
















Reference: 1. Nicorette® Lozenge SmPC.
Nicorette Cools 2 mg Lozenge. Composition: Each lozenge contains 2 mg nicotine (as nicotine resinate). Compressed lozenge (lozenge). Indications: to be used for the treatment of tobacco dependence by relief of nicotine withdrawal symptoms and cravings in smokers 18 years and above. Permanent cessation of tobacco use is the eventual objective. Nicorette Cools 2 mg Lozenges should preferably be used in conjunction with a behavioural support programme. Dosage: Selecting the strength of lozenge to be used will depend on the smoking habits of the individual. Adults: Nicorette Cools 2 mg Lozenges are suitable for smokers with low nicotine dependency e.g. those smoking their first cigarette of the day more than 30 minutes after waking up or those who smoke 20 cigarettes or less per day. Lozenges should not be used for more than 9 months. If users still feel the need for treatment, a healthcare professional should be consulted. Behavioural therapy advice and support will normally improve the success rate. Abrupt cessation of smoking: The patient should make every effort to stop smoking completely during treatment with Nicorette Cools Lozenges. The lozenges should be used whenever there is an urge to smoke. Sufficient lozenges should be used each day and most smokers usually require 8 to 12, not to exceed 15 lozenges. The duration of treatment is individual, but up to six weeks treatment is recommended to break the habit of smoking. The nicotine dose should then be gradually reduced, by decreasing the total number of lozenges used per day. The treatment should be stopped, when the daily consumption is down to 1-2 lozenges. Use a lozenge whenever there is an urge to smoke to maintain complete abstinence from smoking. In the event of sudden cravings any spare lozenges should be retained and used whenever there is a craving or an urge to smoke. Gradual cessation through progressive reduction in smoking: For smokers who are unwilling or unable to quit abruptly. Use a lozenge between smoking episodes to manage the urge to smoke, to prolong smoke-free intervals and with the intention to reduce smoking as much possible. The number of lozenges a day is variable and depends on the patients needs. Nonetheless it should not exceed 15 lozenges per day. If a reduction in number of cigarettes per day has not been achieved after 6 weeks, professional advice should be sought. Reduced tobacco consumption should lead to complete cessation of smoking. A quit attempt should be made as soon as the smoker feels ready, but not later than 6 months after start of treatment. When the number of cigarettes has been reduced to a level from which the user feels able to quit completely, then the schedule for “abrupt cessation” as given above should be started. If the attempt to stop smoking completely has not been started within 6 months after the beginning of treatment, it is recommended to consult a healthcare professional. Paediatric population: Nicorette Cools 2 mg Lozenges should only be used by adolescents (12-17 years inclusive) with advice from a healthcare professional. Nicorette Cools Lozenges are not to be used by children below the age of 12. The safety and efficacy of Nicorette Cools 2 mg Lozenges in children and adolescents has not been investigated. One lozenge should be placed in the mouth and allowed to dissolve. Periodically, the lozenge should be moved from one side of the mouth to the other, and repeated, until the lozenge is completely dissolved (approximately 16-19 minutes). The lozenge should not be chewed or swallowed whole. Users should not eat or drink while a lozenge is in the mouth. Contraindications: Hypersensitivity to nicotine or to any of the excipients. Children under the age of 12 years. Those who have never smoked. Special warnings and precautions for use: The benefits of quitting smoking usually outweigh any risk associated with correctly administered nicotine replacement therapy (NRT). A risk-benefit assessment should be made by an appropriate healthcare professional for patients with the following conditions: Cardiovascular disease: Dependent smokers with a recent myocardial infarction, unstable or worsening angina including Prinzmetal’s angina, severe cardiac arrhythmias, recent cerebrovascular accident and/or who suffer with uncontrolled hypertension should be encouraged to stop smoking with nonpharmacological interventions (such as counselling). If this fails, Nicorette Cools 2 mg Lozenge may be considered but as data on safety in this patient group are limited, initiation should only be under close medical supervision. Diabetes Mellitus. Patients with diabetes mellitus should be advised to monitor their blood sugar levels more closely than usual when smoking is stopped and NRT is initiated as reduction in nicotine induced catecholamine released can affect carbohydrate metabolism. Allergic reactions:
Susceptibility to angioedema and urticaria. Renal and hepatic impairment: Use with caution in patients with moderate to severe hepatic impairment and/or severe renal impairment as the clearance of nicotine or its metabolites may be decreased with the potential for increased adverse effects. Phaeochromocytoma and uncontrolled hyperthyroidism: Use with caution in patients with uncontrolled hyperthyroidism or phaeochromocytoma as nicotine causes release of catecholamines. Gastrointestinal Disease: Swallowed nicotine may exacerbate symptoms in patients suffering from oesophagitis, gastric or peptic ulcers and oral NRT preparations should be used with caution in these conditions. Seizures: Use with caution in subjects taking anti-convulsant therapy or with a history of epilepsy as cases of convulsions have been reported in association with nicotine. Lozenges can represent a choking hazard. Use with caution in individuals with aspiration and swallowing problems. Paediatric population: Danger in children: Doses of nicotine tolerated by smokers can produce severe toxicity in children that may be fatal. Products containing nicotine should not be left where they may be handled or ingested by children. Stopping Smoking: Polycyclic aromatic hydrocarbons in tobacco smoke induce the metabolism of drugs metabolized by CYP 1A2 (and possibly by CYP 1A1). When a smoker stops smoking, this may result in a slower metabolism and a consequent rise in blood levels of such drugs. This is of potential clinical importance for products with a narrow therapeutic window, e.g. theophylline, tacrine, clozapine and ropinirole.
Transferred dependence: Transferred dependence is unusual and is both less harmful and easier to break than smoking dependence. Excipients: This medicine contains less than 1 mmol sodium (23 mg) per lozenge, that is to say essentially ‘sodiumfree’. Undesirable effects: Effects of smoking cessation: Regardless of the means used, a variety of symptoms are known to be associated with quitting habitual tobacco use. These include emotional or cognitive effects such as dysphoria or depressed mood; insomnia; irritability, frustration or anger; anxiety; difficulty concentrating, and restlessness or impatience. There may also be physical effects such as decreased heart rate; increased appetite or weight gain, dizziness or presyncopal symptoms, cough, constipation, gingival bleeding or aphthous ulceration, or nasopharyngitis. In addition, and of clinical significance, nicotine cravings may result in profound urges to smoke. The Nicorette Cools Lozenge may cause adverse reactions similar to those associated with nicotine given by other means. Most of the undesirable effects reported by the subjects occur during the early phase of treatment and are mainly dose dependent. Irritation in the mouth and throat may be experienced, however most subjects adapt to this with ongoing use. Allergic reactions (including symptoms of anaphylaxis) occur rarely during use of Nicorette Cools Lozenge. Immune System Disorders Common Hypersensitivity Not known Allergic reactions including angioedema and anaphylaxis Psychiatric disorders Uncommon Abnormal dream Nervous system disorders Very common Headache Common Dysgeusia, paraesthesia Unknown Seizure* Eye disorders Not known Blurred vision, lacrimation increased Cardiac Disorders Uncommon Palpitations, tachycardia, atrial fibrillation Vascular disorders Uncommon Flushing, hypertension Respiratory, thoracic and mediastinal disorders Very common Cough, hiccups, throat irritation Uncommon Bronchospasm, dysphonia, dyspnoea, nasal congestion, oropharyngeal pain, sneezing, throat tightness Gastrointestinal disorders Very common Nausea, mouth/throat and tongue irritation Common Abdominal pain, dry mouth, diarrhoea, dyspepsia, flatulence, salivary hypersecretion, stomatitis, vomiting, heartburn Uncommon Eructation, glossitis, oral mucosal blistering and exfoliation, paresthesia oral Rare Dysphagia, hypoaesthesia oral, retching Not known Dry throat, gastrointestinal discomfort, lip pain Skin and subcutaneous tissue disorders Uncommon Hyperhidrosis, pruritus, rash, urticaria Not known Erythema General disorders and administration site conditions Common Burning sensation, fatigue Uncommon Asthenia, chest discomfort and pain, malaise. * Cases of seizures have been reported in subjects taking anti-convulsant therapy or with a history of epilepsy. Marketing Autorisation Holder: JNTL Consumer Health I (Ireland) Ltd., Block 5, High Street, Tallaght, Dublin 24, Ireland. PA Number: PA23490/019/011. Date of revision of text: June 2024. Product not subject to medical prescription. Supply through pharmacy and non-pharmacy outlets. Further information available upon request.



Page 5: David appointed Managing Director at United Drug
Page 6: Minister for Health launches new four-year corporate strategy of the PSI
Page 10: The National Immunisation Office Strategic Plan 2024-2027
Page 12: PHX Ireland’s Two-Year Fundraising Journey with ISPCC
Page 16: Getting your Pharmacy Business Ready for Sale
Page 26: What is Parkinson’s Disease
Page 38: Revolutionising the Dispensing Process
Page 48: OTC Retail Pharmacy Product Award winners 2023
PUBLISHER:
IPN Communications
Ireland Ltd.
Clifton House, Fitzwilliam Street Lower, Dublin 2 00353 (01) 6690562
MANAGING DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood: 00353 (87)737 6308 kelly-jo@ipn.ie
SALES MANAGER
Amy Evans | amy@ipn.ie 0872799317
EDITORIAL/ EVENTS & MARKETING EXECUTIVE
Aoife Hunter: aoife@ipn.ie
CONTRIBUTORS
Dr Lucy Jessop
Noel Winters
Sinead Fitzgerald
Mona Thornton
Lisa Wynne
Dr Ella Patchett
Dr Karl Hazel
Martin Hynes
Desmond J. Tobin
Professor Shirley Potter
DESIGN DIRECTOR
Ian Stoddart Design





12

As Irish Pharmacy News was going to press, it was announced that the Pharmacy Regulator, the Pharmaceutical Society of Ireland is responding to a call from the Minister for Health to support enhanced price transparency in pharmacies.
One of the roles of the PSI is promoting good professional practice by pharmacists by raising standards and sharing information for the benefit of patients and the wider health system. They do this in a variety of ways, including the issuing of statutory guidelines.
A statement said, “While the pricing of medicines and the fees set by pharmacies is outside of our remit, the PSI considers that transparency in pricing for patients and members of the public is desirable as an aid to empowering them to make informed decisions about their care. We have previously set out our position on this matter on our website.
“We have been requested by the Minister for Health to consider the development of appropriate guidelines so that patients have an improved understanding of the fees that they pay in respect of the products that are dispensed and the services they use in pharmacies.
“We will now commence the process of developing guidelines for pharmacists and pharmacies on price transparency that will support patient empowerment, while ensuring that any information on pricing, is factual, encourages the safe and rational use of medicines, and in line with relevant legislation. The guidelines will undergo public consultation and will be subject to approval by the Council of the PSI.”
In other news, the PSI has launched the new four-year corporate strategy, which outlines its objectives to ensure continued trust in pharmacy and to safeguard patient and public safety over the next four years. You can read more about this on page 6.
This issue also features the winners of the 2025 OTC & Retail Pharmacy Product Awards. These Awards serve as a platform to celebrate and showcase excellence in product innovation, marketing, and customer value. Since their launch in 2008, they have been committed to highlighting advancements and innovation within the pharmacy sector.
The OTC Awards highlight excellence in product innovation and marketing—key factors that help pharmacists enhance customer value. Our coverage starts on page 48.
Huge congratulations to all the winners!
Irish Pharmacy News is circulated to all independent, multiple Pharmacists and academics in Ireland. All rights reserved by Irish Pharmacy News. All material published in Irish Pharmacy News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd. has taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
FEATURE: PARKINSON’S DISEASE P34
CPD: MANAGEMENT OF COUGH P41
FEATURE: IRRITABLE BOWEL DISEASE P34
TRADE SHOW: COSMETIC ASSOCIATION P74
FEATURE: IRISH MELANOMA FORUM P78
CLINICAL PR: P80
The number of practicing pharmacists in Ireland in 2024 has increased 8.8% from 2023, new research has revealed.

The Minister for Health Jennifer Carroll MacNeill has recently highlighted increases in the healthcare workforce and in healthcare activity as the latest national data tables on nonmonetary healthcare statistics are published. This data allows researchers and the wider public timely access to national information and provides them with a better understanding of Ireland’s data on a range of health topics.
The tables published include national healthcare statistics compiled by the Department of Health as part of the NonMonetary Health Care Statistics questionnaire, administered jointly by Eurostat, the OEC and the World Health Organization (WHO). This will be the third edition of the annually published tables, prior to their release by Eurostat and the Organisation
for Economic Co-operation and Development (OECD).
Minister Carroll MacNeill said, "Data for 2024 shows a steady increase in the healthcare workforce in Ireland. There is a 4% rise in physicians, a 3% rise in midwives, and a 6% rise in nurses compared to 2023. Additionally, there has been a consistent increase in graduates in these fields.
“The increase in the number of practicing healthcare professionals and the compiled surgical procedure statistics reflect this government's commitment to enhance the quality of healthcare services in Ireland, and ensure the best outcomes for patients.
"Publishing these national healthcare statistics also highlights our commitment to transparency and the
dissemination of vital health information. By providing a means of easy access to this data, we are empowering researchers and the public with the tools they need to gain a deeper understanding of Ireland’s health service."
The data shows:
• the number of practicing nurses in Ireland in 2024 was 76,558, an increase of 5.5% from 2023
• the number of practicing pharmacists in Ireland in 2024 was 6,250, an increase of 8.8% from 2023
• graduates in health-related fields of study amounted to a total of 3,455 students in 2023, an increase of 1.6% from the previous year. These fields of study include medicine, nursing, midwifery, dentistry and pharmacy
As of 19 March, the nomination process for pharmacist members interested in appointment to the Pharmaceutical Society of Ireland (PSI) Council has been completed. Five positions are available for pharmacists on the Council in 2025 and 17 nominations have been received. As a result, an election will be held on 16 April, 2025. The candidate names are available along with information about the process of appointment.
Registered pharmacists are entitled, and are encouraged, to participate in the postal ballot. Ballot papers will be sent at the start of April and must be returned, following the instructions provided, to the PSI Returning Officer by Wednesday, 16 April.
The Minister for Health is responsible for the appointment of all Council members in the public interest. Council members are appointed to serve a four-year term.
"From theory to therapy: Balancing evidence in managing common ailments" is the title of a new International Pharmaceutical Federation (FIP) Insight Board report launched recently.
It explores how pharmacists apply scientific evidence in community pharmacy practice, such as in pain management, respiratory care, and reflux treatment.
The report identifies key challenges, such as variability in clinical guidelines, limited access to real-world data, and commercial pressures on medicine adoption. It also outlines strategies to improve decision-making through continuous professional development (CPD), regulatory advancements, and digital tools.
Evidence-based practice is commonly defined as “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients”. In pharmacy practice, this means that medicationrelated decisions are based on a combination of scientific research, professional expertise, and patient preferences, rather than solely on clinical experience or tradition. This ensures that pharmacists deliver care that is both data-driven and patient centred, improving treatment outcomes while adapting to individual patient needs and local healthcare systems.
Recognising the need to translate evidence into actionable pharmacy practice, FIP convened an insight board discussion titled “From theory to therapy: Balancing evidence in managing common ailments— pain, respiratory, and reflux management.” The discussion brought together community pharmacists and experts from FIP’s global network, including representatives from the Community Pharmacy Section (CPS), Hospital Pharmacy Section (HPS), Data and Intelligence Commission (D&I), Academic Pharmacy Section (AcPS), Technology Advisory Group (TAG), and FIP HUB leads.
The objective was to discuss how pharmacists integrate different types of evidence into their daily decision-making processes.
A new Artificial Intelligence (AI) Toolkit for Pharmacy has been launched by the International Pharmaceutical Federation (FIP) to help pharmacists integrate AI into daily practice.
Developed by the FIP Technology Advisory Group and its Working Group on AI, the toolkit provides structured guidance on ethical considerations, implementation strategies, regulatory compliance, and real-world applications to optimise patient care and workflows.
It also addresses key challenges such as variability in clinical guidelines, data privacy concerns, and commercial pressures, offering practical strategies, including continuous professional development, regulatory advancements, and digital tools.
AI is revolutionising healthcare by enabling the analysis of vast amounts of medical data, assisting in clinical decisionmaking, personalising patient treatments, predicting disease outbreaks, and optimising operational workflows. Closely aligned with FIP Development Goal 20 (Digital Health), AI helps build a digitally competent pharmacy workforce, improving clinical care, disease screening, health systems management, and pharmaceutical research. Nonetheless, pharmacists face critical challenges, including data privacy, cybersecurity threats, potential algorithm biases, and ethical concerns.
"This toolkit bridges the gap by offering a clear implementation guide, making AI accessible and actionable," said Chair of the AI Working Group Whitley Yi.
"The AI Toolkit empowers pharmacists with cutting-edge tools to enhance patient care, streamline workflows, and embrace the future of pharmacy practice,” said Chair of the FIP Technology Advisory Group Lars-Åke Söderlund.
This toolkit aims to serve as a comprehensive resource, enabling pharmacists to navigate the complexities of AI, capitalise on its potential, and clearly understand its limitations. By fostering innovation, collaboration, and a proactive approach, FIP seeks to enhance AI's role in pharmacy practice, ultimately driving improvements in patient care and global health outcomes.
David Keyes has been appointed as the new Managing Director of United Drug in Ireland, effective April 1st, 2025.
David has been serving as the Director of United Drug Distributions and Head of Public Affairs for PHX Ireland since 2020, where he played a pivotal role in the development and implementation of the organisation’s pan-European commercial strategy.
Having first joined United Drug in 2012, David has provided strategic leadership across multiple areas of the business, including PreWholesale, Government Contracts, and Public Affairs.
Upon the announcement, Paul Reilly, CEO of PHX Ireland, said: “We are delighted to welcome David into his new role as Managing Director of United Drug in Ireland. David brings with him a wealth of industry knowledge, a steadfast commitment to service excellence, and a proven ability to navigate complex healthcare environments. His expertise and leadership will be instrumental as we continue to drive growth and strengthen our supply chain operations across Ireland.”
On his appointment, David said, “I’m excited to take on this new role and opportunity with the talented team at United Drug. We
David Keyes, Managing Director, United Drug Ireland
David Keyes, Managing Director of United Drug Ireland with colleagues Elaine Condon, Hillary Collins and Jan-Pieter Hallema

have a strong foundation, and I’m committed to continuing to drive the business forward, ensuring we meet the evolving needs of our customers and partners across Ireland. Our priority now is to

ensure that United Drug is best positioned for continued growth, operational excellence, and service delivery, as we meet the evolving needs of our customers and partners across Ireland.”
International Women’s Day was held last month (8 March), and community pharmacies across Ireland reaffirmed their commitment to immediate, expert, and personalised healthcare for women in every community. Pharmacists are the most accessible healthcare professionals in Ireland, providing trusted advice and essential treatments ensuring women receive the education, support, and care they need to lead healthier lives.
According to Susan O’Dwyer, the IPU’s Head of Professional Services, “Women often face challenges in accessing healthcare due to time constraints, affordability, or lack of awareness about available resources.
Community pharmacies address these concerns by offering convenient services that usually do not require an appointment. From emergency and ongoing contraception services to cardiovascular risk assessment and menopause support, pharmacists provide expert guidance tailored to every stage of a woman’s life.”
Safe Pharmacy
Beyond providing essential healthcare services, community
pharmacies also serve as a vital support network for women facing dangerous situations, including domestic abuse. Across Ireland, more than 1,000 ‘Safe Pharmacies’ provide a crucial support to women experiencing domestic abuse and/ or coercive control by offering a secure, discreet place to seek help and support from local services.
Ms. O’Dwyer highlighted the importance of these initiatives, stating, “Unfortunately, violence against women and girls remains a serious issue in our society. For those in domestic abuse and coercive control situations, accessing support services can be challenging, particularly as phones and general movements are often monitored. With pharmacies located in every
town in Ireland, they provide a discrete and accessible location for anyone in need of support. No woman should suffer in silence. Safe Pharmacies are in every community, ready to provide support when it is needed most.”
Ms. O’Dwyer concluded, “Community pharmacies are at the heart of local healthcare, providing women with immediate access to expert advice, essential health services, and preventative care. This International Women’s Day, as we reaffirm our dedication to supporting women’s health, we urge every woman to make her health a priority—because support, advice, and expert care are just a pharmacy visit away.”
The Minister for Health Jennifer Carroll MacNeill TD has launched the new four-year corporate strategy of PSI, the Pharmacy Regulator, which outlines its objectives to ensure continued trust in pharmacy and to safeguard patient and public safety over the next four years.

The strategy covers a period of significant change in the pharmacy and healthcare landscape in Ireland, which is shaping the PSI agenda. A key focus for the regulator is its contribution to the implementation of the recommendations of the Department of Health’s Expert Taskforce to Support the Expansion of the Role of Pharmacy*. The recommendations have sought to align services and practices that can be delivered by pharmacists and pharmacies, providing an enhanced role for pharmacy within an integrated healthcare system.
The strategy also references the roll-out of much-anticipated advances for digital health in Ireland and the impact this will have on pharmacy practice, data management and the patient benefits through initiatives including the National Shared Care Record and ePrescribing. It also outlines how the pharmacy regulator, as part of a multistakeholder approach to future workforce planning, is taking proactive steps to ensure the sustainability of pharmacists available to provide access to safe and effective pharmacy services to the public, now and into the future.
Further to this, it sets out the actions the pharmacy regulator intends to take as part of a commitment to evolve its regulatory approach. Reflecting on the broader landscape, the PSI believes there is a strong imperative to update the regulatory
model for pharmacy, including progressing its programme of reform of the current Pharmacy Act and reviewing its disciplinary and complaint-handling processes to make them more person-centred and efficient.
Speaking at the launch, PSI President Katherine Morrow spoke of the regulator’s focus on continued engagement with all relevant stakeholders to progress its work, including pursuing even greater collaboration with the public in support of positive patient outcomes.
Minister for Health Jennifer Carroll MacNeill TD said, “I welcome the PSI setting out their strategic direction as it continues to provide effective regulation that helps ensure patient safety and public trust in pharmacy.
“The lifetime of the PSI’s new strategy will be an exciting time for pharmacies and pharmacists. I see it as a period with much opportunity for expansion, reform and new possibilities for a wider scope of practice, innovative services, and new business approaches, all leading to better patient care and access.
Minister for Health Jennifer Carroll MacNeill, President of the PSI Council Katherine Morrow and Registrar and Chief Officer Joanne Kissane
recommendations. It is an exciting time, and, together with others, we want to make sure that such changes come to fruition and are successful, sustainable and safe.
MacNeill
Joanne Kissane
“I am committed to working with the PSI and with the sector throughout 2025 and beyond to bring this ambition to life. Together, we can significantly empower both our pharmacy workforce and the public by increasing access to care in the community.”
President of the PSI Katherine Morrow commented, “Our mission is to assure patient safety and public trust in pharmacy through effective regulation. Our new strategy is an ambitious roadmap aligning with a period of significant change for pharmacy within an evolving healthcare landscape. At the heart of what the PSI does is the commitment to ensure the ongoing provision of safe, quality pharmacy care across almost 1,900 pharmacies, and that all pharmacists registered with the PSI, whose number now exceeds 7,700, continue to deliver the high standards of care and professionalism that is expected.
“We are focused on positive patient outcomes. We are committed to supporting pharmacists and pharmacies to provide high quality care and service, including through times of change, such as those envisaged through the Department of Health’s Taskforce
“The PSI embraces the opportunity to support national healthcare initiatives, with the view that doing so will deliver beneficial results for patients and the broader healthcare system, including the people who are providing pharmacy care and services.”
Registrar and Chief Officer PSI Joanne Kissane commented,
“Developed in consultation with stakeholders across pharmacy and health, the PSI Council is keen to see that this strategy prepares PSI, and those registered and regulated by us, for significant changes due to take place. These will ultimately benefit patients in the long term. Our aim is to align our regulatory systems and supports to underpin this evolution in pharmacists’ scope of practice.
“The successful delivery of pharmacy practice changes hinges on a committed, empowered, and engaged patient-facing pharmacist workforce. Our examination of workforce data over the past three years provides crucial data specific to pharmacists in Ireland, supporting strategic workforce planning for the profession into the future. Further to our work with others, including the Department of Health, we are committed to actions that seek to mitigate workforce challenges, with a view to the future of healthcare provision and to meet patient needs.”




Medicines for Ireland (MFI) has warned about the devastating impact of the cost of implementation of the Urban Wastewater Treatment Directive (UWWTD) on the supply of the majority of medicines for Irish patients.
The warning comes after several generic medicines suppliers, supported by Medicines for Europe, filed a legal case with the Court of Justice of the European Union against the creation of an Extended Producer Responsibility (EPR) scheme under the Urban Wastewater Treatment Directive (UWWTD). The legal action seeks to avoid a discriminatory and disproportionate cost burden on generic medicines, therefore safeguarding patient access to vital medicines.
Generic medicines are the mainstay of most patients medicine cabinets, they generally are the lowest cost medications, representing nearly 60 per cent of all prescriptions in 2023. As such, it’s anticipated that generic medicine suppliers may be expected to shoulder the bulk of the financial cost under the EPR scheme for removing micropollutants from urban wastewater. This fails to recognise the extremely tight margins given the pricing models for generic medicines.
Donagh O’Leary, chair of the MFI UWWTD working group said: “Based on the modelling conducted by our colleagues in Medicines for Europe, the real impact of the UWWTD will lead to a tsunami of medicine shortages.
We are extremely concerned that the UWWTD will place disproportionate financial and operational burdens on the generic pharmaceutical industry, hindering the supply of medicines to millions of Irish patients.”
“The directive’s flawed Extended Producer Responsibility (EPR) system unfairly targets pharmaceutical companies, forcing them to cover the lion’s share of costs for removing residues from wastewater. We know that these residues originate from many other sectors beyond pharma and the establishment of an EPR under the Directive must recognise this to spread the cost fairly. We are urgently calling for the Commission to undertake a new impact assessment. In addition, an urgent review of the UWWTD is needed to ensure patient access to essential medicines is not burdened by cost.”
The European Commission estimates ¤1.2 billion annually for EU-wide Extended Producer Responsibility (EPR) in its impact assessment. However, the feasibility study used for the impact assessment only assessed 12 molecules, of which hazardousness data was not available for 7 of them. This is not a reliable sample for the thousands of pharmaceutical molecules.
National regulators and the water industry have estimated much higher costs than the European Commissions. For example, the German Environment Agency estimates costs for Germany alone at ¤885 million – ¤1.025 billion annually, nearly four times the Commission’s projection of ¤238 million. EurEau estimates annual per capita costs at ¤8 – ¤25, translating to EU-wide costs of up to ¤11.3 billion annually. These discrepancies highlight the need for accurate cost assessments to avoid jeopardising medicine supply.
Mr O’Leary concluded: “MFI and our colleagues in Medicines for Europe fully support initiatives to improve environmental sustainability. However, while the EPR framework claims to encourage the development of “greener” medicines, it fails to recognise the complexities of pharmaceutical design, where reformulation is often impractical without compromising the safety and effectiveness of the medicine. MFI remains committed to working with all stakeholders to develop practical and sustainable solutions that safeguard the environment and patient access to essential medicines.”

HSE CEO Bernard Gloster has opened the first of the HSE’s new regional Research Directorates, at St Camillus’ Hospital Limerick, hailing the initiative as a key HSE commitment to advancing the delivery of modern healthcare.
“The best medical care in the world,” he said, “is built upon the latest scientific knowledge, and this knowledge is constantly being added to through research. Healthcare research makes new discoveries, it drives improvement, it makes treatments more effective and processes more efficient. Whether its focus is on technology, surgery, medicine or the patient experience, research helps improve health and social care for our patients and clients, and the HSE, through its Regional Directorates, is committed to supporting research and embedding it across our services.”
The Research Directorate Mid West, led by Prof Declan Lyons, will oversee all aspects of clinical research within the HSE locally, with the aim of promoting the advance of scientific knowledge and medical treatments.
At the official opening Professor Lyons said the opening of the Directorate was, “An important step for the HSE in driving the research agenda forward. In collaboration with our academic partners, our mission in the Mid West is to facilitate reliable, high quality, and innovative research across a range of fields relevant to the people of the locality”
He cited as an exemplar of such research the recent trial of a comprehensive multidisciplinary assessment of older patients living with frailty who present to the Emergency Department (ED) at University Hospital Limerick. Led by consultant geriatrician Dr Aoife Leahy, the research identified key benefits for patients, including reduced wait times in ED, fewer hospital admissions, lower rates of ED re-attendance and fewer nursing home admissions. Patients also reported better quality of life and function at 180 days.
Topical antibiotic treatment of infections, including impetigo, caused by sensitive micro-organisms, in particular Staphylococcusaureus.
Abbreviated Prescribing Information for Finovare can be found below The Summary of Product Characteristics and Patient Information Leaflet can be found at www.hpra.ie

Abbreviated Prescribing Information
Product Name: Finovare 20mg/g Cream. Active Ingedient: Each gram of cream contains 20 mg fusidic acid. Presentation: Cream. A white cream. Indications: Topical antibiotic treatment of infections, including impetigo, caused by sensitive microorganisms, in particular Staphylococcus aureus Posology and method of administration: Apply three times daily or as required. Less frequent application may be adequate for skin covered by a sterile dressing. Cutaneous use. Contraindications and Warnings: Hypersensitivity to the active substance or to any of the excipients .Bacterial resistance among Staphylococcus aureus has been reported to occur with the use of topical fusidic acid. As with all antibiotics, extended or recurrent use of fusidic acid may increase the risk of developing antibiotic resistance. Finovare cream contains cetyl alcohol, butylhydroxyanisole and potassium sorbate which may cause local skin reactions such as contact dermatitis. Butylhydroxyanisole may also cause irritation to the eyes and mucous membranes. If used on the face, care should be taken to avoid contact with the eyes. Finovare can be used during breast-feeding but it is recommended to avoid applying topical Finovare directly on the breast. Undesirable effects: Immune system disorders: Rare: Hypersensitivity. Eye disorders: Rare: Conjunctivitis. Skin and subcutaneous tissue disorders. Uncommon: Dermatitis (including dermatitis contact, eczema), Rash*, Pruritus, Erythema. * Various types of rash reactions such as erythematous, pustular, vesicular, maculo-papular and popular have been reported. Rash generalised has also occurred. Rare: Angioedema, Urticaria, Blister. General disorders and administration site conditions: Uncommon: Application site pain (including skin burning sensation). Application site irritation. Paediatric population: Frequency, type and severity of adverse reactions in children are expected to be the same as in adults. Reporting suspected adverse reactions after authorisation of the medicinal product is important. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Legal Category: POM. Marketing Authorisation Holder: Citrine Healthcare Limited, Orchard Road, Clondalkin, Dublin 22, D22 V4H1, Ireland.Marketing Authorisation Number: PA23214/001/001. Date of Preparation: January 2024
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie). Adverse events should also be reported to Citrine Healthcare Ltd – info@citrinehealthcare.com; 01-4457206




Targeted skin care for dry and flaky skin.




