Ireland’s Dedicated Hospital Professional Publication
Professional 100 Issue

Start
with OMJJARA momelotinib
patients with myelofibrosis and anaemia in a
Omjjara is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with moderate to severe anaemia who have primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis and who are Janus Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. 1 ACVR1 = activin A receptor, type 1; JAKi = Janus Kinase inhibitor.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Abbreviated Prescribing Information
Omjjara 100, 150 and 200 mg film-coated tablets Abbreviated Prescribing Information (Refer to Summary of Product Characteristics (SmPC) before prescribing). PRESENTATONS: Each 100 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 100 mg of momelotinib and 50.8 mg of lactose monohydrate. Each 150 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 150 mg of momelotinib and 76.1 mg of lactose monohydrate. Each 200 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 200 mg of momelotinib and 101.5 mg of lactose monohydrate. INDICATION: The treatment of disease-related splenomegaly or symptoms in adult patients with moderate to severe anaemia who have primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis and who are Janus Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. POSOLOGY AND ADMINISTRATION: Treatment should be initiated and supervised by a physician experienced in the use of anticancer medicinal products. Omjjara should not be used in combination with other JAK inhibitors. The recommended dose is 200 mg once daily. Complete blood cell count and liver function tests must be performed before initiating treatment, periodically during treatment, and as clinically indicated. For dose modifications due to adverse reactions see SmPC. Treatment with Omjjara should be discontinued in patients unable to tolerate 100 mg once daily. Duration of use: Treatment may be continued for as long as the benefit-risk remains positive for patients, as assessed by the treating physician. Missed dose: If a dose of Omjjara is missed, the next scheduled dose should be taken the following day. Two doses should not be taken at the same time to make up for the missed dose. Elderly (≥ 65 years): No dose adjustment necessary. Renal impairment (>15 mL/min): No dose adjustment necessary. Omjjara has not been studied in patients with end-stage renal disease. Hepatic impairment: No dose adjustment is recommended for patients with mild or moderate hepatic impairment. The recommended starting dose is 150 mg once daily in patients with severe hepatic impairment (Child-Pugh Class C). Paediatric population: No data available. Omjjara is for oral use only and can be taken with or without meals. CONTRAINDICATIONS: Hypersensitivity to momelotinib or to any of the excipients. Pregnancy and breast-feeding. WARNINGS/PRECAUTIONS: Omjjara should not be initiated in patients with active infections. Physicians should carefully observe patients for signs and symptoms of infection and initiate appropriate treatment promptly. Patients with chronic HBV infection who receive Omjjara should have their chronic HBV infection treated and monitored according to clinical HBV guidelines. A complete blood count including platelet count should be obtained before initiating treatment with Omjjara, periodically during treatment, and as clinically indicated. Dose interruption or reduction may be required. Liver function tests should be obtained before initiating treatment with Omjjara, periodically during treatment, and as clinically indicated. If increases in ALT, AST or bilirubin related to treatment are suspected, dose interruption or reduction may be required. Prior to initiating or continuing therapy with Omjjara, the benefits and risks for the individual patient should be considered particularly in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately. Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including Omjjara. However, a causal association has not been established Women using systemically acting hormonal contraceptives should add a barrier method during treatment and for at least 1 week after the last dose of Omjjara. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Omjjara. Patients who experience dizziness or blurred vision after taking Omjjara should observe caution when driving or using machines
INTERACTIONS: Co administration of strong CYP3A4 inducers may lead to decreased momelotinib exposure and consequently a risk for reduced efficacy. Therefore, additional monitoring of the clinical signs and symptoms of myelofibrosis is recommended with concomitant use of momelotinib and strong CYP3A4 inducers (including but not limited to carbamazepine, phenobarbital, phenytoin, and St John’s wort [Hypericum perforatum]). Caution and monitoring for adverse reactions are advised with concomitant use of OATP1B1/1B3 inhibitors, including ciclosporin. Momelotinib may increase exposure to other sensitive BCRP substrates, including sulfasalazine; monitor for adverse reactions. Caution is advised when administering momelotinib with P-gp substrates with a narrow therapeutic index. Caution is advised when administering momelotinib with sensitive substrates of OCT1, MATE1 and MATE2-K (e.g., metformin). Narrow therapeutic index or sensitive substrate medicinal products of CYP1A2 (e.g., theophylline, tizanidine) or CYP2B6 (e.g., cyclophosphamide) should be co-administered with momelotinib with caution. Fertility, pregnancy and lactation: Fertility: No clinical data. Omjjara is contraindicated during pregnancy. If Omjjara is used during pregnancy, or if the patient becomes pregnant while taking this medicinal product, the patient should discontinue treatment and be advised of the potential hazard to the foetus. Omjjara is contraindicated during breast-feeding. UNDESIRABLE EFFECTS: Very common (≥ 1/10): Thrombocytopenia, dizziness, headache, cough, diarrhoea, abdominal pain, asthenia, fatigue. Common (≥ 1/100, < 1/10): Urinary tract infection, upper respiratory tract infection, pneumonia, nasopharyngitis, COVID19, cystitis, bronchitis, oral herpes, sinusitis, herpes zoster, cellulitis, respiratory tract infection, sepsis, lower respiratory tract infection, oral candidiasis, rash, skin infection, gastroenteritis, neutropenia, Vit B1 deficiency, syncope, peripheral neuropathy, paraesthesia, blurred vision, vertigo, hypotension, haematoma, flushing, vomiting, constipation, arthralgia, pain in extremity, pyrexia, ALT increased, AST increased, contusion. For more details on undesirable effects, see SmPC. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nrs: EU/1/23/1782/001- 003. Legal category: POM A. Date of preparation of API: April 2025. Code: PI-14515. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
group of companies. @2025 GSK or licensor. PM-IE-MML-JRNA-250002 | August 2025

Call for papers: make your contribution to Hospital Professional News
Articles
Research Papers
Reviews
Programme Descriptions
Reports
Case Reports
Letters to Editor
Support fellow hospital professionals as well as aspiring junior professionals and early-year hospital pharmacists
Practice reports share innovations on any area of practice, including delivering clinical services, pharmacy administration, or new approaches to inform and engage with patients
Perspective articles focus on a specific field or discipline and discuss current advances or future directions, and may include original data as well as expert insight and opinions












Contents Foreword
Grainne Power takes up new position at HPRA P4
World First for Trials Pioneered in Ireland P6
Hospital Pharmacy Workforce Report – Analysis + Expert Commentary P10
A Year of Leadership, Advocacy and Advancing Clinical Excellence for Hospital Pharmacists P21
IHCA Report: Leadership, Reform and the Realities of a System Under Pressure P25
Beaumont RCSI Cancer Centre 2025 Year in Review P32
Men’s Mental Health in Ireland P42
Professional 100 2025 P50
REGULARS
Feature: Preconceptual Care P82
Conference: ESMO 2025 P86
Feature: Atrial Fibrillation P88
Clinical R&D: P96
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only.
All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission.
IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd
Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis natalie@ipn.ie
EDITOR
Kelly Jo Eastwood kelly-jo@ipn.ie
CONTENT AND DIGITAL
CREATOR
Chantal Thurlby-Alexander
chantal@hospitalprofessionalnews.ie
+353 87 337 9258



ACCOUNTS
Fiona Bothwell fiona@ipn.ie
SALES & BUSINESS
DEVELOPMENT LEAD
Sibongile Swan Mude swan@hospitalprofessionalnews.ie
CONTRIBUTORS
Paul Neill
Michele O’Brien
Victoria Spillane
Averil Power
Joanne Kissane
Mary McDonald
Sharon Rice
Ailbhe Rooney
Donal O’Shea
Aisling Looney
Patrick J McGee
Helen Forristal
Dr Usama Boles
DESIGN DIRECTOR
Ian Stoddart Design

Editor
As 2025 draws to a close, this special issue of Hospital Professional News offers a timely reflection on a year defined by innovation, pressure, and progress across Ireland’s hospital system. At European level, momentum continues to build: at the 9th European Commission Multistakeholder Event on Biosimilar Medicines, leaders again called for coordinated EU and national action to fully unlock the value of biosimilars. With more than 160 approvals and 9.2 billion patient-treatment days, Europe remains the global benchmark — and yet, the message is clear: coordinated implementation will determine whether patients and health systems experience the full benefit of these life-changing therapies.
Closer to home, our in-depth workforce report captures the stark reality facing hospital pharmacies nationwide. Behind every controlled-drug check, discharge review, or early-morning oncology round lies a system stretched thin. PSI workforce intelligence confirms a profession under mounting strain — with critical recruitment gaps and role-progression challenges echoed by the HPAI’s latest survey. Departments across the country continue to adapt, innovate, and absorb pressure, but the sustainability of these services remains an urgent national concern.
Against this backdrop, Continuing Professional Development has never been more vital. In this issue, we explore how CPD has evolved into the backbone of safe, modern practice — a structured, evidence-driven model that links reflective learning with real-world patient outcomes. For hospital pharmacists, CPD is not simply a regulatory requirement; it is the catalyst for better decision-making, stronger clinical leadership, and more responsive care.
We also bring you End-of-Year Reports from leading regulatory and representative bodies, including Medicines for Ireland, who spent 2025 strengthening their voice in Dublin and Brussels to ensure that generics, biosimilars and value-added medicines remain central to Ireland’s healthcare strategy.
Finally, our Hospital Professional Top 100 returns — celebrating the most dynamic, influential and forward-thinking clinicians, pharmacists, researchers and healthcare leaders of the year. Their work is shaping hospital medicine in Ireland, and their achievements remind us that innovation thrives even in the most challenging environments.
As we move into 2026, Hospital Professional News remains committed to supporting the hospital community with rigorous reporting, clinical insight, and a platform that champions excellence. Thank you for your readership and continued engagement throughout the year.
Wishing you a peaceful and restorative holiday season, and a successful year ahead.
New Appointment at HPRA

The Health Products Regulatory Authority (HPRA) has announced that Ms Grainne Power has been appointed Chief Executive with effect from 1 January, 2026. Ms Power brings significant experience from across the health products sector and throughout her career has consistently demonstrated a strong commitment to both public and animal health. She is currently Director of Compliance at the HPRA where she is responsible for
an extensive range of licensing, inspection, market surveillance and enforcement activities relating to medicinal products, blood, tissues and cells, controlled substances and cosmetics. Prior to that, she held the position of Director of Human Products Authorisation and Registration where she was responsible for overseeing the assessment of authorisation applications for medicines and clinical trials.
Grainne Power, Chief Executive, HPRA
As Chief Executive, Ms Power will lead the HPRA in its mission to protect and enhance human and animal health through the regulation of health products. Her leadership will also steer the organisation’s strategic ambitions, beginning with the imminent launch of its new 2026–2028 corporate strategy. She succeeds Dr Lorraine Nolan, who’s tenure as Chief Executive is concluding at the end of this year.
Professor Michael Donnelly, Chairman of the Authority, welcomed Ms Power’s appointment. “The Authority is pleased to announce the appointment of Grainne Power as Chief Executive of the HPRA. On behalf of the Authority, I extend our sincere congratulations and a warm welcome to Grainne as she assumes this pivotal leadership role. Her appointment comes at a significant time for the organisation, and we look forward to working together to build on the organisation’s strong position.
Biosimilars 2025: A Turning Point
At the 9th European Commission Multistakeholder Event on Biosimilar Medicines, the Biosimilar Medicines Group urges coordinated EU and national action to fully unlock the value of biosimilar medicines for patients and healthcare systems.
Europe remains the global leader in biosimilar approvals (over 160) and real-world adoption, with 9.2 billion patient-treatmentdays cumulated, to date. In the past year alone, biosimilar medicines delivered 30% more patient-treatment-days EU-wide, demonstrating their essential role in expanding access to critical biologic therapies.
The difference in EU Member states’ biologic treatment rates remains significant. As an example, the ability to provide broader
access to biologic medicines appears constrained in several Central and Eastern European (CEE) countries, with a significant gap remaining in comparison with Western Europe, despite the clear potential of biosimilar medicines to improve outcomes for chronic, immune-mediated and oncological diseases.
The next phase of the EU-funded AUGMENT project is expected to help bridge the gap between policy design and effective implementation. The upcoming Biotech Act presents another major opportunity to reinforce Europe’s leadership in biosimilar development, manufacturing and long-term competitiveness and address the Biosimilar Void trends which see a biosimilar pipeline concentrated on a reduced
number of biologic therapies. To seize it, the Biosimilar Medicines Group calls for:
• Reformed EU medicines procurement rules that enable fast, broad and sustainable biosimilar uptake at loss of exclusivity (LoE), built on multi-winner and pro-competition models;
• An accelerated EMA roadmap to streamline biosimilar development in Europe, drive evidence-based regulatory progress, and lead international biosimilar regulatory convergence to reduce divergence and complexity;
• Robust horizon scanning and monitoring to ensure measurable progress in closing
We look forward to supporting her leadership and wish her every success.”
Ms Power has over 20 years of regulatory, scientific and leadership experience in the health products industry, having held a number of senior management roles across both the public and private sectors. She joined the HPRA in 2018. Before entering the public sector, she held senior quality assurance roles in the pharmaceutical and biopharmaceutical industries, managing systems and processes to ensure compliance with regulatory standards. She also gained earlier experience in quality-related roles within the veterinary medicines and medical devices sectors.
Ms Power holds a degree in Biotechnology from Dublin City University and postgraduate diplomas from both University College Dublin and Trinity College Dublin.
Ms Power is a current member of the Council of the pharmacy regulator, the PSI. Members are appointed by the Minister for Health to act in the public interest in overseeing the regulatory responsibilities of the PSI as the pharmacy regulator.
biologic access gaps across EU Member States.
“The value of biosimilar medicines does not materialise automatically—it depends on coherent and sustained policy action,” said Julie Maréchal-Jamil, Senior Director, Pharmaceutical Policy. “Europe cannot afford a two speed biologics landscape nor a worsening of the Biosimilar Void. The Biotech Act can strengthen EU competitiveness while ensuring all patients benefit from timely, highquality biologic care.”
The Biosimilar Medicines Group is ready to work with the European Commission and all stakeholders to advance evidence-based, outcomes-driven policies that secure equitable access to biologic therapies for every patient in Europe.
IMO Warns: Tackle Childhood Health Inequality Now
The President of the Irish Medical Organisation (IMO) has warned that more needs to be done to address health inequalities from early childhood, noting that a failure to properly focus on early intervention and eradicate existing waiting lists for children will eventually lead to immense – and avoidable – pressure on the health system.
Dr Anne Dee, who is also a public health consultant, was speaking at the IMO’s ‘Health on the Margins’ conference, which took place on Saturday in Limerick.
Dr Dee warned that, more than ever before, an Irish person’s health outcomes were largely dictated by their housing quality, educational and work opportunities, and their lived environment.
She said: “By not doing enough to mitigate the risks of health inequality from birth, we are running the risk of condemning future generations to the same poor health outcomes as their parents, perpetuating the same inequalities that have sadly been allowed to fester in Irish society for far too long.
“We need to see a whole-ofGovernment approach to the problem of children at risk from before they are born, identifying young families and pregnant mothers who are at risk,
Dr Anne Dee, President, Irish Medical Organisation

supporting these families to help address the risks in a holistic way, and working with key stakeholders across all the domains of society towards redressing the imbalance.
Dr Dee added: “More resourcing and staffing are urgently needed in the health service to cater for the increasingly complex needs of a fast-growing and ageing
population living in deprivation, where ageing and frailty occur at a much younger age than their affluent counterparts. But there are other ways we can mitigate this demand and ease the pressure on the system, which is overloaded at present. A comprehensive, social determinants of healthbased approach is needed to help
improve the poor health outcomes which are the reality for far too many people in this country.
“If we do not adequately develop a far-reaching plan to address this problem and focus especially on early intervention, then we will only be passing a bigger issue on to future generations and it will be harder to tackle.
National Action Plan on Antimicrobial Resistance 2026
The Medical Council has welcomed the publication of the third One Health National Action Plan to address Antimicrobial Resistance (AMR) 2026-2030, known as iNAP3.
Published last month by Minister for Health, Jennifer Carroll MacNeill, TD, and Minister for Agriculture, Food and the Marine, Michael Heydon, TD, in collaboration with the Environmental Protection Agency; iNAP3 reinforces Ireland’s commitment to tackling the growing global threat of AMR through a One Health approach, which recognises the interconnection between human health, animal health and the environment.
CEO of the Medical Council, Dr Maria O’Kane, said increasing awareness of AMR is critical to protecting patients, “According to the World Health Organisation, antimicrobial resistance is one of the top ten global threats to human health. Continued planning and action against AMR will be essential for patient safety into the future, and we welcome iNAP3 as an important step forward.
“For doctors, reducing AMR means ensuring that antibiotic medicines are only prescribed and administered when they are genuinely needed. Antimicrobial medicines should only be used where they are required to treat serious infections. For patients, not every illness requires an antibiotic,
and your doctor will advise you on the most effective treatment. Avoiding unnecessary antibiotics helps protect their effectiveness for the future.”
“From a patient safety perspective, knowledge and awareness of this issue is important, as is an understanding of the wider challenges we face. The Medical Council will continue to ensure that expectations relating to safe, appropriate, and evidence-based prescribing are clearly communicated through our Guide to Professional Conduct and Ethics for Registered Medical Practitioners (9th edition), 2024. Doctors who are newly practising medicine or new to Ireland should also familiarise themselves with
the Medical Council’s Safe Start guide.
“AMR cannot be addressed in isolation. The iNAP3 plan provides a clear framework for progress across human health, animal health, agriculture, and the environment. The Medical Council looks forward to working with stakeholders on the implementation of iNAP3 and supporting doctors to deliver care that prioritises patient safety and public health.”
The One Health National Action Plan to address Antimicrobial Resistance (AMR) 2026-2030, known as iNAP3 is available on the Irish Medical Council website –www.imo.ie.
World First for Trials Pioneered in Ireland

RCSI University of Medicine and Health Sciences will contribute to a series of world-first clinical trials aimed at delivering long-term relief for people living with chronic musculoskeletal pain, through a newly funded project supported by the Disruptive Technologies Innovation Fund (DTIF).
The partnership of CrannMed, Salaso Health Solutions, University of Galway’s Institute for Clinical Trials and RCSI University of Medicine and Health Sciences has been awarded ¤6.6million Government investment.
The funding, announced by Minister for Enterprise, Trade and Employment, Peter Burke T.D., is part of the latest tranche of awards from the Disruptive Technologies Innovation Fund.
The consortium will carry out the RECAP project - Resorbable Embolization for Chronic musculoskeletal Pain.
The trial programme combines two complimentary technologies to improve outcomes for people with chronic musculoskeletal pain. Patients will have access to breakthrough pain treatment developed by CrannMed and a recovery programme developed by Salaso with the support of clinical specialists.
In the first trial, a patient will receive an injection which will selectively block blood flow to the site of pain, reducing inflammation and stray nerve endings, which are ultimately the root cause of the pain.
Hospital Pharmacy Congress 2026
Pictured (l-r): Aideen O'Doherty, Head of Programmes, HRB Clinical Research Facility, University of Galway; Liam Farrissey, CEO, CrannMed; Aoife Ní Mhuirí, CEO, Salaso Health Solutions Limited; Minister for Enterprise, Tourism and Employment Peter Burke; and Dawn McGlade, Industry Engagement Business Development Manager, RCSI
human clinical trials conducted here in Ireland. This collaboration shows that Ireland is open for business in clinical research, and RCSI is committed to working with Irish companies to deliver transformative health solutions for patients.”
RCSI’s role in the project is to lead the development and validation of imaging and access protocols for direct injection of the CrannMed technology and conduct the first-in-human clinical use of the technology. Additionally, RCSI will contribute expertise in digital health to optimise patient engagement with the AI-enabled rehabilitation platform.
Patients who have experienced chronic pain need to pursue a structured recovery process aimed at building strength and improving the resilience of musculoskeletal tissues. The second trial assesses the patient’s recovery as they are supported by an AI enabled selfmanagement platform in real-time.
Furthermore, the trials will allow scientists and researchers within the clinical centres to develop new imaging techniques, smart trial designs and pain measurement methodologies that will be needed to make the solution standard within healthcare systems.
Professor Fergal O’Brien, Deputy Vice Chancellor for Research and Innovation at RCSI, said: "RCSI is proud to support innovative Irish medtech through first-in-
The 30th anniversary edition of the EAHP Congress will take place in Barcelona, Spain, between 18-20 March 2026.
Hospital pharmacists across Europe will gather again and seize the moment to explore a broad spectrum of topics—from the latest advancements in pharmaceutical science and technology, to the evolving role of pharmacists in the management of complex diseases via teamwork and collaboration across various roles and responsibilities to achieve the highest standards of patient care.
Over the three-day period, EAHP will engage in discussions about the regulatory landscape, best practices in multidisciplinary medication therapy management, and the ways in which hospital pharmacists can innovate and collaborate to overcome the ever-growing demands of healthcare systems. Teamwork in patient management is essential for ensuring safe, effective, and comprehensive care that involves a collaborative effort among all healthcare professionals, including hospital pharmacists.
The DTIF, administered by the Department of Enterprise, Trade and Employment with support from Enterprise Ireland, aims to drive collaboration between Ireland’s world-class research base and industry to develop disruptive technologies with strong commercial potential and realworld impact.
Professor Fidelma Dunne, Director of the Institute for Clinical Trials at University of Galway, added, "This project is set to revolutionise how chronic joint and muscle pain is treated. The innovative treatment and clinical trial will enable patients to get effective, long-term pain relief from chronic pain without surgery or addictive drugs. This project is an example of academicindustry collaboration at its best and the Institute for Clinical Trails is delighted to support these type of partnerships by providing clinical trial expertise to our industry innovators and ensuring accelerated access to innovative treatments for patients.”


PREVENT
THE DISRUPTION OF MIGRAINE1
The only oral once-daily calcitonin gene-related peptide (CGRP) receptor antagonist for both episodic and chronic migraine patients1,2

Tablet not actual size.
Typical characteristics of migraine are headache of unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity and association with nausea and/or photophobia and phonophobia.3
ABBREVIATED PRESCRIBING INFORMATION. AQUIPTA® (atogepant) 10 mg tablets; 60 mg tablets. Please refer to the Summary of Product Characteristics (SmPC) before prescribing. PRESENTATION: Each tablet contains: 10 mg atogepant in 10 mg tablet, 60 mg atogepant in 60 mg tablet. INDICATION: Prophylaxis of migraine in adults who have at least 4 migraine days per month. DOSAGE AND ADMINISTRATION: The recommended dose is 60 mg taken orally once daily with or without meals; swallowed whole and not split, crushed or chewed. Missed dose to be taken as soon as it isremembered. If forgotten for an entire day, missed dose to be skipped and next dose taken as scheduled. Dose Modification: The recommended dosage of atogepant with concomitant use of strong CYP3A4 inhibitors or strong OATP inhibitors is 10 mg once daily. Special Populations: Elderly: No dose adjustment needed. Renal impairment: In patients with severe renal impairment (creatinine clearance [CLcr] 15-29 mL/min), and in patients with end-stage renal disease (ESRD) (CLcr <15 mL/min), the recommended dose is 10 mg once daily. For patients with ESRD undergoing intermittent dialysis, atogepant should preferably be taken after dialysis. No dose adjustment is recommended for patients with mild or moderate renal impairment. Hepatic impairment: Avoid use of atogepant in patients with severe hepatic impairment. No dose adjustment is recommended for patients with mild or moderate hepatic impairment. Paediatric Population: The safety and efficacy of atogepant in children (< 18 years of age) have not yet been established. CONTRAINDICATIONS: Hypersensitivity to active substance or any of the excipients. SPECIAL WARNINGS AND PRECAUTIONS: Serious hypersensitivity reactions reported, including anaphylaxis, dyspnoea, rash, pruritus, urticaria and facial oedema. Most serious reactions have occurred within 24 hours of first use, however, some hypersensitivity reactions can occur days after administration. Patients should be warned about symptoms of hypersensitivity. If a reaction occurs, discontinue atogepant and institute appropriate therapy. AQUIPTA 60 mg tablets contain 31.5 mg sodium per tablet; this is equivalent to 1.6% of the WHO recommended maximum daily intake of 2 g sodium for an adult. AQUIPTA 10 mg tablets contain less than 1 mmol sodium (23 mg) per dose, that is to say essentially ‘sodium-free’. Atogepant has no or negligible influence on the ability to drive and use machines. However, if affected by somnolence, patients should exercise caution
before driving or using machinery. FERTILITY, PREGNANCY AND LACTATION: Pregnancy: Atogepant is not recommended during pregnancy and in women of childbearing potential not using contraception. Breast-feeding: It is unknown whether atogepant is excreted in human milk. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Fertility: No human data on the effect of atogepant on fertility are available. Animal studies showed no impact on female and male fertility with atogepant treatment. UNDESIRABLE EFFECTS: Common (≥ 1/100 to < 1/10): Decreased appetite, nausea, constipation, fatigue/somnolence, weight decreased. Refer to Section 4.8 of the SmPC for details of other side effects, and for further information.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie.
LEGAL CATEGORY: POM (S1B). MARKETING AUTHORISATION NUMBERS: EU/1/23/1750/001 AQUIPTA 10 mg tablets in blisters, in cartons of 28 tablets; EU/1/23/1750/003 AQUIPTA 60 mg tablets in blisters, in cartons of 28 tablets. MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061, Ludwigshafen, Germany. Further information is available on request from: AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24. DATE OF REVISION: November 2024. PI/1750/002.
References: 1. AQUIPTA® Summary of Product Characteristics, available at www.medicines.ie. 2. Morena-Ajona D, et al. J Clin Med. 2022;11(6):1656. 3. Ferrari MD, et al. Nat Rev Dis Primers. 2022;8(1):2.
Ireland’s First Dedicated Cancer Prevention Theatre

HSE South West has opened Ireland’s first protected theatre resource dedicated to cancer prevention surgery for people at high genetic or clinical risk. This new theatre pathway is one of only a handful of dedicated cancerprevention theatre resources in Europe and marks a major step forward for prevention-led care in the region and nationally.
The protected theatre resource secures regular time and beds for risk-reducing surgery while allowing the theatre to be used by other specialties between booked sessions to maximise utilisation. Importantly, it forms
the final, essential element of a comprehensive regional prevention programme that links rapid genetic testing, specialist risk clinics, radiological surveillance, surgical and reconstructive expertise, and one-to-one psychological and specialist nursing support.
Programme highlights:
• Protected theatre slots for riskreducing surgery at SIVUH in partnership with CUH.
• Integrated risk pathway starting with specialist nursing and genetics assessment, through surveillance or surgical options in breast and gynaecology.
Pictured are Dr Ruth Linehan, Director of Nursing, and Louise Burke, Clinical Nurse Specialist for the Oncological Prevention Service. Ireland's first dedicated cancer prevention theater opens at South Infirmary Victoria University Hospital (SIVUH) in partnership with Cork University Hospital (CUH).
Photography by Gerard McCarthy Photography
• Holistic support including psychology, advanced nursing, and reconstructive plastic surgery options.
• A scalable model designed to be replicated across regions in Ireland and Europe.
This achievement has been driven by regional collaboration across clinical, academic and community partners, including the Transforming Theatre Programme and University College Cork. Key contributors include CUH’s genetics team, surgical oncology, breast, gynaecology, specialist nursing and psychology services, and the CUH Charity.
Professor Mark Corrigan, Surgical Oncologist, Cork University Hospital, commented:
Improvement in Health Services
The Health Information and Quality Authority (HIQA) says it found improvements across the health services it inspects in 2024.
The findings are in HIQA’s Overview Report of the Monitoring and Regulation of Healthcare Services in 2024, published today, which highlights HIQA’s inspection and monitoring activity as healthcare services responded and adapt to a changing landscape.
The findings outlined in this report show that HIQA inspections have been key driving factors in delivering sustained improvement in healthcare services for the benefit of people using services. HIQA has seen a continued effort
from many service providers to use the findings from inspections to drive improvements and positive outcomes for patients.
While good standards of care were observed by HIQA, healthcare remains a challenging environment and a number of key areas require ongoing improvement. The overview report found that hospitals continue to be challenged with suitable infrastructure to support demand, bed capacity and sufficient workforce among other issues.
HIQA’s Director of Healthcare Regulation, Sean Egan, said: “Our inspectors have spoken to many patients who have praised
the healthcare staff who have cared for them. We regularly observe excellent practice from committed staff working in a challenging environment.
“While we are seeing tangible improvements in healthcare services, resourcing and capacity requirements, particularly in acute hospitals, need to be addressed to meet service demand. These shortfalls directly affect both patients and the workforce on a daily basis, and this becomes increasingly crucial as we come into the Winter months.”
HIQA inspections have shown examples of improved levels of compliance in healthcare
“This opening of this new dedicated cancer prevention theatre in Cork is a remarkable achievement. This initiative matters because up to 40% of cancers are preventable; earlier identification and timely risk reducing interventions will reduce cancer incidence, improve outcomes and spare families the concern of a cancer diagnosis for their loved ones. Fewer cancers also mean fewer patients needing complex treatment, lower long term costs and resources freed for other critical health services. Our regional model shows that targeted investment in prevention can deliver operationally sustainable, patient centred care, and with Phases 1 and 2 focused on cancer we are already planning future phases to broaden prevention beyond cancer and a dedicated regional prevention facility opening in 2027.”
Priscilla Lynch, HSE manager for Cork South and West, said:
“This protected theatre resource and integrated prevention pathway is a proud milestone for the South West and for our patients. It gives people clear, timely options to reduce cancer risk and creates a model that can be adopted across Ireland and Europe. The rapid rollout of local genetic testing and the establishment of a coordinated clinical pathway means families can access answers and interventions much sooner than before.”
services in 2024. In situations where findings from previous inspections facilitated comparison on re-inspection in 2024, 41% of national standards assessed on reinspection showed improvements.
In addition, in facilities that provide medical exposure to ionising radiation, the number of regulations assessed as not compliant was 5%, which is significantly lower than the 12% identified between 2021 and 2023. This demonstrates both the value of healthcare regulation and the commitment from health services to address non-compliance in the interest of delivering high-quality care and keeping patients safe.
For healthcare professionals in Ireland only. Abbreviated Prescribing Information can be found below.
Generic Product Launch
Pazopanib Teva
Film-coated Tablets
pazopanib
30 film-coated tablets
High Tech Prescription Medicine
Indications
Renal cell carcinoma (RCC)
Pazopanib Teva is indicated in adults for the first-line treatment of advanced renal cell carcinoma (RCC) and for patients who have received prior cytokine therapy for advanced disease.
Soft-tissue sarcoma (STS)
Pazopanib Teva is indicated for the treatment of adult patients with selective subtypes of advanced soft-tissue sarcoma (STS) who have received prior chemotherapy for metastatic disease or who have progressed within 12 months after (neo) adjuvant therapy.
Efficacy and safety has only been established in certain STS histological tumour subtypes.
Pazopanib Teva Film-Coated Tablets Abbreviated Prescribing Information.
Presentation: Each film-coated tablet contains pazopanib hydrochloride equivalent to 200mg and 400mg pazopanib respectively. Indications: Indicated in adults for the first-line treatment of advanced renal cell carcinoma (RCC) and for patients who have received prior cytokine therapy for advanced disease. Also, indicated for the treatment of adult patients with selective subtypes of advanced soft-tissue sarcoma (STS) who have received prior chemotherapy for metastatic disease or who have progressed within 12 months after (neo) adjuvant therapy. Dosage and administration: Oral use. Adults: The recommended dose of pazopanib for the treatment of RCC or STS is 800mg once daily. Children and Adolescents (Aged <18 years of age): Not suitable for use. Elderly: There are limited data on the use of pazopanib in patients aged 65 years and older. Overall, no clinically significant differences in safety of pazopanib were observed between subjects aged at least 65 years and younger subjects, but greater sensitivity of some elderly patients cannot be ruled out. Renal impairment: No dose adjustment is required in patients with creatinine clearance above 30ml/min. Caution is advised in patients with creatinine clearance below 30ml/min as there is no experience of pazopanib in this patient population. Hepatic impairment: Administration of pazopanib to patients with mild or moderate hepatic impairment should be undertaken with caution and close monitoring of tolerability. 800mg pazopanib once daily is the recommended dose in patients with mild abnormalities in serum liver tests. A reduced pazopanib dose of 200mg once daily is recommended in patients with moderate hepatic impairment. Pazopanib is not recommended in patients with severe hepatic impairment. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: Cases of hepatic failure (including fatalities) have been reported during use of pazopanib and is not recommended in patients with severe hepatic impairment. Serum liver tests should be performed before initiation of treatment with pazopanib, at weeks 3, 5, 7 and 9, then at months 3 and 4, with additional tests as clinically indicated. Periodic testing should then continue after month 4. In clinical studies with pazopanib, events of hypertension including newly diagnosed symptomatic episodes of elevated blood pressure (hypertensive crisis) have occurred, therefore, blood pressure should be well controlled prior to initiating pazopanib. Patients should be monitored for hypertension early after starting treatment (no longer than one week after starting pazopanib) and frequently thereafter to ensure blood pressure control. Pazopanib should be discontinued if there is evidence of hypertensive crisis or if hypertension is severe and persists despite anti-hypertensive therapy and pazopanib dose reduction. Posterior reversible encephalopathy syndrome (PRES)/Reversible posterior leukoencephalopathy syndrome (RPLS) and Interstitial lung disease (ILD)/Pneumonitis have been reported in association with pazopanib and can be fatal. Patients developing PRES/RPLS/ILD/pneumonitis should discontinue treatment with pazopanib. The risks and benefits of pazopanib should be considered before beginning therapy in patients who have pre-existing cardiac dysfunction. The safety and pharmacokinetics of pazopanib in patients with moderate to severe heart failure or those with a below normal left ventricular ejection fraction (LVEF) have not been studied. Interruption of pazopanib and/or dose reduction should be combined with treatment of hypertension in patients with significant reductions in LVEF, as clinically indicated. Patients should be carefully monitored for clinical signs or symptoms of congestive heart failure. Baseline and periodic evaluation of LVEF is recommended in patients at risk of cardiac dysfunction. Pazopanib should be used with caution in patients with a history of QT interval prolongation, in patients taking antiarrhythmics or other medicinal products that may prolong QT interval and in patients with relevant pre-existing cardiac disease. In clinical studies with pazopanib, myocardial infarction, myocardial ischaemia, ischaemic stroke and transient ischaemic attack were observed, with some events being fatal. Pazopanib should be used with caution in patients who are at increased risk of thrombotic events or who have had a history of thrombotic events. In clinical studies with pazopanib, venous thromboembolic events including venous thrombosis and fatal pulmonary embolus have occurred. Thrombotic microangiopathy (TMA) has been reported in clinical studies of pazopanib as monotherapy, in combination with bevacizumab, and in combination with topotecan. Patients developing TMA should permanently discontinue treatment with pazopanib. In clinical studies with pazopanib haemorrhagic events have been reported, including fatal haemorrhagic events. Pazopanib should be used with caution in patients with significant risk of haemorrhage. Before initiating pazopanib, this risk of aneurysm and/or artery dissection formations should be carefully considered in patients with risk factors such as hypertension or history of aneurysms. Pazopanib should be used with caution in patients at risk for gastrointestinal perforation or fistula, as fatal perforation events have occurred in clinical studies. Pazopanib should be discontinued in patients with wound dehiscence. In clinical studies with pazopanib, events of hypothyroidism have occurred. All patients should be observed closely for signs and symptoms of thyroid dysfunction on pazopanib treatment. In clinical studies with pazopanib, proteinuria has been reported. Baseline and periodic urinalysis during treatment is recommended and patients should be monitored for worsening proteinuria. Pazopanib should be discontinued if the patient develops nephrotic syndrome. The occurrence of Tumour lysis syndrome (TLS), including fatal TLS, has been associated with the use of pazopanib. Patients at risk should be closely monitored and treated as clinically indicated. Patients on pazopanib treatment should
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
Product subject to prescription which may be renewed (B)
be observed closely for signs and symptoms of pneumothorax. Cases of serious infections (with or without neutropenia), in some cases with fatal outcome, have been reported. Interactions: Pazopanib metabolism is mediated primarily by CYP3A4, with minor contributions from CYP1A2 and CYP2C8, therefore, inhibitors and inducers of CYP3A4 may alter the metabolism of pazopanib. Coadministration of pazopanib with strong inhibitors of the CYP3A4 family (e.g. ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase pazopanib concentrations. Grapefruit juice contains an inhibitor of CYP3A4 and may also increase plasma concentrations of pazopanib. Concomitant use of pazopanib with a strong CYP3A4 inhibitor should be avoided. If no medically acceptable alternative to a strong CYP34A inhibitor is available, the dose of pazopanib should be reduced during concomitant administration. In such cases there should be close attention to adverse drug reaction, and further dose reduction may be considered if possible drug-related adverse events are observed. Combination with strong P-gp or BCRP inhibitors should be avoided, or selection of an alternate concomitant medicinal product with no or minimal potential to inhibit Pgp or BCRP is recommended. CYP3A4 inducers such as rifampin may decrease plasma pazopanib concentrations. Co-administration of pazopanib with potent P-gp or BCRP inducers may alter the exposure and distribution of pazopanib. In vitro studies with human liver microsomes showed that pazopanib inhibited CYP enzymes 1A2, 3A4, 2B6, 2C8, 2C9, 2C19, and 2E1. Concomitant use of pazopanib and simvastatin increases the incidence of ALT elevations. If a patient receiving concomitant simvastatin develops ALT elevations, follow guidelines for pazopanib posology and discontinue simvastatin. Concomitant use of pazopanib and other statins should be undertaken with caution. Administration of pazopanib with a high-fat or low-fat meal results in an approximately 2-fold increase in AUC and Cmax, therefore, pazopanib should be administered at least 1 hour before or 2 hours after a meal. Concomitant administration of pazopanib with esomeprazole decreases the bioavailability of pazopanib by approximately 40%, and co-administration of pazopanib with medicines that increase gastric pH should be avoided. Pregnancy and lactation: Pazopanib should not be used during pregnancy unless the clinical condition of the patient requires treatment with pazopanib. If pazopanib is used during pregnancy, or if the patient becomes pregnant while receiving pazopanib, the potential hazard to the foetus should be explained to the patient. Patients of childbearing potential should be advised to use adequate contraception during treatment and for at least 2 weeks after the last dose of pazopanib and to avoid becoming pregnant while receiving treatment with pazopanib. Male patients (including those who have had vasectomies) should use condoms during sexual intercourse while taking pazopanib and for at least 2 weeks after the last dose of pazopanib to avoid potential exposure to the medicinal product for pregnant partners and female partners of reproductive potential. Breast-feeding should be discontinued during treatment with pazopanib, as a risk to the breastfed child cannot be excluded. Effects on ability to drive and use machines: No or negligible influence on the ability to drive and use machines. A detrimental effect on such activities cannot be predicted from the pharmacology of pazopanib. The clinical status of the patient and the adverse event profile of pazopanib should be borne in mind when considering the patient’s ability to perform tasks that require judgement, motor or cognitive skills. Patients should avoid driving or using machines if they feel dizzy, tired or weak. Adverse reactions: Thrombocytopenia, neutropenia, leukopenia, thrombotic microangiopathy, hypothyroidism, tumour lysis syndrome, peripheral sensory neuropathy, transient ischaemic attack, cerebrovascular accident, ischaemic stroke, posterior reversible encephalopathy/reversible posterior leukoencephalopathy syndrome, bradycardia, cardiac dysfunction, myocardial ischaemia, venous thromboembolic event, hypertensive crisis, haemorrhage, aneurysms and artery dissections, interstitial lung disease/pneumonitis, pancreatitis, rectal haemorrhage, gastrointestinal haemorrhage, melaena, anal haemorrhage, large intestine perforation, mouth haemorrhage, upper gastrointestinal haemorrhage, enterocutaneous fistula, haematemesis, haemorrhoidal haemorrhage, ileal perforation, oesophageal haemorrhage, retroperitoneal haemorrhage, hepatotoxicity, jaundice, hepatic failure, palmar-plantar erythrodysaesthesia syndrome, haemorrhage urinary tract, vaginal haemorrhage, metrorrhagia. Very Common: Decreased appetite, dysgeusia, headache, hypertension, diarrhoea, nausea, vomiting, abdominal pain, hair colour change, alopecia, rash, proteinuria, fatigue. Common: Infections, hypophosphataemia, dehydration, insomnia, dizziness, lethargy, paraesthesia, vision blurred, flushing, epistaxis, dysphonia, dyspnoea, haemoptysis, stomatitis, dyspepsia, flatulence, abdominal distension, mouth ulceration, dry mouth, hyperbilirubinaemia, hepatic function abnormal, skin hypopigmentation, dry skin, pruritus, erythema, skin depigmentation, hyperhidrosis, arthralgia, myalgia, muscle spasms, musculoskeletal pain, mucosal inflammation, asthenia, oedema, chest pain. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: Pazopanib doses up to 2000 mg have been evaluated in clinical studies. Grade 3 fatigue (dose-limiting toxicity) and Grade 3 hypertension were each observed in 1 of 3 patients dosed at 2000 mg and 1000 mg daily, respectively. There is no specific antidote for overdose with pazopanib and treatment of overdose should consist of general supportive measures. Legal category: POM. Marketing Authorisation Number: PA22579/006/001- 002. Marketing Authorisation Holder: Teva GmbH, Graf- Arco-Str. 3, 89079 Ulm, Germany. Job Code: MED-IE- 00101. Date of Preparation: October 2025
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie.
Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Date of Preparation: October 2025 | Job Code: GEN-IE-00153
Further information is available on request or in the SmPC. Product Information also available on the HPRA website.
Delivering Care Under Pressure - An Analysis of the Hospital Pharmacy Workforce
At 08:00 in the Hospital Pharmacy wing of a major Dublin tertiary centre, a senior pharmacist pauses at the door of the controlleddrugs store. The electronic dashboard shows five unfilled Technician posts, two Junior Pharmacist vacancies and three clinical pharmacy roles deferred. A Technician newly upskilled for discharge review steps into the gap, while the clinical pharmacist heads to the Oncology Ward to start rounds early. It is a quiet snapshot of a much louder national story. Ireland’s Hospital Pharmacy Workforce is evolving, but the pace and pressure of that evolution remain significant.
Recent findings from the Pharmaceutical Society of Ireland (PSI) Workforce Intelligence Report highlight a pharmacist workforce of more than 7,200 and raise concerns about recruitment, retention and role progression in patient-facing positions. The Hospital Pharmacists Association of Ireland (HPAI) adds another layer through its 2024 survey, which found that more than half of responding hospitals had unfilled pharmacist posts and that Technician vacancies ranged from one to four in most departments, with some reporting five to seven senior pharmacist positions vacant. These figures point to a service stretched at both foundational and specialist levels.
A shifting workforce landscape Workforce numbers, qualifications and aspirations
Hospital Pharmacy remains highly specialised. The PSI reports that 86 percent of Hospital Pharmacists have completed additional higher education, and that number reaches 92 percent among those in specialist practice. This reflects long-standing professional ambition. Yet Hospital Pharmacists continue to report limited career progression, gaps in advanced practitioner recognition and outdated pay structures.
Technician recruitment adds another layer of strain. Vacancies are widespread, and without a regulated Technician workforce supporting foundational operational tasks, pharmacists cannot consistently work at the top of their clinical scope. The result is
“Modern hospital pharmacy is no longer defined by dispensing. It is defined by the capacity to deliver medicines-optimisation at every point of care.”
a workforce that is highly qualified but not always optimally deployed.
Reform, expansion and the policy landscape
Hospital Pharmacy reform hinges on three interlinked components. These are the expansion of specialist pharmacist roles, the regulation and formalisation of Pharmacy Technician practice and coordinated workforce planning across acute and community sectors.
The Pharmacy Workforce Working Group, established in 2024 by the PSI and the Department of Health, is now the central mechanism driving this work. Its remit covers strategic workforce planning, leadership, recruitment, innovation, career sustainability and working conditions. Hospital Pharmacy is embedded within these priorities.
Government position on recruitment, retention and Technician regulation
Responding to questions from Hospital Professional News, the Department of Health highlighted PSI data showing that 92 percent of registered pharmacists were practising in 2024, with 14 percent of survey respondents working in hospitals. While the overall pharmacist workforce increased by 24 percent, the public workforce grew by 48 percent. This suggests a strengthening flow of pharmacists entering HSE roles, although local variation remains significant.
The Department confirmed it is responsible for developing a national strategy for the future role of Pharmacy Technicians. This long-term action includes examining scope of practice, regulation, qualification standards and future workforce planning. The Department noted that this work will progress in line with the broader activity of the Workforce Working Group.
PSI response: regulatory and structural direction
The PSI, in response to Hospital Professional News, stated that progress continues across the recommendations outlined in the Workforce Intelligence Report. Actions are being advanced over short, medium and long-term timeframes. Medium-term work underway includes the recognition of Advanced Specialist Pharmacist Grade positions within hospital settings, led by the Department of Health and the HSE. The long-term recommendation relating to the regulation of Pharmacy Technicians lies with the Department of Health, and PSI confirmed it will support any policy or legislative changes required within its regulatory remit.
The PSI also highlighted that monthly statistical updates on its registers are available for those monitoring workforce movements.
The front-line reality
HPAI continues to emphasise that Technician shortages ripple across the entire service. Without adequate Technician staffing, clinical pharmacy services stall, and pharmacists lose time that should be spent on complex medication review, high-risk medicines oversight and wardbased activity. Departments with three or four Technician vacancies, or multiple unfilled senior pharmacist posts, report delays in discharge workflows, bottlenecks in medicines reconciliation and pressure on specialist-led services.
The regulator’s warnings echo this reality. Global pressures on pharmacy recruitment, advances in medicines complexity and the need for consistent skill mix intensify the need for rapid progress in workforce planning.
Professional bodies representing Pharmacy Technicians also note the risks. With the Technician role
largely unchanged since 1994, concerns include inconsistent training standards, barriers to career progression and limits on service redesign where pharmacists and Technicians work as an integrated clinical team.
Why this moment matters
Service-delivery consequences
Technician shortages push foundational tasks upward, reducing the time pharmacists can devote to clinical and patient-facing roles. This affects antimicrobial stewardship, highrisk medicines optimisation and medicines-safety initiatives. Workforce gaps in specialist pharmacist roles limit the capacity of departments to deliver advanced practice services consistently.
Skill mix and progression
Advanced practice pathways for pharmacists exist only partially, and recognition of specialist grades remains a work in progress. Without clear progression, retention becomes a challenge. Technician regulation remains essential to safe service expansion, and its absence delays the development of modern teambased care models.
The wider horizon
Ireland has signalled a clear reform agenda. The foundations now include data, cross-sector collaboration and recognition of structural workforce needs. What remains is the conversion of strategy into service.
Conclusion: evolve or fall behind Ireland’s Hospital Pharmacy Workforce is at a decisive point. Recruitment is improving, but retention, structural recognition and Technician regulation remain essential. Without progress on these long-term actions, clinical pharmacy services risk stagnation. The profession must continue to champion reform, use data to highlight service risks and advocate for coordinated change at system level.
Hospital Pharmacy has moved firmly into the era of clinical medicines-optimisation. The challenge now is ensuring the workforce can match that ambition.
References available on request
Patient Monitoring and Diagnostics
Trusted Solutions for Irish Hospital Systems
Schiller Cardiovit AT-102 G2
The new generation 12 Lead ECG for hospitals and clinics provides superior workflow efficiency with Wi-Fi and advanced interpretation as standard.
• 12 Lead ECG.
• High resolution 8” colour display.
• Trolley available.
Schiller BR-102 Plus Ambulatory BP Recorder
The Schiller BR-102 Plus Ambulatory BP Recorder is a discreet, compact, long term blood pressure monitoring unit which provides the highest level of accuracy while allowing the patient to go about their daily lives undisturbed.
• Recording for 24 or 48 hours.
• Voice recording of patient data.
• Examination of adults and children thanks to a special algorithm.

Ranked on the HSE Dynamic Purchasing System for Non-Invasive Cardiology Equipment
Ranked on the HSE Dynamic Purchasing System

RANKED 1ST on the HSE Dynamic Purchasing System for Non-Invasive Cardiology Equipment

Medilog®AR Holter Recorder
The Schiller Medilog®AR Holter Recorder is a robust, splash and shockproof holter that is easy to clean.
CPD
The Importance of CPD: Why It Matters, How It Supports Career Development and Patient Outcomes
Continuing Professional Development (CPD) is no longer a nice-to-have in Irish hospital practice; it is the engine that keeps complex, multidisciplinary care safe and effective. For pharmacists and their teams, CPD connects individual learning to system improvement — from medicines reconciliation on a Monday ward round to antimicrobial stewardship decisions in the ICU on Friday afternoon.
In Ireland, the CPD model is structured, quality-assured, and increasingly data-driven, with the Irish Institute of Pharmacy (IIOP) and the Pharmaceutical Society of Ireland (PSI) shaping the framework that links reflective learning to demonstrable changes in practice. This feature takes a clinical-frontline view of CPD: what it is, how it works in the Irish system, where the evidence sits on patient outcomes, and how hospital pharmacists can use CPD to accelerate careers while improving care.
What CPD Is in the Irish Context
Definition and purpose. The PSI defines CPD as a continual process of lifelong learning focused on implementing learning in professional practice to improve outcomes for patients. That emphasis on applied learning is important — it is less about collecting certificates and more about changing what clinicians do tomorrow morning on the ward.1
Who runs what. The IIOP, established by the PSI, manages the national CPD system for pharmacists, including the ePortfolio tool, and quality assurance processes for CPD activities. In 2024, the PSI reviewed the CPD model to ensure it remains agile, sustainable, and aligned with future practice needs across care settings.2
Core components you’ll meet in practice:
• Self-directed learning cycle mapped in the ePortfolio, with reflection, self-appraisal, planning, action, documentation and evaluation.3
• Quality assurance of CPD via ePortfolio Review against published standards, with timelines and resubmission windows to support compliance and learning.4
“Ireland’s CPD system is designed to evidence learning in action, not just attendance.”
How
the System Is Being Tightened: Rules, Reviews, and Timelines
The regulatory framework has been modernised to clarify expectations on pharmacists’ participation and submissions. Recently enacted legislation -the PSI (CPD) (Amendment) Rules 2025 requires that every pharmacist will submit prescribed extracts of their ePortfolio to the IIOP for review at defined intervals, moving the model toward clear, consistent annual engagement.6
On the ground, pharmacists have a practical calendar to work to. The IIOP’s 2025-2026 ePortfolio Review timeline sets out notification, submission, review, and resubmission windows across the year, giving hospital teams a realistic runway to gather evidence from audits, MDT projects, and service improvements.7
The ePortfolio Review Policy also states that the process is a profession-wide quality assurance mechanism, with strong confidentiality safeguards and an explicit duty to report falsified extracts to the PSI Registrar under the 2015 regulations.4
For hospital managers, that means CPD engagement and QA are not optional extras. They should be visible in rota planning and PDP conversations, and linked to service objectives such as medicines reconciliation rates, discharge counselling coverage, or IV-to-PO switches.
Where CPD Meets Patient Outcomes: What the Evidence Says
Frontline staff often ask the most important question: does CPD actually change patient care?
The international literature is increasingly clear that interactive, multi-component CPD — particularly when reinforced over time and embedded in practice — improves practitioner knowledge and behaviour and is at least as effective when delivered via e-learning compared with face-to-face formats. Mandatory requirements also improve completion and engagement.8
A 2025 scoping review of CPD interventions across health professionals found positive patient outcomes in most included studies, such as lower hypoglycaemia events and shorter ICU stays when CPD focused on specific practice change with practical skills components.9 These effects were strongest when CPD was not a one-off lecture but part of a supported programme.
The bigger system link is medicines optimisation. NICE’s guideline on safe and effective use of medicines emphasises defined roles, robust transfer of medicines information, and process monitoring across settings. CPD that targets these elements — for example, improving reconciliation within 24 hours of transfer — aligns with international best practice and directly affects error rates and length of stay [10].
Stat Box
• Interactive CPD is most effective, and e-learning performs as well as face-to-face.
• Multi-component CPD programmes have demonstrated patient-level benefits.
• Medicines optimisation standards tie learning to practice.
The IIOP Lens: Quality Assurance and Practical Support
From the Institute’s viewpoint, the CPD system needs to do two things at once — uphold professional standards and make it easy to do the right thing.
Quality assurance guardrails. IIOP policies frame ePortfolio Review as a profession-wide QA
process, with clear standards, timelines, and resubmission options. The model is designed to be supportive while safeguarding public trust.4
Programmes and procurement. The Institute quality assures CPD activities and commissions targeted education where gaps exist, ensuring materials are relevant to practice and meet standards.
Practice-focused resources. Recent IIOP materials on prescription extension provide a neat example, outlining how person-centred care, regulatory guidance, shared decisionmaking, and practice planning come together.12
Expert Perspective:
CPD
Through the Eyes of a CPD Quality Assurance Pharmacist
Dawn Davin, CPD Quality Assurance Pharmacist at the IIOP, brings a hospital-centred perspective, having spent two decades in Tallaght University Hospital with a nephrology focus, national renal leadership roles, and experience in medicines safety at the HPRA. Her current portfolio includes the “In Conversation With…” webinar series.13 This interprofessional webinar series provides a platform which is responsive to the ever-evolving needs of the profession, providing a space for the sharing of knowledge, information and experience in practice.
Why CPD Matters Specifically for Hospital Pharmacists
1. Medicines optimisation and transfer of care. Embedding CPD cycles on reconciliation, high-risk medicines, and deprescribing supports safety and reduces readmissions.10
2. Antimicrobial stewardship and critical care. Interactive CPD linked to guideline updates contributes to measurable patient outcomes.9
3. New and expanded clinical activities. As pharmacist scope expands, CPD is the route to competence and assurance.12
4. Team-based skill-mix and leadership. CPD enables pharmacists to lead QI projects, upskill technicians, and contribute to governance.14
5. System resilience. Global workforce shortages mean CPD maintains quality and capability within existing teams.15
What Effective CPD Looks Like in a Hospital Department
Choose aims that live in your service plan. Align cycles to your hospital’s priorities.
Make it interactive, repeatable, and documented. Interactive formats outperform passive ones; e-learning can be just as effective.8
Build ePortfolio evidence while you work. Reflect, plan, act, document, and evaluate — capturing outcomes as you go.4 Hard-wire medicines optimisation standards. Use NICE guidance to define safe information transfer.10
Use quality assurance to your advantage. Prefer quality assured CPD activities aligned with IIOP QA standards.
Career Development: Using CPD to Build a Clinical Portfolio
Thoughtfully chosen CPD cycles map to specialist skills over time — oncology, renal dosing, or perioperative care. That portfolio becomes your evidence for advanced practice roles and leadership posts.
Participation in HPAI’s Education and Research SIG, which enhances access to quality education and supports practice-based research, offers routes to publish and mentor others.16
CPD That Changes Outcomes: Five Practical Hospital Examples
1. 24-hour medicines reconciliation on transfer –safer handovers, fewer discrepancies.10
2. Hypoglycaemia reduction bundle – reduced adverse events through multicomponent CPD.9
3. Antimicrobial IV-to-PO switch – improved stewardship outcomes.10
4. High-risk anticoagulant counselling – technicianassisted education and adherence improvement.
5. Prescription extension preparedness – local SOPs supported by IIOP resources.12
IIOP/NCCP collaboration – RCSI Charter Week 2026
Save the date! Thursday, 12th February, 2026
Policy Horizon: Why CPD Will Matter More in 2026
• Model refinement. PSI’s review and rule amendments formalise submission cadence.2, 6
• Workforce pressure. With staffing constraints, CPD that enables task-sharing will be critical.14
• Quality and safety regulation. CPD remains the route to assurance in advanced practice.4
• Digital delivery. More blended and e-learning formats will appear.8
For Irish hospital pharmacy, CPD is both a professional duty and a practical lifeline. It is how we keep pace with rapidly evolving therapy, hard-wire medicines optimisation, and build the specialist skills our services need. The model here is mature enough to demand evidence and supportive enough to help deliver it. Used well, CPD is not paperwork — it is patient safety with a paper trail.
References available on request
The Irish Institute of Pharmacy (IIOP) is delighted to announce that it is collaborating with the HSE’s National Cancer Control Programme (NCCP) to host a session entitled ‘Beyond the Prescription – The Pharmacist’s Role in Cancer Care’ as part of RCSI Charter Week 2026. The session will take place on Thursday, 12th February, in-person, in RCSI, from 14:15-16:15.
The interprofessional session will explore how pharmacists, alongside their multidisciplinary colleagues, play a crucial and expanding role across the full continuum of cancer care, from prevention and early detection through to survivorship. The session will spotlight how the provision of cancer care is evolving and how the practice of pharmacy is adapting to meet the needs of patients.
What is RCSI Charter Week? The Royal College of Surgeons in Ireland (RCSI) Charter Meeting is an annual event, celebrating the granting of a Royal Charter and founding of the College in 1784. It is a multidisciplinary event, welcoming each of the faculties and speciality groups in RCSI. The Charter Meeting will take place, in person, from Tuesday, 10 February to Saturday, 14 February 2026 in RCSI, exploring the theme of ‘Cancer’.
Registration - Registration details for the session, other RCSI Charter Meeting sessions, programme of events and speaker bios will be issued closer to the event. Don’t forget to keep an eye on the IIOP website and our social media accounts for further details and updates. Registration for the session will be FREE! for IIOP members wishing to attend the IIOP/NCCP session only. Keep an eye on the IIOP website for registration details. For anyone wishing to attend other events during RCSI Charter Week 2026, please visit the RCSI Charter Meeting webpage.
End of Year Review
Medicines for Ireland

Securing the Medicines Ireland Depends On
For Medicines for Ireland (MFI), 2025 has been a year of intensifying advocacy in Dublin and Brussels to ensure that the generics, biosimilar, and value-added medicines sector is recognised as central to the solutions for the Irish healthcare system.

