CLASSIFICATION OF ELEMENTS CHAPTER 4
Chapter Outline
4.1 Genesis of Periodic Classification
4.2 Modern Periodic Law and Present form of The Periodic Table
4.3 Nomenclature of Elements with Atomic number >100
4.4 Electronic configuration of Elements and the Periodic Table
4.5 Electronic Configuration and Types of Elements: s,p,d,f-blocks
4.6 Periodic Trends in Properties of Elements
4.1 G ENESIS OF PERIODIC CLASSIFICATION
■ By 1865, the number of known elements increased from 31 to 63; 88 occur naturally, while 26 are man-made, with atomic weight becoming a key property after Dalton’s atomic theory.
Dobereiner's Law of Triads
■ John Dobereiner identified triads, sets of three elements with similar chemical properties, where the middle element’s atomic weight is approximately the average of the other two.
Dobereiner’s triads
Telluric Helix
■ In 1862, A.E.B. de Chancourtois arranged elements by increasing atomic weight in a spiral cylindrical table called the Telluric Helix.
■ It did not apply to all known elements.
Newland’s Law of Octaves
■ John Newlands arranged elements by increasing atomic weight and observed that every eighth element showed similarities, calling it the Law of Octaves.
■ Drawback: It worked only for lighter elements (up to calcium) and failed for heavier elements.
CHAPTER 4: Classification of Elements
Lothar Meyer’s Work
■ Lothar Meyer classified by plotting atomic volume, melting point, and boiling point against atomic weight, showing a periodic pattern which is shown in Fig.4.1.
Solved Example
1. What would be the group and period of the element with atomic number 80?
Sol. The element with Z value 80 is mercury (Hg). It is present in period 6 and group II B.
TEST YOURSELF
1. Beryllium follows Newland’s law of octaves. What is its eighth similar element in the classification? (1) K (2) Mg (3) P (4) Si
2. The number of gaseous elements available is (1) 5 (2) 11 (3) 12 (4) 15
3. Which of the following is a Dobereiner's triad? (1) Li, Na, K (2) Fe, Co, Ni (3) Ru, Rh, Pd (4) Os, Ir, Pt
Answer Key (1) 2 (2) 2 (3) 1
4.2 MODERN PERIODIC LAW AND PRESENT FORM OF THE PERIODIC TABLE
■ Periodic Law – "The physical and chemical properties of elements are periodic functions of their atomic weights."
■ Table Structure – Elements are arranged in horizontal rows (periods) and vertical columns (groups), with each group divided into subgroups A and B.
■ Short & Long Periods – The first three periods are short, while the remaining long periods contain two rows (series).
■ Arrangement Criteria – Elements were arranged by atomic weight and chemical properties.
■ Prediction of Missing Elements – Mendeleev left gaps in the table, predicting the properties of undiscovered elements.
Fig. 4.1 Lother Meyer’s curves
Table 4.1 Comparison of properties – Mendeleef’s Eka Elements
Property
of the element
K
Eka Aluminium Eka Silicon As Predicted As Observed As Predicted As Observed
Formula of oxide (EkaAl)2O3 Ga2O3 (EkaSi)O2 GeO2
Formula of chloride (EkaAl)Cl3 GaCl3 (EkaSi)Cl4 GeCl4
Isolation of the elements - - -
■ Predicted Elements – Eka-boron, Eka-aluminium, and Eka-silicon were later identified as Scandium (Sc), Gallium (Ga), and Germanium (Ge).
■ Corrected Atomic Weights – Revised atomic weights of Be, In, U, using Atomic weight = Equivalent weight × Valency.
■ Anomalous Pairs – Some elements were arranged against atomic weight order:
■ Ar (40) & K (39), Co (59) & Ni (58), Te (128) & I (127), Th (232) & Pa (231).
■ Transition Triads (Group VIII) – Elements placed horizontally due to similar properties:
■ Fe, Co, Ni; Ru, Rh, Pd; Os, Ir, Pt.
■ Limitations –Zero group elements were unknown.
■ Cu, Ag, Au were placed with K, Rb, Cs despite different properties.
■ Atomic structure was unknown at Mendeleev’s time.
■ The electronic configuration provided fundamental basis for the properties of elements.
■ Moseley’s Experiments – Bombarding elements with cathode rays produced X-rays with characteristic frequencies. He showed that X-ray frequency is linked to the atomic nucleus charge (atomic number).
( ) aZbυ=−
■ Here, υ is the frequency of X-rays, Z is the atomic number, a and b are constants for a selected type of line. A plot of υ against ‘Z’ gives a straight line, as sh own in Fig.4.2 Y o x Z 0 a
oa Intercept of Y axis is ‘ab’ ν
Fig. 4.2 Plot of υ and atomic number (Z)
■ As atomic number increases, the frequency of characteristic X-rays increases. Hence, atomic number is concluded as the fundamental quantity of element,
original periodic table
TEST YOURSELF
1. Considering the chemical properties, atomic weight of the element ‘Be’ was corrected based on (1) valency (2) configuration (3) density (4) atomic volume
2. Eka silicon is now known as (1) scandium (2) gallium (3) germanium (4) boron
3. The element ‘Sc’ was known long back as (1) eka-aluminium (2) eka-boron (3) eka-silicon (4) eka-mercury
4. Anomalous pair among the following is (1) Boron – Silicon (2) Beryllium – Indium (3) Aluminium – Gallium (4) Cobalt – Nickel
5. Choose the triad not present in group VIII of Mendeleef’s table. (1) Li, Na, K (2) Fe, Co, Ni (3) Ru, Rh, Pd (4) Os, Ir, Pt
6. The frequency of the characteristic X-ray of line of metal target ‘M’ is 2500 cm–1 and the graph between ν vs ‘Z’ is as follows. The atomic number of M is
(1) 49 (2) 50 (3) 51 (4) 25
4.2.1 MODERN PERIODIC LAW
■ In 1913, Moseley demonstrated that atomic number is a more fundamental property than atomic weight, determining an element’s position in the periodic table and explaining anomalous pairs.
■ He stated: "The physical and chemical properties of elements are periodic functions of their atomic numbers."
■ Atomic number (Z) = Nuclear charge = Number of electrons in a neutral atom.
■ Electronic configuration governs element properties, forming the basis of the modern periodic law.
■ Modern Periodic Law: "The physical and chemical properties of elements are periodic functions of their atomic numbers or electronic configurations."
■ The modern periodic table retains the same number of horizontal rows (periods) as Mendeleev’s table.
CHAPTER 4: Classification of Elements
Solved Examples
2. How would you justify the presence of 18 elements in the 5th period of the periodic table?
Sol. When n = 5, l= 0, 1, 2, 3. The order in which the energy of the available orbitals 4d, 5s, and 5p increases is 5s < 4d < 5p. The total number of orbitals available is 9. The maximum number of electrons that can be accommodated is 18 and, therefore, 18 elements are there in the 5 th period.
3. Why is there a break in the third period elements of the long form of the periodic table?
Sol. In the third period, 3s and 3p orbitals are only filled successively. Four orbitals together can hold eight electrons.
TEST YOURSELF
1. The basis of modern periodic law is (1) atomic number (2) atomic size (3) atomic volume (4) atomic mass
2. Which of the following pairs of elements are from the same group of the peri odic table? (1) Mg, Cs (2) Mg, Sr (3) Mg, Cl (4) Na, Cl
3. Elements of a vertical group have (1) same atomic number (2) same electronic configuration (3) same number of valence electrons (4) same number of core electrons
4. The first element of fifth period is (1) K (2) Rb (3) Kr (4) Xe
5. As per the modern periodic law, the physical and chemical properties of elements are periodic functions of their (1) atomic volume (2) electronic configuration (3) atomic weight (4) atomic size
6. The period that contains only gaseous elements is (1) 1 (2) 2 (3) 3 (4) 4
7. The first element and last element in the largest period in modern periodic table are (1) Rb and Xe (2) Cs and I (3) Cs and Rn (4) Fr and Kr
8. Which of the following has both members from the same period of the periodic table? (1) Na, F (2) Mg, Ca (3) Na, Cl (4) Be, Al
Answer Key
(1) 1 (2) 2 (3) 3 (4) 2 (5) 2 (6) 1 (7) 3 (8) 3
4.2.2 LONG FORM OF PERIODIC TABLE
■ Bohr’s Periodic Table – Arranged elements by electronic configuration based on modern periodic law.
Periodic Table of the Elements (Long form) (Representing electronic configurations)
Atomic number Symbol Valence-shell configuration
Main group Elements S-subshell is
Main-Group Elements p-subshell is gradually filled up IA Group 0 (zero) Transition Elements d-subshell is gradually filled up 1 H 1s 1 Inner -Transition Elements f-subshell is gradually filled up
7 Fr Ra AC ** Rf Db Sg Bh Hs Mt Ds Rg Uub Uut
Table 4.7 A modern form of the periodic table (long form)
Salient Features
■ Based on electronic configuration and arranged by increasing atomic number, following the Aufbau principle, as shown in the table 4.3.
■ Consists of 7 periods (horizontal rows) and 18 groups (vertical columns).
■ Periods correspond to the principal quantum number of the outermost orbit, starting with alkali metals and ending with noble gases.
■ First element in a period has its differentiating electron in the s-orbital, while the last element has it in the p-orbital.
■ Fourteen elements from the 6th and 7th periods (3rd group) are placed separately at the bottom are listed in Table 4.4.
Table 4.3 Sub-energy levels filled in periods
Table 4.4 Number of elements present in different periods
7 (20) (Incomplete)
TEST YOURSELF
1. The number of elements present in 2 nd, 3rd, 4th, and 5th periods of the modern periodic table, respectively, are (1) 2, 8, 8, and 18 (2) 8, 8, 18, and 32 (3) 8, 8, 18, and 18 (4) 8, 18, 18, and 32
2. Outer shell octet configuration is observed for the elements of the group (1) 2 (2) 8 (3) 18 (4) 32
3. The element with Z=117 and Z=120 belong to ____and______family, respectively. (1) halogen family, alkaline earth metals (2) nitrogen family, alkali metals (3) halogen family, alkali metals (4) chalcogens family, alkali metals
4. The following statements are related to elements in the periodic table. Which of the following is true?
(1) All the elements in group-17 are gases.
(2) The group-13 elements are all metals.
(3) Elements of group-16 have lower ionisation enthalpy values compared to those of group-15 in the corresponding periods.
(4) For group-15 elements, the stability of +5 oxidation state increases down the group.
5. In a period, elements are arranged in a strict sequence of
(1) decreasing charges in the nucleus (2) increasing charges in the nucleus (3) constant charges in the nucleus (4) equal charges in the nucleus
6. Which of the following pairs has elements containing the same number of electrons in the outermost orbit?
(1) N, O (2) Na, Cl (3) Ca, Cl (4) Cl, Br
Answer Key
4.3 NOMENCLATURE OF ELEMENTS WITH ATOMIC NUMBER >100
■ Element 104 was disputed between American (Rutherfordium) and Soviet (Kurchatovium) scientists.
■ To prevent conflicts, IUPAC introduced a systematic naming system based on numerical roots for atomic numbers (Z > 100).
■ IUPAC Naming Rules: Each digit of the atomic number is replaced with its corresponding root. The roots are combined in sequence, followed by "-ium". are shown in Table 4.5.
Table 4.5 IUPAC nomenclature of heavy elements Atomic
Unniltrium Unt Lawrencium Lr
Unnilquadium Unq Rutherfordium Rf
Unnilpentium Unp Dubnium Db
Unnilhexium Unh Seaborgium Sg
Unnilseptium Uns Bohrium Bh
Unniloctium Uno Hassium Hs
Unnilennium Une Meitnerium Mt
Ununnilium Unn Darmstadtium Ds
Unununnium Uuu Roentgenium* Rg*
Ununquadium Uuq
Ununpentium Uup
Official name and symbol yet to be announced by IUPAC
TEST YOURSELF
1. From the following select, the elements belonging to the same group. (1) Z = 12, 38, 4, 88 (2) Z = 9, 16, 3, 35 (3) Z = 5, 11, 27, 19 (4) Z = 24, 47, 42, 55
2. Rare earths are generally (1) actinides (2) all f-block elements (3) all inner transition elements (4) lanthanides
3. Lanthanum belongs to (1) s-block (2) p-block (3) d-block (4) f-block
4. In the periodic table, transition elements begin with (1) scandium (2) zinc (3) copper (4) mercury
5. The general electronic configuration (n-1)d3ns2 indicates that the particular element belongs to the group (1) VB (2) VA (3) IVB (4) IIB
6. In the sixth period, the orbitals being filled with electrons are (1) 5s, 5p, 5d (2) 6s, 6p, 6d, 6f (3) 6s, 5f, 6d, 6p (4) 6s, 4f, 5d, 6p
7. The representative elements get the nearest inert gas configuration by (1) losing electrons (2) gaining electrons (3) sharing electrons (4) losing or gaining or sharing electrons
8. The period number and group number in which maximum number of elements are placed are, respectively, (1) 4th and IA (2) 5th and zero (3) 7th and IIIA (4) 6th and IIIB
9. The formula of the compound formed by the pair of elements Al and S is (1) AlS (2) Al2S3 (3) Al3S2 (4) AlS2
10. An element has 18 electrons in the outer- most shell. The element is a/an (1) transition metal (2) rare earth metal (3) alkaline earth metal (4) alkali metal
11. Match the columns.
Column-I Column II
A. Polonium I) Liquid metal
B. Mercury II) Liquid non-metal
C. Bromine III) Diamond
D. Carbon IV) VIA group
Choose the correct answer from the options given below.
(1) A-IV, B-I, C-II, D-III (2) A-IV, B-I, C-III, D-II
(3) A-III, B-II, C-I, D-IV (4) A-I, B-IV, C-III, D-II
12. The electronic configuration of an element ‘X’, is 1s2 2s2 2p6 3s2 3p3. What is the atomic number of the element which is just below ‘X’ in the periodic table? (1) 33 (2) 34 (3) 31 (4) 49
Answer Key
(11) 1 (12) 1
4.4 ELECTRONIC CONFIGURATION OF ELEMENTS AND THE PERIODIC TABLE
4.4.1
Electronic Configuration in Periods
1. Period Length & Orbitals – The number of elements in each period is twice the number of atomic orbitals available in that energy level.
2 Period Number & Shells – The period number corresponds to the outermost shell of the atom.
3. Successive Filling – Each period fills the next higher principal energy level.
Period-wise Breakdown:
1st Period (2 Elements) –
■ Fills 1s (K-shell), includes H (1s¹) and He (1s2).
2nd Period (8 Elements) –
■ Starts with Li (2s¹), ends at Ne (2s2 2p⁶).
3rd Period (8 Elements) –
■ Starts with Na (3s¹), ends at Ar (3s2 3p⁶).
4th Period (18 Elements) –
■ Begins with K (4s¹), ends at Kr (4s 24p⁶).
■ Includes 10 transition elements (Sc-Zn) due to 3d filling.
■ Exception: Cr and Cu have one electron in 4s orbital.
5th Period (18 Elements) –
■ Starts with Rb (5s¹), ends at Xe (5p⁶).
■ Includes 10 transition elements (Y-Cd) due to 4d filling.
6th Period (32 Elements) –
■ Starts with Cs (6s¹), ends at Rn (6p⁶).
■ Includes Lanthanides (Ce-Lu, 4f series).
CHAPTER 4: Classification of Elements
7th Period(Incomplete, 32 Elements)
■ Starts with Fr (7s¹).
■ Actinides (Th-Lr, 5f series) included.
■ Expected to end at atomic number 118.
■ Special Placements
■ Hydrogen is placed separately due to its unique properties.
■ Lanthanides (4f) & Actinides (5f) are placed separately to maintain periodic table structure.
4.4.2
Electronic Configuration in Groups
■ Purpose of Classification – Groups elements with similar chemical properties based on their electronic configuration.
■ Groupwise Electron Configuration:
Group 1 (Alkali Metals) – ns¹ (1 electron in outermost shell).
Group 2 (Alkaline Earth Metals) – ns2 (2 electrons in outermost shell).
Group 13 (IIIA) – ns2np¹ (3 valence electrons).
Group 14 (IVA) – ns2np2 (4 valence electrons).
Group 18 (Noble Gases) – ns2np⁶ (full outer shell, stable).
■ Group Number & Valence Electrons – In representative elements, the Roman numeral group number equals the number of outermost shell electrons.
■ Pseudo-Octet Configuration – Nickel group has a unique feature:
Palladium (Pd) has 18 electrons in its outermost shell instead of 8.
■ Exceptions Exist – Though elements in a group share a similar outer shell configuration, some anomalies are observed.
4.5 ELECTRONIC CONFIGURATION AND TYPES OF ELEMENTS:
s, p, d, f -BLOCKS
■ For a systematic study of elements of modern periodic table, further classification of the tabular form is necessary, as shown in the table 4.6.
Classification into Blocks
■ Elements are classified into four blocks based on the entry of the differentiating electron into sub-shells.
■ The s-block is on the left, the p-block on the right, the d-block in the middle, and the f-block at the bottom of the periodic table.
