SOLUTIONS CHAPTER 1
1.1 TYPES OF SOLUTIONS
T he composition and the properties are uniform throughout the homogeneous mixture. The component that is present in a larger quantity is generally called solvent and the component present in a minor quantity is called solute. A binary solution contains only two components. Solvent determines physical state in which the solution exists. Based on the physical state, solutions are classified into three types: 1. solid solutions 2. liquid solutions 3. gaseous solutions. Each type of solution is further classified into 3 more types. Types of solutions and their examples are listed in Table 1.1
Note that the absorption of H 2 gas on palladium is known as occlusion.
Among different types of solutions, solid in liquid type solutions are most frequently studied.
Chloroform
Solid
Ethanol
Sucrose
in water
Solution of hydrogen in palladium
Amalgam of mercury with sodium
Copper dissolved in gold
Table 1.1 Types of solutions and common examples
1.1.1 Aqueous and Non-Aqueous Solutions
Solution prepared by using water as solvent is called aqueous solution. Alcoholic solutions contain ethyl alcohol as solvent. Non-aqueous solutions have benzene, chloroform, ether, carbon tetrachloride, etc., as solvents.
Concentration is the term used to express the amount of solute present in a definite amount of solution. It is also called strength of the solution. The concentration of a solution can be expressed quantitatively in several ways.
1.2 METHODS OF CONCENTRATION
Concentration of a solution represents the composition of the solution. It expresses the relative quantities of solute and solvent. The following methods are used to express the concentration of a solution.
1.2.1 Weight Percent (or) Mass Percent (w/w)
Weight in grams of a solute present in 100 g of a solution is called its weight percent (w/w).
Weight percent of solute =
Weightofsolute 100
Weightofsolution ×
Weight volume percent or percentage of solute (w/v):
Weight in grams of a solute present in 100 mL of a solution is called its weight volume percent (w/v).
Weight volume percent of solute =
Weightofsolute 100
Volumeofsolution ×
1.2.2 Volume Percent (V/V)
Volume in millilitres of a solute present in 100 mL of a solution is called its volume percent.
Volume percent of solute =
Volumeofsolute 100
Volumeof solution ×
1.2.3 Molarity
It is defined as the number of gram moles of the solute present in one litre of solution. It is denoted by ‘M’. Units of molarity are mol L–1. It is dependent on temperature. As temperature increases, the volume of a solution increases and molarity decreases. Molarity (M) is given as,
Number of moles of solute () M
Volume in litres of solution (V) = n (or)
1000 w M
GMWV =× w n GMW =
Here, w is mass of solute in grams and V is volume of solution in millilitres. GMW is gram molecular weight of the solute.
A molar solution is the one in which one gram mole of solute is present in one litre of solution. One millimole of solute present in one millilitre solution is also called one molar solution. Molar (1M), semimolar (0.5M), decimolar (0.1M), centimolar (0.01M), millimolar (0.001M), etc., are in common use.
The mass of solute (w) in grams, present in V litres of a solution, can be calculated from molarity of the solution (M) as, w = M × V × GMW
Molarity also indicates the number of millimoles of the solute dissolved in one millilitre of the solution.
(1 mol = 1000 millimoles)
Some useful relations:
1. Number of millimoles of the solute present in V mL of the solution = M × V
2. Number of moles of solute present in V mL of the solution MV 1000 × =
3. Number of moles of solute present in V L of solution = MV
4. When a solution is diluted,its molarity decreases, but number of moles of solute before and after dilution remains constant.
M1V1 = M2V2
V1 = Volume of the solution before dilution
M 1 = Molarity of the solution before dilution
V2 = Volume of the solution after dilution
M2 = Molarity of the solution after dilution
5. Volume of water added to get a solution of known molarity,
10. When two solutions are titrated against each other as per the following equation: 1212 nAnBmCmD +→+
At the equivalence point of the titration, The molarities of the two solutions are related by the equation 1122 12 MVMV nn =
1.2.4 Concepts of Equivalent Weight
The equivalent weight of a substance is weight in grams of substance which furnishes or combines with one gram of hydrogen ions (H+ion) in a solution
Equivalent Weight of an Acid
6. Molarity = 1000 w GMWV ×
w = weight of the solute in grams
V = volume of the solution in millilitres
GMW = molecular weight of the solute in grams
7. When weight/volume percentage is given, () 10/% wV M GMW × =
8. When weight percentage of solution and specific gravity or density are given, then molarity is given by 10specificgravity(/)% M GMW ×× = ww
10d(/)% M GMW ×× = ww
where(w/w)% = percentage by weight
d = Density in gram/mL
9. For a mixture of two solutions of different molarities, the molarity of the resulting solution is given by
Molecularweightofacid E Basicityofacid =
Number of replaceable hydrogens present in one molecule of acid is called its basicity.
(Its structure is OH | HPOH || O ; it furnishes only two H+ ions)
Equivalent Weight of Base Base
Molecular weight of base E Acidity of base =
Number of replaceable hydroxyl groups present in one molecule of a base is called its acidity.
Example:
1. ()2 BaOH Molecularweight 171.33 E 85.67 22 ===
Molecularweight 171.33 E 85.67 22 ===
2. NaOH Molecularweight 40 E 40 11 ===
3. ()2 CaOH
Molecularweight 74 E 37 22 ===
Equivalent Weight of a Salt
Formula weight of the salt ESalt
Total number of units of charge on cation or anion of the salt =
Example:
1. NaCO23
Formulaweight 106 E 53 22 ===
2. NaCl Formulaweight 58.5 E 58.5 11 ===
3. ()24 3 AlSO
Formulaweight 342 E 57 66 ===
Equivalent Weight of an Oxidising Agent (or) Oxidant
The weight in grams of a substance that gains one mole of electrons is called gram equivalent weight of oxidant.
Formula weight of oxidant E
oxidant
Electrons gained by oxidant (or) Decrease in oxidation state of oxidant per a formula unit = KMnO4 as an oxidant:
i. In acidic medium
MnO4– + 8H+ + 5e–→Mn+2 + 4H2O
Change in oxidation number = 7–2 = 5 or
Number of electrons gained = 5
Formulaweight 158 E 31.6 55 ===
KMnO4
ii. In dilute alkaline medium or in neutral medium
MnO4– +2H2O + 3e–→MnO2 + 4OH–
Change in oxidation number = 7 – 4 = 3
(or) Number of electrons gained = 3 KMnO4
Formulaweight 158 E 52.6 33 ∴===
iii. In strongly alkaline medium: 2 44 MnOeMnO +→
Change in the oxidation state is one unit.
Number of electrons gained is 1.
Potassium Dichromate as an Oxidant: Cr2O7–2+14H+ + 6e– →2Cr+3 + 7H2O
Change in oxidation number for one ‘Cr’ atom = 3
Change in oxidation number for two ‘Cr’ atoms = 6
Number of electrons gained = 6 KCrO227
Formulaweight 294 E 49 66 ===
Equivalent Weight of Reducing Agent (or) Reductant
The weight in grams of substance that loses one mole of electrons is called gram equivalent weight of reductant.
Formulaweightofreductant E Electronslostbyreducantor Increaseinoxidationstateofreducant = Mohr’s salt is ferrous ammonium sulphate. Its formula is FeSO4 (NH4)2 SO4. 6H2O. In its reactions in acidic medium, ferrous (Fe+2) ion gets oxidised to ferric (Fe +3); 23 FeFee ++− →+
so, EMohr’s salt
Formulaweight 392 11 ==
1.2.5 Normality
It is defined as the number of gram equivalents of the solute present in one litre of a solution. It is denoted by ‘N’. Unit of normality is eq L –1. It is dependent on temperature. As the temperature increases, the volume increases and normality decreases.
Normality (N) is given as,
Numberofequivalentsofsolute N (or)
Volumeofsolution inlitres =
Weightofsolute 1000 N GramequivalentweightVolume of solutionin mL =×
Some useful relations:
1) Number of equivalents of solute = N×V; volume V is in litres.
2) Number of milli equivalents of solute= N×V; volume V is in millilitres.
3) The mass of a solute (w) in grams, present in V litres of a solution, can be calculated from normality of the solution (N) as,
w = N × V × GEW
Here,GEW is the gram equivalent weight of solute.
4) Normality of the mixture when two solutions of same solute are mixed;
1122 12 N = NVNV VV + +
Here V 1 + V 2 = Total volume o f the solution.
5) When V a mL of a strong acid of normality N a is mixed with Vb mL of a strong base of normality Nb;
i) If N a V a = NbVb , the solution is neutral.
ii) If N a V a > NbVb, the solution is acidic.
Normality w.r.t H+ = aabb ab NVNV VV +
iii) If N a V a < NbVb, the solution is alkaline.
Normality w.r.t OH– = bbaa ab NVNV VV +
6) Normality–Molarity interrelation:
i) For acids:
Normality = Molarity × basicity of acid
ii) For bases:
Normality = Molarity × acidity of base
iii) For salts:
Normality = Molarity × total number of units of +ve charge or –ve charge of the salt ion
iv) For oxidising (or) reducing agents:
Normality = Molarity × total change in oxidation state per mole of oxidant or reductant
7) For exact neutralisation of a strong acid with a strong base: aa
Weightofbase NV(mL) GEWofbase1000 = bb
WeightofacidNV(mL) GEWofacid1000 =
8) In case of dilution: N1V1 = N2V2
The volume of the solution before and after dilution are V1 and V2, and normalities are N1 and N2 respectively.
9) N = % x specific gravity 100 GEW ×
1.2.6 Molality
Molality is defined as the number of gram moles of the solute present in one kilogram of solvent. It is denoted by ‘ m ’. Units of molality are mol kg –1. It is independent of temperature. Molality is the most accurate and,theoretically,the best method of expressing concentration.
Molality (m) is given as () () Numberofmolesofsoluten m NumberofkgofsolventW =
w1000 m GMWW =× w n GMW =
Here w and W are masses of solute and solvent respectively in grams. GMW is the gram molecular mass of solute.
Parts per Million
Trace quantities of solute in a solution is conveniently expressed in parts per million (ppm).
ppm = 6 Numberofpartsofcomponent 10 Total numberof parts of solution ×
Some useful relations:
1. 10Solubility m Grammolecularweight × = (solubility in g of solute 100 g solvent)
2. ()MolarityVinL m Weightofsolventinkg × =
3. () 1000Molarity
m 1000specificgravityMGMW × = ×−× (here, M = Molarity)
4. Mole fraction of solute = m 1000 m Molecularweightofsolvent
5. M = M × d
a) for aqueous solution d = 1 gm/ml, then M = m.
b) for Non - aqueous solutions:
d = 1 gm/ml then M = m
d > 1 gm/ml : M > m :
d < 1gm/mol \ M < m
1.2.7 Mole Fraction
It is the ratio of number of moles of a component to the total number of moles of the solution. It is denoted by the symbol ‘X’. If number of moles of component substances A and B in a solution are, respectively, nA and nB,
Mole fraction of component,
A = () A A AB n X nn = +
Mole fraction of component,
B = = + B B AB n X (nn)
In a binary solution of A and B, XA+XB = 1. The sum of the mole fractions of all components in a solution is unity.
For very dilute solution: m = 2 1 1000 x m
x2 = mole fraction of solute M1 = Molar . mass of solvent
2 21 x 1000 m 1xm =×
One hundred times mole fraction is called mole percentage. Mole fraction and mole percentage have no units. They do not vary with a change in temperature of solution.
1. What is the molality of a solution of H 2SO4 having 9.8% by mass of the acid?
Sol. 9.8% by mass of H2SO4 contains 9.8 g of H2SO4 per 100 g of solution.
Therefore, if mass of solution = 100 g, mass of solute, H2SO4 = 9.8 g, and mass of solvent = 100 – 9.8, = 90.2 g
Number of moles of HSO Mass of solvent in kg 9.81000 9890.2 =× =1.1 mol kg–1
Molality = 24
Try yourself:
1. In a solution of H 2 SO 4 and water, mole fraction of H2SO4 is 0.9. How many grams of H2SO4 is present per 100 g of the solution? Answer: 98
TEST YOURSELF
1. Calculate normality of 0.98% W V
H2SO4 solution.
(1) 0.1 N (2) 1 N (3) 0.2 N (4) 2 N
2. The mole fraction of a solvent in aqueous solution of a solute is 0.6. The molality of the aqueous solution is (1) 83.25 (2) 13.88 (3) 37 (4) 73
3. Mole fraction of solute in 4.5 molal aqueous solution is
(1) 0.05 (2) 0.025 (3) 0.0375 (4) 0.075
4. 14.3 g of Na2CO3 xH2O completely neutralizes 100 mL of 1 N H 2SO 4 solutions. Value of x is (1) 3 (2) 10 (3) 5 (4) 2
5. The molarity of 1.5 N H3PO4 solution is (1) 1.3 M (2) 0.75 M (3) 0.5 M (4) 4.5 M
6. The number of millimoles of H2SO4 present in 5 L of 0.2 N H2SO4 solution is (1) 500 (2) 1000 (3) 250 (4) 0.5 × 10–3
7. The volume of water that must be added to a mixture to 250 mL of 6 M HCl and 650 mL of 3 M to obtain 3 M HCl solution is (1) 75 mL (2) 150 mL (3) 300 mL (4) 250 mL
8. Molarity of 1 m aqueous NaOH solution (density of the solution is 1.02 g/mL) (1) 1 M (2) 1.02 M (3) 1.2 M (4) 0.98
9. Equivalent weight of Baeyer’s reagent is (M = molecular weight) (1) M (2) M/2 (3) M/3 (4) M/4
Answer Key
(1) 3 (2) 3 (3) 4 (4) 2 (5) 3 (6) 1 (7) 4 (8) 4 (9) 3
1.3 SOLUBILITY
Solubility of a solute in a given solvent represents the maximum quantity of the solute that can be present in dissolved state in the saturated solution at a given temperature.
1.3.1 Solubility of a Solid in a Liquid
When a solid solute is added to a solvent, some solute dissolves and its concentration increases in the solution. This process is known as dissolution. When the solute is continuously added with constant shaking,some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallisation. A stage is reached when the two processes occur at the same rate. Under such conditions, number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
Solute + Solvent Solution
The solution at this stage is said to be saturated solution. (An unsaturated solution is the one in which more solute can be dissolved at the same temperature.) The concentration of the saturated solution is called the ‘solubility’. Thus, solubility may be defined as follows:
The solubility of a solid in a liquid at any temperature is defined as the maximum amount of the solid (solute) in grams which can be dissolved in 100 g of the liquid (solvent) to form the saturated solution at that particular temperature. Molar concentration of saturated solution is called its molar solubility.
Factors Affecting the Solubility of a Solid in a Liquid
The factors on which the solubility of a solid in a liquid depends are: i) nature of the solute and the solvent ii) temperature iii) pressure
Nature of the solute and the solvent: In general, a solid dissolves in a liquid which is chemically similar to it. This is expressed by saying “Like dissolves like”. This statement implies that ionic (polar) compounds,like KCl,dissolve in polar solvents like,water. They are very much less soluble or almost insoluble in non-polar solvents, like benzene, ether, etc. Similarly, non-polar compounds,like naphthalene, anthracene, etc., are soluble in non-polar solvents,like benzene, ether, carbon tetrachloride, etc. They are very less soluble in water.
Temperature: The effect of temperature on solubility depends on heat of solution and it can be explained based on Le Chatelier’s principle.
Heat of solution = Lattice energy + Hydration energy
The solubility of solids which dissolve in liquid solvents with absorption of heat ( D H = + ve) increase with increase of temperature.
The solubility of solids which dissolve in liquid solvents with release of heat ( D H = –ve) decreases with increase of temperature.
In a nearly saturated solution, if the dissolution process is endothermic, the solubility of a substance should increase with rise in temperature.
For salts whose lattice energy is greater than hydration energy, solubility increases with increase in temperature.
Examples: KNO3, NaNO3, Na2SO4.10H2O, CuSO4.5H2O, FeSO4.7H2O, NH4Cl, Na2S2O35H2O, NaClO3, Pb(NO3)2, AgNO3 etc.
In a nearly saturated solution, if the dissolution process is exothermic,The solubility of a sub-stance should decrease with rise in temperature. For such salts hydration energy exceeds lattice energy.
Examples: Ce2(SO4)3, Na2SO4, CaCl2, Li2SO4, CuSO4 etc.
For NaCl lattice energy(184 kcal) is nearly equal to hydration energy (182.8 kcal). Hence, its solubility does not vary much with temperature.
Pressure: Pressure has a very little effect on the solubility of a solid in a liquid because solids and liquids are highly incompressible.
1.3.2 Solubility of a Gas in a Liquid
Almost all gases are soluble in water, though to different extents. The existence of aquatic life in lakes, rivers, sea, etc. is due to dissolution of oxygen gas of the air in water.
Solubility of a gas in a liquid at a particular temperature is also expressed in terms of molarity (moles of the gas dissolved per litre of the solution to form the saturated solution, i.e., in terms of mol L–1) or in terms of mole fraction (X) of the gas.
Factors Affecting the Solubility of a Gas in a Liquid
The important factors on which the solubility of a gas in a liquid depends are:
i) nature of the gas and the solvent
ii) temperature
iii) pressure
Nature of the Gas and the Solvent
Gases like hydrogen, oxygen, nitrogen, etc. dissolve in water only to a small extent,
whereas gases like HCl, NH3, etc. are highly soluble. The greater solubility of the later gases is due to their reaction with the solvent, water.
Oxygen, nitrogen, and carbon dioxide are much more soluble in ethyl alcohol than in water at the same temperature and pressure, while H2S and NH3 are more soluble in water than in ethyl alcohol. Evidently, the greater solubility of a gas in a solvent is again due to the chemical similarity between the gas and the solvent.
Effect of Temperature
The solubility of a gas decreases with increase in temperature. This is expected because the dissolution of a gas in a liquid is always an exothermic process, i.e., it is accompanied by evolution of heat.
Gas + Solvent Solution + Heat
Applying Le Chatelier’s principle, it is evident that increase in temperature would shift the equilibrium in the backward direction, i.e., the solubility would decrease.
Effect of Pressure – Henry’s Law
For the solution of a gas in a liquid, consider a system, as shown in Fig 1.1 (a). The lower part is the solution and the upper part is gaseous system at a pressure P and temperature T. Suppose the system is in dynamic equilibrium, i.e., rate of gaseous particles entering and leaving the solution is the same, which means that rate of dissolution = rate of evaporation. Now, on increasing the pressure over the system, as shown in Fig 1.1 (b) , the gas gets compressed to a smaller volume. Hence, the number of gaseous particles per unit volume increases. As a result, the number gaseous particles striking the surface of the solution and, hence, entering into it also increases, till a new equilibrium is reestablished. Thus, on increasing the pressure of the gas above the solution, the solubility increases.
Fig. 1.1. Pressure increases the solubility of gas
Quantitatively, the effect of pressure on the solubility of a gas in a liquid was studied by Henry and is called Henry’s law. It is stated as follows:
The mass of gas dissolved in a given volume of the liquid at constant temperature is directly proportional to the pressure of the gas present in equilibrium with liquid.
Mathematically, m ∝ p or m = K p . . . . .(i), where m = mass of the gas dissolved in a unit volume of the solvent, p = pressure of the gas in equilibrium with the solvent, K = constant of proportionality whose value depends upon the nature of the gas, the nature of the solvent, and the temperature.
Henry’s law may also be stated as follows:
The solubility of a gas in a liquid at a particular temperature is directly proportional to partial pressure of the gas in equilibrium with the liquid at that temperate.
Dalton, during the same period, had concluded independently that if a mixture of gases are simultaneously in equilibrium with the liquid at a particular temperature, the solubility of any gas in the mixture is directly proportional to the partial pressure of that gas in the mixture.
For a gas A, Henry’s law can be written as:
XA = A Kp ' . . . (ii), where XA is the mole fraction of the gas in the solution, pA is the partial pressure of the gas above the solution, and K’ is proportionality constant whose value depends upon the
Solution
Piston
nature of the gas, nature of the solvent, and the temperature.
For example, the solubility of pure N2 in water at 298 K and a partial pressure 0.78 atm (which is the partial pressure of N2 in air at 1.0 atm) is 5.3 × 10–3 mol L–1 (using molarities in place of mole fractions). If the partial pressure is doubled to 1.56 atm, the solubility of N 2 is doubled to 1.06 × 10–2 M.
From equations (ii) AA 1 Px K' = or PA = KH XA . . . (iii), where H 1 K K' = , is called Henry’s constant.
This is the most commonly used form of Henry’s law and may be defined as follows: The partial pressure of a gas (p) is directly proportional to the mole fraction (X) of the gas in the solution.
Using this expression, the unit K H will be atm or bar (or kbar).
This is a convenient expression for testing the validity of Henry’s law. Plotting equilibrium pressures pA, versus corresponding mole fractions X A , a straight line plot passing through the origin is obtained with slope = K H (in atm or bar), as shown in Fig. 1.2 , for solubility of HCl gas in cyclohexane at 293 K.
Henry Law may be expressed as p = K HC Where C = any concentration term. Unit of KH = P/C . . . . (1)
It may equally written as C = K HP . . . (2) and unit of KH = concentration / pressure for same units of pressure and concentration units of KH from equation (2) is inverse of KH units from equation (1).
Different gases have different values of K H at the same temperature and in the same solvent.
From these values, the following results may be drawn:
i. Henry’s constant, KH, is a function of the nature of the gas.
ii. Greater the value of K H , lower is the solubility of the gas at the same partial pressure [according to eq n . (iii), at a particular temperature].
iii. The value of KH increases with increase in temperature, implying that the solubility decreases with increase in temperature at the same pressure.
It is for this reason that aquatic species feel more comfortable in cold water (in which O2 gas dissolved is more) than in warm water (in which O2 dissolved is less).
