

Healthcare Innovations with Special Features on Oncology, Clinical Trials
“Their work showcased Ireland’s growing multidisciplinary strength in lung cancer care.”
Jarushka Naidoo, Professor of Medical Oncology; Consultant Medical Oncologist at Beaumont RCSI Cancer Centre Page 02

& Research
www.healthnews.ie
“The last decade has transformed multiple sclerosis (MS) care, withan expansion of disease-modifying therapies.”
Dr. Lisa Costelloe, Consultant Neurologist, Beaumont Hospital Page 05


The ARC Hub is helping Ireland realise its promise as a leading biomedical ecosystem ~ARC Hub for Theraputics, Page 06


Results in early-phase clinical trials suggest snake venom as a potential cancer therapy
Irish biotech hopes to advance crotoxin, a protein in rattlesnake venom, for the treatment of cancer following results in early-phase clinical trials.
Research has long pointed to the therapeutic potential of snake venom, but associated stigma has blocked full clinical translation. One company hopes to advance its use, conducting research studies in terminal cancer.
The therapy under investigation, CB-24 (Crotoxin), contains two subunits: A and B. “Once injected into the body via infusion pump, the A subunit actively and selectively locates malignant cells, bypassing healthy tissue. Locking on, it releases its destructive B subunit, triggering cell death,” explains Paul Reid, scientist and Celtic Biotech CEO.
Family foundations and future outlook Paul and John Reid had been looking for a potential therapy for a relative and a friend, who sadly both eventually succumbed to their cancers. Paul, the scientist behind CB-24, had harnessed existing research from a South American company to drive research efforts. Subsequently, a close friend of the brothers, with adult soft tissue sarcoma, was given little hope of survival. He undertook treatment with crotoxin and has now been cancer-free for 20 years. Following this, Celtic Biotech was formed, with earlyphase trials funded by family and angel investors.
but we are highly motivated,” explains John Reid. “We believe this could become an effective, easy-to-administer and easily tolerated treatment that will not adversely impact a patient’s quality of life.”
Clinical progress and milestones
The therapeutic activity was confirmed by early studies from MD Anderson Cancer Center and the US National Cancer Institute, with ongoing Celtic research highlighting promising safety and preliminary efficacy in multiple solid tumours, including lung, brain, breast, prostate and ovarian cancer. Most recently, Celtic Biotech presented their Phase 1b clinical data at ESMO in Berlin.
We believe this could become an effective, easyto-administer and easily tolerated treatment.
“Phase Ia/Ib trials have demonstrated good safety and encouraging signs of activity, and Phase Ic trials with higher dosing are approved and planned for early 2026,” says Paul Reid. Another therapy in their product pipeline, sourced from cobra venom, is CB-6 (Cobramine). CB-6 exhibits anti-inflammatory properties and anti-metastatic activity. Academic institutes in France, the US, Brazil and China are contributing to their accumulation of scientific evidence.


“It has been a long and difficult road to get to this point,
Project Manager: Rebecca Mulcahy rebecca.mulcahy@mediaplanet.com
Project Manager: Sam Kelly sam.kelly@mediaplanet.com
Project Manager: Ali Coogan ali.coogan@mediaplanet.com Business Development Manager: Robert Joyce Managing Director Ellie McGregor | Junior Designer: Ellen Cahill Interim Content Manager: Rachelle Ong | Paid Media Manager: Jonni Asfaha All images supplied by Getty Images, unless otherwise specified
Ireland’s leading role in global lung cancer innovation
Ireland took a leading role at the world’s largest lung cancer conference this year, with insights that will shape both global and Irish oncology research.

