




aggressive forms such as triple-negative breast cancer, is the difficulty in quickly assessing treatment effectiveness. Often, doctors rely on chemotherapy regimens, which are expensive and toxic, continuing with treatment even when it proves ineffective. Typically, doctors cannot determine if a chemotherapy regimen is working until the patient has completed all the prescribed cycles, which can take up to six months. If the treatment is unsuccessful, the patient may need to begin a new regimen, starting the process over. Now, a simple blood test that searches for a biomarker in plasma will allow doctors to assess quickly whether cancer treatments, like chemotherapy, are working. This allows for faster adjustments to the treatment plan, switching to more effective therapies if necessary.
Previously, researchers have focused on nitric oxide production, a molecule that regulates cancer cell growth. Researchers at the University of New England (UNE; Portland, ME, USA; www.une.edu) discovered a biomarker called Nw-hydroxy-LArginine (NOHA) that is a sensitive and reliable indicator for estrogen receptor-negative tumors, which are more aggressive types of breast cancer. Prior research had already linked nitric oxide, inflammatory biology, and breast cancer. The researchers realized that NOHA, being upstream of the nitric oxide process, could be measured through a blood test rather than a tissue sample. NOHA directly correlates with nitric oxide levels, without interference from other biological pathways. With this discovery, they began exploring NOHA as a biomarker for both detecting and monitoring breast cancer and even applied for a patent for a diagnostic tool that uses this blood marker to track aggressive, estrogen-negative tumors.
The researchers conducted a study in Tanzania to test whether NOHA could determine the estrogen receptor status in breast cancer patients. The results from this study were promising, suggesting that NOHA has the potential for improving cancer care. With the NOHA blood test, doctors could monitor NOHA levels after each treatment cycle. If the levels show an unfavorable change, the physician could pause the current drug combination and try another. Building on these findings, the team is now researching NOHA’s role in managing triple-negative breast cancer in the U.S. through clinical trials, which are essential in translating laboratory results into real-world applications.
In addition, the team is exploring NOHA’s potential for early detection of breast cancer, particularly for individuals with genetic predispositions, and its role in drug development. Their work is setting the stage for more dynamic monitoring of treatment effectiveness. Following their success with breast cancer, the researchers have expanded their work to ovarian cancer. The American Cancer Society estimates that nearly 20,000 new cases of ovarian cancer will be diagnosed in the U.S. this year, with almost 13,000 women dying from the disease. Because the symptoms of ovarian cancer are often subtle or absent in its early stages, the disease is frequently diagnosed at a later, more advanced stage. The researchers’ findings indicate that NOHA could also be used to detect and monitor ovarian cancer. This led them to

Image: The test could be a game changer for people with some of the most aggressive forms of cancer (Photo courtesy of UNE)
secure a second patent for a blood diagnostic tool to identify ovarian cancer, broadening the impact of their groundbreaking research.
“It validates to us that (NOHA) is not only going to be centric to breast cancer, but it can also expand to other tumors like ovarian cancer,” said Srinidi (Sri) Mohan, Ph.D., professor at the University of New England School of Pharmacy, who identified NOHA as a potential biomarker for breast cancer detection and monitoring.
• Precise and reliable HbA1c results traceable to IFCC and NGSP
• Fast measurement - results available after 4:30 min
• All in one, pre-calibrated cartridge

• Storage of tests at 2-30°C for up to 18 months
• Maintenance free analyzer


The Simple Western blot system revolutionizes traditional Western blotting by automating the entire process, from protein separation to detection. This system delivers quantitative, reproducible results with minimal hands-on time.


The ID NOW Influenza A & B 2 assay delivers molecular flu results in 13 minutes or less on Abbott’s ID NOW platform, making it significantly faster than other molecular methods and more accurate than conventional rapid tests.