Table 4.6 Configuration of first and last element of each period
Period
1 H(Z= 1) 1s1
2 Li (Z = 3) [He]2s1
3 Na ( Z = 11) [Ne]3s1
4 K ( Z = 19) [Ar]4s1
5 Rb (Z = 37) [Kr]5s1
6 Cs (Z = 55) [Xe]6s1
7 Fr (Z = 87) [Rn]7s1
s - Block Elements(ns¹-2.)
He(Z = 2) 1s2
Ne (Z= 10) [He]2s22p6
Ar (Z = 18) [Ne]3s23p6
Kr (Z = 36) [Ar]3d104s24p6
Xe (Z = 54) [Kr]4d105s25p6
Rn (Z= 86) [Xe]4f145d106s26p6
■ s-block elements have their differentiating electron in the s-orbital of the outermost shell.
■ The first two elements of each period belong to the s-block (Groups 1 & 2: Alkali and Alkaline Earth Metals).
■ Located on the left of the periodic table; helium, though 1s2, is in Group 18 due to its inert nature.
Properties:
■ Highly electropositive, soft metals, low density.
■ Strong reducing agents with low melting and boiling points.
■ Highly reactive, increasing down the group.
■ Form ionic compounds, except Li & Be.
■ Oxidation states: +1 (Group 1), +2 (Group 2).
■ Impart flame colors, except Be & Mg.
p - Block Elements(ns2np¹-⁶)
■ p-block elements have their differentiating electron in p-orbitals of the outermost shell.
■ Includes Groups 13–18 (IIIA to VIIA & Group 18), located on the right side of the periodic table.
■ Helium, though 1s2, is placed in Group 18 due to its inert nature.
■ s-block (except Group 18) + p-block = Representative elements.
Properties of p-block Elements:
Includes metalloids, most non-metals, and some metals.
■ All gaseous elements (except H₂ & He) belong to p-block.
■ Highly electronegative with high electron gain enthalpy.
■ Some act as oxidizing agents, others as reducing agents.
CHAPTER 4: Classification of Elements
■ Reactive, except Group 18 (noble gases).
■ Form mostly covalent compounds (e.g., Cl₂, O₂, HCl) but also ionic halides, oxides, sulfides, nitrides, etc
d - Block Elements((n-1)d1-10 ns1or2)
■ d-block elements have their differentiating electron in d-orbitals of the penultimate shell.
■ Includes Groups 3–12, located in the middle between s-block and p-block.
■ They exhibit properties intermediate to s-block and p-block elements and are called transition elements.
■ Four series: 3d, 4d, 5d (10 elements each, fully filled) and 6d (incomplete, 8 elements).
■ Zn, Cd, Hg do not exhibit typical transition element properties.
Properties of d-block Elements:
■ Electropositive, metallic, solid (except Hg, liquid at room temp.).
■ Show catalytic activity (e.g., Fe in Haber’s process, platinized asbestos in H₂SO₄ contact process).
■ Form cations with different charges.
■ Form both ionic and covalent compounds. Exhibit complex formation (e.g., [Cu(NH₃)₄] SO₄, K₄[Fe(CN)₆]).
■ Form alloys and interstitial compounds. Show colored ions, variable valency (Fe2+, Fe3+), and paramagnetism (Fe2 + = 4.90 BM, Fe3+ = 5.92 BM)
f - Block Elements: [(n-2)f¹-¹⁴ (n-1)d⁰–¹ ns2]
■ f-block elements have their differentiating electron in f-orbitals of the antipenultimate shell.
■ Part of Group 3, located at the bottom of the periodic table.
■ Two series: Lanthanides (Z = 58 to 71) follow La (Z = 57).
■ Actinides (Z = 90 to 103) follow Ac (Z = 89).
■ La & Ac are d-block elements, while lanthanides & actinides are f-block elements.
Properties of f-block Elements:
■ Heavy metals with high density, form colored ions, complexes, and show paramagnetism like d-block elements.
■ Rare earth elements, naturally found in small quantities.
■ Trans-uranic elements (Z > 92) are synthetic.
■ Form complexes and interstitial compounds.
■ Actinides are radioactive and exhibit more oxidation states than lanthanides.
■ Many actinoids exist only in nanogram quantities, synthesized via nuclear reactions.
Classification of Elements into Types
■ Elements are classified into four types based on their electronic configuration and properties:
Type I – Inert gases (noble gases)
Type II – Representative elements
Type III – Transition elements
Type IV – Inner transition elements
Their electronic configuration differences determine their chemical behavior.
Inert Gas Elements
■ Elements with completely filled outermost s and p sub-shells are called inert gases.
■ Group 18 elements are inert due to low reactivity.
■ Xenon-fluorine compounds were discovered in 1962, leading to the term no ble gases.
■ These elements are scarce in nature and also called rare gases.
Representative Elements
■ Elements with incompletely filled outermost s and p sub-shells are called representative elements.
■ Groups 1, 2, 13–17 (all ‘A’ groups) belong to this category.
■ All s- and p-block elements, except Group 18, are representative elements, also called normal elements.
■ Their general electronic configuration is ns¹ -2 np⁰–⁵.
■ They react to achieve inert gas configuration by losing, gaining, or sharing electrons; some attain pseudo-inert gas configuration.
■ They are called representative elements because they participate in most known chemical reactions.
■ Fluorine is the most reactive element.
■ This category includes many non-metals, metalloids, and some metals.
Transition Elements
■ Transition elements have partially filled n and (n–1) shells and belong to the d-block.
■ They exhibit intermediate properties between s-block and p-block elements.
■ General configuration: (n–1)d¹–¹⁰ ns¹–2 with incomplete d-orbitals in higher oxidation states.
■ Group 12 (Zn, Cd, Hg) are not transition elements due to fully filled d-orbitals.
■ Group 11 (Cu, Ag, Au) resemble transition elements in some ionic states.
■ Key properties:
■ Variable oxidation states (e.g., Fe2 +, Fe3+).
■ Colored ions due to d-d transitions.
CHAPTER 4: Classification of Elements
■ Paramagnetism; Fe, Co, Ni are ferromagnetic.
■ Catalytic activity (Ni in oil hydrogenation, Fe in Haber’s process, Mo as a promoter).
■ Forms alloys & interstitial compounds (e.g., brass, bronze, German silver, Pd occluding H₂).
■ High melting/boiling points & densities.
Inner Transition Elements
■ Inner transition elements have partially filled n, (n–1), and (n–2) shells and belong to the f-block.
■ They serve as a transition in physical and chemical properties among transition elements.
■ Two series: Lanthanides (4f) and Actinides (5f), all metals.
■ General configuration: (n–2)f¹ – ¹⁴ (n–1)d⁰ - ¹ ns 2 .
■ Elements in each series have similar properties.
■ Heavy metals, rare or synthetic; actinides, except Th and U, are synthetic.
■ Exhibit variable oxidation states, magnetism, and form complex compounds.
■ Common oxidation state: +3.
Classification into Metals and Non-Metals
■ Elements are classified as metals, non-metals, and metalloids based on properties.
■ Metals (75% of elements) are on the left side of the periodic table.
■ Metals are solid at room temperature (except Hg), have high melting/boiling points, are good conductors, malleable, and ductile.
■ Non-metals (about 12 elements) are on the top right of the periodic table.
■ Non-metals are usually gases but some are solids with low melting/boiling points (except B, C).
■ Non-metals are poor conductors (except graphite) and are brittle, non-malleable, and nonductile.
■ Metalloids exhibit both metallic and non-metallic properties and are found in the p-block (e.g., Ge, As, Sb, Se, Te).
Solved Examples
4. The element Z = 117 has not been discovered. In which group would you place this element? Give the electronic configuration.
Sol. The element with Z = 117 would belong to the halogen family (group 17) and the electronic configuration would be.
5. What would be the IUPAC name and symbol for the element with atomic number 120?
IL ACHIEVER SERIES FOR JEE CHEMISTRY
For (n–1)d Electrons:
■ s = (0.35 × electrons in (n-1)d) + (1.0 × inner shell electrons)
■ ns electrons do not contribute.
Example:
Calculation of Zeff of 3d electron of Cu (z=29)
Electron of Cu = 1s2 2s2 2p6 3s2 3p6 3d10 4s1 18-electrons 10-electrons
s for 3d-electron = 0.35(9) + 1(18) = 21.5
Zeff for 3d-electron of Cu = 29–21.15 = 7.85
Atomic Radius
■ Atomic radius is the distance between the nucleus and the outermost electron cloud.
■ Atomic size refers to the diameter of the atom.
■ Three significant types of radii:
1. Metallic Radius (Atomic Radius)
■ Also called crystal radius, as metals are crystalline.
■ Defined as half of the internuclear distance between two adjacent metal atoms.
■ Example: Sodium (Na) has an internuclear distance of 3.72 Å, so its metallic radius = 1.86 Å.
2. Van der Waals Radius
■ Measured in molecular substances and inert gases.
■ Weak van der Waals forces allow atoms to approach without bonding.
■ Defined as half of the distance between two closest atoms of different molecules in the solid state.
■ Example: Chlorine (Cl₂) has an internuclear distance of 3.6 Å, so its van der Waals radius = 1.8 Å.
3. Covalent Radius
■ Measured for non-metals in covalent molecules.
■ Defined as half of the internuclear distance between two identical atoms in a covalent bond.
■ Determined by electron diffraction, X-ray diffraction, and spectroscopy.
It is measured in Angstrom units, A°.
1A0 = 1 × 10–8 cm = 1 × 10–10 m = 100 pm = 0.1 nm
1nm = 10–9 m = 10–7 cm = 10 A0
■ For example, the internuclear distance between atoms of chlorine molecule is 1.98 A 0. The covalent radius of chlorine is 0.99 A 0 .
■ The covalent radius and van der Waals radius of chlorine are shown diagramatically in Fig.4.3.
Fig.4.3 Radii of chlorine
■ In a heteronuclear molecule, the sum of covalent radii should equal the internuclear distance.
■ Covalent radius is the distance between the nucleus and the mean position of a shared electron pair in a bond.
■ Multiple bonds increase attraction, reducing internuclear distance and decreasing covalent radius.
■ In a heteronuclear molecule, the sum of covalent radii should equal the internuclear distance.
■ Covalent radius is the distance between the nucleus and the mean position of a shared electron pair in a bond.
■ Multiple bonds increase attraction, reducing internuclear distance and decreasing covalent radius.
Comparison of Covalent Radii with Van der Waals' Radii
■ Covalent radius is about 20% shorter than the theoretical atomic radius due to orbital overlap in covalent bonding, which reduces internuclear distance.
■ Van der Waals radius is 40% larger than the covalent radius since non-bonded atoms are held by weak forces.
■ Inert gases are monoatomic and rarely form bonds.
■ For monoatomic elements, the van der Waals radius is considered as the atomic radius.
■ The covalent and vander Waals radii of some non-metallic elements are compared in Table.4.7
Table 4.7 Covalent and van derwaals radii of some elements
Variation of Atomic Radius in Groups
■ Atomic radius increases down a group due to an increase in the number of shells, despite increasing nuclear charge.
■ The effect of additional shells outweighs nuclear attraction, leading to larger atomic size.
■ Shielding effect increases, further reducing nuclear attraction.
■ Metallic (crystal) radius is greater than covalent radius.
■ Hydrogen has the smallest atomic radius, while Caesium has the largest among available elements.
■ The increase in the atomic radius in groups, with increasing atomic number, is shown diagrammatically in Fig.4.4.
Atomic number (Z)
Fig. 4.4 Atomic radii in group
number (Z)
Fig. 4.5 Atomic radii in period
Variation of Atomic Radius in Period
■ Atomic radius decreases across a period from left to right up to noble gases.
■ Reason 1: Increasing atomic number raises effective nuclear charge, pulling electrons closer to the nucleus, reducing atomic size.
■ Reason 2: From halogens to noble gases, atomic radius increases because:
Noble gases use van der Waals radius, which is larger.
Completely filled subshells in noble gases cause inter-electronic repulsions, slightly increasing size.
Radii of second period elements are listed in Table 4.8.
■ The trend in the decrease of atomic radius is valid in any period from alkali element to halogen.
Table 4.8 Radii of 2nd Period Elements
CHAPTER 4: Classification of Elements
■ The decrease in the atomic radius in periods, with increase in atomic number.
■ Every period starts with an electron entering s-sub shell of a new orbit. When the next electron enters in the same s-sub shell, the resulting decrease in the atomic radius is significant.
■ But the decrease in the radius with the p, d, and f-sub shells are being filled is normal.
Variation of Atomic Radius in Transition and Inner Transition Elements
■ Transition elements: Atomic radius decreases slowly across a period.
■ Reason: Increasing nuclear charge pulls electrons closer.
■ (n–1)d-electrons are added, causing poor shielding, leading to a slight decrease in size.
■ Inner transition elements: Atomic radius gradually decreases across the lanthanide and actinide series.
■ Lanthanide Contraction:
■ Electrons are added to (n–2)f orbitals, which provide poor shielding.
■ Causes less size increase between 4d and 5d elements in a group.
■ Actinide Contraction:
■ 5f-electrons provide poor shielding, leading to gradual size reduction across the series.
Radii of trivalent ions of lanthanides is shown in Fig.4.6
Fig. 4.6 Ionic radii (in A°) of lanthanides
Ionic Radius
■ The distance between the nucleus and the point upto which the nucleus shows its influence in an ion is called ionic radius.
Cationic Radius
■ Cation is a positively charged ion formed by the loss of electrons from a neutral atom.
■ Nuclear charge remains unchanged, but fewer electrons experience stronger attraction.
■ Effective nuclear charge per electron increases, pulling electrons closer to the nucleus.
■ Cation radius is smaller than its parent atom due to greater nuclear attraction.
■ This is illustrated by the data in the Table 4.9.
Table 4.9 Atomic and cation radii of some elements
Anionic Radius
■ Anion is a negatively charged ion formed by the gain of electrons by a neutral atom.
■ Effective nuclear charge per electron decreases, reducing nuclear attraction on electrons.
■ Anion radius is larger than its parent atom due to weaker attraction.
■ Cation and anion radii increase down a group due to added electron shells.
Nuclear Charge
■ Anion forms by electron gain in a neutral atom.Lower nuclear attraction increases anion size.
■ Anion radius > Parent atom due to weaker attraction.Cation & anion radii increase down a group due to added shells.
Radii of Isoelectronic Species
■ Ions that have equal number of electrons are called isoelectronic ions.
■ In such ions with decrease of nuclear charge radius increases, Table 4.10.
Table 4.10 Atomic and ionic radii of some isoelectronic series
Solved Examples
6. Compare the radii of H atom, H + ion and H– ion
Sol. H+ is the nucleus of H atom. Its radius is very small.
H– ion has number of electrons more than number of protons. Its size is more than that of H atom.
The radius is in the order: H + < < H < H–
7. Which is a bigger ion among Na +, F–, O2– and Mg2+? Why?
Sol. O2– is bigger ion among the given four. Among isoelectronic ions, the more the negative charge on the ion, the more is its size.
The order is O–2 > F–1 > Na+ > Mg+2
Try yourself:
2. If the van der Waals radius of hydrogen is 120 pm, what should be the intermolecular distance in solid Hydrogen?
Ans:240 pm
Ionisation Enthalpy
Electrons in an atom are attracted by the positively charged nucleus. To remove an electron from an atom, energy has to be supplied in order to overcome the attractive forces. This energy is known as ionisation enthalpy or ionisation energy or ionisation potential. Energy is always required to remove electrons from an atom and, hence, ionisation enthalpies [ D H] are always positive
( ) ( ) +→++− gg MIEMe
■ The minimum energy required to remove electron from an isolated gaseous atom to convert it into a gaseous ion is called ionisation energy.
Here, M is the isolated atom, I is the ionisation, potential and M + is the cation formed by the loss of one electron from M.
The units of ionisation enthalpy = kJmol -1 or kcal mol–1
1eV/atom= 23.06 k cal mol –1
1eV/atom = 96.43 kJ mol –1
1eV/atom = 1.602 × 10 –19 J atom–1
■ IE1, IE2, IE3, IE4, IE5, etc., are collectively known as successive ionisation potential values.
■ In general, the increasing trend in these values is : IE 1 < IE2 < IE 3 ...... IE n .
■ The IE1, IE2, and IE3 values of aluminium are, respectively, 578, 1820, and 2750 kJ/mole.
Table 4.11 Ionisation potential of second period elements
Factors Influencing Ionisation Potential
■ Atomic Radius → Ionisation potential (IP) is inversely proportional to atomic radius.
■ Screening Effect → Inner electrons shield outer electrons, reducing nuclear attraction.
■ Screening efficiency order: s > p > d > f.
■ IP is inversely proportional to the screening effect.
■ Penetrating Power of Orbitals → Greater penetration means stronger nuclear attraction.
■ Penetration order: s > p > d > f.
■ IP increases with penetration power.
■ Electronic Configuration → Half-filled and fully filled orbitals are more stable, requiring higher energy to remove electrons.
■ Inert gases have high IP due to fully filled s and p orbitals.
■ Net Charge on the Ion → Higher positive charge requires more energy for electron removal.
■ IP increases with increasing positive charge.