Ensuring equitable access to immunisation and strengthening vaccine programmes are critical to protecting public health in Ireland. The National Immunisation Office (NIO) Strategic Plan 2024-2027 sets out a targeted approach to enhancing immunisation services, addressing emerging challenges, and supporting healthcare professionals in delivering high quality, evidence based vaccination programmes. With a focus on collaboration, innovation, and equity, the strategy outlines key priorities to improve vaccine uptake, align immunisation governance structures, and strengthen preparedness for emerging infectious threats.

Strengthening Immunisation in Clinical and Hospital Settings
Immunisation remains one of the most effective public health interventions, with significant implications for hospital professionals. The COVID-19 pandemic underscored the importance of strong vaccination strategies, showcasing both successes in uptake and areas requiring continued attention. However, challenges persist, including declining childhood immunisation rates, the emergence of new infectious threats, and pressures on the healthcare system. The strategy underscores the importance of collaboration with healthcare providers across all sectors to enhance vaccine access, address coverage gaps, and improve immunisation uptake.
The 2024-2027 plan focuses on six core objectives, each addressing key areas of vaccine administration and public health protection:
Written by Dr Lucy Jessop, Director, National Immunisation Office
1. Enhancing Childhood Immunisations: Achieving a 95% uptake of routine childhood vaccines is a national priority to prevent outbreaks of vaccine preventable diseases. Healthcare professionals play a crucial role in identifying under immunised patients, supporting catch up vaccination efforts, and promoting vaccine confidence among families.
2. Improving School Based Vaccination Programmes: Strengthening the uptake of school delivered vaccines, including HPV and flu vaccines, is essential to meeting WHO targets for disease prevention. The strategy focuses on maximising vaccine access and ensuring equitable delivery across all regions.
3. Optimising Adult Immunisation Strategies: Hospital based healthcare professionals are central to protecting vulnerable populations, including older adults, pregnant individuals, and those with chronic conditions.
The strategy aims to increase influenza vaccine uptake to 75% in eligible groups and explore opportunities to enhance immunisation efforts for high risk populations such as gay bisexual Men who have sex with Men (gbMSM) in the LGBTQIA+ community.
4. Integrating COVID-19 Vaccination into Routine Care: While COVID-19 is no longer classified as a public health emergency, it remains a significant concern. The NIO Strategic Plan aims to align COVID-19 vaccination with routine immunisation structures where possible, ensuring continued access for at risk groups and supporting healthcare workers in maintaining vaccine uptake.
5. Enhancing Preparedness for Emerging Infectious Threats: The COVID-19 pandemic highlighted the need for strengthened vaccination systems and preparedness strategies. The NIO Strategic Plan emphasises a proactive approach to infectious disease threats, including Mpox and future novel pathogens, through robust surveillance, effective vaccine distribution, and coordinated public health responses.
6. Leveraging Data and Technology for Informed Immunisation Planning: Access to accurate and timely immunisation data is critical for public health decision making. The development of the National Immunisation Information System (NIIS) will enhance real-time tracking of vaccine coverage, facilitate targeted interventions, and support evidence based policy development.
Collaboration is fundamental to delivering effective immunisation programmes. The engagement of hospital staff, general practitioners, nurses, and pharmacists plays a vital role in both vaccine administration and promotion
of and increasing public trust in immunisations. The NIO is committed to supporting healthcare providers with clear, evidence based guidance and training to improve vaccine confidence and counter misinformation.
As Ireland strengthens its immunisation programmes, healthcare professionals across hospital and community settings play a critical role in supporting vaccine uptake and public confidence. By ensuring vaccines are accessible, addressing patient’s questions, and integrating immunisation into routine care where appropriate, healthcare providers contribute to a strong and equitable immunisation system. The NIO remains committed to providing evidence based guidance and resources to support these efforts, including training on how to speak to people about immunisations using motivational interviewing.
For further details, access the full National Immunisation Office Strategic Plan 2024-2027 at www.immunisation.ie.
HSE National Immunisation Office have a new eLearning course 'Talking About Immunisation' to support health professionals and vaccine advocates. The course provides an understanding of how to effectively communicate with people who may be hesitant about vaccinations. Visit: http://hseland.ie
European Immunisation Week, taking place on April 27th, is an opportunity for healthcare professionals to advocate for vaccines and raise awareness about their importance. This year, hospital staff can support the week by engaging with our social media channels and sharing content from our #HumanlyPossible campaign.
Social Media: @hseimm on Twitter & Instagram | National Immunisation Office on YouTube





PHX Ireland have a commitment to supporting the communities they serve, which goes beyond healthcare.

Over the past two years, they have proudly partnered with ISPCC as their chosen charity, working together to make a meaningful impact on the lives of children and young people across Ireland. Through the dedication and generosity of colleagues across all business units, the team are delighted to share that they have raised an incredible ¤50,000 in 2024 for this vital cause.
Selected as the charity partner in 2022, ISPCC’s Childline service resonated deeply with PHX Ireland’s values. As the only 24/7 listening service for children in Ireland, Childline provides a lifeline for those in need of support, comfort, and guidance. Understanding the importance of mental health and well-being, the PHX Ireland team rallied together to fundraise in
David Keyes, Managing Director, Paul Reilly, CEO, PHX Ireland, Hillary Collins, MCIPD, PHX Ireland, Elaine Condon, Managing Director, TCP Clinical Homecare, John Church, CEO, ISPCC and JanPieter Hallema, Managing Director, McCabes Pharmacy
creative and inspiring ways, all in aid of ensuring that Childline can continue its invaluable work.
From charity runs and bake sales to raffles and card sales, teams embraced the challenge with enthusiasm and passion. Each initiative was a testament to the power of collaboration and the willingness of colleagues to give back. The collective effort of PHX Ireland’s teammates across all business units—United Drug, McCabes Pharmacy, and TCP Homecare—demonstrates the strength of the company’s culture of care and responsibility.
Raising ¤100,000 throughout the partnership is a milestone that speaks volumes about the people. This achievement was only possible because of their generosity, determination, and unwavering
commitment to supporting children in need. Every euro raised contributes directly to ISPCC’s mission, helping to ensure that children and young people always have someone to turn to, no matter what challenges they face.
At PHX Ireland, they take pride in living their values—being customer-focused, qualitydriven, collaborative, ambitious, innovative, and inclusive. Their partnership with ISPCC has been a true reflection of these principles, reinforcing the belief that businesses have the power to make a real difference.
As the team celebrate this achievement, they also look forward to continuing their journey of social responsibility, supporting organisations that align with their mission to create a healthier, happier society. To every colleague who took part in this fundraising journey—thank you. Your efforts have helped ensure that Childline remains a lifeline for those who need it most.
Together, we are stronger. Together, we are making a difference.
The HPRA has announced that its Chief Executive, Dr Lorraine Nolan, will step down from her position at the end of this year to pursue new career options.

Dr Nolan has served as the Chief Executive of the HPRA for over nine years and, by the end of 2025, she will have completed two terms in this leadership role. During her tenure, she has successfully guided the organisation through a significant transformation of its key regulatory functions and an expansion of its operations, elevating the standing of the HPRA on national, European and
HPRA Chief Executive, Dr Lorraine Nolan
international levels. In addition, her determined and strategic leadership during Brexit and the Covid-19 pandemic ensured the national regulator for medicines and medical devices made a vital contribution to the successful navigation of these challenges.
Dr Nolan has been a significant contributor to the leadership of both medicines and medical device regulation at European level. Through her former position as Chair of the European Medicines Agency (EMA) Management Board (2022-2025) and her membership of the Heads
of Medicines Agency (HMA) Management Group, she has held many significant roles at this level that have both defined strategy and positively enhanced delivery.
Professor Michael Donnelly, Chair of the HPRA, said, “I wish to commend Dr Nolan for her unwavering leadership and dedication to the organisation. Her tenure has been marked by significant achievements and a hugely positive, enduring legacy. I want to express both my personal thanks and that of the Authority to Lorraine for her service and wish her every success with her future endeavours”.
The Authority is commencing the process for recruitment of a new Chief Executive of the HPRA to take up the position from January 2026. Dr Nolan will continue in her current role until the end of this year and will support the transition in leadership during this period.
Delivering best-in-class products, service and business support for optimum patient care for your customers
Choice Based Buying Group.
Range of quality products including a mix of OTC, branded, generic and cold chain.
Safe and secure sourcing of unlicensed medicines. Bespoke medicines tailored to patients needs.
Ireland’s No1 provider of Ostomy products and services.
Best in class advice, service and support, enabling pharmacists to give patients confidence and peace of mind.
Compliance Based Buying Group
Rewarding you with competitive pricing.
Insights & Education.
Providing meaningful insights to help drive business forward and deliver better patient care.
Call: 01 463 2300
Email: wholesale@united-drug.com
Visit: www.united-drug.com
Login: www.udw.ie
Last month, PHX Ireland brought together over 100 leaders from United Drug, McCabes Pharmacy, and TCP Homecare for a landmark leadership conference focused on collaboration, innovation, and shaping the future of healthcare in Ireland. The event served as a dynamic forum for sharing insights and driving strategic conversations that will define the next phase of growth and excellence across the business units.

PHX Ireland CEO, Paul Reilly set the tone for an engaging and forward-thinking gathering, emphasising the importance of leadership in navigating an evolving healthcare landscape. A series of thought-provoking discussions followed, tackling key opportunities and challenges within our industry.
Elaine Condon, MD TCP Homecare provided a compelling perspective on the complexities of homecare, exploring the evolving needs of patients and providers. Eileen Boland and Hillary Collins brought the PHOENIX group Leadership Competencies to life, reinforcing the critical role of culture, accountability, and leadership in driving success. Meanwhile, David Keyes, MD United Drug, offered valuable insights into the future of United Drug’s supply chain, outlining strategic advancements that will enhance efficiency and service excellence.
Sustainability remains a top priority, and Patrick O’Sullivan highlighted
our ongoing journey towards a greener future, reinforcing that we are the change we want to see. The United Drug Operations Team, led by Alan Pender, showcased Lean initiatives with insights Kaizen, and a cross-functional panel, demonstrating our commitment to operational excellence.
Retail innovation also took centre stage, with Jan-Pieter Hallema, MD McCabes Pharmacy and Brian O’Keeffe, COO McCabes Pharmacy, providing an exciting outlook on the future of the retail operation.
The conference concluded on an inspiring note, with renowned immunologist Professor Luke O’Neill delivering a high-energy session on the future of medicine. His thought-provoking address left attendees energised and ready to embrace the opportunities ahead.
Collaboration, innovation, and leadership are the driving forces behind the progress in PHX Ireland, and together, we are shaping a stronger future for healthcare in Ireland.











Is your pharmacy business at the heart of your long-term investment or retirement plans? Well, with some forethought, you can ensure that all goes to plan and investment return is maximised for your future.

Written by Noel Winters, Corporate Finance Partner, nwinters@fitzgeraldpower.ie

Each expense of the business should be reviewed to see if savings are to be made. However, pharmacy tends to have two primary overheads – wages and rent.
Review regularly and ensure that the business is operating within sector standards.
On labour costs, consider the operating process. The variances we see in the average labour cost of dispensing a prescription item are remarkable.
First things first
To achieve a successful sale, both buyer and seller viewpoints must be considered. If the business is an unattractive proposition from the buyer’s perspective, it simply will not sell. In this way, selling a business is very similar to day-today retail. Would you purchase a faulty item? Of course not. So to sell your business, the question you must ask yourself is: “How can a business be best packaged for sale?”
1. To plan for a successful sale, you must understand how a pharmacy business is valued The method most commonly used is that of an Earnings Basis. Put simply, this considers an appropriate maintainable profit that can be earned by the business and applies an earnings multiple. EBITDA (Earnings Before Interest, Taxes, Depreciation and Amortisation) is the acronym commonly used in valuation as a measure of profit and is essentially the pre-tax cash flow of the business before loan repayment obligations.
2. Current or historical EBITDA may give a false valuation of the business, so care should be taken to review the maintainable EBITDA of the business as the buyer is purchasing the right to a future income stream. Current sector conditions would suggest that multiples of EBITDA between 4 and 6 are appropriate with the principal factors determining the actual multiplier achieved being scale and geography.
3. The final step in the valuation process is to review the assets and liabilities of the company and adjust the value accordingly.
Knowing how a pharmacy business is valued will allow you to work towards making the sale a more attractive proposition to the buyer and lucrative for the seller.
At the risk of oversimplifying the pharmacy business, there are three areas of focus when considering how to increase profitability and therefore the underlying basis of valuation.
Drive turnover
Drive gross profit margin
Control overheads
Driving turnover
Of the three identified headings, we have to appreciate that this may well be the most difficult to achieve in a time where:
a) the sector is continually subject to reduced reimbursement rates from 'State schemes'.
b) and the discretionary spending of the customer continues to be challenged.
However, some areas to consider:
- Using the information systems that are most likely already in your business, spend the time to consider what money is slipping through the net by inaccurate prescription claims. Keep on top of your rejected items. It might be a case of collecting what you are due as much as finding new revenue streams.
- Keep an eye on what the competition is up to. It would be naïve to rely on a stable customer base, as the Irish consumer expects the highest quality product at the best value.
- The retailers’ task of attracting and influencing discretionary spending has become ever more difficult. This is especially relevant to the Front-of-Shop trade where every square inch is important. Substitute stagnant lines with new lines and test success. Don’t be afraid to try things, but make agile decisions where underperformance is identified.
Driving gross profit margin
This should be the primary area of focus. Changing purchasing policies can have a dramatic effect on the profitability of the business. Ensure that you are getting the best terms available. Don’t be shy in negotiating with suppliers and consider onboarding one of the buying platforms
It may be worth assigning a member of staff to a full-time role to source the best discounts available, or even employing a new staff member with this as their sole focus.
Addressing and making improvements in all these focus areas will inevitably lead to increased profitability and a better selling proposition.
Tax Planning – Don’t Shy Away From it and Have
Having put all of your energy into maximising the value of the business, all too often we find that the business is still not ready to be sold because either the business owner / the business itself / the company which owns the business is not set up in the right way to complete a suitable transaction.
Companies and individuals need to plan significantly in advance for the myriad of reliefs –– from entrepreneur and retirement reliefs to participation exemptions, taxeffective interim cash extraction –– and ultimately how to design a tax-efficient exit. Remember: if you are at the altar, it’s probably too late.
The main obstacles that we see at the 11th hour are:
a) Addressing large sums of cash or investments that have built up in the company
b) Being unsure about company share ownership, and deducing who or what achieves the best outcome there
c) The business’ location itself: is the property owned by the business owner, or someone else?
If you would like to explore any of the issues raised, don’t hesitate to get in touch.
Get in touch with Noel Winters nwinters@fitzgeraldpower.ie or visit www.fitzgeraldpower.ie for more information.


For over 35 years, BioGaia has been a trusted pioneer in the probiotic space, earning global recognition for its science-backed innovations that support health at its core: the microbiome. From being the first to introduce a liquid Limosilactobacillus reuteri (formerly Lactobacillus reuteri) probiotic for infants back in 2000, BioGaia has continually led the way in using nature’s good bacteria to solve real health challenges. Today, that same commitment to cutting-edge probiotic solutions is being applied to a long-overlooked yet vital area of health— your mouth.

Meet Prodentis: The Oral Microbiome Game-Changer
BioGaia Prodentis is a dual-action probiotic lozenge formulated specifically for the oral cavity, helping to restore the natural balance of good and bad bacteria in the mouth. While most people think of probiotics as something that supports gut health, science now shows that the benefits extend much further. In fact, the oral microbiome—the community of bacteria living in your mouth— is deeply linked not just to your dental health, but also to digestion and even cardiovascular well-being.
Small Lozenge, Big Benefits
Prodentis contains two patented strains of Limosilactobacillus reuteri, proven in clinical studies to combat harmful bacteria in the mouth. These strains colonize the oral cavity, helping to manage:
• Gingivitis and Periodontal Disease: Prodentis can
reduce inflammation, bleeding gums, and the depth of gum pockets, helping to prevent the progression of periodontal disease.
• Halitosis and Bad Breath: By naturally reducing the bacteria that cause unpleasant odors, Prodentis offers a clean, fresh feeling that lasts.
• Bleeding and Receding Gums: Regular use supports gum health, reduces gum bleeding, and may help slow gum recession.
• Post-Dental Work & Trauma: Ideal for use after cleanings, extractions, or dental surgery, Prodentis helps speed healing and reduce the risk of infection.
• Caries (Cavities): Clinical research has shown Prodentis can reduce Streptococcus mutans, one of the primary bacteria responsible for tooth decay.
Written by Sinead Fitzgerald, Owner, Happy Tummy
The mouth is the entry point to the body, and imbalances here can have far-reaching consequences. Studies increasingly show links between poor oral health and digestive disorders, systemic inflammation, and even heart disease. By nurturing the oral microbiome, Prodentis not only promotes dental health but also helps reduce the burden on your overall system.
Taking care of your oral microbiome is now as simple as enjoying a fresh minty lozenge. Prodentis is easy to incorporate into your daily routine—just dissolve one slowly in your mouth after brushing. There’s
no swallowing, mixing, or complicated dosing. Suitable for anyone over 3 years of age, it’s a seamless addition to any healthconscious lifestyle.
Healthy Mouth, Healthy Life
As people live longer, we are keeping our teeth for longer, too. This means maintaining oral health well into old age is no longer optional—it’s essential. Whether you’re a parent caring for a child, an adult looking to strengthen your dental routine, or a senior managing age-related gum issues, BioGaia Prodentis is designed for you.
Don’t wait until problems arise. Proactively support your mouth from the inside out—with BioGaia Prodentis, the probiotic lozenge trusted by dental professionals worldwide.
Because living longer should include smiling longer




A team from the Centre for Positive Health Sciences at RCSI University of Medicine and Health Sciences led by Dr Jolanta Burke has partnered up with organisations across five EU countries and secured €5.5 million in funding from the Interreg EU programme for an initiative aimed at developing innovative forest-based tools to support adolescents and their families in improving and maintaining their positive health.

The RCSI Centre for Positive Health Sciences team, Dr Jolanta Burke (Principal Investigator), Dr Elaine Byrne, Dr Mary Clarke, Prof. Christian van Nieuwerburgh and PhD Candidates Kate Brassington and Branislav Kaleta, will be involved in this four-year project.
Their work will focus on codesigning a series of forest-based psychological interventions (real-life and virtual reality) that mental health professionals, young people, and their families can engage with to enhance adolescent health and well-being. They will also explore how foresters can adapt Irish forests to create more welcoming environments for young people. By the end of the project, this initiative will deliver a suite of innovative solutions designed to
Dr Jolanta Burke, RCSI Centre for Positive Health Sciences
deepen adolescents’ engagement with nature, helping to safeguard them against anxiety, depression, and other mental health challenges as well as improving their psychological, emotional and social well-being.
Dr Jolanta Burke said: "Many young people are struggling, and our research with nearly 3,000 students reveals a concerning decline in wellbeing as adolescents progress through their education. A cost-effective way to support them is by connecting them with the natural resources of nearby forests, encouraging them to embrace their restorative benefits from an early age. This project has the potential to transform the lives of many young people living in Ireland and their families. It could make our beautiful forests more inviting to them."
Faced with the mental health problems experienced by adolescents, the Forest4Youth project, co-funded by Opens in new windowInterreg North-WestEurope, is proposing an innovative solution: exploring the therapeutic power of forests to improve teenagers' well-being.
Today, 17.5% of young people in north-western Europe suffer from mental disorders such as depression, anxiety or addiction. Mental health services are struggling to meet this growing demand. It is therefore essential to rethink therapeutic support by integrating varied, more accessible and sustainable approaches.
A natural recovery area
Research shows that forest immersion has a direct and beneficial impact on mental health, particularly on symptoms of depression and anxiety, reducing stress, improving mood and promoting concentration. Yet these natural therapies remain under-exploited.
More than 20% of north-western Europe is covered by natural areas that could be used for the benefit of adolescents living with mental health problems. The Forest4Youth project improves the integration of the forest in therapeutic devices thanks to the following results:
• Identification of pilot therapeutic forests in several European countries.
• The development of a care protocol combining real immersion in the forest and virtual reality.
• Accompanying adolescents in therapeutic forest programmes.
• Training for mental health professionals and foresters.
• The creation of reference training centres.
A transnational approach
Forest4Youth is based on an unprecedented collaboration between mental health professionals and the owners and managers of forests and green spaces. The aim is to develop accessible solutions that can be implemented on a large scale, so that young people can benefit from these therapies.
"Reconnecting teenagers with nature gives them a chance to improve their mental health," explain the project's initiators.
The project also calls on public decision-makers to integrate these solutions into health and regional planning policies.
At a time when the mental health of young people is becoming a societal emergency, natural spaces represent a precious and widely available resource: exploiting them for their well-being is a necessity.
Congratulations to the MSc Clinical Pharmacy graduates from the School of Pharmacy at University College Cork, who have celebrated their achievement at the spring conferring ceremony, University College Cork.
The School recognised the following individuals who successfully completed the programme: Caoimhe Bolger, James Crowley, Anne-Marie Doherty, Jane Ensor, Lisa Kehily, Phoebe Kelly, Michael Kilcullen, Rachel MacCarthy, Aoife Maher, James McGuirk, Mary Minehane, Kieran O’Dea, Eve Rodgers, Áine Sweeney.
Caoimhe Bolger received the prize for achieving the highest aggregate score in the programme class 2022-2024.
Jane Ensor received the prize for the best research dissertation in the programme class 2022-2024.
Thank you to McCabe’s Pharmacy, Denis O’Driscoll, and Susan O’Meara for donating the prizes to accompany these awards.
The MSc in Clinical Pharmacy is currently accepting applications for the 2025 September intake. Learn more about the programme and how to apply by visiting www.ucc.ie




Most used brand by dentists worldwide
More powerful magnetic motor with frictionless micro-vibrations delivers a smooth and quiet brushing experience, and even more effective clean, gently removing 100% more plaque and 300% more along the gumline vs a manual toothbrush, resulting in healthier gums in just 1 week.
many years only eventually seeking attention for recurrent ‘sinus’ infections runny nose or a cough. Allergic Rhinitis often begins in early childhood when harder to communicate so parents and HCP need to be aware of all the relevant nasal and extra-nasal and acknowledge the association with poor sleep, fatigue, school performance and frequent infections/ visits to HCW/ hospital. When asthma, often the asthma symptoms dominate however the significant association rhinitis needs to be enquired about and considered when considering management asthma.
Written by Mona Thornton, Co-founder/President, Irish Rhinologic Society
Allergic rhinitis is a chronic inflammatory condition of the nose triggered by an allergic reaction to predominantly inhalant allergens. Its historic reference as ‘hay-fever’, is unfortunate as it unintentionally oversimplifies the broader spectrum of allergic rhinitis which can be triggered by a wide variety of outdoor and year -round allergens and undermines its significance as a chronic illness.
Allergic Rhinitis affects over 400 million people worldwide (1040%,) and is thought to be on the increase. Chronic rhinitis, if poorly controlled, can impact sleep thus can significantly affect quality of life and day to day performance with resultant significant socioeconomic costs, however is often dismissed as ‘just a stuffy nose’. Up to 90% of patients with asthma have rhinitis, whether allergic or non- allergic and when unstable or poorly managed contributes to asthma instability.
In the past few decades, medicines have developed and advanced and the ARIA guidelines have been published and updated regularly to guide Health care workers on this condition yet the medical management of this chronic condition continues to remain suboptimal.
Under or mis diagnosis, inadequate treatment, and inappropriate or delayed referrals to specialist care are some of the many issues that contribute to ineffective control of this condition. However, we cannot ignore data indicating that patients often self -manage their condition, relying on over- the- counter treatments, basing their information on unreliable sources and often without discussion with their general practitioner.
An Australian study demonstrated that 70% of patients self -managed their condition and only 15 % with optimal management. In the USA similar figures were seen and noted to be increased compared to an earlier study in 2009. Patients often recognise their symptoms but do not always discuss it with their health care physician HCP. Frequently information on treatment is sought from many different sources but is not often a HCP and follow up reviews of medications are often not considered thus many patients remain on ineffective treatment and
are somewhat disillusioned about embracing changes in treatment.
Considering that many patients self -manage Allergic Rhinitis or indeed seek over- the -counter treatments, our community pharmacists are well positioned to identify that patient group and advise on management, referral recommendations and are often a trusted source of guidance in the community. Pharmacists should be encouraged to take an active role in identifying patients who may need to be referred to their GP or ENT specialist.
Patient education is also essential to increase awareness, about the implications of this chronic condition, clarify the specifics around diagnosis, change misguided perceptions about treatment and define care pathways for treatment escalation. The Irish Rhinologic Society have recently introduced a podcast series “Follow your Nose’ to guide patients with understanding chronic conditions of the nose.
As mentioned, early and correct diagnosis is key to best management but for many, diagnosis is delayed. Not so for those with grass allergies or indeed any seasonal allergy, as the timing and often intensity of distinctive nasal and allergy symptoms make it easy to recognise and diagnose. Many patients thus often can recognise seasonal Allergic Rhinitis and will seek treatment.
In contrast, non- seasonal Allergic Rhinitis often just presents as chronic nasal blockage but the typical allergy symptoms can be more subtle. This subgroup often go undiagnosed for many years only eventually seeking attention for recurrent ‘sinus’ infections, post nasal drip/ runny nose or a cough. Allergic Rhinitis often begins in early childhood when symptoms are harder to communicate so parents and HCP need to be aware of all the relevant signs both nasal and extra-nasal and acknowledge the association with poor sleep, fatigue, reduced school performance and frequent infections/ visits to HCW/ hospital. When associated with asthma, often the asthma symptoms dominate however the significant association with rhinitis needs to be enquired about and considered when considering management of asthma.
March- April Tree
May-July Grass
June-August Weed
September-October Mould spores
Bartle et al
Clear differentiation from other conditions that share similar symptoms of nasal obstruction and rhinorrhoea is also required to ensure correct diagnosis. Chronic sinusitis has overlapping features and should be included in the differential. The smell fibres are located more posteriorly and higher in the nose and consequently smell function is more significantly affected by conditions involving the skull base or nasal sinuses. Thus moderate to severe loss or reduction in smell is more likely to infer a diagnosis of chronic sinusitis or less commonly tumours of the skull base. For the large majority of patients with Allergic rhinitis, smell loss if present is mild or at most moderate in nature and often fluctuates. (Figure below)
Where a diagnosis is unclear or when response to an appropriate INCS is suboptimal, early referral to a specialist should be considered to facilitate nasendoscopic evaluation for diagnosis and escalation of treatment.
Allergen avoidance, pharmacotherapy, and immunotherapy are indeed the foundations of management, however allergen avoidance should be practical and part of an overall treatment plan that includes pharmacotherapy. When it comes

to pharmacotherapy, the different pathophysiological mechanisms that occur during the early and late phase response provide specific targets for different treatment. Despite this there is significant data that demonstrates for many oral antihistamines are the most common treatment, presumably due to their over -the- counter availability and ease of use. In fact, intranasal corticosteroid treatment INCS is the preferred treatment especially in moderate to severe disease and OAH only play a minor role (Ref ARIA Classification)
Clear differentiation from other conditions that share similar symptoms of and rhinorrhoea is also required to ensure correct diagnosis. Chronic sinusitis overlapping features and should be included in the differential. The smell fibres more posteriorly and higher in the nose and consequently smell function is significantly affected by conditions involving the skull base or nasal sinuses. to severe loss or reduction in smell is more likely to infer a diagnosis of chronic less commonly tumours of the skull base. For the large majority of patients rhinitis, smell loss if present is mild or at most moderate in nature and often Figure )
Safety concerns are a persistent reason for INCS avoidance or patient poor compliance with long term therapy yet widespread evidence should give reassurance about their safety profile. In addition, for many years INCSs were generally considered to have similar efficacy with little strong data to support differences between compounds. Both of these factors have impacted on patient compliance with treatment. However, second generation INCSs have different pharmacokinetic/ pharmacodynamic properties and there are now some studies that show better clinical efficacy however more research is needed to confirm this.
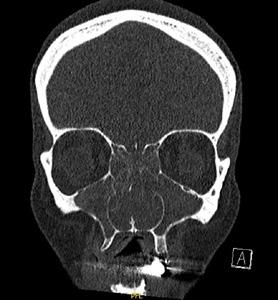
Where a diagnosis is unclear or when response to an appropriate INCS is suboptimal, referral to a specialist should be considered to facilitate nasendoscopic evaluation diagnosis and escalation of treatment.






