







































• Holistic repairs which address current as well as known points of failure
• Trusted by multiple OEMs, dozens of ISOs, and partnered with the most recognized names in healthcare
• Engineering expertise includes proprietary test fixtures based on each coil’s unique design, 3D printing, plastic molding, and state-of-the-art machining enable full mechanical restoration, full-cable harness fabrication, and array manufacturing
• Exclusive repair processes informed by our legacy in FDA registered manufacturing

P.12 SPOTLIGHT
p.12 Department of the Month:
Northwest Territories Health and Social Services Authority
Biomedical Engineering Department
p.14 Professional of the Month: Benjamin Tinsley
p.16 Next Gen: Aaron Febre
p.18 Association of the Month: The Nevada Healthcare Technology Association (NVHTA)
P.20 INDUSTRY UPDATES
p.20 News & Notes
p.28 Tech Choice Award Finalists
p.32 Welcome to TechNation
p.36 Ribbon Cutting: ERD Medical Equipment Solutions
p.38 AAMI Update
p.40 ECRI Update

P.42 THE BENCH
p.42 Biomed 101
p.45 Tools of the Trade
p.46 Webinar Wednesday
p.48 Shop Talk powered by MedWrench
P.50 FEATURE ARTICLES
p.50 Roundtable: CMMS
p.58 Cover Story: Influencing the Future of HTM: Platforms That Promote the Profession
P.65 EXPERT ADVICE
p.65 Career Now
p.66 SPONSORED: MultiMedical System
p.68 Right to Repair
p.72 Networking Notes
p.74 Health-ISAC
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey BUSINESS DEVELOPMENT
EDITORIAL John Wallace
CONTRIBUTORS K. Richard Douglas Joie Marhefka
Steven J. Yelton
Garrett Seeley
Phil Englert
Nathan Proctor
Nadia ElKaissi
ACCOUNT Megan Cabot
EXECUTIVES Emily Hise
ART DEPARTMENT Karlee Gower
Taylor Hayes
Alicia Brown
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
Haley Harris
EVENTS Kristin Leavoy Sydney Krieg
WEBINARS Linda Hasluem
HTMJOBS.COM Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez


Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Nadia ElKaissi, CHTM, Biomedical Engineer, HTM, VA Central Office (19HTM)
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Earl Morris Jr., BMET, Director of Clinical Engineering, Harrison County Hospital
Leticia Reynolds, Clinical Engineering Operations Manager at UCHealth Parkview Medical Center








Our nationally recognized programs include Biomedical Equipment Technology (BMET), Biomedical Equipment Support Specialist (BESS), and the Dental Repair Technician (DRT) Certificate. Each is designed to equip you with the advanced skills needed to thrive in today’s evolving healthcare and dental technology landscape.




Virtual
(VR) labs offer a flexible and immersive learning experience that adapts to your busy schedule. our VR labs allow you to practice and perfect your skills in a controlled, risk-free environment.


BY K. RICHARD DOUGLAS
Far north of the U.S. northern border, high up in Canada and almost to Alaska, are the Northwest Territories. The capital, and largest community, is Yellowknife. It is one of the best places on earth to view the northern lights in the evening, especially in fall and winter.
As the largest city in the Northwest Territories, Yellowknife is also the epi-center for the area’s medical care. The region’s HTM experts comprise the Northwest Territories Health and Social Services Authority (NTHSSA) Biomedical Engineering Department.
The biomed team is made up of 11 professionals. The Territorial Manager Biomedical Engineer is Kevin Taylor, PEng.
“Our department includes Chief Biomedical Engineering Technologist Erwin Sumcad, six senior BMETs (Jason Bell, Jillian Tobin, Shane Simms, Alanna Hinchey, Samantha Powell), one Casual Biomedical Engineer (Lydia Sochan, MASc), and two Casual/Term Senior BMETs (Timothy Ebbeling and Sarah Berg). In terms of scope, we service a wide range of technology in-house and off-contract. This ranges from basic technologies such as pulse oximeters, suction devices, infusion pumps, etcetera, to intermediate technologies such as physiological monitors and defibrillators; as well as high-level technologies such as X-ray, anaesthesia, ventilators and mammography,” Taylor says.
He says that the department also budgets and manages all the Government of the Northwest Territories’ (GNWT) health technology service contracts for areas such as CT, fluoroscopy and lab technology.
“Moreover, not only do we support all health technology in GNWT health facilities, but we also support two-thirds of the Government of Nunavut’s (GNU) health facilities (i.e., specifically the Kivalliq and Kitikmeot Regions). Our service area represents a geographic area of about onefifth of the United States, and more than half of these communities have no road access (i.e. only accessible by air
or in the winter by ice road),” Taylor says.
The team provides equipment management support to one 80-bed tertiary hospital in Yellowknife, one 51-bed secondary hospital, two health centers with in-patient capacities with a combined 26 beds, but no OR and three higher-level health centers in regional hub communities. These facilities will have a few doctors each, but no in-patient capacity.
Their responsibilities also extend to 16 standard health centers, which are staffed by two to five nurses, as well as seven health cabins, which are staffed by a first aid trained community health representative.
Taylor says that the team also manages medical equipment for two primary care facilities in Yellowknife, eight long-term care facilities, which have a total 183 beds across the territory, 12 health centers in Nunavut and the Yellowknife Fire Department.
Team members are well-trained and future recruits will bring with them a firm foundation.
“The goal is that within two years, all BMETs who are hired are factory-trained on dialysis, anaesthesia, X-ray, physiological monitoring, and ventilators. BMETs in the NWT are specialists in being generalists: they must be able to work in all specialty areas and the NWT invests heavily in that training,” Taylor says.
There is good reason why this group needs to be able to work on nearly everything. Taylor says that “service vendors can spend up to three days travelling to do a 30-minute service call or get trapped in a community for three to five days due to bad weather.”
He says that there have been times when a vendor had not set foot in the territory for a year even with a full-service contract.
With such a large and diverse geographical area and diversity of facilities, the need for special projects above and beyond PMs and maintenance is an expected reality.
The department has participated in several, including the replacement of a hospital.
“The Stanton Territorial Hospital (i.e., the Government of the Northwest Territories tertiary care facility) was replaced


with a new facility in 2019 and Biomedical Engineering, along with biomeds at the Department of Health, played a heavy role in the planning and installation of all new health technology,” Taylor says.
The COVID-19 pandemic challenged biomed departments everywhere and the NTHSSA team was not exempt.
“Biomedical Engineering assisted in the planning, and then purchased, an entire year’s capital budget in medical equipment in just one month at the start of COVID. BMETS from the NWT were also the first BMETs in Canada to volunteer to support the Federal Governments National Emergency Strategic Stockpile and two NWT BMETS each spent a month inspecting incoming COVID medical equipment that was purchased by the Canadian Government to distribute throughout Canada,” Taylor says.
The team really went above and beyond when they were called up to help out with a major evacuation.
“The entire capital city of Yellowknife, as well as the major towns of Fort Smith and Hay River, along with other smaller communities were evacuated in August 2023 due to wildfires (two-thirds of the population of the NWT was evacuated more than 1,000 miles south). Biomedical Engineering supported the mothballing of the tertiary hospital and also participated in setting up evacuation centers for patients. We went so far as to support clinical staff in the care of patients at the military hanger due to limited available clinical staff, as well as the loading of patients onto military transports,” Taylor says.
Because of the team’s broad skills and experience working in harsh conditions, members have helped out with Kosovo after the war in 2000s; Argentina with ORBIS International in the early 2000s; Gambia in the late 2000s; and most recently in Sierra Leone in 2023.
The department is skilled at problem-solving and enjoys working under a unique policy that would be the envy of many U.S. biomeds.
“One of the features that makes our biomedical engineering department unique is our health technology right to
repair policy which applies to all health technology purchased for the government of the NWT health facilities,” Taylor says.
He says that the NWT is the first government health system in Canada to have a health technology right to repair policy.
“In short, the policy essentially states that unless there is no clinically acceptable alternative, it is a mandatory procurement requirement that the provision of service manuals, service training, service software, and service parts are all provided at the same level as provided to the original equipment manufacturer service engineers. This policy allows us to best protect patient safety,” Taylor adds.
He says that while the need to ensure patient safety through timely service is true for all areas of North America, because of the remote and rural nature of the team’s territory, sending equipment for repairs can be an incredibly lengthy process, putting patients at risk of delayed care.
“By servicing equipment in-house, we become much more self-sufficient and are able to provide better service to the territory. This policy came out of the challenges faced during COVID-19 and aligns with the Canadian Medical Biological Engineering Society’s (CMBES) Clinical Engineering Standards of Practice and the CMBES position statement on right to repair,” Taylor says.
The team came to the rescue several other times, including during the pandemic when the demand was extra urgent.
Off the job, Taylor is the co-chair of the CMBES’s right to repair committee as well as the national champion on this issue.
“The NWT has presented nationally and internationally on their right to repair program,” he says.
The NTHSSA are a special breed and work far away from OEMs and suppliers. They are highly skilled and take advantage of being able to apply those skills when some biomeds might be blocked. They have shown that, to the greatest degree possible, they are self-sufficient HTM professionals.
In very remote, and often harsh conditions, this team of biomeds reflects the best of the profession.
BY K. RICHARD DOUGLAS
For Benjamin Tinsley, diagnostic imaging support, advanced field service engineer II at Baylor Scott & White Health in Dallas, Texas, the introduction to HTM came out of the realization for the need for biomeds.
“My journey into the HTM field started while I was working with a nonprofit organization in Nicaragua, where I helped coordinate mobile medical clinics. During that time, I noticed that a lot of the equipment we relied on was constantly breaking down – and there was rarely anyone around who could fix it properly. It got me thinking someone out there has to be responsible for keeping this equipment running. That sparked my interest in the technical side of healthcare,” Tinsley says.
He says that when he returned to the U.S., he started looking into how he could get involved in that kind of work and came across the biomedical and imaging technology programs at Texas State Technical College (TSTC) in Waco, Texas.
“I ended up enrolling and earning degrees in both biomed and imaging. That decision set me on the path I’m on today – and I’ve never looked back,” Tinsley says.
While enrolled in the TSTC courses, Tinsley received hands-on training in both fields.
“As part of the program, I also completed an internship with Baylor Scott & White in Plano, which gave me valuable real-world experience working alongside seasoned professionals in a clinical environment,” he says.
After graduation, Tinsley began his career with Siemens Healthineers as an imaging field service engineer.
“That role gave me the opportunity to apply everything I had learned while continuing to expand my knowledge and skills by working directly with



“I’ve focused my experience on keeping this equipment running smoothly. It’s rewarding to know that my work helps support accurate diagnoses and effective treatments,” he says.
As many in HTM have come to realize, scheduling maintenance of high-demand medical equipment can be a challenge.
“One of the main challenges I’ve faced in my current role as an advanced engineer is maintaining a consistent preventive maintenance schedule in the cath labs. These labs are high-demand environments where patient care is always the top priority, so finding the right windows of time to perform thorough maintenance without disrupting critical procedures can be quite challenging,” Tinsley says.
He says that to address this, he has had to work closely with clinical teams to coordinate maintenance during off-peak hours or scheduled downtimes.
“It requires flexibility, strong communication and careful planning to ensure that the equipment remains reliable without interfering with patient care,” Tinsley adds.
He says that in addition to these challenges, he has been involved in projects focused on upgrading and optimizing the imaging systems within the cath, IR, OR, and EP labs.
“These projects have included system updates, integration of new technologies, and workflow improvements aimed at improving operational reliability and supporting the clinical teams in delivering quality care,” Tinsley says.
Away from the job, Tinsley enjoys 3D printing as a hobby and has a corgi named Rooster.
As a member of the HTM community and workforce, Tinsley describes himself as a simple guy who enjoys taking X-ray machines apart and figuring out how they work.
“I like the challenge of troubleshooting and repairing these complex systems because it’s rewarding to know my work helps keep critical medical equipment running smoothly,” he says.
When somebody enjoys their vocation, it means that they likely do excellent work. Diagnostic imaging is made that much more precise thanks to this HTM professional.
FAVORITE BOOK: Harry Potter books
FAVORITE MOVIE: “Star Wars”
FAVORITE FOOD: Pizza
HIDDEN TALENT: 3D printing
FAVORITE PART OF BEING A BIOMED?
What I enjoy most about being a biomed is getting to solve problems every day. Whether it’s fixing a piece of equipment that’s critical to patient care or troubleshooting unexpected issues, it’s rewarding to know that my work directly supports healthcare teams and helps keep things running smoothly.
My manager keeps a stack of TechNation magazines in the shop. It’s a good read that helps me stay connected with what’s happening in biomedical and imaging repair.
Aaron Febre began his healthcare technology management career with education at Durham Tech and UNC Charlotte. He earned both an associate degree and bachelor’s degree before joining Duke University Hospital as a biomedical equipment technician. TechNation recently found out more about his start and his future goals.
Q: WHERE DID YOU GROW UP?
A: Born and raised in Durham, North Carolina
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?
A: I got my associate degree from Durham Technical Community College.
Q: HOW DID YOU FIRST DISCOVER HTM?
A: I did a Google search for healthcare jobs that weren’t a doctor or a nurse. This was one of the first ones to pop up in my search.
Q: WHY (OR HOW) DID YOU CHOOSE TO GET INTO THIS FIELD?
A: I wanted to have a career in healthcare, but I had no aspiration to be a doctor or a nurse. Especially coming from a family full of nurses, so in a way I wanted to rebel in my family. Ha, ha. As I’ve mentioned earlier, I did a Google search of the various healthcare jobs that weren’t those two titles and this was one of the first to come up. From there, I discovered the Better Biomed YouTube Channel and found out that Durham Tech had a 2-year program to get into this field. And the rest of it was history.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: You don’t get bored. You are always doing something different and there’s a ton of things to learn. And I’m happy that I am working with experienced technicians who have a ton of knowledge and are willing to share it.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: One part of HTM I am interested in is integration. There’s one


things. But it’s fun once you get the hang out it.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: Professionally? I hope to acquire my CBET and gain a lot more experience in the field. Personally? I hope to have traveled to places, start/join a band, complete a marathon, etc
FAVORITE HOBBY:
Playing guitar
FAVORITE SHOW OR MOVIE:
My favorite show (anime) is “Yu Yu Hakusho”.
FAVORITE MEAL: Sushi
WHAT WOULD YOUR SUPERPOWER BE?
Running fast (like the Flash)
1 THING ON YOUR BUCKET LIST:
Visit Manchester, England so I can watch my football (soccer) club, Manchester City FC.
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU:
When I was kid, I wanted to be a Formula 1 driver. (It’s a little late to do that now ha ha).


Onsite
On-demand BMET/HTM Technician(s) & Manufacture Remediation/Recall Support
Repair estimate within 48 hours, quick turnaround time & extensive list of loaners available at no charge
MMS will deploy a team of technicians to your facility to complete Annual Periodic Maintenance on your Infusion Pumps All types of medical equipment - new or refurbished
K. RICHARD DOUGLAS
The HTM profession faces some short-term challenges in the coming years as the need to replenish staffing is vital. The retirement of experienced biomeds leaves a void and every avenue must be explored to bring new talent into the field while promoting a little-known profession.
That makes the creation of a new biomed association that much more welcome and needed.
The state of Nevada has not had an organization where biomeds can connect for several years. That has all changed with the formation of the Nevada Healthcare Technology Association (NVHTA).
Kevin Davis, a biomedical service professional and the owner of ERD Medical Equipment Solutions, an ISO-9001 certified independent service organization based in Nevada, founded the new group.
Other Nevada HTM professionals, both active and retired, will hold potential roles as officers, board members or advisor. They include Skylar Bogardus, Candi Robles, Reagan Jordan, Michael Lane, Dr. Binseng Wang, James Knight and Davis.
“The Nevada Healthcare Technology Association (NVHTA) was officially formed in 2025. While our official launch is recent, the vision behind the association has been developing for several years through collaboration with healthcare technology management (HTM) professionals in Nevada,” Davis says.
Davis and his Nevada HTM colleagues recognized a growing gap in professional support, training, and community-building for HTM professionals in Nevada – a state without a dedicated biomed association of its own until now. NVHTA was created to fill that void.
“Our mission is to bring together technicians, educators,

healthcare systems, and industry partners to build a stronger, more connected HTM community – one that promotes innovation, workforce readiness, and long-term career development,” he says.
Davis says that while NVHTA is newly formed in 2025, its roots lie in a much longer conversation about the future of healthcare technology in Nevada.
“For years, local HTM professionals lacked a central hub for networking, peer support, continuing education, and advocacy. As Nevada’s healthcare systems grew – and as retirements created gaps in the workforce – the absence of a unifying association became more visible. NVHTA was created to meet that need. In its founding year, NVHTA’s goals are to begin engaging with hospitals, schools, independent service organizations, and national partners to make NVHTA visible and to gain buzz and membership,” he says.
Davis says that the group’s focus is on building a solid foundation with clear values: community, education, transparency, and workforce development.
“Rather than replicating the model of larger, legacy associations, NVHTA is rooted in action-oriented leadership – developing hands-on BMET training opportunities, planning regional chapter events, and partnering with schools to raise awareness about careers in HTM. We’re proud to be building something new, dynamic and tactile in our approach, and aligned with the evolving needs of our profession,” he says.
Education will be a priority for the new group along with a focus on several other areas that will help grow and strengthen the field.
“While education is absolutely central to NVHTA’s mission, we’re also focused on several unique, high-impact initiatives that set us apart from traditional associations,” Davis says.
He says that one of NVHTAs top priorities is creating entry points into the HTM profession for people who might not otherwise discover it – high school students, veterans,



trade school grads, and career changers.

