






















Finally, the features you love most about your favorite ride share app, food delivery app, Angi, UpWork, or TaskRabbit are available for medical imaging equipment maintenance and repair.
Enter a Trace Ticket with one tap to broadcast your repair needs to a network of qualified technicians.
Review Bids to find the best service option for improved repair outcomes with less equipment down time, resulting in a lower overall cost.
Track progress, issue payments and rate services all in a single dashboard.


Speed
Instantly blast your service request to every qualified and vetted service provider

From response times to uptime, you no longer have to rely on service companies to track their own activities and performance


Like your favorite personal apps, get in-app alerts when a service technician is on their way, arrived, waiting for a part, completed a job, etc.
Competitive bidding among service technicians allows you to get the best price and only pay and track one entity










P.12 SPOTLIGHT
p.12 Professional of the Month: TRIMEDX Biomed Jeff Quid
p.14 Next Gen: Morgan Acquafredda
p.16 Association of the Month: The Kentucky Association of Medical Instrumentation (KAMI)
p.18 Department of the Month: VISN 2 New York/New Jersey VA Healthcare Network HTM Department
P.20 INDUSTRY UPDATES
p.20 TechNation Pulse
p.22 News & Notes
p.27 Ribbon Cutting
p.28 Welcome to TechNation
p.30 AAMI Update
p.32 ECRI Update


P.34 THE BENCH
p.34 Biomed 101
p.36 Webinar Wednesday
p.39 Shop Talk powered by MedWrench
p.41 Tools of the Trade: Claroty xDome
p.42 Roundtable: Tool Kits and Cases
p.50 Guide to AAMI eXchange
p.62 Cover Story: AAMI’s Rapid Release of EQ103
P.67 EXPERT ADVICE
p.67 Careers Now
p.68 Health-ISAC
p.71 Right to Repair
p.72 Networking Notes
p.74 The Future
PUBLISHER John M. Krieg
VICE PRESIDENT Kristin Leavoy
VICE PRESIDENT Jayme McKelvey BUSINESS DEVELOPMENT
EDITORIAL John Wallace
CONTRIBUTORS
Roger Bowles
K. Richard Douglas
Jim Fedele
Joie Marhefka
Manny Roman
Steven J. Yelton
ACCOUNT Megan Cabot
EXECUTIVES Emily Hise
ART DEPARTMENT Karlee Gower
Taylor Hayes
Alicia Brown
DIGITAL SERVICES Cindy Galindo
Kennedy Krieg
Haley Harris
EVENTS
Kristin Leavoy
Kristen Register
Sydney Krieg
WEBINARS Linda Hasluem
HTMJOBS.COM
Kristen Register
Sydney Krieg
ACCOUNTING Diane Costea
CIRCULATION Joanna Manjarrez
Rob Bundick, Director HTM & Biomedical Engineering, ProHealth Care
Carol Davis-Smith, CCE, FACCE, AAMIF, Owner/ President of Carol Davis-Smith & Associates, LLC
Nadia ElKaissi, CHTM, Biomedical Engineer, HTM, VA Central Office (19HTM)
Bryant Hawkins Sr., Site Manager, Children’s Hospital of New Orleans
Earl Morris Jr., BMET, Director of Clinical Engineering, Harrison County Hospital
Leticia Reynolds, Clinical Engineering Operations Manager at UCHealth Parkview Medical Center

P.76 BREAKROOM
p.76 Biomed Brainbuster
p.77 Word Search
p.77 [Contest] What’s on Your Bench?
p.78 Preferred Vendors
p.82 Service Index
p.85 Alphabetical Index
p.86 Time Capsule


Management.
programs, including our newly launched Dental Equipment Technician training, specifically designe d to advance the skills of industry professionals. Our f lexible, expert-led courses enable your staff to stay ahead in this rapidly evolving field.
CBET’s Virtual Reality (VR) labs offer a flexible and immersive learning experience that adapts to your busy schedule. our VR labs allow you to practice and perfect your skills in a controlled, risk-free environment.
Big changes are on the horizon at CBET, and we can' t wait to share the news with you! From innovative partnerships to fresh opportunities designed to advance your career in
CBET’s Virtual Reality (VR) labs offer a flexible and immersive learning experience that adapts to your busy schedule. our VR labs allow you to practice and perfect your skills in a controlled, risk-free environment.



BY K. RICHARD DOUGLAS
TRIMEDX biomed Jeff Quid built and flew model airplanes growing up. He imagined a career in aviation for himself when it came time for higher education. His dad was an electrical engineer, so Quid enrolled in the Avionics Program at Southern Illinois University (SIU) in Carbondale.
“As I progressed through the program, several of my classmates had found another program SIU offered, and that was a B.S. in Advanced Technical Studies with ‘biomed’ as an area of study. I decided after I finished my A.A.S. In Avionics, I was going to move into the biomed degree. I think what changed my mind was a visit to Flying Tigers at O’Hare to see what I would be doing, and with a wind chill at the time of -20, I thought a nice warm hospital would be a much better environment,” Quid says.
When the program ended, Quid was required to do an internship. When an issue prevented the start of the first internship, he landed at his present work location.
“I lived in Schaumburg, Illinois, and so I called a few local area hospitals and the Alexian Brothers shop sounded the friendliest on my initial phone call,” he says.
“I have been a tech within TRIMEDX, still here at Alexian for 29 years now, and I still love what I do. The people here make the job fun, and we have a very family feeling with not only staff but directors and administration. What makes this position so fun and interesting is all the new things I get to learn about. I have learned so much about what other departments do and how we fit in and effect their operations. We also have the advantage here of working directly with administration on capital purchases, projects and ways to improve and integrate our medical equipment,” Quid says.
Over the years, Quid has stayed busy with several projects, utilizing the knowledge of many years in the profession.
“I have been involved in and created many special projects over the years here. Everything from planning telemetry installs, network installs, new equipment distributions in large scale (infusion pumps for example), equipment upgrades [and] capital planning for new construction,” he says.
One example is the installation of new instrument washers in the central sterile department.
“I did not just have to set a date for the vendor, but ensure the physical site was ready prior to install. This started with basics, like will the semi fit into our loading dock – we can handle a 53 foot, but no bigger. Will they fit through the doors and hallways inside the hospital and do I have a route? Elevators? We had to unpack and remove the washers from their crates because they were too tall to move while boxed,” Quid explains.
His next consideration was where to store the units and then scheduling the infection control team to perform an infection control risk assessment (ICRA).
“Meaning, do we need to put up barriers, how to maintain the positive/negative pressure between clean and dirty side. Removal of the units, and then schedule Environmental Services to do a clean/buff and polish of the floor where the old ones were so we have a clean start. I also had to replace the steam valves to each washer prior, because the gates were worn, and they would not shut all the way off. I had to make sure the electrician was available for power requirements and to be able to disconnect the currently hardwired units, and then once installed, reconnect to them,” Quid says.
Even before starting this project, Quid had to ensure that the consumables were available and on a current contract.
“Then, schedule the vendor for the actual install and in-service of staff,” he adds.
Away from work, Quid’s original love of avionics evolved into a hobby along with mountain biking.
“I had always enjoyed building and flying model airplanes, and even met one of our anesthesiologists who was into it as well. I saw a model airplane news magazine on his cart and asked about it, and we got to talking about the hobby,” he says.
“My boss at the time also was into mountain biking, and me and another tech friend of mine in the shop bought ourselves each a mountain bike and competed for several years in the Chequamegon Fat Tire 40. That is a 40-mile, off road mountain bike race from Cable to Hayward, Wisconsin on part of the Birkebeiner trail, logging roads and other places where there is not much of a ‘trail’ to speak of. I did that for about four years,” Quid says.
Quid reflects on his career as a biomed:
“I really enjoy learning, and not just in our little area of equipment, but with processes, procedures, where things are, what to do to if ... I like to understand how things work as a whole, and maybe that includes the corporate process for requesting capital equipment, or why and how we special order consumables for departments. That way, when people ask how do I do something, I may not have access, but I can usually point them to the right person. I don’t think I could ever work in a retail store, or a cubicle where I had the same few hundred square feet to look at for my career. I like the changes that occur as the hospital moves forward. It is really something to look, from where we came when I started, to where we are now,” he says.
The patients and clinicians at Alexian Brothers Medical Center are fortunate to have a caring and dedicated biomed professional who is comprehensive in knowledge and empathetic.
FAVORITE BOOK: James Clavell’s “Shogun”
FAVORITE MOVIE:
“National Lampoon’s Christmas Vacation”
FAVORITE PART OF BEING A BIOMED?
Every day is different, and the people I get to meet are from all walks of life, from all over the world, and in all kinds of situations. I enjoy helping others and solving problems, be it in a policy and procedure, or troubleshooting a difficult case with a patient on the table. I get personal satisfaction for a successful repair, and that being that I was certain the cause of the issue and that I resolved it with my fix.
organ Acquafredda holds an associate of science degree and a Biomedical Equipment Technician certification. Her current role in the HTM field is that of a Service Medical Advisor with iMed Biomedical. TechNation found out more about this budding HTM professional during a recent Q&A.

Q: What do you like most about your position?
A: I appreciate the opportunity to foster strong client relationships, contributing to enhanced customer satisfaction.
Q: What interests you the most about HTM?
Q: Where did you grow up?
A: Suburbs of Dallas, Texas
Q: Where did you receive your HTM training/education?
A: Brown Mackie College, graduated in 2015
Q: How did you first discover HTM?
A: David Francoeur introduced me to the healthcare technology management industry.
Q: Why (or How) did you choose to get into this field?
A: I chose the healthcare technology management industry because I envisioned the opportunity to contribute to patient care while being an integral part of the healthcare sector.
“I am committed to advancing my career within iMed Biomedical and contributing to the expansion of our presence within the DFW metroplex and nationwide.”
A: What interests me most about healthcare technology management is the opportunity to leverage innovative technologies to improve patient care and enhance operational efficiency within healthcare settings. The dynamic nature of the industry, combined with the critical role technology plays in ensuring patient safety and care quality, motivates me to continuously seek solutions that drive both technical and clinical advancements. Additionally, the chance to work at the intersection of healthcare, engineering, and IT allows me to contribute to a field that is both impactful and ever-evolving.

Q: What has been your greatest accomplishment in your field thus far?
A: My biggest accomplishment has been to successfully orchestrate and implement the ground up build of Trinity Regional Medical Center Sachse. I worked closely with regulatory compliance to ensure passing of the Joint Commission Survey.
Q: What goals do you have for yourself in the next 5 years?
A: Over the next five years, I aim to further my education and obtain certification as a healthcare technology manager. Additionally, I am committed to advancing my career within iMed Biomedical and contributing to the expansion of our presence within the DFW metroplex and nationwide. I also aspire to support iMed Biomedical in enhancing and broadening its service offerings to better meet the needs of our customers. I selected iMed Biomedical because of the company’s strong commitment to delivering exceptional value to both its clients and employees through continuous investment in service excellence.
FAVORITE HOBBY:
Motherhood is a significant part of my life, and i find it both fulfilling and enriching.
FAVORITE SHOW OR MOVIE: All the Bachelor franchise shows
FAVORITE MEAL: Chicken Parm
WHAT WOULD YOUR SUPERPOWER BE? Empathetic communication
1 THING ON YOUR BUCKET LIST: Tour Italy and a Mediterranean Cruise
SOMETHING YOUR CO-WORKERS DON’T KNOW ABOUT YOU: I enjoy organizing, designing and remodeling homes.




BY K. RICHARD DOUGLAS
Nicknamed “The Bluegrass State,” Kentucky is the nation’s 15th state, achieving statehood in 1792. Daniel Boone founded one of the state’s first settlements. The state is a major supplier of hardwood, coal and limestone.
Many top-rated healthcare systems can be found in Kentucky. The state has approximately 135 hospitals and healthcare systems. This amount of medical equipment requires a lot of HTM professionals. Those biomeds have been organized under the statewide Kentucky Association of Medical Instrumentation (KAMI).
The association was started in early 2010 when the late Patrick “Pat” Lynch started traveling to Kentucky and meeting with several HTM managers of local shops to see what the interest might be in the state of Kentucky for a biomed association. Lynch’s involvement also helped shorten the learning curve.
“Patrick ‘Pat’ Lynch, the ‘Chief Do-Gooder’ started KAMI. He met with nine other HTM leaders in the area and formed the board. They then filed the paperwork with the state (April 2010) to formally be recognized as a non-profit membership organization,” says KAMI President Jennifer Kizis.
From its beginnings 15 years ago, the organization has found its footing and serves the state’s biomed
community, as well as others. Not all has been smooth sailing as the pandemic threw a wrench into the workings of even the best biomed associations.
“The pandemic was a devastating time and was almost the end of KAMI. We were unsuccessful with virtual meetings and maintaining our regular events. Finally, KAMI was able to reconnect for an event at 3rd Turn Brewing and reorganized their small board into the 12-member board that now exists. The HTM Mixer in 2024 was KAMI’s ‘re-emergence’ and has kickstarted our organization back into our mission,” Kizis says.
She says that in addition to the 12-member board of directors, the group has several committees, including Compliance and Audit, Higher Education, Technology, Programs and Events, Marketing and Promotions.
“We just recently held elections and our fresh new board members are eager to drive our association forward to continue our mission of supporting technicians, engineers, managers, and vendors in the HTM field,” Kizis says.
She says that the organization has almost 50 paying members, but over 300 affiliates through its email communication.
“KAMI also focuses on networking and community service. We partner with SOS International in their mission to improve healthcare access for those in need through recovery and redistribution of surplus medical supplies. We volunteer our medical technology expertise to test the medical equipment, ensuring it is safe and operational,” Kizis says.
Meetings are held quarterly for members and

monthly for the group’s board.
“We also participate in AAMI’s HTM Association Collaborative, where HTMA leaders meet monthly to discuss best practices and association challenges. KAMI is excited to sponsor one of the HTMA’s educational webinars later this summer,” Kizis says.
The association’s inaugural symposium was in November of 2019 at the historic Boone Tavern Inn in Berea, Kentucky.
“The first symposium was just before the pandemic, in 2019. While the attendance was intimate, it was a successful two-day event of education and networking,” Kizis says.
She says that the next annual conference was not

until November 2024, when KAMI partnered with MD Expo to host an HTM Mixer in Lexington, Kentucky.
“The event was a whirlwind of certified education; vendor exhibits and networking with peers. While we may not be able to have an event this large each year, we are looking forward to still providing valuable and engaging events to our HTM community. The HTM Mixer was a blast! The Bluegrass Kick-Off Party made the first night memorable with the live band and bourbon tastings. The venue space was packed as HTMers enjoyed the festivities,” Kizis says.
As associations put some distance in the rearview mirror on the pandemic, some are returning to a normal stride with fresh ideas and perspectives. KAMI is one of those associations.

