SCIENCE
IL Foundation Series - Science Class 7
Legal Disclaimer
This book is intended for educational purposes only. The information contained herein is provided on an “as-is” and “as-available” basis without any representations or warranties, express or implied. The authors (including any affiliated organizations) and publishers make no representations or warranties in relation to the accuracy, completeness, or suitability of the information contained in this book for any purpose.
The authors (including any affiliated organizations) and publishers of the book have made reasonable efforts to ensure the accuracy and completeness of the content and information contained in this book. However, the authors (including any affiliated organizations) and publishers make no warranties or representations regarding the accuracy, completeness, or suitability for any purpose of the information contained in this book, including without limitation, any implied warranties of merchantability and fitness for a particular purpose, and non-infringement. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any errors, omissions, or inaccuracies in the content or information provided in this book.
This book does not constitute legal, professional, or academic advice, and readers are encouraged to seek appropriate professional and academic advice before making any decisions based on the information contained in this book. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any decisions made based on the information provided in this book.
The authors (including any affiliated organizations) and publishers disclaim any and all liability, loss, or risk incurred as a consequence, directly or indirectly, of the use and/or application of any of the contents or information contained in this book. The inclusion of any references or links to external sources does not imply endorsement or validation by the authors (including any affiliated organizations) and publishers of the same.
All trademarks, service marks, trade names, and product names mentioned in this book are the property of their respective owners and are used for identification purposes only.
No part of this publication may be reproduced, stored, or transmitted in any form or by any means, including without limitation, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the authors (including any affiliated organizations) and publishers.
The authors (including any affiliated organizations) and publishers shall make commercially reasonable efforts to rectify any errors or omissions in the future editions of the book that may be brought to their notice from time to time.
Subject to Hyderabad jurisdiction only.
Copyright © 2025 Rankguru Technology Solutions Private Limited. All rights reserved.
ISBN 978-81-985304-7-9
Second Edition
PHYSICS

1.1 INTRODUCTION TO MOTION
When something changes its position as time passes, we say it's in motion. This happens all around us, and there are different ways things can move. Some different types of motion:
Linear motion: When an object moves along a straight line. For example, a bullock cart moving on a straight road.
Circular motion: When an object moves along a circular path. For example, the motion of fan blades.
Periodic motion: When an object repeats its movement at regular intervals of time. For example, the motion of a pendulum, soldiers in march past, etc.
1.2 SLOW OR FAST
We have seen around us that some objects move fast and some slow. Like a man riding a bicycle will be slower than a man driving a car.
We can tell if an object is moving slowly or fast by looking at how far it travels in a certain amount of time.
1.2.1 Slow and fast motion
If something travels a short distance in a given time, it's moving slowly. If an object covers a long distance in the same amount of time, it's moving fast
Bicycle with a rider pedalling slowly
Person walking leisurely
Ant carrying food back to its nest
Table 1.1 Objects that move slowly
Objects that move fast
Car zooming down the highway
Racing bicycle with a cyclist pedalling quickly
Sprinter running in a race
Cheetah chasing its prey
Aeroplane flying overhead
Table 1.2 Objects that move fast

1.3 SPEED
Speed refers to how fast something is moving. It tells us how much distance an object covers in a certain amount of time.
For example, If we say a car is travelling at a speed of 60 kilometres per hour, it means that the car will cover 60 kilometres in one hour.
However, when discussing the motion of a car, it’s essential to recognize that a car rarely maintains a constant speed throughout an entire hour. Typically, a car starts slowly and gradually accelerates. For example, if we state that a car travels at 60 kilometres per hour, we are referring to the average speed over the entire hour. This average speed considers the total distance covered by the car divided by the total time taken. In our discussions, we will consistently use the term 'speed' to refer to this average speed calculation.
Calculation of speed: The formula to calculate speed is:
Speed = Total distance covered
Total time taken
Example: A driver covers 40 km in an hour in a bus. Find its speed.
Solution:
Speed = Total distance covered
Total time taken = 40 km per hour
So, the speed of the driver in the bus is 40 km per hour.
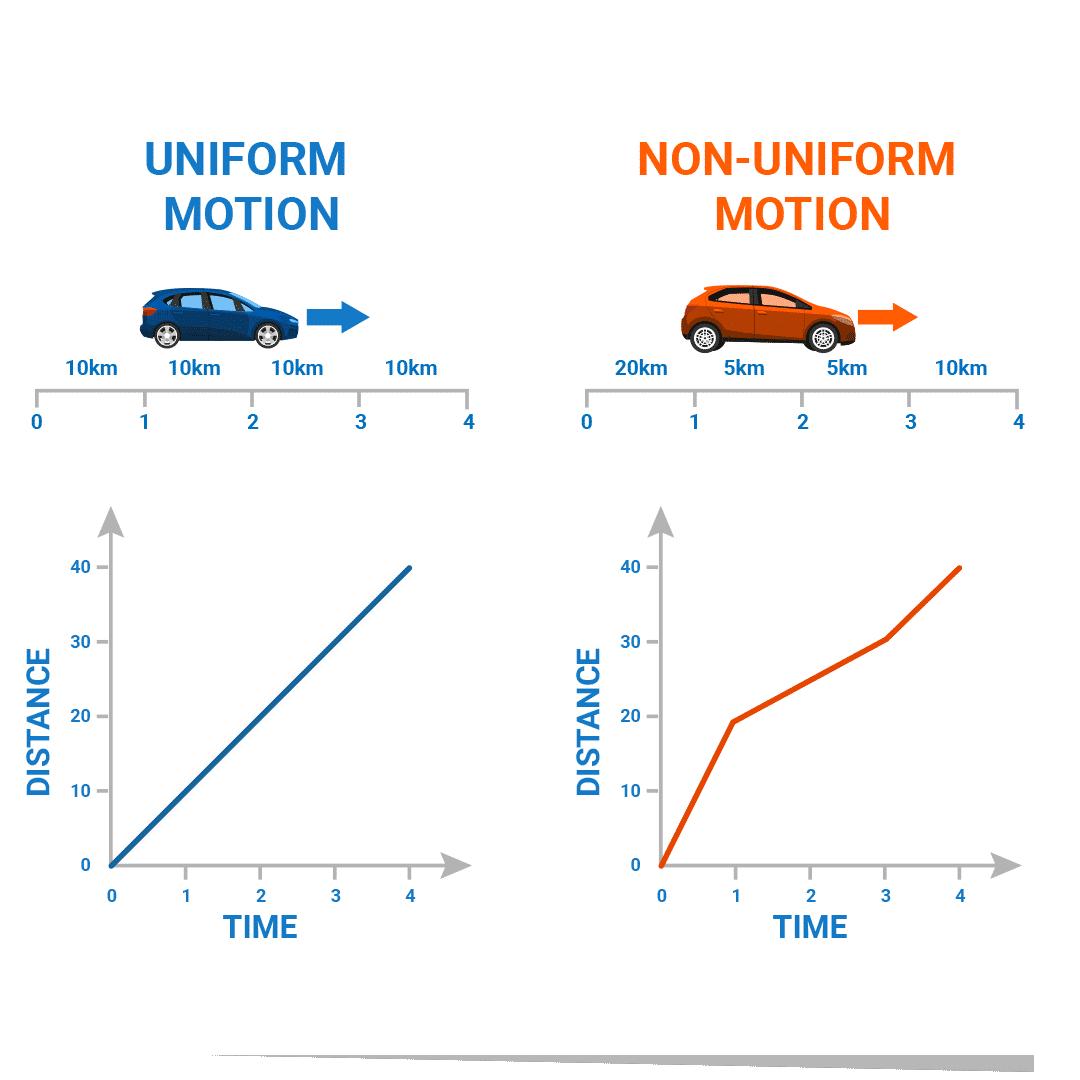
1.3.1 Uniform and non-uniform motion

Uniform motion: When an object moves in a straight line with a constant speed, it's called uniform motion. In uniform motion, the object covers equal distances in equal intervals of time.
For example, if a car covers 1 km in 5 minutes, it will continue to cover the same distance in the next 5 minutes and subsequent intervals.
Non-uniform motion: If the speed of an object changes as it moves along a straight line, it's called non-uniform motion. In non-uniform motion, the object covers unequal distances in equal intervals of time.
For example, if a car covers 1 kilometre in the first 5 minutes, but then its speed changes. In the next 5 minutes, it might cover a shorter or longer distance, depending on how its speed fluctuates.
UNIFORM
1.4 MEASUREMENT OF TIME
Time is how we humans track and record changes happening around us and in the vast universe.
For example, the time from one sunrise to the next gives us a day, while a month is measured from one new moon to the next. A year marks the time it takes for Earth to complete one journey around the sun. But we also need to track the time for a shorter duration than a day for which we use clocks or watches.

1.4.1 Simple pendulum
The clocks make use of some periodic motion. One of the most common examples of periodic motion is that exhibited by a simple pendulum.
Galileo Galilei conducted experiments with a pendulum and discovered that regardless of the swing's width, the pendulum always takes the same time to go back and forth. This implies that the pendulum swings in equal time intervals.
A simple pendulum consists of a weight (often a small metal ball or a piece of stone known as a bob) tied to a string. The other end of the string is attached to a fixed point.
When the pendulum is still and hanging straight down (like in Fig. 1.4(a)), it's at its starting position. But when we push the bob to one side and let go, it starts moving back and forth (like in Fig. 1.4(b)). This back-and-forth movement of the pendulum is called periodic or an oscillatory motion
The pendulum finishes one oscillation when it moves from its starting position (O) to one side (A), then to the other side (B), and then back to the starting position (O) again. It also finishes one oscillation when it moves from one side (A) to the other side (B) and back to side (A) again.
Time period: The time taken by the weight to complete one swing cycle is called the time period.
By increasing the length of the string, you'll notice changes in the time period. Longer strings usually have longer time periods, which implies the weight takes more time to complete one swing
cycle. This helps us understand how pendulums oscillate and how changing variables like string length affect their motion.
1.4.2 Unit of time
Basic unit of time: The basic unit of time is the second (s), represented by the symbol 's'.
Larger units of time include minutes (min) and hours (h), with their relationship known to us.
Some units of time:
• 1 millennium = 10 centuries
• 1 century = 10 decades
• 1 decade = 10 years
• 1 year = 365 days/ 366 days(leap year)
• 1 day = 24 hours
• 1 hour = 60 minutes
• 1 minute = 60 seconds
Basic unit of speed: Speed is measured as distance travelled per unit of time. Therefore, the basic unit of speed is metres per second (m/s). However, it can also be expressed in other units, such as metres per minute (m/min) or kilometres per hour (km/h).
Consistency in unit symbols: It's essential to remember that unit symbols are written in singular form. For example, we write '100 km' and not '100 kms', or '5 cm' and not '5 cms'.
1.4.3 Sundial
In the past, people measured time by watching the movement of the sun during the day and the moon and stars at night. The Jantar Mantar in Central Delhi is a famous example of an ancient sundial, which used the position of the sun's shadow to tell the time.
Today, we use wristwatches and quartz clocks. A mechanical wristwatch has a balance wheel that swings like a pendulum. In a quartz clock, tiny quartz crystals vibrate quickly and precisely, making them very accurate. The most accurate clock is the atomic clock, like the one at the National Physical Laboratory (NPL) in Delhi. It beeps before the morning news to indicate standard time and is accurate up to a millionth part of a second.


1.5 MEASURING SPEED
When you want to know how fast you're moving, you need to measure your speed. You can do this by calculating the distance you travel over a certain amount of time. For example, if you walk from your home to school, you can measure the time it takes and the distance covered to find your speed. Also, if you know your speed and distance, you can estimate how long it will take to reach your destination.
Speedometer: In vehicles like cars, scooters, and motorcycles, you'll often find a metre called a speedometer. This handy tool measures your speed directly in kilometres per hour (km/h). It's usually displayed on the dashboard, making it easy for the driver to keep track of their speed while driving.
Odometer: Another useful metre found in vehicles is the odometer. Unlike the speedometer, which measures speed, the odometer measures the distance travelled by the vehicle. It helps drivers keep track of how far they've travelled, which is handy for various purposes like maintenance or tracking mileage for business or personal reasons.

SOLVED EXAMPLES
Example 1: A boy walks from his home to a post office at a distance of 0.5 km in 20 min. He then returns to his home in 25 min. Find the average speed of the boy.
Solution:
Distance of post office from home = 0.5 km = 500 m
Total distance travelled in going and coming back
D = 500 m + 500 m = 1000 m
Total time taken in going and coming back
t = 20 min + 25 min
=45 min = 45 × 60 s = 2700 s
Average Speed = Total distance covered Total time taken = 1000 m 2700 s = 0.37 m s-1
Example 2: A bus is moving at a speed equal to 20 m/s. How much distance will it cover in 60 s?
Solution:
Speed of bus = 20 m/s
Time taken to travel = 60 s
We know,
Speed = Total distance covered Total time taken
⇒ Distance travelled = speed × time
⇒ Distance travelled = 20 m/s × 60 s
⇒ Distance travelled = 1200 m
1.6 GRAPHS
Graphs are visual representations that show data or mathematical relationships.
1.6.1
Types of graphs
There are different types of graphs. The type of graph shown in Fig. 1.7 (i) is known as a bar graph.
Another type of graphical representation is a pie chart (Fig. 1.7(ii)). The graph shown in Fig. 1.7(iii) is an example of a line graph.


1.6.2
• When we want to describe how an object is moving, one of the simplest ways is to create a distance-time graph. This graph tells us how the distance covered by the object changes as time passes. The distance-time graph is a line graph. In a distance-time graph, the slope represents the speed of the object. A steeper slope means the object is moving faster, while a gentler slope indicates slower motion.
• In a distance-time graph, the distance is always plotted on the y-axis (vertical), while time is plotted on the x-axis (horizontal).
Types of distance-time graphs
(i) When a body is at rest: When an object is not changing its position relative to a reference point, it is at rest. The distance-time graph for a body at rest is a straight line parallel to the time axis (x-axis). This indicates that the object stays at the same distance over time.
(m)
The body is at a distance of 15m from the reference point
(s)
(ii) Uniform speed: When an object covers equal distances in equal intervals of time, it has uniform speed. Its distance-time graph is a straight line. All points on this line represent equal distances covered in equal time intervals.
Fig. 1.9 Distance-time graph when the body has uniform speed
(iii) Non-uniform speed: When an object covers unequal distances in equal time intervals, it is referred to as non-uniform speed. The distance-time graph for non-uniform motion will not be a straight line. It can be either a curve or a zig-zag line which is not parallel to the time axis.
Distance(m)
Time(s)
Fig. 1.10 Distance-time graph when the body has non-uniform speed
Representation of multiple objects
A distance-time graph can represent the motion of more than one object. For instance, if we have two cars, A and B, moving at different speeds, their motion can be depicted on the same graph. The car with a higher speed will have a steeper slope on the graph, indicating greater speed.
1.11 Distance-time graph for more than one object
QUICK REVIEW
• When something changes its position as time passes, we say it's in motion.
• If something travels a short distance in a given time, it is said to be in slow motion.
• If an object covers a long distance in the same amount of time, it is said to be moving fast.
• Speed refers to how fast something is moving.
• Speed =Total distance covered/Total time taken
• When an object moves in a straight line with a constant speed, it's called uniform motion.
• If the speed of an object changes as it moves along a straight line, it's called non-uniform motion.
• A simple pendulum has a weight, like a small metal ball or a piece of stone, tied to a string.
• The back-and-forth movement of the pendulum is called oscillatory motion.
• The time it takes for the pendulum to finish one oscillation is called its time period.
• The basic unit of time is the second (s), and the basic unit of speed is metres per second (m/s).
• Different types of graphs are bar graphs, pie charts, line graphs, etc.
• When we want to describe how an object is moving, one of the simplest ways is to create a distance-time graph.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Slow or fast motion
1. When the position of an object changes with time, it is called ______.
a. Transformation b. Progression c. Evolution d. Motion
2. The __________ of the object determines its fast and slow motion.
a. Mass b. Density
3. Which of the following travels the fastest?
a. Cheetah
b. Wind
c. Speed d. Displacement
c. Sound d. Light
4. If Car A is faster than Car B and Car B is faster than Car C. Which of the following is correct?
a. Car C may be faster than Car A.
b. Car B is the slowest.
c. Car A can cover more distance than Car B and Car C in the same amount of time.
d. Car A and Car C can cover the same distance in the same amount of time.
5. Which of the following must be true if Rohan takes longer than John to finish a running race?
a. Rohan runs slower than John.
b. Rohan is faster than John.
c. Rohan runs at the same speed as John.
d. The speeds of Rohan and John can't be compared.
II. Speed
1. A car covers 60 km with a uniform speed of 120 kmph and the next 60 km with a uniform speed of 80 kmph. The total time taken by the car is
a. 55 min b. 60 min c. 75 min d. 80 min
2. If a car travels 54 km in 90 minutes, then find the speed of the car.
a. 0.6 m/s b. 10 m/s c. 5.4 m/s
3. If you travel a distance of 100 kilometres in 2 hours, what is your speed in m/s?
3.6 m/s
a. 15.5 m/s b. 12.09 m/s c. 20 m/s d. 13.89 m/s
4. Which of the following statements is true:
a. The average speed is the same as the actual speed in case of uniform motion.
b. In the case of non-uniform motion, the speed is constant throughout.
c. The speed of a train is 60 kmph if it has travelled 100 km in one hour.
d. You are in a moving car. The car has already travelled 40 km in 2 hours. Currently, the speedometer shows 60 kmph; hence, the average speed of the car is 60 kmph.
III. Measurement of time
1. What is the basic unit of time?
a. Minute (min)
b. Hour (h)
2. Which of the following is a larger unit of time?
a. Second
3. 1 hour = _______ second
a. 60
b. Hour
b. 36
c. Day (d)
c. Month
d. Second (s)
d. Year
c. 360 d. 3600
4. How many metres does a falcon cover in an hour if he travels at a speed of 320 km/hr?
a. 0.320 m
b. 32000 m
5. Which of the following is not a unit of time?
a. Second
b. Minute
6. An example of oscillatory motion is
a. Motion of a cycle wheel
c. Motion of earth around the sun
c. 320000 m
d. 3.2 m
c. Millisecond
d. Light year
b. Movement of a car on a straight road
d. Motion of a swing
7. The to-and-fro motion of a simple pendulum is called:
a. Circulatory motion
c. Arbitrary motion
8. The time period of a pendulum is defined as:
b. Vibratory motion
d. Oscillatory motion
a. The number of oscillations completed by the pendulum in one second.
b. The time that is taken by the pendulum to complete one oscillation.
c. The time that is taken by the pendulum to go from one extreme to the other extreme.
d. The number of rounds completed in one oscillation.
IV. Measuring speed
1. Sunitha can type 1800 words in half an hour. What is her typing speed in words per minute?
a. 60 b. 600 c. 75 d. 30
2. A train runs from New Delhi to Hyderabad. It covers the first distance of 420 km in 7 hours and the next distance of 360 km in 6 hours. Find the speed of the train.
a. 60 km/h b. 70 km/h
c. 80 km/h d. 50 km/h
3. What is the primary function of a speedometer in a vehicle?
a. To control the vehicle's audio system
b. To display the current speed of the vehicle
c. To measure the amount of fuel consumed
d. To monitor the engine temperature
4. A car moves 100 metres westward in 4 seconds and 80 metres eastward in 2 seconds. Find the average speed of the car.
a. 40 m/s b. 30 m/s
5. What does the odometer of a vehicle record?
a. Time
V. Graphs
b. Speed
c. 20 m/s d. 25 m/s
c. Distance
d. None of these
1. What can you say about the nature of the motion of a body if its distance-time graph is a curved line, as shown in the figure?