■ Variation in Groups → IP decreases down a group due to added shells and increased screening effect.
Table 4.12 Ionisation potential values of elements of group IA
Table 4.13 Ionisation potential of third period
Variation in Periods
■ Ionisation potential increases left to right in a period due to decreasing atomic radius, as shown in the table 4.13 and fig. 4.8.
■ Alkali metals have the lowest IP, while noble gases have the highest in a pe riod.
■ Groups 2, 15, and 18 have higher IP than adjacent elements due to stable configurations.
■ Groups 1, 3, and 16 have lower IP than adjacent elements.
■ Helium has the highest IP, while Caesium has the lowest among available elements.
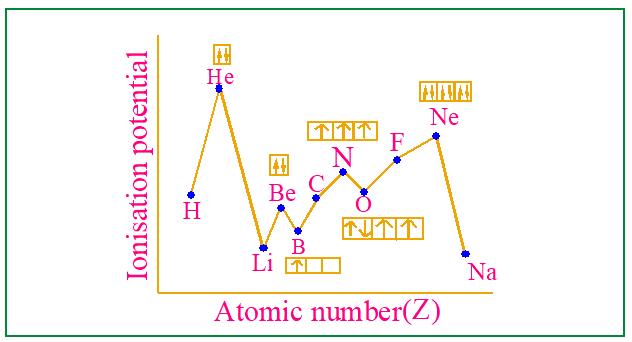
■ Ionisation potential curve is obtained by plotting IP vs. atomic number as shown in Fig.4.8 and Fig.4.9.
Fig. 4.7 Variation of Ionization Enthalpy with Atomic number
Fig. 4.8 Ionisation potential curve for the first eleven elements Atomic Number (Z)
Fig. 4.9 Ionisation potential curve for the first eleven elements
■ Helium has the highest IE (2467.2 kJ/mol) among all elements and inert gases, as shown in the fig. 4.9.
■ Be has higher IE₁ (899 kJ/mol) than B (801 kJ/mol) due to its stable 1s2 2s2 configuration and greater s-orbital penetration.
■ Similarly, Mg (738 kJ/mol) > Al (577 kJ/mol) for the same reason.
■ Nitrogen (1403 kJ/mol) has higher IE₁ tha n Oxygen (1314 kJ/mol) due to its stable halffilled p-subshell.
■ Similarly, Phosphorus (1010 kJ/mol) > Sulphur (999 kJ/mol).
■ Oxygen and Sulphur have lower IE than their preceding group 15 elements due to less stable electron configurations.
Solved Examples
8. The successive ionisation enthalpies of an element M are 5.98, 18.82, 28.44, 119.96, 153.77....eV/atom. What is the formula of chloride of M?
Sol. Observing the IE1, IE2, IE3, IE4, IE5, .... it is noticed that there is a sudden jump from IE 3 and IE4. This observation gives the idea that the element has 3 electrons in the outer most shell, as there is a great difference between 3rd and 4th ionisation enthalpies.
M3+ state is stable and valency is 3.
Formula of chloride of M is MCl 3.
9. The ionisation enthalpy of sodium is 5.14 eV. How many kcal of energy is required to ionise all atoms present in one gram of gaseous Na atoms?
CHAPTER 4: Classification of Elements
Sol. 1 eV atom–1 = 23 kcal mol–1
Energy required to ionise all atoms of 23 g (one mole) of gaseous Na atoms = 23 × 5.14 kcal
Energy required for ionisation of all atoms present in one gram of gaseous Na atoms = 5.14 kcal.
Try yourself:
3. The 1st, 2nd and 3rd ionisation enthalpies of Aluminium are 577, 1816 and 2744 kJmol –1. What is the amount of energy required to convert one gaseous Aluminium atom to gaseous Al 3+?
Ans:8.53 × 10–18 J/atom..
Electron Gain Enthalpy
■ In the process of addition of electron to neutral isolated gaseous atom, a certain amount of enthalpy change is involved. This is called Electron gain enthalpy ( D Heg)
XeX,HH +→∆=∆
( ) ( ) atom ion eg gg
■ Units: Expressed in kJ/mol or kcal/mol.
■ Negative ΔHeg: Energy is released during electron gain (exothermic).
■ Halogens have highly negative Δ Heg due to their strong tendency to gain electrons.
■ Positive ΔHeg: Energy is required for electron addition (endothermic).
■ Atoms reluctant to accept electrons show positive Δ Heg
■ Electron affinity & ionisation potential are defined at absolute zero, but at other temperatures, heat capacities of reactants and products must be considered. 5 , 2 ∆=−− ege HART where eg H∆ = electron gain enthalpy A e = electron affinity
Similarly, 5 HI.PRT 2 ∆=+ , where H∆ is ionisation enthalpy
■ The ionisation potential of a neutral atom A is equal in magnitude with the electron affinity of A+ ion. However, they have opposite signs.
AIAe:AeAE +−+− +→++→+
■ In the above equations, I and E are numerically same but the sign is opposite. Successive Electron Affinities
■ Successive addition of electrons to atomic species involve energy changes.
■ The utilisation of E1 results in the formation of uninegative ion.
XeXE +→+
( ) ( ) 1 gg
■ The utilisation of E2 results in the formation of dinegative ion.
( ) ( ) 2 2 ++→ gg XeEX
■ In a similar manner, the third electron affinity and the fourth electron affinity are defined. E1, E2, E3 etc., are collectively known as successive electron affinity values.
OeO142kJmole +→−
( ) 11
OeO702kJmole +→+
( ) 12 1
Two successive electron affinities of group 16 elements are listed in Table 4.14.
■ Sec ond Electron Gain Enthalpy is positive as energy is required to overcome repulsion between the uninegative ion and added electron.
Table 4.14 First and second electron affinity values of group 16 elements
Element
Factors Influencing Electron Affinity
■ Nuclear Charge → Higher nuclear charge → Higher electron affinity.
■ Atomic Radius → Larger radius → Lower electron affinity.
■ Electronic Configuration → Half-filled & fully filled sub-shells are stable, so they have low electron gain enthalpy.
■ Group Trend → Electron gain enthalpy decreases down a group
■ Variation in Groups
■ In a group, from top to bottom, electron gain enthalpy gradually decreases.
■ Trend in the electron affinity in Table 4.15.
Table 4.15 Electron affinity of halogens
■ The element with most negative electron gain enthalpy is chlorine, and the one with the least negative electron gain enthalpy is phosphorus.
Electron Gain Enthalpy Trends & Anomalies
■ Down a group: F (–328 kJ/mol) < Cl (–349 kJ/mol) due to higher repulsion in F’s small size; O < S follows the same trend.
■ Across a period: Electron affinity increases as nuclear charge increases and atomic radius decreases.
■ Anomalies:
1. Inert gases have high positive enthalpy due to a stable octet.
2. Group 15 elements have lower electron affinity; N has positive gain enthalpy due to half-filled stability.
3. Alkali metals have low negative values, while Be, Mg have positive enthalpy, approximated as zero.
The trend in the electron gain enthalpy values in a period can be observed from the data in Table 4.16.
Table 4.16 Electron gain enthalpy values of some elements (kJ mol–1)
– 46
Solved Examples
10. Write the descending order of electron affinity va lues of chalcogens.
Sol. Decreasing order of electron affinity values of chalcogens: S > Se > Te > O. Electron affinity of oxygen is less because oxygen has small atomic size and the added electron experiences greater repulsion on oxygen atom.
11. Process (A) : F2(g)+ 2e– → 2F–(g)
Process (B) : Cl2(g) + 2e– → 2Cl–(g) Which of these processes is easier? Why?
Sol. F2(g) + 2e– → 2F–(g) is easy. Though electron gain enthalpy of Cl (g) to give Cl–(g) is more than that of F(g) to give F–(g), the bond dissociation of F2(g) is very less, compared to that of Cl2(g).
IL ACHIEVER SERIES FOR JEE CHEMISTRY
Try yourself:
4. The quantity of energy released when one million gaseous iodine atoms are converted into I –(g) ions is 5.0 × 10–16 kJ. In this question: I (g) + e– → I–(g) + Q kJ mol–1 What is the value of Q?
Ans: 301
Electronegativity
■ Electronegativity is the relative tendency of an atom to attract bonded electrons.
■ Defined as an atom’s ability to pull shared electron pairs toward itself.
■ Not directly measurable, so quantitative scales are used for calculation.
Mulliken scale: According to Mulliken, Electronegativity = I.EE.A 2 + ; here, both IE and EA are in eV unit.
Pauling scale: Linus Pauling determined electronegativities with the help of bond dissociation energies.
The Pauling’s equation for the determination of electronegativity is written as,
AB XX0.208−=∆ where D is in k.cals.mol-1
AB XX0.1017−=∆ where D is in kJ.mol-1
■ Here, XA is electronegativity of element A, XB is electronegativity of element B and D is the bond stabilisation or resonance energy
D = Experimental bond energy - Calculated bond energy ( )1/2 ABAABB 1 EEE 2
■ EA–A, EB–B and EA–B are the bond dissociation energy values of the bonds A–A, B–B and A–B, respectively.
■ Pauling arbitrarily assigned a value of 2.1 to hydrogen and determined the electronegativity of fluorine as 4.
■ Based on this electronegativity values of other elements are calculated.
■ Pauling’s values of electronegativity are listed in Table 4.17
Factors Influencing Electronegativity
■ Nuclear Charge → Higher nuclear charge → Higher electronegativity.
CHAPTER 4: Classification of Elements
■ Atomic Radius → Larger atomic radius → Lower electronegativity.
■ Penetration Ability → Greater penetration → Higher electronegativity (Order: s > p > d > f).
■ s-Character in Hybrid Orbitals → More s-character → Higher electronegativity (Order: sp > sp2 > sp3).
Table 4.17 Pauling’s electronegativity values of representative elements
■ Variation in Groups → Electronegativity decreases down a group due to increased atomic radius and shielding effect.
■ Variation in Periods → Electronegativity increases across a period (left to right) up to halogens due to decreasing atomic radius and increasing nuclear charge.
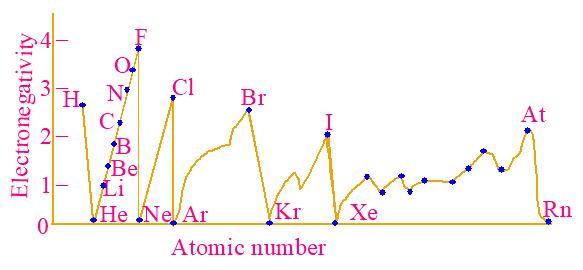
■ Variation of values of electronegativity with atomic number of elements is shown graphically in Fig.4.10.
4.10 Variation of electronegativity
Fig.
■ Halogens have the highest electronegativity in a period.
■ Fluorine (4.0) is the most electronegative, while Cesium (0.7) is the least on Pauling’s scale.
■ Applications of Electronegativity
■ Indicates non-metallic nature → Elements with electronegativity ≥ 2 are generally non-metals.
■ Related to reactivity → Fluorine is the most reactive, while Helium is the least reactive.
■ Elements with similar electronegativity have similar chemical behavior.
■ Predicts bond nature:
■ Electronegativity difference ≥ 1.7 → Ionic bond.
■ Electronegativity difference = 1.7 → 50% ionic, 50% covalent character.
Hanny and Smith Relationship
■ Percentage of ionic character
= 16 (XA – XB ) + 3.5(XA – XB)2, where XA and XB are the electro-negativit ies of two atoms A and B.
Solved Examples
12. How is the nature of covalent bond between two atoms predicted?
Sol. Nature of the covalent bond between two atoms is predicted based on the difference in electronegativity.
If there is no difference in the electronegativity of bonded atoms, the bond is pure covalent.
If electronegativity difference arises between bonded atoms, the bond is polar covalent, and further, increase of difference of electronegativity leads to the formation of ionic bond.
13. Bond energies of H2, Cl2, and HCl are, respectively, 104, 58, and 100 kcal mol –1. Calculate Pauling’s electronegativity of chlorine.
Sol. Average of bond energies of H 2 and Cl2 is the calculated bond energy of HCl
10458 2 + = = 81 kcal mol–1
Experimental bond energy of HCl = 100 kcal mol –1
D = Bond (resonance) stabilisation energy = 100 - 81 = 19 kcal mol–1
X1– X2 = 0.208 = 0.208 = 0.208 × 4.358 = 0.90
Since Pauling’s electronegativity of hydrogen is 2.1, that of chlorine = 2.1 + 0.9 = 3.0.
Try yourself:
5. Is it correct to say that pauling electronegativity of carbon is 2.5 in every compound?
Ans: no
Valency
■ Valency is the combining capacity of an element.
■ Compared to hydrogen, chlorine, or oxygen in bonding.
■ Defined as: Number of hydrogen or chlorine atoms an element can bond with.
■ Twice the number of oxygen atoms it can combine with.
■ Valencies of elements in some compounds are listed in Table 4.18
Table 4.18 Valencies of some elements
Valenc y of group elements is generally same.
■ The periodicity of valency of typical elements is provided in Table 4.21 in the form of hydrides, and oxides
■ Valency remains the same within a group.
■ Valency is zero for uncombined elements and Group 18 (noble gases) under normal conditions.
■ Valency electrons (outermost electrons) determine valency in representative elements.
■ For elements with ≤ 4 valence electrons, valency = number of valence electrons.
■ For elements with ≥ 4 valence electrons, valency = 8 – number of valence electrons.
■ Maximum valency corresponds to group number (in Roman numerals) and is never more than 8.
■ Osmium in OsO₄ and Xenon in XeO₄ have a valency of 8.
■ Valency in a period (for hydrides): Increases from 1 to 4 (Group 1 to 4), then d ecreases back to 1 (Group 5 to 7).
■ Periodic valency trends are evident in hydrides, oxides, and fluorides across periods.
Oxidation Number
■ Oxidation state is the charge present or appearing to be present on an atom in a species.
■ Oxidation states can be positive, negative, zero, or fractional.
■ Examples:
■ Alkali metals (Group IA): Always +1 (lose one electron).
■ Alkaline earth metals (Group IIA): Always +2.
■ Halogens: Always –1 (gain one electron).
■ p-block elements:
■ Oxidation number = Group number or Group number – 8.
■ Example: Group VA elements show +5 and –3 states.
■ Inert Pair Effect: Lower oxidation states become more stable down the group due to the reluctance of ns electrons to bond.
■ Example:
■ Tl (IIIA Group): +1 is more stable than +3.
■ Pb (IVA Group): +2 is more stable than +4.
■ Bi (VA Group): +3 is more stable than +5.
■ d-block elements:
■ Show variable oxidation states (+1 to +8) due to configuration ns 0-2, (n–1)d1-10
■ Common oxidation state = +2.
■ Examples:
■ Mn exhibits +2, +3, +4, +6, +7 states.
■ Cr, Cu, Ag, Au, Hg show +1 state.
■ f-block elements:
■ Exhibit +2, +3, and +4 oxidation states.
■ Lanthanides: +3 is most common.
■ Maximum oxidation state (+8) is shown by Ru, Os, and Xe in RuO₄, OsO₄, and XeO₄.
Variation of oxidation number in transition elements is shown in Table 4.19
Solved Examples
14. Using the periodic table, predict the formula of compound formed between an element X of group 13 and another element Y of group 16.
Sol. The valency of X (group 13) = 3
The valency of Y (group 16) = 2
The compound has 2 atoms of X and 3 of Y.
Hence, the formula = X2Y3
Table 4.19 Variation of oxidation states in transition elements
15. What are the valencies of K in K2O and S in H2S? What should be the formula of compound of K and S in which the above valencies are reflected?
Sol. Two K atoms are in combined state with one oxygen atom. One K-atom combines with 1/2 atom of oxygen. Hence, valency of K is one. One S-atom combined with two H atoms. Hence, valency of S is two. Hence, the formula of the compound is K 2S.
Metallic Nature
■ Electropositivity & Metallic Nature
■ Electropositivity is the opposite of electronegativity, indicating metallic nature.
■ Defined as an element’s tendency to lose electrons and form cations (M →Mⁿ ++ne-).
■ Higher electropositivity means greater metallic nature and lower ionization enthalpy.
■ Electropositivity Trends & Properties
■ Alkali metals are highly electropositive due to:
■ No hydrolysis of their ions.
■ Stable solid bicarbonates (except LiHCO₃).
■ Strong reducing ability.
■ Highly soluble oxides forming strong bases.
■ s-block metals are the most electropositive, followed by p-block metals.
■ All d-block and f-block elements are metals.
■ Metallic nature increases down a group.
■ Metalloids exhibit both metallic and non-metallic properties:
■ Group 14: Arsenic (As) and Antimony (Sb).
■ Group 15: Selenium (Se) and Tellurium (Te).
Nature of Oxides(four types)
■ Generally, metallic oxides are basic. They neutralise acids and they dissolve in water to give bases. e.g., K2O, MgO, Tl2O, etc.
Na2O + H2O → 2NaOH
CaO + H2O → Ca(OH)2
■ Generally, non-metallic oxides are acidic. They neutralise bases like sodium hydroxide. They dissolve in water to give acids. They are called acid anhydrides, e.g., SO 2, P4O10, CO2, etc.,
COHOHCO +→
23 NOHOHNOHNO +→+
■ Oxides of metalloids are generally amphoteric. They react with both acids as well as bases e.g., GeO2, Sb4O6, TeO2, As2O3,etc.
