ADAPTABLE ANATOMY







Learn about the latest dry granulation innovations available only on GERTEIS® roller compactors. Introducing our cutting-edge In-Line Density Measurement Solution, the new Ultra Small Amount Funnel and the Design of Experiments Software Feature!
PAT: In-Line Density Measurement
Real-Time Insights: Say goodbye to off-line testing delays. Our system provides instant density measurements during production, allowing timely adjustments and informed decision-making.
High Precision: Accurate density data is key to robust process control. Whether you’re scaling up or down, our system ensures reliable results.
Closed-Loop Feedback: Linking density measurements to process parameters enables dynamic adjustments. Stay ahead of variations and maintain product consistency.
Design: Ultra Small Amount Funnel


Ideal for Small Batches: Process materials with as little as 10 grams of product — perfect for laboratory development and pilot projects.
No Residuals: Almost no product remains in the equipment, making it an excellent high yield small amount option.
Ease of Use: The funnel seamlessly integrates with the compaction unit of the Mini-Pactor, providing the same superior performance you would find in a full scale production machine.
Software: Design of Experiments (DoE)
Efficient DoE Implementation: Design of Experiments is the fastest and most cost-efficient way to create effective experiments. Tap into the power of DoE without the long learning curve.
Built-in Process Support: This goes beyond ordinary DoE software. It guides you on how to make optimal experimental choices, skipping impossible settings to save product and time.
Complete Guidance: From parameterization to execution, the software provides step-by-step instruction and support.
Technology innovations always feature strongly in Eurolab and this issue is no different. Our cover story mixes art and science and explores the work of Dr Richard Arm, an innovator who creates anatomically correct organs for use by students instead of cadavers - one of his techniques deploys a laser sinter to fuse soft rubber particles together using 3D guided geometry. On a more molecular scale, Sino Biological are manufacturing recombinant proteins, read their interesting story on page 32.
Customer relationship management (CRM) in the life sciences and pharmaceutical sector has seen some upheavel since Salesforce and Veeva parted ways earlier this year. On page 12 we interview the CEO of Veeva's partner Conexus about how the split has affected the industry.
Several additional articles look at pipette technology, and with Pipette Enhanced Workflows on page 20, Integra Biosciences tells us how its customer, BioApp Solutions, used the Viaflo electronic pipette to reduce downtime and improve efficiency.
Political decisions are affecting health care practitioners across the globe and in the news round up (page 6) we cover several funding cuts made by the Trump administration in recent months. At the same time, the European Union is pouring money into the biotech industry - perhaps the UK should rejoin as suggested by Zac Polanski (leader of the Green Party).
We preview the upcoming Lab Innovations show on page 55. Do let me know if you're attending and would like to meet - I'll be there and would love to hear your news and views.
Nicola Brittain Editor

Pipette enhanced workflows
How pipette use can enhance high throughput workflows
Curb your waste How best to dispose of single-use pipette tips
Accelerator free gloves Rethinking cleanroom hand protection
update
investigation of gas uptake rates

reagent purity
A look at an advanced line of recombinant
How AI-enhanced DMTA cycles can help with workflows
A look at lab equipment that helps synthesise genomes
A novel approach
An efficient methodology for PFAS
Why DOE automation is a powerful methodology for practitioners
A perspective on managing punch length

How microscopy is used in clean rooms
A look at a 3D stereoscopic

PUBLISHER
Jerry Ramsdale
EDITOR
Nicola Brittain nbrittain@setform.com
CONTRIBUTING EDITOR
Saskia Henn shenn@setform.com
DESIGN – Dan Bennett, Jill Harris
HEAD OF PRODUCTION
Luke Wikner production@setform.com
BUSINESS MANAGERS
John Abey | Darren Ringer
ADVERTISEMENT EXECUTIVES
John Davis, Iain Fletcher, Paul Maher
e advertising@setform.com
CONTACT US...
t +44 (0) 207 253 2545
e mail@setform.com
Setform’s international magazine for scientists is published twice annually and distributed to senior professionals throughout the world. Other titles in the company portfolio focus on Process, Design, Transport, Oil & Gas, Energy and Mining engineering.
The publishers do not sponsor or otherwise support any substance or service advertised or mentioned in this book; nor is the publisher responsible for the accuracy of any statement in this publication. ©2025. The entire content of this publication is protected by copyright, full details of which are available from the publishers. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner.
Boosting skills with automated microscopy
How microscopy is teaching professionals
Meet the deadline
Encouraging submission of applications
A meeting place for lab professionals A preview of Lab Innovations
Setform Limited | 6 Brownlow Mews, London, WC1N 2LD, United Kingdom

ADAPTABLE ANATOMY
A look at the 3D printed body parts created by Dr Richard Arm


In June this year, 484 staff from the US’s National Institute of Health signed a letter criticising the Trump administration for major spending cuts that they said would harm the “health of people across the globe”. The letter argued said the “life and death nature of [the] work” demanded that changes be “thoughtful and vetted.” It continued: “We are compelled to speak up when our leadership prioritises political momentum over human safety and faithful stewardship of public resources.”
The letter went on to list a series of more detailed concerns stating a shared commitment to academic freedom and that the signatories were unhappy with how the halting of
high-quality peer-reviewed grants and contracts had politicised research.
Separately, a federal watchdog found in early August that the Trump administration had broken the law when it terminated 1,800 grants and interrupted funding for the NIH.
According to the Washington Post, it was the fifth time that the Government Accountability Office, a non-partisan legislative agency, faulted President Trump and his top aides for rearranging the budget in defiance of Congress. Between February and June, investigators estimated, the US government had reduced spend on research and other grants by US$8bn year on year.

The Greater Manchester Commercial Research Delivery Centre, completed in April 2025, has announced its first vaccine studies.
Three new projects aiming to improve the effectiveness of vaccinations for severe acute respiratory infections have launched in Manchester thanks to support from The National Institute for Health and Care Research (NIHR) Greater Manchester Commercial Research Delivery Centre (GM CRDC).
The three studies are the first to be delivered through the GM CRDC, one of 21 CRDCs across the UK, and aim to recruit patients who have been hospitalised with severe acute respiratory infections (SARI).
The studies will enable researchers to evaluate how COVID-19 and Respiratory Syncytial Virus (RSV) vaccines perform outside of clinical trials and across diverse populations. These studies will provide essential real-world evidence into the

degree of protection vaccines offer against serious respiratory illness, hospitalisation, and ongoing healthcare.
Dr Shazaad Ahmad, a Consultant Virologist at Manchester University NHS Foundation Trust (MFT) and the principal investigator for the studies in Greater Manchester, said:
“Vaccination remains one of the most effective interventions for preventing severe outcomes, reducing hospital admissions, and protecting the most vulnerable. These revaluations will help ensure that vaccination programmes deliver maximum benefit to the populations they are designed to protect”.
In July, the European Union launched a strategy to make Europe “the most attractive place in the world for life sciences by 2030”. This strategy is backed by a annual budget of over €10bn from the EU budget. This strategy will be supported by specific funding for key initiatives, including up to €100m for microbiome solutions and €300m for public procurement of life science innovations like new vaccines and cancer treatments, all managed under cross-sectoral coordination. The strategy aims to ‘accelerate innovation, facilitate market access and build public trust in new technologies’. Alongside the strategy was the launch of the EU Biotech Act which aimed to create a more innovation friendly framework across biotech sectors.
At end of September the European

Council approved conclusions on life sciences and said that it welcomes the European Union’s ambition to become the world’s most attractive place for life sciences by 2030, as outlined in the Commission’s strategy. The council also provided guidance that aimed to unlock the Union’s full competitive potential. The Council encourages full support throughout the value chain from
fundamental research and uptake, stronger research in advanced therapy medicinal products (ATMPs), a leading EU role in clinical trials and a broad approach to biotechnology. It also calls for the use of advanced technologies – such as artificial intelligence and quantum computing – in life sciences, while stressing the importance of developing skills, and attracting and retaining talent.
Leading global manufacturer of primary human cells PromoCell GmbH has announced its entry into the Good Manufacturing Practice (GMP) field. At the 32nd ESGCT Congress in Seville, Spain, PromoCell will showcase its Custom GMP Cell Culture Media Services tailored for clinical applications and further manufacturing in the cell-based therapy industry. PromoCell’s Custom GMP Cell Culture Media Services aim to bridge the gap between research and clinical application, offering Excipient GMP-grade cell culture media manufacturing and expert guidance through the research-tocommercialisation journey.

“We noticed an increasing demand for advanced cell culture media in regenerative medicine and cellbased therapy, and we are pleased to introduce our GMP cell culture medium service portfolio to the life science community at the Congress,” says Dr. Irma Börcsök, CEO of PromoCell. “This launch is a testament to our commitment to supporting customers with our
long-standing cell culture medium expertise from discovery to preclinical, clinical, and commercial manufacturing. The company has made both standard and customised Excipient GMP-grade solutions available and claims to deliver “servicedriven offerings that meet high regulatory standards, empowering scientists to scale their innovations with confidence and compliance.”

Dr Richard Arm, a senior research fellow at Nottingham School of Art and Design, is creating organs to replace cadaver parts. Here Saskia Henn explores the benefits of this practice
Traditionally, cadavers have been one of the main tools for surgical training for medical students, surgeons and trainees, emergency medical professionals, researchers and military medical personnel.
The history of cadaver use in medical training dates back to the first half of third century BC in Greece. Ancient Greek physicians Herophilus of Chalcedon and Erasistratus of Ceos were likely the first to use the dissection and vivisection of cadavers for anatomical learning.
By the 4th century CE, much of cadaver use disappeared owing to religious and social taboos. It did not reappear until the 14th century in Bologna, Italy, and from this point on, became increasingly common.
However, the use of cadavers for surgical training and preparation is flawed and has recently waned in popularity.
The process of cadaver acquisition is complex and preservation is expensive. While anatomically accurate, cadavers lack the same physiological functioning such as blood flow and dynamic responses.
Embalming can help to prevent the organs from descending with gravity. However, the use of hazardous chemicals such as formaldehyde not only poses a risk to users but also contributes to tissue discolouration and stiffness.
“When I first started this research in 2012 I was working with a Belgian surgeon who had designed an implantable device for children with spasticity that helped prevent them from having seizures. I made a torso of my young cousin and reproduced it in materials that were able to mimic flesh and bone, albeit rather simplified. This was used by the surgeon for years to teach other surgeons how to implant his new device, helping hundreds of suffering children around the world.” – Dr Richard Arm

Alternatives to cadaver use have recently been gaining traction. Dr Richard Arm, senior research fellow, Nottingham School of Art and Design, NTU has introduced an anatomically accurate solution that offers many advantages over cadavers.
Arm uses a combination of 3D printing and soft materials such as silicone, polyester polyurethanes and polymer waxes to create lifelike organs.
His interest in art and design began with the Renaissance movement and multi-disciplinary creatives such as Di Vinci and Michelangelo, who were not just artists, but designers, inventors, builders, architects and engineers as well.
“Art underpins all these disciplines, and it’s no different for me,” says Arm. “A broad working knowledge of science, medicine, anatomy and material science are all essential to my work. Understanding the human body, how it works and why, can lead the open-minded artist down a plethora of avenues for discovery and inspiration.”
While often overlooked by decisionmakers in education, the arts contribute to the holistic perspective and lateral decision-making necessary to approach the replication of just one human organ, let alone the complexity of the human body.
“Human skin is a great example. Resilient, repairable, and compliant, human skin has some unique characteristics that are controlled by its makeup,” says Arm. “Layers, fibre-filled gel membranes and elastic embedded structures are all replicated in the work I do, inspired by nature’s own design.”
Understanding the human body, how it works and why, can lead the open-minded artist down a plethora of avenues for discovery and inspiration
First, a 3D print of the tissue is created based on a patient’s CT scan. This ensures that the blueprints of the anatomical structures are accurate to the training required. This alone could eliminate the difficulties surgeons experience when training for paediatric patients, for example.
Following the creation of the 3D replica, Arm uses soft materials to recreate the elasticity, flexibility and unique characteristics of specific organs.

