

2025 IMF REGIONAL COMMUNITY WORKSHOP
SAN FRANCISCO, CA

MARCH 29, 2025
Thank you to our sponsors!









IMF REGIONAL COMMUNITY WORKSHOP
SAN FRANCISCO
MORNING AGENDA


Program Evaluations
Please be sure to complete your program evaluation and return your evaluations at the end of today.
We greatly appreciate your time and feedback!

The IMF Support Group Team is






San Francisco Bay Area
Multiple Myeloma Support Group
Meets virtually on the 3rd Saturday of each month at 10AM
City of Hope Myeloma Community Connection
Meets virtually on the 3rd Wednesday of each month at 3PM
Santa Cruz Multiple Myeloma Support Group
Meets virtually on the 1st Monday of each month at 4:30PM
Sacramento Multiple Myeloma Support Group
Meets virtually on the 1st Saturday of each month at 10AM
San Gabriel Valley Multiple Myeloma Support Group
Meets in-person on the 1st Monday of each month at 6:30PM

Orange County Multiple Myeloma Support Group
Meets in a hybrid format on the 1st Thursday of each month at 6PM

Los Angeles Multiple Myeloma Support Group
Meets virtually on the 3rd Saturday of each month at 10:30AM


Upland Multiple Myeloma Support Group
Meets in a hybrid format on the 1st Friday of each month at 10AM

Inland Empire Multiple Myeloma Support Group
Meets in a hybrid format on the 3rd Saturday of each month at 11AM
Westlake Multiple Myeloma Support Group
Meets virtually on the 2nd Saturday of each month at 11AM
Rancho Mirage Multiple Myeloma Support Group
Meets in-person on the 1st Thursday of each month at 3PM
Myeloma Voices at ASH


In Person / 5 Virtual

Myeloma Voices


IMF InfoLine
Connecting Patients to Resources…
Shortening “Time to Hope” for Over 1,000 First-Time Callers Each Year
Assistance with understanding lab results, terminology and disease state
Preparing for medical visits
Access to medical providers
Access to medications
Financial resources
“Thank you so much for the informative conversation and all the time you spent listening and helping me decipher the MM lingo. What an amazing service!”


“Thank you for your response and excellent question suggestions for my hematology team.”





Myelo:
The first generative AI Assistant designed specifically for multiple myeloma.



Written Education Understanding
Booklets
Tip Cards
Myeloma Minute Weekly
Updates
Myeloma Today Quarterly News






































Live Patient Education
4 PATIENT & FAMILY SEMINARS including world-renowned experts
10 MYELOMA COMMUNITY WORKSHOPS including local myeloma experts

Locations



2025 Live Patient Education



Scan for Upcoming Events!





Understanding Myeloma Basics

City of Hope, Duarte, CA
Sarah Lee, MD
OBJECTIVES




How common is Myeloma in the US?








What
Causes Myeloma? How/Why Did I Get This?
Environmental Factors:
• Exposure to some chemicals
• Radiation exposure
Examples:
Agent Orange
Burn pits
Pesticides, Herbicides
Firefighter/First Responder exposures
Individual Factors:
• Age
• Family History of related disorders
• Personal History of MGUS or SMM
• Obesity
VA Study Documents Health Risks for Burn Pit Exposures
Leukemia and Multiple Myeloma Set to Be Added to List of Conditions Linked to Burn Pits
In most cases, the honest truth
WE DON’T KNOW


Multiple Myeloma Diagnosis Can Be Challenging




Kyle RA. Mayo Clin Proc. 2003;78:21-33.



What is the Connection Between Bone Marrow & Myeloma ?






Photo Credit
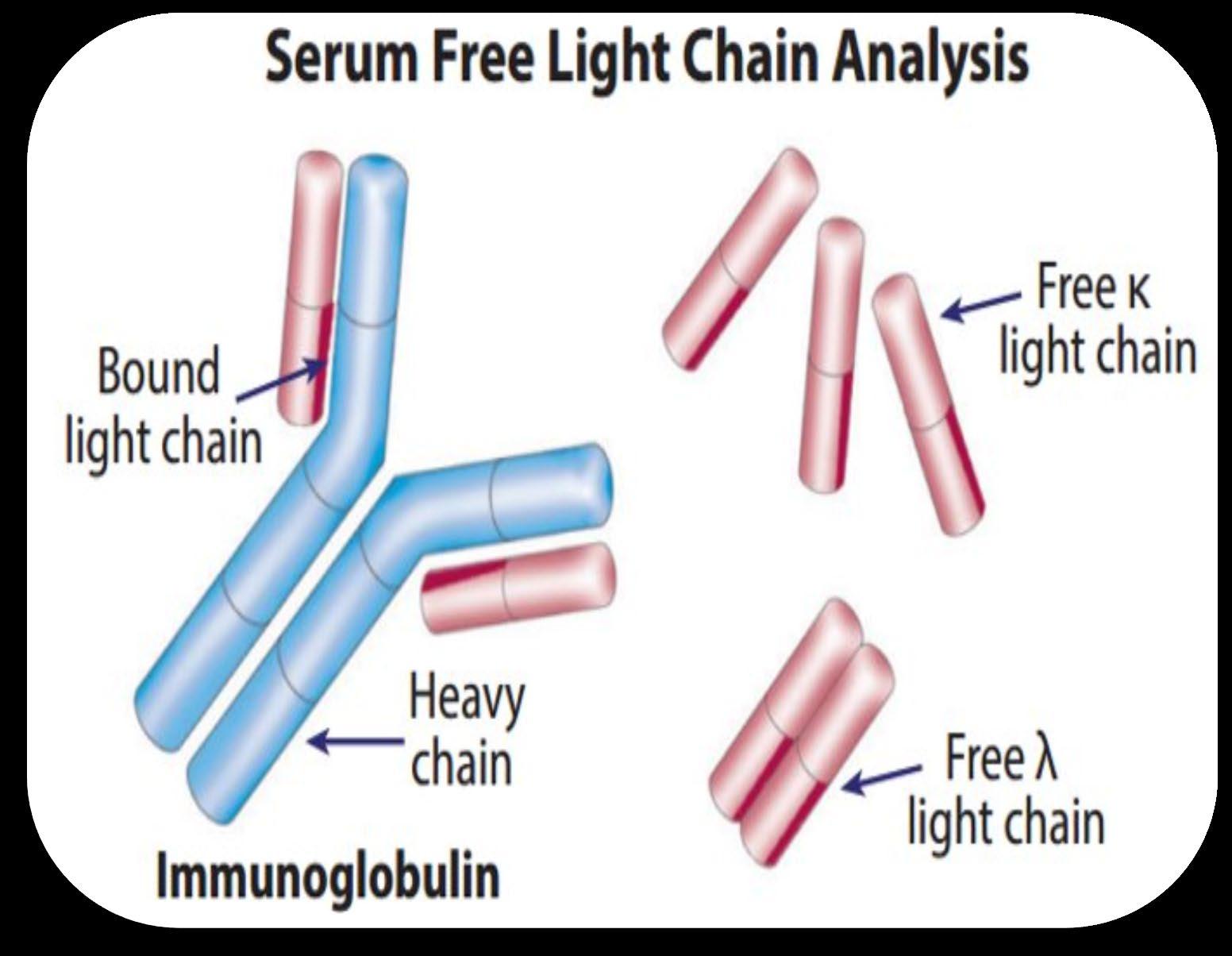
Understanding (Mono)clonal Plasma Cells

Heavy Chain: G, A, M, D, E




Heavy Chain = M-Spike

65% IgG – most common
20% IgA – associated with AL Amyloid
5% to 10% light chain-only (kappa, lambda)
Less common: IgD, IgE, IgM


Is Myeloma the Only Protein Disorder?
• AL-Amyloid
• POEMS
• Light or Heavy Chain Deposition Disease
• MGCS = Clinical
• MGRS = Renal
• MGNS = Neuro
Condition
Clonal plasma cells in bone marrow
MGUS1-4 (Monoclonal Gammopathy of Undetermined Significance)
SMM1-5,8 (Smoldering Multiple Myeloma) Active Multiple Myeloma6-8
Presence of Myeloma
Defining Events
Likelihood

* In clinical trial





Testing For Myeloma: Blood & Urine
Test Name
CBC + differential
Complete metabolic panel
Beta-2 Microglobulin (B2M)
Lactate Dehydrogenase (LDH)
What it means

Hemoglobin, WBC, Platelets
Creatinine, Calcium, Albumin, Liver function
Part of staging and risk stratification
Serum Immunofixation and Protein
electrophoresis (SPEP+IFE)
Immunoglobulins (G, A, M, D, E)
Free light chain assay with kappa/lambda ratio

Urine immunofixation & protein
electrophoresis (UPEP+IFE)
Measures the level of normal and clonal protein
Identifies the type of clonal protein



Measures the level of normal and clonal protein
SV, et al. Lancet Oncol. 2014;15:e538-3548. Ghobrial IM, et al. Blood. 2014;124:3380-3388; mSMART.org; NCCN.org
Identifies the type of clonal protein

