stent Grafting, and How EVAR Conquered the WorldEuropean Journal of Vascular and Endovascular Surgery (origins of EVAR)
Additionally, if this topic has been of particular interest to you, please feel free to contact me for my EPQ, which I wrote on this subject.