Further, as already mentioned, the value of K H depends upon the nature of the gas and the nature of the solvent at the same temperature. The KH values of some gases in different solvents at 298 K are given in Table 1.2.
Table 1.2 Values of Henry’s constant for some gases in different solvents at 298 K
Fig.1.2 Solubility of HCl in cyclohexane at 293 K
Limitations of Henry’s law:
Henry’s law is applicable only if the following conditions are satisfied:
i) The pressure should be low and the temperature should be high, i.e., the gas should behave like an ideal gas.
ii) The gas should not undergo compound formation or dissociation in the solve nt
2. Oxygen gas is bubbled through water at 293 K, exerting a partial pressure of 0.98 bars. Find the solubility of oxygen in g L–1 (given: KH of oxygen gas is 34 kbar).
Sol. Henry’s law: p = KH () O2 X
Compared to the number of moles of water, number of moles of dissolved O2 is negligible. Hence,
Try yourself:
2. How many grams of dissolved CO 2 is expected to dissolve in 500 g of soda water when packed under 2.5 atm of CO 2 at 298 K (KH of CO2 = 1.67 × 108 Pa)?
Ans: 1.6
TEST YOURSELF
1. The Henry’s law constant for O 2 dissolved in water is 4.34 × 10 4 atm at a certain temperature. If the partial pressure of O 2 in a gas mixture that is in equilibrium with water is 0.434 atm, what is the mole fraction of O2 is solution?
(1) 1 × 10–5
(2) 1 × 10–4
(3) 2 × 10–5
(4) 2 × 10–6
2. Two gases ‘A’ and ‘B’ have Henry’s constant (KH) values as 44 kPa and 66 kPa at 293 K. At 293 K, if ‘A’ and ‘B’ are dissolved in water, where the partial pressure ratio of A to B is 1 : 3, then the ratio of solubility of A to B would be
(1) 1 : 2
(2) 9 : 2
(3) 2 : 9
(4) 2 : 1
3. H2S,a toxic gas with rotten egg-like smell,is used for qualitative analysis. If the solubility of H2S in water at STP is 0.195 mol kg–1, then the Henry’s law constant is
Mass of water = 1000 g) p = KH
bars × WO2 1000 32 18
Therefore, mass of O2 dissolved per 1000 mL of water,
2 1 O 0.9832 W 0.05gL 3418 × == ×
(1) 285.6 bar
(2) 324.8 bar
(3) 462.9 bar
(4) 534.8 bar
4. Which of the following gases will greatly deviate from Henry’s law in water?
(1) H2
(2) N2
(3) NH3 (4) CH4
5. Low concentration of oxygen in the blood and tissues of people living at high altitude is due to
(1) Low temperature
(2) Low atmospheric pressure
(3) High atmospheric pressure
(4) Both low temperature and high atmospheric pressure
6. K H of N 2 is 1×10 5 atm. The moles of N 2 dissolved in 10 mole of water is? (Given: Pressure of air is 5 atm and mole fraction of N2 in air is 0.8)
(1) 4 × 10–4 (2) 4 × 10–5
(3) 5 × 10–2 (4) 5 × 10–4
Answer Key
(1) 1 (2) 1 (3) 1 (4) 3
(5) 2 (6) 1
1.4 VAPOUR PRESSURE
The pressure exerted by the vapour molecules of a liquid when they are in equilibrium with the liquid at a given temperature is called vapour pressure of the liquid at that temperature.
Vapour pressure of liquids is measured by barometric method.
Liquids having lower boiling points show higher vapour pressure.
Vapour pressure of a liquid depends upon the following factors:
1) Nature of liquid
2) Temperature
Vapour pressure of a liquid at a given temperature is independent of the quantity of liquid, the surface area, and on the shape of the vessel in which it is kept.
Volatile liquids and non-volatile liquids: Liquids having weaker intermolecular attractions have higher vapour pressures. Such liquids are called volatile liquids. Acetaldehyde, diethylether, pentane, etc., are
examples of volatile liquids. On the other hand, intermolecular forces are strong in a metallic liquid, like mercury. Vapour pressures of such liquids are lower. They are commonly called non-volatile liquids.
Vapour pressure and temperature: With the increase of temperature, vapour pressure of a liquid increases exponentially.
When a liquid is heated, its average kinetic energy of molecules increases. Large number of molecules possess sufficient energy to overcome the intermolecular attractions and escape into the vapour state. Therefore, the rate of evaporation increases and the vapour pressure increases exponentially. Variation of vapour pressures of some liquids with temperature is given graphically in Fig.1.3.
Fig.1.3 Vapour pressure curves of some liquids
The effect of temperature on vapour pressure of a liquid is given by Clausius–Clayperon equation
= Enthalpy of vapourisation of the liquid
Boili ng point: Vapour pressure of a liquid increases with increase in temperature, till its value approaches atmospheric pressure.
solution multiplied by the vapour presure of that component in its pure state.
Let us consider a mixture of two completely miscible volatile liquids A and B, having the mole fractions XA and XB. Suppose at a certain temperature, their partial vapour pressures are pA and pB and the vapour pressures in the pure state are p° A and p°B. According to Roult’s law,
oo AAABBB pxpandpxp == … (1)
totalAB ppp =+ … (2) oo totalABAABB pppxpxp =+=+ or
total BABBBABA p1xpxpppxp =++=−+
()()ooooo total BABBBABA p1xpxpppxp =++=−+ … (3)
The temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure is called boiling point of the liquid (Tb).
Evaporation occurs at all temperatures but boiling occurs only at boiling point. The boiling point of a liquid changes with a change in external pressure and also by addition of volatile or non-volatile substances.
1.4.1 Vapour Pressure of Liquid–Liquid Solution
For a solution of liquid in liquid, as both the components of the solutions are volatile, each component will form vapour above the solution. When equilibrium is reached, each component will exert a vapour pressure, called its partial vapour pressure, whose value depends upon the mole fraction of the component in the solution and the vapour pressure of the component in its pure state. These studies were made by a French chemist, F.M. Raoult, and he put forward the following result known after him as Raoult’s law:
In a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the
Since p° A and p° B are constant at a particular temperature, it is evident from equation (3) that the total vapour pressure is a linear function of the mole fraction X B (or X A as XA = 1 – XB). Thus, a straight line should be obtained, when P is plotted against XA or XB Such a plot is shown in Fig. 1.4 . The lines (I) and (II) give the plots of partial pressure versus mole fraction and the line (III) that of the total pressure versus mole fraction.
Fig.1.4 Vapour pressure versus mole fraction of components
When X A = 1, i.e., the liquid is pure A, p = p ° A, and when X B = 1, i.e., the liquid is pure B, p = p° B
The composition of vapour phase in equilibrium with the solution is determined by the partial pressures of the components. If y A and y B are the mole fractions of the components, ‘A’ and ‘B’ respectively in the vapour phase, from Dalton’s law of partial pressures, or (ptotal=Total vapour pressure of the system)
pA = yA Ptotal; pB = yB Ptotal
In general pi = yi Ptotal
1.4.2 Ideal and Non-ideal Solutions
Liquid in liquid solutions can be classified as ideal solutions and non-ideal solutio ns.
Ideal Solutions
An ideal solution is the solution in which each component obeys Raoult’s law under all conditions of temperatures and concentrations.
An ideal solution will satisfy the following conditions:
i. There will be no change in volume on mixing the two components,
i.e., D V mixing = 0
ii. There will be no change in enthalpy (i.e., no heat is evolved or absorbed) when the two components are mixed, i.e, D H mixing = 0
For example, when we mix 50 cm 3 of benzene with 50 cm3 of toluene, the volume of the solution is found to be exactly 100 cm3, i.e, D V mixing = 0
Hence, the solution obtained is ideal.
An ideal solution may be defined as the solution in which no volume change and no enthalpy change take place on mixing the solute and the solvent in any proportion.
At the molecular level, an ideal solution may be defined as follows:
An ideal solution of the components A and B is defined as the solution in which the intermolecular interactions between the components (A–B attractions) are of the same magnitude as the intermolecular interactions found in the pure components (A–A inter attractions and B–B intermolecular interactions found in the pure components).
A few examples of ideal solutions are given below:
i. Benzene + Toluene
ii. n-Hexane + n-Heptane
iii. Enthyl bromide + Ethyl chloride
iv. Chlorobenzene + Bromobenzene
Non-ideal Solutions
A solution which does not obey Roult’s law at all compositions is called a non-ideal solution.
For such solutions, D Vmixing≠0, D H mixing ≠0. For example,when we mix sulphuric acid (solute) with water (solvent), the amount of heat generated is very large and change in volume is also observed. This is due to formation of a non-ideal solution.
In terms of molecular interactions, a nonideal solution may be defined as follows:
A non-ideal solution is the solution in which solute and solvent molecules interact with one another with a different force than the forces of interaction between the molecules of the pure components.
Types of Non-ideal solutions are divided into two types as explained below.
Non-ideal Solutions Showing Positive Deviations
When a component B is added to another component A, sometimes the partial pressure
of a component A is found to be more than expected on the basis of Raoult’s law. A similar effect is observed for the other component B in the reversed mixing. The total vapour pressure for any solution is thus, greater than that corresponding to an ideal solution of the same composition. Such behaviour of solutions is described as a positive deviation from Roult’s law Fig.1.5.
Vapour pressure of solution
fraction
Fig.1.5 Vapour pressure of binary solutions Showing positive deviation
T he boiling points of such solutions are relatively lower as compared to those of the pure components (because higher the vapour pressure, lower is the boiling point). For one intermediate composition, the total vapour pressure of such a solution will be the highest and the boiling point will be the lowest. This solution acquires the property of boiling at a constant temperature and its composition remains unchanged. Liquid mixtures which distill without any change in composition are called azeotropes or azeotropic mixtures. In case of solutions showing positive deviations, we get minimum boiling (point) azetropes.
The positive deviations are exhibited by liquid pairs for which the A–B molecular interaction forces are weaker than the A–A or the B–B molecular interaction forces. For
example, mixtures of ethanol and cyclohexane (or acetone) show positive deviation. In pure ethanol, a very high fraction of the molecules are hydrogen bonded, as shown below:
CH
On adding cyclohexane (or acetone), its molecules get in between the molecules of ethanol, thus breaking the hydogen bonds and reducing ethanol–ethanol attractions considerably.
Thus, for a non-ideal solution showing positive deviation,
(i) pA > XA p° A,
(ii) D Hmixing=+ve (iii) D V mixing = +ve
A few more examples of non-ideal solutions showing positive deviations are given below:
i. Acetone + Carbon disulphide
ii. Acetone + Ethyl alcohol
iii. Acetone + Benzene
iv. Methyl alcohol + Water
v. Ethyl alcohol + Water
vi. Carbon tetrachloride + Chloroform
vii. Carbon tetrachloride + Benzene
viii. Carbon tetrachloride + Toluene.
Non-ideal Solutions Showing Negative Deviations
If, for the two components A and B, the forces of interaction between the A and B molecules are stronger than the A–A and B–B forces of interaction, the escaping tendency of A and B types of molecules from the solution becomes less than those in the pure liquids. In other words, for any composition of the solution, the partial vapour pressure of each component will be less and the total vapour pressure of the solution will also be less than that expected from Raoult’s law Fig. 1.6.
Fig.1.6 Vapour pressure of binary solutions showing negative deviation
These solutions are said to show negative deviations from Raoult’s law. Such solutions have relatively higher boiling points as compared to those of the pure components (because lower the vapour pressure, higher is the boiling point). For one intermediate composition, the total vapour pressure of the solution will be the least and the boiling point will be the highest. Such a solution will also distil without any change in composition, and it provides an example of another kind of azeotrope. We call it the maximum boiling azeotrope.
For example, negative deviation from Raoult’s law is exhibited by a mixture of chloroform (CHCl3) and acetone, (CH3)2CO. When these are mixed, the hydrogen bonding takes place between the two molecular species, as shown below, due to which the escaping tendency of either of the liquid molecules is decreased. Consequently, the boiling point of solution increases.
In case of solution showing negative deviations, a slight decrease in volume and evolution of heat takes place on mixing, as excepted (i.e., D V and D H both are negative).
Thus, for a non-ideal solution showing negative deviation,
(i) p
(ii) D H mixing = –ve
(iii) D V mixing = –ve
A few more examples of non-ideal solutions showing negative deviations are given below:
1. Chloroform + Benzene
2. Chloroform + Diethyl ether
3. Acetone + Aniline
4. HCl + Water
5. HNO3 + Water
6. Acetic acid + Pyridine
1.4.3 Azeotropic Mixtures
In the process of fractional distillation of liquid mixture, A and B, if we boil the mixture, you can find out the temperature at which it boils, and the composition of the vapour over the boiling liquid. If we boil the liquid mixture at composition C1, notice that the vapour is much richer in the more volatile component B than the original liquid mixture was.
Boiling point
Vapour composition
Boiling point of pure B
Boiling point
Boiling point of pure A
T1
Liquid
Hydrogen bonding between chloroform and acetone
Suppose that we collected and condensed the vapour over the top of the boiling liquid and re-boiled it. We would now be boiling a new liquid which had a composition C2. That would boil at a new temperature T2, and the vapour over the top of it would have composition C3
Boiling point
Vapour composition
Boiling point of pure A
Boiling point of pure B
We can see that we now have a vapour which is getting quite close to being pure B. If you keep on a doing this (condensing the vapour, and then reboiling the liquid produced, you will eventually get pure B. This is the basis for fractional distillation.
A solution which distils without a change in composition at a particular composition is called azeotropic mixture or azeotrope. Azeotrope is a binary mixture of a particular composition, which cannot be resolved by distillation.
Azeotropic mixtures are non-ideal solutions. They have same composition in liquid phase and vapour phase.
They are of two types:
(i) minimum boiling point azeotropes
(ii) maximum boiling point azeotropes
The solutions which show a large positive deviation from Raoult’s law form minimum boiling azeotropes at a specific composition. Rectified spirit obtained from fermented sugars
contains about 95.6% ethanol by volume and is an example of minimum boiling azeotrope.
Minimum Boiling Azeotrope
Enthanol and water mixtures show a large positive deviation from Raoult’s Law and can form minimum boiling azeotrope at certain composition.
The boiling point of this mixture is lower (78.2°C) compared with the boiling point of pure ethanol (78.5°C) and water at 100°C. We might think that this 0.3°C doesn’t matter much, but it has huge implications for the separation of ethanol water mixtures.
Suppose we are going to distil a mixture of ethanol and water with composition C 1 as shown in the given figure. It will boil at temperature given by the liquid curve and produce a vapour with composition C2
When that vapour condenses it will, of course, still have the composition C2. If you reboil that, it will produce a new vapour with composition C3.
The liquid curve and the vapour curve meet at the point corresponding to the composition, 95.6% ethanol the vapour produced will have that same composition of 95.6% ethanol.
This particular mixture of enthanol and water boils as if it were a pure liquid. It has a constant boiling point and the vapour of composition is exactly the same as that of liquid. It is constant boiling mixture or azeotropic mixture or an azeotrope.
Maximum Boiling Azeotrope
Nitric acid and water mixtures show large negative deviation from Raoult’s Law and can form maximum boiling azetropes.
The mixture of nitric acid and water can have boiling point higher than either of the pure liquids because it needs extra heat to break the stronger attractions in the mixture.
In the case of mixture of nitric acid and water, there is a maximum boiling point of 120.5° when the mixture contains 68% by mass of nitric acid, As the acid loses water upon distillation, it becomes more concentrated. Its concentration gradually increases until it approaches to 68% by mass of nitric acid. At that point the vapour produced has exactly the same concentration as the liquid, because the two curves, vapour composition and liquid composition curves meet.
Try yourself:
3. At 80°C, the vapour pressure of liquid A is 520 mm Hg and that of B is 1000 mm Hg. If a mixture of solution of A and B boils at 80°C and 1.0 atm, what will be the mole fraction of A in the solution?
Ans: 0.5
TEST YOURSELF
1. Which statement about the composition of vapour over an ideal solution of 1:1 molar mixture of benzene and toluene is correct?
Assume the temperature is constant (25°C) Vapour pressure data at (25°C):
Benzene = 75 mm Hg
Toluene = 22 mm Hg
(1) The vapour will contain higher percentage of benzene.
(2) The vapour will contain higher percentage of toluene.
(3) The vapour will contain equal amounts of benzene and toluene.
(4) Not enough information is given to make a prediction.
2. The vapour pressure of two liquids P and Q are 80 torr and 60 torr, respectively. The total vapour pressure obtained by mixing 3 moles of P and 2 moles of Q would be (1) 68 torr (2) 20 torr
3. At 88 °C ,vapour pressure of benzene is 900 mm Hg, and vapour pressure of toluene is 360 mm Hg. What is the mole fraction of benzene in the mixture of benzene and toluene that will boil at 88°C?
Sol. oo TotalAABB Ppxpx =+
Here, say liquid (A) is benzene and liquid (B) is toluene. At boiling point, vapour pressure becomes equal to atmospheric pressure, 760 mm Hg.
Therefore, 760 = (900 XA) + (360 XB) = (900 XA) + [360 (1–XA)]
From this equation, XA = mole fraction of benzene = 0.74
(3) 140 torr (4) 72 torr
3. Mixture of volatile components A and B has total vapour pressure (in torr): p = 254–119XA, where xA is mole fraction of A in mixture. Hence, 00 AB p and p are (in torr)
(1) 254, 119 (2) 135, 254 (3) 119, 254 (4) 154, 119
4. The vapour pressure of two pure isomeric liquids X and Y are 200 torr and 100 torr, respectively, at a given temperature. Assuming that a solution of these components obeys Raoult’s law, the mole fraction of component X in vapour phase
in equilibrium with the solution containing equal amounts of X and Y, at the same temperature, is
(1) 0.33 (2) 0.50
(3) 0.66 (4) 0.80
5. The vapour pressure of a pure liquid A is 60 mm, at 25 °C. It forms an ideal solution with another liquid B. The mole fraction of B is 0.6, and total pressure is 64 mm. The vapour pressure of B at 25°C, in mm, is
(1) 75 (2) 66.6
(3) 52 (4) 120
6. The system that forms maximum boiling azeotrope is
(1) Acetone + chloroform
(2) n – hexane + n –heptane
(3) Benzene + Toluene
(4) Carbon disulphide + Acetone
7. The boiling point of C6H6, CH3OH, C6H5NH2 and C6H 5NO 2 are 80°C, 65°C, 184°C and 210.9° C. Which will show highest vapour pressure at room temperature?
(1) C6H6 (2) CH3OH
(3) C6H5NH2 (4) C6H5NO2
8. The vapour pressure of water at 300 K in a closed container is 0.4 atm. If the volume of the container is doubled, its vapour pressure at 300 K will be
(1) 0.8 atm (2) 0.2 atm
(3) 0.4 atm (4) 0.6 atm
9. A solution contains hydrocarbons (A) and (B) in the ratio of 2: 3. The vapour pressures of pure hydrocarbons at 25°C are 300 and 100 mm of Hg, respectively. Mole fraction of ‘B’ in vapour phase is
(1) 2/3 (2) 3/4 (3) 1/3 (4) 3/2
Answer Key
(1) 1 (2) 4 (3) 2 (4) 3 (5) 2 (6) 1 (7) 2 (8) 3 (9) 3
1.5 SOLUTION OF A SOLID IN LIQUID
Consider a solution of cane sug ar or glucose or urea or a salt in water and a solution of sulphur or iodine or naphthalene dissolved in carbon disulphide. These are solutions of a solid in a liquid type. Some physical properties of such solutions are quite different from those of pure solvent.
In a pure liquid, the entire surface is occupied by the molecules of the liquid, as shown in Fig.1.7(a) . If a non-volatile solute is added to a solvent, a homogeneous solution is formed. The vapour pressure of the solution is from the solvent alone. The number of solvent molecules are relatively less at the surface in a solution, as shown in Fig.1.7(b)
Pure solvent
1 mol solvent
1 mol solute
(a) Pure solvent (b) Solution of a solid in liquid solvent
Fig.1.7 Illustration of decrease in vapour pressure
Consequently, the number of solvent molecules escaping from the surface is correspondingly reduced. The vapour pressure is also reduced. The decrease in vapour pressure is directly proportional to quantity of solute.
The difference between the vapour pressure of the pure solvent (P 0) and vapour pressure of solution (Ps) is called lowering of vapour pressure (DP) of solvent in a solution.
The ratio of lowering of vapour pressure of solution to the vapour pressure of pure solvent is called relative lowering of vapour pressure of solution.
s PP P °− ° is rela tive lowering of vapour pressure of solution (RLVP ).
1.5.1 Raoult’s Law
The law states that the relative lowering of vapour pressure of a solution is equal to the mole fraction of its solute.
According to Raoult’s law, at a given temperature, the vapour pressure of solution (P) is directly proportional to mole fraction of solvent.
Let a solution contain nA moles of solvent and n B moles of solute. Then,according to Raoult’s law,
AAAA PX,PkX α= ,
When pure solvent is taken, i.e., X A = 1, then k = PA 0 = vapour pressure of pure solvent.
On substituting the value of k in the above equation, we get,
0 AAA PX P = A A 0 A P X P = = 1 – XB
Subtracting the above two quantities from (1), we get A B 0 A P 11X P −=− AB XX1∴+=
0 AA B 0 A PP X P = = mole fraction of solute.
0 AAB 0 AB A PPn nn P = +
In dilute solutions, n A >>> nB; nnnABA∴+≈
0 AAB 0 A A PPn n P = or o As B o A A pp n n p =
0 AABA 0 BA A PPwM MW P =×
(Here, pA = ps = vapour pressure of solution)
It is called simplified Raoult’s law, where WB, WA are the weights of solute and solvent, M B, M A are the molecular weights of solute and solvent respectively.
If a solution obeys Raoult’s law for all concentrations, its vapour pressure would vary linearly from zero to the vapour pressure of pure solvent. A plot of vapour pressure is linear with mole fraction of the solvent, as shown in Fig.1.8.