In recent years, cancer immunotherapy has emerged as a promising approach where the patient’s immune system is harnessed to fight cancer. One form of immunotherapy, called CAR-T-cell therapy, involves genetic modifications to a patient’s T cells to enable them to target and destroy cancer cells. While this treatment has shown success, particularly in blood cancers, it is not without its risks. One of the severe side effects associated with CAR-T therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), which causes inflammation in the central nervous system. Symptoms of ICANS range from mild issues like headaches and lethargy to more severe manifestations such as impaired consciousness, seizures, or even brain hemorrhages. The incidence of ICANS after CAR-T therapy is high, estimated at around 64%, but there has been no reliable method to predict its severity until now. Researchers have now discovered a method to predict this life-threatening side effect before it occurs.
Researchers at Kyushu University (Fukuoka, Japan; www.med.kyushu-u.ac.jp) analyzed cerebrospinal fluid collected before treatment to identify proteins linked to harmful immune responses that affect the central nervous system following therapy. Published in Leukemia, the study could make cancer immunotherapy safer by helping doctors identify high-risk patients ahead of time, allowing them to implement early interventions or even prevent the condition. In this study, the team analyzed cerebrospinal fluid samples from 29 patients with B-cell non-Hodgkin’s lymphoma before they underwent CAR-T therapy. Out of the cohort, 11 patients developed ICANS, while 18 did not.
The team identified 864 proteins across all spinal fluid samples, narrowing down the list to 46 proteins that displayed significant differences in
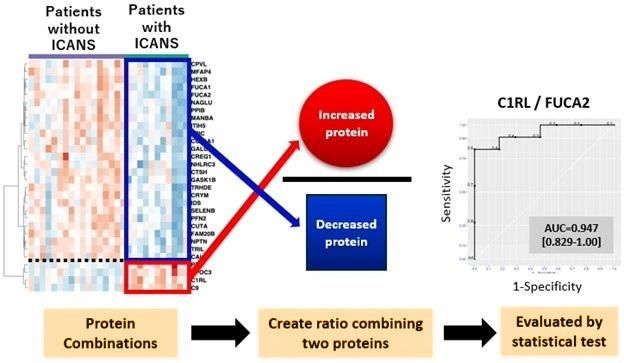
concentration between patients who developed ICANS and those who did not. These proteins became potential biomarkers for predicting ICANS. The researchers pinpointed two key proteins: C1RL, which was elevated in patients who developed ICANS, and FUCA2, which had lower levels in those patients. When combined, the ratio of these two proteins proved to be highly accurate in distinguishing patients at high risk of developing ICANS from those at low risk. To validate their findings, the team tested the C1RL/FUCA2 biomarker on a second group of 10 patients undergoing CAR-T therapy, and in all cases, the protein ratio correctly predicted the risk of developing ICANS. However, the researchers noted that the small sample size means the results are preliminary and need further validation.
Beyond aiding in early detection and timely treatment, the researchers hope their identification of these biomarkers will allow for preventive measures before CAR-T therapy begins. For instance, since C1RL is involved in the comple-
ment system, which is known to trigger inflammation and may contribute to ICANS, patients identified as high-risk could be preemptively treated with drugs that inhibit this system. This predictive test could lead to a more personalized and safer approach to cancer treatment. Additionally, the research team plans to expand their investigation to see if these biomarkers can be applied to other blood cancers beyond B-cell non-Hodgkin’s lymphoma. They are also exploring the potential for using easier-to-collect fluids, such as blood serum, to find more accessible biomarkers for treatment monitoring.
“Spinal fluid collection is an invasive and painful procedure, so most hospitals in Japan and other countries don’t routinely perform it before CAR-T therapy,” said Dr. Tomoko Nomiyama, co-first author and clinical technologist at Kyushu University Hospital. “If we can identify similar biomarkers in blood, our test would become a much simpler and more accessible tool for predicting ICANS.”
Tumor heterogeneity presents a major obstacle in the development and treatment of cancer therapies, as patients' responses to the same drug can differ, and the timing of treatment significantly influences prognosis. Consequently, technologies that predict the effectiveness of anticancer treatments are essential in minimizing side effects and improving treatment efficiency. Current methods, such as gene panel-based tests and patient-derived xenograft (PDX) models, have limitations in their applicability to certain patients, have challenges in predicting drug effects, and require significant time and costs to develop. Now, researchers have successfully created a gastric cancer model using 3D bioprinting technology and patient-derived cancer tissue fragments. This groundbreaking model preserves the characteristics of actual patient tissues and is expected to rapidly assess and predict individual patient drug responses.
In collaborative research by Pohang University of Science & Technology (POSTECH, Gyeongbuk, Korea; www.postech.ac.kr) and The Jackson Laboratory for Genomic Medicine (Farmington, CT, USA; www.jax.org), scientists have developed an in vitro gastric cancer model by utilizing 3D bioprinting technology and tissue-specific bioink that incorporated patient-derived tissue fragments. Notably, they encapsulated cancer tissues within a stomach-derived decellularized extracellular matrix (dECM) hydrogel, which artificially facilitated cell-matrix interactions. By co-culturing these tissues with human gastric fibroblasts, they successfully replicated cancer cell-stroma interactions, thereby recreating the in vivo tumor microenvironment in vitro.
Published in the international journal Advanced Science, the research shows that this model preserved the distinct characteristics of gastric tissues from individual patients by replicating both cell-stroma and cell-matrix interactions. It demonstrated high specificity in predicting the patient's anticancer drug responses and prognosis. Additionally, the model's gene profiles related to cancer development, progression, and drug response closely mirrored those of patient tissues, outperforming conventional PDX models. The rapid fabrication process of this model

