Bradleya 30/2012 pages 111 – 126
In situ analysis of the current conservation status of Mammillaria herrerae Werderm. in the
southern Chihuahuan Desert
Beatriz Maruri Aguilar1, Emiliano Sánchez Martínez1 and Jordan Golubov Figueroa2
1 Jardín Botánico Regional de Cadereyta “Ing. Manuel González de Cosío”, CONCYTEQ. Pasteur 36, Centro Histórico, Santiago de Querétaro, Qro. C.P. 76000, México (email: bmaruri@concyteq.edu.mx, esanchez@conyteq.edu.mx).
2 Laboratorio de Ecología, Sistemática y Fisiología Vegetal, Universidad Autónoma Metropolitana Xochimilco, Departamento El Hombre y Su Ambiente. Calz. del Hueso 1100, Col. Villa Quietud, México, D. F. (email: gfjordan@correo.xoc.uam.mx).
Summary: The semi-arid zone of Querétaro and Hidalgo, in Central Mexico, is an important region for cactus conservation. Mammillaria herrerae Werderm. is a species of local distribution with a threatened conservation status, partly because it has been historically plundered. An exhaustive census of all individuals was made to assess the current status of the species. As a result of the study, a new site is included, which increases the distributional range, and we found that M. herrerae faces adverse factors for its survival, namely: a very small number of wild individuals fragmented into small scattered groups; an extremely reduced area of distribution, and a very specific set of habitat requirements. A clear association of the species with its vegetative and rocky nurses was observed. These characteristics confirm the vulnerability of M. herrerae and suggest its dependence on a specific environment and ultimately the need for protection in its areas of distribution.
Zusammenfassung: Die semiariden Zonen von Querétaro und Hidalgo in Zentralmexiko sind wichtige Gebiete für den Schutz der Kakteen. Mammillaria herrerae Werderm. ist eine gefährdete Art mit lokaler Verbreitung, teilweise wegen historischer Plünderung der Vorkommen. Der heutige Status der Art wurde mit einer umfassenden Zählung bestimmt. Als Resultat der Untersuchungen kann ein neuer Fundort in die Arbeit einbezogen werden, der die bekannte Verbreitung erweitert. Die Daten zeigen, dass das Überleben von M. herrerae von mehreren ungünstigen Faktoren bestimmt ist, nämlich: Eine sehr kleine Zahl von Individuen in fragmentierten, kleinen und zerstreuten Gruppen, ein extrem kleines Verbreitungsgebiet, und sehr spezifische Ansprüche an
das Habitat. Es wurde eine klare Assoziierung der Art mit Ammenpflanzen und Felsblöcken gefunden. Die Beobachtungen bestätigen die Verletzlichkeit von M. herrerae und die Abhängigkeit von spezifischen Umweltbedingungen, und schliesslich die Notwendigkeit von Schutzmassnahmen in ihren Verbreitungsgebieten.
Resumen: La zona semiárida de Querétaro e Hidalgo, en el Centro de México, es relevante para la conservación de las Cactaceae. Mammillaria herrerae Werderm. es una especie endémica y su estatus de conservación se encuentra amenazado, lo que se debe, en parte, al saqueo del que históricamente ha sido objeto. Para conocer su estado actual, se llevó a cabo un censo de los individuos que viven en el medio silvestre. Como resultado del estudio se amplía la zona de distribución conocida, con la inclusión de un nuevo sitio de distribución. Se encontró también que M. herrerae enfrenta circunstancias adversas para su sobrevivencia: existe un número muy reducido de individuos que viven en poblaciones pequeñas y dispersas, en una superficie geográfica extremadamente reducida, y que tienen un conjunto específico de requerimientos de hábitat; se observó también una clara asociación de la especie con nodrizas vegetales y rocosas. Estas características confirman la vulnerabilidad de M. herrerae y apuntan a la necesidad urgente de protegerla en sus áreas de distribución.
Introduction
The Cactaceae are one of the most threatened families of angiosperms (Baillie et al., 2004). Natural populations of many species have been affected by human development, specifically land conversion, cattle rearing and urban growth. The
illegal collection of plants for sale in national and international markets has also worked against the survival of many species (Oldfield, 1984; Robbins, 2003). The whole family is included in CITES Appendix II and many species are in the IUCN list (Hernández & Godínez, 1994). The Cactaceae have been shown to have slow growth rates, long life cycles (Gibson & Nobel, 1986), and low recruitment of new individuals (Hernández & Godínez, 1994). In addition, many species have a restricted distribution as well as highly specific habitat conditions (Zavala-Hurtado & Valverde, 2003). These biological and ecological features are factors that increase their vulnerability, even in the absence of an external disturbing agent.
Even though international reports on threatened species of Cactaceae from the southern Chihuahuan Desert have been already made (Anderson et. al., 1994), and several patterns of distribution for endangered cacti have been studied, there is still a lack of systematic knowledge for many species, so we are in need of current and quantitative information in order to improve the understanding of the family (Gómez-Hinostrosa & Hernández, 2000) and to provide sound proposals for conservation and management. Data such as the geographical distribution, area of occupancy,
population size and structure, habitat characteristics and reproductive behaviour are still unknown for many members of the family (Álvarez et al., 2004), and are integral components of systems that evaluate risk, like IUCN and the Mexican Official Standard NOM-059-SEMARNAT-2010.
Mammillaria is a genus which is almost endemic to Mexico (Craig, 1945; Pilbeam, 1999), represented by 163 species, (Hunt 1987; Pilbeam, 1999). Three main areas in Mexico are important for this genus: the Baja California peninsula, the northwestern section of mainland Sonora, Chihuahua and Sinaloa; and the arid sections of Mexico’s Central Plateau, in the states of Hidalgo, Querétaro, Guanajuato and San Luis Potosí (Craig, 1945). Mammillaria is one of the largest and most popular genera of cacti and is also one of the most studied groups (Anderson, 2001), although the emphasis centres mainly on taxonomic and phylogenetic approaches. Basic demographic and genetic studies are still largely absent (Hernández & Bárcenas, 1995).
