
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
Prof Aziz Makandar1, Mrs. Ayisha Soudagar2*
1Professor and 2*Research Scholar
1&2 Department of Computer Science Karnataka State Akkamahadevi Women’s University Vijayapura, India
Abstract - Therisingrateofskincancercases necessitates sophisticated early detection methodologies. Modern machine learning techniques, especially for image analysis, have emerged as potent tools in this endeavour. These models are designed to process substantial datasets, including high-resolution images of coetaneous lesions, to identifysubtlepatternspotentiallyindicativeofmalignancy. By harnessing machine learning, medical experts have the potential to enhance their ability to detect skin cancer earlier, facilitating timely interventions and potentially improving patient outcomes. This research focuses on applying the support vector machine (SVM) technique, implemented via MATLAB, for skin cancer classification. SVMs are well-suited for this task due to their capacity to manage high-dimensional data and delineate complex decision boundaries. The SVM algorithm's efficacy is evaluated using key performance metrics, including accuracy, sensitivity, and specificity. These metrics assess the algorithm's ability to accurately classify cutaneous lesions, minimize false negatives, and reduce false positives. Through the development of such machine learning approaches, researchers aim to create more reliable and efficient tools for skin cancer detection. This endeavour has offers the capability to redefine how early diagnosis and treatmentareapproachedinskincancer
Key Words: accuracy, sensitivity, classification of skin cancer,specificity,supportvectormachine,skinlesions.
Onemajorworldwidehealthconcerniscancer.According to global predictions, cancer will be responsible for about 10.0 million deaths in 2020 (9.9 million excluding nonmelanomaskincancer).Lungcancer,prostatecancer,and breast cancer in women are the most often diagnosed cancers.Themajorcausesofdeathassociatedwithcancer are stomach, liver, and lung malignancies [1]. Skin cancer is common in Caucasians, including non-melanoma skin cancer (NMSC) and malignant melanoma. [2] The incidenceisrising.
The US Skin Cancer Foundation claims that, Skin cancer affects more Americans, eachyear than by any other type of disease. In recent decades, Skin malignancies have grown more prevalent common, posing serious threats to public health [1]. Melanoma accounts for 80% of skin
cancermortality,makingitoneofthemostdeadlytypesof the disease [2]. The annual incidence of newly diagnosed malignant melanoma cases increased by 31% throughout theprecedingtenyears(2012–2022)[3].
The estimated five-year chances of survival in those suffering from early-detected melanoma is around 99 percent, although early detection is essential for the possibility of successful therapy. When the disease spreads to distant organs, thesurvival rate drops to 30%, and when it affects the lymph nodes, it drops to 68% [3]. Given these numbers, it is critical to accelerate the diagnosis of melanoma and skin-related malignancies. In conclusion, early diagnosis is vital for effective treatment andbetterconsequencesofskinmalignancy.Inaneffortto preserve lives and lessen the financial and medical burdens on patients, automated strategies for reliably diagnosing cancer must be developed because specialists arenotalwaysavailable.Melanomahasaveryvariedlook, andskincancerscanbehardtodifferentiatefromnormal benign skin abnormality. The morbidity and mortality linked to skin cancer may be decreased by using artificial intelligencetohelpdetectthediseaseearly[6].Inaddition to reducing effort, AI-based solutions may improve the Identificationofskinabnormalities [7,8].Oneofthemain drivers of the fourth industrial revolution is artificial intelligence (AI), a branch of computer science that uses computers and software to mimic intelligent human behaviour using a range of technologies [9]. A subset of artificial intelligence known as machine learning, or ML, repeatedly learns from data using statistical models and algorithms. This enables computers to perform certain jobsandforecastthepropertiesofupcomingsamples.Asa result,thecomplexalgorithmsaremadetodo procedures that would likely otherwise be difficult for humans to understand. Convolution neural networks (CNNs), a kind of machine learning that replicates the way biological neurons behave, are the primary architecture for pattern detection in medical image analysis [10]. Millions of peopleworldwideareaffectedbyskincancer,a prevalent and sometimes deadly illness. Improving prognosis and lowering death rates depend on early recognition and accurate differentiation of skin lesions. The majority of Traditional approaches to skin cancer diagnosis rely on the visual inspection of dermatologists, which can be labour-intensive and subjective [11]. Because machine learning (ML) techniques automate the process and

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
increase in the precision of skin cancer diagnosis, they provide a feasible alternative. One possible strategy to enhance the accuracy and efficiency of diagnostic processes is to use machine learning to recognize and categorize skin cancer [12–13].Skin malignanceidentification and treatment might be revolutionized by employingmachinelearningalgorithms indermatologyastechnologyadvances.
