SCIENCE
IL Foundation Series - Science Class 6
Legal Disclaimer
This book is intended for educational purposes only. The information contained herein is provided on an “as-is” and “as-available” basis without any representations or warranties, express or implied. The authors (including any affiliated organizations) and publishers make no representations or warranties in relation to the accuracy, completeness, or suitability of the information contained in this book for any purpose.
The authors (including any affiliated organizations) and publishers of the book have made reasonable efforts to ensure the accuracy and completeness of the content and information contained in this book. However, the authors (including any affiliated organizations) and publishers make no warranties or representations regarding the accuracy, completeness, or suitability for any purpose of the information contained in this book, including without limitation, any implied warranties of merchantability and fitness for a particular purpose, and non-infringement. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any errors, omissions, or inaccuracies in the content or information provided in this book.
This book does not constitute legal, professional, or academic advice, and readers are encouraged to seek appropriate professional and academic advice before making any decisions based on the information contained in this book. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any decisions made based on the information provided in this book.
The authors (including any affiliated organizations) and publishers disclaim any and all liability, loss, or risk incurred as a consequence, directly or indirectly, of the use and/or application of any of the contents or information contained in this book. The inclusion of any references or links to external sources does not imply endorsement or validation by the authors (including any affiliated organizations) and publishers of the same.
All trademarks, service marks, trade names, and product names mentioned in this book are the property of their respective owners and are used for identification purposes only.
No part of this publication may be reproduced, stored, or transmitted in any form or by any means, including without limitation, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the authors (including any affiliated organizations) and publishers.
The authors (including any affiliated organizations) and publishers shall make commercially reasonable efforts to rectify any errors or omissions in the future editions of the book that may be brought to their notice from time to time.
Subject to Hyderabad jurisdiction only.
Copyright © 2025 Rankguru Technology Solutions Private Limited. All rights reserved.
ISBN 978-81-985304-0-0
Second Edition
PHYSICS

MEASUREMENT OF LENGTH AND MOTION
1.1 TRANSPORTATION
Transportation is how people and things get from one place to another. Imagine you want to go visit your friend who lives far away. You can't walk there, so you need some kind of transportation. There are different ways to travel: you could ride a bike, take a bus, hop on a train, get in a car, or even fly in an aeroplane. These are all different types of transportation. They help us move around quickly and easily, whether it's going to school, visiting family, or going on vacation.
1.1.1 Means of transportation
Early Transportation: Long ago, people didn't have any vehicles. They walked or used animals to carry goods.
Boats: For travel on water, people first used simple logs, then learned to build boats from different pieces of wood, mimicking the shapes of water animals.
Invention of the Wheel: The wheel was a big change. It improved over time, and people used animals to pull carts and wagons.
Steam Engine: It was common practice to rely on animals and seafaring vessels for transportation before the 19th century. The invention of the steam engine brought a dramatic change in the mode of transportation. This invention created new transportation options, one of which was the railroad system. Particularly designed to support steam-engine-powered carriages and wagons, the railroads were built.
Modern Transportation: Later we saw vehicles, including trucks, buses, and motor cars developed, revolutionizing travel further. On the water, motorized ships and boats were utilized for transportation. The development of aeroplanes began in the early years of 1900. Later, these were upgraded to accommodate both passengers and cargo. Among the 20th century's innovations are spacecraft, electric trains, monorails, and supersonic jets.

1.2 MEASUREMENT
Measurement is like using a special tool, such as a ruler or a scale, to find out how big or how much of something there is. It's a way to figure out the size, length, quantity, or extent of an object or substance. We do this by comparing what we want to measure to things we already know the size of, which helps us express it in numbers. For example, if we want to know how long a table is, we can use a ruler to measure its length.
1.2.1 Measurement of distance
People used various methods to measure distance:
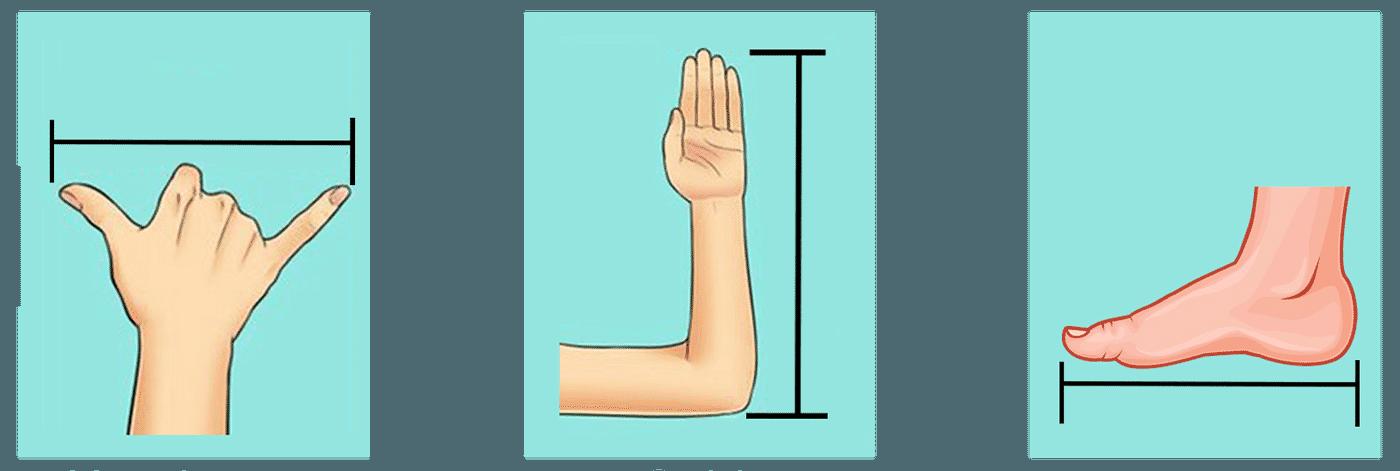
Body Parts: One common method was using body parts as units of measurement. For example, the handspan, the length of a foot or the span of an arm could be used to estimate distances.
Natural Objects: People also used natural objects such as stones, sticks, or ropes to measure distances.
Landmarks: Distances were often estimated based on landmarks or recognizable features in the environment. For instance, the distance between two villages might be described in terms of how long it took to walk between them or the number of hills crossed.
Time and Pace: Some cultures measured distance by time and pace. They would estimate how far they could travel in a certain amount of time or by counting the number of steps taken.
1.2.2 Length and breadth
Using your foot as a unit of measurement is a simple way to assess length and breadth.
Length: It refers to how long something is, like the distance from one end of a room to the other.
Breadth: It refers to the measurement of something from side to side, indicating the extent or span of an object. It represents the distance between two opposite sides of an object or area, often measured across the widest part.
To measure length, walk from one end to the other, counting each step. For breadth, step sideways from one edge to the other. If it's smaller than your foot, use a string to measure part of your foot and count how many times it fits across.
Unit
Measurement involves comparing an unknown quantity to a known quantity, and this known quantity is known as a "unit". This unit is a fixed, predetermined quantity. When measuring, the result is expressed in two parts: a number and the unit of measurement. For instance, if the length of a room is determined to be 15 foot length, then 15 represents the quantity, and 'foot length' is the chosen unit for measurement.
1.2.3 Importance of measurement
Measurement is crucial in many professions and everyday tasks.
• Tailors need it to ensure they have enough cloth to stitch clothes like kurtas.
• Carpenters use measurements to determine the size of furniture they're building.
• Farmers rely on measurements to plan how much seed to sow and how much water to use for their crops.
• Personal tasks, such as determining height or travel distance, also rely on measurements.
1.3 UNITS OF MEASUREMENTS
Historically, measurements were based on body parts like the foot, finger, and step. The Indus Valley civilization showed advanced geometric constructions, indicating precise measurements. The cubit, from elbow to fingertips, was used in Egypt and elsewhere. The foot, varying regionally, was another global unit. A yard was measured from arm to chin. Romans used their pace or steps. In ancient India, the angul (finger) or mutthi (fist) were used, and even today, flower sellers in India use their forearm as a unit. However, the variability in body sizes could have led to measurement inconsistencies. To address this issue we need standard units.
Standard units provide consistency and accuracy in measurements because they are universally agreed upon and widely recognized. This ensures that measurements can be compared accurately regardless of who is measuring or being measured. Unlike using personal body parts like your foot, which can vary in size from person to person, standardized units eliminate discrepancies and minimize errors in measurements. Therefore, using standard units is crucial for precise and reliable communication of measurements.
1.3.1 Metric system

In the late 18th century, the French introduced the metric system, which standardized units of measurement for length, mass, volume, and area. The key units in this system are the metre for length, gram for mass, litre for volume, and square metres for area. The metric system is favoured for its logical structure and convenience for several reasons:
Single Unit Definition: Each physical quantity, such as length and mass, is defined by a single unit. For instance, the metre represents length, and the gram represents mass.
Multiples and Submultiples: The metric system allows for easy creation of larger or smaller forms of a unit by multiplying or dividing it by powers of 10. For example, a kilometre (km) is 1000 times the metre (m), while a centimetre (cm) is 0.01 times the metre.
Prefixes for Clarity: Prefixes are used to denote multiples and submultiples of units, making it clear and concise. For instance, 'kilo-' denotes a factor of 1000, so a kilometre is 1000 metres.
Examples:
1. Large distances, like between towns, are measured in kilometres (km), where 1 km = 1000 m.
2. Smaller lengths, such as the length of an eraser, are measured in centimetres (cm), where 1 cm = 0.01 m.
3. Very small sizes, like the thickness of a hair, can be measured in micrometres (μm), where 1 μm = 0.000001 m.
The metric system's structure enables easy conversion between different units and simplifies calculations in various fields of science, engineering, and everyday life.
1.3.2 Standard units of measurements
Though the metric system was adopted by many countries, scientists soon realized that it needed certain improvements. In 1960, representatives from different nations put together a system of units, based on the metric system, which is now used all over the world. It is called Systeme International d'Unités (International System of Units), SI in short. This system defines the units of seven quantities, called base quantities or fundamental quantities. Out of these, we will require the four given in Table 1.1.
Table 1.1 Some base quantities, their units and symbols
It is enough to define the units for the base quantities because the units for other quantities can be obtained by combining the base units. For example, we can get the unit of area from its formula, which is 'length × length'. Since the unit of length is the metre, the unit of 'length × length' is 'metre × metre', written as metre2 or m2. Similarly, the unit for volume is m3
In SI, the multiples and submultiples of units are formed as in the metric system.
1.4 MEASUREMENT OF LENGTH
You must be familiar with the metre scale and ruler for measuring lengths. A metre scale showcases divisions where each metre (m) is divided into 100 equal parts known as centimetres (cm). Furthermore, each centimetre is further subdivided into ten equal segments, known as millimetres (mm). This relationship can be expressed as:
1 metre (m) = 100 centimetres (cm)
1 centimetre (cm) = 10 millimetres (mm)
For measuring large distances, such as between towns or cities a larger unit of length, the kilometre (km), is defined.
1 kilometre (km) = 1000 metres (m)
1.4.1 Correct and incorrect measurement methods
In daily life, various measuring devices are used for different purposes:
• Metre scale: Used for measuring length, but not suitable for measuring girth or circumference.
• Measuring tape: Preferred by tailors for accurate measurements of cloth length.
• Metre rod: Utilized by cloth merchants to measure large quantities of fabric.
• 15 cm scale: Handy for small measurements like pencil length, found in geometry boxes.
Important considerations when taking measurements
• Placement: Ensure the measuring device makes full contact along the length of the object.
• Zero Mark: If the zero mark is unclear, use another full mark and subtract its reading from the endpoint reading.
• Eye Position: Position your eye directly in front of the measurement point for accurate readings.
Correct eye position is crucial
Place your eye exactly in front of the point where the measurement is taken. Position 'B' represents the correct eye position for accurate measurement readings. Different eye positions ('A' and 'C') may yield different readings, emphasizing the importance of eye alignment for precise measurements.

1.4.2 Indirect measurements of length
In some situations, we can't measure directly, like finding the diameter of a ball or the length of a curved tray edge. For such cases, we use indirect methods.
Measuring the diameter of a sphere
(i) Place the sphere between two blocks.
(ii) Put a scale against the blocks and note the inner edge positions.
(iii) Subtract the two readings to find the diameter.
For example, if the left edge reading is 0.0 cm and the right edge reading is 8.0 cm, then the diameter is 8.0 cm - 0.0 cm = 8.0 cm.
Measuring the length of a curve
For curved surfaces or edges, like a tailor measuring your neck, use a tape measure. Alternatively, use a string and a scale.
• Stretch the string tightly between two points on the curve.
• Mark the string at these points and measure its length on a scale.
For instance, stretch a string along a cup's surface, mark points, straighten the string, and measure it. The difference in readings at the marks gives the curve's length.
1.5 MOTION AND ITS TYPES
1.5.1 Rest and motion
• Motion refers to a change in an object's position over time. For example: Moving car on the road.
• Rest implies an object remains stationary without any change in position. For example: Standing tree in the park.
1.5.2 Rectilinear motion
• Objects move along a straight path, like vehicles on a straight road or soldiers marching in a parade. This type of motion is called rectilinear. Examples include people marching along a straight track or a stone falling vertically.

1.5.3 Circular motion
• Objects move along a circular path, like a stone tied to a string and whirled around is called the circular motion.
• The distance between the object and its center remains constant during rotation.
• Examples include the motion of a point marked on an electric fan blade or the hands of a clock.

1.5.4 Periodic motion
• Objects repeat their motion after a fixed interval of time. This type of motion is called periodic motion.
• Examples include the motion of a pendulum, swinging branches of a tree, or strings of a guitar. The motion of a sewing machine needle or a ball rolling and rotating on the ground also exhibits periodic motion.
Motion can be understood through distance measurements, allowing us to determine how fast or slow motion is. Various examples of motion include the movement of a snail, a butterfly flitting between flowers, rivers flowing, aeroplanes flying, and celestial bodies orbiting. Motion is present everywhere in our surroundings, demonstrating the dynamic nature of our world.
QUICK REVIEW
• Transportation is how people and things get from one place to another.
• Modern Transportation: Motorized boats, aeroplanes, and spacecraft.
• Standardization of measurement is important because it ensures consistency and accuracy.
• Length refers to how long something is.
• Breadth refers to the measurement of something from side to side.
• Measurement involves comparing an unknown quantity to a known quantity, and this known quantity is known as a "unit". This unit is a fixed, predetermined quantity.
• Standard units are universally agreed upon and widely recognized.
• The metric system is a standardized system of measurement used worldwide, introduced in the late 18th century in France.
• International System of Units, SI in short defines the units of seven quantities, called base quantities or fundamental quantities.
MEASUREMENT
• The metre scale showcases divisions where each metre (m) is divided into 100 equal parts known as centimetres (cm).
• Motion refers to a change in an object's position over time.
• Types of motion: Rectilinear motion, circular motion, periodic motion etc.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Transportation
1. Susheel lives 250 km away from his uncle's house. Which of the following means of transportation would be appropriate if his family wants to go to his uncle's house in the minimum time period?
a. Walking
b. On a bicycle
c. Train d. On a horseback
2. What is the primary advantage of the spoked wheel over a solid wheel for transportation?
a. Increased strength
c. Enhanced durability
b. Reduced weight
d. Improved aesthetics
3. How did the invention of the wheel contribute to transportation?
a. Helped in development of steam engines
c. Improvement in land transportation
b. Advancement of water transport
d. Introduction of electric trains
4. In the history of transport, what innovation contributed to making boats more efficient in navigating through water?
a. Invention of the wheel
c. Shaping boats like animals living in water
b. Steam engine
d. Electric engine
5. What technological advancement led to the development of railroads for transportation?
a. Use of animals
c. Invention of the wheel
b. Steam engine
d. Electric trains
6. What is the primary material traditionally used to make wheels for transport vehicles such as bullock carts, horse carts, and camel carts?
a. Plastic
b. Rubber
c. Wood d. Steel
II. Measurement
1. What type of error is associated with unpredictable variations in measurements made with non-standardized units?
a. Precision error
c. Random error
b. Systematic error
d. Human error
2. If the length of a room is determined to be 152 lengths of your foot, what does '152’ represent?
a. The unit of measurement
c. The size of the foot
b. The quantity
d. None of these
3. What are non-standard measures in the context of measurement?
a. Measures that are difficult to understand
b. Measures that vary from person to person
c. Measures approved by international standards
d. Measures only used in scientific experiments
4. The length of a pencil is measured with a ruler which has its edge worn-out. The measurement is done with 1 cm mark as the initial point. If the ruler reads 8 cm, then what is the length of the pencil?
a. 7 cm b. 8 cm c. 9 cm d. 10 cm
5. Which of the following options represents the measurement of length?
a. Number of students in science classroom on a particular day
b. Height of the ceiling of the science classroom
c. The number of wooden chairs in the science classroom
d. Color of the pen used by your science teacher
III. Units of measurement
1. The number of fundamental physical quantities in SI is:
a. 0 b. 5 c. 3 d. 7
2. Which of the following SI units is wrongly matched?
a. Mass – kilogram
c. Length – metre
3. Which of the following statements is incorrect?
b. Temperature-Degree celsius
d. Time – second
a. The standard unit for measuring length is the metre.
b. Kilogram is the standard unit for measuring mass.
c. The standard unit for measuring capacity is the foot.
d. The standard unit for measuring temperature is Kelvin.
4. Which unit would you use to measure the distance between two cities?
a. Centimetres
b. Kilometres c. Millimetres d. Metres
5. Which of the following is used to measure weight in the metric system?
a. Ounces
IV. Measurement of length
b. Pounds
c. Kilograms d. Tons
1. Which of the following will be the most suitable unit for determining a coin's thickness?
a. Kilometre
b. Light year
c. Millimetre d. Mile
2. Which of the following can be used to measure the girth of a tree?
a. Metre rod
b. Rope
c. Ruler
d. Plastic rod
3. The position of which of the following does NOT impact the result while measuring the length of an object?
a. Scale
b. Object
c. Eye
4. Which of the following cannot be used to measure the height of a person?
a. Measurement tape
c. Metre scale
5. A metre scale can't be used to directly measure:
a. The length of a table cloth
c. The length of a curve
V. Motion and its types
d. Light source
b. Small scale of 30 cm
d. Protractor
b. The width of a wall

d. The length of a wall portrait
1. What is the change in the position of an object with respect to its surroundings over a particular time interval called?
a. Dislocation
b. Motion
c. Application d. Displacement
2. What is the motion that is repeated at regular intervals of time called?
a. Periodic motion
c. Translatory motion
b. Non-periodic motion
d. Oscillatory motion
3. When an object moves along a circular path, it is called...
a. Rotational motion
c. Circular motion
b. Spinning motion
d. None of these
4. A moving stem of a tree, the motion of a child on a swing, the strings of a guitar, or the membrane of drums (tabla) are all examples of:
a. Periodic motion
c. Rectilinear motion
b. Random motion
d. None of these
5. A powerful shot by a batsman makes the ball move in:
a. Rectilinear motion
c. Curvilinear motion
WORKSHEET - 2
b. Circular motion
d. Periodic motion
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Find transport based on a water route.
a. Train
b. Cycle
c. Boat
2. Which of the following inventions made a change in the mode of transport?
a. Cycle
b. Wheel
c. Watch
d. Bus
d. Train
3. What type of transportation device was likely one of the earliest applications of the wheel?
a. Boats
b. Chariots
c. Bicycles
4. Where was the wheel likely first used for transportation purposes?
a. Carts and wagons
c. Sledges on boats
d. Aeroplanes
b. Pottery making
d. Ships and boats
5. Which of the following modes of transport was used during ancient times?
a. Foot
c. Boat
b. Animals
d. Foot, animals and boat
6. Which of the following is a standard unit of measurement?
a. Handspan
b. Cubit
c. Inch
d. Pace
7. Rahul wants to measure the circumference of a cricket ground. Which of the following objects should he use for the measurement?
a. Metre rod
b. Ruler
c. Plastic rod
d. Rope
MEASUREMENT OF LENGTH AND MOTION
8. What is/are the precaution(s) that need to be taken while taking measurements with a ruler?
a. The ruler should be very close to the object.
b. The eye should be in front of the point of measurement.
c. Avoid measuring with a broken ruler.
d. All of the above.
9. Three students measured the length of a corridor and reported their measurements. The values of their measurements were different. Which of the following could be the reasons for their differences?
a. Their measurement scales may not be standard, or they may use different scales of measurement.
b. The length of the scale may not be proper, i.e., the length of the scale may be shorter than the length they want to measure.
c. There may be some errors in the scale they are using, or they may not be using the correct method of observing the scale.
d. All of the above.
10. At present the system of measurement adopted by all scientists in the world is
a. MKS
b. CGS
c. FPS d. SI
11. What is the definition of the unit of measurement from the given options?
a. The predefined measure of length used as a standard base for measuring things.
b. Measuring the length of an object from one end to the other.
c. Measuring the length and breadth of an object.
d. The measure of length used for measuring spherical things.
12. 500 cm is equal to _____ m
a. 5 b. 1/5
13. What does SI in SI unit stand for?
a. Standard Indian
c. Standard International
c. 100 d. 50
b. Système International
d. System Indian
14. The distance between Rina's house and Tina's house is 4000 m. Represent it in km.
a. 40 km b. 4 km c. 4000 km d. 400 km
15. A thread is 3 m long. Express its length in mm.
a. 300 mm b. 30 mm c. 3000 mm
16. Identify the correct option for cases mentioned in below mentioned statement.
Height of a boy = 3 m = 300 cm
A: Here unit is m, numerical value is 3
B: Here unit is cm, numerical value is 300
a. Only A, Only B
b. Both A & B
c. Both A & B
d. Neither A nor B
17. Which of the following can be used to measure the length of a curved line?
30000 mm
a. Metre rod b. Plastic ruler c. Plastic rod d. Thread
18. Which is the oldest means of transport?
a. Bi-cycle b. Bus c. Car
Bullock cart
19. Karan wants to measure the length of a box using a ruler as shown in the illustration given below. Where should he place his eyes while measuring the length of the box?
a. At position A
At position B c. At position C
20. What is an important precaution to take when measuring length accurately?
a. Use a ruler made of flexible material.
b. Ensure the starting point is aligned with the zero mark.
At position D
MEASUREMENT
c. Measure from the curved edge of an object.
d. Use a ruler with faded markings.
21. Which of the following is also a periodic motion?
a. Rotatory motion
c. Random motion
22. Which of the following is not an oscillatory motion?
a. Motion of the hammer of an electric bell
b. Motion of your hands while running
c. Motion of a child on a see - saw
d. Motion of a horse pulling a cart
23. A spinning top has
a. Translatory motion
c. Oscillatory motion
24. Which among the following shows circular motion?
a. Simple Pendulum
c. Pendulum of a wall clock
b. Oscillatory motion
d. Linear motion
b. Rotatory motion
d. Rectilinear motion
b. Blades of a moving fan
d. Falling apple
25. Assertion (A): Wheel of a bicycle and ceiling fan both shows circular motion.
Reason (R): In circular motion an object moves such that its distance from a fixed point remains same.
a. Both A and R are correct and R is the correct explanation of A
b. Both A and R are correct, but R is not the correct explanation of A
c. A is correct and R is incorrect
d. A is incorrect and R is correct

LIGHT, SHADOWS, AND REFLECTION
2.1 LIGHT
In our daily lives, we see various objects like buses, cars, trees, and animals. But have you ever thought about how we see these objects?
At night, when it is dark, it is hard to see things around us. Also, when there is no light in a room, we cannot see anything inside it. But when we bring in a light source like a candle or a torch, we can see everything. This shows us how important light is for us to see things.
Luminous objects: Objects like the Sun and light bulbs, which emit their own light, are known as luminous objects.
Non-luminous objects: Objects like tables or bags do not emit light. We see these objects when light falls on them. Such objects are called non-luminous objects.
2.1.1
Different types of optical media
Any material that allows the light to pass through it, either completely or partially, is called an optical medium. There are three main types of optical mediums based on how they allow light to pass through.
Transparent: These mediums allow light to pass through them without any hindrance. As a result, objects viewed through transparent substances appear clear and distinct. For example, glass, water, air, and certain types of plastics, etc.
Translucent: Translucent mediums partially obstruct the light rays, causing objects viewed through them to appear blurry or diffused. For example, frosted glass, butter paper, etc.
Opaque: Opaque mediums completely block the light rays, preventing any visibility through them. For example, wood, metal, concrete, and cardboard.
2.1.2
Properties of light
• Light from the Sun is a natural example of white light.
• Light always travels in a straight line.
• In a vacuum, light travels at a speed of approximately 299,792,458 meters per second (m/s).
2.2 SHADOW
A shadow is a dark area that appears when something blocks light. It happens when an object is placed in front of a light source, causing the light to not reach certain areas. This creates a darker shade on the surface behind the object. Sometimes shadows can also help in identifying the object.