Mammillaria herrerae Werderm. was reported in the Mexican state of Queretaro, near Vista Hermosa, in the municipality of Cadereyta de Montes (Pilbeam, 1999), central Mexico, on the southern portion of the Chihuahuan Desert. The historical

distribution of M. herrerae was restricted to the Ejido Vista Hermosa, specifically surrounding community of “El Arbolito” (20º 40’ 28”N, 99º 32’ 23” W) between an altitude of 1,300 and 1,920 masl. This site is located in a recently modified area, due to the construction, in the early 1990’s, of the “Fernando Hiriart Balderrama, Zimapán” dam. According to Sánchez-Velez (1987), the environmental impacts originated by the construction of a dam were habitat loss and rapid modifications of the environment, factors that were difficult to cope with for many species. Even though the construction of the “Zimapán” Dam did not fall in the distribution of M. herrerae, it did have an unexpected direct impact on the species, as local inhabitants plundered individuals and sold them to the workers of the construction (Ortega, 2004) and at the local market in Cadereyta de Montes, during Christmas. Given the risk, M. herrerae was among the priority species for rescue efforts at this site. The effort included the ex situ conservation of four individuals taken from the dam’s vessel (Ortega, 2004), and taken to the Botanical Garden at the National Autonomous University of Mexico (UNAM). Ortega (2004) also considers M. herrerae as a highly threatened species, mainly because of illegal extraction of individuals, classifies it as a “micro endemic”, according to Rabinowitz (1981) and as “under risk of extinction” according to the Mexican Method for Risk Assessment (NOM-059ECOL-2001). M. herrerae is also listed as critically endangered (CR) in the IUCN List of Threatened Species, of the Species Survival Commission of the International Union for Conservation of Nature (Fitz-Maurice & Fitz-Maurice, 2002), due to its very small population size.
In 2009, a new locality was found, in the southern limestone slopes of “El Doctor” mountain due northwest of the already known locations (Sánchez et al., 2009). This new site of M. herrerae is also under significant pressure because of the construction of the project known as “Acueducto II”, a 108 km dam and water system, designed to carry water from the border of Querétaro and Hidalgo, to the city of Queretaro (Comisión Estatal del Agua de Querétaro, 2008). The water system runs through the southern slopes of the mountain, in the vicinities of the plants.
In the absence of demographic, genetic and reproductive data on any given species, the term “threatened” often refers to a species or taxa whose survival is potentially or actually in danger, due to the following circumstances: anthropogenic or natural agents; very restricted distribution area and a very low density of individuals in their populations (Hernández &
Godínez, 1994). In the semi-arid region of Querétaro some of the circumstances that contribute to threat are habitat destruction and modification (Hernández-Oria et al. 2007). M. herrerae seems to have several conditions that have driven it to the brink of extinction: they have been removed from the wild, its habitat has been decimated and only two sites are known (Ortega, 2004; Sánchez et al., 2009; Ramírez, 2010). Even though M. herrerae can only be found on rocky steep slopes that are relatively inaccessible, illegal collection (Ortega, 2004) has depleted populations and threatened the survival of the species.
Even though the perils of M. herrerae have been known for some time, we are still lacking basic biological information such as a systematic census and exploration of existing populations (Sánchez et al., 2009). The purpose of this study was therefore to quantify population attributes from all the known sites (the plundered ones and the newly reported location). We evaluated population size and structure, density and spatial distribution, nurse association and community structure in a subdivision of the “Mesa de León” and “Altamira” (Hernández-Oria et al., 2007) subgrids of the “Cuadrante Tolimán” study area (Hernández & Bárcenas, 1995). These sites have already been identified as priority sites for conservation of flora (Hernández-Oria et al., 2007).
Materials and methods
We focused effort on the M. herrerae populations still remaining in the eastern portion of the Cadereyta de Montes municipality, in the State of Querétaro, Central Mexico.
The species
M. herrerae has a solitary globose 35 mm wide body with a flattened and a small sunken apex, which can be occasionally caespitose in small groups. Tubercles are arranged in 8 and 13 spirals, closely set, cylindrical, terete, with a nearly truncate tip, 5–6 mm long, 2 mm wide at the base. Areoles are round to oval, 1.5 mm, with scant short white wool below the spines when young, and later becoming naked. Axils are naked with no central spines and have several series of superimposed radial spines c. 100, 1–5 mm long, setaceous, straight, base not enlarged, pure white to whitish ashen-grey, horizontal to a little appressed, interlacing so as to completely cover the body. Flowers are funnelform, lateral 20–25 mm long. The outer perianth segments have an olivegreen mid-stripe, with rose margins while the inner perianth segments are pale rose to violet (Figure 1). It is locally known as “Bolita de hilo” (Little ball of thread) and “Bola de golf” (Golf ball).

Figure 2. (a) General geographical location of the studied sites. (b) Arrangement of the four sites in the historical locality.
Localities
Due to its extremely scarce distribution, an exhaustive search was made in the field, in the surroundings of the original site in the small town of “El Arbolito”, to locate and identify every single individual. Even when all groups of plants are in the vicinities of “El Arbolito”, they are spatially distinct so we considered them four different sites (A1, A2, A3 and A4; Figure 2). A similar search was made in the surroundings of the Acueducto II dam construction road, where we found that individuals of M. herrerae were all closely distributed and there was no further need to name separate sites (hereafter site B). Sites A1 through A4 were located at 20º 41’ N, 99º 31’ W, 1880 masl. Site B was located at 20º76’ N, 99º59’ W, 2030 masl.
Population size and structure
No plots were established as all individuals were included in the census and the information was used as a criterion to designate priorities for conservation areas (Margules & Sarkar, 2009). From February to June 2010, a series of exhaustive searches were made to locate every individual of M. herrerae growing in the field. Each individual was located, tagged and measured to the nearest 0.1 mm (mean diameter from three different stem measurements) with a caliper. For individuals growing in clusters, each stem was measured individually and the total diameter of the cluster
was obtained by summing individual stems. Each group of stems was considered a single individual as we cannot know for certain if they are joined under the soil. Diameter size categories were then determined in centimeters (0–1.30, 1.31–3.30, 3.31–5.30, 5.31–7.30, 7.31–9.30, 9.31–11.30, 11.31–13.30, 13.31–15.30, 15.31–17.30, 17.31–19.30, 19.31 and above). Pairwise comparisons between distribution of coverage among sites were performed with the Kolmogorov-Smirnov statistic (D’Agostino & Stephens, 1986).