oesophagus
“We use a variety of materials to simulate living soft tissues,” says Arm. “For example, for complex vasculature that needs interlinked vessels with specific wall thicknesses that would be impossible to cast, we use a laser sintering technique to fuse soft rubber particles together using 3D guided geometry.”
Every aspect of the body is considered, including the way the blood sinks into the body following an incision, rather than puckering upward, as is often seen in media.
“It’s important that any materials we use are compatible with one another, such as PDMS (silicone gels) polymer waxes and thermoplastic polyurethanes,” says Arm, who rarely uses materials straight off the shelf.
“Liquid and solid additives are used in combinations that are essential to change and control the fundamental mechanical characteristics in most materials, to emulate the native biological tissues properly,” he says.
One advantage that Arm’s method has over cadavers is its availability. For

a host of cultural, legal and religious reasons, the availability of cadavers around the world is inconsistent and controversial. In the UK, for example, cadavers are only available as a main teaching method in about 60% of British medical schools.
Arm’s organs are also reusable, portable and independent from any power source. While cadavers must be properly preserved and respectfully disposed of within a certain time frame, these artificial organs can be left out and even transported without being damaged.
Since Arm’s organs are created from scratch with the purpose of mimicking life-like physiological responses, they bypass the complications and costs that arise from preserving cadavers through embalming, which alters the appearance and composition of soft tissue.
The customisable nature of Arm’s approach makes it suitable for a wide range of situations. He has previously assisted in the training of heart transplant surgeons and first responders treating serious chest
trauma that occurs from gun shots, stabbings, shrapnel penetration and road traffic accidents.
While Arm has several projects underway and still under wraps, he has already begun manufacturing his own manikins and organs at NTU with the help of his research assistant Andrea.
“The reason for us manufacturing our own models rather than licencing our technology is to help drive down inflated prices of surgical models because we want everyone to be able to afford them, not just the privileged few,” says Arm. “We also want to improve and control the quality of the currently available models by designing and building everything in-house.”
Arm’s manikins and organs are available for purchase through NTU’s catalogue of products.
Lowest plunger force.


This familiar lab tool is anything but ordinary. With INTEGRA's SWITCH you choose; pipette manually or 'SWITCH' to repeat dispense mode and gain productivity while enjoying the lowest plunger force possible. SEE IT IN ACTION >

Akshay Kapadia CEO of Conexus Solutions

In this Q&A, Akshay Kapadia CEO of Conexus Solutions talks about the Veeva and Salesforce split and how the Veeva platform meets the specific CRM requirements of the healthcare sector
Why was the split between long-time collaborators Veeva and Salesforce such a big deal for life-science companies?
The Veeva and Salesforce relationship has been a defining force in life sciences customer relationship management (CRM) for more than 15 years.
When Veeva launched in 2007, it built its CRM on Salesforce’s Force. com platform, designing it specifically for the processes and compliance requirements of life sciences companies. This was a pivotal shift that helped move the industry away from traditional on-premises installations and toward a cloud-based, SaaS model. Veeva’s product was an excellent fit for life sciences, and its adoption accelerated quickly. It captured approximately 80 percent of the market in a decade.
The product now sits on a Veeva designed architecture. For the first time in years, organisations are able to choose a CRM and platform that have been designed specifically for their needs as healthcare specialists. It’s a moment of disruption, but also a chance to reimagine what CRM can deliver – not just as a sales tool, but as a driver of intelligent customer engagement.
What does the life sciences industry need from a CRM that might differ from a standard CRM?
The pharmaceutical and life sciences (P&LS) industry has fundamentally different needs from traditional retail or B2B industries when it comes to CRM. These differences are deeply tied to the way the industry operates, the regulatory landscape, and the
unique roles of its stakeholders. Here are some of the key distinctions:
1. A complex selling model: In most industries, the customer who is sold to is also the one who pays for and uses the product. In pharma, the model is far more complex. The healthcare provider (HCP) is the one who ‘sells’ the product they prescribe but doesn’t buy or consume it. The patient consumes the product but typically doesn’t pay for it. The payer (insurance or government) covers the cost, but neither prescribes nor uses the medication. This dynamic creates a multi-layered engagement model that standard CRMs aren’t built to manage.
2. Regulatory compliance requirements: Standard CRMs operate in relatively
Clients want to see measurable results –whether that’s faster approvals, improved field performance, or more efficient content management – they’re looking for service partners who align with those goals

low-regulation environments. In contrast, pharma companies must comply with stringent regulations such as FDA guidelines, HIPAA, the Sunshine Act in the US and GDPR in the EU. Every interaction must be documented, auditable, and compliant –from data capture to content delivery.
3. Sample management:
Sample distribution is a tightly regulated activity in pharma. Reps must collect detailed information, including physician licenses (SLNs), digital signatures, and maintain a chain of custody for each transaction. State-specific laws also apply. Standard CRMs don’t typically have the rigor or built-in processes to support this level of oversight.
4. Field activity tracking:
While standard CRMs offer basic
activity logging and lead tracking, pharma field teams require much more. They need tools for call planning, frequency tracking, capturing detailed call notes, recording product discussions, and tracking the use of approved CLM (Closed Loop Marketing) materials –all while staying compliant.
5. A specialised field force structure:
Pharma field teams are diverse and highly specialised. Unlike traditional models where territories are often geographic, pharma teams include sales reps, Medical Science Liaisons (MSLs), Key Account Managers (KAMs), and Field Reimbursement Managers (FRMs), each with distinct responsibilities and reporting needs. CRM systems must support these nuanced roles and coordinate
interactions across the enterprise. When Veeva launched its CRM in 2007 on Salesforce’s platform, it was purpose-built to address these complexities. It offered life sciences companies a tailored solution right out of the box – something Salesforce itself never provided as a native offering - that has changed with Veeva’s new platform.
Conexus is a service provider that implements and optimises Veeva’s cloud-based software for companies in the life sciences industry. It was built specifically to serve pharmaceutical and life sciences companies. The company understands the unique challenges these organisations face, especially emerging and mid-sized companies. What are customers asking for now that they weren’t say a year or two years ago?
There’s been a noticeable shift in what life sciences companies are prioritising in their service partners. A few years ago, the focus was often on finding experts for a specific application or functional area. Today, customers are looking for broader, more integrated support – they’re looking for partners who can work across the complete Veeva ecosystem, from commercial to clinical to quality.
Another significant shift is in the way value is measured. Traditional time-and-materials models or fully dedicated support teams are giving way to outcome-based models. Clients want to see measurable results –whether that’s faster approvals, improved field performance, or more efficient content management – and they’re looking for service partners who align with those goals.
And of course, AI is at the forefront of many conversations now. What we’re seeing is a growing demand for practical enablement – companies aren’t just looking for high-level strategy or experimental use cases.
Micro-Epsilon’s Glenn Wedgbrow provides insight into how the company’s high precision sensors are used in the medical, biotechnology and pharmaceutical sectors
Medical technology, the pharmaceutical industry and biotechnology are sectors in which sensors and measurement technology are increasingly being used to improve quality and efficiency. MicroEpsilon offers measurement solutions for these industries to solve numerous tasks. Here is a selection of successful projects that help illustrate the range of possible applications.
Stents play a critical role in the treatment of cardiovascular diseases. To ensure their functionality, precise adherence to the diameter is crucial. Optical micrometer sensors from Micro-Epsilon perform diameter checks and wire inspections of the stents during and after production, ensuring the highest level of quality assurance. The shadow cast principle even allows transparent materials to be measured accurately.
The thickness of medical technology products can be measured using confocal chromatic sensors. The confocal sensors measure the wall thickness of transparent materials such as tubes, membranes and balloons, from just one side and deliver accuracy in the micrometer range. Uniform wall thickness is essential for the durability and function of tubes. The sensors measure the thickness without contact
and so ensure consistently high quality during production, as they have no influence on the material.
The confocalDT sensor series from Micro-Epsilon defines the standard for maximum precision and dynamics in confocal chromatic measurement technology. Owing to a wide range of sensors and interface options, this series is suitable for various measurement applications in medical technology. The confocalDT systems are characterised by their fast measuring rates, which are specially designed for dynamic measurement tasks. Dynamic control of the exposure of the CCD line in the controller continuously adapts to the colour and reflectivity changes of the measuring object, which significantly

Draw-wire displacement sensors are used for position monitoring, for example, when moving patient couches or taking measurements in computer tomographs

In medical technology, the colorSENSOR CFO is used, for example, for the colour detection of interior parts such as headrests for couches, packaging control and for sorting medical components by colour, such as closures or labels
optimises the measurement accuracy even at high speeds. With sensor sizes starting at a diameter of just 4 mm, the confocalDT systems enable easy integration even in confined installation spaces. The availability of models with a 90° angle also reduces the required installation depth, which facilitates installation in complex machine arrangements. The measuring systems offer different aperture angles, whereby models with a large aperture angle or a high numerical aperture enable a particularly small light spot as well as excellent lateral (X-Y) and vertical (Z) resolution. Even the smallest of details can be detected with extreme precision.
Draw-wire displacement sensors are used for position monitoring, for example, when moving patient couches. Sensors with potentiometer output are used for tasks that require medium accuracy. Draw-wire sensors with absolute or incremental encoders are used for high precision measurements such as those required in computer tomographs.
Draw-wire sensors provide an effective solution for measuring displacement, distance and position with an impressive measuring range of up to 50,000 mm. These sensors are particularly well suited for use in hard-to-reach places, which makes them indispensable in many industrial applications. They enable accurate measurements even under demanding


DIAGNOSTIC DISPENSING SOLUTIONS
Precision in Every Dispense, Accuracy in Every Test
• Bond medical device housing
• Dispense and freeze reagents for later use
• Highly precise uid volume accuracy and repeatability at ±1%





conditions. In addition, they are characterised by simple, quick and flexible installation, which makes them ideal for various applications, including customer-specific configurations.
During pharmaceutical tablet production, different concentrations of active ingredients influence the tablet colours. The ACS7000 colour measuring system from MicroEpsilon precisely detects subtle colour differences, which enables the composition of the active tablet ingredients to be continuously checked during production.
colorCONTROL ACS7000 is one of the most advanced inline colour measuring systems in the world. The measuring system not only recognises reference colours by comparison, but also identifies individual colours clearly from their coordinates in the colour space. Owing to its very high measurement speed, the colorCONTROL ACS7000 is suitable for applications where colours and shades must be examined on-the-fly and to very high accuracies. The high measuring accuracy means the system is also used in laboratories. Different sensor models are available to suit various measurement tasks.
There are numerous other applications in medical technology where colours are measured with high precision in order to automate and accelerate processes while maximising the level of quality. The colorSENSOR CFO is a highly developed controller, specially designed for precise colour detection tasks. This series impresses with its high colour accuracy and modern interfaces, combined with intuitive user guidance. CFS sensors are connected to a controller with integrated fibre optic cables and can be specially configured for different measurement tasks.
In medical technology, the colorSENSOR CFO is used, for example, for colour detection of interior parts such as headrests for couches, packaging control and for
The highest standards of quality and repeatability apply in medical and pharmaceutical technology. Sensor products from MicroEpsilon are used in a wide range of measurement tasks
sorting medical components by colour, such as closures or labels. It offers a high measuring speed of up to 30 kHz, making it ideal for dynamic processes. Fast output of measured values (Lab/ XYZ) up to a frequency of 500 Hz and a large colour memory enable the management of different test batches with extraordinary precision, with a colour accuracy of ΔE ≤ 0.3.
The controller is also characterised by a robust aluminium housing and IP65 protection rating, which makes it particularly resistant to environmental influences. Customerspecific calibrations are also possible in order to meet specific requirements in medical technology.
For more information visit: www.micro-epsilon.co.uk
To ensure the functionality of stents, precise adherence to the diameter is crucial
The revolutionary FLEXI-FLOW™ Mass Flow & Pressure Controllers are ideally suited for applications in the analytical and biotech market. The compact, lightweight instruments allow easy integration in (portable) devices. Their versatility is second to none:

• Manufactured from non-toxic high grade LDPE
` Shatterproof and safe to use
` Inert to prevent sample contamination
• Responsive control for ease-of-use & reliable performance
• Low-affinity surface for complete sample delivery
• Available sterile or non sterile to suit your application
• No mould release agents used during manufacturing









Samantha Ogles, product manager at Alpha Laboratories, provides insight into simple transfer pipettes as designed for today’s scientific and medical fields
Despite the rapid advancement of technology in scientific fields, the simple plastic transfer pipette remains an essential tool in research, industrial and clinical laboratories, for the easy and safe transfer of liquids. Evolving from the traditional glass Pasteur pipette and rubber bulb, first used in the 1940’s, modern polypropylene single piece pipettes, such as the renowned, high quality Pastette, remain a convenient and cost-effective solution for an extensive range of liquid handling applications.
These disposable transfer pipettes have been developed to meet the demands of various scientific and medical applications. Available in a variety of sizes and styles, they are designed for the controlled transfer of liquids in general liquid handling and also more specialised procedures. They provide precision, safety, convenience, and versatility. Sterile and non-sterile options cater to both
general laboratory use and sensitive applications in microbiology or medical environments. In clinical environments transfer pipettes have many functions, from handling a variety of patient samples, small volume drop work, to preparing slides for analysis. The fine tip and controllable bulb make them ideal for dispensing into sampling cups, without creating bubbles that can disrupt the efficiency of the analyser. They have multiple uses in blood sciences. The narrow stem fits tubes and donor bag tubing and

allows calibrated transfers through graduations and controlled drop size.
Bob Jones of the Department of Microbiology and Infection Control at Victoria Hospital, Kirkcaldy has been using Pastettes since the 1980s, after moving on from homemade glass ones (quite a tricky and dangerous practice). He says “Working in a microbiology laboratory, transfer pipettes are used in abundance for many things. Jumbo Pastettes are ideal for removing sediment from early morning urine for TB examination. Fine Tip Pastettes are
used for filling cell counting chambers with CSF. The serology section is probably the biggest user, transferring serum and plasma from centrifuged blood containers into tubes and vials.”
For self-test and Point of Care (POC) diagnostic kits, where users may not have experience of exact volume pipetting, there are disposable pipettes that will dispense a precise measure of liquid. They offer foolproof simplicity – simply squeeze
the upper bulb to fill and squeeze again to dispense the exact volume required for the test.
Pastettes are extensively used in cell culture laboratories for transferring culture media, reagents, and cells between containers. Their disposable nature eliminates the risk of cross-contamination between samples. In stem cell research, where delicate cells are handled, the flexible tips of Pastettes help ensure that cells are not damaged during transfer. There are even bulbless aspirator Pastettes that can be used with all types of Pipette Aid devices to aspirate and dispense media and reagents. They are ideal for drawing off media from culture flasks and are safer to use than traditional glass or polystyrene pipettes. Being flexible and soft they also protect the cell cultures. They offer the best choice for delicate and valuable cell lines and are a ‘must have’ for work with infectious and hazardous materials.
Even in industrial research and testing laboratories Pastettes are a valuable tool for tasks such as sample preparation for: LC, GC, HPLC, TLC using 1ml auto sampler vials. Robertet, a leading supplier of fragrances, flavours, and natural raw materials, uses transfer pipettes in the applications laboratory, where they formulate trial batches of fragrances that are under development. They work to very precise formulations, occasionally using up to 70 different raw materials for each trial batch. These components are often very expensive, and they cannot afford to waste any, so the accuracy of dispensing is essential. They have found that the 1ml graduated Pastette provides the precision needed for this task.
So, while your laboratory may continue to be enhanced by the addition of increasingly sophisticated equipment, there will always be a place for the multipurpose, humble plastic pipette.
Tom Bentivegna from Integra Biosciences explains how the company expands bioanalytical capabilities with high throughput liquid
BioApp Solutions – based in Leeds, UK – is a specialist provider of training, consultancy and analytical support covering all aspects of bioanalysis. The company was established in 2015 to address the market gap for lab-based bioanalytical consultancy, and to train the next generation of analytical chemists. Unlike officebased consultancies or high throughput laboratories, BioApp draws on decades of combined experience in leading contract research organisations (CROs) to tackle complex challenges that larger organisations may be unable to address owing to their scale or focus on volume. Its clients range from start-ups requiring guidance on establishing new workflows to major pharmaceutical companies seeking tailored, innovative bioanalytical solutions.
BioApp provides expert consultancy services across the full bioanalytical workflow, from exploratory and regulated bioanalysis to method development, validation and biomarker strategies. The company also offers flexible outsourcing options that help sponsors adapt to shifting priorities, manage costs and accelerate research timelines, offering dedicated

full-time equivalent and instrument access, applications support, and comprehensive data interpretation. Its laboratory services focus on earlystage, non-regulated work, combining robust analytical method development with expert project management.
BioApp moved out of the incubator lab it had initially used into its own dedicated CRO facility in 2021 to dramatically expand its analytical
capacity and incorporate mass spectrometry capabilities. This enabled the team to provide a broader spectrum of bespoke sample analyses, as well as offer early-stage method development, workflow optimisation and validation services in house. When establishing its new laboratory, the team was keen to invest in automation products that would suit its workflows, as manual pipetting in high throughput formats can be repetitive, labour intensive and prone to errors, particularly when working

with large batches in 96 well plate format. Tasks including sample dilutions and mixing and extracting supernatants after centrifugation can be time consuming to perform by hand, increasing the risk of variability between operators and placing strain on staff.
The BioApp team was already familiar with Integra’s Viaflo 96 handheld electronic pipettes, having investigated multichannel pipettes for a consultancy client several years earlier. Instruments from several brands were trialled by 20 of the client’s analysts over a week, and Integra’s solution was unanimously preferred for its intuitive design, robustness and minimal downtime. This experience – coupled with BioApp’s policy to invest only in proven, established technologies –made the Viaflo 96 the natural choice for its laboratory. BioApp therefore selected the Viaflo 96 with a 96 channel pipetting head and 300 μl automation Griptips pipette tips to streamline repetitive manual pipetting tasks and boost productivity.
The Viaflo 96 allows the BioApp team to set up entire 96 well plates in seconds. The pipette is used for a range of applications – including sample loading during supported liquid extraction protocols –contributing to significant time savings and improved productivity. Pipetting protocols can be preprogrammed, enabling precise control over cycle numbers and aspirate/ dispense speeds, ensuring each liquid handling step is performed consistently. Eliminating manual errors and inter-operator variability leads to more reproducible results and helps safeguard the mental and physical wellbeing of staff by reducing monotonous, repetitive tasks.
BioApp also selected single- and multichannel Evolve manual pipettes with a range of volumes, from 2 to 300 μl, for handling smaller sample numbers and lower volumes,
particularly during tasks such as spiking samples with known analyte concentrations in preliminary studies. Having multiple pipettes dedicated to specific applications reduces the risk of cross-contamination, adds flexibility and ensures redundancy, helping to overcome throughput limitations. Integra’s ECO rack Griptips pipette tips – which fit securely without leaking or falling off – further enhance productivity, as they are colour matched to the pipettes, allowing staff to quickly select the correct tip without interrupting workflows. This streamlines daily liquid handling tasks and supports sustainability by reducing tip wastage and associated plastic waste.
Reliable liquid handling tools are essential for maintaining productivity, safeguarding data quality and meeting project deadlines in bioanalytical research and development. For a small, highly specialised team working to tight schedules, dependable
equipment minimises the risk of unplanned downtime and ensures that complex workflows can be carried out efficiently from start to finish. BioApp’s suite of Integra tools enables consistent, reproducible pipetting, supporting a variety of method development and sample analysis tasks. They will also play a critical role in helping BioApp to expand its in-house analytical and method development capabilities, diversifying its client base and adapting to evolving industry needs.

Reliable liquid handling tools are essential for maintaining productivity

The single-use pipette tip, an unavoidable consumable in modern biological and chemical research, presents a significant and growing global environmental challenge. While essential for precise liquid handling and preventing cross-contamination, their sheer volume generates a staggering waste stream. Estimates suggest billions of tips are discarded annually, contributing to plastic pollution and resource depletion.
The environmental impact extends beyond mere landfill accumulation. These tips, often made from polypropylene, a petroleum-based plastic, require substantial energy for production. Furthermore, their disposal, particularly incineration, releases harmful greenhouse gases and potential toxins. The reliance on single-use items fosters a linear ‘takemake-dispose’ model, unsustainable in a resource-constrained world.
The sheer volume of discarded pipette tips contributes significantly to the global plastic waste crisis. As highlighted by sources such as the Babraham Institute, laboratory plastic waste is a very large contributor to the overall plastic waste problem. Studies have shown that items like pipette tip boxes make up a very large percentage of laboratory plastic waste. (Babraham Institute).
This waste can persist for centuries,
breaking down into microplastics that contaminate soil and water, posing risks to wildlife and potentially entering the human food chain.
Efforts to mitigate this impact are gaining momentum. Autoclaving and reusing tips, while feasible for some applications, raise concerns regarding accuracy and sterility. However, innovations in biodegradable and reusable pipette tips and packaging offer promising alternatives.
Research into bioplastics derived from renewable resources, such as polylactic acid (PLA), presents a potential solution. Though challenges remain regarding cost and material properties, advancements in biopolymer technology are paving the way for more sustainable options.
Furthermore, implementing robust recycling programs within laboratories is crucial. Closed-loop systems, where used tips and packaging are collected, processed, and repurposed, can significantly reduce waste. Education and awareness campaigns are also vital to encourage responsible consumption and promote sustainable laboratory practices.
In the meantime, a shift towards a circular economy in laboratory consumables is imperative. This requires collaboration between
researchers, manufacturers, and policymakers to develop and implement sustainable solutions. This includes encouraging the development and adoption of reusable or biodegradable pipette tips and packaging as well as supporting manufacturers who prioritise sustainable production and packaging. As seen from Alpha Laboratories, there is a push to use recycled and recyclable materials in the production of the FastZAP pipette tips that are designed with a minimal amount of packaging while preserving product quality.
The environmental cost of scientific progress cannot be ignored. By embracing innovation and adopting responsible practices, the scientific community can minimise its environmental footprint and contribute to a more sustainable future.
References: U.S. Environmental Protection Agency. (n.d.). Sustainable Materials Management: Non-Hazardous Materials. Retrieved from epa.gov
The journey and impact of a pipette tip - Babraham Institute: https://www.babraham.ac.uk/blog/pipette-tips
Rizan, C., & Hankemeier, T. (2021). Towards sustainable analytical chemistry: A critical review on greening liquid chromatography. Analytical and Bioanalytical Chemistry, 413(16), 4065–4085.
Arnott, J. T., & Pilon, L. (2020). Environmental life cycle assessment of single-use and reusable laboratory plasticware. Journal of Cleaner Production, 258, 120677.