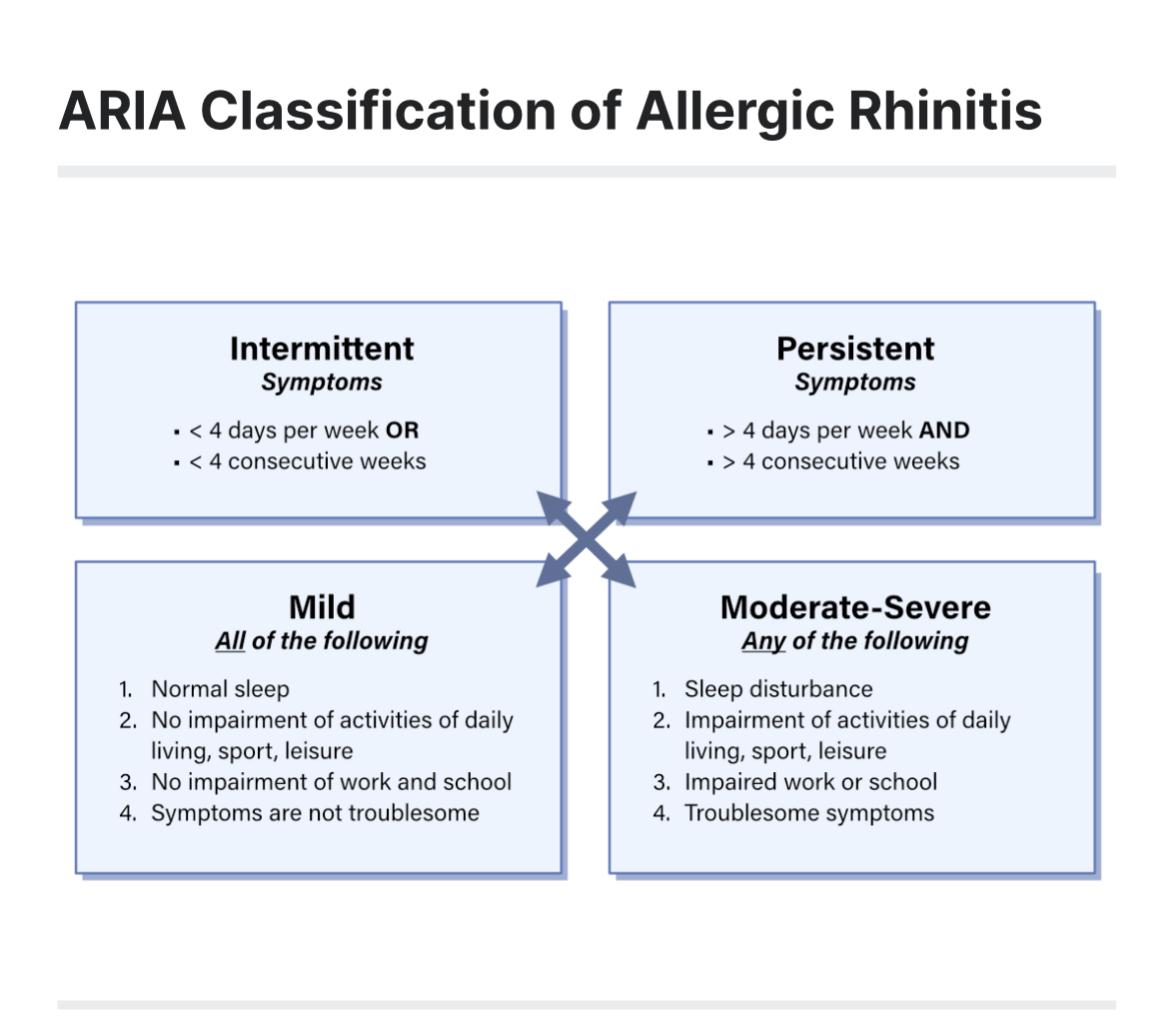
ARIA Classification of Allergic Rhinitis
Keep windows/ doors closed/ no flowers indoors
Vacuum often/ dust with damp cloth
Check pollen count dailyKeep pets outside if you can as they carry pollen indoors
patient on an individual basis, acknowledging the severity of symptoms, patient age and also the cost. Nasal spray technique should also be demonstrated to ensure maximum benefit and minimal complications. The patient is the key player in this so should be included in all discussions about treatment plans.
also a critical consideration.
complications. The patient is the key player in this so should be included in all discussions about treatment plans.
SLIT Sublingual immunotherapy is a promising new treatment in the management of allergic rhinitis. When OAH/ INCS treatment fails, immunotherapy is recommended. It is the only current treatment that directly addresses the cause however is more expensive and requires a 3- year commitment to daily treatment.
Montelukast is a leukotriene receptor antagonist that blocks cysteinyl leukotrienes involved in allergic inflammation which can be used as an alternative therapy especially for patient s with asthma but its role primarily in allergic rhinitis management is limited compared to INCS. Mast cell stabilisers are best as a preventative treatment but are inferior to OAH and INCS so not commonplace in treatment. Oral decongestants are often used by patients to relieve severe congestion but their ready availability until recently meant there was an overuse in patients with chronic disorders which was not without consequence. More recently it has been also noted that, phenylephrine now added to decongestant medications, when taken orally is ineffective.
Montelukast is a leukotriene receptor antagonist that blocks cysteinyl leukotrienes involved in allergic inflammation which can be used as an alternative therapy especially for patients with asthma but its role primarily in allergic rhinitis management is limited compared to INCS. Mast cell stabilisers are best as a preventative treatment but are inferior to OAH and INCS so not commonplace in treatment. Oral decongestants are often used by patients to relieve severe congestion but their ready availability until recently meant there was an overuse in patients with chronic disorders which was not without consequence. More recently it has been also noted that, phenylephrine now added to
In summary, Allergic Rhinitis is a significant public health issue that demands a more robust pathway for care. Many recognise the familiar symptoms of seasonal rhinitis however diagnosis of non -seasonal rhinitis is often delayed. Diagnosis is also strengthened by understanding the differentiation from other chronic conditions including chronic rhinosinusitis where early referral for surgical management is often necessary. Management continues to be suboptimal and so we need to consider the relevant factors that contribute to poor control for patients. There are a number of key players that need consideration in the pathway each with important distinctive roles. Undoubtedly patient education
The role of the pharmacist cannot be underestimated when we consider that for many sufferers,self -management with over the counter medication is commonplace. Pharmacists are ideally placed to identify these patients and guide them in appropriate management and early referral for escalation of care. Not all patients choose to interact with their pharmacist for guidance on medication thus public awareness and education around this chronic condition needs to improve if we wish to make any significant improvements in management of this condition in the future.
SLIT Sublingual immunotherapy is a promising new treatment in the management of allergic rhinitis. When OAH/ INCS treatment fails, immunotherapy is recommended. It is the only current treatment that directly addresses the cause however is more expensive and requires a 3- year commitment to daily treatment.
Practical Advices
Check pollen count daily
Place Vaseline round nostrils to trap pollen
Wear wraparound sunglasses to protect your eyes
Stay indoors when pollen count high/ don't cut or walk on grass
Shower/ change clothes after you've been outside
Don't hang clothes outside if you can/ if you do hang clothes outside avoid peak times ie hang in the middle of the day and bring in before dusk
References
Hayfever 2017
The future of allergic rhinitis management: A partnership between healthcare professionals and patients
Cvetkovski B, Muscat D, Bousquet J, Cabrera M, House R, Katsoulotos G, Laurenco O, Papadopoulos N, Price DB, Rimmer J, Ryan D, Smith P, Yan K, Bosnic-Anticevich S
World Allergy Organisation Journal 2024, vol 13, Issue 3, 100873
Cvetkovski B, Tan R, Kritikos V, Yan K, Azzi E, SRour P and Bosnic-ANitcevich SB
A patient-centric analysis to identify key influences in Allergic management
Nature September 2018
Daley-Yates P, Larenas-Linneman D, Bhargave C, Verma M J Asthma
Allergy 2021 Sep 8; 14: 1093-1104
Euforea.eu: ARIA guidelines
Table: Pharmacotherapy access in Allergic Rhinitis
Radulovic S, Calderon MA, Wilson D, Durham S Sublingual immunotherapy for allergic rhinitis
Cichrane Database 2010 Dec 8; 2010
Cvetkovski B, Tan R, Kritikos V, Yan K, Price D, Smith P, BosnicAnticevich S Management of allergic rhinitis in the community pharmacy: identifying the reasons behind medication self selection Pharm Pract 2018 Sep 26; 16(3): 1332
Table: Pharmacotherapy access in Allergic Rhinitis In summary, Allergic Rhinitis is a




Abbreviated prescribing information
Abbreviated prescribing information
Abbreviated prescribing information
Product Name: Fexo Allergy Relief 120 mg Film-coated tablets
Product Name: Fexo Allergy Relief 120 mg Film-coated tablets
Product Name: Fexo Allergy Relief 120 mg Film-coated tablets
Composition: Each tablet contains 120 mg of fexofenadine hydrochloride equivalent to 112 mg of fexofenadine. Description: Peach coloured oblong, bi-convex film-coated tablet. Dimensions of 14.9-15.3 mm x 6.4-6.8 mm; plain on both sides. Indication(s): Adults and children 12 years and older: Relief of symptoms associated with seasonal allergic rhinitis. Dosage: Adults and children aged 12 years and over: One tablet (120mg) once daily taken before a meal. Children under 12 years: Efficacy and safety of fexofenadine hydrochloride 120 mg has not been studied in children under 12. Children from 6 to 11 years of age: Administer fexofenadine hydrochloride 30 mg. Special populations: No need to adjust the dose confirmed by studies in special risk groups (older people, renally or hepatically impaired patients). Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and Precautions for Use: Limited data in the elderly and renally or hepatically impaired patients. Administer with care in these special groups. Warn patients with a history of or ongoing cardiovascular disease that, antihistamines have been associated with the adverse reactions, tachycardia and palpitations. Interactions: Fexofenadine does not undergo hepatic biotransformation and therefore will not interact with other medicinal products through hepatic mechanisms. Fexofenadine is a P-glycoprotein (P-gp) and organic-anion-transporting polypeptide (OATP) substrate. Concomitant use with P-gp inhibitors or inducers can affect the exposure to fexofenadine. Co-administration with P-gp inhibitors erythromycin or ketoconazole resulted in 2-3 times increase in the level of fexofenadine in plasma. The changes were not accompanied by any effects on the QT interval and were not associated with any increase in adverse reactions compared to the medicinal products given singly. A clinical drug-drug interaction study showed that co-administration of apalutamide (a weak inducer of P-gp) and a single oral dose of 30 mg fexofenadine resulted in a 30 % decrease in AUC of fexofenadine. No interaction with omeprazole. Administration of an antacid containing aluminium and magnesium hydroxide gels 15 minutes prior to fexofenadine hydrochloride caused a reduction in bioavailability, most likely due to binding in the gastrointestinal tract. It is advisable to leave 2 hours between administration of fexofenadine hydrochloride and aluminium and magnesium hydroxide containing antacids Pregnancy and Lactation: Pregnancy: Do not use unless clearly necessary. No adequate data from the use of fexofenadine hydrochloride in pregnant women. Limited animal studies do not indicate direct or indirect harmful effects with respect to effects on pregnancy, embryonal/foetal development, parturition or postnatal development. Breast-feeding: Not recommended. No data on the content of human milk after administering fexofenadine hydrochloride. However, when terfenadine was administered to nursing mothers’ fexofenadine was found to cross into human breast milk. Fertility: No human data available. Ability to Drive and Use Machinery: Based on the pharmacodynamic profile and reported adverse reactions it is unlikely that fexofenadine hydrochloride tablets will produce an effect on the ability to drive or use machines. In objective tests, Fexo Allergy Relief has been shown to have no significant effects on central nervous system function. This means that patients may drive or perform tasks that require concentration. However, in order to identify sensitive people who have an unusual reaction to medicinal products, it is advisable to check the individual response before driving or performing complicated tasks. Undesirable Effects: Nervous system disorders: Common: headache, drowsiness, dizziness. Gastrointestinal disorders: Common: nausea. General disorders and administration site conditions: Uncommon: fatigue. Refer to the SPC for other undesirable effects. Marketing Authorisation Holder: Rowa Pharmaceuticals Limited., Newtown, Bantry, Co. Cork. Marketing Authorisation Number: PA0074/096/001 Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417 E-mail: rowex@rowa-pharma.ie
Composition: Each tablet contains 120 mg of fexofenadine hydrochloride equivalent to 112 mg of fexofenadine. Description: Peach coloured oblong, bi-convex film-coated tablet. Dimensions of 14.9-15.3 mm x 6.4-6.8 mm; plain on both sides. Indication(s): Adults and children 12 years and older: Relief of symptoms associated with seasonal allergic rhinitis. Dosage: Adults and children aged 12 years and over: One tablet (120mg) once daily taken before a meal. Children under 12 years: Efficacy and safety of fexofenadine hydrochloride 120 mg has not been studied in children under 12. Children from 6 to 11 years of age: Administer fexofenadine hydrochloride 30 mg. Special populations: No need to adjust the dose confirmed by studies in special risk groups (older people, renally or hepatically impaired patients). Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and Precautions for Use: Limited data in the elderly and renally or hepatically impaired patients. Administer with care in these special groups. Warn patients with a history of or ongoing cardiovascular disease that, antihistamines have been associated with the adverse reactions, tachycardia and palpitations. Interactions: Fexofenadine does not undergo hepatic biotransformation and therefore will not interact with other medicinal products through hepatic mechanisms. Fexofenadine is a P-glycoprotein (P-gp) and organic-anion-transporting polypeptide (OATP) substrate. Concomitant use with P-gp inhibitors or inducers can affect the exposure to fexofenadine. Co-administration with P-gp inhibitors erythromycin or ketoconazole resulted in 2-3 times increase in the level of fexofenadine in plasma. The changes were not accompanied by any effects on the QT interval and were not associated with any increase in adverse reactions compared to the medicinal products given singly. A clinical drug-drug interaction study showed that co-administration of apalutamide (a weak inducer of P-gp) and a single oral dose of 30 mg fexofenadine resulted in a 30 % decrease in AUC of fexofenadine. No interaction with omeprazole. Administration of an antacid containing aluminium and magnesium hydroxide gels 15 minutes prior to fexofenadine hydrochloride caused a reduction in bioavailability, most likely due to binding in the gastrointestinal tract. It is advisable to leave 2 hours between administration of fexofenadine hydrochloride aluminium and magnesium hydroxide containing antacids Pregnancy and Lactation: Pregnancy: Do not use unless clearly necessary adequate data from the use of fexofenadine hydrochloride in pregnant women. Limited animal studies do not indicate direct or indirect harmful effects with respect to effects on pregnancy, embryonal/foetal development, parturition or postnatal development. Breast-feeding: Not recommended. No data on the content of human milk after administering fexofenadine hydrochloride. However, when terfenadine was administered to nursing mothers’ fexofenadine was found to cross into human breast milk. Fertility: No human data available. Ability to Drive and Use Machinery: Based on the pharmacodynamic profile and reported adverse reactions it is unlikely that fexofenadine hydrochloride tablets will produce an effect on the ability to drive or use machines. In objective tests, Fexo Allergy Relief has been shown to have no significant effects on central nervous system function. This means that patients may drive or perform tasks that require concentration. However, in order to identify sensitive people who have an unusual reaction to medicinal products, it is advisable to check the individual response before driving or performing complicated tasks. Undesirable Effects: Nervous system disorders: Common: headache, drowsiness, dizziness. Gastrointestinal disorders: Common: nausea. General disorders and administration site conditions: Uncommon: fatigue. Refer to the SPC for other undesirable effects. Marketing Authorisation Holder: Rowa Pharmaceuticals Limited., Newtown, Bantry, Co. Cork. Marketing Authorisation Number: PA0074/096/001 Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417 E-mail: rowex@rowa-pharma.ie
Composition: Each tablet contains 120 mg of fexofenadine hydrochloride equivalent to 112 mg of fexofenadine. Description: Peach coloured oblong, bi-convex film-coated tablet. Dimensions of 14.9-15.3 mm x 6.4-6.8 mm; plain on both sides. Indication(s): Adults and children 12 years and older: Relief of symptoms associated with seasonal allergic rhinitis. Dosage: Adults and children aged 12 years and over: One tablet (120mg) once daily taken before a meal. Children under 12 years: Efficacy and safety of fexofenadine hydrochloride 120 mg has not been studied in children under 12. Children from 6 to 11 years of age: Administer fexofenadine hydrochloride 30 mg. Special populations: No need to adjust the dose confirmed by studies in special risk groups (older people, renally or hepatically impaired patients). Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and Precautions for Use: Limited data in the elderly and renally or hepatically impaired patients. Administer with care in these special groups. Warn patients with a history of or ongoing cardiovascular disease that, antihistamines have been associated with the adverse reactions, tachycardia and palpitations. Interactions: Fexofenadine does not undergo hepatic biotransformation and therefore will not interact with other medicinal products through hepatic mechanisms. Fexofenadine is a P-glycoprotein (P-gp) and organic-anion-transporting polypeptide (OATP) substrate. Concomitant use with P-gp inhibitors or inducers can affect the exposure to fexofenadine. Co-administration with P-gp inhibitors erythromycin or ketoconazole resulted in 2-3 times increase in the level of fexofenadine in plasma. The changes were not accompanied by any effects on the QT interval and were not associated with any increase in adverse reactions compared to the medicinal products given singly. A clinical drug-drug interaction study showed that co-administration of apalutamide (a weak inducer of P-gp) and a single oral dose of 30 mg fexofenadine resulted in a 30 % decrease in AUC of fexofenadine. No interaction with omeprazole. Administration of an antacid containing aluminium and magnesium hydroxide gels 15 minutes prior to fexofenadine hydrochloride caused a reduction in bioavailability, most likely due to binding in the gastrointestinal tract. It is advisable to leave 2 hours between administration of fexofenadine hydrochloride and aluminium and magnesium hydroxide containing antacids Pregnancy and Lactation: Pregnancy: Do not use unless clearly necessary No adequate data from the use of fexofenadine hydrochloride in pregnant women. Limited animal studies do not indicate direct or indirect harmful effects with respect to effects on pregnancy, embryonal/foetal development, parturition or postnatal development. Breast-feeding: Not recommended. No data on the content of human milk after administering fexofenadine hydrochloride. However, when terfenadine was administered to nursing mothers’ fexofenadine was found to cross into human breast milk. Fertility: No human data available. Ability to Drive and Use Machinery: Based on the pharmacodynamic profile and reported adverse reactions it is unlikely that fexofenadine hydrochloride tablets will produce an effect on the ability to drive or use machines. In objective tests, Fexo Allergy Relief has been shown to have no significant effects on central nervous system function. This means that patients may drive or perform tasks that require concentration. However, in order to identify sensitive people who have an unusual reaction to medicinal products, it is advisable to check the individual response before driving or performing complicated tasks. Undesirable Effects: Nervous system disorders: Common: headache, drowsiness, dizziness. Gastrointestinal disorders: Common: nausea. General disorders and administration site conditions: Uncommon: fatigue. Refer to the SPC for other undesirable effects. Marketing Authorisation Holder: Rowa Pharmaceuticals Limited., Newtown, Bantry, Co. Cork. Marketing Authorisation Number: PA0074/096/001 Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417 E-mail: rowex@rowa-pharma.ie
Legal Category: Not Subject to medical prescription. Date of Preparation: September 2024 CCF FOR API: 26741
Legal Category: Not Subject to medical prescription. Date of Preparation: September 2024 CCF FOR API: 26741
Legal Category: Not Subject to medical prescription. Date of Preparation: September 2024 CCF FOR API: 26741 Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie
Date of preparation: (02/2025) CCF: 26995
Supply status: Supply through pharmacies only
Date of preparation: (02/2025) CCF: 26995 Supply status: Supply through pharmacies only
What is Parkinson’s Disease? Parkinson’s Disease is the fastest growing neurological condition, and it is estimated that up to 18,000 people are living with the condition in Ireland. This number is expected to double by 2050, which means demand for Parkinson’s services will significantly increase over the coming years.

Parkinson’s Disease (PD) is a progressive neurological disorder, and is classified as a Movement Disorder, as it primarily affects movement. Although Parkinson’s is a movement disorder, there are both motor and non-motor symptoms associated with it. It is variable in its progression, i.e. some people progress more slowly than others, and the symptoms can be effectively controlled with medication for many years.
A common misconception surrounding Parkinson’s is that is a condition diagnosed only in the older person, while the average age of diagnosis is over 65 there are increasing numbers of people being diagnosed with early or young onset, under the age of 60, and there is no age too young to be diagnosed with PD.
Parkinson’s Disease is caused by a loss of a chemical called dopamine. We all lose some of this chemical as we get older, however, people with Parkinson’s lose this chemical at a faster rate than others. It is only when 7080% of dopamine has been lost do symptoms tend to be revealed. The concept of prodromal phase in Parkinson’s is evident in many research papers, this phase is prior to motor symptoms being displayed and may be the existence of non-motor symptoms for between 5 –15 years before a diagnosis occurs. In existence on their own, these symptoms do not lead to a diagnosis of PD but when
Written by Lisa Wynne, Parkinson’s Nurse Specialist, Parkinson’s Ireland
taken alongside other symptoms, a picture of PD may gradually be pieced together.
A tremor is a widely recognised motor symptom of Parkinson’s, 70% of people have a tremor, so it is not experienced by all. Tremor, bradykinesia (a slowness in movement), rigidity and a change in posture are the cardinal symptoms of PD but we must also be aware that Parkinson’s is a multifaceted condition and those living with PD may experience 40+ symptoms.
The non-motor symptoms are often unknown, underdiagnosed, and hidden, but for some, they prove to be the more troublesome symptoms to manage. Constipation, bladder issues and sleep issues are just some of the more common examples. The psychiatric symptoms including anxiety, apathy and depression are the more dominant symptoms affecting both people with PD and their families. With little awareness, these often have the biggest impact.
There is currently no cure for Parkinson’s. There have however been significant improvements in the treatment of the symptoms of Parkinson’s in the last 20 years. Treatments include drug therapies, surgery, exercise component, and an interdisciplinary team input.
There is extensive research currently being carried out into the causes of Parkinson’s and into developing new treatments for Parkinson’s. The goal of this research is to develop biomarkers to assist in the diagnosis of Parkinson’s, slow down progression or even cure Parkinson’s altogether. As research advances, there are likely to be better treatments available over time which will improve the quality of life of people with Parkinson’s. The research surrounding Parkinson’s is moving at an expediential pace globally and within Ireland on many different
areas, such as treatments, role of our gut, use of pesticides in farming communities and exercise, certainly a growth in the last decade.
Each person with Parkinson’s Disease should have an individualised tailored regime depending upon his/her age, physical state, level of disease etc, thus no two patients’ regimes will be the same. A tailored and personalised approach to all therapies, be they pharmacological or non-pharmacological, should be the foundation of care for a person living with PD.
The management of Parkinson’s Disease is very much a multidisciplinary team approach. Consultants, nurse specialists, physiotherapists, occupational therapists, speech and language therapists, social workers, pharmacists, and many other health care workers play a key role in the overall management of the patient’s condition.
There is a misconception out there that the medications only last for 5 or 6 years or so. This is not necessarily true, as medications continue to work for as long as they are taken. Due to the progressive nature of the condition medications may need
to be reviewed will have to be increased or decreased every so often depending upon the level of progression. Levodopa remains the ‘gold standard’ treatment for the majority, initial use of which was in the 1960’s, with a variation of regimes that can be prescribed concomitantly.
Parkinson’s can be effectively managed with oral medications for a significant amount of time but in some people, the medication no longer controls these symptoms, and they may experience motor fluctuations. As the disease progresses, the tailoring of medication regimes and optimisation of these regimes can become a little more difficult with a narrowing therapeutic window and increased wearing off of medication causing more ‘off’ periods and an increase of symptoms, directly impacting mobility, additional symptoms and quality of life of the person with PD.
Complex therapies may be the next line of treatment considered in this case. Complex therapies include surgical and device treatments. Recent advancements in Parkinson’s treatment from an Irish perspective include the availability of Deep Brain

Salicylic acid, lactic acid.

Supplied in a complete treatment kit, with a simple once daily application, Salatac Gel offers a very convenient and painless way of treating warts and verrucas. Salatac Gel dries to a water-resistant film... no need for plasters.






Prescribing information
Salicylic acid 12.0% w/w, lactic acid 4.0% w/w. Uses: For the topical treatment of warts, verrucas, corns and calluses.
Directions: Adults, children and the elderly: Apply once daily until the wart, verruca, corn or callus has disappeared (treatment may take up to 12 weeks). Soak the affected area in warm water for 2-3 minutes and dry with patient’s own towel. Carefully apply one or two drops to the lesion avoiding the surrounding skin. (Peel off the previous day’s gel before reapplying). Once a week gently rub away the treated surface with an emery board to remove any hard skin before re-application of the gel.
Contra-indications, warnings, side effects etc: Please refer to SPC for full details before prescribing. Do not use if sensitive to any of the ingredients. Not to be used on or near the face, intertriginous or anogenital regions, or by diabetics or individuals with impaired peripheral blood circulation. Not to be used on moles or on any other skin lesions for which it is not indicated. Keep away from the eyes, mucous membranes and from cuts and grazes. Some mild, transient irritation may be expected, but in cases of more severe or persistent pain/irritation, the treatment should be suspended or discontinued. Avoid inhaling vapour. Highly fl ammable, keep away from fl ames.
Package quantity, trade price and MA number: 8g tube with applicator. €6.22, PA23128/007/001
Legal category: Supply through pharmacy only.












Further information is available from: Dermal Laboratories (Ireland) Ltd, Head Offi ce Tatmore Place, Gosmore, Hitchin, Herts, SG4 7QR, UK.
Date of preparation: October 2023.
‘Salatac’ is a trademark.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Dermal.