Density and spatial distribution
Every individual of M. herrerae was geographically referenced and mapped using x-y Cartesian coordinates in an ArcGIS 9.3 environment. A spatial interpretation of the distribution of individuals was made. We estimated density of individuals taking as a basis the sub-grids of the “Cuadrante Tolimán” (Hernández & Bárcenas, 1995; Figure 3a). Within the “Cuadrante Tolimán”, M. herrerae was found in two sub-grid squares, named “Mesa de León”, and “Altamira” (Hernández-Oria et al., 2007; Figure 3b). Since the “Cuadrante Tolimán” is too large (30’ per side), we considered smaller 6’ per side subquadrats which, in turn, were also divided into equal portions, giving 25 1.2’ sized quadrats of 4.61 km2 (Figure 3). We generated a series of hypothetical quadrants drawn arbitrarily around
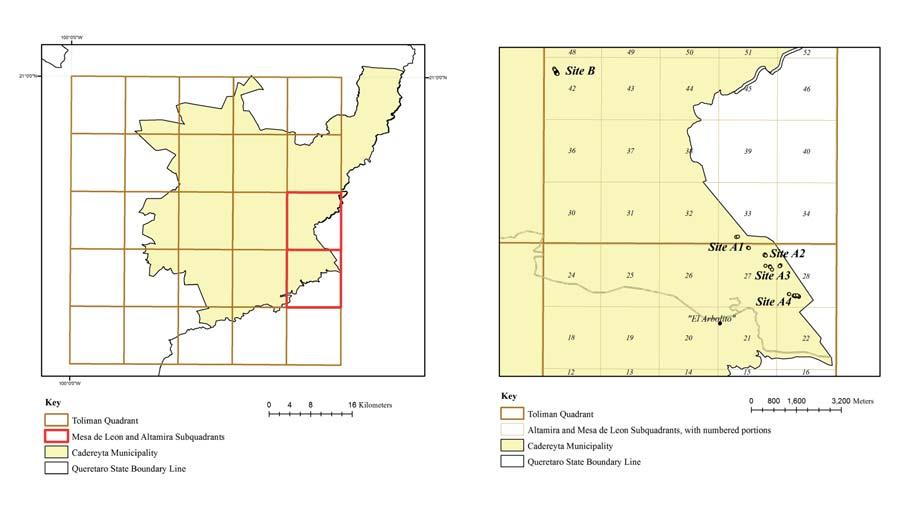
Figure 3. (a) The municipality of Cadereyta de Montes, yellow polygon, in the state of Queretaro showing the “Tolimán” graticule. The “Altamira” and “Mesa de León” sub-quadrants are shown in red. (b) Distribution of sites inside the 1.2’ quadrat portions of the “Altamira” and “Mesa de León” sub-quadrants
each site, and with different areas to estimate density (Site A1, 2,596 m2; A2, 665 m2; A3, 33,656 m2; A4, 11,148 m2; B, 7,956 m2).
Nurse association
To determine if M. herrerae requires a nurse for its establishment, we observed and registered for each individual if the plants were growing under a plant or partially buried under a rock. Nurse species were identified in order to determine preferences of association. Association was analyzed using a Chi-square test (Fowler et al., 1998).
Structure of the community
The composition of the community in the areas of distribution of M. herrerae was described. Each individual shrub and tree inside a 50 × 2 m transect at M. herrerae sites was measured (two orthogonal diameters and height) and taxonomically identified following the same areas of distribution where M. herrerae was found. We compared plant diversity between sites using Rényi diverity profiles (Tómthérész, 1995) and values of importance (Mostacedo & Fredericksen, 2000). When the diversity values of one community throughout the profile is greater than another, we can then state that the former is more diverse than the latter (i.e. they are separable).
When the curves intersect at some point in the profile we cannot say that one community is more diverse than the other (i.e. they are inseparable). In addition, specific values of the scale parameter α of the Rényi profile at 0, 1, 2 and ∞ are related to species richness S, the Shannon diversity index H, the Simpson diversity index D-1 and the Berger–Parker diversity index d-1 respectively (Legendre and Legendre, 1998; Kindt et al., 2006). We compared across sites using a Jaccard’s binary coefficient (Henderson, 2008).
Results
Population size and structure
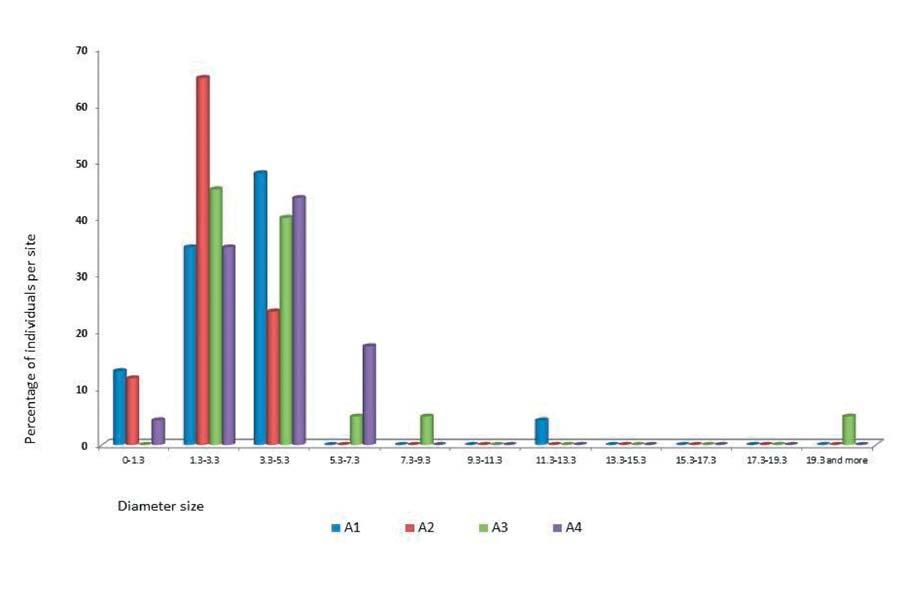
At “El Arbolito” (Sites A1 through A4), small populations were found distributed in an area of a few square meters. In contrast, site B had the highest number of individuals growing close to each other in a single location (Table 1). For all sites, we found a high proportion of individuals (93.02%) with diameters below 5.30 cm. Sites A1, A2 and A3 had the highest proportion of plants below the 5.30 cm threshold (A1, 95.65%, A2, 100%, A3, 85%, A4, 82.61%) (Figure 4a). Site B as in the sites close to “El Arbolito”, had a high proportion of individuals below 5.3 cm diameter (93.78%), with lower values for the percentage of individuals of larger diameters (Figure 4b).