In this article Jo Fabb from safety specialist manufacturer Ansell encourages us to rethink cleanroom hand protection
Working in a cleanroom is like completing a long, detailed puzzle – it requires focus, patience, and stamina. When gloves aren’t designed for lasting comfort, discomfort can set in quickly, leading to skin irritation, loss of concentration, and potential contamination risks. That’s why glove materials and formulation are so important.
For a long time, natural rubber latex gloves were everywhere – in labs, cleanrooms, and hospitals. But they came with a big downside: Type I latex allergies. These happen when your immune system overreacts to proteins found in natural latex, causing reactions that can be serious – like hives, swelling, difficulty breathing, and in extreme cases, anaphylaxis. This type of allergy is an immediate hypersensitivity reaction mediated by the immune system’s antibodies.
That’s why many places switched to non-latex gloves, like nitrile or neoprene. This change helped cut down on latex-related allergic reactions. But here’s the thing: even without latex, some people still
experience skin problems when wearing gloves. Why? The answer lies in the chemicals used to make the gloves stronger and more durable.
Even if you’re using latex-free gloves, you might still run into issues – especially if you have sensitive skin. That’s because many gloves (especially nitrile ones) are made using chemical accelerators. These are

Many gloves are made using chemical accelerators
ingredients like thiurams, carbamates, and mercaptobenzothiazoles (MBTs) that help ‘cure’ the glove material during manufacturing, so the gloves stay stretchy and strong. Accelerators are commonly used in glove manufacturing to speed up the vulcanisation process, making production more efficient.
But for some people, these chemicals can cause what’s known as a Type IV allergy, or allergic contact dermatitis. Unlike latex allergies, this type of reaction doesn’t happen right away. It can show up hours or even days after wearing the gloves. The symptoms are usually redness, itching, dryness, and blisters. Because the reaction takes a while to appear and looks like other common skin conditions (like eczema), it can be easy to miss or misdiagnose. Estimates indicate that more than 13 million workers in the US are potentially exposed to chemicals that can be absorbed through the skin. Contact dermatitis can also result when chemicals are absorbed through a worker’s skin. Contact dermatitis is one of the most common chemically induced causes of occupational illness, accounting for 10 to 15 percent of all occupational illnesses at an estimated annual cost of at least $1bn.1
Allergy Prevents Type I allergy
Prevents Type IV allergy protection from latex proteins from chemical accelerators
Material Synthetic (nitrile, vinyl, or others)
Potential No latex protein, but may
Nitrile without chemical accelerators
Free from both latex proteins allergens contain chemicals and chemical accelerators
Ideal for
Typical usee cases
Those with latex sensitivity
Frequent glove changes
Those with sensitivity to glove chemicals
Long-duration wear (e.g., healthcare) (e.g., cleanrooms, labs)
GLOVE COMPARISON
SUMMARY: LATEX-FREE VS. ACCELERATOR-FREE NITRILE
Choosing the right glove depends on the type of allergy risk you’re managing. Above is a simple breakdown to help you understand the key differences between latex-free and accelerator-free nitrile gloves.
Just as the industry shifted to latex-free gloves to protect against Type I allergies, today’s leading manufacturers are responding to
Type IV sensitivities with acceleratorfree nitrile gloves. These gloves have gained widespread popularity among users owing to their performance, quality, and skin-friendly formulation.
Accelerator-free nitrile gloves are made without chemical accelerators in the manufacturing process. They use the same raw materials as standard nitrile gloves but follow a slightly modified formula that excludes these accelerators. As a result, they offer a safer alternative for users with sensitive skin or chemical allergies. These gloves eliminate common chemical triggers while maintaining the integrity, cleanliness, and compliance standards demanded
Estimates indicate that more than 13 million workers in the US are potentially exposed to chemicals that can be absorbed through the skin
An image showing the impact of chemical accelerants vs no chemical accelerants

by critical environments such as cleanrooms, laboratories, and pharmaceutical facilities. Additionally, their low-risk composition makes them a better choice for both users and the environment.
When it comes to protecting workers who wear gloves for extended periods, skin health is just as important as barrier protection. Accelerator-free nitrile gloves offer a solution that helps reduce these risks while maintaining the high-performance standards expected from nitrile.
The benefits of accelerator-free nitrile gloves include:
• Reduced risk of allergies
• Uncompromised barrier protection and film permeation properties
• Environmental sustainability
• Compliance with regulatory standards
• Versatility in applications
For more info on free nitrile glove solutions click the QR code below.
For more information on free nitrile glove solutions scan the QR code Sign up to Ansell’s newsletter
For more information visit: www.ansell.com

A team from Biotech Fluidics provides details of a practical investigation of gas uptake rates through different tubing types connecting an inline degasser and liquid pump in precision fluidic systems
Inline vacuum degassers (see Figure 1) are vital components within many types of equipment for laboratory analysis, including liquid chromatography, HPLC, UHPLC, ion chromatography, and mass spectrometry. Similarly, degassers are also critical constituents in fluidics systems used in immunology, haematology, in-vitro diagnostics, and semi-conductor manufacturing. In all these fluidic systems, inline degassers serve the role of silent guardians of precision and reliability by removing dissolved gases that could form bubbles which would disturb the fluid flow or recording of
The experiment set up
To compare materials across different tubing dimensions, we calculated gas uptake rate normalised to 1.0 mm tubing wall thickness
measurement signals. Inline degassing is consequently considered the most efficient and convenient ways to eliminate troubles with bubbles [1].
An aspect that often may be overlooked, however, is the choice of tubing interconnecting the units throughout the fluidic system [2]. If the gas permeability across these flow lines is high, the influx of gases into the liquid could even nullify the benefit of the degasser and thus risk


that the output shows poor precision and low accuracy. The factors that would be expected to influence to which extent this re-gassing could occur are the material properties, residence time, tube wall thickness, and exposed surface area.
To investigate gas permeability, we conducted an experiment that continuously determined the changes in oxygen levels after passing different transfer tubing that were placed between a degasser and a withdrawing piston pump. The investigated tubing materials were polyetheretherketone (PEEK), polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and Ethylene tetrafluoroethylene (ETFE), all which had the same length (1 m) and outer diameter (1/16”), but varying inner diameters of 1.0 or 0.75 mm. Additional tubing materials, with other dimensions were also included, namely stainless steel, silicon and PVC (Tygon).
The content of Table 1 summarises the tested tubing materials and their dimensions plus recorded oxygen uptake through the walls of these flow paths. The increase in oxygen levels
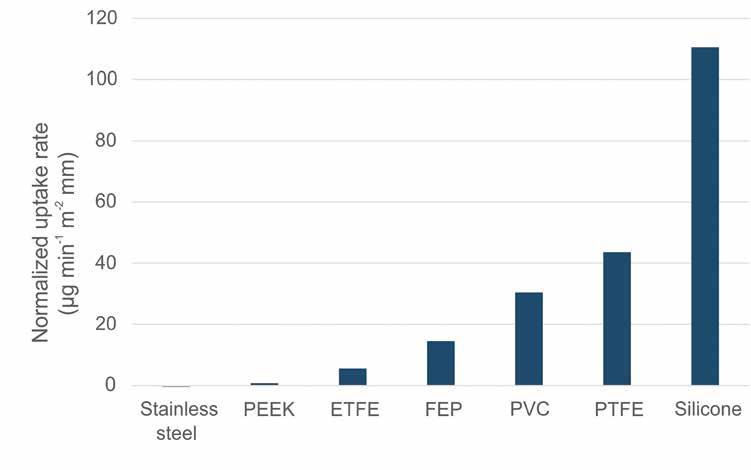

Conditions: Levels of dissolved oxygen were continuously monitored before and after passing though tubing of the stated materials and dimensions, using two optical oxygen FireSting-O2 OXFLOW flow-through sensors. The liquid was air-saturated tap water, withdrawn at 0.2 mL/min from a 1 L bottle at room temperature (24 ± 2 °C) using a Bishoff Compact HPLC pump, via a DEGASi Classic inline vacuum degasser that reduced the oxygen levels to 1 ± 0.3 ppm before entering the tubing to be tested. Values for difference of dissolved oxygen were collected after 25 minutes equilibration for each setup and represent the average during 20 seconds. Uptake rates were calculated by dividing the measured difference in oxygen concentration with internal liquid volume and tubing outer surface area, multiplied by residence time and wall thickness.
ranged from almost 7 ppm down to slightly negative values; the latter likely indicating the uncertainties in the present measurements. It was obvious from the recorded oxygen levels that thicker tubing walls significantly decreased the amount of penetrated gas. To compare materials across different tubing dimensions, we calculated gas uptake rate normalised to 1.0 mm tubing wall thickness. To allow quick comparison of gas uptake rate for different tubing materials, data from Table 1 was averaged for the different materials and plotted in a bar graph, see Figure 2.
The choice of tubing material did have a strong impact on the amount of gas that entered into the liquid flow path, thereby counteracting the benefits of degassing. Tubing wall thickness was also shown to be an important parameter to minimise re-gassing. Silicone tubing showed particularly high tendencies of liquid re-gassing, followed by PTFE and PVC (Tygon) tubing. These types of tubing
materials are therefore not suitable for precision fluidic systems other than in limited sections since their high gas permeability risk resulting in bubble formation even if an inline vacuum degasser is installed.
Although stainless steel tubing displayed zero gas permeability and PEEK only slightly higher, their rigidity and cost might limit the applicability in several applications. The tubing material that best met the criteria of low gas permeability, high flexibility, biocompatibility, and
The tubing material that best met the criteria of low gas permeability, high flexibility, biocompatibility, and chemical inertness, was ETFE
chemical inertness, was ETFE which displayed almost eight times lower gas uptake than PTFE tubing, and five time less than PVC tubing. A good compromise would also be FEP tubing which has limited gas permeability combined with an attractive price.
For more information visit: www.biotechfluidics.com
References
[1] “The Evolution of LC Troubleshooting – Degassing”, LCGC Europe, Dec. 2023, 36 (10), 397-401.
[2] Tubing Materials. https:// biotechfluidics.com/products/ tubing/ (accessed 2025-09-18)
Authors:
Dr. Magda Nyström, Product Specialist, Biotech Fluidics AB, Onsala, Sweden.
Anders Grahn, Chairman & Founder, Biotech Fluidics AB, Onsala, Sweden.
Dr. Tobias Jonsson, Diduco AB, Umeå, Sweden.

An expert from CN Bio explains how preclinical testing is transformed with Organ-on-a-chip technology
At the core of the FDA Modernization Act 2.0 (2022) was the acceptance of data derived using New Approach Methodologies (NAMs), like AI, in-silico tools and Organ-ona-chip, into regulatory filings. At the core of their subsequent roadmap to reduce animal testing in 2025 is an actionable phased strategy to reduce, refine, and ultimately phase out routine preclinical safety testing in animal studies in favor of a NAMsbased approach by encouraging and incentivising their use.
Since then, funding initiatives across the US and Europe have arisen to build research facilities, set up universal standards, train, develop and accelerate the adoption of Organ-on-a-chip (OOC) technology – also known as microphysiological systems (MPS).
Companies that have embraced OOC technology have the early advantage. They utilise it to improve the efficiency of their workflows by addressing gaps where traditional in-vitro assays are not physiologically relevant enough, but animal use is less suited owing to inherent interspecies differences. Some have widely adopted the approach, others have dabbled, many are yet to get started. But there’s no doubt that the field is gaining momentum!
If you ask AI what is at the core of Organ-on-a-chip (OOC) technology, it lists “microfluidics to perfuse cultures, 3D cell culture, dynamic mechanical cues and the integration of multiple cell types, enabling users to create labgrown mimics of human organs and tissues that behave and respond to drugs, toxins, or disease in a way that is more physiologically relevant than traditional 2D cell culture or many animal models”.
While this is true, there are many OOC technologies available and each is different. When the time’s right to modernise your workflows with OOC, it is important to match each system’s core strengths to your needs before choosing. Incorporating any new approach into your workflow takes time, so choose a solution whose core design prioritises ease of setup and use, helping you onboard quickly and sustain momentum.
Remember, the purpose of OOC is to enhance physiological relevance but OOC systems themselves don’t have to be prohibitively complex, they can be plate-based and familiar and utilise
CN Bio’s PhysioMimix Core microphysiological system mimics human physiology
Recombinant Human B7-H3 Protein (ECD, His Tag), Endotoxin-Free Cat#: 11188-H08H-UE


