Welcome!
Thank you for joining us today for the September 13, 2023, International Myeloma Foundation’s Regional Community Webinar


Thank you for joining us today for the September 13, 2023, International Myeloma Foundation’s Regional Community Webinar











September 13, 2023, Agenda
5:30 – 5:35 PM Welcome and Announcements
Kelly Cox, Director of Support Groups - Senior Director Regional Community Workshops

5:35 – 5:55 PM Myeloma 101


Joseph Mikhael, MD - Chief Medical Officer, International Myeloma Foundation
5:55 – 6:15 PM Life is a Canvas, You are the Artist
Rebecca Lu, MS, FNP-C - MD Anderson Cancer Center; IMF Nurse Leadership Board
6:15 – 6:45 PM Frontline Therapy
Al-Ola Abdallah, MD - University of Kansas Medical Center
6:45 – 7:05 PM Q&A with Panel 7:05 – 7:15 PM BREAK
7:15 – 7:25 PM Maintenance Therapy
September 13, 2023, Agenda
Al-Ola Abdallah, MD – University of Kansas Medical Center
7:25 – 7:55 PM Relapsed Therapies & Clinical Trials

Joseph Mikhael, MD - Chief Medical Officer, International Myeloma Foundation
7:55 – 8:25 PM Q&A
8:25 – 8:30 PM Closing Remarks


. The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:
Frequent topics:

• Treatment questions along the spectrum of care
• Clinical Trial access and understanding
• Side effect management and health issues
• Financial resources for myeloma-related expenses

• Myeloma Specialist Referral contact information



• Support group information
• Caregiver Support
Paul






















A core mission of the IMF is to provide thorough and cutting- edge education to the myeloma community.














• Review the basics of blood and cancer
Define multiple myeloma and its key features
Discuss the staging and classification of myeloma
Outline the approach to therapy of myeloma
Appreciate the importance of health disparities in myeloma
• The blood is an “organ” made up of both cells and liquid “plasma”

• Think of wine (red/white/rose)
1. Red Cells – carry Oxygen…trucks
2. White Cells – immune system…army
3. Platelets – help with clotting…ambulance
All produced in the blood factory = Bone Marrow
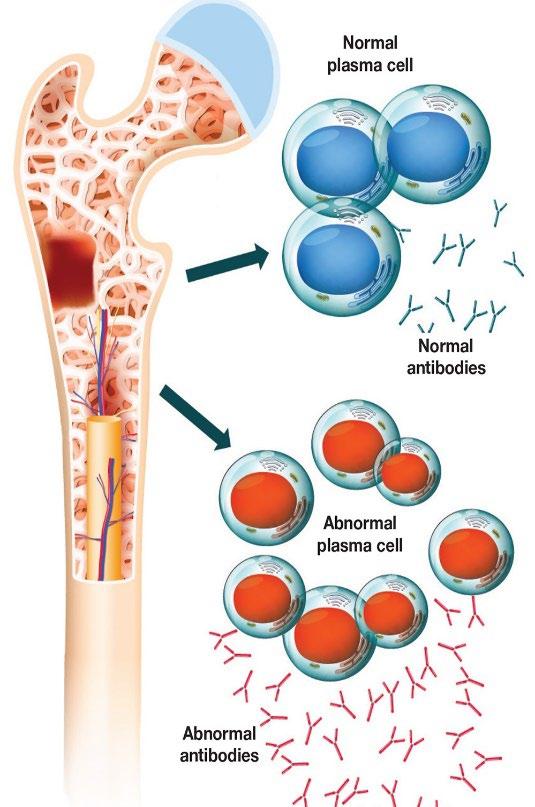
Multiple Myeloma* is a blood cancer that starts in plasma cells from the center of bones (bone marrow).
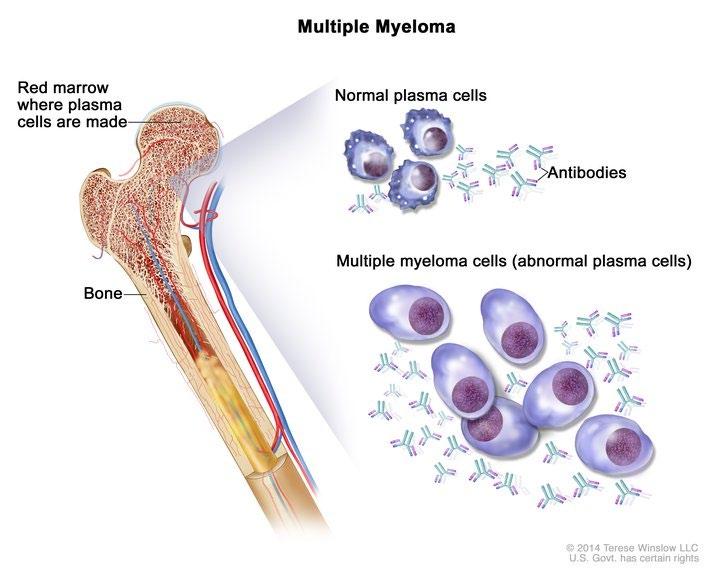
– This is where stem cells mature into red blood cells, white blood cells, and platelets – Myeloma cells are abnormal plasma cells that make an abnormal antibody called “M protein” – M = monoclonal (“identical” or cancerous)
* Myeloma is NOT a bone cancer or skin cancer (melanoma), it is a type of blood cancer.










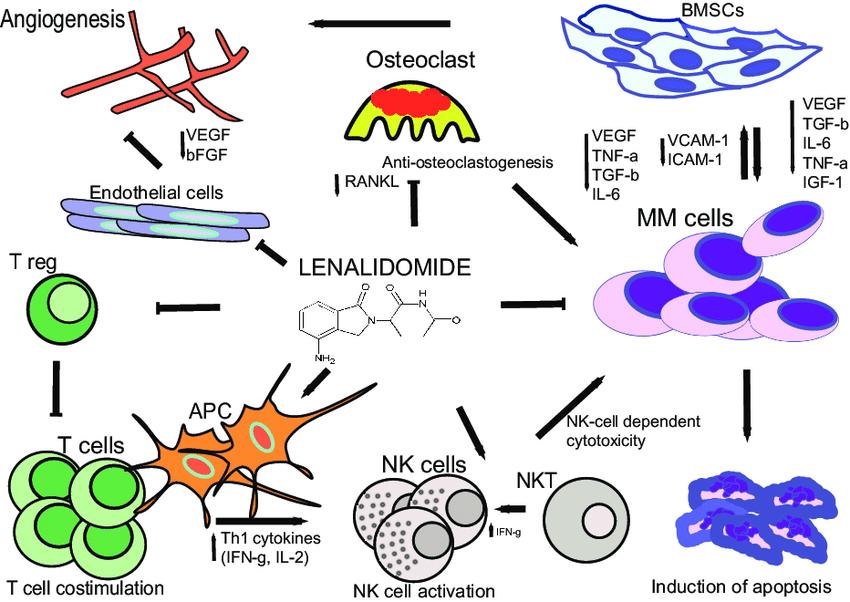
Increase in cytokine production and adhesion molecules





NFkB–IkB
















cFLIP/FADD IL-6 TNF
complex Protein kinases LFA1 FADD cFLIP Cell organelles Fibronectin




NFkB Binding Site
BM Stromal Cells



Dendritic Cells
Monocytes
Inhibition of Anti-Myeloma Immunity

• Cancer of plasma cells
• Healthy plasma cells produce immunoglobulins G, A, M, D, and E
• Myeloma cells produce abnormal immunoglobulin “paraprotein” or monoclonal protein (=M protein)
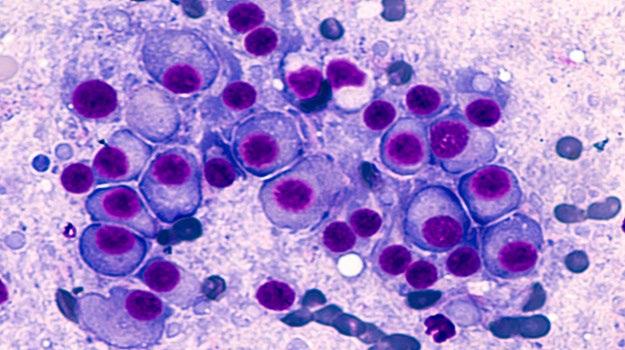
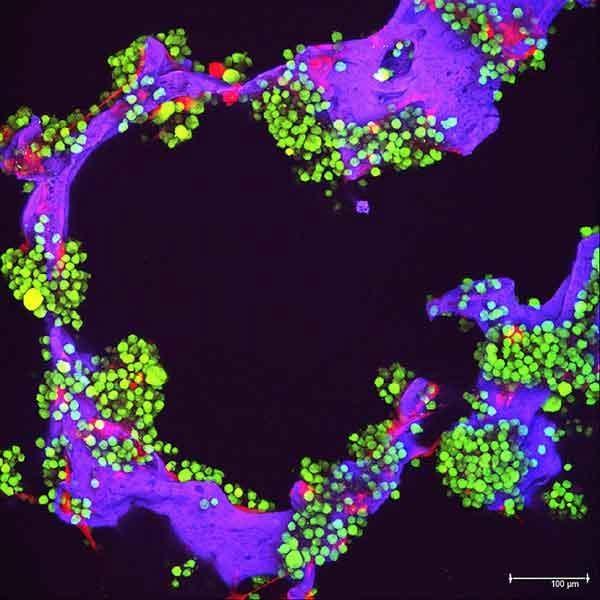
Bone marrow of patient with multiple myeloma
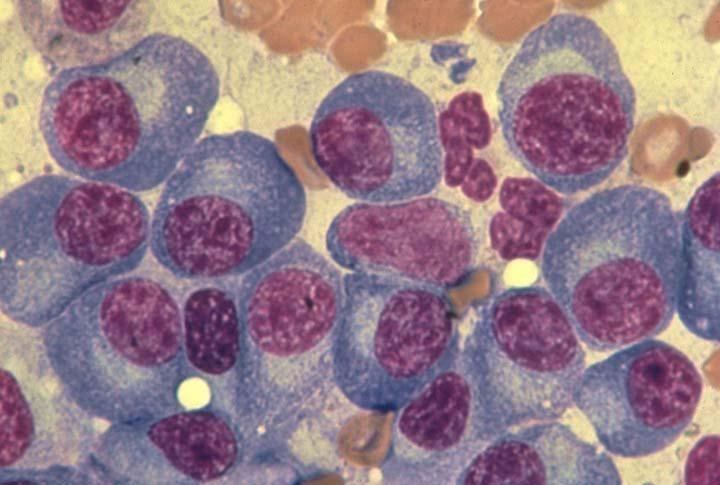
Image courtesy of American Society of Hematology
Kyle et al. Mayo Clin Proc. 2003;78:21-33;
1.8% of all cancers;



17% of hematologic malignancies in the United States
Most frequently diagnosed in ages 65 to 74 years (median, 69 years)
The average age of diagnosis of 4-5 years younger in African American and Hispanic patients
Approx 35,000 Estimated New Cases in 2023
Approx 13,000 Estimated Deaths in 2023
The Average Survival of patients with myeloma is IMPROVING!

MM Incidence Higher incidence in AA vs White patients:
• 15.9 vs 7.5 cases per 100,000 per year
MM Mortality Higher mortality in AA vs White patients:
• 5.6 vs 2.4 MM deaths per 100,000
5-year relative survival evolution from 1973 to 2005
The expected survival is nearly 10 years for all patients, but still less than 5 years in patients with high risk disease
MM Survival
• Survival for White patients increased significantly from 26.3% to 35%
• Survival for AA patients increased from 31% to 34.1%
• IgG+kappa
• IgG+lambda
• IgA+kappa
• IgA+lambda
• etc…
• 80% of myeloma cases
Jones protein
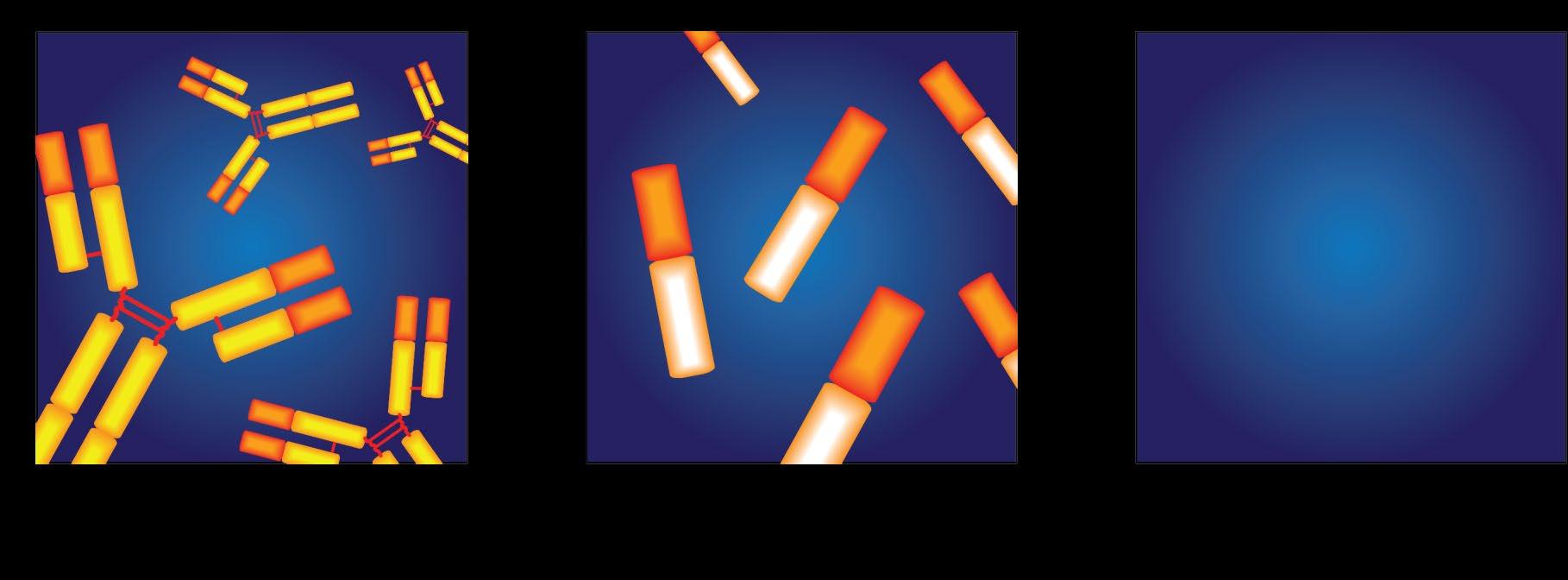
• 18% of all myeloma cases
• Renal failure more common in light chain multiple myeloma; creatinine >2 mg/dL in 1/3 of cases
protein present
• Less than 3% of cases of multiple myeloma


• Subtypes of MM are determined based on the kind of abnormal protein IgG – 55% IgA – 25% IgD – 1-2% IgM – 1% Light Chain Disease only – 20% Non Secretors 1-2 %
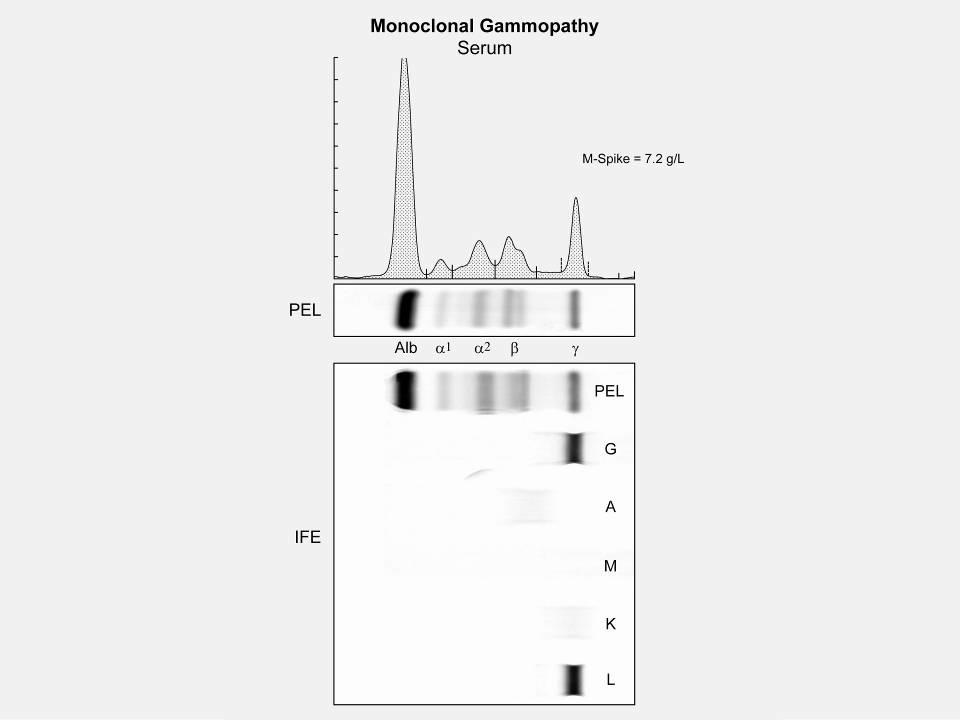 M spike in gamma region
M spike in gamma region
- both “heavy” and ”light” chains
* In clinical trial (preferred) or offer treatment for those likely to progress within 2 years
1. Kyle RA, et al. N Engl J Med. 2007;356:2582-90.
2. International Myeloma Working Group. Br J Haematol. 2003;121:749-57.
3. Jagannath S, et al. Clin Lymphoma Myeloma Leuk. 2010;10(1):28-43.
4. Kyle RA, et al. Curr Hematol Malig Rep. 2010;5(2):62-69.
5. Mateos M-V, et al. Blood. 2009;114:Abstract 614.
6. Durie BG, Salmon SE. Cancer. 1975;36:842-854.
7. Durie BG, et al. Leukemia. 2006;20(9):1467-1473.
8. Rajkumar SV, et al. Lancet Oncology 2014; 15:e538e548.


Fatigue



Kyle RA. Mayo Clin Proc. 2003;78:21-33.
Anemia
Clonal bone marrow ≥10% or bony/extramedullary plasmacytoma
AND any one or more Myeloma-Defining Events
Now SLiM CRAB

C
R A B
alcium elevation
• S (60% Plasmacytosis)
enal complications
nemia one disease
Clonal bone marrow ≥60% BM FLC MRI
sFLC ratio >100 >1 focal lesion by MRI
• Li (Light chains I/U >100)
• M (MRI 1 or more focal lesion)
• C (calcium elevation)
• R (renal insufficiency)
• A (anemia)
• B (bone disease)
BM, bone marrow; FLC, free light chain; MRI, magnetic resonance imaging; sFLC, serum free light chain. Rajkumar et al. Lancet Oncol. 2014;15:e538-e548. Kyle et al. Leukemia 2010;24:1121-1127.
Low Blood Counts
• May lead to anemia and infection
• Anemia is present in 60% at diagnosis
Weakness
Fatigue
Infection
Decreased Kidney Function
• Occurs in over half of myeloma patients
Weakness
Bone Damage
• Affects 85% of patients
• Leads to fractures
Bone pain
Bone Turnover
• Leads to high levels of calcium in blood (hypercalcemia)
Loss of Appetite & Weight loss
About 10% to 20% of patients with newly diagnosed myeloma will not have any symptoms.