through bioprinting also allows drug evaluation within two weeks of tumor tissue extraction from the patient. This efficient platform is expected to make a significant contribution to the development of personalized cancer treatments.
“By reproducing cancer cell-stroma and cell-matrix interactions, this model enhances the accuracy of drug response predictions and reduces unnecessary drug administration to non-responsive patients,” said Professor Charles Lee from The Jackson Laboratory for Genomic Medicine, who led the study.
“This is a critical preclinical platform not only for developing patient-specific treatments but also for validating new anticancer drugs and combination therapies,” added Professor Jinah Jang of POSTECH.
Endometrial cancer, which develops in the lining of the uterus, is the most prevalent gynecologic cancer in the United States, affecting over 66,000 women annually. Projections indicate that in 2025, around 69,120 new cases will be diagnosed, with approximately 13,860 deaths resulting from the disease. While immunotherapy plays a significant role in treatment, it is not effective for all patients, and many experience a recurrence of the cancer. However, researchers have made a key discovery that could guide doctors in selecting more effective treatments for patients with recurrent endometrial cancer. Their study, published in the Journal for ImmunoTherapy of Cancer (JITC), identified specific proteins in the blood (biomarkers) that might predict how well a patient will respond to a combination of two cancer drugs, cabozantinib and nivolumab.
This study, conducted by scientists at the Icahn School of Medicine at Mount Sinai (New York, NY, USA; icahn.mssm.edu), is the first to explore blood markers that could aid in personalizing treatment and improving patient outcomes. The team discovered that certain proteins in the blood exhibited different behaviors in patients receiving nivolumab alone versus those on the combination therapy.
Patients who had lower levels of specific proteins linked to macrophages (a type of white blood cell critical to the immune system) prior to treatment responded more favorably to the drug combination. Additionally, some patients showed particular immune activation markers, which helped enhance their cancer-fighting response, resulting in longer survival.





The study also found that elevated levels of proteins associated with neutrophils (another type of white blood cell involved in the immune response) were connected to increased side



















SYPHILIS RAPID TEST

The OnSite Syphilis Ab Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies including IgG, IgM, and IgA to Treponema pallidum (Tp) in human serum or plasma.

