JAPACVS
INFERIOR MYOCARDIAL INFARCTION RESULTING IN CARDIOGENIC SHOCK REQUIRING MULTIMODAL MECHANICAL SUPPORT




INFERIOR MYOCARDIAL INFARCTION RESULTING IN CARDIOGENIC SHOCK REQUIRING MULTIMODAL MECHANICAL SUPPORT



Journal of the Association of PAs in Cardiothoracic and Vascular Surgery
Editor-in-Chief
Aaron R. Morton, DMSc, MMSc, PA-C, ATC, FAPACVS
Emory University, Atlanta, GA
Associate Editor International
Anitha Chandrasekhar, BS, PA, FAPACVS Medanta the Medicity – Delhi, India
Associate Editor Writer Development
Edward A. Ranzenbach, PA-C, MPAS, CAQ-CVTS, FAPACVS, DFAAPA Forest Ranch, CA
Editor Emeritus
Doug Condit, PA-C Montefiore Medical Center – New York, NY
Editorial Board
JoAnn Montecalvo, MPAS, PA-C Winthrop University Hospital, Mineola, NY
Mitesh Patel, MSHS, PA-C Baylor Scott and White, Plano, TX
David Tecchio, MPAS, MBA, PA-C Vassar Brothers Medical Center, Poughkeepsie, NY
Publisher
David E. Lizotte, Jr. MPAS, PA-C, FAPACVS Executive Director APACVS Fenton, MO
EDITORIAL MISSION:
The JAPACVS is the official clinical journal of the Association of PAs in Cardiothoracic and Vascular Surgery. The mission of the JAPACVS is to improve Cardiac, Vascular and Thoracic Surgical and CVT Critical Care patient care by publishing the most innovative, timely, practice-proven educational information available for the physician assistant profession.
PUBLISHED CONTENT IN THE JAPACVS: Statements and opinions expressed in the articles and communications herein are those of the authors and not necessarily those of the Publisher or the Association of PAS in Cardiothoracic and Vascular Surgery (APACVS). The Publisher and the APACVS disclaim any responsibility or liability for such material, including but not limited to any losses or other damage incurred by readers in reliance on such content. Neither Publisher nor APACVS verify any claims or other information appearing in any of the advertisements contained in the publication and cannot take responsibility for any losses or other damage incurred by readers in reliance on thereon. Neither Publisher nor APACVS guarantees, warrants, or endorses any product or service advertised in this publication, nor do they guaranty any claim made by the manufacturer of such product or service.
SALES OFFICE
APACVS
1208 Victoria Crossing Festus, MO 63028 Phone (502) 321-6155 admin@apacvs.org
JAPACVS/Journal of the Association of PAs in Cardiothoracic and Vascular Surgery is published quarterly (4 issues per volume, one volume per year) by APACVS 1208 Victoria Crossing, Festus, MO 63028. Volume 5, Number 1, Winter 2023. One year subscription rates: $40 in the United States and Possessions. Single copies (prepaid only): $10 in the United States
© 2023 APACVS, INC. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including by photocopy, recording, or information storage and retrieval system, without permission in writing from the publisher.
Editorial
4 From the Editor’s Desk
Aaron R. Morton, MMSc, PA-C, ATC, FAPACVS Editor -In-Chief
Peer Reviewed Content
7 Case Study: Inferior Myocardial Infarction Resulting in Cardiogenic Shock Requiring Multimodal Mechanical Support
Richard H. Bengel PA-C
Claudine Pasquarello PA-C
Qiong Yang MD
Hitoshi Hirose MD
Peer Reviewed Content
34 Effect of Endoscopic Radial Artery Harvest on Grip and Pinch
Strength Short Title: Endoscopic Radial Artery Harvest Effect on Grip Strength
Jared Blackmore, PA-C
Casey T. Walk, MD
Ronald Markert, PhD
Jose Rodriguez, MD
APACVS is the only association representing Cardiac, Thoracic and Vascular Surgery and CTV Critical Care PAs. By PAs, For PAs!


As the field of medicine continues to grow more complex and subspecialized, the need for quality peer reviewed contributions to medical literature also continues to increase. This continued sub-specialization creates a vacuum for evidence-based literature to drive clinical decision making, treatment algorithms and applications. HJ Coolidge first coined the now ubiquitous phrase “publish or perish,” in 1932. This nearly 100-year-old phrase still rings true today, most certainly in contemporary medical practice. Physician Assistants continue to be on the forefront in medical applications and care delivery yet have a limited footprint in medical literature. The benefits of in participation of PA’s in medical publishing are far reaching.
The presence of PA’s on the future edges of medicine continues in various ways and it is imperative we participate in the publication of these advances .Physician Assistants continue to participate in care delivery and investigational teams driving the future edges of medicine, it is imperative this gets captured in the form of publication. By publishing and taking part in original research, case reports and other forms of medical writing, PAs can better demonstrate our expertise in patient care and enhance our roll as scholarly experts. Sharing our contributions to advances in medicine via publication cements our role in medical advances, care delivery, research, and ultimately patient outcomes. PA participation in publication will continue to increase our collective credibility as a profession. This will be imperative as we continue to face greater market pressures from other providers or allied health disciplines.
One of the major barriers in the past has been access to journals and academic publications that are in our specialty space that accept publications by nonphysicians. The Journal of the Association of Physician Assistants in Cardiothoracic and Vascular Surgery (JAPACVS) was created to help eliminate that barrier. The JAPACVS provides access to peer reviewed and referred publication for our colleagues with an engaged specialty focused audience. Additionally, we have established the role of an Associate Editor for Writer Development and created educational content to learn how to go about manuscript creation to aid our colleagues into authorship.
In conclusion, the sub-specialization of medicine and the importance of quality peer-reviewed contribution to the medical literature creates an opportunity for PAs to demonstrate their important contributions to healthcare delivery via medical publishing. By publishing our works, PAs can demonstrate their expertise in patient care, enhance our presence as scholarly experts, and contribute to the collective repository of medical knowledge. This will continue to increase the professional credibility of the PA profession at large and help secure our role in participation with medical advances, care delivery and research. The JAPACVS stands ready with educational materials and author support to assist with increased PA participation in publication.
Keep writing!

-AM
Aaron Morton, DMSc, MMSc, PA-C, ATC, FAPACVS JAPACVS Editor-in-Chief editor@japacvs.org
The result of more than 20 years of continuous advancement, the Vasoview Hemopro 2 Endoscopic Vessel Harvesting System is the latest generation of Vasoview simultaneous cut-and-seal technology.
An embodiment of the Getinge commitment to highly refined technology and robust design, the Vasoview Hemopro 2 virtually eliminates thermal spread* and helps harvesters safely acquire high-quality conduits for coronary artery bypass graft surgery.1

• Insulated jaws provide maximum protection for the saphenous vein or radial artery.
• Enhanced cutting capability for easier fasciotomy.
• Advanced system design helps to enhance the efficiency and safety of vessel harvest.
• Superb visualization and exceptional maneuverability to enable harvesters to work more efficiently.
Key words: cardiogenic shock, mechanical, ECMO, RVAD, shock team