a. The body is in uniform motion. b. The body is in non-uniform motion.
c. Speed is constant. d. The body is at rest.
2. What can you say about the nature of the motion of a body if its distance-time graph is a straight line parallel to the time axis?
a. The body is not in motion.
c. The body has speed.
b. The body is in motion.
d. The body has velocity.
3. In the given distance-time graph, what is the time corresponding to 3 km?
4. The distance-time graph for a butterfly's motion is shown below. For how many seconds does the butterfly remain stationary?
5. The motion of a toy car is depicted in the graph below as a function of time and distance. How far does the toy car travel in 16 seconds?
6. The given figure shows the distance-time graph for the motion of two vehicles, A and B. Which one of them is moving faster?
a. A is moving faster than vehicle B. b. B is moving faster than vehicle A.
c. A is moving at the same speed as B. d. A is not moving.
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which one of the following is an example of uniform motion?
a. A car is moving on a road with traffic
b. A train entering a railway station
c. Motion of a butterfly
d. An aeroplane flying at a speed of 750 km/h towards the north
2. In a distance-time graph, if the speed of the object is changing continuously, what would the graph look like?
a. A straight line b. A square c. A circle d. A curved line
3. When observing the distance-time graph of an object, how can you determine the distance travelled at a specific time instant?
a. Measure the slope of the line.
b. Draw a vertical line from the time on the x-axis to intersect the graph.
c. Draw a horizontal line from the distance on the y-axis to intersect the graph.
d. Calculate the area under the graph
4. A runner makes one lap around a 200 m circular track in 25 s. The average speed of the runner is
a. 0 ms-1
b. 4 ms-1
c. 8 ms-1
16 ms-1
5. Given the distance between the Earth and the sun is 1.5×108 km and the velocity of light is 3×105 kms-1. The time taken for sunlight to reach the earth is
a. 300 s b. 400 s c. 500 s
6. Which of the following is an oscillatory motion?
a. Vibrating wire of a guitar
c. Cycle wheel
600 s
b. Rotatory top
d. Wings of fans
7. Which of the following does not show oscillatory motion?
a. Swing
b. Fan
c. See – Saw d. Pendulum
8. If the scale chosen for a distance-time graph is 2 km = 1 cm, what would be the length of the x-axis for a total distance of 40 km?
a. 10 cm
b. 20 cm
c. 40 cm d. 80 cm
9. In a distance-time graph, what does the slope of the line represent?
a. Time
b. Distance
c. Speed
d. Acceleration
10. In a distance-time graph, what does a straight line indicate about the object's motion?
a. Constant speed
c. Acceleration
b. Changing speed
d. Deceleration
11. Which of the following statements is false about uniform and non-uniform motion?
a. Uniform motion covers equal distance at an equal time interval
b. Non-uniform motion covers unequal distance at an equal time interval
c. In uniform motion, the distance-time graph shows a curved line.
d. In non-uniform motion, the distance-time graph shows a curved line.
12. A cyclist covers a distance of 750 m in 2 min 30 sec. What is the cyclist's speed in km/hr?
a. 18 km/hr
b. 50 km/hr
c. 5 km/hr d. 180 km/hr
13. What is the relation between distance and speed?
a. Distance = speed × time
c. Distance = time / speed
14. How many seconds are there in a day?
a. 60 b. 3600
b. Distance = speed / time
d. Distance = time + speed
c. 86400 d. 216000
15. The device used for measuring time intervals in sports activities is called
a. Wristwatch
b. Stopwatch c. Stop clock d. Quartz watch
16. The distance-time graph for the motion of an object moving with a constant speed is
a. A curved line leaving towards the x-axis
b. A curved line inclined towards
c. A straight line inclined on the x-axis
d. None of these
17. The distance-time graph of the non-uniform motion is a _________________ line.
a. Curved
c. First straight line and then curved
b. Straight
d. First curved line and then straight
18. When can we say the motion of an object is uniform?
a. Equal distances in different intervals of time
b. Unequal distances in different intervals of time
c. Equal distances in equal intervals of time
d. None of these
19. The odometer of a car reads 57321.0 km when the clock shows the time 08:30 AM. If at 08:50 AM, the odometer reading has changed to 57336.0 km. Calculate the speed of the car in km/h.
a. 40 km/h b. 35 km/h c. 30 km/h d. 45 km/h
20. Which of the following statements must be true if car A covers a longer distance than car B in the same amount of time?
a. Car A is slower than car B.
b. Car A is faster than car B.
c. Car A and car B are travelling at the same speed.
d. The speeds of both cars can't be compared.

ELECTRIC CURRENT AND ITS EFFECTS 2
2.1 ELECTRIC COMPONENTS
2.1.1 Symbols of electric components
The pathway through which electric current flows is referred to as an electric circuit
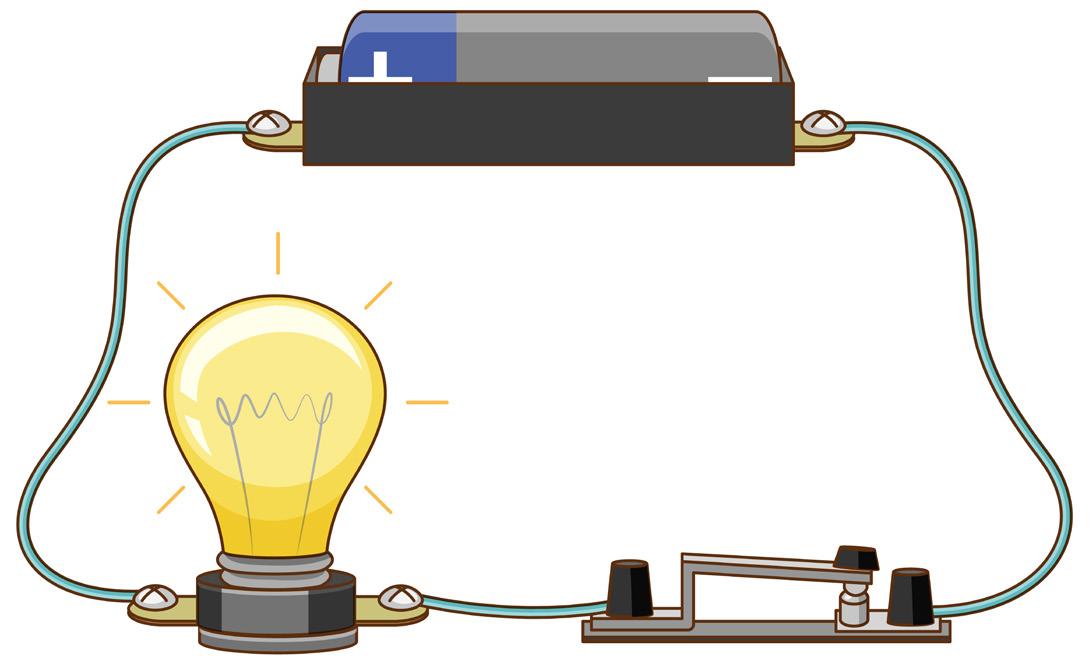

To illustrate the electrical connections via a circuit diagram, typical electrical components are symbolically represented. Below are symbols for several commonly utilized components.
An electric cell works as a source of electrical energy, converting chemical energy into electrical energy, visually denoted by Fig 2.2.
Within this symbol, the longer vertical line denotes the 'positive terminal', while the shorter vertical line denotes the 'negative terminal'. Typically, symbols such as '+' and '–' are inscribed on the cell for identification.
In a circuit diagram, wires used to link various components are depicted by straight lines.
Multiple cells may be connected to enhance current strength for certain applications. When cells are interconnected, the positive terminal of one cell connects to the negative terminal of the next, forming a battery, as symbolised in Fig 2.2.
A switch functions to either close or open an electric circuit and can be positioned at any point within the circuit.
2.1.2 Electric circuit
When the switch is toggled to the 'ON' position, the circuit is complete, extending from the positive terminal of the battery to the negative terminal. This closed circuit allows the current to flow throughout instantaneously.
Conversely, when the switch is set to 'OFF', the circuit remains incomplete, resulting in an open circuit where no current flows through any segment.
2.1.3 Bulb
In the bulb, there exists a thin wire known as the filament, which illuminates upon the passage of an electric current. When the filament of the bulb is broken, the circuit will not be complete because the filament serves as a vital component for the current to flow through the bulb. Therefore, the bulb would not glow because the electrical circuit is interrupted.
Also, sometimes you might have noticed that a glowing electric bulb becomes warm. Let’s understand the reason for that in the next section.
2.2 HEATING EFFECT OF ELECTRIC CURRENT
When a wire/device is connected to a cell/battery for a long duration, it undergoes heating, and this phenomenon is known as the heating effect of electric current. During this process, electrical energy is transformed into heat energy.
2.2.1 Uses of heating effect
In the heating effect of current, the electrical energy is converted into heat energy when electricity flows through a conductor. This effect has both advantages and disadvantages.
It has widespread application in various appliances such as electric irons, heaters, ovens, and filament bulbs. These devices utilize a coil of wire, known as a heating element, which becomes hot when electricity passes through it.
The heating effect also poses some risks. If not controlled properly, it can lead to overheating and potential hazards such as fires. In some instances, when a substantial current flows through a wire, it can become sufficiently heated to melt and eventually break the wire, which may lead to fire or damage to devices.

The amount of heat generated in a wire depends on its length, thickness, and material.
Different materials are employed for wires based on specific requirements. For example, the filament of an electric bulb reaches such high temperatures that it begins to emit light. In contrast, wires used in electric circuits typically do not heat up too much under normal circumstances.
2.2.2 Electric fuses and MCBs
Special wires, composed of specific materials, possess the property of melting rapidly and breaking when subjected to high electric currents. These wires find application in the construction of electric fuses, which are installed in all electrical circuits within buildings or electrical appliances.



Electrical circuits have a maximum safe limit for current flow. If the current exceeds this safe threshold, the wires may overheat, potentially leading to a fire hazard. However, with a proper fuse installed in the circuit, it acts as a safety device by blowing off and interrupting the circuit. Consequently, fuses serve as crucial safety mechanisms, preventing damage to electrical circuits and potential fires. Various types of fuses are utilized for different purposes.
MCBs: Miniature Circuit Breakers (MCBs) can also be used instead of fuse wires. MCBs function as switches that automatically deactivate when the current within a circuit surpasses the designated safe threshold. Upon activation, these switches can be easily reset to restore the circuit's functionality, but on the other hand, once a fuse wire is damaged, it cannot be used again and needs to be replaced with a new one.
Note: MCBs utilise the magnetic effect of electric current
2.3 MAGNETIC EFFECT OF ELECTRIC CURRENT
We know that in a compass, the needle of the compass is a magnet, aligning itself along the northsouth direction. When a magnet is brought close to it, the needle experiences deflection. Similarly, the compass needle deflects when the compass is brought near the current flowing wire.
Insulated
Uninsulated
Compass (Compass Deflection)
Fig. 2.9 Magnetic effect of electric current
In 1800 CE, Hans Christian Oersted made the initial observation that a conducting wire exhibits magnetic properties when an electric current flows through it. This phenomenon is referred to as the magnetic effect of electric current. He was the first to notice that the compass needle consistently deflected whenever current passed through a wire.
Therefore, when electric current traverses through a wire, it effectively behaves like a magnet.
2.3.1 Electromagnet
When a wire is wrapped around a cylinder, the coil surrounding the cylinder shows magnetic properties when an electric current flows through it, which is termed as an electromagnet.
S E
To enhance the strength of the electromagnet, we can increase the current passing through the coil or the number of turns of the coil.
Applications of Electromagnets
• Electromagnets are used in cranes to lift heavy loads efficiently.
• Electromagnets can help separate magnetic materials from waste or junk.
• Doctors utilize electromagnets to extract small metallic fragments that may have inadvertently entered the eye.
• An electric bell incorporates an electromagnet as a vital component for its operation.
In the electric bell, an electromagnet is used as a very crucial component. A soft iron strip featuring a hammer at one end is positioned in proximity to the electromagnet. Upon the passage of current through the coil, it exhibits magnetic properties, attracting the iron strip. As the iron strip moves towards the coil, the attached hammer strikes the gong, generating a sound.
When the iron strip is drawn towards the coil, it disengages from the screw (interrupter), resulting in an open circuit. With the circuit interrupted, the coil stops functioning as an electromagnet, and the attraction between the coil and the iron strip breaks. Consequently, the iron strip returns to its initial position, reconnecting with the contact screw. This action completes the circuit once more, initiating the process repeatedly.
QUICK REVIEW
• The pathway through which electric current flows is referred to as an electric circuit.
• Different symbols are used to represent electrical components.
• Current can flow through only closed circuits, not open circuits.
• The filament is a thin wire used in bulbs, which illuminates upon the passage of an electric current.
• When a wire/device is connected to a cell/battery for a long duration, it undergoes heating, and this phenomenon is known as the heating effect of electric current.
• Fuses or MCBs are used as safety devices in circuits/appliances.
• When an electric current traverses through a wire, it effectively behaves like a magnet, and this phenomenon is termed as the magnetic effect of an electric current.
• When a wire is wrapped around a cylinder, the coil surrounding the cylinder shows magnetic properties when an electric current flows through it, which is termed as an electromagnet.
• In an electric bell, electromagnet is used for its proper working.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Electric components
1. What does the given symbol represent?
a. Ammeter
c. Variable resistor
b. Voltmeter
d. Electric cell
2. In the below diagram, the electric switch is _______ and _______, respectively.
a. On and Off b. Off and On c. Off and Off d. On and On
3. Below are electric symbols of:
a. Cell, Switch and bulb, respectively
b. Switch, electric wire and bulb, respectively
c. Cell, electric wire and switch, respectively
d. Cell, electric wire and bulb, respectively
4. If the circuit is complete, what will happen to the bulb in terms of electricity flow and illumination?
a. The electricity will flow, and the bulb will glow.
b. The bulb will flicker on and off due to intermittent circuit completion.
c. The bulb will not glow unless the circuit is incomplete.
d. The bulb will glow if the circuit is complete or incomplete.
5. In a battery,
a. Two or more cells are connected to each other in any manner
b. The positive terminal of one cell is connected to the positive terminal of the next cell
c. Two or more cells are connected in such a way that the positive terminal of one cell is connected to the negative terminal of the next cell
d. There is only one cell
6. The filament of an electric bulb glows on passage of current because
a. Of the heating effect of electric current
b. It is very thin
c. A chemical reaction takes place in the bulb
d. Of the magnetic effect of electric current
7. ___________ is a device which can break the circuit or make the circuit.
a. A torch
b. A switch
c. A conductor d. An insulator
8. In a bulb, the part which converts electric energy into light energy
a. Terminals
c. Metal base
II. Heating effect of electric current
b. Filament
d. Glass chamber
1. The amount of heat produced in a wire depends on its i) Thickness ii) Material iii) Length
a. ii only
b. i and ii only
2. The heating elements are made from
a. Gold
c. Nichrome alloy
c. ii and iii only d. i, ii, and iii
b. Copper alloy
d. Nickel
3. The device is connected to the circuit for the protection of both the appliances and the people using them.
a. Fuse
b. Bulb
4. The primary function of a fuse is:
a. To open the circuit
c. To reduce the flow of current
c. Battery
d. Resistor
b. To prevent high current flow
d. To close the circuit
5. Which metal is the filamnet of an electric bulb made up of?
a. Tungsten
b. Copper
6. Which of these is false about an electric fuse?
a. The two terminals are made of metal
c. Iron
b. The fuse wire is made of zinc, copper, silver or aluminium
c. The fuse wire is used as a safety device
d. It increases the flow of current in case of a short circuit
d. Silver
7. What is the Full form of MCB?
a. Miniature Circle Breaker
c. Miniature Circuit Breaker
b. Miniature Cycle Breaker
d. Magnetic Cycle Breaker
8. Which of the following devices is nowadays used in place of a fuse?
a. Electromagnet
c. Electric bell
III. Magnetic effect of electric current
b. Galvanometer
d. MCBs
1. What happens when a compass is taken near a current-carrying conductor?
a. The needle of the compass starts pointing in the north direction.
b. The needle of the compass starts pointing in a south direction.
c. The needle of the compass shows deflection.
d. The needle of the compass remains as it is.
2. When an electric current passes through a wire, it behaves like a
a. Magnet
b. Fuse
c. Bulb
d. Switch
3. An electromagnet is based on which of the following effects of electric current?
a. Heating effect of electric current
c. Chemical effect of electric current
4. A doorbell uses a/an
a. Bar magnet
c. Horseshoe magnet
b. Magnetic effect of electric current
d. Physical effect of electric current
b. Electromagnet
d. Disc magnet
5. In which of the following devices is the magnetic effect of electric current not used?
a. An Electric bell
b. A heavy machine used to lift up a car
c. A crane to separate iron materials from junk
d. An electric iron
6. Which of the following statements is false for an electric bell?
a. The iron strip moves away from the electromagnet as soon as the current flows through the circuit.
b. Electric bell works on the concept of electromagnetism.
c. The striker strikes the gong to create the sound.
d. The electric circuit breaks when the iron strip moves towards the electromagnet.
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. The symbols in an electric circuit will help us in identifying
a. Electric current
c. Magnetic effect
b. Electronic device
d. Resistance of circuit
2. In the following, which is not a reason for excessive currents in electrical circuits?
a. Direct touching of wires
c. Switch in off position
3. The combination of two or more cells is called as:
b. Short-circuiting
d. Overloading
a. Switch b. Battery c. Rheostat d. Torch
4. What does the following image represent?
a. Open electric circuit
c. Electrical device
5. The important property of fuse wire
a. High melting point
c. Low boiling point
6. The part of the bulb that glows is
a. Filament
b. Electrode
b. Closed electric circuit
d. Electrical appliance
b. Low melting point
d. High boiling point
c. Electric wire
7. Which of these is not an example of the heating effect of electric current?
a. A light bulb
c. An electric bell
d. Spring
b. An electric iron
d. Electric water heater
8. In the heating effect of electric current, electrical energy is converted into
a. Kinetic energy
c. Chemical energy
9. What are the effects of electric current?
a. Magnetism
c. Magnetism and heat
b. Electric energy
d. Heat energy
b. Sound and heat
d. Sound and light
10. Which of the following principles is an electric fuse based on?
a. Chemical effect of electric current
b. Magnetic effect of electric current
c. Heating effect of electric current
d. Mechanical effect of electric current
11. Which of these is false about an electric fuse?
a. Fuses are designed to interrupt or break the circuit when there is an excessive current flow
b. Fuses can be reused multiple times without any limitations
c. A fuse wire has a low melting point
d. A fuse wire is made of 37 % lead and 63 % tin
12. What happens when the insulation on electrical wires wears off?
a. The circuit becomes more efficient
b. The circuit becomes safer
c. The circuit becomes overloaded
d. The circuit may experience a short circuit
13. Who found the magnetic effect of electric current?
a. Thomas Edison
c. Albert Einstein

b. Hans Christian Oersted
d. Alfred Nobel
14. The most suitable material for making the core of an electromagnet is
a. Iron b. Brass
15. Which of the following is a temporary magnet?
a. Bar magnet
c. Electromagnet
16. An electromagnet loses its magnetism when:
a. The electric current is switched on
b. The electric current is switched off
c. The electric current is increased
d. None of the above
17. An electric bell has:
c. Aluminium d. Steel
b. Horseshoe magnet
d. Disc magnet
i) An interrupter ii) An electromagnet iii) A hammer
a. i and ii only
c. ii and iii only
b. i and iii only
d. i, ii, and iii
18. Three bulbs, A, B, and C, are connected in a circuit, as shown in the figure below. When the switch is 'ON'
A B C
a. Bulb C will glow first.
b. Bulbs B and C will glow simultaneously, and bulb A will glow after some time.
c. All the bulbs A, B and C will glow at the same time.
d. The bulbs will glow in the order A, B and C.
19. Akash wants to increase the strength of his electromagnet. Which of the following can he do to make the electromagnet stronger?
I) Increase the number of times the magnet is stroked.
II) Increase the number of batteries.
III) Increase the number of coils used around the electromagnet.
IV) Change the thickness of the wire.
a. I and III only
c. II and III only
b. II, III and IV only
d. I, II and IV only
20. Why is electrical wiring usually covered with a layer of plastic?
a. To make it strong
c. To make it safe
b. To help electricity flow in it
d. To make it beautiful
21. Which of the following does not belong to the group formed by the others?
a. Copper coin b. Steel spoon c. Wooden ruler d. Iron nail
22. Iron is used for making electromagnets, not steel, nickel, or cobalt, because
a. Iron is cheap and easily available.
b. Iron is a good conductor of electricity.
c. When current is switched off in the coil of an electromagnet made of iron, iron loses all its magnetism quickly.
d. None of these.
23. Mark the correct statement.
a. Fuses and MCBs are safety devices.
b. Fuses are safety devices but MCBs are not.
c. MCBs are safety devices, but fuses are not.
d. Neither fuses nor MCBs are safety devices.
24. When the switch is in the 'ON' position, the electric circuit is said to be
a. Closed
c. Complete
25. When the bulb gets fused, the electric current
a. Flows in the circuit
c. Sometimes flows and sometimes not
26. The key or switch in a circuit is placed
a. Left side of the battery
c. Anywhere in the circuit
27. An electromagnet acts like a magnet
a. When a current is passed through the coil
b. All the time
c. Only if a current does not pass through the coil
d. None of these
b. Open
d. Both (a) and (c)
b. Does not flow in the circuit
d. None of these
b. Right side of the battery
d. Near the positive terminal of the cell