2.2.1 Formation of shadow
Have you ever noticed your shadow following you, or have you ever tried to chase it? Shadows form because light travels in straight lines. Sometimes, on overcast days, it might seem like there are no shadows at all. However, on a sunny day, if you stand with your back to the sun, your shadow becomes visible.


Shadows form when an opaque object blocks the path of light rays. This opaque object does not allow light to pass through it. The light rays that pass the object's edges create the shadow's outline. The darkest part of the shadow, where there is no light at all, is called the umbra. The rest of the shadow appears lighter because it receives light from other parts of the light source, and it is called the penumbra.
The size of shadows changes depending on how close or far the screen is from the object. If you move the screen closer to the object, the main dark part (umbra) gets smaller, and the lighter edges (penumbra) get bigger. If you move the screen away, it's the opposite: the dark part gets bigger, and the lighter edges get smaller.
Note: Shadows can often provide insights into the form of various objects. However, they can occasionally create illusions about the object's actual shape. For example, shadow play.
2.2.2 Pinhole camera
A pinhole camera is a simple device that uses the principle of light to form images. It is a box-like structure with a tiny hole on one side and a screen on the opposite side.
Pinhole camera works on the rectilinear propagation of light, which states that light travels in a straight line. When light from an object passes through the small hole, it forms an inverted image on the screen inside the camera. This is because light rays from the top and bottom of the object cross over as they pass through the hole.
2.2.3 Eclipse

Solar eclipse














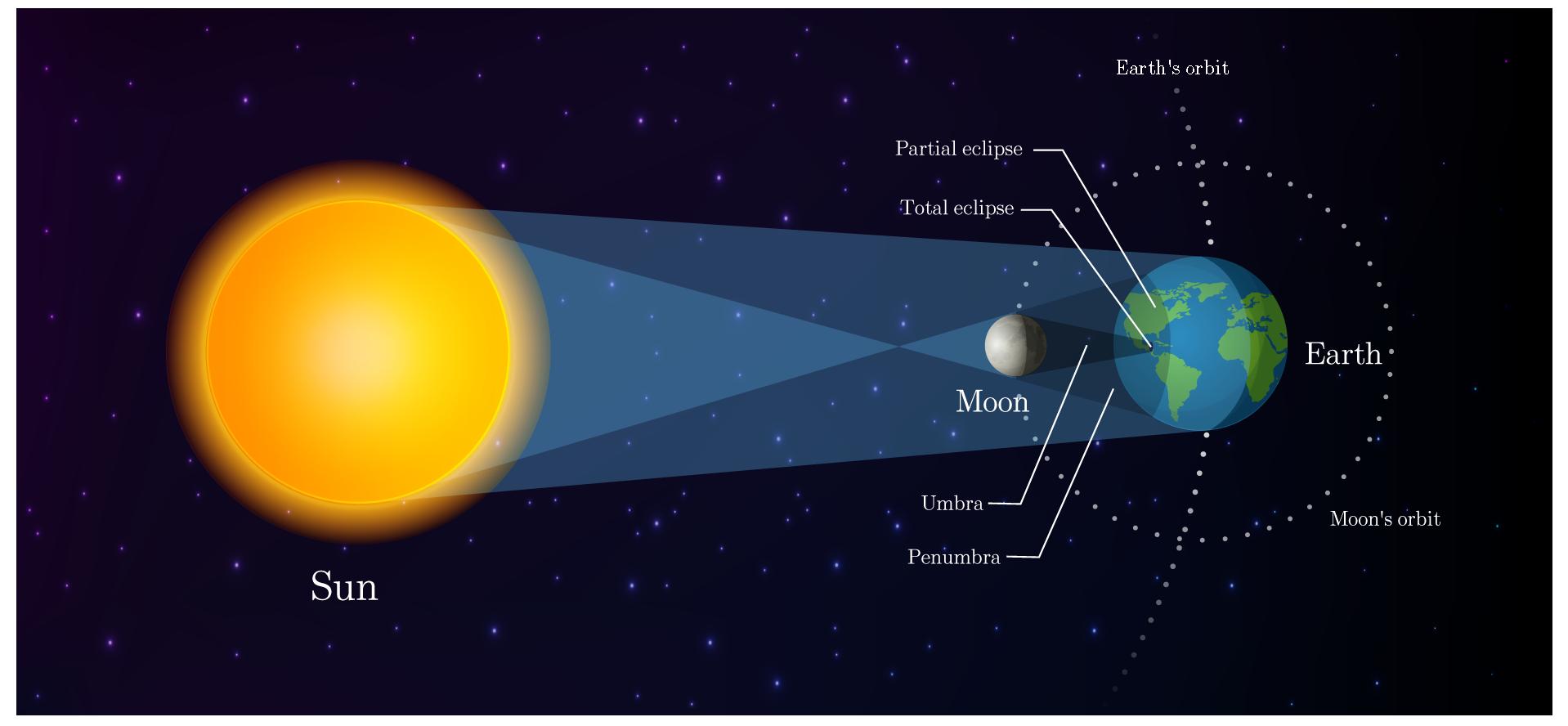
An eclipse happens when a celestial body, like a moon or a planet, passes into the shadow of another celestial body. Two main types of eclipse can be observed from Earth: lunar and solar. In a lunar eclipse, the Earth is between the Sun and the Moon. In a solar eclipse, the Moon is between the Sun and Earth.
When the moon comes between the Sun and the Earth, it casts a shadow on the Earth, making it darker during the day. Because the Sun is big, this shadow has two parts: an umbra and a penumbra. The umbra is the region of the shadow where the Sun is completely blocked by the Moon, leading to a total eclipse, whereas the penumbra is the region where only a part of the Sun is obscured by the Moon, resulting in a partial eclipse.

Lunar eclipse
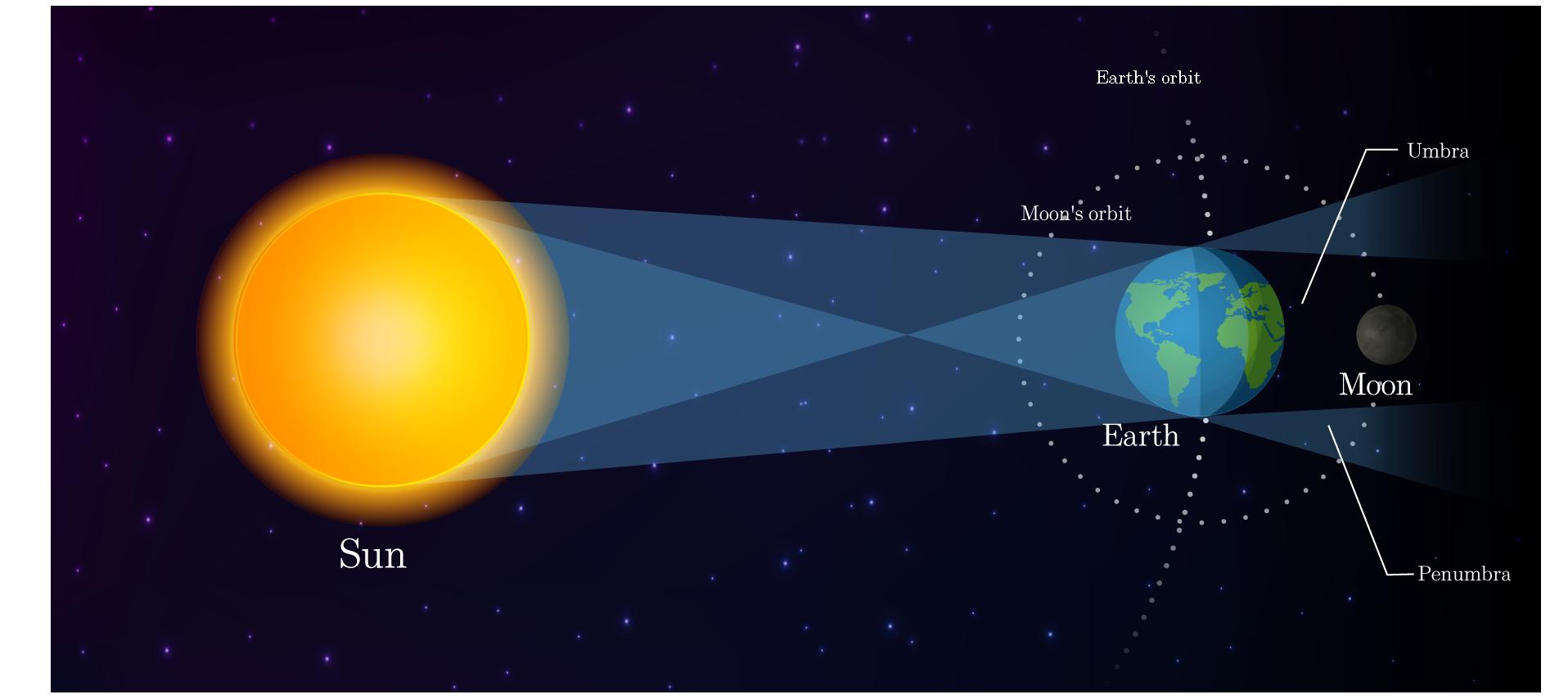
When the Moon, Sun, and Earth align in a straight line with Earth in between the Sun and the Moon, the Earth's shadow falls on the Moon, making it reddish in colour, known as a total lunar eclipse. Also, because of its reddish colour, a total lunar eclipse is sometimes called a “blood moon”.
Partial lunar eclipse: When the Sun, Earth, and Moon don’t line up perfectly, the Moon only travels through a portion of Earth’s darkest shadow, known as the umbra. As this happens, we see the shadow increase in size on the Moon, but it never fully covers it before it starts to decrease again.

Note: Solar and lunar eclipses are only visible in certain regions of the world. They do not occur simultaneously everywhere. Depending on your location, you may or may not be able to witness these celestial events.
2.3 MIRRORS AND REFLECTION
Imagine yourself standing by a calm lake on a peaceful day. Have you ever observed how the lake reflects everything nearby-the trees, the sky, and even your own reflection? Well, mirrors do something quite similar! When you stand in front of a mirror, you see a reflection of yourself, like a duplicate. It is like how water bodies create a mirrored image of their surroundings.
2.3.1 Reflection
Reflection occurs when light bounces off a shiny surface. For instance, when we look into a mirror, see our reflection in the water, or observe our image on any glossy surface, we see a reflection appear.

2.3.2
Lateral inversion
In an image created by a mirror, the left side of the object is on the right, and the right side appears on the left. This phenomenon is called lateral inversion.
For example, if we hold up a placard with the word 'AMBULANCE' written on it in front of a plane mirror, the image of the word 'AMBULANCE' in the mirror will look like it's flipped, with the 'A' on the right side and the 'E' on the right side compared to the original.
Lettering on paper Mirror
AMBULANCE AMBULANCE
QUICK REVIEW
• Luminous objects emit their own light, while non-luminous objects do not emit their own light.
• Objects like tables or bags don't emit light. We see them when light from luminous sources falls on them.
• Optical medium allows the light to pass through it, either completely or partially. There are three main types of optical mediums viz, transparent, translucent, and opaque.
• Light always travels in a straight line.
• Shadow is the dark area that appears when something blocks light.
• A partial or complete blocking of light by one celestial object by another is called an eclipse Two main types of eclipse can be observed from Earth: lunar and solar.
• Reflection occurs when light bounces off a shiny surface.
• The phenomenon of the left side appearing as the right side and the right side appearing as the left side on reflection in a plane mirror is called lateral inversion.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Light
1. If light can be passed completely through object 'A’, then 'A’ is:
a. Translucent
c. Transparent
2. Which one is the natural source of light?
a. Tubelight
c. Sun
b. Opaque
d. None of these
b. Electric bulb
d. Moon
3. A body that does not allow light to pass through it is called:
a. Transparent
c. Opaque
b. Translucent
d. All of the above
4. Which one of the following is a non-luminous object?
a. Moon
b. Book
c. Earth
5. The objects that emit light on their own are called:
a. Light
c. Non-luminous
6. The speed of light approximately is:
d. All of the above
b. Luminous
d. Opaque
a. 3×108 m/s b. 3×105 m/s c. 300 m/s d. 3000 m/s
7. Light travels in a ________________.
a. Curved path
c. Zig-zag path
8. A metal box is an example of:
a. A translucent material
c. An opaque material
b. Random direction
d. Straight line
b. A transparent material
d. Both translucent and opaque material
9. Which among the following options accurately represents the correct set of optical mediums from the given figure?
(i) (ii) (iii)
a. (i) – Opaque, (ii) – Transparent, (iii) – Translucent
b. (i) – Transparent, (ii) – Opaque, (iii) – Translucent
c. (i) – Translucent, (ii) – Transparent, (iii) – Opaque
d. (i) – Opaque, (ii) – Translucent, (iii) – Transparent
10. ____________ main types of optical media exist based on the passage of light.
a. 6
II. Shadow
b. 5 c. 3 d. 2
1. Total lunar eclipse is also called:
a. Blue Moon
c. White Moon
b. Yellow Moon
d. Blood Moon
2. A region of partial darkness formed behind an opaque body is called:
a. Umbra
b. Penumbra
3. An eclipse is an example of:
a. Image
c. Shadow
4. Which of the following cannot make a shadow?
a. Notebook
c. A concrete wall
c. Image
b. Reflection
d. Both (a) and (c)
d. Fringe
b. A clean, transparent window
d. A pen
5. The darkest part of the shadow, where there is no light at all, is called the:
a. Penumbra
c. Both (a) and (b)
b. Umbra
d. None of these
6. Shadows are formed because ______ cannot travel through ______ objects.
a. Light, transparent
c. Transparent objects, light
b. Light, opaque
d. Opaque objects, light
7. A dark area where light from a light source is blocked by an opaque object is called:
a. Blind spot
c. Shadow
b. Space
d. Spotless space
8. When the extended source is smaller than the opaque body, if the source of light moves away from the opaque body, then:
a. Both umbra and penumbra increases
b. Both umbra and penumbra decreases
c. Penumbra increases, but umbra decreases
d. Umbra increases, but penumbra decreases
9. When the extended source is bigger than the opaque body:
a. The size of the umbra is bigger than the penumbra
b. The size of the umbra is smaller than the penumbra
c. The size of the umbra is equal to the penumbra
d. Only a penumbra is formed
10. The size of the umbra is very large compared to the penumbra when:
a. The extended source is bigger than the opaque body
b. The extended source is smaller than the opaque body
c. The point source of light forms a shadow
d. The extended source is equal to the opaque body
III. Mirrors and reflection

1. A reflective surface that bounces off light, producing an exact image of the object, is called:
a. Glass
b. Mirror
c. Bottle d. Pen
2. The bouncing back of light from any shiny surface is known as:
a. Mirror
c. Eclipse
b. Lateral inversion
d. Reflection
3. Which one of the following represents lateral inversion?
a. The image becomes inverted.
b. The image bends laterally.
c. The right side of the object appears on the left side in the mirror.
d. The left side of the object appears on the left side in the mirror.
4. A shadow is formed by an opaque object, but a mirror is formed by:
a. Mirror
c. Transparent object only
5. Mirror is a reflecting surface, but glass is a:
a. Non-luminous object
c. Opaque object
b. Translucent object
d. Opaque object
b. Transparent object
d. Luminous object
6. Which set of letters of the English alphabet will not show lateral inversion?
a. I, O, U
b. N, Z, X
c. I, X, E
d. A, E, I
7. Which of the following is a device to image the Sun?
a. Plane Mirror
c. Glass slab
8. Which of the following statements are correct?
a. Stars are luminous bodies.
b. A butter paper is a translucent object.
b. Pinhole camera
d. All of these
c. Reflection occurs when light bounces off from a reflected shiny surface.
d. All of the above
9. In the mirror, due to lateral inversion, the letter 'b’ appears as the letter:
a. p
c
10. A ________________ reflects a beam of light.
d
b
a. Mirror b. Board c. Glass d. Bowl
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Light reflects in the _________ medium after falling on a mirror.
a. Different
b. Water
c. Air
2. Which of the following materials are transparent in nature?
a. Smoke
c. Muddy Water
3. The lighter region of the shadow is called:
a. Penumbra
c. Both (a) and (b)
4. A mountain rock is an example of:
a. A transparent object
c. An opaque object
5. A colourless cellophane paper is an example of:
a. Transparent objects
c. Translucent objects
b. Thin glass
d. Wood
b. Umbra
d. None of these
b. A translucent object
d. A reflecting object
b. Opaque objects
d. Reflecting objects
d. Same
6. Natural luminous objects are:
a. Oil lamp
b. Sirius
7. During shadow formation, the penumbra is seen:
a. Inside the umbra
c. Away from the umbra
8. Statement (A): A star is a luminous body.
c. Candle
b. Outside the umbra
d. All of these
d. Tubelight
Statement (B): A body which emits light of its own is called a luminous body.
a. Both (A) and (B) are true.
b. Both (A) and (B) are false.
c. A is true, and B is false.
d. A is false, and B is true.
9. The shadow of a red object will be:
a. Red b. White
c. Yellow d. Black
10. Which of the following is not necessary to observe a shadow?
a. Source of light
c. Sun
b. Screen
d. Opaque object
11. Which of the following can never make a circular shadow?
a. A ball
c. A shoe box
12. Which of the following is a living luminous object?
a. Sun
c. Firefly
b. A flat disc
d. An ice-cream cone
b. Moon
d. Burning candle
13. The branch of studying properties and behaviour of light is called:
a. Sonometry
c. Thermometry
b. Kinematics
d. Optics
14. Which of the following is formed because of obstruction of light?
a. Image
b. Object
c. Shadow
d. Light
15. The light coming from the Sun is completely blocked by the Moon in a _____________ eclipse.
a. Total Solar
b. Partial Solar
c. Total lunar
16. Small patches of sunlight under the tree are the:
a. Shadow of tree
c. Images of leaves
d. Partial Lunar
b. Shadow of leaves
d. Images of the Sun
17. Burning candle cannot be seen through a bent tube because:
a. Light cast shadow
c. Light travels in a straight line
b. Light can bend in a metallic pipe
d. Light is a form of energy
18. A paint behind the mirror is used for which of the following purposes?
a. Stops light from passing through the mirror
b. Protects the glass
c. Reflects light
d. Protects the layer of the metal
19. Assertion (A): Rubber is an opaque object.
Reason (R): The objects which do not allow light to pass through them are called opaque.
a. Both Assertion (A) and Reason (R) are correct, and R is the correct explanation of A.
b. Both Assertion (A) and Reason (R) are correct, but R is not the correct explanation of A.
c. Assertion (A) is correct, but Reason (R) is incorrect.
d. Assertion (A) is incorrect, but Reason (R) is correct.
20. Which of the following objects form an unclear shadow?
a. Iron chair
c. Wooden bench
b. Stone
d. Oil paper

ELECTRICITY AND CIRCUITS 3
The discovery of electricity dates to approximately 600 BC, credited to the Greek philosopher Thales de Miletus. Electricity has a significant role in our daily lives, serving various purposes and streamlining numerous tasks. Nowadays, we cannot imagine our day without electricity. Common electrical appliances like heaters, televisions, fans, air conditioners, refrigerators, and more rely on electricity supplied from power stations to our homes. These power stations generate electricity through large machines known as generators.
Electricity, or electric current, is the flow of electric charges over time. It embodies a form of energy essential for powering devices. While many appliances operate on electricity sourced from power stations, some utilise alternative sources such as cells or batteries like a torch.
3.1 ELECTRIC CELL
An electric cell is a device that converts chemical energy into electrical energy and functions as a source of electricity. These cells come in various sizes and shapes, finding applications in devices like clocks, remote controls, cameras, toys, and more.
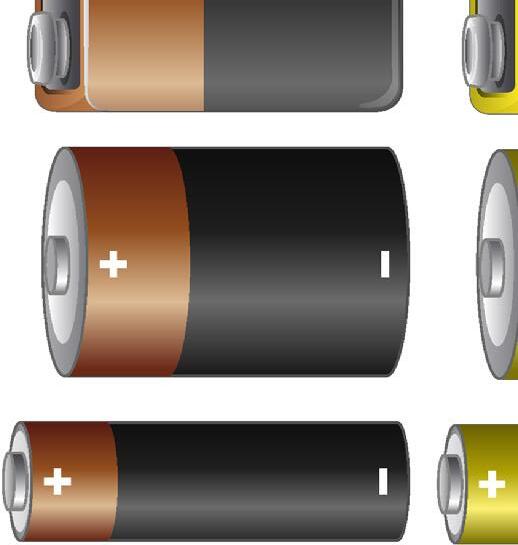
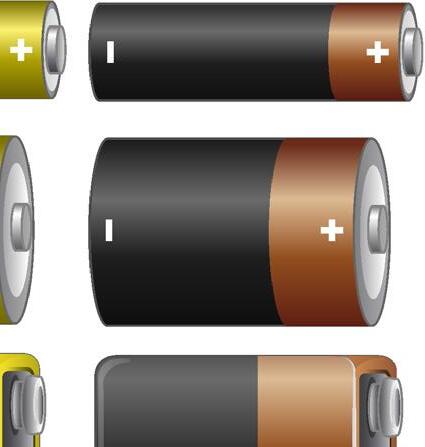
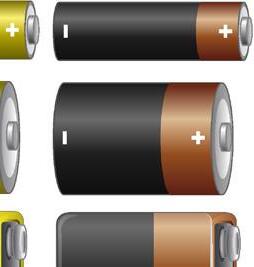
All electric cells have two terminals: a positive terminal and a negative terminal.
Among the most utilised electric cells is the dry cell, which comprises a zinc container containing a paste of ammonium chloride. Enclosed within this paste is a container holding powdered manganese dioxide and carbon, facilitating a chemical reaction through microscopic holes. Once sealed, the chemicals within the dry cell generate electricity, with its positive terminal located at the tip of the metal cap and the negative terminal at the base.