Table 1. Number, mean (standard deviation) diameter and cover of M. herrerae individuals at the five study sites.
Table 2. Significance for the pairwise comparisons between sites (i, j). Values in bold are statistically significant (α = 0.1).
Table 3. Density (individuals/m2) of M. herrerae individuals in portions of “Cuadrante Tolimán”.
Sub-quadrant Portion n δ × 10-6
We compared the distribution of plant cover between sites (Table 2) having as a null hypothesis no differences in cover between sites. We used an α=0.1 to compensate the fact that the Kolmogorov-Smirnov test has a slightly smaller statistical power than other tests. Multiple comparisons show that the cover distribution did not differ between sites (A1, A3), (A1, A4), (A3, A4) and (A2, B). A Cumulative Distribution Function (CDF) for measurements of coverage in square centimeters in sites A1 to B was consistent with the Kolmogorov-Smirnov test (Figure 5). Two groups are clearly identified; those of cluster (A1, A3, A4) and those of cluster (A2, B). The means per site (Table 1) also show a grouping for sites.
Density and spatial distribution M. herrerae seems to grow under very specific conditions: all sites were found between 1,820 and 2,060 m above sea level, on steep slopes in portions of submontane shrub; over a limestone, rocky, scarce Lithosol (FAO-UNESCO classification in INEGI, 1986). The northern plants were found growing on intrazonal Rendzinas. In
addition, all sites had a N–NE orientation, even site B, which was located on the southern slopes of “El Doctor” limestone, and had a N–NE micro orientation. Sites A1 through A4 are located in 1.2’ per side “portions” 27 and 28 of the “Mesa de León” sub-quadrant; and 33 of the “Altamira” sub-quadrant; site B is entirely situated in “portion” 42 of the “Altamira” sub-quadrant. The other parameter was a series of squares arbitrarily outlined for each site, forming a regular figure, square or rectangle, in the surroundings of the area where the plants were found (Figure 6). When individuals of a site were dispersed, more than one hypothetical quadrant was drawn and surfaces were added in order to have a single estimated density value for each one. The highest density per portion occurs at portion 42 of “Altamira” sub-quadrant, where site B is entirely located. The following values are found at portions 28 and 27 of “Mesa de León” sub-quadrant, where sites A2, A3, A4 and a part of A1 exist. The other part of site A1 is located at portion 33 of “Altamira” sub-quadrant, which has the lowest density value (Table 3). The other sub-quadrants, arbitrarily traced surrounding the places with individuals of M. herrerae, have different surfaces which made difficult the comparison among them. However, some facts can be drawn. The highest density occurs at site B. Site A3 has the largest surface, occupied by one if the smallest populations, which results in the lowest density. Sites A4 and A1 are also vast surfaces, although lower than A3, and have also small densities. Site A2, having the smaller number of plants, had a remarkably higher density than sites A1, A3 and A4, because the plants were found in a small area (Table 4).
Nurse association
Taking the total number of plants at the five sites, 68.54% were growing under some nurse species or

individuals
Table 4. Estimated density (individuals/m2) of M. herrerae in quadrants drawn arbitrarily around each site.
Table 5. Total number of individuals and number of individuals under nurse (percentage of total) at the five study sites.
nurses), stones (23.40%), Dasylirion quadrangulatum (12.77%), Agave xylonacantha (6.38%), Painteria revoluta (6.38%), Hechtia glomerata (4.26%) and Pterostemon mexicanus (4.26%). At site B, a higher proportion of plants were found growing under a nurse, and less were partially buried or in the vicinity of stones (40.49%). The most common nurses, sometimes combined with rocks, were Flourensia resinosa (19.51%), Hechtia glomerata (10.24%), Selaginella sp. (9.26%), Dasylirion quadrangulatum (4.39%), Pinus pinceana (3.90%), Agave xylonacantha (1.46%) and Gochnatia hypoleuca (1.46%).
partially buried under or close to a rock. Sites close to “El Arbolito” (A1–A4) were highly variable in nurse association (Table 5). At these sites, 44 individuals were found growing under a nurse, mainly Perymenium berlandieri (29.79% of all
There were significantly more individuals of M. herrerae growing under a nurse than those growing in open space (χ2 = 47.20, P<0.01, df =1). At sites A1–A4, results varied between sites (A1: χ2 = 1.09, P = 0.2964; A2: χ2 = 7.12, P = 0.007; A3: χ2=1.80, P=0.170; A4: χ2 = 1.09, P = 0.584) in which only the site A2 showed a significant difference between plants without a nurse, which was more than plants covered by a nurse. When applied to the whole population at site A the test indicates no significant difference between individuals growing under a nurse and those growing in open spaces (A1–A4: χ2 = 0.30, P = 0.2269, df = 1). At site B, the results showed that the number of individuals growing under a nurse was significantly different than the number of individuals growing in open spaces (B: χ2 = 56.81, P<0.01, df =1).