Stimulation service in Ireland and additional pump therapies. The National DBS (deep brain stimulation) service was established in 2021, providing the neurosurgical aspect in Beaumont Hospital and the neurology management in the Mater Hospital Dublin. In July 2024, the license was approved for levodopa in liquid formulation, delivered via a pump system, providing an additional treatment option for management of advanced Parkinson’s. These recent developments are welcomed following a hiatus in new treatment options for those with PD. Parkinson’s medication is time critical. The delay of medication or a missed dose can result in immediate deterioration of symptoms. Consistency in dosing helps maintain optimal symptom control and reduces the risk of complications like falls and hospitalisations. Missed doses can increase hospital stays, heighten the risk of other health complications, and delay discharge, complicating overall care and treatment plans. Timing and types of medication can vary with each person with Parkinson's.
• Speak with family members to get an accurate medication history.
• Coordinate with the care team to ensure medications are given on time, especially across shifts.
• Utilise tools like reminders and educate patients and caregivers on the importance of strict adherence to dosing schedules.
• Consider use of stickers or visual prompts at bedside, patient chart, electronic records.
Some specific considerations to bear in mind are if the patient is NPO or has an impaired swallow or in the case of peri-operative stages.
Timely medication = Better outcomes. Ensure timely Parkinson's care for improved patient quality of life and reduced hospital stay.
Parkinson’s Ireland will be launching a clinical awareness campaign ‘Meds On Time, Every Time’ later in the year to highlight the importance and increase awareness among many areas. Engagement from clinical staff, people with PD and families will be pivotal in the success of the campaign.
In 2024, Parkinson’s Ireland and Irish Medication Safety Network (IMSN) collaborated on two safety alerts for PD. Alert one focused on ‘Reducing harm from omitted and delayed Parkinson’s Disease Medications in Irish hospitals’ while alert 2 focused on ‘Contraindicated Medication in Parkinson's Disease’. For further information please see www. parkinsons.ie and IMSN website for the most recent publications.
Patient Perspective
Organisations such as Parkinson’s Ireland offer support to those living with Parkinson’s and their families. The core of the work provided by Parkinson’s Ireland is to increase awareness of this condition to all, to provide education and support to optimise therapies and to encourage self-advocacy and empowerment to those living with PD and family members. Selfmanagement for those living with a chronic condition is vital to allow the person to live well and maintain health life and health brain.
Celebrating the Inaugural Flagship Fundraiser with our Donegal Branch
Parkinson’s Ireland is a branchbased charity, with 21 branches across Ireland. Each branch offers a range of services and supports to their members, such as exercise classes, information meetings and social elements such as coffee mornings, Christmas lunches and summer outings. Alongside the in-person branch activities, Parkinson’s Ireland provides a nurse call back and a dietician call back service. Our nurse callback service is available 9am to 5pm, Monday to Friday, and although our helpline is not an emergency one, our nurse specialists always aim to get back to you within one working day. Our helpline manager can also signpost you to other support services available, depending on your situation and needs.
Working with multiple different therapists, we offer virtual classes Monday to Friday on various aspects such as yoga, mindfulness, singing and psychological wellness. Parkinson’s Ireland currently has three PD nurse specialists, providing in person appointment for our Cork members and national phone support line for education and support. This is a valuable and critical lifeline for those either newly diagnosed or living with Parkinson's for a much greater period. A nurse led zoom is facilitated weekly and our nurses also provide a virtual four week newly diagnosed programme with the aim of empowering those with PD and giving them the essential nuggets of information necessary in the earlier days of diagnosis. Throughout the year we provide webinars on topics such as women with PD, carers, and assistive technology, bringing awareness to all, in all corners of the country and
further afield, dispel the myths and associated stigma experienced and provide a network of support in many avenues.
“Since I was diagnosed with Parkinson’s in July 2023, Parkinson’s Ireland has given me enormous help in managing my symptoms.” – Brenda O’Connell, person with PD.
Bringing it all together for awareness month in April, the events will kick off with our flagship fundraiser ‘A Walk in the Park for Parkinson’s’ on April 5th. Taking place in 14 locations nationwide, following a hugely successful launch in 2024 it is hoped this year will surpass our expectations. Our awareness campaign for 2024 was titled ‘Different for Everyone’ to highlight the 40+ symptoms associated with the condition. This year, we wanted there to be a focus on personal stories to help spread further awareness on how Parkinson’s manifests itself differently person to person. During April, Parkinson’s Ireland will share videos from patients, loved ones and health professionals, each person describing one of the many symptoms of Parkinson’s Disease and how Parkinson’s Ireland helps them manage it. “Time to Talk About Parkinson’s” is the title of this year’s annual online conference, where we will have a range of speakers such as dieticians, neurologists and physiotherapists joining us to share advice on how to live positively with PD. Visit www.parkinsons.ie to join our conference on April 12th. If you are unable to join us on the day, a recording will be available from our website soon after.
Please keep an eye on Parkinson’s Ireland’s website and social media during April to find out more about our awareness campaigns, education conference and help us celebrate Parkinson’s Awareness month and World Parkinson's Day on April 11th.
Summary of trial results
In a recent trial of children with moderate atopic eczema, conducted in NHS GP practices (to reflect real-life settings), the mean disease severity score (SCORAD) improved significantly:
• from 37.14 (moderate atopic eczema) at baseline
• to 22.56 (mild atopic eczema) after 2 weeks
• and to 18.48 (mild atopic eczema) after 4 weeks, per protocol analysis of 41 children.
Bridges the gap between plain emollients and topical corticosteroids.
Adex Gel is an emollient with an ancillary anti-inflammatory, nicotinamide 4%, to help reduce inflammation.
Adex Gel can be used continuously, for as long as necessary, all over the body including on the face, hands and flexures. Available on NHS prescription and suitable for patients aged 1 year+.
In addition, the mean children’s dermatology life quality index score (CDLQI) improved significantly from 9.3 (moderate effect on child) at baseline, to 3.7 (small effect on child) after 4 weeks.
Application of Adex Gel in the trial
Three times daily, for 4 weeks, instead of usual emollient or as the first-line treatment for moderate atopic eczema, in both scenarios, without supplementary use of any oral or topical steroids or immunomodulators.
Adex Gel has been shown to be an effective treatment for moderate atopic eczema in children in a real-world setting.

SCORAD is a tool used in clinical trials to assess atopic dermatitis severity based on disease area, intensity and subjective symptoms (itch and sleeplessness). The CDLQI is designed to measure the impact of any skin disease on the lives of children.
Product name: Adex™ Gel. Key ingredients: Isopropyl myristate 15%, liquid paraffin 15%, nicotinamide 4%. Uses: Highly moisturising and protective emollient with an ancillary anti-inflammatory medicinal substance for the treatment and routine management of dry and inflamed skin conditions such as mild to moderate atopic dermatitis, various forms of eczema, contact dermatitis and psoriasis. Package sizes: 100g tube and 500g pump pack. Further information is available from: Dermal Laboratories Ltd, Tatmore Place, Gosmore, Hitchin, Herts, SG4 7QR, UK. ‘Adex’ is a trademark.
Adverse Events/Incidents should be reported. Reporting forms and information for the UK can be found at yellowcard.mhra.gov.uk, and for the Republic of Ireland at www.hpra.ie. Adverse Events/Incidents should also be reported to Dermal.
SCORAD, SCORing Atopic Dermatitis. CDLQI, Children’s Dermatology Life Quality Index.
Reference: 1. Gallagher J. et al. Evaluation of a nicotinamidecontaining emollient for moderate atopic eczema in paediatric patients:
A prospective, multi-centre GP study reflecting real-life settings. Data presented at the Annual Meeting of the Austrian Society of Dermatology and Venereology (ÖGDV), November 2024, Graz, Austria.


Style and celebration took centre stage as Life Pharmacy members attended the 2025 Life Pharmacy Awards

The 4th Annual Life Pharmacy Awards honoured the exceptional contributions of Life Pharmacy professionals across Ireland, recognising individuals and teams who go above and beyond in customer and patient care. Held at the Killashee Hotel, Co. Kildare, the gala
event brought together Life Pharmacy members to celebrate excellence in pharmacy services and was hosted by RTÉ broadcaster and comedian Oliver Callan.
Welcoming attendees, Mairead Reen, Chair of the Life Pharmacy Board, said
“These awards provide an opportunity to pause and reflect on the outstanding work and support that our pharmacy teams across Ireland deliver for their communities every day. It’s so important to recognise and celebrate the commitment and impact of each of you, and whose efforts truly make a difference in the lives of those you serve.”
In total, 12 awards were presented on the night, celebrating excellence across a range of categories, from health services and customer engagement to sales and pharmacy standards. The awards were each sponsored by suppliers of Life Pharmacy and chosen by a jury made up of Life Pharmacy board non-executive directors Andy Sharkey and Seamus Halford as well as Focus Advertising Managing
Director, Daragh Cafferkey and Retail Excellence
Ireland’s Michelle McBride. The People’s Choice Award was decided by customer votes, making it a particularly meaningful recognition for the winners.
With fierce competition across each category, Laura Garrett, Life Pharmacy Operations Manager commented on the tough

job that the jury had in choosing the winners,
“It’s so encouraging to see our members really embrace the awards year after year and setting an incredibly high standard. Every nominee is deserving of recognition, and I don’t envy the job the jury had in selecting the winners!”
2024 was a particularly successful year for Life Pharmacy. The brand marked a major milestone, welcoming its 100th store in Killeagh, Co. Cork, and this momentum shows no signs of slowing down, with 103 pharmacies now
teams and support office staff to our valued industry partners. It’s through this collective effort that we continue to grow, improve, and deliver exceptional care to the communities we serve.”
Cathy Quaid from McCarthy’s Life Pharmacy was awarded Pharmacy Technician of the Year.
“ I’m truly honoured to be named Pharmacy Technician of the Year.
A huge thank you to my incredible team at McCarthy’s Life Pharmacy—this accolade reflects our shared dedication to patient
and resources that enable us to better serve our community every day. This is not just a personal honour but a reflection of the collective support and guidance that I’ve received through Life Pharmacy.”
This year’s People’s Choice Award, a category which was voted for by customers, was awarded to Mulcahy’s Life Pharmacy MPHC, in Mallow, Cork for the second year in a row. It was a double celebration on the night for the team with Artur Zhukavetz picking up the special recognition award. Owner Henry Murphy,

serving communities across Ireland. Life Pharmacy was also honoured as Ireland’s No. 1 retail brand for customer experience and ranked No. 2 overall in the national Customer Experience Insights Survey (CXi). Mairead Reen added,
“Reaching these milestones, like our 100th store and highly impressive ranking in the national Customer Experience Insights survey is the result of the dedication, hard work, and collaboration of so many—from our pharmacy
care. I’m grateful for the support, inspiration, and teamwork every day! ”
Ronan Good, of Good’s Life Pharmacy in Kimmage, Dublin took home the Pharmacist of the Year award. He was very clear about the important role that being part of the Life Pharmacy symbol group has played for him:
“Being part of the Life Pharmacy network has been invaluable to me and my team. It provides a strong support network, access to shared expertise,

to serve. At Mulcahy’s Life Pharmacy, we strive to provide not only expert care but also a welcoming and supportive environment for everyone who walks through our doors. We’re incredibly grateful to our customers for their trust, loyalty, and continued support.”
Speaking at the awards, Jane Brennan, board member of Life Pharmacy and recipient of the Life Pharmacy Team of the Year Award said,
“This award means so much to our entire team because it comes directly from the community that we are so proud
“We’re so proud to be named Pharmacy Team of the Year! Huge thanks to our incredible team at Brennan’s Life Pharmacy and to our wonderful customers for their support. This award reflects our dedication to delivering the best care every day!”

Life Pharmacy is always looking to expand its community of independent pharmacists. To learn more about joining, contact Laura Garrett at lgarrett@uniphar.ie or speak to any Life Pharmacy board member.
Steroid hormone levels are also influenced by other lifestyle choices and factors such as biological sex and age, according to new research that has just been published in leading international journal Science Advances.

The objective of the research was to expand knowledge and understanding of steroid hormone levels, including corticoids and sex hormones, in healthy women and men over a broad age range. This is the first study to analyse such a large number of hormones in nearly 1,000 healthy people, filling a major gap in the knowledge of molecules that are important for our day-to-day well-being.
The work was conducted by members of the Milieu Interieur consortium and led by Dr Darragh Duffy (Institut Pasteur) and Dr Molly Ingersoll (Institut Pasteur and Institut Cochin (Inserm U1016, CNRS, Université Paris Cité). Dr Jamie Sugrue, a Trinity College Dublin graduate, now a Marie Curie-funded postdoctoral researcher at the Institut Pasteur, is the co-first author. Dr Sugrue also worked with Trinity’s Professor Cliona O’Farrelly to secure a Research Ireland Ulysses grant, which kickstarted the ongoing collaboration between Trinity researchers and the Pasteur and Milieu Interieur consortium teams.
The team involved in this current study found that hormone levels vary according to an individual’s age and sex, but that they are also associated with many other factors, such as genetics and common behaviours.
Notably, many steroid hormone levels, beyond sex hormones, are influenced by oral contraceptive use in women, while in men, smoking was associated with altered levels of nearly every steroid hormone measured.
Additionally, measurement of hormones in the same donors
Cliona O’Farrelly, Professor of Comparative Immunology in Trinity’s School of Biochemistry and Immunology
10 years after the original visit showed that decreases in specific androgens were associated with diverse diseases in aging men, implying that these hormones – which are associated with physical characteristics, and supporting strong bones and red blood cell production – play a role in disease development.
This finding – among others – gives the team numerous avenues to pursue in future research.
Dr Sugrue said, “Even in healthy people, immune responses can vary dramatically. As a first step towards understanding how hormones impact immunity, we worked to understand how hormones themselves vary among people. Our study provides a significant resource for the research community, and generates many new hypotheses for further research. Our next steps will focus on understanding how variation in hormone levels contribute to differences in the immune response between people.”
“My current project is specifically focused on variation in antiviral immune responses in healthy people, and in many ways is a continuation of work that I started during my PhD with Prof. Cliona O'Farrelly at Trinity, where we worked on how antiviral immune system variation can confer resistance to hepatitis C virus
infection. By incorporating the hormone measures into my current analysis I hope to uncover exciting new insights.”
Cliona O’Farrelly, Professor of Comparative Immunology in Trinity’s School of Biochemistry and Immunology, added, “If the COVID pandemic taught us anything it was how different all our immune systems are, and being part of the Milieu Interieur collaboration is giving us Trinity researchers a wonderful opportunity to study the molecular and genetic mechanisms responsible for these differences at scale.”
Co-first author, Dr Léa G Deltourbe, Institut Pasteur & Institut Cochin,added, “This study brings much needed data to a subject that is receiving a lot of interest in the mainstream news and on social media platforms, providing a strong basis for investigating the role of steroid hormones in health and disease, including the impact of endocrine disruptors, the link between stress and cortisol, and the role of sex hormones on our well-being.”
As one example, understanding the potential effects of the contraceptive pill on physical and mental health should lead to a better quality of life for women choosing to use this form of medication.
The exciting results in this paper were first presented to an international audience at the Sex Differences in Immune Health conference recently hosted by Trinity.

Ahead of Bowel Cancer Awareness Month (April), the HSE announced that from 1 April 2025, the eligible age range for BowelScreen is extending to include 70-yearolds. Free bowel screening is now available to everyone aged 59 to 70.
BowelScreen invites eligible people to take part every two years using a simple FIT kit test sent to your home. The instructions are easy to follow and it’s done in the privacy of your own bathroom. Using the kit, you take a sample of your poo and post it back to BowelScreen in a plain freepost envelope. The test looks for a level of blood in your poo. The results will be sent out to you within four weeks.
Professor Padraic MacMathuna, Clinical Director of BowelScreen, said, “BowelScreen is a valuable health check that can prevent cancer developing and save lives by finding cancer early. For most people, the FIT kit will be the only test needed. About 3%* of people who do the BowelScreen test are referred for a follow-up colonoscopy.
“This is where we look for and remove pre-cancerous changes, called polyps, from the lining of the bowel. We know that most bowel cancers develop from polyps so by doing this we can prevent cancer.
“Bowel screening is an important part of your normal healthcare routine, but it won’t find all changes.
“Cancer can develop at any time. If you are experiencing symptoms, including changes in your bowel habits, unexplained weight loss or blood in your poo then you should see a GP, even if you have recently had screening or you’re due to take your next screening test. Don’t wait for screening, see your GP if you have symptoms.”
For more information, visit hse.ie/bowelscreen.




Our Medicare Insect Repellent Sprays are available in a fully stocked CDU



Medicare’s Insect Repellent DEET Spray provides protection against stings, bites and ticks when spending time outdoors or by water. Effective protection against mosquitoes, ticks and midges.
Medicare Insect Repellent Spray 30% Citriodiol ® is a strong insect-repellent based on lemon-eucalyptus extracts (without DEET).
Mosquitoes: 4-6 hours Ticks: 4-6 hours
Mosquitoes: 6 hours Ticks: 3 hours
8 hours Ticks: 4 hours
Mosquitoes: 10 hours Ticks: 5 hours
Freephone: 1800 307777 Email: pharmacy@flemingmedical.ie
Written by
Ella


Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel conditions characterised by periods of relapsing and remitting disease and progressive bowel damage. The complexity of IBD management and diagnosis lies in its heterogeneous presentation, unpredictable disease course, and the need for lifelong monitoring to prevent complications such as strictures, fistulas, and colorectal cancer. The aims in management of inflammatory bowel disease include amelioration of patients’ symptoms, to promote endoscopic and histological remission in order to support long-term clinical remission and avoidance of IBD complications.1 Traditionally, this was achieved through regular surveillance endoscopy; the gold standard for assessing mucosal inflammation and guiding therapeutic decisions. Colonoscopy allows for direct visualisation of the intestinal mucosa, enabling biopsies for histopathological evaluation, which remains essential for confirming disease activity and dysplasia surveillance.2 However, it is not without its drawbacks. Endoscopy is an invasive, time-consuming, and costly procedure and creates a burden on ever-growing Irish waiting lists. It may also be associated
with lower patient tolerability, anxiety and discomfort.3 In addition to colonoscopy, crosssectional imaging modalities such as magnetic resonance enterography (MRE) and computed tomography (CT) are frequently employed to assess disease extent, complications, and extramural involvement. While these imaging techniques provide crucial insights, they can also be expensive, time-consuming, and, in the case of CT, expose patients to ionizing radiation.4
In recent years, intestinal or bowel ultrasound (IUS) has emerged as a valuable, non-invasive tool for evaluating IBD. Intestinal ultrasound utilises high-frequency sound waves to generate realtime images of the bowel wall and surrounding structures.5 IUS visualises all bowel wall layers (mucosa, submucosa, muscularis propria, serosa), enabling assessment of transmural inflammation, a hallmark of CD.
Unlike endoscopic procedures, IUS is non-invasive, requires no bowel preparation or sedation, and can be performed at the bedside. Key parameters assessed during IUS include:6
- Bowel Wall Thickness (BWT): Increased thickness (>3 mm in the small bowel and right colon, >4 mm in the left colon) often correlates with active inflammation
- Bowel Wall Hyperaemia using doppler ultrasound: marker of active inflammation
- Wall Stratification: Loss of the normal five-layer bowel wall structure suggests severe inflammation or fibrosis
- Surrounding lymphadenopathy and free fluid: can indicate more severe disease
- Complications associated with IBD: strictures, abscesses and fistula
Unlike colonoscopy, which primarily evaluates mucosal disease, IUS provides additional information on transmural inflammation and extramural complications, making it a complementary modality rather than a replacement.5 The integration of IUS into routine clinical practice has the potential to enhance patient care by providing immediate insights during consultations, thereby supporting a treat-to-target approach in IBD management.
Global Use
Numerous studies internationally, particularly those performed in the US and central Europe, have shown promising results for the introduction of IUS as a standard of care for IBD. The TRUST study was a large multicentre project conducted in over 45 German hospitals which found that IUS can be used to effectively monitor treatment response in patients with active CD.8
Whilst information can be extrapolated from these studies, it is potentially more useful to look at similar studies performed in the UK – slightly closer to home with similar patient cohorts and demographics to an Irish population. Literature of note includes the 2018 METRIC trial.17 In a cohort of 284 patients, the authors conclude both MRE and ultrasound have high sensitivity for detecting the presence of small bowel disease and both are valid first-line investigations in the assessment of IBD. In a further UK study in 2022, 260 patients were investigated via colonoscopy or MRE to assess
lower gastrointestinal symptoms, IBD severity or clinical response to treatment.7 Patients were classified into either IUS-suitable or nonsuitable based on clinical factors e.g. IBD patients without dysplasia surveillance or stricture dilatation. This study determined that 28% of endoscopy patients and 55% of MRE patients were deemed IUS suitable. The primary objective of this work was to assess the cost analysis of introducing IUS as a replacement for endoscopy/MRE for those deemed suitable. They concluded that a projected annual saving of £500,000 could be achieved with the implementation of a IUS integrated programme.
Transabdominal ultrasound has been used as a modality for assessing IBD in central Europe; namely Germany and Italy for the preceding decades but has recently been utilised more in other European countries, Canada and the US via a centralised training programmed through the International Bowel Ultrasound Group (IBUS).8 The use of IUS in Ireland has gained acceptance and is now utilised across a small number of sites and will likely have a significant and positive impact on the future management of IBD and other lower GI conditions.9
Firstly, it can provide an accessible point of care investigation which can be performed by an IBD specialist in the outpatient setting. This has the potential to provide immediate treatment altering decisions in the case of an acute IBD flare, aid in endoscopic triage based on severity of findings and reduce the number of invasive tests in cases with a low pretest probability.7 Of note this may be particularly useful in ruling out non-inflammatory conditions such as irritable bowel syndrome and reducing endoscopy demand. Reassuring or normal ultrasound parameters could also be used to justify de-escalation of some immunosuppressants and therefore reduce medication exposure for patients. It also serves to escalate therapy in patients on biologics who have not fully responded to treatment and while not listed as a formal
target in the updated STRIDE-II treat-to-target guidelines for IBD, IUS is recommended as an adjunct to endoscopy, cross-sectional imaging and biomarkers.a
By providing real time information at the patient’s bedside, IUS can have a positive effect on patient understanding of their own condition. Patients with IBD have been shown in the literature to potentially have a poor level of knowledge surrounding their condition and this is particularly prevalent in the pregnant population.11, 12 Misconceptions and false information can lead to detrimental actions such as stopping biologic medications, increasing the risk of a flare during pregnancy. Patients who have undergone point of care IUS have been shown to have a significant self-reported active disease reported better understanding of all aspects of their disease and disease symptoms and were more confident in their ability to make informed decisions about managing their disease.10 There are pregnancy-specific IBD clinics in Ireland and by introducing IUS, particularly in these clinics,
we can help improve patient knowledge, provide reassurance and also prevents unnecessary radiation to pregnant individuals if further investigations are required. While there are limited studies investigating the use of IUS in pregnancy, the studies thus far have shown that IUS is a useful tool in pregnancy and can detect subclinical inflammation and stratify active inflammation in symptomatic patients.13
Lastly, despite improvements in biological therapy, surgical intervention is required in a significant proportion of patients with IBD.14 Long term inflammation increases the risk of developing strictures, abscesses and fistulae and may ultimately result in emergency surgery.15 While CT and MRI remain the gold standard for postoperative assessment, IUS offers a rapid, bedside, and radiation- and bowel preparation-free alternative for early detection of postoperative recurrence. Furthermore, this use of IUS, in combination with faecal calprotectin, has been proven to be a valid and reliable tool for monitoring postoperative
patients with CD, predicting the risk of postoperative recurrence, allowing for earlier escalation or reinstatement of biologics.16
Limitations
With every imaging modality, there are limitations. CT can provide high resolution images of bowel and extraintestinal complications whilst also being readily available particularly in emergency situations such as suspected perforation.14 As mentioned, CT is associated with radiation exposure which is a concern for the predominantly young demographic of patients who require frequent scanning. MRE can provide a radiation free alternative to CT and can provide high diagnostic accuracy for detecting the presence and activity of CD with reasonable interobserver agreement.4 This is in direct comparison to IUS which has the potential to be limited by operator skill and inter-operator variability. Patient preference and experience is also a crucial role in determining a choice of modality.
The METRIC trial found that while the overall burden of MRE was relatively low, it was still notably
higher than that of IUS and patient willingness to undergo repeat testing was less when compared to IUS.9
In conclusion, the field of inflammatory bowel disease continues to evolve and the role of IUS is becoming increasingly significant due to its non-invasive nature, real-time assessment and radiation-free advantages. While traditional imaging modalities such as MRI, CT, and endoscopy remain important in IBD diagnosis and management, IUS offers a costeffective, accessible, and patientfriendly alternative, particularly for disease monitoring and treatment response evaluation. Increased training, investment, awareness of IUS’s diagnostic accuracy and patient benefits will see it become a key component in Ireland’s IBD care strategy. Moving forward, a multimodal approach combining endoscopy, cross-sectional imaging, and IUS will likely optimise disease management, ensuring patients receive timely and effective care.
References available on request
A community pharmacy environment that fosters teamwork ensured high levels of consumer satisfaction. This series of articles is designed for you to use as guide to assist your team in focusing on meeting ongoing CPD targets and to identify any training needs in order to keep the knowledge and skills of you and your team up to date.

The below information, considerations and checklist provides support to enable you to run a team training session and identify opportunities for learning within the topic of Urinary Tract Infections
Urinary tract infections (UTIs) are among the most common infections affecting adult women. One-half of all women will experience at least one UTI in their lifetime. Recurrence of UTIs is common, with a reported rate of 25% within 6 months of the first occurrence.
Urinary tract infection (UTI) is the collective term used to describe infections involving any part of the urinary tract, which includes the kidneys, ureters, bladder and urethra. Infections of the bladder (cystitis) and urethra (urethritis) are known as lower UTIs and infections of the kidneys (pyelonephritis) or ureters are classed as upper UTIs.
Diagnosis of UTI is based primarily on signs and symptoms.
The incidence of UTIs in adult males under age 50 years is low.
Consider:
Up to 40% of women develop a UTI at some point in their life, compared to 12% in men. In men, cystitis is often associated with infection and inflammation of the prostate gland (prostatitis). Anatomically, the female urethra is shorter and located closer to the anus than in males, which makes it easier for bacteria to reach the female urethra and bladder.
Adult women are 30 times more likely than men to develop a UTI, with almost half of them experiencing at least one episode during their lifetime and one-inthree women experiencing their first episode by the age of 24 years. UTIs are most commonly seen in sexually-active young women.
UTIs can be classified as ‘uncomplicated’ (sometimes referred to as a ‘simple’ UTI) or ‘complicated.’ Uncomplicated infections present most frequently in women without any structural or functional abnormality of the urinary tract, any history of renal disease, or other comorbidity (e.g. immunocompromised patients or those with diabetes), which may contribute to more serious outcomes. Complicated UTIs are
Is your knowledge up-to-date on UTI symptoms, causes and treatments?
Can you identify other conditions that result in the same symptoms?
Does the team follow WWHAM protocol at all times?
Can you identify cases where GP referral is necessary?
Are all staff confident and comfortable giving advice and treatment recommendations for patients presenting with symptoms of UTIs?
Do all members of staff know the patients who should avoid cranberry juice or capsules?
associated with a condition or underlying disease that interferes with the patient’s immune mechanisms and increases the risk of acquiring infection.
Recurrent UTIs, defined as at least two UTIs in 6 months or three UTIs in 1 year, are a significant burden for the patient and result in high costs to the health system. The most commonly prescribed regimens are trimethoprimsulfamethoxazole (or trimethoprim alone), nitrofurantoin, cephalexin and the fluoroquinolones at a quarter of the usual daily dose for 6 months.
Symptoms may include:
• Pain or a burning sensation when passing urine
• Needing to urinate more often during the night
• Urine that looks cloudy
• Needing to urinate more urgently than usual
• Blood in the urine
Children with UTIs may also:
• Have a high temperature
• Appear generally unwell
Check your pharmacy team are aware and understand the following key points:
The symptoms of different UTIs
The appropriate OTC products and what symptoms they can help with
The additional measures than can ease symptoms
The potential side-effects of each product and for whom they are unsuitable
The steps that should be taken to prevent discomfort during infection and reduce risk of infection
WWHAM protocol and which patients must be referred to the pharmacist
All patient conversations are conducted with discretion, sensitivity, and appropriate tone.
• Wet the bed or themselves
• Be sick
When to Refer:
• Someone who has symptoms of a UTI for the first time
• A child has symptoms of a UTI
• A man with symptoms of a UTI
• Pregnant women with symptoms of a UTI
• Symptoms of a UTI after surgery
• Symptoms get worse or do not improve within 2 days
Those suffering with UTIs can be advised to take paracetamol up to four times a day to help reduce pain and a temperature. Children can take liquid paracetamol. They should also be advised to rest and drink fluids so that they can pass urine more regularly during the day.
It may also help for sufferers to avoid sexual intercourse until symptoms have passed.
Cranberry juice and capsules
Drinking cranberry juice may help to prevent UTIs. For those with recurring UTIs, higher strength cranberry capsules are recommended.
However, advise customers that they should not consume cranberry juice or cranberry capsules if they are taking warfarin.
Team Your own knowledge is up-to-date, on UTI symptoms, causes, treatments and referral guidelines
Refresh your knowledge of other conditions which may result in the same symptoms
The appropriate training has been provided to all team members, and assessed, for example through observation or role play
You have the appropriate range of products available
WWHAM Protocol forms the basis of all interactions at the OTC counter
Update your CPD record.



