TOP TOOLS TECHS WANT
























































Instead of waiting for problems to arise, scheduled service keeps your anesthetic vaporizers reliable.

Protects your patients by ensuring accurate, consistent anesthetic delivery and vaporizer reliability.
Stay compliant with regulations from the FDA, Joint Commission, and other governing bodies.






Minimize disruptive downtime and avoid the high costs of last-minute emergency repairs with scheduled service.
Investing in preventative maintenance protects your assets, ensures patient safety, and provides peace of mind.








P.12 SPOTLIGHT
p.12 Department of the Month:
GE HealthCare/AdventHealth
Fish Memorial Hospital Biomedical Department
p.14 Professional of the Month: Jonathan Hatley, CBET
p.16 Next Gen: Michael Miller
p.18 Shifting Gears: Mike Chuma, Getting Past the Rail
P.20 INDUSTRY UPDATES
p.20 News & Notes
p.25 TechNation Pulse
p.27 Ribbon Cutting: AdmarNeuro
p.28 AAMI Update
p.30 ECRI Update 46

P.32 THE BENCH
p.32 Biomed 101
p.34 Webinar Wednesday
p.35 Tools of the Trade
p.36 How I Use MedWrench in my Classroom
P.39 FEATURE ARTICLES
p.39 Roundtable: Test Equipment
p.46 Cover Story: Unveiling the Latest Trends & Tech
p.50 Top Tools Techs Want

P.58 EXPERT ADVICE
p.58 Careers Now
p.60 Cybersecurity
p.62 The Future
p.64 Right to Repair
p.66 SPONSORED CONTENT: Multimedical Systems
p.68 The Massey Method
p.70 Networking Notes
p.72 Health-ISAC
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey BUSINESS DEVELOPMENT
EDITORIAL John Wallace
CONTRIBUTORS
K. Richard Douglas
Joie Marhefka
Steven J. Yelton
Garrett Seeley
Phil Englert
Nathan Proctor
Nadia ElKaissi
ACCOUNT Megan Cabot
EXECUTIVE
ART DEPARTMENT Karlee Gower
Taylor Hayes Alicia Brown
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
Haley Harris
EVENTS Kristin Leavoy Sydney Krieg
WEBINARS Linda Hasluem
HTMJOBS.COM Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez

p.80 Biomed Brainbuster
p.81 Word Search
p.81 [Contest] What’s on Your Bench?
p.82 NCBA Scrapbook
p.84 Preferred Vendors
p.88 Service Index
p.91 Alphabetical Index
p.92 Time Capsule
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Nadia ElKaissi, CHTM, Biomedical Engineer, HTM, VA Central Office (19HTM)
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Earl Morris Jr., BMET, Director of Clinical Engineering, Harrison County Hospital
Leticia Reynolds, Clinical Engineering Operations Manager at UCHealth Parkview Medical Center




Our nationally recognized programs include Biomedical Equipment Technology (BMET), Biomedical Equipment Support Specialist (BESS), and the Dental Repair Technician (DRT) Certificate. Each is designed to equip you with the advanced skills needed to thrive in today’s evolving healthcare and dental technology landscape.






CBET’s Virtual Reality (VR) labs offer a flexible and immersive learning experience that adapts to your busy schedule. our VR labs allow you to practice and perfect your skills in a controlled, risk-free environment.

and are available through
formats. These programs can include foundational, advanced, product-specific, or professional development training and education specifically designed to serve the diverse needs of the HTM industry.


BY K. RICHARD DOUGLAS
Orange City, Florida, is a city of about 13,000 known for Blue Spring State Park. It is located approximately 30 miles northeast of Orlando or about halfway to Daytona Beach.
Serving the healthcare needs of the community is AdventHealth Fish Memorial Hospital. In 2021, the hospital added a new four-story patient tower.
M anaging the hospital’s medical equipment inventory is the GE HealthCare/AdventHealth Fish Memorial Hospital Biomedical Department. The biomed team consists of four members including GE HealthCare apprentice Adrian Figueroa, GE HealthCare Lead Tech Ruben Sebastian, GE HealthCare Tech Robert Cherry and GE HealthCare Program Coordinator Rana Helou.
Because of the small size of the team, the techs are all generalists.
“ We all learn all departments and get exposed to all equipment to be able to provide service to the facility and not have a delay. I always say you never know what happens so always have a plan and do not depend on one person for everything. That is why technicians tag team with lead technicians and program coordinators to learn and advance in the field. The service manual, tech support and an amazing team working together to learn performs a faster route to success. Never specialize or put yourself to a limit,” Helou says.
The team handles Fish Memorial with 253 operating beds, a AdventHealth Deltona ER with 12 beds as well as doctor offices offsite.
“ With AdventHealth growing rapidly in Florida, we continue to help our surrounding areas. GE HealthCare has imaging field service engineers for imaging, and with the
amazing apprentice program, many apprentices get to join the lead techs and program coordinators to understand the field and responsibilities of a hospital on a day-to-day basis, but as well as how important we are in the field making a difference in the hospital and helping save lives alongside the hospital staff,” Helou says.
She says that with the different experiences the team members have in the field, they help each other tackle anything from every-day correctives to projects and maintenance.
“One hand helps the other with success. I like when technicians get exposed to everything in the field and the challenges that they can face, and how to approach it. This is why we do things as a team, but if a technician is specialized on a special unit, we all tag team to learn and expand. Always have a backup plan just in case one person is out of the office the next person can take over and not affect the operation of the facility with a delay,” Helou says.
She says that the team was created to improve preventive maintenance completion, reduce the turnaround time for corrections, help the hospital with cost savings and bring awareness to how much biomed is involved in every department.
“ By having every technician dedicated to a department and contribute to the projects for the hospital as they grow, we are able to help ensure the safety, reliability, security and availability of medical equipment,” Helou says.
H ow is data collection accomplished?
“ The different dashboards created help manage collecting data across the board being able to run reports, data sheets and numbers in real time which helps in providing the ability to review metrics, operations, time, performance as well as decisions,” Helou says.
The biomed team has also cemented its relationship with its IT counterparts.
“ We have built a strong and wonderful relationship

with AdventHealth IT, which has also helped us support medical equipment. But also working with each nurse leader in each department, attending management as well as staff meetings, listening to concerns and compliments helped with how to approach the work force,” Helou says.
H elou is certain that with the expansion of AdventHealth, the team will continue to have projects and challenges.
A solution to a recent challenge was accomplished through the resourcefulness of the team.
“ Bed rentals are a budget by themselves. We were able to help the facility get bed repairs completed on a timely manner by having the staff tagging beds with a red tag


explaining the defect and it has helped with a quicker turnaround time limiting down time. Having a process in place was the solution to all problems,” Helou says.
T he four-member team may be small, but they keep up with involvement in the biomed world. They also support their leader.
“As a team we always keep up with AAMI information, Central Florida Biomed Society, and MD Expo within our state, as well as Friday I-HTM. This most amazing team of gentlemen has been very supportive of my WIL (Women in Leadership) involvement,” Helou says.
I n central Florida, the GE HealthCare biomed team at AdventHealth Fish Memorial is getting the work done and ready to tackle future projects.



BY K. RICHARD DOUGLAS
The U.S. economy impacts people in many ways including a higher cost-of-living, the ability to buy a house or a rising or falling stock market.
It can also lead some people to the HTM profession.
“I became interested in the biomed profession when I was between electrical jobs. I was doing electrical work, but when the housing market crashed in 2008, very few homes were being built and very few electricians were needed, except for service calls, which was no longer steady work,” says Jonathan Hatley, CBET, technical manager, biomedical services, McLeod Health in South Carolina.
He says that he held out for about a year before he had to find another means for steady work. A friend helped him get a job at a place that makes and sharpens saw blades for giant sawmills found in North and South Carolina.
“I was the delivery driver, and I hated that job, but it was work. The saws can be quite large, some with a diameter of six feet; they were very heavy, it was hot in the summer, cold in the winter and I bled on a daily basis,” Hatley adds.
He says he worked at that job for a couple years, and during that time, he met with a guy through his father-in-law who said he was a biomedical equipment technician.
“I was like what’s that? He asked me, ‘Have you ever thought about what happens when equipment breaks in the hospital? It’s all machines, they break sometimes and need maintenance, and don’t you perform maintenance on your car by changing the oil periodically, your lawnmower or other things to help prevent them from breaking?’ As we talked, I found that the job mostly consisted of troubleshooting problems with equipment, repairing those problems, performing maintenance and customer service; all of which I was good at. My thoughts were that as long as hospitals are open, there will always be a need for people like him to repair equipment. I could do that,” Hatley says He enrolled in the biomedical equipment technology
program at Stanly Community College in Albemarle, North Carolina.
Hatley worked his way up in the field from a BMET I to entering his current position as a technical manager of biomedical services in 2023.
In the challenges/projects department, Hatley says infusion pump remediation stands out as a task that requires an understanding of patient flow.
“As manager for the biomedical department, I am involved in many projects, some of them over a year out. By far, some of the toughest projects that I have led, and been involved with, are the infusion pump remediations. They seem to happen


department because it involves every department in the hospital,” he says.
Hatley says that each time it is difficult because of the sheer volume of devices that have to be found and swapped out across each of the facilities; usually simultaneously.
“It takes great coordination between biomed and nursing staff, as well as the vendors to see that each and every aspect of it is calculated and purposeful. We can’t take too many devices out of service at one time without having new/ replacement devices to fill their place. Because it usually takes a week or more at the main hospital, we can’t bank up devices in the evening to have for swap out the following morning because it will cause a shortage on the floors for the night staff and the on-call biomed would get called non-stop throughout the night,” he says.
Hatley says that during the remediations they rely heavily on nursing staff to help swap the devices that are in use on patients.
“We have to circle back through the same departments multiple times because patients are always moving, and new patients are always coming in. That’s why it helps to understand patient flow and having a good working relationship with the
FAVORITE BOOK:
I still read the Goosebumps original set. I’m a ’90s kid.
FAVORITE MOVIE:
“Back to the Future” and all the Marvel movies and “Tombstone”
FAVORITE FOOD:
BBQ
HIDDEN TALENT:
When I close my eyes, I can make people disappear until I open them again.
FAVORITE PART OF BEING
Although my calling is not direct patient care, I take great pride in being able to provide comfort to staff and patients by using my skills to give them assurance by letting them know i am here to support them in their equipment needs, and in most cases, we can fix it if it breaks.
WHY DO YOU READ TECHNATION?
TechNation is a great magazine for the biomed career field that provides useful and insightful information about products from vendors and different perspectives from biomeds across the country. I also enjoy the webinars hosted by TechNation on Webinar Wednesday.
nurses and nursing administration,” he says.
Away from work, Hatley enjoys the simple things in life.
“Grilling, smoking meat and cooking food is a hobby that I have had for many years. I have three kids and a wife, so my hobbies mostly revolve around and benefit them. Mowing grass and lawncare is also a hobby because it’s the only time I am left to my own thoughts without interruptions. Other than that, I like to collect antiques,” he says.
Hatley says that his wife and he have been together for 16 years and married for 11 and they have three kids together; two girls ages seven and six and a boy who is three.
Hatley’s efforts received recognition from his employer.
“In 2018, I received the McLeod Merit Award, which is only given out to about four recipients per quarter in our organization and I am very proud of it. It is ‘For consistently exceeding the standards of excellence and quality set by McLeod and demonstrating cheerful and compassionate service to others,’ ” he explains.
It was well-deserved for this HTM professional who has been in the field since 2011, obtained CBET certification in 2018 and worked his way into leadership. The field is better with people like this.

Michael Miller is a College of Biomedical Equipment Technology alum achieving great things in HTM. He is currently a Clinical Engineering Equipment Technician II with Intermountain Health McKay-Dee Hospital. TechNation recently had a chance to catch up with Miller.
Q: WHERE DID YOU GROW UP?
A: Smithfield, Utah
Q: WHERE DID YOU RECEIVE YOUR HTM TRAINING/ EDUCATION?
A: Online classes through College of Biomedical Equipment Technology.
Q: HOW DID YOU FIRST DISCOVER HTM?
A: My dad started in HTM before I was born.
Q: WHY (OR HOW) DID YOU CHOOSE TO GET INTO THIS FIELD?
A: My dad suggested I look into it after I mentioned medical problems from working in construction.
Q: WHAT DO YOU LIKE MOST ABOUT YOUR POSITION?
A: The great group of people that I work with is what I like the most about my current job.
Q: WHAT INTERESTS YOU THE MOST ABOUT HTM?
A: What interests me the most about HTM is being a part of the healthcare community.
Q: WHAT HAS BEEN YOUR GREATEST ACCOMPLISHMENT IN YOUR FIELD THUS FAR?
A: My greatest accomplishment thus far is taking on three different models of scope washers for two hospitals.
Q: WHAT GOALS DO YOU HAVE FOR YOURSELF IN THE NEXT 5 YEARS?
A: One goal I have for myself is to become a Tech III before 2030.


FAVORITE HOBBY: Riding motorcycles
FAVORITE SHOW OR MOVIE: Gladiator
FAVORITE MEAL: Anything I make on my smoker
WHAT WOULD YOUR SUPERPOWER BE? Wolverines healing
1 THING ON YOUR BUCKET LIST: See Greece, Italy, and Israel
K. RICHARD DOUGLAS
How many people consider leaping off of a 486-foot tall bridge as a celebratory act on their 73rd birthday? This is something that HTM professional Mike Chuma had planned on, but due to weather conditions and other conflicts it was delayed.
Chuma’s plan was to do a tandem BASE jump from the Perrine Bridge over the Snake River in Twin Falls, Idaho. The location is a famous BASEjumping location because it’s the only man-made structure in the United States where BASE jumping is permitted yearround without a permit.
BASE jumping is an extreme sport where jumpers leap from high, fixed objects and deploy a parachute to descend safely to the ground. The name “BASE” is an acronym for the four types of objects jumpers leave from: Buildings, Antennas, Spans (bridges) and Earth (cliffs).
“My son called me and asked me the day before Father’s Day if I wanted to jump. So, we combined it into a birthday/ Father’s Day jump and that’s how we did it. This jump was my third jump off of Perrine Bridge. I have also done two jumps off of KL Tower in Malaysia, both tandems with my son,” Chuma says.
Chuma’s son just happens to be one of the premier BASE jumpers globally.
“My son, Sean, is the pioneer of tandem BASE jumping. He is known as one of the best in the world with over 9,000 BASE jumps. I believe over 1,500, maybe more, are tandem,” Chuma adds.
What is required to do a tandem BASE jump?
“You have to be less than 175 pounds and that’s about it. He goes through the safety routine, what you need to do and all that stuff and then you go out to the bridge. He goes over everything again at the bridge and then you go out there and
you try to stay home which is impossible and you jump. It is one of the most fascinating things I think I have done; everybody will enjoy it. You can also go to Moab, Utah, and jump and also Monte Brento, Italy, and jump,” Chuma says.
The Italian location has established strict requirements, although self-enforced, in order to jump there.
For solo jumping, Chuma says that taking a class would be a good starting point. He says classes require students to have many skydives. His son’s class requires 200 skydives.
“There are some people out there that teach BASE jumping with no experience at all; that is crazy. I have watched many people hit the trees and get hurt by not having any experience whatsoever. I did solos because my son is a base jumper. I did two and that’s about all I’ll ever do, because as other people watched, they wanted to let their friends do BASE jumps and it was too dangerous, so my son said stop and we did,” Chuma says.
In terms of safety protocols, Chuma says that with BASE jumping, there are no reserve chutes, so you really can’t make a mistake.
“Off of Perrine Bridge, six seconds you hit the water or the ground if you’re chute doesn’t open. There have been many people that get line tangled, they have to get out of it quick enough to not get hurt; some do, some don’t. The best thing to do in BASE jumping is not be stupid; most people that get hurt bad, or don’t make it, do stupid things,” Chuma says.
He says that there are very few that have fatalities that keep their head straight and stay calm.
“Not saying that you’re not going to get hurt. It is one of the most dangerous sports in the world. The biggest thing is packing your chute,” Chuma says.
He says that this is the primary safety protocol, and his son is very meticulous about it.
Did Chuma’s previous jumps prepare him for the Father’s Day jump; especially at 73?
“I’m not sure a whole lot prepared me for my Father’s Day/
birthday jump. Getting over the rail of the bridge every time is scarier than hell. It’s difficult because I am old and my body doesn’t bend and my legs were terrible getting over that rail. Your mouth gets dry, you look down you’re scared,” Chuma says.
He says that the experience with the rail was the same with the previous jumps.
“But once we jump, it’s the most fascinating sensation you’ll ever feel. I would do it again and again. I’m going to be scared as hell getting over that rail. Now I do plan to go to Moab, Utah, and jump off of a cliff and I do plan someday hopefully, before I get too old, to go to Brento, Italy and jump out there off of a 5,000-foot cliff. Will I be scared; hell yes!” Chuma says.
What extra challenges are there, even with a tandem jump, for a 73-year-old?
“I think the biggest challenge is, no matter who you jump with on a tandem skydive, a tandem BASE jump, you have to trust that person with your life. I used that analogy in a safety huddle to give a perceptive of a safety moment and I said patients need to trust us in the hospital as I had to trust my son on that tandem BASE jump,” Chuma says.
He says weather conditions are also a big factor.
“There are times we stood on the bridge for 45 minutes watching the wind. One of the good things about my son is, if he doesn’t feel comfortable, we walk off the bridge; that’s why I’m safe and alive today,” Chuma says.
What advice would Chuma give to someone contemplating their first BASE jump?
“If you’re going to do a tandem, investigate the person you’re going to jump with. There are several out there. Check them out, check their history. As far as doing your first BASE jump, have skydives and take a class from some reputable instructor. I highly recommend that before attempting to BASE jump,” he says.
Chuma has been in the biomed business for 54 years. He started out in the Air Force and then worked for ServiceMaster. He entered the management ranks, but later went back to work as a technician, which he considers a good decision.
“I have enjoyed my career; I wouldn’t trade it for anything. I would urge anybody that loves healthcare and being part of the system to go for it,” he says.
Take it from a 73-year-old who jumps off bridges; biomed is a good career. Anyone with the guts to jump off a 486-foot bridge is worth listening to.


