2023 Boca Raton Patient and Family Seminar
March 17 & 18, 2023

Thank you to our Sponsors!












Taking The Reins of Your Multiple Myeloma Care

Beth Faiman, PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO
Cleveland Clinic Taussig Cancer Institute, IMF
Nurse Leadership Board Member
Beth Faiman PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO
 Clinic, Cleveland, OH USA
Clinic, Cleveland, OH USA
Objectives
STABLE OF TREATME

FINDING YOUR GAIT
ENJOYING THE RIDE
STABLE OF TREATMENT


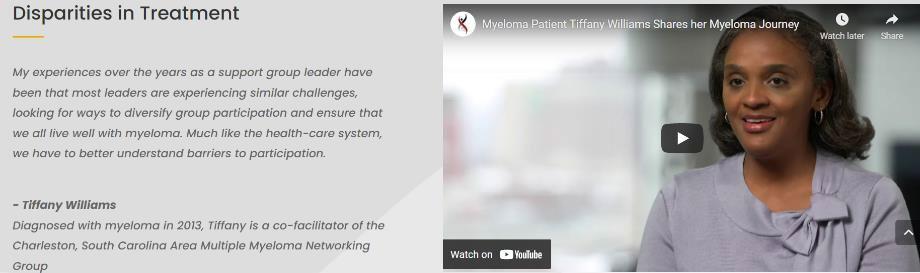
• Myeloma and treatment options, side effects, symptom management, & supportive care
FINDING YOUR GAIT

• Know your care team, telehealth & meeting prep, & shared decision making
GOING THE DISTANCE
• Healthful living, infection prevention, renal and bone health
Stable of Treatment
Treatment options, side effects, symptom management, and supportive care

Gallery of Goals
Myeloma Treatment


• Prevent disease- and treatment-related side effects
• Optimize symptom management
• Allow for good quality of life
Supportive Therapies
• Rapid and effective disease control
• Durable disease control
• Improved overall survival
• Minimize side effects
• Allow for good quality of life
Discuss goals and priorities with your healthcare team.
Myeloma Therapy Combinations
Myeloma Treatment Pallet Common Combinations
Velcade® (bortezomib) VRd, Vd
Revlimid® (lenalidomide) VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab) DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Tecvayli (teclistimab --
Xpovio® (Selinexor) Selinexor-Vd, Selinexor-dex
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
ABECMA ® Idecabtagene Vicleucel, CARVYTKI ® ciltacabtagene autoleucel --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are always an option
Don’t change horses midstream

It is important to stay on myeloma treatment to control myeloma cells and get the most from each treatment.
Responses deepen over time.
Talk to your team if side effects are bothersome. Your team may be able to help but only if they know.
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax *Withdrawn from FDA but still available in certain situations . Prescribing information for each drug listed in the table. NCCN Guidelines. Multiple Myeloma. V3.2023. Accessed January 13, 2023.
Additional Options to Address Side Effects
Non-medication

Patient-Reported Symptoms
A meta-analysis identified the most common patient-reported symptoms and their impact on QOL. Symptoms were present at all stages of disease. Symptoms resulted from both disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy
• Impaired Physical Functioning
• Sexual Dysfunction

Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790.
Psychological
• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial
• Financial burden (80%)
• Financial toxicity (43%)
Steroids: The Good, The Bad, The Ugly
Steroid Synergy
• Steroids are a backbone and work in combination to enhance myeloma therapy

Managing Steroid Side
Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections
Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes
GI Symptoms: Prevention & Management
Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication if recommended
• Imodium®, Lomotil®, or Colestid® or Welchol®
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Constipation may be caused by medications and supplements
• Opioid pain relievers, antidepressants, heart or blood pressure medications
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin

B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Fluid intake can help with both diarrhea and constipation and helps kidney function. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.
Pain Prevention and Management
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management –
Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
– Interventions depends on source of pain
–
May include medications, activity, surgical intervention, radiation therapy, etc
–
Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)

–
Scrambler therapy for neuropathy
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled.
Peripheral Neuropathy Management
Peripheral neuropathy:
Damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
– B-complex vitamins (B1, B6, B12)
– Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes

If neuropathy worsens, your provider may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from neuropathy can be permanent if unaddressed.
Why the Long Face?

98.8%
Fatigue is the most commonly reported symptom. Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression

>35% of patients
~25% of patients
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self harm. Help is available.
Rest and Relaxation Contribute to Good Health
• Adequate rest and sleep are essential to a healthful lifestyle
– Shortened and disturbed sleep increase risk of
– Heart related death
– Increase anxiety
–
Weakened immune system
–
Worsened pain
–
Falls and personal injury
• Things that can interfere with sleep
–
Medications: steroids, stimulants, herbal
supplements
–
Psychologic: fear, anxiety, stress
–
Physiologic: sleep apnea, heart issues, pain

Sleep hygiene is necessary for quality nighttime sleep and daytime alertness
–
Engage in exercise but not too near bedtime
– Increase daytime natural light exposure
– Avoid daytime napping
– Establish a bedtime routine - warm bath, cup of warm milk or tea
• Associate your bed ONLY with sleep
• Sleep aid may be needed
– Avoid before bedtime:
• Caffeine, nicotine , alcohol and sugar
• Large meals and especially spicy, greasy foods
• Computer screen time
Rod NH et al 2014. PloS one. 9(4):e91965; Coleman et al. 2011. Cancer Nurs. 34(3):219-227.National Sleep Foundation. At: http://sleepfoundation.org/ask-the-expert/sleep-hygiene
Mustian et al. Journal of clinical Oncology. Sep 10 2013;31(26):3233-3241; Stan DL, et al. Clin J Oncol Nurs. Apr 2012;16(2):131-141; Zeng Y et al., Complementary therapies in medicine. Feb 2014;22(1):173-186.
Financial Burden
• Financial burden comes from
• Medical costs
– Premiums
– Co-payments
– Travel expenses
– Medical supplies
• Prescription costs
• Loss of income
– Time off work or loss of employment
–
Caregiver time off work
• Funding and assistance may be available
–
Federal programs
–
Pharmaceutical support
–
Non-profit organizations
– Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.

Finding Your Gait
Know your care team, telehealth & meeting prep, & shared decision making

Don’t Ride Alone
YOU are central to the care team
Be empowered
• Ask questions, learn more
• Participate in decisions
Primary Care Provider (PCP)





Communicate with your team
• Understand the roles of each team member and who to contact for your needs
• Participate in support network
Subspecialists
General Hem/Onc
Myeloma Specialist










You and Your Caregiver(s) Support Network


Prepare for Visits and Consider Telemedicine
Come prepared
Bring a list of current medications, prescribed and over the counter
Write down your questions and concerns. Prioritize them including financial issues
Have there been any medical or life changes since your last visit?
Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along

Communicate effectively: your health care team can’t help if they don’t know
Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –
Is telemedicine an option?
• Similar planning for “in-person” appointment PLUS:
What is the process and what technology is needed?
Plan your labs: are they needed in advance? Do you need an order?
Plan your location: quiet, well-lit location with strong wi-fi is best
Plan yourself: consider if you may need to show a body part and wear accessible clothing
Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase
Shared Decision-Making
Be empowered to be part of the treatment decision-making
• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
– Use reliable sources of information
– Use caution considering stories of personal experiences
– Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog
–
My top priority is [goal/value]; additional [preferences] are also important.
–
I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together


















Going the Distance
Healthful living, infection prevention, renal and bone health

Healthful Living Strategies: Prevention
Maintain renal health

• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function
• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight-bearing activity and/or walking
• Bone strengthening agents
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement
• Complementary therapy
Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain a healthy weight
• Nutrition
• Activity / exercise
An ounce of prevention is worth a pound of cure.
Benjamin FranklinFaiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
Infection Awareness & Prevention
Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])
Immunizations (NO live vaccines)
Medications (antibacterial, antiviral)
As recommended by your health care team
COVID: The Best Way to Prevent Illness Is to Avoid Being Exposed to the Virus
Spread mainly through respiratory droplets that are produced by cough, sneezing and talking. More droplets with louder talking, yelling, singing

• Get COVID Vaccine + Booster: Excellent protection against severe disease, but vaccine effectiveness may be lower in people with compromised immune systems
• Wear a High-quality Mask: Respiratory droplets can spread disease; a high-quality mask can prevent exposure to airborne viral particles
• Avoid Crowds & Sick People
• Physical Distance & Outdoors: Close contact and indoor locations increases risk of spread
• Wash Your Hands: Less common to get from a hard surface
• Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
• Compromised immune function comes from multiple myeloma and from treatment.
• Infection is serious for myeloma patients!
Healthful Living Strategies: Keep Active
Movement therapies can reduce stress, promote sleep
Yoga, Pilates, Tai Chi
• Shown to improve sleep and sleep quality
• Improved quality of life & mood
Do:
• Keep a log or journal of your activity
• Notify your healthcare provider about sudden onset of pain, progressive weakness, headaches, blurred vision, numbness, and tingling

• Dehydration can lead to low blood pressure, falls
• Consider weightlifting limits
Do Not:
• Overdo it
• Force exercise
• Try things without discussing with provider Boullosa
Myeloma bone disease may affect your ability to do certain movement activities. Review your activity interests with your health care provider!
Don’t Put the CAR T Before the Horse: CAR T cell therapy, Bi-specific Antibodies, and Clinical Trials in Multiple Myeloma


Stable of Goals
Myeloma Treatment

• Rapid and effective disease control
• Durable disease control
• Minimize side effects
• Allow for good quality of life
• Improved overall survival
Supportive Therapies
BI - SPECIFIC ANTIBODIES
CLINICAL TRIALS
• Prevent disease- and treatmentrelated side effects
• Optimize symptom management
• Allow for good quality of life
Discuss goals and priorities with your healthcare team.
CAR T: A New Treatment Approach

STABLE OF TREATME
CAR T CELL THERAPY
FINDING YOUR GAIT
BI - SPECIFIC ANTIBODIES
ENJOYING THE RIDE
CLINICAL TRIALS
CAR T: Tips
• Different CAR T-cell therapy products have differences in efficacy, safety, manufacturing process, and centers where they are available
• Ask for a referral to CAR T-cell therapy center as soon as it is possible as next treatment option (ie, before relapse)



– Insurance preauthorization required (weeks, maybe longer)
– Must have sufficient blood count and organ function to be eligible
• Your own T cells are engineered and grown, which takes ≈ 4 to 6 weeks

– Must be able to wait or have bridging therapy
– Manufacturing CAR T-cell therapy is limited: center-specific “wait list” processes
• Need to be “known” by the center. How frequently do you or your myeloma doctor need to check in with the CAR T center to let them know your status? How will this happen?
Opportunity for telehealth visits?
• How are slots allocated?
• Advocate for yourself—ask for help navigating the process
• In patient for ~1 week when CAR T administered
• Patients need a caregiver and must stay within proximity of CAR T-cell therapy center for ≈ 1 month after the infusion
• No driving for 8 weeks after CAR T
• One and done…BUT will need ongoing monitoring; some patients need transfusion support
Horse of Another Color:
BiSpecific Antibodies also Target BCMA

• Different bispecific antibodies have differences in efficacy, side effects


– About 7 in 10 patients responded
– CRS is common
– Some had skin/nail disorders
• Tecvayli™ (teclistamab) is the first but more expected
• Off-the-shelf treatment; no waiting for engineering cells

• Infusion (every 2 weeks by may vary)
• CRS, Neurotoxicity, infection are possible
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; MM = multiple myeloma; scFV = single chain fragment variable.
CAR
THERAPY
CLINICAL TRIALS
BISPECIFIC ANTIBODIES

Cytotoxic cytokines
CAR T and Bi-specific Antibodies: Unique Side Effects
CRS is a common but usually mild side effect

CAR T and Bi-specific Antibodies: Unique Side Effects

Seizures
Neurotoxicity is a rare but serious side effect
Clinical Trials: Early Access to Promising Treatments
Pre-clinical
ANIMAL STUDIES FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
Phase 1
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of effectiveness, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
Phase 2
EVALUATION OF EFFECTIVENESS IN A CERTAIN TUMOR TYPE
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically

Phase 3
GATHER ADDITIONAL EFFECTIVENESS AND SAFETY INFORMATION COMPARED TO STANDARD OF CARE
• Placebo may be involved if no standard of care exists; 100s to several thousand patients
• Often multiple institutions; single or double blind
Phase 4
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS
AE = adverse event; DLT = dose-limiting toxicity; MTD = maximum tolerated dose; PD = pharmacodynamics; PK = pharmacokinetics.
Oncol. 2016;7:17-29.
FINDING
How to Find Clinical Trials
Clinicaltrials.gov
https://clinicaltrials.gov/


IMF Infoline
CLINICAL TRIALS
ENJOYING THE RIDE
US & Canada 800-452 CURE (2873)
Worldwide: 1-818-487-7455
infoline@myeloma.org

Clinical Trial Myths: Dispelling Inaccuracies

MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
• Phase 1 and 2, everyone gets active treatment
BI - SPECIFIC ANTIBODIES CLINICAL TRIALS
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time
MYTH: Patients (whatever demographic/ distance from clinic/etc) never participate in clinical trials so I won’t mention it
• Mention the option and give the patient the opportunity; implicit and explicit biases can limit participation
• Some groups may need more information about clinical trials to feel comfortable with participation
MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone though testing to indicate that the drug is likely to be safe and effective for human use
MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel may not be reimbursed and are paid by patient
Importance of Diversity in Clinical Trials

People from racial and ethnic minority and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
Regnante JM, et al. J Oncol Pract. 2019;15(4):e289-e299. FDA website. Clinical Trial Diversity. Accessed March 31, 2022. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity. International Myeloma Foundation website. Accessed March 31, 2022. https://www.myeloma.org/node/4797.





Nurse Leadership Board
In 2006, the IMF founded the Nurse Leadership Board® as a professional partnership to represent oncology nurses who are experts in the care of multiple myeloma patients at leading medical centers. The NLB is improving the nursing care and self- care of patients with multiple myeloma via publications, symposia, multimedia, and research.



Disparities in Myeloma Joseph Mikhael,
MD
International Myeloma Foundation

Health Disparities in Myeloma
Boca Raton Patient and Family Seminar
March 17, 2023
Dr. Joseph Mikhael Chief Medical OfficerInternational Myeloma Foundation


• A word of caution when we discuss issues of Diversity, Equity and Inclusion…

• Personal, Professional and Political
• Equity means to guarantee of fair treatment, access, opportunity, and advancement for all while striving to identify and eliminate barriers that have prevented the full participation of some groups.
• Valuing diversity means that we recognize and respect everyone’s unique qualities and attributes.
• Inclusion means that all individuals feel respected, accepted and valued.
• The history of the IMF compels us to continue to advocate for all

What are Health Disparities?
• Health disparities are preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations - Centers for Disease Control (CDC)

• Health equity generally refers to individuals achieving their highest level of health through the elimination of disparities in health and health care

1. Systemic racism
2. The Healthcare system
3. Social Determinants of Health
4. Biology of the disease and concomitant comorbidities


5. Delayed Diagnosis
6. Access to Care – Triplets, Transplants, Trials and Car T
7. Lack of diversity, cultural sensitivity and optimal communication in healthcare professionals
What are the DRIVERS of Disparities in MM?What are the FACTS about Disparities in MM?
Multiple myeloma is one of the malignancies with the greatest disparity in incidence and prevalence between African Americans and White Americans. It is the most common blood cancer in African Americans (twice the incidence than White Americans). The average age of diagnosis is 4-5 years younger in African Americans and Hispanic Americans

Studies show that biologic differences in myeloma in African Americans may lead to lower-risk disease whereas there may be more high risk disease in Hispanic Americans. African Americans and Hispanic Americans are less likely to receive life-extending therapies. Mortality is TWICE as high in African Americans with myeloma than White Americans

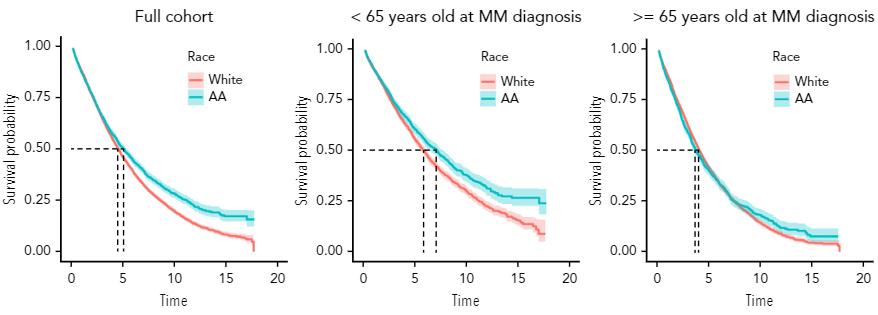
African Americans achieve equal or better outcomes compared to White Americans when they receive therapy.
Ailawadhi et al. (2012); Baker et al. (2013); Greenberg et al. (2015); Hari et al. (2010); Rhotagi et al. (2007); Saraf et al. (2013); Waxman et al. (2010); Data derived by calculating the ratio of the average African American to white age - adjusted incidence rates from 2000 - 2013 for the 8 most common malignancies in African Americans, plus all cancer sites and multiple myeloma. Incidence rates were obtained from: National Cancer Institute. Fast stats. Surveillance, Epidemiology,
1. Myeloma is

in African Americans And the incidence is

the most common blood cancer
growing….
By 2034 it is estimated that African Americans will make up roughly 24% of the newly diagnosed MM population1
2. Myeloma is TWICE as common in African Americans

African Americans have >2x the incidence rate of MM compared to white Americans1
1. American Cancer Society. Cancer Facts and Figures for African Americans 2019-2021.
3. African Americans are younger at diagnosis by about 5 years
Hispanic African American Asian White














66 65
71 69 YEARS YEARS YEARS YEARS
The median age at diagnosis for all patients is 69 years



4. African Americans comprise about 20% of all patients with MM
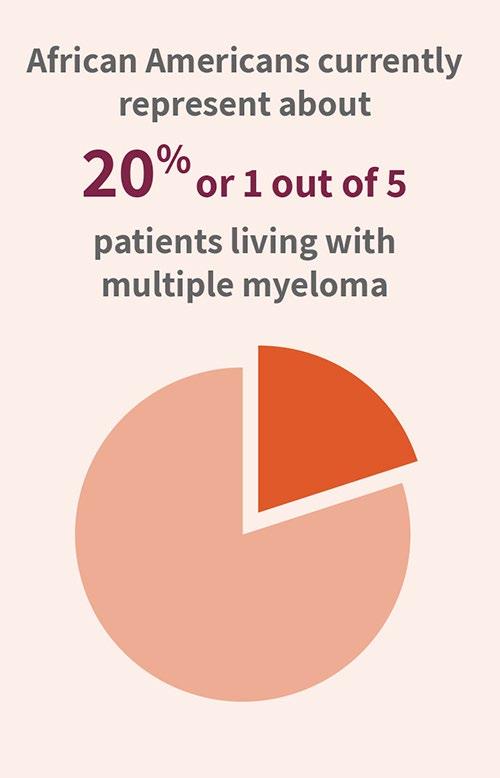
Currently, African Americans comprise 14% of the total population of the USA

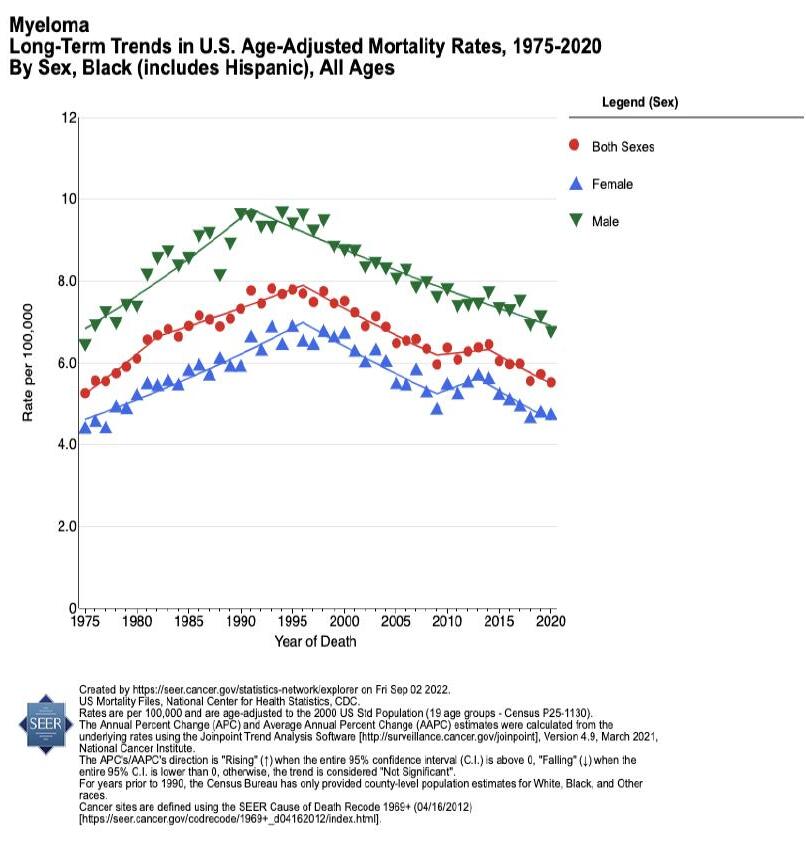
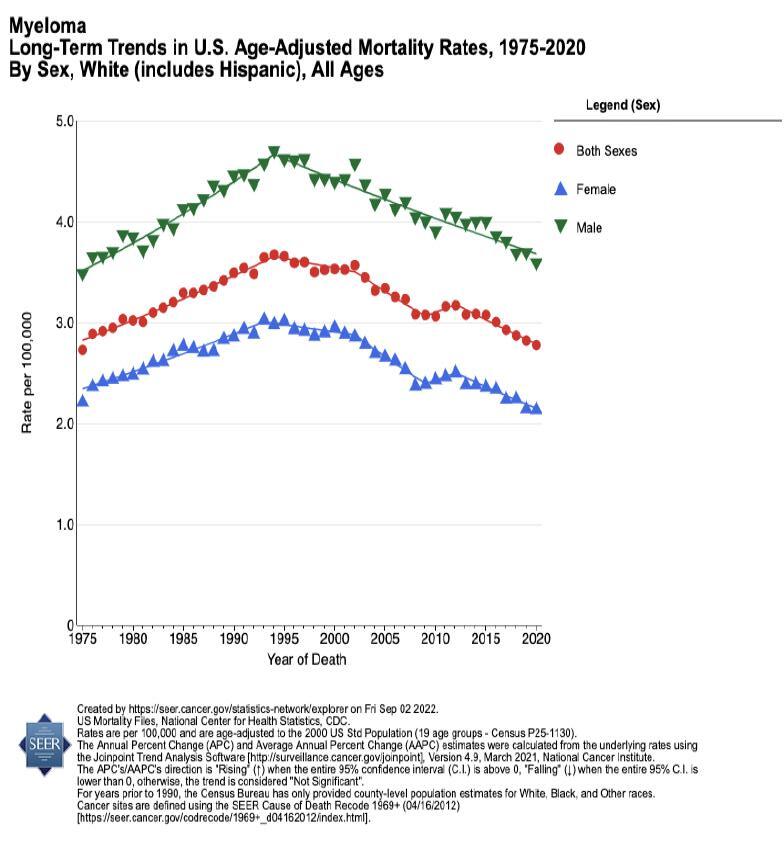
Mortality is TWICE as high in Black Americans with myeloma

6. There is a LONGER time from symptom onset to diagnosis in African Americans
The average myeloma patient sees their primary care doctor THREE times with symptoms and signs consistent with MM. The delay is even longer in African Americans, for many reasons:

Confounding diagnoses (like diabetes)
Access to diagnostics and care
Awareness in primary care providers
Timely referral to specialists…

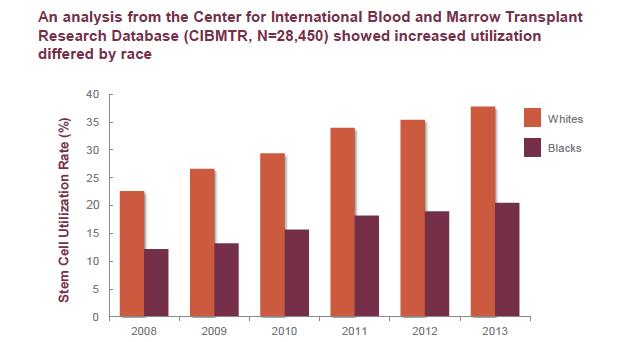
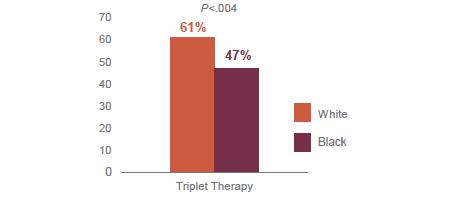


8. African Americans are much less likely to participate in Clinical Trials (the 4th T)
Blacks/African Americans and Myeloma Clinical Trials



Blacks/African Americans are underrepresented in clinical trials, including Myeloma trials. Blacks/African Americans represent 15% of the general cancer population; however, only comprise 4-6% of trial participants.