Once the chemicals are depleted, the dry cell stops producing electricity, necessitating replacement, hence categorised as a primary cell. In contrast, secondary cells are rechargeable, enabling repeated use through a reversible chemical reaction when connected to an external electricity source.
Another type of cell is the button cell, characterised by its small, button-like size. These cells find application in compact electronic devices such as wristwatches, calculators, and hearing aids. Some button cells function as primary cells, while others operate as secondary cells.
Furthermore, there are cells that do not rely on chemical reactions for electricity production, such as solar cells. Solar cells directly convert solar energy into electricity. Harnessing the entirety of solar energy reaching the Earth could potentially supply electricity to homes, offices, and schools at no cost.
Note: It is important to distinguish between a cell and a battery; a cell is a singular unit, while batteries are formed by connecting two or more cells end to end, with the positive terminal of one cell linked to the negative terminal of another.

3.2 ELECTRIC BULB
3.2.1 Parts of an electric bulb
An electric bulb functions as a source of light powered by electricity. Encased in glass, it features a metallic base to which the glass is affixed. Inside the glass casing lies a thin wire, serving as the filament of the bulb, which gives off light. Tungsten, a hard grey metallic element with an exceptionally high melting point, is employed in manufacturing electric bulb filaments.











The inner space is filled with a mixture of gases such as argon, neon, and nitrogen. The base and metal case serves as the two terminals of the bulb, designated as positive and negative.
Structure of a Torch Bulb
Filament
Terminals
Fig. 3.4 Terminals of a bulb
Upon connecting the terminals to an electric cell, the electric current passes through the filament, causing it to heat up and emit light. The two terminals are positioned in a manner that prevents them from making contact.
3.2.2 Connection of bulb to electric cell
The bulb can be connected to cells in six distinct ways, as depicted in figures 3.4 (a) to (f)







Fig. 3.5 Connection of bulb to cell
Only in scenarios (a) and (f) does the electric bulb illuminate.

3.3 ELECTRIC CIRCUIT
The arrangement of components such as an electric cell, an electric bulb, an electric switch, and electric wires interconnected to allow the flow of electric current along a closed loop is referred to as an electric circuit.


Fig. 3.6 Electric circuit
Within an electric circuit, the electric current travels from the positive terminal to the negative terminal of the electric cell.
When the terminals of the bulb are connected with that of the electric cell by wires, the current passes through the filament of the bulb, which makes the bulb glow. Also, occasionally, an electric bulb may fail to illuminate despite being connected to a cell. This occurrence typically arises from the bulb becoming fused. The primary cause of bulb fusion is the breakage of the filament. When the filament breaks, the pathway for electric current is disrupted, resulting in the bulb failing to emit light, hence referred to as a fused bulb.
3.4 ELECTRIC SWITCH
3.4.1 Electric circuit with a switch
One of the most important components of an electric circuit is a switch. A switch is a pair of metal contacts used to open or close a circuit. When the switch is ON, it allows the current to flow, and when the switch is OFF, it prevents the flow of electric current.


In other words, a switch is a simple device that either breaks the circuit or completes it.

An electric torch, a man-made light source, is encased in a plastic shell for insulation. It houses two cells, with the positive terminal of one linked to the other’s negative terminal. When these cells are properly connected, they form a circuit that allows electricity to flow.
A slider is incorporated into the design to turn the torch on or off. A metal spring at the base ensures contact with the switch, completing the electrical circuit. This spring is connected to the lower cell’s negative terminal and links it to the torch bulb’s base terminal.
To enhance the torch’s brightness and reach, a reflector is positioned around the bulb. The operation of a torch bulb is similar to that of an electric bulb. The cells within the torch transform their chemical energy into electrical energy, illuminating the bulb.
3.5 ELECTRIC CONDUCTORS AND INSULATORS
A material that allows electric current to pass through it is known as a conductor. Common examples of conductors include copper, iron, gold, silver, and aluminium. Examples of conductors























Fig. 3.10 Conductors


On the contrary, a material that does not allow electric current to pass through it is known as an insulator. Examples of insulators encompass glass, leather, ebonite, paper, rubber, mica, and wood. Examples of insulators

Fig. 3.11 Insulators


Both conductors and insulators play crucial roles in utilising electricity. When constructing circuits to utilise electric current for various purposes, such as illuminating a bulb or operating a fan, wires composed of metals renowned for their conductivity, like copper, are employed. However, bare wires pose potential hazards; hence, they are invariably encased in insulating materials, such as plastic.
Additionally, the exterior covering of many electrical devices, such as fans and switches, is crafted from insulating materials to mitigate the risk of electric shocks.
QUICK REVIEW
• An electric cell is a device that converts chemical energy into electrical energy and functions as a source of electricity.
• An electric bulb functions as a source of light powered by electricity.
• The path through which electric current can flow is known as an electric circuit.
• A switch is a simple device that either breaks the circuit or completes it.
• An electric torch is a man-made light source.
• A material that allows electric current to pass through it is known as a conductor
• A material that does not allow electric current to pass through it is known as an insulator.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Electric cell
1. Electric cell converts ____________.
a. Electrical energy into chemical energy
b. Chemical energy into electrical energy
c. Solar energy into electrical energy
d. Mechanical energy into electrical energy
2. What are the two terminals of an electric cell?
a. North and South
c. Positive and Negative
3. What is a dry cell made of?
b. East and West
d. Alpha and Beta
a. A zinc container with a paste of ammonium chloride inside
b. A copper container with a paste of sodium chloride inside
c. A silver container with a paste of potassium chloride inside
d. A gold container with a paste of calcium chloride inside
4. What happens when the chemicals in a dry cell are depleted?
a. The cell explodes
c. The cell recharges itself
b. The cell stops producing electricity
d. The cell turns into a battery
5. What is the difference between a primary cell and a secondary cell?
a. Primary cells are rechargeable, but secondary cells are not
b. Secondary cells are rechargeable, but primary cells are not
c. Primary cells are larger than secondary cells
d. None of these
6. How do solar cells produce electricity?
a. By converting mechanical energy into electrical energy
b. By converting chemical energy into electrical energy
c. By converting solar energy into electrical energy
d. By converting thermal energy into electrical energy
II. Electric bulb
1. What is the function of an electric bulb?
a. To convert electricity into heat

b. To convert electricity into light
c. To convert light into electricity d. To convert heat into electricity
2. Which of the following metals is used to make the filament of an electric bulb?
a. Aluminium b. Chromium c. Platinum d. Tungsten
3. What is the purpose of the filament in an electric bulb?
a. It serves as the positive terminal b. It serves as the negative terminal
c. It gives off light when heated
d. It cools down the bulb
4. What gases are typically found in the inner space of an electric bulb?
a. Oxygen and Hydrogen
b. Argon, Neon, and Nitrogen
c. Helium and Xenon d. Carbon dioxide and Methane
5. What happens when the terminals of an electric bulb are connected to an electric cell?
a. The electric current passes through the filament, causing it to cool down
b. The electric current passes through the filament, causing it to heat up and emit light
c. The electric current passes through the filament, causing it to break
d. The electric current passes through the filament, causing it to become magnetic
6. How are the two terminals of an electric bulb positioned?
a. They are positioned in a manner that allows them to make contact
b. They are positioned at the top and bottom of the bulb
c. They are positioned on the same side of the bulb
d. They are positioned in a manner that prevents them from making contact
III. Electric circuit
1. What is an electric circuit?
a. A device that converts chemical energy into electrical energy
b. An arrangement of components interconnected to allow the flow of electric current along a closed loop
c. A device that emits light when connected to an electric cell
d. A device that breaks the filament of a bulb
2. In an electric circuit, where does the electric current travel from and to?
a. From the negative terminal to the positive terminal of the electric cell
b. From the positive terminal to the negative terminal of the electric cell
c. From the bulb to the electric cell
d. From the electric cell to the bulb
3. What happens when the terminals of the bulb are connected with that of the electric cell by wires?
a. The current passes through the filament of the bulb, which makes the bulb glow
b. The filament of the bulb breaks
c. The bulb becomes fused
d. The bulb cools down
4. What is a fused bulb?
a. A bulb that emits light when connected to an electric cell
b. A bulb that fails to emit light due to the breakage of the filament
c. A bulb that glows when the filament is heated
d. A bulb that is connected to an electric cell by wires
5. What is the role of electric wires in an electric circuit?
a. They convert chemical energy into electrical energy
b. They emit light when heated
c. They connect the terminals of the bulb with that of the electric cell
d. They break the filament of a bulb
6. In an electric circuit, the wires are connected to the:
a. The positive terminal of the cell only
b. The negative terminal of the cell only
c. Both positive and negative terminals of the cell
d. None of these
IV. Electric switch
1. What is the function of a switch in an electric circuit?
a. To convert electrical energy into light
c. To break the filament of a bulb
2. What happens when a switch is ON?
a. It prevents the flow of electric current
c. It breaks the circuit
3. What happens when a switch is OFF?
a. It allows the current to flow
c. It completes the circuit
4. What does a switch control in an electric circuit?
a. The flow of electric current
c. The temperature of the circuit
b. To open or close the circuit
d. To emit light when heated
b. It allows the current to flow
d. It cools down the circuit
b. It heats up the circuit
d. It prevents the flow of electric current
b. The emission of light
d. The colour of the circuit
V. Electric conductors and insulators
1. The material that allows an electric current to pass through it is known as:
a. Conductor b. Insulator
c. Electric resistance d. Semiconductor
2. Which of the following is a conductor?
a. Copper b. Glass c. Rubber d. Wood
3. What is an insulator?
a. A material that allows electric current to pass through
b. A material that does not allow electric current to pass through
c. A device that converts electrical energy into light
d. A device that breaks the filament of a bulb
4. Which of the following is an insulator?
a. Iron b. Gold c. Leather d. Silver
5. Why are wires encased in insulating materials?
a. To allow an electric current to pass through
b. To prevent electric current from passing through
c. To prevent potential hazards posed by bare wires
d. To make the wires look attractive
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. What is the location of the positive terminal in a dry cell?
a. At the base
c. Inside the zinc container
2. What type of devices typically use button cells?
a. Clocks and remote controls
b. Wristwatches, calculators, and hearing aids
c. Cameras and toys
d. Solar panels and inverters
b. At the tip of the metal cap
d. Inside the paste of ammonium chloride
3. What is the purpose of the metallic base in an electric bulb?
a. It serves as a terminal
c. It emits light
b. It holds the glass casing
d. It conducts electricity
4. What is the characteristic of tungsten that makes it suitable for manufacturing electric bulb filaments?
a. It has a low melting point
c. It is a good insulator
b. It has a high melting point
d. It is a soft metal
5. What is the difference between a cell and a bulb in terms of their function?
a. A cell converts chemical energy into electrical energy, while a bulb converts electrical energy into light
b. A cell converts electrical energy into light, while a bulb converts chemical energy into electrical energy
c. A cell and a bulb both convert chemical energy into electrical energy
d. A cell and a bulb both convert electrical energy into light
6. What is the role of the glass casing in an electric bulb?
a. It conducts electricity
c. It protects the filament
b. It emits light
d. It cools down the bulb
7. What happens to the light bulb when the switch in its circuit is turned OFF?
a. The bulb glows brighter
c. The bulb changes colour
b. The bulb stops glowing
d. The bulb cools down
8. Why is the exterior covering of many electrical devices made of insulating materials?
a. To allow electric current to pass through
b. To prevent electric current from passing through
c. To mitigate the risk of electric shocks
d. To make the devices look attractive
9. What is the difference between a conductor and an insulator in terms of their use in electrical devices?
a. Conductors are used for the exterior covering of devices, while insulators are used for the wires
b. Insulators are used for the exterior covering of devices, while conductors are used for the wires
c. Conductors and insulators are both used for the exterior covering of devices
d. Conductors and insulators are both used for the wires
10. What would happen to the light bulb if one more cell is connected in a simple electric circuit?
a. The bulb will fuse
c. The brightness of the bulb will increase
b. The brightness of the bulb will decrease
d. The bulb will stop glowing
11. In the following circuit, the bulb will not glow if ends A and B are connected with
a. Metal clip
c. Copper wire
12. Bulb consists of
a. A glass chamber
c. Two terminals
b. Tap water
d. Plastic clip
b. Filament
d. All of these
13. Look at the given figure. It consists of a cell, a bulb with the two terminals X and Y and wires, with ends P and Q; as well as S and R. The direction of the current will be

14. Ravi connected three bulbs with the cells and a switch, as shown. When the switch is moved to ON position




a. The bulb X will glow first
c. The bulbs Z and X will glow first


b. The bulb Y will glow first
d. All the bulbs will glow simultaneously
15. The given figure is a ' Material tester'. Which of the following objects will make the bulb glow when put on the gap shown?



16. An electric switch can be connected:
a. To the positive terminal of the battery only.
b. Anywhere in the circuit.
c. To the negative terminal of the battery only.
d. To the electric bulb only.
17. If we touch a naked current-carrying wire, we get a shock, this is because our body is
a. Insulator of electricity
c. Conductor of electricity
18. Which is not a good conductor of electricity?
b. Source of electricity
d. Made up of non-metals
a. Mercury b. Copper c. Cloth d. Aluminium foil
19. What is the difference between a cell and a battery?
a. A cell is a singular unit, while a battery is formed by connecting two or more cells
b. A battery is a singular unit, while a cell is formed by connecting two or more batteries
c. A cell is rechargeable, but not a battery

d. A battery is rechargeable, but not a cell

20. Choose from the options a, b, c, and d given, which shows the correct direction of the current.























EXPLORING MAGNETS 4
4.1 DISCOVERY OF MAGNETS
Long ago in ancient Greece, there was a shepherd named Magnes. He took his sheep and goats to the mountains for grazing. He carried a stick with him to help control his animals, and this stick had a small piece of iron at one end.
One day, while walking in the mountains, Magnes found that his stick had stuck to a rock. He had to pull hard to free it. It seemed like the rock was pulling the iron tip of his stick towards it, almost as if it had a magical power. This rock turned out to be a natural magnet, and this simple event is said to be how people first discovered magnets in nature for the first time. Such rocks were given the name magnetite, after the name of that shepherd.
4.1.1
What are magnets?
The substances having the property of attracting iron are known as magnets.
There are two types of magnets:
Natural magnets
Natural magnets are naturally occurring magnetic minerals. The most common type of natural magnet is magnetite, which is an iron oxide mineral. Magnetite contains iron. Some people believe that magnetite was first discovered at a place called Magnesia.
Natural magnets can attract certain metals, such as iron, nickel, and cobalt, due to their strong magnetic properties. All natural magnets are permanent magnets; hence, they never lose their magnetic strength. The Lodestone is an extremely rare form of magnetite that occurs naturally as a permanent magnet.
Artificial magnets
After discovering rocks that could attract iron, people noticed that even small pieces of these rocks had special features. Later, they figured out how to make magnets from iron pieces, and those are called artificial magnets.
Artificial magnets are typically created using materials such as iron, nickel, cobalt, or various alloys. They have magnetic properties. Nowadays, artificial magnets come in various shapes, such as bar magnets, horseshoe magnets, cylindrical magnets, and ball-ended magnets.




Poles of magnet: When you spread iron filings on a sheet of paper and place a bar magnet on top of them, you’ll notice:
• The iron filings stick to the magnet.
• More iron filings are attracted to some specific parts of the magnet than others.
• The concentration of iron filings is higher near the ends of a bar magnet. These ends are also known as the poles of the magnet, where the magnetic force is stronger.

4.2 MAGNETIC AND NON-MAGNETIC MATERIALS
Imagine you are organizing a science fair exhibit on magnetic and non-magnetic materials. For the magnetic section, you will display items like iron nails and steel paperclips attracted to a magnet while for the non-magnetic section, you will showcase objects like wooden blocks and plastic toys that don't respond to magnets.
4.2.1 Magnetic materials
The materials that get attracted towards a magnet are magnetic materials. Examples of magnetic materials include iron, nickel, cobalt, and certain alloys.
When you put a magnet near the magnetic materials, they get attracted towards the magnet. We can use magnetic materials to make things like fridge magnets or compasses.
4.2.2 Non-magnetic materials
Non-magnetic materials are not attracted to magnets and cannot be magnetized easily. NonMagnetic materials do not stick to magnets at all.
Examples of non-magnetic materials include wood, plastic, glass, copper, and aluminium.
4.3 MAGNETIC PROPERTIES
4.3.1 Finding directions
For a long time, travellers have relied on magnets to help them find their way. In ancient times, travellers used to carry natural magnets with them and hang them from a thread to determine directions.
To understand this, let’s take a bar magnet and mark one of its ends for identification. Tie a thread to the middle of the magnet, allowing it to suspend freely from a stand. Ensure that the magnet can rotate without any hindrance. Let the magnet come to rest naturally.


4.2 A suspended magnet
Mark two points on the ground to indicate the position of the magnet’s ends when it settles. When the magnet comes to rest, draw a line connecting the two marked points. This line represents the direction in which the magnet points. Now, gently rotate the magnet by pushing one end in any direction and let it come to rest again. Repeat this process by rotating the magnet in different directions and noting the final resting position. You’ll find that the magnet always comes to rest in the same direction, the north-south direction. Even if you disturb the magnet repeatedly, it will eventually settle back to this consistent orientation.
North Pole and South Pole: The end of the magnet that points toward the north is called its north-seeking end or the North Pole of the magnet. The other end that points towards the south is called the south-seeking end or the South Pole of the magnet. All magnets have two poles, whatever their shape may be. Usually, north (N) and south (S) poles are marked on the magnets.
Later, a compass tool was invented based on this magnetic property. Let’s understand a compass tool in detail.
4.3.2 Magnetic compass
A compass is a small box with a glass cover and a magnetized needle that can freely rotate. Inside the box, there is also a dial with directions marked on it. To use the compass, it is placed where direction is needed. The needle settles in the north-south direction, and the compass is rotated until the north and south markings on the dial align with the needle. To make it easier to spot the north end of the needle, it is often painted in a different colour.