This Photo by Unknown Author is licensed under CC BY-SA-NC

Testing For Myeloma: Imaging
Imaging:
– Skeletal survey: Series of X-rays; less sensitive than other techniques
– Whole body low dose (CTWB-LD CT )
– Positron Emission Tomography (PET/CT)
– Magnetic Resonance Imaging (MRI)

Healthy bone versus myeloma bone disease




This Photo by Unknown Author is licensed under CC BY-NC-ND

Testing For Myeloma: Bone Marrow


Bone marrow biopsy & aspirate • Bone marrow plasma cells (%) • Congo Red staining if concern
Bone marrow genetics
• Cytogenetics
• Fluorescence in situ hybridization (FISH)
• Next generation sequencing (NGS)



This Photo by Unknown Author is licensed under CC BY-SA

What
is (the importance of) Myeloma Staging & Risk Stratification?
• Updated as new information becomes available
• Helps to guide therapy and measure response to treatment
• Provides some prognostic value
• Standardizes terminology in medical practice

High Risk FISH Results*






What is the Myeloma Treatment Landscape?
Initial Therapy (a.k.a. Frontline, Induction)
Quad Therapy (ex. CD38+ MoAb + VRd)
HD-Melphalan + Stem Cell
Transplant (ASCT)
Maintenance
Treatment for Relapse
Consolidation
Therapy
Supportive Care and Living Well
Treatment for Relapse

Treatment for Relapse
Treatment for Relapse
Treatment for Relapse


Drug Class Overview

(thalidomide)
(lenalidomide)
(pomalidomide)
Rev, Len
or Pom
(daratumumab)
(isatuximab)


Drug Class Overview

Peptide Drug Conjugate* Pepaxto (Melphalan Flufenamide)
BCMA Targeted Antibody Drug
Conjugate (ADC)*
Blenrep (belantamab mafodotinblmf )
Abecma (idecabtagene vicleucel)
Belamaf, or B
Bispecific Antibodies
Carvykti (ciltacabtagene vicleucel)
Tecvayli (teclistimab)
Talvey (Talquetamab)
Elrexfio (Elranatamab)
Cevostamab, Iberdomide, Mezigdomide, Venetoclax Linvoseltamab, LCAR-B38M, ABBV-383

Measuring Disease Response: IMWG Response Criteria























Negative by next generation flow (NGF) (minimum sensitivity 1 in 10-5 nucleated cells or higher)*
mCR AND normal Free Light Chain ratio, Bone Marrow negative by flow, 2 measures
CR AND negative PCR
Complete Response: Negative immunofixation (IFE); no more than 5% plasma cells in BM; 2 measures
Very Good Partial Response: 90% reduction in myeloma protein
Partial Response: at least 50% reduction in myeloma protein
Minimal Response
Stable Disease: Not meeting above criteria
Progressive Disease: At least 25% increase in identified myeloma protein from lowest level
MRD = Minimal Residual Disease
sCR = Stringent Complete Response; BM = Bone Marrow
Kumar, S., Paiva, B., Anderson, K. C., Durie, B., Landgren, O., Moreau, P., ... & Dimopoulos, M. (2016). International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The lancet oncology, 17(8), e328-e346.


When Do I Need A New Treatment?
• Not every relapse requires immediate therapy
• Each case is different
Symptomatic or extramedullary disease


Asymptomatic biochemical relapse on 2 consecutive assessments

Asymptomatic high-risk disease or rapid doubling time or extensive marrow involvement Consider Observation Monitor Carefully Consider Treatment
Patient-/Disease-Specific Monitor Carefully
Initiate Treatment

Targets on the Myeloma Cell Surface and Therapeutic Antibodies

Bi-Specific Antibodies
Talvey (Talquetamab) CAR-T



Antibody Drug
Empliciti (Elotuzumab)
Bi-Specific Antibodies



Bi-Specific Antibodies
CAR-T






Monoclonal Antibodies
Daratumumab and Darzalex Faspro
Sarclisa (Isatuximab)
TAK-079 MOR202

Immune Therapies
Abecma (Ide-cel CAR-T)
Carvykti (Cilta-cel CAR-T)
Tecvayli (Teclistamab)
Elrexfio (Elranatamab)




Other CAR Ts



Other Bi Specific Antibodies



Antibody Drug Conjugates



How it works:
An antibody directed at a target (BCMA) combined with a cytotoxic agent (chemotherapy)
ADC = Antibody-Drug Conjugate
BCMA = B-Cell Maturation Antigen


ADCP/ADCC = Antibody-Dependent Cellular Cytotoxicity & Phagocytosis
Image Credit: https://creativecommons.org/licenses/by-nc/3.0/


Bispecific Antibodies: Mechanism of Action
• Incorporates 2 antibody fragments to target and bind both tumor cells and T cells
• Brings target-expressing MM cells and T cells into close proximity, enabling T cells to induce tumor-cell death
Targets of Bispecific Molecule Vary


FcRH5
“Off the Shelf” Advantage
• No manufacturing process, unlike CAR T-cell therapy (but like ADC/belantamab therapy)
• Thus, no delay between decision to treat and administration of drug ADC = Antibody-Drug Conjugate; BCMA = B-Cell Maturation Antigen; CD3 = Cluster of Differentiation 3; FcRH5 = Fc receptor-homolog 5; GPRC5D = G-protein coupled receptor family C group 5 member D


The Process of CAR T Cell Therapy
CAR T therapy recommended. Insurance approved and ready to move forward.













The Evolution of Myeloma Therapy
VD
Rev/Dex
CyBorD
VTD
VRD
KRD
D-VMP
DRD
ASCT
Tandem ASCT (?)
Nothing
Thalidomide?
Bortezomib
Ixazomib
Lenalidomide
Combinations
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin*
Melphalan flufenamide*
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Teclistamab, Talquetamab
Elranatamab
D-VRD
Isa-VRD
D-KRD
Isa-VRD “More” induction?
Daratumumab?
Carfilzomib?
Lenalidomide + PI
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib.
Speaker’s own opinions.
CAR T Cell Therapy
Bispecific/Tri-specific
Antibodies
Cell Modifying Agents
Venetoclax
PD/PDL-1 Inhibition?
Small Molecules
* These agents are currently off the market but available through special programs
Anito-cel
Cevostomab
Linvoseltamab
Iberdomide, Mezigdomide
Sonrotoclax


Second/Expert Opinion
• You have the right to get a second opinion. Insurance providers may require second opinions.
• A second opinion can help you:
– Confirm your diagnosis
– Give you more information about options
– Talk to other experts
– Introduce you to clinical trials

– Help you learn which health care team you’d like to work with, and which facility



















Closing the Gap: Health Disparities in Myeloma (Video)
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation

Advancing Treatment Options Through Clinical Trials
Alfred Chung, MD
University of California San Francisco Medical Center


Advancing Treatment Options Through Clinical Trials
Alfred Chung, MD
3/29/2025

What are clinical trials?
Goal of clinical trials are to determine new treatments or combinations of treatments to prevent, detect, or treat disease.
Clinical trials have specific eligibility criteria that must be met for a patient to participate
Clinical trials follow a strict protocol to ensure safety and the validity of scientific method.
There are different phases and designs of clinical trials, depending on specific aims of the trial.

Advantages of participating in clinical trials
All FDA-approved therapies started out as investigational agents on clinical trials!
Provides access to new therapies, which may be better than existing agents.
Close monitoring and care with a multi-disciplinary team.
Help contribute to the science and research for the field of multiple myeloma.
Study medications are generally paid for by the study sponsor

Disadvantages of participating in clinical trials
Dependent on the details of specific study.
Depending on phase of the study, unknowns regarding safety and effectiveness of the therapy.
Trial option should be weighed against available standard options.
As trials are protocol-driven, there may be more frequent visits and procedures than normal practice.
Often requires care at trial center

How to participate in clinical trials?
Reach out to clinical trial site Consult with your oncologist
Most crucial timepoint is at the time when myeloma is starting to progress BUT before the start of new treatment
In California, most health plans must cover "routine patient care costs" associated with cancer clinical trials

Ideal candidates for clinical trials
Able to adhere to required treatment protocols
Accepts degree of uncertainty with treatments
Have adequate organ function and strong enough to proceed
Can benefit from the clinical trial

Areas of unmet needs in multiple myeloma therapy
Newly diagnosed Multiple Myeloma
• What are the best initial strategies for treatment of multiple myeloma?
• Can we cure multiple myeloma with the use of very active agents upfront
Autologous Stem Cell Transplant
• Is transplant needed in the era of increasingly effective frontline therapies?
Induction
Therapy
• Are there alternatives to transplant that may be better?
• What is the best maintenance strategies to achieve safe and longterm remission?
• Can we stop maintenance after certain duration or depth of response?
Maintenance
Therapy

Areas
of unmet needs in multiple myeloma therapy
Relapsed/Refractory Myeloma
Early
Relapses (1-3 prior lines of therapy)
• Are there new therapies that are safer and/or more effective than current established treatments? (e.g. novel CAR-T cell therapies)
• Can therapies approved in later line relapses be more effective in earlier lines or as part of new combinations? (e.g. bispecific antibodies)
Late Relapses (>4 prior lines of therapy and/or exposure to prior T-cell engaging therapies)
• After exhaustion of available approved therapies, are there novel targets or treatment modalities that can safe and effective?
• Are there novel combinations of established therapies that can work?

