



Check out by 12

Lunch is provided
















60-year-old man
- Presented with backache for 2 months, worsening steadily
- Partially relieved by analgesia
- Feeling more tired than usual
Examination: mild pallor, no other significant abnormalities
Investigations: Hb 10.4 g/dL, WBC 4.2 x10^9/L, Platelets 151 x10^9/L
Creatinine 72 umol/L, Corrected calcium 2.44 mmol/L, Albumin 38 g/L, Globulin 53 g/L
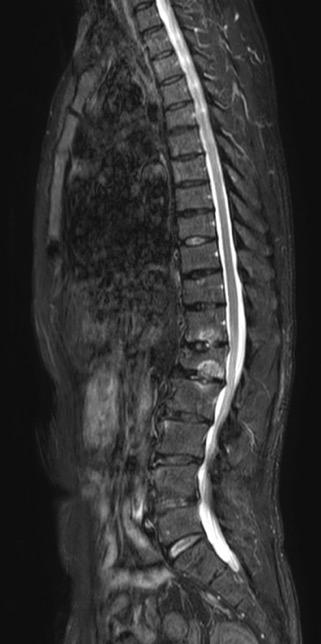
MRI spine: multiple compression fracture from T5 to L2
CT thorax-abd-pelvic: diffuse multiple ill defined lytic lesions at thoracic, lumbar and sacral spines
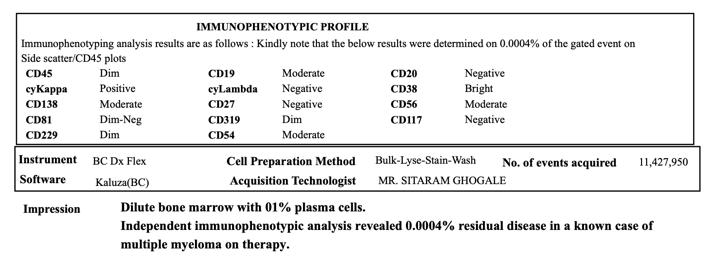
Bone marrow examination: 93% plasma cells positive for CD138, CD56, and cytoplasmic lambda
Karyotype: 46, XY; FISH: no evidence of fusion gene rearrangement or gene deletion
Diagnosis: Multiple myeloma IgG Lambda, ISS I
Newly diagnosed myeloma
M protein 22 g/L, started on Bortezomib -Cyclo -Dexa 1# then Borte -Lenalidomide -Dexa (BRD)
3#
Feb 2019
Aug 2019
Maintenance
M protein not detected, started on Lenalidomide maintenance
Feb 2020
Autologous HSCT
M protein <1, ASCT performed then consolidated with BRD 2#, complicated with PCP + drug allergic reaction
Nov 2023
2nd relapse
CR after 8# DVd, on monthly Dara but developed cytopaenias with M protein 2.4, repeat marrow 39% plasma cells
April 2025
Feb 2025
Disease relapsed
M protein 5, developed pancytopaenia since June 2023 ? MDS, repeat marrow noted 85% plasma cells with TP53 detected, started on Daratumumab -Borte -Dex (DVd)
CAR- T therapy
Ineligible for Teclistastamab trial, patient went for CAR-T therapy
Now 67-year-old, clinically well, ECOG 1
M protein < 1 g/L
Hb 7.3 g/dL, WBC 3.1 x10^9/L, Platelets 27 x10^9/L
Kappa free light chain 12.1 mg/L, Lamda 29.4 mg/L, ratio 0.41 (0.31-1.56)
Repeat marrow: hypercellular marrow with erythroid hyperplasia, no increased in plasma cells
PETCT: interval reduction in metabolic activity at diffusely intense FDG uptake involving the axial and appendicular bones - good partial response
Any role of 2nd autologous haematopoeitic stem cells transplant (stem cells harvested) in this patient (and the timing)?
- How about other myeloma patients?
Is there any ‘maintenance/ consolidation’ therapy to be given post CAR -T?
What would be recommended next line of therapy in 3rd relapse?
- Pomalidomide-based
- Carfilzomab-based
- Selinexor
- Teclistamab (not readily accessible outside trial)
- Chemotherapy: DT-PACE/ Bendamustine??
- Venetoclax???
- Melphalan-Pred-Thalidomide