commonly used consumables such as Transwells. So, before you commit, consider the following key criteria:
1. Make sure that you are comfortable handling the consumables for assay setup and sample extraction.
2. Ensure that the system is easy to program. Don’t waste valuable time over maintenance routines.
3. More importantly, make sure you have adequate training and support to get on the right path. New researchers typically need 5–6 weeks to confidently run PhysioMimix Core experiments.
Deborah Lenart, MSc, Charles River, said: ‘Using the PhysioMimix OOC System is really straightforward; with a little practice, any tissue culture scientist can master the skills needed.’ Access to highly characterised and validated assays is key to your initial success. Most vendors provide validated all-in-one assay or model kits, or protocols that enable new users to get to grips with OOC basics fast but rarely does one size fit all. Therefore, to meet one’s immediate and future needs, another core element to consider is a system’s adaptability.
Multi-chip plates are familiar, accessible and customisable
Confocal image of an Liveron-a-chip model of Metabolic dysfunction-associated steatohepatitis (MASH) disease. Blue (Nuclei) Green (Collagen Type-1), pink (αSMA) and red (Steatosis)
Futureproofing also relates to scalability and the cost of OOC experiments. Consider the throughput you will require once OOC becomes embedded as a core technology and ensure this can be met. Explore the cost per chip and options for cost reduction via miniaturisation without compromising data integrity. Ensure you can extract the widest range of endpoint analysis from each experiment to maximise value and remember, the benefits of perfused organ or tissue cultures can also be applied to more cost-effective cell types, such as iPSCs.
So, what are you waiting for?
To enhance preclinical efficiency, physiologically relevant OOC models and assays represent the future, enabling you to gain deeper and more human-relevant insights into drug responses to inform decisions about which candidates to take forward. To future-proof your investment, ensure you have a strong foundation. Look for an intuitive core system that will grow with your research needs so that you can adopt, adapt and scale with ease.
Examples of how PhysoMimix customers have benefitted from its open architecture:
In a recent webinar, BMS described their work incorporating endothelial cells into a Drug-induced liver injury (DILI) assay, whilst scientists at Sanofi have presented their development of a fully immunocompetent DILI assay at conferences.
Others have applied existing Liver-on-a-chip models to a different context of use (e.g. antisense oligonucleotide delivery at GSK (1), progressive human hepatic resistance MIT & Novo Nordisk collaboration (2).
Charles River utilised CN Bio’s core technology to design their own genotoxicity model and assay (3).
Roche initially utilised a single-organ liver model for quantitative drug metabolism studies (4) before progressing to a dual-organ gut/liver model to simulate the process of first pass metabolism in vitro (5).
Learn more about PhysioMimix Core:

1. DOI: 10.1039/D4LC00504J
2. DOI: 10.1101/2025.01.08.631261
3. https://doi.org/10.1016/j. mrgentox.2024.503762
4. https://doi.org/10.1039/D1LC01161H
5. DOI: 10.1039/D2LC00276K
The only Organ-on-a-chip system with validated performance across single-, multi-organ and higher throughput configurations - all in one system!


An expert from biotechnology company Sino Biological explains how the company’s advanced line of recombinant proteins ensures the purity of reagents
In biomedical research and biomanufacturing, the purity of reagents directly impacts the reliability of experimental outcomes and the safety of downstream therapeutic products. Endotoxins are notorious contaminants in protein preparations. Even trace amounts of endotoxin in recombinant proteins can trigger potent immune responses, distort results, and compromise patient safety, especially in sensitive applications such as immunology, cell and gene therapy, and vaccine production. Biotechnology company Sino Biological’s recent launch of ProPure, an advanced line of endotoxin-free recombinant proteins. aims to address this need.
ProPure represents a new industry standard in the manufacture of recombinant proteins with ultra-low endotoxin content. Unlike conventional preparations, ProPure products are engineered to achieve endotoxin levels below the limit of quantification (LOQ). They are entirely produced in the US at Sino Biological’s centre for bioprocessing (C4B) in Houston, Texas. This facility leverages mammalian expression systems and proprietary multi-step purification protocols to deliver proteins with exceptional purity, stability, and biological integrity.
Sino Biological’s ProPure prduct benefits from minimum endotoxin levels during protein production
Endotoxin-free plasmids
Endotoxin-free buffer with filtration
Low endotoxin-affinity plastic containers
Regular clean-in-place (CIP)
Endotoxin contamination can derail sensitive experiments and therapeutic applications in the following ways:
Distorted data: Even low levels of endotoxin can activate immune pathways, leading to false signals in cell-based assays and immunological studies.
Safety concerns: Contaminated proteins used for animal studies or clinical research may induce harmful inflammatory reactions, endangering animal welfare or patient safety.
Regulatory compliance: Biomanufacturing and therapeutic protein production require strict endotoxin control to meet regulatory standards and ensure product safety.
Features and advantages of ProPure:
Ultra-low endotoxin content: Advanced purification techniques ensure endotoxin levels below the limit of quantification (LOQ), surpassing industry requirements and minimising experimental risk.
Mammalian expression systems: These systems, paired with tailored protocols, minimise bacterial endotoxin introduction and yield proteins with native-like post-translational modifications (PTMs), folding, and functionality.
High consistency and quality: The proprietary manufacturing process ensures batch-to-batch consistency, essential for reproducible results in sensitive applications such as immunology, vaccine research, and animal studies.
Customisation options: In addition to standard catalog proteins, ProPure provides made-to-order custom protein development to serve unique research and production requirements.
Made in the US: The US-based C4B facility underscores Sino Biological’s commitment to supporting the worldwide life sciences community with rapid production, reliable logistics, and robust quality assurance.
Sino Biological’s philosophy goes beyond shipping ultra-pure proteins. The C4B also partners with research labs and biotech firms to offer:
Custom protein production: Tailored to meet specific research needs, including production scale, tag design, and formulation.
Fast turnaround: The integration of cutting-edge technologies, strong expertise in recombinant production, and rapid shipping leads to shorter lead times and increased reliability, crucial during urgent projects like pandemic response or immunotherapy development.
Technical Support: Expert consultation and technical guidance improve experimental success.
A strong example of endotoxinfree protein quality is the Human B7-H3/CD276 protein, which achieves over 90% purity by SDS-PAGE and SEC-HPLC. Its purity and correct molecular size (about 60.4 kDa by SECMALS) are confirmed, while its bioactivity is verified by ELISA with an EC50 of 6.7 ng/ mL. This case underscores the importance of rigorous testing for purity, identity, and bioactivity to guarantee highquality, contamination-free proteins for sensitive research.
Fig.1: Premium ProPure endotoxin-free protein
Optimised mammalian expression platforms and rigorous QC ensure high quality and consistency
Customised proteins and antibodies with an approximate two week delivery window.
24 well plates to 25 L bioreactors for your screening to production level protein needs
Advanced equipment and minimal endotoxin during processing
Sino Biological argues that ProPure marks a new life sciences benchmark for the following reasons:
Researchers and biomanufacturers have a trustworthy source for recombinant proteins, enhancing accuracy, reproducibility, and safety.
The launch of the ProPure product line marks Sino Biological’s ongoing investment in both technological innovation and service to the global scientific community.
In today’s landscape of advanced biomedical research and therapeutics, using endotoxin-free proteins has never been more important. Sino Biological’s endotoxin-free recombinant proteins mean researchers and industry professionals can perform at a high standard, bolstering scientific excellence while minimising risk, according to the company. As research becomes ever more sophisticated and regulatory requirements tighten, the innovation heralds a new era of safety, consistency, and scientific integrity for life science applications.

A whitepaper from ACD/Labs examines how AI-enabled DMTA cycles can overcome fragmented workflows in drug discovery and development
Aseries of two whitepapers produced by ACD/Labs discusses how pharma and biotech organisations can leverage automation and informatics technologies to digitalise workflows, empowering faster, robust, and more cost-effective innovation.
Informatics company ACD/ Labs develops and commercialises software in support of digitalised R&D and has released a new twopart white paper series, AI-DigitalPhysical Convergence: The Future of DMTA in Drug Discovery and Development. The series explores how pharmaceutical organisations can accelerate innovation by modernising the design-make-test-analyse (DMTA) cycle through AI applications and scientific software working in concert with scientists and the physical experiments they undertake.
The papers describe the transformative potential of an ‘AIdigital-physical DMTA cycle’— which can help organisations reduce data preparation time for predictive modeling and AI/ML applications from 80% to zero.


Part one focuses on drug discovery and outlines how digital twins and AI can accelerate lead optimisation, reduce the burden of manual synthesis design, and improve decision-making across exploratory and confirmatory experimentation. By unifying design, synthesis, testing, and analysis, researchers can shorten the path to identifying viable clinical candidates while maintaining scientific rigour.
Part two discusses the implementation of these principles in pharmaceutical development focusing on Chemistry, Manufacturing, and Controls (CMC). Innovations that accelerate pharmaceutical development can significantly reduce the cost of developing an API into a drug product. AI-augmented DMTA cycles enable organisations to implement quality by design (QbD) principles more effectively; leverage design of experiments (DoE) and Bayesian optimisation for iterative and robust design; apply process digital twins for continuous optimisation and regulatory readiness; and improve drug substance characterisation and drug product formulation with higher reproducibility and compliance.
The whitepapers argue that AI can significantly reduce data preperation time for predictive modelling
‘The scientific method is being redefined,’ said Andrew Anderson, white paper author and vice president of innovation and informatics strategy at ACD/Labs. “While many R&D organisations are well into their digitalisation journeys, most continue to operate in fragmented environments that rely heavily on manual data transfer between systems. This creates inefficiencies, increases the risk of errors, and slows down the transition from scientific insight to clinical reality.”
“We’re increasingly seeing machinereadable data being the work product of experiments to help shorten project timelines. Leaders in pharmaceutical R&D are striving to enable collaborations with well-structured data—by their scientists, between scientists and machines, and machineto-machine. In this white paper series, we’re highlighting best practices from the world’s most innovative R&D organisations.”
The white paper series “AIDigital-Physical Convergence: The Future of DMTA in Drug Discovery and Development” is now available for download at acdlabs.com/ FutureofDMTA

From our founding in a former elementary school in 1985 to becoming a global, 100% employee-owned company, we’ve come a long way in our 40year history. We’re proud of our legacy of innovation, our commitment to close partnerships, and our continual investment in the future. Above all, we’re proud of the work we’ve accomplished with you.
Join the celebrations at restek.com/ restek40 A legacy of innovation, a blueprint for the future.
Dr Jakob Maciejko from Nippon Genetics explores how nextgeneration lab equipment supports the synthesis of human genomes
Anew initiative, SynHG (synthetic Human Genome) will develop scalable tools and technologies for synthesising human genomes. This five-year research project marks a bold step forward in genomics. In this article, Dr. Jakob Maciejko, product manager at Nippon Genetics Europe explores recent genomics progress and how modern lab equipment supports cuttingedge research.
Advancements in genomic research have brought the development of nucleic acid drugs (NADs), which regulate and translate nucleic acid functions. These therapies have demonstrated longlasting efficacy in gene repression, replacement and editing.
Numerous studies now discuss the feasibility of NADs in both the prevention and treatment of diseases, the most widely-recognised application of which is the COVID-19 mRNA vaccines
RNA-targeting NADs have gained significant attention in the pharmaceutical industry. Companies from AstraZeneca to Moderna are leveraging RNA-based therapies to address neurological, cardiovascular, genetic and infectious diseases, as well as metabolic disorders.
One particularly active area is oncology. As of January 31, 2024, there were at least 131 RNA-based therapies in clinical trials, with many more in pre-clinical development.
An exciting drug in the pipeline is mRNA-4359, an mRNA drug for the treatment of advanced solid tumours. The first UK patients received the experimental mRNA therapy at