4.3.3 Attraction and repulsion between magnets
Let’s place one bar magnet flat on a table and hold another bar magnet vertically above it with opposite poles facing each other. Slowly bring them closer and observe them. The magnets will attract and stick together. Now, flip the second magnet, so like poles face each other and observe. Now, the magnets will repel, pushing away from each other.
In the above experiment, when different poles of magnets face each other (the North Pole facing the South Pole), they pull towards each other, which is known as magnetic attraction. Conversely, when similar poles face each other (the North Pole facing the North Pole or the South Pole facing the South Pole), they push away from each other, causing magnetic repulsion. This phenomenon is a fundamental characteristic of magnetism.
The fundamental behaviour of magnets is directly related to their alignment when suspended freely. When a magnet is freely suspended, it naturally aligns itself in the north-south direction because the magnetic South Pole of the Earth lies in the geographic north direction, while the magnetic North Pole of the Earth lies in the geographical south direction. Consequently, the North Pole of the magnet is attracted to the magnetic south of the Earth, and the South Pole of the magnet will be attracted to the magnetic north of the Earth. In other words, the magnet aligns along the line, joining the North and South Poles of the Earth.
4.4 CARE OF MAGNETS
4.4.1 Cautions
Magnets can lose their properties if they are exposed to heat, hammered, or dropped from a height. Improper storage can also weaken magnets over time. To keep them safe and maintain their strength, we should take the precautions given below.
• Store bar magnets in pairs with their unlike poles facing each other. Separate them with a piece of wood and place two pieces of soft iron across their ends.
• For horseshoe magnets, place a piece of iron across the poles.
It is also important to keep magnets away from electronic devices such as cassette players, mobile phones, televisions, stereo systems, CDs, and computers.
QUICK REVIEW
• The substances having the property of attracting iron are known as magnets.
• Natural magnets are naturally occurring magnetic minerals.
• Artificial magnets are typically created using materials such as iron, nickel, cobalt, or various alloys.
• The materials that get attracted towards a magnet are magnetic materials.
• Non-magnetic materials are not attracted to magnets and cannot be magnetized easily.
• A compass is a small box with a glass cover, housing a magnetized needle that can freely rotate.
• Magnets can lose their properties if they are exposed to heat, hammered, or dropped from a height.
• Magnets should be kept away from electronic devices.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Discovery of magnets
1. The most common type of natural magnet is:
a. Silicon b. Magnetite c. Magnesium d. Copper
2. Which of the following are naturally occurring magnetic materials?
a. Iron b. Nickel c. Cobalt d. All of these
3. Natural magnet is also called:
a. Moonstone b. Sandstone c. Sunstone d. Lodestone
4. Which of the following shapes of a magnet are possible?
P
a. Only P and Q
c. Only Q and R
Q R
b. Only R and S
d. All P, Q, and R
5. Where is the maximum force of attraction in a magnet?
a. At the centre
b. At the centre
c. Same everywhere d. At the poles
6. How many poles are there in a magnet?
a. 4 b. 6 c. 2 d. 8
7. The end that points towards the south is called:
a. North Pole
c. South-seeking end
8. The end that points towards the north is called:
a. South Pole
b. South Pole
d. Both (b) and (c)
b. South-seeking end
c. North Pole d. None of these
9. Which of the following statements about magnetite is/are correct?
i) It is iron ore.
ii) It is black in colour.
iii) It is non-magnetic.
iv) It was discovered in Magnesia.
a. Only (i) and (iv)
c. Only (ii), (iii), and (iv)
10. ____________ poles attract each other.
a. Unlike
c. Both (a) and (b)
II. Magnetic and non-magnetic materials
b. Only (ii) and (iii)
d. Only (i), (ii), and (iv)
b. Like
d. None of these
1. The materials that get attracted towards a magnet are called:
a. Non-magnetic b. Plastics
c. Magnetic d. Wood
2. Wood, plastic, and glass are ________________________ materials.
a. Magnetic
c. Artificial magnets
3. Iron, cobalt, and nickel are examples of:
a. Magnetic materials
c. Both (a) and (b)
b. Natural magnets
d. Non-magnetic
b. Non-magnetic materials
d. None of these
4. The materials which are not attracted to magnets and cannot be magnetized easily are:
a. Magnetic materials
c. Both (a) and (b)
b. Non-magnetic materials
d. None of these
5. Which of the following materials do not exhibit magnetic properties?
a. Cobalt
b. Iron
c. Nickel d. Lead
III. Magnetic properties
1. How is a compass used to find directions?
a. By detecting the Earth's magnetic field b. By using radio signals
c. By measuring the speed and direction of the wind d. By analyzing the position of stars in the sky
2. In a compass, the magnetic needle always points in which direction?
a. East-West b. South-East c. North-South d. West-North
3. If iron fillings are poured on a magnet, then we observe that:
a. More fillings are attracted at the ends of magnet
b. Fewer fillings are attracted at the ends of magnet
c. Fewer fillings are attracted at the middle of magnet
d. Both (a) and (c)
4. When a magnet is suspended freely, it aligns itself in:
a. N-S direction
c. E-W direction

b. S-E direction
d. Both (b) and (c)
5. The North Pole of the magnet is attracted to magnetic _____________ of the Earth.
a. North b. East c. South d. West
6. The South Pole of the magnet is attracted to magnetic _____________ of the Earth.
a. North b. East c. South d. West
7. _________________ occurs when similar poles face each other.
a. Magnetic attraction b. Magnetic repulsion
c. Both (a) and (b)
d. None of these
8. _________________ occurs when opposite poles face each other.
a. Magnetic repulsion
c. Both (a) and (b)
b. Magnetic attraction
d. None of these
9. The property of the magnet that differentiates a magnet from other substances is its:
a. Attractive property
c. Directive property
b. Repelling property
d. All of the above
10. Attraction is observed between the poles of two bar magnets in the case of:
a. S-pole of one magnet with S-pole of other
b. N-pole of one magnet with S-pole of other
c. N-pole of one magnet with N-pole of other
d. All these cases will show attraction
IV. Care of magnets
1. Heating a magnet leads to:
a. Demagnetisation
c. No change
2. Choose the wrong statement.
b. Increase in magnetic strength
d. Both (a) and (b)
a. Heat can destroy the magnetic properties of magnets.
b. Different magnets are made up of different materials and come in different shapes.
c. There is a maximum attraction in the middle area of a magnet.
d. Magnetite shows magnetic properties.
3. If you break a magnet into 6 pieces, how many North and South Poles will be there in all?
a. N = 6, S = 6
b. N = 3, S = 3
c. N = 6, S = 12 d. N = 12, S = 12
4. The magnetic properties of a magnet cannot be destroyed by:
a. Hammering
c. Heating
b. Dropping on hard surface
d. None of these
5. Which material is generally used to protect a horseshoe magnet?
a. Wooden rod
b. Silicon rod
c. Plastic rod
d. Iron rod
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which alloy is used to make powerful magnets?
a. Steel b. Alnico
2. Which of the following objects is/are magnetic?
a. A nickel coin
c. An iron nail
c. Bronze d. Ferrites
b. A brass screw
d. Both (a) and (c)
3. Which of the following does NOT contain a magnet in it?
a. A torch
c. A fan
b. A radio
d. Both (b) and (c)
4. Two magnets are placed close to each other, as shown below. Which set will attract each other?
5. The magnet used in a magnetic compass is:
a. Electromagnet
c. Temporary magnet
6. Where are the poles of a bar magnet located?
a. At the centre of the magnet
c. On the sides of the magnet
b. Permanent magnet
d. All of these
b. At the ends of the magnet
d. At random locations
7. How can you make two magnets repel?
a. Bring opposite ends together
c. Bring the same pole together
b. Place the magnets in the water
d. Place the magnet in the sand
8. Which of the following objects is attracted to a magnet?
a. Gold ring
c. Steel cabinet
9. Same poles of the magnet ________ each other.
a. Attract
c. First, attract, then repel
10. Which of the following is not a natural magnet?
a. Magnetite
b. Loadstone
b. Silver dollar
d. Aluminium bat
b. Repel
d. None of these
c. Iron oxide d. Copper
11. Ferrite is used for making very powerful permanent magnets. It is a mixture of:
a. Ferric oxide and copper oxide
c. Ferric oxide and zinc oxide
b. Ferric oxide and barium oxide
d. Ferric oxide and aluminium oxide
12. Which of the following materials exhibits magnetic properties?
a. Iron b. Rubber
13. Which of the following is a non-magnetic material?
a. Iron
c. Sand d. Diamond
b. Wood c. Nickel d. Steel
14. Glass is a ___________ material, but cobalt ___________ is a material.
a. Magnetic, non-magnetic
c. Magnetic, magnetic
b. Non-magnetic, non-magnetic
d. Non-magnetic, magnetic
15. Which of the following does not stick to the magnets at all?
a. Rubber
c. Aluminium
b. Nickel
d. Both (a) and (c)
16. _________________ are not attracted to magnets and cannot be magnetized easily.
a. Non-magnetic materials
c. Magnetic materials
b. Fridge magnets
d. None of the above
17. Which of the following substances are repelled by a magnet?
a. Nickel b. Iron
c. Cobalt d. Gold
18. When a bar magnet is brought near iron dust, most of the dust sticks:
a. At the middle
c. At the two ends
b. Equally everywhere
d. Near the middle
19. Two bar magnets were placed close to each other. Which of the sets given below will attract each other?
a. Only P
c. Only P and S
20. A compass needle is a/an:
a. Iron piece
c. Small magnet
b. Only Q and R
d. Only R
b. Electromagnet
d. None
21. Which of the following is indicated by a magnetic compass?
a. Volcano
b. Altitude
c. Direction d. Sea-level
22. The instrument which is used to identify the geographical direction is:
a. Manometer
c. Periscope
23. Which of the following instruments use magnets?
a. Tape recorders
c. Microphones
b. Barometer
d. Magnetic compass
b. ATM cards
d. All of these
24. What is the primary purpose of a magnetic compass?
a. Attracting magnetic materials
c. Aligning with the Earth’s magnetic field
b. Finding the magnetic poles
d. Indicating directions
25. What is the significance of the red tip on the North Pole of a magnetic compass needle?
a. Indicates danger
c. Enhances visibility
b. Represents the North Pole
d. Aesthetic purpose
26. Which of the following gets demagnetized when a powerful magnet is kept near it?
a. Compact disc
c. Both (a) and (b)
b. Cellphone
d. None of these
27. Which of the following may be destroyed when kept near a magnet?
a. Switch
b. Hard disk
c. Tubelight d. Bulb
28. The bar magnets are kept in pairs with their unlike poles on the same side and separated by wood to:
a. To increase the strength of the magnets
c. To facilitate easy storage
b. To reduce the risk of demagnetisation
d. To prevent them from attracting metal objects
29. A bar magnet is hammered for some time, its magnetic strength will:
a. Increase
c. Decrease
30. Magnets lose their properties significantly if:
a. Left in open
c. Dropped from a height
b. Remain the same
d. Increase, then decrease
b. Touched with iron
d. None of these

BLANKPAGE
CHEMISTRY

MATERIALS AROUND US 1
1.1 OBJECTS AROUND US
In our everyday lives, we interact with many items surrounding us. Materials serve as the fundamental components of these items. The objects in our surroundings vary in shape and size, making identification important. Items such as doors, fans, balls, books, pens, pencils, and tables are just a few examples of the objects that are part of our environment. Each of these objects possesses unique characteristics that distinguish them from one another.
Generally, we have observed in the kitchen that some of the objects will break if they fall from a certain height where as some of the objects will not break.
Examples: Crockeries like dishes, cups, and bowls will break if they fall from a certain height, whereas steel plates, bowls, cups, and glasses will not break even after they fall on the floor.

1.1 Objects around us
What is an object?
• Materials are those which are used to make different objects.
Examples: Wood is used to make furniture; plastic is used to make toys, etc.
• A material thing can be seen and touched.
Examples: Pen, paper, chair, book, pencil, rubber, scale, plate, bag, etc.
• All these materials are a form of matter, and each matter possesses some different properties.
1.2 CLASSIFICATION OF MATERIALS
Objects that are seen around can be sorted out into groups based on their appearance, shape, size, colour, materials used, and common properties. This process of grouping various objects according to their common properties is called classification.
Let’s sort the below objects according to the materials they are made up of Scale, chalk, duster, pencil, pen, plate, rubber, sponge, phone, book, bag, handkerchief, key, water bottle, etc.
Scale Wood, plastic, steel
Pen Steel, plastic Pencil Wood Books Wood pulp Bag Leather, fibre (any fabric) Key Metal
Water bottle Metal, plastic
Table 1.1 Materials
1.3 PROPERTIES OF MATERIALS

We choose a material to make an object depending on its properties and the purpose for which the object is to be used.
Examples: Cloth cannot be used to store water, whereas plastic containers or steel containers can be used to store water.
1.3.1 Appearance
Appearance means how an object looks like when you see it for the first time. Based on their appearance, materials can be classified into two categories:
a. Lustrous
b. Non-Lustrous
a. Lustrous
Lustre is a gentle shining light that is reflected from a surface, for example, from polished metal.
Example: Gold retains its lustre for far longer than other metals.
Whereas other metals lose their shine when they come in contact with moisture, depending on their reactivity

b. Non-lustrous
They are dull in appearance and do not have any shining surface.
Examples: Wood, sandpaper, sulphur, phosphorous, etc.
When we see wood, it looks brown, dull, and with a somewhat uneven surface, whereas when we see a metal like iron, it looks shiny.
• Both the materials appear to be different.
• Iron appears to be lustrous, whereas wood appears non-lustrous.










Let’s understand the concept of appearance by collecting different material from your surrounding which you can get easily.
• Iron is reactive in the presence of moisture and oxygen, and the colour of iron will change to brown, which indicates that iron is being rusted
• Whereas the reddish brown shiny appearance of copper turns to bluish green when it gets corroded.
1.3.2 Hardness
We can identify different materials by means of touch or by pressing different objects. How can we say whether the substance is hard or soft?
To understand this, we should know what hardness is:
Hardness is the property of materials, which can be identified by deforming the shape of the object or by compressing the object.
• If the substance is hard, we cannot compress and if it is soft, we can easily compress and the objects regains it shape.
• Some materials are very hard, while some are very soft.
Examples: 1. Hard: Diamond, stone, wood, steel, etc.

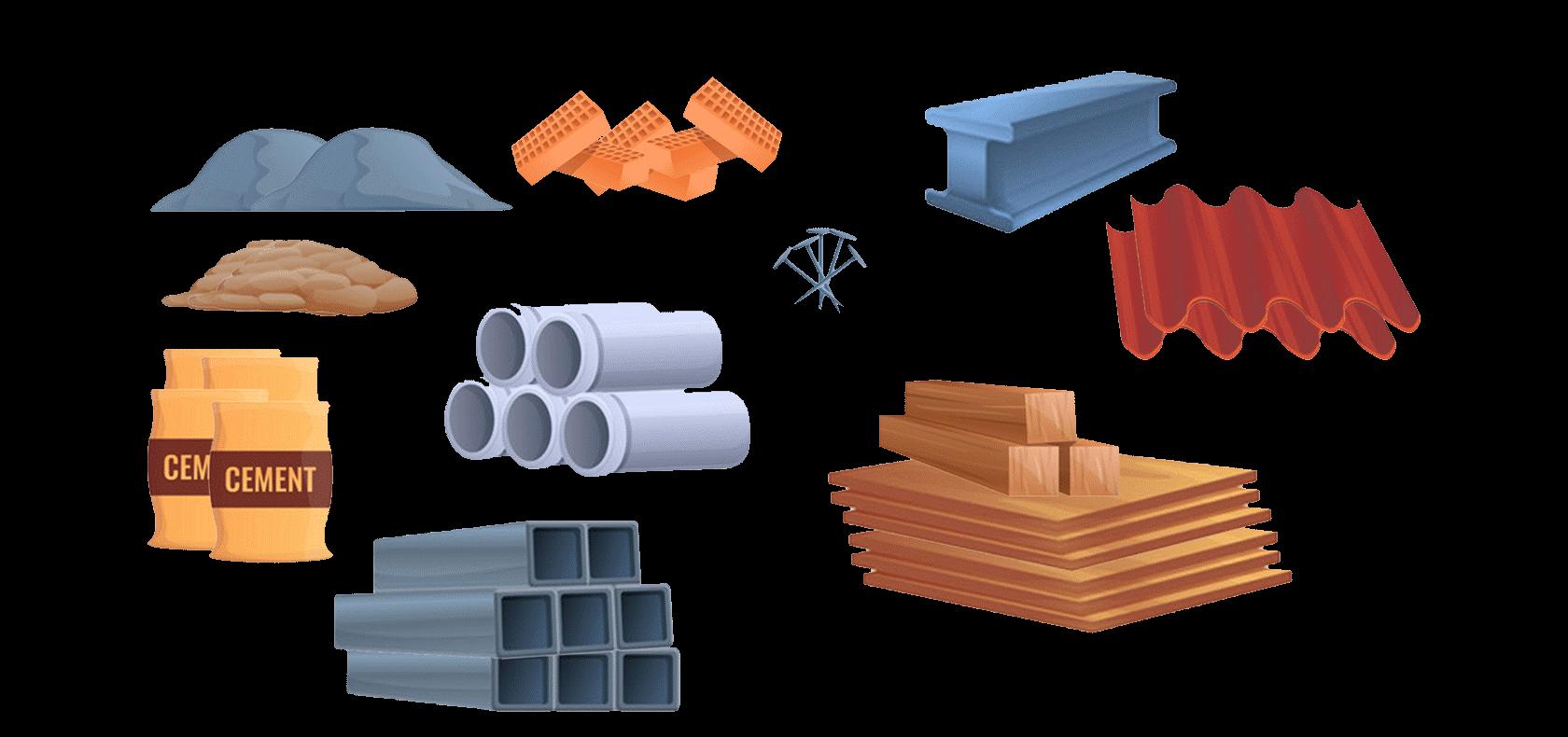
a. Smoothness
Smoothness is the property of the substance which we can find by means of touch.
• The smoothness of the object can be understood by its even surface.
• The quality of being perfectly regular with no holes, lumps, or areas that rise or fall suddenly.
• Smooth objects-
1.
2.
3.
4.
5.
b. Roughness
Roughness is also the property of a substance, which can be identified by means of touch.
• The roughness of an object can be understood by an uneven surface.
• The quality of being irregular, having bumps, or holes.
Examples: When we touch a piece of paper, we feel that the surface of the paper is smooth, but at the same time, when we touch the ground with an uneven surface, we feel that it’s rough.
• Rough objects-
1. Crumbled paper
2. Road
3. Grooves in shoes.
4. Scrubber Sponge
5. Tires




1.3.3 Solubility
• An important property of water is its ability to dissolve many substances in it.
• Most of the solids, liquids and gases dissolve in water.
• Substances which dissolve in water are said to be soluble, and the property is called solubility
• Substances which do not dissolve in water are said to be insoluble.
• For example, common salt, sugar, and glucose dissolve in water, whereas sand, sawdust, oil, and wax are insoluble in water.
1.8 Solubility of different substances
Solubility of liquid in water
Vinegar or lemon juice will get dissolved in water, whereas edible oil will not get dissolved in water.

Fig. 1.9 Solubility of liquid in liquid
Solubility of gas in water
• Gases are also soluble in water.
• As we know, oxygen gets dissolved in water.
• Oxygen dissolves in surface water due to the aerating action of winds.
• Oxygen is also introduced into the water as a by-product of aquatic plant photosynthesis.
• It helps aquatic plants and animals to survive in water.
• Colder water can hold more oxygen in it. As the water becomes warmer, less oxygen can be dissolved in the water.

1.3.4 Floating or sinking
• Few of the substances float over a liquid, and few of the substances will sink.
• Oil floats over water, whereas sand sinks in water.

1.3.5 Density and buoyant force
Density is a characteristic property of a substance, which is the relationship between the mass of the substance and how much space it takes up, i.e., the volume. The mass of atoms, their size, and how they are arranged, forming a substance, determine its density.
• Density equals the mass of the substance divided by its volume.
• Density formula-
D = m/v
• Unit of density is kg per cubic meter [kg/m3].
• Objects with the same volume but different masses have different densities.
What does buoyancy mean?
When an object is placed in liquid, it exerts an upward force on the object. This makes the object appear to be lighter, this upward force is called buoyancy.
Let’s understand how density and buoyancy are correlated and how they make an object float or sink.
• Sinking objects: If the density of an object is greater than that of water, then the objects sink in water. This happens because the buoyant force is less than the force exerted due to the weight of an object.
Example: Iron sinks in water.
• Floating objects: If the density of an object is less than that of water, then objects float on water. Objects float on water because buoyant force is more than the force exerted due to the weight of an object.
Example: Cork floats on water.
1.3.6 Transparency
The property that determines how much light can pass through a material is called transparency. Based on this property materials can be classified as transparent, translucent and opaque.
Transparent materials
Materials which allow light to completely pass through them are called transparent materials.
Examples: Glass, clear water, clean plastic.