Abstract:
Introduction: Cardiogenic Shock initiatives throughout the country have come to the forefront of tertiary care centers. Cardiogenic shock presents in different ways; however, a post myocardial infarction hospital course is a common pathway yet results in nearly a 40% mortality rate. The course of this case study is an outlier in the setting of having a total of four different mechanical circulatory support devices placed ensuing survival.
Case Presentation: 61-year-old male with end-stage-renal-disease presented to the emergency department with an acute inferior wall myocardial infarction. He underwent revascularization; however, he had a complicated hospital course that required the utilization of an intra-aortic balloon pump, veno-arterial ECMO, and percutaneous left and right ventricular assist devices. He survived his course and was discharged.
Discussion: The necessity of each of these devices is important to explore and understand because of their individual indications and how they impact patient survival.
Cardiogenic Shock initiatives throughout the country have come to the forefront of tertiary care centers. Cardiogenic shock presents in different ways; however, a post myocardial infarction hospital course is a common pathway yet results in nearly a 40% mortality rate.1 The course of this case study is an outlier in the setting of having a total of four different mechanical circulatory support devices placed ensuring survival. Among the variety of mechanical circulatory support (MCS) devices available for cardiogenic shock, decision making of the appropriate device is critical. The different MCS devices are used for different pathologies and carry their own possible complications. Implantation and explantation of MCS must be constantly evaluated and executed with timely sensitivity. In this case study, the study institution successfully implanted and explanted four MCS devices during the course of a patient who experienced cardiogenic shock after acute myocardial infarction.
A 61-year-old male with a past medical history of hypertension, end-stage renal disease (ESRD) requiring peritoneal dialysis (PD), and active tobacco use who initially called emergency services for chest pain with associated nausea and insomnia. While in the emergency department, he was diagnosed with an inferior wall ST-elevation myocardial infarction (STEMI). He was taken emergently to the cardiac catheterization laboratory.
The left sided cardiac catheterization (LHC) showed an acute thrombotic proximal right coronary artery (RCA) occlusion of 100%. The interventional cardiologist performed a balloon angioplasty three times prior to regaining coronary flow. The interval “Thrombolysis in Myocardial Infarction” (TIMI) flow revealed extensive clot burden throughout the RCA.2 Following balloon angioplasty, the guidewire was advanced down the RCA into the posterior left ventricular artery (PLV) which caused profound bradycardia. He subsequently went into ventricular fibrillation (Vfib). Per protocol, cardiopulmonary resuscitation (CPR) and advanced cardiac life support (ACLS) were initiated.
The arrhythmia required three rounds of defibrillation before return of spontaneous circulation (ROSC) was achieved. He remained severely bradycardic and a transvenous pacing wire was placed in the right ventricle via the right femoral vein and ventricular pacing was initiated. The patient regained consciousness and followed commands promptly after these events. He then developed profound worsening hypotension requiring norepinephrine, vasopressin, and epinephrine infusions. He was intubated by anesthesia in a controlled fashion. Despite ROSC and being on the ventilator his arterial blood gas (ABG) continued to show a severe metabolic acidosis, presumably a lactic acidosis. The acidosis was treated medically while the mechanical thrombectomy of the RCA was completed. Finally, a drug eluting stent was placed in the RCA. Following revascularization, the patient continued to require high dose vasopressor and inotropic support and a right heart catheterization (RHC) was performed. The patient’s Swan Ganz values were 29/16 with a central venous pressure (CVP) of 24, and a thermodilution cardiac index of 1.2. The calculated pulmonary artery pulsatility index (PAPi) was 0.54 and the calculated cardiac power output (CPO) was 0.17. These values indicated biventricular failure.3 A percutaneous left ventricular assist device was considered; however, his left ventricle was underfilled and an intraaortic balloon pump (IABP) was placed. Following IABP placement, a transthoracic echocardiogram (TTE) was performed which showed an ejection fraction (EF) of 35- 40% with inferior, inferoseptal akinesis, and anterior hypokinesis. The right ventricle was akinetic and dilated. A Cardiogenic Shock consult was placed at that time.4 Via conference call, the cardiac surgeon, cardiac intensivist, interventional cardiologist, cardiologist, and cardiothoracic surgery physician assistant discussed the case and appropriate intervention. Based upon current Extracorporeal Life Support Organization (ELSO) guidelines, the patient was deemed an appropriate candidate for veno -arterial extracorporeal membrane oxygenation (VA ECMO).5 VA ECMO was cannulated with a 22 French arterial cannula in the right femoral artery and a 25 French venous cannula was placed in the left femoral vein. Following successful initiation of ECMO, the IABP was removed in favor of a percutaneous left ventricular assist device (Impella CPTM, Abiomed, Danvers, MA) to act as a left ventricle vent (LV vent). The Impella CP was set to level P2 for a flow of 1.8 liters per minute (l/min).
Figure 1
fluoroscopy were also utilized to ensure proper placement. Next, 5 French antegrade distal perfusion catheters were placed in bilateral superficial femoral arteries spliced from the VA ECMO circuit to provide perfusion to the lower extremities. Despite adequate mechanical flow rates, the patient developed ventricular fibrillation requiring three more defibrillation attempts prior to success. Unfortunately, the echo after this event showed complete cardiac standstill. He was transferred to the Cardiac Care Unit (CCU).
On arrival to the CCU, a full set of laboratory values were assessed, and imaging was obtained to ensure correct placement of the current devices. He required blood transfusion, which is not uncommon for ECMO. The laboratory values demonstrated a continued lactic acidosis and hyperkalemia; meanwhile, he was oliguric trending towards anuria. A central venous hemodialysis catheter was placed, nephrology was consulted, and he was started on

continuous veno-venous hemodialysis (CVVHD).6 Again, he had recurrent episodes of Vfib for which he was defibrillated successfully. On post-operative day (POD) #1 from VA ECMO insertion, sedation was removed to assess brain activity, and he followed commands. Because of the acute nature of this event, and required transplant workup time a consult was placed to a heart transplant center; unfortunately, due to multi-organ system failure, he was deemed too high of a surgical risk and was denied.7 A palliative medicine consultation was performed, as per protocol for all mechanical circulatory support patients at the investigating institution.8 The family continued to pursue aggressive measures. Over the course of next several days, aggressive fluid removal was performed with CCVHD, tube feeds were initiated, the ventilator was weaned to minimal settings and a sedation vacation was performed assuring he remained neurologically intact.9
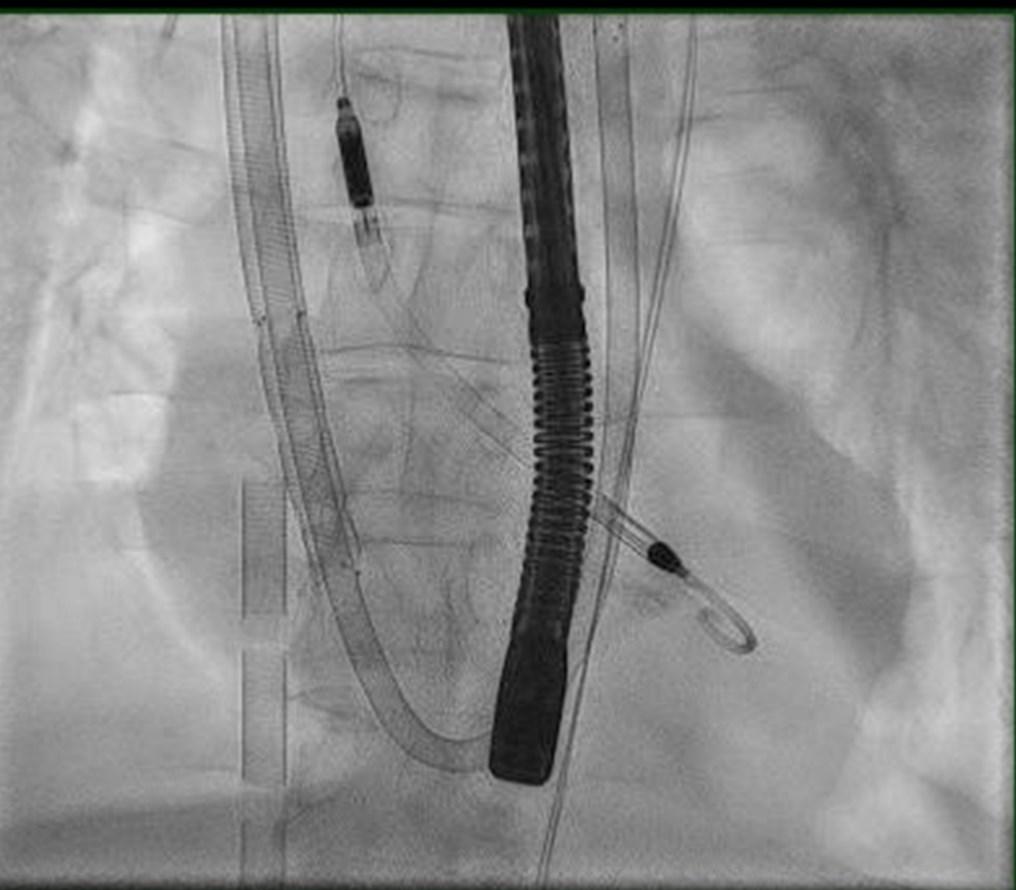
The first attempted ECMO wean was performed three days after implantation, which was successful. On the following day, a formal ECMO wean was performed.10 The heart function improved enough to consider decannulation on the following day; however, on the day of planned decannulation, the patient developed diffuse alveolar hemorrhage. Anticoagulation was held and decannulation was postponed as ECMO was supporting the ventilation and oxygenation of the patient.
Over the course of the next several days, continued hemoptysis delayed decannulation and the patient required multiple bronchoscopies. At that time, the Shock Team reconvened and collectively decided that the risk of continuing ECMO support outweighed the benefit due to the anticoagulation goals of operating this type of mechanical support.11 On POD #7, the team developed a plan to keep the Impella CP and decannulate the VA ECMO in the hybrid OR. Interventional cardiology presence was coordinated and simultaneous cannulation of a right ventricular assist device with an oxygenator was inserted via the right internal jugular vein (ProtekDuo TM , Tandem Life, Pittsburgh, PA). A ProtekDuo was placed, and the VA ECMO was removed, refer to Figure 2 and Figure 3 for reference. The removal of the VA ECMO arterial cannula was complicated by acute limb ischemia requiring vascular surgery intervention including right lower extremity angiogram, repair of the right common femoral artery and femoral artery thrombectomy. Following this, he returned to the CCU in critical yet stable condition. Over the course of the following 24 hours, he remained grossly stable despite frequent suction alarms from the Impella CP, and severely elevated liver function studies concerned the Shock Team of hemolysis. At that time, another TTE was performed which showed a significantly improved left ventricle EF of 4045%. The Impella CP was removed on POD #9 as risk of hemolysis outweighed the benefit of continued mechanical left ventricular support.12 The patient’s course required bilateral chest tube insertion for increasing pleural effusions and repeated bronchoscopies were performed for lavage of remnant hemorrhage.
Over the course of the next several days, his sedation remained light so that he could participate in rehabilitation, but remained bound by renal replacement therapy. His ventilator settings remained minimal. He remained on aggressive CVVHD volume removal goals and his wound vacuum dressings were changed at standard times. On POD #13, he began to “ pressuresupport ventilate” per ventilator weaning strategies.13 Furthermore, his LFTs and myoglobin peaked and began trending down. On POD #19, after serial echocardiograms, the right ventricle regained life sustaining function and the ProtekDuo was removed.
As the acute phase of his hospital course concluded, he required implantation of a permanent pacemaker for sick sinus syndrome on Day #21. He had a tracheostomy and percutaneous