Vapour pressure
0 1 Mole fraction of solvent
Vapour pressure of pure solvent
Fig.1.8 Vapour pressure as a function of mole fraction RLVP is generally used to calculate molecular weight of non-volatile solute.
Limitations of Raoult’s law: It is applicable to 1. dilute solutions only; 2. if the solute is non-volatile and is in molecular state
3. solutions containing solut es, which undergo neither dissociation nor association
1.6 COLLIGATIVE PROPERTIES
The p hysical and chemical properties of aqueous solutions containing solutes in general depends on the nature and also on the structure of solutes. But there are some properties which depend on number of solute particles (ions or molecules) but not on their nature. Such properties are called colligative properties. Properties of solutions which depend on the number of particles of solute, irrespective of their nature, are called colligative properties. There are four colligative properties. They are:
1. relative lowering of vapour pressure of solution;
2. elevation of boiling point of solution;
3. depression of freezing point of solution;
4. osmotic pressure of solution;
Colligative properties are generally used to determine molecular weights of non-volatile solutes.
1.6.1 Relative Lowering of Vapour Pressure
Vapour pressure of a solvent in solution is less than that of the pure solvent. The lowering of vapour pressure depends only on the concentration of solute particles and is independent of their size or molecular weight. If po is vapour pressure of pure solvent, X1 and X2 are mole fractions of solvent and solute in a solution, respectively, the vapour pressure of solution ‘p’ is gi ven by Raoult’s law as:
ps = po . X1 .............................................(1)
The lowering of the vapour pressure of solvent ( D p) is given as:
D p = p° – ps = p° – p°X1 = p°(1–X1) ...(2)
But the sum of mole fractions of both solvent and solute in a solution is unity. Hence, D p = p° X2...........................................(3) o s oo22 pp p X;X pp ∆ == ...................... (4)
solute solutesolvent n nn = +
For dilute solution, nsolute < < nsolvent.
Therefore, ssolute solvent ppn pn °− = °
solutesolvent
solutesolvent WM MW =× ... (5)
Thus, RLVP is directly proportional to number of particles; so, it is a colligative property.
If solute undergoes association or ionisation, the above equation (5) cannot be applied. The lowering of vapour pressure is almost twice for a solution containing 58.5 g (one mole) of sodium chloride in one litre aqueous solution than for a solution containing 342 g (one mole) of sucrose in one litre water. This is because the number of solute particles in 342 g of sucrose is No, (molecules) but the number in 58.5 g of sodium chloride is 2N o , (ions) as sodium chloride ionises almost completely in aqueous solutions (Here, N o = Avogadro’s number).
4. The vapour pressure of an aqueous solution of sucrose at 373 K is found to be 750 mm Hg. What is the molarity of the solution?
Sol. Raoult’s law, s solute solute solutesolvent ppn x pnn °− == °+
This can be simplified as ssolute ssolvent ppn pn °− = s solute solvent s pp nn p °− =×
Molality = nsolute in 1000 g of water x s pp 1000 p18 °− =× 7607501000 0.74m 75018 =×=
Try yourself:
4. Find the mass of non-volatile solute (molecular mass = 40) that should be dissolved in 57 g of octane (Molecular mass = 114) to decrease its vapour pressure to 80%
Ans: 4 gm
1.6.2 Elevation of Boiling Point
The boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure. The boiling point of a solution containing non–volatile solute is always greater than that of pure solvent. This increase in boiling point is called the elevation in boiling point. The elevation in boiling point of solution is due to lowering of vapour pressure of solution. Hence, the solution has to be heated more to make the vapour pressure equal to the atmospheric pressure.
Alternatively, the elevation in boiling point may be explained on the basis of plots of vapour pressure versus temperature, as shown in Fig.1.9
Boiling point of solvent
Fig. 1.9 Elevation of boiling point
As at any temperature, vapour pressure of the solution is less than that of the solvent, the curve for the solution lies below that of the solvent, as shown by the curve CD. The temperatures at which the vapour pressure of the solvent and the solution become equal to the atmospheric pressure are T b 0 and T b , respectively. Obviously, T b > T b 0 . The difference, called the elevation in boiling point, is given by = Tb – Tb 0
It is evident that greater the lowering in vapour pressure (Dp), higher is the elevation in boiling point (DTb), i.e., according to Raoult’s law, the Δp is directly proportional to the mole
fraction of the solute in the solution.
Hence, D Tb ∝ X2 (or) D Tb = kX2 where k is a constant of proportionality.
But 2 2 12 n X nn = +
if the solution is dilute 2 2 1 n X n = 22 21 11 1 b nn XTkM wMw =∴∆=
If the mass of solvent, w 1 = 1 kg, then evidently, 2 1 n m w = , molality of the solution.
Also, for a given solvent, its molecular mass M 1 is constant so that kM 1 = K b , an other constant. Hence, the above result reduces to Tbb = Km ∆ .
As molality is the number of moles of the solute dissolved per 1000 g of the solvent, if w2 grams of the solute of molecular mass M 2 are dissolved in w1 grams of the solvent,
2 21 w 1000 m Mw =×
Hence, the above formula becomes 2 bb 21 w 1000 TK Mw ∆=××
w2 = weight of solute
M2 = molecular weight of solute
w1 = weight of solvent in grams
∴ o 2 bbbb 21 w 1000 TTTK Mw ∆=−=××
This formula is often used for the calculation of molecular masses of non–ionic solutes (i.e., non–electrolytes) where K b is called the boiling point elevation constant or ebullioscopic constant, and ‘m’ is the molality of the solution. If m = 1, then D Tb = Kb
Molal elevation constant may be defined as the elevation in boiling point when the molality of the solution is unity, (i.e, 1 mol of the solute is dissolved in 1 kg (1000 g) of the solvent). The units of kb are degree/molality or K/m or 0C/m or K kg mol –1. Calculation of molal elevation constant from enthalpy of vapourisation is as follows.
where L v = enthalpy vapourisation per gram of the solvent
D H vap = enthalpy vapourisation per mole of the solvent
M1 = molecular mass of the solvent
R = 8.314 K –1 mol –1, if L v or D H vap are in joule.
R = 2 cal deg–1mol–1, if L v or D H vap are in calorie.
The value of K b depends only upon the nature of solvent.
Molecular elevation constant.
It is expressed as K mol –1 for 100 gm of solvent = 10 × molal constant.
5. An aqueous solution containing one gram of urea (molecular mass = 60) boils at 100.25 °C. Calculate the boiling point of the aqueous solution containing 3 g of glucose (molecular mass = 180) in the same volume of the solution.
Sol. bb TKm∆=
In urea solution: b T0.25C∆=°
Therefore, b 1 0.25K 60W =× × . . . . . (1)
In glucose solution: bb 3 TK 180W ∆=× × . . .(2)
Fr om the above equation D T b of glucose solution = 0.25°C.
Therefore, boiling point of the given aqueous solution of glucose = 100°C + 0.25°C = 100.25°C.
Try yourself:
5. When 0.4 g of a solute is dissolved in 40 g of diethyl ether (k b = 2.16 K kg mol–1), its boiling point is increased by 0.17 K. Then find the molar mass of the solute.
Ans: 127
1.6.3 Depression in Freezing Point
Freezing point of a substance is the temperature at which the solid and the liquid forms of the liquid are in equilibrium with each other, i.e., the solid and liquid forms of a substance have the same vapour pressure, e.g., ice and water at 0°C have the same vapour pressure.
The freezing point of a solution containing non- volatile solute is always less than that of pure solvent. This decrease is called the depression in freezing point. The depression in freezing point of solution is due to lowering of vapour pressure of solution.
The depression in freezing point may be explained on the basis of plots of vapour pressure versus temperature, as shown in Fig.1.10.
On cooling, the vapour pressure of the liquid solvent decreases along the curve AB. At the point B, the solid starts appearing and the vapour pressure decreases steeply along the path BC (because solids have lower vapour pressure). At B, the liquid and the solid solvent are in equilibrium and have the same vapour pressure. Thus, B is corresponding to the freezing point Tf 0 of the pure solvent. As the vapour pressure of solution is less than that of the solvent, the curve for the solution lies below that of the solvent. On cooling, it will follow the path DE. At E, the solid appears. Hence, E is corresponding the freezing point Tf of the solution. Obviously, Tf is less than Tf0. The difference is called the depression in freezing point, D Tf
Fig. 1.10 Depression in Freezing Point
It is given by DTf = Tf ° –Tf = Kf m, where Kf is a constant, known as freezing point depression constant or cryoscopic constant of the solvent, and ‘m’ is the molality of the solution, i.e., the number of moles of the solute dissolved in 1000 grams (1 kg) of the solvent.
If m = 1, DTf = Kf. Hence, molal depression constant may be defined as the depression in freezing point when the molality of the solution is unity, i.e, one mole of the solute is dissolved in 1000 g (1 kg) of the solvent.
The units of K f are degrees/molality, i.e, K/m or K kg mol–1; the value Kf depends only upon the nature of solvent.
2 ff 21 w 1000 TK Mw ∆=××
where w2 = weight of the solute
w1 = weight of the solvent
M2 = molecular weight of solute
Calculation of molal depression constant from enthalpy of fusion is as follows:
2 2 1o o f ff
where D Hf = enthalpy of fusion per mole
T0 = freezing point of the liquid (pure solvent)
Lf = latent heat of fusion per gram of the solvent
M1 = molecular mass of the solvent
R = 8.314JK–1mol–1 if Lf or are in joule
= 2 cal deg–1mol–1 if Lf or are in calorie
Molecular depression constant:
It is expressed as K mol –1 for 100 gm of solvent = 10 × molal depression constant.
6. When 36 g of a solute is dissolved in 1.2 kg of water, the solution freezes at –0.93°C. What is the molecular mass of the solute?(K f of water = 1.86 K kg mol–1)
Sol. Freezing point of water = 0°C
So, the depression in freezing point of the solution = D Tf = 0 – (–0.93) = 0.93°C.
D Tf = kf m
36 0.931.86 M1.2 =× × 1.8636 M 60 0.931.2 × == ×
Hence, molecular mass of the solute = 60 amu
Arifreeze Compounds
Water is used in car radiators for cooling. In cold countries water gets freezed in radiators. In such countries water and ethylene glycol is used as coolant in radiators. Addition of ethylene glycol lower freezing point of water and prevents water from freezing. Such compounds are known as Antifreeze compounds.
Try yourself:
6. The temperature of a city was –93°C. A car was used wherein radiator was filled with 5 L of water. What weight of ethyleneglycol (molecular mass = 62) were added to the water of the radiator in order the use the car for travelling (kf of water = 1.86 kg molal–1)
Ans: 15.5 kg
1.6.4
Osmotic Pressure
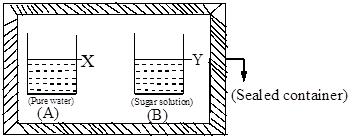
When a dilute solution of a solute is separated from its solvent by a semipermeable membrane, the solvent flows into the solution through the membrane slowly. Such a phenomenon also occurs when two solutions of the same solute with different concentrations are separated by a semipermeable membrane.
The membrane which allows only the molecules of solvent to pass through it, but not the solute molecules, is called a semipermeable membrane.
Parchment paper, cellophane paper, pig’s bladder, some animal membranes, and inorganic precipitate membranes (copper ferrocyanide) are commonly used as semipermeable membrane, in osmosis.
The process of solvent flowing into the solution when the solution and solvent are separated by a membrane is called osmosis. In other words, osmosis is inflow of solvent from dilute solution to concentrated solution.
The hydrostatic pressure developed on the diluted aqueous solution at equilibrium state due to inflow of water when the solution is separated from the water by a semipermeable membrane is also called osmotic pressure.
Osmosis is also called endosmosis because of the inflow of solvent molecules. The osmosis process can be illustrated, as shown in Fig.1.11.
The funnel is filled with a dilute solution of a solute. This is placed in a beaker containing water. The liquid level in the stem of the funnel gradually increases for some time due to entry of water present in the beaker into the funnel by osmosis. After some time the level of solution in the funnel remains constant as it reaches an equilibrium state. As the water level increases in the stem, the pressure exerted by the column of water (hydrostatic pressure) also increases. This acts on the solution in the direction opposite to that of the direction of the flow of water (osmotic pressure) from the beaker into the funnel through the membrane.
The flow of water into the funnel is due to the osmotic pressure of the solution. The pressure that just stops the flow of solvent is called osmotic pressure of the solution.
The osmotic pressure depends on the concentration of the solution. Osmotic pressure is illustrated in Fig.1.12
Fig. 1.11 Phenomenon of osmosis
A semipermeable membrane (parchment paper) is tied to a thistle funnel.
Fig.1.12 Illustration of osmotic pressure
Molar mass of the solute can be calculated from the experimental determination of osmotic pressure ( π ). RT ., π==CRTw MV where M is molecular weight of solute and w is weight of solute. V = volume of solution in litres.
Osmotic pressure method is used for deter-mining molecular masses of proteins, polymers, and other macromolecules.
Solutions having same osmotic pressure at a given temperature are called isotonic solutions. Osmosis does not occurs when such solutions are separated. Osmotic pressure associated with the fluid inside the blood cell is equivalent to that of 0.9% (w/v) sodium chloride solution, called normal saline solution.
If we place cells in a solution containing more than 0.9% ( w / v ) sodium chloride, water will flow out of the cells and cells would shrink. Such a solution is called hypertonic. Hypertonic solutions have higher concentration and possess greater osmotic pressure. If the salt concentration is less than 0.9% (w/v), the solution is called hypotonic. In this case, water will flow into the cells and they would swell. Hypotonic solutions have less concentration and possess lesser osmotic pressure compared to concentrated solutions. Considering 1 M glucose solution and 2M gl ucose solution, 1 M glucose solution is hypotonic with respect to 2 M glucose solution and 2M glucose solution is hypertonic with respect to 1M glucose solution.
7. A 6% aqueous glucose (molecular mass = 180) solution and 2% aqueous solution of an unknown non-electrolytic and non-volatile compounds are isotonic. Find the molecular mass of the unknown compound.
Sol. Since, the given solution are isotonic, their osmotic pressures must be the same, π1 = π2. The solutes are non-electrolytes and non-volatile; hence, their particles concentrations must be the same.
Therefore, C1 = C2
62 180M = M = 60
The molecular mass of the unknown solute = 60 amu.
Try yourself:
7. A solution of urea (molecular mass = 60) of strength, 8.6 g L –1 is isotonic with 5% (W/V %) solution of a non-volatile organic compound. Find the molecular mass of the organic compound.
Ans: 348.8
1.6.5 Reverse Osmosis
If pressure higher than the osmotic pressure is applied to the solution side, solvent will flow out of the solution. This process is called reverse osmosis. Reverse osmosis is used in desalination of sea water. A schematic set up is shown in Fig.1.13. for desalination.
Fig.1.13 Desalination Using Reverse Osmosis
The following phenomena take place due to osmosis:
i) A raw mango placed in concentrated salt solution shrivels.
ii) Wilted flowers revive when placed in fresh water.
iii) A carrot becomes limp when placed in salt water.
iv) People taking a lot of salt or salty food experience water retention in tissue cells and intercellular spaces,result in puffiness (or) swelling,called edema.
v) Water movement from soil into plant roots and subsequently into upper portion of the plant
vi) The preservation of meat by salting and fruits by adding sugar protects against bacterial action because bacterium on
Piston
Semipermeable membrane
salted meat or candied fruit loses water, shrivels, and dies.
vii) If the osmotic pressure of the contents of the living cell is not equal to that of the contents surrounding it outside, two phenomena take place. They are haemolysis and plasmolysis.
Haemolysis is a process of entering of contents into the cell. The cell bulges and, finally, bursts.
Plasmolysis is a process of losing contents from the cell. The cell collapses.
TEST YOURSELF
1. Which of the following is colligative property?
(1) Vapour pressure
(2) Boiling point
(3) Freezing point
(4) Osmotic pressure
2. Relative lowering in vapour pressure of a solution containing 1 mole urea in 54 g H2O is
(1) 1/55
(2) 3/55
(3) 3/4
(4) 1/4
3. Relative lowering of vapourpressure is maximum for
(1) 0.1 m urea
(2) 0.1 m NaCl
(3) 0.1 m Al2(SO4)3
(4) 0.1 m MgCl2
4. The correct order of freezing point for 10 g aqueous solution each of urea, glucose and sucrose is
(1) ()()() fglucosefureafsucrose TTT >>
(2) ()()() fsucrosefglucosefurea TTT >>
(3) ()()() fureafglucosefsucrose TTT >>
(4) ()()() fsucrosefureafglucose TTT>>>
5. How many grams of methyl alcohol should be added to 10 L tank of water to prevent its freezing at 268 K?
(Kf of H2O = 1.86 K–kg/mol)
(1) 880.07 g
(2) 899.04 g
(3) 860.21 g
(4) 878.06 g
6. Two moles of glycol is dissolved in 891.24 g of water. The solution is cooled to –4.8°C. How many gram of ice is formed in the cooling process?
[Kf of water = 1.86 K kg mol –1]
(1) 106.24
(2) 116.24
(3) 126.24
(4) 136.24
7. At room temperature, a dilute solution of urea is prepared by dissolving 0.60 g of urea in 360 g of water. If the vapour pressure of pure water at this temperature is 35 mm Hg, lowering of vapour pressure will be: ( molar mass of urea =60 g mol–1)
(1) 0.027 mm Hg
(2) 0.031 mm Hg
(3) 0.017 mm Hg
(4) 0.028 mm Hg
8. The molal depression constant depends upon (1) nature of the solute
(2) nature of the solvent
(3) heat of solution of the solute in the solvent
(4) vapour pressure of the solution
9. An aqueous solution containing nonvolatile, non -electrolyte freezes at –0.186°C
(Kf = 1.86 K kg mol–1, kb = 0.512 K mol–1). The elevation of boiling point of the same solution is
(1) 0.186 (2) 0.152
(3) 0.512 1.86 (4) 0.0512
10. Equimolal dilute solutions containing different non-volatile, non-electrolyte solutes in the same solvent have
(1) Same boiling point but different freezing point
(2) Same freezing point but different boiling point
(3) Same boiling and same freezing points
(4) Different boiling and different freezing points
11. Yg of non – volatile organic substance of molecular mass M is dissolved in 250 g benzene. Molal elevation constant of benzene is Kb. Elevation in its boiling point is given by
(1) b M KY (2) 4 b KY M
(3) 4 b KY M (4) b KY M
12. The relationship between osmotic pressure(P1) at 273 K when 10 g glucose, 10 g urea (P2) and 10 g sucrose (P3) are dissolved in 250 ml of water is
(1) P1 > P2 > P 3
(2) P 3 > P1 > P2
(3) P2 > P1 > P 3
(4) P2 > P 3 > P1
Answer Key
(1) 4 (2) 4 (3) 3 (4) 2 (5) 3 (6) 2 (7) 3 (8) 2 (9) 4 (10) 3 (11) 2 (12) 3
1.7 ABNORMAL MOLECULAR MASSES
I onic compounds when dissolved in water, d issociate into cations and anions. If we dissolve one formula weight of sodium chloride, (58.5 g) in water, we expect one mole each of Na+ and Cl– ions to be released in the solution. If this happens, there would be two moles of solute particles in the solution.
If interionic attractions are ignored, one mole of sodium chloride in one kg of water would be expected to increase the boiling point by 1.04 K instead of 0.52 K. If we assume that NaCl is completely dissociated in water and increase in boiling point of water is 1.04 K, the molar mass of NaCl would be 29.25 g mol –1 .
This conclusion brings to light that experimentally determined molar mass is always lower than the true value when there is dissociation of solute into ions.
Similarly, some solutes, like acetic acid, when dissolved in solvents, may associate to form dimers due to hydrogen bonding. This happens in solvents with low dielectric constant. Therefore, the number of solute particles decreases. Molar masses of solute determined from such solutions will be higher than that of true mass.
Molar mass that is determined either lower or higher than the expected value is called abnormal molar mass.
A factor ‘i’, known as the Van’t Hoff factor was introduced to account for the extent of dissociation or association. This (i) is defined as follows:
van’t Hoff’s factor ( i) = Observedcolligativeproperty
Calculatedcolligativeproperty i =
van’t Hoff’s factor, Normalmolarmass i Abnormalmolarmass =
Calculated molar mass of solute Observed molar mass of solute i = (or)
Totalnumberofmolesofparticles
afterdissociationorassociation
Numberofmolesofparticles beforedissociationorassociation i =
1.7.1 Solute Dissociation or Ionisation
If a solute is dissociated or ionised in solutions to give ‘n’ ions and ‘ a ’ is the degree of ionisation, then: n nAA
Initial moles 1 0
Number of moles after dissociation 1 a n a
Number of particles before ionisation = 1
Number of particles after ionisation = 1n1(n1) −α+α=+−α
van’t Hoff factor, 1(n1) i 1 +−α =
Degree of ionisation, i1 n1 α=
range of i for dissociation 1 < i < n
1.7.2 Solute Association
If a solute is associated in solutions, n molecules associate and is the degree of association, n nAA
Initial moles 1 0
Number of moles after dissociation 1– aa/ n
Number of particles before association = 1
Number of particles after association =
1(/n) −α+α
van’t Hoff factor, 1(/) 1 n i −α+α =
Degree of association, () 11 1 1(1/) 1 ii or n n α=α=
range of i for association 1 i1 n <<
1.7.3
Colligative
Properties
with Van’t Hoff Factor
When the van’t Hoff factor is included in the mathematical equations of colligative properties and used for molecular mass calculations, correct molecular masses of solutes are obtained. The modified equations are as follows.