■ Some of the metallic oxides are also amphoteric, e.g., ZnO, Al 2O3, SnO2, etc.,
ZnO2HClZnClHO +→+
ZnO2NaOHNaZnOHO +→+
AlO6HCl2AlCl3HO +→+
AlO2NaOH2NaAlOHO +→+
■ Some of the non-metallic oxides are neutral. They do not react with acids as well as with bases e.g., CO, N2O, NO, etc.
■ Basic nature of the oxides increases generally with an increase in the electropositivity of metal forming oxide.
■ Oxides of all elements of group1 are basic and of group 17 are acidic. Down the group, basic nature of oxides increases and acidic nature decreases, as shown in Table 4.20.
Table 4.20 Nature of trioxides of group-15
Element Nature of the element Formula of trioxide Nature of oxide
N non-metal N2O3 acidic
P non-metal P4O6 acidic
As metalloid As4O6 weakly acidic
Sb metalloid Sb4O6 amphoteric
Bi metal Bi2O3 basic
■ Trends in Oxide Nature Across a Period
■ Basic nature of oxides decreases, while acidic nature increases across a period.
■ Oxide acidity increases with higher oxygen content in multiple oxides of the same element.
■ Most basic oxide: Caesium oxide (Cs₂O) → Forms the strongest base, CsOH.
■ Most acidic oxide: Chlorine heptoxide (Cl₂O₇) → Forms the strongest acid, HClO₄ (Perchloric acid).
Periodic Trends and Chemical Reactivity
■ Periodic Trends & Chemical Reactivity
■ Chemical properties are determined by electronic configuration.
■ Across a period (left to right): Atomic radius decreases → Ionization enthalpy increases → Electron gain enthalpy becomes more negative.
■ Reactivity Trends: Alkali metals (Group 1) are highly reactive due to low ionization potential, forming cations (electropositive, good reductants).
■ Halogens (Group 17) are highly reactive due to high electron affinity, forming anions (nonmetallic, good oxidants).
■ Reactivity peaks at period extremes:
■ Left (alkali metals) → React by losing electrons (cation formation, electropositivity).
■ Right (halogens, not noble gases) → React by gaining electrons (anion formation, nonmetallic nature).
■ The periodic trends in the properties of elements are diagramatically given in Fig.4.11.
Solved Examples
16. Is hydrogen electropositive?
Sol. Hydrogen has electropositivity. It is evidenced by the formation of proton. H → e – + H+
However, hydrogen is not a metal. It is a common non-metal.
17. In aqueous solutions lithium is the best reductant. Why?
Sol. Lithium cation is small and its hydration ability is high. The stronger reduction ability of lithium is also reflected in least standard potential of Li +/Li.
Anamolous properties of second period elements
■ Second-period elements show anomalous behavior due to:
■ Small atomic size.
■ High electronegativity.
■ Large charge-to-radius ratio.
■ Absence of d-orbitals for bonding.
■ Second-period elements have a maximum covalency of four.
■ First p-block elements readily form multiple bonds.
■ Oxide behaviour of third period elements is shown in table 4.21.
Fig.4.11 Periodic trends of elements
Table 4.21 Nature of oxides of elements of third period
Group IA Group IIA Group IIIA
VIA Group VIIA
Na2O MgO Al2O3 SiO2 P4O10 SO3 Cl2O7
Strong base Basic Amphoteric Weak acid
Moderately acidic Strong acid Strong acid
NaOH Mg(OH)2 Al(OH)3 H2SiO3 H3PO3 H2SO4 HClO4
Strong base Basic Amphoteric Weak acid
Examples:
1) CC,CC,NN,NN =≡=≡
2) CO,CN,CN,NO ==≡=
Moderately acidic Strong acid Strongest acid
■ Lithium (IA) & Beryllium (IIA) form covalent compounds, unlike heavier group members.
■ Boron forms [BF4]–, while heavier group members expand their valence shells (e.g., [AlF6]3–).
■ Chemical similarities exist in groups, but diagonal relationships also occur.
■ Diagonal relationship: occurs when lighter second-period elements resemble third-period elements diagonally placed in the periodic table.
■ Diagonal relationship of elements is shown in Table 4.22.
■ This similarity is called diagonal relationship.
■ The phenomenon of diagonal relationship does not appear after group 4 and also below third period of the periodic table.
■ Diagonal relationship arises due to similar electronegativity of elements.
■ Elements showing diagonal relationships have similar polarizing power.
■ Polarizing power is the cation’s ability to attract an anion’s charge cloud.
■ Formula: Polarizing po wer = Ionic charge /(Ionic radius)2.
Polarising power = ( ) Chargeofion 2 Ionicradius
■ Across a period: Charge increases, size decreases, leading to higher polarizing power.
■ Down a group: Size increases, causing polarizing power to decrease.
■ Be2+ and Al3+ have similar polarizing power, leading to similar properties.
■ Both dissolve in caustic soda, form amphoteric oxides, and their carbides hydrolyze to produce methane.
Be2C + 4H2O → 2Be(OH)2 + CH4
Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
■ Diagonal similarities are most prominent among lighter electropositive elements.
■ The metal-nonmetal boundary in the periodic table also follows a diagonal t rend.
Table 4.22 Diagonal relationship of elements
Solved Examples
18. Compare the oxidation ability of sulphur and chlorine.
Sol. 2 CleCl
S2eS +→ +→
Chlorine is better oxidant than sulphur. Electron gain enthalpy is more for chlorine. Chlorine accepts electron easily and becomes stable chloride.
19. Lithium is monovalent. Magnesium is divalent. But Li and Mg are diagonally related pair of elements. Why?
Sol. Lithium and magnesium have certain similarities in their properties. Hence, they are called a diagonally related pair of elements.
The reasons are:
(a) Li and Mg have similar electronegativity.
(b) Li+ and Mg2+ have similar polarising power.
TEST YOURSELF
1. The correct order of van der Waals radius of F, Cl, and Br is (1) F > Br > Cl (2) Br > Cl > F (3) F > Cl > Br (4) Br > F > Cl
2. The correct arrangement of O, P, and N in order of increasing radii is (1) O < N < P (2) P < O < N (3) O < P < N (4) N < O < P
3. Covalent bond length of chlorine molecule is 1.98 Å. Covalent radius of chlorine is (1) 1.98 Å (2) 1.7 Å (3) 2.05 Å (4) 0.99 Å
4. The covalent and van der Waals radii of chlorine, respectively, are (1) 1.80 Å and 0.99 Å (2) 0.99 Å and 1.80 Å (3) 1.80 Å and 1.80 Å (4) 0.99 Å and 0.99 Å
5. Electrons with the highest penetrating power are (1) p-electrons (2) s-electrons (3) d-electrons (4) f-electrons
6. The species with largest ionisation potential (1) Li+ (2) Mg+ (3) Al+ (4) Ne
7. Second ionisation energy is higher than first ionisation energy for an element. This is because (1) nuclear charge is high in cation (2) size of cation is higher than neutural atom (3) effective nuclear charge is more for cation (4) bond energy changes with charge
8. In modern periodic table, the groups table that possesses the highest and lowest ionisation energies, respectively, are (1) IA, VIIA (2) zero, IA (3) IA, IIA (4) VIIA, IA
9. In the lithium atom, screening effect of valence shell electron is caused by (1) electrons of K and L shell (2) electrons of K shell (3) two electrons of 1st and one of 2nd shell (4) electrons of L-shell
10. First four ionisation energy values of an element are 191, 578, 872, and 5972 kcals. The number of valence electrons in the element is (1) 4 (2) 3 (3) 1 (4) 2
11. The element with highest electron affinity is (1) fluorine (2) cesium (3) helium (4) chlorine
12. Ionisation of energy of F – is 320 kJ mol–1. The electron gain enthalpy of fluorine would be (1) – 320 kJ mol–1 (2) –160 kJ mol–1 (3) + 320 kJ mol–1 (4) 160 kJ mol–1
13. Which of the following is an endothermic process? (1) First electron affinity of chlorine (2) Second electron affinity of oxygen (3) Formation of NaCl from gaseous ions (4) Hydration of MgCl2
14. In a period from left to right, electron affinity (1) increases (2) decreases (3) remains constant (4) first increases and then decreases
15. Configuration that shows the highest energy released, when an electron is added to the atom, is (1) 1s2 2s2 2p3 (2) 1s2 2s2 2p4 (3) 1s2 2s2 2p5 (4) 1s2 2s2 2p6
16. Pauling’s electronegativity is based on (1) electron affinity (2) ionisation potential (3) both IP and EA (4) bond energies
17. The electronegativity value of chlorine and bromine are, respectively 3 and 2.8. Formula of a binary compound is best represented as (1) BrCl (2) ClBr3 (3) ClBr (4) ClBr5
18. Reference element for Pauling’s electronegativity is (1) H (2) C (3) Cl (4) He
19. What is the correct order of electronegativity?
(1) M+1 < M+2 < M+3 < M+4
(2) M+1 > M+2 > M+3 > M+4
(3) M+1 < M+2 > M+3 < M+4
(4) M+4 < M+2 < M+3 < M+1
20. In a period, electronegativity is highest for (1) chalcogen (2) halogen (3) inert gas (4) alkali metal
21. All the following elements show both positive and negative oxidation states, except (1) N (2) H (3) O (4) F
22. An element with electronic arrangement as 2, 8, 18, 1 will exhibit the following oxidation states (1) + 2 and + 4 (2) + 1 and + 2 (3) + 2 only (4) + 1 only
23. Oxidation state and covalency of Al in [AlCl(H 2O)5]2+ are (1) +2, 6 (2) +3, 6 (3) +2, 4 (4) +3, 4
24. Basic nature of the oxides of a period from left to right (1) increases (2) decreases (3) remains constant (4) first increases and then decreases
25. Oxide that is most acidic is (1) Cl2O7 (2) SO3 (3) P4O10 (4) N2O5
26. Generally, the nature of non-metal oxides is (1) basic (2) acidic (3) amphoteric (4) neutral
27. Most acidic oxide in the periodic table is formed by an element in (1) 2nd period, group VI A (2) 4th period, group VII A (3) 3rd period, group VI A (4) 3rd period, group VII A
28. The correct increasing order of metallic nature of Si, Be, Mg, Na, P is
(1) P < Si < Be < Mg < Na
(3) Si < P < Mg < Be < Na
(2) Si < P < Be < Mg < Na
(4) Na < Mg < Be < Si < P
29. The diagonal relationship phenomenon is not observed after (1) I A group
(2) II A group (3) III A group
(4) IV A group
30. Which of the following is not correct in the case of Be and Al?
(1) Both are rendered passive by conc. HNO 3
(2) Carbides of both give methane on hydrolysis.
(3) Both give hydroxides that are basic.
(4) Both give covalent chlorides.
31. The correct order of polarisability of ion is
(1) Cl– > Br– > I– > F– (2) F– > I– > Br– > Cl–
(3) I– > Br– > Cl– > F– (4) F– > Cl– > Br– > I–
32. The chemistry of lithium is very similar to that of magnesium, even though they are placed in different groups. This is because
(1) both are found together in nature (2) both have nearly the same size
(3) both have similar electronic configuration
(4) the ratio of charge and size is nearly same
33. Beryllium and aluminium exhibit many properties that are similar. But, the two elements differ in
(1) forming covalent halides
(2) forming polymeric hydrides
(3) exhibiting maximum covalency in compounds
(4) exhibiting amphoteric nature in their oxides
Answer Key
3 (32) 4 (33) 3
# EXERCISES
JEE MAIN
Level I
Genesis of periodic classification
Single Option Correct MCQs
1. Which of the following is Dobereiner triad
(1) Li, Na, K
(2) Fe, Co, Ni
(3) Ru, Rh, Pd
(4) Os, Ir, Pt
2. Which is not a Dobereiner’s triad?
(1) Fe, Co, Ni
(2) Li, Na, K
(3) Ca, Sr, Ba
(4) Cl, Br, I
3. Law of Octave is not applicable to (1) Li,Na,K
(2) Be,Mg,Ca
(3) B,Al,Ga
(4) All of the above
4. The Newland’s law of octaves for the classification of elements was found to be applicable only up to the element
(1) Potassium
(2) Calcium
(3) Cobalt
(4) Phosphorus
5. Elements which occupied position in the Lother Meyer curve, on the peaks, were:
(1) alkali metals
(2) highly electropositive elements
(3) elements having large atomic volume
(4) all of the above
6. Lothar Meyer obtained the curve for the known elements by plotting their atomic volumes against (1) atomic numbers
(2) atomic masses
(3) densities
(4) ionisation energies
7. Considering the chemical properties, atomic weight of the element ‘Be’ was corrected based on
(1) Valency
(2) Configuration
(3) Density
(4) Atomic volume
8. Eka silicon is now known as (1) Scandium
(2) Gallium
(3) Germanium
(4) Boron
9. Anomalous pair among the following is
(1) Boron - Silicon
(2) Beryllium - Indium
(3) Aluminium - Gallium
(4) Cobalt - Nickel
10. Number of short periods in short form of periodic table
(1) 3 (2) 2
(3) 4 (4) 6
11. According to Mendeleev’s periodic law
(1) the properties of the middle element were in between those of the other two members
(2) three elements arranged according to increasing weights have similar properties.
(3) the properties of the elements are a periodic function of their atomic weights
(4) the elements can be grou ped in the groups of six elements.
12. The number of elements known at that t ime when Mendeleev arranged them in the periodic table was :
(1) 63 (2) 60
(3) 70 (4) 65
Numerical Value Questions
13. The group of Mendeleev’s periodic table consisting of maximum elements is ____
14. The elements A, B and C form a Dobereiner’s Triad. If the sum of atomic mass of A, B and C is 180, then atomic mass of B is:
[The order of atomic masses is A < B < C]
[Divide your answer by 10]
15. Number of elements present in second series of Mendeleev's periodic table are ___
Modern periodic law and the present form of the periodic table
Single Option Correct MCQs
16. Henry Moseley plotted a graph between ν and Z, where ν was the frequency of X–ray emitted by an atom and Z was its atomic number. This graph showed that
(1) the atomic mass is a fundamental property of an element.
(2) the atomic number is a fundamental property of an element.
(3) Both (1) and (2)
(4) the frequency (ν) was independent of atomic number.
17. Henry Mosely studied characteristic X− ray spectra of elements. The graph which his observation correctly is (1) υ
18. The frequency of the characterstic X ray of K α line of metal target ‘M’ is 2500 cm−1 and the graph between v Vs ‘z’ is as follows, then atomic number of M is (1) 49 (2) 50 (3) 51 (4) 25
19. According to Moseley, a straight line graph is obtained on plotting: Where, ν is frequency and Z is atomic number.
(1) ν vs Z (2) ν2 vs Z
(3) v vs Z (4) 1 v vs Z
Numerical Value Questions
20. The moseleys expression for iron metal would be v = a (X – b), the value of X is ___.
21. The atomic number of metal preceding nickel metal based on Moseley's experiment will be _________
Nomencl ature of elements with atomic number more than 100
Single Option Correct MCQs
22. The IUPAC name of an element with atomic number 119 is
(1) unnilennium (2) unununium
(3) ununoctium (4) ununennium
23. Identify the incorrect match
Name IUPAC official name
A. unnilunium I) Mendelevium
B. unniltrium II) Lawrencium
C. unnilhexium III) Seaborgium
D. unununium IV) Darmstadtium
(A) (B) (C) (D)
(1) IV I II III
(2) III I IV II
(3) III I II IV
(4) III IV I II
24. Ununseptium is the systematic name for element having atomic number
(1) 113 (2) 115
(3) 117 (4) 119
25. The name of the element having atomic no. 104 is/are
(1) Rutherfordium (2) Unnilquadium
(3) Kurchatovium (4) All of the above
26. The IUPAC nomenclature of an element with electronic configuration [Rn]5f146d107s2 is
(1) ununbium
(2) Unnilunium
(3) Unnilquadium
(4) Unniltrium
27. Identify the incorrect statement from the following
(1) The IUPAC name of an element with atomic number 101 is Unnilunium
(2) The IUPAC name of an element with atomic number 102 is Ununseptium
(3) The IUPAC name of an element with atomic number 103 is Unniltrium
(4) The IUPAC name of an element with atomic number 104 is unnilquadium
Numerical Value Questions
28. If IUPAC name of an element is “Unununnium” then the element belongs to nth group of periodic table. The value of n is______
29. The atomic number of Unnilunium is
30. Sum of atomic numbers of Unununium and Unnilennium are?
Electronic configurations of elements and the periodic table
Single Option Correct MCQs
31. The element with ns2np4 as outer electron configuration is a ____________
(1) Alkali Metal (2) Chalcogen
(3) Noble gas (4) Halogen
32. Match column I (electronic configuration) with column II (group)
Column I Electronic configuration
Column II Group name
A. ns2np3 I) Halogens
B. ns2np6 II) Noble gases
C. ns1 III) Group 15 elements
D. ns2np5 IV) Transition elements
V) Alkali metal
(A) (B) (C) (D)
(1) III II V I
(2) III I V IV
(3) II III V I
(4) III IV I V
33. Elements A, B, C, D and E have the following electronic configurations:
A. 1s2, 2s22p1
B. 1s2, 2s22p6, 3s23p1
C. 1s2, 2s22p6, 3s23p3
D. 1s2, 2s22p6, 3s23p5
E. 1s2, 2s22p6, 3s23p6
Which among these will belong to the same group in the periodic table?