In regions where access to clinics for routine blood tests presents financial and logistical obstacles, HIV patients are increasingly able to collect and send a drop of blood using paper-based devices that absorb and preserve the sample for analysis in distant laboratories. While these devices have been beneficial for monitoring medication adherence and tracking disease progression, many of the most commonly used options do not regulate the amount of blood they collect, which can lead to inaccurate results regarding a patient’s infection. Recognizing this limitation, researchers have developed a new paper-based device with wax-printed patterns that form precise channels and collection spots, ensuring a consistent volume of blood is collected every time.
A team from Tufts University School of Medicine (Medford, MA, USA; www.medicine.tufts.edu) collaborated with the National Institute for Communicable Diseases (NICD, Johannesburg, South Africa; www. nicd.ac.za) to carry out a clinical pilot involving 75 HIV-positive patients in South Africa. The NICD provided valuable real-world data, enabling Tufts researchers to compare their plasma spot cards in a clinical environment where they would be actively used. The plasma spot card developed by Tufts’ research team demonstrated a more accurate measurement of a patient’s HIV infection than the widely used Roche plasma spot card (90.5% vs. 82.7%).
Published in the Proceedings of the National Academy of Sciences, the study also found that the Tufts device was better at detecting drug-resistant viral mutations (63% vs. 42%), which can inform physicians about whether to continue or change a patient’s medication regimen. The researchers are now working to expand the use of this technology by forming partnerships with laboratories and researchers both in the U.S. and internationally. They are also refining the device to improve its accuracy and functionality while progressing toward its commercialization.
“We intentionally focus on developing technologies that are simple, both in construction and operation,” said Charlie Mace, an associate professor at Tufts University’s Department. “Those kinds of restrictions can make research more difficult, but ultimately we believe in that approach, because simplicity should lead to accessibility and affordability, which are both clearly needed in health care.”

The cobas c 703 analytical unit enhances efficiency with a throughput of up to 2000 tests/hour and 70 onboard reagent positions. Ideal for high-volume labs, it reduces manual intervention while maintaining operational precision.


Bacteremia, also known as blood poisoning, occurs when bacteria manage to overcome the body’s immune defenses. This condition can progress into sepsis, a serious illness that is responsible for over a third of hospital-related deaths each year. While individuals are frequently exposed to bacteria from the environment, they often fight off these infections without experiencing this deadly progression. Scientists are working to understand how bacteria spread throughout the body to cause systemic infections, with the ultimate goal of halting this process before it escalates.
Researchers at U-M Medical School (Ann Arbor, MI, USA; medschool. umich.edu) have been investigating this issue, focusing on gram-negative bacteria such as Klebsiella pneumoniae, a common cause of pneumoniarelated bacteremia. In their previous studies, they identified three stages in the spread of bacteria: initial infection at a site like the lungs, entry into the bloodstream, and replication while avoiding filtration by the liver and spleen. Traditionally, bacterial infections are analyzed by culturing tissue and counting the resulting bacteria. While it’s easy to
Cont’d on page 15
cute kidney injury (AKI) is a common complication in COVID-19 patients, contributing to a higher risk of mortality. Early detection of kidney-related issues in COVID-19 cases is crucial for improving patient outcomes. Traditional markers of kidney function, such as creatinine and cystatin C, often lack the specificity and sensitivity needed for accurate diagnosis. These markers can be influenced by factors like muscle mass, sex, nutrition, and other variables, while the estimated glomerular filtration rate (eGFR) can vary based on the method used. Now, a new study suggests that serum uromodulin (sUmod) could serve as a promising biomarker for diagnosing AKI in COVID-19 patients.
The study conducted by EUROIMMUN (Lübeck, Germany; euroimmun.com), St George Hospital (Leipzig, Germany; Leipzig, Germany; klinik-st-georg.de), and various other research institutes, has found that sUmod may overcome the limitations of traditional kidney function markers. Uromodulin, a glycoprotein produced exclusively in the kidneys, is directly linked to kidney function. Compared to creatinine and cystatin C, sUmod offers greater sensitivity, allowing for earlier detection of kidney injury. The study analyzed the relationship between sUmod levels and AKI, as well as in-hospital mortality, in a cohort of 378 COVID-19 patients, including subsets with various comorbidities. AKI was diagnosed using standard laboratory parameters and sUmod, with serum uromodulin levels measured through a sensitive ELISA technique based on monoclonal antibodies, which is CE-marked for serum analysis (EUROIMMUN). Out of the 378 patients, 151 (40%) developed AKI, with 116 of these patients showing AKI at hospital admission, and 35
Cont’d from page 14
measure the initial infection phase by observing how bacteria invade the lungs, and similarly, the third phase by assessing how bacteria survive in the liver and spleen, the transition from the lungs into the bloodstream has been difficult to track.
Using an innovative barcoding system, the researchers labeled bacteria with short DNA sequences in mouse models and employed computer analysis to track the movement of K. pneumoniae throughout the body. They initially hypothesized that the bacteria would replicate in the lungs until they overwhelmed the local immune defenses, eventually spilling into the bloodstream. This type of spread, which they called metastatic dissemination, was observed in some mice. However, they also uncovered an unexpected pattern. About half of the mice showed this metastatic pattern, while the other half exhibited a form of bacterial spread where the bacteria entered the bloodstream on their own without first replicating in large numbers, a process they termed direct dissemination.
The findings, published in Nature Communications, revealed that the metastatic pathway was associated with a more severe infection compared to the direct dissemination route. Moreover, over time, the infection tended to follow the metastatic pattern. The discovery of the direct route suggests that bacteria might be establishing low-level reservoirs in other parts of the body, which could offer new targets for treating blood infections. Additionally, the researchers introduced mutations in both the K. pneumoniae bacteria and the mice, which affected the mode of bacterial dissemination. This hinted that the interaction between the bacteria and the host’s immune system could play a key role in determining the course of the infection.
“The project began with a very basic question—how does bacteria leave the lungs—that we have now provided some insight into, closing a significant gap in our knowledge,” said Michael Bachman, M.D., Ph.D., clinical associate professor of pathology and microbiology and immunology at U-M Medical School.
developing it during their stay.