Relative lowering of vapour pressure of solvent, o s solute o pp iX p =
Depression of freezing point, ff TiKm∆=
Elevation of boiling point, bb TiKm∆=
Osmotic pressure of solution, iCST π=
In case of dissociation of solute, i > 1
In case of association of solute, i < 1
When there is no dissociation or association, i = 1
The van’t Hoff factor increases upon dilution and approaches 2 for electrolytes like NaCl and MgSO 4. It gets close to 3 for K2SO4 and 5 for K4[Fe(CN)6]. The van’t Hoff factor for some electrolytes are listed in Table 1.3.
Table 1.3 The van’t Hoff factor, (i) for some electrolytes
Salt
Values of i van’t Hoff factor, i for complete dissociation of solute
8. 75.2 g of phenol is dissolved in 1.0 kg of a solvent (kf = 14 K molal–1). If depression in freezing point of the solution is 7 K, what percentage of phenol that dimerises in the solvent > (Molecular mass of phenol = 94)?
Sol. D Tf = i kf m f f T 794 i 00514 km1475.2 ∆× === ××
At equilibrium, 1 – aa /2 i1 2 α =−α+
0.6251 2 α =−
a = 0.75
Therefore, phenol undergoes 75% dimersation.
Try yourself:
8. In 0.01 M aqueous solution, if BaCl 2 undergoes 49% dissociation, find its van’t Hoff factor.
Ans: 1.98
TEST YOURSELF
1. What is the van’t Hoff factor of ferric sulphate (Assume 100% ionisation) (1) 3 (2) 4 (3) 5 (4) 3
2. van’t Hoff factor is highest for _____ molal K2SO4 (1) 1 (2) 0.001 (3) 0.1 (4) 0.01
3. The van’t Hoff factor for 0.1 m KCl aqueous solution is 1.85. The degree of ionisation of KCl solution is
(1) 0.15 (2) 0.17 (3) 0.85 (4) 0.3
4. The van’t Hoff factor of aq. K2SO4 at infinite dilution has value equal to (1) 1 (2) 2 (3) 3 (4) Between 2 and 3
5. The degree of dissociation (α) of a weak electrolyte, Ax B y is related to van’t Hoff factor (i) by the expression
(1) i1 xy1 α= ++ (2) xy1 i1 +− α= (3) xy1 i1 ++ α= (4) () i1 xy1 α= +−
6. If BaCl 2 ionises to an extent of 80% in aqueous solution, the value of van’t Hoff factor is
(1) 0.4 (2) 2.6 (3) 0.8 (4) 2.4
7. 54% of a solute is dimerized in a solution. van’t Hoff factor of the solute in the solution is
(1) 0.73 (2) 0.63 (3) 0.42 (4) 0.84
8. The freezing point of benzene decreases by 0.45°C,when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid associates to form a dimer in benzene, the percentage association of acetic acid in benzene will be: (Kf for benzene = 5.12 K kg mol −1)
(1) 74.6% (2) 94.6%
(3) 64.6% (4) 80.4%
9. 0.004 M Na 2 SO 4 is isotonic with 0.01 M glucose. The degree of dissociation of Na2SO4 is
(1) 75% (2) 50%
(3) 25% (4) 85%
10. Which of the following aqueous solutions has the highest boiling point?
(1) 0.1 M KNO3
(2) 0.1 M Na3PO4
(3) 0.1 M BaCl2
(4) 0.1 M K2SO4
Answer Key
(1) 3 (2) 2 (3) 3 (4) 3
(5) 4 (6) 2 (7) 1 (8) 2
(9) 1 (10) 2
CHAPTER REVIEW
Types of Solutions
■ A solution is a homogeneous mixture of two or more non–reacting components. Formation of solution is a physical process.
■ A solution of a solid in another solid is known as a solid solution. Many alloys are solid solutions.
■ An alloy of a metal with mercury is called an amalgam.
■ Aqueous solutions are those prepared using water. Non–aqueous solutions have other solvents.
■ The components do not lose their identity during the formation of a solution.
■ Dynamic equilibrium is established between dissolved solute and undissolved solute in a solution.
■ During formation of solution entropy of system increases due to greater disorder.
Methods of Concentration
■ A solution whose molar concentration is definitely known is a standard solution.
■ The most ideal method of expressing concen-tration is the molality ( m).
■ The commonly used method of expressing concentration is molarity ( M).
■ Mass by volume percentage (w/v) indicates the mass of a solute in 100 mL of solution.
■ Mass by weight (w/w) indicates the mass of a solute in 100 g of solution.
■ Molarity indicates the number of moles of the solute dissolved in one litre of the solution or the number of millimoles of the solute dissolved in one millilitre of the solution.
■ Number of millimoles of the solute present in V mL of the solution is given as product of volume and molarity. It is given as V×M.
■ Number of moles of solute present in V litres of solution is given as MV.
■ When a solution is diluted, its molarity decreases.
■ V1M1 = V2M2, where V1 = Volume of the solution before dilution, M 1 = Molarity of the solution before dilution, V2 = Volume of the solution after dilution and M 2 = Molarity of the solution after dilution
■ Molarity, w1000 GMWV ×
■ w = weight of the solute in grams and
■ V = volume of the solution in millilitres
■ M = 10(%w/v) GMW (or) M = () () ()10d%w/w GMW , where d is density in grams per mL.
■ Equivalent weight of a substance expressed in grams is known as gram-equivalent weight or gram-equivalent or equivalent.
■ Number of gram-equivalents = Weight GEW
■ Equivalent weight = Molecular weight n , where n is acidity of a base or basicity of an acid or valency or charge of ion or number of electrons transfered or number of faradays.
■ Normality indicates the number of gram–equivalents of solute present in one litre of the solution.
■ Number of gram equivalents of solute in V litres of solution is given as, NV
■ A normal solution means 1N solution.A decinormal solution is N/10 or 0.1 N solution. A centinormal solution is N/100 or 0.01 N solution.
■ Normality, N = w1000 GEWV ×
■ = 10(%w/v) N GEW (or) N = () () ()10d%w/w GEW
■ Normality is given as, N = Molarity × n.
■ When a solution is diluted, its normality decreases.
■ V 1 N 1 (before dilution) = V 2 N 2 (after dilution)
■ Molarity and normality decrease with increase in temperature, except from 0 °C to 4°C.
■ Normality of the mixture when two solutions of same solute are mixed.
N = 1122 12 NVNV VV + +
■ Molality ( m ) indicates the number of moles of a solute dissolved in 1000 g or one kilogram of the solvent.
■ Molality (m) = w1000 GMWW × × where w is weight of the solvent in grams.
■ Mole fraction of the solute = 1 solute
12 X = + n nn
Mole fraction of the solvent = Xsolvent
2 12 = + n nn , where n1 and n2 are number of moles of solute and solvent.
■ For a binary solution, Xsolute + Xsolvent = 1.
■ Weight percentage, molality, and mole fraction are independent of temperature.
Solubility
■ Solubility represents the number of moles of solute present in a litre of saturated solution, at a given temperature.
■ In a solution of gas in liquid, the solubility of gas increases with decrease of temperature.
■ In the solution of gas in liquid, the solubility of gas increases with increase of its partial pressure.
■ Henry’s law states that the mass of a gas dissolves in a solvent is directly proportional to its pressure.
pgas = KH m or pgas = KH X gas m = masses of gas, X = mole fraction of gas in solution.
Vapour Pressure
■ The process of escape of liquid molecules into space is called evaporation or vapourisation.
■ Rate of evaporation depends on nature of liquid, surface area of liquid, temperature, and flow of air current over the surface.
■ Rapid evaporation results in decrease in temperature, leading to intense cooling.
■ When a liquid and its vapour are in equilibrium with each other, the pressure exerted by the vapour over the liquid surface is known as vapour pressure of the liquid.
■ Volatile liquids with high vapour pressure at a given temperature have low boiling points. Less volatile liquids have high boiling points.
■ The vapour molecules have a higher potential energy than the liquid molecules at the same temperature.
■ The vapour pressure of a liquid depends upon the nature of the liquid and temperature.
■ With an increase in temperature, the vapour pressure of a liquid increases exponentially.
■ A liquid boils at that temperature when its vapour pressure becomes equal to the atmos-pheric pressure.
■ When the external pressure is decreased, the boiling point of liquid decreases.
■ The vapour pressure of a solution of a non-volatile solute is less than the vapour pressure of the pure solvent at the same temperature.
■ If P ° is the vapour pressure of the pure solvent and P S is the vapour pressure of the solution at the same temperature, then, lowering of vapour pressure is Po – P s
■ The vapour pressure of a solution of a nonvolatile solute,is directly proportional to the mole fraction of the solvent in the solution.
PS = Po Xsolvent
Solution of a Solid in Liquid
■ Lowering of vapour pressure is directly proportional to mole fraction of solute
■ Raoul’t law states that the relative lowering of vapour pressure of a solution is equal to the mole fraction of the solute in the solution.
■ Raoult’s law equation is given as,
° = + PPn s PNn .
■ Relative lowering of vapour pressure is independent of temperature.
■ An ideal solution is one which obey Raoult’s law at all concentrations and temperatures.
■ In an ideal solution the volume changes are additive.
■ In the formation of an ideal solution no heat is evolved or absorbed.
■ Chemically similar liquids form ideal solutions.
■ An ideal solution can be separated into two pure components by fractional distillation.
■ Raoults law is applicable to dilute solutions only.
■ When the solute is non-volatile, if the solute does not undergo either ionisation (or) association, Raoult’s law is good to apply.
■ Relative lowering of vapour pressure is determined by Ostwald and Walkers method.
■ In a dilute solution n is very small compared to N, 0S 0 PP w/mw.M PW/MW.m ==
w = weight of the solute,
m = molecular weight of the solute,
W = weight of the solvent and M = molecular weight of the solvent
Colligative Properties
■ The properties which depend on the number of particles of the solute but not on the nature of solute are called colligative properties.
■ Lowering of vapour pressure, elevation of boiling point, depression of freezing point and osmotic pressure are the four colligative properties.
■ All the colligative properties can be used to determine the molecular weight of non–volatile solute. The best method is using Osmotic pressure.
■ When a non-volatile solute is dissolved in the pure solvent its boiling point increases. e.g. sea water boils at greater than 100°C.
■ The difference between boiling points of solution containing a non-volatile solute ( T b ) and the pure solvent T o is called elevation of boiling point D Tb =Tb –T o .
■ Liquids with high boiling point have low vapour pressures and are less volatile.
■ Elevation of boiling point is directly proportional to molality of the solution.
■
where K b is molal elevation constant of the solvent or ebullioscopic constant
■ K b value changes from one solvent to another solvent. Kb = 0.52 degree kg/mol for water
■ 2 b b v RT K 1000 . L = where, L v is Latent heat of vapourisation.
■ The temperature at which the pressure of a liquid is equal to that of the solid is called freezing point.
■ When a non - volatile solute is dissolved in a solvent, the freezing point of the solvent decreases. eg., Sea water freezes below 0°C.
■ Water taken in automobile radiators is mixed with glycerol (or) glycol to decrease its Freezing point (F.P.) to prevent the formation of ice when surrounding temperature falls.
■ The depression in Freezing point is given by Tf∆ is T o –T s, where T o is F.P. of pure solvent and T s is F.P. of solution.
■ Depression in freezing point is directly proportional to molality of the solution.
■ Cellophane paper, cell walls, pig’s gall blader, Copper ferrocyanide Cu2 [Fe(CN)6], etc., are examples of membranes.
■ Egg free from outer shell placed in distilled water enlarges due to endosmosis and when placed in NaCl solution shrinks (plasmolysis) due to exosmosis respectively.
■ Pressure developed due to osmosis is called osmotic pressure.
■ Osmotic pressure is the excess pressure which must be applied to a solution to prevent the flow of the solvent into the solution.
■ Osmotic pressure is directly related to molar concentration and temperature.
■ Osmotic pressure ( π ) in terms of concentration (C) is, π = CRT
■ Molecular weight of solute in terms of osmatic pressure ( π ) is given as, wRT Vπ
■ Solutions having same osmotic pressure are known isotonic solutions.
■ Isotonic solutions generally have the same molar concentrations at a given temperature.
where Kf is molal depression constant of the solvent or cryoscopic constant
■ 2 f f f RT K 1000L = where, Lf is Latent heat of fusion.
■ The passage of solvent molecules from a solution of low concentration into a solution of higher concentration through a semi-permeable membrane is known as osmosis.
■ Semipermeable membrane is one which allows the solvent molecules to pass through but not solute particles.
■ The solution having lower osmotic pressure is known as hypotonic solution and that having higher osmotic pressure is known as hypertonic solution.
■ Osmotic pressure method is widely used to determine molecular masses of proteins, polymers and other macro molecules.
■ The colligative properties of solutions depend on the total number of solute particles present in solution.
■ For different molar concentrations of the same solute, the colligative property has greater value for the more concentrated solution.
Abnormal Molecular Masses
■ For different solutes of same molar concentration, the colligative properties have the greater value for the solution which gives more number of particles on ionisation.
■ Certain solutes in solution are found to associate, leads to a decrease in the number of particles in the solutions. Thus, it results in a decrease in the values of colligative properties.
■ The colligative properties are inversely related, in general to the molecular mass of solute.
■ Electrolytes dissociate in solution to give two or more ions. Such solutions exhibit higher values of colligative properties. The molecular masses of such substances as calculated from colligative properties will be less than their normal values.
■ Certain solutes that undergo dissociation (or) association in solution are found to show abnormal molecular mass. The extent of dissociation (or) association of solutes in solution is determined by Van’t Hoff factor.
■ Van’t Hoff factor ( i) is given as,
i = Normal molar mass
Observed molar mass
Theoritically,
i Observed colligative property
Normal colligative property =
■ For ideal solutions with no association or dissociation of solute the Van’t Hoff factor i = 1. For solutes showing association, i < 1 and showing dissociation, i > 1.
■ If a molecule of solute on dissociation gives ‘ n’ ions and a is the degree of dissociation,
i = 1 + a (n – 1)
Degree of dissociation ( a) = 1 1 i n
■ If a solute forms associated molecules (A) n and is the degree of association,
i = 1 11 −α−
n
Degree of association
■ In terms of Van’t Hoff factor ( i)
Elevation of boiling point. D Tb = iKbm
Depression of freezing point. D Tf = iKfm
Osmotic pressure freezing point a = iCRT
Exercises
JEE MAINS LEVEL
Level – I
Types of Solutions
Single Option Correct MCQs
1. If air is taken as a binary solution, the solvent is
(1) N2
(2) O2
(3) CO2
(4) H2
2. A homogeneous system among the following is
(1) milk
(2) sand in water
(3) urea in water
(4) benzene in water
3. Which among the following is a physical change?
(1) Burning of coal
(2) Burning of sulphur
(3) Dissolution of glucose in water
(4) Burning of white phosphorus
4. Choose the characteristic property/properties of a solution.
a) Formation of a solution is a physical change.
b) Solute and solvent in the solution can be separated by filtration.
c) Solute and solvent in the solution can be separated by decantation.
d) A solution can be represented with a chemical formula.
(1) a, b
(2) a
(3) b, d
(4) c, d
5. A mixture of salt and water can be separated by
(1) filteration
(2) decantation
(3) crystallisation
(4) kept long standing
6. Which of the following differs from the others?
(1) Ruby
(2) Magnalium
(3) Brass
(4) Amalgam
7. Occlusion of hydrogen on palladium is an example of which type of solution?
(1) Gas in solid
(2) Solid in gas
(3) Gas in liquid
(4) Liquid in gas
8. Match the following lists and choose the correct option.
List I
List II
A. Gaseous solution I) German silver
B. Liquid solution II) Milk
C. Solid solution III) Sand in water
D. Colloidal solution IV) Aqueous alcoholic solution
V) Air
(A) (B) (C) (D)
(1) V IV I II
(2) I III II V
(3) IV II V I
(4) II III I IV
Expressing concentration of solution
Single Option Correct MCQs
9. Match the following lists and choose the correct option.
List I
List II
A. Mole fraction I) Number of g equivalents in 1000 ml of solution
B. Molarity II) Always less than one
C. Normality III) Greater than or equal to molarity
D. Molality IV) Number of g moles present in 1000 ml solution
V) Number of g moles of solute in 1 kg of solvent
(A) (B) (C) (D)
(1) I II III IV
(2) II III I V
(3) I IV II III
(4) II IV III V
10. Which of the following solutions contains more number of milli moles of solute?
(1) 0.5 L of 0.2 M aqueous solution
(2) 0.02 L of 0.1 M aqueous solution
(3) 250 mL of 0.04 M aqueous solution
(4) 100 mL of 0.05 M aqueous solution
11. The unit of molarity is
(1) g. lit–1
(2) mole. lit–1
(3) equivalent. lit–1
(4) mole. kg–1
12. To halve the molarity of a solution, which of the following should be adopted?
(1) Weight of the solute to be doubled
(2) Weight of the solvent to be doubled
(3) Volume of the solvent to be doubled
(4) Volume of the solution to be doubled
13. The molarity of liquid HCl, if the density of the solution is 1.17 g/cc, is
(1) 36.5
(2) 18.25
(3) 32.05
(4) 42.10
14. A student has 100 mL of 0.1 M KCl solution. To make it 0.2 M, he has to
(1) evaporate 50 mL of the solution
(2) add 0.01 mol of KCl
(3) both (a) and (b) can be used
(4) neither (a) nor (b) can be used
15. The unit of normality is (1) mole. lit–1 (2) mole. kg–1
(3) equivalent. lit–1
(4) equivalent. kg–1
16. In the reaction 2NaOH + H3PO4 → Na2HPO4 + 2H2O, the equivalent weight of the acid is (1) 49 (2) 98
(3) 32.6 (4) 36.5
17. Which of the following acids has the same molecular weight and equivalent weight?
(1) H3PO2
(2) H3PO3
(3) H3PO4
(4) H2SO4
18. The molecular weight of KMnO 4 is M. In a reaction, KMnO4 is reduced to K2MnO4 The equivalent weight of KMnO 4 is (1) M (2) M/2
(3) M/3 (4) M/5
19. The equivalent weight of hypo in the reaction Na2S2O3 + Cl2 + H2O → Na2SO4 + 2HCl + S is [M = mol. wt.]
(1) M/2 (2) 3M 8
(3) M/3 (4) 2M
20. The number of millimoles of H2SO4 present in 5 litres of 0.2N H2SO4 solution is (1) 500 (2) 1000 (3) 250 (4) 0.5 ×10–3
21. The unit of molality is (1) mole lit–1 (2) mole ml–1
(3) mole kg–1 (4) equivalent kg–1
22. A one-molal solution is one that contains (1) 1 g of the solute in 1000 g of solvent
(2) 1 g mole of the solute in 1000 ml of solution
(3) 1 g mole of the solute in 22.4 litres of solution
(4) 1 g mole of the solute in 1000 g of solvent
23. Regarding molarity, which of the following statements are correct?
a) Unit of molarity is mol kg –1 .
b) Molarity of dibasic acid is half of its normality.
c) Normality × GEW / GMW
d) Molarity is always equal to its molality.
(1) a, b (2) a, b, c
(3) b, c (4) a, c
24. At 25 °C, for a given aqueous solution, M = m. Then, at 50 °C, the correct relationship is
(1) M = m (2) M > m
(3) M < m (4) M = 2m
25. To change the molal conc. to one-half, which of the following should be adopted?
(1) The weight of the solute should be doubled.
(2) The weight of the solvent should be doubled.
(3) The volume of the solvent should be doubled.
(4) The weight of the solution should be doubled.
26. 0.1 gram mole of urea is dissolved in 100g of water. The molality of the solution is (1) 1 m (2) 0.01 M (3) 0.01 m (4) 1.0 M
27. The mole fraction of I2 dissolved in different solvents to prepare different solutions is the same. The molar mass of the solvent having the highest molality is equal to (1) 100 (2) 154 (3) 78 (4) 119.5
Numerical Value Questions
28. The number of moles of phosphate ions present in 4 L of 10–5 M calcium phosphate is y × 10–n. The value of y + n is _______.
29. The molality of iodine in benzene solution is 3.205. The mole fraction of iodine in benzene solution is x. Find the value of 40x.
30. The solubility of Ba(OH)2.8H2O (Molar mass = 315) is 2.52 g per 100 g of water. Molality of OH– is y m. Find the value of 500y.
31. The molarity of a 9.8% 24 W HSO W
of density 1.1 g/cc is ______.
32. If two substances A and B have 00 P:PAB = 1 : 2 and have mole fraction in solution 1 : 2, then the mole fraction of A in vapour is
33. How many of the following concentration units are independent from tempeerature? (1) Molarity (2) Normality (3) Formality (4) Molality (5) w/w % (6) ppm
34. 6 g of urea is dissolved in 90 g of water. The mole fraction of solute is x. Find the value of 1503x.
35. 0.1 mol of NaCl is dissolved in 100 g of water. The mole fraction of water is x. Find the value of 112x.
Solubility
Single Option Correct MCQs
36. Choose the incorrect statement. (K H = Henry’s constant) (1) K H is a characteristic constant for all gas-solvent systems.
(2) Higher the value of KH , lower is the solubility of a gas for a given partial pressure of the gas.
(3) KH has temperature dependence.
(4) KH decreases with temperature.
37. Which of the following statements is correct about Henry’s law?
(1) It is applicable only when pressure is high.
(2) It is applicable only when the temperature is very high.
(3) It is applicable only when gas reacts with the solvent.
(4) KH (Henry’s constant) is a function of the nature of the gas.