Grounded in our Vision Strategy 2024–2029, we have consistently made the case that smarter use of off-patent medicines is the most practical route to sustainable access for patients and sustainable budgets for the Irish health sector.
As we look back on 2025, three EU-level files have defined much of that work: the revision of the EU pharmaceutical legislation, the Critical Medicines Act, and the recast Urban Waste Water Treatment Directive (UWWTD). Alongside these, our push for digital first communications has shown how regulatory frameworks can support better care, lower waste and a smaller environmental footprint.
The Pharma Package is the opportunity to get the foundations right for access and competition
In June, the Council agreed its position on the Pharma Package, a once-in-a-generation overhaul of the EU’s pharmaceutical rules, with an explicit focus on making the sector fairer, more competitive and more resilient. For MFI, the direction of travel is clear. We have argued that any final agreement must:
• Protect and expand the role of generic, biosimilar and valueadded medicines in delivering the “three As” – availability, accessibility and affordability.
• Recognise that off-patent medicines already supply 60% of the medicines Irish patients rely on day-to-day, and that this model only remains viable if pricing, reimbursement and shortage-prevention rules are aligned.
• Avoid creating additional administrative burdens that unintentionally make generic, biosimilar and value added medicines unsustainable in smaller markets like Ireland.
Through Medicines for Europe (MFE), of which MFI is an active member, we have supported efforts to ensure that the legislation is based on a framework that allows companies to keep supplying essential medicines at viable prices.
As Ireland prepares for its EU Council Presidency in the second half of 2026, the implementation and followthrough of this reform will be a critical test of Europe’s ability to turn legislative ambition into practical, patient-centred outcomes.
The Critical Medicines Act is an opportunity to move from dependency to resilience
In March 2025, the European Commission unveiled its proposal, aiming to reduce the EU’s dependence on third-country
Written by Paul Neill, Chair, Medicines for Ireland
suppliers, diversify supply chains, and boost EU based manufacturing for approximately 270 critical medicines.
For generics and biosimilars, the CMA goes to the heart of the economic model. The Commission has explicitly recognised that decades of tenders based solely on lowest price have created a “race to the bottom”, driving manufacturing out of Europe and contributing to the fragility now visible across multiple therapeutic areas.
Throughout 2025, MFI has amplified Medicines for Europe’s position that a “daring” CMA is needed, one that couples security of supply criteria in procurement with dedicated EU funding for essential medicines and API manufacturing, and more flexible state-aid rules.
A well designed Critical Medicines Act can give Ireland and Europe a more stable, predictable foundation for medicines policy. But that outcome is not guaranteed. Securing it will remain a priority for MFI in 2026.
The Urban Waste Water Treatment Directive is the major risk to the availability of medicines for Irish patients
MFI supports the objectives of the EU Urban Waste Water Treatment directive (UWWTD) to protect the environment and advance waste water treatment facilities. However, we are calling for an immediate pause of the implementation of the Directive in its current form, a suspension of the EPR scheme and the revision of the EPR provisions to ensure a science-based and proportionate alternative for funding quaternary treatment that does not harm patient and supply security.
The Directive, which focuses on patient consumption rather than manufacturing emissions already controlled by companies, only applies to the pharmaceutical and cosmetics industries.
• The Directive and its EPR scheme impose disproportionate costs on the pharmaceutical sector while claiming to incentivise “greener” medicines – something prevented by regulatory frameworks and also by the requirement that certain medicines must have a specific effect to treat diseases.
• The data used by the European Commission to justify this proposal is fundamentally flawed and significantly overestimates the toxic load of the pharmaceutical sector.
• The Directive ignores that micropollutants can also come from other sectors, such as food additives, plant protection products, biocides, industrial chemicals, and household cleaning products.
These inaccuracies and assumptions have also led to a seriously underestimated impact of the costs of the proposed EPR scheme on medicines used by millions of patients in Ireland every day.
MFI has taken legal action in the hope that the courts can clarify that this incorrect application of an EPR scheme is not applicable in the case of medicines, and we will continue to advocate for better ways to achieve the objectives set out in the Directive.
Digital-first safety communications is a practical win-win
In March, MFI hosted “Digital Solutions for a Greener Industry”, bringing together representatives from the Irish Pharmaceutical Union, the HPRA and wider stakeholders. The event was expertly chaired by the late Prof Pat O’Mahoney, fondly remembered by all of us across the sector.
Patient safety is the number one priority for all of us operating in

Our ask is straightforward - move to a digital-first, regulator-approved negotiations to update Ireland’s existing Framework Agreement cycle moves from design to implementation, and as Ireland and security of supply are not taken for granted.
NDP Sectoral Plan for Health 2026-2030 News
Minister for Health, Jennifer Carroll MacNeill has published the Department of Health’s Sectoral Plan for 2026-2030 under the National Development Plan (NDP). The Sectoral Plan sets out infrastructure investment priorities for the next five years, building on a strong track record of delivery to support the provision of equitable, accessible and high-quality healthcare across Ireland.
The NDP provision for the health sector for 2026-2030 is €9.25 billion. This represents the largest ever investment in public health infrastructure in the State and will help to deliver a stronger, more modern and digitised healthcare service, providing better and faster care for patients, improved services for families, and a safer, more supportive environment for healthcare workers.
This increased investment for 2026-2030 will be used to progress and deliver a range of health sector priorities throughout the country, including:
• Health digitalisation, such as progressing the HSE Health App, National Shared Care Record, Electronic Health Records (EHR), ePrescribing, Virtual Care and modern workforce, and workplace digital technologies.
• Opening the National Children's Hospital of Ireland and progress the construction of the new National Maternity Hospital.
• Delivering more Surgical Hubs to provide elective care across the regions.

• Complete detailed design and statutory planning for the Elective Care Programme with the ambition to progress these to get construction underway.
• Constructing new acute beds and projects in acute hospitals through the regions (including theatres and Emergency Departments).
• Investing in Cancer Care including Radiation Oncology.
The HSE’s 2026 National Service Plan (NSP) and associated
Capital Plan are currently being finalised and will reflect the priorities and ambition set out in this Sectoral Plan.
Minister Carroll MacNeill said, "I secured an allocation of ¤9.25 billion under the NDP Review to fund our capital and infrastructure programmes over the next five years. This Health Sectoral Plan will ensure that every euro allocated to health infrastructure is invested with value for money and regional balance to the fore.
"Through carefully planned investment in infrastructure, we can advance Sláintecare’s vision of a modern, integrated and patientcentred health service – one that prioritises health outcomes and delivers the most ambitious digital transformation in the history of our health service.
"Our goal is clear: wherever you live in Ireland, we want you to have timely access to high-quality care."
End of Year Review
Osteoporosis Society

Irish Osteoporosis Society End of Year Review Hospital Professional News
It has been another busy year for the Irish Osteoporosis Society Charity, as we continue to raise awareness of this Silent but deadly disease, IF not diagnosed and treated.

The charity has two employees to cover Ireland, which at times can make it very challenging; however, we continue to forge on with our vision, which is to decrease the number of people losing their independence, from a disease which is preventable and treatable in most cases.
Osteoporosis is unique in many ways compared to other diseases. With most diseases, a person is taking medication to slow down the progression of the disease or to help calm symptoms. With Osteoporosis treatments, they either slow down bone loss, which will help to decrease a person's risk of fracturing, or they increase bone formation, which helps to decrease a person's risk of fracturing or both!
Unlike most other diseases, there are no signs and symptoms of Osteoporosis, which is why it is called the Silent killer. People die from the secondary effects of a fracture, which are blood clots, pneumonia or an infection.
A major development is the new treatment for Osteoporosis called Evenity, the chemical name is romosozumab. There are very strict criteria for it, which is a shame, but it has finally been approved in Ireland, as it has been available in the EU for many
years. It is a prescription medicine used to treat severe osteoporosis in postmenopausal women at high risk of fracture. It is a bonebuilding medication given as a monthly injection for 12 months, which works by both increasing bone formation and decreasing bone breakdown. It is prescribed for those who can be proven to have had a major fracture within the last two years.
Irish Osteoporosis Society Charity continued in 2025 to help dispel the many myths regarding this Silent disease. Many people are unaware that only 2% of nutrition information online is accurate. We do not have a figure regarding the accuracy of osteoporosis information online, but based on our helpline queries, there continues to be a lot of misinformation out there. Additionally, what people are being told in person can be quite alarming.
One of the major issues we have faced this year is that many people are being advised to follow the Liftmor study, which suggests lifting heavy weights to help increase bone strength.
The Irish Osteoporosis Society run the national Osteoporosis helpline, and the following are examples of advice given to people with bone
Written by Michele O'Brien, CEO, Irish Osteoporosis Society
loss, including severe bone loss and fractured vertebrae, which the Osteoporosis society would NOT recommend:
• Lift 5kg and higher above their head
• Lift your grandchildren to increase your overall strength and your bone health
• A farmer's carry which is a person carrying heavy weights in each hand around a room, which in our view and from calls received, can and have been detrimental to causing shoulder, neck and back issues, as well as fractures
• Do yoga
• Jump on trampolines
• Stomp your feet to build up bone
The Irish Osteoporosis Society (IOS) does not recommend that anyone with bone loss lift weights unless they are guided by a regulated professional who specialises in bone health. The issue is that there appears to be many people out there who believe they are experts in this area. Considering we run the national helpline, we are very aware of these issues, and the majority of people who have been injured
admit they just never went back to the class. We would encourage people to contact us for help. We offer an excellent service to charity members, which is a 30-minute phone appointment to discuss their bone health. A one-year membership is a donation of ¤4.16 a month.
We have found that many people with fractured vertebrae do not actually realise they have broken bones in their spine. A crushed, wedge or fractured vertebra means a broken bone in a person's back, and the majority of them are not diagnosed.
The IOS would recommend extreme caution following the Liftmor Study, where people are encouraged to lift their maximum weight.
It is widely accepted that the majority of broken bones in the back are due to osteoporosis; 70% of vertebral fractures go undiagnosed. Often, most broken bones in the back are mistaken for muscle strains. Many people feel that they have "pulled a muscle" when in fact, they have actually broken a bone in their back.
The secondary effects of fractures lead to loss of independence and can lead to premature death. This occurring to even one person is not acceptable. Senior citizens who have never lifted weights should begin with active exercises, slowly progress to low-level weights, and then weight increases gradually over time. This allows their bodies to adjust to the stress and strain placed on them by the exercises. This would minimise the muscle and joint soreness associated with beginning or increasing an exercise program. These are noted from calls received by the Irish Osteoporosis Society as the main reasons people do not return to exercise classes or remain compliant with a home exercise program. Ongoing education regarding the anticipated effects of exercise is very important to maintain compliance with the program and to minimise the risk of injuries.
Touching your toes while in the sitting position or standing position, excessive weights, kettle bells, yoga, jumping off boxes, stopping your feet when walking, trampolines or swiss balls.
World Osteoporosis Day
– Time

Written by Michele O'Brien, CEO,
With World Osteoporosis having Society are asking everyone to

Irish Osteoporosis Society
Why prevention of Fractures
The images show the positions that those with bone loss and senior citizens should avoid, as many senior citizens have undiagnosed vertebral fractures and bone loss.
The IOS’s concern with this trial is that they relied on self-reporting for adverse outcomes. We believe it is misleading to say that there were no adverse outcomes, given the accepted lack of reliability with self-reporting, along with the fact that the exclusions for the trial caused a significant cohort of people to be excluded from participating in the trial.
• Thyroxine
• Thiazides
• Antiretroviral agents
Worldwide, up to 37 million fragility equivalent of 70 fractures per minute. that if the persons bones were
With World Osteoporosis having taken place on 20th October, the Irish Society are asking everyone to check to see if they have risk factors for
The Liftmor trial excluded people who had any of the following:
• Lower limb joint injury or surgery
• Recent fracture (within the last 12 months) or localised back pain
We have reviewed the literature, and, based on this, the Irish Osteoporosis Society would not recommend high-intensity resistance exercise for anyone with bone loss, as we believe it is not worth taking the risk of fractures occurring.
monitored closely due to the fact that there are NO signs or symptoms of bone loss occurring or improving. We do not recommend that a person have repeated DXA scans every 5 years; we recommend, whenever possible, annual DXA scans as bone loss is silent and people can and have lost up to 20% of bone in one year.
Unfortunately, the Irish Osteoporosis Society continue to receive a significant amount of calls from people who have developed disc and sciatic nerve problems, as well as fractured vertebrae, when performing forward flexion stretches.
Did you know that all senior citizens need to ensure that they make their homes safe to reduce their risk of falling in their home?
Why prevention of Fractures is common sense as well as economical
Normal bone Image
Worldwide, up to 37 million fragility fractures occur annually in people over equivalent of 70 fractures per minute. A fragility fracture is a broken bone that if the persons bones were healthy, the fracture would not have occurred.
• Less than 5 years since menopause
• Malignancy (cancer)
• Uncontrolled cardiovascular disease
• Cognitive impairment
It is very important that throw rugs are removed from floors, that people wear a good pair of walking shoes in their home versus slippers as many give no support. A handrail should be placed on steps into homes and handrails on both side of your stairs can make it much easier and safer for you. Clothes should be between hip and eye level in cupboards and any clothes that have not been worn in 5 years should be donated. The reason being that if you or a loved one has trouble finding clothes or when trying to pull an item out, you have difficulty, you have too many items. There are so many people who are not in this position and would be forever grateful to receive clothing. There have been multiple incidences where senior citizens with bone loss have actually fractured vertebrae while attempting to manoeuvre clothes out of a wardrobe.
• Recent X-ray or radiation treatment
• Contraindications for participating in heavy physical activity
• Conditions known to affect bone health, such as:
• Thyrotoxicosis or hyperparathyroidism
• Paget’s disease
• Renal disease
• Diabetes
Research shows that 70% of fractures occur in the Osteopenia range (A T score of -1.5 to -2.49), which is why anyone with bone loss should be progressed with caution. Muscles may be strong, but when they pull on a weak bone, it can easily break.
The IOS recommends slow, cautious, supervised progression in exercise programs for older adults new to weightlifting. Exercise should begin gently and increase gradually to prevent soreness and improve long-term compliance.
The following are the main messages that the charity highlighted throughout this past year
• Yoga/forward flexion should not be done by anyone with bone loss, as it can and has caused vertebral fractures.
• The importance of normal vitamin D levels, we recommend a result of 70nmol/L for bone health. Many labs consider a result of 50nmol/L as a normal result.
• Pharmacists can make a significant difference in the bone health area by picking a medication which causes bone loss and red flagging all customers who are taking this medication and suggesting they check out the Irish Osteoporosis Society website. The following are examples of the many that cause bone loss: Aromatase inhibitors, proton pump inhibitors, Arimidex, DepoProvera, progesterone-only contraceptives and one of the major causes of bone loss are corticosteroids.
1 in 2 women over age 50 will experience 50, even though research shows A recent survey in Ireland showed experienced a fragility fracture. expected/assumed by most people
More than one out of four older people fall each year, but less than half actually tell their doctor. The Irish Osteoporosis Society believe from feedback this is due to embarrassment and also many are concerned they may be sent to a nursing home. Research shows that falling once doubles the person’s risk of falling again and that more than 95% of hip fractures are caused by falling, usually by falling sideways. What makes walking with a cane or rollator much more difficult?
• Immobility
• Taking medications (other than osteoporosis meds) known to influence bone, e.g.
• Prolonged corticosteroid use
• Anyone who has broken a bone from a trip and fall or less should be assumed to have Osteoporosis unless proven otherwise.
• Osteopenia should not be considered a “touch of bone loss”
• Everyone with bone loss needs their bone health
• FYI, the only cancer that surpasses Osteoporosis in death is lung cancer, which is why everyone needs to be proactive and help the Irish Osteoporosis Society raise awareness of this silent disease.
Why the Irish Osteoporosis Society does not recommend Chair Yoga
Bending forward puts excessive stress on the anterior aspect of the vertebrae, as well as the discs between them and the sciatic
Our hope for 2026 is that all of you reading this piece will make a conscious effort to help to increase awareness of this Silent disease. By just passing on the National helpline phone number 01 637 5050 or email info@irishosteoporosis.ie, you will make a significant difference in helping to reduce the number of people suffering from this treatable disease.
One of the major issues with those who use canes or rollators is that they are adjusted to high. If either are too high, they can cause, neck, shoulder and back pain and make it more difficult for a person to walk.
There are some in Ireland who older, as they are the highest risk survey was highest amongst 40 common sense, but financially living in Ireland. This is to help independence and their care costing
1 in 2 women over age 50 will experience osteoporosis fractures, and 1 50, even though research shows that most fractures are preventable. A recent survey in Ireland showed that over one third of Irish adults over experienced a fragility fracture. The level of fractures in the survey would expected/assumed by most people to be amongst the older age groups.
How to check if your cane or rollator is too high – you will need someone to help you
There are some in Ireland who believe that DXA scans should only be
The Irish Osteoporosis Society
End of Year Review
Crohn’s & Colitis Ireland

Progress, Pressures, and Priorities within the IBD community in Ireland

Major Developments
2025 was a year of progress and persistent challenges for the inflammatory bowel disease (IBD) community in Ireland. Crohn’s & Colitis Ireland (CCI) strengthened advocacy, expanded patient supports, and deepened its role in research collaboration.
A key highlight was CCI’s growing partnerships with academic and industry leaders, reinforcing its role as a bridge between patients and science. The upcoming launch of the IBD Research Hub will provide a central platform for patient involvement and accessible research updates. Importantly, CCI published its first joint report with Johnson & Johnson, exposing the hidden costs of living with IBD, which average ¤3,252 annually and often lead to delayed or avoided care. This evidence will shape policy discussions on financial supports and equity in chronic disease management.
Challenges and Pressures
Despite these steps forward, systemic gaps remain:
• Financial Burden: Nearly half of patients avoid care due to cost.
• Access Delays: Waiting times for diagnostics and treatment remain excessive, compounded by limited GP access to faecal calprotectin testing.
• Workforce Shortages: Under-resourcing of IBD nurse specialists, dietitians, and psychologists continues to strain services.
• Policy Gaps: Critically, IBD is still not recognised under the Long-Term Illness Scheme or the Chronic Disease Management Pathway, leaving thousands without structured supports or financial relief.
• Systemic Pressures: Broader healthcare challenges—bed shortages, waiting lists, and uneven Sláintecare implementation—impact chronic disease care.
Progress and Achievements
Amid these pressures, notable gains were made:
• Innovation in Diagnostics: Capsule endoscopy expanded across ten centres, reducing reliance on invasive procedures and improving patient experience.
• Clinical Research Leadership: Ireland strengthened its role in global IBD trials through the INITIative IBD Network, opening pathways to novel therapies.
• Support Expansion: CCI introduced in-person support groups across four counties, creating safe spaces for
Written by Victoria Spillane, Interim Chief Operating Officer, Crohn’s & Colitis Ireland
connection and shared experience. Online stoma support groups also grew significantly, offering tailored advice and peer support for those living with an ostomy.
• Breaking Stigma: CCI launched its first Ostomy Awareness Day media campaign, challenging misconceptions and promoting understanding of life with a stoma as a result of IBD. This campaign marked a major step toward normalising conversations and reducing stigma.
Areas of Concern
Progress has stalled in:
• Mental Health Access: Psychological support remains minimal despite clear need.
• Equity in Care: Lack of inclusion in chronic disease frameworks perpetuates financial and access inequities.
• Workforce Planning: Absence of sanctioned posts for CNS, ANPs, dietitians, and psychologists limits care delivery.
Importantly, IBD does not discriminate by age. It can affect children, adolescents, adults, and older people alike, often striking during formative years and continuing throughout life. This lifelong nature means that care must be holistic and adaptable, addressing education, employment, family life, and
ageing. The absence of structured supports for families is a growing concern, IBD impacts not just the individual but their entire support network, creating emotional and financial strain that current systems fail to address.
Looking Ahead to 2026
CCI’s priorities for the year ahead are clear but challenging. Our goal is to grow supports for an ever-expanding IBD community to keep pace with rising needs and demands, particularly around family supports. IBD does not just affect the individual, it impacts their entire support network. Families often face emotional, financial, and practical challenges, and CCI aims to strengthen resources for them.
However, funding constraints remain a major barrier. Without secure, ongoing funding, we are limited in the areas we can grow. This means some services cannot be expanded, and staffing remains insufficient to meet demand. Despite these limitations, CCI will continue to advocate for systemic change.
From a national perspective, priorities must include:
• Addressing Financial Burden: Implementing measures to reduce out-of-pocket costs and recognising IBD under the Long-Term Illness Scheme.
• Building Multidisciplinary Teams: Ensuring access to dietitians, psychologists, and IBD nurse specialists to deliver integrated care.
• Policy Integration: Including IBD in chronic disease management pathways to enable structured, proactive care.
• Innovation and Equity: Leveraging digital health and precision medicine while ensuring equitable access for all patients.
For the 50,000+ people living with IBD in Ireland, 2026 must be a year of action - both in policy and practice.
For
WEZENLA: USTEKINUMAB BY AMGEN
WEZENLA CAN BE ADMINISTERED WITH †ConfiPen™, AMGEN’S ADVANCED PRE-FILLED PEN DESIGNED FOR EASE OF USE7,8
• Available in 45 mg & 90 mg formulations7,8
• Also available in Pre-filled syringe & Vial presentations3-6


WEZENLA is indicated for: (refer to the Summary of Product Characteristics and Patient Information leaflets for full indications, dosage and administration).
WEZENLA demonstrated pharmacokinetic comparability and has been shown to be as well-tolerated when administered with †ConfiPenTM as by a pre-filled syringe9 ADULT CROHN’S DISEASE3-8
PLAQUE PSORIASIS3-5,7,8 PAEDIATRIC PLAQUE PSORIASIS3–5
WEZENLA is not currently approved in Ireland for ulcerative colitis (since the originator still has exclusivity for this indication). Pre-filled syringe or †
Subcutaneous injection with PFS or ConfiPen™
*Patients weighing at least 40 kg, †pre-filled pen has not been studied in the paediatric population and is not recommended for use in paediatric patients CD: Crohn’s Disease, I.V.: intravenous References: 1. Blauvelt et al, BJD, 2025, 192:(5) 826-836 2. Chow V, et al. Clin Pharmacol Drug Dev. 2023;12(9):863–873 3. WEZENLA 45 mg solution for injection. Summary of Product Characteristics 4. WEZENLA 45 mg solution for injection in pre-filled syringe. Summary of Product Characteristics 5. WEZENLA 90 mg solution for injection. Summary of Product Characteristics 6. WEZENLA 130 mg concentrate for solution for infusion. Summary of Product Characteristics 7. WEZENLA ConfiPenTM 45 mg pre-filled pen. Summary of Product Characteristics 8. WEZENLA ConfiPenTM 90 mg pre-filled pen. Summary of Product Characteristics 9. Chow et al. Pharmacokinetic comparability of ABP 654 administered either via prefilled syringe or autoinjector in healthy adult subjects: Results from a randomized, open-label, parallel-group study. Presented at United European Gastroenterology (UEG) Congress, Vienna, Austria, 12-15 October 2024. PP0638.
WEZENLA ▼ (ustekinumab) Abbreviated Prescribing Information
Please refer to the Summary of Product Characteristics (SPC) before prescribing WEZENLA. Pharmaceutical Forms: 130 mg vial concentrate for solution for infusion; 45 mg vial solution for injection; 45 mg and 90 mg solution for injection in pre-filled syringes; 45 mg and 90 mg solution for injection in pre-filled pens (ConfiPenTM). WEZENLA Indications: 45 mg vial solution for injection, 45 mg and 90 mg solution for injection in pre-filled syringes, 45 mg and 90 mg solution for injection in pre-filled pens (ConfiPen): Adult Plaque psoriasis (PsO) - Moderate to severe PsO in patients who failed to respond to, or who have a contraindication to, or are intolerant to other systemic therapies including ciclosporin, methotrexate (MTX) or PUVA (psoralen and ultraviolet A). 45 mg vial solution for injection, 45 mg and 90 mg solution for injection in pre-filled syringes: Paediatric PsO - Moderate to severe plaque PsO in patients from the age of 6 years and older, who are inadequately controlled by, or are intolerant to, other systemic therapies or phototherapies. The pre-filled pen has not been studied in the paediatric population and is not recommended for use in paediatric patients. See section 4.2 of the pre-filled syringe SmPC for posology and method of administration in paediatric patients. 45 mg and 90 mg solution for injection in pre-filled syringes, 45 mg and 90 mg solution for injection in pre-filled pens (ConfiPen): Adult Psoriatic arthritis (PsA) - Alone or in combination with MTX, indicated for the treatment of active PsA patients when the response to previous non-biological disease-modifying antirheumatic drug (DMARD) therapy has been inadequate. 130 mg vial concentrate for solution for infusion, 45 mg vial solution for injection, 45 mg and 90 mg solution for injection in pre-filled syringes, 45 mg and 90 mg solution for injection in pre-filled pens (ConfiPen):Adult Crohn’s Disease (CD) - Patients with moderately to severely active CD who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a TNFα antagonist. 130 mg vial concentrate for solution for infusion, 45 mg vial solution for injection, 45 mg and 90 mg solution for injection in pre-filled syringes:Paediatric CD - treatment of moderately to severely active CD patients weighing at least 40 kg, who have had an inadequate response to, or were intolerant to either conventional or biologic therapy. Dosage and Administration: For use under the guidance and supervision of physicians experienced in the diagnosis and treatment of conditions for which WEZENLA is indicated. After proper training in subcutaneous (SC) injection technique, patients or their caregivers may inject WEZENLA if a physician determines that it is appropriate. Physician should ensure follow-up of patients. Adult PsO - Initial dose of 45 mg administered subcutaneously (SC), followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter. Patients with a body weight > 100 kg, initial dose is 90 mg administered SC, followed by a 90 mg dose 4 weeks later, and then every 12 weeks thereafter. Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks. Paediatric PsO 6 years and older - Recommended dose is based on body weight administered SC at Weeks 0 and 4, then every 12 weeks thereafter. Body weight and recommended dose < 60 kg - 0.75 mg/kg; ≥ 60 - ≤ 100 kg - 45 mg; > 100 kg – 90 mg. Refer to SPC for further details on dosing. The 45 mg vial is available for paediatric patients < 60 kg who need to receive less than the full 45 mg dose. Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks. Adult PsA - Initial dose of 45 mg administered SC, followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter. Alternatively, 90 mg may be used in patients with a body weight > 100 kg. Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks. Adult CD - First dose administered intravenously (IV) over at least one hour. Body weight and recommended dose: ≤ 55 kg – 260 mg; > 55 kg to ≤ 85 kg – 390 mg; > 85 kg – 520 mg. The first SC administration of 90 mg should take place at week 8 after the IV dose. Dosing every 12 weeks is recommended, thereafter. Patients who have not shown adequate response at 8 weeks after the first SC dose, may receive a second subcutaneous dose at this time. Patients who lose response on dosing every 12 weeks may benefit from an increase in dosing frequency to every 8 weeks. Patients may subsequently be dosed every 8 weeks or every 12 weeks according to clinical judgement. Consider discontinuing treatment in patients who show no therapeutic benefit 16 weeks after the IV induction dose or 16 weeks after switching to the 8-weekly maintenance dose. Immunomodulators and/or corticosteroids may be continued. In patients who have responded to treatment, corticosteroids may be reduced or discontinued in accordance with standard of care. In CD, if therapy is interrupted, treatment can be resumed with subcutaneous dosing every 8 weeks. Paediatric CD (patients weighing at least 40 kg) - initiate with a single IV over at least one hour. Body weight and recommended dose: ≥ 40 kg to ≤ 55 kg – 260 mg; > 55 kg to ≤ 85 kg – 390 mg; > 85 kg – 520 mg. The first SC administration of 90 mg should take place at week 8 after the IV dose. Dosing every 12 weeks is recommended, thereafter. Patients who lose response on dosing every 12 weeks may benefit from an increase in dosing frequency to every 8 weeks. Patients may subsequently be dosed every 8 weeks or every 12 weeks according to clinical judgement. Consider discontinuing treatment in patients who show no evidence of therapeutic benefit 16 weeks after the IV induction dose or 16 weeks after dose adjustment. Immunomodulators, 5-aminosalicylate (5-ASA) compounds, antibiotics, and/or corticosteroids may be continued. In patients who have responded to treatment, these medications may be reduced or discontinued in accordance with standard of care. Contraindications: Hypersensitivity to the active substance or to any of the excipients (see section “Special Warnings and Precautions for use” for information on polysorbate and SPC for the full list of excipients and further details). Clinically important, active infection (e.g. active tuberculosis). Special Warnings and Precautions for use: Traceability - The tradename and the batch number should be clearly recorded. Infections - Ustekinumab may have the potential to increase the risk of infections and reactivate latent infections. In PsO, serious bacterial, fungal, and viral infections have been observed. Opportunistic infections including reactivation of tuberculosis (TB), other bacterial infections, fungal infections, viral infections and parasitic infections have been reported. Use with caution in patients with a chronic infection or a history of recurrent infection. Prior to treatment, patients should be evaluated for TB infection. Must not be given to patients with active TB. Initiate anti-TB therapy in patients with latent TB, a history of latent or active TB in whom an adequate course of treatment cannot be confirmed, prior to WEZENLA treatment. Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur. Closely monitor patients with serious infection, suspend WEZENLA until the infection resolves. Caution should be used in treating the elderly. Malignancies - Immunosuppressants like ustekinumab have the potential to increase the risk of malignancy. Some patients with PsO receiving ustekinumab developed cutaneous and non-cutaneous malignancies. The risk of malignancy may be higher in PsO patients who have been treated with other biologics.
Use with caution when considering therapy in patients with a history of malignancy or in patients who develop malignancy while receiving WEZENLA treatment. All patients should be monitored for the appearance of skin cancer (particularly those > 60 years of age, patients with a medical history of prolonged immunosuppressant therapy or those with a history of PUVA treatment). Systemic hypersensitivity reactions - Serious hypersensitivity reactions have been reported, in some cases several days after treatment. If an anaphylactic or other serious hypersensitivity reaction occurs, appropriate therapy should be instituted and administration of WEZENLA should be discontinued. Infusion related reactions - Infusion-related reactions, serious infusion-related including anaphylactic reactions have been reported. Discontinue WEZENLA if serious or life-threatening reactions observed and institute appropriate therapy. Respiratory hypersensitivity reactions - Allergic alveolitis, eosinophilic pneumonia, and non-infectious organising pneumonia have been reported, some leading to respiratory failure and prolonged hospitalisation. Improvement has been reported after discontinuation of ustekinumab and in some cases, administration of corticosteroids. If infection has been excluded and diagnosis is confirmed, discontinue ustekinumab. Cardiovascular events - Cardiovascular events including myocardial infarction and cerebrovascular accident have been observed in patients with PsO exposed to ustekinumab. Risk factors for cardiovascular disease should be regularly assessed during treatment with WEZENLA. Vaccinations - It is recommended that live viral or live bacterial vaccines (such as Bacillus of Calmette and Guérin (BCG)) should not be given concurrently with WEZENLA – refer to SPC for additional information and guidance. Before live viral and live bacterial vaccination, WEZENLA treatment should be withheld for at least 15 weeks after the last dose and can be resumed at least 2 weeks after vaccination. Administration of live vaccines (such as the BCG vaccine) to infants exposed in utero to ustekinumab is not recommended for twelve months following birth or until ustekinumab infant serum levels are undetectable. Patients receiving WEZENLA may receive concurrent inactivated or non-live vaccinations. Long term treatment with ustekinumab does not suppress the humoral immune response to pneumococcal polysaccharide or tetanus vaccines. Concomitant immunosuppressive therapy - In PsO, the safety and efficacy of ustekinumab in combination with immunosuppressants, including biologics, or phototherapy have not been evaluated. In PsA, concomitant MTX use did not appear to influence the safety or efficacy of ustekinumab. In CD and Ulcerative Colitis (UC), concomitant use of immunosuppressants or corticosteroids did not appear to influence the safety or efficacy of ustekinumab. Caution should be exercised when considering concomitant use of other immunosuppressants and WEZENLA, or when transitioning from other immunosuppressive biologics. Serious skin conditions - In PsO, physicians should be alert for symptoms of erythrodermic psoriasis or exfoliative dermatitis. If these symptoms occur, appropriate therapy should be instituted. Discontinue WEZENLA if a drug reaction is suspected. Lupus-related conditions – Lupus-related conditions reported. If lesions occur, especially in sun exposed areas of the skin or if accompanied by arthralgia, the patient should seek medical attention promptly. If these symptoms occur, appropriate therapy should be instituted. Discontinue WEZENLA if a drug reaction is suspected. Polysorbate 80 – WEZENLA contains polysorbate 80 (E 433) - 10.4 mg in 130 mg/26 mL vial equivalent to 0.40 mg/mL, 0.02 mg in 45 mg/0.5 mL vial, 0.02 mg in 45 mg/0.5 mL PFS/PFP and 0.04 mg in 90 mg/1.0 mL PFS/PFP equivalent to 0.04 mg/mL. Polysorbates may cause allergic reactions. Interactions: Refer to SPC for further details. In vitro study and a phase 1 study in subjects with active CD suggest no dose adjustments required in patients who are receiving concomitant CYP450 substrates. Pregnancy, lactation and fertility: Women of childbearing potential should use effective methods of contraception during treatment and for at least 15 weeks after treatment. It is preferable to avoid the use of WEZENLA in pregnancy. Ustekinumab crosses the placenta and has been detected in the serum of infants born to female patients treated with ustekinumab during pregnancy. The clinical impact of this is unknown, however, the risk of infection in infants exposed in utero to ustekinumab may be increased after birth. See sub section “vaccinations” in Special Warnings and Precautions for use for further details. Limited data suggest ustekinumab is excreted in human breast milk in very small amounts. It is not known if ustekinumab is absorbed systemically after ingestion. A decision on whether to discontinue breast-feeding during treatment and up to 15 weeks after treatment or to discontinue therapy with WEZENLA must be made taking into account the benefit of breast-feeding to the child and the benefit of WEZENLA therapy to the woman. The effect of ustekinumab on fertility has not been evaluated. Undesirable Effects: Refer to the SPC for the full list of undesirable effects. Common (≥ 1/100 to < 1/10) - upper respiratory tract infection, nasopharyngitis, sinusitis, dizziness, headache, oropharyngeal pain, diarrhoea, nausea, vomiting, pruritus, back pain, myalgia, arthralgia, fatigue, injection site erythema, injection site pain. Other side effects reported: Serious infections: pneumonia, anal abscess, cellulitis, diverticulitis, gastroenteritis and viral infections. Malignancies: Excluding non-melanoma skin cancer, prostate, melanoma, colorectal and breast cancers. Hypersensitive reactions: Serious infusionrelated reactions including anaphylactic reactions, rash and urticaria. Pharmaceutical Precautions: Store in a refrigerator (2°C – 8°C). Do not freeze. Keep in the outer carton in order to protect from light. If needed, individual 45 mg vial/45 mg and 90 mg pre-filled syringes/pre-filled pens may be stored at room temperature up to 30°C for a maximum single period of 30 days in the original carton and should not be returned to the refrigerator. Legal Category: POM. Presentations and Marketing Authorisation Numbers: WEZENLA 130 mg concentrate for solution for infusion: EU/1/24/1823/004 – 1 pack of 1 vial; WEZENLA 45 mg solution for injection: EU/1/24/1823/001 – 1 pack of 1 vial; WEZENLA 45 mg solution for injection in pre-filled syringe: EU/1/24/1823/002 – 1 pack of 1 syringe; WEZENLA 90 mg solution for injection in pre-filled syringe: EU/1/24/1823/003 – 1 pack of 1 syringe; WEZENLA 45 mg solution for injection in pre-filled pen: EU/1/24/1823/005 – 1 pack of 1 pen; WEZENLA 90 mg solution for injection in pre-filled pen: EU/1/24/1823/006 –1 pack of 1 pen. Marketing Authorisation Holder: Amgen Technology (Ireland) UC, Pottery Road, Dun Laoghaire, Co Dublin, Ireland. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31, Ireland. WEZENLA is a trademark owned by Amgen Inc. Date of PI preparation: October 2025 (IRL-654-25-80006).
▼ This medicinal product is subject to additional monitoring. Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse reactions/events should also be reported to Amgen Limited on +44 (0) 1223 436441 or Freephone 1800 535 160.
End of Year Review
Irish Cancer Society

Irish Cancer Society 2025 Year in Review: Confronting Delays, Championing Equity and Supporting Patients Through Rising Demand

2025 was a pivotal and challenging year for cancer care in Ireland. The Irish Cancer Society continued to act as the leading national voice highlighting critical gaps in the system, championing equity in access, and providing essential services to thousands of patients at a time of growing need. From campaign-driven advocacy to major service demands and policy interventions, the Society played a central role in calling for urgent investment and systemic reform.
A National Wake-Up Call on Cancer Delays
The most urgent warning of the year came in July, when the Society published stark new data showing extensive delays in cancer diagnosis and treatment. These delays—affecting chemotherapy, radiotherapy, and urgent breast and prostate assessments— varied dramatically depending on where patients live. The Society condemned this “postcode lottery” and pointed to serious systemic
causes: insufficient staffing, outdated facilities, and inadequate equipment in cancer centres.
CEO Averil Power warned that treatment delays are directly costing lives. Research shows that every four-week delay in starting treatment can raise mortality by 10%, and Ireland already has the third-highest cancer mortality rate in Western Europe. With cancer incidence projected to double by 2045, the Society’s message was unequivocal: Budget 2026 must significantly expand investment in cancer staff, infrastructure and equipment.
Budget 2025: Exposing Years of Underfunding
In June, the Society launched its Budget 2025 submission, revealing that the National Cancer Strategy has been underfunded by almost ¤180 million since 2017. Underinvestment has hindered screening expansion, delayed
surgery, limited radiotherapy capacity, slowed clinical trial development and impeded access to new cancer medicines.
The country’s leading oncology bodies united behind the Society’s call for a ringfenced annual investment of at least ¤20 million for the National Cancer Control Programme, alongside multiannual funding to plan effectively for a growing cancer burden.
Breast Screening and Tackling Inequality
In October, ahead of Breast Cancer Awareness Month, the Society warned that screening uptake remains too low— particularly among marginalised and underserved communities. While Ireland reached a 71% uptake in 2023, leading countries exceed 80%. The Society pressed Government to expand targeted outreach and remove barriers faced by people with disabilities, migrants and
Irish Cancer Society CEO Averil Power
economically disadvantaged groups. Early detection saves lives, and the Society stressed that Ireland must strive for world-leading performance.
A Year of Extraordinary Patient Support Demand
2025 also saw dramatic increases in demand for the Society’s free patient support services. Building on record figures from 2023, the Society again saw rising use of its Support Line, Daffodil Centres, and Night Nursing programme. Transport Service drivers covered millions of kilometres helping patients access chemotherapy— support that many describe as “a lifeline” during the hardest periods of their illness.
With the State providing only 5% of its funding, the Society relied heavily on public generosity for Daffodil Day. Averil Power appealed to communities to “go all in against cancer,” underscoring the ¤25 million needed yearly to sustain and expand these essential services.
Looking Ahead: A Call for Equity, Investment and Action
Across 2025, one message was constant: Ireland cannot continue to accept delays, inequalities and underfunding in cancer care. As incidence rises and services strain, the Society is doubling down on its mission—ensuring every person affected by cancer receives timely treatment, compassionate support and the best chance of survival.
With targeted government investment, expanded screening, stronger cancer staffing and modernised infrastructure, Ireland can reverse mortality trends and deliver the high-quality cancer care its population deserves. The Irish Cancer Society ends 2025 with a clear mandate for change—and a renewed commitment to advocate, support and fight for every patient in 2026 and beyond.

End of Year Review HPAI
HPAI End of Year Report 2025: A Year of Leadership, Advocacy and Advancing Clinical Excellence
2025 has been a defining year for the Hospital Pharmacists Association of Ireland (HPAI), marked by strong advocacy on behalf of our profession, increased visibility at national and European levels, and continued commitment to clinical upskilling and education. Against a backdrop of persistent medicines and medical device shortages, growing healthcare pressures, and evolving scopes of practice, HPAI members have once again demonstrated leadership, adaptability and dedication to patient care.
European Medicines Shortages: A Growing Challenge
In November, the European Association of Hospital Pharmacists (EAHP) released the results of its latest Shortages Survey. As active contributors to this important work, HPAI members helped highlight the deeply concerning reality facing hospital teams across Europe.
The findings show that shortages of medicines and medical devices remain widespread and disruptive.
• 89% of hospital pharmacists reported that shortages affected patient care.
• 79% noted significant effects on pharmacy operations.
• All respondents experienced shortages of critical medicines at least once to three times in 2024.
• Medical device shortages also remained problematic, affecting patient care (53%) and workflows (45%).
The main causes identified — global API shortages, manufacturing delays, pricing pressures and supply chain instability — reflect challenges familiar to Irish hospitals. The consequences are serious: delayed or cancelled care, suboptimal treatment, longer hospital stays and increased clinical risk.
EAHP has urged policymakers to prioritise stronger coordination, transparent information sharing, national taskforces addressing shortages, enhanced riskassessment frameworks and support for European API
manufacturing. The call to safeguard compounding capacity and develop procurement practices that go beyond lowest price is especially relevant for Irish hospital pharmacists, who continue to play a critical role in maintaining continuity of care for vulnerable patients.
Promoting Independent
Prescribing: A Highlight of the 2025 HPAI Annual Conference
One of the year’s most important professional milestones was the HPAI Annual Educational Conference, held in May. The keynote address from Dr Briegeen Girvin, Senior Lecturer and Independent Prescribing Lead at Queen’s University Belfast, provided an invaluable exploration of the future of pharmacist-led prescribing in hospital settings.
Dr Girvin outlined how independent prescribing empowers pharmacists to assess undiagnosed conditions, initiate treatment autonomously and ensure follow-up — all within their competence and with a clear focus on patient safety. International comparisons highlighted how Ireland lags behind peer countries that have already expanded autonomous prescribing roles.
Evidence presented from the UK demonstrated the profound impact pharmacists can make. A Leeds Teaching Hospital Trust study showed prescribing error rates of 0.7% for pharmacist independent

prescribers, compared with 9.8% for doctors (Turner 2021). This dramatic reduction speaks to pharmacists’ deep medicines expertise and their capacity to enhance patient safety, reduce system pressure, and deliver more efficient care pathways.
The conference reinforced that pharmacist prescribing offers clear benefits to practitioners, organisations and patients — from improved job satisfaction and expanded autonomy to reduced error rates, greater access to care, and better chronic disease management. However, legislative change, medical mentorship, clinical system access and sustained investment in training will be essential for progress in Ireland.
Education, Skills and Professional Development
HPAI remains committed to supporting members with robust clinical education and peer networking opportunities. In January 2026, the Association will host its Clinical Skills Day — a focused upskilling event for hospital pharmacists with fewer than three years’ experience. Registration will open in November 2025 and, given high demand in previous years, is expected to sell out quickly. Attendance will be limited to HPAI and Fórsa members, with a nominal fee to secure places.
Looking ahead, we are also pleased to promote the HPAI
Annual Conference 2026, taking place from 24–26 April 2026. This conference continues to be the premier national event for hospital pharmacists, offering high-quality workshops, clinical sessions and professional networking. Designed to meet the needs of both newly qualified pharmacists and experienced specialists, the programme will once again showcase innovation, best practice and the expanding clinical role of hospital pharmacists.
Looking Forward to 2026
As we close 2025, it is clear that hospital pharmacy is entering a period of significant transformation. Persistent shortages, growing system pressures and an evolving clinical landscape have underscored the essential role of hospital pharmacists in safeguarding patient care. The progress made this year — in advocacy, education and professional development — reflects a profession committed not only to excellence but to continuous improvement.
HPAI will continue to champion the expertise of hospital pharmacists at national and European levels, promote expanded clinical roles, and provide high-quality educational opportunities for our members. Together, we look forward to shaping a safer, more sustainable and more patientcentred hospital pharmacy landscape in 2026 and beyond.
Fresenius Kabi is introducing Azacitidine
Kabi
25 mg/ml powder for suspension for injection (azacitidine)

Fresenius Kabi
Azacitidine Kabi is indicated for the treatment of adult patients who are not eligible for haematopoietic stem cell transplantation (HSCT) with: 1
• Intermediate-2 and high-risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System (IPSS),
• Chronic myelomonocytic leukemia (CMML) with 10-29% marrow blasts without myeloproliferative disorder
• Acute myeloid leukemia (AML) with 20-30% blasts and multi-lineage dysplasia, according to World Health Organization (WHO) classification,• AML with > 30% marrow blasts according to the WHO classification.
* Please refer to the abbreviated prescribing information overleaf forfull details on the indications

Azacitidine Kabi
Abbreviated prescribing information
PRESCRIBING INFORMATION – Azacitidine Kabi 25 mg/mL powder for suspension for injection. Consult the Summary of Product Characteristics (SmPC) for full information. Additional information is available on request. Active ingredients: Each vial contains 100 mg azacitidine. After reconstitution, each mL of suspension contains 25 mg azacitidine Indications: Treatment of adult patients who are not eligible for haematopoietic stem cell transplantation (HSCT) with: Intermediate-2 and high-risk myelodysplastic syndromes (MDS) according to the international prognostic scoring system (IPSS), chronic myelomonocytic leukaemia (CMML) with 10-29% marrow blasts without myeloproliferative disorder, acute myeloid leukaemia (AML) with 20-30% blasts and multi-lineage dysplasia, according to World Health Organisation (WHO) classification, AML with > 30% marrow blasts according to the WHO classification. Dosage and administration: Initiate and monitor under supervision of a physician experienced in the use of chemotherapeutic agents. Patients should be premedicated with anti-emetics for nausea and vomiting. Recommended starting dose for the first treatment cycle, for all patients regardless of baseline haematology laboratory values, is 75 mg/m2 of body surface area, injected subcutaneously, daily for 7 days, followed by rest period of 21 days (28-day treatment cycle). Recommended that patients be treated for a minimum of 6 cycles. Treatment should be continued for as long as the patient continues to benefit or until disease progression. Monitor for haematologic response/toxicity and renal toxicities (see section 4.4 of SmPC for further guidance) a delay in starting next cycle or dose reduction may be necessary. Should not be used interchangeably with oral azacitidine. Dose and schedule recommendations for oral azacitidine differ to injectable azacitidine – verify medicinal product name, dose and administration route. Determine liver function tests, serum creatinine and serum bicarbonate prior to therapy initiation and each treatment cycle. Perform complete blood counts prior to therapy initiation and as needed to monitor response and toxicity, but at a minimum, prior to each treatment cycle. Elderly patients - No specific dose adjustments recommended. May be useful to monitor renal function. Renal impairment - Can be administered to patients with renal impairment without initial dose adjustment. If unexplained reductions in serum bicarbonate levels to <20 mmol/L, reduce dose by 50% on next cycle. If unexplained elevations in serum creatinine or blood urea nitrogen (BUN) to ≥ 2-fold above baseline values and above upper limit of normal (ULN) occur, delay next cycle until values return to normal or baseline and reduce dose by 50% on next treatment cycle. Hepatic impairment - No formal studies conducted in patients with hepatic impairment. Carefully monitor patients with severe hepatic organ impairment for adverse events. No specific modification to starting dose recommended for patients with hepatic impairment prior to starting treatment; subsequent dose modifications should be based on haematology laboratory values. Contraindicated in patients with advanced malignant hepatic tumours. Paediatric population - Safety and efficacy in children aged 0-17 years not yet been established. Currently available data described in sections 4.8, 5.1 and 5.2 of SmPC but no recommendation on posology. Method of administration - Inject reconstituted Azacitidine Kabi subcutaneously into upper arm, thigh or abdomen. Rotate injection sites. Give new injections at least 2.5cm from previous site and never into areas where site is tender, bruised, red, or hardened. Suspension should not be filtered after reconstitution. For instructions on reconstitution of the medicinal product before administration, see section 6.6 of SmPC Contraindications: Hypersensitivity to active substance or to any of the excipients, advanced malignant hepatic tumours, breast-feeding. Special warnings and precautions for use: Haematological toxicity Azacitidine treatment is associated with anaemia, neutropenia and thrombocytopenia, particularly during first 2 cycles. Perform complete blood counts as needed to monitor response and toxicity, but at least prior to each treatment cycle After administration of recommended dose for first cycle, reduce dose for subsequent cycles or delay administration based on nadir counts and haematological response. Advise patients to promptly report febrile episodes. Hepatic Impairment Patients and physicians also advised to be observant for signs and symptoms of bleeding. Patients with extensive tumour burden due to metastatic disease reported to experience progressive hepatic coma and death during azacitidine treatment, especially where baseline serum albumin <30g/L. Azacitidine is contraindicated in patients with advanced malignant hepatic tumours Renal impairment Renal abnormalities ranging from elevated serum creatinine to renal failure and death reported in patients treated with intravenous azacitidine in combination with other chemotherapeutic agents. Renal tubular acidosis, defined
as a fall in serum bicarbonate to <20 mmol/L in association with an alkaline urine and hypokalaemia (serum potassium < 3 mmol/L) developed in 5 subjects with chronic myelogenous leukaemia (CML) treated with azacitidine and etoposide. If unexplained reductions in serum bicarbonate (< 20 mmol/L) or elevations of serum creatinine or BUN occur, dose should be reduced or administration delayed. Advise patients to report oliguria and anuria to the health care provider immediately. Closely monitor patients with renal impairment for toxicity since azacitidine and/ or its metabolites are primarily excreted by the kidney. Laboratory tests Determine liver function tests, serum creatinine and serum bicarbonate, prior to therapy initiation and each treatment cycle. Perform complete blood counts prior to therapy initiation and as needed to monitor response and toxicity, but at a minimum, prior to each treatment cycle Cardiac and pulmonary disease Safety and efficacy of azacitidine in patients with history of severe congestive heart failure, clinically unstable cardiac disease or pulmonary disease has not been established. Recent data from a clinical study in patients with a known history of cardiovascular or pulmonary disease showed a significantly increased incidence of cardiac events with azacitidine; exercise caution when prescribing azacitidine to these patients. Consider cardiopulmonary assessment before and during treatment. Necrotising fasciitis, including fatal cases, reported in azacitidine treated patients. Therapy should be discontinued if necrotising fasciitis develops and appropriate treatment promptly initiated. Patients with high tumour burden prior to treatment are at risk of tumour lysis syndrome; monitor closely and take appropriate precautions. Cases of differentiation syndrome (also known as retinoic acid syndrome) reported in patients receiving injectable azacitidine. Consider treatment with high-dose IV corticosteroids and haemodynamic monitoring at first onset of symptoms or signs suggestive of differentiation syndrome. Differentiation syndrome may be fatal. Please see SmPC for further details. Consider temporary discontinuation of injectable azacitidine until resolution of symptoms and if resumed, caution advised. Caution recommended when driving or operating machines. Fertility, pregnancy and lactation: Women of childbearing potential have to use effective contraception during and for at least 6 months after treatment. Men should be advised not to father a child while receiving treatment and must use effective contraception during and for at least 3 months after treatment. Due to the potential serious adverse reactions in the nursing child, breast-feeding is contraindicated. Undesirable effects: Very common (≥1/10) – Pneumonia (bacterial, viral, fungal), nasopharyngitis, febrile neutropenia, neutropenia, leukopenia, thrombocytopenia, anaemia, anorexia, decreased appetite, hypokalemia, insomnia, dizziness, headache, dyspnoea, epistaxis, diarrhoea, vomiting, constipation, nausea, abdominal pain, petechiae, pruritus, rash, ecchymosis, arthralgia, musculoskeletal pain, pyrexia, fatigue, asthenia, chest pain, injection site erythema, injection site pain, injection site reaction. Common (≥ 1/100 to < 1/10) – Sepsis, neutropenic sepsis, respiratory tract infection, urinary tract infection, cellulitis, diverticulitis, oral fungal infection, sinusitis, pharyngitis, rhinitis, herpes simplex, skin infection, pancytopenia, bone marrow failure, dehydration, confusional state, anxiety, intracranial haemorrhage, syncope, somnolence, lethargy, eye haemorrhage, conjunctival haemorrhage, pericardial effusion, hypotension, hypertension, orthostatic hypotension, haematoma, pleural effusion, dyspnoea exertional, pharyngolaryngeal pain, gastrointestinal haemorrhage, haemorrhoidal haemorrhage, stomatitis, gingival bleeding, dyspepsia, purpura, alopecia, urticaria, erythema, rash macular, muscle spasms, myalgia, renal failure, haematuria, elevated serum creatinine, bruising, haematoma, induration, rash, pruritus, inflammation, discoloration, nodule and haemorrhage at injection site, malaise, chills, catheter site haemorrhage. Uncommon (≥1/1,000 to <1/100) – Hypersensitivity reactions, pericarditis, hepatic failure, progressive hepatic coma, acute febrile neutrophilic dermatosis, pyoderma gangrenosum, renal tubular acidosis. Rare (≥1/10,000 to <1/1,000) – Tumour- lysis syndrome, interstitial lung disease, injection site necrosis. Frequency not known – Necrotising fasciitis, differentiation syndrome, cutaneous vasculitis. Legal category: POM Marketing authorisation number: EU/1/23/1777/001 Marketing
Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroener-Straβe-1, Bad Homburg 61352, Germany. Further information: Available from Fresenius Kabi Limited, Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire, WA7 1NT. Tel +44 (0)1928 533 533. Date of preparation: July 2025 IE--2500022
Adverse events should be reported. Reporting forms and information can be found at: https://www.hpra.ie/report-an-issue
Adverse events should also be reported to Fresenius Kabi Limited, Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire, WA7 1NT Tel +44 (0)1928 533 533.
Fresenius Kabi Limited
Fresenius Kabi Ireland
Unit 3B Fingal Bay, Balbriggan, Co. Dublin, Ireland
Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com
Phone: +353 (0)1 841 3030
Job Code::IE--2500025
Date of Preparation: August 2025
End of Year Review
IPHA