Image: Serum uromodulin has been associated with kidney function and outcome in hospitalized COVID-19 patients (Photo courtesy of EUROIMMUN)
The study revealed that patients with AKI had significantly lower mean sUmod levels (125 ng/ml) compared to those without AKI (215 ng/ml). Additionally, there was a strong correlation between sUmod and other AKI markers, such as serum creatinine and cystatin C. AKI patients had a higher probability of in-hospital death, with a mortality rate of 15%. Among those who died, the mean sUmod levels were significantly lower (129 ng/ml) compared to survivors (188 ng/ml). This study, published in Scientific Reports, further emphasizes the association between AKI and in-hospital death in COVID-19 patients and underscores the importance of early detection and management of AKI. The stable nature of sUmod and its analytical advantages over traditional kidney markers make it a promising candidate for clinical use. Further research is needed to explore its full clinical potential and improve risk stratification in this patient group.


STERILIZABLE INSTRUMENT & WORK-SURFACE MATS
Thermo-Resistant (- 60 °C to 300 °C)
Fully Washable & Flexible
Suitable for central sterilization services
Sterilizable

















EThe Phadia 200 is a fully automated immunoassay system for allergy and autoimmunity diagnostics. Supporting up to 650 ImmunoCAP and EliA tests, it processes up to 42 samples per run with a peak capacity of 96 results per batch.


The LIAISON® Mumps panel (IgG, IgM) is a CLIAbased automated solution for diagnosing mumps virus infections and assessing immune status. It assists in differential diagnosis, particularly in immunized or high-risk individuals.

