






Myeloma Action Month is a global social awareness campaign that takes place every March to raise awareness of multiple myeloma. Every March, we urge you to champion Myeloma Action Month to make an impact on those living with the disease. Will you take action for the myeloma community?




Visit www.myelomaactionmonth.org to learn how you can join the movement.






Assistance with understanding lab results, terminology and disease state
Preparing for medical visits
Access to medical providers
Access to medications
Financial resources
“Thank you so much for the informative conversation and all the time you spent listening and helping me decipher the MM lingo. What an amazing service!”


“Thank you for your response and excellent question suggestions for my hematology team.”





Tip Cards
Myeloma Minute Weekly Updates
Myeloma Today Quarterly News





































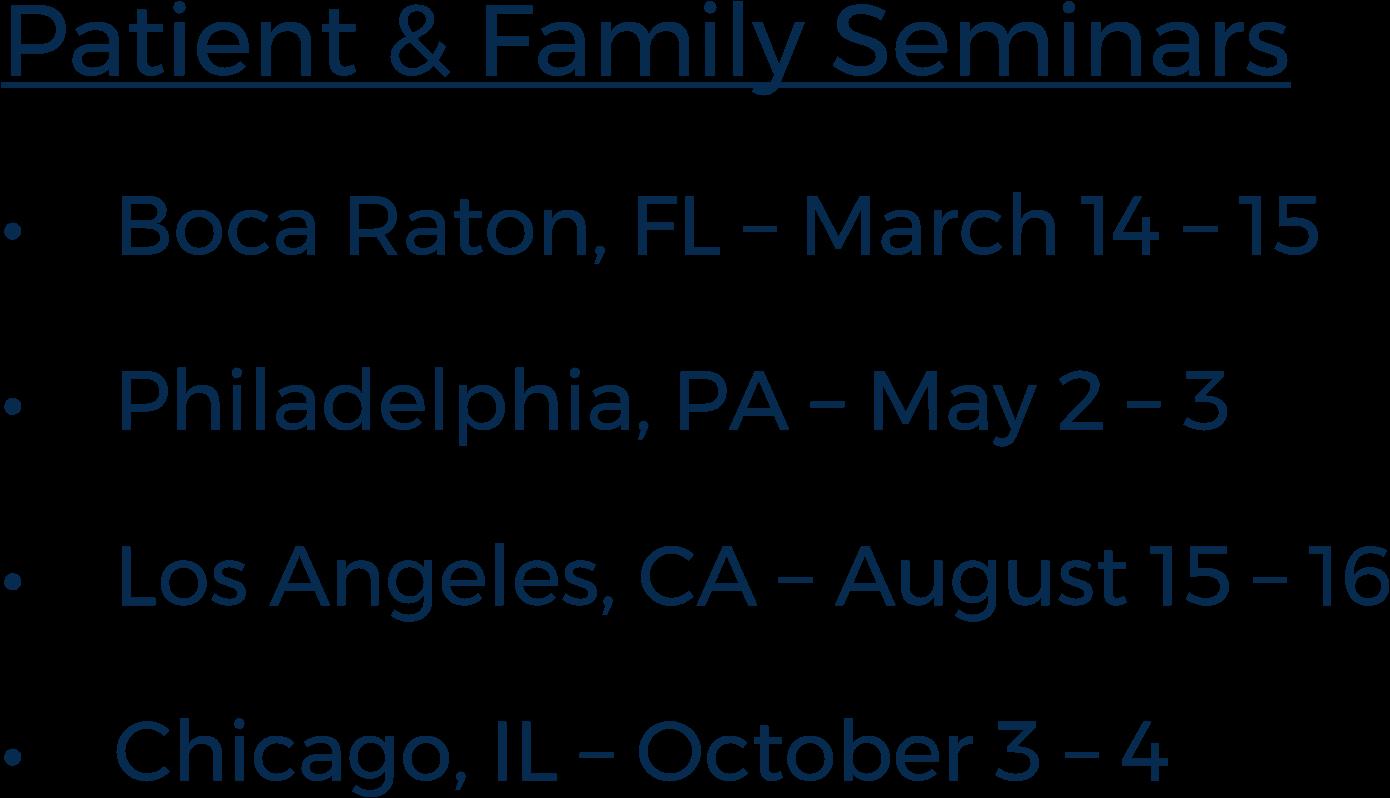
4 PATIENT & FAMILY SEMINARS including world-renowned experts
10 MYELOMA COMMUNITY WORKSHOPS including local myeloma experts

Locations





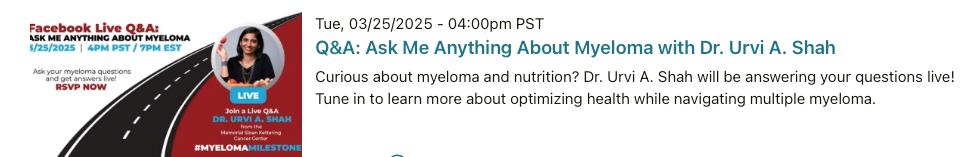




Mark your calendars for these virtual and in person Events.
Scan below QR code for more:



Joseph Mikhael MD, MEd, FRCPC Chief Medical Officer, International Myeloma Foundation Professor, Translational Genomics Research Institute, City of Hope Cancer Center




Some of the important principles of clinical trials:
The drive of research has brought us to where we are
No one is expected to be a “guinea pig” with no potential benefit to them
Research is under very tight supervision and standards
Open, clear communication between the physician and the patient is fundamental Driving research forward!






