Removal of KAPs for Initial Keratin Biomaterial Preparation from Hair Mariana Cabral, Roche C. de Guzman Fred DeMatteis School of Engineering and Applied Science, Hofstra University, Hempstead, NY 11549 INTRODUCTION
METHODS
Keratins are classified as intermediate filaments (IFs) of animal cytoskeletal proteins. The IFs of interest to our lab are the type I and type II: acidic and basic keratins, respectively. Intermediate filaments are remarkably diverse structures and unique to each cell. The main function of IFs is to maintain cell integrity by conferring organization and support. Specifically, IFs provide mechanical strength, biocompatibility, gelation, and degradability, making keratins good biomaterials for tissue engineering applications. Hair keratins (Fig. 1) are formed due to keratinization and cornification processes, self-assemble into bundles, and are embedded in matrix keratin associated proteins (KAPs), held together by disulfide bonds. To obtain keratins, KAPs can initially be removed via reduction of disulfides in the presence of alcohol which renders keratin IFs insoluble.
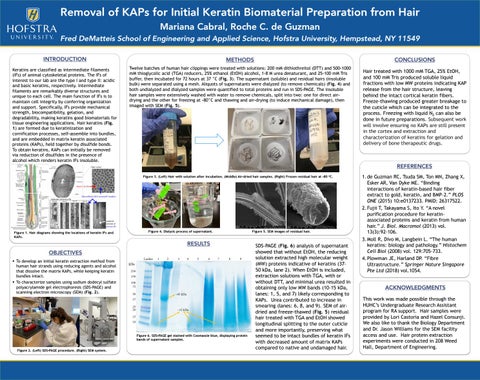
Twelve batches of human hair clippings were treated with solutions: 200 mM dithiothreitol (DTT) and 500-1000 mM thioglycolic acid (TGA) reducers, 25% ethanol (EtOH) alcohol, 1-8 M urea denaturant, and 25-100 mM Tris buffer, then incubated for 72 hours at 37 °C (Fig. 3). The supernatant (soluble) and residual hairs (insoluble bulk) were separated using a mesh. Aliquots of supernatants were dialyzed (to remove chemicals) (Fig. 4) and both undialyzed and dialyzed samples were quantified to total proteins and run in SDS-PAGE. The insoluble hair samples were extensively washed with water to remove chemicals, split into two: one for direct airdrying and the other for freezing at -80°C and thawing and air-drying (to induce mechanical damage), then imaged with SEM (Fig. 5).
CONCLUSIONS
REFERENCES Figure 3. (Left) Hair with solution after incubation. (Middle) Air-dried hair samples. (Right) Frozen residual hair at -80 °C.
Figure 1. Hair diagrams showing the locations of keratin IFs and KAPs.
Figure 4. Dialysis process of supernatant.
RESULTS OBJECTIVES § To develop an initial keratin extraction method from human hair strands using reducing agents and alcohol that dissolve the matrix KAPs, while keeping keratin bundles intact. § To characterize samples using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and scanning electron microscopy (SEM) (Fig. 2).
Figure 6. SDS-PAGE gel stained with Coomassie blue, displaying protein bands of supernatant samples. Figure 2. (Left) SDS-PAGE procedure. (Right) SEM system.
Hair treated with 1000 mM TGA, 25% EtOH, and 100 mM Tris produced soluble liquid fractions with low MW proteins indicating KAP release from the hair structure, leaving behind the intact cortical keratin fibers. Freeze-thawing produced greater breakage to the cuticle which can be integrated to the process. Freezing with liquid N2 can also be done in future preparations. Subsequent work will involve ensuring no KAPs are still present in the cortex and extraction and characterization of keratins for gelation and delivery of bone therapeutic drugs.
Figure 5. SEM images of residual hair.
SDS-PAGE (Fig. 6) analysis of supernatant showed that without EtOH, the reducing solution extracted high molecular weight (MW) proteins indicative of keratins (3750 kDa, lane 2). When EtOH is included, extraction solutions with TGA, with or without DTT, and minimal urea resulted in obtaining only low MW bands (10-15 kDa, lanes: 1, 5, and 7) likely corresponding to KAPs. Urea contributed to increase in smearing (lanes: 6, 8, and 9). SEM of airdried and freeze-thawed (Fig. 5) residual hair treated with TGA and EtOH showed longitudinal splitting to the outer cuticle and more importantly, preserving what seemed to be intact bundles of keratin IFs with decreased amount of matrix KAPs compared to native and undamaged hair.
1. de Guzman RC, Tsuda SM, Ton MN, Zhang X, Esker AR, Van Dyke ME. “Binding interactions of keratin-based hair fiber extract to gold, keratin, and BMP-2.” PLOS ONE (2015) 10:e0137233. PMID: 26317522. 2. Fujii T, Takayama S, Ito Y. “A novel purification procedure for keratinassociated proteins and keratin from human hair.” J. Biol. Macromol (2013) vol. 13(3):92-106. 3. Moll R, Divo M, Langbein L. “The human keratins: biology and pathology.” Histochem Cell Biol (2008) vol. 129:705–733. 4. Plowman JE, Harland DP. “Fibre Ultrastructure.” Springer Nature Singapore Pte Ltd (2018) vol.1054.
ACKNOWLEDGMENTS This work was made possible through the HUHC’s Undergraduate Research Assistant program for RA support. Hair samples were provided by Lori Castoria and Hazel Consunji. We also like to thank the Biology Department and Dr. Jason Williams for the SEM facility access and use. Hair protein extraction experiments were conducted in 208 Weed Hall, Department of Engineering.