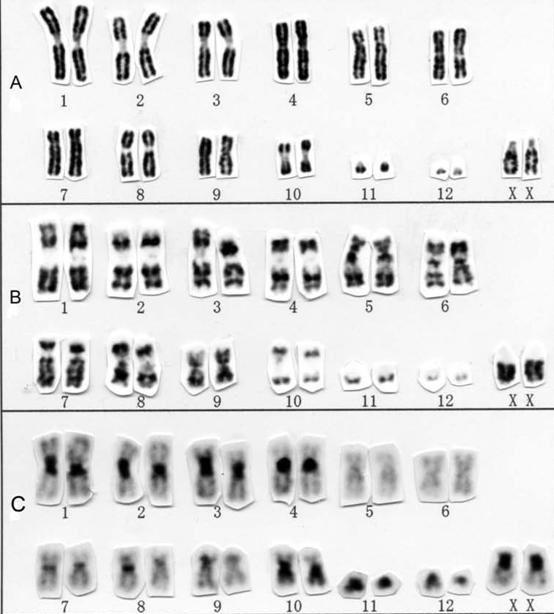
Conventional cytogenetic analysis (karyotyping)




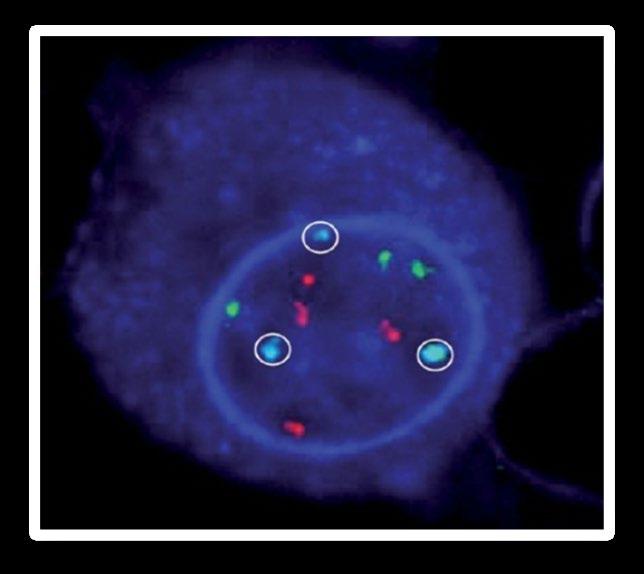
FISH
(fluorescence in situ hybridization)
DNA
Advances
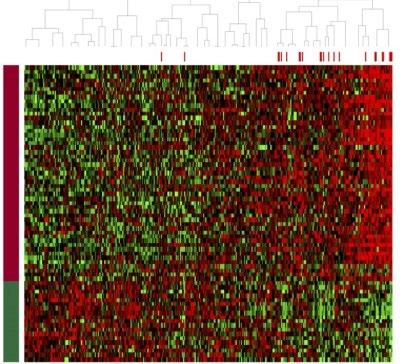
• Genetic expression profiling [GEP]
• Whole-genome/ whole-exome sequencing
• Plasma cell next generation sequencing
Risk Category
Findings on Chromosome (FISH) Analysis Results in the Bone marrow
FISH:
High Risk
• Deletion 17th chromosome
Standard Risk
• Gain of chromosome 1q
• Translocation 4 and 14
FISH:
• Hyperdiploid: More than 1 pair of chromosomes (Trisomies)
• Translocation 11 and 14
• Translocation 6 and 14
• Translocation 14 and 16
• Translocation 14 and 20
• Others
• Normal
NGS: p53 mutation (on chrom 17)

• Double Hit Myeloma: 2 high risk
genetic abnormalities
• Triple Hit Myeloma: 3 or more high risk genetic abnormalities
Myeloma Stage:
Staging refers to the degree to which the cancer has progressed. Most important at time of diagnosis.
Stage 1
β2-microglobulin under 3.6 mg/L


































Stage 2
β2-microglobulin Between 3.5 & 5.4mg/L
Stage 3
β2-microglobulin over 5.5 mg/L
Normal Lactate Dehydrogenase (LDH)
AND
NO High Risk Cytogenetics (FISH)
NO High Risk Cytogenetics (FISH)
HIGH Lactate Dehydrogenase (LDH)
AND/OR
High Risk Cytogenetics (FISH)
Deletion 17 chromosome
Translocation 4 and 14
Translocation 14 and 16th
Translocation 14 and 20th
CBC Counts the number of red blood cells, white blood cells, and platelets


CoMP
Measures levels of albumin, calcium, and creatinine to assess kidney and liver functions, bone status ,and the extent of disease
Beta2 MicroG


Determines the level of a protein linked to MM and kidney function: USED FOR STAGE
LDH Lactate Dehydrogenase

















Determines the level of myeloma cell production and extent of MM : USED FOR STAGE
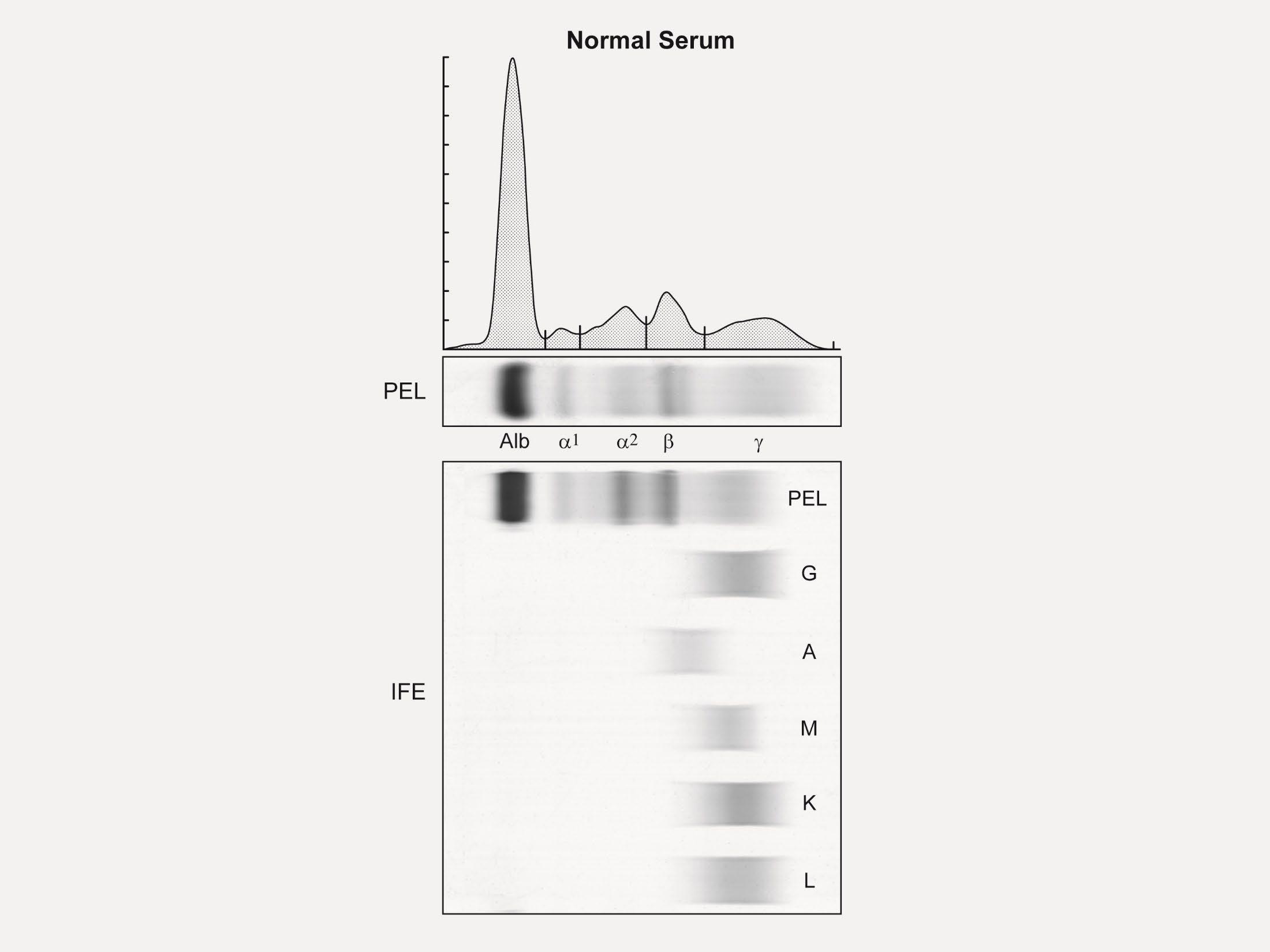
Serum Protein EP Detects the presence & level of M protein = how much myeloma. No Heavy Chain = No M-Spike
Immuno Fixation
Identifies the type of abnormal antibody proteins: IgG, IgA, IgM
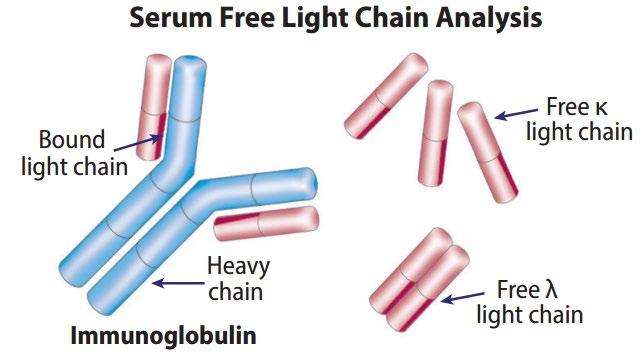
Serum Free Light Chain Measures myeloma free light chains (kappa or lambda) in blood = how much myeloma
Urine Protein EP Detects Bence-Jones proteins (otherwise known as myeloma light chains) in urine (to determine if it’s present or not present)

24 hr Urine Analysis
24 hours of urine collected to test the presence and levels of Bence Jones protein in the urine = how much myeloma

Treatment Planning is the process of thinking about the treatment steps you can take with your doctor, based on your goals and preferences. Treatment decisions are based on:
The results of biomarker tests, cytogenetic (FISH) test, and the stage of multiple myeloma
Your values, goals, and preferences
Your age
Your health and symptoms (if you have kidney disease, heart disease, anemia, or other issues)
Your medical history and past treatments for multiple myeloma
Lifestyle Goals of Therapy
Age

Patient Preference
Myeloma Symptoms

• You have the right to get a second opinion. Insurance providers may require second opinions.
• A second opinion can help you:
–
Confirm your diagnosis
–
Give you more information about options
–
Talk to other experts
–
Introduce you to clinical trials
–
Help you learn which health care team you’d like to work with, and which facility

10
5
SYMPTOMATIC REFRACTORY RELAPSE ACTIVE MYELOMA MProtein g/L 2
ASYMPTOMATIC PLATEAU REMISSION Therapy Time

MGUS = monoclonal gammopathy of undetermined significance
Clone 1.1
Clone 1.2
Clone 2.1 Clone 2.2
Misc

Class
IMiD immunomodulatory drug
Drug Name
Abbreviation Administration
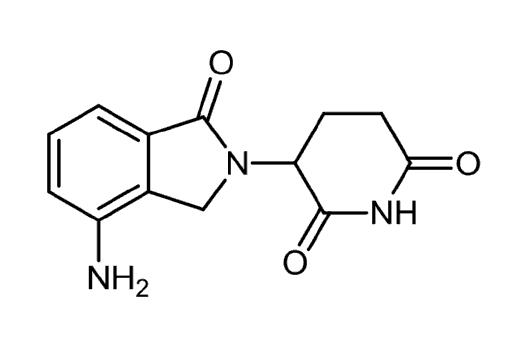
Pomalyst (pomalidomide) P or Pom Oral Revlimid (lenalidomide)
or Rev
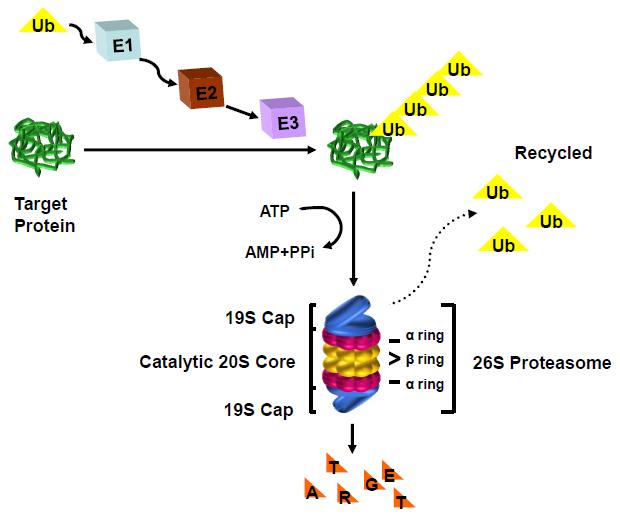
Proteasome inhibitor
Thalomid (thalidomide)
Chemotherapy
Steroids
Monoclonal Antibodies
XPO1 Inhibitors
Kyprolis (carfilzomib) C or K or Car
Velcade (bortezomib) V or Vel or B Intravenous (IV) or subcutaneous injectionSC (under the skin)
Ninlaro (ixazomib) N or I
Cytoxan (cyclophosphamide)

Alkeran or Evomela (melphalan) M or Mel
Decadron (dexamethasone) Dex or D or d
Prednisone P or Pred
Darzalex (daratumumab)
Sarclisa (isatuximab)
Empliciti (elotuzumab)
Xpovio (selinexor)
Dara Isa Elo
Oral or intravenous
Oral or intravenous
Intravenous (IV or SC)
Class
Drug Name
Abbreviation Administration
Peptide Drug Conjugate* Pepaxto (Melphalan Flufenamide) Melflufen Intravenous
BCMA Targeted Antibody Drug Conjugate (ADC)*
Blenrep (belantamab mafodotin) Bela, Belamaf, or B Intravenous
CAR T Cell therapy
Bispecific Antibodies
Abecma (idecabtagene vicleucel)
Carvycti (ciltacabtagene vicleucelel) Cilta-cel

Ide-cel Intravenous (IV) or subcutaneous injectionSC (under the skin)
Tecvayli (teclistimab)
Talvey (Talquetamab)
Elrexfio (Elranatamab)
Tec Talq Elra SC or IV ??? MORE TO COME!!
* these agents are currently off the market but available through special programs
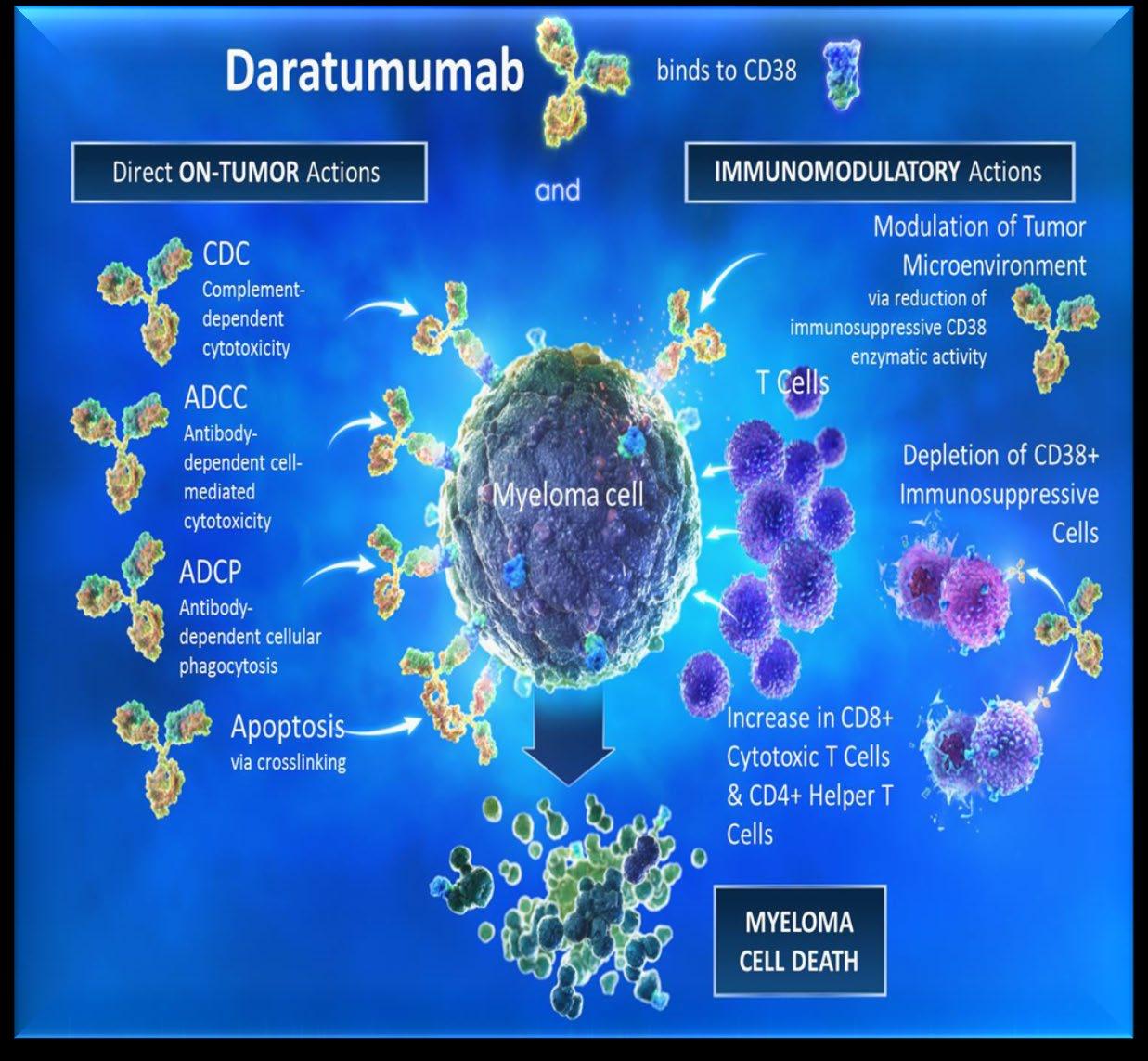
ADCC
Antibody-dependent Cellular cytotoxicity (ADCC)
Effector cells: MM
Complement-dependent Cytotoxicity (CDC) CDC MM
C1q C1q
Apoptosis/growth arrest via targeting signaling pathways MM
FcR
• Lucatumumab or Dacetuzumab (CD40)
• Elotuzumab (CS1; SLAMF7)
• Daratumumab, SAR650984/Isatuximab (CD38)
• XmAb 5592 (HM1.24)
• Daratumumab
• SAR650984/Isatuximab (CD38) Adapted from Tai & Anderson Bone Marrow Research 2011
• huN901-DM1 (CD56)
• nBT062-maytansinoid (CD138)
• Siltuximab (1339) (IL-6)
• BHQ880 (DKK1)
• RAP-011 (activin A)
• Daratumumab, SAR650984/Isatuximab (CD38)
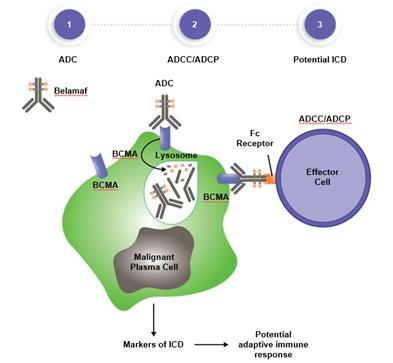 Effector cell
Effector cell
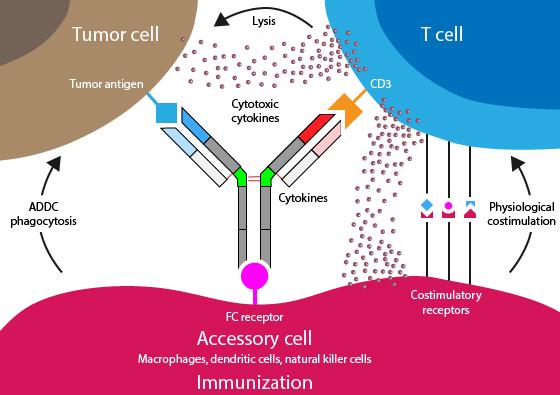
• Incorporates 2 antibody fragments to target and bind both tumor cells and T cells
• Brings target-expressing MM cells and T cells into close proximity, enabling T cells to induce tumor-cell death
Bispecific Molecule Targets Vary
“Off the Shelf” Advantage
• No manufacturing process, unlike CAR T-cell therapy (but like ADC/belantamab therapy)
• Thus, no delay between decision to treat and administration of drug
ADC = Antibody-Drug Conjugate; BCMA = B-Cell Maturation Antigen; CD3 = Cluster of Differentiation 3; FcRH5 = Fc receptor-homolog 5; GPRC5D = G-protein coupled receptor family C group 5 member D
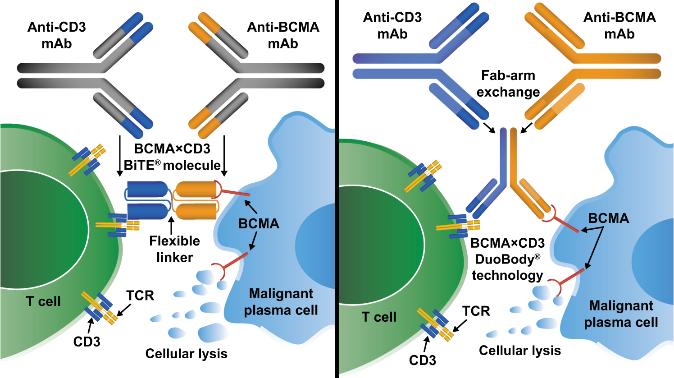
Image Source: Shah N, et al. Leukemia. 2020;34:985–1005. Creative Commons License: CC BY 4.0.
al. Pharmaceuticals (Basel). 2021;14(1):40.
Targets on the Myeloma Cell Surface and Therapeutic Antibodies
Bi-Specific Antibodies
Talquetamab
CAR-T
CD38 GPRC5D SLAMF7 FcRH5


Bi-Specific Antibodies
CAR-T
Antibody Drug
Elotuzumab
Bi-Specific Antibodies
BCMA
Antibody Drug
Daratumumab and Darzalex Faspro
Isatuximab





























TAK-079
MOR202
Immune Therapies

Ide cel CAR T
Cilta cel CAR T
Teclistamab
Other Bi-Specific Antibodies
Other CAR-Ts
Induction
• Velcade/Revlimid/Dex:(VRD)

• Velcade/Thalomid/Dex:(VTD)
• Velcade/Cytoxan/Dex:(CyBorD)
• Darzalex/Revlimid/Dex:(DRD)
• Darzalex/Velcade/Melphalan/Dex
• Darzalex/Velcade/Thalidomide/Dex
• Kyprolis/Revimid/Dex(KRD)
• Darzalex/Velcade/Revlimid/Dex: Dara-RVD
• Ninlaro/Revimid/Dex(IRD)
• Clinical trials
Consolidation
Maintenance
• Stem Cell Transplant
• Continue Induction
• Clinical trial
• Revlimid
• Velcade
• Ninlaro
• Observation
• Thalidomide
• Revlimid/Dara
• Clinical trial
Rescue
Dara+Pomalyst+Dex
Kyprolis+Pomalyst+Dex
Cytoxan+Pomalyst+Dex
Ninlaro+Pomalyst+Dex
Elo+Pomalyst+Dex
Elo+Thaliomide+Dex
Dara+Kyprolis+Dex
Kyprolis+Revlimid+Dex
Elo+Revlimid+Dex
Dara+Revlimid+Dex
Dara+Velcade+Dex
Elo+Velcade+Dex

Cytoxan+Kyprolis+Thalidomide+dex
4 drug therapies of novel agents
Ninlaro+Cytoxan+Dex
Velcade+ Cytoxan+Dex
Velcade+ Pomalyst+Dex
Chemotherapy
Selinexor+Dex
Selinexor+Velcade
Selinexor+ Dara
Isatuximab(Sarclisa)+ Pomalyst+Dex
Darzalex Faspro (under skin)
Ide-cel CAR-T (FDA approved 3/26/2021)
Cilta-cel CAR-T (FDA approved 2/28/2022)




Teclistamab (FDA approved 10/25/2022)


autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib. Speaker’s own opinions.

