IL Foundation Series - Chemistry Class 10
Legal Disclaimer
This book is intended for educational purposes only. The information contained herein is provided on an “as-is” and “as-available” basis without any representations or warranties, express or implied. The authors (including any affiliated organizations) and publishers make no representations or warranties in relation to the accuracy, completeness, or suitability of the information contained in this book for any purpose.
The authors (including any affiliated organizations) and publishers of the book have made reasonable efforts to ensure the accuracy and completeness of the content and information contained in this book. However, the authors (including any affiliated organizations) and publishers make no warranties or representations regarding the accuracy, completeness, or suitability for any purpose of the information contained in this book, including without limitation, any implied warranties of merchantability and fitness for a particular purpose, and non-infringement. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any errors, omissions, or inaccuracies in the content or information provided in this book.
This book does not constitute legal, professional, or academic advice, and readers are encouraged to seek appropriate professional and academic advice before making any decisions based on the information contained in this book. The authors (including any affiliated organizations) and publishers disclaim any liability or responsibility for any decisions made based on the information provided in this book.
The authors (including any affiliated organizations) and publishers disclaim any and all liability, loss, or risk incurred as a consequence, directly or indirectly, of the use and/or application of any of the contents or information contained in this book. The inclusion of any references or links to external sources does not imply endorsement or validation by the authors (including any affiliated organizations) and publishers of the same.
All trademarks, service marks, trade names, and product names mentioned in this book are the property of their respective owners and are used for identification purposes only.
No part of this publication may be reproduced, stored, or transmitted in any form or by any means, including without limitation, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the authors (including any affiliated organizations) and publishers.
The authors (including any affiliated organizations) and publishers shall make commercially reasonable efforts to rectify any errors or omissions in the future editions of the book that may be brought to their notice from time to time.
Subject to Hyderabad jurisdiction only.
Copyright © 2025 Rankguru Technology Solutions Private Limited. All rights reserved.
ISBN 978-81-985539-7-3
Second Edition
CHEMICAL REACTIONS AND EQUATIONS 1
1.1 INTRODUCTION
Reflect upon the provided day-to-day life situations and consider the possible occurrences in each scenario: when milk is left at room temperature during summer, when an iron tawa/pan/nail is left exposed to a humid atmosphere, when grapes get fermented, when food is cooked, when food gets digested in our bodies, and when we respire.
In all the mentioned scenarios, a common theme is the occurrence of chemical or biological processes. Each situation involves a transformation or change, whether it be the fermentation of substances, the reaction of elements with the environment, the cooking or digestion of food, or the respiratory processes within living organisms.
A process that involves rearrangement of the molecular (or) ionic structures of a substance, as distinct from a change in physical form (or) a nuclear reaction. Change in state, change in colour, the evolution of a gas, change in temperature and formation of a precipitate, these observations help us determine whether a chemical reaction has taken place or not. Chemical changes are caused by chemical reactions. A chemical reaction represents the change of the species (atoms, molecules) taking part in the reaction into new species. The reacting species are known as the reactants, and those formed because of the reaction are called products
1.2 CHEMICAL EQUATIONS
When a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide. This description of a chemical reaction in sentence form is quite long. It can be written in a shorter form. The simplest way to do this is to write it in the form of a word equation. The word equation for the above reaction would be:
→ Magnesium+OxygenMagnesiumOxide
However, chemical equations can be made more concise and useful if we use chemical formulae instead of words. So, the above word equation can be written as:
2 → Mg +OMgO
1.2.1 Writing a chemical equation
A chemical equation is a shorthand representation of a chemical reaction in terms of symbols and formulae.
Reactants: The substances that undergo chemical change in the reaction are called reactants. In the above reaction, magnesium and oxygen are the reactants.
Products: The new substance(s) formed during the reaction are called product(s). In the above reaction, magnesium oxide is the product. Mg2+OMgO →
Here, Mg and O2 are the reactants, and MgO is the product. The reactants are written on the left-hand side (LHS) with a plus sign (+) between them. The products are written on the right-hand side (RHS) with a plus sign (+) between them. The arrowhead points towards the products and shows the direction of the reaction.
In a chemical equation, if the number of atoms of each kind is not equal on both sides, such a chemical equation is a skeletal chemical equation for that reaction.
Example: 22MgHClMgClH +→+
It is a skeletal chemical equation which represents a true reaction, which is unbalanced. The equation is unbalanced because the mass is not the same on both sides of the equation.
In a chemical equation, if the number of atoms of each element is the same on both sides, then it is a balanced chemical equation, which obeys the law of conservation of mass.
Law of conservation of mass
The law of conservation of mass is also called the law of indestructibility of matter. It was proposed by Lavoisier. Lavoisier is named the father of modern chemistry because of his experimental studies and quantitative measurements. This is verified by Landolt.
The law states that, the sum of the masses of reactants is equal to that of the products in all physical and chemical changes. In other words, matter can neither be created nor destroyed. The total mass is conserved in any reaction.
Let us consider the example: Potassium nitrate decomposes on heating to form potassium nitrite and oxygen.
322 O →+KNOKNO
The above equation is an unbalanced equation because the total number of oxygen atoms of reactants is 3, and the products are 4.
The number of oxygen atoms in the reactants is not equal to that of the products. So, it is called an unbalanced chemical equation
The above equation can be written as:
322 22O →+KNOKNO
In this equation, the number of atoms of various elements on the side of reactants is equal to the number of atoms of various elements on the side of products.
Such an equation is called a completely balanced equation (or) stoichiometric equation.
Atomic equation: A balanced equation in which the elementary substances are expressed in the atomic form is an atomic equation. Elementary substances mean hydrogen, oxygen, nitrogen, etc.
Example: Na + HCl → NaCl+H
Molecular equation: In this reaction, all elementary gases are represented in the form of molecules.
Example:
Essentials of a chemical equation:
32 223O →+KClOKCl
• All the reactants and products must be written in the form of formulae.
• It must represent a true chemical reaction.
• It must be carried out in a laboratory.

• The number of atoms of each kind should be equal on both sides of the equation.
• The equation should be molecular, i.e., the elementary gases are represented in molecular form.
• Metals like Na, Cu, Mg, etc., and some non-metals like C, S, etc. are written in atomic form.
Significance of a chemical equation: A chemical equation has qualitative and quantitative significance.
Qualitative Significance: It tells us about -
• The names of reactants that entered a chemical reaction.
• The names of products formed after the chemical change.
Example:
222 2HO2HO +→
Qualitative information: Hydrogen combines with oxygen to form water.
Quantitative information: Two molecules of H2 react with one molecule of oxygen to produce two molecules of water.
1.2.2 Introduction to balanced chemical equations
A chemical equation is said to be balanced if the atoms of different elements on both sides of the equation are equal. According to the law of conservation of mass, the total mass of products must be equal to the mass of reactants. This is possible only if the number of atoms of each element is the same on two sides of the reaction. Hence, we should balance a chemical equation. Balancing of equations is done in accordance with the law of conservation of mass.
Total mass of (R) = Total mass of (P)
Where R represents reactants, and P represents products.
In a balanced equation, the number of atoms of each element on both sides of the equation is equal.
1.2.3
Balancing a chemical reaction
The quantity of atoms for each element remains constant before and after a chemical reaction. Consequently, the necessity to balance a skeletal chemical equation arises. Let us delve into the step-by-step process of balancing a chemical equation.
Hit and trial method (or) Frequency number method
Without following the systematic methods of balancing chemical equations, certain coefficients of chemicals are assumed, and assumptions are verified. Such a method is commonly called the hit and trial method. Such a method is useful to avoid the length of the solution and minimise the time consumed for balancing the equation. However, accuracy is doubtful in every trial.
Hit and trial method may be used for simple reactions, especially those equations which do not involve electron transfer.
There are some methods to balance a chemical equation. Here, we are dealing with one of those methods.
Frequency number method: The frequency of occurrence of various elements in an equation is called F-number. 322
The potassium atom is present once in the reactants and once in the products. Thus, its frequency in the whole equation is 2.
Similarly, the nitrogen atom is present at one place in the reactants and at one place in the products. Thus, its frequency is 2. Oxygen atom is present at one place on the reactant's side and at two places on the product's side. Thus, its frequency is 3.
Note: While calculating F-numbers of various elements, just count the number of places where the given element occurs. Do not count the actual number of atoms, as they do not represent places. In the above equation, the F-number of oxygen atoms is 3, not 7.
Examples for finding F-numbers:
Rules for balancing a chemical equation
• Frequency numbers are to be written for all the elements in the given chemical equation.
• Start balancing with the element having the lowest frequency number from the equation.
• In the equation, other elements should be balanced in the increasing order of their frequency numbers.
• If two or more elements have the same frequency number, then first balance the metal.
• If there are two or more metals with the same frequency number, then first balance the metal with the higher atomic number.
• If there are two or more non-metallic elements with the same frequency number, then first balance the non-metallic element with the higher atomic number.
Solved examples for balanced chemical equations:
Example 1: Balance the following chemical equation.
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
Solution
Step 1: Writing the skeletal chemical equation:
The above word equation may be represented by the following chemical equation2442 ZnHSOZnSOH +→+
Step 2: Let us examine the number of atoms of different elements on both sides of the arrow.
As the number of atoms of each element is the same on both sides of the arrow, the above equation is a balanced chemical equation.
Example 2: Calcium bicarbonate solution reacts with calcium hydroxide solution to form calcium carbonate and water.
) 3232 2 CaHCOCa(OH)CaCOHO +→+
Solution
Step 1: List the number of atoms of different elements present in the given equation.
Step 2: List the F-number and give the balancing order for each element.
Balancing order of the elements
Step 3: Carbon has the least F-number, so we start balancing the equation with carbon. 2 carbon atoms are present in reactants, but only one carbon atom is present in products. So, we multiply 3 CaCO by 2.
Step 4: Calcium and hydrogen have the same F-number, 3. However, calcium should be balanced first because it is a metal. In the above equation, there are 2 calcium atoms in the reactants and in the products. Thus, calcium is balanced.
Step 5: In the above equation, 4 hydrogen atoms are present in the reactants, but 2 hydrogen atoms are present in the products. Thus, we multiply H2O by 2.
Step 6: The F-number of oxygen is 4. It is balanced last. 8 oxygen atoms are present in the reactants and 8 oxygen atoms are present in the products. Thus, it is balanced.
Step 7: Ultimately, to verify the accuracy of the balanced equation, it is essential to count the atoms of each element on both sides of the equation.
The number of atoms of all elements on the reactant and product side is equal. Hence, the above equation is balanced.
Example 3: Potassium dichromate on heating with conc. H2SO4 forms potassium sulphate, chromium sulphate, water, and oxygen.
Step 1: List the number of atoms of different elements present in the given equation.
Step 2: List the F-number and give the balancing order for each element.
Balancing order of elements
Step 3: Balancing of chromium
There are 2 chromium atoms in the reactants and in the products. So, it is already balanced.
KCrOHSOSOCrSKOHOO + +++ →
22724242422 3
Step 4: Balancing of potassium
( )
There are 2 potassium atoms in the reactants and in the products. So, there is no need to balance it.
KCrOHSOSOCrSKOHOO + +++ →
22724242422 3
Step 5: Balancing of hydrogen
( )
There are 2 hydrogen atoms in the reactants and in the products. So, there is no need to balance it.
KCrOHSOSOCrSKOHOO + +++ →
22724242422 3
Step 6: Balancing of sulphur
( )
There is one sulphur atom in the reactants and 4 sulphur atoms in the products. So, H2 SO4 is multiplied by 4, and H2 O is multiplied by 4 (at the same time) to balance hydrogens.
KCrO4HSOSOCrSO4HKOO + +++ →
22724242422 3
Step 7: Balancing of oxygen
( )
23 oxygen atoms are present in the reactants (7 + 16) and 22 oxygen atoms are present in the products (4 + 12 + 4 + 2)
The number of oxygen atoms in the reactants is odd, and in the products, it is even. Oxygen occurs in pure elementary form. Thus, we multiply the whole equation by 2 except O2
2227242424322 KCrO+8HSO2+2Cr(SO)+8HOKSO+O →
There are 46 atoms of oxygen present in the reactants, and 40 atoms are in the products. So, 6 oxygen atoms are less in the products. Then multiply 02, which is present in the products, by 3.
2K2Cr(SO) 2KCrO8HSOSO8HO+3O + → ++
227242422 432
Step 8: Ultimately, to verify the accuracy of the balanced equation, it is essential to count the atoms of each element on both sides of the equation.
The number of atoms of all elements on the reactant and product side is equal. Hence, the above equation is balanced.
Limitations of a balanced chemical equation
• It does not give information about the physical state of reactants and products.
3222 CaCO2HClCaClHOCO +→++
Note: To overcome these limitations, use symbols like 's' for solids, 'l' for liquids, and 'g' for gases. When the reaction takes place in a solution, then a symbol 'aq’ is used.
Example: ( ) 3(s)2(g)2() 2(s)g 2PbNO2PbO+4NO+O →
• A balanced chemical equation does not indicate the time of completion of a reaction.
• A balanced chemical equation does not indicate the speed of a chemical reaction.
• A balanced chemical equation does not indicate the conditions which are maintained.
Note: This problem is solved by writing conditions of reaction on the arrowhead.
Example: 1. CO(g) + 2H2(g) 340 atm CH3OH(l)
2. 6CO2(aq) + 12H2O(l) Sunlight C6H12O6(aq) + 6O2 (aq) + 6H2O(l) Chlorophyll Glucose
• A balanced chemical equation does not indicate the change in colour, evolution of heat, etc.
Oxidation number rules
Oxidation number is an apparent charge on an atom in a compound (or) ion.
OR
Oxidation number is also defined as a number which indicates the loss or gain or sharing of electrons by an atom during the formation of a chemical compound.
Oxidation numbers can have positive, negative (or) zero values depending upon the state of combination in the compound (or) ion.
Example: HCl:HClHCl −→++−
Oxidation number of H is +1 and
Oxidation number of Cl is -1.
Similarly, in CCl4, oxidation number of each Cl is - 1, and that of carbon is + 4.
Determination of oxidation number
Rule-1: The sum of oxidation numbers of all the atoms in a molecule is equal to zero.
Example: In 4 KMnO
Oxidation number of K+ oxidation number of Mn+ 4 (oxidation number of Oxygen)= 0.
Rule-2: The oxidation number of an atom in its elementary form is always zero.
Example: The oxidation number of H, O, N, P, S, Cu, and Ag in their elementary forms, i.e., H2, O2, N2, P4, S8, Cu, and Ag, respectively, is zero.
Rule-3: The oxidation number of alkali metals (Li, Na, K, Rb, Cs) in their compounds is always +1.
Example: In NaCl, the oxidation number of Na is +1.
Rule-4: The oxidation number of alkaline earth metals (Be, Mg, Ca, Sr, Ba)in their compounds is always +2.
Example: The oxidation number of Mg in MgO is +2.
Rule-5: The oxidation number of H in its compound is always +1 except in metal hydrides.
Example: In HCl, the oxidation number of H is +1.
In NaH (sodium hydride), the oxidation number of H is -1.
Rule-6: The oxidation number of fluorine in all of its compounds is -1.
Example: In NaF, the oxidation number of F is - 1.
Rule-7: The oxidation number of oxygen in most of its oxides is - 2 except in peroxides, super oxides, oxyfluorides and ozonides.
Example: In Na2O, the oxidation number of O is - 2.
In MgO, the oxidation number of O is - 2.
Exceptional cases:
Case - (i) Peroxides ( ) 2 2 O :
Two oxygen atoms are bonded by a single bond 2 (OO) or ( ) 11OO
The oxidation number of each oxygen in peroxide ion is - 1.
Example: The oxidation number of oxygen in H2O2 is - 1.
The oxidation number of oxygen in Na2O2 is - 1.
Case - (ii) Superoxides ( ) 1 2 O :
The oxidation number of each oxygen in superoxides is - 1/2.
Example: KO2
Rule-8: In a compound ion, the sum of the oxidation states of all atoms is equal to its charge.
Example: In 2 4 SO , the oxidation number of sulphur + 4 (oxidation number of oxygen) = - 2.
Rule-9: The maximum oxidation number of any element is equal to its group number except in the case of oxygen and fluorine.
Example: The oxidation number of sulphur in 222822828 HSO,KSO,SO and 25HSO is +6 due to the presence of peroxy bond.
Rule-10: In some compounds, all the atoms of the same element may not have the same oxidation number. When we calculate the oxidation number for that element in such compounds, we get the average value.
Example: In 223NaSO , the oxidation number of one sulphur atom is +6, and that of the other sulphur atom is -2. So, the average oxidation number of sulphur in 223NaSO , is +2.
Rule-11: In organic compounds, carbon can have any oxidation number from -4 to +4.
Example: In HCHO, the oxidation number of carbon is zero.
Rule-12: From IA to IV A group, the common oxidation number of an element is equal to its group number. From VA group to VIIIA the common oxidation number of any element is given by the formula (Group no. 8).
Example:
• Oxidation number of IA group elements = +1
• Oxidation number of IIA group elements = +2
• Oxidation number of IIIA group elements = +3
• Oxidation number of IVA group elements = +4
• Oxidation number of VA group elements = -3
• Oxidation number of VIA group elements = -2
• Oxidation number of VIIA group elements = -1
• Oxidation number of VIIIA group elements = 0
Rule-13: In all carbides, nitrides, phosphides, and sulphides, the oxidation number of C, N, P, and S are -4, -3, -3 and -2, respectively.
• In 32MgN , the oxidation number of nitrogen is - 3.
• In SiC, the oxidation number of carbon is - 4.
• In 32CaP , the oxidation number of phosphorus is - 3.
• In 2 HS , the oxidation number of sulphur is - 2.
Rule-14: The oxidation number of a metal in all metal carbonyls is zero.
Example: In Ni(CO)4, the oxidation number of Ni is zero.
Note: In NCl3, the oxidation number of N is - 3 and Cl is +1 because nitrogen is smaller in size when the component is chlorine.
Oxidation number method/ Half-reaction method
Oxidation number method is also called the electron transfer method. This method is useful for balancing equations of both ionic as well as molecular reactions. The method focuses on the atoms of the elements undergoing a change in oxidation number. The different steps involved in balancing are listed below:
• Write the skeletal equation representing a redox reaction.
• Write the oxidation numbers of each atom at the top of it.
• Locate the atoms undergoing changes in the oxidation numbers.
• Determine the change in the oxidation numbers per molecule or atom and write these on the lines connecting the species undergoing oxidation and reduction,
• Invert these numbers for the species involved so that the total increase in oxidation number is equal to the total decrease in oxidation number. In this step, the number of electrons in the redox reaction would be balanced.
• Balance the equation on both sides for atoms other than hydrogen and oxygen atoms.
• Finally, balance the hydrogen and oxygen atoms by inspection.
Example 1: Manganese dioxide oxidises hydrochloric acid to chlorine and gives manganous chloride and water.
Solution:
1. The skeletal equation is written as:
2. Focusing on the oxidation numbers:
3. Locating atoms undergoing change in oxidation numbers:
4. Determining the change in oxidation numbers
5. Cris-crossing these changes in the oxidation numbers. 222 MnO+2HClMnCl+Cl →
6. Balancing the atoms other than hydrogen and oxygen
2222 MnO+2HClMnCl+Cl+HO →
7. Balancing the atoms hydrogen and oxygen
2222 MnO+4HClMnCl+Cl+2HO →
Now, this is the balanced equation.
Example 2: Chlorine reacts with cold dilute caustic soda to give sodium chloride, sodium hypochlorite, and water.
Solution:
1. The skeletal equation is written as: 22 Cl+NaCIO NaOH+NaOCl+H →
2. Focusing on the oxidation numbers 0 + 1 -2 + 1 +1 -1 +1 -2 + 1 +1 -2 Cl2 + NaOH NaCl + NaOCl + H2O
3. Locating atoms undergoing change in oxidation numbers
4. Determining the change in oxidation numbers
+ NaOCl Cl 2 increase is 1 decrease is 1
5. Cris-crossing these changes in the oxidation numbers
2l NaCI ClNaOC → +
6. Balancing the atoms other than hydrogen and oxygen
22 Cl+NaClO 2NaOH+NaOCl+H →
7. Balancing the atoms hydrogen and oxygen
22 Cl+NaClO 2NaOH+NaOCl+H →
This is the balanced equation.
1.3 TYPES OF CHEMICAL REACTIONS
1.3.1 Exothermic reactions
Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. Therefore, to show these reactions (also called thermochemical reactions), the word or term 'heat’ is written on the product side.
Reactants → Products + heat (exothermic reaction)
Examples: 2(g)2(g)3(g) N+3H2NH+92kJ → (s)2(g)2 C+OCO+Heat →
Few more examples of exothermic reactions:
• Burning of natural gas
• Respiration
• The decomposition of vegetable matter into compost
1.3.2 Endothermic reactions
Reactions in which heat is absorbed by reactants are called endothermic reactions. Therefore, to show these reactions, the term 'heat’/ ’the value of heat energy’ is written on the reactant side.
Reactants + heat → Product (endothermic reaction)
Examples:
1.3.3 Combination reaction
The reactions in which two or more substances combine to form a single substance are called combination reactions. Combination reactions are quite often known as synthesis. A synthesis reaction is a reaction in which two elements combine to form a new compound.




Examples:
• Take some black lead sulphide in a test tube and heat it. The black sulphide will combine with oxygen to form white lead sulphate.
24 PbS+2OPbSO(Element-compoundcombinationreactions) →
• Put a mixture of fine iron powder and sulphur in a test tube and heat it strongly. A large amount of heat will be produced. In the test tube, there will be signs of melting. Iron sulphide (a greyish-black solid substance) will be formed.
Fe+SFeS(Element-elementcombination) →
• Hold a piece of magnesium ribbon over a flame. It will burn with a dazzling light, forming magnesium oxide.
2 2Mg+O2MgO(synthesis)(Element-elementcombination) → CaO + H2O → Ca(OH)2 (compound; compound combination)
• A combination reaction may (or) may not be a redox reaction. A combination reaction may be an exothermic (or) endothermic reaction.
Example 1: 222 2H+O2HO →
Hydrogen → Undergoing oxidation
Oxygen → Undergoing reduction
Example 2: (s)2(aq)2(s)CaO+CHO(OH) a →
There is no change in the oxidation number of elements. Hence, it is not a redox reaction.
1.3.4 Decomposition or dissociation reaction
Decomposition (or) dissociation reaction is a type of reaction in which a compound breaks down into either elements or a simple compound such that these products of decomposition do not recombine to form the original compound. A decomposition reaction may be brought about by the presence of either heat or light or the passage of electricity. Such a type of reaction that is brought about by heat is known as thermal decomposition.
A


Example:

A
+ B B

A reaction where a more complex molecule breaks down to form two or more simpler products
Fig. 1.2 Decomposition reaction
FeSO4 .7H 2 O(s) (Green)
2FeSO4 (s)
FeSO 4(s) + 7H 2 O(l) (Dirty white)
Fe2O3(s) + SO2(g) + SO3(g) (Brown )
A decomposition reaction may (or) may not be a redox reaction.
Example:
1. 2NaCl → 2Na+Cl2
Na1 →+ to zero oxidation number - reduction
Cl1 →− to zero oxidation number - oxidation
2. CaCO3
CaO + CO2
There is no change in the oxidation number of elements. Hence, it is not a redox reaction.
Types of decomposition reactions
Thermal decomposition: A decomposition reaction may be brought about by the presence of either heat or light or the passage of electricity. Such a type of reaction that is brought about by heat is known as thermal decomposition.
Example:
Photochemical decomposition: Chemical reactions that take place only when light energy is supplied to the reactant molecules are called photochemical reactions. In other words, in photochemical reactions, the reactant molecules absorb light. The rate of a photochemical reaction that takes place depends on the energy associated with the light absorbed by the reactant molecules.
Example:
Electrochemical decomposition: We have seen, from some of the above reactions, that reactant molecules react together only when heat or light energy is supplied to them. However, there are many other reactions which take place only when electric energy is supplied to the reactant molecules. Such reactions, which depend on electric energy, are called electrochemical reactions.
Electrochemical reactions are defined as reactions which proceed when electric energy is absorbed by the reactant molecules.
Example:
1.3.5 Displacement reaction
When an element displaces another element from its compound in an aqueous form, a displacement reaction occurs. In general, a more reactive element displaces a less reactive element from its aqueous solution.
Reactivity series: K>Na>Ca>Mg>Al>Zn>Fe>Pb>[H]>Cu>Hg>Ag>Au






Fig. 1.3 Displacement reaction
Example: (s)4(aq)(s)4(aq) FeCuSOCuFeSO +→+
All displacement reactions are considered as redox and exothermic reactions.
Example: 44 ZnCuSOZnSOCu +→+ Ex: Zn → zero to + 2 oxidation number - Oxidation
Cu2 →+ to zero oxidation number - Reduction
The chemical reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
1.3.6 Double displacement reaction
The chemical reactions in which there is an exchange of ions between the reactants are called double displacement reactions. These are reactions in which two compounds in a solution react with two other compounds by exchanging their radicals. This reaction can be represented by the following general equation.
AB + CD → AD + CB
Example: 2()24()4()() :BaClNaSOBaSO2NaCl +→↓+ aq aq s aq
Examples
A double decomposition reaction is not a redox reaction.
Examples
Example: 2244 2 :BaClNaSOBaSO2NaCl HClNaOHNaCIHO +→↓+ +→+
There is no change in the oxidation number of the elements. Hence, it is not redox. Precipitation, neutralisation reactions are examples of double displacement reactions.
The difference between precipitate and residue:
In the context of chemistry language, the difference between residue and precipitate is that residue is the substance that remains after evaporation, distillation, filtration (or) by any physical or chemical process, while precipitate is a solid that exists in the solution (or) in the liquid phase and is formed mostly by a chemical process.
Residue - Whatever remains after something else has been removed. Precipitate - Is an insoluble product resulting from a chemical process.
1.3.7
Redox reactions
The reactions in which both oxidation and reduction occur are called redox reactions.
Oxidation
• The term oxidation was first used to describe chemical reactions in which oxygen is added to an element or a compound. Because of the presence of oxygen in the atmosphere, many elements combine with it, and this is the principal reason why they occur in the form of oxides.
Oxidation of sulphur in oxygen gives sulphur dioxide.
• Oxidation of sulphite in hydrogen peroxide gives sulphate.
2322242 NaSOHONaSOHO +→+
Removal of hydrogen, or addition of an electronegative element, or removal of an electropositive element is also known as oxidation.
• Hydrogen is removed from hydrogen sulphide on oxidation with chlorine (or) oxygen.
22 HSCl2HClS +→+
• Metals are oxidised by adding more electronegative fluorine or chlorine
• More electropositive potassium is removed by bromine from potassium iodide, which is oxidised to iodine.
22 2KIBr2KBrI +→+
In the modern concept, loss of electron or electrons is oxidation. Oxidation is de-electronation.
A substance which undergoes reduction acts as an oxidant. Oxidant is an electron acceptor. The best oxidant is fluorine. Ozone is also a good oxidant. The other important oxidising agents are potassium permanganate, manganese dioxide, potassium dichromate, chlorine, oxygen, sodium hypochlorite, hydrogen peroxide, cupric oxide, etc.
Reduction
The term reduction is just the reverse of oxidation. The removal of oxygen or addition of hydrogen is called reduction. Similarly, it can involve the removal of an electronegative element or addition of an electropositive element.
a) Reduction of copper oxide with coke gives metal.
CuOCCuCO +→+
b) Ethylene is reduced to ethane upon addition with hydrogen.
24226 CH+HCH →
c) Chlorine is removed from ferric chloride by stannous chloride to give ferrous chloride.
3224 2FeClSnCl2FeClSnCl +→+
d) Cupric chloride is reduced to cuprous chloride upon addition with copper.
In the modern concept, a gain of electron or electrons is reduction. It is also known as electronation.
A substance which undergoes oxidation acts as a reductant. Reductant is an electron donor, and an oxidant is an electron acceptor. The redox reactions and reagents are given in the following table.
Reaction Process involved Reagent Process performed
Oxidation
Reduction
Addition of oxygen or a more electronegative element. Oxidation is the loss of electrons.
Addition of hydrogen or a more electropositive element.
Reduction is the gain of electrons.
Oxidant
Reductant
A substance which undergoes reduction is an oxidising agent. It is a substance which gains electrons.
A substance which undergoes oxidation is a reducing agent. It is a substance which loses electrons.
Table 1.1 Redox reactions and reagents
1.4 EFFECTS OF OXIDATION REACTIONS IN EVERYDAY LIFE
1.4.1 Corrosion
When a metal is attacked by substances around it, such as moisture, oxygen, acids, etc., it is said to be corroded, and this process is called corrosion. Corrosion is the slow eating of the surface of a metal by gases and water vapour present in the air. Rust can harm things like cars, bridges, iron railings, ships, and anything made of metal, especially iron. It’s a big issue because it damages iron a lot. Each year, a lot of money is used to fix or replace the iron that’s been damaged by rust. The most common example of corrosion is rusting, i.e. corrosion of iron. When an iron article remains exposed to moist air for a long time, its surface is covered with a brown, flaky substance called rust.

Aluminium Moist air 23AlO Silvery white
Copper Damp air and CO2
Magnesium Damp air and CO2
Silver Air and H2S
32 CuCO,Cu(OH) and moisture
32 MgCO,Mg(OH) and moisture
Greenish yellow
Greyish white
2 AgS Black
Table 1.2 List of metals and their corrosion compounds
• Electroplating is the most effective method to check corrosion or rusting. The surface of iron is coated with metals like Chromium, Nickel, or Zinc.
Reactivity series: K>Na>Ca>Mg>Al>Zn>Fe>Pb>[H]>Cu>Hg>Ag>Au
• Metals present lower in the activity series are very unreactive.
• Substances which prevent oxidation are called antioxidants.
• Commonly used antioxidants are: BHA-Butylated Hydroxy Anisole, BHT-Butylated Hydroxy Toluene
1.4.2 Rancidity
When fats and oils are oxidised, they become rancid and their smell and taste change. We have often noticed that food containing oil or fat, if left for long, develops a bad taste and bad smell. This is because the oils and fat present in food get oxidised
Storing food in airtight containers is an effective measure to decelerate the oxidation process. Additionally, it is noteworthy that manufacturers of chips often use nitrogen gas to flush bags of chips, preventing oxidation and maintaining the freshness of the product.
QUICK REVIEW
• A complete chemical equation serves as a symbolic representation, delineating the reactants, products, and their respective physical states.
• Chemical equations must be balanced, ensuring an equilibrium in the number of atoms for each type involved in a chemical reaction on both the reactant and product sides. This balance is a fundamental requirement for accurate chemical representations.
• Exothermic reactions release heat along with the products, while endothermic reactions absorb energy.
• Precipitation reactions generate insoluble salts.
• Chemical reaction is the process in which new substances with new properties are formed in a chemical reaction and rearrangement of atoms or elements takes place.
• Reactants: Substances which take part in a chemical reaction.
• Products: New substance produced because of a chemical change.
• Characteristics of chemical reaction: Change in the state: solid, liquid, gas, evolution of a gas, formation of precipitate: insoluble product, change in colour, change in temperature and absorption/liberation of gases.
• A chemical equation is a method of representing a chemical reaction with the help of symbols and formulae.
• In an unbalanced equation, there is an unequal number of atoms of one or more elements in reactants and products.
• In a balanced equation, there is an equal number of atoms of different elements in reactants and products.
• Balancing of an equation can be done by the hit-trial method and by the oxidation number method.
• Chemical equations do not talk about reaction feasibility (or) about the speed of reaction.
• Combination reaction: Two (or) more substances combine to form a single substance.
• Decomposition reaction: A compound splits up into two (or) more simpler substances.
• Displacement reaction: A more reactive element displaces a less reactive element from its salt solution.
• Double displacement reaction: Two compounds react by an exchange of ions to form two new compounds.
• Reactions may also entail the gain or loss of oxygen or hydrogen by substances. Oxidation denotes the gain of oxygen or the loss of hydrogen, whereas reduction involves the loss of oxygen or the gain of hydrogen.
• Oxidation is the process of addition of oxygen, (or) removal of hydrogen, (or) loss of electrons, (or) increase in oxidation number, (or) addition of more electronegative element.
• Reduction is the process of removal of oxygen (or) addition of hydrogen (or) addition of electrons (or) decrease in oxidation number (or) addition of more electropositive element.
• The substance which undergoes reduction is called an oxidizing agent. The substance which undergoes oxidation is called a reducing agent.
• Rancidity is the process by which the food containing oil and fats is marked by an unpleasant smell and taste due to aerial oxidation.
• Corrosion is the process by which the metals are eaten up by the action of air, moisture (or) a chemical.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Basic concepts
1. The chemical equations are balanced to satisfy one of the following laws in chemical reactions. This law is known as
a. Law of conservation of momentum
c. Law of conservation of motion
b. Law of conservation of mass
d. Law of conservation of magnetism.
2. Three beakers labelled A, B, and C, each containing 25 mL of water, were taken. A small amount of NaOH, anhydrous CuSO4, and NH4Cl were added to the beakers, respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in the case of beaker C, the temperature of the solution falls. Which one of the following statement (s) is/are correct?
i. In beakers A and B, exothermic process has occurred
ii. In beakers A and B, endothermic process has occurred
iii. In beaker C, exothermic process has occurred
iv. In beaker C, endothermic process has occurred
a. (i) only b. (ii) only c. (i) and (iv) d. (ii) and (iii)
3. The number of valence electrons in neon is a. 0 b. 8 c. 3
6
4. The oxidation number of the elements is mostly equal to a. Period number b. Group number c. Both a and b d. Atomic number
5. Assertion (A): d-block elements exhibit only one oxidation state in their compounds.
Reason (R): The common oxidation state of d-block elements is +II
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
6. Assertion (A): d-block elements exhibit more than one oxidation state.
Reason: d-block elements show the common oxidation state of +II due to the loss of ns2 electrons.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct.
7. Statement A: Elements often gain or lose enough electrons to attain noble gas electronic configuration.
Statement B: The oxidation number of the element is mostly equal to its group number.
Statement C: The valence electronic configuration of III B group is 21 (1) nsnd
a. All the above statements are correct b. All the above statements are incorrect c. A and B are correct, but C is incorrect d. A and B are incorrect, and C is correct.
8. 'X' is an element which belongs to VA group and 3rd period. The oxidation state and name of the element are
a. + 5 and P b. - 3 and N c. + 5 and N d. - 3 and S
9. Valence electronic configuration of VIII B group elements is a. ( ) 26 1 nsnd b. ( ) 28 1 nsnd c. Both a and b d. ( ) 23 1 nsnd
10. The outermost electronic configuration of chromium is a. [Ar]3d4 4s2 b. [Ar]3d5 4s1
c. [Ar]3d6 4s2
11. Which of the following is not a characteristic of a chemical change?
d. [Ar]3d6 4s1
a. A new substance is formed b. Chemical composition of the original substance changes
c. It is reversible d. Involves absorption or liberation of energy
12. Which of the following is a chemical change?
a. Melting of butter
c. Chopping of carrots
b. Rusting of iron
d. Boiling of water
13. Burning of a candle is a. Physical change b. Chemical change c. Both A and B d. None of these
14. An unbalanced equation is also known as a a. Skeletal equation b. Bone Equation c. Basic equation d. Rough equation
II. Balancing chemical equations
1. In the equation, ( ) 3322 2 CuxHNONOO CuyNO2H → + ++ , the values of x and y are a. 3 and 5 b. 8 and 6 c. 4 and 2 d. 7 and 1
2. In the following equations: 2322 2 x +→++ NaCOHClNaClCOHO , the value of x is a. 1 b. 2 c. 3 d. 4
3. In the chemical reaction, ( ) 22324224242 3 xyz + → +++ KCrOHSOSOSOCrSOHO K , the values of x, y, and z are a. 1, 3, 1 b. 4, 1, 4 c. 3, 2, 3 d. 2, 1, 2
4. Which of the following is an example of a balanced chemical equation based on the word equation: hydrogen + oxygen → water?
a. 222 2HOHO +→ b. 222 HO2HO +→ c. 222 2HO2HO +→ d. 222 HOHO +→
5. 223 SOOSO +→ What is the stoichiometric ratio of SO2 and SO3 in the given equation?
a. 3, 2
b. 2, 2 c. 1, 1
d. 1, 3
6. When a candle burns, which of the following best represents the law of conservation of mass?
a. The candle gets lighter as it burns.
b. The mass of these products is the same as the melted wax.
c. The total mass of the products is less than the mass of the reactant.
d. The total mass of the products is more than the mass of the reactant.
7. Which of the following is an example of an unbalanced chemical equation?
a. 222 2HO2HO +→ b. 4222 CH2OCO2HO +→+
c. 33 NaClAgNONaNOAgCl +→+ d. 2FeSFeS +→
8. Which of the following are the values of a, b, c, d, e, and f, respectively, for the given equation?
4242442 aKMnObHSOcKSOdMnSOeHOf[O] +→+++
a. a = 2, b = 3, c = 1, d = 2, e = 3, f =5
b. a = 3, b = 2, c = 4, d = 2, e = 3, f = 5
c. a = 2, b = 5, c = 1, d = 2, e = 3, f = 1
d. a = 3, b = 2, c = 1, d = 1, e = 3, f = 5
9. Which of the following arrows is used for the indication of precipitate formation during a chemical reaction?
a. ↑ b. → c. ↓ d. ←
10. The oxidation number of O in 22NaO is a. +1
b. +2 c. - 1 d. - 2
11. The oxidation number of Na in NaH is
a. -1 b. +1
c. - 2
12. The oxidation number of H in MgH2 is a. -1 b. +1 c. - 2
13. In CCl4, the oxidation number of carbon is
a. +4
+2
+2
b. - 4 c. -1 d. +1
14. The oxidation number of Ca in all its compounds is a. +1
b. +2 c. +3 d. 0
15. The oxidation number of Mn in KMnO4 is a. +3 b. +4 c. 0 d. +7
16. The oxidation number of K in KCl is a. +6 b. +4 c. +1 d. +7
III. Chemical reactions and types of chemical reactions
1. Assertion (A): Photosynthesis is the opposite reaction of respiration.
Reason (R): A combination reaction is always the opposite of its decomposition reaction.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
2. Assertion (A): hv 2AgCl2Ag2 +Cl → is a photochemical decomposition reaction.
Reason (R): The decomposition of silver halides is used in black and white photography.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
3. Assertion (A): All displacement reactions are exothermic in nature.
Reason (R): In all displacement reactions, a less reactive element displaces a more reactive element.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
4. Assertion (A): A double decomposition reaction can be a neutralization reaction.
Reason (R): A double decomposition reaction can be a precipitation reaction.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
5. Which of the following statements are correct for the given chemical reaction?
2 2PbO(s)C(s)2Pb(s)CO(g) +→+
a. Lead is reduced b. Lead is oxidized c. Carbon is reducedd. d. Both lead is oxidized and carbon is reduced
6. What is true about the following equation?
2342 3Fe(s)4HO(g)FeO(s)4H(g) +→+
i. Iron metal is being oxidized ii. Water is being reduced ii. Water is acting as a reducing agent iv. Water is acting as an oxidizing agent a. i, ii, and iii b. ii and iv c. i, ii, and iv d. i, iii, and iv
7. Below reaction is,
a. Decomposition reaction
c. Combination reaction
2442 ZnHSO(dil)ZnSOH+→+↑
b. Single displacement reaction
d. Synthesis reaction
8. Which of the following statement(s) about the given reaction is/are correct?
223 3MnO(s)4Al(s)3Mn(l)2AlO(s)Heat +→++
i. It is an exothermic reaction ii. It is being oxidised iii. It is a displacement reaction a. (i) only b. (ii) only c. (i) and (ii) d. (i), (ii), and (iii)
IV. Corrosion and rancidity
1. When some metals are exposed to moisture, acids, etc., they tarnish due to the formation of respective metal oxides on their surface. This process is called a. Sublimation b. Corrosion c. Rancidity d. Galvanisation
2. Which of the following compound is formed on the corrosion of silver? a. Silver sulphate b. Silver nitride c. Silver sulphide d. Silver chloride
3. Assertion (A): Oxidation is involved in both corrosion and rancidity. Reason (R): During this process, both metals and food undergo a reduction. a. Both A and R are true, and R is the correct explanation of A b. Both A and R are true, and R is not the correct explanation of A c. A is correct, and R is incorrect d. A is incorrect, and R is correct
4. Which of the following statements about the given reaction are correct?
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g) i. Iron metal is getting oxidised ii. Water is getting reduced iii. Water is acting as a reducing agent iv. Water is acting as an oxidising agent a. (i), (ii), and (iii) b. (iii) and (iv) c. (i), (ii) and (iv) d. (ii) and (iv)
5. In the context of redox reactions, the removal of hydrogen from a substance is known as a. Oxidation b. Dehydration c. Reduction d. Dehydrogenation
6. The chemical reaction involved in the corrosion of iron metal is that of a. Oxidation as well as displacement b. Reduction as well as decomposition
c. Oxidation as well as combination
WORKSHEET - 2
d. Reduction as well as displacement
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Which of the following word equations represents the formation of sodium chloride (table salt) from its elements?
a. Sodium + Chlorine → Sodium Chloride
c. Sodium Chloride → Sodium + Hydrogen
b. Sodium Chloride → Sodium + Chlorine
d. Sodium + Hydrogen → Sodium Chloride
2. In the word equation 'Methane + Oxygen → Carbon Dioxide + Water', how many different elements are involved in the reaction?
a. 1
b. 2
c. 3
d. 4
3. Determine the oxidation number of N in the given compound NH2NH2.
a. + 2
b. -2
c. -1
4. Which of the following is an unbalanced equation?
a. 38222 CHOCOHO +→+
c. 2232 Ca(OH)COCaCOHO +→+
5. The oxidation number of K in KMnO4 is a. +3
b. +2
d. -4
b. 2323 FeO2Al2FeAlO →→+
d. 33 AgNONaClAgClNaNO +→+
c. +1 d. +4
6. Which of the following represents a balanced chemical equation?
a. 226126 6CO6HOCHO +→ b. 4244 SiCl4HOSiOHCl H +→+
c. Na2ClNaCl +→
d. 322Al6HCl2AlCl3H +→+
7. The increasing order of the reactivity of Zn, Cu, Fe, and Ag is
a. Ag < Cu < Fe < Zn
c. Cu < Ag < Fe < Zn
b. Zn < Fe < Cu < Ag
d. Fe < Cu < Zn < Ag
8. What are the coefficients of the following reaction:
2323 FeOAlAlO(s)2Fe(s) Fe +→++

a. 1, 2, 2, 1
b. 2, 1, 1, 2
c. 2, 2, 1, 1
9. ( ) 234342 2 3Hg(OH)2HPOHgPO6HO +→+ is a/an
a. Balanced equation
c. Not feasible
d. 1, 1, 2, 2
b. Unbalanced equation
d. None of the above
10. A chemical equation is made more illuminating by including the chemical formulas of the reactants and products along with the _______ of the reactants and products.
a. Physical states b. Chemical state c. Normal state d. Standard state
11. By substituting chemical formulae for words in chemical equations, we can make them shorter and more useful. An equation for chemistry represents a _________.
a. Physical reaction b. Chemical reaction c. Exothermic reaction d. Physical change
12. Combustion of propane is: C3H8 + O2 → CO2 + H2O . Identify the coefficients of reactants and products.
a. 5, 1, 3, 4
b. 1, 5, 3, 4
c. 3, 4, 1, 5
13. Sulphur + sulphuric acid → sulphur dioxide + water
d. 3, 4, 5, 1
When the given equation is written in molecular form and balanced completely, the coefficient of sulphur dioxide is
a. 1 b. 2 c. 3
14. i. Electricity 222 2HO2HO + →
ii. h 22AgBr2AgBr ϑ →+
The above reactions are examples of
a. Chemical combination
4
b. Chemical decomposition
c. Chemical displacement d. Double displacement
15. A strip of copper was placed in a beaker containing ZnSO4 solution. On observing the strip, the next day, it was noticed that
a. The copper strip became thicker b. The copper strip became thinner
c. The copper strip remained as it was d. The colour of the strip changed
16. You are given the following chemical equation:
Mg(s)CuO(s)MgO(s)Cu(s) +→+
This equation represents:
a. Decomposition reaction as well as displacement reaction
b. Combination reaction as well as double displacement reaction
c. Redox reaction as well as displacement reaction
d. Double displacement reaction as well as redox reaction
17. Consider the following equation of the chemical reaction of a metal 223 M:4M3O2MO +→ . This equation represents
a. Combination reaction as well as reduction reaction
b. Decomposition reaction as well as oxidation reaction
c. Oxidation reaction as well as displacement reaction
d. Combination reaction as well as oxidation reaction
18. Which among the following is (are) double displacement reaction(s)?
i. 22 PbCuClPbClCu +→+ ii. 2424 NaSOBaClBaSO2NaCl +→+
iii. 22 COCO +→ iv. 4222 CH2OCO2HO +→+
a. (i) and (iv) b. (ii) only
c. (i) and (ii) d. (iii) and (iv)
19. Which one of the chemical reactions are incorrect?
i. 44 FeSOZnZnSOFe +→+ ii. 44 ZnSOFeFeSOZn +→+
iii. 2 2Ag2HCl2AgClH +→+ iv. 44 MgCuSOMgSOCu +→+
a. i and ii b. ii and iii
c. iii and iv d. i and iv
20. The reaction in which two compounds exchange their ions to form two new compounds is called
a. Combination reaction
c. Double displacement reaction
b. Decomposition reaction
d. Displacement reaction.
21. In the balanced chemical equation (a lead nitrate +b aluminum chloride → c aluminum nitrate + d lead chloride) Which of the following alternatives is correct?
a. a1,b2,c2,d1 ====
c. a2,b3,c2,d3 ====
22. MgCuOMgOCu +→+
b. a4,b3,c3,d4 ====
d. a3,b2,c2,d3 ====
Which of the following is wrong relating to the above reaction?
a. CuO gets reduced
c. CuO acts as a reducing agent
b. Mg gets oxidised
d. It is a redox reaction
23. Based on the reaction given below, what is the correct decreasing order of reactivity of metals?
i. (s)4(aq)4(aq)(s) ZnCuSOZnSOCu +→+
ii. (s)4(aq)4(aq)(s) FeCuSOFeSOCu +→+
iii. ( ) (s)3(aq)3(s) 2(aq) Cu2CNAgNOuO2Ag ++ →
iv. (s)4(aq)4(qq)(s) ZnFeSOZnSOFe +→+
a. Zn > Fe > Cu > Ag
c. Zn > Fe > Ag > Cu
24. CABCBA +→+ is a
a. Combination reaction
c. Displacement reaction
b. Ag > Cu > Fe > Zn
d. Cu > Ag > Fe > Zn
b. Decomposition reaction
d. Double displacement reaction
25. XYXY →+ is a
a. Combination
c. Displacement
26. XYABXBYA +→+ is a
a. Combination reaction
c. Displacement reaction
27. A + B → AB is a
a. Combination reaction
c. Displacement reaction
b. Decomposition
d. Double displacement
b. Decomposition reaction
d. Double displacement reaction
b. Decomposition reaction
d. Double displacement reaction
28. Aluminium, copper, calcium, and tin, when kept in decreasing order of their reactivity, the most reactive metal is
a. Aluminium
c. Tin
29. Choose the correct option:
i. ABCACB +→+
iii. PQRSPSRQ +→+
b. Copper
d. Calcium
ii. XYZ →+
iv. 2323 AO2O2A BB ++ →
a. Displacement (i), Double decomposition, (ii) Double displacement (iii), Decomposition (iv)
b. Decomposition (i), Double decomposition (ii), Displacement (iii), Double displacement (iv)
c. Double displacement (i), Displacement (ii), Decomposition (iii), Decomposition (iv)
d. Displacement (i), Decomposition (ii), Double displacement (iii), Displacement (iv)
30. Select an example of a redox reaction.
a. 44 MgZnSOMgSOZn +→+
c. 2 Z2n neZ +− + →
31. In the equation, ( ) 3322 2 CuxHNONOO CuyNO2H → + ++
b. 2 MgMg2e →++−
d. None of the above
The values of x and y are a. 3 and 5 b. 8 and 6 c. 4 and 2 d. 7 and 1
32. P6126(aq)2(g)2(g)2(l) CHOQORCOSHO+→++ Energy
The values of P, Q, R, and S, respectively, in a balanced chemical reaction would be a. 1, 6, 1, 6 b. 1, 6, 6, 6 c. 6, 1, 6, 6 d. 6, 6, 2, 6
33. Consider the following reaction:
X calcium carbonate +Y phosphoric acid → calcium phosphate +X water +X carbon dioxide. What is the value of X and Y?
a. X-3, Y-1 b. X-3, Y-4
c. X-1, Y-3 d. X-3, Y-2
34. Write a balanced chemical equation for the following word equation: Nitrogen + Oxygen → Nitric oxide
a. 22 NO2NO +→
c. 223 NONO +→
35. Choose the correct statement.
a. Turning milk into curd is a physical change.
b. 222 NONO +→
d. 22 N2O2NO +→
b. The evaporation of water is a physical change.
c. The burning of camphor is a physical change.
d. The melting of chocolate is a chemical change.
36. Consider the following reactions:
Which one of the following statement is correct?
a. Zn is the least reactive, and Fe is more reactive
b. Zn is the most reactive, and Cu is less reactive
c. Cu is more reactive, and Zn is less reactive
d. Fe is less reactive, and Zn is more reactive