Imperial College Healthcare NHS Trust as part of a phase 1/2 clinical trial in October 2024.
Dr David Pinato, a clinician scientist at Imperial College London’s Department of Surgery Cancer states, “This research is still in the early stages and may be a number of years from being available to patients, but this trial is laying crucial groundwork that is moving us closer towards new therapies.”
Traditional UV-based gel documentation systems, which have remained largely unchanged for over two decades, still dominate despite their limitations. These systems expose lab personnel to harmful UV radiation and can degrade nucleic acid samples, compromising accuracy.
Modern alternatives such as Blue/ Green LED-based systems offer
significant improvements. The LED light is emitted in the visible spectrum and causes no damage for the sample or for the user.
Planet A Foods chose the gel doc system with Blue/Green LED technology from Nippon Genetics Europe as part of their project.
This system provides faster, safer and more precise DNA imaging, crucial in workflows like those at Planet A Foods.
Importantly, these advanced systems are not more expensive. In fact, their lower energy consumption reduces utility costs. Their customisable design, adaptable in complexity and size by Nippon Genetics Europe, makes them suitable for any laboratory, whether large commercial or smaller specialised.
In this article, Dr. Shun-Hsin Liang from Restek details an efficient methodology for direct, simultaneous determination of ultrashortchain, alternative, and legacy PFAS
As interest in monitoring a wider range of PFAS in both potable and nonpotable waters grows, efficient methodology becomes more important. Here, a team from Restek describes a unique approach that provides concurrent ultrashort-chain PFAS analysis along with alternative and legacy PFAS, allowing C2, C3, C4, C6, C8, and alternative compounds to be tested together instead of through separate methods. Results from verification experiments are presented.
Ultrashort-chain, or C2 and C3, per- and polyfluoroalkyl substances (PFAS) are small and very polar compounds that contribute to at least 40% of the total PFAS detected in environmental waters (e.g., rain, river, and groundwaters) [1, 2, 3]. Ultrashortchain PFAS include trifluoroacetic acid (TFA), perfluoropropanoic acid (PFPrA), perfluoroethane sulfonate (PFEtS), and perfluoropropane sulfonate (PFPrS), with TFA being the most abundant and difficult to analyse chromatographically. Current practices for PFAS monitoring do not address the analysis of these newly trending ultrashort-chain compounds owing to their insufficient retention on typical reversed-phase (RP) columns. On the other hand, analytical methods implementing anion-exchange chromatography often show too much retention and poor chromatographic

performance for ultrashort-chain PFAS. The challenge becomes even greater for simultaneous monitoring of ultrashort-chain, alternative, and legacy PFAS in a single method. To overcome this limitation, we used a unique hybrid HILIC/ion-exchange column (Raptor Polar X) to develop a fast and simple LC-MS/MS method for comprehensive analysis of C2, C3, C4, C6, C8, and alternative PFAS. Because the column employs balanced, multimode retention mechanisms, ultrashort-chain PFAS and long-chain PFAS can all be analysed in a single isocratic run. This direct injection method was evaluated by precision and accuracy analysis of fortified water samples, including tap water, river water, groundwater, and water from publicly owned treatment works (POTW, sewage effluent). As demonstrated here, the method provides convenient setup and high-throughput conditions for water testing labs interested in adding ultrashort-chain PFAS analysis to the same workflow used to measure alternative and legacy PFAS.
Because the column employs balanced, multimode retention mechanisms, ultrashort-chain PFAS and long-chain PFAS can all be analysed in a single isocratic run
Chromatographic method:
The chromatographic conditions were as follows.
Column: Raptor Polar X (2.7 µm, 50 mm x 2.1 mm ID [cat.# 9311A52])
Column temp.: 40 ºC
Injection volume: 10 µL
Mobile phase A: Water, 10 mM ammonium formate, 0.05% formic acid
Mobile phase B: Acetonitrile:methanol (60:40), 0.05% formic acid
Time (min) %B
0.00 85
8.00 85
Flow rate: 0.5 mL/min
Ion mode: Negative ESI
Mode: MRM
In a polypropylene vial (used to mitigate background contamination), 250 µL of each water sample was mixed with 250 µL of methanol and 5 µL of internal standard solution (10 ng/mL of 13C2-PFHxA, 13C2PFOA, 13C3-PFBS, 13C4-PFOS in methanol). The vial was capped with a polyethylene cap (again, to reduce background contamination) for injection and analysis.
Calibration standards were prepared by using deionised water (generated by a Thermo Scientific Barnstead E-Pure system) and fortifying it with 14 analytes at a range of 10–800 ng/L. The calibration standard solutions were then diluted 1:1 in methanol following the sample preparation procedure above.
A tap water sample from the Restek facility and three water samples (Chicago river water, groundwater, and POTW effluent water) supplied by the United States Environmental Protection Agency (U.S. EPA) were fortified at 40 and 160 ppt. Blank and fortified water samples were diluted 1:1 in methanol as above for chromatographic analysis and quantified with the calibration standards. For TFA measurement in groundwater, the sample was diluted fivefold with deionised water before fortification at 40 and 160 ppt due to its high TFA concentration.
An isocratic elution was established that produced a fast, simple ultrashort-chain PFAS analysis along with alternative and legacy PFAS in water samples. All analytes eluted in four minutes with balanced retention and good peak shapes (Figure 1). No matrix interference was observed in any of the water samples using an eight-minute cycling time. As will be discussed below, the approximately four-minute hold after the last eluting compound was shown to be necessary to avoid possible matrix interferences.
A simplified isocratic method was developed and verified for ultrashortchain PFAS analysis along with alternative and legacy compounds in water samples. Due to the balanced, multimode retention of these analytes on a Raptor Polar X (2.7 µm) 50 x 2.1 mm column, the analytical method was demonstrated to be fast, rugged, and sensitive with acceptable accuracy and precision. This method is suitable for analytical labs wanting to expand their existing PFAS assays for potable or non-potable water to include C2 and C3 compounds. For the full details of this application note including information on transition times and internal standards, please visit https://www.restek.com/articles/anovel-approach-for-ultrashort-chainpfas-analysis-in-water-samples.
Figure 1:
Chromatographic performance in a single isocratic run


For more information visit: www.restek.com







Michael Schupp from Gerteis explains why design of experiment has become a powerful statistical methodology, and how automation of this can speed up development
The pharmaceutical industry operates within a highly regulated environment that demands rigorous standards for product quality, safety, and efficacy. As the complexity of drug formulations and manufacturing processes continues to increase, traditional trial-and-error approaches to development have proven inefficient and often insufficient. In this context, Design of Experiment (DoE) has emerged as a powerful statistical methodology that enables systematic, efficient, and data-driven exploration of the relationships between critical process parameters and quality attributes.
DoE facilitates a deeper understanding of both formulation and process variables by allowing simultaneous investigation of multiple factors and their interactions. This approach not only accelerates development timelines but also supports regulatory expectations outlined in Quality by Design (QbD) frameworks. By leveraging DoE, pharmaceutical scientists can optimise formulations, enhance process robustness, reduce development costs, and build a strong foundation for scale-up and technology transfer.
However, despite its recognised advantages, the successful implementation of DoE in a pharmaceutical setting presents several challenges that can affect the quality and interpretability of experimental outcomes. These challenges are multifactorial, involving technical, organisational, and resource-related constraints.
One of the primary challenges is the complexity of experimental design. As the number of investigated factors and levels increases, the number of required experimental runs can grow rapidly, especially when interactions and higher-order effects are included. This often leads to substantial demands on materials, instrumentation, and personnel. In early-stage development, where resources are typically limited, such demands may necessitate compromises that impact the robustness of the experimental design.
Another significant challenge lies in the identification and selection of critical factors and appropriate experimental ranges. The omission of relevant variables or the use of impractical or narrow factor ranges may result in models that
do not accurately reflect real-world process behavior. This step requires comprehensive prior knowledge of the system under investigation and is frequently complicated by the inherent uncertainty present during the early phases of product and process development.
The presence of process variability and uncontrolled sources of noise also complicates DoE execution. Variability arising from environmental factors, equipment performance, and operator techniques can obscure true factorresponse relationships, reducing the signal-to-noise ratio and potentially leading to misleading results. This is particularly problematic in small-scale or non-GMP environments where control strategies may be limited.
To address the practical challenges associated with manual DoE implementation, the pharmaceutical industry is increasingly turning to automation as a transformative enabler. The automation of the DoE sequence software tools capable of executing predefined experimental protocols with high precision and repeatability. By doing so, they significantly reduce the risk of
human error, streamline experimental throughput, and enhance data integrity.
One of the primary advantages of DoE automation is the dramatic increase in execution speed, allowing teams to explore a broader design space within a shorter timeframe. In addition to speed, automation enhances reproducibility and consistency across experiments. Human-induced variability—stemming from differences in technique, timing, or interpretation—is virtually eliminated when experimental steps are executed by automated systems. This reduction in procedural variability improves the signal-to-noise ratio of experimental data, leading to more reliable model fitting and more confident interpretation of factorresponse relationships.
The DoE software feature for advanced Gerteis roller compactors perfectly implements all these benefits for dry granulation. Press force and gap are scientifically proven to be the most influential parameters in dry granulation. This knowledge is implemented in the software feature, allowing to run automatic sequences of different press force and gap combinations to find the right parameter settings and identify the influence on product characteristics.
The parameter range can be configured individually to adjust to the DoE strategy. By using the automatic sequence input errors can be avoided and operators can focus on sampling instead of configuring the equipment. Furthermore, the DoE software feature indicates steady state for sampling to reduce variability by different operator sampling techniques.
Figure 1 symbolically shows how the system distinguishes between steady state and adjustment phase. Sampling is performed in steady states only to avoid any out of specification material in the sample. The operator does not have to decide when the process is considered steady; this is determined automatically based on fixed criteria, thus ensuring repeatability and reducing human error.
Physical limitations during execution of the trials, for example draw-in issues, are automatically detected. This allows to skip more challenging steps, helping to save valuable product instead of losing it by running impossible trial settings.
The working principle is shown in Figure 2. If any physical limitations are detected, the current step will end and all more challenging steps, which are
One of the primary advantages of DoE automation is the dramatic increase in execution speed, allowing teams to explore a broader design space within a shorter time frame

steps at higher press force and gap combinations are automatically skipped. In conclusion, DoE plays a critical role in advancing pharmaceutical development by enabling efficient, data-driven process optimization. While manual DoE implementation faces several practical challenges, automation offers a robust solution by improving precision, reducing variability, and increasing throughput. The integration of automated DoE features, such as those in advanced Gerteis roller compactor software, exemplifies how technology can enhance both the reliability and efficiency of experimental workflows.
For more information visit: www.gerteis.com
Authors: Michael Schupp, Head of Technology & Process Engineering, Gerteis Maschinen + Processengineering AG, Stampfstrasse 85, 8645 Jona, Switzerland, +49 55 222 55 22, www.gerteis.com
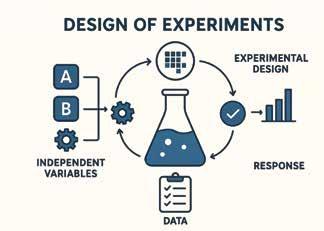

Bill Turner from Natoli provides a pragmatic perspective for European manufacturers looking to ensure tablet consistency and punch length
Standards are a part of our lives. Electrical plugs, petrol nozzles, lamp fixtures, and even mobile phone manufacturers have finally agreed on a plug standard that reduces waste and excessive inventory. In the pharmaceutical industry, standards are governed by organizations and institutes such as NSF, ISO, PDA, USP and ASTM, for example. Standards are established for many reasons and affect every sector and sphere.
In the pursuit of pharmaceutical excellence, tablet consistency remains a cornerstone of quality assurance. Yet, as many seasoned manufacturers know, achieving uniformity in tablet parameters is a multifaceted challenge. While punch length tolerance often takes centre stage in discussions, it is but one of many influential factors. This editorial aims to provide a balanced, persuasive perspective on the true impact of punch length tolerance, especially within the context of European manufacturing standards, and to highlight other critical contributors to tablet consistency.

