

My Blood My Health
Leukiemia
Myeloma
Chronic Lymphocyte Luekemia
Chronic Myleoid Luekemia
Myleoproliferative Neoplasms

Welcome to My Blood, My Health Digital Magazine
Welcome to this edition of My Blood My Health, I am honoured to welcome you to this special edition of My Blood My Health, dedicated to Blood Cancer Awareness Month. This issue is more than just a publication—it’s a platform to inform, inspire, and empower everyone impacted by blood cancers.
Each article has been carefully crafted to provide practical resources, share the latest research insights and treatment updates, and feature the perspectives of patients, caregivers, and healthcare professionals From strategies to improve quality of life to updates on innovative therapies, our goal is to make complex information accessible and meaningful, helping you make informed choices on your journey.
At Heal Canada, we believe that awareness leads to action. By sharing stories of resilience, raising critical questions, and amplifying the patient voice, we are building a community where knowledge is power and advocacy drives change As you read through this issue, I invite you to reflect, share, and connect with others in our growing global network. Together, we can shine a brighter light on blood cancers and continue the vital work of education, support, and advocacy


Cheryl Petruk, MBA B.Mgt. CEO & Founder of Heal Canada
Heal Canada's mission is to empower patientstoaccessbetterandequitable services. We're deeply grateful to our readersfortheircontinuedinterestand support. Your engagement drives us to produceinsightfulandvaluablecontent that encourages patient-centricity in healthcare.
Thank you for participating in our journey, and welcome to this enlighteningissue!
With great humility for reading out a digitalmagazine, Cheryl.
MyBloodMyHealth
Publisher HealCanada www.healcanadaorg
Email:admin@healcanadaorg
ExecutivePublisher&EditorinChief
CherylPetruk EditorialTeam
HealCanadaStaff/Consultants
ScientificAdvisors
MBMHScientificConsultants
Marketing&Outreach HealCanada
PublishedBy
HealCanada–Anationalnot-for-profitorganizationdedicatedto advancingpatientadvocacythrougheducation,empowerment,and engagement PublicationFrequency Bi-Monthly|DigitalOnly
Copyright ©2025MyBloodMyHealthDigitalMagazine.Allrightsreserved.No partofthispublicationmaybecopied,reproduced,ortransmittedin anyformwithoutpriorwrittenconsentofthepublisher
Disclaimer: The Patient Advocacy Digital Magazine provides general information and resources to promote patient empowerment and awareness. The content is not a substitute for professional medical advice or treatment. Always consult with qualified healthcare professionals for personalized guidance medical condition or situation.


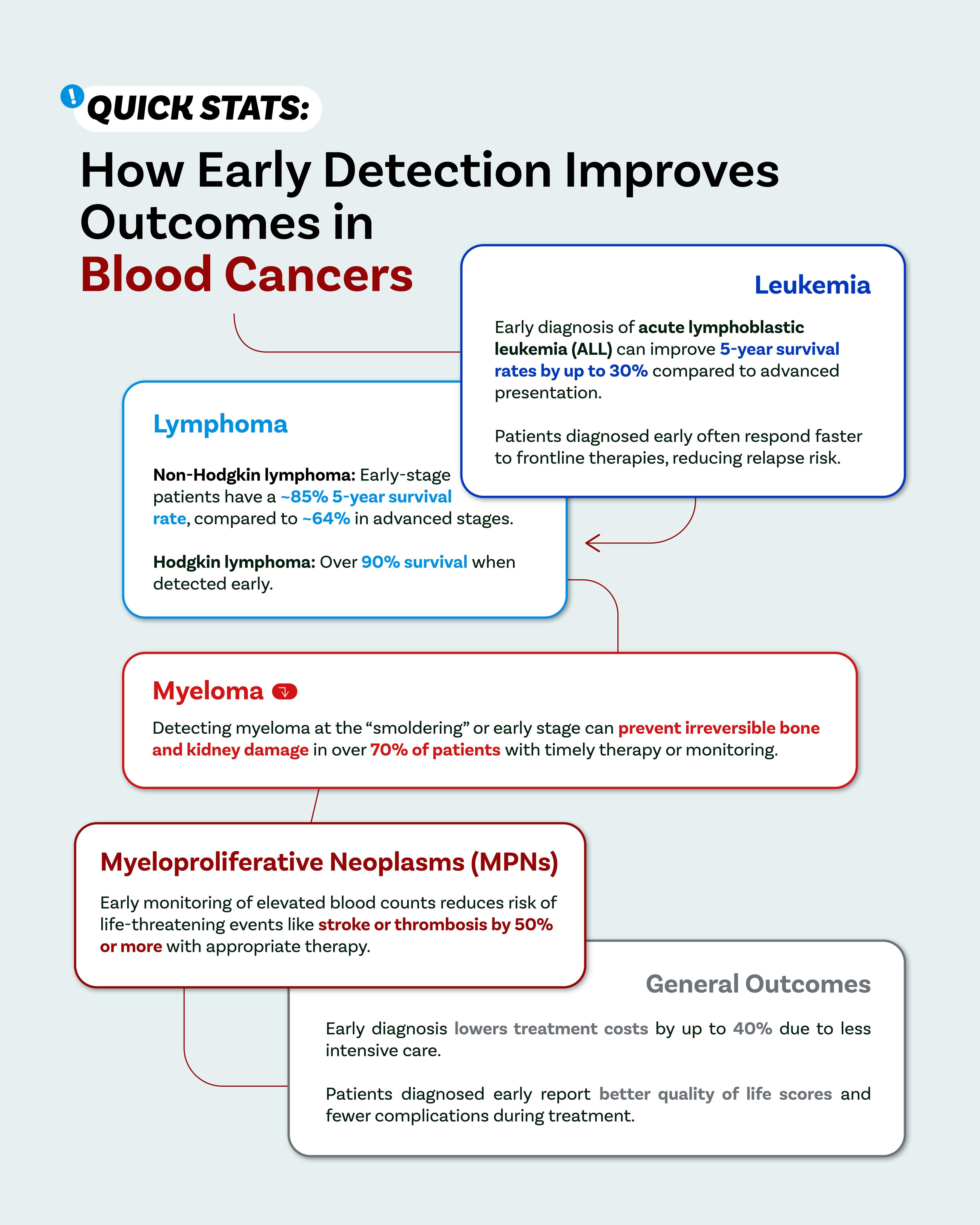
The Power of Awareness: Why Early Detection Matters in Blood Cancers
by Dr. Kemi Okwuegbuna and Cheryl Petruk, MBA
acted on warning signs, provide expert commentary to deepen your understanding, and offer a clear symptom checklist readers can and should use to spark proactive conversations with healthcareproviders.
EarlyDetectionSavesLives:TheWhy
TheAdvantageofCatchingBloodCancersEarly
While blood cancers often progress differently from solid tumours, early detection still offers criticaladvantages:
Treatment flexibility and less toxicity: In early stages, many therapies can be less aggressive while still effective. Early-stage chronic leukemias or indolent lymphoma, for instance, may be managed with watchful waiting or gentle therapies, avoiding the heavier regimens needed for moreadvanceddisease.
Preserving organ function and reducing complications: In diseases like multiple myeloma, early therapycanpreventirreversiblebonedamage,kidneyfailure,oranemia
Accesstocurativeoptions:Somebloodcancers,suchasacuteleukemiasorcertainlymphomas, arepotentiallycurable particularlywhentreatmentbeginsbeforediseaseburdenbecomestoo heavy.
Improvedqualityoflife:Earlydiagnosismeansfewersymptomslikefatigue,infections,orsevere anemia,helpingpatientsremainactiveandfunctional.
Understandingthesestakesmakesawarenessnotjustbeneficial—itcanbetransformational

The Power of Awareness: Why Early Detection Matters in Blood Cancers
ExpertInsights:UnderstandingtheSignals
Todeepenunderstanding,clinicalexpertshavetoldus:
“Bloodcancersoftenbeginsubtly,”“Symptomslikefatigue,nightsweats,weightloss,orenlarged lymph nodes may develop gradually or be mistaken for aging, stress, or infection. But when thesepersist,ordon’tfitatypicalpattern,it’stimetocheck.”
Theimportanceofroutinebloodwork especiallycompletebloodcount(CBC) forearlyclues: Elevatedorlowwhitecellcounts
Anemia(lowredbloodcells/hemoglobin)
Lowplatelets
Presenceofimmatureorabnormalcells
“When such flags appear, even if symptoms seem mild, recommend swift follow-up with a hematologyconsult”
“Akeychallengeisthatearlysignsofbloodcancersoverlapwithmanybenignconditions “Fever, feverishsweats,orswollennodescanbeduetoviralillnesses Butwhatsetsbloodcancersapart ispersistenceorlackofresponsetousualtreatment oracombinationofsymptoms”
Certain red-flag combinations such as fatigue paired with weight loss, or new pain paired with bruising shouldpromptshadowinglabs,imaging,orbiopsy.
“Don’t wait for a dramatic illness. When subtle symptoms persist beyond a few weeks, get a completebloodcounttest evenifit’s‘justtocheck.’Itcouldtrulysavealife.”
WhyAwarenessStillFallsShort—andHowWeCanChangeThat Despiteclearbenefits,earlydetectionstrugglesagainstmultiplebarriers:
Misattribution of symptoms: Many confuse symptoms with aging, stress, depression, or minorinfections—andsodelaymedicalattention
Fear and denial: The thought of “Cancer?” is frightening Some delay seeking care out of anxietyordenial
Access issues: Not everyone has ready access to health care, regular screening, or routine labs barriersthatmaydelaydetection
Awareness gaps: Public recognition of blood cancer symptoms is limited compared to other cancers (like breast or colon). Few remember their blood counts or know to discuss hematologicflags.

The
Power of Awareness: Why Early Detection Matters in Blood Cancers
StrategiestoImproveAwareness
Education campaigns: Organizations, clinics, and communities can spotlight symptom checklists especiallyduringSeptember.
Community outreach: Mobile clinics, health fairs, informational webinars, and posters in communitycentersorpharmacies.
Trainingprimarycareproviders:Makingsurefamilydoctorsandnursepractitionersknowthe subtlesigns andusesimpleCBCtestsgenerously.
Empowering patients: Encouraging readers that asking “Could this be blood cancer?” is a powerfulphrase onethateverypatienthastherighttoask.
YourSymptomChecklist:WhentoTalktoaHealthcareProfessional Belowisahandychecklisttohelppeoplerecognizepotentialearlysignsofbloodcancers Ifyou or someone you know checks more than one box below consistently, consider asking for a medical evaluation. Note: This is not a replacement for medical advice. If you experience any symptoms,pleaseseekmedicaladvicefromalicensedmedicalpractitioner.
Persistent fatigue or weakness
Unusual or excessive bruising or bleeding (gums, nose, skin)
Unexplained bone or joint pain
Elevated blood counts (red cells, white cells, platelets) on lab work
Unexplained weight loss (>5% in weeks –months)
Persistent night sweats, chills
Itching after warm showers or with no rash (aquagenic pruritus)
New or worsening anemia
Frequent or severe infections
Painless lumps or swollen lymph nodes (neck, groin, armpit)
Feeling “full” quickly or achy under ribs
Abnormal protein levels or elevated calcium (from routine labs
Note: None of these symptoms alone confirms cancer—but when paired with lab abnormalities or persistence over weeks, they merit evaluation.

The Power of Awareness: Why Early Detection Matters in Blood Cancers
TheRippleEffect:BeyondDiagnosistoOutcomes
Earlydetectiondoesn’tjustmatterforanindividual’sprognosis—ithasbroaderimpacts:
Reducing treatment intensity: Starting care sooner often spares patients from highly toxic, prolongedtherapies.
Lowering healthcare costs: Early, controlled disease tends to require fewer interventions and hospitalizations.
Protecting families and systems: Timely treatment reduces complications, urgent admissions, andlong-termburdensonfamiliesandhealthcaresystems
Hope and empowerment: Early diagnosis often allows greater control over one’s schedule, energy,andlifeplans andinjectsemotionalandpsychologicalhope.
CalltoAction:WhatYouCanDoThisSeptember—andBeyond
Forindividuals
Familiarizeyourselfwiththesymptomchecklistabove Ifsomethingunusualpersistsmorethanafewweeks,askforbloodtests. Printorsavethechecklist,andsharewithfriends,family,orcaregivers.
Forcaregiversandlovedones
Encourage action—even gentle nudges (e.g. “Hey, maybe get some blood work just in case?”) can sparkcrucialearlyevaluation.
Forcommunitygroupsandworkplaces
Distributesymptomcards,hostawarenesstalks,orinvitehematologistsforQ&Asessions
Forhealthcareproviders
KeepthethresholdfororderingCBCslowwhensymptomspersist.
Empowerstafftoflagsuspiciousresultsswiftly.
ClosingReflections
Blood cancers may lurk quietly, often camouflaged as benign symptoms—but their early warning signs hold immense power As Sarah, David, and Maria remind us: listening to our bodies and pursuing answers can change trajectories. Through collaborative efforts encompassing education, storytelling, and accessible tools like our symptom checklist early detection becomesarealisticandattainablegoalformany.
This September and every month—let’s honour the power of awareness. Let's empower ourselves and our communities with knowledge, drive early conversations with healthcare professionals,andchampionpathstowardtimelydiagnosisandbetteroutcomes
Stayinformed.Stayvigilant.Knowwhattoask.Itmightjustsavealife




CACHEducation is evolving to better serve the needs of patient advocates and healthcare professionals with its rebrand to CACHEducation Academy. This transformation reflects an expanded commitment to delivering high-quality, structured learning experiences tailored to the ever-changing landscape of patient advocacy and healthcare education. As part of this rebrand, CACHEducation Academy will introduce Advanced Curriculum offerings starting in November 2025, providing deeper insights, specialized training, and enhanced skill development for those looking to elevate their expertise. This next phase marks a significant step forward in strengthening the capacity and impact of patient advocates through comprehensive and innovative education.
"Enrolling in CACHEducation was a game-changer for me as a patient advocate. The program provided invaluable knowledge, practical skills, and a supportive community that empowered me to make a real impact in healthcare advocacy."
Understanding the Causes of Blood Cancer by Dr. Kemi Okwuegbuna and Cheryl Petruk, MBA
Currentmedicalandacademictheories,withkeyliterature
Bloodcancers(leukemias,lymphomas,andmyelomas)arisewhenapopulationofblood-forming cellsacquiresadvantagesthatletitexpandabnormally,evadenormalcontrols,andoutcompete healthy cells. No single cause explains all cases; rather, converging factors genetic alterations, agingbiology,environmentalexposures,infections,immuneandmicroenvironmentalinfluences, and inherited predisposition cooperate over time (a “multi-hit,” evolutionary process). Below is acrisptourofwhatweknow(anddon’t),anchoredtohigh-qualityacademicsources.
1)Somaticgenetics:theimmediate“engine”ofdisease
Most blood cancers are driven by acquired (non-inherited) DNA changes in hematopoietic stem/progenitorcells.
Chromosomal translocations create fusion oncogenes that act like stuck accelerators. Classic examples include BCR-ABL1 in chronic myeloid leukemia and PML-RARA in acute promyelocytic leukemia (APL). These aren’t just markers they’re causal drivers and therapeutic targets (e.g., ATRA/arsenicinAPL).
Recurrent point mutations activate growth pathways or derail differentiation In the myeloproliferative neoplasms (MPNs), JAK2 V617F (2005), MPL W515 (2006), and CALR (2013) mutationsareprototypicinitiatinglesionsthatdysregulateJAK–STATsignalling
Hematologic malignancies often reflect cooperating classes of mutations some promote proliferation; others block maturation or alter epigenetic programs fitting long-standing “multistep” models of leukemogenesis. Modern genomic studies extend this to a Darwinian, somatic evolutionframework.(Seesectionsonclonalhematopoiesisandaging.)

Understanding the Causes of Blood Cancer
2)Agingbiology&clonalhematopoiesis:fertilegroundforcancer
With aging, hematopoietic stem cells (HSCs) accumulate “clocklike”mutations(e.g.,SBS1/SBS5signatures)andfunctionaldecline (“inflammaging,” DNA repair stress), creating a background where mutantclonescanexpand.
A central concept is clonal hematopoiesis of indeterminate potential (CHIP/CH) age-related, leukemia-associated mutations (DNMT3A, TET2, ASXL1, etc.) in blood cells without overt cancer. Large cohorts show CH confers a markedly elevated future risk of hematologic malignancy (eg, ~10- to 13-fold in some analyses), thoughmostindividualswithCHneverdevelopleukemia
Why does it matter? CH reframes causation: many blood cancers start years earlier as expanding “pre-leukemic” clones that later acquire cooperating hits under selective pressures (age, inflammation,cytotoxictherapy).
3)Environmentalcarcinogens:radiationandchemicals Someexposureshaverobustcausallinks:
Ionizing radiation (e.g., Hiroshima/Nagasaki Life Span Study) produces a strong, dose-related increase in leukemia risk one ofthefirstandclearestlateeffectsdetected.
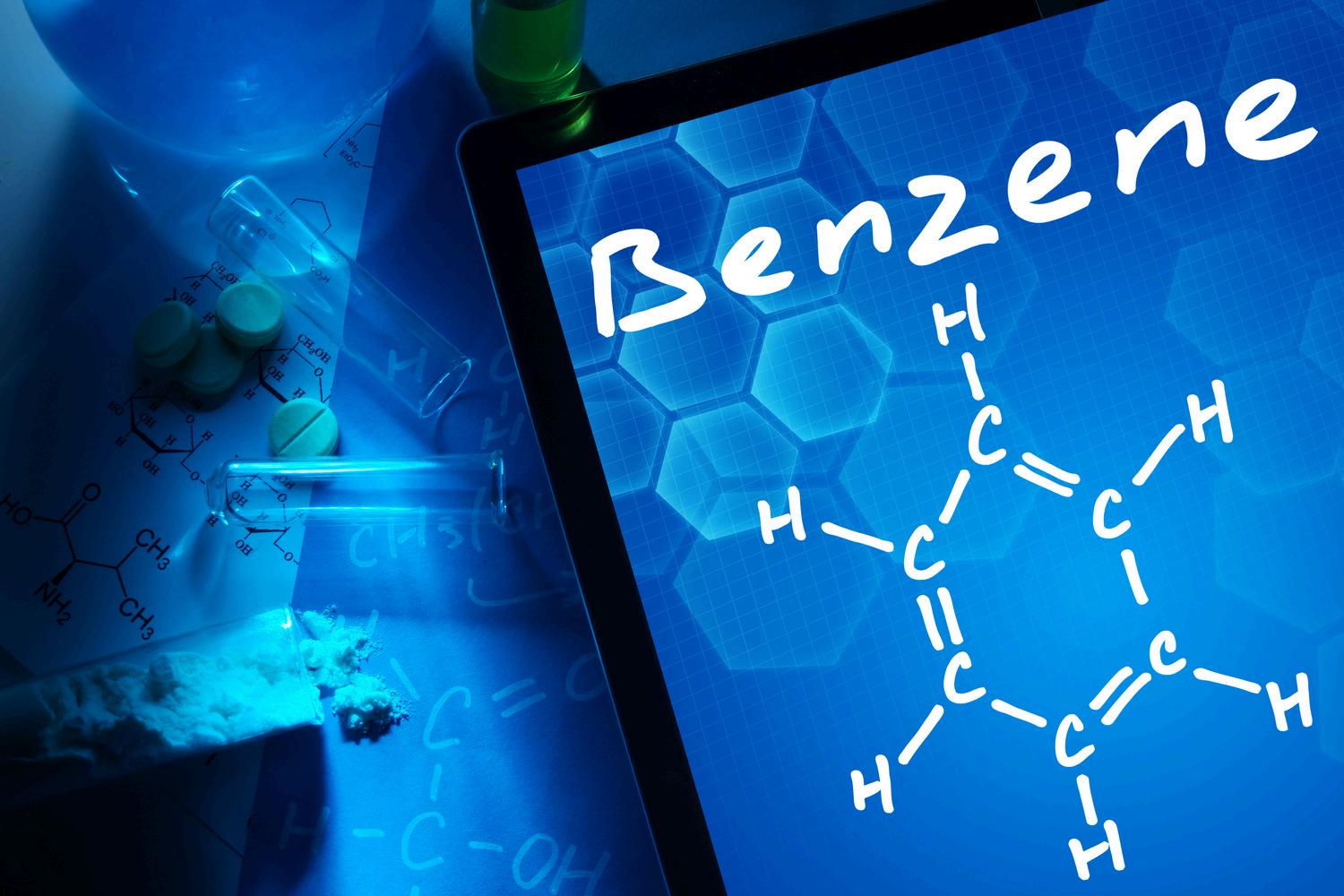
Benzene is a Group 1 carcinogen (IARC) that causes myeloid malignancies; risk relates to cumulative exposure in occupationalandenvironmentalsettings
Formaldehyde is classified as carcinogenic to humans; evidenceforleukemiariskhasstrengthenedovertime,buthas been debated current summaries note sufficient evidence overall,withhistoricalqualifiersregardingleukemia.
Cigarettesmokingincreasestheriskofacutemyeloidleukemia (AML); authoritative reports (e.g., the 2014 U.S. Surgeon General) and public-health agencies list AML among smokingcausedcancers.