Examples of current active MM clinical trials (NorCal)
Newly Diagnosed MM
At diagnosis:
- CARTITUDE-6 (UCSF, Stanford)
Phase 3 randomized trial of autologous stem cell transplant versus ciltacabtagene autoleucel (CART) therapy after quadruplet induction
- aMMbition (UCSF)
Phase 2 curative-intent non-transplant trial utilizing different sequences of immunotherapies including ciltacabtagene autoleucel (CAR-T) therapy upfront
Post-transplant maintenance:
- DRAMMATIC (UC Davis)
Phase 3 randomized trial: lenalidomide versus daratumumab+lenalidomide maintenance with further randomizations for continuation vs. discontinuation of therapy based on minimal residual disease
- Bispecific antibody therapy (UCSF)
TBD/in-start up

Examples of current active MM clinical trials (NorCal)
Relapsed/Refractory
Early Relapse:
- iMMagine-3 (UCSF): Ph3 randomized to anitocabtagene autoleucel (CAR-T) versus standard therapies
- MagnetisMM-32 (UC Davis): Ph3 randomized to elranatamb (BCMA-bispecific) versus standard therapies
- JNJ-79635322 (UCSF): Ph1 Trispecific BCMAxGPRC5DxCD3
Late Relapses/Novel therapies
- Arlocabtagene Autoleucel (UCSF): GPRC5D CAR-T
- BMS-986453 (UCSF, Stanford): Dual (BCMA+GPRC5D) CAR-T
- KTX-1001 (UCSF): MMSET inhibitor for t(11;14) MM
- ISB 2001 (Stanford): BCMAxCD38xCD3 trispecific antibody
- KPG-818 (UC Davis): Cereblon modulator (related to iMIDs)

Thank you!


Q&A WITH PANEL
Program Evaluations
Please be sure to complete your program evaluation and return your evaluations at the end of today.
We greatly appreciate your time and feedback!

BREAK
WHEN YOU RETURN FROM BREAK
PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: NDMM - GETTING STARTED WITH MYELOMA MANAGEMENT
Please move to Flyer AB Room
BREAKOUT B: RRMM - CONTINUING THE MYELOMA TREATMENT JOURNEY
Please remain in this room
Thank you to our sponsors!










Breakout A (Flyer Room)
NDMM: Getting Started with Myeloma Management
Sarah Lee, MD
City of Hope, Duarte, CA


Frontline Therapy
Review the importance of DEPTH of response in early treatment of myeloma and the increasing use of MRD testing
Discuss emerging approaches in transplant eligible patients, including quadruplet therapy and stem cell transplantation
Outline the approach to a patient not going to transplant and how to optimize therapy

Goals of Therapy: The Iceberg Model of Myeloma
>1 Trillion
Disease Burden (# of myeloma cells)
>1 Billion
>10 Million
1 myeloma cell in 100K to 1 million normal cells




Symptomatic Myeloma

At diagnosis

Partial response
50% reduction in M protein

Very good partial response 90% reduction in M protein immunofixation positive only
Complete remission
No M-protein immunofixation negative

Minimal Residual Dis
Flow Cytometry

Minimal Residual Dis
Next Generation Molecular testing


Depth of response matters!
MRD refers to the persistence of residual tumor cells after treatment and is responsible for relapse1
Current techniques can detect MRD with a sensitivity of 10-6 for MM cells2
MR→PR→
VGPR→CR →sCR
10–2 10–6 Induction Consolidation Maintenance Preemptive
MRDhigh
MRDlow
MRDneg
MR, minimal response; neg, negative; pos, positive; R, relapse
MRDpos


1. Adapted from Hauwel M, Matthes T. Swiss Med Wkly 2014:144:w13907

2. Biran N, et al. Curr Hematol Malig Rep 2014;9:368–78
MRD is Prognostic – Both for PFS and OS

JJ,
B, et al. J Clin Oncol. 2017; 35(25): 2900–2910

Lahuerta
Paiva

SMOLDERING MULTIPLE MYELOMA
What about Smoldering Myeloma?
• Recall that all patients prior to having active myeloma, have MGUS (monoclonal gammopathy of undetermined significance) then SMM (smoldering myeloma)
• Historically we have not treated smoldering myeloma, but redefined MM to include “ultra-high risk smoldering myeloma”
• But now clinical trials are being conducted in patients with smoldering myeloma – mostly “high risk”
• High risk SMM is defined in different ways, often including at least 2 out of 3 of the following factors:
• M spike 2 or more
• Light chain ratio (involved/uninvolved) 20 or over
• Bone Marrow Plasmacytosis of 20% or higher

Phase 3 Randomized Study of Daratumumab Monotherapy Versus Active Monitoring in Patients With High-risk Smoldering Multiple Myeloma: Primary Results of the AQUILA Study
Meletios A Dimopoulos1, Peter M Voorhees2, Fredrik Schjesvold3, Yael C Cohen4, Vania Hungria5, Irwindeep Sandhu6, Jindriska Lindsay7, Ross I Baker8, Kenshi Suzuki9, Hiroshi Kosugi10, Mark-David Levin11, Meral Beksac12, Keith Stockerl-Goldstein13, Albert Oriol14, Gabor Mikala15, Gonzalo Garate16, Koen Theunissen17, Ivan Spicka18, Anne K Mylin19, Sara Bringhen20, Katarina Uttervall21, Bartosz Pula22, Eva Medvedova23, Andrew J Cowan24, Philippe Moreau25, Maria-Victoria Mateos26, Hartmut Goldschmidt27, Tahamtan Ahmadi28, Linlin Sha29, Els Rousseau30, Liang Li29, Robyn M Dennis31, Robin Carson32, S Vincent Rajkumar33
1National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece; 2Levine Cancer Institute, Atrium Health Wake Forest University School of Medicine, Charlotte, NC, USA; 3Oslo Myeloma Center, Department of Hematology, Oslo University Hospital, Oslo, Norway; 4Tel-Aviv Sourasky (Ichilov) Medical Center and Tel Aviv University, Tel Aviv, Israel; 5Clínica Medica São Germano, São Paulo, Brazil; 6Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada; 7Kent and Canterbury Hospital, Kent, UK; 8Perth Blood Institute, Murdoch University, Perth, Australia; 9Japanese Red Cross Medical Center, Tokyo, Japan; 10Ogaki Municipal Hospital, Ogaki City, Japan; 11Albert Schweitzer Hospital, Dordrecht, The Netherlands; 12Ankara University, Ankara, Turkey; 13Washington University School of Medicine, St. Louis, MO, USA; 14Institut Català d'Oncologia and Institut Josep Carreras, Hospital Germans Trias I Pujol, Barcelona, Spain; 15South-Pest Central Hospital, National Institute for Hematology and Infectious Diseases, Budapest, Hungary; 16Hospital Alemán, Buenos Aires, Argentina; 17Jessa Hospital, Hasselt, Belgium; 18Charles University and General Hospital, Prague, Czech Republic; 19Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; 20SSD Clinical Trials in Oncol-ematologia e Mieloma Multiplo, AOU Città della Salute e della Scienza di Torino, Torino, Italy; 21Medical Unit Hematology, Karolinska University Hospital, Stockholm, Sweden; 22Institute of Hematology and Transfusion Medicine, Warszawa, Poland; 23Knight Cancer Institute, Oregon Health & Science University, Portland, OR, USA; 24University of Washington and Fred Hutchinson Cancer Center, Seattle, WA, USA; 25University Hospital Hôtel-Dieu, Nantes, France; 26University Hospital of Salamanca/IBSAL/Cancer Research Center-IBMCC (USAL-CSIC), Salamanca, Spain; 27GMMG Study Group at University Hospital Heidelberg, Internal Medicine V, Heidelberg, Germany; 28Genmab US Inc., Plainsboro, NJ, USA; 29Janssen Research & Development, LLC, Shanghai, China; 30Janssen Research & Development, Beerse, Belgium; 31Janssen Research & Development, LLC, Raritan, NJ, USA; 32Janssen Research & Development, LLC, Spring House, PA, USA; 33Mayo Clinic, Rochester, MN, USA.
Presented by MA Dimopoulos at the 66th American Society of Hematology (ASH) Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA, USA
https://www.congresshub.com/ASH2024/ Oncology/Daratumumab/Dimopoulos
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