38. If 0.05 mole of gas is dissolved in 500 grams of water under 1 atm. pressure, 0.1 moles will be dissolved if the pressure is 2 atm. It illustrates
(1) Graham’s law
(2) Dalton’s law
(3) Henry’s law
(4) Boyle’s law
39. In a nearly saturated solution, the solubility increases with an increase in temperature. Then, ∆H sol. is
(1) + ve (2) – ve
(3) + ve (or) – ve (4) 0
40. At 293 K, Henry’s constant for N 2 gas is greater than Henry’s constant for O 2. At 303 K, Henry’s constant for N 2 gas is (1) greater than that of KH of O2 at 303 K
(2) less than that of KH of O2 at 303 K
(3) equal to that of KH of O2 at 303 K
(4) equal to that of KH of O2 at 293 K
41. For a hypothetical gas (X), the Henry’s constant K H is 250 L-atm/mol at 298 K. For the same gas at the same temperature, Henry’s constant KH ,in terms of mol/L-atm, is
(1) 4 × 103
(2) 4 × 10–3
(3) 2 × 103
(4) 4 × 10–4
Numerical Value Questions
42. ‘W’ g of carbon dioxide is dissolved in a one-litre bottle of a carbonated drink, if the manufacturer uses a pressure of 2.94 atm of CO2 to carbonate the drink. If K H is 29.4 L atm/mol, find the value of 10W.
43. Nitrogen gas is bubbled through water at 293 K at a pressure of 0.987 atm. If K H is 19.74 atm, mole fraction of nitrogen gas is y ×10–2. Find the value of 12y.
44. Mole fraction of nitrogen in air is 0.75. The total pressure is one atmosphere and the temperature is 25 0C. K H for nitrogen is 2.5×10–4 M/mm Hg. The concentration of nitrogen in a glass of water at the same temperature is y×10 –4 M. Find the value of y.
Vapour Pressure of Liquid Solutions
Single Option Correct MCQs
45. The vapour pressure of a liquid does not depend on
(1) nature of liquid
(2) temperature
(3) presence of impurities
(4) volume of the liquid
46. Consider the following statements:
(A) Vapour pressure of liquid increases with decrease in temperature.
(B) At the same temperature, all liquids have the same vapour pressure.
(C) Vapour pressure of the liquid decreases when a non-volatile solute is dissolved in a pure liquid.
Among these statements,
(1) only A and B are correct
(2) only A and C are incorrect
(3) only A is correct
(4) A, B, and C are incorrect
47. For a binary ideal liquid solution, the total vapour pressure of the solution is given as
(1) ()ooo tAABB ppppX =+−
(2) ()ooo tBBAA ppppX =+−
(3) ()ooo tBABA ppppX =+−
(4) ()ooo tBBAB ppppX =+−
48. The vapour pressure of any solution containing a non-volatile solute in a volatile solvent is equal to
(1) sum of vapour pressure of the solvent in pure state and its mole fraction in the solution
(2) product of vapour pressure of the solute in pure state and its mole fraction in the solution
(3) product of vapour pressure of the solvent in pure state and the mole fraction of solute present in the solution
(4) product of vapour pressure of the solvent in pure state and its mole fraction in the solution
49. An aqueous solution of 1m glucose is heated in an open vessel till the boiling point is reached. Thereafter, the boiling is continued, and the temperature and the vapour pressure of the solution are recorded at 5 min, 10 min, and 15 min. The corresponding temperatures and pressures are T5, T10, and T15 and p5, p10, and p15, respectively. The correct relationship among them is
(1) T 5 =T 10 =T 15 and p5 < p10 < p15
(2) T 5 < T 10 < T 15 and p5 < p10 < p15
(3) T 5 < T 10 < T 15 and p5 = p10 = p15
(4) T 5 = T 10 = T 15 and p5 = p10 = p15
50.
The initial levels of liquids in two containers are marked X and Y for containers A and
B, respectively. What will happen over time?
(1) The level of liquid will rise above X in container A and drop below Y in container B.
(2) The level of liquid will rise above Y in container B and drop below X in container A.
(3) The level of liquid in both A and B will remain at X and Y, respectively.
(4) The level of liqud will remain at Y in container B and drop below X in container A.
51. The vapour pressure of a solution of nonvolatile solute is
(1) less than that of solvent
(2) equal to that of solvent
(3) more than that of solvent
(4) equal to or more than that of solvent
52. Vapour pressure will be least for
(1) 0.025 m glucose
(2) 0.2 m glucose
(3) 0.05 m glucose
(4) 0.1 m glucose
53. For which of the following mixtures is Raoult’s law not applicable?
(1) C2H5Cl + C2H5Br
(2) n - C6H14 + n - C7H16
(3) H2O + CCl4
(4) CH3OH + C2H5OH
54. Which of the following relations is correct when vapours are in equilibrium with a solution containing two volatile liquids?
P0A = Vapour pressure of pure component A
P0B = Vapour pressure of pure component B
XA = Mole fraction of component A in liquid phase
XB = Mole fraction of component B in liquid phase
YA = Mole fraction of component A in vapour phase
YB = Mole fraction of component B in vapour phase
(1)
SMCQ
Single Option Correct MCQs
55. If liquids A and B form ideal solutions, then (1) the enthalpy of mixing is zero (2) the entropy of mixing is zero (3) the free energy of mixing is zero (4) the free energy and the entropy of mixing are zero.
56. A solution that obeys Raoult’s law is called (1) normal solution (2) non-ideal solution (3) ideal solution
(4) saturated solution
57. Match the following lists and choose the correct option.
List I
A. Lowering of vapour pressure i)
B. Relative lowering of vapour pressure ii)
List II
C. Raoult’s law iii) P°–P
D. Ideal solution iv) Obeying Raoult’s law
v) Boiling point
(A) (B) (C) (D)
(1) iii ii i iv
(2) iv i ii iii
(3) iii i ii iv
(4) i iii iv ii
58. If 15 cm3 of liquid ‘x’ and 20 cm3 of liquid ‘y’ are mixed at 20 ºC, and the volume of
solution was measured to be 35.1 cm3, then the correct reaction is
(1) ΔHmix < 0, solution shows +ve deviation
(2) ΔHmix > 0 solution shows +ve deviation
(3) ΔHmix < 0, solution shows –ve deviation
(4) ΔHmix > 0, solution shows –ve deviation
59. 6 g of urea is dissolved in 90 g of boiling water. The vapour pressure of the solution is
(1) 744.8 mm (2) 758 mm
(3) 761 mm (4) 760 mm
60. As temperature increases, the vapour pressure of a liquid
(1) increases linearly
(2) decreases linearly
(3) increases exponentially (4) decreases exponentially
61. Which one of the following is correct for solutions of components of A and B to follow Raoult’s law?
(1) A-B attractive force is greater than A-A and B-B attractive forces.
(2) A-B attractive force is less than A-A and B-B attractive forces.
(3) A-B attractive force remains the same as A-A and B-B attractive forces.
(4) The volume of solution is different from the sum of volumes of A and B components.
Numerical Value Questions
62. Vapour pressure of a pure component is 400 mm Hg. The ratio of mole fraction of the component in liquid phase to vapour phase is 3 : 2. The total pressure of the solution is __________mm Hg.
63. A solution is prepared by mixing methanol and ethanol. The ratio of vapour pressures of methanol and ethanol in liquid phase is 4 : 1, and the mole fraction ratio in vapour phase is 2 : 1. The mass of ethanol mixed with methanol to prepare the solution is ____________ g.
64. A dilute solution is prepared by dissolving glucose in water. The vapour pressure of the solution is 120 mm Hg and the vapour pressure of water at the same temperature is 360 mm Hg. The molality of the solution is ________m. (Round off to nearest integer)
Colligative Properties and Determination of Molar Mass
Single Option Correct MCQs
65. According to Raoult’s law, the relative lowering of vapour pressure is
(1) equal to the mole fraction of solvent
(2) equal to the mole fraction of solute
(3) independent of the mole fraction of solute
(4) equal to the molality of solution
66. Which of the following is independent of temperature?
(1) Molarity
(2) Normality
(3) % W/V concentration
(4) Relative lowering of vapour pressure
67. 18 grams of glucose is dissolved in 180 grams of water. Relative lowering of vapour pressure of the solution is (1)
68. Which one of the following is not a colligative property?
(1) Osmotic pressure
(2) Lowering in vapour pressure
(3) Depression in freezing point
(4) Viscosity
69. Equimo lal dilute solutions containing different non-volatile and non-electrolytic solutes in the same solvent have
(1) same boiling point but different freezing points
(2) same freezing point but different boiling points
(3) same boiling point and same freezing point
(4) different boiling points and different freezing points
70. Which of the following units is useful in relating the concentration of a solution with its vapour pressure?
(1) Mole fraction (2) Parts per million
(3) Mass percentage (4) Molality
71. Equimolar solutions of electrolytes with different numbers of ions in the same solvent have
(1) same boiling point but different freezing points
(2) same freezing point but different boiling points
(3) same boiling point and same freezing point
(4) different boiling points and different freezing points
72. The ratio of molal elevation constants in aqueous solutions a : b : c is
a) 0.1 m glucose b) 0.2 m urea
c) 0.3 m sucrose
(1) 1 : 1 : 1 (2) 1 : 2 : 3
(3) 3 : 2 : 1 (4) 6 : 3 : 2
73. Ebullioscopy is concerned with (1) osmotic pressure of a solution
(2) elevation of boiling point of a solution
(3) depression in freezing point of a solution
(4) relative lowering in vapour pressure of a solution
74. The study of boiling points is called ebullioscopy. The ebullioscopic constant depends on
(1) solute only
(2) solvent only
(3) both solute and solvent
(4) volume of the solution
75. Aqueous solution of glucose has a freezing point of −0.93 °C. Molality of the solution is (Kf of H2O = 1.86 K kg mole−1)
(1) 1 m (2) 0.2 m
(3) 0.4 m (4) 0.5 m
76. Which of the following aqueous solutions has the lowest freezing point?
(1) 0.05 m glucose (2) 0.2 m glucose
(3) 0.1 m glucose (4) 0.15 m glucose
77. The value of Kf for water is 1.86°, calculated from glucose solution. The value of Kf for water calculated from NaCl solution will be
(1) 3.72 (2) 0.93
(3) zero (4) 1.86
78. During depression of freezing point in a solution, which of the following are in equilibrium?
(1) Liquid solvent, solid solvent
(2) Liquid solvent, solid solute
(3) Liquid solute, solid solute
(4) Liquid solute, solid solvent
79. In countries near polar regions, the roads are sprinkled with CaCl 2. This is done
(1) to minimise snowfall
(2) to minimise pollution
(3) to minimise the accumulation of dust on the road
(4) to minimise wear and tear of roads
80. Kf, molal depression constant, or cryoscopic constant, does not depend on
(1) nature of solvent
(2) amount of solvent
(3) freezing point of solvent
(4) (∆H)fusion of solvent
81. What happens when blood cells are placed in pure water?
(1) The fluid in blood cells rapidly moves into water.
(2) The water molecules rapidly move into blood cells.
(3) The blood cells dissolve in water.
(4) No change takes place.
82. Water transportation in plants takes place through the phenomenon of
(1) diffusion
(2) osmosis
(3) reverse osmosis
(4) reverse diffusion
83. The most suitable colligative property to determine the molar mass of polymers is (1) osmotic pressure
(2) elevation of boiling point
(3) depression of freezing point
(4) lowering of vapour pressure
84. Isotonic solutions will have the (1) same value of van ‘t Hoff’s factor
(2) same solute always
(3) same osmotic pressure
(4) different osmotic pressures
85. An unripe mango, placed in a concentrated salt solution to prepare pickle, shrivels because
(1) it gains water due to osmosis
(2) it loses water due to reverse osmosis
(3) it gains water due to reverse osmosis
(4) it loses water due to osmosis
86. At constant temperature, the osmotic pressure of a solution is
(1) directly proportional to the concentration
(2) inversely proportional to the molecular weight of the solute
(3) directly proportional to the square of the concentration
(4) directly proportional to the square root of the concentration
87. Reverse osmosis is a process
(1) in which applied pressure to the solution side is larger than the osmotic pressure
(2) in which the solvent moves from the solution of higher concentration to the solution of lower concentration
(3) which is used for the desalination of seawater
(4) all of the above
88. During osmosis, the net flow of water through a semipermeable membrane is (1) from both sides of the semipermeable membrane with unequal flow rates (2) from the solution having lower concentration only (3) from the solution having higher concentration only (4) from both sides of the semipermeable membrane with equal flow rates
Numerical Value Questions
89. An ideal solution is prepared by mixing equal masses of benzene and toluene. Find the total vapour pressure of the solution in mm Hg. (Round off to the nearest whole number)
94. Calculate elevation in boiling points for 2 molar aqueous solutions of glucose.
kg mol =
95. K f for water is 1.86 K kg mole -1 . If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol (C2H6O2) must you add to get the freezing point of the solution lowered to –2.8 oC?
96. What will be the temperature (in kelvin) at which 10% (w/v) aqueous solutions of glucose will exhibit the osmotic pressure of 16.4 atm?
97. The osmotic pressure of a solution at 300 K is 73.8 atm. Assuming non-electrolytic solute, what is the molarity of the solution?
98. Which of the following compounds has van ’t Hoff factor ‘i’ equal to 2 for dilute solution?
(1) K2SO4 (2) NaHSO4
(3) Sugar (4) MgSO4
90. A solution containing 30 g of non-volatile solute in 90 g of water has a vapour pressure of 2.8 kPa at 25 0C. The vapour pressure of water at the same temperature is 3.5 kPa. Find the molar mass of the solute in g/mol.
91. The mass of urea (NH2CONH2) required to be dissolved in 1000 g of water to reduce the vapour pressure of water by 25% is ________ g. (Nearest integer)
Given: Molar masses of N, C, O, and H are 14, 12, 16, and 1 g mol -1, respectively.
92. Calculate the percentage composition by weight of an aqueous solution of a solute (Molar mass – 150) that boils at 373.26. (Nearest integer) (Kb = 0.52).
93. 4 g of a non-electrolytic solute dissolved in 200 g of water shows an elevation of the boiling point of 0.026 °C. If the Kb of water is 0.52 K. mol−1 kg, then the molecular weight is x × 100. What is the value of ‘x’?
99. Benzoic acid undergoes dimerisation in benzene solution. The van ’t Hoff factor ( i) is
(1) i = 1 + x (2) i = 1 − x (3) i = 1 + x/2 (4) i = 1 – x/2
100. The van ’t Hoff factor is highest for _____ molal K2SO4.
(1) 1 (2) 0.001
(3) 0.1 (4) 0.01
101 For aqueous NaCl solution, the values of van ‘t Hoff factor at various concentrations are given below.
Concentration (m) van ‘t Hoff factor (i)
0.1 X 0.01 Y 0.001 Z
The correct relation between them is (1) X > Y > Z (2) Z > Y > X
(3) X + Y + Z =2 (4) X = Y = Z
Liquid Mass (g) Vapour pressure of liquid in pure state (mm Hg)
Benzene x 160 Toluene x 60
102. If BaCl 2 ionises to an extent of 80% in aqueous solution, the value of van ’t Hoff factor is
(1) 0.4 (2) 2.6 (3) 0.8 (4) 2.4
103. At infinite dilution, the degree of dissociation for sucrose in aqueous solution is (1) 0 (2) 0.5 (3) 0.99 (4) 1
104. The ratio of any colligative property for KCl solution to that of sugar solution of the same molality is (1) 1 (2) 0.5 (3) 2 (4) 3
105. The van ‘t Hoff factor i for a compound that undergoes dissociation in one solvent and association in another solvent, respectively, is (1) greater than one and greater than one (2) less than one and greater than one (3) less than one and less than one (4) greater than one and less than one
Numerical Value Questions
106. What is the maximum value of van ’t Hoff factor for AlCl3?
107. What is the maximum possible van ’t Hoff factor of CoCl3.xNH3? (Assume all ammonia is co-ordinated)
108. The observed freezing point of an aqueous solution is 0.020488 K, and the calculated freezing point of the same solution is 0.0197 K. If the van ‘t Hoff factor of solute is x, find the value of 100x.
Level - II
Types of Solutions
Single Option Correct MCQs
1. If air is taken as a binary solution, the solvent is (1) N2 (2) O2 (3) CO2 (4) H2
Methods of Expressing Concentration
Single Option Correct MCQs
2. Among the following temperture dependent concentration term is (1) Molality (2) mole fraction (3) w % V (4) w % w
3. A solution is prepared by adding 2 g of ‘X’ to 1 mole of water. Mass percent of ‘X’ in the solution is (1) 5% (2) 2% (3) 20% (4) 10%
4. Mass by volume percentage of 0.1 M NaOH aqueous solution is (1) 8% (2) 4% (3) 0.4% (4) 1.5%
5. The number of glucose molecules present in 10 ml of decimolar solution is (1) 6.0 × 1020 (2) 6.0 × 1019 (3) 6.0 × 1021 (4) 6.0 × 1022
6. H2SO4 is labelled as 9.8% by weight. Specific gravity of H2SO4 is 1.8 g/L The volume of the acid to be taken to prepare 1000 ml of 0.18 M solution is (1) 10 mL (2) 100 mL (3) 740 mL (4) 360 mL
7. The Normality of 1% (w/v)H2SO4 solution is (1) 0.1 (2) 0.2 (3) 0.4 (4) 0.02
8. The equivalent weight of divalent metal is W. The molecular weight of its chloride is : (1) W + 35.5 (2) W + 71 (3) 2W + 71 (4) 2W + 35.5
9. 6 g. of urea is dissolved in 90 g of water. The mole fraction of solute is (1) 1/5 (2) 1/50 (3) 1/51 (4) 1/501
10. A mixture contains equal masses of H2, He, O2 and CH4 at room temperature. The gas having highest mole fraction in the mixture is (1) CH4 (2) H2 (3) He (4) O2
11. At 298 K , Henry’s law constant of a gas ‘A’ dissolved in water is 1.013×10 -2 kbar. Number of mole of ‘A’ dissolved in one kg of water at 1 atm and 298 K will be (1) 11.1 (2) 555 (3) 111 (4) 5.55
12. Vapour pressure of a volatile liquid depends on many factors. Incorrect statement about vapour pressure of liquid among the following is
(1) It depends on nature of the liquid
(2) Its magnitude gets lowered by the addition of non-volatile impurities
(3) It varies with change in temperature
(4) It varies with change in surface area of the liquid
13. When two liquid components A and B are mixed to form an ideal solution, then the correct statement is ( V A and V B are volumes of the two components), Vsol =volume of the solution)
(1) Heat is liberated
(2) Heat is absorbed
(3) Vsol = VA+VB
(4) It does not obey Raoult’s law
14. All the following are non-ideal solutions except (1) Hydrochloric acid + water
(2) n-hexane + n-heptane
(3) Ethyl alcohol + water
(4) Ethyl alcohol + cyclohexane
15. If the total vapour pressure of the liquid mixture A and B is given by the equation: P=180 XA+90 then the ratio of the vapour pressure of the pure liquids A and B is given by
(1) 3:2 (2) 4:1 (3) 3:1 (4) 2:5
16. The aqueous solution with the lowest vapour pressure is: (assume 100% ionisation of solute)
(1) 0.1M BaC 2 (2) 0.1 M urea
(3) 0.1 M Na2SO4 (4) 0.1 M Na3PO4
17. Aluminium Phosphate is 100% ionised in 0.01 molal aqueous solution. Hence, b b T K ∆ is (1) 0.01 (2) 0.015
(3) 0.0175 (4) 0.02
18. The aqueous solution with the lowest freezing point is: (Assume 100% ionization of each solute)
(1) 0.1 M BaCl2 (2) 0.1M urea
(3) 0.1M Na2SO4 (4) 0.1M Na3PO4
19. For 1% solutions of KCl(I), NaCl(II), BaCl2(III) and urea (IV),osmotic pressures at the same temperature in the ascending order will be (Assume 100% ionisation of the electrolytes at this temperature)
(1) I < III < II < IV (2) IV < I < II < III
(3) I < II < II < IV (4) III < IV < I < II
20. Most suitable substance for the preparation of semi permeable membrane used in reverse osmosis is
(1) Cu2[Fe(CN)6] (2) Cellulose acetate
(3) K3[Fe(CN)6] (4) Silica gel
21. Which of the following solution will have the highest boiling point?
(1) 1 M Glucose solution
(2) 1 M Sodium nitrate solution
(3) 1 M Barium chloride solution
(4) 1 M Aluminium chloride solution
22. The ratio of molal elevation constants in aqueous solutions, a : b : c is
a) 0.1 m Glucose
b) 0.2 m Urea
c) 0.3 m Sucrose
(1) 1 : 1 : 1 (2) 1 : 2 : 3
(3) 3 : 2 : 1 (4) 6 : 3 : 2
23. The molal elevation constant is the ratio of the elevation in boiling point to (1) Molarity
(2) Molality
(3) Mole fraction of solute
(4) Mole fraction of solvent
24. K2Hgl4 is 40% ionised in aqeous solutions. The value of it’s Van’t Hoff Factor (i) is (1) 1.8 (2) 2.2 (3) 1.6 (4) 2.0
25. At 25°C for a given solution M = m, then at 50°C the correct relationship is (1) M = m (2) M > m (3) M < m (4) M = 2m
26. Molarity of a solution of density 0.3 g/mL is 2 M. Molality of the same solution under similar conditions will be (Molar mass of solute-25 g.mol–1)
(1) 6.66 m (2) 5 m (3) 8 m (4) 4.5 m
27. The molarity of 0.2 N Na 2CO3 solution is (1) 0.1 M (2) 0 M (3) 0.4 M (4) 0.2 M
28. Which of the following statements is wrong?
(1) Evaporation is a spontaneous process (2) Evaporation is a surface phenomenon (3) Vapour pressure decreases with increase of temperature
(4) The vapour pressure of a solution is always less than the vapour pressure of a pure solvent.