Nguyen Thi Nhung
Stem cell transplantation department,
National Institute of Hematology and Blood Transfusion, Viet Nam
• Male, 58 y/o
• Date of hospital admission: 2020 April
• Past Medical History: Previously healthy
• Reason for Admission: Fatigue and bilateral leg edema.
• History of Present Illness:
– 1 month prior to admission, the patient experienced progressive fatigue and bilateral leg edema and aching pain in the lumbar spine.
– Initial workup at a provincial hospital revealed anemia, renal dysfunction, and hypercalcemia. Multiple myeloma was suspected, and the patient was referred to the NIHBT in April 2020 for further evaluation and management.
Hematology & Biochemistry:
- Hemoglobin: 70 g/L
- Urea: 8.2 mmol/L
- Creatinine: 211 µmol/L, CrCl 32 mL/min/1.73 m²
- Total protein: 120 g/L (Albumin: 23 g/L)
- Beta-2 microglobulin: 12 mg/L
- Calcium: 2.8 mmol/L
- LDH: 570 U/L
Immunoglobulins & Light Chains:
- IgG: 8632 mg/L
- IgA: 21.9 mg/L
- IgM: 16 mg/L
- Kappa light chains: 128 mg/L
- Lambda light chains: 11.8 mg/L
• Serum Protein Electrophoresis and Immunofixation: Monoclonal peak in beta-globulin region, consistent with IgG-Kappa monoclonal protein.
• Bone Marrow Biopsy: >60% plasma cell infiltration
• MRI (Spine): Vertebral compression fractures at L1–L3, multiple lytic lesions in vertebral bodies, lumbar disc herniation.
• FISH Analysis: Negative for t(4;14), t(14;16), t(14;20), dups 1q, del13p,
1. Is the timing of the transplant appropriate? Should we treat more aggressively to achieve CR before transplantation?
2. We had some cases of failed mobilization using Cyclophosphamide (2-4g/m2)? What should we do? (Plerixafor is unavailable)
3. When should we start treatment for relapse MM (asymptomatic biochemical or symptomatic relapse)?
4. What is the suitable treatment for this patient?
Retreat and maintain Daratumumab
Use Ixazomib (IRd)
The second transplant Carfilzomib, Bispecific, CAR T are unavailable





Yi-Wei,
National Taiwan University Hospital

# Resolved HBV infection, under Vemlidy
# Hypertension # Dyslipidemia
2023/05
Foamy urine=> IgA/kappa monoclonal gammopathy (IFE)
2024/01 Smoldering MM
2024/07 Smoldering MM
2024/10 Smoldering MM
2024/12 MM
BM study: 18% cPC
M protein: 2.3g/dl X CRAB or osteolytic lesion
O FISH: 1q amplification, t(4;14)/IGH::FGFR3
O High risk feature*1 (2/20/20)
BM study: 33% cPC
M protein: 2.9g/dl
X CRAB or osteolytic lesion
O Lower back pain BW loss
O High risk feature*2
Light chain ratio: 74.2
M protein: 3.3g/dl
O High risk feature*3
Light chain ratio: 106.2 (SLiM criteria)
Hb: 13 > 10.6 g/dl
Cr: 0.7 > 1.6 mg/dl
Ca: 2.15 mmol/L
LDH: 156 mg/dl
Albumin: 3.4 g/dl
Beta-2 Microglobulin: 3.2 mg/dl
ISS stage II, R-ISS stage II, R2 ISS stage III
ISS stage II, R-ISS stage II, R2 ISS stage III

Multiple myeloma, IgA/kappa type, t(4;14), 1q amplification
ISS stage II, R-ISS stage II, R2 ISS stage III

1. Should cytogenetic high-risk smoldering multiple myeloma be treated actively?
2. What factors predict failure of daratumumab-based therapy despite an initial response?
3. In transplant-eligible patients who relapse prior to autologous stem cell transplantation, is auto-HSCT still a feasible treatment option? or novel agents will be better options?