1.1
The scope of research on Skin Cancer Recognition and Type Differentiation machine learning is extensive, encompassing various aspects of dermatology, medical imaging,andartificialintelligence.Theresearchdomainin skin AI-driven methods for detecting cancer is dynamic and adopts a multidisciplinary approach. It comprises algorithmic development, data considerations, ethical issues, deployment techniques, and an evaluation of the real-world effects on healthcare practices and patient outcomes.
1.2
Skin cancer is a widespread and serious condition that affects millions of people worldwide. The current ways to diagnose it mainly depend on dermatologists visually inspecting the skin. These methods are subjective, take a lot of time, and may not always be accurate. We need an automated and reliablesystem to improve how wedetect and classify skin cancer. This research work aims to bridge the existing gaps in skin cancer diagnosis, revolutionizing the approach to early-stage diagnosis and classificationusingmachinelearningtechniques
Skin cancer is a common and possibly dangerous condition affecting a considerable part of the global population. At present, dermatologists primarily rely on visual examination for skin cancer detection, a method thatindicatethatcanbetime-consumingandoccasionally imprecise. There is an urgent requirement for a more efficient and accurate approach to skin cancer identification. This research endeavors to develop an innovative system utilizing advanced computational technology to enhance the early Detection and differentiationofdermatologicaldisorderscancer.
Theobjectivesoftheworkare:
To investigate the issues and challenges in the earlyidentificationofcutaneouscancer.
Togeneratearobustandaccuraterecognitionand categorization of skin cancer system utilizing machinelearning.
Todivideskinlesionsintovariousgroups(e.g., benign,malignant)forprecisediagnosis
To enhance diagnostic efficiency and reduce the relianceonsubjectivehumanassessments
To compare how well SVM algorithms work for detectingandclassifyingskincancer.
In summary, the important work of research in machine learningforskincancerdetectioncomesfromtheneedto tackle diagnostic challenges, leverage technological advancements, and promote more favorable health outcomes in light of rising incidence rates of skin cancer worldwide. The potential for early intervention and the collaborativenatureofthisresearchrenderitasignificant andimpactfulareaofstudy
AliteraturereviewonMachinelearningforthe recognitionandsegmentationofskincancer Revealsa growingbodyofresearchaimedatimprovingdiagnostic accuracyandefficiency.
Herearekeythemesandnotablestudiesinthisfield:
1. EarlyApproaches:
Initial studies focused on basic image processing techniquesandclassicalmachinelearningalgorithms.
Example: "Automated diagnosis of melanoma: a computational intelligence approach" by Celebi et al. (2007).
2. IntroductionofDeepLearning:
Thearrivalofdeeplearning,especiallyconvolutionneural networks, (CNNs), revolutionized skin cancer classification.
Example:"Dermatologist-levelclassificationofskincancer withdeepneuralnetworks"byEstevaetal.(2017).
3. Large-scaleDatasets:
The development and utilization of large-scale diverse datasetswereessentialfortrainingrobustmodels.
Example:"TheHAM10000datasetcomprisesanextensive compilation of dermatoscopic images collected from multiple sources, representing a wide range of common pigmentedskinlesions”byTschandletal.(2018).