Blacks/African American myeloma patient enrollment steadily increased in the early 1990s, then began to decline in the early 2000s.
Underrepresentation of Blacks/African Americans in clinical trials can limit the generalization and validity of the findings of the clinical trial.

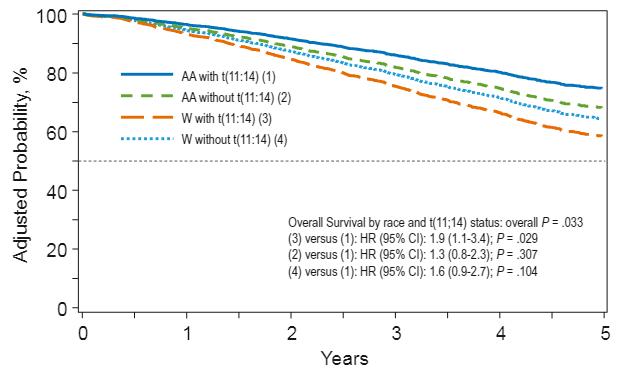
9. There are biologic differences in African Americans with MM that may lead to lower risk disease

African ancestry associated with less aggressive disease
Higher prevalence of [a]:

t(11;14) t(14;16) t(14;20)
Lower prevalence[c]:
13q deletion
17p deletion
• Absence of 17p deletion associated with better survival among younger African Americans vs White counterparts[d]
10. When African Americans receive equal access to care, their survival outcomes are equal, and at times, better than Whites

Fillmore NR, et al. Blood. 2019;133:2615-2618
So what can we do about this?
• It is a complex problem and requires a complex solution
• Key themes of Success:
• Awareness, Education, Advocacy and Empowerment in the lay community


• Education, Cultural Competence, Access in the medical community
• Policy, Expectations, Commitment in the regulatory and corporate community
• This is impossible without genuine collaboration between ALL stakeholders
• We believe the IMF is uniquely poised to address this issue and bring many of these key stakeholders together…
M-Power = Myeloma Power

The core vision of this initiative is to improve the short- and long-term outcomes of African American patients with myeloma.
We want to empower patients and communities to change the course of myeloma…
Increase awareness and reduce stigma
Engage the community
Shorten the time to diagnosis by educating primary care providers
Enhance access to care
Educate providers about culturally sensitive care
The Rationale
• We are very aware of disparities in MANY groups!
• These include race, ethnicity, geography, insurance status, age, gender, and others…
• The disparity in the African American community is the highest in MM and likely the highest in ALL cancers – so we are directing our initial efforts in this community

• Analogy of the house on fire…
• We are already planning other efforts in the Hispanic/Latinx community










Faith Community Nurses
Meeting Communities Where They Are
Levine Cancer
Center & Atrium
A “Surround” Strategy Black Health Matters
Educational Materials
University of MD
M-Power Website & Toolkit
Videos & Podcasts Cancer Center Customize d Education

Print Materials Community Organizations
Emory / Grady
Council
The current or FUTURE patient, family member, or caregiver
Churche s
MSK
Moffit
Involved Support Group Leaders
Michigan State/ Karmanos
Thousands of print publications distributed
165+ US Support Groups Free Community Workshops
Meet the Team













 Robin Tuohy
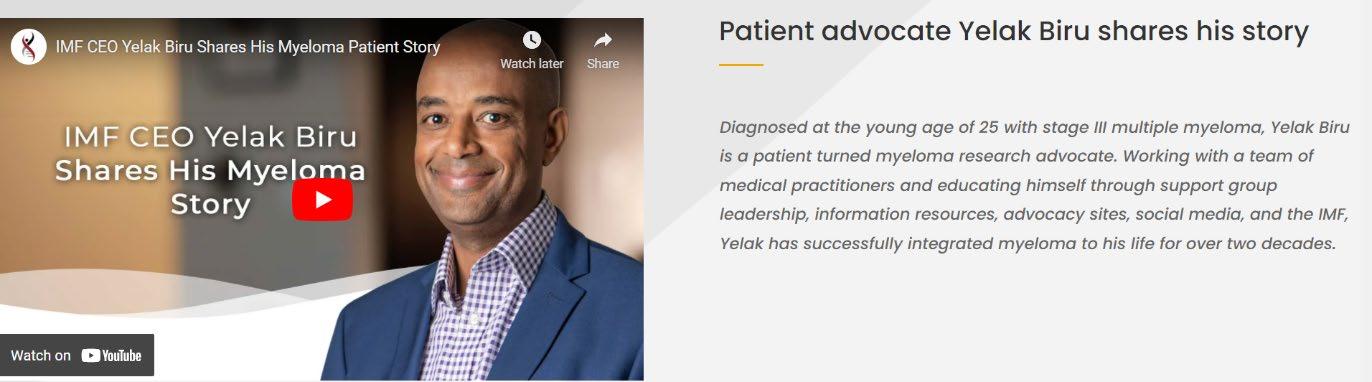
Yelak Biru
Michelle Faber Selma Plascencia
Rafi Stephan
Haleigh Wolfe
Nancy Bruno
Meghan O'Connor
Kelly Cox
Joseph Mikhael
Diane Moran
Peter Anton
Robin Tuohy
Yelak Biru
Michelle Faber Selma Plascencia
Rafi Stephan
Haleigh Wolfe
Nancy Bruno
Meghan O'Connor
Kelly Cox
Joseph Mikhael
Diane Moran
Peter Anton







M-Power Website and Video Reach



…with fresh content, resources added regularly & a page for each city











Meeting Patients and Communities Where They Are Through Engaging, Creative Formats
Healthy Churches 2030 Conference with Doug E Fresh
Customizable Education Options presented to community leaders (e.g. faith nurses, habitat for humanity, divine 9)
Myeloma as the 5th Cancer in community cancer education


Myeloma Added to Family Cancer Education Program


470 Physical Posters + e- version
Myeloma Made Simple in 5 minutes

Cancer Resource Bags delivered directly to neighborhoods
Post-ASH Facebook Live

Health Care Provider Education
Course for PCPs
Wraps up this week
Nurse Course
814 live attendees Linked In Live MM for the Primary Care Provider: A Practical Review to Promote Earlier Diagnosis Among Diverse Populations

<- Publications ->
Student Mentor program with NMA launches next week


Medical Student Scholars for Health Equity in Myeloma






Sponsored by the IMF, Cobb Institute and NMA
Disparities in MM Among African Americans
Education of Primary Care Physicians

Our goal is to reduce DELAYS in diagnosis among African Americans by educating the primary care community with a focus on:

• Recognizing the signs and symptoms of myeloma
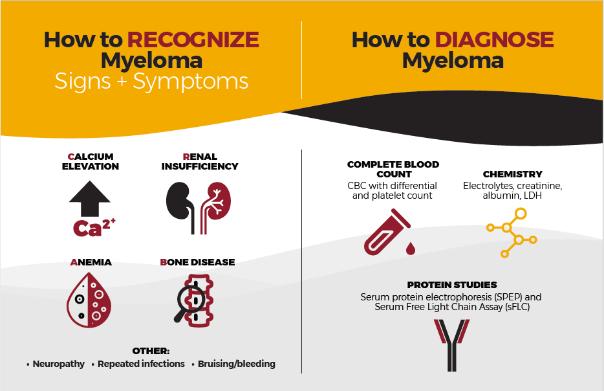

• Discriminating myeloma from other diagnoses such as diabetes
• Capturing an accurate diagnosis through proper use of testing
• Providing referral guidelines for Hematology and Oncology
• Grand Rounds
• Postcards mailed to 6,000+ PCPs in target cities
• Free PCP CME course “Don’t Miss Myeloma”

• Cobb Institute talk
• Talk at NMA Annual Meeting
• Articles and pending publications


359 Live Attendee s
M-Power Community Workshops



Over 10,000 Enduring Replays
Launched March 20, 2021
129 individuals or households attended
50% Black or African American
98% rated it Excellent or Very Good +++ video replays
June 27,2022
56 individuals or households attended
67% Black or African American
100% rated it Excellent or Very Good
2,112 replays
• 87% reported being more aware of the risks and symptoms of multiple myeloma
• 93% plan to share something learned with a family member, friend, or healthcare provider
Launched Sept 25, 2021
48 individuals or households attended 44% African American
100% rated it Excellent or Very Good 49 requests for mailed materials +++ video replays
• 100% reported being more aware of the risks and symptoms of multiple myeloma
• 92% plan to share something learned with a family member, friend, or healthcare provider
Launched Nov 13, 2021
77 individuals or households attended 52 requests for mailed materials
75% African American
90% rated it Excellent or Very Good ++++ video replays
• 86% reported being more aware of the risks and symptoms of multiple myeloma

• 93% plan to share something learned with a family member, friend, or healthcare provider

Launched Oct 1, 2021
49 individuals or households attended 50 requests for mailed materials
60% African American
90% rated it Excellent or Very Good 1,535 replays in 3 weeks
• 90% reported being more aware of the risks and symptoms of multiple myeloma
• 95% plan to share something learned with a family member, friend, or healthcare provider




Medical Student Scholars for Health Equity in Myeloma
Sponsored by the IMF, Cobb Institute and NMA
Medical school students to participate in a health disparities project focused in myeloma alongside an expert in multiple myeloma

GOALS:
• To raise awareness of myeloma in the medical student community and its impact on African Americans
• To support innovative health disparities projects in myeloma and their presentation
• To provide mentorship to medical students with experts in myeloma
• To increase the pool of African American physicians committed to health equity in myeloma.
• To create a community of mentors and mentees from the NMA, the Cobb Institute and myeloma experts that can support each other
Medical Students for Health Equity in Myeloma

• OVER 50 Applications from minority medical students were submitted!
• Selection Committee Selected 12 students (4 from HBCUs)
• Students have been paired with a MM expert to conduct a project in health disparities in myeloma
• Students and mentors will attend the Annual Meeting of NMA in July 2023 where projects will be presented as posters at a special session
• Networking reception will bring together students, mentors, members of SNMA, Cobb Institute and the NMA
• A “community” will therefore be created to further enhance collaboration and career opportunity
Other Collaborative Projects
• Charlotte
• Measurement of burden of disease in African American MM patients prior to and after M-Power

• Capture the number of referrals and percentage of African American patients referred pre/post M-Power
• Old North State Medical Society Chapter of NMA
• Atlanta
• Create an order set in EPIC for suspected MM to include serum protein electrophoresis AND free light chain testing
• Planning a multi-stakeholder roundtable on community engagement
• Black Nurses Association – planning education program for early 2023
• Other scientific studies
• Systematic Review of Health Disparities in MM
• Potential project on measuring time from symptoms to diagnosis over the last several years to capture the potential impact of M-Power in certain cities
• SEER database review of health disparities in MM
"Multiple Myeloma for the Primary Care Provider: A Practical Review to Promote Earlier Diagnosis Among Diverse Populations"


Authors: Joseph Mikhael, Manisha Bhutani, Craig Cole
"Disparities in Multiple Myeloma Among African Americans"

Authors: Joseph Mikhael, Manisha Bhutani, Sagar Lonial

Publications in Progress
1. Culturally Responsive Care Delivery in Oncology: The Example of Multiple Myeloma
Authors – Brandon Blue, Amy Pierre, Joseph Mikhael - under review (submitted Nov 2022)
2. Racial and Ethnic Disparities Influencing Overall Survival in Patients with Multiple Myeloma in the US: A Systematic Literature Review
Authors: Joseph Mikhael, Allie Cichewicz, Elizabeth Mearns, Allicia Girvan, Vicki Pierre, Archibong Yellow-Duke, Frank Cornell, Michael Nixon - being submitted next week!

Publications in Progress contd.
3. Addressing the Disparities: The Approach to the African American Patient with Multiple Myeloma

Authors: Brandon Blue, Manisha Bhutani, Craig Cole, Ashraf Badros, Saad Z. Usmani, Ajay Nooka, Leon Bernal, Joseph Mikhael -
to be submitted shortly
And from the Nurse Leadership Board…
4. Disparities in Clinical Trials
Authors: IMF Nurse Leadership Board Members Rebecca Lu, Donna
Catamero, Kim Noonal, Michaela Hillengass, Joseph Tariman, and Beth
Faiman -
To be submitted shortly
M-Power Website stats



• All Web Visits: Over 250k Page views across main & city sites
Email Stats
• Total Sent: 34 emails
• Total Audience: 218,819
• Open Rate Avg: 28%
“A Day In The Life” Podcast Stats
• Total Listens: Over 4,300
Video Stats:
• Total Views: Over 61k
Health


Disparities is a rapidly moving field…
Facebook Live…

Conclusions
• Health disparities are sadly prevalent across all diseases, but particularly in multiple myeloma
• There are MANY other types of inequity in myeloma, including geography, age, gender, orientation…

• Being aware of these disparities is critical to overcoming them
• The IMF’s M-Power is designed to reduce the inequity with specific emphasis on delayed diagnosis, access to therapy and diversity sensitive clinical care

What Can I Do??
• Be more conscious of the topics of health equity
• Evaluate the opportunities in your experience to reduce disparities
• Support the M -Power movement!
mpower.myeloma.org

 Joseph Mikhael, MD, MEd, FRCPC Chief Medical Officer,
Joseph Mikhael, MD, MEd, FRCPC Chief Medical Officer,
International Myeloma Foundation



Professor, Translational Genomics Research Institute (TGen)

City of Hope Cancer Center
Director of Myeloma Research and Consultant Hematologist, HonorHealth Research Institute
jmikhael@myeloma.org

M-Power Changing the Course of Myeloma

Multiple Myeloma

Did you know that myeloma is the most common blood cancer in people of African descent? But doctors do not typically check people for myeloma during a regular visit because currently there are no national screening recommendations for myeloma. That’s why it’s important to learn the early symptoms of myeloma and let your doctor know that you or a friend or family member are at added risk for the disease.
Because even though myeloma affects African Americans at greater rates, with early diagnosis and treatment, African Americans can have better overall survival in living with the disease.

How You Can Make a Positive Impact in our Myeloma Community
Ilana Kenville, International Myeloma Foundation

How YOU Can Make A Positive Impact In Our Myeloma Community



Pillars of the IMF
Education Support Advocacy Research






Education


You Are Here




Support





Advocacy




Research
Improving Lives, Finding the Cure: The Fight Against Multiple Myeloma Starts Here



Innovating
Groundbreaking Cure

Today, more than 50 projects are occurring to achieve the basic and translational research that is needed to move closer and closer to a cure .



How YOU Can Make An Impact.

• One-Time
• Monthly
• Major Gifts
• Planned Giving


• Peer to Peer
• Corporate Giving
• Stock/IRA Donation
• Tributes and Memorials
• Partners in Progress
• Vehicle Donation
Peer to Peer Fundraising


Virtual Cooking Class

$5,000 100 % 28
IMF Events



Making An Impact











Studio City, CA
Myeloma 101


Myeloma 101: What Patients Need to Know


March 18, 2022

➢ Long Remissions Common

➢ CURE on the Horizon

Importance of Early Diagnosis

➢ Screening can be a new tool
➢ Start discussions with HR SMM

Potential New Myeloma or Smoldering Myeloma
Any Myeloma Defining Events?
• CRAB,
• >60% PC,
• FLC >100,
• MRI >1 focal
No Myeloma Defining Events (SMM)


High Risk SMM (Median TTP ~2 years)
Intermediate or Low Risk SMM
Treat as Myeloma
Early Therapy with Len or Rd x 2 years
Clinical Trials
Observation
Myeloma Tests Needed

➢ Blood counts: anemia?

➢ Chemistry panel: Creatinine and Calcium
➢ SPEP + IFE and UPEP + UFE
➢ Freelite ratio: involved/uninvolved
➢ S B2M; Albumin; LDH
➢ Bone marrow biopsy: FiSH chromosome testing
➢ Bone imaging: CT [WBLDCT]; MRI; PET
Evolution of Treatment Options 15 Years Ago


First Therapy Relapse
VAD (Chemo)
and Revlimid





Treatment Options in 2023




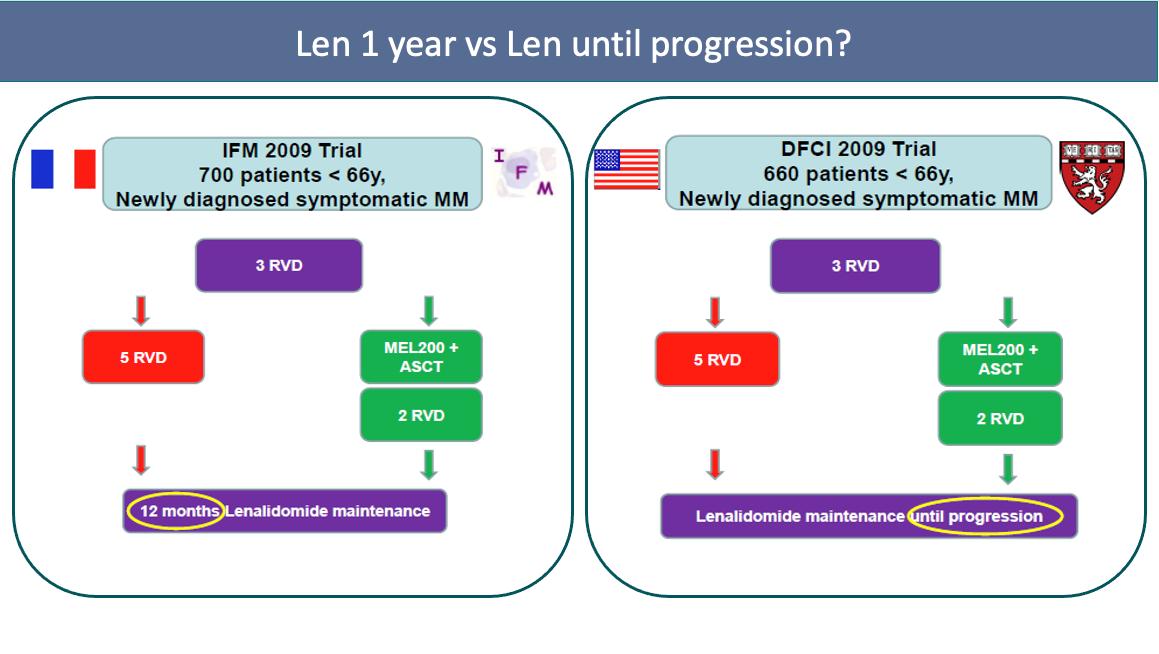

Median PFS: 47.3 vs 35 mo


Median PFS: 67.5 vs 46.2 mo
Benefit: maintenance until PD!






Active Drugs in MM


What Are The Options?



Keep Track of Follow-up Tests
Key Points for Ongoing Care
- CBC; SPEP/UPEP; Freelite; SCANS; BM if needed
- FiSH for t[11;14] or high-risk [17P-; 1q+…]
Alert Doctor to Changes (myeloma/side effects)


Be Aware of Potential Future Options
Be Proactive in Seeking Expert Advice
Set Expectations with Your Primary Doctor


IMF Publications
Download Our Publications




The IMF produces and maintains an extensive library of up-to-date educational publications and periodicals for patients and caregivers, as well as for healthcare professionals. All IMF publications are always free of charge and are available to download by clicking the links below.
A core mission of the IMF is to provide thorough and cutting-edge education to the myeloma community
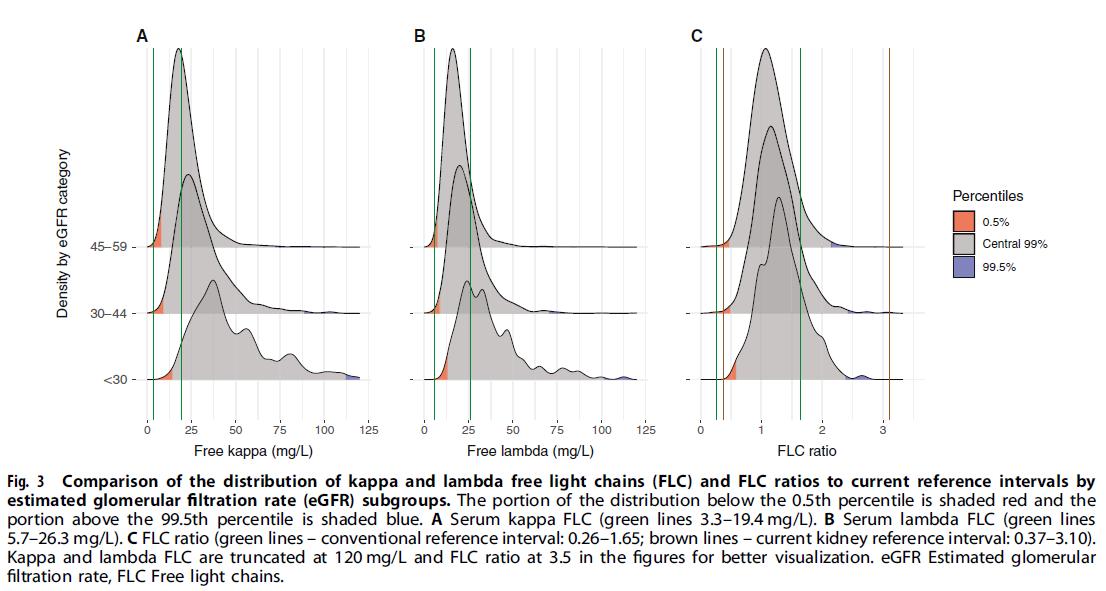
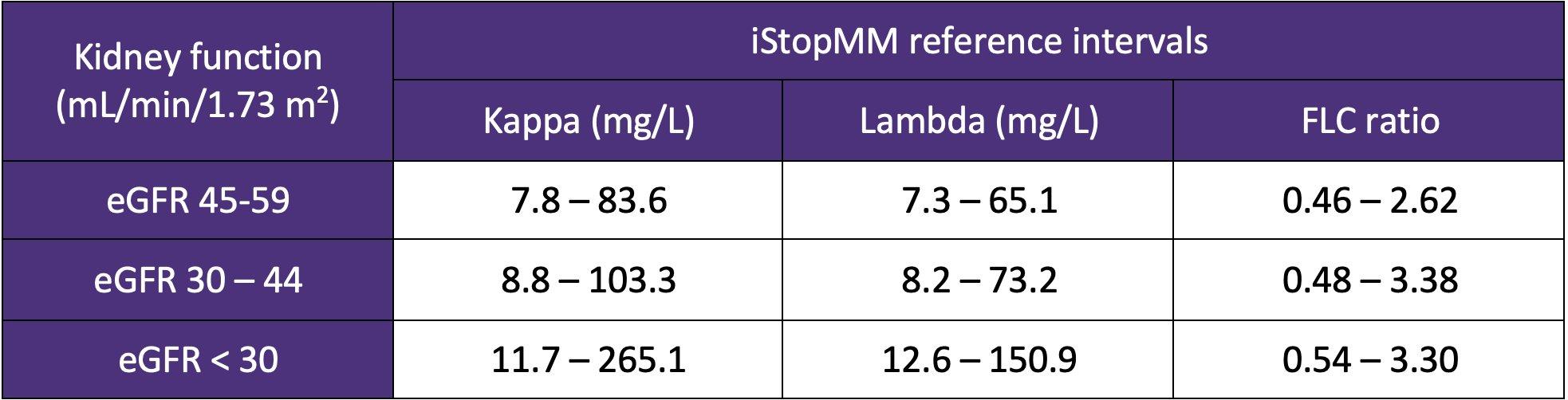
iStopMM: Is Screening the Way to Go?
Sigurður Kristinsson, MD, PhDProfessor of Hematology
University of Iceland

iStopMM
Iceland Screens Treats or Prevents Multiple Myeloma
Professor of Hematology
University of Iceland
Email: sigyngvi@hi.is