ACIDS, BASES, AND SALTS 2
2.1 INTRODUCTION TO ACIDS, BASES, AND SALTS
The word acid is derived from the Latin word 'acidus,' which means sour to taste. In our daily lives, we come across many acidic substances such as citrus fruits, tomatoes, vinegar, tamarinds, cleaning agents used in toilets, medicines like aspirin, folic acid, vitamin C, etc.
There are some other substances known as alkalis and bases, whose properties are different and opposite to that of acids. The word 'alkali' is derived from an Arabic word that means calcined ashes of plants. These were first isolated from the ashes of plants and hence were given the name alkali.
Lavoisier first prepared acids by heating non-metals in oxygen and then dissolving the oxides formed in water. Davy first proved the presence of hydrogen ions (H+) as the main constituent of all acids.
A salt is a compound formed by the reaction of an acid with a base.
The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat.
Acid + Base → Salt + Water (Heat is evolved)
Solution:
A solution is defined as a homogeneous mixture of two or more substances whose composition may be varied within certain limits.
A solution may contain more than one component. The solution may be gaseous, liquid, or solid. The relative amounts of the two components, the solvent and the solute, are expressed in various ways like normality, molarity, molality, mole fraction, etc.
• Binary solution: A solution containing two components (i.e., one solvent and one solute only) is known as a binary solution.
Solvent: Generally, the component present in a larger quantity in any solution is called the solvent.
Example: In a sugar solution, water is the solvent.
Solute: Generally, the component present in lesser quantity in a binary solution is referred to as solute.
Example: In a sugar solution, sugar is the solute.
2.2
UNDERSTANDING THE CHEMICAL PROPERTIES OF ACIDS AND BASES
2.2.1 Identifying acids and bases in the laboratory
Acids and bases in the laboratory are identified using some special chemical substances, which bring out the change in the physical properties of an acid or base. A chemical substance (coloured or dye) which can change its colour or odour in the presence of an acidic or basic medium is called an indicator.
It changes colour when it is put into an acid or a base. An indicator gives different colours in acid and base. Thus, an indicator tells us whether the substance we test is an acid or a base by a change in its colour. The three most common indicators to test for acids and bases are classified as follows.
Indicator
Natural Indicator
Based on colour change
Colour indicators
Litmus
Based on odour change
Red cabbage extract
Turmeric
China rose petals
Hydrangea flowers
Natural indicators
Olfactory indicators
Onion extract Vanilla extract Clove oil
Synthetic Indicator
Methyl orange
Phenolphthalein
Methyl red
Bromo thymol blue
Universal indicator
Fig. 2.1 Classification of indicators
a) Colour indicators: Litmus is a natural indicator obtained from lichens and available in the form of a paper which is in blue and red colours. When dipped in an acidic solution, the blue colour of litmus changes to red. Similarly, when dipped in an alkali solution, the red litmus paper turns blue. Some other natural indicators are listed below.
Indicator Original colour In acidic medium In basic medium
Litmus Purple Blue to red Red to blue Red cabbage Red Red Green
Turmeric Yellow Yellow Red or reddish brown
China rose Light pink Dark pink Green
Hydrangea Blue Blue Pink to purple
Table 2.1 Natural indicators based on colour change
Note: Hydrangeamacrophylla flowers can change colour depending on soil acidity. In acidic soils, chemical reactions occur in the soil that make aluminium available to these plants, turning the flowers blue. In alkaline soils, these reactions cannot occur, and therefore, aluminium is not taken up by the plant. As a result, the flowers remain pink.
b) Olfactory indicators: The term 'olfactory' means 'relating to the sense of smell'. Those substances whose smell (or odour) changes in acidic or basic solutions are called olfactory indicators. An olfactory indicator usually works on the principle that when an acid or base is added to it, then its 'characteristic smell' cannot be detected. Onion and vanilla extracts are olfactory indicators.
Indicator In acidic medium In basic medium
Onion extract
Smell is detected Smell is destroyed
Vanilla extract Smell is detected Smell is destroyed
Clove oil Smell is detected Smell is destroyed
Table 2.2 Olfactory indicators
Synthetic indicators
Methyl orange Orange Red Yellow
Phenolphthalein Colourless Colourless Pink
Table 2.3 Synthetic indicators
a) Action of phenolphthalein: Phenolphthalein is a colourless, weak organic acid. When it dissolves in water, it dissociates to some extent to give colourless hydrogen ions and pink anions.
HPh (colourless) ⇌H+ + Ph - (pink )
Let us consider an alkaline solution and check the colour change with the addition of phenolphthalein. NaOH ionizes as
NaOH → Na+ + OH - (1)
Phenolphthalein (HPh) ionizes as
HPh → H+ + Ph - (2)
H+ from Phenolphthalein combines with OH- from NaOH to give H2O. As H+ ions from the R.H.S of equation (2) are removed, this promotes forward reaction and a greater number of pink Ph- ions are furnished in the solution. This makes the solution pink.
• Let us consider an acidic solution and check the colour change on the addition of phenolphthalein.
In the acidic solution, acid (HA) ionizes as follows.
HA → H+ + A- (1)
Phenolphthalein ( HPh) ionizes as HPh → H+ + Ph- (2)
In (1) and (2), we see the existence of common ion H+. This common ion effect suppresses the dissociation of phenolphthalein by promoting a backward reaction in equation (1). The backward reaction results in the formation of colourless HPh molecules. Hence, when phenolphthalein is added to an acidic solution, the solution remains colourless. Thus, the indicator phenolphthalein appears colourless in acidic and pink in alkaline solutions.
b. Action of methyl orange: Another common acid-base indicator is methyl orange, which is a weak organic base and may be represented as MeOH. When MeOH is dissolved in water, it undergoes dissociation to a very small extent and the undissociated molecules (MeOH) appear yellow in colour, while the cations i.e., Me+ appear red in colour.
MeOH (Yellow) ⇌ Me+ (Red) + OH-
• If the solution to which the indicator is added is acidic, the hydrogen ion furnished by the acid combines with OH- ions furnished by the indicator to form undissociated water, yielding more of the red-coloured Me+ ions. Thus, the solution turns red in colour.
• If the solution is alkaline in nature, then OH- ions suppress the dissociation of methyl orange, and the solution turns yellow in colour.
Hence, Methyl orange in an acidic solution gives red colour and in a basic solution, it gives yellow colour.
Self-indicators
In a redox reaction, KMnO4 acts as an oxidising agent (it gets reduced). Its original colour is purple. It becomes colourless after complete reduction. This colour change indicates KMnO4 acts as a selfindicator (without adding another external indicator).
Examples: KMnO4
Universal indicator
An indicator that covers a very wide range of pH (3-11) and gives different colour changes at different pH values is called a universal indicator. One universal indicator commonly used is a mixture of several indicators with fixed properties.
It is a mixture of -
Phenolphthalein - 0.1gm
Methyl red - 0.2gm
Methyl yellow - 0.3gm
Bromothymol blue - 0.4gm
Thymol blue - 0.5gm
These are dissolved in absolute alcohol to which adequate NaOH is added till a yellow colour is given. This gives characteristic colours at each pH value.
Range of colours of the universal indicator at different pH
ACIDIC NEUTRAL ALKALINE
2.2.2 Acids and bases theories
a. Arrhenius concept of acids and bases
An acid is a substance that produces hydrogen ions (H+)when dissolved in water. A base is a substance that produces hydroxyl ions (OH-)when dissolved in water.
Examples for acids
HCl, H2SO4, HNO3, H2CO3, H3PO4, CH3COOH, etc.
Examples for bases
NaOH, KOH, NH4OH, Ca(OH)2, Mg(OH)2, Al(OH)3, Fe(OH)2, etc.
Limitations of the Arrhenius concept
1. It recognises the dissociation of acids and bases in aqueous medium only.
2. It restricts acids to merely hydrogen-containing compounds and bases to merely hydroxidecontaining compounds.
3. According to the Arrhenius concept, CO2, SO2, SO3, etc., are not regarded as acids and NH3, Na2O, CaO, MgO, Na2CO3, etc., are not regarded as bases.
b. Bronsted-Lowry's concept of acids and bases
Bronsted and Lowry proposed a concept of acids and bases, which is independent of solvents. According to this concept, acids and bases are defined as follows:
An acid is a species (a molecule, a cation or an anion) which can donate one or more protons to any other substance. It is called Bronsted-Lowry acid.
Examples:
A base is a species (a molecule, a cation or an anion) which can accept one or more protons from an acid. It is called Bronsted-Lowry base.
Therefore, an acid is a proton donor, and a base is a proton acceptor.
Examples:

Limitations of Bronsted-Lowry's concept
Bronsted-Lowry's concept of acids and bases cannot explain the acidic nature of CO2, SO2, and SO3 and basic nature of Na2O, CaO, MgO, and BaO.
c. Lewis theory
G.N. Lewis proposed a more general theory of acids and bases. According to this theory, an acid is defined as 'a substance that can accept an electron pair to form a coordinate covalent bond with the donor.'
Examples: H , BF3, SnCl4, Ag⁺, Cu2⁺, CO2, SO2, etc.
A base is defined as 'a substance that can donate a lone pair of electrons to form a coordinate covalent bond with the acceptor.'
Examples: H2O, NH3, Cl , OH , CN , CO, etc.
Limitations of the Lewis theory
1. This theory cannot explain the strength of acids and bases.
2. Acids like HCl, H2SO4, etc. react with bases such as NaOH (or) KOH but do not form a coordinate covalent bond.
3. All the acid-base reactions do not involve coordinate covalent bond formation.
2.2.3 Classification of acids and bases
a. Classification of acids
1. Classification based on source: Based on source, acids are classified into organic acids and inorganic acids.
a. Organic acids: Acids obtained from natural organic matter, such as plants and animals, are called organic acids.
Acid
Source
Oxalic acid Spinach, Tomato
Lactic acid Sour milk
Citric acid Lemon, Orange
Formic acid Stings of Ants and Bees
Acetic acid Vinegar
Malic acid Apple
Tartaric acid Tamarind
Butyric acid Rancid butter
Ascorbic acid Orange
Amino acids Proteins
Table 2.4 Some organic acids
All organic acids are weak acids, and they do not ionise completely in aqueous solution.
b. Inorganic acids: Acids obtained from minerals are called inorganic acids. Inorganic acids are strong acids, and they dissociate completely in aqueous solutions.
Examples: HCl, H2SO4, HNO3, H3PO4, etc.
2. Classification on the basis of molecular composition: On the basis of molecular composition, acids are classified into
a. Binary acids: Acids which contain hydrogen and another element are called binary acids.
Examples: HCl, HI, HF, etc.
b. Oxo acids: Acids which contain hydrogen, oxygen, and atleast one other element are called oxoacids.
Examples: H2SO4, HNO3, HNO2, H3PO4, CH3COOH, etc.
3. Classification based on strength: On the basis of strength, acids are classified into strong acids and weak acids.
a. Strong acids: Acids that undergo ionisation to a large extent in an aqueous solution are called strong acids.
Examples : HCl, H2SO4, HNO3, HBr, HI, HClO4 , etc.
b. Weak acids: Acids that undergo ionisation to a small extent only are called weak acids.
Examples: HF, HCN, HNO2, H2CO3, CH2COOH, HCOOH, H2C2O4, etc.
4. Classification of the solution based on concentration: Based on their concentration, acids are classified into concentrated acids and dilute acids.
a. Concentrated acids: A concentrated acid is almost pure acid with very little water.
b. Dilute acids: A dilute acid has more water and less acid.
5. Classification based on basicity: The basicity of an acid is the number of hydrogen ions that can be produced by the ionisation of one molecule of the acid in its aqueous solution. On the basis of basicity, acids can be classified into
a. Monobasic acids: An acid that produces one hydrogen (or) hydronium ion by the ionisation of one molecule of the acid is called a monobasic acid (or) monoprotic acid.
Examples: HCl, HBr, HI, HNO3, CH3COOH, etc.
b. Dibasic acids: An acid which produces two hydrogen (or) hydronium ions by the ionisation of one molecule of the acid is called dibasic acid (or) diprotic acid.
Examples: H2CO3, H2SO3, H2 SO4, H2C2O4, etc.
c. Tribasic acids: An acid that produces three hydrogen (or) hydronium ions by the ionisation of one molecule of the acid is called tribasic acid (or) triprotic acid.
Example: H3PO4
b. Classification of bases
1. Classification based on strength: Based on their strength, bases are classified into
a. Strong base: A strong base produces a high concentration of hydroxyl ions in aqueous solution.
Examples: NaOH, KOH, Ca(OH)2, etc.
b. Weak base: A weak base produces a low concentration of hydroxyl ions in aqueous solution.
Examples: Cu(OH)2, Fe(OH)2, NH4OH, etc.
2. Classification based on acidity: Bases are also classified as
a. Monoacidic base: A base that produces one hydroxyl ion by the ionisation of one molecule of the base is called a monoacidic base.
Examples: NaOH, KOH, NH4OH, etc.
b. Diacidic base: A base that produces two hydroxyl ions by the ionisation of one molecule of the base is called a diacidic base.
Examples: Ca(OH)2, Mg(OH)2, Cu(OH)2, Zn(OH)2, Fe(OH)2, etc.
c. Triacidic base: A base that produces three hydroxyl ions by the ionisation of one molecule of the base is called a triacidic base.
Examples: Fe(OH)3, Al(OH)3, Cr(OH)3, etc.
2.2.4
Preparation of acids and bases
a. Preparation of acids
1. Synthetic method: Acids are prepared by a direct combination of elements.
a) H2+ Cl2 ⟶ 2HCl
b) S + O2⟶ SO2
2SO2 + O2 ⟶ 2SO3, SO3+H2SO4 ⟶ H2S2O7(oleum)
H2S2O7+H2O ⟶ 2H2SO4(or)
SO3+H2O ⟶ H2SO4
2. By dissolving acidic oxides in water: Some non-metal oxides dissolve in water to give acids.
a) N2O5 + H2O ⟶ 2HNO3
b) CO2 + H2O ⟶ H2CO3
3. By the action of an acid on the salt of another acid: More volatile acids are easily prepared by the action of their salts with less volatile acid.
H2SO4 + NaCl ⟶ NaHSO4 + HCl
b. Preparation of bases
1. By the direct union of a metal with oxygen: Some metals, when heated in air (or) oxygen, form the oxides of metals.
These metal oxides, when dissolved in water, form metal hydroxides.
2. By the action of water (or) steam on some active metals: Some active metals like sodium and potassium react with cold water to form hydroxides with the evolution of hydrogen gas.
3. Magnesium reacts with steam to form magnesium hydroxide with evolution of hydrogen gas.
4. By heating carbonates of some metals: When calcium carbonate is heated, calcium oxide and carbon dioxide are formed.
5. By the action of an alkali on a salt solution: When an aqueous solution of sodium hydroxide is added to an aqueous solution of magnesium sulphate, magnesium hydroxide gets precipitated, and sodium sulphate remains in the solution.
( ) ( ) ( ) 4aq22aq4aq MgSO2NaOHMg(OH)(whiteppt)NaSO + ® +¯
2.2.5 Acids and bases chemical reactions
a. Acids and bases reaction with metals
Metals like potassium, sodium, calcium, magnesium, aluminium, zinc, and iron can react with the aqueous solution of an acid to evolve hydrogen gas.
Examples:
2Na + 2HCl ⟶ 2NaCl + H2↑
Mg + H2SO4 ⟶ MgSO4+ H2↑
Fe + 2HCl ⟶ FeCl2 + H2↑
2Al + 3H2SO4 ⟶ Al2(SO4)3 + 3H2↑
b. Metal carbonates and metal hydrogen carbonates reaction with acids
Acids react with carbonates and bicarbonates to produce salt, water, and carbon dioxide gas.
Examples:
Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2↑
NaHCO3 + HCl ⟶ NaCl + H2O + CO2↑
c. Acids and bases reaction with each other
Acids react with bases to produce salt and water.
Examples:
NaOH + HCl ⟶ NaCl + H2O
2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O
A neutralisation reaction can be defined as a reaction between an acid and a base that produces salt and water.
d. Reaction of metallic oxides with acids
Acids react with oxides of metals to form salt and water.
Examples:
CuO + H2SO4 ⟶ CuSO4 + H2O
MgO + 2HCl ⟶ MgCl2 + H2O
e. Reaction of a non-metallic oxide with base
Bases react with acidic oxides to give salts and water.
Examples:
2NaOH + CO2 ⟶ Na2CO3 + H2O
Ca(OH)2 + CO2 ⟶ CaCO3 + H2O
2.2.6 Do acids produce ions only in an aqueous solution?
Acids can produce ions not only in aqueous solutions but also in other solvents or when they undergo certain chemical reactions. The generation of ions is a characteristic feature of acids, and it can occur in various contexts beyond just aqueous solutions.
One example illustrating that acids can produce ions outside of aqueous solutions is when they react with certain metals. For instance, hydrochloric acid (HCl) reacts with zinc (Zn) to produce zinc chloride (ZnCl2) and liberates hydrogen gas (H2). The reaction can be represented as:
2HCl(aq) + Zn(s) → ZnCl2 (aq) + H2 (g)
In this reaction, hydrochloric acid produces chloride ions (Cl ) in the aqueous solution, and hydrogen ions (H+) are generated because of the reaction with the metal zinc.
2.2.7 Properties of bases
• They are bitter to taste.
• They turn red litmus paper blue.
• The solution of bases in water gives a soapy touch.
• When dissolved in water, it produces hydroxide ion (OH ) in solution.
• They react with acids to produce salt and water.
• Bases react with acidic oxides to give salts and water.
2NaOHCONaCOHO Ca(OH)COCaCOHO + ® + ++ ®
• Base reacts with certain salts to produce another salt and another base.
Examples
Ammonium hydroxide [NH4OH] is added to solution of Aluminium sulphate [Al2(SO4 )3], Aluminium hydroxide [Al(OH)3] and Ammonium sulphate [(NH4)2 SO4] are produced.
6NHOHAlSO2Al(OH)3NHSO ++ →
( ) ( ) 424344 32 (base)(salt)
2.3 COMMON CHARACTERISTICS OF ACIDS AND BASES
2.3.1 The common characteristics between acids and bases
All acids and bases share a common characteristic in that they are classified as electrolytes, meaning they conduct electricity when dissolved in water. This property arises from the presence of ions in their aqueous solutions. Acids release hydrogen ions (H+) into the solution, while bases release hydroxide ions (OH-). The ability to ionise and produce ions in solution is a fundamental similarity shared by all acids and bases.
2.3.2 What happens to an acid or a base in a water solution?
The transformation of an acid or a base in a water solution involves a set of characteristic reactions and behaviours. As the acid dissolves, it undergoes ionisation, leading to the release of hydrogen ions (H+).
HCl + H2O → H3O+ + Cl
Hydrogen ions cannot exist independently; instead, they form by combining with water molecules. Consequently, hydrogen ions are conventionally represented as H+ (aq) or hydronium ions (H3O+). This notation acknowledges the dynamic interaction of hydrogen ions with water molecules in aqueous solutions.
As the base dissolves, it undergoes ionisation, leading to the release of hydroxide ions (OH-) for bases. The degree of ionisation determines the strength of the acid or base. The degree of ionisation determines the strength of the acid or base.
The process of dissolving an acid or a base in water is highly exothermic. Special precautions are necessary when combining concentrated nitric acid or sulfuric acid with water. To ensure safety, the acid should be added gradually to water with continuous stirring.
Introducing water into a concentrated acid may generate excessive heat, leading to splashing and the risk of burns. The container, typically made of glass, might also break due to localised heating. The act of mixing an acid or base with water leads to a reduction in the concentration of ions (H3O+/ OH-) per unit volume. This process is termed dilution, and the acid or base is described as diluted.
2.3.3 Strength of acid or base solutions
We are familiar with the use of acid-base indicators to differentiate between acids and bases.
Scientists have devised a pH scale for measuring the hydrogen ion concentration in a solution. The pH value should be regarded simply as a numerical representation indicating the acidic or basic nature of a solution. A lower pH value corresponds to a higher hydronium ion concentration.
The potency of acids and bases is contingent upon the quantity of H+ ions and OH– ions generated, respectively. For instance, when comparing hydrochloric acid and acetic acid with the same
concentration, such as one molar, they produce varying amounts of hydrogen ions. Acids that yield a higher concentration of H+ ions are categorized as strong acids, while those generating fewer H+ ions are classified as weak acids.
2.4 pH CONCEPT AND pH SCALE
2.4.1 pH scale
It is the easier way to measure the strength of an acidic or basic solution. It was introduced by S.P.L. Sorensen. A scale for measuring hydrogen ion concentration in solution is called the pH scale.
'p' in pH stands for 'potenz' in German. Potenz means power.
Definition of pH
pH of an aqueous solution is the negative logarithm of its H+ ion (hydrogen ion) concentration, expressed in moles/litre.
∴pH = -log[H+] = log10 1 [H+]
⇒[H+] = 10-pH
Similarly, pOH = -log10[OH ]
Where [H+]and [OH-] are the concentrations of H+ and OH ions respectively.
1. pH of a solution is a dimensionless quantity.
2. In a neutral solution, [H⁺] = 1.0×10-7 M
∴pH = -log[1.0×10-7)]
pH = 7
3. In an acidic solution, [H+] > 1.0×10-7 M
If [H+] = 1.0 × 10-6 then pH = -log(1.0 × 10-6)
pH = 6 < 7
So, the pH of an acidic solution is less than 7.
4. In an alkaline solution, [H+] < 1.0 × 10-8 M
If [H+] = 1.0 × 10-8 M, then pH = -log(1.0 × 10-8)
pH = 8 > 7
So, the pH of an alkaline solution is more than 7.
5. On the pH scale, pH can be measured from 0 to 14 at room temperature.
6. pH + pOH = 14
Example :
If [H+] = 1.0 × 10-4 M, then [OH ] = 1.0 × 10-10 M
Then pH = -log(1.0 × 10-4 ) = 4
pOH = -log(1.0 × 10-10) = 10
∴ pH + pOH = 4 +10 = 14.
7. As the pH value decreases from 7 to 0, it represents an increase in H+ ion concentration in the solution and a decrease in OH- ion concentration in the solution.
A log based scale used to keep track of the large change important to acids and bases
When you add an acid, the pH gets smaller. When you add a base, the pH gets larger. Very Acidic
2.3 pH scale
8. As the pH value increases from 7 to 14, it represents a decrease in H⁺ ion concentration and an increase in OH- ion concentration in the solution.
9. Strong and weak electrolytes: All the acids and bases do not ionise to the same extent. The extent of ionisation depends on the polarity of the bond between H and A in the case of acids (HA) and on the polarity of the bond between B and OH in the case of base (BOH).
a. Strong electrolyte: If the extent of ionisation for a compound is large, it is called a strong electrolyte.
Example: NaCl, KCl, etc.
b. Weak electrolyte: If the extent of ionisation for a compound is less, it is called a weak electrolyte.
Example: NH4Cl, CH3 COOH, etc.
Ionic product of water
Pure water is a poor conductor of electricity. But water ionises to a very small extent, producing H+ and OH ions. The ionisation equilibrium is represented as
HOHOHOOH(or)HOHOH) +− ++++−
This shows that water has the dual nature of proton donor and proton acceptor. Then, the equilibrium constant, K, is given by
Since, the ionisation of H2O is negligible, [H2O] can be taken as constant.
Therefore, K[H2O]2 = constant, which can be taken as KW.
KHOOH(or)simplyKHOH +− +−
Here, the constant KW is known as an ionic product of water at a given temperature.
Definition
The ionic product of water, KW, at a given temperature, is defined as "the product of the concentrations of H+ and OH- ions in water or in aqueous solutions".
At
Since, water is a neutral liquid [H+] = [OH-]
Therefore, KW = [H+ ][OH- ] = [H+]2 or [OH-]2
From the ionic product of water, knowing either [H+] or [OH-]the other can be calculated.
As the temperature increases, the ionisation of water increases and hence, the KW value increases. Hence [H+]increase (or) pH of the solution decreases.
Based on the relative amounts of H+ and OH- ions, solutions of various substances can be divided into 3 types. They are
a. Neutral solutions, [H+] = [OH-] = 1×10-7 M
b. Acidic solutions, [H+] > [OH-]
i.e.[H+] > 10-7 M(or)[OH-] < 10-7 M
c. Basic solutions, [H+] < [OH-]
i.e.[H+] < 10-7 M (or) [OH-] > 10-7 M
2.5 IMPORTANCE OF PH IN EVERYDAY LIFE
The pH, denoting the potential of hydrogen, serves as a metric for gauging the acidity or alkalinity of a solution. Its significance extends across numerous facets of daily life. Below are key points underscoring the importance of pH in our everyday experiences.
1. pH role in plants and animals
a. Role of pH in the blood-
The pH of our blood is slightly alkaline and is in the range 7.36-7.42. Our body becomes prone to disease when the pH of our blood deviates from this range.
b. Functions of enzymes at definite pH-
The enzymes function effectively only in the region of biological pH(7-7.5)
c. Function of kidneys against wide variation of pH-
The ability of our kidneys to excrete acids and bases is of great significance as it keeps the kidney working against wide variations in body pH.
2. pH in our digestive system
The pH of gastric juice is 1.0-2.0 due to the secretion of HCl in our stomach. During indigestion the pH of our gastric juice is below 1.4 due to the secretion of a large amount of HCl. This leads to irritation and pain in the stomach. To get relief from acidity, we take antacids such as Gelusil and milk of Magnesia.
3. pH changes as the cause of tooth decay
Carbohydrates and food particles present in our mouth after eating sweet-tasting foods undergo bacterial decomposition, and as a result, HCl is produced as one of the by-products. When the pH of our mouth is below 5.5, tooth decay starts. The tooth decay can be prevented by cleaning the mouth, avoiding eating sweet-tasting food and brushing the teeth, preferably using fluoride toothpaste.
4. pH of the soil in the backyard
Soils with pH values in the alkaline region or highly acidic region are not favourable for the growth of plants. Hence, a farmer should treat the soil of his fields with quick lime/slaked lime/chalk.
5. Self-defense by animals and plants through chemical warfare
Have you ever experienced a honeybee sting? A bee sting leaves an acidic substance that induces pain and irritation. Applying a mild base, such as baking soda, to the affected area provides relief. Nettle is an herbaceous plant that thrives in natural environments. Its leaves are equipped with stinging hairs that can cause discomfort when accidentally touched. The source of this irritation is the methanoic acid secreted by the plant. An age-old remedy for such stings involves rubbing the affected area with the leaves of the dock plant.
Table 2.5 Some naturally occurring acids
2.6 SALTS
2.6.1 Family of salts
A salt is a compound formed by the reaction of an acid with a base in which the hydrogen of the acid is replaced by the metal.
H2 SO4 + NaOH → (NaHSO4) + (H2O) Sodium hydrogen Water sulphate
Salt hydrolysis: Salt hydrolysis may be defined as "the reaction of cation (or) anion (or) both of a salt with water to produce an acidic or basic solution or both"
The process of salt hydrolysis is the reverse process of neutralisation.
Salt + Water → Acid + Base (or) BA + H2O → HA + BOH
Cationic hydrolysis: In some salts, cations are more reactive than anions. Then, cations react with water to produce H+ ions and makes the solution acidic in nature. This is called cationic hydrolysis.
B+ + H2 O → BOH (Weak base) + H+

Anionic hydrolysis: In some salts, anions are more reactive than cations. Then, anions react with water to produce OH- ions, and the solution becomes basic. This is called anionic hydrolysis.
A⁻ + H2O → HA (Weak acid) + OH
Types of salts based on their hydrolysis
Salts are classified into 4 types based on their hydrolytic behaviour. They are
1. Salts of strong acid and strong base: These salts do not hydrolyse. In aqueous solutions, they give cations and anions, and the resulting solution is neutral.
Examples: NaCl, NaNO3, Na2SO4, KCl, KNO3, K2SO4, etc.
Let us consider aqueous solution of NaCl. NaCl dissociates in water to give Na+ and Cl ions. If these ions react with water, the products would be NaOH and HCl, which are strong electrolytes and undergo complete ionisation. Then, H+ and OH⁻ ions recombine to form undissociated water molecules. So, it is neutral.
2. Salts of strong acid and weak base : The aqueous solutions of salts of this type are acidic in nature because the cation of the salt is reactive, and this type of salt undergoes cationic hydrolysis to form weak base and H+ ions.
Examples: NH4Cl, CuSO4, NH4NO3, AlCl3, CaCl2, etc.
Let us consider the aqueous solution of NH4Cl
NH4Cl produces NH4⁺ and Cl ions.
NH4+ ion is more reactive than Cl ion. Then, NH4+ ion react with water, giving a weak base, NH4OH, and H+ ions.
NH4+ + H2O ⇌ NH4OH + H+
∴[H+] > [OH-]. So, the solution is acidic in nature.
3. Salts of weak acid and strong base : The aqueous solutions of salts of this type are basic in nature because the anion of this salt is reactive, and the salt undergoes anionic hydrolysis to form weak acid and OH- ions.
Examples: CH3COONa, Na2CO3, K2CO3, Na3PO4, etc.
Let us consider the aqueous solution of CH3COONa
CH3COONa(aq)→ CH3COO (more reactive ) + Na+ (less reactive)
CH2COO⁻ + H2O ⇌ CH3COOH (weak acid) + OH
∴ [OH-] > [H+], Hence, the solution is basic in nature.
4. Salts of weak acid and weak base : Maximum hydrolysis occurs in this type of salts because both the cation and anion are reactive and react with water to produce H⁺ and OH⁻ ions. Then, the solution may be neutral, acidic, or basic.
Examples: CH3COONH4, (NH4)2CO3, NH4CN, NH4F, AlPO4, etc.
(i) The solution is neutral if the rate of cationic hydrolysis = rate of anionic hydrolysis.
(ii) The solution is acidic if the rate of cationic hydrolysis > the rate of anionic hydrolysis.
(iii) The solution is basic if the rate of cationic hydrolysis < anionic hydrolysis.
Let us consider the aqueous solutions of CH3COONH4.
CH3COONH4(aq) → CH3COO⁻ + NH4⁺
CH3COO + H2O ⇌ CH3COOH + OH
NH4⁺ + H2O ⇌ NH4OH + H⁺
Both reactions take place at the same speed, so the solution is neutral in nature.
Types of salts
1. Normal salt: A salt that doesn't contain any replaceable hydrogen atoms or hydroxyl group is called a normal salt.
Examples :
Salts
Sulphuric acid ( ) 242444244 3 ,,,,, NaSO KSOCaSOMgSOAlSOZnSO , etc.
Hydrochloric acid 22324,,,,,, KClNaClCaClMgClAlClZnClNHCl , etc.
Nitric acid ( ) ( ) ( ) 33333 KNO,NaNO,CaNO,MgNO,ZnNO222 , etc.
Phosphoric acid ( ) ( ) 34343444 23,,, KPONaPOCaPONHPO , etc.
Table 2.6 Examples of normal salts
2. Acidic salt: A salt formed by the partial replacement of the ionisable hydrogen ion of an acid by a cation is called acidic salt. The acidic salts can further react with alkalis to form normal salts.
Examples:
Acids
Acidic Salts
Sulphuric acid ( ) 4442KHSO,NaHSO,CaHSO , etc.
Sulphurous acid ( ) ( ) 333322NaHSO,KHSO,CaHSO,MgHSO , etc.
Phosphoric acid ( ) 24242424 2 NaHPO,KHPO,CaHPO,NaHPO , etc.
Table 2.7 Examples of acidic salts
3. Base salt: A salt formed by the partial replacement of the ionisable hydroxyl ion of a base by an anion is called basic salt.
Cu(OH)2 + HCl ⟶ Cu(OH)Cl + H2O
Examples:
(i) Basic copper nitrate [Cu(OH)NO2]
(ii) Basic lead nitrate [Pb(OH)NO3]
4. Double salt: The salt produced by the crystallisation of two simple salts from a mixture of their saturated solution is called double salt.
Examples:
i) Potash alum: K2 SO4⋅Al2(SO4)3⋅24H2O
ii) Mohr's salt: FeSO4⋅(NH4)2SO4⋅6H2O
5. Mixed salt: A mixed salt contains more than one acidic or basic ions other than hydrogen or hydroxyl ions.
Examples:
i) Sodium potassium sulphate, (NaKSO4)
ii) Disodium potassium phosphate, (Na2KPO4)
6. Complex salt : A complex salt is like a double salt and is formed by the crystallisation from saturated salt solutions of two simple salts.
Examples:
i) Sodium argento cyanide, Na[Ag(CN)2]
ii) Potassium mercuric iodide, K2[HgI4]
iii) Potassium Ferricyanide, K3[Fe(CN)6]
7. Deliquescent salt: Some salts, on exposure to the atmosphere, absorb moisture from the atmosphere, dissolve in it and change into a liquid; such salts are called deliquescent salts.
Examples :
(i) Calcium Chloride, CaCl2
(ii) Magnesium Chloride, MgCl2
(iii) Copper Nitrate, Cu(NO3)2
(iv) Zinc Chloride, ZnCl2
(v) Ferric Chloride (FeCl3)
8. Neutral salt: Salts which are formed by the complete neutralisation of strong acid and strong base are called neutral salt. e.g., Na2SO4, KCl, RbNO3, KClO4, CaCl2, MgSO4, Mg(NO3)2 , etc.
9. Acidic salt: Salts which are formed by the neutralisation of strong acid and weak base are called acidic salt e.g., ZnCl2, FeCl3, SnSO4, PbSO4, PdSO4, PdCl2, FeCl2, etc.
10. Basic salt: Salts which are formed by the neutralisation of strong base and weak acid are called basic salt e.g., Na2CO3, CH3COONa, (HCOO)2Ca, Na3PO4, Ca(C2O4), etc.
2.6.2 General properties of
salts
a. Reaction with an acid: When a salt reacts with an acid, another salt and acid are formed. For example, when sodium chloride is heated with sulphuric acid, sodium hydrogen sulphate (at low temperature), and then sodium sulphate (at high temperature) is produced, and hydrogen chloride gas is evolved.
NaCl + H2SO4 → NaHSO4 + HCl(at low temperature)
2NaCl + H2SO4 → Na2SO4 + 2HCl (at high temperature))
b. Reaction with a base: A salt reacts with a base to produce another salt and base.
(NH4)2SO4 + 2NaOH → Na2SO4 + 2NH4OH
c. Reaction with a metal: Sometimes, a salt solution may react with a metal. For example, when an iron nail is dipped into an aqueous solution of copper sulphate, copper gets deposited on the surface of the nail, and the ferrous sulphate that forms remains in the solution.
CuSO4 + Fe → FeSO4 + Cu(↓)
This reaction shows that iron is more reactive than copper. Thus, a more reactive metal can displace a less reactive metal from a solution of its salt.
d. Behaviour of salts towards water: When a salt is dissolved in water, the solution may be neutral, acidic, or alkaline. This depends upon the nature of the salt used.
1) A normal salt derived from a strong acid and a strong base gives a neutral solution. For example, the aqueous solutions of NaCl and K2SO4 are neutral to litmus.
2) A normal salt derived from a weak acid and a strong base gives an alkaline solution. For example, the aqueous solutions of both sodium carbonate (Na2CO3) and sodium acetate (CH3COONa) are alkaline.
Na2CO3 + 2H2O → 2NaOH + CO2 + H2O
CH3COONa + H2O → CH3COOH + NaOH
2.6.3 Uses of salts
Salts Uses
1) An essential requirement of our food
2) In the preservation of food
3) In preserving fish and meat
Sodium chloride
Sodium carbonate
Sodium bicarbonate
Potassium nitrate
4) In making a freezing mixture, which is used by ice-cream vendors
5) In the manufacture of soaps
1) As washing soda for cleaning clothes
2) Used in the manufacture of glass, paper, textiles, caustic soda, etc.
3) In the refining of petroleum
4) In fire extinguishers
1) used as baking soda
2) In fire extinguishers
3) As an antacid in medicine
1) To make gunpowder, fireworks, and glass
2) As a fertiliser in agriculture
1) Commonly called 'blue vitriol', used as a fungicide to kill certain germs
Copper sulphate
Potash alum
2) In electroplating
3) In dyeing
1) Used to purify water, makes suspended particles in water settle down
2) As an antiseptic
3) In dyeing
Table 2.8 Uses of salts
2.6.4 Some important terms related to salts
Terms Examples
Anhydrous salt
A salt that does not contain any water of crystallisation is called anhydrous salt.
Sodium chloride [NaCl]
Sodium Nitrate [NaNO3] Lead nitrate [Pb(NO3)2]
Hydrated salt
A salt that contains a definite number of molecules of water attached loosely to its one molecule is called hydrated salt.
Water of crystallisation
The number of water molecules that are loosely attached to one molecule of a salt is called water of crystallisation.
Deliquescent substances
The water-soluble substances which absorb the moisture of the air and then dissolve in absorbed moisture to change into a liquid state are called deliquescent substances, and the phenomenon is called deliquescence.
Hygroscopic substances
The substances that absorb moisture from the air, but do not change their state are called hygroscopic substances.
Efflorescent salts
Hydrated crystalline salts, which partly or wholly lose their water of crystallisation when exposed to air to form a powdery mass, are called efflorescent salts, and the phenomenon is called efflorescence.
pH of salts
Copper sulphate pentahydrate [CuSO4.5H2O]
Sodium carbonate decahydrate [Na2CO3.10H2O]
Sodium sulphate decahydrate [Na2SO4.10H2O]
Ferrous sulphate heptahydrate [FeSO4.7H2O]
Zinc sulphate heptahydrate [ZnSO4.7H2O]
Calcium chloride hexahydrate [CaCl2.6H2O]
Anhydrous calcium chloride [CaCl2]
Anhydrous magnesium chloride [MgCl2]
Anhydrous ferric chloride [FeCl3]
Potassium hydroxide [KOH]
Sodium hydroxide [NaOH]
Mercuric nitrate [Hg(NO3)2]
Quick lime [CaO]
Conc. sulphuric acid [H2SO4]
Phosphorus pentoxide [P2O5]
Washing soda [Na2CO3.10H2O]
Blue vitriol [CuSO4.5H2O]
Glauber's salt [Na2SO4.10H2O]
Epsom salt [MgSO4.7H2O]
Green vitriol [FeSO4.7H2O]
Table 2.9 Important terms related to salts
The pH value of salts is influenced by the nature of the acid and base involved in their formation. Salts derived from a strong acid and a strong base are neutral, exhibiting a pH value of 7. Conversely, salts resulting from a strong acid and a weak base tend to be acidic, with a pH value less than 7. On the other hand, salts formed from a strong base and a weak acid are basic in nature, displaying a pH value greater than 7.
2.6.5 Chemicals from common salt
Seawater is a natural reservoir containing various dissolved salts, including sodium chloride, which can be extracted from these saline sources. Additionally, solid salt deposits, often brown in colour due to impurities, are discovered in various regions and referred to as rock salt. These substantial
crystal formations originated from the desiccation of ancient seas, forming rock salt beds that are extracted through mining, like coal.
Common salt as raw material
The common salt obtained through various processes serves as a vital raw material for manufacturing various everyday items, including sodium hydroxide, baking soda, washing soda, bleaching powder, and numerous other products. Examining how a single substance is employed in the production of such diverse materials is intriguing.
2.6.6 Sodium hydroxide
Sodium hydroxide is generated through the electrolysis of an aqueous solution of sodium chloride, commonly known as brine. This electrochemical procedure is termed the Chlor-alkali process, highlighting the production of chlorine and sodium hydroxide as its primary outcomes.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Chlorine gas is released at the anode, while hydrogen gas is generated at the cathode. In proximity to the cathode, a solution of sodium hydroxide is formed. All three products resulting from this process have practical applications.
2.7 SALTS AND THEIR APPLICATIONS
2.7.1 Bleaching powder
Chlorine is produced through the electrolysis of aqueous sodium chloride (brine), as you've learned. This chlorine gas becomes integral in the manufacturing process of bleaching powder. The production of bleaching powder involves the reaction of chlorine with dry-slaked lime [Ca(OH)2]. While represented as CaOCl2, it's important to note that the actual composition of bleaching powder is more intricate.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Uses of bleaching powder
Bleaching powder finds applications in various sectors
(i) In the textile industry, it is employed for bleaching cotton and linen, in paper factories for bleaching wood pulp, and in the laundry for bleaching washed clothes.
(ii) It serves as an oxidising agent in numerous chemical industries.
(iii) It is utilised to purify drinking water by eliminating germs.
2.7.2 Baking soda
Baking soda is a frequently used kitchen ingredient for preparing crispy pakoras and other dishes. Occasionally, it is included to expedite the cooking process. The chemical name of this compound is sodium hydrogen carbonate (NaHCO3), and it is manufactured using sodium chloride as one of its raw materials.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Uses of baking Soda
In the formulation of baking powder, a blend of baking soda (sodium hydrogen carbonate) and a gentle edible acid, like tartaric acid, is employed. When baking powder undergoes heating or is mixed with water, the ensuing reaction is as follows-
NaHCO3 + H+ → CO2 + H2O + Sodium salt of acid
The carbon dioxide produced in this reaction contributes to the rising of bread or cake, resulting in a soft and spongy texture. Furthermore-
• Sodium hydrogen carbonate is a component in antacids. Its alkaline nature allows it to neutralise excess acid in the stomach, providing relief from acidity.
• Additionally, sodium hydrogen carbonate is used in soda-acid fire extinguishers.
2.7.3 Washing soda
Another chemical that can be derived from sodium chloride is commonly known as washing soda. As previously discussed, sodium carbonate can be obtained by heating baking soda, and through recrystallization of sodium carbonate, washing soda is produced. Like sodium carbonate, washing soda is also categorized as a basic salt.
Na2CO3 + 10H2O → Na2CO3.10H2O (Sodium carbonate)
Uses of washing soda
Like sodium hydrogen carbonate, sodium carbonate also holds significance in various industrial processes. The uses of washing soda include
(i) Sodium carbonate (washing soda) finds applications in the glass, soap, and paper industries.
(ii) It is utilised in the production of sodium compounds like borax.
(iii) Sodium carbonate serves as a cleaning agent for domestic purposes. It is employed to eliminate the permanent hardness of water.
2.8 MORE ABOUT SALTS
2.8.1
Water of crystallization
While we commonly associate crystals with being dry, salts, especially in their natural form, often contain water molecules within their crystal structures. This water is known as the water of crystallisation.
Water of crystallisation refers to the fixed number of water molecules present in one formula unit of a salt. For instance, one formula unit of copper sulphate contains five water molecules, as denoted by its chemical formula CuSO4.5H2O.
So, in a strict sense, the crystals of salts are not completely dry. The presence of water molecules in the crystal structure, known as water of crystallization, can give the appearance of dryness, but chemically, these crystals may contain water. This water content is fixed and specific to each salt, and it plays a role in the physical properties of the salt crystals.
For example, one formula unit of Sodium carbonate contains 10 water molecules, as denoted by its chemical formula Na2CO3.10H2O.
Another salt that contains water of crystallization is gypsum. Gypsum has two water molecules as part of its crystalline structure, and its chemical formula is (CaSO4⋅2H2O).
Now, let's explore the applications of this salt.
2.8.2
Plaster of Paris
When gypsum is heated at 373 K, it undergoes dehydration, losing water molecules to form calcium sulphate hemihydrate (CaSO4 1 2 H2O), commonly known as Plaster of Paris. This substance is
utilised by doctors as a plaster for setting fractured bones. Plaster of Paris appears as a white powder, and upon mixing it with water, it reverts to gypsum, forming a hard solid mass.
The chemical reaction representing this transformation is: CaSO4⋅ 1 2 H2O + 1 1 2 H2O → CaSO4⋅2H2O (Plaster of Paris) (Gypsum)
It's important to note that only half a water molecule is represented as attached, indicating water of crystallisation. This representation is employed because two formula units of CaSO4 share one water molecule. Plaster of Paris finds application in crafting toys, making decorative materials, and smoothening surfaces.
The term 'Plaster of Paris' originated from the fact that a large deposit of gypsum was discovered near Paris, France. Gypsum, when heated to a certain temperature, loses water molecules and transforms into calcium sulphate hemihydrate, commonly known as Plaster of Paris. The name
"Plaster of Paris" has since become synonymous with this material, reflecting its widespread use in various applications such as medical casts, sculptures, and construction due to its unique setting properties and versatility.
QUICK REVIEW
• Acid-base indicators are compounds or combinations of compounds with dye properties that are utilised to reveal the presence of acids and bases.
• Types of natural indicators:
Colour Indicators - Red litmus, blue litmus
Olfactory Indicators - Onion extract, Vanilla essence, Clove oil
• Synthetic Indicators - Methyl orange, Phenolphthalein, Methyl red, Thymol blue.
• Physical Properties :
Acids - Sour taste, turns blue litmus to red litmus, provides H+ ions, pH less than 7.
Bases - Bitter taste, turns red litmus to blue, provides OH- ions, pH more than 7 up to 14.
• The acidic nature of a substance stems from the formation of H+ (aq) ions in the solution, while the basic nature results from the formation of OH- (aq) ions.
• In the reaction between an acid and a metal, hydrogen gas is released, and a corresponding salt is generated.
• Similarly, when a base reacts with a metal, hydrogen gas is produced along with the formation of a salt containing a negative ion composed of the metal and oxygen.
• When an acid reacts with a metal carbonate or metal hydrogen carbonate, it yields the corresponding salt, carbon dioxide gas, and water.
• Both acidic and basic solutions in water conduct electricity due to the production of hydrogen and hydroxide ions, respectively.
• Arrhenius acids and bases : HCl, H2SO4, HNO3, H2CO3, and NaOH, KOH, NH4OH, Ca(OH)2
• Bronsted-Lowry's concept of acids and bases : H2O, HCl, H2SO4 and H2O, NH4, HSO4-, OH-
• Lewis’s acids and bases H+, BF3, SiCl4, Ag+ and H2O, NH3, Cl
• The potency of an acid or alkali is assessed through a measurement known as the pH scale, which ranges from 0 to 14, indicating the concentration of hydrogen ions in a solution.
• A solution is considered neutral with a pH of precisely 7, while a solution is acidic if its pH is less than 7 and basic if it is more than 7.
• Living organisms conduct their metabolic activities within an optimal pH range.
• The process of mixing concentrated acids or bases with water is highly exothermic and requires caution.
• Acids and bases neutralise each other, resulting in the formation of corresponding salts and water.
• Water of crystallisation denotes the fixed number of water molecules present in one formula unit of a salt.
• Salts find diverse applications in everyday life and various industries.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Introduction to acids, bases, and salts
1. Among the following, the Arrhenius acid is
a. H2CO3
b. NH4OH
2. Among the following, the Arrhenius base is
a. H2CO3
b. Ca(OH)2
3. Among the following, the Bronsted-Lowry acid is
a. HSO4
b. CN
4. Assertion (A): HNO3 is an acid.
c. Fe(OH)2
c. Na2O
c. CO3-2
d. CO2
d. HNO3
d. NH3
Reason (R): According to the Arrhenius concept, an acid is a substance which produces hydrogen ions when dissolved in water.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
5. Assertion (A): Potassium hydroxide is a base.
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
Reason (R): According to Arrhenius Concept, base is a substance which produces hydroxyl ions when it dissolved in water.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
6. Statement (A): H3PO4 acts as an acid, according to the Arrhenius theory.
Statement (B): Ca(OH)2 acts as a base, according to the Arrhenius theory.
Statement (C): NH3 acts as a base, according to the Arrhenius theory.
a. A, B, and C are correct
b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect
d. A and B are incorrect, and C is correct
7. Statement (A): HSO4⁻ acts as an acid, according to the Bronsted-Lowry theory.
Statement (B): CN acts as a base, according to the Bronsted-Lowry theory.
Statement (C): H2O acts as a base as well as an acid, according to the Bronsted-Lowry theory.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
8. Statement (A): Generally, acids contain hydrogen atoms.
Statement (B): All compounds containing hydrogen are not acids. Statement (C): Glucose contains hydrogen, but it is not an acid.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
II. Understanding the chemical properties of acids and bases
1. Among the following, the monobasic acid is
a. KOH b. HClO4
2. Among the following, the diacidic base is
c. H2SO4 d. H3PO4
a. HCOOH b. NH4OH c. Mg(OH)2
3. Statement (A): H2CO3 is a dibasic acid. Statement (B): HNO3 is a monobasic acid. Statement (C): CH3COOH is a tribasic acid.
a. All A, B, C are correct
c. A, B are correct, and C is incorrect
4. Statement (A): NH4OH is a monoacidic base. Statement (B): Ca(OH)2 is a diacidic base. Statement (C): Al(OH)3 is a triacidic base.
b. A, B, C are incorrect
HNO2
d. A, B are incorrect, and C is correct
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
5. Assertion (A): HCl is a binary acid.
Reason (R): In HCl, one hydrogen atom and one chlorine atom are present.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
6. Assertion (A): HCN is a weak acid.
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct.
Reason (R): HCN undergoes ionisation to only a small extent.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct.
7. Acids react with oxides of metals to form salt and __________.
a. Base b. Water c. H2 gas d. CO2 gas
8. MgO reacts with HCl to form H2O and
a. Mg(OH)2
b. MgH2
9. Acids react with bases to produce
c. MgCl2 d. CO2 gas
a. Acid b. Base c. Salt d. H2 gas
10. Assertion (A) : HCl solution turns blue litmus paper to red.
Reason (R) : HCl is an acid.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
11. Assertion (A) : According to the Arrhenius theory, acids have acidic properties in aqueous solutions.
Reason (R): All acids produce H+ ions in aqueous solution.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
12. Statement (A ) : Acids are sour in taste. Statement (B) : Acids turn blue litmus paper red. Statement (C) : Bases are bitter to taste.
a. A, B, and C are correct
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
13. Statement (A) : Acids react with bases to produce salt and water. Statement (B) : Base reacts with acidic oxides to give salt and water. Statement (C) : Bases turn red litmus paper blue.
a. A, B, and C are correct
b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
14. Statement (A): Bases are bitter in taste. Statement (B): Bases turn red litmus paper blue. Statement (C): NH4OH is an example of a base.
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
15. Statement (A): Acids are sour in taste.
Statement (B): Acids turn blue litmus paper red. Statement (C): Acids show acidic properties only in the absence of water.
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
16. Statement (A): Acids react with metal to produce hydrogen gas. Statement (B): HNO3 is an example of an acid. Statement (C): Alcohol and phenols are acids.
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
17. Statement (A): Metals react with acids and liberate hydrogen gas. Statement (B): Metal carbonates react with acids and liberate carbon dioxide gas.
Statement (C): Metal oxides react with acids to form salt and water.
a. A, B, and C are correct
b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
III. pH concept and pH scale
1. If the H+ ion concentration is 10-4 M, then the pH is
10
4
2. If the H+ ion concentration is 10-3 M, then the pOH is
3
13
3. What is the pH of 0.05M Ba(OH)2
1
14
11
4. The pH of an aqueous solution having 1.825 g of HCl in 500 ml of solution is
0
13
5. Assertion (A): Aqueous HCl is acidic in nature. Reason (R): In HCl solution, [H+] > [OH-].
1
2