AQUILA: Study Design
AQUILA
Screening
Key eligibility criteria:
• ≥18 years of age
• Confirmed SMM diagnosis (per IMWG criteria) for ≤5 years
• ECOG PS score of 0 or 1
• Clonal BMPCs ≥10% and ≥1 of the following risk factors:
- Serum M-protein ≥30 g/L
- IgA SMM
- Immunoparesis with reduction of 2 uninvolved Ig isotypes
- Serum involved:uninvolved FLC ratio ≥8 and <100
- Clonal BMPCs >50% to <60%
All patients were required to have CT/PET-CT and MRI imaging during screening
enrollment period: December 2017 to May 2019 at 124 sites in 23 countries
Treatment/active monitoring phase
DARA monotherapy
1800 mg SCb QW Cycles 1-2, Q2W Cycles 3-6, Q4W thereafter in 28-day cycles until 39 cycles/36 months*
Active monitoring
No disease-specific treatment, with AE monitoring up to 36 months*
Follow -up phase
• Efficacy follow -up until progression by SLiM-CRAB
• Survival follow -up every 6 months until end of study
Primary endpoint:
• PFS by IRC per IMWG SLiM-CRAB criteriac Key secondary endpoints:
• ORR
• Time to first-line treatment for MM
• PFS on first-line treatment for MM
• Overall survival
*Or confirmed disease progression (whichever occurred first).
Stratified by number of risk factorsa for progression to MM (<3 vs ≥3)
Disease evaluation schedule
• Laboratory efficacy – Every 12 weeks by central lab until disease progression
• Imaging (CT/PET-CT, MRI) – Yearly (central review)
• Bone marrow – At least every 2 years
IMWG, International Myeloma Working Group; ECOG PS, Eastern Cooperative Oncology Group performance status; BMPC, bone marrow plasma cell; FLC, free light chain; CT, computed tomography; MRI, magnetic resonance imaging; QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; AE, adverse event; IRC, independent review committee; ORR, overall response rate. aRisk factors included involved:uninvolved FLC ratio ≥8 (yes vs no), serum M-protein ≥30 g/L (yes vs no), IgA SMM (yes vs no), immunoparesis (reduction of 2 uninvolved immunoglobulins vs other), or clonal BMPCs (>50% to <60% vs ≤50%). bDARA SC (1800 mg co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; ENHANZE® drug delivery technology; Halozyme, Inc.]). cPFS was defined as duration from randomization to initial documented progression to active MM or death due to any cause, whichever occurred first.

AQUILA: Progression to MM by IMWG SLiM-CRAB Criteria (IRC Assessment)

AQUILA: Overall Survival
*Deaths due to an event occurring after the AE reporting window (ie, events that happened after patient started subsequent therapy or >30 days after last dose) or deaths with unknown reason.
Early intervention with fixed duration DARA extended overall survival versus active monitoring

AQUILA: Safety Overview

Points for Smoldering Myeloma
• Wow this is a VERY important study and may well change the way we think about and treat high risk smoldering myeloma
• The study was critical to really prove we can delay the progression to active myeloma and even improve survival with 3 years of daratumumab
• It underscores the importance of a DISCUSSION with the healthcare team as many options can be offered to patients with high-risk smoldering MM
• There will be MANY more trials coming in this area, with even more intense therapies like combinations and even CAR T Cell therapy...



NEWLY DIAGNOSED MULTIPLE MYELOMA
Newly Diagnosed MM and Risk
Stratified
Factors to be considered for ASCT
Age, performance status (PS), comorbidities (R-MCI score, HCT-Cl) and organ function

1. Most patients will be given a combination of drugs to control the disease quickly, usually a QUADRUPLET
2. We don’t “save the best for last” because early therapies have a long term effect on survival
3. We seek a DEEP and DURABLE response
4. We mix and match from the 3 major classes of drugs and add steroids:
Proteasome Inhibitors – most often botezomib (Velcade)
Immunomodulatory Drugs – lenalidomide (Revlimid)
Monoclonal Antibodies – daratumumab (Darzalex) and Isatuximab (Sarclisa)
5. We decide early on whether or not someone will have a stem cell transplant

QUADRUPLET therapies are becoming the standard of care for MOST (but not all) patients with newly diagnosed MM
DVRD and Isa-VRD combinations below now FDA approved
Transplant Eligible
Darzalex-Velcade-Revlimid-Dexamethasone (PERSEUS)
Transplant Ineligible
Sarclisa-Velcade-Revlimid -Dexamethasone (IMROZ)
Sarclisa-Velcade-Revlimid -Dexamethasone (BENEFIT)
Darzalex-Velcade-Revlimid-Dexamethasone (CEPHEUS)


PERSEUS: Study Design

Induction

• Transplanteligible NDMM
• Age 18-70 years
• ECOG PS ≤2

V: 1.3 mg/m2 SC
Days 1, 4, 8, 11
R: 25 mg PO Days 1-21
d: 40 mg PO/IV Days 1-4, 9-12

Consolidation

V: 1.3 mg/m2 SC Days 1, 4, 8, 11 R: 25 mg PO Days 1-21
: 40 mg PO/IV Days 1-4, 9-12

Maintenance

DARA: 1,800 mg SCb Q4W R: 10 mg PO Days 1-28 Key eligibility criteria:
10 mg PO Days 1-28 until PD

DARA: 1,800 mg SCb QW Cycles 1-2 Q2W Cycles 3-4
VRd administered as in the VRd group
4 cycles of 28 days

DARA: 1,800 mg SCb Q2W
VRd administered as in the VRd group




Primary endpoint: PFSc
Key secondary endpoints: Overall ≥CR rate,c overall MRD-negativity rate,d OS
Discontinue DARA therapy only

Discontinue DARA therapy only after ≥ 24 months of D-R maintenance for patients with ≥CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
ECOG PS, Eastern Cooperative Oncology Group performance status; V, bortezomib; SC, subcutaneous; PO, oral; d, dexamethasone; IV, intravenous; QW, weekly; Q2W, every 2 weeks; PD, progressive disease; Q4W, every 4 weeks; MRD, minimal residual disease; CR, complete response; OS, overall survival; ISS, International Staging System; rHuPH20, recombinant human hyaluronidase PH20; IMWG, International Myeloma Working Group; VGPR, very good partial response. aStratified by ISS stage and cytogenetic risk. bDARA 1,800 mg co-formulated with rHuPH20 (2,000 U/mL; ENHANZE drug delivery technology, Halozyme, Inc., San Diego, CA, USA). cResponse and disease progression were assessed using a computerized algorithm based on IMWG response criteria. dMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with ≥VGPR post consolidation and at the time of suspected ≥CR. Overall MRD-negativity rate was defined as the proportion of patients who achieved both MRD negativity (10–5 threshold) and ≥CR at any time.
PERSEUS: Overall ≥ CR Rates
Subgroup no. of patients with ≥CR/total no. (%)
Sex
Age
<65 years
≥65 years
Other
143/205 (69.8) 105/149 (70.5)
186/267 (69.7) 62/87 (71.3)
226/323 (70.0) 22/31 (71.0)
129/178 (72.5)
84/125 (67.2) 34/50 (68.0)
122/185 (65.9) 73/96 (76.0)
185/211 (87.7) 127/144 (88.2) 235/261 (90.0) 77/94 (81.9)
(87.6) 23/25 (92.0)
(89.8)
(88.6)
(80.0)
Indeterminate
182/266 (68.4) 59/78 (75.6) 7/10 (70.0) 160/230 (69.6) 88/124 (71.0)
(87.3) 72/78 (92.3) 234/264 (88.6) 63/76 (82.9) 15/15 (100)
195/221 (88.2) 117/134 (87.3)
(0.77-4.58)
3.54 (2.12-5.90) 3.78 (1.45-9.83) 3.60 (2.27-5.70)

Phase
3 Study Results of Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone
(Isa-VRd) Versus VRd for Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma (IMROZ)
Thierry Facon,1 Meletios-Athanasios Dimopoulos,2 Xavier Leleu,3 Meral Beksac,4,5 Ludek Pour,6
Roman Hajek,7 Zhuogang Liu,8 Jiri Minarik,9 Philippe Moreau,10 Joanna Romejko-Jarosinska,11 Ivan Spicka,12
Vladimir Vorobyev,13 Michele Cavo,14 Hartmut Goldschmidt,15 Thomas Martin,16 Salomon Manier,17
Marie-France Brégeault,18 Sandrine Macé,18 Christelle Berthou,18 Robert Z. Orlowski19
1Department of Haematology, University of Lille, and French Academy of Medicine, Paris, France; 2Department of Clinical Therapeutics, National and Kapodistrian University of Athens, Greece; 3Service d'Hématologie et Thérapie Cellulaire, CHU and CIC Inserm 1402, Poitiers Cedex, France; 4Department of Hematology, Ankara University, Ankara, Turkey; 5Istinye University Ankara Liv Hospital, Ankara, Turkey; 6Department of Internal Medicine, Hematology and Oncology, University Hospital Brno, Brno, Czech Republic; 7Department of Hemato-Oncology, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 8Shengjing Hospital of China Medical University (Huaxiang Br), Shenyang, China; 9Department of Hemato-Oncology, University Hospital Olomouc and Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic; 10Department of Hematology, University Hospital Hôtel-Dieu, Nantes, France; 11Department of Lymphoid Malignancies, Marie Sklowdoska-Curie National Research Institute of Oncology, Warszawa, Poland; 12Charles University and General Hospital in Prague, Prague, Czech Republic; 13SP Botkin Moscow City Clinical Hospital, Moscow, Russia; 14IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia "Seràgnoli," Università di Bologna, Bologna, Italy; 15Department of Internal Medicine V, University of Heidelberg, Heidelberg, Germany; 16Department of Hematology, University of California at San Francisco, San Francisco, California, USA; 17Department of Hematology, University Hospital Center of Lille, Lille, France; 18Sanofi, R&D, Vitry-sur-Seine, France; 19Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Copies