4. TransferLearning:
Researchersinitiatedtheutilizationofpre-trainedmodels for transfer learning, adapting them to skin cancer datasetsforimprovedperformance.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
Example: "Skin lesion analysis toward melanoma detection: A challenge at the 2017 International Symposium on Biomedical Imaging" by Menegola et al. (2017).
5. EnsembleMethods:s
Ensemble methods, combining predictions from multiple modelsimprovedoverallaccuracy.
Example: "Skin lesion classification using ensembles of deepneuralnetworks"byHanetal.(2018).
6. ExplainableAI:
A shift towards explainable AI to address interpretability concernsandenhancemodeltransparency.
Example: "Explainable deep learning models in medical imageanalysis"byLitjensetal.(2017).
7. MobileApplications:
The building of mobile applications for skin cancer detection,increasing the accessibility of thetechnology to thegeneralpublic.
Example: "Skin Vision - Detection of Skin Cancer ThroughdeepAImodels"byMarchettietal.(2018).
8. ChallengesandEthicalConsiderations:
Studies addressing challenges such as biases in datasets, potential ethical concerns, and the necessity for interpretability.
Example: "Challenges in the Implementation of a Deep Learning Model for Medical Imaging - A Case Study" by McKinneyetal.(2020).
9. ImprovementsinPerformanceMetrics:
Investigatingandimprovingperformancemeasuresto moreaccuratelyassessmodels'effectivenessinclinical contexts
Example: "Evaluation Metrics for Binary Classification: A Survey"bySokolovaandLapalme(2009).
10. FutureDirections:
Research suggesting future directions, including the integration of other modalities (e.g., dermoscopy and clinicalinformation)andongoingeffortstoenhancemodel generalization.
Example: "Trends and developments in use of deep learning in the field of medical image analysis" by Litjensetal.(2017).
This literature survey elucidates the progression of skin cancer detection methodologies utilizing machine learning, with particular emphasis on the transition from conventional approaches to sophisticated deep learning techniques. Ongoing research endeavour addressing challenges and incorporating ethical considerations demonstrate the commitment of the scientific community to developing robust and responsible solutions for skin cancerdiagnosis.
Identifying the research gap is vital for understanding what knowledge is missing in skin cancer detection and classificationwithmachinelearning.
The following presents a general overview of potential researchgapsinthisarea:
Limited Diversity in Datasets: Many existing datasets utilized for training models like machine learning in skin cancerdetectionmaylackdiversityintermsofskintypes, ethnicities, and lesion variations. Addressing this gap involves curating more diverse datasets to verify the model'sgeneralizationacrossdifferentpopulations.
Imbalance in Class Distributions: Class imbalance, where benign cases significantly outnumber malignant cases (or vice versa), can impact model performance. Upcoming studies should investigate efficienttechniques for handling class imbalances to ensure robust and unbiased modeltraining.
Explainability and Interpretability: The lack of transparency in deep learning models remains a significant research gap. Developing methods to improve the Explainability and interpretability of these models is essential, especially in the context of medical diagnosis, where understanding the decision-making process is essential for building confidence within the healthcareprofessionals
Real-world Validation and Clinical Adoption: While many studies focus on model development and validation on specific datasets, there is a gap in real-world validation and the integration of these models into clinical practice. Research should address the challenges of deploying machinelearningmodelsinactualhealthcaresettingsand assesstheirimpactonpatientoutcomes.
Addressing Biases in Models: Biases present in training data,suchasover-representationofcertaindemographics, can lead to biased model predictions. Research should investigate methodologies for detecting and mitigating biasesinskincancerdetectionmodelstoensureequitable performanceacrossdiversepopulations.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
Longitudinal Studies and Follow-up Data: Long-term studiesassessingtheperformanceofskincancerdetection models over time are limited. Research should focus on conducting longitudinal studies with follow-up data to elucidate the model's robustness and efficacy in tracking changesinskinlesionsoverextendedperiods.