Understanding the Causes of Blood Cancer
4)Infectionsandimmunestimulation
Certain pathogens causally contribute to distinct blood cancers, often via chronic antigenic drive or direct oncogenic programs.
Helicobacter pylori infection can cause gastric MALT lymphoma;antibioticeradicationfrequentlyinduceslymphoma regression, a rare example where removing a microbial driver treatsacancer.
Epstein–Barr virus (EBV) is etiologically linked to several lymphomas (e.g., endemic Burkitt, a subset of Hodgkin lymphoma, post-transplant lymphoproliferative disease) and someepithelialcancers.
Hepatitis C virus (HCV) is associated with increased nonHodgkin lymphoma risk; in indolent B-cell lymphomas, directacting antivirals can trigger lymphoma responses as viremia clears implicatingthevirusindiseasemaintenance
Theoretical note. These links illustrate antigen-driven clonal selection and virus-mediated oncogenesis as credible causal pathwaysinhematologicmalignancy.
5) Therapy-related disease: when cures create risk
Prior chemotherapy/radiotherapy can initiate therapy-related myeloid neoplasms (t-MNs)—now formalized in contemporary classifications (WHO-HAEM5 and ICC) as myeloid neoplasms post-cytotoxic therapy (MN-pCT). Typical mutational patterns reflect alkylating agents, topoisomerase II inhibitors, and prior radiation.

Understanding the Causes of Blood Cancer
6) Bone-marrow “soil”: the niche and microenvironment
Beyond the “seed” (mutant HSC), the bone-marrow microenvironment (BMME) can foster or restrain malignancy. PubMed Haematologica talks about elegant mouse genetics showed that disrupting osteolineage progenitors (e.g., Dicer1 deletion) can initiate MDS-like disease and secondary AML, even though leukemic cells retain wild-type Dicer1 evidence that a defective niche can be causal.
Human and preclinical studies also show that the BMME supports leukemic cell survival, immune evasion, and therapy resistance, making it an emerging therapeutic target.
7)
Inherited predisposition: when risk runs in families
Puttingittogether:aunifyingcausalpicture
A minority of blood cancers arise on a germline foundation. Recognized syndromes include RUNX1, GATA2, ETV6, ANKRD26 and, notably in adults, DDX41 germline variants (often with a second somatic DDX41 hit), typically presenting as MDS/AML in mid-to-late adulthood. Identification affects surveillance, donor selection, and counseling.
Most blood cancers reflect stepwise somatic evolution in long-lived HSCs. Aging seeds the field with CH clones; exposures (radiation/chemicals), chronic immune stimuli or infections, therapy, andnichedysfunctionapplyselectivepressures;germlinevariantssetbaselinerisk.Thedisease youseeintheclinicistheend-productofthisyears-long“competition”

Understanding the Causes of Blood Cancer
What remains uncertain?
Why do some CH clones progress while most remain indolent? (Work continues on clone size, specific genes, and inflammatory stressors.)
Which microenvironmental alterations are initiating versus permissive in humans and how best to therapeutically re-educate the niche.
Practical takeaways
Most blood cancers arise from acquired mutations interacting with ageing biology; a minority are rooted in germline predisposition.
Established external causes include ionizing radiation, benzene, cigarette smoking, and specific infections (e.g., H. pylori, EBV, HCV) in defined entities.
The bone-marrow niche and inflammation are active players—not just bystanders offering new preventive and therapeutic targets.


Heal Canada and Pat ADV Hub in the USA have embarked on a collaborative journey, aiming to revolutionize the realm of patient advocacy across North America. This pioneering partnership brings together two influential organizations from neighbouring countries, combining their extensive expertise and resources.
The objective is to expand and enhance the access to critical information for patient advocates, ensuring that individuals across the continent receive the best possible support and guidance in their healthcare journeys.
By bridging the gap between Canadian and American healthcare advocacy, this alliance promises to foster a more informed, empowered, and connected community of patient advocates, significantly contributing to the improvement of healthcare experiences for countless individuals.
patadvhub@gmail.com

Emerging Treatments for Blood Cancers in Canada (2025) by Dr. Kemi Okwuegbuna and
Cheryl Petruk, MBA
Blood-cancer care is moving fast. In just the last few years, Canada has seen a surge of advanced immunotherapies—especially CAR T-cell therapies and bispecific antibodies—alongside nextgeneration targeted medicines that home in on the mutations or pathways that drive disease. Some of these options are already approved and funded; others are newly authorized and working their way through pan-Canadian review and provincial adoption. For patients and families, the result is more choice, more personalization, and (in some settings) treatment earlier in the journey, not just after many lines of therapy. Health Canada and national health technology bodies have also sharpened their focus on safety and real-world value, which directly shapes what’s offered where you live.
The big immunotherapy shift
CAR T-cell therapy extends to earlier
lines in myeloma
A headline development for Canadians living with multiple myeloma is ciltacabtagene autoleucel (CARVYKTI) being authorized by Health Canada for use as early as second line—for adults who’ve had 1–3 prior therapies and are lenalidomide-refractory. That means some patients can access a one-time, highly active cellular therapy sooner than before, potentially changing the trajectory of their disease and time on continuous therapy. Funding decisions remain provincial, but several jurisdictions have begun working through implementation.
CAR-T safety: honest talk about rare risks
At the same time, Canadian regulators have been transparent about a rare risk of secondary Tcell malignancies after CAR-T. Health Canada completed a safety review and is aligning product information across all CAR-T products; clinicians are advised to monitor long-term. The U.S. FDA added a class-wide boxed warning in 2024, and Canada’s InfoWatch outlined label updates in December 2024. The message isn’t “don’t use CAR-T”—it’s “use CAR-T wisely, counsel clearly, and monitor for years” Benefits still outweigh risks in approved uses
Made-in-Canada manufacturing and access research
Canada continues to build domestic CAR-T capacity and clinical networks (for example, Canadian-led initiatives to manufacture CAR-T in Canada and study access/feasibility). Local capacity can shorten vein-to-vein times, reduce logistical risk, and potentially expand access outside major hubs over time

Emerging Treatments for Blood Cancers in Canada (2025)
Bispecific antibodies: “off-the-shelf” T-cell redirection
While CAR-T re-engineers your T cells, bispecific antibodies act like pre-built matchmakers that bring your T cells to the cancer Canada has seen progress in lymphomas and myeloma with CD20×CD3 and BCMA×CD3 agents (availability and funding vary by province and indication, and some products are still in regulatory or HTA pipelines). These off-the-shelf options can be critical when speed matters or CAR-T isn’t feasible due to comorbidities, geography, or manufacturing slots. As with CAR-T, teams watch closely for cytokine release syndrome (CRS) and neurologic events—but centers have become skilled at prevention and management. (Note: availability and lines of therapy differ by agent and province; ask your team which bispecifics are active where you receive care.)
Disease-by-disease highlights (Canada)
Multiple Myeloma
Earlier-line CAR-T (CARVYKTI): As noted, Health Canada authorized cilta-cel in the second-line setting for lenalidomide-refractory patients with 1–3 prior lines This can offer deep, durable responses after only one infusion. Provincial funding pathways are catching up, so verify local coverage and referral logistics. Innovative Medicine Globalcda-amc.ca
Where bispecifics fit: BCMA- or GPRC5D-directed bispecifics offer “off-the-shelf” immunotherapy. Their place relative to earlier-line CAR-T is an active discussion in Canadian myeloma tumour boards; expect evolving provincial criteria and sequencing guidance What to ask your team
1) Am I potentially eligible for CAR-T earlier than I thought?
2) If CAR-T isn’t right now, is a bispecific appropriate—and how does monitoring work?


Emerging Treatments for Blood Cancers in Canada (2025)
Lymphomas(DLBCLandHodgkinlymphoma)
Second-line CAR-T in DLBCL: Lisocabtagene maraleucel (BREYANZI) has been moving earlier in the DLBCL journey. In January 2025, BMS announced Canadian approval for second-line R/R DLBCL, an “earlier access” milestone that may reduce reliance on high-dose chemo plus transplant in some patients. Provincial listings follow CADTH recommendations and pCPA negotiations askabouttiminginyourprovince.
First-line Hodgkin lymphoma pivot (nivolumab + AVD): A pivotal trial (SWOG S1826) published in NEJM (Oct 2024) showed nivolumab + AVD outperformed BV-AVD with fewer adverse effects, prompting programs to consider N-AVD a new standard in advanced cHL. Ontario Health has alreadycreatedaregimenmonographreferencingtheNEJMdata;broaderCanadianadoptionis inmotionasfundingandguidelinesalign.
Whattoaskyourteam
If I relapse early after R-CHOP, is second-line CAR-T an option instead of transplant? 2) For advancedHodgkinlymphoma,isnivolumab+AVDavailableatmycenter?
AcuteleukemiasandMDS
Menininhibitiononthehorizon:IntheU.S.,revumenib(first-in-classmenininhibitor)gainedFDA approval in Nov 2024 for R/R acute leukemia with KMT2A translocation including pediatric patients. Canadian clinicians are watching closely as Health Canada’s SUR lists evolve; some patients may access menin inhibitors via trials. If your disease harbours a KMT2A translocation (orNPM1mutation),askaboutlocaltrialsorspecialaccessmechanisms.
New therapy for lower-risk MDS (US first): The FDA approved imetelstat (RYTELO) in June 2024 for transfusion-dependent anemia in adults with low- to intermediate-1 risk MDS In Canada, imetelstat remains under evaluation; monitor CADTH and provincial updates If you’re ESArefractoryandtransfusion-dependent,askwhetheratrialorcompassionateaccessispossible
Whattoaskyourteam
1)Hasmyleukemia/MDSbeenprofiledforKMT2AorNPM1?
2)ArethereCanadiantrialsformenininhibitorsortelomeraseinhibition?

Emerging Treatments for Blood Cancers in Canada (2025)
Myeloproliferative neoplasms (MPNs)
Momelotinib for myelofibrosis with anemia: OJJAARA (momelotinib) received a Health Canada Notice of Compliance in November 2024, and in February 2025, Canada’s Drug Agency recommended reimbursement with conditions for MF patients with moderate-to-severe anemia This matters because momelotinib can improve symptoms and anemia, addressing a stubborn need in MF. Ask your hematologist about provincial funding status and referral pathways.
Ropeginterferon (PV): Canada permitted exceptional importation of U.S.-authorized ropeginterferon alfa-2b (BESREMi) in March 2025 to mitigate a peginterferon shortage; that step supports continuity of care while full Canadian regulatory pathways proceed If you’re on interferon or considering it, ask whether ropeginterferon is an option under exceptional importation in your province.
What to ask your team
1) For MF with anemia, am I a candidate for momelotinib now that a Canadian NOC exists?
2) For PV, would interferon (including ropeg under exceptional importation) fit my goals?
How Canadian access decisions get made (and what you can do)
After Health Canada authorizes a therapy, CADTH/pCODR assesses clinical value and costeffectiveness; the pan-Canadian Pharmaceutical Alliance (pCPA) then negotiates with manufacturers; finally, each province decides how and when to fund. This multi-step model explains why a drug can be “approved” nationally but available on different timelines across provinces Patients and clinicians can track CADTH recommendations and ask cancer agencies for program timelines, bridging options, or clinical trials nearby.

Emerging
Treatments for Blood Cancers in Canada (2025)
Safety,survivorship,andlivingwell
Modernimmunotherapiesarepowerful.Theyalsobringspecificmonitoring:
CAR-T: CRS/ICANS in the short term; very rare secondary T-cell malignancies long term so lifetimefollow-upiskey.
Bispecifics:CRSisgenerallylower-gradeandoftenmanageableasteamshavebecomeexpertat premedication,earlydetection,andsupportivecare.
Targeted therapy: Side-effects vary by pathway (e.g., cytopenias, infections, or cardiac signals). Canadiancentreshavestandardizedpathwaystoprevent,detect,andtreatthese.
Fivesmartquestionstobringtoyournextvisit
Eligibility: Given my exact diagnosis, genetics, and prior treatments, which emerging options applytomenowvs.later?
Timing: If a therapy is authorized but not yet funded, are there trials, compassionate access, or out-of-provincereferraloptions?
Safety: How will you monitor and manage CRS, neurologic effects, and rare long-term risks (e.g., secondaryT-cellmalignanciesafterCAR-T)?
Logistics: For CAR-T, what’s the realistic vein-to-vein time and where will I stay during monitoring?Forbispecifics,what’stheinfusioncadence?
Life goals: How will this plan affect work, caregiving, travel, fertility, or finances? Is there a navigatororsocialworkerwhocanhelp?

Bottom line for Canadians
Canada is entering a period where earlier-line cellular therapy, rapid-acting bispecifics, and mutation-targeted medicines are not future ideas—they’re here, expanding, and being integrated into provincial programs. The exact timing and sequencing will differ by province, but the direction is clear: more personalized treatment choices, more emphasis on outcomes that matter to patients, and transparent discussion of safety and long-term monitoring. Bring questions. Your care team is ready to tailor these new tools to your situation.


Discover the Power of Patient Voices!
Join us on the Heal Canada Empowering Voices Podcast, where patients, caregivers, healthcare professionals, and advocates come together to share their journeys, insights, and hope. Whether you’re seeking inspiration, education, or connection, these conversations will empower you to make informed decisions and feel less alone in your healthcare journey.
�� Listen now and be part of the conversation that’s changing lives.
https://www.healcanada.org/empowering-voice-podcast/





Types of Blood Cancers
These article is for education through My Blood My Health and is not a substitute for medical advice. If you have concerns or symptoms, please consult a qualified healthcare professional.
Leukiemia
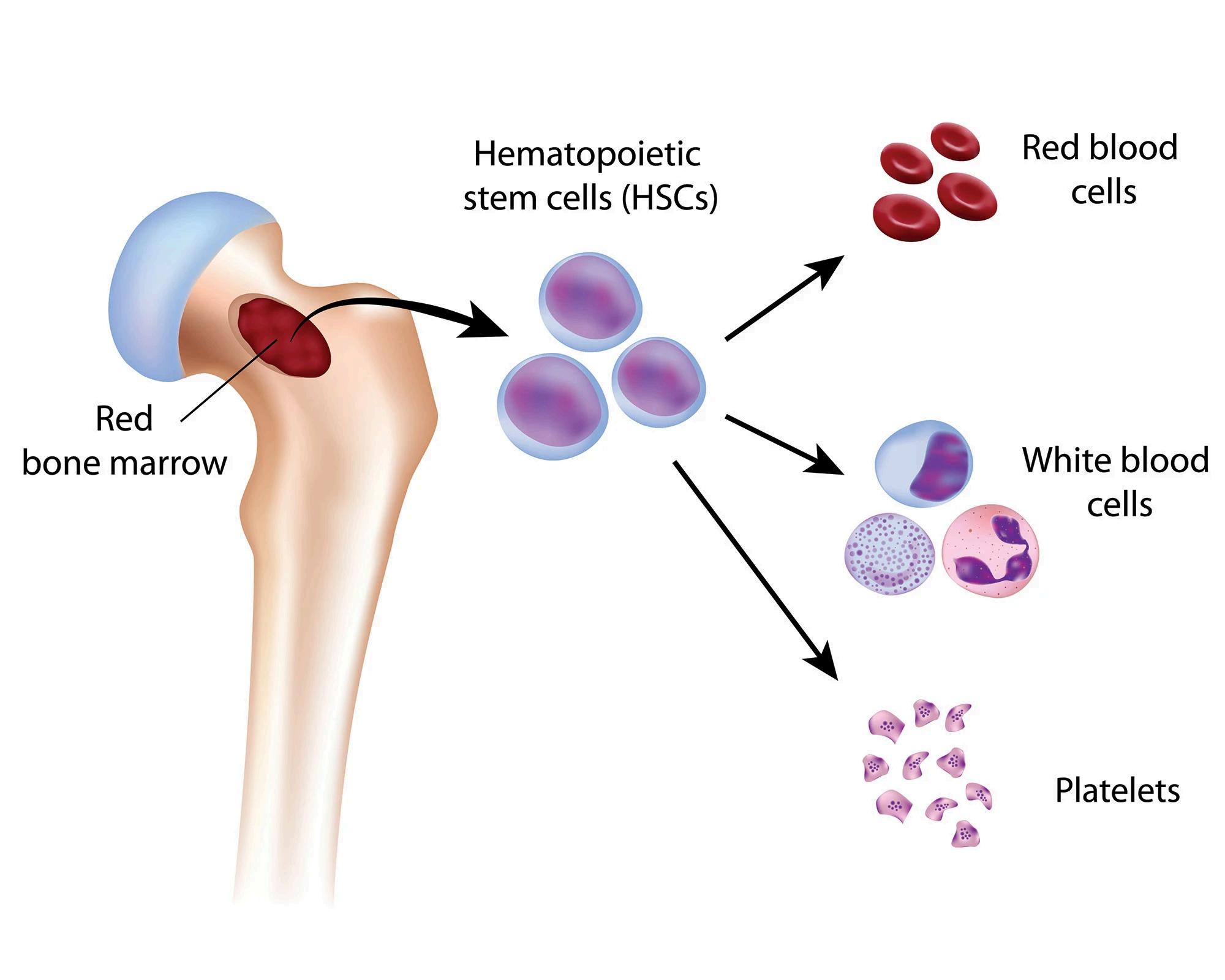
What is Leukemia?
Leukemia is a blood cancer that begins in the bone marrow—the factory for new blood cells and leads to the production of abnormal blood cells that crowd out healthy ones. The word itself comes from the Greek for “white blood,” reflecting how many leukemias start in white blood cells Clinicians classify leukemia by (1) the kind of cell that becomes cancerous—myeloid or lymphoid—and (2) how quickly it develops acute (fast-growing) or chronic (slower-growing). Those two axes create the four major types you’ll hear about: AML (acute myeloid leukemia), ALL (acute lymphoblastic leukemia), CLL (chronic lymphocytic leukemia), and CML (chronic myeloid leukemia).
How leukemia happens (in brief)
Healthy marrow makes red cells (carry oxygen), platelets (clotting), and white cells (immune defence). In leukemia, acquired genetic changes (mutations or chromosomal alterations) push a developing blood cell to multiply abnormally and evade normal maturation and death signals. One well-known example is the Philadelphia chromosome, a swap of DNA between chromosomes 9 and 22 that fuses the BCR and ABL1 genes This driver change produces an overactive enzyme (a tyrosine kinase) and is the hallmark of CML (and present in a subset of ALL).
Over the past few years, expert groups have refined how these diseases are categorized using genetics and molecular features, because biology guides both prognosis and treatment. The World Health Organization’s 5th edition classification (2022–2024) and companion updates in Leukemia reflect this shift to a genetics-first framework.
How common is it?
Leukemia accounts for a meaningful share of blood cancers in both adults and children. In Canada, the Canadian Cancer Society provides up-to-date statistics and trends (incidence and mortality) for leukemia overall and by subtype, which helps patients and policymakers understand the burden across provinces and over time. In the United States, Surveillance, Epidemiology, and End Results (SEER) data show an incidence of roughly 14 4 per 100,000 and a death rate of 5 8 per 100,000 (age-adjusted; most recent periods). Survival varies widely by subtype, age, and biology.