Twitter: @sykristinsson
@iStopMM
Sigurður Yngvi Kristinsson, MD, PhDScreening for monoclonal gammopathy of undetermined significance:
A population-based randomized clinical











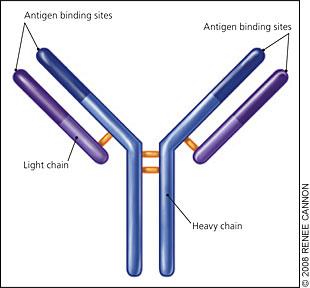
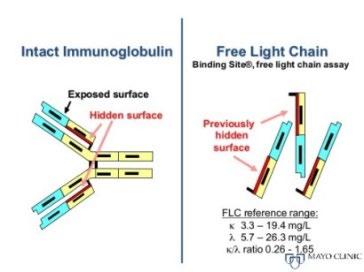
PARAPROTEIN = M PROTEIN
• Monoclonal protein in blood or urine – All are identical

• Free light chains (FLC) in blood
– Kappa or lambda
Protein electrophoresis
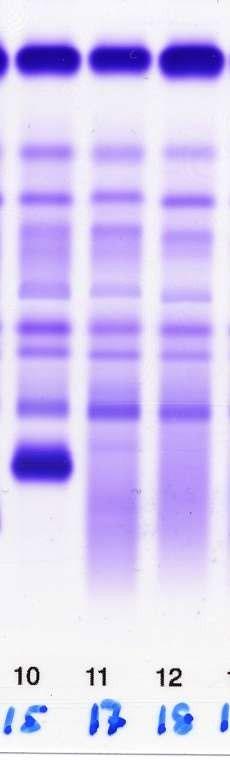

Monoclonal gammopathy of undetermined significance (MGUS)
• Myeloma precursor
• No underlying lymphoproliferative or plasma cell disease
• Prevalence 4% in 50 years and older
– Prevalence increases with age
• 1-1.5% progress to myeloma or realated disaease annually
• Myeloma is always preceded by MGUS

FLC ratio >100
>1 bone lesion on MRI
>60% plasma cells in bone marrow

Risk of progression to MM
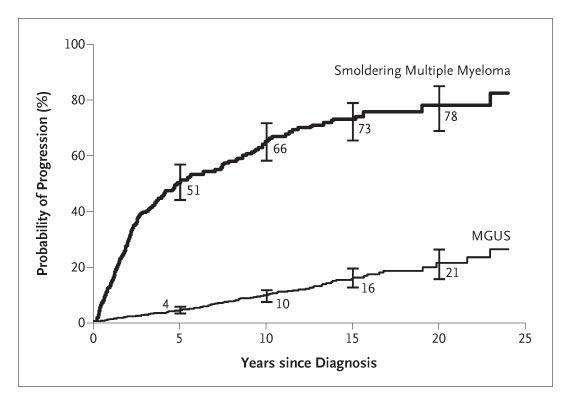



Randomized trials
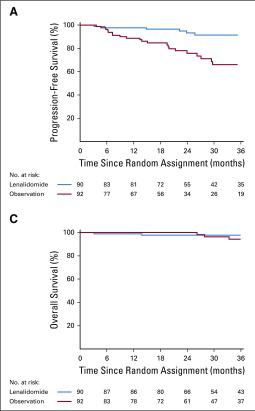
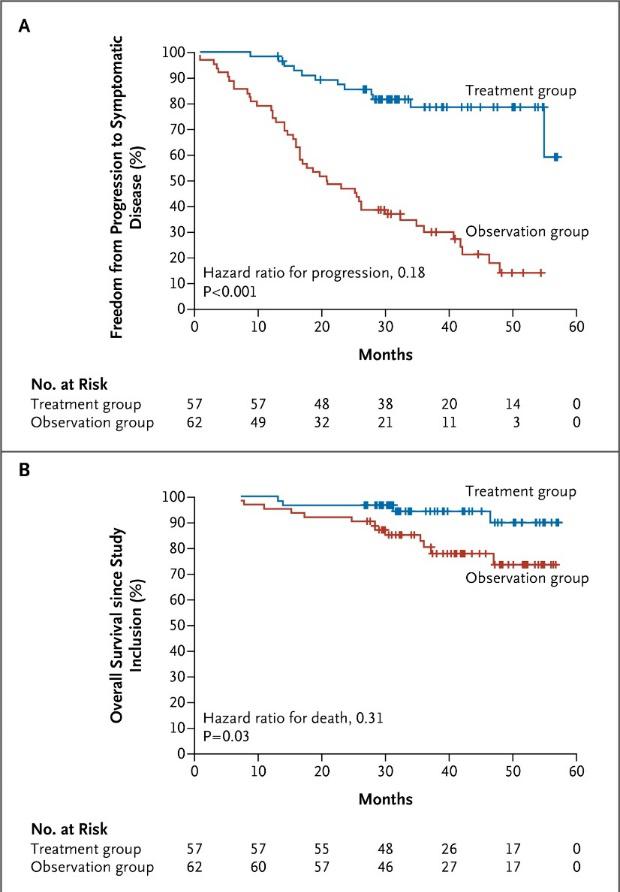

Given what we know – how should we manage SMM?
*Increasing M-protein or FLC ratio in at least two subsequent evaluations,
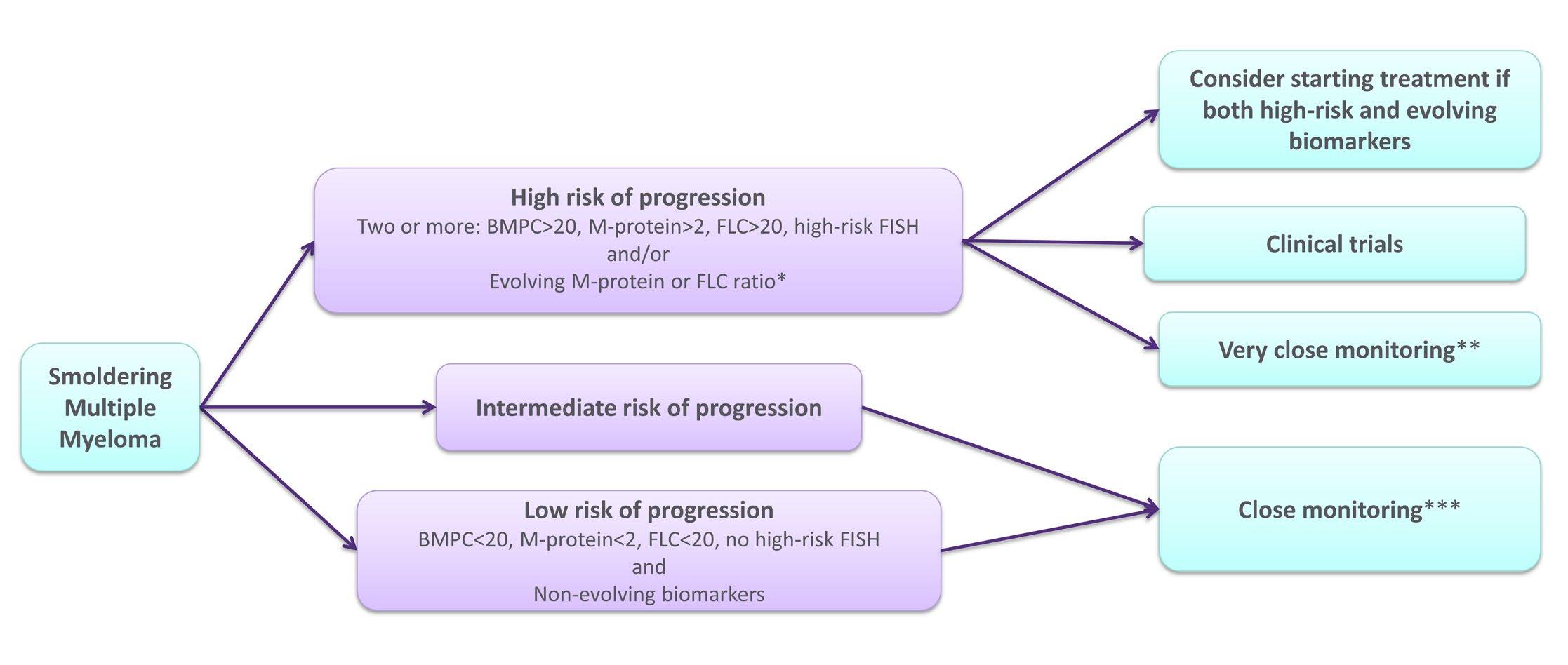
**Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 1-3 months,
**Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 4-6 months.

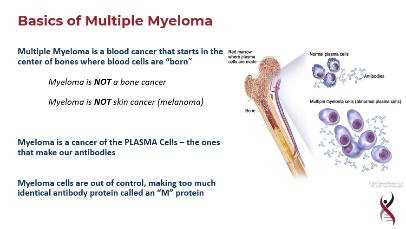
Multiple myeloma
• Malignant disease cause by increase in monoclonal plasma cells in the bone marrow that ususally secrete monoclonal protein (Mprotein)
• Symptoms due to
– Marrow failure
–
Bone disease
–
M-protein
–
Immunodeficiency
–
Kidney disease
–
Plasmacytoma


Survival is really improving
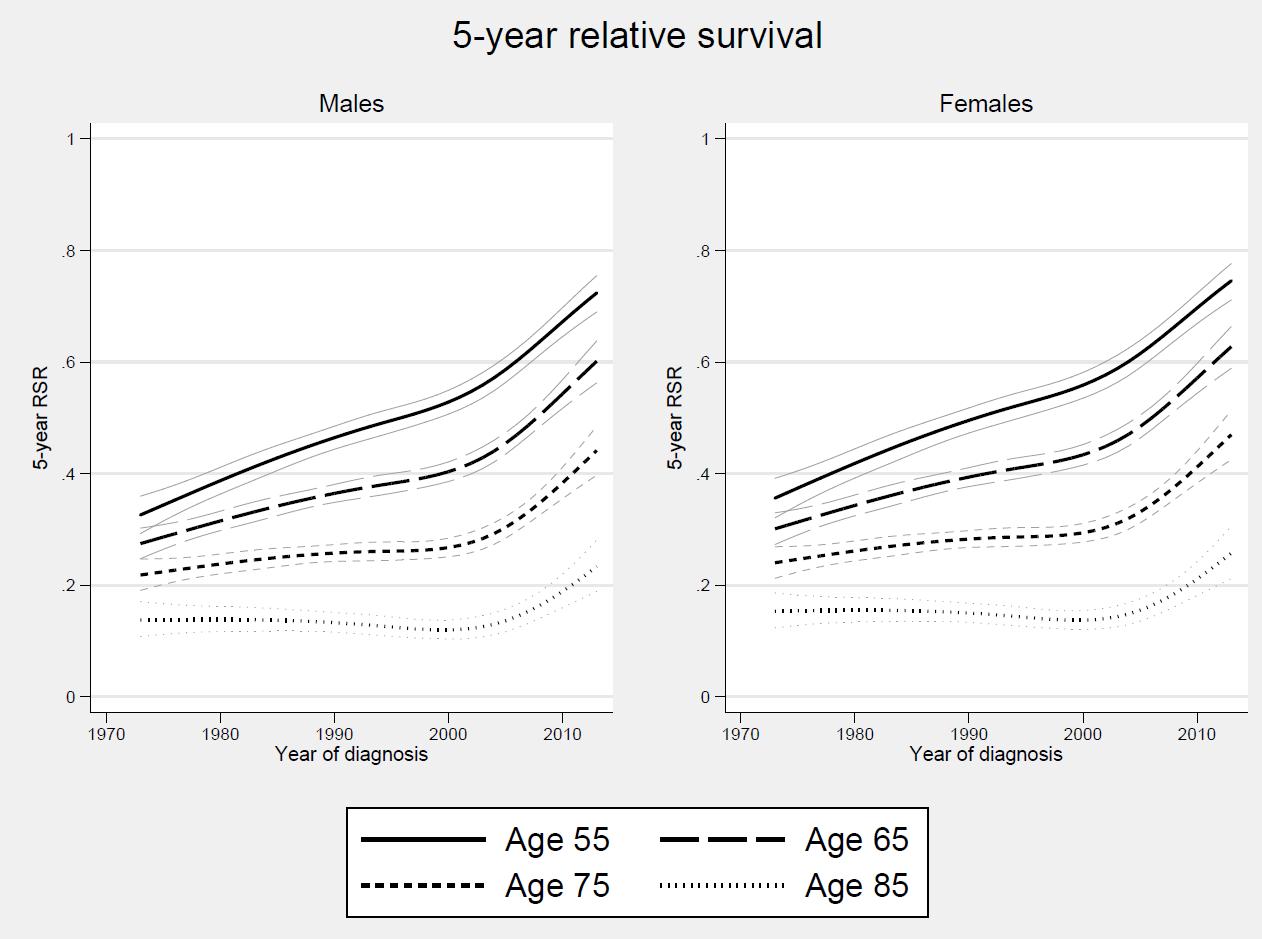
Thorsteinsdottir S et al. Haematologica 2018

Why is survival in myeloma improving?
• High-dose therapy with stem cell support
• Novel drugs
• Improved supportive care

• Prognostic factors
• Earlier treatment?
Does follow-up for MGUS matter?

The Role of Diagnosis and Clinical Follow-up of Monoclonal Gammopathy of Undetermined Significance on Survival in Multiple Myeloma
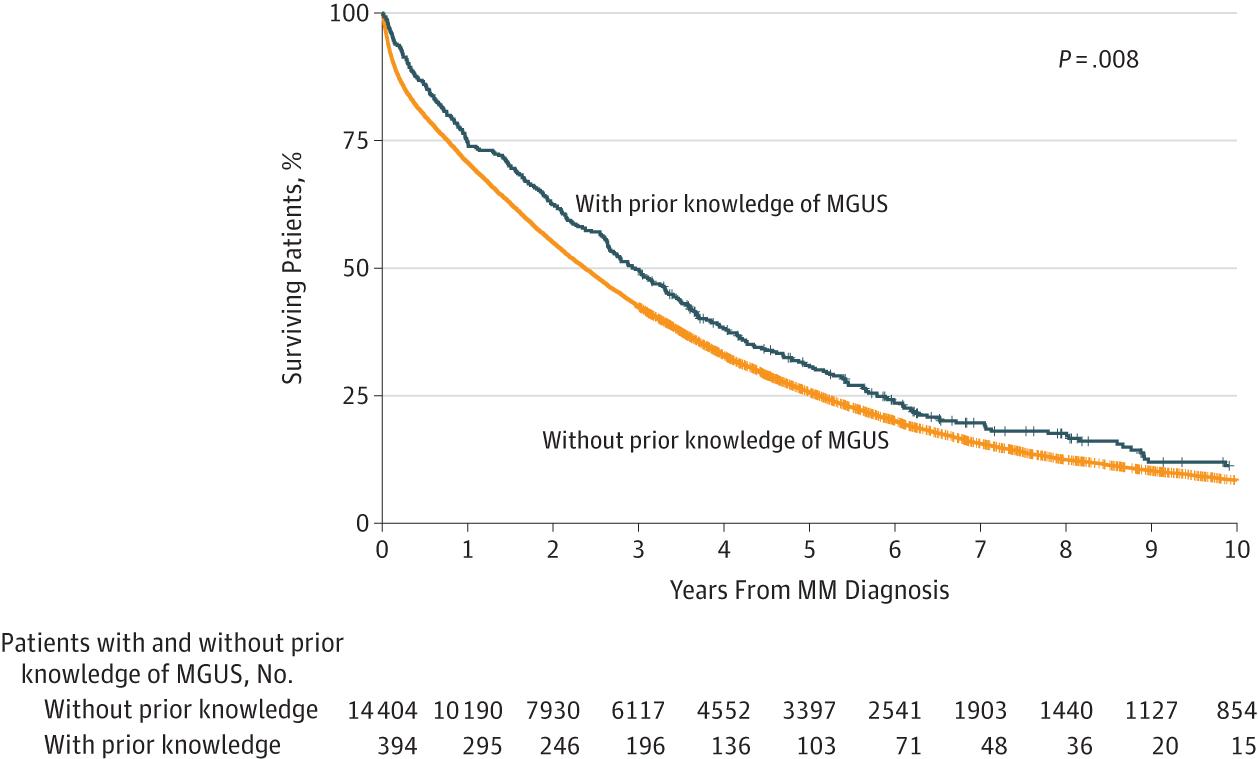

Follow-up

of MGUS is important

How do we find asymptomatic people to treat?

By chance?
By screening?

The iStopMM study

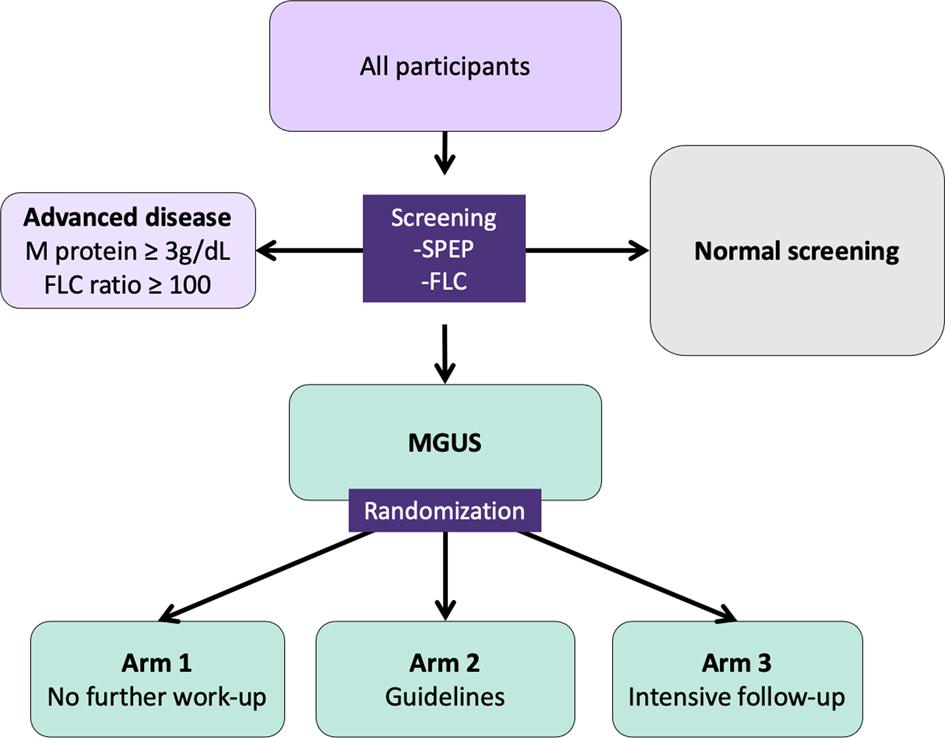


Benefits of screening

• Early detection
• Early treatment
• Prevent rather that treat
• Improved survival?
Potential harm of screening
• Cost
• Psychological harm
• Low risk of progression
• Unnecessary evaluations – Bone marrow, X-ray, MRI etc
• Only non-aggressive disease is captured

Overall aims of iStopMM
• Evaluate the impact of screening for MGUS
• Obtain evidence for optimal work-up and follow-up
• Integration biological, imaging, and germline genetic markers in risk models for progression
• Evaluate the impact of screening on quality of life

• Biobanking
• Evaluate the effect of early detection and early treatment
• All Icelanders born 1975 and earlier
How did this work?
• A purple envelope sent to their home address


All 35 laboratories in Iceland participate


Smoldering myeloma














No further work-up
arm
• Followed for progression to multiple myeloma or another malignancy (Cancer Registry)
• Followed for all diagnoses in inpatient and outpatient clinics throughout Iceland (Patient Registry)
• Followed for vital status every 2 months (Population Registry)
• All prescriptions throughout Iceland (Prescription registry)
• Regular quality of life assessments

• November 2016 to October 2018
Recruitment
• 148 708 Icelanders were eligible to participate
• 80 759 (54.3%) agreed to participate
• 75 422 (93.4%) of these participants have since been sampled

Registries
• Patient Registry
–
8,796,659 primary care visits
–
1,239,285 outpatient clinic visits since 2000
– 182,200 hospital admissions since 1990
• Cancer Registry –

11,139 cancers for our participants
• Laboratory Registry – Every laboratory test performed on participants ever
• Prescription Registry
–
17,734,946 prescriptions in the dataset
An important part of the iStopMM study is to evaluate the psychological aspects of screening:
Regular quality of life, anxiety, and well being assessments in all participants

Participation rate
• Email surveys in general have about a 30% response rate
– When more than 12 questions or longer than 5 minutes may fall to 20%
• We sent 13 questionnaires, taking 20-30 minutes to complete
• Audience was motivated to participate initially
– But signed up for a study where they expected to do nothing more
• Based on this we estimated:
– 20-30% initial response
– Increasing to a little more than 30% with two reminders


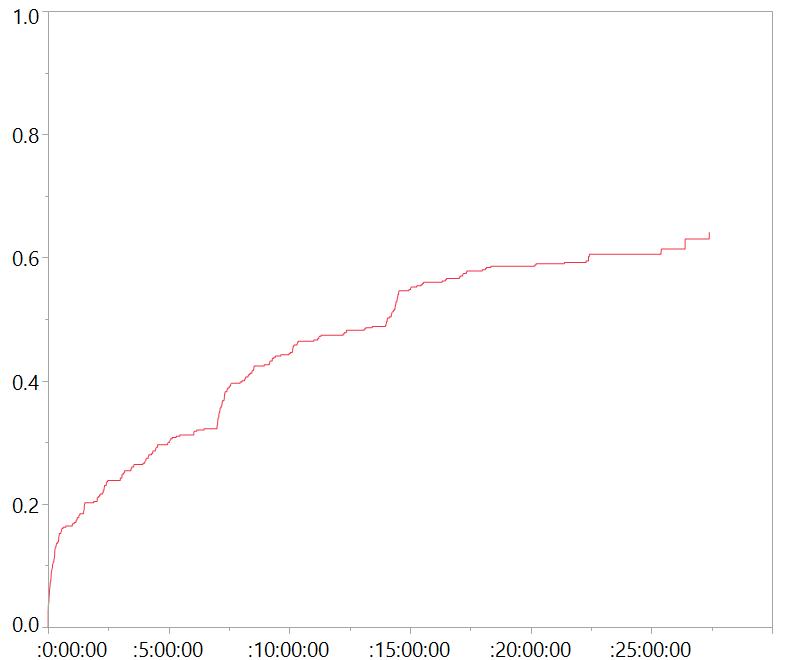

Tenacious participants


Preliminary results on quality of life assessments

Some results….