12
14
a. Both A and R are correct, and R is the correct explanation of A b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, R is incorrect
6. Assertion (A): Aqueous NaOH is basic in nature.
Reason (R): In NaOH solution, [H+] < [OH-].
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
d. A is incorrect, R is correct
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
7. 0.4 g of NaOH (mol. wt = 40) is present in 1 litre of solution. Its pH value is a. 1
13
14
8. The pH of an aqueous solution having 0.49 g of H2SO4 in 100 ml of solution is
1
9. pH of 0.1N NaOH solution is
1
2
2
10. The pH of 10-3 M monoacidic base, if it is 1% ionised, is
5
8
3
12
3
12
4
13
9
11. Assertion (A) : If the concentration of H+ ions increases, then the pH of the solution decreases.
Reason (R) : If the concentration of OH- ions increases, then the pH of the solution decreases.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
12. Statement (A) : Pure water is a good conductor of heat and electricity.
Statement (B) : At 250 C, Kw=1.0×10-24 moles2/lit2
Statement (C) : As the temperature increases, the pH of the solution increases.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
13. Statement (A) : During indigestion, the pH of our gastric juice is below 1.4.
Statement (B) : The pH of our blood is in the range of 7.36-7.42.
Statement (C) : When the pH of our mouth is below 5.5, the teeth start decaying.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
IV. Salts, its applications and more about salts
1. Among the following, the salt is
a. HCl
b. KOH
2. Among the following, the mixed salt is
a. Na2SO4
b. NaHSO4
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
c. NaOH
c. NaKSO4
3. Among the following, the normal salt of hydrochloric acid is
a. NaOCl
b. KCl
4. Assertion (A): K2SO4 is a normal salt.
c. NaOH
Reason (R): K2SO4 does not contain any replaceable hydrogen atoms.
a. Both A and R are correct, and R is the correct explanation of A
d. NaCl
d. H2SO4
d. MgSO4
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, R is incorrect
5. Assertion (A): Cu(OH)NO3 is a basic salt. Reason (R): CuSO4 is an acidic salt.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
6. Statement (A): KHSO4 is an acidic salt. Statement (B): K3PO4 is a normal salt. Statement (C): Cu(OH)Cl is a basic salt.
d. A is incorrect, R is correct
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect d. A and B are incorrect, and C is correct
7. Statement (A): Na[Ag(CN)2] is a complex salt. Statement (B): K2SO4Al2(SO4)324H2O is a double salt. Statement (C): NaKSO4 is a mixed salt.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
8. Among the following,the complex salt is
a. Na2KPO4
c. NaHSO4
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
b. K2[HgI4]
d. KClMgCl2[H2O]
9. The reaction, Pb(OH)2 + HNO3 → Pb(OH)NO3+ H2O shows that Pb(OH)NO3 is
a. Acidic salt
WORKSHEET - 2
b. Basic salt
c. Base
d. Acid
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. An aqueous solution of MgSO4 is acidic due to the hydrolysis of
a. SO4-2
c. Both Mg+2 and SO4-2
2. The nature of the aqueous solution of KCN is
a. Acidic
b. Basic
b. Mg+2
d. Neither Mg+2 nor SO4-2

c. Neutral
3. Among the following, the aqueous solution of the salt having the lowest pH is
a. NaOH
b. Na2CO3
d. Amphoteric
c. NH4Cl d. NaCl
4. Among the following, the aqueous solution of the salt having the lowest pH is
a. 5×10-9
b. 5×10-8
c. 5×10-7
d. 5×10-6
5. Assertion (A): NH4Cl is a salt of strong acid and weak base
Reason (R): The aqueous solution of NH4Cl is basic in nature
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
6. Assertion (A): CH3COONa in aqueous medium is basic in nature.
Reason (R): It is a salt of a weak acid and a strong base.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
7. Statement (A): The aqueous solution of CH3COONH4 is neutral in nature
Statement (B): The aqueous solution of NaNO3 is neutral in nature
Statement (C): The aqueous solution of Na2CO3 is Acidic in nature.
a. A, B, and C are correct
c. A and B are correct, and C is incorrect
8. Among the following, Bronsted-Lowry's base is
a. NaOH
b. NH3
9. Among the following, the false statement is
a. Na2O is a Bronsted-Lowry base
c. An aqueous solution of NH4Cl is acidic
10. Assertion (A): CH3COOH is a dibasic acid.
b. A, B, and C are incorrect
d. A and B are incorrect, and C is correct
c. NH4+ d. H2SO4
b. An aqueous solution of CH3COONa is basic
d. An aqueous solution of FeCl3 is acidic
Reason (R): CH3COOH produces one H+ ion by ionisation.
a. Both A and R are correct, and R is the correct explanation of A
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, R is incorrect d. A is incorrect, R is correct
11. Assertion (A): Boric acid is a monobasic acid. Reason (R): Boric acid ionises to give only one H+ ion in a solution.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
12. Statement (A): Acetic acid is an example of organic acid. Statement (B): HNO3 is an example of inorganic acids. Statement (C): All organic acids are strong acids.
a. A, B, and C are correct b. A, B, and C are incorrect
c. A and B are correct, and C is incorrect
d. A and B are incorrect, and C is correct
13. Calcium hydroxide [Ca(OH)2] reacts with carbon dioxide to produce
a. CaO b. CaC2 c. CaCO3 d. CaCl2
14. Assertion (A): Aqueous solutions of acids conduct electricity.
Reason (R): Acids ionise in water.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
15. Assertion (A): Pure HCl does not turn blue litmus paper to red.
Reason (R): Acids show acidic properties only in the presence of water.
a. Both A and R are correct, and R is the correct explanation of A
c. A is correct, R is incorrect
16. The ionic product of water is represented by
b. Both A and R are correct, and R is not the correct explanation of A
d. A is incorrect, R is correct
a. Kh b. K a c. K w
17. If the H+ ion concentration of the solution is 10-7 M, then the solution is
a. Acidic
Kb
b. Basic c. Neutral d. None
18. A solution turns blue litmus to red. The pH of the solution is probably
8
10
19. pH value of 0.001 M NaOH solution is a. 3 b. 10
12
11
6
13
20. The pH of 0.0001 M NaOH solution is a. 9 b. 10 c. 11 d. 12
21. The pH of 0.001 M HNO 3 solution is a. 0 b. 1
2
22. 5.6 g of KOH (mol. wt = 56) is present in 1 litre of solution. Its pH value is a. 1
13
23. The pH of 10-8 M HCl solution is
7.99
6.99
14
5.99
24. The pH of HCl is 5. It is diluted by 1000 times. Its pH will be a. 5 b. 8
2
3
0
4.99
6-7
25. If the pH of a solution is increased from 3 to 6, its hydrogen ion concentration will be (EAMCET 1996 & 1998)
a. Reduced to half
c. Reduced by 1000 times
b. Doubled
d. Increased by 1000 times
26. The pH of 0.1 molar solution of the acid HQ is 3. The value of the ionization constant, Ka, of this acid is (AIEEE 2014)
a. 3×10-1 b. 3×10-3 c. 3×10-5
3×10-7
27. If the ionic product of Ni(OH) 2 is 1.9×10-15, the molar solubility of Ni(OH)2 in 1.0 M NaOH is (EAMCET 2014)
a. 1.9×10-18 M b. 1.9×10-13 M c. 1.9×10-15 M
1.9×10-14 M
28. Which of the following is Lewis acid? (JEE-MAINS 2018)
a. PH3 b. NF3 c. NaH d. B(CH3)3
29. If you spill a chemical toilet cleaning liquid on your hand, your first aid would be (IIT JEE 2020)
a. Vinegar
c. Aqueous NaHCO3
b. Aqueous NaOH
d. Aqueous NH3
30. The pH of the solution containing 50 mL each of 0.10 M sodium acetate and 0.0 1M acetic acid is [given pKa of CH3COOH = 4.57 ] (NEET -2022)
31. The pH of 0.005M H2SO4 is (EAMCET -1993) a. 2.5 b. 4.5
32. The pH of a 0.001M aqueous solution of sodium hydroxide will be (EAMCET -1993)
5.0
9.0
33. The ionic product of water [H+][OH-] at 25°C is (EAMCET -1993)
a. 1×10-14 b. 1×10-13 c. 1×10-7
1×10-12
34. The nature of a 0.1M solution of sodium bisulphate is (EAMCET -1993)
a. Acidic b. Alkaline c. Neutral d. Weakly acidic
35. The pH of 0.05M H2SO4 is (EAMCET -1994) a. 5 b. 1.3010 c. 2.6990 d. 1
36. The pH of a solution is 5.0. Its hydrogen ion concentration is reduced by a hundred times. The solution will then be (EAMCET -1995)
a. Neutral b. Acidic c. Basic d. Remains same
METALS AND NON-METALS 3
3.1 INTRODUCTION
At present, there are 118 elements known to us, but all of them do not occur in a free state in nature; some of them have been synthesized by using artificial methods, which are known as synthetic elements.
Based on their properties, they are mainly classified into metals and non-metals. Metals elements lose electrons, form positive ions, and show an electropositive nature.
Example: Na ⟶ Na+ + e-, Mg ⟶ Mg2+ + 2e-
Non-metal elements may gain electrons, form negative ions and show an electronegative nature.
Example: Cl + e- ⟶ Cl-, O + 2e- ⟶ O2-
Apart from these, some elements show the properties of both metals and non-metals, and these are known as metalloids.
Metals are used in almost every aspect of life as they are widely used to make defence equipment, ornaments, catalysts, and are used in various synthetic processes. In general, nearly 80% of all elements are metals.
Non-metals are essential for life as oxygen is used by plants and animals for their survival and also in various combustion reactions in homes, factories, aeroplanes and missiles. Sulphuric acid, which consists of sulphur non-metal, is an important industrial chemical and is regarded as king of chemicals.
3.1.1 Bonding in metals
Generally, metals are very hard and have high density as metal atoms are very closely packed by metallic bonds.
Metallic bonding is formed by the electrostatic attractive force between the conduction electrons present in the metallic lattice and the positively charged metal atom core.
Metal lattice consists of two different parts:
i) Electrons that are present in the valence shell, known as valence electrons, which can be easily removed from the atom, are free to move in the lattice.
ii) The positively charged core of the atom, known as the kernel, is the combination of the nucleus and all the shells except the valence shell.
Each kernel is surrounded by a number of valence electrons and vice versa, where the kernels have negligible movements compared to valence electrons. The force of attraction between valence electrons and the positively charged kernels forms metallic bonds.
3.1.2 Physical properties of metals
The metals show physical properties such as electrical conductivity, thermal conductivity, malleability, ductility, lustre, etc. which are the result of these metallic bonds.
Metallic lustre
The property of a metal that shows a shiny surface is called 'metallic lustre'. Metals have lustre, mostly when they are in their pure state.
Example: Cu, Ag, Au, Mg, Al, etc.
When a photon of light strikes the surface of a metal, the atoms absorb it, and the excited electrons start vibrating and release energy as light, which gives lustre to the metal surface. Due to this lustre, metals like gold, silver and platinum have a very high ornamental value.
When the metal surface is exposed to environmental conditions, it undergoes a chemical reaction called corrosion, which causes loss in metallic lustre.
Hardness
Metals are usually very hard and strong, and they can hold large amounts of weight without breaking. The hardness depends on the degree and strength of the metallic bond.
Exceptions: Sodium and magnesium, as they are soft and can be cut by a knife.
Melting and boiling points
Generally, metals have very high melting and boiling points due to strong metallic bonds.
Example: The melting points of iron and copper are 1538° C and 1085° C, respectively.
Exceptions: Elements like gallium (m.pt. = 302K ) and caesium (m.pt. = 301K ) show exceptionally low melting points. Metals such as sodium and potassium also show identical behaviour. Also, mercury metal exists in a liquid state at room temperature.
Malleability
The property that allows the metals to be converted into thin sheets is called malleability. Metals such as gold and silver show highly malleable properties, and they can be easily beaten into thin sheets. Aluminium metal shows a malleable nature, and its sheets/foils are used for packing food items, chocolates, medicines, etc. Copper metal is used to make kitchen utensils and containers due to its malleable nature. Iron metal shows less malleability than the above elements. Still, it can be beaten into thick sheets that are used to make boxes, drums, water tanks, etc., due to their durability.
Ductility
Ductility is the property that allows the metals to be drawn into thin wires. Gold metal is the most ductile metal known, where 1g of gold can be drawn into a 2 km long wire. Copper and aluminium metals also show very high ductility properties as their wires are used in electric fittings. Magnesium, iron and tungsten are also ductile, and tungsten filaments are used in electric bulbs.
Electrical and thermal conductivity
Metals show good electrical conductivity due to the availability of free electrons in the metallic lattice that carries the charge. Silver metal shows high conductivity among all metals. Copper and aluminium metals are used in electric fittings because of their high conductivity and cheap availability. Whereas, metals such as iron and aluminium are used to make utensils and cookware. Metals also show good thermal conductivity because of free electrons that are present in the lattice that arise by conduction. Silver and copper are best known as good conductors, but metals such as mercury and lead are also known for being bad conductors of heat.
Density
Generally, all metals possess high densities except alkali metals. This high-density property of metals is because of the presence of close-packed structures where strong attractive forces exist between the atoms of metals. The metal that has the highest density is osmium and the lowest is lithium.
Tensile strength
Metals exhibit high tensile strength, which refers to their ability to withstand significant loads without breaking.
Exceptions: Zinc, gallium, mercury, sodium, calcium, and potassium, which are not tenacious. Iron shows high tensile strength and is used in the construction of bridges, railway lines, machines, vehicles, etc.
Sonority
When metals are hit by a hammer, they produce a characteristic metallic sound, which is called the sonority property of metals.
Physical state
All metals exist in solid form when kept at normal conditions. [Exception: Mercury]
Metals can form homogeneous mixtures with other metals, which are called alloys. For example, copper and zinc form a homogenous mixture, which is called a brass alloy.
3.1.3 Physical properties of non-metals
Electrical and thermal conductivity
Non-metals generally show covalent bonding, which is formed by the sharing of electrons by more than one atom. Hence, no free electrons are present in the lattice (covalent), which makes them bad conductors of heat and electricity, i.e. insulators.
Exception: Graphite, which is an allotropic form of carbon known for its electrical and thermal conductivity as a single electron per atom, is not involved in bond formation which is free to move throughout the lattice.
Strength and brittleness
Non-metals are brittle, i.e., they break easily upon applying force. Solid non-metals sulphur and phosphorus show a very high brittle nature. Non-metals do not show ductility or malleability properties because of their weak attractive forces between their atoms. The only non-metal diamond shows ductility properties to some extent.
Density
The non-metal molecules are mostly bonded by covalent bonds in a single molecule. They do not show closely packed structures due to large molecule sizes and also have low densities.
Example: i) Phosphorus exists as P4 molecules and has a density of 1.82 gcm-3 ii) Sulphur exists as S8 molecules and has a density of 2.10 gcm-3.
Non-lustrous
Non-metals do not show the shine property, but graphite and iodine are the only non-metals that show metallic lustre.
Low-tensile strength
Solid non-metals show low tensile strength, and they can be easily broken.
Non-sonorous

Non-metals do not produce sound when hit with a hammer, as they are brittle in nature.
Physical state
Non-metals are present in solid, liquid or gaseous state at room temperature. For example, sulphur and phosphorus are present in the solid state, bromine in the liquid state, whereas hydrogen, nitrogen, and oxygen are gases under room temperature conditions.
Allotropy
Allotropy is the property of an element that exists in more than one structural form. Allotropy is shown by non-metals where the different forms of an element are shown, called allotropic forms or allotropes.
Example
• Phosphorus (P) exists in five different forms: white or yellow phosphorus, red phosphorus, violet phosphorus, black phosphorus, and scarlet phosphorus.
• Carbon (C) exists in the following allotropic forms: diamond, graphite, coal, coke, lamp black, etc.
• Sulphur also exists in various allotropic forms, such as rhombic sulphur, monoclinic sulphur, plastic sulphur, etc.
3.2 CHEMICAL PROPERTIES OF METALS
Metals show electropositive nature where they can lose one or more electrons present in the valence shells (subshells) of their atoms to form cations. After losing one or more electrons, metals achieve the electronic configuration of the nearest noble gas element.

3.2.1 Formation of metal oxides
Most of the metals react with oxygen to form metal oxides that show basic nature.
Metal + Oxygen ⟶ Metal oxide
Chemical reactions of some metals
• Sodium reacts with oxygen at room temperature to form its oxides as it reacts vigorously with oxygen and easily starts burning when kept open in the air. Hence, they are stored under kerosene oil to prevent them from reacting with oxygen present in the air. 4Na + O2⟶(2Na2O) Sodium oxide
• Magnesium burns in the presence of oxygen upon heating to form magnesium oxide.
• Iron reacts with oxygen, which gives a mixture of FeO.Fe2O3 (iron II, III oxides) or Fe3O4.
• Copper metal, in reaction with oxygen, forms a solid black mass of copper (II) oxide or cupric oxide.
• Silver and gold metals do not react with oxygen even at very high temperatures; hence, called noble or inert metals.
Burns to form Magnesium Oxide
Burns with sparks to form Iron oxide
Table 3.1 Reaction of metals with oxygen
+ O2 ⟶ 2MgO
+ 3O2 ⟶ 2Fe2O3
Metals like aluminium, iron, zinc, etc., show less reactivity towards oxygen than expected as per their positions in the activity series. They are converted into oxides by reacting with atmospheric oxygen, which gets coated on the metal surfaces and limits the reaction to the surface only.
Most of the metal oxides are insoluble in water, but oxides of group I and II elements form alkalis.
Na2O(s) + H2O(l) ⟶ 2NaOH(aq) Sodium oxide (Basic oxide) Water
Sodium hydroxide (An alkali)
K2O(s) + H2O(l) ⟶ 2KOH(aq)
Potassium oxide (Basic oxide) Water
Potassium hydroxide (An alkali)
3.2.2 Nature of the metal oxides (Reaction of the metal oxides)
Action with water
Oxides of metals such as sodium, potassium, magnesium, etc., dissolve in water to form soluble hydroxides known as alkalis.
MgO(s) + H2O(l)⟶ Mg(OH)2(aq)
Oxides of metals such as calcium and aluminium react with water, forming insoluble hydroxides that remain as suspension.
CaO(s) + H2 O(l)⟶ Ca(OH)2(s)
Al2 O3(s) + 3H2 O(l)⟶ 2Al(OH)3(s)
Action with acids
The metal oxides react with dilute acids to form their salts and water, which are basic in nature.
CaO(s) + 2HCl(aq)⟶ CaCl2(aq)+ H2O(l)
MgO(s) + 2HCl(aq)⟶ MgCl2 (aq) + H2O(l)
Al2 O3(s) + 6HCl(aq)⟶ 2AlCl3(aq)+ 3H2O(l)
ZnO(s) + 2HCl(aq)⟶ ZnCl2 (aq) + H2O(l)
Aluminium oxide and zinc oxide are amphoteric in nature, which means they show both an acidic and basic nature, and they react with alkalis like sodium hydroxide and potassium hydroxide to form salt and water.
Al2O3(s) + 2NaOH(aq) ⟶
ZnO(s) + 2NaOH(aq) ⟶
2NaAlO2(aq) + H2O(l)
Sodium meta-aluminate
Na2ZnO2(aq) + H2O(l)
Sodium zincate
3.2.3 Reaction with water (Formation of oxides and hydroxides)
Highly reactive metals such as sodium and potassium that are placed higher in the series react violently with cold water and form hydrogen gas, releasing some energy.
2K(s)+2H2O(l) ⟶2KOH(aq)+H2(g)+ heat energy
2Na(s)+2H2O(l)⟶2NaOH(aq)+H2 (g) + heat energy
Less reactive metals such as calcium reacts less with water, while magnesium reacts with water only on heating
Ca(s)+2H2O(l)⟶Ca(OH)2(aq)+H2(g)
Mg(s)+2H2O(l) Heat → Mg(OH)2(aq)+H2(g)
Metals such as aluminium, iron and zinc do not react with hot or cold water. But they show a reaction with boiling water (steam) to form the metal oxide and hydrogen.
2Al(s)+3H2O(g)⟶Al2O3(s)+3H2(g)
Zn(s)+H2O(g) ⟶ZnO(s)+H2(g)
3Fe+4H2O(g)⟶Fe3 O4(s)+4H2(g)
3.2.4 Reaction with acids (Formation of metal salts)
Metals that are placed above hydrogen in the activity series react with dilute acids like hydrochloric acid and sulphuric acid to displace hydrogen from acids that form metal salt with the evolution of hydrogen gas.
Metal + Dilute acid ⟶ Metal salt + Hydrogen
2Na(s)+2HCl(dil.)⟶2NaCl(aq)+H2(g)
Mg(s)+H2 SO4 (dil.) ⟶MgSO4(aq)+H2(g)
Ca(s)+2HCl(dil.)⟶CaCl2(aq)+H2(g)
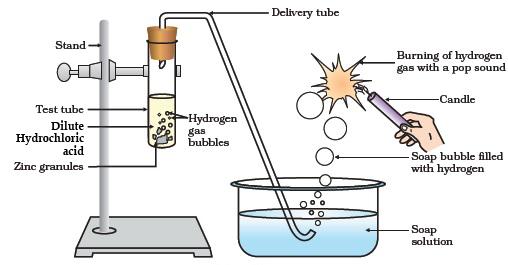
3.2.5 Reaction of metals with salt solutions
More reactive metals can displace less reactive metals from the aqueous solutions of their salts, and these reactions are known as metal displacement reactions.
More reactive metal zinc displaces the metal copper from the aqueous solution of its salts, forming ZnSO4.
Zn(s) + CuSO4(aq) ⟶
Zinc Copper sulphate
ZnSO4(aq) + Cu(s)
Zinc sulphate Copper
Iron displaces copper from the aqueous solution of its salts, forming FeSO4.
Fe(s) + CuSO4(aq) ⟶
Iron Copper sulphate
FeSO4(aq) + Cu(s)
Ferrous sulphate Copper
Copper displaces silver from the aqueous solution of its salts, forming copper nitrate.
Cu(s) + 2AgNO3(aq) ⟶
Copper Silver nitrate
Cu(NO3)2(aq) + 2Ag(s)
Copper nitrate Silver
Magnesium displaces copper from the aqueous solution of its salts, forming magnesium sulphate.
Mg(s) + CuSO4(aq) ⟶
Magnesium Copper sulphate
MgSO4(aq) + Cu(s)
Magnesium sulphate Copper
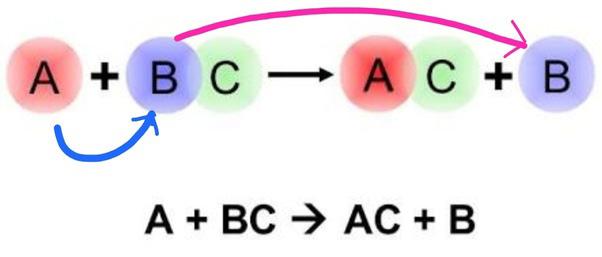
Fig. 3.3 Displacement reaction representation
3.3 ACTIVITY SERIES OF METALS
Metals can be arranged in the order of decreasing reactivity in a series, which is called the activity or reactivity series of metals. The chemical reactivity of metals is linked with their relative positions in the activity series, where a metal placed higher in the series is more reactive as compared to the metal which occupies a lower position.
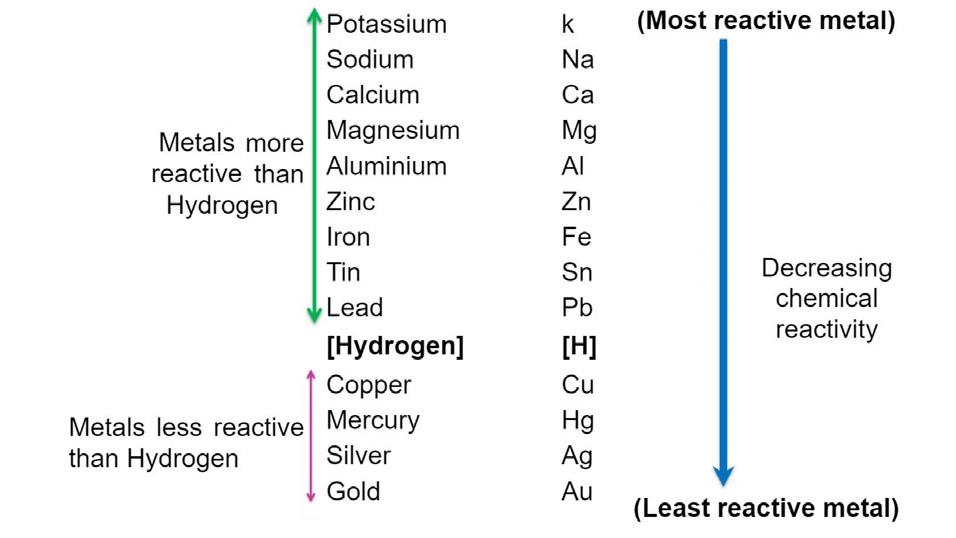
Fig. 3.4 Activity series
Characteristics of activity series of metals
• Metals are arranged in this series on the basis of their reactivity of these metals to give up their electrons to form ions.
• Higher metal in the series shows a greater tendency to form ions in solution.
• The reactivity series provides a classification as the metals that are placed in a decreasing order of reactivity, and the most reactive metals are placed on top, and as we move down, the reactivity of the metals decreases.
• The series also shows which metal will displace the other in a solution, as metals placed above will displace the metals placed below them.
3.4 CHEMICAL PROPERTIES OF NON-METALS
Non-metals show a highly electronegative nature as they have the urge to accept electrons in their valence shells to achieve the configuration of the nearest noble gas. Hence, they act as good oxidising agents forming such anions.
Example: Reaction with Oxygen (Formation of oxides)
Non-metals react with oxygen on heating to form their oxides, and they further react with water to form acids and are therefore acidic in nature.
Reaction of carbon-
Reaction of sulphur-
Reaction of phosphorous-
+ 5O2(g)
Sulphurous acid
Phosphorous pentoxide (Oxide)
P4O10(s) + 6H2 O(aq) ⟶ 4H3 PO4(aq)
Phosphoric acid
3.5 METALS AND NON-METALS REACTION WITH EACH OTHER
3.5.1 lonic compounds
In ionic compounds, ions are held together by specialised bonds called ionic bonds. In an ionic bond, two oppositely charged ions are held through electrostatic forces.
We know that metal atoms have loosely bound valence electrons in their valence shell, and nonmetal atoms need electrons in their valence shell.
Hence, by losing electrons, metal atoms change to cations and by accepting electrons, non-metals form anions. These oppositely charged ions are bound by electrostatic forces, which are called ionic bonds or electrovalent bonds.
Formation of ionic compounds
Metal atom loses electrons by gaining ionisation energy, and these electrons are then accepted by non-metal atom whose electron affinity is positive, which leads to the formation of ionic compound.
Example: Sodium chloride, as sodium (Na) atom has a single electron in the valence shell, which is donated to the chlorine (Cl) atom that has seven valence electrons. By losing one electron, the Na atom changes to Na+cation with electronic configuration 2, 8 that resembles the previous-noble gas neon(Ne). By accepting one electron, the Cl atom changes to Cl-anion with electronic configuration 2,8,8 that resembles the configuration of the next noble gas argon(Ar).
Examples:
i) Formation of sodium chloride:

ii) Formation of calcium fluoride:

iii) Formation of magnesium oxide:

3.5.2 Properties of ionic compounds
Physical states
Most ionic compounds are solids and exist in the form of crystals, as ions are bound by strong attractive forces. The ions are surrounded by oppositely charged ions in the form of crystal structures that show 3-D geometry with a definite number of ions. These ions are held together by strong electrostatic forces, which means they are not free to move. The ions are the fundamental units of ionic compounds, and these crystals differ in their shapes and lustre because of their difference in the nature of the arrangement of the ions in the crystal lattice.
Melting and boiling points
Since the ionic compounds have strong forces of attraction, they show very high melting and boiling points.
Solubility
Generally, ionic compounds are soluble in water, and insoluble in solvents like kerosene, petrol, etc.
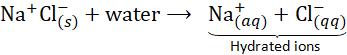
Electrical conductivity
Ionic compounds show electrical conductivity in solutions or in molten state, but not in solid state as solid state ions are very closely packed and are not free to move.
3.6 OCCURRENCE OF METALS
Metallurgy deals with the different methods of extracting metal from its natural sources and then converting them into useful materials so the mankind.
Majority of metals are available in the combined state in the earth's crust. Abundance of various elements in the earth's crust in the order: Oxygen > Silicon > Aluminium > Iron > Calcium > Sodium > Potassium > Magnesium.
3.6.1 Terms related to metallurgy
1. Minerals: Naturally occurring materials from which metals can be extracted.
2. Ores: Minerals from which the metals may be extracted economically in reasonably pure conditions.
Example: Aluminium is the most common metal in earth's crust, occurring in most minerals. However, it cannot be economically extracted from most of these minerals. Instead, the usual ore from which it is profitable to extract is bauxite, which contains 50–70% of Aluminium oxide.
3. Gangue: Rocky and earthly impurities present in ores. Gangue may be acidic (like SiO2) or basic (like FeO).
4. Flux: A substance added in the furnace during smelting to remove gangue.
For haematite (Fe2O3) ore, lime stone (CaCO3) is used as flux to remove SiO2 gangue.
5. Slag: A substance formed when flux reacts with gangue.
SiO2 + CaCO3 → CaSiO3 + CO2 (Gangue) (Flux) (Slag)
6. Roasting: Heating the concentrated ore in the presence of air to remove volatile impurities like S, As, carbon moisture, etc., and to render the ore porous.
Example:
2 ZnS(s) + 3 O2(g)→ 2 ZnO(s) + 2 SO2(g) Zinc blende
7. Smelting: The process of reducing roasted ore and removing the gangue.
8. Calcination: This method is commonly used to convert hydrated oxides or hydroxides and carbonates into respective oxides. It involves heating the ore below its fusion temperature in the absence of air.
9. Heating the ore below its fusion temperature in the absence of air is known as calcination. This method is commonly used to convert hydroxides and carbonates into respective oxides.
Examples:
CaCO3(s) CaO(s)+CO2(g)
MgCO3(s) MgO(s)+CO2(g)
10. Pyro metallurgy: Extraction is done using heat energy. The metals like Cu, Fe, Zn, Pb, Sn, Ni, Cr, Hg, etc., which are found in nature in the form of oxides, carbonates, and sulphides, are extracted by this process.
11. Hydrometallurgy: Extraction of metals involving aqueous solution is known as hydrometallurgy. Silver, gold, etc., are extracted by this process.
12. Electrometallurgy: Extraction of highly reactive metals such as Na, K, Ca, Mg, Al, etc., by carrying electrolysis of one of the suitable compounds in a fused or molten state.
Metals occur mostly as their oxide, carbonate, sulphide, halide, silicate, sulphate, and phosphate minerals. Important types of minerals, their composition and the constituent metals are given in the table. Type of mineral
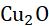
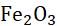
Zincite
Oxide minerals
Bauxite
Cassiterite (or) Tin stone
Pyrolusite
Chromite
Pitch blende
Calamine
Siderite
Lime stone (or) marble
Magnesite
Dolomite
Malachite
Azurite

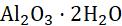
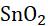
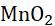
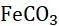

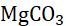
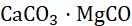
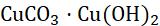





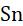
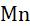
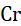



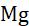
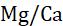


Sulphide minerals
Iron pyrites

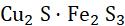
Copper pyrites (or)
Copper glance
Zinc blende (or)
sphalerite
Pentlandite
Galena
Argentite (or) silver glance
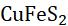
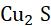
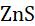

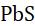





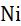


Halide minerals
Sulphate minerals
Phosphate minerals
Silicate minerals
Cryolite (or) 3NaF.AlF3

Common salt (or) rock salt NaCl
Carnalite
Horn silver
Gypsum
Anglesite
Barytes
Monazite
Phosphorite
Monite
Zircon
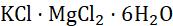

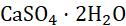
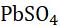
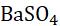

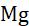



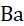
Phosphates of lanthanides and thorium Th
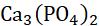
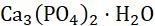



Thorite Th
Kaolinite (a form of clay)
Asbestos
Garnierite
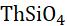

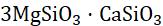
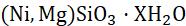
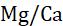
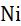
Table 3.2 Important types of minerals and their composition
Extraction of metals from ores involves the following major steps. Concentration of ore, isolation of metal from its concentrated ore and refining of the crude metal.
Compounds
Minerals Ores
Concentration of ore
Hand picking
Electromagnetic separation
Froth floatation
Liquation
Leaching
Methods of extraction
Metallurgy
Extraction of crude metal

Purification or refining
Electrolytic reduction
Reduction by using reducing agents
Self or auto reduction
Hydraulic washing
3.6.2 Concentration of ore
Distillation
Cupellation
Poling
Electrolytic refining
Zone refining
Liquation
The partial purification of ore by removing gangue from it is known as ore dressing or concentration of ore or benefaction.
The following methods are employed for the concentration of ore:
Hand-picking: Lumps of the ore are broken into small pieces. This separation works based on the differences in colour and size of the impure substances.
Hydraulic washing: This method is also called gravity separation or levitation. It is based on the difference in specific gravities of the ore and gangue particles. The sand and mud adhering to the ore are washed away by a stream of water.
Electromagnetic separation: This method is used if the gangue or the ore particles are magnetic in nature.
Froth floatation process: This method is used for the concentration of sulphide ores like galena, Zinc blende, cinnabar, Copper pyrites, iron pyrites, etc. The process is based on different wetting properties of gangue and the ore with water and oil.

Liquation: This method is useful for such ores which contain easily fusible mineral particles and high melting gangue.
Leaching: In this method, the powdered ore is treated with certain reagents in which the ore is soluble, but the impurities are not soluble
3.6.3 Extraction of crude metal
Extraction of crude metal
Based on reactivity series of metals
Metals at top of the reactivity series
Metals in middle of the reactivity series
Metals at bottom of the reactivity series
K, Na, Ca, Mg, AI Zn, Pb, Fe, Cu Hg, Ag, Pt, Au
Electrolytic reduction
Reduction by using reducing agents Heating or Displacement
Electrolytic reduction
The oxides of highly electropositive metals like Na, K, Mg, Ca, Al, etc., cannot be reduced easily with carbon at moderate temperatures. For example, a very high temperature is required at which the metal may combine with carbon to form a carbide. These metals are thus extracted by the electrolysis of their oxides, hydroxides or chlorides in a fused state. Sometimes, a small amount of some other salt is added to lower the fusion temperature, to increase the conductivity or both. Production of sodium metal from a fused mixture of NaCl and CaCl2 is done by electrolytic reduction (Down's process).
The metal is liberated at the cathode. Sodium is obtained by the electrolysis of a fused mixture of NaCl and CaCl2 (Down's process) or by electrolysis of fused sodium hydroxide (Castner's process).
NaCl → Na+ + Cl-
At cathode ∶Na+ + e → Na
At anode ∶ Cl → Cl + e-
2Cl → Cl2
Aluminium is obtained by the electrolysis of alumina mixed with cryolite.
3.6.4 Reduction of metal oxides based on the activity series
The following table shows the various methods of reduction of metallic oxides based on the activity series.
3.6.5
Refining or purification of crude metal
The process of purifying impure metals is called refining of metals. The important methods used are
a) Liquation process: This process is based on the difference in fusibility of the metal and impurities. When the impurities are less fusible than the metal itself, this process is employed. This method is used to purify the metals like Bi, Sn, Pb, Hg, etc.
b) Distillation: This process is used for metals that are easily volatile. This is used for the purification of Zn, Cd, Hg, etc.
c) Pyrometallurgical oxidation process: This process is used when the impurities have a greater affinity for oxygen than the metal itself. This method is usually employed for refining metals like Fe, Cu, Ag, etc. The oxidation is done in various ways.
i) Cupellation: The impure metal is heated in a cupel or oval-shaped crucible made of bone ash or cement, and a blast of air is passed over the molten mass. For example, the impurity of lead present in silver is removed by the cupellation process.
ii) Bessemerisation: The impure metal is heated in a furnace, and a blast of compressed air is blown through the molten mass.
iii) Poling: The impure metal containing oxides as impurity can be purified by this method. The molten, impure metal is stirred with green poles of wood. This method is especially used in the purification of copper (old method).
d) Electrolytic refining of metals: Many metals such as copper, silver, gold, aluminium, lead, etc., are purified by this method. The impure metal is made anode, while a thin sheet of pure metal acts as a cathode.
3.11 Electrolytic refining of metals
i) Zone refining or fractional crystallisation: Elements such as Si, Ga, etc., which are used as semiconductors, are refined by this method. Highly pure metals are obtained. The method is based on the difference in solubility of impurities in the molten and solid states of the metal.
3.7 CORROSION
Metal
Corrosion in the presence of Compound formed after corrosion Colour
Iron Moisture and oxygen
Fe2O3.XH2 O
Aluminium Moist air Al2O3
Copper Damp air, CO2 and moisture CuCO3.Cu(OH)2
Reddish brown
Dull appearance
Green or greenish yellow
Silver Air and H2 S Ag2S Black
Table 3.3 Corrosion of metals
1. Bell metal 80% Copper and 20% Tin In making bells
2. Brass 70-80% Copper and 30-20% Zinc In making gears and bearing
3. Bronze 80-95% Copper and 20-5% Tin In making statues, coins, etc.
4. Duralumin 95% Aluminium, 4% Copper, 0.5% Magnesium, and 0.5% Manganese
In making some parts of aeroplanes
5. Gun metal 88% Copper, 10% Tin, and 2% Zinc In making gears and bearings
6. German silver 25-50% Copper, 24-35% Zinc, and 10-35% Nickel. In making utensils and artistic materials
7. Magnalium 90-98% Aluminium, 2-10% Magnesium In making tools
8. Solder 67% Tin and 33% Lead In soldering metals
9. Rolled gold Cu 95%, Al 5% Artificial jewellery
10. Stainless steel Fe 89.4%, Cr 10%, Mn 0.35%, C 0.25% Utensils, ornamental pieces.
11. Elektron Mg 95%, Zn 5% Construction of aircraft
12. Type metal Pb 82%, Sb 15%, Sn 3% Making printing types
13. Britannia Sn 93%, Sb 5%, Cu 2%
14. Wood's metal Bi 50%, Pb 25%, Sn 12.5%, Cd 12.5%
15. Nichrome Ni 60%, Cr 15%, Fe 25%
16. Constantan Ni40%, Cu60%
17. Monel metal Ni 70%,Cu30%
18. Invar Ni 35%, Steel 65%
Tableware
Electric fuses and other safety devices
Electrical resistances
Electrical resistances
Chemical plants
Surveying instruments, pendulums, chronometer
Table 3.4 Table list of different alloys with composition and uses
QUICK REVIEW
• A mineral from which a metal can be extracted economically and conveniently is called ore.
• The impurity present in the ore is called gangue.
• The substance added to the ore to remove gangue from it is called flux
• The elements or compounds of the metals which occur in nature in the earth's crust are called minerals
• Slag: This is obtained by the reaction of flux and impurity present in the ore.
• Activity series: The descending order of reactivity of metals.
• Calcination is a process of heating the ore strongly in the absence of air or oxygen.
• During calcination, carbonate is converted to its oxide.
• Roasting is a process of heating the ore strongly in a free supply of air or oxygen.
• Calcination and Roasting are carried out in a reverberatory furnace
• Corrosion: The destruction of metal surfaces by the action of air and moisture. Prevented by electroplating, galvanising and painting.
• Alloys: Alloying is a method of improving the properties of a metal. Example: 24 carat gold, bronze.
• Extraction of highly reactive metals such as Na, K, Ca, Mg, Al, etc., by carrying electrolysis of one of the suitable compounds in a fused or molten state.
• Methods of extraction
The extraction of metal from its ore involves mainly three stages: concentration, extraction of crude metal, and refining of the metal.
a) Concentration of ore: Concentration of ore can be done through:
i) Handpicking ii) Hydraulic washing iii) Electromagnetic separation iv) Froth floatation
v) Leaching
b) Extraction of crude metal
i) Electrolytic reduction ii) Reduction by using reducing agents iii) Self or auto-reduction
c) Purification
i) Distillation ii) Cupellation iii) Poling iv) Electrolytic refining v) Zone refining vi) Liquation
• Distillation is used for metals that are easily volatile. This is used for the purification of Zn, Cd, Hg, etc.
• In cupellation, the impure metal is heated in a cupel or oval-shaped crucible made of bone ash or cement, and a blast of air is passed over the molten mass.
• In poling, the impure metal containing oxides as impurity can be purified by this method. The molten, impure metal is stirred with green poles of wood.
• In zone refining, elements such as Si, Ga, etc., which are used as semiconductors, are refined by this method.
• Liquation process is based on the difference in the fusibility of the metal and the impurities.
• Electrolytic refining: Many metals such as copper, silver, gold, aluminium, lead, etc., are purified by this method. The impure metal is made anode, while a thin sheet of pure metal acts as a cathode.
• The destruction of metal surfaces by the action of air and moisture is called corrosion.
WORKSHEET - 1

MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Introduction & physical properties
1. Which metal cannot be used as a reducing agent in smelting?
a. C b. Al
c. Cu d. Na
2. The method used for obtaining highly pure silicon, which is used as a semiconductor material is
a. Oxidation
c. Crystallization
b. Electrochemical
d. Zone refining
3. When metal Z is added to dilute HCl solution, there is no evolution of gas. Metal is
a. K
c. Ag
4. Which of the following is an oxide ore?
a. Bauxite
c. Haematite
5. The poorest conductor of heat is
a. Aluminium
c. Gold
6. Brass is a mixture of
a. Copper and zinc
b. Copper and tin
c. Copper, nickel and zinc
d. Aluminium, copper and traces of Mg and Mn
b. Na
d. Zn
b. Cuprite
d. All of these
b. Silver
d. Lead