ach year in the United States, around 81,000 new cases of bladder cancer are diagnosed, leading to approximately 17,000 deaths annually. Muscle-invasive bladder cancer (MIBC) is a severe form of bladder cancer where tumors invade the detrusor muscle of the bladder. The standard treatment for MIBC has been neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC), but this approach results in significant challenges. Radical cystectomy carries a mortality rate of 0.3–5.7%, along with considerable surgical morbidity, as 64% of patients experience postoperative complications within 90 days. Although around 35% of MIBC patients achieve a complete pathologic response (pCR), meaning no residual tumor remains after NAC treatment, predicting which patients will benefit from this treatment has been difficult due to the tumor’s heterogeneity. Despite numerous studies in this area, developing accurate prediction models and identifying biomarkers that can reliably indicate how patients will respond to treatment have proven challenging. Now, researchers have developed a more effective model to predict the response of MIBC patients to chemotherapy.
This predictive model, created at Weill Cornell Medicine (New York, NY, USA; New York, NY, USA; weill.cornell.edu), utilizes the power of artificial intelligence (AI) and machine learning. It combines whole-slide tumor imaging data with gene expression analyses, improving upon previous models that relied on only one data type. The study, published in npj Digital Medicine, highlights key genes and tumor characteristics that could determine how well patients respond to treatment. By accurately predicting an individual’s response to the standard treatment for MIBC, this model could help clinicians personalize care and potentially spare patients who respond well from undergoing bladder removal surgery.
To enhance the predictive capabilities of the model, the researchers used data from the SWOG Cancer Research Network, which designs and conducts multi-center clinical trials for adult cancers. They specifically integrated data from tumor sample images and gene expression profiles, which show which genes are activated or suppressed. For image analysis, they employed graph neural networks, a specialized AI technique that captures the arrangement and interaction of cancer cells, immune cells, and fibroblasts within the tumor. Automated image analysis was also used to identify the various cell types present in the tumor site. By combining the image-based data with gene expression profiles, the AI-driven model significantly outperformed models using either data type alone in predicting clinical response.

Looking ahead, the researchers plan to incorporate additional data, such as mutational analyses of tumor DNA, which can be detected in blood or urine, as well as spatial analyses to identify the precise cell types present in the bladder. The model also presented new hypotheses for further testing, such as the idea that the ratio of tumor cells to normal cells, like fibroblasts, can impact chemotherapy response predictions. Moving forward, the team aims to validate their findings with other clinical trial cohorts and is open to expanding their collaboration to determine whether their model can predict therapeutic response in a wider range of patients.
“We want to identify the right treatment for the right patient at the right time,” said co-lead Dr. Bishoy Morris Faltas. “The dream is that patients would walk into my office, and I could integrate all of their data into the AI framework and give them a score that predicts how they would respond to a particular therapy. It’s going to happen. But physicians like me will have to learn how to interpret these AI predictions and know that I can trust them—and to be able to explain them to my patients in a way they can also trust.”

On behalf of the IFCC Accreditation Committee: David Kinniburgh (CA, Chair), Nader Rifai (US, member), James O’Connor (IE, member), Sedef Yenice (TR, member)
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) is proud to announce the launch of PROCEEDTM (Providing Continuing Education Excellence and Development), a pioneering accreditation program designed to grant Continuing Education (CE) credits for a broad range of IFCC educational events. This initiative represents a significant milestone in the IFCC’s commitment to advancing education and professional development in the field of laboratory medicine.
Driven by the forward-thinking spirit of the IFCC Accreditation Committee (EBAccC), PROCEEDTM represents a significant step forward in ensuring that the excellent IFCC educational offerings continue to meet rigorous quality benchmarks. By standardizing the process of awarding CE credits, the program reinforces IFCC’s commitment to lifelong learning and continuous improvement in laboratory medicine. PROCEEDTM instills confidence in participants that the educational activities they attend are not only enriching but also recognized under a robust and transparent accreditation framework.
Recognizing the evolving landscape of education, the PROCEEDTM accreditation program has been meticulously designed to grant CE credits for IFCC-endorsed educational events, ensuring compliance with accrediting organizations. The program introduces a comprehensive approach that includes:
• IFCC Worldlab Congresses: High-caliber international events that foster knowledge exchange and collaborative research.
• Distance Learning Initiatives: Online educational activities including “live webi-
nars”, online courses, and recorded webinars for the eAcademy platform and, courses available at https://eacademy. ifcc.org/eacademy and “Adaptive Learning Programme” that have become essential in today’s interconnected world.
In support of these initiatives, the IFCC EB-AccC has developed and published a comprehensive guideline that offers clear and practical recommendations to ensure that all online accredited educational activities adhere to best practices in professional education. The guideline can be accessed at the IFCC Task Force on Global e-Learning/e-Academy (TF-GEL) webpage: https://ifcc.org/executive-board-and-council/eb-task-forces/ taskforce-on-global-elearningeacademy