“NVHTA wants to create exposure to HTM careers that show interested professionals that the HTM field can be agile, affordable, and directly aligned with the needs of hospitals, ISOs, and manufacturers and, most importantly, individual’s skills and passion,” Davis adds.
He says that beyond that, they are invested in publicfacing HTM awareness.
“Through creative outreach efforts we aim to educate not only future biomeds but also parents, school counselors, and the broader community about the critical role HTM plays in safe patient care. We also emphasize partnership development with healthcare systems, equipment manufacturers, and local service providers to bridge gaps between education and employment. By aligning stakeholders early, we create a more sustainable and coordinated pipeline into the profession,” Davis says.
As a brand-new organization, NVHTA has not yet scheduled its first symposium/conference. For now, more routine get-togethers are the norm until a formal conference can be planned.
“Since NVHTA is in its founding year, we are currently in the planning stages for our first annual event, which we expect to host in late 2025 or early 2026. Rather than launching with a large-scale symposium, we’re taking a more focused and grassroots approach – starting with regional chapter meetings, community-driven workshops, and hands-on training events that reflect the needs of our members and partners,” Davis says.
He says that the group’s goal is to create events that are educational as well as deeply collaborative and practical – bringing together biomeds, educators, students, hospital leaders, and vendors in a setting that fosters direct learning, recruiting, and relationship-building.
“For example, providing hands-on training along with networking creates both skill-building and community socializing in one event. We also envision a joint end-ofyear gathering to celebrate our launch year, share insights



from early initiatives and introduce the community to what’s coming in 2026,” Davis adds.
He says that these events will serve as stepping stones to a larger, statewide symposium in the near future – one built on real engagement and measurable value.
For the time being, the new HTM organization is organizing its first round of chapter meetings and planning a calendar of regularly scheduled events. They are also investigating the formation of a scholarship program, which Davis labels a “top priority.”
“We are currently laying the groundwork for a scholarship and sponsorship program tied directly to our BMET workforce training initiative. NVHTA welcomes interested sponsors to help develop scholarship programs and would love interested readers to reach out and collaborate,” he says.
Aligned with this goal is a mission to partner with local biomed training and education programs.
“We are actively working with institutions in northern Nevada, particularly around Carson City and Reno, to bridge the gap between education and employment in the HTM field. Our vision is to work alongside community colleges, not compete with them, by offering flexible, short-format, equipment-based training that prepares students for field work and ISO/hospital-based roles. In the process, we’re also raising awareness of HTM careers among school counselors, educators, and students who may never have heard of biomedical technology,” Davis says.
He says that replacing retiring biomeds is a primary focus and the organization’s strategy focuses on building a pipeline of new talent through outreach, training and awareness.
In the meantime, the group has filed for not-for-profit status and registered with the state. A constitution and bylaws have been outlined. A website has been in development and can be found at: NVHTA.com
There are few advancements within HTM more important than organizing, training and recruiting biomeds, so the newly formed NVHTA is big news.

Ultimo has acquired FSI, a U.S.-based category leader in healthcare facilities and healthcare technology management software. This acquisition marks Ultimo’s second in the U.S., its third overall since 2024, and reflects its strategic commitment to the region’s long-term growth.
FSI has the only computerized maintenance management system (CMMS) platform purpose-built for healthcare service professionals, according to a press release. Its solutions span digital transformation, data management, and in-person asset discovery – serving over a thousand departments across hospitals, healthcare systems and universities.
“Hospitals and healthcare operators are under pressure to digitize fast, stay compliant, and drive down risk in environments where asset performance directly impacts patient care,” said Steven Elsham, CEO of Ultimo. “By bringing FSI’s trusted capabilities together with Ultimo’s EAM capabilities and AI-first vision, we’re delivering a suite
of solutions and intelligence customers need to take control of their assets, reduce operational friction, and drive measurable impact.”
The acquisition builds on Ultimo’s long-standing expertise as the market leader in healthcare asset management in the Netherlands and Belgium and unlocks a deeper presence in the U.S.’s asset-intensive industries. Ultimo will continue the investment to drive market leadership for FSI, with a focus on providing stability for FSI customers.
“This is an exciting new chapter for FSI and its customers as we accelerate investment in solutions that equip customers to tackle regulatory complexity, drive operational efficiency, and accelerate digital transformation” said Zachary Seely, CEO of FSI. “Our customers will have the opportunity to access Ultimo’s AI-first roadmap, including its agentic AI framework.”
This acquisition has been signed and closed by Ultimo.
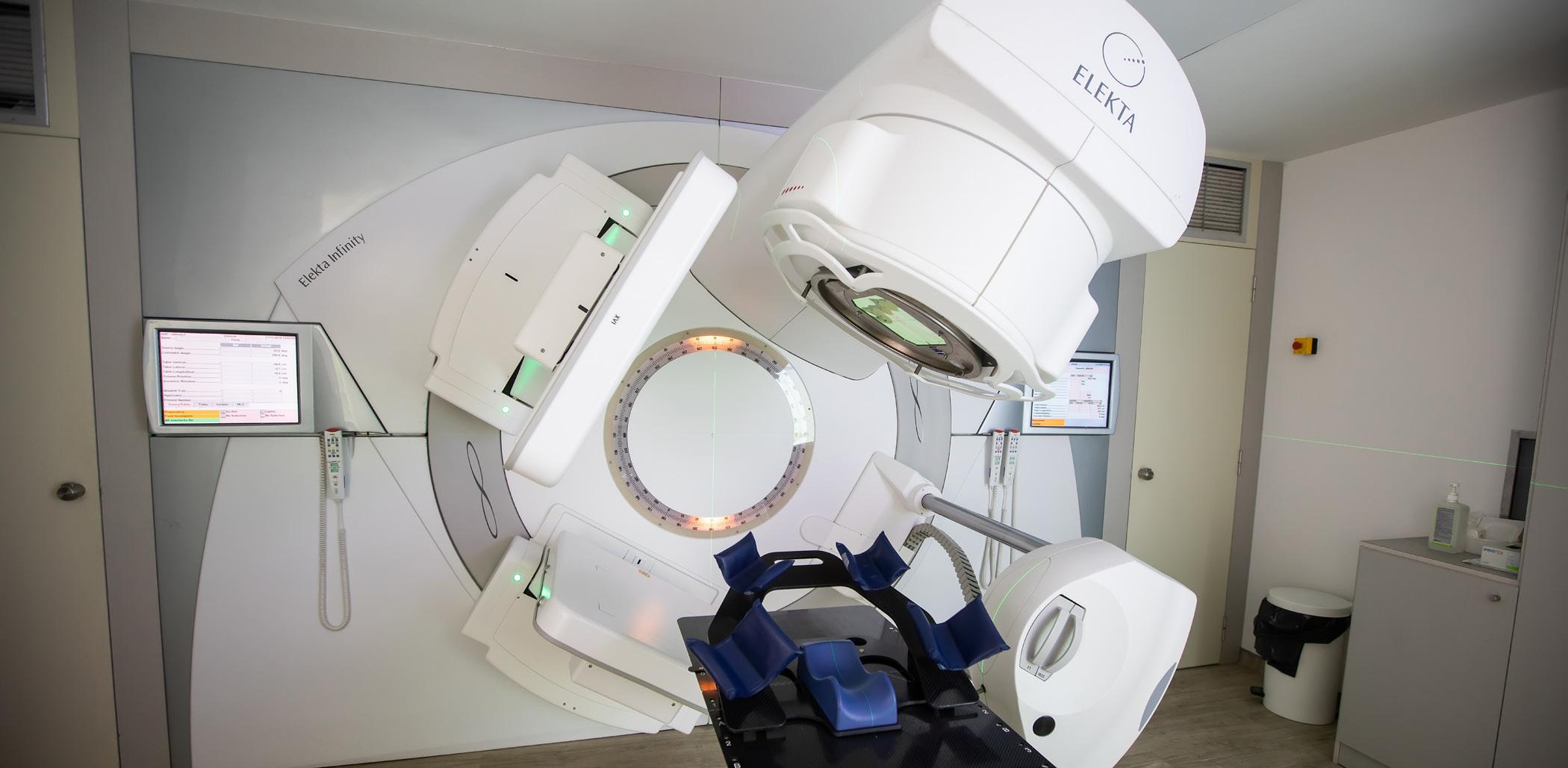
The College of Biomedical Equipment Technology (CBET), Radiology Oncology Systems Inc. (ROS) and Linax Technologies LTD (Linax) have announced a collaboration in establishing and delivering a new and innovative series of online and simulator-based courses designed to teach the technical, operational, and physical principles behind medical linear accelerators used in radiation therapy – a critical technology for current and emerging cancer treatment strategies.
Medical linear accelerators, targeting radiation to treat a broad range of cancers, are among the most complex medical devices routinely used in hospitals. Owing to equipment complexity and the potential dangers associated with radiation errors, it is essential that these devices are operated with the highest degree of precision and maintained by fully qualified and knowledgeable technicians. Providing technicians with high quality and affordable training to maintain and service this equipment with the requisite skill set and accuracy serves an important industry need for the HTM community.
John Vano, president of ROS, a provider of reliable, high quality reconditioned radiation therapy and diagnostic imaging solutions, said, “Developing a strong HTM workforce with expertise in linear accelerator training is not just a technical need – it is a patient safety imperative.”
Noting that training needs are critical in North America as well as globally.
“More and more manufacturers are entering this space, and this new CBET educational track offers a progressive, multi-level curriculum that equips students with the fundamentals to begin or continue a career in medical linear accelerators,” Vano added.
The CBET LINAC Fundamentals courses are designed to provide students with a firm and structured understanding of the medical linear accelerator and its utilization of external beam treatments for high-quality patient care. Preparing students to
effectively triage and manage linear accelerator operations and maintenance, progressing from basic and intermediate levels of LINAC physics, engineering, and application factors of this specialized equipment in a cancer care clinical environment through deeper navigation of LINAC equipment and subsystems, serves an essential HTM industry skills gap and demand.
Marco Carlone, CEO of Linax Technologies, a creator of simulators of complex radiotherapy devices, notes, “Up until now, the only way to learn the principles of medical linear accelerators was to attend a vendor course. With the collaboration between Linax, ROS and CBET, we aim to broaden the medical LINAC service technician community and provide an affordable, accessible, and long-overdue training course for service technicians. Our vision is that of an empowered and flourishing profession, ensuring that cancer patients never have to worry about having access to a high-quality linear accelerator for their treatment.”
The College of Biomedical Equipment Technology provides high quality, stakeholder-based training solutions for the HTM industry, including certificate and associate degree training in biomedical equipment technology and biomedical equipment support specialists, as well as a broad suite of high-quality business training solutions to deliver foundational, advanced, and product specific education to meet the evolving needs of professionals and HTM organizations.
Richard Gonzales, president of CBET, noted that, “Empowering biomeds with linear accelerator training is essential as their expanding roles drive initiatives that enhance quality, patient care, and innovation in healthcare technology.”
By incorporating these courses into its education and training portfolio, CBET continues to expand its robust suite of HTM industry business training solutions in close alignment with the industry’s evolution.

The Army is continuing its transformation effort to bring medical maintenance support closer to operational units without organic maintainers within their ranks.
Under an initiative called Medical Logistics in Campaigning, or MiC, the Home-Station Medical Maintenance Support, or HMMS, program was launched last year at Fort Bragg to begin closing a crucial readiness gap for the warfighter.
Work is ongoing to bring a planned second wave of locations online in the near future, including Fort Benning, Georgia; Fort Campbell, Kentucky; and the U.S. Army Medical Materiel Agency’s Medical Maintenance Operations Division at Tobyhanna Army Depot, Pennsylvania.
“Borne out of MiC, the HMMS program is helping us align medical maintenance with existing sustainment processes of other Army commodities, saving time and money, and ensuring readiness in the process,” said Alfred Zamora, an equipment specialist at USAMMA and acting deputy director for HMMS.
In the Northeast region, units like the 3rd General Support Aviation Battalion, 10th Combat Aviation Brigade, out of Fort Drum, New York, are already utilizing a future HMMS site at MMOD-PA, using Tobyhanna’s helipad recently to drop off over 30 pieces of equipment.
Staff Sgt. Michael Silbart, flight paramedic for the 10th Mountain “DUSTOFF” unit, said the capability to drop off dozens of pieces of equipment at once for recalibration, repair and return to service within a month is “invaluable” to continued operations.
“Being the most deployed unit in the Army requires us to have top-tier maintenance, and that is what USAMMA provides us with,” Silbart said. “Flying directly to USAMMA makes the process seamless and controlled. The lack of a middleman always gives us more control over our equipment and prevents things from getting lost.”
DUSTOFF is used as a callsign by Army air ambulance units, including helicopters dedicated to medical evacuation.
Silbart emphasized the importance of USAMMA’s support, saying it’s the only way to ensure service for their specialized equipment.
“The current facilities here at Fort Drum are not equipped to handle our specific equipment,” he said. “Without USAMMA, we would not be capable of completing our mission here.”
Minimizing downtime and increasing overall readiness are two primary drivers of the planned HMMS program expansion, and the ability to utilize Tobyhanna’s helipad adds more logistical benefits for customers and the team at MMOD-PA alike.
“We’re able to accelerate delivery timelines, reduce ground transport requirements and strengthen relationships with our customer base,” MMOD-PA Director William Wall said. “At the same time, aviation units utilize this resource as part of their training flight hours, making it a mutually beneficial arrangement.”
MMOD-PA technicians successfully completed maintenance and calibration on 18 medical devices, including infusion pumps, ventilators, defibrillators and more, which were exchanged during a coordinated helicopter drop-off for 20 newly arrived devices requiring service.
“This seamless transition, occurring approximately two weeks later, ensured uninterrupted operational readiness and mission support,” Wall said, adding that he expects more use of the Tobyhanna helipad with the future HMMS expansion plans.
Seeking to close critical maintenance gaps that affect readiness, HMMS was developed under MiC, which aims to integrate medical logistics into the Army Sustainment Enterprise as part of the Army’s Campaign Plan 2023-2030.
The first site was established at Fort Bragg during MiC’s proof-of-concept phase in 2024. The North Carolina installation now supports over 100 units, including active-duty and National Guard, throughout the state.
After wave two’s planned rollout later this year, additional proposed sites include Fort Carson, Colorado; Joint Base Lewis-McChord, Washington; and USAMMA’s other two MMODs in Utah and California.
USAMMA is a direct reporting unit to Army Medical Logistics Command, which has been tapped by Army Materiel Command to lead the MiC effort.
The Oregon Biomedical Association (OBA) will host its 2025 Expo & Vendor Fair on Oct. 23-24 at the Holiday Inn-Portland Airport, offering two days of education, networking and industry engagement for healthcare technology professionals.
This year’s theme, “Cascading You Into the Future of Healthcare,” highlights OBA’s commitment to advancing biomedical and imaging technology through training, collaboration and innovation.
The event features three educational tracks with nine

seminars per day, covering imaging, biomedical, and hot-topic discussions. Attendees will also have access to a vendor fair, networking opportunities and door prizes. Breakfast, lunch, and snacks will be provided.
Attendance is limited to 100 tickets per day. Early RSVP is encouraged to secure a spot.
For more information, visit orbmet.org.




A recent Baxter Research Center survey provides an unbiased third-party view of TechNation magazine. The survey examined readers’ habits in regard to editorial content and advertisements. For over 35 years, the Baxter Research Center has provided magazines with valuable feedback and statistics.
Seventy-nine percent of readers stated that TechNation magazine is either important, very important or their personal favorite.
The report states that 78% of respondents read the print issue of the magazine with 22% reading the digital issue of TechNation. A whopping 80% read the magazine at least twice. Not only do HTM professionals read TechNation, but they also share it with colleagues. Baxter found that readers on average share their copy of the magazine with 1.3 people.
TechNation is also a trusted source for decision makers. Eighty percent of respondents indicated that they or someone in their organization took or are likely to take a
2025 MD Expo Dallas just keeps getting better. MD Expo Dallas is approved for CEUs by the ACI. And, MD Expo continues to offer free registration for hospital employees, members of the armed forces and students.
ACI breaks down the available CEUs as follows:
• H.O.T. Workshop: Contrast Injector Operation and PM Training (Session #1) – 4 CEUs
• H.O.T. Workshop: Contrast Injector Operation and PM Training (Session #2) – 4 CEUs
• H.O.T. Workshop: Ultrasound Systems – 7.5 CEUs
• The MD Expo, held November 11-12, 2025, is pre-approved for up to 8 CEUs.
Registration is open for the upcoming fall conference that promises to be one of the biggest MD Expos ever!
MD Expo Dallas is set for Nov. 10-12 at the Renaissance Dallas Addison Hotel in Addison, Texas. Hosted by MD Publishing and TechNation, this premier conference is one of the year’s must-attend events for biomedical technicians, clinical engineers, asset managers and other healthcare technology management (HTM) professionals.
The three-day agenda features industry-leading sessions on HTM best practices, cybersecurity, IT integration,
purchasing actions after seeing an ad in TechNation.
Readers shared their feedback regarding TechNation magazine.
“It keeps me informed on current trends in the industry,” Biomed Supervisor Robert Lampe said.
“I get a lot of insight about so many different areas. I actually have found it very useful for learning and find a lot of the articles very interesting,” Service Technician Terry Absher said.
“TechNation provides articles that are timely and relevant to the field. Challenges faced by biomeds as well as what is on the horizon, are often discussed,” BMET ll Joshua Abner said.
“Keeps me up to date on everything biomedical,” said Biomed Anthony Mack.
Sign up for a free subscription to the industry’s leading publication at 1TechNation.com/subscribe.
compliance and equipment service – delivered by top experts in the field. An expanded exhibit hall will showcase the latest medical technologies and services, offering hands-on demos and direct access to vendors.
The event kicks off on Nov. 10 with H.O.T. (hands-on training) workshops and the invitation - only Leadership Summit, featuring a kickoff session, welcome reception and dinner. On Nov. 11, attendees may also take part in “Lunch & Learn” seminars offering complimentary meals paired with expert insights.
In addition to informal meetups, the event will host exclusive receptions and the highly anticipated “Texas Throwdown” on Nov. 12: a spirited evening featuring live music, Texas-style BBQ, whiskey tasting and line dancing – complete with a build -your- own bandana bar.
Specialized H.O.T. workshops and Leadership Summit events require pre - registration and additional fees; early-bird pricing is available.
For full schedule details, speaker updates and registration information, visit MDExpoShow.com.
Enter the contest and help TechNation celebrate. Fill out the short form at 1technation.com/contest for a chance to win one of 12 prizes, each valued at $150 or more!
Additional entries to win can be acquired by sharing on LinkedIn or submitting a photo. Each month, a winner will be selected and featured in TechNation magazine! August winner is Emily Pleadwell.
Find out more information on Page 92.
A return to Wisconsin for an HTM Mixer supported by the Wisconsin Biomedical Association (WBA) attracted 133 attendees with 49 industry-specific companies showcasing their solutions in a busy exhibit hall.
“This is our third time teaming up with the Wisconsin Biomedical Association and each time keeps getting better and better. They are an exceptional organization to work with,” MD Publishing’s Kristin Leavoy said.
WBA members praised the HTM Mixer for its quality educational sessions, exhibit hall and networking events. It helped that the HTM Mixer was approved for 10 CEUs by the ACI.
WBA Secretary Mitch Von Ruden, who also serves as HTM manager at Mayo Clinic, provided a glowing review.
“The Mixer this year was really excellent, seemed to attract a lot of attendees and vendors. It was great to see and network with so many Women in Leadership (WIL) that came to the event and honestly enhanced the environment and discussion!” Von Ruden exclaimed.
“The amount of vendors at the vendor show was excellent! Great variety and multiple modalities represented,” he said and mentioned that rad, biomed and ultrasound were just some of the modalities represented.
“The HTM Mixer in Milwaukee was a great event, it was really well organized with an upbeat vibe. The food and drinks were excellent, the entertainment was fun, and the vendor show was impressive with lots of variety and chances to connect with people in the field,” Von Ruden said. “It was a perfect mix of networking and having a good time.”
In addition, Von Ruden was thankful for the HTM Mixer.
“I just want to thank John, Kristin, and the entire team at MD Expo for their support of the WBA and the entire HTM community,” he said.
Larry Fennigkoh, Ph.D., P.E., is a professor emeritus, biomedical engineering at the Milwaukee School of Engineering. He has attended several HTM Mixers and MD Expos. He said this HTM Mixer stood out because of its ability to generate excitement among biomeds.