This September, I had the honour of serving as one of the Chairs of the IASLC World Conference on Lung Cancer (WCLC 2025) in Barcelona, the world’s largest meeting dedicated to lung cancer.
Sharing this role with Dr Noemí Reguart, Prof Umberto Malapelle and Prof Isabelle Opitz was a privilege and milestone for Ireland. It highlights the standing of Irish oncology in the international community and our growing contribution through clinical trials and collaborative research.
Key highlights from Barcelona Several breakthroughs unveiled at WCLC 2025 could change lung cancer standards of care.
• Combination therapy in EGFR-mutant lung cancer: The phase III FLAURA2 trial showed improved overall survival with combination therapy. Results suggest combined approaches may offer deeper, longer-lasting benefits for selected patients.
• Long-term survival with immunotherapy: Fiveyear results from the Phase II NADIM study showed exceptional outcomes, with survival rates near 70%. These data confirm that early-stage lung cancer can be curable for some patients.
• Lung cancer screening: A major analysis of high-risk
individuals (with smoking history, lung conditions or advanced age) in Manchester suggested extending screening eligibility. With Ireland’s first lung cancer screening pilot now underway in North Dublin and the Northeast (Lung Health Check), sponsored by the Irish Cancer Society, Beaumont Hospital, RCSI and the EU4Health SOLACE consortium, these findings are timely as Ireland evaluates national screening strategies.
Over 35 Irish delegates attended WCLC, including presenters from clinical, research and advocacy backgrounds. Their work showcased Ireland’s growing multidisciplinary strength in lung cancer care and its role in shaping future clinical trials.
Bringing global insights home
As Chair of the Lung Disease-Specific Subgroup of Cancer Trials Ireland, I lead national collaboration to develop and deliver new lung cancer trials. Lessons from Barcelona will directly inform our portfolio, refining trial design, broadening eligibility and aligning with cuttingedge science.
Our challenge now is to turn momentum into real progress so that international breakthroughs translate into longer lives and better outcomes for people with lung cancer in Ireland.
Celtic Biotech is an Enterprise Ireland HPSU Client and an EU EIC Accelerator grantee.
Paul Reid CEO of Celtic Biotech
John Reid
Co-Founder of Celtic Biotech
WRITTEN BY
Bethany Cooper
Jarushka Naidoo Professor of Medical Oncology; Consultant Medical Oncologist at Beaumont RCSI Cancer Centre Dublin, Chair of the Cancer Trials Ireland Lung DSSG
The importance of attending BreastCheck appointments
With advances in screening, earlier diagnosis and improved treatments for breast cancer, the fiveyear survival rates have improved to up to 87%. BreastCheck plays a vital role in cancer care in Ireland.
Around one-third of all breast cancer cases diagnosed each year are identified through BreastCheck. This invaluable national screening service is provided free to women aged 50–69 years. To avail of this screening service, women should check that they are registered on Registration - Breastcheck. If you are not registered, you will not receive an invitation to the screening service.
Examine your breasts and get checked
The risk of developing breast cancer increases with age and is more common in women aged 50 or older who have been through the menopause. However, cancer can occur at any age. Therefore, it is important that women and girls are aware of the signs and symptoms. We encourage girls from the age of 18 years to carry out a monthly self-breast examination. Women should know what looks and feels normal for them and when changes occur. If changes occur between screening appointments, it is important to get checked. Find more information on how to check your breasts on cancer.ie/breast-cancer.
Signs and symptoms of breast cancer
The following are the signs and symptoms of breast cancer, and you should always consult your doctor to discuss these symptoms.
• A lump or thickening in your breast or armpit.
• A change in the size or shape of one breast.
• A change in the skin of your breast, like puckering or dimpling (the skin may look like orange peel).
• A breast abscess (infected boil); this may appear as a red, tender area on your breast.
• A change in your nipple, like a pulled-in (inverted), sunken or flattened nipple.
• An unusual discharge (liquid) from one or both of your nipples; the discharge may be blood-stained or watery.
• A change on or around the nipple, like a rash or flaky or crusted skin.
• Swelling in your armpit or around your collarbone.
If you are worried or have concerns about breast cancer or any cancer, call the Irish Cancer Society Support Line on Freephone 1800 200 700 or email supportline@ irishcancer.ie

Banking on breakthroughs: how the Biobank Ireland Trust is helping shape Ireland’s future in cancer research
Biobanks are the backbone of cancer research and a vital tool in understanding many illnesses, including rare diseases and cancers that need large numbers of samples to gain crucial insights.
Biobanks play one of the most important roles in medicine. By providing researchers with carefully curated samples and linking them with anonymised clinical data, biobanks help speed up the development of new tests, new treatments and more personalised care for today’s and tomorrow’s cancer patients.
Patient-centred biobanks
For this to be meaningful, it must be done responsibly. Every sample collected in a biobank is stored and handled with strict quality and ethical standards, including consent.
In Ireland, however, biobanks are spread out across the country, which can make it harder for researchers to access the materials they need. The Biobank Ireland Trust, a charity founded in 2004, aims to change that by connecting these collections into a single, coordinated national network while always keeping patient voices at the centre of its work.
Collaborative effort in building tomorrow’s cures
A biobank is more than a room of freezers. It takes a team of professionals to gather, label, store and look after each sample, whether it’s a small piece of tumour tissue, a blood sample, or medical images. These samples are precious, and discoveries that lead to tomorrow’s cures will come from the biobank collections we build today.
The Trust is already making real progress. It helps link biobanks across the Island from Biobanc na Gaillimhe and Trinity St James’s Biobank to Cork University Hospital, St Vincent’s University Hospital and the Northern Ireland Biobank. In October, the Trust and Biobanc na Gaillimhe held the first-ever All-Island Biobanking Symposium in Dublin, bringing experts together to focus on technical advances and the patient experience. The Trust is also working with Health Research Charities Ireland and the University of Galway to publish the first national ‘Biobanking in Ireland’ directory.
Our work is supported by our Biobank Warriors, volunteers who run marathons, compete in triathlons, organise golf events and more to raise vital funds.