CHEMISTRY

ACIDS, BASES, AND SALTS 1
1.1 ACIDS AND BASES
1.1.1 Introduction
We might have tasted lemon while preparing lemon juice, baking soda while baking a cake, common salt while cooking, and we would also have tasted sugar while preparing sweets. Are all these things similar in taste? Some are sweet, some are sour, some are bitter, and some are salty in taste. Substances that taste sour are acids, while those that taste bitter are bases
Examples,
• Lemon and curd contain acids
• Toothpaste and soap, which we use regularly in our day-to-day life, contain base
• Sodium chloride we use to flavour our foods is the common salt.
1.1.2 Acids
• Acids are chemical substances which have a sour taste.
• It is derived from the Latin word acidus/acere, meaning sour.
• Substances which have a sour taste can be considered acidic

• Citrus fruits and vinegar contain acids due to which they are acidic.
Examples: Lemon, oranges, vinegar, etc.
• Acids can be classified into two types-
1. Organic acids
Acids derived from plants and animals
Examples: Citric acid and formic acid
2. Inorganic/mineral acids
Acids derived from minerals present in the earth's crust or from acids prepared in the laboratory
Examples: Sulphuric acid and nitric acid
Organic acids Acids
Acids that occur naturally are called organic acids.
Examples
Organic Acid
Citric acid
Tartaric acid
Lactic acid Occurs in Lemon Tamarind, grapes Milk
Properties of acids
• Acids turn blue litmus to red
Mineral (inorganic) acids
Acids that are prepared from minerals present in the earth are called mineral acids.
Examples
Sulphuric acid - H2SO4
Hydrochloric acid - HCl
Nitric acid - HNO3
• It gives H+ ions when dissolved in water, which are called hydrogen ions.
• Acidic substances have pH values lower than 7.
• Acids react with the base to form salt and water.
1.1.3 Bases
• Bases are chemical substances which have a bitter taste.
• Bases have a slippery feel and are also soapy to the touch.
• These are mostly found in cleaning agents.
• For example: Soaps, detergents, and household cleaners.
Examples of bases:
Detergent
Baking soda
Drain cleaner
Ammonia
Soaps (hand dish)
Bleach
1.3 Examples of bases
Properties of bases
• Basic substances turn red litmus to blue
• It gives OH- ions when dissolved in water, which are called hydroxide ions.
• Bases have a pH value higher than 7.
• Bases react with acids to form salt and water
Uses of bases
Sodium Hydroxide NaOH Making soap, drain cleaner
Potassium Hydroxide KOH Making soap, battery electrolyte
Calcium Hydroxide Ca(OH)2 Leather production, making plaster
Magnesium Hydroxide Mg(OH)2 Laxative, antacid
Ammonium Hydroxide NH4OH Household cleaner
Aluminum Hydroxide Al(OH)3 Antacid, deodorant
Table 1.1 Bases and their uses
1.1.4 Indicators
• Indicators are the substances that are used to check whether the given substance is acidic or basic.
• Indicators will turn one colour in an acidic solution and will turn another colour in a basic solution.
• Dyes in the indicators change colour as soon as they mix with acids or bases.
• These can be broadly classified into two types:
1. Natural indicators
2. Synthetic indicators
Types of indicator
1.2 NATURAL INDICATORS AROUND US
• It is a type of indicator that can be found naturally.
• Helps to determine whether the substance is acidic or basic in nature.
• Common examples of natural indicators are turmeric, litmus, china rose, and red cabbage indicators.
1.2.1 Litmus – a natural dye
• Litmus is the natural indicator which is commonly used to identify the nature of a substance (acidic or basic).
• Litmus is a dye which is obtained from plants called lichens.
• It can be used as a solution or in paper form.
• The natural colour of litmus is purple.
• Litmus papers are available in both red and blue strips of paper.
• In an acidic medium, blue litmus will turn into a red colour.
• In a basic medium, red litmus will turn into blue colour.
• It doesn’t change any colour in a neutral medium, which has a pH equal to 7.
Example : Litmus
1.2.2 Turmeric indicator
• Turmeric is a natural indicator present in yellow colour, which is readily available at our home (for cooking purposes).
• It shows a yellow colour in an acidic and neutral medium.
• It gives red or reddish brown colour in basic medium.
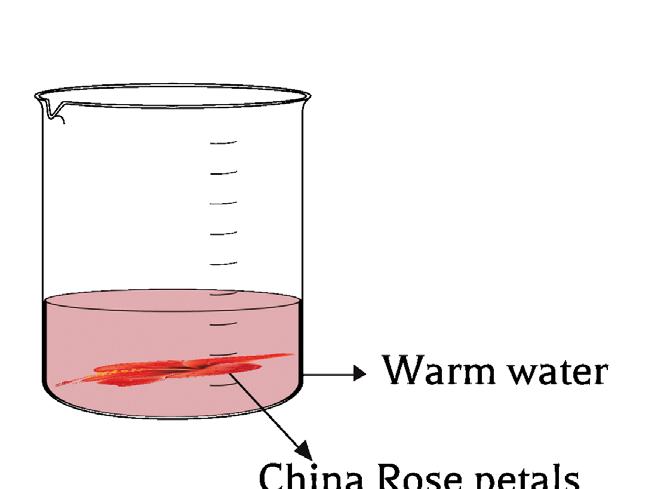
1.2.3 China rose
• China rose is also a natural indicator used to find the substance, whether it is acidic or basic in nature.
• It is prepared from the petals of the China rose flower (hibiscus).
• Warm water is added to China rose petals and can be used as an indicator
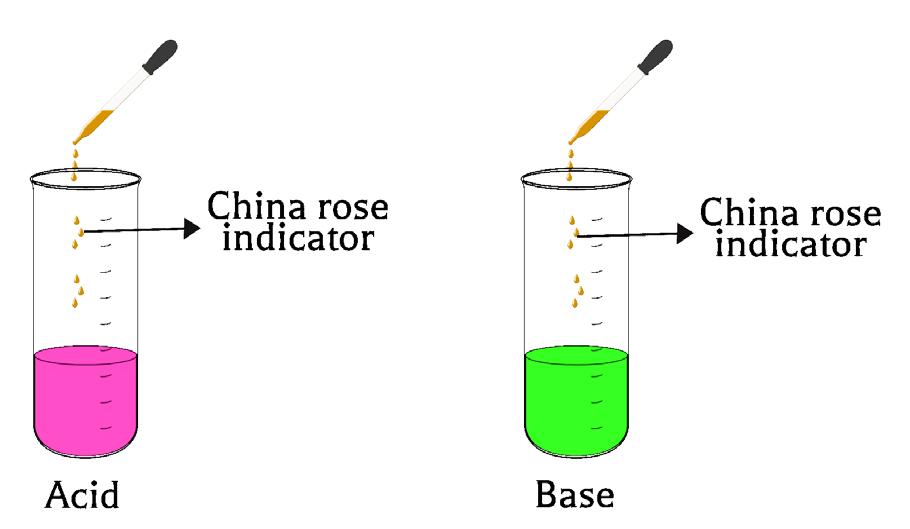
• China rose indicator will give a dark pink (magenta) colour in an acidic medium.
• It will give a green colour in the basic medium.
• No colour change for the neutral solution.

1.2.4 Synthetic indicators
• Indicators that are synthesised in the laboratories are called synthetic indicators. Examples,
1. Methyl orange: Shows red colour in an acidic medium and yellow colour in a basic and neutral medium.
Methyl Orange indicator Acid pH Red orange Yellow Yellow Neutral pH Alkaline pH
1.8 Methyl orange indicator
2. Phenolphthalein: Colourless in acidic medium and pink in basic medium. Phenolphthalein
Acid pH
Neutral pH
Base pH
Phenolphthalein it remains same
Phenolphthalein it remains same
Colour change in acid and base on addition of phenolphthalein
Fig. 1.9 Phenolphthalein indicator
1.3 PROPERTIES OF ACIDS AND BASES
1.3.1 Physical properties of acids and bases
Phenolphthalein it turns into pink
The physical properties of both acids and bases are shown in tabular format.
Properties
Colour
Mineral acids are generally colourless liquids, but sometimes sulphuric acid shows a yellow colour due to impurities. Some organic acids are present in solid form as white-coloured solids.
For example, Benzoic acid
Bases are generally colourless. [Exception: Hydroxides of iron and copper.]
Taste Sour Bitter
Touch - Slippery
Solubility Soluble in water
Some bases, which are called alkalis, are soluble in water.
Table 1.2 Physical properties of acids and bases
1.3.2 Chemical properties of acids and bases
I. Chemical Properties of acids
a. Acids reaction with metals: Some metals displace hydrogen from strong acids (HCl, H2SO4, etc.) based on the strength of the reactivity of metals. Such metals are called active metals.
Zn + H2 SO4 ⟶ ZnSO4 + H2
Mg + 2HCl ⟶ MgCl2 + H2
b. Acids reaction with metal oxides: All dilute mineral acids react with metal oxide compounds to form their respective metallic salts and water.
ZnO + H2SO4 ⟶ ZnSO4 + H2O
CaO + 2HNO3 ⟶ Ca(NO3)2 + H2O
c. Acids reaction with metal carbonates: Metal carbonate reactions with acids can also be called a neutralisation reaction, but it is accompanied by the release of CO2 gas along with the formation of salt and water.
CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2
MgCO3 + H2SO4 ⟶ MgSO4 + CO2 + H2O
II. Chemical properties of bases
Action of heat:
Some bases, on strong heating, undergo melting but they do not undergo decomposition.
Examples: NaOH, KOH
Some other metal hydroxides undergo decomposition on strong heating.
Ca(OH)2 Heat
2Al(OH)3 Heat
CaO + H2O
Al2O3 + 3H2O
Some bases, such as ammonium hydroxide, decompose rapidly on slight heating to give ammonia gas and water as products.
NH4OH Heat → NH3 + H2O
1.4 pH CONCEPT AND pH SCALE
1.4.1. pH concept
The pH value of a solution denotes the concentration of hydrogen ions that is present in the solution. Pure water, which is neutral, shows the pH value of 7. A pH paper is used to find out pH of a solution by matching the change in colour with a universal indicator paper.
pH plays a very important role in our daily life because most of the biochemical processes in our body take place at specific pH values. Generally, the pH of our blood is in the range of 7.35-7.45. Our body becomes susceptible to diseases when there is alteration in normal pH range of blood.
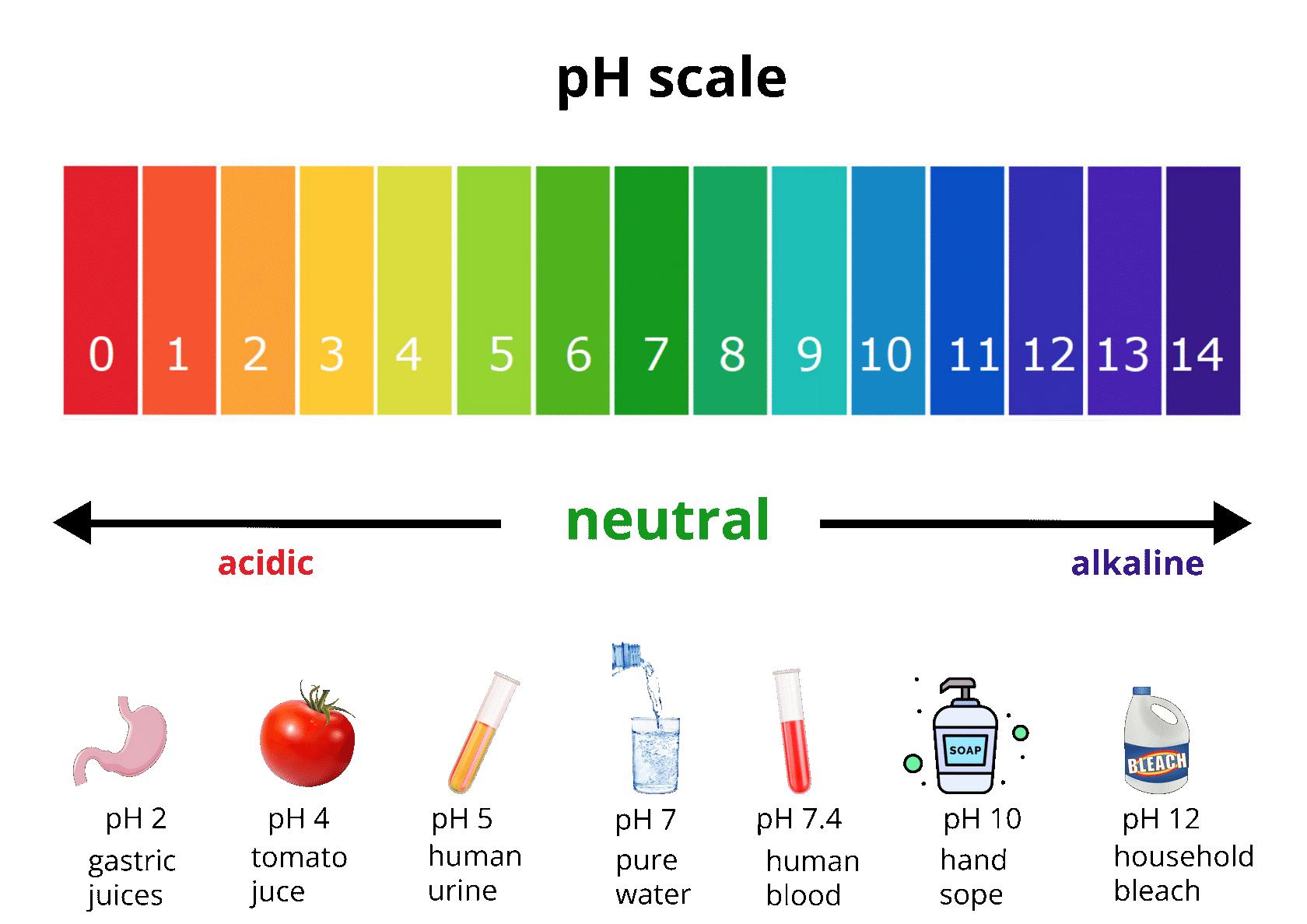
1.4.2 pH scale
The strength of an acid and a base is measured by a scale called the pH scale. Objects that are not very acidic are called basic. The scale has values ranging from 0 (the most acidic) to 14 (the most basic).
In general, lower the pH of a solution, more will be its acidic strength. Similarly, higher the pH of a solution, more will be its basic strength.
• A neutral solution has a pH = 7.
• An acidic solution has a pH < 7.
• A basic solution has a pH > 7. pH scale

1.5 NEUTRALISATION REACTION
1.5.1 Neutralisation
• When an acid reacts with a base, it forms salt and water along with the evolution of heat.
• Salt formed during this reaction is based on the acid and base reacting with each other.
• The product so formed will be a salt which is neutral and has a pH value equal to 7. Hence, the reaction is called a neutralisation reaction.
Examples:
1. HBr +KOH ⟶ KBr + H2O
2. H2SO4 + 2NaOH ⟶ Na2SO4 + 2H2O
3. HCl + Ca(OH)2 ⟶ CaCl2 + 2H2O
4. HNO3 +KOH ⟶ KNO3 + H2O
1.5.2 Phenolphthalein indicator
Phenolphthalein is a chemical compound which is used in acid-base titrations as a pH indicator. It is commonly colourless in acidic solutions and shows a pink colour in basic solutions. This is useful in determining the endpoint of titrations when the solution changes from acidic to basic or vice versa.
Phenolphthalein (pH indicator)
Pipette
Hydrochloric acid
1.12 Acid-Base titration using phenolphthalein indicator
1.6 NEUTRALISATION REACTION IN OUR DAILY LIFE
1.6.1 Indigestion
• The stomach secretes HCl.
• The stomach produces more HCl when we eat very spicy food, which results in acidity. This condition is called indigestion.
• To relieve indigestion, basic substances can be used to destroy its acidic effect
• It can be cured by taking antacid tablets, which contain magnesium hydroxide, and aluminium hydroxide.

Take a basic substance to reduce acid
1.6.2 Insect bite
• The sting of an ant contains formic acid.
• If an ant bites, it injects formic acid into the skin.
• This effect of acid can be treated with a calamine solution (which is basic).
• Calamine solution contains zinc carbonate.
• Or it can also be neutralized by rubbing the moist baking soda, which gives relief.
• Wasp stings are alkaline in nature, which can be neutralized by any acid, such as vinegar.



