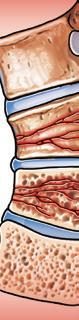
About myeloma bone disease
Approximately 70%–80% of patients with myeloma develop bone disease. Bone disease can cause bones to become thinner and weaker (osteoporosis), and also can create lytic lesions (holes in bone). The weakened bone is more likely to break due to minor pressures or injury (pathologic fracture). The bones most commonly affected are the axial skeleton (spine, pelvis, ribs, and skull) and the upper ends of the long bones of the arms and legs.
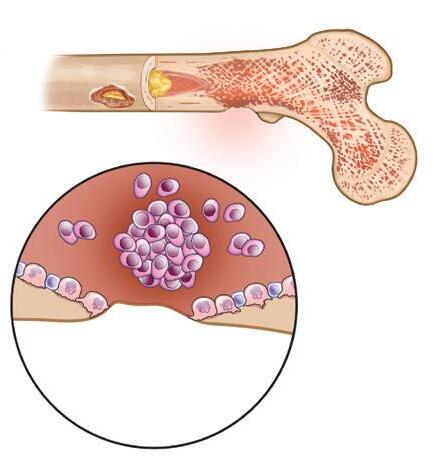
In a healthy skeleton, there is a balance between the breakdown of old bone tissue (performed by cells called osteoclasts) and the building of new bone tissue (formed by osteoblasts). Myeloma cells send signals to osteoclasts, causing them to break down much more bone than what is required for normal skeletal health. Myeloma cells also inhibit the formation of osteoblasts, thus preventing the repair of bone loss.
In addition to giving rise to bone disease, the process of accelerated bone breakdown releases calcium from the bones into the bloodstream, and a condition called hypercalcemia can occur. Both myeloma bone disease and hypercalcemia can be treated with a bone-modifying agent (BMA)
BMAs are not treatment for myeloma, but the use of a BMA does help to prevent skeletal-related events (SRE), which are complications of
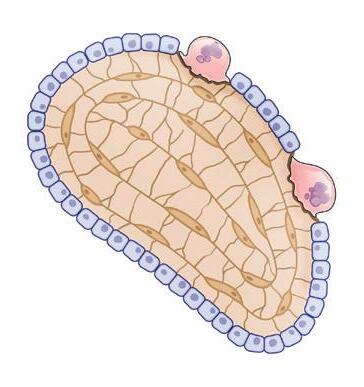
1. Bone-building anatomy
Osteoclast (breaks down bone) Spongy bone containing red marrow Compact bone Yellow marrow
(builds new bone)
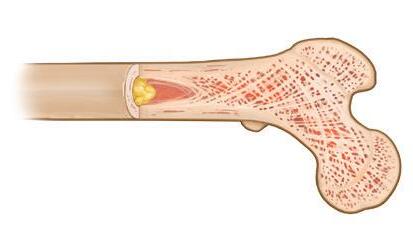
Figure 2. Myeloma bone disease
Figure
myeloma. BMAs are an essential component of supportive care for patients with myeloma bone disease. BMAs include bisphosphonates Aredia® (pamidronate), Zometa® (zoledronate or zoledronic acid), and clodronate, as well as the monoclonal antibody Xgeva® (denosumab).
Treatment with a bisphosphonate
Bisphosphonates are small inorganic molecules that bind to a substance called hydroxyapatite on the surface of damaged bones. At the sites of bone damage, bisphosphonates inhibit and destroy osteoclasts. Since bone damage is caused by the increased numbers and activity of these osteoclasts, treatment with bisphosphonates reduces bone damage and, when the myeloma is well controlled, allows bone healing to occur.
Bisphosphonates have several beneficial effects, including:
¡ Preventing further bone damage,
¡ Reducing bone pain and the need for painkillers,
¡ Correcting and preventing hypercalcemia,
¡ Reducing the need for radiation therapy,
¡ Reducing pathologic fractures due to myeloma (i.e., fracture at a site where myeloma has weakened the bone),
¡ Improving the chances of healing and recovering bone strength,
¡ Improving quality of life.