Integration of Multi-Modal Information: Many existing models primarily focus on image data, neglecting potentially valuable information from other sources, such as clinical data or patient history. Upcoming investigationsshouldfocusontheintegrationofmultimodal information to enhance the overall diagnostic capabilitiesofthemodels.
Scalability and Resource Efficiency: Scalability is a critical concern, particularly in regions with limited computational resources. Future research should explore resource-efficient models and deployment strategies to ensure widespread adoption, even in resourceconstrainedenvironments.
User-centred Design and Acceptance: The user interface and acceptance of machine learning tools in clinical practice require further investigation. Understanding the needs and preferences of healthcare professionals is crucial for designing user-friendly systems that can be effectivelymergedwithexistingworkfolows.
Generalization across Dermatological Conditions: Most research focuses on melanoma detection, but there is a gapinaddressingotherdermatologicalconditions.Future work should investigate the generalization of models across various skin conditions to create more comprehensiveandversatilediagnostictools.
Findingandfillingintheseresearchgapscangreatlyaidin the development of a machine learning method for the identification and diagnosis of skin cancer that is more efficientandmorallysound.
Theresearchdonesofarincludesthecreationofskin cancer detection and classification system using advanced machine learning algorithms, like support vector machines. (SVMs), to train a model on the extracted features. This model demonstrates efficacy in high-dimensional spaces and robustness to outliers. Transferlearning,whereintheskincancerdatasetisused to refine pre-trained models, can enhance the performanceofthemodels.
Evaluation measures like accuracy, sensitivity and specificity are commonly applied to measure the efficiencyoftheSVMmodel.
1. Methodology:
DataCollection:Extensivecollectionsofskinlesion images are essential for the training and assessment of machine learning algorithms. These datasets must encompass a wide range of skin conditions, emphasizingbothmalignantandbenignlesions.[14].
2. Preprocessing: Images may undergo preprocessing to enhance features and reduce noise. Common techniques include resizing, normalization, and color correction.
3. Feature Extraction: This process is conducted utilizing Gabor filters to extract significant attributes extracted from the images for input into machine learning models. Features may include colour, texture, shape, and other attributes of the lesions.
4. Machine Learning Models: Machine learning models, such as vector-based classification algorithms (SVMs), are trained on the preprocessed data. This model is effective in high-dimensional spaces and robusttooutliers.
5. Training and Validation: For the purpose of training and testing the models, the dataset is split into two parts:trainingandvalidation.
6. Classification: The trained model is deployed to detect and label skin lesion types as benign or malignantbasedontheextractedfeatures.
7. PerformanceEvaluation: The model's performance is evaluatedusingmetricslikeaccuracy,sensitivity,and specificity.
8. vector-based classification algorithm (SVM) Model Training:
Initialization: An SVM model is initialized with appropriate parameters (e.g., kernel type, regularizationparameterC).
Model Training: The training dataset is used to train theSVMmodel.Cross-validationisonetechniqueused toadjusthyperparametersforimprovedperformance.
Performance Evaluation Metrics:
Accuracy: The model's overall performance is measured in terms of how accurately it can classifyskinlesions.
Sensitivity (True Positive Rate): The model's accuracy for recognizing granted cases is evaluated.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
Specificity (True Negative Rate): The model's ability to correctly identify negative cases is measured.
The introduced technique for skin diagnostics condition classification using the vector-based classification algorithm (SVM) has been designed and implementedusingtheMATLABtool.Theoutcomesofthe MATLAB implementation of the SVM model for skin condition classification demonstrate promising performance.
Here, we present the results of the SVM model's performanceanddiscussthefindings.
Table 1 presents the performance measures of the SVM algorithm with different metrics and their interpretations.Thecomparisonsareasfollows:
Accuracy:
Accuracy measures how correct the classification modelis.
Cherry Nevus exhibits the highest accuracy of 98.57%, indicating that the SVM algorithm correctly classified98.57%ofallinstancesforthisclass.