29. Which one of the following statements is false?
(1) Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression
(2) The osmotic pressure (π) of a solution containing a non volatile solute is given by the equation π = MRT, where M is the molarity of the solution
(3) Raoult’s law states that the vapor pressure of a component over a solution is proportional to its mole fraction in the solution
(4) The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 > KCl > CH3COOH > sucrose
30. The freezing point of equimolal aqueous solution will be highest for
(1) 653 CHNHCl + (2) Ca(NO3)2
(3) La(NO3)2 (4) C6H12O6
31. Relative lowering of vapour pressure of an aqueous solution containing non-volatile solute is 0.25, Molality of the solution is (1) 13.88 m (2) 9.8 m (3) 18.5 m (4) 21.4 m
32. Cryoscopic constant of benzene is12.5 K kg mol –1. Depression of freezing point for 0.0976 m solution containing a non–electrolyte solute in benzene is (1) 0.75 K (2) 0.9 K (3) 0.5 K (4) 0.3 K
33. Boiling point of one molal NaCl is 373.936 K. Degree of ionization of NaCl is (K b of water = 0.52 K kg mol –1)
(1) 0.75 (2) 0.8
(3) 0.66 (4) 0.92
34. At 27°C , 0.2 M glucose solution is isotonic with all the following except (1) 0.2 M urea
(2) 3.6% (w/v) glucose
(3) 1.2% (w/v) urea
(4) 0.1 M sucrose
Numerical Value Questions
35. The concentration of CO2 in a soft drink that is bottled with a partial pressure of CO 2 of 4 atm over the liquid at 25°C is ____ mol/L (The Henry’s law constant for CO2 in water at 25°C is 3.1 × 10–2 m/atm)
36. If 6 g. of urea is dissolved in 90 g. of boiling water. The vapour pressure of the solution is ___ mm Hg.
37. x gm of urea in 90 g of water, shows same lowering of vapour pressure as 18 g of glucose in 90 g of water. Thus x is ______
38. The weight of urea to be dissolved in 100 g. of water to decrease the vapour pressure of water by 5% is ____ g.(nearest integer)
Level - III
Single Option Correct MCQs
1. 1 part by mass of solute is dissolved in 3 parts by mass of solvent and an aqueous solution is prepared. Mass percentage of solute in the solution is
(1) 25 (2) 33.33
(3) 50 (4) 20
2. 100 ml of an aqueous solution contains 6.023 × 1021 solute molecules. The solution is diluted to 1 L. The number of solute molecules present in 10 ml of the dilute solution is
(1) 6.0 × 1020
(2) 6.0 × 1019
(3) 6.0 × 1018
(4) 6.0 × 1017
3 The value of P0 for benzene is 640 mm of Hg. The vapour pressure of solution containing 2.5 g of a certain substance ‘A’ in 39 g benzene is 600 mm of Hg. The molecular mass of A(g.mol–1) is
(1) 65.25 (2) 130
(3) 40 (4) 80
4. Which will form maximum boiling azeotrope?
(1) C6H6 + C6H5CH3
(2) HNO3+H2O
(3) C6H5OH +H2O
(4) n-hexane and n-heptane
5. Two volatile liquids ‘A’ and ‘B’ mixed in the mole ratio 1 : 1 form an ideal solution. Vapour pressure of pure liquid ‘A’ is 200 mm Hg and pure liquid ‘B’ is 600 mm Hg. Mole fraction of ‘B’ in vapour phase will be
(1) 0.83 (2) 0.66
(3) 0.75 (4) 0.5
6. Liquids A and B form and ideal solution in the entire composition range. At 350 K, the vapour pressures of pure A and pure B are 7×103 Pa and 12 × 103 Pa, respectively. The composition of the vapour in equilibrium with a solution containing 40 mole percent of A at this temperature is
(1) XA = 0.37; XB = 0.63
(2) XA = 0.28; XB = 0.72
(3) XA = 0.4; XB = 0.6
(4) XA = 0.76; XB = 0.24
7. An aqueous solution containing ‘m’ moles of non-volatile non-electrolyte solute freezes at –0.186 °C. The elevation in the boiling point of the same aqueous solution(Kf= 1.86 K kg mol–1, Kb = 0.512 K mol–1) would be
(1) 0.186 (2) 0.512
(3) 0.0512 (4) 0.512/1.86
8. 1.2 g of urea dissolved in 2 kg of water showed a depression of freezing point of ‘X’. 14.4 g of glucose dissolved in 4 kg of water showed a depression of freezing point of ‘Y’. Correct relation between ‘X’ and ‘Y’ is
(1) X = 2Y (2) 2X = Y
(3) X = Y (4) 4X = 5Y
9. An aqueous solution of glucose is made by dissolving 10 g of glucose (C6H12O6) in 90 g of water at 303 K. If the vapour pressure of pure water at 303 K is 32.8 mm Hg, what would be the vapour pressure (in mm Hg) of the solution?
(1) 34.8 (2) 35.5
(3) 31.8 (4) 32.44
10. At 300 K, 3.2 g of naphthalene is dissolved in CCl4 to make a 250 mL solution. Osmotic pressure of the solution is (no association or dissociation of solute occurs in the solution)
(1) 5.223 atm (2) 4.964 atm
(3) 2.463 atm (4) 1.231 atm
11. The aqueous solution with highest freezing point among the following is
(1) 0.02 m fructose (molar mass = 180 g/ mol)
(2) 0.1 m glucose ( molar mass = 180 g/mol)
(3) 0.2 m sucrose ( molar mass = 342 g/mol) (4) 0.05 m urea ( molar mass = 60 g/mol)
12. 4 g of non volatile substance A dissolved in 100 g H2O depressed the freezing point of water by 0.1°C.While 4 g of another non volatile substance B dissolved in 100 g of H2O depressed the freezing point by 0.2°C. What is the relation between molecular weights of A and B.
(1) MA = 4MB (2) MA = MB
(3) MA = 0.5MB (4) MA = 2MB
13. When a solution containing w g of urea in 1 kg of water is cooled to 0.372°C , 200 g of ice is separated. If Kf for water is 1.86 K kg mol–1, w is
(1) 4.8 g (2) 12.0 g
(3) 9.6 g (4) 6.0 g
14. 54% of a solute is dimerised in a solution. Van’t Hoff factor of the solute in the solution is
(1) 0.73 (2) 0.63
(3) 0.42 (4) 0.81
15. Wood’s metal contains 50.0% Bismuth, 25.0% Lead, 12.5% Tin and 12.5% Cadmium by weight. What is the mole fraction of Tin? (Atomic weights : Bi = 209, Pb = 207, Sn = 119, Cd = 112)
(1) 0.202 (2) 0.158
(3) 0.181 (4) 0.221
16. The density of a 56.0% by weight aqueous solution of 1-propanol (CH3CH2CH2OH) is 0.8975 g/cm3. What is the mole fraction of the compound?
(1) 0.292 (2) 0.227
(3) 0.241 (4) 0.276
17. 500 mL aqueous solution of sulphuric acid is containing 500 milli equivalents of acid. Concentration of the solution can be (1) 1 M (2) 0.1 N
(3) 0.5 N (4) 0.5 M
18. Regarding the liquid mixture of 68% Nitric acid + 32% water by mass, incorrect statement is
(1) Boiling point is lesser than 373 K
(2) It shows negative deviations from Raoult’s law
(3) D H mixing = –ve
(4) D V mixing = –ve
19. 0.1 molal aqueous solution of an electrolyte AB 3 is 90% ionised. The boiling point of the solution at 1 atm is (Kb (H2O) = 0.52 K kg mol–1)
(1) 273.19 K
(2) 374.92 K
(3) 373.19 K
(4) 376.4 K
20. Evaluate the following statements for their correctness.
A) The elevation in boiling point temperature of water will be same for 0.1 M NaCl and 0.1 M urea.
B) Azeotropic mixtures boil without change in their composition.
C) Osmosis always takes place from hypertonic to hypotonic solution.
D) The density of 32% H 2 SO 4 solution having molarity 4.09 M is approximately 1 1.26 g mL-1 .
E) A 0.1molal NaCl solution and 0.01molal solution MgCl 2 will have the same osmotic pressure at room temperature. Choose the correct answer from the options given below:
(1) B and D only (2) B, D and E only
(3) A and C only (4) A, B and D only
21. A solution of urea in water has boiling point of100.15 °C Calculate the freezing point of the same solution if Kf and Kb for water are 1.87 K kg mol –1 and 0.52 K kg mol –1 respectively.
(1) –0.54°C (2) –0.44°C (3) –0.64°C (4) –0.34°C
22. At 100 °C, the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm If Kb = 0.52 K kg mol –1, the boiling point of this solution will be
(1) 102°C (2) 103°C (3) 101°C (4) 100°C
23. For an aqueous solution of 0.1M Ba(NO 3)2 osmotic pressure at 300 K is found to be 6.576 atm. Percentage dissociation of Ba(NO3)2 in solution is nearly? (1) 91.3% (2) 84% (3) 74% (4) 100%
24. Two liquids A and B form ideal solutions. At 300 K, the vapour pressure of solution containing 1 mol of A and 3 mol of B is 550 mm Hg. At the same temperature, if one more mole of B is added to this solution, the vapour pressure of the solution increases by 10 mm Hg. Determine the vapour pressure of A and B in their pure states (in mm Hg) (1) 400, 600 (2) 500, 500 (3) 600, 400 (4) 400, 400
Numerical Value Questions
25. A solution of sugar is obtained by mixing 200 g of its 25% solution and 500 g of its 40% solution (both by mass). The mass percentage of the resulting sugar solution is ______ (Nearest integer)
26. 5 g of NaOH was dissolved in deionized water to prepare a 450 mL stock solution. What volume (in mL) of this solution would be required to prepare 500 mL of 0.1 M solution?______ Given : Molar Mass of Na, O and H is 23, 16 and 1 g mol-1 respectively.
27. The number of grams of solute present in 500 g of an aqueous 0.2 m glucose solution is ____g.
28. The number of millimoles of H2SO4 present in 5 litre of 0.2 NH2SO4 solution is ___
29. The mole fraction of a solute in a 100 molal aqueous solution is _____×10−2. (Round off to the Nearest Integer)
30. A company dissolves ‘x’ amount of CO2 at 298 K in 1 L of water to prepare soda water. ‘ x’ =_____×10–3g (nearest integer) (Given: Partial pressure of CO 2 at 298 K = 1.67 k bar, atomic mass of H, C and O is 1, 12 and 16 g/mol respectively)
31. The vapour pressure of 30% ( w/v) aqueous solution of glucose is ____mm Hg at 23°C [Given: The density of 30%( w /v) aqueous solution of glucose is 1.2 g cm–3 and vapour pressure of pure water is 24 mm Hg. [molar mass of glucose is 180 g mol –1]
32. A solution prepared by dissolving 0.8 g of naphthalene in 100 g of CCl 4 has a boiling point elevation of 0.4°C. If 1.24 g of an unknown solute in the same amount of CCl4 produced boiling point elevation of 0.62°C, then molar mass of unknown solute is ___
33. At 27oC, a solution containing 2.5 g of solute in 250.0 mL of solution exerts an osmotic pressure of 400 Pa. The molar mass of the solute is X × 101 g mol–1 (Given: R = 0.83 L bar K–1 mol–1) What is the X value?
34. Lead storage battery contains 38% by weight solution of H2SO4. The van’t Hoff factor is 2.67 at this concentration. The temperature in Kelvin at which the solution in the battery will freeze is ______(Nearest integer). Given Kf = 1.8 K kg mol-1
35. What volume of liquid (in litre) will contain 10 mol? If molar mass of liquid is 280 and its density is 1.4 gm/mL?
36. The vapour pressure of pure water at 25°C is 30 mm. The vapour pressure of 10% (w/w) glucose solution at 25°C is ____ mm.(nearest integer)
37. A decimolar solution of K4[Fe(CN)6] at 300 K is 50% dissociated, then, osmotic pressure of the solution is(in Pascal)
38. The osmotic pressure of solution of PVC in cyclohexanone at 300 K is plotted on the graph. The molar mass of PVC is X × 102, what is the X value in g mol–1 (Nearest integer)
Slope=6×10-4 C π C
(Given: R=0.083 L atm K–1 mol–1)
39. x g of non-electrolytic compound (molar mass = 200) is dissolved in 1.0 L of 0.05 M NaCl solution. The osmotic pressure of this solution is found to be 4.92 atm at 27°C. What will be the value of x ? (Assume complete dissociation of NaCl and ideal behavior of this solution)
40. Solid Lead Nitrate is dissolved in 1 litre of water. The solution was found boil at 100. 15°C. When 0.2 mol of NaCl is added to
THEORY-BASED QUESTIONS
Statement Type Questions
Directions for following questions
Each question has two statements. Statement I and statement II. Mark the correct answer as
(1) if both statement I and statement II are correct,
(2) if both statement I and statement II are incorrect,
(3) if statement I correct but statement II is incorrect,
(4) if statement I incorrect but statement II is correct.
1. S-I : The boiling point of 0.1 M aqueous solution of glucose is less than that of 0.1 M aqueous solution of 0.1 M KCl.
S-II : KCl is an electrolyte and the aqueous solution contains more number of ions.
the resulting solution, PbCl 2 formed which is ______ × 10-6 at 298 K. (Nearest integer) Given: Kb = 0.5 K kg mol–1 and Kf = 1.8 K kg mol -1 , assume molality to be equal to molarity in all cases.
41. The osmotic pressure of blood is 7.47 bar at 300 K. To inject glucose to a patient intravenously, it has to be isotonic with blood. The concentration of glucose solution in g L–1 is _____(Molar mass of glucose = 180 g mol–1]
(R = 0.083 K bar K–1 mol–1) ( Nearest integer)
42. Safrole is contained in oil of sassafrans and was once used to flavor root beer.A 2.4 mg sample of safrole was dissolved in 100 mg of diphenyl ether.The solution had a freezing point of 25.64°C.The freezing point of pure diphenyl ether is 26.84°C and freezing point depression constant Kf is 8°C/m. What is the molar mass of safrole? (nearest integer)
2. S - I : One molar aqueous solution ( d =1 gm/cc) has always higher concentration than one molal.
S-II : The molality of a solution does not depends on temperature whereas molarity depends.
3. S–I : Non-ideal solutions from azeotropic mixture.
S–II : Boiling point of azeotropic mixture is always higher than boiling points of both components.
4. S–I : Weight loss takes place both in solution bulb – I ( w 1 ) as well in solvent (H2O) bulb – II (w2).
S–II : w 1 ∝ P s and w 2 ∝ P 0 – R s where R s is vapour pressure of solution and P 0 is the vapour pressure of solvent.
5. S– I : When a non-volatile solute is added to a solvent, freezing point is lowered.
S–II : At freezing point, equilibrium exist between solid and liquid.
6. S–I : 0.1 molal NaCl solution in water (Kf = 1.86 K kg mol -1) have a freezing point of -1.86.
S-II : van’t Hoff factor of NaCl solution isequal to 2 for 100% ionization.
7. S–I : DVmix and DSmix for an ideal solution is zero.
S–II : A – B interactions in an ideal solution are nearly equal to that of A – A and B – B interactions.
8. S–I : Ethanol + water solution shows positive deviation from Raoult’s law.
S–II : Acetone + Chloroform solution shows positive deviation from Raoult’s law
9. S–I : Total vapor pressure over the solution can be related to mole fraction of any one component.
S-II : Depending on the vapor pressures of the pure components 1 and 2, total vapor pressure over the solutions decreases or increases with the increase of the mole fraction of component 1.
10. S–I : A 0.1 molal aqueous solution of NaCl freezes at the same temperature as a 0.1 molal aqueous solution of urea.
S–II : Colligative properties depends on nature of solute but not on number of solute particles
11. S–I : Rate of evaporation of liquid depends on surface area
S–II : Vapour pressure of liquid depends on surface area
12. S–I : Addition of Hgl2 to aqueous solution of KI increases the freezing point
S–II : A complex K2[Hgl4] is formed
13. S–I : Acetone + Carbon disulphide solution shows positive deviation form Raoult’s law
S–II : Acetone + Aniline solution shows positive deviation from Raoult’s law
14. S–I : The solution having greater vapour pressure has higher boiling point.
S-II : More volatile liquids have low boiling point.
15. S–I : In solution, different parts show different composition.
S-II : Solution is a homogeneous mixture.
16. S–I : The process of flow of solvent through the semi permeable membrane from pure solvent to the solution is called osmosis.
S–II : The process of osmosis can be reversed if a pressure higher than the osmotic pressure is applied on the solution side
17. S–I : At 25°C the vapour pressure of water is greater than that of diethyl ether.
S–II : Sodium amalgam is a solid in liquid type solution
Assertion and Reason Questions
In each of the following questions, a statement of Assertion (A) is given, followed by a corresponding statement of Reason (R). Mark the correct answer as
(1) if both (A) and (R) are true and (R) is the correct explanation of (A),
(2) if both (A) and (R) are true but (R) is not the correct explanation of (A),
(3) if (A) is true but (R) is false,
(4) if both (A) and (R) are false.
18. (A) : freezing point of solvent is greater than that of solution.
(R) : Non-volatile solid is added to the solvent its vapour pressure increases and become equal to solid solvent at the lower temperature
19. (A) : Molality is independent of temperature.
(R) : There is no volume factor in the expression of molality.
20. (A) : At boiling point, vapour pressure of liquid equal to atmospheric pressure
(R) : Volume of a solution increases by increasing the temperature.
21. (A) : Vapour pressure of 0.5 M sugar solution is more than 0.5 M KCl solution
(R) : Lowering of vapour pressure is directly proportional to the number of particles present in the solution.
22. (A) : Helium is used in diving apparatus
(R) : Helium is sparingly less soluble in blood.
23. (A) : Colligative properties are of smaller order for colloidal solutions as compared to values shown by true solutions at same concentration.
(R) : The number of particles in a colloidal solution is comparatively less than in a true solution at same concentration.
24. (A) : Ethylene glycol is used as an antifreeze in the radiator of a car
(R) : Addition of Ethylene glycol to water lowers its freezing point.
25. (A) : Increase in temperature increases vapour pressure of a liquid
(R) : Average kinetic energy of liquid molecules increases by increasing the temperature
26. (A) : The observed molecular mass of acetic acid in benzene is more than it’s theoretical molar mass
(R) : Molecules of acetic acid dimerise in benzene due to hydrogen bonding.
27. (A) : Helium is used to dilute oxygen in diving apparatus.
(R) : Helium has high solubility in O 2
28. (A) : All solutes become more soluble in water at higher temperature
(R) : Solubility of solute does not depend upon temperature
29. (A) : The boiling point of 0.1 M urea solution is more than that of 0.1 M KCl solution.
(R) : Elevation of boiling point is dependent on chemical nature and the number of moles of nonvolatile solute particles present in the solution.
30. (A) : At high altitudes, liquids boil at lower temperatures in comparison to that at sea level.
(R) : At high altitudes, atmospheric pressure is low.
31. (A) : Copper dissolved in gold is a solid solution
(R) : Solute determines the physical state in which solution exists.
32. (A) : A non volatile solute is added in liquid solvent the freezing point of mixture decreases.
(R) : Vapour pressure decreases by addition of non volatile solute, so equilibrium point where vapour pressure of solid and vapour pressure of liquid are equal can reach at lower temperature.
JEE ADVANCE LEVEL
Multiple Option Correct MCQs
1. In a solution 49 g of H2SO4 is dissolved in 2 L of solution. The concentration of solution may be
(1) 0.5 M (2) 0.25 M
(3) 0.5 N (4) 0.25 N
2. For a solution containing nonvolatile solute, the relative lowering of vapour pressure is 0.2, If total moles present in the solution are 5 then select the correct relations
(1) Xsolute = 0.2 (2) nsolute = 1
(3) nsolvent = 4 (4) Xsolute =0.8
3. Correct expressions among the following are: (ρ = density of solution; M = molarity of solution, M 2 = Molar mass of solute; m = molality; X2=Mole fraction of solute)
(1) 2 1000M m 1000MM = ρ−
(2)
4. Vapour pressure of water decreases from one atmosphere to one bar by dissolving glucose. (1 atm = 1.01 bar). Which statement(s) is(are) correct ? (Pbar = 1atm).
(1) The solution contains approximately 1% of glucose by moles.
(2) The solution is nearly 9% w/w
(3) The external pressure must be reduced by nearly 1% to boil the solution.
(4) The prepared solution boils at 100°C.
5. 5.3% (w/v) Na2CO3 solution and 6.3% (w/v) H2C2O4 2H2O solution have same
(1) molality (2) molarity
(3) normality (4) mole fraction
6. A solution of non-electrolyte in a particular solvent has osmotic pressure 2.0 atm at 300 K. Given Kf = 2 K kg mol-1 and R = 0.0821 L atm. K–1 mol–1 (assume molarity and the molality of given solution is the same). Then,
(1) Molality of the solution is 0.0812 mol kg–1
(2) Freezing point of the solution is –0.162 °C
(3) Molarity of the solution is 0.812 mol L–1
(4) Elevation in boiling point of the solution is less than 0.162°C
7. A binary liquid (AB) shows positive deviation from Raoult’s law when (1) oliquidoliquid AAABBB PPandPPχχ>>
(2) Intermolecular forces A – A, B – B > A – B
(3) D Vmix > 0
(4) D Hmix > 0
8. 4 mole of pure liquid A (P0 A = 80 mm of Hg) and 6 mole of pure liquid B ( P0 B = 100 mm of Hg) are mixed. The vapour pressure of the resulting solution is found to be 90 mm of Hg then
(1) It is an ideal solution
(2) The solution shows negative deviation from Raoult’s law
(3) The solution is formed by the absorption of heat
(4) The solution will boil at higher temperature than expected
9. Which of the following has same van’t Hoff factor?
(1) KCl, 50% ionised
(2) K2SO4, 40% ionised
(3) FeCl3, 30% ionised
(4) SnCl4, 20% ionised
10. Which of the following confirm minimum boiling point azeotropic mixture?
(1) CCl4 + CHCl3
(2) CH3COOH + C5H5N
(3) CH3COCH3 + CHCl3
(4) C2H5OH + H2O
11. Cryoscopic constant value depends on
(1) Molar mass of solvent in the solution
(2) Molar mass of solute in the solution
(3) The enthalpy of vapourisation of solvent
(4) Freezing point of solvent
12. Two container each containing liquid water are connected as shown in diagram.
Colorless solution
300 K 350 K Blue Color Solution
Given vapor pressure of H2O(l) at 300 K and 350 K are 22 torr and 40 torr, select correct statement(s):
(1) The final pressure in each container if valve is opened while keeping the containers at the given temperature is 22 torr.