Translucent Materials
Materials which allow some light to pass through them are called translucent materials.
Examples: Butter paper, sunglass, bulb.
Fig. 1.14 Translucent objects
Opaque objects
Materials which do not allow light to pass through them are called opaque materials. This means that you cannot see through an opaque object because it blocks the light from passing through it.
Examples: Wood, metal, etc.
Steel Cupboard
Fig. 1.15 Opaque objects
1.3.7 Heavy or light
Mass
Anything that is heavy or light can be measured using a property called mass. The heavier an object is, the more mass it has. Similarly, lighter objects have less mass.
The terms matter and mass are often confused because they are frequently discussed together. But they are not the same thing. Let’s understand the difference:
• Matter is anything that has mass and takes up space. This means that everything around us like a tree, a table, or even the air we breathe-is matter.
• Mass is the amount of matter in an object. It is a way of measuring how much "stuff" is present inside something.
So, while all matter has mass, mass is a measurement of the matter in an object. For example, a small stone has less mass than a big boulder because the boulder has more matter in it.
1.3.8 Space and volume
When two containers have the same capacity but hold different amounts of water, the water levels will vary. This happens because the amount of water in each container is not the same. The space that a liquid occupies is called its volume. If one container holds less water, the volume of water is smaller because it takes up less space. Volume is a measurement of how much space is filled by a substance, such as water, while space refers to the total area or capacity available, whether it is filled or not.
For example, if one container is half-full and another is completely full, the volume of water in the first container is less, even though both containers have the same capacity. This shows how volume specifically relates to the amount of substance, not just the available space.
QUICK REVIEW
• Objects around us are made up of a large variety of materials.
• Different types of materials have different properties:
• Shine/lustre, non-lustrous materials.
• Hard or soft materials.
• Some objects that are soluble or insoluble.
• Objects may float or sink in water.
• Materials are classified into transparent, translucent, and opaque based on the transmission of light through them.
• Matter is anything that has mass and takes up space.
• Mass is the amount of matter in an object.
• The space that a liquid occupies is called its volume
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Objects around us
1. Which among the following is not a matter?
a. Electricity b. Paper c. Water d. Petroleum
2. Select the odd one out.
a. Aluminium b. Iron c. Silver d. Sand
3. Find the odd one out from the following.
a. Tawa
c. Pressure cooker
b. Spade
d. Eraser
4. When you light an agarbatti in a room, the smoke from the agarbatti _________.
a. Moves upwards
c. Moves randomly in any direction
b. Moves downwards
d. None of these
5. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion (A): Materials can be grouped on the basis of similarities or differences in their properties.
Reason (R): Dividing materials into groups makes it convenient to study their properties and also observe any patterns in these properties.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, but R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
6. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion (A): Glass, water, air, and some plastics are examples of transparent materials.
Reason (R): Wood, cardboard, and metals are examples of opaque materials.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, but R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
7. Rama collected samples of the following items and and mixed them in water separately: (i) Lemon juice (ii) Vinegar (iii) Ink (iv) Groundnut oil
Which of the following substances are completely insoluble?
a. (i) and (ii)
b. (ii) and (iv)
8. Which of the following statements are correct?
1. Plates can be made up of steel and glass.
2. Pens can only be made of plastic.
3. Knives can be made of wood and plastic.
4. A bucket is made up of steel only.
a. 1 and 2
b. 1 and 3
c. (iv) only
d. (i) only
c. 2 and 3
d. 3 and 4
9. I) Substances that break down into smaller pieces or are powdered when hammered are called brittle.
II) Substances that can be spread into sheets when beaten are malleable.
Choose the correct set suitable for the above, respectively.
a. Rock salt, glass
c. Common salt, silver
10. A ⟶ salt, B ⟶ sand, C ⟶ sugar, D ⟶ CO2 gas.
b. Gold, silver
d. Gold, zinc
Sort out the above substances as soluble or insoluble in water.
a. A-Soluble, B-Insoluble, C-Soluble, D-Soluble
b. A-Soluble, B-Soluble, C-Soluble, D-Insoluble
c. A-Insoluble, B-Soluble, C-Soluble, D-Soluble
d. A-Soluble, B-Soluble, C-Insoluble, D-Soluble
II. Classification of materials
1. Select the odd pair.
a. Glass - Transparent
c. Chalk - Lustrous
b. Water - Liquid
d. Sandpaper - Rough
2. Sanjana sits in a chair filled with air. She sticks some pins into the chair. She observed that the size of the chair was reduced. From this, she can conclude that:
a. Air occupies space.
c. Air has some shape.
b. Air is an example of a solid.
d. Air is an example of a liquid.
3. A mixture contains three different substances: X, Y, and Z. They are of the same size, are cubical in shape, and are yellow in colour. X particles are very heavy, insoluble, and nonmagnetic and contribute 50% of the mixture. Y particles are very light, insoluble, and nonmagnetic and contribute 40% of the mixture, while Z particles are iron pieces. Which of the following methods can separate X, Y, and Z?
a. Winnowing, magnetic separation
b. Magnetic separation, sieving
c. Sieving, magnetic separation, filtration
d. Handpicking, sublimation, sieving
4. Material C is stretchy but not an absorbent. Material D is a good absorbent but does not stretch. Which will make the best swimwear?
a. Material D
c. Both material D and C
b. Material C
d. Can’t decide with the given data
5. Which of the following objects can be grouped together based on having a rough surface?
a. Apple and glass
c. Teddy bear and doormat
b. Doormat and sandpaper
d. None of these
6. The table given below shows some objects that have been grouped:
Wooden ruler Coins
Plastic cup Steel spoon
a. Based on their ability to reflect light
b. Based on the surface roughness
c. Based on the surface smoothness
d. Based on their transparency
7. Which of the following materials is used to make an electric fan? (i) plastic (ii) metals (iii) glass
a. Only (i)
c. Only (ii) and (iii)
8. Which of the following is a lustrous material?
a. Wood b. Plastic
III. Properties of materials
b. Only (i) and (ii)
d. (i), (ii), and (iii)
c. Copper d. Chalk
1. There are three liquids: X, Y, and Z. X is colourless but will float on water, Y is also colourless and will not float on water, and Y and Z both are soluble in water. X, Y, and Z can be:
a. Sugar syrup, alcohol, lemon juice
c. Coconut oil, vinegar, alcohol
2. Baking of dough into bread is a kind of change:
a. That can be reversed
b. That cannot be reversed
c. That can be reversed at very hot temperature
d. Cannot say
3. P- Hard and soluble in water.
Q-Liquid and insoluble in water.
R-Hard, opaque, and floats on water. What are P, Q, and R?
a. P- Sugar, Q-Salt, R-Wood
c. P- Salt, Q-Vinegar, R-Tissue Paper
b. Saltwater, alcohol, vinegar
d. Lemon juice, vinegar, alcohol

b. P- Sugar, Q-Coconut oil, R-Wood
d. P- Sugar, Q-Salt, R-Tissue Paper
4. Which of the following has a reddish-brown colour?
a. Gold b. Copper c. Iron d. Brass
5. Which are the following sinks in the water: i) Stone ii) Paper iii) Tomato iv) Iron nail
a. i, iv
6. Select the floatable thing:
b. i, ii c. iv d. i
a. Iron nail b. Wood c. Stone d. Sand
7. Riya has plants in her room. They need a lot of light. What kind of glass windows should she have installed for her indoor garden?
a. Opaque windows
c. Transparent windows
b. Translucent windows
d. No windows, just walls
8. What type of material would be best to cover your windows with that would help keep the sun out of your room so you can sleep?
a. Transparent material
c. Opaque material
b. Translucent material
d. Glass material
9. Our palm becomes _______ when a beam of light passes through it.
a. Opaque
b. Transparent c. Translucent d. Dark
10. When the lighted bulb is seen through oily paper, it is not visible properly. An oily paper is a type of _________ material.
a. Transparent b. Translucent c. Opaque d. Bright
11. In the shops, food items are mostly kept in plastic or glass containers because these are:
a. Transparent materials
c. Opaque materials
b. Translucent materials
d. Hard materials
12. What is the term used to describe the space that a liquid occupies?
a. Mass b. Volume c. Weight d. Density
13. What property is used to measure how heavy or light an object is?
a. Volume
b. Speed c. Temperature d. Mass
14. If a container can hold 1 litre of water but is only half-filled, what is the volume of water in the container?
a. 0.5 litres b. 1 litres c. 1.5 litres d. 0 litres
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which ones are correct?
a. A stone is denser than water. It, therefore, sinks into water.
b. Sponge is soft. It can be compressed easily.
c. Water is transparent. Things can be clearly seen through it.
d. All are correct.
2. ___________ is a translucent substance.
a. Oily paper b. Water c. Sandpaper d. Wood
3. Which of the following is not opaque?
a. Wood b. Frosted glass c. Iron d. Cardboard
4. Which of the following is hard and shines?
a. Wood b. Iron c. Sulphur d. Tin
5. Which of the following is water soluble?
a. Tin b. Iron rust c. Rock d. Salt
6. Which of the following is a translucent material?
a. Simple paper b. Oiled paper c. Wood d. Gold
7. _______ dissolves in water.
a. Gold particles b. Tin c. Oxygen d. Paper
8. Materials that can be easily compressed are ____________.
a. Hard b. Soft c. Rough d. Liquid
9. Select the odd one out.
a. Salt and water
c. Honey and water
b. Sugar and water
d. Oil and water
10. Which of the following types of bodies form shadows when they are placed in the light?
a. Translucent
c. Opaque
b. Transparent
d. Both transparent and translucent
11. Generally, solids are hard in nature, but exceptionally, a sponge is not a hard solid. Then the correct statement regarding sponge is
a. It is not a solid
c. Air is trapped inside the sponge
12. . Choose the opaque object from the following.
a. Charcoal
b. Air
b. It is a gaseous solid
d. All of the above
c. Glass d. Water
13. Match the entries in Column-I with those in Column-II correctly.
Column-I
a) Glass, Air
b) Iron sheet and cardboard
c) Oily paper sheet
d) Sugar and salt
e) Gold and silver
f) Wax
Column-II
i) Floats on water
ii) Translucent
iii) Metallic luster
iv) Transparent
v) Opaque
vi) Soluble in water
a. a⟶iv, b⟶v, c⟶vi, d⟶ii, e⟶i, f⟶iii
b. a⟶iv, b⟶ii, c⟶v, d⟶vi, e⟶i, f⟶iii
c. a⟶v, b⟶iv, c⟶ii, d⟶vi, e⟶iii, f⟶i
d. a⟶iv, b⟶v, c⟶ii, d⟶vi, e⟶iii, f⟶i
14. Shopkeepers prefer to keep biscuits, sweets, and other eatables in the ________ containers such that they are clearly visible to customers.
a. Wood b. Paper c. Cardboard d. Glass
15. Materials that cannot be compressed easily are called __________ materials.
a. Soft b. Rough c. Scaly d. Hard
16. Experiment I: Take some water in a wide-mouthed bowl, put an iron nail in it, and observe.
Experiment II: Put an empty iron tin in the water in a wide-mouthed bowl and observe.
Based on your observation, what can you conclude?
i) Some materials in one shape will sink in water but float on water when they are in another shape.
ii) The materials that can sink can be made to float by changing their shape.
iii) All materials that float can be made to sink by changing their shape
a. i, ii only
c. i, iii only
b. ii, iii only
d. All are correct
17. Examples for solid i) Rubber band ii) Sponge iii) Glass iv) Stone
a. i, ii, iv
c. ii, iii,iv
18. Wood is an example of a/an ________ material.
a. Opaque
b. i, ii, iii
d. All materials are solids
b. Transparent c. Translucent d. Reflecting
19. Which of the following materials allows for the partial visibility of objects?
a. Transparent
b. Translucent c. Opaque d. Solid
20. Which pair of substances among the following would float in a tumbler half filled with water?
a. Cotton thread, Thermocol
c. Pin, oil drops
b. Feather, plastic ball
d. Rubber band, coin
21. Mahesh and Rohan got a chance to visit a ship, but Mahesh was afraid to go on the ship. He said that the ship, being heavier in weight, would sink in the water. But Rohan said it would float on water. Whom do you agree with, and why?
a. Mahesh, as the ship is heavy in weight.
b. Rohan, because the ship floats because of its shape.
c. Mahesh, as the ship can't bear the weight of the people on it.
d. Rohan, as the ship is light in weight.
22. A material which does not dissolve completely into water is called
a. Soluble

b. Insoluble c. Opaque d. Transplant
23. Which one of the following is insoluble in water?
a. Oxygen
b. Sugar c. Salt d. Saw dust
24. Which pair of substances among the following would float in a tumbler half filled with water?
a. Iron sheet, thermocol
c. Pin, oil drops
b. Feather, plastic ball
d. Rubber band, coin
25. While doing an activity in class, the teacher asked Ramu to hand over a translucent material. Which among the following materials will Ramu pick and give the teacher?
a. Glass tumbler
c. Frosted glass
b. Mirror
d. Aluminium foil
26. You are provided with the following materials: i) Magnifying glass ii) Oily paper iii) Stainless steel iv) Glass tumbler
Which of the above materials will you identify as transparent?
a. i and ii
b. i and iii
c. i and iv
d. iii and iv
27. Pick one material from the following which is completely soluble in water.
a. Chalk powder b. Tea leaves c. Glucose d. Saw dust
28. Which of the following will float on water?
a. Iron nails
b. Dry leaves
c. Stone
d. Steel
29. A material that does not dissolve completely into water is called _________.
a. Soluble
b. Insoluble
30. Which of the following best defines "mass"?
a. The amount of space an object occupies.
c. Opaque
d. Translucent
b. The amount of matter in an object.
c. The heaviness of an object due to gravity. d. The shape of an object.
31. Two containers have the same capacity. Container A is filled with water, while Container B is half-filled. Which of the following statements is correct?
a. The space available in both containers is different.
b. The volume of water in both containers is equal.
c. The volume of water in Container B is less because it occupies more space.
d. The volume of water in Container A is less because it occupies less space.
METHODS OF SEPARATION 2
2.1 INTRODUCTION
In our daily lives, we often need different substances for various purposes. However, many times these substances come mixed with other things we don't need. These mixtures can affect the quality of what we use.
Examples,
1. When we have cereals like rice, wheat, or pulses, sometimes there are tiny stones or husks mixed in. Before we cook them, we need to remove these because they can be harmful.
2. Common salt is something we use a lot in our food to make it taste better and to give us nutrients. But it is mixed with a lot of other things when it is in sea water. So, we have to take out the salt from the sea water to use it properly.
We separate mixtures for a few reasons:
(a) Salt with impurities

(b) Cereals with impurities
Fig. 2.1 Mixtures
(i) To get rid of things we don't want or things that can hurt us.
(ii) To get the things we need.
(iii) Sometimes, we need things to be very pure, like water. We need pure water to make medicines, in science labs for making mixtures, in car batteries, and more. So, we have to take out all the impurities from water to make it pure.
Methods of separation
The process by which constituents of a mixture are set apart from one another to get pure substances is called separation.
The choice of method to be used for separation depends upon the properties of the constituents. There are different methods used for the separation of different mixtures.
2.2 SEPARATION OF SOLID-SOLID MIXTURES
2.2.1 Handpicking
This method is used to separate unwanted components that are visible to the naked eye from a mixture. It is helpful when we have a small amount of a mixture and the part we want to remove is easily visible and can be taken out by hand.
Examples,
1. When we find small stones mixed with rice, lentils, or spices, we can simply pick them out by hand.
2. If we have a basket of different coloured balls and we want to separate the red ones, we can do so by hand.
2.2.2 Threshing
Threshing is a method used to separate grains from stalks and other parts of plants. During threshing, the stalks are hit or beaten to release the grain seeds.
This process can be done manually, sometimes with the help of bullocks, or using machines, especially for large amounts of grain.

2.2.3 Winnowing
This is a way to separate grains from their outer covering like husk or hay using the wind. It's a method commonly used by farmers.
For instance, if we have a mixture of rice and husk and we let it fall from a height, the heavier rice grains will fall straight down while the lighter husk gets blown away by the wind and forms a separate heap at a short distance. This way, we can easily separate the rice from the husk.
2.2.4 Sieving


If a mixture has particles of different sizes, we can separate them using a sieve. The holes in the sieve are made to match the sizes of the particles we want to separate.
Examples,
1. Sieving of flour while baking to remove impurities.
2. Separating pebbles and stones from sand.

2.2.5
Sublimation
Sublimation is a process where a solid turns directly into vapour when heated, without going through a liquid phase. This happens with certain solids, like camphor, naphthalene, iodine, and ammonium chloride.
Example: If we have a mixture of salt and ammonium chloride, we can separate them using sublimation.
Here's how we can do it:
When the mixture is heated in a dish covered with an upside-down funnel, ammonium chloride turns into vapour and then condenses back into solid form along the neck of the funnel. Solid ammonium chloride can be scrapped from the funnel, leaving the salt behind in the dish.
2.2.6
Churning
It is a process used to separate butter from buttermilk. This is done using a big churner called a "mathni." When curd is stirred rapidly with the churner, the lighter butter rises to the top, while the heavier buttermilk stays below.
This happens because of a principle similar to what is used in a centrifuge. When liquids are spun or rotated at high speed, the heavier parts move outward, and the lighter parts gather in the center. So, it can be said that churning is a process of separating solid from liquid. For example, when milk is spun, the lighter cream collects in the middle and floats on top of the milk.
2.3 SEPARATION OF SOLID-LIQUID MIXTURES
There are two types of solid-liquid mixtures:
a. Mixture with soluble solids
b. Mixture with insoluble solids
2.3.1 Separation of soluble solids from liquids
a. Evaporation
Evaporation is the process of converting a liquid into its vapour state, either by exposing it to air or by heating. It takes place from the open surface at room temperature. Rate of evaporation increases with increase in temperature.
One common example of evaporation is how salt is obtained from sea water. Sea water is collected in shallow pans and left to evaporate in the sun. As the water evaporates, the salt is left behind.

Can we get the liquid which got evaporated back?
We can get the liquid back by the process of condensation. Condensation is a process of conversion of water vapour into its liquid form on cooling.
Example: Water drops condensed under a plate that has been used to cover a vessel containing water that has just been boiled.
b. Crystallisation
When a solution is evaporated to the point where there is very little solvent left, it is called a concentrated solution. Crystallisation occurs when the concentrated solution is allowed to cool slowly, causing crystals of the dissolved substance to form. This process is used to obtain substances in their pure form. Crystals are solid particles that have a definite shape and size, and they often appear shiny.
Evaporation
Solvent
Impure solid
Stir to dissolve solid
The solution is heated to evaporate most of the solvent
The whole solution is allowed to cool to obtain crystals. Crystals Crystals
Fig. 2.12 Crystallisation process
Filter Paper
The cold solution is poured off to obtain the crystals by pressing them between sheets of filter paper.
To obtain pure sugar from a sugar solution, the solution is first heated to evaporate water quickly. As less water remains in the solution, it is cooled down. During this cooling process, sugar starts to separate out from the solution and forms crystals.
c. Distillation
Distillation is the method of getting a pure liquid from a solution by evaporating and then condensing the vapours.
Clamp Thermometer
Distillation flask
Condenser
Cooling water
Condensed water
Receiving flask
Cold water
Distilled water
Fig. 2.13 Distillation process
It works by heating the solution, causing the liquid part to turn into vapour. This vapour is then cooled down and turns back into a liquid. This new liquid is very pure and is called the distillate. One of the advantages of distillation is that it allows us to separate both parts of a solid-liquid mixture.
Example: Tap water, which has salts dissolved in it, can be purified using distillation. The pure water that is collected after distillation is called distilled water. Doctors use distilled water to make medicines, chemists use it to make solutions, and industries use it for various purposes.
2.3.2 Separation of insoluble solids from liquids
a. Sedimentation and decantation
Sedimentation and decantation are two processes used to separate insoluble solids from solid -liquid mixtures.
Sedimentation happens when heavy solid particles in a mixture settle down over time. The solid that settles at the bottom is called sediment, and the clear liquid above it is called supernatant liquid.
Decantation is when you carefully pour off the clear liquid without disturbing the sediment. This method works for mixtures where the solid is heavier than the liquid and doesn't dissolve in it.
Example: Separation of sand from water, separation of pulses from water.
b. Filtration
The process of separating insoluble solid particles from a liquid by allowing it to pass through a filter is called filtration.
Imagine you have a mixture of sand and water. When you pour the mixture through a filter, the water goes through, but the sand stays behind. The sand that's left on the filter is called the residue, and the water that passes through is called the filtrate.
of liquid and solid
liquid into the container
Filter paper
Semi-permeable paper that separates solids from liquid. Liquid that has passed through filter paper
We can use different materials as filters, like sand, charcoal, cotton, and even paper. Think about when you make tea. The strainer you use to keep the tea leaves out of your cup is a filter!
Example: Separation of mixtures like chalk and water, clay and water, tea and tea leaves, or sawdust and water.
2.4 MODERN TECHNIQUES OF SEPARATION
2.4.1 Chromatography
This is one of the latest techniques to separate the coloured components of a mixture when all the components are very similar in their properties. The word "chromatography" means "colour writing." Earlier it was used to separate mixtures containing coloured components only, but these days this technique is applied to colourless substances too.
The process of separating different dissolved constituents of a mixture by their adsorption on an appropriate material is called chromatography
Fig. 2.16 Chromatography
In this process, common adsorbents used are filter paper, silica gel, etc. and common solvents used are water, ethyl alcohol, acetic acid, etc.
A drop of the mixture is poured on the paper and then the paper is dipped in the liquid. As the liquid moves up the paper, it carries the mixture with it. Some parts of the mixture move faster because they're more soluble, and they leave spots on the paper. These spots show different components of the mixture.
Chromatography can be used to separate pigments from natural colours, drugs from blood (pathological tests), colours in a dye, etc.
2.5 USE OF MORE THAN ONE METHOD OF SEPARATION
We've learned different ways to separate substances from mixtures. Sometimes, one method isn't enough to separate everything. When that happens, we have to use more than one method.
Let us understand this with examples.
Case 1
Imagine we have a mixture of sand, sawdust, and salt. Here's how we can separate them:
The mixture is taken into a glass beaker and water is added to it.
The beaker is allowed to sit for a while without stirring.
The salt dissolves in water creating a salt solution.
Sawdust, being lighter, floats on the water's surface, while sand, being heavier, sinks to the bottom.
Saw dust floats
Salt dissolves in water
Sand settles
Fig. 2.17 Multiple methods used for separation
Salt solution along with the sawdust is poured through a filter paper in a funnel. Sand is left behind in the beaker as sediment.
The salt solution passes through the filter paper, while the sawdust stays on top of it.
Salt is obtained from the salt solution by evaporation.
Case 2
Imagine while attempting to separate salt mixed with sand, we could only recover a small portion of the salt after mixing it with a small amount of sand. What would be the reason behind this? Can you think of a solution?
The issue likely arose because more salt was used than water could dissolve, resulting in a saturated solution where excess salt remained mixed with the sand. Saturated solutions form when no more solute can be dissolved in the solvent.
To resolve this, we can use a larger quantity of water to dissolve all the salt in the mixture. Additionally, heating the saturated solution can increase the amount of salt that can dissolve in the water, allowing for better separation.
QUICK REVIEW
• The process by which constituents of a mixture are set apart from one another to get pure substances is called separation.
• Handpicking is used to separate substances which are different in shape, size, colour and visible to naked eyes.
• Threshing and Winnowing are used to separate lighter components like husk, dirt and dust from heavier substances like grains.
• Sieving is used to separate the substances based on difference in their size.
• Sublimation is a method used to separate the mixture where one of the component is sublime in nature.
• Churning is a process of separating solid from liquid. It works on the principle of centrifugation.
• The conversion of water into water vapours at a temperature less than boiling point is called evaporation. The conversion of water vapours back to water on cooling is called condensation.
• The process of obtaining a substance in its pure form by converting them into crystals is called crystallisation.
• Distillation is the method of getting a pure liquid from a solution by evaporating and then condensing the vapours.
• Sedimentation and decantation are two processes used to separate insoluble solids from solid -liquid mixtures.
• The process of separating insoluble solid particles from a liquid by allowing it to pass through a filter is called filtration.
• The process of separating different dissolved constituents of a mixture by their adsorption on an appropriate material is called chromatography.
• Saturated solutions form when no more solute can be dissolved in the solvent.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Separation of solid– solid mixtures
1. What is the term for the process of separating grains from stalks?
a. Winnowing b. Threshing c. Sieving d. Decantation
2. How can two solid components of different sizes be separated?
a. Sieving b. Filtration c. Evaporation d. Condensation
3. The best technique to separate a mixture of onions and potatoes is:
a. Handpicking b. Sieving c. Winnowing d. Threshing
4. The technique of separating husk from grain is known as:
a. Winnowing b. Threshing c. Sieving d. Decantation
5. What is sublimation?
a. The process of converting a solid directly into a gas without passing through the liquid state.
b. The process of converting a liquid directly into a gas without passing through the solid state.
c. The process of converting a gas directly into a solid without passing through the liquid state.
d. The process of converting a solid directly into a liquid without passing through the gas state.
6. To separate two solids by winnowing, the two solids should have differences in:
a. Weight
b. Colour
c. Size d. Magnetic properties
7. Handpicking, threshing and sieving are methods to remove ______ from ______.
a. Solid, liquid
c. Liquid, solid
b. Solid, solid
d. Gas, liquid
8. A mixture of salt and camphor is heated. The fumes which are evolved will be of
a. Salt
c. Salt and camphor
b. Camphor
d. Water
9. Dry leaves can be separated from sand by the process of
a. Separating funnel
c. Sedimentation
b. Winnowing
d. Handpicking
10. Which of the following methods cannot be used to separate a solid mixture?
a. Hand picking
b. Winnowing
c. Sieving d. Sedimentation
II. Separation of solid– liquid mixtures
1. The transformation of water into water vapour is called:
a. Sedimentation b. Decantation c. Evaporation d. Condensation
2. How can tea leaves be separated from tea?
a. Decantation b. Evaporation c. Filtration d. Sedimentation
3. What is the process of converting water vapours into water?
a. Evaporation b. Condensation c. Dissolution d. Distillation
4. What is the process called when a clear liquid is carefully poured off after sedimentation without disturbing the sediment?
a. Decantation b. Filtration c. Condensation d. Sublimation
5. What is the apparatus used to cool liquid vapours during distillation called?
a. Separating funnel
c. Round bottom flask
b. Condenser
d. Beaker
6. Filtration using a filter paper can be used to separate
a. Water from milk
c. Water from sugar solution
b. Water from oil
d. Water from muddy water
7. The process of obtaining a substance in its pure form by converting them into crystals is called
a. Evaporation b. Crystallisation c. Sublimation d. Sedimentation
8. Salt is obtained from sea water through a process known as:
a. Filtration b. Distillation c. Decantation d. Evaporation
9. What is the term for the process of solid particles settling down in a liquid?
a. Decantation b. Sublimation c. Sedimentation d. Filtration
10. What characteristic allows two components of a mixture to be separated by distillation?
a. Difference in densities b. Difference in boiling points
c. Difference in colour d. Difference in solubilities
11. What is the process of separating butter from buttermilk called?
a. Filtering b. Churning
c. Boiling d. Freezing
12. Why does butter float to the top during the churning process?
a. It is heavier than buttermilk.
c. It dissolves in buttermilk.
III. Modern techniques of separation
b. It evaporates during churning.
d. It is lighter than buttermilk.
1. The process of separating different dissolved constituents of a mixture by their adsorption on an appropriate material is called
a. Distillation
c. Filtration
b. Chromatography
d. Evaporation
2. Which of the following is a common adsorbent used in chromatography?
a. Cotton
c. Aluminum foil
b. Filter paper
d. Plastic wrap
3. What are some common solvents used in chromatography?
a. Water, vinegar, and oil
b. Water, ethyl alcohol, and acetic acid
c. Ethanol, gasoline, and ammonia
d. Hydrochloric acid, bleach, and acetone
4. How does chromatography separate different dissolved constituents of a mixture?
a. By distillation
b. By adsorption on an appropriate material
c. By filtration
d. By condensation
5. In chromatography, why do some parts of the mixture move faster than others?
a. Due to differences in temperature
b. Due to differences in pressure
c. Due to differences in solubility
d. Due to differences in density
IV. Use of more than one method of separation
1. What do you call a solution that cannot dissolve more solute at a specific temperature?
a. Saturated solution
c. Super-saturated solution
b. Condensed solution
d. Unsaturated solution
2. What will happen if a saturated solution of sugar in water is heated?
a. It becomes unsaturated.
c. Sugar starts separating out.
b. It remains saturated.
d. Sugar remains undissolved.
3. Which of the following methods can be used together for the separation of a mixture of sand and salt?
a. Filtration and evaporation
c. Distillation and sublimation
b. Sedimentation and decantation
d. Crystallization and chromatography
4. To separate constituents of a mixture, following methods are adopted: Water + Saw dust + Sugar (X)
Find the methods (X) and (Y) ?
a. (X)⟶ Loading ; (Y)⟶ Condensation
b. (X) ⟶ Filtration ; (Y)⟶ Evaporation
c. (X)⟶ Sedimentation; (Y)⟶ Decantation
d. (X) ⟶ Distillation ; (Y)⟶ Centrifugation
Sugar + Water