• Adopting EVH as a Routine Procedure












• EVH: From the Trainee Perspective


• Dissection with the VirtuoSaph® Plus EVH System
• …and many more Register now and never miss an episode! https://www.terumocv.com/evh_experts Here’s





gastric (PEG) feeding tube placed. On Day #35, he was transitioned from CVVHD to intermittent hemodialysis. His respiratory status improved, and his ventilator support was discontinued on Day #39. Eventually, he passed speech evaluation for oral intake, his tracheostomy was decannulated and he was transferred to an acute rehabilitation unit on Day #46.
The main discussion points surrounding this case study revolve around mechanical circulatory support devices and the implications involved in that process. The device choice matters less about preference of a device compared to another as many of them overlap in some fashion. However, the more important factors in device decision making included patient assessment, identifying patient needs, which device meets the demands, and what is the destination of therapy. An ongoing assessment through the continuum of care is also demonstrated in the presented case study. From the report, it can be identified that certain devices have requirements, limitations, and particular complications associated with them respectively. Regarding patient needs, a full assessment must be performed prior to choosing a device. The assessment must correctly identify cardiogenic shock as opposed to other types of shock. Because mechanical support is primarily indicated for cardiogenic shock, other forms of shock must be effectively ruled out from the differential diagnosis. Although, root-cause determination of shock may be challenging in some patient case scenarios, for example a patient who experiences cardiac arrest and aspirates. It would be difficult in that scenario to solely decide which is the definitive diagnosis for shock as it may be a mixed picture including septic shock. Another common scenario includes septic and cardiogenic shock surrounding endocarditis and bacteremia.14, 15 In the assessment of cardiac function, it is imperative to assess both right heart and left heart function as the device choice can differ. All three; right sided heart failure, left sided heart failure or biventricular failure can lead to different decision-making strategies.
The assessment should include laboratory values such as complete blood counts, basic metabolic panels, arterial and venous blood gas assessment, blood cultures, serum lactic acid levels, and procalcitonin. Imaging during the assessment mainly revolves around echocardiography; although, the sequence of events frequently bypasses this step as patients go directly
to cardiac catheterization upon diagnosis of acute coronary syndrome. In the case where patients go to the catheterization, it is imperative that both a left heart and a right heart assessment are performed. During the assessment of the left heart, a ventriculogram (LV gram) is important to identify ejection fraction and assessment of the coronary arteries to identify intervenable culprit lesions.16
The assessment of the right heart is performed with a Swan Ganz catheter (pulmonary artery catheter). During the RHC, assessment of the central venous pressure (CVP), pulmonary artery systolic pressure, pulmonary artery diastolic pressure, left ventricular end-diastolic pressure (LVEDP) and cardiac index, both thermodilution and mixed venous via Fick calculation are all important to the appropriate decision-making processes. The CVP is the measurement of the pressure at the right atrium, sometimes referred to as the right atrial pressure (RAP). The pressure at the right atrium is correlated to the preload to the right ventricle. An elevated CVP can be attributed to many different causes; however, in the setting of cardiogenic shock an elevated CVP is indicative of RV volume overload or failure. Pulmonary artery pressures are evaluated to assess the function of the left ventricle. These numbers are most relevant when discussed in relation to the patient’s systemic pressures. As the function of the left ventricle deteriorates, the pulmonary artery pressures become in closer ratio to the systemic blood pressure. By measuring the pulmonary artery pressures and the CVP, a calculation can be made to assess the Pulmonary Artery Pulsatility Index (PAPi). Assessment of the PAPi is crucial in identifying right ventricular dysfunction and studies have correlated more aggressive treatment of low PAPi scores and improvement in morbidity and mortality in relation to inferior wall MI patients suffering right ventricle dysfunction. Furthermore, PAPi has also been found to have the highest sensitivity and specificity to right ventricular dysfunction compared to any other current invasive hemodynamic measurement of the right ventricle.16 Once the PAPi is evaluated, the Cardiac Output Power (CPO) should be calculated to evaluate left ventricular dysfunction. In the non-heart failure setting, cardiac output alone may be sufficient to evaluate heart function; however, in the chronic and acute heart failure settings CPO offers measurement of the hydraulic pumping ability of the heart. This evaluates the strength of contraction and the ability to generate pulsatility. CPO has been linked to a strong correlation in
InclusionCriteria
AcuteMyocardialInfarction:STEMIorNSTEMI
• IschemicSymptoms
• EKGand/orbiomarkerevidenceofAMI (STEMIorNSTEMI)
CardiogenicShock
• Hypotension(<90/60)ortheneedforvasopressor orinotropestomaintainsystolicbloodpressure>90
• Evidenceofendorganhypoperfusion (coolextremities,oliguria,lacticacidosis-sendlactate alongwithotherpertinentlabs)
ACTIVATECATHLAB
Notifythe
CardiogenicShock
TeamviaDoc