Venlatev 37.5 mg, 75 mg and 150 mg hard prolonged-release capsules
Treatment of major depressive episodes.
For prevention of recurrence of major depressive episodes.
Treatment of generalised anxiety disorder.
Venlatev (venlafaxine hydrochloride) Hard Prolonged-Release Capsules Abbreviated Prescribing Information. Presentation: Each hard prolongedrelease capsule contains venlafaxine hydrochloride, equivalent to 37.5mg, 75mg and 150mg of venlafaxine respectively. Indications: Treatment of major depressive episodes, generalised anxiety disorder, social anxiety disorder, and panic disorder, with or without agoraphobia. Also indicated for the prevention of recurrence of major depressive episodes. Dosage and administration: For oral use. Adults: For the treatment of major depressive episodes, the recommended dose is 75m/day, up to a maximum of 375mg/day for patients not responding to the initial dose (dose increases can be made at no less than 4-day intervals). For generalised anxiety disorder and social anxiety disorder, the recommended dose is 75mg/day, up to a maximum of 225mg/day (dose increases can be made at intervals of 2 weeks or more). For panic disorder, the recommended dose is 37.5mg/day for 7 days, then increased to 75mg/day, up to a maximum of 225mg/ day (dose increases can be made at intervals of 2 weeks or more). Treatment across all indications should be assessed on a case-by-case basis. Consult the SmPC for full details. Children and Adolescents: Not recommended for use in children and adolescents under the age of 18 years. Elderly: No specific dose adjustments of venlafaxine are considered necessary based on patient age alone, however, caution should be exercised in treating the elderly. The lowest effective dose should always be used. Renal impairment: No change in dose is necessary for patients with glomerular filtration rate (GFR) between 30-70 ml/minute, however, caution is advised. For patients that require haemodialysis and in patients with severe renal impairment (GFR < 30 ml/min), the dose should be reduced by 50%. Because of inter-individual variability in clearance in these patients, individualisation of dosage may be desirable. Hepatic impairment: In patients with mild and moderate hepatic impairment, in general a 50% dose reduction should be considered. However, due to inter-individual variability in clearance, individualisation of dose may be desirable. There are limited data in patients with severe hepatic impairment. Caution is advised, and a dose reduction by more than 50% should be considered. The potential benefit should be weighed against the risk in the treatment of patients with severe hepatic impairment. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Concomitant treatment with irreversible monoamine oxidase inhibitors (MAOIs) is contraindicated due to the risk of serotonin syndrome. Venlafaxine must be discontinued for at least 7 days before starting treatment with an irreversible MAOI. Precautions and warnings: Depression is associated with an increased risk of suicidal thoughts, self-harm and suicide (suicide-related events). This risk persists until significant remission occurs. As improvement may not occur during the first few weeks or more of treatment, patients should be closely monitored until such improvement occurs. It is general clinical experience that the risk of suicide may increase in the early stages of recovery. Patients with a history of suicide-related events, or those exhibiting a significant degree of suicidal ideation prior to commencement of treatment, are known to be at greater risk of suicidal thoughts or suicide attempts and should receive careful monitoring during treatment. As with other serotonergic agents, serotonin syndrome, a potenially life-threatening condition, may occur with venlafaxine treatment, particularly with concomitant use of other substances that affect the serotonergic neurotransmitter system. If concomitant treatment with venlafaxine and other medicinal products that may affect the serotonergic and/or dopaminergic neurotransmitter systems is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and
dose increases. Mydriasis may occur in association with venlafaxine. It is recommended that patients with raised intraocular pressure or patients at risk for acute narrow-angle glaucoma (angle-closure glaucoma) be closely monitored. Dose-related increases in blood pressure have been commonly reported with venlafaxine. In some cases, severely elevated blood pressure requiring immediate treatment has been reported in post-marketing experience. All patients should be carefully screened for high blood pressure and pre-existing hypertension should be controlled before initiation of treatment. Increases in heart rate can occur, particularly with higher doses. Caution should be exercised in patients whose underlying conditions might be compromised by increases in heart rate. Venlafaxine has not been evaluated in patients with a recent history of myocardial infarction or unstable heart disease, therefore, it should be used with caution in these patients. Convulsions may occur with venlafaxine therapy. Venlafaxine should be introduced with caution in patients with a history of convulsions, and concerned patients should be closely monitored. Treatment should be discontinued in any patient who develops seizures. Cases of hyponatraemia and/or the Syndrome of Inappropriate Antidiuretic Hormone (SIADH) secretion may occur with venlafaxine. Venlafaxine should be used cautiously in patients predisposed to bleeding, including patients on anticoagulants and platelet inhibitors. Clinically relevant increases in serum cholesterol were recorded in venlafaxine-treated patients, therefore, measurement of serum cholesterol levels should be considered during long-term treatment. Mania/hypomania may occur in a small proportion of patients with mood disorders who have received antidepressants, including venlafaxine. Venlafaxine should be used cautiously in patients with a history or family history of bipolar disorder. Aggression may occur in a small number of patients who have received antidepressants, including venlafaxine. The use of venlafaxine has been associated with the development of akathisia. In patients with diabetes, treatment with an SSRI or venlafaxine may alter glycaemic control. Insulin and/or oral antidiabetic dose may need to be adjusted. Serotonin-norepinephrine reuptake inhibitors (SNRIs) may cause symptoms of sexual dysfunction. There have been reports of long-lasting sexual dysfunction where the symptoms have continued despite discontinuation of SNRIs. Venlafaxine should be gradually tapered when discontinuing treatment over a period of several weeks or months according to the patient’s needs. Interactions: Venlafaxine must not be used in combination with irreversible non-selective MAOIs. Venlafaxine must not be initiated for at least 14 days after discontinuation of treatment with an irreversible non-selective MAOI. Venlafaxine must be discontinued for at least 7 days before starting treatment with an irreversible non-selective MAOI. Due to the risk of serotonin syndrome, the combination of venlafaxine with a reversible and selective MAOI, is not recommended. may occur with venlafaxine treatment, particularly with concomitant use of other agents that may affect the serotonergic neurotransmitter system. If concomitant treatment with venlafaxine and an SSRI, an SNRI or a serotonin receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases. The concomitant use of venlafaxine with serotonin precursors is not recommended. Caution is advised if a patient’s therapy includes a CYP3A4 inhibitor and venlafaxine concomitantly, as this may lead to an increase in levels of venlafaxine and O-desmethylvenlafaxine Serotonin syndrome may occur with the concomitant use of venlafaxine and lithium. Caution should be exercised with co-administration of venlafaxine and imipramine and metoprolol. Pregnancy and lactation: Venlafaxine must only be
Treatment of social anxiety disorder.
Treatment of panic disorder, with or without agoraphobia.
administered to pregnant patients if the expected benefits outweigh any possible risk. Discontinuation symptoms may occur in the newborns if venlafaxine is used until or shortly before birth. Some newborns exposed to venlafaxine late in the third trimester have developed complications requiring tube-feeding, respiratory support or prolonged hospitalisation. Such complications can arise immediately upon delivery. Epidemiological data have suggested that the use of SSRIs in pregnancy, particularly in late pregnancy, may increase the risk of persistent pulmonary hypertension in the newborn (PPHN). Observational data indicate an increased risk (less than 2-fold) of postpartum haemorrhage following SSRI/SNRI exposure within the month prior to birth. A risk to the suckling child cannot be excluded, therefore, a decision to continue/ discontinue breast-feeding or to continue/discontinue therapy with Venlatev should be made, taking into account the benefit of breast-feeding to the child and the benefit of Venlatev therapy to the woman. Effects on ability to drive and use machines: Venlatev may impair judgment, thinking, and motor skills. Therefore, any patient receiving venlafaxine should be cautioned about their ability to drive or operate hazardous machinery. Adverse reactions: Agranulocytosis, aplastic anaemia, pancytopaenia, neutropaenia, anaphylactic reaction, inappropriate antidiuretic hormone secretion, thrombocytopenia, delirium, neuroleptic malignant syndrome (NMS), serotonin syndrome, convulsion, tardive dyskinaesia, suicidal ideation, suicidal behaviours, Torsade de pointes, ventricular tachycardia, ventricular fibrillation, angle-closure glaucoma, pancreatitis, hepatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, rhabdomyolysis. Very Common: Insomnia, headache, dizziness, sedation, nausea, dry mouth, constipation, hyperhidrosis (including night sweats). Common: Decreased appetite, confusional state, depersonalisation, abnormal dreams, nervousness, libido decreased, agitation, anorgasmia, akathisia, tremor, paraesthesia, dysgeusia, visual impairment, accommodation disorder, including blurred vision, mydriasis, tinnitus, tachycardia, palpitations, hypertension, hot flush, dyspnoea, yawning, diarrhoea, vomiting, rash, pruritus, hypertonia, urinary hesitation, urinary retention, pollakiuria, menorrhagia, metrorrhagia, erectile dysfunction, ejaculation disorder, fatigue, asthenia, chills, weight decreased/increased, blood cholesterol increased. Consult the SmPC in relation to other side effects. Overdose: In post-marketing experience, overdose with venlafaxine was reported predominantly in combination with alcohol and/or other medicinal products, including cases with fatal outcome. Severe poisoning symptoms may occur in adults after intake of approximately 3g of venlafaxine. Published retrospective studies report that venlafaxine overdose may be associated with an increased risk of fatal outcomes compared to that observed with SSRI antidepressants, but lower than that for tricyclic antidepressants. Severe poisoning may require complex emergency treatment and monitoring. Therefore, in event of suspected overdose involving venlafaxine, prompt contact with national poison information centre is recommended. General supportive and symptomatic measures are recommended; cardiac rhythm and vital signs must be monitored. When there is a risk of aspiration, induction of emesis is not recommended. Gastric lavage may be indicated if performed soon after ingestion or in symptomatic patients. Administration of activated charcoal may also limit absorption of the active substance. Legal category: POM. Marketing Authorisation Number: PA1986/099/001-003. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00085. Date of Preparation: September 2024.
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
Prescription Only Medicine.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie.
Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Date of Preparation: January 2025 | Job Code: 00100
Further information is available on request or in the SmPC. Product Information also available on the HPRA website.
Navi Group’s newest dispensary software, DispenSense, is the latest breakthrough in pharmacy technology, designed to revolutionise and simplify the dispensing process and workflow management.

Since its launch in December 2022, pharmacies using DispenSense have dispensed over 4.5 million items nationwide.
Navi Group has consistently led the charge in introducing innovative IT solutions to the pharmacy market in recent years. With DispenSense, the core focus of developing the software was to simplify and enhance the dispensing experience. The incorporation of state-of-the-art technologies within DispenSense ensures that it is not just a software solution; it’s a catalyst for positive change within the pharmacy landscape.
This cutting-edge software with unrivalled features and modules being added continuously, strives to make dispensing more reliable, safe, and efficient. DispenSense empowers pharmacy teams to overcome everyday workflow challenges and efficiency blockers, in turn maximising their focus on patient care.
How it was created:
DispenSense was created based on finding solutions for the challenges our independent pharmacy teams were facing on a daily basis.
We created surveys to get feedback our network of independent pharmacies on the challenges they experience daily with their current PMR systems,
what is important to them in a PMR system such as this, and the value they believe we could add to this area.
The key takeaways from our research found that familiarity, ease of use and functionality matter most to pharmacies when choosing a PMR system, with a clear call out for live stock reports as a gap. We also found huge demand for better daily workflow management and integration with other sites used, as well as:
• Price alerts from PCRS
• Better customer service support
• Formal training
• Interaction management
• Easier editing of scripts
• Scanning prescriptions
• Easier claims management
• Improved reporting functionality
What is it:
DispenSense is the first fullycloud based PMR product to be launched in the Irish pharmacy market, using the latest software technologies which are futureproofed to address the challenges facing a pharmacist today and into the future, while also offering great flexibility and efficiencies. It has been designed by pharmacists and technicians with the sole goal of meeting the needs of the current pharmacy industry, while also showing real progression in a stagnant market.
Setting DispenSense apart is its adeptness at embracing the hybrid nature of modern pharmacy practices, seamlessly handling both Healthmail and paper prescriptions. The team behind DispenSense has introduced a custom-built workflow management tool, providing unparalleled transparency and oversight throughout the entire dispensing process.
Notably, DispenSense caters to traditional pharmacies as well, offering the familiarity of the standard dispensing journey but with enhanced efficiency, bridging the gap between innovation and tradition in the pharmacy landscape.
Features:
Taking all our customer feedback into account, the dynamic team behind DispenSense are dedicated to continually enhancing the dispensary system, striving to simplify tasks and enhance the lives of pharmacy staff. Notable features include:
• Advanced workflow management
• Electronic claiming of free contraception scheme
• Monthly family scheme spend summary
• LTI condition validation
• Advanced integration capability
• Sophisticated stock & price file management
• Real-time updates (clinical & product level) with IPU Webservice integration
• Secure, cloud-based system, accessible 24/7
• Nursing home module now available
• Standard laser A4 printing of claim form receipts
• Fully owings compliant & certified
• Unique Healthmail integration with AI patient matching
• Best-in-class industry recognised Stockley's interaction checker
• Unique intervention logging
• IHI (Individual Health Identifier) number now recorded
Positioned as the system for the present and future, DispenSense anticipates and embraces upcoming challenges in the Irish pharmacy market, including electronic prescriptions and vertical integration in the supply chain. The system is not only poised to adapt but to lead the way in incorporating technical and industry changes for the benefit of its customers.
DispenSense is not just another dispensary system. It’s a partner in supporting you to navigate the ever-evolving landscape of dispensary management.
Pharmacies interested in experiencing the future of pharmacy operations can book a demo or explore more about DispenSense at dispensense.ie or by calling 01 4433884

perindopril arginine/ indapamide/amlodipine


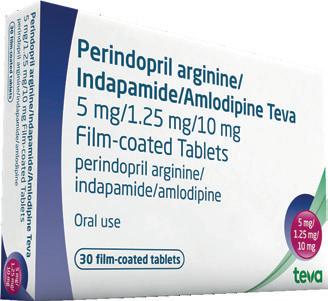

Perindopril arginine/Indapamide/Amlodipine Teva:
As substitution therapy for treatment of essential hypertension, in patients already controlled with perindopril/indapamide fixed dose combination and amlodipine, taken at the same dose level.
Perindopril arginine/Indapamide/Amlodipine Teva Abbreviated Prescribing
Information
Presentation: Each film-coated tablet contains 5mg or 10mg perindopril arginine, 1.25mg or 2.5mg indapamide and 5mg or 10mg of amlodipine. Indications: As substitution therapy for treatment of essential hypertension, in patients already controlled with perindopril/indapamide fixed dose combination and amlodipine, taken at the same dose level. Dosage and administration: For oral use. Adults: One Perindopril/Indapamide/Amlodipine film-coated tablet per day as a single dose. Children: Not recommended for use in children and adolescents. Elderly: Elimination of is decreased in the elderly. Treatment should be initiated after considering blood pressure response and renal function. Renal impairment: Contraindicated in patients with severe renal impairment (creatinine clearance below 30mL/min). In patients with moderate renal impairment (creatinine clearance 30-60mL/min), treatment at doses of 10mg/2.5mg/5mg and 10mg/2.5mg/10mg is contraindicated. In patients with creatinine clearance greater than or equal to 60ml/min, no dose modification is required. Hepatic impairment: Contraindicated in patients with severe hepatic impairment. In patients with mild to moderate hepatic impairment, Perindopril/ Indapamide/Amlodipine should be administrated with caution, as dosage recommendations for amlodipine in these patients have not been established. Contraindications: Hypersensitivity to the active substances, to other sulphonamides, to dihydropyridine derivatives, any other ACE-inhibitor or to any of the excipients. History of angioedema (Quincke’s oedema) associated with previous ACE inhibitor therapy. Dialysis patients. Patients with untreated decompensated heart failure. Severe renal impairment. Moderate renal impairment for Perindopril/ Indapamide/Amlodipine 10mg/2.5mg/5mg and 10mg/2.5mg/10mg. Hereditary/ idiopathic angioedema. Second and third trimesters of pregnancy. Hepatic encephalopathy. Severe hepatic impairment. Hypokalaemia. Combination with nonantiarrhythmic agents causing Torsades de Pointes. Severe hypotension. Shock, including cardiogenic shock. Obstruction of the outflow-tract of the left ventricle (e.g. high-grade aortic stenosis). Haemodynamically unstable heart failure after acute myocardial infarction. Concomitant use with aliskiren-containing products in patients with diabetes mellitus or renal impairment. Concomitant use with sacubitril/valsartan therapy. Extracorporeal treatments leading to contact of blood with negatively charged surfaces. Significant bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney. Precautions and warnings: The combination of lithium and the combination of perindopril/indapamide is usually not recommended. There is evidence that the concomitant use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). If dual blockade therapy is considered necessary, this should only occur under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure. The combination of perindopril and potassium-sparing drugs, potassium supplements or potassium containing salt substitutes is usually not recommended. In patients with normal renal function and no other complicating factors, neutropenia occurs rarely. Perindopril should be used with extreme caution in patients with collagen vascular disease, immunosuppressant therapy, treatment with allopurinol or procainamide, or a combination of these complicating factors, especially if there is pre-existing impaired renal function. There is an increased risk of hypotension and renal insufficiency when patient with bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney are treated with ACE inhibitors. Treatment with diuretics may be a contributory factor. Angioedema of the face, extremities, lips, tongue, glottis and/or larynx has been reported rarely in patients treated with angiotensin converting enzyme inhibitors, including
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
Prescription Only Medicine.
perindopril. This may occur at any time during treatment. In such cases perindopril should be discontinued promptly. Angioedema associated with laryngeal oedema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, which may include subcutaneous epinephrine solution and/or measures to ensure a patent airway, should be administered promptly. There have been isolated reports of patients experiencing sustained, life-threatening anaphylactoid reactions while receiving ACE inhibitors during desensitisation treatment with hymenoptera (bees, wasps) venom. ACE inhibitors should be used with caution in allergic patients treated with desensitisation and avoided in those undergoing venom immunotherapy. Rarely, patients receiving ACE inhibitors during low density lipoprotein (LDL)-apheresis with dextran sulphate have experienced life-threatening anaphylactoid reactions. These reactions were avoided by temporarily withholding ACE-inhibitor therapy prior to each apheresis. Patients with primary hyperaldosteronism generally will not respond to anti-hypertensive drugs acting through inhibition of the reninangiotensin system. Therefore, the use of this product is not recommended. ACE inhibitors should not be initiated during pregnancy; when pregnancy is diagnosed, treatment with ACE inhibitors should be stopped immediately. When liver function is impaired, administration of the diuretic should be stopped immediately if this occurs. If photosensitivity reaction occurs during treatment, it is recommended to stop the treatment. If a re-administration of the diuretic is deemed necessary, it is recommended to protect exposed areas to the sun. Thiazide diuretics and thiaziderelated diuretics may reduce urinary excretion of calcium and cause a mild and transient increase in plasma calcium levels. Thiazides and related diuretics including indapamide have been shown to increase the urinary excretion of magnesium, which may result in hypomagnesaemia. If Perindopril/Indapamide/Amlodipine is prescribed to patients with known or suspected renal artery stenosis, treatment should be started in a hospital setting at a low dose and renal function and potassium levels should be monitored, since some patients have developed a functional renal insufficiency which was reversed when treatment was stopped. A dry cough has been reported with the use of angiotensin converting enzyme inhibitors. If the prescription of an angiotensin converting enzyme inhibitor is still preferred, continuation of treatment may be considered. The risk of hypotension exists in all patients, but particular care should be taken in patients with ischaemic heart disease or cerebral circulatory insufficiency, with treatment being started at a low dose. The safety and efficacy of amlodipine in hypertensive crisis has not been established. Patients with heart failure should be treated with caution. ACE inhibitors should be used with caution in patients with an obstruction in the outflow tract of the left ventricle. In patients with insulin dependent diabetes mellitus (spontaneous tendency to increased levels of potassium), treatment should be started under medical supervision with a reduced initial dose. As with other angiotensin converting enzyme inhibitors, perindopril is apparently less effective in lowering blood pressure in black patients than in non-black patients, possibly because of a higher prevalence of low-renin states in the black hypertensive population. Angiotensin converting enzyme inhibitors can cause hypotension in cases of anaesthesia, it is therefore recommended that treatment with long-acting angiotensin converting enzyme inhibitors such as perindopril should be discontinued where possible one day before surgery. Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Tendency to gout attacks may be increased in hyperuricaemic patients. Interactions: Concomitant use of ACE inhibitors with sacubitril/valsartan, racecadotril, mTOR inhibitors (e.g. sirolimus, everolimus, temsirolimus) and gliptins
(e.g. linagliptin, saxagliptin, sitagliptin, vildagliptin) may lead to an increased risk for angioedema. Hyperkalaemia may occur in some patients treated with Perindopril/ Indapamide/Amlodipine. Some drugs or therapeutic classes may increase the occurrence of hyperkalaemia: aliskiren, potassium salts, potassium-sparing diuretics (e.g. spironolactone, triamterene or amiloride), ACE inhibitors, angiotensin-II receptors antagonists, NSAIDs, heparins, immunosuppressant agents such as ciclosporine or tacrolimus, trimethoprim and cotrimoxazole (trimethoprim/ sulfamethoxazole), as trimethoprim is known to act as a potassium-sparing diuretic like amiloride. The combination of these drugs increases the risk of hyperkalaemia, therefore, the combination of Perindopril/Indapamide/Amlodipine with the abovementioned drugs is not recommended. If concomitant use is indicated, they should be used with caution and with frequent monitoring of serum potassium. Please refer to the SmPC for the full list of known interactions with concomitant products which are either contraindicated, “not recommended”, or “require special care”. Pregnancy and lactation: Not recommended during the first trimester of pregnancy. This medicine is contraindicated during the second and third trimesters of pregnancy. Perindopril/Indapamide/Amlodipine is contraindicated during lactation. A decision should be made whether to discontinue nursing or to discontinue Perindopril/ Indapamide/Amlodipine taking account the importance of this therapy for the patient. Effects on ability to drive and use machines: No studies on the effects of fixed combination of perindopril/indapamide/amlodipine on the ability to drive and use machines have been performed. Perindopril and indapamide have no influence on the ability to drive and use machines but individual reactions related to low blood pressure may occur in some patients. Amlodipine can have minor or moderate influence on the ability to drive and use machines. If patients suffer from dizziness, headache, fatigue, weariness or nausea, the ability to react may be impaired.
Adverse reactions: Agranulocytosis, aplastic anaemia, pancytopenia, leukopenia, neutropenia, thrombocytopenia, hypersensitivity, hyperkalaemia, hypokalaemia, syncope, peripheral neuropathy, angina pectoris, arrhythmia (including bradycardia, ventricular tachycardia and atrial fibrillation), myocardial infarction, Torsade de Pointes (potentially fatal), pancreatitis, gastritis, hepatitis, jaundice, pemphigoid, erythema multiforme, Stevens-Johnson Syndrome, exfoliative dermatitis, toxic epidermal necrolysis, rhabdomyolysis, renal failure, electrocardiogram QT prolongation. Common: Dizziness, headache, paraesthesia, dysgeusia, visual impairment, tinnitus, vertigo, hypotension, cough, dyspnoea, abdominal pain, constipation, diarrhoea, dyspepsia, nausea, vomiting, pruritus, rash, muscle spasms, asthenia. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: There is no information on over-dosage with fixed combination of perindopril/indapamide/amlodipine in humans. For perindopril/indapamide combination the most likely adverse reaction in cases of overdose is hypotension, sometimes associated with nausea, vomiting, cramps, dizziness, sleepiness, mental confusion. For amlodipine, experience with intentional overdose in humans is limited. Gross overdosage could result in excessive peripheral vasodilatation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported. Non-cardiogenic pulmonary oedema has rarely been reported that may manifest with a delayed onset (24-48 hours post-ingestion) and require ventilatory support. Please refer to the SmPC for full guidance on management of overdosage. Legal category: POM. Marketing Authorisation Number: PA1986/108/001-004. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, The Netherlands. Job Code: MED-IE-00091. Date of Preparation: January 2025
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie.
Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Date of Preparation: January 2025 | Job Code: GEN-IE-00114
Further information is available on request or in the SmPC. Product Information also available on the HPRA website.



BENYLIN DAY & NIGHT TABLETS: Composition: Each white (day) tablet contains Paracetamol 500 mg, Pseudoephedrine Hydrochloride 60 mg. Each blue (night) tablet contains Paracetamol 500 mg, Diphenhydramine Hydrochloride 25 mg. Pharmaceutical Form: Day Tablet: Tablet; Night Tablet: Film coated tablet. Indications: For the short-term symptomatic treatment of nasal and sinus congestion associated with daytime symptoms of cold and flu such as pain, headache and/or fever when in combination with bedtime symptoms which are causing difficulty in getting to sleep. Benylin Day & Night is indicated in adult and adolescents aged 15 to 17 years. Dosage: Adults and Adolescents over 15 years: Four tablets should be taken daily. One white tablet (paracetamol and pseudoephedrine) to be taken every 4 to 6 hours during the day (one tablet in the morning, at midday and in the afternoon). Do not take more than 3 white day-time tablets in 24 hours. One blue tablet (paracetamol and diphenhydramine) to be taken at night. Do not take the night-time tablets during the day. Patients should consult their doctor or pharmacist if symptoms persist for more than 3 days or worsen. Not recommended for children and adolescents under 15 years. Elderly: Experience has indicated that normal adult dosage is usually appropriate. However, in frail, immobile, elderly subjects or in elderly patients with renal or hepatic impairment, a reduction in the amount or frequency of dosing may be appropriate. The maximum daily dose should not exceed 60mg/kg/day (up to a maximum of 2g paracetamol per day) in the following situations, unless directed by a physician: Weight less than 50kg, Chronic alcoholism, Dehydration, Chronic malnutrition. Hepatic impairment: In patients with hepatic impairment or Gilbert’s Syndrome, the dose should be reduced or the dosing interval prolonged. The daily dose should not exceed 2g paracetamol/day unless directed by a physician. Renal impairment: Caution should be exercised when administering Benylin Day & Night to patients with mild to moderate renal impairment. It is recommended, when giving paracetamol to patients with renal impairment, to reduce the dose and to increase the minimum interval between each administration to at least 6 hours unless directed otherwise by a physician. See SPC for full details: Contraindications: BENYLIN Day & Night is contraindicated in patients with: hypersensitivity to paracetamol, pseudoephedrine, diphenhydramine or any of the excipients listed in section 6.1. Concomitant use of other sympathomimetic decongestants, beta-blockers or monoamine oxidase inhibitors (MAOIs), or within 14 days of stopping MAOI treatment (see section 4.5). The concomitant use of MAOIs may cause a rise in blood pressure and/or hypertensive crisis. Cardiovascular disease including hypertension, Diabetes mellitus, Phaeocromocytoma, Hyperthyroidism, Closed angle glaucoma, Severe acute or chronic kidney disease /renal failure, Urinary retention in patients at risk of developing respiratory failure. Special warnings and precautions for use: Paracetamol should be administered with caution under the following circumstances: Hepatic impairment, Chronic alcoholism, Renal impairment (GFR ≤ 50ml/min), Gilberts syndrome (familial non-haemolytic jaundice), Concomitant treatment with medicinal products affecting hepatic function, Glucose-6-phosphate dehydrogenase deficiency, Haemolytic anaemia, Glutathione deficiency, Dehydration, Chronic malnutrition, Weight less than 50kg, Elderly patients. Hepatotoxicity at therapeutic dose of paracetamol. Cases of paracetamol induced hepatotoxicity, including fatal cases, have been reported in patients taking paracetamol at doses within the therapeutic range. These cases were reported in patients with one or more risk factors for hepatotoxicity including low body weight (<50 Kg), renal and hepatic impairment, chronic alcoholism, concomitant intake of hepatotoxic drugs and in acute and chronic malnutrition (low reserves of hepatic glutathione). Paracetamol should be administered with caution to patients with these risk factors. Caution is also advised in patients on concomitant treatment with drugs that induce hepatic enzymes and in conditions which may predispose to glutathione deficiency (see sections 4.2 and 4.9). Doses of paracetamol should be reviewed at clinically appropriate intervals and patients should be monitored for emergence of new risk factors for hepatotoxicity which may warrant dosage adjustment. Prolonged or frequent use is discouraged. Paracetamol overdose warning: Taking more than the recommended dose (overdose) may result in severe liver damage. In case of overdose, medical help should be sought immediately. Urgent medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. Patients should be advised not to take other paracetamol-containing products concurrently. Taking multiple daily doses in one administration can severely damage the liver. In such cases, medical assistance should be sought immediately. Caution should be exercised when using the product in the presence of severe hepatic impairment or moderate to severe renal impairment (particularly if accompanied by cardiovascular disease). The hazards of overdose are greater in those with non-cirrhotic alcoholic liver disease. Patients with hepatic disease should consult a doctor before use. In general, medicinal products contains paracetamol should be taken for only a few days without the advice of a physician or dentist and not at high doses.If high fever or signs of secondary infection occur or if symptoms persist for longer than 3 days, a physician should be consulted. Hypersensitivity reactions, including skin rashes, angioedema and anaphylaxis have been reported very rarely with paracetamol. Serious skin reactions such as acute generalised exanthematous pustulosis (AGEP), Stevens-Johnson syndrome (SJS) and Toxic epidermal necrolysis (TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported very rarely in patients receiving paracetamol. Patients should be informed about the signs of serious skin reactions and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity. Ischaemic colitis: Some cases of ischaemic colitis have been reported with pseudoephedrine. Pseudoephedrine should be discontinued and medical advice sought if sudden abdominal pain, rectal bleeding or other symptoms of ischaemic colitis develop. Severe skin reactions such as acute generalized exanthematous pustulosis (AGEP) may occur with pseudoephedrine-containing products. This acute pustular eruption may occur within the first 2 days of treatment, with fever, and numerous, small, mostly non-follicular pustules arising on a widespread oedematous erythema and mainly localized on the skin folds, trunk, and upper extremities. Patients should be carefully monitored. If signs and symptoms such as pyrexia, erythema, or many small pustules are observed, administration of this medicine should be discontinued and appropriate measures taken if needed. Ischaemic optic neuropathy Cases of ischaemic optic neuropathy have been reported with pseudoephedrine. Pseudoephedrine should be discontinued if sudden loss of vision or decreased visual acuity such as scotoma occurs. Although pseudoephedrine has virtually no pressor effects in normotensive patients, this medicine should be used with caution in patients taking antihypertensive agents, tricyclic antidepressants or other sympathomimetic agents (such as appetite suppressants and amphetamine-like psychostimulants). The effects of a single dose on the blood pressure of these patients should be observed before recommending repeated or unsupervised treatment. Posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS): Cases of PRES and RCVS have been reported with the use of pseudoephedrine containing products (see section 4.8). The risk is increased in patients with severe or uncontrolled hypertension, or with severe acute or chronic kidney disease/renal failure. Pseudoephedrine should be discontinued and immediate medical assistance sought if the following symptoms occur: sudden severe headache or thunderclap headache, nausea, vomiting, confusion, seizures and/or visual disturbances. Most reported cases of PRES and RCVS resolved following discontinuation and appropriate treatment.This product is contraindicated for use in patients with pre-existing cardiovascular disease, particularly those with coronary heart disease and hypertension This product is contraindicated for use in patients with thyroid disease, diabetes, glaucoma and, severe renal impairment. Patients with difficulty in urination and/or enlargement of the prostate, should be advised to consult a physician before using pseudoephedrine. This product may act as a cerebral stimulant giving rise to hyperpyrexia, tremor and epileptiform convulsions. Care should be taken when used in epileptic patients. If any of the following occur, this product should be stopped: Hallucinations, Restlessness, Sleep disturbances. Use with caution in occlusive vascular disease. Pseudoephedrine may induce positive results in certain anti-doping tests. Risks of abuse: Pseudoephedrine carries the risk of abuse. Increased doses may ultimately produce toxicity. Continuous use can lead to tolerance resulting in an increased risk of overdosing. The recommended maximum dose and treatment duration should not be exceeded. Diphenhydramine may enhance the sedative effects of central nervous system depressants. While taking this product, avoid alcoholic beverages and consult a healthcare professional prior to taking with central nervous system depressants. Patients with the following conditions should be advised to consult a physician before using diphenhydramine: a respiratory condition such as emphysema, chronic bronchitis, or acute or chronic bronchial asthma; prostate hyperplasia with urinary retention. Concomitant use of other products containing paracetamol or decongestants with Benylin Day & Night could lead to overdosage and should, therefore, be avoided. Night time tablets only: May cause drowsiness. Patients should be advised not to drive or operate machinery if affected. Alcoholic drink should be avoided. Night time tablets only: Patients should be advised not to use with any other product containing Diphenhydramine. The stated dose should not be exceeded. Use in patients with congenital long QT-syndrome should be avoided. Cases of high anion gap metabolic acidosis (HAGMA) due to pyroglutamic acidosis have been reported in patients with severe illness such as severe renal impairment and, sepsis, or in patients with malnutrition or other sources of glutathione deficiency (e.g. chronic alcoholism) who were treated with paracetamol at therapeutic dose for a prolonged period or combination of paracetamol and flucloxacillin. If HAGMA due to pyroglutamic acidosis is suspected, prompt discontinuation of paracetamol and close monitoring is recommended. The measurement of urinary 5-oxoproline may be useful to identify pyroglutamic acidosis as underlying cause of HAGMA in patients with multiple risk factors. The concurrent use of medicinal products, which also prolong the QT interval or result in hypokalemia should be avoided. Immediate medical advice should be sought in an event of an overdose, even if you feel well. Do not take with any other paracetamol containing products. Do not use with any other product containing diphenhydramine, even one used on skin. If symptoms persist or get worse, or if new symptoms occur, patients should stop use and consult a physician. Use when only clearly necessary.
This medicine contains less than 1 mmol sodium (23 mg) per dose, that is to say essentially ‘sodium-free’. Undesirable effects: Immune system disorders: Not known Anaphylactic reaction, hypersensitivity. Investigations: Not known Blood pressure increased; transaminases increased.
Psychiatric disorders: Not known Anxiety, confusional state, euphoric mood, hallucination, irritability. Nervous system disorders: Not known: Agitation, coordination abnormal, convulsion, headache, paraesthesia, psychomotor hyperactivity, sedation, sleep disturbances, tremor, somnolence, Posterior Reversible Encephalopathy Syndrome, Reversible Cerebral Vasoconstriction Syndrome. Eye disorders: Not known Blurred vision, Ischaemic optic neuropathy. Ear and labyrinth disorders: Not known Tinnitus. Cardiac disorders: Not known Palpitations, tachycardia, arrhythmia. Vascular disorders: Not known Hypotension. Respiratory, thoracic and mediastinal disorders: Not known Chest discomfort, dry throat, nasal dryness, dyspnoea. Gastrointestinal disorders: Not known Abdominal pain, constipation, diarrhoea, dyspepsia, vomiting Ischaemic colitis. Skin and subcutaneous tissue disorders: Not known Rash, pruritus, rash pruritic, urticaria, Angiodema, Serios skin reactions, including acute geralised exanthematous pustulosis (AGEP). Renal and urinary disorders: Not known Urinary retention, dysuria. Blood and the lymphatic system disorders: Not known Blood disorders, blood dyscrasias such as thrombocytopenia and agranulocytosis have been reported following paracetamol use, but were not necessarily causally related to the drug. Hepato-biliary disorders: Not known Liver dysfunction. Metabolism and nutrition disorders: Not known High anion gap metabolic acidosis. MAH: JNTL Consumer Health I (Ireland) Ltd. Block 5, High Street, Tallaght, Dublin 24, Ireland. MA Number: PA 23490/006/001. Date of revision of text: March 2025. Product not subject to medical prescription. Supply through pharmacies only. Full prescribing information available upon request BENYLIN NON-DROWSY FOR CHESTY COUGHS SYRUP: Composition: Each 5 mls contains 100 mg guaifenesin and 1.1 mg levomenthol Clear red syrup having a characteristic odour. Indications: The symptomatic relief of productive cough. Dosage: Adults and children
Written by Martin Hynes, Clinical Governance Pharmacist, McCabes Pharmacy
Cough is one of the most common symptoms seen in Irish pharmacies, especially during the colder months. It can range from a benign, self-limiting symptom to an indication of a more serious condition. Classifying coughs by duration (acute, subacute, chronic) and type (dry or productive) helps determine appropriate treatment or the need for referral. Pharmacists can recommend a range of OTC treatments such as antitussives, expectorants, and demulcents, based on the nature of the cough and patient history. With the withdrawal of pholcodine in Ireland, dextromethorphan is now the main suppressant option. Coughs can also be a side effect of medications like ACE inhibitors or arise from conditions like asthma, GORD (gastro-oesophageal reflux disease), or postnasal drip.
Pharmacists must be vigilant for red flags including haemoptysis (coughing up blood), chest pain, or persistent symptoms beyond three weeks. COVID-19 has heightened patient concern about coughs, increasing demand for guidance and reassurance. As highly accessible healthcare professionals, pharmacists play a critical role in managing cough through symptom assessment, patient education, product recommendation, and referral. Staying informed on national guidance ensures safe and effective care in the community setting.