Signsandsymptoms
Becauseleukemiadisruptsnormalbloodcellproduction,manysymptomsaretiedtolowcounts: Anemia(lowredcells):fatigue,shortnessofbreath,paleness
Neutropenia(lowinfection-fightingwhitecells):recurrentorsevereinfections,fevers Thrombocytopenia(lowplatelets):easybruising,nosebleeds,bleedinggums
Some people notice night sweats, weight loss, swollen lymph nodes, a feeling of fullness (enlargedspleen),orbone/jointpain.Symptomscanbenon-specificandmayresemblecommon infections,sopersistentorunusualpatternswarrantmedicalattention.
Howleukemiaisdiagnosed
There’snopopulationscreeningtestforleukemia.Manydiagnosesbeginwithacompleteblood count(CBC)andbloodsmearwhensymptomsorroutinetestsshowsomethingoff.Ifleukemiais suspected, clinicians confirm and classify it using a bone marrow aspiration and biopsy plus specializedtests:
Flowcytometry/immunophenotyping(identifiescellsurfacemarkers)
Cytogenetics(looksforchromosomalchangeslikethePhiladelphiachromosome)
Moleculartesting(detectsmutationsthatinformriskandtreatment)
These studies together determine the exact subtype and risk group, which is essential for treatmentplanning.
Riskfactors:whatweknow
Most leukemias are not inherited. Documented risk factors include ionizing radiation, certain chemicals(notablybenzene),previouschemotherapyorradiationforanothercancer,somerare inherited syndromes (e.g., Down syndrome), and specific prior marrow disorders. Having a risk factordoesnotmeanleukemiaisinevitable;itmeansriskishigherthanaverage.

The major types at a glance
Acute lymphoblastic leukemia (ALL): Rapid-onset lymphoid leukemia seen in children and in older adults. Treatments often combine multi-agent chemotherapy with targeted drugs; CAR Tcell therapy is an option for some relapsed/refractory B-ALL (children/young adults with tisagenlecleucel; adults with brexucabtagene autoleucel).
Acute myeloid leukemia (AML): An aggressive myeloid leukemia of older adults, though it can occur at any age. Care is guided by genetics (e g., FLT3, IDH1/2, NPM1), overall fitness, and goals Diagnostic workup and treatment algorithms are detailed in NCCN patient guidelines and NCI PDQ resources.
Chronic lymphocytic leukemia (CLL): Often found incidentally on routine bloodwork and may be monitored (“watch and wait”) until treatment is needed. Modern targeted therapies (e.g., BTK inhibitors or BCL-2 inhibitor–based combinations) have transformed outcomes; staging uses Rai/Binet systems
Chronic myeloid leukemia (CML): Driven by BCR-ABL1. Daily tyrosine kinase inhibitors (TKIs) like imatinib and newer agents can induce deep remissions; some patients achieve treatment-free remission under strict monitoring.
How leukemia is treated
Treatment plans reflect the leukemia type, genetics, patient age/fitness, and preferences. Broad tools include:
Chemotherapy and combinations (especially for acute leukemias)
Targeted therapy (e g., TKIs for CML; targeted agents in AML and CLL)
Immunotherapies (monoclonal antibodies; CAR T-cell therapy for certain B-ALL)
Stem cell transplantation (allogeneic transplant) for selected higher-risk or relapsed cases
Shared decision-making is key—balancing the chance of long-term disease control with sideeffects and quality of life. National resources like NCI’s PDQ and NCCN’s patient guidelines explain options, potential benefits, and risks in plain language
Living well with leukemia
Beyond disease control, supportive care matters: preventing and treating infections, managing anemia or bleeding, addressing fatigue and mood, and staying current with vaccinations (as advised by your care team). Many adults live for years with chronic forms like CLL or CML, and even in acute leukemias, outcomes continue to improve as precision-guided therapies and cellular therapies evolve. For practical information and peer support, organizations such as the Leukemia & Lymphoma Society offer education and patient services.

When to seek medical advice
See a clinician if you have persistent fatigue, frequent infections, unexplained bruising/bleeding, night sweats, or enlarged lymph nodes or spleen—especially if symptoms last more than a couple of weeks. Prompt evaluation allows earlier diagnosis and, when needed, faster treatment. Remember, these symptoms are not specific to leukemia; many benign conditions can look similar, which is why proper testing is important.
These article is for education through My Blood My Health and is not a substitute for medical advice If you have concerns or symptoms, please consult a qualified healthcare professional.

Myeloma
What Is Myeloma—and Is It a Blood Cancer?
Multiple myeloma (MM)—often simply referred to as myeloma—is indeed a type of blood cancer, one that specifically affects plasma cells, a mature form of white blood cell responsible for producing antibodies. In myeloma, genetically abnormal plasma cells multiply uncontrollably within the bone marrow, producing large amounts of a single type of antibody (a “monoclonal protein”), crowding out healthy blood cells, and causing damage to bones, kidneys, and other organs
It is classified as a hematological lymphoid malignancy of tumour plasma cells. It is unquestionably within the spectrum of blood cancers, along with leukemia and lymphoma.
How Does It Arise? Biology and Classification
Multiple myeloma develops through a multistep biological process driven by genetic changes and interactions with the bone marrow microenvironment. It's genetically heterogeneous and remains incurable mainly due to relapse or treatment resistance.
Diagnosis uses the International Myeloma Working Group (IMWG) criteria, which require:
≥10% of plasma cells in bone marrow being clonal.
At least one myeloma-defining event, which may include end-organ damage captured in the CRAB acronym: Calcium elevation, Renal dysfunction, Anemia, or Bone lesions, or biomarkers indicating high likelihood of progression.
Myeloma is the second most common blood cancer after lymphoma globally, accounting for approximately 10% of hematologic malignancie.
These article is for education through My Blood My Health and is not a substitute for medical advice If you have concerns or symptoms, please consult a qualified healthcare professional.

Myeloma
Epidemiology&CanadianPerspective
Canada:Roughly4,100Canadiansareforecasttobediagnosedwithmultiplemyelomaannually, andaround1,750diefromit.
Prevalencedatashowthatabout1in2,505Canadiansarelivingwithmyelomaasof2022.The annual incidence equates to about 5.2 new cases per 100,000 people, representing approximately1.3%ofallcancersand10%ofbloodcancersinCanada.
AlargepopulationstudyfoundanincidenceinCanadaaveraging54.3casespermillionpeople peryear(≈5.43per100,000),withasteadyannualincreaseofnearly0.96casespermillionper year.Themeanageatdiagnosiswas70years,withabout54%ofcasesinmen.
Worldwide, in 2020, nearly 175,000 people were diagnosed, and 117,000 died from multiple myeloma IntheUS,projectionsestimatearound35,000newcasesand12,000deathsin2023
Approximately 170,405 Americans were living with the disease in 2020 Survival has improved: about60%surviveatleast5years,andaround34%live10yearsormoreafterdiagnosis
Thediseasetypicallyaffectsindividualsaroundage60or69,ismorecommoninmen,andit's rareunderage40
Signs&Symptoms
Myeloma’ssymptomscommonlyreflectbonemarrowinfiltrationandend-organeffects:
Bone pain, particularly in the spine and ribs; osteolytic lesions lead to fractures and are part of theCRABcriteria
Anemia,causingfatigueandpallor
Hypercalcemia(elevatedcalcium)canleadtoconfusion,constipation,anddehydration
Renalimpairmentfromlightchainproteinscloggingthekidneys
Recurrentinfectionsduetoineffectiveantibodyproduction.
Importantly, the disease often starts subtly and without major symptoms, indicated by monoclonal gammopathy of undetermined significance (MGUS) or smouldering myeloma, precursorstatesthatmayprogressovertime.
Diagnosis&Staging
Diagnosis combines blood work (monoclonal proteins), imaging (X-ray, MRI, PET), and bone marrowbiopsy(clonalplasmacells,≥10%).TheIMWGcriteriarefinethresholdstodetectdisease earlierandimproveoutcomes
Staging uses the International Staging System (ISS), later revised (R ISS) to include cytogenetic markersandLDHlevels,offeringrobustprognosticstratification
These article is for education through My Blood My Health and is not a substitute for medical advice If you have concerns or symptoms, please consult a qualified healthcare professional.

Myeloma
Treatment Landscape
Multiple myeloma is considered treatable but not curable in most cases, though remission is often achievable. Therapeutic approaches include: Chemotherapy and steroids.
Targeted therapies: proteasome inhibitors (e.g., bortezomib), immunomodulatory drugs (lenalidomide, thalidomide), and monoclonal antibodies (e.g., elotuzumab)
Stem cell transplantation, especially in eligible younger/fit patients.
CAR T-cell therapies targeting BCMA, such as idecabtagene vicleucel (Abecma), a breakthrough gene therapy now FDA-approved for relapsed/refractory myeloma
Supportive treatment with bisphosphonates to prevent bone fractures and pain.
Palliative care early in advanced stages improves quality of life through symptom management
Ongoing research includes AI-based monitoring of minimal residual disease (MRD) for prognostication and treatment decisions.
Has a significant presence in Canada, with thousands diagnosed annually and a continual rise in prevalence.
Benefitsfromstrongadvocacyeffortsandongoingclinicalresearch helpingpatientslivelonger, healthierlives

Chronic Lymphocyte Luekemia
WhatItIsandHowIt’sTreated
Chronic lymphocytic leukemia (CLL) is a slow-growing cancer of B lymphocytes (a type of white blood cell). In CLL, abnormal B cells build up in the blood, bone marrow, lymph nodes, and spleen. Many people are diagnosed after a routine blood test shows a high lymphocyte count; others notice swollen lymph nodes, fatigue, night sweats, unintentional weight loss, or frequent infections.CLLusuallyprogressesgradually,andtodaytherearemanyeffectivetreatmentsthat cancontrolthediseaseforyears.
HowCLLisDiagnosedandRisk-Stratified
Diagnosistypicallybeginswithacompletebloodcountshowinglymphocytosisandconfirmation by flow cytometry that the cells are clonal B lymphocytes (often CD5+, CD19+, CD23+) Additional tests help guide therapy, including genetic studies of the leukemia cells Two of the most important markers are TP53 disruption (17p deletion and/or TP53 mutation) and IGHV mutation status: TP53 abnormalities signal resistance to chemotherapy, while mutated vs. unmutated IGHV helps predict disease behaviour and response to certain treatments. These factors along with clinical stage and other labs are combined in prognostic tools like the CLLIPItoestimateriskandtailorcare.
“WatchandWait”(ActiveSurveillance)
Becauseearlytreatmenthasnotbeenshowntoimproveoverallsurvivalinasymptomatic,earlystage CLL, the standard approach for many newly diagnosed people is active surveillance (“watch and wait”). This includes regular checkups and labs to monitor for progression. Treatmentgenerallystartsonlywhenspecificcriteriaaremet:significantsymptoms(e.g.,fevers, night sweats, weight loss), bulky or progressive lymph nodes/spleen, rapidly rising lymphocyte counts, autoimmune complications not controlled by steroids, or declining red blood cells/plateletsfrommarrowfailure.
Today’sMainTreatmentOptions
ModernCLL carehasshifteddecisively from chemotherapy totargetedtherapiesandantibodybasedcombinations Themostwidelyusedcategoriesare:

Chronic Lymphocyte Luekemia
BTKinhibitors(BTKi)
BTK inhibitors block Bruton’s tyrosine kinase, a key signal that CLL cells use to survive. They’re pillstakendailyandcanbeusedupfrontorafterrelapse.
Acalabrutinib and zanubrutinib are next-generation (covalent) BTK inhibitors often preferred over ibrutinib due to similar efficacy with fewer certain side effects in many patients. They are commonlyusedaloneorwithananti-CD20antibody.
Pirtobrutinib is a non-covalent BTK inhibitor designed to work even when the cancer has developed resistance to covalent BTKis. In the U.S., it received accelerated approval (Dec 2023) for adults with CLL/SLL previously treated with both a BTK inhibitor and a BCL-2 inhibitor (see below),andongoingstudiescontinuetodefineitsroleearlierintherapy.
Common side effects of BTK inhibitors include bruising/bleeding, high blood pressure (notably with ibrutinib), heart rhythm issues (atrial fibrillation), headaches (more with acalabrutinib), and infections. Your care team will assess cardiovascular risk and drug interactions when choosing amongBTKis
BCL-2inhibitor–based,time-limitedtherapy
Venetoclax inhibits BCL-2, a protein that helps CLL cells avoid cell death. It can be paired with obinutuzumab (frontline) or rituximab (relapsed). A key benefit is fixed-duration treatment often 12 months in the frontline venetoclax-obinutuzumab regimen allowing a defined course with deep responses, including undetectable measurable residual disease (uMRD) for many patients.Carefuldoseramp-upmitigatestumorlysissyndromerisk.
Researchers are actively studying MRD-guided treatment lengths stopping earlier when uMRD isachieved butoutsidetrialsmostguidelinesstillrecommendfollowingthestandard,validated durations.
Anti-CD20monoclonalantibodies
Obinutuzumab and rituximab target CD20 on B cells, aiding immune clearance of CLL cells Today, they are typically used in combination with BTK inhibitors or venetoclax to deepen responses rather than as stand-alone therapies Infusion reactions and infections are the main considerations

Chronic Lymphocyte Luekemia
Chemotherapy (chemoimmunotherapy)
Regimens like FCR (fludarabine, cyclophosphamide, rituximab) or BR (bendamustine-rituximab) used to be standard but are now less common, especially if TP53 is disrupted (where chemo performs poorly). In highly selected younger patients with mutated IGHV, FCR can still induce very long remissions but comes with higher short- and long-term toxicity. Most patients today start with targeted therapies
Cellulartherapyandtransplant(selectsituations)
Allogeneicstemcelltransplantationcanofferlong-termdisease control for a small subset of high-risk, multiply relapsed patients who respond to therapy and are transplant-eligible, balancing potential benefits with significant risks. CAR T-cell therapy has transformed other blood cancers and is under study in CLL; it is available in trials and select settings with encouragingresults,butitisnotyetstandardfrontlinecare.
ChoosingaFirstTreatment
There isn’t a single “best” regimen for everyone Decisions consider:
Genetics: TP53/17p deletion → favor BTKi or venetoclax-based regimensoverchemo
Preference for a daily pill (often indefinite) vs finite therapy: BTKi is typically continuous; venetoclax-obinutuzumab is timelimited.
Comorbidities/risks: Cardiovascular history (BTKi considerations), kidney function (TLS risk with venetoclax), drug interactions,andinfectionrisk.
Age/fitness and logistics: Clinic visits for infusions, monitoring needs,andpersonalpriorities.
Major guidelines (e.g., ESMO) and national resources (e.g., NCI PDQ) list BTKi monotherapy (acalabrutinib or zanubrutinib) and venetoclax + obinutuzumab (fixed duration) among the leading frontline choices for many patients without TP53 disruption, withadjustmentsinspecialpopulations.

Chronic Lymphocyte Luekemia
TreatmentatRelapse
If CLL returns, doctors often switch classes: someone who had venetoclax first might receive a BTKi next, and vice versa After exposure to both classes, pirtobrutinib is an option in the US under accelerated approval, and clinical trials exploring combinations and sequencing are rapidly evolving Supportive care (vaccinations, infection prevention, management of autoimmunecomplications)remainsessentialateverystage
MeasuringSuccessandLong-TermOutlook
Response is assessed by symptoms, blood counts, lymph node size (exam or imaging), and sometimes measurable residual disease (MRD) testing in blood or marrow. Achieving uMRD is associated with longer remissions, especially after fixed-duration therapies, but routine MRDdrivendecisionsoutsidetrialsarestillbeingdefined.Overall,thankstotargetedtherapies,many people with CLL now experience years to decades of good quality of life with sequential treatmentsasneeded.
LivingWellWithCLL
Regularvaccinations(followingyouroncologyteam’sguidance),promptevaluationofinfections, skin cancer screening, and heart-healthy habits are practical steps that complement treatment. Emotional health matters too “watch and wait” can be stressful, and connecting with patient organizations,supportgroups,orcounsellingcanhelp.
KeyTakeaways
CLL is a slow-growing B-cell leukemia; many patients start with active surveillance rather than immediatetreatment.Cancer.gov
When treatment is needed, BTK inhibitors (acalabrutinib, zanubrutinib, and others) and venetoclax-basedcombinationsarethemainstays,oftenpreferredoverchemotherapy. Genetics (especially TP53 status) and IGHV mutation guide choices; TP53-aberrant CLL should avoidchemotherapy.
After both BTKi and BCL-2 therapy, pirtobrutinib is an FDA-approved option in the US under acceleratedapproval(Dec2023)
MRD testing is promising, especially with time-limited venetoclax regimens, but routine MRDguidedstoppingoutsidetrialsisstillevolving.