1. There is a longer time from symptoms to diagnosis among African Americans
2. African Americans are younger by about 5 years on average at diagnosis
3. MM and MGUS are more than 2x as common in African Americans
4. African Americans are less likely to receive the four T’s: Transplant, Triplets, Trials and CAR T
5. African Americans have biologic differences with more t(11;14) and less high-risk cytogenetics with deletion 17p
6. Survival outcomes in African Americans are HALF of what is seen in White Americans
7. African Americans can achieve equal or better outcomes when they receive therapy

The core vision of this initiative is to improve the short- and longterm outcomes of African American patients with myeloma.
Engage the community to increase awareness and provide support
We want to empower patients and communities to change the course of myeloma…

Enhance access to optimal care by educating myeloma providers about the disparity and how to reduce it
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests
You can learn more about this at m-power.myeloma.org



September 13, 2023
COLOR WHEEL OF TREATMENT

Myeloma treatment
Infection and Side Effect Management
Know your care team, Telehealth & Meeting Prep, & Shared Decision Making

• Rapid and effective disease control

• Durable disease control

• Minimize side effects
• Allow for good quality of life
• Improved overall survival
• Prevent disease- and treatmentrelated side effects
• Optimize symptom management
• Allow for good quality of life
DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM
Transplant
Eligible Patients
Transplant
Ineligible patients
Initial Therapy
Transplant
Consolidation
Maintenance
Treatment of Relapsed disease
Everyone
Consolidation/ Maintenance/ Continued therapy
Supportive Care

• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
Patient Preference
Adapted from Philippe Moreau, ASH 2015



A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy

• Impaired Physical Functioning
• Sexual Dysfunction


Psychological

• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial
• Financial burden (80%)
• Financial toxicity (43%)
All can affect quality of life and relationships
• Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression


• Anxiety reported in >35%

• Depression nearly 25% Financial concerns, disease progression, end-of-life, and change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
&
Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications

• Medications to prevent shingles, thrush, or other infections
Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue

• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention
Rajkumar
• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increase in blood sugar levels, diabetes

SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus highdose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 11(1):29–37.
T, Faiman B. Steroid-Associated Side Effects: A Symptom Management
Shortness of Breath
CRS is a common but usually mild side effect

Headache
Encephalopathy
Confusion

Seizures
Tremors
NEUROTOXICITY
Altered wakefulness
Neurotoxicity is a rare but serious side effect
Hallucinations
Facial nerve palsy
Ataxia Apraxia
CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy screening tool; MRI = magnetic resonance imaging.
Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
7-10 fold increased risk of bacterial and viral infections for people with myeloma
• Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
• Compromised immune function comes from multiple myeloma and from treatment.
• Infection is serious for myeloma patients!


Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])

Immunizations (NO live vaccines)
Medications (antibacterial, antiviral)
As recommended by your health care team
COVID: The Best Way to Prevent Illness Is to Avoid Being Exposed to the Virus
Spread mainly through respiratory droplets that are produced by cough, sneezing and talking. More droplets with louder talking, yelling, singing

• Get COVID Vaccine + Booster: Excellent protection against severe disease, but vaccine effectiveness may be lower in people with compromised immune systems
• Wear a High-quality Mask: Respiratory droplets can spread disease; a high-quality mask can prevent exposure to airborne viral particles
• Avoid Crowds & Sick People
• Physical Distance & Outdoors: Close contact and indoor locations increases risk of spread
• Wash Your Hands: Less common to get from a hard surface
Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
• Imodium®, Lomotil ®, or Colestid if recommended
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
• Welchol ® if recommended
Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®

Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain can significantly compromise quality of life

Sources of pain include bone disease, neuropathy and medical procedures
Management
• Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
• Interventions depends on source of pain
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT)
• May include medications (eg bone modifying agents), activity, surgical intervention, radiation therapy, etc

• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled
Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
• B-complex vitamins (B1, B6, B12)
• Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes


If PN worsens, your HCP may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed
Financial burden comes from
• Medical costs
• Premiums
• Co-payments

• Travel expenses
• Medical supplies

• Prescription costs
• Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
• Federal programs
• Pharmaceutical support
• Non-profit organizations
• Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement

• Complementary therapy
Maintain a healthy weight
• Nutrition
• Activity / exercise
Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain renal health
• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function
• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight-bearing activity / walking
• Bone strengthening agents
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
“An ounce of prevention is worth a pound of cure.” Benjamin Franklin
Care partner support is essential for the entire transplant and CAR T processes particularly
• Sedated procedures; Education sessions
• Assistance with daily activities, managing medications and alerting the medical team of changes
• Continued support and assistance is often needed in the early days after returning home. Less assistance will be needed as time goes on.



Care partner can be one person or a rotation of many people.

You are central to the care team








Be empowered
• Ask questions, learn more
• Participate in decisions
Communicate with your team
• Understand the roles of each team member and who to contact for your needs

• Participate in support network








Come prepared:
• Bring a list of current medications, prescribed and over the counter
• Write down your questions and concerns. Prioritize them including financial issues

• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively: your health care team can’t help if they don’t know
• Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?
Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?

• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase


























Chair, US Myeloma Innovations Research Collaborative (USMIRC)
Director, Plasma Cell Disorder Clinic
Division of Hematologic Malignancies and Cellular Therapeutics

Department of Internal Medicine



The University of Kansas Cancer Center

I



have NO financial relationships to disclose
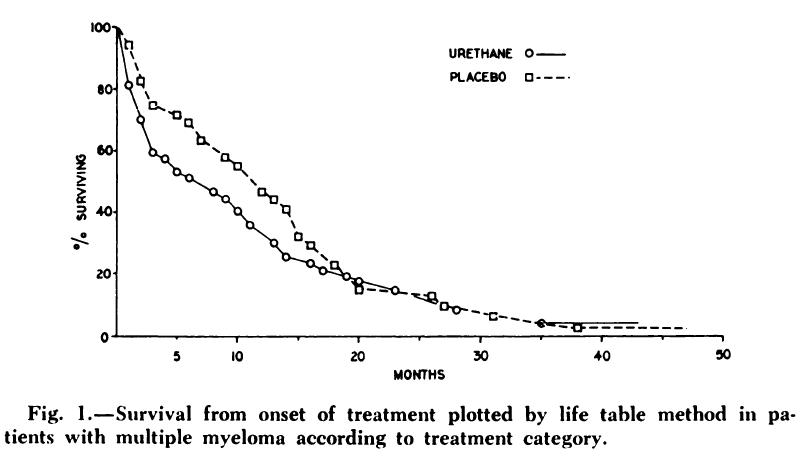
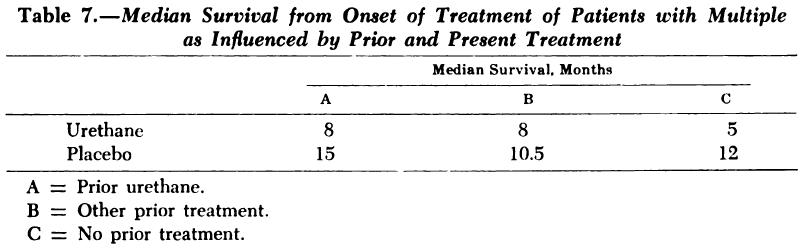
• “A controlled trial of urethane treatment in multiple myeloma.”





Holland JR, Hosley H, Scharlau C, Carbone PP, Frei E 3rd, Brindley CO, Hall TC, Shnider BI, Gold GL, Lasagna L, Owens AH Jr, Miller SP
• Randomized 83 patients with treated or untreated multiple myeloma to receive urethane or a placebo consisting of a cherry- and cola flavoured syrup.


• No difference was seen in objective improvement or in survival in the two treatment groups. In fact, the urethane-treated patients died earlier.
Blood 1966; 27: 328-342


• Achieve disease response

• Reduce Active symptoms
• Prevent any additional morbidity
• Prolong the patient’s overall survival
• Minimize toxicity of therapy
• Use agents with predictable and manageable side effects
• Maximize Quality of life
• Administer medically & cost- effective therapies

• Cure?

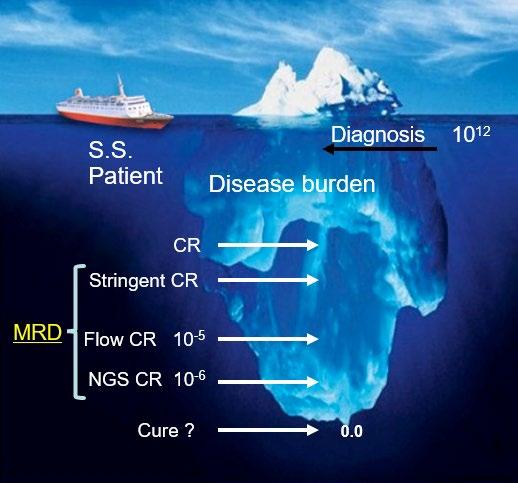

















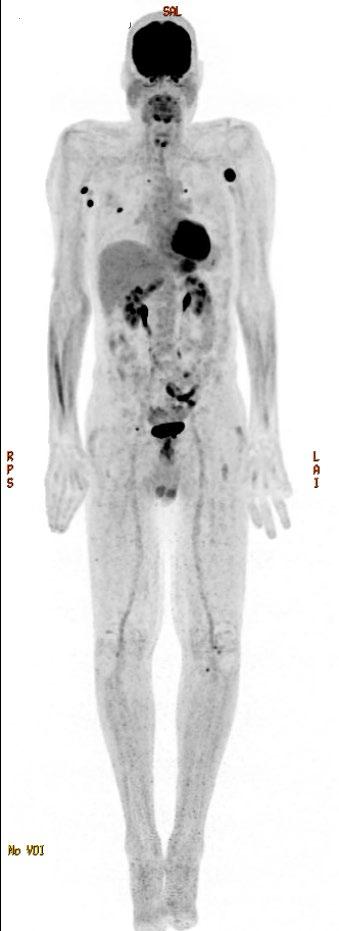








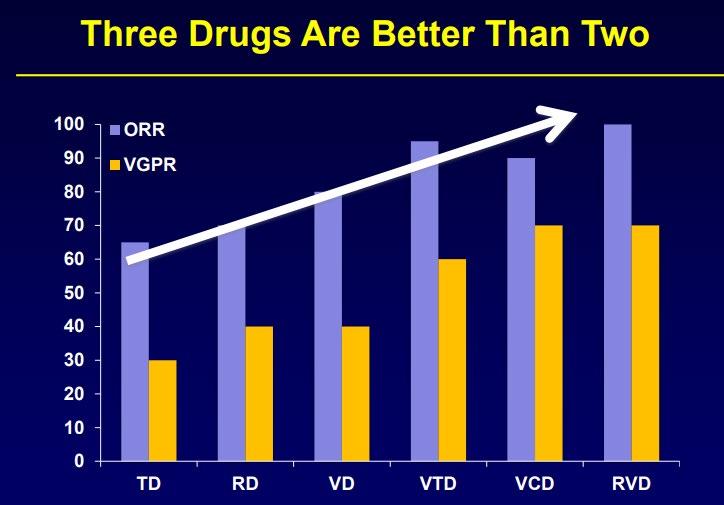

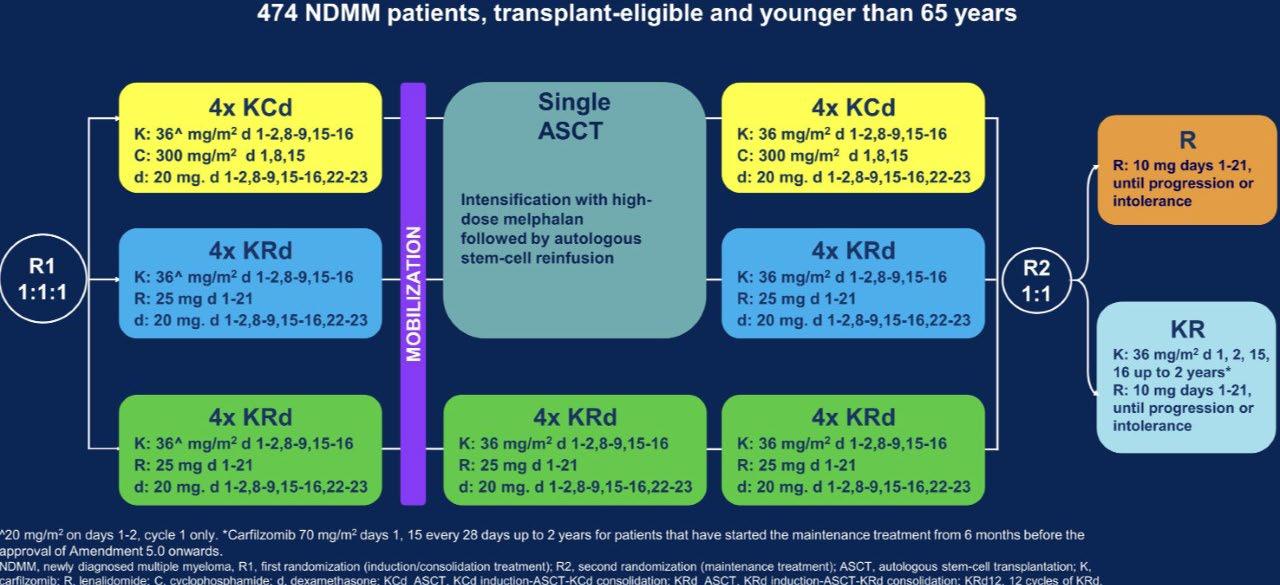
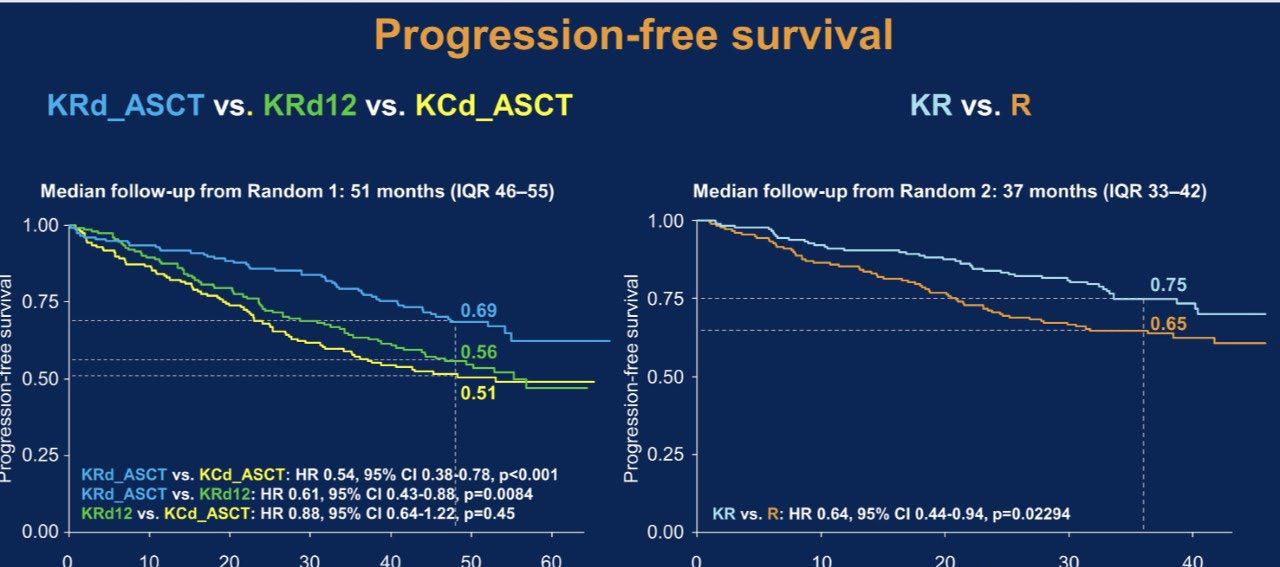
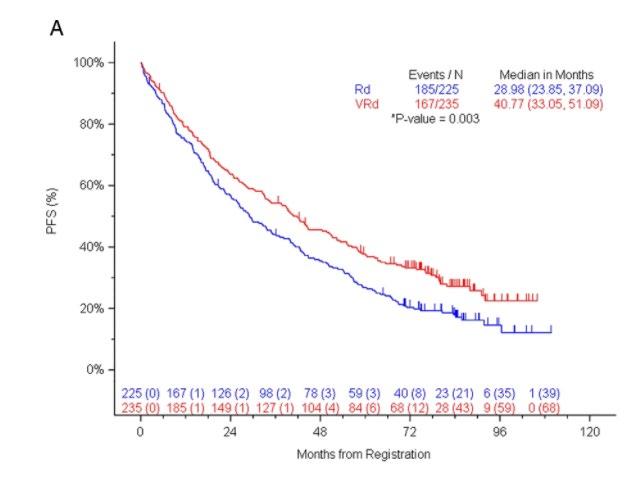
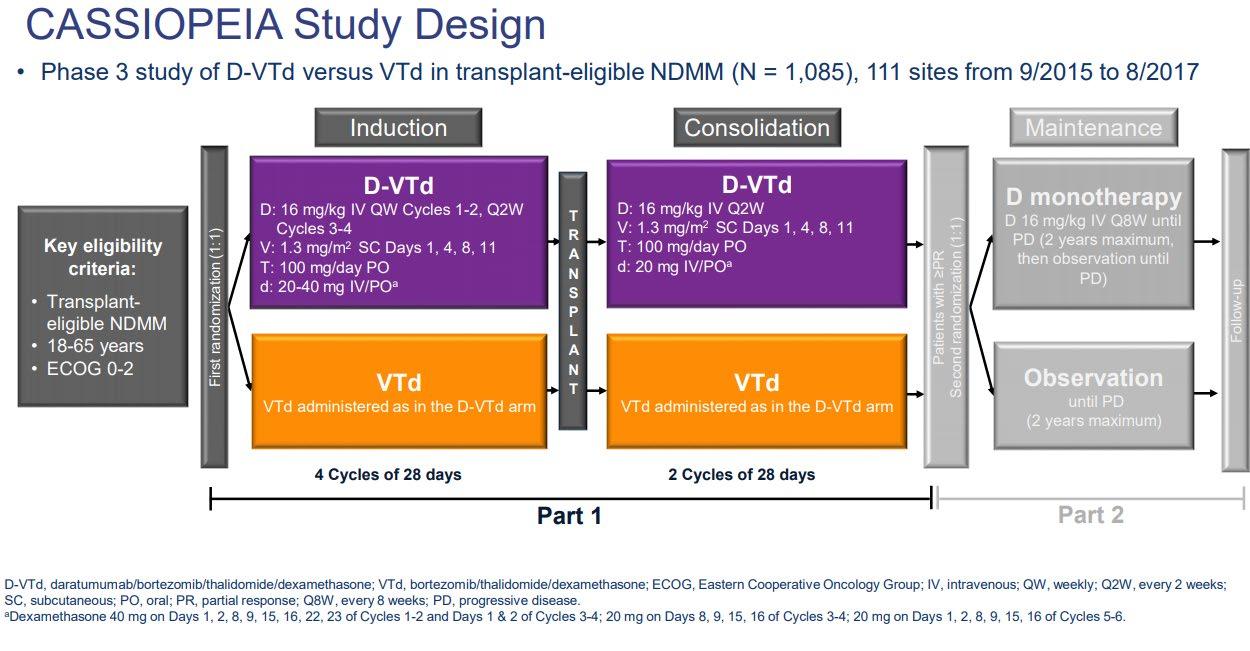
ENDURANCE: VRD vs KRD as induction therapy in NDMM