(2) The final pressure in each container if valve is opened while keeping the containers at the given temperature is 40 torr.
(3) Color of solution in Y contains X turns blue.
(4) Mass of H2O(l) is decreased in container Y.
Numerical Value Questions
13. The vapour pressure vs. temperature curve for a solution solvent system is shown below:
81 82 83
The boiling point of the solvent is ____°C.
14. The molal freezing point constant for water is 1.85 K kg mole–1. The freezing point of 0.1m NaCl solution is -x ×10–3°C. Find the magnitude of ‘x’.
15. 224 mL of SO2(g) at 298 K and 1 atm is passed through 100 mL of 0.1 M NaOH solution. The non-volatile solute produced is dissolved in 36 g of water. The lowering of vapour Pressure of solution (assuming the solution is dilute) P° (H2O) = 24 mm of Hg) is x × 10−2 mm of Hg, the value of x is ________
16. 718.2 g of sucrose is dissolved in 1 kg of water. If ‘100+x’ is the difference between the melting and boiling point of the solutions, then report the value of ‘ x’.
17. In a solvent 50% of an acid HA dimerizes and the rest dissociates. The van’t Hoff factor of the acid is ________×10−2 (nearest integer)
18. The boiling point of an aqueous solution is found to be 100. 28°C. Then the freezing point of the same solution is –x°C. What is the value of ‘x’?
19. When 9.45 g of ClCH 2COOH is added to 500 mL of water; it’s freezing point drops by 0.5°C.
The dissociation constant of ClCH 2COOH is X × 10–3 The value of X is_____ [Kf(H2O) = 1.86 K kg mol−1]
20. For [CrCl3.xNH3], elevation in boiling point of one molal solution is double of one molal urea solution. Hence, the value of x is ___ (assuming complete dissociation)
21. The vapor pressure of benzene at a certain temperature is 640 mm of Hg. A non-volatile and non-electrolyte solid weighing 2.175 g is added to 39.08 g of benzene. If the vapor pressure of the solution is 600 mm of Hg, what is the molecular weight of solid substance?(nearest integer)
22. A mixture of two immiscible liquids, nitrobenzene and water boils at 99°C. Partial vapour pressure of water 733 mm and that of nitrobenzene 27 mm. Calculate the ratio of weights of water to nitrobenzene in the distillate.
23. Vapour pressures of pure liquids, A and B are 20 and 24 mm of Hg respectively. The total vapour pressure of a solution containing 1 mole of A and 3 moles of B is ______
24. At a certain temperature pure liquids, A and B have vapour pressures 10 torr and 37 torr respectively. For a certain ideal solution of A and B, the vapour is in equilibrium with the liquid. Mole fraction of A in the solution is 0.346. Then B A P P in the solution is.
25. The osmotic pressure of blood is 7.65 atm at 310 K. How much glucose (C 6 H 12 O 6 ) in grams should be used per litre for an intravenous injection to be isotonic to blood.
26. Vapour pressuresof ethyl alcohol and methyl alcohol are 45 mm and 90 mm respectively. An ideal solution is formed at the same temperature by mixing 46 g of C2H5OH with 40 g of CH3OH. Total vapour pressure of the solution is approximately x cm. The value of x is __________.
27. The osmotic pressure of a solution of NaCl is 0.10 atm and that of a glucose solution is 0.20 atm. The osmotic pressure of a solution
formed by mixing 1 L of the sodium chloride solution with 2 L of the glucose solution is x ×10–3 atm, the value of ‘x’ is….(nearest integer).
28. An aqueous solution containing 5% by weight of urea and 10% by weight of glucose. What will be the ΔTf of the solution (Kf for H2O is 1.860 K kg mol−1)
29. A mixture of an organic liquid A and water distilled under one atmospheric pressure at 99.2°C. How many grams of steam will be condensed to obtain 1.0 g of liquid A in the distillate? (Vapour pressure of water at 99.2°C is 739 mm Hg. Molecular weight of A = 123).
30. 29.2% (w/w) HCl stock solution has a density of 1.25 g m l –1 . The molecular weight of HCl is 36.5 g mol –1. The volume (mL) of stock solution required to prepare a 200 mL solution of 0.4 M HCl is
31. Weight of solute (M=60) that is required to dissolve in 180 g water to reduce the vapour pressure to 4/5th of pure water is 50 x g. The value of ‘x’ is
32. An aqueous sol: containing 10 g of mixture of urea and glucose boils at 100.58°C. Addition of a further 6.0 g glucose to the above solution causes it to boil at 100.77 ° C mass percentage of urea in the original mixture is 7 y, then y =?
Integer Value Questions
33. The vapour pressures of two volatile liquids A and B at 25°C are 50 torr and 100 torr respectively. If the liquid mixture contains 0.7 mole fraction of B, then the mole fraction of liquid A in the vapour phase is 17 x . The value of x at 25 °C is ____________
34. The mole fraction of urea in an aqueous urea solution containing 900 g of water is 0.05. If
the density of the solution is 1.2 g/cm 3 the molarity of urea solution is ______
35. What is the number of mole of benzene () 0 150 B Ptorr = per mole of toluene
() 0 50 T Ptorr = in vapour phase if the given solution has a vapour pressure of 120 torr in equilibrium with its vapour?
36. Two liquids A ( P° A = 500 mm ) and B(P° B = 750 mm) are mixed at temperature T in a certain ratio such that their partial vapour pressures become equal. The total vapour pressure above the liquid mixture at T is x × 102 mm where x is
37. MX2 dissociates in M+2 and X- ions in aqueous solution, with a degree of dissociation (α) of 0.01. The ratio of the observed depression of the freezing point of the aqueous solution to the value of the depression of the freezing point in the absence of ionic dissociation is:
38. If K3[Fe(CN6)] gets ionised completely in aqueous solution, number of particle in the solution from 1 molecule solute is………
39. Find the number of correct statements about azeotropic mixture
1. When a sizeable deviation from ideality occurs, the boiling point curve for a binary mixture may exhibit a maximum or minimum.
2. Mixtures having maximum or minimum boiling point are known as azeotropic mixture.
3. These mixture distilled without change in composition
4. The azeotropic composition may vary on change in temperature.
40. A 2 M aqueous solution of solute (180 g/ mol) has density 1.10 g/mL. The molality of solution is _______ (round off answer to nearest integer)
Passage-based Questions Q(41-43)
1.24 M aqueous solution of KI has density 1.15 g/cm3
41. Percentage composition of solute in the solution is (1) 17.89 (2) 27.89 (3) 37.89 (4) 47.89
42. Molality of this solution is (1) 2.61 (2) 1.31 (3) 4.12 (4) 3.12
43. Normality of this solution is (1) 0.62 (2) 1.24 (3) 2.48 (4) 3.72
Q(44-46)
Vapour Pressure of a mixture of benzene and toluene is given by P = 179XB + 92, Where XB is mole fraction of benzene.
44. Vapour pressure of the solution obtained by mixing 936 g of benzene and 736 g of toluene
(1) 199.4 mm (2) 271 mm (3) 280 mm (4) 289 mm
45. If vapours are removed and condensed into liquid then what will be the ratio of mole fraction of benzene and toluene in first condensate (1) 2.8 (2) 1.5 (3) 3.5 (4) 4.5
46. This condensed liquid again brought to the same temperature then what will be the mole fraction of benzene in vapour phase. (1) 0.07 (2) 0.93 (3) 0.65 (4) 4.5
Q(47-49)
The addition of 3 g of substance to 100 g CCl 4 (M = 154 g mol –1) raises the boiling point of CCl4 by 0.6°C. Kb (CCl4) is 5.03 K kg mol–1 , Kf (CCl4) is 31.8 K kg mol–1 and density of solution is 1.64 g/mL.
47. Calculate the depression in freezing point
(1) 3.79°C (2) 0.018°C
(3) 4.65°C (4) 251.5°C
48. The osmotic pressure at 298 K is:
(1) 3.79 atm (2) 0.018 atm
(3) 4.65 atm (4) 251.5 atm
49. The molar mass of the substance is:
(1) 37.9 (2) 18
(3) 46.5 (4) 251.5
Q(50-52)
FeCl3 on reaction with K4[Fe(CN)6] in aqueous solution gives a blue colour. These two solutions are separated by a semipermeable membrane as shown in the figure. Both the solutions are sufficiently dilute and osmosis will take place as there is a difference in concentration of the solutions. Imagine the experiment is carried out at 27°C. The osmotic flow will take place in one direction and not in both.
A B
50. Which of the following observations may be recorded after a while?
(1) No change in colour in either of the compartments
(2) Blue colour appears in compartment A
(3) Blue colour appears in compartment B
(4) Blue colour appears in both A and B
51. In the given experiment, with passage of time, the concentrations are expected to change as
(1) Concentration of FeCl3 decreases.
(2) Concentration of K4[Fe(CN)6] increases.
(3) Concentration of FeCl3 increases.
(4) Concentration of both remain unchanged.
52. The osmotic pressure (π) of FeCl 3 solution can be expressed as
(1) 6 R (2) 18 R (3) 24 R (4) 5 R
Q(53-54)
1292.5 g of aquesous solution of 5 m NaCl is kept in a large bucket. The bucket is placed under a tap from which 2 m aqueous solutions of NaCl is flowing. Rate of flow of solution from tap is 0.5 g/s
53. The total amount of solutions (in g) finally present in bucket when solution present in bucket have concentration of NaCl 4 m is
(1) 558.5 (2) 1851
(3) 1351 (4) 1938.75
54. The time (in seconds) after which the bucket will have 4 m concentration of NaCl.
(1) 117 (2) 2000
(3) 1117 (4) 1292.5
Q(55-57)
In a mixture of closely related liquids (such as benzene and toluene), the Raoult law states that the ratio pA is proportional to the mole fraction of A in the liquid, PA = P°XA. Mixtures that obey the law throughout the composition ranges from pure A to pure B are called ideal solutions. In ideal solution the solute also obeys Raoult’s law. Then the total pressure is given by
0 0 totalABB A PPxPx =+
55. Each of the following solution obeys Raoult’s law except
(1) n-hexane + n-heptane
(2) Methanol + Ethanol
(3) carbon tetrachloride + carbon disulphide
(4) chloroform + Acetone
56. For the solution which obeys Raoult’s law, which of the following is incorrect?
(1) D Vmix = 0
(2) D Hmix = 0
(3) D Smix = 0
(4) 00 totalAABB PPxPx =+
57. Benzene and toluene forms nearly an ideal solutions in which the mole fraction of the benzene is 0.4. If vapour pressure of pure benzene is 40 mmHg and toluene is 30 mmHg at a given temperature, then what is the mole fraction of toluene in the vapour phase?
(1) 0.6 (2) 0.47
(3) 0.53 (4) 0.74
Q(58-59)
Answer the questions given below which are based on the following diagram.
59. This type of deviation is also expected in the following mixture
(1) C2H5OH + C6H12
(2) C2H5Br + C2H5Cl
(3) C6H5CN + C2H5CN
(4) C2H5 – O – C2H5 + CHCl3
Q(60-62)
The freezing point of 0.2 molal solution of acetic acid in benzene is 277.65 K. Freezing point of pure benzene is 278.4 K and heat of fusion of benzene is 10.042 kJ/mol. If molarity of solution is equal to molality, and acetic acid is found in equilibrium with its dimmer, then answer the following based on this data
60. Kf for benzene is (1) 3 K × molality–1 (2) 2 K × molality–1 (3) 5 K × molality–1 (4) 10 K × molality–1
61. The degree of association of acetic acid should be
(1) 0.60 (2) 0.5
(3) 0.92 (4) 0.75
62. Calculate the equilibrium constant for dimerisation of acetic acid
58. Solution containing compounds A and B shows this type of deviation from ideal behaviour when
Attraction A .... B DVmix
(1) Larger than average of A...A, B...B attraction +ve +ve larger than expected
(2) As in (a) –ve –ve As in (a)
(3) Smaller than average of A...A, B...B attraction +ve +ve Smaller than expected
(4) As in (c) –ve –ve As in (c)
(1) 10 (2) 5 (3) 20 (4) 40
Q(63-64)
Properties such as boiling point, freezing point and vapour pressure of a pure solvent change when solute molecules are added to get homogeneous solution. These are called colligative properties, Applications of colligative properties are very useful in day-to-day life. One of its examples is the use of ethylene glycol and water mixture as an anti-freezing liquid in the radiator of automobiles. A solution, M is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is 0.9. Given, freezing point depression(Kf)constant of water is 1.86 K kg mol−1 and ethanol is 2.0 K kg mol−1. Boiling point elevation (K b) constant of water is 0.52 K kg mol−1 and ethanol is 1.2 K kg mol−1
Standard freezing point of water=273K
Standard freezing point of ethanol = 155.7 K
Standard boiling point of water= 373K
Standard boiling point of ethanol= 351.5 K
Vapour pressure of pure water= 32.8 mm Hg
Vapour pressure of pure ethanol= 40 mm Hg
Molecular weight of water=18 g mol −1
Molecular weight of ethanol= 46 g mol −1
In answering the following questions consider the solution to be ideal dilute solutions and Solutes to be non-volatile and non-dissociative.
63. The freezing point of the solution, M (in kelvin scale) is
64. The vapour pressure of the solution, M(in kelvin scale) is
Matrix Matching Questions
65. Match List I (concentration term) with List II (property)
List I (concentration term) List II (property)
A. Mole fraction I) Number of gram equivalents in 1000 mL of solution
B. Molarity II) Always less than one
C. Normality III) Greater than or equal to molarity
D. Molality IV) Number of g mole present in 1000 ml solution
V) Number of g mole of solute 1 kg of solvent
(A) (B) (C) (D)
(1) I II III IV
(2) II III I V
(3)
66. Ma tch List I (Molarity) with List II (Normality)
List I (molarity )
List II (normality)
A. 0.5M H2SO4 solution I) 0.1N solution
B. 0.1M NaCl solution II) 1 N solution
C. 0.2M AlCl3 solution III) 1.5 N solution
D. 0.5M H3PO4 solution IV) 2.0 N solution
V) 0.6 N solution
(A) (B) (C) (D)
(1) II IV I III
(2) IV II I V
(3) II I V III
(4) V IV II I
67. Match List I (property) with List II(formula) List-I (property) List-II (formula)
A. Lowering of vapour pressure I) o o PP P
B. Relative lowering of vapour pressure II) o o PPwM PmW =×
C. Raoult’s law III) P0−P
D. Ideal solution IV) Obeying Raoult’s law
V) Boiling point
(A) (B) (C) (D)
(1) III I II IV
(2) IV I II III
(3) I II III IV
(4) II III IV I
68. Match List I (Mixture) with List II (property)
List I (Mixture) List II (property)
A. n-hexane, n-heptane I) Dimerisation
B. CO2, H2O II) Ionisation
C. C6H5COOH, Benzene III) Raoult’s law (obey)
D. C6H5COOH, H2O IV) Henry’s law (obeys)
(A) (B) (C) (D)
(1) III IV I II
(2) IV I II III
(3) I II III IV
(4) II III IV I
69. Match List I (graph) with List II (suitable variables)
List-I (graph) List-II (suitable variables)
A. x y I) Vapour pressure vs. composition
B. y x II) Boiling point of non-ideal mixture vs. composition
C. x y III) log (vapour pressure) vs (temperature)–1
D. y x IV) Vapour pressure vs. temperature.
(A) (B) (C) (D)
(1) III IV I II
(2) IV I II III
(3) I II III IV
(4) IV III II I
70. Match List I (Experimental method) with List II (property measured)
List-I (method) List-II (property measured)
A. OstwaldWalker I) Osmotic pressure
B. Cotrell’s method II) Depression of freezing point
C. Rast’s camphor method III) Elevation of boiling point
D. Berkeley and Hartley’s method IV) Lowering of vapour pressure
(A) (B) (C) (D)
(1) IV III II I
(2) IV III I II
(3) III IV II I
(4) IV II III I
71. Match List I with List II
List I (Solution) List II (property)
A. van’t Hoff factor, i I) Cryoscopic constant
B. Kf II) Isotonic solutions
C. solutions with same Osmotic pressure III) Normal molar mass Abnormal molar mass
D. Azeotropes IV) Solution with same composition of vapour above it
(A) (B) (C) (D)
(1) III II I IV
(2) III I II IV
(3) I III II IV
(4) III I II IV
72. Match List-I with List-II and select the correct answer using the codes given below the lists:
List-I (solution mixture) List-II (mixture property)
A. Benzene + Toluene I) formation of azeotropic mixture
B. Chloroform + acetone (propanone) II) non-ideal solution with positive deviation from Raoult’s law
C. Ethanol + Acetone III) ideal solution
D. HCl+ Wataer IV) D Hmix = 0
(A) (B) (C) (D)
(1) III,IV I I I, II
(2) III, IV I I, II I
(3) I III, IV I I, II
(4) I III, IV I, II I
73. Match the Column – I with Column – II and mark the appropriate choice Column-I (Mixture) Column-II (property)
A. Ethyl alcohol + water I) P = P0X
BRAIN TEASERS
1. Consider equimolal aqueous solutions of NaHSO4 and NaCl with ΔTb and ΔTb as their respective boiling point elevations. The value of 1 b V b T Lt T
will be
B. Benzene + Toluene II) Effect of pressure on gas solutions
C. Henry’s law III) Ideal solution
D. Raoult’s law IV) Azeotropic mixture
(A) (B) (C) (D)
(1) I II III IV
(2) I III II IV
(3) IV III II I
(4) III II I IV
74. Match List-1 (compound) with List-II (Vant Hoff factor) and select the correct answer using the codes given below the lists:
List I (compound) List II (van’t Hoff factor)
(A) Al2(SO4)3; α = 0.7 I) i = 3.4
(B) Na3PO4; α = 0.9 II) i = 2.8
(C) CaCl2; α = 0.9 III) i = 3.8
(D) FeCl3; α = 0.8 IV) i = 3.7
(A) (B) (C) (D)
(1) III IV II I
(2) I II IV III
(3) II I III IV
(4) III II IV I
2. The vapour pressure of an aqueous solution is found to be 750 torr at certain temperature T, if T is the temperature at which pure water boils under atmospheric pressure and the same solution show elevation in boiling point, Δ T b =1.04 K, find the atmospheric pressure in torr (Kb = 0.52 K kg mol−1)
3. A solution of a non-volatile solute in ethanol (B.P of ethanol = 78.4°C) has a vapour pressure of 730 mm of Hg at 78.4°C. To what temperature, the above solution must be heated have the pressure of 760 mm of Hg? Kb of ethanol = 1.22 K kg mol–1. Express temperature of 10x. The value of x is __
4. An organic compound (Mol.wt = 100) exist in “Monomeric form” in its neutral aqueous solution at 25°C with every one unit decrease in pH of the substance dimerises by 50% extent out of the present monomers. With every one unit increase in pH, it ionises into two ions by 50% extent. (ignore impact of H+)
form of hydrogen fluoride molecules present is 4: 1. If 1/x is the fraction of total molecules present in the final sample as H 2 F 2 molecules, what is the value of x ? (Report the value to nearest integer) Hint: Initially there are only HF molecules. Atomic mass of F and H are 19 and 1 respectively.
6. Given below are two statements. One is labeled as Assertion (A) and the other is labeled as Reason (R).
I. What is the molecular weight of the substance at pH = 9 determined by colligative methods?
(1) 57.1
(2) 62.8
(3) 44.5
(4) 79.2
II. At what pH, the molecular weight of the substance is determined to be 133.3 through colligative property experiments?
(1) 6
(2) 5.3010
(3) 4.690
(4) 4
III. At pH = 8, at 1 atm, for 1 molal aqueous solution (Kb = 0.52 K kg mol−1), here, Tb = ?
(1) 100.52°C
(2) 100.26°C
(3) 100.78°C
(4) 101.04°C
5. Hydrogen fluoride gas is collected in a vessel and left for some time. Then, a constant molar mass of the sample is experimentally determined as 34 g/mol. This abnormal molar mass is due to dimerization as well as trimerization of some HF molecules. The mole ratio of monomeric and trimeric
(A) : At 10°C, the density of a 5 M solution of KCl [atomic masses of K and Cl are 39 and 35.5 g mol–1]. The solution is cooled to -21 °C . The molality of the solution will remain unchanged.
(R) : The molality of a solution does not change with temperature as mass remains unaffected with temperature.
In light of the above statements, choose the correct answer from the options given below.
(1) Both (A) and (R) are true and (R) is the correct explanation of (A).
(2) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(3) (A) is true but (R) is false.
(4) (A) is false but (R) is true.
7. Two 5 molal solutions are prepared by dissolving non-electrolyte, non-volatile solute separately in the solvents X and Y. The molecular weights of solvents are M x and M y respectively where M x = 4 3 y M .The relative lowering of vapour pressure of the solution in “X” is m times that of the solution in “Y”. Given that the number of moles of solute is very small in comparison to that of solvent, the value of m is:
(1) 4/3 (2) 3/4
(3) 1/2 (4) 1/4
8. Using vapour pressure versus temperature graph match the following: 12 , 33
Column I (temperature)
Column II (vapour pressure and mole faction)
A. P at 90° I) 0.23
B. Xchlorobenzene in vapour phase II) 0.73
C. Xbenzene in vapour phase at 130°C III) 733.33
D. P at 130°C IV) 433.33
(A) (B) (C) (D)
(1) III IV I II
(2) IV I II III
(3) I II III IV (4) II III IV I
9. Henry’s constant (in kbar) for four gases α,β,γ and δ in water at 298 K is given below: gases α β γ δ KH 50 2 2×10–5 0.5 (density of water =103 kg/m3 at 298 K). This table implies that (1) ‘α‘ has the highest solubility in water at a given pressure.