“You’re never too old to feel alive.” — Mike Chuma

Royal Philips and Masimo have renewed a multi-year strategic collaboration. The companies are taking a bold new approach in accelerating the development and delivery of next-generation patient monitoring solutions, according to a press release.
“This expanded agreement reflects our commitment to empower clinicians with smarter, more integrated technologies that support better outcomes across the continuum of care,” the release states.
A central objective of the partnership is a joint effort to integrate Masimo’s advanced monitoring technologies –including SET pulse oximetry, Radius PPG, and a range of sensor technology – into Philips’ multi-parameter patient monitoring platforms.
“This integration supports clinicians with reliable data and actionable insights across bedside monitors, central stations, and wearable solutions, while giving them the flexibility to use their preferred measurement technologies within a single, unified system. By embedding these capabilities into Philips’ monitoring ecosystem, the collaboration aims to reduce complexity, enhance interoperability, and deliver streamlined tools that support timely decision-making and continuity of care,” the release states.
“Building on this foundation, Philips and Masimo also plan to
collaborate on the development and co-promotion of nextgeneration monitoring solutions that reflect emerging clinical needs and evolving market demands such as the need for greater patient mobility,” it adds. “These efforts are focused on advancing smart, connected care and expanding access to innovative technologies, including AI algorithms, that have the potential to improve patient care over time.”
“Our priority is helping clinicians deliver the best possible care to their patients, and that means staying ahead of the curve,” said Julia Strandberg, executive vice president and business leader of connected care at Royal Philips. “This partnership allows us to respond quickly to evolving clinical needs and market trends, integrating proven technologies into solutions that are easy to use, reliable, and scalable.”
“We are excited to continue to partner with Philips to bring Masimo’s newest innovations in wearables and artificial intelligence to Philips’ platforms,” said Katie Szyman, CEO of Masimo. “Expanding our strong, long-standing partnership with Philips allows us to build on our shared legacies of innovation and helps ensure that Masimo’s best-in-class technologies reach even more patients. Together, Masimo and Philips will continue to empower clinicians to transform patient care.”
TRIMEDX has been named one of the Top 100 Global Inspiring Workplaces by the Inspiring Workplaces Group, ranking #45 on the 2025 list. This global recognition follows TRIMEDX’s recent selection as a Top 100 in North America (30th) announced earlier this year.
“Being named among the world’s most inspiring workplaces is a testament to our associates and the culture we’ve built together,” says TRIMEDX Chief Human Resources Officer Dawn Griffin. “Leadership at TRIMEDX means listening, supporting, and growing together. This recognition reflects our shared commitment to making TRIMEDX a place where people thrive.”
TRIMEDX was previously ranked #30 globally and #29 in North America in 2024. The 2025 Global Top 100 list was drawn from the highest-scoring winners across seven regions,
including North America, Europe, Asia, and Australasia. Entrants were evaluated on six key elements:
• Culture and purpose
• Leadership
• Wellbeing
• Inclusion
• Employee voice
• Employee experience
According to a statement from Inspiring Workplaces, “The quality of submissions from each region has raised the bar, making it the strongest year our independent judges have ever seen. It shows that more business leaders are truly putting their people first and seeing real benefits. It also highlights the positive, meaningful changes happening in workplaces around the world, as reflected in entries from every region.”
Kaiser Permanente and Renown Health announced their agreement to jointly own and operate a health plan and outpatient care delivery system that will bring Kaiser Permanente to northern Nevada beginning in 2026.
This relationship brings together two nonprofit organizations with a shared purpose; to bring high-quality, value-based care to more people by improving access and expanding convenient and affordable care options in northern Nevada.
“Our mission calls us to bring high-quality, affordable health care and coverage to as many people and communities as we can. Our members, employers, physicians and community members have been asking us to provide our unique offering of value-based care in Nevada for some time,” said Greg A. Adams, chair and chief executive officer of Kaiser Permanente. “Working with the leading health care provider in northern Nevada, we will now have the opportunity to care for more people, help more employers offer coverage to their employees, and in collaboration with Renown Health, improve the health of this growing community.”
“We are pleased to introduce this relationship in Nevada, enhancing access to affordable care and coverage. By joining our health plan together with Kaiser Permanente, a respected national partner, we can expand access to affordable, highquality insurance coverage for even more Nevadans,” said Brian Erling, MD, MBA, president and CEO of Renown Health. “This collaboration strengthens our ability to keep care local, while bringing the scale, expertise and innovation of a national leader. Together, we are ensuring that individuals, families and employers across the state have more choices and better coverage. Together, we can improve the overall health of the
communities we serve and create additional capacity to care for more people.”
Under this new agreement, pending regulatory approval, Kaiser Permanente Nevada will offer health plan coverage to employers and the people of northern Nevada starting with open enrollment in 2026.
Kaiser Permanente Nevada creates a unique opportunity by pairing Hometown Health, the insurance arm of Renown Health, with Kaiser Permanente’s value-based care model to deliver what local consumers have asked for; high-quality and affordable care and coverage, close to home. Kaiser Permanente Nevada plans to open a multispecialty ambulatory health system with convenient locations in central and northern Reno. Members and patients will access multispecialty care, diagnostic, pharmacy and ancillary services at these locations and at the existing Del Monte Senior Care Plus Clinic in Reno.
Renown Health is a leading health system in Nevada and has owned and operated Hometown Health since 1988. Renown Health is the region’s largest, locally governed, not-for-profit academic health care network serving Nevada, Lake Tahoe, and northeast California. Renown Health will continue to operate as an independent nonprofit organization and health system and will continue to accept all current health insurance plans for patient care.
Through this agreement, Kaiser Permanente and Renown Health will enter into a joint venture. Kaiser Permanente will acquire a majority interest in Hometown Health. There are no immediate changes to Hometown Health/Senior Care Plus plans, networks or points of contact. The transaction is anticipated to close in early 2026 following required regulatory approvals.

Mindray has announced a deepened commitment to Mercy Ships through an expanded partnership that includes its North American organization.
This partnership underscores Mindray’s long-term, global support for the organization, which works tirelessly to deliver life-changing surgeries, medical training, and lasting healthcare improvements across Africa, a press release states. This collaboration further reinforces Mindray’s mission to advance medical technologies to make healthcare more accessible.
Mercy Ships operates two state-of-the-art hospital ships, Global Mercy and Africa Mercy, providing free surgical care and training programs to underserved communities. For regions with limited access to surgical care, including over 95% of sub-Saharan Africa’s population, these hospital ships serve as a beacon of hope, addressing critical healthcare disparities.
“At Mindray, we are driven by a vision of better healthcare for all. Our partnership with Mercy Ships allows us to live out this mission, ensuring that leading-edge medical technologies reach those who need them most, even in the most challenging environments,” said Wayne Quinn, president, Mindray North America. “By delivering robust, reliable solutions and supporting caregivers with advanced tools, we empower clinicians to provide lifechanging care. Through innovation, a steadfast

commitment to quality, and a focus on meeting clinical needs, we are proud to help break barriers and deliver on the promise of equitable healthcare worldwide.”
Through this partnership, Mindray North America is playing a pivotal role by equipping Mercy Ships with innovative medical technologies that elevate patient care and strengthen healthcare capacity, according to the release. The donation of its technology, including multiple cutting-edge ultrasound systems, highlights Mindray’s ongoing investment in empowering medical teams with best-in-class tools enabling high-quality care in the most challenging scenarios.
Since initiating its relationship with Mercy Ships in 2017, Mindray’s global organization has consistently provided devices and technical support that underpin the success of its medical infrastructure. Today, both ships operate with over 350 Mindray systems, including patient monitors, anesthesia machines, ultrasound machines, defibrillators, and central monitoring solutions. The expanded partnership to include Mindray North America solidifies the company’s role as a critical partner in the Mercy Ships’ life-saving work. Together, Mindray and Mercy Ships aspire to deliver over 50,000 life-changing surgeries while building lasting healthcare capacity – continuously reaching higher to expand access to quality care for those who need it most.

AMX Solutions, a company specializing in portable AMX4+ X-ray systems, is offering a full rebuild service designed to restore units inside and out, extending their useful life for healthcare facilities.
Unlike standard refurbishing, the company said its approach involves a complete rebuild from top to bottom, ensuring units look and function like new.
“There is a world of difference between simply refurbishing and actually rebuilding your AMX unit,” said Lee Ready, president of AMX Solutions.
The rebuild process includes installation of new components such as OEM paint, hardware, GE logo and function labels, a nine-battery set with test block, custom reinforced top cover, rebuilt collimator with front panel and cable, caster wheels with an updated bearing design, a main wiring harness, HV cables, rotor and arm brake cables, drive belts, a hand switch with hanger, bumper pads, wear strips, noise suppressors, filters, grounding cables and a hospital-grade cord reel.
Rebuilt items include the vertical column, horizontal arm, drive wheels, drive motors and drive handle assembly. Tubes are tested, cleaned, sanded and repainted.
AMX Solutions has been in the imaging industry for more than 20 years. The company’s technicians perform all rebuilds, painting and service 100% in house. In addition to complete rebuilds, AMX Solutions offers individual replacement parts and batteries for AMX4+ and Optima units.
“Our mission is to let the industry know that AMX Solutions is still here, with the same team who built our reputation for quality service and customer care,” Lee Ready said. “We want to rekindle old partnerships and create new ones while continuing to provide the same level of excellence people have come to expect from our brand.”
AMX Solutions remains family-owned and operated, led by Ready along with his sons, Campbell and Leland.






Baxter International Inc. has launched the Welch Allyn Connex 360 Vital Signs Monitor, its next generation patient monitoring device. As the latest innovation in Baxter’s connected monitoring portfolio, Connex 360 offers an advanced connectivity and security platform, customizable configurations based on a hospital’s clinical routines, and upgraded capabilities that set the stage for future functionality and enhancements, a press release states. With automated clinical documentation, Connex 360 is designed to simplify the clinician experience and allow more time to focus on patients.
“There is nothing more important than keeping patients safe, especially when time is of the essence,” said Julie Foster, president of front-line care at Baxter. “Care teams rely on vital signs monitoring to help detect changes in their patients’ conditions and make informed decisions. With Connex 360, we are advancing patient monitoring to provide care teams with the timely insights they need at the bedside, so they can make the most of every moment with their patients.”
In today’s complex healthcare environment, care teams and hospital staff are being asked to do more with less, and timely, accurate, and secure patient information is critical. Baxter’s Connex 360 monitor captures vital signs for adult, pediatric and neonate patients, including blood pressure, temperature, pulse rate, respiration rate and blood oxygen levels. Designed for optimized workflows, care teams can obtain a full set of patient vitals in less than one minute and automatically send to the electronic medical record, helping to reduce manual data entry errors and support informed care decisions.
This secure data flow is powered by Baxter’s cloud-based DeviceBridge platform, which works behind the scenes to
help enable accurate data transfer from Connex 360 to hospital IT systems, including the EMR. DeviceBridge also helps support secure device access and clinical data interoperability across Baxter’s ecosystem of connected devices.
Additional key features of Connex 360 include:
• Comprehensive security: Enhanced security features, including end-to-end encryption and certificate-based authentication, help protect patient and facility information.
• Intuitive interface: Baxter’s largest and most responsive color touchscreen allows intuitive navigation and the ability to see more patient data on one screen.
• Enhanced customization: Customizable Early Warning Score protocols alert clinicians to patient deterioration or changes in condition, and fully configurable monitoring modes support the individual needs of the patient.
• Easy upgrades and maintenance: Software upgrades for clinical, operational, and cybersecurity enhancements are streamlined, helping to reduce the burden on hospital IT teams and allowing for enhanced functionality over time.
Baxter expanded its presence in the patient monitoring space through its acquisition of Hillrom and the Welch Allyn portfolio. Welch Allyn has a longstanding history of delivering innovative patient monitoring solutions that support patient care across healthcare settings, from hospitals to outpatient care. Baxter has continued to invest in connected monitoring, with Connex 360 joining the company’s trusted lineup of vital signs solutions. The Connex 360 monitor received U.S. Food and Drug Administration 510(k) clearance and is available for hospitals and health systems to order in the U.S.
Dallas is set to host the Fall 2025 MD Expo. The popular HTM conference will once again feature top-notch education, networking events and an exhibit hall filled with industry-leading companies.
The MD Expo, held November 11-12, 2025, is pre-approved for up to 8 CEUs. Additional CEUs can be earned via the H.O.T. Workshops:
• H.O.T. Workshop: Contrast Injector Operation and PM Training (Session #1 or #2) – 4 CEUs
• H.O.T. Workshop: Ultrasound Systems – 7.5 CEUs
The educational lineup features something for everyone from biomed student to seasoned HTM pro and hospital leadership. Topics on tap include cybersecurity, leadership, troubleshooting, imaging devices, hospital inspections and more.
This year, the fall MD Expo will take place during Veterans Day.
TechNation and parent company MD Publishing have announced a special program honoring veterans as part of the fall 2026 MD Expo. And, as always, MD Expo registration if FREE for all active military members. Register today at MDExpoShow. com.
“MD Expo is honored to host our conference over Veteran’s Day. We plan on paying tribute at the event to those in our industry who have served but need your help in making our vision a reality!” the company announced.
Veterans were invited to submit photos of their time in service along with their name, rank, and branch of military. The photos will be displayed for everyone to take a minute and see the men and women who served in the U.S. Armed Forces and continue to take care of a nation as vital members of the healthcare
Enter the contest and help TechNation celebrate. Fill out the short form at 1technation.com/contest for a chance to win one of 12 prizes, each valued at $150 or more!
Additional entries to win can be acquired by sharing on LinkedIn or submitting a photo. Each month, a winner will be selected and featured in TechNation magazine! September winner is Matt Oetker.
Find out more information on Page 92.

industry.
The MD Expo leadership summit and reverse expo are extra options available to advanced HTM professionals.
Additional special events include a new Lunch and Learn set to be held from 12:30-2:30 on Nov. 11. Young HTM pros are invited to attend the popular Young Professionals at MD Expo social event that serves as an outstanding venue for networking with colleagues. Also, the Women In Leadership (WIL) organization will host a women-only W.I.L. Lounge on Tuesday evening.
Everyone is invited to what is sure to be the biggest event at the fall MD Expo – the Texas Throwdown: A Night of Boots, Beats and BBQ.
Afterall, everything is bigger in Texas.
So, mosey on over Wednesday night for a finale party like no other! The Lone Star spirit will be in full effect. Grab your cowboy boots and denim (the dustier the better) and join new and old HTM community members for an unforgettable evening filled with live music, line dancing, BBQ.
Be sure to check out the curated whiskey tasting, then hit the floor for a round of two-steppin’ to the sounds of a live band. Between dances, swing by the Build-Your-Own Bandana Bar to craft a keepsake – the perfect accessory for the night’s festivities.
Whether you’re a seasoned cowboy or just in it for the BBQ and beats, the Texas Throwdown promises a night of good times, great company and the perfect MD Expo celebration.
Find more information and register today at MDExpoShow.com.




Why do hundreds of HTM professionals attended the TechNation Webinar Wednesday series each year? MD Publishing Webinar Marketing Manager Linda Hasluem used a survey to find out!
Survey results show that 60% prefer the live webinars and 88% of respondents said they would appreciate more interaction with presenters via polls, PDF handouts and a longer Q&A session.
“We haven’t conducted a webinar survey since 2023, and I know we have many new attendees since then. So, I thought it would be the ideal opportunity to test some new ideas we have planned for 2026, but at the same time get updated thoughts/suggestions on how we can improve our webinars, etc.,” Hasluem explained when asked how she came up with the idea to do a survey.
“We had a great response, and I’m sure the chance to win a $100 Amazon gift card helped! The suggestions and ideas were interesting and the overall consensus for our two new ideas was very favorable and will certainly help us plan for next year. We had no negative responses, so that’s a plus point in my books, as it means we are doing a good job in providing the HTM industry with what they want from a webinar,” she added.
The two new ideas for 2026 include a Roundtable Spotlight session and “Unlock and Learn” webinars.
The Roundtable Spotlight will provide five companies exclusive access to participate in a live roundtable panel discussion webinar. Survey participants overwhelmingly showed interest in this option with 90% selecting “Likely” or “Maybe” when asked if they found it valuable.
The “Unlock and Learn” series will offer an engaging and structured learning experience where a company presents a series of webinars. Attendees must pass a post-webinar test with a score of at least 75% in order to access the next webinar in the series.
The survey found that 82% selected “Likely” or “Maybe” when asked if they would watch this type of webinar series.
The 2025 TechNation Webinar Wednesday series continues to be popular among HTM professionals. Registrations through August added up to 1,421 with another 948 registrations for the Tools of the Trade Live Demo webinars this year. Find out more


Admar Neuro is a trusted partner for outsourced biomedical and tech support.
It’s goal since 1984 is to provide high-quality, pre-owned EMG/NCV and EEG systems to enhance patient care through precision and reliability. As a leader in neurodiagnostic devices, Admar Neuro is committed to exceptional service and advancing neurological diagnostics worldwide.
The company’s mission is spelled out on its website as, “To empower private practices with reliable biomedical and tech support solutions, ensuring seamless operations through expert service, repair, and replacement of neurodiagnostic equipment.”
Admar Neuro’s vision is, “To be the leading provider of innovative, dependable biomedical services, setting the standard for excellence in neurodiagnostic equipment support and fostering long-term partnerships with healthcare providers.”
TechNation recently interviewed CEO Adriana Muñoz to find out more about Admar Neuro’s 40 years of experience and what is on the horizon going into 2026.
Q: WHAT ARE SOME OF THE SERVICES AND PRODUCTS YOU OFFER? IS THERE A SPECIFIC OR NEW ONE YOU ARE EXCITED ABOUT RIGHT NOW?
Muñoz: At Admar Neuro, we specialize in service, repair,
and refurbishment of neurodiagnostic equipment including EMG, EEG, VNG and TCD systems. We also offer refurbished device sales, rentals and consumables. Right now, we’re especially excited about our expansion into ultrasound repairs.
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
Muñoz: We are 100% focused on neurodiagnostics. With more than 40 years of experience, our deep specialization enables us to deliver faster turnaround times, higher first-time fix rates, and stronger technical support than general repair providers.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY? DO YOU HAVE ANY GOALS YOU WOULD LIKE TO ACHIEVE IN THE NEAR FUTURE?
Muñoz: We’ve launched an asset management program to be the one-stop shop for neurodiagnostic equipment life cycle support – covering everything from preventative maintenance and repairs to rentals and replacements.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE OUR READERS TO KNOW?
Muñoz: We’re eager to partner with industry peers and OEMs, either by bringing our expertise into their organization or serving as an outsourced provider. Our mission is simple: to be the go-to shop for all things neurodiagnostics.
For more information, call 728-900-3969 or email sales@admarneuro.com.