Young Hoon Park, MD
Ewha Womans University Medical Center, Mokdong Hospital, Republic of Korea
Incidentally detected anemia and monoclonal protein on health screening (November 2023)
No comorbidities and no specific symptoms
No family history of malignancies
Married woman (6 months ago) without kids
Lab ==
CBC: 9330-10.8-278K, ANC 5640
Total Ca: 8.7 (ref. 8.6-10.2 mg/dL), Creatinine 0.76 (0.63-1.01 mg/dL)
Total protein 13.0 (6.4-8.3 g/dL), Albumin 3.4 (3.5-5.2 g/dL)
Uric acid 6.4 (2.4-5.7 mg/dL)
Lactate dehydrogenase 302 (135-214 IU/L)
IgG/A/M 7452 (700-1600 mg/dL)/ 61.6 (70-400 mg/dL)/ 57 (40-230 mg/dL)
Serum EP/IFE: 6.66 g/dL, IgG-kappa
24hr urine EP/IFE: 30.5 mg/day, IgG-kappa
Serum free light chain assay: kappa/lambda 376.71/8.04
B2-macroglobulin 2.89 mg/L
Bone marrow: plasma cell 32.2%
NGS: BRAF (Tier1)
FISH: t(4;14)
MRI & PET: Diffusely and focally hypermetabolic bone lesions in left 5th rib, both humerus, both scapulae, spines, both pelvic bones, both proximal fumora
Final diagnosis
#. MM, IgG-kappa, CRAB(-/-/+/+), ISS 2, R-ISS 3, transplant-eligible


D-VTD#1 (Nov 2022)
D-VTD#2 (Dec 2022)
D-VTD#3 (Jan 2023)
D-VTD#4 (Feb 2023)
G-CSF mobilization Autologous stem cell transplantation (Mar 2023) Lenalidomide maintenance (Aug 2024)
Lenalidomide maintenance PR Plan for pregnancy Stop (June 2025)
Follow-up

Q1: For young myeloma patients with high-risk cytogenetics, what do you consider the optimal initial treatment strategy? Should our approach differ significantly from that for older, high-risk patients?
Q2: How do you find the optimal balance between 'treatment intensity' and 'preserving long-term QoL' in these young patients? Specifically, what are your perspectives on the duration of maintenance therapy or strategies for managing treatment-related toxicities?
Q3: Considering the expected long-term survival in young myeloma patients, beyond managing disease recurrence, what are the most crucial long-term complications that we, as clinicians, should focus on to enhance their quality of life?





Navigating Upfront Choices in Young Standard-Risk Myeloma
Presenter: Dr Natasha Fay Anthony
(Singapore Integrated Programme for Haematology, Senior Resident)
Navigating Upfront Choices in Young Standard-Risk Myeloma
Presenter: Dr Natasha Fay Anthony
(Singapore Integrated Programme for Haematology, Senior Resident)
• 32 year old male, ECOG 0
• Presented with back pain and general weakness; noted bone lytic lesions on spine imaging and underwent vertebroplasty + bone biopsy in 2020 – diagnosed myeloma ‘
• Standard risk with t(11;14)
• Started Dara-VRd in private hospital then referred to SGH for ASCT consolidation
• Preference for natural conception (rather than sperm banking) for religious reasons
• Received 3 cycles Dara-VRD (Under Private Oncologist)= utilized all his financial resources
• 2 further VRD cycles in SGH > VGPR M band 2 g/L
• Autologous stem cell transplant
• Lenalidomide maintenance for 2 years > stop for 3 years
3x Dara-VRD
2x VRD
Jan 2020
Melphalan 200mg/m2 ASCT
June 2020
Started on Len maintenance 10 > 25mg
Oct 2020
Reduced Len to 15mg as no change in VGPR status with Len 25mg for 8 cycles
Sep 2021
Stopped Len maintenance
Sep 2022
March 2020
Best response: VGPR
Sep 2020
M Band persisted at 2 g/L. BMAT: Flow: 0.04% atypical plasma cells. FISH negative.
Aug 2021
No M band but IFX positive
Sep 2022
CR. IFX also negative (best response, no BMAT confirmation)
April 2023present
M band detectable 2g/L
Where access to CD38 monoclonal antibodies is limited by cost, how do we best apply current trial data to guide strategic use of these agents in standard risk NDMM?
For patients with long life expectancy, what is the optimal maintenance regimen and duration to balance efficacy with considerations of
1. Family planning
2. Second primary malignancies
3. ‘Future proofing’ (i.e. preserving agents for later relapse)?