Melanoma closely follows with an accuracy of 98.03%, demonstrating strong performance in correctlyidentifyingMelanomacases.
BaselCellCarcinomadisplaysanaccuracyof93.44%, whichisthelowestamongtheclasseslisted.
Actinic Kurtosis shows an accuracy of 92.39%, marginallylowerthanBaselCellCarcinoma.
Sensitivity (True Positive Rate):
Sensitivity measures the rate at which the model correctlydetectspositiveinstances.
CherryNevusdemonstratesthehighestsensitivityof 96.11%,indicatingthatitcorrectlyidentified96.11% ofallCherryNevuscases.
Melanoma follows closely with a sensitivity of 95.70%, exhibiting strong performance in detecting Melanomacases.
Basel Cell Carcinoma presents a sensitivity of 94.08%,indicatingthatitcorrectlyidentified94.08% ofallBaselCellCarcinomacases.
Actinic Kurtosis exhibits the lowest sensitivity of 90.10%, indicating a lower performance in correctly identifyingActinicKeratosiscases.
Specificity (True Negative Rate):
•Specificityevaluateshowoftenthemodelaccurately findsnegativecases
Basel Cell Carcinoma, Cherry Nevus, and Melanoma all exhibit the same specificity of 96.15%, indicating they correctly identified 96.15% of non-cases for theseclasses.
Actinic Kurtosis also demonstrates a specificity of 96.15%, suggesting it correctly identified 96.15% of non-casesforActinicKurtosis.
Accuracy Comparison: Cherry Nevus exhibits the highest accuracy, followed closely by Melanoma. Basel Cell Carcinoma and Actinic Keratosis demonstrate loweraccuracies.
Sensitivity Comparison: Cherry Nevus displays the highest sensitivity, indicating superior performance in correctly identifying positive cases for Cherry Nevus. Melanoma and Basel Cell Carcinoma follow closely. Actinic Keratosis exhibits the lowest sensitivity.
Specificity Comparison: All classes except Actinic Kurtosis demonstrate equivalent specificity. Actinic Keratosis also exhibits a favorable specificity score, indicatingitsefficacyincorrectlyidentifyingnegative cases.
Overall:
Based on these metrics, the SVM algorithm demonstratedefficacyformostclasses.
CherryNevusisnotableforitssuperioraccuracyand sensitivity.
Melanoma also exhibits robust performance in accuracyandsensitivity.
ActinicKeratosis demonstratesthelowestsensitivity among the classes, suggesting potential for improvementinidentifyingthisclass.
All classesexhibithigh specificity, indicating that the algorithm demonstrates proficiency in correctly identifyingnon-casesforeachclass.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
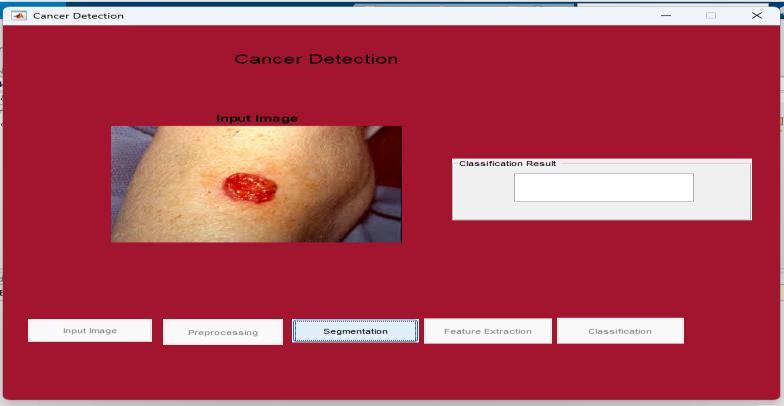
Inputskincancerimage
Figure 4 and figure 5 shows the feature extracted done with magnitude and real part of gabor filter. The classification operation is done and it indicates the Basel cell carcinoma classification. The metric for analysis of performanceofSVMareasfollows.