BY JEANNINE BLAKE, P h .D., RN
It’s 3 a.m. in a busy ICU. Nurses move between rooms, managing a constant stream of patient needs. In one of them, a nurse – working his third shift in a row – is running on four hours of sleep, holding out for 7 a.m. and a few nights off. His patient is critically ill. The room is filled with equipment, a tired family sits vigil, and providers come and go asking questions or performing assessments. Phones ring. Labs are due. Medications need administering.
An infusion pump alarm goes off. He sighs, walks over, and squints at the machine. Another occlusion alarm, again with no clear cause. He opens the pump, taps the tubing, gives it a slight tug, checks the connections, closes the door, and presses start … then mentally crosses his fingers that it will run for more than five minutes. The family watches closely. He offers a reassuring nod and a practiced smile, the kind that says, “Everything’s fine,” even though it doesn’t feel fine because this darn thing won’t stop beeping, but alas, this is just what happens night after night.
This nurse is educated and experienced. He knows the tips and tricks for managing these alarms. But they persist –baffling and disruptive. Is it the pump? The fluid? The patient? The training manuals never cover this in a real-world context. They list what to do, but not why. Not in a way that fits with the reality of clinical care or offers pearls of wisdom to critically think through these kinds of challenges. They don’t account for how fragmented, overloaded and error-prone this environment really is.
Medical device safety cannot rely on users operating at their best. Or even at their mediocre. Real clinical care, especially in high acuity settings like the ICU, happens every day, even on caregivers’ worst days, regularly. We can’t fix this by just adding another training session or telling someone to pay more attention.
The clinical environment is cognitively overloaded, emotionally intense and frequently understaffed.1 Efforts to fill
staffing through shift rotations, floating between units, or travel staff increase the odds that a caregiver will be out of their element, even if they are familiar with the tasks and equipment involved. Mistakes are inevitable, believing otherwise is the biggest error of all.² The system must be designed to prevent human mistakes from becoming patient harm. And in healthcare, part of that system includes the medical devices we use to carry out care.³
Devices must be designed to work with humans, not despite them. We cannot assume that users will recall complex training or be mentally prepared to operate devices “as taught.”⁴ We must start from a different premise: people are fallible, and systems must account for that.
Human factors engineering has long emphasized that education and training should not be the primary method of mitigating use error.³ ⁵ ⁶ And yet, when something goes wrong, clinicians are too often blamed, and then sent to retraining sessions that do little to change outcomes. The message becomes: “Do better.”
Training may be the only option in the short term, but it should NEVER be considered a long-term fix. That fix must come from technology, whether through a software patch, hardware update, or full redesign. Yes, those changes are harder. But they’re also more effective.⁷ And adopting that mindset creates space for innovation rather than defensiveness. It also empowers clinicians to speak up when something doesn’t work, without fearing blame, reprimand or judgement.
Devices must reflect the context in which they’re used. For nurses and other frontline clinicians, this context often includes multitasking, frequent interruptions, fatigue and insufficient support.⁸ And yet, in these environments where the consequences of error are high, the tolerance for uncertainty is low.
Despite these stakes, medical technologies are too-often designed without enough meaningful input from their primary users about how it may fit into their daily workflow. That
disconnect has real consequences, and when systems break down, the burden falls on the human user, in the form of more training on how to navigate the broken system.6 Each system breakdown, and each resulting training burden, is just one more responsibility placed on the human instead of the device.
But human factors principles are clear: if two users make the same mistake, it’s not a user problem. It’s a design problem.
Nurses are highly educated professionals. But they’re still human and human error should be expected, anticipated, and prevented through robust systems, not punished or patched with more training.⁹ We need to shift the way we think about accountability. Errors and lapses are not moral failings or evidence of incompetence.² They are signs that the system, including its devices, is failing to support the people within it and the patients whose care relies upon it.
We don’t need to teach nurses to “try harder.” We need devices built for the messy, high-stakes, beautifully human environments in which nurses operate. Training may patch the system for a while, but only design can truly fix it.
Until we stop blaming individuals and start fixing systems, the same errors will continue, and we’ll keep blaming the same people (or the new ones who replace the ones who’ve burned out). It’s time to build devices for real people, doing real work, under real pressure. Because education is not the answer, design is.
– Jeannine Blake is an assistant professor affiliated with the Elaine Marieb Center for Nursing and Engineering Innovation at the University of Massachusetts Amherst. Opinions and insights are her own.
References
1. Carayon P, Gurses AP. Nursing workload and patient safety — A human factors engineering perspective. In: Hughes R, ed. Patient Safety and Quality: An EvidenceBased Handbook for Nurses. Agency for Healthcare Research and Quality; 2008.
2. Kohn LT, Corrigan JM, Donaldson MS. To err is human:: building a Safer Health System. vol 6. National Academies Press; 2000.
3. Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, et al. Human factors systems approach to healthcare quality and patient safety. Appl Ergon. Jan 2014;45(1):14-25. doi:10.1016/j.apergo.2013.04.023
4. Reason J. Human Error. Cambridge University Press; 1990.
5. Russ AL, Fairbanks RJ, Karsh BT, Militello LG, Saleem JJ, Wears RL. The science of human factors: separating fact from fiction. BMJ Qual Saf. Oct 2013;22(10):802-8. doi:10.1136/bmjqs-2012-001450
6. FDA. Applying human factors and usability engineering to medical devices: Guidance for industry and Food and Drug Administration Staff. 2016. February 3. https://www. fda.gov/regulatory-information/search-fda-guidance-
documents/applying-human-factors-and-usabilityengineering-medical-devices
7. OSHA. Identifying Hazard Control Options: The Hierarchy of Controls. Washington, DC: U.S. Department of Labor.
8. Westbrook JI, Woods A, Rob MI, Dunsmuir W, Day RO. Association of interruptions with an increased risk and severity of medication administration errors. Archives of Internal Medicine. 2010;170(8):683-690.
9. Reason J. Human error: models and management. BMJ. 2000;320:768-70.



As pressures mount for health system leaders nationwide, ECRI convened four virtual roundtables with 17 C-suite healthcare leaders from across the country, representing integrated health systems, children’s hospitals, rural providers, and national associations. The goal was to capture unfiltered insights into what truly drives and sustains patient safety and continuous improvement.
The white paper – published on World Patient Safety Day, September 17 – is the first in a series of articles born from the ECRI C-Suite Roundtables featuring candid insights from healthcare leaders. Download the white paper at www.ECRI.org/News.
ECRI President and CEO Marcus Schabacker, MD, Ph.D., and ECRI Chief Medical Officer Dheerendra Kommala, MD,
moderated the discussions.
“This is a tumultuous time for healthcare leaders and frontline providers in our nation,” said Schabacker. “We launched this roundtable series to dive deeper into the issues they’re facing, and how they elevate patient safety despite these challenges – from shrinking resources to medical misinformation to persistent causes of preventable harm. Together we share what’s truly moving the needle. Only by learning from one another and sharing best practices can we break the cycles that have long held our industry back.”
• Proactive systems prevent harm. Leaders are shifting from reactive models to proactive approaches by hardwiring learning into daily operations and embedding human factors into workflows.
• System design and standardization saves lives. Safety issues can cause longer stays and liability. Welldesigned, standardized systems make the safe choice the easy choice and ensure consistent outcomes across sites.
• Safety is a sound business strategy. Health systems that embed safety and create reliable systems can reduce extended stays, boost staff engagement, strengthen payer negotiations, increase patient satisfaction and improve margins.
There was broad consensus among C-Suite Roundtable participants on the challenges and the path forward. The groups also discussed these challenges, slated for forthcoming publications in the series:
• How organizational culture shapes safety reporting and outcomes
• Navigating care delivery without trusted federal agencies as beacons of truth
• Role of boards and executive leaders in safety and quality
Participants included (in alphabetical order):
• Rick Bassett , MSN, RN, APRN, ACNS-BC, CCRN, FCNS, LSSGB, President of the National Association of Clinical Nurse Specialists, and Adult Critical Care Clinical Nurse Specialist at St. Luke’s Health System;
• Richard Bates , MD, Chief Executive Officer, Thunder Bay Community Health Service;
• Matthew Davis , MD, MAPP, Executive Vice President, Enterprise Physician-in-Chief, and Chief Scientific Officer, Nemours Children’s Health;
• Mindy Dunkerley, MBA, MSN, RN, Chief Quality Officer, Independence Health System;
• Michael Fiorina , DO, FAAFP, Chief Medical Officer, Independence Health System;
• Jonathan Gleason , MD, EVP and Chief Clinical Officer, Prisma Health;
• Djana Harp , MD, MS, MBA, Chief Medical Officer, Norwalk Community Health Center;
• Omar Hasan , MD, MS, MPH, Chief Quality Officer, MaineHealth;
• Mark Leahey, President & CEO, Medical Device Manufacturers Association;
• Kristie Lenze, CEO/CFO, Keystone Rural Health Consortia;
• Tara Jo Manal , PT, DPT, FAPTA, Vice President of Scientific Affairs, American Physical Therapy Association;
• William G. Morice II , MD, PhD, CEO, Mayo Clinic Laboratories;
• Michael Seim , MD, Chief Quality Officer, WellSpan;
• Anne Marie Watkins , DNP, MSHCA, RN, CENP, Chief Nursing Executive, UCI Health;
• Jacqueline Webb , CPNP, Quality, Risk, and Lab Director, Tri-Area Community Health;
• James Werth, Jr. , PhD, ABPP, Chief Executive Officer, Tri-Area Community Health;
For more information, visit ECRI.org.

Mako is a cutting-edge solution that revolutionizes your way of work with plug-and-play simplicity. It’s our most efficient and versatile meter, delivering the highest practical accuracy experienced with the industry’s broadest application range. Beyond the hills of spreadsheets, Ocean NextTM software awaits. Ocean NextTM gathers data from Mako in real-time so you can truly excel. Immerse yourself in a world of streamlined routines and complete traceability, Ocean NextTM.


BY JUSTIN LACKEY
rom hospitals to senior living facilities to medical supply companies, no detail is too small. A single piece of misplaced equipment can delay care, increase operational costs and compromise patient safety. Yet, many facilities still rely on spreadsheets or outdated systems to manage millions of dollars’ worth of medical devices and equipment. For biomedical and healthcare technology management (HTM) professionals responsible for maintaining uptime and compliance, the right tools can prevent daily maintenance challenges.
Modern asset tracking systems offer more than just location monitoring. For hospitals and healthcare networks, the key to effective asset management lies in centralizing life cycle data, streamlining preventive maintenance, and enabling smarter facilities and cleaning protocols. These capabilities are no longer
nice to have; they’re essential in the high-stakes world of patient care.
LIFE
Effective asset tracking provides a full view of the product life cycle, from purchase and deployment to retirement or replacement, enabling teams to go beyond simply knowing a device’s location. Centralized records enable biomedical teams to access a wide range of information, including warranty status, service history, performance benchmarks and cost of ownership.
For example, CHG Healthcare, a leading staffing provider in the medical space, used advanced asset tracking to streamline the management of its equipment across multiple sites. By consolidating monitoring into a single platform, CHG Healthcare not only gained real-time visibility but also reduced the risk of duplicate purchases and unmanaged inventory. Transparency of this kind is particularly critical for hospitals, where purchasing decisions must balance
“When devices are properly tracked, maintained, and optimized, the result is more time for patient care and fewer preventable errors.”
patient care needs on tight budgets.
Life cycle management also plays a vital role in meeting compliance requirements from agencies such as The Joint Commission and the Centers for Medicare & Medicaid Services. Healthcare facilities can avoid last-minute scrambles during audits and maintain a strong regulatory posture year-round with documentation readily available.
One of the most tangible benefits of advanced asset tracking is the transformation of maintenance workflows. Preventive maintenance scheduling shifts operations from being reactive, saving repair and replacement budgets. Immediate benefits include reducing the number of emergency repairs, improving equipment uptime and extending the lifespan of assets.
Biomedical professionals can set up automated alerts for routine maintenance, ensuring that devices are inspected, calibrated, and serviced according to manufacturer recommendations or internal schedules. Maintenance logs are tied to each asset profile, allowing technicians to quickly see what work has been completed, who performed it, and when it was done, thereby avoiding redundant work and enabling better team coordination.
Analyzing maintenance trends enables facilities to identify underperforming equipment and assess total cost of ownership. These insights support more effective capital planning and guide smarter decisions around upgrades or replacements.
While much of the focus in asset tracking centers on clinical equipment, there’s growing recognition of its value in facilities management and infection prevention. Cleaning schedules, disinfection protocols and room turnover procedures can all be standardized and verified through the same asset management system.
When the pandemic heightened scrutiny on infection control, NexClean, a provider of cleaning and disinfection services for healthcare environments, needed greater accountability across its operations. By implementing advanced asset tracking, the team developed digital checklists for cleaning workflows,
monitored equipment usage by location, and documented adherence to CDC-recommended protocols. That level of transparency became critical as environmental services played a direct role in both infection prevention and patient satisfaction.
In-house healthcare maintenance teams can adopt similar models to improve room readiness, monitor HVAC and water filtration systems, and ensure critical areas such as operating rooms or NICUs meet cleanliness standards.
To succeed, asset tracking must be more than a backend system. It needs to be integrated into the daily workflow using mobile-friendly applications and customizable interfaces, with features such as barcode scanning, photo attachments and user-specific access levels. These tools make it easy for teams to update records in real-time, 365 days a year, 24 hours a day, regardless of their location.
Integrating manuals, SOPs and service records into each asset profile provides technicians with all the necessary information at their fingertips. Providing easy access to these assets helps reduce downtime, improves training efficiency and promotes consistency across teams and shifts.
Hospitals face unprecedented challenges, including staffing shortages, supply chain issues and heightened scrutiny of operational efficiency. Asset tracking may not solve all these issues, but it is a foundational piece of the puzzle. When devices are properly tracked, maintained, and optimized, the result is more time for patient care, fewer preventable errors and greater confidence in the equipment that powers modern medicine.
For healthcare professionals, adopting a comprehensive asset management solution is no longer optional. It’s a strategic decision that supports patient outcomes, regulatory compliance and financial stewardship – all from one digital dashboard.

