Periodic Trends of Superalkalkine Earth (HMHC) Sodides Angelo T. Tiglias, René Riedel, Daniel P. Miller.
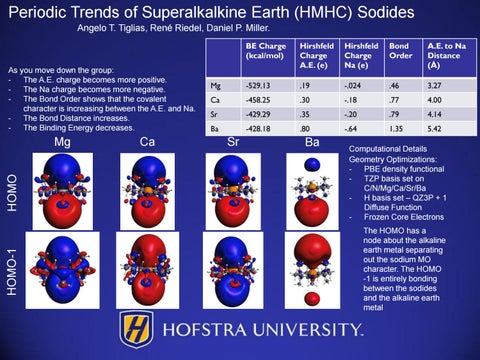
As you move down the group: The A.E. charge becomes more positive. The Na charge becomes more negative. The Bond Order shows that the covalent character is increasing between the A.E. and Na. The Bond Distance increases. The Binding Energy decreases.
HOMO-1
HOMO
Mg
Ca
BE Charge (kcal/mol)
Hirshfeld Charge A.E. (e)
Hirshfeld Charge Na (e)
Bond Order
A.E. to Na Distance (Å)
Mg
-529.13
.19
-.024
.46
3.27
Ca
-458.25
.30
-.18
.77
4.00
Sr
-429.29
.35
-.20
.79
4.14
Ba
-428.18
.80
-.64
1.35
5.42
Sr
Ba
Computational Details Geometry Optimizations: PBE density functional TZP basis set on C/N/Mg/Ca/Sr/Ba H basis set – QZ3P + 1 Diffuse Function Frozen Core Electrons The HOMO has a node about the alkaline earth metal separating out the sodium MO character. The HOMO -1 is entirely bonding between the sodides and the alkaline earth metal