Anyone interested in supporting our vital work and having fun doing it can follow the
Growing Ireland’s clinical trial ecosystem
Ireland is demonstrating leadership on patient involvement in health research. Meaningful impact starts with early, effective engagement with patients, carers and advocates — long before a clinical trial begins.
Irish-based researchers are world-leading in many medical fields. Their endeavours are supported by, among others, the national research and innovation funding agency, Research Ireland.
Investments and partnerships in health research
Much of Ireland’s clinical research investment is through large-scale programmes. A new clinical trial by FutureNeuro Research Ireland Centre for Translational Brain Science, for example, is exploring how advanced brain monitoring can improve epilepsy diagnosis and care.
Similarly, funded strategic partnerships allow researchers to pool resources and work with companies, Government bodies and charities — for example, ELEVATE (Prediction, Early Detection and Intervention in Cerebral Palsy), co-funded by Research Ireland and the Cerebral Palsy Foundation in the US.
The clinical trial ecosystem is like an iceberg, with the trial on the surface. Underneath are enabling components, like biobanks, registries, infrastructure, personnel, patient data systems, approval processes (including ethical, regulatory, legal and contractual), funding and a pipeline of earlystage research. Ireland has been historically weak in some of these areas, including progress towards electronic health records and lengthy approval processes for trials.
Achieving impact and future plans
Incorporating patient views, needs and acceptability from the outset places any clinical trials that might emerge from research on the best possible footing. Increasingly, researchers are encouraged to integrate engagement into all projects, and we’ve focused on building capacity here.
The Government has a stated ambition to increase the number of clinical trials, and to examine how better to support clinicians, researchers and support staff in this regard. Research Ireland is working with Government agencies and departments, the healthcare community, researchers, patients and advocates, to make this a reality.
Pharmaceutical manufacturing — a sector within which Ireland has a large footprint — is increasingly shifting to new advanced therapeutics such as cell therapies. These therapies often have a closer link to research and healthcare systems than traditional pharmaceutical manufacturing. The ensuing benefits from increasing Ireland’s engagement in clinical trials, therefore, would directly impact patients, our economy and society.
Sponsored by BioBank


Professor Seán Hynes Professor in Pathology, University of Galway, Spokesperson, Biobank Ireland Trust
Sonya Carr Cancer Awareness Nurse, Irish Cancer Society
Dr Finnian Hanrahan Scientific Programme Manager, Research Centres, Research Ireland
Without trials, there are no treatments:
how Irish research facility transforms patient care with citizen science

Ireland’s research culture is changing, and patients want to be part of that. The Wellcome HRB Clinical Research Facility at St James’s Hospital stands at the cutting edge of this transformation. The Government’s blueprint for clinical trials is a timely and welcome move to ensure Ireland is positioned as a global leader in clinical research.


For many, clinical trials are something you hear about from a distance, but for some, it’s an everyday reality.
The Wellcome HRB Clinical Research Facility at St James’s Hospital, a state-of-the-art research facility in Ireland, hopes to highlight the real-world impact of clinical trials on everyday people.
“There’s something really comforting about the consistency you get when you participate in a clinical trial,” explains Harry Friel, currently involved in a trial targeting cardiac amyloidosis, a hereditary condition that also affected his brother. “My brother also participated in clinical trials and believed deeply in contributing to new knowledge — that perspective stayed with me.”
Real-world outcomes


Clinical trials represent the difference between the standard of care and therapeutic innovation, often looking at the current therapeutic landscape through a new lens to discover new treatments. While not knowing whether you’re on a placebo or an active drug can be unsettling, tangible clinical outcomes and drug approvals can help address these concerns.
“Because my condition is so common, I expected treatments to be available,” explains Martina Nolan, who has been involved in a Polymyalgia Rheumatica (PMR) clinical trial for the last 18 months. “I recently saw a new drug announced for my condition, and it made me feel proud. Being part of research that helps get these drugs approved is incredibly rewarding.”
David McCarthy, who has worked in St James Hospital for many years, has seen a range of life-changing and measurable clinical outcomes from his involvement in an obesity drug treatment trial for blood pressure. Losing four stone through the trial has significantly improved his BMI, cholesterol and blood pressure. “The investigator-led trial has
genuinely given me my life back; I am healthier and more energised than I’ve ever been.”
For some, clinical trials mean the difference between life and death. With no curative treatments currently available for Muscular Dystrophy, Kate, mother of 13-year-old patient Aonghus, views clinical trial involvement as an act of remarkable courage, paving the way for the discovery of new treatments. “The level of care is definitely higher within a research facility, likely because clinical trials are run to specific standards and procedures,” she explains. “There are so many people working hard behind the scenes to make these trials possible, and everyone who participates in a clinical trial is very courageous, giving up their time and data for the benefit of others facing the same illness.”