Bisphosphonates are not chemotherapy
Bisphosphonates are not a type of chemotherapy, which is used to attack myeloma cells. Bisphosphonates are not a treatment for myeloma. Several clinical trials have demonstrated that bisphosphonates have no anti-myeloma effect and do not prevent progression of early myeloma.
Bisphosphonates are used to treat several types of bone disease, including osteoporosis and the bone-thinning effects of steroid treatment. Bisphosphonates were first introduced more than 20 years ago as an additive to toothpaste to reduce dental decay.
Bisphosphonates are generally safe but they do come with risks, which you must discuss with your doctor.
Myeloma patients can benefit from bisphosphonates
Bisphosphonates are recommended for all patients with myeloma-related bone disease. Bisphosphonates are particularly helpful for myeloma patients being treated with steroids, which reduce bone density. Bisphosphonate therapy improves the negative effects of steroids on bones.
In 2018, the American Society of Clinical Oncology (ASCO) published updated clinical practice guidelines on bonemodifying agents, recommending bisphosphonate therapy for patients with active symptomatic myeloma that requires systemic treatment with or without evidence of lytic lesions or vertebral compression fractures (VCFs) from osteopenia. For more information about VCFs, read the IMF’s publication Understanding Treatment of Myeloma-Induced Vertebral Compression Fractures.
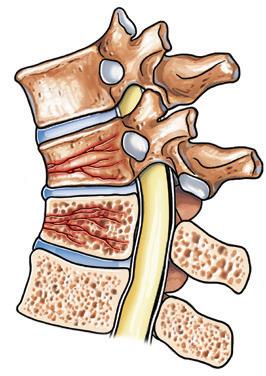
Figure 3. Vertebral compression and vertebral compression fracture
Vertebral compression
Vertebral compression fracture
Bisphosphonates are NOT recommended for patients with monoclonal gammopathy of undetermined significance (MGUS) except in cases of osteopenia or osteoporosis.
Types of bisphosphonates
Several bisphosphonates are approved by the U.S. Food and Drug Administration (FDA) to help achieve effective bone healing in patients with myeloma. However, there are differences in efficacy, administration, and potential side effects.
Aredia
Aredia was approved by the FDA to treat myeloma bone disease in 1995. If your doctor recommends that you should be treated with Aredia, be aware of the following:
¡ Aredia is administered monthly by means of an intravenous (IV) infusion. The standard dose is 90 mg infused during 2–4 hours. Toxicities associated with this medication are related to dosage, frequency of administration, and duration of infusion. Therefore, your doctor may recommend to reduce your dose and/or frequency of administration, and/or increase the duration of infusion.
¡ The kidney toxicity-related concern with the use of Aredia is an excess of a serum protein (albumin) in the urine, known as albuminuria or nephrotic syndrome, that has occurred predominantly if Aredia is given more frequently than monthly or at a dose higher than 90 mg. This side effect is usually reversible with dose and/or schedule adjustments or, in occasional severe cases, by discontinuing Aredia. Very rarely has irreversible damage occurred. Periodic monitoring (e.g., every 3 to 6 months) of urine protein levels with 24-hour urine collection is recommended to prevent any significant kidney damage.
¡ In 2010, Lancet Oncology published the findings from a large, randomized, double-blind, phase III clinical trial by the Nordic Myeloma Study Group (NMSG), which compared Aredia given at 30 mg to the standard dose of 90 mg in patients with newly diagnosed multiple myeloma (NDMM). Given the toxicities associated with long-term bisphosphonate treatment, the aim of the NMSG study was to establish the lowest effective dose of Aredia. The study validated the efficacy of the reduced dose, concluding that “monthly infusion of Aredia 30 mg should be the recommended dose for prevention of bone disease” in myeloma patients. Ask your doctor if Aredia at 30 mg dose is appropriate for your individual case.