“The Milwaukee HTM Mixer, again, provided another great value-added educational, emotionally therapeutic, and networking opportunity for regional biomeds,” Fennigkoh said. “Even though these events are a bit shorter in duration, they are no less valuable in serving and supporting our HTM community. Comparatively, this Mixer captured and preserved all the benefits of the others being just as good or better.”
“The usual and now ‘standard,’ even essential, pre-conference happy hour continues to provide a fantastic opportunity for attendees to connect and reconnect with their peers and old buds. The welcoming atmosphere, great food, and entertainment just sets the stage for a collaborative and learning-filled remainder of the conference,” he added.
Fennigkoh also mentioned the Women in Leadership and the group’s positive impact.
“The women in HTM session was very well attended and received,” he said. “Ann Rovito, her great panel, and especially, the openness of attendees was most enlightening but still sadly revealing on the struggles women continue to face. Nonetheless, the progress, growing numbers of women in HTM, and their contributions continues to be most encouraging. These sessions have been similarly popular and informing at other MD Expo and Mixer events and should be continued.”
Overall, Fennigkoh applauded the ability to consistently deliver quality HTM events.
“Having now attended a number of these Mixers, since their COVID beginnings, the consistent benefits are many and energy is always palpable. Not only do they also give a considerable boost to the co-sponsoring local or state biomed societies, they reach a number of biomeds who may be unable to attend the larger conferences,” he said. “Kudos to MD Publishing and the many consistent and incredibly supportive vendors for making these events happen. The entire HTM community benefits and continues to evolve because of these events.”
See HTM Mixer Wisconsin photos on page 86.




Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking.
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering. This helps maximize uptime and minimize repair costs.
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering. This helps maximize uptime and minimize repair costs.


VOTING ONLINE OPEN THROUGH JANUARY
SCAN TO VOTE
New England Society of Clinical Engineering (NESCE)
HTMA-Ohio
SPONSORED BY

CABMET: Colorado Association of Biomedical Equipment Technicians
California Medical Instrumentation Association (CMIA)
Wisconsin Biomed Association (WBA)
SPONSORED BY

SPONSORED BY

SPONSORED BY
Kevin Curtiss, biomedical equipment specialist III, Renovo Solutions
Jennifer Stilley, biomedical supervisor, Renovo Solutions
Brandi Caton, supervisor of clinical engineering, Trimedx
Eric Massey, regional director, Intelas
Ryan Gonzalez, director, cybersecurity partnerships, Trimedx
James Swandol, manager HTM, Baylor Scott & White
Andrea Brainard, senior director HTM, Children’s Health
Lynne Holland, director HTM, HonorHealth
Daniel Adams, director, Renovo
Bryant Hawkins Sr., site manager, Trimedx
Allison Woolford, clinical engineering operations manager, Duke University Health System
Chris Avila, intern, Eskenazi Health
Jose Zambrano, HTM service delivery manager, Kaiser Permanente
Mayra Becerra, clinical engineering manager, University of Miami Health System
Jamal Guio, biomedical equipment technician III, UT Southwestern Medical Center of the year
Neisa Massey, clinical engineering, periop team lead, Duke University Hospital
Arleen Thukral, VISN 2 healthcare technology manager, U.S. Department of Veterans Affairs of the year of the year of the year of the year
SPONSORED BY

Erika Colombe, biomedical engineering technologist, TRH Services
Jason Garcia, system manager biomedical engineering, HonorHealth
Nathan Manning, senior site manager, Trimedx
SPONSORED BY of the year

Luisa Howell, Air Force, biomedical equipment technician, BMET Instructor, METC BMET training program
Lynnsei Leach, Army, biomedical equipment support specialist, Memphis VA Medical Center
Chris Lopez, Navy, biomedical equipment technician, BMET instructor, METC BMET training program
Greg Palmer, Army, biomedical equipment support specialist, information systems, VA Heart of Texas Healthcare Network
Adrese Atkins, Air Force, 931st Aerospace Medical Squadron medical administration
Pronk Technologies
MW Imaging
J2S Medical
PartsSource
Prescott’s
SPONSORED BY of the year
Steve Cannon
Ed Sloan
Ray Dalton
Mike Busdicker
Sheila O’Donnell
SPONSORED BY


Justin Barbour, Better Biomed Channel
Jennifer Chester, NextJenn TechMom, creator of “Bella the BMET”
Jewel Newell, co-founder I-HTM
Chace Torres, Bearded Biomed, Ohms Law Foundation
April Lebo, president, Women In Leadership (WIL)
award
SPONSORED BY

Jessica Hudson-Rahming, deputy chief healthcare technology manager, U.S. Department of Veterans Affairs
Lisa Fry, region vice president, Intelas Health
Danielle McGeary, vice president HTM, AAMI
Rachel Yarnevic, vice president operations, western region, Renovo Solutions
Lani Bartkowiak, HTM program manager, ProHeath

James Linton, professor & program coordinatorbiomedical engineering, St. Clair College
Todd Boyland, CEO RSTI training, Radiological Service Training Institute (RSTI)
Janet Thomason, director of national procurement Project C.U.R.E.
Clement N.Y.A Appiah Anokye, medical capacity building program partner, Mercy Ships
Deborah Nutsugah, biomedical technician, Mercy Ships
Brian Gore, EP engineering specialist, cardiovascular services, Duke University Health System
Jeff Ruiz, Miles for Myles, senior site manager, Trimedx
James “Jim” Rickner, director of service and training, Advanced Ultrasound Systems
Anntonett Gillins, senior HTM adjunct instructor, College of Biomedical Equipment Technology
Joie N. Marhefka, biomedical engineering technology faculty, Penn State New Kensington
Phoebe Putney Memorial Hospital
Biomedical Engineering Team
Clinical Engineering at University of Kentucky Healthcare
AHS Morristown Medical Center
Biomedical Engineering Team
Sentara Clinical Engineering Department
VISN 2 New York/New Jersey VA
Healthcare Network HTM Department
Memorial Hermann Northeast Hospital Clinical Engineering Department
ProHealth Waukesha Memorial Hospital Biomedical Engineering Department
Western North Carolina VA Health Care
System Biomedical Engineering Department
Reid Health Clinical Engineering Team
Rochester Regional Health’s St. Lawrence Region Biomedical Services Department
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
General Anesthetic Services Inc. is the world’s leading provider of anesthetic vaporizer service and calibration. With over four decades of specialized expertise, we deliver unmatched precision, regulatory compliance, and operational reliability to healthcare institutions and medical device manufacturers across the globe.
In addition to vaporizer calibration, our expanded capabilities include:
• Medical device assembly, tailored to OEM specifications and quality system requirements
• Secure device storage, supporting inventory control, fleet readiness, and logistical efficiency

Whether supporting hospitals, surgical centers, or device innovators, G.A.S. ensures that critical equipment is maintained, assembled, and stored to the highest standards—so our clients can focus on patient care and innovation.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
What sets General Anesthetic Services Inc. apart is our uncompromising commitment to quality, responsiveness, and technical excellence.
• We operate under the highest standards—ISO 13485:2016 and FDA 21 CFR Part 820—ensuring full regulatory compliance across every service touchpoint.
• Our customer service is unmatched: no voicemails, no call centers—just a knowledgeable staff member every time you call.
• With unparalleled expertise in anesthetic vaporizers, we offer industry-leading preventative maintenance programs that reduce downtime and eliminate the need for reactive, corrective repairs.
• G.A.S. maintains the largest vaporizer inventory in the industry, enabling a seamless service/exchange program through our depot repair model—so your facility is never without a vaporizer.
• Every service is backed by our warranty guarantee, reinforcing our commitment to reliability and long-term partnership.
• We don’t just meet expectations—we redefine them.
Q: WHY DID YOU CHOOSE TECHNATION FOR ADVERTISING?
We chose to advertise in TechNation because its audience aligns directly with our mission. General Anesthetic Services Inc. operates at the heart of the biomedical HTM space, specializing in preventative maintenance, service, and supply of anesthetic vaporizers and medical equipment.
TechNation reaches clinical and biomedical engineers who are actively seeking reliable, compliant, and responsive solutions to equipment maintenance challenges. These professionals understand the value of uptime, regulatory alignment, and technical precision—exactly what G.A.S. delivers through our depot exchange model, ISO 13485:2016 systems, and FDA-compliant service programs.
By partnering with TechNation, we’re not just advertising—we’re connecting with the very professionals who rely on our expertise to keep operating rooms safe, efficient, and compliant.
For more information, visit generalanestheticservices.com.
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?

Phoenix Data Systems offers a unified yet flexible approach to healthcare technology management. Celebrating its 40th anniversary this year, Phoenix brings over 300 years of combined direct industry experience. AIMS 3 represents the culmination of that expertise, providing technician-focused usability, operational efficiency, and built-in automation that streamlines tasks, reduces manual effort, ensures complete data capture, and presents essential information at a glance for faster, more effective job execution.
Through its three partners - AIMS 3, Superior Analytics, and HTM Consulting - Phoenix provides solutions that can function independently or together. Superior Analytics ensures data accuracy, normalization, and performance benchmarking, turning information into actionable insights, while HTM Consulting offers management solutions that guide strategy and optimize operations. This integrated approach enables organizations to improve operational efficiency, maintain high-quality data, and achieve long-term strategic outcomes.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?

Phoenix Data Systems, Inc. partners with healthcare organizations through three specialized offerings that deliver complementary solutions for healthcare technology management. AIMS 3 provides leading CMMS software to streamline asset management and operational workflows; Superior Analytics focuses on data cleaning, normalization, and performance benchmarking to ensure actionable insights; and HTM Consulting offers management solutions to guide strategic decision-making and optimize operations. Each company delivers significant standalone value, but together they form a powerful, integrated partnership—helping healthcare organizations overcome operational challenges, improve data integrity, and achieve measurable excellence. It’s the Power of Three: Powerful Alone. Transformative Together.
Q: WHY DID YOU CHOOSE TECHNATION FOR ADVERTISING?
We chose to advertise with TechNation because it is the premier monthly publication and essential resource for over 12,000 medical equipment service professionals. TechNation provides in-depth coverage of healthcare technology management, regulatory updates, and industry trends, making it a trusted source of information in the field. Its authoritative voice and extensive reach ensure that our message is seen by a highly engaged and relevant audience, aligning with our commitment to support the healthcare technology community.
For more information, visit goaims.com.
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
Quality services - We provide end to end services for Nuclear Medicine equipment to support our customers goals and requirements for patient care. We also help customers with camera maintenance, repair, crystal replacement, and detector reburns.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
Experience – particularly with nuclear medicine imaging equipment at end of life who’s use needs to continue to be maintained and can help avoid and/or delay acquisition of costly newer equipment. We support nuclear equipment wherever you are – hospitals, clients and medical centers throughout the US.
Q: WHY DID YOU CHOOSE TECHNATION FOR ADVERTISING?
TechNation’s focus is communicating with medical imaging professionals who make up a large portion of our customer base.
For more information, visit nuclearcameraservices.com.


OUR AD ON PAGE


Kevin Davis, Owner
Q: WHAT PRODUCT, SERVICE, OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
ERD Medical Equipment Solutions is a full-service biomedical organization supporting clinics, surgery centers, and hospitals across the western U.S. We provide preventive maintenance, repair, and ancillary staffing under an ISO 9001:2015 quality management system. While we support a broad range of clinical equipment, our specialization is in three high-impact areas: surgical lights/booms and integration systems, surgical tables, and sterile processing equipment such as sterilizers and washers. By focusing on these mission-critical systems, we help organizations maximize uptime, control capital spending, and remain safe, compliant, and survey-ready.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
ERD stands out for combining deep technical expertise with a responsive, partnership-driven approach. We stay personal and agile, tailoring our services to each client’s needs while maintaining the highest quality standards. We also help health systems safely extend the useful life of mature and end-of-life equipment through disciplined maintenance, legacy parts sourcing, and risk-based assessments — enabling them to direct capital dollars to higher-priority clinical needs. Beyond service, we are deeply committed to workforce development, helping train the next generation of biomeds through hands-on education and industry engagement.
Q: WHY DID YOU CHOOSE TECHNATION FOR ADVERTISING?
TechNation reaches the core of the HTM community — biomedical professionals who are often stretched thin as they balance growing device inventories, expanding facilities, and compliance requirements. These are the professionals we partner with to deliver reliable support. Advertising here allows us to connect directly with biomeds and healthcare organizations who benefit most from having a trusted, quality-focused partner like ERD at their side.
For more information, visit erd-us.com.
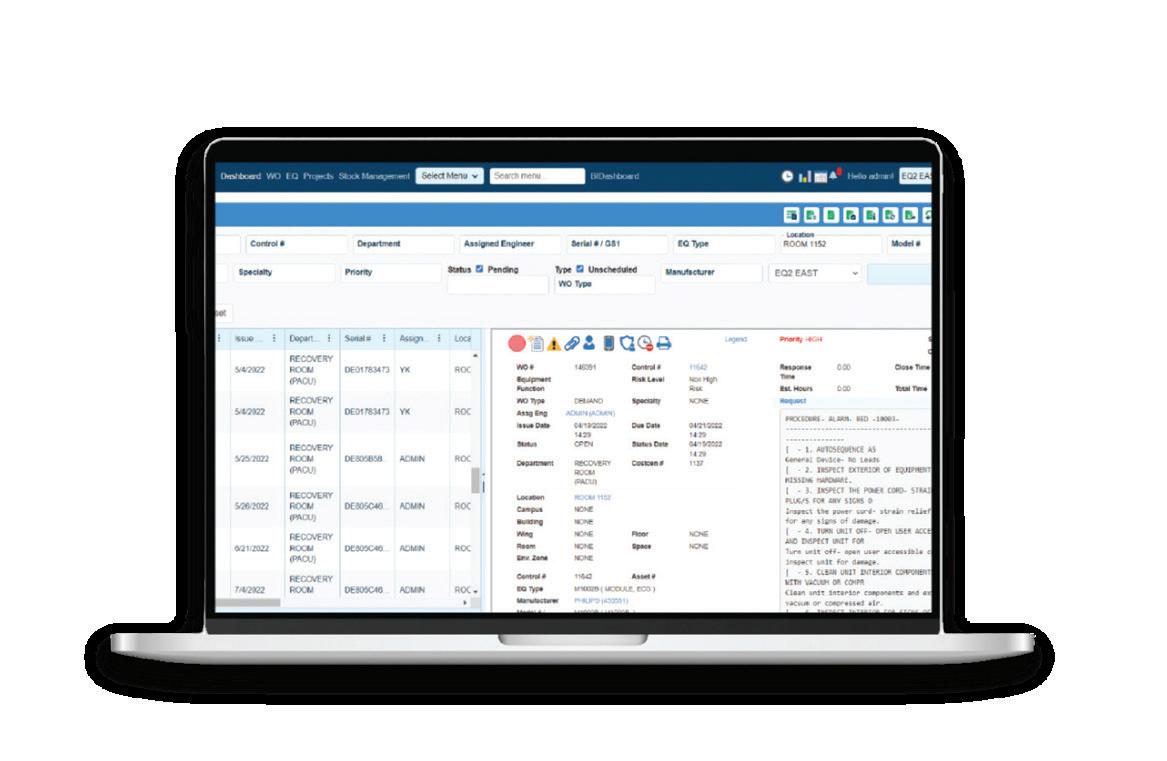
“EQ2
HEMS by TMA Systems is designed specifically for healthcare and the unique needs of HTM....(with) specialized workflows for managing medical equipment and advanced compliance tools and reports, including AEM program management and everything needed to be ready for the next survey.ˮ
Rich Sable, Manager of
Client Success





interdependence or independence are designed for Our offerings
®
• AIMS 3
Keep technicians in the field,
• not in the software
Give managers tools to manage
• departments effectively
Automate routine tasks and
streamline communication
analytics ®
• Superior Analytics
• wide perspectives -industry internal & effectiveness with Improve efficiency and
• foundation of data and deploy based on a solid Strategically plan, purchase,
Standardize & normalize
device definitions, codes,
• HTM Consulting categories, & documentation
Monitor KPIs to track and
• inventory, & contracts labor & budget reporting, Optimize processes for
• productivity, & visibility improve accountability,
Define and deploy targeted
customer surveys for
improvement


At ERD, biomedical technicians bring the skill sets and scheduling flexibility healthcare facilities need to supplement and support the clinical engineering staff. ERD’s biomedical technicians are factory-trained and certified to work on equipment from all leading medical device manufacturers. Healthcare facilities can trust that each item will be tested with the utmost attention to detail. ERD technicians are able to work on a wide variety of equipment including tables, monitors, ESUs, lights and sterilizers.
ERD Owner Kevin Davis recently shared more information about the company and its services.
Q: WHAT ARE SOME OF THE SERVICES AND PRODUCTS YOU OFFER?
A: ERD provides full-scope biomedical support across the western U.S. We specialize in surgical suites and sterile processing – supporting washers, sterilizers, surgical lights/ booms, and surgical tables – and we also offer select medical device sales (including MAVIG). Beyond our specialties, we provide preventive maintenance, corrective maintenance, incoming acceptance checks, compliance documentation, and field service staffing. Lately, we’re excited about our expanded OR/SPD safety assessment program focused on ceiling-mounted equipment and sterility workflows; it pairs hands-on inspections with clear, audit-ready reporting for AAAHC/TJC/DNV/CMS environments.
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
A: ERD stands out by pairing an ISO 9001:2015 quality system with deep OR/SPD specialization (washers, sterilizers, surgical tables and ceiling-mounted lights/booms). Our team leans into the high-impact, high-uptime areas – surgical suites and SPD – where responsiveness, documentation and clear communication matter most. We are vendor-neu-
tral, and pride ourselves on safely extending the useful life of mature/end-of-life systems through disciplined maintenance, legacy parts sourcing and risk-based assessments. This enables health systems to direct capital dollars to other higher-priority clinical needs. Customers report that they value our audit-ready documentation, transparent, timely communication, and an education-first approach that helps teams stay survey-ready while maximizing uptime.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
A: Near term, we’re expanding throughout the West – with growth in Northern & Southern Nevada and Northern, Central, and Southern California, plus project work in Idaho, Colorado and Arizona. Our dedicated efforts enable us to provide reliable, local support to ASCs and health systems. We’re deepening our OR/SPD capabilities (lights/booms, sterilizers, washers, surgical tables) and continuing to mature our ISO 9001:2015 program and safety initiatives, including broader rollout of our OR/SPD Safety & Uptime Assessment.
We’re also laying groundwork for an ERD education & training program aimed at preparing the next generation of HTM technicians – more to share soon. Through our advanced, polished facilities like the ones in Carson City and Sacramento, we aim to provide training for ready-to-hire future biomedical technicians. At our core, we believe that education is power and we live out that mission every day. We are on the brink of exposing ready individuals to a career that is entirely rewarding and in-demand.
A: ERD’s mission is simple: be the trusted partner that keeps critical equipment safe, compliant and ready for care. If your team needs reliable PMs, clean documentation, or experienced support in the OR or SPD, we’d love to collaborate –whether that’s a one-time assessment or ongoing service. I pride myself in creating a company that is approachable for clients, collaborative in creating opportunities that support customers and, most importantly, passionate about not only our company but supporting the growth of healthcare technology management.
For more information, visit erd-us.com.