7. Duralumin is a mixture of the number of components present in a binary solution of
a. Copper and zinc
b. Copper and tin
c. Copper, nickel and zinc
d. Aluminium, copper and traces of Mg and Mn
II. Chemical properties of metals and non-metals
1. Which of the following elements produces basic oxide when reacting with oxygen?
a. Chlorine
c. Phosphorus
b. Sulphur
d. Magnesium
2. Which of the following steps is not involved in the metallurgy of iron?
a. Calcination
b. Smelting
c. Concentration of ore
d. Conversion of ore into oxide
3. Which of the following metals form amphoteric oxide?
a. Copper
c. Aluminium
b. Silver
d. Iron
4. Beakers A, B and C contain Zinc sulphate, silver nitrate and iron (II) sulphate solutions, respectively. Copper pieces are added to each beaker. The blue colour will appear in case of
a. Beaker A
c. Beaker C
5. Pure gold is
a. 14 carat
c. 18 carat
b. Beaker B
d. All the beakers
b. 24 carat
d. 22 carat
6. The process of electrolysis is used for obtaining such metals, which are
a. Highly reactive
c. Highly unreactive
b. Moderately reactive
d. All types of metals
7. Which one of the following is not a method of concentration of metal ores?
a. Gravity separation
c. Electromagnetic separation
8. The zone refining process is used for the
a. Concentration of an ore
c. Purification of metal
9. The purpose of smelting an ore is
a. To oxidise it
c. To separate volatile impurities
b. Froth floatation process
d. Smelting
b. Reduction of a metal oxide
d. Purification of an ore
b. To reduce it
d. To obtain an alloy
10. Metals like copper, mercury and lead are obtained from their oxide ores by
a. Carbon reduction
c. Self-reduction
III. Occurrence of metals
1. Which of the following is true?
a. A mineral need not be an ore
b. Aluminium reduction
d. Electrolytic reduction
b. An ore can’t be a mineral
c. All ores are not minerals
d. All minerals are ores
2. Which of the following is not found in the native state?
a. Pt
c. Au
3. Copper pyrite ore is concentrated by
a. Electromagnetic method
c. Froth floatation method
4. The most abundant metal in the earth’s crust is
a. Oxygen
c. Zinc
b. Cu
d. Na
b. Gravity separation method
d. All the above methods
b. Aluminium
d. Iron
5. Among the following, which is not a concentration technique?
a. Hand-picking
c. Hydraulic washing
b. Froth floatation
d. Distillation
6. Oxides of highly electropositive metals like Na, K, Mg, Ca, Al, etc., are extracted by
a. Self-reduction
c. Reduction by using reducing agents
IV. Extraction of crude metal
b. Electrolytic reduction
d. Heating with C or CO
1. Which of the following metals cannot displace hydrogen from cold water?
a. K
c. Na
b. Al
d. Mg
2. A common metal that is used for the extraction of some metals from their oxides is
a. Cr
c. Mn
b. Fe
d. Al
3. PbS (Galena) ore can be concentrated (physical method) by
a. Magnetic separation
c. Poling
b. Froth floatation
d. Hand-picking
4. Statement (A): In the extraction of Mg from fused anhydrous MgCl2, the air gap of the electrolytic cell is to be replaced by inert gas.
Statement (B): Oxidation of Mg metal is to be prevented during its extraction by electrolytic reduction of anhydrous MgCl2.
Statement (C): Sodium is extracted by electrolysis of aqueous sodium chloride solution.
a. All the above statements are correct
b. All the above statements are incorrect
c. A and B are correct, and C is incorrect
d. A and B are incorrect, and C is correct
5. Assertion (A): Au, Pt, Ag, etc., are found in a free state.
Reason (R): The metals which are noble and chemically less reactive are found in a free state.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
V. Refining or purification of crude metal
1. In electrolytic refining, the pure metal is made to act as
a. Anode
c. Cathode
2. Tin and lead can be refined by
a. Cupellation
c. Poling
b. Oxidizing agent
d. Both (a) & (b)
b. Liquation
d. Bessemerisation
3. During the process of electro-refining of copper, some metals present as impurities settle down as anode mud. These are
a. Sn and Ag
c. Ag and Au
b. Pb and Zn
d. Fe and Ni
4. Assertion (A): Metals of high purity are obtained by zone refining.
Reason (R): Impurities are more soluble in the melt than in pure metal.
a. Both A and R are true, and R is the correct explanation of A
b. Both A and R are true, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
5. Slag is a product of
a. Flux and coke
c. Flux and impurities
6. Silver containing lead as an impurity is purified by
a. Poling
c. Levigation
7. The meaning of cupellation is
a. Removing impurities by heated air
b. Removing impurities by compressed air
c. Stirring with green poles of wood
b. Coke and metal oxide
d. Metal and flux
b. Cupellation
d. Distillation
d. Heating at high temperatures to remove impure metal
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. Upon heating with Cu2S, the reagent that does not give copper metal is [JEE 2014]
a. CuFeS2
c. Cu2O
b. CuO
d. CuSO4
2. What is the role of limestone during the extraction of iron from haematite ore? [EAMCET 2014]
a. Leaching agent
c. Reducing agent
3. Galvanization is applying a coating of
a. Zn
c. Cr
b. Oxidizing agent
d. Flux
b. Pb
d. Cu
4. Which one of the following ores is best concentrated by the froth floatation method?[JEE 2016]
a. Malachite
b. Magnetite
c. Siderite
d. Galena
5. Extraction of gold and silver involves leaching with CN- ion. Silver is later recovered by [NEET 2017]
a. Liquation
c. Zone refining
b. Distillation
d. Displacement with Zn
6. Match items of Column I with the item of Column II and assign the correct code:
Column I
a) Cyanide process
b) Froth floatation process
c) Electrolytic reduction
d) Zone refining
a. a – iv, b – ii, c – iii, d – i
c. a – iv, b – iii, c – ii, d – i
Column II
i) Ultrapure Ge
ii) Dressing of ZnS
iii) Extraction of Al
iv) Extraction of Au
v) Purification of Ni
b. a – i, b – ii, c – iii, d – iv
d. a – iii, b – ii, c – i, d – iv
7. Zinc can be coated on iron to produce galvanized iron, but the reverse is not possible. It is because [NEET 2016]
a. Zinc is lighter than iron.
b. Zinc has a lower melting point than iron.
c. Zinc has a lower negative electrode potential than iron.
d. Zinc has a higher negative electrode potential than iron.
8. The roasting of sulphides gives the gas X as a byproduct. This is a colourless gas with a choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic and acts as a reducing agent, and its acid has never been isolated. The gas X is
a. H2S
c. CO2
b. SO2
d. SO3
9. Calamine, malachite, magnetite and cryolite, respectively are
a. ZnSO4, CuCO3, Fe2O3, AlF3
b. ZnSO4, Cu(OH2), Fe3O4, Na3AlF6
c. ZnCO3, CuCO3. Cu (OH)2, Fe3O4, Na3AlF6
d. ZnCO3, CuCO3, Fe2O3, Na3AlF6
10. Which one is malachite from the following? [NEET2019-20]
a. CuFeS2
c. Fe3O4
11. Identify the correct statement from the following:
b. Cu(OH)2
d. CuCO3.Cu(OH)2
a. Pig iron can be moulded into a variety of shapes.
b. Wrought iron is impure iron with 4% carbon.
c. Blister copper has a blistered appearance due to the evolution of CO2.
d. Vapour phase refining is carried out for nickel by the Van Arkel method.
12. In isolation of which one of the following metals from their ores is the use of cyanide salt not commonly involved? [JEE MAINS -22]
a. Zinc
c. Silver
13. Non-metals generally act as
a. Oxidizing agents
c. Both (a) and (b)
b. Gold
d. Copper
b. Reducing agents
d. None of these
14. An element 'X' forms an oxide XO2, which is a very useful gas used in the photosynthesis process. The element is
a. Sulphur
c. Nitrogen
b. Carbon
d. Phosphorus
15. The acid formed when sulphur trioxide reacts with water is
a. Sulphurous acid
c. Both (a) and (b)
b. Sulphuric acid
d. None of these
16. Which of the following ores cannot be concentrated by electromagnetic separation?
a. Chromite
c. Magnetite
b. Pyrolusite
d. Cuprite
17. Which of the following oxides cannot be reduced with carbon to obtain the metal?
a. MnO2
c. Cr2O3
b. Al2O3
d. None of these
18. The process of zone refining is used in the purification of
a. Si
b. Ag
c. Al d. Cu
19. Flux is used to remove
a. Basic impurities
c. All types of impurities
20. The gravity separation method is based upon
b. Acidic impurities
d. Both acidic and basic impurities
a. Preferential washing of ores and gangue particles
b. The difference in densities of ore particles and impurities
c. The difference in chemical properties of ore particles and impurities
d. None of these
21. The process of removing lighter gangue particles by washing in a current of water is called
a. Levigation
c. Liquation
22. Which ore contains both iron and copper?
a. Cuprite
c. Copper pyrite
b. Leaching
d. Cupellation
b. Chalcocite
d. Malachite
23. Hydrogen gas is not widely used as a reducing agent because-
a. Hydrogen decomposes to atomic hydrogen at higher temperatures.
b. Hydrogen isomerizes to ortho hydrogen at higher temperatures.
c. Many metals form hydrides at lower temperatures.
d. There is a risk of explosion from oxygen and hydrogen in the air.
24. A large volume of copper (II) sulphate solution is left in an iron container overnight. Identify the correct statement.
a. The solution evaporates completely, and some copper (II) sulphate crystals are left behind.
b. The part of the container in contact with the solution is coated with copper.
c. Some fine iron particles are formed in the solution.
d. Atmospheric oxygen reacts with the copper (II) sulphate to give black copper (II) oxide.
25. Metals are refined by using different methods. Which of the following metals are not refined by electrolytic refining?
(i) Au (ii) Cu (iii) Na (iv) K
a. (i) and (ii)
c. (ii) and (iii)
26. Another name for refining technique:
a. Enrichment
c. Purification
27. Both calcination and roasting can be performed in
a. Reverberatory furnace
c. Muffle furnace
28. Carnallite is an ore of
a. Sodium
c. Magnesium
29. Which of the following is acidic flux
a. CaO
c. BaO
30. Calcination is the process of heating the ore
a. In inert gas
c. In the absence of air
31. Roasting is carried out in case of
a. Galena
c. Copper glance
32. Smelting is usually carried out in
a. Blast furnace
c. Muffle furnace
b. (i) and (iii)
d. (iii) and (iv)
b. Concentration
d. Froth floatation
b. Blast furnace
d. Electric furnace
b. Potassium
d. Aluminium
b. MgO
d. SiO2
b. In the presence of air
d. In the presence of CaO and MgO
b. Iron pyrites
d. All of these
b. Open hearth furnace
d. Electric furnace
33. The chemical formula of the compound formed by the rusting of copper metal is
a. CuCO3 Cu(OH)2
c. Cu(OH)2
34. The percentage of chromium in stainless steel is
a. 25%
c. 10 %
b. CuCO3
d. CuCO3 H2O
b. 89.4%
d. 0.35 %
CHEMICAL BONDING 4
4.1 CHEMICAL BOND
Matter is made up of the same or different kinds of elements. Under normal conditions, no other element exists as an independent atom in nature, except noble gases.
Atoms of the same or different types of elements combine to form molecules. Molecules are the aggregates of atoms held together by the forces of attraction, called the chemical bond and behave as a single entity.
4.1.1 Definition of chemical bond
The attractive force that holds two or more atoms or ions together in a molecule or ion is called a chemical bond.
The phenomenon of the union of two or more atoms involving redistribution of electrons, such that each atom involved in bonding acquires a stable electronic configuration in order to gain stability is known as chemical bonding.
4.2 KOSSEL-LEWIS APPROACH
4.2.1 Explanations for the formation of a chemical bond
Atoms combine to form molecules for two reasons. They are:
I. To reduce the energy
1. Every system of the universe tends to lose potential energy and become more stable. That means every system of the universe tends to change from a higher energy state (i.e., a less stable state) to a lower energy state (i.e., a more stable state).
Example: Stretched rubber bands contract; red hot iron radiates heat and cools down; water flows spontaneously from hills to plains.
- Repulsive forces
- Attractive forces
Fig. 4.1 Forces between two atoms
2. In a similar way, when two atoms approach each other, the forces of attraction and repulsion operate between them.
3. The forces of attraction are between the nucleus of one atom and the electrons of another atom.
4. The forces of repulsion are between two nuclei as well as between the electrons of two atoms.
5. If the attractive forces balance the repulsive forces, then the total potential energy of the system decreases and a chemical bond forms as a result.
6. At a distance that is shorter than the equilibrium distance, repulsive forces operate predominantly. Thus, no chemical bond is formed.
7. A chemical bond is said to be formed at the lowest energy of the curve.
Repulsion
Internuclear distance
Bond energy bond length
Equilibrium Attraction
Fig. 4.2 Energy curve in a chemical bond
8. The internal energy of a molecule is less than the sum of the internal energies of individual atoms in that molecule.
Example: In the formation of one mole of hydrogen, 104 K.Cal of energy is released.
H + H → H2 + 104 K.cal
This suggests that hydrogen molecules have less energy than their constituent hydrogen atoms by about 104 K.Cal/mole. So, hydrogen molecules are more stable than hydrogen atoms.
II. Lewis octet rule
1. Noble gas elements except helium have a stable, completely filled ns2 np6 type of electronic configuration (8 electrons) in their outermost shell. This type of electronic configuration is called octet electronic configuration. Helium has a completely filled K shell with two electrons and is also stable (duplet electronic configuration).
2. Because of this stable electronic configuration, noble gases have little or no tendency to combine with each other or with the atoms of any other element.
3. The atoms of all the remaining elements try to attain octet electronic configuration in order to get the maximum stability by participating in the chemical reactions.
'The tendency of atoms to achieve octet electronic configuration in their outermost shell is known as the Lewis octet rule.'
4.2.2 Covalent bond
Complete electron transfer between identical atoms or atoms with very little difference in their electronegativities is not possible.
In such cases, the atoms contribute the electrons in equal numbers and form electron pairs which are shared by both atoms. Bonds formed in this way are referred as covalent bonds.
Covalent bonds were explained by G.N. Lewis.
Definition
A chemical bond formed between two atoms of the same or different elements through equal contribution and mutual sharing of one or more electron pairs in such a way that both atoms acquire the stable electronic configuration of the nearest noble gas is called a covalent bond.
The chemical bond formed between two atoms due to the mutual sharing of electrons is called a covalent bond.
As the electron pairs are shared between two atoms, a covalent bond is also called an electron pair bond.
Classification of covalent bonds
Based on the fact that the electron pair is shared equally or unequally between the atoms, covalent bond is classified into 2 types. They are:
1. Non-polar covalent bond: A covalent bond in which electron pairs are shared equally between two bonded atoms is called a non-polar covalent bond.
Example: H2, O2, N2, F2, Cl2, Br2, I2, CH4, P4, S8, etc.
2. Polar covalent bond: A covalent bond, in which the electron pairs are shared unequally between two atoms such that the bonded atoms acquire partial positive and negative charge is called a polar covalent bond.
Example: HF, HCl, HBr, H2 S, H2 O, NH3, etc.
Characteristic properties of covalent compounds
1. Covalent compounds are often gases, liquids or relatively soft solids at room temperature.
2. Covalent compounds generally have low melting and boiling points.
3. Covalent compounds are neither hard nor brittle.
4. Covalent compounds are soluble in non-polar organic solvents like ether, benzene, etc. Non-polar covalent compounds are insoluble in water, whereas some of the polar covalent compounds are soluble in water.
5. Generally, covalent compounds in their original or molten state or in the solution form are nonconductors of electricity.
6. As the covalent compounds are rigid and directional, covalent compounds exhibit stereoisomerism.
4.2.3 Lewis representation of simple molecules/ions
Chemical bonding mainly depends on the number of electrons present in the outermost shell (valence electrons).
The electrons of the inner shell are well protected and are generally not involved in the chemical bond formation.
G.N. Lewis, an American chemist, introduced simple and convenient notations to represent the valence electrons in an atom. These notations are called Lewis symbols.
Lewis symbols are also known as electron dot symbols.
(or) Zero group
Significance of Lewis symbols
1. The number of dots around a symbol represents the number of valence electrons. This number of valence electrons help us calculate the group number and group valency of an element.
2. From IA to IVA group, valency = group number. From VA to VIIIA group, valency = 8 - group number.
4.2.4 Limitations of the octet rule
1. Incomplete octet of the central atom
In some compounds, the number of electrons surrounding the central atom is less than eight, this is especially the case with elements having less than four valence electrons.
Examples:
Fig. 4.4 Incomplete octet of a central atom
Li, Be, and B have 1,2 and 3 valence electrons only. Other such compounds are AlCl3 and BF3. These compounds are called hypovalent compounds.
2. Odd electron molecules
In molecules with an odd number of electrons, such as nitric oxide (NO) and nitrogen dioxide (NO2), the octet rule is not satisfied for all the atoms.
Note: 3e- bond is equivalent to a half covalent bond.
Example: Superoxide ion(O-2)
3. Expanded octet
Elements in which there are more than eight valence electrons around the central atom. This is termed the expanded octet. Obviously, the octet rule does not apply in such cases.
Some of the examples of such compounds are PF5, SF6, H2SO4, and IF7, etc., including a number of coordination compounds. Such compounds are called hypervalent compounds.
(SF6) (12 e⁻ s around S atom) (PF5) (10 e⁻ s around P atom)
4.2.5 Formal charge
(H2SO4) (12 e⁻ s around S atom)
(IF7) (14 e⁻ s around I atom)
The charge on a molecule is zero. In the case of polyatomic ions, the net charge is possessed by the ion as a whole and not by a particular atom. It is, however, feasible to assign a formal charge to each atom. The formal charge is a factor based on a pure covalent bond formed by the sharing of electron pairs equally by neighbouring atoms.
Definition
The formal charge of an atom in a polyatomic molecule or ion is defined as the difference between number of valence electrons of the atom in a free state and number of electrons assigned to the atom in the Lewis structure.
Formal charge is denoted by Qf and is given by Qf = NA - NM = NA - NLP - 1/2NBP NA is the number of valence electrons in the free atom, NM is the number of valence electrons belonging to the atom in the molecule, NL is the number of electrons in lone pairs, and NB is the number of electrons in bond pairs.
The counting of the number of electrons is based on the assumption that the atom in the molecule owns one electron of each shared bonded pair and both the electrons of the unshared lone pair.
The formal charges do not represent real ionic charges. Formal charges represent a tendency to build up positive or negative charges. It is possible to select a structure of the lowest energy for the given molecule with the help of formal charges. The lowest energy structure is the one with the smallest formal charges on the atoms.
4.2.6 Calculation of formal charge
1. PH3 molecule
The Lewis dot structure is:
4.8 Lewis dot structure of PH3 molecule
The formal charge of P:
Qf = [ NA - NM ] = [ NA - NLP - 1/2NBP ] ={ 5 - 2 - 1/2(6) } = ( 5 - 5 ) = 0
The formal charge of H:
Qf = [ NA - NM ] = [ NA - NLP - 1/2NBP ] = { 1 - O - 1/2(2) } = ( 1 - 1 ) = 0
2. N2O molecule
The Lewis structure is:
Fig. 4.9 Lewis dot structure of N2O molecule
The formal charge of the first N atom is:
Qf = 5 - 4 -1/2(4) = -1
The formal charge of the second N atom is:
Qf = 5 - 0 - 1/2(8) = +1
The formal charge of the O atom is:
Qf = 6 - 4 - 1/2(4) = 0
4.3 IONIC OR ELECTROVALENT BOND
4.3.1 Definition of ionic bond
The electrostatic force of attraction between two oppositely charged ions which is formed by the transfer of one or more electrons from one atom to another is called an ionic bond
An ionic bond is also called an electrovalent bond or polar bond.
Lattice energy
Lattice is a systematic three-dimensional arrangement of oppositely charged ions. This arrangement leads to the stability of the ionic substances. The lattice energy of an ionic crystal is the amount of energy released when one mole of crystal is formed by the oppositely charged gaseous ions. This is denoted by U. It can also be defined as the amount of energy needed to disperse one mole of ionic crystal into isolated constituent gaseous ions.
Lattice energies of ionic crystals cannot be measured directly, but experimental values are obtained from thermodynamic data using the Born-Haber cycle. The cycle is based on Hess's law of constant heat summation, which states that the total energy change in a chemical reaction remains the same whether the reaction takes place in one step or in several steps.
Na+(g) + Cl(g) → NaCl(s); U = -788.0 kJmol-1
4.3.2 Explanation of ionic bond
During the formation of an ionic bond:
1. One atom which has a more electropositive character (metal) should lose one or more electrons to form a cation. Then, that cation attains the electronic configuration of the nearest stable noble gas.
A A+ + e-
Metal Cation
Fig. 4.10 Cation
2. Another atom which has a more electronegative character (non-metal) should gain one or more electrons to form an anion. Then, that anion attains the electronic configuration of the nearest stable noble gas.
B + e- B-
Non-metal Anion
Fig. 4.11 Anion
3. Then, the two oppositely charged ions, i.e., cation and anion, come close and are held together by electrostatic forces of attraction. These electrostatic forces of attraction are called ionic bonds.
Fig. 4.12 Ionic bonds
* The compounds containing ionic bonds are called ionic or electrovalent or polar compounds.
Eg., NaCl, MgCl2, MgO, LiF, AlF3, Na2O, etc.
4.3.3 Examples of ionic bond
Example: Formation of Sodium chloride (NaCl)
The atomic number of sodium is 11, and its electronic configuration is 1s2 2s2 2p6 3s1. It has only one valence electron. Thus, the sodium atom loses its outermost electron to attain the stable electronic configuration of neon and becomes sodium ion Na+. Na Sodium atom
Na+ + 1e-
Sodium ion
1s22s22p63s1 1s22s22p6(Neon electronic configuration)
Fig. 4.13 Electronic configuration of Sodium atom and ion
The atomic number of chlorine is 17, and its electronic configuration is 1s2 2s2 2p6 3s2 3p5. It has 7 valence electrons. It gains one electron, lost by the sodium atom, to acquire the stable electronic configuration of argon and becomes chloride ion (Cl-).
Chlorine atom
Chlorine ion + 1eXCl
1s22s22p63s23p5 1s22s22p63s23p6(Argon configuration)
Fig. 4.14 Electronic configuration of Chlorine atom and ion
The two oppositely charged ions Na+ and Cl- ions come close and are held together by electrostatic forces of attraction called ionic bonds.
The above entire process of transfer of electrons from sodium to chlorine atom can be represented as follows:
4.3.4
Conditions for the formation of ionic bond
The following conditions favour the formation of ionic bond
1. The ionic bond is formed between a metal atom and a non-metal atom. The transfer of electrons takes place from the metal atom to the non-metal atom.
2. The ionic bond is formed between the two atoms with a difference in their electronegativity that is greater than 1.7.
Example: The electronegativity of Na is 0.9, and the electronegativity of chlorine is 3.0. So, the difference is 3.0 - 0.9 = 2.1 > 1.7. So, Na and Cl will form an ionic compound.

3. In the formation of an ionic bond, there must be an overall decrease in energy. That means energy must be released.
4. The ionic bond is formed between an atom of low ionization potential and an atom of high electron affinity or high electronegativity.
5. A strong ionic bond is formed between alkali metals and halogens.
6. The strongest ionic bond is formed between caesium and fluorine.
7. Generally, oxides, halides and sulphides of alkali and alkaline earth metals are ionic in nature.
8. So, ionic compounds are generally obtained by the combination of metallic and non-metallic elements.
Exception: The ionic compound containing only non-metallic elements is NH4Cl.
9. An electrovalent bond cannot be formed between atoms of the same electronegativity.
4.3.5 General characteristic properties of ionic compounds
1. Physical state
Most ionic compounds are crystalline solids at room temperature.
2. Melting and boiling points
All ionic compounds have a high melting and boiling point.
3. Hard and brittle
Ionic compounds are hard in nature. Their hardness is due to strong forces of attraction between oppositely charged ions which keep them in their allotted positions.
4. High density
Ionic compounds are generally heavier than water.
5. High solubility in water
Ionic compounds are generally soluble in water but insoluble in organic solvents. The principle involved is 'like dissolves like', i.e., ionic compounds are polar (opposite ions), and water is a polar solvent.
6. Good conductors of electricity
Solid ionic compounds are poor conductors of electricity because the ions are fixed rigidly in their positions.
In an aqueous solution or in a molten state, ionic compounds are good conductors of electricity due to the presence of free ions.
7. Space isomerism
Since an ionic bond is non-directional, ionic compounds do not show space isomerism or stereoisomerism.
8. Ionic reactions
Ionic reactions are very fast because, in their aqueous solutions, ions are free to move.
Example:
4.4 BOND PARAMETERS
4.4.1 Bond length
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. It is expressed in A0 units (or) pico metre.
Example: 1A0 = 102 picometre
Table 4.2 Bond length
Bond lengths are measured by spectroscopic, x-ray diffraction and electron-diffraction techniques.
4.4.2 Bond energy or Bond enthalpy
There are two types of bond energies. They are
1. Bond dissociation energy
It is the energy required to break a particular bond in a polyatomic molecule in the gaseous state. (molecule) (free radicals)
A-B(g) A(g) B(g) +
2. Bond energy (E)
It is the average bond dissociation energy of all bonds present in a poly-atomic molecule. This bond dissociation energy or bond energy is expressed in kJ/mol.
Explanation
In methane (CH4), all four C-H bonds are equivalent, but the energy required to break the first bond CH CH H 43 is not the same as the energy required to break the second bond CH CH H 32 and so on,
i.e., if the individual dissociation energies of C-H in methane are divided by four, we can get the bond energy.
Table 4.3 Bond energy
4.4.3 Bond angle
• It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule or complex ion. The bond angle is expressed in degrees.
• It gives some idea regarding the distribution of orbitals around the central atom in a molecule, and hence, it helps us determine its shape.
Example
1. The H-O-H bond angle in water is 104.5°.
2. The H-C-H bond angle in methane is 109°281
Table 4.4 Bond angle
4.4.4 Bond order
Bond order is defined as one-half of the difference between the numbers of electrons present in the bonding to the anti-bonding orbitals. Bond order = 1/2 (NB-NA )
• A positive bond order (NB > NA ) means a stable molecule, while a negative (NB < NA ) or zero (NB = NA) bond order means an unstable molecule.
• If a bond order increases, the bond length decreases.
4.4.5 Polarity of bonds
Ionic and covalent bonds represent only two extremes of a continuous spectrum of possibilities. Between these two extremes, we have a large number of bonds in which the bonding electrons are shared unequally between two atoms but are not completely transferred. Such bonds are said to be polar covalent bonds. Polar covalent bonds lie between two extremes of the bonding.
(Full charges)
charges)
covalent (Electronically symmetrical)
Fig. 4.17 Polarity of bonds
Comparing the three substances, NaCl, HCl, and Cl2.
• NaCl: The bond in NaCl is largely ionic, between Na+ and Cl-. NaCl bond is only about 80% ionic, and the electron transferred from Na to Cl still spends some of its time near sodium. Na+ Cl-is an ionic bond.
• HCl: The bond in the HCl molecule is polar covalent. The chlorine atom attracts the bonding electron pair more strongly than hydrogen does, resulting in an asymmetrical distribution of electrons. Chlorine, thus, HCl has a partial negative charge, and hydrogen has a partial positive charge. H-Cl is a polar covalent bond.
• Cl2: The bond in the Cl2 molecule is non-polar covalent, with the bonding electrons centred between the two identical chlorine atoms and attracted equally to both atoms. Cl-Cl is a nonpolar covalent bond.
A covalent bond, in which the electrons are shared unequally, and the bonded atoms acquire partial charges, is called a polar covalent bond. Bond polarity is due to the difference in electronegativity, the ability of an atom in a molecule to attract the shared electrons in a bond.
Polar molecule
A molecule which has oppositely charged poles is called a polar molecule or dipole.
Bond polarity is described in terms of the ionic character in the given table.
Table 4.5 Bond polarity and ionic nature
The ionic character increases with the increasing difference in the electronegativity (EN) between bonded atoms.
The following equation was proposed by Hanny and Smyth for calculating the percentage of the ionic character in the A-B bond, on the basis of the values of electronegativity of the atoms A and B.
Percentage of the ionic character =[16(XA-XB ) + 3.5(XA-XB )2]
XA = Electronegativity of the atom A
XB = Electronegativity of the atom B
4.4.6 Resonance
Definition
The phenomenon in which two or more structures can be written for a compound which involves identical positions of atoms, is called resonance
Conditions of resonance
1. The relative position of all the atoms in each of the resonance forms must be the same. They should differ only in the position of electrons.
2. The number of unpaired and paired electrons in each of the resonance forms must be the same.
For example:
Contributing structures should be of almost equal energy.
The actual structure of the molecule is said to be a resonance hybrid of various possible alternative structures. The alternative structures are referred to as the resonance structures or canonical forms.
For example, the Ozone (O3) molecule can be represented as:
Fig. 4.18 Resonance in an ozone (O3) molecule
Structures I and II represent the two canonical forms, while structure III is the resonance hybrid.
Some more examples:
1. CO3-2 ion
2. CO2
3. Benzene
4.19 Resonance in a CO3-2 ion
4.20 Resonance in a CO2 molecule
I
II
Fig. 4.21 Resonance in a benzene molecule
III
[Structures I and II are resonance structures, and the actual structure of the benzene molecule (III) is represented as the hybrid of these two resonance structures (I and II).]
Note: The resonance hybrid is more stable than any one of the various resonance structures. In compounds exhibiting resonance, the bond order can be calculated as shown below:
Bond order = Total number of bonds between two atoms in all resonating structures
Total number of resonating structures
For example: The C-C bond order in benzene
Resonance energy
The difference in energy between the hybrid and the most stable resonance structure is known as resonance energy. It can be determined by the difference between the calculated and the experimental heat of combustion [energy released when one mole of a compound is burned] of the compound].
For example: In benzene, the calculated heat of combustion is 85.8 kcal/mol, and the experimental heat of combustion is 49.8 kcal/mol. Therefore, the resonance energy of benzene is 85.8 - 49.8 = 36 kcal/mol. This means benzene is said to be stabilised by a resonance energy of 36 kcal/mol.
4.5 VALENCE BOND THEORY (VBT)
4.5.1 Introduction to valence bond theory
To explain the limitations of the Lewis concept of covalent bonds, valence bond theory was proposed. This theory was first presented by Heitler and London in 1927 to explain how a covalent bond is formed. This theory was extended by Pauling and Slater in 1931 to explain the shapes of molecules and the direction of the bonds in the molecules.
4.5.2 Postulates of valence bond theory
1. In the formation of a covalent bond, when two atoms come closer to each other, then there is a minimum energy state. At this energy state, the atomic orbitals of two atoms undergo partial interpenetration.
2. This partial merging of atomic orbitals is called the overlapping of atomic orbitals.
3. Thus, a covalent bond is formed by the overlapping of atomic orbitals of the valence shell of the two atoms.
4. The orbitals which are involved in the overlapping must have:
• Unpaired electrons.
• The electrons of antiparallel spins.
5. As a result of overlapping, there is a maximum electron density between the two atoms.
6. When a covalent bond is formed between two atoms by the overlapping process, some amount of energy is released. So, the molecule is more stable than that of isolated atoms.
7. The energy released during the formation of a covalent bond is called bond enthalpy or bond energy.
8. The greater the overlapping, the lesser will be the bond length. Thus, the attraction will also be greater and so will the bond energy. Thus, the stability of the bond will also be high.
9. The greater the extent of overlapping, the stronger the covalent bond formed.
10. The closer the valence shells are to the nucleus, more will be the overlapping, and the bond energy will also be high.
11. The order of bond energy of different shells is 1-1 > 1-2 > 2-2 > 2-3.
Between two subshells of the same shell, the subshell that is more directionally concentrated shows more overlapping.
12. The order of the bond energy of subshells in the 2nd orbit is s-s < s-p < p-p.
4.5.3 Shapes of orbitals
The shape of s- orbital is Spherical The shape of p- orbital is Dumbbell y z x
Fig. 4.22 Shapes of s & p orbitals
The shape of the d-orbital is a double dumbbell.
4.5.4 Types of covalent bon d based on overlapping
The covalent bond may be classified into 2 types depending upon the types of overlapping. They are:
1) Sigma bond (or) σ bond
2) Pi bond (or) π bond
1. Sigma bond
A covalent bond formed by the end-to-end overlapping of half-filled orbitals of two atoms along the internuclear axis is called σ bond.
σ bond is also called head-on overlap or axial overlap.
CHEMICAL
1. A sigma bond is the first bond formed between two bonded atoms.
2. Only one σ bond should be formed between any two atoms.
3. σ bond is a strong bond because in the formation of a σ bond, overlapping of atomic orbitals takes place to a maximum extent.
4. The electron cloud of a σ bond is symmetrical above the internuclear axis (imaginary line joining the nuclei of two atoms).
5. σ bond has a cylindrical symmetry.
6. There can be free rotation of atoms around this bond
7. σ bond is less reactive.
8. σ electrons are referred to as localised.
9. σ bond has an independent existence.
10. A σ bond may exist either alone or along with π - bonds.
11. The shape of the molecule is determined by the σ bond. A σ bond can be formed by any one of the following types of overlappings.
i) s - s overlapping
ii) s - p overlapping
iii) p - p overlapping
12. Hybridised orbitals are also involved in σ bond formation.
2. Pi Bond
A covalent bond formed by the sidewise overlapping of half-filled p orbitals or p and d orbitals of two atoms which are already bonded through a σ bond and in which the electron clouds are concentrated above and below the internuclear axis is called a π-bond. This is also called lateral overlapping.
1. π-bonds are only formed when σ bond has already formed between two atoms.
2. π-bond is a weak bond when compared to a σ bond because in the formation of a π-bond, the overlapping of atomic orbitals takes place to a smaller extent.
3. The axes of orbitals involved in this type of overlapping should be parallel to one another and perpendicular to the internuclear axis.
4. The orbitals formed due to sidewise overlapping consist of two saucer-type charged clouds above and below the plane of participating atoms.
5. Free rotation of atoms is not possible around this bond.
6. π-bonds are more reactive because the electron cloud is more exposed to attacking species.
7. π-electrons are referred to as mobile electrons.
8. π-bond has no independent existence. It is always present along with a σ bond.
9. π-bonds do not affect the shape of the molecule.
10. A π-bond can be formed by any one of the following two types of overlappings:
i) p-p overlapping
ii) p-d overlapping
π-bond is not formed by s-orbitals.
11. Hybridised orbitals are never involved in π-bond formation.
12. Each additional bond formed between two bonded atoms is a π-bond.
Table 4.6 Types of bond
4.5.5 Types of overlapping
1. s-s Overlapping
In this case, a half-filled s-orbital of one atom overlaps with the half-filled s-orbital of another atom along the internuclear axis.
Due to this overlapping, only the σ bond can be formed between any two atoms.
Example: H2
Formation
of the hydrogen molecule
1. The hydrogen molecule is formed by the combination of two hydrogen atoms.
2. The electronic configuration of hydrogen is 1s1.
3. Each hydrogen atom has one unpaired electron in the 1s orbital.
4. When two hydrogen atoms approach each other, the half-filled 1s orbitals of two hydrogen atoms overlap axially to form a bonding orbital resulting in the formation of an H2 molecule.
5. The covalent bond formed between two hydrogen atoms is represented by s-s.
Fig. 4.24 Covalent bond formed between two hydrogen atoms
2. s-p Overlapping
This type of overlapping occurs between the half-filled s-orbital of one atom and the half filled p-orbital of another atom along the internuclear axis.
Due to this type of overlapping too, only the σ bond can be formed between any two atoms.
Example: HCl, HBr, HI.
Formation of the H-Cl molecule
1. A hydrogen chloride molecule is formed by the combination of one hydrogen atom and one chlorine atoms.
2. The electronic configuration of hydrogen is 1s1 = sp3
3. The electronic configuration of chlorine is 1s2, 2s2, 2p6, 3s2, 3px2, 3py2, 3pz1
4. A hydrogen atom has one unpaired electron in the 1s orbital, and a chlorine atom also has one unpaired electron in the 3pz orbital.
Fig. 4.25 Formation of the HCl molecule
5. As the hydrogen atom and chlorine atom approach each other, the half-filled 1s orbital of hydrogen atom overlaps with the half-filled 3pz orbital of the chlorine atom along the internuclear axis to form an s-p bond between the hydrogen atom and chlorine atom resulting in the formation of the HCl molecule.
3. p-p Overlapping
The p-p overlapping takes place in two ways. They are:
i) p-p overlapping along the internuclear axis or axial p-p overlapping.
ii) Lateral p-p overlapping.
a. Axial p-p overlapping
This type of overlapping occurs between a half-filled p-orbital of one atom and the half-filled p-orbital of another atom along the internuclear axis.
The covalent bond formed due to this type of overlapping can be represented by p-p .
Example: F2, Cl2, Br2, I2
Formation of the fluorine molecule
1. A fluorine molecule is formed by the combination of two fluorine atoms.
2. Electronic configuration of fluorine is 1s2, 2s2, 2px2, 2py2, 2pz1
3. Each fluorine atom has one unpaired electron in a 2pz orbital.
4. As two fluorine atoms approach each other, the half-filled 2pz orbitals of two fluorine atoms overlap axially to form a σ p-p bond between two fluorine atoms resulting in the formation of the F2 molecule.
(or) + - -
Fig. 4.26 Formation of the fluorine molecule
b. Lateral p-p overlapping
This type of overlapping occurs between a half-filled p-orbital of one atom and the half-filled p-orbital of another atom perpendicular to the internuclear axis.
1. In this type of overlapping, electron clouds are concentrated above and below the plane of participating atoms.
2. The axes of two p-orbitals involved in this type of overlapping should remain parallel to each other.
3. The covalent bond formed due to this type of overlapping can be represented by π p-p .
Example: O2, N2
4.6 Hybridisation
4.6.1 Introduction to hybridisation
1. The stability of molecules without octet electronic configuration can be explained by the concept of hybridisation.
2. The concept of hybridisation was put forward by Pauling.
3. Hybridisation is a modification of valence bond theory.
4. The chemical and physical evidence indicates that in methane (CH4), there are four equivalent bonds. It is explained by the concept of hybridisation.
Definition
Hybridisation can be defined as the process of intermixing of the atomic orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shape.
4.6.2 Salient features of hybridisation
1. The new orbitals formed during hybridisation are called hybrid orbitals.
2. The number of hybrid orbitals is equal to the number of atomic orbitals that get hybridised.
3. The hybridised orbitals are always equivalent in energy and shape.
4. The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
5. These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs, and thus, a stable arrangement. Therefore, the type of hybridisation indicates the geometry of the molecules.
Important conditions for hybridisation
• The orbitals present in the valence shell of the atom are hybridised.
• The orbitals undergoing hybridisation should have almost equal energy.
• The promotion of an electron is not an essential condition prior to hybridisation.
• It is not necessary that only half-filled orbitals participate in hybridisation. In some cases, even the filled orbitals of valence shells take part in hybridisation.
i) Only orbitals undergo hybridisation but not electrons.
ii) Hybrid orbitals always form only σ bonds, but they do not form π bonds.
4.6.3 Types of hybridisation
Based on the number of orbitals involved in hybridisation, there are different types of hybridisations. They are sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp Hybridisation
The intermixing of one s-orbital and one p-orbital of an atom to produce two new sp hybridised orbitals of equivalent energy is called sp hybridisation.
1. This hybridisation is also called linear or diagonal hybridisation.
2. One s-orbital and one p-orbital of the same energy merge together.
3. The sp hybrid orbitals have one electron each.
4. The two sp hybrid orbitals are pointed in opposite directions along the axis to get the maximum stability.
5. Each sp hybrid orbital has 50% p-orbital character and 50% s-orbital character.
6. The angle between the axis of the two sp hybrid orbitals is 180
7. It gives a linear shape to the molecule.
hybrid orbitals
Fig. 4.28 Linear sp hybrid orbitals
Example: sp hybridisation is present in BeCl2, CO2, C2, H2, and HCN.
Structure of BeCl2
In the BeCl2 molecule, the central atom is beryllium. It undergoes sp hybridisation.
The ground state electronic configuration of beryllium 1s2, 2s2.
The excited state electronic configuration of beryllium 1s2, 2s1, 2px1, 2py0, 2pz0. 2s sp 2px 2py 2pz
4.29 Electronic configuration of beryllium
In a Be atom, one of the 2s electrons goes to the 2p level. Then, the 2s and 2p orbitals of the Be atom undergo sp hybridisation and produce two sp hybrid orbitals. These sp hybrid orbitals have one electron each. The electronic configuration of chlorine is 1s2, 2s2, 2p6, 3s2, 3p5
The pz orbital of one chlorine and the one sp hybrid orbital of the Be atom overlap to form an sp-p bond. Similarly, the pz orbital of another Cl atom and the other sp hybrid orbital of Be atom overlap to form the sp-p bond.
The shape of the BeCl2 molecule is linear.
The bond angle in the BeCl2 molecule is 180°
sp Hybridisation
Table 4.7 sp Hybridisation
sp2 Hybridisation
The intermixing of one s-orbital and two p-orbitals of an atom to produce three new sp2 hybridised orbitals of equivalent energy is called sp2 hybridisation.
1. This hybridisation is also called trigonal planar hybridisation.
2. Three sp2 hybrid orbitals lying in a plane at a 120° angle to one another.
3. Each sp2 hybrid orbital has 33.33% of s character and 66.66% of p character.
4. It gives a trigonal shape to the molecule.
Trigonal planar sp² hybridisation
Fig. 4.32 Trigonal planar shape
Examples: sp2 hybridisation is present in BCl3, C2H4, SO2, SO3, benzene (C6H6 ), graphite, and HCHO (methanal).
Structure
of BCl3 molecule
In the BCl3 molecule, the central atom is boron; it undergoes sp2 hybridisation.
The ground state electronic configuration of boron 1s2 2s2 2p1
The excited state electronic configuration of boron 1s2 2s1 2px1 2py1 2pz0. sp
In B atom, one of the 2s electrons goes to the 2p level. Then, the 2s and 2px, 2py orbitals of the B atom undergo sp2 hybridisation and produce three sp2 hybrid orbitals. These three sp2 hybrid orbitals have one electron each.
The electronic configuration of chlorine 1s2 2s2 2p6 3s2 3p5 3s 3px 3py 3pz
The pz orbital of one chlorine atom and the one sp2 hybrid orbital of B atom overlap to form an sp2-p bond. Similarly, the two Pz orbitals of two chlorine atoms overlap with two sp2 hybrid orbitals of B atom to form two sp2-p bonds.
The shape of the BCl3 molecule is a trigonal planar. The bond angle in the BCl3 molecule is 120° .
sp2 Hybridisation
Table 4.8 sp2 Hybridisation
sp3 Hybridisation
The intermixing of one s orbital and three p orbitals of an atom to produce four new sp3 hybridised orbitals of equivalent energy is called sp3 hybridisation.
1. This hybridisation is also called tetrahedral hybridisation.
2. The sp3 hybrid orbitals have a tetrahedral orientation in space.
3. The four sp3 hybridised orbitals point towards the four corners of a regular tetrahedron.
4. The tetrahedral angle is 109° 28'.
5. Each sp3 hybrid orbital has 25% s and 75% p characters.
Four sp³ hybrid orbitals
Fig.4.34 sp3 hybrid orbitals
Example: sp3 hybridisation is present in CH4, NH3, H2O, NF3, OF2, CCl4, SnCl4, SiCl4, diamond, silica, alkanes, and cyclo alkanes.
Structure of CH4 molecule
In a CH4 molecule, the central atom is carbon, and it undergoes sp3 hybridisation.
The ground state electronic configuration of carbon 1s2 2s2 2px1 2py1 2pz0.
The excited state electronic configuration of carbon 1s2 2s1 2px1 2py1 2pz1.
sp3
In a carbon atom, one of the 2s electrons goes to the 2p level. Then, the 2s and 2px,2py, and 2pz orbitals of the C atom undergo sp3 hybridisation and produce four sp3 hybrid orbitals. These four sp3 hybrid orbitals have one electron each.
The electronic configuration of H is 1s1
sp3
The 1s orbital of one H atom and one sp3 hybrid orbital of the C atom overlap to form an sp3-s bond. Similarly, the three 1s orbitals of the 3H atoms overlap with three sp3 hybrid orbitals of the C atom to form three sp3-s bonds. The shape of the CH4 molecule is tetrahedral. The bond angle in a CH4 molecule is 109° 281
Structure of CH4
Special cases of sp3 hybridisation
Structure of ammonia molecule
The central atom in the NH3 molecule is nitrogen. The electronic configuration of nitrogen is 1s2 2s2 2px1 2py1 2pz1. In the nitrogen atom, 2s, 2px, 2py and 2pz orbitals intermix to give four equivalent sp3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron. Among the four sp3 hybrid orbitals, one is occupied by a lone pair of electrons, and the remaining three are half-filled. The half-filled sp3 hybrid orbitals overlap with the 1s orbitals of three hydrogen atoms
to form three σ sp 3 -s bonds. The expected shape of NH3 is tetrahedral, but NH3 has a pyramidal shape with three hydrogen atoms occupying the base and the lone pair forming the apex of the pyramid, as shown in Fig. The observed bond angle HN H is 107° 48'.
Structure of water molecule
The central atom in a water molecule is oxygen. The electronic configuration of oxygen is 1s2 2s2 2px2 2py1 2pz1. In an oxygen atom, 2s, 2px, 2py, and 2pz intermix to give four equivalent sp3 hybrid orbitals. Among these sp3 hybrid orbitals, two are occupied by lone pairs of electrons, and two are half-filled. The two half-filled sp3 orbitals of oxygen overlap with the 1s orbitals of two hydrogen atoms in their axes to form two σ(sp3-s) bonds.
Now, the oxygen atom is surrounded by two lone pairs and two bond pairs of electrons. The molecule is expected to have a tetrahedral shape. As a result of the presence of two lone pairs, the water molecule assumes V or angular or bent shape, as shown in Fig. The bond angle in the water molecule HH O is 104° 31'.
Fig. 4.37 Structure of a water molecule
Hybridisation
Table 4.9 sp3 Hybridisation
QUICK REVIEW
• Chemical Bond: The attractive force that holds two or more atoms or ions together in a molecule or ion is called a chemical bond.
• Every system of the universe tends to lose potential energy and become more stable. That means every system of the universe tends to change from a higher energy state (i.e., less stable state) to a lower energy state (i.e., more stable state).
• This suggests that hydrogen molecules have less energy than its constituent hydrogen atoms by about 104 K. Cal/mol. So, hydrogen molecules are more stable than hydrogen atoms.
• All noble gas elements except helium have a stable, completely filled ns2 np6 type of electronic configuration (8 electrons) in their outermost shell. This type of electronic configuration is called octet electronic configuration. Helium has a completely filled the K-shell with two electrons and is also stable (Duplet electronic configuration).
• Odd electron molecules: In molecules with an odd number of electrons, such as nitric oxide (NO) and nitrogen dioxide (NO2), the octet rule is not satisfied for all the atoms.
• The electrostatic forces of attraction between two oppositely charged ions which are formed by the transfer of one or more electrons from one atom to another atom is called an ionic bond.
• A chemical bond formed between two atoms of the same or different elements by equal contribution and mutual sharing of one or more electron pairs in such a way that both the atoms acquire the stable electronic configuration of a noble gas is called a covalent bond.
• Classification of covalent bond: Based on the fact that the electron pair is shared equally or unequally between the atoms, covalent bonds are classified into 2 types. They are non-polar covalent bonds and polar covalent bonds.
• Non-polar covalent bond: A covalent bond in which the electron pairs are shared equally between the two bonded atoms is called a non-polar covalent bond.
• Polar covalent bond: A covalent bond, in which the electron pairs are shared unequally between the two atoms such that the bonded atoms acquire partial positive and partial negative charge is called a polar covalent bond.
• Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. It is expressed in A0 units (or) pico metre.
• Bond dissociation energy: It is the energy required to break a particular bond in a polyatomic molecule in the gaseous state.
• Bond energy (E): It is the average bond dissociation energy of all bonds present in a polyatomic molecule. This bond dissociation energy or bond energy is expressed in K.J/mole.
• Bond angle: It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule or complex ion.
• Bond order is given as:
BondingelectronsAntibondingèlectrons 2
• Bond order in O2 is 2 and in N2 is 3.
• Resonance: The phenomenon in which two or more structures can be written for a compound which involves identical positions of atoms is called resonance.
Example: CO3(-2) ion
• The covalent bond may be classified into 2 types depending upon the types of overlapping. They are:
• Sigma bond (or) σ bond
• Pi bond (or) π bond
• s-s overlapping: In this case, the half-filled s-orbital of one atom overlaps with the half-filled s-orbital of another atom along the internuclear axis.
• s-p overlapping: This type of overlapping occurs between the half-filled s-orbital of one atom and the half-filled p-orbital of another atom along the internuclear axis.
• p-p overlapping: p-p overlapping takes place in two ways. They are:
1. p-p overlapping along the internuclear axis or axial p-p overlapping.
2. Lateral p-p overlapping.
• Hybridisation: Hybridisation can be defined as the intermixing of the atomic orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shape.
• Types of hybridisation: Based on the number of orbitals involved in hybridisation, there are different types of hybridisations. They are sp, sp2, sp3, sp3d, sp3d2, and sp3d3
• The intermixing of one s-orbital and two p-orbitals of an atom to produce three new sp2 hybridised orbitals of equivalent energy is called sp2 hybridisation
• The intermixing of one s-orbital and three p-orbitals of an atom to produce four new sp3 hybridised orbitals of equivalent energy is called sp3 hybridisation
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Chemical bond, covalent bond, and ionic bond
1. How many electrons are present around sulphur in SF6?
a. 10 electrons
c. 12 electrons
b. 14 electrons
d. 8 electrons
2. Lewis dot symbol of an element X is X .. ., and X contains five shells, then X is:
a. Te
b. In c. Sn d. I
3. Among the following, the element that follows the duplet rule is:
a. Helium
b. Sodium
4. According to the Lewis theory, the dots represent:
a. Valence electrons
c. Radicals
c. Oxygen
b. Core electrons
d. Ions
d. Sulphur
5. The pair of elements which forms an ionic bond is:
a. C + Cl b. H + F c. Na + Br d. O + H
6. Among the following, the monovalent electropositive element is:
a. Oxygen b. Potassium c. Chlorine d. Neon
7. Ionic compounds do not show space isomerism due to:
a. Directional nature
c. Ionic compounds are heavier than water
b. Non-directional nature
d. Hard in nature
8. Among the following, the most electropositive element is:
a. Calcium b. Sodium c. Caesium d. Potassium
9. Statement A: Electropositive atoms form cations.
Statement B: Electronegative atoms form anions.
Statement C: Cations and anions combine to form ionic bonds.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. Statements A, B are correct, but C is incorrect.
d. Statements A, B are incorrect, but C is correct.
10. Assertion (A): The ionic bond is the strongest bond.
Reason (R): Strong electrostatic forces of attraction are present between two oppositely charged ions.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
11. Assertion (A): CsCl is more ionic than NaCl.
Reason (R): Cs has a lower ionisation potential than Na.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
12. Statement A: Sulphur is a divalent element.
Statement B: Chlorine is a monovalent element.
Statement C: Aluminium is a trivalent element.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
II. Bond parameters
1. The bond length in a C-F bond is:
a. 142pm
147pm
121pm
2. The bond energy of a triple bond when compared to a double bond is