ID #OA-49
Study design: Isa-VRd vs VRd in transplant-ineligible NDMM
Initiation phase (4 x
Treatment until PD, unacceptable toxicities, patient withdrawal

Primary endpoint: PFS
Key secondary endpoints: CR rate, MRD– CR (NGS, 10-5) rate, ≥VGPR rate, OS
*Patients considered Ti due to age or comorbidities.
†In the maintenance phase, patients randomized to the VRd arm who experience PD may cross over to receive Isa-Rd.
‡10 mg/day if eGFR 30 to <60 mL/min/1.73 m2
§If aged ≥75 years, d was administered on days 1, 4, 8, 11, 15, 22, 25, 29, and 32.
C, cycle; CR, complete response; d, dexamethasone; eGFR, estimated glomerular filtration rate; Isa, isatuximab; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGS, next-generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PO, orally; R, lenalidomide; SC, subcutaneous; Ti, treatment-ineligible; V, bortezomib; VGPR, very good partial response. Orlowski RZ, et al. ASCO 2018.

Baseline characteristics
Patient characteristics were balanced in both arms
*One patient in the Isa-VRd arm had an ECOG PS of 3. †High risk defined as the presence of del(17p) and/or t(4;14) and/or t(14;16), with cutoffs defined in footnote ‡ ‡Abnormality defined as present in at least 30% of abnormal bone marrow plasma cells for t(4;14) and t(14;16) and 1q21+ (at least 3 copies), and at least 50% of abnormal plasma cells for del(17p). Only one patient had 2 high-risk cytogenetic abnormalities: del(17p) and t(4;14). §1q21+ defined as at least 3 copies of 1q21. Amplification 1q21 defined as at least 4 copies of 1q21. ¶In addition, there were 67 (25.3%; Isa-VRd) and 49 (27.1%; VRd) patients with paramedullary disease and 1 patient in each group with both
and
Primary endpoint met: Interim PFS analysis–IRC assessment
population
60-mo PFS rate: 63.2%
mPFS: NR

60-mo PFS rate: 45.2%
mPFS: 54.34 months (95% CI, 45.207 to NR)
*Cutoff date for PFS analysis: September 26, 2023 (median follow-up, ~5 years). †Nominal one-sided P value. CI, confidence interval; HR, hazard ratio; Isa, isatuximab; ITT, intent-to-treat; mPFS, median PFS; NR, not reached; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; VRd, bortezomib, lenalidomide and dexamethasone. Facon T, Dimopoulos MA, Leleu X, et al. Isatuximab, Bortezomib, Lenalidomide and Dexamethasone for Multiple Myeloma.
BENEFIT Study design: Isa-VRd vs Isa-Rd in Ti NDMM

†Cycle 1 only. CR, complete response; Cy, cycle; d, dexamethasone; D, day; Isa, isatuximab; M, month; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGS, next generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; R,
PRESENTED BY: Xavier Leleu, MD, PhD
lenalidomide; SPM, second primary malignancy; Ti, transplant-ineligible; V, bortezomib; VGPR, very good partial response.
Quadruplets are indeed better than triplets in patients going to transplant
They also seem to be better in transplant ineligible patients but with some caveats
we have less evidence in patients over 80
dosing of drugs is CRITICAL to ensure tolerability
Revlimid – we don’t need 25mg in most patients
Velcade – should be given weekly – still unclear as to length of time
Dexamethasone – DOWN with DEX! – we can taper and discontinue in the first 4-6 months
Darzalex-VRD for transplant eligible and Sarclisa-VRD for transplant ineligible now FDA approved!

#DownWithDex
HISTORICAL TREND TOWARD OPTIMAL DEXAMETHASONE DOSING

Dose
Dose
Dose
40 mg x 12 days per month x 6 months
40 mg x 4 days per month x 6 months
Harding and Mikhael, Blood Editorial “Down with dex!” 2025
Optimal dose yet to be defined


DO WE REALLY STILL NEED TRANSPLANT??
RVD +Stem Cell Transplant vs. RVD without Transplant DETERMINATION Trial of Newly Diagnosed MM: DESIGN
-Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of RVD -56 sites within the United States from 2010 to 2018
End Points of Study and Follow -up
ASCT: Melphalan 200 mg/m2 + Stem Cell Support (n = 310)
• Primary end point: progression-free survival (time to next relapse)
• Secondary end points included:
• Response rates, overall survival, quality of life, and adverse events
• Follow-up on participant status : median of 6 years
N Engl J Med. 2022 Jul 14;387(2):132-147. doi: 10.1056/NEJMoa2204925
Primary endpoint: Progression-free survival (PFS)




of Newly Diagnosed MM Quality
of Life

Global Health Status/QoL, Physical Functioning

DETERMINATION Discussion
ASCT remains very relevant and important in prolonging PFS in younger and eligible patients
BUT it may not be mandatory in all eligible patients upfront
As with other agents, we INDIVIDUALIZE the sequencing patterns
ASCT does carry genuine toxicity, short term and long term
We may become callous to these toxicities
Maintenance therapy remains an important part of myeloma therapy
Joseph Mikhael – ASCO Plenary Discussant DETERMINATION

ASCO Guidelines: What criteria are used to assess
eligibility for autologous stem cell transplant (SCT)?
Recommendation
Patients should be referred to a transplant center to determine transplant eligibility
Evidence Rating
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Chronologic age and renal function should not be the sole criteria used to determine eligibility for SCT.
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Mikhael J, et al. J Clin Oncol. April 1, 2019. DOI:10.1200/JCO.18.02096.

As noted before, quadruplets are now becoming the standard of care for most patients
However, some patients may not be “quad eligible”
In these patients, triplets, or even doublets may be considered
the most commonly used regimen is DRD – darzalex, revlimid and dex
this is based on the MAIA trial of DRD vs RD

MAIA Study Design – DRD vs RD
‒ Patients were enrolled in MAIA from March 2015 through January 2017

Key eligibility criteria
• TIE NDMM
• ECOG PS score 0-2
• CrCl
≥30 mL/min
D: 16 mg/kg IV
QW Cycles 1-2, Q2W Cycles 3-6, then Q4W thereafter until PD
R: 25 mg PO Days 1-21 until PD
da: 40 mgb PO or IV
Days 1, 8, 15, 22 until PD
Primary endpoint
• PFS Key secondary endpoints
• MRD (NGS; 10–5) 1:1 randomisation
d: 40 mg PO Days 1, 8, 15, 22 until PD
Cycles: 28 days Rd
End-oftreatment visit (30 days after last dose) Longterm follow-up
R: 25 mg PO Days 1-21 until PD
• OS
• PFS2
• ORR
• CR/sCR rate
Updated PFS

• D-Rd continued to demonstrate a significant PFS benefit, with median PFS not reached with D-Rd
• These data provide a new PFS benchmark in patients with NDMM who are
Overall Survival

D-Rd demonstrated a significant benefit in OS, with a 32% reduction in the risk of death, in patients with NDMM who are transplant ineligible
Key Take-Aways
Although historically we have not treated smoldering myeloma, new evidence suggests we can treat patients with high risk SMM with daratumumab monotherapy for 3 years
D-VRD is the new standard of care in Transplant Eligible Patients
Isa-VRD is a new standard of care in Transplant Ineligible Patients
DRD or Sarclisa+Rd may still be considered in some patients
Although ASCT remains the standard of care, use is likely to decline in patients who are 65-75 or with significant comorbidities
Continuous therapy has resulted in better outcomes
The balance of toxicity and efficacy is particularly important in this population
ESPECIALLY with dexamethasone
Ongoing studies will help us decide if CAR T cell therapy should move upfront and possibly even replace transplant
What we do frontline has an impact in the long term...