(1) A and C
(2) A and D
(3) A and B (4) A and E
34. Match List I with List II
List I (Block) List II (General electronic configuration)
A. Most reactive metals I) ns2np6
B. P-block II) ns2(n−1)d1−10
C. Transition metals III) ns1–2
D. Inner transition metals IV) (n–2)f1–14 (n–1) d0 or 1 ns2
(A) (B) (C) (D)
(1) II III IV I
(2) III II III IV
(3) II III I IV
(4) III I II IV
35. Match the following in view of period and the orbitals being filled
List I
List II
A. Second period I) s, f, d
B. Fourth period II) s, f, d, p
C. Sixth period III) s, p
D. First period IV) s V) s, d, p
(A) (B) (C) (D)
(1) iv i iii v (2) iii v ii iv
(3) ii iii iv v (4) iii i ii v
36. Number of unpaired electrons in Gd(Z =64) and the net electrons spin are (1) 7, 3.5 (2) 8, 3 (3) 6, 3 (4) 8, 4
37. The electronic configuration of an element ‘X’, is 1s2 2s2 2p6 3s2 3p3. What is the atomic number of the element which is just below ‘X’ in the periodic table (1) 33 (2) 34 (3) 31 (4) 49
38. Identify the element that has the following electronic configuration 1s22s22p63s23p64s23d104p65s24d105p66s24f2 (1) Ba (2) At (3) Ce (4) Pr
39. In the long form of the periodic table, the valence shell electronic configuration of 5s25p4 corresponds to the element present in:
(1) Group 16 and period 5
(2) Group 17 and period 6
(3) Group 17 and period 5
(4) Group 16 and period 6
Numerical Value Questions
40. The electronic configuration of an element is 1s22s22p6 5s25p3. What is the atomic number of the element?
41. The element with the lowest atomic number that has a ground state electronic configuration of (n−1)d5ns2 is located in___ period
42. The number of electrons in the valency shell of non-metallic liquid element in the periodic table is _____
Electronic configurations and types of elements s,p,d,f blocks
Single Option Correct MCQs
43. The element having electronic configuration (Xe)4f05d16s2 belongs to (1) d-block (2) f-block (3) p-block (4) s-block
List I (Atomic number)
List II
A. 53 I) d-block
B. 55 II) p-block
C. 57 III) f-block
D. 62 IV) s-block
(A) (B) (C) (D)
(1) I II III IV
(2) II IV I III
(3) II I III IV
(4) I III II IV
45. Match List I with List II.
List I (Atomic number)
List II (Block of periodic table)
A. 37 I) p-block
B. 78 II) d-block
C. 52 III) f-block
D. 65 IV) s-block
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) II IV I III
(2) IV III II I
(3) IV II I III
(4) I III IV II
46. The period in which s-block, p-block and d-block elements not present are
(1) 4 (2) 6 (3) 7 (4) 3
47. Match List I with List II.
List I List II
A. s block I) RareEarths
B. d block II) NobleGases
C. f block III) StrongReducing agents
D. p block IV) n-1s2p6d10
V) present from fourth period
The correct match is (A) (B) (C) (D)
(1) II I IV III
(2) V III I II (3) III V I II (4) I V II III
48. Which of the following sets of atomic number belong to that of alkali metals?
(1) 1, 12, 30, 4, 62 1 (2) 37, 19, 3, 55 (3) 9, 17, 35, 53 (4) 12, 20, 56, 88
49. Element with atomic number 56 belongs to which block?
(1) s (2) p (3) d (4) f
50. Representative elements mainly belongs to (1) s- and p-blocks
(2) p- and d-blocks (3) f-block only (4) d- and f-blocks
Numerical Value Questions
51. The number of elements among the following atomic numbers that are p block elements is ______ 83, 79, 42, 64, 37, 54, 34
52. Find out total number of representative elements in the given elements:
Cd, Nb, Ta, Te, Ra, Mo, Po, Pd, Tc.
53. Number of f-electrons present in the electronic configuration of Thallium(Tl) are ______
Periodic trends in properties of elements
Single Option Correct MCQs
54. The correct order of radii is (1) N < Be < B (2) F < O2− < N3− (3) Na < Li < K (4) Fe3+ <Fe2+ <Fe4+
55. Which of the following is correct order of size of the given species?
(1) I > I > I+
(2) I+ > I > I
(3) I > I+ > I (4) I > I > I+
56. Atomic radius depends upon (A) Number of bonds formed by the atom (B) Nature of the boding (C) Oxidation state of the atom
(1) A, B (2) B, C
(3) A, C (4) A, B, C
57. The correct order of van der waal radius of F, Cl, Br is
(1) F > Br > Cl (2) Br > Cl > F
(3) F > Cl > Br (4) Br > F > Cl
58. Atomic radii of fluorine and neon in Angstrom units are respectively given by:
(1) 0.72, 1.60
(2) 1.60, 1.60
(3) 0.72, 0.72
(4) None of these
59. Covalent radius of Cl is 99pm. Select best representation for Cl2 molecule.
60. The electronic configuration with the highest ionization enthalpy is
(1) [Ne]3s23p1
(2) [Ne]3s23p2
(3) [Ne]3s23p3
(4) [Ar]3d104s24p3
61. Second ionization energy is higher than first ionization energy for an element. This is because
(1) Nuclear charge is low in cation
(2) Size of cation is higher than neutral atom
(3) Effective nuclear charge is more for cation
(4) Bond energy changes with charge
62. The I 1 ,I 2 ,I 3 ,I 4 values of an element ‘M’ are 120, 600, 1000, and 8000 kJ/mole respectively then the formula of its sulphate.
(1) MSO4
(2) M2(SO4)3
(3) M2SO4
(4) M3(SO4)2
63. What is the value of electron gain enthalpy of Na+ if IE1 of Na is 5.1 ev ?
(1) –10.2 ev
(2) –5.1 ev
(3) +2.55 ev
(4) +10.2 ev
64. The correct increasing order of ionization enthalpy of He, Li+ and Be+2 is
(1) He < Li+ < Be+2 (2) Li+ < Be+2 < He
(3) Be+2 < Li+ < He (4) Be+2 < He < Li+
65. Electron affinity of oxygen is less than that of sulphur because
(1) Electronegativity of oxygen is more (2) repulsions with incoming electron (3) sulphur is a stronger oxidant (4) Bond dissociation energy of O 2 is less
66. The electron affinity of chlorine is 3.7eV.
1 g of chlorine is completely converted to Cl ion in the gaseous state (1 eV=23.06 k cal mol −1 ). The energy released in the process is
(1) 7.2 K.Cal (2) 4.8 K.Cal
(3) 8.2 K.Cal (4) 2.4 K.Cal
67. The first ionization potential of K is 3.1 eV, the value of electron gain enthalpy of K+ will be
(1) +1.5 eV (2) –1.5 eV (3) –3.1eV (4) –9.3eV
68. Inert gases have positive electron gain enthalpy. Its correct order is
(1) Xe < Kr < Ne < He
(2) He < Ne < Kr < Xe
(3) He < Kr < Xe < Ne
(4) He < Xe < Kr < Ne
69. 1st electron affinity (EA1) is positive for (1) O (2) F
(3) C (4) N
70. Which of the following property increases down the group
(1) Metalic bond strength
(2) Ionization energy
(3) Electropositivity
(4) Electron affinity
71. Which among the following has high electron affinity?
(1) O (2) S
(3) Se (4) Te
72. Which of the following has the lowest electron gain enthalpy?
(1) F (2) Cl
(3) I (4) Br
73. Which group elements have almost zero affinity for electrons?
(1) VIIA (2) VIA
(3) VA (4) VIIIA
74. The element with highest electronegativity is
(1) O (2) F
(3) Cl (4) Br
75. The correct option with respect to the Pauling electronegativity values of the elements is:
(1) Ga > Ge (2) Si < Al
(3) P > S (4) Te > Se
76. Which of the following factors does not affect electronegativity?
(1) Effective nuclear charge
(2) Screening effect of inner electrons
(3) Type of hybridization of the atom
(4) Strength of bond in which it is participated
77. Fluorine has the highest electro negativity among the group on the pauling scale, but the electron affinity of fluorine is less than that of chlorine because.
(1) The atomic number of fluorine is less than that of chlorine.
(2) Fluorine being the first member of the family behaves in an unusual manner.
(3) Chlorine can not accommodate an electron better than fluorine by utilizing its vacant 3d – orbital.
(4) Small size, high electron density and an increased electron repulsion make addition of an electron to fluorine less favourable than that in the case of chlorine.
78. Pair of elements with equal values of electro negativity.
(1) Be, Al
(2) Mg, Al
(3) Mg, Ca
(4) F, Ne
79. EN of Fluorine in Mulliken scale is (1) 4 (2) 1.428 (3) 11.2 (4) 0.7
80. Which of the following is not a measurable quantity?
(1) Ionization potential
(2) Atomic radii
(3) Electron affinity
(4) Electronegativity
81. Which of the following is incorrect?
(1) Acidic oxides:- CO2,SiO2,GeO2
(2) Amphoteric oxides:- SnO 2,PbO2
(3) Neutral oxides:- CO,GeO,PbO
(4) Lewis Acidic strength:- BCl3 > AlCl3 > GaCl3 > InCl3
82. Which of the following is most acidic oxide?
(1) N2O5 (2) SO3
(3) Cl2O7 (4) P4O10
83. Correct increasing order of acidic character is
(1) Al2O3 < MgO < Na2O < Cs2O
(2) Cs2O < Na2O < MgO < Al2O3
(3) Na2O < MgO < Al2O3 < Ca2O (4) Cs2O < Al2O3 < Na2O < MgO
84. Among the following basic oxide is (1) SO3 (2) SiO2 (3) CaO (4) Al2O3
85. All the following are neutral oxides except (1) N2O (2) NO2 (3) NO (4) CO
86. The oxidation number and covalency of sulphur, in molecule are
(1) 0 and 2 (2) +6 and 8 (3) 0 and 8 (4) +6 and 2
87. Which of the following pair is metalloid (1) Na, Al (2) Si, C (3) Ge, As (4) Sb, P
88. The effect of lanthanoid contraction in the lanthanoid series of element by and large means:
(1) Increase in both atomic and ionic radii
(2) Decrease in atomic radii and increase in ionic radii
(3) decrease in both atomic and ionic radii
(4) Increase in atomic radii and decrease in ionic radii
89. Which of the following metals does not show inert pair effect?
(1) Tl (2) Ga (3) ln (4) Al
90. Similarity in the radius of Zr and Hf is explained on the basis of
(1) Lanthanide contraction
(2) Inert pair effect
(3) Same outershell configuration
(4) Anomalous configuration
91. The order of metalic character is
(1) P < Mg < Si < Na
(2) P < Si < Mg < Na
(3) Mg < Na < Si < P
(4) Si < P < Na < Mg
92. Which pair of symbols identifies two elements that are metalloids?
(1) As and Ge
(2) Mg and Si
(3) P and As
(4) Ti and V
93. Lanthanide contraction means:
(1) Contraction of atom of lanthanum element due to poor shielding d-subshell electron
(2) Contraction of atom of lanthanum element due to high shielding of d-subshell electron
(3) Contraction of atom of elements after lanthanum due to poor shielding of f-subshell electron
(4) Contraction of atom of elements before lanthanum due to poor shielding of f-subshell electron
94. A compound contains atoms X, Y and Z. The oxidation number of X is +3, Y is +5 and Z is –2. The possible formula of the compound is
(1) XYZ2 (2) Y2(XZ3)2 (3) X(YZ4) (4) X3(Y4Z)2
95. Among the following which element exhibits highest number of oxidation state in its compounds.
(1) Osmium (2) Nitrogen
(3) Carbon (4) Chlorine
Numerical Value Questions
96. The C-C single bond length is 1.54 A° and that of Cl−Cl is 1.98A°. If the electronegativity of Cl and C are 3.0 and 2.5 respectively, the Cl−Cl bond – length will be equal to ________A°
97. If 1st Ionization enthalpy of K is 419 kJ/mol then K+ + 1e → K. ΔH = −x. x is equal to
98.The five successive ionization enthalpies of an element are 800, 2427, 3658, 5024 and 32824 kJ mol −1 . The number of valence electrons in the element is:
99. I.E. and E.A. of an element are 13 eV and 3.8 eV respectively. The electronegativity of the element on Pauling’s scale is.
100. The values of electronegativity of atom A and B are 1.20 and 4.0 respectively. The percentage of ionic character of A – B bond is nearly
101.How many of the following are acidic oxides?
SO2,CO2,Cl2O7,Al2O3,MgO,
102. How many of the following are metalloids?
Phosphorus , Arsenic, Antimony, Bismuth, Lead, Nitrogen, Carbon
103. How many oxides are Amphoteric in nature CO2, CO, Na2O, Al2O3, PbO, SnO, ZnO,
CHAPTER 4: Classification of Elements
Level II
Genesis of Periodic Classification
Single Option Correct MCQs
1. Which of the following sets of elements follows Newland’s octave rule?
(1) Be, Mg, Ca (2) F, Cl, Br
(3) Na, K, Rb (4) B, Al, Ga
Modern Periodic Law and Present Form of the Periodic Table
Single Option Correct MCQs
2. The atomic weights of ‘Be’ an d ‘In’ were corrected by Mendeleef using the formula
(1) ( ) v aZb=−
(2) 2 nh mvr π =
(3) Atomic weight = Equivalent weight × valency
(4) Equivalent weight = Atomic weight × valency
3. Eka-silicon is now known as (1) Scandium
(2) gallium
(3) germanium
(4) Boron
4. As per the Mendeleef’s periodic table the physical and chemical properties of elements are periodic functions of their (1) atomic mass
(2) atomic number
(3) atomic volume
(4) atomic radii
5. As per the modern periodic law, the physical and chemical properties of elements are periodic functions of their (1) atomic volume
(2) electronic configuration
(3) atomic weight
(4) atomic size
Long Form of Periodic Table
Single Option Correct MCQs
6. In the periodic table, the elements are arranged in the periods following the (1) Hund’s rule of maximum multiplicity (2) Pauli’s exclusion principle (3) Aufbau principle (4) Both (1) and (2)
7. The long form of the periodic table consists of (1) 8 horizontal and 7 vertical series (2) 7 horizontal and 18 vertical series (3) 7 horizontal and 7 vertical series (4) 8 horizontal and 8 vertical series
8. The position of the element with Z = 106 in the periodic table is (1) d-block (2) s-block (3) f-block (4) p-block
9. Atomic number of nitrogen is 7. The atomic number of the third member in the same family is (1) 23 (2) 15 (3) 33 (4) 51
10. Atomic numbers of actinides are (1) 57 to 71 (2) 80 to 103 (3) 58 to 71 (4) 90 to 103
11. Which of elements, with the following atomic numbers belong to the same group? (1) 9, 16, 35, 3 (2) 12, 20, 4, 38 (3) 11, 19, 27, 5 (4) 24, 47, 42, 55
12. The element that belongs to 3rd period and IVA group of the periodic table is (1) silicon (2) carbon (3) germanium (4) tin
13. An element belongs to group 17 with atomic number 17. What is the atomic number of the element belonging to the same group and present in the fifth period? (1) 25 (2) 33 (3) 35 (4) 53
14. The sub-shells filled one by one for 4 th period elements are (1) 3d, 4s, and 4p (2) 4s, 4p, and 4d (3) 4s, 3d, and 4p (4) 3d, 4p, and 4s
Numerical Value Questions
15. According to Moseley’s equation: ( ) aZb ν =− the graph between vs Z ν is given below, the frequency v (in s–1) for atomic number (Z)=52 is _____.
Ο Z 45° 1
Nomenclature of Elements with Atomic number >100
Single Option Correct MCQs
16. As per IUPAC rule, the atomic number for the element unbiunium is:
(1) 120 (2) 121
(3) 112 (4) 122
17. IUPAC official symbol of the element with the symbol Unu is
(1) No (2) Md (3) Lr (4) Fm
Electronic Configuration of elements and Periodic Table
Single Option Correct MCQs
18. Which group and period does an element belong to if the electronic configuration of an element in its – 2 oxidation state is 1s22s22p63s23p6?
(1) Period 3, group 16
(2) Period 3, group 17
(3) Period 4, group 16
(4) Period 4, group 17
19. The 79th electron of an element ‘X’ with an atomic number 79 enters into
(1) s-orbital (2) p-orbital (3) d-orbital (4) f-orbital
Electronic Configuration of elements and Types of elements
Single Option Correct MCQs
20. An element has 18 electrons in the outer most shell. The element is a/an
(1) transition metal
(2) rare earth metal
(3) alkaline earth metal
(4) alkali metal
21. Which of following is not correctly matched?
(1) d-block element: Electronic configuration is ns0–2(n-1)d–10
(2) p-block element: Electronic configuration is ns1–2np1–6
(3) s-block element: Electronic configuration is ns1–2
(4) Cerium(Ce): The first member in the f-block in modern periodic table.