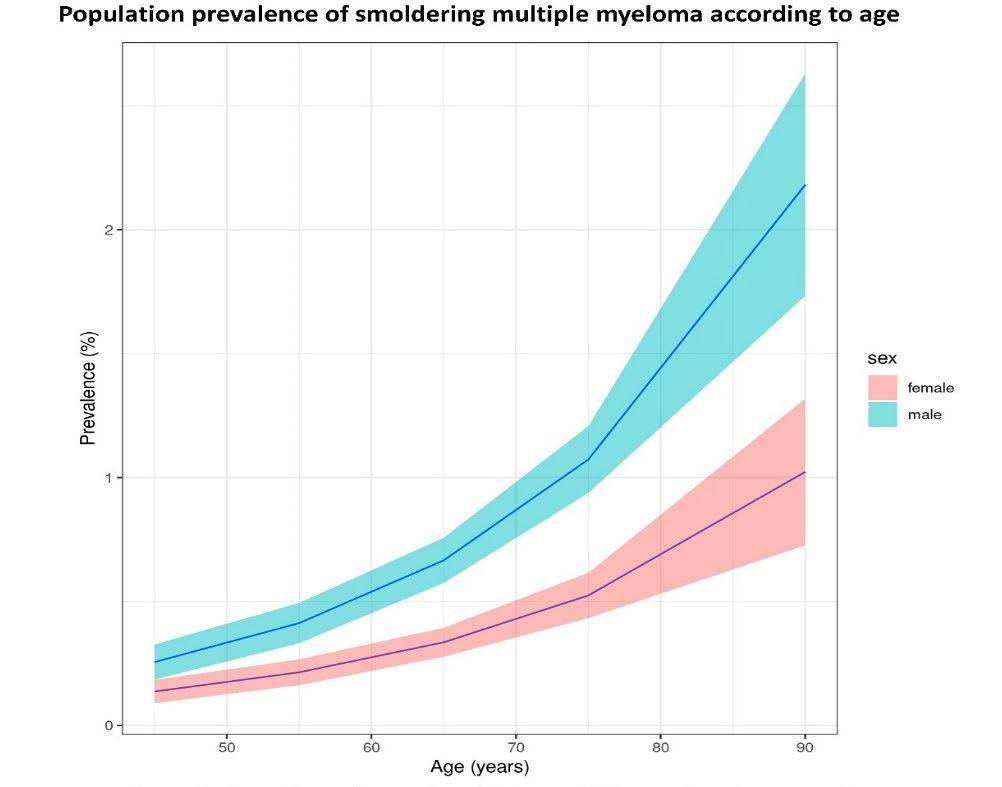
• Prevalence of MGUS in Iceland:
–
4.3%
• 3.6% of females
• 5.0% of males
– Increases with age
• 1.4% in age group 40-49 years
• 2.6% in age group 50-59 years
• 4.8% in age group 60-69 years
• 7.9% in age group 70-79 years
• 10.9% in age group 80-89 years
• 14.0% in age group 90-109 years
Prevalence of Smoldering Multiple Myeloma: Results from the iStopMM study
Sigrún Thorsteinsdóttir Íris Pétursdóttir1, Jón K Sigurðsson1, Guðrún Á Sigurðardóttir1, Ásdís R Þórðardóttir1, Brynjar Viðarsson4, Páll T Önundarson4, Bjarni A Agnarsson4, Margrét Sigurðardóttir4, Ingunn Þorsteinsdóttir4, Ísleifur Ólafsson4, Elías Eyþórsson4, Ásbjörn Jónsson4, Petros Kampanis5, Malin Hulcrantz6, Brian GM Durie7, Thorvardur J Löve1, Stephen Harding5, Ola Landgren8, Sigurður Y Kristinsson1,4
1. Faculty of Medicine, University of Iceland, Reykjavík, Iceland
2. Department of Hematology, Rigshospitalet, Copenhagen, Denmark
3. Public Health Sciences, University of Iceland, Reykjavík, Iceland
4. Landspítali University Hospital, Reykjavík Iceland
5. The Binding Site, Birmingham, West Midlands, UK.
6. Memorial Sloan Kettering Cancer Center, New York, NY, USA.

7. Cedar-Sinai Samual Oschin Cancer Center, Los Angeles, CA, USA
8. Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA






Monoclonal gammopathy of undetermined significance and COVID-19
A population-based study
Sæmundur Rögnvaldsson1,2#, Elías Eyþórsson2#, Sigrún Þorsteinsdóttir1,3, Brynjar Viðarsson2, Páll Torfi Önundarson1,2, Bjarni A
Agnarsson1,2, Margrét Sigurðardóttir2, Ingunn Þorsteinsdóttir2, Ísleifur Ólafsson1,2, Hrafnhildur L Runólfsdóttir2, Dadi Helgason2, Arna
R Emilsdóttir2, Arnar S Agustsson1,2, Aron H Bjornsson1,2, Guðrún Kristjansdottir2, Ásdís Rósa Þórðardóttir1, Ólafur Skúli Indriðason1,2, Ásbjörn Jónsson4, Gauti Kjartan Gíslason1, Andri Ólafsson1, Hlíf Steingrímsdóttir2, Petros Kampanis5, Malin Hultcrantz6, Brian GM
Durie7, Stephen Harding5, Ola Landgren8, Runólfur Pálsson1,2, Thorvarður Jon Love1,2 , Sigurður Yngvi Kristinsson1,2
1: Faculty of Medicine, University of Iceland, Reykjavik, Iceland; 2: Landspítali–The National University Hospital of Iceland, Reykjavik, Iceland; 3: Rigshospitalet, Copenhagen, Denmark; 4:
Akureyri Hospital, Akureyri, Iceland; 5: The Binding Site ltd., Birmingham, United Kingdom; 6: Memorial Sloan-Kettering Cancer Center, New York City, NY, USA; 7: Cedar-Sinai Samual
Oschin Cancer Center, Los Angeles, CA, USA; 8: Sylvester Cancer Center, University of Miami, Miami, FL, USA
#: Equal contribution (co-first author)


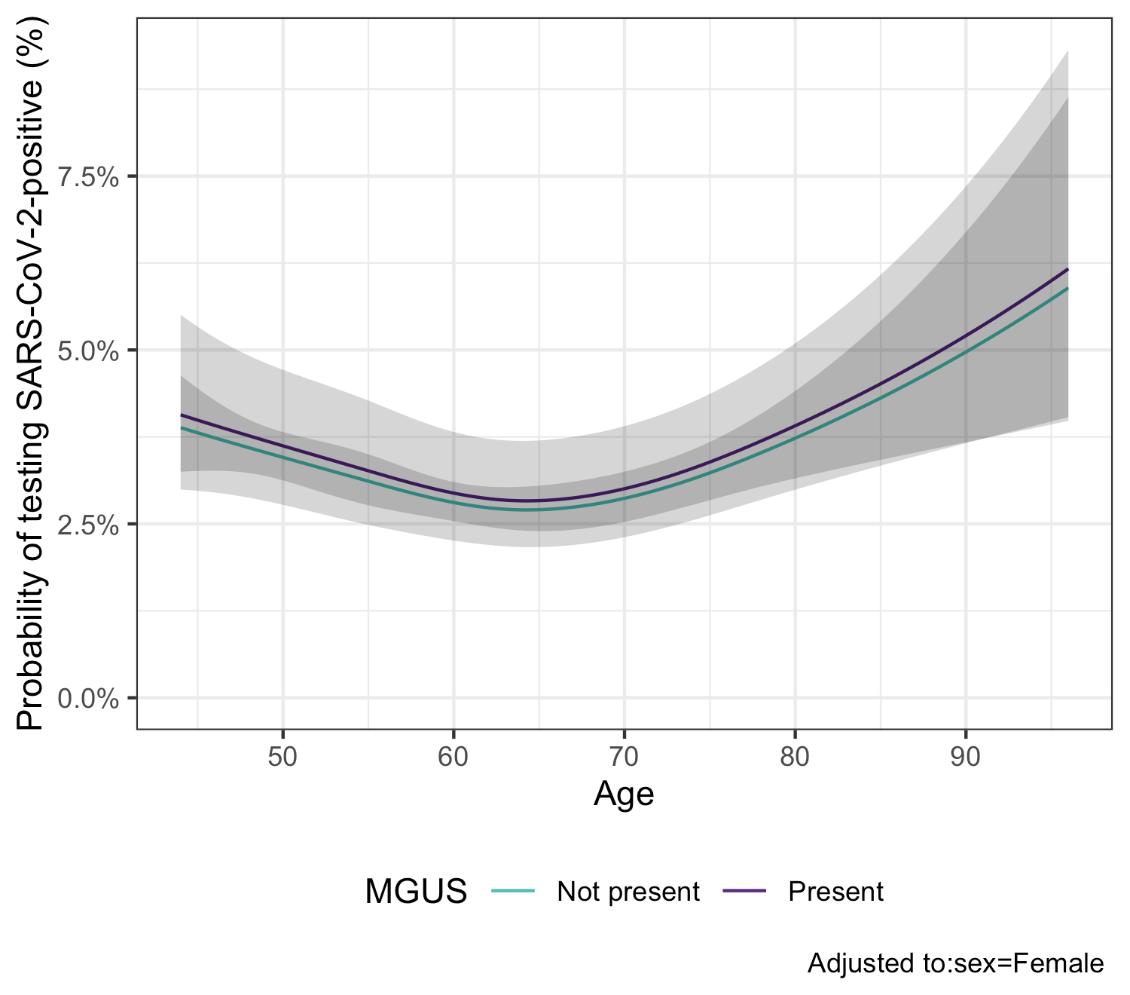

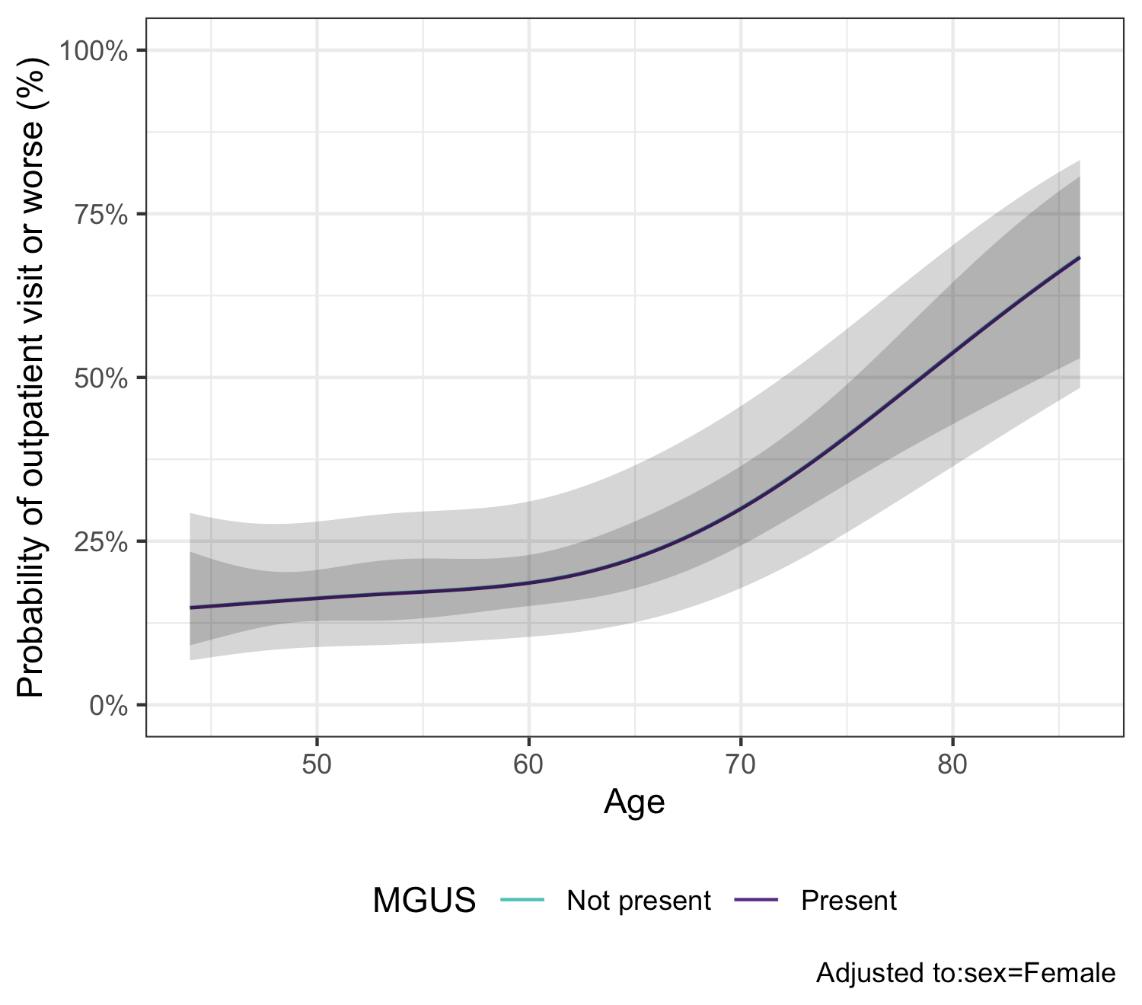
Conclusion
MGUS is not associated with contracting SARS-CoV-2 or increased severity of COVID-19 once infected

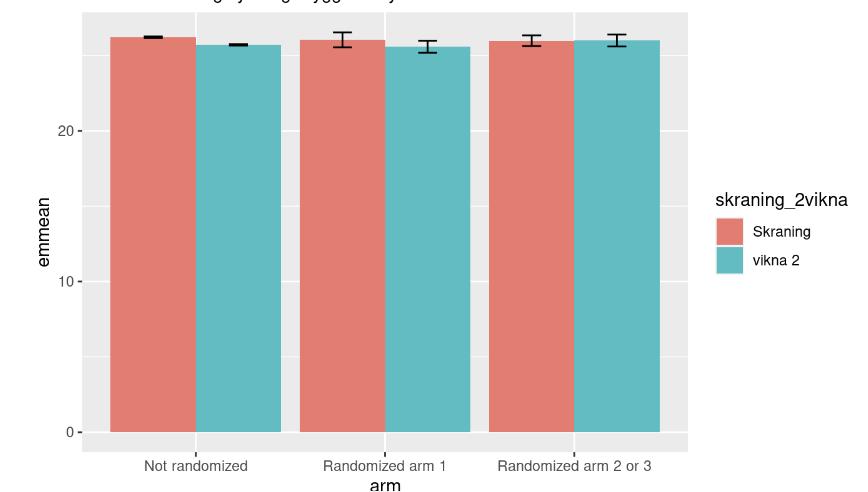
Covid-19 vaccinations and the effect on MGUS development

How the M-protein varies by time since vaccination
Contrasts of predictive margins with 95% CIs
The m-protein is constant over time since last vaccinated (keeping age, sex and year constant)

N=28















*FLC ratio >100 or M-protein >30 g/L
MGUS
• Arm 1
– Traditional health care
• Arms 2 and 3
–
Medical history and clinicial examination
– Blood work-up in all individuals
• Arm 3
–
Extensive blood work, bone-marrow, low-dose CT in all patients
– Annual follow-up
– More intense bone marrow evaluations


Conclusions
• In this large prospective population-based screening study, the iStopMM study, including >75,000 screened persons, we have identified 3,725 individuals with monoclonal gammopathy

• In the RCT, after 3 years of follow-up, we show that active screening identifies significantly higher number of individuals with full-blown malignancy and smoldering disease
• Our findings illustrate the fact that early detection and intervention is achievable
Population-based nationwide clinical trial with early intervention

Early intervention
• Early intervention offered to individuals with SMM –

KRd in high risk SMM –
Rd in intermediate risk SMM
• Balance of treating only those that need treatment
• So far no SMM patient has progressed to MM
• Following changes in incidence of MM
So, should we screen for MGUS?
• Although our findings are encouraging, until final results of the iStopMM study become available, including data on survival and quality of life, we advise against systematic MGUS screening in healthy individuals














The iStopMM team


Frontline Therapy Peter Voorhees, MD Levine Cancer Institute

Multiple Myeloma: Frontline Therapy
Peter Voorhees, M.D. Clinical Professor of MedicineChief, Plasma Cell Disorders Division
Department of Hematologic Oncology and Blood Disorders
Levine Cancer Institute, Atrium Health / Wake Forest School of Medicine
Outline
• The drugs we use to treat newly diagnosed myeloma
• How we choose best therapy for our patients with myeloma
• The evolving role of transplant in frontline therapy
• Treatment of patients who cannot or do not want an upfront transplant
• Treatment of patients who can and want an upfront transplant
• Maintenance therapy
• Conclusions
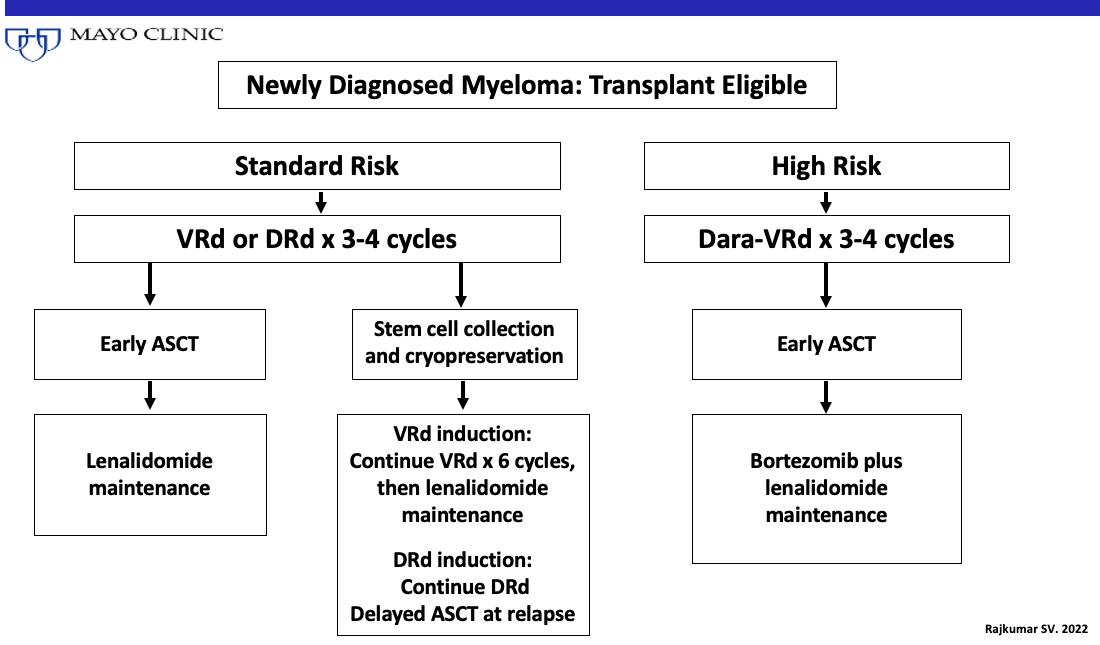
The Myeloma Treatment Paradigm
Transplant candidate
Induction therapy
Consolidation therapy
Maintenance therapy
Non-transplant candidate
Induction therapy
Maintenance therapy
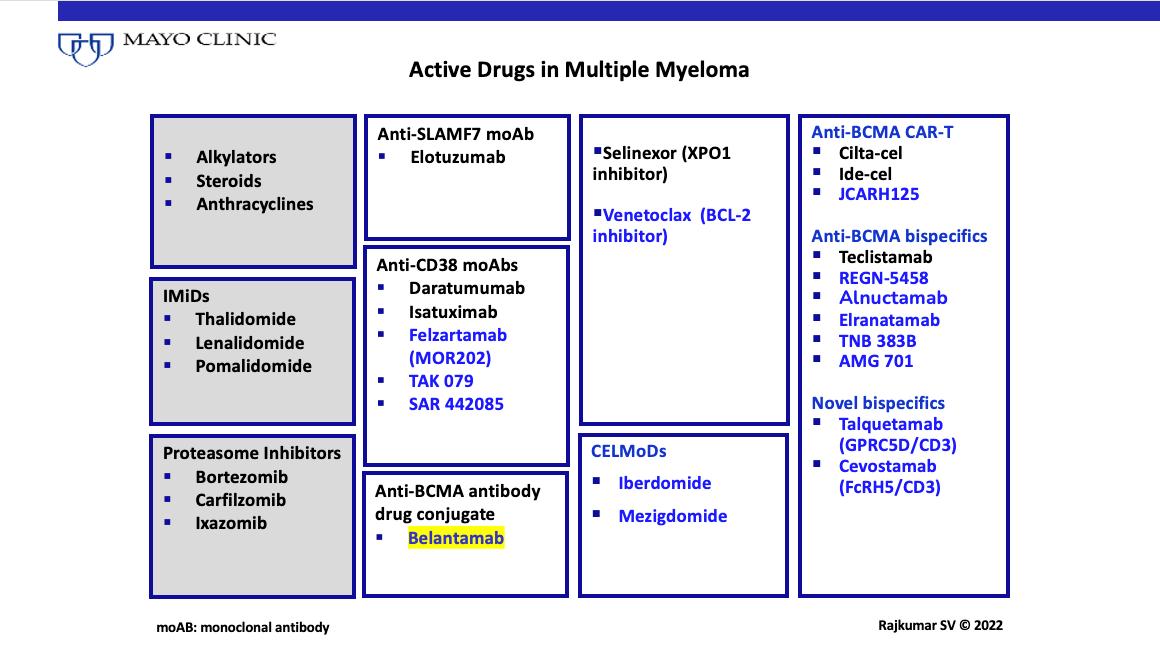
The Drugs
The Core Pillars of Myeloma Therapy
• The Immunomodulatory Drugs (IMiDs)
• Thalidomide, Lenalidomide (Revlimid), Pomalidomide (Pomalyst)
• The Proteasome inhibitors
• Bortezomib (Velcade), Carfilzomib (Kyprolis), Ixazomib (Ninlaro)
• The CD38 antibodies
• Daratumumab (Darzalex), Isatuximab (Sarclissa)
• Alkylating Agents
• Cyclophosphamide (Cytoxan), Melphalan (Transplant)
• Steroids
• Dexamethasone, prednisone
How to Choose
Factors to Consider in Treatment Choice
• Patient preference
• Shared decision-making model
• Transplant or No Transplant
• Other medical conditions
• Neuropathy, heart disease, kidney disease
• Standard or high-risk myeloma
• Logistical factors
• Distance from the infusion center
Treatment Attrition in Multiple Myeloma
• Real-world assessment of lines of therapy and outcomes based on 3 US insurance claims databases
Put your best foot forward
The Evolving Role of Transplant in Myeloma
Autologous Stem Cell Transplant / Rescue
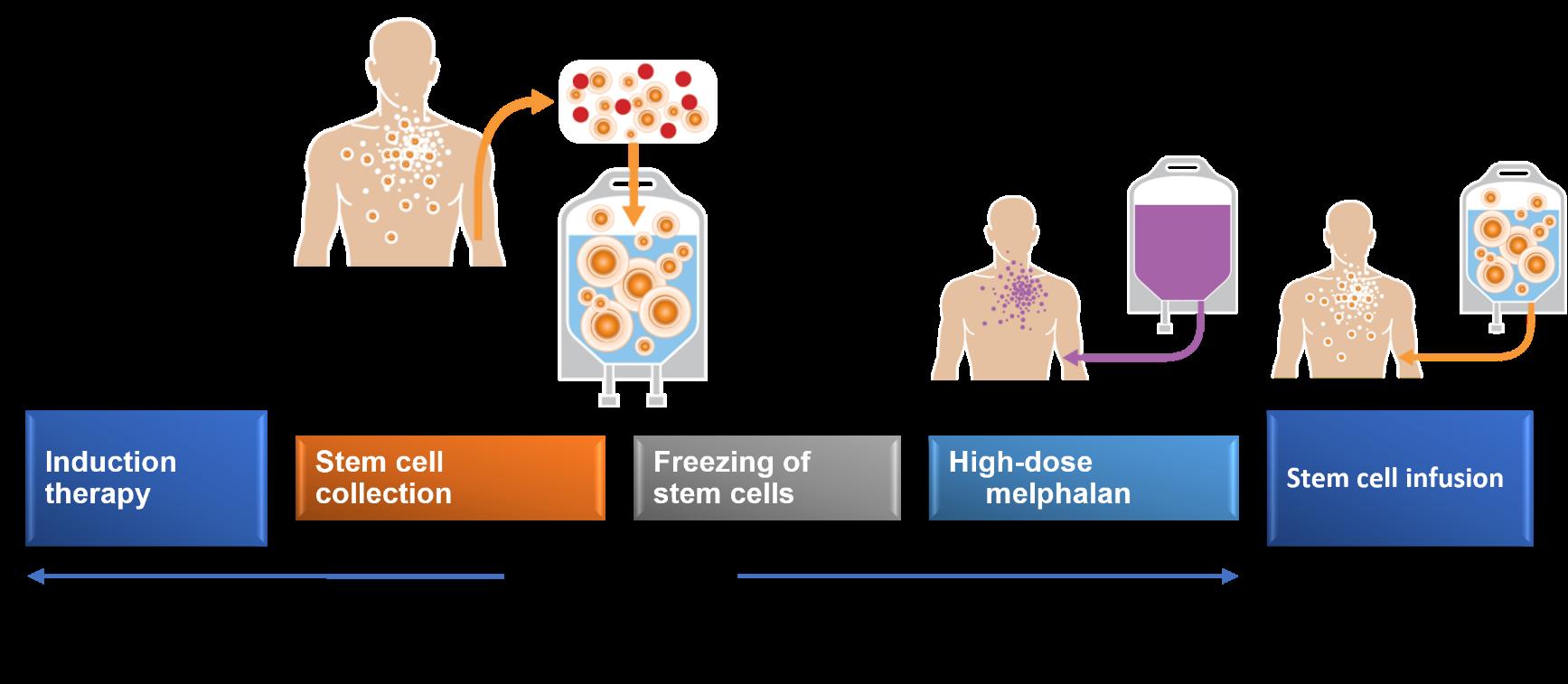
Transplant Outcomes by Age