Bee’s and Red ant’s stings are acidic Wasp stings are alkaline
To Neutralise them we use an alkali, such as

To Neutralise them, we use an acid, such as

1.6.3 Soil treatment
• Plants do not grow well in the soil if it is more acidic or basic.
• If the soil is more acidic, it has to be treated with base [CaO, Ca(OH)2] to neutralize its effect.
• If the soil is more basic, it has to be treated with organic matter to neutralize the effect of the base.
1.6.3 Factory waste
• Factory waste is that which is produced from industrial activities such as manufacturing.
• If they are released into the water bodies, it will kill the fish and other marine organisms.
• Hence, it has to be treated with some bases before releasing it into the water bodies.
1.6.4 Acid rain
• Acid rain is a result of air pollution caused by the emission of sulphur dioxide and nitrogen oxide, which reacts with the water molecules and produces acids.
How does acid rain occur ?
Sulphur dioxide and nitrogen dioxide gets released by factories
Goes up in the atmosphere
Reacts with water vapour present in the atmosphere
Forms acids (Sulphuric acid and nitric acid)
Acid comes down with rain is known as acid rain
1.15 Acid rain
Acid rain can be harmful to
• Human beings.
• Plants, animals, and birds.
• Buildings and monuments.
• Water, soil, and the atmosphere.
Methods to control acid rain
• Less usage of fossil fuels.
• Less usage of sulphur fuels.
• Power plants should be reduced.
• Reforestation.
QUICK REVIEW
• Acids are sour in taste chemicals which turn blue litmus to red, release H+ ions in water, and have a pH value of less than 7.
• Acids can be organic (derived from plants and animals) and inorganic (synthesised from laboratories).
• Bases are bitter in taste chemicals and soapy to the touch. They have a slippery feel, which turns red litmus to blue, releases OH- ions in water, and has a pH value of more than 7.
• Indicators are the substances used to check the nature of the given substance by observing the change of colours.
• Indicators can be natural and are derived from fruits, vegetables, and flowers. (Litmus, China rose, turmeric) and their colour change in acidic and basic medium.
• Synthetic indicators are prepared in the laboratory (phenolphthalein, methyl orange) and their colour change in acidic and basic medium.
• Acid rain is the cause of air pollution and its harmful effects.
• Salt is formed by the result of a neutralisation reaction in which the reaction take place in between an acid and a base to form salt and water.
• Neutralisation reaction plays a vital role in our day to day life, such as indigestion, ant bite, and in treating the soil and also the factory waste.
•
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Acids and bases
1. Which of the following has oxalic acid in it? a. Lemon
Curd
2. Which of the following is acidic in nature?
a. Apple juice
Soap solution
3. Which of the following acids is present in aerated drinks? a. Lactic acid
Maleic acid
4. Example for acids prepared from minerals. a. Citric acid
Sulphuric acid
Tomato
Vinegar
Slaked lime
Lime
Carbonic acid
Citric acid
Oxalic acid
Acetic acid
5. The process of dissolving an acid or a base in water is called a/an ___________ process.
a. Endothermic b. Exothermic c. Photochemical d. Electrical
6. Tannic acid is present in
a. Lemon b. Tea c. Cough syrup d. Tamarind
II. Natural indicators around us
1. Litmus is extracted from
a. Fungi
b. Algae c. Lichens d. Bacteria
2. Which of the following is not a natural indicator?
a. Turmeric
c. Beetroot juice
3. Which of the following is used as an indicator?
b. Red cabbage juice
d. Methyl Orange
a. Potato b. Onion c. Tomato d. Brinjal
4. Juice prepared from Lemon is taken in a glass tumbler and water is added to it. Then, a red litmus paper and a blue litmus paper were dipped into it. What changes were observed?
a. The red litmus paper turns blue.
b. The blue litmus paper turns red.
c. The red litmus paper becomes white. d. The blue litmus paper becomes white.
5. The figure given below shows the colour change in test tubes A, B, and C when the China rose indicator is added to them. The respective solutions in test tubes A, B, and C are

a. Sugar solution, Lime water, Baking powder
b. Lime juice, Sugar solution, Lime water
c. Lime water, Sugar solution, Lime juice d. Sugar solution, Lime juice, Vinegar
6. What is the colour change sequence of the China rose indicator as the pH increases from acidic to alkaline?
a. Red ⟶ Blue
c. Green ⟶ Yellow
III. Properties of acids and bases
b. Blue ⟶ Red
d. Dark pink ⟶ Green
1. Which one of the following is the characteristic of a base?
a. Red litmus changes to blue.
c. Evolve hydrogen gas, when reacting with strong metal.
b. Exothermic when reacting with water.
d. All of the above
2. The correct way of making a solution of acid in water is to ______________.
a. Add water to acid
c. Add base into water
b. Add water to the base
d. Add acid to water
3. Why are acid solutions good conductors of electricity?
a. They evolve hydrogen gas in reaction with the metal.
c. They give hydrogen ions on dissociation in water.
b. They evolve hydrogen gas in reaction with water.
d. They react with a base to produce water.
4. Which of the following oxides will react with acids to form salt and water?
a. SO2
b. SO3
IV. pH concept and pH scale
c. CO d. CuO
1. When air is bubbled through pure water, the pH value of water drops from 7.0 to 5.6 . Which gas is responsible for this change?
a. Argon
b. Carbon dioxide c. Nitrogen d. Oxygen
2. A drop of colourless liquid was placed on blue litmus paper. The litmus paper turned red. The liquid could be
a. Dilute Hydrochloric acid
c. Distilled Water
b. Dilute Sodium Hydroxide Solution
d. Sodium Bicarbonate Solution
3. The pH range of blood is ___________.
a. 7.36-7.42
b. 1.0-2.0
c. 4.5-5.5
4. Among the following, which solution exhibits a pH value of less than 7?
a. HCl
b. CH2COONa c. KOH
5. Among the following, which solution exhibits a pH value of more than 7?
a. Mg(OH)2
b. H2 SO4
6. Statement-A: In a neutral solution, the pH = 7.
Statement-B: In an acidic solution, the pH < 7.
Statement-C: In an alkaline solution, the pH < 7.
a. All the statements A, B, and C are correct.
c. A and B are correct, and C is incorrect.
V. Neutralisation reaction
c. HNO3
d. 2.5-3.5
d. NaOH
d. H2CO3
b. All the statements A, B, and C are incorrect.
d. A and B are incorrect, and C is correct.
1. Colours of phenolphthalein indicator in acidic and basic medium, respectively, are
a. Pink, purple
c. Colourless, pink
b. Purple, pink
d. Pink, colourless
2. On adding phenolphthalein indicator to a colourless solution, no change is observed. What is the nature of this solution?
a. Acidic
c. Either basic or neutral
b. Basic
d. Either acidic or neutral
3. Name the product formed from the neutralisation reaction of potassium hydroxide and hydrochloric acid.
a. Potassium chloride and water
c. Potassium chloride and hydrogen
4. _______ is the product of acid and base reactions
a. Acid
b. Potassium chloride
d. Potassium hydride and water
b. Base c. Salt d. Inert gas
5. Which of the following solutions can be used to neutralize the pain and irritation caused by an ant bite?
a. Vinegar
b. Baking soda c. Lemon juice d. HCl solution
6. Which of the following is NOT a type of antacid?
a. Sodium bicarbonate
c. Hydrogen peroxide
WORKSHEET - 2
b. Calcium carbonate
d. Aluminium hydroxide
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. What is the nature of a gas that evolved during a reaction and turns wet blue litmus red?
a. Acidic
b. Basic
2. Acids generate H+ ions in which form?
a. Solid
b. Aqueous
c. Neutral d. None of the above
c. Both d. None of the above
3. When acid or base is added to water, the reaction is __________ in nature.
a. Endothermic
b. Exothermic
4. The water-soluble bases are called?
a. Acids
5. Alkalis have
a. A soapy texture
c. Corrosive nature
b. Bases
c. No change d. None of the above
c. Alkali
d. None of the above
b. Bitter taste
d. All of the above
6. What are the substances that help in distinguishing acid from the base by changing their colour?
a. Olfactory indicators
c. Indicators
b. Chemicals
d. None of the above
7. What colour is observed when a drop of NaOH is added to red litmus?
a. Green
b. Blue
c. Yellow d. No change
8. The products of the neutralisation reaction are:
a. Salt and water
c. Basic solution
b. Acidic solution
d. None of the above
9. Phenolphthalein changes its colour to _______ when it reacts with a base.
a. Green
b. Yellow
c. Pink
d. Red
10. Metallic oxides are _______, while non-metallic oxides are _______ in nature.
a. Basic, acidic
b. Acidic, basic
c. Neutral, acidic
11. What scale is used to measure the strength of an acid or a base?
a. Richter Scale
b. Regular Scale
c. pH Scale
d. Neutral, basic
d. None of the above
12. A value of 9 shown on a pH scale indicates that the given solution is _________ in nature.
a. Acidic
c. Neutral
b. Basic
d. None of the above
13. The strength of a base increases with the production of ______ ions.
a. H+
b. OH-
c. Both
14. What is the optimum pH range for the proper functioning of our body?
a. 5.0 to 8.0
b. 1.0 to 7.0
15. A change in pH value may affect the
a. Growth of plants.
c. Optimum range for the functioning of the body.
d. None
c. 8.0 to 14 d. 7.0 to 7.8
b. Survival of aquatic organisms.
d. All of the above.
16. How many molecules of water are present in washing soda?
a. 2
b. 5
17. What is the chemical formula for baking soda?
a. NaHCO3
b. NaOH
c. 10
d. 1
c. NaCl
d. CaOCl2
ACIDS, BASES, AND SALTS
18. What is the electrolysis of NaCl called?
a. Fermentation
c. Both fermentation and chlor-alkali process
b. Chlor-Alkali Process
d. None of the above
19. Copper sulphate, when heated, loses its blue colour due to the________.
a. Decolouration
c. Addition of water to crystallisation
20. Evolution of which gas causes a rise in cakes?
a. Oxygen
b. Chlorine
21. The bitter taste of food is imparted by:
a. Acids
b. Bases
b. Loss of water of crystallisation.
d. None of the above
c. Hydrogen d. Carbon dioxide
c. Salts
22. Turmeric and litmus solutions are examples of ____________.
a. Acids
b. Bases
23. Which of the following are synthetic indicators?
a. Litmus Solution
c. Turmeric
d. None of the above
c. Indicators d. Salts
b. Phenolphthalein
d. China rose
24. If a solution turns red litmus blue, the nature of the solution is _________.
a. Acidic
b. Basic
c. Neutral
25. Which colour does methyl orange, upon its contact with acid, impart?
a. Red
b. Yellow
d. None of the above
c. Orange d. Blue
26. Copper sulphate appears blue in colour due to the_______
a. Its acidic nature
c. Due to the presence of sulphate ions
b. Due to the water of crystallisation
d. None of the above
27. The common name of CaOCl2 is_______.
a. Washing powder
c. Baking soda
b. Bleaching powder
d. Common salt
28. The chlor-alkali process results in the formation of______.
a. Chlorine
c. Hydrogen
b. Sodium hydroxide
d. All of the above
29. pH value 7 corresponds to ____________ nature of the solution.
a. Acidic
b. Basic
30. The acid present in nettle sting is ____________.
a. Tartaric acid
31. HCl produces H+ only in?
a. An aqueous solution
c. Both the media
b. Oxalic acid
c. Neutral
d. None of the above
c. Citric acid d. Methanoic acid
b. Dry medium
d. None of the above
32. Which of the following gas evolves during the reaction of metals with acids?
a. Carbon dioxide
c. Hydrogen
b. Oxygen
d. Chlorine
33. Phenolphthalein changes its colour from colourless to pink in the presence of ___________.
a. Acid
c. Neutral Solution
34. The chemical formula of gypsum is _________.
a. CuSO4.5H2O
c. Na2CO3.10H2O
35. Uses of bleaching powder include?
a. In the textile industry
c. To bleach wood pulp
b. Base
d. None of the above
b. CaSO4.2H2O
d. None of the above
b. An oxidising agent
d. All of the above
36. Kanika identified the nature of a few substances with the help of three different indicators. She listed down her observations in the given table with some blanks.
No.
1 Lime water Blue p Green
2 Curd No change No change q
3 Table salt No change No change No change
4 Toothpaste r Red s
5 Lemon juice No change No change Magenta
Identify p, q, r, and s.
a. p-magenta, q-green, r-blue, s-magenta
c. p-blue, q-magenta, r-red, s-green
b. p-red brown, q-magenta, r-blue, s-green
d. p-green, q-red, r-magenta, s-blue

PHYSICAL AND CHEMICAL CHANGES 2
2.1 INTRODUCTION
Change is a very important feature of nature. Every day, you experience numerous changes in the world around you. These changes often involve different substances. Some common changes that we observe in our surroundings are the ripening of fruits, growth of plants and animals, cooking of rice, formation of curd, freezing of water, etc.
Make a list of five changes you have noticed around you. Also, make a list of five objects that do not show any change in them with time.
Have you ever wondered what happens to a substance when it undergoes a change?
Let us try to understand with the help of the following examples:
(i) A raw banana is green, but it turns yellow when ripened. Thus, when a fruit ripens, its colour changes.
2.1 Change in colour due to ripening
(ii) A small sapling grows with time and becomes a large tree. Here, the shape and size of the sapling change.
growing into a tree
Fig. 2.2 Sapling growing into a tree
(iii) Molten wax becomes hard on cooling. Rice becomes soft on cooking. Thus, the hardness of the substance might also change when a change occurs.
Molten wax getting solidi ed Molten wax getting solidified Cooked rice
(iv) Water changes into ice when we freeze it for a few hours in a freezer. Here, the state of the substance changes.
From the above examples, it is clear that when changes take place, the substances might change their colour, shape, size, hardness, state, etc.
Thus, we observe that almost everything around us goes through changes. Since these changes are quite varied, it is important to classify them and study them in detail. In science, these changes can be broadly classified into two types -
(i) Physical change
(ii) Chemical change
2.2 PHYSICAL CHANGE
A physical change is a change in which no new substance is formed, and the chemical composition of the original substance remains the same, even though some of its physical properties, like state, shape, size, etc., may change.
Example 1: Roti made from the ball of the dough.

2.5
When we make a ball of dough and then roll it into a roti, the shape and the size of the original dough keep on changing. No new substance is formed. If the shape of the roti is not round, we can again change it back to the ball of the dough. Since we can get the original substance back, it is also termed a reversible physical change.
Example 2: Salad made from different vegetables.

2.6 Making of salad
When we cut the vegetables, only the shape and size will change. There is no difference in the colour or taste. This means the properties of vegetables are present in their pieces, and no new substances are formed. Since, we cannot get the original vegetables back, it is also termed an irreversible physical change.
Example 3: Sublimation of Ammonium chloride
Ammonium chloride sublimate
Ammonium chloride
Fig. 2.7 Sublimation of ammonium chloride
When solid ammonium chloride is heated gently in a test tube, it changes into vapours. When the vapour touches the upper cooler part of the test tube, it changes back into solid ammonium chloride. This shows that, chemically, no new substance is formed; only the state of the substance changes. We observe an energy exchange takes place to change the state of the original substance.
2.2.1 Characteristics of a physical change
1. No new substance is formed. The chemical composition of the substance remains the same.
2. There may be changes in the state, size, shape, and colour of the original substance during physical changes.
3. Original substance may or may not be obtained. Physical changes can be reversible or irreversible.
4. There may or may not be any exchange of energy during a physical change.
2.3 CHEMICAL CHANGE
A chemical change is a permanent change in which new substance(s) is/are formed whose chemical composition and physical and chemical properties are completely different from those of the original substance(s).
For example, when we cook food daily, raw grains and vegetables change their form. Cooking requires heat energy, which is supplied by cooking gas (L.P.G), kerosene or electric devices like microwaves. Can we get the raw grain or vegetables back once the energy supply is stopped?
The answer is 'no’ because the chemical composition of the original substances has changed during the process of cooking. So, it is a permanent and irreversible change.
Let us look at various demonstrations to understand chemical changes in detail:
1. Burning of magnesium ribbon
When magnesium ribbon is burnt in the air, it burns with a dazzling white flame. It forms a powdery ash when it is burnt completely. A new substance is formed after burning magnesium. This powdery ash is known as magnesium oxide
2Mg + O2 ⟶ 2MgO
Magnesium Oxygen Magnesium oxide
When magnesium oxide is dissolved in water, a new substance called magnesium hydroxide is formed.
MgO + H2O ⟶ Mg(OH)2
Magnesium oxide Water Magnesium hydroxide
When a drop of this solution is kept over litmus paper, it is observed that it turns red litmus blue and blue litmus does not turn red.
So, we can say that the burning of magnesium in the air is a chemical change due to the formation of a completely new compound, magnesium oxide.


Magnesium ribbon
Water + Ash (Solution)
2. Reaction of copper sulphate with iron
When copper sulphate reacts with iron, the colour of the solution changes from blue to green. This is due to the formation of iron sulphate, which is a completely new substance. Also, the iron nail gets covered with a brown-coloured deposit of copper, which is another new substance.
CuSO4 + Fe ⟶ Cu + FeSO4
Copper sulphate (Blue) Iron Copper (Brown) Iron sulphate (Green)
Hence, the reaction of copper sulphate with iron is a chemical change, as two new substances, iron sulphate and copper, are formed.
After some time
Iron nail
CuSO4 solution FeSO4 solution
Cu deposites on the Iron nail
Fig. 2.9 Reaction of copper sulphate with iron
3. Reaction of carbon dioxide with lime water
When vinegar reacts with baking soda in the test tube, carbon dioxide gas is formed. This gas produces a hissing sound.
Vinegar (Acetic acid) + Baking soda (Sodium hydrogen carbonate) ⟶ Carbon dioxide + Other substances
When this carbon dioxide gas is passed through lime water, calcium carbonate is formed, which makes lime water milky.
CO2 + Ca(OH)2 ⟶ CaCO3 + H2O
Carbon dioxide Lime water Calcium carbonate Water
Thus, the reaction of carbon dioxide with lime water is a chemical change, as new compounds, calcium carbonate and water, are formed.
Carbon dioxide
Vinegar + Baking soda Lime water
2.10 Reaction of carbon dioxide with lime water
2.3.1 Characteristics of a chemical change
1. One or more new substances are formed.
2. The chemical composition and properties of the original substance change.
3. The change is permanent and cannot be reversed.
4. A chemical reaction is involved in chemical change.
5. There is an exchange of energy during a chemical change. This means heat, light, or both might be given out or consumed.
2.4 RUSTING OF IRON
Rusting is a chemical change which takes place in the presence of air and moisture only. It is a reddish brown powder, which is formed on the surface of iron. Fe + O2 + H2O
Fe2O3.xH2O Iron Oxygen Water Hydrated iron oxide (Rust)
For rusting, the presence of both oxygen and water (or water vapour) is essential. In fact, if the content of moisture in the air is high, which means if it is more humid, rusting becomes faster.
The saltwater also makes the process of rust formation faster. This is the reason why ships suffer a lot of damage from rusting.