BY K. RICHARD DOUGLAS

According to the U.S. Department of Veteran’s Affairs, “the U.S. is divided into 18 Veterans Integrated Service Networks, or VISNs — regional systems of care working together to better meet local health care needs and provides greater access to care.”
These regional systems are numbered from the northeast to the southwest.
In the New York and New Jersey region, the VISN is VISN 2. The medical equipment in facilities within VISN 2 is managed by the VISN 2 New York/New Jersey VA Healthcare Network Healthcare Technology Management team. The department’s five-member staff include VISN 2 Chief
Healthcare Technology Manager Arleen Thukral, M.S., CCE, CHTM; VISN 2 Deputy Healthcare Technology Manager Jessica Hudson-Rahming, CHTM; VISN 2 Biomedical Equipment Support Specialist-Biomedical Information Systems (BESS-BIS) Matthew Dilla; VISN 2 Biomedical Equipment Support SpecialistBiomedical Information Systems (BESS-BIS) Nicholas Martorano; and VISN 2 Biomedical Engineer Joseph Gucciardi, M.S., CCE.
The VISN 2 New York/New Jersey VA Healthcare Network Healthcare Technology Management team supports nine facilities with over 3,000 beds, $551 million in medical equipment asset value and more than 45,000 medical devices.
“Service contracts are assigned a Contracting Officer Representative (COR) who is responsible for statement of work, independent government cost estimate, market research, FITARA and submission to contracting,” Thukral says.
She says that the Contracting Officer (CO) will review the
package and clarify expectations.
“The contract bids are reviewed and evaluated by the COR. After contract award, the COR is responsible for reviewing reports and certifying invoices and working with the CO if amendments/modifications are required,” Thukral adds.
How is data collection accomplished on the VISN 2 team?
“While there are automated reports and scheduled jobs that run such as upcoming contract expiration reports and medical device incidents opened yesterday, most dashboards require validation by facility and VISN HTM staff to ensure accuracy of CMMS and SMAK data through the HTM Dashboard which includes medical device inventory, patient safety, medical device sustainment, medical device security, resource and operations, equipment planning, fiscal, staffing and misc,” Thukral says.
She says that technician assignments are managed locally by department managers. Local managers provide training opportunities to ensure competencies in specialty areas such as imaging and critical care.
“VISN 2 has approximately 46,000 networked medical devices, 400 servers and over 2,000 devices with naming standard index. Our dedicated team, led by Matt and Nick, is responsible for medical device security, EHR device interface testing and validation, and configuration management and support,” Thukral says of HTM/IT integration.
She says that HTM has deployed its own virtual server infrastructure and it is robust, supporting various clinical applications and services.
“We’re also integrating AI technologies to assist physicians in reading medical images, which is a big step forward in improving diagnostic accuracy and patient outcomes,” Thukral explains.
The department’s experienced staff have been busy with projects, bringing more advanced technology to its facilities, including software solutions.
“VISN BIS staff, Martorano and Dilla, have worked on an exciting project to deploy Microsoft’s System Center Configuration Manager (SCCM) across VISN 2. SCCM is highly leveraged in IT and outside of healthcare environments,” Thukral says.
She says that HTM has had to manually manage software and images on clinical systems in the past.
“SCCM solution allows HTM staff to automate software installs, updates, and even removal all from one dashboard. There are also capabilities to standardize clinical image rollouts for configuring/managing OS level items to better security and accessibility of managed systems,” Thukral says.
She says that the use of a software management solution will exponentially assist staff in keeping clinical systems aligned with their Software Bill of Materials (SBoM) as well as vulnerabilities kept in check for outdated software.
“They use Microsoft SCCM to manage unsupported operating systems, which means even our older systems get the updates and patches they need to stay secure. For tackling vulnerabilities, they use automated tools to spot and fix risks across the network efficiently,” Thukral adds.

Department staff have taken steps to streamline the onboarding process and to bring greater efficiencies through automation.
“For new employees, there is a lot to learn, from understanding the technology landscape to how-to document in Nuvolo CMMS and ensure SOPs are being adhered to,” Thukral says.
She says that VISN 2 staff have worked to ensure there is a one-stop shop for navigating to important links, reviewing the strategic goals and accomplishments and accessing knowledge articles that provide how-to for everything from contracting to installing a system.
“Nick developed the power app tool to make the interface easy to navigate and provide a Google-like search for looking up knowledge articles. The team has also streamlined communications by implementing an automated process of sending emails with specific characteristics, which are then auto-saved into designated folders within our SharePoint. In addition, I am validating a HTM chatbox that can answer questions about VISN 2 standardized SOPs,” Thukral says.
Off the clock, the department’s manager remains active in bettering the HTM community. Thukral is involved with AAMI standards with the Equipment Committee, including revisions of EQ89 and TIR for EQ103 (see cover story) and the newly formed AAMI HTM Advisory Community group which comprises of Healthcare Technology Leadership Committee.
She is also vice-chair of Healthcare Technology Certification Commission (HTCC), which oversees the CCE certification process, procedures and policies.
An involved manager in all things HTM and an impressive biomed team, who have embraced innovation, provide superior management of the medical equipment inventory in VISN 2.
TechNation is celebrating Healthcare Technology Management (HTM) Week, taking place May 18-25, 2025, with a special contest for HTM professionals. Each weekday, one lucky biomed will win a $100 gift card in a contest sponsored by FSI.
To enter, visit 1technation.com/htmweekcontest and create a fun caption for a selected photo. Winners will be announced daily during HTM week on TechNation’s Facebook and LinkedIn pages.
HTM professionals, mark your calendars! Registration is open for HTM Mixer Denver 2025, a premier regional conference designed to provide hands-on education, networking, and industry engagement. The event will take place on May 15-16, 2025, at the Omni Interlocken Hotel in Broomfield, Colorado.
The HTM Mixer Denver has been approved for 12 CEUs by the ACI.
Also, the HTM Mixer Milwaukee is set for July 31-August 1. It is proudly supported by the Wisconsin Biomedical Association (WBA).
HTM Mixers are known for delivering high-impact education and networking in a more personalized environment compared to larger national conferences. Attendees can look forward to:
The next MD Expo is set for this fall in the Dallas area. Join HTM professionals from throughout the nation November 10-12 at the Renaissance Dallas Addison Hotel.
The deadline for Call for Presenters has been extended to May 15.
MD Expo seeks presenters who can offer:
• First-hand experience implementing new technologies
• Case studies of process improvement in healthcare technology management
• Best practices using techniques to maximize efficiency and reduce costs
• Practical ways to facilitate inter-departmental communication and support within the facility
• Effective promotion of patient safety and risk management programs
• Career development tips for biomedical professionals
HTM Week raises awareness and appreciation for professionals who manage and maintain medical devices and health technology across healthcare systems. TechNation, the leading magazine for medical equipment service professionals, offers valuable resources for those in the biomedical, HTM, imaging, and IT fields.
For more details, visit 1technation.com/htmweekcontest and learn about FSI’s CMMS platform at fsiservices.com.

• Continuing Education Credits – Advance your career with valuable CE opportunities.
• Engaging Exhibit Hall – Discover the latest innovations and solutions from top vendors.
• Unparalleled Networking – Connect with peers and leading professionals in the HTM industry. Find out more at HTMmixer.com.
Dallas, TX • November 10-12, 2025
• Proven leadership methods and models and implementation strategies
Benefits of presenting at MD Expo include:
• Sharing your knowledge and experience
• Being recognized as a leader in the HTM community
• Professional development – add your speaking engagement to your list of achievements
• Networking with others in the HTM industry and gaining awareness of available resources
Find out more at mdexposhow.com.
The 2025 TechNation Tour continues this month at the HTM Mixer in Denver. The tour features a long list of stops where TechNation representatives will give away swag and host some reader parties. Be sure to find the TechNation crowd at the 2025 AAMI eXchange in New Orleans!
HTM professionals are invited to come join the TechNation community!
The list of TechNation Tour stops include these conferences:
• HTM Mixer CO, May 15

• AAMI eXchange, June 20
• HTM Mixer WI, July 31
• NCBA, Aug. 25
• NESCE, Oct. 15
• MD Expo Dallas, Nov. 10
• FBS, Dec. 4
Check 1TechNation.com for updates and additional tour stops!
Enter the contest and help TechNation celebrate. Fill out the short form at 1technation.com/contest for a chance to win one of 12 prizes, each valued at $150 or more!
Additional entries to win can be acquired by sharing on LinkedIn or submitting a photo. Each month, a winner will be selected and featured in TechNation magazine!
March’s winner is Paul Collier.
Find out more information on Page 86.


MARCH WINNER Paul Collier


• TOOL CASE, FIELD SERVICE DESOLDER PUMP
• FILE, FLAT NEEDLE
• FILE, ROUND NEEDLE
• FILE, TRIANGULAR NEEDLE FLASHLIGHT, TACTICAL
“AA” FORCEPS, STRAIGHT 6” W/GRIP
• (2) HANDLE FOR BLADES, DRIVE-LOC
• HANDLE FOR FILES AND MANY MORE!






In an ongoing effort to increase operational readiness, Airmen from the 8th Medical Group executed expeditionary medical support health response team (EMEDS HRT) training Feb. 13.
The training focused on familiarizing the Med Hawks with the EMEDS HRT tent setup and equipment checks, furthering the Wolf Pack’s contingency and deployment capabilities.
EMEDS are modular, scalable, rapid-response medical packages that can be used in humanitarian relief, wartime contingencies and disaster response operations. The package contains three unique building blocks: EMEDS HRT, EMEDS+10 and EMEDS+25 personnel and specialized equipment components. EMEDS HRT is the first and smallest of the EMEDS packages and provides surgical and trauma care, acute intervention and primary care to a risk population of up to 3,000 individuals.
“Our training was to ensure our Airmen knew how to set up the medical tents properly, and function test generators and environmental control units,” said U.S. Air Force Lt. Col. Brian Johnson, 8th
Healthcare Operations Squadron commander.
“Most of our medics haven’t seen an EMEDS+25 or HRT setup, so this training provided them with some familiarization in the process so if we ever need to deploy an EMEDS-HRT here, we will be ready,” said Johnson.
Med Hawk logistics Airmen and biomedical equipment technicians coordinated with the 8th Civil Engineer and Logistics Readiness Squadrons to perform site surveys and deliver equipment in less than 24 hours.
“We are all pretty familiar with the equipment and after this training are very confident in our abilities to get set up,” said Senior Airman Alexander Page, 8th HCOS biomedical equipment technician.
The 8th Medical Group’s EMEDS HRT training is crucial in maintaining the Wolf Pack’s ability to accept follow-on forces. By conducting training, the 8th MDG ensures its personnel are prepared to aid the Wolf Pack and follow-on forces so that they can return to the fight.
Dismissing patient, family, and caregiver concerns tops ECRI’s 2025 list of the most significant threats to patient safety. The global healthcare safety nonprofit organization says time and resource constraints make it increasingly difficult for some clinicians to provide empathetic care that addresses patient and caregiver concerns, potentially leading to missed and delayed diagnoses.
More than 94% of patients reported instances when their symptoms were ignored or dismissed by a doctor, according to a survey from HealthCentral. ECRI says when concerns go unaddressed, patients and caregivers feel like they’re experiencing “medical gaslighting,” which the American Journal of Medicine defines as “an act that invalidates a patient’s genuine clinical concern without proper medical evaluation.” Unlike the popular usage of the term “gaslighting,” medical gaslighting is not considered intentional, and clinicians are often unaware they exhibit the behavior, ECRI experts say.
ECRI says medical gaslighting can happen when clinicians are rushed for time, have biases that reflexively attribute symptoms to issues like mental illness, age, or weight, or make cognitive errors like interpreting new information in a way that confirms a previous diagnosis. This can lead to a missed diagnosis, delayed treatment, and decreased trust between patients and their healthcare providers.
“Most clinicians have a deep commitment to healing and protecting their patients and would never intentionally make a patient feel unheard, but it nevertheless happens with alarming
frequency,” said Marcus Schabacker, MD, Ph.D., president and chief executive officer of ECRI. “Providing high-quality healthcare starts with truly listening to patients. When we value their input, we gain critical insights that improve patient outcomes and build trust. A healthcare system that prioritizes patient voices is one that delivers safer, more efficient, and more compassionate care for all. Unfortunately, too many clinicians are operating under time and resource constraints that fuel substandard care.”
ECRI experts say solutions require a holistic approach that considers how all aspects of a health system – including leadership and governance structures, patient engagement, workforce wellness, and training infrastructure – promote safety.
The 2025 concerns in ranked order are:
• Dismissing patient, family, and caregiver concerns
• Insufficient governance of artificial intelligence
• Spread of medical misinformation
• Cybersecurity breaches
• Caring for veterans in non-military health settings
• Substandard and falsified drugs
• Diagnostic error in cancers, vascular events, and infections
• Healthcare-associated infections in long-term care facilities
• Inadequate coordination during patient discharge
• Deteriorating working conditions in community pharmacies
ECRI’s 2025 report includes recommendations for healthcare organizations to create organizational resilience to navigate the identified threats and strive for total systems safety.
Welcome to GMED ONE, where we have been setting the gold standard in biomedical repair services since 2011. As a leader in the field, we pride ourselves on our reputation for excellence, trust, and unparalleled service.
Our mission is to provide fast, reliable repairs on a wide range of medical equipment, ensuring that healthcare professionals can continue to deliver the highest quality care to their patients.
•
•
•
•
•
•


FSI, a leading healthcare CMMS/EAM provider, recently announced the availability of its certified ServiceNow integration to make work order routing, creation, and assignment easier than ever.
For today’s hospitals and healthcare facilities, finding ways to cut costs and increase efficiency continue to be a key area of focus, while needing to adhere to more complex compliance requirements. Within facilities and clinical asset management, key integrations with broadly utilized tools like ServiceNow can help improve knowledge sharing and drive faster response times – leading to better service to internal and external customers.
“The healthcare market continues to focus on digital transformation and interoperability – on having systems that speak to each other to share data that leads to more efficient work,” said Chris Lang, vice president, product and strategy, FSI. “We’ve heard from our customers a ServiceNow integration with our platform would eliminate duplicative work while giving visibility into what’s happening at the asset level.”
ServiceNow helps organizations automate various
TMA Systems, a global leader in enterprise asset and maintenance management software, has acquired EQ2, a provider of computerized maintenance management systems (CMMS) tailored specifically for healthcare organizations to manage their clinical assets and facility equipment.
“This strategic acquisition brings together two industry leaders to deliver cutting-edge technology and comprehensive solutions that drive operational efficiency, compliance, and safety in healthcare facilities worldwide,” according to a press release.
The combination of TMA Systems’ robust enterprise asset management platform with EQ2’s healthcare-focused HEMS platform will revolutionize how hospitals, clinics, and other medical facilities manage their assets, maintenance, and regulatory compliance, the release adds.
“We are thrilled to welcome EQ2 into the TMA Systems family,” said Mark Simner, CEO of TMA Systems. “With EQ2’s deep expertise in clinical equipment management and our cutting-edge EAM and CMMS technology, we are poised to set a new industry standard for facility management in hospitals and medical institutions. This acquisition underscores our
management workflows across departments. This new integration builds a seamless sharing of data with FSI, which provides the leading CMMS platform designed for hospitals and healthcare facilities.
When a user submits an issue via the incident or case module in ServiceNow, the data pushes to FSI’s CMMS. It automatically triggers a work order – and the system can assign and route it to the technician as soon as it comes in. With the ability to leverage other workflow automation technology, it helps users to focus time on what matters most.
“Advancing healthcare asset and facility management rests on the ability to automate manual tasks, to give lift to teams to focus on more strategic outputs,” said Lang. “From seamless integrations with companies like ServiceNow to integrated automations, our customers can rely on our platform to deliver the tools necessary to do what they need to do, more efficiently and at a lower cost.”
The ServiceNow and FSI integration is currently available to all customers using both platforms.
commitment to providing our clients with the most advanced and reliable solutions available.”
EQ2’s flagship platform, HEMS, has long been trusted by leading healthcare organizations to streamline operations, improve patient safety, and ensure regulatory compliance. By integrating EQ2’s clinical engineering capabilities with TMA Systems’ advanced asset and maintenance management technology, customers will benefit from an unparalleled suite of solutions designed to optimize performance, reduce costs, and enhance patient care.
“Joining forces with TMA Systems represents a powerful opportunity to expand our impact in the healthcare sector,” said Navneet Agarwal, general manager and chief technology officer of EQ2. “Our shared commitment to innovation and customer success makes this partnership a natural fit, and we look forward to delivering even greater value to our clients.”
EQ2 represents TMA’s fifth acquisition since 2021.The acquisition marks a significant step in TMA Systems’ strategic growth plan, further cementing its position as a leading provider of intelligent maintenance and asset management solutions across multiple industries.