154pm
a. Half b. Same c. Less d. More
3. Among ethane, ethene, and ethyne, the C-C bond energy is:
a. The same in all the three compounds
c. Greatest in ethene
b. Greatest in ethyne
d. Greatest in ethane
4. The bond length of Alkanes, when compared to Alkenes, is:
a. Less b. More c. Same d. Half
5. Statement (A): The bond angle is expressed in degrees.
Statement (B): The bond dissociation energy or bond energy is expressed in K.J/mole.
Statement (C): The bond lengths are measured by spectroscopic, x-ray diffraction and electron-diffraction techniques.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
6. Among the following, the molecule with the bond order as one is:
a. H2
b. O2
7. Bond polarity is the least in:
a. N-H
c. N2 d. He2
b. O-H c. H-F
d. C-H
8. Bond energy is the highest in the molecule:
a. F2 b. Br2 c. I2 d. Cl2
9. The resonance energy of benzene is:
a. 36 kcal/mol b. 39 kcal/mol c. 40 kcal/mol d. 42 kcal/mol
10. The stability of the molecule is measured by:
a. Resonance energy
c. Non-bonding electrons
11. Bond order in CO3-2 is: a. 1.5
1
III. Valence bond theory (VBT) and hybridisation
1. σ electrons are referred to as:
a. Delocalised
c. Mobile electrons
b. Number of π-bonds
d. Number of dative bonds
1.33
2
b. Localised
d. Both b and c
2. Among the following, the molecule formed by s-p overlapping is
a. HF b. N2 c. F2 d. Cl2
3. Acetylene molecule contains:
a. 5σ bonds
c. 3σ bonds and 2π bonds
b. 4σ bonds and 1π bonds
d. 2σ bonds and 3π bonds
4. The number of σ bonds present in the methane molecule is:
a. 1 b. 2 c. 3 d. 4
5. Among the following, the molecule with the s-s overlapping is:
a. Cl2 b. H2
c. HCl d. I2
6. The covalent bond formed between two hydrogen atoms is represented by: a. σ s-s b. σ s-p c. σ p-p d. π s-p
7. Among the following, the compound in which the sp hybridisation takes place is:
a. OF2 b. NH3 c. C2H2 d. PCl3
8. The number of lone pairs on carbon in HCN is:
a. 4 b. 3 c. 2 d. 0
9. The shape of the BeCl2 molecule is:
a. Trigonal planar b. Tetrahedral
c. Octahedral d. Linear
10. The percentage of s character in sp hybridisation is:
a. 25% b. 50% c. 75% d. 30%
11. The hybridisation of C in CO2 is:
a. sp b. sp2 c. sp3
12. Statement A: Hybrid orbitals always form only σ bonds.
Statement B: Only orbitals undergo hybridisation, not electrons
Statement C: Hybrid orbitals form σ and π bonds.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
13. Statement A: The bond angle in a BeCl2 molecule is 180°.
Statement B: Hybridised orbitals are always equivalent in energy and shape.
sp3d
Statement C: The promotion of an electron is an essential condition prior to hybridisation.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
14. The bond angle in the SO3 molecule is:
a. 180° b. 120° c. 1090 281 d. 90°
15. The geometry of the molecule BF3 is:
a. Linear
c. Trigonal planar
b. Pyramidal
d. Tetrahedral
WORKSHEET - 2
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. The number of π bonds present in tetra cyano ethylene are:
a. 6
b. 7
2. The ratio of σ and π bonds present in butadiene is:
a. 2, 9
b. 5, 2
c. 8 d. 9
c. 9, 2
d. 2, 5
3. The hybridisation of carbon and oxygen in a methanol molecule are, respectively:
a. sp3, sp3
b. sp3, sp2
4. H2O is dipolar, whereas BeF2 is not. It is because:
c. sp3, sp
a. H2O involves hydrogen bonding whereas BeF2 is a discrete molecule
b. H2O is linear, and BeF2 is angular
c. H2O is angular, and BeF2 is linear
d. The electronegativity of F is greater than that of O
5. Assertion(A): The Lewis symbol for aluminium is ∙ .
Reason(R): The outermost shell electronic configuration of C is 2s2 2p2
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
6. Assertion(A): Bond length is measured in Angstrom units.
Reason(R): 1 Angstrom (1 Å)=102 pico metres.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
7. Assertion(A): π bonds are weaker than σ bonds.
d. sp2, sp3
Reason(R): π bonds are formed by the overlapping of p-p orbitals along their axis.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
8. Assertion(A): s-p overlapping is present in the HF molecule.
Reason(R): HF molecule is formed by the overlapping of the s orbital of a hydrogen atom and the p orbital of fluorine atom.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
9. Among the following, the linear molecule is:
a. NO2
b. SO2
10. BF3 adducts with NH3 because:
a. 'N' contains the highest electronegativity
b. 'N' contains the lowest atomic size
c. CO2
d. H2S
c. 'NH3' contains a lone pair of electrons, and BF3 is an electron-deficient molecule
d. The atomic size of boron is less
11. Among the following, the molecule that contain both ionic and covalent bonds are:
a. CH2Cl2
b. K2SO4
c. BeCl2
d. SO2
12. The number and type of bonds between two carbon atoms in CaC2 are: (IIT-JEE)
a. One sigma (σ) and two pi (π) bonds
c. One sigma (σ) and a half pi (π) bonds
b. Three sigma (σ) and two pi (π) bonds
d. One sigma (σ) bond only
13. Among the following, the maximum covalent character is shown by the compound.
(AIEEE - 2011)
a. FeCl2
b. SnCl2
c. AlCl3
d. MgCl2
14. The hybridisation of orbitals of an N atom in NO3-, NO2+, and NH4+are, respectively:
(AIEEE - 2011)
a. sp, sp2, sp3
b. sp2, sp, sp3
c. sp, sp3, sp2
d. sp2, sp3, sp
15. The hybridisation of the underlying atom changes in: (AIEEE - 2004)
a. AlH3 changes to AlH4-
c. NH3 changes to NH4+
b. H2O changes to H3O+
d. In all cases
16. Which one of the following compounds has the smallest bond angle in its molecule?
a. OH2
b. SH2
c. NH3
d. SO2
17. The correct order of bond angles (smallest first) in H2S, NH3, BF3, and SiH4 is: (AIEEE - 2009)
a. H2S < NH3 < SiH4 < BF3
c. H2S < SiH4 < NH3 < BF3
b. NH3 < H2S < SiH4 < BF3
d. H2S < NH3 < BF3 < SiH4
18. Lattice energy of an ionic compound depends on the: (AIEEE -2010)
a. Charge on the ion and size of the ion
c. Size of the ion only
b. Packing of ions only
d. Charge on the ion only
19. Which of the following is a polar molecule? (NEET - 2013)
a. BF3
b. SF4
c. SiF4
d. XeF4
20. Which one of the following molecules contains no π bond? (NEET - 2013)
a. CO2
b. H2 O
c. SO2
d. NO2
21. The covalent alkaline earth metal halide (X = Cl, Br, I) is: (JEEADV2019)
a. BeX2
b. CaX2
c. SrX2
22. Statement (A): The shape of a PCl5 molecule is trigonal bipyramidal.
Statement (B): The shape of an XeF4 molecule is square planar.
Statement (C): The shape of an IF7 molecule is pentagonal bipyramidal.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
23. Statement(A): The bond energy of CH3-H is 426.8 kJ/mole.
Statement(B): The bond energy of O=O is 497.9 kJ/mole.
Statement(C): The bond energy of Cl-Cl is 242.7 kJ/mole.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
24. Assertion (A): The shape of NH3 is pyramidal.
Reason (R): N undergoes sp3 hybridisation in NH3.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
d. MgX2
25. Statement A: The bond angle in H2O is 1040 311
Statement B: Two lone pairs are present in H2O
Statement C: In H2O, Oxygen undergoes sp2 hybridisation, and the shape of the molecule is angular.
a. Statements A, B, and C are all correct.
b. Statements A, B, and C are all incorrect.
c. A, B are correct, but C is incorrect.
d. A, B are incorrect, but C is correct.
26. Match the following: Set - A
A. Covalent compounds 1. HCl
B. Polar covalent bond 2. H2
C. Non polar covalent bond
a. A-3, B-1, C-2
c. A-1, B-2, C-3
27. Match the following:
Hybridisation
3. Soluble in benzene
b. A-2, B-3, C-1
d. A-2, B-1, C-3
A. sp 1. 25%
Percentage of s-character
B. sp2 2. 50%
C. sp3 3. 33.33%
a. A-1, B-2, C-3
c. A-3, B-1, C-2
b. A-3, B-2, C-1
d. A-2, B-3, C-1
28. Assertion (A): The Lewis symbol for aluminium is Al . ..
Reason (R): The outermost shell electronic configuration of Al is 3 s2 3p1
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
29. Assertion (A): The shape of NH3 is pyramidal. Reason (R): N undergoes sp3 hybridisation in NH3.
a. Both A and R are correct, and R is the correct explanation of A.
b. Both A and R are correct, and R is not the correct explanation of A.
c. A is correct, but R is incorrect.
d. A is incorrect, but R is correct.
30. Among the following, the compound in which sp3 hybridisation takes place is:
a. NH3 b. SO3 c. BCl3 d. HCHO
31. The nature of the hybridisation of N in NF3 is:
a. sp b. sp2 c. sp3
32. The bond angle in Ammonia is: a. 180° b. 120° c. 107° 481
33. The type of hybridisation of S in SO2 is:
sp3d
109° 281
a. sp b. sp3 c. sp2 d. sp3d
34. Statement A: sp2 hybridisation is also called linear hybridisation.
Statement B: The percentage of 'P' character in sp2 hybridisation is 33.33 .
Statement C: The excited state electronic configuration of boron is 1s2 2s1 2p2
a. Statements A, B and C are correct.
b. Statements A, B and C are incorrect.
c. A, B are correct, but C is incorrect.
d. A,B are incorrect, but C is correct.

CARBON AND ITS COMPOUNDS 5
5.1 CARBON INTRODUCTION
Carbon is one of the most important non-metallic elements, and it can be found in all living organisms as biological molecules. It exists in both free and combined states. Carbon exists in the Earth’s crust as coal and in trace amounts as diamond and graphite in various allotropic forms. Carbon, when combined, forms oxides (carbon dioxide, carbon monoxide), carbonates (metal carbonates), and a variety of organic compounds such as proteins and carbohydrates. Let us see how carbon forms different molecules and structures.
5.1.1
Introduction to hybridisation
The concept of hybridisation of atomic orbitals was proposed by Pauling. The shapes of molecules and bond angles can be explained in terms of hybridisation.
The phenomenon of intermixing orbitals of the same atom, which have nearly the same energy to form an equal number of new orbitals of equivalent energy, is known as hybridisation. The new orbitals of equivalent energy are called hybrid orbitals
The atomic number of carbon is 6. Its electronic configuration in its ground state is ssppp122 22 xyz 22 11 0 It has only two unpaired electrons; hence, it should be divalent. But all the known compounds of carbon confirm that carbon is tetra-covalent.
In order to account for tetra-valency, it is believed that during the process of bond formation, which is an energy-releasing process, the two electrons in the s2 orbital get unpaired and out of them, one is promoted to an empty orbital. This is the excited state, and then its electronic configuration is 1s2 2s1 2px 1 2py 1 2pz 1
It should be noted, however, that the four half-filled valence orbitals are not equivalent, there being three p-orbitals and one s-orbital, and so all the four bonds could not be equivalent; the bond using the s-orbital would be different from the three bonds using the p-orbital. However, it is found that all the four bonds formed by carbon atoms in methane-like compounds are identical.
5.1.2 sp Hybridisation
Each sp hybrid orbital has 50 per cent s-orbital character and 50 per cent p-orbital character. The type of hybridisation in which one ’s’ orbital and one ’p’ orbital are involved is called ’sp’ hybridisation.
The two sp hybrid orbitals lie along a straight line and thus make an angle of 1800 with each other. The two 2p orbitals, which are left in their original state, lie in different planes at right angles to each other and also to the hybrid orbitals.
Structure of acetylene: One of the sp-hybrid orbitals of each carbon atom overlaps axially with a similar orbital of the other to form a sp-sp sigma bond. The other hybrid orbital of each carbon atom overlaps axially with a half-filled 1s-orbital of a hydrogen atom to form C-H bonds. The unhybridised p-orbitals of the two carbon atoms overlap sidewise to form two bonds r - . The C ≡ C energy is 827.6 kJ mol-1, and the bond length is 1.20 A 0 .
5.1.3 sp2 Hybridisation
Each sp 2 hybrid orbital has 33.33 per cent s-orbital character and 66.67 per cent p-orbital character. The type of hybridisation in which one ’s’ orbital and two ’p’ orbitals are involved is called ’sp2’ hybridisation.
The three sp 2 orbitals lie in one plane, making an angle of 120 0 with each other. Unhybridised p-orbital is oriented in a plane at a right angle to the plane of the three hybridised orbitals.
Structure of ethylene: Each carbon atom of ethylene uses one of its sp 2 hybrid orbitals for the axial overlapping with the similar orbital of the other carbon atom to form a sp sp 22 - sigma bond. The other two sp 2 hybrid orbitals on each carbon overlap axially with half-filled 1s orbitals of hydrogen atoms to form C - H sigma bonds
All the atoms in ethylene lie in the same plane. The unhybridised p-orbital on each carbon is perpendicular to the plane of these atoms, with one lobe above the plane and the other below the plane. These p-orbitals overlap sidewise to form a pi-bond between the carbon atoms.

5.1.4
sp3 Hybridisation
Each sp 3 hybrid orbital has a 25 per cent s-orbital character and 75 per cent p-orbital character. The type of hybridisation in which one ’s’ orbital and three ’p’ orbitals are involved is called ’ sp 3 ’ hybridisation. The four sp 3 hybrid orbitals are directed towards the four corners of a regular tetrahedron. The angle between any two sp 3 hybrid orbitals is 109o28’. This type of hybridisation is present in all saturated hydrocarbons in which carbon forms four single bonds.
Structure of methane: Each of the hybrid orbitals of carbon overlaps with 1 s orbitals of hydrogen to form carbon-hydrogen sigma bonds. The carbon atom lies at the centre of the tetrahedron, while the four hydrogen atoms occupy the four corners or vertices of a tetrahedron
By observing the structure of a molecule, it is possible to predict the state of hybridisation of carbon. A carbon atom that is directly linked to four other atoms or groups is sp3 hybridised. A carbon atom that is directly linked to three other atoms or groups is sp2 hybridised. A carbon atom that is directly linked to two other atoms or groups is sp hybridised.
5.2 BONDING IN CARBON – THE COVALENT BOND
5.2.1 The covalent bond
Complete electron transfer between identical atoms (or) between atoms that have very little difference in their electro-negativities is not possible. In such cases, the atoms contribute the electrons in equal numbers and form electron pairs, which are shared by both atoms. Bonds formed in this way are referred to as covalent bonds. The covalent bond was explained by Lewis.
Definition: A chemical bond formed between two atoms of the same or different elements by equal contribution and mutual sharing of one or more electron pairs in such a way that both the atoms acquire the stable electronic configuration of the nearest noble gas is called a covalent bond.
(or) The chemical bond formed between two atoms due to the mutual sharing of electrons is called a covalent bond.
For example- One atom of carbon shares 4 pairs of electrons, one with each of 4 atoms of hydrogen, to form a molecule of methane.
Fig. 5.4 Covalent bond formed by carbon in methane molecule
As the electron pairs are shared between the two atoms, a covalent bond is also called an electron pair bond
5.3 VERSATILE NATURE OF CARBON
We learned about how atoms share electrons to form covalent bonds. We also looked at methane, a simple carbon compound. Carbon is found in many things, including us. Scientists are aware of millions of carbon compounds. That is far more than the total number of compounds formed by all other elements combined. What makes carbon so unique? It’s because of how it shares electrons, which allows it to create a wide range of compounds.
5.3.1
Catenation
Definition: It is the self-linking tendency of an element in the form of straight chains or cyclic rings. It decreases with a decrease in element bond strength.
Carbon is special because it can connect with other carbon atoms, creating big molecules, which is carbon’s catenation property. These molecules can be long chains, branches, or even arranged in rings. Also, carbon atoms can connect with single, double, or triple bonds. Like-
CH3-CH2-CH2-CH3 or CH3CH = CH2
CH3C ≡ CH
Silicon also shows a catenation property, which allows it to form chains of up to seven or eight atoms.
i) It is a colourless transparent substance with extraordinary brilliance. Graphite is a greenish-black opaque substance.
ii) Structure - hybridisation (Tetrahedral)
Hybridisation (Hexagonal rings)
It is an allotrope of carboncontaining clusters of 60 carbon atoms joined together to form a spherical molecule.
Football-like structure contains 20 hexagonal and 12 pentagonal rings.
iii) Uses: Uses: Uses:
a) Used for cutting and grinding
a) Powdered graphite is used as a lubricant
b) Used for drilling holes
c) Diamond ’dies’ are used for drawing thin wires like the tungsten filament of an electric bulb.
d) Used in Jewellery
e) Sharp-edged diamonds are used by eye surgeons as a tool to remove eye cataracts.
b) Used for making carbon electrodes.
c) Used for preparing pencil leads.
a) Fullerenes have been extensively used for several biomedical applications like MRI, X-ray, drug and gene delivery and photodynamic therapy, etc. ,, Ex:CCC6070120
Note: Graphite is a good conductor of electricity due to the presence of unpaired electrons without involving in bond formation.
Table 5.2 Different allotropes of carbon
5.3.2 Saturated and unsaturated carbon compounds
The organic compounds have been classified on the basis of carbon skeleton (structure) or functional groups or the concept of homology. A general classification based on the carbon chain-
1) Acyclic compounds: Non-cyclic compounds are called aliphatic compounds and consist of single or branched chain compounds. They do not contain rings and are open-chain compounds. They may be saturated, in which carbon atoms form only single bonds (Alkanes), or they may be unsaturated, in which one or more carbon atoms form multiple bonds (alkenes and alkynes).
Saturated Unsaturated
CH3CH3
Ethane
CH3CH2CH3
Propane
CH2 = CH2
Ethylene
Saturated compounds: In acyclic hydrocarbon molecules of this kind, there are no double or triple bonds in the carbon structure. These hydrocarbons are known as alkanes, which fall under the category of saturated hydrocarbons. In saturated hydrocarbons, every carbon atom forms single covalent bonds, fulfilling all their valencies. The general formula for saturated hydrocarbons is CnH2n+2. They are commonly called paraffins. Like-
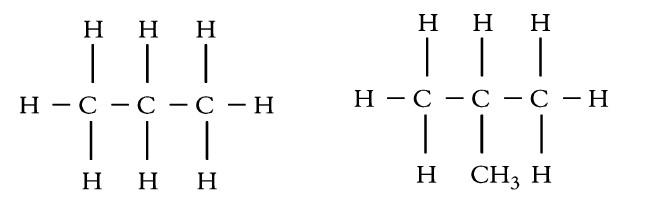
5.6 Alkanes
Unsaturated compounds: When carbon atoms in the structure of acyclic hydrocarbon molecules have more than one bond between them, these molecules are known as unsaturated hydrocarbons. Depending on the specific type of multiple bonds they have, they are categorised as either alkenes or alkynes.
Alkenes: Carbon compounds having double bonds (at least one) are known as alkenes. Their general formula is CnH2n. They are commonly called olefins. Like-
5.7 Alkenes
Alkynes: Hydrocarbons with at least one carbon-carbon triple bond are referred to as alkynes, and their general formula is CnH2n-2, assuming the presence of a single triple bond. These compounds are commonly known as acetylenes. Like-
Fig. 5.8 Alkynes
2) Cyclic compounds or Ring Compounds: These are the compounds in which carbon atoms are linked to each other or to the atoms of other elements in the form of a ring. These are closed-chain compounds. The compounds with only one ring are monocyclic, and those with more than one ring are polycyclic. Compounds having a ring or rings of only carbon atoms in the molecule are called homocyclic or carbocyclic compounds.
Example: cyclohexane cyclohexene cyclohexadiene

5.3.3 Homologous series


A series of compounds having the same functional group but a difference of - CH2 unit (14 unit mass) between two successive members. They show the same chemical properties and gradual variation in physical properties.
Table 5.3 Homologous series
The general characteristics of a homologous series are:
• They exhibit similar chemical properties because of the presence of the same functional group.
• There is a regular gradation in their physical properties.
• They can be represented by a general formula.
• The successive members differ in the molecular formula by aCH 2 - unit and so in molecular mass and their general methods of preparation are almost similar.
5.3.4 Nomenclature of carbon compounds
Nomenclature means the system of naming organic compounds based on certain guidelines. In order to solve the problem of naming organic compounds, an organisation called the International Chemical Congress met for the first time at Geneva in 1892. They developed a certain system called the Geneva system. This system was modified as an I.U.C. system in a meeting of the International Union of Chemistry was held in Liege (Belgium) in 1930, and later on, it gave to another modified system known as the IUPAC system, which is a system adopted by the International Union of Pure and Applied Chemistry. The latest IUPAC system is based on the recommendations made in 1993.
IUPAC system of nomenclature of aliphatic compounds
According to the IUPAC system, the name of an organic compound consists of three parts :
1) Root word
It indicates the basic carbon skeleton, i.e., the number of carbon atoms present in the longest continuous carbon chain of an organic compound. In general, the root word for any carbon chain is ’alk’.
Table 5.4 Root words
2) Suffix
Primary suffix: It indicates the degree of saturation (all single bonds) or unsaturation (double or triple bonds) in the carbon chain.
Nature of carbon chain Primary suffix
One bond - ane Alkane
One bond - ene Alkene
Two bonds - yne Alkyne
Three bonds - adiene Alkadiene
Two bonds - atriene Alkatriene
Name of group
Alkane - ’H’ atom - yl Alkyl
Alkene - ’H’ atom - enyl Alkenyl
Alkyne - ’H’ atom - ynyl Alkynyl
Table 5.5 Primary suffix
Secondary suffix: The secondary suffix indicates the nature of the functional group, which is added after the primary suffix. The terminal ’e’ of the primary suffix is replaced by the secondary suffix. Example: Secondary suffix for the functional groups ’-OH’ and ’-CHO’ are ’ol’ and ’al’ respectively,
Alkane + ol = Alkanol
Alkene + al = Alkenal
E.g. : Butane + ol = Butanol
E.g. : Butene = Butenal
Alkyl groups forming branches of the parent chain are considered side chains. Atoms or groups of atoms such as halo (-X), nitro (-NO2), nitroso (-NO), and alkoxy (-OR) are referred to as substituents. Root words are prefixed with the name of the substituent or side chain. These are arranged as follows while writing the names:
Prefix(es) + root word + primary suffix + secondary suffix
3) Prefix
Primary prefix: The primary prefix is meant only for alicyclic compounds, and it is always ’cyclo’. If the compound is not alicyclic, the primary prefix is absent. For example- Cyclobutane and cyclopentane.
Secondary prefix: The secondary prefix tells us about the substituents or secondary grade functional groups. In polyfunctional compounds, after selecting the principal functional group, all other functional groups are taken as substituents, and while writing them as substituents, there is a change in their designation, such as,
-X halo NH 2 - amino
>CO oxo, keto NO 2 - nitro-R alkyl -NO nitroso
RO- alkoxy -CHO formyl -CN cyano -OH hydroxy -COOR alkoxycarbonyl SO H 3 - sulpho -COX halocarbonyl CONH 2 - carbamoyl
Table 5.6 Secondary prefix
5.4 CHEMICAL PROPERTIES OF CARBON COMPOUNDS
5.4.1 Combustion
Carbon, in its various allotropic forms, burns in the presence of oxygen, producing carbon dioxide and emitting heat and light. Furthermore, many carbon compounds produce considerable quantities of heat and light when combusted. For example-
(i) CO CO 22 " ++ heat and light
(ii) CH OCOH O 42 22 " ++ + heat and light
5.4.2 Oxidation
Combustion greatly aids in the oxidation of carbon compounds. Further from complete oxidation, alcohols can be converted into carboxylic acids via various processes. LikeCH CH OH 32AlkalineKMnOHeat 4 +
5.4.3 Addition reaction
A reaction in which two reactants combine to form a single compound without loss of any atom or group is called an addition reaction. This reaction is characteristic of compounds that contain a double or a triple bond. The reagent is added across the double or triple bond of the substrate. A few examples are given below.
5.4.4 Substitution reaction
A reaction in which an atom or a group of atoms in a molecule is replaced by another atom or group is called a substitution reaction. For exampleCH Br NaOH CH OH NaBr 33 $ -+ +
5.4.5 Esterification
Acetic acid reacts with alcohols to give the corresponding ester. This reaction is known as esterification. CH COOH CH OH CH COOC HH O 32 53 25 2 $ ++
Esters are commonly known for their pleasant fragrance and find applications in perfume production and flavour enhancement. When subjected to sodium hydroxide, an alkali, esters undergo a chemical transformation, reverting to alcohol and the sodium salt of carboxylic acid. This particular reaction is termed saponification and is integral to the soap-making process. Soaps, essentially, are composed of sodium or potassium salts derived from long-chain carboxylic acids.
5.5 STUDY OF FUNCTIONAL GROUPS
The functional group may be defined as an atom or a group of atoms joined in a specific manner in its molecule, which is responsible for the characteristic chemical properties of the compounds. Important functional groups are listed in the following table.
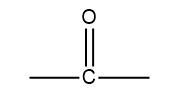
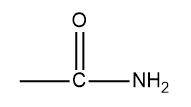

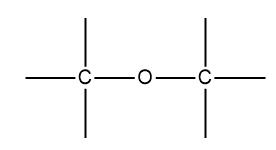
Table 5.7 Functional groups
5.5.1 Alcohols (R-OH)
The fundamental portion of an alcohol is a hydroxy group connected to an alkyl group ( R-OH). The IUPAC names of alcohols are derived from the parent alkanes and end with -ol. Replace - ’e’ of the parent hydrocarbon with -ol. Thus, in general, alcohol is named as alkanol
The next step is to select the longest continuous chain containing C to which the OH group is attached. The C to which the OH group is attached should get a minimum number. The functional group present is an alcohol (OH). Hence, the suffix is ’-ol’. For example-

Fig. 5.9 Alcohols containing one hydroxy group
Alcohols containing two or more hydroxy groups are commonly known as polyhydric alcohols, and in the IUPAC system, suffixes diol, triol, and so on are used instead of ol to indicate the number of hydroxyl groups. In such cases, the vowel ’e’ of alkane is retained in the name. Thus, the general name for an alkane containing two hydroxyl groups is alkanediol.

5.5.2
Aldehydes
(R-CHO)

The longest continuous carbon chain contains the -CHO group and the carbon of the -CHO group gets the lowest number. The aldehydes are named by suffixing ’al’ in place of e of the corresponding alkane. Thus, in general, an aldehyde is named alkanal.
The chain is numbered from the aldehydic carbon, but the position of the aldehyde is specific by the number ’I’, as it is understood that aldehydic carbon is the terminal carbon. Compounds with two aldehydic groups are named Alkanedial and so on, where the vowel ’e’ of alkane is retained. The unsaturated aldehydes are named alkenal (en+al) and alkynal (yne+al)
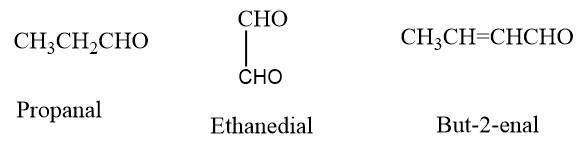
5.11 Aldehydes
5.5.3
Ketone (R-CO-R)
The longest continuous carbon chain must contain a ketonic group.
The ketones are named by suffixing ’one’ in place of e of the corresponding alkane. Thus, the name Alkanone. The carbon chain is numbered in such a way that the ketonic group gets the lowest number.
Further, numbering the positions of the substituents is carried out, and these are placed as prefixes according to the rules explained earlier.
The compounds containing two ketonic groups are termed allkanediones, along with the position number of groups. The vowel ’e’ of the alkane is retained in this case.
The unsaturated ketones are called alkenones (ene + one) or alkynones (yne + one). For example-
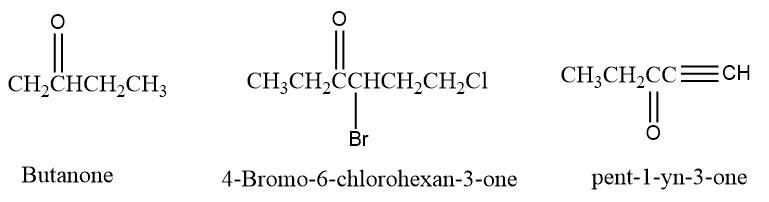
5.5.4 Carboxylic acid (R-COOH)
A carboxylic acid is an organic compound containing the carboxyl group -COOH, -CO2H, -COOH.
The longest continuous carbon chain must contain the carboxylic group, which gets the lowest number. The naming of carboxylic acids is done by adding the suffix ’-oic acid’ in the leave of e of the corresponding alkane. Thus, the name alkanoic acid.
The name of the carboxylic acid is written as two words (unlike others, which are written as a single word.) The general name for the family of unsaturated carboxylic acid is alkenoic acid and alkynoic acid.
In the case of dicarboxylic acids, the longest chain should include both carboxylic groups. For example- CH3COOH and HCOOH.
5.5 ISOMERISM 5.6
5.6.1 Isomerism
The term isomer was first introduced by Berzelius. Organic compounds that have the same molecular formula but differ from each other in some physical or chemical properties or both are known as isomers (Greek: iso - same; meros - part), and the phenomenon is known as isomerism
Isomerism is exhibited by compounds having directional bonds. There are two main types of isomerism: structural isomerism and stereoisomerism.
Structural isomerism is due to the difference in the way in which the constituent atoms or groups are linked to one another within the molecule without any reference to space. Structural isomerism has five types, of which we will read only the first three. They are-
1. Chain isomerism
2. Position isomerism
3. Functional group isomerism
5.6.2 Chain isomerism
Chain isomerism arises due to the difference in the arrangement of carbon atoms constituting the chain. It is also known as nuclear or skeletal isomerism. Chain isomerism is found in compounds containing more than three carbon atoms. For example- Butane has two chain isomers.
In n-butane, 4 carbons are in continuous chain and in isobutane, only 3 carbons are in continuous chain.
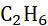

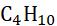
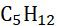




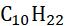
Table 5.8 Number of chain isomers of alkanes
5.6.3 Position isomerism
Isomers which differ in the position of a functional group or multiple bonds or substituents in the same carbon chain are called position isomers. For example-
• CH OH 37 has two position isomers.

Fig. 5.14 Isomers of propanol
• Butene has two position isomers.

Fig. 5.15 Isomers of butene
5.6.4 Functional group isomerism
Compounds having the same molecular formula but different functional groups are called functional group isomers. Some examples are-
• Alcohols and ethers: CH O 26 has two functional isomers:

Fig. 5.16 Functional isomerism in between alcohol and ether
• Aldehydes, ketones, and unsaturated alcohols: CH O 36 has the following functional isomers:
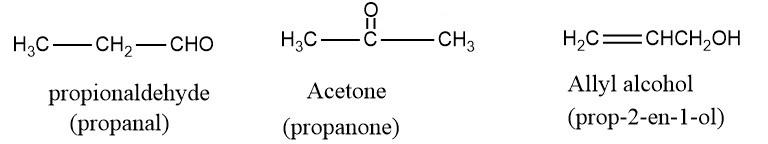
Fig. 5.17 Functional isomerism in aldehydes, ketones and unsaturated alcohols
• Acids, esters and hydroxy carbonyl compounds: CH O 36 2 has the following functional isomers:
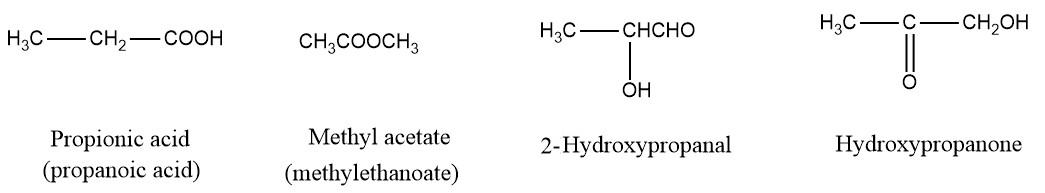
Fig. 5.18 Functional isomerism in acids, esters, and hydroxy carbonyl compounds
5.5
5.7
SOME IMPORTANT CARBON COMPOUNDS
– ETHANOL AND ETHANOIC ACID
5.7.1 Ethanol (C2H5OH)
Ethanol exists as a liquid under normal room temperature conditions. It is widely recognised as alcohol and serves as the primary component in various alcoholic beverages. Due to its effective solvent properties, ethanol finds application in medicinal products such as iodine tinctures, cough syrups, and numerous tonic formulations.
Reactions of ethanol with sodium
When alcohols undergo a reaction with sodium, hydrogen is produced as a byproduct. In the case of ethanol, the additional outcome is the formation of sodium ethoxide.
CH OH Na CH ONaH 22 2 25 25 2 " ++
Sodium ethoxide
Reactions of ethanol to give unsaturated hydrocarbon
When ethanol is heated at 443 K in the presence of an excess of concentrated sulfuric acid, it undergoes dehydration, leading to the formation of ethene.
CH CH OH 32HotConcH SO 24 CH CH HO 22 2 =+ Ethanol Ethene
5.7.2 Ethanoic acid (CH3COOH)
Acetic acid, also known as ethanoic acid, is classified within the carboxylic acids group. Vinegar, a solution containing 5-8% acetic acid in water, serves as a widely utilised preservative for pickles. Due to its tendency to freeze in cold climates during winter, it earned the name ’glacial acetic acid’, with a melting point of 290 K. It’s worth noting that, unlike strong mineral acids such as HCl, carboxylic acids exhibit weak acidic properties as they are not completely ionised.
5.7.3 Esterification reaction
Acetic acid reacts with alcohols to give the corresponding ester. This reaction is known as esterification.
CH COOH CH OH CH COOC HH O 32 53 25 2 $ ++
Esters are commonly known for their pleasant fragrance and find applications in perfume production and flavour enhancement. When subjected to sodium hydroxide, an alkali, esters undergo a chemical transformation, reverting to alcohol and the sodium salt of carboxylic acid.
This particular reaction is termed saponification and is integral to the soap-making process. Soaps, essentially, are composed of sodium or potassium salts derived from long-chain carboxylic acids.
Reaction with a base
Similar to mineral acids, the reaction between ethanoic acid and a base, like sodium hydroxide, results in the formation of a salt, known as sodium ethanoate or, commonly referred to as sodium acetate, along with the production of water.
NaOH + CH3COOH → CH3COONa + H2O
Sodium hydroxide ethanoic acid sodium acetate
Reaction with carbonates and hydrogen carbonates
Ethanoic acid undergoes a chemical reaction with carbonates and hydrogen carbonates, resulting in the formation of salt, carbon dioxide, and water. The commonly referred salt in this reaction is known as sodium acetate.
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
5.8 SOAPS AND DETERGENTS
5.8.1 Soaps
These are sodium or potassium salts of long-chain fatty acids (RCOONa); here, RC H, CH 15 31 17 35 = . These are obtained by alkaline hydrolysis of fats and oils (saponification). They remove dirt and oil from clothes by micelle formation. They form scum with hard water. The alkyl group (R), consisting of a long chain of carbon atoms, is the hydrophobic part, while (COO - Na+) is hydrophilic in nature.
tail
Fig. 5.19 Sodium stearate - A soap
head
Saponification reaction
It is the reaction in which an ester reacts with sodium hydroxide to form sodium salts of acid and alcohol is formed.
32 53 25 ++
Soaps are the sodium or potassium salts of higher fatty acids. They react with the Ca 2+ ions and Mg +2 ions are present in hard water and form corresponding calcium and magnesium salts of the acids. Since these salts are insoluble in water, a lot of soap is wasted as curdy white precipitate, which is also known as scum. Therefore, soaps are not so effective in hard water. Its reaction with soap is
Cleaning action of soaps
Most dirt is oily in nature, and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap dissolves in water, while the carbon chain dissolves in oil. The soap molecules, thus, form structures called micelles. Where, one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water, and we wash our clothes clean.
5.8.2 Detergents
Detergents are the sodium salts of long-chain benzene sulphonic acids or long-chain alkyl hydrogen sulphates. The ionic group in a detergent is SO Na 3 -+ . Detergents can be used for washing even when the water is hard. Some of the detergents are non-biodegradable. Also, detergents have a strong cleansing action in comparison to soaps.

Furthermore, detergents exhibit higher efficacy compared to soaps due to their ability to form soluble calcium or magnesium salts of sulphonic acid in hard water, preventing any undesired precipitation.
QUICK REVIEW
• Hybridisation: The phenomenon of intermixing of orbitals of the same atom which have nearly the same energy to form an equal number of new orbitals of equivalent energy is known as hybridisation.
• There are various types of hybridisation. They are: sp,sp, sp 23 .
• Hydrocarbons: Saturated Hydrocarbons (Alkanes) are the compounds of carbon and hydrogen only. They have only single bonds between any two atoms. Their general formula is CH . nn22 +
• Alkenes or olefins: These have a C = C double bond along with single bonds. Their general formula is CHnn 2
• Alkynes: These have at least one C ≡ C, triple bond along with single bonds. Their structural formula is CHnn22 - .
• Root word: It indicates the basic carbon skeleton, i.e., the number of carbon atoms present in the longest continuous carbon chain of an organic compound. In general, the root word for any carbon chain is ’alk’.
• Primary suffix: It indicates the degree of saturation (all single bonds) or unsaturation (double or triple bonds) in the carbon chain.
• Secondary suffix: The secondary suffix indicates the nature of the functional group, which is added after the primary suffix. The terminal ’e’ of the primary suffix is replaced by the secondary suffix.
• Primary prefix: The primary prefix is only meant for alicyclic compounds (cyclic compounds in which double bonds are not in the alternate position), and it is always ’cyclo’. If the compound is not alicyclic, the primary prefix is absent.
• Secondary prefix: The secondary prefix tells about the substituents or second-grade functional groups. If there are several substituents, they are written in the alphabetical order of their designations.
• Writing the name of the compound: Prefix + root word + primary suffix + secondary suffix
• i. Follow the IUPAC rules described earlier.
ii. The terminal ’e’ of the alkane is replaced by the suffix of the functional group.
iii. The positional number of the functional group may be represented in either of the three ways.
• On the basis of the above rules, the following general procedure is followed in the naming of an
organic compound.
• a. Identify the parent chain containing the principal functional group and as many of the secondary functional groups and multiple bonds as possible.
b. Select the corresponding root word for the chain selected.
c. Number the carbon atoms of the chain selected from the end so that the various groups get as small numbers as possible and the principal functional group gets the lowest number.
d. Attach to the root word the primary suffix (-ane, -ene (or) -yne) representing the nature of the carbon-carbon bonds in the selected chain.
e. Add suitable prefixes (alphabetical order) and secondary suffixes with their respective numbers of attachment to the parent chain to denote the number and position of each substituent or functional group.
f. Write the name of the compound as one word, separating the numbers from the designations or prefixes or suffixes by hyphens and separating the numbers from each other by commas.
• Isomerism: (Iso - same, meros - part) Compounds which have the same molecular formula, but differ in physical and chemical properties are called isomers and the phenomenon is called isomerism.
• Oxidation Reaction: It is the process of intake of oxygen and removal of hydrogen.
• Combustion Reaction: It is the reaction in which CO andH O 22 are obtained by burning an organic compound.
• Addition Reaction: In this, the reagents add completely to the substrate. e.g., hydrogenation (addition of H2).
• Substitution Reaction: In this, an atom or group of atoms replace another atom or group from the substrate.
• Structural isomerism is due to the difference in the way in which the constituent atoms or groups are linked to one another within the molecule.
• Chain isomerism, position isomerism, and functional group isomerism are some types of structural isomerism.
• Ethanol, CH OH 25 : It is obtained from sugar and soluble in water. It is called denatured alcohol if mixed with methanol, pyridine, etc.
• Ethanoic Acid, : CH COOH 3 Also called acetic acid. Its 5-8% aqueous solution is called vinegar.
Soaps: These are sodium or potassium salts of long-chain fatty acids (RCOONa); here
RC H, CH 15 31 17 35 = . These are obtained by alkaline hydrolysis of fats and oils (saponification).
• Detergents: These are ammonium or sulphate salts of long-chain carboxylic acid. They do not
form scum with hard water, so they can be used with it. These are also called soapless soap.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Carbon introduction
1. When the hybridisation state of the carbon atom changes from sp3 to sp2 and finally to, the angle between the hybrid orbitals
a. Decreases gradually
c. No change
2. Hybridisation of one ’s’ and one ’p’ orbitals, we get
a. Two mutually perpendicular orbitals
c. Four orbitals directed tetrahedrally
3. sp2 hybrid orbitals are not present in
a. SO2
b. BF3
c. B2H6
4. The ratio of pure orbitals to hybridised orbitals in ethylene is
a. 2:3
b. 3:1
c. 1:1
b. Decreased considerably
d. Increases progressively
b. Two orbitals at 180o
d. Three orbitals in plane
d. SO3
d. 1:3
5. The type of overlapping not observed in the formation of ethylene molecule is
a. sp sp 22 v -
b. sp p 2 v -
c. sp s 2 v -
d. pprr
6. The hybrid orbitals have a bond angle of 10928' ° . The ratio of the percentage of ’s’ and ’p’ characters is
a. 1:1
b. 1:2
c. 1:3
II. Covalent bond and versatile nature of carbon
d. 2:3
1. A cyclic hydrocarbon having carbon-carbon single bonds as well as carbon-carbon double bonds in its molecule is
a. CH612
b. CH614
c. CH66
d. CH512
2. An unsaturated hydrocarbon having a triple covalent bond has 50 hydrogen atoms in its molecules. The number of carbon atoms in its molecule will be
a. 24
b. 25
c. 26
3. The molecular formulae of a homologue of butane is
a. CH48
b. CH36
c. CH46
d. 28
d. CH38
4. One of the following molecular formulae cannot represent two organic compounds having
different functional groups. This molecular formula is
a. CH O 512 b. CH O 510 c. CH O 5102 d. CH512
5. The number of carbon atoms present in the molecule of the fifth number of the homologous series of alkynes is
a. Four b. Five c. Six d. Seven
6. The molecular formulae of some organic compounds are given below. Which of these compounds contains an aldehyde group?
a. CH O 38
b. CH O 36 2
c. CH O 36
d. CH Cl 37
7. The molecular formula of an organic compound is C48H94. This compound belongs to the homologous series of a. Alkenes b. Aldehydes c. Alkynes d. Alkanes
8. One of the following molecular formulae represents a ketone. This formula is
a. CH O 512",
b. CH O 6122
c. CH O 614
d. CH O 612
9. The molecular formula of the third member of the homologous series of ketones is
a. CH O 48
b. CH O 36
c. CH O 510",
10. Assertion (A): The suffix used for naming an aldehyde is -al
d. CH O 612
Reason(R): Butanone is a carbon compound having the functional group -CO- .
a. Both A and R are correct, and R is the correct explanation of A
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
11. Assertion (A): The compound with formula C3H8 (Propane) is a saturated hydrocarbon.
Reason(R): It corresponds to the general formula CnH2n+2.
a. Both A and R are correct, and R is the correct explanation of A
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
12. Assertion (A): If one hydrogen atom is removed from alkane, its primary suffix name is enyl.
Reason (R): If one hydrogen atom is removed from alkyne, its primary suffix name is ynyl.
a. Both A and R are correct, and R is the correct explanation of A
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
13. Assertion (A): The part of the name that appears before the root word is called ’prefix’.
Reason (R): The primary prefix is only meant for alicyclic compounds.
a. Both A and R are correct, and R is the correct explanation of A
b. Both A and R are correct, and R is not the correct explanation of A
c. A is correct, and R is incorrect
d. A is incorrect, and R is correct
III. Chemical properties of carbon compounds, study of functional groups, isomerism
1. The reaction, CH CBr 3 3 - ^h HO 2 CH COH 3 3 - ^h is
a. Elimination reaction
c. Free radial reaction
b. Substitution reaction
d. Addition reaction
2. The IUPAC name of the compound CH CH CH CH CH (OH) CH 32 52 3 ^h is
a. 4-ethyl-2-pentanol
c. 2-ethyl-2-pentanol
3. The IUPAC name of CH CH COOH 3 2 - ^h is
a. 2- propanoic acid
c. 2-Methylpropanoic acid
4. The correct IUPAC name of Cl CCHCHO 32 -
a. 3, 3, 3-Trichloropropanal
c. 2, 2, 2-Trichloropropanal
5. The IUPAC name of isobutanol is
a. 2-methyl propanol
c. 2-butanol
b. 4-methyl-2-hexanol
d. 3-methyl-2-hexanol
b. Isobutanoic acid
d. 2-Methylbutanoic acid
b. 1, 1, 1-Trichloropropanal
d. Chloral
b. 2-methyl-2-propanol
d. 2-methyl-1-propanol
6. The correct IUPAC name of a compound having a structural formula
CH CH (OH) COOH 3 is
a. Lactic acid
c. α -hydroxy propanoic acid
7. Functional isomerism occurs due to
b. 2-hydroxy propanoic acid
d. Carboxypropanol
a. The difference in nature of functional group b. The difference in position of atom
c. The difference alkyl group d. The difference in carbon atoms
8. In isomerism, iso means
a. Part b. Same c. Same part d. Different part
9. The phenomenon in which compounds have the same molecular formula but differ in physical and chemical properties is called
a. Allotropy
b. Isomerism c. Carbon dating d. Attachment
10. Statement A: Alcohols and ethers are functional isomers.
Statement B: But - 1 - ene and but - 2 - ene are positional isomers.
Statement C: n-Butane and isobutane are chain isomers.
a. All the statements A, B, and C are correct
b. All the statements A, B, and C are incorrect
c. A and B are correct, and C is incorrect
d. A and B are incorrect, and C is correct
IV. Some important carbon compounds – ethanol and ethanoic acid