Rusting affects iron articles and slowly destroys them. Since iron is used in making bridges, ships, cars, truck bodies and many other articles, the monetary loss due to rusting is huge.
2.4.1 Prevention of rusting

To prevent rusting, we have to prevent iron articles from coming in contact with oxygen, water, or both.
Let us look at various methods that help prevent rusting -
1. Painting the surface of iron objects.
2. Greasing or oiling the surface of iron objects.
3. Galvanisation is a process of coating the surface of iron with zinc to prevent the rusting of iron.
For example, the iron pipes we use in our homes to carry water are galvanised to prevent rusting.
4. Alloying is a process in which two or more metals or a metal and a non-metal are mixed in a molten state to improve the properties of metals.
For example, when iron is mixed with a small amount of carbon and metals like chromium or manganese, stainless steel is formed. Stainless steel does not get rusted.
2.5 CHEMICAL REACTION
A chemical change involves a chemical reaction. So, a chemical change is generally called a chemical reaction.
The process by which chemical change takes place, i.e., by which one or more substances react to form new substances having different compositions and different properties, is called a chemical reaction. The substances that take part in a chemical reaction are called reactants, and the substances formed in a chemical reaction are called products
2.5.1 Characteristics of chemical reactions
When a chemical reaction takes place, it is accompanied by one or more of the following characteristics:
1. Change in state
Some chemical reactions are accompanied by changes in state. For example, petrol, which is a liquid, burns to form water vapour and carbon dioxide, which are gaseous.
2. Change in colour
Some chemical reactions are characterised by a change in colour
3. Formation of a precipitate
Some chemical reactions are characterised by the formation of a precipitate. A precipitate is a solid product which separates out from the solution during a chemical reaction and is shown by an arrow pointing downwards (↓).
NaCl + AgNO3 ⟶ AgCl ↓ + NaNO3
Sodium chloride Silver nitrate Silver chloride (White ppt.)
4. Evolution of a gas
Sodium nitrate
A gas may evolve during a chemical reaction if one of the reactants is a liquid or in solution form. The evolution of a gas is confirmed by the appearance of efflorescence (bubbling) and is shown by an arrow pointing upwards (↑).
Zn + H2 SO4 ⟶ ZnSO4 + H2 ↑
Zinc Sulphuric acid
5. Change in temperature
Zinc sulphate Hydrogen
Some chemical reactions are accompanied by changes in temperature, i.e., rise or fall in temperature.
a. Some reactions are accompanied by a rise in temperature in which heat is evolved
b. Some reactions are accompanied by a fall in temperature in which heat is absorbed
2.6 TYPES OF CHEMICAL REACTIONS
Chemical reactions can be grouped into various types on the basis of their nature. Some common types of chemical reactions are:
1. Combination reaction
The reaction in which two or more substances combine to form a single substance is called a combination reaction.
Examples,
a. When magnesium is burnt in the presence of oxygen, magnesium oxide is formed.
2Mg + O2 ⟶ 2MgO Magnesium Oxygen Magnesium oxide
b. Carbon burns in the presence of oxygen to form a gaseous compound, carbon dioxide.
2. Decomposition reaction
The reaction in which a single compound breaks into two or more simple substances is called a decomposition reaction.
Examples,
a. Calcium carbonate decomposes on strong heating to form calcium oxide and carbon dioxide. CaCO3
b. When electric current is passed through water, it decomposes into hydrogen and oxygen.
3. Displacement reaction
The chemical reaction in which one element takes the position or place of another element present in a compound is called a displacement reaction. These reactions occur mostly in solution form, and a more active metal displaces or removes another less active element or metal to form a compound. AB + C ⟶ CB + A
Examples,
a. When copper sulphate reacts with iron, iron displaces copper from copper sulphate and forms green-coloured iron sulphate and reddish brown copper is deposited on iron.
CuSO4 + Fe ⟶ Cu + FeSO4
Copper sulphate (Blue) Iron Copper (Brown) Iron sulphate (Green)
Iron nail
CuSO4 solution
After some time
Cu deposites on the Iron nail
FeSO4 solution
Fig. 2.12 Displacement reaction
b. When copper sulphate reacts with zinc granules, zinc displaces copper from copper sulphate and forms colourless zinc sulphate and reddish brown copper is deposited on zinc.
CuSO4 + Zn ⟶ Cu + ZnSO4
Copper sulphate (Blue)
Zinc
Copper (Brown)
Aqueous solution of copper sulphate
Test tube is stirred
Zinc sulphate (Colourless)
Zinc granules colourless zinc sulphate solution
Copper deposit on zinc granules
Fig. 2.13 Displacement reaction
4. Double displacement reaction
A chemical reaction in which two compounds in their aqueous state exchange their ions or radicals to form new compounds is called a double displacement reaction.
AB + CD ⟶ CB + AD
Examples,
a. When hydrochloric acid reacts with sodium hydroxide, it forms a salt, sodium chloride and water.
NaOH + HCl ⟶ NaCl + H2O
Sodium hydroxide Hydrochloric acid Silver chloride Water
b. When lead nitrate solution is mixed with potassium iodide solution, a yellow precipitate of lead iodide is formed. This reaction is a precipitation reaction and can be expressed as follows : Pb(NO3)2 + 2KI(aq) ⟶ PbI2 ↓ + 2KNO3(aq)
Lead nitrate solution (Colourless)
Potassium iodide solution (Colourless)
5. Oxidation and reduction reaction
Examples,
Lead iodide (Yellow ppt.) Potassium nitrate solution
a. Oxidation involves the addition of oxygen or removal of hydrogen from a substance. C + O2 ⟶ CO2 Carbon Oxygen Carbon dioxide
b. Reduction involves the addition of hydrogen or the removal of oxygen from a substance. H2 + CuO ⟶ Cu + H2 O
Hydrogen Copper oxide Copper Water
6. Redox reaction
The reaction in which both oxidation and reduction occur together is called a redox reaction.
Example,
When ferric oxide reacts with carbon monoxide, iron and carbon dioxide are formed. Here, ferric oxide is reduced, and carbon monoxide is oxidised. So, it is a redox reaction. Reduction
QUICK REVIEW
• Changes can be of two types: physical and chemical.
• Physical changes are changes in the physical properties of substances. No new substances are formed in these changes. These changes may or may not be reversible.
• In chemical changes, new substances are produced. They are permanent and irreversible.
• Rusting is a chemical change which takes place in the presence of air and moisture only.
• Rusting can be prevented by painting, greasing, galvanisation, and alloying.
• The process by which chemical change takes place, i.e., by which one or more substances react to form new substances having different compositions and different properties, is called a chemical reaction.
• Chemical reactions can be grouped into various types on the basis of their nature. Some common types of chemical reactions are:
• Combination reaction
• Decomposition reaction
• Displacement reaction
• Double displacement reaction
• Redox reaction
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Physical change
1. Which of the following is not a physical change?
a. Breaking of a glass tumbler
c. Melting of butter
b. The dissolving of sugar in water
d. Formation of curd from milk
2. A reversible change is a change which __________.
a. cannot be reversed
b. takes place during a displacement reaction
c. is a chemical change
d. can be reversed
3. Identify the physical change among the following.
a. Burning of magnesium ribbon
c. Tearing a paper into pieces
4. Choose the incorrect statement.

b. Photosynthesis
d. Digestion of food
a. Chemical reactions are characterised by a change in colour and smell.
b. Change in chemical compositions of reactants takes place during a chemical change.
c. Physical changes are always reversible.
d. Change in physical state is a physical change.
5. Observe the given experimental set-up.

Which of the following represents the correct observation?
a. Vapours of chlorine are evolved.
b. Ammonium chloride melts after some time.
c. White solid gets deposited on the upper cooler part of the test tube.
d. Violet-coloured vapours are produced as ammonium chloride undergoes sublimation.
6. Dissolve some common salt in water and leave the solution in an open and sunny place for a day. What do you observe?
a. The whole of the solution evaporates.
b. Water evaporates, leaving behind salt.
c. Salt evaporates, leaving behind water.
d. No change of any form in the salt solution.
7. The given reaction is a physical change because
a. New substances are formed.
b. It is an irreversible change.
c. Chemical composition remains the same, and only state is changed.
d. Chemical properties are the same.
8. A few changes are given below:
A. Making furniture from wood
B. Making an aeroplane from a paper
C. Breaking of a vase
D. Rolling roti from dough
Classify these changes into I. Irreversible-physical change, and II. Reversible-physical change.
9. In the following questions, a statement of assertion is followed by a statement of reason. Mark the correct choice
Assertion: Cutting paper into very small pieces is an irreversible change.
Reason: Physical changes are always reversible.
a. Both assertion and reason are true, and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but reason is false.
d. Both assertion and reason are false.
10. Converting ice into water when heating or keeping the ice at room temperature is an example of
a. Physical and reversible change
c. Chemical and reversible change
II. Chemical change
b. Physical and irreversible change
d. Chemical and irreversible change
1. Which gas is produced when vinegar reacts with baking soda?
a. Hydrogen
c. Carbon dioxide
2. Which of the following is a chemical change?
a. Cutting off a cloth
c. Ironing of a cloth
b. Carbon monoxide
d. Oxygen
b. Drying of a cloth
d. Burning of a cloth
3. Carbon dioxide gas turns limewater milky. This change is a
a. Physical change
c. Chemical change
4. Study the given figure carefully.
b. Displacement reaction
d. Double displacement reaction
Blue coloured solution Iron nails Brown red deposition
Green coloured solution
Which among the following reactions explains the above change?
a. ZnSO4 + Cu ⟶ CuSO4 + Zn
c. CuSO4 + Fe ⟶ FeSO4 + Cu
b. FeSO4 + Cu ⟶ CuSO4 + Fe
d. CuSO4 + Zn ⟶ ZnSO4 + Cu
5. Identify the correct statement.
a. Rusting and burning are reversible changes.
b. Physical change is always an irreversible change.
c. Carbon dioxide gas turns limewater milky.
d. Cutting of an apple is an irreversible chemical change.
6. Select the correct statement.
a. Hydrogen gas is evolved when vinegar is added to baking soda.
b. Heat energy is always given out during a chemical change.
c. A new product is always formed during a chemical change.
d. Combining two substances by heating is a physical change.
7. When an iron nail is dipped in copper sulphate solution, the colour of copper sulphate changes to
a. Green
b. Blue
8. Which of the following is not a chemical change?
i. Magnesium burning ii. Cake baking
iii. Cooking of food iv. Cutting of vegetables
v. Melting of ice cream
c. Pink
d. Colourless
a. i and iii b. ii and iv c. iii and v d. iv and v
9. Which of the following will result in a chemical change?
a. Mixing of milk and sugar
c. Mixing of milk and curd
10. When carbon dioxide is passed through limewater
b. Mixing of milk and water
d. Mixing of milk and nuts
a. It turns blue due to the formation of calcium oxide.
b. It turns green due to the formation of iron sulphate.
c. It turns white due to the formation of calcium hydroxide.
d. It turns milky due to the formation of calcium carbonate.
III. Rusting
1. Rusting of iron is
a. An irreversible chemical change
c. A reversible chemical change
2. Galvanisation is a process in which
a. Iron is coated on a zinc metal
c. Zinc and iron are mixed in a molten state
b. An irreversible physical change
d. A reversible physical change
b. Zinc is coated as a layer on iron
d. Carbon is mixed with iron and heated
3. Which of the following methods cannot be used to prevent rusting?
a. Painting
c. Galvanisation
b. Alloying
d. Mixing with sulphur
4. While learning about the topic 'Rusting', Rohan, a class 7 student, wrote the following statements in his book. Select the incorrect statement(s) among the following.
I. Depositing a layer of tin on iron is called galvanisation.
II. Stainless steel rusts more quickly as it contains carbon and metals like chromium, nickel and manganese.
III. Salty water fastens the process of rust formation.
a. III only
b. I only
c. I and II
5. When an iron spade is left open in a moist atmosphere, it
a. Develops a brown powdery layer of iron oxide
b. Develops a green layer of iron oxide
c. Develops a brown layer of oxygen
d. Does not undergo any change
IV. Types of chemical reactions
1. Which type of chemical reaction is the following reaction?
AB + C ⟶ CB + A
a. Decomposition reaction
c. Double Displacement reaction
d. I, II, and III
b. Displacement reaction
d. Combination reaction
2. Which type of reaction is the following reaction?
Fe+CuSO4⟶Cu+FeSO4
a. Redox reaction
c. Double displacement reaction
b. Displacement reaction
d. Combination reaction
3. The equation, Mg(s) + CuO(s) ⟶ MgO(s) + Cu(s) represents
i. Decomposition reaction
ii. Displacement reaction
iii. Combination reaction
iv. Double displacement reaction
v. Redox reaction
a. i and ii
b. iii and iv
c. ii and v
4. Cut vegetables turn brown when exposed to air. This is due to
a. Evaporation
5. In a displacement reaction
d. iv and v
b. Oxidation c. Neutralisation d. Displacement
a. The colour of the original solution does not change
b. A more reactive element displaces a less reactive element from its solution
c. A less reactive element displaces a more reactive element from its solution
d. The elements with the same reactivity displace each other in a salt solution
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which among the following is an example of a physical change?
a. Burning of magnesium ribbon
c. Melting of candle wax
b. Photosynthesis
d. Digestion of food
2. A compound X is formed when the ash formed by the burning of magnesium in the air is dissolved in water. Find the compound X.
a. Magnesium oxide
c. Magnesium hydroxide
b. Magnesium carbonate
d. Magnesium chloride
3. If magnesium oxide is dissolved in water, the resulting solution will
a. Turn red litmus blue
b. Give no colour with phenolphthalein
c. Turn blue litmus red
d. Give back the magnesium ribbon on standing
4. Identify the wrong pair out of the following:
a. Melting of butter - Physical change
b. Shaping of glass by heating - Physical change
c. Burning of paper - Physical change
d. Making a boat from paper - Physical change
5. Keeping a stone in sunlight for a few hours is
a. A physical change
b. Neither a physical nor a chemical change
c. A chemical change
d. Combination of physical and chemical changes
6. In which type of change do we observe changes in specific properties of matter but not in chemical composition?
a. Physical change
b. Both physical and chemical changes
c. Chemical change
d. Neither physical nor chemical change
7. Which of the following statements is correct regarding the formation of rust and ice?
a. Rust is formed by a chemical change, while ice is formed by a physical change.
b. Both rust and ice are formed by physical changes.
c. Both rust and ice are formed by chemical changes.
d. Rust is formed by a physical change, while ice is formed by a chemical change.
8. Rohini has classified a few changes around us, as shown in the table. Sr. No
1. Browning of sliced brinjal
2. Dissolving salt in water
3. Baking of a cake
4. Respiration
5. Making shapes out of clay
The changes classified correctly are
a. 3 and 5 b. 2, 4 and 5
9. Observe the given figure carefully.
Physical, reversible
Physical, reversible
Chemical, reversible
Chemical, irreversible
Physical, reversible
c. 1 and 5
d. 2 and 4
Dilute sulphuric acid Gas ’X’ Zinc granules
Read the given passage and fill in the blanks by selecting an appropriate option. In the given figure, i). the reaction takes place, which is a chemical change. ii). being more reactive than iii). produces gas 'X', which is iv), and it burns with a v). i. ii. iii. iv. v.
a. Oxidation Zn H SO2 Dazzling light
b. Displacement H Zn H Pop sound
c. Displacement Zn H H2 Pop sound
d. Reduction Zn S SO2 Rotten egg smell
10. The following reaction is an example of Ca(OH)2(s) Heat → CaO(s) + H2O(g)
a. Combination reaction
c. Decomposition reaction
b. Double displacement reaction
d. Displacement reaction
11. Observe the given figure and fill in the blanks to complete the sentence.

Magnesium ribbon burning with a dazzling light
In the figure, magnesium reacts with oxygen to form P, which on reaction with water, forms Q.
a. P - MgCl Q - Mg(OH)2
c. P - MgO Q - MgCl
b. P - MgO Q - Mg(OH)2
d. P - MgO Q - MgOH
12. Observe the given figure and determine the chemical formula of the 'colourless solution of Y'.
Blue colour solution of (X) (Y) (X)
Colourless solution of (Y)
13. Tina placed several wire pieces made of different metals into a blue-coloured copper sulphate solution. What will be the colour of the solutions in beakers I, II, and III after half an hour?"
I
II III
a. Green Blue Green
b. Blue Green Green
c. Green Blue Blue
d. Blue Blue Blue
14. The process of mixing a non-metal or metal with a molten metal is known as _______.
a. Alloying
c. Decomposition
b. Galvanisation
d. Electroplating
15. In this section, each question presents two matching lists. Options a., b., c., and d. provide the possible combinations of elements from List-I and List-II, from which one correct combination is to be chosen.
List-I
(P) Folding of paper
(Q) Zinc coating
(R) Baking a cake
(S) Oxidation
List-II
1. Cannot be reversed
2. Cut apples
3. Can be reversed
4. Galvanisation
a. P-3, Q-4, R-2, S-1
b. P-3, Q-4, R-1, S-2
c. P-4, Q-3, R-2, S-1
d. P-1, Q-2, R-4, S-3
16. Which of the following statements is incorrect?
a. The iron gate, if not painted, gets rusted due to the presence of air and moisture.
b. Burning of a candle is a chemical change while melting of wax is a physical change.
c. Rusting of iron is a reversible chemical change.
d. Rusting can be prevented by applying a coat of paint or grease.
17. Which of the following statements is incorrect?
a. A physical change can be reversible as well as irreversible.
b. Oxygen gas is evolved when baking soda and vinegar react.
c. Nitrogen gas turns limewater milky.
d. The green colour of copper sulphate turns blue when an iron nail is added to it.
18. Which of the following changes represents a physical change that is irreversible?
a. Crushing of chalk into powder
c. Melting of ice cream
19. Which part of the ship will rust the fastest?
b. Breaking of an egg
d. Heating a piece of iron wire
z
Y
c. Z
20. The conditions necessary for rusting are
a. Carbon dioxide, oxygen and hydrogen
b. Carbon dioxide and water
c. Oxygen and water
d. Hydrogen and water
d. X, Y, and Z will rust equally
21. In the following questions, a statement of assertion is followed by a statement of reason. Select the correct choice
Assertion: The reaction in which a substance is decomposed into two or more simple substances is known as a decomposition reaction.
Reason: The decomposition can be carried out by giving energy in the form of heat, light, electricity, etc.
a. Both assertion and reason are true, and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but reason is false.
d. Both assertion and reason are false.
22. Rusting can be prevented by
a. Removing a brown layer of rust from the surface of iron
b. Cutting the contact of iron from air and water
c. Keeping iron covered with paper
d. Keeping on pouring water on the surface of the iron
23. In this section, each question presents two matching lists. Options a., b., c., and d. provide the possible combinations of elements from List-I and List-II, from which one correct combination is to be chosen.
List-I
(P) Iron nail + Copper sulphate
(Q) Vinegar + Baking soda
(R) Iron + Moisture + Air
(S) Lime water + Carbon dioxide
1. Carbon dioxide
2. Calcium carbonate
3. Displacement reaction
4. Rust
a. P-3, Q-1, R-2, S-4
b. P-3, Q-1, R-4, S-2
c. P-1, Q-2, R-4, S-3
d. P-2, Q-3, R-1, S-4
24. When water is heated, it is converted into steam. What makes it a physical and reversible change?
a. Water and steam are two different compounds and are interconvertible.
b. Water and steam have the same chemical composition, and on cooling, steam can be condensed back to water.
c. Water and steam have the same physical properties but different chemical properties.
d. Water can be converted to steam, but steam cannot be converted back into water.
25. The process of changing ammonium chloride directly into vapours on heating is called
a. Evaporation b. Melting
c. Condensation d. Sublimation
26. What type of reaction occurs when a compound breaks down into simpler substances?
a. Combination reaction
c. Single displacement reaction
b. Decomposition reaction
d. Combustion reaction
27. Which of the following is an example of a combustion reaction?
a. Rusting of iron
c. Dissolving salt in water
b. Burning of wood
d. Mixing vinegar and baking soda
28. What type of reaction is represented by the equation: 2H₂ + O₂ → 2H₂O?
a. Combination reaction
c. Single displacement reaction
b. Decomposition reaction
d. Double displacement reaction
29. Which of the following is an example of a decomposition reaction?
a. 2H₂ + O₂ → 2H₂O
c. Mg + 2HCl → MgCl₂ + H₂
b. 2KClO₃ → 2KCl + 3O₂
d. Fe + CuSO₄ → FeSO₄ + Cu
30. In the following questions, a statement of assertion is followed by a statement of reason. Select the correct choice.
Assertion: In a displacement reaction between copper sulphate and zinc, copper is deposited. Reason: Zinc is more reactive than copper.
a. Both assertion and reason are true, and the reason is the correct explanation of the assertion.
b. Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
c. Assertion is true, but the reason is false.
d. Assertion is false, but the reason is true.