(2) Solubility of ‘γ‘ at 308 K is lower than at 298 K
(3) The pressure of 55.5 molal solution of ‘γ‘ is 1 bar
(4) The pressure of 55.5 molal solution of ‘δ‘ is 250 bar
10. Solutions containing 23 g HCOOH is/are:
(1) () solution w 46g of 70%HCOOH d=1.40 g/ml V
(2) 50g of 10 M HCOOH, dsolution =1 g/ml
(3) w 50 g of 25%HCOOH w
(4) 46 g of 5MHCOOH dsolution =1 g/ml
11. Number of correct statements is/are ‘a’ and incorrect statements ‘ b’. Then a X b is
1) n–Hexane and n–Heptane form ideal solution
2) Bromoethane and chloroethane form ideal solution
3) Benzene and Toulene form ideal solution
4) ethanol and acetone form non–ideal solution with –ve deviation
5) Carbon disulphide and acetone form non ideal solution with +ve deviation
6) Phenol and aniline form non–ideal solution with –ve deviation
7) Chloroform and acetone form non–ideal solution with +ve deviation
8) van’t Hoff factor (i) = Abnormal molar mass normal molar mass
12. If 0.05 mol of gas are dissolved in 500 g of water under 1 atm pressure, 0.1 mol will be dissolved if the pressure is 2 atm. It illustrates
(1) Graham’s Law
(2) Dalton’s Law
(3) Henry’s Law
(4) Boyle’s Law
I. Consider some facts and select the correct facts.
I) This is observed when A....B attractions are greater than average of A....A and B....B attraction.
II) ΔHmix = +ve, ΔVmix =+ve
III) Boiling point is smaller than expected such that vaporisation is increased
IV) Mixture is called Azeotropic mixture
(1) I, II, III
(2) II, III, IV
(3) I, III, IV
(4) I, II, III, IV
II. Total vapour pressure of mixture of 1 mol of volatile component A(P0 A = 100 mm Hg) and 3 mol of volatile component B(PB 0 = 60 mm Hg) is 75 mm. For such case
(1) There is +ve deviation from Raoult’s law
(2) Boiling point has been lowered
FLASH BACK (Previous JEE Questions)
JEE Main
1. A solution of two miscible liquids showing negative deviation from Raoult's law will have (2024)
(1) increased vapour pressure, increased boiling point
(2) increased vapour pressure, decreased boiling point
(3) decreased vapour pressure, decreased boiling point
(3) Force of attraction between A and B is smaller than that between A and A or between B and B
(4) All the above statements are correct
14. Two volatile liquids X and Y form an ideal binary solution and variation of vapour pressure versus mole fraction is decribed in the following diagram. Which of the following statement regarding pure X, pure Y and the solution of X and Y is correct?
(P x 0>P y0)
Vapour Pressure 240 mm mole fraction
(1) At point Z, the mole fraction of X in the vapour phase, which is in equilibrium with the solution is 0.6.
(2) Vapour pressure of X in pure state is greater than that of Y in pure state at same temperature
(3) Attractive intermolecular interaction between X-X in pure liquid X and Y-Y in pure liquid Y is same
(4) Vapour pressure of solution at point Z is 480 mm of Hg
(4) decreased vapour pressure, increased boiling point
2. The quantity which changes with temperature is (2024)
(1) Molarity (2) Mass percentage
(3) Molality (4) Mole fraction
3. Volume of 3 M NaOH (formula weight 40 g mol–1) which can be prepared from 84 g of NaOH is ________ × 10 –1 dm3. (2024)
4. A solution H2SO4 is 31.4% H2SO4 by mass and has a density of 1.25 g/mL. The molarity of the H2SO4 solution is _______ M (nearest integer) [Given molar mass of H2SO4 = 98 g mol–1] (2024)
5. Molality (m) of 3M aqueous solution of NaCl is: (Given: Density of solution = 1.25 g mL –1 , Molar mass in g mol–1 : Na –23, Cl–35.5) (2021)
(1) 3.85 m (2) 2.79 m (3) 1.90 m (4) 2.90 m
6. When 'x' × 10–2 mL methanol (molar mass = 32g; density = 0.792 g/cm 3) is added to 100 mL of watr (density = 1 g/cm3), the following diagram is obtained.
[Given density of the solution = 1.25 g/mL]
9. At 20°C, the vapour pressure of benzene is 70 torr and that of methyl benzene is 20 torr. The mole fraction of benzene in the vapour phase at 20°C above an equimolar mixture of benzene and methyl benzene is_____ × 10−2. (Nearest integer) (2021)
10. The vapour pressures of A and B at 25°C are 90 mm Hg and 15 mm Hg respectively. If A and B are mixed such that the molefraction of A in the mixture is 0.6, then the mole fraction of B in the vapour phase is x × 10–1. The value of x is _______ . (Nearest integer) (2021)
11. Of the following four aqueous solutions, total number of those solutions whose freezing point is lower than that of 0.10 M C 2H5OH is ………… . (Integer answer) (2021)
[Given, Kw = 1 × 10−14 and Kb = 1.8 × 10−5]
12. 83 g of ethylene glycol dissolved in 625 g of water. The freezing point of the solution is ………K. (Nearest integer) (2021)
7. 0.05M CuSO 4 when treated with 0.01M Cu 2 Cr 2 O 7 given green color solution of Cu2Cr2O7. The two solutions are separated as shown below” [SPM: semi permeable Membrane]
K2Cr2O7 CuSO4
Side X SPM Side Y
Due to osmosis
(1) Molarity of CuSO4 solution is lowered.
(2) Green colour formation observed on side Y.
(3) Molarity of K2Cr2O7 solution is lowered.
(4) Green colour formation observed on side X.
8. Molarity (M) of an aqueous solution containing x g of anhyd. CuSO4 in 500 mL solution at 32°C is 2 × 10–1 M. Its molality will be ____ × 10 –3 m. (nearest integer).
[Use, molal freezing point depression constant of water = 1.86 K kg mol −1 , Freezing point of water = 273 K and Atomic masses: C = 12.0 u, O = 16.0 u, H = 1.0 u]
13. 1 kg of 0.75 molal aqueous solution of sucrose can be cooled up to − 4ºC before freezing. The amount of ice (in g) that will be separated out is ……… . (Nearest integer)
[Given, Kf H2O=1.86 K kg mol−1] (2021)
14. A cylinder containing an ideal gas (0.1 mol of 1.0 dm3 ) is in thermal equilibrium with a large volume of 0.5 molal aqueous solution of ethylene glycol at its freezing point. If the stoppers, S1 and S2 (as shown in the figure) are suddenly withdrawn, the volume of the gas in litres equilibrium is achieved will be________ ( Given, Kf water =2.0 K kg mol−1 R = 0.08 dm3 atm K−1mol−1) (2020)
15. A graph of vapour pressure and temperature for three different liquids X, Y and Z is shown below:
(2) leaves the vapour decreases
(3) leaves the vapour increases
(4) leaves the solution increases
17. For 1 molal aqueous solution of the following compounds, which one will show the highest freezing point? (2018)
(1) [Co(H2O)6]Cl3
(2) [Co(H2O)5Cl]Cl2.H2O
(3) [Co(H2O)4Cl2]Cl.2H2O
(4) [Co(H2O)3Cl3].3H2O
18. The freezing point of benzene decreases by 0.45°C when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid associates to form a dimer in benzene, the percentage association of acetic acid in benzene will be: (Kf for benzene=5.12 K kg mol −1) (2017)
(1) 74.6% (2) 94.6%
The following inferences are made:
A) X has higher intermolecular interactions compared to Y
B) X has lower intermolecular interaction compared to Y
C) Z has lower intermolecular interactions compared to Y
The correct inference(s) is/are: (2020)
(1) B and C (2) A
(3) B (4) A and C
16. An open beaker of water in equilibrium with water vapour is in a sealed container. When a few grams of glucose are added to the beaker of water, the rate at which water molecules. (2020)
(1) leaves the solution decreases
CHAPTER TEST – JEE MAINS
Section – A
(3) 64.6% (4) 80.4%
19. 18 g glucose C 6H 12O 6 is added to 178.2 g water. The vapor pressure of water (in torr) for this aqueous solution is: (2016)
(1) 76.0 (2) 752.4 (3) 759.0 (4) 7.6
JEE Advanced
20. An aqueous solution is prepared by dissolving 0.1 mol of an ionic salt in 1.8 kg of water at 35 ºC. The salt remains 90% dissociated in the solution. The vapor pressure of the solution is 59.724 mm of Hg. Vapor pressure of water at 35 ºC is 60.000 mm of Hg. The number of ions present per formula unit of the ionic salt is _______. (2022)
1. Insulin is dissolved in a suitable solvent and the osmotic pressure (π) of solution of various concentrations (g/cm 3 ) C is measured at 20°C . The slope of π against C is found to be 4.65×10−3 . The molecular weight of the insulin (g/mol) is: (R = 0.0821 L atm mole−1 K−1) (1) 3 × 105 (2) 9 × 105 (3) 4.5 × 105 (4) 5.16 × 106
2. 6 g of a non volatile solute is dissolved in 90 g of water, such that the lowering in vapour pressure is 2%. The molecular weight of the solute is (considered solution is in dilute condition)
(1) 65 (2) 92
(3) 60 (4) 80
3. Molality of an aqueous solution that produces an elevation of boiling point of 1.00 K at 1 atm pressure. (K b for water = 0.512 K kg mol–1)
(1) 0.512 M
(2) 0.19 m
(3) 1.95 m
(4) 5.12 M
4. The degree of dissociation (α) of a weak electrolyte, Ax B y is related to van’t Hoff factor (i) by the expression:
(1)
i
5. The volume of 0.02 M aqueous HBr required to neutralize 10.0 mL of 0.01 M aqueous Ba(OH)2 is (Assume complete neutralisation)
(1) 5.0 mL (2) 10.0 mL
(3) 2.5 mL (4) 7.5 mL
6 What is the mass ratio of ethylene glycol, C2H6O2 (molar mass = 62 g/mol) required for making 500 g for 0.25 molal aqueous solution and 250 mL of 0.25 molal aqueous solution?
(1) 1:1 (2) 2:1
(3) 3:1 (4) 1:2
7. Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is
(1) 2.28 mol kg-1 (2) 0.44 mol kg-1
(3) 1.14 mol kg-1 (4) 3.28 mol kg-1
8. The quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO 2 pressure at 298 K is____ g (Henry’s law constant = 1.67× 108 Pa at 298 K)
(1) 0.64
(2) 1.86
(3) 6.4
(4) 18.6
9. 105g of a sample of water contain 16.2 g of Ca(HCO3)2, it’s hardness is
(1) 200 ppm
(2) 300 ppm
(3) 60 ppm
(4) 100 ppm
10. If Al2(SO4)3 is 80% ionized in water, then the value of vant Hoff’s factor [ i] for Al2(SO4)3 in water is
(1) 3.2
(2) 4.2
(3) 4.8
(4) 3.8
11. At 298 K, Henry’s law constant is highest for (1) CO2 (2) O2
(3) HCHO (4) He
12. Sea water is found to contain 5.85% NaCl and 9.50% MgCl 2 by weight of solution. Calculate its normal boiling point assuming 80% ionization for NaCl and 50% ionization of MgCl2 (Kb(H2O) = 0.51 kg mol–1 K)
(1) Tb = 101.9°C
(2) Tb = 102.3°C
(3) Tb = 108.5°C
(4) Tb = 108.5°C
13. Which of the following show negative deviation from Raoult’s law?
(1) C2H5OH and H2O
(2) HNO3 and H2O
(3) CHCl3 and CCl4
(4) C6H6 and C6H5CH3
14. 11.1 g. of CaCl 2 is present in 100 mL of the aqueous solution. The chloride ion concentration is.
(1) 1 M (2) 2 M
(3) 0.5 M (4) 0.2 M
15. As temperature increases, vapour pressure of a liquid
(1) increases linearly (2) decreases linearly (3) increases exponentially (4) decreases exponentially
16. 6.0230 × 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is
(1) 0.01 M (2) 0.001 M
(3) 0.1 M (4) 0.02 M
17. The number of ions present in 1 mL of 0.1 M CaCl2 solution is
(1) 1.8 × 1020
(2) 6.0 × 1020
(3) 1.8 × 1019
(4) 1.8 × 1021
18. K H of N 2 is 1×10 5 atm. The moles of N 2 dissolved in 10 mol water is (given pressure of air is 5 atm and mole fraction of N2 in air is 0.8)
(1) 4 × 10–4 (2) 4 × 10–5 (3) 5 × 10–2 (4) 5 × 10–4
19. Which is correct about Henry’s law ?
(1) The gas in contact with the liquid should behave as an ideal gas.
(2) There should not be any chemical interaction between the gas and liquid.
(3) The pressure applied should be high.
(4) Henry’s law is valid through out entire rage of pressures.
20. 34. 2 g of cane sugar(Mol.Wt =342) is dissolved in 100 g of water and a solution is prepared. Molality of solution is (1) 1 m (2) 0.1 m (3) 0.01 m (4) 0.2 m
Section – B
21. You are given 500 mL of 2 N HCl and 500 mL of 5 N HCl. What will be the maximum volume of 3 M HCl that you can make from these two solutions
22. The total pressure of a mixture of non-reacting gases X (0.6 g) and Y(0.45 g) in a vessel is 740 mm of Hg. The partial pressure of the gas X is _____ mm of Hg. (Nearest Integer) (Given: molar mass X=20 and Y=45 g mol−1)
23. If a g of non-volatile solute (A) is dissolved in b g of pure liquid (B), the vapour pressure becomes 9 10 th of it’s original value. The molecular weight of the solvent (B) is 1 5 th of the molecular weight of solute (A). Find (b/a)?
24. A solution of 1.73 g of ‘A’ ( non-volatile and non-electrolyte ) in 100 cc of water is found to be isotonic with a 3.42% (wt. /vol.) solution of sucrose (C12H22O11) at same temperature. Molecular weight of ‘A’ is______g/mole. (Molar mass of C12H22O11=342 g/mol)
25. K f for water is 1.86 K. kg/mol. If your automobile radiator holds 1 kg of water, how many grams of ethylene glycol [C2H6O2] must you add to get the freezing point of the solution lowered to −2.8°C?
CHAPTER TEST – JEE ADVANCED
2020 P1 Model
SECTION – A
[Single Option correct MCQ's]
1. Pure water freezes at 273 K and 1 bar and boils at 373 K and 1 bar. Addition of nonvolatile solute changes the freezing and boiling point of water. Boiling point of an aqueous solution is 100.56°C. Latent heat of fusion and latent heat of vaporization of water are 80 cal g −1 and 540 cal g −1 respectively. The figures shown below represent plot of vapour pressure (V.P) versus temperature (T). Identify the correct plot.
(1) Water Ice 1
V.P/bar
Water+Ethanol
270 273 T/K
(2) Water Ice 1
V.P/bar
Water+Ethanol
271 273 T/K
(3) Water Ice 1
V.P/bar
Water+Ethanol
270 273 T/K
(4) Water Ice 1
V.P/bar
Water+Ethanol
271 273 T/K
2. A complex is represented as CoCl 3.x NH 3 Its 0.1 molal solution in water shows ΔTf = 0.5580. Kf for H2O is 1.86 K kg mol-1 . Assuming 100% ionization of complex and coordination number of Co is 6. Calculate the value of x
(1) 3 (2) 4
(3) 5 (4) 6
3. Absolute alcohol cannot be obtained by simple fractional distillation because:
(1) Pure C2H5OH is unstable
(2) C2H5OH do not form hydrogen bonds with water
(3) Boiling point C2H5OH is very close to that of water
(4) Constant boiling azeotropic mixture is formed with water
4. Insulin (C2H10O5)n is dissolved in a suitable solvent and the osmotic pressure π(atm) of solutions of various concentrations (g / ml) C is measured at 20°C. The slope of a plot of π against ‘C’ is found to be 4.65×10 −3 Molecular weight of insulin is
(1) 4.8×105 g
(2) 9 × 105 g
(3) 3 × 105 g
(4) 5.16 × 106g
5. The plots of 1 XA (on y-axis) vs 1 AY (on x-axis)(where X A and YA are the mole fraction of liquid A in liquid and vapour phase respectively) is linear with slope and y-intercept respectively.
(1) () 00 0 00 AAB BB PPP and PP
(2) () 00 0 00 BAB AB PPP and PP
(3) () 00 0 00 ABA BB PPP and PP
(4) () 00 0 00 BBA AB PPP and PP
6. The total concentration of dissolved particles inside red blood cells is approximately 0.30 M and the membrane surrounding the
cells is semi permeable. What would the osm otic pressure (in atmosphere) inside the cells become if the cells were removed from the blood plasma and placed in pure water at 298 K?
(1) 7.34 atm
(2) 1.78 atm
(3) 2.354 atm
(4) 0.74 atm
SECTION – B
[Multiple Option correct MCQ's]
7. Equimolal solutions of NaCl, BaCl 2 and Na3PO4 are prepared in water, then correct statements are
(1) Freezing point of NaCl solution is –2°C if freezing point of BaCl2 solution is –3°C
(2) Freezing point of Na 3 PO 4 is –4°C if freezing point of BaCl2 is –3°C
(3) Elevation in boiling point for BaCl 2 solution is 1.5 times higher than NaCl solution
(4) Elevation in boiling point for NaCl is half of Na3PO4 solution
8. Consider the following arrangement and choose the correct options.
M Na2so4
M KCL
Semipermeable membrane
(1) Osmotic pressure of Na2SO4 solution is lesser than the osmotic pressure of KCl solution
(2) Water will flow from KCl solution to Na2SO4 solution
(3) Water will flow from Na2SO4 solution to KCl solution
(4) Osmotic pressure of Na2SO4 solution is higher than the osmotic pressure of KCl solution
9. One mole of a liquid A (vapour pressure = 300 mm Hg) and 2 mole of a liquid B (vapour pressure = 360 mm Hg) are mixed. The mole fraction of A in the vapour mixture if found to be 5/17. Which of the following is correct?
(1) (ΔH)mix > 0
(2) (ΔS)mix > 0
(3) (ΔV)mix = 0
(4) (ΔG)mix > 0
10. Choose the pairs having identical value of van’t Hoff factor.
(1) 0.05 M K 4 [Fe(CN) 6 ] (50% degree of dissociation) and 0.05 M Mohr’s salt (80% degree of dissociation)
(2) 0.2 M NaCl (80% degree of dissociation) and 0.2 M BaCl 2 (40% degree of dissociation)
(3) 0.05 M Na 3 PO 4 (60% degree of dissociation) and 0.05 M K4[Fe(CN)6] (45% degree of dissociation)
(4) 0.01 M NaNO 3 (90% degree of dissociation) and 0.01 M FeCl3 (30% degree of dissociation)
11. A graph is plotted with temperature of a solution containing benzene and toluene as a function of mole fraction. Choose the correct options.
b Vapour
(1) a → b represents evaporation
(2) b → c represents condensation
(3) c → d represents evaporation
(4) c → d represents condensation
12. Consider the following graphs.
B=0
B=1
A=0
A=1
B=0
Choose the correct statements.
B=0
A=1
(1) According to both graphs mole fraction of A > mole fraction of B in condensate
(2) Graph I belongs to minimum boiling azeotrope
(3) Graph II belongs to maximum boiling azeotrope
(4) Graph II belongs to minimum boiling azeotrope while graph I belongs to minimum boiling azeotrope
SECTION – C
[Numerical Value Questions]
13. Lowering of vapour pressure of 1.00 m solution of a non-volatile solute in a hypothetical solvent of molar mass 40 g at its normal boiling points is ___ torr.
14. For the solution of the gases w,x,y and z in water at 298 K, the Henry’s law constants (KH) are 0.5, 2, 35 and 40 kbar, respectively. Find number of correct plots for the given data___
15. When 3.00 g of a substance ‘x’ is dissolved in 100 g of CCl4. It raises the boiling point by 0.60 K, the molar mass of the substance ‘ x’ is _________ g mol−1(Given: Kb for CCl4 is 5.0 K kg mol−1 )
16. The following phase diagram shows a very small part of the solid–liquid phasetransition boundaries for two solutions of equal concentration. Substance A has i = 1, and substance B has i = 3. (i = van’t Hoff factor) 2 1 0 5 10 15 T(oC) P(atm) 20
What is the melting point of the pure liquid solvent in centigrade scale?
17. Liquids, A and B form ideal solution over the entire range of composition. At temperature T, equimolar binary solution of liquids A and B has vapour pressure 45 Torr. At the same temperature, a new solution of A and B having mole fractions, XA and XB respectively has vapour pressure of 22.5 torr. The value of XA/XB in the new solution is _____.(Given that the vapour pressure of pure liquid A is 20 torr at temperature T)
18. 80 mole percent of MgCl 2 is dissociated in aqueous solution. The vapour pressure of 1.0 molal aqueous solution of MgCl2 at 38°C ________mm Hg. (Nearest integer) Given: Vapour pressure of water at 38°C is 50 mm Hg
ANSWER KEY JEE Mains Level
Level-III
(41) 54 (42) 160
Theory-based Questions (1) 1 (2) 1 (3) 3 (4) 1 (5) 1 (6) 4 (7) 4 (8) 3 (9) 4 (10) 2 (11) 3 (12) 1 (13) 3
JEE Advanced Level
Brain Teasers
Flashback
Chapter Test – JEE Mains
Chapter Test –
JEE
Advanced (1) 3 (2) 5 (3) 4 (4) 4 (5) 3 (6) 1 (7) 1,2,3,4 (8) 2,4 (9) 2,3,4 (10) 2,3,4 (11) 1,2,3 (12) 1,2,3 (13)