BY MARGARET KOGA WARD & MIKE ZIEMELIS
The Food and Drug Administration (FDA) has issued a final rule to amend the requirements of the existing Quality System (QS) Regulation, 21 CFR 820 (Part 820). The amended Part 820 will take effect February 3, 2026, and will be called the Quality Management System Regulation or QMSR.
QMSR incorporates by reference ISO 13485:2016, the well-established, international, quantity management system standard for medical devices. ISO 13485 is used internationally by many regulatory authorities and forms the basis for the Medical Device Single Audit Program (MDSAP). For those unfamiliar with the legal terminology, to “incorporate by reference” means to include a second document within another, in this instance, ISO 13485 will be part of the Part 820 regulation, as if it were fully written out.
FDA will begin to enforce the QMSR requirements upon the effective date, February 2, 2026. Until then, all manufacturers are required to meet the current Part 820 requirements (the QS Reg).
For many years the FDA has recognized the value of global harmonization and has been an active participant in programs to drive harmonization, such as international standard committees (e.g., ISO), the Global Harmonization Task Force (GHTF), and the International Medical Device Regulatory Forum (IMDRF), a voluntary group of medical device regulators from around the world who work together to harmonize medical device regulations.
When ISO 13485:2016 was released, many medical device professionals were surprised by the similarities with the existing FDA QS Regulation, however, ISO 13485 reflected almost 20 years of progress, and placed additional emphasis on risk management and risk-based decisions throughout a
manufacturer’s quality management system (QMS).
In amending the Part 820 regulations, the FDA reviewed ISO 13485 and stated:
“We have determined that the requirements in ISO 13485 are, when taken in totality, substantially similar to the requirements of the QS regulation, providing a similar level of assurance in a firm’s quality management system and ability to consistently manufacture devices that are safe and effective and otherwise in compliance with the Federal Food, Drug, and Cosmetic Act (FD&C Act).”
Industry professionals must not assume that the QMSR is the same as 13485. Although FDA has incorporated 13485 by reference, they have also published additional FDA requirements that organizations must meet. Many of these requirements are listed in QMSR Part 820.10.
The QMSR is available on the FDA website. The layout of the QMSR differs from the existing QS Reg:
• The first section, titled “Supplemental Information” contains the majority of the information in the QMSR, but not the actual regulations. Valuable information is contained within this section, notably the following:
• Executive Summary (a summary of the major provisions of the Final Rule).
• Table of Abbreviations and Acronyms.
• Background (why the change).
• Comments on the Proposed Rule and FDA’s Responses (the preamble).
• Effective Date and Implementation Strategy.
To gain clarity on the FDA’s thoughts on the 13485 requirements, expectations for your Quality Management System (QMS) including risk management read the preamble (Section V. Comments on the Proposed Rule and FDA’s Responses).
FDA received feedback when the proposed rule was published. The preamble lists the comments, and the FDA response. Although the preamble is not enforceable, it clearly outlines FDA’s expectations. For example:
• What are the FDA’s expectations for a culture of quality within
your organization? Refer to comments 27 and 55.
• What are FDA’s expectations for risk management in the QMS? Refer to comment 19 and 32.
• 13485 does not require an independent reviewer in design and development reviews (13485 Clause 7.3.5). FDA discusses their expectations in their response to comment 46.
• 13485 Clause 8.2.2, Complaint handling, does not require a formally designated complaint unit. The FDA discusses their expectations in their response to comment 54.
The word “risk” appears in the QS Reg once under section 820.30(g) “Design Validation shall include software validation and risk analysis, where appropriate.” Compare this to the work risk(s) appearing in the 2016 version of 13485 over 25 times. FDA’s expectations for risk management are now codified in 13485 and are expected to be integrated throughout the quality system. As an example, Section 4.1.2 states “The organization shall … apply a risk based approach to the control of the appropriate processes needed for the quality management system.”
While the mentioning of risk differs between the QS Reg and the 13485 standard, the FDA’s expectation that risk is applied throughout the quality system was explicitly stated in the preamble to the existing QS Reg, and in FDA guidance documents. Having the QMSR incorporate by reference the 13485 standard now explicitly states the application of risk management throughout an organization’s QMS.
The actual regulations for combination products and medical devices are located (or referenced) towards the end of the Final Rule.
21 CFR Part 4 – Regulation of Combination Products
21 CFR Part 4 – Regulations of Combination Products: The FDA has made conforming edits to Part 4 to clarify the device QMS requirements for combination products. (For additional information refer to Supplementary Information section III. Background, C. FDA’s Current Regulatory Framework)
Part 820 – Quality Management System Regulation
Here is where you will find the amended Part 820 regulations for medical devices. There are two Subparts: The General Provisions and the Supplemental Provisions.
This section includes:
• The scope of the regulation (essentially unchanged from the QS Reg)
• The definitions – Pay attention to the definitions! The QMSR also incorporates by reference Clause 3 of ISO 9000:2015 as this is a normative reference for definitions used in 13485. However, the QMSR has a definition hierarchy as follows:
1. A definition from the FD&C Act always takes precedence (e.g., manufacturer).
2. A definition from Part 820.3 of the QMSR (these are listed in the Final Rule, for example, the term “Component” is defined in the QMSR).
3. A definition from ISO 13485:2016.
4. A definition from ISO 9000:2015.
• Incorporation by reference: This section contains the statement that the QMSR is incorporating by reference
ISO 13485:2016, and ISO 9000:2015 Clause 3 (definitions). Note that the actual text of these standards is not included, however, this section contains information for accessing the information (although most organizations have purchased these two standards)
• Requirements for a quality management system: This is a very important section as the QMSR includes more than ISO 13485, and an organization must meet additional FDA regulations. This part clarifies some of the additional regulatory requirements! Remember, ISO 13485 is an international standard, and includes many statements such as “in accordance with applicable regulatory requirements,” when you see that term, please refer to Part 820.10 of the QMSR for guidance on the meeting the applicable FDA regulatory requirements.
FDA determined that some additional regulations are required, these regulations go above and beyond 13485:
• Part 820.35 Control of records: Includes the additional information required for complaint records, service records, unique device identification (UDI), and information for marking documents as confidential.
• Part 820.45 Device labeling and packaging controls. Contains numerous requirements for packaging and labeling, many of which have been carried over from the QS Reg.
The transition date is approaching fast, and a gap analysis should be performed to ensure your organization will be compliant!
FDA will have a new inspection process From FDA’s website: “FDA will develop a new inspection process to align with the requirements of the new QMSR. The process will be developed for implementation when the rule takes effect.” This comes from the Quality Management System Regulation Frequently Asked Questions page that was last updated on August 7, 2024.
FDA will phase out the Quality System Inspection Technique (QSIT) once the QMSR is fully implemented and replace it with a new inspection program. Additionally, the MDSAP Audit model will be updated but is expected to be minimally impacted because the MDSAP program was already aligned with the requirements within 13485.
AAMI has several training courses that can be taken to keep you up to date on the new requirements:
• AAMI Expert Insights: Transitioning to the FDA Quality Management System Regulation
• AAMI Quality System and Risk Management Courses.
This entry for AAMI’s new Insights into Innovation series is authored by Margaret Koga Ward, the principal engineer and owner of Teragram Consulting, and Mike Ziemelis, the Vice President of R&D for Hologic. Their insights and opinions are their own and do not represent the views of AAMI or AAMI Foundation.

ECRI assessed evidence surrounding single-use versus reprocessed medical devices in hospitals.
One way healthcare providers attempt to reduce hospital-acquired infections (HAIs) is to discard medical devices following use rather than disinfect and reuse them – but this practice carries significant cost and environmental implications. ECRI, a global healthcare and patient safety nonprofit, released a series of assessments analyzing the evidence surrounding single-use versus reused or reprocessed medical devices in hospital settings.
The research was conducted by the ECRI-Penn Evidence-based Practice Center and involved a systematic literature review of more than 2,000 studies, primarily focused on devices used for HAI prevention such as hospital gowns, and bedside care devices, including laryngoscopes. The research also includes other types of scopes and surgical items.
Each assessment report includes a chart that indicates whether the available evidence supports single use, reuse, or is inconclusive – across clinical, economic, and environmental categories. The findings emphasize that local factors such as infection rates, supplier agreements, reuse frequency, and reprocessing methods influence outcomes and should be considered.
• Clinical impact: Clinical outcomes sometimes favored single-use disposable devices depending on the specific device, particularly for infection rates.
• Economic considerations: Economic analyses of single-use versus reusable devices gave mixed findings, though in most categories, reusable devices are less costly.
• Environmental footprint: Environmental impact evidence often favored reused or reprocessed devices, as disposable devices are more harmful for the environment.
While single-use devices are often favored for infection prevention, the evidence supporting this is not consistent across all device types, and reusable options often present economic and environmental advantages. The research highlights the complex tradeoffs between clinical outcomes, economic costs, and environmental impact when choosing between single-use disposable and reusable or reprocessed medical devices.
“There’s no one-size-fits-all solution when it comes to choosing between single-use and reusable medical devices,” says ECRI’s Director of Clinical Evidence Evan LeGault. “These assessments are designed to support hospitals, supply chain professionals, infection control teams, and sustainability officers in weighing the tradeoffs and identifying what works best for their organization.”
ECRI searched for primary studies and systematic reviews comparing single-use and reused or reprocessed medical devices for in-hospital use. The systematic literature search captured publications from 1/1/2010 to 1/6/2025 and identified 2,133 citations to screen, ultimately yielding 48 studies in 21 device categories.
Substantial gaps exist in the current evidence base, with clinical outcomes and economic costs reported far less frequently than environmental impact, and most of the 21 device categories were examined by few studies. In the absence of strong direction from clinical or economic evidence, environmental impacts may become a deciding factor.
This study highlights the need for further research as well as increased transparency from manufacturers, reprocessing companies, and healthcare institutions.
Download the full research reports from the ECRI Newsroom at www.ECRI.org/News.





BY ELYSSA EL-HAJJ
enerative artificial intelligence (Gen AI) is becoming increasingly more common and normalized. Surveys indicate up to 40% of the nation’s workforce currently utilizes AI. This number will only increase as more organizations shift to embracing Gen AI. This is how to become more efficient and save time and energy.
Everyone experiences the repetitive or routine tasks that happen day to day. They take time away from larger or more complex projects. Things like filling in paperwork, summarizing meeting notes, and looking up information can easily be streamlined using Gen AI. There are infinite ways of implementing AI in the workplace. My concern with the never-ending possibilities of AI is that we won’t slow down to consider issues like misinformation, privacy violations and inauthenticity.
To understand the pitfalls of Gen AI, first we need to understand how the model is built and where all the information comes from. Gen AI functions based on
the prompts from end users. Then, the technology will source answers from all the data it has access to, which is a lot both in sheer amount and in range of reliability. AI models train on sources like public data sets that pull from books, scientific journals, news sites, and free image libraries – all the avenues one might look at for information. However, Gen AI also trains on sources like user-generated content (UGC), pulling from social media, forums, and other sites where there is little oversight into what is uploaded. Here is where we run into issues. How often does one come across misinformation or opposing opinions while scrolling through the web? I could go and change an entry on Wikipedia right now, that won’t make it accurate or even remotely true, but Gen AI will still take it into their training model. This is a crack in the system where inaccurate information can be the output.
AI trains on the information it’s given, and that can be a problem. Also, the risk of violating privacy is concerning. Consider the example of an individual generating a report internal to the organization. To save some time, they decide to input the report into a Gen AI to create a summary. That internal report could have contained information that wasn’t made public. It may even contain private identification data on a
worker or customer. All that data is now available to AI and can be used in future data sets.
Misinformation and privacy violations are serious outcomes while using Gen AI but don’t happen every day. What does happen when using Gen AI daily? Most concerningly is the loss of authenticity and personal voice. While Gen AI can easily be used to fix grammatical errors, it is just as easy to generate a response to an email or a direct message. When the reply is a simple “Thank you!” or “I will have [task] done by the end of the day.” there is no issue. However, when responding to a customer concern, an AI generated response might fall flat on providing a personal touch. This will affect the quality of services and solutions. Much of customer service is being able to communicate with the individual in a way they understand and feel heard.
AI currently does not have the same context on the individual as the service team does, especially if there is a previously established connection. A service team member is better equipped to explain a technical issue in a way that the customer can understand compared to an AI. Its output can be overly complicated and not specified to the issue at hand. Building a connected and collaborative organization cannot be done with 100% AI generated emails being sent back and forth. This lack of sincerity also extends to connecting with the public. News sites and article publishers have noted that pieces generated by AI receive significantly less viewership. Gen AI can scrub your voice from the dialogue, flattening the tone and taking away unique expressions that are specific to the individual. Gen AI has the ability to sound human at times, but it cannot replace the human touch in writing. That is what generates emotion, tone, and storytelling which makes even scientific journals more interesting to read.
Don’t get me wrong, I use AI, in fact I used it to assist in the creation of this article. However, I’ve avoided the pitfalls. See, Gen AI is developing new abilities every day but understand that Gen AI does not replace all the functions of an individual. Use this new technology. Use it while placing an emphasis on education of its use. Discuss its limitations and consider what data sets are being used. See if there are any privacy safeguards put in place. Treat Gen AI as a first draft and fact check your information. Always edit the output to fit the needs and preserve your voice to increase authenticity. Everyone is adapting to the new age of AI, pushing us to higher levels of productivity at lower effort. It is exciting to see how the tool develops; however, it is still just a tool. As such, please consider the previous advice on making AI a guide, not a crutch in doing office work. Your co-workers and customers will all see the difference if it is used correctly.
For something fun to think about, can you tell where AI was utilized in this article? If you can’t, that was the point. Challenge yourself to work in synchronization with AI.

Elyssa El-Hajj is a biomedical engineer with VA North Texas.

Finally, the features you love most about your favorite ride share app, food delivery app, Angi, UpWork, or TaskRabbit are available for medical imaging equipment maintenance and repair.
Enter a Trace Ticket with one tap to broadcast your repair needs to a network of qualified technicians.
Review Bids to find the best service option for improved repair outcomes with less equipment down time, resulting in a lower overall cost.
Track progress, issue payments and rate services all in a single dashboard.

Speed
Instantly blast your service request to every qualified and vetted service provider

From response times to uptime, you no longer have to rely on service companies to track their own activities and performance


Like your favorite personal apps, get in-app alerts when a service technician is on their way, arrived, waiting for a part, completed a job, etc.
Competitive bidding among service technicians allows you to get the best price and only pay and track one entity



Registration on WebinarWednesday.live. Eligible for 1 CE credit from the ACI.
Downtime Trace and SkillNet are partnering to improve speed, lower pricing, and provide standardization of communication to health care customers and medical equipment service providers. Together, they solve one of the industry’s biggest challenges: matching the right talent to the right need, at the right time.
Downtime Trace delivers an on-demand marketplace that connects hospitals with skilled technicians in moments of urgent downtime. Health systems can immediately locate skilled professionals to keep equipment online and patient care from being interrupted. SkillNet provides the Downtime Trace marketplace with accurate, validated, and standardized transcripts that capture a service technician’s training, certifications, and on-the-job experience in one portable
record. By creating a common language of skills, SkillNet ensures hospitals know exactly what each technician is capable of.
• Speed: Hospitals engage qualified service companies and technicians within minutes
• Trust: Skills are transparent, validated, and consistent across the industry
• Efficiency: Less time spent on paperwork, more time solving other downtime challenges
• Opportunity: Professionals gain visibility and recognition for their expertise
Technology is reshaping how healthcare organizations manage assets, space, and operations. From CMMS integrations to emerging AI capabilities –tech advancements are empowering healthcare maintenance teams with more ways to streamline workflows and leverage operational data.
The recent Webinar Wednesday session “Smart Tools for Smart Operations: AI and Technology in HTM/ HFM” sponsored by FSI, is eligible for 1 CEU from the ACI.
Joe Stockman, director of product experience (FSI), and Justin Ryan, product owner, CMS Analytics (Enstoa), explored the current state of AI and technology in healthcare maintenance management. They also walked attendees through real-world HTM and HFM use cases, and illustrated how integrations and intelligent tools support streamlined workflows across key operational areas. Attendees also heard insights about how the industry may evolve as technology continues to advance, and how to prepare for what’s next. A question-and-answer session generated additional insights for attendees.
“Always great to keep up to date with what’s going on!” said Mike Smit, senior biomedical engineering technologist-renal, Thunder Bay Regional Health Sciences Centre.
“To stay up on the HTM standards,” said Alec Hadley, a field service manager with ReNew Biomedical.
“It has career specific stories and information,” said Bridgett Moshier, a BMET apprentice at Holmes Regional Medical Center.
“To keep informed of upcoming and/or existing policies, software, and devices relevant to the biomedical career field,” said Alan Nicewarner, CBET, BSWH McKinney.