NUTRITION IN PLANTS 1
All living beings need energy to perform their daily tasks.. This energy is derived from the food they eat. After intake, the food is broken down inside the body into simple elements to produce energy. Food includes components such as carbohydrates, proteins, lipids, vitamins, and minerals. These components of food, also referred to as nutrients, produce energy, aid in the development and maintenance of body tissues, and protect against illness. Apart from nutrients, water is also essential for an organism’s body.
Plants are capable of manufacturing their food, but animals and human beings are unable to do so. Instead, they depend on plants to derive their nutrition. Therefore, both human beings and animals directly (by eating plants) and indirectly (by eating plant-eating animals), depend on plants for survival.
1.1 MODE OF NUTRITION IN PLANTS
Nutrition is the process in which the food consumed by an organism is used by its body to perform various life processes. Based on the mode of nutrition, all organisms are categorized as autotrophs and heterotrophs.
1.1.1 Autotrophs and heterotrophs
Green plants are called autotrophs as they can synthesise their own food. This mode of nutrition in which an organism prepares its own food by using simple inorganic substances such as carbon dioxide and water is known as autotrophic nutrition. Autotrophs are also known as producers.
Other organisms like animals and human beings who cannot synthesise their own food but depend on plants or other organisms for their nutrition are called heterotrophs. This mode of nutrition, as seen in heterotrophs, is called heterotrophic nutrition. All heterotrophs are directly or indirectly dependent on plants for food. A few examples of heterotrophs are non-green plants, fungi, most bacteria, animals, and humans.
1.2 PHOTOSYNTHESIS - FOOD-MAKING PROCESS IN PLANTS
The sun is the main source of energy for all living organisms on Earth. Green plants, who are known as producers, prepare food by harnessing the energy of the sun. This process in which green plants synthesize their food in the presence of sunlight is known as photosynthesis. It is the most remarkable phenomenon where the solar energy of the sun is converted into chemical energy of food.
During photosynthesis, complex organic substances like carbohydrates are synthesised from simple inorganic raw materials like carbon dioxide and water with the help of chlorophyll present in the green leaves of plants in the presence of sunlight.

1.2.1 Components necessary for photosynthesis
The components required for photosynthesis include chlorophyll, carbon dioxide and water.
a. Chlorophyll: Numerous green structures called chloroplasts are found in the green leaves and green stems of plants. A green pigment called chlorophyll present in chloroplasts traps solar energy. With the help of solar energy, CO2, and water, plants synthesize food.
b. Carbon dioxide: Carbon dioxide is absorbed through tiny pores called stomata that are found on the underside of the leaf surface. Two guard cells located on either side of the stomata regulate its opening and closing. It acts as the carbon source for sugar formation.
c. Water and minerals: Roots have root hairs that help in the efficient absorption of water and minerals from the soil. Special structures called vessels are found throughout the plant body. They act like pipes and transport water and minerals from the roots to the stems, branches and leaves. These vessels provide a continuous path or passage for the water and nutrients to reach the leaves.
1.2.2
Factors affecting photosynthesis
There are several factors that may affect the rate of photosynthesis.
a. Light: The Sun is the main source of light for photosynthesis in green plants. A very small part of the sun's energy reaching the earth is utilised by plants for photosynthesis. The intensity of light has an impact on the rate of photosynthesis. For e.g. in low-intensity light, photosynthesis will be low, but with an increase in light intensity, the rate of photosynthesis will also increase to a certain limit. However, at very high intensity, the light can damage chlorophyll, the photosynthetic pigment and impact photosynthesis.
b. Temperature: Low temperatures inhibit photosynthesis, while temperatures up to 40° C increase the rate of photosynthetic activity. However, temperatures above 40° C decrease photosynthesis.
c. Carbon dioxide: Increased concentration of carbon dioxide in air accelerates the rate of photosynthesis. However, too much of it can decrease the photosynthetic rate and cause toxicity to plants.
d. Water: Water is an important raw material for photosynthesis, and it affects the rate of photosynthesis. Plants use only 1% of the water absorbed by the roots during photosynthesis. Inadequate hydration causes the stomatal pores to remain close to avoid transpiration, which in turn also blocks the entry of CO2 into the leaves.
1.2.3 Significance of photosynthesis
a. Since all living organisms directly or indirectly depend upon plants for their nutrition and sustenance, photosynthesis is an important phenomenon.
b. Due to photosynthesis, a balance between O2 and CO2 levels is maintained in the atmosphere.
c. The process of photosynthesis releases oxygen as a byproduct, which is used by humans and animals for respiration.
1.2.4 Synthesis of plant food other than carbohydrates
Even though plants synthesise carbohydrates through photosynthesis, they also need other nutrients, such as proteins and fats, for healthy growth and development. The carbohydrates synthesised by plants contain the elements carbon (C), hydrogen (H), and oxygen (O). Apart from this, nitrogen is also necessary for the production of proteins.
Nitrogen, being abundant in the atmosphere, cannot be used by plants directly. There are two methods by which plants obtain nitrogen for metabolism:
a. Some nitrogen-fixing soil bacteria, such as Rhizobium, convert free nitrogen into water-soluble nitrogenous compounds (e.g. ammonia) in the soil. The plants readily absorb these soluble forms along with water.
b. Nitrogen-containing fertilisers, when added to the soil, improve its fertility and help the plants to absorb nitrogen for their growth and development.
1.3 OTHER MODES OF NUTRITION IN PLANTS
Plants exhibiting a heterotrophic mode of nutrition can be broadly classified as either parasitic or insectivorous.
1.3.1
Parasitic plants
An organism that resides in or on the body of another organism (host) and obtains its nourishment from it is called a parasite. Plants that obtain few or all of their nutritional requirements from the host plant are called parasitic plants. They grow specialised root-like haustoria that invade the stem of the host plant and obtain nutrients. There are two types of parasite plants: total parasitic plants and partial parasitic plants.
A.
Total parasitic plants
Some parasitic plants become totally dependent on the host for their nutrition and thus do not require green leaves. Such plants are called total parasitic plants, such as Cuscuta (dodder), Rafflesia (corpse flower), etc.
Cuscuta, being rootless and leafless, is a total stem parasite that cannot perform photosynthesis. Instead, it entangles the stems and branches of the host plant with the help of its yellow, wire-like stems to obtain its food.
Rafflesia is a total root parasite. Its stem is greatly reduced as thin threads, grow in the roots of the host plant to derive its nourishment. The plant lacks chlorophyll and bears the largest flower in the world.


B. Partial parasitic plants
Some green plants lack roots and cannot absorb water and minerals from the soil. However, they have green leaves that can be used to photosynthesise and manufacture food on their own. These plants depend on the host plant for water and minerals and live as partial parasites. Mistletoe is a partial parasite that develops haustoria to absorb water and minerals from the stems of the host plant and perform photosynthesis. It grows on big trees such as Acacia, mango, etc.

1.3.2 Insectivorous plants
Some green plants, despite being able to photosynthesise, feed on insects to fulfil their nutritive requirement for nitrogen. These plants are usually found in nitrogen-deficient soils, and have devised special mechanisms to trap, digest, and absorb the insects. Some examples of insectivorous plants are pitcher plants (Nepenthes), Venus flytraps (Dionaea), sundew (Drosera), bladderworts, etc.
The pitcher-like structure of a pitcher plant is a modified leaf. It has a lid to cover and open the mouth of the pitcher. When an insect enters the pitcher, the lid closes, and the caught insect is moved down the pitcher with the help of hairs inside the pitcher. The digestive juices secreted in the pitcher then digest the insect, and nitrogen is absorbed in the process.




In the Venus flytrap (Dionaea), the leaf is modified into two lobes, each having stiff, spine-like guard hairs on its margin. When an insect comes in contact with the hairs, the lobes close, trapping the insect inside.
Thus, insectivorous plants are also referred to as partial heterotrophs as they exhibit both autotrophic and heterotrophic modes of nutrition.
1.4 SAPROPHYTES
Organisms that feed on dead and decaying plants and animals are called saprophytes. These mostly include chlorophyll-lacking non-green plants.
1.4.1 Saprotrophic nutrition
Organisms that feed on non-living organic matter, such as dead and decaying plants and animals, are known as saprotrophs. They absorb nutrients from dead and decomposing matter by secreting digestive juices on them and converting them into a soluble form. This mode of nutrition, which absorbs nutrients in solution form, is called saprophytic nutrition. Examples include fungi (like bread moulds, mushrooms, etc.) and bacteria (unicellular microorganisms).


1.5 SYMBIOSIS
The term symbiosis or symbiotic relationship refers to the relationship between two organisms that allows them to share nourishment and shelter. Such organisms benefiting and supporting each other are referred to as symbionts. Symbiosis is thus a unique form of nutrition. The following are some common symbiotic relationships observed in nature:
Lichens
A symbiotic relationship, one in which an alga and a fungus coexist and benefit each other, is demonstrated by lichen. Since fungi are saprotrophs, the algae (being autotrophs) perform photosynthesis and provide nourishment to the fungi. In exchange, the fungus gives the algae water, nutrients, and a place to live.
Mycorrhiza


Another example of symbiosis is exhibited by mycorrhiza. Some trees allow specific fungi to live in their roots. The fungi get their nourishment from the photosynthesising tree and, in return, help the plant absorb water and nutrients efficiently from the soil.

Leguminous plants and Rhizobium bacteria
The association between leguminous plants and the bacteria Rhizobium is a classic example of symbiosis. They are nitrogen-fixing soil bacteria. They absorb nitrogen in the atmosphere and convert it into soluble nitrogenous compounds in the soil. Being heterotrophs, Rhizobium live in the roots of leguminous plants (such as peas, beans, grams, and lentils) for food and shelter. They form nodules in the roots and make nitrogen easily available to them for absorption and growth.

1.6 HOW NUTRIENTS ARE REPLENISHED IN THE SOIL
Nutrients required for the growth of plants are naturally present in the soil. However, due to continuous cropping, the soil may lose its fertility and become deficient in essential nutrients like potassium (K), phosphorus (P), and nitrogen (N). To maintain the health of the plants, the soil must be replenished with nutrients periodically.
1.6.1 Recycling of nutrients into the soil
There are several ways by which soil enrichment can be done.
a. Adding manures and fertilisers
Manure contains humus (organic matter), while fertilizers are laden with specific nutrients (nitrogen, phosphorus, potassium). Manure and fertilisers should be added before and after planting the crops to increase soil fertility. This helps the plant grow properly.
b. Leguminous plants
Soil becomes nitrogen-deficient post a crop harvest. Farmers can choose to plant leguminous crops (such as peas, beans, etc.) to replenish nitrogen in the soil. The roots of legume plants harbour Rhizobium bacteria. These bacteria can use the nitrogen present in the air and provide soluble nitrogenous compounds to the soil, which plants readily absorb. This symbiotic relationship is, therefore, very important to farmers.
QUICK REVIEW
• Energy is obtained from the food we eat and is required for the growth and survival of an organism.
• Nutrition is the process in which the food consumed by an organism is used by its body to perform various life processes.
• Based on the mode of nutrition, organisms show autotrophic nutrition or heterotrophic nutrition.
• Green plants (autotrophs) synthesise food (carbohydrate, complex organic substance) from simple inorganic raw materials like carbon dioxide and water with the help of chlorophyll in green leaves and sunlight in a process called photosynthesis.
• Chlorophyll, a green pigment present in the green leaves, traps solar energy.
• Stomata (tiny pores) on the lower surface of leaves open for the intake of carbon dioxide and release of oxygen in the air.
• Photosynthesis absorbs CO2 and releases O2, which helps maintain a balance between both gases in the atmosphere.
• Parasitic plants derive their nutritional requirements from the living host plant and are categorised into total parasitic plants and partial parasitic plants.
• Saprotrophs feed on the non-living organic matter of dead, decaying plants and animals.
• Symbiosis is a unique association of two organisms that benefit and support each other for food and shelter.
• To maintain the health of the plants, the soil must be replenished with nutrients from time to time with the help of manure and fertilisers.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Mode of nutrition in plants
1. Carbohydrates are mainly made up of which of the following elements?
a. Carbon and hydrogen only
c. Carbon, oxygen and nitrogen
b. Oxygen and carbon only
d. Carbon, hydrogen and oxygen
2. Which of the following organisms is not an autotroph?
a. Pitcher plant
c. Alga
b. Mango tree
d. Fungus
3. The mode of nutrition in which an organism cannot prepare its own food and depends on other organisms for its nutrition is referred to as
a. Autotrophic mode of nutrition
c. Symbiotic mode of nutrition
4. Green plants are called as autotrophs because
a. They prepare their own food by photosynthesis
b. They cannot prepare their own food
b. Heterotrophic mode of nutrition
d. None of these
c. They convert organic matter into simple soluble form
d. They depend on bacteria to obtain N2 nutrition
5. What is the role of chlorophyll in photosynthesis?
a. Absorption of carbon dioxide.
b. Release of oxygen
c. Absorption of atmospheric nitrogen and water vapour
d. Conversion of light energy into chemical energy
II. Photosynthesis – food making process in plants
1. Green plants synthesize their food in the form of
a. Starch
c. Proteins
b. Glucose
d. Cellulose
2. The essential gas required for the process of photosynthesis is
a. Carbon dioxide
b. Oxygen
c. Ammonia
d. None of these
3. Which of the following factors will not have an immediate effect on the process which the shown plant is undergoing?
Sunlight
i. Amount of water
ii. Amount of fertiliser
iii. Concentration of CO2
iv. Concentration of O2
v. Intensity of light
a. (i) and (iii)
c. (i), (ii) and (iii)
4. Select the mismatched pair out of the following.
a. CO2 - Raw material of photosynthesis
b. Chlorophyll - Absorbs green light
c. Stomata - Present on leaf surface
d. O2 - Byproduct of photosynthesis
b. (ii) and (iv)
d. (ii), (iii) and (v)
5. Assertion: The survival of almost all living organisms directly or indirectly depends upon the food made by plants.
Reason: Photosynthesis can take place only in the leaves of a plant and not in any other plant part.
a. If both assertion and reason are true and the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
III. Other modes of nutrition in plants
1. The plant which traps and feeds on insects is:
a. Cuscuta
b. China rose
c. Rose d. Pitcher plant
2. Mistletoe is a ____________ parasitic plant that ____________ green leaves.
a. Partial, does not possess
c. Total, possesses
3. Which of the following statements is true?
b. Partial, possesses
d. Total, does not possess
a. Stomata give roots and the ability to convert the sun's energy into food.
b. Cuscuta converts atmospheric nitrogen into soluble nitrogenous compounds.
c. Leaves send food down to the roots through xylem vessels.
d. Parasitic plants have special roots called haustoria that penetrate the stem of the host plant.
4. Refer to the given Venn diagram and select the correct option.
a. P could be pitcher plant whereas R could be Aloe
b. Q could be pea, whereas P could be sundew
c. Q could be bladderwort, whereas R could be Opuntia
d. Both (a) and (b)
5. The table given below represents the organisms and their mode of nutrition.
Organism
Mode of Nutrition
Cuscuta (i) (ii) Insectivorous (iii) Saprotrophic
The information given in which of the following options correctly completes the table?
a. (i) - Parasitic, (ii) - Mosquito, (iii) - Algae
b. (i) - Autotrophic, (ii) - Mushroom, (iii) - Yeast
c. (i) - Parasitic, (ii) - Drosera, (iii) - Yeast
d. (i) - Autotrophic, (ii) - Bladderwort, (iii) - Yeast
IV. Saprophytes
1. Some organisms secrete digestive juices over the decaying matter and absorb nutrients from it. This is called ________________ mode of nutrition.
a. Autotrophic b. Saprotrophic
c. Parasitic
2. Which of the following statements is correct?
a. Most fungi and bacteria are saprotrophs
d. Symbiotic
b. Some mushrooms are edible, whereas some are poisonous
c. Some fungi are used in the preparation of medicines
d. All of these
3. Refer to the given picture. Select the incorrect statement regarding the organism shown in it.

a. It is a fungus.
b. It feeds on dead organic matter.
c. It can synthesize its own food.
d. Some of its varieties are edible whereas some are poisonous.
4. Select the incorrect statement out of the following.
a. Fungal spores germinate and grow when they land on wet and warm surfaces.
b. Insectivorous plants usually grow in nitrogen-deficient soils.
c. Saprotrophs are green in colour due to the presence of chlorophyll.
d. Heterotrophs cannot prepare their own food.
5. Assertion: Mushrooms exhibit a saprotrophic mode of nutrition.
Reason: Mushrooms obtain their nutrition from dead and decaying organic matter.
a. If both assertion and reason are true and the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
V. Symbiosis
1. Lichen is an association between
a. An alga and a fungus
c. A fungus and a tree
2. A symbiotic relationship is one in which
b. An alga and a tree
d. Bacteria and legume plant
a. Organisms feed on dead and decaying organic matter
b. Organisms trap and feed on insects.
c. Two organisms live together and benefit from each other
d. One organism grows as a parasite on the body of another
3. Select the incorrect statement regarding the association between leguminous plants and Rhizobium bacteria.
a. It is a symbiotic relationship.
b. Rhizobium cannot make its own food.
c. Rhizobium converts atmospheric nitrogen into soluble form.
d. This association causes a deficiency of nitrogen in the soil.
4. Assertion: Most of the plants can absorb nitrogen from air except certain leguminous plants.
Reason: Leguminous plants include cereal crops such as peas, beans, etc.
a. If both assertion and reason are true and the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
5. Symbiosis is the phenomenon in which two different kinds of organisms pool together their nutritional requirements. Which one represents such an association from the following figures?