1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by IPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Benylin has no editorial oversight of the CPD programmes included in these modules
Learning Objectives:
• Understand the classification of coughs by duration and cause
• Review the appropriate use of OTC treatments available in Ireland
• Recognise seasonal trends in cough presentations
• Communicate the importance of not ignoring chronic persistent coughs
• Educate patients on appropriate use of antibiotics to treat coughs
• Identify red flags that require referral
• Support patient self-care and safety through effective pharmacy interventions
Introduction
Cough remains one of the most common reasons for patients to visit a community pharmacy. As frontline healthcare professionals, pharmacists are often the first point of contact. While many coughs are self-limiting, they can also indicate underlying conditions requiring medical attention. The main active ingredients in OTC cough medicines have remained relatively constant over the
past 20 years. This CPD article reviews the key aspects of cough management, grounded in current Irish pharmacy practice and patient safety standards.
Types of Cough
Coughs can be classified by their duration, nature, and underlying aetiology (causes). Pharmacists should explore the patient's full history, including associated symptoms, comorbidities, and any use of prescribed medications (e.g. ACE inhibitors).
By Duration:
• Acute cough: Lasts under 3 weeks, typically viral in origin (e.g. common cold or influenza).
• Subacute cough: Lasts between 3–8 weeks, often post-infection. It is common to dispense a prescription for an antibiotic to a patient for a chest infection and for them to return a couple of weeks later with the cough being the last remaining symptom.
• Chronic cough: Persists beyond 8 weeks. A chronic undiagnosed cough should always be referred to the patient’s GP for further investigation.
The most common causes of chronic cough in the community are:
o Postnasal drip. When the nose or sinuses produce extra mucus, it can drip down the back of the throat and cause a cough. This condition also is called upper airway cough syndrome (UACS).
o Asthma. An asthma-related cough may come and go with the seasons. It may appear after an upper respiratory tract infection. Or it can get worse when the patient is exposed to cold air or certain chemicals or fragrances.
o Gastroesophageal reflux disease. In this common condition, also called GORD, stomach acid flows back into the oesophagus. The constant irritation can lead to chronic coughing. The chronic cough can in turn exacerbate GORD.
o Infections. A cough can last long after other symptoms of pneumonia, flu, a cold or another infection of the upper respiratory tract have gone away. A common cause of a chronic cough in adults — but one that often isn't recognized — is whooping cough, also known as pertussis. Chronic



cough also can occur with fungal infections of the lung, as well as tuberculosis infection, also called TB, or lung infection with nontuberculous mycobacteria, also called NTM. NTM is found in soil, water and dust.
o Chronic obstructive pulmonary disease (COPD). Also called COPD, this is a lifelong inflammatory lung disease that limits airflow from the lungs. COPD includes chronic bronchitis and emphysema. Chronic bronchitis can cause a cough that brings up coloured sputum. Emphysema causes shortness of breath and damages the air sacs in the lungs, also known as alveoli. Most people with COPD are current or former smokers.
o Blood pressure drugs. Angiotensinconverting enzyme inhibitors, also called ACE inhibitors, which are commonly prescribed for high blood pressure and heart failure, are known to cause chronic cough in some people.
Coughing persistently throughout each day for weeks or months can affect a person’s quality of life and must not be ignored. An unrelenting cough can lead to:
o Sleep disruption.
o Headache.
o Dizziness.
o Vomiting.
o Sweating
o Unintended bladder loss, also known as urinary incontinence.
o Broken ribs.
Less commonly, chronic cough can also be a sign of:
o Aspiration - when food or other items are swallowed or inhaled and go into the lungs.

Acute cough in children is mainly caused by a self-limiting viral upper respiratory tract infection and resolves without antibiotics within three to four weeks. The other most common causes of cough in children include asthma, respiratory tract infections, and GORD. Associated symptoms can include sneezing and fever in a clinically well child. Antibiotics do not generally improve the overall clinical condition.
For symptom relief, honey can be used in children over one year of age for a soothing effect. Paracetamol and ibuprofen are recommended for associated pain. There are specific children’s cough medicines of the adult versions available, however only for children aged over six years and only on pharmacist or GP advice.
o Bronchiolitis - an infection that causes swelling, irritation and buildup of mucus in the small airways of the lung.
o Pulmonary fibrosis - gradual damage and scarring of the lungs due to a cause that isn't known.
o Lung cancer - cancer that starts in the lungs, including non-small cell lung cancer and small cell lung cancer.
o Non-asthmatic eosinophilic bronchitiswhen airways are inflamed but asthma is not the cause.
o Sarcoidosis - groups of inflamed cells that form lumps or nodules in different parts of the body but most often in the lungs.
In summary, a chronic undiagnosed cough must not be ignored, and patients must be actively counselled to attend their doctor for a referral.
By Nature:
• Dry cough: Non-productive, often viral, allergy-related or triggered by medication.
• Chesty/productive cough: Mucusproducing, common in bacterial infections or chronic respiratory conditions.
General Summary of Causes of Cough in Ireland:
• Upper respiratory tract infections (URTIs): Still the leading cause of acute cough.
• Environmental exposure: Increased pollen in spring, or damp environments during Irish winters.
• Medication-induced: ACE inhibitors (e.g. ramipril, perindopril) are widely prescribed and known to cause dry cough.
• Smoking and vaping: A rising concern, especially among younger patients.
Antibiotics should not be used as the first line of treatment for coughs. Patients with acute cough should be given an explanation about why antibiotics are unlikely to reduce their symptoms and advice about self-care. Antibiotics should not be prescribed for acute cough unless there are systemic features of infection and/or other risk factors present such as chronic lung or heart disease. Here are some tips on explaining why antibiotics are mostly not suitable to treat coughs:
• The common cold and flu, RSV and COVID-19 are all viruses.
• Most sore throats are caused by viruses, especially when there is also a runny nose or cough.
• Chest colds, even when the cough thick, sticky phlegm or mucus last a long time, are most often caused by viruses. Cigarette smoke and pollution can increase the risk getting one of these viruses, but bacteria are not usually the cause.
• Most sinus infections (sinusitis) are caused by viruses. The symptoms are a lot of mucus in the nose and post-nasal drip. Most sinus infections will go away on their own without antibiotics. Mucus that is yellow or green does not necessarily mean a bacterial infection.
• Antibiotic resistance: When antibiotics are not used correctly or used too often, they can cause bacteria to change. That means the medicines won't work as well when they are needed. These antibiotic-resistant infections are more difficult to treat.
Pharmacy colleagues must ensure recommendations comply with HPRA


(Health Products Regulatory Authority) classifications and indications. The goal is to relieve symptoms while supporting the body's natural healing.
1. Antitussives (Cough Suppressants)
Used for dry, irritating coughs.
• Dextromethorphan is widely available and suitable for adults and children over 12.
o Avoid in patients taking Selective Serotonin Reuptake Inhibitors (SSRIs) or Monoamine Oxidase Inhibitors (MAOIs) due to serotonin syndrome risk.
o Not suitable for asthma patients due to potential respiratory depression.
• Pholcodine has been withdrawn in Ireland and across the EU due to safety concerns.
• Codeine carries a risk of sedation, constipation, and dependence
• Cough suppressants should not be used in children under 6 years old
• Products must be used with caution and avoided in productive coughs or in those with underlying respiratory conditions.
2. Expectorants and Mucolytics
Help loosen and expel mucus.
• Guaifenesin is the most commonly available expectorant in Irish pharmacies.
• Often included in combination products
Mucolytics thin and loosen mucus, therefore making it easier to cough it up and clear it. Mucolytics make phlegm less thick and sticky.
• Carbocisteine is available over the counter
3. Demulcents and Soothing Syrups
Provide relief by coating the throat and easing irritation.
• Simple Linctus, honey and lemon preparations, and glycerine-based products are well tolerated and suitable for many age groups.
4. Antihistamines
• Diphenhydramine is a sedating antihistamine and can reduce symptoms associated with upper airway cough syndrome (UACS or postnasal drip). Diphenhydramine can help dry up a cough and runny nose by blocking the effects of histamine.
• Other sedating antihistamines: promethazine, triprolidine
• Sedating antihistamines should not be used in patients with glaucoma or prostate enlargement

• Non-sedating antihistamines (e.g., cetirizine, loratadine) may help in coughs that are caused by allergies .i.e. by targeting the case of the cough.
5. Combination Products
Cough medicines often include multiple ingredients—decongestants (e.g. pseudoephedrine), antihistamines (e.g. diphenhydramine), or analgesics (e.g. paracetamol).
• Pharmacists must counsel on duplication of active ingredients and potential contraindications (e.g. hypertension, pregnancy, glaucoma).
6. Non-Medicinal Measures
• Staying well hydrated
• Using saline nasal sprays or humidifiers
• Avoiding smoking and alcohol
• Rest and good nutrition
• Inhalation of steam (with caution in children and the elderly)
To avoid passing on coughs to others, good cough and hand hygiene steps should be followed and patients should be advised to:
• Wash their hands often with warm water and soap
• Cough into their elbow to stop germs from getting onto their hands and spreading to other people
• Use tissues to trap germs when they cough or sneeze
• Bin all used tissues as quickly as possible
Yearly Trends in Ireland
Cough presentations follow clear seasonal patterns in Ireland. According to HSE surveillance and Irish pharmacy sales data:
• Winter (Oct–Mar) sees peaks due to influenza, RSV, and rhinovirus.
• Spring and Summer trigger allergic coughs due to grass and tree pollens, particularly in the west and midlands.
COVID-19 has altered public perception of cough, with more patients seeking reassurance, antigen tests, and symptom management. Pharmacists have responded by offering extended consultations and symptom screening.
When to Refer (Red Flags)
Pharmacists should be aware of clinical guidelines such as those from the ICGP and HSE.
Immediate GP or A&E referral if:
• Haemoptysis (coughing up blood)
• Chest pain or difficulty breathing
• Unexplained weight loss or night sweats
• Persistent hoarseness
• Oxygen saturation concerns or cyanosis (bluish-purple colour to the skin)
• High-risk groups (e.g. COPD, immunosuppressed, elderly, or infants)
Refer to GP if:
• Cough persists beyond 3 weeks with no signs of improvement
• Suspected asthma, GORD, or post-nasal drip
• Cough worsens or recurs frequently
• Ongoing medication-related cough
• Child under 5 years with significant cough symptoms
• Suspected pertussis (whooping cough), especially during local outbreaks
The Pharmacist's Role in Cough Management
Pharmacists in Ireland are increasingly recognised for their clinical contributions. Cough consultations offer opportunities to:
• Provide accurate self-care advice
• Screen for red flags and ensure appropriate referrals
• Support antimicrobial stewardship by discouraging unnecessary antibiotic use
• Promote vaccination (e.g. flu, pneumococcal, COVID-19 boosters)
• Guide safe product use, particularly in children, older adults, and pregnant women
Putting all the information togetherConsultation Steps in the Pharmacy
Step 1: Ask Open Questions
Pharmacy teams should ask WWHAM:
• Who is displaying the symptoms?
• What are the symptoms?
• How long have they had them?
• What treatments have they tried already?
• Are they taking any other medication?


Leading on from the general WWHAM questions, more specific questions could be asked:
• Duration of cough? Acute (<3 weeks), subacute (3–8 weeks), or chronic (>8 weeks)?
• Productive or dry cough? Assess for mucus production.
• Any associated symptoms? Fever, chest pain, wheezing, weight loss?
• Medication history? Use of ACE inhibitors, sitagliptin, or other drugs?
• Recent travel? Exposure to TB or long-haul flights?
• Smoking history? Chronic cough may indicate COPD or lung disease.
• Occupational/environmental exposure? Smoke, dust, chemicals?
Step 2: Listen
Providing time to listen ensures that the patient feels heard and will be more receptive to advice and treatment recommendations.
Step 3: Determine Type of Cough
• Dry cough: Non-productive, associated with allergies, asthma, or acid reflux.
• Chesty cough: Produces mucus, often caused by infections.
Step 4: Recognise Red Flags
Adults:
• Persistent cough (>3 weeks)
• Unexplained weight loss
• Immunosuppression (e.g., chemotherapy, diabetes)
• Difficulty breathing
• Haemoptysis
• Chest pain
Children:
• Symptoms lasting >3 weeks
• Irritability/drowsiness
• Decreased urine output
• Fever >39°C
• Systemic unwellness
Step 5: Recommend Appropriate Product
Treatment should align with HSE guidelines:
• Self-care: Honey and lemon, hydration, rest.
• Pain relief: Paracetamol, ibuprofen.

• OTC cough medications: Selected based on symptom type and patient preference.
Conclusion
Cough is a multifaceted symptom that requires a thoughtful, patient-centred approach. In the community pharmacy setting, pharmacists have the tools, training, and trust of their patients to provide effective support, ensure safety, and know when to refer. As demand grows for accessible frontline healthcare, the pharmacist’s role in managing cough remains essential and evolving.
References:
https://www.resourcepharm.com/cough-in-thepharmacy-a-practical-approach.html
https://www.pharmacynewsireland.com/cpdmanagement-of-cough-in-pharmacy/ https://ipu.ie/ipu-review-article/coughconsultation-and-treatment-options/ https://pharmaceutical-journal.com/article/ opinion/its-time-to-change-the-way-weapproach-coughs-in-community-pharmacy
https://www.freece.com/blog/overview-ofcough-management/
https://www2.hse.ie/conditions/cough/ https://www.nhs.uk/conditions/antibiotics/
1. What is the most appropriate treatment for a dry, tickly cough in a patient over 12 years old?
A. Guaifenesin
B. Dextromethorphan
C. Amoxicillin
D. Simple Linctus
2. Which of the following symptoms requires immediate referral to a GP or A&E?
A. Mild chesty cough lasting 4 days
B. Hoarse voice for 2 days
C. Blood in sputum
D. Sneezing and nasal congestion
3. Which cough suppressant has been withdrawn in Ireland and the EU?
A. Codeine
B. Pholcodine
C. Dextromethorphan
D. Diphenhydramine
4. What is the most common cause of an acute cough in Ireland?
A. GORD
B. ACE inhibitors
C. Viral Upper Respiratory Tract Infection
D. Asthma
5. Which of the following non-medicinal measures can help relieve a cough?
A. Dehydration
B. Avoiding rest
C. Steam inhalation
D. Taking antibiotics



Ireland’s first dedicated patient stem cell research facility has been opened by RCSI University of Medicine and Health Sciences. The RCSI Patient Stem Cell Discovery Core is a state-of-the-art research lab that will accelerate innovation in stem cell science and play a vital role in advancing personalised and precision medicine.

Minister for Mental Health Mary Butler has announced a significant ¤31 million capital investment for mental health in 2025.
This is the largest ever one-year allocation for mental health infrastructure and forms part of the government’s broader commitment to delivering modern, fit-for-purpose mental health facilities. It will also ensure the development of facilities so that patients can access highquality services in communities across Ireland.
Minister Butler said, “This ¤31 million investment reaffirms our commitment to delivering improved mental health services in line with Sharing the Vision, our national mental health policy. This is the biggest ever investment in mental health infrastructure in a single year. I am confident that our ongoing investment will allow us to continue to upgrade and develop new vital infrastructure, which in turn will ensure people experiencing mental ill-health can access services in safe, therapeutic and recovery-focused environments.”
Located at RCSI’s Smurfit Education and Research Centre at Beaumont Hospital, the new facility marks a significant milestone in patient-centered biomedical research in Ireland. It will enable advanced stem cell research and personalised drug testing, with initial applications focusing on lung disease and extending to other conditions over time.
A unique facility in Ireland, the RCSI Patient Stem Cell Discovery Core is fully integrated within both Beaumont Hospital’s clinical services and RCSI’s research ecosystem. Patient samples can be brought directly from the hospital to the research lab just a short walk away, enabling clinically informed and comprehensive testing of personalised treatments. The vision of this facility is that the proximity of the hospital will mean that the impact of discoveries can be felt more quickly, with insights generated in the lab directly informing patient care back in the hospital. This closed loop of research and treatment is particularly valuable in diseases like pulmonary fibrosis, where precise and tailored interventions are essential.
The facility will also support gene editing to advance novel
therapeutic strategies and partnerships with industry and academic institutions to create a collaborative hub for discovery.
Professor Killian Hurley, Associate Professor of Medicine at RCSI and Consultant in Respiratory Medicine at Beaumont Hospital, emphasised its transformative potential: “Through this unique national resource, we can now test new drugs on patient-specific cells grown in the lab. This will enable clinicians to better predict treatment responses and translate this into patient care, particularly in complex lung diseases where a personalised approach is essential. It is a major step forward in our ability to tailor treatments to the individual.”
David Crosby, a double lung transplant recipient, has been a passionate supporter of the new facility and helped raise funds to make it a reality: “As someone living with pulmonary fibrosis and the recipient of a double lung transplant, I know how critical it is to have access to treatments that are tailored to each patient’s condition. This facility will help make treatments more personalised and evidence-based not just for me but for many others living with complex conditions.
I’m proud to have supported something that puts patients at the heart of discovery and care.”
The launch of the RCSI Patient Stem Cell Discovery Core reinforces RCSI’s role as a national and international leader in translational medicine, placing patient outcomes at the centre of research.
Professor Fergal O’Brien, Deputy Vice Chancellor for Research and Innovation at RCSI, said: “We are proud to lead the way with Ireland’s first fully clinically integrated patient stem cell research lab. By enabling our researchers and collaborators to develop stem-cell-based models of disease, we’re accelerating the translation of laboratory discoveries into treatments. This is a true bench-to-bedside resource, advancing precision medicine that is tailored to the individual needs of patients.”
The RCSI Patient Stem Cell Discovery Core is co-funded by RCSI and the generous support from patient advocates. Future work in the facility will be supported by funding from Research Ireland, the Health Research Board, and the European Research Council.
A range of projects in acute mental health, community services and Child and Adolescent Mental Health Services (CAMHS) and specialist services such as Eating Disorders, are being progressed in 2025. Full details are available in the Health Capital Plan 2025. These include:
• major redevelopment of Adult Acute and Psychiatry of Later Life Units across the country
• continued progress on facilities for CAMHS and community mental health services, within the provision of Primary Care Centres
• ensuring the new Specialist Eating Disorders Hub at Mount Carmel Hospital in Dublin is commissioned and begins service
• beginning the necessary feasibility and preparatory studies for Ireland’s first Mother & Baby Perinatal Mental Health Unit to be colocated with the new National Maternity Hospital
Minister Butler continued, “Re-development projects and continued investment in upgrading facilities will ensure that treatment is delivered in the most appropriate settings.”

Utipro Plus AF:
Improves symptoms of cystitis¹
Reduces UTI recurrences² Reduces antibiotic use² Improves quality of life³






A highly anticipated event in the Irish retail calendar, the Irish Pharmacy News OTC and Retail Pharmacy Product Awards are the only industry accolades dedicated to recognising and rewarding companies and their products within the OTC market.
These Awards serve as a platform to celebrate and showcase excellence in product innovation, marketing, and customer value. Since their launch in 2008, they have been committed to highlighting advancements and innovation within the pharmacy sector.
Pharmacies are the most important distribution channel for consumer health, offering a trusted, accessible, and professional environment for healthcare products and advice. Unlike other retail outlets, pharmacies provide expert guidance from trained pharmacists, ensuring that consumers make informed decisions about over-the-counter (OTC) medications, vitamins, and wellness products.
They serve as a critical touchpoint for public health, offering personalised recommendations, preventative care, and early intervention for minor ailments.
Additionally, pharmacies maintain strict quality control and regulatory compliance, ensuring the safety and efficacy of the products they distribute. Their accessibility, expertise, and commitment to patient care make them an essential link between healthcare manufacturers and consumers.
According to IQVIA, a leading global provider of clinical research services, commercial insights and healthcare intelligence to the life sciences and healthcare industries, the total value of the OTC market in Ireland based on IQVIA’s data for 12 months, from May 2023 to April 2024, was ¤571 million. This market grew by 2.1% (value) and decreased by 3.1% (volume) when comparing year-on-year growth. This was significantly lower than the forecasted growth as Ireland’s value sales growth for 2023 was expected to be 6%.
The five major OTC categories (including Pain Relief, Cough, Cold and Respiratory products (CCR), Vitamins and Minerals, Digestive Health, and Habit Treatment) account for ¤450 million of the total value. Unlike other markets, Cough, Cold and Respiratory Products (CCR) is the second largest category in the Irish OTC market.
According to General Manager with IQVIA, Gwynne Morley, “The Global OTC market is expected to grow at +6.8% CAGR during the next five years, 2024-2028. It is also forecasted that Europe’s OTC market will grow at 6.1% in 2024. Growth within Ireland’s OTC market is not as optimistic based on the performance of the first half of the year – sales value growth is flat, 0.4% PPG 6-month April 20242.”
Over the following pages, we
showcase those products that topped the judges scorecards across categories, including Best Beauty Product, Best Marketing/ Training Campaign, Best Baby Skincare Product, Best Sleep/ Stress Product, Best Children and Baby Product, and much more.
The OTC Awards highlight excellence in product innovation and marketing—key factors that help pharmacists enhance customer value. As external retailers and powerful buying groups expand their influence, continuous innovation in the OTC market is essential. With consumers increasingly turning to the internet for health information and online shopping, many are managing ailments without consulting a doctor or pharmacist. However, pharmacists still play a crucial role in guiding customers toward informed and responsible healthcare choices.


CALPOL® Vapour Plug has won ‘the Best Baby & Children’s Product at the OTC Awards 2025. The CALPOL® Vapour Plug can help soothe and comfort children at night and ease breathing. CALPOL® Vapour Plug & Nightlight releases soothing lavender and camomile vapours that comforts children through the night and help with clear and easy breathing.
“The team at CALPOL is absolutely delighted that the CALPOL® Vapour Plug & Nightlight has won Best Baby & Children’s Product at the OTC Awards 2025! We are proud to showcase a product that can provide comfort to children through the night, helping with clear and easy breathing to aid a restful night's sleep.” - Megan Hearty, Commercial Marketing Lead Kenvue

“The Calpol Vapour Plug and Nightlight is an excellent product offering a dual benefit – a nightlight and soothing vapours. Calpol has strong brand recognition within this group. Extensive investment in marketing, including influencer partnerships and pharmacy promotions.”
“Calpol Vapour Plug and Nightlight are no longer just used for kids, but for all cold and/or flu suffers to ease stuffed noses and sinuses. The product works great in the dual purpose format, and is very easy to set up, use and refills are readily available in a number of sizes, providing value for money too.”


Judges comments










The 2025 Award for Best Irish Pharmacy Brand went to Revive Active, which also won the title in 2024.
Galway-based company Revive Active is Ireland’s No.1 Vitamin and Dietary Supplement Brand*, offering 15 premium products to support health at every stage of life. Proudly Irish and manufactured in Mullingar, Revive Active combines science-led innovation with high-quality ingredients. From all-in-one super supplements to targeted solutions like Joint Complex and the new Capsule Collection, the brand is dedicated to helping people enrich their lives.
“This Award is a testament to the strength of our partnerships with pharmacies nationwide and the trust our customers place in our brand. This Award recognises the dedication, innovation, and hard work behind Ireland’s No.1 Vitamin and Dietary Supplement Brand” - Kevin Barry, Sales Director at Revive Active
“Market Leader. This is a top seller. Irish consumers totally trust this Brand.”
“Revive Active is a game-changer for anyone looking to elevate their health and energy levels. It's a highly effective, all-in-one supplement that lives up to its promises, and I wholeheartedly recommend it to anyone looking to boost their vitality and overall health.”






