Justin Lackey is the president of Asset Panda, a cloud-based asset management platform.
Watch these webinars on-demand
The recent TechNation Tools of the Trade Live Demo webinar “ESA700 Series Electrical Safety Analyzers” drew strong global interest with 41% of attendees joining from outside the U.S. Sponsored by Fluke Biomedical, the session was eligible for 1 CE credit from ACI.
Fluke Biomedical Technical Sales Engineer Jamie Spragis, CBET, led the demo, sharing expertise and answering questions in real time. He noted Fluke teams worldwide are available to provide training and onsite demonstrations for those wanting a deeper dive into the ESA700 Series.
The ESA700 Series Electrical Safety Analyzers are Fluke Biomedical’s easiest-to-use models to date. Combining a safety analyzer and patient simulator, they deliver precision, versatility and portability in one package. Key features include:
• Small and portable – Run procedures on the go
• Auxiliary battery – Avoid power cycling between tests
• Touchscreen interface – Intuitive and efficient
• Standalone operation – Run procedures without a PC using OneQA workflow software
Spragis encouraged attendees to elevate their electrical safety testing with these tools.
The webinar set new benchmarks for registrations (289) and attendees (109), with 27 requesting follow-up from Fluke. A recording remains available at WebinarWednesday.live.
A trivia prize went to Mark Woodruff of California, who won a Swiss Force Meister Multi-Tool.
Survey feedback highlighted the analyzer’s strengths. Thomas Kockler of Technical Life Care cited “ease of use and adaptability.” David Banister of Clinical Engineering Services noted “automatic pushing of quantitative data to a CMMS.”
Todd Walker of Oregon Health & Science praised its “compact and versatile” design, while Bryan Medical Center’s David Miller called it a “cool package” for ECG and ESIs. Athira Ashokan of One Health added that it “simplifies workflows and improves efficiency while being user-friendly.”
As healthcare environments become increasingly connected, the need to secure biomedical devices while ensuring operational efficiency and patient safety has never been greater. A recent Webinar Wednesday presentation offered an in-depth look at how Claroty empowers biomedical engineering teams to integrate robust cybersecurity and device management into their daily workflows.
Webinar co-presenters David Guffrey and Joe Walker explored:
• How to provide a comprehensive and accurate inventory of connected medical devices.
• How teams gain visibility into patch levels, utilization trends, procurement planning and more.
• How actionable insights support smarter decisions for biomedical engineering technicians.
Attendees were also able to submit questions to the presenters for additional insights.
The webinar was sponsored by Claroty and 75 healthcare professionals tuned in for the live presentation. A recording of the webinar is available for on-demand viewing at WebinarWednesday.live.
Don Bass, who works in the biomedical engineering department at Jackson Park Hospital & Medical Center in
Illinois, won a Swiss Force Meister Multi-Tool.
Attendees provided feedback via a survey that asked “Excluding CE credits, why do you attend Webinar Wednesday?”
“To stay informed and updated on changes in the medical field biological equipment testing,” said Martin O’Brien, biomedical technician instructor, RTS-Medical.
“To stay as current as I can on HTM topics,” said Gerald McNeil, BMET III, GE HealthCare.
“Nowadays, AI is booming, and AI is integrated into our medical devices. This trend exposes our devices’ cybersecurity vulnerability to outside and inside. Thus, I need to know how I can prevent any future threats by attending these types of webinars,” said Tedd Koh, MET, Olive View UCLA Medical Center.
“I attend to expand my BMET knowledge and for exposure to topics and modalities I’m not too familiar with,” said Charitable Roosevelt, BMET, Endeavor IO.
For more information about TechNation’s Webinar Wednesday series, including upcoming webinars, visit WebinarWednesday.live. Find details about continuing education opportunities at the fall MD Expo in Dallas at MDExpoShow.com.


Registration on WebinarWednesday.live. Eligible for 1 CE credit from the ACI.
The FlowAnalyser PRO is IMT Analytics’ flagship benchtop gas flow analyzer, designed for biomedical engineers, independent service providers and medical equipment manufacturers. Engineered to test all types of mechanical ventilators, anesthesia machines and a wide range of other medical devices that generate gas flow and pressure, it combines the full capabilities of the industry-leading FlowAnalyser PF-300 series into one powerhouse. With the addition of an ultra-low-flow channel, the FlowAnalyser PRO can precisely measure across the entire spectrum, from the smallest flow rates and vacuum pressures to hospital gas line pressures and flows up to 300 L/min. Its 5-inch highresolution multi-touch display offers an intuitive, modern,
user interface. Advanced features such as profile handling, on-device testing applications, smart automatic trigger detection and data recording help streamline workflows, reduce handling errors and improve measurement accuracy. When paired with the OR-703 MultiGasAnalyser, a highprecision gas analyzer measuring CO₂, N₂O and all common volatile anesthetic agents, the FlowAnalyser PRO transforms into a complete anesthesia machine testing solution. Whatever the testing requirements are, the FlowAnalyser PRO delivers with the highest precision and seamless user experience.
For more information, visit imtanalytics.com


BY JAMES LINTON
As a BMET professor teaching over 100 students annually at St. Clair College, I’ve learned that while textbooks provide the foundation, real-world application is where students truly come alive. One of the most powerful tools I’ve integrated into my classroom isn’t a physical simulator or advanced diagnostic device — it’s MedWrench.
MedWrench is more than just a forum — it’s a living, breathing ecosystem of working professionals, real-time equipment issues, and community-driven problem-solving. For my students, it offers a unique window into the HTM world as it exists today — not how it was when I started my career long ago.
Here’s how I use MedWrench in my program and why I think more educators and technicians should consider doing the same.
Every week, I have begun discussing with my students a discussion post pulled directly from the MedWrench forums. It might be a service issue with a GE monitor, a strange DICOM error, or someone struggling to get a legacy sterilizer to pass a self-test.
Students are then asked to:
• Analyze the reported symptoms
• Research the equipment’s service manual (if available)
• Draft a hypothetical step-by-step troubleshooting plan During these discussions they’re evaluated not on being “right,” but on how well they think through the problem — this teaches structured diagnostic reasoning, something no multiple-choice test can replicate.
ENCOURAGING STUDENTS TO RESEARCH, ASK AND CONTRIBUTE TO THE ONLINE COMMUNITY
I challenge my more advanced students (especially in their final year) to actively contribute to MedWrench. That means:
• Posting their own questions
• Answering others if they have the expertise
• Sharing a tip they’ve learned from co-op or class
This builds:
• Confidence in their knowledge
• Digital professionalism — knowing how to ask smart questions
• A habit of lifelong learning and community involvement
TEACHING DIAGNOSTIC THINKING THROUGH REAL EQUIPMENT FAILURES
MedWrench provides a constant stream of authentic problems techs are facing in hospitals and clinics. These aren’t theoretical failures — they’re gritty, messy and sometimes unsolved.
I use them in:
• Case studies: We’ll break down an issue as a class, roleplaying as in-house techs or field service reps.
• Sim labs: I recreate the problem on similar devices in our lab, adding faults or failures for them to find.
• Assignments: Students write a mock service report with documentation and repair notes. This process teaches them more than just how to fix things — it instills:
• Accountability
• Logical sequencing
• Communication under pressure
When students see an issue on MedWrench and then experience something similar during their co-op or first job, it validates their preparation. They’re not blindsided because they’ve already walked through it intellectually.
Plus, by seeing how real techs document, communicate, and even disagree in the forums, students learn:
• How HTM professionals think in the wild
• That there’s no shame in asking for help
• That community is key in a field that changes daily
Whether you’re an instructor, manager, or working tech, MedWrench isn’t just a help desk — it’s an educational goldmine.
• Use it to find real examples to supplement your lectures
• Have students present cases in class
• Build assignments that mimic what they’ll face in the field
• Post challenges your department is facing and invite student input
• Use it to spot common issues or training gaps
• Take part in various TechNation challenges to build a sense of community
• Mentor without pressure sometimes even a single reply helps a student grow
In an industry as complex and ever-evolving as HTM, building bridges between generations is essential. MedWrench has become a bridge in my classroom — and it can be one in yours too.

For more information, visit medwrench.com.








Detect
Lightweight,
Stay
Helps
As HTM continues to evolve, the demands placed on test equipment are rapidly expanding – from improved automation and digital integration to enhanced portability, accuracy and reliability. In this Roundtable article, TechNation asked leaders across the test equipment landscape to share their insights regarding the latest trends, challenges and innovations shaping how biomedical professionals service and support medical devices.
Participants in this year’s Roundtable article on test equipment are:
• BC Group International Director of Business Development Justin Barbour;
• Datrend Systems Inc. Director of Business Development Owen Liu;
• Pronk Technologies Vice President of Business Development Greg Alkire;
• Rigel Medical Business Development Manager Lewis Lennard;
• RTI Group Vice President of Sales North America Steve Holmes; and
• Southeastern Biomedical Co-Owner Boyd Campbell, CBET, CRES, CHTM.
Q: WHAT TRENDS ARE YOU SEEING IN TEST EQUIPMENT DEVELOPMENT FOR THE SERVICE AND REPAIR OF MEDICAL DEVICES?
BARBOUR: There’s a huge demand for automating technician tasks. Fewer clicks and less user intervention leads to higher efficiency. Most of the largest medical technology service companies in the industry are developing or using automatic data input for CMMS work orders.
LIU: Medical device test equipment is shifting toward greater digitalization and connectivity, enabling more automation, predictive maintenance and integration with hospital IT/CMMS systems. At the same time, regulatory compliance, cybersecurity and interoperability are driving demand for more advanced, automated and traceable
testing tools. Usability, modularity and sustainability are also becoming key differentiators for service and repair applications.
ALKIRE: It’s critical for test equipment to stay current with the latest medical device requirements for performing service. As test equipment manufacturers, we make sure that the test equipment design will meet or exceed the manufacturer’s accuracy requirements as well as specifications and features needed to perform the tests. Beyond this, we provide biomeds with the tools to minimize their workload, such as an app that brings multiple technologies into sync. This allows biomeds to move seamlessly through their service and maintenance responsibilities. Technicians can systematically and efficiently proceed according to the manufacturers’ requirements (or edited versions for spot testing) for both setup and testing with on-the-go smart procedures. These are not simply instructions but dynamic interfaces with ready-to-launch test sequences as well as recordkeeping for CMMS systems or database uploads.
LENNARD: Portability and ease of use are front and center. HTM teams are on the move, and the less bulky the equipment, the better. We’re also seeing more automation and guided workflows built into analyzers, which really helps reduce user error and speed up testing. Displays have improved massively too with clear, color screens that make results easier to interpret at a glance, which makes a big difference in busy HTM environments.
HOLMES: Currently, we see a trend where hospital engineers, ISOs and manufacturers are leveraging the available features in the Ocean software to create automated workflows for service engineers. This reduces the time required for service and minimizes downtime for clinics. We are also receiving more requests for quick turnaround times on equipment calibration to ensure engineers can remain up and running without interruption. Alongside this shift, RTI introduced the Mako X-ray test meter, the most accurate compact platform designed for faster setup and Ocean-driven automated workflows.
CAMPBELL: The biggest trend we see as a test equipment calibration and service company is the growing demand for reliability. More customers are recognizing the value of investing a little more upfront to purchase higher-quality devices. Since most biomedical departments do not have backup test equipment, any downtime for repairs can create serious challenges in completing PMs and service work on schedule. Over time, many users have realized that when the mean time between failure is longer, the total cost of ownership is actually lower. Fewer breakdowns mean less downtime, less disruption, and greater overall efficiency – making reliability one of the most important considerations when selecting test devices.
Q:
BARBOUR: The largest issue seems to be proprietary test equipment ecosystems or existing test equipment software limitations. BC Group has invested hundreds of thousands of dollars into developing software that can be used with any test equipment while providing the flexibility for user customization of their tests. We take feedback from our customers and have a team of engineers constantly improving our software: myBC Mobile app and myBC Connect cloud service.
LIU: HTM professionals often face challenges such as keeping up with rapidly evolving device technologies, ensuring compliance with complex regulations and audits, plus managing a wide range of equipment with limited budgets and staff. They also need test tools that are accurate, easy-to-use and seamlessly integrate with their documentation workflows. Companies like Datrend help address these challenges by developing intuitive, modular and standards-compliant test equipment that automates testing, reporting, supports connectivity with CMMS systems and reduces training burdens – ultimately saving time while ensuring patient safety.
ALKIRE: When selecting test equipment, there are some key criteria to consider. First, performance and accuracy are really important. Make sure the test equipment includes not only the features you need but that its specifications meet your medical device manufacturer’s requirements for accuracy. Second, you need to keep reliability and durability in mind. The equipment needs to be able to handle the wear and tear that occur in the field. Test equipment should meet standards for durability such that biomeds don’t have to deal with unexpected downtime or problems with operational integrity as a unit is subjected to the bumps and bruises of everyday use. This is one key reason we subject all our product designs to drop tests 50 times from 3 feet followed by performance tests that confirm operation within specifications.
LENNARD: The challenge is often finding tools that balance compliance with simplicity. No one has time for equipment that’s overly complicated. With Rigel, we’ve focused on intuitive designs, the Uni-Therm has automated walkthroughs, the SafeTest 60+ keeps electrical safety testing simple and efficient, and the UniPulse 400 is light and portable enough to carry anywhere. It means biomeds can get accurate results quickly, without being slowed down by the tester itself.
HOLMES: One challenge is storing measured data securely. This can be addressed by saving data backups in the cloud. Another challenge is ensuring that everyone follows the same procedure and keeps those procedures up to date. Templates that include all instructions and required parameters provide a solution, and with RTI’s






solutions, these templates can be updated automatically for all users. A positive development in test equipment is that it continues to improve, making measurements easier. However, this can also lead to a loss of knowledge among users. This challenge can likewise be mitigated by using well-structured templates with clear instructions.
CAMPBELL: In today’s environment, where there are so many open positions for biomedical technicians, it has become increasingly important that devices enable technicians to complete their tasks in a timely manner. One way this is being addressed is through automation. For example, the new Fluke Biomedical ESA710 Safety Analyzer allows all PM procedures to be stored in the analyzer and performed automatically, saving valuable technician time. Another critical factor is ensuring that test devices are readily available when needed. At Southeastern Biomedical, we recognized long ago that the more calibrations that can be completed onsite during annual service, the more productive the entire biomedical department can be. As a company founded and operated by certified biomedical technicians, we bring a unique perspective – we’ve been on the technician’s side of the bench. That’s why we equip our calibration team with the broadest range of testing standards to maximize what can be completed onsite. Of course, not every calibration can be performed in the field. For those requiring depot service, we maintain an average turnaround time of less than five days, ensuring that critical equipment is returned quickly and downtime is minimized.
BARBOUR: Most technicians only use 50% of their test equipment features. Through YouTube videos, Teams training sessions, trade show demonstrations, a custom AI assistant, and email support, we are dedicated to ensuring our users get the most from their test equipment.
LIU: Training and support play a vital role in ensuring HTM professionals get the full value from today’s advanced simulators and analyzers. While the technology has evolved far beyond legacy test equipment to highly connected and automated solutions, even the most capable tools only deliver results when users are confident and efficient in operating them. Intuitive product design can help reduce ramp-up time, but ongoing education and refresher training remain essential for keeping teams current with new features, standards and best practices.
ALKIRE: We believe this really begins with our product designs, which focus on intuitive interfaces to make it easy to use. Not having to navigate through lots of menus makes using the equipment much easier at the outset. To complement this, having a library of how-to videos for customers who may have more technical questions about how to set up or use our equipment for some unique tests addresses most questions. Another feature that really assists biomeds in getting the most benefit from using our products is the Pronk Mobilize wireless solution. It’s a great tool in that it gives biomeds the ability to have a library of test procedures with testing automation within them for performing service on a wide range of medical devices, and it also generates a complete test report automatically at the end of the testing to streamline the entire service process and save time.
LENNARD: Training and support are crucial. Even with simpler equipment, people want to feel confident from day one. We put a lot of emphasis on hands-on demos, quick-start sessions, and webinars, so users know how to get the most from the analyzer straight away. And if there are questions later, our support team is there to back them up.
HOLMES: Although the equipment is easy to get started with, many features are often overlooked if users only focus on the basics. Training ensures that professionals gain a deeper understanding of the full capabilities of the system, enabling them to use advanced functions that
can save time, improve accuracy and enhance workflow efficiency. Being part of the purchasing process and offering tailored training also helps customers make more informed decisions by clearly demonstrating the differences between systems and how these align with their specific needs. RTI training, in particular, provides a structured way to transition smoothly to a new X-ray system, ensuring that users feel confident in their daily work. Beyond the initial setup, continuous training and support also help customers adapt to software updates, new features, and evolving requirements – making sure the solution remains valuable long after the initial purchase.
CAMPBELL: As devices incorporate more capabilities and biomedical technician training levels vary, it becomes increasingly important that these devices are used to their fullest potential. To achieve this, all available features must be utilized, which requires accessible and effective training. Training should be concise, easy to understand, and available in multiple formats – whether through onboard user manuals, videos or onsite sessions. Just as we expect medical device manufacturers to provide thorough education and support for their equipment, the same standard should apply to biomedical test device manufacturers. Choosing a company with dedicated people who are willing to come onsite and provide hands-on education is essential to ensure technicians can confidently and efficiently use these tools.
Q: HOW DO YOU SEE TEST EQUIPMENT EVOLVING IN THE NEXT 5-10 YEARS TO MEET THE NEEDS OF THE HTM COMMUNITY?
BARBOUR: Within the next 5 years, we’ll see more predictive maintenance and AI supported diagnostics. Within 10 years, most all of the medical tech companies and hospitals will be utilizing some sort of automation and standardized testing.
LIU: Over the next 5-10 years, test equipment will become more connected, automated and intelligent (AI) with seamless integration into CMMS and hospital IT systems to streamline workflows and documentation. We’ll also see greater emphasis on predictive maintenance, cybersecurity validation and interoperability testing as devices become more networked and software driven. At the same time, usability and training support will remain central, with tools designed to be more intuitive, reducing the learning curve and helping HTM teams do more with fewer resources.
ALKIRE: We are in very exciting times. Technology is expanding very rapidly in many areas, healthcare included. This has generated the capability for test equipment to reach higher standards of portability and
durability as well as great improvements in flexibility whereby biomeds can tailor their particular testing needs to their workflow, i.e., user-configurability. Software solutions that connect all of the HTM workflow pieces together to address the administrative tasks for performing, documenting and storing service activity into a CMMS will be key to success in our industry.
LENNARD: Test equipment will continue to get smaller, faster and more user-friendly. I think we’ll see even more automation in test sequences and clearer guidance built into devices, which helps reduce variability between users. Portability and integration with CMMS will also keep driving design, biomeds want testers that are easy to carry, but still give them all the functionality they need.
HOLMES: The next wave is automated, template-led QA, precisely what Mako and Ocean are built to deliver at scale. Automation of workflows is an increasingly requested feature and represents a trend that will continue to evolve, offering ever-greater support for HTM professionals. By streamlining repetitive tasks and standardizing procedures, automated workflows reduce the risk of human error, shorten service times and ensure consistency across different teams and facilities. For HTMs, this means more efficient use of resources, quicker turnaround for essential services, and ultimately improved uptime and reliability of medical equipment –factors that directly impact the quality of patient care.
CAMPBELL: Test equipment automation is rising to the top of the priority list. As manufacturers release new devices, we’re seeing a strong focus on tools that not only perform functions more quickly, but also streamline and standardize the documentation process. Automation reduces the time required for routine tasks, ensures consistency and gives biomedical technicians more bandwidth to focus on higher-level troubleshooting and patient safety.
Q: WHAT ADVICE WOULD YOU GIVE TO HTM PROFESSIONALS WHEN IT COMES TO EVALUATING AND INVESTING IN TEST EQUIPMENT?
BARBOUR: Limit the models of each type of test equipment in your inventory. Try to keep each analyzer and simulator to 1-2 models which will greatly improve technician proficiency. Reach out to test equipment manufacturers for training opportunities – all test equipment companies have excellent staff training programs.
LIU: I’d recommend HTM professionals to evaluate test equipment in terms of total value – accuracy, compliance, ease of use, integration with existing systems, plus longterm upgradability and support. Prioritize tools that are future-ready (scalable, modular and software-upgradable) so they can adapt as standards, technologies and
hospital needs evolve. Finally, consider the training and support resources offered, since these can greatly impact efficiency, staff confidence, and overall ROI.
ALKIRE: Test equipment is a long-term type of investment. It is important to select a product with high reliability, great durability to reduce downtime, and paths for upgrades to reduce costs in the long run. You want a product that provides great value by including a standard multi-year warranty at no extra cost. Ask about what kind of durability testing has been done and what level of abuse the product can handle when used in the field. Look at the performance specifications for its features and verify it does meet with the medical device manufacturer’s requirements. Does it have a good solution for generating service reports that can be seamlessly integrated into your CMMS? What software solutions are available to improve a biomed’s ability to service medical devices? Not all solutions are the same, so have the manufacturer demonstrate the key capabilities that you need.
LENNARD: Don’t just look at the upfront price or the spec sheet. Think about the bigger picture, how portable and easy to use is it day-to-day, and what does it really cost you over time? Some equipment comes with subscriptions or ongoing fees, and that can add up quickly. It’s worth asking whether you’ll be locked into those extra costs, or if the analyzer is straightforward to own with just calibration and support. The right tester should just make your job easier.
HOLMES: Make sure to fully understand the capabilities of the systems under consideration. Take the opportunity to invite RTI’s team to demonstrate the hardware and software or set aside time for an online presentation. Evaluate the total cost of ownership – not only the initial investment, but also long-term service and support. Ensure that the solution is future-proof and able to evolve with your needs. Avoid investing in outdated platforms that risk being discontinued.
CAMPBELL: Test equipment automation is rising to the top of the priority list. As manufacturers release new devices, we’re seeing a strong focus on tools that not only perform functions more quickly but also streamline and standardize the documentation process. Automation reduces the time required for routine tasks, ensures consistency and gives biomedical technicians more bandwidth to focus on higher-level troubleshooting and patient safety.
Q: IS THERE ANYTHING YOU WOULD LIKE TO ADD?
BARBOUR: Establish a test equipment feedback system within your facility. Technician complaints are the greatest resource to improving your maintenance program. Share that feedback with your test equipment
vendor. Management needs to go through their entire test equipment inventory every year (with senior technicians). Inspect for damages, missing parts/ accessories, expired calibrations, leaking or old batteries, and excessive or unneeded test equipment.
LIU: Only that the role of HTM professionals has never been more critical – balancing patient safety, compliance and efficiency in an increasingly complex healthcare environment. By partnering closely with customers, we aim to design test equipment that not only meets today’s requirements but also evolves with tomorrow’s challenges, making their jobs easier and their impact greater.
ALKIRE: The great thing about our industry is that customers have choices in what is the best test equipment solution, so don’t be afraid to ask the hard questions about their products, reliability, service and innovations that separate them from a competitor. At Pronk, we are very open to demonstrating our products in person, talking about the features and accuracy that set us apart and how the durability testing we do provides a very high reliability, which enables us to include an industry-best 4-year warranty at no extra cost to our customers.
LENNARD: HTM professionals do an incredible job under tight constraints. Our role is to listen and make sure we’re building tools that actually help them in the field. The best feedback we get is from biomeds using our testers day-to-day, and that’s what pushes us to keep improving on portability, simplicity and usability.
HOLMES: Building on the new Mako X-ray meter, RTI Group continues to evolve and will unveil another major development at RSNA’s Annual Meeting, Nov. 30-Dec. 4, 2025. Follow RTI Group on LinkedIn and email us at sales.us@rtigroup.com to be among the first to hear the news. Lastly, did you know RTI is now the exclusive North American distributor for VacuTec’s DAP and AEC Chambers? Let’s connect, we’re ready to help future-proof your department and drive meaningful change.
CAMPBELL: This is an exciting time in our industry, with many new developments shaping the way we work. Take the opportunity to have a knowledgeable representative come onsite to demonstrate the latest technology. They can also assist with future planning by helping you develop a 3- or 5-year budgeting plan. Ask them to review your current inventory to identify any devices that may be out of support or discontinued. Most importantly, ensure that you have the support you need throughout the life of your test equipment, so your biomedical department can continue to operate efficiently and confidently.
• Register for free and apply to any of our listings
• Browse our 350+ open positions across the United States