opment: Allowing professionals to earn CE credits for career advancement and certification requirements.
• Global Accessibility: Offering accredited programs through in-person and digital learning platforms.
• Enhanced Career Opportunities: Strengthening the credentials of laboratory medicine professionals.
Future of PROCEEDTM
Official Recognition and Trademark Registration
To solidify the program’s credibility, IFCC has successfully registered PROCEEDTM as an official trademark under the European Union Intellectual Property Office (EUIPO). This strategic move enhances credibility and ensures that all stakeholders can trust in the quality and consistency of the accreditation process. Why PROCEED Matters?
With the growing need for high-quality education and professional competency validation, PROCEEDTM offers several key benefits:
• Standardization of CE Credits: Ensuring that educational activities meet global accreditation standards.
• Recognition for Professional Devel-
The IFCC EB-AccC is actively working to expand the scope of PROCEEDTM , integrating it into more IFCC educational initiatives and forging collaborations with national societies, national accreditation authorities (NAA), and accrediting organizations. The program will continue to evolve, providing laboratory professionals with access to the highest quality continuing education opportunities while ensuring their professional development efforts receive well-deserved recognition.
For more information about PROCEEDTM and upcoming accredited distance learning events, visit the web pages of the IFCC EB-AccC and IFCC TF-GEL. Join us as we PROCEEDTM toward a future of excellence in laboratory medicine education!
The IFCC along with its Task Force for Young Scientists (TF-YS) invites you to Participate into the 4th Edition of the "IFCC FORUM for Young Scientists” that will be held in Brussels ahead of the EuroMedLab main congress.
At the FORUM the YS will have opportunities for training and improve communication and networking. The scientific program of the FORUM, currently in preparation, will provide the young scientists with an excellent opportunity for an open discussion platform about scientific and
personal experiences, exchange of ideas among colleagues and best practices. Selected Young Scientists will present and discuss their activities in laboratory medicine and benefit from career skills development.
Young Scientists (YS) are the future of laboratory medicine! Future leaders need to be trained and encouraged to succeed in their role, ideally with the support of experienced leaders. Participate in the FORUM to make this feasible!
Don’t miss the event, stay tuned!