Halo/Qliq
WeanOFFVasopressorsandInotropes
ACCESS&HEMODYNAMICSUPPORT
• Obtainfemoralarterialaccess(viadirectvisualizationwithuseofultrasound andfluro)
• Obtainvenousaccess(FemoralorInternalJugular)
• ObtaineitherFickcalculatedcardiacindexorLVEDP IFLVEDP>15ORCARDIACINDEX,2.2ANDANATOMYSUITABLE,PLACE IMPELLA
CoronaryAngiography&PCI
• AttempttoprovideTIMIIIIflowinallmajorepicardiavesselsotherthanCTO
• IfunabletoobtainTIMIIIIflow,consideradministrationofintra-coronary
PerformPost-PCIHemodynamicCalculations
1.CardiacPowerOutput(CPO):MAPxCO 451
2.PulmonaryArteryPulsatilityIndex(PAPI): sPAP-dPAP RA
IfCPOis>0.6andPAPI>0.9,operatorsshouldweanvasopressorsandinotropesanddetermineifImpellacanbeweanedandremovedintheCath LaborleftinplacewithtransfertoICU.
EscalationofSupport-Shouldmonitorhourlyhemo'sanddetermineQ4-6hrsneedformoresupport IfCPOremains<0.6operatorsshouldconsiderthefollowingoptions:
• PAPIis<0.9considerrightsidedhemodynamicsupport
• PAPIis.0.96considerationforadditionalhemodynamicsupport Localpracticepatternsshoulddictatethenextsteps:
• PlacementofmorerobustMCSdevice(s)-ECMO,ProtekDuo,RVAD.
• TransfertoLVAD/Transplantcenter
IfCPOis>0.6andPAPI<0.9considerprovidingrightsidedhemodynamicsupportifclinicalsuspicionforRVdysfunction/failure.
VascularAssessment
• PriortodischargefromtheCathLab,adetailedvascularexamshouldbeperformedincludingfemoralangiogramandDopplerassessment oftheaffectedlimb
• Ifindicated,externalbypassshouldbeperformed.
ICUCare
• Hourlyhemodynamicassessments,includingdetailedvascularassessment-NEEDQ4-6hrsre-evaluationwithTeamtodecideescalationof careorweaningofsupport.
• MonitorforsignsofhemolysisandadjustImpellapositonasindicated
DeviceWeaning
Impellashouldonlybeconsideredforexplanationoncethefollowingcriteriaaremet:
• Weaningofffromallinotropesandvasopressors
• CPO>0.6andPAPI>0.9
DeviceWeaning
Patientswhodonotregainmyocardialrecoverywithin3-5days,asclinicallyindicated,shouldbetransferredtoanLVAD/TransplantCenter.If patientsarenotcandidates,palliativecareoptionsshouldbeimplemented
predicting mortality surrounding chronic heart failure and cardiogenic shock.17 Once the PAPi, CPO, PAWP, and FICK cardiac index are assessed, the treatment for mechanical support should be made. 4, 18 Once the treatment pathway comes to warrant mechanical support, the team will discuss which device would best benefit the patient. The devices range in which side of the heart is supported, how much cardiac output support is offered, if the ventilation and oxygenation are supported, and if cardiac perfusion is supported. Furthermore, accessibility of insertion is also discussed. The ability to insert the correct device in an appropriate amount of time is crucial to decreasing morbidity and mortality in cardiogenic shock.
The Shock Team Approach is a multidisciplinary team that evaluates, discusses, and intervenes on cardiogenic shock, bringing different perspectives to form a unified plan for complex patients. The Shock Team primarily treats cardiogenic shock; however, it is involved in assessing different pathological shock patients. The purpose of the team evaluating all shock patients is to ensure capture of all shock cardiogenic in nature; meanwhile, excluding those patients who would not benefit from cardiac or mechanical support.4, 19
The team primarily consists of a cardiologist, interventional cardiologist (IC), cardiac surgeon, and cardiac intensivist. These providers are typically the on-call providers; therefore, the system is reliant on broad acceptance of the terms and obligations of the team. The ancillary support comes from the catheterization laboratory (cath lab), operating room staff, critical care unit (CCU), advanced providers (APP), perfusionists, nurses, transfer center, and systemic knowledge of the shock pathway to initiate Shock Team involvement. Each facet of the team has a duty that equates to their respective skill sets and abilities. The cardiologist typically holds the longest relationship with a patient, from the time that the alert is initiated until outpatient follow-up. Having a heart failure cardiologist is preferred; although, not necessary at every institution if a relationship with a destination center is bridged. If a patient needs destination therapy through either heart transplantation or long term LVAD, they will require transport to that center after stabilization and ready for further advanced therapy.
The IC holds multiple responsibilities as well and intervention including implantation is heavily decided in conjunction with the cardiac surgeon. The IC can initiate a shock activation, implant devices, explant devices and intervene on coronary arteries causing hemodynamic instability. A shock activation from the IC usually evolves from acute coronary syndrome (ACS) or catheterization complication. Implanting certain devices can be done percutaneously requiring fluoroscopy utilizing a specified skill set by the IC. The main objective of the cath lab is to identify right sided and left sided function, and coronary anatomy. By evaluating the left and right side of the heart, the team can decide which device to choose, which is discussed in further detail in subsequent sections. Through evaluation of the coronary arteries, the IC and cardiac surgeon can determine if percutaneous coronary intervention or coronary artery bypass graft is best suited for the patient. Current literature depicts that during a STEMI, the culprit lesion is intervened upon; however, in the setting of shock, all flow limiting stenosis should be intervened upon. Furthermore, current literature supports hemodynamic stabilization and systemic tissue reperfusion prior to bypass grafting. The cardiac surgeon is involved with multiple responsibilities; typically, the surgeon has two main objectives, supporting implantation of the devices and recognizing surgical candidacy of both revascularization and destination therapy. Implanting devices usually falls into the scope of practice of the surgeon when a conduit graft or cannulation of a cardiopulmonary bypass circuit is indicated. Furthermore, the surgical candidacy of the patient is evaluated at two different levels by the surgeon. Revascularization is only part of the surgical evaluation. During this part of the evaluation bypass targets are identified, conduit limitations and surgical complexity revolving around comorbidities are discussed in relation to risk versus benefit to the patient. Meanwhile, the surgeon also evaluates the likelihood of the patient being a candidate for heart transplantation or destination Left Ventricular Assist Device (LVAD) implantation. The cardiac intensivist holds a role that culminates in the assessment and plan of these other specialists. The intensivist must assess the likelihood of recovery and ability to stabilize the patient given the revascularization strategy and mechanical circulatory support device choice.
The discussion during the shock activation conference call allows the specialists to voice expert opinion and concern from different perspectives. The conversation starts with a case
presentation by the provider who identified shock and called the transfer center. The next several parts of the conversation lead to answers surrounding specific questions led by the IC, surgeon, and intensivist about chronicity of the patient’s heart function, pertinent medical history, patient presentation, and family/patient wishes. Through this discussion a plan is formulated, the patient is assessed including catheterization, and a device is implanted.
Table 1: Mechanical circulatory support devices including indications, contraindications and benefits of devices utilized during this case presentation.
Intraaortic Balloon Pump (IABP) AMI, cardiogenic shock, acute mitral valve regurgitation, cardiac catheterization with or without percutaneous coronary intervention (PCI), refractory unstable angina, cardiac surgery, inability to wean from cardiopulmonary bypass, left ventricular failure, refractory ventricular rhythms.
Impella CP High-risk PCI, post-cardiotomy cardiogenic shock, cardiogenic shock secondary to myocardial infarction and shock states refractory to medical therapy with or without IABP.
ProtekDuo Primary right ventricular failure without left ventricular failure secondary to acute MI.
Absolute: aortic insufficiency, aortic dissection, end-stage heart disease without available destination and aortic stents.
Increase coronary perfusion pressure.
0.5 LPM CO
Mural thrombus, mechanical AV, moderate to severe AI or AS, PAD that does not allow for implantation, significant right heart failure, cardiorespiratory failure, atrial or ventricular septal defects, cardiac tamponade, or LV rupture.
Increase coronary perfusion pressure
4.0 LPM CO
Right sided heart failure with concomitant left sided heart failure. Fully support right ventricular CO with ability to utilize oxygenator.
Veno-arterial extracorporeal membranous oxygenation
(VA ECMO)
Cardiac index less than 2.0 l/min2 and hypotension as defined by systolic blood pressure less than 90 mmHg on high dose inotropic support and/or IABP support.
Cardiogenic shock from acute coronary syndrome, arrhythmia, myocarditis, drug overdose, cardiac trauma, pulmonary embolism, postcardiotomy shock, inability to wean from cardiopulmonary bypass, and bridge to therapy such as LVAD or transplant.
No foreseeable myocardial recovery or noncandidacy for durable LVAD implantation or heart transplantation.
Unrepaired aortic dissection, severe aortic insufficiency, unknown downtime prior to cardiac arrest, inadequate CPR leading to massive tissue malperfusion, brain injury, disseminated malignancy, and severe peripheral vascular disease.
Full right and left ventricle CO support, with ability to support ventilation and oxygenation.
At the study institution, the Shock Team is activated via the transfer center. Once a patient is identified as having tissue malperfusion, the evaluating provider, whether that is an emergency medicine provider or a patient in the cath lab, is informed to call the transfer center. Once the transfer center is aware of the Shock Activation, a conference phone call is made between the activating provider, the cardiologist, IC, cardiac surgeon, and cardiac intensivist. The patient and case are discussed. The plan is developed, and a device is implanted if indicated. Once the device is in and the team is satisfied with the stabilization of cardiogenic shock, the patient is admitted to the CCU for continuous critical care management. The subsequent management is done primarily by the cardiac intensivist; however, a team approach is continued throughout the hospital course, particularly involving transport to a destination center.
The intraaortic balloon pump (IABP) is a counterpulsation heart pump. Counterpulsation is a technique to lower the afterload on the heart during contraction resulting in decreased workload on the left ventricle. The IABP deflates at the end of diastole, just before the left ventricle contracts. This causes a void in the column of blood beyond the left ventricle; therefore, there is less pressure the heart has to overcome to create output. The IABP generates approximately 0.5 liters/minute of increased cardiac output.19 As opposed to intuition because the lack of significant increase in cardiac output, the IABP 30-day mortality rate of cardiogenic shock patients was found to be noninferior when compared to the Impella CP.20 Next, the IABP inflates at the end of systole, just before the aortic valve closes. As the aortic valve closes and the IABP inflates. This causes a rise in coronary perfusion pressure. Lastly, the IABP is the easiest and fastest device to implant, making it enticing in emergent cardiogenic and hemodynamic situations as a first line device.
The indications for IABP are acute myocardial infarction, cardiogenic shock, acute mitral valve regurgitation, cardiac catheterization with or without percutaneous coronary intervention (PCI), refractory unstable angina, cardiac surgery, inability to wean from cardiopulmonary bypass, left ventricular failure, refractory ventricular rhythms, sepsis, and complex congenital heart disease.
The contraindications are divided into absolute and relative lists. The absolute contraindications include aortic insufficiency, aortic dissection, end-stage heart disease without available destination and aortic stents. The relative contraindications are uncontrolled sepsis, abdominal aortic aneurysm, tachyarrhythmias, severe peripheral arterial disease (PAD) with or without complex arterial surgery.21