Zometa
Zometa was approved by the FDA in 2001 based on clinical trial results comparing it with Aredia. Zometa produces more rapid and prolonged reduction in elevated blood calcium than Aredia when elevated levels are present. However, results evaluating effects on bone disease showed that Zometa and Aredia affect SREs equivalently. The major difference between Zometa and Aredia is the infusion time. If your doctor recommends that you should be treated with Zometa, be aware of the following:
¡ Zometa is administered monthly by means of an IV infusion. The standard dose is 4 mg infused during 15–45 minutes. Toxicities associated with this medication are related to dosage, frequency of administration, and duration of infusion. Therefore, your doctor may recommend to reduce your dose and/or frequency of administration, and/or increase the duration of infusion.
¡ The kidney toxicity-related concern with the use of Zometa is an increase in serum creatinine. Reports of both increased serum creatinine and occasionally more severe kidney damage called acute tubular necrosis (ATN) have raised questions if Zometa must be used more cautiously in order to minimize the potential for kidney-related problems. Your serum creatinine level should be checked before each dose of Zometa, especially if there is concern about kidney function, such as with Bence-Jones myeloma, diabetes, long-standing high blood pressure, and in elderly or frail patients.
¡ If you had normal renal (kidney) function at the outset, and your serum creatinine value increases by 0.5 mg/dL, or if you had abnormal renal function at the outset, and your serum creatinine value increases by 1.0 mg/dL, your doctor should hold the next dose of Zometa until your serum creatinine value returns to within 10% of the baseline.
¡ If you experienced a mild elevation in serum creatinine value, which then returns to within 10% of the baseline, your doctor may consider adjustments to your treatment schedule, such as increasing the time of
infusion from 15 minutes to 30 minutes or more, using a larger volume of diluting fluids, or delaying the administration of the next dose. Ask your doctor which option is most appropriate for your individual case.
The Z-MARK clinical trial, conducted from 2007 through 2012 at 67 centers in the U.S., evaluated the effectiveness and safety of a dosing method for Zometa in preventing skeletal complications in 121 patients with advanced myeloma who were on an IV bisphosphonate for about 1–2 years. Participants received the standard dose or a reduced dose of Zometa every 4 weeks or every 12 weeks for up to 96 weeks. Participants in the “every 12 weeks” group who experienced an SRE were switched to the “every 4 weeks” group. Study results published in 2016 showed that “the use of Zometa every 12 weeks compared with every 4 weeks did not result in an increased risk of skeletal events over 2 years.”
The updated ASCO guidelines state that “for patients without active myeloma who are receiving maintenance therapy, receiving bisphosphonates every 3 months, rather than monthly, is an option.” The guidelines note that “there are insufficient data to recommend a specific duration of bisphosphonate therapy” beyond 2 years. Monthly treatment is to be resumed “upon relapse with new-onset SREs.”
Clodronate
Clodronate (clodronic acid or clodronate disodium) is an oral bisphosphonate that is not routinely used in myeloma. However, if you are intolerant of IV infusions, have kidney disease or dysfunction, and/or are using steroids, ask your doctor if you would benefit from taking an oral bisphosphonate.
Xgeva
Xgeva, a monoclonal antibody, was approved by the FDA in 2018 for the prevention of SREs in patients with myeloma. Xgeva is a BMA; it is not the same as the monoclonal antibodies used to treat myeloma. The approval was based on data from the ‘482 clinical trial, a large (1,718-patient), international, randomized, double-blind, multicenter study that demonstrated the non-inferiority of Xgeva when compared to Zometa.
Xgeva is a monoclonal antibody that binds to the cytokine RANKL, an essential factor in initiating bone breakdown by osteoclasts. Like the bisphosphonates, Xgeva is an osteoclast inhibitor, but its mechanism of action, the way it is metabolized by the body, and its route of administration all differ from the bisphosphonates.
¡ Bisphosphonates bind to bone mineral tissue, where they are absorbed by mature osteoclasts, causing osteoclasts to die. Xgeva does not become imbedded in bone tissue; it affects immature osteoclasts, blocking their ability to mature, function, and survive.