Every patient is unique and must be viewed that way
Benefits of trials are numerous and include:
Early access to “new” therapy
Delay use of standard therapy
Contribution to myeloma world – present and future
Financial access to certain agents
Must be balanced with potential risks
“Toxicity” of side effects
Possibility of lack of efficacy

MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time
MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use
MYTH: Clinical trials are expensive and not covered by insurance
website. Accessed March 25, 2024. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/CLINICAL-TRIALS-MYTH-FACTPRINT.pdf?hsCtaTracking=f6689b95-1626-40d9-8c87-c6b8d31600a4%7C35221aa8-d487-4db3-9416-b9c3c35e3bac
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc may not be reimbursed and are paid by patient

Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!

Most agents are tested in lab models
Various “myeloma cell lines”, also known as “in vitro”
Next step is animal model
We are more like mice than you think!!
Earliest study in Phase I is called “First in Human”
Often uses extremely low dose of drug to ensure safety

All patients receive the experimental therapy
Phase 1 trials find the optimal dose of a new drug or drug combination
Patients get higher doses as the study continues
Determine side effects of new drugs or combinations
Explore how the drug is metabolized by the body

Important for all stages of myeloma


Determine if a new drug or combination is effective against the cancer
May be added to a Phase 1 study once the ideal dose is found
Patients usually receive the experimental therapy
In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)

Highest form of clinical evidence. Typically, a large number of patients are required…usually required for full FDA approval
Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
o The patient is randomly assigned to a treatment—a process called “randomization”
o Neither the physician or the patient can determine which treatment is given

May be placebo controlled, if no standard treatments are available
Very closely monitored for effectiveness and side effects

Preclinical

ANIMAL STUDIES: Examine safety and potential for efficacy
PHASE 1
PHASE 2
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of efficacy, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically
PHASE 3
GATHER ADDITIONAL EFFECTIVENESS AND SAFETY INFORMATION
COMPARED TO STANDARD OF CARE
• Placebo may be involved if no standard of care exists; hundreds to several thousand patients
• Often multiple institutions; single or double blind; sometimes open label
PHASE 4
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS

• Patients will receive, at a minimum, the best standard treatment
• If the new treatment or intervention is proven to work, patients may be among the first to benefit
• Patients have a chance to help others and improve cancer care

• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient
• Health insurance and managed care providers do not always cover clinical trials


• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes
• Not have physicians who offer them trials
• Have a disconnect with their healthcare team

There has been a lack of diverse representation in clinical trials in myeloma.
In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
This is significant for the following reasons:
All patients of all races and ethnicities should be able to benefit from clinical trials.
Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
Reasons for underrepresentation in clinical trials are complex and include:
Systemic racism, accessibility of clinical trials, sensitivity to diversity by medical professionals
Misconduct in medicine in the past, the lack of trust in the system, and more.



[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
– FDA

Leadership and commitment
Community engagement practices
Investigator hiring, training, and mentoring practices
Patient engagement practices
US Cancer Centers of Excellence: Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials

1.Identify Myeloma Patients who have been on a trial from historically underrepresented groups
2.Diversity in Clinical Trials Academy
Designed with input from our African American Patient Advocacy Panel, the patients will attend an in-person training on the ABCs of Clinical Trials, unconscious bias, reasons for participation in trials, limitations of trials, informed consent, and how to effectively relate their personal stories.
3.Rollout of the program
This will be incorporated into IMF in-person meetings, videos, the IMF website, articles, podcasts, social media campaigns, and support group curricula
Objective: To promote trust and educate patients regarding clinical trials, particularly those from populations underserved by clinical trials, laying the groundwork for potential future trial participation


How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?
What is currently known about the new drug or combination?
What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?




Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?





Discuss with your physician if you are eligible for a clinical trial
Work with your physician to determine the best trial for you
Meet with the clinical research nurse or trials coordinator to discuss the trial
Carefully review the provided “Informed Consent”
Describes the study and any potential safety concerns related to the experimental medication






cancer.org/home/attend/webinartemplate/2022/07/25/on-demand/just-askincreasing-diversity-in-cancer-clinical-research




Myeloma Action Month is a global social awareness campaign that takes place every March to raise awareness of multiple myeloma. Every March, we urge you to champion Myeloma Action Month to make an impact on those living with the disease. Will you take action for the myeloma community?
Visit www.myelomaactionmonth.org to learn how you can join the movement. Will you




OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

These are the core values we bring to accomplishing our mission each day.
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.