No preference for a particular species of nurse was identified. Twenty one species were found as nurses, and their importance in the area (Table 8) seems to have no connection with the occupancy rate that M. herrerae makes of them. For instance, Perymenium berlandieri, a common species in “A” sites, only has a relevant role as a nurse at site A1. On the other hand, at site B Flourensia resinosa is the most important species, and is also an important nurse plant. At “A” sites, M. herrerae seems to be taking few species as nurse, while at site B, individuals are established under a wider variety of species. On the other hand, rocks seem to have a constant role as nurses in all sites except A2, where the nursing has its lowest rate (Table 6).

Figure 5. Cumulative distribution function (CDF) for cover of M. herrerae at the five study sites.
Structure of the plant community
In the semi-desert area of Queretaro and Hidalgo, southern Chihuahuan Desert, different types of xerophytic scrubs (microphyll, rosetophyll, crassicaule, and the lesser dry submontane scrub) can be

Figure 6. Spatial distribution and size of M. herrerae individuals at the five study sites. Note the different scales for each site.
found (Zamudio et al., 1992). M. herrerae is present in submontane shrub, specifically, transects made at sites A1, A2, A3 and A4, consisted of 43 taxa, belonging to 20 families. There is a predominance of species of the Cactaceae (11 species), followed by Fabaceae (5 species), Euphorbiaceae (4 species), Agavaceae, Anacardiaceae, Asteraceae, Burseraceae, Rhamnaceae, Rubiaceae, with two species each; and Bromeliaceae, Celastraceae, Ephedraceae, Fouquieriaceae, Heliotropiaceae, Krameriaceae, Nolinaceae, Pterostemonaceae, Rutaceae, Scrophulariaceae and Turneraceae with one species each. At site B, we found 23 taxa in 14 families: Fabaceae (4 species) Cactaceae and Asteraceae, with three species; Agavaceae, Nolinaceae and Rhamnaceae, with two species each and Bromeliaceae,
Burseraceae, Orobanchaceae, Pinaceae, Pterostemonaceae, Rubiaceae and Turneraceae with one species each. Ten species were common in all transects (Table 7) and of these, seven were used by M. herrerae. We found differences in the diversity of the plant community between sites (Figure 7). These were divided into two main groups, one having site B and A1, and another containing the rest of the A sites. Site A2 had the highest species richness while site A1 had the lowest. Jaccard’s binary coefficient showed higher similarity of species occurred in spatially closer sites near “El Arbolito” (pairs A3, A4 and A1, A2). Sites A1−A3 and A3−A4 had the same value of similarity. Site B has the lowest values of similarity the highest being with site A3 and the lowest with site A4
Table 6. Percentage of nurse occupancy – rocks and plants, per species – and without nurse, per site.
Species
Agave striata Zucc.
Nurse for M. herrerae Sites
Bauhinia ramossisima Benth. ex Hemsl. × A1
Dasylirion longissimum S. Watson × A3, A4, B
Euphorbia antisiphylitica Zucc. × A2, A4
Havardia pallens Benth.
Hechtia glomerata Zucc. × A3, A4, B
Karwinskia subcordata Schlecht. × B
Opuntia stenopetala Engelm.
Perymenium berlandieri Schrad. × A1, A4, B
Turnera diffusa Willd. × A1, A4, B
(Table 9). The relative ecological importance of species was different between sites (Value of Importance, IVI; Table 8) such that P. berlandieri was a relevant species at sites A and F. resinosa was the relevant species at site B.
Discussion
M. herrerae, has already been reported as a threatened species (Hernández & Godínez, 1994; Ortega, 2004; Sánchez et al., 2009) in the semidesert of Queretaro and Hidalgo, an area that has been identified as having high species richness and a high number of threatened species

(Hernández & Godínez, 1994). Using the criteria of Rabinowitz (1981), M. herrerae has several factors that are common with other threatened species. For example, M. herrerae falls into the rarest category. The entire group of individuals measured in this study lived inside an area of almost 77 km2, divided by a tributary of the Moctezuma River. Localities closer to El Arbolito (A1, A2, A3 and A4) are 17 km from site B. Site A has been subject to illegal collection (Ortega, 2004; Ramírez, 2010), and therefore has a very small number of individuals (the highest numbers were found at Sites A1 and A4 have with a mere 23 individuals). Differences in population structure among these sites were marginal: A1, A3 and A4 seem to have similar structures, with a population devoid of large individuals. The similarities between sites A2 and B, in terms of size of M. herrerae arise for different reasons. For instance, we think site B as being in a relatively pristine condition, whereas site A2 is an area that has been plundered. However, mean and standard deviation of diameter and cover of both sites are the lowest of all studied populations, and Site A2 has the lowest surface of all sites. At sites A1, A3 and A4 the absence of small individuals could mean infrequent recruitment. Other globose species of the same family have been found with low levels of recruitment, namely Strombocactus disciformis and Turbinicarpus pseudomacrochele (Alvarez et al., 2004), which is a common attribute in the Cactaceae. Site A2 had the smallest individuals, which could suggest that recruitment has occurred in
recent years or these individuals may be too small for profit. The small sizes found in Site B are very likely recent episodes of recruitment, as the site benefits from low levels of anthropogenic impact. The patterns of geographical distribution of cacti in the Chihuahuan Desert, as in virtually all Mexican arid and semi-arid lands, are still poorly known, although certain patterns are being revealed, such as species concentration at some places, and extremely narrow distributions (Gómez-Hinostrosa & Hernández, 2000).
Table 8. Value of Importance (IVI) of the first five species at each site. P = Rank in the list.
M. herrerae shows specific growth conditions on steep slopes, a characteristic shared by other species of the same genus such as Mammillaria longimamma (Valverde et al., 1999), and Mammillaria crucigera (Martorell & Patiño, 2006) and other globose species of the same family, for example, Neolloydia pseudopectinata (Martínez et al., 1994). The species grows between 1,820 and 2,060 m above the sea level in portions of submontane scrub over a limestone, rocky Lithosol; all individuals were found growing on slopes oriented N–NE, and the entire area of distribution is reduced. This circumstance adds to threat because it is usually assumed that rare species with very limited geographical ranges are, by definition, more vulnerable than widely distributed ones (Rabinowitz, 1981).