VI. How nutrients are replenished in the soil
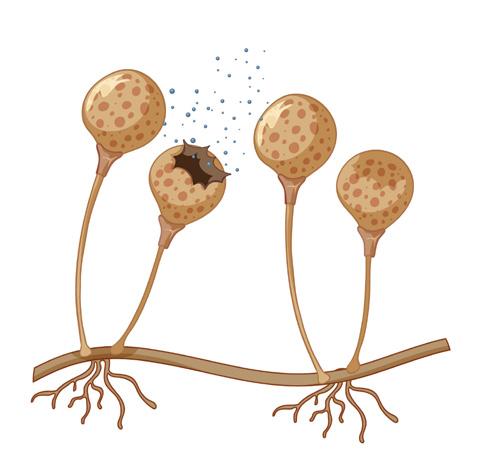
d. None of these
1. Replenishment of nutrients in the soil can be done by
a. Adding manures
c. Growing legume crops
b. Adding fertilizers
d. All of these
2. How can we enhance the level of nutrients and minerals in the soil?
a. By adding fertilizers to the soil
b. By adding manure to the soil
c. By avoiding the use of fertilizers and manure
d. Both (a) and (b)
3. Assertion: Plants absorb mineral nutrients from the soil.
Reason: Manures add or enhance nutrients back into the soil.
a. If both assertion and reason are true and the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
4. What is the primary benefit of recycling nutrients into the soil?
a. It reduces soil erosion
b. It promotes healthy plant growth
c. It increases air pollution
d. It depletes soil fertility
5. Identify the correct pair with respect to support plant growth.
a. Nitrogen-Potassium
c. Phosphorus-Sodium
WORKSHEET - 2

b. Potassium-Sodium
d. Nitrogen-Sodium
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Cuscuta is an example of
a. Autotroph
c. Saprophyte
b. Parasite
d. Host
2. Which part of the plant takes in carbon dioxide from the air for photosynthesis?
a. Root hair
c. Leaf veins
b. Stomata
d. Sepals
3. Which of the following options contains end products of photosynthesis?
a. C6H12O6 + O2
c. C6H12O6 + CO2
4. Select the incorrect statement out of the following.
a. Leaves are the food factories of plants.
b. CO2 + H2O
d. CO2 + O2
b. Water and minerals are absorbed by the roots from the soil and transported to the leaves.
c. Raw materials required for photosynthesis are carbohydrates and oxygen.
d. Chlorophyll pigment of leaves helps to capture the energy of sunlight.
5. Where do plants primarily absorb minerals and nutrients from?
a. Air
c. Water
b. Soil
d. Sunlight
6. Why is Nepenthes considered partially heterotrophic? Select the correct statement out of the following:
a. It chemically synthesises its food from atmospheric nitrogen.
b. It depends entirely on other organisms for nutrients.
c. It combines photosynthesis with insect trapping for nutrient intake.
d. It absorbs nitrogen from soil through roots, supplementing photosynthesis.
7. Which of the following statements is correct regarding the given structure?
a. It is the insect-catching organ of a parasitic plant.
b. It is the modification of the leaf.
c. It is the pitcher of the plant Venus flytrap.
d. It is the haustorium of a parasitic plant.
8. Match column I with column II and select the correct option.
Column I
A. Hydrilla
B. Pitcher plant
C. Cuscuta
D. Bread mould
Column II
i. Parasitic
ii. Saprotroph
iii. Photosynthesis
iv. Insectivorous
a. A - (iii), B - (iv), C - (i), D - (ii)
b. A - (iii), B - (i), C - (iv), D - (ii)
c. A - (ii), B - (iv), C - (i), D - (iii)
d. A - (ii), B - (iv), C - (iii), D - (i)
9. Which of the following statements is true regarding insectivorous plants?
a. They are called partial heterotrophs.
b. They exhibit both autotrophic and heterotrophic modes of nutrition.
c. They trap and digest insects to obtain N2 nutrition.
d. All of these
10. An example of nitrogen-fixing symbiotic bacteria is
a. Rhizobium
c. Mycobacterium
b. E. coli
d. None of these
11. Why do the amounts of minerals and nutrients in the soil tend to decline over time?
a. Plants release excess nutrients
b. Rainfall washes away nutrients
c. Soil naturally replenishes
d. Plants absorb minerals and nutrients
12. Certain fungi live in the roots of trees. The tree provides nutrients to the fungus and, in return, takes its help to absorb water and minerals from the soil. This kind of relationship is referred to as
a. Parasitism
c. Symbiosis
13. Photosynthesis converts
a. Solar energy to mechanical energy
c. Solar energy to chemical energy
b. Competition
d. Predation
b. Mechanical energy to solar energy
d. Chemical energy to solar energy
14. _____________ is a leafless and rootless parasitic plant that derives its nutrition from a living host plant.
a. Nepenthes
c. Cuscuta
b. Venus flytrap
d. Mistletoe
15. Nitrogen (N2) present in the atmosphere is directly absorbed by
a. Autotrophs
c. Insectivorous plants
b. Leguminous plants
d. None of these
16. The production of carbohydrates from CO2 and H2O in the presence of chlorophyll using sunlight energy and releasing O2 is called
a. Respiration
c. Transpiration
b. Photosynthesis
d. Oxidation
17. A mutually beneficial relationship between two living organisms is called __________and an example of this is ___________.
a. Parasitism, mycorrhiza
c. Symbiosis, lichens
b. Predation, mycorrhiza
d. Parasitism, lichens
18. What is the process by which nutrients are returned to the soil to be used again by plants for their growth?
a. Nutrient consumption
c. Nutrient disposal
b. Nutrient extraction
d. Nutrient recycling
19. Which of the following options contains autotrophic organisms only?
a. Rose, Cuscuta, Hydrilla
c. Bougainvillea, rose, hibiscus
20. Select the correct statement.
b. Nepenthes, Rafflesia, hibiscus
d. Cuscuta, pitcher plant, mistletoe
a. Manures and fertilizers are added to increase productivity.
b. Sun is the ultimate source of energy for all living organisms.
c. Cells of the pitcher of Nepenthes plant secrete digestive juices to digest the insect.
d. All of these
21. Which of the following options contains leguminous crops only?
a. Rice, wheat and maize
c. Peas, beans and maize
b. Wheat, rice and peas
d. Peas, beans and gram
22. Match column I with column II and select the correct option.
Column I
A. Algae
B. Lichens
C. Bread mould
D. Rafflesia
a. A - (ii), B - (iii), C - (iv), D - (i)
b. A - (iii), B - (ii), C - (iv), D - (i)
c. A - (i), B - (iii), C - (ii), D - (iv)
d. A - (iv), B - (iii), C - (i), D - (ii)
Column II
i. Parasitic
ii. Autotrophic
iii. Symbiosis
iv. Spoilage of food
23. Assertion: Insectivorous plants are completely heterotrophic organisms.
Reason: Insectivorous plants can synthesise their own food and also feed on insects to obtain their nitrogen nutrition.
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
24. Water and minerals present in soil are absorbed by (A) and transported to (B) by vessels which run like pipes throughout the root, stem, branches and leaves. Select the correct option for (A) and (B) in the above statement.
a. A- Roots B - Leaves
b. A- Leaves B - Roots
c. A- Leaves B - Stem
d. A- Stem B - Leaves
25. Which of the following statements supports the fact that the upper surface of a leaf is usually greener than the lower surface?
i. More chlorophyll is present on the upper surface to absorb more light.
ii. There are more stomata present on the upper surface than on the lower surface.
iii. More sugar is formed on the lower surface.
iv. The green leaves look greener under the hot Sun.
a. (i) only
c. (ii) and (iv)
b. (ii) and (iii)
d. (iv) only
26. Refer to the given flow chart and select the option that correctly identifies organisms P, Q, R and S.
Heterotrophic mode of nutrition
Parasitic mode of nutrition Saprophytic mode of nutrition
a. P - Hydrilla; Q - Hydra; R - Dionaea; S - Amoeba
b. P - Hibiscus; Q - Cuscuta; R - Rhizopus ; S - Drosera
c. P - Elodea; Q - Taenia; R - Bladderwort; S - Hydrilla
d. P - Ocimum; Q - Coleus; R - Mistletoe; S - Spirogyra
27. Assertion: The rate of photosynthesis is affected by several factors, such as light temperature, water, carbon dioxide, etc.
Reason: The rate of photosynthesis increases with the increase in intensity of light and decreases with the decrease in intensity of light.
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
28. Assertion: Mistletoe can prepare its own food by photosynthesis.
Reason: Mistletoe is a parasitic plant and does not possess leaves.
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
29. Assertion: The coloured leaves, other than green, also possess chlorophyll, but they do not appear green in colour.
Reason: The large amount of red, brown and other pigments mask the green colour.
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
30. Assertion: Fungi suddenly appears during the rainy season.
Reason: Fungal spores, which are generally present in the air, get favourable conditions to germinate during the rainy season.
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.

NUTRITION IN ANIMALS 2
2.1 INTRODUCTION
All organisms, including human beings, require food for growth, repair and functioning of the body. Food consists of many components called nutrients. Various nutrients, along with their functions and sources are given in the following table.
Nutrients
Functions
Carbohydrates Give energy to our body
Fats Give energy and insulation to our body
Proteins Help in growth, repairing of tissues, healing of small cuts and wounds
Vitamins
Minerals
Roughage
Water
Help us to resist diseases, increase immunity
Help us to resist diseases, increase immunity
Helps the body to easily get rid of undigested food (waste matter)
Maintains balance of body fluids, helps to remove waste matter, helps to transport nutrients to cells, etc.
Table
2.2 DIFFERENT WAYS OF TAKING FOOD
Various modes of feeding
Sources
Cereals, potatoes, etc.
Ghee, oil, butter, nuts, etc.
Milk, eggs, meat, pulses, fish, etc.
Fruits and vegetables, eggs, fish, etc.
Milk, green leafy vegetables, fruits, etc.
Wholemeal cereals, vegetables, fruits, etc.
Drinking water, juices, soups, fruits with high water content, e.g., watermelon, etc.
Since different animals eat different types of food, they have different types of feeding organs and different modes of feeding. A few are discussed below:
Bees, hummingbirds, and butterflies suck nectar from flowers. Butterflies have coiled mouth parts called proboscis, which unwind into straight tubes while feeding. Bees and hummingbirds use their tongue and beak to obtain flower nectar.
Mosquitoes and leeches have their mouthparts adapted for piercing and sucking blood from the body of human beings. A mosquito sucks the blood of animals and human beings with its proboscis.
The spider spins a sticky web that traps tiny insects. Afterwards, it injects digestive fluids into the insect's body, breaking down its body parts externally. Consequently, the spider's food digestion occurs outside its own body. Finally, the spider ingests the digested food.
2.3 DIGESTION IN HUMANS
2.3.1 Introduction
Digestion is the process by which the complex chemical compounds present in the food are broken down into simpler substances that are readily absorbed and utilised by the body.
The digestive system of human beings consists of two main parts: the alimentary canal (gut or digestive tract) and digestive glands. The food components gradually get digested as the food travels through the various compartments of the alimentary canal.
Alimentary canal
The alimentary canal is a long tube which starts from the mouth and ends at the anus. It can be divided into various compartments, mouth or buccal cavity, food pipe or oesophagus, stomach, small intestine, large intestine, and anus.
Digestive glands
Digestive glands, such as the salivary glands (in the mouth), gastric glands (in the stomach), liver and pancreas, secrete digestive juices to convert complex substances of food into simpler ones. The inner wall of the small intestine also secretes digestive juices.
Oral cavity
Mouth
Sublingual gland
Liver
Gallbladder
Duodenum
Small intestine
Ascending colon
Caecum
Appendix
Rectum
Parotid gland
Pharynx
Submandibular gland
Oesophagus
Stomach
Pancreas
Transverse colon
Descending colon
Sigmoid colon
Anal canal
Anus
Fig. 2.1 Human digestive system
2.3.2 Mouth and buccal cavity
The mouth is bordered by the upper and lower lips. The lips help in closing the mouth during swallowing. Inside the mouth are present the teeth and the tongue. Food is taken through the mouth. This is called ingestion. The food is broken down into smaller pieces or chewed with the teeth.

The first set of teeth grows during infancy, and they fall off at the age of six to eight years. These are termed the milk teeth. The second set that replaces them is the permanent teeth. The permanent teeth may last throughout life or fall off during old age or due to some dental disease.
Based on their different shapes and functions, human teeth are of four kinds:
Table 2.2 Types
1. Incisors are the four front teeth at the middle of each jaw. They are chisel-shaped for biting and cutting.
2. Canines are the ones on either side of the incisors in each jaw. They are pointed for tearing the food.
3. Premolars are the two on each side of the canines in each jaw. They help in crushing and grinding the food.
4. Molars are the last three teeth on each side of each jaw. They have broad, uneven surfaces for finer crushing and grinding of ingested food.
Tongue
The tongue is a fleshy muscular organ that is attached at the back to the floor of the buccal cavity, free at the front, allowing movement in all directions. Its surface is rough due to tiny bumps called taste buds, which detect sweet, salty, sour, and bitter tastes. Although taste buds detect all four tastes, certain regions of the tongue may be more sensitive to particular tastes, detecting them earlier than others.
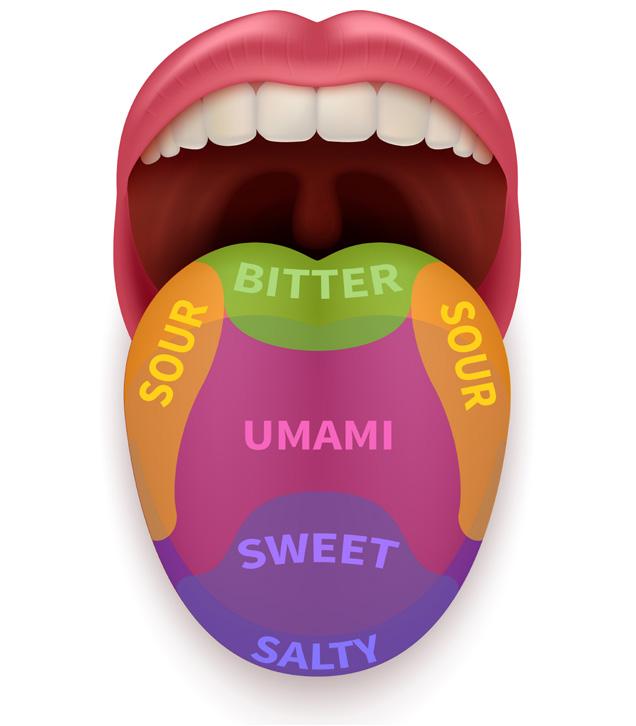
Functions of tongue
(i) Helps in tasting the food.
(ii) Manipulates the food while chewing.
(iii) It mixes saliva with food while chewing.
(iv) Helps in swallowing the food and in speaking.
2.3.3 Pharynx
The tongue pushes the food from the mouth towards the pharynx. The pharynx is a common passage for food and air.
2.3.4 Oesophagus
The moist ball of food called a bolus, when swallowed, reaches the food pipe or oesophagus. It is about a muscular tube that is about 25 cm long. It runs along the neck and the chest. Its function is to transfer the food from the pharynx to the stomach.
Nasal cavity
Larynx
Trachea
Nasopharynx
Oropharynx
Hypopharynx
Oesophagus
Fig. 2.5 Pharynx and oesophagus
Peristalsis: The muscles of the oesophagus gently push the food down to the stomach in a wavelike action (contractions and relaxations) called peristalsis. This movement takes place throughout the alimentary canal to push the food forward.
The direction of the peristaltic movements is reversed during vomiting, when due to some factors (such as taking stale food), food instead of moving downwards, is pushed upwards leading to vomiting.
Area of contraction
Oesophagus
Food bolus
Muscular layer Area of relaxation
2.3.5 Stomach
The stomach is a J-shaped, thick-walled bag-like structure. It receives food from the oesophagus. The inner lining of the stomach secretes digestive juice (or gastric juice), which contains mucus, hydrochloric acid, and the digestive enzymes.
Functions of stomach
(i) The thick muscular walls of the stomach contract to churn the food and mix it with the digestive juice produced by the stomach.
(ii) The mucus protects the lining of the stomach from the action of enzymes and acids.
(iii) Hydrochloric acid (HCl) kills the bacteria that enter the stomach along with the food, and it makes the medium in the stomach acidic to help the digestive enzymes act.
(iv) The digestive juice of the stomach contains digestive enzymes, e.g., pepsin, which cause the breakdown of proteins into simple substances (i.e., amino acids).
(v) Partial digestion of food occurs in the stomach.
(vi) Food is stored in the stomach for a few minutes to a few hours. When food leaves the stomach, it is like a thin paste, which is then moved down to the small intestine, where most of the digestion takes place.
2.3.6 Liver, gall bladder, and pancreas
The liver is reddish-brown and is the largest gland in the body. It is situated on the right side of the body, below the chest region. The liver produces a greenish-yellow fluid called bile, which is stored in the gall bladder, an organ found just below the liver. The bile juice breaks down fats in tiny droplets, which can be digested and absorbed easily, and this process is called emulsification.
Cystic duct
Gallbladder
Common bile duct
Duodenum
Right hepatic duct
Tail of pancreas
Pancreas
Body of pancreas
Left hepatic duct
Pancreatic duct
Head of pancreas
The pancreas is a large, whitish, leaf-shaped gland situated below the stomach. It secretes pancreatic juice, which is poured into the duodenum through the pancreatic duct. The pancreatic duct opens into the duodenum by an aperture common to that of the gall bladder.
The pancreatic juice contains enzymes namely amylase, trypsin and lipase, which help in the digestion of carbohydrates, proteins, and fats, respectively.
2.3.7 Small intestine
The small intestine is the longest portion of the alimentary canal. It is over 7.5 metres long and is highly coiled. The digestion of food is completed in the small intestine. It receives bile juice from the liver and pancreatic juice from the pancreas and secretes several digestive juices (intestinal
juices) of its own. Thus, when the partly digested food from the stomach reaches the small intestine, it is mixed with the bile, pancreatic, and intestinal juices, and the complete digestion of all the components of food takes place.
Small Intestine
Duodenum
Jejunum
Illeum
Functions of small intestine
(i) Proteins are broken down into amino acids.
(ii) Carbohydrates are broken down into simple sugars, such as glucose.
(iii) Fats are hydrolysed into fatty acids and glycerol.
(iv) Absorption of digested food takes place in the intestinal wall.
2.3.8 Absorption in the small intestine
The digested food can be utilised by our body only if it gets absorbed. The digested food can pass into the blood vessels in the wall of the intestine. This process is called absorption
Absorption of food occurs through thousands of finger-like outgrowths in the inner walls of the small intestine. These are called villi. The villi increase the surface area for absorption of the digested food. Each villus has a network of thin and small blood vessels close to its surface. A minute blindended lymph vessel called lacteal also occurs in each villus of the small intestine. The digested fats are absorbed into the lacteals. The food absorbed on the surface of the villus passes into the blood in the capillaries.
Assimilation
The absorbed substances are transported via the blood vessels to different organs of the body, where they are used to build complex substances, such as the proteins required by the body. This is called assimilation. The food that remains undigested and unabsorbed enters the large intestine.
2.3.9 Large intestine
The large intestine is about 1.5 metres long and consists of three regions: the caecum, the colon, and the rectum
The large intestine does not secrete any enzyme. It mainly absorbs water from the undigested food. After much water is absorbed, the undigested waste matter that reaches the rectum is semi-solid.
The rectum is the last part, about 15cm long. It stores the undigested waste matter called faeces. The rectum opens to the outside at the anus. It has a circular muscle (sphincter) to keep it closed. When this muscle relaxes, the anus opens to eliminate the faeces.
The process of eliminating the undigested food through the anus is called egestion.
Transverse colon
Ascending colon
Descending colon
2.3.10 Diarrhoea and ORT
Diarrhoea is the condition of passing three or more loose or liquid stools per day. It is caused due to an infection of bacteria, etc. It spreads through contaminated (unclean or polluted) water and food, poor hygienic conditions, etc. It is very common in India due to poverty and a lack of proper hygiene. It leads to loss of fluids from the body, i.e., dehydration, which may even prove fatal, particularly in young children. Diarrhoea should not be neglected. The patient should be rehydrated by giving an oral rehydration solution (ORS). ORS is a mixture of drinking water, salt, and sugar, which can be easily prepared at home.
Oral rehydration therapy (ORT) is a type of fluid replacement used as a treatment for dehydration. It involves drinking water mixed with sugar and salt while continuing to eat. When dehydration is severe, therapy also includes supplemental zinc.
2.4 DIGESTION IN GRASS-EATING ANIMALS
Ruminants and rumination
Ruminants are the hooved, plant-eating (herbivorous) mammals that include cattle (cow, buffalo), goat, sheep, deer, yak, etc. Ruminants possess a unique digestive system that allows them to use energy from fibrous plant material better than other herbivores. The stomach of these animals consist of four chambers: rumen, reticulum, omasum, and abomasum.
Omasum
Oesophagus
Small intestine
Reticulum
Abomasum
Rumen
Fig. 2.12 Ruminant digestive system
During grazing, they quickly swallow plenty of grass without much chewing. This is an adaptation by which they need very little time for feeding. The food is partially chewed and mixed with saliva. This partially chewed food is sent to the rumen (large sac-like structure). Here, the partial digestion of food takes place as it harbours a large population of anaerobic bacteria, which help in the fermentation of food.
Food through mouth
Rumen
Back to mouth Regurgitation
Rumen (Major digestion)
Reticulum
Omasum
Rumen
Abomasum
Large intestine
Duodenum
Abomasum (Action of gastric juice &enzymes)
Goes to Reticulum (Filtration of food material)
Omasum (Grinding of food)
Fig. 2.13 Process of digestion in ruminants
These microorganisms produce an enzyme (cellulase) that helps in the digestion of cellulose (a complex carbohydrate present in the grass). This partly digested food, called cud, is then pushed to the second chamber called the reticulum. The rumen and reticulum are closely associated, and their contents mix freely. When the animal is resting, the cud is brought back into the mouth in small quantities and the animal chews it further. This process is called rumination, and these animals are called ruminants (cud-chewing mammals). After completely chewed, the food is sent to other parts of the stomach, i.e., abomasum and omasum, and ultimately to the intestine for complete digestion.
2.5 FEEDING AND DIGESTION IN AMOEBA
2.5.1
Pseudopodia
Amoeba is a microscopic single-celled organism found in pond water. Amoeba has a cell membrane, a rounded, dense nucleus, and many small bubble-like vacuoles in its cytoplasm. Amoeba constantly changes its shape and position. It pushes out one or more finger-like projections called pseudopodia or false feet for movement and capture of food.
2.5.2
Food vacuole