Best Women’s Product was voted for by our esteemed judging panel and the winner for 2025 is Optibac For Women.
Optibac For Women is a specialist supplement designed for those seeking high-quality friendly bacteria for women’s intimate flora, from Ireland's most trusted and recommended brand of friendly bacteria supplements.
“Everyone at Optibac is absolutely thrilled that our best-selling ‘For Women’ has won an Irish Pharmacy News OTC Award for Best Women’s Product for the third consecutive year! Optibac For Women has been meticulously researched to support women’s intimate health, and it's truly rewarding to see our dedication to excellence recognised again this year” - Madeleine Walsh Ireland
Regional Manager
“Optibac For Women is an outstanding probiotic supplement that comes highly recommended for supporting women’s health. Customers consistently praise its effectiveness in promoting a healthy balance of gut bacteria, which is essential for digestive health, immunity, and overall well-being. The specially selected strains of probiotics in this formula, such as Lactobacillus and Bifidobacterium, are particularly beneficial for maintaining vaginal health, supporting urinary tract function, and enhancing digestion.”
“Consumers are becoming more educated about probiotics, so providing more detailed information about the specific strains included in the product and how they benefit women's health would help build even more trust. Clear explanations about the science behind the formulation would resonate well with educated consumers.”




Best VMS product was awarded to Zest Active - an innovative super supplement from Irish Supplement company Revive Active.
Zest Active contains a combination of 25 active ingredients, including vitamins, minerals, amino acids, beta glucans and choline all delivered in one daily powdered sachet. It offers multiple benefits, including support for the immune system, energy, brain and muscle function.

“Zest Active is a daily go-to for immune support, energy, brain, and muscle function. This win recognises the growing love for this powerhouse sachet and the incredible community who start their day with Zest Active” - Triona McDonald, Irish Brand and Retail Activations Manager at Revive Active
“Zest Active is an outstanding product that truly delivers when it comes to supporting an active lifestyle. Customers rave about its ability to boost energy, endurance, and overall performance, making it a top choice for those who want to stay active and feel their best. Whether you're tackling a workout, a long day, or recovering after intense physical activity, Zest Active helps keep you energized and focused.”
Judges Comments













The beauty industry is fast-moving and highly saturated. Understanding changing consumer opinions and the behavioural indicators behind global trends is integral to securing the success of any company or brand within the space.
“We’re absolutely thrilled that Sleek N Easy has been named Best Beauty Buy! Created by athletes, for athletes, it delivers sleek, secure hairstyles in seconds—no grease, no crunch, just clean, effective hold.
As more gyms and public facilities move to ban aerosols, Sleek N Easy offers a smart alternative. Perfect for gyms, schools, and on-the-go lifestyles, it’s a standout product your customers will love.
Pharmacies, this is one to stock.
For trade deals and exclusive offers, email us at contact@ sleekneasy.com. Let’s bring sleek, sport-ready haircare to your shelves.” - Katie Nolan, Owner, Sleek N Easy
“The quality of the products is excellent, and each item feels durable and effective. I particularly appreciated the attention to detail in the selection of items – they are practical, easy to use, and provide real value. Whether you're a beginner or someone looking to streamline your workout essentials, this kit has you covered.”
“Not many products are made targeting women in sports, but Sleek Stick both targets these women and girls as well as offering a bespoke solution that sportswomen crave. Not just for athletes, but for anyone with an active lifestyle that wants their hair to stay put, this product needs to be in every gym bag.”



Elave Sensitive Skin took the win for the Best Skincare Product category. Elave Sensitive Care Intensive Ointment is a treatment ointment formulated to protect the skin's natural barrier and reduce flare-ups of eczema, dermatitis, psoriasis and other dry itchy skin conditions.


Regular use reduces flare-ups of eczema, dermatitis, psoriasis and other dry, itchy sensitive skin conditions and can extend breaks between corticosteroid treatments.
“Elave Sensitive skin is delighted to win the Best Skincare Product with Elave Intensive Ointment in the 2025 IPN OTC Awards.
It is our belief that Skin Sensitivity doesn’t have to get in the way of life. By using our gentle and effective skincare regimes, our customers can manage itchy, annoying conditions like psoriasis, dermatitis, rosacea, eczema and sensitive skin, making their daily lives happier.” -
Tom Gardiner, Brand Manager, Gardiner Family Apothecary
“Elave Intensive Ointment is an exceptional product that truly stands out for its ability to provide deep hydration and relief for dry, sensitive skin. Customers rave about how quickly it absorbs and how effective it is in soothing irritated skin, leaving it feeling soft and nourished without being greasy. The fact that it's free from harsh chemicals, fragrances, and parabens makes it a top choice for those with sensitive skin or conditions like eczema and psoriasis.”












Not to damage their winning streak, Scope Healthcare has won the Best Eye Care Product for the fourth consecutive year with new product HYLO DUAL INTENSE®.
HYLO DUAL INTENSE® is the next generation eye drop for the management of symptoms of dry eye disease, providing patients with instant, long-lasting relief. HYLO DUAL INTENSE® contains two dualacting ingredients that work together to break the vicious cycle of dry eye. Working in combination with 2% Ectoin® osmoprotectant, it also contains 0.2% High Molecular Weight Hyaluronic Acid, providing patients with a preservative-free & phosphate-free formulation that gives superior hydrating relief.
HYLO DUAL INTENSE® is conveniently presented in the patented COMOD bottle which offers patients precise dosing with a guaranteed 225 drops and 6 months sterility from first opening.
“Amazing product as it widens the spectrum in eye health! Great advertisement! Appealing packaging! Great work!”
“Hylo Dual Intense Preservative-free Eye Drops are truly exceptional! This product stands out as a top-tier solution for dry, irritated eyes, providing long-lasting relief and comfort. The preservative-free formula is gentle on the eyes, making it an ideal choice for those with sensitive eyes. It’s one of the few eye drops I’ve used that provides immediate hydration and relief without any stinging or discomfort.”
Judges Comments












Alflorex® has scooped the title of Best Digestive Product for 8 years in a row.
Alflorex® is the leading probiotic for IBS, a condition that affects up to 1 in 7 people in Ireland every day. It costs approx. ¤1/day and reduces ALL of the symptoms of IBS including: • abdominal pain • bloating & gas • unpredictable bowel movements like alternating diarrhoea and constipation
“We are thrilled that Irish pharmacists consistently recognise Alflorex as the best digestive health product. We truly appreciate your support! PrecisionBiotics, with its unwavering commitment to rigorous scientific research, has a long-standing reputation for developing high-quality products. Alflorex, a testament to this commitment, has consistently proven to be a top-quality product, endorsed by both the public and industry experts. Thank you for being an essential part of our journey.” - Shirley O’Regan, Marketing Manager, Ireland
“I was really impressed with Alforex®. This product stands out due to its carefully selected ingredients designed to support digestive health effectively. The formulation is well-balanced, focusing on both the gut and overall wellness, which makes it a versatile option for consumers. I appreciate how Alforex combines high-quality ingredients to deliver noticeable benefits, promoting not only healthy digestion but also overall vitality.”
“An excellent product that does get repeat custom. Feel I can sell it with confidence as there is evidence behind it. It has been prescribed on several occasions by GI consultants.”













Taking home their third Award, Revive Active won the Best Immunity Product for 2025.
Revive Active contains a combination of 26 active ingredients including vitamins, minerals and amino acids all delivered in one daily powdered sachet offering multiple benefits, including immune support, energy and heart health.
The perfect way to supplement your body’s natural resources. Revive Active as a brand has never compromised on the quality and quantity of the ingredients it uses and that is why each sachet contains 8 active ingredients that support the immune system. These ingredients include vitamins C, D, B12, and B6, as well as folate, copper, zinc, and selenium. Each daily sachet provides a multifaceted approach to supporting the immune system.
Revive Active is a premium Irish brand that puts the consumer first, ensuring they feel the benefits of taking the product daily.
“This is a proud win for our flagship supplement, Revive Active. Designed to support energy, immune function, and heart health, this product continues to be a favourite among customers. This Award reflects our continued commitment to creating high-quality, effective products that support overall wellbeing and help people feel their best every day” - Joanne Clancy, Senior Brand Manager at Revive Active
“Revive is exceptional its carefully selected blend of vitamins, minerals, and natural extracts. The formula is designed to support both physical and mental health, helping users feel revitalized throughout the day. I’ve noticed a clear improvement in my energy levels and focus after taking it, without the jitters or crashes often associated with other energy supplements. It's the perfect balance of natural ingredients to help maintain energy and mental sharpness, especially during busy or stressful period.”




It was a win for fabÜ SHROOMS MENO & PERI as they took the title of Best Menopause Product 2025.


fabÜ SHROOMS MENO & PERI is an award-winning supplement designed to support women’s well-being during midlife. Formulated by pharmacist Laura Dowling, it blends Lion’s Mane mushroom with essential vitamins and minerals to contribute to hormonal activity, psychological function, metabolism, and bone and muscle function.
“I’m absolutely thrilled to have won these two Awards for my incredible SHROOMS MENO & PERI blend. Hearing from women in pharmacies and online about how much it’s helped them means the world to me. It’s what drives me to keep creating supportive, powerful blends, and to grow fabuwellness into Ireland and the UKs go-to destination for wellness. Huge thanks to the IPN and the judges for this incredible recognition” – Laura Dowling, Founder, fabÜ
“FABÜ SHROOMS MENO & PERI is an exceptional supplement that has earned high praise for its thoughtful formulation and impressive results! Many users rave about how it helps with mood regulation, energy levels, and reducing hot flashes in a natural and effective way.”
“Excellent. A unique Irish product and brand that is super supportive to retailers.”
Judges Comments











Active Iron Pregnancy scooped the Award for Best Pregnancy/Fertility Product 2025.
Active Iron is an Irish iron supplement which is clinically proven to increase iron and energy levels, helping to fight tiredness & fatigue*. Active Iron is specially formulated to avoid constipation, making it kind on the stomach while being strong enough to increase iron levels.
Active Iron Pregnancy is a combination product containing non-constipating Active Iron plus a complete time-release multivitamin, providing all the essential nutrients mum and baby need throughout each trimester.
“We are absolutely delighted to receive the Best Fertility & Pregnancy Product award for Active Iron Pregnancy at the IPN Product Awards. This recognition highlights the hard work and dedication we’ve put into creating a product that truly supports women throughout their pregnancy journey.
“We’re proud to continue offering a clinically proven solution that helps nourish both mum and baby with the highest quality iron.” - Claire Lynch, Head of Marketing, Active Iron
“A good product -the whole range is becoming very well know and recommended by hospitals. Much easier to take than other iron preps and unlike most that are easy to take the iron amount is well absorbed so raises iron sufficiently. A very good product.”
“Active Iron has branched out from being just an Iron supplement, to supporting many different aspects of life. Active Iron Pregnancy being one of the latest, is already find a home in many Medicine Cabinets. Strong Science, backed up with a 360 degree marketing campaign has grown sales of this product immensely.”




Tonstix Throat Pops takes the 2025 Award for Best Children and Baby Supplement. Tonstix Honey Jelly Pops are a breakthrough solution for children’s sore throats and coughs, combining efficacy with child-friendly appeal.



Made from natural ingredients, Tonstix delivers soothing relief while its fun, safe stick design makes this an easy, mess-free option that children love! Recent studies by the Center of Applied Bioscience Research revealed Tonstix honey-based formulation exhibits wound-healing and anti-inflammatory properties, underscoring its dual benefits.
“We are honoured Tonstix Throat Pops has been awarded Best Child & Baby Supplement. Though designed for throat and cough relief, each pop also delivers 15% of a child’s Recommended Daily Allowance (RDA) of Vitamin C and Zinc to support immune health. Thank you to the pharmacy community for your continued support and trust in our growing brand.” - Sinéad & Denise, Tonstix Founders

“The ‘pop’ format is refreshing and easy to consume, making it appealing to both kids and adults. The novelty of the delivery mechanism makes it more user-friendly compared to traditional lozenges or sprays, which can sometimes feel clinical or unappealing.”
“Tonstix has been a marvel since it first appeared. Kids love the taste and texture, parents love the simplicity and ease of use. Ideal for anything from sore throat, to ‘airplane ear’ these have been flying off the shelf since they first landed.”










The 2025 Award for Best Marketing/Training Initiative went to Acnecide 5% Face Gel.
Acnecide 5% Face Gel targets the 3 main causes of acne: bacteria, clogged pores, and excess oil. Containing 5% Benzoyl Peroxide, Acnecide helps control breakouts, unblock pores and reduce blackheads and whiteheads, killing up to 95% of spot-causing bacteria in one week.
Indications: Gel: For the topical treatment of acne vulgaris. Wash: For the cutaneous treatment of acne vulgaris of the face, chest and back, where comedones and papules predominate.
“We are truly honoured to receive this award. With acne becoming more prevalent in adolescents, our goal has been to provide an effective solution that addresses its root causes. We’re thrilled to be expanding into Ireland, helping more teens achieve clearer skin. This recognition reaffirms our commitment to innovation and excellence in the treatment of acne. Thank you for believing in our mission.” –
Nicole Currie, Senior Brand Manager
“The gel is lightweight, non-greasy, and absorbs quickly, making it easy to incorporate into a daily skincare routine. I noticed that it helped reduce redness andinflammation, while also preventing new breakouts from forming, which is a huge plus for anyone struggling with acne.”
“An excellent product with the unique selling point of being a benzoyl peroxide OTC; I recomend this a lot as the last point before prescription items. A very useful product for Pharmacy.”















The title of Best Sleep/Stress Product goes to PrizMAG Pure Magnesium Bisglycinate.
PrizMAG Pure Magnesium Bisglycinate is an advanced magnesium supplement formulated to help alleviate stress, improve sleep quality, and address magnesium deficiency. Made with pure magnesium bisglycinate, it ensures superior bioavailability for effective absorption without digestive upset. Free of magnesium oxide, fillers, and allergens, PrizMAG is the purest choice for relaxation and nervous system support.
“It’s a true honour to have PrizMAG Pure Magnesium Bisglycinate win Best Sleep/Stress Product at the 2025 OTC Awards! We’re so grateful to be recognised for our commitment to quality and transparency. We’ve worked hard to create a genuinely pure, effective formula—no fillers, binders, or manufacturing chemicals—to help people sleep better, manage stress, and feel their best.” - Meg Esser, co-owner, ITL Health
“The product is easy to take, with a smooth texture that dissolves easily, which is a big plus for those who are sensitive to the taste or texture of supplements. The packaging is sleek and convenient, making it easy to incorporate into a daily routine.”
“PrizMag is the best selling Magnesium supplement in out stores, which is a bold claim for a very congested market. The benefits of taking Magnesium are reaching more and more customers everyday, and PrizMag is at the forefront especially for Sleep Aid. An easy to take format, ideal for pre-bedtime routine.”










Solpa Cold and Flu took the top spot in the 2025 Best Cough, Cold, and Flu Product category. Solpa Cold and Flu provides effective relief from cold and flu symptoms, as well as the pain and congestion caused by sinusitis. It helps with aches and pains, headaches, blocked nose, sore throat, chills, lowering of temperature, and loosening stubborn mucus, while providing relief from chesty coughs.
The cough, cold, and flu market is a crucial category for pharmacies, especially during peak seasons. Consumers rely on pharmacies for fast, effective relief from symptoms like congestion, sore throat, and fever, driving strong demand for decongestants, cough syrups, lozenges, and immune-support supplements.
“We're absolutely thrilled and deeply honoured to be recognised as the best cold and flu product! This achievement truly reflects the incredible dedication and hard work of our entire team at Perrigo. Thank you for believing in our unique product.” - Isabella Salazar, Senior Brand Manager, Perrigo
“I am hugely impressed with Solpa Cold and Flu—it is a standout product for relieving the discomfort of cold and flu symptoms. From the moment I started using it, I noticed a significant reduction in my symptoms, including congestion, sore throat, and body aches. The formula is powerful yet gentle, offering fast and effective relief without causing any unwanted side effects like drowsiness.”
“A very well performing product since its launch. The excellent marketing has ensured it has performed well.”






Solpa Cold & Flu Multi Relief Max Powder for Oral Solution, Paracetamol 1000 mg, Guaifenesin 200 mg, Phenylephrine Hydrochloride 12 2 mg
For the relief of symptoms of colds and flu and the pain and congestion of sinusitis, including aches and pains, headache, blocked nose and sore throat, chills, lowering of temperature, and to loosen stubborn mucus and provide relief from chesty coughs Adults, the elderly and adolescents aged 16 years and over: One sachet every 4-6 hours as required to a maximum of 4 sachets (4 doses) in a 24-hour period. Do not give to children and adolescents under 16 years Not to be continued for over 3 days without consulting a doctor. Contraindications: Hypersensitivity to any of the ingredients, severe hepatic impairment, hyperthyroidism, hypertension, diabetes, cardiovascular disease, those taking tricyclic anti-depressants or beta-blockers or other antihypertensive agents, patients who are taking or have taken monoamine oxidase inhibitors within the last two weeks or those currently receiving other sympathomimetics, phaeochromocytoma, closed angle glaucoma, and porphyria. Precautions: circulatory disorders, chronic alcoholism, urinary retention or prostatic hypertrophy, gilbert’s syndrome, concomitant treatment with flucloxacillin and medicinal products affecting hepatic function, glucose-6-phosphate dehydrogenase deficiency, haemolytic anaemia, glutathione deficiency, dehydration, chronic malnutrition, elderly, adults and adolescents weighing less than 50kg, may act as a cerebral stimulant. Use with paracetamol-containing products Do not take with alcohol. Pregnancy and lactation: Not to be taken during pregnancy or whilst breast feeding without medical advice. Side effects: Thrombocytopenia, agranulocytosis, anaphylaxis, severe cutaneous hypersensitivity, bronchospasm, hepatic dysfunction, acute pancreatitis, angioedema, angle glaucoma. See SmPC for full list of side effects and further information. Product not subject to medical prescription PA1186/021/003 MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: Feb 2023 SPC: https://www.medicines.ie/medicines/solpa-cold-flu-multi-relief-max-powder-for-oral-solution-35312/spc#tabs MAT-4747







A new category for this year, Best Sports Product went to O.R.S Sport Hydration Tablets.
O.R.S Sport Hydration Tablets are expertly developed by Pharmacists to meet the hydration needs of athletes and fitness enthusiasts, following the World Health Organisation’s oral rehydration solution guidelines. Approved and recommended by InformedSport.com, they are approved for professional use, ensuring safety and quality for competitive athletes.
“We are delighted that O.R.S Sport has won Best Sports Product at the OTC & Retail Pharmacy Product Awards. A lot of work went into the Sport sku to ensure we had an offering for those needing an additional proven formula to reduce fatigue and support muscle function, and restore electrolyte balance for those working out, in training or just being more active” – Nuala-Anne Curley, Head of Marketing, Pharmed
“Customers consistently praise these tablets for their ability to quickly replenish electrolytes and keep them feeling energised and refreshed, whether after a workout or during a long day of physical activity. The carefully balanced formula of sodium, potassium, and magnesium works effectively to support hydration, prevent cramping, and boost endurance—making it a top choice for athletes and active individual.”
“The tablet form is convenient, easy to carry, and simple to use, making it ideal for on-the- go hydration. This is a significant advantage for busy consumers or athletes who need quick hydration during or after exercise.”
Judges Comments











Formulated by Pharmacists, to follow World Health Organisation oral rehydration solution guidelines O.R.S Hydration Tablets contain a scientific blend of electrolytes glucose, salts and minerals for fast and effective hydration that tastes great.


WINNER O.R.S SPORT - BEST SPORTS PRODUCT Winner at the OTC & Retail Pharmacy Product Awards 2025
O.R.S Sport goes further by adding Magnesium and Vitamin D to help reduce fatigue and support muscle function. It restores the salts and minerals lost during exercise and helps balance electrolytes too.









fabÜ SHROOMS MENO & PERI won the 2025 Best Natural Product Award.
fabÜ SHROOMS MENO & PERI is an awardwinning supplement designed to support women’s well-being during midlife. Formulated by pharmacist Laura Dowling, it blends Lion’s Mane mushroom with essential vitamins and minerals to contribute to hormonal activity, psychological function, metabolism, and bone and muscle function. It is the first supplement on the Irish market to focus on this stage of life. The feedback since launch has been incredible. Women simply love it! Word of mouth and organic user experience content have helped make this one of the top-selling products for women’s wellness in Ireland.
“What truly impressed me is the noticeable improvement in overall well-being. I felt more balanced, both physically and mentally, and it helped reduce the occasional mood swings and fatigue that can accompany this phase of life. The product is not only effective but incredibly easy to incorporate into daily routines, making it a no-fuss addition to any wellness regimen.”
“fabÜ Shrooms Meno & Peri has been a sensation for many women since its launch Backed by the fabulous pharmacist Laura, testimonials for this product speak for themselves, with an amazing blend and tried and trusted vitamins balanced with mushrooms to give customers a natural option to fight the many symptoms of Perimenopause and Menopause.”
Judges Comments



Are you interested in improving breastfeeding training and education among community pharmacists?
A national study* has commenced to explore the breastfeeding knowledge, practices and training among community pharmacists in Ireland. This information will inform future national developments in infant feeding, education and training programmes and support breastfeeding practices in Ireland.
To participate in the study Community Pharmacists will be asked to complete a short online questionnaire. Scan the QR code to get started.
For further information about the study please get in touch via email denise.mcguinness@ucd.ie or niamh.vickers@ucd.ie
*This study has received UCD Ethics approval
www.ucd.ie/nmhs


Survey Open From:
3rd April – 6th June 2025

Scan QR Code to take the Survey
“Forging
the future of healthcare.”


The Cosmetic Association Christmas Trade Show will be held on Sunday May 11th – Tuesday 13th, 2025 at the RDS Dublin.
The Cosmetic Association was formed over 35 years ago to offer the Pharmacy sector a one-stop-shop for their Christmas trade. As the years went by, exhibitors took the opportunity of introducing new brands at the Trade Fair and this was a welcome addition. For Buyers to see a full stand of cosmetics, or a display of perfumes, or a range of jewelry or a display of wrapping / bags, in one day, and in one place, is a huge benefit.
We have extended in a full selection of ‘front of house’ products in Pharmacy. We have included gift retailer, garden center shops and some beauty salons with retail section.
The event will be the most colourful to date and we hope you will all enjoy your visit. Remember, we have free parking within the RDS grounds. Our event is scheduled for May 11th, 12th and 13th 2024 in the Main Hall and Hall 3, in the RDS, Merrion Road, Ballsbridge, Dublin 4, and we will be delighted to see you all there. Registration is available at the door.
Please see a sneak peak of some of the stands and products to look out for.

“Théa Pharma, Ireland’s Number 1 choice for Eye Health, proudly invites you to visit us at Stand D07–D08 at Ireland’s leading Pharmacy Trade Show, May 11–13. With over 150 years of dedication and expertise in eye care, we are global leaders in 100% preservative-free solutions. Discover our wide range of eye care products such as Thealoz Duo (Europe’s Number 1 dry eye drops), Blephaclean (Europe’s Number 1 daily eyelid cleanser), and Nutrof Total (Europe’s number 1 nutritional eye care supplement). Step into the future of eye health with Théa. Your patients deserve nothing less.”


2X LESS REDNESS FINES LINES HEATING SENSATION INFLAMMAGING INNOVATION
1
Limits the inflammaging markers:
3 PHARMACOLOGICAL

Regulates the immune barrier

Reduces inflammation Hydrates
INFLAMMAGING MARKERS


Graham Anthony Distribution
Do you offer your customers choice …
We have brands to suit everyone and all skin types and conditions... Derma, Aesthetic, Clinical, Natural, B-corp. Unbelievable margins, Profitability for shelf space, Consultant lead promos, strong visual Merchandising, assets for Online Platforms, Incentives for you and your staff. Training is key for your pharmacy; it increases sales and motivates your team. Our Training team will discuss which type of training fits your requirements.
Graham Anthony Distribution is an Irish Company bringing you the best brands from Europe and France the home of Skincare for 37 years. Come to our stand at Cosmetic Trade Show in the RDS for unbelievable packages to stock any of our ranges or contact Graham Anthony Distribution on +353 1 8222711 or email sales@gadistribution.ie
This daily anti-inflammaging serum is directly inspired by dermatological research: Uriage Dermatological Laboratories have selected 3 pharmacopoeia-registered molecules: hesperidin, enoxolone and niacinamide These 3 pharmacological molecules, with their soothing and antioxidant action, work in synergy to slow skin ageing by protecting skin cells from chronic inflammation.
A CORRECTIVE TREATMENT - Formulated without fragrance, it gives a sensation of freshness on application. Its green texture neutralises redness. Apply morning and night before your daily skincare routine. For Men and Women
PROVEN IMMEDIATE EFFECTIVENESS - This dermatologist-recommended serum acts on the visible signs of inflammaging. It smoothes fine lines, evens out skin tone and soothes feelings of overheating. Skin is immediately moisturised.
NO. 1 RANGE PRESCRIBED BY DERMATOLOGISTS
*all prescribers combined at CUF 11/24 Available in Pharmacies nationwide.
Photoactivate your Skin, Harness the Sun’s
Martiderm introduces the first range of sunscreens boosted by exposure to UV radiation
Just launched NEW ACTIVE [D] HYDRA GEL SPF50+ which offers very high broad-spectrum protection, is non-comedogenic and water and sweat resistant.
Thanks to exclusive Photo-Active Shield Tech®, the sunscreens are boosted by exposure to the sun, increasing absorbance by 7.7%.1
1Stability of Photo-Active Shield Tech. Study of absorbance over time. Ref: 24/00254 PROTECTION- Lower Concentration of Filters = High Protection
High protection is achieved with a lower concentration of sunscreen. Better for the skin, better for the planet.
Maintains High Protection for Longer


The formula of UV Boost products remains stable with exposure to the sun. With just one application, UV Boost sunscreens maintain an even layer on the skin for longer, ensuring better protection.
Severe hair loss? thinning, fine hair? Stop hair loss, stimulate growth and increase hair density in just 3 months.
The new PHYTOCYANE anti-hair loss dietary supplement is the first formula to act against the three biological causes of progressive hair loss: hormonal, tissue, and vascular. A powerful and concentrated formula against progressive hair loss with active ingredients with scientifically proven effectiveness: pine phytosterols and zinc accelerate hair growth, taurine protects against hair follicle inflammation, and grape polyphenols boost microcirculation to strengthen the fibre. Complementary active ingredients such as L-cysteine, arugula, and a B vitamin complex promote better growth quality.

Men: Severe hair loss, hereditary, thinning hair | Women: Progressive, menopausal, hereditary hair loss Available in Pharmacies nationwide.




Pharmed is a leading healthcare and pharmaceutical services provider across Ireland and the UK. We offer a variety of tailor-made solutions to distribution, sales and marketing for the purpose of representing manufacturers of beauty, pharmaceutical and OTC products. With established global networks, Pharmed provides an extensive range of services to meet the strategic requirements of our partners.
See below some of the great products on display at our stand!
Human+Kind are passionate about caring for the planet and committed to crafting affordable, high-quality skincare products with vegan and natural formulations. Proudly crafted in Ireland, our natural, vegan, and sustainable skincare collection are of the highest quality, carefully formulated to nourish all skin types and ages.


NeoStrata is a dermatologist-founded skincare brand renowned for its commitment to innovation and scientific excellence. NeoStrata offers a wide range of products, using powerful ingredients such as Alpha Hydroxy Acids (AHAs), Polyhydroxy Acids (PHAs), and other advanced compounds, designed to address various skin concerns, from ageing and hyperpigmentation to acne and sensitivity.
Formulated by Pharmacists, to follow World Health Organisation oral rehydration solution guidelines O.R.S Hydration tablets contain a scientific blend of electrolytes, glucose, salts and minerals for fast and effective hydration that tastes great.
Everyday hydration for everyone, O.R.S Hydration Tablets are suitable for adults and kids for everyday wellness, hot weather and travel, sport and exercise.