Tri-Imaging Solutions introduces a cutting-edge platform that enhances supply chain management, engineer performance, and system monitoring. It provides engineers with diagnostic tools and video tutorials, and streamlines parts ordering and tracking. Designed for efficiency, the platform minimizes downtime and optimizes operations, setting a new standard for reliability in medical imaging.
XperTIS proactively monitors system health, supports engineers in repairs, and enhances the supply chain process by giving teams seamless access to parts ordering and order tracking
XperTIS offers step-by-step repair guidance, helping engineers troubleshoot efficiently while ensuring faster, more accurate parts ordering This helps maximize uptime and minimize repair costs

AIV Transmitter Case Kits
Replace all outer plastic and exterior membrane switches in-house
Transmitter Repair
Send to AIV for inclusive at rate repair
Parameter Repair
Send to AIV for at rate repair on TRAM/MMS moduls















Vitalacy provides AI-driven monitoring solutions designed to reduce hospital infections and adverse patient events. Its most popular solutions include hand hygiene compliance monitoring and virtual care for hospitals.
“Currently, we are especially excited about our new AIpowered cameras designed to assist nurses with fall prevention. These cameras generate automatic alerts, allowing staff more time to respond to unattended bed exits,” Vitalacy Vice President of Engineering Dylan Humphrey said.
“The possibilities for AI-assisted care are endless, and we are just beginning to explore them. Our customers often identify the next patient safety need, my team engineers the solution, and as a result, patients receive safer care,” he added.
TechNation recently found out more about Vitalacy Inc.
Q: HOW DOES YOUR COMPANY STAND OUT IN THE MEDICAL EQUIPMENT FIELD?
HUMPHREY: Vitalacy automates data collection on a massive scale for hospitals that traditionally rely on manual tracking. Take dispensers, for example – our system records each dispense event across an entire facility, providing insights into areas that need refilling sooner, identifying popular and underutilized handwashing stations (such as unit entries and nursing stations), and tracking hand hygiene compliance trends.
Additionally, our dispenser sensors detect malfunctioning or unused dispensers, turning what was once a static device into a smart tool that informs decision-making for facilities, operations, and environmental services (EVS) teams.
Q: WHAT IS ON THE HORIZON FOR YOUR COMPANY?
HUMPHREY: At Vitalacy, we are committed to making infection prevention and patient safety solutions more effective, accessible, and user-friendly for hospitals of all sizes. Currently, we are developing lighter, longer-lasting wearables to improve ease of use for staff, ensuring better adoption and sustained compliance.
Beyond our bed monitoring system, we are expanding our patient monitoring capabilities to include chair monitoring, helping hospitals address a broader range of patient safety concerns. We have also been optimizing our compliance dashboard, enabling faster compliance calculations and improving room-level compliance tracking for more precise data.
Additionally, we are enhancing flexible reporting options and moving toward AI-driven compliance reporting, giving hospital leaders predictive insights to proactively improve safety measures. To increase accessibility, we are working on making our solutions available to a broader range of healthcare organizations, ensuring more hospitals can implement infection prevention and patient safety technologies.
These advancements reinforce Vitalacy’s mission to simplify compliance tracking, enhance patient outcomes, and drive innovation in hospital safety technology.
Q: IS THERE ANYTHING ELSE YOU WOULD LIKE OUR READERS TO KNOW?
HUMPHREY: Hand hygiene is one of the most critical components of infection prevention, and it requires a dedicated, data-driven solution – not just an add-on to RTLS tracking. At Vitalacy, our mission is to help hospitals create safer environments by combining real-time compliance monitoring with actionable insights for hospital leaders, infection preventionists, and nursing teams.
Our system provides accurate, real-time hand hygiene data, allowing hospitals to proactively address compliance gaps and improve patient safety. Our solutions were specifically designed to identify patient safety challenges and enhance workflow efficiency. By reducing infections and adverse events, our technology directly supports this mission.
As part of our commitment to advancing healthcare technology, we have launched Vitalacy Virtual Care for AI-powered remote patient monitoring and are developing Workforce Analytics to improve staffing efficiency. By continuously innovating in patient safety and infection prevention, we aim to help hospitals deliver higher-quality care and better outcomes for patients.
For more information, visit vitalacy.ai
Q: WHAT PRODUCT, SERVICE OR SOLUTIONS DOES YOUR COMPANY PROVIDE TO THE INDUSTRY?
Secure Mount provides high-quality, American-made cable management and loss prevention solutions to hospitals nationwide. We are committed to helping hospitals save time and reduce costs by securing expensive equipment that is frequently misplaced within their facilities.
Q: TELL US WHAT DIFFERENTIATES YOUR COMPANY FROM THE COMPETITION?
What distinguishes us from the competition is our over 20 years of experience providing hospitals with a comprehensive range of solutions to prevent the misplacement of cables and lead wires. Additionally, our high end in-house machine shop enables us to deliver custom-built solutions tailored to the specific needs of hospitals.
Q: WHY DID YOU CHOOSE TECHNATION FOR ADVERTISING?


Bryce
Vice President of Operations
We selected TechNation for its extensive reach within our target audience, which will help us raise awareness of our specialized products. By doing so, we aim to assist hospitals in reducing the costs associated with misplaced equipment, ultimately enabling them to reallocate funds toward further advancements that benefit the American public.
For more information, visit securemount.com




BY TRESSA DANIELS
Human factors engineering (HFE), while certainly not new, is having a moment. This article explores the critical importance and methodologies of HFE, highlighting its pivotal role in enhancing safety, efficiency, and user satisfaction in the development of medical devices.
How does HFE accomplish these important goals? Simply put, by designing products that align with human capabilities, expectations, and limitations. HFE is particularly valuable in fields such as healthcare, aviation, and consumer electronics, where it can lead to significant improvements in usability, reduce the risk of use errors, and tailor devices to diverse user needs.
Before conducting usability testing in the design and development phase, several steps are necessary to optimize HFE. Initial research and observation are invaluable to understanding points of user confusion, workflows, workarounds, and environmental considerations involved with predicate or competitor products. This research can assist in identifying user needs as well as understanding user profiles and potential use errors.
Key activities in this phase include:
1. User Research: Understanding user problems and needs through interviews, surveys, and other research methods.
2. Workflow Analysis: Studying how users interact with existing systems and tools to identify pain points and opportunities.
3. Contextual Inquiry: Observing users in their real-world environments to gain insights into their natural behaviors and needs.
In addition to research and observation, a task analysis is an important method used to break down the steps required to complete a task and analyze each step for possible areas of design deficiency. A task analysis can start before a prototype exists by using a similar in-market product or the defined product purpose developed during concept and feasibility.
The Perception, Cognition, and Action (PCA) model is a key part of the task analysis, used to better understand and analyze human interactions with systems, particularly in identifying the root cause of use errors later in design and development formative testing. It involves perceiving relevant stimuli, processing the information to make decisions, and taking actions based on cognitive processing. Here’s a breakdown of PCA:
1. Perception
• Stimuli: Users must perceive (see, hear, touch) relevant stimuli from the environment or device interface.
• Challenges: Issues can arise if stimuli are not noticeable or are misinterpreted.
2. Cognition
• Processing: Users process (understand, comprehend) the perceived information to make decisions.
• Challenges: Cognitive overload, misunderstandings, or incorrect assumptions can lead to errors.
3. Action
• Execution: Users take actions (push, pull, press, twist, etc.) based on their cognitive processing.
• Challenges: Physical limitations, incorrect actions, or delays can cause errors.
By applying the PCA model, potential use errors can be systematically identified and addressed during the design and testing phases, thereby reducing post-market problems. During design and development, the project team will plan a
minimum of two to three formative usability tests. In addition, there are other methods of formative evaluations that can also be conducted. Formative Evaluation Methods involve both analytical and empirical techniques to assess and improve the user interface (user interfaces include software, hardware, IFUs, training material, accessories, and packaging). Analytical methods include task analysis, heuristic evaluations, and expert reviews and do not include feedback from intended users. Empirical methods involve gathering direct user feedback through in-depth interviews, contextual inquiry, cognitive walkthroughs (note: these can be done with or without users), and usability testing.
Formative Testing is an iterative design process to identify and fix user interface problems early. It involves three key steps:
1. First, identifying issues by revealing 90% of design flaws with 8-10 intended users (not employees).
2. Next, modifying the design to eliminate errors and use problems.
3. Finally, confirming effectiveness of the changes by conducting human factors validation testing.
HFE is not complete without Human Factors Validation. Human Factors Validation testing is used to prove that the final design is safe and effective for use. This includes identifying and mitigating potential hazards through risk analysis, iterating on the design to fix issues through formative testing, and proving the design is safe and effective through HF validation testing.
Human Factors Validation Testing requirements include:
• 15 users per intended user group
• All critical tasks be performed
• Final production equivalent product
• Simulated environment of use
• Realistic training scenarios
• Uninterrupted use conditions
Human Factors Validation Testing is essential to demonstrate that the final design is safe and effective for use.
HFE is a specialized skill set that considers all aspects of medical device design holistically. It strikes a balance between safety and usability, focuses on risk reduction, and ensures compliance with global regulations.
And what’s the bottom line on HFE? The return on investment (ROI) for incorporating human factors can be observed in several areas:
1. Preventing costly recalls: Early identification and mitigation of usability issues can prevent expensive recalls.
2. Higher user satisfaction and loyalty: Improved user satisfaction can lead to increased market share.
3. Decreased Ccosts: Addressing usability issues early in the design process can save significant costs associated with redesigns and post-market fixes.
In summary, investing in human factors engineering is not just about meeting regulatory requirements; it’s about creating products that truly meet the needs of your users, customers, and patients.





ECRI partnered with Oxygen CoLab to conduct intensive testing of oxygen devices to identify and catalogue those best for resource-deprived healthcare settings.
More than 1 million deaths occur every year due to hypoxemia in low- and middle-income countries, many of which could be prevented with appropriate access to medical oxygen.
The COVID-19 pandemic exposed major gaps in access to medical oxygen devices, prompting donors, global organizations and supply chain leaders to take action to improve availability long-term. But most of these efforts didn’t address oxygen access for lower-level facilities that serve vulnerable patients in low- and middle-income countries.
ECRI partnered with the Oxygen CoLab – a UK Foreign,
Commonwealth & Development Office-funded initiative – to create a comprehensive directory of oxygen concentrators to aid in the procurement of these devices for low-resource settings.
“We replicated real-world scenarios and tested 11 devices to see how they perform in the harsh conditions where they could potentially be deployed,” said Brad Bonnette, ECRI’s Senior Project Officer, “like in a refugee camp, a field hospital, a community health center, or primary health outpost. Picture a mobile clinic in a remote village without a consistent power supply. We want to ensure patients everywhere have access to safe, quality care – whether they live in a metropolitan area near a high-tech hospital, or in a remote community.”
Oxygen concentrators have a particularly important role in low-resource settings, namely for smaller and more remote health facilities, for both acute and non-critical-care patients. The devices are proven to save lives, yet there are challenges to implementing wide-scale solutions in low-resource settings. Most concentrator models don’t adapt well to settings with unreliable power sources,
and dusty, humid conditions – plus a shortage of staff with specialized training in device maintenance.
With many makes and models available, there has long been a gap in evidence regarding product performance, safety, usability, repairability, electrical resilience and energy efficiency to inform decision-making.
“One of the biggest surprises was the variation in how the units handled the challenging conditions,” said Bonnette. “Some suffered catastrophic failure due to accumulated dust, while others were able to deliver a high concentration of oxygen in the same dusty conditions. Some had a hard time with heat and humidity but did well with dust. We hope buyers can use this information to ensure they acquire equipment that can perform well in their environment.”
Brad Bonnette has been a device safety engineer for ECRI for nearly 20 years. In that time, he has evaluated devices used in infusion therapy, respiratory care, anesthesia, sleep medicine, and consumer health.
ECRI is the only organization in the world to operate independent testing labs where engineers conduct hands-on, rigorous medical device evaluations.
To learn more about this project visit ECRI.org/News


soma@somatechnology.com (800) 438-7662
13485:2016
somatechnology.com @Somatechintl

Lewis Lennard, product manager at Rigel Medical US, shares some insights on the current trends and strategies driving advancements in the biomedical test equipment sector.
There is a trend toward portability in biomedical test equipment to meet the demand for compact, lightweight, and handheld devices. Portable analyzers enable healthcare facility testing and field service applications, expanding access to testing capabilities in remote or resource-limited environments. Several of Rigel’s portable analyzers have been used in harsh, remote conditions, and the durability of the equipment has been recognized.
Integration with IoT technology stands out as another prominent trend. We recently released an app that can generate PDFs and test reports, which helps facilitate real-time data capture and storage, simplifying the management of test results and documentation. As with many other areas in healthcare, workflow automation is becoming a focus in the biomed arena. Most biomedical test equipment companies are now offering some form of automation.
Q. WHAT ARE SOME OF THE BIGGEST CHALLENGES FACING THE BIOMEDICAL TEST EQUIPMENT SECTOR TODAY, AND WHAT STRATEGIES ARE BEING EMPLOYED TO OVERCOME THEM?
Keeping pace with rapidly evolving technology is challenging. Test equipment manufacturers need to be innovative with the next generation of test equipment. Collaborating with biomeds, biomedical equipment technology colleges, and medical device manufacturers keeps us up-to-date on emerging technologies and ahead of the curve.
One of the biggest challenges is connected devices and IoT integration. If biomedical test equipment is on the same networks as patient data, the risk is clearly high. To enhance cybersecurity measures, test equipment must implement encryption protocols and secure network processes in the same way that medical devices do.
Q. HOW DOES YOUR COMPANY ENSURE EFFICIENT, FLEXIBLE SERVICING OF MEDICAL DEVICES, ESPECIALLY IN EMERGENCIES, AMID THE GROWING DEMAND FOR PORTABLE SOLUTIONS IN BIOMEDICAL TESTING?
Rigel is seeing strong growth in testing requirements for smaller, remoter healthcare facilities. The design of portable
test equipment is important so that it can be easily transported to healthcare facilities, clinics, or remote locations during emergencies. These devices are lightweight, compact, and often battery-powered, enabling rapid deployment and onsite testing without the need for a specialized infrastructure. Test equipment solutions need to be diverse, catering to a range of settings, from large metropolitan hospitals to remote communities in Alaska.
Q. WHAT MEASURES DOES YOUR COMPANY TAKE TO ENSURE THE PRECISION AND DEPENDABILITY OF TEST EQUIPMENT CALIBRATION AND SERVICING, PARTICULARLY IN ENVIRONMENTS WHERE ACCURACY IS CRITICAL FOR PATIENT SAFETY?
A test equipment manufacturer must have calibration to standards such as ISO 17025 for calibration laboratories, and at the very least ensure reference standards and calibrated equipment are traceable to national or international standards. This ensures the accuracy and traceability of measurements and minimizes uncertainties in calibration processes. Our service and calibration arm, Calibrationhouse, has [United Kingdom Accreditation Service] (IEC 17025) capabilities for biomedical test equipment and counts worldwide medical device manufacturers and healthcare organizations among its customers.
Q. HOW ARE YOU ASSISTING HTM OFFICIALS IN UTILIZING AND MAINTAINING TEST EQUIPMENT THROUGH TRAINING AND EDUCATIONAL PROGRAMS?
We assist biomedical engineers by providing comprehensive training and education initiatives. These include online webinars, onsite training sessions, and video tutorials covering equipment operation, troubleshooting, and preventive maintenance. Rigel also offers certification programs, continuing education courses, and technical support to address topics, regulatory compliance, and any issues biomeds encounter.
We strongly believe in cultivating the next generation of biomeds. We have donated test equipment to colleges as this greatly benefits students by providing enhanced access to new learning experiences and preparing them for future careers in the field. Through hands-on experience with equipment, students develop crucial technical skills in equipment operation and maintenance. Additionally, networking opportunities with HTM professionals may arise, helping students’ educational experiences.