• A 63-year-old man, government officer presented with foamy urine and leg edema for 6 weeks
• U/D: T2DM (Last HbA1C 2 months ago= 6.7%, no DR), dyslipidemia, hypertensio n, SVD S/P PCI to LAD, OSA
• PE: BMI 35 kg/m2, mild pallor, pitting edema 3+ both legs
• Initial lab tests:
• Hb 10.1 g/dL, Hct 30.8%, MCV 76.9 fL, RDW 15.9%, WBC 10400/µL (N 50%, L41%), platelet 344000/µL
• UPCI 10.78, urine protein 24 hr = 7.67 g, cholesterol 414 mg/dL, LDL 305 mg/dL, Alb 1. 99 g/dL, Cr 0.84 mg/dL, Ca 7.82 mg/dL (9.84 mg/dL)
• Dx. Nephrotic syndrome
• Renal biopsy:
Renal AL amyloidosis


• The glomeruli show moderate to severe matrix expansion with acellular eosinophilic amorph ous material (negative PAS and silver stain) and inhomogeneous glomerular basement memb rane thickening. Arterioles show focal acellular eosinophilic material deposits.
• The Congo red stain shows congophilic staining with apple green birefringence under p olarized light.
• Immunofluorescence shows lambda (3+) smudgy mesangial staining while kappa (trace) sm udgy mesangial staining.
Consult hematologist for type of amyloidosis and further investigation
• SPEP: Monoclonal at B1 zone (M protein 0.4 g/dL); SFLC: kappa 17.41 mg/L, lambda 65.14 mg/L, ratio 0.26 (dFLC 47.7); IFE: IgA lambda monoclonal gammopathy; IgA level: 332 mg/dL (78-311)
• β2-microglobulin 2.33 mg/L, LDH 408 U/L
• Bone marrow biopsy:
• Normocellular marrow with trilineage hematopoiesis, plasmacytosis, and a myloid deposition
• Congo red stain is positive for amyloid
• Immunostains show increased plasma cells 15% of marrow cellularity with lambda light chain predominant
• FISH: positive deletion of 17p13 = 5.5%
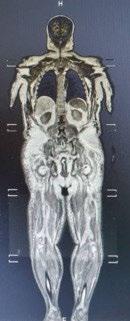
• MRI whole body: no abnormal marrow signaling, no plasmacytoma
• EKG: low voltage in limb lead with antero-septal infarction
• Cardiac MRI: cardiac amyloidosis is diagnosed.
• NT proBNP 11,991 pg/mL, Troponin T 102 ng/L
• Multiple myeloma IgA lambda with systemic AL amyloidosis, R-ISS stage II, Revised Mayo stage II, Renal stage II (Transplant eligible)