• Get directly connected with industry hiring managers
• A talent network of 3600+ actively looking biomedical and imaging professionals
• A variety of listing and advertising options
• Social media, print, and eNews promotion
“HTMjobs.com is a remarkable website that can be used by any personnel looking for employment, internships, or just a good read from their Career Center . The site offers job postings from employers in the HTM industry, as well as resources for job seekers to help them prepare for their job search, including resume tips, interview advice, and more.”

By K. Richard Douglas
The healthcare industry is experiencing a profound transformation, propelled by a wave of technological advancements that are reshaping the way care is delivered, managed and experienced.
As healthcare looks to the future, the landscape is defined by the continued prominence of emerging technologies, with artificial intelligence (AI) remaining a central theme. Breakthroughs in biomedical technology are bridging the gap between scientific discovery and clinical application, ushering in an era of more personalized, efficient and data-driven healthcare systems.
The key trends and innovations poised to redefine healthcare and biomedical science include everything from AI-driven diagnostics to advanced regenerative medicine and enhanced cybersecurity.
Artificial intelligence and machine learning (ML) stand as one of the most exciting and promising technological advancements in healthcare, significantly improving the speed and accuracy of diagnostics. AI algorithms are capable of analyzing vast datasets, including medical imaging, genetic information, and electronic health records. It can identify patterns and predict disease outcomes with unprecedented speed and efficiency.
The AI revolution will shape the future of medicine for clinicians and patients and find applications in HTM that are already changing the way things are done in the biomed shop.
For clinicians, AI is already aiding in patient care. In diagnostics, AI-powered tools are becoming increasingly adept at interpreting medical images like X-rays, MRIs, and CT scans. In some cases, it detects early signs of cancer and other conditions more accurately than human radiologists.
For instance, AI algorithms can swiftly process CT scans to detect pneumonia patterns.
Beyond imaging, AI models can predict wound healing by analyzing various factors and suggest optimal debridement methods for diabetic foot ulcers, leading to tailored treatment plans and reduced risks. AI is also being integrated into platforms for patient appointment scheduling, utilizing electronic health records (EHRs) to match patients with the most appropriate providers.
The biomed department, a repository of technology and tech-focused professionals, is a participant in healthcare innovations, along with trends and applications for new technologies.
“HTM is developing into a proactive, strategic and datadriven field. In addition to being a biomedical equipment technician (BMET), the modern HTM professional also serves as a clinical technology integrator, cybersecurity steward, and strategic asset manager, making sure that the entire clinical technology ecosystem is secure, safe, effective and efficient,” says Naveed Ahmed Khan, assistant maintenance manager at Saudi German Hospital in Riyadh, Saudi Arabia.
In any discussion of trends or emerging technologies in HTM, it is hard to ignore AI as the leading topic.
During the early days of the Internet, it wasn’t recognized that the technology would be world-changing. Today, with more
of an understanding of technology, millions understand that AI is a game changer.
“One of the biggest trends in HTM is the growing application of artificial intelligence (AI) to predict equipment failures, optimize maintenance strategies, and enhance overall reliability. Alongside AI, connected devices and remote diagnostics are making it easier to support equipment across multiple sites, and cybersecurity is now front and center as everything becomes more networked,” says Nadia ElKaissi, CHTM, biomedical engineer with the VHA Office of Healthcare Technology Management.
Khan says one AI trend is predictive maintenance.
“In order to maximize uptime and resource allocation, maintenance is moving away from reactive and scheduled procedures and toward predictive ones,” he says.
He says that HTM’s additional duties will include analyzing warnings produced by AI to rank interventions and overseeing novel predictive analytics.
“I believe AI/ML will become more commonly used for continual monitoring of equipment condition and failure diagnostics with minimal, if any, human intervention, thus leading to remote predictive maintenance,” says Binseng Wang, principal consultant, BSI-Health Technology Consulting.
He says that this will make most planned/scheduled maintenance obsolete and will significantly reduce failure rates and downtimes, with significant benefits to the healthcare delivery organizations.
“Unfortunately, this approach is only available for OEMs due to the access control they have on their embedded software – aka ‘privileged access’ – and therefore, will further limit the service ability of in-house teams and ISOs,” Wang adds.
AI may also lead to more HTM intervention in the home healthcare area.
“AI is being used with remote monitoring to make sure patients who need 24-hour surveillance are being watched by a camera instead of having to pay for a person to sit in the room and watch. This can be used with suicide patients or those who are a fall risk,” says Allison Woolford, M.S., CBET, clinical engineering-operations manager at Duke Raleigh Hospital and Duke Regional Hospital, part of the Duke University Health System.
She says that using AI in telehealth can help streamline which patients need to come to the emergency department or who can be seen at home.
“Their symptoms can be reviewed virtually, and treatment plans can be provided in real time for minor symptoms. No need to crowd the emergency department for the common cold,” Woolford says.
Other innovations in healthcare, which will impact medicine, and have already changed the surgical suite, are robotics and nanotechnology.
Robotics in healthcare is advancing rapidly, particularly in surgery, patient care and rehabilitation. Robotic-assisted surgery, exemplified by systems like the da Vinci Surgical System and a growing list of others, enables surgeons to perform minimally invasive procedures with greater precision and control, leading to reduced complications and shorter recovery times.
Diagnostic robots can accurately guide needles in biopsy


procedures, improving specimen quality.
Beyond surgery, robots are being deployed to support medical staff by automating sanitation-related or supply management chores, protecting human employees from infection risks and burnout.
“The big name in robotics is Intuitive and the da Vinci robot, but Medtronic Hugo or Johnson and Johnson OTTAVA is trying to get into the game. Also, surgeons are opting to use more and more robots for their cases, saying that it minimizes downtime for the patient, which means the HTM community has to push harder for training,” Woolford says.
CYBERSECURITY REMAINS A FOCUS
Cybersecurity has become an unwelcome trend for HTM. The rapid digitalization of healthcare brings significant cybersecurity challenges. Health system ransomware attacks nearly doubled in 2024, affecting over 1,000 U.S. hospitals and impacting millions of people.
The February 2024 cyber-attack on Change Healthcare highlighted the extreme interconnectedness and delicacy of the entire healthcare ecosystem, disrupting operations nationwide and revealing vulnerabilities.
HTM plays an important role in mitigating threats and the safe networking of medical devices.
“The single most important force behind change is cybersecurity. Digital vulnerabilities are being included in device safety, in addition to physical dangers,” Khan says.
He says that HTM can do its part and find unpatched devices by doing vulnerability scans. Also, coordinating patch management with healthcare teams and vendors to guarantee effectiveness and safety. Dividing up medical devices on secure networks in cooperation with IT and joining incident response teams if a cybersecurity intrusion involves a device.
Companies are now beefing up security, scrutinizing technology vendor partners, and increasingly deploying AI and machine learning tools to detect abnormal network activity that could signal a breach. The downside of AI enhancement is its potential exploitation by hackers to create sophisticated malware and hyper-realistic social engineering attacks, necessitating high-end data protection mechanisms.
Adherence to regulations like HIPAA and annual cybersecurity checks are crucial to preventing data breaches.
“Although there are few proven cases of attacks through medical devices, there are numerous gaps and holes that would allow such attacks to happen without warning. Unfortunately, this challenge is draining significant HTM resources everywhere, thus limiting the ability of HTM
professionals to think outside of the box and replace ‘doing the same old, same old’ with what is truly worthwhile doing, and putting the advancement of the field at risk,” Wang says.
In addition to the many potential applications of AI, cybersecurity advances and robotics are non-AI technologies including innovations in replenishing the HTM pipeline and retiring a major OS.
“The trend I am seeing rise is technical schools that offer a certificate for biomedical equipment technology. With more retirements happening, entry-level positions are more common. Employers would like to hire people with some basis for their education,” says Stephanie Drake, HTM manager.
She says that she knows an anesthesia tech who is attending an HTM school online that includes VR training, so he can go through the hands-on training without having to move to another state.
“I anticipate that many of these students will be receiving their certificates within the next year and looking to start working in the HTM field,” Drake says.
OEM support for operating systems has posed a challenge for HTM and IT in the past. That challenge re-emerges with the retirement of active support for another OS.
“End of support for Windows 10 and older models is approaching next month, which can be a problem for medical devices running on Windows 10 or older. Running older operating systems can leave facilities open to security vulnerabilities. However, nobody has the funding to replace all devices that are running on Windows 10 to Windows 11,” Woolford says.
ElKaissi says that AI remains a leading area of focus; but it’s not the only game-changer.
“Hospitals are also investing in smarter asset management to cut downtime, remote support tools to save time on-site, and stronger cybersecurity strategies to keep connected devices safe. These developments are enabling HTM professionals to reduce downtime, improve cost efficiency, and ensure that clinical technology supports safe and effective patient care,” she says.
Providers in the HTM space have their own perspective on what is hot in HTM today.
“At Intelas, we’ve focused on solving one of the biggest challenges HTM leaders face today – visibility and decisionmaking around medical equipment readiness, utilization and cybersecurity. Our solutions such as EquipREADY, EquipTRAX and CyberHub are designed to help hospitals move beyond reactive maintenance toward proactive, data-driven management,” says Marley Pritchard, vice president of client experience & strategy at Intelas.
She says that EquipREADY ensures critical devices are always patient-ready, reducing downtime and preventing care delays. EquipTRAX gives leaders real-time insights into equipment utilization and availability, which helps optimize capital planning and reduce unnecessary rental costs.
“CyberHub addresses the growing cybersecurity threat by providing hospitals with a clear inventory of connected devices, risk scoring and prioritized remediation pathways. By integrating these tools with our HTM service model, we help health systems tackle equipment availability, budget pressures and patient safety risks simultaneously,” Pritchard adds.
Other insights come from provider Renovo Solutions.
“The challenge: Hospitals are juggling dozens of service vendors, driving up costs and creating inefficiencies. Renovo’s solution: We consolidate contracts under one transparent agreement, delivering guaranteed savings and simplified management,” says Matthew Forrest, vice president of business development at Renovo.
Forrest says that another challenge is that many health systems want to keep in-house talent but lack specialized expertise or resources. He says that his company offers
flexible hybrid programs to supplement in-house teams, or fully outsource solutions where needed.
In order to update or create devices or services, Pritchard says that her company is grounded in continuous client feedback loops.
“We gather input directly from frontline biomedical engineers, clinical staff, and health system executives through multiple channels: Day-to-day operator feedback, captured through our service teams embedded on-site,” she says.
Pritchard says that client satisfaction surveys come via platforms like ClientShare Pulse, which provides real-time, structured insights into service performance.
Another solution for HTM departments are automated detailed records.
“We have created smart, automated ‘checklists’ that address several issues that HTM departments face today. These bring huge time savings to very busy departments. They bridge the gap between testing per the manufacturers’ requirements in the service manuals – which can be confusing and very time consuming – and getting through a PM in significantly less time,” says Greg Alkire, vice president of business development at Pronk Technologies.
He says that the Pronk Mobilize checklists are dynamic, sending sequences to the test equipment from a single interface and wirelessly capturing all the work performed.
“The detailed records make it easy sailing during audits and, more importantly, ensure that medical devices are safe to return to the hospital departments,” Alkire adds.
As the biomed community enters 2026, the old observation “change is the only constant in life” will once again be confirmed. It really is healthcare technology with the emphasis on “technology” that remains hot and trending.
AI is not just reshaping healthcare — it’s redefining diagnostics, patient care, and the role of HTM professionals.









In today’s rapidly advancing landscape, healthcare technology management (HTM) professionals continue to play a vital role in keeping medical devices running safely and reliably. As technology grows more complex and demands on departments increase, the right tools have become more important than ever – helping HTM teams boost efficiency, improve accuracy and maximize their impact on patient care.
The 2025 Top Tools Techs Want features the latest and greatest devices to assist healthcare technology management (HTM) professionals achieve success when it comes to preventative maintenance, repairs and more! These tools offer top-notch electrical safety testing, workflow optimization, patient simulation testing, system informatics, tool calibration, mobile report generation, extra tech support, parts procurement and more.