The intraaortic balloon pump (IABP) had historically been a class I recommendation for cardiogenic shock; however, over the past several years the IABP has fallen to a IIa and IIb recommendation in both America and Europe. Despite the downgraded recommendation, it remains the most
commonly used device for cardiogenic shock.22 Current literature is moving to a more unfavorable picture for the IABP in the setting of cardiogenic shock. In the IABP-SHOCK I trial, the researchers were only able to describe a modest improvement in the APCHE II score, which is the most commonly used ICU mortality predictor score.23, 24 Following that study, the IABP-SHOCK II study only confirmed that the use of IABP in the setting of myocardial infarction was only superior in comparison to medical therapy. Furthermore, the study failed to show a difference in 30-day mortality in the setting of cardiogenic shock with myocardial revascularization.25
Complication rates for IABP fall between 9-30% based on current literature. The most common complication is lower extremity limb ischemia requiring emergent revascularization. Other complications include false aneurysm formation, thromboemboli, and femoral artery stenosis. Other non-vascular complications include seromas, spinal cord ischemia, visceral ischemia, groin infection, balloon rupture or entrapment, peripheral neuropathy, and atheroemboli.26
In the presented case study, the IABP was used to temporize hemodynamics and increase myocardial perfusion. Although the IABP can improve LV function, this was not the patient’s main hemodynamic issue. The IABP may have improved coronary perfusion during intervention while the RCA was intervened upon with PCI. Despite intervention to the culprit lesion, the patient continued to hemodynamically deteriorate leading to escalation of mechanical support that better supported the right ventricle.
Percutaneous left ventricular devices are temporary mechanical heart pumps that are inserted to assist in unloading the workload of the heart by generating cardiac output. These devices are inserted via percutaneous catheter in the femoral arteries or surgically via axillary approach. Although other devices are currently on the market, in the present case an Impella CP was utilized; therefore, discussion will focus on this particular device. The Impella CP is a mechanical pump utilizing microaxial flow. This device is inserted percutaneously via a femoral artery catheter. It is inserted retrograde through the aortic valve. The inflow cannula is located in the left ventricle and the outflow cannula resides above the aortic valve. The device pulls blood out of the left ventricle and
expels the blood above the aortic valve. By pulling the blood out of the left ventricle, it decreases the workload of the left ventricle by reducing preload. The Impella CP can provide up to 4.0 liters/ minute of cardiac output. In addition, it can provide increased coronary perfusion pressure.27 By increasing coronary perfusion pressure, the myocardium has a higher likelihood of recovery. The increased probability of recovery is secondary to decreased end diastolic pressures and increased microvascular circulation.28 Because the Impella CP can significantly unload the left ventricle and increase microvascular

circulation, it holds a place in the treatment of ischemic cardiogenic shock. In initial studies such as the IMPRESS trial, the Impella did not show added benefit to myocardial infarction patients in cardiogenic shock. However, more recent data has proven that the Impella improves morbidity and mortality outcomes after cardiogenic shock. The largest cohorts to date, published in The Journal of Interventional Cardiology, provided a clinically significant improvement in patients who received left ventricle off-loading prior to PCI, and it went on to outline the improved outcomes in patients who failed inotropes with and without IABP therapies.28 Furthermore, according to the same study, the likelihood of more complete revascularization is achieved with the Impella compared to IABP.29 In another article published in the Annals of Thoracic Surgery, the Impella CP had superiority in 30 -day mortality at the study institution compared to historical data at the same institution in a similar cohort of patients.30
Indications for Impella CP placement revolve around similar principles as the IABP. The Impella is indicated for high-risk percutaneous revascularization, post-cardiotomy cardiogenic shock, and cardiogenic shock secondary to myocardial infarction. The Impella CP is indicated for shock states refractory to medical therapy with or without IABP. The Impella CP is limited to a four-day time limit of implantation according to the Food and Drug Administration (FDA). The contraindications for the Impella CP include mural thrombus, mechanical aortic valve, aortic valve stenosis with an aortic valve area of <0.6 cm2, moderate to severe aortic insufficiency, peripheral vascular disease that does not allow for implantation, significant right heart failure, cardiorespiratory failure, atrial or ventricular septal defects, cardiac tamponade, or LV rupture.31
Introducing a mechanical circulatory device into the body carries significant risks, from both surgical and medical perspectives. The main categories of risk revolve around implantation, embolization, and hemolysis. The implantation of Impella CP can cause injury to the vasculature in the femoral artery resulting in dissection, retroperitoneal hematoma, or limb ischemia. The aortic valve may be damaged, or the patient may experience left ventricular rupture. Embolization typically occurs during implantation on diseased aortas and is less common in the subsequent duration.
Lastly, hemolysis is the destruction of blood cells caused by axial blood flow. The destruction of platelets can lead to an acquired thrombocytopenia. Furthermore, the hemolysis of red blood cells can lead to anemia, bilirubinemia and, if severe enough, acute renal failure.32
The limitations of the Impella CP were demonstrated in the present case. The patient was experiencing biventricular failure status post cardiac arrest. The Impella CP was able to assist in unloading the LV. However, because the patient presented with an RCA territory infarct, the patient needed right ventricular support. In many settings, left sided heart failure is the most common cause of right sided heart failure. In this case, the right ventricular function was compromised by the MI.