6th DaraVCd in sCR
IFE: no paraprotein detected SFLC: k 9.24, L 7.84, ratio 1.18, dFLC 1.37
No response
Partial response
Very good
partial response
≤30% ↓ in NT-proBNP from baseline ≤30% ↓ in proteinuria from baseline ≤30% ↓ in AP from baseline
31% to 60% ↓ in NT-proBNP from baseline 31% to 60% ↓ in proteinuria from baseline
>60% ↓ in NT-proBNP from baseline to nadir >400 pg/mL
to 60% ↓ in AP from baseline
>60% ↓ in proteinuria from baseline to nadir >200 mg/24 hr >60% ↓ in AP from baseline to nadir >2 x LLN
Complete response Nadir NT-proBNP ≤400 pg/mL Nadir proteinuria ≤200 mg/24 hr Nadir AP ≤2 x LLN
Organ progression
NT-proBNP progression (>30% and >300 pg/mL ↑) or troponin T progression (>33% ↑) or ejection fraction progression (≥10% ↓)
50% ↑ (≥1 g per day) of 24-hr urine protein to >1 g per day or 25% worsening of serum creatinine or creatinine clearance 50% ↑ of AP from nadir
3/7/2023 ASCT
Daratumumab maintenance x 19 cycle
Bone marrow biopsy: no plasma cell
SFLC: 32.39/25.02 = 1.29 (dFLC 7.37)
Ifx: polyclonal
Daratumumab - Only renal pr ogression (rena l biopsy confir med) 5/1/2025
• Treatment: Continued VRD for 5 cycles
• Renal response: partial response
• Questions
• What would be your preferred second-line treatment after Dara-V CD followed by daratumumab maintenance, in a patient with rena l-only progression?
• Would you consider a second autologous stem cell transplant (AS CT) in this situation? What about second-line maintenance?
• Would your treatment approach change if the patient had AL amy loidosis only, without MM?





Takeshi Yoroidaka
Kanazawa University Hospital, Japan
Kanazawa University Hospital, Japan

47 Years, Male IgG-κ type, ISS-I, R-ISS II, t(4;14)-, t(14;16)-, del(17p)-, 1q gainEPd 1cycle DKd 1cycles Ide-cel 5.4×106 cells VRD 2cycles/DRd 2cycles ASCT(Mel200)/Len/Ixa
At initial diagnosis
• hypercalcemia
• renal impairment
• Bone lesion
• lymphocyte apheresis
• Bridging therapy with DKd

Case summary:
• 48 years old, male
• t(4;14)-, t(14;16)-, del(17p)-, 1q gain-
• refractory: bortezomib, lenalidomide, ixazomib, Elotuzumab, pomalidomide
• relapsed after Ide-cel (13 months later) with bone leision
1.What is the best option after Ide-cel relapse?
• Daratumumab+Carfilzomib+dex
• radiation therapy
• Belantamab-mafodotin
• Elranatamab/Teclistamab
• Talquetamab (not available now, in Japan)
Case summary:
• 48 years old, male
• t(4;14)-, t(14;16)-, del(17p)-, 1q gain-
• refractory: bortezomib, lenalidomide, ixazomib, Elotuzumab, pomalidomide
• relapsed after Ide-cel (13 months later) with bone leision
2.Should allogeneic transplantation be considered as a strate gy for long-term survival?






Jing-Xia Li
Dept. of Hematology, The First Affiliated Hospital, Sun Yat-Sen University

• CBC: WBC 13.71×109/L,NEUT 6.85×109/L,
Hb 99g/L,PLT 93 ×109/L;
• LDH 2071U/L
• β2MG: 8582.56ug/L
• ALB 32g/L, GLB 26g/L
• Othet test: unremarkable.
• Ammonia: 185umol/L
• Uro-Protein: mALB 319.89mg/L,
λ chain 3021.6mg/L,κ chain 23.29mg/L.
Ig: IgA 0.26g/L, IgG 3.54g/L, IgM <0.21g/L
IF: IgD-λ; B-J protein: λ+
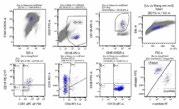
BM: 23% plasma cells; PB: 8% plasma cells
FCM: clonal PC restrictedly expressing cλ.
C: 2.32mmol/L
R: 119umol/L
A: 99g/L
B: No obvious bone destruction. Soft tissue in lower abdomen, with increased metabolic activity: EMD?
FISH: 1q21 amp (4copies), 1p32 del









One cycle of PAD



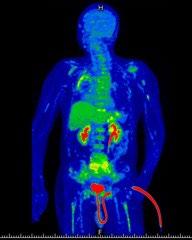


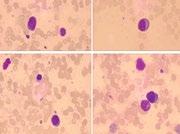










• Extremely rare presentation:
o Underlying mechanism?
• Aggressive biological behavior, short DOR, and poor prognosis:
o CAR-T cell therapy?
o Bi-specific antibodies? Tri-specific antibodies?
o Timing & combination?
o Other...