The SafeTest 60+ is a compact, portable electrical safety analyzer designed for highvolume testing of medical devices including those with patient leads. It performs earth bond, insulation resistance and leakage current tests to international standards such as IEC 60601, IEC 62353 and NFPA99. The analyzer features limit checking with clear pass/fail indication, ensuring fast and accurate verification. Its color display, intuitive interface and automatic detection of secondary earth paths make it easy for technicians to complete reliable safety checks efficiently.

Healthcare technology management technicians are constantly balancing speed, accuracy and compliance. FSI, healthcare’s leading CMMS, delivers three essential tools for seamless workflows – and maximum efficiency in the field.
• Flow FSI’s configurable workflow automation tool, empowers teams with no-code automations that eliminate repetitive manual tasks, reduces errors, and ensures consistent documentation. Whether it’s triggering inspections, locking fields, or routing approvals, Flow keeps processes moving without the need for constant oversight or IT setup.
•View brings clarity to complex environments by integrating asset data and work orders directly into floor plans. With visual overlays and spot shadowing, techs can quickly locate equipment, identify needs and reduce time spent searching or traveling between locations.
• Inbox, FSI’s mobile app, transforms work order management into a fast, intuitive experience at the point of service. Its streamlined, email-style interface makes it easy for any user to log time, add notes and close work orders with a few taps from any mobile device.
These tools form a technician-focused ecosystem that boosts productivity and keeps teams ahead of the curve. With fewer repetitive tasks, improved asset visibility, and mobile work order access, technicians can keep focus on priority work for a safe, efficient healthcare environment.


Tri-Imaging Solutions offers XperTIS, an innovative platform designed to transform how imaging departments manage service, repairs and supply chains. Built with efficiency at its core, XperTIS maximizes uptime, lowers repair costs and extends equipment life while streamlining operations for biomedical engineers and service teams. The platform provides a full suite of intelligent tools, including predictive analytics that monitor system health and anticipate potential failures before they occur. By leveraging historical data, XperTIS identifies the most likely root causes of an issue and recommends the parts needed for repair. Engineers can then quickly order and track those parts directly through the system, reducing delays and minimizing costs. In addition, XperTIS enhances engineer performance with step-by-step repair guidance, video tutorials and multimedia modules that walk technicians through complex fixes. From mapping log events to sub-assemblies for pinpoint accuracy to analyzing tube utilization for life-expectancy predictions, the system offers unmatched technical support. By combining supply chain management, engineer assistance, and advanced analytics into one platform, XperTIS sets a new standard for reliability in medical imaging.

The smallest, most versatile and accurate multichannel pressure meter designed with the range and accuracy to test virtually any type of medical device. It’s capable of positive and negative pressure measurements – including differential pressures – for gas and fluids, providing extreme performance and accuracy. The palm-sized Pressure MAX fits in a shirt pocket and operates on batteries. It is the only pressure meter verified to pass all specifications after 50 drops from 3 feet. Features include:

• Two independent pressure channels: Narrow Port (range of -300 to 400 mmHg, with amazing accuracy of 0.2% of reading for positive pressures) and Wide Port (range of -14 to 100 PSI, capable of accuracy to 1% of reading)
• User-selectable 11 units of measure to meet any application
• Fluid exhaust port, digital timer and peak hold
• Innovative target range capability for results at a glance. The unit illuminates:
o Green when measurement is within programmed target range
o Red or blue when outside of target range (red=above range, blue=under)
• Capture and export electronic data via the Pronk Mobilize App
Pressure MAX is backed by Pronk’s industryleading 4-year warranty. With its incredible range, accuracy, and durability, it may be the last pressure meter you’ll need.

Technicians everywhere trust the ESU2400H for its intuitive design and effortless operation. Built-in auto sequences guide users step-by-step through preventive maintenance on a wide range of common electrosurgical units. Automatic footswitch activation, clearly labeled color coded ports, and a large, vibrant touch screen all combine to deliver an exceptional user experience. The high frequency ESU2400H variant is engineered to precisely measure even the latest advancements in electrosurgical technology.
The Southeastern Biomedical Customer Calibration Portal provides complimentary access to a secure searchable database containing critical equipment information. Customers can easily view calibration history, repair history, calibration certificates, and their test equipment inventory – all in one convenient location. The portal also offers real-time status updates for devices sent to our calibration lab, displaying key milestones such as received, in progress, and shipped. From the homepage, customers can quickly request an RMA for devices that require repair or calibration outside of regularly scheduled onsite visits. The portal also allows users to print the RMA return form and a packing slip, helping to streamline the shipping process and save time. This service is available to all Southeastern Biomedical calibration customers, providing instant access to essential documentation that supports compliance with Joint Commission and DNV requirements. With this information readily accessible, our customers can stay audit-ready and focus on their core responsibilities – confident that their test equipment calibration needs are being expertly managed, whether onsite or at our depot.


Our vision is to revolutionize commerce between customers and service providers by transforming it from a slow, expensive and antiquated phonecall system to a fast, competitive and visual experience.
What is it? How does it work?
Riding the coattails of the most popular ride share apps, food delivery apps and TaskRabbit, Downtime Trace is a mobile app for medical equipment maintenance and repairs. From their mobile phones, healthcare organizations experience the following while the app tracks and stores important data for future access to their service information:
• Blast a service need
• All bids received are displayed on one screen for an easy visual comparison
o All service provider options have been vetted for the following: competence to service the specific make/model of the equipment, proper insurance coverage, motivation to perform, and ratings/reviews from other customers
• Receive in-app communication during the service
• In-app approvals (which are tracked)
• In-app payments and reviews
o Customers pay through the app, and Downtime Trace pays service providers within 3-5 days of their approval of a job.
• Easy access to historical service data for past service events
MedWrench is a product focused support network where medical professionals, purchasing administrators, manufacturers, dealers and industry experts can provide opinions, share ideas, and gather relevant information on medical technology and equipment.
Our platform is built by users, for users — making it simple to get answers for your medical equipment questions.
In the forum section, members can ask about anything — batteries, power issues, replacement parts, service manuals and more. Whatever the need, our community is ready to help with real-world answers.
Want to know the best part? It is all free. That’s right! Simply create a free account on MedWrench.com, add specific pieces of medical equipment to your “bench” and be notified when new information comes in!


Streamline your case repairs with the MX40 Case Surgeon, the ultimate tool for precision and speed. Designed with innovation and reliability, this tool is your new solution for tackling case repairs with ease and efficiency. Built in-house by a team of biomed experts featuring:
• Exceptional Accuracy: Perform precise repairs, every time.
• Enhanced Efficiency: Minimize downtime and maximize productivity.
• Robust Design: Built to withstand demanding use in professional environments
Want to see this solution at work? Join us at the MD Expo in Dallas where our team will be providing a live demo. Check out your MD Expo guide for more information.

PartsSource PRO is more than a software platform – it’s the healthcare industry’s first and only cloud-based marketplace and managed service solution for managing mission-critical equipment. It is a comprehensive portfolio with access to parts, services, training and asset monitoring solutions. Built for hospital HTM teams, PartsSource PRO transforms how healthcare providers buy, manage and maintain critical equipment by boosting uptime, reducing costs and delivering actionable insights that drive smarter decisions. With advanced visual analytics, automated workflows, and a trusted nationwide supplier network, PartsSource PRO helps hospitals operate with greater speed, control and confidence.

Why healthcare leaders choose PartsSource PRO:
• Unlock real-time insights to uncover savings, elevate quality and track performance with precision.
• Consolidate spend across vendors, streamline parts ordering and integrate seamlessly with existing ERP systems.
• Ensure mission-critical equipment stays online with reliable access to parts and proactive service management.
• Automate tedious workflows and accelerate the entire procurement process – saving teams valuable time and cutting delays.
• Standardize purchases, monitor spend and manage device formularies with powerful visual analytics.
• Track every order, measure vendor performance and stay ahead of supply chain risks with the Operations Dashboard and Supply Chain Risk Monitor.
The result? A smarter, stronger medical equipment supply chain that empowers hospitals to deliver better patient care – without the hidden costs, downtime or inefficiencies of traditional procurement.

NOVEMBER 5
SPONSORED BY

WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
NOVEMBER 12
SPONSORED BY

WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
NOVEMBER 19
SPONSORED BY

TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.

CHECK OUT OUR ON-DEMAND PODCASTS AT 1TECHNATION.COM/ CATEGORY/PODCASTS
SPONSORED BY


TOOLS OF THE TRADE Downtime Trace + SkillNet: The Future of HTM Workforce Readiness
SPONSORED BY
Bridging the Gaps in Healthcare Operations: How Digital Procurement Drives Standardization, Savings & Uptime
SPONSORED BY

Reactive Mode to Leader Mode

All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.

BY VICKIE SALEMI
Is it a good or a bad thing to hug your job?
Job hugging, holding onto your job and not leaving, is trending. Think of it as the opposite of job hopping. You’re basically staying put in your job, riding it out, even if you’re unhappy. It makes sense during uncertain times when you hear of layoffs; people may be reluctant to leave their comfort zone. That said, they may be missing opportunities by not even interviewing to evaluate all of their options and career growth and potential financial advancements.
Chris Graham, executive vice president of workforce and community education at National University, said, “Job hugging is largely a response to the uncertainty and shift in the market, causing workers to weigh stability and job security over ambition. Many are asking themselves, ‘Why change the course if everything is OK right now?’
Employees are also assessing their careers through the lens of their personal priorities. This is especially the case for adult learners, working parents and members of the military, who are all balancing work with continuing their education, child care, active duty and more. If a workplace provides flexibility and the work-life balance they need, loyalty will continue to be expressed.”
The pros can bring you a sense of comfort and stability
knowing you already know your job, your boss’s style and the organization. It’s also a win for employers to keep top talent while investing in their skills. Although they may get complacent with compensation (i.e., lower annual raises), figuring you’re not going to leave, they may offer additional retention tools such as generous benefits, sabbaticals and more. So, you may want to leverage this time to ramp up your skills and tap into available resources.
As for cons to job hugging, it’s that same comfort zone. Graham said, “People may find themselves complacent with where they are in their roles, resisting creativity and out-of-the box thinking or ending up feeling stuck within their careers because they aren’t being challenged to see their full potential.”
Just because you’re staying put doesn’t mean you’re fully engaged and productive. You may be going through the motions and just getting by out of fear of leaving. Another con is financial setbacks. If you pursue a new job that would boost your annual salary by 15%, well, that’s a win; if you stay in place, you’re likely getting a much smaller annual increase. Plus, there’s no guarantee in job hugging that your current job won’t be downsized.
To address this con of complacency, employers should be proactive in employee engagement and satisfaction to keep up morale through offering upskilling, mentorship, internal mobility opportunities and more.
Just because turnover is low doesn’t mean everyone is happy and productive in their roles. Another con to job hugging is lack of movement. If fewer people resign and land new jobs, there will be less opportunities available, and with fewer job requisitions, there’s less movement overall. Recruiters will have less work and may be impacted with layoffs.
Remember, at first, during the pandemic no one left their roles; then in 2021 we had the Great Resignation when a staggering number of people hit the job market. Movement can be a healthy sign. When people resign and opportunities are available to replace their roles, more people may be likely to look for a new job.
As for whether or not this trend is here to stay, think of it as a pendulum, similar to the trends we saw over the past several years.
Annie Rosencrans, director of people and culture at HiBob, said, “When employees are back in the driver’s seat, as they were during the Great Resignation, there is a strong possibility the pendulum will swing back and job hopping will pick up again. That said, I don’t think it will be an
immediate change. People will need time to adjust and gain trust in the labor market before they make any career moves that could be seen as risky.”
As for the employer perspective, Rosencrans said employers need to look underneath the surface to identify workers who may be job hugging.
“Left unaddressed, this disengagement can quietly spread and erode team morale. Recognizing who is job hugging is just the first step. From there, HR and business leaders must work together to understand why it’s happening and take action to re-engage these employees. By addressing the root causes, organizations can turn job hugging into an opportunity to strengthen culture and commitment,” said Rosencrans.

Vicki Salemi is a career expert for Monster, an author, a speaker and consultant, TV commentator and former corporate recruiter. Send your questions to hello@vickisalemi.com.




BY NADIA ELKAISSI, CHTM
When the U.S. Food and Drug Administration (FDA) updates its playbook, the healthcare world pays attention. Its recent introduction of 19 new medical device categories may sound technical, but the implications are widereaching. These new classifications aim to reflect modern technologies more accurately – everything from connected wearables to AI-driven diagnostic software. For hospitals, though, these shifts can have real consequences for how devices are evaluated for risk and how HTM and OIT teams manage controls.
WHY THE FDA UPDATED CATEGORIES
Medical devices are no longer just scalpels and scanners.
Today’s devices are often hybrids of hardware, software and connectivity. For instance, a defibrillator is no longer just a box with paddles; it’s a network-enabled tool that records patient data, integrates with electronic health records (EHRs) and can even be updated via software updates. By introducing 19 new categories, the FDA is drawing a sharper distinction that reflects real-world device complexity and risk. Instead of trying to fit modern tools into outdated buckets, the agency is giving hospitals, manufacturers and regulators clearer frameworks for oversight. This makes risk assessments more consistent, device approval more transparent and smoother development for manufacturers.
RISK ASSESSMENT RIPPLE
Risk assessment is the backbone of hospital decisionmaking around new technology. Before adopting any device – whether it’s a bedside monitor or a life-saving infusion pump – hospitals evaluate how failure, misuse, or compromise could effect patients. The new FDA categories will shift those evaluations in important ways.
1. Category=Risk Signal: If a device, such as a defibrillator or infusion pump, is classified in a higher-risk category, hospitals must implement stricter validation, documentation and monitoring procedures. Reclassification isn’t just a bureaucratic detail – it directly affects how much oversight HTM, OIT and clinical teams must apply.
2. Software as a Device: Many modern defibrillators and infusion pumps are controlled by embedded software and often connect to hospital networks. If the FDA explicitly defines these as “software-containing devices,” hospitals must now treat not only the hardware but also the code inside as part of the risk assessment.
3. Operational Impact: A defibrillator failure could mean immediate harm. While an infusion pump delivering the wrong dosage could cause long-term damage. Their placement in higher-risk categories formalizes what hospitals already know instinctively: the stakes are too high for minimal impact.
For hospitals, the immediate result of these new FDA categories may be more work upfront – new training, revised
can move toward risk-based IT control models where the rigor of oversight matches the device’s clinical importance. Instead of one-size-fits-all policies, high-risk devices like infusion pumps, and defibrillators will get the protection they deserve, while lower-risk devices can be managed more flexibly.
Still, it’s important to recognize that while FDA categories are a useful starting point, they are not the whole story. Categories offer a framework, but true risk evaluation must also factor in variables such as device connectivity, multifactor authentication, vendor support and even the threat landscape at a given moment. A reclassified device might look high-risk on paper but have strong built-in safeguards; another might sit in a lower category yet pose a bigger cyber risk if poorly maintained.
In other words, the new FDA categories highlight crucial aspects of device safety, but hospitals must layer that guidance with their own clinical, operational and cybersecurity insights. Only then can they build a complete resilient picture of risk – one that goes beyond mere labels and reclassifications.

Nadia ElKaissi, CHTM, is a biomedical engineer in healthcare technology management with the VA Central Office (19HTM).
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.



Tri-Imaging Solutions introduces a cutting-edge platform that management, engineer performance, and system monitoring. diagnostic tools and video tutorials, and streamlines parts Designed for efficiency, the platform minimizes downtime setting a new standard for reliability in medical imaging.
Objectives
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering. This helps maximize uptime and minimize repair costs
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical
Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering. This helps maximize uptime and minimize repair costs. Solutions
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering This helps maximize uptime and minimize repair costs

XperTIS o helping while en ordering minimize

BY STEVEN J. YELTON, P.E., AAMIF
When I entered the “HTM World” then called clinical engineering, it was common to have a mentor to provide you with leadership and specialized training. Traditionally, at that time as is equally true now, there were not many clinical engineering educational programs. I had an associate degree in electronics technology and worked on laboratory equipment at the Ohio State University as I completed my electrical engineering degree.
I then went to work teaching electronics at the college level. Since I had background in laboratory equipment, I was asked to move to the faculty of the biomedical equipment technology program at Cincinnati State College. Gregory L. Herr, C.C.E., became my mentor. He was then and is currently the director of HTM at The Christ Hospital in Cincinnati, Ohio.
Greg and I have worked together in many different roles for 30-plus years. We were recently discussing a blog post
that he submitted in 2017. This blog discussed what he thought were challenges for those of us working in the HTM field and what we need to do to continue to advance our field.
This was a time when the term “healthcare technology management (HTM)” was starting to take off and we were re-examining our roles. Let me post some excerpts from the blog and look at was happening then and now. I find this post to be very informative and felt it was worth sharing again.
I would like to share the following from the 2017 blog: “As the term ‘healthcare technology management (HTM)’ becomes more prevalent, those of us who work in this field are still challenged with what this name really means. How does one implement the goals of HTM? What are the new opportunities? Embracing HTM as a name should be the first step in a broader goal of bringing greater recognition, respect, and value to the work we do. If it’s just a name change – without an examination of our role – we have missed an opportunity.
Healthcare leadership wants to know what meaningful work HTM does and how that work imparts measurable value. What would happen if HTM did not exist? Or sold as a service to the lowest bidder? What can we do to stay relevant, to be viewed as valued HTM service – and not
identified solely as a necessary evil? Healthcare leaders value quality, cost effectiveness, safety and patient satisfaction. So how does HTM align strongly with those values? What will it take for HTM to thrive?
The HTM professional must expand thinking beyond past practices and norms. Benchmarks, such as the number of devices maintained to the number of FTEs or the cost-ofservice ratio (COSR), are rough gauges, but they don’t show quality or value. What about downtime or IT security costs? Is there effective technology planning? How are these costs incorporated? Or do we let other departments assume these functions? If we do, HTM will lose its role in the future.
The factors of quality, customer and patient satisfaction, overall value (a measurement of customer satisfaction over total costs) are important, but how does HTM align with these factors? How COSR measure quality or overall value? A low COSR implies efficient operations, but it says nothing about quality. It does not measure cost effectiveness since revenue or usage are not accounted. HTM must include specific quality and value factors in its benchmarking.
The very nature of healthcare technology management is changing. There is a growing information technology (IT) component to much of what we do. In some healthcare facilities, there is an emerging clinical engineering (CE)-IT hybrid position to manage the medical IT systems. Such a position involves taking clinical engineering professionals and training them in basic technical support, applications, and IT fundamentals. Such a professional must be able to
interact directly and interact across departments as a peer and with a clear understanding of the workflow. A clear and efficient workflow is important to any organization, and HTM professionals must be involved in setting and understanding the workflow.
The question of how to move HTM into the future hinges on several factors, including each organization’s goals, our own skills in developing and demonstrating our value, and of course the professional relationships we have with leadership and department colleagues. For HTM to thrive, we must think more about our changing role in healthcare and our alignment with the critical success factors the organization has defined.”
I would like to thank Greg for sharing this with us. I find that his vision was very insightful then and still holds true today. He and I discussed areas that seem to be surfacing now and could be added to his vision. The one area that seems to us to be gaining a lot of traction is artificial intelligence (AI). It will be interesting to continue our look back at how HTM was and is.

vc_TechNation_Clr Ad_7x4.5_25Sept25_r1.pdf 1 9/25/25 10:39 AM
Steven J. Yelton, P.E., AAMIF; is a senior HTM consultant for a large health network in Cincinnati, Ohio and is a professor emeritus at Cincinnati State Technical and Community College where he teaches biomedical instrumentation (HTM) courses.