LOOKING
Management and Technicians achieve more in less time
Integrate with all of your existing systems
Compliance and AEM made easier with the right tools and reports
Our system helps you keep your devices protected
Data and information in formats that fit the needs of all levels of technicians, management, and the hospital as a whole
Implementations and support performed by our in-house, US-based staff

Lewis Lennard is a product manager at Rigel Medical US in Tampa, Florida.


The most recent Webinar Wednesday session was a Tools of the Trade Live Demo highlighting the Tri-Imaging Solutions innovative platform – XperTIS. XperTIS is designed to revolutionize supply chain management, engineer performance, and system monitoring. The XperTIS platform enhances system uptime by enabling system monitoring and equipping engineers with diagnostic capabilities to identify and resolve issues.
The live demo highlighted a library of step-by-step video tutorials and instructions that are available to engineers guiding them through installations and repairs. XperTIS also provides the ability to order parts directly, track shipments, and manage RMAs as it streamlines the parts ordering process.
With a focus on efficiency and empowerment, Tri-Imaging’s XperTIS platform ensures equipment runs at peak performance, reduces downtime, and optimizes supply chain operations setting a new standard for the industry.
Fifty HTM professionals logged in for the live presentation. Keith Hall in Utah was the trivia contest winner during the webinar. He won a multi tool.
The presentation was eligible for 1 CE credit from the ACI. A recording of the webinar also provides 1 CE credit when viewed at WebinarWednesday.live.
The session concluded with an informative Q&A session with Tri Imaging Solutions Vice President of Operations John Drew and his team answering more than 10 questions from attendees.
The questions answered included:
• How is it different than other systems?
• What manufacturers and modalities are you currently monitoring?
• What manufacture and modalities will you do next?
• Are you seeing any PHI?
• How do you accomplish the monitoring of the system, what is the installation process?
TechNation’s Webinar Wednesday recently featured a Tools of the Trade Live Demo of the Soma Capital Equipment Service. The session is eligible for 1 CE credit from the ACI.
In the webinar, Soma’s experienced biomedical engineers were seen navigating maintenance and general troubleshooting procedures on popular medical equipment. The panel of subject matter experts demonstrated how to replace batteries on the Steris 4085 and Skytron 3600B surgical tables. They illustrated battery replacements for the Baxter InfusOR, Alaris PC Unit, and Medfusion 3500 infusion pumps, ensuring proper functionality for medication delivery. Another expert demonstrated how to troubleshoot common issues on the GE OEC 9900 Elite and Orthoscan FD Pulse C-arms, helping to reduce downtime in imaging systems. Finally, attendees saw how to balance a surgical microscope and replace the lightbulb on a Lumera 700, ensuring precision and reliable performance.
This educational session offered practical, hands-on guidance for maintaining critical equipment, making it a valuable resource for biomedical engineers and technicians seeking to refine their skills.
The expert panelists also answered questions during the webinar.
HTM professionals were tuned in for the live webinar. Jody Butler, a biomedical equipment technician with Memorial Hospital of Sweetwater County, Wyoming, won a Swiss Force Meister Multi-Tool during the webinar.
Attendees were asked, “What was your single biggest takeaway from today’s product demo?”
“Having an overall knowledge of the biomedical equipment is necessary; however, specializing in certain modalities, can facilitate efficiency in a maintenance program,” said Fernando Trujillo, Senior Clinical Technology Manager, DHA MEDLOG-HTM.
“Some of the most common problems with medical equipment need just quick and easy fixes,” said Narayanaswamy Ramaswamy, Manager, Biomedical Engineering, Hamad Medical Corporation.
“It touched on real life things that happen in the field,” said Patrick Markle, Integration Engineer, Methodist LebonHeur.

The TechNation Webinar Wednesday session “The Hidden Power of Your RTLS Data: Secrets to Unlocking Asset Performance” presented by Mike Tohill, senior director of customer success at Cognosos, is eligible for 1 credit from the ACI. The webinar was sponsored by Cognosos and free to attend. Access the on-demand recording of the session at WebinarWednesday.live and watch the webinar free to obtain 1 CE credit.
In the webinar, Tohill achieved several objectives including an explanation on how biomeds can best understand the breadth of data captured within their RTLS. He also shared how to identify key performance indicators (KPIs) within RTLS data to track asset health, utilization, and efficiency.
After the session, biomeds had the knowledge to discover hidden patterns and trends in their RTLS data to gain insight into operations. They also can explore real-world examples of how organizations are using RTLS data to improve asset performance and achieve business objectives.
An informative question-and-answer session followed Tohill’s presentation. The Q&A provided additional insights that empower biomeds to achieve their goals.
The webinar was well attended and Andrew Warren, QA Manager with Advent Health Orlando, won a Swiss Force Meister Multi-Tool.
Attendees were asked, “Why do you read TechNation magazine?”
“To stay up to date with changes in the technology industry and have knowledge of added values shared by industry leaders,” said Calvin McDowall, Director, Clinical Engineering, Valley Health System.
“To get current news about my chosen profession,” said Ethan Hertz, Clinical Engineer, Duke University Health System.
“Industry information and catching up on how other biomeds tackle daily issues,” said Ricardo Ortiz, Biomed Manager, United Regional.
“Articles about new products and trends in clinical engineering,” said Doug Hershberger, Supervisor Clinical Engineering, Cleveland Clinic Mercy Hospital.

The recent Webinar Wednesday session “Cybersecurity Insights with Scripps Health: Protecting Healthcare Networks” is eligible for 1 credit from the ACI. The webinar was sponsored by Claroty, formerly Medigate by Claroty.
Presenters Ty Greenhalgh, healthcare industry principal, Claroty, and Luke Karkosh, senior director of enterprise architecture, Scripps Clinic, provided insights and answered questions from attendees during the webinar.
HHS has proposed changes to the HIPAA Security Rule requiring new comprehensive network segmentation and enhanced access controls. Karkosh explored how Scripps has strengthened its medical device cybersecurity with advanced network protection strategies.
Attendees were able to learn how to minimize lateral movement, protect electronic health information (ePHI), and comply with evolving regulations. They gained actionable insights to build a resilient, compliant cybersecurity framework focused on securing medical devices and how to address the unique challenges faced by device owners in healthcare environments.
Key takeaways included:
• How changes to HIPAA will impact HTM and Clinical Engineers
• Why visibility is crucial for effective network segmentation
• How segmentation reduces the risk of intruder access to sensitive systems
The webinar drew 80 attendees for the live presentation and a recording of the webinar is available for on-demand viewing at WebinarWednesday.live. Attendee Grant Kelley, MIS & Clinical Engineering Analyst, BTNRH, NE, won a Swiss Force Meister Multi-Tool.
Attendees were asked, “Excluding CE credits, why do you attend Webinar Wednesday?”
“Want to see how other institutions are dealing with biomed devices and cyber risk,” said Jon Helgason, Biomed Cybersecurity Program Director, Nebraska Medicine.
“I am interested in learning more about the different topics discussed in these webinars, which most of the time introduce new regulations and best practices,” said Ounssa Akhayar, Clinical Engineer, UConn Health.
“To keep informed of existing and upcoming and/or existing policies, software, and devices relevant to the biomedical career field,” said Alan Nicewarner, CBET, BSWH McKinney.
“I like being up to date on any trends or new technologies in the industry,” shared Luis Molina, Clinical Engineering Manager, New York Presbyterian-Lower Manhattan.
Struggling with staffing gaps in your biomedical or clinical engineering department? MultiMedical Systems provides expert short- and long-term staffing solutions to keep your operations seamless From preventative maintenance to equipment repair, our skilled technicians integrate quickly and efficiently with your team minimizing downtime and maximizing patient care.






MedWrench is an online resource for medical equipment service professionals to engage with their peers about medical equipment repairs, source parts and to locate service companies. The following are examples of how the MedWrench community members help each other in the website’s forums.
Q: I have a vip6 that displays level sense errors when attempting to start a clean cycle. Process completes with no errors. When inspecting sensors I find paraffin buildup around sensors. Temps are within ranges. Any ideas?
A: It could be bad level sensors, but the front heater on the retort can fail and it will cause these errors. The retort may feel hot to touch and it will not give an heater errors, you can ohm out the heaters to see if they are out of range.
A: Clean the sensors. You don’t want any build-up on or around the level sensors. Each level sensor has a vertical slot on one side or the other. You need a brush that will get into that slot and clean it thoroughly.
Q: I have a Midmark 623 I am currently working on and the base and back functions on the remote are only controlling the back and not the base (back up controls back up the three other functions lower the back). All the fuses are good on the board so everything should be getting power, I have also checked the
remote on other tables and the remote is wired correctly and works right on other tables. The only thing I can think of would be some damage to where the remote plugs into the base of the table. Any ideas or insight would be much appreciated!
A: While you’re in there I’d check to see if the relays are working right. The service manual in the documents section shows you how on page B-20. It sounds like you may be looking at a new PC board though.
A: You may have solved this. If you are seeing damage to the connector. Otherwise check your cables. Functions not working could be bad actuators, cabling, relays, pcb. But as far as an actuator being activated by the wrong button, the signal has to be going to the wrong place. Only 2 ways to do that, pins in the wrong spot or someone moved connectors around
Q: After the self test it shows me faults 35, 36 and 56. and a reduction in the maximum rate, the maximum power
A: Also, I just saw your earlier posts and it could be a brick going bad (there are 4). Usually that throws a brick failure code but maybe it’s partially working.
Good Luck!
To register for a FREE account on MedWrench, visit medwrench.com
MAY 14
SPONSORED BY

TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
MAY 21
SPONSORED BY

WEBINAR WEDNESDAY
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.

Choosing the Best Modern Healthcare CMMS Solution
SPONSORED BY

TOOLS OF THE TRADE Cognosos Asset Management SPONSORED BY An Introduction to Computed Tomography


SPONSORED BY Planning Medical Device Disposal
SPONSORED BY


SPONSORED BY TOOLS OF THE TRADE Downtime Trace Mobile App

All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.

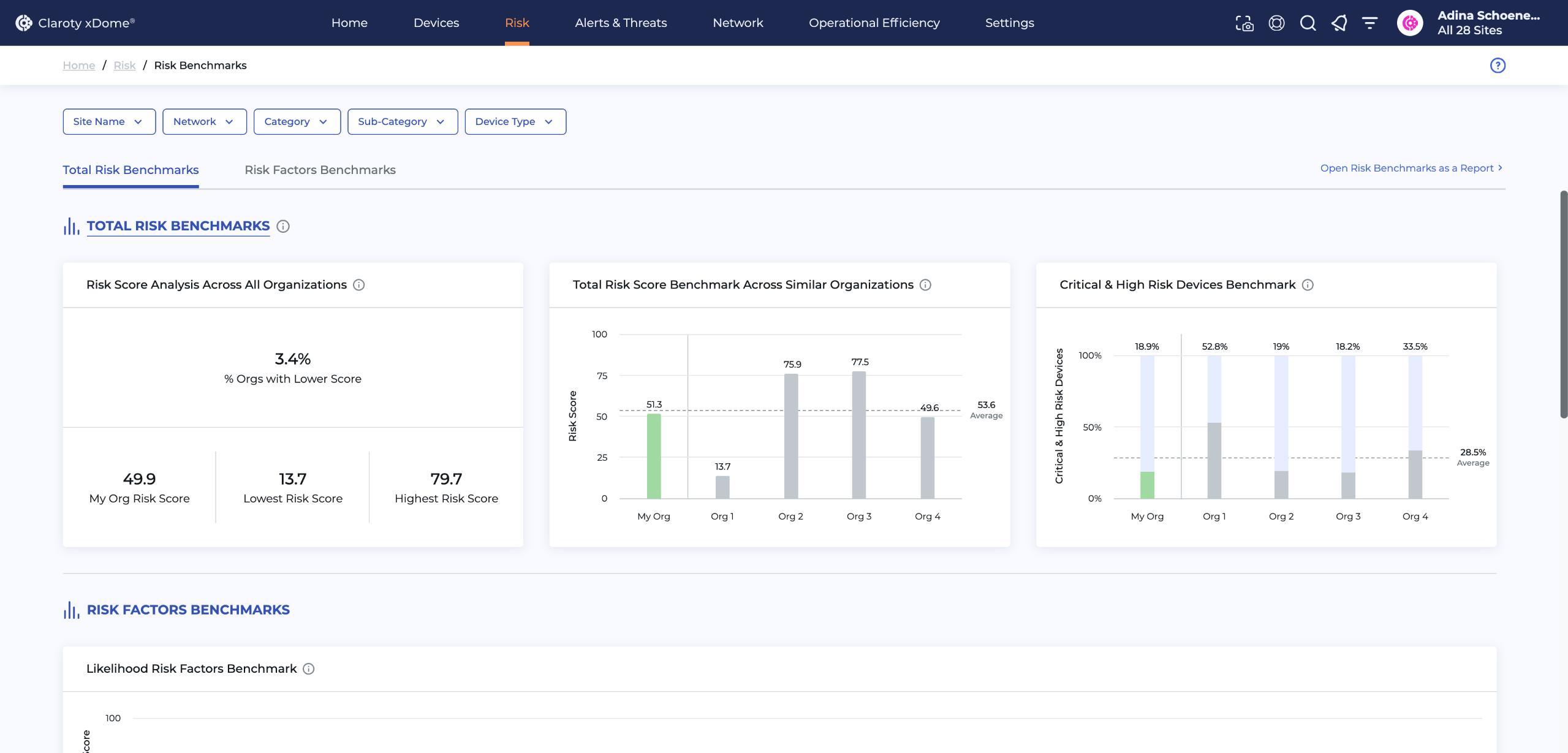
Learn more on how to implement effective and efficient strategies regarding Exposure Management in Claroty xDome for Healthcare. In Claroty xDome, you can benchmark your risk against your industry peers, understand device risk scores and profiles and better manage vulnerabilities and exposures. For more information, visit claroty.com