All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
The Webinar Wednesday Tools of the Trade Live Demo “AAMI’s New CEU Journal and The Importance of ACI Certification” sponsored by AAMI is eligible for 1 CE credit from the ACI.
AAMI Vice President of Healthcare Technology Management Danielle McGeary, CHTM, PMP, delivered an informative presentation and answered questions from attendees during the hour-long webinar. A recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
She provided detailed insight into the value and impact of earning ACI certifications, including the qualifications, expectations and long-term benefits of maintaining certifications. ACI certification recognizes healthcare technology management professionals who demonstrate advanced knowledge of medical devices and clinical practices, along with strong technical and problem-solving skills critical to the maintenance and repair of healthcare technology.
In addition to exploring the professional advantages of certification, this session introduced AAMI’s new CEU journal and tracking process, which now allows certified individuals to update and manage their CEUs in real time. This improvement is designed to streamline recertification and make it easier than ever to stay on top of your professional development.
The webinar was extremely popular with the most registrations and attendees for a webinar since 2023.
Frank Nichols, a biomed manager at Piedmont Medical Center in South Carolina, won a Swiss Force Meister Multi-Tool for correctly answering a trivia question during the webinar.
Attendees provided feedback via a survey that included the question, “What was your single biggest takeaway from today’s product demo?”
“Presenters’ depth of knowledge,” said Robert Smothers, a field service engineer with HSS Inc.
“Good understanding of the process of re-certification and how to complete my CE credits,” said William Roberts, a biomedical technician with Samaritan’s Purse.
“The different educational sources available,” said Joseph Cianciolo, a BESS with the VA Connecticut Healthcare System.
“Refresher on the ACI certifications available, study courses and upcoming testing window,” said Joseph Trujillo, a biomedical engineering manager at Hoag Hospital Newport Beach.
“Good to know the best practice to update my CEUs and really helped guide me,” said Matt Steele, owner of GMAK Mechanical and Biomedical Solutions.
Watch these webinars on-demand
The recent Webinar Wednesday presentation “Laser Safety in Healthcare Technology: Compliance, Maintenance and Risk Mitigation” was presented by Vangie Dennis, MSN, RN, CNOR, CMLSO, FAORN, Perioperative Consulting LLC, and Patti Owens, MHA, BSN, RN, CMLSO, CNOR, AestheticMed Consulting International LLC.
The subject matter experts discussed the need for a Laser Safety Officer (LSO). The webinar covered guidelines, standards, and procedures for safe laser use in medical settings, as well as defined the roles and responsibilities of an LSO.
The webinar clarified whether an LSO is required based on specific guidelines and standards for laser use. The presenters, with expertise in laser safety, addressed the crucial aspects of laser safety, including biophysics, tissue interaction and safety protocols.
The session also included a question-and-answer session. The presenters provided additional insights via thoughtful answers to questions from attendees.
The webinar was well attended with 67 logged in for the live presentation. A recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
Attendees provided positive feedback regarding the webinar via a survey. They were also asked, “Why do you read TechNation magazine?”
“Keeps me current in what is going on in the industry,” said Michael Mele, ACTM, Kaiser Permanente
“It is the leading magazine in our HTM community. There are various articles every month such as cover story, roundtable, department of the month and professional of the month, and so on. There are tons of good articles, thus I read it every month,” said Tedd Koh, MET, Olive View UCLA Medical Center.
“TechNation is informative and current with healthcare topics in the biomedical world as well as other medical areas. I enjoy reading the articles and it makes me feel that I am part of a larger group than just where I work,” said Brett Hayes, CBET, Ochsner LaFayette General Orthopedic.
“To keep up with today’s technology and to keep my CBET certification,” said Gerald McNeil, MBET III, St Joseph’s Hospital.


MedWrench is an online resource for medical equipment professionals (biomeds, HTMs, imaging professionals, etc...) to engage with their peers about medical equipment repairs, source parts and locate a service company. The following are examples of how the MedWrench community members help each other in the website’s forums.
Q: One of my wall mounted belray Belmont 096 x rays is displaying an error code (e.13). Despite the service, install and user manuals being online, I am unable to find e.13 described in those manuals.
A: Manuals floating around online don’t actually list E.13, which makes it confusing. On the Belray 096, error codes in the “E.1x” range are almost always tied to vertical or arm motion faults rather than exposure. For E.13 specifically, it’s typically a limit detection / movement obstruction error. So it could be:
• A limit switch isn’t being seen by the control board,
• There’s physical resistance (arm binding, cable tension, wall mount alignment), or
• The encoder/position sensor isn’t reporting back within expected values.
What you can try onsite:
• Power cycle the unit
• Check if any movement (up/down, in/out) is stiffer than normal
• Inspect the limit switches and wiring harness at the joints If the error clears temporarily, it’s likely a failing position sensor or sticky limit switch.
Q: How do you remove the power cord connection from the back of the Sigma Baxter infusion pump I unscrewed one screw but there are two tiny screws like stubs. Also, how do you remove that so I can change out the power cord?
A: Yes, you need to remove those tiny screws. They take a T6 driver. Take both those off and you should be able to slide the metal clamp off. I bought a cheap set of torx drivers off amazon specifically for these pumps.
Good Luck!
Q: There’s a piercing noise coming out from my hospital bed but it’s working properly when I plug it back on. What might be causing this noise?
A: Is the piercing noise all the time? When you move a section, if so which section. Is ti only up or down or both?
A: That noise could be an alarm from the bed’s control system, often caused by a loose connection, stuck button, or motor strain. If unplugging and plugging back in stops it, it might be resetting the fault. Check cables, connections, and if a specific movement triggers it. If it continues, refer to the manual’s alarm codes or have maintenance inspect it.
For more FREE forum information, visit medwrench.com/forums.






Infusion Pump Support
Fixed or Flat Rate Repair Options on Many Popular Pumps
AIV-Manufactured Replacement Parts Power Cords & AC Adapters
PowerMATE Family
AIV’s Special Purpose Relocatable Power Taps

Check out the PowerMATE -CM with current monitoring! Visit our Website!

AIV specializes in manufacturing new fetal monitor transducers and repairing popular GE/Corometrics and HP/Philips models.






n dustry experts share insights and advice regarding CMMS in this month’s Roundtable article. TechNation invited several leading companies to participate in the article with questions about unique features, regulatory standards, data analytics and more.
Participants in the Roundtable article are:
• ServiceNow Healthcare Solutions Specialist and Consultant Chaitanya Dahagam, MD;
• Renovo Solutions Vice President of Business Development Matt Forrest;
• Phoenix Data Systems Vice President of HTM Consulting Al Gresch;
• TruAsset Director of Business Development Amanda Moser;
• EQ2 HEMS by TMA Systems Manager, Client Success Rich Sable; and
• FSI Director of Product Experience Joe Stockman.
Q: WHAT MAKES YOUR CMMS SOLUTION UNIQUELY SUITED FOR HTM PROFESSIONALS COMPARED TO GENERAL-PURPOSE MAINTENANCE PLATFORMS?
DAHAGAM: ServiceNow’s CMMS stands out because it’s built upon one of the industry leading Enterprise Asset Management apps, including the Medical Asset Workspace tailored specifically for healthcare teams. It empowers HTM professionals with end-to-end workflows for RMAs, recalls,
and calibration management, wrapped in powerful analytics like risk scoring heatmaps, asset performance, total cost of ownership and many more. Mobile first features allow field teams to take action, scan, audit, pick, and remediate right from their mobile device. It’s a compliance ready, full life cycle platform that general purpose systems simply can’t match.
FORREST: RENOVOLive, our CMMS is built specifically for healthcare technology management, not adapted from another industry. It supports HTM-specific workflows like device risk scoring, preventive maintenance scheduling by modality, FDA recall tracking, and complete multi-vendor service history. Unlike other CMMS systems, RENOVO built our platform in-house, drawing directly from decades of real-world HTM experience. This means our customers get a purpose-built, turnkey solution that’s intuitive to use and ready to deploy – without the hidden costs or delays of custom development. We also provide complete cost and operational transparency with clear, real-time visibility into asset performance, service activity, and total program costs.
GRESCH: AIMS 3 was built specifically for the HTM and HFM industries. Our team has over 300 man-years of direct industry experience and understands first-hand the challenges the industry faces, including regulatory compliance, technician efficiency and productivity, cybersecurity, the need for effective integrations and automation, plus meaningful and impactful reporting for executive leadership.
We believe that the work our customers do is critical to the safe and effective delivery of patient care and are committed to being a trusted and capable partner in their success.
MOSER: Many CMMS solutions are repurposed from other industries, often requiring workarounds to meet the unique needs of the HTM industry. In contrast, TruAsset was specifically designed for HTM professionals and remains dedicated to that focus with every new feature and upgrade. TruAsset also streamlines compliance with key industry accreditations, including Joint Commission, DNV, CMS, and various state and federal requirements.
SABLE: EQ2 HEMS by TMA Systems is designed specifically for healthcare and the unique needs of HTM, while general-purpose platforms lack the specialization required for HTM. HEMS One CMMS offers specialized workflows for managing medical equipment and advanced compliance tools and reports, including AEM program management and everything needed to be ready for the next survey. Real-time business intelligence (BI) dashboards, an automated unable to locate (UTL) process, and straightforward handling of device reactivation or retirement are other standout features. Frequently, HTM departments request that the CMMS become the “hub” of all the applications they work with and HEMS One stands out in this area by supporting numerous interfaces and integrations, while adding new ones all the time. Current areas supported include cybersecurity tools, real time locating services (RTLS), ECRI Institute Alerts and Recalls, oneSOURCE, parts ordering and procurement applications, ticketing systems, and calibration and testing applications, among others.
STOCKMAN: FSI’s CMMS is purpose-built for healthcare environments, including HTM departments. It integrates biomedical asset tracking, compliance workflows, and mobile tools that support technician efficiency and regulatory readiness – features not typically found in general-purpose platforms. FSI’s CMMS also has integrations to other systems commonly used by HTM professionals for parts ordering, automated device testing, security and vulnerability, and real-time locater systems (RTLS).
Q: HOW DOES YOUR SYSTEM HELP HTM DEPARTMENTS STAY COMPLIANT WITH REGULATORY STANDARDS LIKE THE JOINT COMMISSION, DNV OR CMS?
DAHAGAM: The solution helps HTM teams stay inspection-ready by embedding compliance into daily workflows. It supports full traceability of medical equipment from onboarding and maintenance to recalls and decommissioning aligned with requirements from TJC, CMS and DNV. Calibrations are managed with documented quality standards, traceable to each calibration event, supporting ISO 13485 aligned quality management systems. Features like risk scoring and asset heatmaps enable proactive prioritization of high-risk equip-
ment, while audit ready records and real-time dashboards simplify external surveyor reviews and internal governance.
FORREST: Compliance is built in. RENOVOLive tracks all maintenance activities against regulatory standards, flags overdue inspections, and creates audit-ready reports instantly. It also documents AEM programs, OEM procedure adherence, and captures technician sign-offs to provide airtight regulatory documentation.
GRESCH: Phoenix is committed to staying up to date with evolving healthcare regulatory requirements by tracking the latest updates, participating in compliance-focused forums, industry events, and sharing best practices with customers. AIMS 3 helps track compliance metrics, provides tools for audit and inspection preparation, and offers dedicated support during these processes. Additionally, I lead several sessions each year through AAMI’s Expert Insights program, titled “Mastering HTM Accreditation Compliance: Stress Less, Achieve More,” where proven methods for using CMMS to achieve compliance are shared.
MOSER: Maintaining compliance with regulatory standards is a top priority. TruAsset offers a variety of customizable service codes to track failures, operator errors, recalls and more. With its flexible data filtering capabilities, you can easily generate detailed reports and custom dashboard widgets to monitor key metrics. The streamlined data layout simplifies inspections, providing quick access to historical data, EOC reports and other essential analyses.
SABLE: HEMS One enables HTM teams to create manufacturer-specific PM procedures, even with multiple frequencies like inspections and battery replacements. Regulatory compliance dashboards track performance and present data for EOC meetings or audits by the Joint Commission, DNV or CMS. The BI Dashboard shows real-time PM completion and highlights trends, while technicians see outstanding PMs directly on their own individual dashboard. The equipment inventory dashboard shows PM procedure type (OEM or AEM), frequency, next due date and risk classification. Additionally, the AEM Dashboard aligns with client policies as well as standards by the Joint Commission and CMS to manage equipment transitions between OEM and AEM procedures.
STOCKMAN: FSI’s platform includes built-in compliance tracking, automated documentation, and audit-ready reporting aligned with standards from Joint Commission, DNV and CMS. It helps HTM teams maintain inspection readiness and streamline corrective actions. Automated workflows can help with documenting 100% completion by excluding unable to locate medical devices.
Q: WHAT ROLE DOES DATA ANALYTICS PLAY IN YOUR CMMS, AND HOW ARE HTM PROFESSIONALS USING IT TO IMPROVE OPERATIONS?
DAHAGAM: Analytics enables HTM teams to shift from






Chaitanya
Dahagam
ServiceNow Healthcare Solutions
reactive to proactive operations. Real-time dashboards track KPIs like availability, MTTR, and failure trends, helping teams prioritize high-risk assets. Calibration analytics allows centralized monitoring of critical assets boosting compliance without missing a single calibration, while failed calibrations allow trigger of fully traceable remediation tasks that ensure compliance with ISO quality standards. Paired with condition assessments and condition scores for qualitative assessments, teams gain visibility into all aspects of asset health, extending equipment life and reducing downtime through smarter maintenance planning.
FORREST: Analytics transform raw service history into actionable strategy. HTM leaders use our dashboards to identify high-cost assets, monitor downtime, and track failure trends by manufacturer, model, or location. Combined with our resolution data, teams can refine training, improve first-time fix rates, and make better capital replacement decisions.
GRESCH: We help HTM teams unlock actionable insights through superior analytics and HTM consulting, enabling excellence in operations. We also:
• Uncover trends hidden in large data sets;
• Develop processes to identify and eliminate inefficiencies;
• Standardize nomenclature and practices to drive quality;
• Benchmark against industry norms confidentially;
• Evaluate alternatives to devices and maintenance schedules;
• Motivate teams to reach new levels of performance; and
• Empower leadership with clear, actionable reporting
MOSER: Data analytics plays a pivotal role in HTM by offering insights across various areas. It helps identify high-failure items, enabling predictive maintenance, root cause analysis and cost reduction through timely replacements. Life cycle analysis can also be performed to optimize asset management. Additionally, reporting on work order efficiency highlights opportunities to refine workflows for greater productivity. Custom dashboards provide each user with the ability to track KPIs relevant to their role, empowering them to make data-driven decisions and pursue continuous improvement.
SABLE: Data analytics in HEMS One empowers HTM teams to drive process improvements and make informed decisions. Leaders can review tracking toward service level agreements (SLAs) and set performance goals based on real-time metrics. The system offers quick access to key indicators like PM failures, work orders for devices in use, and those unable to be located (UTL). The BI dashboard includes valuable insights such as mean-time between failure (MTBF) and cost-of-service ratio (COSR), which help guide budgeting. Overall, analytics in HEMS One enhance departmental performance, compliance, capital planning and strategic planning.
STOCKMAN: Data analytics in FSI’s CMMS enables HTM professionals to monitor asset performance, identify trends in service requests, and optimize preventive maintenance schedules, among other abilities. These insights support better decision-making and resource allocation, including analysis needed for setting up an AEM program.
Q: HOW HAS CUSTOMER FEEDBACK FROM HTM PROFESSIONALS INFLUENCED RECENT UPDATES OR NEW FEATURES IN YOUR SYSTEM?
DAHAGAM: Customer feedback is crucial to the development and evolution of the ServiceNow CMMS solution. For example, customer feedback helps the ServiceNow healthcare and life sciences (HCLS) product development team prioritize key CMMS features (e.g., enterprise asset onboarding, preventive maintenance automation, automated work order assignment and resolution, predictive analytics).
FORREST: Nearly every feature upgrade is a direct result of input from our customers and technicians. Critical device alerts, integration with Power BI, cybersecurity tools and capital equipment planning tools are results from customer feedback. Their insights ensure the system keeps pace with real-world challenges.
GRESCH: This year marks our 40th annual User Conference, where we gather feedback and ideas directly from the AIMS Community to continuously improve our products and services. Customers also share best practices and success stories, showcasing how our CMMS helps drive efficiency and improves outcomes.
MOSER: TruAsset is committed to gathering client feedback throughout the year, ensuring that upgrades and new features align with user needs. Our top priority is maintaining client satisfaction and continuously evolving to meet the demands of the HTM community. Since TruAsset users rely on the system to ensure patient safety and uphold accreditation compliance, it’s essential that the CMMS solution continues to provide the tools and efficiency necessary to support these critical goals.
SABLE: Customer feedback plays a vital role in shaping the EQ2 HEMS One CMMS, as clients offer insights based on real-world use and evolving needs. These suggestions often lead to simplified workflows or entirely new ones that enhance HTM efficiency. Clients also alert EQ2 to regulatory changes from recent surveys, helping the system stay compliant through updated monitoring and reporting tools. When hospital administration assigns new responsibilities to HTM teams, EQ2 adapts HEMS One or its BI Dashboard to support those tasks. This collaboration between users and developers ensures HEMS One continues to evolve with meaningful, practical features.
STOCKMAN: FSI actively incorporates feedback from HTM users into the product roadmap to enhance usability and functionality. Recent updates include streamlined mobile access, configurable workflow automations, and expanded reporting tools – all designed to meet the evolving needs of healthcare technicians.
DAHAGAM: Modern platforms can automate preventive maintenance, manage recalls and calibrations, provide audit-ready documentation, integrate analytics and risk scoring and now, increasingly, leverage AI to handle more complex and heuristic tasks. Before evaluating vendors, HTM teams should first define their ideal workflows, compliance requirements, and reporting expectations, then look for a platform that delivers those outcomes natively not through add-ons or heavy customization. While there will always be opportunities to expand and tailor use cases, choosing a platform that enables rapid time to value for new solutions is just as critical.
FORREST: Select a platform that understands HTM’s unique needs – regulatory compliance, medical device risk scoring, multi-vendor support, and interoperability with hospital systems. Avoid tools that only “track” work; you need a solution that actively improves decision-making.
GRESCH: Most CMMS platforms can help meet compliance requirements and improve efficiency – but will they elevate your department’s visibility and value within your organization? Choose a partner who understands your business, is invested in your long-term success, and is committed to keeping you current with emerging technologies and industry needs.
MOSER: Many HTM professionals face the challenge of evaluating multiple CMMS providers, a process that can be both time-consuming and overwhelming. A recommended approach is to start by outlining key pain points that need addressing, identifying must-have features, and noting “nice-to-have” items that would be beneficial but not critical. With this framework in place, you can reach out to potential vendors and narrow down the list to those that most effectively align with your objectives. From there, scheduling web demos with a core group can help further streamline the options. Additionally, reaching out to client references provides valuable insights into the service experience. Once only two or three candidates remain, involve a broader group of staff in the demos and gather collective feedback to make an informed, final decision.
SABLE: When selecting a new CMMS, HTM leaders should start by identifying current system shortcomings and desired features. The team should then arrange product demos and narrow down the options to a shortlist of candidates. Separate demos for leadership and technicians help ensure the system benefits all users and fits their workflows. Finally, evaluating vendor support approach (what is the turn-around time on different types of support requests; is the support personnel based in the U.S., etc.) is crucial to ensure reliable assistance, minimize downtime, and maximize the long-term value of the investment.
STOCKMAN: Choose a CMMS that’s tailored to healthcare. Look for platforms that support granular compliance tracking, intuitive mobile functionality, and integration with biomedical workflow tools. Scalability and vendor support with HTM department expertise are also key for long-term success.
Q: LOOKING AHEAD, WHAT INNOVATIONS OR TRENDS DO YOU BELIEVE WILL SHAPE THE FUTURE OF CMMS IN THE HTM SPACE OVER THE NEXT 3 TO 5 YEARS?
DAHAGAM: CMMS solutions powered by agentic AI that helps HTM teams orchestrate work across teams, departments, data siloes and beyond. Smart clinical/ medical devices that generate more data (clinical and non-clinical) and that travels to and through the organization’s systems instantaneously and in real-time. Significant security and vulnerability risks for the devices and data that require organizations to have real-time analytics to proactively prevent breaches, downtime, and liability.
• Remote Troubleshooting Integration – CMMS platforms will connect directly with virtual service tools, reducing dispatch needs.
• AI-Driven Predictive Maintenance – Machine learning will anticipate failures before they happen.
• Real-Time Interoperability – Seamless integration with EHR, IoT-connected devices, and hospital data networks will become standard.
GRESCH: Phoenix sees AI playing a transformative role in CMMS over the next 3-5 years. Key innovations will include:
• Predictive maintenance and advanced analytics using historical repair data;
• Real-time equipment location, availability and utilization tracking;
• Integration of device data with clinical systems to support care delivery;
• Automated parts ordering and inventory management to reduce stockouts; and
• AI-driven repair suggestions and remote expert access – fully integrated into your CMMS.
MOSER: In the coming years, technology is poised to significantly shape the future of CMMS solutions. AI and machine learning will empower CMMS systems to analyze vast amounts of real-time data, potentially identifying failures before they occur, which will enhance patient safety and minimize downtime. IoT integrations connecting medical devices and equipment will feed real-time data directly into CMMS platforms for more accurate monitoring. We can also expect a shift away from internally hosted solutions toward cloud-based platforms, which will be complemented by mobile devices that streamline the preventive maintenance (PM) process by enabling live data entry rather than time-consuming post-task updates.
SABLE: Over the next 3 to 5 years, CMMS will evolve with artificial intelligence, transforming how HTM teams schedule maintenance, predict failures and optimize workflows. IoT-enabled medical devices will allow real-time tracking and condition-based maintenance for critical equipment like ventilators and imaging systems. These sensors will help detect anomalies early, reducing downtime and improving patient safety. Mobile CMMS apps will advance with more voice-to-text and barcode scanning functionality, and AR-assisted repairs. All of these innovations will significantly enhance HTM team productivity and efficiency.
STOCKMAN: Expect growth in digital enablement, like AI-driven predictive maintenance, deeper integration with IoT-enabled medical devices, and expanded use of virtual reality for training and facility planning. CMMS platforms will become more proactive and data-centric.
Q:
DAHAGAM: HTM organizations have a tremendous opportunity to focus on what they want their CMMS solution to do instead of limiting their capabilities simply because they can’t find a CMMS solution that does what
they want it to do. As such, HTM organizations should conduct internal discovery to determine what their ideal CMMS solution should do. Then, they should work with companies like ServiceNow, who have experience in the HTM CMMS workflows and solutions space, to design, implement, and deploy CMMS solutions that allow them to realize their CMMS vision today and empower them to be prepared for the future.
FORREST: HTM professionals are the hidden backbone of patient care. Our mission is to give them tools built for their world – ones that reduce downtime, ensure compliance and deliver actionable insights. With RENOVOLive and our HTM-specific CMMS, we’re helping HTM departments go beyond “maintenance” and become strategic, data-driven partners in patient care.
GRESCH: Don’t just choose a CMMS – choose a partner. Understand the total cost of ownership and the long-term operational and financial value your CMMS partner should deliver beyond implementation and the CMMS.
MOSER: Selecting and implementing a CMMS platform requires careful consideration and active involvement. Beyond the selection process, having a reliable and knowledgeable support team is crucial for success after implementation. It takes time to configure the system and establish best practices, but solid support can streamline this learning curve significantly. A well-designed CMMS serves as a powerful tool for managing service requests, preventive maintenance schedules and completion, parts inventory, contracts, and much more. The key to success lies in investing in a solution that balances efficiency for daily users with the comprehensive needs of administration.
SABLE: A strong CMMS helps HTM managers and technicians manage assets, work orders, regulatory compliance, and preventive maintenance scheduling in one platform. It streamlines operations, supports patient safety and improves regulatory compliance. Additional strengths include inventory and parts tracking along with interfaces to supply chain vendors and making data-driven decisions, especially with capital equipment planning using the KPIs from your CMMS data analytics.
STOCKMAN: FSI is committed to empowering HTM professionals with tools that simplify compliance, enhance operational efficiency and support the critical mission of healthcare delivery. We welcome collaboration and feedback to continue evolving our solutions.