• Retrospective CIBMTR analysis
• 15,999 patients
• 2092 ≥70 years old
OS for Pts Treated with MEL200
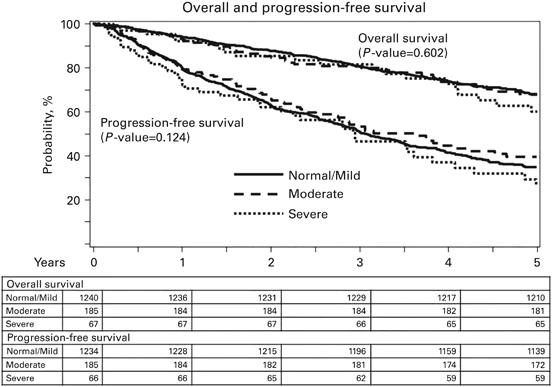
Transplant Outcomes by Kidney Function
• Retrospective CIBMTR analysis
• 1492 patients
• Normal kidney function: 1240 pts
• Moderately impaired kidney function: 185 pts
• Severely impaired kidney function: 67 pts
• Transplant-related mortality 0% in pts with moderate to severe renal impairment
• Kidney function not associated with inferior progression-free or overall survival
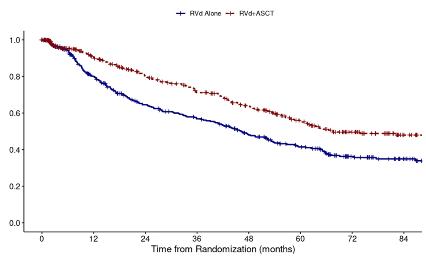
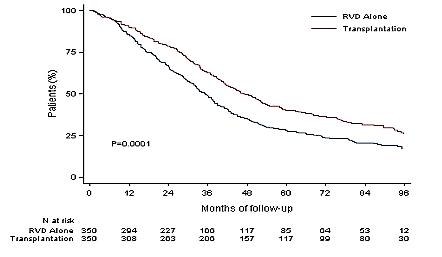
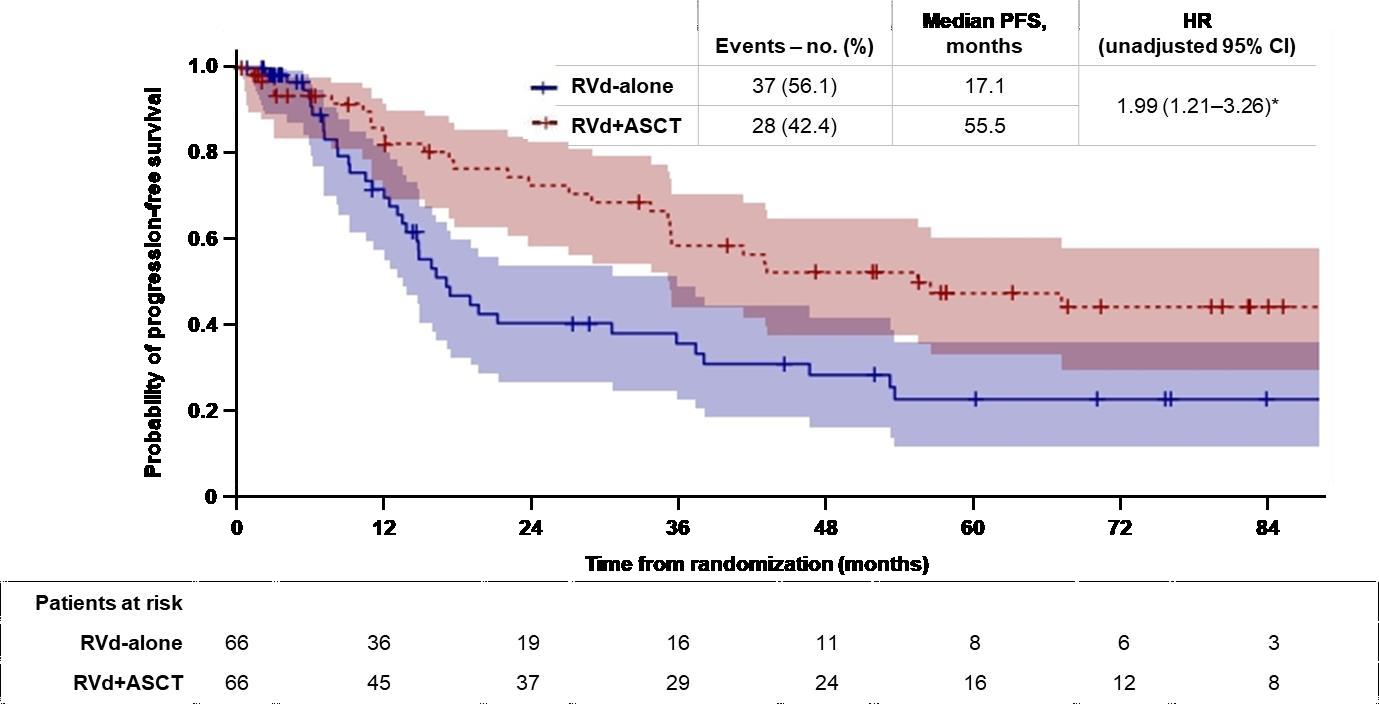
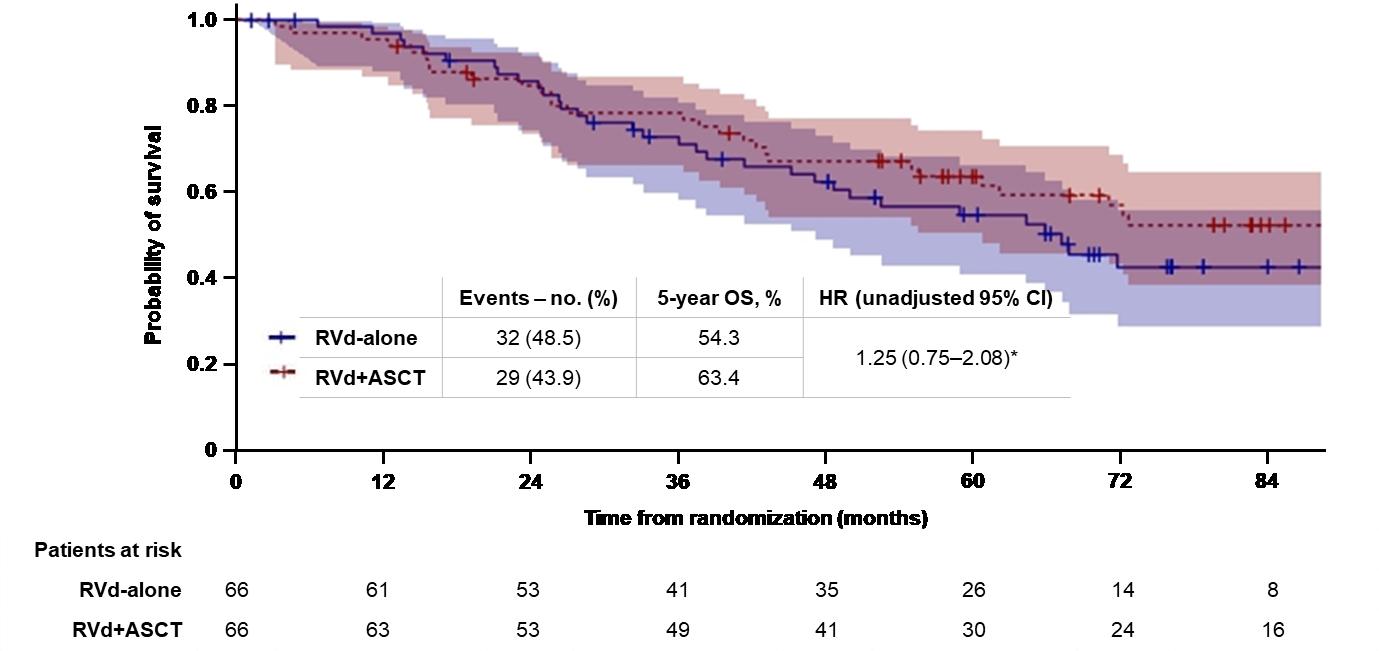
DETERMINATION: Phase III Study of Upfront vs Deferred
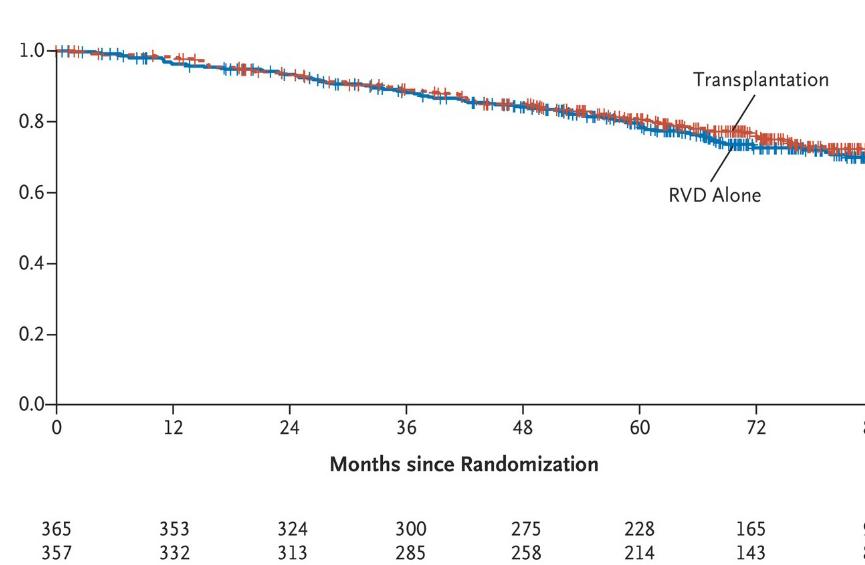
Transplant in Newly-Diagnosed Multiple Myeloma
• 3 cycles of RVd induction ASCT 2 cycles of RVd consolidation len maintenance vs 8 cycles of RVd induction len maintenance
Progression-Free Survival
Median PFS: 46.2 vs 67.5 months (HR 1.53, 95% CI

1.23 – 1.91, P < 0.001)
Overall Survival
5-Year OS: 79.2% vs 80.7% (HR 1.1, 95% CI 0.73 – 1.65, P >0.99)
RVd = Revlimid, velcade and dexamethasone
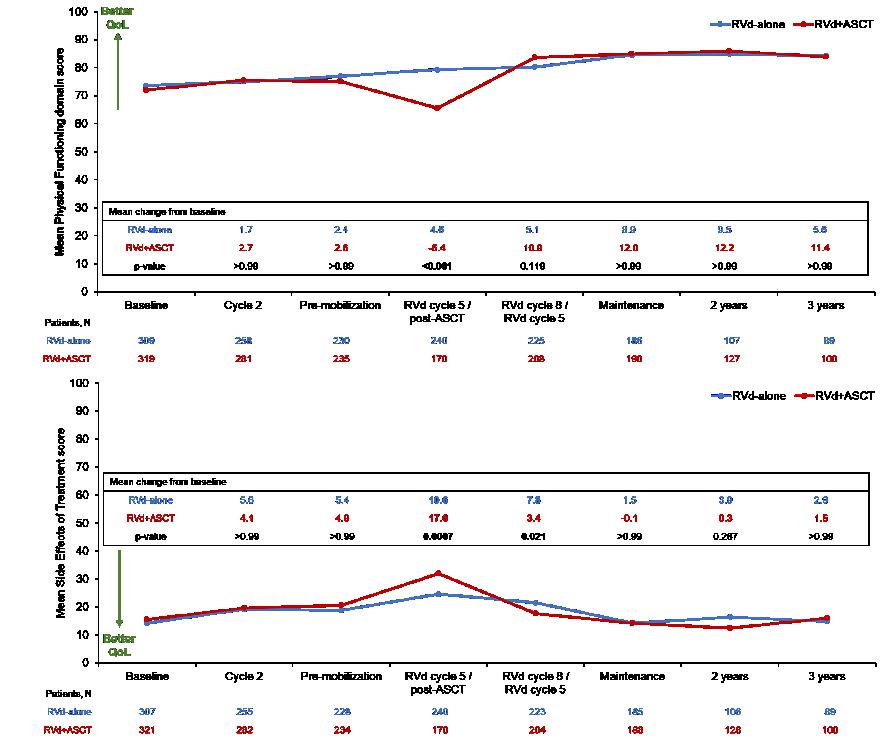
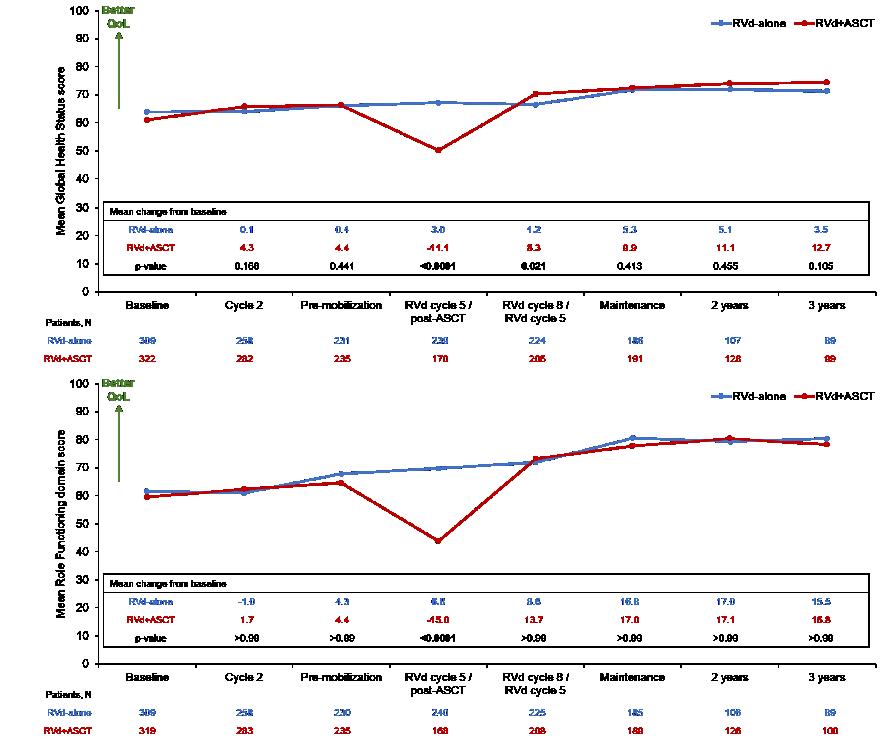
DETERMINATION: Quality of Life
(Baseline N >300 patients per arm)
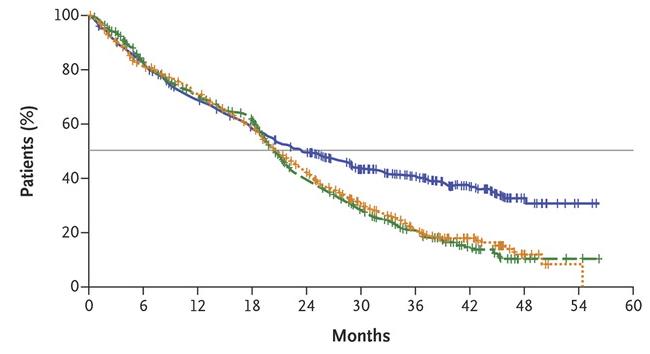
DETERMINATION: Outcomes by High-Risk Cytogenetics
Shaded areas indicate 95% CIs
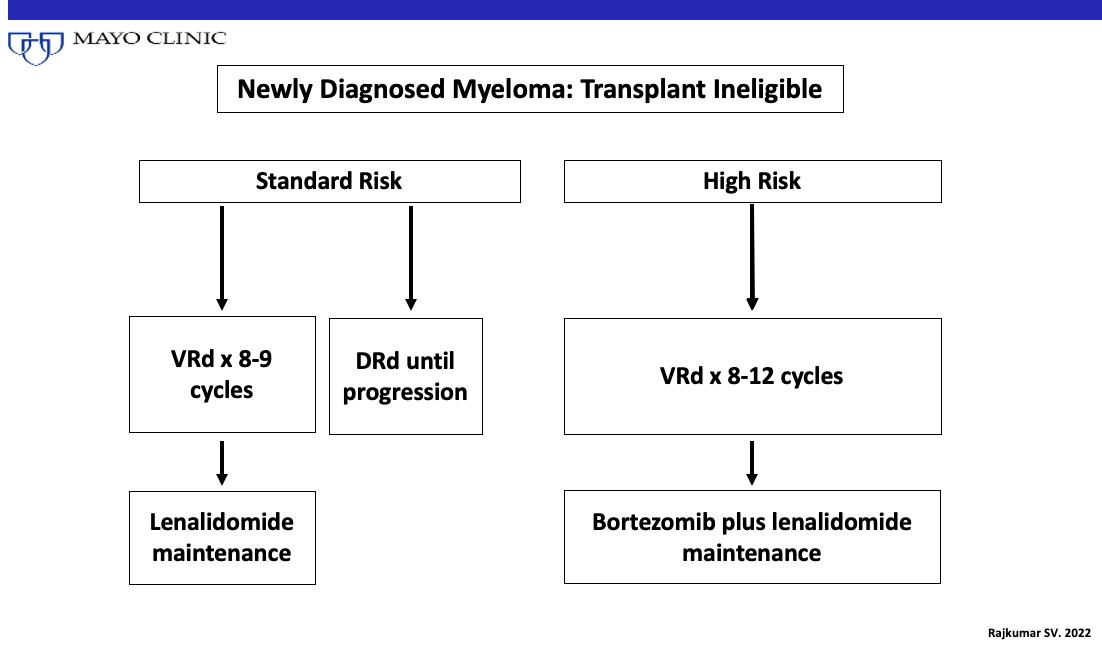
Treatment: Transplant Ineligible or Deferred
Lenalidomide (Revlimid) and Dexamethasone: The Backbone for
Newly-Diagnosed, Patients with Transplant-Ineligible Multiple Myeloma
Rd = Revlimid and dexamethasone; MPT = melphalan, thalidomide and dexamethasone
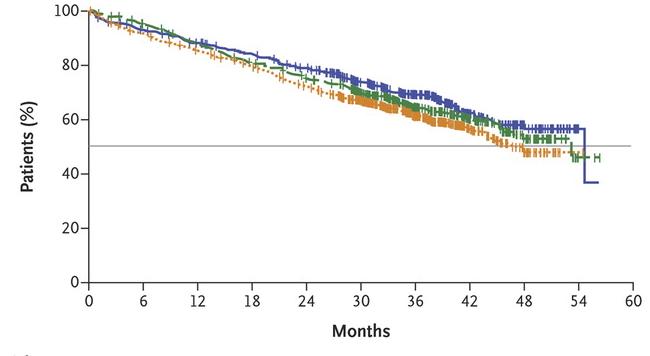
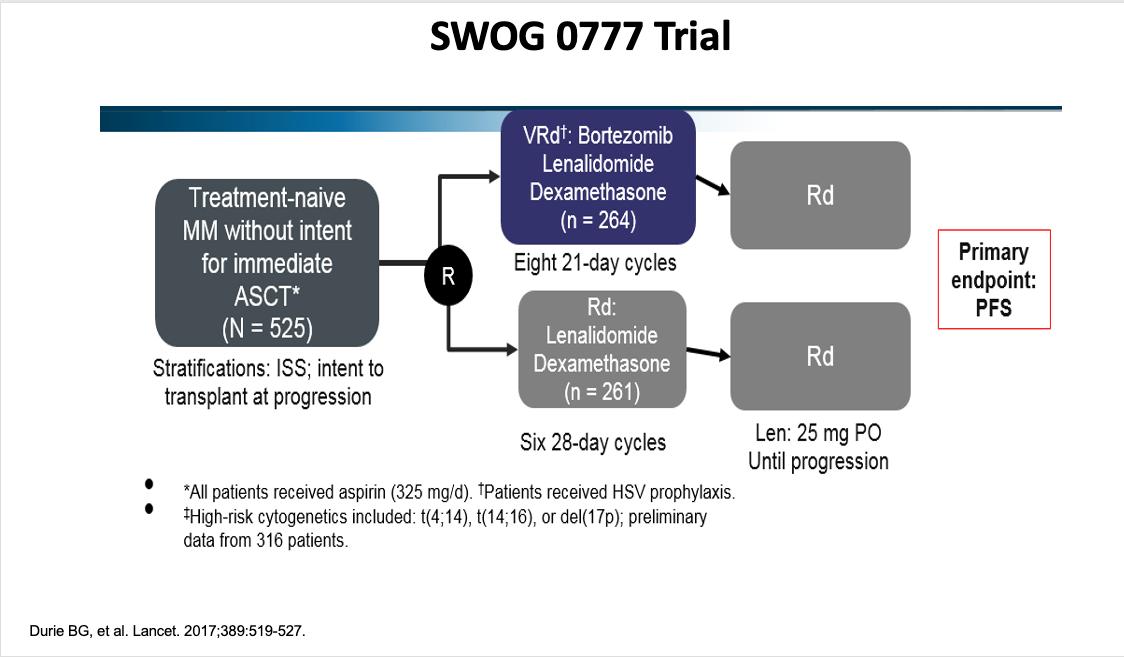
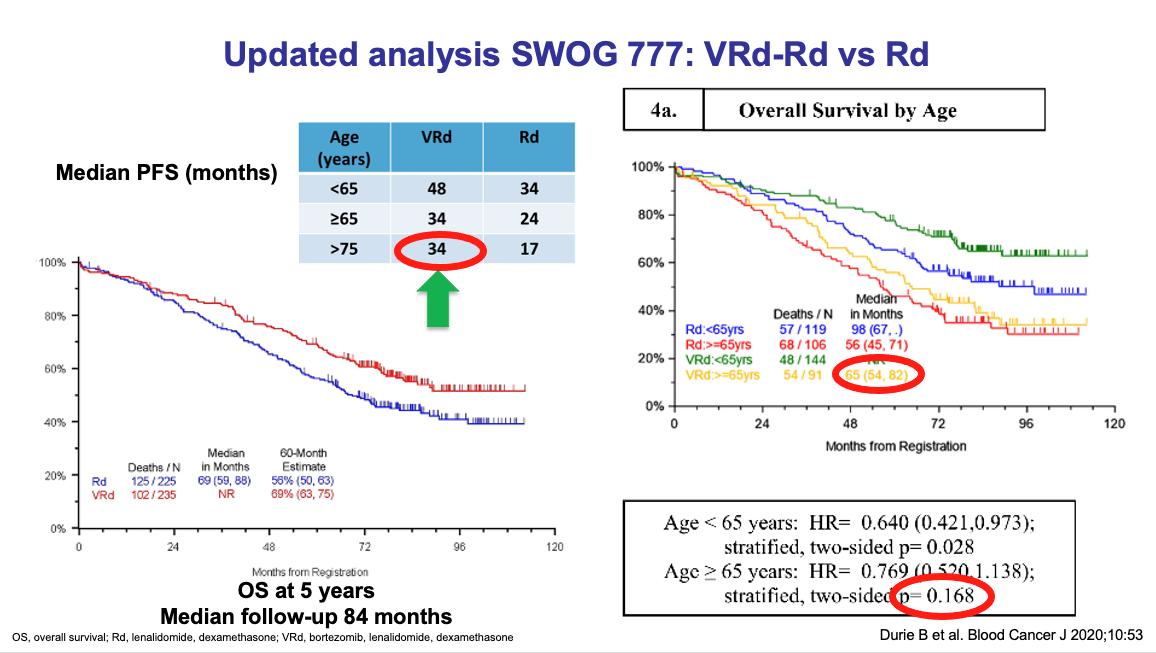
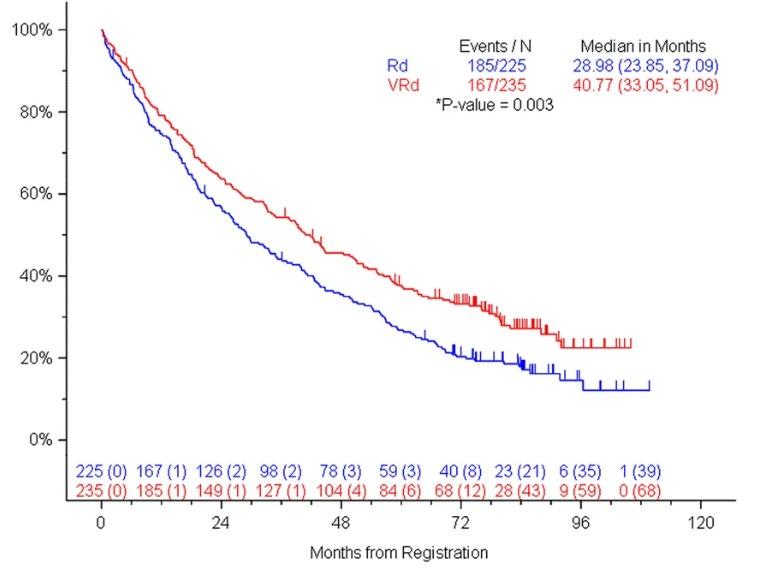
SWOG S0777: Adding Bortezomib (Velcade) to Revlimid and Dexamethasone
Randomized, phase III trial comparing bortezomib, lenalidomide and dexamethasone to lenalidomide and dexamethasone for patients with newly diagnosed myeloma not eligible for or declining upfront transplant
Median OS: 69 months vs Not Reached (>84 months, HR 0.709, 95% CI 0.543 – 0.926)
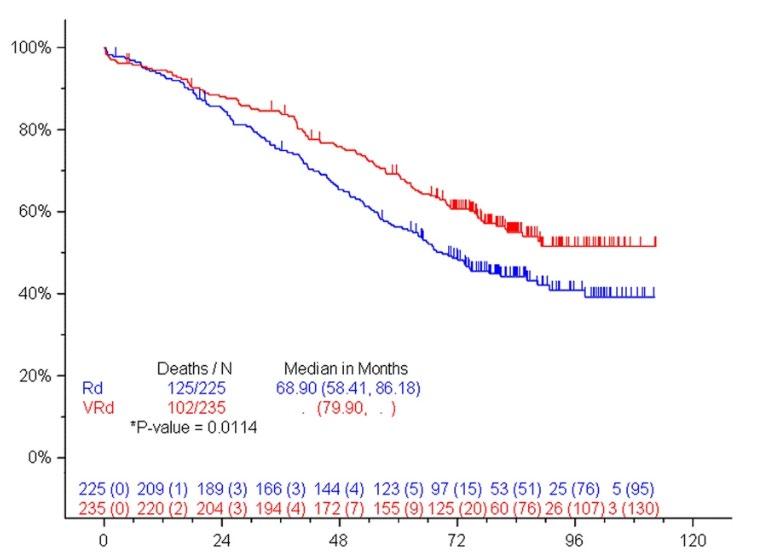
Median PFS: 29 vs 41 months (HR 0.742, 95% CI 0.594 – 0.928)
• 43% ≥65 years old, 57% <65 years old
• ≥Grade 3 Neurologic AEs: 33%
Rd = Revlimid and dexamethasone; VRd = Velcade, Revlimid and dexamethasone
Durie, B, et al. Lancet 2017;389:519-27.
Durie, B, et al. Blood Cancer J 2020;10:53.
RVD-Lite
Induction: Nine 5-Week Cycles •
Consolidation: Six 4-week cycles
•
15; Bort D1, 15
• Median Age 73 (65 –93) • ≥Grade 3 PN: 2%
35.1 mos
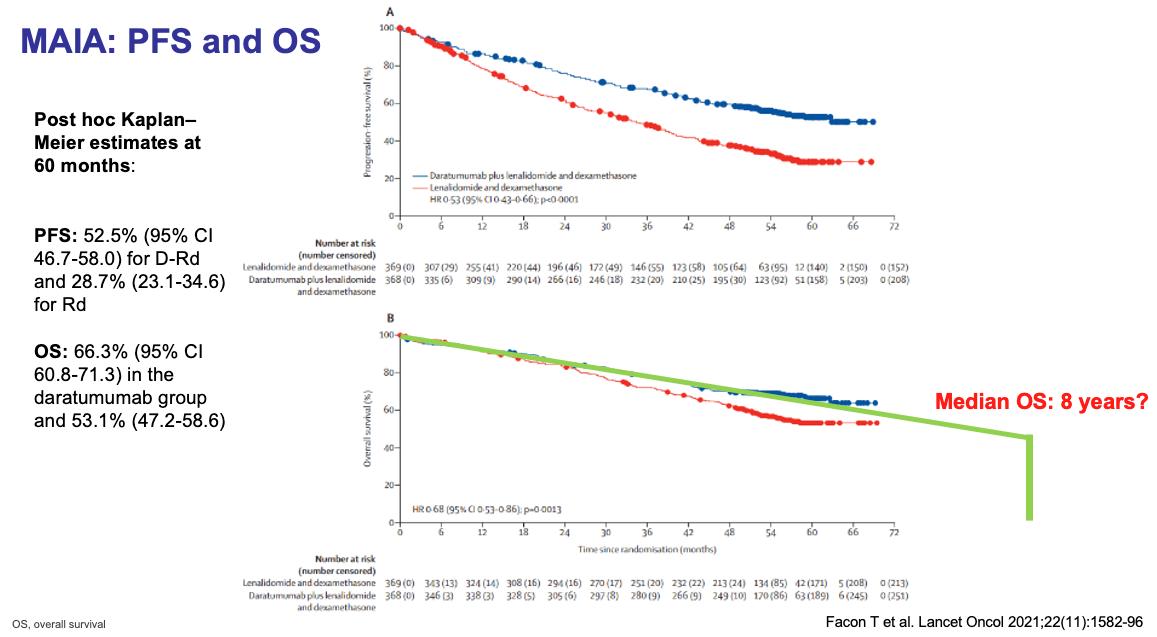
MAIA: Adding Daratumumab (Darzalex) to Revlimid and Dexamethasone
Multicenter, randomized (1:1), open-label, phase 3 study