Possession of the correct tools is just as important as the knowledge needed to maintain and repair medical equipment. One of the most important tools for every healthcare technology management (HTM) professional is a tool kit or case to keep tools safe, close by and ready for use.
TechNation recently asked several industry leaders to share their insights regarding tool kits and cases, including which tools are essential for HTM pros and what are the must-have features every biomed needs when it comes to their tools.
Participants in this roundtable discussion on tool kits and cases are:
• Pronk Technologies Vice President of Business Development Greg Alkire;
• Southeastern Biomedical Co-owner Boyd Campbell, CBET, CRES, CHTM;
• Fluke Biomedical Technical Sales Engineer Jamie Spragis, CBET; and
• South Plains Biomedical Services (SPBS) DFW Branch Manager Chace Torres.
Q: WHAT ESSENTIAL TOOLS SHOULD BE INCLUDED IN AN HTM TOOL KIT FOR BIOMEDICAL ENGINEERS?
ALKIRE: The ideal toolkit will support biomeds with portability, ease of use and multi-functional capabilities. For example, the compact electrical safety analyzer, Safe-T Sim, includes customizable safety autosequences plus ECG and
respiration vital signs simulation. Similarly, a very popular, small, comprehensive multi-parameter patient simulator known as SimSlim offers ECG, respiration, arrhythmia, 4 invasive pressures, YSI 400/700 temperature, and cardiac output – all with an internal battery run time of up to 10 years. For servicing infusion pumps, using an analyzer like FlowTrax that is compatible with any type of infusion device and integrates a very accurate NIST full-featured pressure meter, temperature meter, and digital timer/stopwatch allows biomeds to do more testing while carrying fewer devices.
CAMPBELL: A comprehensive healthcare technology management (HTM) toolkit for biomedical engineers should include a range of essential tools for effective equipment maintenance and repair. The basic hand tools, such as screwdrivers, Torx bits, wrenches, and pliers, are foundational to any toolkit. These tools are commonly found in most electronic toolkits available on the market. However, the key to an effective HTM toolkit lies in selecting tools of high quality and functionality. It’s not just about having the right tools, but ensuring they are durable and reliable for biomedical applications. If a toolkit is only a generic electronics toolkit, it may lack the specific design and features required by healthcare professionals. Thus, a well-curated HTM toolkit should be evaluated and tailored to meet the unique demands of biomedical engineers to ensure optimal performance and safety in healthcare environments.
SPRAGIS: A comprehensive HTM toolkit for biomedical engineers should include essential manual tools like screwdrivers and wrenches, along with advanced diagnostic instruments such as electrical safety analyzers, patient simulators, infusion device analyzers, gas flow analyzers, and defibrillator analyzers. While test instruments are crucial, service manuals, procedural documents, and reliable documentation tools are equally important. A laptop or tablet is necessary for accessing manuals and logging documentation, and network analyzers and communication cables/adapters facilitate troubleshooting networked devices. Additionally, software that automates measurement collection, optimizes workflows, standardizes procedures, and improves documentation and reporting can significantly boost productivity and compliance. Personal protective equipment and cleaning supplies ensure safety.
TORRES: I think overall a tech can be mostly prepared for any job with the following: socket wrench set, bit drive set, screwdriver, assorted pliers set (crimping, needle-nose, tongue & groove, cutter) and, of course, a multimeter.
Q: ARE THERE ANY TOOLS OR CASES THAT ARE FREQUENTLY OVERLOOKED BUT ARE CRITICAL FOR BIOMEDICAL ENGINEERS WHEN SERVICING MEDICAL EQUIPMENT?
ALKIRE: A critical factor is whether or not the devices are capable of having features added in the future. For example, most existing Pronk products in the field can be upgraded with Mobilize wireless connectivity. Our current Mobilize products are also updated with the latest advances in the software. Another vital factor: device durability as
demonstrated by a standard four-year warranty or droptested designs so as to prevent unnecessary downtime. Finally, one would want to look at whether or not the toolkit contains the auxiliary equipment (cables, adapters, etc.) necessary to complete the specific service or maintenance as standard as opposed to an additional purchase.
CAMPBELL: When servicing medical equipment, there are several essential tools and features that are often overlooked in standard toolkits for biomedical engineers.
Some of these include:
• Screwdrivers with insulated shafts – These are critical for safety, as they help prevent electrical shocks when working with powered medical devices.
• Lighted magnifying glass – Useful for reading small, detailed labels or numbers on medical devices, ensuring accuracy during maintenance and repair.
• Magnetizer/demagnetizer – This tool is vital for ensuring that screws and small parts don’t become magnetized, which could interfere with sensitive equipment.
• Cordless screwdriver – A highly practical tool that saves time and effort when dealing with numerous screws in medical devices.
In addition to these tools, the tool case itself plays a crucial role. It should be designed with:
• Large pockets to accommodate various cables, plugs, multimeters, tachometers, and other essential accessories.
• A dedicated space for a tablet or laptop – as these are often used to access manuals, software updates, or diagnostic programs.
• Disinfecting capability – The case and tools should be easy to disinfect to maintain a sterile environment, preventing contamination between different medical devices.
By ensuring these often-overlooked tools and features are included, biomedical engineers can better maintain and service medical equipment in a safe, efficient, and hygienic manner.
SPRAGIS: Critical but often overlooked tools for biomedical engineers include automation platforms that consolidate essential data, enabling seamless integration and efficiency. These systems automatically record results, freeing up engineers to focus on the equipment and enhancing interactions with the clinical team. Additionally, multifunctional test devices that can perform diverse procedures and accurately capture documentation are invaluable. Such devices streamline workflows significantly, offering multiple functionalities in a single unit. This not only improves efficiency but also ensures comprehensive testing and accurate record-keeping, which are crucial for maintaining high standards of patient care and compliance.
TORRES: Carrying cases/tool bags are more personal and preference than anything else, I feel. I like to use a combination. I have a small mechanic tool bag which fits my first look tools for a repair. If I need to get deeper in the trenches, I have a larger utility backpack that holds larger and more unique tools. Overlooked tools include bungie cables, jack stands, spotlight, telescopic pickup magnet,

Greg Alkire
Pronk Technologies



soldering iron/heat shrink, hot glue gun, magnetic screw bin, pick and hook set.
Q: HOW CAN A BIOMED ENSURE THAT THEIR TOOL KITS ARE PORTABLE, YET COMPREHENSIVE ENOUGH FOR THE VARIETY OF MEDICAL EQUIPMENT THEY WORK ON?
ALKIRE: Biomeds can look for tool kits specifically designed with the portability, accuracy and range to complete service on the most common medical devices in a hospital. Pronk’s BMET PACK PRO meets this qualification. Simulators (SimCube NIBP, OxSim SpO2, SimSlim Multi-parameter), automated safety analyzer (Safe-T Sim), and multi-function IV pump analyzer (FlowTrax) all fit within a compact pack along with the standard accessories. Demonstration of compliance to hospital and regulatory standards is also provided with the Pronk Mobilize option of Bluetoothenabled test equipment, such that results are captured instantly in a comprehensive, detailed test report. This allows biomeds to operate more efficiently. For example, Mobilize uses a smart device’s camera to capture the medical device’s barcode, decreasing the opportunity for errors. The final report includes the test equipment’s calibration dates among many other details. Mobilize even transmits PM sticker info (e.g., next inspection due date, etc.) to a Brother portable printer to improve workflow.
CAMPBELL: A biomedical technician should first determine the primary application of their tool kit. If portability is a priority, a soft-sided case is often preferable due to its lighter weight compared to a hard case. When servicing a wide range of medical devices, selecting essential tools while minimizing bulk is crucial. Multi-purpose tools, such as interchangeable handles and bits, such as Wiha tools, can help reduce weight without sacrificing functionality. Larger tools, like socket sets or torque wrenches, may be best carried separately as needed. Organizing tools into modular sections can also enhance efficiency, allowing technicians to customize their kit based on the specific job. Balancing portability with versatility ensures the toolkit remains practical and effective for diverse maintenance and repair tasks.
SPRAGIS: To achieve a balance between
portability and
comprehensiveness, biomedical engineers should rely on a high-quality laptop or tablets, which can be further enhanced by accessing platforms like OneQA workflow automation software. Technological advancements in laptops now enable them to serve as all-in-one devices for performing preventive maintenance tasks efficiently and thoroughly.
TORRES: I actually have two separate tool bags. One for typical repair needs and one that carries the overlooked tools as well. One way to discern what you’re going to need is to gather as much information about the work your about to dive into. If you’re getting into a surgical table you might need to add some tools and subtract others. I personally take the approach, as a field service tech, to run with an ever changing flexible load out. I let the device I’m working on specifically dictate what bag I use. Some do the opposite and just roll with everything at once, to each his own.
Q: HOW IMPORTANT IS CUSTOMIZATION IN TOOL KITS FOR DIFFERENT TYPES OF BIOMEDICAL EQUIPMENT?
ALKIRE: At Pronk, our test equipment solutions are tailormade to meet the wide range of needs for biomedical engineers. We offer different BMET PACK bundles, such as the PACK PRO and PACK ADVANCED. Both include the automated Safe-T Sim Safety Analyzer. Pronk offers over 20 different configurations of equipment bundles to best suit your needs. Choosing cases for kits that can support various configurations with some extra space to fit additional equipment is very useful. For example, the BMET PACK comes with configurable dividers with extra space for tools and laptop/tablet, and our SimCube Patient Simulator kit cases can hold the new Pronk Inflator and Pronk Power Bank, as well. Ultimately, customers customize as they see fit, and we try our very best to support their needs.
CAMPBELL: A well-designed general-purpose toolkit can serve both imaging and standard biomedical applications with the addition of a few specialized tools. A well thought out tool kit will have extra space available to allow technicians to personalize their kits based on their specific responsibilities, ensuring they have the right tools for each job while maintaining portability. This flexibility helps optimize efficiency, reduces unnecessary weight, and
ensures readiness for a variety of service needs.
SPRAGIS: Customization is vital in tailoring tool kits to manage the nuances of various biomedical equipment types effectively. Tools like OneQA can be precisely adjusted to meet specific technician requirements, breaking the limitations of a one-size-fits-all approach. Procedures may require different levels of detail – some necessitating multiple images, others a more streamlined format. Customization not only facilitates standardization among team members but also enhances overall efficiency and time management, ensuring that each piece of equipment is serviced with the appropriate level of care and precision.
TORRES: Custom load outs typically start when you are diving into a specialty device. For a steam bulk sterilizer you might want to ensure that you have heat resistant gloves, channel locks, Teflon tape, and a plumbers wrench. Whereas for an anesthesia device you might run with a breathing circuit ready to go or just rely on a PM kit (seals/Orings) that you have with you to solve most issues.
ALKIRE: The biggest challenge is having enough equipment for what everyone needs. The founders of Pronk Technologies understood 21 years ago the importance of miniaturizing test devices while designing easy-to-use interfaces, and this was crucial towards the mobility and flexibility needed in our market. They also recognized the additional importance of designing budget-friendly bundles – with hundreds of dollars in savings in the more comprehensive kits – ranging from a single device up to 10 devices in a single case with standard accessories. Another challenge biomeds face is evaluating the accuracy of test equipment. Verifying that the test equipment meets the accuracy requirements listed in the medical device service manual or following the general rule of thumb of a 4:1 test accuracy ratio between the test equipment and the device under test (i.e., making sure it is four times more accurate than what is required) is something that should be evaluated prior to purchasing any piece of test equipment.
increases the risk of stripping screws, turning a simple task into a time-consuming problem. Investing in high-quality, reliable tools ensures longevity, efficiency, and fewer disruptions during critical maintenance and repair tasks.
SPRAGIS: Balancing price with proven reliability and quality is a significant challenge for HTM professionals when choosing tools. They often grapple with challenges such as ensuring standardization across their toolkits, selecting the appropriate analyzer for specialized tasks, and maintaining calibration verification and procedure revisions. Minimizing the number of accessories and cables needed through comprehensive test instruments is critical. An HTM professional must be confident in the reliability and accuracy of the test instruments to limit the risk of having to retest a medical device due to an out-of-tolerance test instrument. Addressing these challenges is vital for maintaining high standards of service and patient care.
“One of the biggest challenges is ensuring tool quality. While many necessary tools can be purchased at a local hardware store, they may not offer the durability and precision required for biomedical work. A broken or worn-out tool can cause unnecessary frustration and delays.”
TORRES: I think many of the techs just entering the field think they need to shell out a bunch of cash to get going and that is not the case. I always tell my techs to get the essentials. As you go along, new repairs are going to show you what tool you need to add to the bag. You can certainly be a brand person or go after the sleekest tool load, but it’s not required to be successful. If you want to get tools on a budget there are a couple options: surplus tool store, garage sales/estate sales, Facebook marketplace, and Harbor Freight.
Q: WHAT ADVICE WOULD YOU GIVE TO A NEW BIOMEDICAL ENGINEER WHEN IT COMES TO ASSEMBLING THEIR FIRST TOOL KIT?
CAMPBELL: One of the biggest challenges is ensuring tool quality. While many necessary tools can be purchased at a local hardware store, they may not offer the durability and precision required for biomedical work. A broken or wornout tool can cause unnecessary frustration and delays. For example, a Phillips screwdriver with worn or indented tips
ALKIRE: Look for portability, durability, and that the kit has all of the devices, cables, adapters, etc. necessary to complete the testing plus the capability to allow you to document electronically. As they look more into different products, new biomeds may find that some test equipment requires time-consuming navigation of software menus, and so ease of use becomes a factor to improve workflow. To simplify testing and at the same time improve on accuracy, some products, such as Pronk Mobilize, wirelessly connect test equipment to an organization’s preventive maintenance checklists for automated test sequences and repeatable or adaptable test procedures to minimize chances for human error. Of course, the engineers still need to manage the testing. But the app can be very helpful toward allowing the engineers to focus on testing the medical device rather than searching for needed settings on test equipment, finding procedures, or handwriting test results.
CAMPBELL: Rather than building a tool kit piece by piece, it’s best to start with a high-quality, pre-assembled kit designed specifically for biomedical technicians. These kits typically include essential, durable tools tailored for the field. From there, the technician can customize it as needed based on their specific work requirements. Investing in a quality set from the start is more cost-effective in the long run, as a well-made tool kit can last for decades. A properly selected kit ensures reliability, efficiency, and fewer tool-related frustrations over time.
SPRAGIS: New biomedical engineers should prioritize investing in high-quality, durable tools to avoid issues associated with inferior equipment. They should ensure data accuracy and procedural completeness, or leverage systems designed to handle these verifications, allowing them to focus on their technical role. Choosing advanced, technologically sophisticated test instruments will maximize efficiency and allow new engineers to concentrate on developing their technical skills without being hindered by unreliable tools.
Future-proofing your inventory by selecting test instruments that use technology enabling automation and workflow efficiency, from brands with a long history of support and commitment to quality, that invest in the next generation of biomedical tools, will ensure sustained effectiveness and reliability. Building a tool kit with these considerations in mind will set a strong foundation for a successful career in biomedical engineering.
TORRES: Understand what the essential tools are that you need day one. Consider the budget you have to work with. Think about what’s important to you with your tools – brand, reliability, comfort, cost, etc. Lastly, consider what you’re going to transport your tools with – hardcase w/wheels, multiple compartment toolboxes, simple tool bag, backpack or sling case, and so on. Your tools are an extension of yourself and should be a representation of how you want to tackle the job. Recommendations are always great to consider, but also make sure you are happy with what you get, if not make some changes until you are. Good luck out there!
