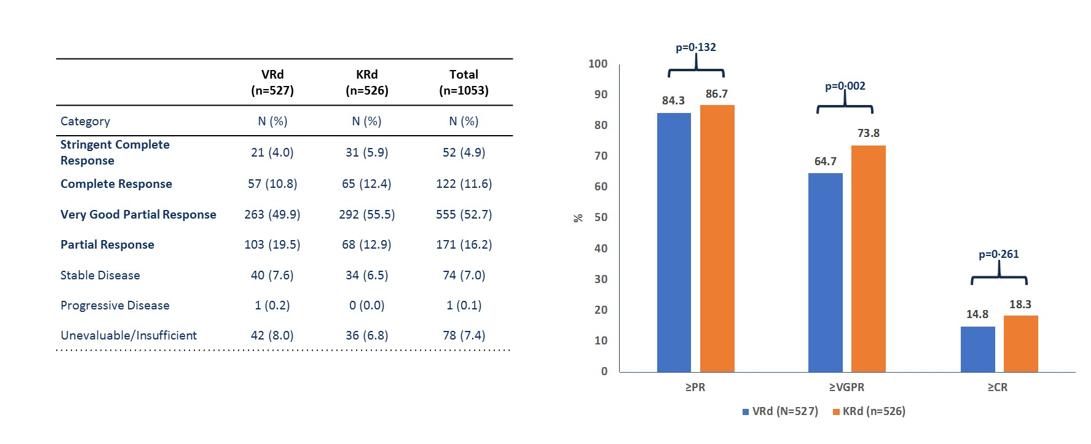


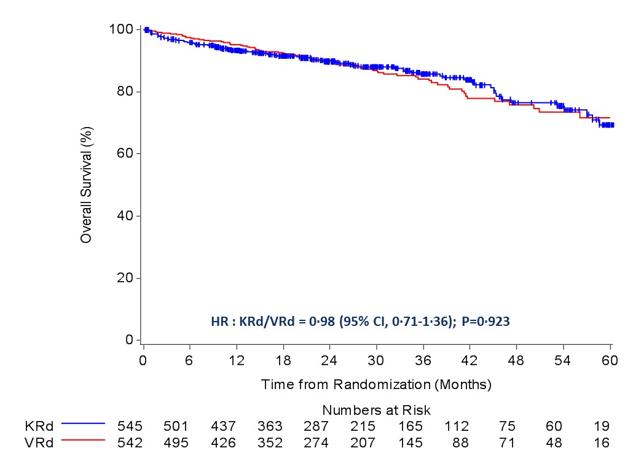
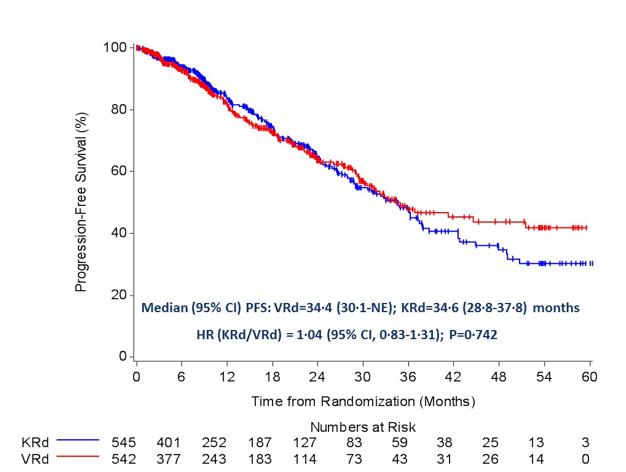




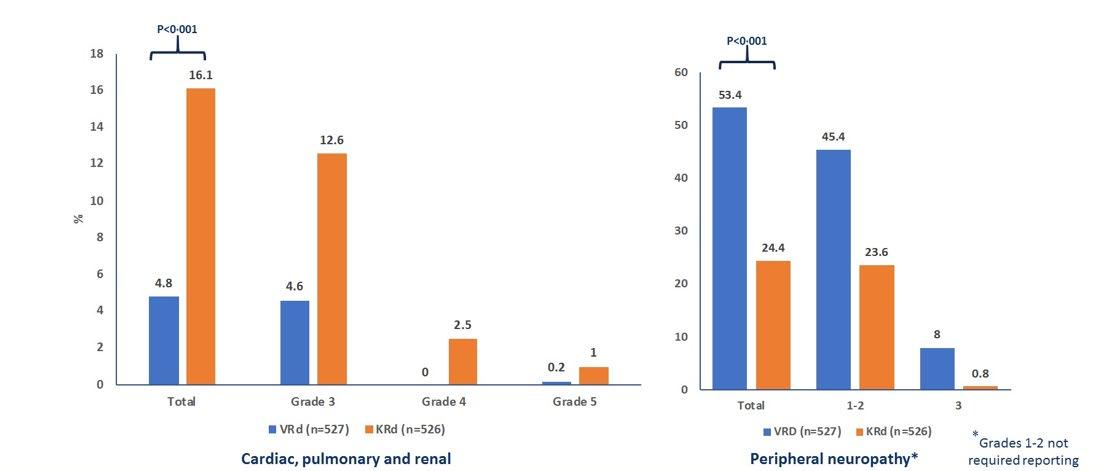





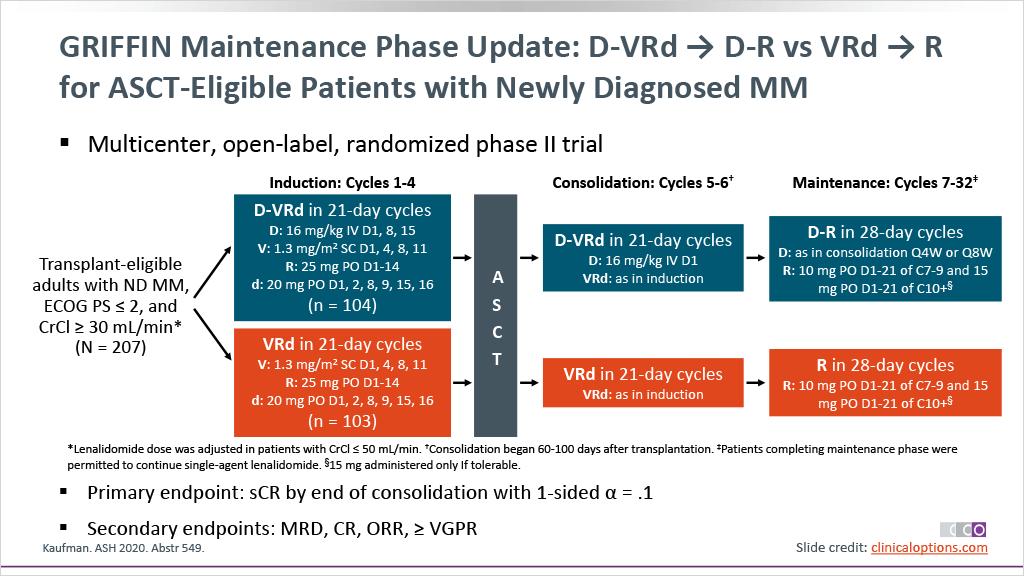


sCR,
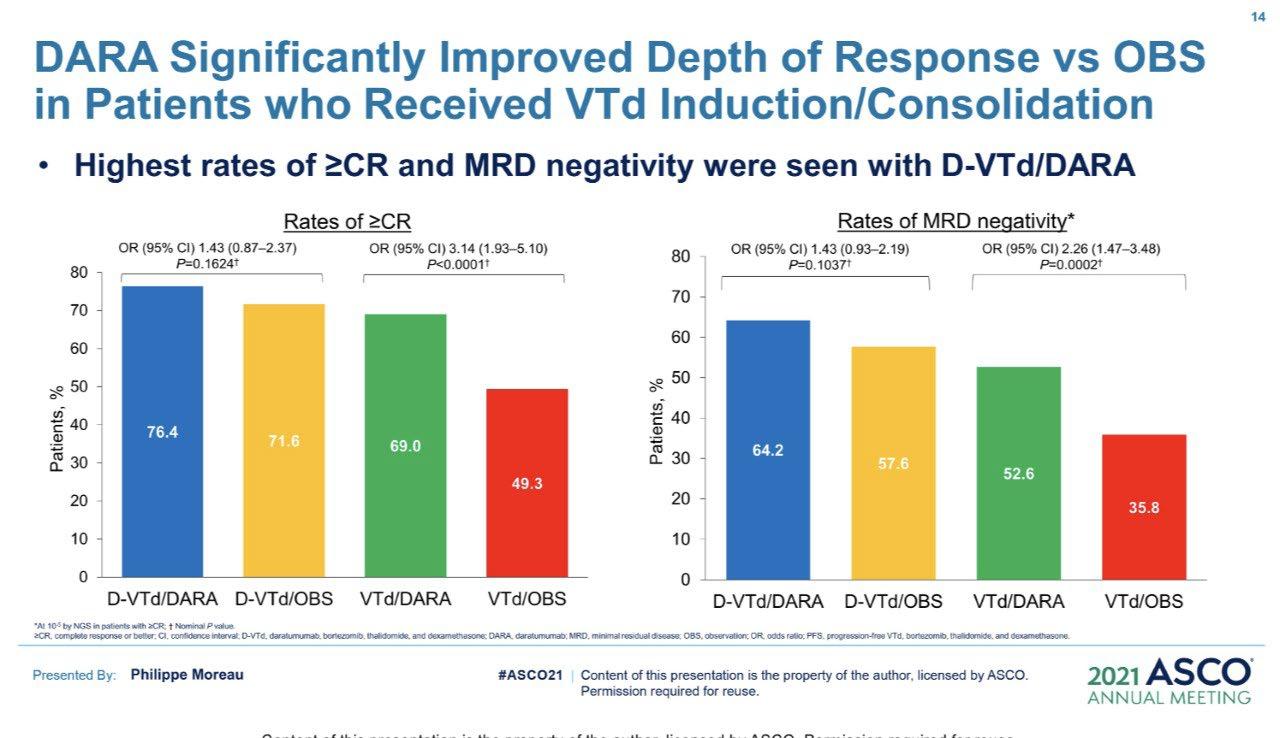
• Rates of ≥CR improved over time and the deepest responses occurred at the end of study maintenance

• At all timepoints, response rates for D-RVd were consistently higher versus RVd

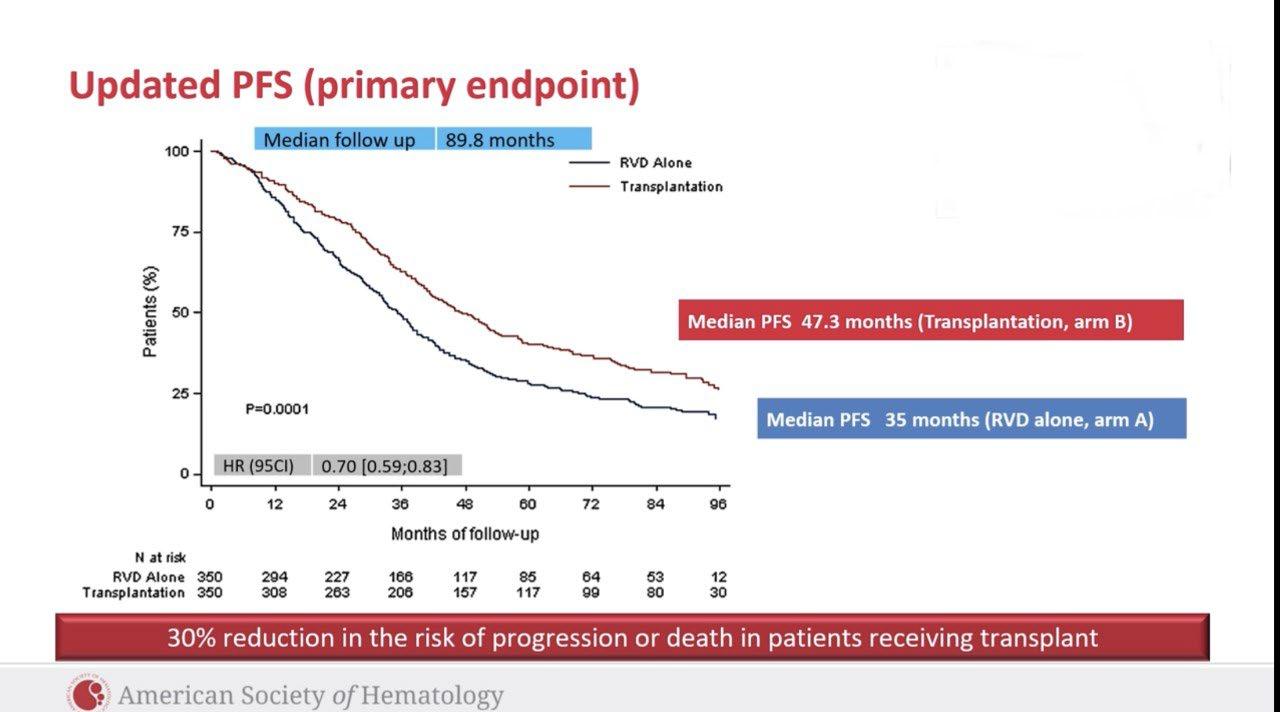
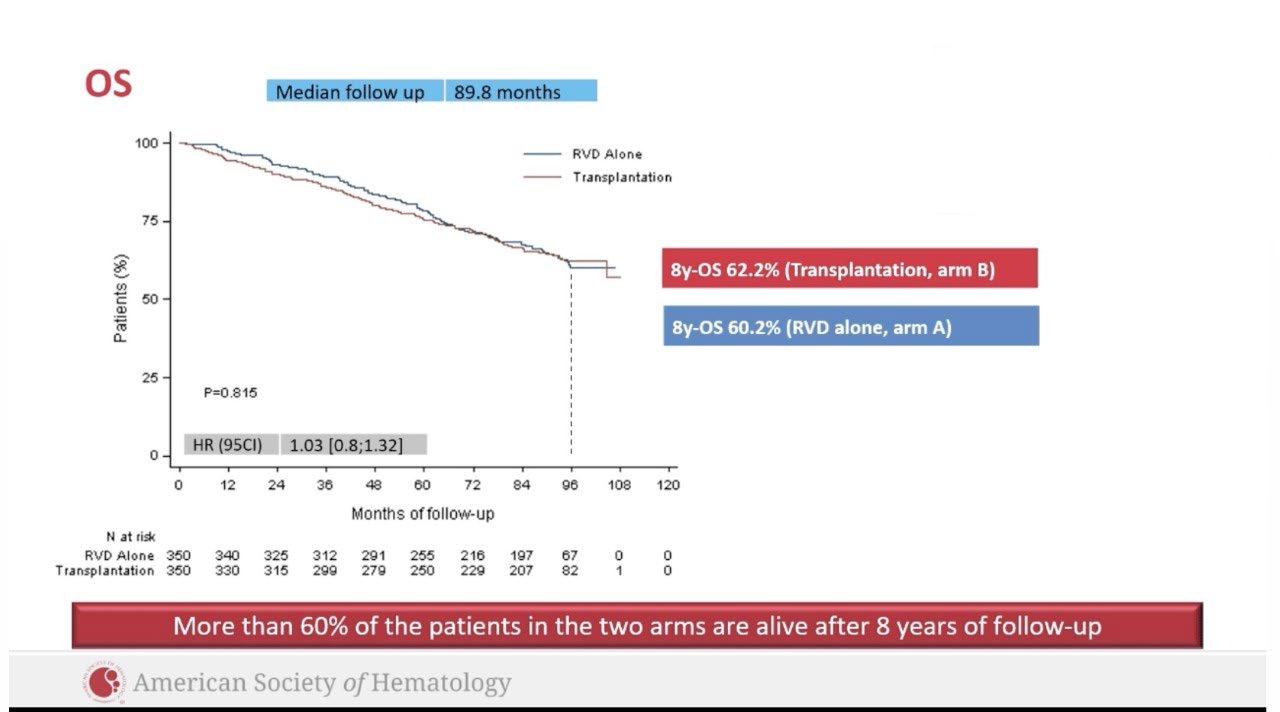
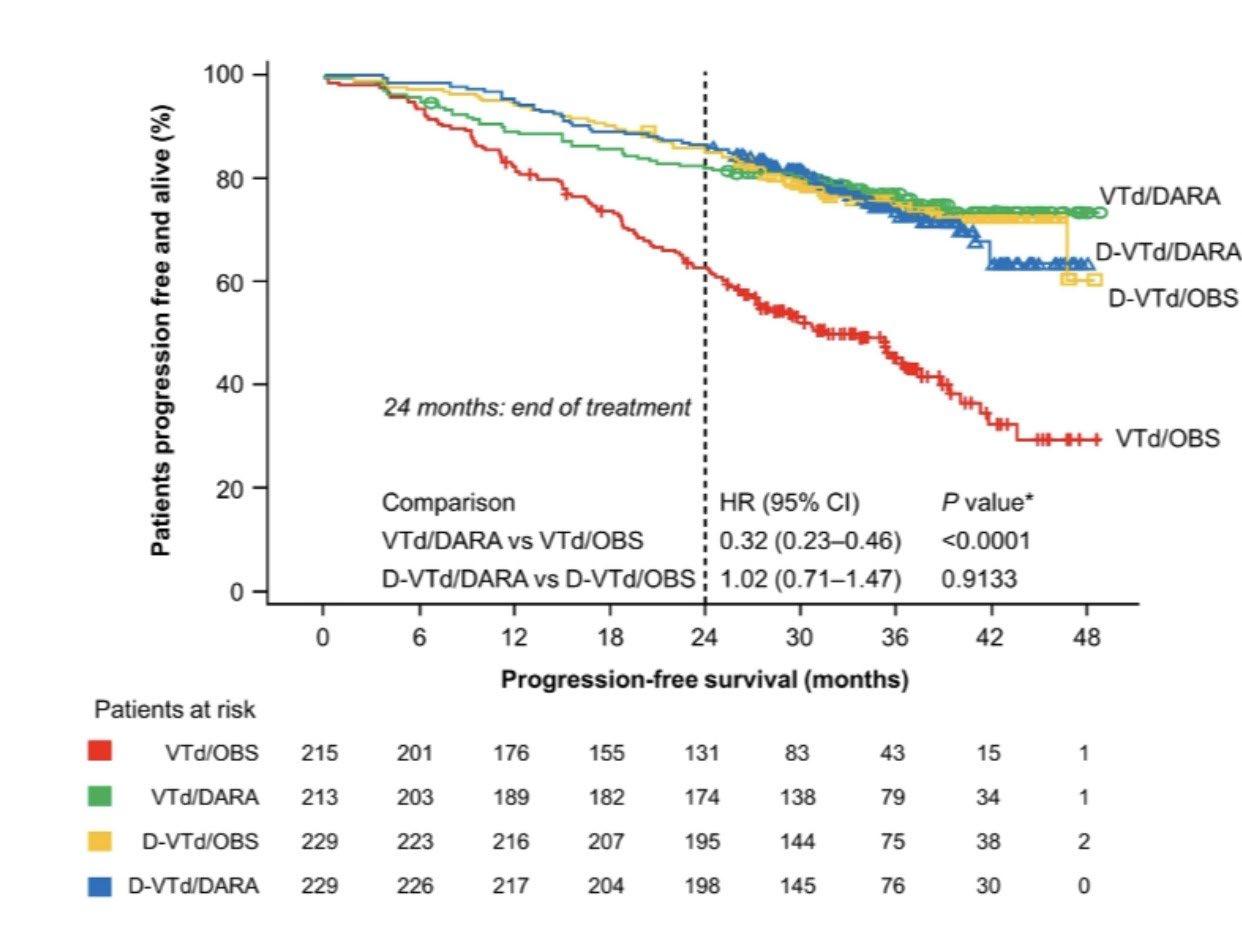
– Median follow-up: 49.6 months