Chronic Myleoid Luekemia
Chronic Myeloid Leukemia (CML) is a type of cancer that originates in the bone marrow, affecting the blood-forming cells responsible for producing white blood cells. Unlike acute leukemias, which progress rapidly, CML develops more slowly and is often detected incidentally through routine blood work Over the past two decades, advances in targeted therapies have transformed CML from a once life-threatening disease into a largely manageable chronic condition for many patients. This article explores the biology of CML, its stages, diagnosis, treatment strategies, and future outlook.
What is Chronic Myeloid Leukemia?
CML is a myeloproliferative neoplasm (MPN), a category of blood cancers characterized by abnormal growth of bone marrow stem cells. The hallmark of CML is the presence of the Philadelphia chromosome, a genetic abnormality caused by a translocation between chromosomes 9 and 22. This translocation creates a BCR-ABL1 fusion gene, which encodes an abnormal tyrosine kinase enzyme. The enzyme drives uncontrolled cell division and survival of myeloid cells, leading to elevated white blood cell counts in the blood and bone marrow CML accounts for about 15% of adult leukemias and has an annual incidence rate of 1–2 cases per 100,000 people worldwide (Hochhaus et al., 2020). It is more common in adults, with a median age of diagnosis around 60 years, though it can occur at any age.
Phases of CML
CML typically progresses through three distinct clinical phases: Chronic Phase: The most common stage at diagnosis (85–90% of patients).
Patients may be asymptomatic or present with fatigue, night sweats, weight loss, or splenomegaly (enlarged spleen). Blood counts reveal elevated white blood cells and platelets. Accelerated Phase: The disease becomes less stable, with rising blast counts (immature white blood cells) and worsening symptoms. Resistance to therapy may begin to emerge. Prognosis worsens without effective treatment.
Blast Phase (Blast Crisis): CML transforms into an acute leukemia, resembling acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Blast cells exceed 20% of blood or marrow cells. This phase is life-threatening and associated with poor outcomes. These article is for education through My Blood My Health and is not a substitute for medical advice If you have concerns or symptoms, please consult a qualified healthcare professional.

Chronic Myleoid Luekemia
Symptoms and Diagnosis
Many patients with CML are diagnosed incidentally through routine blood tests showing high white blood cell counts. When symptoms do occur, they may include:
Fatigue
Abdominal fullness or discomfort (due to splenomegaly)
Night sweats and fevers
Unintentional weight loss
Bone or joint pain
Diagnosis is confirmed through:
Complete blood count (CBC): Elevated white blood cells with a left shift (immature cells).
Bone marrow biopsy: Increased myeloid cells and presence of Philadelphia chromosome
Cytogenetic and molecular testing: Detection of the BCR-ABL1 fusion gene via fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), or karyotyping.
Molecular monitoring of BCR-ABL1 levels by PCR is critical throughout treatment to assess response and guide therapy.
Treatment of CML
Tyrosine Kinase Inhibitors (TKIs)
The discovery of imatinib (Gleevec) in 2001 revolutionized CML treatment. TKIs specifically inhibit the BCR-ABL1 protein, halting the uncontrolled proliferation of leukemic cells. Today, several TKIs are available:
Imatinib (first-generation)
Dasatinib, nilotinib, bosutinib (second-generation)
Ponatinib (third-generation, effective against resistant mutations such as T315I)
These oral drugs have transformed CML into a chronic disease with near-normal life expectancy for many patients. Therapy response is measured through hematologic, cytogenetic, and molecular milestones, such as achieving a major molecular response (MMR) or deep molecular response (DMR).
Allogeneic Stem Cell Transplantation
Before TKIs, bone marrow or stem cell transplantation was the only curative option. Today, transplantation is reserved for patients with TKI resistance, intolerance, or advanced-phase disease.
Interferon-alpha and Chemotherapy
Historically used before TKIs, interferon-alpha can still play a role in certain cases, such as pregnancy, where TKIs are contraindicated. Hydroxyurea may be used to control high white blood cell counts temporarily.

Chronic Myleoid Luekemia
MonitoringandTreatment-FreeRemission
An important development in CML management is the concept of treatment-free remission (TFR). Patients who achieve sustained deep molecular responses on TKI therapy may discontinue treatment under close monitoring, with about 40–60% maintaining remission (Mahon et al., 2018). This strategy offers hope for reducing long-term drug toxicity, financial burden, and quality-of-lifechallenges.
Prognosis
The prognosis of CML has improved dramatically. With effective TKI therapy, 5-year survival rates exceed 90% for patients diagnosed in the chronic phase (Hochhaus et al., 2020). Prognostic scoring systems such as the Sokal, Hasford, and EUTOS scores help predict treatment outcomes, althoughmolecularmonitoringremainsthemostreliablemeasureoflong-termdiseasecontrol.
FutureDirections
Despiteremarkablepr
Resistance
BCR-ABL1
TKIsandcombina
Long-term metabolic,andg
Access and insomeregions
Cure stra vaccines, minimalresidualdiseaseandpr
Conclusion
Chronic My greatest success limited trea chronic disease
Continued and immunother outcomes molecular essentialpillarsinmanagingthisdisease

Myleoproliferative Neoplasms
Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers that arise from abnormal growth of stem cells in the bone marrow, the body’s blood-forming factory They are characterized by the overproduction of one or more types of blood cells—red blood cells, white blood cells, or platelets. While some MPNs may remain stable for years, others can progress to more aggressive forms of blood cancer, including acute myeloid leukemia (AML). Advances in research have greatly improved our understanding of these diseases, leading to new treatment strategies and better outcomes for many patients
What Are Myeloproliferative Neoplasms?
The term myeloproliferative neoplasm describes a group of clonal hematologic cancers in which a genetic mutation causes uncontrolled proliferation of blood cell precursors in the bone marrow. These cells mature but accumulate in excessive numbers, leading to abnormal blood counts and complications
The hallmark feature of most MPNs is the presence of specific driver mutations in genes that regulate blood cell growth and signaling pathways, most notably JAK2, CALR, and MPL. These mutations activate the JAK-STAT signaling pathway, driving constant signals for cell division even in the absence of normal growth factors.
MPNs are classified as Philadelphia chromosome–negative (classic MPNs such as polycythemia vera, essential thrombocythemia, and primary myelofibrosis) or Philadelphia chromosome–positive, represented by chronic myeloid leukemia (CML).
Myleoproliferative Neoplasms
Types of Myeloproliferative Neoplasms
Polycythemia Vera (PV)
PV is characterized by the overproduction of red blood cells, often accompanied by elevated white blood cells and platelets. Symptoms may include:
Headaches, dizziness, and blurred vision
Itching (pruritus), especially after warm showers
Blood clotting events (deep vein thrombosis, stroke, or heart attack)
Enlarged spleen (splenomegaly)
Most patients with PV harbor the JAK2 V617F mutation. Left untreated, PV can increase the risk of life-threatening clots or progress to myelofibrosis or AML.
Essential Thrombocythemia (ET)
ET involves the overproduction of platelets, the blood cells responsible for clotting. Patients are at risk for both blood clots and bleeding complications due to dysfunctional platelets. Common symptoms include headaches, visual disturbances, and tingling in the hands or feet. Mutations in JAK2, CALR, or MPL are most commonly found.
Primary Myelofibrosis (PMF)
PMF is a more aggressive MPN characterized by scarring (fibrosis) of the bone marrow, which disrupts normal blood cell production. As a result, blood formation may shift to organs like the spleen and liver, causing enlargement and related symptoms. Hallmarks include:
Severe fatigue and weight loss
Night sweats and fevers
Anemia and low platelet counts in advanced stages
PMF can progress to acute leukemia and is associated with significant morbidity and mortality
Other Rare MPNs
There are less common subtypes such as:
Chronic neutrophilic leukemia (CNL)
Chronic eosinophilic leukemia (CEL)
MPN-unclassifiable (MPN-U), where features overlap but don’t fit neatly into one category

Myleoproliferative Neoplasms
Symptoms of MPNs
Symptoms vary by subtype but can include:
Fatigue and weakness
Unexplained weight loss
Night sweats
Enlarged spleen causing abdominal fullness or pain
Headaches, dizziness, or visual disturbances
Increased risk of thrombosis or bleeding
Importantly, some patients remain asymptomatic for years and are diagnosed through routine blood tests.
Diagnosis
Diagnosis begins with a complete blood count (CBC) showing abnormal red cells, white cells, or platelets.Furthertestingincludes:
Bonemarrowbiopsy:toevaluatemarrowcellgrowthandfibrosis.
Cytogeneticandmoleculartesting:toidentifykeymutations(JAK2,CALR,MPL,BCR-ABL1). Imaging:suchasultrasoundorMRI,todetectspleenorliverenlargement.
Accuratemoleculardiagnosisisessentialnotonlyforconfirmingthediseasebutalsoforguiding therapy.
TreatmentApproaches
PolycythemiaVera
Phlebotomy:periodicremovalofbloodtoreduceredcellmass.
Low-doseaspirin:tolowerclotrisk.
Cytoreductivetherapy:hydroxyureaorinterferon-alphainhigh-riskpatients.
Targetedtherapy:JAKinhibitorssuchasruxolitinibforresistantorintolerantPV.
EssentialThrombocythemia
Low-doseaspirin:formostpatients.
Cytoreduction:hydroxyurea,interferon-alpha,oranagrelideforhigh-riskpatients.
PrimaryMyelofibrosis
JAKinhibitors:ruxolitinib,fedratinib,andpacritinibhelpreducesymptomsandspleensize. Stem cell transplantation: the only potentially curative therapy, though suitable only for select patientsduetorisks.
Supportivecare:transfusionsandsymptommanagement.

Myleoproliferative Neoplasms
ComplicationsandRisks
UntreatedoradvancedMPNscanleadtoseriouscomplications: Thrombosisandbleeding:commoninPVandET.
Progressiontomyelofibrosis:PVandETcanevolveintosecondarymyelofibrosis. Transformationtoacuteleukemia(blastcrisis):occursinupto20%ofpatientsovertime.
Burden of symptoms: chronic fatigue, pruritus, and psychological distress significantly impact qualityoflife.
LivingwithanMPN
With regular monitoring and appropriate therapy, many patients live for years with controlled disease. Quality-of-life challenges, such as fatigue, emotional stress, and treatment side effects, remain central to patient care Support groups, advocacy organizations, and patient education playanimportantroleinhelpingpatientsandcaregiversnavigatethesechronicconditions
ResearchandFutureDirections
ThefutureofMPNtreatmentisrapidlyevolving:
Next-generationJAKinhibitorsaimtoimproveefficacyandreducetoxicity.
Combinationtherapiesarebeingtestedtoovercomeresistance.
Novel targets beyond JAK-STAT, such as epigenetic regulators and telomerase inhibitors, are underinvestigation.Immunotherapyapproachesmayonedayhelperadicatemalignantclones. Effortstoachievetreatment-freeremissioninCMLandpotentiallyinotherMPNsareongoing.
Myeloproliferative neoplasms represent a complex but increasingly manageable group of blood cancers. From PV and ET, which often remain indolent for years, to MF and CML, which can be life-threatening without treatment, the spectrum of MPNs reflects the importance of early diagnosis, molecular testing, and targeted therapy Advances in understanding the genetic and molecular basis of these diseases have transformed patient care and opened doors to personalized medicine With continued research, the outlook for patients with MPNs continues toimprove,offeringhopeforlonger,healthierlivesandevenpotentialcures

Myleodisplatic Syndrome
Myelodysplastic syndromes (MDS) are a group of rare blood cancers characterized by abnormal development of blood-forming cells in the bone marrow. Once known as “pre-leukemia,” MDS occurs when immature blood cells (called blasts) do not mature properly and fail to function normally. This leads to low counts of red blood cells, white blood cells, and/or platelets, a condition known as cytopenia Although some people with MDS may live with stable disease for years, others may progress to acute myeloid leukemia (AML), a more aggressive form of blood cancer.
MDS is classified as a clonal hematopoietic disorder, meaning it begins with a genetic change in a single bone marrow stem cell that gives rise to defective blood cells. These abnormal cells either die prematurely in the marrow or enter circulation without functioning properly.
As a result, patients with MDS often experience: Anemia (low red blood cells) leading to fatigue, weakness, and shortness of breath. Neutropenia (low white blood cells), which increases susceptibility to infections. Thrombocytopenia (low platelets), causing easy bruising, bleeding, and petechiae. The disease is heterogeneous, ranging from mild forms requiring little intervention to aggressive types that rapidly evolve into AML.
Causes and Risk Factors
The exact cause of MDS is often unknown, but several risk factors are recognized: Age: MDS is most common in individuals over 60 years old.
Previous chemotherapy or radiation: Known as therapy-related MDS, this subtype arises after cancer treatment.
Genetic predisposition: Rare inherited syndromes (e g., Fanconi anemia, Shwachman-Diamond syndrome) can predispose individuals.
Environmental exposures: Long-term contact with benzene, tobacco smoke, or heavy metals may increase risk.
Most cases of MDS, however, occur sporadically without a clear cause.

Myleodisplatic Syndrome
Classification of MDS
MDS is classified by the World Health Organization (WHO 2022) into subtypes based on blood counts, blast percentage, and genetic abnormalities. The main categories include:
MDS with low blasts (MDS-LB) – mild cytopenias, fewer immature cells
MDS with SF3B1 mutation and ring sideroblasts (MDS-SF3B1) – characterized by defective red cell maturation.
MDS with biallelic TP53 inactivation – associated with poor prognosis.
MDS with excess blasts (MDS-EB) – higher risk of progression to AML.
Therapy-related MDS – occurs after exposure to chemotherapy or radiation. This classification guides both prognosis and treatment
Symptoms
Symptomsvarybutofteninclude:
Persistentfatigueandweakness
Paleskin(fromanemia)
Frequentinfections(fromneutropenia)
Easybruisingorbleeding(fromthrombocytopenia)
Enlargedspleenorliver(insomecases)
Because these symptoms overlap with many other conditions, MDS is often diagnosed through detailedbloodandbonemarrowtesting.
Diagnosis
DiagnosisofMDSrequiresacombinationoftests:
CompleteBloodCount(CBC):Showscytopenias.
PeripheralBloodSmear:Revealsabnormalcellmorphology.
BoneMarrowBiopsy:Confirmsdysplasia,blastcount,andcellularity
Cytogenetic and molecular testing: Detects chromosomal abnormalities (e g., deletion 5q, monosomy7,complexkaryotype)andgenemutations(e.g.,TP53,TET2,ASXL1).
PrognosisandRiskStratification
Prognosis varies widely. Some patients live for a decade with low-risk disease, while others progress quickly to AML. Doctors use scoring systems like the Revised International Prognostic ScoringSystem(IPSS-R),whichconsiders:
Cytogeneticabnormalities
Numberandseverityofcytopenias
Blastpercentageinbonemarrow
Thisriskstratificationguidestreatmentdecisions.
This article is informational and not a substitute for medical advice Individual situations vary please discuss your results and options with your hematologist/oncologist

Myleodisplatic Syndrome
PrognosisandRiskStratification
Prognosis varies widely. Some patients live for a decade with low-risk disease, while others progress quickly to AML. Doctors use scoring systems like the Revised International Prognostic ScoringSystem(IPSS-R),whichconsiders:
Cytogeneticabnormalities
Numberandseverityofcytopenias
Blastpercentageinbonemarrow
Thisriskstratificationguidestreatmentdecisions.
TreatmentApproaches
SupportiveCare
Bloodtransfusionsforanemia
Erythropoiesis-stimulatingagents(ESAs)toboostredcellproduction
Antibioticsforinfections
Platelettransfusionsforbleeding
Disease-ModifyingTherapies
Hypomethylating agents (HMAs): Azacitidine and decitabine can improve survival and delay AMLprogression.
Lenalidomide: Effective for patients with an isolated deletion 5q (del(5q)) chromosomal abnormality
Immunosuppressive therapy: Antithymocyte globulin (ATG) and cyclosporine for select patients
CurativeTherapy
Allogeneic stem cell transplantation remains the only potentially curative treatment, but it is oftenlimitedtoyoungerorfitpatientsduetorisks.
LivingwithMDS
MDS can be a chronic condition requiring ongoing monitoring and management Patients benefit from a multidisciplinary approach, including hematologists, transplant specialists, and supportive care teams. Fatigue, emotional stress, and treatment side effects can significantly affectqualityoflife,makingpsychologicalsupportandpatientadvocacycrucial.

Myleodisplatic Syndrome
FutureDirections
ResearchinMDSisadvancingtoward:
Targetedtherapies:Drugsdesignedtoinhibitspecificmutations(e.g.,IDH1/2inhibitors). Combinationtherapies:HMAsplusvenetoclaxornovelimmunecheckpointinhibitors. Genomicprofiling:Personalizingtreatmentbasedonindividualmutationalprofiles. Improvedtransplantapproaches:Reducingcomplicationsandexpandingeligibility.
Myelodysplastic syndrome is a complex group of bone marrow cancers that disrupt normal blood cell development and function With its variable course, ranging from mild to aggressive, MDS presents both clinical challenges and opportunities for innovation in treatment Advances in molecular genetics and targeted therapy are offering new hope, and ongoing research continuestotransformtheoutlookforpatientslivingwiththisdisease.

Iron Deficiency Anemia
Iron Deficiency Anemia: Understanding the Most Common Blood Disorder
Iron Deficiency Anemia (IDA) is the most common type of anemia worldwide, affecting billions of people regardless of age, gender, or geography. It occurs when the body doesn’t have enough iron to produce hemoglobin—a protein in red blood cells that carries oxygen throughout the body. Without adequate hemoglobin, the body’s tissues and organs don’t get the oxygen they need to function optimally, leading to fatigue, weakness, and a range of other symptoms
This article explores what IDA is, who it affects, the symptoms to watch for, treatment options, and what the long-term outlook looks like for patients living with this condition.
What Is Iron Deficiency Anemia?
Iron is an essential mineral that plays a critical role in producing hemoglobin. When iron stores are depleted or when the body’s demand for iron exceeds supply, red blood cell production drops This leads to fewer red blood cells, smaller cell size, and reduced oxygen-carrying capacity.
Causes of IDA
Iron deficiency anemia can result from one or a combination of these factors:
Inadequate dietary intake
Diets low in iron-rich foods such as red meat, leafy greens, or fortified grains.
Blood loss
Heavy menstrual periods, gastrointestinal bleeding (from ulcers, hemorrhoids, or cancers), or frequent blood donation.
Increased demand
Pregnancy, breastfeeding, rapid growth during childhood or adolescence, or endurance training. Poor absorption
Conditions like celiac disease, inflammatory bowel disease, or bariatric surgery can limit iron absorption.