• Register for free and apply to any of our listings
• Browse our 350+ open positions across the United States

• Get directly connected with industry hiring managers
• A talent network of 3600+ actively looking biomedical and imaging professionals
• A variety of listing and advertising options
• Social media, print, and eNews promotion
“HTMjobs.com is a remarkable website that can be used by any personnel looking for employment, internships, or just a good read from their Career Center . The site offers job postings from employers in the HTM industry, as well as resources for job seekers to help them prepare for their job search, including resume tips, interview advice, and more.”
BY K. RICHARD DOUGLAS

People often decide early that they want to be a biologist, a teacher, a nurse, a firefighter or enter the military. This is a decision that many make in high school and the occupation is one they are familiar with.
But how many young people think;
“I’m going to be a biomedical technician?” or “I’m going to be a
clinical engineer?” Very few consider these options because few high school students have heard of these careers.
As a procession of aging biomeds exit the field, the need to fill vacancies requires a proactive approach. The need for experienced and well-trained HTM professionals is a constant.
It’s not just that high school students aren’t asking the question “What is a biomed?” They don’t even know to ask the question. Unless the field enters their tangential knowledge at some point, they remain as unaware as the general public. This presents a challenge to those tasked with staffing biomed departments and independent

service organizations as well as OEM service teams.
The same challenge goes for introducing the field to those who have completed some core requirements in a junior or community college. At the point of making a career decision, how do these students know to consider HTM? For those in the most advanced HTM educational programs; how do they learn networking and soft skills?
Thankfully, there are those within HTM who are working to promote and grow the field and expand its visibility to potential future candidates. Also, there are individuals providing the newer biomeds and biomed students a leg up by teaching them valuable skills to ensure success.
With future biomeds scattered among thousands of high schools, technical schools and colleges, the opportunity for those in HTM to reach out and influence someone is great. This may be easier said than done. However, AAMI created a tool to assist biomeds in spreading the word.
“HTM in a Box was developed as a strategic response to the growing workforce shortage in the HTM field, especially as a large portion of the current workforce nears retirement. As part of AAMI’s broader goal to support the HTM personnel pipeline, this initiative was created to make it easier for HTM professionals to actively promote their field without the added burden of building their own presentation materials,” says Danielle McGeary, CHTM, PMP, vice president, HTM at AAMI.
High school presentations are more focused on career paths, education options, and job stability – providing a more concrete vision of how to enter the field. College and beyond presentations are built for technical audiences (like biomedical engineering or IT students) and adult learners. These presentations include information on certifications, career ladders and real-world roles like BMETs and clinical engineers,” McGeary says.
She says that each section is flexible as presenters can adapt talking points and choose to spend more time on slides that resonate with their specific audience (e.g., BMETs for community college students, clinical engineering for biomedical engineering majors).
“If younger people relate to technology, then they are certainly going to use tech to help them make decisions on critical life choices like careers. We need to meet the new audience on their terms.”
- Justin Barbour
The idea behind HTM in a Box is to offer a pre-packaged presentation to allow HTM professionals, and others, to present the field in a concise and positive way. It provides the choice of three HTM career presentations, designed around the age group of the audience.
“The concept was designed in collaboration with a small project team from the Technology Management Council (TMC). The goal was to create a virtual, easy-to-access set of tools that would empower HTM professionals to conduct outreach across multiple age groups – whether at a local middle school career day or a college fair for students exploring health technology careers,” McGeary says.
She says that the Prezi-based presentation is segmented into three distinct tracts: middle school, high school and college and beyond (which also covers adults and career changers). Each track conveys the same core message: what HTM is, why it matters, and what a career in the field looks like. Each presentation is customized in tone, language and visual design to match the audience:
“Middle school presentations are simple, visually engaging, and designed to spark curiosity. They emphasize the cool factor of technology and the idea of helping others.
McGeary says that the tool is particularly effective for career days at schools, community or STEM events, college classroom presentations and career fairs and community organizations.
For more than six years, HTM professional Justin Barbour, CBET, has produced content for “The Better Biomed Channel,” a YouTube channel with more than 1,100 videos that promote the biomed profession, technical skills, events, tools and many other topics.
When creating biomed content over the Internet, the content creator doesn’t always know who their content is reaching. Yet, through Barbour’s efforts, he has heard back from prospective biomed students considering the field and others.
“I receive one to two emails weekly from people sharing their stories. I love hearing stories because everyone’s story is different. I met a guy in San Antonio who used to repair cellphones in Chicago. He watched a video I did with Louis Rossmann and became curious about this career. He moved to San Antonio and became a biomed. He’s doing pretty good now,” Barbour says.
He says that another story sticks with him on this topic.
“A person wrote me when they were graduating with their four-year degree in biomedical engineering. They heard about the career in one of my videos and wanted to reach out to thank me for the introduction. The completion of a four-year degree made me realize how long I’ve been doing videos,” Barbour adds.
Barbour is aware of the various audiences his videos reach.
“The coolest thing about Google owning YouTube is that they keep stats on everything. I can see viewer age, gender, geographic location, etcetera, and I can see trends over time. Since the start of the Better

Biomed channel, I’ve had a majority of viewership of younger people. It makes sense. They relate directly to technology and are most likely to be invested into tech careers. I always meetup with younger techs at events, and they’ll always have my time,” he says.
Barbour says that one of the demographics he was not expecting was the female technician audience.
“Over the years, I’ve seen an upward trend from 1.5 percent to now almost 10 percent viewership, which tracks directly with attendance that I’ve witnessed at biomed training programs around the country,” he says.
Barbour truly believes that “people buy into what they relate to.”
“If younger people relate to technology, then they are certainly going to use tech to help them make decisions on critical life choices like careers. We need to meet the new audience on their terms. I have even changed some of my content to relate more directly to the new viewers: short form content being recorded in portrait mode rather than long form in landscape,” he says.
He says that there are many technical people around the world who might be in deadend jobs – like an electrical engineering graduate repairing cellphones for $28/hour.
especially when it comes to educating and inspiring the next generation. With Bella the BMET, I’ve found a way to make the world of healthcare technology management (HTM) approachable, fun, and relatable. Bella allows us to tell real stories from our field in a way that resonates with children and adults. It’s not just about fixing equipment; it’s about showing the heart behind the work we do,” Chester says.
Chester’s Bella character has gained traction in several age groups beyond grade schoolers.
“What’s been most surprising, and incredibly rewarding, is how many adults have shared that they learned about the biomed field for the first time through Bella. Parents, educators, and even clinicians have told me, ‘I had no idea what a biomed was until I read this book to my child.’ That ripple effect has extended into high schools and colleges too, sparking conversations about career paths in HTM that wouldn’t have happened otherwise. The reach has gone far beyond what I ever imagined,” she says.
In addition to her books, Chester takes the HTM profession directly to the public in an engaging way.

“We need to continue to be ambassadors for this career to all potential candidates,” Barbour adds.
When not producing content, Barbour is the director of business development for BC Group International.


“Elevate HTM’s vision is bold, it’s to ensure that every young person, especially those in underserved communities, is aware of healthcare technology management and sees it as a pathway to purpose, stability and impact.”
- Bryant Hawkins Sr.
A kid’s book about a fictional biomed has reached an even wider audience of teens and adults who have learned about the HTM profession. That has been the experience of author; Jennifer Chester, BMET III. Chester wrote the book “Bella and the Big Fix Biomeds: Wired for Greatness.” She is also the author of “Bella the BMET’s Adventure in Healthcare Technology,” “Bella’s Big Fix: The Story of a Smart Girl and Her Tools” and “Big Ben and the Heart of New Orleans.”

“Storytelling is one of the most powerful tools we have,
“Promoting HTM is at the heart of everything I do with my Mobile Discovery Museum. Bella and the Big Fix Biomeds have become the official mascots of the experience. We’ve taken the museum into schools, summer camps, and even onto the expo floor at AAMI, where kids and adults alike get hands-on with the tools and tech that keep hospitals running,” she says.
She says that while she is not necessarily adding more medical devices, she has started weaving BMET storytelling into the exhibits to reinforce problem-solving and critical thinking.
“For example, I redesigned our Indi robot game to mirror the way a biomed might travel through a hospital to troubleshoot equipment, turning coding and decision-making into a narrative kids can understand and apply. This fall, I’ll also be giving a keynote and book reading at the LEAD Girls event in North Carolina. Just one of many opportunities to share the magic of biomed through storytelling,” Chester adds.
HTM ADVOCATE LAUNCHES NONPROFIT
Trimedx Site Manager Bryant Hawkins Sr., not to be confused with TechNation 40 Under 40 honoree Bryant Hawkins Jr., is an advocate for HTM via his LinkedIn page, HTM on the Line podcasts and the Elevate HTM program. Hawkins said the official mission statement for is: “Elevate HTM exists to educate, inspire, and empower the next generation of HTM professionals. We introduce
students, especially in underserved communities, to careers in healthcare technology management through classroom visits, media, mentorship and hands-on exposure. At the same time, we uplift HTM professionals by reminding them that their work matters.”
“Elevate HTM’s vision is bold, it’s to ensure that every young person, especially those in underserved communities, is aware of healthcare technology management and sees it as a pathway to purpose, stability and impact,” he adds. “The organization’s work goes far beyond just exposing kids to the HTM industry, it’s about transforming lives through exposure, opportunity and mentorship. Every classroom visit, career day and conversation is a chance to spark curiosity, plant seeds of possibility and help the next generation discover a career they can be proud of.”
This mission is already moving nationwide. Elevate HTM has reached students in Florida, North Carolina, New York and California, with a goal of impacting 3,000 students in Louisiana and another 1,000 across the country. The team has grown from one person to 12 members in seven different states, making it the only nonprofit in the United States specifically targeting young people to bring awareness to the HTM industry.
The expansion plans are ambitious. In October 2025, Elevate HTM will launch NextGen Pathways, a podcast designed for youth, co-hosted by an HTM professional and a schoolteacher. It will highlight lesser-known healthcare careers, with HTM leading the conversation. Spring 2026 will see the release of the Elevate HTM app, connecting students, parents and teachers to career exploration resources. In fall 2026, though possibly sooner, the Elevate HTM Online Academy will launch as a virtual career exploration platform, delivering engaging, real-world HTM content to middle and high school students nationwide. Monthly motivational videos will also be produced to inspire and encourage students to envision a brighter future.
“Elevate HTM exists to not only create awareness but to build a lasting pipeline of leaders who will protect patients, advance healthcare, and carry this profession forward. The work is growing louder, bigger and farther than ever, and it’s only just getting started,” Hawkins says.
The mission reflects how a chance decision continues to provide positive impact.
“After graduating high school in 1990, I didn’t have a plan. No blueprint. No clear next step. I just knew I needed to do something. I grabbed a college catalog from Delgado Community College in New Orleans, closed my eyes, and pointed. My finger landed on ‘Biomedical Equipment Technology.’ No big strategy.
No dream I’d been chasing since childhood. So, I actually happened upon the biomed industry,” Hawkins explains.
His career inspires him to be an advocate for the HTM field.
“It’s simple, this work changes lives. Early in my career, I was repairing dialysis machines when it hit me, this wasn’t just about fixing equipment. This was about keeping people alive,” Hawkins says. “That moment flipped a switch for me.”
“Over the years, I’ve seen how critical HTM professionals are to healthcare, yet most people don’t even know we exist,” he says. “We’re the invisible force that makes patient care possible, and I’ve made it my mission to change that. I know firsthand that young people, especially in communities like the one I grew up in, rarely hear about this career. I found it by chance, and I want the next generation to find it on purpose.”
When asked if he wanted to add anything, Hawkins said, “I’ve met so many great people in this industry and I keep meeting more. I’m far from a perfect HTM professional, but I love what I do, despite the ups and downs that come with it. I found HTM by accident, and now my life’s work is making



from discovering a career that changes their life and the lives of the patients they’ll one day serve. Together, we can make HTM impossible to ignore.”
For information or to volunteer, email info@elevatehtm.com. Elevate HTM is also interested adding corporate sponsors to support this one-of-a-kind mission to bring nationwide awareness to the HTM industry.
Once students are beyond the orientation process and have committed to the HTM profession as a career, there is still much to know, even at the graduate level. Internships are an integral component for those entering the field to get hands-on knowledge while picking up key traits for a successful career. This includes getting the most out of HTM conferences.
At the University of Connecticut, interns receive pointers that go beyond their textbooks and labs via a program for students enrolled in the Master of Science in Biomedical Engineering (MSBME) degree program.
“The internships are integral to the UCONN CE Internship program. This program is 40-plus years old and was designed by hospital clinical engineering leaders who wanted/needed to develop a pipeline of clinical engineering talent. The original design assumed that students would augment their academic studies with related work assignments at the founding hospitals. Therefore, all interns accepted into the program are assigned to a hospital for the four academic semesters and the intervening summer as a condition of completion,” says Carol Davis-Smith, MS, CCE, FACCE, AAMIF, operations director of the Clinical Engineering Internship Program at the University of Connecticut’s College of Engineering and president of Carol Davis-Smith & Associates LLC.
She says that the interns obtain real world experience
since they are assigned to real world problems with real world responsibilities at their host health system.
“The UCONN adjunct faculty – all of whom are still working in the field – have gone to great efforts to ensure the course content aligns well with the typical internship experience, so interns learn the theory and then ‘do it’ at the hospitals,” Davis-Smith says.
Students also get a leg-up by way of some guidance Davis-Smith has provided taking advantage of opportunities through professional conferences.
“Each academic semester, I convene the University of Connecticut Clinical Engineering (UCONN CE) interns for CE Week. During this time together, I provide the interns with extracurricular learning – i.e., concepts that are not covered in their academic courses and not likely explained during their working internship,” she says.
Davis-Smith says that last fall, she had the opportunity to align CE Week with the MD Expo New England conference.
“We only had one day together as a group before the conference, so I decided the extracurricular learning should focus on how to get the most out of attending the conference. The first component required the interns to register for the conference, which included selecting the educational sessions they wanted to attend. During CE Week, I lined the meeting room with posters with the title of each session and the interns indicated their choices with sticky dots,” Davis-Smith says.
She then facilitated a discussion where the interns described what they thought each session would be about – because the descriptions aren’t always clear –and why the interns made their choices.
“In the end, several interns changed their choices based on the discussions. I explained that this happens frequently