¡ While bisphosphonates are excreted via the kidneys, and can cause kidney toxicity, Xgeva is not cleared by the kidneys, and produces a low level of kidney-related side effects when compared to the bisphosphonates. The ASCO guidelines indicate that Xgeva may be preferable to bisphosphonates in patients with kidney damage.
¡ While bisphosphonates approved for myeloma in the U.S. are given as an IV infusion, Xgeva is given as a subcutaneous (SQ) injection.
Common side effects of Xgeva
The most common side effects of Xgeva are diarrhea, nausea, low red blood cells, thrombocytopenia, low calcium levels, back pain, swelling of the lower legs or hands, upper respiratory tract infection, rash, and headache.
The most common serious adverse reaction in the phase III ‘482 clinical trial was pneumonia, which occurred in 8% of patients in both the Xgeva and Zometa arms of the study.
The current ASCO guidelines warn that Xgeva should not be stopped abruptly because severe bone breakdown can occur in this situation. The Xgeva package insert warns of multiple vertebral fractures (MVF) following treatment discontinuation, and counsels the treating doctor to “evaluate the individual patient’s risk for vertebral fractures.”
Females of reproductive potential and males with female partners of reproductive potential should discuss with their doctor the risk of embryofetal toxicity and if the use of effective contraception is necessary before treatment with Xgeva begins, during treatment, and/or after the last dose of treatment is administered.
Possible side effects of bone-modifying agents
Be sure to discuss with your doctor the following possible side effects:
Osteonecrosis of the jaw
Osteonecrosis of the jaw (ONJ) is a problem that occurs in 3%–4% of patients with myeloma or other cancers who have been treated with either bisphosphonates or Xgeva. This condition is often preceded by inflammation or an infection in the mouth, and produces pain, swelling, and bone damage around the tooth sockets in the jaws. There is bone necrosis or loss of bone, which can lead to loose teeth, sharp edges of exposed bone, bone spurs, and the breaking loose of small bone spicules or dead bone. Symptoms may not be obvious at first, or may include pain, swelling, numbness, a “heavy jaw” feeling, or loosening of a tooth.
¡ Prior to treatment with any BMA, all myeloma patients should have a dental evaluation, preferably with an oral surgeon or dental oncologist familiar with ONJ. Careful monitoring and follow-up are required.
¡ Prevention can help avoid or reduce the scope of ONJ. Be sure to inform your dentist if you are receiving treatment with a BMA. Maintain excellent oral hygiene and visit your dentist regularly.
¡ If an infection is present, treatment with an antibiotic is recommended. The choice of antibiotic depends upon the type of infection that is documented. An oral rinse may be appropriate.
¡ If required, proceed with preventive dental care before starting treatment with a BMA.
¡ Management without surgery is recommended as a first step. Minor dental work to reduce sharp edges or remove injured tissue may be required. A protective mouth guard may be helpful.
¡ If possible, avoid tooth extraction and any elective jaw surgery. If dental surgery is required, interruption of BMA therapy is recommended. Current data indicate poor healing with continued BMA therapy in this setting.
¡ If problems persist or if healing is slow, consideration can be given to stopping bisphosphonate therapy for 2 to 4 months to facilitate recovery.
¡ Dentures can be worn, but may need adjustment. Dental implants appear to be contraindicated. Use of hyperbaric oxygen does not appear to be helpful.
Kidney dysfunction
All bisphosphonates are potentially toxic to the kidneys. Since myeloma itself can impact kidney function (through damage caused by myeloma light chain protein and/or by elevated blood calcium), the possibility of kidney-related side effects is of particular concern.
Before you begin treatment with a BMA, ask your doctor if the BMA in combination with other medications you are receiving may be more likely to have a negative impact on your kidney function. This includes myeloma therapies as well as nonsteroidal anti-inflammatory drugs (NSAIDs).
Atypical femur fractures (AFFs)
Atypical fractures of the femur (thigh bone) have been reported in patients who received treatment with either a bisphosphonate or with Xgeva. AFFs can occur as a result of no or minimal trauma. AFFs are rare and they can also occur in patients with no exposure to BMAs. The risk of atypical fractures is included in the “Warnings and Precautions” section of the package inserts for all FDA-approved bisphosphonates, as well as for Xgeva.