Breakout B (Main Room)
RRMM: Continuing the Myeloma Treatment Journey
Alfred Chung, MD
University of California San Francisco Medical Center


RRMM: Continuing the Myeloma Treatment Journey
Alfred Chung, MD
03/29/25

Relapsed/Refractory Myeloma
- Relapsed: Myeloma previously under control that is now returning or getting worse“progressing”
- Refractory: Myeloma that is not responding to specific treatment (while on therapy or within 60 days of last therapy)


RRMM: Definition/types of relapses
Progressive Disease: worsening or return of myeloma based on formal IMWG response criteria
• Biochemical progression: return of disease based on blood markers only – without new damage/symptoms
• Symptomatic progression: typically, new lesions in the bone or other organs usually with concurrent biochemical progression
IMWG Progression Criteria
Any one or more of the following criteria: 25% increase from lowest confirmed response value in one or more of the following criteria:
• Serum M-protein (absolute increase must be ≥0.5 g/dL);
• Serum M-protein increase ≥1 g/dL, if the lowest M component was ≥5 g/dL;
• Urine M-protein (absolute increase must be ≥200 mg/24 hours);
• In patients without measurable serum and urine M-protein levels, the difference between involved and uninvolved FLC levels (absolute increase must be >10 mg/dL);
Lines of therapy: 1 or more cycles of planned therapy. A new line of therapy starts when planned course of therapy is modified to include other agents as a result of disease progression, relapse, or toxicity
• In patients without measurable serum and urine M-protein levels and without measurable involved FLC levels, bone marrow plasma-cell percentage irrespective of baseline status (absolute increase must be ≥10%);
• Appearance of a new lesion(s), ≥50% increase from nadir in >1 lesion, or ≥50% increase in the longest diameter of a previous lesion >1 cm in short axis;
• ≥50% increase in circulating plasma cells (minimum of 200 cells per microL) if this is the only measure of disease.

RRMM: Goals
To prevent damage to organs – such as bone and kidneys
To re-achieve a deep disease response and try to sustain that response as long as possible
To try to maximize effectiveness of the therapy and minimize side-effects
To try to prolong overall survival – how long patients live

RRMM: Treatment Selection Considerations
Timing of the relapse
• “Functional high risk” – if early relapse after initial therapy
Response to prior therapy (sensitive or refractory)
• Consideration of medication class switching (different drug target)
Aggressiveness of the relapse (biochemical vs. symptomatic)
• Disease related risk factors such as FISH/cytogenetics status
• Extramedullary myeloma and plasma cell leukemia
Performance status/Patient factors
• Functional status/frailty/fitness
• Other health conditions
• Social support system
Strength/Safety of Therapy
• Considering the most effective treatments based on available clinical data
• Considering the side-effect profile of the selected therapy

Myeloma Toolbox
Immunomodulator Imide Drugs (IMiDs)


Proteasome Inhibitor (PI)



Antibodies



Bispecific antibodies/T-cell engagers:
High-dose chemo (hyperCD, D-CEP, VD-PACE)


(antiBCMA)
Anti- BCMA Chimericantigen Receptor T-cell Therapies (CAR-T cell therapy)
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)



Other FDA approved therapies:



Idecabtagene vicleucel (ide-cel)
Ciltacabtagene autoleucel (cilta-cel)

Lenalidomide
Pomalidomide
Thalidomide
Bortezomib
Bendamustine
Teclistamab
Elotuzumab (anti-CS1 antibody)
Selinexor (XPO inhibitor)
Off-label
Venetoclax (for t(11;14) myeloma)


Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)
Yellow = Commonly used and available in earlier lines of therapy (<4) Red = Commonly used and available only in late relapse currently (after 4 lines of therapy)

Bortezomib
Off-label
Venetoclax (for t(11;14) myeloma)
IMiDs – Convenient/effective oral drugs
Lenalidomide
- Less useful in RRMM – as most patients are refractory but can be considered if not refractory/unexposed
Pomalidomide
- Very commonly used in RRMM as an effective oral medication
- Can be combined with various agents to increase effectiveness (e.g. carfilzomib, cyclophosphamide, anti -CD38 antibody)
- Has penetration into central nervous system
Thalidomide
- Not used commonly in the US
Class benefits/side-effects
- May help activate T-cells
- Fatigue
- Cytopenias – lowering of the blood counts
- GI issues – diarrhea
- Risk of blood clots/strokes

Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Bortezomib
Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)
Yellow = Commonly used and available in earlier lines of therapy (<4) Red = Commonly used and available only in late relapse currently (after 4 lines of therapy)

Off-label
Venetoclax (for t(11;14) myeloma)
Proteasome Inhibitors (PI) – Generally, once a week treatments
Bortezomib (given under the skin)
- Less useful in RRMM – as most patients exposed; however, is an options as less patients are refractory
- Highest rates of neuropathy
Carfilzomib (given in IV)
- Most potent of the PIs and deepest responses
- Downsides:
Cardiovascular risks (high blood pressure, heart failure, arrhythmias)
Fatigue/dyspnea
Risk of kidney injury
Low blood counts
Ixazomib (given as capsule)
- Not commonly used as lower efficacy seen in studies; however, may be a good option for some patients as can be combined with pomalidomide/dexamethasone as an all -oral option
- May have higher rates of gastrointestinal side-effects

Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)
Yellow = Commonly used and available in earlier lines of therapy (<4) Red = Commonly used and available only in late relapse currently (after 4 lines of therapy)

Bortezomib
Off-label
Venetoclax (for t(11;14) myeloma)
Anti-CD38 antibodies – Daratumumab/Isatuximab
Among MM drugs, likely has one of the highest benefit-to-risk ratio; if not received in the 1st line of therapy – would strongly consider at first-relapse
Generally, Daratumumab and Isatuximab considered with regards to safety and efficacy
Dosing frequency:
- Daratumumab – once a week x 8 doses, every other week x 8 doses, and then monthly ongoing
- Isatuximab – once a weeky x 4 doses, and then every other week ongoing

Side-effects:
• Infusion reaction – usually with just the first dose
• Risks of infection/lower immunoglobulin levels – some patients may require IVIG supplementation
Novellis D et al. Int. J. Mol. Sci. 2023, 24(1), 645

Pomalidomide / CAR-T / Carfilzomib Randomized Phase 3 Studies
Attal M et al. Lancet. 2019;394(10214):2096-2107. 2. Dimopoulos MA et al. ASH 2020. Abstract 412. 3. Sebag M et al. ASH 2020. Abstract 413. 4. Rodriguez-Otero P et al. N Engl J
. 2023;388(11)1002-1014. 5. Leaked EHA abstract shows

Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)
Yellow = Commonly used and available in earlier lines of therapy (<4)
= Commonly used and available only in late relapse currently (after 4 lines of therapy)

Bortezomib
Off-label
Venetoclax (for t(11;14) myeloma)
Conventional chemotherapy
Cyclophosphamide
- Can commonly used at beginning as can be dosed relatively full strength even in setting of bad kidney function
- At high-doses – can be administered in the hospital as high-dose chemotherapy when needed for very aggressive disease (“hyper-CD”)
- Advantages: can be given orally, relatively well tolerated at weekly doses, can be combined with other myeloma therapies
- Disadvantages: low blood counts, GI side-effects, may affect cellular therapies downstream when given at high doses.
Others are used in special circumstances:
- High-dose chemotherapy for very aggressive disease
- Bendamustine can be considered as late line option
- 2nd melphalan autologous transplant sometimes considered in select patients

Commonly used “off-the-shelf” regimens after relapse from Dara-RVD
Carfilzomib/Pomalidomide/Dexamethasone
- Useful with prior Dara-RVD induction
Carfilzomib/Cyclophosphamide/Dex
- Useful for Pom-refractory patients
Pomalidomide/Cyclophosphamide/Dex
- Useful for carfilzomib-refractory patients -
1. Sonneveld P et al. Hemasphere. 2022;6(10):e786. 2. Shah JJ et al. Blood. 2015;126(20):2284-2290. 3. Derman BA et al. Blood Adv. 2023;7(19):5703-5712. 4. Venner CP et al. Am J Hematol. 2021;96(5):552-560. 5. Garderet L et al. Blood. 2018;32(24):2555-2563. 6. Baz RC et al. Blood. 2016;127(21):2561-2568.

Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Yellow = Commonly used and available in earlier lines of therapy (<4)
Red = Commonly used and available only in late relapse currently (after 4 lines of therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)

Bortezomib
Off-label
Venetoclax (for t(11;14) myeloma)
Bispecific Antibodies

Targeting BCMA - Teclistamab - Elranatamab
Targeting GPRC5D - Talquetamab


Bispecific Administration
Currently all approved products are subcutaneous (under the skin) injections
Only approved for RRMM after 4 prior lines of therapy, including PI, an iMiD, and an anti-CD38 monoclonal Ab.
Generally, requires an initial inpatient hospitalization for “step-up” dosing before reaching full-doses (~7 – 9 day hospital stay for teclistamab; 9 – 11 days for talquetamab)
This is to monitor and treat for cytokine release syndrome “CRS” and immune effector associated neurotoxicity “ICANS” – which can commonly happen

Cytokine Release Syndrome


Shimabukuro-Vornhagen. j. immunotherapy cancer 6, 56 (2018).