love to feature you, your colleague or the entire department in one of TechNation’s monthly features!
Think of a Biomed/CE Department deserving of recognition.
THE MONTH TheHoustonMethodistBiomedicalEngineeringDepartment knownuston,TexasisoneofthebestcitiesinAmerica.Thecity andsurroundingcommunitiesforma tonmetropolisthatextendstotheGalvesBay.Withapopulationexceedingcity2.3million,itisthefourthlargest miles.intheU.S.,covering637square Houstonwasfoundedin1836andoffersattractionslikeSpace CenterHouston,thelargestfinearts
andthe downtownaquarium. Acityofthissizedependsonhealthcaresystemswiththe capacitytoservesuchalargepopulation.Thatcapabilityis andembracedbyHoustonMethodistHospital,affiliatedhospitals other
Werecentlyprofiledasmalltwo-techniciandepartment,butit totakesabiomeddepartmentattheotherendofthespectrum network.managemedicaldevicesforsuchalarge-scalehealth operatingWitheighthospitals,anacademicinstituteand2,509 beds,HoustonMethodistincludes27,947employcalees,withmanyworkinginbiomed.The85-memberbiomediAnthonyengineeringdepartmentisledbySystemDirector Maroulis.Othermembersofleadershipinclude CampusDirectorofClinicalEngineeringMarcBateman, Ruiz,ManagersofClinicalEngineeringTerresaEverhart,Javier CoordinatorsMiguelMezaandCoreaunJacksonandProgramBrandonHightandNathanStathos.Theteam’sclinicalengineersincludeZachSmith,CristianDelgado, BailynPiecewiczandHaniKhalil.Thedepartmentalso consistsoftwoadministrationassistants,fivebiomed equipmenttechleads,23biomedequipmenttechIIs,23 biomedequipmenttechIIIs,15biomedequipmenttechIs,4 biomedradiologyserviceengineersand dataentry specialist. 91,000Batemansaysthatthebiomedteamservicesmorethan medicaldevicesin-housethankstotheexceptional
trainingoptionsavailabletothedepartment’stechnicians. Inadditiontomanagingequipmentattheeighthospitals, andthelargebiomedteamalsoservesmorethan20orthopedic tionalsportsmedicinelocationsofferingphysicalandoccupaTheytherapyacrosstheGreaterHoustonarea. PhysicianarealsoresponsibleforequipmentattheSpecialty Groupwith848physiciansat191locationsandthePrimaryCareGroupwith169physiciansat42locations. NewYork-PresbyterianThehospitalisaffiliatedwithWeillCornellMedicine, HospitalandtheTexasAnnual
educationinstitute,hasafacultyof742,with2,110credentialed researchers.
BIGTEAM,BIGPROJECTS ThechallengestoaddressCOVID-19surgestaxedthe resourcesofhealthcarefacilitiesacrossthenation,butit requiredinnovationonagrandscaleforthebiomedteamat Houston Methodist. “AttheonsetoftheCOVID-19pandemic,there was a glaringneedtoreduceexposuretopatientswithinthe hospitalforourRTdepartment.Thankfully,ourHamiltonG5 haveventilatorshaveadetachableinteractionpanel.RTaskedto panelventsinsidetheCOVIDroomandplacetheinteraction outsidetominimizeexposurefortheRTtherapisttogo insidetheroom.Theinteractionpanelwasattachedtoa tableoutsidethepatientroomandwehadtodothisfor 90-100G5patientrooms,”Smithsays. tionHesaysthattogetherwiththeITmedicaldeviceintegrateam,theysuccessfullyopenedavirtualICU(vICU). “Wehadakeyroletoplayinensuringthepatientphysiological monitorswerecapableofbeingusedinthisnew,innovative setting,”Smithsays.TheteamtackledmanyotherprojectsrelatedtothevICU tomeettheneedsofpatientsandvisitorsduringthe pandemic.AnotherprojectwascenteredaroundAlarispump interoperabilityandtyingthemtopatientrecords. “Pharmacy,nursing,biomed,multipleITgroups,andthe
TQ:WHEREDIDYOUGROWUP?
TechNationEditorJohnWallace recentlyquizzedHardingabouther educationandoutlookforthefuture.
A:Lexington,Kentucky
Q:CANYOUTELLUSABOUTYOUREDUCATIONAND TRAINING?A: master’sBachelorofArtsinArtHistory,BachelorofArtsinHistory, Management.inhumanresourcesandAAMILeadershipin
Q:WHEREDIDYOURECEIVEYOURHTMTRAINING/ EDUCATION?A: USCBachelor’sfromtheUniversityofKentucky,master’sfrom …butHTMfromexperience!
Q:HOWDIDYOUFIRSTDISCOVERHTM?A: Aftergraduateschool, receivedajobofferfromAramarkHCT.Ihadnoideawhatitwas,buttheysaid wouldbeworkingintraining,soIwasexcitedandinterested. amstill learningsomethingneweveryday.
Q:HOWDIDYOUCHOOSETOGETINTOTHISFIELD?A: gotintoHTMbyaccident,but amchoosingtostay becauseofthepeople supportandtheamazingthingsthey aredoing.Thefieldisourfrontlineandtheyseemtooftenbe un-sungheroes– wouldliketotakepartinchangingthat.
Q:WHATDOYOULIKEMOSTABOUTYOURPOSITION?A:Evenonharddays,knowingthat amimpactingthehealthandsafetyofothersinapositiveway,even indirectly,iswhat keepsmegoing.


Q:WHATINTERESTSYOUTHEMOSTABOUTHTM?A: manyForsucha“smallindustry,”itisalsoveryvast;thereareso though,typesofrolesandspecialties.Withthat“small”industry, IreallyappreciatethecamaraderieamongstthoseintheHTMfield.Weareallonehappylittlefamily! INQ:WHATHASBEENYOURGREATESTACCOMPLISHMENT YOURFIELDTHUSFAR? A: trainingThecreationoftheTRIMEDXTrainedprogram–aninternal TRIMEDXprogramforTRIMEDXAssociates.Thisisabigwinfor andwillcontinuetogrow,but couldnothavedone fromanyofitwithoutthesupportfrommyteam,fromTRIMEDXand theamazingvendorswepartneredwith. NEXTQ:WHATGOALSDOYOUHAVEFORYOURSELFINTHE YEARS? A: leadershipamveryinterestedindataanalyticsandefficiency,soa rolerelativetothatwouldbeveryinterestingtome.

ARE EQUIPPED FOR BETTER?
TechNation is celebrating Healthcare Technology Management (HTM) Week, taking place May 18-25, 2025, with a special contest for HTM professionals.
Each weekday, one lucky biomed will win a $100 gift card in a contest sponsored by FSI.
TO ENTER:
Scan this QR code or visit 1technation.com/htmweekcontest and create a fun caption for a selected photo.
Winners will be announced daily during HTM week on TechNation’s Facebook and LinkedIn pages.












AAMI’s biggest conference for healthcare technology management (HTM) professionals is coming to New Orleans!
From June 20-23, the 2025 AAMI eXchange will convene the HTM community, as well as some sterile processing professionals, for an unforgettable weekend. But AAMI is not content to simply play the hits. The programming at the 2025 eXchange was chosen based on your feedback from past years and is sure to provide exceptional value. Read on to learn about the education sessions, available CEUs, HTM leadership training, career fair, charity golf tournament, and much more!
REGISTRATION AND NEW EXHIBITORS
Register today! Early bird rates for full conference and one-day registration are available until April 19, 2025. AAMI members enjoy a discounted rate, as do students, active military personnel, and government workers. You can find more information on registration rates for the 2025 AAMI eXchange here. Questions? Contact us at exchange@ aami.org. Registration for the 2025 eXchange is graciously sponsored by TRIMEDX.
eXchange KEYNOTES
The opening keynote will be delivered by Aaron Horowitz,
CEO of Empath Labs. Empath Labs specializes in developing guides for pediatric clinical research called Clinical Companions, which are used by more than 175,000 children worldwide to alleviate anxiety and improve their medical treatment. Join us to learn about ‘Empathy as a Superpower!’
The 2025 AAMI award winners will be recognized in New Orleans.
Jinesh Patel, CEO and Co-Founder of Uptime Health, will deliver a keynote on ‘Expanding Horizons: How Biomedical Technicians Can Diversify and Capture New Markets.’ Patel will discuss how clinical engineers and technicians already have the skills to enter new markets and expand their services to non-traditional settings.
The expo hall will feature more than 150 exhibitors, including medical device manufacturers, independent service organizations, third-party servicers, non-profits, professional associations, and members of the trade press. Attendees with expo hall passes have access to 10 hours of exhibit time over three days, including a welcome reception on Friday, June 20. Various exhibitors will also host dozens of in-booth sessions, presentations, and product demos throughout the weekend.
The AAMI Studio, generously sponsored by GE Healthcare, is also returning at this year’s eXchange. As in past years, we will interview leading figures from around the HTM field, recognize AAMI award winners, and host livestream programming including Live @eXchange!



There are a total of 80 education sessions at this year’s eXchange, divided into the following tracks. Each track includes multiple sessions over the three-day show. You can earn CEUs for your AAMI certifications by attending these courses. Notable topics within each track are listed below.
Sterilization Track: Featuring sessions on steam sterilization, sterile packaging, isolation gowns, borescope inspection, and more.
Patient Safety and Regulatory Track: Focusing on incident investigations, lessons learned from hurricane response, and the debate over remanufacturing.
Cybersecurity Track: Addressing enterprise risk, the aftermath of cyberattacks, and ITO cybersecurity.
Artificial Intelligence (AI) Track: Discussing AI in preventative maintenance, clinical integration issues, and using chatbots in HTM.
Home Health and Hospital at Home Track: Addressing planned maintenance and the integration of digital technology into biomedical engineering.
Career and Professional Development Track: Including sessions on disability in HTM, career progression, vendor relations, and how female professionals can navigate the HTM field.
Equipment Management Track: Featuring topics like department financials, navigating equipment end-of-life, and more.
HTM Recruitment: Including discussions on how to change HTM recruitment, strategic talent acquisition, and attracting new BMETs to the field.
Virtual and Augmented Reality: Incorporating sessions on how the VA is using VR/AR tools and how to use AR to deliver precision-guided service.
Are you ready to apply for that management position? HTM professionals interested in taking the next step in their careers are encouraged to sign up for the HTM Leadership Training course. This 7-hour course is taught by staff from the College of Biomedical Equipment Technology and includes material on vendor negotiations, business ethics, new technologies, team building, company culture, change management, and much more.
Attendees can also sign up for this year’s Technical Training course. Entitled Introduction to Servicing Radiologic and Fluoroscopic Imaging Services, it focuses on the systems and technologies found in most medical imaging departments. Biomedical equipment technicians, managers, and students alike are encouraged to attend.




The eXchange will also feature several concurrent symposiums on June 21, including the annual symposium of the American College of Clinical Engineering (ACCE) and an Industry Symposium presented by Philips.
After receiving rave reviews in 2024, the AAMI Career Fair is back! Held on June 22-23, the career fair is a one-stop resource for job seekers and employers alike.
Job seekers will have access to representatives from some of the best employers in HTM. Representatives from the Department of Veterans Affairs, Kaiser Permanente, GE Healthcare, Sodexo HTM, the Mayo Clinic, and TRIMEDX will be at the fair, and more organizations are expected to be announced soon. Job seekers may sign up for a free professional headshot courtesy of AAMI, and one-onone resume review sessions with AAMI’s human resources staff are also available.
On Friday, June 20, AAMI will also host an event for high school students from the New Orleans area. This event will introduce them to the HTM field, allowing them to talk to HTM professionals and explore opportunities in the field.


The HTM field is vast, but if you find yourself on the list below, consider joining us in New Orleans! Typical eXchange attendees include:
• HTM leaders, clinical engineers and biomedical equipment technicians.
• Management staff from hospitals, clinics, and other healthcare facilities.
• Members of U.S. government agencies like the Department of Defense and Department of Health and Human Services.
• Experts in cybersecurity, risk management, and systems engineering.
• Students in biomedical engineering, biomedical technology and related fields.
• Professionals involved with standards development with AAMI or ISO.
• Sterile processing professionals.
• Trade press reporters covering medical devices, sterilization, or standards.
For all these groups and others, attending eXchange is a great chance to meet colleagues from across the field and discover new professional opportunities. Register today!
Education Sessions, Available CEUs, HTM Leadership Training, Career Fair, Charity Golf Tournament, And Much More!
Come along with Bryant
takes us on an indepth journey of all the great things to do and see in New Orleans: A CITY THAT CLAIMS YOU


WEDNESDAY, JUNE 18
1:00 PM - 5:00 PM—Exhibitor Set-up (by appointment only, for booths 600 sq. ft. or larger ONLY)
THURSDAY, JUNE 19
8:00 AM - 4:30 PM—Set-up for all Exhibitors
8:00 AM - 5:30 PM—Registration Open
FRIDAY, JUNE 20
7:30 AM - 7:00 PM—Registration Open
7:30 AM - 2:00 PM—3rd Annual Golf TournAMent (buses to depart at 6:00AM from the convention center)
8:00 AM - 12:00 PM—Set-up for all Exhibitors
9:00 AM - 5:00 PM—HTM Leadership Training
3:00 PM - 5:15 PM—Concurrent Education Tracks/Sessions
5:30 PM - 7:00 PM—Welcome Reception in the Expo Hall
SATURDAY, JUNE 21
6:30 AM - 5:30 PM—Registration Open
7:15 AM - 10:15 AM—Concurrent Education Tracks/ Sessions
7:30 AM - 10:15 AM—Clinical Engineering Symposium - Presented by ACCE
7:30 AM - 11:00 AM—AAMI's Career Fair
8:00 AM - 10:00 AM—Technical Training: Intro to Servicing Radiologic and Fluoroscopic Imaging Services
10:30 AM - 12:00 PM—Keynote Speaker & Awards Presentations Part I
12:00 PM - 1:00 PM—Lunch in the Expo Hall
12:00 PM - 5:00 PM—Exhibit Hall Open - click to view list of exhibitors


12:00 PM - 5:00 PM—Exhibitor In-Booth Theaters, Exhibitor Innovation Hub, and AR/VR Theater in the Exhibit Hall
3:30 PM - 4:00 PM—AAMI's Annual Business Meeting
4:00 PM - 5:00 PM—Happy Hour in the Expo Hall
5:00 PM - 6:15 PM—Industry Symposium - Sponsored by Philips - More Information Coming Soon
6:00 PM - 8:00 PM—AAMI PARTY! @ Mardi Gras World & The Grand Oaks Mansion
SUNDAY, JUNE 22
6:45 AM - 8:00 AM—Industry Symposium: TBD
7:30 AM - 5:30 PM—Registration Open
7:30 AM - 11:00 AM—AAMI's Career Fair
8:00 AM - 10:15 AM—Concurrent Education Tracks/ Sessions
10:30 AM - 2:00 PM—Keynote Speaker & Awards Presentations Part II
12:00 PM - 1:00 PM—Lunch in the Expo Hall
12:00 PM - 4:00 PM—Exhibit Hall Open - click to view a list of exhibitors
12:00 PM - 4:00 PM—Exhibitor In-Booth Theaters, Exhibitor Innovation Hub, and AR/VR Theater in the Expo Hall
4:15 PM - 5:15 PM—Concurrent Education Tracks/Sessions
5:30 PM - Vendor Night - Open Night for Vendors and Attendees to Connect on their Own in the "Big Easy"
MONDAY, JUNE 23
7:30 AM - 12:00 PM—Registration Open
8:00 AM - 10:15 AM—Concurrent Education Tracks/ Sessions
10:30 AM - 12:00 PM—Keynote Speaker
Schedule current as of March 20, 2025 - subject to change.