“Our family owes a huge debt of gratitude to St James’s for having the vision and courage to repeatedly seek the funding needed to bring its facilities to the highest standard. Their commitment has helped make it possible for organisations and pharmaceutical partners to invest in Ireland, and, most importantly, to invest in the people of Ireland.”
Martina Hennessy Director of the Wellcome HRB CRF at St James’s Hospital
Harry Friel Patient in Phase 3 Cardiology Trial
David McCarthy Patient in InvestigatorLed Blood Pressure Trial
Kate Coyle Parent of Patient in a Duchenne Muscular Dystrophy Phase 1 clinical trial
Martina Nolan Patient in Phase 3 Clinical Trial relating to Polymyalgia Rheumatica
To find out more, go to: www.sjhcrf.ie
WRITTEN BY Bethany Cooper
Image provided by Wellcome-HRB Clinical Research Facility
Irish neurosurgery and neurology teams leading global trials and advancing patient care
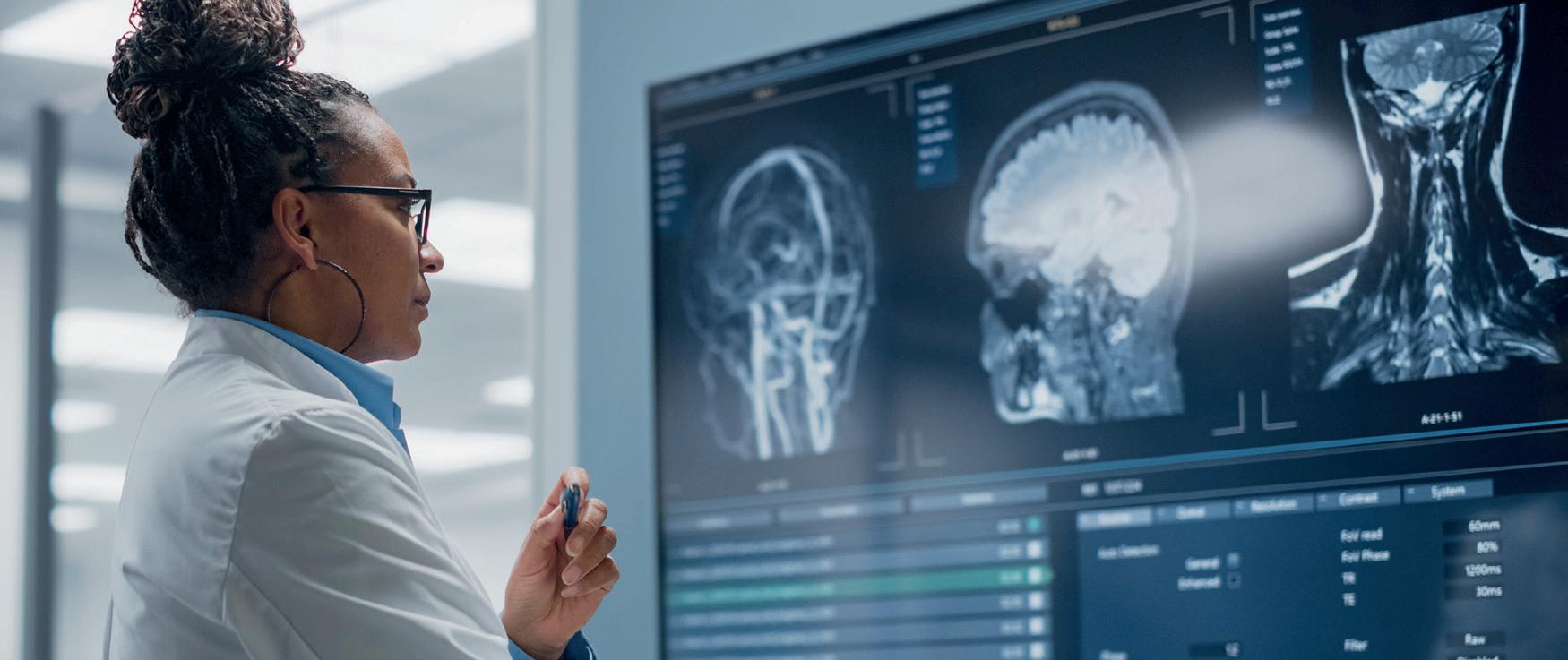
Professor Mohsen Javadpour and Dr Lisa Costelloe discuss how breakthrough studies in brain aneurysms and Multiple Sclerosis (MS) monitoring are helping clinicians deliver safer, smarter and more effective neurosurgical and neurological care.
Consultant neurosurgeon Professor Mohsen Javadpour speaks of his special interest in brain aneurysm research, highlighting its tangible impact on risk factor consultation and interventional treatment pathways.
Brain aneurysm research
“About 3% of the population harbour an aneurysm, most of which never rupture. So, what makes an aneurysm rupture?” he asks.
To answer this, Javadpour and his team built a prospective risk model database at Beaumont Hospital, alongside local audit nurses and nurse specialists who’ve helped to collect data on over 1,000 cases of subarachnoid haemorrhage (SAH).
“Analysing cases of ruptured aneurysm helped us to clearly identify major risk factors: hypertension increases risk 2.5-fold, smoking eightfold, but combined, the risk rises thirtyfold. This changes the way we speak to our patients about prevention.”