Bill Turner, Natoli
Tablet consistency begins with formulation. Variations in active pharmaceutical ingredients (APIs), excipients, particle sizes, shapes, and preparation methods all play pivotal roles. Since tablet dies are filled volumetrically, blend density must remain consistent to ensure uniform
weight and thickness. Particle size distribution (PSD) is particularly impactful – smaller particles compress more tightly, increasing compacted density and hardness. However, excessive fines can lead to defects such as sticking and flashing. Batch-tobatch variability in PSD, and particle morphology can significantly alter tablet properties, underscoring the need for rigorous formulation control.
The tablet press itself introduces variables that affect consistency. Die table run-out – vertical movement during rotation – can alter fill volume and weight, as the dosing scraper interacts with the die table at a critical moment. Pressure rolls may also exhibit run-out or lack perfect roundness, leading to inconsistent tablet thickness. These mechanical nuances, though often overlooked, warrant attention during troubleshooting and process optimisation.

TOOLING TOLERANCE FACTORS: RETHINKING THE PUNCH LENGTH PARADIGM
In the arena of solid dose manufacturing, we are aware of the many standards that exist that assure product quality and safety. The standards for tablet compression tooling are relatively new and govern the basic tool configuration for the most common “B” and “D” type tools. The tool standards not only govern the basic tool configuration, such as tool length, punch head configuration, and barrel diameter, but they also affect the mating surfaces, such as the tablet press cams, turret guides, and die pockets. Although a governing body does not govern the latter, tablet press manufacturers respect the tool standards as a guide for engineers tasked with developing tablet presses for optimum performance.
Tooling, particularly punch dimensions, is frequently scrutinised when tablet inconsistencies arise. While variations in working length (W.L.), cup depth (C.D.), and overall length (O.L.) do influence tablet parameters, their impact is often overstated.
European manufacturers typically adhere to a ±0.01mm W.L. tolerance, as per ISO 18084 standards. By contrast, U.S. TSM specifications allow a broader ±0.025mm tolerance. Despite these differences, data shows that even a ±0.02mm tolerance yields tablets well within acceptable weight and thickness ranges.
DATA ANALYSIS: QUANTIFYING THE IMPACT OF TOLERANCE
Consider a 10mm diameter standard concave tablet with a target weight of 350mg and thickness of 4.80mm. A ±0.01mm W.L. tolerance results in a thickness variation of ±0.4% and a weight variation of ±0.15%.

Tolerance of ±0.01mm


Doubling the tolerance to ±0.02mm increases thickness variation to ±0.8% and weight variation to ±0.3% – still well within specification. These figures represent worst-case scenarios at tolerance extremes; actual deviations are typically smaller owing to machining practices.
Consistency in working length across a punch set is more critical than absolute dimensional values. As punches wear, deviations may occur, but punch sets with ≤0.04mm T.I.R. remain viable for consistent tablet
production. Matching reports, which pair punches based on W.L., combine specific upper and lower punches to result in the least possible combined W.L. variation. This minimises tablet thickness deviation and enhances uniformity. These reports can be generated for both new and used tooling using automated inspection systems like Natoli’s TMII.
Tighter tolerances demand more sensitive, costly equipment and frequent calibration. Environmental factors, such as temperature, can
affect measurements due to thermal expansion and contraction of tool steels. Manufacturers must weigh the benefits of tighter tolerances against the logistical and financial implications of maintaining them.
Tablet consistency is influenced by a constellation of factors –formulation, press mechanics, and tooling dimensions. While punch length tolerance plays a role, it should not be overemphasised at the expense of other variables. European manufacturers can confidently adopt a ±0.02mm W.L. tolerance, achieving in-spec tablets while optimising tooling costs and inspection efficiency. A holistic approach, grounded in data and practical insights, is the key to consistent, high-quality tablet production. For more information visit: www.natoli.com

Meet the NEW ProteQ VISO from Vision Engineering - a revolutionary digital stereo microscope set to redefine precision workflows. Its groundbreaking ‘autostereo’ display delivers true 3D imaging on a flat screen - no eyepieces or glasses required - dramatically enhancing user perception while improving comfort and reducing fatigue.
Engineered for inspection, design and manufacturing, ProteQ VISO combines exceptional 3D imaging performance with powerful digital tools. Users can effortlessly capture and share 3D images, collaborate in real time, and integrate with networks.
Boost productivity and achieve unparalleled clarity for applications in electronics, medical device development, and more .
Experience the next generation of microscopy.
Contacting Vision Engineering as follows. Or scan the below QR code.
Tel: +44 (0) 1483 248300
Email: enquiries@visioneng.co.uk
Web: www.visioneng.com/proteqviso

Al2O3 and a pure aluminium particle on a carbon background. The contrast can be seen clearly
In this article, Prerna Sudera from Thermo Fisher explores how advanced microscopy helps manufacturers improve cleanliness and product reliability
Not every product failure starts with a faulty part or design flaw. Often, the problem is something much smaller: microscopic contamination. Tiny particles such as dust, metallic debris or fibres can quietly compromise the performance of final products. Here, Dr. Prerna Sudera, industry specialist at Thermo Fisher Scientific, discusses how advanced microscopy techniques can help manufacturing teams to master technical cleanliness for more reliable products.
Even at the scale of a few microns, foreign particle contamination can be detrimental to the functionality and reliability of products. For example, microscopic debris can scratch surfaces, obstruct microfluidic channels or nozzles and can cause electrical contacts to short-circuit, especially in densely packed electronic assemblies.
Successfully applying coatings, adhesives and paints also relies on contaminant-free surfaces. Residual contamination can act as a barrier, preventing proper bonding or film formation. This can result in delamination, reduced corrosion resistance or mechanical failure — all of which compromise product durability and performance.
For these reasons, many industries enforce technical cleanliness standards as a regulatory requirement. In the medical device sector, for example, guidelines such as ISO 19227 help to ensure surgical implants and instruments are free from particulate contamination that could trigger inflammation or infection. In the automotive industry, standards such as

ISO 16232, or VDA 19 for the German market, help to guarantee high-quality fluid-bearing components.
A major obstacle to technical cleanliness is the complex workflows and resources it typically demands. Expert operators are needed to complete manual cleaning and inspection processes, which can introduce delays into overall production. Additionally, different contaminants require different approaches to cleaning. For example, ultrasonic cleaning is used to remove metallic particles, while solvent treatments are needed to eliminate chemical residues.
Prior to cleaning, precise particle detection and analysis is a critical first step. Advanced technologies must accurately determine not only particle size, but also shape and chemical composition, ensuring contaminants are identified and addressed effectively.
For example, larger particles with sharp, irregular edges can cause abrasion or mechanical blockage, while smaller particles can penetrate lubrication films, accelerate wear and trigger fatigue or corrosion. Additionally, knowing whether a particle is metallic, polymer-based, glass or an organic residue can help identify its source and inform process improvements to prevent recurrence. Using a combination of microscopy techniques, manufacturers can unlock these insights.
Optical microscopy and scanning electron microscopy (SEM) are two of the most common technical cleanliness techniques. After washing
components, the particles collected on a filter membrane can be analysed using either method. Each brings its own advantages.
The primary benefit of optical microscopy is its speed. It takes only five to ten minutes for optical microscopes to scan whole filters. However, for this method to be successful, the contaminants analysed must be a different colour than the background, as optical microscopes use visual contrast to detect particles. Light-coloured or transparent particles such as glass are likely to be missed.
In contrast, SEM offers enhanced imaging capabilities that go beyond simple visual inspection. Instead of colour contrast, SEMs detect variations in atomic number by capturing backscattered electrons. SEM also produces high-resolution images of a particle’s surface structure by detecting low-energy secondary electrons emitted from the sample. This surface detail helps operators to understand the physical characteristics that may contribute to functional issues, such as irregular edges.
Another key advantage of SEM lies in its ability to integrate energy-dispersive X-ray spectroscopy (EDS) capabilities. Advanced systems that combine both SEM and EDS analysis allow users to generate compositional data in parallel with imaging. This makes it possible to identify the elemental composition of particles, helping to track the root causes of contamination and therefore implement more targeted process controls.


SUSTAINABILITY • AI & AUTOMATION • QUALITY & COMPLIANCE • BIOTECHNOLOGY • SKILLS & DEVELOPMENT
Meet with your peers across the UK lab community, share knowledge with industry leaders in the conference, get-hands on with tech from leading suppliers - and ultimately save time when levelling up your lab practices.


4,500+
200+
NEW lab professionals suppliers networking opportunities
5 conference streams




Phillip Townend from Vision Engineering explores how a digital stereo microscope enables eyepiecefree 3D image viewing, capture and share
AA digital stereo microscope that has been designed for inspection, design and laboratory applications
new type of digital stereo microscope has been developed for detailed inspection, design, and laboratory applications. The system’s primary technical innovation is a fully integrated autostereoscopic display, which generates true three-dimensional images on a flat screen without requiring the operator to wear special glasses or use traditional eyepieces. This development aims to improve operator ergonomics and workflow efficiency in precision-focused fields such as electronics, prototyping, and medical device manufacturing. The technology includes the capability for 3D image sharing, allowing distributed teams to collaborate in new ways. Live streaming, picturein-picture and headset-free sharing support rapid decisions and effective communication across laboratories, departments and sites.
The system delivers a glasses-free 3D image by combining an advanced IPS LCD display with an integrated eyetracking system. Twin cameras built into the monitor track the operator’s eye position in real-time. This data is processed by software that continually adjusts the image projected to each eye, ensuring a clear and stable stereo image is maintained even as the user moves their head.
By eliminating the need for eyepieces, this technology allows operators to maintain an upright, natural posture, which can reduce musculoskeletal strain and fatigue during extended use. The enhanced depth perception provided by

The system is suitable for operation in cleanroom environments, including laminar flow cabinets
the stereo image allows for more intuitive interaction with the subject being inspected or manipulated. For collaborative work, the display can be switched from 3D to a conventional 2D mono view, allowing multiple viewers to see a shared image simultaneously, either locally or via remote streaming.
To support contemporary technical environments, the microscope integrates a suite of digital tools accessible directly through its interface. Operators can capture, play back, and stream high-resolution images and video to a USB drive or a connected PC. The software includes
The digital microscope system features an integrated autostereoscopic display that creates a 3D image without the need for eyepieces

tools for on-screen annotation and digital dimensioning, allowing users to add notes and perform measurements on the live or captured image to streamline reporting.
Further integration features include picture-in-picture and image overlay capabilities, enabling a live view to be compared against a reference image or diagram. The system can interface with external analysis software and provides network connectivity, allowing operators to access files and communication platforms directly from the microscope unit.
The microscope system, known as ProteQ VISO, incorporates several features for use in specialised environments. A 10:1 optical zoom is controlled electronically, with the current magnification level displayed live on screen. The lighting system uses low-heat LEDs to protect sensitive samples, and lighting components can be changed quickly to suit different applications.
For safety-critical or cleanroom applications, the display unit can be separated from the main microscope body and mounted externally. Optional modules, including a 360-degree rotating viewer and sub-stage
illumination for translucent samples, provide additional flexibility for complex inspection tasks.
When inspection results need to be shared, VISO makes it simple for distributed teams to see the same detail in 3D at the same time. Live streaming, picture-in-picture and headset-free sharing support rapid decisions and effective communication across departments, sites and supply chains.
• Live 3D streaming for instant feedback and approvals – 3D image viewing in multiple locations simultaneously
• Picture-in-picture for two-way visual interaction
• Headset-free remote sharing without specialist equipment
• Multilingual interface for international teams
• Ideal for collaboration in contract manufacturing approvals, life science and distributed laboratory expertise evaluations, and design reviews across remote teams.