5. Select the correct sequence for the separation of mixture of camphor, sand and sugar.
a. Sublimation - Sedimentation and filtration - Evaporation
b. Evaporation - Sublimation - Sedimentation and filtration
c. Sedimentation and filtration - Evaporation - Sublimation
d. Filtration - Sublimation - Evaporation
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which types of materials can be separated by handpicking?
a. Solids with different sizes and colours
b. Solids with the same size and colour
c. Solids which are soluble in water
d. Solids which settle down when dissolved in water
2. What is the process of separating insoluble and suspended solid particles of different sizes from a liquid using a porous separator?
a. Filtration b. Decantation c. Evaporation d. Sedimentation
3. In the following questions, a statement of assertion is followed by a statement of reason.
Assertion: Husk and flour can be separated by the process of sieving.
Reason: Sieving is used when two components of mixture differ in size. Mark the correct choice as:
a. Both assertion and reason are true and reason is the correct explanation of assertion.
b. Both assertion and reason are true but reason is not the correct explanation of assertion.
c. Assertion is true but reason is false.
d. Assertion is false and reason is true.
4. Separation of common salt from sea water is by _________.
a. Sieving
c. Sedimentation
b. Evaporation
d. Winnowing
5. Which of the following properties can affect dissolution of sugar in water?
a. Quantity of sugar
c. Type of water
6. Which technique is used to purify dirty water?
a. Chromatography
c. Winnowing
b. Temperature
d. Humidity
b. Distillation
d. Sublimation
7. Which method is used to separate an insoluble solid from a liquid?
a. Chromatography
c. Filtration
b. Sublimation
d. Distillation
8. Which method can be used to separate a mixture of rice grains and sand?
a. Decantation
c. Chromatography
b. Winnowing
d. Sublimation
9. What method is used to separate ink into its different colours?
a. Sedimentation
c. Evaporation
b. Chromatography
d. Distillation
10. What method can be used to separate a mixture of sand and water?
a. Sedimentation
c. Chromatography
b. Filtration
d. Threshing
11. Which method can be used to separate a mixture of oil and water?
a. Distillation
c. Chromatography
b. Filtration
d. Decantation
12. What method is used to separate a mixture of camphor and sand?
a. Sublimation
c. Filtration
b. Distillation
d. Magnetic separation
13. Which method is suitable for separating a mixture of alcohol and water?
a. Filtration b. Distillation c. Sublimation d. Decantation
14. The rate of evaporation increases with
a. Decrease in temperature
b. Increase in temperature
c. Increase in volume of liquid
d. Decrease in volume of liquid
15. Which of the following mixtures is not solution?
a. Sea water
c. Soda water
16. Mark the incorrect statement.
b. Muddy water
d. Sugar water
a. Distillation can be used to separate a mixture of soluble solid and liquid.
b. Stone pieces from rice can be handpicked.
c. Naphthalene can be separated from a mixture by dissolving it in water.
d. Chromatography is based on the principle of adsorption.
17. Which of the following methods is not correctly matched to give method of separation of the mixture?
a. Common salt and camphor-Sublimation
b. Sugar and water-Evaporation
c. Stones and wheat flour-Handpicking
d. Sawdust and stones-Decantation
18. Match the correct method
List-I
(P) Separate stones from rice
(Q) Separate drugs from blood
1. Chromatography
2. Handpicking (R) Getting sugar in its pure form
(S) Separate chalk powder from water
3. Filtration
4. Crystallisation
a. P-3, Q-4, R-1, S-2
b. P-1, Q-3, R-4, S-2
c. P-2, Q-1, R-4, S-3
d. P-2, Q-4, R-3, S-1
19. Which of the following undergoes sublimation?
a. Iodine
b. Chlorine c. Sulphur
d. Iron
20. What happens when a mixture containing a sublimate is heated?
a. Sublimate is left in China dish.
b. Non-sublimate is left in China dish.
c. Sublimate is deposited on the outer end of funnel.
d. Non-sublimate is deposited on the inner cold surface of the funnel.
21. Paneer or cottage cheese is separated from liquid by using
a. A muslin cloth
c. Alum
b. Charcoal
d. Sieve
22. Which of the following cannot be separated by filtration?
a. Sand and water
c. Salt and water
b. Mud and water
d. Sawdust and water
23. Filtration using a filter paper can be used to separate
a. Water from milk
c. Water from sugar solution
b. Water from kerosene oil
d. Water from muddy water
24. In the following questions, a statement of assertion is followed by a statement of reason.
Assertion: The process of conversion of water vapour into its liquid form is called condensation.
Reason: A mixture of camphor and common salt can be separated by sublimation.
Mark the correct choice as:
a. Both assertion and reason are true and reason is the correct explanation of assertion.
b. Both assertion and reason are true but reason is not the correct explanation of assertion.
c. Assertion is true but reason is false.
d. Assertion is false and reason is true.
25. In the following questions, a statement of assertion is followed by a statement of reason.
Assertion: When the heavier component in a mixture settles after water is added to it, the process is called decantation.
Reason: Sand and water can be separated by sedimentation and decantation.
Mark the correct choice as:
a. Both assertion and reason are true and reason is the correct explanation of assertion.
b. Both assertion and reason are true but reason is not the correct explanation of assertion.
c. Assertion is true but reason is false.
d. Assertion is false and reason is true.
26. During filtration the solid left on the filter paper is called
a. Residue b. Filtrate c. Adsorbent d. Solvent
27. Sieving cannot be used to separate two solids with ______ size.
a. Greater b. Smaller
c. Same d. Different
28. Water is considered as a very good solvent as water can dissolve
a. Only solids
c. Only liquids
b. Solids and liquids
d. Solid, liquids and gases
29. Which of the following cannot be used as a solvent in chromatography?
a. Water
c. Acetic acid
b. Ethyl alcohol
d. Silica gel
30. Pebbles and stones can be removed from sand by _______
a. Distillation b. Winnowing c. Sieving d. Filtration
31. Churning is a process of separating ______ from liquid.
a. Liquid b. Gas c. Solid
d. None of the above
32. What happens to the components of milk when it is spun at high-speed using a centrifuge?
a. The lighter cream collects toward the center.
b. The heavier cream moves outward.
c. Both cream and milk mix together.
d. The milk evaporates completely.

BIOLOGY

COMPONENTS OF FOOD 1
1.1 INTRODUCTION
Food is essential for all living beings, including humans. It provides the energy and nutrients needed for growth and development. In simple terms, food is any substance that living organisms consume to sustain life. Food comes in many forms such, as fruits, vegetables, grains, meat, dairy products, and more. The main functions of food include providing energy for our bodies to function, supporting the growth and repair of tissues, and maintaining body health.
1.2 WHAT DO WE EAT?
1.2.1
Food in different regions
India is known for its rich diversity in food, which is closely linked to the crops grown, climate, soil types, and cultural practices of each region. Traditional foods are based on locally grown crops. In Punjab, wheat and maize are staples, leading to dishes like makki di roti and sarson da saag. Karnataka’s cuisine features rice and ragi, forming dishes like idli, dosa, and ragi mudde. While rice and wheat-based dishes are common nationwide, some foods remain unique to specific regions. Cooking methods have changed significantly over time. Earlier, people used clay stoves (chulha) and stone grinders (sil-batta), which were required a lot of efforts. Modern tools like gas stoves and electrical grinders now make cooking faster and easier.
1.3 WHAT DO DIFFERENT FOOD ITEMS CONTAIN?
Food consumed daily is composed of one or more ingredients. These ingredients are obtained from plants and animal sources that are essential for our bodies. These essential components are known as nutrients. Nutrients are substances found in food that are essential for the growth, development, and maintenance of our bodies. These substances provide the energy and raw materials needed to support various bodily functions, such as metabolism, growth, and repair. The different types of nutrients present in food are carbohydrates, proteins, vitamins and minerals, roughage or fibres and water.
Carbohydrates are macronutrients that provide energy. Proteins are essential for tissue building and repair. The vitamins and minerals support overall health and various biological functions Dietary fibre, or roughage, aids in digestion and promotes a feeling of fullness, found in fruits, vegetables, whole grains, and legumes. Water regulates body temperature, transports nutrients, lubricates joints, and helps our body to be hydrated.
Fig. 1.1 Nutrients obtained from food
Mostly, the food items contain carbohydrates or starch, proteins, and fats. There are certain simpler test methods to estimate the presence of these nutrients. These tests can be performed on raw ingredients or cooked food to find out the amount of nutrients present in it.
1.3.1
Test for starch (carbohydrates)
• Take a sample of the substance you want to test for starch.
• Add a few drops of iodine solution to the sample.
• If starch is present in the sample, the iodine solution will change colour from brown to blueblack.
• This colour change indicates a positive test result for the presence of starch.
• If the sample remains brown after adding iodine, it shows that starch is not present in it.
1.3.2
Test for proteins
• Take a sample of the substance you want to test for proteins.
• Add a few drops of dilute copper sulfate solution to the sample.
• If proteins are present in the sample, a colour change will occur to form a violet or purple colour complex.
• A violet or purple colour change indicates proteins are present.
• No colour change suggests proteins are absent in the sample.
1.3.3
Test for fats
• Take a small amount of the food item and crush it on a piece of paper.

• Check for any oily patch left behind by the food on the paper after crushing.
• Hold the paper against light to see if light passes faintly through the patch.
• If an oily patch is seen, it indicates the presence of fat in the food.
• Let the paper dry to rule out any water content in the food.
• If no oily patch is visible after drying, it suggests the food does not contain fat.
1.4 WHAT DO VARIOUS NUTRIENTS DO FOR OUR BODY?
Each nutrient has different functions in our body to keep us healthy and strong.
1.4.1 Functions of nutrients
Carbohydrate
Carbohydrates serve as the primary energy source, providing fuel for daily activities. Most of the carbohydrates are obtained from plant sources. The plant sources that are rich in carbohydrates are rice, sweet potatoes, maize, sugarcane and more.
Fig.1.2 Sources of carbohydrate
Proteins
Proteins play an important role in building and repairing tissues while contributing to metabolic processes. Food proteins are otherwise known as "body-building foods", which are obtained from plants and animal sources. Some of the plant sources are gram, tuar dal, moong, beans, soya beans and peas. Some of the animal sources are meat, fish, paneer, milk, eggs and more.
Fats
Fats offer energy, facilitate cell growth, protect organs, and aid in the absorption of essential vitamins. The plant sources that are rich in fats are groundnuts, coconut, nuts and so on. The animal sources that are rich in fats are ghee, butter, meat, fish, eggs and so on.
Vitamins
Fig. 1.5 Plant sources of fats
Fig. 1.6 Animal sources of fats
Vitamins are organic compounds that regulate key metabolic functions. There are 13 vitamins that are categorized as water-soluble vitamins, such as vitamin B and vitamin C and fat-soluble vitamins, such as vitamins A, D, E, and K.
Vitamin Role
Food Sources
Vitamin A Keeps skin and eyes healthy. Carrots, papaya, mango
Vitamin C Helps the body fight against diseases. Orange, lemon and amla
Vitamin D Helps the body use calcium for bones and teeth. Fish, egg, liver and milk
Vitamin E Acts as an antioxidant to protect cells. Nuts, seeds, leafy green vegetables
Vitamin K Essential for blood clotting and bone health. Green leafy vegetables, broccoli
Vitamin B-complex Important for energy production, brain function, and metabolism. Wheat, rice and liver


Minerals
Minerals, inorganic elements, support bodily functions such as bone formation, nerve function, and fluid balance. Some of the minerals are iron, calcium, iodine, and so on.
Mineral
Iodine
Phosphorus
Iron
Calcium
Food Sources
Fish, iodized salt, ginger
Meat, dairy products, nuts, whole grains
Red meat, poultry, fish, beans, dark leafy greens
Milk and eggs
Table 1.2 Minerals and their sources
Roughage
Roughage, also known as dietary fibre, is derived from plant foods like whole grains, pulses, fruits, and vegetables. Although roughage does not provide nutrients, it plays an important role in maintaining digestive health by adding bulk to our diet. This fibre helps promote regular bowel movements and aids in the removal of undigested food, supporting overall digestive function.
Water
Water is essential for absorbing nutrients and removing waste from the body. We mainly get water from liquids like water, milk, and tea, as well as from cooked foods. Fruits and vegetables also provide water to our bodies. Drinking enough water is important for staying hydrated and keeping our bodies functioning well.
1.5 BALANCED DIET
1.5.1 Need for a balanced diet
A balanced diet, which consists of the food consumed in a day providing essential nutrients in proper quantities to support growth and overall health, is essential. It is important to ensure that the diet is neither excessive nor deficient in quantity. There must be adequate roughage and water in a balanced diet that aids digestion and hydration.
Nutrient-rich foods such as pulses, groundnuts, soybeans, sprouted seeds, fermented foods, mixed flours, fruits, vegetables, contribute to meet nutritional needs.
COMPONENTS
Eating too much unhealthy food high in fats, sugars, and calories and not enough fruits, vegetables, and whole grains can lead to weight gain and obesity. Obesity is a condition characterized by excessive body weight due to an accumulation of fat that can have negative effects on health. Eating a balanced diet with the right amount of healthy food helps our bodies to get rid of obesity.
1.5.2 Cooking of food
Cooking of food such as cereals, vegetables, and meat enhances taste and aids digestion. However, incorrect cooking methods can diminish food nutrients. Avoid overheating, overboiling, and deep frying, as they can deplete food nutrients. Washing fruits and vegetables after cutting may strip away essential vitamins and minerals in the peels. Excessive washing of rice and pulses can lead to nutrient loss. It is advisable to cook food in minimal water to preserve nutrients, as discarding excess water can result in nutrient loss.
Vitamin C is heat-sensitive and can be lost during cooking, so consuming raw fruits and vegetables is recommended for vitamin C intake.
1.5.3 Nutrition deficiency and diseases

Nutrient deficiency refers to a condition that occurs when the body does not receive an adequate amount of essential nutrients such as vitamins, minerals, proteins, carbohydrates, and fats required for proper functioning and health. When the body lacks these necessary nutrients, it can lead to various health issues and deficiencies, impacting growth, development, immunity, and overall well-being.
Deficiency diseases are health conditions that occur when the body lacks essential nutrients such as vitamins, minerals, and proteins.
Deficiency of carbohydrate
Carbohydrate deficiency, also known as carbohydrate malnutrition, occurs when the body does not receive enough carbohydrates for energy production. This can result in fatigue, weakness, low blood sugar levels, and impaired physical and mental performance.
Deficiency of protein
Protein deficiency, not having enough protein in your body, can cause issues like weak muscles, getting sick more often, slow healing of cuts, and difficulty growing properly.
Protein deficiency can lead to two severe conditions: marasmus and kwashiorkor.
1. Marasmus is a form of severe malnutrition characterized by energy deficiency, causing extreme weight loss, muscle wasting, and overall weakness.
2. Kwashiorkor is a type of protein malnutrition that results in symptoms like swelling, skin issues, and bloated stomach due to fluid retention.
Deficiency of vitamins and minerals
Some diseases caused by deficiency of vitamins and minerals are listed below.
Deficiency Disease Cause Symptoms
Scurvy
Rickets
Anaemia
Goitre
Deficiency of Vitamin C Swelling and bleeding gums, slow wound healing
Deficiency of Vitamin D Softening, bending, and deformity of bones
Deficiency of Iron/B12 Fatigue, paleness, loss of appetite
Deficiency of Iodine Swollen thyroid gland, slow growth, mental retardation
Table 1.3 Deficiency diseases and their symptomsisease
Most of the deficiency diseases can be prevented by taking a balanced diet in day-to-day life.
1.6 MILLETS: NUTRITION-RICH CEREALS
Millets, such as jowar, bajra, ragi, and sanwa, are native crops of India and can be grown in various climatic conditions. These small grains have been part of the Indian diet for centuries and are highly nutritious. They are rich in vitamins, minerals like iron and calcium, and dietary fibers, which are essential for a balanced diet and the normal functioning of the body. Due to their high nutritional value, millets are also referred to as nutri-cereals.
1.7 FOOD MILES: FROM FARM TO OUR PLATE
Food travels through several steps before reaching our plates. This journey, known as food miles, includes farming, threshing, winnowing, storage, grinding, packing, and transportation to shops. Reducing food miles is important as it lowers transportation costs, minimizes pollution, supports local farmers, and ensures fresher, healthier food.
Food on our plate