Jing-Xia Li, jxleeee@qq.com
Dept. of Hematology, The First Affiliated Hospital, Sun Yat-Sen University





Dr. Julian Jomell
Known case of Breast Cancer – 2022; AC x 6cycles from June to October 2022
Currently maintained on Letrozole 2.5mg tab OD
Patient was initially well until follow-up last May 2025 noted with pancytopenia
associated with
• Hgb 74 Hct 24 WBC 3.33 (N-33, L-61, M-2, E-1) Plt 58
• Hgb 74 Hct 24 WBC 3.37 (N-34, L-55, M-3, E-1) Plt 59
• Crea 1.2 K 4.9
The core biopsy shows a markedly hypercellular bone marrow with 8090% overall cellularity. There are infiltrate of plasma cells in large aggregates comprising approximately 50-60% of the marrow cellularity.
Erythrogranulopoiesis is present with maturation with a myeloid to erythroid ratio of 3:1. Megakaryocytes with medium-to large-sized hypolobated forms are seen. Special stain shows absent to mild reticulin fibrosis (Grade 0)
SPEP: Consistent with monoclonal gammopathy; Monoclonal peak
concentration 53.4% (6.3 g/dl)
B2 microglobulin 4.1 mg/L (1.0-2.4mg/L) Albumin 3.3 TP 11.8 g/dl
FISH for Myeloma: (+) 1q21 amplification
Immunohistochemistry results
• CD 138: Positive diffuse and strong cytoplasmic membrane expression in plasmic cells comprising 50-60% of the marrow cellularity
• Kappa and Lambda: Positive, strong, cytoplasmic expression of KAPPA is seen in the majority of the neoplastic plasma cells supportive of KAPPA restriction
• Which regimen is best to be used with the case given with poorrisk cytogenetic results?






SR-2,
Dr Amrit Dhar MBBS,MD
Department of Medical Oncology and Hematology
Tata Memorial Hospital, Mumbai,India

• 63 y/o, Female, No known comorbidities
• Baseline complaints: Low backache in 2020
• Diagnosed as:
✓ Multiple Myeloma- Lambda Light chain disease
✓ C-R+A+B+; ISS-II
✓ CTG: 13q deletion
✓ Baseline SPEP- Distorted beta region, Free Lambda 8620, Free Kappa 21.0
✓ K/L ratio: 0.002

3. DFI 3 years
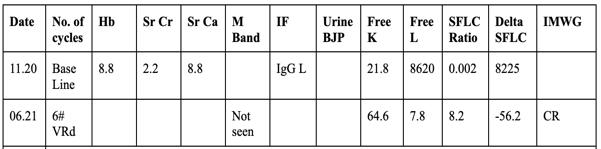
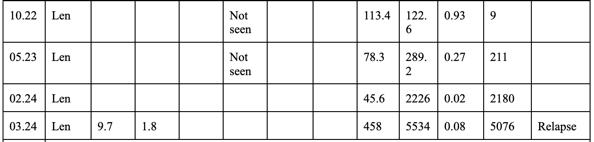

4. Dara-VD —>Progression in 6 months
5. KPd—-> Progressed in 3 months
✴ Screened for BCMA-CART trial - Not eligible
✴ Two PI, Two ImiDs and 1 AntiCD38 had been used
✴ Discussed further options with patients
• Planned for Teclistamab Inital Step up dosing


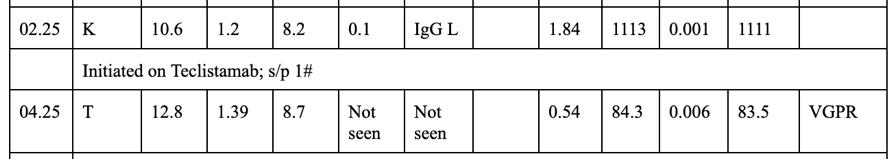

Post cycle 3 Teclistamab


• CRS Grade 1 in week 1 only.
• LRTI- Likely viral in week 3
• Rest no issues
• Recieved IVIG concurrenlty [IgG: 320]
• Maintains CR