The 12th Annual Scientific Meeting of the Irish Melanoma Forum (IMF) was held on May 2, 2024, at O'Reilly Hall, University College Dublin (UCD). This significant event brought together experts from research, clinical practice, and patient advocacy to discuss the latest advancements in melanoma research, diagnosis, and treatment. Co-chaired by Professor Shirley Potter and Professor Desmond J. Tobin, the conference highlighted the increasing importance of collaboration in addressing Ireland’s growing melanoma rates.
Professor Potter, a Consultant Plastic and Reconstructive Surgeon at St James’s Hospital and Blackrock Clinic, and Clinical Professor at UCD School of Medicine, emphasised the necessity of a multidisciplinary approach in melanoma management. Her clinical expertise spans melanoma, advanced skin cancer, and head and neck microvascular reconstruction. Professor Tobin, Full Professor of Dermatological Science and Director of The Charles Institute of Dermatology at UCD, brings a robust research background in skin and hair sciences, particularly focusing on pigmentation in health and disease, including melanoma. Their combined leadership reflects a strategic integration of clinical practice and basic laboratory research, aiming to foster comprehensive advancements in melanoma understanding and treatment, as well as melanoma research capacity-building in Ireland.
Key Themes and Discussions
Several key themes shaped the discussions at the forum, focusing on personalised medicine, novel therapeutic approaches, and emerging biomarkers:
- Clinical Research in Phenotypes, Biomarkers, and Histopathology:
Chaired by Prof. Aurelie Fabre and Dr Paula Calvert, this session included a keynote from Prof. Sapna Patel of MD Anderson Cancer Centre, who discussed perioperative systemic therapy in melanoma. Presentations by Mr Tim Arentsen and Ms Katie Hanratty explored gene expression profiling and molecular analysis in both cutaneous and uveal melanoma, emphasising early detection and risk assessment.
- Basic Research: Led by Prof. Breandán Kennedy and Dr Cristina Casalou, this session delved into the mechanisms of melanoma progression.
Prof. Colin Goding from the Ludwig Institute for Cancer research at University of Oxford discussed phenotypic plasticity in melanoma and its role in
therapy resistance. Researchers such as Ms Marta Valenti and Ms Marzia Pendino presented novel research, including the role of oestrogen stimulation and cannabinoid-based therapies for uveal melanoma.
- Melanoma Management:
Chaired by Prof. Derek Power and Prof. Paul Donnellan, this session covered both the psychological and clinical aspects of treatment. Dr Sinéad Lynch highlighted the importance of psycho-oncology in post-cancer care, while Prof. James Larkin from The Royal Marsden NHS Foundation Trust presented on cellular therapy and its potential to reach a clinical inflection point. Dr Garret O’Connell and Dr Chris Cronin addressed surgical excision variations and the development of consensus guidelines for neoadjuvant immunotherapy.
- Sentinel Node Biopsy and Systemic Therapy: Chaired by Dr Aoife Lally and Mr Fiachra Martin, this session featured Prof. Marc Moncrieff, who questioned the relevance of sentinel node biopsy in light of advances in systemic therapy. The session concluded with a Multidisciplinary Team (MDT) discussion, allowing experts to collaborate on complex melanoma cases.
As melanoma rates continue to rise in Ireland, experts highlighted several challenges and areas for future improvement:
- Access to Advanced Therapies: Despite advancements in melanoma treatments, Irish patients face delays in accessing immunotherapies and targeted treatments due to regulatory and funding challenges. Forum attendees called for streamlined approval processes and better access to oncology drugs.
- Public Awareness and Prevention: Experts emphasised the importance of public education on sun safety and early melanoma detection, especially given Ireland's high proportion of fairskinned individuals. Raising
Desmond J. Tobin and Professor Shirley Potter
awareness about the dangers of unprotected UVR exposure remains crucial in reducing the disease burden.
- Research and Biobanking Infrastructure: Prof. Desmond Tobin called for increased investment in melanoma research, particularly in strengthening the country’s biobanking infrastructure. Current bio-banking regulations restrict access to human melanoma tissue samples, hindering translational research.
The IMF 2024 meeting was an invaluable platform for collaboration, where researchers, clinicians, and patient advocates could exchange ideas, share research findings, and work together on challenges. As melanoma cases continue to rise, efforts in research, policy reform, and patient education will be essential in improving patient outcomes and alleviating the disease's impact on Ireland’s healthcare system.
The next Irish Melanoma Forum is scheduled for May 23, 2025, in Dublin, Ireland. The event will feature prominent keynote speakers, including:
- Professor John Kirkwood (University of Pittsburgh) – A world leader in melanoma research, particularly in melanoma biology and targeted therapies.
- Professor David Fisher (Harvard Medical School) – Known for his ground breaking research on molecular and genetic events that underlie melanoma formation
- Professor John Crown (St. Vincent’s Private Hospital & Dublin City University) –

One of the most prominent Irish oncologists, known for his expertise in cancer treatment and his advocacy for personalised medicine approaches in oncology.
- Professor Myles Smith (Royal Marsden Hospital) – Specialising in melanoma surgical oncology, he has contributed significantly to the surgical treatment of melanoma.
In addition to these keynotes, the 2025 meeting will feature parallel sessions such as the Melanoma Clinical Nurse Specialist Parallel Meeting, focusing on patient management, education, and support, and the Cancer Trials Ireland Parallel DSSG Meeting, discussing clinical trials. The Patient Advocate Parallel Meeting will empower advocates with the latest strategies to support melanoma patients and their families.
The 2025 forum promises to build on the successes of 2024 by addressing cutting-edge research and clinical practices in melanoma care and treatment. As the IMF continues to evolve, it serves as a vital platform for healthcare professionals, researchers (incl. early career researchers via travel bursaries etc.) and patient advocates to come together in the fight against melanoma.
For the most up-to-date information on the goals and agenda of the 2025 meeting, it is advised to consult official sources closer to the event date.
ALK’S ALLERGY TREATMENT
ACARIZAX®(12 SQ-HDM SLIT) - NOW APPROVED FOR CHILDREN AGED 5+ IN IRELAND
ALK has announces that ACARIZAX® has received a paediatric indication extension for the treatment of moderate to severe house dust mite allergic rhinitis disease, making it available for children aged 5 years and older in Ireland. Previously approved for patients aged 12+, this development marks a milestone in allergy treatment, broadening access to an innovative therapy that addresses the underlying cause of house dust mite allergic rhinitis.
Studies suggest that allergic rhinitis is the most common allergic condition, affecting approximately 26% of Irish people, with house dust mites being the most common allergen.The condition can lead to disturbed sleep, impaired concentration, worsened asthma symptoms, school absenteeism and increased psychosocial burden.
ACARIZAX® is a sublingual immunotherapy (SLIT) tablet designed to treat the root cause of house dust mite allergy rather than just alleviating symptoms. By gradually desensitising the immune system, SLIT helps reduce the severity of allergic reactions over time, leading to long-term symptom relief and reduced reliance on symptomatic medications such as antihistamines and nasal steroids.
Dr Juan E Trujillo Wurttele and Dr Sadhbh Hurley together as the paediatric allergy team in Cork University Hospital and coordinators of the MSc of allergy and clinical immunology in UCC said, “House dust mite allergic rhinitis is often overlooked but can profoundly impact children’s daily lives. Expanding Acarizax’s indication to younger children represents a major step forward in addressing this unmet medical need, providing a long-term treatment option that can improve both symptoms and overall quality of life.”
ACARIZAX® has been approved for reimbursement in the UK through the NHS for the treatment of moderate to severe house dust mite allergic rhinitis disease in people 12 to 65 years that is persistent despite symptom relieving medicine, following a positive recommendation from NICE (National Institute for Health and Care Excellence). This expands access for eligible patients, providing them with a clinically proven, long-term treatment option that can
significantly improve quality of life while also relieving a financial burden for patients.
ALK General Manager Emil Stage Olsen added, “This extension brings the benefits of Acarizax to younger children. This approval reflects our ongoing dedication to transforming allergy treatment and providing healthcare professionals with advanced solutions for paediatric allergic rhinitis management. It aligns with ALK’s broader mission to improve allergy management and enhance patient outcomes.”
For more information about ACARIZAX® and ALK’s commitment to allergy care, visit www.alk.net.
AYA EXTENDS THREE YEAR BRAND AMBASSADOR PARTNERSHIP WITH ROBBIE HENSHAW THROUGH TO 2028
AYA, a leading name in the health and wellness supplement space, is proud to an-nounce the extension of its partnership with Irish International Rugby Player Robbie Henshaw for an additional three years. Having first joined the brand in 2023, Robbie will continue to serve as the official brand ambassador through 2028, reinforcing the shared commitment to promoting health, fitness, and overall well-being.
Robbie Henshaw, known for his sporting achievements, has been an integral part of AYA’s journey over the past two years. Through inspiring collaborations, engaging campaigns, and authentic endorsements, Robbie has helped elevate the brand’s presence within retail pharmacies and impact within the wellness community. The re-newed partnership solidifies
the mutual success and continued vision of both parties.
AYA is six years in market and has seen double digital growth every year since launch. Last year +37% growth. It is the number one vitamin brand in Allcare and Hickeys and is sold in over 1500 pharmacies nationwide.
“We are excited to extend our partnership with Robbie Henshaw for another three years,” said Jackie Kelly, Business Development Director of Uniphar. Robbie’s passion for wellness and dedication to excellence have played a crucial role in AYA’s brand’s growth within pharmacies over the last two years. We look forward to continuing this successful collaboration and inspiring even more people on their health and fitness journeys’’.
As part of the extended agreement, Robbie Henshaw will remain a key figure in mar-keting campaigns, social media promotions, and upcoming product launches. Robbie will continue to share his personal experiences using AYA’s products, offering fans and followers a genuine perspective on their benefits.
Robbie Henshaw comments on partnership, “I am honoured to continue my partner-ship with AYA. Since 2023, I have seen firsthand their dedication to quality and innova-tion in the wellness space. Being a professional athlete requires stamina, concentra-tion, and a very intense training regime, AYA’s vitamins help me to keep on top of my health and well-being. And I’m excited to keep working together to motivate and empower individuals to prioritize their health and well-being too.
"We were delighted to have brought AYA and Robbie together two years ago, and their three-

year extension proves our belief that when excellence meets excellence in au-thentic partnerships, everyone wins!" –commented Trevor Twamley, CEO of Sport En-dorse.
AYA an Irish owned company is committed to delivering only the highest quality food supplements and vitamins to support health and well-being for all the family. The range of 44 products caters for adults, children, and babies. Exclusive to Irish phar-macy AYA is widely available across the country including Allcare Pharmacy, Life Pharmacy, Hickeys Pharmacy, McCauley Pharmacy, and other leading Groups and Independent Pharmacies, where staff are trained to give advice and guidance on appropriate products. AYA’s researchbacked products are formulated by scientists to deliver noticeable health benefits to customers and are gluten-free and vegan-friendly or vegetarian.
The distinctive blue bottles of AYA’s adult range reflect the iconic apothecary of the old world, with contents that have been formulated based on the latest cutting-edge research. While many of AYA’s colourful kids and baby range are unique products that contribute to the overall development in healthy children, and others help to support certain requirements or conditions.
A HAPPY MOUTH IS A HAPPY MIND – WORLD ORAL HEALTH DAY MESSAGE, 20TH MARCH 2025
Looking after your oral health is a positive step to taking better care of yourself. Each small step added to your daily brushing routine can lead to a brighter smile and lessen the risk of dental and gum disease. From infancy to old age.
World Oral Health Day is an annual event to raise awareness of the importance of oral care of your mouth, teeth, gums, and yourself.
The Dental Health Foundation endorses this important message. Oral health and overall health and well-being are interconnected. Poor oral health increases the risk of developing noncommunicable diseases, for example, cancer, diabetes, respiratory, cardiovascular, and Alzheimer's disease. The World Health Organisation points to 3.5 billion people worldwide suffering from oral disease (half the world population), and 90% of adults will experience cavities at some stage in their lives.
The Dental Health Foundation Ireland’s recent survey discovered that the Irish adult has room for improving their oral hygiene and care practices. In a study of 1,000
people nationwide, 59% of adults brushed twice daily; however, only 49% brushed before bed, despite this time being the most important time in the day.
Spit out toothpaste and don’t rinse after brushing is the best advice, to keep the fluoride in the paste protecting your teeth. Of the cohort that are brushing daily, only 17% follow this advice, and 31% rinse after brushing.
ASTELLAS’ PADCEVTM (ENFORTUMAB VEDOTIN) PLUS KEYTRUDA® (PEMBROLIZUMAB) SHOWS LONG-TERM EFFICACY IN FIRST-LINE TREATMENT OF LOCALLY ADVANCED OR METASTATIC UROTHELIAL CANCER (LA/MUC)
Astellas Pharma Inc. CEO: Naoki Okamura, recently announced additional follow-up results from the Phase 3 EV-302 clinical trial (also known as KEYNOTE-A39) evaluating the efficacy and safety of enfortumab vedotin, a Nectin-4 directed antibody-drug conjugate, plus pembrolizumab, a PD-1 inhibitor, in patients with previously untreated locally advanced or metastatic urothelial cancer (la/ mUC). The results showed a sustained overall survival (OS) and progression-free survival (PFS) benefit consistent with the findings of the primary analysis after an additional 12 months of follow-up (median follow-up of 29.1 months). This data was presented during a rapid oral session (Abstract 664) at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) 2025.
Thomas Powles, M.R.C.P., M.D., Professor of Genitourinary Oncology at Queen Mary University of London; Director, Barts Cancer Center, London; EV-302 Primary Investigator said, “These latest findings from the EV-302 trial reaffirm the primary results, which demonstrated survival improvements for patients treated with enfortumab vedotin and pembrolizumab that were previously unprecedented in locally advanced or metastatic urothelial cancer. These data show that the potential survival benefit has become even more robust with extended follow up and further solidify the combination as standard of care.”
Results showed enfortumab vedotin plus pembrolizumab reduced the risk of death by 49% versus chemotherapy (hazard ratio [HR] = 0.51, 95% confidence interval [CI], 0.430.61). The median OS was 33.8 months for the combination versus 15.9 months for chemotherapy. The OS benefit was observed in all prespecified subgroups,
including cisplatin eligible and ineligible subgroups. Enfortumab vedotin plus pembrolizumab also reduced the risk of disease progression or death by 52% versus chemotherapy (HR = 0.48, 95% CI, 0.41-0.57). The median PFS was 12.5 months for the combination versus 6.3 months for chemotherapy. The safety profile was consistent with previous findings and no new safety concerns were identified.
In addition to longer follow-up data, an exploratory analysis evaluating treatment outcomes and safety profile in patients with confirmed complete response (cCR) will also be presented. Among patients evaluable for response, confirmed objective response rate (cORR) was 67.5% for enfortumab vedotin plus pembrolizumab compared to 44.2% for chemotherapy. Median duration of response (DOR) was 23.3 months (95% CI, 17.8-not estimable [NE]) for the combination and 7.0 months (95% CI, 6.2-9.0) for chemotherapy. A cCR was achieved in 30.4% of patients treated with enfortumab vedotin plus pembrolizumab and 14.5% of patients treated with chemotherapy. Median duration of cCR was not reached for the combination and 15.2 months (95% CI, 10.3-NE) for chemotherapy. In patients with cCR, grade ≥3 treatment-related adverse events occurred in 61.7% of patients in the enfortumab vedotin plus pembrolizumab arm compared to 71.9% in the chemotherapy arm. There were no treatment-related deaths in the cCR subgroup.
SKIN CANCER IN IRELAND: NEW REPORT SHOWS NEARLY 100% SURVIVAL RATE FOR MOST CASES.
A new report published by the National Cancer Registry of Ireland shows good news for skin cancer patients with most people (close to 100%) surviving at least five years after being diagnosed with non-melanoma skin cancer. For melanoma, the more serious type of skin cancer, survival rates have improved significantly to 92% (meaning about 9 out of 10 patients survive at least five years).
Key facts:
• More than 11,000 people in Ireland are diagnosed with skin cancer each year, making skin cancer the most common cancer in Ireland.
• About 9 out of 10 cases are nonmelanoma skin cancers (NMSC), other types include melanoma.
• Almost all non-melanoma patients survive at least 5 years, whereas about 9 out of 10
melanoma patients survive at least 5 years.
• About 270 people die from skin cancer each year in Ireland. What the numbers tell us about different groups:
The incidence rate of certain NMSC cancers (squamous cell carcinoma or SCC) is decreasing in women, and the rate for other forms (basil cell carcinoma or BCC) is stabilising. However, the incidence rate for melanoma in women continues to increase.
For men, the incidence rate of SCC is stabilising, while the BCC incidence rate is increasing at a slower pace. For melanoma, the incidence rate in men stopped increasing since 2015.
Men are more likely to die from skin cancer than women:
• 1.6 times more likely to die from melanoma.
• 2.3 times more likely to die from non-melanoma skin cancer.
Why survival rates are so good:
Most skin cancers (both melanoma and non-melanoma) are caught early (at stage I or II), which makes them much easier to treat successfully.
Commenting on the report, Professor Deirdre Murray, Director of the National Cancer Registry said, "Understanding the trends in skin cancer is vital for public health awareness and prevention strategies. Many of these cancers could be prevented with wider adoption of safe sun practices in our population and avoidance of sun beds."
WEGOVYTM(SEMAGLUTIDE 2.4 MG) AVAILABLE IN IRELAND TO TREAT OVERWEIGHT AND OBESITY
Wegovy® (semaglutide 2.4 mg) is a once-weekly subcutaneous injection that belongs to a class of medicines called glucagonlike peptide-1 (GLP-1) receptor agonists. It is approved by the European Medicines Agency (EMA) for weight management as an adjunct to diet and physical activity to help adults and adolescents, living with a prespecified Body Mass Index (BMI) and/or certain weight-related conditions to lose weight and maintain this weight loss.
Ireland becomes one of more than 15 countries around the world to make Wegovy® available. This treatment, which must be prescribed by a healthcare professional on prescription, and will be dispensed through
pharmacies, is indicated for chronic weight management. It is intended as an adjunct to a reduced-calorie diet and increased physical exercise for:
• Weight control, including weight loss and maintenance, in adults with a BMI of ≥30 kg/m2 (obesity).
• For weight management, including weight loss and maintenance, in adults with a BMI of ≥27 kg/m2 to <30 kg/ m2 (overweight) and at least one weight-related comorbidity (e.g., blood glucose disturbances such as pre-diabetes or type 2 diabetes mellitus, hypertension, dyslipidemia, obstructive sleep apnoea, or cardiovascular disease).
The clinical evidence for the drug is based on the STEP (Semaglutide Treatment Effect in People with Obesity) clinical trial programme, conducted by Novo Nordisk, which has shown that semaglutide at a weekly dose of 2.4 mg enables weight reduction in one out of three patients by 20%, as well as a clinically significant average weight reduction of ~17%, maintained over 2 years. Additionally, the clinical trial named SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) conducted by Novo Nordisk, has demonstrated that semaglutide 2.4 mg leads to a statistically significant reduction of major adverse cardiovascular events (MACE) by 20% compared to a placebo.
Dr Donna Sexton, Clinical, Medical & Regulatory Director, Novo Nordisk Ireland outlines that: “Glucagon-like peptide-1 (GLP1) is a hormone involved in the physiological regulation of appetite and calorie intake. Semaglutide acts as a GLP-1 receptor agonist, i.e., it selectively binds to the GLP1 receptor and activates it. This molecule has a dual effect. On the one hand, clinical trials show that it increases the feeling of satiety and fullness and reduces the frequency and intensity of the feeling of hunger, consequently decreasing caloric intake. On the other hand, it has also shown the ability to lower the level of glucose in the blood, which implies a slight delay in gastric emptying after eating.”
Nina Hovland, General Manager and Vice President of Novo Nordisk Ireland said: “In Ireland, we are seeing positive developments addressing the high prevalence of obesity; there is recognition that obesity is a chronic disease; we have an ‘Obesity Model of Care’ and ‘Clinical Guidelines’ in place and importantly, a commitment to a new ‘Obesity Policy and Action
Plan’, included in the Programme for Government.
“It is very exciting to launch a product that doesn’t just deliver a loss of weight, but a gain in health. Weight is not just about a number, but the benefits that a treatment can provide alongside the loss of weight.
“I am also encouraged to see that one of the first official events by our new Minister for Health, Jennifer Carroll MacNeill T.D., was to host the World Health Organisation (WHO), ‘Demonstration Platform on the Prevention and Treatment of Obesity’, to learn from obesity initiatives in Ireland. This is a significant step for Ireland, signalling a clear intent to address the chronic disease of obesity and builds on the progress that has already been made.
As a company that is dedicated to bringing innovative treatments to people living with obesity, being part of that change in Ireland is exciting and makes me very proud.”
Professor Carel Le Roux said:
“Today’s announcement of the availability of semaglutide 2.4 mg1 for people living with the disease of obesity in Ireland represents a significant development in obesity management. Semaglutide 2.4 mg has demonstrated clinically meaningful weight loss when combined with diet and exercise.
“Semaglutide 2.4 mg was evaluated in the STEP phase 3 clinical trial programme, which included more than 5,000 study participants. Trial data showed that semaglutide 2.4 mg resulted in significantly greater weight loss and health gains versus comparator arms.
“Additionally, in patients with pre-existing cardiovascular disease and a BMI ≥ 27 kg/m2 , but without diabetes, semaglutide 2.4 mg was superior to placebo in reducing the incidence of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke.”
DUBLIN RESEARCH TEAM DEVELOPS NEW INTERNATIONAL GUIDELINES
A groundbreaking new guideline developed by an international team of researchers, led by Irish experts from UCD, RCSI, and St John of God Hospital, has been recognised as providing a clear framework for preventing significant weight gain associated with antipsychotic medications.
Published in Schizophrenia Bulletin, the guideline addresses
a critical gap in clinical practice by offering evidence-based recommendations on the use of metformin, a well-established diabetes medication, to mitigate this distressing side effect.
About 1,500 people develop a psychotic disorder for the first time in Ireland every year and one percent of the population will experience an episode of psychosis in their lifetimeequivalent to 54,000 people across the Irish population.
Most individuals experiencing a first episode of psychosis gain a substantial amount of weight within months of starting antipsychotic treatment, a side effect that can severely impact their long-term physical health.
While lifestyle interventions like diet and exercise are often recommended, the rapid onset of weight gain makes it challenging to counteract with these measures alone. Research has shown that metformin is most effective when introduced alongside antipsychotic treatment or at the earliest signs of weight gain.
“For decades, studies have highlighted metformin’s potential to manage antipsychotic-induced weight gain, but there was no clear guidance on how and when to use it,” says Aoife Carolan, first author of the study and a pharmacist at St John of God Hospital, as well as a PhD student at RCSI. “Our guideline seeks to bridge this gap, providing clinicians with a practical tool to safely and effectively prescribe metformin to prevent excessive weight gain.”
The guideline is distilled into a one-page algorithm containing three core recommendations and essential prescribing information. It is part of the PROGRESS research initiative, led by Prof Brian O’Donoghue from UCD, and is funded by the Health Research Board as part of a broader effort to improve national psychosis treatment protocols. The research team includes psychiatrists, endocrinologists, general practitioners, pharmacists, people with lived experience of psychosis, and international experts.
Michael Norton, lead PPI representative with PROGRESS, emphasised the importance of prioritising physical health in psychiatric treatment. “It is vital that clinicians consider the physical effects of antipsychotic medications. This guideline encourages a holistic approach to treatment, ensuring service users receive comprehensive support in their recovery journey.”
Professor Donal O’Shea, National Lead for Obesity with the HSE, welcomed the publication, stating, “Obesity prevention is critical, and we now understand that medication plays a significant role in weight gain. These guidelines will help identify those most at risk and ensure timely intervention with metformin to mitigate this impact.”
The Irish Cancer Society has warned that lifesaving bowel cancer screening uptake is going backwards. That’s according to the latest available data released by the HSE for 2024.
Marking the beginning of Bowel Cancer Awareness Month (April), the charity is concerned that in 2024, Ireland’s already low target of 45% uptake in bowel cancer screening was missed, with just 41% of those eligible participating.
Around 2,500 people are diagnosed with bowel cancer each year and it is the second most common cause of cancer-related death in Ireland. The earlier bowel cancer is detected the better the outcome for the patient.
Steve Dempsey, Director of Advocacy & Communications at the Irish Cancer Society said: “Screening saves lives. It can detect bowel cancer before signs or symptoms are even present, and that means better outcomes and less harsh treatment pathways. But in 2024, only 2 in 5 people who were eligible to participate in BowelScreen actually took part. The Irish Cancer Society is deeply concerned that the uptake in lifesaving bowel cancer screening is going backwards. Ireland’s target uptake is just 45%, which is nowhere near the EU recommended bowel cancer screening target uptake of 65%.”
He continued: “There is good news. From today, the age eligibility of BowelScreen has been expanded from 59-69 to 59-70. That’s about 48,000 more people eligible over the next two years. But without greater uptake, eligibility means little.”
Tom McHugh, aged 64, from Dublin was diagnosed with bowel cancer in July 2023. It was only after his wife, who is younger than him, received her home test kit, he realised he hadn’t received his own. Tom proactively phoned the service to make sure he was registered and shortly afterwards received his home test kit. It was through BowelScreen that his bowel cancer was detected.
Calling for more people to take part in bowel cancer screening,

Tom McHugh said: “It is quite worrying to me that well over half of people who could be participating aren’t doing so. I am proof that screening works, and for anyone hesitating, I would strongly encourage you to please participate. I was fit and healthy. I had no symptoms whatsoever and yet bowel cancer was there, and it would have gotten a lot worse had it not been caught early through the screening programme.”
Prof. Barbara Ryan, Consultant Gastroenterologist, Tallaght University Hospital & Clinical Professor, Trinity College Dublin, said: “This Bowel Cancer Awareness Month, and every month, it is so important for people to be aware of the possible signs and symptoms of bowel cancer. What we are particularly asking people to look out for is persistent changes – changes that last longer than 3 weeks. Symptoms such as changes in your toilet habits, blood in your poo, weight loss, pain or bloating in your abdomen area and so on can be caused by other things. But if your symptoms have gone on for more than 3 weeks, go to your GP. You can get referred for further testing to either rule out bowel cancer or to detect it as early as possible.”
Signs and symptoms of bowel cancer include:
• Changes in your poo that last longer than 3 weeks.
• A lump, pain, or bloating in your tummy.
• Feeling you have not emptied your bowel fully.
• Losing weight for no reason.
• Blood in your poo, which may look red or black.
• Feeling very tired or breathless.
• Bleeding from your bottom.
For anyone with queries related to bowel cancer, the Irish Cancer Society’s freephone Support Line can be reached at 1800 200 700 or by email at supportline@irishcancer.ie.
CELEBRATING A WIN FOR SUSTAINABILITY IN IRISH HEALTHCARE:
Health Innovation Hub Ireland (HIHI), with the HSE and the Irish College of GPs has announced the winners of its GreenTech in healthcare call today. The three leading healthcare organisations have identified six innovative products and services that promote environmental sustainability in both primary and secondary healthcare. The call was the first step in tackling the research and innovation gap in sustainable product pathways in the Irish health sector. Now, the winning products and services in green healthcare, will be trialled. This allows Irish health providers to pilot, refine and adapt sustainable solutions effectively, before wider implementation. HSE and Enterprise Ireland partnership, HIHI, will lead collaborations across Irish healthcare sites to deliver trialling on the ground.
Winners:
Aerogen: This HIHI GreenTech winner pitched a unique long term multi-disciplinary project developing sustainable medical devices, transitioning to a circular economy model and reducing the environmental footprint of Aerogen’s Solo Nebuliser
products. There are several stages to this work and HIHI looks forward to beginning the first stage, supporting research and problem definition, throughout Irish healthcare sites.
Vanguard AG: This HIHI GreenTech winner pitched a proven solution for the remanufacturing of single-use medical devices. HIHI looks forward to trialing this solution as part of a sustainability pilot in Irish healthcare.
HaPPE: This HIHI GreenTech winner pitched a full cycle biodigestion system, creating a sustainable solution for healthcare waste, specifically compostable PPE and food waste. The solution leverages compostable materials, on-site bio-digestion and advanced decontamination technology. HIHI looks forward to trialing this solution as part of a sustainability pilot in Irish healthcare.
EccoSpray: This GreenTech winner pitched an eco-friendly alternative to traditional ultrasound gels, to measure sustainability, waste reduction and efficiency benefits. The product has previously been trialed by HIHI with positive results on usability and image quality. HIHI now looks forward to trial this solution as part of a sustainability pilot in Irish healthcare.
Offerre: This HIHI GreenTech winner, a new consortium of Irish companies—Offerre, Envetec, DeltaQ, Enva - pitched a multifaceted solution focused on

medical waste treatment and recovery. HIHI now looks forward to mapping this solution to trial it as part of a sustainability pilot in Irish healthcare.
Medfirst Supplies - Safe clean box: This HIHI GreenTech winner, pitched a closed, sealed cabinet system that automates the manual cleaning of RIMDs. Uses sodium bicarbonate (non-abrasive, noncorrosive and water-soluble) with low-pressure compressed air for effective pre-cleaning of medical device. HIHI now looks forward evaluating this solution as part of its sustainability portfolio in Irish healthcare.
The pitches were judged by HIHI Clinical Sustainability Advisors (CSA) from across Ireland who

Some of the partners and winners of the first national HSE, ICGP, HIHI GreenTech in Healthcare call: Will Hogan Ecco Spray, Declan Lacey MedFirst, Eimear Galvin Health Innovation Hub Ireland, Philip Crowley, HSE, Roisin Breen, HSE, Oran Murphy, VanGuard AG and Conor Connelly, Offerre
work on the frontline, and a panel of experts from the HSE, Irish College of General Practitioners and Irish healthcare siteshealthcare sites, including St James’s, TUH, CUH, CUMH, UHG.HIHI, the HSE and the Irish College of GPs will now support the development and trialling of these innovative products and services.
HIHI National Director Dr Tanya Mulcahy said, “Today marks a significant step toward integrating sustainable innovation into Irish healthcare. By identifying and trialling these six pioneering solutions, Health Innovation Hub Ireland, with the HSE and the Irish College of GPs, is fostering real-world impact in environmental sustainability.
“The diversity of the selected products - from circular economy medical devices to eco-friendly ultrasound gels - demonstrates a broad commitment to tackling healthcare’s environmental footprint. This initiative sets a strong precedent for future collaborations in green healthcare innovation."
Some of the winners of the national HSE, ICGP, HIHI GreenTech in Healthcare call: Declan Lacey MedFirst, Will Hogan Ecco Spray, Lisa O’Riordan, HaPPE Earth, Conor Connelly, Offerre and Oran Murphy, VanGuard AG
COSMETIC & GIFT TRADE FAIR

SUNDAY 11TH MAY
10am - 6pm
MONDAY 12TH MAY
9am - 8pm
TUESDAY 13TH MAY
9am - 6pm
The Main Hall, RDS, Ballsbridge, Dublin