IPHA’s 2025 Review of Medicines Access, Trials and Health Policy
2025 was a year defined by both challenge and momentum for Ireland’s research-based biopharmaceutical industry. Across medicines access, clinical research and public health policy, the sector continued to highlight persistent structural issues while also contributing constructively to major national reforms. From significant delays in access to innovative treatments to encouraging progress on clinical trials and new insight into the economic and health value of Self-Care, the year underscored a central truth: Ireland stands at an inflection point. With the right policy choices, patients could benefit from faster access to innovation and a more efficient, sustainable healthcare system.
Faster and Fairer Access to Medicines
At the heart of IPHA’s policy focus this year was the urgent need to reform Ireland’s medicines access and reimbursement system. In February, the association published its position paper on Programme for Government commitments relating to medicines access, benchmarking timelines for the first time against the statutory standards set out in the Health Act 2013. The findings were stark: patients continue to wait nearly two years on average to access newly reimbursed medicines.
Of the 88 medicines reimbursed between 2022 and 2024, the average time from application to access was 617 days—more than triple the legal 180-day decision timeline. Cancer patients faced even longer waits, averaging 694 days, while orphan medicines took 655 days. For medicines requiring a full health technology assessment, the average wait stretched to 916 days, with state processes accounting for almost two-thirds of that timeline.
The consequences for patient care are profound. Clinicians often cannot prescribe treatments already recognised internationally as standard of care, and private patients can sometimes access therapies months or even years before public patients. IPHA’s assessment was unequivocal: the issue is no longer legislative but operational. The existing statutory framework can deliver timely access—if processes are resourced, governed and managed to meet the legal requirements. As negotiations approach on a new Framework Agreement in 2025, IPHA has set out five key principles to ensure the reimbursement pathway becomes more predictable, transparent and aligned with patient needs. The objective is clear: to finally
deliver the “as quickly as possible” commitment contained in the Programme for Government.
Ireland Falling Behind Europe on Cancer Medicines
Parallel to IPHA’s own analysis, a new report by data analysts IQVIA for EFPIA highlighted Ireland’s worsening position relative to European peers. Of 56 oncology medicines licensed by the European Medicines Agency since 2020, only 14—just 25%— are available in Ireland, the lowest availability rate in Western Europe. Irish cancer patients also wait longer than the EU average for access to newly licensed treatments: 644 days compared with the European mean of 586 days. This represents a deterioration of almost two months on last year’s figure, and a significant increase over the 477-day average recorded in 2020. Reduced availability and longer timelines translate into diminished treatment options and, ultimately, poorer clinical outcomes.
The experience of other countries shows what is possible when State authorities and industry work in partnership: faster timelines, predictable pathways, and rigorous value assessments can coexist. For Ireland, the upcoming renegotiation of the Framework Agreement presents a critical opportunity to align policy with patient needs.
Clinical Trials: Encouraging Progress but Work Ahead
In May, IPHA published its latest Clinical Trials Activity Comparison Report, setting out a mixed but hopeful picture. Ireland saw a 34% increase in industry-sponsored trials initiated in 2024, rising from 32 to 43 studies. The time to recruit the first patient—a key measure of system efficiency— also improved dramatically, falling from 67 to 46 days. These gains
reflect the growing impact of the National Clinical Trials Oversight Group, as well as cross-sector efforts to streamline processes. However, challenges persist. Ireland ranked 18th out of 27 EU countries in clinical trials per capita in 2024. When compared to Denmark—similar in population and economic profile—the disparity is striking: 75 industrysponsored trials here across 2023–2024 versus 229 in Denmark.
Former Minister for Health Stephen Donnelly expressed an ambition last year to double clinical trials activity, which could position Ireland among Europe’s top performers. IPHA recognises the foundational work underway and continues to collaborate with partners including the State Claims Agency and Health Service Executive to remove barriers, standardise documentation, and accelerate trial initiation.
Unlocking the Potential of Self-Care
A further milestone in 2025 was the publication of new research on the value of Self-Care ahead of SelfCare Awareness Day in July. The findings underscore the substantial health and economic impact of empowering patients to manage minor ailments. Self-Care currently saves ¤727 million annually, with ¤495 million accruing to the HSE alone. With targeted policy support, an additional ¤166 million in savings could be achieved.
Beyond the financial benefits, expanded Self-Care could relieve significant pressure on general practice. The research shows that one GP working hour per day could be freed up nationwide—a meaningful gain at a time of chronic GP shortages. IPHA set out five recommendations, including integrating Self-Care into national policy, reclassifying appropriate medicines to overthe-counter status, expanding
pharmacist-led services, and introducing new digital supports for patients.
Budget 2026:
A Positive Step for New Medicines
The year concluded with encouraging signals from Government. Budget 2026 allocated ¤30 million specifically for new medicines within the wider ¤217 million PCRS medicines budget. This will support access for up to 6,000 patients to 32 innovative treatments expected to seek reimbursement next year. These therapies span oncology, immunology, metabolic disease, women’s health and rare disorders.
Importantly, Minister for Health Jennifer Carroll MacNeill provided the strongest commitment yet that the State intends to meet the statutory 180-day reimbursement timeline. This explicit policy position marks a significant step forward and aligns with IPHA’s long-standing call for adherence to the 2013 legislation.
In addition, the increase of the R&D Tax Credit from 30% to 35% strengthens Ireland’s competitiveness and supports continued investment in innovation—a vital factor in maintaining Ireland’s global leadership in biopharmaceutical manufacturing and research. 2025 demonstrated both the scale of the challenge and the potential for real progress. IPHA’s priorities—timely access to medicines, a high-performing clinical research ecosystem, and an empowered, patient-centred healthcare model—are now firmly on the national policy agenda. With political will, cross-sector collaboration and a renewed commitment to delivering on the law’s promise, Ireland can move closer to ensuring patients receive the best care as quickly and fairly as possible.

End of Year Review IHCA
IHCA 2025 Year in Review: Leadership, Reform and the Realities of a System Under Pressure
2025 was a defining year for the Irish Hospital Consultants Association (IHCA) — a year marked by principled advocacy, national leadership, and a renewed focus on supporting sustainable, safe, and patientcentred care within an increasingly strained health system. Across major policy debates, national conferences, and public health reform, the Association continued to champion constructive, evidence-based solutions while highlighting the growing pressures facing consultants and the patients they serve.
Driving the National Conversation on Sustainable Healthcare
The year opened with the IHCA’s landmark symposium, Implementing Sustainable Healthcare Solutions, where leading international experts convened to address one of healthcare’s most urgent challenges: delivering high-quality care within a climate-resilient, environmentally responsible system. With contributors such as Professor Hugh Montgomery, Dr Brian O’Connell, Dr Cathy Burke and Dr Philip Crowley, the event showcased practical, evidencebased strategies that hospitals can implement today.
Speakers emphasised that up to one-third of healthcare spending is “low value,” often driven by unnecessary tests or inappropriate prescribing. Solutions such as the “gloves off” initiative — proven successful in other countries — highlighted how small behavioural changes can reduce waste, cut emissions, and improve patient care. The symposium reaffirmed the IHCA’s commitment to embedding sustainability into the core of Irish healthcare planning.
Mental Health Reform: A Call for Workable, Patient-Centred Legislation
In April, the IHCA published its position paper on the Mental Health Bill 2024, building on extensive engagement with the Oireachtas and mental health stakeholders. While supporting the Bill’s broad principles, the Association raised critical concerns around unintended barriers to care,
IHCA President, Professor Gabrielle Colleran

disproportionate legal processes, and risks to patients’ timely access to treatment.
IHCA Vice President and Consultant Psychiatrist Professor Anne Doherty highlighted the need to ensure that legislation is workable in practice and aligned with international best standards. The Association warned that without amendments, excessive administrative and legal requirements could divert consultant psychiatrists from urgent clinical duties, further straining mental health services already under severe pressure. Throughout 2025, the IHCA continued to advocate for a balanced, fair, and patient-focused legislative framework.
Medical Workforce Pressures Laid Bare
A pivotal moment arrived in July when the Medical Council’s Medical Workforce Intelligence Report 2024 confirmed what consultants had long been warning: specialists and NCHDs continue to work excessive hours far beyond contractual limits. Nearly half of all specialists routinely exceed 40 hours per week, and almost one in five work more than 48 hours — a breach of the European Working Time Directive.
Most concerning is that 45% of those working beyond 48 hours spend this time in direct patient care, often in high-pressure specialties such as surgery and obstetrics. Coupled with burnout,
high attrition, and over 1,000 doctors leaving the register in 2024, the data underscored the urgency of workforce reform. The report also reinforced the need for system-wide digital transformation. Excessive administrative burdens ranked among the top barriers to good patient care, strengthening the IHCA’s call for accelerated implementation of electronic health records and modern digital infrastructure as outlined in the National Development Plan.
Annual Conference 2025: Leading from the Frontline
In October, the IHCA brought together over 200 consultants and healthcare leaders at its Annual Conference under the theme Leading from the Frontline: Medical Negligence & Clinical Leadership. President Professor Gabrielle Colleran set the tone, urging a shift toward clinicianled leadership and solutions that prioritise compassion, fairness, and patient safety.
The morning session examined Ireland’s medical negligence processes — an area where patients and clinicians alike often face prolonged, traumatic experiences. Building on constructive engagement with justice and legal stakeholders, the IHCA explored pre-action protocols and the strengthening of domestic expert-witness capacity.
The afternoon session focused on clinical leadership as a catalyst for meaningful reform. Contributions from senior national figures underscored the essential role of empowered clinical directors in shaping safer, more sustainable services.
Professor Colleran described the Association’s role succinctly: clinicians must not only deliver care, but also “share our insights, our hopes, and our dreams for a better, fairer health service.”
The IHCA’s evolving mission, she emphasised, is to “light a candle, not curse the darkness.”
A Constructive Voice for 2026 and Beyond
Throughout 2025, the IHCA continued to position itself as a credible, solutions-driven advocate for patients and hospital consultants. From sustainability and mental health reform to workforce realities and clinical leadership, the Association’s voice remained consistent and clear: better resourcing, stronger governance, and clinician-led reform are essential to building the health service Irish patients deserve.
As Ireland moves into 2026 with ongoing challenges — understaffing, escalating demand, and system inefficiencies — the IHCA remains firmly committed to leading the national discussion with integrity, expertise, and determination.
End of Year Review
IIOP Supporting Pharmacists, Shaping the Future

The Irish Institute of Pharmacy reflects on a year of progress and looks ahead to 2026 with new resources, expanded CPD, strengthened peer networks, and enhanced communications.
For the Irish Institute of Pharmacy (IIOP), 2025 was more than just another busy year — it’s been a year of transformation, collaboration, and laying the foundations for the future. From supporting pharmacists through professional CPD cycle reviews to preparing for regulatory change, the Institute’s work has touched most corners of the profession.
A Year of Connection and Support
At the heart of the IIOP’s calendar sits the ePortfolio Review project, a cornerstone initiative that runs from January to May, with a lot happening behind the scenes! The rhythm of the project is cyclical — once one year’s review closes, preparations for the next begins almost immediately; reviewers are trained, peer support pharmacists deliver information webinars and IT systems are maintained and updated. All year round, our team are delighted to speak directly with pharmacists, answering their questions, and ensuring they feel supported throughout the process. In 2026 we look forward to strengthening engagement with pharmaceutical assistants, as we work with them to begin to establish their ePortfolio Review process.
Training for an Expanded Role
2025 also saw the delivery of two new online programmes to support pharmacists in continuing to provide quality patient care. The Open Disclosure training programme supports pharmacists to understand their requirements under the new Open Disclosure Framework. The Common Conditions Service training equips pharmacists with the knowledge to embrace an expanded role whereby they can supply certain prescription-only medicines through established clinical protocols, where this is the most appropriate option.
During 2025 the IIOP was delighted to see the continued growth of
peer networks. The Mentoring Programme provides guidance and encouragement by pairing experienced pharmacist mentors with mentees. While initiatives like the Pharmacist Antimicrobial Stewardship Network (PAMSNet) create spaces for discussion and shared learning. These networks are more than professional groups — they are communities, and in 2026 the IIOP will continue to strengthen them, supporting pharmacists’ connections.
Advancing International Collaboration
Collaboration also extended beyond Ireland’s borders in 2025. The Executive Director’s participation in Workshops in LLLP in Sydney — focused on burnout and professional identity — helped advance the IIOP’s work with international colleagues on global pharmacy challenges. These efforts were reinforced through a series of national and international partnerships across the year, further embedding the Institute’s role in shaping practice at both local and global levels.
Sharing Knowledge Through Webinars
Another highlight of 2025 is the continued success of the IIOP’s In Conversation With… webinar series. First launched in May 2020 in response to the needs of the profession during the COVID-19 pandemic, the series has become a trusted space for learning and dialogue. In June 2025, the IIOP celebrated its 100th webinar, delivered by Nikki Holmes, Director of Pharmacy at NHS Dumfries & Galloway, on the topic “Clozapine: What’s it all about?”.
Since its inception, the series has grown to 110 webinars, attracting almost 14,000 attendees and generating over 41,000 rewatches. This milestone reflects the profession’s appetite for accessible, high-quality learning opportunities and the Institute’s commitment to meeting that need.
Preparing for Change Behind the Scenes
Much of the Institute’s work goes unseen. In 2025, significant effort went into preparing for new regulations: the PSI (CPD) (Amendment) Rules 2025 (S.I. 508/2025). These rules require a shift from accreditation to a policybased system of quality assurance for CPD activities delivered through the IIOP.
Extensive desk research informed the development of a draft Quality Assurance of CPD Activities policy, including proposed Quality Standards, and 2026 will see this work come to life. It’s a change that underscores the IIOP’s commitment to maintaining the highest standards in professional development.
Welcoming New Practitioners
Looking to the horizon, early 2026 will also bring the launch of the New to Practice resource. Designed for pharmacists joining the Irish register from abroad, it will serve as both a welcome guide and a refresher for those already practicing in Ireland. In a profession that is increasingly global, this resource will help ensure that every pharmacist feels confident and prepared to deliver care in the Irish context.
they are, reinforcing the sense of community and accessibility at the heart of the Institute’s mission.
• Bluesky: @IIOPharmacy
• Twitter: @IIOPharmacy
• Linkedin: https://www.linkedin. com/company/irish-institute-ofpharmacy
• Instagram: @irish_institute_of_pharmacy
• Facebook: https://www. facebook.com/iiopinfo

Looking Ahead
Strengthening Communication Channels
Alongside these initiatives, the IIOP has expanded its communication platforms to keep pharmacists connected and informed. The Institute now has a presence across Bluesky, X (Twitter), LinkedIn, Instagram, and Facebook, and has recently launched a WhatsApp channel to provide timely updates on events and resources. These channels ensure that pharmacists can engage with the IIOP wherever
Reflecting on its achievements during 2025, the vision for 2026 is clear: to continue building a profession that is resilient, collaborative, and future focused. Whether through expanding the ePortfolio Review, strengthening networks, embedding quality assurance, welcoming new practitioners, or enhancing communication, the Institute remains dedicated to driving positive change for pharmacists — and ultimately, for the patients they serve.
As 2026 unfolds, the Institute again invites pharmacists to engage with its programmes, share their experiences, and contribute to the networks that make the profession stronger. Whether through ePortfolio Review, mentoring, webinars, or new resources, every voice matters in shaping a future where pharmacy continues to expand and thrive.

End of Year Review APPEL
APPEL in 2025: Shaping the Future of Pharmacy Through Experiential Learning
Written by Conor Walsh, APPEL Operations Pharmacist
As 2025 comes to a close, the pharmacy profession in Ireland stands at a pivotal moment. From the rollout of expanded clinical services in community settings to the growing integration of pharmacists into multidisciplinary hospital teams, this year has underscored the profession’s adaptability and innovation. Against this backdrop, APPEL’s experiential learning placements have played a vital role in preparing the next generation of pharmacists for these evolving demands.

This year, APPEL facilitated over 640 pharmacy student placements across Ireland, an achievement only made possible through the dedication of our placement providers and their collegiate commitment to supporting and shaping the future of our profession. With this support, our 4th year students, placements spanned diverse practice areas; with 80 students undertaking their four-month placement in community pharmacies, 42 students in hospital pharmacies, 35 students in industrial settings and 35 students in innovative fields within Role Emerging Practice. Our 5th-year students completed 142 placements in community pharmacy settings and 41 placements in hospital pharmacy settings.
These figures tell a story of opportunity and variety. Students were exposed to the realities of frontline care in community pharmacies, in a sector that is ever-changing to keep up with the demands of the expanded role of the pharmacist. In hospitals, they witnessed the complexity of clinical decision-making and multidisciplinary collaboration. Industry placements offered insights into regulatory affairs, clinical trials, and the development of innovative therapies, while role-
emerging settings showed our students the expanding footprint of pharmacists in non-traditional roles in areas that while nonpatient-facing, still are integral to patient care.
2025 was not a year without its’ challenges. Workforce shortages in community pharmacy continued to dominate headlines, while hospitals faced mounting pressures from increased patient acuity and resource constraints. These realities made securing sufficient placement capacity a complex task. Yet, thanks to the commitment of our pharmacy colleagues, students were welcomed into busy practices and given meaningful learning experiences, and allowed to develop their own professional competencies.
For placement providers, supervising students during such demanding times required flexibility and creativity. But the rewards were clear, as one Senior Preceptor of a 5th Year student reflected “I enjoy learning from students and hearing what they learn in college and seeing how they apply that information. I learn new ways to do things.”
This sentiment captures the essence of experiential learning; that it is a two-way street.
Students gain confidence and practical skills, while preceptors can benefit from fresh perspectives and renewed enthusiasm for their profession.
Experiential learning placements are not just a curriculum requirement, but they are an investment in the future of pharmacy. For students, they provide the chance to contextualise academic knowledge, develop clinical judgment, and understand the realities of patient care. For placement providers, the benefits are equally compelling:
• Shape the Future: Inspire and mentor the next generation of pharmacists.
• Build Your Talent Pipeline: Gain early access to motivated individuals who may become future colleagues.
• Enhance Professional Development: Supervising students fosters reflection and creates opportunities for CPD cycles within the IIOP ePortfolio.
In a year where pharmacy practice has continued to expand - the need for well-prepared graduates has never been greater. The future is bright, but it requires collective effort. APPEL is now accepting expressions of interest for 4th-year placements, running from 31st August to 18th December 2026. These placements will span community, hospital, industry, and roleemerging practice areas, reflecting the dynamic nature of pharmacy in Ireland. You can register to offer one of these placements by scanning the below QR code or contacting eoi@appel.ie.
Our goal for 2026 is to expand placement capacity across all sectors, ensuring that every student can access a high-quality experiential learning opportunity. With pharmacy in Ireland continuing to embrace digital health solutions, advanced clinical roles, and public health initiatives, students must be equipped to thrive in diverse and innovative settings. Placement providers are key to making this vision a reality.
As we reflect on 2025, we are proud of what we have achieved together. Despite challenges, the commitment of our placement providers and trainers has ensured that hundreds of students experienced the realities of pharmacy practice. This does not go unnoticed, and APPEL would like to take this opportunity to thank each and every person who has engaged with our experiential learning programme this year. Looking forward, we see a profession that is innovative, patient-centred, and collaborative and APPEL placements will continue to play a pivotal role in shaping that future.

End of Year Review
PSI - The Pharmacy Regulator

Significant Progress for Patients and Pharmacy
2025 has been a year of significant progress and transformation for the PSI, marked by key milestones and collaborative achievements across the pharmacy sector. This year, we marked several milestones that are meaningful for pharmacists, their teams, and the public.

Our daily work is concerned with ensuring our core regulatory functions are conducted so that we support the availability of safe, appropriate and effective pharmacy care in Ireland and, when required, take actions to address failings. Throughout the year, we balanced this core regulatory work with ongoing improvement initiatives, while also supporting the expansion and evolution of pharmacy and healthcare. The recommendations of the Expert Taskforce for expanding the role of pharmacy have been particularly influential in shaping our agenda. We have been contributing considerably to progress on several significant strategic initiatives to ensure pharmacy fulfils its potential as part of an integrated healthcare system for greater patient benefit.
New Corporate Strategy
In April, we were pleased to be joined by the Minister for Health, Jennifer Carroll MacNeill, for the launch of our Corporate Strategy for 2025-2028. The launch was a great opportunity to bring together many from across the pharmacy sector and to hear from the Minister about her vision and expectations for pharmacy and patient care. The strategy itself
was developed in consultation with our stakeholders, and our actions against the three strategic objectives are now well underway. These objectives outline how we will approach the changes expected for pharmacy and the broader health landscape, our proposals for continued regulatory change and advancement, and our plans for internal improvement.
Registration updates
One of the core functions of the PSI is the registration of pharmacists, pharmaceutical assistants, and pharmacies, and maintaining the register, including online as a searchable database available to all. At the end of this year, there are over 7,900 pharmacists registered in Ireland, along with over 1,900 pharmacies. Both registers have grown this year, with the number of pharmacists continuing to increase year-on-year.
As part of our work in this area, the PSI assesses the qualifications and practical experience of pharmacists who want to work in Ireland, to ensure the high standards required under Irish and EU law are met. This year, I am very pleased about the changes that have come
Written by Joanne Kisssane, Registrar and Chief Officer, PSI-The Pharmacy Regulator
into effect for our route to registration for pharmacists from outside of the EU, providing a more holistic approach to qualification assessment.
For pharmacies, our work involves checking compliance with legal provisions to ensure they are safe to open and operate during visits by PSI inspectors, including for new pharmacy openings. By the end of this year, we will have conducted c.300 inspections.
We are at an important juncture for pharmacy, with a significant focus on the implementation of several important policy projects that are expanding pharmacy practice. Ensuring that we have a sustainable, competent pharmacy workforce available is integral to meeting health service and patient needs, and to maintaining the pharmacy as a trusted and accessible environment where high-quality care is provided to communities across Ireland.
Workforce matters
We continue to pursue our commitment to the current and future pharmacy workforce, along with the Department of Health and others on the Pharmacy Workforce Working Group. The work of this group is grounded in
the recommendations of our 2023 Workforce Intelligence Report. This evidence-based report is a vital document for pharmacy workforce considerations in Ireland. This report highlights the importance of ensuring the sustainability of the professional pharmacy workforce to provide continued safe and effective pharmacy services to the public.
We benefit from the insights gathered through our registers and, once again this year, from our annual workforce survey of pharmacists. We are pleased with the positive and engaged response to this year’s survey—our fourth to date—and look forward to sharing the data in the new year. In parallel, we are progressing a delineated operating model for pharmacies, designed to support rest breaks within the current pharmacy structure. Over the summer, we collected feedback on the changes required to our registration rules to enable this model.
Accreditation of MPharm programmes
The approval of three new MPharm programmes at new locations across the country has been another highlight of the year, significantly expanding educational opportunities for future pharmacists nationwide. We were very pleased to see the successful accreditation of the programmes for the University of Galway, Atlantic Technological University, and South East Technological University earlier this year. Alongside the schools of pharmacy at Trinity College Dublin, University College Cork, and the Royal College of Surgeons in Ireland, where programmes were also accredited again this year, these programmes and their future graduates will make important contributions to the academic, pharmacy and healthcare landscape over the coming years, including adding capacity for future sustainability of the pharmacy workforce.

highlights an organisational commitment to the continued modernisation of our regulatory approach and to embrace digital technologies, to enhance service delivery and communications to all stakeholders.
Expanding role of pharmacy
After many years of consideration, reports and discussion, a milestone change is taking place for pharmacy and for the public, with a Common Conditions Service commencing in community pharmacies. Legislation to enable the service, allowing pharmacists to prescribe where needed, was signed by the Minister for Health at the end of October and training for the new service is now available through the IIOP.
Over the past two years, the PSI has been involved both in
to support the new service by specifying the training required for pharmacists, and developed by a team at RCSI and the IIOP. We have also published guidance as a resource to support compliance as pharmacies establish this new service safely.
This is an exciting time, and there has been collective and collaborative working by many in getting to this stage. Pharmacists and pharmacies can now prepare by considering the enablers that are set out on the PSI website.
Continuing Professional Development
Earlier this year, we consulted on proposed changes to legislation on the Continuing Professional Development (CPD) system for pharmacists and a new CPD system for pharmaceutical
of relevance to a pharmacist’s professional practice.
Pricing transparency
Following a request by the Minister for Health to the PSI to enhance availability of information for the public about the price of pharmacy fees and services, the PSI developed principle-based guidance for pharmacies this summer. Following consultation and consideration by the PSI Council, this guidance has been published for use by pharmacists and pharmacies. While we do not determine the prices charged by pharmacies, we do expect that pharmacies are transparent about the costs to patients and fees associated with the dispensing of medicines and pharmacy services and that they meet the responsibilities outlined in this new guidance.

financial sustainability. We very much appreciate all who take the time to share their views with us. We gather this information into consultation reports that the PSI Council considers as they make their decisions. These reports are published on our website.
We have also benefitted from the input of our Pharmacist Panel to ensure that regulatory changes and pharmacy initiatives are informed by practical experience and expertise from the profession. We met with panel members several times this year. We look forward to maintaining this panel and to offering opportunities for others to join next year.
The PSI was also pleased to host a meeting of the HPRA’s Patient Forum in Spring this year, which provided a valuable opportunity to discuss the expanded scope of pharmacists, prescribing for common conditions, and the delineated operating model.
We appreciate also the opportunities extended to us to meet and present to those working across pharmacy at conferences and other events, and to pharmacy students during the academic year, to share details about our role and the focus of our ongoing work.
In conclusion, it’s been a truly interesting year. I want to acknowledge the work of PSI Council, new and old, and colleagues in PSI for their unwavering commitment, dedication, and professionalism. As we look ahead to 2026, we remain committed to working collaboratively with our stakeholders to further strengthen pharmacy practice and ensure the highest standards of care for patients and the public.
Joanne Kissane's presentation at the Corporate Strategy launch
End of Year Review
Asthma Society

Ireland’s Asthma Crisis: What 2025 Revealed and What Must Change in 2026

Asthma is one of Ireland’s most common chronic respiratory conditions, affecting around one in ten people. In 2025, major progress was made in understanding and managing the disease through innovative strategies aimed at improving outcomes and reducing preventable deaths. National healthcare reforms and updated GINA guidelines are driving personalised community care, emphasising early intervention, better inhaler technique, and reduced reliance on short-acting relievers to improve control and cut hospital admissions. These developments reflect a growing emphasis on proactive care, education, and community support.
The Asthma Society of Ireland plays a central role in advancing patient-centred care within this evolving landscape. We empower patients through our free services, education campaigns, and tailored resources. Our policy priorities –including free MART inhalers and a severe asthma registry – focus on equity, efficacy and prevention. Ireland’s collaborative approach – uniting policymakers, clinicians, and patient organisations – offers a promising path forward. With targeted action and investment in key areas, we can build a healthier future for those living with asthma. This review highlights the challenges and opportunities ahead in asthma care.
Challenges and pressures
Asthma affects an estimated 450,000 people in Ireland and remains a major public health challenge, compounded by under-diagnosis, poor symptom control, and unequal access to care. Environmental shifts, an ageing population, the effects of poor housing, and overreliance on
Written by Mary McDonald, Patient Services Manager, Asthma Society Ireland
treatments to relieve symptoms are adding pressure to the healthcare system. Asthma-related deaths have increased by 42% in five years – from 66 in 2019 to 94 in 2024 – while emergency visits and hospital admissions consistently exceed the EU average.
Despite welcome Sláintecare reforms, pressures on hospital respiratory services continued to intensify throughout 2025. Many hospitals experienced sustained demand for urgent reviews during exacerbations, alongside long waiting lists for pulmonary function testing and challenges in accessing specialist severe asthma pathways. These pressures increasingly affect continuity of care between hospital and community settings, highlighting the importance of integrated care and consistent guideline implementation. The HSE’s Adult Asthma Model of Care aims to tackle these challenges. Ambulatory respiratory hubs, focusing on chronic disease prevention and management, have significantly reduced waiting times in 2025 for consultant-led diagnostic clinics. Direct GP referrals for diagnostics and education bring treatment closer to home.
GP feedback shows integrated community systems deliver high-quality asthma care, with 87% reporting better outcomes. However, the phased rollout prioritises areas with the longest waits and highest disease burden, leaving some rural regions without hubs. Further pressures arise from shortages of respiratory nurse specialists and physiologists in some hubs.
Critically, Ireland is an outlier among our European counterparts in not having a national severe asthma registry to support service planning, outcome tracking, and research. Approximately 5% of asthma patients develop severe asthma. The Asthma Society raised this need in this year’s pre-budget meetings with the Minister for Health and Oireachtas health committee members, along with the need for equitable access to severe asthma care and treatments.
Continued investment into the Chronic Disease Management (CDM) Programme has yielded favourable results. Yet certain challenges are evident. While GP participation has grown steadily in 2025, some practices – particularly rural – continue to face capacity challenges. Resource constraints hinder consistent 2025 GINA and BTS guideline implementation. Studies indicate that GPs would welcome further training and support in implementing them, particularly around Pulmonary Function Test interpretation, inhaler device selection, and distinguishing COPD from asthma.
Regrettably, there is no dedicated paediatric asthma model of care. This limits timely access to specialist care for children and risks inconsistent management due to the absence of a standardised pathway across care levels.
In 2025, intermittent shortages of certain asthma medications occurred, but the HPRA, GPs, and pharmacists provided excellent guidance and advice on suitable alternatives to support patients.
Medication accessibility was further hindered by cost-of-living pressures in 2025. In an Asthma Society survey, over half of respondents reported struggling to make ends meet in the previous 12 months and 24% reported going without asthma medication in the previous three months due to financial constraints.
Persistent health inequalities and environmental factors, such as poor air quality, remain major concerns. Ireland’s housing crisis also impacts asthma patients, with substandard accommodation, poor ventilation and air quality exacerbating – and even causing – asthma.
These combined pressures result in increased morbidity and mortality, school and work absences, and reduced quality of life. They also contribute to rising system costs and strain across healthcare services.
Progress and achievements
In 2025, asthma management in Ireland advanced through updated international guidelines and national reforms. GINA 2025 advised discontinuing SABA-only treatment due to links with severe
asthma and mortality. The updated BTS/NICE/SIGN guideline, published in late 2024 promotes accurate diagnosis, monitoring, and long-term management for all age groups to improve control and reduce attack risk.
The rollout of Integrated Respiratory Hubs under Sláintecare and the HSE’s Enhanced Care in the Community programme has transformed asthma care. The Programme prioritises community-based, patient-centred approaches to reduce hospital admissions and improve quality of life. Previously, patients faced waits of up to three years for specialist care. In 2025, many regions reported substantial reductions in specialist wait times through community-based diagnostic services.
Patients are now supported to manage their asthma at home and in the community, reducing reliance on specialist care and empowering greater control over their health. Asthma action plans, inhaler technique training, symptom monitoring, and self-management strategies are key priorities.
Pharmacists played a particularly important role in 2025 by supporting patients with correct inhaler technique, medicines optimisation, and adherence monitoring — areas where errors remain highly prevalent across all age groups. Pharmacists also contributed to antimicrobial stewardship by helping patients distinguish between asthma exacerbations and respiratory infections. Further integration of pharmacists into multidisciplinary asthma care presents an opportunity to reduce exacerbations and improve longterm disease control.
Growing demand for our patient services in 2025 reflects the continued need for accessible support and education to strengthen self-management. Our Asthma Adviceline (1800 44 54 64) offers free 30-minute calls, as required, with our nurse or physiotherapist. We will handle close to 5,000 calls by year’s end – an increase of 50% on last year. Our webinar series was viewed by 1,040 patients and healthcare professionals. These services are delivering measurable system value: evaluation data from

asthma.ie. Our Nurse WhatsApp messaging service (086 059 0132) is ideal for busy parents – with our short videos and infographics delivered straight to their phone. All information booklets were updated and approved by the Medical Advisory Group to reflect the latest asthma management guidelines.
The Asthma Society has through our ongoing advocacy influenced
asthma registry, reviewing asthmarelated deaths, and improving access to high-tech medications. National priorities align, focusing on strengthening communitybased services, and integrating digital health tools for better monitoring and continuity of care.
In 2026, the Asthma Society will continue to build engagement across healthcare settings, including working with GPs to
The Society also plans to expand our multidisciplinary patient services team, enable seamless referrals by healthcare professionals to our Adviceline and extend our reach into underserved communities. We will leverage digitalisation, automations and strategic partnerships to reach more patients and improve outcomes.
In 2026, for example, we will begin a multi-stakeholder research programme funded by the European Commission to develop an asthma fitness app for coaches and patients.
Moving into 2026, the Society remains committed to advocacy, education, and policy development to improve asthma and quality of life outcomes for the 450,000 people we represent
2025: A Year of Transition, Collaboration, and Purpose End of Year Review The PMI
As we conclude another year marked by profound transformation across Ireland’s pharmaceutical industry, it is a privilege to reflect on where we stand - as an industry, a professional community, and as partners in a shared mission to improve patient outcomes.
The PMI has always been there to support in leadership, learning, and collaboration across the sector. Over the years we have grown into a vibrant membership organisation representing professionals from across the industry in Ireland. Our purpose has remained constant: to support those who steer this industry forward by fostering a culture of continuous development and cultivating shared understanding. Through our Stakeholder Briefings, expert-led Masterclasses, and our flagship Annual Pharma Summit, we bring together the people whose decisions shape Ireland’s healthcare future.
Throughout 2025, the PMI has continued to support our members in navigating the global turbulences alongside local challenges in this rapidly changing environment.
At our Pharma Summit earlier this year, we reflected on the unprecedented pace of innovation, the evolving role of payors, and the importance of leadership in shaping a patient-centered, sustainable healthcare ecosystem. Across all our engagements, one message has been clear: no single organisation can meet today’s challenges alone. Only through open dialogue, evidence-based and future-focused discussions can we collectively deliver the solutions that patients and health systems need.
As we look to 2026 and beyond, I remain deeply optimistic. Ireland has a fifty-year legacy of excellence in biopharmaceutical innovation, underpinned by talent and trust. The fundamentals remain strong. However, sustaining our success will require continued investment, regulatory agility, commitment to timely access, and unwavering collaboration across stakeholders. This year has been pivotal for the pharmaceutical industry in Ireland, marked bystrategic transitions and renewed commitment to innovation and sustainability.
In Ireland, our access to medicines remains an urgent priority if we are to maintain our competitiveness and ensure patients continue to receive groundbreaking drugs. Reimbursement timelines remain a concern. The upcoming renewal of the IPHA Framework Agreement on Pricing and Supply represents a pivotal opportunity to modernise and strengthen the process that enable timely access to innovative treatments for patients.
At a broader level, our industry continues to grapple with the lasting impact of the pandemic and the growing complexity of patient journeys. Diagnosis and treatment pathways have lengthened, backlogs have increased, and the shift toward personalised and rare-disease therapies has made patient involvement more critical than ever. We are entering an era where collaboration is not optional - it is the mechanism through which new care pathways will be designed, digitised, and delivered.
On behalf of the PMI, thank you for your leadership, your partnership, and your dedication throughout the year. Together, we will continue to

Written by Sharon Rice, President, The Pharmaceutical Managers’ Institute
navigate change with purpose— and ensure that innovation reaches the patients whose lives depend on it.
We wish you and your families a happy and safe Christmas and we look forward to welcoming you to events throughout 2026.
End of Year Review
Beaumont RCSI Cancer Centre

Beaumont RCSI Cancer Centre 2025 Year in Review: Advancing Excellence, Innovation and Equity in Cancer Care

2025 marked a transformative year for Beaumont Hospital and the Beaumont RCSI Cancer Centre, defined by strategic ambition, collaborative leadership and a renewed commitment to delivering world-class, equitable cancer care. From the launch of a far-reaching five-year strategy to hosting a major international cancer conference, the organisation strengthened its role as a national leader in complex care, research, and innovation.
A Strategic Roadmap for the Next Era of Care
In February, Beaumont Hospital unveiled Building Excellence in Care, Together, a comprehensive strategic plan that will guide the hospital through 2025–2030. Rooted in seven core principles, the plan sets out a blueprint for delivering patient-centred, sustainable and future-focused healthcare. It reflects extensive analysis of population trends, healthcare demand and stakeholder engagement.
As one of Ireland’s largest model-4 hospitals, serving a local population of over half a million and providing national cancer,
transplant and neuroscience services, Beaumont’s mission is expanding in complexity and scale. The strategy commits to:
• strengthening patient-centred care and enhancing patient experience;
• building an inclusive, empowered workforce culture;
• reinforcing strong clinical and corporate governance;
• investing in modern diagnostic, physical and digital infrastructure;
• deepening clinical, educational and research partnerships;
• improving performance, sustainability and service integration across the new regional health structures.
The plan also places a strong emphasis on digital transformation — including enhanced electronic records, digital pathology expansion and modernised diagnostic platforms — recognising that technologydriven healthcare is essential to meeting rising patient needs. With these changes, Beaumont aims to deliver more streamlined patient
pathways, better data-informed decision-making and faster access to specialist care.
The strategic plan is underpinned by the hospital’s core values — Excellence, Compassion, Respect and Quality Care — reflecting a continued dedication to the people and communities it serves. Welcoming the strategy, Professor Cathal Kelly, Vice Chancellor of RCSI, highlighted the longstanding partnership between RCSI and Beaumont in delivering impactful research, innovation and clinical education.
Showcasing Innovation and Equity at the 2025 Beaumont RCSI Cancer Centre Conference
In May, cancer experts from Ireland and around the world gathered at the annual Beaumont RCSI Cancer Centre Conference to explore the theme Transforming Cancer Care: Innovation, Integration, and Equity. Hosted at the RCSI Education and Research Centre, the event reinforced the Centre’s commitment to advancing cutting-edge cancer research and ensuring equity of access to diagnostics and treatment.
Pictured at the Beaumont RCSI Cancer Centre Conference were Professor Cathal Kelly, Vice Chancellor & Registrar RCSI, Professor Patrick Morris, Consultant Medical Oncologist & Anne Coyle, CEO Beaumont Hospital
Professor Patrick Morris, Medical Director of the Beaumont RCSI Cancer Centre, emphasised the importance of understanding the diverse backgrounds and needs of patients to tailor effective treatment strategies. The conference highlighted the growing global momentum behind personalised care, digital innovation, and the integration of artificial intelligence into oncology practice.
Keynote speakers included Professor Linda Sharpe, a leading cancer epidemiologist, and Professor Arsela Prelaj, an international expert in thoracic oncology and AI-driven cancer research. Their contributions showcased emerging global trends in cancer science, datadriven diagnostics, and innovative clinical techniques.
Distinguished speakers across radiation oncology, psychooncology, robotic surgery, pharmacy and digital pathology reflected the Cancer Centre’s interdisciplinary strength. Chief Operations Officer Claire Noonan noted that the conference continues to serve as a vital platform for shaping national and
EMPOWER

Train Patients to Self Administer the XGEVA® Pre-Filled Syringe at Home1*
Self-administration with XGEVA® (denosumab) pre-filled syringe can empower patients to receive their bone-targeted agent treatment in the comfort of their own home, potentially reducing the need to travel to and spend time in hospital compared with in-hospital administration.
XGEVA® is indicated for the prevention of skeletal related events [pathological fracture, radiation to bone, spinal cord compression or surgery to bone) in adults with advanced malignancies involving bone.2
XGEVA® (denosumab) Brief Prescribing Information
Please refer to the Summary of Product Characteristics (SmPC) before prescribing XGEVA® Pharmaceutical Forms: 1.7 mL solution for injection presented as a single use vial or 1.0 mL solution for injection presented as a pre-filled syringe, both containing 120 mg of denosumab. Contains sorbitol (E420). Indication: Prevention of skeletal related events (pathological fracture, radiation to bone, spinal cord compression or surgery to bone) in adults with advanced malignancies involving bone. Dosage and Administration: Single subcutaneous injection of XGEVA® 120 mg given once every 4 weeks. The administration of the 120 mg/1.7 mL vial should only be performed by a healthcare professional. The administration of the 120 mg/1.0 mL pre-filled syringe can be administered by a patient or caregiver who has been trained by a healthcare professional. The first self-administration with the pre-filled syringe should be supervised by a healthcare professional. No dosage adjustment is required in patients with renal impairment or in elderly patients (age ≥ 65). Patients must be supplemented daily with at least 500 mg calcium and 400 IU vitamin D unless hypercalcaemia is present. Not recommended in paediatric patients (under 18 years of age). Give XGEVA® patients the package leaflet and patient reminder card. Contraindications: Severe, untreated hypocalcaemia; hypersensitivity to the active substance or to any of the excipients; unhealed lesions from dental or oral surgery. Special Warnings and Precautions: Pre-existing hypocalcaemia must be corrected prior to initiating therapy with XGEVA®. Hypocalcaemia can occur at any time during therapy. Monitor calcium prior to initial dose, within two weeks of initial dose and if suspected symptoms of hypocalcaemia occur. Severe symptomatic hypocalcaemia has been reported. Consider additional monitoring of calcium level in patients with risk factors for hypocalcaemia or if otherwise indicated based on clinical condition of the patient. Patients with severe renal impairment (creatinine clearance < 30 ml/min) or receiving dialysis are at greater risk of developing hypocalcaemia; this risk and accompanying elevations in parathyroid hormone increases with increasing degree of renal impairment. Regular monitoring of calcium levels in these patients is especially important. If hypocalcaemia occurs while receiving XGEVA®, additional calcium supplementation and additional monitoring may be necessary. Osteonecrosis of the jaw (ONJ) has occurred commonly in patients treated with XGEVA®. Delay treatment in patients with unhealed open soft tissue lesions in the mouth. A dental examination with preventive dentistry and an individual benefit-risk assessment is recommended prior to treatment. Refer to the SmPC for risk factors for ONJ. Patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups and immediately report oral symptoms during treatment with XGEVA®. While on treatment, invasive dental procedures should be performed only after careful consideration and avoided in close proximity to XGEVA® administration. The management plan of patients who develop ONJ should be set up in close collaboration between the treating physician and a dentist or oral surgeon with expertise in ONJ. Osteonecrosis of the external auditory canal has been reported in patients receiving XGEVA® Refer to the SmPC for risk factors. Atypical femoral fracture (AFF) has been reported in patients receiving XGEVA® and the risk increases with longer duration of treatment. Discontinuation of XGEVA® therapy in patients suspected to have AFF should be considered pending evaluation of the patient based on an individual benefit risk assessment. Patients being treated with XGEVA® should not be treated concomitantly with other denosumab containing medicinal products (for osteoporosis indications) or with bisphosphonates. The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. Patients with Phenylketonuria (PKU) should be administered XGEVA® from the single use vial as it does not contain phenylalanine. Phenylalanine may be harmful to patients with PKU because the body cannot remove it properly. Interactions: No interaction studies have been performed. Pregnancy and lactation:
There are no adequate data on the use of XGEVA® in pregnant women. Not recommended for use in pregnant women or women of childbearing potential not using contraception. It is unknown whether XGEVA® is excreted in human milk. A risk/benefit decision should be made in patients who are breast-feeding. No data are available on the effect of XGEVA® on human fertility. Undesirable Effects: Adverse reactions in patients receiving XGEVA® to prevent the occurrence of skeletal related events: Very common (≥ 1/10) hypocalcaemia, dyspnoea, diarrhoea and musculoskeletal pain; Common (≥ 1/100 to < 1/10) new primary malignancy, hypophosphataemia, tooth extraction, hyperhidrosis and osteonecrosis of the jaw; Uncommon (≥ 1/1,000 to <1/100) atypical femoral fracture, lichenoid drug eruptions; Rare (≥ 1/10,000 to < 1/1000) drug hypersensitivity, anaphylactic reaction. In a phase III clinical trial in patients with multiple myeloma, the highest incidence of ONJ was observed. ONJ was confirmed in 5.9% of patients treated with XGEVA® and 3.2% of patients treated with zoledronic acid. In 3 phase III clinical trials in patients with advanced malignancies involving bone, ONJ was confirmed in 1.8% of patients treated with XGEVA® and 1.3% of patients treated with zoledronic acid (primary treatment phase) Among subjects with confirmed ONJ, most (81% in both treatment groups) had a history of tooth extraction, poor oral hygiene, and/or use of a dental appliance. Higher incidence of hypocalcaemia among subjects treated with XGEVA® compared to zoledronic acid has been observed in SRE prevention clinical trials. In a phase III trial in patients with multiple myeloma, hypocalcaemia was confirmed in 16.9% of patients treated with XGEVA® and 12.4% of patients treated with zoledronic acid. In 3 phase III clinical trials in patients with advanced malignancies involving bone, hypocalcaemia was reported in 9.6% of patients treated with XGEVA® and 5.0% of patients treated with zoledronic acid. In the post-marketing setting, severe symptomatic hypocalcaemia (including fatal cases), hypersensitivity (including rare events of anaphylactic reaction) and musculoskeletal pain (including severe cases) have been reported. In four phase III clinical trials new primary malignancy was reported in 1.5% of patients treated with XGEVA® and 0.9% of patients treated with zoledronic acid. The cumulative incidence at one year was 1.1 % for denosumab and 0.6 % for zoledronic acid, respectively. No treatment-related pattern in individual cancers or cancer groupings was apparent. Neutralizing antibodies have not been observed in clinical studies. Please consult the SmPC for a full description of undesirable effects. Pharmaceutical Precautions: Do not mix with other medicinal products. Store at 2°C to 8°C (in a refrigerator). XGEVA® may be stored at room temperature (up to 25°C) for up to 30 days in the original container, do not put it back in the refrigerator. It must be used within this 30 day period. Do not freeze. Keep vial or pre-filled syringe in outer carton to protect from light. XGEVA® solution should be inspected visually before administration. Do not inject the solution if it is cloudy, discoloured or if it contains many particles or foreign particulate matter. Legal Category: POM. Presentation, Basic Costs and Marketing Authorisation Number: XGEVA®120 mg/1.7 mL: Pack of 1, EU/1/11/703/001. XGEVA®120 mg/1.0 mL: Pack of 1, EU/1/11/703/004. Price in Republic of Ireland is available on request. Marketing Authorisation Holder: Amgen Europe B.V., Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31, Ireland. XGEVA® is a registered trademark of Amgen Inc. Date of PI preparation: May 2024 (Ref: IRL-162X-0524-80001) Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie Adverse reactions/events should also be reported to Amgen Limited on +44 (0)1223
End of Year Review
Beaumont RCSI Cancer Centre

international practice, encouraging collaboration that accelerates innovation and improves patient outcomes.
A Year of Progress and Collective Ambition
Building on its strong academic partnership with RCSI, Beaumont continued in 2025 to enhance its profile as a centre of excellence in cancer care, education and research. The year also saw significant progress in expanding robotic surgery capabilities, strengthening psycho-oncology supports and embedding new digital workflows across cancer services. These initiatives are laying the foundation for more personalised treatment pathways and improved patient experience. With cancer incidence rising and treatment becoming more complex, Beaumont’s 2025 achievements demonstrate the power of strategic clarity, collaboration and sustained innovation. As the hospital moves into 2026, its focus remains on expanding equitable access, accelerating diagnostic capacity and driving research that will shape the next generation of cancer therapies.
Armed with its new strategic plan and strengthened partnerships with RCSI and global experts, the Beaumont RCSI Cancer Centre enters the coming year with renewed purpose: to lead nationally and internationally in
delivering transformative, equitable and patient-centred cancer care.
A Decade of Care
Beaumont Hospital FITT Team recently marked the 10th anniversary of its Frailty Intervention Therapy Team (FITT), a decade of pioneering, personcentred care for older adults in our community living with frailty.
Established in 2015, FITT was created to provide early assessment and intervention for frail and older patients presenting to the Emergency Department and aims to ensure older people access the right services and appropriate care pathways.
Over the past decade, the team has transformed how Beaumont supports older patients, helping them maintain independence, avoid unnecessary hospital admissions, and return home safely whenever possible. FITT has been running in Beaumont Hospital Emergency Department (ED) since 21st of September, 2015.
The multidisciplinary team includes physiotherapists, occupational therapists, medical social workers, Speech and language Therapists, Dieticians, Advanced nurse specialists, and geriatricians, all working collaboratively to deliver holistic, evidence-based care.
Since its inception, FITT has had a 37% increase in patients referred to their service (2020 versus 2024) and supported thousands of patients in the Emergency
Department and Acute Medical Assessment Unit, significantly reducing admissions and improving functional outcomes. The service has also influenced frailty care models across Ireland, serving as a blueprint for similar initiatives nationwide.
The success of FITT is a testament to the dedication and collaboration of the entire multidisciplinary team. Ten years on, their work continues to make a real difference in the lives of our most vulnerable patients.
As Beaumont Hospital reflects on this milestone, the FITT team remains committed to continuing its mission — innovating, educating, and delivering compassionate care for older adults well into the next decade.
In other news for Beaumont Hospital, the Information & Communications Technology Department (ICT) has been recognised with the prestigious Imprivata Clinical Impact Award for its successful implementation of Single Sign-On (SSO) technology across its health system. This award recognises healthcare organisation’s commitment to innovation, security, and improving the patient experience.
The new single sign-on solution streamlines access to clinical applications, enabling physicians, nurses, and a wide range of hospital staff to securely log in with just one set of credentials. By reducing the time spent on
NEWS - New Report on Irish Mental Health
repeated logins, clinical staff can spend more time focused on patient care.
This major ICT project involved deploying Imprivata’s industryleading identity and access management platform throughout Beaumont Hospital. Through extensive planning, training, and a phased rollout, Beaumont’s ICT department ensured a smooth transition with minimal disruption on day-to-day operations. Early feedback from clinical staff has been overwhelmingly positive, with many citing faster access to critical applications and less frustration with password management. Based on a conservative estimate of around 5 seconds per password entry, it is estimated that the rollout of the Imprivata SSO potentially saved approximately 649 staff hours throughout August 1st –31st 2025. While the actual time saved will vary by application and workflow, these figures illustrate the scale of authentication activity supported by the platform and the possible efficiency gains.
Mark Graham, Director of ICT said; “This award reflects the dedication and expertise of our IT team, to deliver technology that truly supports our frontline staff. Beaumont is the first healthcare provider in Ireland to recognise the value of the SSO platform and trial it in various departments across the hospital. With the support of the Technology and Transformation Team in the HSE, Beaumont was able to expand the trial and roll-out the platform hospital wide”.
The combined use of restrictive practices in Irish mental health centres has fallen to its lowest level since national reporting began in 2008, according to a new report published by the Mental Health Commission (MHC).
Entitled The Use of Restrictive Practices in Approved Centres – Activities Report 2024, the report documents the use of seclusion, physical restraint and mechanical restraint across 66 approved inpatient mental health centres nationwide during 2024. This is the MHC’s sixteenth report on the use of seclusion, mechanical means of bodily restraint and physical restraint in approved centres and forms part of its statutory remit to report independently on the quality and safety of mental health services in Ireland.
The report shows that total restrictive practices decreased by 18% in one year, from 3,467 episodes in 2023 to 2,836 episodes in 2024. Over a five-year period, the number of restrictive practice episodes has reduced by almost half, falling by 48.64% from 5,830 in 2020 to 2,836 in 2024.
This represents the lowest annual total since reporting commenced in 2008.
As part of this ongoing downward trend, the report highlights a sustained reduction in both physical restraint and seclusion, alongside the continued rarity of mechanical restraint. The findings reflect the impact of strengthened oversight, enhanced reporting requirements and the impact of the revised Rules and Codes of Practice introduced by the MHC in January 2023, underpinned by human rights and person-centred care principles. The MHC also acknowledges the ongoing efforts of service providers to adopt a human-rights approach to care and treatment which is resulting in reduction and elimination of restrictive practices.
The introduction by the MHC of the revised Rules and Code of Practice on 1 January 2023 was informed by international developments in human rights, advances in trauma-informed and person-centred care, and growing evidence that restrictive practices can cause physical and psychological harm.
For healthcare professionals in Ireland only. Abbreviated Prescribing Information can be found below.
Smoking Cessation Medicine
Varenicline Teva
Film-coated Tablets
varenicline
Available on private prescription only.
Indications
Varenicline Teva 0.5 mg and Varenicline Teva 1 mg Film-coated Tablets (initiation pack) and Varenicline Teva 1 mg
Film-coated Tablets
Varenicline Teva is indicated for smoking cessation in adults.
Varenilcine 0.5mg and 1mg Film-Coated Tablets Abbreviated Prescribing Information Presentation: Each film-coated tablet contains varenicline citrate equivalent to 0.5mg and 1mg varenicline. Indications: Varenicline is indicated for smoking cessation in adults. Dosage and administration: Oral use. Adults: The recommended dose is 1mg Varenicline twice daily following a 1-week titration (see SmPC for details). Children: Not recommended for use. Elderly: No dosage adjustment is necessary. Elderly patients are more likely to have decreased renal function, prescribers should consider the renal status of an elderly patient. Renal impairment: No dosage adjustment is necessary for patients with mild (estimated creatinine clearance >50ml/min and ≤80ml/min) to moderate (estimated creatinine clearance ≥30ml/min and ≤50ml/min) renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30ml/min), the recommended dose of Varenicline is 1mg once daily. Hepatic impairment: No dosage adjustment is necessary. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: Physiological changes resulting from smoking cessation, with or without treatment with Varenicline, may alter the pharmacokinetics or pharmacodynamics of some medicinal products, for which dosage adjustment may be necessary (examples include theophylline, warfarin and insulin). As smoking induces CYP1A2, smoking cessation may result in an increase of plasma levels of CYP1A2 substrates. Changes in behaviour or thinking, anxiety, psychosis, mood swings, aggressive behaviour, depression, suicidal ideation and behaviour and suicide attempts have been reported in patients attempting to quit smoking with Varenicline. Depressed mood, rarely including suicidal ideation and suicide attempt, may be a symptom of nicotine withdrawal. Clinicians should be aware of the possible emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking with or without treatment. If serious neuropsychiatric symptoms occur whilst on Varenicline treatment, patients should discontinue Varenicline immediately and contact a healthcare professional for re-evaluation of treatment. Smoking cessation, with or without pharmacotherapy, has been associated with exacerbation of underlying psychiatric illness (e.g. depression). In clinical trials and post-marketing experience there have been reports of seizures in patients with or without a history of seizures, treated with Varenicline. Varenicline should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. At the end of treatment, discontinuation of Varenicline was associated with an increase in irritability, urge to smoke, depression, and/or insomnia in up to 3% of patients. In such instances, tapering should be considered. Patients taking Varenicline should seek immediate medical attention if they experience signs and symptoms of myocardial infarction or stroke. Hypersensitivity reactions including angioedema (swelling of the face, mouth neck and extremities) have been reported in patients treated with varenicline. Some rare life-threatening reports required urgent medical attention due to respiratory compromise. Rare and severe cutaneous reactions (Stevens-Johnson-Syndrome and
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
Freephone: 1800 - 201 700 | Email: info@teva.ie
Prescription Only Medicine.
Erythema Multiforme) have also been reported in post-marketing reports. Due to the life-threatening nature of these conditions, varenicline should be discontinued and a healthcare provider should be contacted immediately. Interactions: Varenicline has no clinically meaningful drug interactions (see SmPC for further details). No dosage adjustment of Varenicline or co-administered medicinal products listed below is recommended. In vitro studies indicate that Varenicline is unlikely to alter the pharmacokinetics of compounds that are primarily metabolised by cytochrome P450 enzymes. Furthermore, since metabolism of Varenicline represents less than 10% of its clearance, active substances known to affect the cytochrome P450 system are unlikely to alter the pharmacokinetics of Varenicline, therefore a dose adjustment of Varenicline would not be required. Varenilcine is not known to affect the pharmacokinetics of metformin, digoxin, bupropion and warfarin. Co-administration of cimetidine, with Varenicline increased the systemic exposure of varenicline by due to a reduction in varenicline renal clearance. In patients with severe renal impairment, the concomitant use of cimetidine and Varenicline should be avoided. Pregnancy and lactation: As a precautionary measure, it is preferable to avoid the use of varenicline during pregnancy. A decision on whether to continue/discontinue breast-feeding or to continue/ discontinue therapy with varenicline should be made taking into account the benefit of breast-feeding to the child and the benefit of varenicline therapy to the woman. Effects on ability to drive and use machines: Varenicline may have minor or moderate influence on the ability to drive and use machines. Varenicline may cause dizziness, somnolence and transient loss of consciousness, and therefore may influence the ability to drive and use machines. Adverse reactions: Diabetes mellitus, suicidal ideation, depression, hallucinations, psychosis, seizure, cerebrovascular accident, transient loss of consciousness, myocardial infarction, angina pectoris, tachycardia, atrial fibrillation, electrocardiogram ST segment depression, gastritis, haematemesis, severe cutaneous reactions including Stevens Johnson Syndrome and Erythema Multiforme, angioedema. Very Common: Nasopharyngitis, abnormal dreams, insomnia, headache, nausea. Common: Bronchitis, sinusitis, weight increased, decreased appetite, increased appetite, somnolence, dizziness, dysgeusia, dyspnoea, cough, gastrooesophageal reflux disease, vomiting, constipation, diarrhoea, abdominal distension, abdominal pain, toothache, dyspepsia, flatulence, dry mouth, rash, pruritus, arthralgia, myalgia, back pain, chest pain, fatigue, liver function test abnormal. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: In case of overdose, standard supportive measures should be instituted as required. Legal category: POM. Marketing Authorisation Number: 0.5mg PA1986/129/001, 1mg PA1986/129/002, 0.5mg & 1mg Initiation Pack PA1986/129/003. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00093. Date of Preparation: May 2025.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie.
Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Date of Preparation: October 2025 | Job Code: GEN-IE-00155
Further information is available on request or in the SmPC. Product Information also available on the HPRA website.