1. Predictors of Outcomes in Teclistimab?
1. Role of T-cell subset analysis?
2. sBCMA as a marker of response?
2.Infectious complications? Are they different from CART?
3.How to dose post-achievement of CR?
4. Approach to Teclistimab failure?
5.Role of BCMA-CART post teclistimab?

• Dr Bhausahab Bagal, DM, Professor, Hematology Unit, Department of Medical Oncology,TMH
• Department of Medical Oncology
• All staff and colleagues

Thank you for your patience






• 49/M IgG kappa myeloma
• Diagnosed in Thailand in 4/2022. Presented with PRB
• Noted anemia and thrombocytopenia
• No renal impairment nor hypercalcemia
• SPE: IgG kappa 52.5 g/L
• SFLC: kappa 399, K/L ratio: 167.65
• BM: 69% plasma cells
• FISH: Gain in CKS1B (1q21), loss of CDKN2C (1p32.3)
• B2M: 9.81
• R-ISS stage II
• DaraKT x 2 cycles, Dara-KRCD, DaraVTD x 2 cycles
• DaraRD x 2 cycles PD with extensive extramedullary involvement including NP, neck LN, liver, duodenum, paraspinal soft tissue mass
• DT-PACE x 3 cycles
• Disease progressed with extensive disease involving NP, cervical LN, right hilar mass; peritoneal mass and soft tissue mass overlying T10 and T8, bilateral humeri, femurs, spine.
Dara-KT, DaraKRCD, Dara VTD, DaraRD

Teclistamab cycle 1-15


• PET CT after 6 months of teclistamab (1/2024)
• previous R NP mass, cervical LN, R hilar mass, peritoneal mass, peritoneal deposit, paraspinal soft tissue mass, epidural mass from T9-11 resolved
• Presented with 3 episodes of transient left sided numbness (11/2024)
• Transcranial doppler: unremarkable
• EEG: No epileptiform discharge
• Contrast MRI brain:Suspected diffuse supratentorial leptomeningeal enhancement and nodular thickening.
• CSF shows involvement by plasma cell myeloma
• PET-CT (12/2024):no disease involvement
• Managed as isolated CNS relapse
CNS relapse (leptomeningeal disease) while on teclistamab 11/2024
12/2024
Switch to Pom / Dex and started IT chemotherapy
5/2025
CNS relapse (both leptomeningeal and parenchymal disease)



received Intrathecal chemotherapy with IT MTX and hydrocortisone
CSF clear for plasma cell after 4 doses of IT chemotherapy then patient decline further IT chemotherapy in view of intolerance to post-LP headache
Withheld teclistamab and switched to Pomalidomide and
Dexamethasone since 12/2024
Patient developed headache in 5/2025
CSF shows involvement by plasma cell myeloma
MRI brain (6/2025):
- Leptomeningeal enhancement are detected at bilateral parasagittal and bilateral cerebral hemispheres.
- Multiple focal enhancing cerebral lesions have been detected, most likely indicative of brain metastasis. These are associated with perilesional vasogenic edema.
With Penta-refractory multiple myeloma with extensive extramedullary disease, received 15 cycles of teclistamab , then developed CNS relapse (currently on IT chemotherapy and pomalidomide/dexamethasone up to 7 cycles, pending workup for PET-CT and BM also discussed with oncologist for craniospinal RT)
1. At 1st isolated CNS relapse while on teclistamab, should we continue teclistamab for systemic disease control?
2. If workup confirm both systematic and CNS relapse, what will be the possible treatment option?
3. If it is an isolated CNS relapse and the CNS disease is under control, what should be next line of treatment?





Thank you to our Sponsors!





Scan to share your thoughts and feedback with us