22. The outer most electronic configuration of the most electronegative element is
(1) ns2np3
(2) ns2np6(n-1)d2
(3) ns2np5
(4) ns2np6
23. Two elements ‘X’ and ‘Y’ have the following configuration X=1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 , Y=1s 2 2s 2 3s 2 3p 5 . The compound formed by the combination of ‘X’ and ‘Y’ will be (1) XY2 (2) X 3Y2 (3) X2Y3 (4) XY 5
Numerical Value Questions
24. Find the total number of 6th period elements from the given atomic numbers : 81, 63, 80, 50, 54, 48, 86
25. Number of elements present in shortest period is ___.
26. The VII B group of Mendeleef’s periodic table consists of ____ elements.
27. When 4f level is completely filled with electrons, the next electron will enter into a subshell whose (n+l) is equal to ____.
28. The longest period in which s-block, p-block, d-block, and f-block elements are present is ___.
29. The total number of gaseous elements is ___.
Periodic Trends in Properties of Elements
Single Option Correct MCQs
30. The correct increasing order of the ionic radii is
(1) Cl–<Ca2+<K+<S2–(2) K+<S2–<Ca2+Cl–(3) Ca2+<K+<Cl–<S2–(4) S2–<Cl–<Ca2+<K+
31. The correct order of atomic radii of the elements O, N, S, and P is (1) N < P < S < O (2) N < O < P < S (3) O < N < P < S (4) O < N < S < P
32. Atomic radii of fluorine and neon in angstrom units are respectively (1) 0.72, 1.62 (2) 0.72, 0.72 (3) 1.2, 1.2 (4) 1.62, 0.72
33. Screening by inner electrons will be more effective in (1) Mg (2) K (3) Sr (4) Cs
34. The first ionisation enthalpies of the elements C, N, P, and Si are in the order of (1) C < N < Si < P (2) N < Si < C < P (3) Si < P < C < N (4) P < Si < N < C
35. The correct option for the 1 st ionisation energy (kJ/mol) of Li, Na, K, and Cs, respectively is (1) 496, 520, 419, 374 (2) 374, 419, 496, 520 (3) 520, 496, 419, 374 (4) 374, 419, 520, 496
36. The correct order of increasing first ionisation energy is
(1) Ca < K < Ne < P < F
(2) F < Ca < Ne < P < K
(3) K < Ca < P < F < Ne (4) Ne < F < P < Ca < K
37. Which of the following is the correct order of ionisation energy?
i) Be+ > Be ii) Be > Be+ iii) C > Be iv) B > Be
(1) ii, iii (2) iii, iv (3) i, iii (4) i, ii
38. Which of the order for ionisation energy is correct?
(1) Be<B<C<N<O (2) B<Be<C<O<N (3) Be>B>C>N>O (4) B>Be>C>O>N
39. The five successive ionisation energies of an element ‘X’ are 800, 1427, 2658, 25024, and 32824 kJ mol−1, respectively. The valency of ‘X’ is
(1) 1 (2) 2 (3) 3 (4) 4
40. The first, second, and third ionisation energies are 737, 1045, and 7733 kJ/mol, respectively. The element can be (1) Na (2) B (3) Al (4) Mg
41. The element with highest IP1 in the modern periodic table is (1) Cl (2) He (3) N (4) O
42. The group of elements with highest second ionisation energy is
(1) II A (2) VIII A (3) VII A (4) I A
43. The correct order of electron gain enthalpy is
(1) O > S > Se > Te (2) Te > Se > S > O (3) S > O > Se > Te (4) S > Se > Te > O
44. Which of the following is the correct order of electron affinity?
(1) I > Br > F > Cl (2) F > Cl > Br > l
(3) Br > I > F > Cl (4) Cl > F > Br > l
45. Which of the following has the highest electron gain enthalpy?
(1) [Ne]3s23p3 (2) [Ne]3s23p4
(3) [Ne]3s23p5 (4) [Ne]3s23p2
46. The correct order of electron affinity is (1) Be < B < C < N (2) Be < N < B < C
(3) Be < N < B < C (4) N < C < B < Be
47. Energy is liberated in which of the following processes?
(1) Cl (g)→ Cl+ (g) +e–
(2) HCl(g) → H+ (g) +Cl– (g)
(3) Cl (g) + e– → Cl– (g)
(4) O– (g) + e– → O2– (g)
48. What is the value of electron gain enthalpy of Na+ if IE1 of Na is 5.1 eV?
(1) –10.2 eV (2) –5.1 eV
(3) +2.55 eV (4) + 10.2 eV
49. 3.55 g of Cl (g) liberates Q kJ of heat by gaining electrons. Electron gain enthalpy of chlorine is ______ kJ/mol.
(1) –5 Q (2) –10 Q
(3) –20 Q (4) –2.5 Q
50. The electronegativity of the following elements increases in the order
(1) S < P < N < O (2) P < S < N < O
(3) N < O < P < S (4) N < P < S < O
51. Which list includes elements in order of increasing metallic character?
(1) Si, P, S (2) As, P, N
(3) Al, Ge, Sb (4) Br, Se, As
52. Maximum valency with respect to hydrogen shown by a third period element is (1) 3 (2) 4
(3) 7 (4) 6
53. Among a) Na2O, b) MgO, c) Al2O3 , d) P2O5 , and e) Cl2O7 the most basic, most acidic,
56 and amphoteric oxides, respectively, are (1) a, b, c (2) b, e, c (3) a, e, c (4) e, c, a
54. Among Al 2 O 3 , SiO 2 , P 2 O 3 , and SO 2 , the correct order of acid strength is (1) SO2< P2O3< SiO2< Al2O3 (2) SiO2 < SO2< Al2O3 < P2O3 (3) Al2O3 < SiO2< SO2< P2O3 (4) Al2O3< SiO2< P2O3< SO2
55. In which pair of oxides, both are acidic in nature?
(1) CaO, SiO2 (2) B2O3, SiO2 (3) B2O3, CaO (4) N2O, BaO
56. The diagonal relationship phenomenon is not observed after
(1) I A group (2) II A group (3) III A group (4) IV A group
57. Diagonal relationship is present between the lighter elements of ____ and ___periods.
(1) second, third (2) second, fourth (3) third, fourth (4) third, fifth
58. The polarising power of which of the following pairs is similar?
(1) Li, Mg (2) Li+, Mg2+ (3) Li2+, Mg2+ (4) Li+, Mg+
Numerical Value Questions
59. The IP1, IP2, IP3, IP4 , and IP5 of an element are 7.1, 14.3, 103.4, 166.8, and 262.2 eV, respectively. The element belongs to ___ group.
60. The element with highest electron affinity belongs to period number _____.
61. The period with maximum electronegativity belongs to _____ .
CHAPTER 4: Classification of Elements
Multiple Concept Questions
Single Option Correct MCQs
62. Which of the following is an incorrect set?
Group Period
(1) Z = 38 IIA 5th
(2) Z =58 IIIB 6th
(3) Z = 25 VIIB 4th
(4) Z = 22 VIB 4th
63. The element with atomic number 56 is likely to have the same outer shell configuration as the element with atomic number .
(1) 12 (2) 19
(3) 14 (4) 22
64. The electronic configuration of seventh element in lanthnoid series is (1) [Xe]4f35d56s2 (2) [Xe]4f75d26s1 (3) [Xe]4f75d16s2 (4) [Xe]4f35d66s2
65. The element having electronic configuration [Xe]4f145d06s2 belongs to (1) d-block, 12th group (2) f-block, 3rd group (3) s-block, 2nd group (4) f-block, 12th group
66. Atomic radius depends upon i) number of bonds formed by the atom ii) nature of the bonding iii) oxidation state of the atom
(1) i and ii (2) ii and iii
(3) i and iii (4) i, ii, and iii
67. The pair that has similar atomic radii is ? (1) Sc and Ni (2) Ti and Hf (3) Mo and W (4) Mn and Rc
68. A sudden jump between the values of second and third ionisation energies of an element is associated with configuration (1) 1s22s22p63s1 (2) 1s22s22p63s23p1 (3) 1s22s22p63s23p2 (4) 1s22s22p63s2
69. Which of the following elements have electron affinity greater than fluorine?
(1) O (2) S (3) Se (4) Cl
70. The correct order of acidic strength is (1) K2O>CaO>MgO (2) CO2>N2O5>SO3 (3) Na2O>MgO>Al2O3 (4) Cl2O7>SO2>P4O10
71. Amphoteric oxide combinations are in (1) ZnO, K2O, SO3 (2) ZnO, P2O5, Cl2O7 (3) SnO2, Al2O3, ZnO (4) PbO2, SnO2, SO3
Numerical Value Questions
72. From the given compounds, number of compounds that give acidic solution when dissolved in water is __.
CaO, SO 2, SO 3, Fe 2O 3, Cl 2O 7, CO 2, NaO, NO2
73. An element ‘X’ belong to 4th period and 4th group in the modern periodic table. Then, the atomic number of ‘X’ is ___.
74. How many of the following exist with +2 as the most common oxidation state?
Al, Si, Pb, B, Zn, Ca, Ba, Cr, Sc, and N
75. Common oxidation state of inner transition elements is ___.
76. Atomic number of fifth member of noble gas is ____.
77. The number of elements present in the group-3 in the modern periodic table is ____.
78. CO2, N2O, BaO, Cl2O7, Al2O3, N2O3, NO, CaO in above oxides, the number of amphoteric oxides ____.
79. In a period, the element with largest atomic volume belongs to which of the groups
Level III
Single Option Correct MCQs
1. Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set up of the long form of the periodic table?
(1) 107 (2) 118
(3) 126 (4) 102
2. The period number in the long form of the periodic table is equal to
(1) magnetic quantum number of any element of the period
(2) atomic number of any element of the period
(3) maximum principal quantum number of any element of the period
(4) maximum azimuthal quantum number of any element of the period
3. What is the position of the element in the periodic table satisfying the electronic configuration (n-1)d1ns2 for n=4?
(1) 3rd period and 3rd group
(2) 4th period and 4th group
(3) 3rd period and 2nd group
(4) 4th period and 3rd group
4. Choose the statement that is not correct for periodic classification of elements is:
(1) The properties of elements are periodic function of their atomic numbers.
(2) Non-metallic elements are Iess in number than metallic elements.
(3) For transition elements, the 3d-orbitals are filled with electrons after 3p-orbitals and before 4s-orbitals.
(4) The first ionisation enthalpies of elements generally increase with increase in atomic number as we go along a period.
5. The first ionisation potentials of Na, Mg, and Si, respectively, are 496, 737, and 786 kJ/mol. The first ionisation potential of ‘Al’ in kJ/mol is
(1) 577 (2) 487
(3) 856 (4) 768
6. Consider the following changes:
A(g)→A+(g) + e– is E1 and A+(g)→A2+(g)+e– is E2
The energy required to pull out the two electrons are E 1 and E 2, respectively. The correct relationship between two energies would be
(1) E1<E2 (2) E1>E2
(3) E1=E2 (4) E1≥ E2
7. Elements X, Y, and Z have atomic numbers 19, 37, and 55, respectively. Which of the following statements is true about them?
(1) Their ionisation potential would increase with increasing atomic number.
(2) Y would have an ionisation potential between those of X and Z.
(3) Z would have the highest ionisation potential.
(4) Y would have the highest ionisation potential.
8. Choose the correct statement about an element having electronic configuration 1s22s22p63s23p63d54s2
(1) It belongs to s-block.
(2) Its highest oxidation state is +7 in its compounds.
(3) It is a non-metal.
(4) It belongs to 3 rd period of modern periodic table.
9. Ionisation energy values of an atom are 495, 767, 1250, and 4540 kJ mol-1. The formula of its sulphate is (1) MSO4 (2) M2SO4 (3) M2(SO4)3 (4) M(SO4)2
10. Which of the following orders is not correct?
(1) IE(I) of Be > IE(I) of B but IE(II) of Be < IE(II) of B
(2) IE(I) of Be < IE(I) of B but IE(II) of Be < IE(II) of B
(3) IE(II) of O > IE(II) of N
(4) IE(I) of Mg > IE(I) of Al
11. Screening effect influences
A) atomic radius
B) ionisation enthalpy
C) electron gain enthalpy
(1) A, B only (2) B, C only
(3) A, C only (4) A, B, and C
12. Which of the following is incorrect?
(1) Cesium is the most electropositive element while F is the most electronegative element.
(2) Chlorine has the highest –ve electron gain enthalpy of all the elements.
(3) Electron gain enthalpy of N as well as that of noble gases is positive.
(4) In any period, the atomic radius of the noble gas is lowest.
13. Electronic configuration of four elements
A, B, C, and D are given below:
(A) 1s22s22p6 (B) 1s22s22p4
(C) 1s22s22p63s1 (D) 1s22s22p5
Which of the following is the correct order of increasing tendency to gain electron?
(1) A < C < B < D (2) A < B < C < D
(3) D < B < C < A (4) D < A < B < C
14. Fluorine has the highest electronegativity among the ns 2np 5 group on the Pauling scale, but the electron affinity of fluorine is less than that of chlorine because
(1) the atomic number of fluorine is less than that of chlorine
(2) fluorine, being the first member of the family, behaves in an unusual manner.
(3) fluorine can accommodate an electron better than chlorine by utilising its vacant 3d–orbital
(4) small size, high electron density and an increased electron repulsion makes addition of an electron to fluorine less favourable than in the case of chlorine in isolated state
15. A, B, and C are hydroxyl compounds of the elements X, Y, and Z, respectively. X, Y and Z, are in the same period of the periodic table. A gives an aqueous solution of pH less than seven. B reacts with both strong acids and strong alkalies. C gives an aqueous solution which is strongly alkaline.
Which of the following statements is/are true?
I: The three elements are metals.
II: The electronegativity decreases from X to Y to Z.
III: The atomic radius decreases in the order X, Y, and Z.
IV: X, Y, and Z could be phosphorus, aluminium, and sodium, respectively.
(1) I, II, and III are correct.
(2) I and III are correct.
(3) II and IV are correct.
(4) II, III, and IV are correct.
16. Which of the following statements are not correct?
1) The electron gain enthalpy of ‘F’ is more negative than that of ‘Cl’
2) Ionisation enthalpy decreases in a group of periodic table.
3) The electronegativity of an atom depends upon the atoms bonded to it.
4) Al 2 O 3 and NO are examples of amphoteric oxides.
(1) 1, 3, and 4
(2) 1, 2, and 3
(3) 3 only
(4) 1, 2, 3, and 4
17. Which of the following is not true about Pauling scale electronegati vity
(1) ( ) 0.1017/ AB XXkJmol −=∆
(2) 2.8 mulliken pauling X X =
(3) reference element for determination of EN is hydrogen
(4) ( ) 0.208/ AB XXkJmol −=∆
18. Percentage of ionic character in the covalent bond A-B is (given XA= 2, XB= 3)
(1) 12.5% (2) 30%
(3) 19.5% (4) data is insufficient
19. In which of the following arrangements the order is not according to the property indicated against it?
(1) Al3+<Mg2+<Na+<F– increasing ionic size
(2) B<C<O<N increasing first ionisation energy
(3) I<Br<F<Cl increasing electronegativity
(4) Li<Ca<Al<Si valency with respect to hydrogen
20. Which of the following statements is incorrect?
(1) H+ is the smallest cation in the periodic table.
(2) van der Waals radius of chlorine is more than its covalent radius.
(3) Ionic mobility of hydrated Li+ is greater than that of hydrated Na +
(4) He has the highest ionisation enthalpy in the periodic table.
21. Consider the following points:
(a) Cs is the strongest reducing agent among IA group elements (in aqueous solution).
(b) Be(OH)2 is amphoteric.
(c) The density of potassium is less than sodium.
(d) Among the alkali metals Li, Na, K, and Rb, lithium has the maximum value of melting point.
Choose the correct statements
(1) (a), and (b) are correct
(2) (a), (b), and (c) are correct
(3) (b), and (c) are correct
(4) (b), (c), and (d) are correct
22. Which of the following statements about 4BF and 3 6AlF are correct?
(a) B and Al differ in their oxidation states.
(b) B, Al differ in their covalency.
(c) B obeys octet rule.
(d) B and Al are in diagonal relationship.
(1) a, b (2) b, c, d
(3) a, b, c (4) b, c
23. Which of the following properties is true regarding diagonal relationship between Li and Mg?
(1) Li and Mg salts do not impart colour in oxidising flame.
(2) Li or Mg nitrate liberates only one paramagnetic gas on heating.
(3) Their bicarbonates are highly stable.
(4) Their carbonates decompose on heating to form a gas whose molecule has net zero dipole moment.
Numerical Value Questions
24. Calculate the electronegativity of fluorine from the following data. Express your answer to the nearest possible integer.