Men's Health: Obesity
Obesity in Men: An Overview

The prevalence of obesity in Irish adults is 25%. Obesity affects men and women equally in Ireland, however just 20% of people who present for treatment are male. This is despite the risks of obesity to health being more profound in men than women, including having a higher prevalence of sleep apnoea, cardiovascular risk and type two diabetes. In this article we discuss barriers to accessing treatment for men, the impact of testosterone deficiency on obesity, and advances in pharmacological management of obesity. The availability of pharmacological treatment is changing attitudes toward obesity as a disease. There is an ever increasing need – and potential benefit - to highlighting the impact of obesity in men and to encourage men to seek treatment. Based on the 2022 Healthy Ireland Survey (HIS) numbers, conservative estimates are that 150,000 men currently live with Class II and III obesity in the Republic of Ireland and 450,000 with Class I obesity, equalling over half a million men in the Republic of Ireland living with obesity.
Barriers to access and engagement in treatment for men
Societal stigma is probably the primary reason men are less likely than women to address
their obesity. This is despite hospitalisation rates between men and women being equal.i Men tend not to view obesity as a complex disease – so they are less likely than women to seek medical treatment. This may be due to the belief that men are expected to appear stoic and self-reliant. The societal belief that patients living with obesity are ‘not trying hard enough’ negatively impacts treatment-seeking behaviour in both men and women.ii It has been shown that those who believe obesity is controllable display more negative attitudes towards people living with obesity. Men have been found to display more anti-fat attitudes than women. iii Overall, men living with obesity may be more likely than women to believe that their obesity is due to lack of willpower, resulting in reduced treatment seeking behaviour in men with obesity. The evidence that obesity is a pathophysiological process rather than an issue of behaviour and self-control needs to be further communicated to society.
Socioeconomic status is another factor contributing to accessing care for obesity. Individuals who have more obesity have less healthcare insurance, and therefore less access to bariatric surgeries or GLP1 medications.
Ultimately, we need obesity to be recognised as a long term illness to allow these patients to equally access GLP1 agonists.
Obesity drives over 220 chronic diseases and makes a major contribution to the development of cardiovascular disease, type two diabetes and certain cancers. Obesity more negatively impacts health in men as the distribution of adipose differs between the genders, with men usually having central adipose distribution and women having a peripheral distribution. Central adiposity has been found to better predict all-cause mortality risk than BMI, as it contributes to visceral adiposity.iv Higher visceral fat contributes to increased cardiovascular risk and increased pharyngeal collapsibility resulting in obstructive sleep apnoea.v It also contributes to elevated postprandial insulin levels, which over time can result in insulin resistance and subsequently the development of type two diabetes mellitus.ii
Endocrine factors influencing obesity and metabolic dysfunction
There is a linear correlation between decreased testosterone levels in the circulation and increasing obesity. The expression
Written
by Ailbhe Rooney and Donal O’Shea, Department of Endocrinology, St Vincents University and St Columcilles Hospitals, University College Dublin, Ireland
of the aromatase enzyme is increased in the subcutaneous adipose tissue of men with obesity. This results in the aromatisation of testosterone to oestradiol (E2) in subcutaneous adipose tissue. This increased E2 acts on both oestrogen receptors ESR1 and ESR2. The ESR1 receptor is associated with increased insulin sensitivity, and is negatively correlated with BMI however ESR1 expression is down regulated in response to increased aromatase levels seen in obesity. Thus, increased aromatase levels contribute to insulin resistance and increasing obesity. The result is a cyclical testosterone to oestradiol shunt and suppressed ESR1 levels, further driving adipogenesis.vi
Testosterone is the main naturally produced anabolic steroid contributing to muscle mass. Decreased testosterone due to aromatization puts patients with obesity at increased risk of sarcopenia which in turn could reduce exercise tolerance.vii Increased adiposity also causes reduced metabolism via proinflammatory cytokines including increased IL6 and TNFα. These factors result in the tendency for individuals with obesity to continue to increase their weight despite little or no changes to caloric intake and exercise. As a result the
advice to eat less and move more is insufficient in the management of obesity.
Financial Cost of Obesity
There is a rising cost of obesity, with the 2025 estimation of direct healthcare cost of obesity and overweight to be ¤1.1 billion, in comparison with the 2015 estimate (based on 2009 data) of ¤202.6 million. Direct healthcare cost refers to general practitioner visits, hospital inpatient stays, consultant visits and emergency department attendances. Obesity related complications are more common in class two and three obesity than those who have overweight or have class one obesity.viii There is an obvious need therefore to prevent patients from progressing to class two and three obesity in order to reduce the development of complications.
Established and emerging pharmacological treatments
GLP1 receptor agonists are established pharmacological therapies for the treatment of obesity. Semaglutide (Ozempic) is a GLP1 Receptor agonist, Tirzepatide (Mounjaro) is a dual receptor agonist (GLP1 and GIP) while Retatrutide (not yet available clinically) is a tri- receptor agonist targeting GLP, GIP and glucagon. A major concern regarding the use of GLP1 agonists is contribution to future frailty, as both fat and muscle are lost when on these treatments. Lean body mass loss can account for 15-40% of weight loss using GLP1 therapies in vulnerable patient cohorts, such as those with chronic kidney disease and liver disease.ix This highlights the need to integrate sufficient protein intake and resistance training whilst on GLP1 treatment to optimise weight loss and muscle maintenance. Emerging treatments are now addressing this sarcopenia/ frailty threat. The BELIEVE phase 2b trial shows the monoclonal antibody Bimagrumab, when used with semaglutide, preserves lean mass whilst increasing weight loss in comparison to the use of either agent alone. Combination therapy led to fat loss accounting for 92.8% of weight loss in comparison to semaglutide only (71.8%).ii In the cohort who received Bimagrumab alone, 100% of weight loss was due to fat loss, and there was a 2.5% increase in muscle mass. Bimagrumab is an activin type two receptor inhibitor, resulting in down regulation of
pathways which contribute to muscle atrophy – basically it prevents myostatins from inhibiting muscle growth.
In the popular press it has been reported that this approach could be abused by athletes and the world anti-doping agency have added Ozempic to its 2025 monitoring list – some headlines are claiming that the Olympic games in 2028 could be the Ozempic games.
Obesity Model of Care in Ireland
The model of care for Obesity in Ireland was approved by the HSE in 2020 and was subsequently adapted by the World Health Organisation and used as their recommended tool for countries looking to establish a holistic obesity prevention and management framework. It is currently being funded and gradually stood up – having received specific funding in each of the last three budgets. In the adult space, this involves establishing an obesity treatment program (this is called The Best Health Programme) in the community across all chronic disease hubs. This is a multidisciplinary team (MDT) designed programme that runs over 12 months, delivered by dieticians, and is currently available in about 50% of the chronic disease hubs. Referral can be made by any health professional and based on the patients progress, they may be referred to one of six level three hospital based MDT’s for the management of severe and complex obesity. There is a plan to have one MDT in each health region. Three of these are currently funded and we will be advocating for further funding for the other three in the next number of years. If a decision is made that an individual requires bariatric surgery then the patient will be referred to one of three bariatric surgical centres - one in Dublin and one in Galway (both funded) and plans for one in Cork. At childhood level there is also a model of care which again runs through identification in the community, sign posting to community based interventions and social prescribing. In addition, there are multidisciplinary based teams running a programme called “Changing Together” in three of the health regions. These will need to be fully developed and then expanded to reach all of the health regions. There will be a provision for obesity treatment in the general paediatric setting in the hospital

but the exact nature of this and how it will be delivered has not been fully scoped out. Finally in Childrens Health Ireland there is an MDT for the management of children with severe and complex obesity. In time this team will have access to bariatric care. Globally, the number of children living with overweight and obesity now exceeds the number of children living with underweight. The GLP-1 treatments have been studied from the age of 5 upwards and their use in children will need to be increased.
Key Takeaways for Improving Outcomes and Quality of Care
Both stigmatisation of obesity and low “help seeking behaviour” in men contribute towards the disparity between men and women in seeking treatment for obesity.
The Irish Coalition for People Living With Obesity (ICPO) have an episode wherein five men share their personal experiences of obesity. They discuss the impact of language used in medical and social settings as well as testify to the benefits of seeking treatment. Further conversations will help to alleviate shame and highlight the impact of treatments such as GLP1s and bariatric surgery but also emphasise the importance of a holistic approach in ensuring successful outcomes.
In conclusion, addressing attitudes towards obesity as a disease process will increase the propensity of men to engage in obesity care. Bridging the gap between medical knowledge and societal perspectives of obesity will help to improve patient outcomes. Thus, investments into further sociological research to explore the barriers faced by men in seeking treatment for obesity is required. In the hospital setting all teams dealing with conditions which are driven by obesity should aim to address it, and not just manage the disease they are treating. This is of particular relevance for oncologists, cardiologists, sleep specialists and diabetologists.
The continued expansion of national services will streamline obesity care pathways. Continual advancements in pharmacotherapies will help to personalise treatments to improve outcomes. Ultimately, while there is much work to be done in addressing obesity in men, shifting perspectives and medical advances will help to alleviate
the disease burden and improve patient outcomes.
References
i Golinelli D, Sanmarchi F, Guarducci G, Palombarini J, Benetti P, Rosa S, Lenzi J. Gender differences in healthcare utilization across Europe: Evidence from the European Health Interview Survey. Health Policy. 2025 Sep 22:105448.
ii Schreurs L, De Smedt C, Goris E, Unuane D, Schoneveld M, Steenackers N, Pazmino S, Gies I, Van der Schueren B, De Cock D. “Just put in a little more effort”: the help-seeking experience of patients living with obesity: Behavior, Psychology and Sociology. International Journal of Obesity. 2025 Aug 14:1-8.
iii Flint SW, Hudson J, Lavallee D. UK adults’ implicit and explicit attitudes towards obesity: a cross-sectional study. BMC obesity. 2015 Sep 4;2(1):31.
iv Muscogiuri G, Verde L, Vetrani C, Barrea L, Savastano S, Colao A. Obesity: a genderview. J Endocrinol Invest. 2024 Feb;47(2):299-306. doi: 10.1007/ s40618-023-02196-z. Epub 2023 Sep 23. PMID: 37740888; PMCID: PMC10859324
v Lam JC, Mak JC, Ip MS. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012 Feb;17(2):223-36.
vi Ahmed F, Hetty S, Laterveer R, Surucu EB, Mathioudaki A, Hornbrinck E, Patsoukaki V, Olausson J, Sundbom M, Svensson MK, Pereira MJ. Altered expression of aromatase and estrogen receptors in adipose tissue from men with obesity or type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2025 Jan 21:dgaf038.
vii Fui MN, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian journal of andrology. 2014 Mar 1;16(2):223-31.
viii Healthy Ireland Survey
ix Memel Z, Gold SL, Pearlman M, Muratore A, Martindale R. Impact of GLP- 1 Receptor Agonist Therapy in Patients High Risk for Sarcopenia. Curr Nutr Rep. 2025 Apr 28;14(1):63. doi: 10.1007/s13668-025-00649-w. PMID: 40289060.


In eBC &
aBC
KISQALI is not recommended for concomitant use with tamoxifen2 Powerful consistency1-17
Early breast cancer:
NATALEE1
HR+/HER2- eBC
HR+/HER2- aBC
KISQALI in combination with an aromatase inhibitor is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer at high risk of recurrence. In pre- or perimenopausal women, or in men, the aromatase inhibitor should be combined with a luteinising hormone-releasing hormone (LHRH) agonist.2
Advanced or metastatic breast cancer:
KISQALI is indicated for the treatment of women with HR-positive, HER2-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy. In pre- or perimenopausal women, the endocrine therapy should be combined with a LHRH agonist.2
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing. Kisqali (ribociclib) 200 mg film-coated tablets
Presentation: Film coated tablets (FCT) containing 200 mg of ribociclib and 0.344 mg soya lecithin. Indications: Early breast cancer - Kisqali in combination with an aromatase inhibitor is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer at high risk of recurrence (see section 5.1 for selection criteria). In pre- or perimenopausal women, or in men, the aromatase inhibitor should be combined with a luteinising hormone-releasing hormone (LHRH) agonist. Advanced or metastatic breast cancer - Kisqali is indicated for the treatment of women with HR-positive, HER2-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy. In pre- or perimenopausal women, the endocrine therapy should be combined with a LHRH agonist. Dosage and administration: Patient selection for treatment with Kisqali based on the tumour expression of HR and HER2 should be assessed by a CE-marked in vitro diagnostic (IVD) medical device with the corresponding intended purpose. If the CE-marked IVD is not available, an alternative validated test should be used. Adults: Early breast cancer - The recommended dose is 400 mg (two 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days. In patients with early breast cancer, Kisqali should be taken until completion of 3 years of treatment or until disease recurrence or unacceptable toxicity occur. When Kisqali is used in combination with an aromatase inhibitor (AI), the AI should be taken orally once daily continuously throughout the 28-day cycle. Please refer to the Summary of Product Characteristics (SmPC) of the AI for additional details. In pre- or perimenopausal women, or in men, the aromatase inhibitor should be combined with a LHRH agonist. Advanced or metastatic breast cancer - The recommended dose is 600 mg (3 x 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days. When Kisqali is used in combination with an AI, the AI should be taken orally once daily continuously throughout the 28 day cycle. Please refer to the Summary of Product Characteristics (SmPC) of the AI for additional details. When Kisqali is used in combination with fulvestrant, fulvestrant is administered intramuscularly on days 1, 15 and 29, and once monthly thereafter. Please refer to the SmPC of fulvestrant for additional details. Treatment of pre and perimenopausal women with the approved Kisqali combinations should also include an LHRH agonist in accordance with local clinical practice. Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of Kisqali. Please see section 4.2 of SmPC for recommended dose modification guidelines. Kisqali can be taken with or without food (see section 4.5 of SmPC). The tablets should be swallowed whole and should not be chewed, crushed or split prior to swallowing. Special populations: ♦Renal impairment: Mild or moderate: No dose adjustment is necessary. Severe: A starting dose of 200 mg is recommended in patients with severe renal impairment. Kisqali has not been studied in breast cancer patients with severe renal impairment. Caution should be used in patients with severe renal impairment with close monitoring for signs of toxicity. ♦Hepatic impairment: No dose adjustment is necessary in patients with early breast cancer with hepatic impairment (see section SmPC 5.2). In patients with advanced or metastatic breast cancer, no dose adjustment is necessary in patients with mild hepatic impairmentModerate or severe: Dose adjustment is required, and the starting dose of 400 mg once daily is recommended. ♦Elderly (>65 years): No dose adjustment is required. ♦Pediatrics(<18 years): Safety and efficacy have not been established. Contraindications: Hypersensitivity to the active substance or to peanut, soya or any of the excipients. Warnings/Precautions: ♦Neutropenia was most frequently reported AR. A complete blood count (CBC) should be performed before initiating treatment. CBC should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. Febrile neutropenia was reported in 1.7% of patients exposed to Kisqali in the phase III clinical studies. Patients should be instructed to report any fever promptly. Based on the severity of the neutropenia, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 2 (see section 4.2 of SmPC). ♦Hepatobiliary toxicity increases in transaminases have been reported. Liver function tests (LFTs) should be performed before initiating treatment. LFTs should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. If grade ≥2 abnormalities are noted, more frequent monitoring is recommended. Recommendations for patients who have elevated AST/ALT grade ≥ 3 at baseline have not been established. Based on the severity of transaminase elevations, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 3 (see section 4.2). ♦QT interval prolongation has been reported with Kisqali. The use of Kisqali should be avoided in patients who have already or who are at significant risk of developing QTc prolongation. This includes patients with long QT syndrome, with uncontrolled or significant cardiac disease, including recent myocardial infarction, congestive heart failure, unstable angina and bradyarrhythmias and patients with electrolyte abnormalities. The use of Kisqali with medicinal products known to prolong QTc interval and/or strong CYP3A4 inhibitors should be avoided as this may lead to clinically meaningful prolongation of the QTcF interval (see SmPC sections 4.2, 4.5 and 5.1). If co-administration of Kisqali with a strong CYP3A4 inhibitor cannot be avoided, the Kisqali dose should be changed as described in SmPC section 4.2. QT interval prolongation in early breast cancer –study O12301C (NATALEE), a QTcF interval increase >60 msec from baseline was observed in 19 (0.8%) patients receiving Kisqali plus AI. The ECG should be assessed prior to initiation of treatment. Treatment with Kisqali should be initiated only in patients with QTcF values <450 msec. The ECG should be repeated at approximately Day 14 of the first cycle then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended. Appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should be performed prior to initiation of treatment, at the beginning of the first 6 cycles, and
AEs for eBC were: neutropenia, abnormal liver function tests and leukopenia and for aBC were, neutropenia, leukopenia, abnormal liver function tests, lymphopenia, infections, back pain, anaemia, fatigue, hypophosphataemia

REFERENCES: 1. Crown JP, et al. Presented at the European Society For Medical Oncology Congress 2025, 17–21 October, Berlin, Germany. 2. KISQALI (ribociclib). Summary of Product Characteristics. 3. Hortobagyi GN, et al. Ann Oncol. 2024:S0923-7534(24)04064-X. 4. Hortobagyi GN, et al. N Engl J Med. 2022;386(10):942–950. 5. Neven P, et al. Breast Cancer Res. 2023;25:103. 6. Im S-A, et al. N Eng J Med. 2019;381:307–316. 7. Yardley DA, et al. Ann Oncol. 2022; 33(S7):S629. 8. Verma S, et al. Br Cancer Res Treat. 2018;170:535–545. 9. Beck JT, et al. Cancer Res. 2019;79 (4_Supplement):P6-18-14. 10. Fasching PA, et al. Breast. 2020;54:148–154. 11. Harbeck N, et al. Ther Adv Med Oncol. 2020;12:1–8. 12. Fasching PA et al., Annals of Oncology, Volume 34, Issue 10, 2023, Pages 951-953. 13. Lu et al., JCO 42, 2812-2821(2024). 14. Burris et al.Br J Cancer. 2021 Aug;125(5):679-686. 15. Slamon DJ, et al. Ther Adv Med Oncol. 2023;15:1–16. 16. Slamon D, et al. N Engl J Med. 2024; 390:1080-1091. 17. Fasching PA, et al. Oral LBA13. Presented at the European Society for Medical Oncology Congress 2024, 13–17 September, Barcelona, Spain.
before the start of Kisqali treatment.
Any abnormality
on the observed QT prolongation during treatment, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 4 (see section 4.2 of SmPC). Based on the E2301 study QTcF interval data, Kisqali is not recommended for use in combination with tamoxifen. ♦Critical visceral disease. The efficacy and safety of ribociclib have not been studied in patients with critical visceral disease. ♦Severe cutaneous reactions Toxic epidermal necrolysis (TEN) has been reported with Kisqali treatment. If signs and symptoms suggestive of severe cutaneous reactions (e.g. progressive widespread skin rash often with blisters or mucosal lesions) appear, Kisqali should be discontinued immediately. ♦Interstitial lung disease/pneumonitis ILD/ pneumonitis has been reported with CDK4/6 inhibitors including Kisqali. Patients should be monitored for pulmonary symptoms indicative of ILD/ pneumonitis which may include hypoxia, cough and dyspnoea and dose modifications should be managed in accordance with Table 5 (see section 4.2 of SmPC) Based on the severity of the ILD/pneumonitis, which may be fatal, Kisqali may require dose interruption, reduction or discontinuation as described in Table 5 (see section 4.2 of SmPC). ♦Blood creatinine increase ribociclib may cause blood creatinine increase – if this occurs it is recommended that further assessment of the renal function be performed to exclude renal impairment. ♦CYP3A4 substrates ribociclib may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates (see section 4.5 of SmPC). Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co administration with CYP3A4 inhibitors. Pregnancy, Fertility and Lacation ♦Pregnancy: Pregnancy status should be verified prior to starting treatment as Kisqali can cause foetal harm when administered to a pregnant woman. Kisqali is not recommended during pregnancy and in women of childbearing potential not using contraception. ♦Women of childbearing potential who are receiving Kisqali should use effective contraception (e.g. double-barrier contraception) during therapy and for at least 21 days after stopping treatment with Kisqali. ♦Breast‑feeding: Patients receiving Kisqali should not breast feed for at least 21 days after the last dose. ♦Fertility: There are no clinical data available regarding effects of ribociclib on fertility. Based on animal studies, ribociclib may impair fertility in males of reproductive potential. ♦Effects on ability to drive and use machines Patients should be advised to be cautious when driving or using machines in case they experience fatigue, dizziness or vertigo during treatment with Kisqali. Interactions: ♦Concomitant use of strong CYP3A4 inhibitors should be avoided, including, but not limited to, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil, and voriconazole. Alternative concomitant medicinal products with less potential to inhibit CYP3A4 should be considered. Patients should be monitored for ARs. If concomitant use of a strong CYP3A4 inhibitor cannot be avoided, the dose of Kisqali should be reduced (see section 4.2 of SmPC). ♦Grapefruit or grapefruit juice should be avoided. ♦Concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John’s Wort (Hypericum
perforatum). An alternative medicinal product with no or minimal potential to induce CYP3A4 should be considered. ♦Caution is recommended when Kisqali is administered with sensitive CYP3A4 substrates with narrow therapeutic index (including, but not limited to, alfentanil, ciclosporin, everolimus, fentanyl, sirolimus, and tacrolimus), and their dose may need to be reduced. ♦Concomitant administration of Kisqali with the following CYP3A4 substrates should be avoided: alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam, triazolam. ♦Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pitavastatin, pravastatin, rosuvastatin and metformin. ♦Co-administration of Kisqali with medicinal products with known potential to prolong the QT interval should be avoided such as anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other medicinal products known to prolong the QT interval including, but not limited to, chloroquine, halofantrine, clarithromycin, ciprofloxacin, levofloxacin, azithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide and intravenous ondansetron. Kisqali is not recommended for use in combination with tamoxifen. Adverse reactions –advanced or metastatic breast cancer: ♦Very common: Infections, neutropenia, leukopenia, anaemia, lymphopenia, decreased appetite, headache, dizziness, dyspnoea, cough, nausea, diarrhoea, vomiting, constipation, stomatitis, abdominal pain, dyspepsia, alopecia, rash, pruritus, back pain, fatigue, peripheral oedema, asthenia, pyrexia, abnormal liver function tests. ♦Common: thrombocytopenia, febrile neutropenia, hypocalcaemia, hypokalaemia, hypophosphataemia, vertigo, lacrimation increased, dry eye, syncope, dysgeusia, hepatotoxicity, erythema, dry skin, vitiligo, dry mouth, oropharyngeal pain, blood creatinine increased, electrocardiogram QT prolonged, interstitial lung disease (ILD)/pneumonitis. ♦Rare: Erythema multiforme ♦Not known: Toxic epidermal necrolysis (TEN) ♦ Please refer to SmPC for a full list of adverse reactions. Adverse reactions - early breast cancer: ♦Very common: Infections, neutropenia, leukopenia, headache, Cough, Nausea, diarrhoea, constipation, abdominal pain, alopecia, fatigue, asthenia, pyrexia, abnormal liver function tests ♦Common: Anaemia, thrombocytopenia, lymphopenia, hypocalcaemia, hypokalaemia, appetite decreased, dizziness, dyspnoea, interstitial lung disease (ILD) / pneumonitis, vomiting, stomatitis, hepatotoxicity, rash, pruritus, peripheral oedema, oropharyngeal pain, blood creatinine increased, electrocardiogram QT prolonged ♦Uncommon: Febrile neutropenia ♦ Please refer to SmPC for a full list of adverse reactions. Legal Category: POM. Pack sizes: Unit packs containing 21, 42 or 63 FCTs. Not all pack sizes may be marketed. Marketing Authorisation Holder: Novartis Europharm Limited Vista Building Elm Park, Merrion Road Dublin 4 Ireland Marketing Authorisation Numbers: EU/1/17/1221/003 & 005. Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Dublin 4. Tel: 01 2601255 or at www. medicines.ie Prescribing information last revised: Nov 2024

Men's Health: Fertility & Oncology
Towards a National Fertility Preservation Pathway
Fertility Preservation Options for Individuals Assigned Male at Birth Receiving Oncology Care in Ireland: Current Landscape and Future Directions

Establishing a coordinated, inclusive national fertility preservation (FP) service is now both clinically and ethically imperative. Ireland’s healthcare system already possesses the multidisciplinary expertise — in oncology, andrology, reproductive medicine, and laboratory science — to deliver such a programme. What is needed is unified national infrastructure, dedicated funding, and clinician education to ensure that every patient, regardless of age, diagnosis, or gender identity, is offered timely information and access to fertility preservation. Integrating FP into routine oncology care would not only align Ireland with international best practice but would affirm a fundamental principle of survivorship: that the opportunity for future parenthood should not be an unintended casualty of successful cancer treatment.
Introduction
Advances in cancer treatment have dramatically improved survival rates, particularly among the young in Ireland, where more than 82% of children, adolescents and young adults (CAYA) survive their cancer diagnosis (NCRI, 2023). As survival improves, attention has rightly shifted to quality-of-life outcomes, including the ability to have genetically related children in the future. Cancer therapy can of course result in temporary or permanent infertility — an often-overlooked consequence of lifesaving care, but one with potentially profound sequalae. Fertility is deeply connected to identity, relationships, and future planning. Fertility-related distress is prevalent and enduring among cancer survivors (Logan et al., 2019), underscoring the need for timely, sensitive, and equitable access to fertility preservation (FP).
Written by Dr Aisling Looney, MB BCh BAO FECSM FRCS(Urol) MSc, Consultant Urologist and Andrologist, St Vincent's University Hospital and St Columcille's Hospital
Impact of Cancer Therapy on Fertility
Spermatogenesis & Cancer Treatments
In the testes, sperm production (spermatogenesis) is sustained by a pool of spermatogonial stem cells (SSCs) within the seminiferous tubules. These cells, supported by Sertoli and Leydig cells, divide rapidly on the pathway to becoming mature spermatozoa - a feature that makes them highly susceptible to cytotoxic agents. The prepubertal testis lacks sperm, so sperm retrieval is not a viable option in this cohort.
Discussing fertility, and FP early on a cancer journey — ideally at diagnosis — improves satisfaction and reduces anxiety of patients and their families. Clinicians must tailor conversations to the individual’s age, health status, and gender identity as fertility discussions can be complex and emotionally charged.
For minors, consent and assent are key ethical considerations. Parents or guardians may authorise procedures, but ongoing communication with the young person is essential. The establishment of Irish regulatory mechanisms similar to the UK HTA framework would provide clear governance for tissue handling and storage over time.
In Ireland, FP provision for this population is evolving: adult sperm cryopreservation is publicly funded for those with an oncological need, but services for those who are under 18, or over 18 and cannot produce a semen specimen, or do not have semen of adequate quality, remain fragmented. This article reviews current practices, emerging technologies, and policy developments in the FP domain for those assigned male at birth (AMAB) receiving oncology care in Ireland.
Chemotherapy targets rapidlydividing cells and can therefore disrupt sperm production. While Leydig cell function (and thus testosterone levels) may remain intact, SSC depletion can lead to temporary or permanent infertility. In prepubertal individuals, this risk is amplified, as SSC loss before sexual maturation eliminates the potential for future sperm production. Stukenborg et al. (2018) demonstrated that spermatogonial quantity in prepubertal testes correlates directly with adult fertility potential. Of course it is not just chemotherapeutic agents that negatively impact spermatogenesis. The testis is among the most radiosensitive tissues in the body, and the relationship between reproductive capacity and radiation sensitivity, first described by Bergonié and Tribondeau, still applies. Thus, pelvic radiotherapy can result in absolute infertility. Furthermore, cranial irradiation can also affect fertility, via disruption of the hypothalamic–pituitary–gonadal axis, reducing gonadotrophin release. While hormonal replacement therapy can restore testosterone and support partial recovery, germ cell loss may be irreversible.
Risk conferred by cancer treatments
The risk of infertility assigned to the individual depends on their diagnosis and treatments. Guidelines exist as to estimating the risk conferred to the testes by the patient’s treatment (e.g. Oncofertility Consesnus
Document). Typically, select prepubertal patients, whose cancer treatment convey a >50% of infertility, should be offered invasive FP therapies, such as TTC.
Established Fertility Preservation Approaches
Sperm cryopreservation for the post-pubertal cohort
Sperm cryopreservation is the cornerstone of FP for postpubertal individuals. Semen samples collected before chemotherapy or radiotherapy can be stored for future use, enabling conception through assisted reproductive technologies (ART) such as In-Vitro Fertilisation (IVF).
Since 1998, the Irish Department of Health has funded semen cryopreservation for adults undergoing gonadotoxic therapy. The Health Service Executive (HSE) currently covers a 10year storage period for those over 18, while charitable and research partnerships such as the Childhood, Adolescent and Young Adult (CAYA) Fertility Project, housed in the Merrion Fertility Clinic, provides support for some younger patients.
Timing is critical — gametes collected even weeks after treatment begins may be compromised. However, acutely unwell patients, those experiencing psychological distress, or individuals with disabilities or cultural concerns may struggle to provide samples or their samples may be inadequate. Sensitive counselling and prompt coordination with andrology teams are essential to overcome these barriers - to assist with the production of a semen sample or to provide surgical sperm retrieval.
Surgical sperm retrieval (SSR) When ejaculation is not possible or semen samples contain inadequate spermatozoa, healthy sperm can be retrieved by urologists directly from the reproductive tract. Techniques include:
• Electroejaculation – electrical stimulation of ejaculation under general anaesthetic (GA).
• Testicular sperm extraction (TESE) or microdissection TESE (mTESE) to extract healthy seminiferous tubules. This is typically day-case GA surgery comprising of a small scrotal incision, testicular delivery and the extraction of seminiferous tubules, with or without the use of an operating microscope.
• OncoTESE, a prodecure similar to mTESE but performed during orchidectomy for testicular malignancy.
All of these procedures require support from an embryological laboratory. Outcomes are encouraging. For example, the UCLH cohort (BJU Int, 2022) of 39 patients showed a 68% success rate for patients requiring SSR for oncological purposes (having failed semen cryopreservation) - with at least one viable sample obtained regardless of cancer type. Crucially, these procedures can be arranged rapidly on emergency theatre lists in relevant institutions to avoid delaying oncological treatment. Operators must remain hyper-vigilant regarding the risk of scrotal haematoma, which might delay curative oncological treatments, and so meticulous haemostasis intraoperatively is critical.
Experimental and Emerging Approaches
Testicular tissue cryopreservation (TTC)
For prepubertal individuals lacking mature sperm, TTC offers a promising pathway for preserving fertility for those at high, or very high risk of infertility due to their cancer treatments. A small testicular biopsy, containing spermatogonial stem cells is extracted prior to cancer treatment, and frozen for future use.
The goal is to either mature SSCs in vitro (i.e. culture stem cells into mature spermatozoa) for use in IVF; or reimplant the patients own stem cells after cancer remission, in order to restore fertility. International progress has been striking: Fayomi et al. (2019) reported the first live birth from autologously grafted prepubertal testicular tissue in an animal model, and in 2023, the first human SSC transplant to the rete testis was achieved. Although clinical translation remains in early stages, TTC represents a realistic avenue for fertility restoration in future generations of cancer survivors, and already forms the standard of care in many developed nations.
Innovative research in Ireland
Ireland is contributing to this global effort. An ERC-funded University of Limerick project, led by Associate Professor Eoghan Cunnane, is developing in vitro models of the human testis to explore testicular cell maturation and microenvironmental support systems for FP. These advances align with emerging regenerative
medicine strategies and underscore the country’s potential to participate in international oncofertility networks.

The Irish Context: Policy, Access, and Future Directions
As outlined above, FP provision in Ireland has historically centred on adult semen cryopreservation. However, the National Cancer Care Programme (NCCP), the Health Information and Quality Authority, and the Irish Cancer Society have each emphasised the urgent need for inclusive, age-appropriate services. Compared with peer nations, Ireland remains at an early stage of FP policy implementation.
The Department of Health recently tasked HIQA to review international FP practices. This scoping review, published in 2024, found that all ten comparator countries publicly fund gamete (sperm) cryopreservation prior to cancer therapy, and eight of the ten (Denmark, France, Portugal, England, Sweden, Northern Ireland, Wales and Australia) offer some level of tissue cryopreservation. In contrast, the Republic of Ireland currently lacks a formal domestic pathway for testicular tissue storage and biobanking.
Yet, on this island, the expertise exists — among Irish urologists, embryologists, oncologists, haematologists, and researchers — to deliver these services safely. The confluence of the AHR Bill (2024), the NWIHP fertility programme (2023), and growing NCCP support offers a critical opportunity to embed FP into oncology pathways.
A proposed national protocol for individuals assigned male at birth with cancer would, in the view of the author, entail:
1. Early identification by paediatric cancer clinicians of potentially suitable pre/peri pubertal patients (at high or very high gonadotoxic risk due to their cancer or its treatment) - namely in Children’s Health Ireland (CHI).
2. Prompt referral to andrology services for an urgent in-house review in CHI.
3. Baseline clinical review by andrologist – including hormonal, infectious, and pubertal assessment.
4. Coordinated sperm or tissue cryopreservation within 24–48 hours in CHI (typically carried out in conjunction with other pre-chemotherapy procedures).
5. Commencement of cancer treatment 24-48 hours post-operatively.
6. Post-treatment andrological follow-up and survivorship planning.
Implementation of this pathway would align Ireland with international standards and ensure equitable, timely access for all.
Survivorship and Long-Term Care
Fertility care is part of a continuum of cancer and survivorship care rather than a discrete event. Long-term follow-up should include hormonal monitoring, sexual function assessment, and reproductive counselling. Cryopreserved sperm can remain viable for decades, allowing conception through assisted reproduction even many years after treatment. Those unable to preserve gametes may explore options such as surgical sperm retrieval, donor sperm, adoption, or emerging SSC-based therapies as they become available.
Importantly, fertility preservation intersects with broader survivorship domains — including endocrine and sexual health — that shape quality of life. Multidisciplinary survivorship clinics, integrating oncology, urology, and reproductive health, are essential to the holistic care that our cancer survivors deserve.
Conclusion
Fertility preservation for individuals assigned male at birth represents a cornerstone of equitable oncology care. While Ireland’s existing public funding for semen cryopreservation is commendable, substantial gaps persist for adolescents, prepubertal patients, and those patients unable to provide adequate semen specimens. Surgical sperm retrieval (post-puberty) and testicular tissue cryopreservation (pre-puberty) offer hope for the future, but progress depends on policy development, infrastructure investment, and inclusive practice.
The expansion of fertility services under NWIHP, coupled with the AHR Bill’s regulatory framework, positions Ireland to now develop comprehensive, inclusive fertility preservation services comparable to international best practice. In doing so, the health system can truly support every patient’s right to future reproductive optionsensuring that survivorship includes not just life after cancer, but life with possibility.
References available on request

Men's Health: Mental Health
Men’s Mental Health in Ireland

One in every four people in Ireland will experience a mental health condition during their lifetime; with depression being the most common occurring in one in ten of us.1 Factors like stress, loneliness, financial struggle, alcohol/ substance misuse as well as the psychological impacts of major life events (eg - parenthood/ separation/ retirement) contributing to this. The likelihood of dying from suicide is four times higher for men.2 Men are also less likely to seek support or engage in services due to unique challenges that discourage them.3 These include societal expectations of stoicism, selfreliance and perceived gender norms.2
Mental health literacy which describes someone’s knowledge and beliefs around mental health has been found to be significantly lower in men.4 Men may believe asking for help is an admission of failure or weakness (it can actually be a sign of strength).5 Men may worry how they will be perceived by family, friends or neighbours and fear judgement by others if they seek help. Despite significant efforts and campaigns to improve this, considerable stigma still exists around mental health. Men also hold more stigmatising attitudes in this respect.3
The National Men’s Action Plan (2024) which superseded the National Men’s Health Policy identifies isolation, access to services (especially in rural areas) and a lack of awareness of available resources and supports as barriers to men’s mental health.2 Although women have higher rates of suicidal ideation, self-
Written by Patrick J McGee; Advanced Specialist PharmacistMental Health, Beaumont Hospital
Contact and Rapport
harm and suicide attempts, men have substantially higher rates of completed suicide. Of the 512 deaths by suicide in Ireland in 2021 78.3% were men and men aged between 45 to 49 were the highest cohort of all suicides (9.4%).6 Men also have an increased likelihood of dying by suicide from more lethal methods (eg - hanging, carbonmonoxide poisoning or firearms).2
Sub groups of men at increased risk of poor mental health include the unemployed, homeless, prisoners, LGBTQI+, veterans, ethnic minorities and men with existing mental illness.7 In the traveller community where masculinity is fostered and celebrated men are even less likely to seek support.2 There exists an even greater stigma and cultural sensitivity around mental health and suicide within this group.2, 8 In people with autism suicidality is common (66%) and people with ADHD are five times more likely to attempt suicide.9, 10
Another at risk group of men are the farming community; the majority of whom are selfemployed males. Irish farmers can experience a higher level of mental health issues and suicide due to factors such as the isolative nature of farming and the reduced opportunities for social interaction.11 Farmers are often faced with significant financial pressures and have considerable responsibilities to both their farms and their families and often prioritise the interests of their farms over their own health.11
Community Pharmacist Role
Pharmacists are highly educated, readily accessible and trusted healthcare professionals who are well placed to support men’s mental health, especially for the harder to reach groups like rural farmers and travellers. Such men may be less inclined to make an appointment with their GP but may prefer a less formal chat with their pharmacist. They are likely to visit their local pharmacy more regularly than a GP and this presents opportunities for engagement.
Building good relationships with male patients allows pharmacists more opportunities to make positive interventions around wellness and mental health. Pharmacists should be aware of early warning signs of mental health deterioration or psychological distress, such as subtle changes in appearance (reduced self-care, weight changes) or behaviour (excessively talkative/ less talkative/ overly pessimistic/ withdrawn) or changes in activity levels. Asking patients about and taking an interest in a patient’s mental wellbeing is a positive intervention. Asking how they are doing or how their medication is going is a simple conversation opener. In fostering open conversations it is important to be supportive and nonjudgemental, to ask questions, to listen and to signpost if necessary. Offering privacy to male patients (in the consultation room) may encourage men who are reluctant to engage. Early intervention is one of the key factors in suicide prevention and if concerned someone is thinking about suicide it is important to ask them clearly, directly and sensitively.
Stigma
Pharmacists have a role in challenging mental health stigma by creating a supportive environment that promotes positive mental health and emotional wellbeing. Pharmacists should challenge stigmatizing attitudes (eg - people’s views or sensationalised media headlines) around mental health and promote open conversations around mental wellbeing among customers, patients and staff. Improved public knowledge around mental health will reduce the negative attitudes held by society and enable more men with mental health challenges to seek support. There are large gaps in mental health literacy in Ireland and especially among men.4 Pharmacists can be proactive within their community by engaging in positive mental health initiatives and awareness campaigns (egholding a pharmacy event for world mental health day).
Medications
Pharmacists also clearly have important roles in managing patients’ medications by providing information and
counselling and giving advice on minimising and managing side-effects of medications for mental health. They are also well placed for monitoring and advising on patients’ physical health (especially for patients on medication that cause metabolic side-effects like weight gain or increased lipids/ glucose such as antipsychotics). Pharmacists (and patients) can access the HSE’s subscription to the “Choice and Medication” service. This is an invaluable resource containing detailed information, fact sheets and printable leaflets covering all mental health medications and mental illnesses for patients with a range of literacy levels in multiple languages. www. choiceandmedication.org/ireland.
Vigilance
Pharmacists have responsibility in monitoring and restricting the sale of drugs with potential for abuse or harm. These include laxatives, codeine containing products and cough and cold remedies. They should also be vigilant to repeated purchases of products containing paracetamol which may indicate self-harm/ suicide intent. Another intervention may be to follow up with patients where there may be non-compliance and to liaise with their GP/ CMHT (Community Mental Health Team) where there is an obvious mental health deterioration.
Continued Professional Development
To ensure pharmacists can support men with mental health challenges they may require upskilling in the area. Mental health first aid is an excellent course that can be undertaken by all pharmacy staff. It is an evidence based, early intervention training programme that teaches people the signs and symptoms of common mental health disorders and how to provide relief, support and signposting. It is a paid course provided by a range of institutions in Ireland (St John of God; Redcross; MHFA Ireland). There are also many accredited CPD resources for pharmacists wishing to build expertise and confidence in this area. These include:
Mental Health First Aid
www.mhfaireland.ie; www.stjohnofgodhospital.ie
Irish Institute of Pharmacy (IIOP): e-learning and webinars IIOP Mental Health and Wellbeing Resource Hub www.iiop.ie
IPU Academy: e-learning and live courses www.ipuacademy.ie
Maudsley Learning www.maudsleylearning.com/ elearning/
BMJ Learning https://new-learning.bmj.com
Choice and Medication www.choiceandmedication.org/ ireland
National Office for Suicide Prevention www.hse.ie/eng/ services/list/4/mental-healthservices/nosp/
Connecting for Life - Ireland’s national suicide reduction strategy 2015 to 2020 www.hse.ie/eng/services/ list/4/mental-health-services/ connecting-for-life/nationalstrategy-to-reduce-suicide/ Signposting
It is important to be aware of the resources available to men and encourage them to seek support when necessary. Requesting and displaying highly visible leaflets/ posters/ signage from signposting resources is also beneficial.
Men’s Sheds – Community based groups where men gather to share skills, work on projects,
and socialize. Open to men of all ages. www.menssheds.ie
HSE Mental Health –Information on common difficulties and self-help tips. www.yourmentalhealth.ie
Mental Health Ireland – Charity dedicated to promoting mental health and supporting people with mental health challenges. www.mentalhealthireland.ie
Sheds for Life – Health promotion programme for supporting physical, mental and social wellbeing of Irish Men’s Sheds members. www. menssheds.ie/sheds-for-life/
Men’s Aid (formerly AMEN) –For men experiencing domestic violence. www.mensaid.ie
Samaritans – Provide a dedicated confidential emotional support helpline for anyone in distress or despair. www.samaritans.ie
Aware – Provide support and information for people who experience depression or bipolar illness and their families. www.aware.ie
Pieta House – Provide free, professional support for people experiencing suicidal thoughts, self-harm or who are bereaved by suicide. www.pieta.ie
Text About it – Anonymous HSE funded SMS/ WhatsApp
service for people requiring mental health support. www.text50808.ie

HSE Drugs and Alcohol Helpline – Confidential service providing support, information and available services. www. hse.ie/eng/services/list/5/ addiction/drugshivhelpline/
Drug and Alcohol Information and Support – Education, Training, Information, Support and Treatment and Rehabilitation Services. www.drugs.ie
Alcoholics Anonymous –Fellowship where alcoholics help each other and reach out to others struggling with alcoholism. www.alcoholicsanonymous.ie
Gamblers Anonymous –Fellowship where people help each other recover from gambling addiction. www.gamblersanonymous.ie
Grow – Provide community based, peer support groups and deliver mental health programmes within the community and workplace. www.grow.ie
Shine – Support and education for individuals, families and communities impacted by mental illness and stigma. www.shine.ie
Bodywhys – Support organisation for people with eating disorders. www.bodywhys.ie
Lighthouse – Holistic support to constriction community on emotional, physical and financial wellbeing. www.lighthousecharoty.org
HUGG – Support organisation for people bereaved by suicide. www.hugg.ie
CAIRDE – Programme to enhance knowledge of mental health issues and reduce stigma in the construction industry. www.hsa.ie/eng/your_industry/ construction
First Fortnight – Arts based mental health charity that challenges mental health stigma and promotes mental wellbeing. Arts festival held every January. www.firstfortnight.ie
Practitioner Mental Health Matters Programme –Confidential service for health professionals. www.practitionerhealth.ie
Traveller Counselling Service –Counselling service for members of the travelling community. www.travellercounselling.ie
In a crisis situation where there is imminent concern then contacting a relative or referring to the GP or emergency services is appropriate.
References available on request


Men's Health: Renal Carcinoma
Renal Cell Carcinoma in Ireland: What Hospital Teams Need to Know Now
At 07:45 on a Monday in a Dublin theatre complex, a partial nephrectomy is being briefed. The urology consultant walks the team through ischaemia time and margins while, two floors down, oncology pharmacists are finalchecking a first-cycle prescription for pembrolizumab plus axitinib. On the inpatient ward, a nurse has just escalated a patient’s blood pressure after day-10 of a VEGFTKI. This is modern renal cell carcinoma (RCC) care in Ireland: robotic surgery next door to immunotherapy-TKI combinations, with hospital pharmacists holding the safety line on dose, interactions and immune-related toxicities. The pace of change is welcome — but it demands precision, joined-up pathways and informed pharmacy leadership.
RCC in Ireland — the numbers that matter
RCC accounts for the majority of kidney cancers and is most commonly the clear-cell subtype. While Ireland’s absolute case numbers are modest compared with larger neighbours, the burden is meaningful for acute hospitals. Public-facing patient information from the Irish Cancer Society estimates roughly ~630 new kidney cancer diagnoses annually in Ireland, a figure that aligns with historical trends in NCRI reporting and confirms a steady service demand across surgical, medical oncology and pharmacy pathways.1, 2
Beyond incidence, the international guideline picture has moved quickly. Both EAU (2025) and ESMO (2024/25 living guidelines) have refreshed recommendations across localised, locally advanced and metastatic disease. The take-home for Irish hospital teams is straightforward: risk-stratified systemic therapy and vigilant toxicity management are now core, not optional, features of care — and service models must reflect that reality.3, 4
Surgical bedrock, systemtherapy surge
Surgery remains the curative cornerstone for localised disease, with partial nephrectomy prioritised when oncologically appropriate. But metastatic and high-risk settings increasingly hinge on systemic therapy choices
— and those choices bring practical workload to pharmacy.
Adjuvant therapy: the postnephrectomy pivot
For adults at increased risk of recurrence following nephrectomy (with or without metastasectomy), adjuvant pembrolizumab is an authorised option in the EU. Hospital pharmacies should anticipate adjuvant pathways that mirror other immuno-oncology programmes: MDT selection, clear eligibility documentation and early toxicity-education for patients leaving surgical wards.5
First-line metastatic/advanced RCC (clear-cell)
Across ESMO/EAU guidance and pivotal trials, IO–TKI combinations dominate first-line therapy. Commonly used or referenced regimens include:
• Pembrolizumab + axitinib — a standard first-line option across risk groups, with NCCP regimen support in Ireland.
• Avelumab + axitinib — an alternative IO–TKI combination supported by JAVELIN Renal 101 data.
• Cabozantinib + nivolumab — for treatment-naïve advanced RCC, with EU authorisation and Irish health-technology assessment activity noted.
• Ipilimumab + nivolumab — IO–IO, particularly in intermediate/ poor risk.
For hospital pharmacists, the nuances are practical: starting doses, blood-pressure thresholds, hepatic/thyroid surveillance, and immune-related adverse event (irAE) playbooks. Ireland’s NCCP SACT library provides high-value, operational regimens that align with these international standards and should anchor local order sets.6, 7, 8 VEGF-TKIs and IO agents: what pharmacists have to catch
The backbone agents in Irish practice include axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib and tivozanib, often paired with pembrolizumab or avelumab in the first-line, or used as targeted monotherapy in subsequent lines. Each brings a distinct toxicity “accent” that pharmacy must anticipate.
• Axitinib (often with pembrolizumab or avelumab): watch for hypertension, hand–foot syndrome, diarrhoea and hypothyroidism. Doseinterruptions and step-downs are common; ensure home BP monitoring and drug–drug interaction checks (notably CYP3A4).
• Cabozantinib (monotherapy or with nivolumab): hypertension, mucositis, diarrhoea and palmar–plantar erythrodysaesthesia drive dose modifications; NCCP explicitly flags stopping rules for severe, refractory hypertension.
• Lenvatinib (often with pembrolizumab): robust PFS benefit in CLEAR/KEYNOTE-581 came with additive hypertension, proteinuria and fatigue risk — pre-emptive counselling and urine dipstick routines help.
• Pazopanib: daily oral dosing with liver enzyme surveillance and dose titration in 200mg steps per EU product information.
• Sorafenib: now used selectively; historical EPAR data still inform expectations on skin toxicity and diarrhoea.
• Tivozanib: a well-tolerated VEGFR TKI option with EU approval; be mindful of bloodpressure escalation and fatigue profiles.
Immune-checkpoint inhibitors (pembrolizumab, nivolumab, ipilimumab, avelumab) require proactive irAE pathways: gradebased steroid algorithms, endocrinology referral routes for thyroid/adrenal events, and clear stop-restart criteria captured on the order set. The operational challenge is embedding them in day-to-day ward practice.9, 10
What’s uniquely Irish about RCC pathways?
NCCP regimens, NCPE decisions and hospital formulary flow Ireland’s oncology infrastructure runs through three levers that hospital pharmacists navigate daily:
1. NCCP SACT regimens — the practical “how” of dosing, monitoring and supportive measures.
2. NCPE/HTA assessments — the “should we reimburse?” lens, highlighting Ireland’s costeffectiveness scrutiny.
3. NCRI perspective — helps size the population need and survival trends, informing service capacity and stock planning.11, 12
Urology–oncology interface
RCC care in Ireland is concentrated in high-volume centres, with robotic partial nephrectomy entrenched. Prof Barry McGuire, a Dublin-based consultant urological surgeon with a subspecialty interest in kidney cancer and robotic surgery, exemplifies the surgical end of that pathway. For hospital pharmacists, knowing the local surgical caseload and adjuvant referral pattern helps predict IO starts and discharge counselling needs.13
Second-line and beyond: sequencing with purpose
When disease progresses:
• Switch-class strategies are common: from IO–TKI to a different TKI (e.g., cabozantinib, tivozanib, pazopanib) or to lenvatinib + everolimus in selected scenarios.
• Prior IO exposure raises nuanced questions about further IO; international reviews in 2024 emphasise tailoring to prior response and toxicity history.
• Pharmacy should expect dosefinding periods on second-line TKIs; patient-held diaries and early telephone reviews reduce unplanned admissions for dehydration, diarrhoea and hypertensive crises.14
The horizon
Expect iterative EAU/ESMO updates as combinations mature and biomarkers inch forward. Locally, NCCP regimen revisions and NCPE determinations will continue to shape what’s usable and fundable. Keep one eye on sequencing data and the other on service-level quality: RCC outcomes depend on getting today’s basics right — blood pressure, endocrine monitoring, dose-holds and patient education. References available on request
Men's Health: Prostate Cancer
PSA for proactive prostate health management
Every year, 620 men in Ireland die from prostate cancer. Most of those deaths could be prevented with awareness and early detection.
Unfortunately, a lot of men avoid or delay speaking to their GP about Prostate health because they assume that it will lead to something awkward and invasive.
What they don’t realise is that the first step in being proactive about your prostate health is much simpler than that. Early detection can start as a simple PSA blood test.
Prostate cancer is the most common male cancer in Ireland. Around 4,067 men are diagnosed annually in Ireland. 1 in 6 people born with a prostate will be diagnosed in their lifetime with rates of prostate cancer higher in black men with 1 in 4 getting diagnosed with prostate cancer. Men are two and a half times more likely to get prostate cancer if their father or brother had it. Inherited genes especially BRCA 2, being overweight or obese can also be risk factors to be aware of.
However, prostate cancer responds well to treatment and, if detected early, it can be treated successfully. With early detection, over 93% of men survive.
Like many other forms of cancer, these survival rates are all dependant on when the cancer is detected. Early detection saves lives allowing for better treatment options that will include a non-
invasive Active Surveillance plan if suitable and can ensure a more straightforward cancer journey – and most importantly improve survival outcomes and quality of life.
The Marie Keating Foundation is urging men to take charge of their prostate health by talking to their GP about a simple PSA blood test once they turn 50 — or from 45 if there’s a family history of prostate or breast cancer.
A PSA or prostate-specific antigen test is a simple blood test that monitors possible changes in your prostate which may need further investigations and follow up. By having regular PSA levels checked as instructed by your GP or Urologist, it can help to detect prostate problems or cancer in its earliest stages, even if you are experiencing no symptoms.
Prostate cancer is often asymptomatic, meaning there are no obvious changes or signs that something might not be right with your body. This is why open and honest communication between men, and their GPs is so vital and why we urge you to ask about that important PSA blood test.
We want men to feel confident speaking openly about prostate health, recognising the signs and symptoms if they are present, and starting that important conversation with their GP. A PSA test is quick, non-invasive, and takes less than five minutes — but it could be the first step in prioritising your prostate health.