The left ventricular dysfunction was secondary to post-cardiac arrest stunning of the myocardium.
The Impella assisted in LV output; however, the patient required more support leading to prolonged Impella utilization in this case. VA ECMO was placed emergently to support biventricular failure and respiratory compromise. The Impella CP was left in place as a left ventricle vent, to ensure complete decompression of the left ventricle. The concomitant use of Impella CP with VA ECMO has shown in a randomized trial to have clinically significant lower hospital mortality and a higher rate of bridge to recovery or advanced therapy such as permanent LVAD.33

Veno-Arterial Extracorporeal Membrane Oxygenation (VA ECMO)
Veno-Arterial Extracorporeal Membrane Oxygenation (VA ECMO) is a cardiopulmonary bypass circuit that drains blood from the venous system, passes the blood through a semi-permeable membrane allowing for diffusion of carbon dioxide (CO2) and oxygen, and returns the blood in retrograde flow into the aorta. The venous blood is drained by the inflow cannula. By draining the blood from the venous system, it reduces the preload to the RV thus not giving preload to the LV. Subsequently, the workload of the heart is reduced by decreasing the myocardial stress of the LV. By removing preload to the right ventricle, it does not allow for blood to pass through the pulmonary vasculature which does not support native ventilation and oxygenation. The blood drained from the venous system must pass through a semi-permeable membrane, referred to as the oxygenator, allowing for CO2 removal and oxygen diffusion into the blood. The blood is then returned to the patient via the outflow cannula into the abdominal aorta via retrograde flow providing antegrade perfusion to the cerebral and coronary circulation.
Indications for VA ECMO
Indication for VA ECMO is based on two key factors: hemodynamic support needs and underlying diagnosis. Hemodynamic support factors include a cardiac index less than 2.0 l/min2 and hypotension as defined by systolic blood pressure less than 90 mmHg on high dose inotropic support and/or IABP support. Underlying diagnosis is important in initiation of ECMO because reversing the cause or bridge therapy needs to be determined, as cannulation of ECMO without those parameters is futile. Underlying diagnoses include but are not limited to cardiogenic shock from acute coronary syndrome, arrhythmia, myocarditis, drug overdose, cardiac trauma, pulmonary embolism, postcardiotomy shock, inability to wean from cardiopulmonary bypass, and bridge to therapy such as LVAD or transplant.34
The most discussed contraindication for VA ECMO is the recoverability of heart function or the ability to bridge to a long-term device or transplant candidacy. The other contraindications include unrepaired aortic dissection, severe aortic insufficiency, unknown down-time prior to cardiac arrest, inadequate CPR leading to massive tissue malperfusion, brain injury, disseminated
malignancy, and severe peripheral vascular disease.34
Complications of ECMO are very common and are highly correlated with increased rates of morbidity and mortality. Complications can be divided into a few sections. The first is insertion. Insertion can be complicated by vascular injury, embolism, and bleeding. Management of ECMO has two major contributors: anticoagulation and pump tubing. Anticoagulation goals can rise and fall between individualized patients. If a patient is hemorrhaging, anticoagulation can be stopped; however, this may lead to circuit thrombosis or other thrombotic events. If the patient is hypercoagulable, increasing anticoagulation goals may lead to bleeding, disseminated intravascular coagulopathy (DIC) or heparin induced thrombocytopenia (HIT). The ECMO tubing has been associated with systemic inflammatory response syndrome (SIRS) presents with profound refractory hypotension and capillary leak. Profound hypotension requires increased vasopressor support which causes ischemia, particularly mesenteric ischemia precipitates a cascade of decompensation. Meanwhile, third spacing through capillary leak may lead to extreme volume overload with a prolonged course of mobilizing fluids, pulmonary edema, effusions, and a protracted rehabilitation course. 32, 34
In this present case, VA ECMO was initiated after the patient failed stabilization with revascularization and hemodynamic support with the IABP. The IABP did not provide enough cardiac output support. After the RHC was performed, the patient was noted to have a PAPi that indicated the need for right ventricular support. In the setting of an emergency, with biventricular failure, implantation of VA ECMO is the most appropriate device to be implanted. VA ECMO offered this patient right sided hemodynamic support, left sided hemodynamic support and the ability to utilize acute respiratory distress syndrome (ARDS) ventilator settings during the recovery of his aspiration pneumonia that occurred during his cardiac arrest.35 Once VA ECMO was initiated, removal of the IABP in favor of the Impella CP was performed. Utilization of the Impella CP at this time was indicated as a LV vent. By venting the left ventricle, the ventricle is decompressed allowing for decreased myocardial oxygen demand and lower incident of LV thrombus if the heart is not generating enough pulsatility to eject blood through the aortic valve. Concomitant use of the Impella CP in the setting of VA ECMO has been proven to improve mortality.36

In the presented case, the percutaneous right ventricular device utilized was the ProtekDuo. The ProtekDuo is a dual-lumen percutaneous device that is inserted via Seldinger technique, through the internal jugular vein, into the superior vena cava (SVC), right atrium, in the right ventricle and resides in the pulmonary artery.37 The ProtekDuo drains blood from the SVC. The drained blood passes through a semipermeable membrane where diffusion of CO2 and oxygen occurs. Then the blood is returned through the outflow cannula that resides in the pulmonary artery. Essentially, the preload is drained from the right ventricle, bypassing it and delivering the preload to the left ventricle. Thus, the RVAD hemodynamically supports right sided dysfunction without giving left sided hemodynamic support. This particular product also supports ventilation and oxygenation. At the present time, there are two widely available percutaneous right ventricular support devices. In the presented case the decision to utilize the ProtekDuo over the other available device is because of the availability to splice in an oxygenator to the circuit. In addition to RV support, the patient required ventilation and oxygenation support due to severe hemoptysis. The hemoptysis was attributed to pulmonary hemorrhage and coagulopathy secondary to severe liver dysfunction. As the pulmonary hemorrhaging resolved, more reliance on the ventilator could be achieved; therefore, leading to the decreased need of the gas exchange component provided by the ProtekDuo. Furthermore, the liver slowly returned to baseline secondary to adequate continued hemodynamic support. In the present case, the ProtekDuo was utilized as a long-term RV mechanical support device. The patient’s LV status regained normal function; however, the patient’s RV and pulmonary status were still compromised. The RVAD provided this patient the ability to receive the hemodynamic support that was needed, without the sequela of extended utilization of VA ECMO. Furthermore, by shifting to the RVAD, the patient’s mobility status improved as a patient has an increased ability to be out-ofbed and ambulate compared to VA ECMO. By utilizing this device during this patient’s hospital course, the study institution was able to improve other aspects of patient recovery such as mental status, ambulation, return of bowel function, and decannulation from the ventilator while still maintaining hemodynamic support.
In summary, the case presented offers a detailed look into the decision making, indications and limitations of different mechanical circulatory devices that are utilized in a complex cardiogenic shock course. Each circulatory device offers its own strengths, weaknesses, indications, and complications that must be navigated by the Shock Team in order to increase chances of patient survival. By understanding the process of identifying shock, evaluating patient needs, and executing decision making, proper patient-device matching can be achieved in hope of improving overall mortality and morbidity in cardiogenic shock.