DRd (n = 368)

Key Eligibility Criteria
• NDMM
• Transplant ineligible
• CrCl ≥30 mL / min
Stratification factors
• ISS stage
• Age < 75 vs ≥75
• Geographic location
28-day cycle
Daratumumab 16 mg/kg IV

• Days 1, 8, 15 and 22 in Cycles 1-2, days 1 and 15 Cycles 3-6, day 1 Cycle 7+ until PD
R 25 mg PO
• Days 1-21 of each cycle until PD
D 40 mg PO weekly until PD
Rd (n = 369)
28-day cycle
R 25 mg PO
• Days 1-21 of each cycle until PD
D 40 mg PO weekly until PD
Primary endpoint
• PFS
Secondary endpoints

• TTP
• ORR
• sCR/CR
• MRD
• OS
• PFS2
• Safety
MAIA: Demographic and Baseline Disease Characteristics
D-Rd, daratumumab/lenalidomide/dexamethasone; Rd, lenalidomide/dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; MM, multiple myeloma. a2 patients had an ECOG PS score >2 (1 patient each with an ECOG PS score of 3 and 4). bIncludes IgD, IgE, IgM, and biclonal.
•
MAIA: Updated Efficacy Results
Phase III Study of Lenalidomide and
Facon, T, et al. Lancet Oncol 2021;22:1582-96.
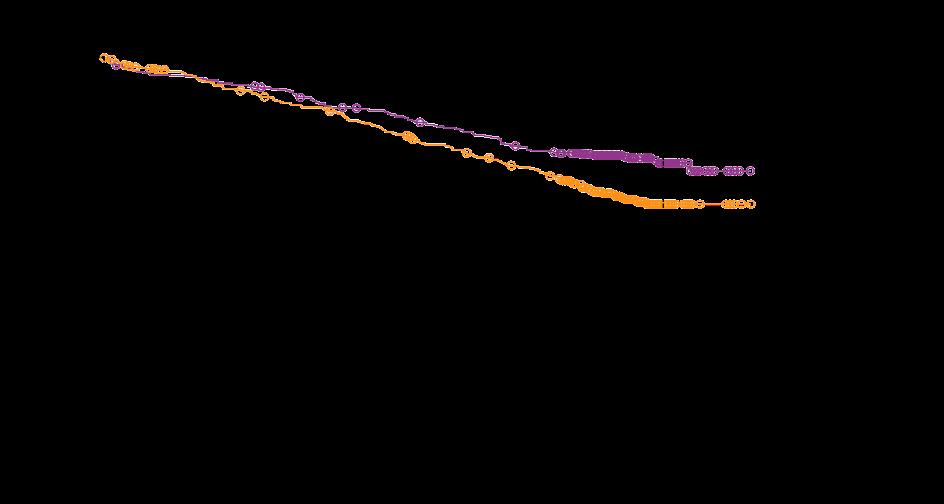
Facon, T, et al. NEJM 2019;380:2104-15.
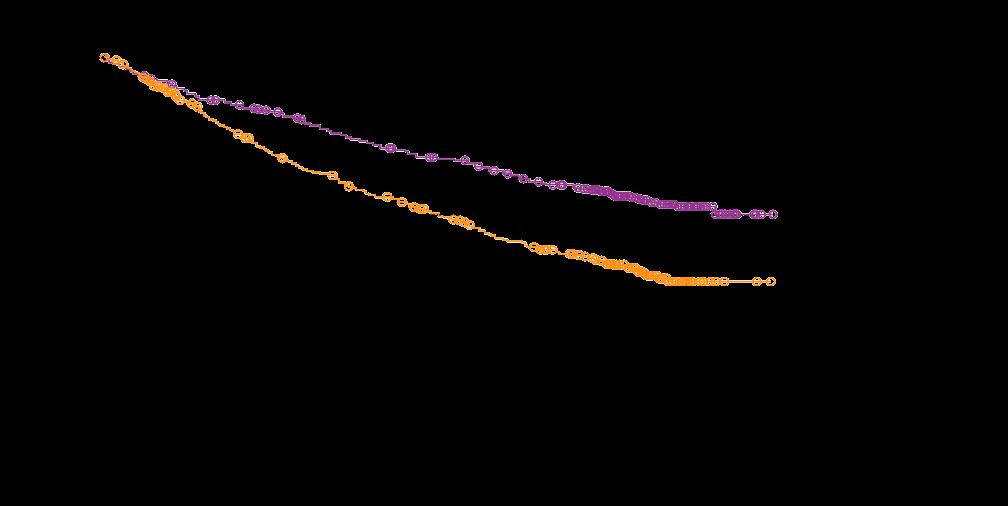
Progression-Free Survival






Treatment: Transplant Eligible
Dexamethasone for Transplant-Eligible Patients with Newly Diagnosed Myeloma
Randomized, phase III trial comparing bortezomib, thalidomide and dexamethasone to thalidomide and dexamethasone as induction therapy before and consolidation therapy after tandem autologous stem cell transplantation
CASSIOPEIA: Adding Darzalex to Velcade, Thalidomide and Dexamethasone for Patients Undergoing Upfront Transplant


Induction Four 28-day Cycles



Consolidation Two 28-day Cycles
Darzalex + Velcade, Thalidomide and Dexamethasone
Velcade, Thalidomide and Dexamethasone
Darzalex + Velcade, Thalidomide and Dexamethasone


Velcade, Thalidomide and Dexamethasone
every 8 weeks for up to 2 yrs


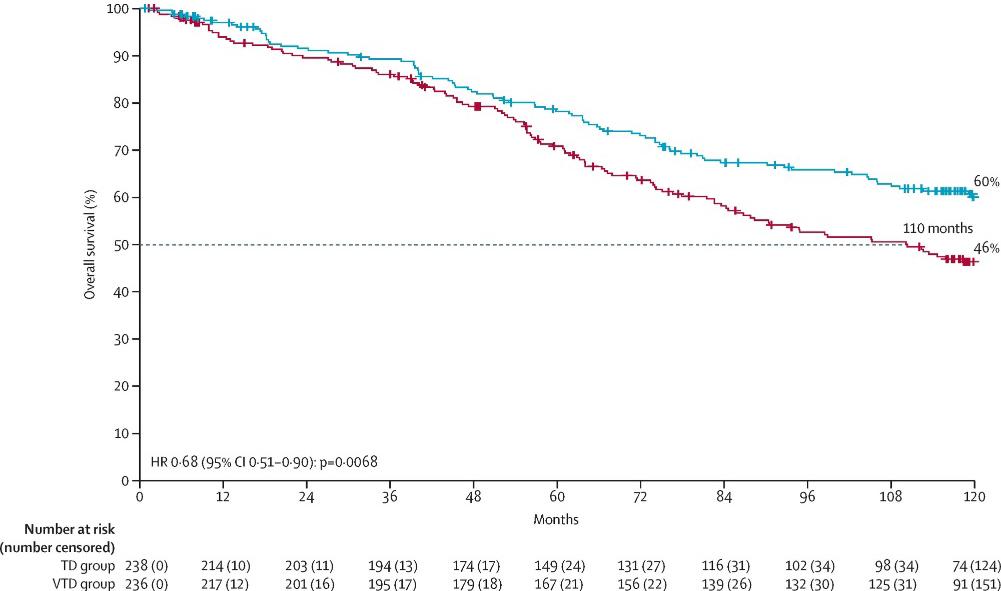
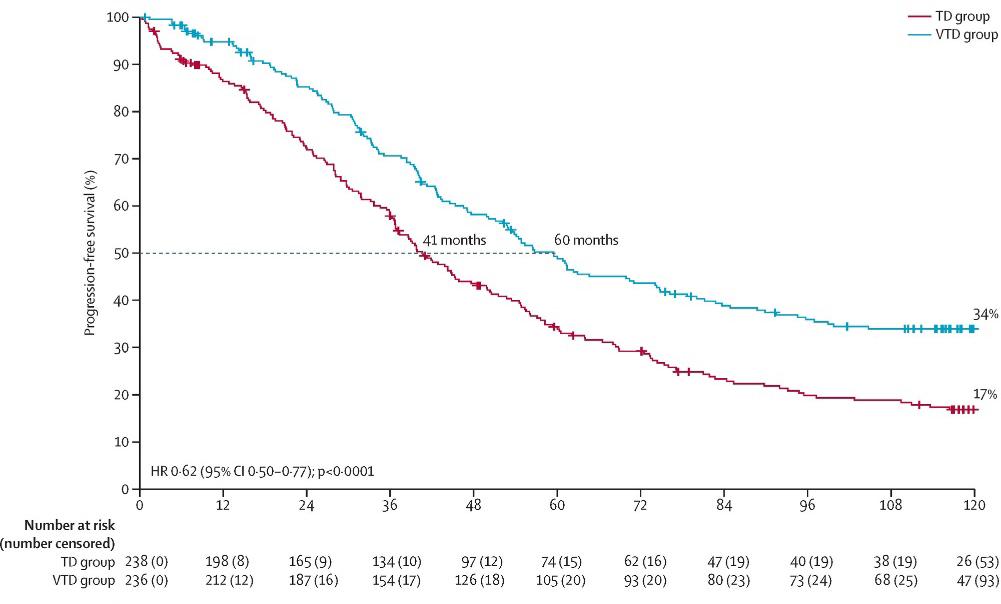
CASSIOPEIA: Progression-Free and Overall Survival
0.58 (95% CI 0.47–0.72)
Median follow-up: 44.5 months
PFS: not reached
HR 0.54 (95% CI 0.37–0.79) D-VTd: 41 deaths VTd: 73 deaths
Velcade and Dexamethasone for Patients with Newly Diagnosed Myeloma
Undergoing Upfront Transplant
Induction Four 21-day Cycles


Consolidation Two 21-day Cycles
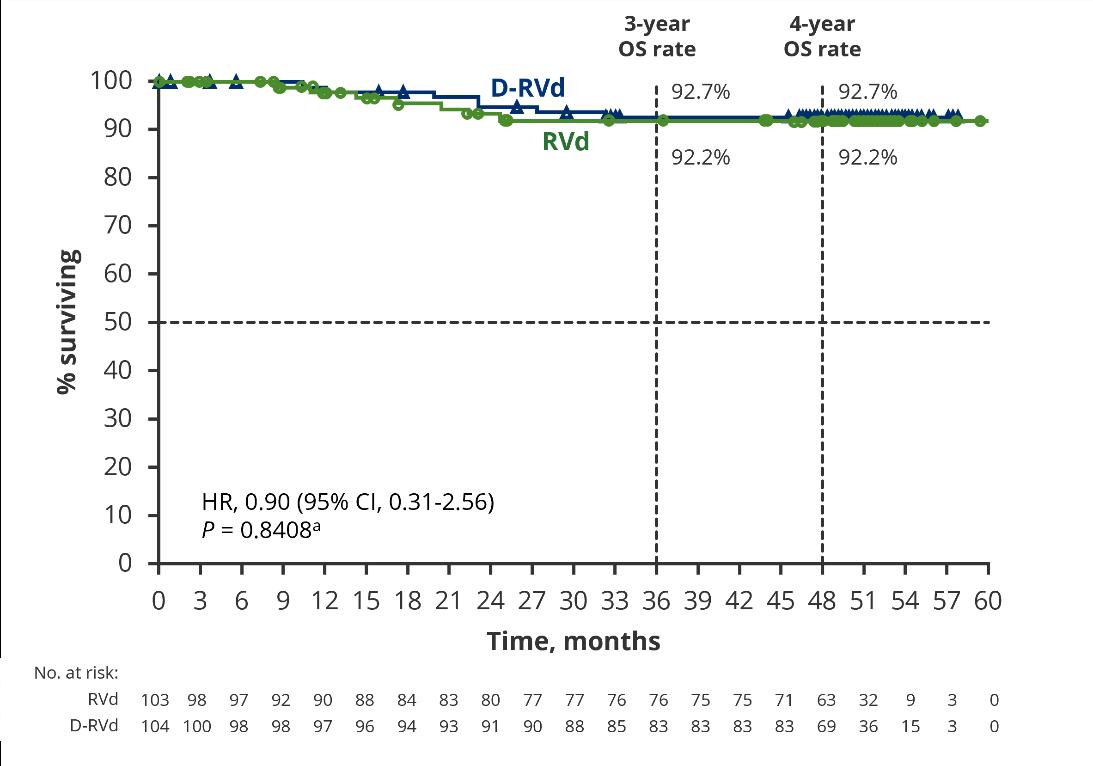
Maintenance


Darzalex + Velcade, Revlimid and Dexamethasone
Darzalex + Velcade, Revlimid and Dexamethasone




Velcade, Revlimid and Dexamethasone
Velcade, Revlimid and Dexamethasone
GRIFFIN: Baseline Disease Characteristics
ITT, intent-to-treat; ISS, International Staging System; MM, multiple myeloma. aECOG PS is scored on a scale from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability.
bThe ISS disease stage is based on the combination of serum β2-microglobulin and albumin levels. Higher stages indicate more advanced disease. cCytogenetic risk was assessed by fluorescence in situ hybridization (locally tested) among patients with available cytogenetic risk data; high risk was defined as the presence of del(17p), t(4;14), or t(14;16). Laubach, J
GRIFFIN: Longitudinal Outcomes

Patient Reported Outcomes: Quality of Life in GRIFFIN
Quality of life improvements seen in multiple domains for both groups but numerically favoring Dara-RVd, including global health score, physical functioning, EQ-5D-5L visual analog scale, EQ-
5D-5L utility score, disease symptoms, and future perspective
Maintenance Therapy
Thank you to our Sponsors!












Approaches to Relapse Panel Discussion
 led by Brian G.M. Durie, MD
International Myeloma Foundation
led by Brian G.M. Durie, MD
International Myeloma Foundation
The Approach to Relapse Panel Discussion
Saturday, March 18, 2023—Boca Raton, FL
Beth Faiman, PhD, MSN, APN-BC, AOCN®, BMCTN®, FAAN, FAPO


IMF Nurse Leadership Board

Cleveland Clinic
Taussig Cancer Institute
Cleveland, OH
Nikhil Munshi, MD

Harvard Medical School
Dana-Farber Cancer Institute
Boston, MA
Peter Voorhees, MD

Atrium Health
Levine Cancer Institute
Charlotte, NC
Therapy Options for RRMM Patients





First Relapse



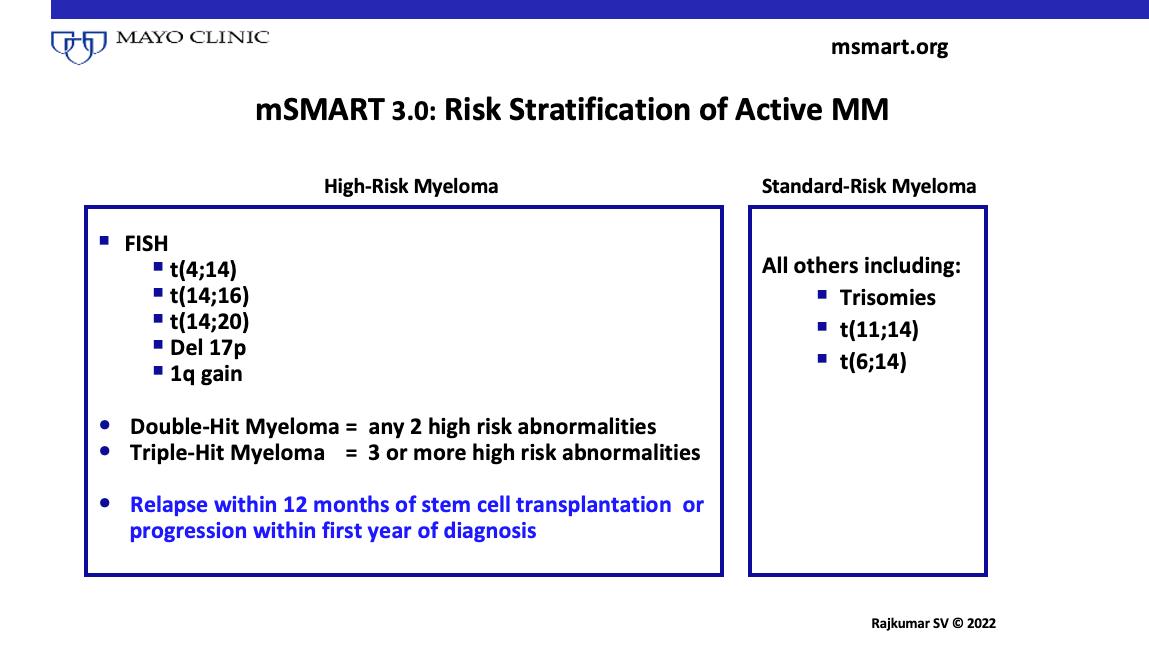
Risk Stratification of Active MM


Myeloma Treatment Paradigm

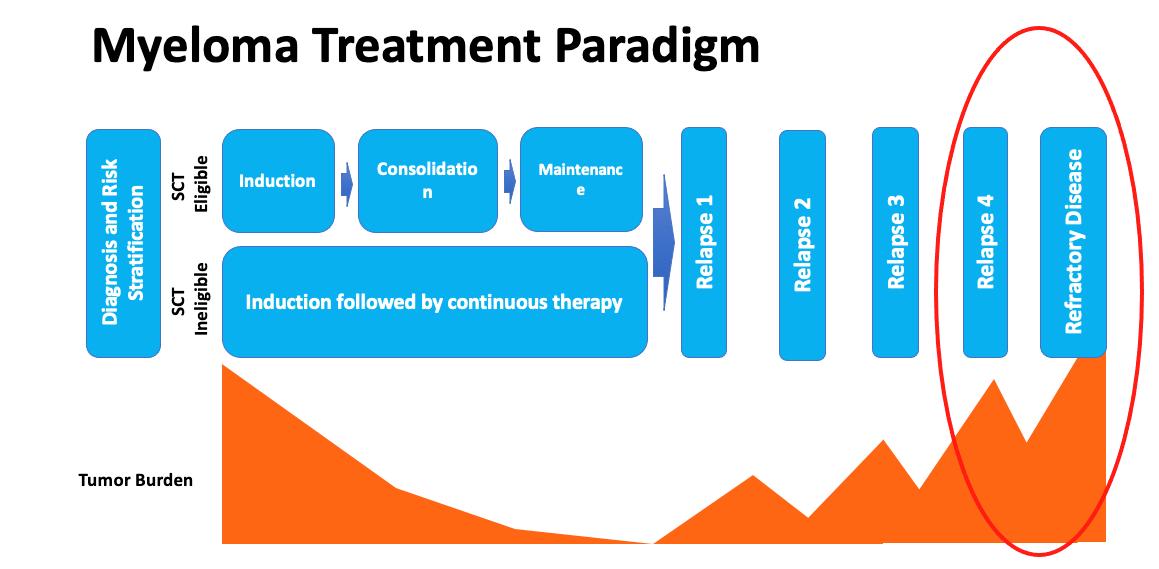

Active Drugs in MM



What Are The Options?