– Median PFS was not reached in either group

– PFS was longer for D-RVd/D-R versus RVd/R, with a clinically meaningful 55% reduction in the risk of disease progression or












Recommendation
Patients should be referred to a transplant center to determine transplant eligibility

Evidence Rating

Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Chronologic age and renal function should not be the sole criteria used to determine eligibility for SCT.
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate

RVD x 3
CY (3 g/m2)
MOBILIZATION
Goal: 5 x 106 cells/kg
Stratification ISS, FISH
RVD x 3
CY (3 g/m2)
MOBILIZATION
RGoal: 5 x 106 cells/kg
Melphalan


200 mg/m2 + ASCT
Systemic GEP, CGH risk-adapted strategy
RVD x 5
RVD x 2
Lenalidomide 12 mo
Cy = cyclophosphamide; SCT = stem cell transplantation

Lenalidomide 12 mo
SCT at relapse
MEL 200 mg/m2 if <65 y, ≥65 y 140 mg/m2









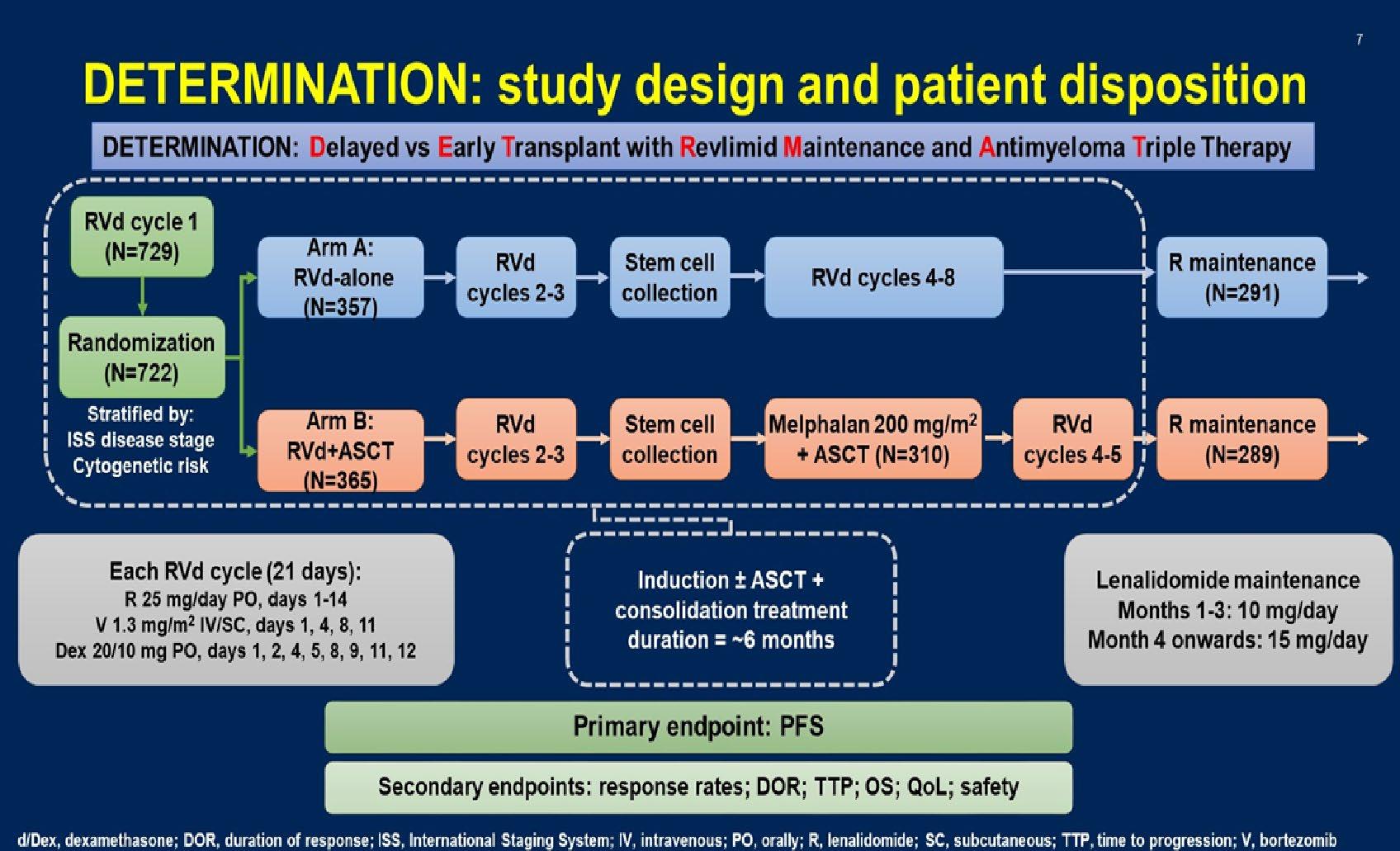









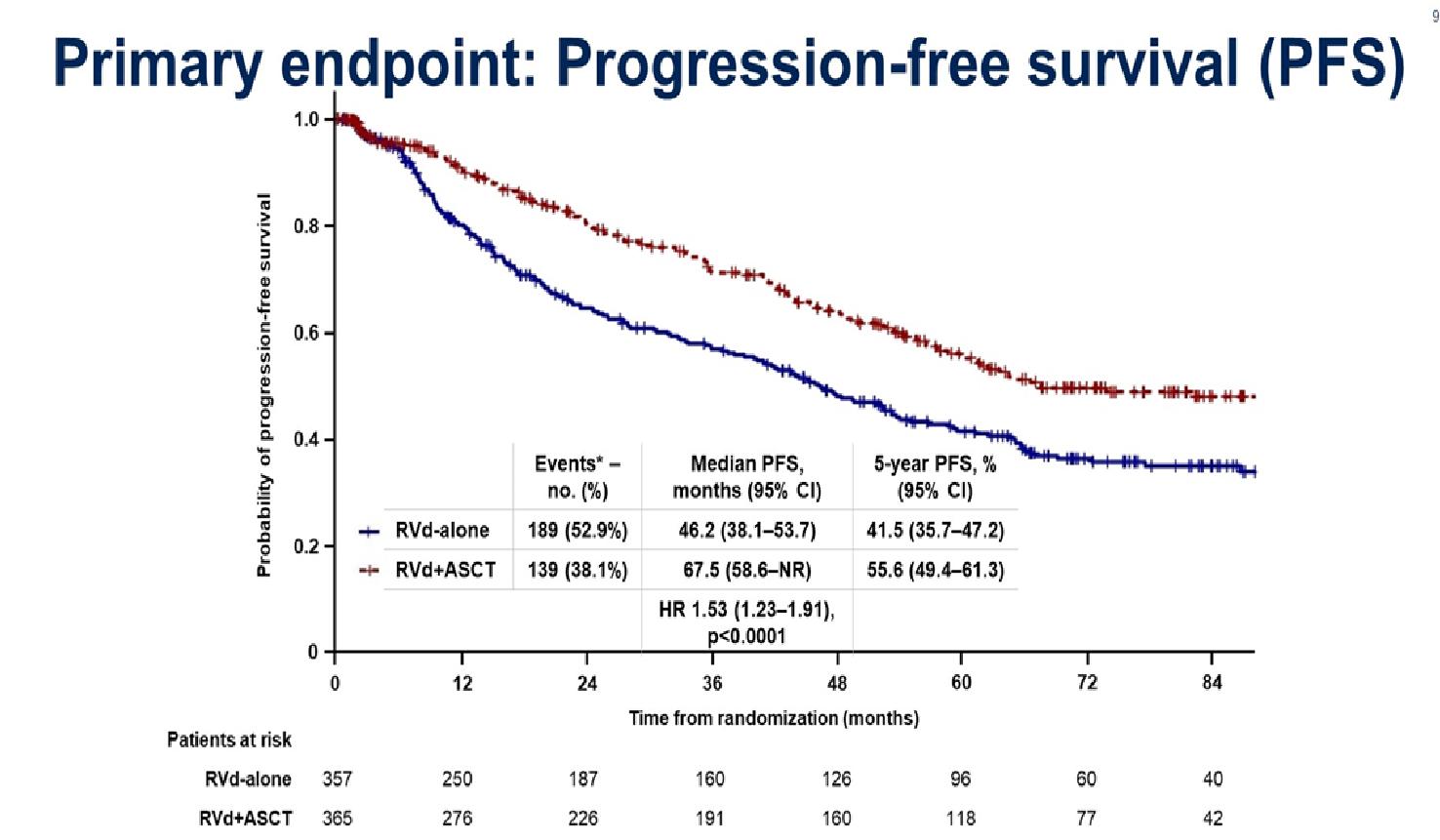
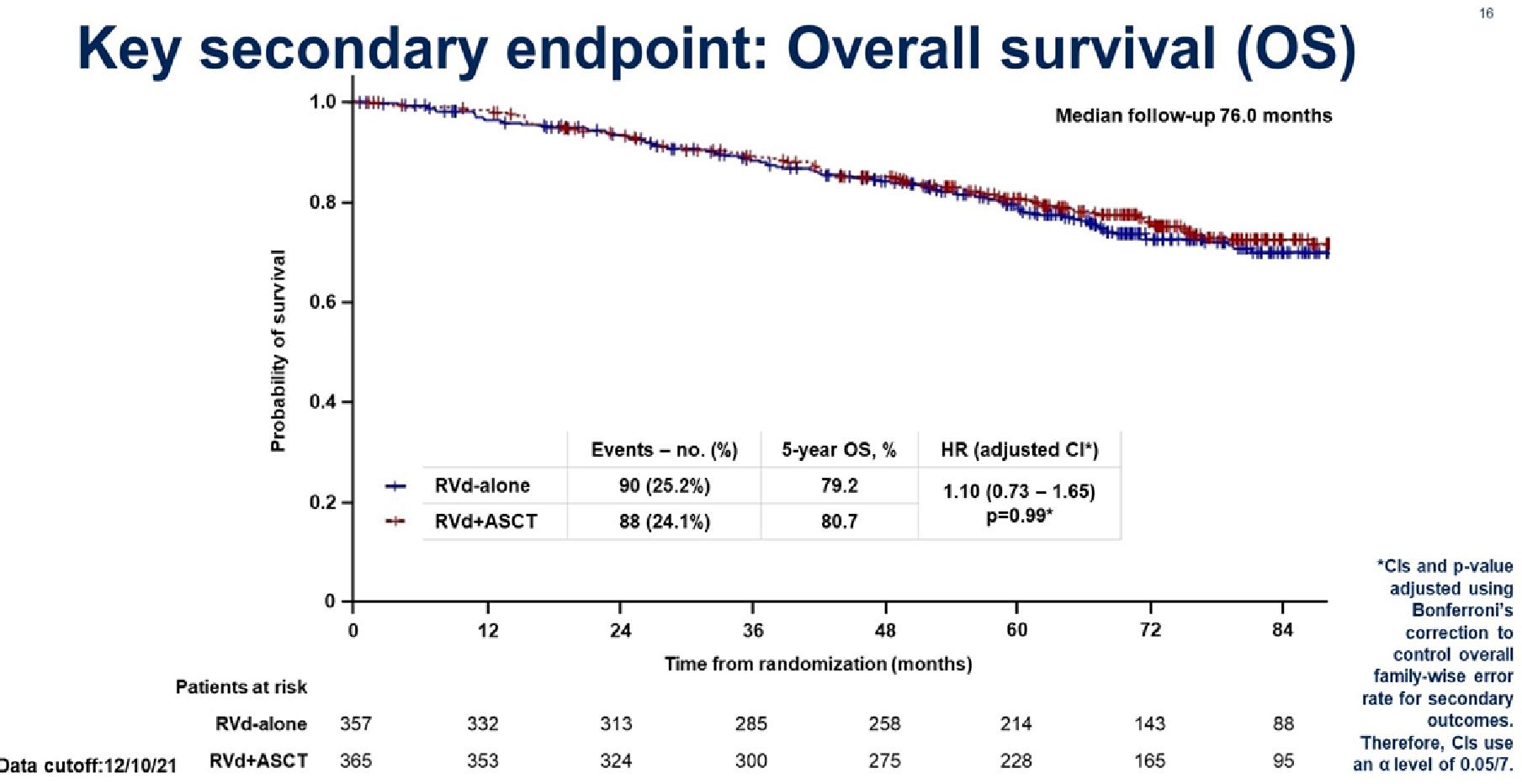



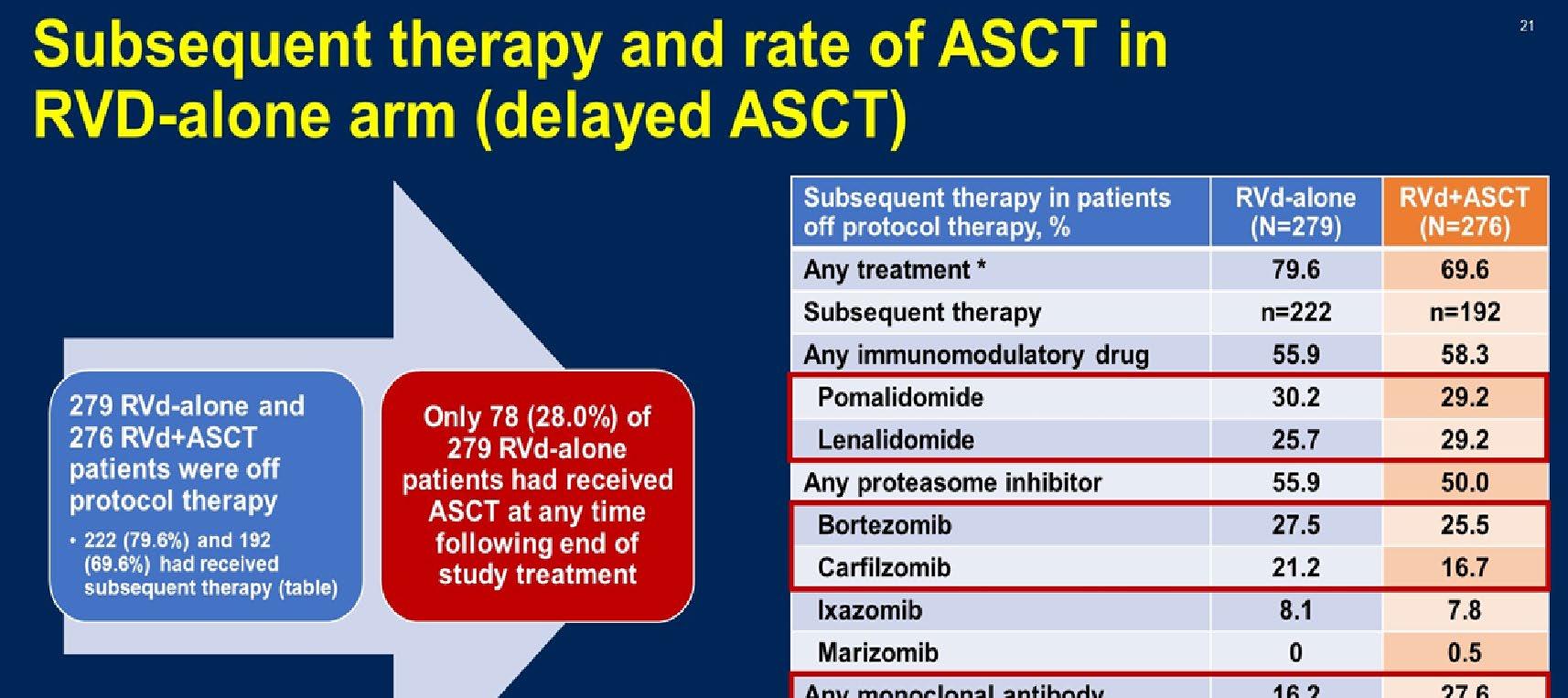
















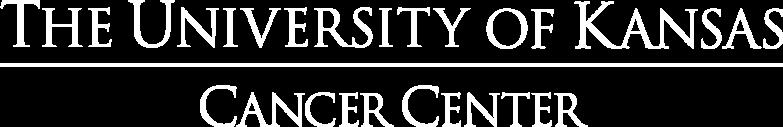








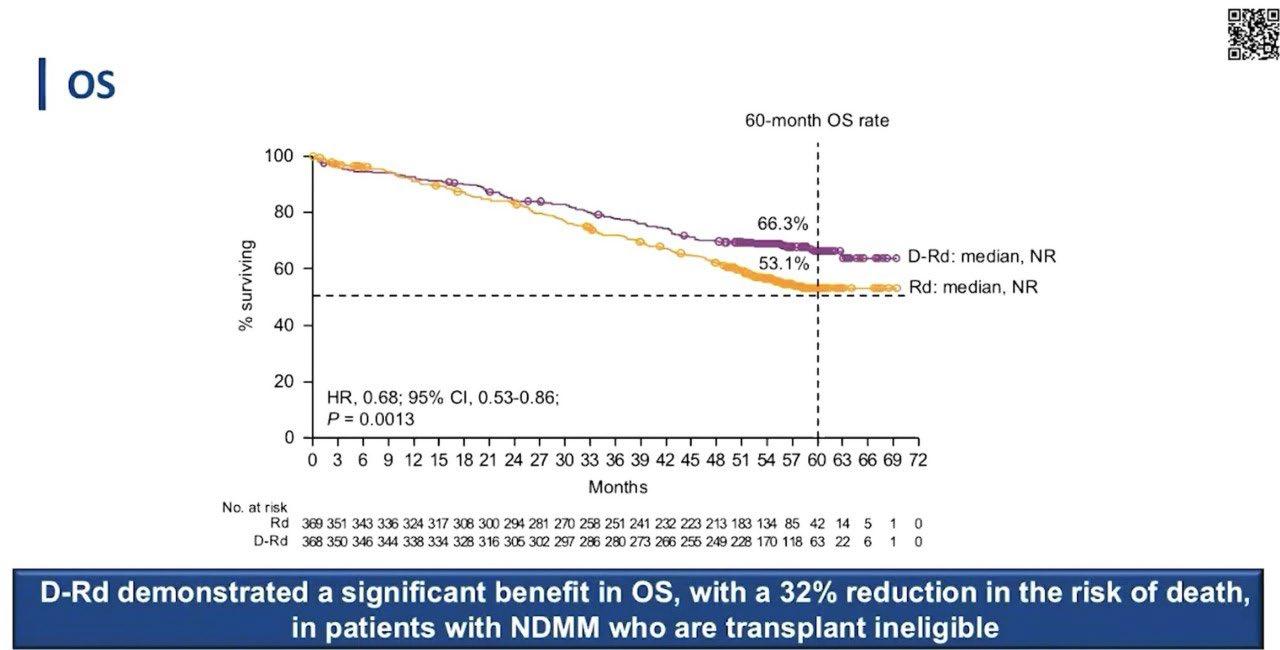
Stratified by ISS (I vs II vs III), region (N America vs other), age (< 75 vs ≥ 75 yrs)
Daratumumab 16 mg/kg IV
(QW cycles 1-2, Q2W cycles 3-6, Q4W cycle 7+) +
Patients with ASCTineligible NDMM, ECOG PS 0-2, CrCl ≥ 30 mL/min

(N = 737)

Lenalidomide 25 mg/day PO on Days 1-21 +
Dexamethasone 40 mg/wk* PO or IV
(n = 368)


Lenalidomide 25 mg/day PO on Days 1-21 +
Dexamethasone 40 mg/wk* PO or IV
(n = 369)
*Reduced to 20 mg/wk if > 75 yrs of age or BMI < 18.5.
Primary endpoint: PFS
28-day cycles until progression
Secondary endpoints : ≥ CR rate, ≥ VGPR rate, MRD negativity, ORR, OS, safety

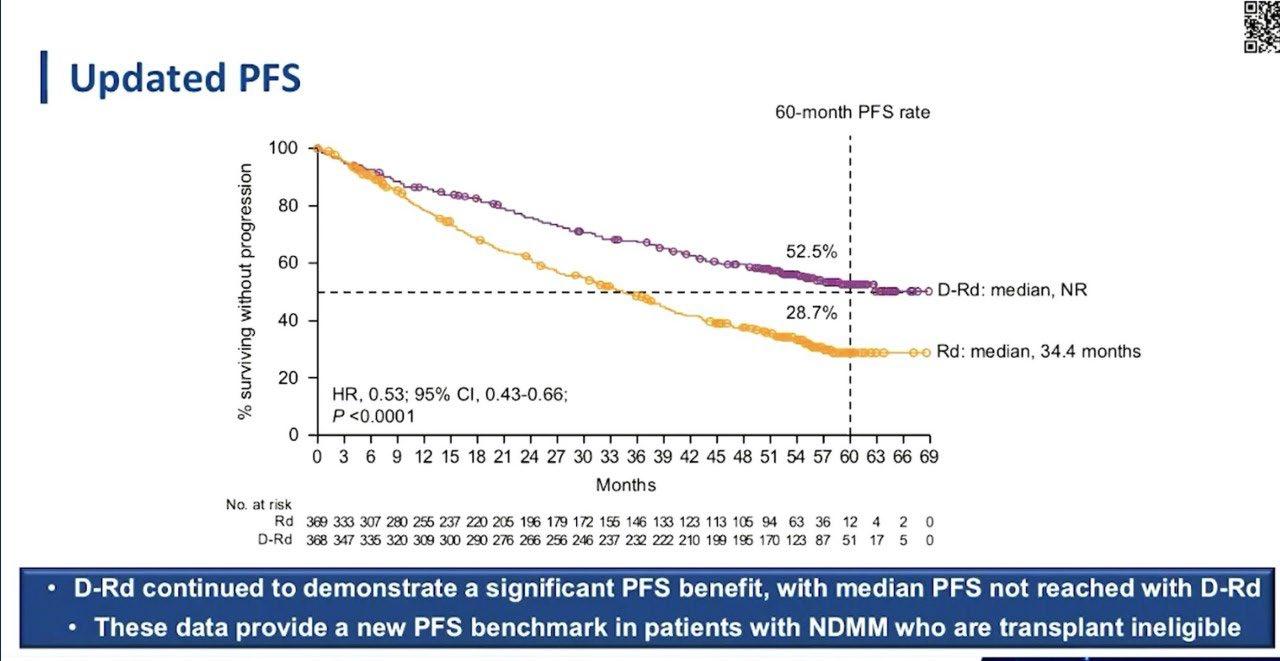





-

ASCT is still a vital treatment in Newly diagnosed myeloma

-
Recommend to consider ASCT upfront (Unlikely to receive it later in RW)
-
Combination therapy is considered an optimal therapy as induction therapy

-
Trials are needed to focus on patients for High-risk Myeloma
-
How I treat Standard Risk Myeloma who are ineligible for transplant? DRd
-
How I treat High Risk Myeloma Ineligible for transplant? VRd
• Open the Q&A window, allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A window or answer your question live.