Transport to retails shop


Story of chapati: from farm to plate
Threshing and winnowing of grains
Storage of grains
Grinding of grains and packing
Fig. 1.8 From farm to plate
We should be mindful of food wastage, as significant effort goes into producing and delivering food. By taking only as much as we can consume, we can respect the hard work of farmers and others involved in the process. This small step can reduce wastage and promote sustainable practices.
QUICK REVIEW
• Food is essential for providing energy and essential nutrients like carbohydrates, proteins, vitamins, minerals, roughage, and water for growth and maintenance of health.
• Tests like iodine for starch, dilute copper sulfate for proteins, and observation of oily patches for fats can be used to detect nutrient presence in food.
• Carbohydrates serve as energy sources; proteins aid in tissue repair and growth, and fats play several bodily roles, including energy storage.
• Vitamins such as A, B, C, D, E and K and minerals like iodine, iron, and calcium have specific roles in bodily functions and are essential for a balanced diet.
• Roughage aids in digestion, while water is necessary for hydration and bodily functions.
• Proper cooking methods and nutrient-rich food choices are key to preserving nutrients.
• Deficiencies in nutrients can lead to various deficiency diseases like scurvy, rickets, anaemia, and goitre, emphasizing the importance of a balanced diet for overall health and well-being.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Introduction and what do we eat?
1. Which of the following components of food provide us with energy?
a. Vitamins
c. Proteins
2. Find the odd one out.
a. Photosynthesis
c. Fat
b. Minerals
d. Carbohydrates and fats
b. Respiration
d. Excretion
3. Which of the following nutrients is required in small quantities?
a. Vitamins and minerals
c. Carbohydrates
4. Find out the correct statement about food.
a. Food provides nutrients that provide energy to our body for performing various activities.
c. It helps build immunity to fight against different diseases.
5. The food components provide our body with:
b. Protein
d. Fats
b. It helps in body growth.
d. All of the above
a. Ingredients b. Nutrients c. Fragments d. Ornaments
6. Which statement best describes the change in cooking methods over time?
a. Traditional tools like sil-batta and chulha required effort, while modern gas stoves and grinders have simplified cooking.
b. Only wheat and rice were cooked traditionally, but modern tools introduced more ingredients.
c. Modern cooking methods have made food preparation more expensive and timeconsuming.
d. Clay stoves have completely disappeared due to modern technology.
7. Which of the following correctly identifies a dish that matches its region?
a. Idli – Punjab
b. Makki di roti – Karnataka
COMPONENTS OF FOOD
c. Ragi mudde – Karnataka
II. What do different food items contain?
d. Sarson da saag – Tamil Nadu
1. When iodine is placed on a piece of potato, what colour does it turn?
a. Blue-black
b. Violet
2. Which of the following food contain fats?
a. Mango
b. Potato
c. Red-brown d. Yellow-red
c. Groundnuts d. Radish
3. What nutrient is indicated by the appearance of an oily spot on paper?
a. Fats
b. Protein
c. Starch d. Minerals
4. Which of the following is used for testing the presence of starch in a given food item?
a. Sugar
c. Iodine
b. Salt
d. None of the above
5. Statement A: Starch gives blue-black while adding iodine solution to it.
Statement B: The presence of fats is tested using the iodine solution.
a. Both the statements are true.
c. Statement A is correct, and statement B is incorrect.
b. Both the statements are false.
d. Statement A is incorrect, and statement B is correct.
6. A colour indicates the presence of proteins in the food item:
a. Violet
b. Green
c. Black d. None
7. Which of the following food items shows a positive result for the protein test?
a. Bread b. Apple c. Egg d. Rice
III. What do various nutrients do for our body?
1. Which nutrient acts as a building block and helps in the body’s repair?
a. Proteins b. Minerals c. Vitamins d. Fats
2. Which is not a fat-soluble vitamin?
a. Vitamin A
Vitamin B
3. Which vitamin has an important role in eyesight?
a. Vitamin A
Vitamin D
4. Which vitamin helps the body defend against disease?
a. Vitamin A b. Vitamin C
Vitamin D
Vitamin K
Vitamin K
Vitamin E
Vitamin E
Vitamin E
5. Which of the following is an animal source of fat?
a. Pulses b. Coconut c. Meat d. Nuts
6. Among the following, which of the minerals is responsible for bone formation?
a. Iron b. Calcium c. Iodine d. Phosphorus
7. Which of the following does not provide nutrients but has an essential role in digestion?
a. Vitamins
b. Minerals c. Proteins d. Roughage
8. Identify the major carbohydrate source from the following.
a. Fish
IV. Balanced diet
b. Eggs
c. Spinach d. Rice
1. Which food group should you eat the most for a healthy diet?
a. Fruits and vegetables
c. Chips and candy
b. Dairy products
d. Fried foods
2. What is a recommended practice to preserve nutrients in food while cooking?
a. Overheating and overboiling food items
c. Washing fruits and vegetables vigorously after cutting
3. What does nutrient deficiency refer to?
a. Consuming the required amount of essential nutrients
c. Eating a balanced diet rich in essential nutrients
b. Deep frying meals for enhanced taste
d. Cooking food in minimal water to prevent loss of nutrients
b. Inadequate intake of essential nutrients required for proper functioning
d. Exercising regularly to maintain nutrient levels
4. What is the result of carbohydrate deficiency in the body?
a. High blood sugar levels b. Improved physical and mental performance
c. Fatigue, weakness, and low blood sugar levels
d. Increased energy production
5. What can occur when the body experiences a deficiency of vitamins and minerals?
a. Improved immune function
c. Development of deficiency diseases
b. Enhanced cognitive abilities
d. Increased energy levels
COMPONENTS OF FOOD
V.
Millets and food miles
1. Which of the following best explains why millets are referred to as "nutri-cereals"?
a. They contain high amounts of carbohydrates and fats.
b. They are rich in vitamins, minerals, and dietary fibers essential for the body's normal functioning.
c. They are easier to cultivate than other grains like rice and wheat.
d. They have been a part of the Indian diet for centuries.
2. Which statement accurately reflects the significance of reducing food miles?
a. It helps increase the export of local produce, making it globally competitive.
b. It ensures that food is cheaper by avoiding taxes on imported items.
c. It supports local farmers, reduces transportation costs, and minimizes pollution.
d. It allows food to be preserved for longer periods, increasing its shelf life.
3. What makes millets adaptable to different climatic conditions?
a. Their ability to retain water and grow in extreme drought conditions.
b. Their ability to retain water and grow in extreme drought conditions.
c. Their small size, which allows for quicker germination and harvesting.
d. The use of genetically modified seeds that can withstand harsh climates.
4. Which of the following correctly describes the sequence of steps food undergoes before reaching consumers, as per the passage?
a. Threshing → Farming → Winnowing → Storage → Packing → Grinding → Transportation
b. Farming → Winnowing → Threshing → Storage → Grinding → Packing → Transportation
c. Farming → Threshing → Winnowing → Storage → Grinding → Packing → Transportation
d. Winnowing → Threshing → Farming → Storage → Grinding → Packing → Transportation
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. When iodine is placed on a piece of bread, what colour does the solution turn?
a. Yellow-red b. Violet c. Red-brown d. Blue-black
2. Which of the following food makes an oily patch on a paper?
a. Fruits b. Rice
c. Green leafy vegetables d. Butter
3. Which of the following is not a vitamin deficiency disease?
a. Marasmus b. Scurvy c. Ricket d. Beriberi
4. Which of the following statements is incorrect?
a. Marasmus is a disease caused by the deficiency of proteins and carbohydrates.
b. Iron is required for the formation of haemoglobin.
c. Calcium and potassium are examples of vitamins.
d. Excessive loss of water from the body causes dehydration.
5. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion (A): Vitamins are essential nutrients needed by the body in small amounts.
Reason(R): Anaemia, goitre, and marasmus are health conditions caused by a lack of vitamins.
a. Both assertion (A) and reason (R) are true, and the reason (R) is the correct explanation of the assertion (A).
b. Both assertion (A) and reason (R) are true, but the reason (R) is not the correct explanation of the assertion (A).
c. Assertion (A) is true, but the reason (R) is false.
d. Assertion (A) is false, but the reason (R) is true.

COMPONENTS
6. Identify the nutrient we get from the food in the given image.
a. Carbohydrates and fats
c. Proteins and fats
7. How does water assist the body in waste removal?
a. By increasing body temperature
c. By aiding in urine and sweat production
b. Vitamins and minerals
d. Carbohydrates only
b. Through the formation of muscles
d. By slowing down metabolic processes
8. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion(A): Both animal and plant sources provide proteins in our diet.
Reason(R): Plant sources like pulses, soybeans, grams, and nuts also offer proteins for consumption.
a. Both assertion(A) and reason(R) are true, and the reason (R) is the correct explanation of the assertion (A).
b. Both assertion(A) and reason (R) are true, but the reason (R) is not the correct explanation of the assertion (A).
c. Assertion (A) is true, but the reason (R) is false.
d. Assertion (A) is false, but the reason (R) is true.
9. Identify the function of the nutrient that is highlighted in a different colour.
a. Body building
c. Digestion of food
b. Defense against disease
d. Provides energy
10. Consider the following characteristics of a food component:
i. Found in rice and potatoes
ii. Converts to glucose in the body
Which of the following food components matches the description?
a. Roughage
b. Protein
c. Carbohydrate d. Vitamin
11. Which of the following diseases occurs due to the deficiency of vitamin D?
a. Rickets
c. Night blindness
b. Scurvy
d. Anaemia
12. Which of the following serves as the source of immediate energy?
a. Glucose
b. Fats
13. Which mineral is necessary for the transport of oxygen?
a. Iron
b. Calcium
c. Starch
c. Sodium
d. Proteins
d. Potassium
14. Which of the following conditions would you recommend consuming iodized salt for?
a. Beri-beri
b. Goitre
15. What nutrient deficiency leads to anaemia?
a. Vitamin B1
c. Iron
16. Select the correct match out of the following.
a. Vitamin C -Seafood
c. Protein -Potatoes
c. Scurvy
b. Vitamin B12
d. Both (b) and (c)
b. Calcium -Milk
d. Iron -Grapes
d. Rickets
17. Rita made a paste of two food items, P and Q each, and put them in two test tubes. She added dilute iodine solution to food item P and copper sulphate solution to food item Q. She observed that P turned blue-black and Q turned violet. Which of the following is correct regarding this?
a. Q is a starch-rich food
c. Q is a fat-rich food
b. P is a starch-rich food
d. P is a protein-rich food
18. Identify the rich sources of roughage from the following options:
a. Milk, meat, soybean, grains
c. Broccoli, fruits, carrot, spinach
b. Egg, curd, pulses, fruit juice
d. Onion, yoghurt, cheese, pulses
19. For what reason is it recommended to consume some vegetables without cooking?
a. They are fresh.
b. They are cold.
COMPONENTS
c. Vitamins are preserved.
20. Identify the incorrect pair.
a. Vitamins - Protect against disease
c. Proteins - Prevent constipation
d. Germs grow in cooked food.
b. Fats - Provide energy
d. Minerals - Keep bones healthy
21. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion(A): Milk, fruits, vegetables, and juices are considered good sources of water.
Reason(R): The role of water involves regulating the body temperature.
a. Both assertion (A) and reason (R) are true, and the reason (R) is the correct explanation of the assertion (A).
b. Both assertion (A) and reason (R) are true, but the reason (R) is not the correct explanation of the assertion (A).
c. Assertion (A) is true, but the reason (R) is false.
d. Assertion (A) is false, but the reason (R) is true.
22. Select whether the given statement is true or false:
1. Soybeans and peas aid in the construction and repair of body tissues.
2. Papaya and oranges are abundant in vitamin C.
3. Dietary fibres provide nutrition and are vital components of food.
a. 1- True, 2- true, 3-true.
c. 1- False, 2- True, 3- True.

b. 1- True, 2- false, 3-false.
d. 1- True, 2- false, 3-true.
23. Identify the mismatched pair from the options provided:
a. Vitamins and minerals - Protective foods
b. Anaemia - Caused by deficiency of iron
c. Pulses and eggs - Rich sources of proteins
d. Roughage - Easily digested by our body
24. In the following question, a statement of assertion is followed by a statement of reason. Mark the correct choice.
Assertion(A): Rickets in children are caused by the deficiency of vitamin D.
Reason(R): Vitamin A is present in carrots.
a. Both assertion (A) and reason (R) are true, and the reason (R) is the correct explanation of the assertion (A).
b. Both assertion (A) and reason (R) are true, but the reason (R) is not the correct explanation of the assertion (A).
c. Assertion (A) is true, but the reason (R) is false.
d. Assertion (A) is false, but the reason (R) is true.
25. Which of the following is a fat-soluble vitamin?
a. Vitamin C
c. Vitamin D
b. Vitamin B2
d. Vitamin B12
GETTING TO KNOW PLANTS 2
When we step outside and look around, we notice many different types of plants. Some are small, others are tall, and some are just small patches of green on the ground. They come in various shapes and sizes. We might see plants with green leaves or reddish ones. Some plants have big red flowers, while others have tiny blue ones, and some may not have any flowers at all. Plants are everywhere around us. Now, let's learn about the different parts of a plant.
2.1 CLASSIFICATION OF PLANTS
Plants can be classified based on the thickness of their stems and the point from which their branches grow. These groups include:
1. Herbs
2. Shrubs
3. Trees
4. Climbers
5. Creepers
2.1.1 Herbs
• Herbs are characterised by their soft, tender, and non-woody green stems.
• Typically, herbs complete their life cycle within a year, making them annual plants.
• Herbs generally do not grow very tall, typically not more than a meter.
• Herbs are known for their aromatic leaves. It serves various purposes, including culinary, medicinal, and ornamental uses.
• Examples of herbs include tomato, sunflower, basil, parsley, mint, coriander, oregano, and rosemary.




2.1.2 Shrubs
• Shrubs are woody plants. Their branches generally originate from the base of their stems.
• They are typically smaller than trees but larger than herbs, ranging from a few feet to several meters in height.
• Shrubs have a longer lifespan compared to herbs, often lasting for many years.
• These plants are characterised by their dense foliage, which may include leaves, flowers, and sometimes fruits.
• Shrubs are commonly used in landscaping to provide structure, texture, and colour to gardens and outdoor spaces.
• Examples of shrubs include rose, jasmine, croton, holy basil (tulsi), Bougainvillea, china rose, pomegranate, and henna.




2.1.3 Trees
• Trees are large, woody plants characterised by a single main trunk that supports branches and foliage.
• They are typically taller than shrubs, often reaching several meters or more.
• Trees have a long lifespan, often lasting for many years or centuries.
• These plants play vital ecological roles, providing oxygen, habitat, and food for various organisms.
• Trees come in a wide variety of shapes, sizes, and forms, ranging from towering evergreens to spreading deciduous species.
• They contribute to the beauty of landscapes and are often planted for shade, timber, or ornamental purposes.
• Examples of trees include neem, mango, palm, teak, oak, sandalwood, coconut, Eucalyptus, and banyan.




(a) (b) (c) (d)
Characteristics
Size
Nature of stem
Type of branches
Examples
2.1.4 Creepers
These are short plants with a height of less than 1m
Shrubs are mediumsized plants with a height of around 1-3m
Green and soft stem, have a few branches Hard and woody stem, not very thick.
Branches are either absent or rarely present
Mint, tomato, coriander, wheat, spinach, paddy, grass etc.
Branches arise from the base of the stem; hence, it has a bushy appearance
Rose, Hibiscus, lemon, henna, croton, etc.
Tallest plant with a height of more than 3-4 m
Thick and woody stem.
Branches arise from the upper part of the stem at some distance from the ground
Banyan, coconut, mango, neem, oak, cashew, etc.
Table 2.1 Difference between herbs, shrubs, and trees
• Creepers are plants that have soft, weak, and green stems and hence cannot stand straight; instead, they spread on the ground.
• Some creepers use tiny roots to cling onto surfaces or climb upwards.
• Creepers are found in gardens, where they cover the ground and add greenery.
• Some creepers even produce flowers or fruits.
• Examples of creepers include watermelon, pumpkin, and strawberry.


2.1.5 Climbers
• Climbers are plants that have soft and weak stems.
• They grow upwards by attaching themselves to other structures or plants for support.
• They have special structures like tendrils or hooks that help them cling to surfaces.
• Climbers can be found in forests, gardens, and even indoors, where they use walls, fences, or trees to climb.
• Examples of climbers include grapevines, money plants, morning glories, and peas.


Plants have two main parts: the part above the ground, called the shoot system, and the part below the soil, known as the root system.
The shoot system consists of the stem, branches, leaves, flowers, and fruits, while the roots make up the root system.
2.2 ROOTS
The root is the part of the plant found under the soil. They are responsible for absorbing essential minerals and water from the soil and transferring them to the stem. This process enables the stem to distribute these nutrients to all parts of the plant. An equally important role of roots is anchoring the plant firmly in the soil, which supports its upright position. Additionally, roots store important nutrients and food necessary for the plant's growth and development.
2.2.1 Types of roots
Roots are classified into two types:
1. Taproot
2. Fibrous root
Taproot

• In plants with a taproot system, there is a main root that grows deep into the soil called the primary root, accompanied by smaller roots branching out from it, known as lateral roots.
• Plants with taproots are firmly anchored in the soil, making them difficult to pull out.
• Examples of plants with tap roots are carrots, radishes, sweet potatoes, turnips, and tapioca.
Primary root
Secondary root
Tertiary root
Rootlets
Fibrous root
• In this type of root system, a group of similar-sized roots emerge from the base of the plant.
• They do not have a main root.
• Plants with fibrous roots are relatively easy to pull out as they do not go very deep in the soil.
• Examples of plants with fibrous roots are banana, wheat, maize, onion, bamboo, etc.
Tap Roots
Taproot has one prominent and long root (primary root) and a bunch of smaller roots that grow from this main root called lateral root.
It is hard to pull out plants with tap roots as they penetrate deep within the soil.
This root system is seen to exist in plants with leaves displaying reticulate venation.
Examples of plants with tap roots are carrots, turnips, gram, China rose, etc.
Fibrous Roots
Fibrous roots have similar-sized roots that emerge from the base of the plant. They do not have a main root.
These plants are easier to pull out as the roots are not very deep in the soil.
This root system is seen to exist in plants with leaves displaying parallel venation.
Examples of plants with fibrous roots are banana, wheat, maize, onion, bamboo, etc.
Table 2.2 Difference between taproot and fibrous root
2.2.2 Modifications of root
Plants grow in different areas of the world and in many different climates. They need to adapt themselves to survive in different conditions. The roots of some plants modify themselves to adapt to these areas and climates. Modification of roots takes place both in taproots and fibrous roots.
Some of these modifications are as follows-
i. Storage roots: The roots of many plants, like carrots, radishes, turnips, sweet potatoes, etc., are edible. These roots store food in them and, therefore, are swollen and fleshy. The food made by the green leaves travels downwards and is stored in these roots. Plants use this food when conditions are not favourable.
ii. Supporting roots: The banyan tree has roots that grow vertically downwards from its branches. As these roots reach the ground, they fix themselves in the soil and give support to the spreading branches of the huge tree. Such roots are called prop roots. In sugarcane, maize, etc., stilt roots arise obliquely downwards from the stem and penetrate the soil to provide extra support to the plants.
iii. Climbing or clinging roots: Some climber plants, e.g., money plant, betel, ivy, etc., possess climbing or clinging roots. These roots penetrate the cracks of the support and hold the support firmly by forming claws, swollen discs, or secreting a sticking juice at their tips.
2.3 STEM
The stem is part of the plant that supports branches, leaves, buds, flowers, fruits, and seeds. It helps transport fluid from roots to other parts of the plant. The stem grows towards the source of light in most of the plants. A node is a point from where the branches and leaves arise on the stem. In between two nodes, the portion is called an internode. The angle present between the stalk of a leaf and the stem is called the axil.
2.3.1 Functions of stem
• The stem keeps the plant upright.
• It holds leaves in position and helps them to spread out as the stem and its branches grow.
• The stem helps to conduct water and minerals from the roots to the leaves.
• It carries the food manufactured by the leaves to other parts of the plant.
2.3.2 Modifications of stem
Stem modifications help them to perform different functions. Some of the stem modifications are as follows-
Storage of food: In some plants, the stem stores food prepared by the leaves underground. For e.g., tuber in potato, rhizome in ginger and turmeric, etc. These underground stems are different from roots as they have nodes and internodes, scale leaves, buds, and adventitious roots.
Preparation of food: In some plants like cactus, the stem is green and succulent. The stem carries out photosynthesis as the leaves are reduced to spines.
Stem tendrils: Tendrils are thread-like sensitive structures that coil around a support to help the plant climb up, e.g. a grapevine.
Multiplication: New plants arise from the stem cuttings, e.g., rose, jasmine, and Hibiscus.
Protection: In some plants, stems are modified into thorns and prickles to protect the plant from being eaten by herbivores, e.g., roses and Bougainvillea
Storage of water: Stems of plants like cactus and jade swell up to store water in them.
Photosynthetic
Succulent stem of cactus
Stem tendril of grapevine
Fig. 2.11 Modifications of stem
2.4 LEAVES
Leaves are vital organs of plants, serving as the primary sites for photosynthesis and playing a crucial role in the plant's overall health. A leaf consists of three main parts: the leaf blade (or lamina), the petiole, and the veins.
1. The leaf blade is the flat, green part of the leaf responsible for capturing sunlight.
2. The petiole is a narrow stalk that connects the leaf blade to the stem, providing support and allowing for movement. The leaves attached to the stem through a petiole are called a petiolate leaf. The leaves directly attached to the stem without petiole are called sessile leaves
3. Veins are vascular tissues that run through the leaf, comprising the midrib and side veins. Midrib is a thick vein that runs along the centre of the leaf blade, providing structural support and transporting water and nutrients. The side veins branch off the midrib, and the side veins distribute water and nutrients to different parts of the leaf. The veins perform the dual function of transporting water and food throughout the leaves and provide structural support.
2.4.1 Types of leaves
The leaves may be simple or compound.
Simple leaves
In simple leaves, the lamina of the leaf is undivided and connected to the petiole, which grows from the bud of a stem. Plants like mango, Hibiscus, and banana have simple leaves.
Compound leaves
In compound leaves, the lamina is divided into small leaves called leaflets. Leaflets do not have a bud, and they grow from one common bud of a stem. Compound leaves are further classified into two types: pinnately compound and palmate compound leaflets.
Pinnately compound leaflets have a common axis known as midrib. Palmate compound leaflets are attached to the tip of the petiole, forming a palm-like structure. Plants like Neem, Mimosa pudica, and Acacia have compound leaves.
2.4.2
Leaf venation
The arrangement of veins in a leaf is called venation. There are two main types of venation:
Reticulate venation
In this type, veins form a net-like pattern on both sides of the midrib of the leaf. It's commonly found in plants with taproots like peepal, mango, neem, Petunia, rose, Hibiscus, and many others.