As described for other species of the Cactaceae, for example, Ariocarpus scaphirostris (Mandujano et al., 2007), M. herrerae shows, at site B, a clumped distribution pattern because of factors that favour establishment and growth. Some authors have stated that the favourable microclimate provided by the verticality of the slopes could help plants to avoid very high temperatures; cliffs have been mentioned as cool refuges for succulent plants in tropical drylands (Martorell & Patiño, 2006). Estimated density at site B was the highest of all sites. At A sites, near “El Arbolito”, the pattern of distribution is not that obvious, probably due to the fact that few individuals are still left in their habitat. Estimated density, calculated within the surface of hypothetical quadrants of the surroundings in every site shows extremely low values in all A sites. Even the highest site density of M. herrerae is still lower than densities of other globose species with a threatened status such as Mammillaria pectinifera (Valverde et al., 2009), and is consistent with other species having local distribution: Hernández-Oria et al. (2007) reported M. herrerae, Echinocactus grusonii and Thelocactus hastifer as vulnerable species with estimated low densities in this area of the Chihuahuan Desert.
Nurse association of M. herrerae is clear for both sites. The most important nurse species is Perymenium berlandieri, which holds the highest Value of Importance (IVI) at sites A1, A2 and A3, and the second at site A4. On the other hand, site B has Flourensia resinosa as its important nurse species, and, in the same manner, this species has the highest Value of Importance at the site. That would probably mean that there is low specific association with nurse species. Nonetheless, for the Cactaceae, recruitment of new individuals is strongly
influenced by microenvironmental conditions, frequently provided by the presence of nurses. Sosa & Fleming (2002) have suggested that adequate places for germination and survival are placed under perennial plants and rock cracks. RojasAréchiga & Vázquez-Yanes (2000) mention that germination and recruitment under the shade provided by associate plants or rocks provide extra moisture and protection against excessive radiation during early stages of growth, which have been shown to be a basic requirement for seedling survival. That process could be happening at site B. At A sites the fact that plants have been severely looted has affected seedling recruitment and nurse association. Experienced collectors can easily find the plants under their nurses.
The community at site A2 has the highest value of species richness. This circumstance, combined with the fact that the population at this site could have a slightly higher recruitment than at other A sites could support the idea of an urgent need for conservation of this specific site, as long as this richness is not due to a greater degree of disturbance. At site A4, the highest diversity value was found. This probably means that the species of the community at this site are more evenly distributed than at the other ones, although this site has the smallest number of families and is located in the southwest corner of the distribution zone, relatively close to disturbed areas, so the high value of H’ perhaps is not relevant. However, P. berlandieri was found among its top five species; this species has been reported as a synanthropic one (López-Pérez et al., 2011), which could indicate some degree of disturbance in the zone. At the other sites – A1 and A3, which happen to be similar in population structure – the same species (P. berlandieri) is the only nurse present among the important species for M. herrerae. According to the Jaccard’s binary coefficient, there is at least 25% similarity in the vegetation of all sites, which reinforces the idea that the vicinities of “El Arbolito” had, in the past, a broader distribution of species. The vicinity of the zone has been modified for agricultural purposes, which may have had an impact on the species. Site B does not have a high value of species richness and diversity; in fact it has the lowest value of the Shannon Wiener diversity index and has the lowest similarity with A sites, which could reinforce the idea that its steep topography is limiting species diversity. Nevertheless, coincidence in nurse species exists between site B, and all sites closer to “El Arbolito”; for instance, H. glomerata, and A. xylonacantha Specifically, sites B and A2 share H. glomerata as a species with a high Value of Importance.
Table 9. Jaccard’s binary coefficient for similarity between sites of distribution of M. herrerae
This could suggest that the H. glomerata and M. herrerae association could have previously been more widespread and is now restricted to these two sites.
Under the premise that plants with small distributions are potentially or actually more exposed to extinction than those species with a wider distribution (Hernández & Godínez, 1994), M. herrerae becomes a typical example of a threatened cactus whose conservation requires immediate action. It shows three typical features associated with risk: (1) small populations, (2) reduced distribution and (3) specificity in its habitat. The analysis made in this study confirms the relevance of “Mesa de León” and “Altamira” subgrid squares of the “Cuadrante Tolimán”, as priority areas for conservation. It also shows differences in population structure between historically plundered sites, and a new locality that has not yet been altered. Even if it was not possible to protect the whole area, key locations are identified, in places that coincide with previous papers (Sánchez et al., 2006; Hernández-Oria et al., 2007) that highlight the relevant areas for the conservation of flora. M. herrerae is a visible indicator of a bad use of a charismatic species: its extensive use and looting has been documented and the size and distribution of its population is known. Drastic differences in sizes of population of disturbed and non-disturbed sites are undeniable. However, the entire panorama is still incomplete; we are still missing studies that could include population dynamics, recruitment, ex situ conservation, reintroduction programmes and community wide information that would definitely help to improve this species status. With current practices, the status of the species is perilous and urgent conservation measures are needed in the short term. M. herrerae is definitely one of the species in the Cactaceae whose conservation should be a priority at a local and national scale.
A multifocal strategy is required for the conservation of the species that should include educational, socioeconomic and biological approaches.
A sustained programme of environmental education is required that reinforces the knowledge and importance of biodiversity and local conservation, oriented to the local inhabitants, is a priority. Previous experiences exist on this issue in the area (Jardín Botánico Regional de Cadereyta, 2006). However, it is necessary to enlarge educational activities to a wider group of the region’s residents, and to the visitors. Secondly, some authors associate environmental degradation to poverty. Schteingart (2000) says that poverty per se is not the direct cause of this damage, but it is a complex mechanism where economic, educational and health conditions – among others –have a lack of resources and are not responsive, which translates into actions that degrade the environment, such as plant looting. At “El Arbolito” (site A), human population has a “High” degree of marginalisation (CONAPO, 2005), a level linked to poverty and low incomes; therefore, an improvement in living conditions of the local inhabitants is urgent (Maruri et al., 2011). Education and improvement of life conditions should lead to the establishment of an environmental governance scheme and to the execution of its five steps (World Resources Institute, 2003): participation of those interested and/or affected; development of the capacities of the society for supervision of those who decide on environmental issues; decentralisation of the process so that local people can make decisions and face the consequences; access to environmental information, and to the conscience that ecosystems are the backbone of life and of human social ecosystems, and that every action has an environmental impact. The case of M. herrerae could be a candidate for ex situ plant conservation through propagation that can be used for reintroduction, reforestation and restoration of historic endangered species.