Be
626 Holdings weare626.com
Booth #2524

Alco Sales & Service Co. alcosales.com Booth #2529

BC Group International, Inc. bcgroupstore.com Booth #2618

Claroty claroty.com/healthcare-cybersecurity/ medigate Booth #2632


College of Biomedical Equipment Technology cbet.edu Booth #2437

CPN Power, Inc cpnpower.com Booth #2408

ECRI ecri.org Booth #2613

Elite Biomedical Solutions elitebiomedicalsolutions.com Booth #2842

EQ2, LLC eq2llc.com
Booth #2528 Innovatus Imaging innovatusimaging.com

Booth #2402

Integrity Biomedical Services, LLC integritybiomed.com
Booth #2432
MedWrench medwrench.com Booth #2944





RS&A
Booth #2314
MultiMedical Systems, LLC multimedicalsystems.com
Booth #2737

PM Biomedical pmbiomedical.com
Booth #2531

Pronk Technologies pronktech.com
Booth #2612

QRS Solutions / Datrend Systems Inc qrs-solutions.com
Booth #2629

TechNation 1technation.com
Booth #2845

Tenacore LLC tenacore.com
Booth #2513

TruAsset, LLC truasset.com
Booth #2400
USOC Medical usocmedical.com
Booth #2409

VIZZIA Technologies vizziatech.com
Booth #2536

Get ready for an adventure! AAMI eXchange attendees who take on MedWrench’s Scavenger Hunt have the chance to win over $2,400 in prizes


HERE’S HOW TO PLAY:
• Grab your Scavenger Hunt card at the MedWrench booth 2944 or from any of our awesome participating sponsors.
• Complete the hunt and turn in your finished card at the MedWrench booth before June 22nd at 2:00 PM CT.
• You must be present for the prize drawing at the MedWrench booth to be one of our lucky winners!
• AAMI #2719
• ALCO Sales and Service Co. #2529
• Block Imaging #2414
• FSI #2913
• Innovatus Imaging #2402
• Integrity Biomedical Services, LLC #2432
• Interlight #3045
• MedWrench #2944
• MultiMedical Systems, LLC #2737
• PartsSource #2837
• PM Biomedical #2531

• Prescott’s #2423
• Pronk Technologies #2612
• QRS Solutions/Datrend Systems, Inc. #2629
• Rigel Medical #2441

• Sage Services Group #3029
• TechNation #2845
• United Infusion #2523
• USOC Medical #2409





• The fastest growing HTM talent network in the country.
• 350+ open opportunities throughout the United States.

» A variety of posting options ranging from single-job postings to 12-month unlimited memberships.
“My HR department advertised on various government sites and our web site but we did not get a single applicant in over 120 days. Fairbanks Alaska is hard to recruit for but I took out an ad on HTM Jobs and got two good applicants in less than 30 days. I am hiring them both. Thanks HTM Jobs.”
- D. Anderson, Tanana Chiefs Conference
Featured Employers: Agiliti, Renovo Solutions, TRIMEDX, Erbe USA Inc., Dartmouth-Hitchcock, Sutter Health, and more!


BY K. RICHARD DOUGLAS
Among AAMIs many offerings is its role as a standards development organization, so a standard for AEM is clearly within its wheelhouse. While very well known for certifications and education, AAMI, in collaboration with other organizations, develops standards recognized across HTM.
Since 2014, AEM programs have been developed based on internal data sets developed by HTM departments using evidence-based maintenance. The goal has been to determine the most reasonable frequency for maintenance and testing based on data sets that also track failure rates.
The Centers for Medicare and Medicaid Services (CMS) allowed HTM departments to deviate from OEM maintenance recommendations, with some categories of equipment, when the HTM department has evidence that their alternate schedule does not impact patient safety or equipment function.
The interpretation of what is suitable in these programs, along with the best approach to the development of a compliant AEM program, has been the subject of debate and discussion.
Healthcare systems must meet the CMS Conditions of Participation. A healthcare delivery provider must meet these standards to receive compensation from Medicare and Medicaid. The standards must be met for every patient, even if that patient was not a Medicare or Medicaid patient.
Although efficiency and cost savings might be a part of an AEM program, the regulators are not interested in efficiency or cost savings; they are only focused on patient safety. Although, in CMS’s 2013 memo titled “Hospital Equipment Maintenance Requirements,” the agency did state that hospitals: “may through experience have identified more efficient or effective maintenance activities which do not reduce the safety of the equipment.”
CMS also expects that the standards of accrediting organizations meet or exceed those same standards. Healthcare providers must comply with federal, state and local laws.
Against this backdrop is the issue of AEM programs that satisfy the exacting standards of the regulators. AAMI’s expert working group focused on this specific area of concern to develop a standard to help align AEM programs and allow them to stand up against scrutiny.
The EQ103 standard provides clarification in order to respond to accrediting bodies with justification and explanation for an AEM program’s parameters and guidelines.
The process of creating a standard requires discussion, debate, research, consensus, feedback and documentation.
The ANSI/AAMI EQ103: 2024 standard on alternative equipment management (AEM) programs in healthcare delivery organizations resulted from that process. The standard attempts to bring more broad standardization to AEM programs. The new standard was developed by a diverse group of participants in the working group, bringing a number of perspectives and experience to the process.
EQ103 provides a structured framework for what equipment should be eligible for the program, a risk-based assessment and the correct way to document the program. It includes sections on types of AEM activities, evaluating the performance of an AEM program, evaluating the safety and effectiveness of an AEM program and the decision to place equipment in an AEM program.
It lays out a best-practices approach for the management of an AEM program.
AAMI’s Medical Equipment Management Committee (EQ) develops standards on aspects of medical equipment management for healthcare technology managers. The EQ committee includes five working groups. The working groups include subject matter experts. There is cross-collaboration between the groups.
Many of the locally developed internal AEM programs have had a lot of thought and data put into their creation to produce greater levels of efficiency, but the paradigm developed through a national standard would offer a framework as guidance.
To provide more solid guidelines that can be implemented across the healthcare continuum and to satisfy CMS as well as those HTM professionals implementing an AEM program, the AAMI Alternate Equipment Management Working Group developed the EQ103 standard.
The two co-chairs of the committee, who spearheaded the effort, are Maggie Berkey, biomedical equipment specialist at Bio-Electronics, and Colleen Haugen-Ortiz, healthcare technology management quality specialist at GE HealthCare.
In developing the standard, the working group compared their framework to “what’s currently out there, including requirements from CMS and The Joint Commission, and other AAMI standards such as EQ56 and EQ89.”
The standard was approved by the American National Standards Institute (ANSI) in November of 2024; an important goal of the AAMI EQ103 working group.
Berkey says that there are many ways to implement an AEM program and the standard “makes it as easy as possible to get it right.”
Haugen-Ortiz says that uniformity is important in the new standard so that there is no conflicting information so that
comparing the standard to existing standards like EQ56 or EQ89 does not result in any conflicts. Also, reviewing what CMS or The Joint Commission has published on the topic of AEM programs, was reviewed to avoid any conflict.
ANSI/AAMI EQ56 is an HTM-specific standard to create a robust maintenance program.
Elements of the standard, which will be important to those obtaining a copy, include its guidance for dealing with regulators, consistency with other standards and ANSI approval.
What will be some of the most important standardized practices to come out of EQ103?
“The annual evaluation to ensure that safety has not been compromised,” Berkey says.
“A better definition of what new equipment is, such as new tech versus new to that hospital,” says Haugen-Ortiz.
She says that the standard will provide direction for what equipment is usable for AEM and how it’s used for AEM. Berkey says that the standard is useful when HTM is talking to an accreditation body in order to point to the standard as a reference for why an action was determined.
The co-chairs say that it is important to be able to defend your AEM program. Was a risk-assessment carried out and how is the program maintained? These elements allow an AEM program to

be more robust and, as a consequence, more defendable.
The co-chairs say they are developing a technical information report (TIR) to support the new EQ103 standard. Training on the new standard is also being developed. That effort would be aided by feedback from the regulators.
AEM programs must be continuously re-evaluated and tweaked as time goes on.
How does the standard comply with CMS requirements?
“The CMS S&C: 14-07-Hospital was our main source of information and we built this standard to provide clarity on everyday processes in HTM,” Haugen-Ortiz says.
How does the standard allow for variations/divergences from the OEM recommendations? What are some examples?
“We used CMS’ ‘S&C: 14-07-Hospital’ memo as our guide. We didn’t want to contradict the current guidelines but expand on them to help answer some of the burning questions that tend to bubble up. In the section titled ‘Elements of an AEM Program,’ we included several notes that delve into various scenarios,” Haugen-Ortiz says.
ANSI approves AAMI standards and the previous experience of many in the AAMI AEM working group, who worked on other standards, had input.
“While creating this standard, we followed ANSI guidelines to build a consensus document. The definitions are consistent with current standards as we didn’t want to include any conflicting

The process of creating a standard requires discussion, debate, research, consensus, feedback and documentation.
information. Many of the participants were also involved in creating or very familiar with EQ56 and EQ89 which helped ensure all the information was current,” Berkey says.
The process for developing a standard like EQ103 can take years. How were the committee and stakeholders able to accelerate this timeline? Many in the AAMI alternate equipment management (AEM) working group put in lots of time and energy and worked tirelessly to bring the standard to fruition. Haugen-Ortiz described members of the group as a “passionate group of people.”
“We had an awesome group of professionals who donated so much of their time, knowing that this standard was needed some time ago. We had multiple meetings per week and people willing to meet rigorous deadlines. They were the true drivers of this standard. Yes, there were hours when we would argue over one word or a sentence. It’s unavoidable but that is the point of having a consensus body, to make sure there is representation and that everything is worded correctly,” she says.
There were stakeholders who had a history in standards development who lent their AEM experience and their standards experience to the group’s work.
Berkey said the goal was to create a standard that “would make sense to everybody, anybody, everywhere, anywhere.” The insights of the experienced group members ensured this outcome.
CREATED FOR EXISTING OR NEW AEM PROGRAMS
How difficult will the standard be to implement for those who have set up parameters and criteria based around their own AEM programs, and will it potentially require major changes to documentation, inputs, procedures and timetables?
“If you already have a great AEM program, you’ll see where you stand and be confident when your surveyor comes. For those who are not sure, or are just getting started with AEM and want help or clarification, this standard is a great tool for them to make their program more robust, knowing all the t’s are crossed and i’s dotted,” Berkey says.
Does the new standard offer any flexibility?
The co-chairs say that the flexibility is with the authorities having jurisdiction such as state and local laws.
“We wrote this with a group of people from various backgrounds and job titles to ensure that there was representation to meet the needs of the entire HTM community,” Haugen-Ortiz says.
Adoption of the standard by CMS or The Joint Commission, and feedback from those bodies, would reinforce the confidence that AEM programs have in bringing their programs in line with the new standard.
Aligning the elements of an AEM program into commonly accepted standards that satisfy surveyors and others, while assuring the highest degree of patient safety and equipment performance is a big lift. ANSI/AAMI EQ103 endeavors to make that feat possible.









Choose the right capital equipment, supplies, and health IT for your facility with guidance from ECRIʼs team of biomedical and cybersecurity engineers, clinicians, and human factors experts.
Improve the quality and cost-effectiveness of care
Get test results and ratings, free of vendor bias
Stay on top of risks with hazard and recall notifications
Compare technology with overviews and specifications



Learn more at www.ecri.org/solutions/device-evaluations or contact us today at 610-825-6000, x5891.


BY VICKI SALEMI
Whether you’re negotiating your salary, meeting a new client or interviewing for a new job, reading the room is an important skill. How can you learn it?
Soft skills are valuable not only at work, but in life as well. And figuring out how to read the room is an important skill to build.
Andrew Brodsky, a management professor at the University of Texas at Austin and author of “PING: The Secrets of Successful Virtual Communication,” said, “Effectively reading the room and engaging in impression management can make the difference between getting a promotion or landing a major contract versus losing out on most every work opportunity. For example, if you are meeting with a potential client and you don’t notice they’ve lost interest in a topic you’re talking about, the odds of you getting that deal are slim. Similarly, if you don’t realize your manager isn’t looking for feedback on an idea they’re presenting, yet you decide it is a good idea to criticize that idea during a meeting, you are unlikely to receive a positive evaluation from that manager.”
Important meetings can have even more gravitas when they’re online and it’s not as easy to read the room. Plus, there are limited hand gestures and body language to read; on the phone, there’s a lack of facial expressions. You may be focused on how you appear and may not notice how participants react.
Brodsky recommended asking for clarification instead of making assumptions. “If you can’t tell whether your coworker feels positively or not about a new initiative you proposed, explicitly ask them. This step is one of the most effective for minimizing the misinterpretations that often plague virtual interactions,” said Brodsky.
Whether you’re building and honing this skill in person or online, both mediums involve two steps. First, question your approach. Brodsky said, “This could be as simple as realizing within a current email interaction that email might not be the ideal mode, and asking the other person if they’d mind hopping on a quick phone call to resolve the issue.”
For the second step, get familiar with research on nonverbal behaviors. There are so many new cues that are used by people for interpreting information – from emojis to typos to what time of day a message is sent – that if you are trying to simply go on gut instinct, you won’t achieve ideal outcomes.”
If you need further specific directions, Brodsky suggested mimicry by using a similar tone, communication modes, nonverbal behavior and jargon; but don’t take it too far to copy everything the other person does – that could backfire and feel forced.
“For example, if they use exclamation marks or emojis, then you should too. Not only will this help you to be more attentive to your communication partner, but it will also help build trust with the other person, as research shows we are drawn to people who communicate similarly to us,” said Brodsky.
If you’ve ever watched “Hot Ones,” host Sean Evans is a proficient master at reading the room and mirroring behavior, in my opinion. In turn, it appears that he builds trust with celebrities and helps put them at ease as they venture into discomfort while eating spicy hot wings.
Similarly, you may want to identify leaders in your organization who are also adept at this skill so you can learn by observing.

Vicki Salemi is a career expert for Monster, an author, a speaker and consultant, TV commentator and former corporate recruiter. Send your questions to hello@vickisalemi.com.