Further research includes leading an international multicentre study examining risk factors for aneurysm recurrence following treatment and the prospective use of aggressive, early intervention for poor-grade SAH. “Poor grade SAH
has traditionally been managed in a delayed fashion, but our study of 250 patients showed that early treatment allowed over half of patients to survive and remain independent, particularly younger patients.”
“Neurosurgical research involves assessing high-risk procedures, making trial design and patient recruitment challenging,” continues Javadpour. “Despite this, neurosurgery has been at the forefront of randomised controlled trials, with our clinical research centre running and participating in multiple international studies.”
Novel outcome measure for multiple sclerosis
The last decade has transformed multiple sclerosis (MS) care, with an expansion of disease-modifying therapies providing hope to the 2.9 million people affected globally. To measure the effectiveness of potential drug candidates, novel outcome measures are urgently needed to capture subtle changes in disease activity and progression.
Dr Lisa Costelloe, consultant neurologist, explains.
“Outcome measures for MS need to reflect the full diversity of the symptoms experienced,” she explains. “We often see patients with MS who feel their condition
has worsened, but their MRI hasn’t changed. In addition, cognitive difficulties, fatigue, visual changes and subtle disease progression cannot always be captured with standard MRI monitoring.”
Driving effective patient management, Dr Costelloe’s team is investigating a novel potential biomarker, ocular microtremor (OMT), alongside measures of gait and cognition, as well as standard clinical monitoring. With funding from Enterprise Ireland and collaboration with local medtech company, Head Diagnostics, the research hopes to provide more comprehensive, accurate and realtime data on MS disease activity.
“We’re lucky to receive funding for this project, without which, it wouldn’t be possible,” continues Costelloe. “This is an extremely exciting and fruitful area of research at the moment, with a real potential to drive meaningful change for MS management.”
The RCSI CRC supports research within Beaumont Hospital, providing the infrastructure, support and high-level quality management needed to run complex clinical trials in-house, allowing principal investigators at Beaumont Hospital to “engage ethically and effectively in research,” explains Javadpour. “We have a strong track record of research at Beaumont, and we’re well equipped for clinical trials.”
“The clinical research centre provides the perfect infrastructure to conduct high-quality clinical research,” explains Dr Costelloe. “We have the expertise, allied services on site and a large population of neurological patients, making it an excellent platform for securing research funding.”
The expertise and infrastructure of both Beaumont Hospital and the RCSI CRC combined make it the perfect partner for international clinical research. This is evidenced by involvement in the recent neurosurgical study — the BONANZA trial — within which Beaumont Hospital is the highest recruitment site in Europe.


WRITTEN
BY
Bethany Cooper

Professor Mohsen Javadpour Consultant Neurosurgeon, Beaumont Hospital
Dr. Lisa Costelloe Consultant Neurologist, Beaumont Hospital Sponsored by
Irish research hub continues to foster national ecosystem for innovative therapeutics
National research hub bridges Ireland’s biopharmaceutical sector with the academic ecosystem, accelerating research towards commercial success and cultivating the next generation of research entrepreneurs.
Ireland has the highest number of third-level graduates in Europe per population among people aged 25–34, with 25% of PhD graduates pursuing employment in the biopharmaceutical sector.
“Ireland may seem small, but it’s the 3rd largest exporter of pharmaceuticals worldwide, with a strong foundation of technology, making it well placed to innovate within the therapeutic space,” explains Professor Vincent Kelly, Academic Director.
The ARC Hub for Therapeutics is leveraging Ireland’s biomedical infrastructure to support early-career researchers. “The sheer depth of knowledge required to develop a therapeutic is enormous,” explains Kelly. “We’re supporting early-career researchers to realise their commercial ideas, connecting them to an ecosystem of support far wider than their academic institutions.”
Multi-institution partnership and mentorship