Amoeba feeds on some microscopic organisms. When it senses food, it pushes out pseudopodia around the food particle and engulfs it. The food becomes trapped in a food vacuole. Digestive juices are secreted into the food vacuole. They act on the food and break it down into simpler substances. Gradually, the digested food is absorbed. The absorbed substances are used for growth, maintenance, and multiplication. The undigested food residue is expelled outside by the vacuole.
QUICK REVIEW
• Since different animals eat different types of food, they have different types of feeding organs and possess different modes of feeding, e.g., sucking in bees and butterflies, tentacles for capturing food in Hydra, etc.
• Ingestion is the process of taking in food through the mouth opening.
• Digestion is the process of breaking down food (complex insoluble organic compounds) into simple, soluble forms with the help of digestive juices made in the body.
• Absorption is the process by which food in the simple and soluble form passes into the body fluids, such as blood.
• Assimilation is the process of using the absorbed nutrients by the body cells to produce energy and for the growth of the body.
• Egestion is the process of removal of undigested food or waste matter from the body.
• The alimentary canal and the associated digestive glands together constitute a human digestive system.
• Ruminants, such as cattle and sheep, have a four-chambered stomach comprising the rumen, reticulum, omasum, and abomasum.
• Ruminants practice rumination, where the partially digested food (cud) from the rumen is regurgitated, chewed, and re-swallowed for further digestion before passing to the other stomach chambers and intestines.
• Amoeba feeds on some microscopic organisms. When it senses food, it stretches out pseudopodia (finger-like projections) around the food particle and engulfs it. The food becomes trapped in a food vacuole for digestion with the help of digestive juices.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Different ways of taking food
1. What is the primary mode of feeding in rabbits, rats, and squirrels?
a. Sucking b. Gnawing c. Piercing d. Scraping
2. Among these animals, which one commonly swallows its whole food?
a. Snake b. Ant c. Cow d. Lion
3. Which of the following statements is not true regarding feeding behaviours?
a. Methods of ingesting food vary among different organisms.
b. Bees and hummingbirds extract nectar from flowers.
c. Hydra captures its prey using its tentacles.
d. Lice and mosquitoes grind their food with teeth.
4. Which of the following animals is known to be a filter-feeder?
a. Snake b. Housefly c. Flamingo d. Rabbit
5. Choose the pair where both the animals are known for blood-sucking.
a. Tick and mite
c. Leech and rabbit
II. Digestion in humans
b. Mosquito and housefly
d. Bedbug and honeybee
1. The process of breaking down complex components of food into simpler substances is called:
a. Absorption b. Ingestion c. Digestion d. Assimilation
2. Which option correctly depicts the sequential path of food from the mouth to the anus?
a. Mouth → Oesophagus → Stomach → Small intestine → Large intestine → Anus
b. Mouth → Large intestine → Oesophagus → Stomach → Small intestine → Anus
c. Mouth → Small intestine → Large intestine → Oesophagus → Stomach → Anus
d. Mouth → Stomach → Small intestine → Large intestine → Oesophagus → Anus
3. Which part of the digestive system assists in combining food with saliva?
a. Oesophagus b. Salivary glands c. Tongue d. Liver
4. The teeth responsible for tearing food are known as:
a. Incisors b. Canines c. Premolars d. Molars
5. The last molar tooth in human beings is referred to as:
a. Adult tooth b. Wisdom tooth c. Child tooth d. Elder's tooth
6. Which substance in your body is the hardest?
a. Dentine b. Bone c. Cement d. Enamel
7. What does saliva transform starch into?
a. Glucose b. Sucrose c. Maltose d. Lactose
8. Where does the mechanical digestion of food begin?
a. Small intestine b. Mouth c. Oesophagus d. Stomach
9. Which enzyme converts proteins in milk into curd?
a. Trypsin b. Rennin c. Pepsin d. Erepsin
10. Where is the bile juice produced?
a. Stomach b. Liver c. Pancreas d. Gall bladder
11. Which combination of organs is engaged in the process of digesting food?
a. Pancreas, liver, and stomach
c. Heart, pancreas, and stomach
b. Stomach, oesophagus, and kidney
d. Liver, pancreas, and lungs
12. Which part of the digestive system do the liver and pancreas release their secretions into?
a. Large intestine
c. Stomach
b. Small intestine
d. Oesophagus
13. Which organ is the largest gland in the human body?
a. Pancreas
b. Stomach c. Kidney d. Liver
14. Assertion: Peristaltic movement of the food pipe walls propels food towards the stomach. Reason: Peristaltic motions reverse during vomiting episodes.
a. Both assertion and reason are true, and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but reason is false.
d. Assertion is false, but reason is true.
15. Assertion: Carbohydrates & proteins are broken down into simpler forms during digestion. Reason: They are complex substances that cannot be utilised as such by the body.
a. Both assertion and reason are true, and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but reason is false.
d. Assertion is false, but reason is true.
16. Which of the following statements is correct about the role of villi?
a. Increase the surface area for absorption of food
b. Decrease the surface area for absorption of food
c. Help in the digestion of fat
d. None of these
17. Which part of the alimentary canal secretes acid?
a. Small intestine b. Large intestine c. Mouth d. Stomach
18. Which of the following carries the digested food to all parts of the body?
a. Blood b. Mucus c. Bile d. Saliva
III. Digestion in grass-eating animals
1. Which of the following statements about digestion in ruminants is correct?
a. Ruminants eat food quickly and store it temporarily in the rumen.
b. When ruminants stop feeding, cud returns to the buccal cavity to be chewed again.
c. Ruminants can digest cellulose present in grass.
d. All of these
2. Which of the following options comprises the four chambers in the digestive tract of ruminating animals?
a. Rumen, reticulum, omasum, abomasum
b. Rumen, reticulum, small intestine, large intestine
c. Small intestine, large intestine
d. Small intestine, large intestine, rumen
3. Match the terminologies in column I with their correct definition in column II.
Column I
a. Cud
Column II
i. The process of storing and partial digestion of grass to form cud
b. Rumen ii. Partially digested food
c. Ruminants
d. Rumination
a. a-i, b-ii, c-iii, d-iv
c. a-iii, b-ii, c-i, d-iv
iii. Animals that eat grass and have a rumen
iv. The place where the animals store grass
b. a-iv, b-iii, c-ii, d-i
d. a-ii, b-iv, c-iii, d-i
IV. Feeding and digestion in Amoeba
1. Digestion of food inside the food vacuole occurs in
a. Hydra b. Cockroach c. Amoeba d. Birds
2. Pseudopodia help in the ingestion of food in
a. Amoeba b. Earthworm c. Paramecium d. Snake
3. Which of the following structures is NOT found in Amoeba?
a. Cell wall b. Nucleus c. Cytoplasm d. Pseudopodia
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. In the small intestine, the food is broken down into simpler forms. Match the components of food in column I with their simpler forms in column II.
Components Simpler forms
A. Carbohydrates i. Glycerol
B. Fats
C. Proteins
ii. Amino acids
iii. Glucose
a. A-i, B-ii, C-iii, b. A-iii, B-i, C-ii, c. A-ii, B-iii, C-i, d. A-ii, B-i, C-iii,
2. Which of the following options is the right order of the parts of the alimentary canal?
a. Buccal cavity, stomach, oesophagus, small intestine, large intestine, rectum, anus
b. Buccal cavity, oesophagus, stomach, large intestine, small intestine, rectum, anus
c. Buccal cavity, oesophagus, stomach, small intestine, large intestine, rectum, anus
d. Oesophagus, buccal cavity, stomach, large intestine, small intestine, rectum, anus
3. Assertion (A): Fatty acids and glycerol cannot be absorbed directly into the blood from the intestine.
Reason (R): They are insoluble in water.
a. Both A and R are true, and R is the correct explanation for A.
b. Both A and R are true, but R is not the correct explanation for A.
c. A is true, but R is false.
d. Both A and R are false.
4. Which organ is primarily responsible for reabsorbing water from the undigested food?
a. Liver
b. Stomach c. Large intestine d. Small intestine
5. Raj ate a sandwich containing carbohydrates, proteins, and fats. Among the following organs, which one will play a crucial role in breaking down these nutrients?
a. Kidney b. Brain c. Mouth d. Small intestine
6. Assertion (A): The primary function of the stomach is to absorb nutrients from food.
Reason (R): The stomach has a highly basic environment that aids in the digestion of proteins.
a. Both A and R are true, and R is the correct explanation for A.
b. Both A and R are true, but R is not the correct explanation for A.
c. A is true, but R is false.
d. Both A and R are false.
7. Read the given statements and select the option which correctly fills the blanks in any two of these statements.
(i) Paramecium has stiff hair-like structures called __________ all over its body, which are used for _________________.
(ii) Hydra has a number of ______ around its mouth that entangle small aquatic animals and kill them with their ______ cells.
(iii) Frog uses its long, sticky _______ to catch insects.
(iv) Mosquito sucks up the blood of animals with its _______.
a. (ii) Cilia, absorptive; (iii) limb
b. (i) Tentacles, ingestion; (iv) feeding tube
c. (i) Cilia, ingestion; (ii) tentacles, stinging
d. (iii) Tongue; (iv) pseudopodia
8. Which of the following statements is incorrect?
a. The saliva lubricates the food.
b. The tongue helps in tasting food.
c. The bile breaks down fat into small droplets.
d. The saliva helps digest proteins.
9. Select the incorrect statement regarding the human digestive system.

a. The digestive tract and associated glands together constitute the digestive system.
b. The tongue helps mix the saliva with the food and helps in swallowing.
c. The pancreatic juice acts only on carbohydrates and converts these into simpler forms.
d. Digestion of food is completed in the small intestine.
10. The digestive juice of the stomach contains pepsin enzyme, which breaks down
a. Complex carbohydrates into sugars
c. Fats into fatty acids
11. Hydrochloric acid in the stomach
a. Helps in the digestion of fat
c. Converts starch into sugars
b. Proteins into simpler form
d. All of these
b. Kills bacteria ingested with food
d. Converts proteins into amino acids
12. The digestion of carbohydrates starts in ____(i)____ with the help of enzyme ____ (ii)____.
Which of the following options correctly fills the blanks and completes the given statement?
a. (i) buccal cavity; (ii) pepsin
c. (i) buccal cavity; (ii) amylase
b. (i) stomach; (ii) amylase
d. (i) stomach; (ii) pepsin
13. The table below illustrates the food components and their digested products. Select the correct option for P and Q.
Food components Digested products P Amino acids
Carbohydrates Q
a. P - Fats; Q - Glycerol
c. P - Fats; Q - Glucose
b. P - Proteins; Q - Glucose
d. P - Proteins; Q - Glycerol
14. Match column I with column II and select the correct option.
Column I
A. Mechanical breakdown of food by chewing i. Stomach
B. Passage that connects mouth to stomach ii. Gall bladder
C. Chemical digestion of protein starts iii. Mouth
D. Stores bile iv. Oesophagus
a. A - (iii), B - (iv), C - (i), D - (ii)
c. A - (iv), B - (iii), C - (i), D - (ii)
Column II
b. A - (iii), B - (iv), C - (ii), D - (i)
d. A - (ii), B- (iii), C - (iv), D - (i)
15. What happens to the food when it enters from the oesophagus to the stomach?
a. The food mixes with intestinal juices and the digestion of carbohydrates starts.
b. The food mixes with gastric juices and digestion of protein starts.
c. The food quickly moves into the small intestine.
d. The food quickly moves into the large intestine.
16. _____(i)_____ digestion involves the breakdown of large, _____(ii)_____ food molecules into smaller soluble ones with the help of _____(iii)_____.
Which of the following options fills the blanks correctly and completes the given statement?
a. (i)-Physical, (ii)-soluble, (iii)-enzymes
b. (i)-Chemical, (ii)-insoluble, (iii)-enzymes
c. (i)-Thermal, (ii)-insoluble, (iii)-heat
d. (i)-Mechanical, (ii)-soluble, (iii)-heat
17. Match column I with column II and select the correct option.
Column I
A. Maximum absorption of nutrients i. Anus
B. Absorption of water from undigested food ii. Small intestine
C. Opening through which waste matter is expelled out iii. Stomach
D. Secretion of HCl iv. Large intestine
a. A - (ii), B - (iii), C - (i), D - (iv)
c. A - (iv), B - (ii), C - (i), D - (iii)
Column II
b. A - (ii), B - (iv), C - (i), D - (iii)
d. A - (i), B - (iii), C - (ii), D - (iv)
18. Which of the following structures closes the windpipe to prevent the entry of food into it during eating?
a. Pharynx b. Food pipe c. Epiglottis d. Tongue
19. Which of the following statements is incorrect?
a. The large intestine is called large because its length is greater than that of the small intestine.
b. Minerals and vitamins do not need to be broken down during digestion.
c. Mucus of the stomach protects the lining of the stomach from the action of enzymes and acids.
d. The small intestine is smaller in diameter than the large intestine.
20. Which of the following statements is correct regarding oral rehydration therapy (ORT)?
a. It is used as a treatment for dehydration.
b. The patient is given drinking water mixed with sugar and salt.
c. It has an important role in reducing the number of deaths due to dehydration in severe cases.
d. All of these
21. After undergoing gall bladder removal surgery due to gallstones, which problem is Rahul most likely to encounter?
a. Difficulty digesting carbohydrates and proteins due to the absence of the gall bladder, which stores bile containing amylase and pepsin enzymes.
b. Difficulty digesting fats because the gall bladder stores bile juice, which aids in fat emulsification.
c. Difficulty digesting fats because the gall bladder secretes bile juice, essential for fat digestion.
d. Difficulty absorbing vitamins B and C due to the absence of the gall bladder, which secretes bile, aiding in the absorption of these vitamins.
22. Assertion: The liver produces bile, which is stored in the gall bladder.
Reason: Bile aids in the breakdown of fats into simpler substances, like fatty acids and glycerol.
a. Both assertion and reason are true, and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but reason is false.
d. Assertion is false, but reason is true.
23. Amylolytic enzymes are produced from
a. Salivary gland and liver
c. Salivary glands and pancreas
b. Stomach and pancreas
d. Stomach and liver
24. The absorption of ___________ is the main function of lacteals.
a. Amino acids
c. Fatty acids and glycerol
b. Glucose and vitamins
d. Lactic acid
25. The characteristics of Organ A and Organ B of the human body are given below.
Organ A: 1) Food is completely digested here.
2) The substance produced is carried to all parts of the body.
Organ B: 1) Water absorption takes place here.
2) The substance produced is expelled out of the body.
Select the option which correctly identifies Organs A and B.
a. Organ A - Small intestine; Organ B - Oesophagus
b. Organ A - Stomach; Organ; B - Large intestine
c. Organ A - Large intestine; Organ B - Liver
d. Organ A - Small intestine; Organ B - Large intestine

Physics
1: MOTION AND TIME
Worksheet 1
I. Slow or fast motion
1.
II. Speed
III. Measurement of time
IV. Measuring speed
V. Graphs
Chemistry
1: ACIDS, BASES, AND SALTS
Worksheet 1
I. Acids and bases
II. Natural indicators around us
III. Properties of acids and bases
IV. pH concept and pH scale
V Neutralisation reaction
Worksheet 2
Worksheet 2
2: ELECTRIC CURRENT AND ITS EFFECTS
Worksheet 1
I. Electric components
II. Heating effect of electric current
III. Magnetic effect of electric current
2: PHYSICAL AND CHEMICAL CHANGES
Worksheet 1
I. Physical change
Worksheet 2
II. Chemical change
III. Rusting
IV. Types of chemical reactions
1. d 2. b 3. d 4. b 5. b
Worksheet 2
1. c 6. a 11. b 16. c 21. b 26. b 2. a 7. a
d
Biology
1: NUTRITION IN PLANTS
Worksheet 1
I. Mode of nutrition in plants
1. d 2. d 3. b 4. a 5. d
II. Photosynthesis – food making process in plants
1. b 2. a 3. b 4. b 5. c
III. Other modes of nutrition in plants
1. d 2. b 3. d 4. d 5. C
IV. Saprophytes
1. b 2. d 3. c 4. c 5. a
V. Symbiosis
1. a 2. c 3. d 4. d 5. a
VI. How nutrients are replenished in the soil
1. d 2. d 3. b 4. b 5. a
Worksheet 2 1. b 6. c 11. d 16. b 21. d 26. b 2. b 7. b 12. c 17. c 22. a 27. a 3. a 8. a 13. c 18. d 23. d 28. c 4. c 9. d 14. c 19. c 24. a 29. a 5. b 10. a 15. d 20. d 25. a 30. a
2: NUTRITION IN ANIMALS
Worksheet 1
I. Different ways of taking food
1. b 2. a 3. d 4. c 5. a
II. Digestion in humans
1. c 2. a 3. c 4. b 5. b
b 13. d 14. b 15. a 18. a
III. Digestion in grass-eating animals 1. d 2. a 3. d
IV. Feeding and digestion in Amoeba 1. c 2. a 3. a
Worksheet 2