As states move forward with “right to repair” laws intended to give consumers and independent technicians more access to parts, tools and manuals, medical devices remain a contested segment – and legal barriers could prevent states from enforcing such measures.
Over the past several years, more than half of U.S. states have introduced some form of right to repair legislation. Many of the enacted laws focus on consumer electronics, farm equipment and appliances. In most cases, lawmakers included exemptions for medical equipment, citing safety concerns.
Advocates argue that the exemptions put hospitals and patients at risk. Gay Gordon-Byrne, executive director of The Repair Association, and a strong advocate of right to repair said smaller hospitals often face long delays waiting on manufacturer technicians.
Speaking at a recent industry forum, she recalled one case where a patient’s treatment was compromised because of repair wait times.
“It took six months before the MRI machine was
repaired, and by that time, the cancer present in his system was far advanced,” Gordon-Byrne said.
Consumer advocates also warn that hospitals are often unable to use their in-house engineers to maintain equipment because manufacturers restrict access to software, replacement parts and repair manuals. Nathan Proctor, who leads the national right to repair campaign for the Public Interest Research Group (PIRG), made that point in congressional testimony last year.
“The inability of a health delivery organization to use its own in-house engineers to make timely repairs negatively impacts patient treatment,” Proctor said.
Manufacturers argue that independent technicians may not have the specialized training required to repair highly regulated equipment such as ventilators, MRI machines and defibrillators.
Jim Jeffries, senior vice president of public affairs with AdvaMed, emphasized the risks in a policy briefing.
“There is no credible evidence that providers have had any delays to medical device repairs,” Jeffries said.
The legal question: Can states enforce medical device repair laws?
Whether states could even enforce right to repair mandates for medical devices is uncertain. Federal law, particularly the Medical Device Amendments (MDA) to the Food, Drug, and Cosmetic Act, creates what is
known as “express preemption.” This clause prohibits states from imposing requirements that differ from or add to federal standards for device safety or effectiveness.
In the 2008 Supreme Court case Riegel v. Medtronic, justices ruled that state laws or lawsuits could not impose stricter requirements on devices that had already received FDA approval through the premarket approval process. That decision confirmed the strength of federal preemption in this area.
Still, there is a narrow exception. Courts have allowed state actions that “parallel” federal requirements –essentially, those that mirror existing FDA obligations rather than creating new ones. The scope of that exception remains contested, and courts have interpreted it differently.
The legal framework means that if a state were to pass a law requiring manufacturers to share repair materials for medical devices, it could face preemption challenges. If the requirements imposed additional safety obligations or conflicted with FDA standards, courts would likely strike them down.
Some experts say states could have more success by targeting categories of medical equipment not subject
to the FDA’s most rigorous approval process, or by focusing on access to non-safety-critical information such as basic manuals and training materials.
Other state laws have sidestepped the issue by excluding medical devices entirely, while a few have included limited categories such as powered wheelchairs. Advocates argue that more sweeping federal action may ultimately be needed to create uniform standards.
The right to repair debate continues to intensify across the country, with consumer groups, hospitals and manufacturers all staking firm positions. For medical devices, however, the question is not only one of safety but also one of law.
Because of federal preemption, state legislatures are constrained in what they can require. Unless Congress or federal regulators step in, states may find themselves limited to incremental changes, leaving hospitals and patients caught between repair delays and regulatory boundaries.
“Right now, states are doing what they can,” Gordon-Byrne said. “But when it comes to medical devices, the real solution is going to have to come from the federal level.”



BY NICOLE PALMER
The HTM industry is facing a quiet but critical turning point. Across the country, experienced HTM professionals – including biomedical equipment technicians (BMETs), clinical engineers and HTM leaders – are approaching retirement age. With decades of institutional knowledge walking out the door, hospitals and health systems are grappling with how to preserve this expertise and ensure seamless continuity in patient care technology management.
This challenge is not abstract; it’s already here. As retirements accelerate, the shortage of skilled HTM professionals is widening. Organizations that do not prepare risk losing essential knowledge, compromising compliance and creating inefficiencies that directly impact patient care. One company helping health systems meet this challenge is MultiMedical Systems (MMS), a nationwide provider of supplemental and on-demand biomedical and imaging technicians. MMS not only fills immediate staffing needs, but also provides training, knowledge transfer and management oversight to strengthen HTM programs for the long term.
According to surveys from AAMI and other industry groups, a significant portion of the HTM workforce is over the age of 55. These professionals have been instrumental in managing
the life cycle of medical equipment, ensuring compliance with safety standards and responding to increasingly complex technology integration challenges. Their retirement leaves not only staffing gaps but also profound knowledge vacuums – from vendor relationships to legacy system management.
Many hospitals underestimate how much of this expertise is undocumented. A senior BMET may know exactly which vendor representative to call when an obscure imaging system alarm appears, or how to coax an aging ventilator through one more inspection cycle. Without structured knowledge transfer, these practical insights disappear the moment a retirement party is over.
When veteran HTM leaders retire without a proper succession or knowledge transfer plan, organizations risk:
• Equipment downtime due to undocumented procedures;
• Compliance and audit vulnerabilities if testing protocols are not clearly recorded;
• Inefficient troubleshooting and maintenance workflows that frustrate staff and delay care;
• Loss of vendor negotiation strategies and contract insights; and
• Weakened leadership pipelines with no prepared successors.
Such gaps directly affect patient safety and operational performance. A single day of downtime for critical imaging equipment, for example, can delay care for dozens of patients and cost thousands of dollars.

Forward-thinking health systems are building structured succession plans to prepare for this generational shift. Key elements include:
1. Identifying Critical Roles and Knowledge Areas
Conducting a skills audit to determine which roles and individuals hold “tribal knowledge.”
2. Documenting Processes and Workflows
Developing SOPs (standard operating procedures), troubleshooting guides and equipment histories that capture both the “what” and the “why” of daily operations.
3. Mentorship and Cross-Training
Pairing retiring professionals with junior staff to foster hands-on learning.
4. Investing in Talent Development
Supporting certifications (CBET, CHTM), leadership training, and partnerships with colleges to build HTM pipelines.
5. Using Technology for Knowledge Capture
Leveraging CMMS platforms, wikis and video tutorials to preserve expertise in real time.
This is where MultiMedical Systems (MMS) becomes an invaluable partner. MMS provides far more than temporary staffing – its services directly support succession planning and knowledge retention in HTM departments:
• Supplemental and On-Demand Staffing: MMS deploys experienced BMETs and imaging technicians across the country, bridging gaps when senior staff retire or during periods of turnover. This ensures patient-ready equipment and uninterrupted operations.
• Training and Mentorship: MMS technicians don’t just fill seats; they share expertise. By working alongside in-house teams, they provide real-time training and mentoring, helping newer staff gain hands-on skills and confidence.
• Management Oversight: MMS offers program-level support, bringing structure, standardization, and best practices gathered from working with diverse health systems. This oversight helps preserve institutional knowledge while raising the overall quality of HTM operations.

• Knowledge Documentation: Through its programs, MMS helps hospitals establish consistent documentation practices. Service histories, asset data and vendor insights are captured in organized, accessible formats – so knowledge doesn’t walk out the door with a retiree.
• Flexible HTM Support: Whether for short-term projects, ongoing coverage, or leadership transitions, MMS adapts to each hospital’s needs. This flexibility allows organizations to manage the uncertainty of workforce changes without compromising safety or compliance.
Succession planning cannot be a one-time project. It requires continuous commitment from HTM leadership, HR and hospital administration. Partnering with MMS ensures that this commitment is backed by real expertise, additional capacity and structured processes that make knowledge retention achievable.
By combining internal initiatives – like mentorship programs and leadership development – with the external support MMS provides, hospitals can safeguard critical knowledge while preparing the next generation of HTM professionals.
The HTM industry sits at a crossroads. The wave of retirements presents both a serious risk and an opportunity to rethink how knowledge is preserved. With the right strategy, hospitals can transform this challenge into a chance to build stronger, more resilient teams.
MultiMedical Systems (MMS) plays a pivotal role in this transformation. By supplying skilled technicians, training in-house staff, and providing oversight that embeds best practices, MMS helps hospitals not only retain essential knowledge but also create a sustainable path for the future of HTM.
In short: succession planning is no longer optional – and MMS is the partner that makes it possible.

Nicole Palmer is vice president of operations with
Multimedical
Systems LLC.

BY ERIC MASSEY
In healthcare technology management (HTM), certifications are the gold standard. Whether it’s a CBET proving technical mastery or a CHTM validating management expertise, certifications demonstrate rigorous preparation and achievement. But here’s the truth: leadership itself goes beyond any single exam. It’s not a one-time test. It’s a curriculum you build, renew and practice throughout your entire career.
Too often, leaders are promoted without that same intentional preparation. The result? Brilliant technicians find themselves struggling to manage teams, delegate effectively, and set a vision. Leadership, like any certification, requires structured growth and ongoing renewal.
Communication is the foundation of leadership mastery. But it’s more than clarity it’s about connection. Great leaders don’t just deliver information; they inspire, influence and create belonging. They make their teams feel seen, heard and valued.
• Practice Drill: Before every meeting, write down not only your key message but also a story or example that brings it
to life. Then ask yourself: “How can I invite my team into this vision, so they feel like co-creators?”
Delegation isn’t about passing off tasks it’s about building trust. Every time you delegate, you’re saying, “I believe in you.” Done well, it empowers your people, strengthens accountability, and develops future leaders. Done poorly, it erodes trust and engagement.
• Practice Drill: When delegating, always provide three things: the why behind the task, the resources for success, and your clear belief in their ability. Follow up with support, not micromanagement.
Technical certifications prepare us for solving known problems. Leadership prepares us to create the future. Vision-setting is more than defining what’s next it’s aligning the team around a clear direction, why it matters, and how each person contributes.
• Practice Drill: Each quarter, write a “Future State” paragraph describing where your department will be in six months. Share it with your team and connect each individual’s role to that vision.
Like technical and management certifications, leadership mastery builds in levels. Here’s a curriculum to guide your growth:
• Level 1 Communication Fundamentals: Active listening, feedback, storytelling and clarity.
• Level 2 Delegation & Empowerment: Defining success, providing resources and building trust.
• Level 3 Vision & Strategic Direction: Goal setting, prioritization and rallying the team around purpose.
• Level 4 Advanced Competencies: Emotional intelligence, change management, conflict resolution and courage under pressure.
Overlay this with six habits that drive high performance clarity, energy, necessity, productivity, influence and courage and you’ve got a structured framework for lifelong leadership mastery.
Don’t just read practice. Here’s your 30-day roadmap:
• Week 1: Observe & Reflect
Where are you strong? Where do you struggle?
• Week 2: Set a Specific Goal
Define what improvement looks like in one competency.
• Week 3: Practice Intentionally
Use drills like storytelling in communication, trust-building in delegation, or visionsharing in meetings.
• Week 4: Assess & Adjust
Ask your team for feedback. What improved? What needs more work?
Treat this like a true certification exam: structured, intentional and measured.

Leadership isn’t just about personal growth it’s about impact. When leaders fail to grow, it’s not only teams that suffer it’s hospitals, patients and the entire HTM industry.
The world needs you to lead with clarity, courage and care. So, ask yourself: “What’s at stake if I don’t step up as a leader?”
When leaders commit to mastering their craft, everyone benefits. That’s a certification worth earning and renewing.

BY GARRETT SEELEY
Recently, our team worked on a 3D reconstruction system without a monitor, yet it contained a powerful video card. This type of configuration is called a “headless” system, as there is no way to see what the reconstruction system is doing without additional equipment. Normally we add a monitor or use remote desktop access to see the headless system’s operations. Regardless, it is a system without a direct display. Let’s discuss why a high-end video card is present in a headless system.
Consider normal data flow for X-ray imaging systems with reconstruction. In such X-ray systems, an X-ray detector captures a digital image and forwards the information to the 3D reconstructor. The reconstructor then assembles the raw data into the required 2D or 3D image. Afterwards, it sends the results to the control system for final processing, technologist review and archiving. This is all accomplished using a headless 3D reconstructor with an open-source operating system (Linux) and a high-quality, expensive video card.
The odd part about this model is that the video card has just as much or even more processing power than the system it is connected to. This explains why a computer that doesn’t use a display may still need a powerful video card. This could even be true if the video card itself costs half as much as the overall computer system.
The most important concept with 3D reconstruction, and with AI-powered systems, is that they do not handle data in the same way a typical desktop computer would. To put it into an analogy: A CPU, the main processor of a computer, is big and powerful. It can perform intense calculations and process extremely difficult tasks. It is raw power, like a bulldozer. This is impressive and great for heavy calculations.
However, this is not what video systems require.
In video systems, processing must focus on the needs of each pixel in a display. It takes a lot of small calculations to reconstruct pixel data. It is best to approach this task with an army of small processors, called video cores. These are not like CPU cores in that they are not designed for large processing tasks but rather for small tasks. In a video card, there are over 1,000 cores. These may be called GPUs or Graphics Processing Units. A CPU, by contrast, currently has about 4 to 16 cores.
The GPUs are far less powerful per core, but they excel in numbers. The theoretical paths for data in a processor, called threads, are usually around 1.5 to 2 times the threads compared to the number of cores. This means that while a CPU can perform dozens of complex tasks simultaneously, a GPU can perform tens of thousands of simple tasks in the same timeframe. This is the processing advantage of a video card. It excels in performing smaller calculations.
For this reason, GPUs are also very good at working with large language models for AI systems. GPUs started out as strict video task processors. Recently, programmers have found that it is possible to write software for video cards, giving them tasks that were previously only for a CPU. For example, bitcoin mining is usually performed on several video cards as opposed to a powerful CPU. The CPU was simply the wrong tool for the job. The same is true for AI systems. To simulate thought in AI systems, it requires computing threads to mimic minds. The work is not difficult, but the number of information paths required is staggering. This is why video card companies have recently made trillions on AI systems. Video card GPUs are far better than CPUs for AI systems.
The question is about what type of processing is best for the application. Does the task require a CPU to come in like a dozen bulldozers, or is it best for a video card to come in like an army of 10,000 shovels? This is why some tasks are best performed by a video card rather than a CPU.
As a video card is traditionally added to a computer system,

they are expandable, and several cards can be installed and even linked together. The effect is that it is relatively easy to expand the computer system for GPU tasks. Additionally, video cards themselves have built-in memory. Due to video card manufacturers using proprietary, custom card builds, video cards are usually equipped with faster RAM than the main computer system, often leading CPU systems by one or two generations (DDR6 vs. DDR4 RAM). This means that it is possible for a computer system to have more VRAM than main system memory. Given the requirements faced in 3D reconstruction, AI, and even virtual reality systems, a video card becomes a clear choice for expanding and upgrading a system. This explains why video card demand and costs have ballooned over the past decade.
In the future, we will see more systems functioning with
multiple video cards, even if there is no need to run a display on the system. The cards may not be connected to anything visible, because that isn’t their primary task. The system software will utilize the card for additional, small-scale computational tasks other than displaying information. It’s all about the number of simultaneous tasks over the size of the task. Ironically, a video card was originally designed to assist a computer in its display tasks. Video cards are slowly being integrated into main computer system operations. Expect to see this in more medical applications in the future.



Garrett Seeley, MS, CBET, is a biomedical equipment support specialist-imaging with VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.