Who Does IDA Affect?
IDA is widespread, but certain populations are more at risk:
Women of Childbearing Age: Due to menstrual blood loss, women between 15 and 50 are at high risk. Up to 20% of women in North America may have low iron levels, according to the Centers for Disease Control and Prevention (CDC, 2023).
Pregnant Women: Iron requirements double during pregnancy to support fetal growth and maternal blood volume expansion.
Children and Adolescents: Rapid growth increases iron demand.
Dietary habits (picky eating or lack of iron-rich foods) often compound the risk.
Older Adults: Chronic conditions, medications, and age-related changes in absorption increase the risk.
Patients with Chronic Diseases: Conditions like kidney disease, cancer, inflammatory bowel disease, or chronic bleeding disorders frequently lead to secondary IDA
Athletes: Endurance sports can increase iron losses through sweat, urine, or gastrointestinal microbleeding.
Signs and Symptoms of IDA
Iron deficiency anemia often develops slowly, and symptoms may be mild or overlooked until anemia becomes severe.
Common Symptoms
Persistent fatigue and weakness
Pale skin, especially noticeable in the face or inner eyelids
Shortness of breath during routine activities
Rapid heartbeat or palpitations
Cold hands and feet
Other Indicators
Headaches, dizziness, or lightheadedness
Brittle nails or hair loss
Pica (craving non-food items like ice, dirt, or starch)
Restless legs syndrome (RLS)

If you suspect iron deficiency, it’s critical to see a healthcare provider for a blood test. Diagnosis typically involves:
Complete Blood Count (CBC)
Serum ferritin and iron studies
Additional testing to find the cause of iron loss if needed

DiagnosisofIDA
Accurate diagnosis is essential to guide treatment and rule out underlying causes, especially gastrointestinalbleedingorchronicdisease.
Keytestsinclude:
HemoglobinandHematocritlevels–toassessthedegreeofanemia. Ferritin–ameasureofstorediron(lowlevelsindicatedeficiency).
SerumironandTotalIronBindingCapacity(TIBC)–toevaluateavailableiron. Insomecases,additionaltesting,suchasacolonoscopyorupperendoscopy,maybeorderedto investigategastrointestinalsourcesofbloodloss.
TreatmentOptionsforIDA
Effectivetreatmentaddressesbothirondeficiencyanditsrootcause. DietaryChanges
Inmildcases,dietalonemayhelprestoreironlevels.
Iron-richfoods:
Redmeat,poultry,fish
Lentils,beans,tofu
Darkleafygreens(spinach,kale)
Fortifiedcerealsandgrains
Pai
Avo

OralIronSupplements
First-linetherapyformostpatients.
Commonforms:ferroussulfate,ferrousgluconate,orferrousfumarate. Usuallytakenfor3–6monthstorebuildstores.
Side effects: gastrointestinal upset, constipation, or dark stools. Slow-release formulations or alternate-daydosingcanhelp.
Intravenous(IV)IronTherapy
Forpatientswho:
Cannottolerateoraliron
Havemalabsorptionsyndromes
Needrapidreplenishment(e.g.,severeanemia,latepregnancy,chronicdisease)
IVformulations(likeferriccarboxymaltoseorironsucrose)quicklyrestorelevels,ofteninone ortwoinfusions.
AddressingtheRootCause
Treatgastrointestinalbleedingorulcers.
Manageheavymenstrualbleedingwithhormonaltherapies.
Addressunderlyingchronicdiseases.
5.Erythropoiesis-StimulatingAgents(ESAs)
Inpatientswithchronickidneydiseaseorcancer,ESAscombinedwithirontherapymaybeused tostimulateredbloodcellproduction.
Long-Term Outlook
Iron deficiency anemia is highly treatable, and the prognosis is generally excellent once the underlying cause is addressed.
Recovery Timeline
Symptom improvement – within a few weeks of starting therapy.
Normal hemoglobin – typically within 2–3 months
Iron stores – may take 6 months or longer to replenish fully Challenges
Non-adherence due to side effects from oral supplements.
Unidentified or ongoing blood loss leading to recurrent anemia.
Complications of Untreated IDA
Severe fatigue is impacting daily function.
Increased risk of infections
Heart complications, such as a rapid heartbeat or heart failure, can occur in severe cases. In pregnancy, risks include low birth weight or premature birth.

LivingWellwithIDA
Withpropermanagement,mostpeoplewithIDAcanleadfull,healthylives.
Self-ManagementTips:
Takesupplementsasprescribedandfollowupwithyourhealthcareprovider
Eatabalanceddietwithiron-richfoods
Reportanyrecurringsymptomspromptly
Scheduleregularbloodteststomonitorprogress
IDAandSpecialPopulations
Children: Early detection is critical as iron deficiency can affect cognitive development and behaviour.Pediatriciansoftenrecommendironsupplementationinat-riskchildren.
PregnantWomen:Screeningforanemiaisroutine.Supplementationimprovesoutcomesforboth motherandbaby.
Older Adults: Regular screening and nutritional assessments help prevent complications related tocognitivefunctionandcardiovascularhealth.
Research and Emerging Therapies
Recent advancements focus on improving the safety, efficacy, and convenience of iron therapies:
New IV iron formulations with fewer side effects
Alternate-day dosing regimens have been shown to improve absorption and reduce side effects of oral iron.
Digital tools and apps for monitoring symptoms and treatment adherence.
Key Statistics
Approximately 1.2 billion people worldwide are affected by IDA (World Health Organization, 2023).
Up to 50% of anemia cases globally are due to iron deficiency
In Canada, about 4–6% of men and 12–15% of women have low iron levels (Statistics Canada, 2023).

Iron Deficiency Anemia may be common, but it is far from trivial Recognizing symptoms early, understanding risk factors, and seeking timely medical care are essential steps toward recovery With proper treatment and lifestyle adjustments, patients can restore their iron levels, reclaim their energy, and prevent long-term complications.
Quality of Life Survey for IDA
If you have Iron Deficiency Anemia, please take the survey https://www.surveymonkey.com/r/MRQ3VXL
Podcast - for IDA
https://www.healcanada.org/podcasts
Patient Advocacy Support Group IDA
If you would like to join an IDA Support Group, please email us at admin@healcanada.org






Overview of Iron Deficiency Anemia Treatment in Canada
First-line Therapy: Oral Iron Supplementation
Standard of Care: Oral iron salts—like ferrous sulphate, fumarate, or gluconate—remain the primary treatment for IDA due to their affordability, effectiveness, and widespread availability
Dosing Considerations: Traditional dosing (e.g., ferrous sulphate 325 mg three times daily) is still used, though emerging evidence supports lower or alternate-day dosing to reduce gastrointestinal side effects while maintaining efficacy.
Challenges: Around 30–70 % of patients report side effects such as constipation, nausea, abdominal pain, or diarrhea, limiting adherence KHSC Kingston Health Sciences.
Monitoring: Hemoglobin and ferritin levels are typically reassessed in 2–4 weeks for response, and treatment continues for 4–6 months after anemia correction to replenish iron stores—target ferritin levels are often set above 100 µg/L in Canadian guidelines.
When Oral Iron Isn't Enough: Intravenous Therapy
New Approval—Ferinject® (Ferric Carboxymaltose): As of March 2024, Health Canada approved Ferinject for use in adults and children aged one year and older in cases where oral iron is intolerable or ineffective. It's also indicated for adults with iron deficiency associated with heart failure (NYHA class II/III) to improve exercise capacity.
Other IV Options in Canada: Health Canada had previously approved ferumoxytol (marketed as Feraheme) for IDA treatment in patients with chronic kidney disease.
Clinical Use of IV Iron: IV infusions provide fast, effective iron replenishment and are particularly helpful when oral therapy fails or when quick correction is necessary, such as in pregnancy or severe anemia. Common IV formulations in clinical use include iron sucrose (Venofer®), ferric carboxymaltose (Ferinject), ferumoxytol (Feraheme), and iron isomaltoside (Monofer). Each has different dosing protocols and safety profiles.
Safety Considerations: Serious allergic reactions are rare, especially with newer IV preparations, but mild reactions like dizziness or metallic taste may occur Most institutions monitor patients during infusion to ensure safety.

Dietary and Public Health Measures
Food Fortification & Dietary Counseling: Promoting iron-rich foods (e g., red meats, legumes, leafy greens) and dietary counseling is a complementary strategy. Fortified foods—like ironfortified flour or cereals are widely utilized public health approaches to help prevent IDA. Supplements for At-Risk Groups: Preventive supplementation is sometimes recommended for those with increased iron needs (e.g., pregnant individuals, those with restrictive diets, or malabsorption conditions).
Diagnostics and Broader Clinical Pathway
Identifying Underlying Causes: Diagnosis involves CBC, ferritin testing, and investigation of potential etiologies especially with microcytic anemia presentations. If ferritin is normal but anemia persists, hemoglobinopathies like thalassemia may be considered.
Further Investigations: If there’s an inadequate response to iron treatment, guidelines recommend GI evaluations (upper/ endoscopy, colonoscopy) and, if these are negative, exploring small bowel causes using capsule endoscopy or CT/MR enterography Medsc


Summary Table: Treatment Landscape in Canada
Treatment Modality Use Case / Benefits Limitations / Monitoring Requirements
Oral Iron Supplements First-line, cost-effective, convenient GI side effects; adherence challenges; ongoing monitoring
IV Iron (Ferinject®, Feraheme, etc.) Effective when oral therapy fails or is urgent; newly approved options are expanding treatment access. Requires a clinical setting; potential infusion reactions
Dietary & Fortification Strategies Population-level prevention; supports therapy. May not address existing anemia; adherence relies on education
Diagnostics / Investigations Essential for identifying causes and guiding therapy
Resource-dependent; may delay treatment initiation temporarily

FinalThoughts
In Canada, the primary approach remains oral iron supplementation, supported by evidencebased guidelines. However, treatment is becoming more flexible and effective with the introduction of new IV iron options like Ferinject, expanding the capacity to manage patients whocannottolerateorrespondtooraliron.
Public health measures—such as improved dietary intake and food fortification—continue to play a key role in prevention, while robust diagnostic pathways help ensure that underlying conditionsaren’tmissed
Shouldyoulike,Icandivedeeperintodosingprotocols,compareoutcomesbetweenIVagents, orexploreregionalaccessandreimbursementissuesacrossCanadianprovinces.


Spotlight on Innovation: Advances in Blood Cancer Treatment (2025)
Blood cancer care is changing fast and for the better. In just the last couple of years, new immune therapies, smarter targeted drugs, and practical digital tools have opened real options for people living with leukemia, lymphoma, myeloma, myeloproliferative neoplasms (MPNs), and related conditions. This spotlight walks you through the biggest developments—what they are, why they matter, and how to talk about them with your care team—using plain language and a patient-first lens.
CAR T-cell therapy: Powerful, expanding, and easier to access
What it is (in a sentence): Doctors collect some of your T-cells, engineer them to recognize a marker on your cancer cells, multiply them, and infuse them back to hunt the cancer.
What’s new
Earlier use in myeloma. In 2024, the FDA moved BCMAdirected CAR T earlier in treatment: ciltacabtagene autoleucel (Carvykti) became available in the second line, not just after many prior therapies, and idecabtagene vicleucel (Abecma) was cleared for earlier use in relapsed/refractory myeloma. That’s a big shift patients may no longer have to wait through multiple regimens to access a one-and-done cell therapy. More lymphomas and now CLL/SLL In 2024, the CD19 CAR T lisocabtagene maraleucel (Breyanzi) gained new indications: for follicular lymphoma and mantle cell lymphoma, and crucially for CLL/SLL after both a BTK inhibitor and venetoclax offering a cell therapy option where choices have been limited.
Access improvements In June 2025, the FDA eliminated the special REMS programs that used to be required for all CAR Ts (the boxed warnings for CRS/ICANS remain). This reduces administrative friction for hospitals and may help expand the number of centers offering these treatments.
Safety clarity In April 2024, the FDA added a class boxed warning about rare cases of T-cell malignancies (new Tcell cancers) after CAR T, across both CD19- and BCMAdirected products. The overall risk appears low, but it’s important to discuss it alongside the well-known risks of cytokine release syndrome (CRS) and neurotoxicity.

Whatthismeansforyou
If you or your loved one has myeloma and has relapsed after initial therapy, you may be eligible for CAR T soonerthanbefore.

If you have DLBCL, FL, MCL, or CLL/SLL, ask whether CARTisafitnoworasaplannedoptionlater.
Because manufacturing takes weeks, teams often use “bridging” therapy to control disease while your cells are prepared Knowing timelines helps you plan supports around work, caregiving, travel, and infection prevention.
Bispecificantibodies:“Off-the-shelf”immunetherapies What they are: Antibodies that latch onto a T-cell with one“hand”andyourcancercellwiththeother,bringing themtogethertokillthecancer withoutcollectingand engineeringyourowncells.
Highlights
Epcoritamab (Epkinly), a CD20×CD3 bispecific, added an FDA indication in follicular lymphoma in June 2024 (building on its DLBCL indication) It’s given subcutaneously with step-up dosing and careful monitoringearlyon
Teclistamab (Tecvayli), a BCMA bispecific for myeloma, earned a reduced dosing frequency option (every two weeks) for patients in deep remission; that can ease clinicburdenonceyou’restable.JNJ.
Talquetamab (Talvey), first-in-class GPRC5D×CD3 for myeloma, received accelerated approval in 2023 and remains a key option, especially for patients previously exposedtoBCMA-directedtherapies.
Safetynotes
Bispecifics can cause CRS and neurologic effects, especially in the first cycles, plus infection risk (including opportunistic viruses) Your team will do step-up dosing, premedication, and infection prevention; calling early about fevers or confusion is crucial.

Why patients like them
They’re available immediately (no manufacturing wait), and many are subcutaneous or IV on a clinic schedule that may become less frequent over time—useful if travel to a CAR T center is hard.
Precision & targeted therapies: Sharper tools for specific mutations or pathways
Blood cancers are driven by different genetic “wiring.” Matching drugs to those drivers can deepen responses and reduce broad toxicity
Breakthroughs and updates
Menin inhibition for acute leukemia. In November 2024, the FDA approved revumenib (Revuforj) for KMT2A-rearranged relapsed/refractory acute leukemia in adults and children ≥1 year—the first approval in this class. Another menin inhibitor, ziftomenib, is under FDA priority review in 2025 for NPM1-mutant AML. Ask about mutational testing if you or a loved one has AML
Next-gen BTK inhibition in CLL/SLL. Pirtobrutinib (Jaypirca), a non-covalent BTK inhibitor, gained an FDA indication in December 2023 for adults who’ve already had both a BTK inhibitor and a BCL-2 inhibitor an important option when resistance emerges. Zanubrutinib continues to mature with additional data and new combinations (including an FL indication with obinutuzumab in 2024).
Anemia-focused advances in MDS and MPNs
Imetelstat (Rytelo), the first-in-class telomerase inhibitor, was approved in June 2024 for lowerrisk MDS with transfusion-dependent anemia after ESAs, offering meaningful transfusion independence for some patients.
Momelotinib (Ojjaara) became the first myelofibrosis therapy specifically indicated for patients with anemia (approved September 2023). Managing anemia and spleen/symptoms together can improve everyday functioning. Pacritinib (Vonjo) emains option thrombocytopenia


CAR-T beyond DLBCL: more doors opening. Beyond the myeloma expansions above, Breyanzi also added follicular lymphoma and mantle cell lymphoma indications in 2024 evidence that celltherapyisbecomingaplatformacrossB-cellcancers.
Bottom line: Ask your clinician whether your cancer has a target (e.g., KMT2A rearrangement, NPM1 mutation in AML; BTK resistance in CLL; cytopenias in MF) that matches one of these neweroptions.
Digitalhealth&symptomtracking:Smallhabits,bigimpact
Whytracksymptomsdigitally?Becausewhatyounoticeday-to-day fatigue,fevers,neuropathy, mood, sleep, pain often predicts complications before labs or scans do When clinics invite patientstoreportsymptomsregularlythroughanapporwebportal(ePROs),outcomesimprove: Randomizedtrialsshowbetterqualityoflife,fewerERvisits/hospitalizations,andevenimproved survival when patients use electronic symptom reporting with rapid team feedback, compared withusualcare.
Practicaltoolsandframeworks
Validated instruments matter. In MPNs, the MPN-10 / MPN-SAF Total Symptom Score is a short, validated questionnaire used in clinics and recommended in guidelines; digital versions are increasinglyusedforroutinetracking.
Clinic portals and stand-alone apps. Many cancer centers activate ePRO modules inside the patient portal (e.g., weekly check-ins that page your nurse if a score spikes). Disease-specific platforms such as HealthTree Cure Hub help myeloma and other blood-cancer patients track labs, symptoms, and treatments in one place and contribute anonymized real-world data to research.
Plain-language care pathways. The NCCN launched a refreshed Guidelines Navigator to make high-quality, plain-language guidance easier to use on mobile handy to prepare for appointmentsandconfirmwhatyou’veheard.



Howtostart(amini-playbook)
1.AskyourclinicwhethertheyofferePROsymptommonitoringandhowalertsarehandledafter hours.(Whocallsyouback?Howfast?)
2.Pickonetrackeranduseitconsistently ideally,theoneyourcareteamcansee.Ifyouusean outsideapp,bringtheprintoutorscreenshottovisits.
3. Track what matters: fatigue/energy, pain, fevers, weight change, mood/sleep, bowel patterns, neuropathy,bleeding/bruising,newlumpsorswelling,infections,medicationsideeffects.
4. Set a weekly check-in. Five minutes on Sunday night can catch trends you might otherwise miss.
5)Puttingittogether:Choosingamongoptions
There’s no single “best” therapy the right next step depends on your disease type, prior treatments, genetics, age, other health issues, and your priorities (e.g., fewer clinic days vs. deepestresponse).Here’saquickcomparisontosparkproductiveconversationswithyourteam: Option;Howitworks;Whoithelpsmosttoday;Pros;Watch-outs
CAR T-cell therapy; Retrain your own T-cells to target CD19 or BCMA Many B-cell lymphomas; myeloma (now earlier); CLL/SLL after BTK + BCL-2; One-time infusion, high response rates, durable remissions CRS/ICANS risk; manufacturing wait; rare T-cell malignancy warning; caregiver/timecommitments.
Bispecificantibodies“Off-the-shelf”T-cellengagers(eg,CD20×CD3,BCMA×CD3,GPRC5D×CD3) DLBCL/FL (epcoritamab), myeloma (teclistamab/talquetamab) Available immediately; can transition to less-frequent dosing Early-cycle CRS/neurologic toxicity; infection risk; regular clinictime
Targeted therapies: Aim at specific mutations/pathways (menin, BTK, JAK/ACVR1) AML with KMT2A rearrangement (revumenib); CLL with BTK resistance (pirtobrutinib); MF with anemia (momelotinib); MDS anemia (imetelstat). Oral options in some cases; precision matching; can be combined.Needmutationtesting;somearenewwithevolvinglong-termdata



Questionstobringtoyournextappointment
“Where am I on my treatment journey?” (First line? Secondline?Later?)
“Do my labs or genetics open doors to targeted options?” (e.g., KMT2A/NPM1 in AML; BTK resistanceinCLL;cytopeniaprofileinMF.)
“Could CAR T or a bispecific be right for me now orasaplannednextstep?”
“How do we monitor and prevent infections?” (Vaccines,antivirals,IVIG,growthfactors.)
“Can we use ePRO symptom tracking?” (What app/portal?Whowatchesmyalerts?)
“What’s the clinic time commitment and caregiver supportneeded?”

“Aretheretrialsnearbythatfitmysituation?”
g well through treatment: small choices, big ends
ever therapy you and your team choose, patient-testedhabitsmakeadifference: symptomsweeklyandcallearlyaboutfevers, ness of breath, confusion, chest pain, ing, or new neurologic symptoms. (ePRO amsexistbecauseearlyactionworks.)
nt infections: hand hygiene, masks in ded indoor spaces, and keep vaccines up-toperyourclinician.
Protect energy: fatigue is common; plan “big energy” tasks for better days, take short walks to fight deconditioning, and ask about sleep or mood supports

Bring a buddy (in person or by speakerphone) to important visits to help with notes and shared decisions.
Ask about financial and logistical help: social work, travel programs, and assistance foundations can reducetheburdenofhigh-touchtherapies.