• If the host answers live, you may see a notification in the Q&A window.


• If the host replies via the Q&A box – you will see a reply in the Q&A window.






7:15 – 7:25 PM Maintenance Therapy
September 13, 2023, Agenda
Al-Ola Abdallah, MD – University of Kansas Medical Center
7:25 – 7:55 PM Relapsed Therapies & Clinical Trials

Joseph Mikhael, MD, Chief Medical Officer, International Myeloma Foundation
7:55 – 8:25 PM Q&A
8:25 – 8:30 PM Closing Remarks





Chair, US Myeloma Innovations Research Collaborative (USMIRC)
Director, Plasma Cell Disorder Clinic
Division of Hematologic Malignancies and Cellular Therapeutics

Department of Internal Medicine



The University of Kansas Cancer Center

I



have NO financial relationships to disclose



• Discuss maintenance therapy and its application in myeloma

• Outline the major options for maintenance therapy
• Introduce the newer trend for the use of dual maintenance

What is Maintenance:
Less intense longer-term therapy with goal of better PFS and OS
What does the Ideal Maintenance therapy look like?
- Deepen remission
- Prolong remission


- Easy to administer (Oral is preferable)
- Minimal toxicity






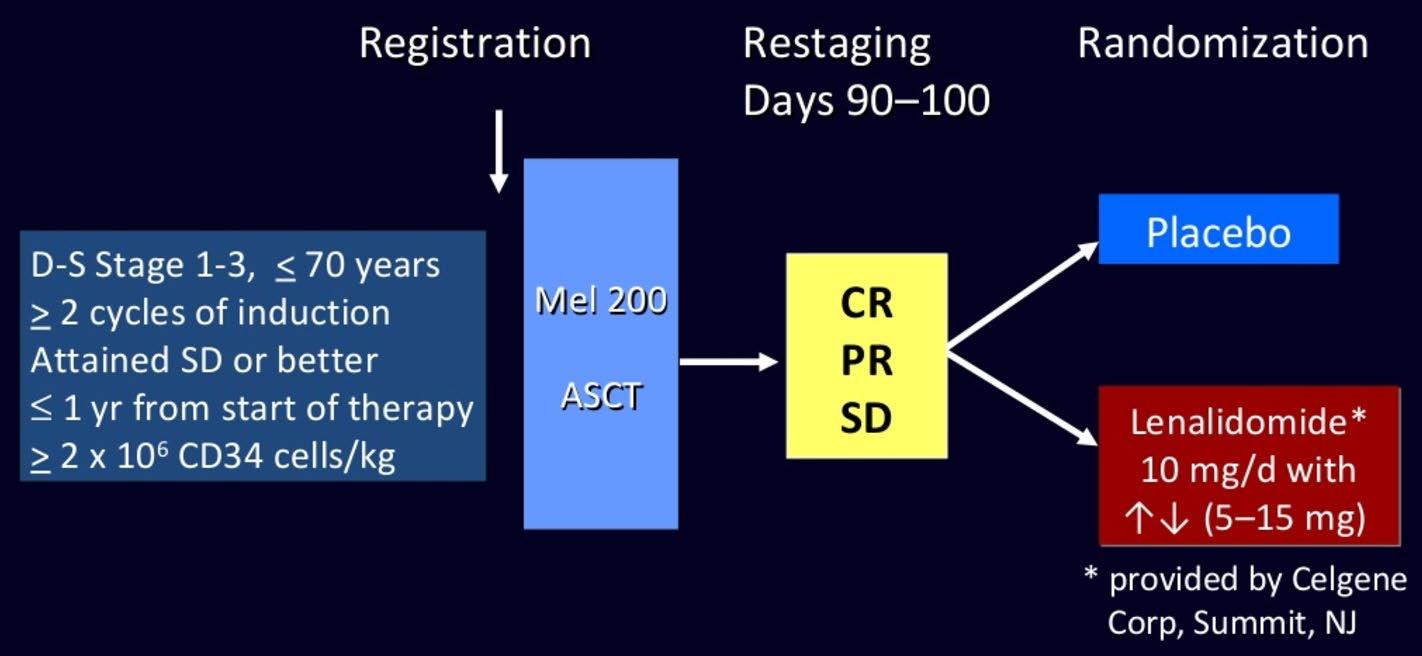










Induction
NDMM
Treated on Myeloma XI induction protocols

Maintenance
Lenalidomide

10 mg/day, days 1‒21/28
R 1:1
Observation
N=1551 (TE=828; TNE=723)
Median follow-up: 27 months (IQR 13‒43)
Exclusion criteria
• Failure to respond to lenalidomide as induction IMiD, or development of PD
• Previous or concurrent active malignancies
IQR, interquartile range; NDMM, newly diagnosed multiple myeloma; PD, progressive disease
Jackson et al., Lancet 20:57-73, 2019

Significant improvement in PFS from 18 to 36 months, HR=0.45

(n=857) 36 [31, 39]

(n=694) 18 [16, 20]





There is a 26% reduction in risk of death, representing an estimated 2.5-year increase in median survival

Cumulative incidence of developing a second cancer and cumulative probability of death because of competing causes, after multiple myeloma.
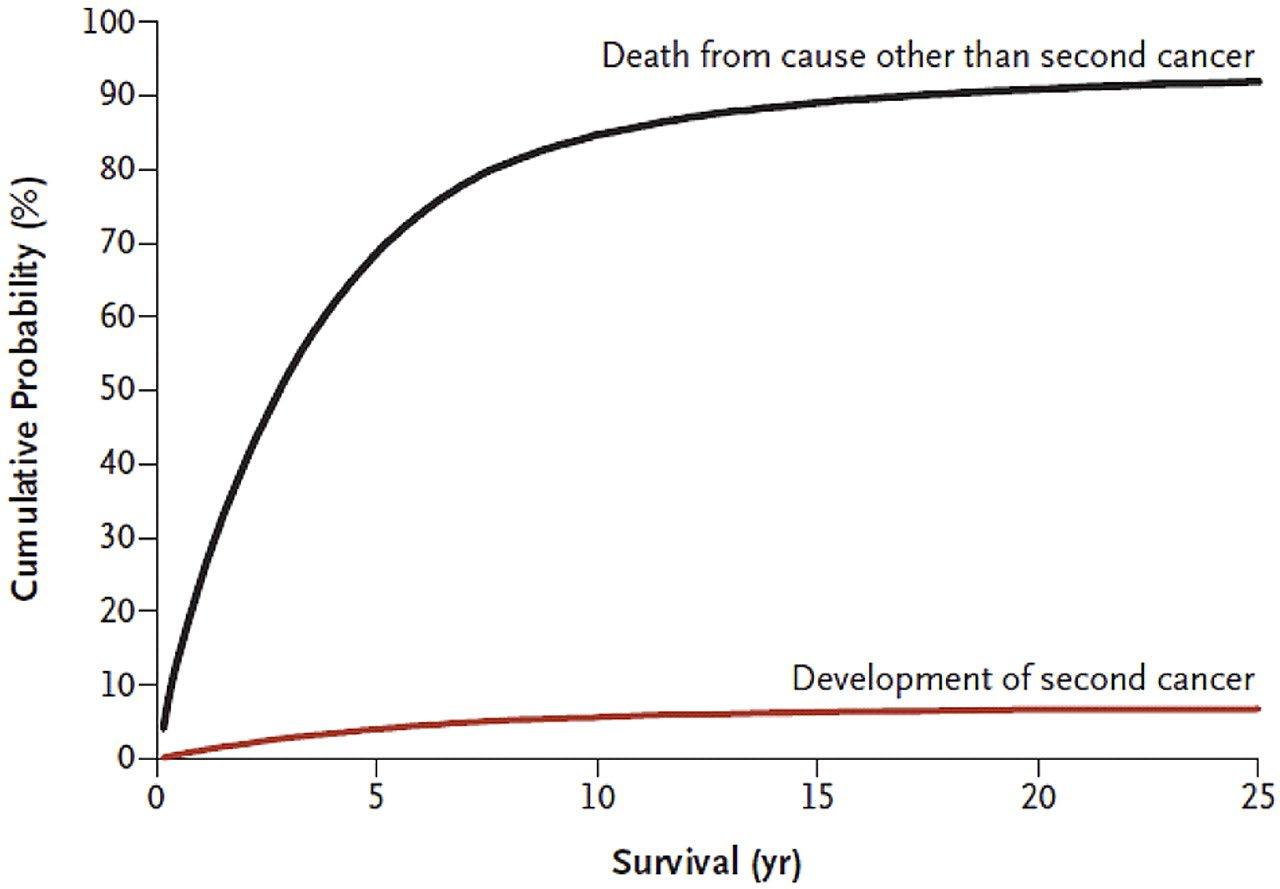
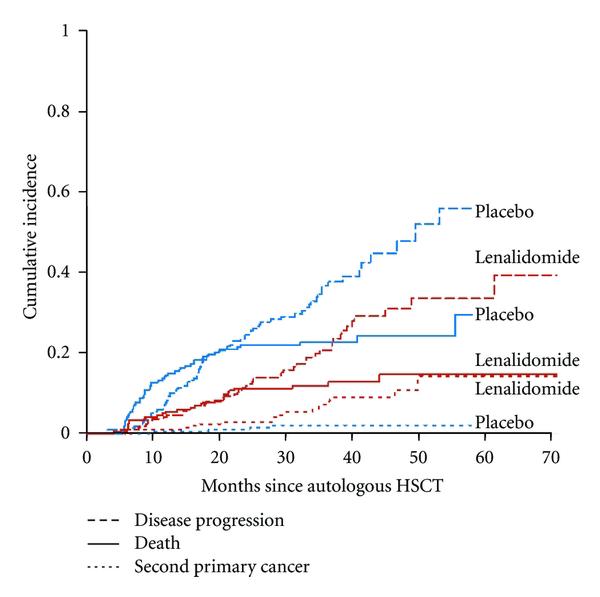





Subgroup analysis: bortezomib + double cycle HDM before ASCT improved OS vs single cycle of HDM before ASCT
– No significant difference in PFS between subgroups



















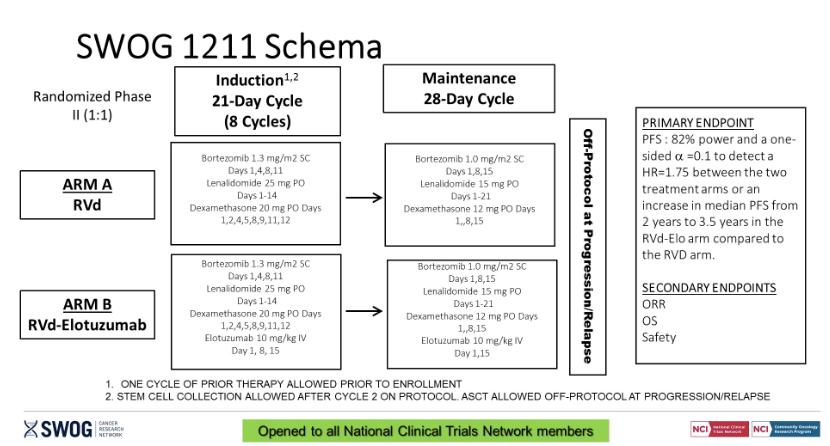


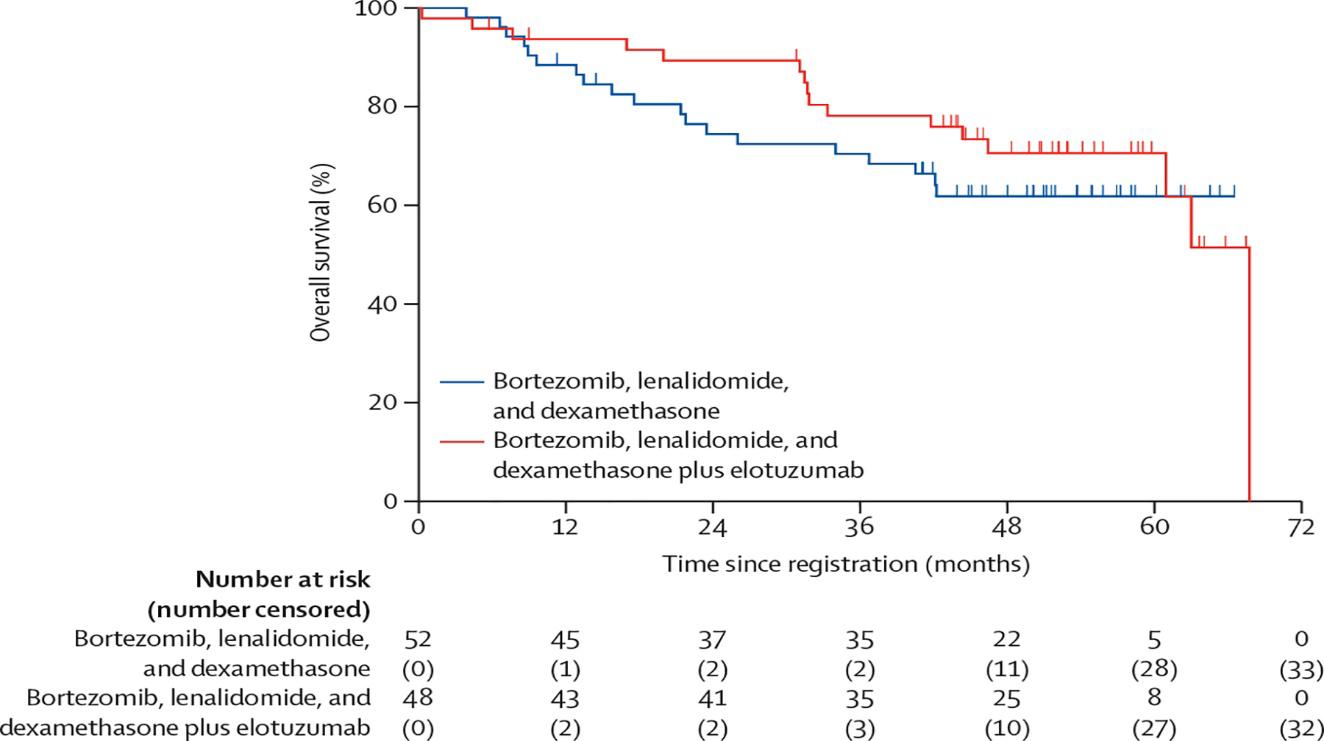



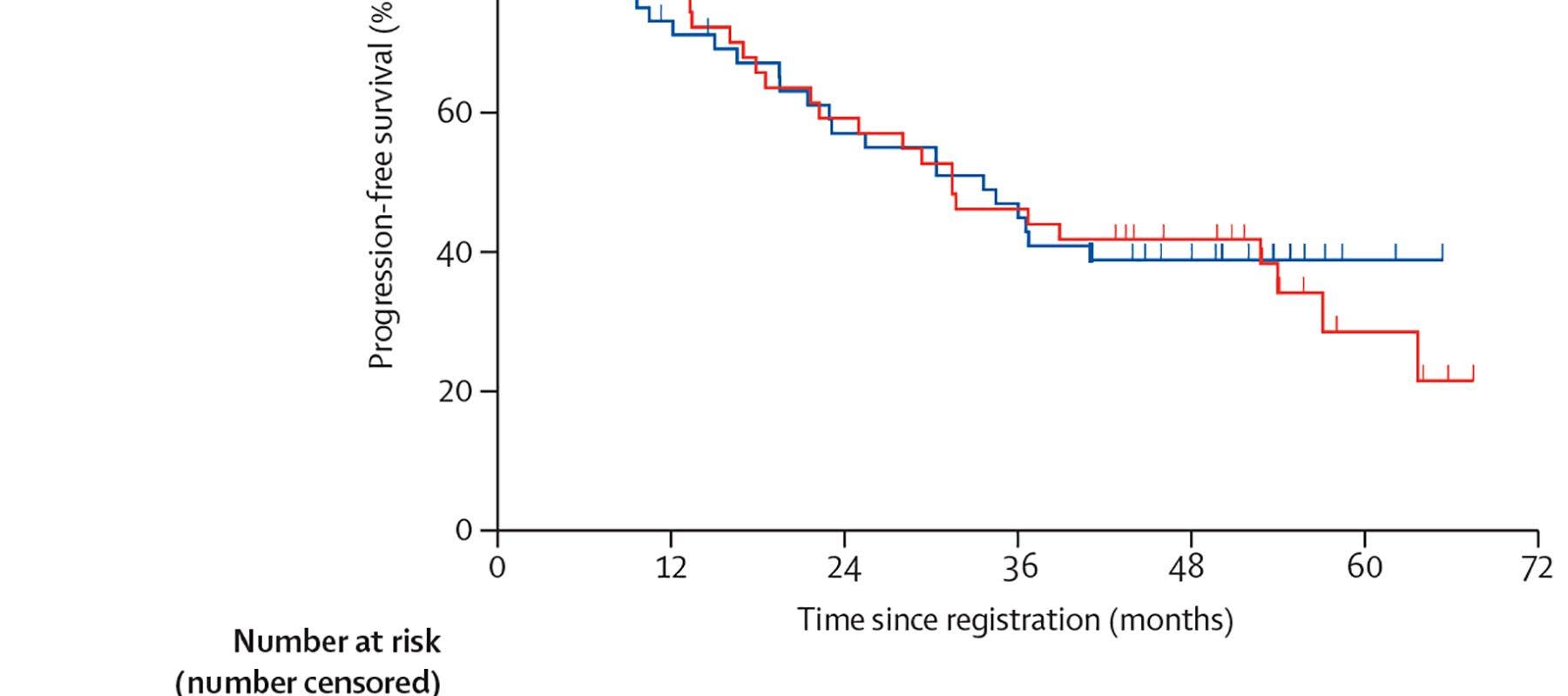

• Should post-transplant maintenance therapy be recommended for all patients?
– Yes
• Which agent should be used?
– Lenalidomide remains the standard of care
• What is the optimal duration?
– Treatment until progression remains the standard of care
• What should patients with high-risk cytogenetics receive?
– Consider lenalidomide + proteasome inhibitor; clinical trial

• Should MRD status dictate maintenance therapy?