Unique side-effect profiles for bispecifics
BCMA bispecifics – Teclistamab, Elranatamab
- Hypogammaglobulinemia – low immunoglobulins -> warrants monthly infusion for IVIG (intravenous immunoglobulins) to prevent infections
- Higher risks of infections GPRC5D bispecific - Talquetamab
- Skin rash/skin peeling
- Nail changes
- Dysgeusia = loss of taste
- Weight loss

Bispecifics - Efficacy


Bispecifics - Efficacy
Talquetamab


18 mos
CAR-T Cell Therapy





CAR-T Process



127 Sheykhhasan, M., Ahmadieh-Yazdi, A., Vicidomini, R. et al. CAR T therapies in multiple myeloma: unleashing the future. Cancer Gene Ther 31, 667–686 (2024).
Bispecifics vs. CAR-T: Factors

Advantages:
• “off-the-shelf”
• Easy to coordinate administration
• Lower upfront toxicity (lower grade CRS/no lymphodepletion)
• More tolerable in frail/elderly patients
• Potentially translatable to community practice
• Titratable
Disadvantages:
• Ongoing B-cell aplasia (for BCMA-bispecifics)
• Need for ongoing therapy/unclear duration of therapy
• Potentially lower durability of remissions
• Potential for T-cell exhaustion
• Currently only approved after > 4 lines of therapy
Disadvantages:
• Requires specialized centers to administer
• Need for apheresis and ex vivo T-cell modification
• Time to manufacture/risk of nonviable product
• Need for lympodepletion/risk of cytopenias
• Risks of delayed adverse toxicities (e.g. neurotox, colitis, etc)
• Higher risks/grades of CRS/IEC-HS
Advantages:
• “one-and-done”
• Preferable sequencing before bispecifics
• Potentially longer duration of response
• Potentially better for extramedullary disease compared to single-agent bispecifics


Candidate for CAR-T Therapy?
Access/Availability
Social/Economic Support
Adequate cardiovascular/renal/liver function/functional status
Able to receive adequate bridging/tolerate manufacturing

Important considerations/side-effects of CAR-T Therapy
1st month is intensive including frequent lab checks and possible transfusions – (at UCSF – twice a week clinic visits after initial hospital stay of 10 days post-CART for cilta-cel or 7 days post-CART for ide-cel)
Main risks during hospital stay:
- CRS/ICANS
- Low blood counts
Main risks after discharge
- Low blood counts
- Infections/low immunoglobulin levels => may need IVIG monthly for first 6 months
- (Rare) – delayed neurotoxicities – parkinsonism-like syndrome, nerve palsies
For first 4 weeks, must be within 2-hours of treatment center
For first 8 weeks, no driving or operating heavy machinery

BCMA Sequencing – CART before Bispecific Better
Elranatamab: 54% ORR (7/13) with prior BCMA
1. Anderson L et al. 2021 ASCO. Abstract 8016. 2. Ferreri CJ et al. 2021 ASH. Abstract 766. 3. Berdeja J et al. Lancet. 2021;398(10297);314-324.
4. Lin Y et al. 2022 EHA. Abstract P961. 5. Cohen AD et al. 2022 ASH. Abstract 2028. 6. Moreau P et al. N Engl J Med. 2022;387(6):495-505. 7. Touzeau C et al. 2022 ASCO. Abstract 8013. 8. Raje NS et al. 2022 ASH. Abstract 895.

Candidates for CAR-T Therapy -Early referral important
Success depends on good planning!
Fitness of T-cells likely important:
- Consideration of CAR-T in earlier lines
Treatment exposures can affect T-cell fitness and potentially CAR-T manufacturing success
- Alkylator therapy (e.g. bendamustine, high-dose cyclophosphamide, etc)
- Prior bispecific therapies
BCMA sequencing matters
- Prior BCMA exposure appears to decrease PFS/duration of responses to CAR-T
Washout periods may be needed for prior high-dose therapies and bispecifics before collection
- >6 – 8 week washout from prior high-dose alkylator, bendamustine, bispecific therapy
High burden of disease warrants debulking with effective bridging
- Ideally with effective conventional therapies, but may need high-dose therapies at times (e.g. VDPACE, hyper-CD)
- Talquetamab: bridging new approach

Myeloma Toolbox
Immunomodulator
Imide Drugs (IMiDs)


Lenalidomide
Proteasome Inhibitor (PI)
Pomalidomide
Thalidomide


Carfilzomib
Ixazomib


Anti-CD38 Antibodies Daratumumab

Conventional chemotherapy


Bispecific antibodies/T-cell engagers:

Cyclophosphamide
Isatuximab
Melphalan
High-dose chemo (hyperCD, D-CEP, VD-PACE)
Teclistamab (antiBCMA)
Anti- BCMA Chmericantigen Receptor Tcell Therapies (CART cell therapy)
Yellow = Commonly used and available in earlier lines of therapy (<4)
Red = Commonly used and available only in late relapse currently (after 4 lines of therapy)
Bendamustine
Elranatamab (antiBCMA)
Taqluetamab (antiGPRC5D)


Idecabtagene vicleucel (ide-cel)
Other FDA approved therapies:
Ciltacabtagene autoleucel (ciltacel)




Elotuzumab (antiCS1 antibody)
Selinexor (XPO inhibitor)

Bortezomib
Off-label
Venetoclax (for t(11;14) myeloma)
Other agents
Elotuzumab (anti-CS1 antibody) - IV
- Only effective when combined with iMiD; no single agent activity
- Only marginal added benefit of response rates and duration of response
Selinexor (XPO inhibitor) – tablets
- Selinexor/dexamethasone only modest response rates (~26%) but can improve to >70% when combined with other agents (e.g. bortezomib, carfilzomib, pomalidomide)
- May be useful in patients who have otherwise exhausted other options or as bridging to or while awaiting immunotherapies
- Side-effects: low sodium, notable nausea/vomiting, lowering platelet counts
Venetoclax – tablets (OFF-LABEL)
- Have shown very high-response rates in patients with t(11;14) on FISH testing, including deep responses
- Most effective when combined with PI or anti-CD38 antibodies
- Side-effects: lowering of blood counts, GI side-effects

Therapies on the horizon – future outlook
Return of belantamab mafadotin (anti-BCMA antibody-drug conjugate)
CAR-T
- Utilizing earlier (potentially frontline)
- Availability of more potent CAR-Ts, dual targeting and new antigens
Bispecific therapies
- Finding optimal combinations to prevent resistance and prolong response/aim for cure
- Tri-specific antibodies
Expanding the arsenal
- CELMODs: iberdomide, mezigdomide
- Cevostamab: anti-FCRH5 bispecific antibody
- ISB2001: CD3xBCMAxCD38 trispecific antibody
- BCL2 inhibitors for t(11;14) MM

Choosing the right treatment strategy depends on the patient! There is no one-size fits all!
Questions?


LUNCH
Please move to the Astrojet Room
IMF REGIONAL COMMUNITY WORKSHOP
SAN FRANCISCO
AFTERNOON AGENDA



IMF Update
Robin Tuohy, Vice President, Support Groups International Myeloma Foundation

Managing Side Effects and Living Well with Myeloma
Kevin Brigle, PhD, NP
Massey Comprehensive Cancer Center
Virginia Commonwealth University, Richmond, VA

Seasons of Multiple Myeloma
Kevin Brigle, PhD, NP
Oncology Nurse Practitioner
VCU Massey Comprehensive Cancer Center




Wintery Mix of Treatment Options Spring into Managing Side Effects

Summer of Success











Wintery Mix of Treatment Options

Diverse and Complex Treatment Combinations









Myeloma Treatment Common Combinations
Velcade® (bortezomib)
Lenalidomide
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab)
DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel)
Carvykti (ciltacabtagene autoleucel)


Elrexfio (elranatamab) --

Tecvayli® (teclistamab) --
Talvey (talquetamab) --

Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options


Stem Cell Transplant
ELIGIBILITY
1
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization Collecting Stem Cells
Duration: Approximately 2 weeks
TRANSPLANT
High Dose Chemotherapy
Stem Cell Infusion
Supportive Care Engraftment
2 PHASE 3
Duration: Approximately 3-4 weeks
POST-TRANSPLANT
Restrengthening
Appetite recovery
Location: HOME PHASE
Location: Transplant Center
Location: Transplant Center
“Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks

CAR T: Another Treatment Approach
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)
Manufacturing takes
≈ 4 to 6 weeks
Bridging therapy may be needed
T-Cell Collection


No driving for 8 weeks
“One & Done” with continued monitoring
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support


Bispecific Antibodies
• Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
• BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
BISPECIFIC ANTIBODIES

– Elrexfio (elranatamab)

• GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss





– Talvey (talquetamab)




CAR T and Bispecific Antibodies: Unique Side Effects





CRS is a common but often mild & manageable side effect


CAR = chimeric antigen receptor; CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.