Glossary (quick & friendly)
CAR T-cell therapy: Your own T-cells are engineered to recognize a cancer marker (like CD19 in many lymphomas or BCMA in myeloma) and infused back.
Bispecific antibody: An antibody that binds to a T-cell and a cancer cell simultaneously to bring them together.
CRS/ICANS: Common early CAR T/bispecific side effects—fevers, low blood pressure (CRS), and temporary brain-related symptoms (ICANS).
Menin inhibitor: A drug class (e g., revumenib) that disables a protein complex driving certain leukemias (KMT2A-rearranged, NPM1-mutant).
ePRO: Electronic patient-reported outcomes your symptoms, tracked regularly via a portal or app. These programs improve outcomes when teams respond to alerts.
The horizon: what we’re watching
Earlier-line use of CAR T and bispecifics across more blood cancers, including combinations (e g., dual bispecifics in myeloma).
More precision drugs (e.g., additional menin inhibitors for AML under review in 2025).
Smarter digital tools that integrate symptom alerts, lab trends, and home vitals in one patientfriendly dashboard—now appearing in major guideline apps and portals.




New Treatements for Polycthemica Vera
Author: Lindsay Kaun, PharmD, BCPS
Current treatment strategies are aimed at reducing hematocrit and symptom burden through phlebotomy,aspirin,andcytoreductiveagents,whileemergingtherapiesofferpromiseforbetter diseasecontrolandqualityoflife.
Polycythemia vera (PV) is a rare and chronic blood cancer that is classified as a myeloproliferative neoplasm (MPN) The 3 classical Philadelphia chromosome-negative MPNs, consisting of PV, essential thrombocythemia (ET) and myelofibrosis (MF), originate in the hematopoieticstemcellsofthebonemarrowandarecharacterizedbyincreasedproliferationof themyeloidlineagesowingtoanoveractiveJAK-STATpathway
Bonemarrowstudyofamyeloproliferativedisorder
MPNs were once considered disorders rather than cancers and were largely ignored9 This identification changed in 2008 once it was understood that these diseases involve all types of myeloidcellsandareclonallydrivenbyspecificdrivermutations
TherecognitionofMPNsasbloodcancersin2008broughtlong-overdueattentionandresearch investment.2PVisthemostcommonMPNandtheonlyMPNinwhicherythrocytosisoccurs.
Acquired somatic gain-of-function mutations of JAK2, primarily JAK2V617F and less commonly exon12,arepresentinvirtuallyallpatientswith ThesemutationsleadtoanoveractiveJAK-STAT signalling pathway, which is responsible for transmitting signals from growth factors, cytokines andotherligandsoutsideofastemcelltoitsnucleus.Thisleadstoexcessiveerythropoiesisand a subsequent increase in red blood cell (RBC) mass, which may lead to clinically significant thrombotic and hemorrhagic complications. Because pluripotent stem cells are involved, PV is considered to be a tri-lineage disease and can overproduce white blood cells and platelets as well.
PV is estimated to affect 44 to 57 per 100,000 people, equating to an estimated 150,000 to 190,000patientsintheUS.
PV is usually diagnosed between 50 and 70 years of age and is more common in men than women.ThemediansurvivalforpatientswithPVisapproximately14years,withthosediagnosed atanearlieragemorelikelytosurvivelonger.
These patients have a 1.6-fold higher mortality. Despite its later diagnosis and indolent course, PVisstillexpectedtoshortensurvivalbyapproximately5to10years

PVsymptomsareoftennon-specificbutcangreatlyimpactqualityoflife(QOL).Fatigueisoften reportedbypatientsasthemostprevalentandseveresymptom,with84%ofpatientsreporting fatigueintheinternationalMPNLandmarksurvey.
Inaddition,ofpatientswithPVwithhighsymptomburden,94%hadreducedQOLand483%had decreasedworkproductivity Othersymptomsincludedrenchingsweats,weightloss,abdominal discomfort, bone pain, sexual dysfunction, headache, shortness of breath, blurry/double vision, eye pain, dizziness, brain fog, lack of concentration, and aquagenic pruritus Many of these symptoms (ie, fatigue and pruritus) are thought to be caused by pro-inflammatory cytokines fromtheJAK-STATpathway
Clinical signs may include hypertension and splenomegaly
It is important to note that patients with PV may not always exhibit obvious signs of illness but often feel “sick” as these non-specific symptoms compound and impact QOL This may make their experience difficult to understand for others.
The complications of PV can be serious and lead to significant morbidity and mortality The increased blood viscosity caused by erythrocytosis, qualitative abnormalities of RBCs, and leukocytosis has been associated with an increased risk of thromboses and other microcirculatoryabnormalities
Thromboses can be either venous or arterial and may occur in any area of the body. The incidence range of arterial and venous thrombosis is 12% to 39% in PV. Arterial thrombosis, includingacutemyocardialinfarction,cerebrovascularischemicepisodes,andperipheralarterial occlusion,represent60%to70%ofallcardiovasculareventsinPV.
Deep vein thrombosis and pulmonary embolism may also occur in these patients. Additionally, patients with PV show a high prevalence of rare forms of thrombosis, such as abdominal vein thrombosis,includingextrahepaticportalveinocclusion,Budd-Chiarisyndrome,andmesenteric veinthrombosis
These events tend to occur most frequently shortly before or after diagnosis and decrease over time, likely due to PV treatment. Cardiovascular mortality accounts for 41% of deaths (1.5 death events per 100 persons per year), with an incidence of non-fatal thrombosis of 3.8 events per 100patient-years.
Another serious complication of PV is the risk of progression to MF or transformation to acute myeloidleukemia(AML).Approximately20%ofpatientswithPVprogresstoMForanaggressive formofAMLknownasMPNblastphase(MPN-BP).

Elevatedhematocrit(HCT)isahallmarkofPV,indicatingoverproductionofRBCsandhighblood viscosity. Other lab abnormalities include increased hemoglobin (HGB), abnormal red cell morphology (mean corpuscular volume, mean corpuscular hemoglobin), increased lactate dehydrogenase,decreasederythropoietin,andirondeficiency.
The 2016 World Health Organization diagnostic criteria for PV require the presence of either all threemajorcriteriaorthefirsttwomajorcriteriaandtheminorcriterion
ThemajorWHOcriteriaare:
HGB >165 g/dL in men and >16 g/dL in women, or HCT >49% in men and >48% in women, or red cellmass>25%abovemeannormalpredictedvalue
Bonemarrowbiopsyshowinghypercellularityforagewithtrilineagegrowth,and PresenceofaJAK2V617Forexon12mutation.
Theminorcriterionisasubnormalerythropoietinlevel WorkupforPVtypicallyincludesabone marrow biopsy to detect the JAK2 mutation and look for the presence of fibrosis, which may indicateprogressiontoMF.
Risk stratification per the National Comprehensive Cancer Network (NCCN) classifies patients with PV under 60 years of age and no history of thrombosis as low risk and those over 60 years ofageand/orwithapriorhistoryofthrombosisashighrisk.
The main goals of treatment are to reduce the risk of thrombosis, which is the most common causeofmortality,preventtransformationtoMForMPN-BP,andtotreatsymptoms. Atthistime,nodrugtherapyhasbeenshowntoprolonglifeorpreventtransformationtoMFor AML. The mainstays of PV treatment are low-dose aspirin and therapeutic phlebotomy (bloodletting) to mitigate thrombotic risk. The purpose of phlebotomy is to reduce the red cell massandsubsequenthyperviscosity,whichcandirectlyleadtothrombosis.
Current guidelines recommend that patients with PV maintain a target HCT under 45%.16 This cutoffisbasedonthe2013CYTO-PVstudy,whichrandomized365patientswithPVtreatedwith phlebotomyand/orhydroxyureatoeitheraHCTtargetoflessthan45%or45%to50%18Aftera median follow-up of 31 months, death from cardiovascular causes or major thrombotic events wasrecordedin3%ofpatients(5of182patients)withaHCTleveloflessthan45%comparedto 10%(18of183patients)ofpatientswithaHCTlevelof45%to50%(P= 007)18Somephysicians useaHCTtargetof42%inwomenorinthosewithsymptoms

An argument can be made that because men have higher testosterone levels, which boost RBC production, and a higher normal red cell mass than women, women should have lower HCT targets. While there is no data to support this practice, HCT targets can be individualized for those who may feel better at lowerHCTs.
Phlebotomy is not a benign intervention as it can exacerbate disease-related iron-deficiency Recurrent phlebotomy may lead to or worsened iron-associated symptoms, such as fatigue, lack of concentration, shortness of breath, depression, paresthesia, and restlesslegssyndrome.
It also results in volume shifts which can lead t hypotension, lightheadedness and dizzines
Phlebotomies are also time-consuming and resource intensive,andaccessmaybedifficultforpatientslivin inmoreruralareas ThetreatmentofPVisdrivenbyth patient’s risk stratification Low-risk patients ar managed with phlebotomy and low-dose aspiri
Cytoreductive therapy may be added in the case o frequent phlebotomies, phlebotomy intoleranc disease-related symptoms, progressive thrombocytos and/orleukocytosis,orsplenomegaly.
Cytoreductive agents used in PV include hydroxyurea (HU), pegylated interferon-alpha (INF-α) and ruxolitinib.HU was first used as an antineoplastic in the 1960s and, despite not being FDAapproved for PV, it is the most commonly used cytoreductive agent It has the dual advantages of being oral and inexpensive. Important adverse effects (AEs) that are often dose-related and may lead to discontinuation include leg ulcers and other mucocutaneous manifestations such as mouth sores, gastrointestinal symptoms, pneumonitis, and fever.
There is a risk of HU-induced secondary malignancies, particularly non-melanoma skin cancers, and approximately 25% of patients with PV become intolerant or resistant to HU.
Two INF-α products are used to treat PV: peginterferon alfa-2a (Pegasys; pharma& GmbH) and ropeginterferon alfa-2b (Besremi; PharmaEssentia Corporation). Peginterferon alfa-2a is not FDAapproved for PV but has been used off-label for many years. Ropeginterferon alfa-2b is a longeracting formulation that requires less frequent dosing than peginterferon alfa-2a. Both are administered as subcutaneous injections and typically take several months to reach full therapeutic effect.

A recent update to the NCCN Guidelines for PV recommends ropeginterferon alfa-2b as a preferred cytoreductive therapy, while HU and peginterferon alfa-2a are recommended as alternatives. The AE profile of INF-α is extensive and includes a black box warning for neuropsychiatric,autoimmune,ischemicandinfectiousdisorders.
Other AEs include injection site reactions, flu-like symptoms, headache, fatigue, nausea, diarrhea,musculoskeletalpain,dizziness,arthralgias,andcytopenias
Ruxolitinib (Jakafi; Incyte Corporation) is a JAK1/2 inhibitor that is approved for use in patients with PV who are intolerant to or refractory to HU. This drug may be especially useful in those withsymptomsofpruritusandsplenomegaly.
The phase 2 MAJIC-PV (ISRCTN61925716) study demonstrated that ruxolitinib had a superior complete response within 1 year compared to best available treatment (BAT) in patients who were resistant to or intolerant of HU: 40 (43%) patients on ruxolitinib vs 23 (26%) on BAT (OR 2.12; 90% CI 1.25-3.60, P=0.02).24 Ruxolitinib is associated with cytopenias, particularly anemia and thrombocytopenia. Its immunosuppressive effects and propensity for weight gain can make itdifficulttotolerateformanypatients.
There are new developments on the horizon for PV that have the potential to alleviate phlebotomy burden, consistently maintain HCT under 45% and improve patient QOL. Rusfertide, a hepcidin mimetic, is the furthest along in development. Rusfertide binds to an iron exporter on key cells involved in iron metabolism called ferroportin, reducing iron availability in the bone marrow that is needed for erythropoiesis.
The ongoing phase 3 VERIFY study achieved its primary endpoint, showing a significantly higher proportion of clinical responders defined as patients no longer meeting phlebotomy eligibility among those treated with rusfertide for PV (77%) compared to those who receivedplacebo(33%)duringweeks20to32(P<00001)

Givinostat (Duvyzat; Italfarmaco SpA) is a histone deacetylase inhibitor specific for JAK2 V617Fmutatedcells.Thephase3GIV-INPVtrialinvestigatinggivinostatiscurrentlyinprogress.
There are several other molecules currently in phase 2 development. Sapablursen (ISIS 702843; Ono Pharmaceutical) is a ligand-conjugated antisense molecule that targets the TMPRSS6 gene to modulate hepcidin production and subsequent regulation of iron metabolism20 Bomedemstat inhibits LDS1, an enzyme involved in increasing cell proliferation and hematopoieticdifferentiation
Divesiran is a double-stranded small interfering RNA targeting TMPRSS6 mRNA.20 Lastly, the combination of 2 frequently used cytoreductive therapies, ruxolitinib and ropeginterferon alfa2b, are being studied in a phase 2 trial in HU-resistant patients with PV; results are expected in 2028.

Pharmacistsareinauniquepositiontoimprove the care of patients with PV. They can ensure that patients are treated to guideline recommendations, ie, that all patients with PV are on low-dose aspirin and high-risk patients additionally receive cytoreductive therapy, if notcontraindicated.
Pharmacists can intervene in cases where HCT is not maintained at less than 45% and recommend the addition of therapy or dose adjustments as needed to achieve this goal. As many new PV diagnoses are missed, pharmacists can also help identify cases of untreatederythrocytosisforfurtherworkup.
Lastly, pharmacists may serve a role in educating patients and providers on the importance of recognizing and managing PV symptoms, as well as managing HCT levels to mitigatethromboticrisk.

Interferon Therapy for MPNs in Canada in 2025: Shortages,
Stopgaps, and Why BESREMi Matters for PV
Interferons have long been part of the therapeutic backbone for Philadelphia–chromosome–negative myeloproliferative neoplasms (MPNs) especially polycythemia vera (PV) because they control blood counts and, uniquely, can drive down the JAK2 V617F mutation burden over time(apotentialmarkerofdiseasemodification).Contemporaryreviewsandguidelinescontinue to position pegylated interferon-α alongside hydroxyurea as a first-line cytoreductive option in PV,particularlyforyoungerpatientsandthoseaimingtoavoidorcomeoffhydroxyurea.
TheCanadiancontext:what’sbeenused andwhat’smissing
Historically in Canada, peginterferon alfa-2a (PEGASYS) authorized for hepatitis B/C has been prescribed off-label for PV and related MPNs. Other interferon products have dwindled: interferonalfa-2b(IntronA)hasbeendiscontinuedinCanada(andabroad),leavingpeginterferon alfa-2aasthepracticalinterferonoptionformanyhematologists.
Theshortage:aTier3supplycrunch
Canada entered a Tier 3 (most serious) shortage of PEGASYS beginning October 29, 2024, with theshortagelistedasongoingthroughDecember31,2025 TheDrugShortagesCanadadatabase and Health Canada’s InfoWatch both flagged this as critical, noting real risks for MPN patients whodependoninterferontherapy eventhoughPEGASYSisnotformallyauthorizedforMPNs.
To mitigate the impact, Health Canada (March 17, 2025) permitted the exceptional, temporary importation of US-authorized BESREMi (ropeginterferon alfa-2b-njft). Crucially, Health Canada emphasizes that BESREMi is “similar but not identical” to PEGASYS; it carries different dosing, indication,andmonitoringrequirementsandshouldnotbeconsideredadirectsubstitute.Italso lacksaCanadianDINandmaynotappearinstandardpharmacysystems,requiringspecialbilling processes.
RecallsandSafetyAlerts
Professional bodies and pharmacy groups circulated practical guidance on converting appropriate patients, while also clarifying important exceptions (eg, pregnant patients and thosewithCTCLshouldgenerallyremainonPEGASYSwhenpossible)

Following Health Canada’s temporary pathway, distributor FORUS Therapeutics announced USauthorized BESREMi is available in Canada via Exceptional Importation, with technical details (e.g.,OntarioPIN09858357andQuebecRAMQcode99114432)toenablebilling/logistics.
BESREMiremainsnotyetreviewedunderHealthCanada’sstandarddrugreviewprocessin2025, butalicensing/registrationtrackwiththeoriginatorcompany(PharmaEssentia)isunderway
Whatmakesinterferon andBESREMi importantforPV?
Diseasecontrolthatcanbe“disease-modifying.”
Randomized and long-term extension studies demonstrate that ropeginterferon alfa-2b (BESREMi) achieves durable complete hematologic responses and deep molecular responses (reductionsinJAK2V617F alleleburden),withoutcomesthatstrengthenovertime.ThePROUDPV trial and CONTINUATION-PV extension (to 5–6 years) showed sustained efficacy and, in later follow-up,signalssuchasimprovedevent-freesurvivalcomparedwithcontrolarms.PMCNature Emerging analyses (and real-world/clinical experience reports) continue to link deeper JAK2 burden reductions with favourable clinical trajectories, underscoring why many clinicians view interferonastheonlycytoreductiveoptionwithrobust,prospectivemolecular-responsedatain PV.
Tolerabilityandcadence.
Ropeginterferon’s long half-life enables dosing every 2–4 weeks (after titration), which many patients find easier than weekly regimens associated with older interferons Several reviews highlight comparatively lower long-term discontinuation rates with ropeginterferon versus historicalinterferonformulationsinPVcohorts
Aviablefirst-lineoption especiallyforyoungerPVpatients
Reviews and expert algorithms place pegylated interferon-α alongside hydroxyurea as first-line cytoreduction in PV. For younger patients who may be on therapy for decades, the potential for sustainedhematologiccontrolandmolecularremissionsmakesinterferonparticularlyattractive.
PracticalimplicationsofthePEGASYSshortage Whoshouldswitch?
Canadian hematology groups and pharmacy bodies recommend considering BESREMi for appropriate patients affected by the shortage, while noting specific groups should not be switched(e.g.,pregnancyandCTCL,wherePEGASYSremainspreferred).Thedecisionbelongsto the treating hematologist, who will individualize based on prior response, comorbidities, and goals(e.g.,fertility,molecularresponse).
Dosingandmonitoringarenotinterchangeable.
Health Canada’s risk communication stresses that BESREMi has different dosing and monitoring thanPEGASYS;cliniciansaredirectedtotheUSPrescribingInformationandrelevantguidelines, andpatientsrequirecounselingonsyringeformatandhandling(BESREMiarrivesasasingle-use prefilledsyringewithEnglish-onlylabels).

Coverageandlogistics
Because BESREMi’s importation is exceptional (without a DIN), ordering and billing require special processes and may vary by province/plan. FORUS has published provincial billing identifierstofacilitatedispensing;patientsandprovidersshouldworkwithspecialtypharmacies andinsurersoncase-by-casearrangements.
HowBESREMichangesthenear-termlandscapeforCanadianPVpatients
WhileCanadaaddressesthePEGASYSshortagethroughatleastDecember2025,theavailability of BESREMi offers a credible, evidence-based interferon path for many PV patients who otherwise faced therapy disruption or suboptimal alternatives It is particularly impactful for patients aiming for long-term disease control with potential molecular remissions, for those intolerant or resistant to hydroxyurea, and for clinicians striving to reduce phlebotomy burden whileadheringtohematocritandthrombotic-risktargets.
That said, not every patient should be switched, and interferon choice in pregnancy remains a special case (PEGASYS preferred when feasible). The message from Canadian advisories is consistent: coordinate closely with your hematology team to determine suitability, dosing, and monitoring andtonavigatethetemporarylogisticsofanimportedproduct.
Bottomline
Interferon remains a cornerstone for PV in Canada, valued for durable hematologic control and theuniquepotentialtoreduceJAK2V617Falleleburdenovertime.WileyOnlineLibrary CanadaisexperiencingaTier3PEGASYSshortage(Oct2024→Dec2025),officiallyrecognizedby HealthCanada.
In response, US-authorized BESREMi is temporarily available through exceptional importation, withdistinctdosing/monitoringandspecialhandling(noDIN)
Why it matters for PV: BESREMi brings long-term, disease-modifying potential with convenient dosing and robust trial data helping preserve interferon access during the shortage and potentiallyimprovinglong-runoutcomesformanyCanadianPVpatients.