Written by Helen Forristal, Director of Nursing, Marie Keating Foundation

Signs and symptoms to be aware of
Dysuria – difficulty and pain passing urine.
Urgency – difficult to postpone passing urine.
Frequency – going to the toilet more frequently than before.
Nocturia – getting up more than twice a night to pass urine.
Hesitancy – taking time to get going when passing urine.
Flow – has become weak or intermittent.
Incomplete emptying – feeling that the bladder has not emptied.
Blood – present in the urine.
Less common symptoms include:
• Trouble having or keeping an erection.
• Lower back pain or pain in hips or upper thighs.
It is important to note that the prostate enlarges with age. It wraps around the urethra (waterpipe) and this enlargement (benign prostatic hyperplasia –BPH) can cause bladder outlet obstruction which can lead to the above symptoms. BPH is more common than prostate cancer, but the conditions may exist together. BPH is not cancerous but can be troublesome.
Do not wait for signs and symptoms to present before taking
action, as Prostate Cancer is very often symptom free, particularly in its early stages. If you do however have urinary symptoms speak to your GP, it could be for a variety of reasons not necessarily prostate cancer, but the sooner you address it, the better the outcome. Get proactive for peace of mind and more positive treatment paths when issues are detected early. If you experience any of the listed symptoms your GP will determine the severity of your symptoms and examine your prostate gland. Examination involves a digital rectal examination (DRE) where the prostate is examined and felt by the doctor by using a finger in the back passage. The examination itself will be quick and painless, and will assess the size, texture and consistency of the gland and look for any irregularities.
If discovered early, prostate cancer can be treated successfully.
In closing, we urge you to Stand Up for Your Prostate.
All men, but especially those whose father, brother or grandfather has or had prostate cancer, need to engage in conversations earlier because we know that early cancer detection from the age of 40- 45 years of age onwards can save lives. A simple prostate blood test, the PSA, is the best way to assess your risk.
For more information on the Marie Keating Foundations Stand Up for Your Prostate 2025 campaign go to mariekeating.ie

AMGEVITA® (adalimumab) Prescribing Information
Please refer to the Summary of Product Characteristics (SmPC) before prescribing Pharmaceutical Form: Single dose pre-filled syringe contains 20 mg adalimumab in 0.4 mL (50 mg/mL) solution, 20 mg adalimumab in 0.2 mL (100 mg/mL) solution, 40 mg adalimumab in 0.8 mL (50 mg/mL) solution, 40 mg adalimumab in 0.4 mL (100 mg/mL) solution, 80 mg adalimumab in 0.8 mL (100 mg/mL) solution, and single dose pre-filled pen (SureClick®) contains 40 mg adalimumab in 0.8 mL (50 mg/mL) solution, 40 mg adalimumab in 0.4 mL (100 mg/mL) solution and 80 mg adalimumab in 0.8 mL (100 mg/mL) solution. Indications and Dosage: please refer to SmPC for full information. For subcutaneous injection. Treatment should be initiated and supervised by specialist physicians experienced in conditions for which AMGEVITA® is indicated. Ophthalmologists should consult with an appropriate specialist before starting treatment. Give patients a Patient Reminder Card. After training in injection technique, patients may self-inject with medical follow-up as necessary. Optimise other concomitant therapies (e.g. corticosteroids and or/immunomodulatory agents). Rheumatoid arthritis (RA): In combination with methotrexate (MTX) for: 1. moderate to severe, active RA in adult patients with inadequate response to disease-modifying anti-rheumatic drugs (DMARDs) including MTX; 2. Severe, active and progressive RA not previously treated with MTX. Can be given as monotherapy if intolerance to or when continued treatment with MTX is inappropriate. AMGEVITA® reduces rate of progression of joint damage when given in combination with MTX. Dosage: 40 mg every other week as a single dose via subcutaneous injection. Continue MTX. Glucocorticoids, salicylates, non-steroidal anti-inflammatory drugs, or analgesics can be continued during treatment. In monotherapy, patients may require 40 mg every week or 80 mg every other week if there is a decrease in clinical response. Reconsider treatment beyond 12 weeks if no clinical response. Consider need for dose interruption, e.g. before surgery or if serious infection occurs. Polyarticular juvenile idiopathic arthritis (pJIA): In combination with MTX for active pJIA in patients from the age of 2 years with inadequate response to one or more DMARDs. Can be given as monotherapy if intolerance to or when continued treatment with MTX is inappropriate. Dosage: 10 kg to < 30 kg: 20 mg every other week. ≥ 30 kg: 40 mg every other week. Reconsider treatment beyond 12 weeks if no clinical response. Enthesitis-related arthritis (ERA): treatment of active ERA in patients 6 years and older with inadequate response to, or intolerance to conventional therapy. Dosage: 15 kg to < 30 kg: 20 mg every other week. ≥ 30 kg: 40 mg every other week. Ankylosing spondylitis (AS): treatment of adults with severe active AS with inadequate response to conventional therapy. Axial spondyloarthritis without radiographic evidence of AS: treatment of adults with severe axial spondyloarthritis without radiographic evidence of AS but with objective signs of inflammation by elevated CRP and/or MRI, and an inadequate response to, or intolerance to nonsteroidal anti-inflammatory drugs. Psoriatic arthritis (PsA): treatment of active and progressive psoriatic arthritis in adults with inadequate response to previous DMARD therapy. Dosage: (AS, nr-axSpA, PsA): 40 mg every other week. Reconsider treatment beyond 12 weeks if no clinical response. Psoriasis: treatment of moderate to severe chronic plaque psoriasis in adults wo are candidates for systemic therapy. Dosage: 80 mg at week 0, followed by 40 mg
every other week from week 1. Reconsider treatment beyond 16 weeks if no clinical response. Paediatric Plaque Psoriasis: treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age with inadequate response to or if topical therapy and phototherapies are inappropriate. Dosage: 15 kg to < 30 kg: 20 mg followed by 20 mg every other week starting one week after initial dose. ≥ 30 kg: 40 mg then 40 mg every other week starting one week after initial dose. Reconsider treatment beyond 16 weeks if no clinical response. Safety has been assessed in paediatric patients with plaque psoriasis for a mean of 13 months. Hidradenitis suppurativa (HS): treatment of active moderate to severe HS (acne inversa) in adults and adolescents from 12 years of age with inadequate response to conventional systemic HS therapy. Dosage: adult: 160 mg at Day 1, followed by 80 mg at Day 15. At day 29 continue with 40 mg every week or 80 mg every other week. Reintroduction after treatment interruption: 40 mg every week or 80 mg every other week. Dosage: adolescent (≥ 30 kg): 80 mg at week 0 then 40 mg every other week starting at week 1. If response is inadequate, consider increasing to 40 mg every week or 80 mg every other week. Dosage: adult and adolescent: Antibiotics may be continued if necessary. Use concomitant topical antiseptic wash on a daily basis. Reconsider treatment beyond 12 weeks if no improvement. Periodically evaluate the benefit and risk of continued treatment. Crohn’s disease: treatment of moderately to severely active Crohn’s disease in adults with no response despite a full and adequate course of therapy with a corticosteroid and/or an immunosuppressant or intolerance to or have medical contraindications for such therapies. Dosage: Induction: 80 mg at week 0, then 40 mg at week 2. For a more rapid response: 160 mg at week 0, then 80 mg at week 2; risk of adverse events higher during rapid induction. Maintenance: 40 mg dose every other week. Corticosteroids may be tapered in accordance with clinical guidelines. If decrease in clinical response, can increase to 40 mg every week or 80 mg every other week. If no response by week 4 there may be benefit from continued therapy to week 12. Reconsider treatment beyond 12 weeks if no clinical response. Paediatric CD, ≥ 6 years: Moderately to severely active CD with inadequate response to, intolerance to or contraindication to conventional therapy including primary nutrition therapy and a corticosteroid and/or an immunomodulator. Dosage: < 40 kg: Induction: 40 mg at week 0 then 20 mg at week 2. For a more rapid response: 80 mg at week 0 then 40 mg at week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 20 mg dose EOW. If insufficient response, consider 20 mg every week. Dosage: ≥ 40 kg: Induction: 80 mg at week 0 then 40 mg at week 2. For a more rapid response: 160 mg at week 0, then 80 mg at week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 40 mg dose EOW. If insufficient response, consider 40 mg every week or 80 mg EOW. Reconsider treatment beyond 12 weeks if no clinical response. Paediatric Crohn’s disease: treatment of moderately to severely active Crohn’s disease in paediatric patients from 6 years of age with inadequate response to conventional therapy including primary nutrition therapy and a corticosteroid and/or an immunomodulator, or intolerant to or have contraindications for such therapies. Dosage: < 40 kg: Induction: 40 mg at week 0 then 20 mg at week 2. For a more rapid response: 80 mg at week 0 then 40 mg at week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 20 mg dose every
other week. If insufficient response, consider 20 mg every week. Dosage: ≥ 40 kg: Induction: 80 mg at week 0 then 40 mg at week 2. For a more rapid response: 160 mg at week 0, then 80 mg at week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 40 mg dose every other week. If insufficient response, consider 40 mg every week or 80 mg every other week. Reconsider treatment beyond 12 weeks if no clinical response. Ulcerative colitis: treatment of moderately to severely active ulcerative colitis in adult patients with inadequate response to, intolerance to or contraindication for conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA).
Dosage: Induction: 160 mg at week 0 and 80 mg at week 2. Maintenance: 40 mg every other week. Corticosteroids may be tapered in accordance with clinical guidelines. If insufficient response, consider increasing to 40 mg every week or 80 mg every other week. Do not continue treatment beyond 8 weeks if no clinical response. Paediatric ulcerative colitis: treatment of moderately to severely active ulcerative colitis in paediatric patients from 6 years of age with an inadequate response to conventional therapy including corticosteroids and/or 6-MP or AZA, or who are intolerant to or have medical contraindications for such therapies. Dosage: < 40 kg: Induction: 80 mg at week 0 and 40 mg at week 2. Maintenance starting at week 4: 40mg EOW. Dosage: ≥ 40 kg: Induction: 160 mg week 0 and 80 mg week 2. Maintenance starting at week 4: 80mg every other week. Patients reaching 18 years should continue their prescribed maintenance dose. Reconsider treatment beyond 8 weeks if no clinical response. No relevant use in children <6 years. Uveitis: treatment of non-infectious intermediate, posterior and panuveitis in adults with inadequate response to corticosteroids, in patients in need of corticosteroid-sparing, or in whom corticosteroid treatment is inappropriate. Dosage: 80 mg at week 0 and 40 mg every other week from week 1. There is limited experience in initiation of treatment with adalimumab alone. Treatment can be initiated in combination with corticosteroids and/or with other non-biologic immunomodulatory agents. Concomitant corticosteroids may be tapered starting two weeks after initiating treatment with AMGEVITA®. Evaluate on a yearly basis the benefit and risk of continued treatment. Paediatric uveitis: treatment of paediatric chronic non-infectious anterior uveitis in patients from 2 years of age who have had an inadequate response to or are intolerant to conventional therapy, or in whom conventional therapy is inappropriate. Dosage: ≥ 30 kg: 40 mg every other week in combination with MTX. A loading dose of 40 mg (< 30 kg) or 80 mg (≥ 30 kg) may be administered 1 week in advance of maintenance therapy. No clinical data on use of loading dose in patients < 6 years. Evaluate benefit and risk of continued treatment on a yearly basis. Contraindications: Hypersensitivity to the active substance or to any excipients; Active tuberculosis (TB) or other severe infections such as sepsis and opportunistic infections; Moderate to severe heart failure (NYHA class III/IV). Warnings and Precautions: Traceability: Clearly record the name and batch number. Infections: Patients taking TNF-antagonists are more susceptible to serious infections, especially if impaired lung function. Monitor for infections, before, during and for 4 months after treatment. Do not initiate treatment during an active infection, until infection is controlled. Consider risk/benefit prior to treatment in patients exposed to TB or who have travelled in areas of high risk of TB or endemic mycoses. Evaluate new infections and monitor closely. Stop



WHAT’S BEHIND AMGEVITA® (ADALIMUMAB) MAKES THE DIFFERENCE
Track record
AMGEVITA® is backed by nearly four decades of experience in biologics, quality Irish manufacturing and a history of reliable product supply.1
Tried and tested
Similar safety, efficacy and immunogenicity to the originator proven in multiple trials# of AMGEVITA® in over 800 patients. 2,3
Patient friendly
AMGEVITA® is citrate-free, latex-free with patient friendly SureClick® pre-filled device.4,5
>40
treatment if new serious infection or sepsis and treat. Exercise caution in patients with a history of recurring infections or who are predisposed to infections, including the use of concomitant immunosuppressive medications. Serious Infections: Consult SmPC for details. Serious infections, including those associated with hospitalisation or death, were reported in patients receiving treatment. TB: Reactivation and new onset TB, both pulmonary and extra-pulmonary (disseminated). Screen all patients before therapy initiation for active or inactive (latent) TB. Perform detailed medical assessment and appropriate screening tests for TB in all patients and results recorded on patient reminder card. If latent TB is suspected, consult physician with appropriate expertise Despite prophylaxis, TB reactivation has occurred on adalimumab. If active TB is diagnosed, do not initiate AMGEVITA® treatment. Other opportunistic infections: Opportunistic infections were observed in patients receiving adalimumab. Stop AMGEVITA® in patients with signs and symptoms of such infections. Consult with physician with appropriate expertise for diagnosis and administration of empiric antifungal therapy. Hepatitis B reactivation: Reactivation of HBV has occurred in chronic carriers; some cases were fatal. Test patients for HBV infection before initiating treatment. HBV carriers should consult a specialist physician and be closely monitored for reactivation of HBV infection throughout therapy and for several months after treatment. If reactivation occurs, stop treatment and initiate appropriate antiviral and supportive treatment. Neurological events: Caution in patients with pre-existing or recent-onset central or peripheral nervous system demyelinating disorders. Consider discontinuation if any of these develop. Perform neurologic evaluation in patients with non-infectious intermediate uveitis prior to initiation of treatment and regularly during treatment. Allergic reactions: Reports of serious allergic reactions including anaphylaxis. For serious allergic or anaphylactic reaction, stop AMGEVITA® immediately and initiate appropriate therapy. Malignancies and lymphoproliferative disorders: A possible risk of malignancy, some fatal, including lymphomas and leukaemia, cannot be excluded (see SmPC). Examine all patients, especially those with a medical history of extensive immuno-suppressant therapy or psoriasis patients with a history of PUVA treatment, for non-melanoma skin cancer prior to and during treatment. Melanoma and Merkel cell carcinoma have also been reported. Caution in COPD patients, and in patients with increased risk for malignancy due to heavy smoking. Consider the potential risk with the combination of AZA or 6-MP and AMGEVITA® (hepatosplenic T-cell lymphoma has occurred). Caution in patients with a history of malignancy. Risk of developing dysplasia or colon cancer unknown. Screen patients with UC, history of dysplasia or colon carcinoma, for dysplasia before and during treatment. Haematologic reactions: Adverse events of the haematologic system, including medically significant cytopenia, have been reported. Advise patients to seek immediate medical attention if signs and symptoms of blood dyscrasias develop. Vaccinations: Patients may receive concurrent vaccinations, except for live vaccines. Bring paediatric patients up to date with all immunisations prior to initiating treatment. Congestive heart failure: See contraindications. Caution in mild heart failure (NYHA class I/II). Discontinue treatment if new or worsening symptoms of congestive heart failure. Autoimmune processes: Autoimmune antibodies may form. Stop treatment if development of a lupus-like syndrome with positive antibodies against double-
stranded DNA. Surgery: Consider the long half-life of AMGEVITA® for planned surgical procedures. Monitor closely for infections. There is limited safety experience in patients undergoing arthroplasty or surgical procedures. Elderly patients: Serious infections were higher in patients over 65, some of which were fatal. Consider risk of infections in these patients. Interactions: Antibody formation was lower when AMGEVITA® was given together with MTX rather than as monotherapy. Combination of AMGEVITA® with other biologic DMARDs (e.g. anakinra and abatacept) or other TNF-antagonists is not recommended. Fertility, pregnancy and lactation Only use during pregnancy if clearly needed. Women of childbearing age to consider use of adequate contraception during and for at least 5 months after the last treatment. Administration of live vaccines (e.g. BCG) to infants exposed to AMGEVITA® in utero is not recommended for 5 months following the mother’s last injection during pregnancy. AMGEVITA® can be used during breastfeeding. Effects on ability to drive and use machines: AMGEVITA® may have a minor influence on the ability to drive and use machines. Vertigo and visual impairment may occur. Adverse Reactions: Very common ≥ 1/10: Respiratory tract infections; including lower and upper respiratory tract infection, pneumonia, sinusitis, pharyngitis, nasopharyngitis and pneumonia herpes viral, leukopenia; including neutropenia and agranulocytosis, anaemia, lipids increased, headache, abdominal pain, nausea and vomiting, elevated liver enzymes, rash; including exfoliative rash, musculoskeletal pain, injection site reaction; including erythema. Common ≥ 1/100 to < 1/10: Systemic infections; including sepsis, candidiasis and influenza intestinal infections; including gastroenteritis viral skin and soft tissue infections; including paronychia, cellulitis, impetigo, necrotising fasciitis and herpes zoster ear infections, oral infections; including herpes simplex, oral herpes and tooth infections, reproductive tract infections; including vulvovaginal mycotic infection, urinary tract infections; including pyelonephritis, fungal infections, joint infections, skin cancer excluding melanoma, benign neoplasm, leucocytosis, thrombocytopenia, hypersensitivity, allergies, hypokalaemia, uric acid increased, blood sodium abnormal, hypocalcaemia, hyperglycaemia, hypophosphataemia, dehydration, mood alterations, anxiety, insomnia, paraesthesia; including hypoaesthesia, migraine, nerve root compression, visual impairment, conjunctivitis, blepharitis, eye swelling, vertigo, tachycardia, hypertension, flushing, haematoma, asthma, dyspnoea, cough, GI haemorrhage, dyspepsia, gastroesophageal reflux disease, Sicca syndrome, worsening or new onset of psoriasis; including palmoplantar pustular psoriasis, urticaria, bruising; including purpura, dermatitis; including eczema, onychoclasis, hyperhidrosis, alopecia, pruritus, muscle spasms; including blood creatine phosphokinase increased, renal impairment, haematuria, chest pain, oedema, pyrexia, coagulation and bleeding disorders; including including activated partial thromboplastin time prolonged, autoantibody test positive; including double stranded DNA antibody), blood lactate dehydrogenase increased, impaired healing. Serious adverse reactions have been reported; AMGEVITA® may affect the immune system and the body’s defence against infection and cancer. Fatal and life-threatening infections; including sepsis, opportunistic infections and TB, HBV reactivation and various malignancies (including leukaemia, lymphoma and HSTCL) have also been reported. Serious haematological, neurological and autoimmune reactions have also been reported. These include rare reports of
pancytopenia, aplastic anaemia, central and peripheral demyelinating events and reports of lupus, lupus-related conditions and Stevens-Johnson syndrome. Pharmaceutical Precautions: Store in a refrigerator (2°C – 8°C). Do not freeze. Keep the prefilled syringe or pre-filled pen in the outer carton in order to protect from light. The prefilled syringe or pre-filled pen may be stored up to a maximum of 25C for up to 14 days. The pre-filled syringe or pre-filled pen must be protected from light and discarded if not used within the 14-day period. Legal Category: POM.
Presentation and Marketing Authorisation Number: AMGEVITA® 20 mg solution for injection in pre-filled syringe: EU/1/16/1164/001 – 1 pack, AMGEVITA® 40 mg solution for injection in pre-filled syringe: EU/1/16/1164/003 – 2 pack AMGEVITA® 40 mg solution for injection in pre-filled pen: EU/1/16/1164/007 – 2 pack; AMGEVITA ® 20 mg/0.2ml solution for injection in pre-filled syringe: EU/1/16/1164/010 – 1 pack, AMGEVITA® 40 mg/0.4ml solution for injection in pre-filled syringe: EU/1/16/1164/012 – 2 pack, AMGEVITA® 40 mg/0.4ml solution for injection in pre-filled pen: EU/1/16/1164/015 – 2 pack, AMGEVITA® 80 mg/0.8ml solution for injection in pre-filled pen: EU/1/16/1164/020 – 1 pack,. Marketing
Authorisation Holder: Amgen Europe B.V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. AMGEVITA® is a registered trademark of Amgen Inc. Date of PI preparation: May 2024 (Ref: IRL-501-0424-80003)
1. Amgen Data on File REF-101991 (last accessed 30th of August 2024).
2. Papp K, et al. J Am Acad Dermatol 2017. 3. Cohen S, et al. Ann Rheum Dis 2017. 4. AMGEVITA® (adalimumab) summary of product characteristics including supporting information. 5. Amgen Data On File Hub-AMB-2000001 (last accessed 30th August 2024). Date of prep to May 2024 IRL-501-0524-80001

Health: Andropause
The Silent Shift: Exploring Andropause Through an Irish Clinical Lens
Across Ireland, discussions about men’s hormonal health remain muted. While women’s menopause is widely recognised and supported, its male counterpart, often referred to as andropause or late-onset hypogonadism, is still surrounded by uncertainty. The condition involves a gradual reduction in testosterone levels that can, in some men, cause fatigue, low mood, loss of libido and reduced muscle mass. It is not universal and not inevitable, but where it occurs, it can have a profound effect on quality of life. For Ireland’s hospitals and pharmacists, the challenge lies in bringing structured, evidence-based care to an area often clouded by stigma and misinformation.
Testosterone decline begins subtly. On average, levels drop by about one per cent each year from the age of forty onwards, but age alone does not make a diagnosis. The Health Service Executive (HSE) advises that in many cases, symptoms thought to stem from “male menopause” are in fact related to stress, lifestyle, or other medical issues rather than hormones. Irish clinicians agree that it is vital to distinguish between normal ageing and genuine testosterone deficiency. Consultant urologist Dr John Sullivan of St James’s Hospital, Dublin, describes the reality he sees in his clinics: “Irish men don’t go down the pub and talk about how their sex drive, or that their energy has decreased. There’s a lot of silence and often embarrassment, but there is support and structured treatment when it’s truly needed.”
Symptoms that prompt review typically include persistent tiredness, low libido, erectile dysfunction, mood changes, and physical deconditioning. Dr Emmett Byrne, GP and men’s health specialist at the Preventive Health Clinic in Bray, highlights the pattern he sees most often: “The three lead symptoms are low libido, loss of earlymorning erections and erectile dysfunction. Fatigue and anxiety are common too.” His view reflects a broader shift among Irish GPs and hospital teams who are becoming increasingly aware of the condition, though not all cases warrant testosterone therapy.
Before treatment, assessment must be rigorous. Morning blood tests, ideally taken between 7 am and 11 am after fasting, are the foundation. If testosterone levels are low or borderline, testing should be repeated. Total testosterone is measured alongside sex hormonebinding globulin (SHBG) and albumin, which help calculate free testosterone. Additional investigations such as luteinising hormone (LH), follicle-stimulating hormone (FSH), thyroid function and prolactin levels help identify primary or secondary causes. Importantly, results are always interpreted in conjunction with symptoms and clinical context.
Testing that stands up to scrutiny
1. When to test: Morning samples after adequate rest and fasting, avoiding periods of acute illness.
2. What to order: Total testosterone, SHBG, and albumin for free testosterone calculation; LH, FSH, prolactin and thyroid tests where appropriate.
3. Thresholds: Consistent low results with clear symptoms before considering therapy.
Lifestyle factors must always be addressed first. Sleep, diet, exercise, and mental wellbeing all have measurable effects on testosterone. Irish clinicians frequently see men whose hormone levels normalise after weight loss, improved sleep or treatment of sleep apnoea. Dr Byrne notes: “We often find that once weight, stress and sleep are managed, testosterone levels rise naturally. It’s about treating the whole man, not just the lab result.”
Hospital pharmacists occupy a vital role in this continuum. Many medicines can suppress testosterone, including opioids, glucocorticoids, and certain psychotropics. Pharmacists can identify these risks, advise prescribers and educate patients. They also manage the safe use of replacement therapies, ensuring that monitoring is consistent and that formulations are used correctly.
When replacement is appropriate
Once reversible causes are
addressed and testosterone deficiency is confirmed on repeat testing, therapy may be appropriate. Treatment must be individualised and evidence based. Indications include confirmed low testosterone with compatible symptoms that persist despite lifestyle modification.
Contraindications include active or high-risk prostate cancer, severe untreated sleep apnoea, uncontrolled heart failure, elevated haematocrit, and a desire for fertility.
In Ireland, transdermal gels and patches are commonly prescribed, offering steady absorption and easier dose adjustment. Intramuscular injections remain an option for some men, though they require close follow-up due to fluctuations in hormone levels. Whichever formulation is chosen, adherence and patient education are critical. Pharmacists often lead on this, advising patients about correct gel application, preventing skin transfer, and ensuring consistent timing of doses.
Monitoring is non-negotiable. Testosterone levels, haematocrit, prostate-specific antigen (PSA), blood pressure, and lipids should be reviewed regularly. Dr Sullivan warns: “There’s a lot of underground prescribing of testosterone online, and that’s dangerous. A man showing symptoms must be thoroughly assessed and followed up by a doctor.” Ongoing surveillance ensures that benefits are maintained while minimising risks such as erythrocytosis, hypertension, or prostate complications.
The cardiovascular and prostate safety of testosterone therapy has been debated internationally, but recent evidence is reassuring when therapy is used appropriately. Large trials have shown no increase in major cardiovascular events in men treated for clinically confirmed hypogonadism under supervision. Nevertheless, the Irish clinical community remains cautious. As Dr Forde, an Irish GP quoted in Farmers Journal, explains: “If you give a man testosterone who doesn’t need it, you risk stimulating too much
red blood cell production, which can lead to clots. More studies are needed, but used correctly, it’s safe and effective.”
From an Irish hospital perspective, the ideal pathway is clear. Primary care flags suspected cases: endocrinology or urology confirms diagnosis and initiates treatment if appropriate; pharmacy provides patient education, dosage oversight, and safety monitoring. The collaboration between these departments ensures that testosterone therapy is used judiciously and safely. The ultimate aim is to support men’s health in a way that reflects the same clinical seriousness and compassion applied to women’s hormonal care.
A patient story from Dublin illustrates the point. In his early fifties, a man presented with low energy and loss of libido. His BMI was 32, placing him in the obese category, and he had early type 2 diabetes. Tests revealed low testosterone, but a sleep study uncovered moderate obstructive sleep apnoea. Continuous positive airway pressure (CPAP) was introduced, and weight management support began. After six months his testosterone levels had risen naturally and his symptoms resolved, without the need for hormone therapy. Treating the cause rather than the number proved to be the key.
Ireland is beginning to talk more openly about male hormonal health. Awareness campaigns by men’s health advocates and clinicians are slowly dismantling stigma. “Irish men are far less likely to discuss sexual or hormonal issues,” says Dr Sullivan, “but we are starting to see a change in attitude. Understanding andropause is the next step in ensuring men receive the care they deserve.”
For hospitals and pharmacists, the responsibility now lies in structure and safety. Clear referral pathways, consistent monitoring, and public education are essential. Andropause may never attract the same visibility as menopause, but it deserves the same rigour, empathy and professionalism in care.
Start with OMJJARA
momelotinib
See patients with myelofibrosis and anaemia in a
Omjjara is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with moderate to severe anaemia who have primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis and who are Janus Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. 1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
ACVR1 = activin A receptor, type 1; JAKi = Janus Kinase inhibitor.

Learn more about Omjjara:
Prescribing Information and Important Safety Information Omjjara Summary of product characteristics, available at www.medicines.ie. Accessed June 2025
Abbreviated Prescribing Information
Omjjara 100, 150 and 200 mg film-coated tablets Abbreviated Prescribing Information (Refer to Summary of Product Characteristics (SmPC) before prescribing). PRESENTATONS: Each 100 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 100 mg of momelotinib and 50.8 mg of lactose monohydrate. Each 150 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 150 mg of momelotinib and 76.1 mg of lactose monohydrate. Each 200 mg tablet contains momelotinib dihydrochloride monohydrate equivalent to 200 mg of momelotinib and 101.5 mg of lactose monohydrate. INDICATION: The treatment of disease-related splenomegaly or symptoms in adult patients with moderate to severe anaemia who have primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis and who are Janus Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. POSOLOGY AND ADMINISTRATION: Treatment should be initiated and supervised by a physician experienced in the use of anticancer medicinal products. Omjjara should not be used in combination with other JAK inhibitors. The recommended dose is 200 mg once daily. Complete blood cell count and liver function tests must be performed before initiating treatment, periodically during treatment, and as clinically indicated. For dose modifications due to adverse reactions see SmPC. Treatment with Omjjara should be discontinued in patients unable to tolerate 100 mg once daily. Duration of use: Treatment may be continued for as long as the benefit-risk remains positive for patients, as assessed by the treating physician. Missed dose: If a dose of Omjjara is missed, the next scheduled dose should be taken the following day. Two doses should not be taken at the same time to make up for the missed dose. Elderly (≥ 65 years): No dose adjustment necessary. Renal impairment (>15 mL/min): No dose adjustment necessary. Omjjara has not been studied in patients with end-stage renal disease. Hepatic impairment: No dose adjustment is recommended for patients with mild or moderate hepatic impairment. The recommended starting dose is 150 mg once daily in patients with severe hepatic impairment (Child-Pugh Class C). Paediatric population: No data available. Omjjara is for oral use only and can be taken with or without meals. CONTRAINDICATIONS: Hypersensitivity to momelotinib or to any of the excipients. Pregnancy and breast-feeding. WARNINGS/PRECAUTIONS: Omjjara should not be initiated in patients with active infections. Physicians should carefully observe patients for signs and symptoms of infection and initiate appropriate treatment promptly. Patients with chronic HBV infection who receive Omjjara should have their chronic HBV infection treated and monitored according to clinical HBV guidelines. A complete blood count including platelet count should be obtained before initiating treatment with Omjjara, periodically during treatment, and as clinically indicated. Dose interruption or reduction may be required. Liver function tests should be obtained before initiating treatment with Omjjara, periodically during treatment, and as clinically indicated. If increases in ALT, AST or bilirubin related to treatment are suspected, dose interruption or reduction may be required. Prior to initiating or continuing therapy with Omjjara, the benefits and risks for the individual patient should be considered particularly in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately. Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including Omjjara. However, a causal association has not been established Women using systemically acting hormonal contraceptives should add a barrier method during treatment and for at least 1 week after the last dose of Omjjara. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Omjjara. Patients who experience dizziness or blurred vision after taking Omjjara should observe caution when driving or using machines INTERACTIONS: Co administration of strong CYP3A4 inducers may lead to decreased momelotinib exposure and consequently a risk for reduced efficacy. Therefore, additional monitoring of the clinical signs and symptoms of myelofibrosis is recommended with concomitant use of momelotinib and strong CYP3A4 inducers (including but not limited to carbamazepine, phenobarbital, phenytoin, and St John’s wort [Hypericum perforatum]). Caution and monitoring for adverse reactions are advised with concomitant use of OATP1B1/1B3 inhibitors, including ciclosporin. Momelotinib may increase exposure to other sensitive BCRP substrates, including sulfasalazine; monitor for adverse reactions. Caution is advised when administering momelotinib with P-gp substrates with a narrow therapeutic index. Caution is advised when administering momelotinib with sensitive substrates of OCT1, MATE1 and MATE2-K (e.g., metformin). Narrow therapeutic index or sensitive substrate medicinal products of CYP1A2 (e.g., theophylline, tizanidine) or CYP2B6 (e.g., cyclophosphamide) should be co-administered with momelotinib with caution. Fertility, pregnancy and lactation: Fertility: No clinical data. Omjjara is contraindicated during pregnancy. If Omjjara is used during pregnancy, or if the patient becomes pregnant while taking this medicinal product, the patient should discontinue treatment and be advised of the potential hazard to the foetus. Omjjara is contraindicated during breast-feeding. UNDESIRABLE EFFECTS: Very common (≥ 1/10): Thrombocytopenia, dizziness, headache, cough, diarrhoea, abdominal pain, asthenia, fatigue. Common (≥ 1/100, < 1/10): Urinary tract infection, upper respiratory tract infection, pneumonia, nasopharyngitis, COVID19, cystitis, bronchitis, oral herpes, sinusitis, herpes zoster, cellulitis, respiratory tract infection, sepsis, lower respiratory tract infection, oral candidiasis, rash, skin infection, gastroenteritis, neutropenia, Vit B1 deficiency, syncope, peripheral neuropathy, paraesthesia, blurred vision, vertigo, hypotension, haematoma, flushing, vomiting, constipation, arthralgia, pain in extremity, pyrexia, ALT increased, AST increased, contusion. For more details on undesirable effects, see SmPC. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nrs: EU/1/23/1782/001- 003. Legal category: POM A. Date of preparation of API: April 2025. Code: PI-14515. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Trademarks are owned by or licensed to the GSK group of companies. @2025 GSK or licensor. PM-IE-MML-JRNA-250001 | July 2025
References: 1. Omjjara Summary of product characteristics, available at www.medicines.ie. Accessed August 2025









Hospital Professional
As we look ahead into 2026, now is the time to reflect on those who have been, and who will be, empowering others moving into the future.
We’re marking the end of 2025 with our unique HPN Professional Top 100 – a prestigious annual list which celebrates Ireland’s top hospital and healthcare achievers.
The HPN Top Professional Top 100 is a carefully constructed network of the most influential hospital professionals across a range of disciplines, both in the healthcare and pharma industries, who act as ambassadors and role models for their peers and colleagues.
Over the past many months, our team of writers has reached out to pharmacists, consultants, clinical specialists, representative bodies and organisations throughout the sector and encouraged them to put forward those colleagues they feel are redrawing the landscape for the future.
Those who have made our final list have displayed motivating behaviour and inspirational leadership affecting the development of hospital services across Ireland.



























































































Professional 100

Dr Peter Barrett, HRB Clinician Scientist Research Fellow/Consultant, Public Health Medicine, HSE
Dr Peter Barrett, HRB Clinician Scientist Postdoctoral Fellow and Consultant in Public Health Medicine (HSE South West), continues to reshape Ireland’s public health research landscape in 2025. The first public health physician ever awarded a HRB Clinician Scientist Fellowship, he leads groundbreaking work at the INFANT Research Centre, UCC, using international maternal health records to predict and prevent agerelated chronic diseases in women.
A UCC medical graduate with Masters degrees from Karolinska Institutet and the University of Cambridge, Dr Barrett is also the first alumnus of the prestigious Wellcome Trust–HRB ICAT Programme, completing a PhD in large-scale epidemiology. His dynamic portfolio spans maternal health, infectious disease, substance use and sexual health, while he actively mentors students and trainees, strengthening Ireland’s future public health workforce.
Dr Bibi Ayesha Bassa, Clinician-Scientist & National Leader in VTE Research
Dr Bibi Ayesha Bassa is one of Ireland’s most dynamic early-career clinician-scientists, driving major advances in venous thromboembolism (VTE) understanding within trauma and emergency care. Her doctoral research at RCSI—focused on VTE risks in spinal cord injury and major trauma—has produced influential, practice-changing publications across The Surgeon, Injury, EMJ and more. Her work has shaped national guidelines and informed clinical practice within the Mater Major Trauma Centre. As Co-Chair of INViTE and Evidence Synthesis Lead for the National VTE Clinical Advisory Group, she plays a pivotal role in translating research into policy and education. Recognised with multiple national awards, Dr Bassa is positioning Ireland as a global leader in VTE research and trauma-care innovation.


Dr Eimear Brannigan, Clinical Lead AMRIC, HSE
Dr Eimear Brannigan is a nationally recognised leader in infectious diseases and antimicrobial stewardship, serving as Consultant in Infectious Disease and National Clinical Lead for Antimicrobial Resistance and Infection Control (AMRIC) within the HSE. At a time when antibiotic resistance poses one of the greatest threats to modern medicine, Dr Brannigan has been at the forefront of safeguarding the effectiveness of these life-saving treatments. Through clinical leadership, national policy development and public engagement, she has championed responsible antibiotic use across healthcare, agriculture and the wider community. Her work underpins patient safety in everything from routine care to complex surgery, cancer treatment and organ transplantation, ensuring Ireland remains aligned with global efforts to combat antimicrobial resistance and protect the future of healthcare.
Professional 100
Professor Stephen Byrne, Deputy President/Registrar, School of Pharmacy, University College Cork
In 2025, Prof Stephen Byrne has been dynamic in shaping the academic and professional landscape of pharmacy. His leadership in advancing pharmacy education, promoting evidence-based practice, and strengthening research output has had a significant impact across the sector. Stephen has been instrumental in modernising curricula to prepare students for expanded clinical roles, ensuring graduates enter the workforce with advanced skills in prescribing, digital health, and patient care. He has also championed collaborations between academia, healthcare providers, and policymakers to drive innovation and workforce development. Through his mentorship and vision, he continues to inspire the next generation of pharmacists. His influence, energy, and strategic direction make him a truly dynamic contributor to the profession in 2025.


Professor Karen Cadoo, Cancer Geneticist and Medical Oncologist, St James’s Hospital
In 2025, Professor Karen Cadoo continued to shape the future of oncology in Ireland through her leadership in hereditary cancer, precision medicine and clinical trials. A Consultant Medical Oncologist at St James’s Hospital and Associate Professor at Trinity College Dublin, Professor Cadoo is recognised for driving national advances in early detection and personalised cancer therapy. Her research in genomics, immunotherapy and targeted therapeutics continues to influence international practice, while her leadership within Cancer Trials Ireland has expanded access to cutting-edge studies for Irish patients. A passionate advocate for equity, she works to ensure that every patient — regardless of background — benefits from scientific progress. Prof Cadoo remains one of Ireland’s most impactful voices in modern cancer care.
Professor Ronan Cahill, Consultant Surgeon at the Mater Misericordiae University Hospital/Professor of Surgery, University College Dublin
Professor Ronan Cahill, UCD Professor of Surgery and Consultant Colorectal Surgeon at the Mater Misericordiae University Hospital, was honoured in 2025 as a Fulbright Irish Award recipient — recognising his outstanding contribution to surgical science and global academic collaboration. As Director of the UCD Centre for Precision Surgery and the Mater’s Digital Surgery Unit, he is internationally regarded for pioneering advancements in minimally invasive cancer surgery. His Fulbright research at the University of Pennsylvania will focus on accelerating the integration of artificial intelligence, telesurgery and computer-assisted systems into operative practice. Professor Cahill’s work is helping to shape the next era of surgical care, enhancing precision, safety and outcomes for patients in Ireland and beyond.


Professor Mary Cannon, Consultant Psychiatrist, Beaumont Hospital/Professor of Psychiatric Epidemiology & Global Leader in Youth Mental Health, Royal College of Surgeons Ireland
Professor Mary Cannon, Consultant Psychiatrist at RCSI and Beaumont Hospital, continues to be one of Ireland’s most influential mental health researchers. In 2025, her internationally recognised work on early-life risk factors, psychosis, and youth mental health informed national policy and global research agendas. Prof Cannon leads pioneering studies examining how childhood adversity, neurodevelopmental factors and social environments shape long-term mental health outcomes. Her research has directly contributed to improved prevention strategies, earlyintervention services and public awareness campaigns. A regular expert voice in national mental health reform, she champions evidence-based practice and accessible care for young people. Through groundbreaking publications and mentorship, Prof Cannon has helped position Ireland at the forefront of psychiatric epidemiology.
Professional 100

Dr Aine Carroll, Professor of Integrated Care and Improvement at University College Dublin
Dr Áine Carroll, Consultant in Rehabilitation Medicine at the National Rehabilitation Hospital and Professor of Healthcare Integration at UCD, remains one of Ireland’s most influential health-system reform leaders. In 2025, her work advancing integrated care models, clinical redesign and person-centred pathways continued to guide national policy and HSE transformation programmes. As former National Clinical Lead for Integrated Care, Dr Carroll has shaped how services for chronic disease, disability and complex needs are delivered across the country. Her research and international consultancy in implementation science and system design are widely recognised. A champion of co-production, digital enablement and workforce innovation, Dr Carroll is driving a more coordinated, efficient and compassionate Irish health service.
Dr Nicholas Clarke, Programme Director, Converge: Centre for Chronic Disease and Population Health Research.
Dr Nicholas Clarke, Research Fellow and Coordinator of the Converge Programme, is a rising leader in epidemiology and population health. With an MSc in Applied Social Research (TCD) and a PhD in Epidemiology and Public Health (UCC), he was awarded a prestigious HRB Emerging Investigator Award in 2022. He now oversees four major Converge research streams, spanning chronic disease epidemiology, intervention effectiveness, health-impact modelling and global health.
Dr Clarke has led influential research in cancer screening, head and neck cancer survival and health-services improvement. As principal investigator on multiple national studies, he is advancing strategies to increase BowelScreen uptake, address screening barriers among marginalised women, and understand defensive information processing across Ireland’s screening programmes—driving more equitable, evidence-based cancer prevention.


Professor Richard Costello, RCSI Department of Medicine/ Consultant Respiratory Physician at Beaumont Hospital
Professor Richard Costello is a globally recognised respiratory specialist whose groundbreaking research has led to the launch of Phyxiom, one of Ireland’s most exciting digital health innovations. A Professor of Respiratory Medicine at RCSI and Consultant at Beaumont Hospital, he has spent over a decade developing clinically validated digital tools that transform asthma and COPD management. His work—published in Lancet Respiratory Medicine and supported by over ¤15 million in competitive funding—has shown that digitally informed care reduces medication use, prevents hospitalisations, and improves diagnostic accuracy. As Co-Founder and CMO of Phyxiom, he is driving a new era of objective, real-time respiratory monitoring that empowers clinicians, enhances adherence, and delivers better patient outcomes.
Dr Conor Costigan, Gastroenterology Specialist Registrar, Tallaght University Hospital
Dr Conor Costigan has helped pioneer a major innovation in gastrointestinal diagnostics at Tallaght University Hospital, leading research that demonstrates the transformative impact of telemedicine and at-home testing for Helicobacter pylori. His study—published in the Journal of Clinical Medicine—showed that the at-home C13 Urea Breath Test offers a fast, accurate, patient-friendly alternative to traditional clinic-based testing. Reviewing 423 virtual appointments, Dr Costigan and the TUH GI Laboratory Team reported outstanding engagement, with zero non-attenders, minimal cancellations, and diagnostic accuracy matching in-person services. By enabling earlier diagnosis, reducing hospital visits, and improving patient experience, Dr Costigan’s work highlights how virtual care can meaningfully enhance modern gastroenterology and shape the future of community-based diagnostics in Ireland.


Elmarie Cottrell, Chief II Pharmacist & Operational Lead, HMMS
Elmarie Cottrell is a key national leader in digital medicines management within the HSE. As Operational Lead for the Hospital Medicines Management System (HMMS) within the AIDMP, she is driving one of Ireland’s most significant pharmacy digital-transformation projects. HMMS replaces outdated stock-control systems with a modern, data-driven platform that delivers real-time visibility of medicine use, procurement, and cost. Under Elmarie’s leadership, hospitals across acute and non-acute settings have successfully transitioned to HMMS, with six sites already live. Her strategic vision, strong collaboration with pharmacy teams, and commitment to modernising medicine processes make her an influential force in Ireland’s digital-health future. In 2025, she further strengthened her leadership credentials by completing the IMI Professional Diploma in Strategic Transformational Leadership.
Dr Miriam Coughlan, Chief II Pharmacist, St James’s Hospital Dublin
Dr Miriam Coughlan has been pivotal in advancing digital medication safety at St James’s Hospital in 2025. Under her leadership, the pharmacy team rolled out closed-loop medication administration (CLMA) technology, reducing dispensing errors and streamlining patient record integration. She also co-ordinated a training initiative across clinical wards to embed safe-prescribing culture. Dr Coughlan’s vision and data-driven approach exemplify how pharmacy leadership enhances quality and governance in major tertiary hospitals.


Dr Rebecca Cramp, Director of Code and Regulatory Affairs, IPHA
As Director of Code and Regulatory Affairs at IPHA, Dr Rebecca Cramp has been a leading voice for overhauling Ireland’s clinical trials system in 2025. Her message is clear: Ireland must move faster. Drawing on comparisons with Denmark and Finland—where industry trial activity far outpaces Ireland—she argues that predictable, standardised and efficient trial processes are essential if patients are to access breakthrough medicines sooner. Under her guidance, IPHA’s 2025–2026 workplan prioritises smarter trial design, streamlined hospital sign-off procedures, and dedicated clinical research resources. Dr Cramp believes that with the right reforms, Ireland can become a European leader in clinical research, giving patients earlier access to life-saving innovation.
Professional 100

Dr Suzanne Crowe, Consultant, Paediatric Intensive Care, CHI Crumlin/Clinical Senior Lecturer, Paediatrics, Trinity College Dublin
Dr Suzanne Crowe, one of Ireland’s foremost paediatric intensive care specialists and President of the Irish Medical Council, continued to shape national healthcare leadership in 2025 with the publication of her acclaimed memoir, Intensive Care: True Stories of Healing, Heartache and Hope from Inside Irish Children’s Medicine. A Clinical Associate Professor at UCD School of Medicine and Consultant Paediatric Intensivist at CHI Crumlin, Dr Crowe brings three decades of clinical expertise, research excellence and deep compassion to her work. Her book offers rare insight into the emotional and ethical complexities of caring for critically ill children, reflecting her lifelong commitment to patient advocacy, healthcare improvement and the humanity at the heart of medicine. A respected educator and author of over 50 peer-reviewed papers, she remains a powerful voice for safe, ethical and compassionate care.
Professor James Crowley, Consultant Cardiologist/ President, Irish Cardiac Society
Professor James Crowley is a leading Consultant Cardiologist whose extensive clinical and research work continues to shape cardiovascular care across Ireland. With over 140 peer-reviewed publications and a long track record at Galway University Hospitals, he remains at the forefront of interventional cardiology and cardiac research. As a former President and current Council director of the Irish Cardiac Society, he has helped guide national cardiology policy, education and training — strengthening the Irish cardiology workforce and fostering excellence. His efforts in pioneering novel interventional strategies, mentoring the next generation of cardiologists, and contributing to major cardiovascular studies underscore his commitment to improving patient outcomes and elevating Ireland’s position in global cardiology.


Professor Sally-Ann Cryan, Pharmacist, School of Pharmacy, Royal College of Surgeons Ireland
Professor Sally-Ann Cryan has been a dynamic leader in 2025, advancing pharmaceutical innovation and strengthening academic–practice partnerships. Her work in drug delivery science and translational research continues to shape national priorities in advanced therapeutics. As an educator, she champions experiential learning, interdisciplinary collaboration, and the development of practice-ready graduates. In 2025, her leadership has driven curriculum enhancement, research capacity building, and impactful engagement with regulators, policymakers, and industry. Through her mentoring, strategic vision, and commitment to scientific excellence, she continues to inspire students, researchers, and practitioners while elevating Ireland’s profile in pharmaceutical science.
Professor Martin Curley, Professor of Innovation, Maynooth University/Director, Digital Health Ecosystem
Professor Curley stands at the forefront of innovation, shaping the future with his insights, experience and transformative ideas. As the Professor of Innovation at Maynooth University and the Director of the Digital Health Ecosystem at the Innovation Value Institute, Martin drives forwardthinking innovation and transformative advancements in the digital age.
In the advisory realm, Martin has chaired the European Commission Open Innovation Strategy Policy Group, contributing to the pioneering Open Innovation 2.0 paradigm. His involvement with the European Commission Connect Advisory Forum and various governmental advisory boards underlines his significant role in shaping policies on sustainability, future workforce, and highperformance computing.

Dr Caroline Curtin, RCSI University of Medicine and Health and Sciences
Dr Caroline Curtin is a leading researcher in regenerative medicine whose innovative work is advancing the next generation of therapies for bone and cartilage repair, as well as targeted treatments for primary bone cancer. A Principal Investigator in the Tissue Engineering Research Group at RCSI, her research focuses on gene-activated scaffold systems and 3D tumour models that enable precise, localised drug delivery. In 2025, she received a prestigious award for her project developing controlled-release, non-viral gene delivery platforms for primary bone cancers—aggressive tumours that disproportionately affect children and adolescents. Dr Curtin’s work represents a transformative leap in personalised oncology, offering the potential to simultaneously eradicate tumour cells while promoting bone regeneration.


Dr Anne Dee, President, Irish Medical Organisation
Dr Anne Dee, Public Health Consultant and President of the Irish Medical Organisation (IMO), has emerged as one of Ireland’s strongest voices on health inequality in 2025. At the IMO’s Health on the Margins conference, she delivered a powerful call for Government action to address deprivation-driven health gaps from early childhood. Dr Dee warned that without urgent investment in early intervention, vulnerable children risk repeating the poor health outcomes of previous generations—placing avoidable pressure on an already overstretched health system. Her leadership emphasises whole-of-government collaboration, evidence-based prevention, and compassionate public health policy. Dr Dee’s advocacy is shaping the national conversation on social determinants of health and ensuring equity remains central to Ireland’s healthcare reform agenda.
Professor Norman Delanty, Consultant Neurologist, Beaumont Hospital/Clinical Professor of Neurology, Royal College
of Surgeons Ireland
Professor Norman Delanty, Consultant Neurologist at Beaumont Hospital and Clinical Professor of Neurology at RCSI, continues to drive major advances in epilepsy diagnostics and precision therapeutics. A leading investigator in genomic epilepsies, Professor Delanty’s 2025 work has focused on integrating real-world genomic data into clinical pathways, enabling earlier identification of rare syndromes and more personalised treatment strategies. As Director of the Epilepsy Programme at Beaumont, he champions multidisciplinary care and digital innovation to improve outcomes for complex patients. Internationally recognised for contributions to epilepsy genetics and biomarker discovery, Professor Delanty’s leadership is helping position Ireland at the forefront of next-generation neurology, where targeted therapies and precision diagnostics are transforming patient lives.