– Not outside of a clinical trial
• What about Second Primary Malignancies?
– They are real, require a discussion and monitoring, but are outweighed by benefit
•

Recommend to consider maintenance therapy in post-transplant (even if pts achieve MRD negative)
• Lenalidomide is the drug of choice for all myeloma patients
• Benefits of Lenalidomide outweighs its risk as maintenance therapy (SPC)

• Duration of treatment (continuous vs fixed) needs more studies and to utilize use of MRD status when to stop treatment

• Other options for maintenance: Velcade, daratumumab (strong data), less preferable is use of Carfilzomib (not convenient)
• High risk myeloma maintenance: No strong evidence what to use, though many institutions are using doublet (PI+IMiDs)








• Provide the rationale for clinical trials
• Outline the phases of clinical trials
• Discuss the risks and benefits of clinical trials
• Preview important clinical trials in myeloma
Remember some of the important principles of clinical trials:
• The drive of research has brought us to where we are
• No one is expected to be a “guinea pig” with no potential benefit to them
• Research is under very tight supervision and standards
• Open, clear communication between the physician and the patient is fundamental
• Every patient is unique and must be viewed that way
• Benefits of trials are numerous and include:
• Early access to “new” therapy
• Delay use of standard therapy
• Contribution to myeloma world – present and future
• Financial access to certain agents
• Must be balanced with potential risks
• “toxicity” of side effects
• Possibility of lack of efficacy

• Clinical trials translate results of basic scientific research into better ways to prevent, diagnose, or treat cancer
• The more people that take part, the faster we can:
• Answer critical research questions
• Find better treatments and ways to prevent cancer

Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!
• Most agents are tested in lab models
• Various “myeloma cell lines” = in vitro
• Next step is animal model
• We are more like mice than you think!!

• Earliest study in phase I is called “First in Human”
• Often uses extremely low dose of drug to ensure safety
Tests safety Tests how well treatment works Compares new treatment to standard treatment
• All patients receive the experimental therapy
• Phase 1 trials find the optimal dose of a new drug or drug combination
• Patients get higher doses as the study continues
• Determine side effects of new drugs or combinations
• Explore how the drug is metabolized by the body
• Important for all stages of myeloma
• Determine if a new drug or combination is effective against the cancer
• May be added to a phase 1 study once the ideal dose is found
• Patients usually receive the experimental therapy • In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)
• Highest form of clinical evidence. Typically a large number of patients are required…usually required for full FDA approval
• Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
• The patient is randomly assigned to a treatment—a process called randomization
• Neither the physician or the patient can determine which treatment is given
• May be placebo controlled, if no standard treatments are available
• Very closely monitored for effectiveness and side effects
• Long-term studies with a large number of patients
• Usually to track outcomes of a large “cohort” of patients


• Patients are treated using available therapies
• Efficacy and safety are analyzed following treatment

• Typically involve a large number of patients


• Allow early access to experimental therapies when no alternatives are available
• Often precedes formal approval of a drug
Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol
Possible benefits:
•Patients will receive, at a minimum, the best standard treatment
•If the new treatment or intervention is proven to work, patients may be among the first to benefit
•Patients have a chance to help others and improve cancer care
Possible risks:
• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient • Health insurance and managed care providers do not always cover clinical trials
Patients may: • Be unaware of clinical trials
Lack access to trials
Fear, distrust, or be suspicious of research
Have practical or personal obstacles
Face insurance or cost problems
Be unwilling to go against their physicians’ wishes
Not have physicians who offer them trials
Have a disconnect with their healthcare team
• There has been a lack of diverse representation in clinical trials in myeloma. In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
• This is significant for the following reasons: All patients of all races and ethnicities should be able to benefit from clinical trials.
Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
• Reasons for underrepresentation in clinical trials are complex and include systemic racism, accessibility of clinical trials, sensitivity to diversity by medical professionals, misconduct in medicine in the past, the lack of trust in the system, and more.
Doctors might:
• Lack awareness of appropriate clinical trials
• Be unwilling to “lose control” of a person’s care
• Believe that standard therapy is best
• Be concerned that clinical trials add administrative burdens


How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?







What is currently known about the new drug or combination?
What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?

Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?


I may get a sugar pill (placebo) instead of real therapy.
No placebos are given alone every patient receives treatment.
I’ll be treated like a guinea pig.
Clinical studies are for people with no other options.

Most patients receive care that exceeds expectations.
Many involve an adjustment to a standard of care that may improve outcome or quality of life
The more people who participate, helps to speed drug development.



• Discuss whether or not you are eligible for a clinical trial with your physician
• Work with your physician to determine the best trial for you
• Meet with the clinical research nurse or trials coordinator to discuss the trial
• Carefully review the provided “Informed Consent”
• Describes the study and any potential safety concerns related to the experimental medication

• SO many areas being studied right now including…
• CAR T Cell therapy (used earlier in myeloma, new targets, faster manufacturing, even allo CAR T!)
• Novel Bispecific Therapies (with new targets)
• New molecules – CelMods, Modakafusp…
And MANY more!

• Discuss an approach to treating relapsed myeloma based on patient, disease and treatment characteristics

• Review the important trend of using an aggressive approach in early treatment of myeloma
• Outline the key results from recent trials in early relapse
• Discuss the approach to late relapse and the use of novel therapies such as CAR T and bispecific antibodies
Fewer patient are eligible for therapy in each subsequent line of therapy (LOT)
Figure adapted from: Yong, K et al. Br J Haematol 2016;175(2):252-264.It is not a simple algorithm of treatment #1 then 2 then 3…
• Leverage the benefit of multiple mechanisms of action in combination therapy
Categories:
1-3 prior lines • Later Relapse
Refractory to PI, IMiD and MoAb = Triple Class Refractory
Principles
1. Depth of Response matters…likely incorporate MRD soon
2. High risk vs standard risk…more aggressive Rx in high risk
3. Balance efficacy and toxicity…initially and constantly assess
4. Overcome drug resistance…change mechanism of action when possible
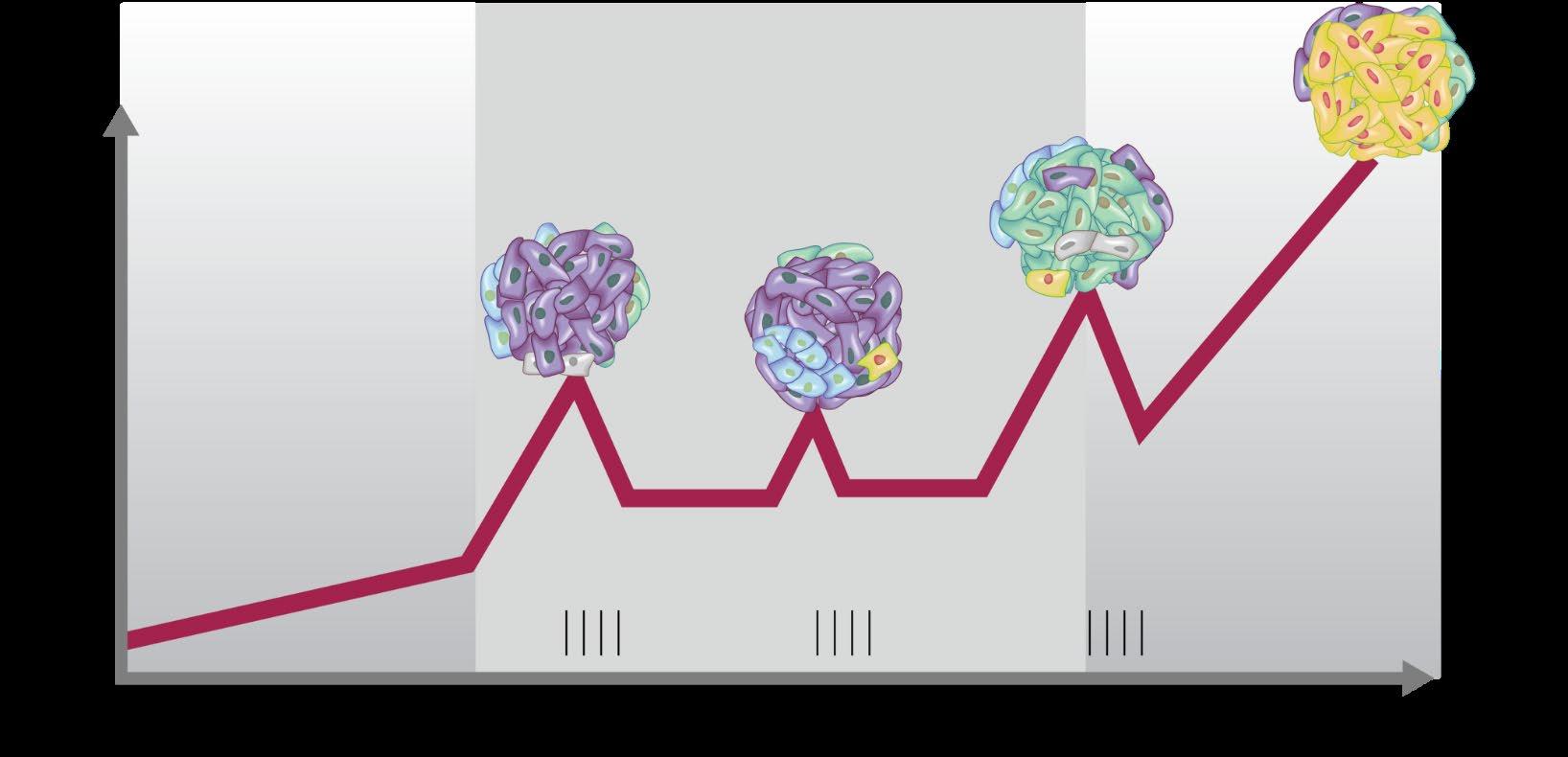
Definitions: What is relapsed/refractory disease and a line of therapy?
• Relapsed: recurrence (reappearance of disease) after a response to therapy
• Refractory: progression despite ongoing therapy

• Progression: change in M protein/light chain values
• Line of therapy: change in treatment due to either progression of disease or unmanageable side effects
• Note: initial (or induction) therapy + stem cell transplant + consolidation/ maintenance therapy = 1 line of therapy

Disease-Related

• Nature of the relapse
– Biochemical vs symptomatic
• Risk stratification
– High-risk chromosomal abnormalities: del(17p), t(4;14), t(14;16)
• Disease burden
Therapy-Related
• Previous therapies
• Prior treatmentrelated adverse event
• Regimen-related toxicity
• Depth and duration of previous response
• Cost to patient
Patient-Related
• Renal insufficiency
• Hepatic impairment
• Comorbidities
• Preferences
• Social factors
– Support system
–
Accessibility to treatment center
– Insurance coverage
Time: Years!

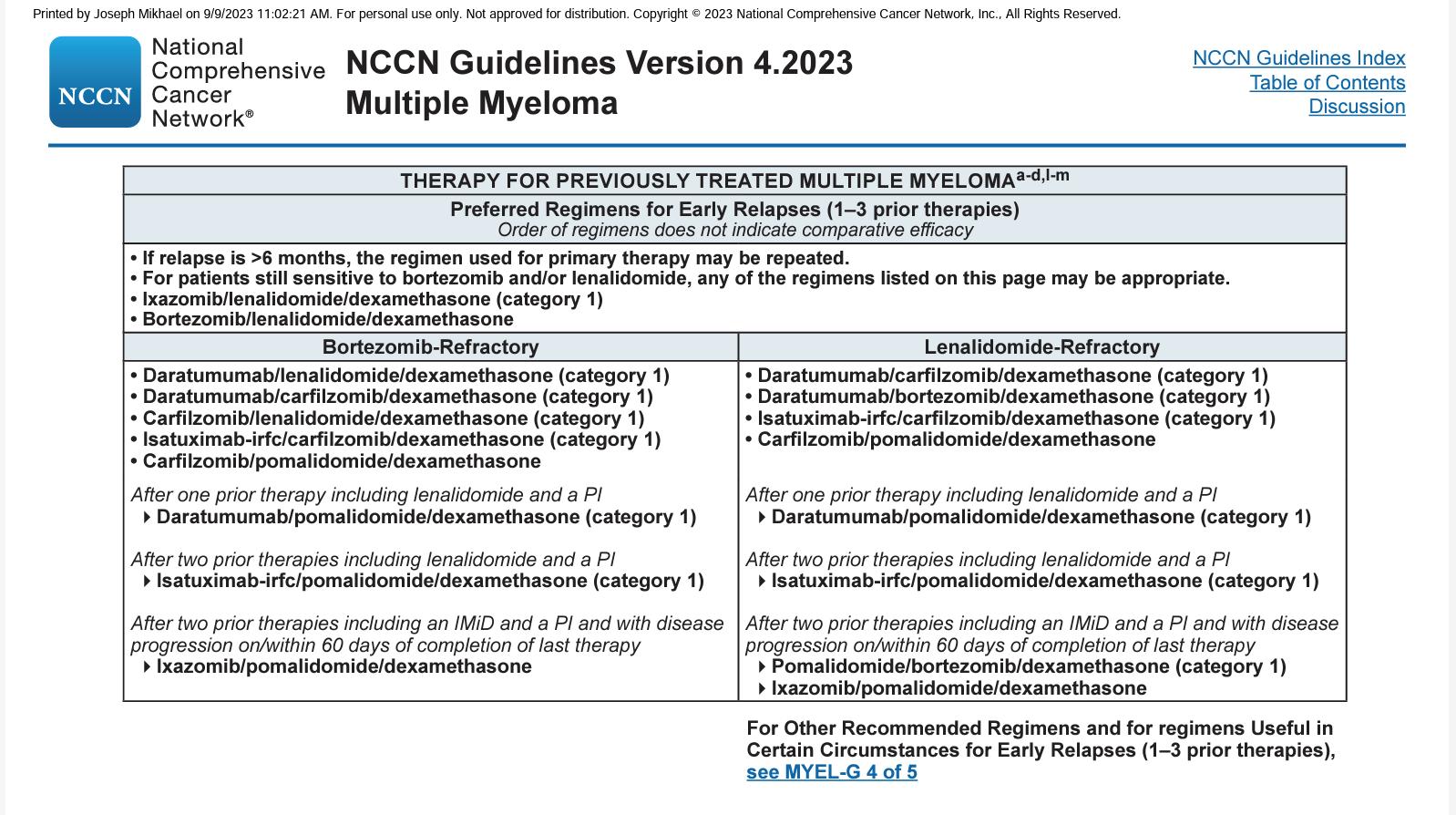
Preferred options: DRd (or KRd)
Not Refractory to Lenalidomide
Refractory to Lenalidomide
Alternatives: DVD, Kd, DaraKd, IsaKd, IRd, Erd
When Dara, Isa, K not available: Rd, Vd, VTD, VCD, VMP
Preferred options: PVd DaraKd IsaKd
Second options
DaraVd;Kd
Other options: KPd; DaraPd; Ipd
When Dara, isa, K or P not available: VCD, Vd, VMP
Moreau P, et al. IMWG Recommendations for RRMM. Lancet Oncol. 2021;22(3):e105-e118.
DKd,daratumumab/carfilzomib/dexamethasone; DPd, daratumumab/pomalidomide/dexamethasone; DRd, daratumumab/lenalidomide/ dexamethasone; DVd, daratumumab/bortezomib/dexamethasone; Elo–Rd, elotuzumab/lenalidomide/dexamethasone; Ipd, ixazomib/pomalidomide/dexamethasone; Ird, ixazomib/lenalidomide/dexamethasone; Isa–Kd, isatuximab/carfilzomib/dexamethasone; Kd, carfilzomib/dexamethasone; KPd, carfilzomib/pomalidomide/dexamethasone; KRd, carfilzomib/lenalidomide/ dexamethasone; PVd, pomalidomide/bortezomib/dexamethasone; Rd, lenalidomide/dexamethasone; SVd, selinexor/bortezomib/dexamethasone; VCd, bortezomib/cyclophosphamide/dexamethasone; Vd, bortezomib/dexamethasone; VMP, bortezomib/melphalan /prednisone; VTd, bortezomib/thalidomide/dexamethasone.
General Principles
Use mechanisms of action not previously used
Do not continue to use lenalidomide if progressing on len maintenance
Triplets are preferred over doublets
In real practice - most patients receiving VRD (Bortezomib-Lenalidomide-Dex) like regimens, 1st relapse is typically
Daratumumab + Pomalidomide + Dex (APOLLO)
Isatuximab + Pomalidomide + Dex (ICARIA)
Daratumumab + Carfilzomib + Dex (CANDOR)
Isatuximab + Carfilzomib + Dex. (IKEMA)
Selinexor + Bortezomib + Dex (BOSTON)

Not Refractory to Lenalidomide* Refractory to Lenalidomide*
Not refractory to CD38 moAB
Dara-refractory or Relapse while on CD38 moAB
Not refractory to CD38 moAB
Dara-refractory or Relapse while on CD38 moAB
DRd
KRd (preferred)
ERd, IRd
(Alternatives)
DKd or Isa-Kd Or
DPd or Isa-Pd
KCd or KPd
(preferred)
VCd or EPd
(Alternatives)
*Consider salvage ASCT in patients eligible for ASCT who have not had transplant before; Consider 2nd auto SCT if eligible and had >36 months response duration with maintenance to first ASCT
Drug Formulation Approval
Velcade (bortezomib)
Kyprolis (carfilzomib)

Ninlaro (ixazomib)
Revlimid (lenalidomide)*
Pomalyst (pomalidomide)*





XPOVIO (selinexor)
• IV infusion
• SC injection
• IV infusion
• Weekly dosing
• For relapsed/refractory myeloma
• For relapsed/refractory myeloma as a single agent, as a doublet with dexamethasone, and as a triplet with Revlimid or Darzalex plus dexamethasone
Once-weekly pill
• For relapsed/refractory myeloma as a triplet with Revlimid and dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-weekly pill
• For relapsed/refractory myeloma as a triplet with Velcade and dexamethasone
*Black box warnings: embryo-fetal toxicity; hematologic toxicity (Revlimid); venous and arterial thromboembolism
IV, intravenous; SC, subcutaneous
• Velcade-Pomalystdex (VPd) vs Vd Regimens compared
• Kyprolis-Revlimiddex (KRd) vs Rd





• Ninlaro-Rd (IRd) vs Rd
• XPOVIO-Velcade-dex (XPO-Vd) vs Vd

Median progression-free survival favored
• VPd: 11 vs 7 months
• KRd: 26 vs 17 months
• IRd: 21 vs 15 months
• XPO-Vd: 14 vs 9 months
Clinical considerations
• Consider for relapse on Revlimid
• VPd associated with more low blood counts, infections, and neuropathy than Pd

• KRd associated with more upper respiratory infections and high blood pressure than Rd
• IRd an oral regimen
• Gastrointestinal toxicities and rashes
• Lower incidence of peripheral neuropathy



• XPO-Vd associated with low platelet counts and fatigue with triplet, but less neuropathy than the Vd

Drug Formulation
Approval
Darzalex (daratumumab)

SC once a week for first 8 weeks, then every 2 weeks for 4 months, then monthly
• For relapsed/refractory myeloma as a single agent and as a triplet with Revlimid or Velcade or Kyprolis or Pomalyst plus dexamethasone
Empliciti (elotuzumab)


IV once a week for first 8 weeks, then every 2 weeks (or every 4 weeks with pom)
• For relapsed/refractory myeloma as a triplet with Revlimid or Pomalyst and dexamethasone
Sarclisa (isatuximab)
IV once a week for first 4 weeks, then every 2 weeks
• For relapsed/refractory myeloma as a triplet with Pomalyst or Kyprolis and dexamethasone

Regimens compared
• Darzalex-Revlimiddex (DRd) vs Rd
• Darzalex-Velcade-dex (DVd) vs Vd




• Darzalex-Kyprolis-dex (DKd) vs Kd
• Darzalex-Pomalystdex (DPd) vs Pd
Median progressionfree survival favored
• DRd: 45 vs 18 months
• DVd: 17 vs 7 months
• DKd: 29 vs 15 months
• DPd: 12 vs 7 months
• Consider for relapses from Revlimid or Velcade maintenance
Clinical consider-ations
• DRd associated with more upper respiratory infections, low blood white blood cell counts, and diarrhea
• Consider for patients who are Revlimid-refractory without significant neuropathy
• DVd associated with more low blood cell counts
• Consider for younger, fit patients who are doublerefractory to Revlimid and Velcade
• DKd associated with more respiratory infections
• Sever side effects (possibly fatal) in intermediate fit patients 65 and older
• Consider in patients who are double-refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Severe low white blood cell counts