1. Thiele H, Freund A, Gimenez MR, et al. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock - Design and rationale of the ECLS-SHOCK trial. Am Heart J. 2021;234:1-11.
2. Durante A. Role of no reflow and microvascular obstruction in the prognostic stratification of STEMI patients. Anatolian Journal of Cardiology. 2018;19:346-349.
3. Korabathina R, Heffernan KS, Paruchuri V, et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593-600.
4. Tehrani B, Truesdell A, Singh R, Murphy C, Saulino P. Implementation of a cardiogenic shock team and clinical outcomes (INOVA-SHOCK Registry): Observational and Retrospective Study. JMIR Research Protocols. 2018;7:e160.
5. Gajkowski EF, Herrera G, Hatton L, Velia Antonini M, Vercaemst L, Cooley E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO journal. 2022;68:133-152.
6. Tandukar S, Palevsky PM. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest. 2019;155:626-638.
7. Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014;6:1120-1128.
8. Godfrey S, Sahoo A, Sanchez J, et al. The Role of Palliative Care in Withdrawal of Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. J Pain Symptom Manage. 2021;61:1139 -1146.
9. Khan B, Fadel W, Tricker J, et al. Effectiveness of Implementing a Wake Up and Breathe Program on Sedation and Delirium in the ICU. J Crit Care Med. 2014;42:e791-e795.
10. Cavarocchi NC, Pitcher HT, Yang Q, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg. 2013;146:1474-1479.
11. Sy E, Sklar MC, Lequier L, Fan, Eddy, Kanji, Hussein D. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation (VA-ECMO): A systematic review and meta-analysis. J Crit Care. 2017;39:87-96.
12. Sibbald M, Džavík V. Severe hemolysis associated with use of the impella LP 2.5 mechanical assist device. Catheter Cardiovasc Interv. 2012;80:840-844.
13. Rose L. Strategies for weaning from mechanical ventilation: A state of the art review. Intensive Crit Care Nurs. 2015;31:189-195.
14. Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166-E176. doi:10.3978/j.issn.2072-1439.2015.07.17
15. Tartavoulle T, Fowler L. Cardiogenic Shock in the Septic Patient: Early Identification and Evidence-Based Management. Crit Care Nurs Clin North Am. 2018;30:379-387.
16. Thiele H., Desch S, Piek, Jan J., et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: Design and rationale of CULPRIT-SHOCK trial. Am Heart J. 2015;172:160-169.
17. Basir MB, Kapur NK, Patel K, et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173-1183.
18. Doll JA, Ohman EM, Patel MR, et al. A team-based approach to patients in cardiogenic shock. Catheter Cardiovasc Interv. 2016;88:424-433.
19. Janda M, Scheeren TWL, Bajorat J, et al. The Impact of Intra-aortic Balloon Pumping on Cardiac Output Determination by Pulmonary Arterial and Transpulmonary Thermodilution in Pigs. J Cardiothorac Vasc Anesth. 2006;20:320-324.
20. Ouweneel DM, Engstrom AE, Sjauw KD, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol Cardiovasc Dis. 2016;202:894-896.
21. Krishna M, Zacharowski K. Principles of intra-aortic balloon pump counterpulsation. Continuing Education in Anaesthesia, Critical Care & Pain. 2009;9:24-28.
22. Fuernau G, Thiele H. Intra-Aortic Balloon Pump (IABP) in cardiogenic shock. Curr Opin Crit Care. 2013;19:404-409.
23. Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152-160.
24. Kramer, A. A., Zimmerman, J. E., & Knaus, W. A. (2021). Severity of illness and predictive models in society of critical care medicine’s first 50 years: a tale of concord and conflict. Critical Care Medicine, 49(5), 728-740.
25. Thiele H, MD, Schuler G, MD, Neumann F, MD, et al. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock: Design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163:938-945.
26. Stone GW, Ohman EM, Miller MF, et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The benchmark registry. JACC. 2003;41:1940-1947.
27. Remmelink M, Sjauw KD, Henriques JPS, et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70:532-537.
28. Watanabe, Fish, K., Kovacic, J. C., Bikou, O., Leonardson, L., Nomoto, K., Aguero, J., Kapur, N. K., Hajjar, R. J., & Ishikawa, K. Left Ventricular Unloading Using an Impella CP Improves Coronary Flow and Infarct Zone Perfusion in Ischemic Heart Failure. J Am Heart Assoc, 7(6). https:// doi.org/10.1161/JAHA.117.006462
29. Remmelink M, Sjauw KD, Henriques JPS, et al. Effects of mechanical left ventricular unloading by impella on left ventricular dynamics in high-risk and primary percutaneous coronary intervention patients. Catheter Cardiovasc Interv. 2010;75:187-194.
30. Lemaire A, Anderson MB, Lee LY, et al. The Impella Device for Acute Mechanical Circulatory Support in Patients in Cardiogenic Shock. Ann Thorac Surg. 2014;97:133-138.
31. Monteagudo Vela M, Simon A, Riesgo Gil F, et al. Clinical Indications of IMPELLA Short-Term Mechanical Circulatory Support in a Tertiary Centre. Cardiovasc Revasc Med. 2020;21:629-637.
32. Ali JM, Abu-Omar Y. Complications associated with mechanical circulatory support. Ann Transplant. 2020;8:835.
33. Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella® on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J H Fail. 2017;19:404-412.
34. Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166-E176. doi:10.3978/j.issn.2072-1439.2015.07.17
35. Nakamura M, Imamura T, Hida Y, Kinugawa K. Pulmonary Artery Pulsatility Index and Hemolysis during Impella-Incorporated Mechanical Circulatory Support. J Clin Med. 2022;11:1206.
36. Del Sorbo L, Goffi A, Goligher E, Fan E, Slutsky AS. Setting mechanical ventilation in ARDS patients during VV-ECMO: where are we? Minerva anestesiologica. 2015;81:1369-1376.
37. Stepanenko A, Potapov EV, Krabatsch T, Hetzer R. Simple Implantation of a Temporary Right Ventricular Device for Right Ventricular Failure After Left Ventricular Device Implantation via a Left Lateral Thoracotomy. ASAIO journal. 2011;57:17-18.
TITLE: EFFECT OF ENDOSCOPIC RADIAL ARTERY HARVEST ON GRIP AND PINCH STRENGTH SHORT TITLE: ENDOSCOPIC RADIAL ARTERY HARVEST EFFECT ON GRIP STRENGTH
Jared Blackmore, PA-C1, Casey T. Walk, MD2, Ronald Markert, PhD3, Jose Rodriguez, MD1