Parallel venation
In this type of venation, veins run parallel to each other along the length of the leaf. This type of venation is typical in plants with fibrous roots, such as wheat, tulip, banana, palm, grasses, etc.
2.4.3 Functions of leaves
The leaves serve the following functions in a plant-
• The main function of leaves is photosynthesis, i.e., the synthesis of food for the whole plant. All living organisms depend directly or indirectly on photosynthesis to obtain their food.
• Stomata present on leaves carry out the process of transpiration.
Photosynthesis
In most plants, leaves are typically green due to the presence of a pigment called chlorophyll. Through the process of photosynthesis, leaves utilise sunlight, water from the soil, and carbon dioxide from the air to produce food. Chlorophyll captures sunlight, providing the energy necessary for this process. As a result of photosynthesis, oxygen is released as a byproduct.
During photosynthesis, leaves produce sugar, primarily in the form of glucose. This glucose may further convert into starch and is stored within various parts of the plant, such as fruits, roots, stems, or leaves. Plants utilise some of this stored food to sustain their life processes.
Additionally, the stored food serves as sustenance for herbivores, which are, in turn, consumed by carnivores. Thus, leaves play a crucial role in providing sustenance to the entire ecosystem.
Transpiration
Transpiration is the process through which plants lose water in the form of water vapour with the help of stomata present on the surface of their leaves. This phenomenon is vital in the water cycle as it contributes significantly to the release of water into the atmosphere. Moreover, transpiration has a cooling effect on the plant's body.
Transpiration also facilitates the transport of nutrients within the plant by pulling water, along with essential nutrients, upwards from the roots. This upward movement of water and nutrients is crucial for the overall health and growth of the plant.
GETTING
2.4.4
Modifications of leaf
In some plants, the leaf or part of the leaf is modified to perform some special functions. Some of the modifications of the leaf are as follows-
Support: Leaves are modified into tendrils that coil around a support and help the plant to climb. E.g., peas, lentils, etc.
Storage of food: Leaves of some plants store extra food and are mostly eaten as vegetables, e.g., spinach, cabbage, lettuce, etc.
Multiplication: The leaves of some plants have buds that can grow into new plants. E.g., Bryophyllum
Protection: In some plants, e.g., cactus, leaves are modified to form spines. This prevents the loss of water from the leaves by the process of transpiration. Spines also protect the plant from grazing animals, as in prickly poppy.
2.5 FLOWER
The flower is the most attractive part of flowering plants (angiosperms). Flowers are important plant parts as they help plants in reproduction. In different plants, flowers vary in shape, size, and colour. A typical flower consists of a stalk called a pedicel that joins it to the stem. The uppermost part of the pedicel is wider and is known as the thalamus. On the top of the thalamus, the different parts of the flower are arranged in four whorls. These four whorls are as follows:
1. Sepals: The outermost whorl consists of green, leaf-like structures called sepals. These protect the flower during its development, i.e., in the bud stage, and also support the petals when the flower blooms. The whorl formed by sepals is called calyx.
2. Petals: Petals may be white or brightly coloured and often scented to attract insects, birds, etc., which help in pollination. The whorl formed by petals is called the corolla.
3. Stamens: Stamens are the male reproductive parts of the flower. Each stamen consists of a thin, green stalk called filament with a bilobed sac-like structure called anther. The filaments are attached to the thalamus. The anther contains pollen grains that contain male gametes and help in reproduction. Stamens are collectively called androecium
4. Pistil: In the centre of the flower is the female reproductive part called the pistil or carpel. It is also called gynoecium. The pistil consists of three parts: the basal swollen part of the pistil is called an ovary, the short tube-like upper part is style, and the knob-like structure at the tip of the style is the stigma. Stigma receives pollen grains from anthers. Inside the ovary are small bead-like structures called ovules. Ovary and ovules finally develop into fruit and seeds, respectively.
Flowers show a wide range of structures across different plant species. The arrangement of sepals, petals, stamens, and carpels (pistils) can vary greatly.

Most flowers contain all four whorls: sepals, petals, stamens, and carpels, and are referred to as complete flowers. Examples include Petunia, pea, mustard, Hibiscus, and gulmohar.
However, some flowers lack one or more of these whorls and are termed incomplete flowers. Examples of such flowers include those of the date palm and mulberry.
The calyx and corolla, consisting of sepals and petals, respectively, are considered accessory or nonessential whorls as they are not directly involved in reproduction.
On the other hand, stamens and pistils form the essential whorls. These structures serve as the male and female reproductive organs of the flower, playing a direct role in the process of reproduction.
Functions of flower
• The flower is the reproductive organ of a plant and leads to the formation of fruits and seeds.
• Flowers provide nectar to insects.
• Some flowers are also edible.
2.6 STRUCTURE OF OVARY
The ovary is the swollen basal part of the pistil, located at the bottom of the flower. It contains ovules that develop into seeds after fertilisation. The ovary is surrounded by one or more carpel walls. Within the ovary, there are one or more compartments called locules, each containing one or more ovules.
2.7 POLLINATION
The process of transfer of pollen grains from the anther to the stigma of the same flower or another flower of the same kind by wind, water, insects, birds, bats, etc., is known as pollination.
After pollination, the ovary swells up and changes into a fruit, and the ovules become seeds. A fruit contains a fruit wall and seeds. Pollination is of two types self pollination and cross pollination.
QUICK REVIEW
• Plants are typically categorised into several groups based on their height, stem structure, and branching patterns, namely herbs, shrubs, trees, climbers and creepers.
• Roots play a vital role in absorbing water and minerals from the soil and provide anchorage to the plant. They are generally classified into taproots and fibrous roots.
• The stem of a plant serves as a support structure for leaves, flowers, and fruits.
• The stem facilitates the transportation of water from roots to leaves and other plant parts while also transporting food synthesised in the leaves to other areas of the plant.
• A typical leaf consists of a petiole and a lamina, with the arrangement of veins on the lamina termed venation, which can either be reticulate or parallel.
• Green leaves perform photosynthesis, utilising carbon dioxide and water in the presence of sunlight to produce food for the plant.
• Plants exhibiting leaves with reticulate venation typically possess tap roots, while those with parallel venation tend to have fibrous roots.
• The essential parts of a flower include sepals, petals, stamens, and pistils.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Classification of plants
1. What type of plant is characterised by having a soft, non-woody stem and typically grows close to the ground?
a. Herb b. Shrub c. Tree d. Climber
2. Which type of plant is known for its woody stem and relatively small size compared to trees?
a. Herb b. Shrub c. Tree d. Creeper
3. Which type of plant typically requires support structures to grow vertically and often wraps itself around other plants or objects for support?
a. Herb b. Shrub c. Tree d. Climber
4. What distinguishes a shrub from a tree?
a. Height b. Leaf size
c. Stem thickness
d. Flower colour
5. Which of the following is the correct match between the characteristics of the stem and the category of plant?
a. Weak stem which cannot stand upright: Creeper
b. Green tender stem: Shrub
c. Thick, hard stem with branching near the base: Tree
d. Thick, hard stem with branches high on the plant: Herb
II. Roots
1. Which of these plants has a taproot?
a. Maize
b. Wheat
2. Which of these plants store food in their roots?
a. Pea and radish
c. Potato and pea
c. Grass
d. Carrot
b. Onion and garlic
d. Carrot and turnip
3. Fibrous roots are present in which of the following plants?
a. Wheat
b. Sunflower c. Basil d. Mango
4. The underground plant part which fixes the plant to the soil is
a. Root
b. Trunk c. Stem
5. Which of the following plants is eaten by us as a modified root?
a. Cassava
c. Potato
6. Which of the following are functions of roots?
A: Roots make food for the plant.
B: Roots anchor the plant firmly to the soil.
C: Roots take in carbon dioxide from the water.
D: Roots take in water and nutrients from the soil.
a. A and C only
c. A, B, and D only
b. Sweet potato
d. Both (a) and (b)
d. Leaf
b. B and D only
d. A, C, and D only
7. ' A ' comes out first from the seed when it is kept on wet cotton wool. Which of the following is not a function of ' A '?
a. Anchoring the plant
c. Absorption of minerals
8. Which of the following is classified correctly?
a. Banana: fibrous root: reticulate venation
b. Banyan: tap root: parallel venation
c. Maize: fibrous root: reticulate venation
d. Rose: tap root: reticulate venation
III. Stem
b. Prevention of soil erosion
d. Conduction of food

1. The underground stem of the onion modified for storing food is
a. Tuber b. Bulb c. Rhizome d. Corm
2. Stem of a plant
a. Conducts water and minerals
c. Supports the plant
b. Conducts food
d. All of these
3. Which of the following are not the functions of the stem?
(i) Conduction of water and minerals from leaves to the roots.
(ii) Anchoring the plant firmly into the soil by binding with soil particles.
(iii) Keeping the plant upright.
(iv) Conduction of food in the plant.
a. (i) and (iii) only
c. (i) and (ii) only
b. (ii) and (iii) only
d. (ii) and (iv) only
4. In some plants like cucurbit, gourd, etc., the stem is weak and needs support. Some thread-like structures arise from the stem that helps the plants to climb. These structures are called:
a. Stem tendrils
c. Spines
b. Leaf tendrils
d. Thorns
5. Which stem modification is commonly seen in plants like strawberry and spider plants?
a. Stolon
b. Rhizome
1. The flat green part of a leaf is called
a. Petiole
b. Lamina
2. Transpiration mainly takes place through
a. Roots
b. Leaves
3. Which of the following is not a function of leaves?
a. Photosynthesis
c. Water absorption
4. Which of the following is not a correct match?
a. Petiole: attaches the leaf to stem
c. Margin: gives shape to the leaf
c. Bulb
d. Tuber
c. Vein
c. Stems
b. Transpiration
d. None of these
d. Midrib
d. Sepals
b. Lamina: green flat part of leaf
d. Veins: transpiration
5. Which of the following would you observe in grass?
a. Parallel venation and fibrous root
c. Reticulate venation and fibrous root
b. Parallel venation and tap root
d. Reticulate venation and tap root
6. Which of the following combinations of features will be observed in the rose plant?
a. Parallel venation and fibrous root
c. Reticulate venation and taproot
1. Which of these is the male part of a flower?
a. Sepal
b. Petal
2. Which of the following parts turns into a fruit?
a. Thalamus
b. Anther
b. Parallel venation and taproot
d. Reticulate venation and fibrous root
c. Stamen
d. Pistil
c. Ovary
d. Ovule
3. Select the incorrect pair out of the following.
a. Petals - five in number in China rose
b. Stamen - consists of filament and stigma
c. Pistil - innermost whorl of a flower
d. Ovary - contains ovules
4. Select the incorrect statement.
a. All the flowers possess sepals, petals, stamens, and carpels.
b. The number of sepals is equal to the number of petals in all the flowers.
c. Stamens are always less than the pistils in a flower.
d. All of these
5. The part of the flower that is usually brightly coloured and attracts insects is
a. Sepal
b. Petal
c. Stamen d. Carpel
6. Refer to the given diagram and select the correct option regarding the labelled parts.
a. Part P are green leaf-like structures.
b. Part Q is usually brightly coloured.
c. Part R is the female part of a flower.
d. Part S changes into fruit after fertilisation.
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. A medium-sized plant with a hard, brown but thin stem is known as
2. Which of the following is incorrect regarding climbers?
a. They cannot stand upright on their own.
b. They have weak stems.
c. They are medium-sized plants with thick woody stems.
d. Pea and grapevine are climbers.
3. Select the option that contains only herbs.
a. Rose and Hibiscus
c. Rose and lemon
b. Money plant and beanstalk
d. Coriander and wheat
4. Which among the following is correct regarding the type of venation and the plant in which it is present?
a. Reticulate venation in peepal
c. Parallel venation in China rose
b. Reticulate venation in maize
d. Parallel venation in pea
5. Which of the following statements correctly describes transpiration?
a. Synthesis of food in the presence of sunlight
b. Absorption of water and minerals from soil
c. Conduction of water and minerals
d. Release of water vapours by leaves
6. In which of the following plants are prop roots commonly found?
a. Wheat b. Maize
7. The main function of roots in a plant is to:
a. Carry out photosynthesis
c. Store food
c. Sunflower d. Corn
b. Anchor the plant in the soil
d. Produce flowers
8. What feature distinguishes a climber from a creeper?
a. Leaf shape
c. Mode of attachment
b. Flower colour
d. Stem thickness
9. In plants like sweet potatoes, which part is modified to store food?
a. Stem b. Leaf c. Root d. Flower
10. Which of the following is a modified stem that grows underground and consists of short, thickened storage leaves?
a. Rhizome b. Stolon c. Bulb d. Tuber
11. During the process of photosynthesis
a. Carbon dioxide is absorbed
c. Oxygen is not required
12. In reticulate venation,
a. Midrib is not present
c. Veins form a network
b. Sunlight is required
d. All of these
b. Veins run parallel to each other
d. None of these
13. Which among the following leaves shows parallel venation?
a. Banana
b. Mango
c. Banyan
14. The point on the stem from where the leaf arises is:
a. Petiole
b. Lamina
c. Node
15. Which of the following sets of flowers contains incomplete flowers?
a. Hibiscus and Petunia
c. Pea and mulberry
d. Guava
d. Trunk
b. Gulmohar and mustard
d. Date palm and mulberry
16. Mayuri enclosed the leafy branch of a well-watered potted plant in a polythene bag and kept it in sunlight for 5-6 hours to demonstrate the process of transpiration. However, water droplets did not appear inside the polythene bag. What could have been the reason for the failure of the experiment?
a. She kept the plant in sunlight
c. She watered the plant
b. She selected a leafy branch
d. None of these
17. Deepa was given some plants or twigs of plants like coriander, wheat, neem, maize, banana, and China rose. From them, she was asked to select two plants, one of which is an herb with taproot and leaves showing reticulate venation and the other an herb with leaves showing parallel venation and fibrous roots. Which two plants would you select for the same?
a. Coriander, wheat
c. Wheat, maize
b. Neem, maize
d. China rose, neem
GETTING TO KNOW PLANTS
18. Assertion: Stem is like a two-way street in a plant.
Reason: Stem conducts water and minerals from roots to leaves and other plant parts (upward) and also conducts food from leaves to roots and other plant parts (downward).
a. If both assertion and reason are true, then the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
19. Assertion: The flat, green portion of the leaf is called lamina.
Reason: Lamina is attached to the stem by a narrow stalk called a petiole.
a. If both assertion and reason are true, then the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
20. Assertion: We cannot easily pull out a plant from the soil.
Reason: The roots of a plant hold it firmly in the soil.
a. If both assertion and reason are true, then the reason is the correct explanation of assertion.
b. If both assertion and reason are true, but reason is not the correct explanation of assertion.
c. If the assertion is true but the reason is false.
d. If the assertion is false but the reason is true.
21. Which of the following is not the primary function of the stem?
a. Conduction of water
c. Formation of branches
b. Photosynthesis
d. Bears flowers and fruits
22. Which of the following terms constitutes the female part of the flower?
a. Sepals, petals, and stamen
c. Ovary, stamen, and stigma
b. Stigma, style, and ovary
d. Ovary, style, and stamen
23. Which of the following is a function of flowers in a plant?
a. Absorb sunlight
c. Attract pollinators
b. Conduct photosynthesis
d. Anchor the plant
24. The green pigment responsible for photosynthesis in plants is called:
a. Chlorophyll b. Anthocyanin c. Xanthophyll d. Carotene
25. Which of the following is a characteristic feature of monocotyledonous plants?
a. Parallel venation in leaves
c. Taproot system
26. Pollination is the process of:
a. Seed formation in plants
b. Fertilisation in plants
b. Netted venation in leaves
d. Flower parts in multiples of four or five
c. Transfer of pollen from male to female reproductive organs
d. Absorption of water by plant roots
27. Which of the following agents is not involved in pollination?

a. Wind b. Insects c. Water d. Fungi
28. In a compound leaf, what is the structure that attaches the leaflets to the main stem?
a. Petiole b. Blade c. Rachis d. Vein
29. Which of the following is a non-flowering plant?
a. Rose b. Mango c. Pine d. Tulip
30. Identify the incorrect statement:
a. The stem is the ascending part of the axis bearing branches, leaves, flowers, and fruits.
b. Stem develops from the radicle of the embryo of a germinating seed.
c. Stem is divisible into nodes and internodes.
d. Dry membranous scale leaves of the bulb are called tunics.
ANSWER KEY
Physics
1: MEASUREMENT OF LENGTH AND MOTION
Worksheet 1
I. Transportation
1. c 2. b 3. c 4. c 5. b
6. c
II. Measurement
1. c 2. b 3. b 4. a 5. b
III. Units of measurement
1. d 2. b 3. c 4. b 5. c
IV. Measurement of length
1. c 2. b 3. d 4. d 5. c
V. Motion and its types
1. b 2. a 3. c 4. a 5. c
Worksheet 2
1. c 2. b 3. b 4. a 5. d
6. c 7. d 8. d 9. d 10. d 11. a 12. a 13. b 14. b 15. c
16. c 17. d 18. d 19. b 20. b
21. b 22. d 23. b 24. b 25. a
2: LIGHT, SHADOWS, AND REFLECTION
Worksheet 1
I. Light
1. c 2. c 3. c 4. d 5. b
6. a 7. d 8. c 9. b 10. c
II. Shadow
1. d 2. b 3. c 4. b 5. b 6. b 7. c 8. d 9. b 10. b
III. Mirrors and reflection
1. b 2. d 3. c 4. d 5. b
6. a 7. b 8. d 9. c 10. a
Worksheet 2
1. d 2. b 3. a 4. c 5. a
6. b 7. b 8. a 9. d 10. c
11. c 12. c 13. a 14. c 15. a
16. d 17. c 18. d 19. a 20. d
3: ELECTRICITY AND CIRCUITS
Worksheet 1
I. Electric cell
1. b 2. c 3. a 4. b 5. b
6. c
II. Electric bulb
1. b 2. d 3. c 4. b 5. b
6. d
III. Electric circuit
1. b 2. b 3. a 4. b 5. c
6. c
IV. Electric switch
1. b 2. b 3. d 4. a
V. Electric conductors and insulators
1. a 2. a 3. b 4. c 5. c
Worksheet 2
1. b 2. b 3. a 4. b 5. a
6. c 7. b 8. c 9. b 10. c
11. d 12. d 13. a 14. d 15. d
16. b 17. c 18. c 19. a 20. b
4: EXPLORING MAGNETS
Worksheet 1
I. Discovery of magnets
1. b 2. d 3. d 4. d 5. d
6. c 7. d 8. c 9. d 10. a
II. Magnetic and non-magnetic materials
1. c 2. d 3. a 4. b 5. d
III. Magnetic properties
1. a 2. c 3. a 4. a 5. c
6. a 7. b 8. b 9. a 10. b
IV. Care of magnets
1. a 2. c 3. a 4. d 5. d
Worksheet 2
1. b 2. d 3. a 4. d 5. b
6. b 7. c 8. c 9. b 10. d
11. c 12. a 13. b 14. d 15. d
16. a 17. d 18. c 19. d 20. c
21. c 22. d 23. d 24. d 25. b
26. c 27. b 28. b 29. c 30. c
Chemistry
1: MATERIALS AROUND US
Worksheet 1
I. Objects around us
1. a 2. d 3. d 4. c 5. a
6. b 7. c 8. b 9. c 10. a
II. Classification of materials
1. c 2. a 3. a 4. b 5. b
6. a 7. b 8. c
III. Properties of materials
1. c 2. b 3. b 4. b 5. a
6. b 7. c 8. c 9. c 10. b
11. a 12. b 13. d 14. a
Worksheet 2
1. d 2. a 3. b 4. d 5. d
6. b 7. c 8. a 9. d 10. c
11. c 12. a 13. d 14. d 15. d
16. a 17. d 18. a 19. b 20. b
21. b 22. b 23. d 24. b 25. c
26. c 27. c 28. b 29. b 30. b
31. a
2: METHODS OF SEPARATION
Worksheet 1
I. Separation of solid-solid mixtures
1. b 2. a 3. a 4. a 5. a
6. a 7. b 8. b 9. b 10. d
II. Separation of solid-liquid mixtures
1. c 2. c 3. b 4. a 5. b
6. d 7. b 8. d 9. c 10. B
11. b 12. d
III. Modern techniques of separation
1. b 2. b 3. b 4. b 5. c
IV. Use of more than one method of separation
1. a 2. a 3. a 4. b 5. a
ANSWER KEY
Worksheet 2
1. a 2. a 3. a 4. b 5. b
6. b 7. c 8. b 9. b 10. b
11. d 12. a 13. b 14. b 15. b
16. c 17. d 18. c 19. a 20. b
21. a 22. c 23. d 24. b 25. d
26. a 27. c 28. d 29. d 30. C
31. c 32. b
Biology
1: COMPONENTS OF FOOD
Worksheet 1
I. Introduction and what do we eat?
1. d 2. c 3. a 4. d 5. b
6. a 7. c
II. What do different food items contain?
1. a 2. c 3. a 4. c 5. c
6. a 7. c
III. What do various nutrients do for our body?
1. a 2. b 3. a 4. b 5. c
6. b 7. d 8. d
IV Balanced diet
1. a 2. d 3. b 4. c 5. c
V. Millets and food miles
1. b 2. c 3. a 4. c
Worksheet 2
1. d 2. d 3. a 4. c 5. c
6. d 7. c 8. a 9. d 10. c
11. a 12. a 13. a 14. b 15. d
16. b 17. b 18. c 19. c 20. c
21. b 22. a 23. d 24. b 25. c
2: GETTING TO KNOW PLANTS
Worksheet 1
I. Classification of plants
1. a 2. b 3. d 4. a 5. a
II. Roots
1. d 2. d 3. a 4. a 5. d
6. b 7. d 8. d
III. Stem
1. b 2. d 3. c 4. a 5. b
IV. Leaves
1. b 2. b 3. c 4. d 5. a
6. c
V. Flower
1. c 2. c 3. b 4. b 5. b
6. d
Worksheet 2
1. c 2. c 3. d 4. a 5. d
6. b 7. b 8. c 9. c 10. d
11. d 12. c 13. a 14. c 15. d
16. d 17. a 18. a 19. a 20. a
21. b 22. b 23. c 24. a 25. a
26. c 27. d 28. c 29. c 30. d