E-mail: info@globetech.net
AZLTK & Lab Expo – 3rd International Azerbaijan Laboratory Medicine Congress & Lab Expo. May 1-3; Baku, Azerbaijan; azklmib.az
Immunology 2025 – Annual Meeting of the American Association of Immunologists (AAI). May 3-7; Honolulu, HI, USA; Web: immunology2025.aai.org
ISLH 2025 Congress – International Society for Laboratory Hematology. May 7-9; Halifax, NS, Canada; Web: islh.org
22nd International Congress of Cytology. May 11-15; Florence, Italy; iccflorence2025.com
26th IFCC-EFLM EuroMedLab Congress of Clinical Chemistry and Laboratory Medicine. May 18-22; Brussels, Belgium; Web: euromedlab2025brussels.org
SLAS Europe 2025 Conference and Exhibition - Society of Laboratory Automation and Screening. May 20-22; Hamburg, Germany; slas.org
Hospitalar 2025. May 20-23; Sao Paulo, Brazil; hospitalar.com
ESHG 2025 – European Human Genetics Conference. May 24-27; Milan, Italy; 2025.eshg.org
ISBT Milan 2025 – 35th Regional Congress of the International Society of Blood Transfusion. May 31 - Jun 4; Milan, Italy; isbtweb.org
JUNE
WHX Labs Lagos 2025. Jun 2-4; Lagos, Nigeria; worldhealthexpo.com
LabMedUK25 – National Meeting of the Association for Laboratory Medicine (UK). Jun 9-11; Manchester, UK; Web: labmed.org.uk
WHX Miami 2025 (formerly FIME). Jun 11-13; Miami, FL, USA; Web: worldhealthexpo.com
108th Annual Meeting of the German Society for Pathology. Jun 12-14; Leipzig, Germany; Web: pathologie-dgp.de
EAACI 2025 – Annual Congress of the European Academy of Allergy & Clinical Immunology. Jun 13-16; Glasgow, Scotland; eaaci.org
50th CBAC – Congress of the Brazilian Society of Clinical Analysis. Jun 15-18; Campinas, Brazil; sbac.org.br
ASM Microbe 2025 – American Society for Microbiology. Jun 19-23; Los Angeles, CA, USA; asm.org
ISTH 2025 Congress – International Society on Thrombosis and Haemostasis. Jun 21-25, Washington, DC, USA; isth2025.org
FOCIS 2025 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 24-27; Boston, MA, USA; focisnet.org
ESHRE 2025 – 41st Annual Meeting of the European Society of Human Reproduction and Embryology. Jun 29 - Jul 2; Paris, France; eshre.eu
JULY
FEBS 2025 – 49th Congress of the Federation of European Biochemical Societies. Jul 5-9; Istanbul, Turkey; 2025.febscongress.org
ASV 2025 – 44th Annual Meeting of the American Society for Virology (ASV). Jul 14-17; Montreal, Canada; asv.org
FEMS MICRO 2025 – Federation of European Microbiological Societies. Jul 14-17; Milan, Italy; femsmicro.org
WHX Labs Kuala Lumpur (formerly Medlab Asia). Jul 16-18; Kuala Lumpur, Malaysia; Web: worldhealthexpo.com
2025 ADLM Annual Scientific Meeting & Clinical Lab Expo. Jul 27-31; Chicago, IL, USA; Web: meeting.myadlm.org
AUGUST
32nd Congress of the Latin American Society of Cytopathology (SLAC). Aug 5-8; Buenos Aires, Argentina; citologiala.org
23rd CNB International Congress 2025 – Colombian National College of Bacteriology. Aug 15-18; Bogota,
Colombia; congresocnb.com
IUIS 2025 – International Union of Immunological Societies. Aug 17-22; Vienna, Austria; iuis2025.org
ICBMBLM 2025 – Joint Conference of the Malaysian Association of Clinical Biochemists (MACB) & Malaysian Society for Biochemistry and Molecular Biology (MSBMB). Aug 25-27; Petaling Jaya, Malaysia; conference.macb.org.my
SEPTEMBER
WHX Labs Cape Town 2025 (formerly Medlab Africa). Sep 2-4; Cape Town, South Africa; worldhealthexpo.com Thailand Lab International 2025. Sep 3-5; Bangkok, Thailand; thailandlab.com
ECP 2025 – 36th Congress of the European Society of Pathology. Sep 6-10; Vienna, Austria; esp-pathology.org
47th Mexican National Congress of Clinical Chemists and Expoquím 2025. Sep 8-13; Oaxaca, Mexico; conaquic.com
CAP25 – Annual Meeting of the College of American Pathologists. Sep 13-16; Orlando, FL, USA; cap.org
EUROTOX 2025 – 59th Congress of the European Societies of Toxicology. Sep 14-17; Athens, Greece; eurotox2025.com
ESCV 2025 – 27th Annual Meeting of the European Society of Clinical Virology. Sep 17-20; Thessaloniki, Greece; escv2025.org
23rd International Congress of Therapeutic Drug Monitoring & Clinical Toxicology. Sep 21-24; Singapore; iatdmct2025.org
MASCL 2025 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Sep 21-26; Montreal, Canada; msacl.org OCTOBER
54th Mexican Congress of Clinical Pathology – Mexican Federation of Clinical Pathology. Oct 1-4; Guadalajara; Mexico; fempac.org.mx
WHX Labs Nairobi 2025. Oct 6-8; Nairobi, Kenya; worldhealthexpo.com
ASHI 2025– 51st Annual Meeting of the American So-