AFFs can occur in one or both thighs and usually present as a dull aching pain weeks to months before a complete fracture occurs. Myeloma patients who were also receiving therapy with dexamethasone or another glucocorticoid
(a class of corticosteroids) have reported atypical fractures. If you notice any new or unusual thigh, hip, or groin pain, report this to your doctor right away.
Fever
Pyrexia (medical term for fever) is defined as a temperature of 100.4°F (38°C) or higher. Fever may occur as an infusion-related reaction (IRR) associated with the use of bisphosphonates. The fever is usually mild; each treatment center has its own definition for “mild” temperature. You may experience a fever during your IV infusion or during the hours after, and the fever may last for several hours. Typically, fever occurs with the first or second infusion, and less frequently (if at all) with subsequent infusions. Patients who have severe recurrent fevers may not be able to tolerate IV bisphosphonates. As with other possible side effects, promptly report your fever to your doctor.
Vein irritation
With IV infusions of bisphosphonates, mild phlebitis (vein irritation) can occur at the site of the infusion. It is usually mild, and patients typically recover within 1 to 2 days. Careful monitoring is recommended during each infusion in order to avoid any leakage of medication around the vein. A short infusion of saline at the end of the bisphosphonate infusion is recommended to clear the Aredia or Zometa from the area and reduce the chance of phlebitis.
General aches and pains
These effects sometimes occur briefly, along with fever, with infused bisphosphonates. Back pain has been reported in patients who are receiving therapy with Xgeva. Ask your doctor if receiving medication prior to your infusions may be beneficial for you.
Combining BMAs with myeloma therapies
In general, BMAs can be safely combined with most myeloma therapies. Your doctor may decide not to give these therapies on or close to the same day as administration of intravenous treatment for myeloma. Caution about potential kidney toxicity with bisphosphonates has been discussed earlier in this booklet. Talk to your doctor to be sure that the BMA you are receiving is not being combined with another drug that can harm your kidneys.
Insurance coverage
In the U.S., the Centers for Medicare & Medicaid Services (CMS), a federal agency that administers the Medicare program, reimburse for bisphosphonate therapy. Most other insurance programs in the U.S. also reimburse for bisphosphonate therapy. Xgeva is significantly more expensive than generic Aredia or Zometa, so unless there are medical reasons why a patient cannot receive bisphosphonate therapy (e.g., kidney disease), there may be
insurance issues with receiving reimbursement for Xgeva. If you experience any problems with insurance coverage or reimbursement, speak with your healthcare team.
Since hospitals and clinics set up contracts for one product versus another, it’s important to double-check which agent, from which source, is being administered to you. Some patients may experience a new side effect or be intolerant of the solution into which the active ingredients are added. If this happens to you, be sure to find out what drug you received and which company manufactured it, and report your side effects to your doctor.
Other approaches to bone care
Kyphoplasty provides a tool that may have an impact on bone care for myeloma patients. This procedure involves the injection of liquid cement using the balloon technique in an attempt to provide acute pain relief and to stabilize collapsed vertebrae or other damaged bones. The results of the CAFE clinical trial, a randomized clinical trial of kyphoplasty versus non-surgical intervention, concluded that patients randomized to have kyphoplasty had improved pain relief, back function, and quality of life. For more information about kyphoplasty, read the IMF’s publication Understanding Treatment of Myeloma-Induced Vertebral Compression Fractures.
Contingent on your doctor’s approval, suggested measures to improve bone health include the following:
¡ Adequate pain control to allow for movement and exercise.
¡ Radiation therapy and/or orthopedic surgery to restore structural integrity of bones and to recover full mobility. Since radiation therapy can impair local bone healing, it is used sparingly for acute problems such as spinal cord compression, severe refractory pain, and treatment or prevention of a pathologic fracture.
¡ Exercise, especially walking and/or swimming, to enhance bone strength, flexibility, and endurance.