Acknowledgements
We thank The British Cactus and Succulent Society who granted funds for this study. Dr. Fabiola Magallán Hernández, from the Regional Botanical Garden of Cadereyta, and Dr. Hugo Maruri Aguilar, from Queen Mary University of London, reviewed the manuscript and made important contributions. Biol. Armando Bayona Celis, from the Queretaro Center for Nature Resources made the geographical definition of the sub-quadrants. Biol. Pablo Gallardo Fernández and Mr. Joel Vargas, from CIAQSA, provided support during field work at site B. M.Sc. José Hernández Oria and Biol. Luis Enrique Torres Galeana contributed in the field work. Álvaro Ramírez Ramírez, a local resident, was our companion and guide.
References
ÁLVAREZ, R., GODÍNEZ, H., GUZMÁN, U., & DÁVILA, P. (2004). Aspectos ecológicos de dos cactáceas mexicanas amenazadas: implicaciones para su conservación. Bol. Soc. Bot. Mex 75: 7−16.
ANDERSON, E. F. (2001). The cactus family Timber Press, Portland, Oregon.
ANDERSON, E. F., MONTES, S.A. & TAYLOR, N.P. (1994). Threatened cacti of México. Succulent Plant Research Vol. 2. Royal Botanic Gardens, Kew.
BAILLIE, J.E.M., HILTON-TAYLOR, C., STUART, S.N. (eds.). (2004). A global species assessment. IUCN Red List Programme Office.
COMISIÓN ESTATAL DEL AGUA DE QUERÉTARO. (2008). Ficha Técnica sobre el Proyecto Acueducto II (http://www.ceaqueretaro.gob. mx/acueducto2/). (Reviewed: June 1st, 2011.)
CONSEJO NACIONAL DE POBLACIÓN (CONAPO). (2005). Índice de Marginación. http://www.conapo.gob.mx/publicaciones/IndiceMargLoc2005.pdf.(Reviewed: June 13th, 2010.)
CRAIG, R. T.(1945). The Mammillaria Handbook Abbey Garden Press, Pasadena.
D’AGOSTINO, R.B. & STEPHENS, M. (1986). Goodness of fit techniques. Marcel Dekker, New York.
FITZ MAURICE, W.A. & FITZ MAURICE, B.(2002). Mammillaria herrerae. 2002 IUCN Red List of Threatened Species.
FOWLER J., COHEN, L. & JARVIS, P.(1998). Practical statistics for field biology. John Wiley and Sons, Chichester, West Sussex.
GIBSON, A.C. & NOBEL, P.S.(1986). The cactus primer. Harvard University Press, Cambridge, Mass.
GÓMEZ-HINOSTROSA, C. & HERNÁNDEZ, H.M. (2000). Diversity, geographical distribution and conservation of Cactaceae in the Mier y Noriega region, Mexico. Biodivers. Conserv. 9: 403−418.
HENDERSON, P. A. (2008). Practical methods in ecology. Blackwell Publishing, Oxford.
HERNÁNDEZ M. H. & GODÍNEZ A. H.(1994). Contribución al conocimiento de las Cactáceas mexicanas amenazadas. Act. Bot. Mex. 26: 33−52.
HERNÁNDEZ H. M. & BÁRCENAS, R.T.(1995). Endangered cacti in the Chihuahuan Desert: I. Distribution patterns. Cons. Biol. 9:1176−1188.
HERNÁNDEZ-ORIA, J. G., CHÁVEZ, R. & SÁNCHEZ, E. (2007). Factores de riesgo en las Cactaceae amenazadas de una región semiárida en el sur del Desierto Chihuahuense, México. Interciencia 32: 728−734.
INSTITUTO NACIONAL DE ESTADÍSTICA, GEOGRAFÍA E INFORMÁTICA (INEGI). (1986). Síntesis Geográfica, Nomenclátor y Anexo Cartográfico del Estado de Querétaro Instituto Nacional de Estadística y Geografía, Querétaro, Mexico.
JARDÍN BOTÁNICO REGIONAL DE CADEREYTA. (2006). Las tres erres que tú eres –Conservación de cactáceas amenazadas con la participación de las comunidades locales. Consejo de Ciencia y Tecnología del Estado de Querétaro. Querétaro, Mexico.
KINDT, R., & ROE, R.(2005). Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi, Kenya.
LEGENDRE, P. & LEGENDRE, L. (1998). Numerical ecology. Elsevier, Amsterdam.
LOPEZ-PEREZ, Y., TEJERO-DIEZ, D., TORRES-DÍAZ, A. N. & LUNA-VEGA, I. (2011). Flora del bosque mesófilo de montaña y vegetación adyacente en Avándaro, Valle de Bravo, Estado de México. Bol. Soc. Bot. Méx. 88:35−53.
MAGURRAN, A. E.(2004). Measuring biological diversity. Blackwell Publishing, Oxford.
MANDUJANO, M.C., VERHULST, J.A.M., CARRILLO ANGELES, I.G. & GOLUBOV, J.(2007). Population dynamics of Ariocarpus scaphirostris Bödeker (Cactaceae): evaluating the status of a threatened species. Int. J. Plant Sci. 168: 1035−1044.
MARGULES, C. & SARKAR, R. S.(2009). Planeación Sistemática de la Conservación. Translated by SÁNCHEZ CORDERO, V. & FIGUEROA, F.Universidad Nacional Autónoma de México, Comisión Nacional de Áreas Naturales Protegidas y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, D. F.