1. When ethanol reacts with acetic anhydride in the presence of a catalyst, which of the following compounds does it form?
a. Ethyl benzoate b. Ethyl acetate
c. Ethyl chloride d. Ethyl bromide
2. When ethanoic acid reacts with sodium hydroxide, what is the product formed?
a. Sodium bicarbonate and water
b. Sodium chloride and water
c. Sodium ethanoate and water d. Sodium acetate and water
3. Which process can ethanoic acid be converted into ethyl ethanoate?
a. Saponification b. Esterification c. Hydrolysis d. Oxidation
4. Which of the following is a byproduct formed when ethanoic acid reacts with an alcohol to form an ester?
a. Water b. Carbon dioxide c. Hydrogen gas d. Ammonia
5. Which chemical component of synthetic detergents is responsible for breaking down grease and oil stains?
a. Fragrances b. Surfactants c. Preservatives d. Colourants
6. What is the chemical process by which soap is formed?
a. Condensation
c. Saponification
7. Which reagent is used in the following reaction?
CH3COOH " CH3COONa + H2O + CO2
a. Alkaline KMnO4 b. conc. H2SO4
b. Oxidation
d. Combustion
c. dry HCl d. NaHCO3
8. Ethanol reacts with sodium and forms two products, which are?
a. Sodium ethanoate and hydrogen
c. Sodium ethoxide and hydrogen
b. Sodium ethanoate and oxygen
d. Sodium ethoxide and oxygen
9. When ethanoic acid and washing soda react, which of the following products are not formed?
a. Carbon Dioxide b. Sodium ethanoate c. Water d. Hydrogen chloride
10. The dehydration of ethanol results in the formation of
a. Methane b. Ethane c. Ethene d. Butyne
11. What change will you observe if you test soap with litmus paper (red and blue)?
a. Red litmus paper turns blue
c. Red litmus paper turns green
WORKSHEET - 2
b. Blue litmus paper turns red
d. No change is observed
I. MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. _______ is inserted between the locant and the substituent name.
a. Comma b. Hyphen c. Dot d. Any one of the above
2. The bond between carbon atom (1) and carbon atom (2) in compound NC CH CH 12 2 involves the hybridisation
a. sp 2 and sp 2 b. sp 3 and sp c. sp and sp 2 d. sp and sp
3. The ratio of the total number of sp, sp andsp 23 orbitals in the compound is CH CH CCHC CCH 33 / -= =- - ≡ C - CH3
a. 1 : 1 : 1
2 : 2 : 1
4. Which compound given below has sp ,sp 32 , and sp orbitals in the ratio of 6 : 3 : 2?
a. CH CH CH CH CC CH 32 3 / -= - ≡ C - CH3 b. CH CH CH CH CCH 32 / -= ≡ CH
b. CH CH CC CH CH 32 2 / -= ≡ C - CH = CH2 d. CH CH CH CCH 3 / -= - ≡ CH
5. The ratio of the number of sp, sp 2 and sp 3 carbons in the compound given below is HC CCHCH 23 == -
a. 1 : 2 : 1 b. 2 : 1 : 1 c. 1 : 1 : 2
1 : 2 : 3
6. How many ’methyl groups’ are present in 2, 3-dimethyl-4-ethyl heptane?
a. 2
b. 8
c. 4
d. 5
7. In which of the following species is the underlined carbon having sp 3 hybridisation?
a. CH COOH 3
b. CH CH OH 32
c. CH COCH 33
d. CH CH CH 23 =-
8. The IUPAC name of the compound CH CH CH CH 23 2 =- ^h is: [JEE 1987]
a. 1,1-dimethy-propene
c. 2-vinyl propane
9. IUPAC name of n-amyl alcohol is
a. butan-1-ol
b. pentan-2-ol
b. 3-methyl-1-butene
d. None of the above
c. pentan-3-ol
d. pentan-1-ol
10. Among the following, which one represents the correct name of the compound?
a. methyl pentane
b. 2-propene c. 1-pentanone d. 2-butane
11. Fat + Alkali Soap + Glycerol
What name is given to the above-shown process?
a. Saponification
c. Esterification
b. Carboxylation
d. Hydrogenation
12. CH OH 25 on oxidation with acidified KCrO 22 7 gives CH COOH 3 . Which of the following statements is /are correct regarding these two compounds?
I) They both react with sodium metal to evolve a combustible gas.
II) They both react with NaHCO 3 to evolve a gas which turns lime water milky.
III) They both turn blue litmus red
a. I and II only b. II only c. I only d. I, II and III
13. We know that alcohols can be oxidised to carboxylic acids in the presence of an oxidising agent like KCrO 22 7 (or) KMnO 4 CH OH X' HCOOH Y' KOH CH OH P' CH COOH Q' H H 3 25 3 '' '' K KOH
The X, Y, P, and Q respectively is/are
a. X-KMnO4, Y-MnO2, P-K2Cr2O7, Q-Cr2O7
b. X-KMnO4, Y-MnO2, P-KMnO4, Q-MnO2
c. X-K2Cr2O7, Y-Cr2O7, P-MnO4, Q-MnO2
d. X-K2Cr2O7, Y-Cr2O7, P-K2Cr2O7, Q-Cr2O7
14. Which organic structure among the following is not an isomer of the compound
CH CO CH CH CH CH 3 222 3 ?
a. CH CH OCHCHCHCH 32 23 =
b. CH CH CO CH CH 3 2 23 ^h
b. CH CH CHCH CH CHO 32 2 =
d. CH CH COCH CH CH 32 22 3
15. The number of isomers possible for CH O 78 is
a. 2
b. 3
c. 4 d. 5
16. The number of geometrical isomers of CH CH CH CH CH CH CH Cl 3 =-=-=-
a. 2
b. 4
c. 6
d. 8
17. The number of possible isomers for the compound with molecular formula CBrClFI 2 is (IIT)
a. 3
4
5
6
18. When ethanol is dehydrated, which of the following is not a possible product?
a. Ethylene b. Diethyl ether c. Ethanol d. Water
19. What is the primary product when ethanol (C2H5OH) undergoes dehydration in the presence of concentrated sulfuric acid?
a. Ethylene (C2H4)
b. Ethane (C2H6)
c. Diethyl ether (C4H10O) d. Ethanol (C2H5OH)
20. Which of the following compounds leads to the formation of the oxidation of ethanol using potassium dichromate?
a. Ethane b. Ethanoic acid c. Ethyl chloride d. Ethene
21. Which of the following is a common use of ethanoic acid in the food industry?
a. Preservatives in canned foods b. The leavening agent in bread-making c. Sweetener in beverages d. Emulsifier in salad dressings
22. When ethanoic acid reacts with sodium hydroxide, what is the product formed?
a. Sodium bicarbonate and water b. Sodium chloride and water
c. Sodium ethanoate and carbon dioxide d. Sodium acetate and water
23. Among the following the compound that is always taken as substitutent in IUPAC nomenclature?
a. -NH2 b. -CN c. -NO2 d. -COOH
24. Which of the following compounds is an example of positional isomerism?
a. Ethane and propane b. Methanol and ethanol
c. Butane and isobutene
d. Methylamine and ethylamine
25. Which of the following compounds is an example of a positional isomer of 2,2-dimethylpropane?
a. 2-methylpentane
c. 2,3-dimethylpentane
b. 2-methylbutane
d. 3-methylpentane
26. Which of the following statements are correct?
(I) A pair of positional isomers differs in the position of the same functional group.
(II) A pair of structural isomers have the same relative molar mass.
(III) A pair of functional group isomers belong to different homologous series.
a. II and III
b. I and III
c. I and II
d. I, II and III
27. The difference between 2-methylpentane and 3-methylpentane is in the-
a. Number of carbon atoms
c. Number of double bonds
b. Position of the methyl group
d. Position of the functional group
28. Which of the following is NOT a suitable oxidising agent for oxidising a primary alcohol to a carboxylic acid?
a. Potassium permanganate
c. Jones reagent
b. Chromic acid
d. Sodium borohydride
29. What is the product obtained when butan-2-ol is oxidised?
a. Butanal b. Butanone c. Butanoic acid
d. Butyl acetate
30. Which of the following correctly represents the chemical equation for the oxidation of primary alcohol?
a. CH3CH2OH + [O] $ CH3CHO + H2O
b. CH3CH2OH +[O] $ CH3CH2OH2+ +2e-
c. CH3CH2OH + [O] $ CH3CH2COOH + H2O
d. CH3CH2OH + [O] $ CH3CH2CHO + 2 H2O
31. When a hydrocarbon undergoes incomplete combustion, why is the formation of carbon monoxide (CO) considered a potential health hazard?
a. CO is a greenhouse gas and contributes to global warming.
b. CO is highly flammable and can cause explosions.
c. CO is a strong oxidising agent.
d. CO is toxic and can bind to haemoglobin, reducing oxygen transport.
32. During the combustion of methane (CH4), which factor contributes most significantly to the production of carbon monoxide (CO) rather than carbon dioxide (CO2)?
a. High oxygen concentration
c. Incomplete combustion
b. Low reaction temperature
d. Presence of a catalyst
33. The IUPAC nomenclature of CH3-CH(OH)-CH2-CH2-COOH is
CARBON
a. 1-Carboxy-3-butanoic acid
c. 1-Carboxy-4-butanol
b. 4-Hydroxypentanoic acid
d. 4-Carboxy-2-butanol
34. Which of the following elements oxidised in the conversion of ethanol to ethanoic acid?
a. Carbon is oxidised
b. The hydrogen oxidation number is changed
c. Carbon is reduced
d. Carbon undergoes disproportionation
35. In vinegar, the percentage of acetic acid is
a. 5-8 per cent
b. 6-10 per cent
36. Bath soap is a mixture of
c. 7-12 per cent
a. Sodium and calcium salts of higher fatty acids
b. Sodium stearate
c. Sodium salts of higher fatty acids
d. Potassium salts of higher fatty acids
d. 8-14 per cent
37. Soaps and detergents are kept in the family of which of the following types of compounds?
a. Surface inactive compound
c. Acidic compounds
b. Surface active compounds
d. Water insoluble
38. During the micelle’s formation, the hydrophilic part is ______.
a. Polar b. Non-polar
c. Cannot be predicted d. Non-ionic
39. Which of the following is not an example of soap?
a. Sodium stearate
c. Sodium palmitate
b. Alkyl benzene sulphonate
d. Sodium oleate
40. Detergents are generally sodium salts of _______.
a. Phosphoric acid
c. Sulphonic acid
b. Hydrochloric acid
d. Nitric acid
41. Select the incorrect statement from the following options.
a. In the micelle formation, the water-soluble heads are directed towards the centre.
b. In the micelle formation, the water-soluble heads are on the surface in contact with the water.
c. In the micelle formation, the water-insoluble tails are directed towards the centre.
SOME BASIC CONCEPTS OF CHEMISTRY
6.1 IMPORTANCE OF CHEMISTRY
Chemistry deals with the study of materials, their preparations, their properties, their accurate, supermicroscopic structures, their mutual interactions, and their uses in all possible walks of life.
Chemistry plays an important role in meeting human needs for food, healthcare products, and other materials aimed at improving the quality of life. Many life-saving drugs have been isolated from plant and animal sources or prepared by synthetic methods.
Chemistry is responsible for designing and synthesising new materials having specific magnetic, electric, and optical properties. Safer alternatives to environmentally hazardous refrigerants like CFCs, which are responsible for ozone depletion in the stratosphere, have been successfully synthesised.
Nature of matter
Matter is anything that has mass and occupies space. Substance or material is another common term used in chemistry. All the things around us, such as air, water, books, tables, pencils, etc., are matter. Matter is physically classified into three states: solids, liquids, and gases. The plasma state is also referred to as matter.
Description of matter nearly in terms of states of aggregation does not suffice and it becomes necessary to make use of other properties to obtain a more useful classification of matter.
One such useful way is to classify matter into three categories: Elements, compounds, and mixtures.
6.1.1
Atoms and molecules
The finest particle of matter is called an atom. Two or more atoms of the same or different elements combine to give a molecule. Atoms may lose or gain electron or electrons to form ions
Fig. 6.1 Scheme of categories of matter: Chemical classification
• Pure substances are of two types. The substance that gives the same atoms on fine division is called an element. Molecules of elements are homoatomic.
Example: H2, Cl2, O2, O3, P4, S8, Cn, etc.
• The substance that gives different atoms on division is called a compound. Molecules of compounds are heteroatomic.
Example: HCl, H2O, NH3, NaOH, H2SO4, C6H12O6, etc.
• Compounds may be ionic or covalent. Compounds have a definite composition of the combined elements.
• Mixtures are usually two types. They may be homogeneous or heterogeneous. Air is an example of a homogeneous mixture. Similarly, salt solution or sugar solution is a homogeneous mixture.
• Colloidal solutions, emulsions, and suspensions are examples of heterogeneous solutions. Blood, milk, cloud, and smoke are heterogeneous mixtures.
Atomicity
Atom is the smallest particle of the element, which represents the reactivity of the element. A molecule is the smallest particle of the substance which exists in a free state. Number of atoms constituting a molecule is called atomicity. Noble gases are monoatomic. Each molecule has only one atom.
Example: He, Ne, Ar, Kr, Xe, and Rn.
Elementary gases are diatomic. Each molecule has two atoms.
Example: H2, N2, O2 and Cl2
Atomicity of F2, HCl, LiH is also 2. Ozone, O3 is triatomic. White phosphorous P4 is tetraatomic Rhombic sulphur S8 is octaatomic.
The atomicity of some familiar substances is given in the table below.
Note: Noble gases are monoatomic. Elementary gases are diatomic. However, one gaseous element is even available as triatomic, O3.
Mixtures have no definite composition of the constituents. Mixtures are mostly physical in nature. Components of mixtures can be separated by methods like crystallisation, sublimation, evaporation, distillation, solvent extraction, chromatography, etc.
Differences between compound and mixture are listed in the table below.
Sr. No.
Compound
1) The constituents of a compound are in a fixed mass ratio.
Mixture
The constituents of a mixture are in any mass ratio.
2) Compound is always homogeneous in nature. Mixture may be homogeneous or heterogeneous.
3) The properties of a compound are different from those of its constituents.
The properties of a mixture are almost midway from those of its constituents.
4) Compound formation involves a chemical change. Mixture formation generally involves a physical change.
5) The constituents of a compound cannot be easily separated. Heat or light is normally required for the separation.
The constituents of a mixture can be separated by simple physical methods or by mechanical means.
6) Compounds possess distinct melting and boiling points. Melting and boiling points of mixtures are usually non-stop.
7) Example: NaHCO3
Example: Salt and sand
Table 6.2 Differences between compound and mixture
Atoms combine to give molecules by mutual sharing of electrons. Atoms can also combine by the transfer of electrons to give ionic substances
6.2 PROPERTIES OF MATTER AND THEIR MEASUREMENT
6.2.1 Atomic weight
An individual atom is very small and assumed to be spherical. The radius of an atom is in the order of 10-10 m. If ten grams of iron or ten grams of copper is taken and made into 1023 small pieces of the same size, we may get each fine particle as an atom. The real mass of an individual atom is as small as about 10-26 kg. The real masses are not practicable to measure. Hence, relative masses are used.
The concept of relative masses of atoms was first introduced by Dalton, by taking the water molecule. The water molecule has two H atoms and one O atom. If the mass of the H atom is taken as one unit, then that of the O atom will be 16 units.
The unit of relative mass is the atomic mass unit. It is familiarly known as 'amu', but now denoted by 'u' and is called unified mass. The present reference of relative masses is the 12C isotope. The relative mass of 12C is taken as 12 amu. Hence, 'amu' is defined as one-twelfth of the mass of 12C isotope:
1 amu = Mass of 12C 12
Example: If the relative mass of He is taken as one unit, what is that of calcium?
Solution:
Relative masses of He and Ca are 4 and 40, respectively.
Compared to He as 1, that of Ca is 40/4 = 10
Atomic weight is stated as the number of times the weight of one atom is heavier to the unified mass.
Atomic weight =
6.2.2 Average atomic mass
Weight of one atom of element 1/12 × Weight of 12C
Many naturally occurring elements exist in more than one form, called isotopes.
When we take into account the existence of isotopes, and their per cent abundances, the average mass of that element can be computed.
The average isotopic mass of chlorine is 35.5. This is because, naturally, chlorine is available in two stable isotopic forms, 35Cl, and 37Cl in the approximate mass ratio 3:1.
Example: The relative abundances of 12C and 13C, respectively, are 98.892 and 1.108. If the atomic masses of 12C and 13C are 12 u and 13.00335 u, respectively, calculate the average atomic mass of carbon.
Solution:
Average atomic mass of carbon = (98.892×12) × (1.108 × 13.00335)/(98.892 + 1.108) = 12.011 u
Example: Neon is naturally available as 20Ne and 22Ne with an average atomic mass of 20.2. Calculate the relative abundance of the heavier isotope.
Solution:
Let the percentage abundance of 22Ne be x. The percentage abundance of 20Ne is 100 - x
20.2 = (100 - x)20 + 22x 100
Per cent abundance of 20Ne = 10%
Atomic masses are fractional, but mass numbers are whole numbers.
A mass number is usually given for elements. It is also given for the fundamental particles. The mass numbers of electron, proton, and neutron are 0, 1, and 1, respectively.
The mass number of an element denotes the sum of the number of protons and neutrons present in the nucleus of its atom.
6.2.3 Molecular mass
Molecular mass is the sum of masses of all sub-atomic particles present in a molecule. It is generally calculated as sum of the atomic masses of the elements constituting the molecule.
Molecular weight is also measured relatively. It has no units.
Molecular weight = Weight of one molecule of substance 1/12 × Weight of 12C
The molecular weight of water is the sum of the atomic weight of O and twice the atomic weight of H. It is equal to 18.02u.
Example: What is the formula mass of gypsum?
Solution:
Formula of gypsum is CaSO4.2H2O.
Formula mass of CaSO4.2H2O = (1×40) + (1×32) + (4×16) + (2×18) = 172u
Example: Calculate the mass of a fructose molecule.
Solution:
The molecular formula of fructose is C6H12O6
Molecular mass of C6H12O6 = (6×12u) + (12×1u) + (6×16u) = 180u
While atomic masses are determined using Dulong and Petit's law, molecular masses are determined by Cannizzaro's method and vapour density method.
If the specific heat of a metal is given in calg-1, the product of the atomic mass of the element and specific heat is approximately 6.4 .
Atomic mass = 6.4
Specific heat
If the valency of an element and its equivalent mass are known, the product gives atomic mass.
Atomic mass = Valency × Equivalent mass
If the vapour density of a volatile substance is obtained, molecular weight is taken as twice the vapour density.
Molecular mass = 2 × Vapour density
In Victor Mayer's method, the volume of air displaced by a fixed mass of a volatile substance is determined. The volume is translated to standard temperature and pressure conditions. The mass corresponding to 22400 cc of air gives the molecular mass.
Molecular mass = 22400 × Weight of substance
Volume of air displaced at STP
The molecular weight of substances can also be determined based on the following:
1. Raoult's law method,
2. Depression in the freezing point method,
3. Elevation in boiling point method,
4. Osmotic pressure method,
5. Measurement of density, etc.
The mass spectroscopic method, however, is the most accurate experimental method for the molecular masses.
The molecular weight of a homogenous mixture is called effective molecular weight. If the number of moles of different substances in the mixture is n1, n2, … then,
Effective molecular weight of mixture = (n1 M1+n2 M2+...) (n1+n2+…)
6.2.4 Avogadro constant
The characteristics of elements and compounds are represented by atoms, molecules and ions. Though we understand the concepts through microscopic particles, we take practically measurable quantities of substances. In view of this, gram atomic weight, gram molecular weight, and gram ionic weight are introduced.
Gram Atomic Weight (GAW) is the weight of an element in grams, numerically equal to its atomic weight. It is also represented as 'gram atom'.
Gram Molecular Weight (GMW) is the weight of a substance in grams, numerically equal to its molecular weight. It is also represented as 'gram mole'.
The atomic weight of chlorine is 35.5, and the gram atomic weight of chlorine is 35.5 g. Similarly, the molecular weight of chlorine is 71, and the gram molecular weight of chlorine is 71 g.
There are many atoms present in one gram of an element's atomic weight. Similarly, there are many molecules present in one gram of the molecular weight of a substance. The number is denoted by NA
or N0 and is called Avogadro constant in honour of Amedeo Avogadro. It is also called the Avogadro number. Avogadro number is the number of atoms present in one gram atom or the number of molecules present in one gram mole.
To really appreciate the largeness of Avogadro constant, it is written as-
NA= 602213670000000000000000
In the exponential fashion, the Avogadro constant is written as NA= 6.022×1023
Avogadro constant represents the number of fundamental entities in GAW, GMW or GIW (gram ionic weight).
Example 1: How many atoms are present in 2 grams of calcium? (GAW of Ca is 40 g )
Solution:
40 grams of Ca has 6.022 × 1023 atoms
2 grams of Ca has = v?
Number of calcium atoms =2/40 × 6.02 × 1023 = 3.011 × 1022
Example 2: If a person spends one million rupees per sec continuously, how many years would it take to spend Avogadro number of rupees?
Solution:
Number of rupees to spend = 6.022 × 1023
Time required = (6.022 × 1023)/106 = 6.022 × 1017 sec
Number of years required = (6.022 × 1017)/(60 × 60 × 24 × 365) = 1.91 × 1010).
6.2.5
Properties of chemicals
The characteristic properties are broadly classified into two categories: physical properties and chemical properties.
Physical properties are those properties which can be measured or observed without changing the identity of the composition of the substance. Colour, odour, density, freezing point, boiling point, vapour pressure, etc., are some examples of physical properties.
Chemical properties are those properties which are measured or observed during a chemical change. Acidity, basicity, combustibility, oxidation, reduction, etc., are some examples of chemical properties. These properties are characteristic reactions of different substances.
The SI units: Many properties, such as length, area, volume, etc., are quantitative in nature. Any quantitative measurement or observation is represented by a number followed by units in which it is measured.
English system and metric system of measurement were being used in different parts of the world. The metric system was more convenient as it was based on the decimal system. This allows the use of prefixes to indicate the multiples or submultiples of a unit. These prefixes are listed in the table.
nano n
micro
milli m
peta P
exa E
10-2 centi c 1021 zeta Z 10-1 deci d 1024 yotta Y
Table 6.3 List of prefixes
The International System of Units, Le systeme International d'unit's- abbreviated as SI, in Metre convention held in Paris, 1875 adopted the SI system of units. The system has seven base units representing length, mass, time, electric current, temperature, quantity of substance and luminous intensity. These base units are listed in the table.
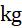
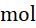

Table 6.4 Base quantities and their units
6.2.6
Mass and volume
Mass of a substance is the amount of matter present. Weight is the force exerted by gravity on an object. The mass of a substance is constant, whereas its weight may vary from one place to another due to changes in gravity. Though mass and weight are different by definition, chemists commonly use weight to denote the amount of matter. The SI unit of mass is kilogram. However, its fraction gram is used in the laboratory because smaller amounts of chemicals are used in reactions.
The mass of a substance can be determined accurately in the laboratory using an analytical balance. Volume is a term to denote the space occupied by a certain amount of matter. The mathematical expression of volume is V = lbh. The derived SI unit of volume is m3. In laboratories, smaller volumes of chemicals are used. Hence, volume is often denoted in cm3 or dm3 units.
The most common unit of volume of a gas or solution is litre (symbol L). In the laboratories, the volume of liquids or solutions can be measured by a graduated cylinder, burette, pipette, volumetric flask, etc., 1 L = 1000 mL = 1 dm3;1 mL = 1 c.c. = 1 cm3
Volumes are commonly obtained from density measurements. The density of a substance is the amount of mass per unit volume. Hence, volume is the ratio of mass and density.
The density of solids and liquids is often expressed in gcc-1 and gases in gL-1
Example 1: Write the derived unit of density.
Solution:
SI unit of density = SI unit of mass SI unit of volume =kgm-3
Example 2: How many mL is one m3?
Solution:
1 m3 = (10 dm)3 = 103 dm3 = 103 L; 1 L = (10 cm)3 = 103 cm3 = 103 mL
Therefore 1 m3 = (100 cm)3 = 106 cm3 = 106 mL
One m3 of gas is 106 mL
6.2.7
Significant figure
Every experimental measurement has some amount of uncertainty with it. However, one would always like the results to be precise and accurate. Precision refers to the closeness of various measurements for the same quantity. However, accuracy is the agreement of a particular value to the true value of the result.
The uncertainty in the experimental and the calculated values is indicated by mentioning the number with certain meaningful digits called significant figures. The uncertainty is indicated by writing certain digits and the last uncertain digit. If we write a result as 1234 mL, we say 123 is certain, and 4 is uncertain. The uncertainty would be ±1 in the last digit. Therefore, the result may be 1233 mL or 1235 mL.
The rules for determining the number of significant figures are stated below:
• All non-zero digits are significant. There are two significant figures in 85 cm and three significant figures in 0.258 mL.
• Zero preceding the first non-zero digit is not significant. Such zero indicates the position of the decimal point. There is one significant figure in 0.05 and two significant figures in 0.0026.
• Zeros between two non-zero digits are significant. There are four significant figures in 50.02.
• A zero at the end (or right) of a number is significant, provided they are on the right side of the decimal point. There are four significant figures in 0.2800 g.
• The terminal zero is not significant if there is no decimal point. Very large and too small values are generally represented in scientific and exponential notation.
There is one significant figure in 200
There are three significant figures in 200.
There are four significant figures in 200.0
There are two significant figures in 2.0×102
There are three significant figures in 2.00×102
• Counting the number of objects, for example, 3 balls or 20 eggs, have infinite significant figures, as these are exact numbers.
• The result of addition or subtraction cannot have more digits to the right of the decimal point than either of the original numbers.
• The result of multiplication or division must be reported with no more significant figures, as there are few significant figures in the measurement.
• While limiting the result to the required number of significant figures, the rounding off of the number is done as follows-
1. If the rightmost digit to be removed is less than 5, the preceding number is not to be changed.
2. If the rightmost digit to be removed is more than 5, the preceding number is increased by 1.
3. If the rightmost digit to be removed is 5, the preceding number is not changed when it is an even number, but it is to be increased by one when it is an odd number.
4. When 12.0 has one digit after the decimal point, the result should be reported only up to one digit after the decimal point. Hence, round off the result to 32.1. Number of significant figures is 3.
6.2.8 Dimensional analysis
Often, while calculating, there is a need to convert units from one system to another. The method used to accomplish this is called dimensional analysis, factor label method or unit factor method. It should be noted that in the dimensional analysis, units can be handled just like other numerical parts. It can be cancelled, divided, multiplied, squared, etc.
Example 1: A m3 vessel at STP has oxygen gas. How many moles of oxygen are present?
Solution:
22.4 L at STP = 1 mol
1 m3 = 1000 L
Number of moles of oxygen = 1 m3 × 1000 L 1 m3 × 1 mol 22.4 L = 1000 22.4 = 44.6
Example 2. How many seconds are there in 7 days?
Solution:
1 Day = 24 hr; 1 = 24 h/1 day; 1 hr = 60 min;
1 = 60 min 1 hr ; 1 min = 60sec; 1 = 60 s 1 min
The unit factors can be multiplied in series in one step only.
6.3 MOLE CONCEPT
Mole is the amount of a substance containing the same number of elementary chemical units as the number of atoms present in 0.012 kg of carbon-12 isotope
One mole of carbon is one gram atomic weight of carbon-12 isotope. One mole of carbon is taken as 12 grams of carbon-12 isotope. The number of atoms in one mole of carbon is 6.022 × 1023. Similarly, one mole of water is taken as 18 grams, and the number of molecules in one mole of water is also equal to 6.022 × 1023.
However, one mole of sodium chloride is 58.5 grams. One mole of sodium chloride has 6.022 × 1023 formula units of NaCl. The total number of ions present in one mole of sodium chloride is 1.2044 × 1024
The mass of one mole of a substance is called its molar mass. Mole is nothing but the gram molecular weight or gram mole of a substance.
The volume occupied by one mole of a gaseous substance is called gram molar volume (GMV). The GMV depends on both temperature and pressure. Temperature of 273 K and pressure of 1 atm are called standard temperature and pressure (STP) conditions. At STP, the value of GMV is 22.4 lit or 22400 cc.
The charge carried by one mole of electrons is called one Faraday

6.5 Molar masses of some chemicals
atoms
molecules
molecules
molecules
atoms
atoms
ions
electrons Silver ion Ag+
Table 6.6 Examples of mole relationships
electrons
molecules
atoms
formula units
ions
ions
atoms
molecules
atoms
The formation of ammonia from its elements in terms of the mole concept is summarised in the table below.
L of N2 at STP
L of H2 at STP
Table 6.7 Formation of ammonia
L of NH3 at STP
The importance of mole
Mole concept is important and useful as it accounts for the following.
• One mole of an element represents one gram of atomic weight of the element. It is called a gram atom.
• One mole of a substance represents one gram of molecular weight of the substance. It is called a gram mole or gram molecule.
• One mole of an element means the mass of 6.022 × 1023 atoms of the element.
• One mole of a covalent substance means the mass of 6.022 × 1023 molecules of the substance.
• One mole of an ionic substance means the mass of 6.022 × 1023 formula units of the substance.
• One mole of a gas or vapour occupies a volume called GMV. GMV at STP conditions is 22.4 L.
• The charge of one mole of electrons is called Faraday. One Faraday is given as 96,500 C.
• The concept of mole represents the Avogadro constant.
• The density of a gas in gL-1 is the ratio of GMW and GMV.
• Vapour density is one-half of the molecular weight of the substance. Vapour density is the mass of 11.2 L of a gas at STP.
• The ratio of Avogadro constant and gram molar volume (GMV) in 'cc' is called Loschmidt number. The value of Loschmidt number is given as 2.69 × 1019.
• The number of molecules in a substance is the product of the number of moles and Avogadro constant.
• The number of atoms in a substance is the product of the number of molecules and atomicity.
• The number of moles of a given mass of substance is the ratio of mass in grams and GMW.
• Mole is extremely useful in calculations because it simplifies the work of a chemist.
Example 1: Calculate the mass of one mole of electrons.
Solution:
Mass of one electron = 9.1095×10-31 g
Mass of one mole electrons = Mass of electrons × Av. number = 9.1095 × 10-31 × 6.022 × 1023 = 5.5 × 10-7 Kg
Mole of electrons has 0.55mg mass.
Example 2: How many moles are present in 108 grams of glucose?
Solution:
Weight of glucose = 108 g
GMW of glucose = 180 g
Number of moles = Weight /GMW = 108/180
Moles of glucose = 0.6
Example 3: The atomic mass of mercury is 200, and the density is 13.6 gcc-1. How many moles of metal are present in one litre?
Solution:
Weight of Hg = Volume × Density = 1000 × 13.6 = 13600 g
Number of moles = weight /GAW = 13600/200 = 68
One litre of mercury contains 68 moles
Example 4: 3.011 ×1022 molecules are removed from a vessel containing 1680 cc of nitrogen at STP. How many moles are remaining?
Solution:
Number of moles of N2 originally present = Volume / GMV = 1680/22400 = 3/40
Number of moles of N2 removed = Number of molecules / Avogadro number = (3.011 × 1022)/(6.022 × 1023) = 1/20
Number of moles remaining = 3/40 - 1/20 = 1/40 = 2.5 × 10-2 = 0.025
6.4 PER CENT COMPOSITION
Chemical formulae are an application of the law of constant proportions. The composition of a pure chemical component is always fixed. The weight fraction or the per cent weight of a constituent in a given compound is constant. The fraction of the weight of a constituent is given as,
Weight fraction = Weight of the constituent/Formula weight of the compound
Per cent weight = Weight fraction ×100
Example 1: An organic compound has 8 per cent of sulphur by weight. What is its molecular weight of the compound?
Solution:
8 parts of sulphur =100 parts of compound
32 parts of sulphur = ?
The minimum molecular weight of the compound = 32/8 × 100 = 400. Molecular weight may be a multiple of 400, like 800, 1200, 1600, 2000, etc.
Example 2: The weight percentage of iron in haemoglobin is 0.33. If the approximate molecular weight of haemoglobin is 68000, how many iron atoms are present in the molecule? (At.wt. of Fe is 56)
Solution:
100 parts of haemoglobin = 0.33 parts iron
68000 parts of haemoglobin= ?
Weight of Fe in haemoglobin = 68000/100 × 0.33 = 224 gm
56 g of Fe = one Fe
224 g of Fe = ?
Number of iron atoms = 224/56 = 4
6.4.1 Weight per cent
The weight percentages of carbon and hydrogen in organic compounds is determined by Liebig method. Cupric oxide is used as an oxidant. Carbon is oxidised to carbon dioxide, and hydrogen is oxidised to water. Carbon dioxide is absorbed in potash solution and water in concentrated sulphuric acid or anhydrous calcium chloride. The weights of carbon dioxide and water are determined experimentally, and the percentage weights are calculated.
Per cent weight of carbon = w1 w × 12 44 × 100
Per cent weight of hydrogen = w2 w × 2 18 × 100
Where w is the weight of the organic compound, w1 is the weight of carbon dioxide, and w2 is the weight of water.
Nitrogen present in a compound can be estimated using Duma's method or Kjeldahl's method. In Duma's method, the nitrogen content of organic compound is made as free nitrogen, N2. The volume of nitrogen V1 is measured at the experimental conditions of pressure, P1 and temperature T1.
The volume is translated to STP conditions using the equation = (P1 V1)/T1 = (P2 V2)/T2
Per cent weight of nitrogen = V2 w × 28 22400 × 100
where w is the weight of the substance, and V2 is the volume of nitrogen at STP.
In Kjeldahl's method, the compound is heated with concentrated sulphuric acid to convert nitrogen to ammonium sulphate. The ammonia liberated by treating it with sodium hydroxide is estimated using a back titration method.
Per cent weight of nitrogen = w3 w × 14 17 × 100 where w3 is the weight of NH3 liberated.
Sulphur present in an organic compound is estimated using the Carius tube method. When the compound is heated with barium chloride, barium sulphate is precipitated. The precipitate is separated by filtration, dried and weighed.
Per cent weight of sulphur = w4 w × 32 233 × 100 where w4 is the weight of barium sulphate.
Halogen present in an organic compound is estimated by the Carius tube method using silver nitrate as a reagent. The weight of silver halide precipitated is experimentally determined.
Phosphorus present in a compound is also estimated using the Carius tube method by precipitating magnesium ammonium phosphate. The precipitate is ignited to pyrophosphate and weighed. There is no suitable direct method for the estimation of oxygen. Generally, the weight per centage of oxygen is obtained by subtraction.
Example 1: On heating 0.2 g of an organic compound with a mixture of barium chloride and nitric acid, 0.466 g of barium sulphate was obtained. Calculate the percentage of sulphur.
Solution:
Weight of substance = w = 0.2 g; weight of barium sulphate = w4 = 0.466 g
weight percentage of sulphur = w4 × 32 × 100 w × 233 = 0.466 × 32 × 100 0.2×233 = 32%
6.4.2 Empirical formula
The empirical formula is the simplest formula of a chemical substance. It is the formula of the substance that gives the relative whole number ratio of atoms of each element present in the molecule of a substance.
Example: CH is the empirical formula of benzene. This formula indicates that the ratio of number of 'C' and number of 'H' atoms is 1:1. It also indicates that the ratio of mass of 'C' and 'H' is 12:1. It suggests that the weight percentages of carbon and hydrogen in benzene are respectively 92.3% and 7.69%. Compounds with the same empirical formula possess the same percentage composition of elements.
The empirical formula can be determined from the mass percentages of various elements present in a compound.
The sequence of steps in the determination of the empirical formula are:
• The weight percentage (or weight) of each constituent element is taken.
• The per cent weight of each constituent is to be divided by its atomic weight.
• The simplest whole number ratio of the values of step (2) is to be obtained. This may be done by dividing all values with the smallest among them.
• Write the whole number as a suffix against the symbol of each element.
• Ionic substances have no molecules. They are represented only by their empirical formula.
Example 1: An organic compound contains carbon, hydrogen, oxygen, and nitrogen in the weight ratio 3:1:8:3.5. Calculate its empirical formula.
Solution:
Ratio of atoms, C : H : O : N = 3 12 : 1 1 : 8 16 : 3.5 14 = 0.25 : 1 : 0.5 : 0.25 = 1 : 4 : 2 : 1 The empirical formula is CH4O2N.
Example 2: A compound contains 26.6% potassium, 35.4% chromium and the remaining oxygen. What is its empirical formula? (At.wt. of K = 39.1, Cr = 52, O = 16 )
Solution:
Ratio of K : Cr : O = 26.6 39.1 : 35.4 52
The empirical formula is K2Cr2O7.
6.4.3 Molecular formula
The molecular formula of a compound is one which expresses the actual number of atoms of each element present in one molecule.
The molecular formula of benzene is C6H6. This indicates that the benzene molecule has six carbon atoms and six hydrogen atoms.
Molecular formula = n × Empirical formula,
Here, n is the number of empirical formula units repeated in the molecular formula.
The number of repeating empirical units is obtained as a ratio of molecular mass and mass represented by the empirical formula.
Molecular formula mass
n =
Empirical formula mass
The empirical formula may be the same for different compounds. Formaldehyde, acetic acid, lactic acid, and glucose all have the same empirical formula, CH2O. The difference is n = 1 for formaldehyde, n = 2 for acetic acid, n = 3 for lactic acid and n = 6 for glucose.
The empirical formula is the same for all alkenes or cycloalkanes, but the individual members differ in their molecular weights and structural formula.
Oxalic acid
2 H2C2O4
Hydrogen peroxide HO H2O2
Sodium carbonate Na2CO3 -
Table 6.8 Empirical and molecular formula of some substances
Example 1: A brominated alkane analysis gave 12.8% carbon and 2.1%H. If its vapour density is 93.95, what is its molecular formula?
Solution:
Per cent weight of bromine = 100 - (%C + %H) = 100 - 14.9 = 85.1
C:H:Br = 12.8 12 : 2.1 1 : 85.1 80 = 1:2:1
Empirical formula is CH2Br.
Molecular weight = 2 × V.D. = 2 × 93.95 = 187.9 n = 187.9/94 = 2
Molecular formula = 2 × CH2Br = C2H4Br2
6.5 STOICHIOMETRIC CALCULATIONS
6.5.1 Stoichiometric coefficients
In general, a reaction between reacting substances is described by a balanced chemical equation. aA + bB ⟶ cC + dD
In the above equation, A and B are reactants and C and D are products. A balanced equation is also called a stoichiometric equation. The coefficients a, b, c, and d are called stoichiometric coefficients or mole coefficients of the substances. These coefficients give the idea of the quantities of the substances consumed and also the substances produced in chemical reactions.
This area of study is known as 'stoichiometry' (pronounced as stoy-key-om-uh-tree), a name derived from the Greek word, which means measure of element.
A balanced equation provides the following information:
a) The reactants and products involved in the chemical change.
b) The relative number of moles or molecules of the reactants and products.
c) The relative number of parts of the mass of reactants and products.
d) The relative volumes of gaseous reactants and products.
The quantitative relationship existing between the quantities of the reactants and the products in a chemical reaction is known as stoichiometry.
Chemical reactions are represented concisely by chemical equations using formulae of the reactants and products. The mole coefficients must be kept in mind in calculations based on equations. The exact quantities of reactants and products that appear in the balanced chemical equation are known as stoichiometric quantities.
Mole is translated to weight or volume or number of particles, which is helpful in stoichiometric calculations. The important relationships in calculations based on chemical equations are:
a) Weight-weight relationships
b) Weight-volume relationships
c) Volume-volume relationships
d) Weight-number relationships
e) Volume-number relationships
In order to use the above relationships, one must recollect the following three concepts.
1. One mole is a gram molecular weight of a substance. In the case of monoatomic substances and metal vapours, mole is taken as gram atomic weight. If the substance is in the form of ions, mole is taken as gram ionic weight.
2. One mole represents a gas whose volume is called gram molar volume. Gram molar volume of a gas at standard temperature and pressure is 22.4 L or 22,400cc.
3. One mole denotes Avogadro number of elementary chemical units. Avogadro constant is written as 6.022×1023.
6.5.2 Weight-weight relationship
It is possible to calculate the amounts of products formed in a chemical reaction if the amounts of the reactants participating in the reaction are known. This is possible only if the stoichiometric equation is known. Such calculations help us to know beforehand whether a particular chemical reaction under consideration is economically viable for the production of a required product or not.
Example 1: Calculate the weight of calcium carbonate required to produce carbon dioxide that is sufficient for the conversion of one decimole of sodium carbonate to sodium bicarbonate.
Solution:
Step 1: Regarding the requirement of carbon dioxide:
The balanced equation for the conversion of sodium carbonate to sodium bicarbonate is
Na2CO3 + H2O + CO2 ⟶ 2NaHCO3
Decimal = 0.1 mole
1 mole of Na2CO3 ≡ 1 mole of CO2
Carbon dioxide required for 0.1 moles of Na2CO3 = 0.1 mole (or 4.4 grams)
Step 2: Regarding the requirement of calcium carbonate :
The balanced equation for the decomposition of calcium carbonate is
CaCO3 ⟶ CaO + CO2
1 mole of CO2 ≡ 1 mole of CaCO3
44 grams of CO2 = 100 grams of CaCO3