MM: Second or Higher Relapse

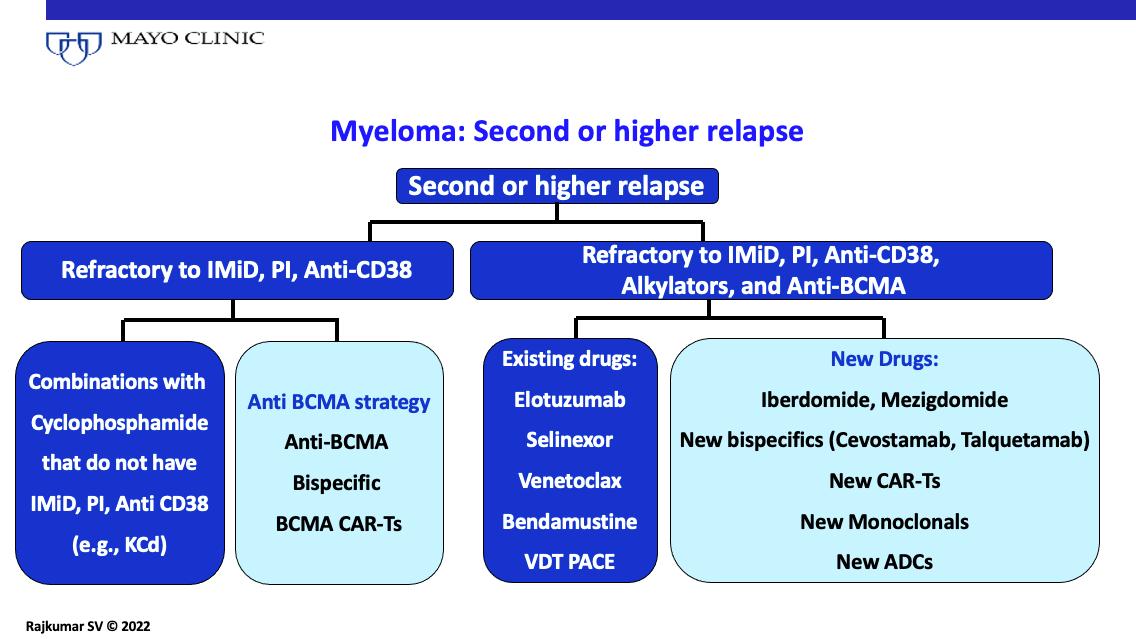

Therapeutic advances have led to prolonged survival in MM, but it remains a chronic disease
Newer drugs increase the options available
Treatment of myeloma requires a long-term strategy


Key is delivering the best “package” of treatment at a given stage
Optimal combinations and sequencing are key
Risk-stratified approach in clinic
Future will be developing more individualized approaches


Immune Therapies Nikhil Munshi, MD
Dana-Farber Cancer Institute

Immunotherapy of multiple myeloma
Nikhil C. Munshi, MDProfessor of Medicine
Harvard Medical School

Kraft Family Chair
Director, Basic and Correlative Science
Jerome Lipper Multiple Myeloma Center
Dana-Farber Cancer Institute

Boston VA Healthcare System

Disclosures
Advisory Board/Consultant: Adaptive, AbbVie, Amgen, BMS, Celgene, DCT, Janssen, Karyopharm, Legend, Novartis, Oncopep, Takeda
Scientific Founder: Oncopep, DCT
Immune-therapies under investigation in Hematological Malignancies
Immunotherapy1,2
Active (Designed to act on the immune system itself)
I-O therapies
Therapeutic cancer vaccines
• Immune effector cell modulators
• Checkpoint Inhibitors
• Co-stimulatory agonists
Unspecific
• Cell-based
• DC-based cancer vaccines
• Single antigen/ peptide-based
• Cytokines
• Interleukins
• Interferons
• IMiDs
Passive (Designed to act on the tumor)
Antitumor mAbs Adoptive
• Tumordirected mAbs
• Ab-drug Conjugates (ADC)
• Cell therapies
• Adoptive T-cell therapy
DC, dendritic cell; IMiD, immunomodulatory agent; I-O, immuno-oncology; mAb, monoclonal antibody. 1. Finn OJ. Ann Oncol. 2012;23(suppl 8 ):viii6-viii9.
Mellman I et al. Nature. 2011;480:480-489.
What Immune-based Therapies Are Available
• Immunomodulatory agents – Thalidomide, Lenalidomide and Pomalidomide – iberdomide, CC92480
• Antibodies – Daratumumab, Elotuzumab, Isatuximab
• Checkpoint inhibitors
• Antibody drug conjugates - Belantemab
• CAR-T cell therapies – Ide-cel, Cilta-cel. Anti-GPRC5D
• BiTES – anti-BCMA, Anti-GPRC5D, anti-FCRL-5 •
Chimeric Antigen Receptor




T cells (CAR T Cells)
• Exploit native antibody or T cell recognition and signaling pathways
• Introduction of unique genes through viral vectors to allow recognition of tumor cells
• Targeting – A unique tumor specific antigen
• Effective tumor cell killing
• Dramatic expansion after infusion

Image courtesy of Stephan Grupp, UPenn
Tumor-specific BCMA CAR construct
T cell Native TCR Tumor cell BCMA CAR-T cell Dead tumor cellCAR T Production

Promising MM antigens for current targeted immunotherapies
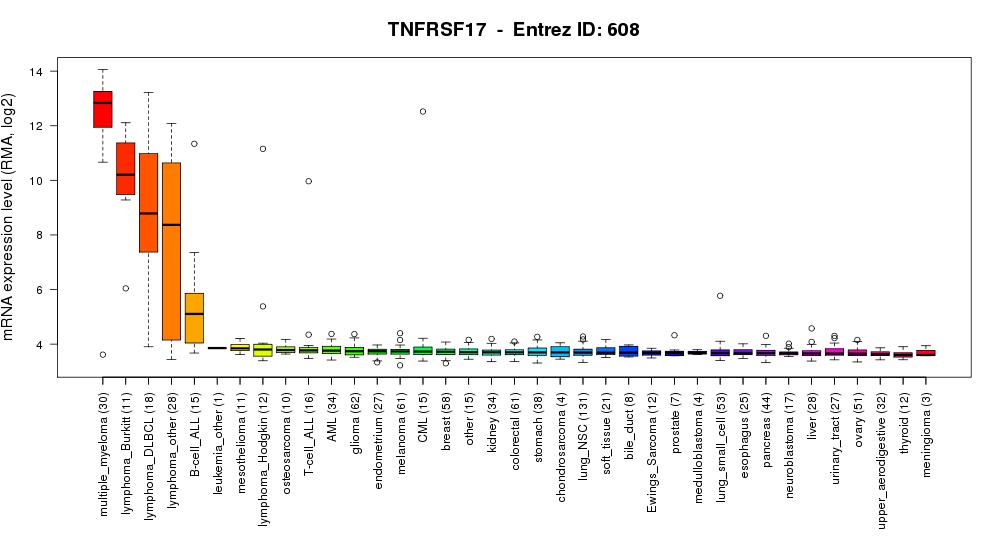
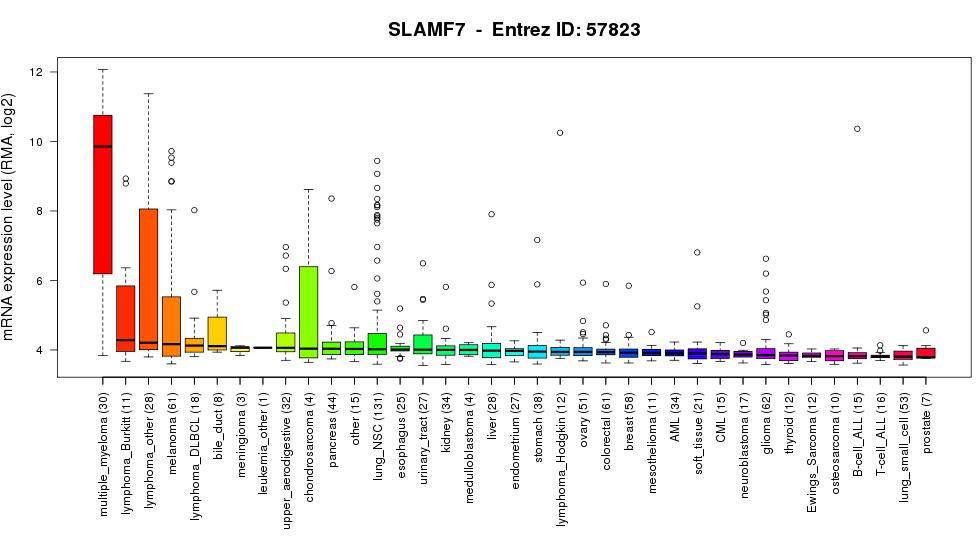
BCMA SLAMF7 1 3 6
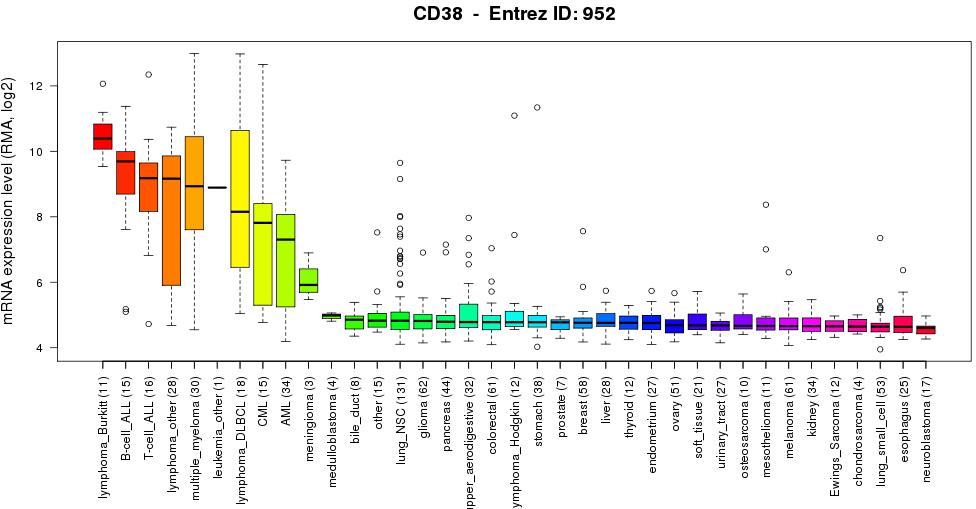
CD38
Phase 2 KarMMa Study: Ide-cel in Relapsed Refractory Multiple Myeloma
Ide-cel efficacy: longer follow-up (median 24.8 months)
Patients treated at 450 × 106 cells target dose had an ORR of 81% and a CR/sCR of 39%
• Median time to first response of 1.0 month (range 0.5−8.8); median time to CR
(range 1.0−15.8)
• Median follow-up of 24.8 months (range 1.7−33.6) across target dose levels
• Of those patients with a complete response or better, 79% were MRD negative
KarMMa: Robust PFS and OS Outcomes With Ide-Cel
PFS: Overall and According to Target Dose1
OS by Number of Prior Lines of Therapy and in All Ide-Cel–Treated Patients2
• PFS increased by depth of response; median PFS was 20 months in patients with CR/sCR
• ASH 2022: patients achieving early and sustained undetectable MRD after ide-cel had prolonged PFS3 (Oral abstract Monday, December 12, 2022, 3:30 PM)
Ciltacabtagene Autoleucel in RRMM (CARTITUDE)1
Binding Domains
Cilta-cel structure: two
BCMA-targeting domains
designed to confer avidity plus a 4-1BB costimulatory domain
CARTITUDE-1: tested cilta-cel in patients with PS ≤1 and receiving ≥3 prior therapies, including a PI, an IMiD, and an anti-CD38 therapy, or doublerefractory to PIs and IMiDs
JNJ-4528
LCAR-B38M
JNJ-68284528
Ciltacabtagene autoleucel
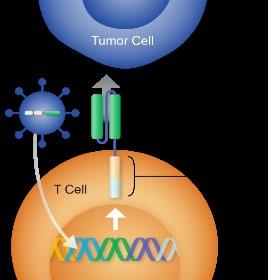
Median time to first response: 1 month (0.9–8.5)



Responses ongoing in 70 (72.2%) patients
Of evaluable patients, 93.0% achieved MRD 10-5 negativity
Median time to MRD 10-5 negativity: 1 month (0.8–7.7)
Among patients with 6 months individual follow-up, most had cilta-cel CAR+ T cells below the level of quantification (2 cells/µL) in peripheral blood
CAR, chimeric antigen receptor; CR, complete response; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response. PR or better, Independent Review Committee assessed. No patient had CR or stable disease as best response. cMRD was assessed in evaluable samples at 10-5 threshold by next-generation sequencing (clonoSEQ, Adaptive Biotechnologies) in all treated patients at Day 28, and at 6, 12, 18, and 24 months regardless of the status of disease measured in blood or urine; patients were not evaluable primarily due to lack of an identifiable clone in the baseline bone marrow sample. dAll treated patients.
Cilta-cel Achieves Long Duration of Response in RRMM
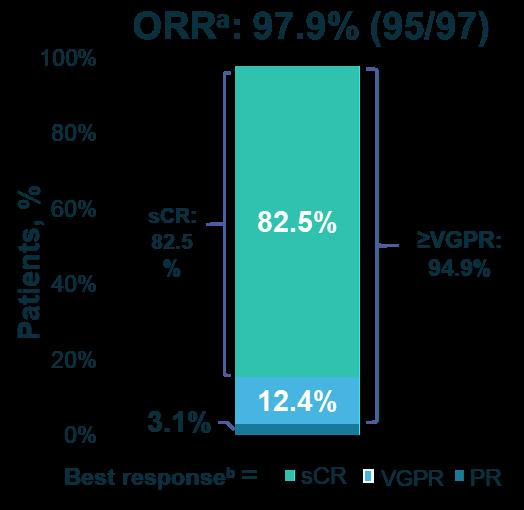
Cilta-cel is associated with sustained MRD negativity and better long-term outcomes for a broad range of patients with specific characteristics
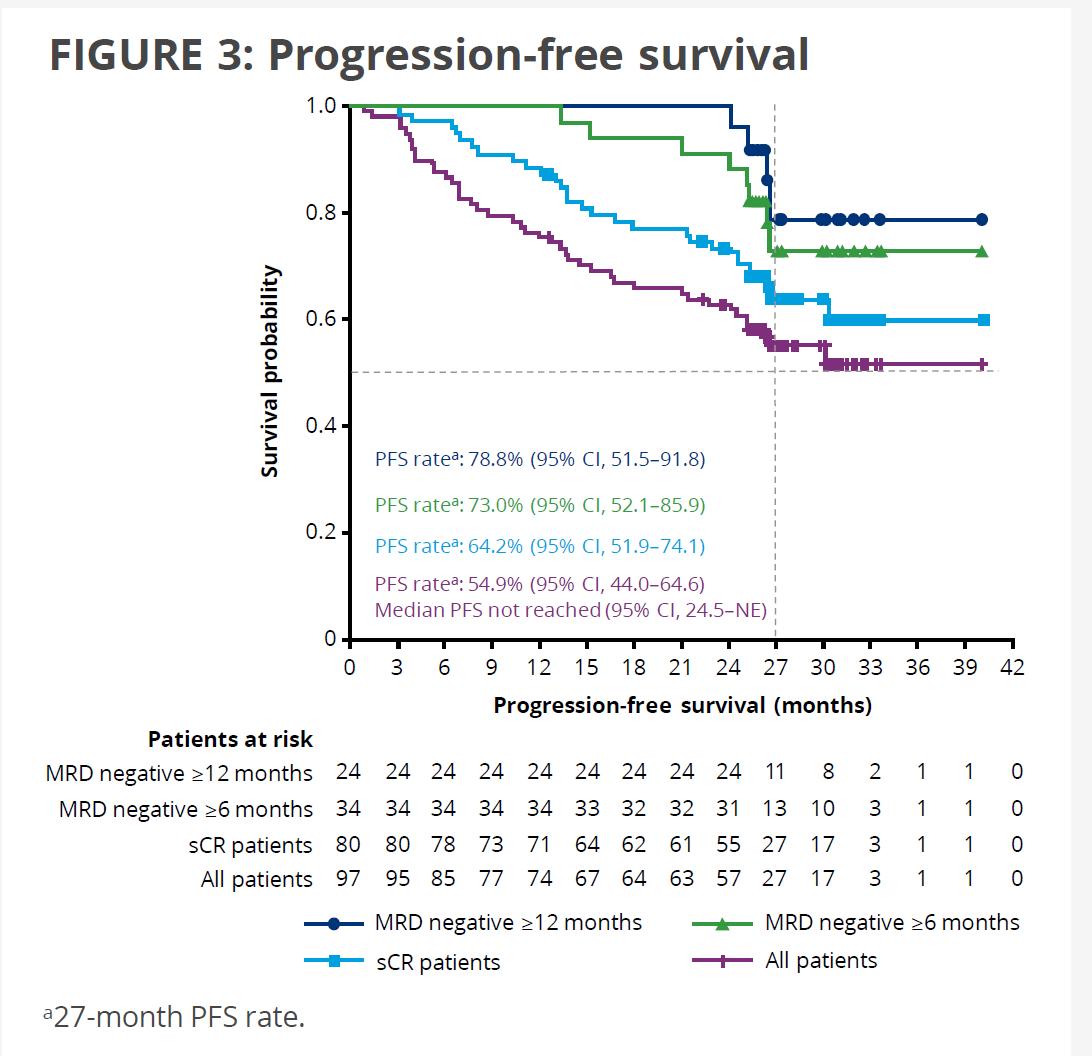
27-month PFS 54.9% (95% CI, 44.0-64.6)
Median PFS Not reached (95% CI, 24.5–NE)
27-Month OS 70.4% (95% CI, 60.1–78.6)
Median OS Not reached (95% CI, 27.2 months–NE)
27-mo PFS – MRD- >12 months 78.8% (95% CI, 51.5 – 91.8)
27-Mo OS – MRD- >12 months 90.8% (95% CI, 67.7-97.6)
ASCO 2022 UPDATEKarMMa subgroup analysis: Ide-cel yielded high response rates in most subgroups, including high-risk patients
• ORR was ≥65% and CR rate was ≥20% across all high-risk subgroups except R-ISS disease stage III
• Presence of extramedullary disease and baseline tumor burden did not substantially affect ORR
Data cutoff date: 14 Jan 2020.
CR, complete response; ORR, overall response rate; PR, partial response; R-ISS, revised International Staging System; sCR, stringent complete response; VGPR, very good partial response. aSum of CR/sCR, VGPR, and PR rates may differ from the ORR rate due to rounding. Raje N, et al. Presented at 62nd ASH Meeting 2020. Abstract 3234.
• Median time to first response was 1.0 month in both elderly groups and in the overall treated populationb
• Median duration of response was consistent across age groups, ranging from 10.7 to 11.0 months b
• Ide-cel yielded high response rates in most subgroups, including high-risk patients2
• ORR was ≥65% and CR rate was ≥20% across all high-risk subgroups except R-ISS disease stage III2
Ide-cel is currently approved by the FDA. The safety and efficacy of ide-cel is still under investigation by other regulatory authorities. Data cut-off date: 14 January 2020. aValues may not add up to total due to rounding; bTime to first response and duration of response were assessed in responders: n = 38 for ≥ 65 years group, n = 18 for ≥ 70 years group, and n = 94 for overall ide-cel treated population. cCRS graded according to Lee criteria (Lee DW, et al., Blood 2014;124:188-195); dInvestigator-identified NT events were graded according to the NCI CTCAE v4.03. 1. Berdeja J, et al. Presented at ASH 2020; abstract 1367. 2. Raje N, et al. Presented at ASH 2020; abstract 3234.
The overall response and safety profile for the elderly groups were comparable with those observed in the overall population1
Efficacy and Safety of Cilta-cel in Lenalidomide-Refractory Patients with Progressive Multiple Myeloma after 1–3 Prior Lines of Therapy: CARTITUDE-2
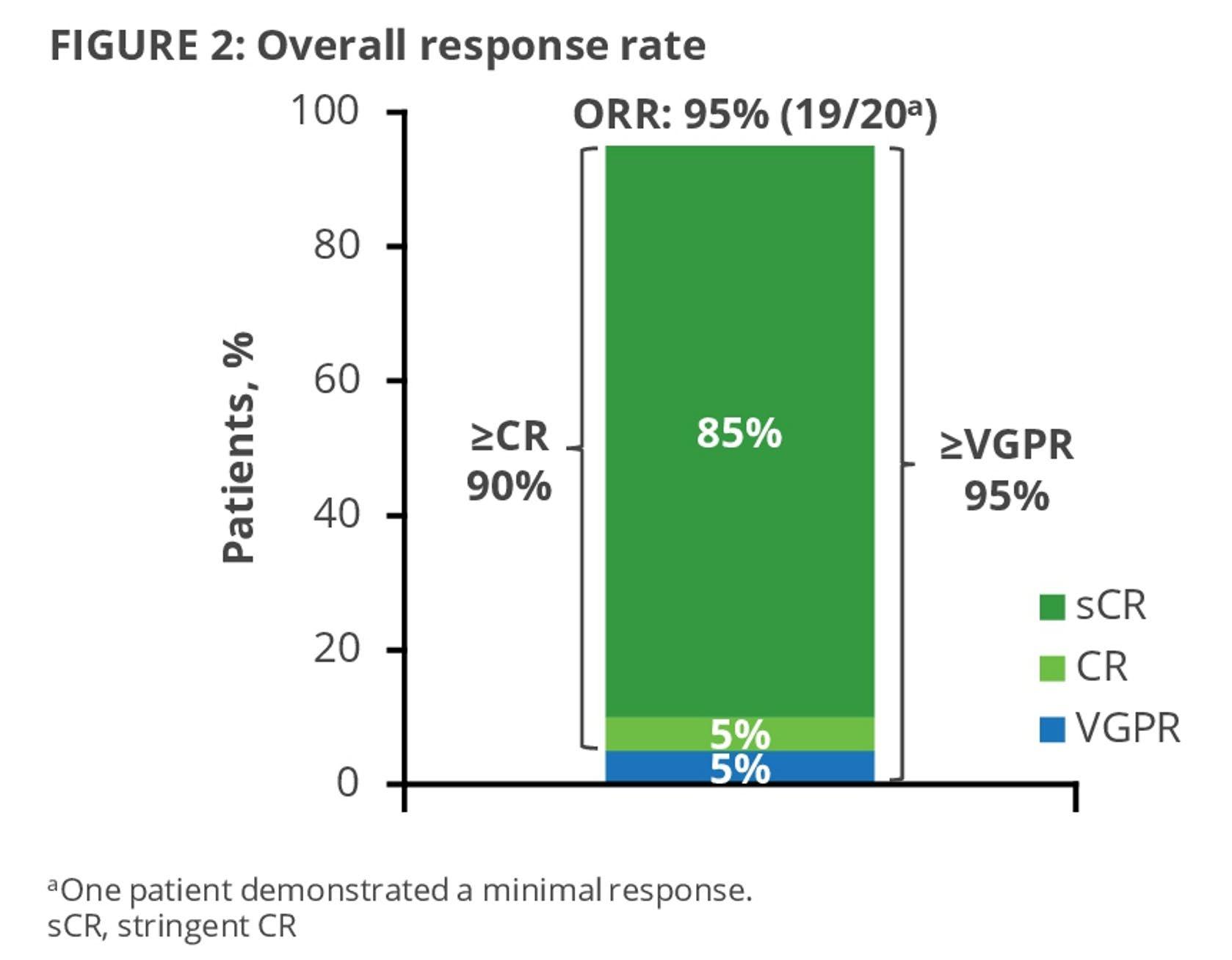
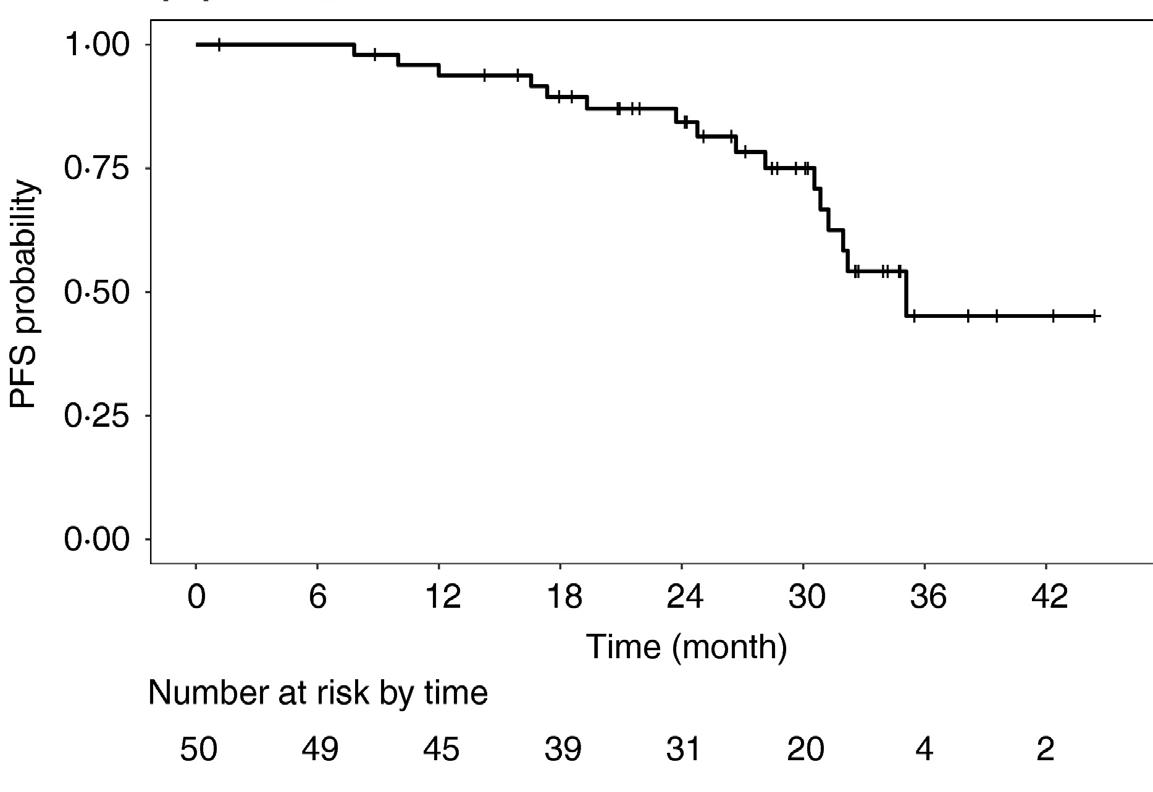
CARTITUDE-2: Cilta-Cel Appears Active in High-Risk RRMM
Cohort B of the phase 2 CARTITUDE-2 study assessed cilta-cel in patients with MM who had early relapse (≤12 months after ASCT or ≤12 months after start of initial treatment with antiMM therapy)1
ORR: 100% (19/19)
• High response rate in this challenging population
• 90% remained progression-free at 1 year post cilta-cel infusion
• Results at 18 months of follow-up show durability and deepening of response, maintenance of PFS
≥CR value does not sum appropriately due to rounding.
Toxicity Profile
• Cytokine Release Syndrome – CRS
• Neurotoxicity
• Cytopenia
• Infections and Hypogammaglobulinemia
• Other Toxicities
Cytokine Release Syndrome (CRS)
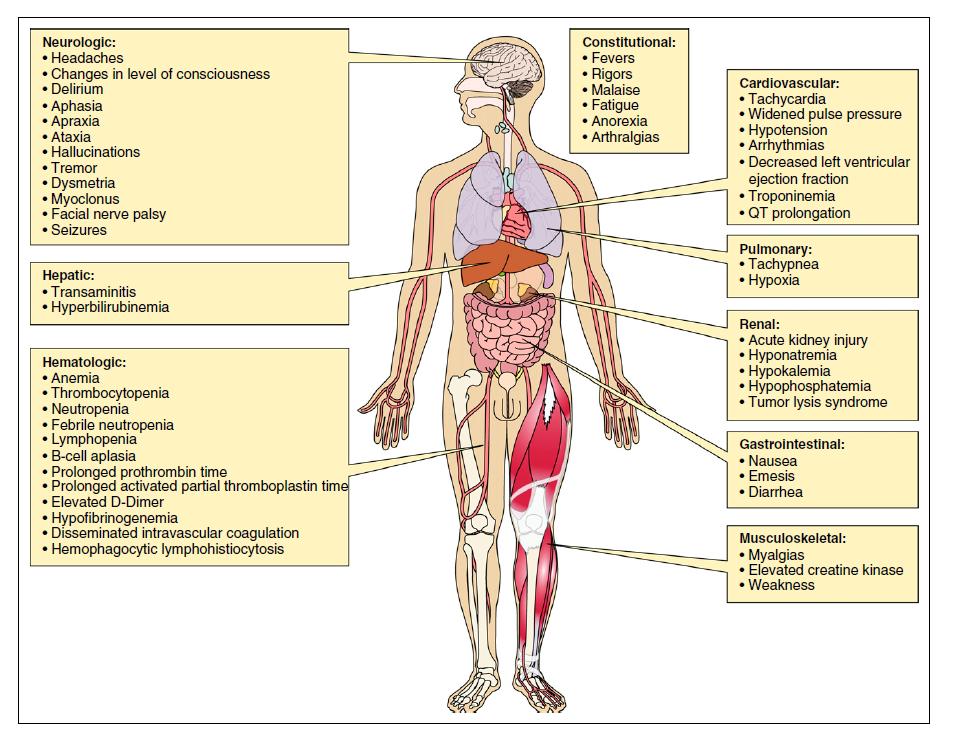
CAR-T related neurotoxicity, aka ICANS: Immune effector
cell-associated neurotoxicity syndrome
• (Headache)
• Lethargy
• Agitation
• Tremor • Aphasia
• Delirium
• Encephalopathy
• Seizures
• Cerebral edema
• Usually after CRS