(E = bond dissociation energy and X= electronegativity)
EH–H=104.2 k cal/mol
EF–F=36.6 k cal/mol
EH–F=13.4 k cal/mol
XH=2.1
25. How many of the following factors influence the electronegativity of the atom of an element?
i) Effective nuclear charge
ii) Screening effect of inner electrons
iii) Size of the atom.
iv) Oxidation state of the atom in bond
v) Type of hybridisation of the atom
vi) Strength of bond in which it is participated
vii) Number of surrounding atoms on the other atom with which it is bonded
viii) Nature of bond
26. How many of the following orders/ statements are correct?
a) IP1of nitrogen is not less than that of oxygen.
CHAPTER 4: Classification of Elements
b) IP1 of Pb is less than that of Sn.
c) Electronegativity order.
d) IP2 order B > C > Be.
e) Electron gain enthalpy order Ne > Ar > Kr > Xe > He > Rn.
f) Electron affinity of Be+4 is 217.6 eV/ion nearly.
g) Electronegativities of K and Rb are same
h) Order of sizes Tl > In > Al > Ga > B
i) Official IUPAC name of element with atomic number 104 is Unnilquadium
27. A metal has electronic configuration [Ar]183d74s2. On the basis of this electronic configuration, find out the group number of metal.
28. The ionisation energy and electron affinity of an element are 12.0 eV and 3.8 eV, respectively. Its electron negativity on Milliken scale is __.
29. Among the following species, how many have their ionic size greater than O 2–?
Se2–, F–, N3–, P3–
THEORY-BASED QUESTIONS
Statement Type Questions
Each question has two statements. Statement I and statement II. In light of the given statements, choose the most appropriate answer from the options given below:
(1) if both statement I and statement II are correct.
(2) if both statement I and statement II are incorrect.
(3) if statement I correct but statement II is incorrect.
(4) if statement I is incorrect but statement II is correct.
1. S-I : 5th period of the long form periodic table is the longest period.
S-II : The longest period of the periodic table includes 32 elements.
2. S-I : van der Waals’ radius of an element is always larger than its c ovalent radius.
S-II : Two molecules can never be closer than two atoms in a molecule.
3. S-I : In Cl2 molecule, the covalent radius is double the atomic radius of chlorine.
S-II : Radius of anionic species is always greater than their parent atomic radius.
4. S-I : Ionisation enthalpy difference from B to Al is more than that of Al to Ga.
S-II : Ga has completely filled d-orbital.
5. S-I : Electron gain enthalpy becomes less negative as we go down the group.
S-II : Size of atom increases on going down the group and the addition of electron would be farther from the nucleus.
6. S-I : Li and Mg show similar chemical properties.
S-II : Li + and Mg 2+ have nearly same polarising power.
7. S-I : For transition elements, on moving from left to right in a transition series, ionisation energy increases.
S-II : As the atomic number increases, the effective nuclear charge also increases.
8. S-I : Metallic or electropositive character of elements increases as the value of ionisation potential decreases.
S-II : In a group, moving from top to bottom, metallic or electropositive character increases.
9. S-I : The first IP of nitrogen is greater than oxygen, while the reverse is true for the second IP values.
S-II : Oxygen unipositive ion has a halffilled electronic configuration.
Assertion and Reason Type Questions
In each of the following questions, a statement of Assertion (A) is given, followed by a corresponding statement of
Reason (R). Mark the correct answer as
(1) if both (A) and (R) are true and (R) is the correct explanation of (A)
(2) if both (A) and (R) are true but (R) is not the correct explanation of (A)
(3) if (A) is true but (R) is false
(4) if both (A) and (R) are false
10. (A) : According to modern periodic law, the physical and chemical properties of elements are periodic functions of their atomic numbers.
(R) : Atomic number is equal to the number of electrons present in any species (atom or ion).
11. (A) : ‘He’ and ‘Be’ both have the same outer electronic configuration, like ns2 type.
(R) : Both are chemically inert.
12. (A) : Mg2+ and Al3+ are isoelectronic but ionic radius of Al3+ is less than that of Mg2+
(R) : The effective nuclear charge on the outer shell electrons in Al3+is more than that in Mg2+
13. (A) : The first ionisation enthalpy of 3d series elements is more than that of group 2 metals.
(R) : In 3d series of elements, successive filling of d-orbitals takes place.
14. (A) : The first ionisation enthalpy for oxygen is lower than that of nitrogen.
(R) : The four electrons in 2p orbitals of oxygen experience more electron–electron repulsion due to small size.
15. (A) : The first ionisation enthalpy for oxygen is lower than that of nitrogen.
(R) : The four electrons in 2p orbitals of oxygen experience more electron–electron repulsion.
16. (A) : Helium has the highest value of ionisation energy among all the elements known.
(R) : Helium has the highest value of electron affinity among all the elements known.
17. (A) : The first electron gain enthalpies for all the elements is exothermic.
(R) : Electron gain enthalpy is the amount of energy accompanied when an electron is added to neutral isolated atom.
18. (A) : Lithium, having maximum negative E° (SRP) value, is the strongest reducing agent among all alkali metals in solution.
(R) : Lithium is the lightest metal in the periodic table.
19. (A) : Both Be and Al exhibit similar properties in their compounds.
(R) : Due to almost the same polarising power of cations, some pairs of elements, like Be and Al, are diagonally related in their properties of compounds.
20. (A) : Tl, in its +1 oxidation state, is more stable than its +3 oxidation state.
(R) : Tl exhibits inert pair effect.
21. (A) : The decreasing order of acidic character of CO2, N2O5, SiO2, and is SO3>N2O5>CO2>SiO2
(R) : As the electronegativity difference between the central atom and oxygen decreases, the acidic character of the oxide increases.
JEE ADVANCED LEVEL
Multiple Option Correct MCQs
1. The electron affinity of nitrogen is very low, which can be attributed to
(1) ‘N’ atom has stable filled 2p 3 configuration
(2) addition of electron to 2p orbital increase spin paired repulsion
(3) energy released due to attraction between the nucleus and incoming electron is offset by the inter-electron repulsion by pairing
(4) due to small size, inter-electron repulsions are more than the other elements in the group
2. Consider the following ionisation steps: ( ) ( ) ;100; gg MMeHeV +− →+∆=+
( ) ( ) 2 2;250 gg MMeHeV +− →+∆=+
select correct statement(s):
(1) IE1 of M(g) is 100 eV
(2) IE1 of M+ (g) is 150 eV
(3) IE2 of M (g) is 250 eV
(4) IE2 of M (g) is 150 eV
3. The correct order of radii is (1) Mg+2<Li+2<N–3 (2) Li<Na<K
(3) Fe+2<Fe+3<Fe+4 (4) Be>B>N
4. Which of the following statements is/are incorrect?
(1) IE 2 of group VA<IE 2 of group VI elements.
(2) IE2 of group IA<IE2 of group II elements.
(3) IE2 of group VIA<IE2 of group VII A elements.
(4) IE2 of 4d series <IE1 of 5d series.
5. Which of the following have a diagonal relationship?
(1) Li→Mg (2) B→Mg (3) Be→Al (4) Be→Na
6. In which of the following arrangements, the order is not correct according to the property indicated against it?
(1) Increasing size: Cu2+<Cu+<Cu
(2) Increasing IE1: B<C<N<O
(3) Increasing IE1: Na<Al<Mg<Si
(4) Increasing IE1: Li<Na<K<Rb
7. Which of the following orders of atomic / ionic radius is/are correct?
(1) I– > I >I+ (2) Mg2+>Na+>F–
(3) P5+<P3+ (4) Li>Be>B
8. How many of the following are endothermic?
(1) ( ) ( ) gg MM → (2) ( ) ( ) gg MM + → (3) ( ) ( ) 2 gg MM++ → (4) ( ) ( ) 23 gg MM++ →
9. Generally, the ionisation potential in a period increases but there are some exceptions. Such as (1) Be and B (2) N and O (3) Mg and Al (4) Na and Mg
10. Select the correct statement(s)
(1) Sulphur has a lower electron affinity than chlorine.
(2) Iodine has a lower electron affinity than bromine.
(3) Boron has a lower first ionisation energy than Be.
(4) Sulphur has a lower first ionisation energy than phosphorus.
11. Which of the following sequences is/are incorrect with respect to the property indicated?
(1) Oxidising power: F2<Cl2>Br2
(2) Bond energy: F2>Cl2>Br2
(3) Electronegativity: F>Cl>Br
(4) Electron affinity: F>Cl>Br
12. If 0 2 N atoms of X(g) are converted into X+(g) by absorbing energy E1, 0 2 N atoms of X are converted to X–, and E2 energy is released, then,
(1) ionisation potential of X would be 1 0 2 E N
(2) ionisation potential of X would be 2E 1
(3) electron affinity of X would be 2 2 0 E N
(4) electron affinity of X would be 2E 2
13. Consider three elements with the following abbreviated electronic configurations: X=[Ar]4s2; Y=[Ne]3s23p4; Z=[Ar]3d104s24p4
Then, which of the following statements is/are correct?
(1) X is metal; Y is non-metal.
(2) The element having highest atomic size is X.
(3) The element having highest ionisation energy is Z.
(4) The element having highest electron affinity is Y.
14. The covalent radius of an element depends on
(1) number of bonds formed between atoms of that element
(2) type of hybridisation involved by its atoms in the covalent bond formation
(3) ionic character of the covalent bond formed by its atom with the atoms of other element
(4) oxidation state of the atom in its covalent compounds
15. Ionisation energy of atoms A and B are 350 and 250 kcal/mol, respectively. If the electron affinity of these atoms are 70 kcal/ mol and 90 kcal/mol, respectively, then
(1) electron cloud is more attracted by A
(2) electron cloud is more attracted by B
(3) electronegativity of A is more than B
(4) electronegativity of A is less than B
16. Which of the following is/are true order(s)?
(1) B+<B<B– → size
(2) I<Br<Cl<F→ electron affinity
(3) O2–<O–<O+→Zeffective
(4) Na<Al<Mg<Si→ ionisation potential
17. The first( D r H 1 ) and the second ( D r H 2 ) ionisation enthalpies (in kJ mol–1), and the ( D egH) electron gain enthalpy (in kJmol –1) of a few elements are given below:
Elements D r H1 D r H1 D eg H
I 520 7300 –60
II 419 3051 –48
III 1681 3374 –328
IV 1008 1846 –295
V 2372 5251 +48
VI 738 1451 –40
Choose the correct option(s):
(1) The most reactive metal is II.
(2) III and IV are both non-metals, III being the most reactive non-metal.
(3) VI is a metal, and it can form a stable binary halide of the formula MX (X=halogen).
(4) V is the most unreactive element.
Numerical Value Questions
18. The electronic configuration of thorium is [Rn]5fx6dy7sz. What is the value of y+z–2x?
19. IP1 and IP2 of Mg are 178 and 348 kcal mole-1 , The energy required for the reaction. Mg → Mg2++2e– is ____ kcal.
20. The amount of energy released when 10 6 atoms of iodine in vapour state are converted to I – ions is 4.9 ×10 –13 J. The electron affinity of iodine in eV per atom is___.
21. Among the following, the total number of orders that are correct with respect to the property indicated against each is
(i) Mg>Al>Si>P: covalent radius
(ii) Na+<O2-<F-<N3-: ionic size
(iii) Al3+<Mg2+<Li+<K+: ionic size
(iv) C<Si>P>N: electron affinity value
(v) N<C<O<F: electron affinity value
(vi) F>Cl>Br>I: electron affinity value
(vii) Si>Mg>Al>Na: first ionisation energy
(viii) O>F>N>C: second ionisation energy
(ix) N>P>Sb>As: third ionisation energy
CHAPTER 4: Classification of Elements
22. The number of species having higher first ionisation energy than Ca from the following is ___.
Ge, Ga, Br, Se, Kr, As, K
23. The number of pairs, in which electron affinity of the second element is more than that of the first element is ____.
(F, Cl) (C, N) (O, N) (F, Ne) (B, C), (O, S)
24. If the atomic radius of non-metal bromine is 1.14 Ao , its covalent radius is ____ A o .
25. Zeff for the last electron in carbon atom is __________.
26. Number of possible ionisation potential values for C-atom is ____.
Integer Value Questions
27. Covalent bond length of chlorine molecule is 2 Ao. The covalent radius of chlorine is __ Ao
28. The first four ionisation energy values of an element are 191, 578, 872, and 8982 kcal. The number of chlorine atoms per molecule of the compound formed by it is ___.
29. Ionisation potential and electron affinity of an element are 17.92 eV and 4.48 eV, respectively. The electronegativity of the element is ___.
30. Common oxidation state of d-block elements is +x. The value of x is ____.
31. In how many pairs, first species has lower ionisation energy than second species?
(i) N and O
(ii) Br and K
(iii) Be and B
(iv) I and I–
(v) Li and Li+
(vi) O and S
(vii) Ba and Sr
32. How many of the following elements have positive or zero electron gain enthalpy values?
Be, Mg, B, N, He, Ne, O, P
33. The bond energies of H–H; X–X, and H–X are 104 kcal, 38 kcal, and 138 kcal, respectively. The electronegativity of X i s
Y. Then, find Y 3.8. Given: ( ) 67=8.18
Passage-based Question
(Q: 34-35)
In the modern periodic table, the elements are placed in order of increasing atomic number. There have been numerous designs of the table over the years but the most common is the long form of periodic table. The long form of periodic table shows all the elements in numerical order.
34. The atomic number of second element in period-3 is
(1) 10 (2) 20 (3) 12 (4) 13
35. The number of d-electrons in copper atom is
(1) 10 (2) 12 (3) 9 (4) 4
(Q: 36-38)
In the modern periodic table, the elements are placed in order to increasing atomic number. There have been numerous designs of the table over the years but the most common is the long form of periodic table. The long form of periodic table shows all the elements in numerical order.
36. What is the atomic number of the (as yet undiscovered) alkali earth metal after radium?
(1) 120 (2) 121 (3) 124 (4) 118
37. In which of the following inert gases electrons are occupying in 4f-orbitals but
no electron in 6d-orbitals in ground state electronic configuration?
(1) Kr (2) Xe (3) Rn (4) Uuo
38. Total number of S-block elements in the periodic table is ___ (1) 14 (2) 12 (3) 13 (4) 18
(Q: 39-40)
Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option.
Ionisation energies of three hypothetical elements are given below (in kJ/mole): I II III X 122 340 1890 Y 99 931 1100 Z 118 1220 1652
39. What is the value (magnitude only) of ( ) ( ) ( ) 2 gg ZeZkJ +−+ +→ ?
40. Energy (in kJ/mol) required for the process ( ) ( ) 2 2 gg ZZe →++− will be _____.
(Q: 41-42)
The tendency of the atom of an element to attract the shared electron pairs, more towards itself in a hetero nuclear diatomic molecule or in a polar covalent bond is called electronegativity. Pauling calculated the electronegativities of different elements from the bond energies using the equation:
0.208/ AB xxkcalmol −=∆
41. Calculate the electronegativity of chlorine from the bond energies of ClF(66 kcal/mol), F2(40 kcal/mol), and Cl2(62.5 kcal/mol).
42. From the bond energies of HF, H 2, and F2 are 135, 100, and 36 kcal/mol, respectively. Calculate the extra ion resonance energy in the HF molecule.
Matrix Matching Questions
43. Match the Column I (element) with Column II (group to which the element belongs)
Column I (Element) Column II (Group to which element belongs to)
A. N I) VI A group
B. F II) VA group
C. O III) VII A group
D. C IV) IV A group
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) II III IV I
(2) II III I IV
(3) III II I IV
(4) I `II III IV
44. Match Column I (electronic configuration) with Column II (element)
Column I (Electronic configuration) Column II (Element)
A. 1s2,2s2,2p2,3s2,3p6,4s1 I) d-block element
B. 1s2,2s2,2p2,3s2,3p6 II) Halogen
C. 1s2,2s2,2p2,3s2,3p6, 3d6,4s2 III) Alkali metal
D. 1s2,2s2,2p5 IV) Noble gas
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) I II III IV
(2) III IV I II
(3) I III II IV
(4) II IV III I
45. Match the Column I(atomic number) with Column II(period).
Column I (Atomic number)
Column II (Period)
A. 31 I) 5
B. 50 II) 3
C. 56 III) 4
D. 14 IV) 6
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) I II III IV
(2) II I IV II
(3) III IV I II
(4) III I IV II
46. Match Column I(element) with Column II (atomic radii).
Column I (Element)
Column II (Atomic radii in pm)
A. O I) 88
B. C II) 74
C. B III) 66
D. N IV) 77
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) IV III II I
(2) I IV III II
(3) III IV I II
(4) II I IV III
47. Match the Column I(Oxide) with Column II (Nature)
Column I (Oxide)
Column II (Nature)
A. MgO I) Acidic
B. BeO II) Neutral
C. Cl2O7 III) Basic
D. CO IV) Amphoteric
Choose the correct answer from the options given below:
(A) (B) (C) (D)
(1) II IV I III
(2) II III I IV
(3) II III IV I
(4) III IV I II
48. Match the List I (oxide) with List II (nature)
List I (Oxide) List II (Nature)
A. N2O5 I) Amphoteric
B. BaO II) Basic
C. BeO III) Neutral
D. NO IV) Acidic
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) IV III I II
(2) IV II I III
(3) II IV III I
(4) I II III IV
49. Match Column I (Element) with Column II (Characteristic property)
Column I (Element) Column II (Characteristic property)
A. Li I) Forms acidic oxide
B. P II) Has diagonal relation with Mg
C. Be III) Has highest electronegativity among all elements and exhibits only one oxidation state
D. F IV) Electronegativity is equal to Al
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) II I IV III
(2) II III I IV
(3) III I II IV
(4) II I IV III
FLASH BACK (Previous JEE Questions)
JEE Main
1. Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R: (2024)
Assertion (A) : The first ionisation enthalpy decreases across a period.