Professional 100

Ciara Ni Dhubhlaing, Pharmacist, St Patrick’s Mental Health Services
Ciara Ní Dhubhlaing is one of Ireland’s leading specialist psychiatric pharmacists, driving innovation, education and excellence in mental healthcare. With over 20 years’ experience across community and hospital settings in Ireland and the UK, she now serves as Pharmacist Executive Manager at St Patrick’s Mental Health Services. Ciara is credentialed at advanced level with the College of Mental Health Pharmacy (CMHP), where she has also served as President, and she plays a national leadership role as Chair of the Irish Institute of Pharmacy Working Group on Mental Health and as an Executive Member of HPAI.
A qualified Independent Prescriber, Ciara has published impactful research on clozapine counselling, benzodiazepine optimisation, antipsychotic stewardship and metabolic health in mental illness. She is a committed mentor, lecturer and peer reviewer, fostering a culture of continuous learning and research within St Patrick’s. Her work continues to elevate the role of psychiatric pharmacy and improve outcomes for people living with mental health conditions.
Professor Seán Dinneen, Consultant Endocrinologist, Galway University Hospital/Professor of Diabetic Medicine, University of Galway
Professor Seán Dinneen remains a driving force in reshaping Ireland’s diabetes services. In 2025, his leadership of the National Clinical Programme for Diabetes continues to deliver meaningful improvements in integrated care, structured education, and equitable service access. An internationally respected researcher, Prof Dinneen is known for championing patient-centred systems redesign, coproduction with service users, and evidence-driven policy. His ongoing work to modernise diabetes pathways—particularly in Type 1 diabetes and technologysupported care—has positioned Ireland as a model for collaborative chronic disease management. Prof Dinneen’s vision, grounded in compassion and scientific rigour, continues to influence national strategy and elevate diabetes care standards.


Professor Frank Doyle, Professor of Psychology and Behavioural Medicine, Royal College of Surgeons Ireland
Professor Frank Doyle continues to shape national healthcare through rigorous, policy-relevant research. In 2025, he secured ¤219,960 in Research Ireland COALESCE funding to lead RENOVATE, a major new study designed to transform how depression is treated across the Irish health system. Working with co-investigator Dr Fiona Boland, Prof Doyle will evaluate the clinical effectiveness and cost-efficiency of pharmacological and non-pharmacological treatments, generating robust evidence to inform patient-centred, financially sustainable care pathways. His work will also identify optimal approaches for specific populations, ensuring real-world impact. A leader in health psychology, behavioural science, and chronic disease research, Prof Doyle continues to anchor Ireland at the forefront of evidence-driven healthcare innovation.
Professor Fidelma Dunne, Professor in Medicine at the School of Medicine and Associate Director of the Clinical Research Facility (CRF) at University of Galway
In 2025, Professor Fidelma Dunne continues to lead internationally recognised research into diabetes in pregnancy and women’s metabolic health. Her team’s recent clinical studies have influenced HSE guidelines on gestational-diabetes management and glucose monitoring. As a strong advocate for collaboration, she has worked closely with pharmacists and dietitians to embed holistic diabetes-care pathways across Irish hospitals. Prof Dunne’s dual focus on clinical excellence and research translation ensures that Ireland remains at the forefront of endocrine medicine and patient-centred innovation.


Professor Joe Eustace, Professor of Medicine, Consultant Nephrologist/Director, Clinical Research Facility, Cork
Professor Joe Eustace, one of Ireland’s most respected figures in health research and clinical trial infrastructure, continued to shape national policy and innovation in 2025 through his leadership as Director of the Health Research Board Clinical Research Facility (HRB-CRF) and Chair of the National Research Ethics Committees. A Consultant Nephrologist and former Dean of Graduate Studies at UCC, Prof Eustace has been instrumental in transforming Ireland’s capacity for high-quality, patientcentred research, strengthening governance, ethical oversight, and national trial networks. His work has accelerated access to cutting-edge therapies, expanded multidisciplinary research training, and driven Ireland’s position as a competitive, internationally recognised hub for clinical research excellence. Prof Eustace’s commitment to integrity, collaboration and innovation continues to influence healthcare outcomes nationwide.
Professor Mark Ferguson, Director General of Science Foundation Ireland
Professor Mark Ferguson, Director General of Science Foundation Ireland and Chief Scientific Adviser to the Government, has overseen one of the most significant transformations in Ireland’s research landscape in 2025. A key milestone was the launch of SFI’s Shaping Our Future strategy by An Taoiseach Micheál Martin and Minister Simon Harris, setting a bold vision to unlock Irish research potential and strengthen national innovation. This year also marked the formal transition into Taighde Éireann – Research Ireland, unifying Ireland’s research funding system. Under Ferguson’s leadership, new initiatives such as New Foundations 2025 and Innovate for Ireland advanced talent development, basic research, interdisciplinarity and Ireland’s global scientific competitiveness.

Professional 100

Professor Fidelma Fitzpatrick, Consultant Microbiologist, Beaumont Hospital/Head of Department, Clinical Microbiology, Royal College of Surgeons Ireland
Professor Fidelma Fitzpatrick remains one of Ireland’s most influential voices in infection prevention, antimicrobial stewardship and healthcare quality improvement. In 2025, her leadership continued to shape national policy as she advanced evidence-based strategies to reduce healthcare-associated infections and combat antimicrobial resistance. A former Chair of the National Clinical Programme for Healthcare-Associated Infection and AMR, Professor Fitzpatrick is widely recognised for her pioneering research, her role in establishing Ireland’s IPC frameworks, and her ability to translate emerging science into actionable clinical practice. Through frontline collaboration, public advocacy and international research partnerships, she continues to drive safer, more resilient healthcare systems for patients across Ireland and beyond.
Dr John Fitzsimons, Consultant Paediatrician, Children’s Health Ireland/National Clinical Director, HSE National Quality and Patient Safety Directorate
Dr John Fitzsimons has been a driving force behind Ireland’s modern patient-safety agenda. In 2025, his leadership continued to promote system-wide improvements in clinical governance, incident management and safety culture across hospitals and community care. Dr Fitzsimons has spearheaded national frameworks that strengthen learning from adverse events, embed human-factors principles, and support healthcare teams in delivering consistently safer care. A respected clinician, educator and reformer, he advocates for transparent, person-centred systems that empower staff and protect patients. His commitment to measurable improvement and evidence-driven policy has significantly advanced Ireland’s progress towards a high-reliability health service.


Professor William
Gallagher,
Professor of Cancer Biology, School of Biomolecular & Biomedical Science UCD
Professor William Gallagher, Co-Lead of Precision Oncology Ireland and Director of the UCD Conway Institute, continues to be one of Ireland’s most visionary cancer researchers. In 2025, he played a pivotal role in advancing Phase 2 of Ireland’s largest-ever cancer research collaboration, uniting universities, industry and charities to accelerate personalised cancer diagnostics and therapeutics. Internationally recognised for his groundbreaking work on biomarkers and imaging in breast cancer, Professor Gallagher has been central in positioning Ireland as a global leader in precision oncology. His commitment to translating cutting-edge science into real-world patient benefit—combined with his entrepreneurial leadership in spin-outs and innovation—ensures that Irish cancer research not only keeps pace with global advances but helps shape the future of cancer care.
Professional 100
Dr Emer Guinan, Associate Professor, Cancer Rehabilitation & Survivorship, Trinity College Dublin
Dr Emer Guinan is a national leader in cancer rehabilitation and survivorship whose work is reshaping post-treatment care in Ireland. An Associate Professor in Trinity College Dublin’s School of Medicine, she drives education, research and clinical collaboration across the Trinity St James’s Cancer Institute. A CORU-registered physiotherapist, her research focuses on the role of exercise and rehabilitation in improving outcomes for cancer patients. As Principal Investigator of the HRB-funded PERCS 2.0 programme, she is advancing how exercise is integrated into routine cancer care. Dr Guinan’s team also co-developed cancerrehabilitation.ie, a national implementation resource for patients and clinicians. With over 100 peer-reviewed publications and ¤4.5 million in research funding, she continues to influence national cancer-care policy and service design.


Professor Gerry Hanna, Consultant, St Luke’s Radiation Oncology Network/ Marie Curie Chair of Clinical Oncology, Trinity College Dublin
Professor Gerry Hanna is driving a new era of innovation in Irish radiation oncology in 2025. Professor Hanna opened the year with his powerful inaugural lecture, “From Monotone to Symphony: The Transformation of Lung Cancer Radiotherapy.” In it, he traced the evolution of radiotherapy from Marie Curie’s foundational discoveries to today’s AI-enabled precision approaches, highlighting his own global contributions across PET-guided planning, stereotactic ablative radiotherapy, and novel drug–radiation combinations.
A former Director of Radiation Oncology at the Peter MacCallum Cancer Centre in Melbourne and now Vice Clinical Lead at Cancer Trials Ireland, Professor Hanna is advancing a national mission: to raise Ireland’s fiveyear cancer survival rate from 50% to 75% within a decade. In 2025, he launched AITRON, a groundbreaking all-Ireland translational research network connecting radiation oncology scientists to accelerate discovery and improve outcomes for lung cancer and beyond.
Professor Orla Hardiman, Professor of Neurology at Trinity College Dublin/Consultant Neurologist, Beaumont Hospital
In 2025 Professor Hardiman’s neurology research achieved major recognition with a breakthrough in early biomarkers for motor-neuron disease. Her work also includes patient-family education programmes where pharmacists collaborate to optimise disease-modifying therapy access. She continues to elevate the profile of pharmacy-neurology interactions in Ireland, underscoring how pharmacists contribute to complex neurological pathways.
Also this year Professor Hardiman was conferred with a Higher Doctorate Degree from the National University of Ireland. Throughout her career, Professor Hardiman has worked to further the understanding and awareness of the heterogenous pathological mechanisms underlying the neurodegenerative condition Amyotrophic Lateral Sclerosis (ALS) and frontotemporal dementia (FTD), to develop novel processes and technologies that can characterise disease heterogeneity and to contribute to the development of effective precision-based treatments.


Professor Anne Marie Healy, Professor, Pharmacy, School of Pharmacy, Trinity College Dublin
In 2025, Professor Healy has been a dynamic leader in advancing pharmaceutical sciences, research excellence, and academic innovation. Her work continues to shape Ireland’s pharmacy education landscape through her commitment to high-impact research, interdisciplinary collaboration, and the development of future-focused curricula. Professor Healy is widely recognised for her contributions to drug formulation and delivery science, leading research teams whose work influences both academia and industry. This year, she has championed initiatives that strengthen research capacity, support postgraduate training, and foster strong partnerships with national and international collaborators. Her dedication to nurturing emerging scientists and her strategic vision for the future of pharmacy education solidify her reputation as a dynamic, influential, and highly respected leader in the field.
Professional 100

Professor Mary Higgins, UCD School of Medicine
Associate Professor Mary Higgins, an obstetrician at the National Maternity Hospital and academic at the UCD School of Medicine, was honoured with the prestigious UCD University Award for Teaching Excellence in 2025. A nationally respected clinician–educator, Professor Higgins is celebrated for her compassionate, evidence-based approach to women’s health and her deep commitment to medical education.
Having recently completed a Masters in Clinical Education, a Masters in Patient Safety and Human Factors, and a Diploma in Healthcare Simulation, she brings first-hand insight into the student experience, shaping her mission to support learners at every stage.
Students praise her for her generosity, clarity, and ability to create memorable, practice-centred, research-led teaching moments that leave a lasting impact.
Professor Hilary Hoey, Director of Professional Competence, Royal College of Surgeons Ireland
Professor Hilary Hoey has been awarded the prestigious RCPI St Luke’s Medal, one of the highest honours in Irish medicine, recognising her exceptional contribution to paediatrics and transformative leadership within the Royal College of Physicians of Ireland. A pioneering figure in children’s health, Prof Hoey has shaped national practice through her work in clinical care, medical education, research, and healthcare policy.
As RCPI’s Director of Professional Competence, she oversees programmes supporting almost 7,000 doctors, ensuring the highest standards of lifelong learning and professional development. Celebrating her achievements, Dr Judith Meehan described her as “a trailblazer for women in medicine and a champion for children’s health,” reaffirming her profound impact on paediatric care in Ireland and internationally.


Professor Mary Horgan, interim Chief Medical Officer/Professor of Infectious Diseases at UCD/Mater Misericordiae University Hospital
Professor Horgan continues to champion antimicrobial stewardship and infection control in 2025, publishing new guidance on hospital-community transitions of care. Her national leadership in infectiousdisease policy influences how pharmacists are embedded in antimicrobial decision-making. She also mentors early-career clinicians and pharmacy colleagues in interdisciplinary programmes, bridging medicine and pharmacy in Ireland.
Professor Horgan was the first woman to be appointed as Dean of the Medical School in UCC, serving a 4-year term. She has a research interest in the fields of medical education and Infectious Diseases. She is a Director of Education of the European Society of Infectious Diseases and Clinical Microbiology (ESCMID).
Professor Alan Irvine, Consultant Dermatologist, Children’s Health Ireland/St James’s Hospital/ Professor of Dermatology, School of Medicine, Trinity College Dublin
Professor Alan Irvine, Consultant Dermatologist at Children’s Health Ireland and St James’s Hospital and Professor of Dermatology at Trinity College Dublin, has been named among the Clarivate Highly Cited Researchers 2025 — a global distinction recognising the world’s most influential scientists. A pioneering clinician–scientist, Professor Irvine has transformed international understanding of skin disorders through groundbreaking research into their genetic, environmental and immunological causes. His landmark discovery of filaggrin loss-of-function mutations as the major genetic risk factor for eczema reshaped dermatology worldwide and continues to guide precision therapies for patients.
With hundreds of peer-reviewed publications in leading journals such as Nature Genetics and the New England Journal of Medicine, his work—supported by CHI’s research team and the Children’s Health Foundation—continues to improve outcomes for children and adults living with skin disease.

Jo Hanna Ivers, Associate Professor, Addiction/Associate Dean, Civic Engagement, Trinity College Dublin
Associate Professor Jo-Hanna Ivers, a leading addiction scientist and Associate Dean of Civic Engagement at Trinity College Dublin, has been appointed to the Scientific Committee of the European Union Drugs Agency (EUDA) — a prestigious role that places her at the centre of Europe’s evidencebased response to drug use, addiction, and recovery.
As Head of the Neurobehavioural Addiction Research Group and Director of Trinity’s M.Sc. in Addiction Recovery, Dr Ivers brings deep national and international expertise to the EUDA. In her new role, she will help shape research priorities, advise on methodological standards, identify emerging drug-related threats, and ensure scientific evidence drives European policy. Her appointment underscores Trinity’s leadership in addiction science and strengthens Ireland’s voice in shaping Europe’s public health strategy.


Grainne Johnston, Hospital Pharmacist, Mater Misericordiae University Hospital
Gráinne Johnston Gráinne Johnston, Hospital Pharmacist at the Mater Misericordiae University Hospital, continues to distinguish herself as one of Ireland’s most innovative clinical pharmacy leaders. A finalist for the 2024 PharmaSource Hospital Pharmacist of the Year Award, she has now gained international recognition through her publication at the European Association of Hospital Pharmacists Congress.
Her study, Impact of an Integrated Medicines Optimisation Pharmacist (IMOP) on Biosimilar Uptake, demonstrated how targeted pharmacist-led education and patient engagement can dramatically increase Best Value Biologic (BVB) adoption. Following her intervention, 64% of Humira® and Enbrel® patients were successfully switched to biosimilars — delivering major cost savings while maintaining high-quality care.
Gráinne’s work showcases the transformative value of hospital pharmacists in driving medicines optimisation, empowering patients, and ensuring sustainable use of healthcare resources.
Dr Paul Kavanagh, Specialist, Public Health Medicine/Health Advisor, HSE Tobacco Free Ireland
Dr Paul Kavanagh is a medical doctor, specialist in public health medicine, and holds postgraduate qualifications in Community Health, Health Economics, Medical Education, Healthcare Leadership and Data Science. He was appointed to the Board of the National Cancer Registry Ireland in July 2025. He is currently a Consultant in Public Health Medicine in the HSE National Health Intelligence Unit where he works across the health system, using health information and analytics to frame, explore, and inform decisions for better population health and health services in Ireland. He is also Public Health Advisor for the HSE Tobacco Free Ireland Programme, Chair of the RCPI Cinical Advisory Group on Smoking and E-Cigarettes and has advised the World Health Organization on clinical guidance for tobacco-use cessation. In the past, Paul has led a wide range of strategic initiatives across different health system settings in areas including population-based health planning, health improvement, disease prevention, clinical and professional governance, and medical education.


Professor Brendan Kelly, Head of Discipline, Psychiatry, Trinity College Dublin
Professor Brendan Kelly is one of Ireland’s most influential voices in psychiatry, human rights, and mental health law. A Visiting Full Clinical Professor at UCD School of Medicine and Professor of Psychiatry at Trinity College Dublin, he is an internationally recognised scholar whose work bridges clinical care, ethics, history, and social justice.
Author of 23 books — including Buddhism and Psychiatry (2025) and The Handbook of Compassion in Healthcare (2025) — and more than 400 peer-reviewed papers, Professor Kelly is a Fellow of the Royal Irish Academy and Editor-in-Chief of the International Journal of Law and Psychiatry.
His research focuses on mental health legislation, civic participation, and the rights of people with mental illness, while his frequent media commentary continues to shape national and international mental health discourse.
Professional 100

Professor Cathal Kelly, Registrar, Royal College of Surgeons Ireland
Professor Kelly, Vice Chancellor, Chief Executive Officer and Registrar of RCSI, has been awarded an Honorary Fellowship of the American College of Surgeons (ACS), one of the most prestigious distinctions in international surgery. In conferring this honour, the ACS acknowledges Professor Kelly’s contribution as an international leader who has transformed surgical education and care quality in Ireland and beyond.
A graduate and Fellow of RCSI, Professor Kelly served as Dean of the Faculty of Medicine and Health Sciences (2006-2009) before his appointment as Chief Executive and Registrar in 2009. Prior to taking up these leadership roles, he was a consultant general and vascular surgeon with a special interest in endovascular surgery in Beaumont Hospital. He combined his career in surgical practice with chairmanship of the surgical division and an academic position in RCSI as ViceDean for curriculum change.
Dr Helena Kelly, Head of School of Pharmacy & Biomolecular Sciences, Royal College of Surgeons Ireland
Dr Helena Kelly continues to bridge academia and clinical practice in 2025, leading RCSI’s Pharmaceutical Technology research in advanced drug-delivery systems. Her team’s work on long-acting injectable formulations has significant potential for hospital-based therapies in oncology and endocrinology. Dr Kelly also mentors postgraduate researchers through RCSI’s translational-medicine initiatives, ensuring the next generation of scientists understand the hospital-pharmacy interface. Her commitment to research excellence and innovation reinforces Ireland’s global reputation in pharmaceutical technology.
Prior to being appointed Head of School, she was Deputy Head (Programmes Innovation) in PBS with responsibility for the implementation of two new programmes, the MSc in Technologies and Analytics in Precision Medicine (TAPM) and BSc in Advanced Therapeutic Technologies (ATT). The development of these programmes was funded through a ¤7.8m HEA Human Capital Initiative award titled 'Enabling Future Pharma -Beyond the Pill'.


Anne Marie Kiernan, Croom Orthopaedic Hospital’s Pain Excellence Centre
Anna Marie Kiernan, Advanced Nurse Practitioner at Croom Orthopaedic Hospital’s Pain Excellence Centre, has been recognised nationally for her leadership in digital innovation, winning the Best Use of Innovative Technology Award at the 2025 HSE Spark Summit.
Her project, Patch Me If You Can/Virtually Pain Free, piloted a gamified augmented reality tool designed to ease procedural pain during Qutenza patch treatments — a creative, patient-centred solution that showcases how technology can transform comfort, experience, and outcomes in clinical care.
Selected as one of just 12 standout innovations nationwide, Anna Marie’s work demonstrates how frontline ingenuity can harness existing resources in novel ways, improving processes, reducing pressure on healthcare teams, and setting new standards in pain management across the HSE.
Maura Kinahan, PIER Committee
Maura Kinahan is a highly respected pharmaceutical leader and a driving force behind the recognition and advancement of non–patient-facing pharmacists in Ireland. With a career spanning major global companies including Élan, Bristol-Myers Squibb and Pfizer, she has held senior roles across pharmaceutical development, regulatory affairs, supply chain, commercial operations and medical affairs. A pharmacy graduate of the University of Strathclyde with a PhD from the University of Manchester, Maura also completed the prestigious BioInnovate Fellowship in medical device innovation.
As a founding member of PIER, she has been instrumental in championing education, mentorship and professional development for pharmacists working in industry, academia and regulatory roles. Now applying her leadership expertise in the renewable energy sector, Maura continues to bridge science, innovation and strategic growth across industries.


Fionnuala King, National Lead, Access & Integration Drug Management Programme (AIDMP), HSE
Fionnuala King is a transformational leader in hospital medicines management, driving national improvement across procurement, clinical optimisation and digital infrastructure. Since 2019, she has expanded the AIDMP into a central force for safer, more efficient medicines use in Irish hospitals. Her programme leads national guideline development, supports medicine-shortage management and provides clinical leadership to the National Pharmacy Procurement Support Team. Fionnuala also oversees HMMS, the national pharmacy digital-transformation project, with nine hospitals now live—a milestone aligned with the National Service Plan 2025 and Digital Health Strategy. She has been a key architect of the new hospital pharmacy career structure, including the introduction of Advanced Specialist Pharmacists, ensuring a modern, sustainable workforce for the future of secondary-care pharmacy.
Ciara Kirke, Clinical Lead, HSE Medication Safety Programme
Ciara Kirke, Clinical Lead of the HSE National Medication Safety Programme, has been recognised as the HSE West North West regional Poster Winner at the HSE Integrated Healthcare Conference 2025 for her impactful work on the iSIMPATHY project. Her winning poster, “Safety and Savings,” highlighted the powerful benefits of pharmacist-led, person-centred medicines reviews in general practice. Using the “7 Steps to Appropriate Polypharmacy” approach, pharmacists reviewing high-risk or heavily prescribed patients delivered significant clinical and economic gains, with annual savings of ¤124,968 to ¤347,844 per pharmacist. Ciara also champions the HSE Health App and its My Medicines List feature, emphasising how keeping an accurate medicines record improves safety, communication and patient empowerment across Ireland’s healthcare system.


Professor Aonghus Lavelle, Lecturer, Department of Anatomy & Neuroscience, University College Cork/APC Microbiome Ireland
Professor Aonghus Lavelle, Clinical Anatomist, Gastroenterologist, and researcher with APC Microbiome Ireland at University College Cork, has been awarded ¤613,556 under the prestigious Research Ireland Frontiers for the Future Programme for his groundbreaking work on inflammatory bowel disease (IBD).
His project, Characterising uncultured bacteria associated with bile acid dysmetabolism and disease severity in IBD, aims to uncover how specific gut bacteria influence bile acid metabolism and contribute to disease progression in ulcerative colitis and Crohn’s disease. Building on earlier discoveries linking severe IBD to the loss of key secondary bile acids and associated bacteria, Professor Lavelle will recruit patients to isolate and culture these microbial species for the first time.
The long-term goal: developing novel microbiome-based therapies that could transform the treatment of IBD for more than 40,000 people in Ireland.
Professor Carel le Roux, Director of the Metabolic Medicine Group, University College Dublin, Ireland
Professor Carel le Roux, Professor of Chemical Pathology at UCD School of Medicine, has added another significant contribution to metabolic and obesity research with the publication of the paper “Glucagon-like peptide-1 analogues reduce alcohol intake” in Diabetes, Obesity and Metabolism
Co-authored with Dr Maurice O'Farrell and Dr Faisal I. Almohaileb, the study provides compelling evidence that GLP-1 analogues—already transformative in obesity and diabetes care—may also reduce harmful alcohol consumption by altering neurobiological reward pathways.
An internationally renowned clinician–scientist, Prof le Roux is recognised for pioneering research on metabolic disease, bariatric mechanisms, and incretin-based therapies. His work continues to shape global obesity guidelines and advance novel treatments that address the biology—not the stigma—of chronic disease.


Professor Mark Little, Director of the Trinity Translational Medicine Institute
Professor Little, newly appointed Director of the Trinity Translational Medicine Institute (TTMI), brings transformative leadership to one of Ireland’s foremost centres for patientcentred biomedical research. A Professor of Nephrology at Trinity’s School of Medicine and an internationally recognised clinician-scientist, he takes over from Professor Aideen Long, whose six-year tenure strengthened TTMI through the challenges of the COVID era.
Prof Little will drive TTMI’s new strategy to deepen integration with key clinical partners— CHI, Tallaght University Hospital, and St James’s Hospital—and with leading research hubs such as the Trinity St James’s Cancer Institute, TBSI, and the Wellcome-HRB Clinical Research Facility.
Under his leadership, TTMI will continue to accelerate translational research that delivers real-world impact, advancing novel diagnostics, therapies, health-service innovations, and education shaped directly by clinical need.
Professor Declan Lyons, Director, HSE Research Directorate Mid West
Professor Lyons, Director of the newly launched HSE Research Directorate Mid West, is leading a transformative step forward for Irish healthcare research. Opened in March 2025 by HSE CEO Bernard Gloster, the Directorate is the first of the HSE’s new regional research hubs, designed to embed innovation across frontline services. Prof Lyons now oversees all clinical research activity in the region, providing a single point of entry for regulatory support, expert guidance, and research infrastructure.
He emphasises the Directorate’s mission to deliver high-quality, reliable, and impactful studies that benefit local communities — citing recent frailty research led at UHL that significantly reduced admissions, wait times, and re-attendance rates. Prof Lyons aims to cultivate a thriving research culture that drives better patient outcomes locally, nationally and internationally.


Professor Ignacio Martin-Loeches, Academic Professor of Intensive Care Medicine, Trinity College Dublin
Professor Ignacio Martin-Loeches, newly appointed as Trinity College Dublin’s first Academic Professor of Intensive Care Medicine, continues to redefine Ireland’s global impact in critical care research. In 2025, he led two landmark international studies published in The Lancet and Nature Medicine, cementing Ireland’s role at the forefront of innovation in sepsis, respiratory failure, and ICU medicine. A clinician scientist at St James’s Hospital, Prof Martin-Loeches directs large-scale research programmes that integrate bedside practice with cutting-edge translational science to improve survival and long-term outcomes for critically ill patients. His leadership has strengthened Ireland’s international research collaborations and expanded evidence-based approaches in intensive care, driving advancements that directly shape global clinical practice.
Zoe McCrudden, Advanced Nurse Practitioner, Heart Failure
Zoe McCrudden is an Advanced Nurse Practitioner in heart failure whose leadership is transforming community cardiac care in the North West. With nearly two decades of cardiovascular expertise, she has developed an integrated CHO1 community heart failure service that reduces hospital admissions, accelerates access to specialist diagnostics, and empowers patients through education and selfmanagement. In 2024, Zoe received the prestigious ESC Nursing Societies Award for her contribution to cardiovascular care. Her clinical innovation includes advancing point-of-care and lung ultrasound, establishing joint respiratory–diabetes clinics, and planning new breathlessness pathways. As an Adjunct Lecturer at the University of Galway, she is shaping the next generation of advanced practitioners while driving evidence-based, patient-centred cardiac care.

Professional 100

Professor
Kingston
Mills, Professor of Experimental Immunology, Trinity College Dublin
Professor Mills, Professor of Experimental Immunology at Trinity College Dublin and Academic Director of TBSI, continues to lead groundbreaking advances in global immunology. In 2025, he co-led the development of a needle-free nasal vaccine against whooping cough — a major breakthrough published in Nature Microbiology — that not only prevents severe disease but also halts bacterial transmission by stimulating strong mucosal immunity at the respiratory tract. This could redefine how we protect against respiratory infections worldwide. Under his leadership, Trinity’s immunology research remains at the forefront of scientific innovation. His work bridges basic science, translational research and public health, delivering solutions that could reshape vaccine development for generations.
Mr Daragh Moneley, Consultant Vascular Surgeon, Beaumont Hospital
In 2025, Mr Daragh Moneley was appointed Surgical CPD Programme Lead at RCSI, a new role reflecting the growing importance of structured, high-quality lifelong learning in surgical practice. A Consultant Vascular Surgeon at Beaumont Hospital, Mr Moneley is also RCSI Training Programme Director for Vascular Surgery and Chair of the clinical governance group overseeing the RCSI Hospital Group Robotic Surgery Programme.
RCSI’s CPD programme has expanded dramatically in recent years, growing from 32 courses to more than 134 annually, now engaging over 1,200 participants across multiple learning formats. In his new role, Mr Moneley will lead the development of responsive, specialty-led CPD aligned with health service needs, workforce development and patient outcomes. Working with specialty advisors and surgical educators, he will ensure future CPD programmes remain relevant, rigorous and fit for a rapidly evolving surgical landscape.

Professor
Frank Moriarty,

Associate Professor, School of Pharmacy, Royal College of Surgeons Ireland
In 2025, Frank Moriarty has been a dynamic leader in advancing data-driven healthcare through his work at the Royal College of Surgeons in Ireland (RCSI). He played a central role in developing RxTrends, an innovative real-time prescribing analytics platform that provides clinicians, policymakers, and researchers with unprecedented insight into national prescribing behaviour. This interactive tool enables users to track trends, identify emerging concerns, and support evidence-based decisionmaking across Ireland’s health system. Frank’s commitment to improving medication safety, transparency, and population-level understanding of prescribing patterns positions him at the forefront of digital health innovation. His work on RxTrends not only strengthens clinical practice but also empowers the wider healthcare community with meaningful, accessible data.
Dr Catherine Motherway, Voluntary Health Insurance Board
Dr Catherine Motherway is a leading Irish intensive-care physician and advocate for organ donation, recently appointed to the Voluntary Health Insurance Board (2025–2029), reflecting her enduring commitment to national healthcare governance and patient access. As Clinical Lead with Organ Donation Transplant Ireland (ODTI), she continues to champion ethical transplant practices and raise public awareness on the importance of donating life-saving organs. With decades of experience in critical care and anaesthesia — including a consultant post at University Hospital Limerick — Dr Motherway has contributed significantly to intensive care standards, national training, and health-service resilience. Her presence on both clinical and policy fronts reinforces vital connections between frontline care and healthcare system stewardship.

Tanya
Mulcahy, Director,
Health Innovation Hub Ireland
Dr Tanya Mulcahy, Director of Health Innovation Hub Ireland, is a driving force behind Ireland’s expanding health innovation ecosystem. Since joining HIHI in 2013, she has helped transform it from a single-site pilot into a national programme with four partner locations, supporting companies navigating the healthcare system and empowering frontline innovators to develop, test and scale new ideas. With a research career spanning University College Cork, Trinity College Dublin, and biotech roles in the US at ScheringPlough and Orchid Biosciences, she brings deep scientific and commercial expertise. Tanya also contributes to initiatives such as the BioInnovate Programme and founded the Women’s Ideas Network, championing innovation, leadership and diversity across Ireland’s health and research landscape.


Dr Deidre Mulholland, Area Director of Public Health, HSE
Dr Deirdre Mulholland, Area Director of Public Health for HSE Dublin & North East, continues to shape national healthcare standards and quality improvement in 2025. A former GP and public health physician with HIQA, she led the creation of national safety standards and helped establish Ireland’s healthcare quality reporting system. In early 2025, she chaired a key national conference session at NOCA, underlining her commitment to data-driven audit and patient safety across acute and community care. Her ongoing public health leadership — grounded in evidence, governance, and clinical insight — reinforces crucial connections between frontline care delivery, quality assurance, and national health-services transformation.
Eibhlín
Mulroe, CEO,
Cancer Trials Ireland
Eibhlín Mulroe, CEO of Cancer Trials Ireland since 2015, has led the organisation through a decade of transformation, strengthening Ireland’s capacity for world-class cancer research. She oversees a 50-strong cross-functional team and works closely with Chair Dr Jonathan Westrup and Clinical Lead Prof Bryan Hennessy to deliver its mission of giving Irish patients early access to cutting-edge cancer treatments. Under her leadership, the group rebranded, expanded its national infrastructure, commissioned its first economic impact report and established a Patient Advocate Advisory Group. Eibhlín also contributes nationally through roles with EHealth Ireland, the Royal Irish Academy and multiple research and policy committees. Her career spans patient advocacy, health research, and leadership of major national and European initiatives.

Professional 100

Professor Jarushka Naidoo, Consultant Medical Oncologist, Beaumont Hospital, Dublin
Professor Jarushka Naidoo has been named one of the world’s most influential scientists on the 2025 Clarivate Highly Cited Researchers list — a recognition reserved for the top 1% of researchers globally. An internationally renowned authority in lung cancer and immunotherapy, Prof Naidoo serves as Ireland’s national lung cancer lead for Cancer Trials Ireland and holds an adjunct faculty position at Johns Hopkins University, where she previously pioneered groundbreaking clinical trials.
A leader in immunotherapy innovation, she contributes to global ASCO and SITC guideline panels and has secured prestigious awards from IASLC, LCFA and the NIH. Her commitment to mentorship has shaped the careers of numerous early-stage oncologists. Trained in Trinity College Dublin and Memorial Sloan Kettering Cancer Center, Prof Naidoo continues to drive transformative advances in cancer care and research in Ireland and worldwide.
Dr Siobhán Ní Bhriain, National Clinical Director, Integrated Care, HSE
Dr Siobhán Ní Bhriain is a national healthcare leader driving integrated care strategy as the HSE’s National Clinical Director for Integrated Care. In 2025, she continues to lead clinical design and innovation that shapes how care pathways are developed and delivered across hospital, community and primary care settings. Her work brings clinicians together with patients, educators and policymakers to embed evidence-based, person-centred models of care that align with Sláintecare and improve outcomes across the health system. Dr Ní Bhriain’s voice features prominently in national forums and HSE clinical discussions on improving quality, access and continuity of care. Her emphasis on partnership, data-driven pathways and health service transformation positions her at the forefront of healthcare design in Ireland. HSE.ie


Dr Aoibhinn Ní Shúilleabháin, Associate Professor, School of STEM Education, Innovation & Global
Studies, Dublin City University
Dr Aoibhinn Ní Shúilleabháin is an award-winning academic, science communicator and Associate Professor at Dublin City University. Her research in mathematics education champions inclusive and innovative teaching practices, particularly for learners from underrepresented backgrounds. In 2025 she leads national advisory work on Ireland’s Nature Restoration Plan, positioning science and evidence at the heart of environmental policy. A familiar voice in media, Dr Ní Shúilleabháin brings complex scientific and educational issues to broad audiences, advocating for STEM engagement and gender equity. Her contributions to policy, public dialogue and science communication amplify the connection between research, education and societal impact, while her leadership in advisory committees underscores her influence on Ireland’s science-policy landscape.
Professor Philip Nolan, Research Professor, Kathleen Lonsdale Institute for Human Health Research
Professor Nolan, one of Ireland’s most influential scientific leaders, continues to shape national research and innovation strategy in 2025. A physiologist by training and former President of Maynooth University, Prof Nolan has long championed excellence in STEM, evidence-informed policy, and the advancement of Irish research on the global stage. As former Director General of Science Foundation Ireland, he oversaw major national programmes supporting frontier discovery, early-career researchers, and large-scale strategic research centres. In 2025, his leadership remained a focal point of public discourse as he completed his tenure at SFI amid the transition to Taighde Éireann –Research Ireland, highlighting his legacy in strengthening Ireland’s research ecosystem. A widely cited academic and respected public communicator, he continues to contribute as an authoritative voice in science, policy and higher education.


Ms Orla O’Brien, Chief Executive, Irish Blood Transfusion Service/Children’s Health Ireland
Orla O’Brien was appointed to the Children’s Health Ireland (CHI) Board in 2025, bringing decades of health-care leadership and governance expertise to the national stage. She currently serves as Chief Executive of the Irish Blood Transfusion Service and has held senior executive roles across the Irish healthcare system, from acute hospital operations to strategic healthcare transformation. At CHI, O’Brien chairs key committees and contributes to shaping national policy and governance frameworks that support safe, high-quality care for children across Ireland’s pediatric hospitals. Her extensive experience in both clinical and corporate health leadership strengthens stakeholder collaboration and governance, reinforcing CHI’s mission to deliver integrated, patient-centred services for the nation’s youngest patients.
Professor Fergal O’Brien, Deputy Vice Chancellor, Research & Innovation and Professor of Bioengineering & Regenerative Medicine,
RCSI
Professor Fergal O’Brien is Deputy Vice Chancellor for Research & Innovation and Professor of Bioengineering & Regenerative Medicine at RCSI, leading world-class work in tissue engineering and regenerative biomaterials. He drives innovation in advanced scaffold technologies and biomaterials with applications spanning bone, cartilage, cardiovascular, respiratory, and neural repair, translating laboratory breakthroughs into clinical impact. O’Brien has published extensively, filed numerous patents, and secured major research awards, including European Research Council grants. His leadership in regenerative medicine and biomechanical research continues to position Ireland at the forefront of biomedical innovation, with multidisciplinary collaborations that bridge fundamental science and therapeutic solutions. Prof O’Brien’s work exemplifies transformative research with realworld healthcare benefits.

Professional 100

Professor Donncha O’Brien, Consultant Neurosurgeon, Beaumont Hospital/National Clinical Advisor in Neurosurgery
Professor Donncha O’Brien, Consultant Neurosurgeon at Beaumont Hospital and RCSI Associate Professor, has been appointed National Clinical Advisor in Neurosurgery for the RCSI National Clinical Programme in Surgery. As Clinical Director of the Neurocent Directorate and National Clinical Lead for Epilepsy Surgery (adults and paediatrics), he brings deep clinical and strategic expertise to a role created at a time of intense pressure on neurosurgical services, with over 11,000 patients awaiting outpatient appointments.
Professor O’Brien will lead a programmatic, pathway-focused approach to tackling waiting lists, strengthening governance, and integrating neurosurgery within the national trauma network. His work will drive the development and standardisation of new patient pathways, protocols and guidelines—aimed at delivering faster, fairer, and more coordinated neurosurgical care nationwide.
Mr Cian O’Caheny, Hospital Pharmacist, Mater Misericordae
University Hospital
Cian O’Caheny is a hospital pharmacist actively advancing practice in geriatric and emergency medicine settings, with a professional interest in medicine optimisation, deprescribing, and frailty. Working clinically while undertaking a part-time PhD exploring adverse drug reactions and prescribing challenges in older adults, O’Caheny contributes to improving medication safety and outcomes for vulnerable patients. He has been involved in multidisciplinary quality improvement initiatives, bringing pharmacy perspectives into emergency care research and practice. His work bridges frontline pharmacy practice with evidence-based improvements, emphasising tailored medicines management and enhanced care for older populations—reflecting a growing focus on pharmacy’s role in multidisciplinary hospital care.


Lauren V. O’Connell, Specialist Registrar in General & Colorectal Surgery, Mater Misericordiae University Hospital
Ms Laura V. O’Connell has been named the 2025 recipient of the RCSI PROGRESS Women in Surgery Fellowship, recognising her exceptional clinical, academic and leadership achievements. A Specialist Registrar in General and Colorectal Surgery at the Mater Misericordiae University Hospital, she will undertake an advanced colorectal cancer fellowship at the Peter MacCallum Cancer Centre in Melbourne, gaining world-class expertise in complex pelvic, robotic and cytoreductive surgery.
A UCD honours graduate, Ms O’Connell achieved FRCSI in 2024 and was awarded the Professor W.A.L. McGowan Medal as the top-performing candidate across all surgical subspecialties. With over 35 peer-reviewed publications and a strong commitment to surgical education, she represents the next generation of Irish surgical leaders driving excellence, equity and innovation in cancer care.
Professor Rosemary O’Connor, Professor of Cell Biology and Head of the School of Biochemisty and Cell Biology, University College Cork
Professor Rosemary O’Connor is a leading cell biologist and Principal Investigator at University College Cork, renowned for her research on insulin-like growth factor-related signalling in cancer and cell biology. With a distinguished academic career spanning decades, she established and leads research programs that explore the molecular underpinnings of cancer progression and metabolic regulation. Her work has contributed foundational insights into signal transduction pathways relevant to cancer biology and neurodegenerative processes. A mentor to many postgraduate researchers, Prof O’Connor’s scientific leadership advances understanding of complex cellular mechanisms with broad implications for biomedical research and therapeutic development.


Professor Caitriona O’Driscoll, Prof/Chair of Pharmaceutics, School of Pharmacy, University College Cork
Professor Caitríona O’Driscoll, coordinator of the GENEGUT project and a pioneering leader in RNA therapeutics, has been elected to the Royal Irish Academy, recognising her exceptional contributions to pharmaceutical science in Ireland and beyond. Appointed in 2003 as the first Head of UCC’s School of Pharmacy, she has built an internationally acclaimed research programme in non-viral RNA delivery, with major collaborations across SFI centres including CURAM, SSPC, AMBER and APC. Now Emeritus Professor and Director of UCC Futures –Future Medicines, she continues to shape the future of advanced therapeutics. O’Driscoll also ranks among the top 2% of most-cited researchers worldwide, underscoring her global impact. A visionary academic and research leader, she exemplifies innovation at UCC.
Professor Lorraine O’Driscoll, Prof/Chair of Pharmacology & Biomedicine, Trinity College Dublin
Professor Lorraine O’Driscoll is Professor/Chair of Pharmacology and Biomedicine at Trinity College Dublin’s School of Pharmacy and Pharmaceutical Sciences. She is also Research Director of Trinity St. James Cancer Institute accredited, in June 2025 by the Organisation of European Cancer Institutes (OECI), as the first Comprehensive Cancer Centre on the island of Ireland. Professor Lorraine O’Driscoll is also Vice-President of the Royal Irish Academy. She leads the Government of Ireland All-Ireland Cancer Liquid Biopsies Consortium (CLuB) and in 2025 again she was named among Stanford University/ Elsevier World’s Top 2% Scientists.


Dr Maria O’Kane, CEO, Irish Medical Council
Earlier this year, Dr Maria O’Kane was appointed CEO of the Medical Council, bringing over 30 years of clinical and executive leadership to one of Ireland’s most important regulatory bodies. A Consultant Psychiatrist by training and former Chief Executive of the Southern Health and Social Care Trust in Northern Ireland, she has led complex, high-performing health services serving almost 400,000 people. Dr O’Kane has also held senior roles within the British Medical Association and the Royal College of Psychiatrists, reflecting her long-standing commitment to professional standards and patient safety.
Stepping into this national role, Dr O’Kane emphasises the importance of a highly skilled medical workforce and the Council’s role in supporting doctors throughout every stage of their careers—education, training, registration, professional development, and regulation.
Professional 100

Professor Derek O’Keeffe, Consultant Endocrinologist, University Hospital Galway
Professor O’Keeffe’s 2025 work focuses on digital and robotic innovation in endocrine care, including a pilot robotics-assisted insulin-delivery programme in a regional hospital. His collaboration with pharmacy teams supports implementation of digital health tools and AI-driven adherence monitoring. By integrating pharmacy-led interventions with robotics and data analytics, he’s helping redefine chronic-disease management pathways in Ireland.
Professor O'Keeffe is a distinguished Consultant Endocrinologist and the Professor of Medical Device Technology at the University of Galway, known as a "Physicianeer" for blending medicine and engineering, holding dual doctorates, working with NASA, and pioneering digital health innovation at University Hospital Galway. He leads the Health Innovation via Engineering (HIVE) Lab, researches digital health, and excels in numerous activities like piloting, Taekwondo, and marathon running.
Professor Risteárd Ó Laoide, National Director, National Cancer Control Programme
Professor Risteárd Ó Laoide, National Director of the National Cancer Control Programme, has marked a significant milestone in 2025 with the publication of the updated National Competency Framework for Pharmacists Providing Cancer Care. First developed in 2015 following the NCCP Oncology Medication Safety Review, the Framework has now been modernised to reflect the rapidly evolving role of pharmacists across hospital and community settings. With rising use of systemic anti-cancer therapies, greater reliance on oral anti-cancer medicines, and increasingly complex treatment protocols, pharmacists require enhanced skills to support safe, high-quality care. Under Professor Ó Laoide’s leadership, the updated Framework ensures pharmacists remain equipped, future-focused and central to delivering excellence in Ireland’s cancer services as treatment models and technology continue to advance.


Professor Anthony O'Regan, Dean, Institute of Medicine, Royal College of Physicians of Ireland
Professor Anthony O’Regan is one of Ireland’s leading respiratory physicians and a national champion for excellence in clinical care, education, and healthcare leadership. As Consultant Respiratory Physician and Dean of the Irish Institute of Medicine, he has played a pivotal role in shaping postgraduate medical training and strengthening clinical standards across the health service.
Professor O’Regan is internationally recognised for his contributions to respiratory medicine, including innovative work in interstitial lung disease, complex asthma, and multidisciplinary models of care. A respected educator and researcher, he has been instrumental in advancing integrated respiratory pathways that improve outcomes and reduce hospital burden.
Driven by a commitment to patient-centred innovation, Professor O’Regan continues to influence national health policy and inspire the next generation of Irish clinicians.
Dr Niamh O’Rourke, Head of Standards, Health Information & Quality Authority
Dr Niamh O’Rourke is Deputy Director of Health and Social Care Standards at HIQA and a leading voice in shaping quality and person-centred care in Ireland and across Europe. In 2025, she has represented Ireland at senior European policy forums, speaking at the European Parliament Roundtable on Quality in Social Services in Brussels and the European Social Network Members Forum in Warsaw. Dr O’Rourke highlighted Ireland’s approach to developing robust national standards for home support and long-term care, focused on enabling people to live independently for longer. Her work has directly informed the emerging European Framework on Quality in Social Services, underlining the impact of Ireland’s evidence-based, outcomes-focused standards model. Dr O’Rourke continues to drive innovation in health and social care regulation at both national and EU level.

Dr Diarmuid O’Shea, Consultant Geriatrician & National Leader in Ageing Care/President, Royal College of Physicians in Ireland
Dr Diarmuid O’Shea is one of Ireland’s most influential voices in geriatric medicine, renowned for championing excellence in care for older people. As Consultant Geriatrician at St Vincent’s University Hospital and former President of the Irish Gerontological Society, he has led transformative national initiatives to improve ageing services, frailty pathways, and age-attuned models of care. Dr O’Shea is a trusted advisor on health policy and a passionate advocate for dignity, independence, and integrated community support for older adults. A sought-after speaker and educator, he continues to shape national dialogue on healthy ageing, workforce development, and the future of geriatric medicine. His leadership has helped embed evidence-based, compassionate care across Ireland’s health system.


Dr Suzanne O’Sullivan, Consultant Neurologist, Author & Global Authority on Psychosomatic Illness
Dr Suzanne O’Sullivan, Consultant Obstetrician and Urogynaecologist at Cork University Maternity Hospital, has been appointed Chair of the RCPI Institute of Obstetricians & Gynaecologists. A national leader in women’s health, she has spent over a decade shaping specialist education, serving as National Director of Education and Training and previously as National Specialty Director for both BST and HST programmes. Dr O’Sullivan is Clinical Lead of the National Mesh and Complex Pelvic Floor Centre and co-director of Ireland’s EBCOG-recognised subspecialty training programme in urogynaecology. A respected advocate on recruitment, retention, and litigation reform, she brings deep expertise and vision to the role. “I am honoured to represent our specialty and advance excellence in care for women and girls,” she said.
Muriel Pate, Chief II Pharmacist, Pharmacy Workforce Development, Access & Integration, HSE
Muriel has played a pivotal leadership role in one of the most significant milestones in Irish hospital pharmacy in more than four decades: the introduction of the first cohort of Advanced Specialist Pharmacists. Fourteen years after the McLoughlin Report called for a senior clinical grade to retain expertise and strengthen frontline care, Muriel and her team helped drive this long-awaited reform from concept to implementation. Her work brought together national stakeholders, advanced practice standards, and international guidance to shape a competency-based framework aligned with modern healthcare needs. With 235 pharmacists validated in Phase 1, this transformative initiative is redefining career pathways, elevating clinical practice, and enhancing patient outcomes across the HSE. Muriel’s vision and dedication have ushered in a new era for the profession.

Professional 100

Gráinne Power, Chief Executive, Health Products Regulatory Authority
Gráinne Power has been appointed Chief Executive of the Health Products Regulatory Authority, effective 1 January 2026 — a role that reflects more than 20 years of regulatory, scientific and leadership experience across the health products sector. Currently the HPRA’s Director of Compliance, she oversees licensing, inspections, market surveillance and enforcement for medicines, clinical products, controlled substances and cosmetics, having previously led Human Products Authorisation and Registration. As Chief Executive, she will guide the organisation into its new 2026–2028 strategy, succeeding Dr Lorraine Nolan. Welcoming her appointment, HPRA Chair Prof Michael Donnelly praised her deep expertise and commitment to public and animal health. She also serves on the Council of the Pharmaceutical Society of Ireland.
Dr Karen Power, Clinical Risk Researcher at National Treasury Management Agency
Dr Karen Power is a leading Irish obstetrician whose work is driving innovation and improved outcomes in maternity and women’s health services. Based at the National Maternity Hospital, she has been central to advancing evidence-based care, improving pathways for high-risk pregnancies, and strengthening multidisciplinary teamwork across obstetrics and gynaecology. Dr Power is widely recognised for her clinical leadership, research contributions, and commitment to patient safety. She plays an active role in shaping national guidelines and is a trusted educator for trainee doctors and midwives. Passionate about advocacy, equity, and respectful maternity care, she continues to champion system improvements that support mothers, infants, and families across Ireland.


Audrey Purcell, Chief II Pharmacist, St John of God Hospital, Dublin
Audrey Purcell has emerged as a national leader in medication safety through her awardwinning work on improving patient education for Direct Oral Anticoagulants (DOACs) and warfarin. At the Irish Medication Safety Network Conference, she delivered both a poster and an oral presentation—earning the Best Oral Presentation Prize for her impactful contribution to anticoagulation safety. Audrey’s project delivers clear, accessible, and standardised patient information developed through extensive collaboration with the HSE National VTE Clinical Programme, where she serves on the patient information group. Supported by ongoing implementation research with the NCP VTE and RCSI, her work is poised to influence national practice and empower patients to safely manage high-risk therapies through better understanding and informed decision-making.
Dr Emma Regan, Clinical Psychologist/President, International Association of Correctional and Forensic Psychology
In 2025 Dr Regan was appointed President of the International Association of Correctional and Forensic Psychology. Dr Regan is a Clinical Psychologist and Director of Care and Rehabilitation with the Irish Prison Service (IPS). She began working for the IPS in 2012, beginning as a prison psychologist, then national Head of Psychology Services for eight years, and more recently Director with responsibility for Primary Healthcare, Psychology, and Prison Services for people in custody including Education, Work and Training, Chaplaincy, Integrated Sentence Management, Incentivised Regimes and Resettlement.

Mr John Rice, Consultant Orthopaedic Surgeon, University Hospital Kerry
Mr John Rice is playing a pivotal role in advancing orthopaedic care in Ireland through the successful rollout of the Irish National Orthopaedic Register (INOR) at University Hospital Kerry (UHK), making it the eighth hospital nationally to implement the system. As Clinical Lead for the INOR Project at UHK, Mr Rice has championed the integration of this national electronic register, which for the first time centralises hip and knee replacement data across Ireland. INOR enables the secure collection of patient information at pre-operative, surgical and post-operative stages, supporting early detection of implant performance and more efficient recall and review processes. Under Mr Rice’s leadership, UHK is strengthening patient safety, improving longterm outcomes, and ensuring its joint replacement services meet nationally and internationally recognised standards of excellence.
Professor Tracy Robson, Deputy Vice Chancellor for Academic Affairs, RCSI University of Medicine and Health Sciences
Professor Tracy Robson, Head of Pharmacology and Therapeutics at RCSI University of Medicine and Health Sciences, is internationally recognised for her groundbreaking work in developing next-generation cancer therapeutics. Her research focuses on the thrombospondin (TSP-1) pathway and novel peptide-based treatments with the potential to overcome resistance and improve outcomes for patients with hard-to-treat cancers. In 2025, her team advanced several promising discoveries toward clinical translation, strengthening RCSI’s position as a global leader in oncology innovation. A dedicated mentor and research strategist, Prof Robson plays a pivotal role in shaping Ireland’s cancer research landscape through collaborative partnerships, high-impact publications, and successful competitive funding. Her commitment to scientific excellence continues to drive transformative change in cancer care.

Dr Tomás Ryan, Professor In, Biochemistry/Professor In, Trinity Inst. of Neurosciences (TCIN)
Dr Tomás Ryan, Associate Professor in Trinity College Dublin’s School of Biochemistry & Immunology and Principal Investigator at the Trinity Biomedical Sciences Institute, is one of Ireland’s most influential neuroscientists. His pioneering research on memory engrams and neural circuit plasticity has reshaped understanding of how memories are stored, lost, and recovered. In 2025, Dr Ryan’s work gained major international attention for its insights into cognitive dysfunction and long COVID, positioning his lab at the forefront of global neurobiology. A compelling public communicator, he continues to play a prominent role in national science advocacy and policy discussions. His innovative contributions bridge fundamental neuroscience with real-world clinical challenges, driving advances in diagnostics, therapeutics, and brain health.
Professional 100

Nuala Scanlon, Chief 2 Antimicrobial Pharmacist, Mater Misericordiae University Hospital
Nuala Scanlon is a pioneering Antimicrobial Stewardship (AMS) Pharmacist who has shaped national best practice for over 20 years at the Mater Misericordiae University Hospital (MMUH). Appointed at a time when specialist AMS pharmacists were rare in Ireland, she quickly defined the vital contribution of pharmacists in optimising antimicrobial use and helped drive the expansion of AMS pharmacy services.
Embedded at the point of prescribing, Nuala plays a central role on consultant-led ward rounds across critical care, transplantation, trauma, haematology and surgical specialties, ensuring safe, evidence-based antimicrobial selection, dosing and duration. Her expertise supports complex patient cohorts in Ireland’s largest critical care centre.
A respected educator and committee lead, Nuala has consistently kept MMUH antimicrobial KPIs within national HSE targets — championing safer, smarter prescribing for every patient.
Professor Helen Sheridan, Professor in Natural Product Science at the School of Pharmacy and Pharmaceutical Sciences (SoPPS) - Trinity College Dublin
Professor Sheridan, Ireland’s first Professor in Pharmacognosy, has reshaped how we understand medicines from nature through her groundbreaking work at Trinity College Dublin. As former Director of Research at the university’s School of Pharmacy and academic founder of the NatPro, she has championed innovation that links traditional knowledge with modern healthcare needs. A Fellow of Trinity College Dublin and the Royal Society of Chemistry, Helen has secured over ¤21 million in competitive funding and advanced natural-product research spanning global health, ethnopharmacology, and bioeconomy solutions. Remarkably, she identified and progressed a nature-derived lead molecule from discovery to human clinical trials. Her work continues to inspire community pharmacists, highlighting the vital connection between natural medicines, scientific excellence, and patient-centred care.