BY PHIL ENGLERT
This column builds on last month’s discussion of cyber resiliency metrics. You can read last month’s column at 1TechNation.com.
Clinicians are focused on delivering safe, uninterrupted care. They need metrics that translate technical risks into operational realities, connect cybersecurity to clinical outcomes, ensure operational continuity, and directly affect patient outcomes.
The first metric is the number of devices with unsupported operating systems. Unsupported operating systems no longer receive security patches or updates from vendors, making them highly vulnerable to cyberattacks. Devices running outdated operating systems are more likely to experience downtime because of malware, ransomware or compatibility issues. Knowing the number and location of these devices helps clinical leaders plan for contingencies and minimize disruption. The data for this metric comes from the CMMS and is tagged by location and risk classification.
Highlighting the number of devices with unsupported operating systems typically aligns with a standard. By making clinical leaders aware of this technology debt and the higher risk of compromise that could directly impact patient safety in high-acuity environments, it ensures that clinical leaders are aware of the risks and may lead to support for capital replacement, especially if executive leadership holds clinical leaders accountable for the risks of operating outdated technology.
The next metric for clinical leaders is the percentage of
devices with secure configuration and access controls. This metric is like the first in that it looks at a dimension of technical debt. In medical devices, secure configuration and access controls refer to settings and safeguards that prevent unauthorized access, misuse or exploitation of the device. Like devices with known vulnerabilities or unsupported operating systems, those lacking secure configurations represent a latent risk accumulating over time and can compromise clinical operations when exploited. The data for this metric will come from configuration management databases and network access control systems. Poorly configured devices are more likely to be targeted in cyberattacks, resulting in downtime or degraded performance. This affects scheduling, throughput and staff efficiency. Combined with the first metric, clinical leaders are empowered to prioritize device upgrades or replacements, support funding requests for cybersecurity improvements and collaborate with HTM and IT teams to protect clinical workflows. These metrics foster collaboration between clinical and technical teams, helping clinicians advocate for cybersecurity as a patient safety issue.
The third metric for clinical department leaders is the average downtime from cybersecurity-related device incidents derived from incident response logs and CMMS service records. Organizations must look for, recognize and record cyber incidents in medical devices. This requires well-trained staff who know how to identify software-related issues on medical devices and how to retain logs for investigation. Medical devices are essential to diagnostics, monitoring and treatment. When a device is taken offline because of a cybersecurity incident – such as malware infection, unauthorized access or ransomware – the
resulting downtime can delay critical procedures or diagnostics, force clinicians to use less effective backup methods and increase the risk of adverse patient outcomes.
HTM professionals are on the frontlines of medical device management. They need metrics that guide day-to-day decisions and long-term planning. Collecting and utilizing data to support cybersecurity resilience in healthcare facilities is a complex endeavor, especially in organizations with multiple stakeholders, each with distinct roles, responsibilities and priorities. While metrics are essential for guiding decisions and improving security posture, several challenges can hinder their effectiveness. Challenges include a mix of legacy systems, vendor-managed platforms, and siloed databases. When integrating these disparate systems to produce unified, actionable cybersecurity metrics, careful planning and persistent collaboration will be needed to tackle this technically complex and resourceintensive activity. The journey will be worth it.
HTM should develop a device risk classification metric incorporating cybersecurity risk and impact on clinical operations. Expand upon the traditional HTM risk elements of patient safety risk, maintenance requirements, and environment of use by adding acuity setting, cyber risk posture, PHI, and lateral access to develop a more comprehensive assessment of clinical impact in the event of device failure, regardless of the cause. The data source includes the CMMS with integrated risk scoring based on device type, connectivity and patient impact. Expanding beyond traditional HTM risk elements, this enhanced metric becomes a powerful tool for day-to-day decisionmaking and strategic planning. By incorporating a multidimensional view through which device risk is assessed, HTM professionals can play a central role in building a resilient, patient-safe and cyber-secure healthcare environment.
The second HTM metric is the percentage of devices with up-to-date software/firmware. Collecting and utilizing this data presents both technical and organizational challenges. Firmware update schedules vary widely across manufacturers. Some vendors do not provide visibility into current software versions or patch status. Devices may require manual checks or proprietary tools to verify update status. HTM teams may struggle to obtain accurate, timely data across a diverse device fleet. Passive monitoring tools may be able to provide patch availability information. Health-ISAC provides a software bill of materials (SBOM) link webpage for HTM professionals to access multiple manufacturer webpages or portals providing cybersecurity artifacts. CMMS, vendor portals, passive monitoring tools and software inventory tools can provide data for this metric. Overcoming these challenges requires cross-functional collaboration, enhanced CMMS capabilities and clear policies for updated tracking and validation.
The percentage of devices with up-to-date software/ firmware reflects the medical device fleet’s cyber hygiene, operational reliability and patient safety readiness. Outdated
software is a common attack vector. This metric is a strategic indicator of how well HTM teams manage cybersecurity risks, support clinical operations, maintain regulatory compliance and help HTM teams track and prioritize updates.
The final metric is the HTM staff cybersecurity training completion rate, a vital metric because it reflects the preparedness, awareness and capability of the HTM team to manage and mitigate cybersecurity risks associated with medical devices. This data may come from the learning management system (LMS), obtaining and maintaining cybersecurity credentials and self-study reporting. HTM professionals are often the first to interact with medical devices – installing, configuring, maintaining and troubleshooting them. A well-trained team is essential for maintaining device security and responding effectively to threats. This metric indicates how well-equipped the HTM team is to protect medical devices and support safe, uninterrupted patient care.
For HTM professionals, cybersecurity metrics are more than performance indicators; they are decision-making tools that help prioritize daily tasks, plan strategically, advocate effectively and collaborate across departments. These metrics are essential for maintaining safe, secure and resilient technology systems in a healthcare environment where medical devices are increasingly connected and vulnerable.
Cybersecurity in healthcare is a clinical and operational imperative, not just an IT issue. Medical devices are critical to patient care, and their compromise can have life-threatening consequences. Healthcare systems can provide executive leaders with actionable insights to guide strategic investments by adopting a structured, metric-driven approach grounded in the MITER framework. A metric-driven model empowers clinical leaders to understand and support mitigating cyber risks to patient care and equips HTM teams with the tools and data needed to strengthen device security.
These metrics are more than numbers – they are a language that bridges technical and clinical domains, enabling informed decision-making and shared accountability. With leadership support, healthcare organizations can operationalize these metrics, integrate them into existing workflows, and build a culture of cyber resilience that protects patients, staff and systems. This program will reduce the vulnerability footprint and foster a culture of shared responsibility and continuous improvement.
The time to act is now. Cyber threats are evolving, but so is the ability to anticipate, withstand, recover and adapt. Measure what matters and use those measurements to drive meaningful change. By leveraging MITRE’s guidance, you can align your cyber resilience efforts with industry’s best practices and ensure that your metrics are actionable, relevant and tailored to the unique challenges of medical device ecosystems.





















THE SHOW FOR BIOMEDICAL/HTM PROFESSIONALS
HANDS-ON TRAINING OFFERED AT OUR H.O.T. WORKSHOPS!








The words can appear horizontally, vertically, diagonally, and may be spelled forwards or backwards. 1. Top Tools Techs Want
Rigel Medical
Pronk Technologies
Elite Biomedical Solutions
FSI
BC Group
Southeastern Biomedical
Tri Imaging Solutions
Lifespark Medical
Downtime Trace
PartsSource 12. TechNation
MedWrench
Ramsey Worman
Clinical Engineer III
Primary Children’s Hospital
• International Biomedical Aeronox 2.0 nitric oxide
delivery analyzer with nitric oxide tank
• Abbott iStat 300 blood gas analyzer
• Philips MX450 physiological monitor
• Life Flight neonatal transport incubator sled:
- International Biomedical Voyager neonatal transport incubator
- Zoll X Series defibrillator monitor
- Percussionaire TXP-2D high frequency ventilator
- Hamilton Medical Hamilton-T1 ventilator
- Sentec SDM transcutaneous PCO2 monitor
- Medfusion 3500 syringe infusion pumps
- Eitan Group Sapphire infusion pumps (almost visible at right end of incubator hood)


The North Carolina Biomedical Association (NCBA) recently held its 47th annual Symposium at the Cherokee Casino and Resort in Cherokee, North Carolina. This HTM event featured expert-led educational sessions, vendor showcases of the latest technology and valuable networking opportunities.



1 4 2 3

1. The NCBA symposium teed off on August 25, with the NCBA Mike McCoy Golf Tournament, sponsored by PartsSource. The tournament concluded with the Golf Awards and Opening Reception, sponsored by Probo Medical.
2. TechNation’s Jayme McKelvey (left) and Megan Cabot were all smiles at the magazine’s booth in the NCBA exhibit hall.
3. NCBA leadership welcomed HTM professionals to the annual symposium.
4. A packed house was on hand for the NCBA awards presentation.
5. Vizient’s Aaron Weinbaum presents one of the more than 30 educational sessions offered at the 2025 NCBA Symposium.


6. Live music added to the amazing networking atmosphere at this year’s NCBA Symposium.
7. NCBA proudly displayed its 2025 Association of the Year TechNation Tech Choice Award at the symposium.
8. A packed exhibit hall brought HTM vendors together for insightful conversations amid the latest technology and solutions on display at vendor booths.
9. The TechNation Happy Hour, sponsored by FSI, continues to be a popular destination at the annual symposium.
10. Legends and newcomers break bread and raise a glass to the future of HTM at the TechNation Happy Hour.




Admar Neuro admarneuro.com • 728-900-3969
AMX Solutions amxsolutionsinc.com • 866-630-2697
BC Group bcgroupstore.com.com • 314-638-3800
FiberTech fibertechmedical.com • 866-628-6888
Interlight interlightus.com • 800-743-0005
Jet Medical jetmedical.com • 714-937-0809
Metropolis International metropolismedical.com • 718-371-6026
Nuclear Camera Services nuclearcameraservices.com • (630) 780-6363
Ozark Biomedical ozarkbiomedical.com • 800-457-7576
PM Biomedical pmbiomedical.com • 800-777-6474
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Tri-Imaging Solutions triimaging.com • 855-401-4888








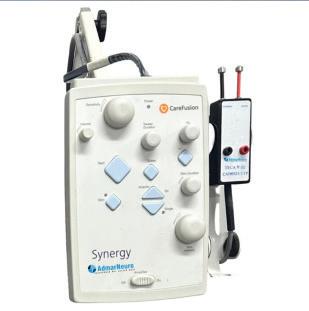

















at is Xpe
Tri-Imaging

Tri-Imaging

Tri-Imaging
monitors system ealth, supports engineers in repairs, and nhances the supply chain process by ving teams seamless access to parts rdering and order tracking



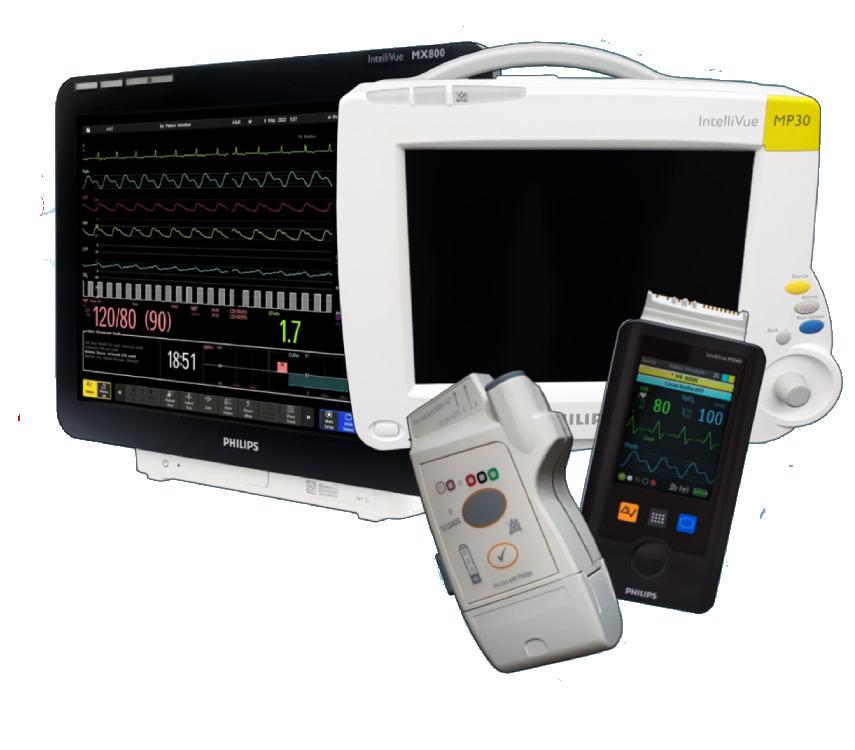



enhances supply
provides engineers
tutorials, ordering and Designed for efficiency, the platform minimizes downtime and optimizes operations, setting

for reliability in medical imaging.

Tri-Imaging

...Since you are still flipping through this magazine, it simply means you still have not found an

XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate pa ordering This helps maximize
management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
reliable, pre-owned imaging company that sells working systems.
helping engineers troublesho while ensuring faster, more a ordering. This helps maximiz minimize repair costs








Admar
General
Cardiac
Jet Medical Electronics Inc
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Contrast Media Injectors
Defibrillator
Diagnostic Solutions diagnostic-solutions.com
FDM Enterprises fdmenterprises.com • 615-479-5572
Innovatus Imaging innovatusimaging.com • 844-687-5100
Oxygen Blender
Tenacore LLC tenacore.com • 800-297-2241
Patient Monitors
aiv-inc.com • 888-587-6759
BC Group International, Inc BCGroupStore.com • 314-638-3800
PM Biomedical pmbiomedical.com • 800-777-6474
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Rigel Medical, Seaward Group rigelmedical.com • 813-886-2775
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Tenacore LLC tenacore.com • 800-297-2241
USOC Medical usocmedical.com • 855-888-8762
Refurbish
Admar Neuro admarneuro.com • 728-900-3969
AMX Solutions
www.amxsolutionsinc.com • 866-630-2697 84
General Anesthetic Services generalanestheticservices.com • 800-717-5955 5
Innovatus Imaging innovatusimaging.com • 844-687-5100 8
Nuclear Camera Services nuclearcameraservices.co • (630) 780-6363 85
General Anesthetic Services generalanestheticservices.com • 800-717-5955 5
Repair
Admar Neuro admarneuro.com • 728-900-3969
• 888-587-6759
AMX Solutions www.amxsolutionsinc.com • 866-630-2697
BC Group International, Inc BCGroupStore.com • 314-638-3800
CM Parts Plus cmpartsplus.com • 877-267-2784
FDM Enterprises fdmenterprises.com • 615-479-5572 23
General Anesthetic Services generalanestheticservices.com • 800-717-5955 5
Innovatus Imaging innovatusimaging.com • 844-687-5100 8
Nuclear Camera Services nuclearcameraservices.co • (630) 780-6363 85
PM Biomedical pmbiomedical.com • 800-777-6474 85
Soma Tech Intl somatechnology.com • 800-438-7662 91 Tenacore
General Anesthetic Services generalanestheticservices.com • 800-717-5955
Telemetry
aiv-inc.com • 888-587-6759
USOC
BC Group International, Inc BCGroupStore.com • 314-638-3800
General Anesthetic Services generalanestheticservices.com • 800-717-5955
Healthmark Industries hmark.com • 800-521-6224
IBA Radcal Corporation www.radcal.com • 800-423-7169
Pronk Technologies, Inc. pronktech.com • 800-609-9802
QRS Solutions qrs-solutions.com/ • 877-254-7086
Rigel Medical, Seaward Group rigelmedical.com • 813-886-2775
RTI Group North America rtigroup.com
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Tubes/Bulbs
Interlight interlightus.com
Ultrasound
Admar Neuro admarneuro.com • 728-900-3969
Innovatus Imaging innovatusimaging.com
General Anesthetic Services generalanestheticservices.com • 800-717-5955 5 Nuclear Camera Services nuclearcameraservices.co • (630) 780-6363
Biomedical pmbiomedical.com
AMX Solutions
Metropolis International, LLC Metropolismedical.com • 718-371-6026
rsti-training.com • 800-229-7784
RTI Group North America rtigroup.com • 800-222-7537
Tri-Imaging Solutions triimaging.com • 855-401-4890









TechNation cover articles addressed important topics in 2024.

The HTM Mixer in Lexington, Kentucky, was proudly supported by KAMI and provided all the benefits of an MD Expo on a smaller scale.











The 2024 Biomed Wishlist and MD Expo Scrapbook provided informative and fun content for TechNation readers!





MD Expo New England delivered networking, education and more.



Finally, the features you love most about your favorite ride share app, food delivery app, Angi, UpWork, or TaskRabbit are available for medical imaging equipment maintenance and repair.
Enter a Trace Ticket with one tap to broadcast your repair needs to a network of qualified technicians.
Review Bids to find the best service option for improved repair outcomes with less equipment down time, resulting in a lower overall cost.
Track progress, issue payments and rate services all in a single dashboard.


Speed
Instantly blast your service request to every qualified and vetted service provider

Take Control of Tracking

From response times to uptime, you no longer have to rely on service companies to track their own activities and performance Standardized Communication
Like your favorite personal apps, get in-app alerts when a service technician is on their way, arrived, waiting for a part, completed a job, etc.

Pay Through the App
Competitive bidding among service technicians allows you to get the best price and only pay and track one entity











Choose the right capital equipment, supplies, and health IT for your facility with guidance from ECRI Instituteʼs team of biomedical and cybersecurity engineers, clinicians, and human factors experts.



ECRI is a trusted authority on healthcare technologies that improve the quality and cost-effectiveness of patient care.
Get test results and ratings, free of vendor bias


Stay on top of risks with hazard and recall notifications
Compare technology with overviews and specifications
Learn more at www.ecri.org/solutions/device-evaluations or contact us today at 610-825-6000, x5891.