Hosted by Trinity College Dublin, in partnership with RCSI University of Medicine and Health Sciences and University College Dublin (UCD), The ARC Hub will strengthen collaboration and knowledge transfer between universities, investors, industry and clinicians to create new therapeutic products, industry licenses and highquality spin-outs indigenous to Ireland.
“The risk is high in therapeutics, and community is important,” explains Dr Araz Raoof, Executive Director. “The Hub brings together a diverse skillset of expertise and comprehensive operational models to help researchers with concept validation, clinical development
R&D tax credit increase will help medtech transform lives
Medtech’s ability to transform lives and grow the economy depends on its culture of innovation. Recent policy changes, specifically changes in the R&D tax credit, may impact this.

Gand competitive analysis, helping to shape and mature ideas.”
The Hub connects key stakeholders in therapeutic development, combining experts in project management, discovery research and business development with leading academic institutions, which Kelly refers to as Ireland’s “powerhouses of research.”
Strategic investment in therapeutic innovation
The ARC Hub for Therapeutics is co-funded by the Government of Ireland and the European Union through the ERDF Southern, Eastern and Midland Regional Programme 2021-2027, and aligned with national priorities to affirm Ireland as a hub for therapeutic discovery. “We’re catalysts and connectors, a key player in driving therapeutic innovation at both a national and European level,” explains Raoof, “We hope to have a voice in driving the national life science strategy.”
The ARC Hub is helping Ireland realise its promise as a leading biomedical ecosystem, creating a pool of researchers with therapeutic discovery mindsets, and promoting discovery in high-priority areas such as smallmolecule programmes, gene therapy, biomaterials and therapeutic biomarkers.
To mark the progress made since inception, The ARC Hub for Therapeutics held a ‘launch event’ bringing together academics, investors, business leaders, researchers and international accelerator programmes to showcase the projects underway and the possibilities for future innovation.

lobal headwinds are seeing competition for medical technology innovation rise as this dynamic industry grows. It’s one of the most pioneering sectors in Europe, and the recent increase in R&D tax credit to 35% marks a milestone that Irish Medtech has lobbied for. Additionally, the upcoming review of outsourcing and qualifying expenditure definitions, along with the announcement that the threshold for first-year refunds under the R&D tax credit scheme will increase to €87,500 to support smaller projects, marks a positive step forward.
Strengthening Ireland’s global edge Ireland’s medtech sector is a global leader, directly employing 50,000 people, contributing €20 billion in exports and delivering life-saving technologies worldwide. There are over 450 medtech companies in Ireland, of which 80% are SMEs or start-ups, underscoring the scale of innovation-led entrepreneurial activity. Ireland is also home to 9 of the world’s top 10 medtech companies and a thriving indigenous base.
Our neighbours, France, have an R&D tax credit at 43%, followed by the Netherlands at 32%, with the UK also strengthening support with a focus on SME R&D relief. This means that the Budget 2026
announcement will undoubtedly support our position as an internationally recognised leader.
Legacy of breakthrough medical innovations
Medtech is one of Europe’s most innovative industries, and if measured in patents, Ireland has a proud record with over 2,000 patents filed over the past decade and more than 60% of businesses actively investing in R&D here. Targeted improvements to our R&D support will ensure we stand out both locally and globally, as well as further enhance Ireland’s competitiveness.
Ireland has made a big impact from the first bi-aural stethoscope developed by Trinity College Dublin graduate Arthur Leared in 1851, to the portable defibrillator developed by Dr James Francis ‘Frank’ Pantridge in partnership with NASA in 1965, and beyond.
Irish Medtech is confident that, with the right business environment, we’ll continue to reinvent the way we view health and treat for a better future.