in real-time at conferences when we engage in conversations with other attendees – aka, a networking benefit,” Davis-Smith adds.
She says that the second component required the interns to participate in the MD Expo exhibit hall BINGO game.
“This resulted in them visiting most, if not all the vendor booths, and learning about the products and services. I know they participated because several of the interns won prizes! The New England-based interns may have derived the most benefit from this exercise because many vendors staff their booths with local sales and service representatives. Consequently, the New England-based interns had the opportunity to meet their local vendors and begin building relationships. Other interns benefited from the exercise because I saw them interacting in the exhibit hall of the MD Expo SoCal conference,” Davis-Smith says.
She also provides the students with the resources and opportunities to enrich their soft skills.
“One of their first assignments is a LinkedIn learning module about critical thinking. The objective is to learn what questions to ask, when to ask them, and who they should be asking before drawing conclusions. This prepares them for the 40 clinical rotations they will complete over their four academic semesters,” Davis-Smith says.
She says that the rotations require them to schedule time away from their internship work with clinical users to observe them using the medical devices and systems with patients.
“The interns are expected to ask questions, so they understand the clinical workflows, why and how the technology is used, what the clinical user likes/dislikes about the technology, etcetera. Each month, the interns submit their logbooks with their observations. I grade not only the content but also spelling and grammar,” Davis-Smith adds.
From grade school through graduate programs, the message of the HTM profession, as well as skills to succeed in the profession, are communicated in resourceful ways to inform the youngest future biomeds to the most advanced masters-level recruits. The platforms and methods used all benefit the profession and those entering the field. Thankfully, there are those who have taken the initiative to take on this mission.
BY JOHN WALLACE
Radiological Service Training Institute (RSTI) Director of Strategic Partnerships and Military Programs Kim Rowland is well known throughout the HTM community. Years of dedicated service to HTM combined with leadership skills earned her the votes of her peers and the 2025 Tech Choice Award in the Industry Influencer category.
to fill the pipeline of skilled professionals, it’s essential to not only recruit but to inspire,” Rowland says. “Whether it’s through military transition programs, mentorship, or industry visibility, my goal is to make sure that when the next generation asks, ‘What’s a biomed?’ – that we are there with the answer, the resources, and to welcome the next gen into the field.”
influencer exceeds the description of a social media influencer. She is an active participant at national, regional and state HTM conferences. Rowland helped launch the Women in Leadership (WIL) organization. She also contin ues to be an advocate for HTM as a rewarding career field. Yet, HTM is a career field she was unaware of until she found herself in it.

existed,” Rowland says. “My career began in a few different non-health care industries, with a focus on customer service, marketing, and business development. I’ve always had a drive to succeed and, for me, success is measured by believing in the product, being part of the right team and making a meaningful impact.”
Once she was aware of HTM, Rowland almost immediately began promoting it as a great career field.
“I’ve seen firsthand the dedication and skill it takes to succeed in HTM and also how under-recognized the profession can be,” she shares. “The people in this field quietly make a huge impact on healthcare every day, and I want the next generation to see HTM as a rewarding, stable and meaningful career path. Being named TechNation’s Tech Choice Award 2025 winner for Industry Influencer was an honor, but more importantly, it’s a platform to amplify the stories, challenges and opportunities within HTM.”
Read a more in-depth article on how this Tech Choice Award winner is promoting HTM online at 1technation.com/wrenchie-kim-rowland.








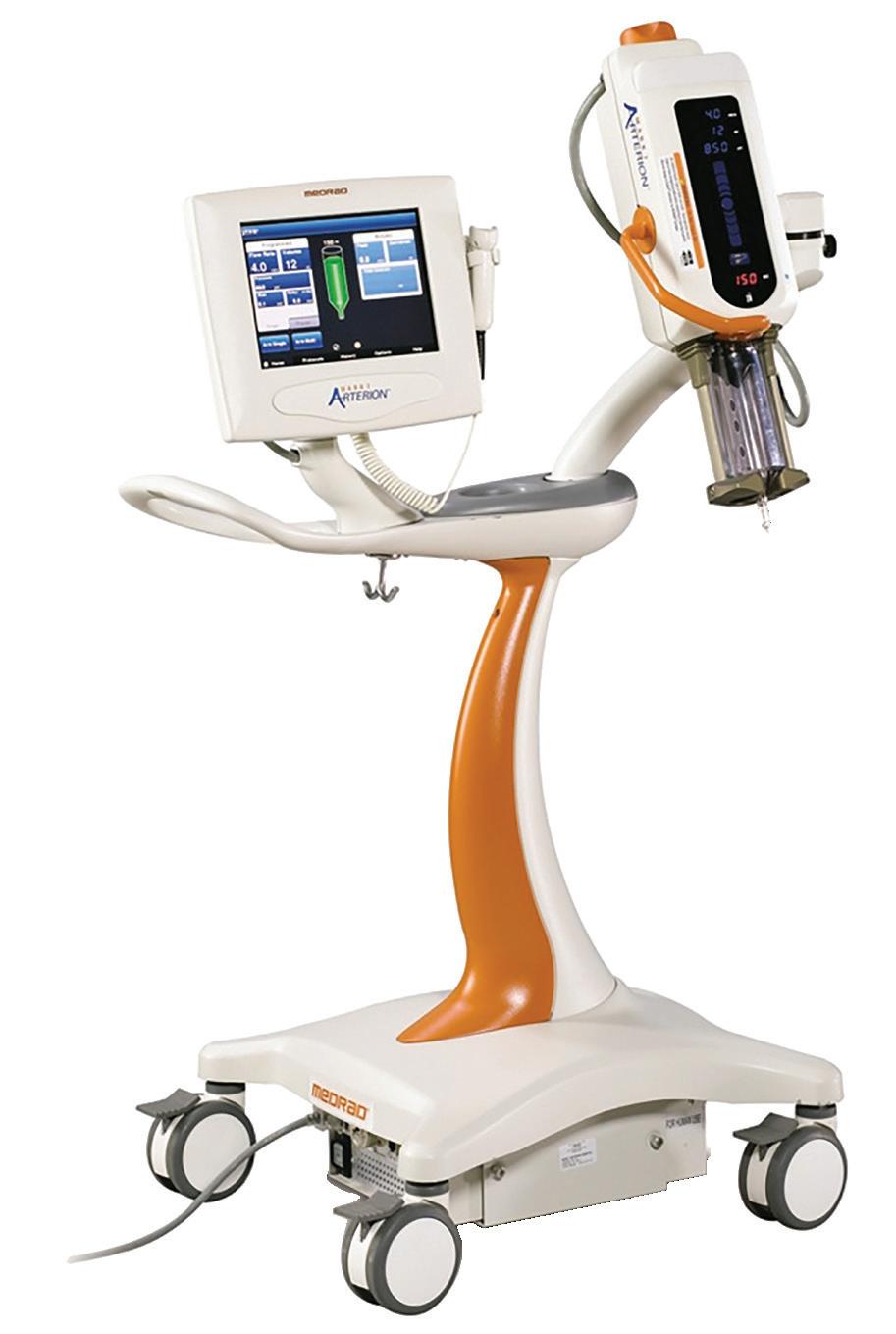

BY KATHLEEN FURORE
If you’re looking for a new job, why not consider a former employer?
OK, so I know what you’re thinking. If your former employer was toxic, there’s no way you’d consider working there again. That’s a valid assessment, but what if it wasn’t toxic? You accomplished the goals of your job and needed new opportunities, so you landed jobs elsewhere. Now that you’re seeking another new opportunity, that former company may be able to support you in your new career goals as you command a higher salary than when you left.
You aren’t alone in this strategy of becoming a boomerang employee. According to ADP payroll data, in March, 35% of all new hires were former employees. That’s an increase from 31% in 2024.
It makes sense to consider returning to your old stomping grounds, and from the employment perspective it makes good business sense. When I worked in recruiting, the first thing I did was ensure the potential rehire did not have poor performance and they weren’t terminated for negative issues. Once those aspects were clear, proceeding with a former employee often equated to a faster time-to-fill the vacant position.
Boomerangs have knowledge of the culture and, over time, although tech systems may have changed and the organizational charts moved around with new leadership, they’ll have a swifter ramp up time that often has less hiccups as new hires.
In addition, if they worked for competitors during their absence, as a boomerang employee, they may be able to bring valuable business acumen and insider industry knowledge with them, along with members of their former
external teams.
From an employee perspective, it’s potentially a win-win situation too. Rather than going to a new employer, especially if it has just laid off workers, returning to a former employer can be a breath of fresh air and relief, comfort and growth.
Nela Richardson, Ph.D., ADP’s chief economist and ESG officer, said in the research results, “There are several reasons employers might be eager to rehire former employees. In the case of the information sector, for example, a worker with the right skill set can be difficult to find. Certain skills might be geographically concentrated, for example, which makes luring back former employees one good way to obtain needed expertise.”
In addition, boomerang employees have a swift onboarding process.
“This ability could be highly sought after by employers looking to make efficiency gains as the economy slows and the threat of higher prices increases,” said Richardson. “Returning employees, particularly those lured to new organizations during and after the pandemic, might have learned that the grass wasn’t greener at their new job. Moreover, lower U.S. rates of residential mobility suggest that workers today are less inclined to move for new opportunities, which limits their options.”

Vicki Salemi is a career expert for Monster, an author, a speaker and consultant, TV commentator and former corporate recruiter. Send your questions to hello@vickisalemi.com.
BY CHRIS MENDOZA
Consider this brief conversation:
“A re you ISO certified?”
“ Yes. 13485.”
“ Great. You just went up another peg in my book.”
That 10-second exchange with a DNV auditor said everything about how certifications shape perception, and the weight they carry with clients and regulators alike.
For an independent service provider, ISO 13485 is more than a logo on a website. It’s a globally recognized quality management standard that tells a customer: “We meet the same rigorous process controls and documentation requirements as leading manufacturers.”
Why does this matter? Because ISO 13485 builds trust before the first work order, giving auditors and administrators immediate confidence that our processes have been independently verified. It reduces client risk by allowing hospitals to demonstrate that their vendors operate under proven quality systems. It actively supports compliance by aligning with CMS, TJC, DNV, HFAP, AAAHP, and CIHQ standards. Most importantly, it proves consistency, “say what you do, do what you say … and prove it.”
And here’s the key: compliance isn’t something to prepare for before an audit. It’s how we run our operation every single day. Stay ready, so you don’t have to get ready.
In healthcare technology management (HTM), compliance
is often treated like an event, a scramble to prepare for inspection. But a reactive approach creates stress, wastes time, and increases the chance something gets missed. Worse, it pulls attention away from what truly matters: delivering safe, reliable equipment to the people who need it most.
The alternative is to treat compliance, your quality management system (QMS), as the operating system of your shop. In this model, the same processes that keep you audit ready also improve turnaround time, resource utilization and employee morale. Passing inspections and running an efficient operation aren’t competing priorities, they become one and the same.
An effective QMS is built on the same principles that drive Lean and Six Sigma: eliminate waste, reduce variation, and create flow. Applied to compliance, these principles translate into daily practices: documentation stays current, preventive maintenance is scheduled and completed on time, corrective actions (CAPAs) are tracked through closure, and key performance indicators (KPIs) are monitored continuously.
At MMS, this model has been part of our DNA for years. Supporting a client during a survey doesn’t require us to shift gears, it simply means showing auditors what we already do. This approach isn’t an industry secret. It’s the standard every shop should strive to meet.
1. Map your regulatory touchpoints. List every compliance requirement you touch. CMS, accrediting bodies, even local fire marshal inspections. Identify which
activities generate records (PMs, repairs, training) and where those records live.
2. Align daily workflows with requirements. Structure work orders, PM schedules, and documentation templates to meet audit expectations by default. Document these workflows in writing and specify the records they generate.
3. Make KPIs visible. A dashboard showing PM completion, CAPA closures, and turnaround times creates accountability and momentum. If a metric slips, it’s corrected immediately, not discovered months later. Review KPIs often and eliminate reports that don’t add value. Clutter hides what matters.
4. Conduct micro-audits. Monthly spot checks of random records help identify documentation gaps before they grow. Better yet, use technology to perform full-scope reviews, verifying the accuracy and completeness of every record.
5. Integrate CAPA into problem-solving. Treat every issue, from a missed PM to a missing screw, as a chance to find root cause and prevent recurrence. Done well, CAPA not only satisfies auditors but also improves efficiency, reduces costs, and boosts staff confidence.
6. Train for readiness, not the event. Don’t wait for mock audits or boot camps. Build compliance awareness into regular staff meetings: review KPIs, share audit findings, and explain the “why” behind requirements. When everyone understands purpose as well as process, compliance becomes second nature.
It’s easy to think of compliance as a burden. But when it’s integrated into daily workflows, compliance becomes a relief instead of a stressor, technicians no longer drop everything for paperwork, and managers aren’t stuck in firefighting mode.
The ultimate question in quality and compliance, the end of every policy and procedure; “Is this device safe for patient use?” When you analyze root causes, when you ask about proper PMs, you’re protecting patients who will never know your name. You’re making sure the next family in that ICU room doesn’t have to worry about whether the equipment will work. Compliance is how we keep the promise to patients we’ll never meet.
When MMS supports a client during a regulatory audit, we’re not “consulting” in the traditional sense. We’re simply extending the same ISO 13485-certified processes we use internally.
When compliance becomes your operating system, it stops functioning as a cost center and transforms into a competitive advantage. New client onboarding moves faster because processes already align with accreditor

expectations. Operational risks are reduced because problems are identified and solved early. Clients gain confidence knowing certification, transparent KPIs, and proven processes back every decision. And employees benefit from a less stressful, more predictable workflow that fosters pride in their work.
Whether you’re a small shop or a large regional service provider, building compliance into your daily operating system is the most effective way to meet regulatory demands, improve operations, and protect patients.
In the end, compliance isn’t just about satisfying auditors, it’s about creating a culture where quality, efficiency, and accountability move in the same direction. When compliance becomes the operating system of an HTM shop, audits stop being events and start becoming validations of the good work already being done.
For technicians, that means less stress and more pride. For managers and executives, it means fewer risks and stronger performance. And for patients, it means safe, reliable equipment. Every time. At MMS, our daily commitment to readiness has proven that when you treat compliance as a way of life, you don’t just meet standards. You raise them.

Chris Mendoza, CHTM, is the director of quality & systems administration with MultiMedical System LLC.
BY NATHAN PROCTOR

One of the most common opposition arguments against Right to Repair is that it violates the Intellectual Property (IP) rights of the manufacturer.
Devlin Hartline, Legal Fellow at the Hudson Institute’s Forum for Intellectual Property, presented this argument during a congressional hearing on Right to Repair in 2023. He claimed that while proponents claim Right to Repair is about property owners’ rights, he sees the manufacturers intellectual property as the relevant rights, not the owner of the device – and that Right to Repair undermines key IP rights.
As it seems that the U.S. military is pursuing a Right to Repair for all the equipment they purchase, with support from a bipartisan coalition of lawmakers, these arguments are once again being put forward by manufacturer-aligned lobbying entities.
In response, 11 law professors and experts in intellectual property law from some of the most prominent U.S. law schools have sent a letter to both the House and Senate Armed Services Committee chairs and ranking members in support of the Warrior Right to Repair Act.
The letter details that, in fact, the bill is consistent with longstanding IP law and presents no conflict with their rights, noting “As early as 1901, courts have recognized a ‘right of repair or renewal’ under U.S. copyright law. Doan v. American Book Co., 105 F. 772
(7th Cir. 1901). Since then, courts have repeatedly brushed back efforts to use copyright law to control the markets for repair parts and information.”
Sens. Tim Sheehy (R-MT) and Elizabeth Warren (D-MA) introduced the Warrior Right to Repair Act in early July. This important legislation would codify and extend to other branches of the military the Right to Repair provisions in Defense Secretary Pete Hegseth’s Army Transformation and Acquisition Reform memorandum from April 30.
The bill, as proposed, would significantly reform the inefficient U.S. Department of Defense (DOD) procurement process. Allowing the military to repair its own systems and equipment would save taxpayers billions of dollars in sustainment costs, improve military readiness and save servicemembers lives. Given the support for this legislation, similar language was added to the base text of the federal National Defense Authorization Act – the funding package for the U.S. military that is considered “must pass” legislation.
Servicemembers and veterans who wish to support military Right to Repair can join well over 100 veterans in signing a letter of support at https://forms.gle/1ioifSFtk9qdeu3c6. Businesses and repair advocates can sign on to this letter https://forms.gle/TiCnQsjQEnMHbTMS9. Both letters will be shared with Congress to demonstrate the deep public support for Military Right to Repair.