¡ Avoidance of risky activities (e.g., bicycling, skiing, skating, climbing ladders), which can increase the likelihood of falls and/or fractures.
¡ Regular re-evaluation and follow-up testing to rule out new bone disease and assess the impact of treatment.
Solitary plasmacytoma of bone
Solitary plasmacytoma of bone (SPB) is a rare cancer of unknown origin. Fewer than 1,000 people in the U.S. are diagnosed with SPB each year. SPB is not multiple myeloma. SPB can be treated, and sometimes cured, with the use of radiation therapy or surgery.
SPB is a single mass of monoclonal plasma cells in a bone or bone marrow. The diagnosis of SPB requires a solitary bone lesion (a biopsy of which shows infiltration by plasma cells), negative imaging results for other bone lesions, absence of clonal plasma cells in a random sample of bone marrow, and no evidence of anemia, hypercalcemia, or renal involvement suggesting systemic myeloma.
It is important to note that the majority of people with SPB are eventually diagnosed with myeloma. While the use of serum M-protein testing to assess response in patients with SPB requires further study, regular follow-up with serum M-protein testing is highly recommended (except for patients with non-secretory disease).
In closing
This booklet is not meant to replace the advice of your doctors and nurses who are best able to answer questions about your specific healthcare management plan. The IMF intends only to provide you with information that will guide you in discussions with your healthcare team.
To help ensure a good quality of life through effective treatment of your myeloma, you must play an active role in your own medical care. We encourage you to visit myeloma.org for more information and to join the Myeloma Knowledge Platform at myprofile.myeloma.org.
To receive the most up-to-date information about myeloma in a caring and compassionate manner, call the IMF InfoLine at 1.818.487.7455, email InfoLine@myeloma.org, or schedule a time to talk with an IMF InfoLine Coordinator at mmsm.link/infoline.
To get answers to your questions without having to wait, ask Myelo® anytime 24/7 at myeloma.org. This generative AI assistant is designed to help you find the right resources.
Use the hyperlinks and web addresses included in this publication for quick access to resources from the IMF. Sign up at subscribe.myeloma.org for our quarterly journal Myeloma Today and weekly e-newsletter Myeloma Minute, as well as alerts about IMF news, events, and actions.
The International Myeloma Foundation (IMF) is the global leader in myeloma. Our mission is to improve the quality of life of myeloma patients while working toward prevention and a cure. Since 1990, the IMF has been serving the myeloma community through the following four pillars:
RESEARCH At the IMF, finding a cure for myeloma is our top priority. The IMF Scientific Advisory Board (SAB) of leading myeloma experts identifies key opportunities to drive research forward. The IMF Black Swan Research Initiative® (BSRI®) is pushing the boundaries with early screening for a precursor condition of myeloma as well as cure-focused myeloma clinical trials. The IMF International Myeloma Working Group (IMWG) provides trusted guidelines for diagnosing, treating, and managing myeloma. We also fund innovative research through the IMF Brian D. Novis Research Grants.
EDUCATION Myeloma is a complex and unique journey for each patient. The IMF offers hundreds of videos and free publications in multiple languages to inform and empower patients and care partners to navigate their myeloma journey. All IMF seminars, webinars, and workshops are free-of-charge and designed to directly connect the patient community with expert myeloma clinicians. The IMF Nurse Leadership Board (NLB) provides recommendations for the management of myeloma. The IMF M-Power Project works to break down barriers and ensure health equity in underserved populations.
SUPPORT Studies show that social support can greatly improve the quality of life of people with cancer. The IMF offers more than 160 myeloma support groups across North America, including specialized groups for Spanish-speakers, people with smoldering myeloma, care partners of patients with myeloma, and patients who do not have care partners. The IMF InfoLine answers myeloma-related questions. Myelo®, the IMF’s generative AI assistant, is available 24/7 to help you find the right resources.
ADVOCACY In the U.S., the IMF Advocacy team represents your interests at the federal and state levels. Internationally, the IMF Global Myeloma Action Network (GMAN) works to improve patient access to treatments.