CAR T and Bispecific Antibodies: Unique Side Effects













Spring Into Managing Side Effects



The Early Bird Gets the Worm: Communicate Proactively
with Your Healthcare Team
Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue

How You Feel
Side Effects of Treatment can
cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Neuropathy
• Fatigue

Tip: Keep a Symptom Diary and bring it to appointments
Tip: proactively discuss common side effects and what to do if they occur



Are Steroids Messing With Your Sunny Disposition?
Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider
Managing Steroid Side Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections

Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes

Infection Can Be Serious for People With Myeloma
Preventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Infection Prevention Tips
Good personal hygiene (skin, oral)

Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines

Preventative and/or supportive medications (next slide)


Medications Can Reduce Infection Risk
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV
Medication Recommendation(s) for Healthcare Team Consideration
Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection Consider prophylaxis with levofloxacin
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)

Some
Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IVIg recommended
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain treatment dose intensity
people receiving BCMA-targeting therapies have experienced infections that are less common like CMV, PJP and fungal infections


GI Symptoms: Prevention & Management
Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
• Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency Increase fiber
• Stay well hydrated
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Anorexia, the inability to eat, is
common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake
Diarrhea is common during transplant and long-term maintenance therapy. Other medications and supplements
• Hydration is very important
• Electrolyte replacement is common
• Good skin care will help prevent irritation
• Stool exam may be needed to rule-out infection
• If no infection, anti-diarrheal medication may be prescribed

Discuss GI issues with healthcare providers to identify causes and make adjustments to medications and supplements


Management of Oral Side Effects

Mouth
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate anti-fungal therapy for oral thrush
Taste Changes
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended.
Weight Management
Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Dental Care
Attention to oral hygiene.
Regular
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia.
Meet with a Nutritionist
Consider diet changes, supplements
Monitor weight
Education and emotional support are key strategies to manage oral toxicities.

Dry
Dysphagia
Catamero D, Purcell K, Ray C, et al. Presented at the 20th International Myeloma Society (IMS) Annual Meeting Nurse Symposium; September 27–30, 2023; Athens, Greece.

Skin and Nail Side Effects
Possible side effect to some treatments and supportive care medications


Skin Rash:
• Prevent dry skin; apply lotion
• Report changes to your care team
• Medication interruption or alternative, as needed
• Steroids:
– Topical for grades 1-2,
– Systemic and topical for Grade 3
• Antihistamines, as needed
Nail Changes:

• Keep your nails short and clean. Watch for “catching and tearing”

• Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
• A nail hardener may help with thinning
• Tell the team if you have signs of a fungal infection, like thickened or discolored nails

Photos: Mount Sinai Hospital, NY, NY
Feel Like a Spring Chicken: Prevent and Manage Pain
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy


Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled


Peripheral Neuropathy Management
Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness

Prevention / management:
• Bortezomib once-weekly and/or subcutaneous administration
• Massage area with cocoa butter regularly
• Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
• Safe environment: rugs, furnishings, shoes
If neuropathy worsens, your provider may:
• Adjust your treatment plan
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed
B, et al. CJON. 2017;21(5)suppl:19-36. Tariman, et al. CJON.2008;12(3)suppl:29-36. Zhao T, et al. Molecules. 2022;27(12):3909.



Understanding Changes to Kidney Function
• Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
• Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
• Treatment
– Treatment for myeloma
– Hydration
– Dialysis


Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important

Additional Supportive Care


Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.


Summer of Success



Let the Sun Shine In


Fatigue is the most reported symptom.
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression 98.8%
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm.
>35% of patients
≈25% of patients



Bee an Empowered Patient
Ask
questions
– What are my treatment options?
– What are the pros and cons of the different options?
– How will we know if treatment is working?
– What do the different labs mean?
– Who will be monitoring my labs?
– How can I access my test results (eg, patient portal)?
– What will we do if my treatment doesn’t work or quits working?
Participate in decisions
– Share your priorities and preferences
– Include care partner(s) in your discussion



Speak up if something seems different or unusual
– Normally 4 vials of blood but only drawing 3?
– Normally specialty pharmacy confirms delivery but haven’t heard from them this month?
– When is my next appointment?
Live in the sunshine, swim the sea, drink the wild air.
Communicate with your healthcare team
– Understand the roles of each team member
– Who to contact for your needs (eg, side effects, insurance issues, other)
Develop a support network
– Learn from others: IMF has many support groups or you can start one (IMF’s can help)

– Ralph Waldo Emerson

Care Partners Are Vital for Success


If you want to go fast, go alone, if you want to go far, go together
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)



African Proverb
• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners




Cultivate A Care Network


If you want to go fast, go alone, if you want to go far, go together
• Multiple studies demonstrate that strong social ties are associated with longevity, improved adherence to medical treatment and overall improved health outcomes
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions


• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners




Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466 -475. Yang YC, et al. Proc Natl Acad Sci U S A. 2016;113(3):578 -583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol 2010; 75(2):122–137.
African Proverb
IMF Care Giver Tip Cards

Enjoy Life’s Bounty


Harvest Good Health
Have a Primary Care Provider & Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Women specific: mammography, pap smear
• Men specific: prostate
• Vision
• Hearing
• Dermatologic evaluation
• Dental checkups & cleaning

Develop & maintain healthy behaviors
• Good nutrition
• Regular activity
• Quit tobacco use
• Sufficient Sleep (next slide)
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56.
An ounce of prevention is worth a pound of cure. Benjamin Franklin

Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.





























Q&A


Living the Myeloma Life: Local Patient & Care Partner
Alan Plisskin (Patient Advocate) &
Mary Kay Yamamoto (Care Partner)

Living the Myeloma Life: Alan and Mary Kay
















Beyond Myeloma Therapy:
Understanding Infection - Risk, Prevention and Management
Peter Chin-Hong, MD
University of California San Francisco Health
Beyond myeloma therapy: infectious diseases
Peter Chin-Hong MD Professor of Medicine & Associate Dean
March 29, 2025


Questions

For myeloma patients:
• Why increased risk of infectious diseases?
• What are common infectious diseases?
• What are common prevention strategies for infectious diseases?

Questions
For myeloma patients:
• Why increased risk of infectious diseases?
• What are common infectious diseases?
• What are common prevention strategies for infectious diseases?

Why infections in myeloma?
• The disease – Plasma cells affected – Normally make antibodies to fight infection
• Treatment of disease – Longer survival also increases infection risk
• Infection more common early on – Especially in the first year

Why infections in myeloma?
• The disease
– Plasma cells affected – Normally make antibodies to fight infection
• Treatment of disease
– Longer survival also increases infection risk
• Infection more common early on
– Especially in the first year
During stem cell transplants, drugs and radiation kill cancer cells but may also damage the immune system

Then the new healthy cells move in
Why infections in myeloma?
• The disease – Plasma cells affected – Normally make antibodies to fight infection
• Treatment of disease – Longer survival also increases infection risk
• Infection more common early on – Especially in the first year

Blimark C et al, Haematologica.
Questions
For myeloma patients:
• Why increased risk of infectious diseases?
• What are common infectious diseases?
• What are common prevention strategies for infectious diseases?

Common infections
Early: bacteria and viruses

Pneumonia
• Streptococcus pneumoniae
• Influenza
• RSV
• COVID-19

Skin infections
• Varicella zoster virus (Bortezomib)
• Staphylococcus aureus

Urine infections
• E Coli
Hepatitis B
• (Bortezomib)
Common infections
Later: fungus & others

Pneumonia
• Pneumocystis jiroveci/PCP (dexamethasone)

Skin/throat/vaginal
• Candida albicans

Pneumonia/Sinusitis
• Aspergillus fumigatus
Questions
For myeloma patients:
• Why increased risk of infectious diseases?
• What are common infectious diseases?
• What are common prevention strategies for infectious diseases?

General strategies
• Wash hands • Mask/Ventilation • Sleep/nutrition/exercise • Vaccinate – Pneumococcus, RZV zoster, COVID, RSV, influenza, Hep B
• Immunoglobulin replacement
• Antibiotic prophylaxis

Common infection prevention
Early: bacteria and viruses

Pneumonia
• Streptococcus pneumoniae [Levofloxacin for at least 3 mo] [Vaccine]
• Influenza [Vaccine]
• RSV [Vaccine]
• COVID-19 [Vaccine]

Skin infections
• Varicella zoster virus (Bortezomib) [acyclovir] [Vaccine]
• Staphylococcus aureus

Urine infections
• E Coli
Hepatitis B
• (Bortezomib) [Vaccine]
Common infection prevention
Later: fungus & others

Pneumonia
• Pneumocystis jiroveci/PCP (dexamethasone) [TMP/SMX]

Skin/throat/vaginal
• Candida albicans

Pneumonia/Sinusitis
• Aspergillus fumigatus
Questions
For myeloma patients:
• Why increased risk of infectious diseases?
• What are common infectious diseases?
• What are common prevention strategies for infectious diseases?







Thank you

Q&A WITH PANEL

Closing Remarks
Robin Tuohy, VP Patient Support
International Myeloma Foundation
Program Evaluations
Please be sure to complete your program evaluation and return your evaluations at the end of today.
We greatly appreciate your time and feedback!

Thank you to our speakers & our sponsors!









OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

IMF Core Values:
These are the core values we bring to accomplishing our mission each day.
Patient Centric
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
Respect All
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
Excellence and Innovation
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
Honor differences
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.