Automated microscopy technology can help industrial teams keep pace with change.
In this article Dr. Anna Prokhodtseva from Thermo Fisher Scientific tells us how
Advanced materials, such as multiphase alloys and layered composites, are constantly being engineered to meet performance, weight and durability goals in manufacturing. However, implementing these materials requires characterising them in high resolution to understand their properties and behaviour. This forces materials engineers and quality control professionals to familiarise themselves with several specialised instruments.
Across automotive, aerospace and additive manufacturing, materials are becoming more complex. For example, nanostructured coatings are now standard in many applications. For all their performance advantages, such as improved strength or durability, these materials are challenging to analyse because of their intricate micro- and nanoscale features.
Researchers must record specific information about the internal structures of these materials to assess how they will perform in their intended applications. However, obtaining
1) SEM image of a cross-section array created on a Zn-Mg-Al-coated lowcarbon steel.
2) SEM image showing a cross-section of a Zn-Mg-Al-coated low-carbon steel. 3) SEM tilt-corrected image showing microstructural features of the ZnMg-Al coating on the low-carbon steel substrate.
thorough, high-resolution material characterisation is laborious and technically challenging.
This is not helped by the growing skills gap. UK manufacturers continue to face challenges when hiring skilled workers, with 75 per cent recording skills shortages as one of their top three obstacles to growth, according to a report from Barclays Corporate and The Manufacturer. Furthermore, a 2024 paper published by the International Monetary Fund noted that advanced manufacturing is among the sectors with the greatest skills gap increase.
Because of this skills deficit, many quality control and failure analysis teams lack the in-house expertise to operate advanced characterisation equipment, like focused ion beam (FIB) and scanning electron microscopes (SEM). These are required to reveal subsurface structures and generate high-resolution cross sections. However, owing to the shortage of skilled operators, they are generally considered too complex or specialist for routine use in industry.
Thermo Scientific developed the Scios 3 FIB-SEM to help combat these challenges. It is designed for high-throughput, precise materials analysis, combining FIB and SEM technologies into one integrated system. Most importantly, it includes advanced automation features to reduce the learning curve that normally accompanies FIB-SEM systems, meaning the instrument is significantly more accessible to those with limited microscopy experience.
The ability to generate reliable, high-quality cross sections and sub-
surface images with little manual input is central to the Scios 3 FIBSEM’s design.
Finally, the Scios 3 FIB-SEM supports various configurations, including secondary ion mass spectrometry (SIMS), energy dispersive X-ray spectroscopy (EDS) and electron backscatter diffraction (EBSD) detectors. It also offers flexible configurations for lamella preparation and 3D analysis. This enables different teams to tailor the system to meet their unique application requirements, whether it’s failure analysis, quality control or materials development.
The automotive industry frequently uses zinc-based coatings to prevent corrosion on steel parts. It is difficult to conduct a thorough analysis of these coatings owing to their intricate microstructures, which include fine intermetallic layers and several alloy phases.
Cross sections of these coatings traditionally require significant manual labour and skilled operators to prepare and image. This procedure can now be substantially automated with the Scios 3 FIB-SEM and its Auto
Cross Section (AXS) software. Users can designate areas of interest and instruct the system to mill and image these successively without constant supervision, automatically creating and imaging cross sections at multiple sites on a sample. As well as reducing the strain on operators, this automation improves consistency across samples and speeds up the overall workflow. This is especially helpful when analysing zinc-based coatings because the feature sizes vary significantly, ranging from large primary zinc dendrites that are over 100 μm in diameter to much finer eutectic phase structures of around 100 nm.
In this application and many others, automated microscopy systems with integrated FIB-SEM capabilities can go a long way in helping close the manufacturing industry’s skills gap. They make advanced materials characterisation more accessible, even for industrial teams lacking specialised knowledge, by simplifying complex processes and producing precise, repeatable results.

The looming changes to the UK Apprenticeship Levy are causing ripples across industries. For the healthcare sector, which often relies on Level 7 apprenticeships to address leadership skills gaps and workforce challenges, these changes are particularly significant.
“With Levy funding for new Level 7 apprenticeship starters aged 22 and over ending in January 2026, healthcare organisations need to act swiftly to capitalise on current funding opportunities before the December cut-off,” explains Steven Hurst, director of corporate learning, Arden University.
Here, Steven explores the potential impact of these changes, highlights the opportunities still available and proposes practical solutions to ensure healthcare organisations can maximise the remaining funding to future-proof their workforce.
Level 7 apprenticeships, equivalent to postgraduate qualifications, have become an invaluable tool for the healthcare sector. They fill critical skills gaps, upskill existing talent and develop leaders who are equipped to tackle some of the industry’s most pressing challenges.
For instance, the senior leader apprenticeship has helped healthcare organisations to address issues such as:
• Leadership diversity: Leadership in healthcare remains predominantly white and male at board level. Level 7 apprenticeships foster diversity by offering training pathways to underrepresented groups.
• Accidental managers: Many healthcare professionals find themselves in leadership roles without formal training. Leadership apprenticeships bridge this gap, providing essential management and strategic skills.
• Digital transformation: Legacy systems and tight budgets have slowed efforts to adopt and scale digital technologies. Training through apprenticeships can prepare leaders to drive successful digital initiatives.

However, with the government’s impending restrictions on Level 7 funding, the healthcare industry faces a narrowing window to maximise these benefits.
From January 2026, government Levy funding for Level 7 apprenticeships will no longer be available for new starters aged 22 and over, which is likely to cause a key pain point for those wanting to formally upskill more senior staff. However, there’s a silver lining for organisations that act quickly, as businesses can still utilise the Levy funding for new level 7 learners, as long as they’re enrolled before the 31 December deadline.
This urgency presents both a challenge and an opportunity. Left unchecked, skills gaps and leadership deficits may exacerbate already critical workforce shortages, but organisations that proactively plan can not only mitigate these risks but also strengthen their position for the future.
To make the most of the remaining
Level 7 funding, healthcare organisations should consider these four key actions:
1. Audit skills needs and workforce requirements
Conduct a thorough assessment of the current workforce to identify skills gaps and forecast future needs. For example, consider areas like leadership, digital transformation and clinical management, where advanced training will be essential. Use this information to prioritise staff who are ready to enrol in Level 7 apprenticeships while funding is still available.
2. Accelerate recruitment for cohorts
With the December cut-off fast approaching, ramping up recruitment efforts for apprenticeships is crucial. Partnering with education providers now can help to ensure smoother enrolment processes. Many training providers are already working with healthcare employers to fast-track applications and onboarding.
3. Focus on age-eligible candidates
With the age cap for funding set to change, targeting talent before the end of the year, who will be ineligible
From January 2026, government levy funding for Level 7 apprenticeships will no longer be available for new starters aged 22 and over
to access funding from 2026, makes strategic sense. Prioritise candidates aged 22 and over for enrolment this year, ensuring that funding supports this group before eligibility shifts.
4. Explore Level 6 apprenticeships as alternatives
For organisations where Level 7 funding may no longer be feasible from 2026 owing to budget restrictions, Level 6 apprenticeships remain a valuable alternative.
Equivalent to degree-level qualifications, these programmes still deliver high-quality training while addressing workforce diversity and retention goals.
While the funding restrictions may signal a shift in apprenticeship opportunities, they also highlight the importance of future-proofing workforce development strategies.
Employers committed to investing in apprenticeships can take steps such as:
• Adjusting training budgets to cover the full cost of Level 7 programmes post-2026, where possible.
• Collaborating with universities and training providers to explore tailored solutions and alternative programmes.
• Staying informed about evolving government funding options, tax incentives and sector-specific initiatives.
Proactively adopting these strategies can ensure that healthcare trusts maintain access to vital skills and leadership development in the years ahead.
The clock is ticking for healthcare organisations to make the most of current funding. Acting decisively over the next few months can help secure the training and leadership development necessary to address workforce challenges and prepare for future demands.
For more information visit: www.arden.ac.uk

Creative Biolabs
A leading global supplier, offers highquality biological products like recombinant antibodies, membrane proteins, and cell lines, and custom services in therapeutics discovery to support your research needs.
T 1-631-416-1478
E info@creative-biolabs.com
W www.creative-biolabs.com

INTEGRA is a leading provider of high quality laboratory tools and consumables for liquid handling. We are committed to fulfilling the needs of laboratory professionals in research, diagnostics, and quality control within the life sciences industry.
T +44 (0) 1635 797000
E info-uk@integra-biosciences.com
W www.integra-biosciences.com

A Swiss manufacturer of advanced highquality pharmaceutical roller compactors. Our understanding of the dry granulation process combined with patented roller compaction systems make Gerteis® the technology leaders in the field of dry granulation.
T +41 (0)55 222 55 22
E sales@gerteis.com
W www.gerteis.com

L.B.Bohle
Headquartered in Germany, a leading system supplier for the pharmaceutical industry. We provide sustainable machines and processes for efficient batch and continuous manufacturing, ensuring high-quality solutions for demanding production needs.
T +492524 – 93 23 0
E info@lbbohle.de
W www.lbbohle.com

Hamamatsu Photon
Manufacturer of optoelectronic components and systems. Including sensors and systems for spectroscopy (inc. ultrafast), scientificgrade cameras, beam monitoring systems, photon-counting detectors and systems, photomultipliers, photodiodes and IR detectors.
T (49)8152-375-0
E info@hamamatsu.eu
W www.hamamatsu.com

PHC Europe
As part of PHC Group, we provide high-quality laboratory equipment for biomedical and diagnostic applications, ensuring precision, reliability and performance. We offer expert service, certification and innovative solutions for life science, healthcare and industrial sectors.
T +31 76 5433833
E marketing@eu.phchd.com
W www.phchd.com/eu/phceu

Restek
Chromatography is what we do and who we are. From LC and GC columns to sample preparation, standards to accessories, Restek is your first and best choice. Restek is Pure Chromatography.
T 1-814-353-1309
E crm@restek.com
W www.restek.com
Sino Biological
A global leader in manufacturing affordable, high-quality reagents, including recombinant proteins, antibodies and cDNA clones in-house.
T +49 (0)6196 9678656
E marketing@sinobiological.com
W www.sinobiological.com
e advertising@setform.com



The 10th Lab Innovations, to take place on the 29th and 30th of October, will act as a meeting place for all lab sectors in industry and research. The show will see attendees present, explore and discover pioneering technologies that are propelling the business of science.
The show will see the following key themes explored:
• Sustainability
• Robotics & automation
• Quality, accreditation and compliance
• Biotech innovation
• Technical product advice & demos
• Professional development
One of the most lauded elements of the show is the lab innovations awards which aims to celebrate pioneers of industry. The ceremony will take place on the final day of the show and aims to recognise the achievements, hard work and commitment of those in the Lab Innovations community. Reply to the call for entries on the website if
Robotics and automation will form one of five key themes at the show

you have a project you’re proud of or a colleague you’d like to see recognised. The themes of the show were decided upon during an annual advisory board in March which examined feedback and reflected on industry advances. This year the organisers decided to focus on content and the the conference agenda.

• Content is key
• Attendees are looking to gain knowledge on topics such as AI, automation, regulation and importantly sustainability, as an umbrella topic across the whole event.
• Content as a whole should be varied and cover interests of the broadest range of attendees.
Delegates will be able to choose from five conference theatres with talks that aim to provide food for thought - they cover innovation, sustainability, automation, data, AI, regulation, accreditation, biotech, upskilling, careers and more.
They will hear case studies from scientific thought leaders who have driven breakthroughs,
A digital hub on the website offers a podcast, media gallery, news and blogs providing insights and information on the industry’s new innovations.

• Temperature compensated
• High pressure tolerance (20 bar)
• Fast response time (1-10 Hz)

• Small and bio-compatible
• Digital USB communication
• Automatic pH range log

Biotech Fluidics has now launched a new flowmeter kit that provides fast, precise, and reliable validation of the ongoing performance of any HPLC pump.