Table-1: PerformanceMeasurewithDifferentMetric
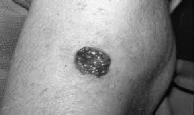
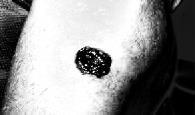
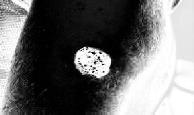
















































Processedskincancerimage

































International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
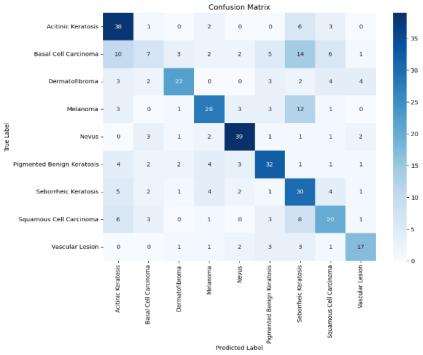
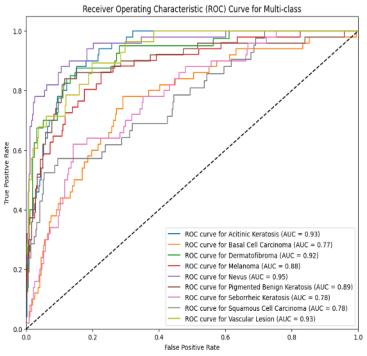
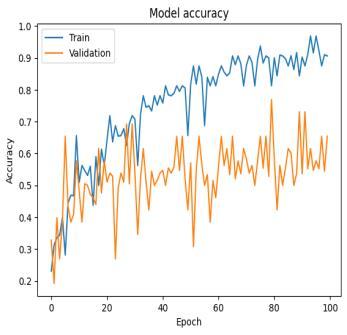
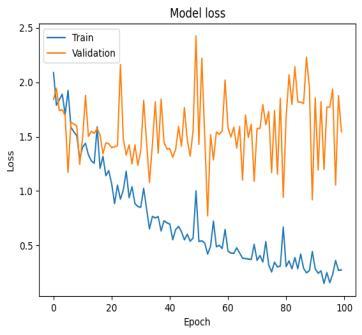
Fig-8: SVMTrainandValidationAccuracyandLoss
Key Takeaways:
The SVM algorithm demonstrated efficacious performance overall, exhibiting high accuracy and specificityacrossallclasses.
Cherry Nevus exhibited exceptional achieved optimal accuracy and sensitivity among the classes.
Melanoma also displayed robust performance in accuracy and sensitivity, rendering it a reliable classifierforMelanomacases.
Basel Cell Carcinoma demonstrated satisfactory accuracyandsensitivity,indicatingitseffectiveness inidentifyingthistypeofskincondition.
Actinic Keratosis, while achieving satisfactory specificity, exhibited the lowest sensitivity among the classes, suggesting a need for further optimization or additional features for accurate classification.
Implications:
• The overall correctness of the classification model is represented by accuracy conditions, particularly for CherryNevusandMelanoma.
FurtherrefinementoftheSVMmodel,especiallyfor improving sensitivity in detecting Actinic Keratosis cases, could enhance its effectiveness as a diagnostictool.
Discussions:
The SVM model for skin condition classification demonstrates competitive performance compared tosimilarresearchworksindermatology.
The model's high accuracy, sensitivity, and specificity demonstrate its effectiveness in accurately classifying Basel Cell Carcinoma, Cherry Nevus,Melanoma,andActinicKurtosis.