BY PHIL ENGLERT
On January 30, 2025, the Cybersecurity and Infrastructure Security Agency (CISA) released medical advisory ICSMA-25-030-01, highlighting critical vulnerabilities in the Contec CMS8000 patient monitors. These vulnerabilities – which include an out-ofbounds write, hidden backdoor functionality, and privacy leakage – pose significant risks to patient safety and data security. The U.S. Food and Drug Administration (FDA) issued a safety communication on the same day, emphasizing the risks associated with these vulnerabilities. The FDA highlighted that the Contec CMS8000 and relabeled versions, such as the Epsimed MN-120, may be remotely controlled by unauthorized users, potentially compromising patient data and device functionality. The CMS8000 came on the market around 2005 and obtained FDA 510(k) clearance in June 2011.
The FDA’s recommendations for healthcare providers and patients were twofold: Unplug and discontinue the use of device if you rely on remote monitoring features. Second, the FDA recommended using local monitoring features only, such as disabling wireless capabilities and unplugging ethernet cables. Physiological monitors do not provide lifesaving or life-sustaining treatment, but they are essential in monitoring the condition of at-risk patients. Patient monitors are monitored centrally to promptly notify caregivers of patient condition changes. Rapid response can be the difference between good and bad outcomes.
The Contec CMS8000 patient monitor is widely used in healthcare settings to track vital signs such as heart rate, oxygen saturation, and blood pressure. I have two concerns.
The first is patient safety. These monitors are designed to operate in a high acuity setting such as an ICU or CCU and to serve adult, pediatric, and neonatal patients. Most deployments would be connected to a patient monitoring network and central monitoring. Disconnecting these monitors from the network would reduce the effectiveness of patient monitoring in those settings where the patients are least resilient.
The vulnerabilities identified by CISA are particularly concerning because of the potential impact on patient care and data integrity.
• Out-of-Bounds Write (CVE-2024-12248): This vulnerability allows an attacker to send specially formatted UDP requests to write arbitrary data, potentially leading to remote code execution.
• Hidden Backdoor Functionality (CVE-2025-0626): The firmware contains a hard-coded IP address that bypasses existing network settings, enabling remote control and file manipulation.
• Privacy Leakage (CVE-2025-0683): Patient information and sensor data can be leaked to an unknown external network.
Claroty’s Team82 conducted an in-depth analysis of the Contec CMS8000 firmware and concluded that the issue is more likely an insecure design flaw rather than a hidden backdoor. The hard-coded IP address in the device’s firmware is listed in the operator manual, suggesting it is intended for internal network configuration rather than malicious purposes. Despite this, the insecure design poses significant risks, as it can be exploited to collect patient data or perform insecure firmware updates. Claroty’s research underscores the importance of addressing these vulnerabilities promptly to mitigate potential security threats.
Cylera’s threat intelligence research team also investigated the vulnerabilities and confirmed the presence of the hard-coded IP address linked to a Chinese university. Their analysis also supports the notion that the behavior
observed in the Contec CMS8000 is not an intentional backdoor but a result of poor design choices. Cylera’s research highlights the potential consequences of these vulnerabilities, including the risk of compromised patient monitors being used as entry points for broader network attacks. The team recommends immediate remediation to prevent unauthorized access and data leakage.
The Claroty and Cylera research teams also noted that the IP address 202.114.4[.]119 is registered to the China Education
or malicious IP addresses is another effective protection. The FDA is unaware of any cybersecurity incidents, injuries, or deaths related to these cybersecurity vulnerabilities. The disclosure of these vulnerabilities has significant implications for healthcare providers. Ensuring the security of medical devices is crucial to maintaining patient safety and protecting sensitive health information. Healthcare facilities should consider proactive measures to address these vulnerabilities, including conducting thorough security assessments of









BY NATHAN PROCTOR
For the last decade, Repair.org — working closely with my organization, PIRG, and others — has championed reforms to remove barriers from independent repair technicians and product owners that prevent people from fixing modern electronic products.
Model legislation, which requires manufacturers to make the parts, tools and information they use to facilitate repairs available, has been filed hundreds of times around the country, and has broken through over the last four years. Here’s my roundup of the latest, especially on our efforts to extend Right to Repair to medical devices.
As of this year, all 50 U.S. state legislatures now have considered Right to Repair bills over the last eight years. Clearly, the idea is resonating with Americans from coast to coast.
“This is more than a legislative landmark — it’s a tipping point. We’ve gone from a handful of passionate advocates to a nationwide call for repair autonomy,” said Kyle Wiens, CEO of iFixit as we marked the milestone. “People are fed up with disposable products and locked-down devices. Repair is the future, and this moment proves it.”
If manufacturers were hoping Right to Repair was going to fade away, they sorely miscalculated. Currently at least 26 states have introduced or carried over legislation so far in 2025 (so far, there
will be additional states that refile legislation over the course of the year).
In addition to progress on Right to Repair for other industries, our coalition continues to work to find a way to advance Right to Repair for medical devices — despite fierce opposition from the medical device manufacturers.
Vermont’s H. 160, put forward by Reps. Anne Donahue and Monique Priestley, would require manufacturers of medical devices to provide access to any repair documentation, parts or tools to health care facilities or independent service organizations. The impetus of the legislation is the increasing costs of running health care facilities in Vermont, and concerns about the loss of rural health care facilities.
Over the last 5 years, PIRG has organized more than 600 biomedical professionals to back Right to Repair. This spring, we are launching a new effort to sign on supporting organizations, including hospitals, state biomedical associations and other entities. You can see our statement of principles, and sign as an individual or bring this letter to organizations you are a part of.
Scan the qr code to find our letter:

Nathan Proctor is senior director of the U.S. PIRG Campaign for the Right to Repair.
BY SAUL RAMIREZ AND GARRETT SEELEY
After the article on OEM software compliance, a question came up. How do biomeds maintain existing software?
The answer requires piecing together several of the previous Networking Notes articles and expanding on a new position in the healthcare technology management field; an IT/Biomed hybrid. We covered the tasks of procurement in the articles on NMDD and ERA and set up in articles for TCP/IP. All of this is important to the new position of Biomedical Equipment Systems Specialist in Information Systems; the Information Systems BESS, ISBESS, or simply “Info Sys.”
In the past decade, this field has grown to include more technicians with specific computer networking and server skills. They often save the day only to disappear to some limited access server room. The question is, what do ISBESS techs do?
One of the most confusing issues is that Info Sys technicians focus on medical computers. Unfortunately, this definition can be facility specific. In general, if a computer or server deals with data other than text, if it operates with a medical device, or interfaces the patient; it is a medical computer. Basically, anything not used to access the patient record can be considered a medical computer and therefore not under IT management. Info Sys techs work on these systems. Additionally, Info Sys will work on medical device networking. They may be asked to contribute their expertise to anything that other biomeds need help on, particularly with networking these devices.
Info Sys technicians set up and maintain medical computer systems. They do this the same way an IT technician maintains networked computer systems. Info Sys are concerned with hardware and software, permissions and logins, and overall computer functionality. There are network concerns and security issues like the ones covered in the ACL and ICE articles. There
are setup issues, such as in the TCP/IP or the Network Troubleshooting articles. Please read these articles for foundational material on these tasks. IT switches seem to be the only thing IT network admins will not give an Info Sys tech direct access to. However, IT has no idea about what to set in the ACL for a medical device communication. An Info Sys must work closely with IT to guide and help compile the ACL lists. This requires documentation and communication to the network admins, so please do read the articles on ACL, ICE, and NMDD for these tasks.
INFO SYS PROFESSIONALS SPEND 1/3 OF THEIR TIME
On top maintaining medical computer systems and networked medical devices, there is also the maintenance of the software itself. Software requires patches to fix and maintain its functionality. These patches are provided by the software manufacturer, listed on their websites, go through the 510K vetting process, and are safe for the computer they are being loaded on. These usually occur every year or two and maintain the application software. Additionally, there are operating system updates that need to be installed to a medical device. These are made by the company that programmed the main operating system (such as Microsoft) and released generally every month. Then, finally, there are system upgrades. This is where the application and operating system will be updated to newer versions. A good example of this is the upgrade from Windows 10 to Windows 11. The entire software of the computer is replaced with a newer version offering more features, better usage of hardware, and more functionality. This usually is accompanied with an application upgrade from the medical device manufacturer. Upgrades happen around every 3 years. The client end computers (used by the medical staff) can usually be updated automatically, but most still need to be verified after the upgrade. The server updates must be manually deployed and inspected to prevent service interruptions. Preferably, upgrades are tested on a system that is not being used or in a simulated VM environment called a Sandbox. We will have to explain Virtualization in a separate article due to the complexity and flexibility of this concept. More on this subject will follow this
article. Let’s just say that there is something to update every month, and this is all the responsibility of the ISBESS. All this work is performed while maintaining other corrective repairs and installs. Just maintaining and updating the software can take up to a third of an Info Sys job tasks.
We will have to change. Telehealth is an example of a grey area. It involves home health, IT, and biomed. Smart phones and Software as Medical Devices (SaMD) aren’t even a consideration to the average Info Sys right now. HTM will probably not touch a system unless it is directly dumping data to one of our machines. If it is Internet-based or cloud-based, or it its EMR related, then that is maintained by IT. We may only work on a back-end server and not the actual device or the software that is the source of the information. There really is no way of knowing what is around the bend, but we know it will involve IT and biomed working together in the future. This is before we involve new technologies from companies like Meta and Google, Cloud and AI applications. Stuff like that is best for another article.
When describing the Info Sys position, I found myself saying “another article” often. This is proof that Info Sys is an exciting and somewhat confusing hybrid position. It is something that
most HTM professionals do not understand. As a testament to the Information Systems BESS, this article references no less than 6 other articles. That is a record in cross referencing for the Networking Notes team. That really means that it took the entire team at the Dallas VA team more than half a year of monthly articles to describe what the Info Sys does. That’s how multifaceted the skill set is to do this job. Therefore, if you have that ISBESS network wizard or Info Sys guru at your work, please thank them for all that is done behind the scenes to do their job. Honestly, they deserve it.


Garrett Seeley, MS, CBET, is a biomedical equipment support specialistimaging with VISN 17: VA North Texas Health Care System, Dallas Veterans Affairs Medical Center.
Saul Ramirez is a Lead BESS Biomedical Information Systems at VA NTXHCS.


BY STEVEN J. YELTON, P.E., AAMIF
I’ve recently been approached to help with the possible development of a college HTM program. I feel this is encouraging to our field in that it shows that college administrations are seeing this as a viable program for their college. This is also encouraging since we are constantly trying to figure out ways to get more students and graduates into the HTM field. I feel that it is timely to revisit this topic as we move into the time of year when we are looking at new programs to propose at the college level.
In our area, when a new program is considering opening, it is normally a minimum of around 50 miles away from existing programs. This way there is little competition between institutions. We have generally found that community college students tend to live at home and
commute to school. This means that it is very important for them to have an available program relatively close to home. We are always open to helping our colleagues at other colleges.
Another aspect of having multiple colleges with HTM programs is the availability of students for co-operative education (co-op) placements or internships. As I mentioned before, the proximity of the placement to a student’s home is very important to them.
Cincinnati State Technical and Community College has always been a co-op school where our students are required to complete cooperative education assignments in order to graduate. As such, we have co-op coordinators for each program. They recruit and place students, search for positions, and teach classes on interviewing skills, resume writing, job responsibilities and communications. In conjunction with our other faculty, industry advisors and
employers, our co-op coordinators provide advice to students to make sure early on that they are in the field that best fits them. One very frustrating situation for our co-op coordinators is when they don’t have students available to place in co-op or full-time positions. Their main goal is to accommodate students and employers. We generally have more openings than available students, so additional programs in our area could help that situation.
When I help colleges start new HTM programs I always refer them to AAMI and the resources that they have available. I see the resources at AAMI as closing the loop for the educational process.
Now that the ANSI/AAMI EQ110:2024 standard for healthcare technology management (HTM) educational programs is published, I recommend it as a good starting point for programs. This standard will give a baseline for what a viable HTM program should be. As I always mention, it is also a great resource for HTM technicians and employers to compare to needed skills for entering the field.
This standard in conjunction with the college’s accreditation agency requirements should guide them in the development of their program. At Cincinnati State, we are accredited by the North Central Association of Schools and Colleges. We achieved this accreditation in 1976 and it remains the benchmark for all of our programs. We then use the AAMI standard and guidelines from The Technology Accreditation Commission of the Accreditation Board for Engineering and Technology (TACABET) to further “tune” our engineering technology programs.
In addition to these resources, we rely heavily on industry advisory committees and employers for guidance on the program curriculum. In this article, I’m suggesting ways to foster cooperation between learning institutions which would help
fill HTM roles without infringing on each other’s immediate areas. I always like to give a shout out to the apprenticeship program within AAMI since I feel that this is very innovative and provides opportunities to a potentially underserved group. I feel that this is a great feeder program to our programs. The hope is that these students would choose to complete their associate or possibly a bachelor’s degree. This program has been very successful and has helped fill the need for HTM technicians. The following statement is from the AAMI Array website: “Because of the shortage of qualified healthcare technology management (HTM) professionals in the hiring pool, some hospitals and other employers are training their own biomedical equipment technicians (BMETs) from the ground up with no formalized way to ensure these BMETs are trained consistently or to a minimum standard. This apprenticeship program will bring structure to that process and contribute to a solution for bridging the current skill gap in the field for entry-level employees. The intent is that the availability of a formalized BMET apprenticeship that offers formalized training in a real work environment will attract new professionals to the field.”
The final piece to closing the loop in HTM training is certification. I feel strongly about graduates attaining a certification from the AAMI Certification Institute (ACI). I feel that this is a great way to demonstrate a graduate’s education and experience and could be used as a factor for promotion within the field.

Steven J. Yelton, P.E., AAMIF;







































and time saving solution for ordering imaging replacement parts, equipment moves, ultrasound probe repair and on-site service.

The words can appear horizontally, vertically, diagonally, and may be spelled forwards or backwards.
Dina Nordin, BMET 2
AdventHealth Wesley Chapel
• Water
• Coffee
• A Tool Bag
• My Work Phone
• PM Stickers
Submit your bench to be featured in TechNation at 1technation.com/my-bench/. You could win a $25 Amazon gift card via the “What’s On Your Bench” Contest!


AM Bickford ambickford.com • 800-795-3062
BC Biomedical bcgroupstore.com.com • 314-638-3800
Interlight interlightus.com • 800-743-0005
J2S Medical jsmedical.com •844-DIAL-JS2
Jet Medical jetmedical.com • 714-937-0809
Metropolis International metropolismedical.com • 718-371-6026
Ozark Biomedical ozarkbiomedical.com • 800-457-7576
PM Biomedical pmbiomedical.com • 800-777-6474
Pronk Technologies, Inc. pronktech.com • 800-609-9802
Renovo Solutions renovo1.com • 844-4RENOVO
Secure Mount securemount.com • 517-784-9613
Tri-Imaging Solutions triimaging.com • 855-401-4888

















































Anesthesia
A.M. Bickford
Cardiac Monitoring
Cardiology
Southeastern Biomedical, Inc sebiomedical.com/ • 828-396-6010
Interlight
BC
Defibrillator

























The Professional of the Month feature continues to be a popular article that spotlights the men and women of HTM.

Elite Biomedical representatives are all smiles as they prepare to interact with HTM professionals at MD Expo in Seattle.

The Vault feature included photos of old medical devices and asked readers to guess what they were to win a prize.

USOC is a fixture at HTM trade shows sharing their knowledge and services with attendees. Look for them at the next MD Expo.