Prof Vincent P. Kelly Trinity College Dublin, ARC Hub for Therapeutics Academic Director
Dr Araz Raoof University College Dublin, ARC Hub for Therapeutics Executive Director
Dr Eoghan Ó Faoláin Director, Irish Medtech Association Get involved and enable discovery: therapeutics. archub.ie
Sponsored by ARC Hub for Therapeutics
WRITTEN BY Bethany Cooper
Making AI work safely for GP practices
AI can support safer, more efficient general practice. With clear oversight, it can help teams work smarter and ease pressure across the system.
AI is starting to play a tangible role in Irish general practice. New tools can generate coded note summaries, produce patient document summaries and use voice capture to create accurate, structured consultation records. These tools help GPs complete records efficiently, improving accuracy, continuity of care and freeing time for patient contact in the face of rising demand.
AI in practice operations
In the UK, digital triage has been introduced nationally under NHS policy, with mixed results so far, while other tools such as ambient listening and referral drafting are being encouraged and show early promise in improving efficiency and record quality. Similar developments are emerging in Ireland, with features like automated task creation and document tagging expected over time. Looking ahead, AI tools that support clinicians in interpreting lab results and chatbased patient communication are starting to appear. When supported by clear oversight, they can help ease administrative workload and improve communication.
AI is also helping practice managers draft SOPs, policies and workflow documents more efficiently, supporting rather than replacing professional judgement and experience.
Safe and ethical use
Maintaining patient safety and data protection is essential. Teams should use only secure, employer-approved systems with paid licences, as free public versions often share data externally and lack the necessary safeguards. Seeking guidance from your indemnifier can also help ensure AI is used safely and responsibly.
In Ireland and across Europe, work is underway to guide safe AI adoption in healthcare. The HSE is leading early governance efforts, while HIQA is developing national guidance. The EU AI Act will apply full regulatory standards for high-risk tools from 2026.
Practical guidance for GP teams
• Develop an internal AI policy outlining acceptable use
• Use AI to streamline work, not make decisions
• Review and verify all outputs before sharing or storing
• Avoid free or public AI tools for patient or practice data
With clear boundaries and good oversight, AI can help general practice work smarter while keeping patient care at its heart.

Healthcare’s carbon challenge: Ireland’s path to sustainable care
Healthcare accounts for approximately 4-5% of global CO2 emissions, and Ireland is no exception. Like health systems worldwide, Ireland needs to balance delivering high-quality care while reducing its environmental footprint.
The HSE Climate Action Strategy 20232050 stresses that meaningful progress requires collective action, meaning hospitals, suppliers, clinicians and communities must work together.
The urgency of sustainable healthcare Reducing emissions is inseparable from improving population health, as climate-driven factors such as heat, air pollution and shifting disease patterns are already influencing clinical demand.
Innovation and transformation
Within Ireland, the most carbon-intensive aspects of healthcare are hospital buildings, energy consumption and the broader supply chain. Imaging particularly contributes significantly due to its high-power requirements and complex materials. However, technological innovation is reshaping sustainability in this area.
Modern MRI platforms now incorporate helium-efficient systems, represented by sealedmagnet designs like Philips BlueSeal, which use less than 0.5% of the helium previously required. This breakthrough not only reduces dependence on scarce resources but also strengthens the resilience and sustainability of healthcare services.
Similarly, next-generation CT systems offer workflow efficiencies that can reduce scan setup times by over 50%, lowering energy use per patient and streamlining care pathways. Digital transformation is accelerating these changes. Remote clinical support, virtual training and connected-care pathways are reducing travel for both patients and clinicians, a key contributor to healthcare emissions. This shift aligns with the HSE’s growing emphasis on digital innovation, service redesign and community-centred models of care.
Ireland’s opportunity
Sustainability is now central to healthcare planning in Ireland. The country has a unique opportunity to lead by adopting circulareconomy principles, investing in longer-life equipment, ensuring transparent reporting and developing low-carbon digital pathways. Through collaboration across the Irish health ecosystem, Ireland can build a cleaner, more resilient system that’s better prepared for climate risks and supports healthier people and a healthier planet.
Partnering for a sustainable future
Philips is proud to support Ireland’s healthcare system in its journey toward a low-carbon future. From helium-free MRI technology like BlueSeal to energy-efficient CT platforms and digital solutions that reduce travel emissions, Philips delivers innovations that make healthcare greener, smarter and more resilient.


Empowering clinicians with AI: a new era of smarter, safer healthcare
The integration of artificial intelligence (AI) and automation into healthcare promises to enhance the quality of care, streamline operations and provide more personalised and efficient patient experiences
Under Digital for Care, the Irish health service is evolving, and innovative technology is playing a key role in supporting this.
Empowering healthcare personnel with advanced technological solutions can help to enhance clinical decision-making, streamline administrative processes and improve patient care.
HSE AI initiatives and their benefits
Several AI initiatives are underway across the HSE, including AI-assisted radiography interpretation, predictive modelling, ambient scribing, AI-based translation and process automation.
Integrating AI in healthcare has a range of benefits, including improved diagnostic accuracy, personalised treatment plans, more efficient resource allocation and analysis of vast amounts of data quickly and accurately. It also provides healthcare professionals with valuable insights that can lead to better patient outcomes and algorithms that can identify patterns in patient data, which may indicate the early onset of diseases, allowing for timely intervention and treatment.
AI strategy for healthcare to be launched
The Department of Health and the HSE will shortly launch their first AI strategy for healthcare in Ireland, AI for Care. The strategy will set out the healthcare system’s aspiration for AI in healthcare and opportunity areas for AI deployment in Ireland’s Health Service to 2030. An accompanying AI implementation framework will outline how AI for Care will be implemented and provide a toolkit for implementing AI projects across the HSE to guide the safe, ethical and effective deployment of AI technologies, and to ensure regulatory adherence.
Tom Laffan, HSE Chief Data and Analytics Officer, commented, “AI can transform how we deliver efficient and innovative healthcare for patients and the workforce. Our deployment of AI will be underpinned by appropriate governance that ensures safe, responsible and ethical deployment, enabling us to harness the full potential of AI to enhance healthcare delivery.”
Richard Greene, HSE Chief Clinical Information Officer, said, “Across any AI effort, we will ensure a human approach is taken to use AI to further enable, not replace, healthcare professionals in their work, and that we lean on lived experience to guide continuous learning.”
Sponsored by Philips