MARTÍNEZ AVALOS, J.G., SUZÁN AZPIRI, H. & SALAZAR OLIVO, C.(1994). Aspectos ecológicos y demográficos de Neolloydia pseudopectinata (Backeberg) E. F. Anderson. Cact. Suc. Mex. 34: 27−33.
MARTORELL, C. & PATIÑO, P.(2006). Globose cacti (Mammillaria) living on cliffs avoid high temperatures in a hot dryland of Southern Mexico. J. Arid Environ 67: 541−552.
MARURI, A. B., GUIMONT FITZ, A., SÁNCHEZ MARTÍNEZ, E., GRAVEL, N. & DUBÉ, M.(2011). La protección de los recursos naturales en la comunidad de El Arbolito: Íncipit de un proceso de gobernanza ambiental para el Semidesierto Queretano. In Gobernanza Ambiental para el Manejo Sustentable de Recursos: La experiencia de Canadá en México. El Colegio de Veracruz, Mexico (unpublished).
MOSTACEDO, B. & FREDERICKSEN, T. S.(2000). Manual de métodos básicos de muestreo y análisis en ecología vegetal. Proyecto de Manejo Forestal Sostenible, BOLFOR, Santa Cruz, Bolivia.
NORMA OFICIAL MEXICANA NOM-059SEMARNAT-2001 (2001). Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. DOF 06-03-2002.
NORMA OFICIAL MEXICANA NOM-059SEMARNAT-2010 (2010). Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. DOF 30-12-2010.
OLDFIELD, S. (1984). The Cactaceae, a family threatened by trade. Oryx 18: 148−151.
ORTEGA, R.(2004). Rescate y caracterización ecológica de especies vegetales en estatus crítico de conservación, en el área del proyecto hidroeléctrico Zimapán, México. Tesis profesional. Universidad Michoacana de San Nicolás de Hidalgo, Mexico.
PILBEAM, J.(1999). Mammillaria. The Cactus File Handbook 6. Cirio Publishing Services, Southampton.
RABINOWITZ, D.(1981). Seven forms of rarity. In SYNGE, H.(ed.) The Biological Aspects of Rare Plant Conservation. Wiley, New York, pp. 205−217.
RAMÍREZ A. (2010). On illegal collection of M. herrerae individuals. Personal communication.
ROBBINS, C. S.(ed.). (2003). Prickly Trade: Trade and Conservation of Chihuahuan Desert Cacti. Washington D.C./Fondo Mundial para la Naturaleza, Mexico.
ROJAS-ARÉCHIGA, M. & VAZQUEZ-YANES, C. (2000). Cactus seed germination: a review. J. Arid Environ. 44: 85−104.
SÁNCHEZ MARTÍNEZ, E., CHÁVEZ MARTÍNEZ, R. J., HERNÁNDEZ-ORIA, J.G. & HERNÁNDEZ MARTÍNEZ, M.M.(2006). Especies de Cactaceae prioritarias para la conservación en la zona árida Queretano Hidalguense. Manual del Proyecto Consejo de Ciencia y Tecnología del Estado de Querétaro, Mexico.
SÁNCHEZ MARTÍNEZ, E., HERNÁNDEZ MARTÍNEZ, M. M., HERNÁNDEZ-ORIA, J. G., RUIZ CAMPOS, G., MARTÍNEZ VARA, P. & TORRES GALEANA, L. E. (2009). M. herrerae Werderm., a Mexican species on the brink of extinction. Mitteilungsbl. AfM. 33: 26−37, 70−79.
SÁNCHEZ-VELEZ, A. (1987). Conservación Biológica en México. Perspectivas. Colección Cuadernos Universitarios. Agronomía No. 13. Universidad Autónoma Chapingo, Mexico.
SCHTEINGART, M.(2000). Pobreza y alternativas de equidad social. En: Seminario: El CIID en la gestión del desarrollo urbano sostenible en América Latina: lecciones aprendidas y demandas de nuevos conocimientos. Document consulted at http://www.idrc.ca/competition/ev-22823-201-1-DO_TOPIC.html (accessed 3rd February 2011).
SOSA, V.J. & FLEMING, T.H.(2002). Why are columnar cacti associated with nurse plants? In FLEMING, T. H. & VALIENTE-BANUET, A. (eds.) Columnar cacti and their mutualists. Evolution, ecology and conservation. The University of Arizona Press, Tucson, pp. 306−323. TOMTHÉRÉSZ, B.(1995). Comparison of different methods for biodiversity ordering. J. Veg. Sci 6: 283−290.
VALVERDE, P.L., ZAVALA-HURTADO, J.A., JIMÉNEZSIERRA, C., RENDÓN-AGUILAR, B., CORNEJOROMERO, A., RIVAS-ARANCIBIA, S., LÓPEZORTEGA, G. & PÉREZ-HERNÁNDEZ, M.A.(2009).
Evaluación del riesgo de extinción de Mammillaria pectinifera, cactácea endémica de la región de Tehuacán-Cuicatlán. Rev. Mex. Biodiv 80: 219−230.
VALVERDE, T., TREJO, M. L. & CASTILLO, S.(1999). Patrón de distribución y abundancia de Mammillaria magnimamma en la reserva del Pedregal de San Ángel, México, D. F. Cact. Suc. Mex. 44: 64−74.
WORLD RESOURCES INSTITUTE. (2003). Decisions for Earth: Balance, voice and power. UNEP, WB, World Resources Institute. In PIÑEIRO, D. Movimientos sociales, gobernanza ambiental y desarrollo territorial rural. Departamento de Sociología, Facultad de Ciencias Sociales, Universidad de la República, Uruguay.
ZAMUDIO, R.S., RZEDOWSKI, J., CARRANZA, E. & CALDERÓN, G.(1992). La vegetación en el Estado de Querétaro. Consejo de Ciencia y Tecnología del Estado de Querétaro, Mexico.
ZAVALA-HURTADO, J.A. & VALVERDE, P.L.(2003). Habitat restriction in Mammillaria pectinifera, a threatened endemic Mexican cactus. J. Veg. Sci. 14: 891−898.