ELOQUENT-2

ELOQUENT-3

Regimens compared
• Empliciti-Revlimiddex vs Rd
ICARIA-MM IKEMA
• EmplicitiPomalyst-dex vs Pd
• Sarclisa-Pomalyst-dex vs Pd
• Sarclisa-Kyprolis-dex vs Kd
Median progressionfree survival favored
• Empliciti-Rd: 19 vs 15 months
• Empliciti-Pd: 10 vs 5 mos
• Sarclisa-Pd: 12 vs 7 mos
• Sarclisa-Kd: 41 vs 19 mos
Clinical considerations
• Consider for non-Revlimid refractory, frailer patients
• Overall survival benefit with Empliciti-Rd

• Empliciti-Rd associated with more infections




• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Sarclisa-Pd associated with severe low white blood cell counts, more dose reductions, upper respiratory infections, and diarrhea




• Consider for patients refractory to Revlimid and Velcade
• Sarclisa-Kd associated with higher MRD negativity rates
• Sarclisa-Kd associated with severe respiratory infections
Refractory to IMiD, PI, Anti-CD38
Refractory to IMiD, PI, Anti-CD38, Alkylators, and Anti-BCMA
Existing drugs:
Combinations with Cyclophosphamide
that do not have IMiD, PI, Anti CD38 (e.g., KCd)
Anti BCMA strategy
Anti-BCMA
Bispecific
BCMA CAR-Ts
Elotuzumab
Selinexor
Venetoclax
Bendamustine
VDT PACE
New Drugs: Iberdomide, Mezigdomide
New bispecifics (Cevostamab, Talquetamab)
New CAR-Ts
New Monoclonals
New ADCs
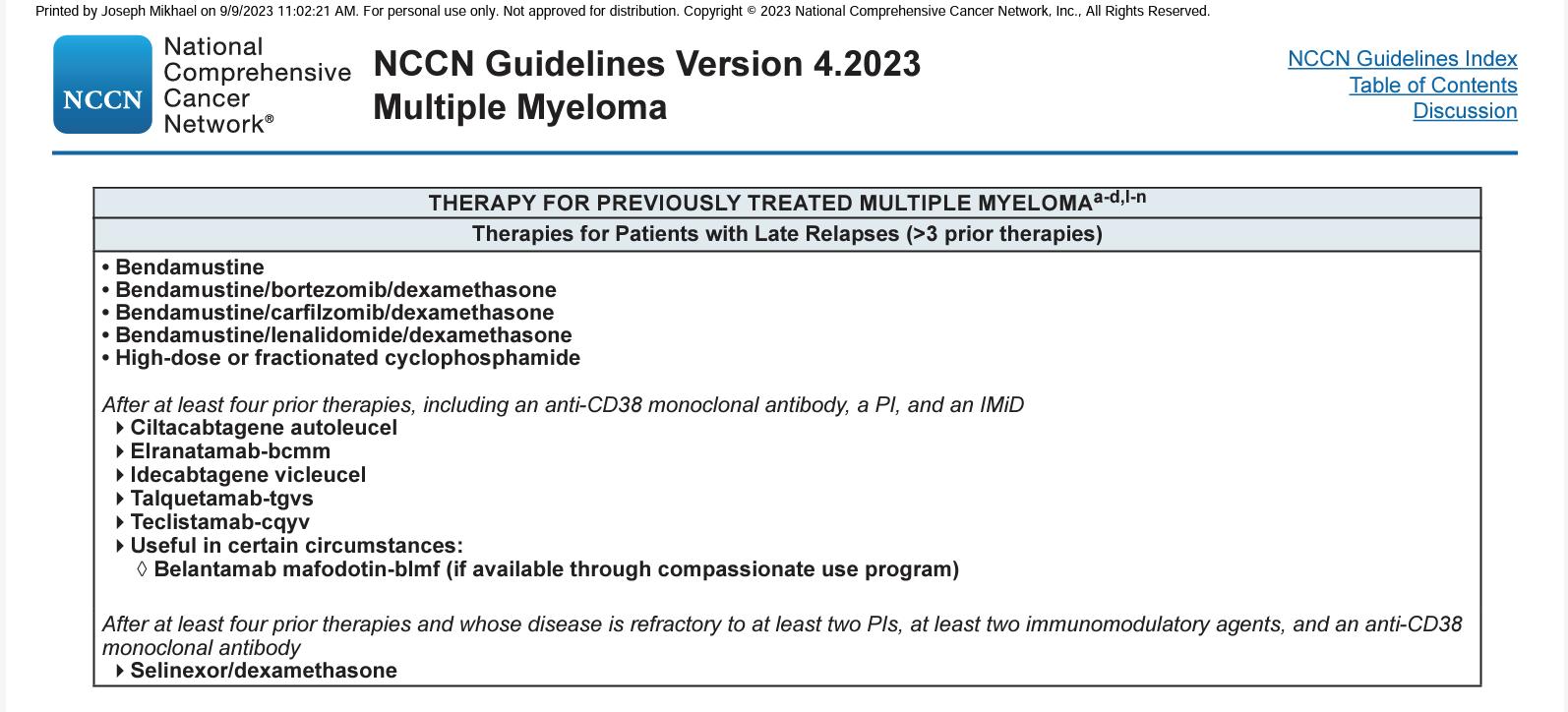
Class
Drug
Formulation
Approval
Nuclear export inhibitor
XPOVIO (selinexor)
Twice-weekly pill
• For relapsed/refractory myeloma in combination with dexamethasone (after at least 4 prior therapies and whose disease is refractory to at least 2 PIs, at least 2 IMiDs, and an anti-CD38 mAb
Antibody-drug conjugate
Blenrep (belantamab mafodotin)*
2.5 mg/kg IV over approximately 30 minutes once every 3 weeks
• For relapsed/refractory myeloma (after at least 4 prior therapies including an anti-CD38 mAb, a PI, and an IMiD
Chimeric antigen receptor (CAR)
T cell
Abecma (idecabtagene vicleucel)
300 to 460 × 106 genetically modified autologous CAR T cells in one or more infusion bags
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and an anti-CD38 mAb
CAR T cell
Bispecific antibody
Carvykti (ciltacabtagene autoleucel)
0.5 to 1.0 × 106 genetically modified autologous CAR T cells/kg of body weight
Tecvayli (Teclistamab) Step up dosing then weekly SQ
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb
For relapsed/refractory myeloma (after 4 or more prior Talquetamab lines of therapy, (PI, an IMiD, and an anti-CD38 mAb
Elranatamab
Bispecific antibodies
• Cevostamab, Alnuctamab, ABBV383, and others
• Target BCMA, GPRC5D, or FcRH5 on myeloma cells and CD3 on T cells
• Redirects T cells to myeloma cells
Cereblon E3 ligase modulators (CELMoDs)
• Iberdomide
• Targets cereblon
• Enhances tumoricidal and immune-stimulatory effects compared with immunomodulatory agents
Small molecule inhibitors
• Venetoclax
• Targets Bcl-2
• Induces multiple myeloma cell apoptosis
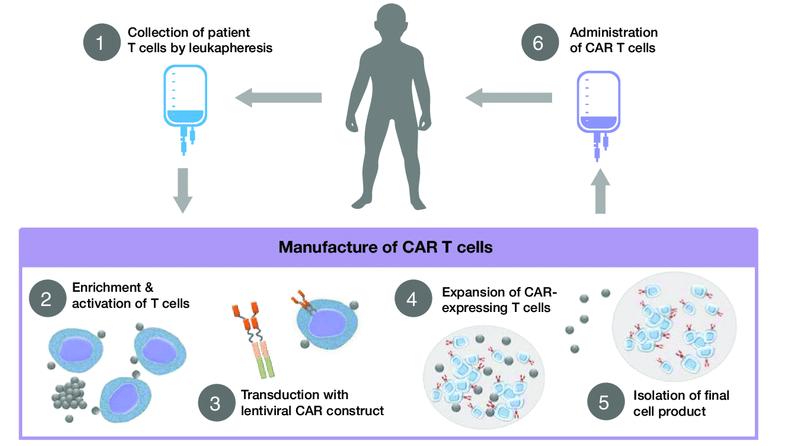
Collect patient’s white blood cells
Isolate and activate T cells Engineer T cells with CAR gene
Tumor cell
Targeting element (eg, CD19, BCMA, CD20)
Spacer
Transmembrane domain
Expand CAR T cells Infuse same patient with CAR T cells
CD19
Viral vector with CAR DNA
CARengineered T cell

Costimulatory domain (eg, CD28 or 4-1BB)
CD3���� (essential signaling domain)
Median manufacturing time: 17-28 days
Patients undergo lymphodepleting (and possibly salvage/bridging) therapy
1 day
4–
Standard of care therapy is permitted until CAR T cells are ready for infusion
3 days
Fludarabine and Cytoxan are used to create “immunologic space” to CAR T cells to expand
CR or sCR and MRD NE CR or sCR and MRD-
ORR, overall response rate; PR, partial response; VGPR, very good partial response; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; PFS, progression-free survival
KarMMa Trial. Munshi NC et al. N Engl J Med. 2021;384:705.
CARTITUDE-1 Trial. Berdeja JG et al. Lancet. 2021;398:314; Martin T et al. J Clin Oncol. June 4, 2022 [Epub ahead of print].
Onset 1−9 days after CAR T-cell infusion
2−9 days after CAR T-cell infusion
Duration 5 11 days 3 17 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
Management
• Actemra (tocilizumab)

• Corticosteroids
• Supportive care
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years;
§For children ≤12 years; ‖Only when concurrent with CRS
Xiao X et al. J Exp Clin Cancer Res. 2021;40(1):367. Lee DW et al. Biol Blood Marrow Transplant. 2019;25:625; Shah N et al. J Immunother Cancer. 2020;8:e000734.
Survey of 20 centers. Responses from 17 centers.
Cellular therapies CAR T-cell therapy
Patient’s cells collected
Types
Patient given chemotherapy before cells are infused back into patient Yes,
When in the course of myeloma is this usually done?
After multiple relapses As part of initial treatment
Side effects of treatment
Cytokine release syndrome; confusion
*An immune cell that is the “business end” of the system, in charge of maintaining order and removing cells. †Precursor cells that give rise to many types of blood cells. We actually collect CD34+ve cells.
Fatigue, nausea, diarrhea
There are currently 3 approved bispecific antibodies:
Teclistamab (Tecvayli)
Talquetamab (Talvey)
Elranatamab (Elrexfio)


Bispecific antibodies are also referred to as dual specific antibodies, bifunctional antibodies, or T-cell engaging antibodies
Bispecific antibodies can target two cell surface molecules at the same time (one on the myeloma cell and one on a T cell)
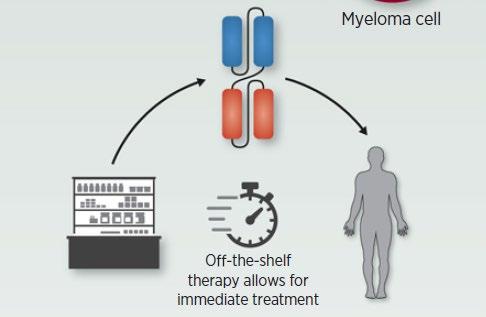
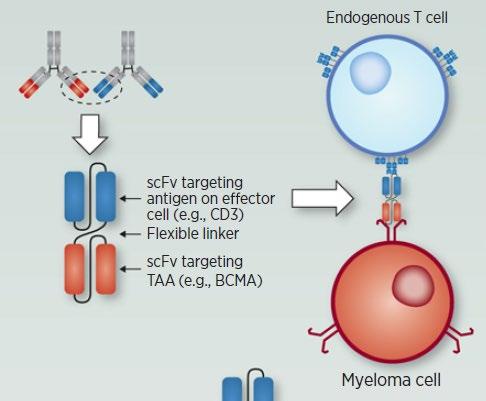
Many different bispecific antibodies are in clinical development; none are approved for use in myeloma
Availability is off-the-shelf, allowing for immediate treatment
Cohen A et al. Clin Cancer Res. 2020;26:1541.
Examples:
• Elranatamab
• Teclistamab
• TNB-303B (ABBV-383)
• REGN5458
• Cevostamab
• Talquetamab
BCMA, GPRC5D, or FcRH5Drug Formulation
Approval
Tecvayli (teclistamab)*
Step-up dosing† the first week then once weekly thereafter by subcutaneous injection

• For relapsed/ refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and an anti-CD38 mAb)
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
*Black box warning: cytokine release syndrome; neurologic toxicities
†Patients are hospitalized for 48 hours after administration of all step-up doses.
Tecvayli is available only through a restricted distribution program.
Median duration of response 18.4 months
CR or better median DOR not reached (95% CI: 16.2–NE)
• Overall median DOR of 18.4 months (95% CI: 14.9–NE), and was not yet mature with data from 71 patients (68.3%) censored
• 12-month event-free rate:
• Overall:
Overall median DOR 18.4 months (95% CI: 14.9–NE)
• Patients with CR or better:
68.5% (95% CI: 57.7–77.1)
80.1% (95% CI: 67.6–88.2)
• 2 patients (1.2%) discontinued due to AEs (grade 3 adenoviral pneumonia; grade 4 PML)
• 1 patient had dose reduction at cycle 21
• The most common AEs were CRS and cytopenias
• Infections occurred in 126 (76.4%) patients (grade 3/4: 44.8%)
• 123 patients (74.5%) had evidence of hypogammaglobulinemiaa
• There were 19 deaths due to AEs, including 12 COVID-19 deaths
• 5 deaths due to teclistamab-related AEs:
• COVID-19 (n=2)
• Pneumonia (n=1)
• Hepatic failure (n=1)
• PML (n=1)
• Median duration of followup was 12.0 mos (range 0.3–32.3)
• ORR = 64% (95% CI, 50–75); CR/sCR rate = 38% (21/55)
• 54% (7/13) of patients with prior BCMA-directed therapy achieved response

• For responders (n = 35), median TTR = 36 days (range 7–262)
Data cutoff 9/30/2022
Swimmer plot depicts disease assessments relevant to first response, confirmation of response, deepening of response, and best response. Mutational analysis was filtered on functional mutations annotated in OncoKB and normal allele frequency <5% in paired peripheral blood mononuclear cell samples. * Prior anti-BCMA ADC. * Prior BCMA-targeted CAR-T.
MR = minimal response; NE = not evaluable; PD = progressive disease; Q2W = every 2 weeks; REL = relapse; SD = stable disease; TTR = time to response.
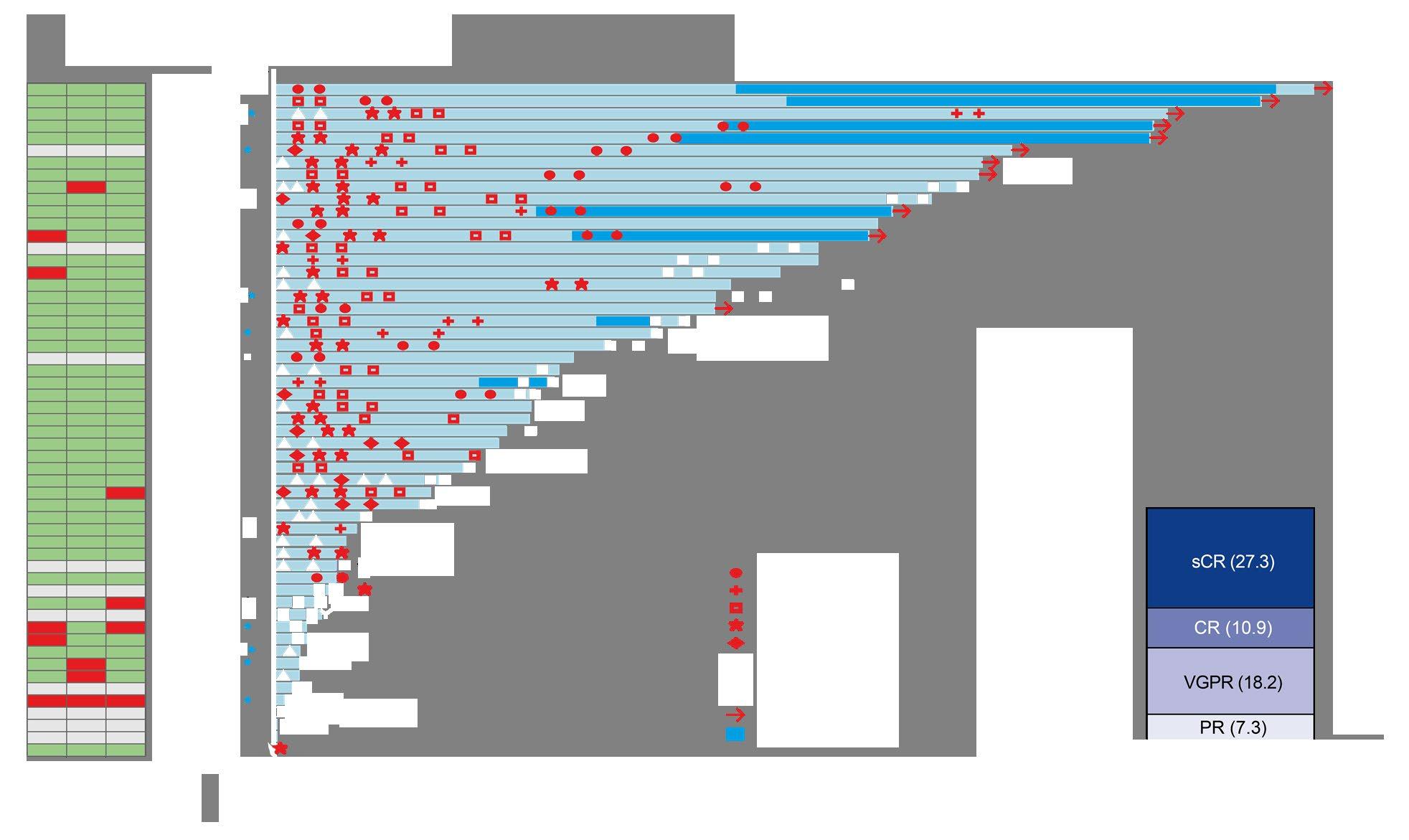
ORR was similar for QW and Q2W schedules
• Triple-class refractory: 72.6% and 71.0%
• Penta-drug refractory: 71.4% and 70.6%

• ORR was consistent across subgroups including baseline ISS stage III disease, baseline cytogenetic risk, number of prior therapies, and belantamab exposure, except among patients with baseline plasmacytomas
*Calculated from n =106 responders in each group.
CRS events
• Most CRS events were grade 1/2 and largely confined to step-up doses and first full dose
Non-hematologic AEs
• Low rates of grade 3/4 nonhematologic AEs
• Low discontinuation rates due to AEs Most common AEs were CRS, skin-related events, nail-related events, and dysgeusia

Hematologic AEs
• Most high-grade AEs were cytopenias
• Cytopenias were generally limited to first few cycles
*AEs were graded by CTCAE v4.03 with 2–3 step-up doses.
*Based on a recent sampling
• Cytokine release syndrome (CRS)
• Neurotoxicity (ICANS)
• Usually occurs within first 1–2 weeks
• Frequency (all grade and grade 3–5) higher with CAR T
• Cytopenias
• Target unique
• For example, rash, taste disturbance seen with GPRC5D, but not with BCMA
• Infections
• Incidence for bispecifics at RP2D not yet known
• Viruses: CMV, EBV
• PCP/PJP
• Ongoing discussions regarding prophylactic measures IVIG
Anti-infectives
CAR T and bispecific antibodies are very active even in heavily pretreated patients.
Side effects of CAR T cells and bispecific antibodies include cytokine release syndrome, confusion, and low blood counts, all of which are treatable.
Abecma and Carvykti are only the first-generation CAR T cells and target the same protein. Different CAR Ts and different targets are on the way.
Bispecific antibodies represent an “off-the-shelf” immunotherapy; Tecvayli was approved in October 2022, and now Talvey and Elrexfio in August 2023
Several additional bispecific antibodies are under clinical evaluation.
autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib. Speaker’s own opinions.
• Open the Q&A window, allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A window or answer your question live.



• If the host answers live, you may see a notification in the Q&A window.


• If the host replies via the Q&A box – you will see a reply in the Q&A window.














M-Power Tampa – September 23, 2023 in Tampa, FL
M-Power Detroit – November 4th, 2023 in Detroit, MI
You can find all details for upcoming events on our website!



Thank you for attending today's program!
September 13, 2023, International Myeloma Foundation’s Regional Community Webinar


As follow up to today's workshop, we will have the speaker slides and a video replay available.

These will be provided to you shortly after the workshop concludes.


At the close of the meeting a feedback survey will pop up.
This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.