1 Division of Cardiothoracic Surgery, Miami Valley Hospital, 30 E. Apple St., Suite 1480, Dayton, OH 45409
2 Wright State University Department of Surgery, Miami Valley Hospital, Weber Center for Health Education 7th Floor, 128 E. Apple St., Dayton, OH 45409
3 Wright State University Boonshoft School of Medicine, Professor and Vice Chairman for Research, Department of Internal Medicine, 128 E. Apple St., Dayton, OH 45409
Keywords: Endoscopic radial artery harvesting, CABG, pinch strength, grip strength
Abstract:
Background: Coronary artery bypass grafting (CABG) utilizes autologous conduits, preferentially the internal mammary artery followed by the greater saphenous vein (GSV). Studies have shown that radial artery (RA) conduits, when combined with appropriate protocols, have improved outcomes compared to GSV. Harvesting the RA prompted concern for prolonged weakness in the donor hand; literature search showed that this has not been evaluated to date. Objective of this study is to evaluate the effect of endoscopic RA harvesting on grip and pinch strength.
Methods: A retrospective chart review was performed. Patients undergoing CABG with planned RA harvest who had pre/post operative grip and pinch strength recorded since 2016 were included. Patients with incomplete data were excluded. A dynamometer and pinch gauge were utilized to evaluate grip, tip pinch, key pinch, and palmar pinch strength. Preoperative data was compared to data collected at three weeks and three months postoperative follow up.
Results: Population of study included 23 men (88%) and 3 women (12%), mean age 60 years old, range of 47-71. No statistically significant differences were found among all strength assessments by the three month follow up. There was a down trend in grip strength at 3 weeks, but this equilibrated by the 3 month follow up. There was minimal change in all pinch strength assessments.
Conclusion: Endoscopic RA harvesting does not have a significant effect on grip or pinch strength, although there is a down trend of grip strength at 3 weeks that appears to equilibrate by the 3 month follow up.
Background
Coronary artery bypass grafting (CABG) is performed using various conduits to provide blood flow beyond a coronary artery stenosis or occlusion. Across the United States, two vessels are predominantly utilized for the surgery: the Internal Mammary Artery (IMA) and the Greater Saphenous Vein (GSV). The Radial Artery (RA) was first used by Carpentier in 1971 but stopped shortly after due to a 35% rate of occlusion and narrowing of the artery1,2 . In 1976, Fisk et al. stated, "the radial artery should not be used for coronary bypass3.” In the early 1990's, Acar et al., re-established the RA as a suitable conduit after incidentally discovering previous RA grafts that remained patent, and subsequently modified the harvest techniques to avoid spasm and intimal
hyperplasia4. These studies also utilized calcium channel blockers during and after surgery, which were not available during the early attempts at using the RA as a CABG conduit. More recent studies have noted the superiority of the RA as a second-line conduit compared to the GSV, noting the multi -artery approach to CABG resulting in a lower incidence of cardiac events, lower risk of graft occlusion, lower incidence of myocardial infarction, a lower incidence of repeat revascularization and an improved survival benefit of the RA compared to GSV in patients younger than 705,6 .
Despite these publications, the GSV remains in favor as the second conduit following the IMA, instead of the RA. There are risks associated with radial artery harvesting including limb ischemia, limb loss, nerve damage, vasospasm, poor cosmesis pen technique, compression syndrome, increased surgical time and concern for residual hand and grip weakness. As endoscopic vessel harvesting has evolved, the RA can be harvested endoscopically in the same technique as the GSV. Endoscopic RA harvesting has shown to have fewer complications compared to open RA harvesting7,8. When compared for graft quality, patency, overall mortality, Endoscopic RA harvest was found to be non-inferior to open technique9. For the same quality, patency, and mortality the patient receives less complications at harvest site and improved cosmesis.
The only remaining concern is the question of residual strength after RA harvest. There is a gap in the literature relating to this concerned risk of decrease in hand strength in the donor arm. Many patients requiring CABG are relatively young and have significant concerns with losing grip or pinch strength in a donor hand after RA harvest. We have hypothesized that there is no loss of function or strength in the hand from the donor arm following endoscopic RA harvesting.
An IRB was obtained, and a retrospective chart review was performed. Patients undergoing CABG with planned RA harvest who had pre/post operative grip and pinch strength recorded since 2016 were included. Exclusion criteria for the study was previous cerebrovascular accident with residual deficit of the upper extremity and insufficient data for analysis.
A dynamometer and pinch gauge were used to assess the patient's grip and pinch strength prior to surgery, and then approximately three weeks and three months postoperatively. The dynamometer assesses the grip strength of the hand. The pinch gauge is used to assess the tip pinch,
key pinch, and palmar pinch – all various techniques to test pinch strength. Tip pinch is when then thumb opposes the pad of the index finger. Key pinch is when the thumb opposes the side of the index finger. Palmar pinch is when the thumb, index, and middle finger all assert a force on an object. Each test is performed three times on each hand for an accurate mean. The non-donor arm was tested to use as the control.
Data analysis was performed using the statistical software program, SPSS. A p value of less than 0.05 was considered significant. All radial arteries were harvested using the endoscopic technique, as described by Dr. Navia, et al., with a minor difference of a more distal incision10. The procedure was performed using the Vasoview Hemopro 2 Endoscopic Vessel Harvesting System (Getinge US Sales, LLC, Wayne, NJ). Average tourniquet time of our procedure was 22 minutes. The patients were started on a diltiazem drip of 2.5mg/hr intraoperatively and this was continued until postoperative day 1, if tolerated. On postoperative day 1, the patients were started on amlodipine 5mg PO QD and continued upon discharge.
The patients were 23 men (88%) and 3 women (12%). The mean age was 60 years old, with a range of 42-71. Two patients were excluded from final data calculations as they were found to have chronic unilateral weakness from previous CVAs. Two patients did not receive testing at their first post-op visit but did receive testing at the three-month follow-up visit. Three patients did not show up to their three-month follow-up visit, but their first post-op visit results were included in the statistical analysis. Mean first and second visit post op day were 21.5 and 94.17 days. Grip and pinch strength compared to control are outlined in Table 2. One patient did not receive pre-operative grip strength testing but was tested with the pinch gauge; for this patient, only the pinch gauge was used at follow-up visits.
Compared to First Follow Up Evaluation
Strength Assess-
Preoperative Evaluation Compared to Second Follow Up Evaluation
This study followed grip and pinch strengths of 24 patients from preoperative to postoperative settings after endoscopic RA harvesting and noted no statistical difference over time compared to preoperative assessments. There is a down trend in strength when compared to the control (nondonor arm), noted mostly at grip strength at first follow up. This notes approximately a 5.7 lb. difference between the donor and control arms at this time interval, that we found to be on average of 21.5 days. This difference was noted to be equilibrating at the second follow up (avg. 94.17 days) as the grip strength difference was 2.9 lbs. All other pinch grip strength assessments had no statistical difference with observed minimal change throughout the entirety of the study.
While no statistical significance was noted, this change in grip strength demonstrates a clinical change to the patients, bringing into question how to determine clinical significance. Studies on the minimal clinically important difference in grip strength have noted that any change greater than 6.5 kg (14.33 lbs) are clinically significant11,12. While there was a demonstrated decrease in grip strength post op, it not only was statistically insignificant, but clinically insignificant as well. Also, it equilibrated over time to within 3 lbs of preoperative testing. Long term studies could be performed to ensure this trend continues.
The literature supports the RA to be the preferred second choice conduit over the GSV when utilized in appropriate scenarios and has noted multiple benefits including overall survival5,6. There is a concern for risks and complications associated with harvesting the RA 1-3. However, endoscopic harvesting of RA is much preferred over open approach as it has been shown to be non-inferior in overall mortality, fewer complications, non-inferior conduit quality, as well as overall improved cosmesis 7-9 .
This study is not without limitations. The data collection is not randomized, but this is unable to be designed in this fashion due to the nature of the procedures. Another limitation is the overall number of patients; a better analysis could be completed with a larger population. Larger studies at longer intervals would improve understanding of the complete effect of endoscopic RA harvesting on grip strength. However, our study was sufficiently powered with the number of patients used, therefore we stopped further data collection.
Our study has shown no significant decrease in grip or pinch strength in the short- or longterm settings. Following cessation of data collection, we have applied the results of the study to our practice and have endoscopically harvested over 250 radial arteries without complication, regardless of hand dominance. Our criteria for use of RA in CABG are as follows; complete palmar arch with a RA diameter of at least 2mm per ultrasound measurement, left sided lesions of at least 70%, right sided lesions of at least 90%, adequately sized coronary with good runoff per subjective discretion. Exclusion criteria includes incomplete palmar arch, RA measuring less than 2mm, atherosclerotic disease of the RA, End Stage Renal Disease requiring hemodialysis, subclavian stenosis, Raynaud
disease, Scleroderma, and rheumatoid arthritis. We have harvested two RA that were not suitable for use as a conduit in CABG due to extensive atherosclerotic changes. Therefore, we have no reluctancy with harvesting the radial artery from the dominant hand. This in turn provides the opportunity for bilateral radial artery harvesting in the appropriate clinical scenario.
1. Carpentier A, Guermonprez JL, Deloche A, Frechette C, DuBost C. The Aorta-to-Coronary Radial Artery Bypass Graft. Ann Thorac Surg. 1973;16(2):111-121. doi:10.1016/S0003-4975(10)65825-0
2. Geha AS, Krone RJ, McCormick JR, Baue AE. Selection of coronary bypass. Anatomic, physiological, and angiographic considerations of vein and mammary artery grafts. J Thorac Cardiovasc Surg. 1975;70(3):414-431.
3. Fisk RL, Brooks CH, Callaghan JC, Dvorkin J. Experience with the Radial Artery Graft for Coronary Artery Bypass. Ann Thorac Surg. 1976;21(6):513-518. doi:10.1016/S0003-4975(10)63919-7
4. Acar C, Jebara VA, Portoghese M, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54(4):652-660. doi:10.1016/0003-4975(92)91007-V
5. Gaudino M, Benedetto U, Fremes S, et al. Radial-Artery or Saphenous-Vein Grafts in CoronaryArtery Bypass Surgery. N Engl J Med. 2018;378(22):2069-2077. doi:10.1056/NEJMoa1716026
6. Tranbaugh RF, Dimitrova KR, Friedmann P, et al. Radial Artery Conduits Improve Long-Term Survival After Coronary Artery Bypass Grafting. Ann Thorac Surg. 2010;90(4):1165-1172. doi:10.1016/j.athoracsur.2010.05.038
7. Patel AN, Henry AC, Hunnicutt C, Cockerham CA, Willey B, Urschel HC. Endoscopic radial artery harvesting is better than the open technique. Ann Thorac Surg. 2004;78(1):149-153. doi:10.1016/ j.athoracsur.2004.03.001
8. Kim G, Jeong Y, Cho Y, Lee J, Cho J. Endoscopic Radial Artery Harvesting may be the Procedure of Choice for Coronary Artery Bypass Grafting. Circ J. 2007;71(10):1511-1515. doi:10.1253/ circj.71.1511
9. Lei JJH, Ravendren A, Snosi M, Harky A. In patients undergoing coronary artery bypass grafting, is endoscopic harvesting superior to open radial artery harvesting? Interact Cardiovasc Thorac Surg. 2021;32(3):447-451. doi:10.1093/icvts/ivaa291
10. Navia, José L., Gabriel Olivares, Paul Ehasz, A. Marc Gillinov, Lars G. Svensson, Nicolas Brozzi, & Bruce Lytle. "Endoscopic radial artery harvesting procedure for coronary artery bypass grafting." Annals of Cardiothoracic Surgery, 2.4 (2013): 557-564. doi: 10.3978/j.issn.2225319X.2013.07.09
11. Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. 2019;31(1):75-78. doi:10.1589/jpts.31.75
12. Kim JK, Park MG, Shin SJ. What is the Minimum Clinically Important Difference in Grip Strength? Clin Orthop. 2014;472(8):2536-2541. doi:10.1007/s11999-014-3666-y