Nathan Proctor is senior director of the U.S. PIRG Campaign for the Right to Repair.
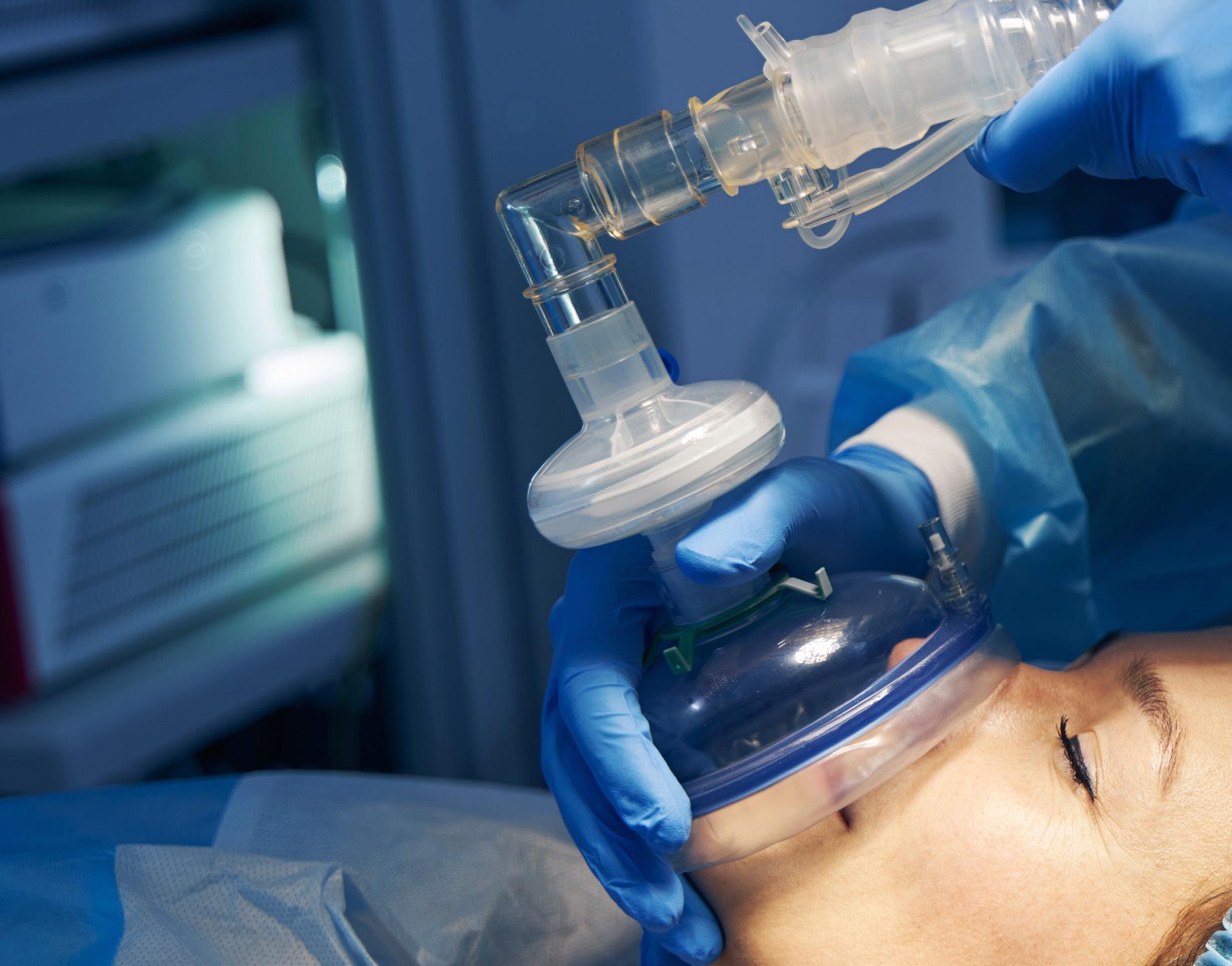





Set Pressure Targets & Pass/Fail Tolerance. Color indicates results: blue = , red = above tolerance. FITSIN SHIRTPOCKET!






MedWrench has you covered with thousands of manuals, user guides, and expert insights right at your fingertips.




When time is critical and patient care depends on reliable equipment, there’s no room for digging through drawers or chasing down missing manuals. MedWrench is your one-stop destination for medical equipment resources - INSTANTLY SEARCHABLE, ALWAYS AVAILABLE.
Need a manual? Browse thousands of equipment pages on
No account? No problem. Sign up in seconds and start exploring.
BY GARRETT SEELEY
couple of articles ago, I wrote about the differences between hard drive technologies and the potential for them to cause issues when booting separate systems. This reminded me of the many times the concept has been misunderstood throughout my career. One thing I have consistently seen cause confusion among starting technicians is the difference between RAM (random access memory) and hard drives/storage. People often refer to RAM as storage, and vice versa. They are not the same thing.
Memory (RAM) is a temporary workspace where the CPU, the main processing unit, does its actual work. Think of it like a counter space in a kitchen, where the CPU is the chef. This should not be confused with hard drives or storage in general. Both RAM and storage have sizes measured in gigabytes (GB) and both have access speeds; however, memory is much faster than drives due to the connection style. The CPU needs to access memory very quickly, so the memory is connected to the motherboard using a bus. The number of pins RAM uses is like the CPUs, usually 288 pins for data transfer in a 64-bit system. This means both the RAM and CPU can load or unload 64 parallel bits (1s or 0s) simultaneously. About a quarter of the CPU pins are used just to connect to memory.
Currently, the typical memory is around 16 GB, with RAM speeds significant enough to match the CPU itself, moving at 2 to 4 GHz (billions of inputs or outputs per second). This high speed allows memory to handle the CPU’s working information needs efficiently.
As a side note, there is memory on the CPU itself called “cache.” This is for the quick access of data that the CPU uses repeatedly. The most frequently used information is stored in cache because it offers the fastest possible access without the CPU leaving its own chip. Think of it like a chef wearing an apron with their most used tools in their pockets. Although it’s the fastest way for the CPU to get information, cache is not really for main work.
Despite being the primary working areas of the computer, memory and cache are temporary and volatile, meaning data does not remain if power is removed. For long-term data retention, we use static or persistent storage devices like hard drives. All drives act as long-term storage and do not facilitate the CPU’s main work. Using the kitchen analogy again, a drive is like a refrigerator or freezer – not a workspace, but a storage space.
Modern hard drives have storage sizes typically in the terabyte (TB) range – like 1 or 2 TB – a hundred times more than RAM. However, the connection used by hard drives is usually serial, leading to slower speeds. For instance, a SATA hard drive often operates at 1.5 Gbps (less than one-sixth the speed of RAM). Hard drives use few connectors, such as three wires for data communication, unlike the 64-bit RAM bus which can handle more data simultaneously. Therefore, randomly accessing data on a hard drive can be much slower, up to 100,000 times slower than RAM for some tasks. Thus, it’s best to consider drives strictly for storage.
There are exceptions, such as Virtual Memory, which is called swap files on Linux/Unix systems. These are part of the hard drive used as RAM overflow. This setup allows the CPU to move less frequently needed data from RAM to the hard drive, effectively expanding the system’s usable memory. Items in this area operate as if they are still in RAM, and for practical purposes they are. However, the hard drive provides a small relief for the RAM,
effectively expanding its usable area. The application to older medical devices
• CPU and RAM: Older systems used large central processing units (CPUs), often housed under a heat sink, connected to RAM via multiple parallel bus lines. This configuration allowed fast data transfer and efficient processing.
• EEPROM Storage: Electronically erasable programmable read-only memory (EEPROM) served as the storage device for the onboard computer. The EEPROM chip is typically socketed and located near the CPU, identifiable by a version number or part number, and is often secured with zip ties or clips to ensure it remains in place.
Modern medical equipment has evolved to employ architecture like that of modern desktop computers. This often uses consumer off the shelf (COTS) hardware. This can include:
• CPUs: Modern CPUs are more capable and feature integrated, larger heat sinks for better thermal management.
• High-Speed RAM: Utilization of high-speed RAM modules such as DDR4 or DDR5, which are present on small cards or “sticks” next to the CPU.
• Advanced Storage Solutions: More advanced storage solutions like solid state drives (SDDs), which provide higher storage capacity and faster access times are
mounted on SATA buss to the motherboard, usually farther away from the CPU, next to the card expansion slots.
Newer M.2 drives look like USB storage but are placed on a special slot next to the memory. This is because M.2 Drives use a similar slot to the video card bus.
The transition from older to modern systems represents a significant advancement in technology, improving the efficiency, performance and reliability of medical equipment. Understanding these components and their interactions is crucial for maintaining and upgrading medical systems. Using these techniques will help you quickly recognize the type of board and infer its purpose during initial troubleshooting. These concepts help demystify internal components within medical devices. While the CPU uses RAM as a small yet fast workspace, drives serve as long-term storage. That is the most important concept that is present in all computers and yes, even medical systems. Look for these elements to better understand the hardware you are working on.

Garrett Seeley, MS, CBET, is a biomedical equipment support specialistimaging with VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
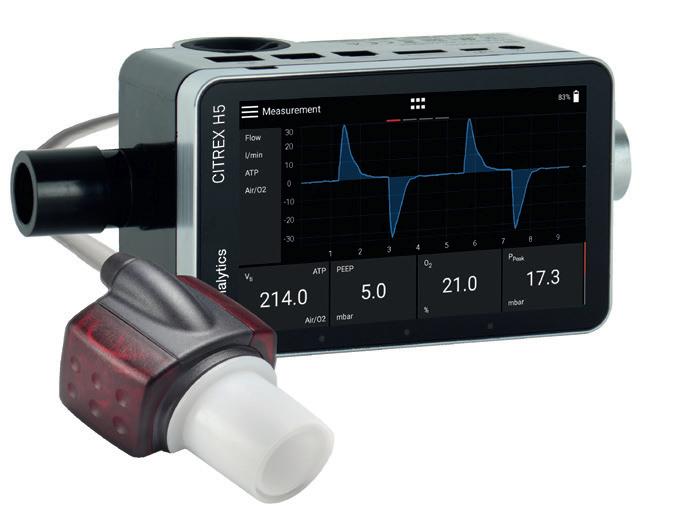

BY PHIL ENGLERT

In today’s healthcare landscape, medical devices are no longer isolated instruments. They are networked, data-rich systems integral to patient care. As these technologies become more connected, they become more vulnerable to cyber threats. The risks are real and growing, from ransomware attacks that disable imaging systems to vulnerabilities in patient monitoring systems that could be exploited remotely and render remote monitoring inoperable. Though not identified in the wild, it has been demonstrated that it is possible to alter the medication flow of infusion pumps.
Healthcare technology management (HTM) leaders must move beyond reactive security measures and build a proactive, measurable cyber resilience program to address this challenge. But how do we measure resilience meaningfully to executives, clinicians and technical teams?
The answer lies in metrics. In 2011, MITRE published the Cyber Resiliency Engineering Framework, which offers a structured approach to evaluating an organization’s ability to anticipate, withstand, recover from, and adapt to cyber threats. The framework’s principles (tinyurl.com/mtpaznj9) were updated in 2015 and still apply today. MITRE, in partnership with the National Institute of Standards and Technology (NIST), created the original cyber resiliency framework, NIST Special Publication Developing Cyber-Resilient Systems: A Systems Security Engineering Approach (NIST SP 800- 160v2r1).
The report defines how cyber resiliency metrics can inform investment and design decisions. It establishes a scoring methodology to assess cyber resiliency across different systems. The framework assists in evaluating alternative cybersecurity solutions and helps ensure that cyber resiliency assessments are repeatable and reproducible. This article explores how to apply MITRE’s principles to medical device ecosystems and identifies nine key metrics tailored to three critical stakeholder groups.
MITRE’s framework defines cyber resiliency as the capacity of systems to continue operating under adverse conditions, including cyberattacks. It emphasizes four core goals: anticipate threats before they occur, withstand attacks while maintaining essential functions, recover quickly from disruptions, and adapt to evolving threats and system changes. The framework also outlines metrics that span technical, operational, and mission domains, making it ideal for healthcare environments where patient safety, clinical workflows, and IT infrastructure intersect.
Senior leaders, including the chief information security officer (CISO), need metrics that provide a high-level view of organizational risk and resilience. These metrics should inform investment decisions, regulatory compliance, and enterprise risk posture.
The first executive metric might be the Percentage of Networked Medical Devices with Known Exploitable Vulnerabilities or KEVs. While technical, this metric allows for tracking over time, hopefully a reduction, and a current organizational status, as new vulnerabili -

ties are discovered and exploits are developed. The data for this metric will be derived from vulnerability management platforms (e.g., Tenable, Qualys) integrated with computerized maintenance management systems (CMMS) or IT asset inventories and Software Bill of Materials to identify the components present in the medical device technologies. This metric quantifies exposure across the device fleet. A high or increasing percentage signals systemic risk and helps prioritize remediation efforts.
The Time to Patch Critical Vulnerabilities in Medical Device Technologies is a second metric. Due to its regulated nature, patch availability significantly lags behind the patching cycles of traditional IT endpoints. Tracking this metric and understanding the drivers in the lag may lead to more informed procurement decisions and investment decisions of protective measures, such as more granular segmentation capabilities or network access controls. The data for this metric will come from patch management logs, vendor service records, and CMMS. This metric measures operational agility. Shorter patch times reflect a mature response capability and reduce the exposure window.
The final executive metric, a Medical Device Cyber Risk Score by Facility, is a strategic tool that empowers executive leaders to manage risk, protect patients, and guide the organization toward greater cyber resilience across multiple locations. The data for this metric will be derived from aggregated device risk assessments, network segmentation audits, and vulnerability scans. Providing an organization-wide but site-specific view enables benchmarking across hospitals, guiding targeted investments and interventions.
These metrics help executives understand where the organization stands and where it needs to go. Cybersecurity metrics empower healthcare executives to lead with confidence. They transform abstract threats into actionable insights, enabling leaders to protect patients, safeguard data, and ensure the continuity of care. In an era where cyber resilience is synonymous with patient safety, these metrics are strategic imperatives to drive the resourcing needed to protect patient safety and ensure the mission of healthcare delivery.
Editor’s Note: next month, this column will explore key metrics for clinical leaders and HTM teams, focusing on transforming risk into meaningful improvements in patient care.

Phil Englert is the director of medical device security for Health-ISAC.






The words can appear horizontally, vertically, diagonally, and may be spelled forwards or backwards.
1. Innovation
2. Interoperability
3. Cybersecurity
4. Automation
5. Connectivity
6. Analytics
7. Integration
8. Future of HTM
9. Sustainability
10. CMMS
11. Data
12. Efficiency
13. Standardization
Randy Ozminkowski
Radiology Service Engineer, Clinical Engineering Advocate Christ Medical Center
• Fuji collimator
• MRI Filtrine unit pump
• MRI injector
• toolbox
• Bayer/Medrad PC
• Tee probe
• video converter Submit your bench to be featured in TechNation at 1technation.com/my-bench/. You could win a $25 Amazon gift card via the “What’s On Your Bench” Contest!












Admar Neuro admarneuro.com • 728-900-3969
AMX Solutions www.amxsolutionsinc.com • 866-630-2697
BC Group bcgroupstore.com.com • 314-638-3800
FiberTech fibertechmedical.com • 866-628-6888
Interlight interlightus.com • 800-743-0005
Jet Medical jetmedical.com • 714-937-0809
Metropolis International metropolismedical.com • 718-371-6026
Nuclear Camera Services nuclearcameraservices.com • (630) 780-6363
PM Biomedical pmbiomedical.com • 800-777-6474
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Secure Mount securemount.com • 517-784-9613
Tri-Imaging Solutions triimaging.com • 855-401-4888




















Tri-Imaging

platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.





and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking


XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering This helps maximize uptime and minimize repair costs
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering This helps maximize uptime and minimize repair costs.














OCTOBER 1
Reactive Mode to Leader Mode

WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
OCTOBER 15
SPONSORED BY
WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
OCTOBER 22
SPONSORED BY


TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.

SPONSORED BY Why Training is Key to a Strong Workforce

SPONSORED BY AAMI’s New CEU Journal and The Importance of ACI Certification

SPONSORED BY Enhancing Biomedical Device Security and Patient Safety with Claroty
SPONSORED BY

TOOLS OF THE TRADE ESA700 Series Electrical Safety Analyzers

All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
Wisconsin welcomed the 2025 HTM Mixer supported by the Wisconsin Biomedical Association (WBA). The conference attracted 133 attendees with 49 industryspecific companies showcasing their solutions in an active exhibit hall. WBA members and attendees enjoyed the HTM Mixer for its quality educational sessions, exhibit hall and networking events.

2 5



3 6

4

1. Enjoying all the sights, sounds, and fried delights at the Wisconsin State Fair - because business connections and cheese curds make the perfect combo.
2. WBA Secretary Mitch Mitch Von Ruden graciously accepts a generous donation from FSI in support of their scholarship fund.
3. Nothing like walking away a winner. Attendees had a blast playing door prize bingo and taking home some amazing prizes.
4. Smiles at the start - the Welcome Reception was a great event at Vue, a rooftop ballroom located on the 21st floor.
5. The exhibit hall buzzed with energy as vendors showcased HTM’s latest products and technology.
6. Attendees striking a pose with a neon glow.


7. Nothing beats prizes and surprises inside the exhibit hall.
8. One of the most talked-about sessions: Advancing Women in HTM– Empower, Innovate, Strengthen.
9. The famous Brat—one of the Milwaukee Brewers’ Racing Sausages—was the hit of the show, even posing for a complimentary caricature!
10. Education was front and center! The HTM Mixer Milwaukee was officially approved for 10 CEUs by the ACI.
11. Conversations and connections: Trimedx inside the exhibit hall.
12. Networking was in full swing during the exhibit hall hours.
13. Fun is synonymous with the HTM Mixer! Attendees enjoyed out-of-the-box entertainment, including live Irish music, Irish dancing, and even a visit from the Milwaukee Brewers’ Racing Sausage, The Brat!

9 10 8



12 13

Anesthesia
General
Inc.
Prescott’s, Inc.
Monitors/CRTs BMES
Parts Plus cmpartsplus.com
Diagnostic Solutions diagnostic-solutions.com
Innovatus Imaging innovatusimaging.com • 844-687-5100
International X-Ray Brokers internationalxraybrokers.com
508-559-9441
LLC
Tri-Imaging Solutions triimaging.com • 855-401-4891
Neurodiagnostics
Admar Neuro admarneuro.com
Prescott’s, Inc. https://prescottsmed.com/ • 833-423-3786
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Tenacore LLC tenacore.com • 800-297-2241
USOC Medical usocmedical.com • 855-888-8762
Refurbish
Admar Neuro admarneuro.com • 728-900-3969
Tubes/Bulbs



TechNation cover articles addressed important topics in 2023.



Attendees and exhibitors enjoy a Halloween party during the 2023 fall MD Expo.




The first-ever TechNation 40 Under 40 class was a big hit with the HTM industry.
The inaugural Tools of the Trade Live Demo presented by Elite Biomedical was a popular new concept.


The 2023 spring MD Expo featured education, exhibit hall, prizes, networking events and a photo booth!





















Choose the right capital equipment, supplies, and health IT for your facility with guidance from ECRI Instituteʼs team of biomedical and cybersecurity engineers, clinicians, and human factors experts.



ECRI is a trusted authority on healthcare technologies that improve the quality and cost-effectiveness of patient care.
Get test results and ratings, free of vendor bias


Stay on top of risks with hazard and recall notifications
Compare technology with overviews and specifications
Learn more at www.ecri.org/solutions/device-evaluations or contact us today at 610-825-6000, x5891.