The wt. of calcium carbonate to produce the required carbon dioxide =100/44 × 4.4 = 10 g
Example 2: Calculate the weight of calcium carbonate required to produce carbon dioxide that is sufficient for the conversion of one decimal sodium carbonate to sodium bicarbonate.
Solution:
Step 1: Regarding the requirement of carbon dioxide
The balanced equation for the conversion of sodium carbonate to sodium bicarbonate is
Na2CO3 + H2O + CO2 ⟶ 2NaHCO3
Decimol = 0.1 mole
1 mole of Na2CO3 ≡ 1 mole of CO2
Carbon dioxide required for 0.1 moles of Na2CO3 = 0.1 mole (or 4.4 grams)
Step 2: Regarding the requirement of calcium carbonate
The balanced equation for the decomposition of calcium carbonate is CaCO3 ⟶ CaO + CO2
1 mole of CO2 ≡ 1 mole of CaCO3
44 grams of CO2 = 100 grams of CaCO3
The wt. of calcium carbonate to produce the required carbon dioxide = 100/44 × 4.4 = 10 g
6.5.3 Weight-volume relationship
This method of calculations based on equations is employed when one or more of the reactants or products of the reaction are present in a gaseous state. This method makes use of converting the mole to gram molecular weight of a substance and to the gram molar volume of the other substance.
Example 1: When 50 grams of sulphur is burnt in the air, 4% of the impure residue is left over. Calculate the volume of air required at STP containing 21% of oxygen by volume.
Solution:
Weight of sample of sulphur taken = 50 g
Percentage of impurity = 4%
Weight of impurity in sample = (4 × 50)/100 = 2 grams
Weight of sulphur in sample = 50-2 = 48 grams
Combustion of sulphur gives sulphur dioxide
S + O2 ⟶ SO2
1 mole of S ≡ 1 mole of O2
32 grams of S = 22.4 L of O2 at STP
48 grams of S = ?
Volume of oxygen required at STP = 48/32 × 22.4 = 33.6 L
21 L of O2 is present in 100 L of air
33.6 L of O2 present in?
Volume of air required at STP = 33.6/21 × 100 = 160 L
6.5.4 Volume-volume relationship
If gases are used as reactants and gases are produced in the reaction, Gay-Lussac's law of combining volumes may be used for calculations based on equations. The mole coefficients of gaseous substances directly give the volumes or volume ratio of the substances. Stoichiometric calculations are very simple if the law of combining volumes is useful.
Example 1: What is the minimum volume of carbon monoxide at STP needed to react completely with 0.112 L of oxygen at 127° C and 1.5 atm.
Solution:
Carbon monoxide is oxidised in oxygen to give carbon dioxide
2CO + O2 ⟶ 2CO2
1 mole of O2 ≡ 2 moles of CO
1 volume of O2 = 2 volumes of CO
0.112 L O2 at STP = 0.224 L CO at STP
STP conditions Given conditions,
P1 = 1 atm P2 = 1.5 atm
T1 = 273 K T2 = 400 K
V1 = 0.224 L V2 = ?
Volume of carbon monoxide needed in the given conditions V2 = P1V1 T1 × T2 P2 = 1 × 0.224 × 400 273 × 1.5 = 229.3 L
Other relationships
Instead of translating mole into the two common measurable quantities mass and volume, some times the number of particles is also taken into consideration. Gram molecular weight and gram molar volume are related to Avogadro constant
Example 1:100 mL of phosphorus pentachloride is totally decomposed to its trichloride at 1 atm and 546°C. How many molecules of chlorine are formed?
Solution:
Decomposition of phosphorus pentachloride is given as
PCl5 ⟶ PCl3 + Cl2
1 mole (PCl5 ) = 1 mole of Cl2
1vol of PCl5 = 1 vol of Cl2
100 mL of PCl5 = 100 mL of Cl2
Given conditions
P1 = 1 atm
T u = 819 K
V1 = 100 mL
SOME BASIC CONCEPTS OF CHEMISTRY
STP conditions
P2 = 1 atm
T2 = 273 K
V2 = ?
The volume of Cl2 measured at STP
V2 = P1V1 T1 × T2 P2 = 1 × 100 × 273 819 × 1 = 33.33 mL
22400 mL of Cl2 at STP = 6.022 × 1023 molecules
33.33 mL of Cl2 at STP = ?
Number of molecules of chlorine obtained = 33.33 22400 × 6.022 × 1023 = 8.96 × 1020 molecules
6.5.5 Limiting factor
The reactant that limits the product formation is called a limiting reagent, and the concept is termed a limiting factor. The limiting factor must also be kept in mind when performing calculations based on chemical equations.
Example 1: In a gas phase reaction, 50 kg of nitrogen and 10 kg of hydrogen are mixed to produce ammonia. Identify the limiting reagent. Calculate the maximum amount of ammonia produced.
Solution:
The gaseous phase chemical equation for the production of ammonia is given as
N2 + 3H2 ⟶ 2NH3
1 mole of N2=3 moles of H2
28 kg of N2=(2×3)kg of H2
46.67 kg of N2=10 kg of H2
50 kg of N2=10.71 kg of H2
Hence, hydrogen is the limiting reagent.
3 moles of H2=2 moles of NH3
(3×2)kg of H2=(2×17)kg of NH3
10 kg of H2=?
Maximum amount of ammonia produced is = (2 × 17 × 10)/(3 × 2) = 56.6 kg
Example 2: 11.2 L of oxygen STP and 8 grams of calcium are allowed to react. What weight of which chemical is left unreacted?
Solution:
The balanced chemical equation is 2Ca+O2⟶2CaO
2 moles of Ca ≡ 1 mole of O2
(2×40) grams of Ca = 22.4 L of O2 at STP
8 grams of Ca = 2.24 L of O2 at STP
The limiting reagent is calcium.
Volume of O2 at STP required = 2.24 L
Volume of O2 left unreacted = 11.2-2.24 = 8.96 L at STP
6.6 EQUIVALENT WEIGHTS
6.6.1 Concept of equivalent
From the experimental illustration of oxidation-reduction, it was noticed that copper displaces silver from silver nitrate. The balanced chemical equation for the reaction between copper and aqueous silver nitrate is given as
Cu(s) + 2AgNO3(aq) ⟶ 2Ag(s) + Cu(NO3)2(aq)
One gram of copper can reduce two gram ions of silver. Each gram atom of silver can be obtained by using only one-half mole of copper (or 31.75 grams), which is called equivalent
Substances in a chemical reaction need not react in 1:1 ratio of their moles or masses or volumes.
The reactants in a reaction always react in an equal ratio to their equivalents, and the products are also formed in an equal ratio to their equivalents. The concept of equivalent, equivalent weight or equivalent mass is an outcome of the law of reciprocal proportions.
For an element of atomic mass 'A' and valency 'n', the equivalent weight 'E' is given as E = A/n.
The atomic mass of copper is 63.50. The valency of copper is 2. Therefore, the equivalent weight of copper in its reaction with silver cation is 31.75.
Gram equivalent weight (GEW) is also proposed to be similar to gram molecular weight (GMW), gram atomic weight (GAW), and gram ionic weight (GIW). The gram-equivalent weight of copper is 31.75 g. Equivalent weights of some elements are given in the table below.
Table 6.9 Equivalent weights of some elements
The equivalent weight of an ion is the number of grams of the ion which can be discharged by the loss or gain of one mole of electrons. It is the ratio of ionic weight and charge magnitude of ion.
Equivalent weights of important cations and anions are given in the tables below.
Table 6.10 Equivalent weights of some cations
Table 6.11 Equivalent weights of some anions
Equivalent weight of an acid is the number of grams of the acid, which gives one mole of protons in water. It is the ratio of the molecular weight of acid and the basicity of acid. Basicity of acid is the number of H+ ions that the acid furnishes.
Equivalent weight of acid =
Formula weight of acid
Basicity of acid
Equivalent weight of a base is the number of grams of the base, which gives one mole of hydroxyl ions in water. It is the ratio of the molecular weight of the base and the acidity of the base. The acidity of the base is the number of OH- ions that the base furnishes.
Equivalent weight of base = Formula weight of base
Acidity of base
Equivalent weight of a salt is the number of grams of salt formed by one mole of electrons. It is the ratio of formula weight of salt and total positive or negative charges of the ionic substance.
Equivalent weight of salt =
Formula weight of salt Number of electrons transferred
Equivalent weight of acid = Formula weight of salt Total number of positive (or) negative charges
The equivalent weight of a salt in practice is taken as the sum of equivalent weights of ions represented in the empirical formula of salt.
Equivalent weight of salt = Equivalent weight of cation + Equivalent weight of anion.
Equivalent weights of important acids, bases, and salts are given in tables below.
Table 6.12 Equivalent weights of some acids
Table 6.13 Equivalent weights of some bases
Table 6.14 Equivalent weights of some salts
The equivalent weight of a redox reagent is the number of grams of the reagent involved in the loss or gain of one mole of electrons. It is the ratio of the formula weight of the reagent and the change in oxidation number.
Equivalent weight of oxidant =
Formula weight
Number of electrons gained
Equivalent weight of reductant = Formula weight
Number of electrons lost
Formula weight
Equivalent weight in electrolysis =
Number of Faradays
Number of electrons transferred in a redox reaction is generally obtained by the change in oxidation number. The change in oxidation number of oxidant or reductant some times is dependent on medium. Change in oxidation state and equivalent weights of potassium permanganate in different media are given in the table.
Table 6.15 Equivalent weights of potassium permanganate in different media
Example 1: Calculate the equivalent weights of ferrous sulphate (a) as salt and (b) as reductant.
Solution:
Formula weight of ferrous sulphate, FeSO4 = 56 + 32 + 64 = 152
a) As salt, the number of electrons transferred in the formation of salt is 2.
Equivalent weight =152/2 =76
b) As reductant, the number of electrons transferred Fe2+ ⟶ Fe3+ is 1.
Equivalent weight = 152
6.6.2 Determination of equivalent weights
The equivalent weight of a metal is the number of parts of the metal which can displace one gram of hydrogen from a mineral acid.
The equivalent weight of an element is the number of parts of that element which can combine with 8 parts of oxygen or 35.5 parts of chlorine by weight.
The equivalent weight of a substance is that amount of substance liberated using one Faraday of electricity.
The equivalent weight of a binary substance is the sum of the equivalent weights of the components. The equivalent volume of a gas is the volume occupied by the one-gram equivalent weight of the substance.
Equivalent volume = Molar volume X
Where 'x' is a factor by which the molecular weight of the substance is divided to get its equivalent weight. Isomorphous compounds have the same 'x'.
Isomorphism is a phenomenon of compounds having similar molecular formulas as well as crystal geometry.
Example i): MgSO4⋅7H2O, FeSO4⋅7H2O and ZnSO4⋅7H2 O ii): K2SO4⋅Al2(SO4)3⋅24H2O and (NH4 )2SO4⋅Fe2(SO4 )3⋅24H2O
Equivalent weights of volatile chlorides can be obtained by the measurement of vapour density. Equivalent weights of metals can be obtained by displacement of hydrogen. Dulong and Petit's law is used to determine atomic weights from specific heats, thereby obtaining equivalent weights of solid heavy elements.
The product of valency and equivalent weight gives the atomic weight of an element. The ratio of weights of two substances involved in a reaction is nothing but the ratio of their equivalent weights.
If w1 is the weight of substance 1 and w2 is the weight of substance 2.
w1 w2 = E1 E2
where E1 is the equivalent weight of substance 1 and E2 is the equivalent weight of substance 2
w1
E1 = w2 E2 = w3 E3 (and so on)
Example 1: The equivalent weight of element X is 3. If the vapour density of volatile chloride of X is 77, find out the molecular formula of chloride.
Solution:
Equivalent weight of chloride of X = Equivalent weight of Cl + equivalent weight of X = 35.5 + 3 = 38.5
Molecular weight of chloride = vapour density × 2 = 77 × 2 = 154
Valency of the element X = 154/38.5 = 4
Chlorine is taken as monovalent since it is chloride.
The molecular formula of chloride of X is XCl4
6.7 CONCENTRATION OF SOLUTIONS
6.7.1 Solution state
The solution state is a pseudo-state of matter. It refers to mixtures but not pure substances. Mixtures may be homogeneous or heterogeneous. A homogeneous mixture of two or more components is called a homogeneous solution or solution. The components of the solution do not react chemically, and the mixture exists in a single phase.
When the solution contains only two chemical constituents, it is termed a binary solution. Similarly, with three components, it is called a ternary solution.
The constituents of a solution can not be separated by filtration. A solution is characterised by homogeneity and the molecular state or ionic state of the components.
The major constituent of a solution is called a solvent. The solvent in a solution is the constituent which has the same state of aggregation as that of the solution. One or more remaining components of a solution are called solutes.
The amount of the solute dissolved in the unit quantity of the solution is termed as concentration. Solutions containing relatively higher amounts of solute are called concentrated solutions, and lower amounts of solutes are called dilute solutions. A solution whose concentration is known is termed a standard solution. Solutions prepared using water as a solvent are called aqueous solutions. Nonaqueous solutions are prepared using alcohol, ether, acetone, hexane, benzene, etc., as solvents.
6.7.2
Concentration terms
The concentration of a solution can be expressed in a number of ways. Weight fraction, volume fraction, mole fraction, molality, molarity, normality, formality, parts per million, etc., are important methods of concentration.
Strength: The strength of a solution is the amount of the solute in grams present in one litre of the solution.
Strength ( or concentration )=
Mass of solute in grams
Volume of the solution in litres
Mass percentage: The mass or weight fraction of a solution is the ratio of the weight of the solute to the total weight of solution
Weight fraction =
Weight of solute
Sum of weights of components in solution
Per cent weight = weight fraction × 100
Volume percentage: The volume fraction of a solution is the ratio of the volume of the solute to the total volume of solution.
Volume fraction =
Volume of solute
Sum of volumes of components in solution
Per cent volume = volume fraction ×100
Per cent mass by volume = (Weight of solute ×100)
Volume of solution
Mole fraction: Mole fraction is the ratio of the number of moles of a given component to that of all components. It is denoted by 'X'.
If 'n' is the number of moles of solute and 'N' is the number of moles of solvent
Xsolute = n (n+N) ; Xsolvent = N (n+N)
The sum of mole fractions of all components in a solution is unity.
Xsolute + Xsolvent = 1
Per cent mole = mole fraction ×100
Molality: Molality is the number of gram moles of the solute present dissolved in one Kg of solvent. It is denoted by 'm'
Molality =
Number of gram moles of solute
Number of kilograms of solvent
If w is the weight of solute (in g), W is the weight of solvent (in g) and GMW is the gram molecular weight of solute, molality is given as
m = w/GMW × 1000/W
If the solubility of solute is given as 'S' (weight of solute that can saturate 100 grams of solvent), molality is given by the expression.
m = 10 S/GMW
The relationship between molality (m) and mole fraction of solute (X) in an aqueous solution is given as
X = m/(m+55.5)
Molarity: Molarity is the number of gram moles of the solute present dissolved in one L of solution. It is denoted by 'M'
Molarity =
Number of gram moles of solute
Number of litres of solution
If w is the weight of solute (in g), V is the volume of solution (in ml ), and GMW is gram molecular weight of solute, molarity is given as
M = w/GMW × 1000/V
If 'x' is per cent weight (w/W), 'y' is per cent weight by volume (w/v), and 'd' is the specific gravity of a solution, molarity is given as
M = (10 × x × d)/GMW or M = (10 × y)/GMW
Molality or molarity of water is often given, respectively, as 55.5 mol kg -1 and 55.5 mol L-1.
Generally, the numerical value of the molal concentration of a solution is greater than that of the molar concentration of the same solution. For a dilute solution, molality and molarity are taken as the same. These two methods of concentration are related to each other through the density of a solution as:
m = (M×1000)
(1000d-M×GMW)
Normality: Normality is the number of gram equivalents of the solute present dissolved in one L of solution. It is denoted by 'N'.
Normality =
Number of gram equivalents of solute
Number of litres of solution
If w is the weight of solute (in g ), V is the volume of solution (in mL ), and GEW is the gram equivalent weight of solute
N = w/GEW × 1000/V
Normality is given as,
N= (10 × x × d) GEW or N = (10× y) GEW
Molarity and normality are one and the same for a solution whose gram molecular weight and gram equivalent weight are same.
Molarity and normality are interrelated as N = n × M,
Here, n is the valency of an element, or n is the charge magnitude of an ion, basicity of an acid, acidity of a base, or the total number of positive or negative charges in the formula of ionic substance or the number of electrons gained by an oxidant molecule or the number of electrons lost by a reductant molecule or the number of Faradays in an electrochemical reaction.
Parts per million: The number of parts of solute present in one million parts of the solution by weight is called parts per million (ppm).
In practice, parts per million is the number of milligrams of solute present in one kilogram of aqueous solution. Generally, the hardness of water, concentration of pollutants, etc. are denoted in this method.
Example 1: Calculate the mole fraction of glucose in 10% aqueous solution.
Solution:
100 g of solution =10 g glucose + 90 g water
Number of moles of glucose = 10 180 = 1 18
Number of moles of water = 90 18 = 5.
Mole fraction of glucose = Xsolute = 1 18 / 1 18 + 90 18 19 = 1 91 = 0.01099
6.7.3
Calculations involving concentration
Molar or normal solutions: One gram mole of a solute present dissolved in one litre solution is called one molar solution. One millimole of a solute present dissolved in one millilitre solution is also called one molar solution.
Similarly, one gram equivalent of a solute present and dissolved in one litre solution is called one normal solution. One milli equivalent of a solute present dissolved in one millilitre solution is also one normal solution.
Product of volume and concentration: The quantity of solute can be obtained by multiplying molarity with its volume. Molarity is given in molL-1, and volume is given in L.
The product of the molarity of a solution and its volume gives the number of moles of solute. If V is the volume and M is the concentration:
VM = L × mol/L = number of moles of solute
VM = Number of moles of solute (volume V is in L )
VM = Number of millimoles of solute (volume v is in mL )
VN = Number of equivalents of solute (volume V is in L )
VN = Number of milli equivalents of solute (volume v is mL )
Dilution of solutions: Dilution is a process of adding more solvent to the solution. During dilution, though the amount of solvent and solution increases, the amount of solute remains constant. The concentration of solution decreases upon dilution.
The number of moles or the number of equivalents of solute remains the same before and after dilution of a given solution.
The equation useful for the concentration during dilution of molar solutions is V1 M1=V2 M2, where V1 is the volume of the solution before dilution, and V2 is the volume of the solution after dilution.
M1 is the molarity of the solution before dilution.
M2 is the molarity of the solution after dilution.
Similarly, the equation useful for normal solutions:
V1N1 = V2N2
where N1 is the normality of solution before dilution, and N2 is the normality of solution after dilution.
Mixture of solutions: Mixing is a process of adding a solution with another solution of the same or different nature. During mixing solutions of similar nature, milli equivalents are additive and for solutions of opposite nature, milli equivalents are subtractive.
For a mixture of acids, bases, or oxidising agents or reducing solutions of two or more agents, the resulting normality of the mixture is given as
N= (V1N1+V2N2+...) (V1+V2+...)
where V1, V2, … are the volumes of the solutions mixed and N1, N2… are the normalities of the solutions mixed.
For a mixture of solutions of an acid with a base or an oxidising agent with a reducing agent, the resultant normality of the mixture is given as N = (V1N1 - V2N2)/4
Example 1: How many moles are present in 1500 mL of semi-molar sucrose solution? What is the weight of the solute in the solution?
Solution:
The number of moles of solute present in V Lit solution is given as VM, where V = 1.5 lit and M = 0.5 molL-1
Number of moles of solute = 1.5 × 0.5 = 0.75
Gram molecular weight (GMW) of sucrose =342 g
Weight of solute = number of moles × GMW = 0.75 × 342 = 256.5 g
Example 2: Two grams of pure caustic soda is present dissolved in 1.5 Lit solution. If 10 mL of this is diluted to 150 mL, what is the normality of diluted base?
Solution:
Number of moles of caustic soda
Weight/GMW = 2/40 = 0.05
Normality = 0.05/1.5 = 0.0333 eqL-1
Equation for dilution = V1N1 = V2N2
Normality of dilute solution = N2 = (V1N1)/V2
=(10×0.0333)/150 = 0.00222 eqL-1
QUICK REVIEW
• Mole: A mole represents 6.023 × 1023 particles.
• The actual meaning of a mole is a heap, but here it represents the number of atoms or molecules or particles present in a fixed amount of the substance.
• Atoms and Molecules: The fixed amount of the substance is known as atomic mass for atoms and molecular mass for molecules.
• Atomic mass unit (a.m.u.): The quantity of mass equal to 1/12th of the mass of carbon-12 atom.
• Relative atomic mass: The ratio of mass of one atom of an element to 1/12th of the mass of one atom of Carbon - 12 isotope.
• Atomic mass of an element = Mass of one atom of an element /(1/12th part of mass of an atom of C-12 isotope)
• Atomic mass can be expressed as 1 a.m.u (or) one unified mass-(U) (or) one Avogram (or) one Aston (or) one Dalton.
• 1 a.m.u.= 1.66×10-24 g = 1.66 × 10-27 kg
• Gram atom: The amount of substance equal to the atomic weight of an element expressed in grams.
• Relative molecular mass: The average relative mass of a molecule as compared to the 1/12th mass of a carbon-12 atom.
• Determination of molecular weight-
a. Homoatomic molecules: Molecular weight = Atomic weight × Atomicity.
b. Heteroatomic molecules: The molecular weight of a heteroatomic molecule is equal to the sum of atomic weights of atoms of all the elements present in one molecule.
• Gram molecular mass: The amount of substance equal to the molecular weight of that substance expressed in grams is called a gram molecule or gram molecular mass.
• Mole: The amount of the substance that contains as many particles as atoms exactly present in 12 g of the C-12 Isotope.
• Avogadro’s number (NA): The number of entities in one mole of a substance is known as Avogadro’s number.
• Applications of Mole Concept
a. The chemical formula represents one mole of the substance.
b. The formula mass in grams represents the mass of one mole of that substance.
c. One mole of any substance contains 6.023 × 1023 particles.
d. At STP, one mole of any gas occupies 22.4 litres of volume
• Gram molar volume (GMV): At STP, one mole of any gas occupies 22.4 litres of volume.
• STP means Standard Temperature and Pressure. i.e., 00 C or 273K and a standard pressure of 1 atm.
• The reactant that is present in the lesser amount gets consumed after some time, and then no further reaction occurs even though the other reactant is present in the large amount.
• Hence, the reactant which gets consumed limits the amount of product formed and is, therefore called the limiting reagent.
• If a reaction involves two or more reactants, then the reactant consumed first, limiting the amount of product, is called a limiting reagent or limiting reactant.
• Knowing the actual yield and theoretical yield, the per cent yield can be calculated using the given formula.
• Per cent yield = Actual yield/Theoretical yield × 100
• Mass % of an element = Mass of that element in the compound/Molar mass of the compound × 100
• Equivalent Weight: A number which denotes the number of parts by weight of the element required to combine with or displace 8 parts by weight of oxygen or 1.008 parts by weight of hydrogen or 35.5 parts by weight of chlorine.
• Equivalent weight = Atomic weight of the element/Valency of the element
• Equivalent weight of an element in terms of electrons: Weight of the element which loses or gains the Avogadro number (NA) of electrons.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Importance of chemistry, properties of matter and mole concept
1. The number of molecules present in 4.4 g of CO2 gas is
a. 6.023×1023
b. 5.023×1023
2. Present atomic weight determination is based on
a. O16
b. C12
c. 6.023×1024
c. H1
3. Which of the following contains Avagadro number of atoms?
a. One mole of helium gas
c. 22.4 ltr of CO2 at STP
4. Equal masses of oxygen and ozone have equal
a. Number of gram molecules
c. Number of gram atoms
d. 6.023×1022
d. O17
b. 11.2 ltr of hydrogen gas at STP
d. 3.2 gms of methane
b. Volumes at STP
d. Number of electrons
5. If we assume 1/24th part of the mass of carbon instead of 1/12th part of it as 1 amu., the mass of 1 mole of a substance will
a. Remain unchanged
c. Get halved
6. Maximum number of electrons are present in
a. 2.24 lit of SO2 at STP
c. 0.2 moles of NH3
b. Get doubled
d. Can't be predicated
b. 1.5 gm moles of oxygen
d. 2 mole atoms of sulphur
7. The gas having the same number of molecules as 16g. of oxygen is
a. 16 g of O3
c. 16 g of SO3
b. 48 g of SO3
d. 1 g of hydrogen
8. Avogadro number is the number of molecules present in
a. 1 g of molecule
c. 1 atom of molecule
b. 1 gram molecular mass
d. 1 litre of molecule
9. The atomicity of a species is x, and its atomic weight is y. The molecular weight of the species is
a. x + y
b. y + x
c. xy d. x - y
10. Which of the following samples contains 0.1 mole glucose
a. 18 gms of glucose
c. 100 ml of 1M glucose solution
b. 6.023 × 1022 molecules glucose
d. 500 ml of 10% W/W glucose solution
11. 20 gms of sulphur on burning in air produced 11.2 lts of SO2 at STP. The percentage of unreacted sulphur
a. 80% b. 20% c. 60% d. 40%
12. 60 gms of limestone on heating produced 22 gms of CO2. The percentage of CaCO3 in limestone is a. 80%
60%
83.3%
13. The mass of Na2CO3 required to prepare 500ml of 0.1M solution is a. 10.6 g b. 5.3 g c. 2.65 g
87.66%
7.95 g
14. 'X' grams of calcium carbonate was completely burnt in the air. The weight of the solid residue formed is 28 g. What is the value of 'X' (in grams)
44
50
15. 'X' litres of carbon monoxide is present at STP. It is completely oxidised to CO2. The volume of CO2 formed is 11.207 litres at STP. What is the value of 'X' in litres? a. 22.414 b. 44.828
16. How many litres of oxygen (at STP) are required for the complete combustion of 39 gms of liquid benzene? (Atomic weights : C = 12, H = 1, O = 16 ) a. 22.4
84
42
11.2
17. What is the volume (in lit) of carbon dioxide liberated at STP when 2.12 grams of sodium carbonate (mol. wt. =106 ) is treated with excess dilute HCl? a. 2.28
0.448
44.8
18. How many litres of CO2 at STP will be formed when 100ml. of 0.1MH2 SO4 reacts with an excess of Na2CO3?
a. 22.4
b. 2.24
c. 0.224 d. 5.6
19. How much volume of CO2 at S.T.P is liberated by the combustion of 100 cm3 of propane (C3H8)?
a. 100 cm3
b. 200 cm3
c. 300 cm3 d. 400 cm3
20. The amount of zinc required to produce 224 ml of H2 at NTP on treatment with dilute H2 SO4 solution will be
a. 0.65 g b. 0.065 g c. 65 g d. 6.5 g
21. What weight of sodium hydroxide is required to neutralise 100 ml of 0.1 NHCl?
a. 4 g b. 0.4 g c. 0.04 g d. 40 g
22. What volume of hydrogen gas, at 273 K and 1 atm pressure, will be consumed in obtaining 21.6 g of elemental boron (atomic mass = 10.8 ) from the reduction of boron trichloride by hydrogen?
a. 89.6 L b. 67.2 L c. 44.8 L d. 22.4 L
23. At STP, one litre of gas weighs 1.25 grams. The gas contains 85.71% of carbon and 14.29% of hydrogen. The formula of the compound is
a. CH4
b. C2H6
c. C3H8
d. C2H4
24. A certain compound contains magnesium, carbon and nitrogen in the mass ratio 12:12:14. The formula of the compound is
a. MgCN b. Mg2CN c. MgCN d. Mg(CN)2
25. The mass of hydrogen at STP that is present in a vessel which can hold 4 grams of oxygen under similar conditions is
a. 1 gram
b. 0.5 grams c. 0.25 gms d. 0.125gm
26. How many moles of barium carbonate will contain 1.5 moles of oxygen atoms
a. 1 mole
b. 0.5 mole
27. The number of electrons in 1.8 grams of H2O is
a. 6.02×1023
b. 3.01×1023
c. 0.25 mole d. 0.4 mole
c. 0.602×1023 d. 60.22×1023
28. From 320 mg. of O2, 6.023 ×1020 molecules are removed, the number of moles remaining is
a. 9×10-3 moles
b. 9×10-2 moles c. zero d. 3×10-3 moles
29. The density of water is 1 gm/ml. The volume of a water drop is 1.8ml. The number of molecules present in one water drop are
a. 6.023×1023
b. 6.023×1021
c. 3.011×1020 d. 6.023×1022
30. A gaseous mixture contains oxygen and nitrogen in a 1:4 ratio by weight. The ratio of their number of molecules is
a. 1:4 b. 1:8 c. 7:32 d. 3:16
31. The total number of species present in 1 mole of potash alum in terms of Avogadro number, 'N', is
a. 3 N
b. 5 N
c. 8 N d. 32 N
32. An oxide of nitrogen has a molecular weight of 92. Find the total number of electrons in one gram mole of that oxide.
a. 4.6 N
b. 46 N
c. 23 N d. 2.3 N
33. The number of moles of water in 488.6 gms of BaCl2 2H2O is (molecular weight of BaCl2.2H2O=244.33)
a. 2 moles
b. 4 moles
c. 3 moles
II. Per cent composition and stoichiometric calculations
1. Equivalent weight of calcium metal is
a. 12
b. 24
c. 36
d. 5 moles
d. 20
2. For which of the following compound equivalent weight is equal to molecular weight
a. H2SO4
b. H3PO2
c. H3PO4
3. What is the mole percentage of O2 in a mixture of 7 g of N2 and 8 g of O2?
a. 25%
b. 75%
d. H3PO3
c. 50% d. 40%
4. An aqueous solution of glucose is 10%(w/v). The volume in which 1 mole of glucose is dissolved will be
a. 18 litres
b. 9 litres
c. 0.9 litres
d. 1.8 litres
5. 0.115 gm of sodium metal was dissolved in 500 ml of the solution in distilled water. The normality of the solution would be
a. 0.010 N
b. 0.0115 N
c. 0.023 N d. 0.046 N
6. 1 kg of 2 m urea solution is mixed with 2 kg of 4 m urea solution. The molality of the resulting solution is
a. 3.33 m
b. 10 m
c. 1.67 m
d. 5 m
7. What will be present in the solution when 50 ml of 0.1 M HCl is mixed with 50 ml of 0.1 M NaOH solution?
a. 4.5 millimole of H+
c. 0.1 M NaCl
b. 0.05 millimole of OH-
d. 10-7 M of H+ion
8. 0.66 g of H3PO2 will require xml of 0.1 M NaOH for complete neutralisation. x is
a. 100 ml b. 200 ml
c. 300 ml d. none of these
9. 2H2O → 4e- + O2 + 4H+: The equivalent weight of molecular oxygen is
a. 32 b. 16 c. 8 d. 4
10. The equivalent weight of Hypo in the reaction [M= molecular weight] 2Na2S2 O3 + I2 → 2NaI + Na2S4O6 is
M
M/2 c. M/3 d. M/4
11. The average concentration of Na+ ion in human body serum is 3 to 4 gm per litre. The molarity of Na+ ion is about
a. 0.15M
b. 3.4M
c. 2.3M
d. 0.68M
12. The mole fraction of ethanol in an aqueous solution of ethanol and water is 0.1. The mass per cent of ethanol is approximately a. 10
90
22
78
13. 0.84 g of an acid (mol.wt.150) was dissolved in water, and the volume was made up to 100ml. 25ml of this solution required 28ml of (N/10)NaOH solution for neutralisation. The equivalent weight and basicity of the acid
a. 75,2 b. 150,1 c. 75,4
150,2
14. One litre of a solution contains 18.9gm of HNO3 and one litre of another solution contains 3.2gm of NaOH. In what volume ratio must these solutions be mixed to obtain a neutral solution?
a. 3:8 b. 8:3 c. 15:4 d. 4:15
15. The maximum amount of BaSO4 precipitated on mixing equal volumes of BaCl2(0.5 M) with H2SO4(1 M) will correspond to
a. 0.5 mol
b. 1.0 mol
c. 1.5 mol d. 2.0 mol
16. H2C2O4.2H2O(Mol.wt = 126) can be oxidised into CO2 by acidified KMnO4. 6.3gms of oxalic acid can not be oxidised using which of the following options?
a. 3.16 gms of KMnO4
c. 0.1 mole of KMnO4
b. 200 ml of 0.1 M KMnO4
d. 0.02 moles of KMnO4
17. A mixture containing one mole of BaF2 and two moles of H2SO4 will be neutralised by
a. 1 mole KOH
c. 4 mole KOH
WORKSHEET - 2
b. 2 mole Ca(OH)2
d. 2 mole KOH
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. The number of significant figures in N0 = 6.022 × 1023 i.e. Avogadro's number are
a. Three
c. Five
b. Four
d. Can be any of these
2. A compound has 20% of nitrogen by weight. If one molecule of the compound contains two nitrogen atoms, the molecular weight of the compound is
a. 35 b. 70 c. 140
280
3. The empirical formula of an organic compound is CH2O. Its vapour density is 45. The molecular formula of the compound is
a. CH2O
b. C2H4O2
c. C3H6O3 d. C6H12O6
4. 15 cc of gaseous hydrocarbon requires 45cc of oxygen for complete combustion. If 30cc if CO2 is formed, the formula of the gaseous compound is
a. C3H6
b. C2H2
5. The empirical formula of acetic acid is
c. C6H10 d. C2H4
a. CH3-COOH b. C2H4O c. CH2O d. CHO
6. The empirical formula weight of a compound containing carbon and hydrogen is 13. The molecule of the compound is 39 times heavier than a molecule of hydrogen. The molecular formula of the compound is
a. CH
b. C3H3
c. C13H13 d. C6H6
7. An organic compound containing carbon, hydrogen, and oxygen contains 52.20% carbon and 13.04% hydrogen. The vapour density of the compound is 23. Its molecular formula will be
a. C2H6O
b. C3H8O
c. C4H8O
8. An alkane has a C/H ratio (by mass) of 5.1428. Its molecular formula is
a. C5H12
b. C6H14
c. C8H18
d. C5H10O
d. C7H16
9. The percentage of silica in sodium silicate is approximately (Atomic weight of Si = 28 )
a. 25
b. 40
c. 50 d. 60
10. The empirical formula of acetic acid is the same as that of
a. Sucrose
c. Oxalic acid
b. Glucose
d. Formic acid
11. A and B are two elements which form AB2 and A2B3, if 0.18 mole of AB2 weighs 10.6 g, and 0.18 mole of A2B3 weighs 17.8 g. Then:
a. Atomic weight of A is 21.2
c. Atomic weight of A is 18.8
b. Atomic weight of B is 21.2
d. Atomic weight of B is 18.8
12. A mixture of the metals A and B, where equivalent weights are 12 and 9, displaces H2 from an acid. If 0.39 g of mixture displaces 448cc of hydrogen at STP. The mixture contains
a. 0.12 g A
b. 0.27 g B
c. 0.12 g B d. 0.27 g A
13. The molar mass of haemoglobin is about 65000 g mol-1. Every haemoglobin contains 4 iron atoms. Thus,
a. Iron content in haemoglobin is 0.35% by mass
b. 1 mole of haemoglobin contains 56 g iron
c. 1 mole of haemoglobin contains 224 g iron
d. If iron content is increased to 0.56%, the molar mass of haemoglobin would be higher than 65000 gmol-1
14. Which compound has a lower percentage of nitrogen by mass than in N2H4?
a. N3H b. NH3
c. HNO3 d. N2O5
15. In which of the following reactions no change in gaseous volume occurs when measured at similar T and P?
a. Combination of N2 and O2 to give NO
b. Combination of N2 and H2 to form NH3
c. Combustion of carbon to give CO
d. Combustion of carbon monoxide
16. If 0.80 mole of MnO2 and 146 g of HCl react MnO2 + 4HCl → MnCl2 + Cl2 + 2H2 O then which of the following is incorrrect?
a. 0.80 mole of Cl2 is formed
b. 0.80 mole of HCl is reacted
c. MnO2 completely reacts
d. MnO2 is the limiting reactant
17. 10ml N2 is reacted with 20 ml H2 to form NH3. The correct statement is
a. 13.3 ml NH3 is formed
b. 20 ml NH3 is formed
c. 3.4 ml N2 is left after the completion of the reaction
d. 16.7 ml NH3 of the mixture is left after the completion of the reaction
18. Equivalent weight of H2S is equal to the equivalent weight of
a. HCl

b. H2O2 c. H2SO4 d. H2O
19. The equivalent mass of H3PO4 in the reaction given below is H3PO4 + NaOH → NaH2PO4 + H2O
49
98
32.6
40
20. 25.5 g of H2O2 solution on decomposition gave 1.68L of O2 at STP. The percentage strength by weight of the solution is
a. 30 b. 10 c. 20 d. 25
21. How much Ca(NO3)2 in mg must be present in 50 ml of a solution with 2.35 ppm of Ca?
a. 0.1175 b. 770.8 c. 4.7
22. '20 volume' of H2O2 is equal to
a. 20% H2O2 by mass
c. 1.764 N
0.48
b. 16% H2O2 by mass
d. 3.571 N
23. 500 ml of a 0.1 N solution of AgNO3 added to 500 ml of 0.1 N solution of KCl. The concentration of nitrate ions in the resulting mixture is
a. 0.05 N
c. 0.2 N
b. 0.1 N
d. Reduced to zero
24. 0.70 g of a sample of Na2CO3.xH2O was dissolved in water, and the volume was made to 100 ml. 20 ml of this solution required 19.8 ml of (N/10)HCl for complete neutralisation. The value of x is
a. 2 b. 1
4
10
25. Equivalent weight and formula weight of reactants are not same in the conversions
a. AgNO3 → Ag metal
c. H2O2 → H2O
b. CuO → Cu2O
d. Na2S2O3 → Na2S4O6
26. 2Na2S2O3 + Cl2 → 2NaCl + Na2S4O6: As per this reaction
a. Hypo is oxidised
b. Equivalent weight of Hypo is half of its formula weight
c. Only sulphur and chlorine are involved in redox reaction
d. Sulphur, oxygen, and chlorine are involved in redox reaction
27. One mole of chlorine combines with a certain weight of metal, giving 111 gm of its chloride. The same amount of metal can displace 2 gm of hydrogen from an acid. The equivalent weight of metal is. a. 40
20
80
10
28. H2SO4 + NaOH → NaHSO4 + H2O: In this reaction, the equivalent weight of the acid is a. 98 b. 49
29. The equivalent weight of metal is 20. If the metal forms a tri-positive ion, its atomic weight will be
20
40
30. The equivalent weights of 'S' in SCl2 and S2Cl2 are in the ratio a. 1:2
2:1
31. The following is not a fixed quantity
a. atomic weight of an element
b. equivalent weight of an element (or) compound
c. molecular weight of a compound
d. formula weight of a substance
32. Equivalent weight of K2Cr2O7 in an acidic medium is a. 24.5
49
1:1
1:4
33. If x g is the mass of NaHC2O4 required to neutralise 100 ml of 0.2 M NaOH and gg required to reduce 100 ml of 0.02 M KMnO4 in an acidic medium, then
a. x = y
b. 2x = y
c. x = 4y d. 4x = y
34. A mixture containing 0.05 mol of K2Cr2O7 and 0.02 mol of KMnO4 was treated with excess of KI in an acidic medium. The liberated iodine required 2.0 L of Na2S2O3 solution for titration. The concentration of Na2S2O3 solution was
a. 0.125 molL-1
c. 0.25 molL-1
b. 0.20 molL-1
d. 0.30 molL-1
35. What volume of 0.01 M K2Cr2O7 would be required to oxidise Fe (II) in 50 ml of 0.03 M solution of ferrous ammonium sulphate in an acidic medium?
a. 150 ml
b. 75 ml
c. 50 ml

d. 25 ml
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
7.1 INTRODUCTION
7.1.1
Genesis of periodic classification
After the proposal of John Dalton’s atomic theory, scientists took atomic weight as an important property of the elements and tried to seek a relationship between the properties of the elements and their atomic weights.
At present, 118 elements are known. Of these elements, 92 are available in the elemental form and the remaining 26 elements are man-made. Efforts to synthesise new elements are continuing. To study such a large number of elements, their chemistry, and their innumerable compounds separately is a difficult task. To ease out this problem, scientists started to systemise the knowledge about the elements and their innumerable compounds by classifying them. The classification may help to study the elements better and to correlate the properties of elements with some fundamental properties that are characteristic of all the elements.
Classification of elements, development of periodic law, and the periodic table are the consequences of systemising the knowledge and contributions of many scientists like - John Dalton (1808), Lavoisier (metals and non-metals), John Doebereiner (1829), De Chancourtos (1862), John Alexander Newlands (1865), Lother Meyer (1869), Dmitri Ivanovich Mendeleev (1905), Moseley (1913), Rang, Werner, Bury, etc.
7.1.2 Dobereiner’s classification
In 1829, John Wolfgang Dobereiner, a German scientist was the first to consider the idea of trends among the properties of elements.
He noted the physical and chemical properties of several sets of three elements, arranged in the increased order of their atomic weights called triads. The law of triads states that when elements are arranged in order of their increasing atomic mass, the mass of the middle element is approximately the arithmetical mean of the remaining two elements of the triad.
Table 7.1 Examples of Dobereiner's triads
Limitations of Dobereiner’s system
Few elements were known at the time of Dobereiner. The law of triads seemed to work only for a few elements. Quite a large number of similar elements could not be grouped into triads. For example, Fe, Mn, Ni, Co, Zn, and Cu are similar elements but cannot be placed in the triads. Similarly, it was possible that quite dissimilar elements could be grouped into triads.
Example: Carbon (12), nitrogen (14), and oxygen (16) can form a triad, but their properties are entirely different from each other.
As this classification failed to arrange the known elements in the form of triads, it was dismissed as a coincidence.
7.1.3 Newlands’s classification
The English chemist John Alexander Newlands (1865), a lover of music, propounded the law of octaves. He arranged many of the known elements in the increasing order of their atomic masses and noticed that the properties of every eighth element were a repetition of the first element, just like the eighth note of octave in Indian as well as Western music.
Table 7.2 Newland’s arrangement of elements
Newland’s law of octaves
When elements are arranged in the increasing order of their atomic mass, the eighth element resembles the first in physical and chemical properties, just like every eighth note that resembles the first in octaves of music.
From Newland's classification, a very important conclusion was made, that there is some systematic relationship between the order of atomic mass and repetition of properties, which gives rise to a new term periodicity
7.1.4 Lother Meyer’s curve
Lother Meyer in Germany and Mendeleev in Russia quite independently evolved, in 1869, identical classifications of elements. Both these classifications emphasised the periodicity of the properties of the elements with their atomic masses
Lother Meyer emphasised the physical characteristics of the elements. Lother Meyer presented the classification in the form of a curve, while Mendeleev presented it in the form of a table. Lother Meyer calculated the atomic volumes of the known elements by the applying formula,
Atomic Volume= Atomic mass Density
When he plotted these atomic volumes against corresponding atomic masses, a curve taking the form of sharp peaks and broad minima was obtained.
Lother Meyer pointed out that elements having similar properties occupy similar positions on the curve, such as:
• Alkali metals, having the largest atomic volumes, occupy the maxima of the curve.
• The alkaline earth metals (Mg, Ca, Sr, Ba) occupy positions at about the midpoints on the descending portion of the curve.
• The halogens (F, Cl, Br, I) occupy positions on the ascending portions of the curve before inert gases.
• The transition elements occupy minima of the curve.
On the basis of the above observations, Lother Meyer proposed that the physical properties of the elements are a periodic function of their atomic masses
7.1.5 Mendeleev’s periodic law
In 1869, Dmitri Ivanovich Mendeleev (Father of periodic classification), an eminent Russian chemist, was the first to successfully arrange elements.
Mendeleev relied on the similarities in the empirical formulae and properties of compounds by the elements. Mendeleev put forward the periodic law, stating that the physical and chemical properties of the elements and their compounds are a periodic function of their atomic weights
In Mendeleev’s periodic table elements, are arranged in the increasing order of their atomic weights.
1. H = 1
2. Li=7 Be=9.4 B=11 C=12 N=14 O=16 F=19
3. Na=23
4. K=39 Ca=40 ........=44 Ti=48 V=51 Cr=52 Mn=55
5. (Cu=63) Zn=65 .......=68 .......=72 As=75 Se=78 Br=80
6. Rb=85 Sr=87 *Yt =88 Zr=90 Nb=94 Mo=96 ....=100
7. (Ag=108) Cd=112 In=113 Sn=118 Sb=122 Te=128 I=127
Fe=56,Co=59 Ni=59, Cu=63
Ru=104, Rh=104 Pd=106, Ag=108
8. Cs=133 Ba=137 *Di=138 *Ge=140 - - - -
9. - - - - - - - -
10. - - *Er=140 *La=180 Ta=182 W=184Os=195, Ir=197 Pt=198
11. (Au=199) Hg=200 T1=204 Pb=207 Bi=208 - - -
12. - - - Th=231 - U=240 - -
Table 7.3 Mendeleev’s original periodic table
The properties shown by these are similar to those predicted by Mendeleev. In this, elements are arranged in horizontal rows called periods and vertical columns called groups. Each group is divided into two subgroups, namely 'A' and 'B'. The first three periods are called short periods, and the remaining are called long periods. Each long period contains two rows of elements called series.
While arranging the elements in the periodic table, he not only followed the increasing order of atomic weights but also considered their properties. If the properties of elements did not correspond to what is expected for that place, he left blank places and proposed the properties of elements to be present in blanks. Accordingly, these elements were discovered later.
of the element
point, K
Formula of Oxide (EkaAl)2O3 Ga2O3 (EkaSi)O2 GeO2
Formula of Chloride (EkaAl)Cl3 GaCl3 (EkaSi)Cl4 GeCl4
Isolation of the elements
Table 7.4 Comparison of properties- Mendeleev’s Eka elements
No one had any idea of the structure of an atom in the days of Mendeleev. Several decades had to pass before the electron was discovered and atomic structure was developed. The electronic configuration provided a fundamental basis for the properties of elements.
7.2
MODERN PERIODIC LAW AND THE PRESENT FORM OF THE PERIODIC TABLE
7.2.1 Modern periodic table by Mosely
Moseley bombarded (in 1913) various elements or their compounds with cathode rays in the discharge tube. This resulted in the emission of X-rays with characteristic frequencies. Moseley showed that the frequency is related to the charge present on the nucleus of an atom of the element used as anode in the discharge tube. Then, the frequencies are related to atomic numbers by the equation: (),=− vazb where v is the frequency of the characteristic X-rays emitted.
Z is the atomic number (nuclear charge), and a and b are constants for a given line. Moseley observed the regularities in the characteristic X-ray spectra of the elements and found that the plot vsZ v is a straight line. No such relationship was obtained when frequency and atomic mass (A) were plotted.
As the atomic number (serial number of elements in the periodic table) increases, the wavelength of characteristic X-rays decreases (frequency increases). From this, Moseley concluded that the charge present on the positive nucleus is the atomic number because, with the increase in the atomic number, this fundamental property also increases.
7.2.2 Introduction of modern periodic table
The empirical evolution of the periodic table reached its peak in 1913 when Moseley showed that atomic number is a more fundamental property of an element than its atomic weight. The position of an element in the periodic table depends on its atomic number, and the reason for the anomalies in the original periodic table becomes clear at once. Moseley modified Mendeleev’s periodic law and stated that the physical and chemical properties of the elements are periodic functions of their atomic numbers
The atomic number is equal to the nuclear charge or the number of electrons in the neutral atom. Further, it was recognised that the periodic law is essentially the consequence of the periodic variation in electronic configurations. The configurations indeed determine the properties of elements and their compounds and are the basis for the modern periodic law.
The modern periodic law states that the physical and chemical properties of the elements are periodic functions of their atomic numbers or their electronic configurations.
The original form of the periodic table has since been modified as a result of structural elucidation of atom and the discovery of noble gas elements. Numerous forms of the periodic table have been devised from time to time. Moseley constituted the periodic table by unfolding Mendeleev’s table in which the elements are arranged according to the atomic numbers. This is closely similar to that of Bohr-Thomson table
The most convenient version of the periodic table was constructed by Bohr based on modern periodic law, and the elements were arranged in the order of their electronic configurations
Main group Elements s-subshell is gradually filled up
Periodic Table of the Elements (Long Form) (Representing Electron Configuration)
Main group Elements p-subshell is gradually filled up
Atomic number
Symbol
Valence-shell Configuration
Elements d-subshell is gradually filled up
The elements are arranged in a table, which is called the long form of the periodic table. The table consists of 7 horizontal rows called periods and 18 vertical columns called groups.
The long form of the periodic table contains-
a. Periods
There are 7 horizontal rows in the periodic table. These are called periods
First period: It contains two elements, hydrogen and helium. So, it is called the shortest period.
Second period: It contains 8 elements: Li, Be, B, C, N, O, F, and Ne.
Third period: It contains 8 elements: Na, Mg, Al, Si, P, S, Cl, and Ar.
The second and third periods are called short periods.
Fourth period: It contains 18 elements from K to Kr (Z = 19 to 36).
Fifth period: It contains 18 elements from Rb to Xe (Z = 37 to 54).
The fourth and fifth periods are called long periods
Sixth period: It contains 32 elements from Cs to Rn and is called the longest period (Z = 55 to 86).
Seventh period: It is an incomplete period which contains 31 elements.
b. Groups
There are 18 vertical columns in the periodic table called groups. They are IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIII (three groups), IB, IIB, IIIA, IVA, VA, VIA, VIIA, VIIIA (zero groups), respectively. According to the IUPAC nomenclature, the groups are numbered 1, 2, 3,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, and 18 respectively from left to right
On the basis of the differentiating electron, the periodic table is divided into four main blocks: s, p, d, and f.
i) Elements of groups 1 and 2 are s-block elements.
ii) Elements of groups 13, 14, 15, 16, 17, and 18 are p-block elements.
iii) Elements of groups 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 are d-block elements.
iv) The f-block elements comprise two horizontal rows placed at the bottom of the periodic table.
Fig. 7.5 s, p, d, and f block
d-Block elements (except IIB) are called transition elements, while the f - block elements are called inner transition elements.
Note: f-block elements range from Ce58 to Lu71 - Lanthanides, and Th90 to Lr103 - Actinides.
7.3 NOMENCLATURE OF ELEMENTS
The naming of the new elements had traditionally been the privilege of the discoverer (or discoverers), and the suggested name was ratified by the IUPAC. In recent years, this has led to some controversy.
To avoid such problems, the IUPAC has made a recommendation that until a new element’s discovery is proved and its name is officially recognized, a systematic nomenclature be derived directly from the atomic number of the element using the numerical roots for 0 and number 1-9. These are shown in the table.
Table 7.5 IUPAC notation for naming elements with Z > 100
7.3.1 Nomenclature of elements with atomic numbers > 100
The roots are put together in order of digits, which make up the atomic number, and 'ium' is added at the end. The IUPAC names for elements with Z and above 100 are shown in the table below.
ununnilium
Hassium Hs
Meitnerium Mt
Darmstadtium Ds
Roentgenium Rg
Copernicium Cn
Nihonium Nh
Flerovium Fl
Moscovium Mc
Livermorium Lv
Tennessine Ts
ununoctium Uuo Oganesson Og
Table 7.6 IUPAC Nomenclature of heavy elements
So, now when a new element is found, it initially gets a temporary name with a three-letter symbol. Later, representatives from different countries vote to decide on a permanent name and symbol. The final name might show where the element was discovered or honour a famous scientist. Currently, we’ve found elements with atomic numbers up to 118. The International Union of Pure and Applied Chemistry (IUPAC) officially announces the names of all elements.
Periodic Table of Elements: Electron Configurations
The vertical columns of the periodic table are called groups or families. There are eighteen groups. Among these groups, eight are important. These groups are 1, 2, 13, 14, 15, 16, 17, and 18. A part of group 3 elements are separately shown at the bottom of the periodic table in the form of Lanthanides and Actinides. Fourteen elements coming after lanthanum are called lanthanides. Lanthanides are from cerium (Z = 58) to lutetium (Z = 71). Fourteen elements coming after actinium are called actinides. Actinides are from thorium (Z = 90) to lawrencium (Z =103). Lanthanides are commonly called rare earths. Most of the actinides are mainly synthetic elements.
Hydrogen is the only element that can be placed in two different groups in the periodic table: group 1 and group 17.
7.4 ELECTRONIC CONFIGURATION OF ELEMENTS AND THE PERIODIC TABLE
In this part, we’ll see how the arrangement of atoms in the periodic table is directly correlated with the arrangement of their electrons..
7.4.1 Electronic configurations in periods and groups
In periods
A critical observation of the long form of the periodic table suggests the following:
The number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
The period number corresponds to the shell number in which the distant electrons from the nucleus of the atom are present. Each successive period in the periodic table is associated with the filling up of the next higher principal energy level.
The first period starts with the filling of the lowest energy level, 1s (K shell). The period has two elements. Hydrogen has the electronic configuration 1s1 and helium has 1s2.
The second period starts with lithium. The differentiating electron of lithium enters the 2s (L shell) and has the electronic configuration 1s22s1. The 2 p orbitals are filled starting from boron. The second shell is completely filled at neon (2s22p6). Thus, there are eight elements in the second period.
The third period starts with sodium with a differentiating electron entering the 3s orbital (M shell) Successive filling of 3s and 3p sub-shells gives rise to the third period of eight elements from sodium to argon.
The fourth period starts at potassium (2, 8, 8,1 ) with a filling up of 4s (N shell). The filling of 4s is complete with calcium. Before the 4p orbitals, the filling up of 3d orbitals (M shell) becomes energetically favourable. Ten elements from scandium to zinc are filled with differentiating electrons in a 3d sub-shell. The period ends with krypton with the filling up of the 4p orbitals. Thus,
there are eighteen elements in the fourth period. In this period, exceptionally, Cr and Cu have one electron in the outer shell, i.e. 4s orbital.
The fifth period begins with rubidium filling up of 5s orbital. Similar to the fourth period, the 4d sub-shell is filled starting at the yttrium and up to cadmium. The period ends at xenon with the filling up of the 5 p orbitals. Thus, the fifth period also has eighteen elements.
The sixth period starts with caesium. The period has thirty-two elements and electrons that are filled successively in 6s, 4f, 5d, and 6p orbitals. The filling up of the 4f orbitals begins with cerium and ends at lutetium to give the 4f series, also called the lanthanide series. There are thirty-two elements in the sixth period (longest period).
The seventh period starts with francium. This period is incomplete and is expected to end at the element with atomic number 118. Filling up of the 5f orbitals after actinium gives the 5f series, also called the actinide series.
In groups
The main reason for the classification is to group together elements which are chemically similar. Elements present in the same group have similar electronic configurations and have the same number of electrons in the outermost shells.
The elements of group 1 or alkali metals have only one electron in the outermost shell of each atom. The general electronic configuration of alkali metals is ns1.
The elements of group 2 or alkaline earth metals have two electrons in the outermost shell of each atom. The general electronic configuration of alkaline earth metals is ns2
The group number in the Roman symbol for representative elements directly denotes the number of electrons present in the outermost shell of the atom of each element.
Atoms of the elements of group IIIA (or group 13) have three electrons in the outermost shell. Their general electronic configuration is ns2np1. Similarly, group IVA (or group 14) elements have general configuration ns2np4, etc.
Noble gas elements (group 18), however, have filled octet in their outermost shells with general electronic configuration ns2np6. Group-wise, the general electronic configuration of the important groups is listed in the table.
5 Rb(Z = 57) [Kr]5s1 Xe(Z = 54) [Kr]4d105s25p6
6 Cs(Z = 55) [Xe]6s1 Rn(Z = 86) [Xe]4f145d106s26p6
7 Fr(Z = 87) [Rn]7s1 - -
Table 7.7 Configuration of the first and the last element of each period
Nickel group has an exceptional feature. This group is called the pseudo-octet configuration group. The element palladium has the electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d10, with eighteen electrons in the outermost shell. In general, elements of the same group have similar outer shell electronic configurations, though there are few anomalies.
ELECTRONIC CONFIGURATIONS AND TYPES OF ELEMENTS: s-, p-, d-, f-BLOCKS
Based on the entry of the differentiating electron into the sub-shells, elements are classified into four blocks. They are:
1. s-block elements
3. d-block elements
2. p-block elements
4. f-block elements
Different blocks of the elements in the long form of the periodic table are given in the following fig.
Fig. 7.7 s, p, d, and f-block elements in the long form of periodic table
s-block is present at the left side, p-block is at the right, d-block is at the middle and f-block at the bottom of the long form of the periodic table.
Atomic number of aluminium is 13. The thirteenth electron of aluminium enters into the 3p-orbital. Hence, aluminium is called a p-block element. Similarly, the atomic number of titanium is 22, with the configuration of the differentiating electron. Titanium is called a d-block element. He, due to configuration, belongs to the 's’ block but is placed in the zero group due to its inert nature.
7.5.1 The s-block elements
The elements in which the differentiating electron enters the s-orbital of the outermost shell are called s-block elements.
The first two elements of each period belong to the s-block. Group 1 and 2 (alkali and alkaline earth metals) constitute the s-block. These elements are located at the left side of the long form of the periodic table. The general electronic configuration of the s-block elements is ns1-2.
Characteristic properties of s-block elements are:
• The elements are highly electropositive and are soft metals with lower densities.
• They are very good reducing agents.
• They have low melting and boiling points.
• They are very reactive and form ionic substances, except lithium and beryllium.
• They exhibit an oxidation state of +1 or +2.
• They show characteristic colours in the flame.
7.5.2 The p-block elements
The elements in which the differentiating electron enters the p-orbitals of the outermost shell are called p-block elements. Groups 13, 14, 15, 16, 17, and 18 (IIIA to VIIA and Zero groups) constitute the p-block. The group 18 element that is misplaced in the p-block is helium. These elements are located on the right side of the periodic table. The p-block elements together with s-block elements (except zero group) are referred to as representative elements or main group elements.
The general electronic configuration of p-block elements is ns2np1-6 or ns2npx (x = 1 to 6).
Characteristic properties of p-block elements are:
• The elements include all metalloids, most of the non-metals and some metals.
• All gaseous elements (except H2 and He) are p-block elements.
• Most of these elements are highly electronegative and have high electron gain enthalpy.
• Some elements are good oxidising agents. Some of them act as reducing agents.
• Except for group 18, these elements are very reactive.
• They mostly form covalent compounds e.g. Cl2, O2, HCl, though other compounds such as ionic halides, oxides, sulphides, nitrides, etc., are also known.
7.5.3 The d-block elements (Transition elements)
The elements in which the differentiating electron enters the d-orbitals of the penultimate shell are called d-block elements. Groups 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 constitute the d-block. These elements are located in the middle, in between the s-block and p-block of the long form of the periodic table.
They have the properties intermediate to those of the s-block and p-block elements. They are referred to as transition elements. The d-block contains four series of elements. The 3d, 4d, and 5d-series are completely filled with ten elements each. The 6d-series is incomplete and has only eight elements. Zinc, cadmium and mercury, which have the electronic configuration (n-1)d10ns2, do not show most of the properties of transition elements. The general electronic configuration of d-block elements is (n-1)d1-10ns1 or 2 .
Characteristic properties of d-block elements are:
1. The elements are all electropositive and are metals.
2. They are all solids except mercury, which is a liquid at room temperature.
3. Most of the elements possess catalytic activity.
Examples:
(i) In the preparation of NH3 by Haber’s process, the catalyst is Fe, and the promoter is Mo.
(ii) In the preparation of H2SO4 by Contact process, the catalyst is platinised asbestos.
4. They form cations with different magnitudes of the charge.
5. They form ionic as well as covalent compounds.
6. They form complex compounds.
Example: [Cu(NH3)4SO4, K4[Fe(CN6)]]
7. They form alloys and interstitial compounds.
8. They mostly form coloured ions, exhibit variable valency (e.g. Fe2+, Fe3+), and Para magnetism. (μ = 4.90 BM for Fe+2, μ = 5.92 BM for Fe+3)
7.5.4 The f-block elements (Inner transition elements)
The elements in which the differentiating electron enters the f-orbitals of the anti-penultimate shell are called f-block elements.
A part of group 3 constitutes the f-block. These elements are located at the bottom of the periodic table. There are two series of f-block elements. The first series follows lanthanum (Z = 57) and is called lanthanides [Ce(Z = 58) to Lu (Z = 71)].
The second series follows actinium (Z = 89) and is called actinides [Th(Z = 90) to Lr (Z = 103)].
The general electronic configuration of f-block elements is (n-2)f1-14(n-1)d0 or 1ns2. La and Ac are d-block elements, but lanthanides and actinides are f-block elements.
Characteristic properties of f-block elements are
• These elements are heavy metals with high density and form coloured ions, complexes andshow paramagnetic property, similar to d-block elements.
• They are naturally available in very small quantities and are called rare earths.
• Trans-uranic elements (Z > 92) are all synthetic.
• They also form complexes and interstitial compounds.
• They show a great deal of similarity among themselves in their properties.
• Actinide elements are radioactive.
• Actinides show a greater number of oxidation states compared to lanthanides.
7.5.5 Metals, non-metals and metalloids
In addition to displaying the classification of elements into s, p, d, and f-blocks, and other broad classification of elements given on the basis of their properties, elements can also be divided into metals, non-metals, and metalloids based on their properties. Among the elements in periodic table more than 75% are metals. These metals appear on the left side of the periodic table
Metals are usually solids at room temperature. They usually have high melting and boiling points. They are good conductors of heat and electricity. Metals are malleable and ductile. About one dozen elements are non-metals. They are placed at the top right-hand side of the periodic table. Nonmetals are usually gases at room temperature. Some of them are also solids with low melting and boiling points. These are poor conductors of heat and electricity (except graphite). Most of the nonmetallic solids are brittle and are neither malleable nor ductile.
Some other elements exhibit both metallic and non-metallic properties. Such elements are called metalloids. Metalloids are placed in the p-block. Examples include Ge, As, Sb, Se, Te, etc. Metals usually react with dilute acids, while non-metals react with alkali.
7.6 PERIODIC TRENDS IN PROPERTIES OF ELEMENTS
The properties which are directly or indirectly dependent on the electronic configuration of the elements are called atomic properties or periodic properties. The meaning of the word periodic is that a particular property is repeated in a system at regular intervals. In the modern periodic table, these intervals are 2, 8, 8, 18, 18, and 32, i.e., similar properties are observed with elements belonging to the same group, which have been arranged in groups after the difference of either 2 or
8 or 18 or 32 in atomic numbers as similar valence shell electronic configurations recur after certain regular intervals of atomic number. This is the cause of periodicity in atomic properties.
Properties such as atomic radii, ionization energy, electron gain enthalpy, electronegativity, electropositivity, valency, oxidation number, etc., show periodicity.
The screening effect or shielding effect
In a multi-electron atom, the electrons of the valence shell are attracted towards the nucleus, and these electrons are also repelled by the electrons present in the inner shells. On account of this, the actual force of attraction between the nucleus and the valence electrons is somewhat decreased by the repulsive force acting in the opposite direction. This decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is called the screening effect or shielding effect
The magnitude of the screening effect depends upon the number of inner electrons; i.e., the higher the number of inner electrons, the greater the value of the screening effect.
shell (valence shell)
shell
7.6.1 Effective nuclear charge
Due to the screening effect, the valence electron experiences less attraction toward the nucleus. This brings a decrease in the nuclear charge. The resultant nuclear charge is termed as effective nuclear charge.
It is represented by Z ZZZ , effective nuclear charge, z = actual nuclear charge, σ= screening constant]
7.6.2 Atomic radius
Atomic radii are defined as the distance between the centre of the nucleus and the outermost shell where electrons are present.
It is expressed in nano metre (1nm = 10-9 m) or in Angstrom units (1A0 = 10-10 m) or in pico (1pm = 10-12m) metre
Various names have been proposed for atomic radii depending on the nature of the atoms. They are:
a) Covalent radii
b) Van der Waals’ radii
c) Crystal radii (or) Metallic radii
Covalent radii: In a homo nuclear di-atomic molecule, it is one-half of the distance between the centre of the two nuclei bonded by a single covalent bond.
covalent radii
Fig. 7.9 Homo nuclear di-atomic molecule
Van der Waals’ radii: It is one-half of the internuclear distance between the two atoms of A and B, in the two neighbouring molecules of the substance in the solid state.
Note: Van der Waals’ radii of an element are always larger than its covalent radii.
Crystal Radii: It is also called metallic radii. It is defined as 'half of the distance between the nuclei of two adjacent metal atoms in the metallic closely packed crystal lattice.' It is used for metal atoms which are assumed to be closely packed spheres in the metallic crystal.