Difficulty concentrating
CAR T cells Fever Hospitalization Dexamethasone Fludarabine
CRS and ICANs With BCMA CAR-T Constructs1
What Are the Emerging other CAR T Studies in MM?
Construct Current Status
Comments
CT-053
Phase 2
Humanized CAR T cells
P-BCMA-01
Phase 2
Autologous T stem cell
memory (Tscm) CAR-T cells; also an Allo program
CRB-402
Phase 2
BB21217 – Memory CAR T cells
ALLO-715
Phase ½
Allogeneic CAR T cell
CT-103A
Phase 1
Fully humanized CAR T cells
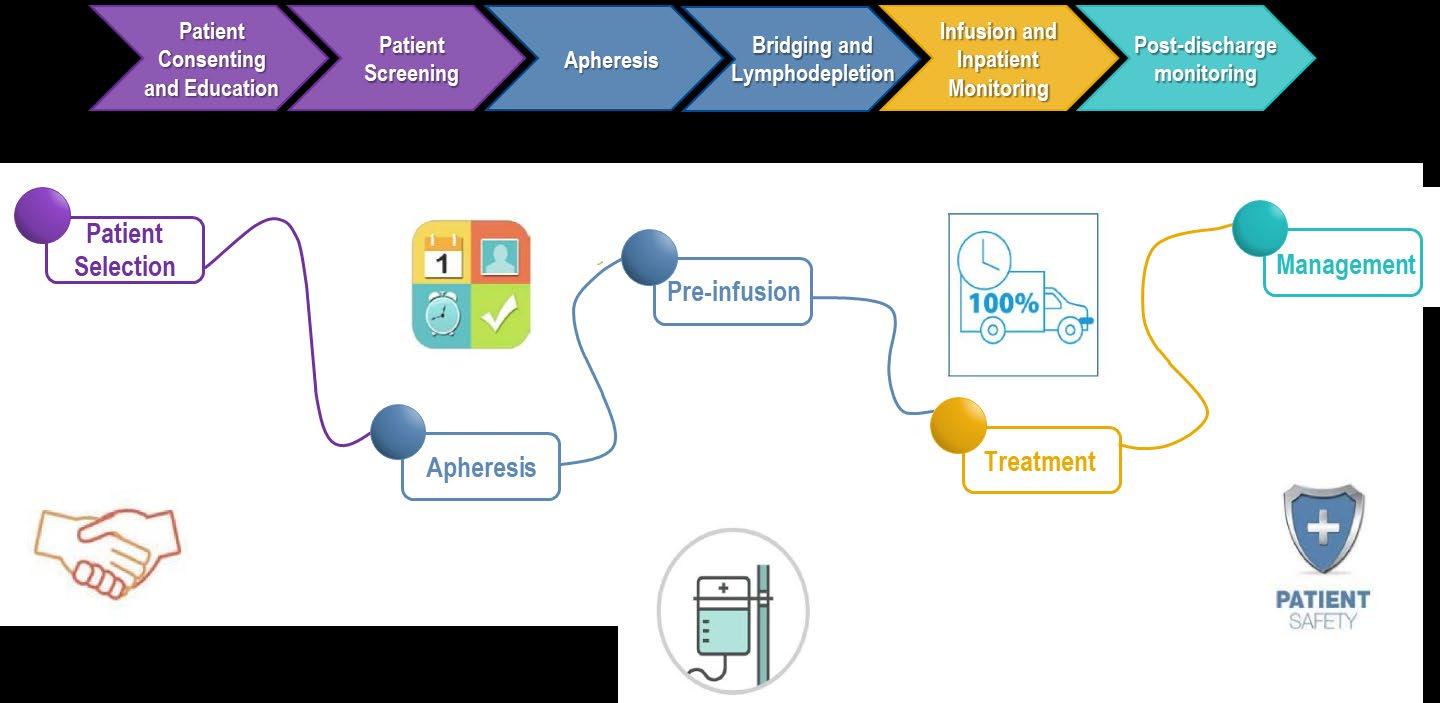
Overview of the CAR T cell administration process1,2
Ex vivo CAR T cell manufacturing Collection Infusion
Grow and expand number of T cells

Identify patients eligible for CAR T cell therapy
Evaluation and selection of the patient in the CAR T cell therapy center
Apheresis and transport of cells to CAR T cell manufacturing site
Isolate and activate T cells


Engineer T cells with CAR gene
Bridging therapy (post-apheresis, and typically stopped 2 weeks prior to infusion)2
Lymphodepletion therapy
(3 consecutive injections 3–5 days before CAR T cell infusiona)3,4
Infusion and monitoring (infusion to Day 28)3
Monitoring medium-term (Day 28–100)3 and long-term (> Day 100)3 complications
Image extracted from: Dana-Farber Cancer Institute, How CAR T cell therapy works. Available from: https://www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/how-car-t-cell-therapy-works/. Accessed February 2020. Flowchart extracted from: Moran D. The potential of CAR T-cell therapy and the myeloma patient journey. Myeloma Today. Available from: https://indd.adobe.com/view/07583bc3-3af4-4a8d-a142-47cb2c8a6402. Accessed February 2021. aTypically fludarabine/cyclophosphamide.
CAR, chimeric antigen receptor.
1. Moran D. The potential of CAR T-cell therapy and the myeloma patient journey. Myeloma Today. Available from: https://indd.adobe.com/view/07583bc3-3af4-4a8d-a142-47cb2c8a6402. Accessed February 2021.
2. Protocol for: Raje N. N Engl J Med 2019;380:1726-37. 3. Yakoub-Agha I, et al. Haematologica 2020;105:297–316. 4. Turtle CJ, et al. Sci Transl Med. 2016;8:355ra116.
Which patient to Consider?
Identifying patients eligible for CAR T cell therapy
Patient eligibility should be determined prior to leukapheresis
Which patient to Consider? Identifying patients eligible for CAR T cell therapy
Treatment characteristics: Disease characteristics: Patient characteristics:
Patients who have received at least four prior MM treatment regimens:a,1,2
• Including a PI, an IMiD® agent and an anti-CD38 mAb
Patients who have progressive disease:1,2
• Do not need to be refractory to the last treatment regimen; stable disease or minimal response are acceptable
• Do not need traditional measurable disease; imaging is adequate
No age limit for eligibility to receive CAR T cell therapy:1,3
• If patients are over 75 then they will be judged on an individual basis
Patients must be willing and able to adhere to the clinic visit schedule and other requirements:1,4
• Patients must agree to continued follow-up for gene therapy trials (as mandated by the regulatory guidelines)
aReflecting inclusion criteria in pivotal clinical trials – approved indication may vary. CAR, chimeric antigen receptor; IMiD® agent, immunomodulatory drug; mAb, monoclonal antibodies; MM, multiple myeloma; PI, proteasome inhibitor. 1. Personal opinion of speaker based on expert panel manuscript pending publication. 2. Shah N, et al. J Immunother Cancer. 2020;8:e000734. 3. Berdeja J, et al. Presented at ASH 2020; abstract 1367. 4. Protocol for: Raje N. N Engl J Med 2019;380:1726-37.
Comorbidities and relevant considerations
Cardiorespiratory
Well managed and compensated cardiorespiratory comorbidities are acceptable.1,2 No fixed EF requirement which are liberal than those required for high-dose therapy and transplant.
Renal function
Patients with adequate renal function defined as CrCl ≥ 30 mL/min using Cockcroft-Gault equation, will be included1–3
Decreased renal function would require dose reduction for fludarabine and cyclophosphamide during lymphodepletion1–3
Viral
CAR T cell therapy should be deferred for patients with active ongoing viral infection, e.g. HCV, HBV or HIV.1,2
Immune status
Patients considered for CAR T cell therapy irrespective of recurrent, non-severe infections1,2
CAR, chimeric antigen receptor; CrCl, creatine clearance.
Factors impacting CAR T cell therapy outcomes and the risk of toxicities
Patient on chronic immunosuppressantsa should be considered with a possibility to hold it during CAR T cell therapy.1,2
Ongoing treatment with intermittent topical, inhaled or intranasal corticosteroids is allowed
Patients on anticoagulation should have no active bleeding and should be safe to be taken off anticoagulation1,2
Adequate bone marrow function is not a prerequisite for consideration for CAR T cell therapy1,3
• There are minimal blood count requirements for a patient to be considered for therapy1,2
• A low count (ANC < 1000 cells/mm3 and/or platelet count < 50,000 mm3) may impact production of adequate CAR T cells, and may also increase risk of more prolonged cytopenia following lymphodepletion1,2
Cilta-cel is not approved by any regulatory agency. Ide-cel is currently approved by the FDA only. AE, adverse event; ANC, absolute neutrophil count; CAR, chimeric antigen receptor. 1. Personal opinion of speaker based on expert panel manuscript pending publication. 2. Protocol for: Raje N. N Engl J Med 2019;380:1726-37. 3. Shah N, et al. J Immunother Cancer. 2020;8:e000734. 4. Munshi NC, et al. Presented at ASCO 2020; abstract 8503. 5. Madduri D, et al. Presented at ASH 2020; abstract 177.
CAR T cell therapy differs from ASCT
• It is important to consider patient eligibility and treatment characteristics when deciding between CAR T cell therapy and ASCT:1
CAR T cell treatment ASCT
Patient characteristics
Patients ≥ 70 years2,3
Cardiorespiratory or renal comorbidities2–4
At risk of infection4,5
Treatment characteristics
Need for induction2,3
Need for T/PBS cell collection2,5
Short recovery time4–6
ASCT, allogeneic hematopoietic stem cell transplantation; CAR, chimeric antigen receptor. 1. Personal opinion/experience of the speaker. 2. Protocol for: Raje N. N Engl J Med 2019;380:1726-37. 3. Al Hamed R, et al. Blood Cancer J. 2019;9:44. 4. Shah N, et al. J Immunother Cancer. 2020;8:e000734. 5. Multiple Myeloma Research Foundation. The difference between cellular therapy and autologous stem cell transplant. Available from: https://themmrf.org/2020/02/24/the-difference-between-cellular-therapy-and-autologous-stem-celltransplant/#:~:text=The%20interaction%20between%20the%20engineered,a%20lot%20of%20interest%20recently.&text=In%20ASCT%2C%20the%20cells%20are,not%20modified%20in%20any%20way. Accessed February 2020. 6. Martin T, et al. Presented at ASH 2020; abstract 2291.
What is Bispecific Antibody?
Cell Death
T cell activation
Cytokine secretion
Cytotoxicity
Bispecific Antibody Constructs Facilitate Cell-to-Cell Interactions via Dual Antigen Specificity
Redirects CD3+ T cells to BCMA + MM cells
Induces T cell-mediated killing of MM patient cells
BCMA Teclistamab CD3Teclistamab: An Off-the-Shelf, T-Cell–Redirecting Bispecific Antibody1
• Binds to CD3 on T cells and BCMA on plasma cells
• Mediates T-cell activation and subsequent lysis of BCMA-expressing MM cells
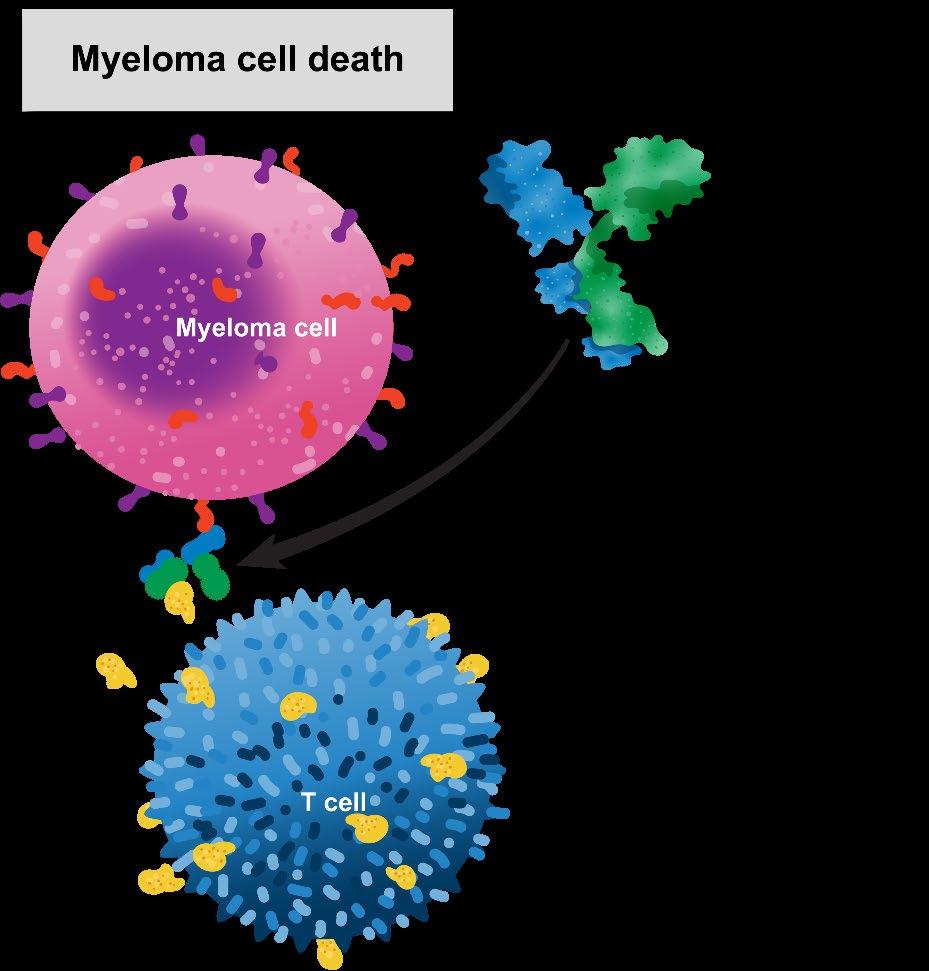
• Teclistamab was tested in the MajesTEC-1 study
– RP2D for teclistamab monotherapy: 1.5 mg/kg SC QW with step-up doses of 0.06 and 0.3 mg/kg
MajesTEC-1: Teclistamab Is Highly Active in Triple-Class–Refractory MM1,2
Screening Treatment
Cohort A
Triple-class exposed
Key eligibility criteria
• Documented, measurable RRMM
• ≥3 prior lines, including prior PI, IMiD, and anti-CD38 therapy
• No prior BCMA-targeted therapy
• Primary endpoint: ORR
Week 1
• Step-up doses of teclistamab SC (0.06 and 0.3 mg/kg)
Cycles ≥1
• Weekly teclistamab SC 1.5 mg/kg
• Continue until PD
• ORR of 63% in patients with triple-class–exposed disease
• Median DOR of 18.4 months
• MRD negativity rate: 24.7% (10-5)
– 26.7% in the all-treated (N = 165) patient population
–
81.5% of MRD-evaluable patients (44 of 54) were MRD negative
– Almost half (46.2%) of patients with ≥ CR were MRD negative
MajesTEC-1: Robust PFS and OS Outcomes in RRMM1
Median: 11.3 mo (95% CI, 8.8-17.1)
Median: 18.3 (95% CI, 15.1-NE)
ASH 2022: Baseline correlatives for pivotal RP2D patients support clinical combinations of teclistamab with agents such as daratumumab or checkpoint inhibitors. Updated oral abstract Saturday, December 10, 9:30 AM2
Practical Points: The Safety Experience With Teclistamab1
Maximum CRS Grade
• All CRS events were grade 1/2, except for 1 transient grade 3 CRS event that occurred in the context of concurrent pneumonia (resolved in 2 days)
• All CRS events fully resolved without treatment discontinuation or dose reduction
• 5 patients (3.0%) had a total of 9 ICANS events (7 events were concurrent with CRS)
• All ICANS events were grade 1/2 and fully resolved
• No treatment discontinuations or dose reductions due to neurotoxic events, including ICANS
MagnetisMM-1: Elranatamab Is Active in RRMM1
• Elranatamab: a humanized BsAb targeting BCMA and CD3
• Preclinical studies have demonstrated antitumor activity and delayed tumor progression
• Elranatamab was given SC at doses from 80 to 1,000 mcg/kg either weekly or every 2 weeks
• Confirmed OR was 64% among 55 patients receiving elranatamab at a dose >215 mcg/kg and 35% (19 of 55) of patients achieved ≥ CR
1. Jakubowiak AJ et al. ASCO 2022. Abstract 8014.New Directions: Targeting GPRC5D and CD3Talquetamab Is Active in RRMM
Both RP2Ds of talquetamab have comparable safety, efficacy, and pharmacokinetic profiles
New Directions: Targeting FcRH5 and CD3 Cevostimab is Active in RRMM
Cevostamab is a humanized IgG1-based T-cell–engaging BsAb that targets FcRH5 on myeloma cells and CD3 on T cells
• Dual binding induces T-cell activation and potent T-cell–directed killing of myeloma cells
Phase 1 dose-escalation and dose-expansion study evaluating the safety and efficacy of Q3W IV cevostamab in patients with RRMM1
• Cevostamab was given as a fixed-duration treatment for up to 17 cycles (≈1 year)
• Cycle 1 step-up dosing was evaluated to mitigate CRS
ASH 2022: enduring responses after 1-year, FD cevostamab therapy in patients with RRMM; early experience from a phase 1 study2 (Oral abstract Saturday, December 10, 5:30 PM)

Plans to Improve the Outcome of CAR-T Cell Therapy
Improve the CAR T cell product
• Improve response and overcome resistance:
– Dual antigen binding1
– RNA CARs2
– Peptide stimulated T cells with vaccine3
– Find novel targets ( e.g. GPRC5D4)
• Improve expansion and reduce risk of toxicities:
– Suicide genes/safety switches as a means to deactivate CAR T cells2
– Gene editing (e.g. PD-1 knockdown2)
– Select CD4:CD8 ratios2
– PI3K inhibitors5
• Reduce turnaround time:
– Gene editing of allogeneic CAR T cells2,6
Increase target expression
• Gamma-secretase inhibitors for BCMA7
Improve the CAR T cell treatment protocol
• Optimize infusion schedule:
– Retreatment at progression8
• Improve patient selection:
– Treat at an earlier line of therapy9
– Treat patients with a low disease burden9
– Treat patients regardless of high-risk disease9
• Use rational combinations OR maintenance therapy:
– Checkpoint inhibitors1, IMiDs10
Conclusions
• Immune and biologic therapies are achieving an unprecedented level of responses in late stages of myeloma
• Tumor intrinsic and microenvironmental factors both at diagnosis and at relapse, have significant role in outcome
• Task to develop therapies that sustains the deep response – to lead to cure !
Thank you to our Sponsors!