Laffan HSE Chief Data and Analytics Officer
Chris Taylor Sustainability Lead UKI Government and Public Affairs, Philips
Rachel Bothwell Founder and Director, GP Practice Ally
GP Practice Ally supports practice managers across Ireland with the tools, training and community they need to run confident, wellorganised practices. Our focus is on helping teams build healthier, more sustainable practices through streamlined operations and empowered teams. Learn more at gppracticeally.ie
Tom

AI technologies are also improving the clinician experience, reducing administrative burden and giving time back to patient care.
Digitisation: the tech revolution reshaping Irish healthcare
Ireland’s push toward digitisation is accelerating, with electronic health records (EHRs) forming the backbone of community-focused care.

While technology adoption might feel like a step away from human-led care, Ireland’s Digital Health Strategy places people firmly at the heart of its transformation.

Digitisation is expected to eliminate the delays and duplication caused by patient records being held across multiple locations. In the longer term, sharing data seamlessly between acute hospitals, primary care and community services can be essential to the Health Service Executive’s (HSE) shift toward more community-based care.
The Strategy is guided by a roadmap running from 2024 to 2030, with much of its success dependent on EHRs, a secure digital version of a patient’s health record.
Decades of groundwork toward digitisation shift
The shift towards digitisation may feel like a leap, but organisations like MEDITECH have been laying the groundwork for decades.
longitudinal record. Today, it’s the EHR supplier to more than 80% of the private healthcare market in Ireland.
Unified records shape patient care Among the first to adopt EHRs is Mater Private Network, which provides care in 12 locations in Ireland. They took an all-ornothing approach, implementing MEDITECH’s Expanse platform in 2024 in two phases, with less than three months between phases.
The move from manual documentation to a single unified patient record started years earlier and involved training around 2,500 staff, from finance and administration to nurses and independent consultants.
But, as Nikki Kane, Chief Operating Officer of Mater Private Network, explains, the benefits are already evident. “From a patient point of view, it has definitely improved safety, because you have all the information there when it’s needed.”
Kerley continues, “We have a genomics solution that continues to evolve. For example, if a patient has a genetic factor, there may be specific medications that work more effectively for them, or others that should be avoided and that will be flagged. This enables physicians to identify the best medication and dosage based on a patient’s genetic information, which ultimately leads to better outcomes and fewer side effects.”
Integrated AI-enabled analytics can help predict patient no-shows and streamline appointment scheduling. AI technologies are also improving the clinician experience, reducing administrative burden and giving time back to patient care.
Sponsored by MEDITECH

MEDITECH Executive Director, International, Gina Kerley explains, “We began work in Ireland back in 2002 when patient records were stored in filing cabinets and departments had little to do with each other. Over time, the sector has moved towards end-to-end EHRs that span the entire continuum of care.”
MEDITECH has developed tools such as its Expanse platform — a web-based EHR that brings patient information into a single
EHRs’ real-time data and ability to access information instantaneously can enable teams to respond in emergencies, track referrals and monitor clinical outcomes.
“In terms of what we can do with that data from a patient-experience perspective, that can ultimately transform healthcare,” concludes Kane, who views this as just the start of a digital journey. With the EHR in place, the scope for future integrations such as AI-driven tools is huge.
As the HSE aims to shift care from hospital settings to communitybased support, joined-up services and interoperability become more critical. Adopting standardisation among EHRs will be key to ensuring a connected healthcare system with patient needs at the heart. It will also open opportunities for greater transparency and autonomy via the HSE Health App — dubbed the ‘Digital Front Door.’
Having worked on implementing EHRs in healthcare settings worldwide, Kerley believes Ireland is poised to reap the benefits. “The foundation is just getting the right EHR in. The journey really begins once the platform is in place and its tools, data and interoperability come together to support better decisions and improve care.”
WRITTEN BY
Kate Sharma
Gina Kerley Executive Director, International, MEDITECH
Nikki Kane
COO, Mater Private Network