Switching from PEGASYS® to BESREMI®: What Patients Should Ask

Ask About Your Suitability
Am I a good candidate for BESREMi®?
• Are there reasons I should stay on PEGASYS®, such as pregnancy or other conditions?
• How will switching impact my treatment goals, such as controlling hematocrit or reducing my JAK2 mutation burden?

Know the Side Effects
What side effects should I expect with BESREMi®?
• How do I report side effects or concerns early?
• What should I do if I miss a dose?

Understand the Differences
How is the dose and schedule different from PEGASYS®?
• What new monitoring (labs, visits) will I need?
• Will I be able to self-inject, and how often
Learn About Logistics
Which pharmacy will dispense BESREMi®?
• How is the medication stored and handled?
• How is insurance coverage or reimbursement arranged, and who can help with paperwork?
Plan for Follow-Up


How often will we check my blood counts and liver function after the switch?
• How will we measure whether the medication is working for me?
• What are the steps if BESREMi® isn’t effective or tolerated?

Clinical Trials and Surveys
Surveys
AML - Acute Myleod Leukemia
Patients Quality of Life
PV - Polycythemia vera
Patients Quality of Life
Quality of life - Blood
Cancer Patients
Iron Deficiency Anemia
Quality of Life
















If you are living with a hematological condition or care for someone who is, consider exploring clinical trials. Talk to your healthcare team, reach out to advocacy organizations and use platforms such as ClinicalTrials.gov or Canadian Cancer Trials to learn more. Your voice and experience matter—more than you know.

Clinical Trial Search - Canada
HealthCanada'sClinicalTrialsDatabase-Website
Health Canada, through its Clinical Trials Database, is providing the public with a listing of specific information relating to phase I, II, and III clinical trials in patients. The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving humanpharmaceutical andbiological drugs
AccesstheClinicalTrialsDatabase
Patients can access the database to determine if a clinical trial has met the regulatory requirements. The database may also assist Canadians in finding clinical trials that might be relevanttotheirmedical condition.
The Clinical Trials Database is not a registry, and therefore, it does not contain comprehensive information about each clinical trial. To maximize the use of the database and available information, users are advised to link to external resources, including publicly available registries,toobtainfurtherinformationsuchastrial objectivesandpatienteligibility.
Note, however, that not all clinical trials are necessarily registered and thus found in these registries.
Health Canada continues to encourage sponsors to register their clinical trials in publicly accessibleregistriessuchasClinicalTrials.gov andISRCTN.
A Canadian-based registry for cancer trials is also available at Canadian Cancer Trials Additionally, the search portal provided by the World Health Organization (WHO) can be used to access a central database that contains information about trials registered in several international registries.
Health Canada is the federal regulator responsible for authorizing the importation and sale of drugs for the purpose of clinical trials This responsibility is fulfilled through the review of clinical trial applications (CTAs) for phase I, II, and III clinical trials, filed by clinical trial sponsors One of the objectives of Health Canada's review is to ascertain that subjects participating in the trial will notbeexposedtounduerisks.

Clinical Trial Search - Canada
Every year, Health Canada authorizes approximately 900 clinical trials in patients. The database lists trials that were authorized by Health Canada starting April 1, 2013. The database will be populated with information about each clinical trial after Health Canada issues its No-Objection Letter (see terminology section for a definition). Thus, following the launch, the number of clinicaltrialsavailableinthedatabaseisexpectedtobesmall,butthenumberwillincreasewith timeasthedatabaseispopulated.
InformationListedintheDatabase
The database will provide the following information on clinical trials for which a CTA has been authorized:
Protocol Number;
Protocol Title;
Drug Name;
Medical Condition; Study population; Date of No Objection Letter;
Sponsor Name; Control Number; Study Start Date; Study End Date; Trial Status
Health Canada's Clinical Trials Database serves as an essential resource for patients, healthcare providers, and researchers by offering regulatory insights into authorized clinical trials in Canada. While not a comprehensive registry, the database helps users identify trials that have met Health Canada’s safety and regulatory standards, offering a level of trust and transparency forCanadiansexploringparticipationinclinicalresearch.
By supplementing searches with additional global registries such as ClinicalTrialsgov, ISRCTN, and the Canadian Cancer Trials portal, individuals can gain a more complete understanding of trial options and eligibility As the database continues to expand over time, it reinforces Health Canada's commitment to safeguarding patient well-being while promoting access to innovation andinformeddecision-makinginclinicaltrialparticipation


Resources

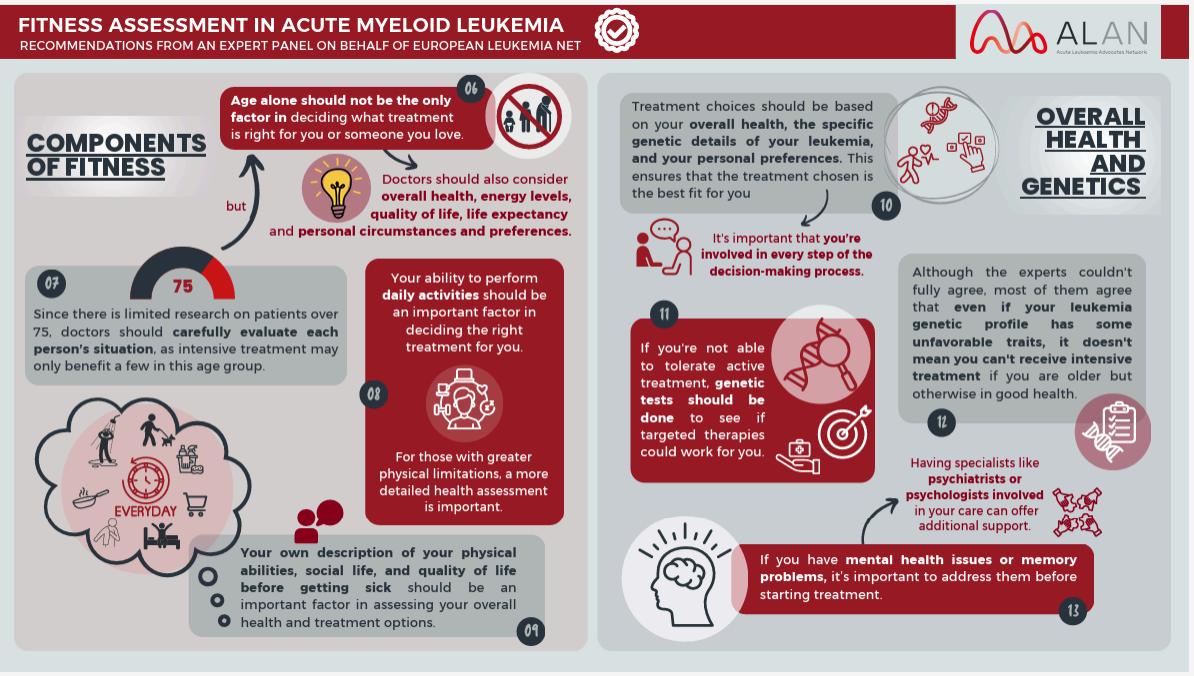

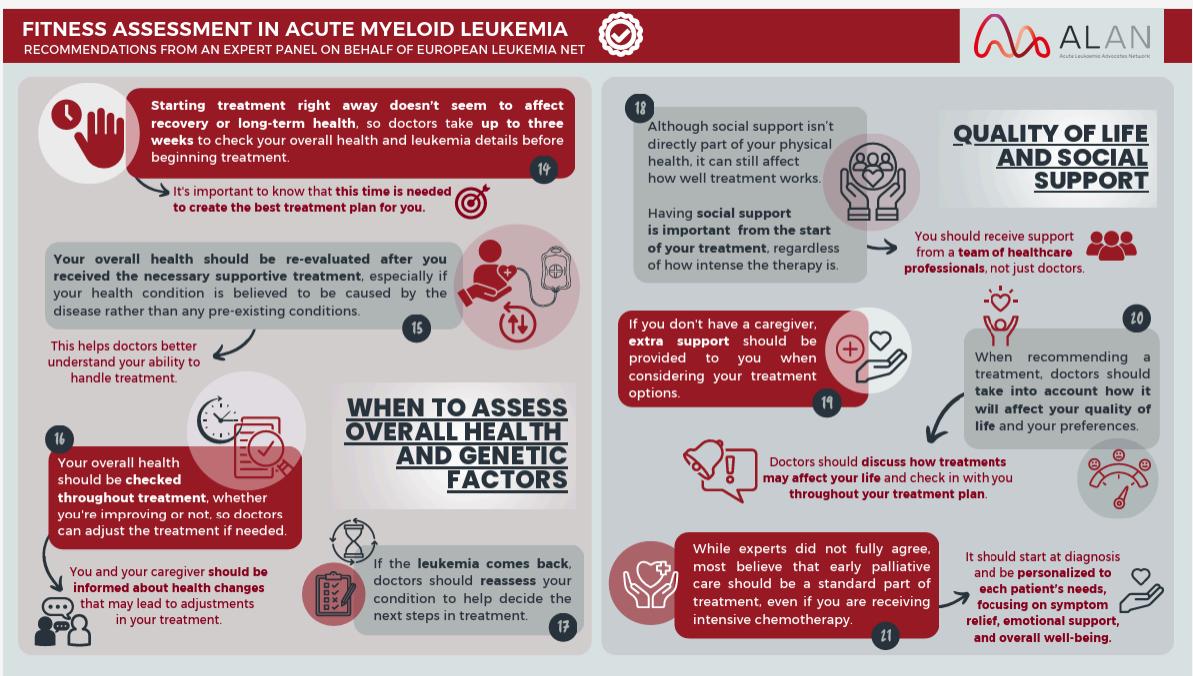
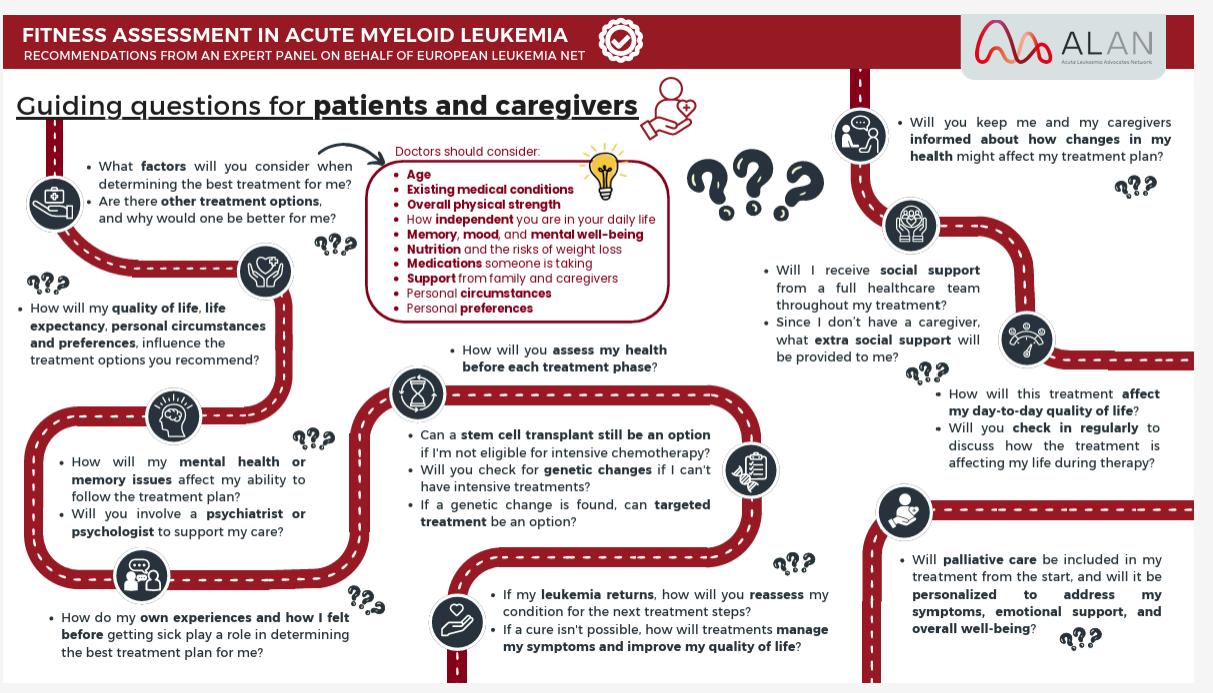
















Hematolgy Advocacy Groups
List of Hematology Advocacy Groups - Canada
National & Regional Hematology Advocacy Organizations
Network of Rare Blood Disorder Organizations (NRBDO)
A pan-Canadian coalition representing patients with rare blood disorders such as hemophilia, thalassemia, aplastic anemia, and von Willebrand disease. NRBDO focuses on advocacy, healthcare access, and best practices in care delivery

Canadian Hemophilia Society (CHS)
Founded in 1953, CHS supports individuals with inherited bleeding disorders through education, research, and advocacy. It operates chapters in every province
Aplastic
Anemia & Myelodysplasia Association of Canada (AAMAC)
Provides education, peer support, and research funding for Canadians living with aplastic anemia, myelodysplastic syndromes (MDS), and paroxysmal nocturnal hemoglobinuria (PNH). Offers local support meetings and webinars.
Leukemia
& Lymphoma Society of Canada (LLSC)
A national charity dedicated to curing leukemia, lymphoma, Hodgkin's disease, and myeloma. LLSC provides patient support services, education, and funds research across Canada.
Lymphoma Canada
Focuses on education, support, and advocacy for lymphoma patients. Offers virtual and in-person support groups across provinces, including Ontario.





List of Hematology Advocacy Groups - Canada
Myeloma Canada
The only national organization exclusively focused on multiple myeloma. Provides educational resources, promotes clinical research, and advocates for patient access to new therapies.
CLL Canada
A national advocacy group supporting Canadians affected by Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Lymphoma (SLL). Offers education and works to improve treatment access
Alpha-1 Canada
Provides peer-to-peer support and disease management services for Canadians diagnosed with Alpha-1 Antitrypsin Deficiency. Engages in national and international advocacy efforts.
aHUS Canada
Advocates for individuals with atypical Hemolytic Uremic Syndrome (aHUS), focusing on education, support, and access to treatment.
Canadian Hemochromatosis Society
Founded by Marie Warder, this organization raises awareness and provides support for those affected by hereditary hemochromatosis. Offers educational materials and advocacy.
The Canadian CML Network
is a community of people living with Chronic Myeloid Leukemia. We are dedicated to providing emotional, social and educational support to people living with CML and their families.The Canadian CML Network works with patients and healthcare providers from all across Canada, and is focused on building CML patient groups nationally







Canadian MPN Network Patient Advocacy Group
A Formal Canadian network of patients and caregivers Provides a platform for sharing best practices and connecting with local support groups.
Know your Blood
A Formal Canadian organization dedicated to the Black/Caribbean community. Provides a platform for sharing and connecting for specific diseases to this community


Informal Groups (Facebook, other social media)
CLL/SLL Information & Support (Canada)
A Canadian community for individuals living with Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Lymphoma (SLL), their families, and professionals. Members share experiences, ask questions, and offer mutual support. Facebook CML Friends of Canada
A group for Canadians living with or supporting someone with Chronic Myeloid Leukemia (CML). It serves as a space for sharing experiences, information, and support among members. Lymphoma Canada – Facebook Page
While not a group, Lymphoma Canada's Facebook page offers resources, updates, and information on virtual support groups, including monthly sessions co-hosted with Wellspring for lymphoma and CLL patients. Lymphoma Canada

List of Hematology Advocacy Groups - USA






Leukemia & Lymphoma Society (LLS)
LLS is the largest nonprofit dedicated to curing leukemia, lymphoma, Hodgkin's disease, and myeloma. It provides patient support, funds research, and advocates for policies to ensure access to quality, affordable care
National Bleeding Disorders Foundation (NBDF)
Formerly the National Hemophilia Foundation, NBDF focuses on awareness, care, and treatment of inheritable blood and bleeding disorders like hemophilia and von Willebrand disease
International Myeloma Foundation (IMF)
IMF is dedicated to improving the quality of life for myeloma patients through research, education, support, and advocacy. It offers resources for patients, caregivers, and healthcare professionals
Leukemia Research Foundation
This foundation's mission is to cure leukemia by funding research and supporting patients and families. It provides educational resources and financial assistance.
Lymphoma Research Foundation
As the nation's largest lymphoma-focused health organization, it is devoted to funding lymphoma research and providing patients with education and support services
CancerCare
CancerCare offers free, professional support services for people affected by cancer, including counselling, support groups, educational workshops, publications, and financial assistance

List of Hematology Advocacy Groups - USA







National Marrow Donor Program (NMDP)
NMDP manages the Be The Match Registry, facilitating bone marrow and umbilical cord blood transplants for patients with life-threatening blood cancers
Aplastic Anemia and MDS International Foundation (AAMDSIF)
AAMDSIF provides support and education for patients with aplastic anemia, myelodysplastic syndromes (MDS), and related bone marrow failure diseases.
MDS Foundation
The MDS Foundation offers support and resources for patients with myelodysplastic syndromes, including information on support groups and treatment options.
International Waldenstrom's Macroglobulinemia Foundation
This foundation provides support and education for patients with Waldenström macroglobulinemia, a rare type of non-Hodgkin lymphoma, and funds research for improved treatments.
Children's Cancer and Blood Foundation (CCBF)
CCBF is the first and largest charitable organization in the U.S. dedicated to supporting the care of children with cancer and blood diseases
MPN Advocacy & Education International
This organization offers educational programs, patient and caregiver conferences, and resources tailored to MPN patients. They aim to empower patients through knowledge and support.
MPN Education Foundation
The foundation provides information and support for MPN patients, including the MPN-NET online support group, which facilitates discussions among patients, caregivers, and healthcare professionals.

List of Hematology Advocacy Groups - Global
The Acute Leukemia Advocates Network (ALAN)
A global patient advocacy organization dedicated to improving the lives of people affected by acute leukemias. By connecting patients, caregivers, and advocates, ALAN promotes awareness, facilitates access to information, and supports research to ensure patient voices shape leukemia care and policy worldwide.
CML Advocates Network
An informal global network moderated by Chronic Myeloid Leukemia (CML) patients and caregivers. Provides a platform for sharing best practices and connecting with national support groups
The Global MPN Scientific Foundation (Global MPNSF)
A non-profit organization committed to improving the lives of individuals affected by myeloproliferative neoplasms (MPNs), a group of rare blood cancers. Through education, advocacy, and research support, Global MPNSF raises awareness, connects patients with specialists, and promotes advancements in treatment and care.