Professor Breda Smyth, Professor Public Health, Royal College of Surgeons in Ireland/Former Chief Medical Officer
Professor Breda Smyth is a nationally recognised public health leader and Chief Medical Officer whose work has shaped population health policy and disease prevention strategies across Ireland. With a distinguished career in health protection, surveillance and health promotion, she has guided national responses to major public health challenges and championed equity in healthcare access. A strong advocate for evidence-based practice, Professor Smyth has amplified the voice of public health in policy, education and community engagement, while promoting systems thinking that improves population outcomes. Her leadership strengthens professional collaboration and ensures public health remains central to Ireland’s healthcare strategy.
Richard Sykes MPharm MBA MPSI, Chief Pharmacist, Portiuncula University Hospital
Richard is current Vice-President of the Hospital Pharmacists Association of Ireland. Working through acute, specialist mental health and primary care trust roles in the NHS whilst undertaking postgraduate education in both clinical and management areas, Richard moved to Ireland in 2014, initially as Chief Pharmacist at the Galway Clinic and finally at Portiuncula within the HSE.
Richard has contributed to the development of hospital pharmacy both within his own department and in the wider profession through his work with HPAI and the European Association of Hospital Pharmacists. He represents Ireland at European hospital pharmacy practice meetings in the role of EAHP implementation ambassador.
A keen advocate of the recognition and development of the specialism of hospital pharmacy, Richard has worked as a member of a small team to ensure that the profession has a tool to communicate and share practice ideas through the establishment of the HPAI online resource www.hpai.ie.


Professor Gábor Széplaki, Head of Cardiac Electrophysiology, Mater Private Hospital
Earlier in 2025, Professor Széplaki and the Cardiac Electrophysiology Team at Mater Private Network hosted the inaugural meeting of Europe’s leading cardiology experts in the field of pulsed field ablation (PFA). Professor Gábor Széplaki is an internationally respected cardiologist and researcher whose work bridges advanced clinical practice and high-impact cardiovascular science. With a distinguished academic and clinical career, he has contributed to cutting-edge research in interventional cardiology, heart failure and translational cardiovascular medicine. A sought-after speaker and collaborator, Prof Széplaki’s expertise extends across global research networks, shaping clinical guidelines and innovations that improve patient outcomes. His leadership in cardiovascular education and mentorship helps build capacity in the next generation of clinicians and researchers, ensuring that breakthrough discoveries continue to translate into improved care standards worldwide.
Professor Mark Tangney, Principal Investigator, Cancer Research, University College Cork
Professor Mark Tangney, a leading cancer biologist at Cancer Research@UCC, has played a pivotal role in one of Ireland’s most ambitious cancer research initiatives of 2025. As a key investigator in Precision Oncology Ireland Phase 2 (POI-2)—a ¤28 million national partnership uniting universities, charities and industry—Prof Tangney is spearheading OncoBioMaps Ireland, a groundbreaking project mapping the cancer microbiome to transform personalised care. His team will build Ireland’s first standardised national platform for collecting and analysing multi-layer biological data from cancer patients, enabling tailored diagnostics and treatments. Recognised for advancing microbiome-driven oncology, Prof Tangney’s leadership places UCC at the centre of a major national effort to accelerate innovation and deliver faster, more precise cancer therapies to Irish patients.

Professional 100

Professor Cormac Taylor, Professor of Cellular Physiology, UCD Conway Institute
Professor Cormac Taylor is one of Ireland’s foremost figures in diabetes research and clinical innovation, with international recognition for his work on the pathophysiology, prediction and prevention of type 1 diabetes. His leadership of the Irish Centre for Vascular Biology and contributions to large-scale cohort studies have informed understanding of disease mechanisms and risk stratification. A visionary in precision medicine and translational research, Prof Taylor’s work integrates clinical insight with population health approaches to improve outcomes for people with metabolic disease. Through interdisciplinary collaboration and public engagement, he continues to drive forward scientific discovery and evidence-based strategies that shape national and global diabetes care.
Professor Ines Thiele, Professor for Systems Biomedicine, National University of Ireland, Galway
Professor Ines Thiele is redefining how science understands the human microbiome. In 2025, the University of Galway–led team she directs unveiled APOLLO, the world’s largest digital collection of microbes, comprising almost 250,000 advanced computer models that map the metabolic behaviour of bacteria living in and on the human body. Built on more than a decade of pioneering work, APOLLO enables researchers to simulate microbiome activity at unprecedented scale, accelerating discovery without reliance on slow, costly laboratory experiments. The platform has already generated powerful insights into conditions such as Crohn’s disease, Parkinson’s disease and child undernutrition. As a principal investigator with APC Microbiome Ireland, Professor Thiele’s leadership places Ireland at the forefront of computational biology, opening new pathways for diagnostics, personalised medicine and microbiome-based therapies worldwide.


Jayne Tuthill, Drug Safety Facilitator, Mater Misericordiae University Hospital
Jayne is the Drug Safety Facilitator at the Mater Misericordiae University Hospital, where she exemplifies leadership, strategic communication, and effective project management. She plays a key role on the Drug Safety and Drugs & Therapeutics Committees and chairs the Insulin Subgroup, while also contributing nationally through the Irish Medication Safety Network. Her initiatives include launching a hospital drug-information mobile app in January 2025 and leading a major quality-improvement project on missed medicines doses, developing the “Drug Administration ON TIME” framework and a TimeCritical Medicines patient education video. She has also introduced digital tools to distinguish IR and SR opioids and supported a CATO® oncology software upgrade. Awarded Young Hospital Pharmacist of the Year 2024, Jayne is deeply committed to advancing patient safety and pharmacy excellence.
Dr Gintarė Valentelytė, Postdoctoral Researcher, School of Population Health, Royal College of Surgeons Ireland
In 2025, Dr Gintare Valentelyte of RCSI University of Medicine and Health Sciences emerged as a leading voice in tackling health inequalities through data-driven research. As lead author of a major national study analysing Healthy Ireland survey data from 2015–2023, Dr Valentelyte uncovered stark socio-economic disparities in diabetes prevalence—revealing that individuals in disadvantaged communities and those with lower educational attainment face diabetes rates almost three times higher than their more affluent peers. Her work highlights an urgent need for targeted, accessible prevention programmes and a more individualised approach to chronic disease management. Supported by Science Foundation Ireland and the Research Ireland Professorship Programme, Dr Valentelyte’s research is already shaping policy discussions and guiding more equitable public health strategies across Ireland.


Professor Christian Waeber, Head of Pharmacology and Therapeutics at University College Cork
Professor Waeber has been awarded the 2025 John Feely Medal by the Irish Association of Pharmacologists—the organisation’s highest honour. Presented at its annual meeting in University College Dublin, the medal recognises his sustained, island-wide impact on pharmacology. Over thirteen years at UCC, Prof Waeber has strengthened research, teaching, and postgraduate training, contributing major advances in drug development, migraine and stroke science, and controlled-release drug-delivery platforms. He led the creation of the Munster Integrated Stroke Research and Education Catalyst in 2023 and has authored over 150 papers with 31,000+ citations. His award highlights a career defined by leadership, innovation, and exceptional scientific contribution.
Professor Leonie Young, Lead, The Endocrine Oncology Research Group
Professor Leonie Young, a leading breast cancer researcher at RCSI, continued to shape international precision oncology in 2025 through landmark discoveries in endocrineresistant breast cancer. As head of the Young Laboratory, she leads pioneering research examining tumour biology, metastasis, and treatment resistance—work that has influenced global therapeutic strategies. In 2025, Professor Young advanced groundbreaking studies on circulating tumour cells and molecular signatures that predict response to therapy, strengthening Ireland’s reputation at the forefront of cancer research. A passionate mentor and advocate for women in STEM, she plays a central role in national research infrastructure, clinical collaboration, and EU-funded innovation programmes. Her scientific leadership continues to transform patient care and drive the future of personalised breast cancer treatment.

Preconception Care
Preconceptual care reduces risk of spontaneous preterm birth for select high-risk populations: A prospective study
Gillian A Corbett1 2, Larissa Luethe2, Aoife McEvoy1, Maggie O'Brien1, Brian McDonnell1, Silvia Carlotta Rosalia2, Fionnuala M McAuliffe1 2 and Siobhan Corcoran2
1National Maternity Hospital, Dublin, Ireland.
2Preterm Birth Service, National Maternity Hospital, Dublin, Ireland.
Preterm birth, defined as birth before 37 weeks’ gestation, is the leading global cause of mortality for children under five.1 Spontaneous preterm birth (sPTB) accounts for most preterm births. Preterm birth is associated with significant health, psychological and financial cost for the individual, their family and society as a whole.2-4 Despite this, strategies to effectively predict and prevent spontaneous preterm birth are lacking. Although advances in research surrounding progesterone therapy, cervical and abdominal cerclage,5 the global rate of preterm birth has not decreased in the last decade.6 Preterm birth remains the single greatest c ontributor to loss of human capital of any disease across the human lifecourse.7
Preconceptual care is defined by the WHO as interventions provided before pregnancy to promote the health and wellbeing of women and families, as well as improved pregnancy and childhealth outcomes.8 Preconceptual counseling has many acknowledged benefits for those at risk of preterm birth including positive patient experience and enhanced patient knowledge.9, 10 Qualitative work with experts by experience in preterm birth shows that pre-conceptual counseling, anticipation of risk and an active approach to risk reduction can improve sense of control, mitigate the trauma of an unanticipated spontaneous preterm birth. This can improve the psychological burden for parents in subsequent pregnancy.11 Preconceptual counseling is associated with improved pregnancy outcomes including greater disease control and reduced rates of adverse pregnancy outcomes including preterm birth in patients with
chronic medical disorders.12 13 Yet, impact of preconceptual counseling on rates of PTB in those with risk factors is not yet well established.14
Preconceptual prevention of preterm birth may encompass several strategies. Preconceptual supplementations with folate and omega 3 reduces risk of preterm birth.5 15 Mitigation of predisposing factors such as smoking cessation is associated with reduced risk of PTB.16 Most conventional preterm prevention therapies such as vaginal progesterone, cervical cerclage and cervical pessary are not offered until the end of the first trimester of pregnancy. Abdominal cerclage can be offered either in pregnancy or ahead of pregnancy with judicious population selection. Recent cohort studies have found that laparoscopic pre-pregnancy abdominal cerclage may be associated with higher rates of full-term birth.17 The Born Too Soon movement 2013 report recommends optimisation of medical conditions, nutritional factors, mental health, smoking patterns, sexually transmitted infections and intimate partner violence for all women of reproductive age to reduce population risk of PTB.18 It recommends evaluation of impact of preconception care programs on rates of preterm birth but since this report, there have been limited data specifically dedicated to preconceptual preterm birth prevention care.
At the Preterm Birth Clinic of this center, formal preconceptual care has been delivered for women at risk of PTB since 2012, with service explansion from 2018. The present study examined the structure, assessment and management of these consultations, as well as impact on preterm birth rates in subsequent pregnancies.
MATERIALS AND METHODS
This was a prospective quasiexperimental study of all preconceptual counseling consultations and subsequent pregnancy outcomes at the Preterm Birth Clinic at a tertiary referral maternity center, Dublin, Ireland over a 5-year period (2018–2023). The center is a tertiary-level university maternity hospital. Inclusion criteria was any woman who attended the Preterm Birth Clinic for prepregnancy counseling with risk factors including prior PTB, spontaneous mid-trimester loss (sMTL), recurrent PTB or significant cervical surgery such that the cervix is noted to be short on clinical examination. These patients are referred for counseling from a variety of sources where their risk of PTB was deemed to be significant: internal and external obstetric referral, bereavement services, neonatology colleagues, colposcopy colleagues, or referral from general practice or patient themselves. Preterm birth categories are defined according to the WHO as extremely preterm (less than 28 weeks), very preterm (28 to less than 32 weeks), late preterm (32–37 weeks),7 with midtrimester loss defined as delivery between 14+0 and 22+6 weeks gestation (inclusive). The threshold of viability in this unit is 23+0 weeks gestation, differentiating late second trimester loss and extreme early preterm birth.
The decision-making tree used for preconceptual consultations is presented in Figure 1
Preconceptual counseling was conducted by a maternal-fetal medicine consultant with special interest in preterm birth. Content of preconceptual counseling is described in detail in Appendix S1 Counseling was conducted over a one 30-min consultation, with the option for a second or
third visit if additional follow up discussion is required. Factors important for decision making included significant history of sPTB/sMTL and cervical length on transvaginal ultrasound. Women with a history of prior sMTL, perinatal death resulting from sPTB before 28 weeks or recurrent sPTB are deemed particularly high-risk, with the first two criteria also experiencing the trauma associated with perinatal loss. This is a major factor in subsequent pre-pregnancy decision making. At pre-pregnancy consultation, four management options are outlined to the patient, and a joint decision is made based on individualised risk assessment of sPTB. Management options include:
1. Preterm Birth Clinic care with cervical length surveillance, reserving progesterone or cerclage for a cervical length <25mm.
2. Preterm Birth Clinic care with progesterone (if history of sPTB or cervical length <25 mm in pregnancy) and consideration for cerclage if cervical length <25 mm on serial cervical scanning.
3. Preterm Birth Clinic care with elective cervical cerclage in early pregnancy, based on history and pre-pregnancy cervical length.
4. Abdominal cerclage prepregnancy.
For those with a subsequent pregnancy, pregnancy outcomes were analysed and compared to a contemporary sPTB cohort of consecutive patients attending the Preterm Birth Clinic (2021–2023) who did not receive preconceptual counseling. The criteria for identification of these women were the same as for preconceptual counseling, being either a prior preterm birth or significant cervical surgery and difference in risk
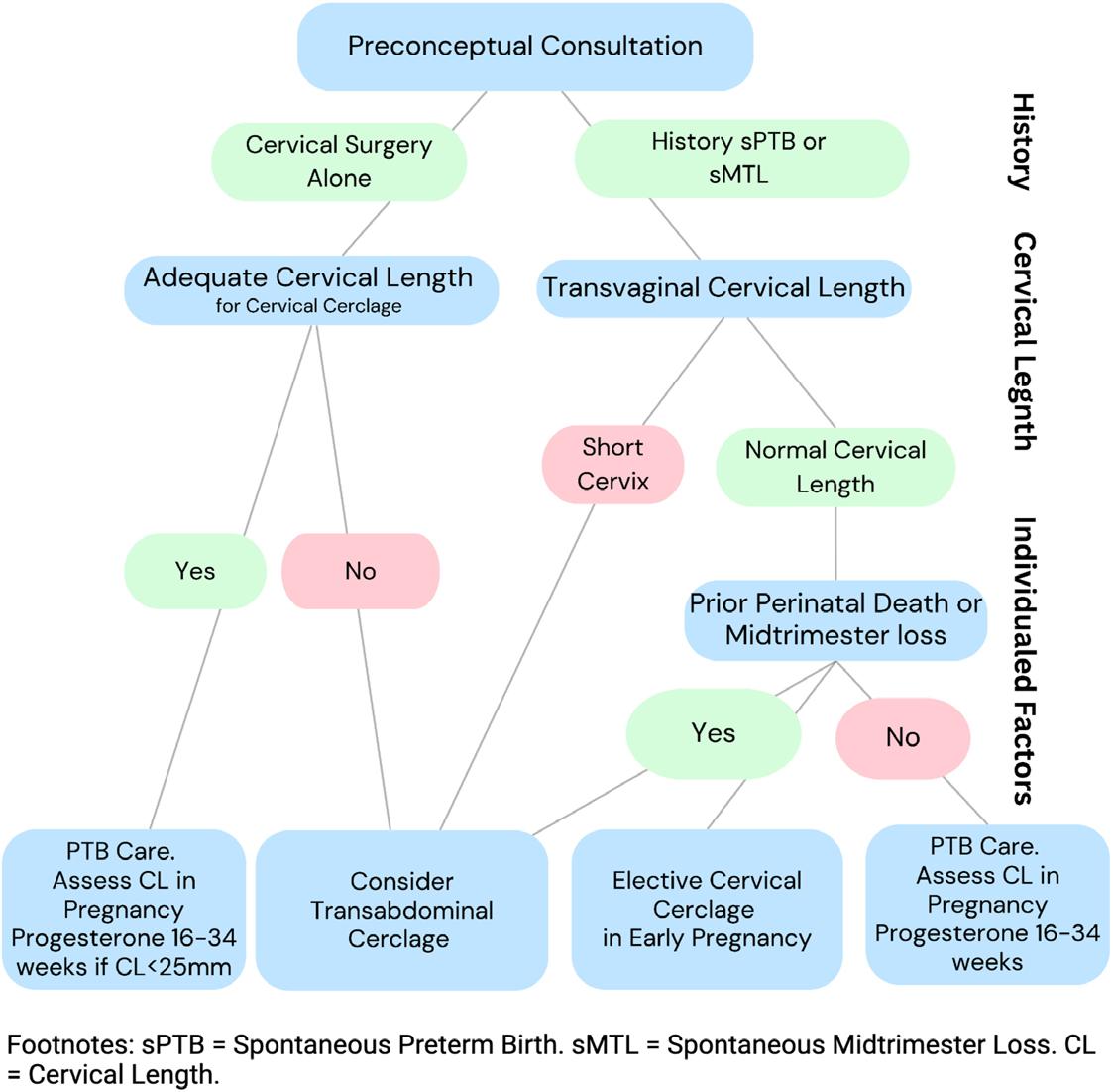
Figure 1: Preconceptual consultation decision tree, illustrating key factors in decision making including exact preterm birth history, pre-pregnancy cervical length and individualised situational factors.
factor profile including high-risk criteria (prior MTL, PTB <28 weeks or recurrent PTB) was examined between groups. Maternal demographics, preterm birth risk factor profile, sPTB prevention strategy and pregnancy outcomes were compared between pregnancy cohorts. Cervical length is measured at booking
for all women, except those with a pre-pregnancy abdominal cerclage in place.
At this Preterm Birth Clinic, booking occurs at 10–12 weeks’ gestation and cervical length assessment and individualised preterm birth risk assessment is performed, after screening and treatment of vaginal infection. Vaginal progesterone (200 mg nocte until 34 weeks’ gestation) is recommended for anyone with prior sPTB or sMTL or if cervical length is <25 mm. Cervical cerclage is considered if cervical
length is <25 mm at booking or at subsequent serial cervical length.
A cervical pessary is considered for those with a very short cervix where there is technical challenge associated with placement of cervical cerclage or disputed benefit of cervical cerclage. Abdominal cerclage is considered in the first trimester if there is history of recurrent sPTB/MTL refractory to cervical cerclage. All women having cervical cerclage, abdominal cerclage or pessary for short cervix are commenced on vaginal progesterone, if not already commenced. The cervical cerclage
or pessary is removed at 36 weeks’ gestation or sooner if labor or preterm rupture of membranes. Serial cervical length assessment is performed every 3 weeks from booking until 28 weeks. If a cerclage or abdominal cerclage, or cervical pessary is in place in, serial cervical length assessment is not performed. Vaginal progesterone is coadministered if there is a significant history of sPTB (recurrent MTL or sPTB, particularly if perinatal loss). Women with abdominal cerclage are delivered by prelabour cesarean section at 38 weeks’ gestation.
Preconception Care
Descriptive statistics were used to analyse all preconceptual consultations. To compare pregnancy cohorts, data were assessed for normality and log transformed where non-normal data was present. Normally distributed continuous outcomes were compared using an independent t-test and expressed using mean and standard deviation (SD). Non-normally distributed continuous outcomes were compared using a Wilcoxon rank test and expressed using median and interquartile range (IQR). Categorical variables were compared using the chi-square test. Sensitivity analysis was performed based on risk factor profile for cervical surgery alone and for very high risk of preterm birth, defined as prior spontaneous mid-trimester loss, preterm birth before 28 weeks or recurrent sPTB. Statistical significance was taken as a P value less than 0.05. Given the exploratory nature of the study and relatively small sample size, correction for multiple testing was not performed.19 20 All statistical analysis was performed using R studio (R version 4.3.2 [2023-1031]). Ethical approval was granted by the Institutional Review Board (study ID E15.2022).
RESULTS
In the 5-year period, 97 women attended for preconceptual counseling. Source of referral for preconceptual counseling included internal colposcopy (25/97, 27.5%), internal neonatology (6/97, 6.6%), internal obstetric (38/97, 39.2%), external obstetric (9/97, 9.9%), general practitioners (8/97, 8.8%), fertility centers (8/97, 8.8%) and self-referral from patients (3/97, 3.3%). There was a prior history of sPTB or sMTL in 62/97 (64%), with 36/40 (36.0%) having a history of cervical surgery alone. Of the women with previous sPTB/ MTL, 20 had significant risk factors prior to the sPTB/MTL including multiple pregnancy, fully dilated cesarean section or previous cervical surgery. Median (IQR) cervical length at pre-pregnancy consultation was short at 19.0 (13.0) mm. After consultation and joint decision making (Figure 1), 48/97 (49.5%) chose reassessment in early pregnancy and consideration of vaginal progesterone and cerclage, 15/97 (15.5%) chose elective cerclage in early pregnancy and 34/97 (35.0%) chose pre-pregnancy abdominal cerclage.
Of the women who attending preconceptual counseling, 47/97 (48.5%) had a subsequent pregnancy. Management at pre-pregnancy counseling versus ultimate sPTB prevention management in pregnancy is
presented in Figure 2. These pregnancies were compared to comparison pregnancy cohort with similar risk profile who did not have preconceptual counseling, with no significant difference in risk factors for preterm birth between the two cohorts. Pregnancy outcomes for 165 women were analysed, 47 who attended for preconceptual counseling and 118 who did not. Women in pregnancy after preconceptual counseling were slightly younger and less multiparous than the comparison group (Table 2) with no difference in BMI, ethnicity or educational attainment. There was no difference in sPTB risk factor profile between the pregnancy cohorts, including similar prevalence of high-risk populations between both groups (with a history of sMTL, sPTB before 28 weeks or recurrent sPTB).
Cervical length at booking was similar between groups, where cervical length was not measured for anyone with a pre-pregnancy abdominal cerclage.
Women without preconceptual counseling were more likely to have surveillance without need for progesterone or cervical cerclage (34/118, 28.8% vs. 2/47, 4.3%, P = 0.001), and were less likely to have abdominal cerclage (4/118, 3.4% vs. 17/47, 36.2%, P < 0.001). Gestation at delivery was significantly lower in the preconceptual care group (median, IQR 38.1.[1.8] vs. 38.9
[2.6], P = 0.041). There was no significant difference in rates of term birth, or preterm birth or mid-trimester loss of any category. No sPTB before 32 weeks’ gestation occurred for women who had preconceptual care prior to pregnancy.
Sensitivity analysis was performed for all women with very highrisk history. These women were significantly more likely to have abdominal cerclage in pregnancy (5/19, 26.3% vs. 0/32, 0.0%, P = 0.003). There was no significant difference in gestation at delivery or rate of full-term birth. However, there was a significant reduction in the rate of sPTB before 32 weeks in women who had preconceptual counseling (0/19, 0.0% vs. 6/32, 18.8%, P = 0.044). On regression analysis of impact of intervention on gestation at delivery and adjusted for maternal confounders and preterm birth treatment, preconceptual counseling did not significantly alter gestation at delivery (ß coefficient 0.49, 95% CI: 2.11, 3.03). Of the high-risk women with sMTL after preconceptual care (n = 3), all had cervical cerclage in pregnancy with mid-trimester loss refractory to cervical cerclage (delivery gestation ranging from 14 to 21 weeks of pregnancy). All three of these women went on to have abdominal cerclage after this loss and had a subsequent full-term delivery. For women with previous
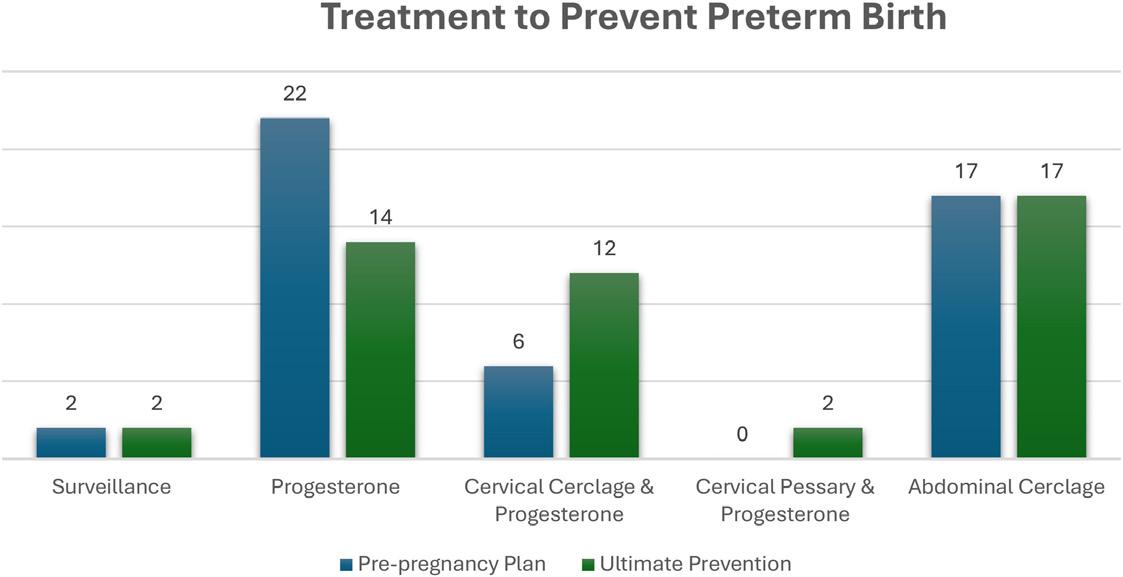
excisional cervical surgery but no history of preterm birth (n = 60), preconceptual counseling did not impact sPTB or sMTL rates at any gestation.
DISCUSSION
The present study details preconceptual consultations for women at risk of sPTB and found that for a select highrisk subgroup, preconceptual counseling significantly reduced the risk of sPTB before 32 weeks. Women receiving preconceptual counseling for risk of sPTB were more ethnically diverse and overweight than the general hospital population21 and had a short cervix at consultation. Almost two-thirds had a prior sPTB or MTL, with 45% having a birth before 28 weeks and 24.7% having prior perinatal death or periviable loss. There was a high rate of uptake of pre-pregnancy abdominal cerclage. Most women did not change ultimate prevention of preterm birth management in pregnancy. Of women with preconceptual counseling, those who had a subsequent pregnancy were slightly younger, normal weight, less diverse and had a higher educational attainment.
Many studies have found reduced risk of preterm birth with preconceptual care and optimising nutritional factors, tobacco exposure, summarised by a recent FIGO checklist for preconceptual care.22 These studies are based mainly on data from general obstetric populations. Specific to preterm birth cohorts, a review of 92 pre-pregnancy consultations in a New Zealand preterm birth service found similar rate of opting for pre-pregnancy cerclage (31/92,
33.7%,) but were a combination of abdominal cerclage high transvaginal cerclage with circumferential dissection.23 There was a similar rate of subsequent pregnancy (39/92, 42.4%) but pregnancy outcomes were not detailed.23 Review of the literature found a lack of contemporary data examining outcomes after preconception counseling for women at high-risk of preterm birth, underlining the importance of data presented in this study.
As highlighted in many documents on preterm birth prevention, population selection is paramount.24 This study found that preconceptual care did not reduce preterm birth rates for all patients. For women with history of cervical surgery but no history of preterm birth, preconceptual care does not reduce preterm birth rate. However, for women with a particularly high-risk histories, preconceptual counseling is associated with a significant reduction in rate of sPTB under 32 weeks compared to a control cohort with similar risk factor profile who did not receive pre-pregnancy counseling. This reduction appears to be actioned through the uptake of prepregnancy abdominal cerclage, which is significantly higher in the pre-pregnancy consultation group. Rates of recurrence of preterm birth are highest with preterm birth before 28 weeks or mid-trimester loss, ranging from 21% to 27%.2527 Indeed, data presented here found that for women with sPTB <28 weeks, mid-trimester loss or recurrent sPTB, the recurrence rate was 37.5% in the subsequent pregnancy (Table 3). As such, effective interventions such as
NEWS - University Hospital Galway Emergency Department Wins Bronze
preconceptual counseling to reduce subsequent preterm birth hold significant potential for impact both on health of the mother and child, as well as the healthcare facility and wider society.
This study identifies a high-risk subgroup of women (prior sPTB <28, sMTL or recurrent sPTB) in whom preconceptual care reduces risk of early PTB <32 weeks’ gestation. These women will benefit from referral to a preterm birth clinic or local obstetric with equivalence expertise ahead of subsequent pregnancy. In our center, this consultation is typically timed for 6–12 months after the preterm birth, which allows for recovery from birth and pregnancy, neonatal care and events to evolve and spans the minimum recommended interpregnancy interval of 6 months. This study assists in standardising referral criteria for pre-pregnancy care for those at significant risk of preterm birth. This has a role in promoting access equity by offering prepregnancy consultation to all, including those who often miss the opportunity for pre-pregnancy optimisation including women of non-European descent, higher deprivation scores and lower socioeconomic status.
Limitations of this study include the small cohort, with approximately twenty prepregnancy consultations are performed per year at our Preterm Birth Clinic. This sample size limits data modeling and extrapolation, particularly for subgroups and adjusting for confounders The possibility of chance findings because of multiple testing should be considered and our results
The Emergency Department (ED) at University Hospital Galway (UHG) took part in the Royal College of Emergency Medicine (RCEM) GreenED initiative and has been awarded Bronze Accreditation by recognising the team’s commitment to environmental responsibility and innovative waste-reduction practices in one of the hospital’s busiest clinical areas.
GreenED is an RCEM initiative which aims to measure and reduce the environmental impact in the ED. This is the first sustainability framework developed specifically for secondary-care settings and aims to reduce the carbon emissions of a department through sustainable and cost-saving practices, whilst maintaining or improving patient care. It provides a structured set of evidence-based actions, organised into Bronze, Silver, and Gold levels, along with the supporting guidance and resources needed to implement and achieve them.
The ED at University Hospital Galway alongside the ED at Mayo University Hospital, are among the first emergency departments in Ireland to receive this prestigious accreditation. The department now looks forward to building on this success as it works toward achieving Silver and ultimately Gold status.
should be interpreted in the context of this. Future multicenter studies with larger study samples and prospective concurrent control groups are required to improve power and validation of findings of these single center findings. Another limitation is absence of confounders including socioeconomic group, stress factors, smoking and chronic comorbid diseases, which are known modifiable factors for preterm birth. They are included in preconceptual counseling discussion and would be salient to include in any future analysis. These at-risk preterm birth cohorts have high uptake of cervical and abdominal cerclage, which impacts subsequent pregnancy outcomes and lengths subsequent pregnancy. Any impact of preconceptual care on pregnancy outcome is likely to be acting through these interventions but small numbers mean regression analysis controlling for these factors is challenging. There is real value in national registries such as the UK Preterm Birth Network to pool data and outcomes for preconceptual care in preterm birth cohorts.28
CONCLUSIONS
Preconceptual counseling is an effective intervention to reduce risk of early spontaneous preterm birth for select high-risk populations. Patients with prior spontaneous preterm birth before 28 weeks, spontaneous mid-trimester loss or recurrent spontaneous preterm birth should be referred for preconceptual counseling ahead of a subsequent pregnanc
References available on request

James Foley, Consultant in Emergency Medicine at University Hospital Galway said, “This achievement reflects a truly inclusive, multi-disciplinary effort across the department. Our team has embraced innovation and sustainability and these changes have not only reduced waste and improved efficiency but have also supported more patient-centred care.”
ESMO 2025: Stabilisation or Step Change?
A Clinical Look at Mixed but Meaningful Signals Across Tumour Types

The 2025 Congress of the European Society for Medical Oncology (ESMO) held in Berlin last month brought together the world’s leading oncologists, scientists and healthcare professionals to share the most up-to-date developments in cancer care. The meeting was less about shock and awe and more about steady, deliberate progress. Rather than a flurry of breakthroughs, the congress presented a cohesive narrative of refinement. Across tumour types and trial phases, the science pointed towards sharper patient selection, earlier adoption of emerging therapies, and a decisive shift from discovery to delivery. For hospital professionals, the take-home message is that the future of oncology lies not in dramatic reinvention but in meticulous integration. Precision is the new progress, and system readiness is the differentiator.
ADCs once lived on the fringes of clinical practice. At ESMO 2025 they firmly occupied centre stage. The standout presentation came from the phase 3 trial evaluating Disitamab Vedotin combined with toripalimab in untreated HER2-
expressing advanced urothelial cancer. Median progression-free survival exceeded twelve months, with overall survival curves separating impressively against platinum-based chemotherapy. Toxicity was manageable, dominated by predictable ocular and dermatological events that are increasingly familiar to experienced ADC centres. For Irish hospitals, the implications are immediate. HER2 testing in bladder cancer must transition from optional to standard. Pathology teams will need clear reflex testing policies, and pharmacy departments must review handling procedures to accommodate this new therapeutic class. Infusion units, in turn, should anticipate longer observation times and new side-effect profiles. This is the type of progress that demands infrastructure, not applause. In thoracic oncology, the congress captured both the maturity and the humility of modern immunotherapy. The HARMONi-6 study, evaluating ivonescimab plus chemotherapy for newly diagnosed squamous non-smallcell lung cancer, demonstrated
a median progression-free survival improvement of just over four months compared with tislelizumab plus chemotherapy. While not transformational, the data validate dual immuneangiogenic blockade as a tangible next step in first-line therapy. Equally important were the negative results. Multiple TIGIT-
“ESMO 2025 was not fireworks. It was wiring. The lights are about to get much brighter in the clinic.”
pathway studies once touted as “the next checkpoint revolution” failed to achieve meaningful endpoints. That honesty marked a refreshing shift away from hype and toward realism. For clinicians, this means investing in timely, comprehensive molecular profiling—EGFR, ALK, KRAS, PD-L1, and beyond—before committing to treatment. For hospital administrators, it signals the need to resource laboratory turnaround times that match clinical urgency. Delayed results translate directly into delayed therapy, and in thoracic oncology, time is tissue.
Prostate cancer provided one of the week’s most compelling glimpses of the near future. The PSMAddition trial formally introduced radioligand therapy into the metastatic hormonesensitive setting. Patients receiving lutetium-177 vipivotide tetraxetan (Pluvicto) alongside standard androgen deprivation and androgen receptor pathway inhibitors experienced a 28 per cent reduction in radiographic progression or death compared with standard care alone.
Though overall survival data remain immature, the trend is clear: radioligands are migrating upstream. For hospitals, that evolution is logistical as much as clinical. Radiopharmacies must evaluate isotope supply security, licencing compliance, and radiation safety protocols. Nuclear medicine departments will need close scheduling collaboration with oncology wards to prevent bottlenecks. These may sound like administrative details, but in practice they determine whether a promising treatment remains theoretical or becomes accessible to Irish patients.
Gastrointestinal oncology sessions carried less glamour but perhaps more immediate relevance to day-to-day multidisciplinary teams. Minimal residual disease and circulating tumour DNA dominated discussions, with multiple colorectal and gastric cancer trials confirming that ctDNA negativity strongly correlates with improved recurrence-free survival. MRD monitoring is moving rapidly from research to reality. Adjuvant treatment decisions will soon hinge on whether microscopic disease is molecularly detectable. For hospital professionals, now is the moment to establish pathways that integrate ctDNA results into tumour board deliberations. Radiology departments should coordinate with molecular labs to align scanning intervals and blooddraw timing. The consistency of process will soon matter as much as the sophistication of testing.
Hepatocellular carcinoma and biliary tract cancers, often the quiet underdogs of the oncology agenda, finally secured renewed attention. Combination strategies pairing immunotherapy with targeted agents yielded incremental but clinically meaningful gains in progression-free survival. Modest improvements, yes, but consistent across subgroups and increasingly translatable outside trial settings. For clinicians, the immediate value lies in expectation management— helping patients understand that success sometimes looks like stability rather than cure. Incremental control is still progress, particularly in diseases long associated with poor prognoses.
Breast oncology continued to blend innovation with pragmatism.
DESTINY-Breast05 and DESTINYBreast11 extended ADC use into earlier lines of HER2-positive and HER2-low disease. Selective oestrogen receptor degraders such as giredestrant generated discussion around endocrine resistance, while mature monarchE
data reinforced adjuvant CDK4/6 inhibition as a mainstay in highrisk early disease. Yet it was survivorship research that most captured clinicians’ attention. Among over six thousand BRCApositive women diagnosed during pregnancy, those who conceived again later experienced no difference in survival outcomes compared with those who did not. This finding dismantles longstanding fears about recurrence risk and demands an evolution in patient counselling. Fertility and survivorship are no longer peripheral topics—they are central to comprehensive oncology. Irish oncology clinics should ensure that reproductive counselling is embedded in treatment planning from the outset, with pharmacists and psychologists contributing alongside oncologists.
Neuroendocrine tumours, a traditionally underrepresented group, found fresh momentum in Berlin. Data on a novel ribonucleotide reductase inhibitor combined with lutetium Lu-177 for progressive gastroenteropancreatic NETs signalled renewed optimism in overcoming treatment resistance. While still early-phase, the durability of disease stabilisation was encouraging. The Irish NET community, small but highly specialised, can take these findings as a cue to expand multidisciplinary collaboration. Enhanced access to molecular imaging and referral pathways to national centres will ensure that suitable patients are not left behind as new trials open.
Beyond the science, ESMO 2025 also highlighted the human side of progress. Awards this year carried symbolic weight. Professor Natasha Leighl received the Women for Oncology Award for her leadership in mentorship and gender equity. Professor Christina Curtis earned the Translational Research Award for bridging cancer genomics with clinical application, reminding the field that precision medicine begins with data integrity. The Lifetime Achievement Award went to Professor Rolf Stahel, honoured for decades of collaborative work that shaped international lung cancer standards. These recognitions echo a message relevant to every Irish hospital: building a sustainable oncology ecosystem depends not only on trials and treatments but on people, mentorship and shared vision.
Operationally, the congress left little doubt about what hospitals must prioritise next. Pathology
Stat Box
• 12-month median progression-free survival with Disitamab Vedotin + Toripalimab in HER2positive urothelial cancer
• 4-month PFS gain with ivonescimab + chemotherapy in squamous NSCLC
• 28 % reduction in disease progression in metastatic hormone-sensitive prostate cancer with Pluvicto
• ctDNA negativity emerging as a key predictor of adjuvant success in colorectal and gastric cancers
departments should expand biomarker testing capabilities, embracing multiplex and nextgeneration sequencing platforms that can deliver actionable data within days. Pharmacy teams must upskill in the preparation and safety monitoring of ADCs and radiopharmaceuticals. Nursing services will need targeted training on handling, infusion management and radiation protection. Tumour boards must evolve to reflect modern medicine, with nuclear medicine specialists, genetic counsellors and molecular biologists becoming regular contributors rather than occasional guests. Integration across departments is no longer optional—it is the essence of safe, equitable cancer care.
There was also a subtle but important discussion around data stewardship. As ctDNA, MRD and real-world evidence begin to influence national reimbursement frameworks, data accuracy becomes a patient safety issue. Hospitals must ensure consistent sample handling, secure digital infrastructure and interoperability between laboratory and clinical information systems. A delayed or misfiled MRD result can alter treatment timing and potentially affect survival. The digital backbone of oncology is becoming as vital as the drugs themselves.
For hospital professionals in Ireland, the 2025 congress reaffirmed that the country’s oncology landscape must evolve quickly but carefully. Larger centres such as St Vincent’s, Beaumont, and Cork University Hospital are already positioned to lead the rollout of advanced diagnostics and radioligand therapy. Regional hospitals will
need clear referral networks and shared care agreements to ensure equity of access. Funding conversations will inevitably follow. While ADCs and radioligands are resource-intensive, their targeted nature means fewer nonbenefitting patients and reduced toxicity-related admissions— savings that offset initial cost. Advocacy from clinical leaders will be essential to ensure the HSE and Department of Health see these treatments as investments rather than expenditures.
ESMO’s focus on precision also redefined patient communication. Many patients now arrive quoting trial names and drug acronyms from social media, armed with halffacts and full hopes. The challenge for healthcare professionals is to translate that enthusiasm into informed consent. Clear, transparent conversation about eligibility criteria, data maturity and regulatory timelines builds trust. It also anchors expectations, reducing frustration when access delays arise. Communication, as one oncologist quipped during a panel, has become “the most personalised medicine of all.”
The final verdict from Berlin is neither stabilisation nor revolution—it is calibration. Oncology is being recalibrated to operate with more precision, more data, and more humanity. ADCs are no longer the future; they are the present. Radioligands are advancing steadily, ctDNA is shaping adjuvant decisions, and mentorship is shaping the profession itself. The systems that support this science must now catch up.
Irish hospitals face a pivotal choice: prepare now for integration or play catch-up later under pressure. That preparation is not abstract—it means investing in diagnostics, training, and workflow redesign before new licences land. The momentum of oncology has never been more aligned with operational planning. Stability, in this context, is not stagnation but readiness.
ESMO 2025 was a congress of realism. It reminded the field that progress is cumulative, not cataclysmic. Oncology’s greatest achievements rarely arrive overnight; they build quietly, study by study, guideline by guideline. The lesson for healthcare professionals is to stay connected, stay adaptable and, above all, stay collaborative. The revolution in cancer care is already happening—just in quieter, more disciplined tones.
References available on request
Heart Disease
Atrial Fibrillation associated with Heart Failure; Elucidating the clinical and economic implication of rhythm strategy
Written by and with contributions from:
Prof Usama Boles, Consultant Cardiologist and Electrophysiologist, Senior Lecturer RCSI Cardiology Department, Tipperary University Hospital, Clonmel
Dr Joseph P Morris, Cardiology SpR in TUH
Dr Lee Dobson (MB, BCh, BAO), SHO in Cardiology TUH
Dr Shikha Bu Sharar, Intern in Cardiology TUH
Introduction
Atrial fibrillation (AF) is an increasing healthcare challenge, with profound implications for patient outcomes and resource utilization. In Ireland, AF affects approximately 11% of adults aged ≥65 years1 and its prevalence continues to rise in parallel with population ageing. Within 15 years, the European population living with AF will reach 14–17 million, with 120,000–215,000 new diagnoses annually.2
AF is often underappreciated in clinical practice and mistakenly regarded as a relatively benign rhythm disturbance. The clinical presentation of AF is heterogeneous, with many patients experiencing atypical symptoms such as dyspnoea, fatigue, or exercise intolerance rather than the classic symptom of palpitations. This diagnostic challenge contributes to delayed recognition and treatment.
Given that stroke is both the leading cause of acquired disability and the second leading cause of death in Ireland,4, 5 the stroke prevention aspect of AF management has traditionally dominated clinical attention and guideline recommendations. However, heart failure (HF) represents an equally important and increasingly recognised consequence of AF.
This review examines the contemporary evidence for managing AF in the context of heart failure, with particular emphasis on rhythm control strategies including catheter ablation.
HFrEF and AF
AF and HFrEF frequently coexist and share a complex, bidirectional relationship in which each condition can precipitate or exacerbate the other through mechanisms such
as structural cardiac remodelling, neurohormonal activation, and rate-related left ventricular dysfunction.6,7
Atrial arrhythmia induced HFrEF shows worse mortality compared with other aetiologies of HFrEF.
Early studies into the influence of mortality of HFrEF patients with showed that AF is independently associated with increased 5 mortality in patients with symptomatic or asymptomatic HFrEF with risk ratio of 1.34.23
A 2009 meta-analysis of 16 RCT and observational studies of patients with HF showed mortality was 46% in AF patients compared to 32% in patients in SR during follow up.24 Patients with atrial fibrillation/ arrhythmia induced HFrEF have increased mortality with RR of 2.9; compared with patients who develop AF as a consequence of HF whereby mortality is still increased compared to SR but less so with RR of 1.8525 Rhythm control shows significant improvement in outcomes for this high-risk cohort.
EASTAFNET compared rate and rhythm control (with both pharmacotherapy and catheter ablation) looking at composite end-point of mortality from cardiovascular cause, stroke or hospitalisation due to acute coronary syndrome or worsening HF. This outcome occurred significantly less in the rhythm control group (3.9 per 100 person years) than the rate control group (5.0 per 100 person years). This equated to RR of 0.79 with rhythm control proving clear superiority. AF is ubiquitously known as a leading risk factor for stroke. A UK cohort study of electronic health records of >5,000,000 patients compared patients with and without AF, but notably without any other risk factors for stroke. Over 5 years stroke occurred in 3.8% of AF patients compared to 1.5%
in control. Interestingly all cause dementia was increased by RR of 1.17 in those with AF.10
Rhythm control for AF
In the acute setting, rhythm control is typically reserved for haemodynamically unstable patients. For stable patients who are already anticoagulated >3 weeks,[13] pharmacological or electrical cardioversion should be considered as first line. The ESC recommends rhythm control strategy within 12 months for all patients with AF who are at risk of a thrombotic event, ie CHADSVA >0.14, 15
The ESC recommends catheter ablation in patients with HFrEF and AF where there is clinical suspicion of tachycardia induced cardiomyopathy to improve LVEF, as a class I recommendation with grade A evidence. Pulmonary vein isolation is the cornerstone technique for AF ablation.16 ESC further recommend catheter ablation in all appropriate patients with both HFrEF and AF to reduce hospital admissions and improve survival.14
CASTLE-AF17 is a landmark trial in defining the success of catheter ablation, comparing medical management of AF induced HFrEF AF (rate and/or rhythm control) and catheter ablation. Both groups received guideline based therapy for HFrEF. CASTLE-AF demonstrated a 15% reduction in hospitalisation secondary to worsening HFrEF, 11% reduction in mortality from cardiovascular causes and 12% all cause mortality reduction at 3 years in patients who underwent catheter ablation. Likewise, CAMERA-MRI compared catheter ablation with rate control; for patients treated with catheter ablation the Left Ventricular Ejection Fraction (LVEF) improved by 18% on average after 6 months compared to only 4.4% in the rate control arm. This represented normalisation of LVEF (i.e. from LVEF <50% to LVEF >50%) in 58% of patients who underwent catheter ablation compared with 9% who received rate control alone.
Rhythm strategy has clear benefits in reduction of hospitalisation, morbidity and HF related mortality. The concept of AF induced HF should be more clear and treated with a focus on rhythm strategies including ablation options. The
latter is getting quite robust approach in many contemporary systems thanks to increasing expertise, Economical viability as well as innovations in technology used making it extremely safe and effective than before.
Advanced Strategies in AF Ablation and HF: Timing, Guidelines, and Refractory Cases
Timing of Ablation: Does Earlier Intervention Matter?
Initiating an early rhythm control in the natural history of AF is aimed at preventing the progressive pathophysiological changes that is seen in persistent AF which is more difficult to treat and associated with worse outcomes (Ogawa et al., 2018). The EARLYAF RCT (randomised control trial) 3-year follow-up demonstrated a 75% reduction in progression from paroxysmal to persistent AF compared with AADs (Andrade et al., 2023). The preventive role that catheter ablation has for AF-induced cardiomyopathy needs to be considered its management strategy for when AF-induced HF occurs.
In patients with AF and heart failure, the specific timing of ablation appears critical to outcomes. A retrospective study found that in acute decompensated heart failure patients with persistent AF, early ablation within 90 days of HF admission reduced all-cause mortality by 53% (HR 0.47; 95% CI 0.25-0.88, p=0.014), with similar reductions in cardiovascular and HF-specific mortality (Calvert, 2024; Sakamoto et al., 2023). These findings suggest that deferring ablation after acute HF stabilisation may miss a critical therapeutic window when the arrhythmia substrate is most amenable to intervention.
There is recent evidence supporting catheter ablation use in advanved AF-induced heart failure. The CASTLE-HTx trial demonstrated a mortality benefit with catheter ablation in endstage heart failure patients with AF (Sohns et al., 2023). This study included patients with severely symptomatic AF and end-stage HF who were being evaluated for heart transplantation or ventricular assist device implantation. The trial was terminated early after a data safety monitoring board identified an unexpectedly large mortality benefit favouring ablation, with a
hazard ratio of 0.32 (p<0.001) for the primary composite endpoint of death, LVAD implantation, or urgent heart transplantation
Updated Guidelines: ESC 2024 Recommendations for Persistent AF and HF
The 2024 ESC Guidelines for the management of atrial fibrillation significantly strengthen recommendations for catheter ablation in patients with HF (Rienstra et al., 2024; Van Gelder et al., 2024).
Class I Recommendations (Should be performed):
• Catheter ablation is now recommended as a firstline rhythm control option for suitable patients with paroxysmal AF (upgraded from IIa in the previous guideline) (Hindricks et al., 2021).
• Ablation should be performed in patients with AF and HFrEF where tachycardia-induced cardiomyopathy is suspected, with the aim of reversing left ventricular dysfunction (Demarchi et al., 2024)
• Implementation of rhythm control strategy should be considered within 12 months of AF diagnosis in selected high-risk patients to reduce cardiovascular death or hospitalisation (Kirchhof et al., 2020).
Class IIa Recommendations (To be considered):
• Catheter ablation should be considered in all appropriate patients with both HFrEF and AF to reduce hospital admissions and improve survival.
• For patients with persistent AF refractory to antiarrhythmic drug therapy, endoscopic and hybrid ablation procedures should be considered to prevent symptoms, recurrence, and AF progression.
Refractory AF with HF: Role of Physiologic pacing and AV Node Ablation
For patients with refractory permanent AF and HF who cannot achieve or maintain sinus rhythm despite optimal therapy, atrioventricular node (AVN) ablation combined with cardiac resynchronization therapy (CRT) offers an alternative strategy.
The APAF-CRT Trials; Ablate and Pace for Atrial Fibrillation—Cardiac Resynchronization Therapy) trial had two trial phases (Brignole et al., 2021; Brignole et al., 2018). The morbidity phase randomized 102 patients with severely symptomatic permanent AF, narrow QRS (≤110 ms), and recent HF hospitalisation to AV node (AVN) ablation plus CRT versus
Several comprehensive economic analyses have examined the cost-effectiveness of catheter ablation in AF-HF populations:
Region / Study ICER (Cost per QALY gained)
U.S. (Chew et al., 2020) $38,500
Canada (Lau et al., 2021)
Australia (Gao & Moodie, 2019)
CABANA Economic Analysis (Chew et al., 2022)
pharmacological rate control. AVN ablation plus CRT demonstrated 61% reduction in death or HF hospitalization, improved LVEF from 32% to 39% at 24 months, and enhanced functional capacity and quality of life.
$42,000 CAD (~$33k USD)
Conclusion
Highly cost-effective (<$50k threshold); longterm savings from fewer HF admissions.
Cost-effective; 0.36 QALY gain with ablation.
$55,900 AUD Borderline at $50k threshold
$57,900 USD Economically attractive when QoL benefits are included.
These improvements translated into meaningful cost savings that largely offset the initial procedural expenses within two to three years.
Lifetime Cost-Effectiveness
Implications for the Irish Healthcare System
Across all models, mortality reduction, QoL improvement, and lower HF hospitalization rates were the strongest drivers of cost-effectiveness. Repeat ablation procedures, procedural complications, and shorter follow-up durations can diminish its economic advantage. Overall, catheter ablation demonstrates excellent economic value, with incremental cost-effectiveness ratios typically ranging between $40,000 and $60,000 per quality-adjusted life year (QALY), making it comparable to or even more cost-effective than many other established cardiovascular interventions.
The mortality phase was stopped early after enrolling 133 patients due to survival benefit. All-cause mortality occurred in 11% with ablation & CRT versus 29% with rate control (HR 0.26; 95% CI 0.10-0.65; p=0.004), with the composite endpoint of death or HF hospitalisation showing HR 0.50 (95% CI 0.31-0.81).
Several comprehensive economic analyses have examined the cost-effectiveness of catheter ablation in AF-HF populations (see above).
Implications for the Irish Healthcare System
The HSE estimates €700 million in annual HF admissions and €650 million in GP visits related to HF [22]. In average , one third of those patient is expected to have AF as a main drive to HF worsening. Expanding access to ablation could substantially reduce this burden. Cost-effectiveness data from similar systems (UK, Canada, Australia) suggest Ireland would see comparable benefits, especially if combined with early AF detection and rhythm strategy adoption
These benefits likely derive from rate regularization improving ventricular filling, guaranteed 100% biventricular pacing without intrinsic conduction competition, avoidance of RV-only pacing dyssynchrony, and reduced neurohormonal activation [64]. Current ESC 2024 guidelines provide Class IIa recommendation for AVN ablation combined with CRT in patients with severely symptomatic AF despite rate control attempts, permanent AF where rhythm control has failed, or need for ventricular pacing (Van Gelder et al., 2024).
The CAAN-AF trial, currently ongoing, is investigating AVN ablation in patients with AF and HFrEF who have wider QRS and already have CRT devices, which will further inform optimal patient selection (Sanders et al., 2025).
Health
Economic
Impact
of Ablation in AF-HF
Catheter ablation for AF in patients with HF has also shown economic benefits. A U.S-based study of 1,568 patients with both AF and HF demonstrated substantial reductions in healthcare use within three years following catheter ablation (Field et al., 2020). AFrelated admissions decreased by 64%, HF-related admissions by 22%, emergency department visits by 51%, cardioversion procedures by 52%, and overall hospital length of stay by 65%.
Across all models, mortality reduction, QoL improvement, and lower HF hospitalisation rates were the strongest drivers of cost-effectiveness. Repeat ablation procedures, procedural complications, and shorter follow-up durations can diminish its economic advantage. Overall, catheter ablation demonstrates excellent economic value, with incremental cost-effectiveness ratios typically ranging between $40,000 and $60,000 per quality-adjusted life year (QALY), making it comparable to or even more cost-effective than many other established cardiovascular interventions.
The HSE estimates ¤700 million in annual HF admissions and ¤650 million in GP visits related to HF.22 In average , one third of those patient is expected to have AF as a main drive to HF worsening. Expanding access to ablation could substantially reduce this burden. Cost-effectiveness data from similar systems (UK, Canada, Australia) suggest Ireland would see comparable benefits, especially if combined with early AF detection and rhythm strategy adoption. While upfront investment in equipment, staffing, and training is required, even modest reductions in HF hospitalisations can rapidly offset costs. For instance, preventing a single 10-day HF admission (~¤15,000–¤20,000) and a few ED visits can nearly neutralize the initial interventional expense. The improved QOL with SR maintenance is another huge constraint and to be discussed in future correspondence.
While upfront investment in equipment, staffing, and training is required, even modest reductions in HF hospitalizations can rapidly offset costs. For instance, preventing a single 10-day HF admission (~€15,000–€20,000) and a few ED visits can nearly neutralize the initial interventional expense. The improved QOL with SR maintenance is another huge constraint and to be discussed in future correspondence.
Recent head-to-head comparisons for treatment of AF with HFrEF
Recent head-to-head comparisons for treatment of AF with HFrEF
CAMERA MRI