Note: Metallic radii are always larger than covalent radii. Comparison of three radii of an element: Van der Waals’ radii > Metallic (crystal) radii > Covalent radii
Ionic radius
It is the distance of the outermost shell of an anion or cation from its nucleus. The radius of the cation is always smaller than the atomic radius of the parent atom.
Examples: Na > Na+
The radius of the anion is always larger than the atomic radius of the parent atom.
Examples: Cl- > Cl
Iso electronic species
Some atoms and ions which contain the same number of electrons are known as isoelectronic species. Examples: C-4, N-3, O-2
The size of isoelectronic ions decreases with an increase in the atomic number. C-4 > N-3 > O-2 >F> Ne > Na+ > Mg+2 > Al+3 > Si+4
Variation of atomic radii in period
The atomic radii decrease from left to right along a period in the periodic table. In a period, the atomic number increases, and distinguishing electrons enter the same outer shell. Hence, the nuclear charge increases. This increases the attraction between the nucleus and the extra nuclear electrons as the number of orbitals remains the same. Due to this, all electrons in orbitals are pulled closer to the nucleus. This goes on from atom to atom in a period. The atomic radius of inert gas is shown to be the largest in a period because of its Vanderwaal’s radius, which is generally larger than the covalent radii.
Example
2nd period
Atomic radii A (in) 1.23, 0.89, 0.82, 0.77, 0.75, 0.73, 0.72, 1.60
Variation of atomic radii in group
In a group from top to bottom, the atomic number increases, and the valence shell increases hence, the atomic radii increase. Example: Li (1.23), (1.57), K (2.03), Rb (2.16), Cs (2.35). The increase in size is due to the presence of extra energy shells in the elements as we go down the group.
In vertical columns of transition elements, there is an increase in the size from the first member to the second member. But from the second member to the third member, there is a small change in the size and sometimes sizes are the same. This is due to lanthanide contraction
7.6.3 Ionization energy
It is the minimum amount of energy required to remove an electron from the valence shell of an isolated neutral gaseous atom. It is represented by I.E.
The ionization energy required to remove the first electron from a neutral gaseous atom is termed the first ionization energy (I.E1).
Similarly energy required to remove second and third electrons is referred as second ionization energy (I.E2) and third ionization energy (I.E3) respectively.
M(g) ⟶ M+(g) + e- ΔH=IE1
M+(g) ⟶ M2+(g) + e-
M2+(g) ⟶ M3+(g) + e-
Generally, IE3 > IE2 > IE1
Variation of ionization energy in period
ΔH=IE2
ΔH=IE3
Generally, ionization energy increases as we move from left to right along a period, because with increase in atomic number in a period, nuclear charge increases and hence electrons are more tightly held.
Note: Nitrogen has a half-filled p orbital. So, it requires more ionization energy to remove an electron.
Variation of ionization energy in group
On moving down the group, the valence shell becomes far away from the nucleus, and thus, nuclear attraction towards valence electrons decreases, which results in a decrease in ionization energy.
7.6.4 Electron gain enthalpy (Electron affinity)
Ionisation energy is the energy associated with the removal of electrons. Another important property that influences the behaviour of elements is the ability to accept one or more electrons. This ability is measured in terms of electron affinity. When a neutral atom gains an electron, it liberates energy. This energy is known as electron gain enthalpy or electron affinity.
Electron affinity is defined as the amount of energy released when an electron is added to a neutral, gaseous atom to convert it into a negative ion.
X(g) + e-⟶X-(g) + E
Variation of electron gain enthalpy (electron affinity) in period
Electron gain enthalpy increases across a period from left to right. The nuclear charge increases from left to right across a period, and consequently, it will be easier to add an electron to a smaller atom.
Variation of electron gain enthalpy (electron affinity) in groups
The electron affinity decreases from top to bottom as the atomic size increases and added electron would be farther from the nucleus. But generally the second element in a group i.e., third period element (IIA to VIIA groups) has greater electron enthalpy than the first element i.e., second period element due to Electronic repulsions of the small size of the first element.
The order of electron affinity of various groups is:
Si > C > Ge > Sn > Pb (IVA group)
P > N > As > Sb > Bi (VA group)
S > Se > Te > Po > O (VIA group)
Cl > F > Br > I (VIIA group)
7.6.5
Electronegativity

The tendency of an atom of an element to attract the shared pair of electrons in a covalent bond towards its own direction is called electronegativity. Electronegativity is the property of a bonded atom, and it has no units
Variation of electronegativity in period
On moving from left to right in a period, the electronegativity value increases because atomic size decreases and nuclear charge increases.
Period Li Be B C N O F Ne
1.0
Variation of electronegativity in group
On moving down the group, the group's electronegativity decreases because atomic size increases. Note: The highest electronegative element is fluorine (F), while the least electronegative element is Cs. However, for noble gases, the EN value is zero (0).
7.6.6 Electropositivity
Variation of electropositivity in period
In a period from left to right, electropositivity decreases. This is due to an increase in ionization energy along the period, which makes the loss of electrons difficult.
Variation of electropositivity in group
In a group from top to bottom, electropositivity increases. This is due to a decrease in ionization energy on going down a group.
7.6.7 Metallic and non-metallic property
Metallic property
Metals have the tendency to form cations by losing electrons. Metals are electropositive in nature. Metals are good reducing agents. Metals have low IP and low EN values.
Example: Na, K, Al, etc.
Variation of metallic property in period
The Metallic property decreases along the period from left to right, and the non-metallic property increases along the period due to the following reasons:
i) Nuclear charge increases in a period.
ii) IP increases, EN increases.
Example: IA and IIA group elements are metals, whereas VIIA and VIIIA group elements are nonmetals.
Variation of metallic property in the group
The metallic property increases down the group, and the non-metallic property decreases down the group, due to the following reasons:
i) IP decreases down the group.
ii) Shielding effect increases.
iii) Nuclear charge decreases.
Example: In IVA group, carbon is a non-metal whereas tin and lead are metals.
Non-metallic property
Non-metals have the tendency to pull the shared pair of electrons towards themselves. Non-metals are highly EN and possess high IP.
Non-metals are good oxidising agents.
Example: C, N, O, S, P,...etc.
7.6.8
Periodic trends in chemical properties
Valency
Valency is the combining capacity of an element. (OR)
According to the new concept, valency may be defined as the number of electrons that are lost, gained or shared with one atom of that element to acquire the stable configuration of the nearest noble gas element.
The valency of metals is given by the number of valence electrons present in an atom. The valency of non-metals is given by subtracting the number of valence electrons present in an atom from 8, i.e., (8 - number of valence electrons).
Valency of s and p-block elements
In the case of representative elements, the number of valence electrons increases from 1 to 8 from left to right in a period, so the valency increases from left to right and then decreases to zero.
Valency of d-block elements
These elements have the outer most electronic configuration ns1-2(n-1)d1-10. These elements show variable valency involving valence electrons and d electrons of the penultimate shell.
The common valency of the transition elements is either 2 or 3.
Example: Iron has a variable valency of 2 and 3.
Valency is zero for any element in its non-combined state. The valency of zero group elements under laboratory conditions is zero. The electrons present in the outermost shell of a representative element generally denote its valency. These electrons are called valency electrons or valence electrons:
Generally the valency of elements with fewer electrons in the valence shell is the number of valence electrons. In case the number of valency electrons is four or more, the general valency of the elements is eight minus the number of valence electrons.
Oxidation number (oxidation state)
The oxidation number or oxidation state of an element is defined as the possible electronic charge which the atom of the element appears to have acquired in the given chemical form of the element. The common oxidation states of the elements are related to their valence electronic configuration.
The oxidation state may be +ve, - ve, zero, or fractional. For example, alkali metals use one electron and exhibit +1, and halogens gain one electron and exhibit -1 oxidation state.
1. For Group IA elements, the oxidation no. is +1, and for Group IIA elements, the oxidation no is +2.
2. For p-block elements, the oxidation no. = G.No (or) G-8. e.g., VA group elements can exhibit +5 and -3 states.
3. In the p-block elements down the group, a higher oxidation state becomes less stable due to the inert pair effect.
The reluctance of ns electrons to participate in bond formation is known as the inert pair effect.
Example: In IIIA group, +1 oxidation state of Tl is more stable than +3 oxidation state. Similarly, in IVA group, for Pb +2 oxidation state is more stable than +4 oxidation state, and in VA group, for Bi +3 oxidation state is more stable than +5 oxidation state.
4. d- block elements exhibit variable oxidation numbers ranging from +1 to +8 , due to their configuration. ns0 to 2,(n-1)d1 to 10 .
Example: Manganese exhibit +2, +3, +4, +6 and +7 states.
5. The common oxidation number for d-block elements is +2.
6. In d-block elements, +1 state is shown by Cr, Cu, Ag, Au, and Hg.
7. f-Block elements exhibit +3, +2, +4 states due to their configuration. For lanthanides, the common oxidation number is +3.
8. The maximum oxidation number, +8, is shown by Ru, Os, and Xe in their oxides (RuO4, OsO4, XeO4).
7.7 DIAGONAL RELATIONSHIP
Definition
There exists a characteristic analogy in elements of 2nd and 3rd period. The first element of a group resembles closely with second element of next successive group. This phenomenon is known as diagonal relationship. Therefore, the first three elements of second period (Li, Be and B) not only show resemblance with the elements of their own groups but show resemblance with the elements diagonally placed in the higher groups.
Fig 7.14 Diagonal relationship
Lithium shows a close resemblance with magnesium, beryllium with aluminium, and boron with silicon. The resemblance, however, disappears after these pairs.
On moving far away in period or in group, the relationship disappears since the elements located diagonally show large differences in their atomic radii because the increasing or decreasing trend in atomic radii does not occur in the same order.
7.7.1 Chemical reactivity
The reactivity of elements depends on two properties of elements. They are:
a) Electropositivity
b) Electronegativity
If the elements are highly electropositive in nature, they can lose the electrons easily and react with other elements. If the elements are highly electronegative in nature, they can easily pull the electrons towards themselves and easily react with other elements.
In a group: The electropositivity character increases down the group, and electronegativity decreases. Therefore, the reactivity of elements increases down the group.
Example: Caesium is the last element in the IA group. Hence, it is highly reactive. However, in the case of p-block elements, as the electronegativity nature decreases down the group, the reactivity decreases too.
Example: Fluorine is highly reactive as the electronegativity of fluorine is 4. Its reactivity decreases down the group.
In a period: The electropositivity decreases from left to right, but electronegativity increases from left to right. From this, the electropositive nature is more to the extreme left in the period. So, reactivity is high in IA and IIA group elements; then, it decreases to the right. Therefore, halogen group elements are highly reactive as electronegativity is high to the extreme right of the period.
Example: Fluorine is the first element in VIIA group which has the highest electronegativity.
QUICK REVIEW
• First periodic table was constructed by Mendeleev. The periodic law states that the physical and chemical properties are periodic functions of their atomic weights.
• Long form of the periodic table is called Bohr table. Here the elements are arranged in the increasing order of atomic numbers.
• There are 18 vertical columns in the long form, called groups and 7 horizontal rows called periods.
• First period is the shortest period and has only 2 elements. Second and third periods are short periods with eight elements each. Fourth and fifth periods are normal periods with eighteen elements each. Sixth period is longest period with 32 elements. Seventh period is incomplete.
• The period number indicates the valence shell, and the group number in Roman letters denotes the number of electrons in the outermost shell. However, elements of the zero group generally have eight electrons in the outermost shell.
• s-Block elements have ns1-2 configuration. They include alkali and alkaline earth elements (groups IA and IIA).
• p-Block elements have ns2np1-6 configuration. The elements present are IIIA to VIIA groups and zero groups. Many gases, non-metals, and metalloids are present in the p-block.
• The general configuration of d-block elements is (n-1)d1-10ns1 or 2. There are four series of d-block elements.
3d series from Sc(Z = 21) to Zn(Z = 30)
4d series from Y(Z = 39) to Cd(Z = 48)
5 d series from La(Z = 57) to Hg(Z = 80)
• The general configuration of f-block elements is (n-2) f1-14 (n-1)d0 or 1ns2. There are two series of f-block elements. 4f-Series are called lanthanides, from Ce(Z = 58) to Lu(Z = 71). 5fSeries are called actinides, from Th(Z = 90) to Lr(Z = 103)
• Noble gases belong to the zero group. They have the outer s and p orbitals completely filled (ns2np6). They are chemically inactive and are attributed to octet configuration. They exist as monoatomic gases.
• IUPAC: International Union of Pure and Applied Chemistry.
• IUPAC nomenclature for super heavy elements: The elements after fermium are called super heavy elements and at present these are called 'trans fermium elements’.
• The following rules are proposed by IUPAC for naming elements with atomic numbers more than 100.
Root word nil un bi tri quad pent hex sept oct enn
• Electronic configuration: The systematic arrangement of electrons in various atomic orbitals in the increasing order of their energies is known as electronic configuration.
The increasing order of various atomic orbitals is as follows:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p
• Properties such as atomic radii, ionization energy, electron gain enthalpy, electronegativity, electropositivity, valency, oxidation number, etc., show periodicity.
16. This decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is called the screening effect or the shielding effect
• Due to the screening effect, the valence electron experiences less attraction towards the nucleus. This brings a decrease in the nuclear charge. The resultant nuclear charge is termed as effective nuclear charge.
• Atomic radii is defined as the distance between the centre of the nucleus and the outer most shell where electrons are present.
• Various names have been proposed for the atomic radii depending on the nature of the atoms. They are:
a) Covalent radii
b) Van der Waals’ radii
c) Crystal radii (or) Metallic radii
• Ionic radii: It is the distance of the outermost shell of an anion or cation from its nucleus.
• Definition of ionization energy: It is the minimum amount of energy required to remove an electron from the valence shell of an isolated neutral gaseous atom.
Generally, IE3> IE2> IE1
• Electron affinity of an element is equal to the energy released when an electron is added to valence shell of an isolated neutral gaseous atom.
• The tendency of an atom of an element to attract the shared pair of electrons in a covalent bond towards its own direction is called electronegativity.
• The tendency of an atom of an element to lose one or more electrons and form a positive ion is known as electropositivity.
• Diagonal relationship: A characteristic analogy exists in the elements of 2nd and 3rd periods. The first element of a group resembles closely with second element of next successive group. This phenomenon is known as diagonal relationship.
• Chemical reactivity: The reactivity of elements depends on two properties of elements. They are electropositivity and electronegativity.
WORKSHEET - 1
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
I. Genesis of periodic classification
1. The maximum number of elements available in the elemental form
a. 26
b. 92
2. Which of the following is Dobereiner triad
a. Li, Na, K
c. 111
d. 118
b. Fe, Co, Ni c. Ru, Rh, Pt d. Os, Ir, Pd
3. Lother Meyer constructed a curve to classify the elements by studying the following properties
a. Atomic mass
b. Density
c. Atomic volume
d. All
4. Identify the set of elements showing similar properties according to Newlands
a. F and Cl
b. Al and Si
c. Mg and K
d. All
5. Statement (A): In theLother Meyer curve, the highly electropositive elements occupy the maxima of curve.
Statement (B): In the Lother Meyer curve, the less electropositive elements (alkaline earth metals) occupy the descending curve.
a. Statement A is correct, and B is incorrect
b. Both statements, A and B, are correct
c. Statement B is correct, and A is incorrect
d. Both statements A and B are incorrect
II. Modern periodic law and the present form of the periodic table
1. According to Mendeleev's periodic law, the physical and chemical properties of the elements are periodic functions of their
a. Atomic number
c. Both 1 and 2
2. Anomalous pair among the following is
a. Boron - Silicon
c. Aluminium - Gallium
b. Atomic mass
d. None
b. Beryllium - Indium
d. Cobalt - Nickel
3. The triad not present in Group VIII of Mendeleev's table is
a. Li, Na, K
b. Fe, Co, Ni
c. Ru, Rh, Pd d. Os, Ir, Pt
4. Which of the following pairs of atomic numbers represents elements belonging to the same group?
a. 11, 20 b. 12, 30
c. 13, 31 d. 14, 33
5. As per the modern periodic law, the physical and chemical properties of elements are periodic functions of their
a. Atomic number
c. Atomic weight
b. Electronic configuration
d. Atomic size
6. By taking chemical properties into consideration, the atomic weights of which of the following elements were corrected?
a. Te and I
c. Co and Ni
b. Ar and K
d. Be, Au, and Indium
III. Nomenclature of elements with atomic numbers > 100
1. The symbol of an element is Uno. The atomic number and name of this element at present are
a. 108, Uno
b. 108, Hassium
c. Hassium, 108 d. Uno, 108
2. The symbol of an element is Uqn. Its atomic number is
a. 143
b. 141
c. 142 d. 140
3. Which of the following atomic numbers is named ununtrium?
a. 103 b. 104 c. 110 d. 113
4. The IUPAC name of 104Rf is
a. Unnil hexium
c. Unun quadrium
5. The IUPAC name of 107Bh is
a. Unnil heptium
c. Unnil hexium
b. Unnil quadium
d. Unun pentium
b. Unnil septium
d. Unnil bium
6. The following are some statements about transition elements. i) The II B group belongs to transition elements. ii) In these elements, the last two shells, ns and (n-1)d, are partially filled. iii) They show variable valencies.
a. All are correct
c. Only ii, iii are correct
b. Only iii is correct
d. Only i and ii are correct
7. The IUPAC name of an element was unnilnilium. Its present name is
a. Nobelium
b. Fermium
c. Einstenium d. Mendeleevium
IV. Electronic configuration of elements and the periodic table
1. The electronic configuration of group III elements is
a. ns2 np3
b. ns2 np5
2. The total number of gaseous elements are
c. ns2 np1 d. ns2 np2
a. 8 b. 9 c. 10 d. 11
3. The sub-shells filled one by one for 4th period elements are
a. 3d, 4s, and 4p
b. t4s, 4p, and c. 4s, 3d, and 4p
d. 3d, 4p, and 4s
4. The starting element and last element in the largest period in the modern periodic table are
a. Rb and Xe
b. Cs and I
c. Cs and Rn d. Fr and Kr
5. Configuration that does not denote a transition element
a. 3d1 4s2
b. 3d10 4s1
c. 3d10 4s24p2
V. Electronic configurations and types of elements: s, p, d, f-blocks
1. The general electronic configuration of d-block elements is
a. ns1-2 (n-1)d1-10
c. ns1-2 (n-1)d1-9
2. Lanthanum belongs to
a. s-block
b. p-block
d. 3d8 4s2
b. ns2 (n-1)d1 (n-2)f1-14
d. ns1-2 np6 (n-1)d1-10
c. d-block
3. Inert gas element which has a different valence shell configuration is
a. Xe
b. Ne
4. Atomic numbers of actinides are
a. 57 to 71
b. 80 to 103
c. Kr
c. 58 to 71
d. f-block
d. He
d. 90 to 103
5. The period, in which s-block, p-block and d-block elements are present, is
a. 1
6. Elements of p-block are
a. Only non-metals
b. 6
c. Metalloids and non-metals
c. 7
b. Only metalloids
d. 3
d. Metalloids, non-metals, and metals
7. Following are some statements about the modern periodic table.
i) It consists of s, p, d, and f-blocks.
ii) The energy levels filling order in 6th period is 6s, 4f, 5d and 6p.
iii) III A group contains the maximum number of elements.
a. Only i & ii are correct
c. Only ii & iii are correct
VI. Periodic trends
b. Only i is correct
d. All are correct
1. Which of the following does not reflect periodicity?
a. Bonding behaviour of elements
c. Ionisation energy
2. Covalent radii is used for
a. Metals
b. Non – metals
b. Electronegativity

d. Neutron/proton ratio
c. Both a and b d. Metalloids
3. Among the following, the correct order of radii is
a. Van der Waals' radii < metallic radii
c. Covalent radii > van der Waals' radii
b. Metallic radii < Van der Waals' radii
d. Crystal radius < Covalent radius
4. Statement (A): Repeating the properties of elements in regular intervals is known as periodicity.
Statement (B): Having a similar valence electronic configuration is the cause of periodicity.
Statement (C): The higher the inner electrons the greater the value of the screening effect.
a. All the above statements are correct
c. A and B are correct, but C is incorrect
5. High ionization potential is exhibited by
a. Metals
c. Metalloids
6. The element with the highest ionization energy is
a. Oxygen
b. Nitrogen
7. The electron affinity for inert gases is likely to be
a. High
b. Low
8. The element with the highest electron affinity is
b. All the above statements are incorrect
d. A and B are incorrect, but C is correct
b. Non-metals
d. Radioactive elements
c. Helium d. Carbon
c. Zero d. Positive
a. O b. F c. Cl d. N
9. Statement A: The magnitude of EA depends on the atomic size and electronic configuration.
Statement B: Energy is released is when an electron is added to the valence shell of an isolated neutral gaseous atom.
Statement C: Electron gain enthalpy decreases with an increase in the atomic number across the period.
a. All the above statements are correct
c. A and B are correct, but C is incorrect
10. Electropositivity is seen in
a. Metalloids b. Metals
b. All the above statements are incorrect
d. A and B are incorrect, and C is correct
c. Non-metals d. Zero group elements
11. The most electropositive element in the periodic table is
a. Cs b. Ca c. Li d. Pb
12. Among the following, the element with metallic properties is
a. P
b. N c. K d. S
13. As the electropositivity increases, the metallic nature
a. Increases
c. Remains constant
b. Decreases
d. No change
14. Statement (A): The metallic property decreases along the period from left to right. Statement (B): The non-metallic property increases along the period. Statement (C): Metals have the tendency to form cations by gaining electrons.
a. All the statements are correct
c. A and B are correct, and C is incorrect
b. All the statements are incorrect
d. A and B are incorrect, and C is correct
15. The valence electronic configuration of the oxygen family is
a. ns2 np1
b. ns2 np2
c. ns2 np3
16. The oxidation number of the elements is mostly equal to
a. Period number
c. Both a and b
d. ns2 np4
b. Group number
d. Atomic number
17. ' X ' is an element which belongs to the VA group and 3rd period. The oxidation state and name of the element are
a. +5 and P
VII. Diagonal relationship
b. -3 and N
1. Diagonal relationship is shown by
a. All elements with their diagonally opposite elements
c. Some of the elements of 2nd and 3rd periods
2. Aluminium is diagonally related to
a. Li
WORKSHEET - 2
b. Si
c. +5 and N
d. -3 and S
b. All elements of 3rd and 4th periods
d. Elements of d-block
c. Be d. B
MULTIPLE CHOICE QUESTIONS WITH SINGLE CORRECT ANSWER
1. In lanthanides, the differentiating electron enters the [EAMCET 1993]
a. d-subshell
b. f-subshell
c. p-subshell
d. s-subshell
2. The non-transition metal among the following is [EAMCET 1993]
a. Ag
b. Zn
c. Os
d. Pt
3. The 100th numbered element is named in honour of [EAMCET 1996]
a. Einstein b. Bohr
c. Fermi
d. Curie
4. Among the following pairs of atomic numbers, the elements belonging to the same group are
a. 11, 20
b. 12, 30
c. 13, 31
d. 14, 33
5. The electronic configuration of chromium is [EAMCET 1996]
a. [Ar]3d4 4s2
c. [Ar]3d4 3s2
b. [Ar]4d5 4s1
d. [Ar]3d5 4s1
6. The number of periods present in the long form of the periodic table is [EAMCET 1999]
a. 6
b. 7
c. 8
d. 18
7. The electronic configuration that corresponds to an inert gas is [EAMCET 1999]
a. 1s22s22p5
b. 1s22s22p6
c. 1s22s1
d. 1s22s22p63s1
8. The general electronic configuration (n-1)d3 ns2 indicates that the particular elements belong to the group [EAMCET 2002]
a. VB
b. VA
c. IVA
d. IIB
9. The electronic configuration of Cu( Atomic number = 29) is [AIEEE 2004]
a. [Ar] 4s1 3d5
c. [Ar] 4s2 3d5
10. The electronic configuration of aluminium is
a. 1s22s2p63s1
b. 1s22s2p63s23p1
b. [Ar] 4s1 3d10
d. [Ar] 4s2 3d9
c. 1s22s2p63s23p5 d. 1s22s22p4
11. According to the Periodic Law of elements, the variation in properties of elements is related to their [AIEEE - 2003]
a. Nuclear masses
c. Nuclear neutron-proton number ratios
b. Atomic numbers
d. Atomic masses
12. Which one of the following ions has the highest value of ionic radius? [AIEEE - 2004]
a. O2-
b. B3+
c. Li+
d. F-
13. In which of the following arrangements the order is NOT according to the property indicated against it [AIEEE - 2005]
a. Li<Na<K<Rb: Increasing metallic radius
b. I <Br<F<Cl: Increasing electron gain enthalpy (with negative sign)
c. B<C<N<O: Increasing first ionisation enthalpy
d. Al3+<Mg2+<Na+<F-: Increasing ionic size
14. The electronic configuration of 59Pr (praseodymium) is [EAMCET - 2017]
a. [54Xc]4f2 5d1 6s2
c. [54Xc]4f3 6s2
b. [54Xc]4f1 5d2 6s2
d. [54Xc]4f3 5d2
15. Which of the following elements has the lowest melting point? [EMCET - 2017]
a. Sn
b. Pb
c. Si
d. Ge
16. The correct order of N-compounds in their decreasing order of oxidation states is
a. HNO3, NH4Cl, NO, N2
c. HNO3, NO, N2, NH4Cl
17. The Lanthanoide that would show colour is
a. Gd3+ b. La3+
b. HNO3, NO, NH4Cl, N2
d. NH4Cl, N2,NO, HNO3
c. Lu3+ d. Sm3+
18. Identify a molecule which does not exist. [NEET 2020-21]
a. O2
b. He2
c. Li2 d. H2
19. The number of protons, neutrons, and electrons in 175Lu71, respectively, are [NEET 2020-21]
a. 175, 104, and 71
c. 104, 71, and 71
b. 71, 104, and 71
d. 71, 71, and 104
20. 4d, 5p, 5f, and 6p orbitals are arranged in the order of decreasing energy. The correct option is [NEET 2019-20]
a. 5f > 6p > 5p >4d
c. 6p > 5f > 4d > 5p
21. The radii of F, F-, O, O2- are in the order
a. O2- > F- > O > F
c. O2- > F- > F > O
b. 6p > 5f > 5p > 4d
d. 5f > 6p > 4d > 5p
b. O2- > F > O > F-
d. O2- >F- > F > O
22. The correct arrangement of increasing atomic radius among Na, K, Mg, Rb is
a. Mg < K < Na <Rb
c. Mg < Na < Rb < K
b. Mg < Na < K < Rb
d. Na < K < Rb < Mg
23. When an electron is added, energy is absorbed in which of the following?
a. C
b. N
c. F d. O
24. The first four ionisation energy values of an element are 191, 578, 872, and 5972 k.cal. The number of valence electrons in the element is
a. 4
b. 3
c. 1
d. 2
25. Electron affinity is
a. Energy required to take out an electron from an isolated gaseous atom
b. The tendency of an atom to attract an electron towards itself
c. Energy absorbed when an electron is added to an isolated atom in a gaseous state
d. Energy released when an electron is added to an isolated atom in a gaseous state
26. In a period from left to right, electron affinity
a. Increases
c. Remains constant
b. Decreases
d. First increases, then decreases
27. Configuration that shows the highest energy released when an electron is added to the atom is
a. 1s2 2s2 2p3 b. 1s2 2s2 2p4 c. 1s2 2s2 2p5 d. 1s2 2s2 2p6
28. Atomic radius depends upon i) Number of bonds formed by the atom ii) Nature of the bonding
iii) Oxidation state of the atom
a. i and ii
b. ii and iii
29. The correct statement among the following is
c. i and iii
a. Covalent radius is 40% more than Van der Waal's radius.
b. Van der Waals radius is less than covalent radius.
c. Van der Waal's radius is 40% more than covalent radius.
d. Radii cannot be compared.
30. Which of the following has the largest atomic radius?
d. i, ii, and iii
a. Al b. Si c. Cl d. Na
31. The first ionisation energy of lithium will be
a. Greater than Be
c. Equal to that of Na
b. Less than Be
d. Equal to that of F
32. Element that has the highest first ionisation energy among the following is
a. Ca
b. Mg
33. Screening effect is not observed in
c. Al d. Si
a. He+ b. Li+ c. H d. All of these
34. Which one of the following sets of ions represents the collection of isoelectronic species? (AIEEE)
a. K+, Ca2+, Sc3+, Cl-
c. K+, Cl-, Mg2+, Al3+, Sc3+
b. Na+, Ca2+, Sc3+, F-
d. Na+, Mg2+, Al3+, Cl-
35. In which of the following arrangements the order is not according to the property indicated against it? (AIEEE)
a. Li < Na < K < Rb (Increasing metallic radius)
b. I < Br < F < Cl (Increasing electron gain enthalpy - with negative sign))
c. B < C < N < O (Increasing first ionisation enthalpy)
d. Al3+ < Mg2+ < Na+ < F-(Increasing ionic size)
36. Of the following sets, which one does not contain isoelectronic species? (AIEEE)
a. BO33-, CO32-, NO3-
c. CN-, N2, C22-
b. SO32-, CO32-, NO3-
d. PO43-, SO42-, ClO4-
37. The increasing order of the first ionisation enthalpies of the elements B, P, S, and F (lowest first) is (AIEEE)
a. F< S < P < B b. B < P < S < F
c. P < S < B <F d. B<S<P<F
38. Which one of the following sets of ions represents a collection of isoelectronic species? (AIEEE)
a. K+,Cl-,Ca2+,Sc3+
c. N3-,O2-,F-,S2-
39. The ion that is isoelectronic with CO is
a. CN-
b. O2+
b. Ba2+,Sr2+,K+,Ca2+
d. Li+,Na+,Mg2+,Ca2+
c. O2- d. N2+
40. Element with atomic number 38, belongs to (EAMCET)
a. IIA group and 5th period
c. VA group and 2nd period
41. Electron gain enthalpy of X would be equal to
a. Electron affinity of X-
c. Ionisation potential of X
42. Which has the largest atomic size?
a. Al
b. Al2+
b. IIA group and 2nd period
d. IIIA group and 5th period
b. Ionisation potential of X-
d. None of these
c. Al3+ d. Al+
43. Among the isoelectronic species, K+, S2-, Cl-, and Ca2+ the radii of the ions decrease as
a. Ca2+ > K+ > Cl- > S2-
b. Cl- > S2- > K+> Ca2+
c. S2- > Cl- > K+> Ca2+ d. K+> Ca2+> S2- > Cl-
44. Statement A: Metals are electropositive in nature.
Statement B: With the increase in atomic size, electropositivity increases.
Statement C: As the shielding effect increases, EP decreases.
a. All the statements are correct
c. All the statements are incorrect
b. A and B are correct, C is incorrect
d. A and B are incorrect, C is correct
45. Observe the following statements: (EAMCET)
I)The physical and chemical properties of elements are periodic functions of their electronic configuration.
II)Electronegativity of fluorine is less than the electronegativity of chlorine.
III)Electropositive nature decreases from top to bottom in a group.
The correct answer is:
a. I, II, and III are correct
c. Only I and II are correct
b. Only I is correct
d. Only II and III are correct

ANSWER KEY
1: CHEMICAL REACTIONS AND EQUATIONS
Worksheet 1
I. Basic concepts
1. b 2. d 3. b 4. b 5. d
6. b 7. a 8. a 9. c 10. b
11. c 12. b 13. b 14. a
II. Balancing chemical equations
1. c 2. b 3. a 4. c 5. b
6. b 7. d 8. a 9. c 10. c
11. b 12. a 13. a 14. b 15. d
16. c
III. Chemical reactions and types of chemical reactions
1. b 2. b 3. c 4. b 5. a
6. c 7. b 8. d
IV. Corrosion and rancidity
1. b 2. c 3. c 4. c 5. a
6. c
Worksheet 2
1. a 2. c 3. b 4. a 5. c
6. d 7. a 8. a 9. a 10. a
11. b 12. b 13. c 14. b 15. c
16. c 17. d 18. b 19. b 20. c
21. d 22. c 23. d 24. c 25. b
26. d 27. a 28. d 29. d 30. a
31. c 32. b 33. d 34. a 35. b
36. b
2: ACIDS, BASES, AND SALTS
Worksheet 1
I. Introduction to acids, bases, and salts
1. a 2. b 3. a 4. a 5. a
6. c 7. a 8. a
II. Understanding the chemical properties of acids and bases
1. b 2. c 3. c 4. a 5. a
6. a 7. b 8. c 9. c 10. a
11. c 12. a 13. a 14. a 15. c
16. c 17. a
III. pH concept and pH scale
1. b 2. c 3. b 4. c 5. a
6. a 7. d 8. a 9. d 10. d
11. c 12. b 13. a
IV. Salts, it’s applications and more about salts
1. d 2. c 3. b 4. a 5. b
6. a 7. a 8. b 9. b
Worksheet 2
1. b 2. b 3. c 4. d 5. c
6. a 7. c 8. b 9. a 10. d
11. c 12. c 13. c 14. a 15. a
16. c 17. c 18. d 19. c 20. b
21. d 22. b 23. b 24. d 25. c
26. c 27. c 28. d 29. c 30. b
31. c 32. d 33. a 34. a 35. d
36. a
3:
METALS AND NON-METALS
Worksheet 1
I. Introduction & physical properties
1. c 2. d 3. c 4. d 5. d
6. a 7. d
II. Chemical properties of metals and nonmetals
1. d 2. d 3. c 4. b 5. b
6. a 7. d 8. c 9. b 10. c
III. Occurrence of metals
1. a 2. d 3. c 4. b 5. d
6. b
IV. Extraction of crude metal
1. b 2. d 3. b 4. c 5. a
V. Refining or purification of crude metal
1. c 2. b 3. c 4. a 5. c
6. b 7. d
Worksheet 2
1. a 2. d 3. a 4. d 5. d
6. a 7. d 8. b 9. c 10. d
11. a 12. d 13. a 14. b 15. b
16. d 17. d 18. a 19. d 20. b
21. a 22. c 23. d 24. b 25. b
ANSWER KEY
26. c 27. a 28. c 29. d 30. c
31. d 32. a 33. a 34. c
4: CHEMICAL BONDING
Worksheet 1
I. Chemical bond, covalent bond, and ionic bond
1. c 2. b 3. a 4. a 5. c
6. b 7. b 8. c 9. a 10. a
11. a 12. a
II. Bond parameters
1. c 2. d 3. b 4. b 5. a
6. a 7. d 8. d 9. a 10. a
11. c
III. Valence bond theory (VBT) and hybridization
1. b 2. a 3. c 4. d 5. b
6. a 7. c 8. d 9. d 10. b
11. a 12. c 13. c 14. b 15. c
Worksheet 2
1. d 2. c 3. a 4. c 5. d
6. b 7. c 8. a 9. c 10. c
11. b 12. a 13. c 14. b 15. a
16. b 17. a 18. a 19. b 20. b
21. a 22. a 23. a 24. a 25. c
26. a 27. d 28. a 29. d 30. a
31. c 32. c 33. c 34. d
5: CARBON AND ITS COMPOUNDS
Worksheet 1
I. Carbon introduction
1. d 2. b 3. c 4. c 5. b
6. c
II. Covalent bond and versatile nature of carbon
1. c 2. c 3. d 4. c 5. c
6. c 7. c 8. d 9. c 10. b
11. a 12. d 13. b
III. Chemical properties of carbon compounds, study of functional groups, isomerism
1. b 2. b 3. c 4. a 5. d
6. b 7. a 8. b 9. b 10. a
IV. Some important carbon compounds –ethanol and ethanoic acid
1. b 2. d 3. b 4. a 5. b
6. c 7. d 8. c 9. d 10. c
11. a
Worksheet 2
1. b 2. c 3. d 4. a 5. a
6. d 7. b 8. b 9. d 10. d
11. a 12. c 13. b 14. b 15. d
16. d 17. d 18. c 19. a 20. b
21. a 22. d 23. c 24. c 25. b
26. c 27. b 28. d 29. b 30. a
31. d 32. c 33. b 34. a 35. a
36. d 37. b 38. a 39. b 40. c
41. a
6: SOME BASIC CONCEPTS OF CHEMISTRY
Worksheet 1
I. Importance of chemistry, properties of matter & mole concept
1. d 2. b 3. a 4. c 5. a
6. d 7. d 8. b 9. c 10. a
11. b 12 c 13. b 14 b 15 d
16 b 17. b 18. c 19. c 20. a
21. b 22. b 23. d 24. d 25. c
26. b 27. a 28. a 29. d 30. c
31. d 32. b 33. b
II. Per cent composition & stoichiometric calculations
1. d 2. b 3. c 4. d 5. a
6. a 7. d 8. a 9. c 10. a
11. a 12. c 13. a 14. d 15. a
16. c 17. d
ANSWER KEY
Worksheet 2
1. b 2. c 3. c 4. d 5. c
6. d 7. a 8. b 9. c 10. b
11. a 12. a 13. a 14. b 15. a
16. a 17. a 18. b 19. a 20. c
21. d 22. d 23. a 24. a 25. c
26. b 27. b 28. a 29. c 30. a
31. b 32. b 33. c 34. b 35. d
7: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
Worksheet 1
I. Genesis of periodic classification
1. d 2. a 3. d 4. a 5. b
II. Modern periodic law and the present form of the periodic table
1. b 2. d 3. a 4. c 5. a
6. d
III. Nomenclature of elements with atomic numbers > 100
1. b 2. d 3. d 4. b 5. b
6. c 7. b
IV. Electronic configuration of elements and the periodic table
1. c 2. d 3. c 4. c 5. c
V. Electronic configurations and types of elements: s, p, d, f-blocks
1. a 2. c 3. d 4. d 5. b
6. d 7. a
VI. Periodic trends
1. d 2. b 3. b 4. a 5. b
6. c 7. c 8. c 9. c 10. b
11. a 12. c 13. a 14. c 15. d
16. b 17. a
VII. Diagonal relationship
1. c 2. c
Worksheet 2
1. b 2. b 3. c 4. c 5. d
6. b 7. b 8. a 9. b 10. b
11. b 12. a 13. c 14. c 15. a
16. c 17. d 18. b 19. b 20. a
21. a 22. b 23. b 24. b 25. d
26. b 27. d 28. d 29. c 30. d
31. b 32. d 33. d 34. a 35. c
36. b 37. d 38. a 39. a 40. a 41. b 42. a 43. c 44. c 45. b