Reason (R) : The increasing nuclear charge outweighs the shielding across the period.
In the light of the above statements, choose the most appropriate from the options given below:
(1) Both A and R are true and R is the correct explanation of A
(2) A is true but R is false
(3) A is false but R is true
(4) Both A and R are true but R is NOT the correct explanation of A
2. The element having the highest first ionization enthalpy is (2024)
(1) Si (2) Al
(3) N (4) C
3. Given below are two statements:
Statement I : Fluorine has most negative electron gain enthalpy in its group.
Statement II : Oxygen has least negative electron gain enthalpy in its group.
In the light of the above statements, choose the most appropriate from the options given below.
(1) Both Statement I and Statement II are true
(2) Statement I is true but Statement II is false
(3) Both Statement I and Statement II are false
(4) Statement I is false but Statement II is true
4. Given below are two statements: (2024)
Statement I : Along the period, the chemical reactivity of the element gradually increases from group 1 to group 18.
Statement II : The nature of oxides formed by group 1 element is basic while that of group 17 elements is acidic.
In the the light above statements, choose the most appropriate from the questions given below:
(1) Both Statement I and Statement II are true
(2) Statement I is true but Statement II is false
(3) Statement I is false but Statement II is true
(4) Both Statement I and Statement II are false
5. The first ionisation enthalpies of Be, B, N, and O follow the order (2022)
(1) O < N < B < Be (2) O < N < B < Be
(3) B < Be < N < O (4) B < Be < O < N
6. The IUPAC nomenclature of an element with electronic configuration [Rn]5f146d17s2 is (2022)
(1) unnilbium
(2) unnilunium
(3) unnilquadium
(4) unniltrium
7. Given below are two statements
Statement I : In Cl2 molecule the covalent radius is double the atomic radius of chlorine.
Statement II : Radius of anionic species is always greater than their parent atomic radius.
In light of the above statements, choose the most appropriate answer from the options given below:
(2022)
(1) Both statement I and statement II are correct.
(2) Both statement I and statement II are incorrect.
(3) Statement I is incorrect but statement II is correct.
(4) Statement I is correct but statement II is incorrect.
8. The electronic configuration of Pt (atomic number 78) is
(1) [Xe]4f145d96s1
(2) [Kr]4f145d10
(3) [Xe]4f145d10
(4) [Xe]4f145d86s2
9. Give below are two statements.
One is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : The ionic radii of O2- and Mg2+ are same.
Reason (R) : Both are isoelectronic species.
In light of the above statements, choose the correct answer from the options given below.
(2022)
(1) Both (A) and (R) are true and ( R) is the correct explanation of (A).
(2) Both (A) and (R) are true but ( R) is not the correct explanation of (A).
(3) (A) is true but (R) is false.
(4) (A) is false but (R) is true
10. Which of the following statements are not correct?
A. The electron gain enthalpy of F is more negative than that of Cl.
B. Ionisation enthalpy decreases in a group of periodic table.
C. The electronegativity of an atom depends upon the atoms bonded to it.
D. Al 2 O 3 and NO are examples of amphoteric oxides.
Choose the most appropriate answer from the options given below. (2022)
(1) A, B, C, and D
(2) A, C, and D only
(3) A, B, and D only
(4) B and D only
11. Given below are two statements:
Statement I: The decrease in first ionisation enthalpy from B to Al is much larger than that from Al to Ga.
Statement II : The d orbitals in Ga are completely filled. (2022)
In light of the above statements, choose the most appropriate answers from the options given below.
(1) Statement I is incorrect but statement II is correct.
(2) Both the statements I and II are incorrect.
(3) Both the statements I and II are correct.
(4) Statement I is correct but statement II is incorrect .
CHAPTER TEST – JEE MAIN
Section - A
Single Option Correct MCQ's
1. The triad not present in group VIII of Mendeleef table is
(1) Li, Na, K (2) Fe, Co, Ni (3) Ru, Rh, Pd (4) Os, Ir, Pt
2. The IUPAC symbol for the element with atomic number 119 would be (1) Unh (2) Uue (3) Uun (4) Une
3. The number of elements present in 2 nd , 3rd, 4th, and 5th periods of modern periodic table, respectively are
(1) 2, 8, 8, and 18 (2) 8, 8, 18, and 32
(3) 8, 8, 18, and 18 (4) 8, 18, 18, and 32
4. Following are some statements about modern periodic table. Pick the correct statement(s).
i) It consists of s, p, d, and f- blocks.
ii) The energy levels filling order in 6th period is 6s, 4f, 5d, and 6p.
iii) IIIA group contains maximum number of elements.
(1) i and ii (2) only i (3) ii and iii (4) i, ii, and iii
5. Which one of the following is correct order of the size?
(1) I–>I>I+ (2) I>I–>I+ (3) I>I+>I– (4) I+>I–>I
6. In which of the following sets, elements have nearly same atomic radii?
(1) Li, Be, B (2) Mg, Ca, Sr (3) Fe, Co, Ni (4) O, S, Se
7. The lanthanoid contraction is responsible for the fact that
(1) Zr and Y have about the same radius
(2) Zr and Nb have similar oxidation state
(3) Zr and Hf have about the same radius
(4) Zr and Zn have same oxidation state
8. Consider the following ionisation reaction: I.E (kJ/mol) I.E (kJ/mol) A(g)→A+(g)+e–,A1 B(g)→B+(g)+e–,B1 B+(g)→B+2(g)+e–,B2 C(g)→C+(g)+e–,C1 C+(g)→C+2(g)+e–,C2 C+2(g)→C+3(g)+e–,C3
If uni-positive ion of A, di-positive ion of B
and tri-positive ion of C have zero electron. Then, incorrect order of corresponding IE is
(1) C3>B2>A1 (2) B1<A1>C1
(3) C3<C2>B2 (4) B2<C2>A1
9. In which of the following arrangement the order is incorrect according to the property indicated against it?
(1) Al+3<Mg2+<Na+<F– = increasing ionic size
(2) B<C<N<O= increasing first ionisation enthalpy
(3) I<Br<F<Cl= increasing electron gain enthalpy (with negative sign)
(4) Li<Na<K<Rb= increasing metallic radius
10. Which one of the following arrangements of the incorrect representation of the property indicated with it?
(1) Br<Cl<F: electronegativity
(2) F<Br<Cl: electron affinity
(3) F2<Br2<Cl2: bond energy
(4) Br2<Cl2<F2: oxidising strength
11. The elements ‘X’, ‘Y’, and ‘Z’ form oxides, which are acidic, basic, and amphoteric, respectively.The correct order of their electronegativity is
(1) X > Y > Z (2) Z > Y > X
(3) X > Z > Y (4) Y > X > Z
12. Calculate the percentage ionic character for molecule AB when electronegative difference is 2.0.
(1) 46% (2) 36% (3) 30% (4) 50%
13. Which of the following oxides is amphoteric?
(1) CrO (2) Cr2O3 (3) CrO3 (4) CrO5
14. Which of the following orders presents the correct sequence of the increasing basic nature of the given oxides?
CHAPTER 4: Classification of Elements
(1) Al2O3<MgO<Na2O<K2O
(2) MgO<K2O<Al2O3<Na2O
(3) Na2O<K2O<MgO<Al2O3
(4) K2O< Na2O<Al2O3<MgO
15. An element A of group-1 shows similarity with an element B belonging to group-2. If A has maximum hydration enthalpy in group-1, then B is ___.
(1) Mg (2) Be
(3) Ca (4) Sr
16. The ionisation potential and electron affinity of fluorine are 17.42 and 3.45 eV, respectively. Calculate the electronegativity of fluorine in Mulliken scale
(1) 3.726 (2) 4.726
(3) 2.726 (4) 1.726
17. The electron affinity of chlorine is 3.7 eV. How much energy in kcal is released when 2 g of chlorine is completely converted to C in ion in a gaseous state? (1 eV = 23.06
kcal /mol)
(1) 48 kcal (2) 2.8 kcal
(3) 8.4 kcal (4) 4.8 kcal
18. The correct order of ionic radius is (1) Ti4+<Mn7+
(2) 35Cl– > 37Cl–(3) K+ > Cl–(4) P3+ > P5+
19. Which of the following processes do not involve absorption of energy?
(A) S(g)+e– →S– (g)
(B) O– (g) +e–→O2– (g)
(C) Cl (g) +e– →Cl– (g)
(D) O (g) +e– →O– (g)
(1) (A), (C), (D) (2) (A), (B), (D) (3) (B), (C), (D) (4) (A), (B), (C), (D)
20. Mar k the correct statement out of the following.
(A) Helium has the highest first ionisation enthalpy in the periodic table.
(B) Fluorine has less negative electron gain enthalpy than chlorine.
(C) In any period, the atomic radius of the noble gas is the highest.
(D) Hg and Br are liquids at room temperature.
(1) (A), (B)only (2) (A), (C) only (3) (A), (B), (C) only (4) (A), (B), (C), and (D)
Section-B
Numeric Value Questions
21. Calculate the electronegativity of fluorine from the following data. Express your answer to the nearest possible integer.
(E= bond enthalpy and X = electronegativity)
EH–H = 104.2 kcal/mol
EF–F = 36.6 kcal/mol
EH–F = 13.4 kcal/mol
XH = 2.1
22. From the given compounds, number of compounds acidic in water is ____.
CaO, SO2, SO3, Fe2O3, Cl2O7, CO2, Na2O, No2
23. How many of the following factors influence the electronegativity of the atom of an element?
i) Effective nuclear charge
ii) Screening effect of inner electrons
iii) Size of the atom
iv) Oxidation state of the atom in bond
v) Type of hybridisation of the atom
vi) Strength of bond in which it is participated
vii) Number of surrounding atoms on the other atom with which it is bonded
viii) Nature of bond
24. An element ‘X’ belongs to 4th period and 4th group in the modern periodic table. Then, the atomic number of ‘X’ is ___.
25. How many of the following orders/ statements are correct?
a) IP1 of nitrogen is not less than that of oxygen.
b) IP1 of Pb is less than that of Sn.
c) Electronegativity order of B > Tl > In > Ga > Al.
d) IP2 of order B > C > Be.
e) Electron gain enthalpy order Ne < Ar < Kr < Xe < He < Rn.
f) Electron affinity of Be+4 is 217.6 eV/ ion.
g) Electronegativities of K and Rb are same.
h) Order of sizes is Tl > In > Al > Ga > B.
i) Official IUPAC name of element with atomic number 104 is unnilquadium.
CHAPTER TEST – JEE ADVANCED
2022 P-1 Model Section-A
[Numerical Value Questions]
1. How many of the following statements are correct?
(i) Each period consists of a series of elements having the same valence shell.
(ii) Each period corresponds to a particular principal quantum number of the valence shell present in it.
(iii) Each period starts with an alkali metal having the outermost electronic configuration ns1 .
(iv) Each period ends with a noble gas with the outermost electronic configuration ns2np6 ,except helium, having outermost electronic configuration 1s 2 .
(v) Each period starts with the filling of a new energy level.
(vi) The number of elements in each period is twice the number of atomic orbitals available in energy level that is being filled.
2. How many of them are metalloids in the modern periodic table?
B, Si, Ge, As, Sb, Te, Po, At,Cs, Fr, Ga, Hg, and Br,
3. The bond energies of H–H, X–X, and H–X are 104 kcal, 38 kcal, and 138 kcal, respectively. The electronegativity of X is p. Then, p–2.8 is ____. Given: ( ) 67=8.18
4. How many of the following elements have positive or zero electron gain enthalpy values?
Al, Be, Mg, B, N, He, Ne, O, P, F
5. The common oxidation state of the first transition series is +x. The value of x is ___.
6. If covalent bond length of fluorine molecule is 20 pm, the covalent radius of chlorine is ____ pm.
7. Among CO 2, CO, SO 2, Al 2O 3, SO 3, P 4O 6, B2O3, BeO, CaO, BaO, N2O5, and NO
the number of acidic oxides = x
the basic oxides = y
the amphoteric oxides = z
the neutral oxides = w. What is the value of xy zw + + ?
8. Among the following number of oxides which is/are more basic as compared to Na2O is _____.
Li2O, K2O, Cs2O, Rb2O, MgO, CaO, Al2O3
Section-B
[Multiple Option Correct MCQs]
9. Select the correct statements about the element Uuo.
CHAPTER 4: Classification of Elements
(1) This element belongs to period-7.
(2) This element belongs to group-18.
(3) This is a p-block element.
(4) This is a metal.
10. Stability of ions of Ge, Sn, and Pb will be in the order
(1) Ge+2<Sn+2<Pb+2
(2) Ge+4<Sn+4<Pb+4
(3) Sn+4>Sn+2
(4) Pb+2>Pb+4
11. Following statements regarding the periodic trends of chemical reactivity of alkali metals and halogens are given. Which of these statements gives the correct picture?
(1) Chemical reactivity increases with increase in atomic number down the group in both alkali metals and halogens.
(2) In alkali metals, the reactivity increases, but in the halogens, it decreases with increases in atomic number down the group.
(3) The reactivity decreases in alkali metals but increases in halogens with increases in atomic number down the group.
(4) In both alkali metals and halogens, chemical reactivity decreases with increase in atomic number down the group.
12. In which of the following arrangements, the order is correct according to property indicated?
(1) Al 3+<Mg 2+<Na +<F –: increasing ionic size
(2) B<C<O<N: increasing first ionisation enthalpy
(3) CI<F<Br<I: increasing electron affinity
(4) Li<Na<K<Rb: increasing metallic radius
13. Which of the following sets of elements have more similarities in their properties?
(1) S, Na (2) Li, Mg
(3) Be, Al (4) Zr, Hf
14. Which of the following statements are true?
(1) In a multi-electron atom, the effect of nuclear charge experienced by the outermost electron is less than the theoretical value of the nuclear charge (Z).
(2) On moving from second to third transition series in a group [except Y(39) and La (57)] electronegativity increases due to the increase of +18 units in nuclear charge.
(3) Pauling assumed that the electronegativity value of fluorine is 4 and calculated the electronegativity values of other elements from this value.
(4) Higher ionisation potential and higher electron affinity values implies lower electronegativity value.
Section -C
[Matrix Matching Questions]
15. Match the elements in List I with Lis t II.
List I (Elements) List II (Classification)
A. He, Ne, Ar I) Representative elements
B. Fr, Ra, At II) Lanthanoids
C. Ce, Gd, Yb III) Noble gases
D. Rb, Ga, Cl IV) Radioactive elements
Choose the correct answer from the options given below:
(A) (B) (C) (D)
(1) III IV II I
(2) IV III II I
(3) I IV II III
(4) III IV I II
16. Match the electronic configuration in Column I with the ionisation energy in Column II ( kJ mole)
Column I
Column II
A. ns2 I) 2100
B. ns2np1 II) 1400
C. ns2np3 III) 800
D. ns2np6 IV) 900
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) III I II IV
(2) IV I III II
(3) IV III I II
(4) IV III II I
17. Match the List I with List II
List I List II
A. I>Br>Cl>F I) First ionisation energy
B. F>CI>Br>I II) Atomic radii
C. CI>F>Br>I III) Electron affinity
D. O>F>N>C IV) Electronegativity V) Second ionisation energy
Choose the correct match:
(A) (B) (C) (D)
(1) II I,IV,V V V
(2) II I,V III V
(3) II I,V IV,III V
(4) II,IV I,V III V
18. Match Column I(element) with Column II (group to which element belongs)
Column I (Element)
Column II (Group to which element belongs)
A. P I) VI A group
B. Br II) VA group
C. Se III) VII A group
D. La IV) IIIB group
Choose the correct answer from the options given below.
(A) (B) (C) (D)
(1) II III IV I
(2) II III I IV
(3) III II I IV
(4) I II III IV
ANSWER KEY
JEE Main
- I
- III
(12) 4 (13) 1 (14) 4 (15) 3 (16) 1 (17) 4 (18) 3 (19) 3 (20) 3 (21) 4 (22) 4 (23) 4 (24) 4 (25) 5 (26) 7 (27)
Theory-Based Questions
(21) 1
JEE Advanced Level
(11) 3
Chapter Test-JEE Main
Chapter Test-JEE Advanced (1) 6 (2) 8 (3) 1 (4) 5 (5) 2 (6) 10 (7) 2 (8) 3 (9) 1,2,3 (10) 1,4 (11) 2 (12) 1,2,4 (13) 2,3,4 (14) 1,2,3 (15) 1 (16) 4 (17) 1 (18) 2