List of Hematology Advocacy Groups - Online
MPN-NET Online Support Group
Hosted by the MPN Education Foundation, MPN-NET is an email-based discussion group where patients and caregivers share experiences, information, and support related to MPNs.
Facebook Groups
Several Facebook groups offer informal support for MPN patients: Myeloproliferative Neoplasms (MPN) Support Group: A global community where members discuss various MPN-related topics.
Polycythemia Vera (PV) Support Group: Focused on individuals diagnosed with PV, sharing experiences and coping strategies.
Essential Thrombocythemia (ET) Support Group: A space for those with ET to connect and support each other.
Myelofibrosis (MF) Support Group: Dedicated to discussions around living with MF, treatments, and research updates.




References
James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005. Nature
Pikman Y, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis. PLoS Med. 2006. PMC
Nangalia J, et al Somatic CALR mutations in MPN with nonmutated JAK2. N Engl J Med. 2013
New England Journal of Medicine
De Thé H, Chen Z. APL pathogenesis/therapy (PML-RARA). Haematologica/Reviews. 2010–2017 overview. Haematologica
Jaiswal S, et al. Age-related clonal hematopoiesis and risk of hematologic cancer. N Engl J Med. 2014. New England Journal of Medicine
Genovese G, et al Clonal hematopoiesis as a strong risk factor for hematologic cancer N Engl J Med. 2014. New England Journal of Medicine
Warren JT, et al. Clonal hematopoiesis and risk for hematologic malignancy (review). Blood. 2020. PMC
COSMIC Signatures SBS1/SBS5 (clock-like). COSMIC database. cancer.sanger.ac.uk
Hsu WL, et al. Leukemia/lymphoma/myeloma incidence in atomic-bomb survivors. Radiat Res. 2013. PMC
IARC Monographs Vol. 120. Benzene. 2018. InChem
U.S. HHS (Surgeon General). The Health Consequences of Smoking—50 Years of Progress. 2014. HHS.gov
CDC. Health effects of cigarettes Cancer (includes AML). 2024. CDC
Wotherspoon AC, et al. Regression of gastric MALT lymphoma after H. pylori eradication. Lancet. 1993. PubMed
Parsonnet J, et al. H. pylori infection and gastric lymphoma risk. N Engl J Med. 1994. New England Journal of Medicine
Young LS, et al. Epstein–Barr virus: oncogenic roles review. Nat Rev Cancer. 2016. PubMed Cohen JI, et al. EBV and cancer (state-of-the-art review). Ann Rev Pathol. 2019. Annual Reviews
Merli M, et al Interferon-free direct-acting antivirals in HCV-associated indolent NHL Blood. 2016. ASH Publications
Zhu X, et al. Meta-analysis: HCV infection increases NHL risk. Medicine (Baltimore). 2019. Lippincott Journals
Raaijmakers MH, et al. Bone progenitor dysfunction induces MDS and secondary leukemia (Dicer1). Nature. 2010. PubMed
Pimenta DB, et al Bone-marrow niche mechanisms in AML (review). Cancers (Basel). 2021 PMC

References
Khoury JD, et al. WHO 2022: myeloid/histiocytic neoplasms overview. Leukemia. 2022. Nature
Churpek JE & Godley LA. How I diagnose/manage inherited myeloid malignancy risk. Blood. 2016/2017. PMC
Makishima H, et al Germline DDX41: unique subtype of adult myeloid neoplasms Blood. 2022/2023. P
National Cancer Institute. Leukemia—Patient Version. Accessed 2025. Cancer.gov
Khoury JD, et al. The 5th edition WHO classification of myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022. Nature
Alaggio R, et al. WHO 5th edition classification of lymphoid neoplasms overview. Leukemia. 2022. Nature
Canadian Cancer Society. Leukemia statistics (Canada). Updated 2025. Canadian Cancer Society
SEER (NCI). Cancer Stat Facts: Leukemia (incidence, mortality). Updated 2024–2025. SEER
NCI Dictionary of Cancer Terms. Philadelphia chromosome. Cancer.gov
Haider MZ, et al. Philadelphia Chromosome. StatPearls. Updated 2023. NCBI
American Cancer Society Tests for AML (diagnosis & staging). Updated 2025 American Cancer Society
NCI PDQ®. Adult AML—Patient/Health Professional Versions. Updated 2024–2025. Cancer.gov+1
NCCN Guidelines for Patients®. Acute Myeloid Leukemia. Version 1.2025. NCCN
NCI PDQ®. CLL—Patient Version. Updated 2024. Cancer.gov
NCI PDQ® & ACS Leukemia risk factors (benzene, radiation, therapy-related; Down syndrome). Cancer.govAmerican Cancer Society
Goldstein BD. Benzene and hematologic cancers (IARC/NCBI Bookshelf). 2019. NCBI FDA & NCI Cancer Currents. CAR T approvals in B-ALL (tisagenlecleucel; brexucabtagene autoleucel). U.S. Food and Drug AdministrationCancer.gov
World Health Organization. (2023). Global Prevalence of Anemia and Iron Deficiency. Centers for Disease Control and Prevention. (2023). Iron and Iron Deficiency. Pasricha, S.-R., et al. (2021). Diagnosis and management of iron deficiency anemia: a clinical update. The Medical Journal of Australia, 214(5), 219–226. Short, M. W., Domagalski, J. E. (2013). Iron Deficiency Anemia: Evaluation and Management. American Family Physician, 87(2), 98–104
Tolkien, Z., Stecher, L., Mander, A. P., Pereira, D. I., & Powell, J. J. (2015). Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One, 10(2): e0117383.

References
Nature Reviews Disease Primers: MM is a hematological lymphoid malignancy with complex genetics, incurable but treatable
Canadian Cancer Society/Myeloma Canada statistics on incidence, prevalence, mortality in Canada Canadian Cancer Societymyeloma.cachaire-myelome-canada.org.
Epidemiology study in Canada: incidence, age, trends Pub MedCanadian Research Data Centre Network.
JAMA Hematology: plasma cell cancer characterization JAMA Network.
Overview/human primer: multiple myeloma as plasma cell malignancy, symptoms Mayo Clinic
Cleveland Clinic: confirmation that myeloma is a rare blood cancer causing bone and tissue damage Cleveland Clinic+1
Wikipedia: global and US incidence, survival, staging, diagnostic criteria, treatment developments including CAR‑T, prognosis Wikipedia.
Elotuzumab approval as immunostimulatory antibody for MM Wikipedia.
Idecabtagene vicleucel (Abecma) CAR T approval JAAD
AI based MRD evaluation study Verywell Health+8arXiv+8Wikipedia+8.
Myeloma Canada organization overview American Journal of Medicine Health.com/Verywell distinction of myeloma among blood cancers Mayo Clinic
British Columbia Ministry of Health. (2023). Iron deficiency – diagnosis and management. Government of British Columbia. https://www2.gov.bc.ca/gov/content/health/practitionerprofessional-resources/bc-guidelines/iron-deficiency
CMAJ. (2025). Intravenous iron in the management of iron deficiency anemia. Canadian Medical Association Journal, 197(24), E680. https://www cmaj.ca/content/197/24/E680
CSL Vifor. (2024, March 19). Ferinject® approved by Health Canada for the treatment of iron deficiency anemia in adult and pediatric patients and iron deficiency in adult patients with heart failure. https://newsroom.csl.com/2024-03-19-Ferinject-R-approved-by-Health-Canadafor-the-treatment-of-iron-deficiency-anemia-in-adult-and-pediatric-patients-and-irondeficiency-in-adult-patients-with-heart-failure
British Columbia Ministry of Health. (2023). Iron deficiency – diagnosis and management Government of British Columbia. https://www2.gov.bc.ca/gov/content/health/practitionerprofessional-resources/bc-guidelines/iron-deficiency
CMAJ. (2025). Intravenous iron in the management of iron deficiency anemia. Canadian Medical Association Journal, 197(24), E680. https://www.cmaj.ca/content/197/24/E680

References
CSL Vifor. (2024, March 19). Ferinject® approved by Health Canada for the treatment of iron deficiency anemia in adult and pediatric patients and iron deficiency in adult patients with heart failure. https://newsroom.csl.com/2024-03-19-Ferinject-R-approved-by-Health-Canadafor-the-treatment-of-iron-deficiency-anemia-in-adult-and-pediatric-patients-and-irondeficiency-in-adult-patients-with-heart-failure
Health Canada. (2024). Ferinject® product monograph. https://labeling.cslbehring.com/PRODUCT-DOCUMENT/CA/CSLBehring/Ferinject-HCapproved-PM-March-11-2024.PDF
Medscape. (2025). Iron deficiency anemia: Guidelines. In emedicine. Retrieved September 1, 2025, from https://emedicine.medscape.com/article/202333-guidelines
National Center for Biotechnology Information. (2024). Management of iron deficiency anemia: Current guidelines and evidence. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC10939879/ PubMed. (2025). Parenteral iron: New trends in clinical use and safety. Haematologica, 110(5), 1234–1241. https://pubmed.ncbi.nlm.nih.gov/40603803/
FirstWord Pharma. (2025, March 11). Accrufer® (ferric maltol) receives Health Canada approval for adults with iron deficiency anemia intolerant to other oral iron therapies https://firstwordpharma.com/story/5941391
Canada access & approvals: CADTH/pCODR process; pCPA role. Innovative Medicine Globaldhpp.hpfb-dgpsa.ca
Canada—myeloma CAR-T earlier line: Health Canada NOC for CARVYKTI (second line). Innovative Medicine Globalcda-amc.ca
Canada—DLBCL second-line CAR-T: Breyanzi earlier-line approval press release Bristol Myers Squibb
Canada—MF with anemia: Health Canada NOC for momelotinib; CDA-AMC reimbursement recommendation. dhpp.hpfb-dgpsa.caca.gsk.com
Canada CAR-T safety: HC summary safety review & InfoWatch. dhpp.hpfbdgpsa caGovernment of Canada
Canada—Hodgkin lymphoma first-line: NEJM Nivo-AVD and OH regimen update PMCcancercareontario.ca
Marcellino BK, Hoffman R. Recent advances in prognostication and treatment of polycythemia vera. F1000Res. 2021;10:29. doi:10.12703/r/10-29. Barbui T, Thiele J, Gisslinger H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J 2018;8(2):15
Myeloproliferative neoplasms, version 1.2024. NCCN. Accessed January 30, 2024. https://www.nccn.org/patients/guidelines/content/PDF/mpn-patient.pdf

References
Tefferi A, Barbui T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;1‐23. doi:10.1002/ajh.27002
Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33.
Scherber RM, Geyer HL, Dueck AC, et al The potential role of hematocrit control on symptom burden among polycythemia vera patients: insights from the CYTO-PV and MPN-SAF patient cohorts. Leuk Lymphoma. 2017;58(6):1481-1487. doi:10.1080/10428194.2016.1246733.
Duminuco A, Harrington P, Harrison C, Curto-Garcia N. Polycythemia vera: barriers to and strategies for optimal management. Blood Lymphat Cancer. 2023;13:77-90. Published December 21, 2023. doi:10.2147/BLCTT.S409443.
Kiladjian JJ, Winton EF, Talpaz M, Verstovsek S Ruxolitinib for the treatment of patients with polycythemia vera. Expert Rev Hematol. 2015;8(4):391-401. doi:10.1586/17474086.2015.1045869
Besremi Package insert.https://us.pharmaessentia.com/wpcontent/uploads/2021/11/BESREMi-USPI-November-2021-1.pdf
Pegasys. Package insert. Accessed September 18, 2024. https://www gene com/download/pdf/pegasys_prescribing.pdf
Tremblay D, Yacoub A, Hoffman R. Overview of Myeloproliferative Neoplasms: History, Pathogenesis, Diagnostic Criteria, and Complications. Hematol Oncol Clin North Am. 2021;35(2):159-176. doi:10.1016/j.hoc.2020.12.001.
Lawrence L. How the name change to myeloproliferative neoplasms affected people with the disease. Cure. 2022;21(3).
Spivak JL Polycythemia vera Curr Treat Options Oncol 2018;19(12):52. doi:10.1007/s11864018-0529-x.
NORD Rare Disease Database. Polycythemia vera. Accessed September 11, 2024. https://rarediseases.org/rare-diseases/polycythemia-vera/#affected
Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer J. 2018;8(3):3. doi:10.1038/s41408-017-0042-7.
Passamonti F, et al Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117(10):755-761. doi:10.1016/j.amjmed.2004.06.032.
Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y.

References
Cuthbert D, Stein BL. Polycythemia vera-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. J Blood Med. 2019;10:359-371. doi:10.2147/JBM.S189922.
Gerds AT, Mesa R, Burke JM, Grunwald MR, Stein BL, Squier P, Yu J, Hamer-Maansson JE, Oh
ST Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL. Blood. 2024;143(16):1646-1655.
doi:10.1182/blood.2023020232.
Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2005;128:275-290. doi:10.1111/j.1365-2141.2004.05277.x.
Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(5):1071-1082. doi:10.1007/s00277-019-03625-x
Grenier JMP, El Nemer W, De Grandis M. Red blood cell contribution to thrombosis in polycythemia vera and essential thrombocythemia. Int J Mol Sci. 2024;25(3):1417. doi:10.3390/ijms25031417.
Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176-2184. doi:10.1182/blood-2013-03-460154.
Harrison CN, Nangalia J, Boucher R, et al Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J Clin Oncol. 2023;41:3534-3544. doi:10.1200/JCO.22.01935.
Protagonist and Takeda Announce Positive Topline Results from Phase 3 VERIFY Study of Rusfertide in Patients with Polycythemia Vera. March 3, 2025. Accessed April 14, 2025. https://feeds.issuerdirect.com/news-release.html?newsid=7353096637891738
National Cancer Institute (NCI) PDQ®: Chronic Lymphocytic Leukemia Treatment (health professional and patient versions; updated 2024–2025). Comprehensive, regularly updated overview of CLL staging and treatment options. Cancer.gov+1
European Society for Medical Oncology (ESMO): 2024 interim update on first-line and relapsed CLL therapies; evidence-based guideline on diagnosis, treatment, and follow-up Annals of Oncology+1
American Cancer Society: CLL treatment by risk group; targeted therapy drug summaries (updated March 2025). Clear explanations of frontline and relapsed options. American Cancer Society+1
U.S. FDA (Dec 1, 2023): Accelerated approval of pirtobrutinib (Jaypirca) for adults with CLL/SLL after prior BTKi and BCL-2 inhibitor Regulatory label and indication details U.S Food and Drug Administration
CLL Society (Feb 2025): Updates on MRD-guided venetoclax-obinutuzumab strategies and context for “watch and wait” in newly diagnosed patients.

References
Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C., Apperley, J. F., Cervantes, F., … Hehlmann, R. (2020). European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia, 34(4), 966–984. https://doi.org/10.1038/s41375-020-0776-2
Mahon, F. X., Réa, D., Guilhot, J., Guilhot, F., Huguet, F., Nicolini, F., … Rousselot, P. (2018). Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. The Lancet Oncology, 11(11), 1029–1035. https://doi.org/10.1016/S14702045(09)70233-3
American Cancer Society. (2024). Chronic Myeloid Leukemia (CML). Retrieved from: https://www.cancer.org/cancer/chronic-myeloid-leukemia.html
National Cancer Institute (2023). Chronic Myeloid Leukemia Treatment (PDQ®)–Patient Version. Retrieved from: https://www.cancer.gov/types/leukemia/patient/cml-treatment-pdq
Tefferi, A., & Barbui, T. (2019). Polycythemia vera and essential thrombocythemia: 2020 update on diagnosis, risk-stratification and management. American Journal of Hematology, 94(1), 133–143. https://doi.org/10.1002/ajh.25303
Verstovsek, S., Mesa, R. A., Gotlib, J., Gupta, V., DiPersio, J F., Catalano, J V., Kantarjian, H. (2012). A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. New England Journal of Medicine, 366(9), 799–807. https://doi.org/10.1056/NEJMoa1110557 National Cancer Institute. (2023). Myeloproliferative Neoplasms Treatment (PDQ®)–Patient Version. Retrieved from: https://www.cancer.gov/types/myeloproliferative American Cancer Society (2024). What Are Myeloproliferative Neoplasms?. Retrieved from: https://www cancerorg/cancer/types/myeloproliferative-neoplasms.html
Fenaux, P., Haase, D., Santini, V., Sanz, G. F., Platzbecker, U., Mey, U., … List, A. F. (2021). Myelodysplastic syndromes Nature Reviews Disease Primers, 7(1), 83 https://doi.org/10.1038/s41572-021-00323-3
Malcovati, L., Hellström-Lindberg, E., Bowen, D., Adès, L., Cermak, J., del Cañizo, C., … Greenberg, P. (2013). Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood, 122(17), 2943–2964. https://doi.org/10.1182/blood-2013-03-492884
National Cancer Institute (2023). Myelodysplastic Syndromes Treatment (PDQ®)–Patient Version. Retrieved from: https://www.cancer.gov/types/myeloproliferative/patient/myelodysplastic-treatment-pdq American Cancer Society. (2024). What Are Myelodysplastic Syndromes (MDS)?. Retrieved from: https://www.cancer.org/cancer/types/myelodysplastic-syndrome.html




Alliances





















Alliances

















Partners









My Blood, My Health Team
Cheryl Petruk, MBA B.Mgt.

Cheryl A. Petruk is a multifaceted professional whose career spans across patient advocacy,business,andpost-secondaryeducation,showcasingherdedicationtomakinga significantimpactineachoftheseareas.
Cheryl's transition into patient advocacy was driven by a passion from her family circumstances and a deep commitment to ensuring patients' rights and access to care Cheryl has worked tirelessly to bridge the gap between the healthcare system, patients, andpharmastakeholders,ensuringthatpatients'voicesareheardandtheirneedsaremet Her work involves collaborating with Stakeholders and Patient Advocacy Organizations, lobbying for patient centricity, and providing patient support and guidance Cheryl's empatheticapproachanddedicationtoadvocacyhavemadeherarespectedfigureinthis field,admiredbypatients,healthcareprofessionals,andfellowadvocates

Cheryl also leads and is the lead faculty member at CACHEducation, Patient Advocacy Training CherylisacertifiedgreenbeltinVBHC,acertifiedTrainerinVBHC,andisaDBA Student My Blood My Health Digital Magazine –
Heal Canada and its signature initiative, My Blood My Health, collaborate with a diverse array of consultants who bring specialized expertise to support our mission of advancing patient advocacy, education, and engagement in hematological health. These consultants include healthcare professionals, patient engagement strategists, medical writers, clinical research advisors, digital health experts, legal and regulatory specialists, and communications and media consultants. Each contributes uniquely—whether it's crafting patient-centered educational content, guiding ethical and regulatory compliance for clinical outreach, developing digital tools for patient engagement, or ensuring that advocacy messaging resonates across platforms. This multidisciplinary approach ensures our programs are informed, inclusive, and impactful, amplifying the patient voice while driving meaningful change in healthcare.
Statement © 2025 My Blood My Health Digital Magazine All rights reserved
This publication is intended for informational and educational purposes only. No portion of this magazine may be copied, republished, or shared without express written permission, except as allowed under fair use for non-commercial educational purposes For inquiries or permissions, contact: admin@healcanada org
My Blood My Health is a patient-focused initiative of Heal Canada, dedicated to raising awareness of blood cancers and supporting the patient voice.