Catheter Ablation "CA" vs rate control [22]

EAST-AFNET 4

Newly diagnosed AF: Rate control vs rhythm control [15]

CASTLE-AF: Rhythm Control: Catheter Ablation vs. Pharmicotherapy [17]
Primary endpoint: Change in LVEF from baseline to 6 months post-CA
Mean improvement of 18% in CA group compared to 4.4% in only rate control group
Normalisation (>50%) of LVEF was significantly improved from 58% in CA group compared to 9%


Primary endpoint: composite of CVS death, stroke and hospitilisation for either HF or ACS
Main inclusion criteria: AF diagnosis <12 months
Rhythm control reduced risk of endpoint by 22%
Primary endpoint: composite of hospitalisation and mortality.
Absolute risk reduction of primary endpoint of 16.1% in CA group
Median improvement in LVEF of 8% in CA group

Heart Disease
Current evidence base of HFrEF and AF
Current evidence base of HFrEF and AF
Study/Year
Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure: The EASTAFNET 4 Trial
(Rillig et al, 2021)
Aim
To evaluate whether early rhythm control (ERC) improves outcomes in patients with HF and recently diagnosed AF.
Causal Effects Between Atrial Fibrillation and Heart Failure: Evidence from a Bidirectional Mendelian Randomization Study
(Zhang et al, 2023)
Under-recognition of Heart Failure in Patients with Atrial Fibrillation and the Impact of Gender: A UK Population-Based Cohort Study
(Zakeri et al, 2021)
Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial
(Brachmann et al, 2024)
To assess the bidirectional causal relationship between atrial fibrillation (AF) and heart failure (HF) through Mendelian randomisation analysis.
To assess the recognition rates of HF in patients with AF and examine gender differences.
Patient Randomisation
A total of 798 patients with AF and HF (NYHA class II–III or LVEF <50%) were randomised to either ERC (396 patients) or usual care (402 patients). Most had preserved LVEF (n=442), while others had midrange (n=211) or reduced (n=132) LVEF. The median follow-up was 5.1 years.
Data from large-scale genome-wide association studies involving AF (n=1,030,836) and HF (n=1,665,481) were examined.
UK primary and secondary care records from 2004 to 2016 were used to identify adults with incident AF.
Results
ERC reduced the primary composite outcome (CV death, stroke or HF/ACS hospitalisation) with a hazard ratio of 0.74 (P=0.03), consistent across HF subtypes. Safety outcomes and LVEF improvement were similar between groups.
Conclusion
Early initiation of rhythm control therapy within one year of AF diagnosis provides clinical benefits in patients with HF, including those with preserved EF, by reducing CV events without increasing adverse effects.
To evaluate the impact of AF recurrence and burden on clinical outcomes among patients with both HF and AF.
Of 363 patients with HF and AF randomised to either catheter ablation (n = 179) or pharmacological therapy (n = 184), this subanalysis was conducted on 280 patients (128 ablation, 152 pharmacological). All had implanted defibrillators with home monitoring to track AF burden.
Genetic predisposition to AF was associated with a higher risk of developing HF (OR=1.18), and vice versa, genetically determined HF increased the risk of AF (OR=1.44).
A significant number of patients with AF had unrecognised HF, with notable gender disparities in diagnosis.
AF and HF share a reciprocal causal relationship, indicating common genetic factors that may guide therapeutic development.
Improving HF recognition in AF patients, particularly among women, is crucial for timely management and better outcomes.
Clinical impact of atrial fibrillation progression in patients with heart failure with preserved ejection fraction: A report from the CHART-2 Study
(Ito et al, 2024)
To investigate the incidence and clinical impact of AF progression, specifically the transition from paroxysmal AF to persistent AF, in patients with HF with preserved ejection fraction (HFpEF).
The study enrolled 718 patients with HFpEF and paroxysmal AF from the CHART-2 registry. Patients had a median age of 72 years, with 36% being female. They were followed for a median of 6 years.
Baseline AF burden did not predict the primary composite outcome or mortality. AF recurrence was not linked to mortality or HF hospitalisations.
However, patients with an AF burden <50% at 6 months post-ablation had a significantly lower risk of adverse outcomes, with a hazard ratio of 0.33 for the primary endpoint and 0.23 for allcause mortality. A higher AF burden (≥50%) at 6 months was associated with worse clinical outcomes.
AF progression occurred in 14.6% of patients over 10 years (cumulative incidence of 16.7%). Prior HF hospitalisation (HR 1.74, P = 0.007) and left atrial diameter (per 5 mm increase, HR 1.37, P < 0.001) predicted AF
6-month AF burden was a strong predictor of clinical outcomes in patients with HF and AF. However, AF recurrence after ablation was not linked to improvements in mortality or HF hospitalisation. Reducing AF burden after ablation is crucial for improving long-term outcomes.
AF progression in HFpEF patients is associated with an increased risk of adverse outcomes, particularly worsening HF. This risk is most pronounced early after progression to persistent AF, highlighting the
Current evidence base of HFrEF and AF
Study/Year
of adverse outcomes, with a hazard ratio of 0.33 for the primary endpoint and 0.23 for allcause mortality. A higher AF burden (≥50%) at 6 months was associated with worse clinical outcomes.
Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure: The EASTAFNET 4 Trial
Clinical impact of atrial fibrillation progression in patients with heart failure with preserved ejection fraction: A report from the CHART-2 Study
(Rillig et al, 2021)
(Ito et al, 2024)
Causal Effects Between Atrial Fibrillation and Heart Failure: Evidence from a Bidirectional Mendelian Randomization Study
Management of Atrial Fibrillation Across the Spectrum of Heart Failure With Preserved and Reduced Ejection Fraction
(Zhang et al, 2023)
(Reddy et al, 2022)
Under-recognition of Heart Failure in Patients with Atrial Fibrillation and the Impact of Gender: A UK Population-Based Cohort Study
(Zakeri et al, 2021)
Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial
(Brachmann et al, 2024)
Atrial Fibrillation Ablation in Heart Failure with Reduced vs Preserved Ejection Fraction: A Systematic Review and Meta-Analysis
(Oraii et al, 2024)
Conclusion
evaluate whether early rhythm control (ERC) improves outcomes in patients with HF and recently diagnosed AF.
To investigate the incidence and clinical impact of AF progression, specifically the transition from paroxysmal AF to persistent AF, in patients with HF with preserved ejection fraction (HFpEF).
To assess the bidirectional causal relationship between atrial fibrillation (AF) and heart failure (HF) through Mendelian randomisation analysis.
To review the interplay between AF and HF, examine the role of atrial myopathy and evaluate emerging evidence supporting catheter ablation as a treatment strategy in selected patients. The review also aims to offer practical guidance on managing AF in HF and identify areas for future research.
To assess the recognition rates of HF in patients with AF and examine gender differences.
To evaluate the impact of AF recurrence and burden on clinical outcomes among patients with both HF and AF.
To compare the effects of catheter ablation for AF in patients with HFrEF versus HFpEF.
The study enrolled 718 patients with HFpEF and paroxysmal AF from the CHART-2 registry. Patients had a median age of 72 years, with 36% being female. They were followed for a median of 6 years.
A total of 798 patients with AF and HF (NYHA class II–III or LVEF <50%) were randomised to either ERC (396 patients) or usual care (402 patients). Most had preserved LVEF (n=442), while others had midrange (n=211) or reduced (n=132) LVEF. The median follow-up was 5.1 years.
Data from large-scale genome-wide association studies involving AF (n=1,030,836) and HF (n=1,665,481) were examined.
Analysis of existing studies on AF, atrial myopathy and HF to assess treatment approaches, particularly the role of rhythm control strategies like catheter ablation.
UK primary and secondary care records from 2004 to 2016 were used to identify adults with incident AF.
Of 363 patients with HF and AF randomised to either catheter ablation (n = 179) or pharmacological therapy (n = 184), this subanalysis was conducted on 280 patients (128 ablation, 152 pharmacological). All had implanted defibrillators with home monitoring to track AF burden.
Systematic review and meta-analysis of 12 RCTs and 4 substudies, involving 2,465 participants.
ERC reduced the primary composite outcome (CV death, stroke or HF/ACS hospitalisation) with a hazard ratio of 0.74 (P=0.03), consistent across HF subtypes. Safety outcomes and LVEF improvement were similar between groups.
AF progression occurred in 14.6% of patients over 10 years (cumulative incidence of 16.7%). Prior HF hospitalisation (HR 1.74, P = 0.007) and left atrial diameter (per 5 mm increase, HR 1.37, P < 0.001) predicted AF progression. Progression was associated with worsening HF (HR 1.68, P = 0.004); 26% of
events occurred within 1 year of AF progression.
Genetic predisposition to AF was associated with a higher risk of developing HF (OR=1.18), and vice versa, genetically determined HF increased the risk of AF (OR=1.44).
AF and HF have a bidirectional relationship, where AF can be either a cause or a consequence of HF. Initial trials did not show a benefit of systematic rhythm control in HF with reduced ejection fraction (HFrEF), but newer data suggest that selected patients may benefit from catheter ablation. Additionally, emerging evidence indicates a link between AF, left atrial myopathy, mitral regurgitation and HFpEF, with catheter ablation showing potential benefits in this population. Management of AF in HF should consider atrial myopathy severity, AF chronicity and the underlying left ventricular HF phenotype.
A significant number of patients with AF had unrecognised HF, with notable gender disparities in diagnosis.
Baseline AF burden did not predict the primary composite outcome or mortality. AF recurrence was not linked to mortality or HF hospitalisations.
Catheter ablation reduced HF events and cardiovascular death in HFrEF patients but showed no benefit in HFpEF patients.
However, patients with an AF burden <50% at 6 months post-ablation had a significantly lower risk of adverse outcomes, with a hazard ratio of 0.33 for the primary endpoint and 0.23 for allcause mortality. A higher AF burden (≥50%) at 6 months was associated with worse clinical outcomes.
Early initiation of rhythm control therapy within one year of AF diagnosis provides clinical benefits in patients with HF, including those with preserved EF, by reducing CV events without increasing adverse effects.
AF progression in HFpEF patients is associated with an increased risk of adverse outcomes, particularly worsening HF. This risk is most pronounced early after progression to persistent AF, highlighting the importance of timely identification and intervention in at-risk patients.
AF and HF share a reciprocal causal relationship, indicating common genetic factors that may guide therapeutic development.
The modern management of AF in HF should be individualised, taking into account atrial myopathy, AF chronicity, and HF phenotype. Catheter ablation may provide benefits for select patients, particularly those with HFrEF and possibly those with HFpEF. Further research is needed to refine treatment strategies and determine which patient subgroups benefit the most from rhythm control interventions.
Improving HF recognition in AF patients, particularly among women, is crucial for timely management and better outcomes.
6-month AF burden was a strong predictor of clinical outcomes in patients with HF and AF. However, AF recurrence after ablation was not linked to improvements in mortality or HF hospitalisation. Reducing AF burden after ablation is crucial for improving long-term outcomes.
Catheter ablation for AF is beneficial in HFrEF but not in HFpEF, highlighting the need for tailored treatments based on ejection fraction.
Conclusion - Atrial fibrillation and heart failure are common, interlinked conditions that require early recognition and evidence-based management across all levels of care. Timely rhythm control, particularly through catheter ablation, has been shown to improve symptoms, QOL, and reduce mortality. Early referral for rhythm control strategies should therefore be strongly encouraged, especially in patients with AF-induced or AF-exacerbated heart failure. Broader access to ablation services and continued investment in public electrophysiology infrastructure are likely to be both clinically beneficial and economically justified, representing a high-value approach to improving cardiovascular outcomes. References available on request
Clinical impact of atrial fibrillation progression in patients with heart failure with preserved ejection fraction: A report from the CHART-2 Study
Atrial fibrillation and heart failure are common, interlinked conditions that require early recognition and evidence-based management across all levels of care. Timely rhythm control, particularly through catheter ablation, has been shown to improve symptoms, QOL, and reduce mortality. Early referral for rhythm control strategies should therefore be strongly encouraged, especially in patients with AF-induced or AF-exacerbated heart failure. Broader access to ablation services and continued investment in public electrophysiology infrastructure are likely to be both clinically beneficial and economically justified, representing a high-value approach to improving cardiovascular outcomes.
(Ito et al, 2024)
To investigate the incidence and clinical impact of AF progression, specifically the transition from paroxysmal AF to persistent AF, in patients with HF with preserved ejection fraction (HFpEF).
The study enrolled 718 patients with HFpEF and paroxysmal AF from the CHART-2 registry. Patients had a median age of 72 years, with 36% being female. They were followed for a median of 6 years.
AF progression occurred in 14.6% of patients over 10 years (cumulative incidence of 16.7%). Prior HF hospitalisation (HR 1.74, P = 0.007) and left atrial diameter (per 5 mm increase, HR 1.37, P < 0.001) predicted AF
AF progression in HFpEF patients is associated with an increased risk of adverse outcomes, particularly worsening HF. This risk is most pronounced early after progression to persistent AF, highlighting the





*Updated PFS at 6.2-year median follow-up is an investigatorassessed exploratory descriptive ad hoc endpoint. The 5-year PFS rates are based on estimates derived from the 6.2-year median follow-up Kaplan-Meier curves: overall population HR 0.66 (95% CI, 0.55-0.78); HRd population HR 0.51 (95% CI, 0.40-0.66); HRp population HR 0.67 (95% CI, 0.50-0.89). PFS results from the long-term follow-up of the PRIMA study were concordant with the primary analysis. In the PRIMA primary analysis, the primary endpoint was PFS in the HRd population followed by PFS in the overall population, as determined on hierarchical testing. PFS was evaluated by BICR. HRd population HR 0.43 (95% CI, 0.31-0.59, P<0.001); overall population HR 0.62 (95% CI, 0.50-0.76, P<0.001).1,2 †ZEJULA capsules and tablets can be taken at any time of day, at approximately the same time each day.3
1LM, first-line maintenance; BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; HRd, homologous recombination deficient; HRp, homologous recombination proficient; HRQoL, health-related quality of life; PFS, progression-free survival.
References:
1. Monk BJ, Barretina-Ginesta B, Pothuri I, et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: final overall survival results from PRIMA/ENGOTOV26/GOG-3012 trial. Ann Oncol. 2024 Sep 14:S0923-7534(24)03762-1. doi:10.1016/j.annonc.2024.08.2241. Online ahead of print.
2. González-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019;381(25):2391-2402.
3. ZEJULA (niraparib). Summary of Product Characteristics. GlaxoSmithKline; 2024. Available at: https://www.medicines.ie/ medicines/zejula-100-mg-film-coated-tablets-35495/spc [Accessed: October 2025].
4. González-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019;381(25)(suppl):2391-2402.
Zejula 100 mg film-coated tablets
Abbreviated Prescribing Information (Refer to Summary of Product Characteristics (SmPC) before prescribing).
PRESENTATION: Each tablet contains niraparib tosylate monohydrate equivalent to 100 mg of niraparib. Each tablet contains 34.7 mg lactose. INDICATIONS: 1. Monotherapy for the maintenance treatment of adult patients with advanced epithelial (FIGO Stages III and IV) high-grade ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. 2. Monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. POSOLOGY AND ADMINISTRATION: Treatment should be initiated and supervised by a physician experienced in the use of anticancer medicinal products. Zejula tablets should be taken without food (at least 1 hour before or 2 hours after a meal) or with a light meal. Patients should be encouraged to take their dose at approximately the same time each day. Bedtime administration may be a potential method for managing nausea. First-line ovarian cancer maintenance treatment: 200 mg taken once daily. However, for those patients who weigh ≥ 77 kg and have baseline platelet count ≥ 150,000/μL, the recommended starting dose is 300 mg, taken once daily. Recurrent ovarian cancer maintenance treatment: 300 mg once daily. If patient weighs less than 58 kg a starting dose of 200 mg may be considered. Treatment should be continued until disease progression or toxicity. Missing dose: If patients miss a dose, they should take their next dose at its regularly scheduled time. For dose modifications due to adverse reactions see SmPC. Elderly (≥ 65 years): No dose adjustment necessary; limited clinical data in patients aged >75 years. Renal impairment: No dose adjustment with mild to moderate renal impairment. Use with caution in severe renal impairment or end stage renal disease undergoing haemodialysis. Hepatic impairment: No dose adjustment with mild hepatic impairment (either aspartate aminotransferase (AST) > upper limit of normal (ULN) and total bilirubin (TB) ≤ ULN or any AST and TB > 1.0 x – 1,5 x ULN). For patients with moderate hepatic impairment (any AST and TB > 1.5 x - 3 x ULN) the recommended starting dose of Zejula is 200 mg once daily. Use with caution in patients with severe hepatic impairment (any AST and TB > 3 x ULN) patients. Patients with ECOG performance status 2 to 4: No clinical data. Paediatric population: No data available. CONTRAINDICATIONS: Hypersensitivity to niraparib or to any of the excipients. Breast-feeding. WARNINGS/PRECAU-

Sustained progression-free survival at 6.2 years
with once-daily ZEJULA 1LM monotherapy treatment1-3*†

TIONS: Haematologic adverse reactions: Complete blood counts should be done weekly for the first month, followed by monthly monitoring for the next 10 months and periodically after this time is recommended to monitor for clinically significant changes in any haematologic parameter. If a severe persistent haematologic toxicity develops that does not resolve within 28 days following interruption, Zejula should be discontinued. Myelodysplastic syndrome/acute myeloid leukaemia (MDS/AML): For suspected MDS/AML or prolonged haematological toxicities the patient should be referred to a haematologist. If MDS/AML is confirmed, Zejula should be discontinued, and the patient treated appropriately. Hypertension, including hypertensive crisis: Pre-existing hypertension should be adequately controlled before starting Zejula treatment. Blood pressure should be monitored at least weekly for two months and monitored monthly afterwards for the first year and periodically thereafter during treatment with Zejula. Hypertension should be medically managed as well as adjustment of the Zejula dose if necessary. Zejula should be discontinued in case of hypertensive crisis or if medically significant hypertension cannot be adequately controlled. Posterior Reversible Encephalopathy Syndrome (PRES): PRES is a rare, reversible, neurological disorder which can present with rapidly evolving symptoms including seizures, headache, altered mental status, visual disturbance, or cortical blindness, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging (MRI). In case of PRES, it is recommended to discontinue Zejula and to treat specific symptoms including hypertension. The safety of reinitiating Zejula therapy in patients previously experiencing PRES is not known. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Zejula. Patients who take Zejula may experience asthenia, fatigue, dizziness or difficulties concentrating. Patients who experience these symptoms should observe caution when driving or using machines. INTERACTIONS: Caution in combination with vaccines, immunosuppressant agents or with other cytotoxic medicinal products. Caution when combined with active substances the metabolism of which is CYP3A4-dependent and, notably, those having a narrow therapeutic range (e.g. ciclosporin, tacrolimus, alfentanil, ergotamine, pimozide, quetiapine, and halofantrine). Caution when combined with active substances the metabolism of which is CYP1A2-dependent and, notably, those having a narrow therapeutic range (e.g. clozapine, theophylline, and ropinirole). Caution when combined with substrates of BCRP (irinotecan, rosuvastatin, simvastatin, atorvastatin, and methotrexate). In-




Hypothetical patient representations. HRQoL was preserved in patients receiving ZEJULA throughout treatment (exploratory analysis; data cut-off: primary analysis), while delivering sustained PFS rate benefit vs placebo at 6.2-year median follow-up (per investigator-assessed exploratory descriptive ad hoc analysis).1,2,4*
creased plasma concentrations of co-administered medicinal products that are substrates of MATE1 and -2 (e.g. metformin) cannot be excluded. Caution when niraparib is combined with active substances that undergo an uptake transport by OCT1 such as metformin. Fertility, pregnancy and lactation: Fertility: No clinical data. Zejula should not be used during pregnancy or in women of childbearing potential not willing to use highly effective contraception during therapy and for 6 month after receiving the last dose. A pregnancy test should be performed on all women of childbearing potential prior to treatment. Pregnancy: Zejula should not be used during pregnancy. Breast-feeding: Contraindicated during administration and for one month after last dose. UNDESIRABLE EFFECTS: Very common (≥ 1/10): UTI, thrombocytopenia, anaemia, neutropenia, leukopenia, decreased appetite, insomnia, headache, dizziness, palpitations, hypertension, dyspnoea, cough, nasopharyngitis, nausea, constipation, vomiting, abdominal pain, diarrhoea, dyspepsia, back pain, arthralgia, fatigue, asthenia. Common (≥ 1/100, < 1/10): Bronchitis, conjunctivitis, MDS/AML, hypersensitivity, hypokalemia, anxiety, depression, cognitive impairment, dysgeusia, tachycardia, epistaxis, dry mouth, abdominal distension, mucosal inflammation, stomatitis, myalgia, photosensitivity, rash, peripheral oedema, increased GGT, AST, ALT, AKK Phos, creatinine, decreased weight. For more details on undesirable effects, see SmPC. Marketing Authorisation (MA) Holder: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA Nr: EU/1/17/1235/004. Legal category: POM A. Date of preparation of API: July 2025. Code: PI-12578. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255 or via online form https://gsk.public.reportum.com.
Date of preparation: November 2025 | PM-IE-NRP-JRNA-250001. Trade marks are owned by or licensed to the GSK group of companies. ©2025/2026 GSK group of companies or its licensor.
¤28M Programme to Deliver Next Generation Cancer Care
Minister for Further and Higher Education, Research, Innovation and Science, James Lawless TD, launched Phase 2 of Ireland’s leading and largest ever cancer research programme, Precision Oncology Ireland (POI).

Siobhan Gaynor, Advanced Cancer Patient Council member and patient researcher; Prof Donal Brenan, UCD Professor of Gynae-Oncology, Consultant Gynaecological Oncologist at the Mater Hospital, and Deputy-Director of POI; Prof Walter Kolch, Director of Precision Oncology Ireland and Systems Biology Ireland (based at UCD); Dr Siobhan Roche, Director of Science for the Economy, Taighde Éireann–Research Ireland; Prof Kate Robson Brown, UCD Vice-President for Research, Innovation and Impact. Photo credit: Angela Halpin, photographer
POI is a cancer research consortium dedicated to advancing personalised cancer research and care. Its mission is to develop tailored diagnostic and therapeutic solutions using cuttingedge technologies and data, ensuring every patient receives the right treatment at the right time.
The programme is an ambitious and unique strategic partnership model, co-funded through Research Ireland, industry and the
not-for-profit sector, coordinated by the Systems Biology Ireland centre based at University College Dublin (UCD), which is led by Professor Walter Kolch.
Minister Lawless said: “Breakthroughs in cancer research are most likely when clinicians, scientists, industry leaders, patients, charities and other stakeholders come together with a shared purpose. The first phase of Precision Oncology Ireland stands
as proof that strong, sustained collaboration can unlock innovation and accelerate transformative solutions. I congratulate the team on the advances made to date and wish them every success as they embark on this next phase of funded research.”
Professor Walter Kolch said, “Research is the engine behind new medicines. POI-2 is all about linking top notch cancer research to clinical translation. This includes the development of advanced computer simulations of the disease, so that we can design the best diagnostic and treatment approaches for each individual patient.”
UCD Professor of GynaeOncology, Consultant Gynaecological Oncologist at the Mater Hospital, and DeputyDirector of POI, Professor Donal
Brennan said: “The first phase of this programme delivered significant achievements. We now aim to strengthen collaboration with clinical partners and move towards embedding research within standard cancer care, so that patients and society across Ireland – and beyond – can benefit from improved outcomes, enhanced healthcare innovation, and more sustainable delivery of cancer services.”
The first phase of POI established a pioneering cross-sector collaborative model, bringing together academics, industry partners, charities, and patients.
Dr Diarmuid O’Brien, CEO, Research Ireland, said: “The highly-impactful Precision Oncology Ireland strategic partnership is enabling breakthroughs in cancer

“The challenge has moved to focus on quality of life needs as well as the need for more real-world data to be gathered to better inform service delivery and hopefully improve our care. In addition, we are hoping to better educate the public and the health service about the realities of living with an advanced cancer diagnosis.”
biology, supporting the development of new treatments, training the next generation of researchers, nurturing biotech start-ups, and strengthening critical research infrastructure.
“This programme has built a unified community that is creating a lasting impact on how we approach cancer research in Ireland. Phase 2, now, represents a substantial increase in investment, scale and ambition, as we work to deliver the next generation of cancer care.”
Patient advocate and researcher Siobhan Gaynor supports the launch of POI-2. Siobhan lives with advanced breast cancer which is a life-limiting disease. She volunteers on several cancer research committees and conducted a survey via Cancer Trials Ireland in 2023 to capture never beforeseen input from stage IV cancer patients. Her research was
Professor Walter Kolch, Director of Precision Oncology Ireland and Systems Biology Ireland (based at UCD)
POI-2 will build on the success of Phase 1 by deepening interdisciplinarity through stronger clinical engagement, broadening scientific scope and infrastructure, enhancing patient and public involvement, expanding training opportunities, and fostering collaboration across the cancer research and healthcare communities from the outset.
UCD Vice-President for Research, Innovation and Impact, Professor Kate Robson Brown said: “The launch of POI-2 marks a pivotal step in realising our collective strategic vision to advance precision medicine, accelerate interdisciplinary collaboration, and harness the transformative power of AI and digital technologies. Most importantly, it reflects our unwavering commitment to translating groundbreaking research into tangible improvements in patient care and outcomes. This programme exemplifies the future of healthcare research and innovation and we are proud to be at the forefront.”
New report highlights only three of 28 targets met in National Cancer Strategy
The Irish Cancer Society has launched new figures that highlight only three of 28 defined targets have been met in the National Cancer Strategy. With Ireland’s cancer mortality rate the third highest in Western Europe, the Society has warned that things will get worse unless increased investment is provided in budget 2026.
The Irish Cancer Society’s NCS Scorecard highlights performance against the 28 defined targets in the National Cancer Strategy in 2024 and 2025.
Of these targets only three have been met. 14 have not been met; seven have no data available; 1 is considered unlikely to meet its 2026 target given its current trajectory, and three have a 2026 deadline which cannot yet be assessed.
CEO, Averil Power said, “One in two of us will get cancer in our lifetime. When we do, we deserve to be given the best possible chance of surviving the disease. But as the National Cancer Strategy Scorecard highlights, target waiting times for vital cancer tests and treatment are not being met. As a result, our cancer death rate is the third highest in Western Europe.
“The National Cancer Strategy was launched in 2017 and will expire in 2026. As we head into the final year of the Strategy, it's clear most targets are not being met. That will only change if Government provides additional investment in the National Cancer Strategy in next week’s budget.”
awarded the Irish Cancer Society Public and Patient Involvement (PPI) project of the year in 2025.
Following the survey, Siobhan was also behind the creation of an Advanced Cancer Patients Council, which ensures dissemination of the survey results and campaigns for changes in Irish advanced cancer care by patients and for patients.
Speaking at the launch, Siobhan said: “Most Stage IV cancers are incurable, although thanks to science and research, we are now living longer. The challenge has moved to focus on quality-of-life needs as well as the need for more real-world data to be gathered to better inform service delivery and hopefully improve our care. In addition, we are hoping to better educate the public and the health service about the realities of living with an advanced cancer diagnosis.”
Professor John Kennedy, Chair of the last National Cancer Strategy, said, “We are deeply concerned about the impact lack of investment in the National Cancer Strategy is having on people with cancer in Ireland. The fact of the matter is that patients are being let down.
“Without Government investment in cancer services, staff, and infrastructure, it is cancer patients who continue to pay a too high price. Budget 2026 must prioritise the National Cancer Strategy, with ringfenced new recurrent development funding for the National Cancer Control Programme of at least ¤20 million each year. The huge challenges posed by the combination of a growing and increasingly aged population along with ever more complex, expensive and effective therapy, can only be addressed by coherent planning for, and multiannual funding of, the next National Cancer Strategy over its lifetime.”
The Irish Cancer Society is calling for increased investment in Budget 2026 towards cancer staff, buildings, and equipment to ensure target waiting times are met. Specifically:
• Provide sufficient investment in staff, buildings, and equipment to enable targets of the current National Cancer Strategy to be met.
• Plan and resource the next National Cancer Strategy.
• Improve the availability of data to support transparency and decision-making.
Clinical R&D
ALEXION, ASTRAZENECA RARE DISEASE, WILL TRANSITION TO 100% RENEWABLE ENERGY FOR IRELAND OPERATIONS SITES IN 15-YEAR BIOMETHANE GAS PURCHASE AGREEMENT
Alexion, AstraZeneca Rare Disease, has announced that it will transition to biomethane to provide 100% of its heating needs at its Dublin and Athlone, Ireland sites, making it the first pharmaceutical company in the country to fully embrace this sustainable energy source to fuel the research, development and manufacturing of medicines. This landmark initiative will be achieved through a fifteenyear partnership with Carbon AMS, a leading biomethane producer and will contribute to the Company’s flagship decarbonisation programme, Ambition Zero Carbon.
This agreement is the first largescale biomethane contract with ‘additionality’ in Ireland, meaning the project will add renewable capacity to Ireland’s national gas grid. Biomethane will be sourced from a new state-of-the-art plant strategically located in Duleek, Co. Meath— approximately 30km from Alexion’s College Park site and 120km from its Athlone operations—and will use grass silage obtained through longterm contracts with local farmers. Construction of the Duleek facility is expected to be completed, and the first supply of biomethane generated to sites, in 2026.
The Duleek facility will supply at least 32GWh of biomethane per year to the Alexion Operations sites, equivalent to 6.3% of AstraZeneca’s global gas consumption and displacing approximately 5,800 tonnes of carbon dioxide equivalent (CO2e) emissions per year. The transition and contractual agreement are key elements of AstraZeneca’s flagship Ambition Zero Carbon programme, which aims to reduce greenhouse gas (GHG) emissions from the Company’s global operations and fleet (Scopes 1 and 2) by 98% in 2026, compared to a 2015 baseline, on the way to becoming science-based net zero by 2045.
The agreement will see Carbon AMS construct a purpose-built anaerobic digestion system, in which microorganisms break down organic material, such as grass, in a sealed, oxygen- free enclosure. The anaerobic digestion process produces biogas, which is then turned into biomethane, a renewable source of energy. The nutrient-rich by-product will be returned to farmland, enhancing soil health, promoting further grass growth and reducing the need for synthetic fertilisers, contributing to
sustainable Irish agriculture and a circular economy.
Ireland’s abundant grasslands and temperate climate provide ample agricultural residues and grass silage for anaerobic digestion, ensuring a sustainable biomethane supply.
Minister for Transport and Minister for Climate, Energy and the Environment, Darragh O’Brien TD, said: “The commitment by Alexion, AstraZeneca Rare Disease to transition their Irish operations to 100% biomethane marks a significant contribution to the National Biomethane Strategy. The project’s ‘additionality’ facilitated by the partnership with Carbon AMS and involving the development of new renewable infrastructure in Duleek and longterm contracts with local farmers illustrates how private-sector initiatives can deliver tangible benefits for both environmental goals and the agricultural community. Such collaborations are vital for contributing to our national decarbonisation targets, strengthening our energy independence and fostering a more sustainable future for Ireland.”
Shane Doyle, Senior Vice President, Global Operations and Sustainability, Alexion, said: “Our partnership with Carbon AMS marks a pivotal moment in Alexion and AstraZeneca's sustainability journey. By transitioning to 100% biomethane for our heating needs in Ireland, we are not only significantly reducing our operational emissions but also pioneering a new source of energy for the pharmaceutical industry in Ireland. This substantial, longterm commitment underscores our dedication to environmental stewardship and our role in fostering a more sustainable future for Ireland.”
FIRST CAR T-CELL THERAPY TECARTUS® (BREXUCABTAGENE AUTOLEUCEL) FOR ADULT ACUTE LYMPHOBLASTIC LEUKAEMIA PATIENTS NOW REIMBURSED IN IRELAND
Gilead Sciences Ltd. and Kite, a Gilead company, has announced that eligible patients in Ireland 26 years of age and above with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL) will now be able to access Tecartus® (brexucabtagene autoleucel; brexu-cel) through the public health system. Brexucabtagene autoleucel is the first CAR T-cell therapy to be reimbursed in Ireland for this patient population.
Acute lymphoblastic leukaemia (ALL) is an uncommon acute
haematological malignancy, representing approximately 11% of all leukaemias in Ireland. While advances in chemotherapy and targeted immunotherapies have improved overall survival, relapsed or refractory (r/r) adult ALL is associated with “poor outcomes”.
Julian Cole, Executive Director of Medical Affairs at Gilead Sciences, said, “This approval marks a major milestone for those aged 26 or more with relapsed or refractory ALL. Brexucabtagene autoleucel is now available to eligible patients offering a personalised and important treatment option where few options existed. We’re proud to help bring this innovation to patients who need it.”
The approval is supported by data from the pivotal ZUMA-3 single-arm trial in adult patients with relapsed or refractory B-precursor ALL.
CAR T-cell therapies are individually made starting from a patient’s own white blood cells, called T-cells. The cells are collected through a process similar to donating blood platelets or plasma and sent to specialised manufacturing facilities where they are engineered to target the patient’s cancer, expanded and then returned to the hospital for infusion back into the patient.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
ABBVIE SCOOPS MAJOR GONGS AT PHARMA INDUSTRY AWARDS
Global biopharmaceutical company, AbbVie, was recently honoured in two major categories at the Pharma Industry Awards
Pictured accepting the Pharma Company of the Year award from Matt Moran, Judging Coordinator Pharma Industry Awards 2025, are (left to right) AbbVie Ireland’s Mary Moran, Yvonne Rochford, and Susan O'Hehir.
Ireland 2025, winning Pharma Company of the Year (Large) and Biopharma Company of the Year. The awards, which were presented at a gala ceremony at the Dublin Royal Convention Centre, celebrate innovation, business excellence and societal impact, and recognise the combined effort of all six AbbVie sites in Ireland in bringing life-changing treatments to patients.
OFFICIAL OPENING OF BLACKROCK HEALTH WOMENS HEALTH CENTRE
Blackrock Health, a cornerstone of Irish healthcare for over 40 years, has proudly announced the official opening of the Blackrock Health Womens Health Centre. This state-of-the-art facility, backed by a previously announced ¤16 million investment, is now open at 2-5 Warrington Place, Dublin 2, marking a pivotal moment in delivering truly holistic and integrated healthcare specifically for women in Ireland. The Blackrock Health Womens Health Centre stands as the nation's most comprehensive private, consultant-led centre dedicated to women’s health. With a team of 28 consultants, four women’s medicine specialists and 30 ancillary healthcare professionals and support staff, the collective team will provide unparalleled access to a vast array of specialist services, all designed with the unique needs of women at their core.
What truly sets the Blackrock Health Womens Health Centre apart is its integrated approach. Diagnostics and treatments across each disease area are delivered with a deep understanding of how these health concerns can co-exist. This enables the centre's expert, consultant-led team, spearheaded by Professor Donal Brennan, Clinical Director and Gynaecological Oncologist and joined by many experts in women’s health amongst whom


Blackrock Health, a cornerstone of Irish healthcare for over 40 years, today proudly announces the official opening of the Blackrock Health Womens Health Centre. This state-of-the-art facility, backed by a previously announced ¤16 million investment, is now open at 2-5 Warrington Place, Dublin 2, marking a pivotal moment in delivering truly holistic and integrated healthcare specifically for women in Ireland. Pictured were Prof. Donal Brennan, Clinical Director and Gynaecological Oncologist, Blackrock Health Womens Health Centre, Dr Caoimhe Hartley, Clinical Lead Women’s Medicine and GP, Blackrock Health Womens Health Centre with Lorraine Keane, TV Personality and Brand Ambassador for the Women’s Health Centre Dr Caroline Whelan, CEO of Blackrock Health and Gordon Dunne, CEO of Blackrock Clinic. Picture Jason Clarke
are Dr. Gerry Agnew, Clinical Lead Urogynaecology, Dr Caoimhe Hartley, Clinical Lead Women’s Medicine and Prof Kirk Levins, Pain Medicine, to provide an unrivalled breadth of care under one roof.
“We are incredibly proud to be officially opening the Blackrock Health Womens Health Centre today.” said Dr. Caroline Whelan, Group CEO of Blackrock Health. “This state-of-the-art facility represents a pivotal milestone in our unwavering commitment to providing comprehensive, compassionate, and truly specialised care that empowers women at every stage of their health journey. We are not just opening a centre; we are opening a new chapter in dedicated women's healthcare.”
Blackrock Health Womens Health Centre will offer an extensive array of specialist services, including consultant-led
This initiative aligns with the Women’s Health Action Plan 20242025, addressing critical gaps in current services and empowering women to take control of their health journey with convenient access to specialist care.
Healthlink referrals are welcome for services available at Blackrock Health Women’s Health Centre. For more information and to book appointments, please visit blackrockhealth.com.
CROÍ URGES GOVERNMENT TO PRIORITISE HEART HEALTH AS CHARITY HOSTS HEART HEALTH CHECKS AND AWARENESS EVENT IN LEINSTER HOUSE
Croí, the Heart and Stroke Charity, today held a Heart Health Check and Awareness Event in Leinster House to highlight the urgent need for a new national cardiovascular plan to tackle cardiovascular disease (CVD), which is Ireland’s leading cause of death and disability.
The event, hosted by Minister Noel Grealish offered simple health checks for things like blood pressure, pulse, and cholesterol, and also included a test for Lp(a) –a genetic factor that can increase the risk of heart disease. One in five people worldwide have high Lp(a) levels. Knowing your level can help you take steps to protect your heart health, or that of a loved one. New European guidelines recommend that every adult should have this test at least once in their lifetime. However, it’s not yet available to the public in Ireland – something Croí hopes will change.
extends across the health service and the wider economy: one in two families will be affected by heart disease or stroke; while CVD is responsible for 40% of hospital admissions and 75% of hospital bed days; and costs the State an estimated at €3.4 billion annually.
Despite these stark figures, Ireland has had no national cardiovascular strategy since 2019. With an ageing population and rising prevalence of risk factors such as hypertension, obesity, diabetes and high cholesterol, Croí is warning that the burden of CVD will continue to escalate unless prevention and early detection become national policy priorities.
Meeting with Oireachtas members throughout the day, Croí called for the urgent implementation of three priority policy actions:
1. Publish a new National Cardiovascular Health Plan with measurable targets to reduce deaths and hospitalisations, expand prevention and rehabilitation programmes, and strengthen early detection and treatment services.
2. Implement the National Review of Adult Specialist Cardiac Services (2023), which offers a clear pathway for delivering safe, equitable and high-quality cardiac care nationwide.
3. Provide dedicated funding to ensure full delivery of the National Stroke Strategy. This would improve patient outcomes, reduce long-term disability and lower overall healthcare costs.
clinics in gynaecology, urology, breast health, endocrinology, gastroenterology, pain medicine, cardiology, dermatology, psychology, health screening, and rheumatology. These services will be complemented by a full suite of nursing and allied health offerings, including physiotherapy, urodynamics, phlebotomy, and radiology diagnostics including DXA scanning, mammography, and ultrasound.
Dr Caoimhe Hartley, Clinical Lead Women’s Medicine and GP, Blackrock Health Womens Health Centre said, “The Blackrock Health Womens Health Centre brings together an exceptional team of specialists across a wide range of disciplines. This collaborative approach allows us to provide truly integrated care, addressing the interconnected nature of women’s health needs. We are delighted to open our doors today to offer a new healthcare solution to women for now and into the future.”
In addition to testing, the event offered tailored advice on cardiovascular risk reduction, including stroke prevention, to some 100 members of the Oireachtas and staff.
Cardiovascular disease currently claims more than 9,000 lives every year in Ireland, accounting for one in every three deaths. Its impact
Croí, the Heart and Stroke Charity, held a Heart Health Check and Awareness Event in Leinster House to highlight the urgent need for a new national cardiovascular plan to tackle cardiovascular disease

Clinical R&D
Croí’s Medical Director, Professor Jim Crowley said, “Today’s heart health event in Leinster House, and the high volume of demand showed just how seriously members of the Oireachtas take their own heart health. We were delighted to provide checks to some 100 members and staff over the course of the day. The level of engagement we saw shows both the scale of concern and the appetite for early detection when it comes to cardiovascular health and stroke prevention. It also underlines, in a very real way, how vital accessible prevention services are.”
“Cardiovascular disease remains Ireland’s biggest killer, but a more proactive, preventative model of healthcare can save lives. What we need now is a coordinated national plan to ensure every community in Ireland can access the same level of support. Hopefully today will show our politicians just how easy it is to prevent cardiovascular incidents – all we need is the supporting policies to put these checks in place, and ensure they are accessible across all of Ireland,” Professor Crowley added.
SANOFI AND REGENERON’S DUPIXENT APPROVED AS THE FIRST TARGETED MEDICINE IN THE EU IN OVER A DECADE FOR CHRONIC SPONTANEOUS URTICARIA
The European Commission has approved Dupixent (dupilumab) for the treatment of moderateto-severe chronic spontaneous urticaria (CSU) in adult and adolescent patients 12 years and above with inadequate response to histamine-1 antihistamines (H1AH) and who are naive to antiimmunoglobulin-E (IgE) therapy for CSU. Eligible patients can use Dupixent as a first-line targeted treatment option.
“The unpredictable nature of chronic spontaneous urticaria leaves patients guessing when they’ll have their next outbreak of disruptive, debilitating hives and itch, which can make life challenging,” said Tonya Winders, President & CEO, Global Allergy & Airways Patient Platform. “Dupixent is proven to reduce these intense symptoms and has the potential to make a positive impact on people struggling to control this disease.”
“Standard-of-care, firstline treatment options like antihistamines offer limited relief for many people living with uncontrolled chronic spontaneous urticaria, leaving them to face unrelenting cycles of itch and hives,” said Alyssa Johnsen, MD, PhD, Global Therapeutic Area Head, Immunology Development at Sanofi. “Dupixent significantly
reduced these symptoms of CSU and led to more patients experiencing well-controlled disease or a complete response compared to placebo in two phase 3 studies. Now, eligible patients with CSU in the EU have a new option that is proven to reduce itch and hives.”
The approval is based on data from two phase 3 clinical studies in the LIBERTY-CUPID program (NCT04180488). Study A and Study C included 284 patients aged 12 years and older who were symptomatic despite the use of antihistamines and who were naïve to anti-IgE therapy. Both studies assessed Dupixent as an add-on therapy to standard-of-care antihistamines compared to antihistamines alone and demonstrated Dupixent significantly reduced urticaria activity (a composite of itch and hives), and individual measures of itch and hive severity compared to placebo at 24 weeks. Dupixent also increased the percentage of patients with well-controlled disease and complete response at 24 weeks compared to placebo. Study B (n=108) provided additional safety data and evaluated Dupixent in patients aged 12 years and older who were inadequate responders or intolerant to anti-IgE therapy and symptomatic despite antihistamine use.
Safety results from Study A, Study B and Study C were generally consistent with the known safety profile of Dupixent in its approved indications. The most common adverse reactions for Dupixent overall are injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes, and eosinophilia. Additional adverse reactions of injection site induration, injection site dermatitis, and injection site hematoma were reported in the CSU adult and adolescent studies. Adverse events more commonly observed with Dupixent (≥5%) than placebo in patients with CSU were injection site reaction, COVID-19, hypertension, CSU, and accidental overdose.
“The approval of Dupixent for certain adults and adolescents with chronic spontaneous urticaria in the European Union represents the first innovation for patients with this disease in over a decade,” said George D. Yancopoulos, MD, PhD, Board co-Chair, President and Chief Scientific Officer at Regeneron. “Physicians now have a new approach for CSU with Dupixent, as the only treatment that inhibits IL4 and IL13, two key drivers of type 2 inflammation, and can offer patients significant improvement in debilitating itch
and hives. This approval further demonstrates the ability of Dupixent to advance the treatment landscape for yet another chronic type 2 inflammatory disease, with a well-established safety profile across its indications.”
Beyond the EU, Dupixent is also approved for CSU in certain adults and adolescents in several countries including the US and Japa
MATER PRIVATE
NETWORK WINS “HEALTHCARE EDUCATION EVENT OF THE YEAR”
Mater Private Network and CVRI Dublin are proud to announce that they have been awarded “Healthcare Education Event of the Year” at the Irish Healthcare Awards 2025 for their flagship event, the Dublin Heart Summit. This recognition celebrates the organisations’ commitment to advancing cardiovascular education, clinical excellence, and innovation in heart health across Ireland.
A panel of over 30 expert judges, including leading physicians, educators, patient advocates, medical journalists, and representatives from the pharmaceutical and life sciences industries, carefully reviewed this year’s entries. The judges said that this flagship educational event raised awareness of cardiac conditions and treatments, achieving high-impact education with large attendance and very high delegate satisfaction.
Now in its second year, the Dublin Heart Summit is a collaboration between Mater Private Network and the Cardiovascular Research Institute (CVRI) Dublin, supported by the Royal College of Surgeons in Ireland (RCSI) and the Irish
Cardiac Society. The 2025 Summit brought together over 700 healthcare professionals including cardiologists, GPs, and allied health specialists, to explore the latest developments in cardiovascular medicine, patient care, and research.
The event was designed to foster collaboration, encourage knowledge-sharing, and inspire new approaches to improving outcomes in cardiovascular disease. This year’s programme featured expert-led discussions on emerging treatments, ground-breaking technologies, and evolving models of care, underscoring Ireland’s growing reputation as a leader in heart health innovation.
Adopting a hybrid delivery model, the Summit provided both inperson and virtual participation, ensuring broad accessibility and engagement across the healthcare community. All attendees received Continuing Professional Development (CPD) certification and access to on-demand educational materials to support continued learning.
In addition to its clinical impact, the Dublin Heart Summit played an important role in raising public awareness around cardiovascular disease. Through national broadcast coverage and expert interviews, Mater Private Network helped bring key messages about prevention and heart health to a wider audience.
Speaking about the award, Professor Robert Byrne, Director of Cardiology and Director of the CVRI at Mater Private Network said “We are honoured to receive this recognition from the Irish Healthcare Awards. The Dublin Heart Summit reflects our ongoing commitment to clinical excellence,

education, and innovation. Through collaboration with our partners in RCSI and the Irish Cardiac Society, we are helping shape the future of cardiovascular care while improving outcomes for patients across Ireland.”
The Dublin Heart Summit has now established itself as Ireland’s leading cardiovascular education forum, uniting professionals from across disciplines to share insights, exchange ideas, and advance the understanding of heart health. Looking ahead to 2026, a new symposium – the Women’s Heart Summit – will take place on 24th January, followed by the 3rd Dublin Heart Summit on 18th April.
TEVA RECEIVES EUROPEAN COMMISSION APPROVALS FOR PONLIMSI® (DENOSUMAB) BIOSIMILAR TO PROLIA® AND DEGEVMA® (DENOSUMAB) BIOSIMILAR TO XGEVA®
Teva Pharmaceuticals International GmbH, a subsidiary of Teva Pharmaceutical Industries Ltd. (NYSE: and TASE: TEVA) announced that the European Commission (EC) has granted marketing authorizations for its two denosumab biosimilar candidates – PONLIMSI, a biosimilar to Prolia®1 and DEGEVMA, a biosimilar to Xgeva®, following the positive opinion of the Committee for Medicinal Products for Human Use (CHMP) earlier this year.
These approvals mark another significant milestone in Teva’s industry-leading biosimilars portfolio and its ongoing commitment to improving patient access to essential biologic therapies across Europe. Teva plans to launch both products in key European markets in the coming months.
The approval represents an important step in advancing Teva’s Pivot to Growth strategy, reinforcing the company’s dedication to broadening access to biosimilar medicines for patients.
Steffen Nock, SVP Head of Biosimilars & Chief Science Officer, said: “This approval represents an important step forward in increasing patient access to biosimilar therapies for serious bone conditions, underscoring our commitment to supporting better care for patients."
Michal Nitka, SVP Head Generics Europe & Global Head OTC, added: "Through product launches like these, we remain committed to providing additional treatment options for healthcare systems across Europe – especially in countries where access to biosimilars can still be improved."
THE HEALTH SERVICE EXECUTIVE (HSE) APPROVES BIMZELX®(BIMEKIZUMAB) FOR ADULTS LIVING WITH HIDRADENITIS SUPPURATIVA AND ADULTS LIVING WITH AXIAL SPONDYLOARTHRITIS
UCB is pleased to announce that the Health Service Executive (HSE) has approved BIMZELEX® (bimekizumab) in Ireland for the treatment of adults with active moderate-to-severe hidradenitis suppurativa (HS)*, and adults with active non-radiographic axial spondyloarthritis (nr-axSpA)† or ankylosing spondyloarthritis (AS), who have responded inadequately to conventional therapy‡.
“We are thrilled that bimekizumab has been approved by the HSE for patients in Ireland living with two separate chronic diseases,” said Nadege Feeser, Head of Immunology UCB UK & Ireland. “Both hidradenitis suppurativa and axial spondyloarthritis can impact daily life. We firmly believe that every patient deserves to live as free as possible from the challenges and uncertainty of disease, and this recommendation underscores our dedication to supporting people affected by HS and axSpA.”
Estimated to affect over 50,000 people in Ireland, HS is a chronic, recurrent, and painful condition characterised by inflammation, which can appear in any skin area but is commonly found in areas containing apocrine sweat glands. This appears as redness, swelling, and lumps in the armpits, breasts, abdominal fold, groin, genital area and buttocks.
“Hidradenitis suppurativa creates painful abscesses and scarring on the skin. For some patients, existing treatments are not effective, and they are forced to live with a poorly controlled, painful skin disease,” said Professor Brian Kirby, Consultant Dermatologist at St. Vincent's University Hospital and a Full Clinical Professor at University College Dublin. “It is fantastic to see a much-needed treatment option for individuals impacted by this difficult-to-treat condition, which has very few licensed treatment options available.”
The approval of bimekizumab for the treatment of HS is supported by data from two Phase 3 studies, BE HEARD I and BE HEARD II, which evaluated the efficacy and safety of bimekizumab in 1,014 adult patients with moderate-tosevere HS. Results showed that a significantly higher proportion of patients treated with bimekizumab versus placebo achieved a 50 percent or greater improvement in HS symptoms at Week 16, as measured using HiSCR50, the
primary endpoint in both trials. The safety profile of bimekizumab was consistent with safety data seen in previous trials with no new observed safety signals.
“HS can have profound impacts on many basic activities of daily life (e.g., bathing, dressing, moving, walking). Furthermore, HS can also affect people in many other areas of life including relationships, work and education, sleep, and mental health,” said Barry McGrath, PhD, Co-Founder and Chairperson of HS Ireland, “As there are limited treatment options for HS, this approval provides another possible safe and effective option for certain people living with moderate to-severe HS.”
Up to 33,000 people in Ireland are living with a form of axSpA, which can include non-radiographic axial spondyloarthritis and ankylosing spondyloarthritis. AxSpA is a chronic inflammatory condition affecting the spine and other areas of the body, typically developing in teenagers and young adults. The symptoms are varying, but typically include pain and stiffness, which can impact quality of life.
“This approval means that rheumatologists have another option to offer patients in Ireland,” said Barry O’Shea, Consultant Rheumatologist in St James's Hospital and Clinical Associate Professor at Trinity College Dublin. “With no cure available, providing options to people living with axSpA is a significant step towards relieving the burden of the condition.”
The approval of bimekizumab for the treatment of axSpA is supported by data from two Phase 3 studies, BE MOBILE 1 and BE MOBILE 2, which evaluated the safety and efficacy of bimekizumab in 254 with active nr-axSpA, and 332 patients with active AS, respectively. Results showed significant improvement in the symptoms and measures of disease activity compared to placebo at week 16 in both nraxSpA and AS patient populations.
Bimekizumab will be available for these chronic inflammatory diseases from December 2025.
Marketing authorisation of bimekizumab was previously obtained for axSpA from the HSE in June 2023.1 It received approval from the Medicines and Healthcare products Regulation Agency (MRHA) August 2023, and from the European Commission (EC) in June 2023. For HS, marketing authorisation of bimekizumab was previously obtained from the HSE in April 2024, subsequently receiving approval from the MHRA in June 2024, and from the European Commission (EC) in April 2024.
CLINICIAN PHD PROGRAMME MARKS SIGNIFICANT IMPACT ON HEALTH RESEARCH
Specialist clinical training that integrates PhDs has driven significant advances in health research and patient care over the past decade, according to speakers at an All-Island event held at the Mansion House in Dublin.
The Irish Clinical Academic Training (ICAT) Programme, a unique allIreland, cross-institutional, and multi-disciplinary initiative, marked the outstanding impact of the programme on health research at its flagship event, “Celebrating Excellence and Impact of Clinical Academic Training”.
71 clinicians have participated in the programme to date, making contributions across a range of clinical research fields including: diagnosis of early Alzheimer’s disease; cardiac care for cancer patients; blood clotting in Covid-19; a bleeding assessment tool; and e-cigarette use in adolescents.
Launched in 2016, ICAT is a collaboration between the University of Galway, Trinity College Dublin, Queen’s University Belfast, RCSI University of Medicine and Health Sciences, University College Cork, and University College Dublin.
The programme has secured over ¤40 million in funding from the Health Research Board (HRB), Wellcome, the Department of Agriculture, Food and the Marine, the HSE National Doctors Training and Planning, the Health and Social Care R&D Division NI, the Northern Ireland Medical and Dental Training Authority, the College of Anaesthesiologists of Ireland, and its six partner universities.
Professor David Williams, ICAT Programme National Director, RCSI University of Medicine and Health Sciences, said: “A cornerstone of ICAT’s success is its all-island model, which brings together institutions and health systems north and south to position Ireland as a globally recognised hub for clinical academic research. The programme fosters cuttingedge science and innovation in healthcare-related research, aligned with the strategic priorities of both Ireland and Northern Ireland.”
The event was opened by Dr Gráinne Gorman, Chief Executive Officer of the HRB who commented: “The HRB is proud to support ICAT in developing high calibre clinician researchers. It is essential we work with the health system to create the right conditions to harness their potential and ensure they can apply their research expertise in practice to benefit people’s health and care.”