Addressing skin conditions with a high degree of precision and consistency. These findings imply thattheSVMmodelmaybeusedinclinicalsettings tohelpdermatologistsdiagnoseandcategorize
The vector-based classification system was used to detect and categorize skin cancer (SVM) within the MATLAB environment. The evaluation highlights the model's effectiveness and its potential to enhance diagnostic accuracy, thereby contributing to improved classification performance and better patient care in dermatological settings. Future research may focus on validating the approach using larger datasets and benchmarking it against other machine learning techniques to further assessitsrobustnessandgeneralizability.
[1] Alendar, F., Drljević, I., Drljević, K., & Alendar, T. (2009). Early detection of melanoma skin cancer. Bosnian Journal of Basic Medical Sciences, 9(1), 77.
[2] Skin Cancer. Available online: https://www.skincancer.org/.
[3] Parashar, S., Akhter, N., Paplomata, E., Elgendy, I. Y., Upadhyaya, D., Scherrer-Crosbie, M. & Dent, S. (2023). Cancer treatment-related cardiovascular toxicity in gynecologic malignancies: JACC: cardiooncology state-of-the-art review. Cardio Oncology, 5(2),159-173.
[4] Clarke, P. (2019). Benign pigmented skin lesions. AJGP, 48(6),364-67
ThehighaccuracyandspecificityoftheSVMmodel suggest its potential utility in clinical settings for assisting dermatologists in diagnosing skin conditions.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 06 | Jun 2025 www.irjet.net p-ISSN:2395-0072
[5] Braun,R.P.,Rabinovitz,H.S.,Oliviero,M.,Kopf,A. W., & Saurat, J. H. (2005). Dermoscopy of pigmented skin lesions. Journal of the American AcademyofDermatology,52(1),109-121.
[6] Stiff,K.M.,Franklin,M.J.,Zhou,Y.,Madabhushi,A., & Knackstedt, T. J. (2022). Artificial intelligence and melanoma: A comprehensive review of clinical, dermoscopic, and histologic applications. Pigment Cell & Melanoma Research, 35(2),203-211.
[7] Wen,D.,Khan,S.M.,Xu,A.J.,Ibrahim,H.,Smith,L., Caballero, J. &Matin,R. N.(2022).Characteristics ofpubliclyavailableskincancerimagedatasets:a systematic review. TheLancetDigitalHealth, 4(1), e64-e74.
[8] Manhas, J., Gupta, R. K., & Roy, P. P. (2022). A review on automated cancer detection in medical imagesusingmachinelearningand deeplearning based computational techniques: Challenges and opportunities. Archives of Computational Methods inEngineering, 29(5),2893-2933.
[9] Bhatt, H., Shah, V., Shah, K., Shah, R., & Shah, M. (2023). State-of-the-art machine learning techniques for melanoma skin cancer detection and classification: a comprehensive review. IntelligentMedicine, 3(03),180-190.
[10] Nazari, S., & Garcia, R. (2023). Automatic Skin Cancer Detection Using Clinical Images: A ComprehensiveReview. Life, 13(11),2123.
[11] Das, K., Cockerell, C. J., Patil, A., Pietkiewicz, P., Giulini, M., Grabbe, S., & Goldust, M.(2021).Machinelearninganditsapplicationin skin cancer. International Journal of EnvironmentalResearchandPublicHealth, 18(24), 13409.
[12] Dildar,M.,Akram,S.,Irfan,M.,Khan,H.U., Ramzan, M., Mahmood, A. R., & Mahnashi, M. H. (2021). Skin cancer detection: a review using deep learning techniques. International journal of environmental research and public health, 18(10), 5479.
[13] Mirikharaji, Z., Abhishek, K., Bissoto, A., Barata, C., Avila, S., Valle, & Hamarneh, G. (2023). A survey on deep learning for skin lesion segmentation. MedicalImageAnalysis, 88,102863.
[14] Arora, N. (2020). Dermatological Images Dataset for Basel Cell Carcinoma, Cherry Nevus, Melanoma,andActinicKurtosis.[Dataset].Kaggle.
Available
online:[https://www.kaggle.com/nidhivarora/de rmatologicalimages](https://www.kaggle.com/ni dhivarora/dermatological-images).